 ,中国农业科学院植物保护研究所植物病虫害生物学国家重点实验室,北京 100193
,中国农业科学院植物保护研究所植物病虫害生物学国家重点实验室,北京 100193Identification and Expression Analysis of the Halloween Gene Family in Agasicles hygrophila
LIU YiRan, ZHANG Hong, JIN JiSu, ZHOU ZhongShi, GUO JianYing ,State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193
,State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193通讯作者:
责任编辑: 岳梅
收稿日期:2019-09-27接受日期:2019-10-29网络出版日期:2020-05-16
| 基金资助: |
Received:2019-09-27Accepted:2019-10-29Online:2020-05-16
作者简介 About authors
刘亦然,E-mail:liuyiran0907@163.com。

摘要
关键词:
Abstract
Keywords:
PDF (1351KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
刘亦然, 张虹, 靳继苏, 周忠实, 郭建英. 莲草直胸跳甲Halloween基因家族鉴定及表达分析[J]. 中国农业科学, 2020, 53(10): 2009-2019 doi:10.3864/j.issn.0578-1752.2020.10.008
LIU YiRan, ZHANG Hong, JIN JiSu, ZHOU ZhongShi, GUO JianYing.
0 引言
【研究意义】空心莲子草(Alternanthera philoxeroides)为世界性恶性入侵杂草[1],原产于南美洲,20世纪30年代传入我国[2],对我国农林牧渔业产生了巨大危害[1,3-4]。莲草直胸跳甲(Agasicles hygrophila)隶属鞘翅目叶甲科,是空心莲子草的专食性天敌,其幼虫、成虫取食空心莲子草的叶片及嫩茎,控草效果较为显著[5]。蜕皮激素(ecdysone)参与调控昆虫中多种生命活动,如繁殖、滞育、先天免疫等[6,7]。Halloween基因家族是蜕皮激素合成通路中重要成员[8,9,10],开展莲草直胸跳甲Halloween家族基因研究,将有助于了解蜕皮激素对昆虫的调控机制,为生防天敌的扩繁提供新思路。【前人研究进展】激素调节贯穿于昆虫的整个生活史,昆虫体内的激素包括保幼激素、蜕皮激素和脑激素等;其中,蜕皮激素最早由BUTENANDT等于1954年报道[11],可参与调节昆虫的蜕皮、变态、滞育和繁殖等生理过程[12],具体包括调节昆虫的细胞增殖、分化与凋亡、蜕皮及变态、神经系统的重塑、滞育及免疫应答等多项生命活动[13]。蜕皮激素的主要活性物质为20-羟基蜕皮酮(20E),其在昆虫的幼虫期由前胸腺产生,而在昆虫羽化为成虫后,其体内的蜕皮激素由卵巢合成,参与卵巢的发育与繁殖[14]。研究发现,在烟草天蛾(Manduca sexta)和黑腹果蝇(Drosophila melanogaster)幼虫期蜕皮激素参与启动幼虫不同龄期之间的蜕皮及变态[15];用20E处理结扎的家蚕(Bombyx mori)雌蛹,血淋巴中卵黄原蛋白(Vg)含量明显提高,卵巢的发育速度明显加快[16]。在20E生物合成方面的突破性进展是细胞色素P450超家族编码的Halloween基因家族的发现[17]。如已证实,在黑腹果蝇中Halloween家族基因表达产物经过一系列生化反应生成蜕皮激素;同时,当CYP302A1被敲除后,黑腹果蝇出现蜕皮激素滴度降低的现象[18]。此外,在家蚕化蛹前对其Halloween基因家族成员CYP314A1进行干扰,出现家蚕不能正常化蛹以及雌蛾卵巢发育不正常的现象[19]。在植食性昆虫中,Halloween基因家族主要包括CYP307(spook)、CYP306A1(phantom)、CYP302A1(disembodied)、CYP315A1(shadow)以及CYP314A1(shade)[20];其中,CYP307、CYP306A1、CYP302A1、CYP315A1通过一系列的生化反应参与合成无活性的蜕皮酮,最终由CYP314A1催化合成活性物质20E[20,21]。但不同物种中其体内Halloween基因家族存在一定差异,如瓦螨(Varroa destructor)和烟草天蛾中均只鉴别到3个Halloween基因家族成员[22,23]。莲草直胸跳甲作为空心莲子草的生防天敌,我国于1987年从美国佛罗里达州引进该跳甲并在江苏、云南、四川和湖南等地释放[24]。田间调查发现,该跳甲成虫和幼虫的虫口密度越大,其控草速度越快,控草效果越显著[25]。【本研究切入点】目前,对于昆虫细胞色素P450基因的研究主要集中在其对农药的抗药性以及对不利环境的抵抗等方面,迄今在莲草直胸跳甲中未有关于P450基因参与蜕皮激素合成通路的报道,对其蜕皮激素合成通路中Halloween基因家族的数量、表达模式等尚不清楚。【拟解决的关键问题】田间调查表明,在我国湖南地区,莲草直胸跳甲的种群密度呈季节性波动趋势,夏季数量明显减少[26],这是环境因素对其卵孵化、幼虫发育、蛹羽化和成虫繁殖影响的综合体现。由于蜕皮激素参与调节昆虫的蜕皮、变态、滞育和繁殖等生理过程[14],本研究分析参与蜕皮激素合成的Halloween基因家族在莲草直胸跳甲中的表达模式,解析Halloween基因家族对莲草直胸跳甲种群动态的影响,为进一步应用RNA干扰技术分析其基因功能打下基础,进而为探寻促增莲草直胸跳甲种群数量的方法提供新思路,从而更好地对入侵杂草空心莲子草进行生物防治。1 材料与方法
1.1 供试植物
空心莲子草根茎于2018年6月采自福建省福州市福建省农业科学院植物保护研究所南通中试基地,携带至河北省廊坊市中国农业科学院植物保护研究所廊坊科研中试基地温室,在塑料盒(30 cm×30 cm×30 cm)中用营养土(黑土﹕蛭石=1﹕1)栽培,并采用扦插方式扩大栽种规模。空心莲子草茎秆达4—7节间长时用于试验。1.2 供试昆虫
莲草直胸跳甲成虫于2018年10月采自湖南省长沙市湖南省农业科学院附近,携带至中国农业科学院植物保护研究所廊坊科研中试基地进行室内饲养,饲养温度为(25±1)℃,相对湿度为75%±5%,光周期为12L﹕12D,连续饲养3代后用于试验。1.3 样品采集
为测定Halloween基因在莲草直胸跳甲不同发育阶段的表达量,自产卵第1天起,每隔24 h逐日收集莲草直胸跳甲各个时期的样品,分别包括卵期第0—4天、幼虫第1—13天、蛹期第1—7天和成虫期第1—6、8、10天的雌虫。上述各天数的样品包括2—20个个体,3个生物学重复。为测定Halloween基因在莲草直胸跳甲不同组织的表达量,将雌成虫在1×PBS磷酸缓冲液(pH 7.4)中进行解剖,分别取头、胸、中肠、卵巢以及脂肪体,每份组织从5—15个个体中收集,3个生物学重复。
收集的所有样品立即在液氮中冷冻,-80℃中保存备用。
1.4 总RNA提取及第一链cDNA合成
利用Trizol法提取莲草直胸跳甲不同发育时期以及各组织的总RNA。采用超微量紫外分光光度计Nanophoto meter P330(Implen,Germany)和1%琼脂糖凝胶电泳鉴定RNA的纯度和完整性。参照TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix反转录试剂盒说明书,RNA定量为1 μg,反转录合成第一链cDNA并于-20℃保存备用。1.5 目的片段的扩增
将本实验室前期建立的莲草直胸跳甲转录组数据与NCBI库进行比对,得到莲草直胸跳甲中共有6个Halloween基因家族成员,分别为CYP302A1、CYP306A1、CYP307A1、CYP307A2、CYP314A1、CYP315A1,利用Primer Premier 5.0软件设计,Oligo软件分析引物,并由上海生工生物工程公司合成所需引物。以反转录得到的cDNA为模板,用表1中特异性引物进行PCR扩增。扩增体系:10×PCR buffer 2.5 μL,dNTPs(2.5 mmol·L-1)0.5 μL,引物(10 μmol·μL-1)1 μL,Taq酶0.5 μL,cDNA模板0.5 μL,ddH2O补至25 μL。反应程序:94℃预变性5 min;94℃变性30 s,55℃退火30 s,72℃延伸1 min,34个循环;72℃延伸10 min。产物用1%琼脂糖凝胶电泳检测,切取目的条带进行PCR产物回收,连接至PEAST- T3载体并转化至大肠杆菌感受态细胞,得到阳性克隆后测序。Table 1
表1
表1用于克隆莲草直胸跳甲中Halloween基因的完整或部分ORF引物
Table 1
| 基因 Gene | 正向引物 Forward primer (5′-3′) | 反向引物 Reverse primer (5′-3′) |
|---|---|---|
| AhCYP302A1 | CACAGTTTTGGAAAAATACC | TTATCTTCTAATAAACTC |
| AhCYP306A1 | ATGATGATTACTTATCTAGTGG | CTAACCTGCTTGAAAAGGCA |
| AhCYP307A1 | GCACATACATTTCCACGAGC | TTATCTTTGTGCAAATTT |
| AhCYP307A2 | ATGCTCGCGCTCGTTGTG | TCAAGATCTGGGAGTTAGC |
| AhCYP314A1 | ATGATAGACGAAAGCCTGTAC | CTAAACTCTTTCTCTAA |
| AhCYP315A1 | ATGTTTTTTCACGTAAAACAATT | TTAATCGATTTTACGAAACACC |
新窗口打开|下载CSV
1.6 保守性分析与进化分析
通过在线分析软件Emboss Transeq将上述得到的核酸序列转换为氨基酸序列,后将其编码的氨基酸序列在NCBI中进行保守结构域预测以及Blastp分析,分别找到与Halloween家族基因具有同源性的昆虫Halloween基因序列。使用MEGA6.0软件采用邻接法(neighbor-joining,NJ)进行1 000次重复计算后构建系统进化树。1.7 实时荧光定量PCR(qRT-PCR)检测目的基因的表达水平
根据扩增得到的目的基因设计特异性定量引物,同时选择在莲草直胸跳甲体内表达稳定的β-actin作为内参基因(表2),并以上述提取的不同发育历期、不同组织的总RNA经反转录得到的cDNA为模板,利用qRT-PCR技术检测莲草直胸跳甲Halloween基因家族成员在不同组织以及不同发育阶段的表达变化。选取TransStart Tip Green qPCR SuperMix荧光定量试剂盒进行实时荧光定量。扩增体系:2×TransStart Tip Green qPCR SuperMix 10 μL,Passive Reference Dye(50×)0.5 μL,引物(10 μmol·μL-1)0.5 μL,cDNA 1 μL,补充RNase-free water至20 μL,扩增程序采用两步法。Table 2
表2
表2用于检测莲草直胸跳甲中Halloween基因表达水平所用引物
Table 2
| 基因 Gene | 正向引物 Forward primer (5′-3′) | 反向引物 Reverse primer (5′-3′) |
|---|---|---|
| AhCYP302A1 | GATGAGAGCGATGTAACTGAGG | TCGGGTATGAATTTGTCTGGAG |
| AhCYP306A1 | CGTAAGCCATCCATAGAAG | AGGTCTGATTCGCATAGT |
| AhCYP307A1 | ACTTTGGCGTTTTGTGATTGG | TGGGTTTCTGTCAAGATAACGG |
| AhCYP307A2 | ACTTGCAGAGGACGCG | GGAGGGGAATTGTAGTCC |
| AhCYP314A1 | GACTTCTTGTTCGTTGGTTC | TGTAGATGGTCTCTTCGCTA |
| AhCYP315A1 Actin | TCGGTGGTTCTAACTATAAG AGGTATTGCCGACAGAAT | AGGACAACAGTATCATCTAAT TGCTGGAAGGTGGATAAG |
新窗口打开|下载CSV
1.8 数据分析
利用2-ΔΔCT法对测定出的目的基因的相对表达量进行计算,计算公式:2-ΔΔCT=2- [(CT待测组-CT待测组(内参))-(CT对照组-CT对照组(内参))] [27]。以该基因表达量最低的时期或组织作为对照,测定的基因表达量定为1。采用R version 3.5.1软件进行数据分析,同一基因在不同日龄间的表达量差异采用单因素方差分析(one-way ANOVA)的LSD法。2 结果
2.1 Halloween基因家族系统发育树分析
基于前期得到的转录组数据并结合NCBI分析,在莲草直胸跳甲中共鉴定得到6个Halloween基因家族成员,设计引物(表1)扩增得到其中间片段。为了明确莲草直胸跳甲中Halloween基因家族的进化情况,分别进行了结构域分析和系统进化树分析(图1、图2)。通过分析Halloween家族基因氨基酸序列可以看出其均属于细胞色素P450超家族;AhCYP306A1、AhCYP314A1、AhCYP315A1在5′端存在跨膜区域。由图2中可以看出,Halloween基因家族各基因可以分别与其他物种对应的基因聚为一支,形成6个分支,表明莲草直胸跳甲中Halloween基因在漫长的进化过程中符合P450超家族的特点,即具有一定的保守性;6个Halloween基因分开聚成6支,表明它们可能分别存在不同的进化模式并各自具有不同的生物学功能。图1
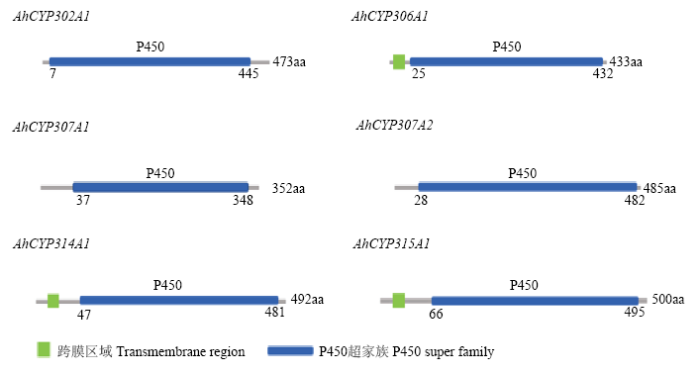 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1莲草直胸跳甲Halloween基因家族的结构域
Fig. 1Domain architecture of A. hygrophila Halloween gene family
图2
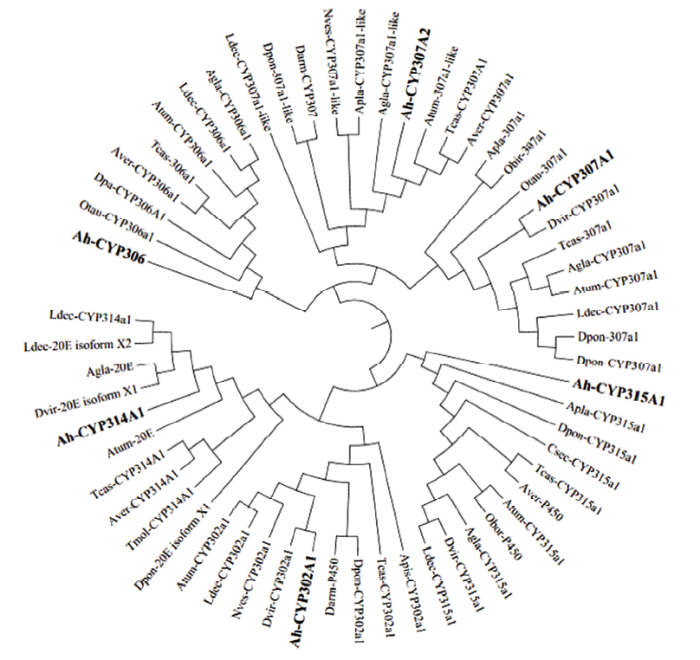 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2基于氨基酸序列构建的Halloween基因家族的系统进化树(邻接法)
Fig. 2Phylogenetic tree of the Halloween gene family based on amino acid sequence (neighbor-joining)
2.2 Halloween基因家族时空表达分析
2.2.1 不同时期Halloween基因家族的表达分析 由图3可知,Halloween基因家族成员在莲草直胸跳甲不同发育阶段的表达量差异较大。其中,AhCYP302A1的mRNA表达量在1日龄卵期较高,蛹期第6、7天最高,在成虫期急剧下降;AhCYP306A1的mRNA表达量呈波动趋势,在幼虫末龄及蛹前期表达量较高,其中以幼虫第10天表达量最高,显著高于其他时期的表达量;AhCYP307A1表达量在卵期第2、3天最高,之后呈波动下降趋势,在成虫期表达量趋于稳定;AhCYP307A2表达量整体呈波动趋势,其中以蛹期第2天表达量最高;AhCYP314A1在产卵初期的表达量显著高于其他时期,在卵开始发育后的整个生活史中,AhCYP314A1的表达量整体呈先波动下降后波动上升的趋势;AhCYP315A1在卵期第0天表达量最高,之后持续低表达直至雌虫羽化第2天,在雌成虫发育期间均高表达。图3
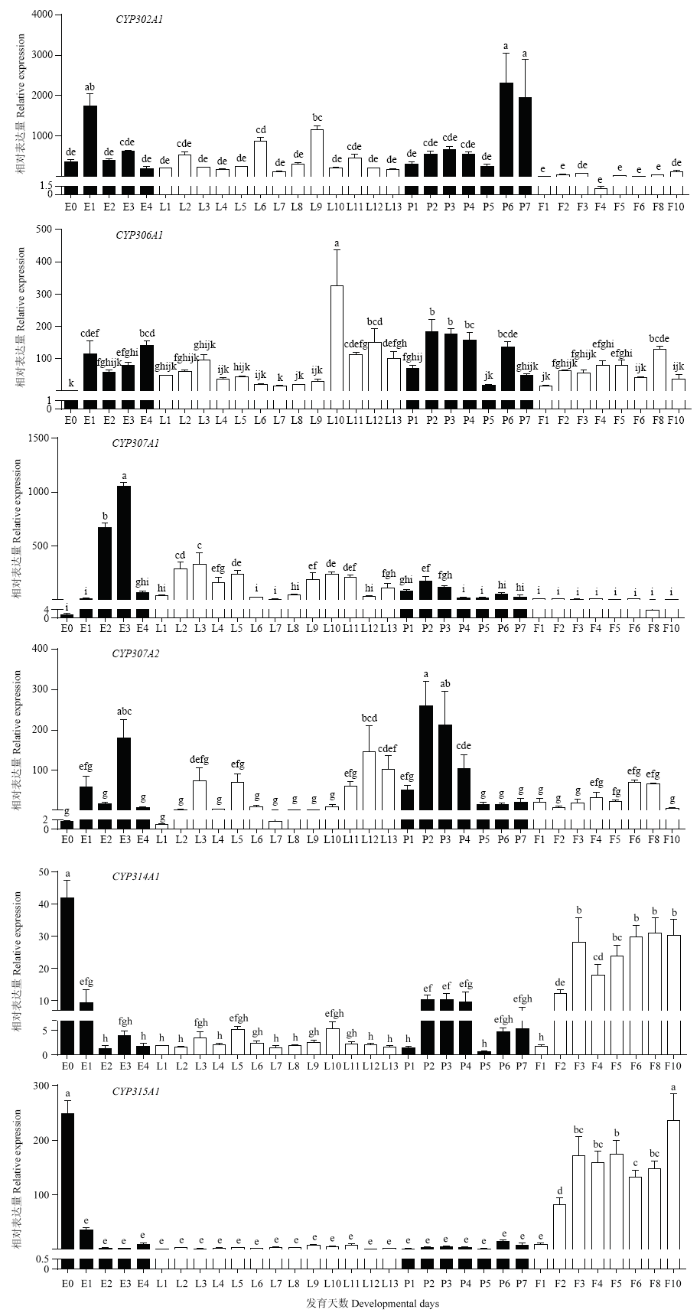 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3莲草直胸跳甲Halloween基因家族在不同发育时期的相对表达量
E:卵Egg;L:幼虫Larva;P:蛹Pupa;F:雌成虫Female adult。图中数据为平均值±标准误,柱上不同字母表示同一基因表达量在不同日龄间差异显著(P<0.05,LSD法) All values are shown as the mean±SE. Different letters on the column indicate that the expression level of the same gene at different ages is significantly different at P<0.05 by LSD test
Fig. 3Relative expression of the Halloween gene family at different developmental stages of A. hygrophila
2.2.2 不同组织Halloween基因家族的表达分析 对Halloween基因家族6个基因在莲草直胸跳甲雌成虫头、胸、中肠、卵巢、脂肪体5种组织中表达量的检测表明(图4),Halloween基因家族成员在中肠中表达量均极低。AhCYP302A1在胸部表达量最高,其次为头部和卵巢,但在这3种组织中的表达量差异不显著。AhCYP306A1在卵巢和脂肪体内高表达,显著高于在头部和中肠的表达量。AhCYP307A1、AhCYP307A2分别在头部和卵巢表达量极高,在其余组织中表达量均较低。AhCYP314A1在卵巢中表达量最高,其次为脂肪体,在头部和中肠中表达量极低。AhCYP315A1在卵巢中表达量最高,在胸部和脂肪体中表达量相近,在中肠表达量最低,仅为在卵巢中表达量的千分之一。
图4
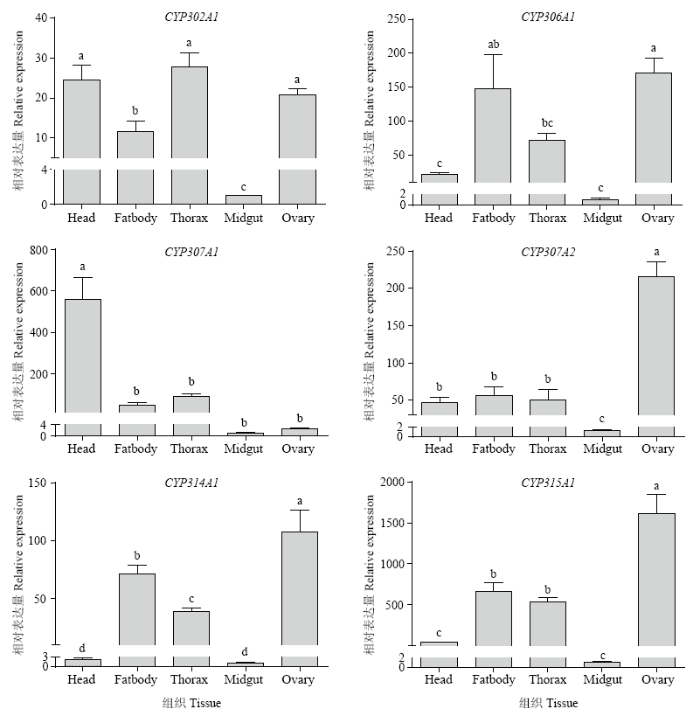 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4莲草直胸跳甲Halloween基因家族在不同组织的相对表达量
Head:头;Fatbody:脂肪体;Thorax:胸;Midgut:中肠;Ovary:卵巢。图中数据为平均值±标准误,柱上不同字母表示同一基因表达量在不同组织间差异显著(P<0.05,LSD法)All values are shown as the mean±SE. Different letters on the column indicate that the expression level of the same gene in different tissues is significantly different at P<0.05 by LSD test
Fig. 4Relative expression of the Halloween gene family in different tissues of A. hygrophila
3 讨论
莲草直胸跳甲是入侵杂草空心莲子草的专一性天敌,其成虫和幼虫均取食叶片,若在同一地点连续释放2—3年,空心莲子草基本可得到控制[28]。目前,在我国湖南长沙等地空心莲子草一年四季均大量发生,而莲草直胸跳甲的种群动态却呈波动趋势。Halloween基因家族作为蜕皮激素合成通路中重要的一支,在昆虫生长发育和繁殖中具有重要作用,因此会对昆虫种群动态产生影响[29]。虽然已在多种昆虫中发现并鉴定Halloween家族基因,但目前尚无有关莲草直胸跳甲中该基因家族的研究报道。本研究利用RT-PCR技术克隆获得莲草直胸跳甲蜕皮激素合成途径Halloween家族6个基因AhCYP302A1、AhCYP306A1、AhCYP307A1、AhCYP307A2、AhCYP314A1、AhCYP315A1的中间片段,其中AhCYP306A1、AhCYP307A1、AhCYP307A2属于细胞色素P450超家族中CYP2集团,AhCYP302A1、AhCYP314A1、AhCYP315A1属于线粒体集团。进化分析发现莲草直胸跳甲中Halloween基因家族具有一定的保守性,表明其在不同昆虫中可能具有相似的功能;但在不同昆虫中Halloween基因家族不同,有些酶在昆虫进化过程中已被消除、复制或发生改变;如CYP307家族中的spot在鳞翅目昆虫以及果蝇科中不存在,但在冈比亚按蚊(Anopheles gambiae)和埃及伊蚊(Aedes aegypti)中检测到此基因[12];此外,在莲草直胸跳甲中发现的CYP307A2,在家蚕以及烟草天蛾中却不存在[30],表明蜕皮激素的合成路径在不同昆虫中存在物种特异性和进化差异。
蜕皮激素可在昆虫整个生活史中表达[31]。本研究采用实时荧光定量PCR技术测定了Halloween基因在莲草直胸跳甲不同虫态和雌成虫不同组织中的表达量。结果发现,Halloween基因在莲草直胸跳甲各虫态均有表达,这与家蚕中的表达模式相似[19],表明Halloween基因家族对莲草直胸跳甲整个生活史均有影响。在莲草直胸跳甲中AhCYP302A1、AhCYP306A1、AhCYP307A2在幼虫以及蛹期表达量相对卵期、成虫期较高,表明三者可能主要参与了莲草直胸跳甲的蜕皮过程;其中,AhCYP306A1、AhCYP307A2在末龄幼虫及化蛹初期的表达量显著上调可能为其蜕皮与变态化蛹作准备。AhCYP307A1、AhCYP314A1、AhCYP315A1在成虫以及卵期表达量相对较高,三者可能主要与莲草直胸跳甲的卵巢发育、卵发育等繁殖过程有关;AhCYP307A1在卵期第2、3天高表达以及AhCYP302A1在卵期第1天高表达的模式,推测其可能参与了莲草直胸跳甲卵期的胚胎发育过程,这与黑腹果蝇中缺陷CYP302A1(Dib)以及CYP307A1(Spo)时胚胎发育不正常的现象相符[18]。莲草直胸跳甲Halloween基因家族中的6个成员在同一发育时期、同一组织中表达存在差异,推测其可能在莲草直胸跳甲中所行使的功能不同。如对白背飞虱(Sogatella furcifera)中若虫Halloween基因时空表达分析表明,CYP307A1(Spo)在其2—4龄均有表达,其中在4龄以胸部表达量最高;给白背飞虱饲喂dsSpo后,若虫发育显著延迟,其中24%的若虫停留在3龄[32]。在不同组织中,莲草直胸跳甲中Halloween基因家族除AhCYP307A1外大多在卵巢中高表达,这与NIWA等报道的类固醇类激素参与调节蜕皮动物成虫阶段的生殖过程(如种系发育、先天发育等)[12]以及成虫蜕皮激素的初级来源为卵巢相符[33,34]。昆虫头部作为感知嗅觉、触觉、听觉的部位,AhCYP307A1在莲草直胸跳甲头部的特异性高表达是否表明其与头部行使这些功能有关还有待进一步验证。同样值得关注的是,在一些非内分泌的组织中,如中肠,也检测到Halloween基因的表达,这些组织是否可以作为原发性或继发性合成蜕皮激素的部位有待进一步研究。
4 结论
克隆并鉴定得到莲草直胸跳甲中6个Halloween基因家族成员,分析发现其均属于P450超家族且具有一定保守性。实时荧光定量PCR结果表明,莲草直胸跳甲6个Halloween基因家族成员的表达在不同发育时期存在差异,但大多在卵巢中高表达;表明其在不同发育时期及不同组织中可能具有不同的功能,根据表达模式推测可能对莲草直胸跳甲的蜕皮与繁殖具有一定影响。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.ibmb.2016.05.003URL [本文引用: 1]
//
[本文引用: 1]
DOI:10.7717/peerj.7428URL [本文引用: 1]
DOI:10.1242/dev.045641URL [本文引用: 1]
DOI:10.1016/j.chemosphere.2019.124676URL [本文引用: 1]
DOI:10.1515/znb-1954-0601URL [本文引用: 1]
DOI:10.1080/09168451.2014.942250URL [本文引用: 3]

Steroid hormones are responsible for the coordinated regulation of many aspects of biological processes in multicellular organisms. Since the last century, many studies have identified and characterized steroidogenic enzymes in vertebrates, including mammals. However, much less is known about invertebrate steroidogenic enzymes. In the last 15 years, a number of steroidogenic enzymes and their functions have been characterized in ecdysozoan animals, especially in the fruit fly Drosophila melanogaster. In this review, we summarize the latest knowledge of enzymes crucial for synthesizing ecdysteroids, the principal insect steroid hormones. We also discuss the functional conservation and diversity of ecdysteroidogenic enzymes in other insects and even non-insect species, such as nematodes, vertebrates, and lower eukaryotes.
[本文引用: 1]
[本文引用: 1]
DOI:10.1146/annurev-ento-020117-043258URL [本文引用: 2]
DOI:10.1016/j.ibmb.2003.06.001URL [本文引用: 1]

Abstract
This short review summarizes our current knowledge about the role of transcription factors regulated by ecdysteroids and juvenile hormone (JH) in larval molting and metamorphosis in the tobacco hornworm, Manduca sexta, and Drosophila melanogaster. We show new evidence that EcR-A/USP-2 and E75A contribute to the down-regulation of MHR3 after the peak of ecdysteroid. Also, there is suggestive evidence that both MHR4 and βFTZ-F1 may regulate the expression of dopa decarboxylase as the ecdysteroid titer declines. We summarize the regulation by JH of the Broad transcription factor that normally appears in the epidermis in the final larval instar and specifies pupal cuticle formation at the metamorphic molt. Premature expression of different Broad isoforms also is shown to cause precocious degeneration of the prothoracic glands as well as to prevent ecdysteroid release during its presence.[本文引用: 1]
[本文引用: 1]
DOI:10.1146/annurev.ento.44.1.507URL [本文引用: 1]
[本文引用: 2]
DOI:10.3864/j.issn.0578-1752.2014.03.019URL [本文引用: 2]

【Objective】Insect metamorphosis is initiated by ecdysone, which is synthesized in prothoracic gland through cytochrome P450 enzymes-catalyzed enzymatic reactions. Silkworm (Bombyx mori) is a silk-producing insect. Interrupting or delaying the pupa-adult transition in silkworm will help to reform the flow of silkworm cocoon-processing and to further improve the quality of silk. The objective of this study is to identify and characterize P450 genes involving in ecdysteroidogenesis in silkworm and to provide candidate genes for genetic controlling of silkworm metamorphosis. 【Method】 Ecdysteroidogenesis-related P450 genes were identified in silkworm and other insects through sequence homology search. Phylogenetic tree of insect ecdysteroidogenesis- related P450 genes was constructed by ClustalW program. The mRNA expression profiles of ecdysteroidogenesis-related P450 genes from silkworm were checked by microarray and RT-PCR analysis. RNAi experiment was used to examine the effects of down- regulation of expression level of ecdysteroidogenesis-related P450 genes on silkworm metamorphosis. 【Result】Four P450 genes involving in ecdysteroidogenesis, including Cyp306a1, Cyp302a1, Cyp315a1, and Cyp314a1, were identified in the silkworm genome. Comparative analysis revealed that each edysteroidogenesis-related P450 gene existed as a single copy in the surveyed insects and all its orthologs in insects were grouped together well in the phylogenetic tree, indicating edysteroidogenesis-related P450 genes and edysteroidogenesis pathway were very conserved during insect evolution. Spatio-temporal expression profiling found that Cyp302a1, Cyp315a1, and Cyp314a1 showed a high expression in the ovary, testis, and head of silkworm larvae and were continually expressed during the late stages of silkworm metamorphosis. Cyp314a1 expression could be highly detected before wandering, pupation, or eclosion, showing a consistency with the stage-specific changes of ecdysone pulses. RNAi of Cyp314a1 resulted in not only larval death before silkworm pupation but also developmental abnormality of adult ovary. Two key genes involving in ecdysone signaling, HR3 and Ftz-f1, were also down-regulated after Cyp314a1 RNAi.【Conclusion】Silkworm P450 genes involving in ecdysteroidogenesis are evolutionarily conserved and down-regulating the expression level of the selected P450 gene interrupts normal pupation, suggesting that ecdysteroidogenesis-related P450 genes might be used in altering genetically the progress of silkworm metamorphosis.
DOI:10.3864/j.issn.0578-1752.2014.03.019URL [本文引用: 2]

【Objective】Insect metamorphosis is initiated by ecdysone, which is synthesized in prothoracic gland through cytochrome P450 enzymes-catalyzed enzymatic reactions. Silkworm (Bombyx mori) is a silk-producing insect. Interrupting or delaying the pupa-adult transition in silkworm will help to reform the flow of silkworm cocoon-processing and to further improve the quality of silk. The objective of this study is to identify and characterize P450 genes involving in ecdysteroidogenesis in silkworm and to provide candidate genes for genetic controlling of silkworm metamorphosis. 【Method】 Ecdysteroidogenesis-related P450 genes were identified in silkworm and other insects through sequence homology search. Phylogenetic tree of insect ecdysteroidogenesis- related P450 genes was constructed by ClustalW program. The mRNA expression profiles of ecdysteroidogenesis-related P450 genes from silkworm were checked by microarray and RT-PCR analysis. RNAi experiment was used to examine the effects of down- regulation of expression level of ecdysteroidogenesis-related P450 genes on silkworm metamorphosis. 【Result】Four P450 genes involving in ecdysteroidogenesis, including Cyp306a1, Cyp302a1, Cyp315a1, and Cyp314a1, were identified in the silkworm genome. Comparative analysis revealed that each edysteroidogenesis-related P450 gene existed as a single copy in the surveyed insects and all its orthologs in insects were grouped together well in the phylogenetic tree, indicating edysteroidogenesis-related P450 genes and edysteroidogenesis pathway were very conserved during insect evolution. Spatio-temporal expression profiling found that Cyp302a1, Cyp315a1, and Cyp314a1 showed a high expression in the ovary, testis, and head of silkworm larvae and were continually expressed during the late stages of silkworm metamorphosis. Cyp314a1 expression could be highly detected before wandering, pupation, or eclosion, showing a consistency with the stage-specific changes of ecdysone pulses. RNAi of Cyp314a1 resulted in not only larval death before silkworm pupation but also developmental abnormality of adult ovary. Two key genes involving in ecdysone signaling, HR3 and Ftz-f1, were also down-regulated after Cyp314a1 RNAi.【Conclusion】Silkworm P450 genes involving in ecdysteroidogenesis are evolutionarily conserved and down-regulating the expression level of the selected P450 gene interrupts normal pupation, suggesting that ecdysteroidogenesis-related P450 genes might be used in altering genetically the progress of silkworm metamorphosis.
DOI:10.1016/j.ibmb.2011.01.005URL [本文引用: 2]

We previously reported preferential expression of genes for ecdysteroid signaling in the mushroom bodies of honeybee workers, suggesting a role of ecdysteroid signaling in regulating honeybee behaviors. The organs that produce ecdysteroids in worker honeybees, however, remain unknown. We show here that the expression of neverland and Non-molting glossy/shroud, which are involved in early steps of ecdysteroid synthesis, was enhanced in the ovary, while the expression of CYP306A1 and CYP302A1, which are involved in later steps of ecdysone synthesis, was enhanced in the brain, and the expression of CYP314A1, which is involved in converting ecdysone into active 20-hydroxyecdysone (20E), was enhanced in the brain, fat body, and ovary. In in vitro organ culture, a significant amount of ecdysteroids was detected in the culture medium of the brain, fat body, and hypopharyngeal glands. The ecdysteroids detected in the culture medium of the fat body were identified as ecdysone and 20E. These findings suggest that, in worker honeybees, cholesterol is converted into intermediate ecdysteroids in the ovary, whereas ecdysone is synthesized and secreted mainly by the brain and converted into 20E in the brain and fat body. (C) 2011 Elsevier Ltd.
DOI:10.1016/j.ibmb.2007.02.012URL [本文引用: 1]

Abstract
The insect molting hormone, 20-hydroxyecdysone (20E), is a major modulator of the developmental processes resulting in molting and metamorphosis. During evolution selective forces have preserved the Halloween genes encoding cytochrome P450 (P450) enzymes that mediate the biosynthesis of 20E. In the present study, we examine the phylogenetic relationships of these P450 genes in holometabolous insects belonging to the orders Hymenoptera, Coleoptera, Lepidoptera and Diptera. The analyzed insect genomes each contains single orthologs of Phantom (CYP306A1), Disembodied (CYP302A1), Shadow (CYP315A1) and Shade (CYP314A1), the terminal hydroxylases. In Drosophila melanogaster, the Halloween gene spook (Cyp307a1) is required for the biosynthesis of 20E, although a function has not yet been identified. Unlike the other Halloween genes, the ancestor of this gene evolved into three paralogs, all in the CYP307 family, through gene duplication. The genomic stability of these paralogs varies among species. Intron–exon structures indicate that D. melanogaster Cyp307a1 is a mRNA-derived paralog of spookier (Cyp307a2), which is the ancestral gene and the closest ortholog of the coleopteran, lepidopteran and mosquito CYP307A subfamily genes. Evolutionary links between the insect Halloween genes and vertebrate steroidogenic P450s suggest that they originated from common ancestors, perhaps destined for steroidogenesis, before the deuterostome–arthropod split. Conservation of putative substrate recognition sites of orthologous Halloween genes indicates selective constraint on these residues to prevent functional divergence. The results suggest that duplications of ancestral P450 genes that acquired novel functions may have been an important mechanism for evolving the ecdysteroidogenic pathway.DOI:10.1111/imb.12155URL [本文引用: 1]
DOI:10.1016/j.ibmb.2005.12.002URL [本文引用: 1]
DOI:10.1016/j.actao.2006.03.003URL [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1006/meth.2001.1262URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0129541URL [本文引用: 1]
DOI:10.1016/j.ydbio.2006.07.023URL [本文引用: 1]
DOI:10.1146/annurev.ento.43.1.545URL [本文引用: 1]
DOI:10.1186/1471-2199-14-19URL [本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0055131URL [本文引用: 1]
