 ,, 贾琦, 巫蔚, 赵路评, 薛冰, 刘欢欢, 尚静
,, 贾琦, 巫蔚, 赵路评, 薛冰, 刘欢欢, 尚静 ,, 雍太文, 李庆, 杨文钰四川农业大学农学院/四川省作物重大病害重点实验室/带状复合种植工程技术研究中心,成都 611130
,, 雍太文, 李庆, 杨文钰四川农业大学农学院/四川省作物重大病害重点实验室/带状复合种植工程技术研究中心,成都 611130Species Identification and Virulence Determination of Beauveria bassiana Strain BEdy1 from Ergania doriae yunnanus
ZHANG Lei ,, JIA Qi, WU Wei, ZHAO LuPing, XUE Bing, LIU HuanHuan, SHANG Jing
,, JIA Qi, WU Wei, ZHAO LuPing, XUE Bing, LIU HuanHuan, SHANG Jing ,, YONG TaiWen, LI Qing, YANG WenYuCollege of Agronomy, Sichuan Agricultural University/Sichuan Key Laboratory for Major Crop Diseases/Sichuan Engineering Research Center for Crop Strip Intercropping System, Chengdu 611130
,, YONG TaiWen, LI Qing, YANG WenYuCollege of Agronomy, Sichuan Agricultural University/Sichuan Key Laboratory for Major Crop Diseases/Sichuan Engineering Research Center for Crop Strip Intercropping System, Chengdu 611130通讯作者:
责任编辑: 岳梅
收稿日期:2019-11-20接受日期:2019-12-25网络出版日期:2020-07-16
| 基金资助: |
Received:2019-11-20Accepted:2019-12-25Online:2020-07-16
作者简介 About authors
张磊,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (2570KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张磊, 贾琦, 巫蔚, 赵路评, 薛冰, 刘欢欢, 尚静, 雍太文, 李庆, 杨文钰. 大豆高隆象致病球孢白僵菌菌株BEdy1的鉴定及毒力测定[J]. 中国农业科学, 2020, 53(14): 2974-2982 doi:10.3864/j.issn.0578-1752.2020.14.020
ZHANG Lei, JIA Qi, WU Wei, ZHAO LuPing, XUE Bing, LIU HuanHuan, SHANG Jing, YONG TaiWen, LI Qing, YANG WenYu.
0 引言
【研究意义】大豆是重要的经济和粮油作物,具有较高的营养价值。在大豆生长过程中,多种病虫害严重限制其产量。鞘翅目象甲科害虫在全世界大豆种植区均有发生与危害,危害我国大豆的象甲科害虫主要为大豆高隆象(Ergania doriae yunnanus)[1]。从2015年起,四川省大豆高隆象发生危害严重,已经成为主要害虫之一,而大豆高隆象的假死特性增加了防治难度。现有化学药剂针对性不强,即使大量施用仍达不到理想的防治效果。目前,未有针对大豆高隆象的有效生物防治学手段。因此,探究一种安全、有效且环保的大豆高隆象生物防治技术具有重要意义。【前人研究进展】在美国部分地区的大豆植株发生严重落叶,调查发现主要由进口长角象甲(Calomycterus setarius)危害造成[2,3]。长角象甲在20世纪40年代自日本传入美国,田间杂草增多会导致其大量发生并造成严重危害[4,5]。此外,在热带地区如巴西、阿根廷等国家,大豆茎秆象甲(Sternechus subsignatus)严重危害大豆茎秆,损伤的茎秆阻断了营养物质输送,导致产量下降[6,7]。最近,一种墨西哥大豆荚象甲(Rhyssomatus nigerrimus)被发现,其主要危害大豆荚,发生严重的地区豆荚损害率达48%[8]。不同种的大豆象甲取食植株部位存在差异,但最终都导致了大豆产量的严重降低。在我国,造成田间危害的象甲种类主要为大豆高隆象。该虫从6月上旬开始发生,至10月上旬结束。成虫蛀食茎秆、豆荚;卵被产在豆荚内部,孵化以后,幼虫在豆荚内取食豆粒,造成严重危害[1]。近年来,随着玉米-大豆复合种植技术的大面积推广,套作大豆种植面积逐年上升[9]。据调查,在复合种植模式下大豆高隆象造成的危害远高于其他害虫,危害的田块一般损失10%—20%,危害严重的田块损失高达60%—70%[1]。目前,主要以化学防治手段来控制大豆高隆象。尚静等[10]利用三唑磷或氯虫苯甲酰胺进行田间防治,取得一定的效果,但会造成一定程度的叶片氧化胁迫损伤。相关研究表明,使用白僵菌防治象甲,如红棕象甲(Rhynchophorus ferrugineus)[11]、玉米象甲(Sitophilus zeamais)[12]、茶大灰象甲(Sympiezomias citri)[13]和紫薇梨象(Pseudorobitis gibbus)[14]等,得到了较好的效果。白僵菌是一种常见的昆虫病原真菌,寄主范围广泛。其中,球孢白僵菌(Beauveria bassiana)可寄生15个目700多种昆虫,容易大规模生产,是目前研究和应用最多的昆虫病原菌之一[15]。与化学农药相比,其对非靶标生物危害小,更加环保。【本研究切入点】田间观察到一头自然感染真菌死亡的大豆高隆象成虫僵虫,采集后对该虫生真菌进行分离、鉴定,并探究其生物学特性以及对大豆高隆象成虫的毒力。【拟解决的关键问题】明确自然致大豆高隆象死亡的虫生真菌的种类和生物学特性,测定其对大豆高隆象成虫的毒力,为防控大豆高隆象提供生物防治新材料。1 材料与方法
1.1 供试昆虫
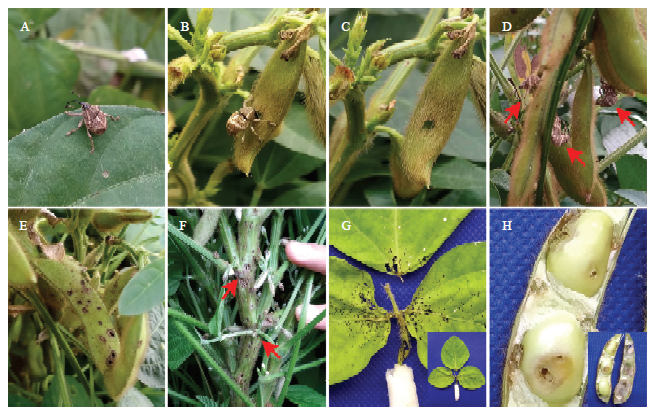
大豆高隆象每年7—9月发生率高,对茎秆和豆荚危害严重(图1)。大豆高隆象成虫收集自四川省玉米-大豆带状复合种植仁寿科研基地。在人工光照培养箱中饲养,温度控制在(25±1)℃,光周期为14 L﹕10 D。饲养食物为采摘的新鲜豆荚,每2 d更换一次新鲜食物。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1大豆高隆象的发生与危害
A—F:田间发生与危害 ;G、H:室内取食危害2 d后的叶片与豆荚
Fig. 1The occurrence and harm of E. d. yunnanus
A—F:The occurrence and harm in the field;G、H:The feeding damage of soybean leaf and pod after 2 days in the laboratory
1.2 昆虫病原真菌的收集、分离和鉴定
2018年9月,在四川省仁寿县的大豆田中采集到一头被真菌感染的大豆高隆象成虫僵虫。用消毒接种针从僵虫虫体上挑取霉层,接种于含100 μg·μL-1医用链霉素的PDA平板上,置于26℃恒温培养箱中暗培养。5 d后,用直径5 mm的灭菌打孔器在菌落边缘打取菌饼,转接到PDA平板中央进行纯化培养。定期观察菌落形态特征,并利用蔡司显微镜Gmbh37081对分子孢子拍照观察。利用真菌DNA快速提取试剂盒(DN41,艾德莱,北京)对纯化后的菌株进行DNA提取。使用引物ITS1和ITS4进行PCR扩增,反应总体系为25 μL:2×Taq Master Mix 12.5 μL、DNA模板1 μL、上下游引物各1 μL、ddH2O 9.5 μL。反应程序:94℃ 3 min;94℃ 30 s,54℃ 30 s,72℃ 1 min,35个循环;72℃ 10 min。PCR产物经1%的琼脂糖凝胶电泳检测后,送至生工生物工程(上海)股份有限公司进行测序。在NCBI数据库对测序获得的序列进行BLAST比对,确定其生物学分类。下载相近菌株的rDNA-ITS序列,在MEGA 7软件中通过MUSLE比对,基于邻接法构建系统发育树。该菌种已由本实验室申请专利菌种保藏。
1.3 不同培养温度对菌株BEdy1的生长速率和产孢量的影响
将菌株在PDA培养基纯化培养10 d,用直径5 mm的灭菌打孔器在菌落边缘打取菌饼,转接至PDA培养基中央,置于不用温度(16、20、22、25、26、28、30和35℃)的恒温培养箱中培养,每个处理3个重复。10 d后,采用十字交叉法测量菌落直径,计算菌落生长速率。并用直径5 mm的灭菌打孔器在菌落中心至边缘1/2处打取菌饼,每个平板取3个菌饼,接入15 mL含0.2%的吐温-80溶液中,均匀振荡后制成孢子悬浮液,用血球计数板测定孢子浓度,计算每平方毫米的产孢量。计算公式:菌落增长直径=菌落直径-菌饼直径(5mm);菌落生长速率(mm·d-1)=菌落增长直径/培养时间(10 d);产孢量(个/mm2)=孢子数/mL×15/(π×半径2)。
1.4 菌株BEdy1对大豆高隆象成虫的毒力测定
纯化后的菌株于PDA平板上培养15 d,收集分生孢子,加入0.2%的吐温-20溶液搅拌均匀,配制浓度梯度为1.0×105、1.0×106、1.0×107和1.0×108孢子/mL的孢子悬浮液。采用浸虫处理法[14]进行接种,具体操作:将大豆高隆象健康成虫浸入孢子悬浮液3—5 s,迅速取出,在无菌滤纸上吸掉多余菌液,放入直径为15 cm的培养皿。培养皿底部垫有无菌水湿润的滤纸,以保持湿度,顶部覆盖通有小孔的保鲜膜来维持气体出入。用新鲜大豆荚喂食,每2 d更换一次新的食物和湿润滤纸。每个浓度处理50头,重复3次,0.2%的吐温-20溶液处理作为对照。每日定时观察,统计高隆象的死亡和发病数据。购买一个商品化的球孢白僵菌产品(山西绿海农药科技有限公司),用于比较菌株对大豆高隆象的毒力差异。根据产品使用说明,配置最佳的使用浓度(1.0×108孢子/mL)进行如上所述的生物测定。使用1.0×108孢子/mL孢子悬浮液喷施大豆植株(V4期),1 d后取叶片作为食物饲喂高隆象,测定真菌寄生植物对高隆象的毒力。叶柄部位用湿润无菌棉包裹以保持水分供应,置于直径为15 cm的培养皿中,培养皿底部垫有无菌水湿润的滤纸保湿。每2 d更换一次新的食物和湿润滤纸。每个处理30头昆虫,设置3个重复,0.2%的吐温-20溶液喷施大豆叶片作为对照。
1.5 数据处理与分析
利用SPSS 24的概率统计分析计算致死中时间(LT50)。利用Excel 2007对孢子悬浮液浓度作对数转换和死亡率做线性回归,计算致死中浓度(LC50)和LC95。利用SPSS 24的ANOVA方法进行方差分析,通过Duncan法比较差异显著性。2 结果
2.1 昆虫病原真菌BEdy1的形态学和分子生物学鉴定
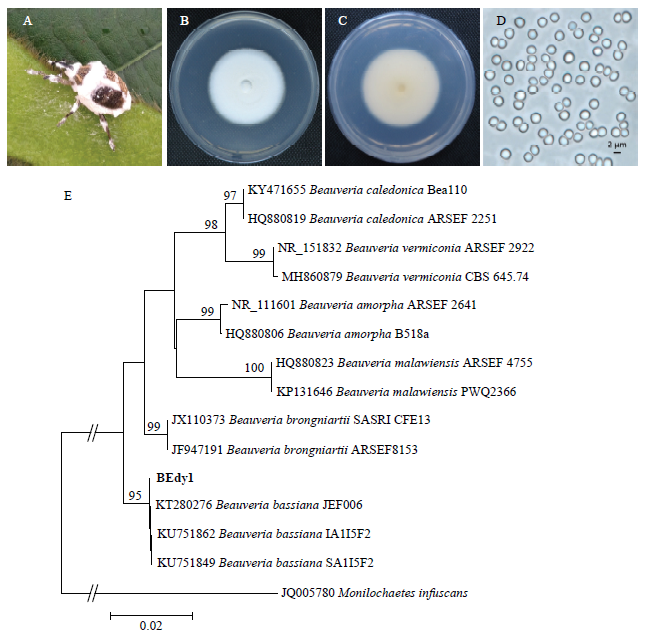
从自然感病死亡的大豆高隆象僵虫虫体分离获得纯化菌株,命名为BEdy1(图2)。菌株在PDA平板上的菌落呈白绒状,菌落背面为淡黄色。分生孢子近球形,单孢,透明,表面光滑,直径大小约2 μm。对菌株的rDNA-ITS进行PCR扩增,测序获得基因片段长度为544 bp。通过BLAST比对,发现BEdy1与多个已经公布的球孢白僵菌rDNA-ITS存在很高的序列相似性,其中与菌株JEF006(登录号:KT280276)的rDNA-ITS相似性达99%。系统发育树分析发现,菌株BEdy1以95%的支持率与球孢白僵菌聚集在一支(图2)。结合菌株形态学结果,最终将致使大豆高隆象田间死亡的病原菌BEdy1鉴定为球孢白僵菌,GenBank登录号为MK345993。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2BEdy1的形态学特征和系统发育分析
A:自然感染真菌BEdy1死亡的高隆象成虫僵虫;B:BEdy1菌落正面 ;C:BEdy1菌落背面;D:分生孢子显微照片;E:系统进化分析。苏格兰白僵菌;蠕孢白僵菌;多形白僵菌;马拉维白僵菌;布氏白僵菌;球孢白僵菌
Fig. 2Morphological characteristics and phylogenetic analysis of BEdy1
A:The cadaver of E. d. yunnanus infected with fungus BEdy1 in the field;B:Front of BEdy1 colony;C:Back of BEdy1 colony;D:Micrograph of conidia;E:Phylogenetic analysis。Beauveria caledonica;Beauveria vermiconia;Beauveria amorpha;Beauveria malawiensis;Beauveria brongniartii;Beauveria bassiana
2.2 温度对球孢白僵菌BEdy1生长和产孢的影响
在26℃下,球孢白僵菌BEdy1菌落生长速率最高,达4.34 mm·d-1。在16—30℃,菌落均能生长,而35℃下则停止生长。有利于BEdy1菌株生长的温度为22—28℃,生长速率均在4 mm·d-1以上。该菌株在22℃下产孢量最大,为4.63×106孢子/mm2。20—26℃之间菌株产孢量较高,20、25和26℃下产孢量分别为2.95×106、3.63×106和3.55×106孢子/mm2,3个温度下产孢量差异不显著(表1)。Table 1
表1
表1不同培养温度下BEdy1的生长速率和产孢量
Table 1
| 温度 Temperature (℃) | 生长速率 Growth rate (mm·d-1) | 产孢量 Sporulation (×106孢子/mm2) |
|---|---|---|
| 16 | 2.09±0.10d | 0.93±0.22cd |
| 20 | 3.32±0.04c | 2.95±0.35b |
| 22 | 4.01±0.14b | 4.63±0.63a |
| 25 | 4.02±0.04b | 3.63±0.30b |
| 26 | 4.34±0.12a | 3.55±0.35b |
| 28 | 4.21±0.04ab | 1.47±0.12c |
| 30 | 2.01±0.02d | 0.33±0.09de |
| 35 | 0e | 0e |
新窗口打开|下载CSV
2.3 球孢白僵菌BEdy1对大豆高隆象成虫的毒力
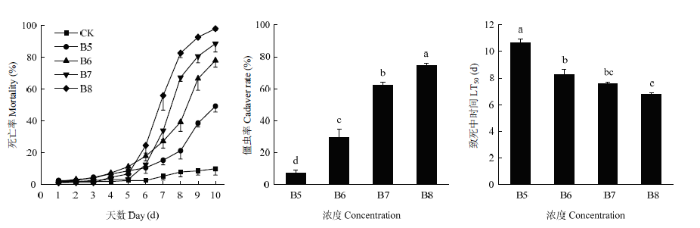
不同浓度的BEdy1孢子悬浮液浸虫处理后,第6天起试虫死亡数开始快速增加;浓度为1×108孢子/ mL的孢子悬浮液处理,第10天死亡率接近100%;浓度为1×107、1×106和1×105孢子/mL的孢子悬浮液处理10 d后的死亡率分别88.67%、77.33%和49.33%,而对照组10 d的死亡率仅为10%;1×108孢子/mL孢子悬浮液处理的LT50为(6.79±0.13)d;随着孢子浓度的降低,LT50值逐渐增加。僵虫率与孢子悬浮液的处理浓度呈正相关(图3)。1×108孢子/mL的孢子悬浮液处理下,试虫发病最严重,僵虫率达74.67%。图4为处理10 d后试虫的发病情况,随着孢子浓度的增加发病越来越重,对照组处理未见僵虫出现。第10天的 LC50、LC95分别为4.80×104、3.57×107孢子/mL(表2)。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3不同孢子浓度的球孢白僵菌BEdy1对大豆高隆象的毒力
B5: 1.0×105 spores/mL; B6: 1.0×106 spores/mL; B7: 1.0×107 spores/mL; B8: 1.0×108 spores/mL
Fig. 3Virulence of B. bassiana BEdy1 to E. d. yunnanus with different spore concentrations
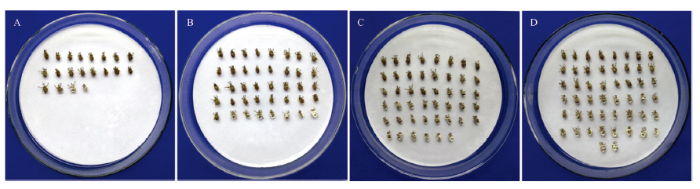
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4不同孢子浓度的球孢白僵菌BEdy1处理大豆高隆象10 d的发病情况
A: 1.0×105 spores/mL; B: 1.0×106 spores/mL; C: 1.0×107 spores/mL; D: 1.0×108 spores/mL
Fig. 4Pathogenesis of E. d. yunnanus treated with different spore concentrations of B. bassiana BEdy1 for 10 days
Table 2
表2
表2不同时间下球孢白僵菌BEdy1对大豆高隆象的毒力
Table 2
| 时间 Time (d) | 毒力回归方程 Toxicity regression equation (y=) | LC50 (孢子/mL) | LC95 (孢子/mL) | 相关系数 Correlation coefficient |
|---|---|---|---|---|
| 7 | 0.1287x-0.5047 | 6.43×107 | 2.02×1011 | 0.9470 |
| 8 | 0.2120x-0.8513 | 2.37×106 | 3.14×108 | 0.9879 |
| 9 | 0.1760x-0.4473 | 2.41×105 | 8.70×107 | 0.9560 |
| 10 | 0.1567x-0.2333 | 4.80×104 | 3.57×107 | 0.9196 |
新窗口打开|下载CSV
孢子浓度为1×108孢子/mL的商品化球孢白僵菌产品LH处理大豆高隆象10 d后,死亡率仅为58.00%,僵虫率为4.67%,极显著低于同浓度的BEdy1处理。LT50为(9.00±0.52)d,与BEdy1处理相比差异显著(表3)。
Table 3
表3
表3商品化球孢白僵菌LH对大豆高隆象的毒力
Table 3
| 时间 Time (d) | 死亡率 Mortality (%) | 僵虫率 Cadaver rate (%) | LT50 (d) |
|---|---|---|---|
| 8 | 38.67±4.81 *** | 0 *** | - |
| 9 | 50.67±6.36 *** | 2.00±1.15 *** | - |
| 10 | 58.00±3.06 *** | 4.67±1.76 *** | 9.00±0.52 ** |
新窗口打开|下载CSV
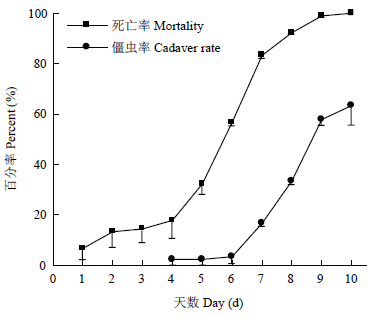
大豆植株外施浓度为1×108孢子/mL的BEdy1孢子悬浮液,叶片喂食大豆高隆象。第5天开始,大豆高隆象死亡数快速增加;至第10天,死亡率达100%。LT50为(5.27±0.35)d,第10天僵虫率为63.33%(图5)。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5叶面喷施BEdy1对大豆高隆象的毒力
Fig. 5Virulence of spraying BEdy1 on leaves to E. d. yunnanus
3 讨论
对害虫的防治方法主要包括化学防治、农业防治、生物防治等措施。化学药剂虽然防治效果显著,防治成本低,但长期大量使用对非靶标生物和环境产生一定影响,且害虫产生抗药性,导致再猖獗[16]。栽培措施的改变,抗(耐)虫品种的选育,一定程度上可以减轻虫害的发生,但仍然难以完全控制害虫。近年来,人们对于食品安全愈加重视,绿色农业已经成为未来农业的发展趋势。生物防治对人畜安全,对环境影响小,具有较好的发展前景,其中利用昆虫病原真菌防治害虫,方法简便,效果较好,引起了广泛的重视[17]。昆虫病原真菌可以寄生于昆虫体内,导致昆虫死亡。对虫生真菌的分离鉴定、生物学特性研究是开发运用的首要环节。传统的真菌分类以生物学特征进行,由于真菌种类众多,个体多态性等原因易造成假阳性的结果。随着分子生物学的快速发展,使真菌的分类鉴定快速、准确。REHNER等[18]利用ITS和EF-1α对86株白僵菌进行分类,确定了6种白僵菌的分类地位。本研究中,通过形态学观察,结合ITS序列比对,构建系统发育树,确定了致使大豆高隆象田间死亡的真菌BEdy1为球孢白僵菌。温度对真菌的生长十分重要[19],本研究中球孢白僵菌菌株BEdy1在22—28℃之间生长和产孢较好,与之前的研究结果基本一致[20,21]。刘召等[22]研究发现,温度和光照对白僵菌的菌丝生长和孢子产生存在影响,一定条件下的低温有利于孢子产生,22℃下孢子产生密度最大,这与本文的研究结果一致。
王定锋等[23]利用1.0×108孢子/mL的球孢白僵菌孢子悬浮液处理柑橘灰象甲(Sympiezomias citri)成虫7 d后,累计死亡率达100%;王定锋等[13]测定了布氏白僵菌对茶大灰象甲的毒力,结果表明5.0×107孢子/mL的孢子悬浮液处理7 d,茶大灰象甲累计死亡率达100%;宋晓兵等[24]研究表明,一株分离自柑橘木虱(Diaphorina citri)的球孢白僵菌在孢子悬浮液浓度为1.0×108孢子/mL时,处理柑橘木虱成虫7 d,其校正死亡率为95.7%。本研究中获得的球孢白僵菌菌株BEdy1在浓度为1.0×108孢子/mL的孢子悬浮液处理10 d后,大豆高隆象的死亡率近100%,同时有较高的僵虫率,毒力显著高于商品化球孢白僵菌。研究发现,球孢白僵菌可以在葡萄叶片内生,有效降低粉蚧等刺吸昆虫取食[25]。也可以内生于玉米叶片,从而影响蚜虫生长和繁殖[26]。本研究中,球孢白僵菌BEdy1孢子悬浮液喷施大豆叶片,对大豆高隆象同样有较好的毒杀效果。RUSSO等[27]研究发现,球孢白僵菌处理大豆叶片,7 d内真菌定殖率达100%,并且改善了多项大豆生长参数,可增加大豆产量。因此,利用球孢白僵菌防治大豆高隆象具有较大的潜力。
4 结论
致使大豆高隆象田间死亡的虫生真菌菌株BEdy1为球孢白僵菌,该菌株在适当环境条件下生长速度快,产孢量高,并对大豆高隆象具有较高的致死率,有望开发成为高效防治大豆高隆象的生防药剂。致谢:
本研究得到四川农业大学郭铭老师、严雳和李倩倩同学的帮助,在此一并致谢!参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
URLPMID:14503588 [本文引用: 1]
DOI:10.1017/S0007485308005798URL [本文引用: 1]
DOI:10.1111/j.1439-0418.2007.01240.xURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/enr.2019.49.issue-1URL [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
URLPMID:29427636 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3852/mycologia.97.1.84URLPMID:16389960 [本文引用: 1]

Beauveria is a globally distributed genus of soil-borne entomopathogenic hyphomycetes of interest as a model system for the study of entomopathogenesis and the biological control of pest insects. Species recognition in Beauveria is difficult due to a lack of taxonomically informative morphology. This has impeded assessment of species diversity in this genus and investigation of their natural history. A gene-genealogical approach was used to investigate molecular phylogenetic diversity of Beauveria and several presumptively related Cordyceps species. Analyses were based on nuclear ribosomal internal transcribed spacer (ITS) and elongation factor 1-alpha (EF1-alpha) sequences for 86 exemplar isolates from diverse geographic origins, habitats and insect hosts. Phylogenetic trees were inferred using maximum parsimony and Bayesian likelihood methods. Six well supported clades within Beauveria, provisionally designated A-F, were resolved in the EF1-alpha and combined gene phylogenies. Beauveria bassiana, a ubiquitous species that is characterized morphologically by globose to subglobose conidia, was determined to be non-monophyletic and consists of two unrelated lineages, clades A and C. Clade A is globally distributed and includes the Asian teleomorph Cordyceps staphylinidaecola and its probable synonym C. bassiana. All isolates contained in Clade C are anamorphic and originate from Europe and North America. Clade B includes isolates of B. brongniartii, a Eurasian species complex characterized by ellipsoidal conidia. Clade D includes B. caledonica and B. vermiconia, which produce cylindrical and comma-shaped conidia, respectively. Clade E, from Asia, includes Beauveria anamorphs and a Cordyceps teleomorph that both produce ellipsoidal conidia. Clade F, the basal branch in the Beauveria phylogeny includes the South American species B. amorpha, which produces cylindrical conidia. Lineage diversity detected within clades A, B and C suggests that prevailing morphological species concepts underestimate species diversity within these groups. Continental endemism of lineages in B. bassiana s.l. (clades A and C) indicates that isolation by distance has been an important factor in the evolutionary diversification of these clades. Permutation tests indicate that host association is essentially random in both B. bassiana s.l. clades A and C, supporting past assumptions that this species is not host specific. In contrast, isolates in clades B and D occurred primarily on coleopteran hosts, although sampling in these clades was insufficient to assess host affliation at lower taxonomic ranks. The phylogenetic placement of Cordyceps staphylinidaecola/bassiana, and C. scarabaeicola within Beauveria corroborates prior reports of these anamorph-teleomorph connections. These results establish a phylogenetic framework for further taxonomic, phylogenetic and comparative biological investigations of Beauveria and their corresponding Cordyceps teleomorphs.
DOI:10.1080/09583157.2018.1564812URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
