 ,1, 何颖婷1, 周小枫1, 辛晓萍1, 张爱玲2, 袁晓龙1, 张哲1, 李加琪
,1, 何颖婷1, 周小枫1, 辛晓萍1, 张爱玲2, 袁晓龙1, 张哲1, 李加琪 ,1
,1Effect of KISS1 Interference on the Function of Porcine Granulosa Cells in Porcine Ovary
LU SiYu ,1, HE YingTing1, ZHOU XiaoFeng1, XIN XiaoPing1, ZHANG AiLing2, YUAN XiaoLong1, ZHANG Zhe1, LI JiaQi
,1, HE YingTing1, ZHOU XiaoFeng1, XIN XiaoPing1, ZHANG AiLing2, YUAN XiaoLong1, ZHANG Zhe1, LI JiaQi ,1
,1通讯作者:
责任编辑: 林鉴非
收稿日期:2019-11-28接受日期:2020-10-29网络出版日期:2020-12-01
| 基金资助: |
Received:2019-11-28Accepted:2020-10-29Online:2020-12-01
作者简介 About authors
陆思羽,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (833KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
陆思羽, 何颖婷, 周小枫, 辛晓萍, 张爱玲, 袁晓龙, 张哲, 李加琪. 干扰KISS1基因对猪卵巢颗粒细胞功能的影响[J]. 中国农业科学, 2020, 53(23): 4940-4949 doi:10.3864/j.issn.0578-1752.2020.23.018
LU SiYu, HE YingTing, ZHOU XiaoFeng, XIN XiaoPing, ZHANG AiLing, YUAN XiaoLong, ZHANG Zhe, LI JiaQi.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】卵巢是母畜繁育的基础,其主要功能是产生和排出成熟的卵子。卵巢卵泡中的颗粒细胞能够分泌性激素,促进母畜性征的发育及维持,卵巢能否健康发育关系着母畜繁殖能力的强弱及利用年限的长短[1]。卵泡生长发育的本质是颗粒细胞增殖和分化[2],卵泡闭锁的主要标志是颗粒细胞凋亡,颗粒细胞能够为卵巢和卵泡的生长发育提供促性腺激素受体、类固醇激素、细胞因子、生长因子等物质[3],颗粒细胞过度凋亡会导致雌激素分泌减少,孕激素分泌增加[4]等。综上所述,颗粒细胞作为卵泡的重要组成部分,其功能会直接影响卵泡的生长发育,进而影响雌性动物的繁殖能力。本研究将颗粒细胞作为探究卵泡体外发育的细胞模型,通过设计KISS1的干扰片段,基于实时定量PCR(quantitative real time PCR, qRT- PCR)试验证明干扰KISS1能够影响PI3K/AKT通路的信号传导,并进一步在细胞水平和分子水平探究了干扰KISS1对猪卵巢颗粒细胞凋亡、周期及雌激素分泌能力的影响,为完善KISS1调控颗粒细胞功能的机制研究提供了一定的理论基础。【前人研究进展】1997年Lee通过原位杂交和差异显示技术首次发现并命名了KISS1,该基因位于人类1号染色体1q32-41区,能够抑制肿瘤生长[5],因此可作为区分转移性黑色素瘤和非转移性黑色素瘤的标志物[6]。近年来有研究表明,KISS1基因在母畜发情、卵泡生长发育、排卵等生理过程中起着重要的作用[7]。对成年大鼠使用Kisspeptin治疗后,KISS1的表达量与大鼠的排卵数成正相关[8,9],而突变KISS1,会导致大鼠和小鼠的卵巢功能衰退,卵泡发育异常,进而阻碍卵泡成熟,影响小鼠卵巢黄体组织的形成[9,10]。此外,当小鼠到达初情期时,KISS1和GPR54在下丘脑-垂体-性腺轴中的表达量显著增高,表明KISS1/GPR54信号可由性腺轴传导,在哺乳动物中枢和神经内分泌系统中发挥作用[8]。这些研究结果表明,KISS1在一定程度上影响着母畜的繁殖性能。前期研究已经证实过表达KISS1能够促进卵巢卵泡的发育和排卵[8],且KISS1在卵泡生长发育的过程中起到重要性作用[7],但目前干扰KISS1在猪卵泡颗粒细胞中的作用尚不清楚。磷脂酰肌醇3-激酶(phosphoinositide 3-kinase, PI3K)可以磷酸化磷脂酰肌醇[11],其作为细胞中典型的信号转导通路[12,13],参与调节细胞的生长、分化、增殖和凋亡。PI3K/AKT是调控原始卵泡激活和发育的经典信号通路,PTEN是PI3K/AKT通路的终止信号,在初级和次级卵泡中敲除卵母细胞的该基因,PI3K/AKT信号通路仍能够正常激活[14],敲除成年小鼠卵母细胞中的PTEN,小鼠的原始卵泡在卵泡池就会发生早衰,且FSH和LH分泌异常,使小鼠丧失生育能力[15],但是敲除颗粒细胞中的PTEN,能够减缓颗粒细胞凋亡[16],并促进颗粒细胞增殖。此外蔡竞等证实P-AKT、p-mTOR、p-p70S6等表达水平下调能够抑制PI3K/AKT/mTOR信号通路的活性,抑制颗粒细胞增殖,进而影响卵泡发育[17],综上可知卵巢卵泡的发育与PI3K/AKT通路活性相关。【本研究切入点】PI3K信号通路与卵巢卵泡的生长发育密切相关,笔者前期研究表明过表达KISS1能够显著降低猪卵巢颗粒细胞的凋亡率,促进颗粒细胞分泌雌二醇(estradiol, E2),显著上调PI3K信号通路关键基因及雌激素受体ESR1和ESR2的mRNA水平[18],基于干扰猪卵巢颗粒细胞中KISS1基因的表达,能够影响PI3K信号通路部分标志基因的表达,我们推测干扰KISS同样也能够影响颗粒细胞功能,因此本研究拟通过干扰KISS1,进一步阐释KISS1对猪卵巢颗粒细胞功能的影响机制。【拟解决的关键问题】本研究以长大二元杂母猪卵巢卵泡颗粒细胞为试验材料,在转录水平证实干扰KISS1能够影响PI3K信号通路相关基因的表达,并拟在细胞和分子水平研究干扰KISS1对颗粒细胞凋亡、周期及E2分泌的影响。1 材料与方法
1.1 试验材料
母猪卵巢颗粒细胞:采集广州市嘉禾望岗屠宰场的长大二元杂母猪卵巢,迅速置于4℃含2%双抗(青霉素和链霉素)的PBS中,尽快返回实验室,并迅速分离卵巢颗粒细胞。主要试剂:DMEM基础培养基、含EDTA的胰酶稀释液、PBS缓冲液、胎牛血清、青霉素和链霉素购自Hyclone(美国);Lipofectamine? 3000转染试剂盒、Opti-MEM?试剂购自Invitrogen(美国);KISS1的干扰片段KISS1-siRNA(CAACAAGAAGTGGGA GCAGAA)及对照Scrambled- siRNA由广州锐博生物科技有限公司(中国)合成;Trizol、PrimeScriptTM RT Reagent Kit试剂盒购自TAKARA(日本);无水乙醇、4%甲醛、氯仿、异丙醇购自广州鼎国生物有限公司(中国);SYBR Green qPCR Mix购自Thermo(美国);Annexin V-FITC/PI双染细胞凋亡检测试剂盒和细胞周期检测试剂盒购自江苏凯基生物技术股份有限公司(中国);猪雌二醇试剂盒购自江苏酶免实业有限公司(中国);其他均为常规化学试剂。
1.2 原代颗粒细胞的分离与培养
从屠宰场采集的母猪卵巢,迅速置于4℃含2%双抗(青霉素和链霉素)的PBS试剂中,迅速带回实验室,在准备室用PBS清洗数次。在细胞房超净台取5 mL DMEM于15 mL离心管,使用镊子夹取母猪卵巢,用1 mL注射器小心多次吸取卵泡液共2 mL于15 mL离心管(卵泡直径为3—5 mm);25℃ 800 r/min离心5 min,弃上清,再加入预热的PBS(含1%双抗),轻柔吹打以重悬沉淀细胞,清洗沉淀细胞两次。先加入10 mL完全培养基(含10%小牛血清和1%双抗)于75 cm2细胞培养瓶,再加入5 mL细胞悬液,轻柔摇晃均匀。将培养瓶置于37℃,5% CO2培养箱,48 h后观察颗粒细胞的生长状况,用PBS清洗颗粒细胞两次,加入15 mL的完全培养基,再培养24 h可进行后续相关试验。1.3 颗粒细胞的接种和转染
铺板:观察细胞状态,当细胞汇合度达到80%左右,用预热的PBS(含1%双抗)清洗颗粒细胞两次;使用5 mL 0.25%的胰蛋白酶消化75 cm2培养瓶中的细胞3—5 min,快速加入5 mL不完全培养基(含10%胎牛血清的DMEM培养液)终止消化,25℃ 800 r/min离心5 min;用预热的PBS(含1%双抗)清洗细胞两次。将细胞悬液均匀加入细胞培养孔中,用完全培养基补充体积,轻柔地摇晃均匀,置于37℃,5% CO2培养箱继续培养。转染:铺板后细胞的汇合度达到80%左右,即可转染。用预热的PBS清洗颗粒细胞两次,转染方法参照Lipofectamine? 3000试剂盒说明书(每个试验组设计4个重复,使用24孔细胞培养板进行),步骤如下:在灭菌的1.5 mL离心管中加100 μL Opti-MEM和4 μL Lipofectamine? 3000试剂,离心混匀;在新的离心管中依次加入95 μL Opti-MEM、5 μL稀释好的Scrambled-siRNA和KISS1-siRNA(此时干扰片段及其对照终浓度为50 nmol·L-1)及4 μL P3000TM,离心混匀;将Lipofectamine? 3000混合液加到P3000TM混合液中,离心混匀,在室温下孵育10 min;在每孔细胞中加入450 μL不完全培养基及50 μL混合液;转染后将细胞培养板置于37℃,5% CO2培养箱继续培养,6—12 h后换液(防止转染试剂对细胞影响过大)。
1.4 颗粒细胞RNA抽提
从细胞中抽提RNA,不需要对细胞进行消化,直接加入Trizol(10 cm2·mL-1)在冰上放置10—15 min,4℃ 12 000 r/min离心5 min,将含RNA的上清转移至新的RNase-free管,加入氯仿(氯仿:Trizol为 1:5),激烈震荡,室温下放置5 min,4℃ 12 000 r/min离心15 min,将上层水相小心移至新的RNase- free管,加入异丙醇(异丙醇:Trizol为1:2),轻微颠倒混匀,室温下放置10 min,4℃ 12 000 r/min离心15 min,去上清。加入与Trizol等量的75%乙醇,清洗抽提的RNA,4℃ 12 000 r/min离心15 min,去上清,此步骤重复两次。将RNA于室温干燥5—10 min,加入30 μL的DEPC水溶解RNA沉淀。RNA质检:制备浓度为1%的琼脂糖凝胶,将2 μL RNA样品与2 μL Loading Buffer混匀,15 V/cm电压电泳20 min,使用凝胶成像仪拍照,观察条带,使用紫外分光光度计检测样品OD260/OD280的比值;-80℃保存备用。
1.5 检测关键基因的转录水平
RNA反转录参照TAKARA的PrimerScriptTM RT Reagent Kit说明书。RNA反转录反应体系(10 μL):5×PrimeScriptRT Master Mix,2 μL;Total RNA≤500 ng;补充RNase Free dH2O至总体积达10 μL。RNA反转录反应条件:37℃ 15 min,85℃ 5 s。参照NCBI公布的序列信息,使用Primer primer 5.0设计qRT-PCR引物(表1)。以母猪卵巢颗粒细胞的cDNA为模板,GAPDH为内参。qPCR反应体系(20 μL):2×SYBR Green PCR Master Mix,10 μL;PCR Forward Primer,0.6 μmol·L-1;PCR Reverse Primer,0.6 μmol·L-1;cDNA,2 μL;补充dd H2O至总体积达20 μL。qPCR反应程序:95℃预变性10 min;95℃预变性5 s,60℃延伸1 min,42个循环。引物由上海捷瑞生物科技有限公司合成。
Table 1
表1
表1定量引物
Table 1
| 引物名称 Primer name | Gene ID | 引物序列 Primer sequence | 产物大小 Size (bp) |
|---|---|---|---|
| qRT-KISS1 | 100145896 | F: AACCAGCATCTTCTCACCAGG | 192 |
| R: CTTTCTCTCCGCACAACGC | |||
| qRT-PIK3CG | 396979 | F: AACGGGCTTTGAGATAGTGAA | 184 |
| R: AAGTTGCTTGGTTGGTGGATA | |||
| qRT-PIK3C1 | 5290 | F: CAAGTGAGAATGGTCCGAATG | 152 |
| R: GTGGAAGAGTTTGCCTGTTTT | |||
| qRT-PDK1 | 100286871 | F: AAATCACCAGGACAGCCAATA | 190 |
| R: CTTCTCGGTCACTCATCTTCAC | |||
| qRT-AKT1 | 100126861 | F: CTGCACAAACGAGGCGAGT | 89 |
| R: CGCTCCTTGTAGCCGATGAA | |||
| qRT-FOXO3 | 733621 | F: ACAAACGGCTCACTCTGTCCCA | 85 |
| R: GAACTGTTGCTGTCGCCCTTATC | |||
| qRT-TSC2 | 100505408 | F: CGAGGTGGTGTCCTACGAGAT | 115 |
| R: GAGCAGGCGTTCAATGATGTT | |||
| qRT-BAD | 100521065 | F: AGTCGCCACTGCTCTTACCC | 172 |
| R: TCTTGAAGGAACCCTGGAAATC | |||
| qRT-ESR1 | 10013276 | F: GATGCCTTGGTCTGGGTGAT | 124 |
| R: AGTGTTCCGTGCCCTTGTTA | |||
| qRT-ESR2 | 396697 | F: AAGGGAAAAGGAGGATGGGACA | 202 |
| R: CAGATAGGGACTGCGTGGAGGT | |||
| qRT-Star | 396597 | F: GGAAAAGACACAGTCATCACCCAT | 121 |
| R: CAGCAAGCACACACACGGAAC | |||
| qRT-CYP17 | 100157047 | F: AAGCCAAGACGAACGCAGAAAG | 228 |
| R: TAGATGGGGCACGATTGAAACC | |||
| qRT-3BHSD | 445539 | F: GGGGCTTCTGTCTTGATTCCA | 284 |
| R: GGTTTTCAGTGCTTCCTTGTGC | |||
| qRT-17BHSD | 397574 | F: CCCAACGCAGGAGACTCAAAAT | 149 |
| R: CCAGAGCCCATAACGAAGACAGA | |||
| qRT-CYP19A | 403332 | F: GCTGGACACCTCTAACAACCTCTT | 91 |
| R: TTGCCATTCATCAAAATAACCCT |
新窗口打开|下载CSV
1.6 颗粒细胞凋亡检测
参照江苏凯基生物技术股份有限公司的Annexin V-FITC/PI双染细胞凋亡检测试剂盒说明书进行颗粒细胞凋亡检测。取细胞接种于6孔板,当细胞汇合度达到80%左右,转染Scrambled-siRNA和KISS1-siRNA于颗粒细胞培养24 h,PBS清洗细胞,使用不含EDTA的胰酶消化细胞,再次用PBS溶液清洗细胞。取0.5 mL 细胞悬液(约5×105个细胞),加入500 μL 1×Binding Buffer,加入5 μL Annexin V-FITC,再加入室温预热的5 μL Propidium Iodide,室温避光孵育5 min,立即用流式细胞仪检测分析(每个试验组设计3个重复)。1.7 颗粒细胞周期检测
使用江苏凯基生物技术股份有限公司的流式检测细胞周期试剂盒,取细胞接种于6孔细胞板,当细胞汇合度达到80%左右,转染Scrambled-siRNA和KISS1-siRNA于颗粒细胞培养24 h,PBS清洗细胞,使用不含EDTA的胰酶消化细胞,再次用PBS溶液清洗细胞。使用70%的乙醇溶液固定细胞,4℃固定细胞4 h以上,将固定好的颗粒细胞用预冷的PBS洗两次,室温2 000 r/min离心5 min,用400 μL的碘化丙啶轻柔重悬细胞,加入100 μL RNase,4℃避光孵育30 min。24 h内用流式细胞仪检测分析(每个试验组设计3个重复)。1.8 颗粒细胞上清中E2含量检测
使用ELISA检测细胞上清中E2的含量,参照江苏酶免实业有限公司的猪雌二醇ELISA试剂盒说明书。确定标准品孔和样本孔,在标准品孔中加不同浓度的标准品50 μL;取细胞接种于6孔板,当细胞汇合度达到80%左右,转染Scrambled-siRNA和KISS1- siRNA于颗粒细胞培养24 h,吸取细胞上清1 000 r/min离心20 min,取50 μL于96孔板即为样本孔,空白孔不加;用封板膜封住反应孔,37℃孵育30 min,弃去液体,在吸水纸上拍干,每孔加入350 μL的洗涤液,静置1 min,弃去液体,在吸水纸上拍干,该步骤重复5次;每孔加入100 μL的辣根过氧化物酶标记的检测抗体(除空白孔),用封板膜封住反应孔,37℃孵育30 min,弃去液体,在吸水纸上拍干;每孔加入350 μL的洗涤液,室温静置1 min,弃去液体,在吸水纸上拍干,该步骤重复5次;每孔加入各50 μL A和B底物,用锡箔纸包裹以避光,37℃孵育15 min;每孔加入50 μL的终止液,在15 min内用酶标仪A450测定OD值。1.9 数据的统计分析
每个试验3—4个生物学重复,数据均用平均值±标准差表示,并经双尾T-test检验差异是否具有统计学意义。**代表P<0.01,*代表P<0.05。2 结果
2.1 干扰猪KISS1的效果
转染Scrambled-siRNA和KISS1-siRNA(序列:CAACAAGAAGTGGGAGCAGAA)至颗粒细胞后的qRT-PCR结果如图1,KISS1-siRNA组的KISS1的表达水平显著低于Scrambled-siRNA对照组(P<0.01),结果表明干扰片段KISS1-siRNA具有良好的干扰效果,可以用于后续试验。图1
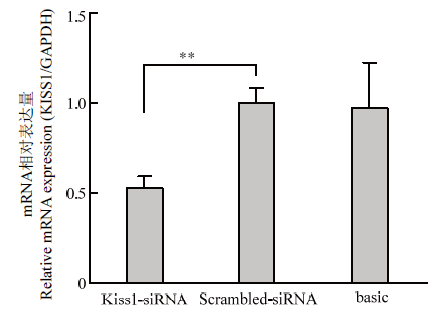 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1颗粒细胞KISS1的干扰效果
Fig. 1Interference effect of porcine KISS1 in granulosa cells
* P<0.05,** P<0.01。下同 The same as below
2.2 干扰KISS1抑制PI3K信号通路
干扰KISS1后,激活PI3K信号通路的AKT1转录水平显著降低(P<0.05),PIK3CG、PI3CI、PDK1转录水平降低,但差异不显著(图2-A);抑制PI3K通路的FOXO3和BAD转录水平显著降低(P<0.05),TSC2的mRNA表达水平降低,但差异不显著(图2-B)。结果表明,干扰KISS能够抑制PI3K信号通路部分标志基因的表达。图2
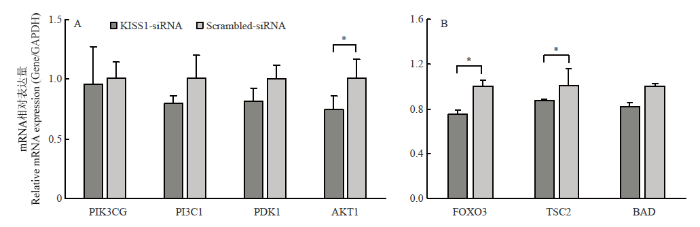 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2干扰KISS1抑制PI3K信号通路
A. PI3K信号通路激活相关基因的表达;B. PI3K信号通路抑制相关基因的表达
Fig. 2Interfering KISS1 inhibits PI3K signaling pathway
A. PI3K signaling pathway activates expression of related genes. B. PI3K signaling pathway inhibits the expression of related genes
2.3 干扰KISS1的表达促进颗粒细胞的凋亡
颗粒细胞Annexin FITC/PI检测结果如图3,其中Q1区域为机械损伤细胞,Q2(FITC+/PI+)区域为晚期凋亡或坏死细胞,Q3(FITC+/PI-)区域为早期凋亡细胞,Q4(FITC-/PI-)区域为活细胞(图3-A和B)。KISS1-siRNA组颗粒细胞的凋亡率显著高于Scrambled- siRNA对照组(P<0.01)(图3-C),结果表明,干扰KISS1促进颗粒细胞凋亡。图3
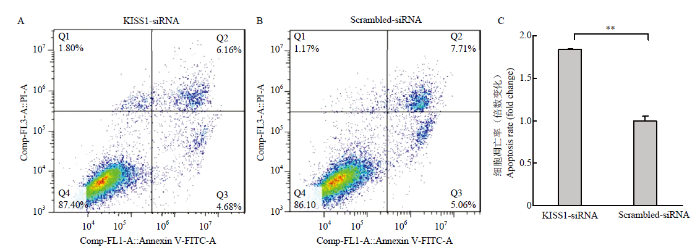 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3干扰KISS1促进颗粒细胞凋亡
A.转染KISS1-siRNA的流式检测结果;B.转染Scrambled-siRNA的流式检测结果;C.干扰KISS1促进颗粒细胞凋亡
Fig. 3Interfering KISS1 promotes granulosa cell apoptosis
A. Flow cytometry of KISS1-siRNA transfection. B. Flow cytometry of Scrambled-siRNA transfection. C. Interfering KISS1 promotes granulosa cell apoptosis
2.4 干扰KISS1的表达影响颗粒细胞的细胞周期
为探究KISS1对颗粒细胞周期的影响,流式检测结果如图4,单因素方差分析证实KISS1-siRNA组处于G1/G0期的颗粒细胞比例显著高于Scrambled- siRNA对照组的颗粒细胞(P<0.01),即干扰KISS1后,颗粒细胞阻滞在分裂间期。图4
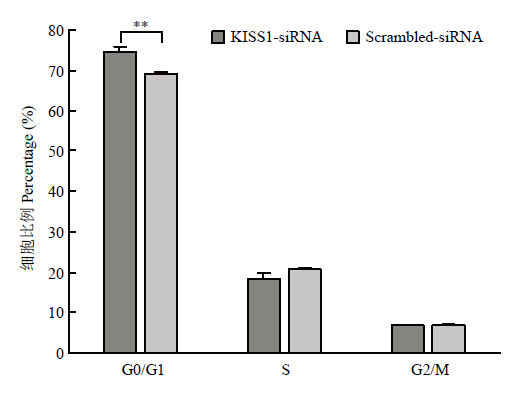 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4干扰KISS1能够将颗粒细胞阻滞在分裂间期
Fig. 4Interfering KISS1 arrests granulosa cells in interphase
2.5 干扰KISS1的表达抑制颗粒细胞分泌E2
颗粒细胞的主要功能之一是分泌E2,为研究KISS1对颗粒细胞分泌E2能力的影响,ELISA法检测结果如图5,KISS1-siRNA组细胞上清中E2的浓度显著低于Scrambled-siRNA对照组(P<0.01),即干扰KISS1的表达,可以抑制颗粒细胞分泌E2。图5
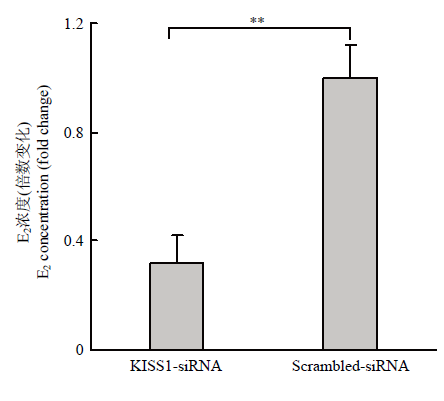 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5干扰KISS1抑制颗粒细胞分泌E2
Fig. 5Interfering KISS1 inhibits granulosa cell secretion of E2
2.6 干扰KISS1的表达能影响雌激素信号通路相关基因的表达
干扰KISS1能够影响颗粒细胞的凋亡和周期,并影响颗粒细胞分泌E2的能力,为进一步探究KISS1能否影响雌激素合成相关基因及雌激素受体的转录水平,qRT-PCR结果证实,干扰KISS1,雌激素受体ESR1的转录水平显著低于Scrambled-siRNA组(P<0.05),ESR2的转录水平低于Scrambled-siRNA组,差异不显著(图6-A),说明干扰KISS1能够抑制ESR1和ESR2表达。此外,qRT-PCR结果证实,干扰KISS1后,雌激素合成通路基因Star(P<0.05)、CYP17(P<0.01)、3B-HSD(P<0.05)和17B-HSD(P<0.05)转录水平显著低于Scrambled-siRNA组,CYP19A的转录水平低于Scrambled-siRNA组(图6-B),说明干扰KISS1能够影响雌激素合成通路基因转录水平。图6
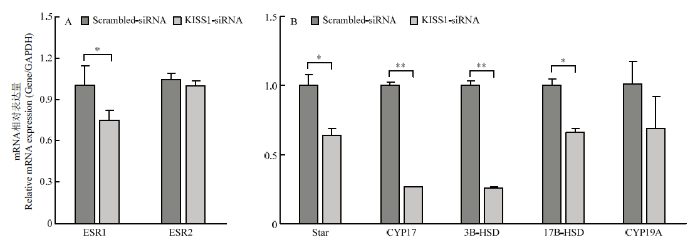 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6雌激素分泌相关通路基因转录水平
A. 干扰KISS1抑制ESR1和ESR2表达;B. 干扰KISS1抑制雌激素通路基因的表达
Fig. 6Gene transcription level of estrogen secretion related pathway
A. Interfering KISS1 inhibits ESR1 and ESR2 expression; B. Interfering KISS1 inhibits the expression of estrogen pathway genes
3 讨论
3.1 KISS1与PI3K信号通路的联系
猪的KISS1位于9号染色体[19],包含2个外显子,CDS区长度约为417 bp,编码138个氨基酸[20],广泛分布于人和动物的各个组织中,在人的胎盘中表达量最高[6, 21],在大鼠的下丘脑中表达量最高[22],在猪的下丘脑、垂体、卵巢等组织中都有不同水平的表达[20]。早期有关KISS1的研究主要集中在癌细胞的转移抑制[23],之后的研究更多集中在KISS1对哺乳动物中枢调节和神经内分泌的作用[24],近期已有研究证实,KISS1在哺乳动物的卵巢中表达,但关于KISS1在猪卵巢中表达调控机制的研究较少。此外,PI3K/AKT信号通路是调控原始卵泡激活和发育的经典信号通路,于是本研究首先探究了干扰KISS1对PI3K信号通路中部分基因转录水平的影响。又因为卵泡是卵巢的组成单位,圆形泡状卵泡位于卵巢皮质部,主要由卵母细胞、颗粒细胞和膜细胞组成,根据卵母细胞及颗粒细胞不同时期的生长状况,可将卵泡分为原始卵泡、初级卵泡、次级卵泡、三级卵泡及成熟卵泡。原始卵泡的卵母细胞仅包裹着单层扁平状的颗粒细胞,随着卵泡的发育,包裹卵母细胞的颗粒细胞逐渐增至数层,形态也由扁平状变为柱状,并在卵泡排卵前逐渐分化为包裹卵母细胞的卵丘颗粒细胞和紧贴卵泡内壁的壁层颗粒细胞[25,26,27]。颗粒细胞的发育优先于卵母细胞的发育[28],因而颗粒细胞的生长分化是原始卵泡生长和启动的关键因素,而颗粒细胞的凋亡是卵泡闭锁的主要标志,于是本研究将母猪卵巢颗粒细胞作为探究卵泡体外发育的细胞模型。干扰KISS1后,发现能够激活PI3K信号通路的AKT1转录水平显著降低(P<0.05),抑制PI3K信号通路激活的FOXO3转录水平也显著降低(P<0.05),该结果初步说明干扰KISS1可能微调PI3K信号通路的基因转录水平,并影响PI3K信号通路活性。3.2 KISS1与颗粒细胞功能的联系
基于干扰KISS1能够影响PI3K/AKT信号通路标志基因的表达量,于是进一步检测干扰KISS1对颗粒细胞功能的影响。前期研究发现,过表达KISS1能够促进颗粒细胞增殖,抑制颗粒细胞凋亡[18];本研究发现,干扰KISS1,凋亡的颗粒细胞比例显著增加,这部分结果与前期研究结果相呼应,证明KISS1的转录水平能够影响颗粒细胞的增殖和凋亡,于是进一步研究干扰KISS1对颗粒细胞周期的影响。已知细胞周期分为G0/G1、S和G2/M期,其中G0/G1期为细胞处于阻留的状态,细胞在未受刺激时会暂时停止分裂,但DNA合成与细胞分裂的潜力仍然存在,在一定适宜刺激下,又可进入周期进行分裂增殖[29],G0/G1期阻滞是机体调节细胞周期的重要手段[30]。S期为DNA合成时期,DNA数目在此时期加倍,一般情况下细胞一旦进入S期,细胞分裂就会继续进行下去,直到下一周期[31]。本研究发现干扰KISS1,阻滞在G0/G1期的颗粒细胞显著高于对照组(P<0.01),而处于S期的颗粒细胞则低于对照组,但差异不显著,细胞周期结合细胞凋亡的结果,也说明干扰KISS1能够显著影响颗粒细胞的功能。3.3 KISS1与颗粒细胞E2分泌的联系
KISS1/GPR54信号传导是下丘脑回路的一部分,该信号能够促性腺激素释放,进而促进卵巢中E2释放[32]、性器官发育[33]等,于是本研究拟通过干扰KISS1,探究KISS1与颗粒细胞E2分泌的联系,结果表明体外干扰颗粒细胞中的KISS1,培养基上清中E2的浓度显著降低(P<0.01),该部分结果与前人研究结果一致。同时已有研究表明,雌激素受体ESR1和ESR2在动物机体内具有重要的生理作用[34,35],E2与受体ESR1和ESR2结合才能发挥作用[36],且在卵巢发育的过程中,随着颗粒细胞的生长,ESR1的表达水平也随之增加[37,38]。当雌激素受体与其配体结合时,可协同PI3K通路,激活原始卵泡,促进卵泡成熟[39],但当小鼠体内的雌激素受体缺失时,小鼠血清中FSH、LH、E2等含量显著高于正常小鼠,小鼠排卵功能发生障碍[40]。综上所述,ESR1和ESR2基因的转录水平,能够反映颗粒细胞分泌E2的能力。此外,雌激素受体的表达除了可以调节相关激素的分泌,还能够升高雌激素合成相关基因CYP17和HSD17b的转录水平,促进排卵[41]。于是本研究将Scrambled-siRNA和KISS1-siRNA转染至颗粒细胞,抽取细胞RNA,并使用qRT-PCR分析颗粒细胞中雌激素受体及E2信号通路关键基因的转录水平。结果证实,干扰KISS1,E2受体ESR1表达量显著下调,E2信号通路关键基因Star、CYP17、3B-HSD和17B-HSD也显著下调,该结果与ELISA结果一致,从分子水平佐证了干扰KISS1能够抑制颗粒细胞E2分泌。
4 结论
干扰KISS1能够抑制猪卵巢颗粒细胞中PI3K信号通路的激活,同时能够促进颗粒细胞的凋亡,将颗粒细胞阻滞于分裂间期。干扰KISS1能够抑制颗粒细胞中雌激素受体ESR1和ESR2及雌激素合成信号通路基因Star、CYP17、3B-HSD和17B-HSD的转录水平,抑制E2的分泌。(责任编辑 林鉴非)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1095/biolreprod40.4.720URLPMID:2752073 [本文引用: 1]

Immature oocytes were collected from immature female rats (60-65 g) 40 h after injection with 6 IU pregnant mare's serum gonadotropin (PMSG). Oocytes were matured cumulus-intact (CI) or cumulus-free (CF) in medium supplemented with 0.5% bovine serum albumin (BSA) or 5-20% serum for periods of up to 24 h. After assessment for nuclear maturation, the oocytes were exposed to epididymal sperm for fertilization in vitro. In vitro-matured and ovulated oocytes undergoing fertilization were transferred to unilaterally pregnant recipients for embryonic and fetal development. The presence of cumulus cells and serum shortened (by 2 h) the time required for polar body emission by in vitro-matured oocytes and also helped to increase significantly the penetrability of the oocytes by spermatozoa. A high proportion (45.6%) of fertilized oocytes showed evidence of abnormal fertilization following maturation in the absence of cumulus cells. Oocytes matured CI before fertilization were able to develop to viable fetuses (57.8%) in proportions similar to ovulated oocytes (55.0%) after in vitro fertilization. These findings indicate an essential role for cumulus cells in promoting normal cytoplasmic maturation of oocytes necessary for pronuclear formation and subsequent developmental capability.
DOI:10.1007/s00441-015-2257-xURLPMID:26254045 [本文引用: 1]

In this review, we discuss the way that insights from evolutionary theory and systems biology shed light on form and function in mammalian reproductive systems. In the first part of the review, we contrast the rapid evolution seen in some reproductive genes with the generally conservative nature of development. We discuss directional selection and coevolution as potential drivers of rapid evolution in sperm and egg proteins. Such rapid change is very different from the highly conservative nature of later embryo development. However, it is not unique, as some regions of the sex chromosomes also show elevated rates of evolutionary change. To explain these contradictory trends, we argue that it is not reproductive functions per se that induce rapid evolution. Rather, it is the fact that biotic interactions, such as speciation events and sexual conflict, have no evolutionary endpoint and hence can drive continuous evolutionary changes. Returning to the question of sex chromosome evolution, we discuss the way that recent advances in evolutionary genomics and systems biology and, in particular, the development of a theory of gene balance provide a better understanding of the evolutionary patterns seen on these chromosomes. We end the review with a discussion of a surprising and incompletely understood phenomenon observed in early embryos: namely the Warburg effect, whereby glucose is fermented to lactate and alanine rather than respired to carbon dioxide. We argue that evolutionary insights, from both yeasts and tumor cells, help to explain the Warburg effect, and that new metabolic modeling approaches are useful in assessing the potential sources of the effect.
DOI:10.1530/rep.0.1220829URLPMID:11732978 [本文引用: 1]

A new perspective on ovarian follicular development has emerged over the last decade. Whereas the oocyte was previously considered only a passive recipient of developmental signals from oocyte-associated granulosa cells, it is now clear that communication between oocytes and granulosa cells is bidirectional. A complex interplay of regulatory factors governs the development of both types of cell. This interplay is essential not only for oocyte development but also for follicular development, beginning with the initial assembly of the primordial follicle and continuing throughout ovulation. The existence of an oocyte-granulosa cell regulatory loop, essential for normal follicular differentiation as well as for the production of an oocyte competent to undergo fertilization and embryogenesis, is proposed. Although gonadotrophins are essential for driving the differentiation of granulosa cell phenotypes, within its sphere of influence, the oocyte is probably the dominant factor determining the direction of differentiation and the function of the granulosa cells associated with it.
DOI:10.1093/hmg/ddh124URLPMID:15056605 [本文引用: 1]

FOXL2 mutations cause gonadal dysgenesis or premature ovarian failure (POF) in women, as well as eyelid/forehead dysmorphology in both sexes (the 'blepharophimosis-ptosis-epicanthus inversus syndrome', BPES). Here we report that mice lacking Foxl2 recapitulate relevant features of human BPES: males and females are small and show distinctive craniofacial morphology with upper eyelids absent. Furthermore, in mice as in humans, sterility is confined to females. Features of Foxl2 null animals point toward a new mechanism of POF, with all major somatic cell lineages failing to develop around growing oocytes from the time of primordial follicle formation. Foxl2 disruption thus provides a model for histogenesis and reproductive competence of the ovary.
DOI:10.1006/geno.1998.5566URLPMID:9806840 [本文引用: 1]

The identification and sequence of KiSS-1 (HGMW-approved symbol, KISS1), a human malignant melanoma metastasis-suppressor gene, was recently published. In this report, we present a corrected genomic sequence, genomic structure, and refined chromosomal location for KiSS-1. The genomic organization of the sequence reveals a gene consisting of four exons. The first two exons are not translated; the third exon contains 38 5' noncoding bases followed by the translational start site and another 100 translated bases. The terminal exon contains a further 332 translated bases, the translational stop codon, and the polyadenylation signal. The gene maps to chromosome 1q32 as determined by radiation hybrid mapping and FISH analysis. The relatively simple organization of this gene will facilitate analyses for mutations and abnormal expression in human tumors.
DOI:10.1093/jnci/88.23.1731URLPMID:8944003 [本文引用: 2]

BACKGROUND: Microcell-mediated transfer of chromosome 6 into human C8161 and MelJuSo melanoma cell suppresses their ability to metastasize by at least 95% without affecting their tumorigenicity. This observation demonstrates that the ability to metastasize is a phenotype distinct from tumor formation and suggests that tumorigenic cells acquire metastatic capability only after accumulating additional genetic defects. These results also imply that mutations of genes on chromosome 6 are among those late genetic changes responsible for metastatic potential. They further suggest that a melanoma metastasis-suppressor gene(s) is encoded on chromosome 6 or is regulated by genes on chromosome 6. PURPOSE: Our objective was to identify the gene(s) responsible for the suppression of metastasis in chromosome 6/melanoma cell hybrids. METHODS: A modified subtractive hybridization technique was used to compare the expression of messenger RNAs (mRNAs), via an analysis of complementary DNAs (cDNAs), in metastatic cells (C8161 or MelJuSo) and nonmetastatic hybrid clones (neo6/C8161 or neo6/MelJuSo). RESULTS: A novel cDNA, designated KiSS-1, was isolated from malignant melanoma cells that had been suppressed for metastatic potential by the introduction of human chromosome 6. Northern blot analyses comparing mRNAs from a panel of human melanoma cells revealed that KiSS-1 mRNA expression occurred only in nonmetastatic melanoma cells. Expression of this mRNA in normal heart, brain, liver, lung, and skeletal muscle was undetectable by northern blot analysis. Weak expression was found in the kidney and pancreas, but the highest expression was observed in the placenta. The KiSS-1 cDNA encodes a predominantly hydrophilic, 164 amino acid protein with a polyproline-rich domain indicative of an SH3 ligand (binds to the homology 3 domain of the oncoprotein Src) and a putative protein kinase C-alpha phosphorylation site. Transfection of a full-length KiSS-1 cDNA into C8161 melanoma cells suppressed metastasis in an expression-dependent manner. CONCLUSIONS: These data strongly suggest that KiSS-1 expression may suppress the metastatic potential of malignant melanoma cells. IMPLICATIONS: KiSS-1 may be a useful marker for distinguishing metastatic melanomas from nonmetastatic melanomas.
[本文引用: 2]
DOI:10.1152/ajpendo.90895.2008URLPMID:19141682 [本文引用: 3]

Kisspeptins, the products of the KiSS-1 gene acting via G protein-coupled receptor 54 (GPR54), have recently emerged as pivotal signals in the hypothalamic network triggering the preovulatory surge of gonadotropins and, hence, ovulation. Additional actions of kisspeptins at other levels of the hypothalamic-pituitary-ovarian axis have been suggested but remain to date scarcely studied. We report herein the pattern of expression of KiSS-1 and GPR54 in the human and nonhuman primate ovary and evaluate changes in ovarian KiSS-1 expression in a rat model of ovulatory dysfunction. KiSS-1 and GPR54 mRNAs were detected in human ovarian tissue and cultured granulosa-lutein cells. In good agreement, kisspeptin immunoreactivity was observed in cyclic human and marmoset ovaries, with prominent signals in the theca layer of growing follicles, corpora lutea, interstitial gland, and ovarian surface epithelium. GPR54 immunoreactivity was also found in human theca and luteal cells. Administration of indomethacin to cyclic female rats disturbed ovulation and resulted in a dramatic drop in ovarian KiSS-1, but not GPR54, cyclooxygenase-2 (COX-2), or progesterone receptor, mRNA levels at the time of ovulation; an effect mimicked by the selective COX-2 inhibitor NS398 and rescued by coadministration of PGE(2). Likewise, the stimulatory effect of human choriogonadotropin on ovarian KiSS-1 expression was partially blunted by indomethacin. In contrast, KiSS-1 mRNA levels remained unaltered in another model of ovulatory failure, i.e., the RU486-treated rat. In summary, we document for the first time the expression of KiSS-1/kisspeptin and GPR54 in the human and nonhuman primate ovary. In addition, we provide evidence for the ability of inhibitors of COX-2, known to disturb follicular rupture and ovulation, to selectively alter the expression of KiSS-1 gene in rat ovary. Altogether, our results are suggestive of a conserved role of local KiSS-1 in the direct control of ovarian functions in mammals.
[D].
[本文引用: 2]
[本文引用: 2]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1126/science.296.5573.1655URLPMID:12040186 [本文引用: 1]

Phosphorylated lipids are produced at cellular membranes during signaling events and contribute to the recruitment and activation of various signaling components. The role of phosphoinositide 3-kinase (PI3K), which catalyzes the production of phosphatidylinositol-3,4,5-trisphosphate, in cell survival pathways; the regulation of gene expression and cell metabolism; and cytoskeletal rearrangements are highlighted. The PI3K pathway is implicated in human diseases including diabetes and cancer, and understanding the intricacies of this pathway may provide new avenues for therapuetic intervention.
DOI:10.1038/nrg1879URLPMID:16847462 [本文引用: 1]

Phosphatidylinositol 3-kinases (PI3Ks) evolved from a single enzyme that regulates vesicle trafficking in unicellular eukaryotes into a family of enzymes that regulate cellular metabolism and growth in multicellular organisms. In this review, we examine how the PI3K pathway has evolved to control these fundamental processes, and how this pathway is in turn regulated by intricate feedback and crosstalk mechanisms. In light of the recent advances in our understanding of the function of PI3Ks in the pathogenesis of diabetes and cancer, we discuss the exciting therapeutic opportunities for targeting this pathway to treat these diseases.
DOI:10.1371/journal.pone.0006186URLPMID:19587782 [本文引用: 1]

Immature ovarian primordial follicles are essential for maintenance of the reproductive lifespan of female mammals. Recently, it was found that overactivation of the phosphatidylinositol 3-kinase (PI3K) signaling in oocytes of primordial follicles by an oocyte-specific deletion of Pten (phosphatase and tensin homolog deleted on chromosome ten), the gene encoding PI3K negative regulator PTEN, results in premature activation of the entire pool of primordial follicles, indicating that activation of the PI3K pathway in oocytes is important for control of follicular activation. To investigate whether PI3K signaling in oocytes of primary and further developed follicles also plays a role at later stages in follicular development and ovulation, we conditionally deleted the Pten gene from oocytes of primary and further developed follicles by using transgenic mice expressing zona pellucida 3 (Zp3) promoter-mediated Cre recombinase. Our results show that Pten was efficiently deleted from oocytes of primary and further developed follicles, as indicated by the elevated phosphorylation of the major PI3K downstream component Akt. However, follicular development was not altered and oocyte maturation was also normal, which led to normal fertility with unaltered litter size in the mutant mice. Our data indicate that properly controlled PTEN/PI3K-Akt signaling in oocytes is essential for control of the development of primordial follicles whereas overactivation of PI3K signaling in oocytes does not appear to affect the development of growing follicles. This suggests that there is a stage-specific function of PTEN/PI3K signaling in mouse oocytes that controls follicular activation.
DOI:10.1126/science.1152257URLPMID:18239123 [本文引用: 1]

In the mammalian ovary, progressive activation of primordial follicles from the dormant pool serves as the source of fertilizable ova. Menopause, or the end of female reproductive life, occurs when the primordial follicle pool is exhausted. However, the molecular mechanisms underlying follicle activation are poorly understood. We provide genetic evidence that in mice lacking PTEN (phosphatase and tensin homolog deleted on chromosome 10) in oocytes, a major negative regulator of phosphatidylinositol 3-kinase (PI3K), the entire primordial follicle pool becomes activated. Subsequently, all primordial follicles become depleted in early adulthood, causing premature ovarian failure (POF). Our results show that the mammalian oocyte serves as the headquarters of programming of follicle activation and that the oocyte PTEN-PI3K pathway governs follicle activation through control of initiation of oocyte growth.
DOI:10.1210/me.2008-0095URLPMID:18606860 [本文引用: 1]

FSH activates the phosphatidylinositol-3 kinase (PI3K)/acute transforming retrovirus thymoma protein kinase pathway and thereby enhances granulosa cell differentiation in culture. To identify the physiological role of the PI3K pathway in vivo we disrupted the PI3K suppressor, Pten, in developing ovarian follicles. To selectively disrupt Pten expression in granulosa cells, Ptenfl/fl mice were mated with transgenic mice expressing cAMP response element recombinase driven by Cyp19 promoter (Cyp19-Cre). The resultant Pten mutant mice were fertile, ovulated more oocytes, and produced moderately more pups than control mice. These physiological differences in the Pten mutant mice were associated with hyperactivation of the PI3K/acute transforming retrovirus thymoma protein kinase pathway, decreased susceptibility to apoptosis, and increased proliferation of mutant granulosa cells. Strikingly, corpora lutea of the Pten mutant mice persisted longer than those of control mice. Although the follicular and luteal cell steroidogenesis in Ptenfl/fl;Cyp19-Cre mice was similar to controls, viable nonsteroidogenic luteal cells escaped structural luteolysis. These findings provide the novel evidence that Pten impacts the survival/life span of granulosa/luteal cells and that its loss not only results in the facilitated ovulation but also in the persistence of nonsteroidogenic luteal structures in the adult mouse ovary.
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 2]
DOI:10.1095/biolreprod.109.079863URLPMID:19828777 [本文引用: 1]

Kisspeptin-GPR54 signaling plays an essential role in normal reproduction in mammals via stimulation of gonadotropin secretion. Here, we cloned the porcine KISS1 cDNA from the hypothalamic tissue and investigated the effect of estrogen on the distribution and numbers of KISS1 mRNA-expressing cells in the porcine hypothalamus. The full length of the cDNA was 857 bp encoding the kisspeptin of 54 amino acids, with the C-terminal active motif designated kisspeptin-10 being identical to that of mouse, rat, cattle, and sheep. In situ hybridization analysis revealed that KISS1-positive cell populations were mainly distributed in the hypothalamic periventricular nucleus (PeN) and arcuate nucleus (ARC). KISS1 expression in the PeN of ovariectomized (OVX) pigs was significantly upregulated by estradiol benzoate (EB) treatment. On the other hand, KISS1-expressing cells were abundantly distributed throughout the ARC in both OVX and OVX with EB animals. The number of KISS1-expressing neurons was significantly lowered by EB treatment only in the most caudal part of the ARC, but other ARC populations were not affected. The present study thus suggests that the PeN kisspeptin neurons could be responsible for the estrogen positive feedback regulation to induce gonadotropin-releasing hormone/luteinizing hormone (GnRH/LH) surge in the pig. In addition, the caudal ARC kisspeptin neurons could be involved in the estrogen negative feedback regulation of GnRH/LH release. This is the first report of identification of porcine KISS1 gene and of estrogen regulation of KISS1 expression in the porcine brain, which may be helpful for better understanding of the role of kisspeptin in reproduction of the pig.
[本文引用: 2]
[本文引用: 2]
DOI:10.1038/35079135URLPMID:11385580 [本文引用: 1]

Metastasis is a major cause of death in cancer patients and involves a multistep process including detachment of cancer cells from a primary cancer, invasion of surrounding tissue, spread through circulation, re-invasion and proliferation in distant organs. KiSS-1 is a human metastasis suppressor gene, that suppresses metastases of human melanomas and breast carcinomas without affecting tumorigenicity. However, its gene product and functional mechanisms have not been elucidated. Here we show that KiSS-1 (refs 1, 4) encodes a carboxy-terminally amidated peptide with 54 amino-acid residues, which we have isolated from human placenta as the endogenous ligand of an orphan G-protein-coupled receptor (hOT7T175) and have named 'metastin'. Metastin inhibits chemotaxis and invasion of hOT7T175-transfected CHO cells in vitro and attenuates pulmonary metastasis of hOT7T175-transfected B16-BL6 melanomas in vivo. The results suggest possible mechanisms of action for KiSS-1 and a potential new therapeutic approach.
DOI:10.1159/000083140URLPMID:15665556 [本文引用: 1]

The KiSS-1 gene codes for a family of neuropeptides called kisspeptins which bind to the G-protein-coupled receptor GPR54. To assess the possible effects of kisspeptins on gonadotropin secretion, we injected kisspeptin-52 into the lateral cerebral ventricles of adult male rats and found that kisspeptin-52 increased the serum levels of luteinizing hormone (p < 0.05). To determine whether the kisspeptin-52-induced stimulation of luteinizing hormone secretion was mediated by gonadotropin-releasing hormone (GnRH), we pretreated adult male rats with a GnRH antagonist (acyline), then challenged the animals with intracerebroventricularly administered kisspeptin-52. The GnRH antagonist blocked the kisspeptin-52-induced increase in luteinizing hormone. To examine whether kisspeptins stimulate transcriptional activity in GnRH neurons, we administered kisspeptin-52 intracerebroventricularly and found by immunocytochemistry that 86% of the GnRH neurons coexpressed Fos 2 h after the kisspeptin-52 challenge, whereas fewer than 1% of the GnRH neurons expressed Fos following injection of the vehicle alone (p < 0.001). To assess whether kisspeptins can directly act on GnRH neurons, we used double-label in situ hybridization and found that 77% of the GnRH neurons coexpress GPR54 mRNA. Finally, to determine whether KiSS-1 gene expression is regulated by gonadal hormones, we measured KiSS-1 mRNA levels by single-label in situ hybridization in intact and castrated males and found significantly higher levels in the arcuate nucleus of castrates. These results demonstrate that GnRH neurons are direct targets for regulation by kisspeptins and that KiSS-1 mRNA is regulated by gonadal hormones, suggesting that KiSS-1 neurons play an important role in the feedback regulation of gonadotropin secretion.
DOI:10.1016/j.bbrc.2003.11.066URLPMID:14652023 [本文引用: 1]

GPR54 is a G-protein-coupled receptor that displays a high percentage of identity in the transmembrane domains with the galanin receptors. The ligand for GPR54 has been identified as a peptide derived from the KiSS-1 gene. KiSS-1 has been shown to have anti-metastatic effects, suggesting that KiSS-1 or its receptor represents a potential therapeutic target. To further our understanding of the physiological function of this receptor, we have generated a mutant mouse line with a targeted disruption of the GPR54 receptor (GPR54 -/-). The analysis of the GPR54 mutant mice revealed developmental abnormalities of both male and female genitalia and histopathological changes in tissues which normally contain sexually dimorphic features. These data suggest a role for GPR54/KiSS-1 in normal sexual development, and indicate that study of the GPR54 mutant mice may provide valuable insights into human reproductive syndromes.
URLPMID:21576407 [本文引用: 1]

The Kiss-1 gene encodes a secreted protein that is proteolytically cleaved to produce a number of structurally related peptides, with high interspecies conservation, globally termed kisspeptins. The original niche for the role of kisspeptin in human physiology is derived from cancer biology, with the loss of Kiss-1 expression being associated with poor prognosis in several malignancies. However, kisspeptin has recently emerged as a fundamental player in the field of reproductive biology. Genetic analysis of large consanguineous pedigrees by two independent groups led to the association of inactivating mutations of GPR54, the receptor which mediates kisspeptin action, with idiopathic hypogonadotropic hypogonadism. In the present paper the most salient aspects of the multifaceted role of kisspeptin in the reproductive system are reviewed, including the association of kisspeptin with the gonadal steroid feedback loop and the triggering of puberty onset.
DOI:10.1530/ror.0.0050122URLPMID:10864857 [本文引用: 1]

Oocyte quality in pigs is defined as the potential of that oocyte to develop into a viable offspring. There is increasing evidence that the programming of the oocyte must be completed before leaving the ovarian follicle, including both nuclear and cytoplasmic maturation. Pig oocytes matured in vitro under basic conditions are deficient in some, as yet unidentified, cytoplasmic factors and thus developmentally incompetent. This developmental incompetence can be overcome to some extent by follicular supplementation (with follicular fluid or granulosa cells) of the culture medium, emphasizing the importance of somatic signals during oocyte maturation. Furthermore, evidence is accumulating that the status of the follicle has a critical impact on the competence of the oocyte in vitro and in vivo and this has been demonstrated by co-culture with follicles at different maturational stages or from breeds with enhanced embryo survival. It now also appears that manipulation of maternal nutrition before mating or oocyte collection can enhance embryo survival in vivo and oocyte developmental competence in vitro, presumably by altering follicular secretions and hence the environment in which the oocyte is nurtured. Identification of both the key follicular factors influencing pig oocyte quality and reliable markers of oocyte quality will undoubtedly yield major improvements in embryo survival in vivo and embryo production in vitro.
DOI:10.1210/edrv.21.2.0394URLPMID:10782364 [本文引用: 1]

Mammalian ovaries consist of follicles as basic functional units. The total number of ovarian follicles is determined early in life, and the depletion of this pool leads to reproductive senescence. Each follicle develops to either ovulate or, more likely, to undergo degeneration. The dynamics of ovarian follicle development have interested endocrinologists and developmental biologists for many years. With the advent of assisted reproductive techniques in humans, the possibility of regulating follicle development in vivo and in vitro has gained clinical relevance. In this review, we focus upon key branching points during the development of ovarian follicles as well as factors involved in determining the eventual destiny of individual follicles. We discuss inconsistencies in the literature regarding the definitions of follicle recruitment and selection and propose to name the two major steps of follicle development as initial and cyclic recruitment, respectively. Because some of these disparities have arisen due to differences in the animal systems studied, we also compare the development of the ovarian follicles of both humans and rats. We also review the status of knowledge of several puzzling clinical issues that may provide important clues toward unlocking the mechanisms of follicle development.
DOI:10.1146/annurev.physiol.64.081601.142703URLPMID:11826265 [本文引用: 1]

There is growing awareness that androgens and estrogens have general metabolic roles that are not directly involved in reproductive processes. These include actions on vascular function, lipid and carbohydrate metabolism, as well as bone mineralization and epiphyseal closure in both sexes. In postmenopausal women, as in men, estrogen is no longer solely an endocrine factor but instead is produced in a number of extragonadal sites and acts locally at these sites in a paracrine and intracrine fashion. These sites include breast, bone, vasculature, and brain. Within these sites, aromatase action can generate high levels of estradiol locally without significantly affecting circulating levels. Circulating C19 steroid precursors are essential substrates for extragonadal estrogen synthesis. The levels of these androgenic precursors decline markedly with advancing age in women, possible from the mid-to-late reproductive years. This may be a fundamental reason why women are at increased risk for bone mineral loss and fracture, and possibly decline of cognitive function, compared with men. Aromatase expression in these various sites is under the control of tissue-specific promotors regulated by different cohorts of transcription factors. Thus in principle, it should be possible to develop selective aromatase modulators (SAMs) that block aromatase expression, for example, in breast, but allow unimpaired estrogen synthesis in other tissues such as bone.
[本文引用: 1]
[本文引用: 1]
DOI:10.2478/s11658-014-0219-zURLPMID:25424911 [本文引用: 1]

Peripheral T cells are in G0 phase and do not proliferate. When they encounter an antigen, they enter the cell cycle and proliferate in order to initiate an active immune response. Here, we have determined the first two cell cycle times of a leading population of CD4(+) T cells stimulated by PMA plus ionomycin in vitro. The first cell cycle began around 10 h after stimulation and took approximately 16 h. Surprisingly, the second cell cycle was extremely rapid and required only 6 h. T cells might have a unique regulatory mechanism to compensate for the shortage of the gap phases in cell cycle progression. This unique feature might be a basis for a quick immune response against pathogens, as it maximizes the rate of proliferation.
DOI:10.1074/jbc.M116.773226URL [本文引用: 1]
DOI:10.1007/BF00364018URLPMID:53130 [本文引用: 1]

Chinese hamster cells were grown for 1+ and 2+ cell cycles in the presence of BrdU and then treated by the sister chromatid differential staining technique (SCD). Those regions of a chromosome which had replicated twice in the presence of BrdU were pale staining and by selecting appropriate metaphase cells an accurate reconstruction of the DNA synthetic patterns was possible. A direct correlation between the staining intensity of the G bands and the order in which they replicate was found. Dark staining G bands were always the last region of a chromosome to replicate while G negative bands were first. It is concluded that each G band may be a cluster of replicons capable of initiating DNA synthesis simultaneously.
DOI:10.1210/en.2004-0431URLPMID:15217982 [本文引用: 1]

Kisspeptins are products of the KiSS-1 gene, which bind to a G protein-coupled receptor known as GPR54. Mutations or targeted disruptions in the GPR54 gene cause hypogonadotropic hypogonadism in humans and mice, suggesting that kisspeptin signaling may be important for the regulation of gonadotropin secretion. To examine the effects of kisspeptin-54 (metastin) and kisspeptin-10 (the biologically active C-terminal decapeptide) on gonadotropin secretion in the mouse, we administered the kisspeptins directly into the lateral cerebral ventricle of the brain and demonstrated that both peptides stimulate LH secretion. Further characterization of kisspeptin-54 demonstrated that it stimulated both LH and FSH secretion, at doses as low as 1 fmol; moreover, this effect was shown to be blocked by pretreatment with acyline, a potent GnRH antagonist. To learn more about the functional anatomy of kisspeptins, we mapped the distribution of KiSS-1 mRNA in the hypothalamus. We observed that KiSS-1 mRNA is expressed in areas of the hypothalamus implicated in the neuroendocrine regulation of gonadotropin secretion, including the anteroventral periventricular nucleus, the periventricular nucleus, and the arcuate nucleus. We conclude that kisspeptin-GPR54 signaling may be part of the hypothalamic circuitry that governs the hypothalamic secretion of GnRH.
[本文引用: 1]
DOI:10.1210/en.2003-1462URLPMID:14670986 [本文引用: 1]

Until 1996, when estrogen receptor (ER)-beta was discovered, life seemed simple. The gonadal steroid hormone 17 beta-estradiol had one receptor, the ER, a ligand-inducible nuclear transcription factor. ER variants, the result of base pair insertions, transitions, and deletions, as well as alternative splicing, were considered abnormal and a prominent feature of breast cancer. Since then, like many other scientific beliefs, this concept has increased dramatically in complexity, and we are now faced with an ever-increasing array of estrogen-binding proteins, putative ERs, in the brain as well as in the extraneural targets of estrogen. The end is unlikely to be in sight. Some of these putative receptors have been localized to plasma or nuclear membranes, and others to the cytoplasm and/or nucleus. The molecular characteristics of membrane ERs are still in question, and, in most instances, the proteins have not been sequenced or cloned. However, based on transfection and immunohistochemistry, the generally held view, if not dogma, maintains that both nuclear and plasma membrane-associated ERs probably originate from the same gene and transcript that produce the classical intranuclear receptors ER-alpha and ER-beta. However, the physiological relatedness of this observation remains open to question. This review addresses evidence that, in addition to ER-alpha and ER-beta, there exist a variety of non-ER-alpha/non-ER-beta nuclear, cytoplasmic, and plasma membrane ERs in the brain, including G protein-coupled receptors; a novel, developmentally regulated, membrane-associated ER, ER-X; a functional, truncated ER-alpha variant, ER-46; and a putative ER that is immunochemically, structurally, and functionally completely distinct from ER-alpha and ER-beta.
DOI:10.1016/s0022-4804(03)00215-4URLPMID:14697301 [本文引用: 1]

Cardiovascular disease is the number one cause of death among women, accounting for nearly 50% of female deaths. Statistics show that women on average develop cardiovascular disease 10 to 15 years later in life than men, and that the risk may increase after menopause. This observation has led to much speculation as to what physiological change(s) associated with menopause is responsible for the higher risk of atherosclerosis. Estrogen, with its potential as a cardioprotective agent and as an immunomodulator of the inflammatory response in atherosclerosis, has received the most attention. Understanding the mechanisms that lead to these differences may allow beneficial therapeutic intervention to enhance this effect in females and evoke this protection in males. This review will do the following: (1) characterize mechanisms of atherosclerosis, (2) explore the role of estrogen-replacement therapy, (3) define the effect of gender on inflammation, (4) compare and contrast the effects of estrogen and testosterone on endothelial functional, and (5) suggest mechanistic based therapeutic opportunities.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]

雌激素在卵泡发育过程中所起的作用,已经得到人们的普遍认可.雌二醇(E2)及其类似物在卵泡的体细胞的增殖和分化中起重要作用,但其作用机理目前还不完全清楚.一些最新研究结果雌激素受体β(ERβ)的发现、雌激素受体基因敲除以及雌激素缺失动物模型的建立等,使我们能够进一步探讨雌激素在卵泡发育过程中的作用机理.结合这些最新研究动态,探讨雌激素在卵泡发育过程中的作用及雌激素对卵巢内的体细胞表型分化的影响.
URL [本文引用: 1]

雌激素在卵泡发育过程中所起的作用,已经得到人们的普遍认可.雌二醇(E2)及其类似物在卵泡的体细胞的增殖和分化中起重要作用,但其作用机理目前还不完全清楚.一些最新研究结果雌激素受体β(ERβ)的发现、雌激素受体基因敲除以及雌激素缺失动物模型的建立等,使我们能够进一步探讨雌激素在卵泡发育过程中的作用机理.结合这些最新研究动态,探讨雌激素在卵泡发育过程中的作用及雌激素对卵巢内的体细胞表型分化的影响.
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]

雌激素通过雌激素受体(ER)在雌性生殖活动中起着重要作用,雌激素受体是配体依赖的转录调节因子,属核受体超家族成员.目前认为存在2种亚型:即经典的ERα和新发现的ERβ.利用基因敲除技术研究表明,ERα和ERβ对于卵巢的发育不是必需的,但ERα可通过负反馈回路调节排卵,而ERβ通过卵巢内和卵巢外2条途径调节卵泡的发育和排卵,两者共同作用以维持雌性性征、卵泡发育和排卵等生理活动;在子宫中,ERα占主导作用,在子宫成熟和胚胎附植过程中起着重要作用,ERβ则能以反应特异性和剂量依赖性的方式调节ERα的作用.
URL [本文引用: 1]

雌激素通过雌激素受体(ER)在雌性生殖活动中起着重要作用,雌激素受体是配体依赖的转录调节因子,属核受体超家族成员.目前认为存在2种亚型:即经典的ERα和新发现的ERβ.利用基因敲除技术研究表明,ERα和ERβ对于卵巢的发育不是必需的,但ERα可通过负反馈回路调节排卵,而ERβ通过卵巢内和卵巢外2条途径调节卵泡的发育和排卵,两者共同作用以维持雌性性征、卵泡发育和排卵等生理活动;在子宫中,ERα占主导作用,在子宫成熟和胚胎附植过程中起着重要作用,ERβ则能以反应特异性和剂量依赖性的方式调节ERα的作用.
[本文引用: 1]
[本文引用: 1]
