 ,1, 党志红1, 朱伟旗2, 高占林
,1, 党志红1, 朱伟旗2, 高占林 ,1, 韩秀英
,1, 韩秀英 ,1, 赵建江1, 王文桥1, 路粉1, 吴杰1
,1, 赵建江1, 王文桥1, 路粉1, 吴杰1Identification of Major Pathogenic Fungi of Soybean in Hebei Province and Screening of Control Fungicides
BI QiuYan ,1, DANG ZhiHong1, ZHU WeiQi2, GAO ZhanLin
,1, DANG ZhiHong1, ZHU WeiQi2, GAO ZhanLin ,1, HAN XiuYing
,1, HAN XiuYing ,1, ZHAO JianJiang1, WANG WenQiao1, LU Fen1, WU Jie1
,1, ZHAO JianJiang1, WANG WenQiao1, LU Fen1, WU Jie1通讯作者:
责任编辑: 岳梅
收稿日期:2020-04-14接受日期:2020-06-25网络出版日期:2021-01-01
| 基金资助: |
Received:2020-04-14Accepted:2020-06-25Online:2021-01-01
作者简介 About authors
毕秋艳,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3153KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
毕秋艳, 党志红, 朱伟旗, 高占林, 韩秀英, 赵建江, 王文桥, 路粉, 吴杰. 河北省大豆主要病原真菌鉴定及防治药剂筛选[J]. 中国农业科学, 2021, 54(1): 71-85 doi:10.3864/j.issn.0578-1752.2021.01.006
BI QiuYan, DANG ZhiHong, ZHU WeiQi, GAO ZhanLin, HAN XiuYing, ZHAO JianJiang, WANG WenQiao, LU Fen, WU Jie.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】大豆是全世界最重要的农作物之一,在农业生产和社会发展中发挥着重要作用。根据国家统计局数据显示,2019年中国大豆播种面积933万公顷(1.4亿亩)。据农情调查,2019年河北省大豆种植面积10.4万公顷。河北省处于黄淮海夏大豆产区和北方春大豆产区交界地带[1],四季分明,光照充足,适宜大豆生产[2]。张家口、承德、唐山和秦皇岛等地区为春大豆的主要产区[3]。其他地区以夏大豆为主,占整个河北省大豆种植面积的80%左右。随着大豆种植面积的扩大,病害成为影响其产量的主要因素之一。目前,我国已报道的大豆病害约40余种[4],病原主要包括真菌、细菌、线虫和病毒等,其中70%—80%为真菌病害,一般会造成10%—30%减产,严重时可达50%以上,更严重时甚至会导致绝产[5]。大豆真菌病害不仅直接造成产量与品质降低,部分病原真菌在侵染过程中还分泌多种对人畜有害的毒素与代谢物,对农产品的安全性构成极大威胁[6]。河北省作为全国的大豆主产区,及时科学诊断新病害并提出具体的防治药剂,对于提高大豆产量和品质具有十分重要的现实意义[7]。【前人研究进展】大豆真菌病害种类多、分布广、危害大,各种病害从症状上难以区分,导致防治困难。常见的大豆病害包括大豆根腐病、菌核病、灰斑病、褐纹病、黑斑病、锈病、白粉病等[4]。随着大豆种植面积的扩大,自20世纪90年代,病害逐渐被认为是大豆生产的限制因子[8]。大豆病害在不同地区的病原菌种类复杂,有些种的病原菌只在国外或中国真菌志上报道。大豆上同一种病害在不同国家、不同区域可能为同一属不同种病原菌所致。炭疽菌属(Colletotrichum)是一类重要的植物病原菌,其地理分布和寄主范围均较为广泛。该菌变异较多,种内存在不同的生理小种[9]。对巴西进境大豆进行病害分离,参考Ainsworth分类系统鉴定出4种炭疽菌,证实了大豆炭疽菌具有多样性。大豆灰斑病菌也具有非常明显的生理分化现象[10]。阿根廷大豆曾被鉴定出主要病原菌分别为核盘菌(Sclerotinia sclerotiorum)、大雄疫霉大豆专化型(Phytophthora megasperma)、大豆猝死综合症病菌(Fusarium tucumaniae)、叉丝壳菌(Microsphaera diffusa)。巴西大豆曾被鉴定出主要病原菌有胶孢炭疽菌(Colletotrichum gloeosporioide)、辣椒炭疽菌(Colletotrichum capsici)、平头炭疽菌(Colletotrichum truncatum)和黑线炭疽菌(Colletotrichum dematium)[9]。从俄罗斯种植区采集的大豆植株的叶片和茎杆样品中共分离到6种病原菌,分别为链格孢(Alternaria sp.)、大豆北方茎溃疡病菌(Diaporthe phaseolorum var. caulivora)、黑附球菌(Epicoccum nigrum)、镰孢菌(Fusarium sp.)、立枯丝核菌(Rhizoctonia solani)、毛霉(Mucor fragilis)[11]。对美国进境大豆鉴定出主要病原菌包括细极链格孢(Alternaria tenuissima)、樱桃链格孢(Alternaria cerasi)、苘麻链格孢(Alternaria abutilonis)、百日菊链格孢(Alternaria zinniae)、落葵链格孢(Alternaria basellae)[12]。阿根廷、巴西、俄罗斯和美国鉴定出的大豆主要病原菌可以在辣椒、杂草、小麦等植物上存在共生现象。在大豆病害化学防治方面,根腐病一般采用50%多菌灵可湿性粉剂+50%福美双可湿性粉剂(3﹕2),用药总量为种子重的0.5%进行拌种防治[7]。由于药剂拌种只能控制前期根部病害,成株期一旦发病应尽早喷广谱性杀菌剂防治。大豆灰斑病菌群体结构发生变化,导致某些主栽品种在多雨年份时会大量发生灰斑病[10]。在灰斑病发病初期,应及时喷洒1—2次杀菌剂减轻发病。70%甲基硫菌灵可湿性粉剂1 000倍液或75%百菌清可湿性粉剂700—800倍液对灰斑病具有抑制作用[13]。在大豆疫病发病初期,60%氟吗啉·代森锰锌可湿性粉剂1 500倍或50%烯酰吗啉可湿性粉剂1 500倍喷雾对其具有抑制作用[14]。腐霉利、菌核净、三唑酮和苯醚甲环唑等对大豆白粉病和菌核病均有很好的防治效果[15]。大豆锈病的防治药剂有三唑酮、萎锈灵、多菌灵和代森锰锌等[16]。此外,大豆炭疽病防治药剂以苯醚甲环唑或百菌清为主;大豆紫斑病、黑斑病、灰星病、茎枯病防治药剂均以多菌灵或代森锰锌为主[15]。综上,大豆病害的防治使用比较频繁的杀菌剂以多菌灵、甲基硫菌灵、福美双、菌核净、腐霉利、三唑酮、萎锈灵、百菌清、退菌特、烯酰吗啉和代森锰锌老品种为主,偶见苯醚甲环唑、氟吗啉·代森锰锌药剂的使用。【本研究切入点】近年河北省大豆新增几种不常见病害[6,15],由于对其病害及病原菌种类不十分了解[17],导致药剂种类的选用比较困难。【拟解决的关键问题】对河北省主要大豆真菌病害和病原菌种类进行系统鉴定,并针对所鉴定病害和病原菌展开不同种类杀菌剂的筛选,为河北省大豆真菌病害的有效防治提供依据。1 材料与方法
试验于2019年在河北省农林科学院植物保护研究所完成。1.1 病样的采集与病原菌分离、保存
笔者课题组于2019年5—9月自河北省大豆主产区采集具有不同典型、明显病症的地上部大豆叶片、夹和茎秆,带回实验室,用清水将其冲洗并晾干,在病健交界处剪取长5 mm、宽2—3 mm的长方形标本组织,用0.1%升汞溶液消毒5 min,再用无菌水漂洗3次,直接移至马铃薯葡萄糖琼脂(potato dextrose agar,PDA)培养基平板上。每个培养皿中接种3—5块标本组织,置于温度25℃、相对湿度70%、黑暗培养箱内恒温培养,待菌落长出后选取每种病原菌形态一致的菌落进行反复纯化,挑取边缘菌丝再接于PDA平板上,于相同条件下培养7—10 d,待产孢后挑取单孢进行培养3—7 d,获得纯培养菌株。单孢菌株接种到常规PDA培养斜面上,4℃保存备用。1.2 主要试剂、仪器、杀菌剂及培养基
Fungal DNA Kit D3390试剂盒,Omega Bio-Tek公司;PCR扩增试剂盒、2×Taq PCR Mix、DNA Marker DL2000,日本TaKaRa公司;引物由生工生物工程(上海)股份有限公司合成;其他试剂均为国产分析纯。LRH-250F型生化培养箱,上海一恒科学仪器有限公司;正置BX63显微镜,日本Olympus公司;超景深三维VHX-1000立体显微镜,KEYENCE基恩士(中国)有限公司;Thermo NanoDrop 2000紫外分光光度计,美国NanoDrop科技有限责任公司;SimpliAmpTM Thermal Cycler PCR仪,美国Life technologies公司;DYY-8C型电泳仪,北京六一生物科技有限公司。杀菌剂:95%苯醚甲环唑原药(利尔化学股份有限公司);99.5%氟菌唑原药(江苏禾本生化有限公司);95%叶菌唑原药(江苏辉丰生物农业股份有限公司);98%肟菌酯原药(山东省联合农药工业有限公司);95%嘧菌酯原药(江苏辉丰生物农业股份有限公司);97.5%吡唑醚菌酯原药(浙江禾本科技有限公司);95%氰烯菌酯原药(江苏省农药研究所股份有限公司);96%氟吡菌酰胺原药(拜耳股份公司);96%啶酰菌胺原药(安徽广信农化股份有限公司);99.4%辛菌胺原药(陕西省西安嘉科农化有限公司);95%乙蒜素原药(南阳神圣农化科技有限公司)。制剂:10%苯醚甲环唑水分散粒剂(瑞士先正达作物保护有限公司);30%氟菌唑可湿性粉剂(日本曹达株式会社);60 g·L-1叶菌唑水乳剂(江苏辉丰生物农业股份有限公司);50%肟菌酯悬浮剂(青岛奥迪斯生物科技有限);250 g·L-1嘧菌酯悬浮剂(先正达南通作物保护有限公司);250 g·L-1吡唑醚菌酯乳油(巴斯夫欧洲公司);25%氰烯菌酯悬浮剂(江苏省农药研究所股份有限公司);41.7%氟吡菌酰胺悬浮剂(拜耳股份公司);50%啶酰菌胺水分散粒剂(巴斯夫欧洲公司);1.8%辛菌胺醋酸盐水剂(陕西省蒲城美尔果农化有限公司);80%乙蒜素乳油(南阳神圣农化科技有限公司)。
PDA培养基:马铃薯200 g、葡萄糖20 g、琼脂20 g,蒸馏水定容至1 L;不加琼脂为马铃薯葡萄糖(potato dextrose,PD)液体培养基。AEA培养基:酵母浸粉10 g、NaNO3 6g、KH2PO4 1.5 g、KCl 0.5 g、MgSO4·7H2O 0.25 g、丙三醇20 mL、琼脂粉20 g、蒸馏水1 L;YBA培养基:酵母浸粉10 g、蛋白胨5 g、醋酸钠20 g、琼脂粉20 g、蒸馏水1 L。
1.3 病原菌的鉴定
1.3.1 河北省大豆主要真菌病害病症及病原菌分离纯化 观察不同植株病害病症(A—E),记录病斑颜色、发病程度、发病规律等,将典型病症照相并带回实验室对病原菌进行分离培养。1.3.2 病原菌的形态学鉴定及回接致病性验证 菌落特征观察:将纯化后的病原菌菌株分别接种于PDA平板上,置于25℃、黑暗培养箱内恒温培养5 d,进行菌落特征的观察和拍照,记录菌落形态、颜色。
菌丝、孢子形态观察:对纯化菌株平板培养5 d后在超景深三维VHX-1000立体显微镜下观察菌丝形态、颜色,之后继续平板培养至10—15 d后产孢;对平板上不能产孢的病原菌A,将其于PDA平板上培养5 d后在菌株边缘打孔,取其直径5 mm菌柄至30 mL PD培养液进行摇床160 r/min振动培养7 d至产孢;正置BX63显微镜下观察孢子的形态、颜色及结构特征等,并进行显微形态观测、拍照,参照有关资料进行鉴定。
经预试验后,对于不能很好使叶片致病的A、B病原菌,在PDA培养基上25℃培养5 d后,用灭菌牙签蘸取打孔直径菌落2 mm,与豆茎秆成45°针刺植株第一茎节,刺穿后旋转牙签,并缓慢抽出[18]。其他病原菌回接致病性验证,选取室内无菌培养的健康大豆叶片喷雾接种病原菌孢子悬浮液,每片叶子喷浓度为1×103个/mL的孢子悬浮液0.5 mL。待接种发病后,观察致病力,并从病部再分离病原菌,比较所分离病原菌与原接种病原菌是否一致。
1.3.3 病原菌的分子生物学鉴定 病原菌菌株在PDA培养基25℃黑暗培养7 d后,用灭菌手术刀刮取培养基表面菌丝50—200 mg置于离心管中,采用Fungal DNA Kit D3390试剂盒抽提病原菌基因组DNA,于超微量紫外分光光度计测定DNA浓度及纯度,其余的DNA溶液置于-20℃冰箱保存备用。真菌物种鉴定使用rDNA-ITS 作为Marker片段,使用ITS1:TCCGTAGGTGAACCTGCGG和ITS4:TCCTCCGCTTATTGATATGC通用引物扩增。PCR扩增反应体系:10×Ex Taq buffer 2.0 μL,5 U Ex Taq 0.2 μL,2.5 mmol·L-1 dNTP Mix 1.6 μL,引物ITS1 1 μL,引物ITS4 1 μL,DNA 0.5 μL,ddH2O 13.7 μL,总体积20 μL。PCR扩增反应程序条件:95℃预变性5 min,95℃ 30 s,56℃ 30 s,72℃ 90 s,25个循环,72℃ 10 min。用1.0%琼脂糖凝胶电泳检测,120 V电压电泳30 min PCR扩增结果,观察图像是否有特异性目的片段。将含有目的条带的PCR产物交由生工生物工程(上海)股份有限公司。用各基因相对应的PCR扩增引物进行3730XL一代双末端测序,对测序结果质控与组装、拼接。将所得序列校对后于GenBank(http://www.ncbi.nlm.nih.gov)核酸序列数据库进行BLAST同源性比对,然后用MEGA 7.0软件根据各菌株rDNA-ITS基因序列进行系统发育分析,用NJ法(neighbor-joining method)构建分子进化树,并进行1 000次自展抽值检验分子进化树可靠性。分析病原菌菌株与数据库菌株的亲缘关系,进行近源物种信息的确认。同时,将1.3.2室内接种的致病力菌株分离鉴定,rDNA-ITS序列检测其与原接种病原菌序列是否一致。根据1.3.1、1.3.2和1.3.3初步分析的病原菌种类结果,对已有特异性引物的病原菌进行特异性保守序列分子验证鉴定。使用特异性引物TEF1-αF:ATGGGTAAGG ARGACAAGAC和TEF1-αR:GGARGTACCAGTSAT CATGTT对病原菌A[19]、ACT-512F:ATGTGCAAGGC CGGTTTCGC和ACT-783R:TACGAGTCCTTCTGGC CCAT对病原菌B[20]、EF-1F:CATCGAGAAGTTCG AGAAGG和EF-1R:TACTTGAAGGAACCCTTACC对病原菌D[21]进行特异性扩增。PCR扩增反应体系、反应程序条件、电泳、测序、分子进化树的构建和分析同上。
1.4 河北省大豆主要真菌病害防治药剂筛选
1.4.1 室内杀菌剂的毒力测定 采用菌丝生长速率法测定5种不同种类杀菌剂的11个代表品种对河北省大豆主要病原菌的抑制作用。供试原药分别用适量丙酮溶解,配制成质量浓度为1 000 μg·mL-1的母液,加入无菌水进行系列稀释,通过预试验得到供试药剂对不同病原菌的有效浓度范围。苯醚甲环唑和氟菌唑为3、1.5、0.75、0.375、0.1875、0.0938、0.0469、0.0234 μg·mL-1;叶菌唑为1、0.5、0.25、0.125、0.0625、0.0313、0.1563、0.0078 μg·mL-1;肟菌酯和嘧菌酯为200、100、50、25、12.5、6.25、3.125、1.5625、0.7813 μg·mL-1;吡唑醚菌酯为20、10、5、2.5、1.25、0.625、0.3125、0.1563 μg·mL-1;氰烯菌酯和氟吡菌酰胺为200、50、12.5、3.125、0.7813、0.1953、0.0488、0.0122、0.0061 μg·mL-1;啶酰菌胺为800、200、50、12.5、3.125、0.7813、0.1953 μg·mL-1;辛菌胺为80、40、20、10、5、2.5、1.25 μg·mL-1;乙蒜素为600、300、150、75、37.5、18.75、9.375、4.6875 μg·mL-1。酰胺类杀菌剂(氟吡菌酰胺、啶酰菌胺)用YBA含药平板[22],甲氧基丙烯酸酯类杀菌剂(肟菌酯、吡唑醚菌酯、嘧菌酯、氰烯菌酯)用AEA+水杨肟酸(100 μg·mL-1)含药平板[23],其他药剂用PDA含药平板,同时设置丙酮和空白对照。将分离鉴定好的木贼镰孢(Fusarium equiseti)、大豆炭疽菌(Colletotrichum chlorophyti)、茎点霉菌(Phoma herbarum)、链格孢(Alternaria alternata)、嘴突凸脐蠕孢(Exserohilum rostratum)分别接种于PDA平板上,用直径5 mm打孔器在预培养5 d的各大豆病原菌菌落边缘打取菌饼,正面朝下分别接种到各含药和空白对照平板上。每处理重复4次,置于25℃、相对湿度70%、黑暗培养箱内恒温培养。培养5 d后,对照组病原真菌菌落接近长满平板,采用十字交叉法分别测量各处理的菌落直径。计算各处理浓度的抑制率[22,23],抑制率(%)=100×(对照菌落增长直径-处理菌落增长直径)/对照菌落增长直径。
1.4.2 杀菌剂在离体叶片或幼茎上对大豆主要病害的防治效果 不同种类杀菌剂代表品种按照其使用说明的推荐剂量分别设置处理浓度:30 μg·mL-1苯醚甲环唑、75 μg·mL-1氟菌唑、6 μg·mL-1叶菌唑、150 μg·mL-1肟菌酯、150 μg·mL-1吡唑醚菌酯、50 μg·mL-1嘧菌酯、60 μg·mL-1氰烯菌酯、50 μg·mL-1氟吡菌酰胺、500 μg·mL-1啶酰菌胺、12 μg·mL-1辛菌胺、100 μg·mL-1乙蒜素。预试验后对于不能很好使叶片致病A、B病原菌,选择大豆健康幼苗采取针刺茎节接种病原菌的方法。大豆第一片真叶平展后针刺第一茎节接种。将病原菌在PDA 培养基上25℃培养5 d后,用灭菌牙签蘸取打孔直径菌落2 mm,与豆茎秆成 45°针刺植株第一茎节[18],刺穿后旋转牙签,并缓慢抽出,以未蘸取菌落的灭菌牙签针刺植株作为对照。接种后植株置于25℃、相对湿度95%—100%培养室保湿48 h,再用压力0.1 MPa的空压机带动喉头喷雾器将不同浓度杀菌剂均匀喷施到供试植株上,每株喷施1 mL药液。所有杀菌剂处理各重复5次,空白对照喷蒸馏水,试验重复4次。之后25℃,相对湿度75%—100%继续培养,7 d后调查发病情况并计算各杀菌剂对病害的防治效果。
杀菌剂在离体叶片上对其他大豆主要病害的影响:选择健康的大豆叶片,每两片叶子背面朝上置于直径15 cm培养皿中的滤纸上,每皿加15 mL蒸馏水保湿,用湿润的脱脂棉包裹叶柄。将病原菌培养至产孢,每皿叶子喷浓度为1×103个/mL的孢子悬浮液0.5 mL;于25℃下恒温培养1 d后,用压力0.1 MPa的空压机带动喉头喷雾器将不同浓度杀菌剂均匀喷施到供试叶片上,以雾滴均匀欲滴而不落为度,每皿叶子1 mL药液。所有杀菌剂处理各重复5皿,空白对照喷蒸馏水,试验重复4次,所有处理于25℃恒温光照培养,7 d后调查发病情况并计算各杀菌剂对病害的防治效果。
病害分级调查参照大豆根部病害和叶部病害分级标准改进进行[24]。0级:无病斑;1级:病斑面积占植株/叶片总面积1/4以下;2级:病斑面积占植株/叶片总面积1/4—1/2;3级:病斑面积占植株/叶片总面积1/2以上;4级:病斑连片,但没有坏死;5级:植株/叶片萎蔫或枯死。计算公式:病情指数=Σ(各级病株/叶数×相对级数值)/(调查总株/叶数×5)×100;防治效果(%)=100×(对照病情指数-处理病情指数)/对照病情指数。
1.5 数据分析
应用Excel和SPSS 16.0软件对数据进行统计分析,采用Duncan氏新复极差法进行不同杀菌剂对同种病害防治效果的差异显著性分析。2 结果
2.1 大豆主要真菌病害症状
病害A:初期茎基部表皮出现淡红褐色不规则病斑,病斑逐渐变成灰褐色凹陷坏死,并绕根茎扩展,地上部植株矮小瘦弱,叶色淡绿,后期分枝、结荚干枯,形成灰黑色坏死斑(图1-A-1),症状似土传病害。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1河北省大豆主要真菌病害田间和室内症状及其病原菌形态
病害A、B、C、D、E后数字分别代表:1田间病症;2致病性验证病症;3菌落;4菌丝(10×40倍镜,标尺=200 μm);5孢子(10×40倍镜,标尺=20 μm)
Fig. 1Main fungal disease symptoms and pathogen morphology from field and indoor of soybean in Hebei Province
The numbers after disease A, B, C, D and E respectively represent: 1 field disease; 2 pathogenicity verification; 3 colony; 4 hypha (10 × 40 times, scale=200 μm); 5 spore (10 × 40 times, scale=20 μm)
病害B:大豆荚部病斑为圆形,褐至浅灰色(图1-B-1),严重时小黑点呈轮纹状排列,病荚无种子或皱缩、干瘪,症状似炭疽病。
病害C:发病初期叶片出现黄褐色稍凹陷斑点,病斑逐渐扩大,凹陷加深,边缘变褐色,病斑边界清晰(图1-C-1),后期多个病斑融合成不规则大斑,症状似叶斑病的一种。
病害D:叶片发病初期圆形至不规则形病斑,中央褐色,四周略隆起,有时呈暗褐色轮纹状,后期病斑扩展或破裂,叶片卷曲干枯(图1-D-1),湿度大时表面有密集黑色霉层,症状也似叶斑病的一种。
病害E:叶片感病后,初期出现黑色小点(图1-E-1),边缘褪绿,病斑逐渐扩展接近圆形,后期形成不规则灰褐色至黑色病斑,症状也似叶斑病的一种。
2.2 大豆主要病原菌形态学及回接致病性验证
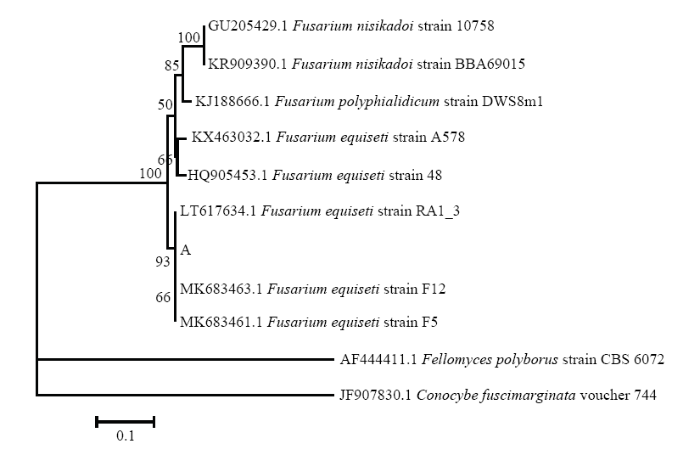
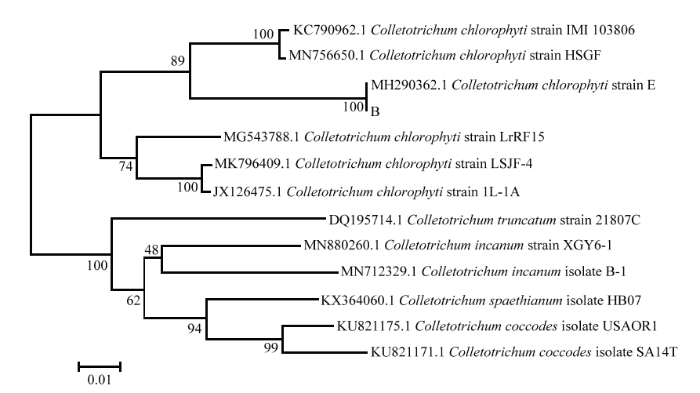
病原菌A:在PDA培养基上,菌落形状圆形,米白色至淡黄色,气生菌丝较短,棉絮状,不能长至培养皿盖(图1-A-3、1-A-4)。孢子形态:大型分生孢子似月牙形,3—6个分隔,分隔明显,顶孢呈锥形(图1-A-5)。通过形态特征观察该菌被初步鉴定为镰孢菌的一种。病原菌回接验证结果表明,该病原菌具有致病力(图1-A-2)。室内回接病原菌致病组织进行再分离,获得的病原菌形态特征与原接种菌株一致。病原菌B:在PDA培养基上,菌落圆形,气生菌丝毡状或绒状,紧贴培养基生长,菌落边缘整齐,呈灰白色,培养3 d后菌丝开始变黑(图1-B-3、1-B-4)。培养3 d后开始产生厚垣孢子,厚垣孢子单生或串生于菌丝顶端或中间,褐色至黑褐色,光滑。分生孢子单胞,无色,新月形或镰刀状,一端尖锐,另一端较钝,无附着胞产生(图1-B-5)。通过形态特征观察该菌被初步鉴定为大豆炭疽菌的一种。病原菌回接验证结果表明,该病原菌具有致病力(图1-B-2)。室内回接病原菌致病组织进行再分离,获得的病原菌形态特征与原接种菌株一致。
病原菌C:在PDA培养基上,菌落形态呈近圆形,有轮纹,边缘不均匀,菌落中央稍隆起,菌丝灰白色,生长旺盛,棉絮状(图1-C-3、1-C-4)。分生孢子器呈椭圆形至圆形,黑褐色;分生孢子呈卵圆形或椭圆形,无色单胞(图1-C-5)。观察其形态特征与茎点霉描述相符。病原菌回接验证结果表明,该病原菌具有致病力(图1-C-2)。室内回接病原菌致病组织进行再分离,获得的病原菌形态特征与原接种菌株一致。
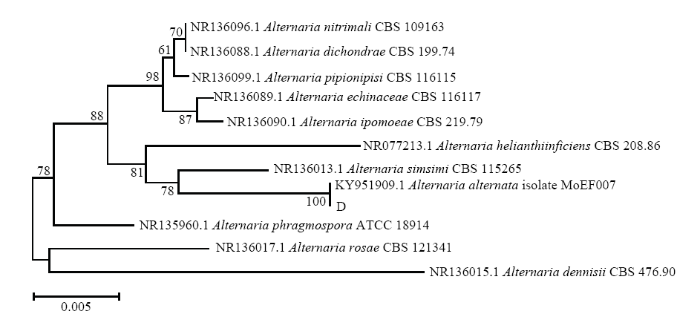
病原菌D:在PDA培养基上,菌落簇生,褐色或青褐色(图1-D-3、1-D-4)。分生孢子梗单生或数根束生,暗褐色;分生孢子倒棒形(图1-D-5),3—6个串生,有纵隔膜1—2个,横隔3—4个,横隔处偶有缢缩现象。通过形态特征观察该菌被初步鉴定为链格孢的一种。病原菌回接验证结果表明,该病原菌具有致病力(图1-D-2)。室内回接病原菌致病组织进行再分离,获得的病原菌形态特征与原接种菌株一致。
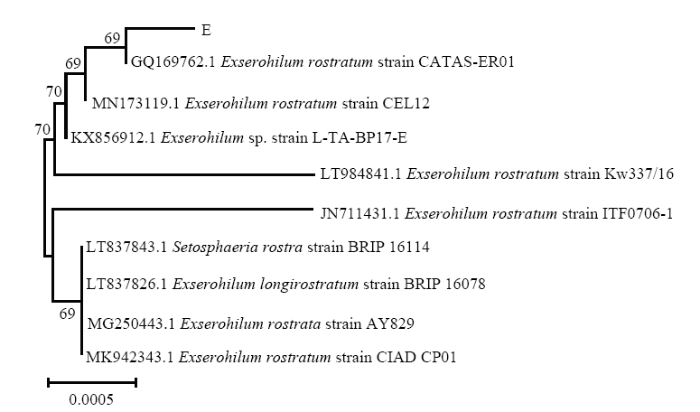
病原菌E:在PDA培养基上,菌落灰褐色,中央隆起,棉絮状,边缘气生菌丝绒毛状,背面褐色(图1-E-3、1-E-4)。分生孢子梗单生或簇生于菌丝,褐色,光滑,多隔膜。分生孢子淡褐色至中等褐色,椭圆形或近圆柱形,端部钝圆,基部有明显突出的脐点,常在两端有隔膜增厚现象,中等褐色,两端细胞颜色稍浅(图1-E-5)。通过形态特征观察该菌被初步鉴定为凸脐蠕孢的一种。病原菌回接验证结果表明,该病原菌具有致病力(图1-E-2)。室内回接病原菌致病组织进行再分离,获得的病原菌形态特征与原接种病原菌一致。
2.3 河北省大豆主要病原菌分子生物学鉴定及对应病害种类确定
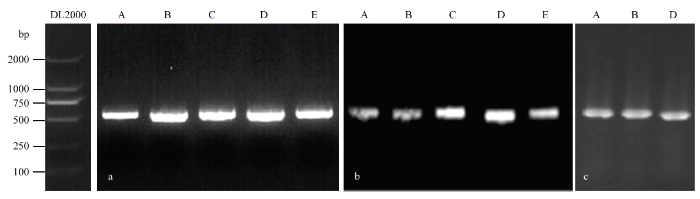
以ITS1和ITS4为引物,对田间分离出的5种病原菌进行PCR扩增,结果均能扩增出约600 bp的单一条带,大小在500—750 bp(图2-a)。用GenBank核酸序列数据库和MEGA 7.0软件的NJ法根据各菌株扩增出的ITS分子序列进行多重序列比较,构建分子系统发育树(图3—图7)。根据LANDEWEERT等对真菌的分子分类鉴定原则[25],即通过分子序列比对,序列相似性≥99%鉴定为相同种;序列相似性>95%且<99%,鉴定为相同属;序列相似性≤95%,鉴定为相同科。病原菌A与木贼镰孢LT617634.1相似性为100%,同时2.2分析该病原菌为镰孢菌的一种,初步确定引起病害A的病原菌为木贼镰孢;病原菌B与大豆炭疽菌MH290362.1相似性为100%,同时2.2分析该病原菌为大豆炭疽菌的一种,初步确定引起病害B的病原菌为大豆炭疽菌;病原菌C与茎点霉菌KJ767079.1相似性为100%,同时2.2分析该病原菌为茎点霉菌的一种,初步确定引起病害C的病原菌为茎点霉菌;病原菌D与链格孢KY951909.1相似性为100%,同时2.2分析该病原菌为链格孢的一种,初步确定引起病害D的病原菌为链格孢;病原菌E与嘴突凸脐蠕孢GQ169762.1相似性为100%,同时2.2分析该病原菌为凸脐蠕孢的一种,初步确定引起病害E的病原菌为嘴突凸脐蠕孢。利用真菌通用引物ITS1和ITS4对分离的5种室内回接致病菌基因组DNA进行扩增,鉴定结果与原病原菌一致(图2-b)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2田间分离病原菌与室内接种病原菌的电泳比较鉴定
a、b、c分别代表田间分离病原菌rDNA-ITS、室内回接致病病原菌rDNA-ITS、特异保守序列验证
Fig. 2Electrophoretic comparative identification of the pathogens isolated from the field and inoculated in the laboratory
a, b and c represent rDNA of field isolated pathogens, rDNA of laboratory inoculated pathogens, and specific conservative sequence verification, respectively
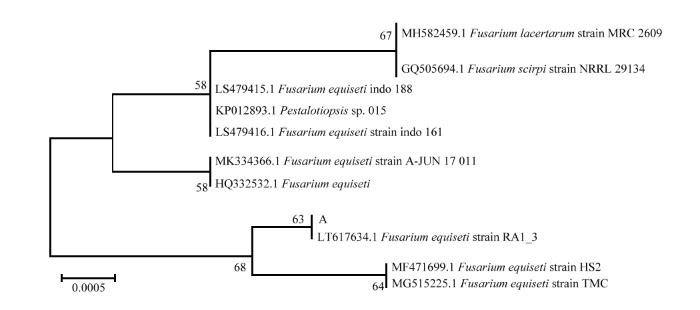
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3基于病原菌A的ITS序列的发育树(NJ)
Fig. 3The neighbor-joining (NJ) tree of pathogen A based on sequences of ITS
图4
 新窗口打开|下载原图ZIP|生成PPT
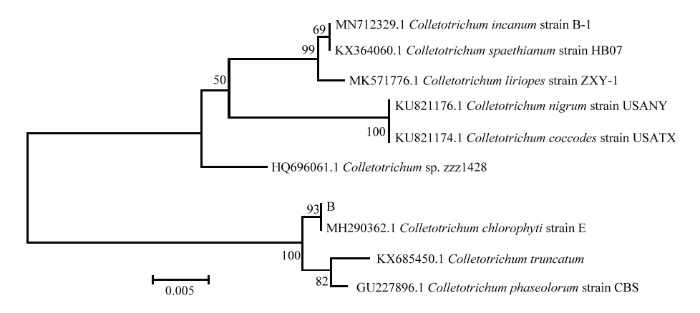
新窗口打开|下载原图ZIP|生成PPT图4基于病原菌B的ITS序列的发育树(NJ)
Fig. 4The neighbor-joining (NJ) tree of pathogen B based on sequences of ITS
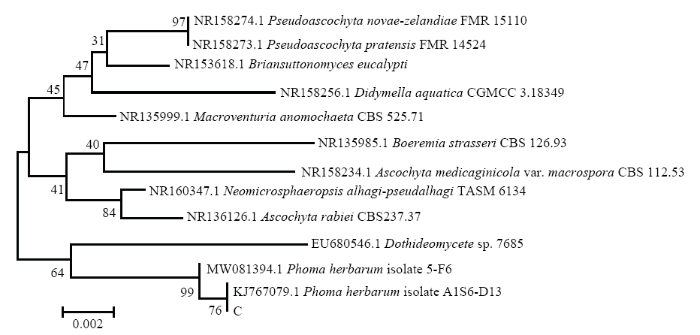
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5基于病原菌C的ITS序列的发育树(NJ)
Fig. 5The neighbor-joining (NJ) tree of pathogen C based on sequences of ITS
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6基于病原菌D的ITS序列的发育树(NJ)
Fig. 6The neighbor-joining (NJ) tree of pathogen D based on sequences of ITS
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7基于病原菌E的ITS序列的发育树(NJ)
Fig. 7The neighbor-joining (NJ) tree of pathogen E based on sequences of ITS
同时,以特异性引物TEF1-αF和TEF-1αR对病原菌A、ACT-512F和ACT-783R对病原菌B、EF-1F和EF-1R对病原菌D进行特异性PCR扩增验证,结果也均能扩增出约600 bp的单一条带,大小在 500—750 bp(图2-c)。用GenBank核酸序列数据库和MEGA 7.0软件的NJ法根据各菌株扩增出的TEF1-α、ACT、EF-1α分子序列进行多重序列比较,构建分子系统发育树(图8—图10),病原菌A 与木贼镰孢LT617634.1、病原菌B与大豆炭疽菌MH290362.1、病原菌D与链格孢KY951909.1仍分别聚类在一起。分子序列验证结果表明,病原菌A、B、D分别为木贼镰孢、大豆炭疽菌、链格孢。
图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8基于病原菌A的TEF1-α序列的发育树(NJ)
Fig. 8The neighbor-joining (NJ) tree of pathogen A based on sequences of TEF1-α
图9
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9基于病原菌B的ACT序列的发育树(NJ)
Fig. 9The neighbor-joining (NJ) tree of pathogen B based on sequences of ACT
图10
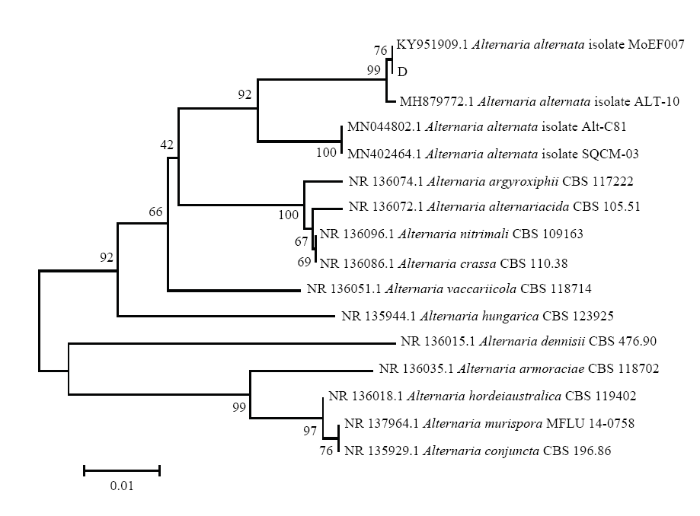 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10基于病原菌D的EF-1α序列的发育树(NJ)
Fig. 10The neighbor-joining (NJ) tree of pathogen D based on sequences of EF-1α
结合病害病症、病原菌菌落形态、菌丝及孢子显微形态和基于ITS、TEF1-α序列系统发育分析,明确病害A是由木贼镰孢引起的大豆根腐病,病害B是由大豆炭疽菌引起的炭疽病,D是由链格孢引起的大豆黑斑病。初步明确病害C是由茎点霉菌引起的茎点霉叶斑病,病害E是由嘴突凸脐蠕孢引起的凸脐蠕孢叶斑病。
2.4 不同种类杀菌剂对5种病原菌的毒力
5种病原菌对三唑类杀菌剂敏感,苯醚甲环唑EC50值0.0466—1.1102 μg·mL-1,氟菌唑EC50值0.0955—1.5024 μg·mL-1,叶菌唑EC50值0.0112—0.4206 μg·mL-1。5种病原菌对甲氧基丙烯酸酯类杀菌剂吡唑醚菌酯敏感,EC50值分别为2.8012、0.1775、0.5791、9.3865和2.0022 μg·mL-1;仅木贼镰孢对肟菌酯敏感,EC50值1.8804 μg·mL-1;木贼镰孢和大豆炭疽菌对氰烯菌酯敏感,EC50值分别为0.0109和4.4280 μg·mL-1。大豆炭疽菌、茎点霉菌、链格孢、嘴突凸脐蠕孢对酰胺类氟吡菌酰胺敏感,EC50值2.5741—7.2185 μg·mL-1;大豆炭疽菌、茎点霉菌、嘴突凸脐蠕孢对啶酰菌胺敏感,EC50值0.4857—2.5139 μg·mL-1。大豆炭疽菌和嘴突凸脐蠕孢对烷基多胺类杀菌剂辛菌胺敏感,EC50值分别为8.4571和7.7361 μg·mL-1。大豆炭疽菌对有机硫仿生杀菌剂乙蒜素敏感,EC50值8.1840 μg·mL-1(表1)。Table 1
表1
表1不同种类杀菌剂对分离自大豆的5种病原菌的毒力
Table 1
| 杀菌剂种类 Fungicide species | 杀菌剂名称 Fungicide name | 病原菌Pathogen (EC50, μg·mL-1) | ||||
|---|---|---|---|---|---|---|
| 木贼镰孢 F. equiseti | 大豆炭疽菌 C. chlorophyti | 茎点霉菌 P. herbarum | 链格孢 A. alternata | 嘴突凸脐蠕孢 E. rostratum | ||
| 三唑类 Triazole | 苯醚甲环唑Difenoconazole | 1.1102 | 0.3129 | 0.9125 | 0.5928 | 0.0466 |
| 氟菌唑Triflumizole | 1.5024 | 0.2600 | 0.8647 | 0.8581 | 0.0955 | |
| 叶菌唑Metconazole | 0.0112 | 0.2216 | 0.4206 | 0.3907 | 0.0283 | |
| 甲氧基丙烯酸酯类 Strobilurin | 肟菌酯Trifloxystrobin | 1.8804 | 26.3545 | 138.9770 | 32.0614 | 75.1247 |
| 吡唑醚菌酯Pyraclostrobin | 2.8012 | 0.1775 | 0.5791 | 9.3865 | 2.0022 | |
| 嘧菌酯Azoxystrobin | 83.0320 | 27.0851 | 11.6454 | 10.0925 | 36.3156 | |
| 氰烯菌酯Phenamacril | 0.0109 | 4.4280 | 154.9974 | 164.0990 | 39.9152 | |
| 酰胺类 Amide succinate dehydrogenase inhibitors | 氟吡菌酰胺Fluopyram | 42.1129 | 5.8304 | 2.8306 | 7.2185 | 2.5741 |
| 啶酰菌胺Boscalid | 40. 4705 | 2.5139 | 0.4857 | 155.0532 | 2.2585 | |
| 烷基多胺类 Alkyl polyamines | 辛菌胺 Xinjunan | 12.0130 | 8.4517 | 34.3226 | 21.7385 | 7.7361 |
| 有机硫仿生类 Organic sulfur bionic fungicide | 乙蒜素 Ethylicin | 10.3906 | 8.1840 | 416.1457 | 180.9062 | 45.9120 |
新窗口打开|下载CSV
2.5 杀菌剂在离体叶片或幼茎上对大豆主要病害的防治效果
三唑类杀菌剂苯醚甲环唑、氟菌唑、叶菌唑和甲氧基丙烯酸酯类杀菌剂肟菌酯、吡唑醚菌酯对5种病害防治效果显著,酰胺类杀菌剂氟吡菌酰胺对除大豆根腐病之外的其他4种病害防治效果显著,酰胺类杀菌剂啶酰菌胺对茎点霉叶斑病防治效果显著,烷基多胺类杀菌剂辛菌胺、有机硫仿生杀菌剂乙蒜素对炭疽病和凸脐蠕孢叶斑病防治效果显著,其防治效果分别达到90%以上;其他杀菌剂对5种病害表现不同程度的防治作用,防治效果为55.41%—89.36%(表2)。结合2.4不同种类杀菌剂对5种病原菌的毒力测定结果,三唑类杀菌剂苯醚甲环唑、氟菌唑、叶菌唑和甲氧基丙烯酸酯类杀菌剂吡唑醚菌酯推荐作为河北省大豆主要真菌病害的优选兼治药剂。Table 2
表2
表2不同种类杀菌剂在离体叶片或幼茎上对5种大豆真菌病害的防治效果
Table 2
| 杀菌剂种类 Fungicide species | 杀菌剂名称 Fungicide name | 病害Disease(病情指数Disease index /防治效果Control effect (%)) | ||||
|---|---|---|---|---|---|---|
| 大豆根腐病 Soybean root rot | 炭疽病 Anthracnose | 茎点霉叶斑病 Leaf spot of P. herbarum | 大豆黑斑病 Black spot | 凸脐蠕孢叶斑病 Leaf spot of E. rostratum | ||
| 三唑类Triazole | 苯醚甲环唑Difenoconazole | 5.00/93.24a | 5.00/93.15a | 6.00/91.49a | 5.50/92.20a | 6.00/91.49ab |
| 氟菌唑Triflumizole | 6.00/91.89a | 7.00/90.41a | 6.50/90.78a | 4.00/94.33a | 4.00/94.33a | |
| 叶菌唑Metconazole | 7.00/90.54a | 6.00/91.78a | 5.00/92.21a | 4.50/93.62a | 7.00/90.07ab | |
| 甲氧基丙烯酸酯类 Strobilurin | 肟菌酯Trifloxystrobin | 6.00/91.89a | 5.00/93.15a | 5.50/92.20a | 6.50/90.78a | 5.50/92.20ab |
| 吡唑醚菌酯Pyraclostrobin | 4.00/94.59a | 4.00/94.52a | 7.00/90.07a | 6.00/91.49a | 5.00/92.91ab | |
| 嘧菌酯Azoxystrobin | 9.00/87.84ab | 12.00/83.56b | 9.00/87.23ab | 11.50/83.69ab | 7.50/89.36b | |
| 氰烯菌酯Phenamacril | 10.00/86.49ab | 15.00/79.45b | 12.50/82.27b | 20.00/71.63c | 12.50/82.27c | |
| 酰胺类 Amide succinate dehydrogenase inhibitors | 氟吡菌酰胺Fluopyram | 26.00/64.86c | 6.00/91.78a | 6.00/91.49a | 6.00/91.49a | 6.50/90.78b |
| 啶酰菌胺Boscalid | 33.00/55.41d | 11.00/84.93b | 5.50/92.20a | 13.50/80.85b | 8.00/88.65b | |
| 烷基多胺类 Alkyl polyamines | 辛菌胺 Xinjunan | 20.00/72.97b | 6.00/91.78a | 11.00/84.40b | 11.00/84.40ab | 7.00/90.07b |
| 有机硫仿生类 Organic sulfur bionic fungicide | 乙蒜素 Ethylicin | 18.00/75.68b | 5.00/93.15a | 12.50/82.27b | 14.00/ 80.14b | 4.50/93.62a |
| 对照Control | - | 74.00/- | 73.00/- | 70.50/- | 59.50/- | 36.00/- |
新窗口打开|下载CSV
3 讨论
不同病原菌侵染植物后其病症存在不同程度的相似性,给病害诊断及病原菌鉴定带来较大困难。本研究通过病原菌的宏观形态、菌丝和孢子显微结构、ITS及特异性分子序列综合分析,确定河北省大豆主产区的病原菌分别为木贼镰孢、大豆炭疽菌、茎点霉菌、链格孢、嘴突凸脐蠕孢,对应病害分别为大豆根腐病、炭疽病、茎点霉叶斑病、大豆黑斑病、凸脐蠕孢叶斑病。根部病害是危害豆类的重要病害之一,发生普遍,镰孢菌是引起我国大豆根腐病的主要病原菌。镰孢菌分多种,种群复杂,近年来常发生不同种镰孢菌引起的大豆根腐病[26],本研究发现引起河北省大豆根腐病的病原菌为木贼镰孢。大豆炭疽病是世界各大豆产区重要病害之一,在乌拉圭大豆上曾发现类似炭疽病症状的豆秆,确认其病原菌为大豆炭疽菌[27]。本研究中导致河北省大豆炭疽病的病原菌同样为大豆炭疽菌。据中国真菌志记载茎点霉菌在杂草上曾有发生[28],本研究在河北省大豆叶片上发现茎点霉菌。链格孢是一类适应性强、极为常见的真菌。全世界已描述的近500个链格孢种级分类单位中,95%以上兼性寄生在植物上,引起多种经济植物病害,如黑斑病、褐斑病、茎枯病、赤星病等。目前国内外关于大豆中链格孢的研究较少。据报道,美国大豆分离出的链格孢属于5个不同的种,同时也证实了大豆中链格孢菌的多样性[12],本研究鉴定出链格孢是引发河北省大豆黑斑病的病原菌。嘴突凸脐蠕孢在国外报道其广泛寄生,寄主主要有稗属、稻属、小麦属、各种杂草等[29],据中国真菌志记载该病原菌也可侵染大豆,本研究发现凸脐蠕孢叶斑病由该病原菌引发。河北省大豆真菌病害种类复杂,防治药剂种类的科学选用成为有效防治大豆真菌病害的关键技术之一。生产上往往由于不了解病原菌种类和杀菌剂特性,盲目用药,造成防治效果降低,同时影响环境[15]。因此,应合理开展大豆病害科学、高效对靶药剂防治。根据本研究中5种杀菌剂11个代表品种对河北省5种大豆主要病原菌的抑制作用及对相应病害的防治效果,推荐三唑类杀菌剂苯醚甲环唑、氟菌唑、叶菌唑和甲氧基丙烯酸酯类杀菌剂吡唑醚菌酯作为河北省大豆主要真菌病害的优选兼治药剂。其田间防治效果有待于进一步验证。
所选用杀菌剂的种类、作用机制与病原菌种类存在一定对应关系。三唑类杀菌剂,其作用机理为影响甾醇类生物合成,使菌体细胞膜功能受到破坏[30],对子囊菌亚门、担子菌亚门和半知菌亚门的病原菌均有活性。本研究中木贼镰孢和大豆炭疽菌无性时期属于半知菌亚门,有性时期为子囊菌亚门;茎点霉菌属于半知菌亚门;链格孢属于子囊菌亚门;嘴突凸脐蠕孢属于半知菌亚门。以上对应关系也可以看出,三唑类甾醇抑制剂苯醚甲环唑、氟菌唑、叶菌唑可以作为河北省大豆主要真菌病害兼治的首选杀菌剂种类之一。
甲氧基丙烯酸酯类杀菌剂通过抑制植物病原真菌中细胞色素bc1间的电子传递,从而抑制线粒体的呼吸作用,能有效防治子囊菌、担子菌、半知菌和卵菌等引起的病害,其不同代表品种防治对象及抑制作用存在差异[31]。氰烯菌酯对半知菌亚门、子囊菌亚门的镰孢菌属真菌病害有显著的防治效果。肟菌酯对子囊菌亚门白粉病、半知菌亚门叶斑病有特效。嘧菌酯高效、广谱,对几乎所有真菌界的子囊菌亚门、担子菌亚门、鞭毛菌亚门和半知菌亚门等病害均有良好的活性,但由于该杀菌剂生产上使用普遍,存在抗药性水平上升、防治效果下降的现象[32]。吡唑醚菌酯防治子囊菌、担子菌、半知菌和卵菌纲引起的多种病害,同时它又是一种新型杀菌剂,能诱导许多作物的生理变化,增强硝酸盐(硝化)还原酶的活性提高对氮的吸收,降低乙烯的生物合成,延缓作物衰老,促进作物的生长。可见,甲氧基丙烯酸酯类呼吸抑制剂吡唑醚菌酯也可作为河北省大豆主要真菌病害兼治的首选杀菌剂之一。
由于气候变化等多种因素的作用,病害的发生逐渐呈现出种类多、频率高的趋势,对农业生产的危害日益增强。大豆真菌病害与温度、湿度及降雨、耕作制度等变化关系密切,因此,随着各种条件的不断变化,应及时调查和鉴定田间病害和病原菌种类的发生、发展程度,根据病害的种类合理选用杀菌剂,达到有效防治的目的。
4 结论
河北省大豆主产区致病病原菌分别为木贼镰孢、大豆炭疽菌、茎点霉菌、链格孢、嘴突凸脐蠕孢,对应病害分别为大豆根腐病、炭疽病、茎点霉叶斑病、大豆黑斑病、凸脐蠕孢叶斑病。推荐三唑类甾醇抑制剂、甲氧基丙烯酸酯类呼吸抑制剂吡唑醚菌酯作为河北省大豆主要真菌病害综合防治的优选兼治药剂。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URL [本文引用: 1]

利用 4 9对SSR引物和 14个农艺性状对 96份黄淮夏大豆进行遗传多样性分析 ,以了解黄淮夏大豆产区的资源多样性状况 ,为种质资源的利用和开发提供帮助。结果表明 ,SSR的遗传多样性指数的分布范围为 1.0 70 0~2 .7310 ,Simpson指数分布范围为 0 .5 313~ 0 .92 0 3;14个农艺性状的遗传多样性指数平均值为 1.1912 ,Simpson指数的平均值为 0 .6 0 79,可见黄淮夏大豆具有丰富的遗传变异。 96份材料的农艺性状和分子数据聚类结果均呈现一定的地理分布规
URL [本文引用: 1]

利用 4 9对SSR引物和 14个农艺性状对 96份黄淮夏大豆进行遗传多样性分析 ,以了解黄淮夏大豆产区的资源多样性状况 ,为种质资源的利用和开发提供帮助。结果表明 ,SSR的遗传多样性指数的分布范围为 1.0 70 0~2 .7310 ,Simpson指数分布范围为 0 .5 313~ 0 .92 0 3;14个农艺性状的遗传多样性指数平均值为 1.1912 ,Simpson指数的平均值为 0 .6 0 79,可见黄淮夏大豆具有丰富的遗传变异。 96份材料的农艺性状和分子数据聚类结果均呈现一定的地理分布规
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
URL [本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
[本文引用: 4]
DOI:10.7505/j.issn.1007-9084.2014.05.018URL [本文引用: 1]

Asian soybean rust (SBR), caused by the airborne fungus, Phakopsora pachyrhizi Syd. & P. Syd, is an important soybean yield deterrent of great economic importance to soybean growers around the world since last century. Previously, a management strategy to combat the pathogen was to spray fungicide; however, this substantially increased the cost of soybean production, being also inhospitable to the environment. Therefore, the most effective long-term strategy is to breed lines resistant to Asian soybean rust. Genetic resistance will decrease fungicide applications. Understanding the basic research of the most devastating rust pathogen to date and its advances is necessary to plan strategies to reduce the damage caused by SBR. This review provides detailed information on the disease taxonomy, its epidemiology, molecular biology of the pathogen, and provides a summary of strategies to combat the threat of this devastating disease.
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 2]

Common bacterial blight, caused by Xanthomonas axonopodis pv. phaseoli and Xanthomonas fuscans subsp. fuscans, is one of the major constraints to common bean production and causes heavy yield losses and reduces the commercial value of seeds. Contaminated seeds are the primary mean of disease dissemination. This study aimed to detect the pathogens of common bacterial blight on seeds of 60 common bean samples collected from five main production regions. The pathogen colonies of common bacterial blight were isolated from seed extracts of 36 samples on MT medium by dilution-plating method, and the bacteria populations were from 2.49×102 to 5.20×107 CFU per seed. The 36 representative strains were pathogenic on bean cultivar “Yingguohong” by stem inoculation. Based on PCR detection with specific primer pairs, 20 isolates were identified as X. fuscans subsp. fuscans, while 16 were identified as X. axonopodis pv. phaseoli. The results of seed detection showed that common bean seeds used in commerce and research had been contaminated with pathogens of common bacterial blight, it suggests that it is necessary to establish pathogen-free seeds production base and strengthen seed regulation.
URL [本文引用: 2]

Common bacterial blight, caused by Xanthomonas axonopodis pv. phaseoli and Xanthomonas fuscans subsp. fuscans, is one of the major constraints to common bean production and causes heavy yield losses and reduces the commercial value of seeds. Contaminated seeds are the primary mean of disease dissemination. This study aimed to detect the pathogens of common bacterial blight on seeds of 60 common bean samples collected from five main production regions. The pathogen colonies of common bacterial blight were isolated from seed extracts of 36 samples on MT medium by dilution-plating method, and the bacteria populations were from 2.49×102 to 5.20×107 CFU per seed. The 36 representative strains were pathogenic on bean cultivar “Yingguohong” by stem inoculation. Based on PCR detection with specific primer pairs, 20 isolates were identified as X. fuscans subsp. fuscans, while 16 were identified as X. axonopodis pv. phaseoli. The results of seed detection showed that common bean seeds used in commerce and research had been contaminated with pathogens of common bacterial blight, it suggests that it is necessary to establish pathogen-free seeds production base and strengthen seed regulation.
[本文引用: 1]
[本文引用: 1]
DOI:10.1094/PDIS-04-13-0402-PDNURLPMID:30708473 [本文引用: 1]

Colletotrichum chlorophyti was first reported in the United States in 2009 on soybean petioles (Glycine max [L.] Merr.) collected from Alabama, Illinois, and Mississippi (4). This species has not been reported to infect seed, unlike other Colletotrichum spp. (2). From the 2012 growing season, soybean seeds obtained from the National Agricultural Statistics Service representing 151 seed lots from growers' fields in 11 states were assayed by plating them on acidified potato dextrose agar (APDA). Before plating, seeds were surface disinfected by sequential immersion in 50% ethanol for 30 s, 20% commercial bleach for 1 min, two 1 min rinses in sterile distilled water, and kept at 25 degrees C in the dark for 1 week. Infected seeds from one seed lot from Arkansas produced colonies similar to Colletotrichum spp. This seed lot was visually examined and divided into asymptomatic or discolored symptomatic seeds. Because of the limited number of seeds in the seed lot, 20 seeds that asymptomatic and 40 seeds that appeared symptomatic were assayed on APDA as previously described. Asymptomatic seeds did not produce any dark fungal colonies. Among the symptomatic seeds, five appeared to have flecked light gray seed coats with some larger grayish to black and irregular spots where cracks were sometimes formed, and they developed small black fungal masses or became entirely dark on the surface. Five fungal isolates were obtained from these infected seeds. On APDA, the isolates initially produced white to pink smooth-margined colonies, turned black with age, produced no aerial growth, and filled a 9 cm diameter petri dish within 10 days. DNA of one isolate was extracted for PCR and sequencing of the ITS region with ITS1 and ITS4 primers (3). From the BLAST analysis, the sequence was 100% identical to C. chlorophyti isolates, IMI 103806, and CBS 142.79 (Accession Nos. GU227894 and GU227895, respectively). To test for pathogenicity, the fungus was sub-cultured on APDA and eight APDA discs (4 mm diameter) were set into 50 ml potato dextrose broth inside a 250-ml flask and shook at a speed of 100 rpm at room temperature (24 +/- 1 degrees C) for 10 days. The mycelium was then weighed, fragmented with a blender, and resuspended in sterile distilled water to a final concentration of ~40 mg/ml. The mycelial suspension was sprayed on soybean seedlings of cv. Williams 82 (two plants/pot) at growth stage V1 to V2 until runoff. The inoculated plants were kept in a moist chamber (>90% relative humidity) for 48 h at 24 +/- 1 degrees C in the dark, and then transferred to normal plant growing conditions. At 5 days post-inoculation (dpi), the leaves showed typical symptoms caused by C. chlorophyti, including necrosis on the edge of young leaves and petioles, formation of irregular dark brown lesions, and leaves became scrolled (4). Setose acervuli, curved conidia with tapered ends (21.4 +/- 1.1 x 3.8 +/- 0.3 mum), and chlamydospores were found on the detached symptomatic leaves after 12 dpi. No perithecia formed. The morphology matched the description of C. chlorophyti (1,4). To our knowledge, this is the first report of C. chlorophyti in Arkansas and the first time that this species has been reported infecting seed of any plant. References: (1) S. Chandra and R. N. Tandon. Curr. Sci. 34:565, 1965. (2) G. L. Hartman et al. Page 13 in: Compendium of Soybean Diseases, APS Press, St. Paul, MN, 1999. (3) T. J. White et al. Page 315 in: PCR Protocols. A Guide to Methods and Applications. Academic Press, San Diego, CA, 1990. (4) H.-C. Yang et al. Plant Dis. 96:1699, 2012.
DOI:10.1080/00275514.1999.12061051URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
URL [本文引用: 1]
DOI:10.1128/aem.69.1.327-333.2003URLPMID:12514012 [本文引用: 1]

Molecular identification techniques based on total DNA extraction provide a unique tool for identification of mycelium in soil. Using molecular identification techniques, the ectomycorrhizal (EM) fungal community under coniferous vegetation was analyzed. Soil samples were taken at different depths from four horizons of a podzol profile. A basidiomycete-specific primer pair (ITS1F-ITS4B) was used to amplify fungal internal transcribed spacer (ITS) sequences from total DNA extracts of the soil horizons. Amplified basidiomycete DNA was cloned and sequenced, and a selection of the obtained clones was analyzed phylogenetically. Based on sequence similarity, the fungal clone sequences were sorted into 25 different fungal groups, or operational taxonomic units (OTUs). Out of 25 basidiomycete OTUs, 7 OTUs showed high nucleotide homology (> or = 99%) with known EM fungal sequences and 16 were found exclusively in the mineral soil. The taxonomic positions of six OTUs remained unclear. OTU sequences were compared to sequences from morphotyped EM root tips collected from the same sites. Of the 25 OTUs, 10 OTUs had > or = 98% sequence similarity with these EM root tip sequences. The present study demonstrates the use of molecular techniques to identify EM hyphae in various soil types. This approach differs from the conventional method of EM root tip identification and provides a novel approach to examine EM fungal communities in soil.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s10658-018-1463-2URL [本文引用: 1]
