 ,, 李任建, 张琦, 张育铭, 韩渊怀, 张宝俊
,, 李任建, 张琦, 张育铭, 韩渊怀, 张宝俊 ,山西农业大学植物保护学院,山西太谷 030801
,山西农业大学植物保护学院,山西太谷 030801Identification of Co-Expression Genes Related to Endogenous Abscisic Acid in Response to the Stress of Sclerospora graminicola by WGCNA in Foxtail Millet
CHANG GuoRong ,, LI RenJian, ZHANG Qi, ZHANG YuMing, HAN YuanHuai, ZHANG BaoJun
,, LI RenJian, ZHANG Qi, ZHANG YuMing, HAN YuanHuai, ZHANG BaoJun ,College of Plant Protection, Shanxi Agricultural University, Taigu 030801, Shanxi
,College of Plant Protection, Shanxi Agricultural University, Taigu 030801, Shanxi通讯作者:
责任编辑: 岳梅
收稿日期:2020-02-17接受日期:2020-03-4网络出版日期:2020-08-16
| 基金资助: |
Received:2020-02-17Accepted:2020-03-4Online:2020-08-16
作者简介 About authors
常国蓉,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (4207KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
常国蓉, 李任建, 张琦, 张育铭, 韩渊怀, 张宝俊. 利用WGCNA鉴定谷子内源脱落酸响应禾生指梗霉胁迫的共表达基因[J]. 中国农业科学, 2020, 53(16): 3280-3293 doi:10.3864/j.issn.0578-1752.2020.16.007
CHANG GuoRong, LI RenJian, ZHANG Qi, ZHANG YuMing, HAN YuanHuai, ZHANG BaoJun.
0 引言
【研究意义】脱落酸(ABA)作为一类“逆境激素”,在响应干热、高盐、低温、重金属及辐射等非生物胁迫过程中起着重要作用。同时,脱落酸可直接或通过与水杨酸(SA)、茉莉酸(JA)和乙烯(ETH)等激素的互作或调控等参与寄主的生物胁迫[1]。脱落酸信号转导依赖其受体蛋白PYR/PYL/PCAR及负调控因子2C类蛋白磷酸酶(PP2C)和正调控因子SNF1相关的蛋白激酶(SnRK2)组成的双重负调控系统的调控,激活其下游基因的表达[2,3]。据报道,在拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)等作物中,约10%的基因受脱落酸的调控[3]。谷子(Setaria italica)是我国北方地区一种重要的杂粮作物,具有较强的抗旱、抗逆能力,且生育期短,经济效益高[4]。但谷子生产上常受到多种生物胁迫及非生物胁迫因素的影响,特别是近年来,随着谷子种植规模扩大,由卵菌纲禾生指梗霉(Sclerospora graminicola)引起的谷子白发病在各地频发,对谷子产量和品质的提高造成严重威胁,分析脱落酸在谷子响应禾生指梗霉侵染中的调控作用,有利于解析谷子白发病的发生特点及规律,并为其防治提供新思路。【前人研究进展】稻瘟病菌(Magnaporthe oryzae)在水稻感染部位能够激活脱落酸信号,抑制水稻中最初入侵的免疫信号,从而负调控水稻对稻瘟病菌的抗病性[5,6]。SONG等[7]研究发现,对番茄叶面外施脱落酸,可有效降低番茄早疫病菌(Alternaria solani)对番茄植株的病害程度。ARF10可诱导甘蓝型油菜对脱落酸的敏感性,提高对黑斑病菌(Alternaria brassicicola)的抗性[8]。PYL和SnRK2是脱落酸信号转导途径中两个关键的正反馈调节的家族基因。当植物体内脱落酸大量积累时,脱落酸与其受体PYR/PYLs/RCARs结合后,其以单体形式与磷酸酶PP2Cs结合,通过抑制PP2Cs,从而释放SnRK2s蛋白激酶,SnRK2s磷酸化激活ABFs及RAV1等转录因子、激活下游脱落酸响应基因[9,10,11,12,13,14]。反之当脱落酸含量较低时,PYR/PYLs/RCARs以二聚体的形式存在,SnRK2的活性受到抑制,阻止脱落酸信号传导[15]。ZHANG等[16]研究发现,SnRK2家族的OsSAPK9可与OsSGT1相互作用,提高水稻的抗白叶枯病能力;YAO等[17]通过GWAS群体和转录关联的方法,鉴定到PYL和SnRK2在玉米早期抗穗腐病中显著上调。【本研究切入点】现有研究表明,脱落酸在寄主响应病菌侵染、扩展、致病过程中发挥重要作用[9],且PYL和SnRK2是脱落酸信号转导途径中两类关键因子,在脱落酸代谢过程中发挥着重要作用。然而目前尚未有脱落酸代谢在谷子抗病中的相关研究。【拟解决的关键问题】通过基因家族、加权基因共表达网络(WGCNA)、转录组分析对PYL和SnRK2家族基因进行鉴定和分析,探索谷子响应禾生指梗霉侵染过程中脱落酸发挥的作用及关键的调控因子,为后续谷子抗白发病或抗其他生物胁迫的调控机制研究提供参考。1 材料与方法
1.1 试验材料
感病品种晋谷21号种子由山西农业大学生物工程研究所种质资源库提供,病菌孢子于2016年在山西农业大学试验田的病株上分离获得。2017年5月初将种子拌菌处理后种植于山西农业大学谷子杂粮育种基地,以消毒不接种谷子为对照组,采取随机区组设计方法划分试验小区,每个小区面积为3 m×2 m。在谷子叶片刚表现出“灰背”症状后,每隔7 d取材一次,样品经液氮速冻后于-80℃保存,用于脱落酸含量测定和RNA提取,共取材5次,以健康叶片为对照,3次重复。1.2 谷子PYL和SnRK2家族全基因筛选
PYL家族筛选:在Phytozome数据库(https:// phytozome.jgi.doe.gov/pz/portal.html)中获取谷子(v2.2)、水稻(v7_JGI)和拟南芥(TAIR10)的基因组数据。在Pfam数据库(http://pfam.xfam.org/)中下载PYL (Polyketide_cyc2)HMMER模型(PF10604),通过HMMER软件[18]中的hmmsearch命令搜索谷子全基因蛋白质数据,选取比对值E-value<0.01。SnRK2家族筛选:利用蛋白激酶模型(PF00069)通过hmmsearch对谷子基因组进行搜索,随后利用拟南芥数据库和水稻数据库下载的SnRK2家族基因对上一步具有蛋白激酶结构的基因进行同源比对,选取比对值E-value<0.01,利用MEGA计算筛选的基因与水稻、拟南芥SnRK2的遗传距离,筛选谷子SnRK2家族基因。使用Pfam中的Batch search对检索到PYL和SnRK2蛋白序列结构域进行验证。使用TBtools软件[19]对谷子的基因组注释gff3文件的基因定位结果可视化分析。
1.3 谷子PYL和SnRK2蛋白理化性质和基因定位分析
采用在线工具ExPASY-ProtParam(https://web. expasy.org/protparam/)对谷子PYL和SnRK2家族基因的氨基酸数目、分子量、等电点和总平均疏水指数进行分析[20]。1.4 谷子PYL和SnRK2的系统进化分析
采用MEGA 7.0[21]中的ClustalW将拟南芥(9个)、水稻(8个)的PYL序列与谷子PYL序列进行多重比对,将拟南芥(5个)、水稻(9个)SnRK2序列与谷子SnRK2序列进行多重比对,构建系统进化树,矫正后,通过Test Neighbor-Joining Tree(邻近法)构建进化树。利用进化树在线美化网站ITOL进行进化树美化。1.5 谷子PYL和SnRK2的顺式作用元件分析
采用TBtools软件[19]提取谷子PYL和SnRK2家族基因的启动子序列,将提取的启动子序列到在线数据库PlantCARE(http://bioinformatics.psb.gent.e/ eboolslantcare/html)[22]分析谷子PYL和SnRK2家族基因顺式作用元件。1.6 脱落酸含量的测定
取0.5 g叶片材料在液氮中研磨成粉末,将粉末与3 mL 80%甲醇在4℃下混合4 h,4℃,12 000×g离心20 min。收集上清液,用作激素提取液[23]。采用ELISA试剂盒(上海酶联)测定内源激素脱落酸含量[24]。用酶标仪(SP-Max2300A2)在450 nm处测定吸光度,用标准曲线法计算待测激素的含量。以同时期健康叶片为对照,3次重复。1.7 共表达网络的构建和模块鉴定
利用同时期转录组表达矩阵,过滤FPKM值<1的基因。使用R软件(R version 3.4.4)和WGCNA(R version 1.6.6)包,构建加权基因共表达网络与划分相关模块[25]。使用WGCNA包中的pickSoftThreashold计算软阈值,选取最佳网络构建的power值。使用blockwiseModules构建无尺度网络,参数按照默认值设置[25]。1.8 脱落酸及其下游信号转导关键模块鉴定和功能分析
利用moduleTraitCor<-cor(mergedMEs,datTraits,use=“p”)对脱落酸含量和共表达模块进行相关性分析,鉴定关键模块。分析PYL和SnRK2基因家族在共表达网络中的分布情况,鉴定其信号转导调控的关键模块。提取模块中的基因,利用TBtools进行GO富集[19],以谷子基因组为参考数据库,P-value<0.05,利用Cytoscape对模块进行可视化[26]。1.9 谷子PYL和SnRK2在禾生指梗霉胁迫下的表达模式分析
对转录组数据进行分析后,利用pheatmap对谷子PYL和SnRK2家族成员的表达水平进行可视化。1.10 实时荧光定量PCR(qRT-PCR)
选取4个基因的转录本进行qRT-PCR验证。使用primer 3设计定量PCR引物,利用M-MuLV第一链cDNA合成试剂盒(TaKaRa)对提取的RNA进行纯化并反转录成cDNA。采用2×SG快速qPCR混合试剂盒(TaKaRa)进行荧光定量分析。qRT-PCR扩增的反应体积为15 μL,包括7.5 μL 2×SG快速qPCR主混合物、0.6 μL每个引物、40 ng cDNA模板、3.3 μL ddH2O。PCR流程:95℃变性30 s,95℃变性5 s,60℃退火/延伸30 s,40个循环,以actin为内对照基因,用2-ΔΔCT法计算相关基因表达。3次重复。2 结果
2.1 谷子PYL和SnRK2的筛选
基因家族分析共鉴定到PYL和SnRK2家族基因各11个(表1)。谷子PYL和SnRK2家族基因除6号和8号染色体外,其余染色体均有分布,其中4号染色体上只有PYL基因Seita.4G239500,2号和7号染色体上只有SnRK2,分别为Seita.2G394500和Seita. 7G100500,3号和9号染色体上数目最多,分别有6个。PYL家族基因氨基酸长度介于175—220 aa,等电点介于4.39—8.88,分子量较小,分子量最大为23.67 kD。SnRK2家族基因氨基酸数目在333—454 aa,等电点介于4.73—8.49,分子量较大,最高可达51.77 kD。Table 1
表1
表1谷子PYL和SnRK2基因家族成员基本信息
Table 1
| 类型 Type | 基因编号 Gene ID | 染色体 Chromosome | 基因组位置 Genomic location | 氨基酸长度 Amino acid (aa) | 等电点 pI | 分子量 Molecular weight (kD) | 总平均疏水指数 Average of hydropathicity |
|---|---|---|---|---|---|---|---|
| PYL | Seita.1G013900 | 1 | 1185261-1188981 | 211 | 5.92 | 23.67 | -0.393 |
| Seita.1G030500 | 1 | 2860241-2862675 | 201 | 5.97 | 21.72 | -0.338 | |
| Seita.3G072600 | 3 | 4600680-4602575 | 193 | 4.39 | 20.38 | -0.011 | |
| Seita.3G076200 | 3 | 4851939-4854302 | 205 | 5.91 | 22.16 | -0.233 | |
| Seita.3G207900 | 3 | 16201030-16202684 | 204 | 8.88 | 21.58 | -0.132 | |
| Seita.4G239500 | 4 | 36338582-36339202 | 206 | 6.71 | 22.14 | -0.220 | |
| Seita.5G140800 | 5 | 12377439-12378609 | 206 | 5.25 | 21.84 | -0.101 | |
| Seita.5G302400 | 5 | 35644268-35645327 | 175 | 4.93 | 18.81 | -0.210 | |
| Seita.5G369100 | 5 | 40592575-40593198 | 207 | 6.75 | 22.70 | -0.252 | |
| Seita.9G311900 | 9 | 36003871-36005478 | 207 | 5.24 | 22.13 | -0.195 | |
| Seita.9G437300 | 9 | 48993016-48994206 | 220 | 6.58 | 22.92 | 0.036 | |
| SnRK2 | Seita.1G190000 | 1 | 27251818-27256180 | 454 | 8.49 | 51.77 | -0.605 |
| Seita.2G394500 | 2 | 45956776-45961725 | 339 | 5.30 | 38.47 | -0.177 | |
| Seita.3G003200 | 3 | 157270-159276 | 380 | 5.99 | 43.11 | -0.547 | |
| Seita.3G230400 | 3 | 19092668-19097929 | 360 | 5.68 | 41.77 | -0.546 | |
| Seita.3G369900 | 3 | 47387035-47389674 | 374 | 4.94 | 41.46 | -0.224 | |
| Seita.5G395400 | 5 | 42440116-42444731 | 362 | 6.06 | 42.33 | -0.619 | |
| Seita.7G100500 | 7 | 20312278-20317276 | 358 | 6.00 | 40.98 | -0.558 | |
| Seita.9G318200 | 9 | 36623246-36626468 | 333 | 5.48 | 37.93 | -0.467 | |
| Seita.9G079800 | 9 | 4716246-4721220 | 366 | 4.81 | 41.48 | -0.308 | |
| Seita.9G169200 | 9 | 11473484-11476494 | 362 | 4.73 | 40.73 | -0.283 | |
| Seita.9G379000 | 9 | 43841287-43845704 | 344 | 5.10 | 39.13 | -0.238 |
新窗口打开|下载CSV
2.2 谷子PYL和SnRK2的系统进化分析
谷子PYL家族在系统进化中分为4类,橙色分支中的Seita.3G072600、Seita.5G302400和Seita.5G140800较为保守,蓝色分支中的谷子PYL与水稻PYL聚集在一起,表明这部分谷子PYL可能与水稻PYL亲缘性较高。谷子SnRK2家族在系统进化中可分为3类,在灰色、紫色和黄色3个分支中,水稻SnRK2均与谷子SnRK2位于同一小分支上,而拟南芥SnRK2在分支上较独立,表明SnRK2在禾本科间较为保守(图1)。图1
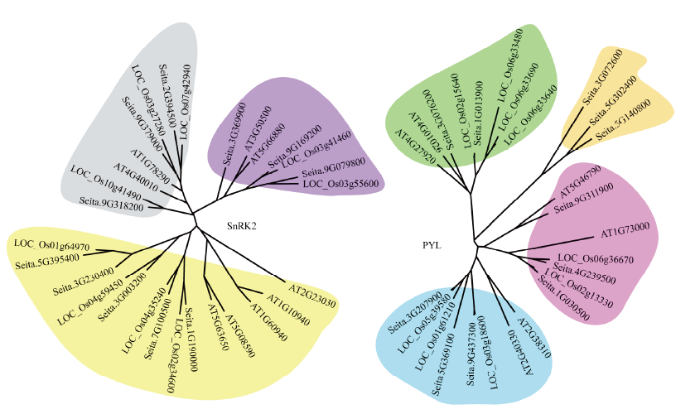 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1谷子PYL和SnRK2家族系统进化树
每一种颜色覆盖的基因为亲缘性较近的一类基因
Fig. 1Phylogenetic tree of PYL and SnRK2 family genes in S. italic
Each color covered gene is a kind of gene with close relationship
2.3 顺式作用元件预测
谷子PYL和SnRK2家族基因顺式作用元件分析表明(图2),PYL和SnRK2家族基因中在127个区域预测到光响应元件。其中,22个家族基因都预测到脱落酸响应元件;谷子PYL家族基因Seita.1G013900、Seita.9G311900和SnRK2家族基因Seita.3G230400和Seita.3G369900被预测到防御和胁迫响应元件;PYL和SnRK2家族基因同时预测到其他激素类响应元件,其中与茉莉酸甲酯响应有关的基因最多,PYL家族9个,SnRK2家族10个。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2谷子PYL和SnRK2家族基因的启动子顺式作用元件分析
图中每个彩色方块表示图例对应的响应元件,每个方块的位置表示在启动子上的位置
Fig. 2Functions of promoter cis-acting elements in PYL and SnRK2 family genes in S. italic
Each color block in the figure represents the response element corresponding to the legend, and the position of each block represents the position on the starter
2.4 脱落酸含量的测定
受侵染不同时期叶片中脱落酸含量测定表明,脱落酸在病菌侵染前期显著积累,TG1和TG2时期脱落酸含量显著高于对照组,分别为22.50和18.08 ng·mL-1,随后含量显著减少。在TG3时期降低到与对照组同等水平13.79 ng·mL-1,在TG4时期达到最低值13.18 ng·mL-1,且含量显著低于对照组21.82 ng·mL-1(图3)。图3
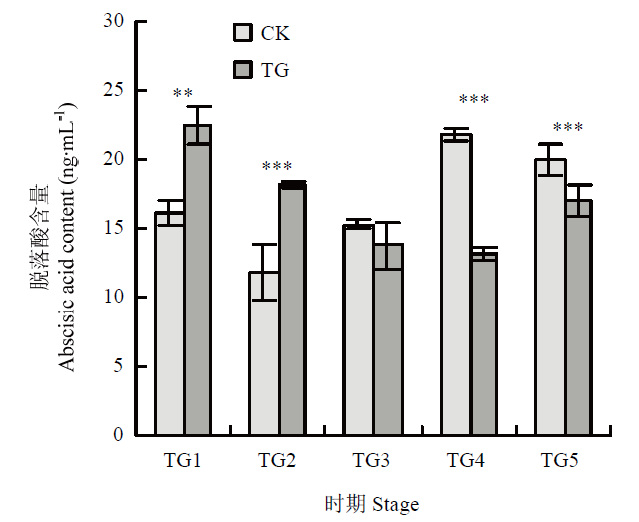 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3脱落酸含量测定
**: P<0.01; ***: P<0.001
Fig. 3Determination of abscisic acid content
2.5 谷子脱落酸共表达网络鉴定
利用gsg=goodSamplesGenes(datExpr,verbose= 3),gsg$allOK参数测试数据的合理性,在所测RNA-seq数据中筛选软阈值β=12构建共表达网络(图4-A),利用18 535个基因共得到34个基因共表达模块(图4-B)。其中MEturquoise模块的基因数目最多有3 924个,MEdarkolivegreen模块的基因数目最少,仅有43个。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4软阈值确定和共表达网络构建
A:左图纵坐标表示网络模型指数,右图表示平均连通度;横坐标表示构建网络的软阈值,红色横线表示选取的最佳软阈值;B:不同的颜色表示构建的网络模块,系统进化树表示不同样本的层次聚类,每个样本对应模块的一种颜色
Fig. 4Determination of soft threshold and construction of co-expression network
A:Different colors represent the built network modules, cluster dendrogram indicates hierarchical clustering of different samples, and each sample corresponds to a color of the module;B:The vertical coordinate on the left represents the index of the network model, the right represents the mean connectivity, and the horizontal coordinate represents the soft threshold of the network, the red line indicates the best soft threshold selected
2.6 谷子脱落酸共表达网络鉴定
通过共表达网络与脱落酸含量进行关联,鉴定到与脱落酸高度正相关的模块为MEpaleturquoise模块,相关性值为0.56,其次为MEbrown和MEdarkolivegreen模块,相关性值均为0.48(图5-A)。其中负相关性中值最高的为MEpink模块,相关性值为-0.54(图5-B),相关性绝对值最低的为MEdarkorange模块,相关性值为0.024。对脱落酸信号转导途径中的SnRK2和PYL家族基因所在模块的鉴定中发现(图5-C),SnRK2和PYL所在模块数目最多的为MEbrown模块,占总数目的27%,其中该模块中有4个PYL和2个SnRK2,表明该模块也是脱落酸调控及发挥生理效应的关键模块。在MEturquoise模块中有5个SnRK2,但该模块与脱落酸含量的相关性绝对值仅为0.026,不存在相关性。有5个PYL和1个SnRK2不属于任何模块。故MEpaleturquoise和MEbrown为脱落酸及其信号转导响应禾生指梗霉侵染的关键模块。图5
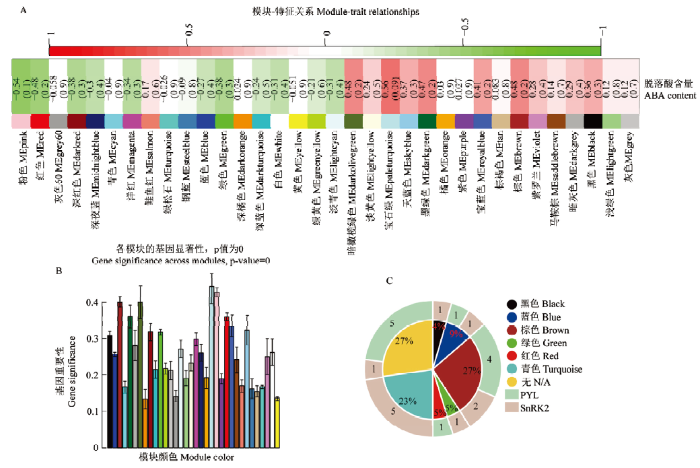 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5关键模块的鉴定
A:共表达模块与脱落酸含量关联的热图;B:模块与脱落酸含量的相关性;C:谷子PYL和SnRK2家族基因在模块的分布情况,内圆扇形表示属于该模块的家族基因所占总基因数目的比例,外部扇形表示属于该模块的家族基因数目
Fig. 5Identification of key modules
A:The heat map of co-expression module related to the content of abscisic acid;B:Relativity of module to the content of abscisic acid;C:The distribution of PYL and SnRK2 family genes in the module, the inner circle sector represents the proportion of family genes belonging to the module in the total number of genes, and the outer sector represents the number of family genes belonging to the module
2.7 模块的功能富集
对筛选的MEpaleturquoise和MEbrown模块进行功能富集分析(表2),其中MEpaleturquoise模块显著富集到1-3-beta-D-葡聚糖生物合成过程(GO: 0006075)、类黄酮代谢过程(GO:0051552)和类黄酮生物合成过程(GO:0051553),还富集到了钙离子平衡(GO:0055074)和磷酸转移酶活性(GO:0016780)等。MEbrown模块富集到响应真菌(GO:0009620)、生物刺激反应(GO:0009607)、免疫系统的过程(GO:0002376)等与胁迫相关的功能;激素响应(GO:0009725)、激素介导的信号通路(GO:0009755)、乙烯激活的信号通路(GO:0009873)和油菜素内酯(GO:0009742)介导的信号通路等与激素相关的一些功能;以及蔗糖响应(GO:0009744)、脂肪酸代谢(GO:0006631)和调节氮化合物代谢过程(GO:0051171)等与基础代谢相关的功能。Table 2
表2
表2模块GO富集情况
Table 2
| 类型 Type | 基因编号 Gene ID | GO富集 GO term | P-value |
|---|---|---|---|
| MEpaleturquoise | GO:0000775 | 染色体、着丝粒区域Chromosome, centromeric region | 0.02291 |
| GO:0008135 | 翻译因子活性、核酸结合位点Translation factor activity, nucleic acid binding | 0.03575 | |
| GO:0006075 | 1-3-beta-D-葡聚糖生物合成过程 1-3-beta-D-glucan biosynthetic process | 0.0002 | |
| GO:2000112 | 调控细胞大分子生物合成过程Regulation of cellular macromolecule biosynthetic process | 0.0015 | |
| GO:0051552 | 类黄酮代谢过程Flavone metabolic process | 0.0249 | |
| GO:0051553 | 类黄酮生物合成过程Flavone biosynthetic process | 0.0249 | |
| GO:0055074 | 钙离子平衡Calcium ion homeostasis | 0.0249 | |
| GO:0016780 | 磷酸转移酶活性,用于其他取代磷酸基Phosphotransferase activity, for other substituted phosphate groups | 0.02825 | |
| MEbrown | GO:0003995 | 脂酰CoA脱氢酶活性Acyl-CoA dehydrogenase activity | 0.001849608 |
| GO:0051171 | 调节氮化合物代谢过程Regulation of nitrogen compound metabolic process | 1.95E-05 | |
| GO:0009755 | 激素介导的信号通路Hormone-mediated signaling pathway | 7.67E-05 | |
| GO:0071383 | 细胞对类固醇激素刺激的反应Cellular response to steroid hormone stimulus | 8.79E-04 | |
| GO:0009725 | 激素响应Response to hormone | 0.002160987 | |
| GO:0009742 | 油菜素类内酯介导的信号通路Brassinosteroid mediated signaling pathway | 0.002538882 | |
| GO:0002376 | 免疫系统的过程Immune system process | 0.003433683 | |
| GO:0043207 | 对外界生物刺激的反应Response to external biotic stimulus | 0.018615083 | |
| GO:0009873 | 乙烯激活的信号通路Ethylene-activated signaling pathway | 0.018737534 | |
| GO:0009607 | 生物刺激反应Response to biotic stimulus | 0.019137736 | |
| GO:0080134 | 应激反应的调节Regulation of response to stress | 0.019394085 | |
| GO:0006631 | 脂肪酸代谢过程Fatty acid metabolic process | 0.022668677 | |
| GO:0009744 | 蔗糖响应Response to sucrose | 0.023618658 | |
| GO:0044038 | 细胞壁大分子生物合成过程Cell wall macromolecule biosynthetic process | 0.033696381 | |
| GO:0009620 | 响应真菌Response to fungus | 0.043398934 |
新窗口打开|下载CSV
对与脱落酸关联的MEpaleturquoise模块和MEbrown模块中以谷子PYL、SnRK2家族基因构建了共表达网络。其中MEplaeturquoise中鉴定到Seita. 4G105600、Seita.6G218100和Seita.9G138400为该模块的枢纽基因(图6-A),Seita.4G105600在水稻和拟南芥中都被鉴定为含有WD结构域的超家族蛋白,Seita.6G218100在水稻中的同源基因为WRKY57转录因子,在拟南芥中同源基因为包含VQ基序的蛋白;Seita.9G138400在水稻和拟南芥中都注释的为植物特有的响应胁迫相关的转录因子TIFY(表3)。在脱落酸信号转导途径中的PYL和SnRK2家族基因有4个处于网络调控的枢纽位置(图6-B),其中PYL家族基因的Seita.1G013900和SnRK2家族基因的Seita. 2G394500在网络中权重值较高,可能起主要调控作用。
图6
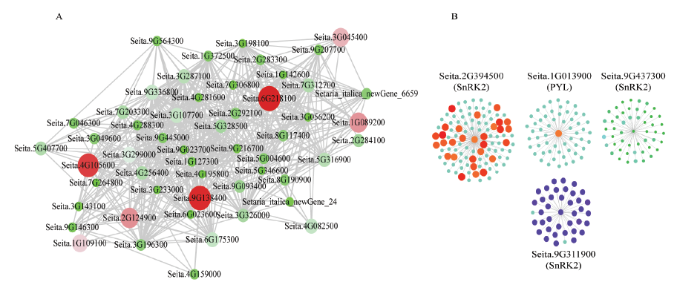 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6MEpaleturquoise模块内基因和MEbrown模块内PYL、SnRK2家族基因共表达网络
图中点的大小表示该基因在网络中的权重大小,图A中红色表示核心基因,图B中红色表示权重值较高的基因,蓝色表示权重值中等的基因,绿色表示权重值较小的基因
Fig. 6Gene co-expression network of the MEpaleturquoise module and PYL, SnRK2 family genes in MEbrown module
The size of the midpoint in the figure indicates the weight of the gene in the network. The red in figure A indicates the core gene. In figure B, the red indicates the gene with higher weight value, the blue indicates the gene with medium weight value, and the green indicates the gene with lower weight value
Table 3
表3
表3模块核心基因功能注释
Table 3
| 候选基因 Candidate gene | 水稻同源基因 Homologous genes in O. sativa | 基因功能 Gene function | 拟南芥同源基因 Homologous genes in A. thaliana | 基因功能 Gene function |
|---|---|---|---|---|
| Seita.4G105600 | LOC_Os06g33480 | 包含蛋白质的WD结构域、G-beta重复结构域 WD domain, G-beta repeat domain containing protein | AT1G24130 | 转导蛋白/WD40重复超家族蛋白 Transduction/WD40 repeat-like superfamily protein |
| Seita.6G218100 | LOC_Os12g01180 | WRKY57转录因子 WRKY57 transcription factors | AT5G46780 | 包含VQ基序的蛋白 VQ motif-containing protein |
| Seita.9G138400 | LOC_Os06g36670 | TIFY 转录因子 TIYF transcription factors | AT1G51600 | TIFY2A转录因子 TIYF2A transcription factors |
新窗口打开|下载CSV
2.8 表达模式分析
在响应禾生指梗霉侵染的表达模式分析中,处理组中的PYL家族基因Seita.1G030500、Seita.3G207900、Seita.9G311900和Seita.9G437300均在前期表达水平较高,SnRK2家族基因Seita.2G394500、Seita.3G003200在TG1时期表达水平较高(图7-A)。对谷子PYL和SnRK2家族基因与脱落酸含量进行相关性分析,鉴定到基因Seita.1G030500、Seita.2G394500和Seita.3G003200显著性最高,分别为0.80、0.65和0.72(图7-B)。图7
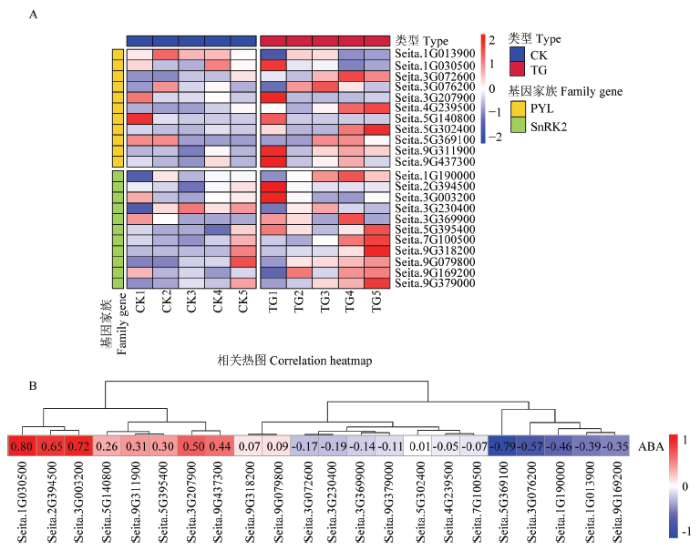 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7PYL和SnRK2家族基因表达模式及相关性分析
A:转录组中谷子PYL和SnRK2家族基因的表达热图;B:谷子PYL和SnRK2家族基因与脱落酸含量的相关性系统热图
Fig. 7Gene expression pattern and correlation analysis of PYL and SnRK2 families
A:The expression heat map of PYL and SnRK2 family genes in the transcriptome;B:The systematic heat map of correlation between PYL and SnRK2 family genes and abscisic acid content in the transcriptome
2.9 实时荧光定量PCR
4个基因Seita.2G394500、Seita.4G105600、Seita.6G218100和Seita.9G138400的荧光定量分析表明,基因Seita.2G394500在TG1、TG2时期的表达量显著上调;基因Seita.4G105600和Seita.6G218100在TG2、TG3、TG4时期的表达量均高于对照组的表达量,且TG2、TG3时期表达量显著上调;基因Seita.9G138400 5个时期表达量显著下调(图8)。图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8不同时期基因表达量变化
*: P<0.05; **: P<0.01
Fig. 8Changes in gene expression at different stages
3 讨论
脱落酸可作为抗病性的抑制因子或增强剂,VLEESSCHAUWER等[27]研究发现,通过对水稻叶片施加脱落酸,可增加水稻对褐斑病的抗性,但ULFERTS等[28]发现脱落酸含量的升高导致稻瘟病菌对大麦叶片的危害加重。脱落酸在植物的防御反应中扮演着复杂而矛盾的角色。本研究中,在禾生指梗霉早期侵染阶段,脱落酸含量较对照组显著增加,表明禾生指梗霉的侵染可能引起寄主内源脱落酸含量的积累。鉴定出的11个谷子SnRK2基因被分为3类,与水稻[29]、拟南芥[30]的研究结果一致,而且与玉米[31]、高粱[32]的研究结果类似,主要包括不依赖于脱落酸、不依赖于激酶或对脱落酸的依赖性弱和对脱落酸具有很强的依赖性3类。谷子Seita.1G013900、Seita.4G239500基因与水稻中OsPYL3(LOC_Os02G15640)和OsPYL9(LOC_Os06g36670)同源性较高,这两个基因在水稻中可以正向调控脱落酸信号,是真正的水稻脱落酸受体[33],通过PYL和SnRK2的同源聚类分析验证了其在脱落酸信号转导通路上的重要作用。在共表达网络分析中,筛选到与脱落酸含量高度相关的MEpaleturquoise模块,通过对该模块的富集分析鉴定到了槲皮素、类黄酮和钙离子平衡相关的功能。贾振华[34]用槲皮素处理野生型拟南芥后,活性氧含量增加,同时还出现活性氧爆发、过敏性细胞坏死、胼胝质沉积等多种抗性反应,进而增强拟南芥对丁香假单胞菌(Pseudomonas syringae)的抗性。PYL和SnRK2在脱落酸信号转导过程中具有重要作用,在顺式作用元件分析中,鉴定到PYL和SnRK2家族基因中存在与防御和胁迫响应、脱落酸、水杨酸、茉莉酸甲酯、类黄酮等响应元件,表明PYL和SnRK2家族基因在参与激素响应胁迫方面可能发挥重要作用。在对PYL和SnRK2高度相关的MEbrown模块富集中,富集到了大量与生物胁迫相关功能的基因,如免疫系统过程、细胞壁大分子生物合成过程、脂肪酸代谢、油菜素内酯、乙烯及生物刺激反应等。脂肪酸代谢物甘油-3-磷酸(G3P)的积累可以提高拟南芥对炭疽病菌(Colletotrichum higginsianum)的抗性[35],植物角质层中的主要成分也是脂肪酸及其衍生物,对抵御病菌侵染具有重要作用[36]。油菜素内酯可诱导水稻对烟草花叶病毒(Tobacco mosaic virus,TMV)、稻瘟病菌和水稻白叶枯病菌(Xanthomonas oryzae)的抗性[37]。通过对谷子不同侵染、扩展阶段脱落酸含量的测定、生物信息学分析及不同时期表达量的测定,推测脱落酸在禾生指梗霉侵染过程中可能发挥着非常重要的作用。
本研究通过表达模式和相关性分析,鉴定到基因Seita.1G030500、Seita.2G394500和Seita.3G003200在脱落酸响应过程中发挥着重要作用。通过共表达网络分析预测到的3个核心基因中,Seita.4G105600在水稻和拟南芥中的同源基因均为含有WD40重复结构的家族蛋白,WD40家族蛋白在植物生长发育、响应胁迫方面具有重要作用,李宝燕[38]鉴定到含有WD40基序的NtTTG2通过协调生长素和水杨酸提高抗病性。Seita.6G218100在水稻中的同源基因为WRKY57,WRKY转录因子在番茄上被报道为负调控番茄对灰霉病的抗性[39]。Seita.9G138400的同源基因在水稻和拟南芥中均被鉴定为TIFY家族蛋白基因,TIFY家族蛋白在植物生长发育、激素应答和逆境胁迫方面具有重要作用,过表达VvTIFY9可提高葡萄对白粉病的抗性[40]。笔者推测,谷子内源脱落酸在受到禾生指梗霉胁迫时,可能通过Seita.6G218100、Seita.4G105600和Seita.9G138400基因编码的WRKY57、WD40重复结构蛋白以及TIFY蛋白激活抗病响应途径。
4 结论
脱落酸在禾生指梗霉侵染谷子叶片早期大量积累,通过加权基因共表达网络进行脱落酸及其信号转导基因PYL和SnRK2关联分析,共筛选到6个基因参与谷子内源脱落酸响应禾生指梗霉侵染过程,包含1个PYL家族基因、2个SnRK2家族基因、1个WD40家族蛋白基因、1个WRKY57转录因子和1个TIFY转录因子基因。经qRT-PCR验证发现1个SnRK2家族基因、1个WD40家族蛋白基因和1个WRKY57转录因子基因共3个基因可能在谷子脱落酸响应禾生指梗霉侵染过程中发挥重要作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:20192755 [本文引用: 1]
URLPMID:31598026 [本文引用: 1]
URLPMID:31598026 [本文引用: 1]
URLPMID:16901781 [本文引用: 2]
[D].
[本文引用: 1]
[本文引用: 1]
DOI:10.1094/MPMI-23-6-0791URLPMID:20459318 [本文引用: 1]

Plant hormones play pivotal signaling roles in plant-pathogen interactions. Here, we report characterization of an antagonistic interaction of abscisic acid (ABA) with salicylic acid (SA) signaling pathways in the rice-Magnaporthe grisea interaction. Exogenous application of ABA drastically compromised the rice resistance to both compatible and incompatible M. grisea strains, indicating that ABA negatively regulates both basal and resistance gene-mediated blast resistance. ABA markedly suppressed the transcriptional upregulation of WRKY45 and OsNPR1, the two key components of the SA signaling pathway in rice, induced by SA or benzothiadiazole or by blast infection. Overexpression of OsNPR1 or WRKY45 largely negated the enhancement of blast susceptibility by ABA, suggesting that ABA acts upstream of WRKY45 and OsNPR1 in the rice SA pathway. ABA-responsive genes were induced during blast infection in a pattern reciprocal to those of WRKY45 and OsPR1b in the compatible rice-blast interaction but only marginally in the incompatible one. These results suggest that the balance of SA and ABA signaling is an important determinant for the outcome of the rice-M. grisea interaction. ABA was detected in hyphae and conidia of M. grisea as well as in culture media, implying that blast-fungus-derived ABA could play a role in triggering ABA signaling at host infection sites.
[本文引用: 1]
DOI:10.1016/j.plaphy.2011.03.018URLPMID:21530290 [本文引用: 1]

The plant hormone abscisic acid (ABA) is an important regulator in many aspects of plant growth and development, as well as stress resistance. Here, we investigated the effects of exogenous ABA application on the interaction between tomato (Solanum lycopersicon L.) and Alternaria solani (early blight). Foliar spraying of 7.58 muM ABA was effective in reducing disease severity in tomato plants. Previously, increased activities of phenylalanine ammonia-lyase (PAL), polyphenol oxidase (PPO) and peroxidase (POD) were observed in exogenous ABA-treated tomato leaves. Moreover, these enzyme activities were maintained at higher levels in ABA-pretreated and A. solani challenged tomato plants. Tomato defense genes, such as PR1, beta-1, 3-glucanase (GLU), PPO, POD, and superoxide dismutase (SOD), were rapidly and significantly up-regulated by exogenous ABA treatment. Furthermore, a subsequent challenge of ABA-pretreated plants with the pathogen A. solani resulted in higher expression of defense genes, compared to water-treated or A. solani inoculated plants. Therefore, our results suggest that exogenous ABA could enhance disease resistance against A. solani infection in tomato through the activation of defense genes and via the enhancement of defense-related enzymatic activities.
DOI:10.1094/MPMI-05-19-0132-RURLPMID:31184524 [本文引用: 1]

Concomitant increase of auxin-responsive factors ARF16 and ARF17, along with enhanced expression of ARF10 in resistant Sinapis alba compared with that in susceptible Brassica juncea upon challenge with Alternaria brassicicola, revealed that abscisic acid (ABA)-auxin crosstalk is a critical factor for resistance response. Here, we induced the ABA response through conditional expression of ARF10 in B. juncea using the A. brassicicola-inducible GH3.3 promoter. Induced ABA sensitivity caused by conditional expression of ARF10 in transgenic B. juncea resulted in tolerance against A. brassicicola and led to enhanced expression of several ABA-responsive genes without affecting the auxin biosynthetic gene expression. Compared with ABI3 and ABI4, ABI5 showed maximum upregulation in the most tolerant transgenic lines upon pathogen challenge. Moreover, elevated expression of ARF10 by different means revealed a direct correlation between ARF10 expression and the induction of ABI5 protein in B. juncea. Through in vitro DNA-protein experiments and chromosome immunoprecipitation using the ARF10 antibody, we demonstrated that ARF10 interacts with the auxin-responsive elements of the ABI5 promoter. This suggests that ARF10 may function as a modulator of ABI5 to induce ABA sensitivity and mediate the resistance response against A. brassicicola.
[本文引用: 2]
[本文引用: 2]
URLPMID:19880399 [本文引用: 1]
[本文引用: 1]
URLPMID:19893533 [本文引用: 1]
URLPMID:21658606 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:31712918 [本文引用: 1]
URLPMID:32542992 [本文引用: 1]
URLPMID:21593126 [本文引用: 1]
URLPMID:32817944 [本文引用: 3]
[本文引用: 1]
[本文引用: 1]
URLPMID:27004904 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1385/0-89603-268-X:461URLPMID:7951745 [本文引用: 1]
URLPMID:19114008 [本文引用: 2]
[本文引用: 1]
URLPMID:20130100 [本文引用: 1]
[本文引用: 1]
DOI:10.1105/tpc.019943URLPMID:15084714 [本文引用: 1]

To date, a large number of sequences of protein kinases that belong to the sucrose nonfermenting1-related protein kinase2 (SnRK2) family are found in databases. However, only limited numbers of the family members have been characterized and implicated in abscisic acid (ABA) and hyperosmotic stress signaling. We identified 10 SnRK2 protein kinases encoded by the rice (Oryza sativa) genome. Each of the 10 members was expressed in cultured cell protoplasts, and its regulation was analyzed. Here, we demonstrate that all family members are activated by hyperosmotic stress and that three of them are also activated by ABA. Surprisingly, there were no members that were activated only by ABA. The activation was found to be regulated via phosphorylation. In addition to the functional distinction with respect to ABA regulation, dependence of activation on the hyperosmotic strength was different among the members. We show that the relatively diverged C-terminal domain is mainly responsible for this functional distinction, although the kinase domain also contributes to these differences. The results indicated that the SnRK2 protein kinase family has evolved specifically for hyperosmotic stress signaling and that individual members have acquired distinct regulatory properties, including ABA responsiveness by modifying the C-terminal domain.
URLPMID:15292193 [本文引用: 1]
URLPMID:18797872 [本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
URLPMID:18567828 [本文引用: 1]
URLPMID:17257167 [本文引用: 1]
URLPMID:12609030 [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1104/pp.16.00747URLPMID:27268959 [本文引用: 1]

Although necrotrophic pathogens cause many devastating plant diseases, our understanding of the plant defense response to them is limited. Here, we found that loss of function of WRKY57 enhanced the resistance of Arabidopsis (Arabidopsis thaliana) against Botrytis cinerea infection. Further investigation suggested that the negative regulation of WRKY57 against B cinerea depends on the jasmonic acid (JA) signaling pathway. Chromatin immunoprecipitation experiments revealed that WRKY57 directly binds to the promoters of JASMONATE ZIM-DOMAIN1 (JAZ1) and JAZ5, encoding two important repressors of the JA signaling pathway, and activates their transcription. In vivo and in vitro experiments demonstrated that WRKY57 interacts with nuclear-encoded SIGMA FACTOR BINDING PROTEIN1 (SIB1) and SIB2. Further experiments display that the same domain, the VQ motif, of SIB1 and SIB2 interact with WRKY33 and WRKY57. Moreover, transient transcriptional activity assays confirmed that WRKY57 and WRKY33 competitively regulate JAZ1 and JAZ5, SIB1 and SIB2 further enhance these competitions of WRKY57 to WRKY33. Therefore, coordinated regulation of Arabidopsis against B cinerea by transcription activators and repressors would benefit plants by allowing fine regulation of defense.
[本文引用: 1]
