 ,1, 王擎
,1, 王擎 ,1, 朱行1, 王建霞1, 申珅1, 刘宁1, 郝志敏
,1, 朱行1, 王建霞1, 申珅1, 刘宁1, 郝志敏 ,1, 董金皋
,1, 董金皋 ,1,2
,1,2Identification and Expression Pattern Analysis of Septin Gene Family of Setosphaeria turcica
LONG Feng ,1, WANG Qing
,1, WANG Qing ,1, ZHU Hang1, WANG JianXia1, SHEN Shen1, LIU Ning1, HAO ZhiMin
,1, ZHU Hang1, WANG JianXia1, SHEN Shen1, LIU Ning1, HAO ZhiMin ,1, DONG JinGao
,1, DONG JinGao ,1,2
,1,2通讯作者:
责任编辑: 岳梅
收稿日期:2020-03-28接受日期:2020-05-10网络出版日期:2020-12-16
| 基金资助: |
Received:2020-03-28Accepted:2020-05-10Online:2020-12-16
作者简介 About authors
龙凤,E-mail:
王擎,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (604KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
龙凤, 王擎, 朱行, 王建霞, 申珅, 刘宁, 郝志敏, 董金皋. 玉米大斑病菌Septin基因家族的鉴定与表达模式分析[J]. 中国农业科学, 2020, 53(24): 5017-5026 doi:10.3864/j.issn.0578-1752.2020.24.005
LONG Feng, WANG Qing, ZHU Hang, WANG JianXia, SHEN Shen, LIU Ning, HAO ZhiMin, DONG JinGao.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】玉米大斑病是严重威胁玉米安全生产的真菌性病害之一[1],其病原玉米大斑病菌(Setosphaeria turcica)主要侵害玉米叶片、叶鞘和苞叶,以叶片受害最重,严重影响玉米的产量和质量。但由于该病菌具有明显的生理分化现象,且变异频繁,使主要依赖抗病品种的病害防控工作面临极大威胁,因此,充分解析病原菌致病性的调控机制将成为寻找病害防控新策略的重要突破口[2]。【前人研究进展】隔膜蛋白Septin广泛存在于除植物以外所有真核生物中,是高度保守的GTP结合蛋白家族[3],Septin被认为是继微管、微丝和中间纤维之后的第4种细胞骨架蛋白[4]。迄今为止,已鉴定了酿酒酵母(Saccharomyces cerevisiae)中Septin基因家族具有7个成员,其中5个(Shs1p/Sep7p、Cdc3p、Cdc10p、Cdc11p和Cdc12p)在有丝分裂期间表达并参与胞质分裂,在芽颈处形成环形结构[5]。它们参与芽位置的选择、有丝分裂纺锤体的定位、极化生长和胞质分裂[6],而Spr3p和Spr28p是孢子形成时期所特有的[7]。值得注意的是,Septin环结构是伴随细胞周期的发生而出现的,只有在G2/M转变期子芽形成到细胞分裂结束时,Septin环结构才能被检测到,在其他时期,Septin以一种无序状态存在,在细胞中很难被检测到[8,9]。Septin以蛋白复合体的形式参与Septin环的构建,任何主要的Septin基因 Cdc3、Cdc10、Cdc11或Cdc12的突变都会阻止Septin环的发育,从而导致有丝分裂延迟和芽伸长[10]。动植物真菌病原体存在酿酒酵母中未发现的Septin形式,并在动力学和功能上表现出复杂性,使真菌病原体成为揭示Septin行为多样性的重要模型。稻瘟病菌(Magnaporthe oryzae)中,位于附着胞孔内环形的F-肌动蛋白网络由4个Septin鸟苷三磷酸酶(Sep3、Sep4、Sep5和Sep6)组装,并聚合成一个动态的异寡聚环,从而支撑F-肌动蛋白形成细胞骨架,以形成侵入钉破坏叶片表面[11,12]。因此,Septin对依赖附着胞侵染寄主的病原微生物至关重要。在大多数病原真菌中,Septin的缺失导致极性生长的增加和毒力的降低。在构巢曲霉(Aspergillus nidulans)中,ΔAspB突变体形成多个异常、发育不良的菌丝分支以及多个胚芽管,而野生型菌株只形成一个胚芽管[13]。在灰葡萄孢(Botrytis cinerea)中,ΔBcSep4突变体芽管增长,不能形成附着胞和侵染垫,致病力完全丧失[14] 。在棉阿舒囊霉(Ashbya gossyipii)中,Aghsl1或Agcdc12缺失菌株其菌丝具有额外极性生长一个弯曲菌丝[15]。Septin蛋白以细胞周期蛋白/Cdk依赖性方式直接控制菌丝形态发生,在白色念珠菌(Candida albicans)中,Cdk Cdc28通过调控细胞周期蛋白Ccn1和Hgc1,从而快速建立并维持细胞骨架蛋白Cdc11的磷酸化,以促进菌丝发育,这些磷酸化位点的诱变会导致异常的菌丝形态[16],其中Δcdc10和Δcdc11突变体与野生型白色念珠菌相比,其生长速度正常但产生弯曲菌丝的频率更高,细胞壁沉积的轻微不一致,并且在感染小鼠模型中毒力减弱[17,18]。【本研究切入点】大量研究表明,Septin蛋白缺失严重影响对宿主的致病力,特定Septin蛋白的功能丧失伴随的后果在不同物种间有较大差异。目前,对于玉米大斑病菌Septin蛋白功能,尤其是在病菌侵染结构发育过程中发挥的作用尚未进行过系统研究。【拟解决的关键问题】利用生物信息学手段明确玉米大斑病菌Septin家族的组成,及其成员基因和编码产物的结构特征、理化性质;分别将病原菌接种于人造疏水介质及感病寄主叶片,检测Septin家族各基因的表达水平,揭示Septin在病菌侵染结构发育过程中的表达模式,为深入阐明Septin家族不同成员在病菌致病过程中的功能打下基础。1 材料与方法
试验于2019年在河北农业大学生命科学学院/河北省植物生理与分子病理学重点实验室完成。1.1 供试菌株及寄主
玉米大斑病菌野生型菌株01-23和玉米品种B73自交系均由河北农业大学真菌毒素与植物分子病理学实验室保存。1.2 主要培养基及试剂
PDA培养基、1.2% Agar培养基,RNA提取Trizol试剂盒购于北京索莱宝科技有限公司。Prime Script ? RT Reagent Kit试剂盒购自TaKaRa(大连)公司,UEIrisII RT-PCR system for First-Strand cDNASynthesis(with dsDNase)反转录试剂盒和Super EvaGreen? qPCR Master Mix试剂盒购自US Everbright? Inc(江苏),引物在生工生物工程(上海)股份有限公司合成。1.3 病菌样本收集
采用气生菌丝涂断法收集在PDA培养基上25℃培养13 d的玉米大斑病菌分生孢子,配制浓度为20个/μL的玉米大斑病菌孢子悬浮液,用移液枪吸取2 mL孢子悬浮液点在铺有玻璃纸的水琼脂平板上,25℃保湿孵育,诱导分生孢子萌发。参考申珅等的方法分别于接种后6 h(分生孢子萌发形成芽管)、12 h(芽管末端膨大形成附着胞)、24 h(附着胞基部产生纤细的侵入钉)[19,20]收集菌体材料,湿重约0.5 g,分别提取其RNA。将相同浓度的玉米大斑病菌孢子悬浮液接种于感病寄主B73玉米叶片表面,于接种后1 h(分生孢子萌发早期)、6 h(芽管形成后期)、12 h(附着孢发育早期)、18 h(附着孢形成后期)、24 h(侵入钉形成后期)从叶片上收集病菌材料[19,20],置于50 mL管中,分别提取其RNA。1.4 RNA的提取
参考申珅等的方法用Trizol试剂盒分别提取人造疏水介质诱导下玉米大斑病菌不同发育阶段菌体和接种感病寄主叶片不同时间后菌体的总RNA[19,20],采用UEIrisII RT-PCR system for First-Strand cDNA Synthesis(with dsDNase)反转录试剂盒合成cDNA,用于RT-qPCR检测Septin的转录水平。所有试验均设3次生物学重复。1.5 Septin基因及编码产物的生物信息学分析
利用MEGA7.0软件邻接法对已报道的其他真菌物种的Septin序列与玉米大斑病菌Septin序列进行多重序列比对,构建系统发育树,重复次数设置为1 000。利用GSDS2.0(
1.6 Septin表达水平的RT-qRCR分析
利用Primer 5设计各基因特异性引物(表1),以 β-Tubulin为内参基因,以各时期cDNA为模板,Super EvaGreen? qPCR Master Mix试剂盒进行RT-qPCR扩增,采用美国Bio-Rad CFX96 TouchTM型荧光定量PCR系统进行试验(表2)。Table 1
表1
表1RT-qPCR试验所用引物
Table 1
| 名称Primer | 序列Sequence (5′ to 3′) |
|---|---|
| β-Tubulin-F | GGGAACTCCTCACGGATGTTG |
| β-Tubulin-R | TAACAAVTGGGCAAAGGGTCA |
| StSep1F | ACTGCTGCCTGTTCTTCA |
| StSep1R | TGGAACTCCTCCTTTATCC |
| StSep2F | GATTCGGAGACCAGATTGA |
| StSep2R | AGTAGGCGTGATGAAGTAGAG |
| StSep3F | TGGCTCGGAGAAGGATGT |
| StSep3R | GGGTTCGGATGAGGATGG |
| StSep4F | GCTCATCCGCACCCACATG |
| StSep4R | CATCTTCTGGAGCTTGGC |
新窗口打开|下载CSV
Table 2
表2
表2RT-qPCR反应体系
Table 2
| 反应组分 Reaction component | 反应体系 Reaction system | 终浓度 Final concentration |
|---|---|---|
| 2×Fast Super EvaGreen Master Mix | 10 μL | 1× |
| F, R primers | 1 μL | 0.1-0.5 μmol·L-1 each |
| cDNA (500 ng·μL-1) template | 1 μL | |
| H2O | 补足至20 μL Up to 20 μL |
新窗口打开|下载CSV
RT-qPCR程序:95℃ 3 min;95℃ 15 s,60℃ 60 s(收集荧光),45个循环;每个样品均设3个生物学重复。以分生孢子时期的基因表达水平为对照,采用基因相对表达量分析方法2-ΔΔCt和Origin软件,对试验数据进行差异显著性分析,GraphPad Prism6绘制图表。
2 结果
2.1 玉米大斑病菌候选Septin的获取
以玉米小斑病菌(Bipolaris maydis)中的6个Septin(ChCdc3、ChCdc10、ChCdc11、ChCdc12、ChCdc100和ChCdc100)的氨基酸序列为探针,对玉米大斑病菌数据库(Table 3
表3
表3玉米大斑病菌Septin的信息
Table 3
| 基因 Gene | 基因登录号 Database accession number | 基因长度 Gene length (bp) | 蛋白质ID Protein ID | 大小 Size (aa) | 基因组定位 Genomic mapping |
|---|---|---|---|---|---|
| StSep1 | XP_008021140.1 | 1588 | 162710 | 341 | scaffold_1:3755824-3757411 (+) |
| StSep2 | XP_008027140.1 | 1883 | 163440 | 375 | scaffold_3:81842-83724 (+) |
| StSep3 | XP_008028687.1 | 1406 | 93639 | 381 | scaffold_4:2483454-2484859 (+) |
| StSep4 | XP_008026163.1 | 1424 | 110191 | 432 | scaffold_21:190124-191547 (+) |
| StSep5 | XP_008030793.1 | 1977 | 165795 | 475 | scaffold_8:185341-187317 (-) |
| StSep6 | XP_008030826.1 | 2554 | 99177 | 793 | scaffold_8:379528-382081 (-) |
新窗口打开|下载CSV
图1
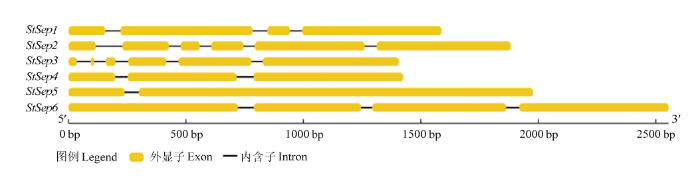 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1玉米大斑病菌Septin基因结构
Fig. 1The gene structure of Septin homologues in S. turcica
2.2 Septin的系统进化分析
不同植物病原真菌Septin同源基因在氨基酸水平上呈现高度的相似性,系统发育树分成两个大的进化支,玉米大斑病菌的StSep1、StSep2、StSep3、StSep4与不同病原真菌的核心Septin(Cdc3、Cdc10、Cdc11和Cdc12)聚于同一进化支,而StSep5、StSep6分别与玉米小斑病菌的Septin同源,构成另一个进化支(图2)。玉米大斑病菌中没有与酿酒酵母SPR3、SHS1、SPR28同源的Septin。图2
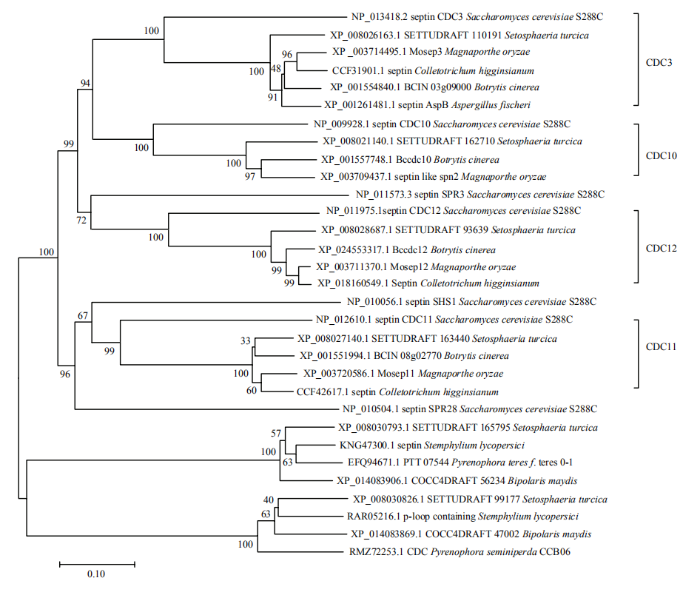 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2Septin的系统发育分析
Fig. 2The phylogenetic tree of Septins from different fungi
2.3 Septin的结构特征分析
NCBI-CDD(conserved domain database)数据库在线比对结果表明,StSep1、StSep2、StSep3、StSep4 为CDC/Septin GTPase家族成员,StSep5、StSep6主要为P-loop-NTPase域的成员,用于结合核苷酸的β-γ磷酸部分(通常为ATP或GTP)和Mg2+。通过DNAMAN对4个候选Septin的氨基酸序列进一步进行比对分析,结果表明,候选Septin均具有GTP-CDC结合域(G1、G3、G4 motif)[22],构成保守序列PFAxvGs特征的3个残基vGs已映射到保守域CDC-Septin上的G5 motif。PAN等[23]使用Weblogo程序分析Septin基序发现序列WgxEV、YexYR中的ExxxxR和基序WG中度保守。在G3 motif与G4 motif之间的氨基酸序列FIxPtGHxL和在G4 motif的氨基酸序列ExDD[22],尚无功能注释(图3),这些保守结构域在希金斯炭疽菌(Colletotrichum higginsianum)的Septin中也存在,但具体的生物学功能,有待今后进一步的探究与解析。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3Septin的保守结构域分析
Fig. 3Conservative domain analysis of Septins
2.4 核心Septin的生物信息学分析
2.4.1 理化性质 总体而言,相对分子质量最大的是StSep4,相对分子质量最小的是StSep1。StSep1的PI为7.20,属于中性蛋白质;StSep2、StSep4的PI分别为5.07、6.56,属于酸性蛋白质;StSep3的PI为8.54,属于碱性蛋白质(表4)。4个Septin的脂肪族氨基酸指数范围为78.87—86.53,总平均亲水性均为负值,因此玉米大斑病菌中的4个核心Septin均属于亲水性蛋白。Table 4
表4
表4Septin的基本理化性质
Table 4
| 特征蛋白 Characteristic protein | StSep1 | StSep2 | StSep3 | StSep4 |
|---|---|---|---|---|
| 相对分子质量 Relative molecular mass (kD) | 39.1454 | 42.6070 | 44.0992 | 49.7026 |
| PI | 7.20 | 5.07 | 8.54 | 6.56 |
| (Asp+Glu) | 44 | 72 | 60 | 68 |
| (Arg+Lys) | 44 | 53 | 63 | 66 |
| 分子式Molecular formula | C1733H2735N495O520S10 | C1851H2985N535O595S11 | C1938H3107N573O580S12 | C2179H3514N624O671S16 |
| 不稳定性系数 Coefficient of instability | 49.91 | 47.37 | 48.84 | 42.73 |
| 脂肪族氨基酸指数 Aliphatic amino acid index | 84.31 | 86.53 | 78.87 | 81.62 |
| 总平均亲水性 Total average hydrophilicity | -0.498 | -0.608 | -0.175 | -0.681 |
新窗口打开|下载CSV
2.4.2 二级结构 Septin二级结构由折叠、α螺旋、β转角以及无规则卷曲4种结构组成,以Stsep1所含折叠比例最高,为15.84%;StSep3所含无规则卷曲的比例最高,为49.87%;StSep4所含α螺旋的比例最高为51.16%(图4)。
图4
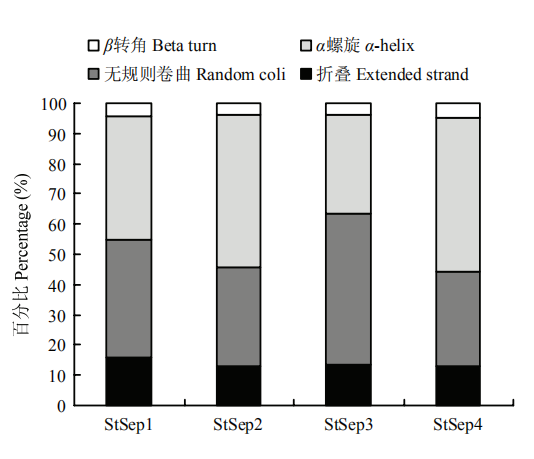 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4玉米大斑病菌Septin的二级结构特征
Fig. 4The secondary structure characteristics of Septins in S. turcica
图5
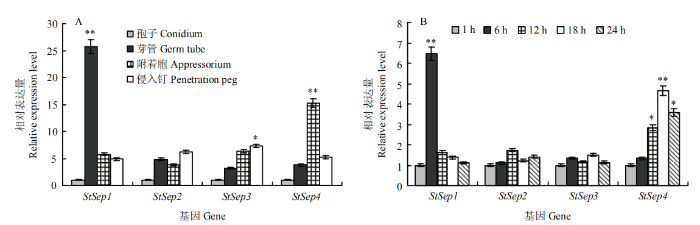 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5Septin表达模式分析
A:疏水介质诱导下玉米大斑病菌不同发育时期的表达量 Expression at different developmental stages induced on hydrophobic medium;B:接种后不同时间的表达量 Expression in different times after inoculation of maize leaves。*:0.01<P<0.05;**:P<0.01
Fig. 5Expression pattern analysis of Septins
2.4.3 亚细胞定位、跨膜区结构预测和信号肽预测 Septin均定位于细胞质中,无跨膜区结构,不具有信号肽位点,为非分泌蛋白。
2.5 Septin表达模式分析
以人造疏水介质为诱导,模拟病菌侵染结构的发育过程,以分生孢子时期的基因表达水平为对照。StSep1在芽管形成后期表达活跃(P<0.01),其表达量是分生孢子时期的25.69倍,StSep4在附着胞形成后期活跃表达(P<0.01),其表达量是分生孢子时期的15.22倍,随后侵入钉形成,StSep4表达水平下调,但其表达量仍高于分生孢子时期。StSep2、StSep3随诱导时间延长,表达水平持续上调,在侵染丝时期其表达量分别达到分生孢子时期的6.21和7.32倍。由此推测StSep1可能主要参与病菌孢子萌发过程,StSep2、StSep3可能主要参与病菌侵染结构形成过程,而StSep4可能主要在病菌的附着胞发育及侵染丝的形成中发挥调控作用。分析Septin基因家族在病菌侵染感病寄主叶片过程中的转录水平,以接种初期1 h的基因表达水平为对照。结果表明,StSep1和StSep4的转录水平均呈现先上调后下调的变化趋势。StSep1在接种后6 h转录水平极显著上调(P<0.01),随后迅速下降,至接种后18 h和24 h基本下调至与起始阶段相当的水平,StSep4在附着胞形成阶段表达活跃,随着时间延长,继续上调至18 h达到高峰,之后表达量下调,至24 h后仍高于萌发初期。StSep2、StSep3在接种后18 h和24 h表达活跃,表达量略有上调,无显著性差异。
3 讨论
20世纪80年代末,由HARTWELL首次在酿酒酵母的胞质分裂缺陷(CDC)突变体中发现Septin,最近在绿藻、褐藻及纤毛虫中也发现了Septin,而在高等绿色植物中尚未发现Septin的存在[24]。在对Septin系统发育和进化起源的研究中发现,Septin家族在进化上相对保守,各家族成员之间其N端和C端稍有不同,位于中央的鸟苷三磷酸(GTP)结合结构域和多碱性氨基酸结构域都是高度保守的[9,25]。大量研究表明,Septin是从酵母到人类高度保守的真核细胞骨架蛋白家族,所有真菌均具有4种核心Septin:Cdc3、Cdc10、Cdc11及Cdc12[14]。在哺乳动物中Septin家族有很多变异剪接本,翻译后修饰保守性差,使得该家族基因功能更为复杂。相对于其他细胞骨架GTP酶、真核生物的Tubulin蛋白及细菌中的FtsZ蛋白,Septin家族的这些保守序列与Ras家族更为相近[26]。典型Septin具有以下结构特征:(1)所编码的蛋白质分子量约30—65 kD;(2)包含GTP酶域所特有的3个保守序列:G1、G3、G4基序(motif)。Septin蛋白G1基序(Gxxxx GK[S/T])高度保守,G3基序(Dxx G)中度保守,其共有序列为DTPG,G4基序(xKxD)严谨保守[27];(3)N端为多元区域,C端为螺旋卷曲结构,这一结构被认为可能与Septin家族成员的同源与异源聚合有关[28]。不同亚型的Septin可聚合形成隔膜丝,隔膜丝进一步可形成纤维束、环状、网格状、笼状和沙漏状等复杂的高级结构[27]。
大量研究表明,Septin在真菌病原体中起着重要的作用。在稻瘟病菌中,MoSEP3-GFP定位于附着胞孔处,缺失后导致侵染钉不能形成,致病力完全丧失,并且囊外复合体(Exocyst)在附着胞孔处的组装依赖于隔膜蛋白,Septin表达和Septin环形成之前,Exocyst在附着胞孔处不会发生积累[12,29]。在禾谷镰孢(Fusarium graminerarum)中FgCdc3、FgCdc11或FgCdc12的靶向缺失导致菌丝体生长速度减慢,分生孢子形成减少且形态异常,其敲除突变体毒力降低,而ΔFgcdc10突变体与野生型的生长形态和毒性无明显差别[30]。在禾谷镰孢中正常的成熟子囊孢子由4个细胞簇组成,具有4个单核区室,而ΔFgcdc3和ΔFgcdc12突变体的子囊孢子形态缺陷,大多为椭圆形的单细胞,子囊孢子每个隔室包含多个核,表明在子囊孢子发育过程中,Septin基因的缺失影响细胞核分裂、分隔[31]。玉米黑粉菌(Ustilago maydis)中的Septin可以组装成芽颈环,生长尖端的带状结构以及长的Septin纤维,3种不同结构可在同一细胞中共存。Septin基因UmSep4的缺失严重影响玉米黑粉菌的细胞形态,并引起热敏感性和对细胞壁应激源的敏感性增强,突变体毒力减弱,但UmSep4的缺失似乎对其他Septin定位的影响较小,仅改变了UmSep3的芽颈定位,表明Septin在玉米黑粉菌中显示出一定程度的相互独立性[32]。而在酿酒酵母中,当缺失一个Septin时,通常会破坏Septin环。在构巢曲霉中,野生型菌株具有规则的梗基、瓶梗以及分生孢子链,而ΔAspA和ΔAspC突变体形成不规则且融合的梗基、瓶梗,产生的分生孢子较少,菌丝中的横隔减少,并且ΔAspA、ΔAspC突变体的生长发育似乎比野生型更快,分生孢子萌发速度更快,芽管早期出现,菌丝分支增加[33]。揭示了在不同病原真菌中Septin行为的多样性。此外,病原菌效应蛋白的分泌和转运同样也依赖Septin。例如,大丽轮枝菌(Verticillium dahliae)Septin5和F-actin以成环的形式(Septin环)定位于菌丝颈环,其在菌丝颈环的有序组织是分泌蛋白穿透界面所必需的,Septin5缺失导致分泌蛋白滞留在附着枝及菌丝颈环内,病菌的致病力降低[34],说明Septin参与囊泡运输。
本研究表明,Septin在玉米大斑病菌侵染过程中表达模式发生了明显变化,且4个核心Septin呈现明显不同的表达模式,其中StSep1在芽管时期表达最活跃,StSep4在附着胞形成阶段和侵染丝时期表达量显著上调。StSep2、StSep3表达模式相近,均在芽管和附着胞形成时期表达上调。因此推测,StSep1可能主要参与孢子萌发早期的芽管形成,StSep2、StSep3可能与附着胞发育关系密切,而StSep4可能在病菌侵染过程中附着胞形成、成熟以及侵入钉的形成发挥重要调控作用。结合隔膜蛋白Septin在调控稻瘟病菌、禾谷镰孢和玉米黑粉菌致病性中的重要作用及玉米大斑病菌不同发育时期Septin的表达规律,推测玉米大斑病菌Septin与其功能相似,均参与调控病菌极性生长及致病力。在玉米大斑病菌中Septin是否作为一个复合体发挥作用,多个Septin高级结构如何在同一细胞质中以致病形态共存,再者,隔膜蛋白Septin在病菌中是否显示出一定程度的相互独立性等所涉及的生物学问题有待于进一步深入研究。
4 结论
玉米大斑病菌基因组中共有6个Septin基因,随机分布,不存在连锁关系;其中4个为核心Septin,均具有GTP-CDC结合域(G1、G3、G4 motif)。StSep1、StSep4分别在芽管及附着胞形成阶段活跃表达,StSep2、StSep3在侵入丝形成时期表达上调。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1186/1471-2229-12-56URLPMID:22545925 [本文引用: 1]

BACKGROUND: Setosphaeria turcica is a fungal pathogen that causes northern corn leaf blight (NCLB) which is a serious foliar disease in maize. In order to unravel the genetic architecture of the resistance against this disease, a vast association mapping panel comprising 1487 European maize inbred lines was used to (i) identify chromosomal regions affecting flowering time (FT) and northern corn leaf blight (NCLB) resistance, (ii) examine the epistatic interactions of the identified chromosomal regions with the genetic background on an individual molecular marker basis, and (iii) dissect the correlation between NCLB resistance and FT. RESULTS: The single marker analyses performed for 8 244 single nucleotide polymorphism (SNP) markers revealed seven, four, and four SNP markers significantly (alpha=0.05, amplicon wise Bonferroni correction) associated with FT, NCLB, and NCLB resistance corrected for FT, respectively. These markers explained individually between 0.36 and 14.29% of the genetic variance of the corresponding trait. CONCLUSIONS: The very well interpretable pattern of SNP associations observed for FT suggested that data from applied plant breeding programs can be used to dissect polygenic traits. This in turn indicates that the associations identified for NCLB resistance might be successfully used in marker-assisted selection programs. Furthermore, the associated genes are also of interest for further research concerning the mechanism of resistance to NCLB and plant diseases in general, because some of the associated genes have not been mentioned in this context so far.
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/nrm2407URLPMID:18478031 [本文引用: 1]

Septins comprise a conserved family of proteins that are found primarily in fungi and animals. These GTP-binding proteins have several roles during cell division, cytoskeletal organization and membrane-remodelling events. One factor that is crucial for their functions is the ordered assembly of individual septins into oligomeric core complexes that, in turn, form higher-order structures such as filaments, rings and gauzes. The molecular details of these interactions and the mechanism by which septin-complex assembly is regulated have remained elusive. Recently, the first detailed structural views of the septin core have emerged, and these, along with studies of septin dynamics in vivo, have provided new insight into septin-complex assembly and septin function in vivo.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1016/S1369-5274(01)00269-7URL [本文引用: 1]

Abstract
A specialized cortical domain is organized by the septins at the necks of budding yeast cells. Recent findings suggest that this domain serves as a diffusion barrier and also as a local cell-shape sensor. We review these findings along with what is known about the organization of the septin cortex and its regulation during the cell cycle.DOI:10.1128/MCB.01529-09URLPMID:20123972 [本文引用: 1]

During yeast sporulation, a forespore membrane (FSM) initiates at each spindle-pole body and extends to form the spore envelope. We used Schizosaccharomyces pombe to investigate the role of septins during this process. During the prior conjugation of haploid cells, the four vegetatively expressed septins (Spn1, Spn2, Spn3, and Spn4) coassemble at the fusion site and are necessary for its normal morphogenesis. Sporulation involves a different set of four septins (Spn2, Spn5, Spn6, and the atypical Spn7) that does not include the core subunits of the vegetative septin complex. The four sporulation septins form a complex in vitro and colocalize interdependently to a ring-shaped structure along each FSM, and septin mutations result in disoriented FSM extension. The septins and the leading-edge proteins appear to function in parallel to orient FSM extension. Spn2 and Spn7 bind to phosphatidylinositol 4-phosphate [PtdIns(4)P] in vitro, and PtdIns(4)P is enriched in the FSMs, suggesting that septins bind to the FSMs via this lipid. Cells expressing a mutant Spn2 protein unable to bind PtdIns(4)P still form extended septin structures, but these structures fail to associate with the FSMs, which are frequently disoriented. Thus, septins appear to form a scaffold that helps to guide the oriented extension of the FSM.
DOI:10.1099/13500872-142-10-2897URL [本文引用: 1]
DOI:10.1016/S1534-5807(03)00061-3URL [本文引用: 1]

Abstract
Septins are GTPases involved in cytokinesis. In yeast, they form a ring at the cleavage site. Using FRAP, we show that septins are mobile within the ring at bud emergence and telophase and are immobile during S, G2, and M phases. Immobilization of the septins is dependent on both Cla4, a PAK-like kinase, and Gin4, a septin-dependent kinase that can phosphorylate the septin Shs1/Sep7. Induction of septin ring dynamics in telophase is triggered by the translocation of Rts1, a kinetochore-associated regulatory subunit of PP2A phosphatase, to the bud neck and correlates with Rts1-dependent dephosphorylation of Shs1. In rts1-Δ cells, the actomyosin ring contracts properly but cytokinesis fails. Together our results implicate septins in a late step of cytokinesis and indicate that proper regulation of septin dynamics, possibly through the control of their phosphorylation state, is required for the completion of cytokinesis.[D].
[本文引用: 2]
[D].
[本文引用: 2]
DOI:10.1091/mbc.e04-07-0640URLPMID:15385632 [本文引用: 1]

Septins are GTP binding proteins important for cytokinesis in many eukaryotes. The Schizosaccaromyces pombe genome sequence predicts orthologues of four of five Saccharomyces cerevisiae septins involved in cytokinesis and these are named Spns1-4p. That spns1-4 are not essential genes permitted the application of a combined genetic and proteomics approach to determine their functional relationships. Our findings indicate that Spns1-4p are present throughout interphase as a diffusely localized approximately 8.5S complex containing two copies of each septin linked together as a chain in the order Spn3p-Spn4p-Spn1p-Spn2p. Septin recruitment to the medial region of the cell is genetically separable from ring formation, and whereas it is normally restricted to mitosis, it can be promoted without activation of the mitotic cell cycle machinery. Coalescence into ring structures requires Spn1p and Spn4p associate with at least one other septin subunit and the expression of Mid2p that is normally restricted to mitosis. This study establishes the functional requirements for septin complex organization in vivo.
DOI:10.1126/science.1222934URLPMID:22723425 [本文引用: 1]

To cause rice blast disease, the fungus Magnaporthe oryzae develops a pressurized dome-shaped cell called an appressorium, which physically ruptures the leaf cuticle to gain entry to plant tissue. Here, we report that a toroidal F-actin network assembles in the appressorium by means of four septin guanosine triphosphatases, which polymerize into a dynamic, hetero-oligomeric ring. Septins scaffold F-actin, via the ezrin-radixin-moesin protein Tea1, and phosphatidylinositide interactions at the appressorium plasma membrane. The septin ring assembles in a Cdc42- and Chm1-dependent manner and forms a diffusion barrier to localize the inverse-bin-amphiphysin-RVS-domain protein Rvs167 and the Wiskott-Aldrich syndrome protein Las17 at the point of penetration. Septins thereby provide the cortical rigidity and membrane curvature necessary for protrusion of a rigid penetration peg to breach the leaf surface.
DOI:10.1105/tpc.15.00552URLPMID:26566920 [本文引用: 2]

Magnaporthe oryzae is the causal agent of rice blast disease, the most devastating disease of cultivated rice (Oryza sativa) and a continuing threat to global food security. To cause disease, the fungus elaborates a specialized infection cell called an appressorium, which breaches the cuticle of the rice leaf, allowing the fungus entry to plant tissue. Here, we show that the exocyst complex localizes to the tips of growing hyphae during vegetative growth, ahead of the Spitzenkorper, and is required for polarized exocytosis. However, during infection-related development, the exocyst specifically assembles in the appressorium at the point of plant infection. The exocyst components Sec3, Sec5, Sec6, Sec8, and Sec15, and exocyst complex proteins Exo70 and Exo84 localize specifically in a ring formation at the appressorium pore. Targeted gene deletion, or conditional mutation, of genes encoding exocyst components leads to impaired plant infection. We demonstrate that organization of the exocyst complex at the appressorium pore is a septin-dependent process, which also requires regulated synthesis of reactive oxygen species by the NoxR-dependent Nox2 NADPH oxidase complex. We conclude that septin-mediated assembly of the exocyst is necessary for appressorium repolarization and host cell invasion.
DOI:10.1128/EC.05164-11URL [本文引用: 1]

In yeast, septins form rings at the mother-bud neck and function as diffusion barriers. In animals, septins form filaments that can colocalize with other cytoskeletal elements. In the filamentous fungus Aspergillus nidulans there are five septin genes, aspA (an ortholog of Saccharomyces cerevisiae CDC11), aspB (an ortholog of S. cerevisiae CDC3), aspC (an ortholog of S. cerevisiae CDC12), aspD (an ortholog of S. cerevisiae CDC10), and aspE (found only in filamentous fungi). The aspB gene was previously reported to be the most highly expressed Aspergillus nidulans septin and to be essential. Using improved gene targeting techniques, we found that deletion of aspB is not lethal but results in delayed septation, increased emergence of germ tubes and branches, and greatly reduced conidiation. We also found that AspB-green fluorescent protein (GFP) localizes as rings and collars at septa, branches, and emerging layers of the conidiophore and as bars and filaments in conidia and hyphae. Bars are found in dormant and isotropically expanding conidia and in subapical nongrowing regions of hyphae and display fast movements. Filaments form as the germ tube emerges, localize to hyphal and branch tips, and display slower movements. All visible AspB-GFP structures are retained in Delta aspD and lost in Delta aspA and Delta aspC strains. Interestingly, in the Delta aspE mutant, AspB-GFP rings, bars, and filaments are visible in early growth, but AspB-GFP rods and filaments disappear after septum formation. AspE orthologs are only found in filamentous fungi, suggesting that this class of septins might be required for stability of septin bars and filaments in highly polar cells.
[D].
[本文引用: 2]
[D].
[本文引用: 2]
DOI:10.1091/mbc.e06-03-0215URLPMID:16899511 [本文引用: 1]

Nuclei in the filamentous, multinucleated fungus Ashbya gossypii divide asynchronously. We have investigated what internal and external signals spatially direct mitosis within these hyphal cells. Mitoses are most common near cortical septin rings found at growing tips and branchpoints. In septin mutants, mitoses are no longer concentrated at branchpoints, suggesting that the septin rings function to locally promote mitosis near new branches. Similarly, cells lacking AgSwe1p kinase (a Wee1 homologue), AgHsl1p (a Nim1-related kinase), and AgMih1p phosphatase (the Cdc25 homologue that likely counteracts AgSwe1p activity) also have mitoses distributed randomly in the hyphae as opposed to at branchpoints. Surprisingly, however, no phosphorylation of the CDK tyrosine 18 residue, the conserved substrate of Swe1p kinases, was detected in normally growing cells. In contrast, abundant CDK tyrosine phosphorylation was apparent in starving cells, resulting in diminished nuclear density. This starvation-induced CDK phosphorylation is AgSwe1p dependent, and overexpressed AgSwe1p is sufficient to delay nuclei even in rich nutrient conditions. In starving cells lacking septins or AgSwe1p negative regulators, the nuclear density is further diminished compared with wild type. We have generated a model in which AgSwe1p may regulate mitosis in response to cell intrinsic morphogenesis cues and external nutrient availability in multinucleated cells.
DOI:10.1016/j.devcel.2007.06.011URL [本文引用: 1]
DOI:10.1128/iai.71.7.4045-4051.2003URLPMID:12819094 [本文引用: 1]

Hyphal growth of Candida albicans is implicated as an important virulence factor for this opportunistic human pathogen. Septin proteins, a family of cytoskeletal elements that regulate membrane events and are important for proper morphogenesis of C. albicans, were examined for their role in tissue invasion and virulence in the mouse model of systemic infection. In vitro, septin mutants are only mildly defective for hyphal growth in liquid culture but display pronounced defects for invasive growth into agar. In vivo, the septin mutants were found to exhibit attenuated virulence. However, mice infected with the mutants displayed high fungal burdens in their kidneys without obvious symptoms of disease. Histological examination of infected kidneys revealed defects in organ invasion for the cdc10 Delta and cdc11 Delta deletion mutants, which displayed both reduced tissue penetration and noninvasive fungal masses. Thus, the septin proteins are necessary for invasive growth, which appears to be more important to the successful pathogenesis of C. albicans than hyphal growth alone.
DOI:10.1128/EC.00216-12URL [本文引用: 1]

Septins were identified for their role in septation in Saccharomyces cerevisiae and were subsequently implicated in other morphogenic processes. To study septins in Candida albicans hyphal morphogenesis, a temperature-sensitive mutation was created that altered the C terminus of the essential Cdc12 septin. The cdc12-6 cells grew well at room temperature, but at 37 degrees C they displayed expected defects in septation, nuclear localization, and bud morphogenesis. Although serum stimulated the cdc12-6 cells at 37 degrees C to form germ tube outgrowths, the mutant could not maintain polarized hyphal growth and instead formed chains of elongated cell compartments. Serum also stimulated the cdc12-6 mutant to induce a hyphal reporter gene (HWP1-GFP) and a characteristic zone of filipin staining at the leading edge of growth. Interestingly, cdc12-6 cells shifted to 37 degrees C in the absence of serum gradually displayed enriched filipin staining at the tip, which may be due to the altered cell cycle regulation. A striking difference from the wild type was that the cdc12-6 cells frequently formed a second germ tube in close proximity to the first. The mutant cells also failed to form the diffuse band of septins at the base of germ tubes and hyphae, indicating that this septin band plays a role in preventing proximal formation of germ tubes in a manner analogous to bud site selection. These studies demonstrate that not only are septins important for cytokinesis, but they also promote polarized morphogenesis and selection of germ tube sites that may help disseminate an infection in host tissues.
[本文引用: 4]
[本文引用: 4]
DOI:10.3864/j.issn.0578-1752.2017.16.008URL [本文引用: 3]

【Objective】The objective of this study is to clarify the function of cAMP phosphodiesterase (PDE) and cAMP signal pathway in regulating the pathogenicity of Setosphaeria turcica, the genes encoding cAMP phosphodiesterase were cloned and the expression pattern of these genes were analyzed during the development of infective structures and the early stage of infecting the host. 【Method】Based on conserved sequence of PDE genes from Saccharomyces cerevisiae, Candida albicans, Botrytis cinerea, Magnaporthe oryzae, Metarhizium anisopliae and Aspergillus niger, the homologous fragments were amplified by degenerate primers PCR, and the full length PDE genes and flanking sequences were obtained by combining RACE and Genome Walking. Multiple sequence alignment for prediction coding product of the protein was constructed by MEGA 5.0 software and phylogenetic tree was constructed by adjacent method. Gene structure was analyzed by GSDS. Physical and chemical properties were analyzed by ProtParam. Secondary structure was predicted by SOMPA. Conserved domains were analyzed by SMART on line. The material of different development periods from S. turcica was collected under the induction on artificial hydrophobic medium and on the susceptible host surface. Quantitative real-time PCR (qRT-PCR) was used to analyze the gene expression patterns.【Result】There were one high-affinity phosphodiesterase gene (StH-PDE) and one low-affinity phosphodiesterase gene (StL-PDE) in the genome of S. turcica. Full-length of StH-PDE was 3 208 bp, which consisted of 5 introns and 6 exons, the coding region was 2 898 bp. The full length of StL-PDE was 5 054 bp, which contained 4 introns and 5 exons, with the coding region of 3 090 bp. StH-PDE protein contained conservative type I cAMP phosphodiesterase, and StL-PDE contained L-PDE specific type II cAMP phosphodiesterase catalytic domain. PDE homologous genes in different plant pathogenic fungi presented a high degree of similarity. StH-PDE and the high affinity phosphodiesterase gene from Magnaporthe grisea, Cordyceps militaris, Metarhizium acridum were clustered in the same evolution. StL-PDE and the low affinity phosphodiesterase gene from Ascochyta rabiri, Scedosporium apiospermum, Fusarium oxysporum, Metarhizium album were clustered in the same evolution. Compared with the mycelia, the expression levels of StL-PDE and StH-PDE in conidia were significantly up-regulated, and StL-PDE and StH-PDE were up-regulated by about 52-fold and 2-fold under the induction on artificial hydrophobic medium, respectively. The expression of both genes at the early stage of spore germination was significantly down-regulated. With the germination process, the expression level gradually increased, and the second peak appeared at the appressorial formation stage, and then the expression level was down again. However, in the whole process, the highest expression level of StH-PDE appeared in the appressorial formation period, reaching nearly 7-fold and 2-fold of that in the mycelia and the conidia, respectively, and the expression level of StL-PDE was always lower than that in conidia. During the interaction of S. turcica with the susceptible host, the expression of StH-PDE and StL-PDE in the process of pathogen infection was consistent with that under the induction of artificial hydrophobic material. The expression of both genes in the early stage of spore germination was significantly down-regulated. With the germination process, the expression level gradually increased. The expression of StH-PDE was down-regulated in 18 and 24 h post-inoculation beyond the early stage of spore germination. 【Conclusion】StH-PDE and StL-PDE showed a down-regulation-up-regulated-down-regulated expression pattern in the development of pathogen infection. The relative expression level of StH-PDE was the highest during the appressorial formation, and the expression level of StL-PDE in conidia was the highest.
DOI:10.3864/j.issn.0578-1752.2017.16.008URL [本文引用: 3]

【Objective】The objective of this study is to clarify the function of cAMP phosphodiesterase (PDE) and cAMP signal pathway in regulating the pathogenicity of Setosphaeria turcica, the genes encoding cAMP phosphodiesterase were cloned and the expression pattern of these genes were analyzed during the development of infective structures and the early stage of infecting the host. 【Method】Based on conserved sequence of PDE genes from Saccharomyces cerevisiae, Candida albicans, Botrytis cinerea, Magnaporthe oryzae, Metarhizium anisopliae and Aspergillus niger, the homologous fragments were amplified by degenerate primers PCR, and the full length PDE genes and flanking sequences were obtained by combining RACE and Genome Walking. Multiple sequence alignment for prediction coding product of the protein was constructed by MEGA 5.0 software and phylogenetic tree was constructed by adjacent method. Gene structure was analyzed by GSDS. Physical and chemical properties were analyzed by ProtParam. Secondary structure was predicted by SOMPA. Conserved domains were analyzed by SMART on line. The material of different development periods from S. turcica was collected under the induction on artificial hydrophobic medium and on the susceptible host surface. Quantitative real-time PCR (qRT-PCR) was used to analyze the gene expression patterns.【Result】There were one high-affinity phosphodiesterase gene (StH-PDE) and one low-affinity phosphodiesterase gene (StL-PDE) in the genome of S. turcica. Full-length of StH-PDE was 3 208 bp, which consisted of 5 introns and 6 exons, the coding region was 2 898 bp. The full length of StL-PDE was 5 054 bp, which contained 4 introns and 5 exons, with the coding region of 3 090 bp. StH-PDE protein contained conservative type I cAMP phosphodiesterase, and StL-PDE contained L-PDE specific type II cAMP phosphodiesterase catalytic domain. PDE homologous genes in different plant pathogenic fungi presented a high degree of similarity. StH-PDE and the high affinity phosphodiesterase gene from Magnaporthe grisea, Cordyceps militaris, Metarhizium acridum were clustered in the same evolution. StL-PDE and the low affinity phosphodiesterase gene from Ascochyta rabiri, Scedosporium apiospermum, Fusarium oxysporum, Metarhizium album were clustered in the same evolution. Compared with the mycelia, the expression levels of StL-PDE and StH-PDE in conidia were significantly up-regulated, and StL-PDE and StH-PDE were up-regulated by about 52-fold and 2-fold under the induction on artificial hydrophobic medium, respectively. The expression of both genes at the early stage of spore germination was significantly down-regulated. With the germination process, the expression level gradually increased, and the second peak appeared at the appressorial formation stage, and then the expression level was down again. However, in the whole process, the highest expression level of StH-PDE appeared in the appressorial formation period, reaching nearly 7-fold and 2-fold of that in the mycelia and the conidia, respectively, and the expression level of StL-PDE was always lower than that in conidia. During the interaction of S. turcica with the susceptible host, the expression of StH-PDE and StL-PDE in the process of pathogen infection was consistent with that under the induction of artificial hydrophobic material. The expression of both genes in the early stage of spore germination was significantly down-regulated. With the germination process, the expression level gradually increased. The expression of StH-PDE was down-regulated in 18 and 24 h post-inoculation beyond the early stage of spore germination. 【Conclusion】StH-PDE and StL-PDE showed a down-regulation-up-regulated-down-regulated expression pattern in the development of pathogen infection. The relative expression level of StH-PDE was the highest during the appressorial formation, and the expression level of StL-PDE in conidia was the highest.
URLPMID:17681935 [本文引用: 1]

We developed a web server GSDS (Gene Structure Display Server) for drawing gene structure schematic diagrams. Users can submit three types of dataCDS and genomic sequences, NCBI GenBank accession numbers or GIs, exon positions on a gene. GSDS uses this information to obtain the gene structure and draw diagram for it. Users can also designate some special regions to mark on the gene structure diagram. The output result will be PNG or SVG format picture. The corresponding sequence will be shown in a new window by clicking the picture in PNG format. A Chinese version for the main page is also built. The GSDS is available on http://gsds.cbi.pku.edu.cn/.
URLPMID:17681935 [本文引用: 1]

We developed a web server GSDS (Gene Structure Display Server) for drawing gene structure schematic diagrams. Users can submit three types of dataCDS and genomic sequences, NCBI GenBank accession numbers or GIs, exon positions on a gene. GSDS uses this information to obtain the gene structure and draw diagram for it. Users can also designate some special regions to mark on the gene structure diagram. The output result will be PNG or SVG format picture. The corresponding sequence will be shown in a new window by clicking the picture in PNG format. A Chinese version for the main page is also built. The GSDS is available on http://gsds.cbi.pku.edu.cn/.
URL [本文引用: 4]

希金斯炭疽菌可以侵染菜心、萝卜等众多十字花科蔬菜引起炭疽病,给农业生产造成巨大的经济损失。septin是一个广泛存在于除植物以外其他生物的基因家族,其功能与细胞分裂、细胞内物质运输以及细胞周期的调控与细胞凋亡等关系密切,将成为新开发药物的潜在靶标。基于酿酒酵母中7个典型Septin蛋白的氨基酸序列,对炭疽菌属蛋白质数据库进行Blastp比对和关键词搜索,并通过SMART在线分析最终明确希金斯炭疽菌中含有5个典型的Septin。同时,从理化性质、跨膜区结构、二级结构以及亚细胞定位等方面对Septin进行生物信息学分析,结果显示,上述Septin均为亲水性蛋白,且不具有跨膜结构,其二级结构及亚细胞定位情况不同。
URL [本文引用: 4]

希金斯炭疽菌可以侵染菜心、萝卜等众多十字花科蔬菜引起炭疽病,给农业生产造成巨大的经济损失。septin是一个广泛存在于除植物以外其他生物的基因家族,其功能与细胞分裂、细胞内物质运输以及细胞周期的调控与细胞凋亡等关系密切,将成为新开发药物的潜在靶标。基于酿酒酵母中7个典型Septin蛋白的氨基酸序列,对炭疽菌属蛋白质数据库进行Blastp比对和关键词搜索,并通过SMART在线分析最终明确希金斯炭疽菌中含有5个典型的Septin。同时,从理化性质、跨膜区结构、二级结构以及亚细胞定位等方面对Septin进行生物信息学分析,结果显示,上述Septin均为亲水性蛋白,且不具有跨膜结构,其二级结构及亚细胞定位情况不同。
DOI:10.1186/1471-2148-7-103URLPMID:17601340 [本文引用: 1]

BACKGROUND: Septins are cytoskeletal GTPase proteins first discovered in the fungus Saccharomyces cerevisiae where they organize the septum and link nuclear division with cell division. More recently septins have been found in animals where they are important in processes ranging from actin and microtubule organization to embryonic patterning and where defects in septins have been implicated in human disease. Previous studies suggested that many animal septins fell into independent evolutionary groups, confounding cross-kingdom comparison. RESULTS: In the current work, we identified 162 septins from fungi, microsporidia and animals and analyzed their phylogenetic relationships. There was support for five groups of septins with orthology between kingdoms. Group 1 (which includes S. cerevisiae Cdc10p and human Sept9) and Group 2 (which includes S. cerevisiae Cdc3p and human Sept7) contain sequences from fungi and animals. Group 3 (which includes S. cerevisiae Cdc11p) and Group 4 (which includes S. cerevisiae Cdc12p) contain sequences from fungi and microsporidia. Group 5 (which includes Aspergillus nidulans AspE) contains sequences from filamentous fungi. We suggest a modified nomenclature based on these phylogenetic relationships. Comparative sequence alignments revealed septin derivatives of already known G1, G3 and G4 GTPase motifs, four new motifs from two to twelve amino acids long and six conserved single amino acid positions. One of these new motifs is septin-specific and several are group specific. CONCLUSION: Our studies provide an evolutionary history for this important family of proteins and a framework and consistent nomenclature for comparison of septin orthologs across kingdoms.
DOI:10.1515/BC.2011.086URL [本文引用: 1]

Until recently, it had appeared that the septin family of proteins was restricted to the opisthokont eukaryotes (the fungi and animals and their close relatives the microsporidia and choanoflagellates). It has now become apparent that septins are also present in several other widely divergent eukaryotic lineages (chlorophyte algae, brown algae, and ciliates). This distribution and the details of the non-opisthokont septin sequences appear to require major revisions to hypotheses about the origins and early evolution of the septins.
DOI:10.1186/s12862-018-1297-8URLPMID:30616529 [本文引用: 1]

BACKGROUND: Septins are cytoskeletal proteins important in cell division and in establishing and maintaining cell polarity. Although septins are found in various eukaryotes, septin genes had the richest history of duplication and diversification in the animals, fungi and protists that comprise opisthokonts. Opisthokont septin paralogs encode modular proteins that assemble into heteropolymeric higher order structures. The heteropolymers can create physical barriers to diffusion or serve as scaffolds organizing other morphogenetic proteins. How the paralogous septin modules interact to form heteropolymers is still unclear. Through comparative analyses, we hoped to clarify the evolutionary origin of septin diversity and to suggest which amino acid residues were responsible for subunit binding specificity. RESULTS: Here we take advantage of newly sequenced genomes to reconcile septin gene trees with a species phylogeny from 22 animals, fungi and protists. Our phylogenetic analysis divided 120 septins representing the 22 taxa into seven clades (Groups) of paralogs. Suggesting that septin genes duplicated early in opisthokont evolution, animal and fungal lineages share septin Groups 1A, 4 and possibly also 1B and 2. Group 5 septins were present in fungi but not in animals and whether they were present in the opisthokont ancestor was unclear. Protein homology folding showed that previously identified conserved septin motifs were all located near interface regions between the adjacent septin monomers. We found specific interface residues associated with each septin Group that are candidates for providing subunit binding specificity. CONCLUSIONS: This work reveals that duplication of septin genes began in an ancestral opisthokont more than a billion years ago and continued through the diversification of animals and fungi. Evidence for evolutionary conservation of ~ 49 interface residues will inform mutagenesis experiments and lead to improved understanding of the rules guiding septin heteropolymer formation and from there, to improved understanding of development of form in animals and fungi.
DOI:10.3724/sp.j.1005.2008.01097URLPMID:18779165 [本文引用: 1]

The septins are a family of proteins that are broadly distributed in almost all of eukaryotes except plants. septin was first identified in yeast as a protein that played a role in cytokinesis. With the recent advances in the field, the functions of these proteins become diverse in many organisms. In particular, the number of known mammalian septin family members has increased dramatically. They are now known to have many cellular roles such as cytokinesis, polarity determination, vesicle trafficking and membrane dynamics. Recently, more and more data suggest that some septin family members participate in the pathogenesis of different diseases including neoplasia, neurodegeneration and infections. These make the research of septins a hallmark in cell biology and pathology. In this review, we will summarize the major research progresses about septins in their classification, structure, biological function and the relationship with human diseases.
DOI:10.3724/sp.j.1005.2008.01097URLPMID:18779165 [本文引用: 1]

The septins are a family of proteins that are broadly distributed in almost all of eukaryotes except plants. septin was first identified in yeast as a protein that played a role in cytokinesis. With the recent advances in the field, the functions of these proteins become diverse in many organisms. In particular, the number of known mammalian septin family members has increased dramatically. They are now known to have many cellular roles such as cytokinesis, polarity determination, vesicle trafficking and membrane dynamics. Recently, more and more data suggest that some septin family members participate in the pathogenesis of different diseases including neoplasia, neurodegeneration and infections. These make the research of septins a hallmark in cell biology and pathology. In this review, we will summarize the major research progresses about septins in their classification, structure, biological function and the relationship with human diseases.
[D].
[本文引用: 2]
[D].
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/s41586-019-1637-xURLPMID:31597961 [本文引用: 1]

The blast fungus Magnaporthe oryzae gains entry to its host plant by means of a specialized pressure-generating infection cell called an appressorium, which physically ruptures the leaf cuticle(1,2). Turgor is applied as an enormous invasive force by septin-mediated reorganization of the cytoskeleton and actin-dependent protrusion of a rigid penetration hypha(3). However, the molecular mechanisms that regulate the generation of turgor pressure during appressorium-mediated infection of plants remain poorly understood. Here we show that a turgor-sensing histidine-aspartate kinase, Sln1, enables the appressorium to sense when a critical turgor threshold has been reached and thereby facilitates host penetration. We found that the Sln1 sensor localizes to the appressorium pore in a pressure-dependent manner, which is consistent with the predictions of a mathematical model for plant infection. A Deltasln1 mutant generates excess intracellular appressorium turgor, produces hyper-melanized non-functional appressoria and does not organize the septins and polarity determinants that are required for leaf infection. Sln1 acts in parallel with the protein kinase C cell-integrity pathway as a regulator of cAMP-dependent signalling by protein kinase A. Pkc1 phosphorylates the NADPH oxidase regulator NoxR and, collectively, these signalling pathways modulate appressorium turgor and trigger the generation of invasive force to cause blast disease.
DOI:10.1016/j.fgb.2016.07.005URLPMID:27387218 [本文引用: 1]

Septins are GTP-binding proteins that regulate cell polarity, cytokinesis and cell morphogenesis. Fusarium head blight (FHB), caused by Fusarium graminearum, is one of the most devastating diseases worldwide. In this study, we have functionally characterized the core septins, Cdc3, Cdc10, Cdc11 and Cdc12 in F. graminearum. The loss of FgCdc3, FgCdc11, FgCdc12, but not FgCdc10, mutants showed significant reduction in growth, conidiation and virulence. Microscopic analyses revealed that all of them were involved in septum formation and nuclear division. Moreover, disruption of septin genes resulted in morphological defects in ascospores and conidia. Interestingly, conidia produced by DeltaFgcdc3, DeltaFgcdc11 and DeltaFgcdc12 mutants exhibited deformation with interconnecting conidia in contrast to their parent wild-type strain PH-1 and the DeltaFgcdc10 mutant that produced normal conidia. Using yeast two-hybrid assays, we determined the interactions among FgCdc3, FgCdc10, FgCdc11 and FgCdc12. Taken together, our results indicate that septins play important roles in the nuclear division, morphogenesis and pathogenicity in F. graminearum.
DOI:10.1111/mpp.12492URLPMID:27666337 [本文引用: 1]

Septins are a highly conserved family of GTP-binding proteins that contribute to many cellular and metabolic functions, including cell polarity, cytokinesis, cell morphogenesis and pathogenesis. In this study, we characterized the septins FaCdc3 and FaCdc12 in the filamentous fungus Fusarium asiaticum. The functions of FaCdc3 and FaCdc12 were evaluated by constructing deletion mutants of FaCdc3 and FaCdc12, designated DeltaFaCdc3-5 and DeltaFaCdc12-71, respectively. The deletion mutants exhibited a reduced rate of mycelial growth, increased aerial hyphae formation, irregularly shaped hyphae, reduced conidiation and a lack of sexual reproduction in wheat kernels. Histochemical analysis revealed that the conidia and hyphae of DeltaFaCdc3-5 and DeltaFaCdc12-71 formed large lipid droplets (LDs). DeltaFaCdc3-5 and DeltaFaCdc12-71 also exhibited increased resistance to agents that induce osmotic stress and damage the cell membrane and cell wall. In addition, the hyphae and conidia of the two mutants formed fewer septa than those of the wild-type and exhibited aberrant nuclear distribution. Pathogenicity assays showed that DeltaFaCdc3-5 and DeltaFaCdc12-71 exhibited reduced virulence on wheat spikelets, which was indirectly correlated with a reduced level of deoxynivalenol accumulation. All of these defects were restored by genetic complementation of the two mutants with the parental FaCdc3 and FaCdc12. These results indicate that FaCdc3 and FaCdc12 play a critical role in various cellular processes in F. asiaticum.
DOI:10.1371/journal.pone.0012933URLPMID:20885997 [本文引用: 1]

BACKGROUND: Septins are a highly conserved family of GTP-binding proteins involved in multiple cellular functions, including cell division and morphogenesis. Studies of septins in fungal cells underpin a clear correlation between septin-based structures and fungal morphology, providing clues to understand the molecular frame behind the varied morphologies found in fungal world. METHODOLOGY/PRINCIPAL FINDINGS: Ustilago maydis genome has the ability to encode four septins. Here, using loss-of-function as well as GFP-tagged alleles of these septin genes, we investigated the roles of septins in the morphogenesis of this basidiomycete fungus. We described that septins in U. maydis could assemble into at least three different structures coexisting in the same cell: bud neck collars, band-like structures at the growing tip, and long septin fibers that run from pole to pole near the cell cortex. We also found that in the absence of septins, U. maydis cells lost their elongated shape, became wider at the central region and ended up losing their polarity, pointing to an important role of septins in the morphogenesis of this fungus. These morphological defects were alleviated in the presence of an osmotic stabilizer suggesting that absence of septins affected the proper formation of the cell wall, which was coherent with a higher sensitivity of septin defective cells to drugs that affect cell wall construction as well as exocytosis. As U. maydis is a phytopathogen, we analyzed the role of septins in virulence and found that in spite of the described morphological defects, septin mutants were virulent in corn plants. CONCLUSIONS/SIGNIFICANCE: Our results indicated a major role of septins in morphogenesis in U. maydis. However, in contrast to studies in other fungal pathogens, in which septins were reported to be necessary during the infection process, we found a minor role of septins during corn infection by U. maydis.
DOI:10.1128/EC.00269-09URLPMID:19949047 [本文引用: 1]

Septins are cytoskeletal proteins found in fungi, animals, and microsporidia, where they form multiseptin complexes that act as scaffolds recruiting and organizing other proteins to ensure normal cell division and development. Here we characterize the septins AspA and AspC in the multicellular, filamentous fungus Aspergillus nidulans. Mutants with deletions of aspA, aspC, or both aspA and aspC show early and increased germ tube and branch emergence, abnormal septation, and disorganized conidiophores. Strains in which the native aspA has been replaced with a single copy of aspA-GFP driven by the native septin promoter or in which aspC has been replaced with a single copy of aspC-GFP driven by the native promoter show wild-type phenotypes. AspA-GFP and AspC-GFP show identical localization patterns as discrete spots or bars in dormant and expanding conidia, as rings at forming septa and at the bases of emerging germ tubes and branches, and as punctate spots and filaments in the cytoplasm and at the cell cortex. In conidiophores, AspA-GFP and AspC-GFP localize as diffuse bands or rings at the bases of emerging layers and conidial chains and as discrete spots or bars in newly formed conidia. AspA-GFP forms abnormal structures in DeltaaspC strains while AspC-GFP does not localize in DeltaaspA strains. Our results suggest that AspA and AspC interact with each other and are important for normal development, especially for preventing the inappropriate emergence of germ tubes and branches. This is the first report of a septin limiting the emergence of new growth foci in any organism.
DOI:10.1371/journal.ppat.1006275URLPMID:28282450 [本文引用: 1]

Successful infection of the host requires secretion of effector proteins to evade or suppress plant immunity. Secretion of effectors in root-infecting fungal pathogens, however, remains unexplored. We previously reported that Verticillium dahliae, a root-infecting phytopathogenic fungus, develops a penetration peg from a hyphopodium to infect cotton roots. In this study, we report that a septin ring, requiring VdSep5, partitions the hyphopodium and the invasive hypha and form the specialized fungus-host interface. The mutant strain, VdDeltanoxb, in which NADPH oxidase B (VdNoxB) is deleted, impaired formation of the septin ring at the hyphal neck, indicating that NADPH oxidases regulate septin ring organization. Using GFP tagging and live cell imaging, we observed that several signal peptide containing secreted proteins showed ring signal accumulation/secretion at the penetration interface surrounding the hyphal neck. Targeted mutation for VdSep5 reduced the delivery rate of secretory proteins to the penetration interface. Blocking the secretory pathway by disrupting the vesicular trafficking factors, VdSec22 and VdSyn8, or the exocyst subunit, VdExo70, also arrested delivery of the secreted proteins inside the hyphopodium. Reduced virulence was observed when cotton roots were infected with VdDeltasep5, VdDeltasec22, VdDeltasyn8 and VdDeltaexo70 mutants compared to infection with the isogenic wild-type V592. Taken together, our data demonstrate that the hyphal neck is an important site for protein secretion during plant root infection, and that the multiple secretory routes are involved in the secretion.
