 ,, 朱菲菲, 王轶敏, 郭宏, 张林林, 李新, 郭益文, 丁向彬
,, 朱菲菲, 王轶敏, 郭宏, 张林林, 李新, 郭益文, 丁向彬 ,天津农学院动物科学与动物医学学院/天津市农业动物繁育与健康养殖重点实验室,天津 300384
,天津农学院动物科学与动物医学学院/天津市农业动物繁育与健康养殖重点实验室,天津 300384Effects of PSMB5 on the Proliferation and Myogenic Differentiation of Skeletal Muscle Satellite Cells
LAI YuTing ,, ZHU FeiFei, WANG YiMin, GUO Hong, ZHANG LinLin, LI Xin, GUO YiWen, DING XiangBin
,, ZHU FeiFei, WANG YiMin, GUO Hong, ZHANG LinLin, LI Xin, GUO YiWen, DING XiangBin ,Tianjin Key Laboratory of Agricultural Animal Breeding and Healthy Husbandry/College of Animal Science and Veterinary Medicine, Tianjin Agricultural University, Tianjin 300384
,Tianjin Key Laboratory of Agricultural Animal Breeding and Healthy Husbandry/College of Animal Science and Veterinary Medicine, Tianjin Agricultural University, Tianjin 300384通讯作者:
责任编辑: 林鉴非
收稿日期:2019-11-13接受日期:2020-04-2网络出版日期:2020-10-16
| 基金资助: |
Received:2019-11-13Accepted:2020-04-2Online:2020-10-16
作者简介 About authors
来裕婷,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3010KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
来裕婷, 朱菲菲, 王轶敏, 郭宏, 张林林, 李新, 郭益文, 丁向彬. PSMB5蛋白对牛骨骼肌卫星细胞增殖与成肌分化的影响[J]. 中国农业科学, 2020, 53(20): 4287-4296 doi:10.3864/j.issn.0578-1752.2020.20.016
LAI YuTing, ZHU FeiFei, WANG YiMin, GUO Hong, ZHANG LinLin, LI Xin, GUO YiWen, DING XiangBin.
0 引言
【研究意义】 肌肉是动物躯体的重要组成部分,骨骼肌大约占体重的40%,骨骼肌在调节动物新陈代谢、机体运动、能量储存和健康等方面至关重要,是机体功能正常运转的必要组分。骨骼肌卫星细胞作为肌源性的干细胞,在肌肉发育分化和肌损伤修复中都发挥了重要的作用。泛素-蛋白酶体系统(Ubiquitin- roteasome system, UPS)是真核生物降解蛋白的主要途径。泛素(ubiquitin, Ub)先标记要降解的蛋白质,然后由蛋白酶体识别和降解[1]。泛素蛋白酶体介导的蛋白降解由泛素(Ub)、泛素活化酶(ubiquitin-activating enzyme, E1)、泛素结合酶(ubiquitin-conjugating enzyme, E2)、泛素-蛋白连接酶(ubiquitin-protein ligating enzyme,E3)、去泛素化酶(deubiquitinating enzymes, DUBs)、26S蛋白酶体及其底物(蛋白质)构成[2,3]。泛素-蛋白酶体对底物的降解过程主要包括泛素化、蛋白酶体降解、去泛素化3个过程。由于底物蛋白在细胞内广泛存在,泛素化参与调节细胞周期进程、细胞增殖与分化、以及信号传导等多种生物学过程[4]。在肌肉发育分化过程中,泛素-蛋白酶体系统可能也发挥了重要的调节作用,具体调控机制亟需进行深入研究。【前人研究进展】26S蛋白酶体是降解泛素化底物的一个ATP依赖型蛋白水解复合体,由20S核心蛋白酶体和19S调节颗粒构成[5,6,7]。19S调节颗粒可以识别泛素化的蛋白,20S是泛素-蛋白酶体系统的中枢蛋白水解结构[8,9]。20S是一个圆筒形结构,由α亚基和β亚基构成的4个环层叠组成,内侧的2个β环具有蛋白酶催化水解活性[10],β1、β2和β5为活性亚基,具有特异性肽切割位点,是整个泛素-蛋白酶体通路降解蛋白的关键位点[11,12]。其中,蛋白酶体β5亚基(PSMB5)具有糜蛋白酶样活性[13]。在肌肉中,泛素-蛋白酶体系统与肌原纤维蛋白的降解密切相关[14,15]。有研究表明,过表达PSMB5可增强人骨髓间充质干细胞20S蛋白酶体活性,提高细胞增殖能力[16]。也有研究表明,PSMB5过表达能够促进人骨髓间充质干细胞向神经元样细胞分化[17]。还有研究表明免疫蛋白酶体 β5i 亚基可通过升高钙调神经磷酸酶的蛋白水平和活性,促进 DOCA 盐诱导的心肌肥厚[18]。沉默PSMB5基因可降低神经干细胞蛋白酶体活性抑制新生小鼠神经干细胞的增殖分化能力[19]。也有研究表明在心脏肥大的动物模型中,心脏蛋白酶体亚基的蛋白表达水平与心肌肥厚呈正相关,可能是由于蛋白酶体促进了心脏肥大信号通路中相关蛋白的表达[20]。【本研究切入点】上述研究提示蛋白酶体亚基PSMB5可能在细胞分化和肌肉发育过程中发挥了重要的调控作用,但PSMB5对牛肌肉发育分化是否具有调控作用还不清楚。【拟解决的关键问题】本研究利用牛骨骼肌卫星细胞体外诱导成肌分化模型,模拟牛骨骼肌生长发育过程,通过改变牛骨骼肌卫星细胞中PSMB5基因的表达水平,研究PSMB5蛋白对牛骨骼肌卫星细胞体外增殖和成肌分化的影响。1 材料与方法
1.1 细胞
牛骨骼肌卫星细胞由天津市农业动物繁育与健康养殖重点实验室分离冻存。1.2 试验进行时间及地点
试验于2019年1—9月在天津市农业动物繁育与健康养殖重点实验室完成。1.3 主要仪器
二氧化碳培养箱(SANYO);荧光倒置显微镜(Leica);通用酶标仪(Biotek)电泳仪、转膜仪、高灵敏化学发光成像分析系统(Bio-Rad)等。1.4 主要试剂
DMEM;FBS(Fetal Bovine Serum),HS(Horse Serum),Opti-MEM,0.25%胰蛋白酶购自美国Gibco公司;si-RNA和EdU细胞增殖检测试剂盒购自广州锐博公司;RIPA裂解液,电泳液,电转液,TBS和发光液购自北京索莱宝公司;Protease inhibitor cocktail购自美国SIGMA公司; BCA试剂盒购自北京康为公司;LipofectamineTM 3000 Reagent和proteasome 20S Antibody抗体购自赛默飞公司;MyHC一抗购自DSHB公司;GAPDH一抗,羊抗鼠二抗和羊抗兔二抗购自北京中杉金桥公司;pcDNA3.1购自武汉淼灵生物科技有限公司;RNA快速提取试剂盒购自艾德莱生物科技有限公司;反转录试剂盒购自Takara biotechnology Corporation;All-in-OneTM qPCR Mix,购自GeneCopoeia。2 方法
2.1 牛骨骼肌卫星细胞的复苏、传代及诱导分化
牛骨骼肌卫星细胞体外诱导成肌分化模型的建立参考天津市农业动物繁育与健康养殖重点实验室已建立的方法进行[21]。细胞在37 ℃水浴中复苏,用1 mL完全培养基(含20% FBS的DMEM)中和,室温,1 000 r/min离心10 min,移除上清,加入适量的完全培养基重悬,接种于60 mm的培养皿中。待细胞融合度达到80%时传代,用37 ℃预热的PBS清洗细胞两次,加入1 mL 0.25%胰酶室温消化2 min,加入1 mL完全培养基终止消化,室温1 000 r/min 离心5 min,移除上清,加入适量的完全培养基重悬,接种于细胞培养板中。待细胞汇合度达到80%,加入分化培养基(含2% HS的DMEM)进行体外诱导分化。2.2 试验设计及处理
牛骨骼肌卫星细胞分化前后PSMB5表达量的检测:将牛骨骼肌卫星细胞传代后接种于24孔板,在完全培养基中培养24 h,诱导分化培养72 h,利用RNA快速提取试剂盒分别提取增殖期和分化期牛骨骼肌卫星细胞总RNA,qRT-PCR检测牛骨骼肌卫星细胞分化前后PSMB5的mRNA表达差异,Western blotting 法检测PSMB5在牛骨骼肌卫星细胞分化前后蛋白表达水平的差异。PSMB5的干扰及过表达效果检测:设计合成PSMB5小干扰RNA si-RNA-PSMB5 (si-PSMB5,序列为:AGAGGAGCCAAGAATTGAA);构建PMSB5的表达载体pcDNA3.1-PSMB5(pcDNA-PSMB5)。将牛骨骼肌卫星细胞在24孔培养板内进行增殖培养,当细胞融合度达80%时,按生产商家说明书利用Lipofectamine 3000转染已构建的si-PSMB5及过表达载体pcDNA-PSMB5,以si-NC及空质粒pCDNA3.1(pcDNA-NC)转染细胞作为对照。qRT-PCR及Western blotting法检测转染处理后48 h的过表达和干扰效果。
PSMB5对牛骨骼肌卫星细胞增殖的影响:转染后24 h,按Cell-Light EdU Apollo In Vitro Imaging Kit试剂盒说明书检测牛骨骼肌卫星细胞增殖期细胞数。
PSMB5对牛骨骼肌卫星细胞成肌分化的影响:光学显微镜观察干扰和过表达PSMB5后诱导分化72h牛骨骼肌卫星细胞肌管形成状态,Western blotting检测分化标记因子MyHC的蛋白水平表达。
2.3 qRT-PCR检测PSMB5的基因表达水平
取5 μL总RNA利用All-in-OneTM First-Strand cDNA Synthesis Kit试剂盒将RNA反转录为cDNA,再采用qRT-PCR检测PSMB5基因的表达水平。qRT-PCR反应体系:10 μmol·L-1上游引物、10 μmol·L-1下游引物、2.0 μL 5倍稀释cDNA、10 μL 2×All-in-OneTM qPCR Mix,RNase-free water 将体系补至20 μL。qRT-PCR反应引物信息如表1所示。
Table 1
表1
表1qRT-PCR引物信息
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence | 片段长度 Fragment length |
|---|---|---|
| PSMB5-F | 5′-TCAAGATGTCAGCCACACCC -3′ | 112 |
| PSMB5-R | 5′-ACCACGCCTCCATACACAAG -3′ | |
| MyHC-F | 5′- CTGGAATCCGGAGGCAGAA-3′ | 105 |
| MyHC-R | 5′- TTTTCGAAGGTAGGGAGCGG-3′ | |
| GAPDH-F | 5′- TGTTGTGGATCTGACCTGCC-3′ | 135 |
| GAPDH-R | 5′- AAGTCGCAGGAGACAACCTG-3′ |
新窗口打开|下载CSV
2.4 Western blotting检测PSMB5和牛骨骼肌卫星细胞分化标记基因蛋白表达水平
用蛋白裂解液裂解6孔板中增殖期细胞或分化期肌管细胞,收集蛋白,用BCA法测定蛋白浓度,再通过Western blotting检测PSMB5和分化标志基因MyHC的蛋白的表达量,具体步骤参考本实验室已建立的方法进行[22]。2.5 EdU法检测牛骨骼肌卫星细胞增殖状态
将牛骨骼肌卫星细胞接种在96孔板中,转染后24 h,按 Cell-Light EdU Apollo In Vitro Imaging Kit试剂盒说明书检测各处理细胞的增殖期细胞数。每组试验均设置3个生物学重复,每个生物学重复至少采集3个技术重复或视野。EdU增殖效率=EdU阳性细胞数/Hoechst 33342标记细胞数,分别记数3个200×视野的细胞总数及EdU阳性细胞数。2.6 统计分析
每组试验均设置3个生物学重复,所有数据均以“平均数±标准差”表示。EdU结果采用χ2检验进行分析;qRT-PCR结果按2-ΔΔCt法计算,采用t检验进行差异显著性分析,以GAPDH基因作为内参基因对检测的目的基因进行归一化。“*”表示差异显著(P<0.05),“**”表示差异极显著(P<0.01)。3 结果
3.1 牛骨骼肌卫星细胞分化前后PSMB5表达量的检测
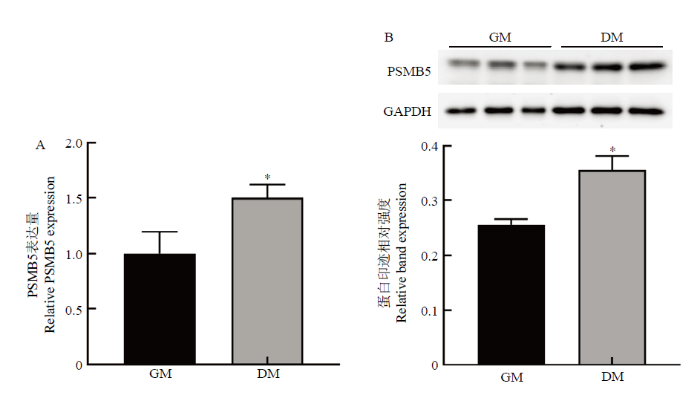
将牛骨骼肌卫星细胞传代后接种于24孔板,在增殖培养基中培养24 h,诱导分化培养72 h,qRT-PCR和Western blotting检测牛骨骼肌卫星细胞分化前后PSMB5的表达差异,(图1)。可知,PSMB5在牛骨骼肌卫星细胞分化前后表达水平存在显著差异,诱导分化后牛骨骼肌卫星细胞中PSMB5的mRNA和蛋白表达量均显著高于增殖期(P<0.05)。结果提示PSMB5可能对牛骨骼肌卫星细胞的成肌分化有一定的调控作用。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1牛骨骼肌卫星细胞分化前后PSMB5表达量的检测
GM表示牛骨骼肌卫星细胞增殖期;DM表示牛骨骼肌卫星细胞诱导分化72 h
Fig. 1Expression of PSMB5 in proliferation and differentiation of bovine skeletal muscle satellite cells
Comment: GM indicates the period of proliferation of bovine skeletal muscle satellite cells; DM indicates the differentiation of bovine skeletal muscle satellite cells for 72 hours
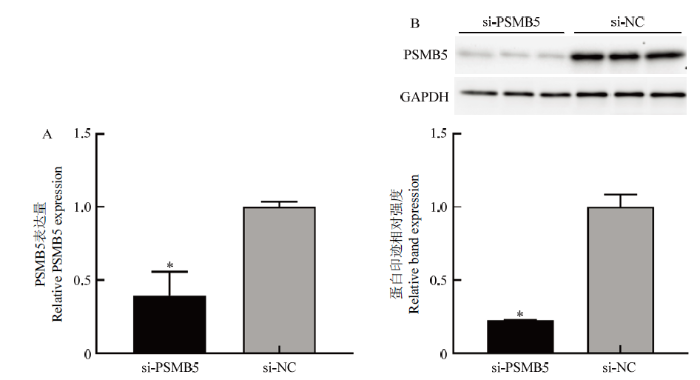
3.2 PSMB5干扰及过表达效果检测
当24孔培养板内增殖期细胞融合度达80%时,转染小干扰RNA si-PSMB5和PSMB5过表达质粒pcDNA-PSMB5,转染处理后48 h后,qRT-PCR及Western blotting检测细胞中PSMB5的表达水平变化。结果如图2和图3所示,转染si-PSMB5后,PMSB5的mRNA表达水平(图2-A)及蛋白表达水平(图2-B)显著低于对照组(P<0.05);转染pcDNA-PSMB5过表达质粒后,PSMB5的mRNA水平(图3-A)及蛋白表达水平(图3-B)显著高于对照组(P<0.01)。结果表明小干扰RNA以及构建的PSMB5过表达质粒分别对牛骨骼肌卫星细胞具有明确的干扰和过表达效果。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2siRNA的干扰效果检测
Fig. 2Detection of interference effects of si-PSMB5
图3
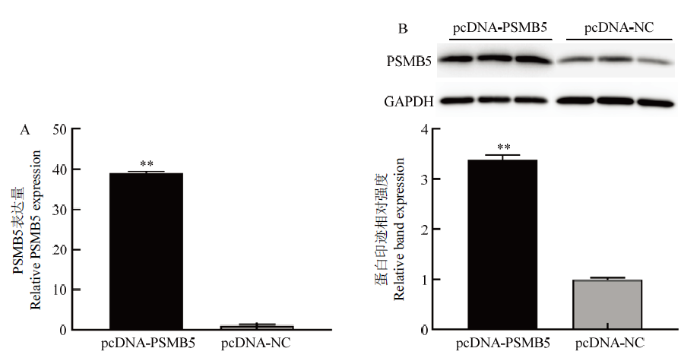 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3PSMB5质粒载体的过表达效果检测
Fig. 3Detection of pcDNA-PSMB5 overexpression effect
3.3 PSMB5对牛骨骼肌卫星细胞增殖的影响
将96孔板中细胞转染si-PSMB5和pcDNA- SMB5,转染处理24 h后,采用EdU法检测牛骨骼肌卫星细胞的增殖状态。结果如图4所示,干扰或过表达PSMB5后,牛骨骼肌卫星细胞EdU阳性细胞率与对照组相比差异均不显著(P>0.05)。结果表明PSMB5对牛骨骼肌卫星细胞的增殖没有明显的影响。图4
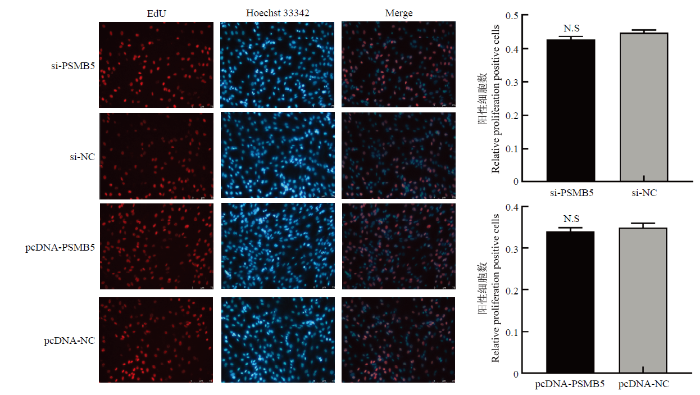 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4EdU法检测干扰及过表达PSMB5对牛骨骼肌卫星细胞增殖的影响
Fig. 4EdU detects proliferating cells of bovine skeletal muscle satellite cells after knockdown and overexpression of PSMB5
3.4 PSMB5对牛骨骼肌卫星细胞成肌分化的影响
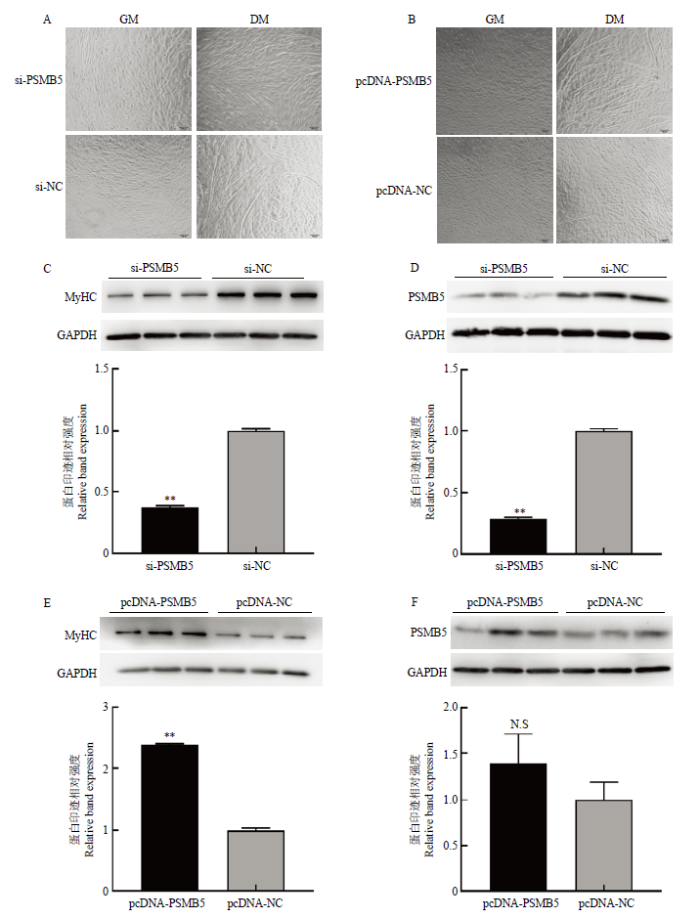
干扰或过表达PSMB5后,将牛骨骼肌卫星细胞诱导分化72 h,然后采用光学显微镜观察牛骨骼肌卫星细胞肌管形成状态,Western blotting 检测分化72 h后PSMB5以及分化标记因子MyHC的蛋白水平表达。如图5所示,干扰PSMB5后,从细胞形态图来看,试验组形成的肌管数量明显少于对照组(图5-A);Western blotting检测结果显示,干扰PSMB5表达后,分化标志因子MyHC的蛋白水平显著降低(P<0.01)(图5-C);诱导分化72 h时,PSMB5蛋白水平仍显著低于对照组(P<0.01)(图5-D)。过表达PSMB5后,试验组中形成的肌管数量明显多于对照组(图5-B);Western blotting检测结果显示,过表达PSMB5后,分化标志因子MyHC的蛋白表达水平显著高于对照组(P<0.01)(图5-E);诱导分化72 h,试验组PSMB5的蛋白表达水平仍高于对照组但差异不显著(P>0.05)(图5-F)。研究结果表明干扰PSMB5的表达会抑制牛骨骼肌卫星细胞体外成肌分化进程,而过表达PSMB5可以促进牛骨骼肌卫星细胞体外成肌分化进程,PSMB5对牛骨骼肌卫星细胞的成肌分化过程具有显著的调控作用。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5干扰和过表达PSMB5对牛骨骼肌卫星细胞分化的影响
(A-B)转染siRNA-PSMB5和pcDNA-PSMB5及对照组牛骨骼肌卫星细胞诱导成肌分化的效果(100×);(C-D)Western blotting检测干扰PSMB5对分化标志因子MyHC及PSMB5蛋白表达水平的影响 (E-F) Western blotting检测过表达PSMB5对分化标志因子MyHC及PSMB5的蛋白表达水平的影响
Fig. 5Effects of interference and overexpression of PSMB5 on the differentiation of bovine skeletal muscle satellite cells
Comment: (A-B)Effect of transfection of siRNA-PSMB5 and pcDNA-PSMB5 and control group bovine skeletal muscle satellite cells on myogenic differentiation (100×)(C-D)Western blotting to detect the effect of interfering with PSMB5 on the expression levels of PSMB5 and differentiation markers MyHC(E-F)Western blotting to detect the effect of over-expressing PSMB5 on the protein expression levels of PSMB5 and differentiation markers MyHC
4 讨论
骨骼肌发育过程极其复杂,主要包括体节细胞增殖分化、成肌细胞增殖分化、肌管成熟以及肌纤维形成等环节。骨骼肌卫星细胞是骨骼肌中具有分化增殖潜能的肌源性干细胞,通常以静息状态存在于肌纤维肌膜与基底膜之间,在一定的条件下可以被激活,发生增殖和分化。卫星细胞在动物出生后肌肉的生长发育和再生过程中发挥着十分重要的作用[23,24]。肌卫星细胞的体外成肌分化过程很好地模拟了体内的发育过程,成为研究细胞分化和肌肉发育发生机制的良好细胞模型。泛素-蛋白酶体途径在细胞内普遍存在,从细胞膜、细胞质到细胞核等处的蛋白质都受到泛素化酶和蛋白酶体的监控[25]。有研究表明,对于处于活跃增殖期的细胞如血管平滑肌,泛素-蛋白酶体主要通过NF-κB抑制细胞凋亡,引起血管平滑肌细胞发生增殖性病变;对于已经分化并且处于非分裂期的细胞,如在不稳定的动脉粥样硬化斑块中的细胞,泛素-蛋白酶体抑制细胞凋亡的作用明显减弱[26]。此外,蛋白酶体可以降解凋亡相关蛋白,抑制蛋白酶体活性,可以促使p53、凋亡蛋白Bax和蛋白激酶C(PKC)等集聚,进而促使心肌细胞凋亡[27]。在小鼠骨骼肌卫星细胞的再生过程中,蛋白酶体的活性上升[28]。另外,泛素-蛋白酶体系统在肌肉萎缩和神经损伤变性过程中都发挥了重要作用。应用蛋白酶体抑制剂MG-132能抑制离体神经肌细胞蛋白降解,延缓离体神经轴突变性的发生[29]。综合以上研究发现,泛素-蛋白酶体系统在细胞增殖、分化中起着非常重要的作用。PSMB5为蛋白酶体中具有水解活性的亚基,在泛素-蛋白酶体中发挥着重要作用,它可能在细胞分化和肌肉发育过程中发挥了重要的调控作用。目前,PSMB5在牛骨骼肌卫星细胞增殖分化中的表达模式和调控作用还不清楚,因此,笔者检测了牛肌卫星细胞增殖分化过程中PSMB5的mRNA和蛋白表达水平的变化,检测结果发现PSMB5在牛骨骼肌卫星细胞分化前后表达水平存在显著差异,PSMB5在牛骨骼肌卫星细胞分化期的表达水平较高,推测其对牛骨骼肌卫星细胞的增殖和分化过程可能具有一定的影响。有研究报道,选择性敲除小鼠肌卫星细胞特异性19S蛋白酶体调节亚基(Rpt3)基因,会阻碍小鼠体内肌再生过程,同时还会抑制肌再生过程中肌卫星细胞的增殖与分化[30]。PSMB5基因沉默可降低神经干细胞蛋白酶体活性抑制小鼠神经干细胞的增殖分化能力[19]。PSMB5过表达能够促进人骨髓间充质干细胞向神经元样细胞分化[17]。在本研究中,通过模拟体内肌卫星细胞增殖分化过程,成功建立体外牛肌卫星细胞增殖分化模型,设计si-RNA抑制PSMB5的表达,干扰PSMB5后发现,细胞分化能力减弱,肌管数量明显减少,同时牛骨骼肌卫星细胞分化标志基因MyHC的蛋白表达量下调,试验结果表明,干扰PSMB5表达会抑制牛肌卫星细胞的分化;构建PSMB5过表达载体,转染细胞后发现,过表达PSMB5的牛骨骼肌卫星细胞分化能力增强,肌管数量增多,且MyHC蛋白表达量上调,说明过表达PSMB5后能够促进牛骨骼肌卫星细胞的成肌分化。本研究结果表明,PSMB5对牛骨骼肌卫星细胞的成肌分化过程具有显著的调控作用,与骨髓间充质干细胞和小鼠神经干细胞上研究类似,PSMB5在牛骨骼肌卫星细胞上表现出了对细胞分化的调控作用,但PSMB5在牛骨骼肌卫星细胞上没有表现出在骨髓间充质干细胞和神经干细胞上类似的对增殖的调控作用,可能是其在不同细胞类型中发挥作用的途径不一致,具体的调控作用机制还有待进一步研究。
5 结论
本研究发现PSMB5蛋白对牛骨骼肌卫星细胞的成肌分化过程具有显著的调控作用。干扰PSMB5表达可以抑制牛骨骼肌卫星细胞的成肌分化进程,而过表达PSMB5可以促进牛骨骼肌卫星细胞体外成肌分化进程。研究探明了PSMB5蛋白对牛骨骼肌卫星细胞增殖和成肌分化的具体调控作用,为进一步开展PSMB5在牛成肌分化中的调控机制研究奠定了基础。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1002/jcb.20130URLPMID:15352157 [本文引用: 1]

Ubiquitination is an increasingly common post-translation modification that controls both the expression and activity of numerous proteins in the eukaryotic cell. One frequent target of the ubiquitin (Ub) modification machinery is transcription factors. Although ubiquitination generally modulates their function by inducing proteasome-dependent degradation, past and recent studies indicate that ubiquitination also regulates nuclear-cytoplasmic trafficking of transcriptional regulators. Ubiquitination is known to modulate transcription factor localization by destroying sequestering proteins and by directly promoting nuclear import and export of modified substrates. This review discusses old and new paradigms relating Ub modification and the control of transcription factor shuttling in and out of the nucleus.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:25599644 [本文引用: 1]
[本文引用: 1]
.
[本文引用: 1]
DOI:10.1093/cvr/cvp225URLPMID:19578074 [本文引用: 1]

The primary intracellular protein degradation systems, including the ubiquitin-proteasome and the lysosome pathways, have been emerging as central regulators of viral infectivity, inflammation, and viral pathogenicity. Viral myocarditis is an inflammatory disease of the myocardium caused by virus infection in the heart. The disease progression of viral myocarditis occurs in three distinct stages: acute viral infection, immune cell infiltration, and cardiac remodelling. Growing evidence suggests a crucial role for host proteolytic machineries in the regulation of the pathogenesis and progression of viral myocarditis in all three stages. Cardiotropic viruses evolve different strategies to subvert host protein degradation systems to achieve successful viral replication. In addition, these proteolytic systems play important roles in the activation of innate and adaptive immune responses during viral infection. Recent evidence also suggests a key role for the ubiquitin-proteasome and lysosome systems as the primary effectors of protein quality control in the regulation of cardiac remodelling. This review summarizes the recent advances in understanding the direct interaction between cardiotropic viruses and host proteolytic systems, with an emphasis on coxsackievirus B3, one of the primary aetiological agents causing viral myocarditis, and highlights possible roles of the host degradation systems in the pathogenesis of viral myocarditis and its progression to dilated cardiomyopathy.
DOI:10.1038/386463a0URLPMID:9087403 [本文引用: 1]

The crystal structure of the 20S proteasome from the yeast Saccharomyces cerevisiae shows that its 28 protein subunits are arranged as an (alpha1...alpha7, beta1...beta7)2 complex in four stacked rings and occupy unique locations. The interior of the particle, which harbours the active sites, is only accessible by some very narrow side entrances. The beta-type subunits are synthesized as proproteins before being proteolytically processed for assembly into the particle. The proforms of three of the seven different beta-type subunits, beta1/PRE3, beta2/PUP1 and beta5/PRE2, are cleaved between the threonine at position 1 and the last glycine of the pro-sequence, with release of the active-site residue Thr 1. These three beta-type subunits have inhibitor-binding sites, indicating that PRE2 has a chymotrypsin-like and a trypsin-like activity and that PRE3 has peptidylglutamyl peptide hydrolytic specificity. Other beta-type subunits are processed to an intermediate form, indicating that an additional nonspecific endopeptidase activity may exist which is important for peptide hydrolysis and for the generation of ligands for class I molecules of the major histocompatibility complex.
DOI:10.1006/geno.2000.6231URLPMID:10945464 [本文引用: 1]

PSMC3 and PSMC4, components of the 19S complex of the 26S proteasome, show a significant degree of amino acid similarity, especially in the conserved ATPase domain (CAD). In this study, we characterized the mouse Psmc3 and Psmc4 genes. The genomic structures of both genes showed a significant degree of similarity. The Psmc3 gene was composed of 12 coding exons, whereas the Psmc4 gene had 11 exons. Exons encoding the leucine zipper domain and CAD were identical in number between the Psmc3 and Psmc4 genes. The Psmc3 gene mapped to mouse chromosome 2, whereas Psmc4 mapped to chromosome 7. We further addressed the biological roles of Psmc3 and Psmc4 through the generation of gene targeted mice. Both Psmc3- and Psmc4-deficient mice died before implantation, displaying defective blastocyst development. These findings indicate that Psmc3 and Psmc4 have similar and essential roles in early embryogenesis and further that both ATPases have noncompensatory functions in vivo.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1006/bbrc.2001.5849URLPMID:11779124 [本文引用: 1]

Muscle cachexia induced by sepsis, severe injury, cancer, and a number of other catabolic conditions is mainly caused by increased protein degradation, in particular breakdown of myofibrillar proteins. Ubiquitin-proteasome-dependent proteolysis is the predominant mechanism of muscle protein loss in these conditions, but there is evidence that several other regulatory mechanisms may be important as well. Some of those mechanisms are reviewed in this article and they include pre-, para-, and postproteasomal mechanisms. Among preproteasomal mechanisms, mediators, receptor binding, signaling pathways, activation of transcription factors, and modification of proteins are important. Several paraproteasomal mechanisms may influence the trafficking of ubiquitinated proteins and their interaction with the proteasome, including the expression and activity of the COP9 signalosome, the carboxy terminus of heat shock protein 70-interacting protein (CHIP) and valosin-containing protein (VCP). Finally, because the proteasome does not degrade proteins completely into free amino acids but into peptides, postproteasomal degradation of peptides by the giant protease tripeptidyl peptidase II (TPP II) and various aminopeptidases is important in muscle catabolism. Thus, multiple mechanisms and regulatory steps may influence the breakdown of ubiquitinated muscle proteins by the 26S proteasome.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1016/j.yjmcc.2014.12.007URLPMID:25526681 [本文引用: 1]

Proteasomal degradation is critical to maintaining cardiac function and is altered in various diseases. Angiotensin II (Ang II) acts as a growth factor to induce cardiac growth. Here we aimed to test whether proteasome is involved in the development of Ang II-induced cardiac hypertrophy and dissect its molecular mechanisms. Cardiac hypertrophy was induced by Ang II infusion (1000 ng/kg/min) using mini-osmotic pumps. Starting 1 day before implantation, the mice were injected with the proteasome inhibitor bortezomib (BTZ, 50 mug/kg, 3 times per week) or with vehicle. After 14 days, the pool of ubiquitinated proteins was reduced but the protein expression of proteasome subunits (including beta1i, beta2i and beta5/beta5i) was markedly up-regulated in left ventricular hypertrophy versus control, which was accompanied by a significant increase in proteasome activities. Furthermore, Ang II-treated mice showed a significant increase in blood pressure but decrease in cardiac contractile function, and significant left ventricular hypertrophy, fibrosis and inflammation, which were all attenuated in BTZ-treated mice. Mechanistically, these beneficial effects were associated with the inhibition of degradation of angiotensin II type 1 receptor-associated protein (ATRAP) and inactivation of AT1R-mediated p38 MAPK and STAT3 signaling pathways. In conclusion, our data indicate that the activation of proteasome is required for the Ang II-induced cardiac hypertrophy, and suggest that the inhibition of proteasome activity by BTZ could be a potential therapeutic strategy for the treatment of cardiac hypertrophy and other heart diseases.
URL [本文引用: 1]

本研究对牛骨骼肌卫星细胞进行体外分离培养、诱导分化和鉴定,采用胶原酶和胰酶联用的酶消化法分离肌卫星细胞,应用差速贴壁法进行纯化,观察卫星细胞及诱导分化后肌管的形态结构,并利用标志基因的反转录PCR(RT-PCR)和免疫荧光染色方法对分化前后细胞进行鉴定。结果显示,分离出的肌卫星细胞呈梭形生长,生长状态良好,RT-PCR和免疫荧光染色显示肌卫星细胞Pax7和MyoD呈阳性表达,纯化后的肌卫星细胞纯度大于93%;诱导分化后,卫星细胞融合生长,形成的肌管状态良好,分化标志基因MyoG和MHC呈阳性表达。本研究建立了一套从牛肌肉组织中分离和鉴定肌卫星细胞的方法,可以为肌肉的发育分化和肉牛肉质改良研究提供良好的细胞模型。
URL [本文引用: 1]

本研究对牛骨骼肌卫星细胞进行体外分离培养、诱导分化和鉴定,采用胶原酶和胰酶联用的酶消化法分离肌卫星细胞,应用差速贴壁法进行纯化,观察卫星细胞及诱导分化后肌管的形态结构,并利用标志基因的反转录PCR(RT-PCR)和免疫荧光染色方法对分化前后细胞进行鉴定。结果显示,分离出的肌卫星细胞呈梭形生长,生长状态良好,RT-PCR和免疫荧光染色显示肌卫星细胞Pax7和MyoD呈阳性表达,纯化后的肌卫星细胞纯度大于93%;诱导分化后,卫星细胞融合生长,形成的肌管状态良好,分化标志基因MyoG和MHC呈阳性表达。本研究建立了一套从牛肌肉组织中分离和鉴定肌卫星细胞的方法,可以为肌肉的发育分化和肉牛肉质改良研究提供良好的细胞模型。
DOI:10.1007/s11626-015-9953-4URLPMID:26424132 [本文引用: 1]

MicroRNAs (miRNAs) have been found to play essential roles in muscle cell proliferation and differentiation. MicroRNA-1 (miR-1) and microRNA-206 (miR-206), which are similar and have the same seed sequence, have specific roles in modulating skeletal muscle proliferation and differentiation in vitro and in vivo. However, there is no information about their function during bovine skeletal muscle satellite cell development. In this study, the profiles of miR-1 and miR-206 and their biological functions in bovine skeletal muscle cell development was investigated. The target genes were predicted, and we used a dual-luciferase reporter assay to demonstrate that miR-1 and miR-206 directly targeted the 3' untranslated region (3'UTR) of paired-box transcription factor Pax7 and histone deacetylase 4 (HDAC4). We showed that miR-1 and miR-206 facilitate bovine skeletal muscle satellite cell myogenic differentiation by restricting the expression of their target gene and that inhibition of miR-1 and miR-206 increased the Pax7 and HDAC4 protein levels and substantially enhanced satellite cell proliferation. Therefore, our results revealed the mechanism in which miR-1 and miR-206 positively regulate bovine skeletal muscle satellite cell myogenic differentiation via Pax7 and HDAC4 downregulation.
DOI:10.1016/j.cell.2005.05.010URLPMID:16051152 [本文引用: 1]

Satellite cells are situated beneath the basal lamina that surrounds each myofiber and function as myogenic precursors for muscle growth and repair. The source of satellite cell renewal is controversial and has been suggested to be a separate circulating or interstitial stem cell population. Here, we transplant single intact myofibers into radiation-ablated muscles and demonstrate that satellite cells are self-sufficient as a source of regeneration. As few as seven satellite cells associated with one transplanted myofiber can generate over 100 new myofibers containing thousands of myonuclei. Moreover, the transplanted satellite cells vigorously self-renew, expanding in number and repopulating the host muscle with new satellite cells. Following experimental injury, these cells proliferate extensively and regenerate large compact clusters of myofibers. Thus, within a normally stable tissue, the satellite cell exhibits archetypal stem cell properties and is competent to form the basal origin of adult muscle regeneration.
DOI:10.1126/science.1114758URLPMID:16141372 [本文引用: 1]

Muscle satellite cells contribute to muscle regeneration. We have used a Pax3(GFP/+) mouse line to directly isolate (Pax3)(green fluorescent protein)-expressing muscle satellite cells, by flow cytometry from adult skeletal muscles, as a homogeneous population of small, nongranular, Pax7+, CD34+, CD45-, Sca1- cells. The flow cytometry parameters thus established enabled us to isolate satellite cells from wild-type muscles. Such cells, grafted into muscles of mdx nu/nu mice, contributed both to fiber repair and to the muscle satellite cell compartment. Expansion of these cells in culture before engraftment reduced their regenerative capacity.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.2174/156652410791065426URLPMID:20205676 [本文引用: 1]

Cardiovascular disease is the leading cause of death in the western world. The major contributor of all cardiovascular deaths is myocardial infarction, which often progresses into end-stage heart failure. The loss of cardiomyocytes is a key problem in the development of cardiovascular disease. Two main processes mediate cardiomyocyte loss: necrosis and apoptosis. In contrast to necrosis, apoptosis is a well regulated process essential in normal development and tissue homeostasis. Tight regulation of this process is crucial, especially in post mitotic cells lacking regenerative capacity, like cardiomyocytes. The ubiquitin-proteasome system, accounting for 80 to 90% of intracellular protein degradation, appears to be involved in the regulation of apoptosis. In this process, regulation is performed through the degradation of pro- and anti-apoptotic proteins involved in cell cycle control and specific apoptotic pathways. On the one hand, disturbances in this normally well regulated process are associated with a number of cardiovascular diseases. On the other hand, proteasomal dysfunction may result from ischemia, hypertrophy and heart failure, and a number of cardiomyopathies. This paper reviews the current knowledge on the role of the ubiquitin-proteasome system-mediated regulation of cardiomyocyte apoptosis in cardiovascular disease. Finally, within the ubiquitin-proteasome system new molecular targets for treatment of cardiovascular disease are suggested.
DOI:10.1016/j.stemcr.2018.10.009URLPMID:30416048 [本文引用: 1]

Adult muscle stem cells (satellite cells) are required for adult skeletal muscle regeneration. A proper balance between quiescence, proliferation, and differentiation is essential for the maintenance of the satellite cell pool and their regenerative function. Although the ubiquitin-proteasome is required for most protein degradation in mammalian cells, how its dysfunction affects tissue stem cells remains unclear. Here, we investigated the function of the proteasome in satellite cells using mice lacking the crucial proteasomal component, Rpt3. Ablation of Rpt3 in satellite cells decreased proteasome activity. Proteasome dysfunction in Rpt3-deficient satellite cells impaired their ability to proliferate, survive and differentiate, resulting in defective muscle regeneration. We found that inactivation of proteasomal activity induced proliferation defects and apoptosis in satellite cells. Mechanistically, insufficient proteasomal activity upregulated the p53 pathway, which caused cell-cycle arrest. Our findings delineate a critical function of the proteasome system in maintaining satellite cells in adult muscle.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1242/jcs.150961URL [本文引用: 1]

The ubiquitin-proteasome and autophagy-lysosome pathways are the two major routes of protein and organelle clearance. The role of the proteasome pathway in mammalian muscle has not been examined in vivo. In this study, we report that the muscle-specific deletion of a crucial proteasomal gene, Rpt3 (also known as Psmc4), resulted in profound muscle growth defects and a decrease in force production in mice. Specifically, developing muscles in conditional Rpt3-knockout animals showed dysregulated proteasomal activity. The autophagy pathway was upregulated, but the process of autophagosome formation was impaired. A microscopic analysis revealed the accumulation of basophilic inclusions and disorganization of the sarcomeres in young adult mice. Our results suggest that appropriate proteasomal activity is important for muscle growth and for maintaining myofiber integrity in collaboration with autophagy pathways. The deletion of a component of the proteasome complex contributed to myofiber degeneration and weakness in muscle disorders that are characterized by the accumulation of abnormal inclusions.
