 ,1, 李鹏飞2, 许勤智1, 安清明1, 孟金柱
,1, 李鹏飞2, 许勤智1, 安清明1, 孟金柱 ,1
,1Screening and Analysis of Follicular Development Related Genes in Goat
ZHAO YuanYuan ,1, LI PengFei2, XU QinZhi1, AN QingMing1, MENG JinZhu
,1, LI PengFei2, XU QinZhi1, AN QingMing1, MENG JinZhu ,1
,1通讯作者:
责任编辑: 林鉴非
收稿日期:2019-06-24接受日期:2020-07-10网络出版日期:2020-09-01
| 基金资助: |
Received:2019-06-24Accepted:2020-07-10Online:2020-09-01
作者简介 About authors
赵园园,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1506KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
赵园园, 李鹏飞, 许勤智, 安清明, 孟金柱. 山羊卵泡发育相关基因的筛选及分析[J]. 中国农业科学, 2020, 53(17): 3597-3605 doi:10.3864/j.issn.0578-1752.2020.17.016
ZHAO YuanYuan, LI PengFei, XU QinZhi, AN QingMing, MENG JinZhu.
0 引言
【研究意义】哺乳动物卵泡发育是一个受多种因素调控的复杂生物学过程,在这个过程中有众多激素或者调控因子直接或间接参与[1]。通过基因敲除,在小鼠的卵巢中已经发现有 1 000 多个基因共同调控着卵泡的发育[2]。然而目前对促进山羊卵泡的优势化或导致其闭锁的分子机理尚不清楚。通过对山羊第一卵泡波中DF和SF颗粒细胞进行高通量测序,筛选出影响卵泡发育的关键基因,为深入探究卵泡发育的调控机制具有重要意义。【前人研究进展】在哺乳动物的一个发情周期中,一簇卵泡经过募集、选择及优势化过程发育成为排卵卵泡[3]。在卵泡的优势化过程中,细胞色素P450侧链裂解酶(Pytochrome P450 side chain lyase, P450scc)和细胞色素P450芳构化酶(cytochrome P450 aromatase, P450arom)mRNA能够促进卵泡的发育,推测P450arom和P450scc可能促进卵泡颗粒细胞分泌雌二醇(E2),从而参与负反馈调节作用并促进卵泡的优势化[4]。胰岛素样生长因子(insulin-like growth factor,IGF)会显著促进颗粒细胞分泌E2,并加快卵泡选择与优势化过程[5],然而成纤维细胞生长因子(fibroblast growth factor, FGF)与IGF的作用却相反,它通过抑制卵泡颗粒细胞分泌E2和抑制卵泡LH受体的表达从而抑制卵泡的选择与优势化过程[6]。卵泡一旦确定了优势化地位后,优势化卵泡会通过分泌E2和INH等调控因子维持自己的优势化地位[7]。LI等[8]通过对水牛不同大小卵泡的颗粒细胞进行高通量测序,发现免疫系统可能在卵泡的成熟和排卵过程中起了重要作用。TERENINA等[9]通过对猪的正常卵泡与闭锁卵泡颗粒细胞进行转录组测序,筛选出了CAMTA2等11个基因可能在卵泡颗粒细胞的增殖过程中起了抑制作用,从而引起卵泡闭锁。【本研究切入点】山羊第一卵泡波中的DF最终可能发育成为成熟卵泡,直到排卵;而SF则会受到各种调控因子而作用而走向闭锁,其中颗粒细胞的凋亡是导致卵泡发生闭锁的关键因素[10]。然而目前对促进卵泡的优势化或导致其闭锁的分子机理尚不清楚。【拟解决的关键问题】通过对山羊第一卵泡波中DF和SF颗粒细胞进行高通量测序,并通过qRT-PCR进行验证分析,筛选出影响卵泡发育的关键基因,为深入探究其调控卵泡发育机制提供理论依据。1 材料与方法
本试验于2018年12月至2019年4月在铜仁学院完成。1.1 试验动物及样品采集
在贵州省铜仁市沿河土家族自治县华珍牧业有限公司,选取10只1岁龄健康的贵州白山羊分别注射前列腺素F2α,使其同期发情,此后每天用B超检测并记录卵泡的生长情况,发情3 d后,统一屠宰并采集第一卵泡波中DF(直径4.5—6 mm)与SF(直径3—4.5 mm),迅速置于4℃灭菌杜氏磷酸缓冲液(DPBS)中,迅速运输到铜仁学院动物学实验室。将处于DPBS中的DF和SF分别放到盛有0.9%的生理盐水的培养皿上,使用眼科剪刀剪开并用细胞刮刀刮取位于卵泡内膜上的颗粒细胞(GCs)后置于-80℃ 冰箱中保存。1.2 试验方法
1.2.1 总RNA提取、文库构建及测序 将保存在-80℃冰箱中的GCs取出后置于冰盒中解冻,加入1 mL Trizol(购自Invitrogen公司,美国)分别提取两种卵泡中GCs的总RNA,经RNeasy mini kit(购自QIAGEN公司,德国)纯化,Agilent Bioanalyzer 2100完整性检测,Qubit 2.0 Flurometer测量浓度后,交由北京诺和致源生物信息科技有限公司进行文库构建并通过Illumina Hiseq 2500平台测序。1.2.2 数据处理及分析 FastQC(http://www. bioinformatics.babraha m.ac.uk/projects/fastqc/)用于对测序产出原始数据进行质量评估,然后清除原始数据(raw reads)中带接头的、低质量的 reads,从而获得品质较高的有效读段(clean reads)。使用Trinity(http://trinityrnaseq.sourceforge.net/)对得到的clean reads进行重新组装,以便得到单一序列(singleton)及重叠群(contigs)此时得到的序列称为unigenes。使用CLC Genomics Workbench将unigenes与山羊RefSeq数据库进行比对。基于负二项分布的DESeq2软件用于差异表达mRNA的分析,之后使用goseq软件对得到的差异表达基因进行GO分析,使用kobas软件进行KEGG信号通路分析。
1.2.3 反转录及引物合成 通过EasyScript? One-Step gDNA Removal and cDNA Synthesis SuperMix(购自北京全式金生物技术有限公司)将提取到的总RNA反转录成cDNA,条件为:42℃孵育15 min,85℃加热5 s失活TransScript RT与gDNA Remover。Primer 5.0设计引物(表1),使用18S rRNA作为内参基因,引物合成委托生工生物工程(上海)股份有限公司完成。
Table 1
表1
表1荧光定量引物基因列表
Table 1
| 基因名称 Gene name | 引物(5′→3′) Primer sequences (5′→3′) | 产物大小 Size (bp) |
|---|---|---|
| PRLR | F:ACCAGTTCCAGGGCCAAAAA R:GCATCAGGTGTTGGTCCTCA | 173 |
| PTX3 | F:CTCTCTGGTCTGCAGTGTCG R:TGAAGAGCTTGTCCCACTCG | 153 |
| RGN | F:GGGTCCCTGTACTCCCTCTT R:TGCGGTTGGAGATCTTTCCC | 178 |
| DKK3 | F:GTGGCCTCTTCCCAGTACAC R:GACCAGTTTAGCAGCCCTGT | 160 |
| ALDH1A2 | F:TGGAATCCCTCAATGGTGGC R:AGCCCAGCCTGCATAATACC | 95 |
| RARRES1 | F:TAAAAGCCCCTTGAACGCAG R:ACGTAAGAGCTGCCCAGAAA | 102 |
| 18S rRNA | F:CCCACGGAATCGAGAAAGAG R:TTGACGGAAGGGCACCA | 117 |
新窗口打开|下载CSV
1.2.4 qRT-PCR分析 qRT-PCR用于验证贵州白山羊DF与SF GCs中差异表达mRNA的相对表达水平。采用3个样本重复,3个技术重复,通过TransStart? Tip Green qPCR SuperMix(购自北京全式金生物技术有限公司)对各基因进行相对定量分析,根据产品使用说明书构建20 μL PCR反应体系:2×Transstart? Tip Green qPCR super mix 10 μL,上下游引物各0.4 μL,cDNA 4 μL(100 ng),RNA-free H2O 5.2 μL。反应程序为:94℃预变性1 min;94℃ 10 s,60℃ 30 s,72℃ 10 s,45个循环。结果使用2-△△CT法来计算各基因的相对表达情况。
2 结果
2.1 RNA-seq数据分析
高通量测序得到的raw reads,经过滤带接头的、含N的及低质量的reads,最终在DF中获得43 217 934条clean reads,占95.19%;在SF中获得40 766 348条clean reads,占95.35%(图1)。将得到的clean reads比对到山羊RefSeq数据库中,共得到33 896条带有注释的转录本。设定FPKM >1, q value<0.05,在两种卵泡颗粒细胞中共获得13 644个基因,其中438个基因高表达(FPKM≥1),表2中列出了表达量排名前20的基因。图1
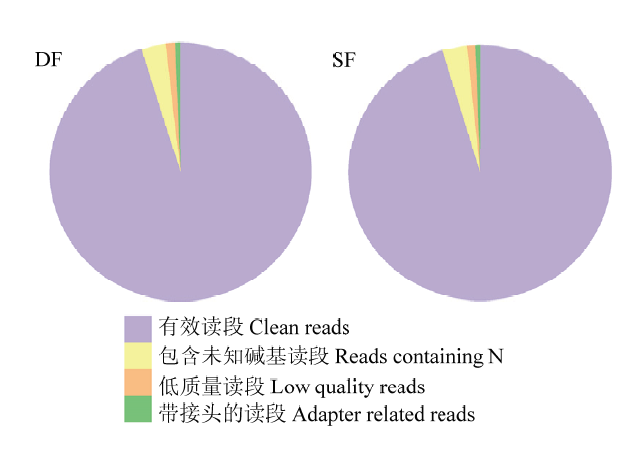 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1DF和SF颗粒细胞中原始数据的分类
Fig. 1Classification of raw reads in DF and SF granulosa cells
Table 2
表2
表2SF和DF颗粒细胞中表达量最高的20个基因
Table 2
| 基因名称Gene name | SF-FPKM | DF-FPKM |
|---|---|---|
| Eukaryotic Translation Elongation Factor 1 Alpha 1(EEF1A1) | 7 110.13 | 8 928.81 |
| Tumor Protein, Translationally-Controlled 1(TPT1) | 3 655.98 | 4 714.90 |
| TIMP Metallopeptidase Inhibitor 1(TIMP1) | 3 412.45 | 859.27 |
| Ribosomal Protein L37(RPL37) | 3 144.41 | 3 930.05 |
| Cytochrome C Oxidase Assembly Factor COX1(COX1) | 2 528.75 | 1 340.44 |
| Ribosomal Protein S14(RPS14) | 2 440.53 | 2 907.19 |
| Ribosomal Protein S18(RPS18) | 2 304.26 | 2 709.49 |
| Ribosomal Protein S11(RPS11) | 2 132.58 | 2 437.76 |
| Ribosomal Protein Lateral Stalk Subunit P0(RPLP0) | 2 129.79 | 2 196.60 |
| Ribosomal Protein L21(RPL21) | 2 054.80 | 2 740.17 |
| Ribosomal Protein S19(RPS19) | 2 054.79 | 2 494.83 |
| Ribosomal Protein L19(RPL19) | 2 045.15 | 2 609.62 |
| Ribosomal Protein S23(RPS23) | 2 005.29 | 2 171.69 |
| Oxytocin/Neurophysin I Prepropeptide (OXT) | 1 907.67 | 559.09 |
| Ribosomal Protein Lateral Stalk Subunit P2(RPLP2) | 1 869.02 | 2 217.82 |
| Ribosomal Protein S27a (RPS27A) | 1 862.11 | 2 363.39 |
| Ribosomal Protein S17(RPS17) | 1 841.97 | 2 247.31 |
| Receptor For Activated C Kinase 1(RACK1) | 1 805.27 | 1 950.70 |
| Ribosomal Protein L26(RPL26) | 1 738.19 | 2 382.36 |
| Ribosomal Protein S16(RPS16) | 1 737.56 | 2 035.36 |
新窗口打开|下载CSV
2.2 差异表达基因筛选
在差异转录本分析过程中,将DF和SF的FPKM (Fragments per kilobase of transcript per million fragments mapped)进行标准化,使用DESeq2软件对获得13 644个mRNA进行差异表达分析,设定参数:FPKM≥1,SF-FPKM/DF-FPKM>1,P<0.05,共获得695个差异表达mRNA,其中233个在从属卵泡颗粒细胞中表达显著上调,462个则表达下调(图2)。图2
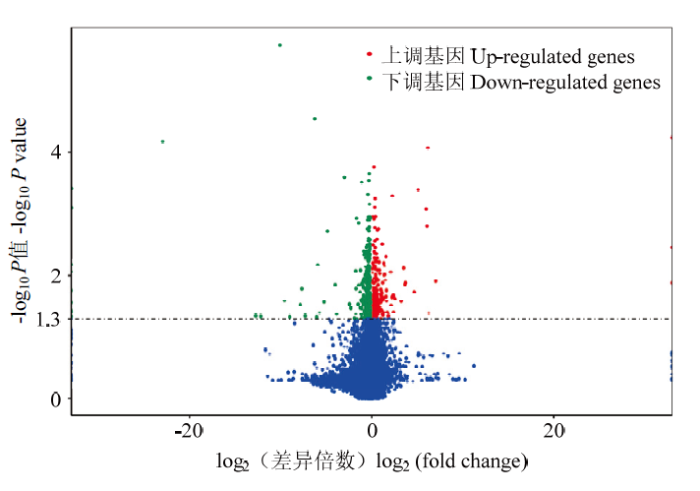 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2DF和SF颗粒细胞中差异表达基因火山图
Fig. 2Volcano plot of differentially expressed genes in DF and SF granulosa cells
2.3 GO功能富集
通过goseq软件对得到的695个差异表达基因进行GO功能富集分析, 共分为三大类42组:其中生物学过程占47.6%,细胞组分占47.6%,分子功能占4.8% (图3)。图3
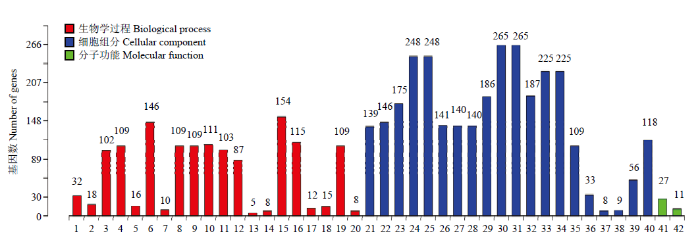 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3DF和SF颗粒细胞中差异表达基因GO分析
1:RNA加工;2:RNA拼接;3:核酸代谢过程;4:含碱基化合物的代谢过程;5:核糖核蛋白复杂生物合成;6:细胞大分子代谢过程;7:核糖体生物合成;8:杂环代谢过程;9:细胞芳香族化合物的代谢过程;10:细胞氮化合物代谢过程;11:基因表达;12:RNA代谢过程;13:核糖体小亚基生物合成;14:核糖体RNA加工;15:大分子代谢过程;16:氮化物代谢过程;17:ncRNA加工;18:ncRNA代谢过程;19:有机循环化合物代谢过程;20:核糖体RNA代谢过程;21:下拨核内腔;22:细胞核部分;23:细胞核;24:细胞内的细胞器;25:细胞器;26:细胞膜内腔;27:细胞内细胞器内腔;28:细胞器内腔;29:细胞内细胞器的部分;30:细胞内的;31:细胞内的部分;32:细胞器的部分;33:细胞内膜上细胞器;34:膜上细胞器;35:核质;36:核糖核蛋白复合体;37:胞质核糖体小亚基;38:胞质核糖体;39:核质部分;40:大分子复合体;41:RNA结合;42:mRNA结合
Fig. 3GO analysis of differentially expressed genes in DF and SF granulosa cells
1: RNA processing; 2: RNA splicing; 3: nucleic acid metabolic process; 4: nucleobase-containing compound metabolic process; 5: ribonucleoprotein complex biogenesis; 6: cellular macromolecule metabolic process; 7: ribosome biogenesis; 8: heterocycle metabolic process; 9: cellular aromatic compound metabolic process; 10: cellular nitrogen compound metabolic process; 11: gene expression; 12: RNA metabolic process; 13: ribosomal small subunit biogenesis; 14: rRNA processing; 15: macromolecule metabolic process; 16: nitrogen compound metabolic process; 17: ncRNA processing; 18: ncRNA metabolic process; 19: organic cyclic compound metabolic process; 20: rRNA metabolic process; 21: nuclear lumen; 22: nuclear part; 23: nucleus; 24: intracellular organelle; 25: organelle; 26: membrane-enclosed lumen; 27: intracellular organelle lumen; 28: organelle lumen; 29: intracellular organelle part; 30: intracellular; 31: intracellular part; 32: organelle part; 33: intracellular membrane-bounded organelle; 34: membrane-bounded organelle; 35: nucleoplasm; 36: ribonucleoprotein complex; 37: cytosolic small ribosomal subunit; 38: cytosolic ribosome; 39: nucleoplasm part; 40: macromolecular complex; 41: RNA binding; 42: mRNA binding
2.4 KEGG信号通路分析
通过kobas软件对差异表达基因进行KEGG信号通路分析,共发现20条通路(图4),其中与核糖体通路相关的基因最为显著富集,达到50个;另外,还发现5个基因参与了卵母细胞的减数分裂。图4
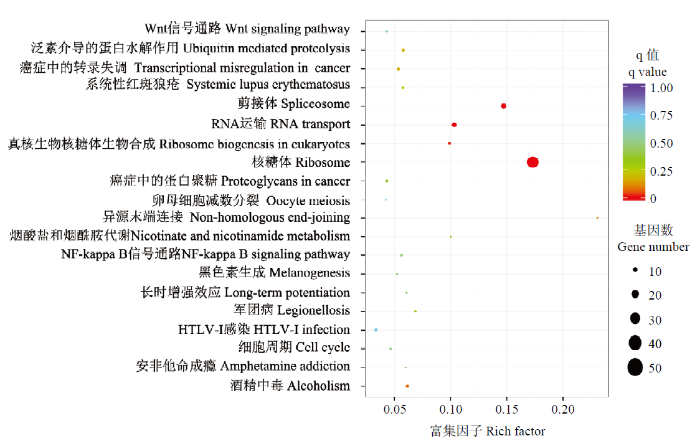 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4DF和SF颗粒细胞中差异表达基因KEGG通路富集散点图
Fig. 4Scatter plot of enriched KEGG pathway in DF and SF granulosa cells
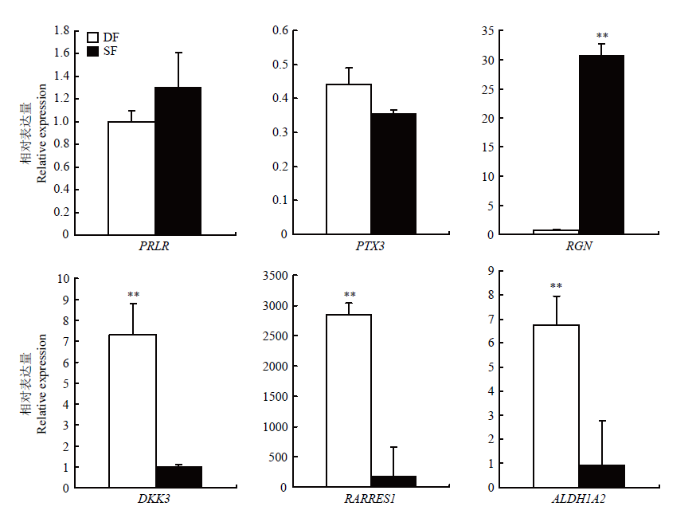
2.5 qRT-PCR验证分析
通过功能分析,我们筛选出6个可能与山羊卵泡发育密切相关的基因(表3)。其中PRLR、PTX3、RGN在SF颗粒细胞中表现为上调;DKK3、ALDH1A2、RARRES1则表现为下调。QRT-PCR结果显示PRLR、RGN、DKK3、ALDH1A2、RARRES1的表达趋势与高通量测序结果一致,且RGN在SF颗粒细胞中的表达量极显著地高于DF(P<0.01);DKK3、ALDH1A2、RARRES1在DF颗粒细胞中的表达量极显著地高于SF(P<0.01),PTX3的荧光定量结果虽然与高通量测序结果的表达趋势相反,但不存在显著差异(P>0.05)(图5)。Table 3
表3
表3可能与山羊卵泡发育相关的候选基因
Table 3
| 基因名 Gene name | SF-FPKM | DF-FPKM | log2差异倍数 log2 fold change | 基因功能 Gene function |
|---|---|---|---|---|
| Prolactin receptor (PRLR) | 37.46 | 4.11 | 3.19 | 刺激卵泡LH受体生成 Stimulate the production of LH receptors in the follicle |
| Pentraxin 3 (PTX3) | 39.92 | 6.16 | 2.70 | 影响雌性动物生殖能力 Affecting females’ reproductive ability |
| Regucalcin (RGN) | 49.39 | 8.13 | 2.60 | 调节Ca2+信号传导,以及Ca2+依赖的细胞过程和酶活性 Regulates Ca2+ signaling, and Ca2+ dependent cellular processes and enzyme activity |
| Dickkopf WNT Signaling Pathway Inhibitor 3 (DKK3) | 15.92 | 74.57 | -2.23 | 影响胚胎发育 Affect embryonic development |
| Aldehyde Dehydrogenase 1 Family Member A2 (ALDH1A2) | 3.80 | 34.23 | -3.17 | 建立局部胚胎视黄酸水平 Establishing local embryonic retinoic acid levels |
| Retinoic Acid Receptor Responder 1 (RARRES1) | 110.41 | 527.73 | -2.26 | 调节管状酪氨酸循环 Regulating tubular tyrosine cycle |
新窗口打开|下载CSV
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5候选基因在山羊DF和SF颗粒细胞中的相对表达量(**表示P<0.01)
Fig. 5Relative expression of candidate genes in DF and SF granulosa cells of goat (** indicates P<0.01)
3 讨论
雌性哺乳动物卵巢上的DF持续生长、成熟并排出卵子是依赖于颗粒细胞中FSH通过激活cAMP-蛋白激酶A通路,进而诱导芳香化酶将膜细胞分泌的雄激素转化为E2,而SF由于生长速率和E2分泌降低,从而走向闭锁[11]。HUSSEIN等[10]研究发现,颗粒细胞的凋亡是引起卵泡闭锁的关键因素;颗粒细胞受到凋亡蛋白酶激活坏死因子(TNF)、Fas等刺激后,使促凋亡蛋白的构象发生变化,从而由细胞液转移到线粒体外膜上,并与膜上及膜内的抗凋亡蛋白相互作用,最终导致其凋亡[12,13]。LI等[14]通过对牛发生偏差前的最大卵泡(PDF1)和发生偏差后的最大卵泡(ODF1)颗粒细胞进行转录组测序,获得83个差异表达基因,其中MAPK13、CPXM1和ARID4B在卵泡发育过程中可能起抑制作用。本研究通过对贵州白山羊DF和SF颗粒细胞进行高通量测序筛选出695个差异表达基因,其中233个在SF颗粒细胞中表达显著上调,462个表达下调。结合功能分析,筛选出具有代表性的6个可能与山羊卵泡发育密切相关的基因,qRT-PCR验证结果表明,PRLR、RGN、DKK3、ALDH1A2和RARRES1在DF和SF颗粒细胞中的表达趋势与高通量测序结果完全一致,PTX3在DF和SF颗粒细胞中的表达趋势与高通量测序结果虽相反,但差异不显著(P>0.05)。在哺乳动物中,促乳素受体(PRLR)对维持黄体以及孕酮的分泌起着重要的作用[15]。PRL可能通过对子宫内膜的直接作用在妊娠中发挥作用[16]。PRL能够促进排卵、着床及胎盘发育的过程[17], 敲除小鼠卵泡中的PRL,使得卵母细胞释放延迟,成熟受损进而导致排卵减少[18]。颗粒细胞中正五聚蛋白3 (PTX3) 基因的表达也与卵母细胞及胚胎的发育能力有关,是预测胚胎发育能力的可靠指标[19,20]。在小鼠排卵前卵泡腔内,PTX3 mRNA表达主要位于卵丘细胞中,而在颗粒细胞中表达很少[21],进一步证实了笔者的数据准确性。钙调素(Regucalcin,RGN)在牛大卵泡(直径>10 mm)中的表达量是小卵泡(直径<5 mm)中的9.8倍,被认为是参与了优势卵泡的形成及提高颗粒细胞的存活率[22]。这与本研究的结果产生分歧,可能是物种之间表达的差异所致。
Dickkopf WNT信号通路抑制剂3(dickkopf WNT signaling pathway inhibitor 3,DKK3)是一种旁分泌蛋白,它可以通过Wnt信号通路参与胚胎发育[23]。DKK3表观遗传沉默,会破坏正常 Wnt/β-catenin 信号传导和细胞凋亡调控[24]。醛脱氢酶1家族成员A2 (Aldehyde Dehydrogenase 1 Family Member A2,ALDH1A2)启动子中存在雌激素反应元件位点[25],在切除大鼠卵巢的子宫内,E2可以促进ALDH1A2表达,但却抑制ALDH1A1表达[26]。ALDH1A2是调节性腺中肾RA合成的主要酶,通过释放全反维甲酸(RA)的酶来启动细胞的减数分裂。构建ALDH1A2和ALDH1A3双敲除小鼠与全反维甲酸反应元件(RARE)报告小鼠杂交表明,在缺乏这两种酶产生RA情况下,雌性小鼠可发生减数分裂,雄性小鼠不可发生减数分裂[27,28,29,30]。目前对全反维甲酸受体应答器 1(retinoic acid receptor responder 1,RARRES1)的报道较少,RARRES1作为RA释放的下游基因,可能在细胞的减数分裂过程中起重要作用。
4 结论
本研究在贵州白山羊DF与SF颗粒细胞中共获得695个差异表达mRNA,其中233个在SF颗粒细胞中表达显著上调,462个则表达下调。通过功能分析,筛选出6个可能与山羊卵泡发育密切相关的基因,qRT-PCR结果发现,DKK3、ALDH1A2、RARRES1和RGN在DF和SF颗粒细胞中表达量存在极显著差异,推测在山羊卵泡发育过程中可能促进卵泡的优势化或导致闭锁,对深入探究调控卵泡发育机制具有重要意义。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1186/s40709-019-0093-yURL [本文引用: 1]
DOI:10.1261/rna.754207URLPMID:17951331 [本文引用: 1]

Small noncoding RNAs have been suggested to play important roles in the regulation of gene expression across all species from plants to humans. To identify small RNAs expressed by the ovary, we generated mouse ovarian small RNA complementary DNA (srcDNA) libraries and sequenced 800 srcDNA clones. We identified 236 small RNAs including 122 microRNAs (miRNAs), 79 piwi-interacting RNAs (piRNAs), and 35 small nucleolar RNAs (snoRNAs). Among these small RNAs, 15 miRNAs, 74 piRNAs, and 21 snoRNAs are novel. Approximately 70% of the ovarian piRNAs are encoded by multicopy genes located within the repetitive regions, resembling previously identified repeat-associated small interference RNAs (rasiRNAs), whereas the remaining approximately 30% of piRNA genes are located in nonrepetitive regions of the genome with characteristics similar to the majority of piRNAs originally cloned from the testis. Since these two types of piRNAs display different structural features, we categorized them into two classes: repeat-associated piRNAs (rapiRNAs, equivalent of the rasiRNAs) and non-repeat-associated piRNAs (napiRNAs). Expression profiling analyses revealed that ovarian miRNAs were either ubiquitously expressed in multiple tissues or preferentially expressed in a few tissues including the ovary. Ovaries appear to express more rapiRNAs than napiRNAs, and sequence analyses support that both may be generated through the
DOI:10.2174/1574893612666170403165746URL [本文引用: 1]
DOI:10.1095/biolreprod56.5.1158URLPMID:9160714 [本文引用: 1]

The objective of this study was to investigate changes in expression of mRNAs encoding FSH receptor (FSHr), LH receptor (LHr), cytochrome P450 side-chain cleavage (P450(scc)), cytochrome P450 17alpha-hydroxylase (P450(c17)), and cytochrome P450 aromatase (P450(arom)) during recruitment and selection of bovine ovarian follicles. Dairy heifers (4-5 per group) were ovariectomized at 12, 24, 36, 48, 60, 72, 84, or 96 h after initiation of the first follicular wave following estrus as determined by ultrasonography (Time 0 = initiation of follicular wave; mean +/- SEM = 42.0 +/- 2.6 h after estrus). Expression of mRNAs encoding FSHr, LHr, P450(scc), P450(c17), and P450(arom) was detected by in situ hybridization and quantified by image analysis. Antral follicles were classified as healthy or atretic. Healthy follicles expressed higher (p < 0.01) amounts of mRNAs for gonadotropin receptors and steroidogenic enzymes than did atretic follicles, and expression of LHr, FSHr, P450(scc), P450(c17), and P450(arom) increased (p < 0.01) with follicular size and stage of the follicular wave. Expression of mRNAs for P450(scc), P450(arom), and LHr was time- and size-dependent during recruitment and selection. During recruitment, expression of mRNAs for P450(scc) and P450(arom) was first detected in granulosa cells of 16 of 21 of the follicles 4-6 mm in diameter at 12 h. At 24 and 36 h, almost all follicles 6-9 mm in diameter, but not those 4-5 mm in diameter, expressed both P450(scc) and P450(arom) mRNA in the granulosa cells. At 48 h and thereafter, P450(scc) and P450(arom) mRNA were expressed predominantly in one healthy large follicle per cow with a few exceptions. Expression of LHr mRNA was first detected in granulosa cells at 36 h and was always found in granulosa cells of one follicle > or = 8 mm per cow with exception of one cow at 36 h (no expression) and another two cows, one each at 36 and at 84 h (expression in 2 follicles). In addition, LHr mRNA expression in the granulosa cell layer was limited to follicles that also expressed mRNAs for P450(scc) and P450(arom) in the granulosa cells. In summary, follicular recruitment in cattle was associated with expression of P450(scc) and P450(arom) mRNA within granulosa cells, and the process of follicular selection was associated with initiation of LHr mRNA expression in granulosa cells.
DOI:10.1016/j.fertnstert.2013.03.008URLPMID:23548939 [本文引用: 1]

OBJECTIVE: To identify resistin in human ovarian follicles and investigate the effect and the molecular mechanisms associated with resistin on steroidogenesis in human granulosa cells (GCs). DESIGN: The effects of recombinant human resistin on the secretion of progesterone (P) and estradiol (E2) by cultured human GCs were investigated. SETTING: Academic institutions. PATIENT(S): Twenty infertile and healthy women undergoing IVF. INTERVENTION(S): Primary human GC cultures stimulated with recombinant human resistin (10 ng/mL). MAIN OUTCOME MEASURE(S): Determination of messenger RNA (mRNA) and protein expression of resistin in fresh human GCs by reverse transcriptase-polymerase chain reaction (RT-PCR), immunoblot and immunohistochemistry, respectively; measurement of P and E2 levels in the conditioned media by radioimmunoassay; determination of cell proliferation by tritiated thymidine incorporation; and analysis of signaling pathways activation by immunoblot analysis. RESULT(S): Human GCs and theca cells express resistin. In primary human GCs, resistin decreases P and E2 secretion in response to insulin-like growth factor I (IGF-I). This was associated with a reduction in the P450 aromatase and P450scc (cholesterol side-chain cleavage cytochromes P450) (P450scc) protein levels but not those of 3beta-hydroxysteroid dehydrogenase (3beta-HSD) or steroidogenic acute regulatory protein (StAR) and with a decrease in IGF-I-induced IGF-I receptor and mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation. Resistin treatment does not affect IGF-I-induced cell proliferation and basal steroidogenesis (there is no IGF-I or follicle-stimulating hormone stimulation). In the basal state, resistin rapidly stimulates Akt and MAPK ERK1/2 and p38 phosphorylation in primary human GCs. CONCLUSION(S): Resistin is present in human GCs and theca cells. It decreases P and E2 secretion, P450scc and P450 aromatase protein levels, and IGF-IR signaling in response to IGF-I in primary human GCs.
DOI:10.1111/joim.12580URLPMID:27878865 [本文引用: 1]

Fibroblast growth factor (FGF) 21 belongs to a hormone-like subgroup within the FGF superfamily. The members of this subfamily, FGF19, FGF21 and FGF23, are characterized by their reduced binding affinity for heparin that enables them to be transported in the circulation and function in an endocrine manner. It is likely that FGF21 also acts in an autocrine and paracrine fashion, as multiple organs can produce this protein and its plasma concentration seems to be below the level necessary to induce a pharmacological effect. FGF21 signals via FGF receptors, but for efficient receptor engagement it requires a cofactor, membrane-spanning betaKlotho (KLB). The regulation of glucose uptake in adipocytes was the initial biological activity ascribed to FGF21, but this hormone is now recognized to stimulate many other pathways in vitro and display multiple pharmacological effects in metabolically compromised animals and humans. Understanding of the precise physiology of FGF21 and its potential medicinal role has evolved exponentially over the last decade, yet numerous aspects remain to be defined and others are a source of debate. Here we provide a historical overview of the advances in FGF21 biology focusing on the uncertainties in the mechanism of action as well as the differing viewpoints relating to this intriguing protein.
DOI:10.1016/S0093-691X(99)00138-7URLPMID:10734375 [本文引用: 1]

Ovarian changes determined by daily transrectal ultrasonic scanning, and its correlation with serum progesterone (P4) and estradiol (E2) concentrations were studied in seven cyclic Saanen goats. Estrous cycles were synchronized with 2 injections of a PGF2 alpha analogue 9 d apart. All follicles > or = 2 mm in diameter and CL were measured each day. One goat showed a longer interestrous interval, associated with development of a cystic-luteinized structure. The mean interovulatory interval for the other 6 goats was 20.8 +/- 0.4 d. The incidence of goats with 4, 3, and 2 follicular waves was 3, 1 and 2 respectively; follicular waves emerged on Days 0.5 +/- 0.6, 7.2 +/- 0.7, 10.7 +/- 0.5 and 13.7 +/- 0.8 for Wave 1, 2, 3 and the Ovulatory wave, respectively. The largest follicle of Wave 2 was smaller (4.9 +/- 0.1 mm) than the largest follicles of Wave 3 (6.2 +/- 0.1 mm; P < or = 0.01) and of the Ovulatory wave (7.0 +/- 0.5 mm; P < or = 0.01), and tended to be smaller than the largest follicle of Wave 1 (6.3 +/- 0.6 mm; P < or = 0.09). Interval between emergence of Wave 1 and Wave 2 was longer than interval between emergence of Wave 2 and Wave 3 (7.3 +/- 0.9 d vs 4.0 +/- 0.4 d; P < or = 0.01), and between Wave 3 and the Ovulatory wave (3.8 +/- 1.1 d; P < or = 0.05). Two days before ovulation, the diameter of the ovulatory follicle was larger (P < or = 0.01) than the first subordinate follicle. Serum E2 concentrations increased from the day of ovulation (2.7 +/- 0.3 pg/mL) to Day 2 (7.6 +/- 0.9 pg/mL; P < or = 0.01), associated with the early-mid growing phase of the largest follicle of Wave 1, and then decreased to basal levels on Day 5 (P < or = 0.01) and peaked again (16.5 +/- 2.4 pg/mL) 2 d before ovulation. The CL were detected ultrasonically on Day 3 post ovulation and attained a mean maximum diameter of 13.5 +/- 0.8 mm between Days 8 and 14. The following characteristics were observed: 1) ovarian follicular development in goats is wave-like; 2) increased P4 concentrations may be promoting follicular wave turnover; 3) it is suggested that the presence of follicular dominance and the production of E2 are different among waves. While in Wave 1 and in the Ovulatory wave, follicular dominance is present and production of E2 is consistent, no changes in serum E2 concentrations were found in other stages of the interovulatory interval. In the intervening waves, no indicators of follicular dominance could be firmly documented.
DOI:10.1016/j.anireprosci.2017.11.004URLPMID:29126830 [本文引用: 1]

The normal maturation and ovulation from ovarian follicles is important in ensuring conception and improving fertility of buffalo. The molecular regulation mechanism of buffalo follicles growth, however, remains unknown. This study analyzed the gene expression profiles associated with buffalo ovarian follicle growth. According to the analysis of RNA sequencing, 17,700 unigenes and 13,672 differentially expressed genes (DEGs) were detected. A total of 30 common DEGs were identified during four stages of follicle growth, and the expression patterns are basically synchronized, suggesting the products as a result of expressions of these genes may cooperate to regulate follicular development. Furthermore, GO and KEGG enrichment analyses revealed that the majority of DEGs in early stage of follicular growth were enriched in ribosomal and oxidative phosphorylation signaling pathways, and the expression patterns of these DEGs are basically up-regulated at the beginning of follicular growth (<8mm, diameter), and then down-regulated (8-12mm) in the following stages of follicular development. The pathway of immune signaling, including allograft rejection, chemokine signaling pathway, natural killer cell mediated cytotoxicity, phagosome, and antigen processing and presentation, was significantly enriched in the last stage of follicular development (>12mm), which indicates that the immune system has an important role in the last stage of follicular maturation and ovulation. This study provided a gene expression profile of buffalo follicle growth, and provided an insight into biological processes associated with molecular regulation of ovarian follicle growth.
DOI:10.1152/physiolgenomics.00069.2016URLPMID:27940565 [本文引用: 1]

Ovarian folliculogenesis corresponds to the development of follicles leading to either ovulation or degeneration, this latter process being called atresia. Even if atresia involves apoptosis, its mechanism is not well understood. The objective of this study was to analyze global gene expression in pig granulosa cells of ovarian follicles during atresia. The transcriptome analysis was performed on a 9,216 cDNA microarray to identify gene networks and candidate genes involved in pig ovarian follicular atresia. We found 1,684 significantly regulated genes to be differentially regulated between small healthy follicles and small atretic follicles. Among them, 287 genes had a fold-change higher than two between the two follicle groups. Eleven genes (DKK3, GADD45A, CAMTA2, CCDC80, DAPK2, ECSIT, MSMB, NUPR1, RUNX2, SAMD4A, and ZNF628) having a fold-change higher than five between groups could likely serve as markers of follicular atresia. Moreover, automatic confrontation of deregulated genes with literature data highlighted 93 genes as regulatory candidates of pig granulosa cell atresia. Among these genes known to be inhibitors of apoptosis, stimulators of apoptosis, or tumor suppressors INHBB, HNF4, CLU, different interleukins (IL5, IL24), TNF-associated receptor (TNFR1), and cytochrome-c oxidase (COX) were suggested as playing an important role in porcine atresia. The present study also enlists key upstream regulators in follicle atresia based on our results and on a literature review. The novel gene candidates and gene networks identified in the current study lead to a better understanding of the molecular regulation of ovarian follicular atresia.
DOI:10.1093/humupd/dmi001URLPMID:15705959 [本文引用: 2]

Cell death was first described in rabbit ovaries (Graaffian follicles), the phenomenon being called 'chromatolysis' rather than apoptosis. In humans, the ovarian endowment of primordial follicles is established during fetal life. Apoptotic cell death depletes this endowment by at least two-thirds before birth, executed with the help of several players and pathways conserved from worms to humans. To date, apoptosis has been reported to be involved in oogenesis, folliculogenesis, oocyte loss/selection and atresia. Several pro-survival and pro-apoptotic molecules are involved in ovarian apoptosis with the delicate balance between them being the determinant for the final destiny of the follicular cells. This review critically analyses the current knowledge about the biological roles of these molecules and their relevance to the dynamics of follicle development. It also presents the existing literature and assesses the gaps in our knowledge.
[本文引用: 1]
DOI:10.1007/s10815-017-1031-2URLPMID:28866821 [本文引用: 1]

PURPOSE: The purpose of this study was to evaluate the capacity of bovine granulosa cells to generate reactive oxygen intermediates in response to lipopolysaccharide. We hypothesized that granulosa cells increase reactive oxygen intermediates in response to Gram-negative lipopolysaccharide in a similar manner to immune cells. METHODS: Bovine peripheral blood mononuclear cells and granulosa cells were cultured in the presence of lipopolysaccharide. Oxidative stress was evaluated using the fluorescent marker dye CellROX, and oxidative stress-related genes were measured using real-time RT-PCR. RESULTS: As expected, peripheral blood mononuclear cells increased oxidative stress in response to lipopolysaccharide as measured by accumulation of the fluorescent marker dye CellROX. While granulosa cells demonstrate the capacity to increase accumulation of CellROX dye in response to a positive control menadione, lipopolysaccharide had no effect on accumulation of CellROX dye. The expression of GSR, SOD1, and SOD2 were variable in peripheral blood mononuclear cells treated with lipopolysaccharide but were consistently upregulated when co-incubated with the antioxidant, N-acetyl cysteine. The expression of oxidative stress-related genes was not altered in granulosa cells, with the exception of elevated SOD1 following lipopolysaccharide exposure in the absence of antioxidant. CONCLUSIONS: Combined, these data suggest that while reactive stress is important in pathogen killing and inflammation in immune cells, granulosa cells do not increase oxidative stress in response to lipopolysaccharide.
DOI:10.1016/j.theriogenology.2017.04.048URLPMID:28601152 [本文引用: 1]

X-linked inhibitor of apoptosis protein (XIAP), an endogenous of inhibitor of caspases, plays crucial roles in regulating ovarian granulosa cell apoptosis during follicular atresia. The aim of the present study was to determine the presence and localization of XIAP in the goat ovary and its expression level during follicular development. The full length cDNA of XIAP from goat ovary cells was cloned using reverse transcription PCR. A total of 497 amino acid residues were encoded by open reading frame and had high identity with homologous sequences from other mammals. XIAP was widely expressed in adult goat tissues as determined by real-time PCR and it demonstrated higher expression in propagative organs. High level of XIAP was detected in large healthy follicles and corpus luteum in comparison with that in small antral follicles, which was in accordance with the immunohistochemistry results and atretic follicles had very low expression. XIAP was localized in both granulosa and theca cells in antral follicles but not in primordial follicles. Furthermore, luteinizing hormone stimulated the proliferation of mRNA encoding XIAP in granulosa cells in vitro. The present study demonstrated that XIAP was expressed in a follicular-stage-dependent manner in goat ovaries.
[本文引用: 1]
[本文引用: 1]
DOI:10.1210/endo.136.3.7867584URLPMID:7867584 [本文引用: 1]

Pubertal mammary development in the rat is largely dependent upon GH and estrogen. We recently showed that insulin-like growth factor-I (IGF-I) can substitute for GH in inducing mammary development in male rats, suggesting that IGF-I mediates GH action. The present study investigated whether IGF-I, like GH, required estradiol (E2) to act or whether IGF-I could substitute for both GH and E2. The effects of IGF-I were tested in the presence and absence of E2. Elvax pellets containing IGF-I or des(1-3) IGF-I were implanted into right lumbar mammary glands of sexually immature, hypophysectomized, oophorectomized female rats, with control BSA-containing pellets in the contralateral glands. After 5 days, both lumbar mammary glands were removed and examined in whole mounts for mammary development by counting terminal end buds and alveolar structures. E2, administered in SILASTIC brand capsules, had no independent effect on mammary development. In the absence of E2, des(1-3) IGF-I had a small, but significant, independent effect on mammary development; native IGF-I was ineffective. The addition of E2 significantly enhanced the effects of IGF-I and des(1-3) IGF-I on mammary development, similar to that noted when E2 was given along with GH. We also studied the effects of E2 and/or hGH on mammary gland IGF-I messenger RNA (mRNA) in hypophysectomized castrated male animals. E2 alone did not increase mammary gland IGF-I mRNA concentrations, but E2 enhanced the effect of hGH on IGF-I mRNA by 4- to 6-fold. These studies indicate that IGF-I can have a small independent effect on mammary development, but like GH, E2 is required for a full effect. They also indicate that E2 is capable of synergizing with GH in the production or expression of IGF-I mRNA, and that the action of E2 on mammary development may take place at multiple sites. If locally produced IGF-I does indeed mediate the action of GH in mammary development, then although E2 is capable of enhancing the effect of GH on IGF-I mRNA, its major effect in mammary development occurs after IGF-I is produced.
URLPMID:14645190 [本文引用: 1]
DOI:10.1007/978-3-319-12114-7_6URLPMID:25472537 [本文引用: 1]

The output of prolactin (PRL) is highly dynamic with dramatic changes in its secretion from the anterior pituitary gland depending on prevailing physiological status. In adult female mice, there are three distinct phases of output and each of these is related to the functions of PRL at specific stages of reproduction. Recent studies of the changes in the regulation of PRL during its period of maximum output, lactation, have shown alterations at both the level of the anterior pituitary and hypothalamus. The PRL-secreting cells of the anterior pituitary are organised into a homotypic network in virgin animals, facilitating coordinated bouts of activity between interconnected PRL cells. During lactation, coordinated activity increases due to the changes in structural connectivity, and this drives large elevations in PRL secretion. Surprisingly, these changes in connectivity are maintained after weaning, despite reversion of PRL output to that of virgin animals, and result in an augmented output of hormone during a second lactation. At the level of the hypothalamus, tuberoinfundibular dopamine (TIDA) neurons, the major inhibitors of PRL secretion, have unexpectedly been shown to remain responsive to PRL during lactation. However, there is an uncoupling between TIDA neuron firing and dopamine secretion, with a potential switch to enkephalin release. Such a process may reinforce hormone secretion through dual disinhibition and stimulation of PRL cell activity. Thus, integration of signalling along the hypothalamo-pituitary axis is responsible for increased secretory output of PRL cells during lactation, as well as allowing the system to anticipate future demands.
DOI:10.1186/s12958-015-0091-3URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1186/1471-2407-13-603URL [本文引用: 1]
[本文引用: 1]
DOI:10.1006/geno.2001.6546URLPMID:11386761 [本文引用: 1]

The mouse RALDH2 gene spans >50 kb, has a structure similar to that of human class 1 aldehyde dehydrogenase genes, and localizes to the central region of chromosome 9 by single-strand polymorphism analysis. Expression of mouse RALDH2 was detected in testis, lung, brain, and heart (Northern blot) and in liver and kidney (RNase protection assays). Expression was not detected by RNase protection assay in testis of vitamin A-deficient rats, and all-trans-retinoic acid dosing did not increase expression in vitamin A-deficient rat testis. A 2.3-kb section of the gene 5' to the transcription start site included neither retinoic acid nor retinoid X response elements, but included TATA and CCAAT motifs and AP, AHR, CREB, ER, Ets, and SREBP sites. The promoter initiated transcription of a luciferase reporter in human embryonic kidney cells (EBNA) and mouse Leydig- (TM3) and Sertoli-derived (TM4) cell lines, but neither all-trans-retinoic acid nor 9-cis-retinoic acid affected reporter transcription. These data suggest that relatively weak RALDH2 expression in vitamin A-deficient testis reflects vastly decreased numbers of germ cells, the major site of expression.
DOI:10.1210/en.2004-0514URLPMID:15205379 [本文引用: 1]

Estrogen (E2) has been shown to induce the biosynthesis of retinoic acid (RA) in rat uterus. Here we examined whether E2 could directly induce the enzymes involved in this process by using the ovariectomized rat. A retinol dehydrogenase that we have previously described, eRolDH, and the retinal dehydrogenase, RalDH II, were found to have markedly increased uterine mRNA levels within 4 h of E2 administration, independent of the prior administration of puromycin. eRolDH and RalDH II and their mRNAs were also increased in uteri of rats during estrus. This indicated that RA biosynthesis in rat uterus is directly controlled by E2 and provides a direct link between the action of a steroid hormone and retinoid action. We also examined the cell-specific localization of RalDH II by immunohistochemistry. The enzyme was observed in the stromal compartment, particularly in cells close to the uterine lumenal epithelium. eRolDH was observed only in the lining epithelial cells. Taken together with the previous observations of cellular retinol-binding protein and cellular retinoic acid-binding protein, type two also being expressed in the lumenal epithelium, we propose that RA production is compartmentalized, with retinol oxidation occurring in the lumenal epithelium and subsequent oxidation of retinal to RA occurring in the underlying stromal cells.
[本文引用: 1]
DOI:10.1016/j.ydbio.2011.05.658URLPMID:21621532 [本文引用: 1]

Dmrt1 belongs to the DM domain gene family of conserved sexual regulators. In the mouse Dmrt1 is expressed in the genital ridge (the gonadal primordium) in both sexes and then becomes testis-specific shortly after sex determination. The essential role of DMRT1 in testicular differentiation is well established, and includes transcriptional repression of the meiotic inducer Stra8. However Dmrt1 mutant females are fertile and the role of Dmrt1 in the ovary has not been studied. Here we show in the mouse that most Dmrt1 mutant germ cells in the fetal ovary have greatly reduced expression of STRA8, and fail to properly localize SYCP3 and gammaH2AX during meiotic prophase. Lack of DMRT1 in the fetal ovary results in the formation of many fewer primordial follicles in the juvenile ovary, although these are sufficient for fertility. Genome-wide chromatin immunoprecipitiation (ChIP-chip) and quantitative ChIP (qChIP) combined with mRNA expression profiling suggests that transcriptional activation of Stra8 in fetal germ cells is the main function of DMRT1 in females, and that this regulation likely is direct. Thus DMRT1 controls Stra8 sex-specifically, activating it in the fetal ovary and repressing it in the adult testis.
DOI:10.1093/hmg/ddq027URLPMID:20106871 [本文引用: 1]

Germ cells are the foundation of an individual, since they generate the gametes and provide the unique genome established through meiosis. The sex-specific fate of the germline in mammals is thought to be controlled by somatic signals, which are still poorly characterized. We demonstrate here that somatic Wnt signalling is crucial for the control of female germline development. Wnt-4 maintains germ cell cysts and early follicular gene expression and provides a female pattern of E-cadherin and beta-catenin expression within the germ cells. In addition, we find that Stra8 expression is downregulated and the Cyp26b1 gene is expressed ectopically in the partially masculinized Wnt-4-deficient ovary. Wnt-4 may control meiosis via these proteins since the Cyp26b1 enzyme is known to degrade retinoic acid (RA) and inhibit meiosis in the male embryo, and Stra8 induces meiosis in the female through RA. Reintroduction of a Wnt-4 signal to the partially masculinized embryonic ovary, in fact, rescues the female property to a certain degree, as seen by inhibition of Cyp26b1 and induction of Irx3 gene expression. Wnt-4 deficiency allows only 20% of the germ cells to initiate meiosis in the ovary, whereas meiosis is inhibited completely in the Wnt-4/Wnt-5a double mutant. These findings indicate a critical role for Wnt signalling in meiosis. Thus, the Wnt signals are important somatic cell signals that coordinate presumptive female follicle development.
DOI:10.1016/j.devcel.2010.08.010URLPMID:20833365 [本文引用: 1]

Sex determination of mammalian germ cells occurs during fetal development and depends on signals from gonadal somatic cells. Previous studies have established that retinoic acid (RA) triggers ovarian germ cells to enter meiosis and thereby commit to oogenesis, whereas in the developing testis, the enzyme CYP26B1 degrades RA and germ cells are not induced to enter meiosis. Using in vitro and in vivo models, we demonstrate that fibroblast growth factor 9 (FGF9) produced in the fetal testis acts directly on germ cells to inhibit meiosis; in addition, FGF9 maintains expression of pluripotency-related genes and upregulates markers associated with male germ cell fate. We conclude that two independent and mutually antagonistic pathways involving RA and FGF9 act in concert to determine mammalian germ cell sexual fate commitment and support a model in which the mitosis/meiosis switch is robustly controlled by both positive and negative regulatory factors.
