2
Study of Genotyping and Performance in Late-Feathering Qingyuan Partridge Cocks
LI Hua1,2, FANG GuiJun1, HUA GuoHong2, TAN ShuWen1,3, ZHANG ZhengFen2, HONG YuYu1, YU Hui1,2责任编辑: 林鉴非
收稿日期:2017-11-15接受日期:2020-03-11网络出版日期:2020-05-16
| 基金资助: |
Received:2017-11-15Accepted:2020-03-11Online:2020-05-16
作者简介 About authors
李华,E-mail:okhuali@fosu.edu.cn

摘要
关键词:
Abstract
Keywords:
PDF (524KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李华, 方桂军, 华国洪, 谭淑雯, 张正芬, 洪煜宇, 于辉. 清远麻慢羽公鸡的基因分型与生产性能研究[J]. 中国农业科学, 2020, 53(9): 1913-1920 doi:10.3864/j.issn.0578-1752.2020.09.017
LI Hua, FANG GuiJun, HUA GuoHong, TAN ShuWen, ZHANG ZhengFen, HONG YuYu, YU Hui.
0 引言
【研究意义】快慢羽杂合子公鸡的分子鉴别、慢羽鸡缺失体抗病品系组建以及候选基因的甄别对家禽分子育种具有重要理论和应用价值,快慢羽品系生长和羽速差异对生产具有重要意义。【前人研究进展】快慢羽为伴性遗传性状,慢羽鸡主尾羽短于快羽鸡,邱祥聘等把快羽分为R1型(主翼羽长于覆主翼羽5mm 以上)和R2型(主翼羽长于覆主翼羽2 mm 而短于5 mm 以内),慢羽分为微长型(主翼羽长于覆主翼羽在2mm 以内,L1)、倒长型(主翼羽短于覆主翼羽,L2 )、等长型(主翼羽与覆主翼羽等长,L3 )和未出型(主翼羽未长出或主翼羽与覆主翼羽均未长出,L4)[1]。快慢羽初生雏鸡的雌雄鉴别已广泛应用于生产,具有极大的市场价值,国内已广泛开展了快慢羽的表型与生产性能的研究[2],其中大部分研究为不同品种快慢羽品系不同阶段对体重等生产性能有不同的影响[3,4,5];有关快慢羽各亚型对生产性能的影响研究甚少,如尹华贵等研究了120日龄长型、微长型、等长型、短型、未出型5种不同羽型泸州黄羽乌鸡的体重之间并无显著差异[3,4,5,6]。慢羽基因座K与内源性病毒21(ev21)基因紧密连锁,可通过对ev21的插入与否检测区分快慢羽,准确率一般达99% 左右[7,8,9]。对杂合慢羽公鸡的鉴别,大多数育种场目前主要采用测交依据表型鉴别,对其分子鉴别处于探讨阶段,且尚无报道慢羽公鸡以及其亚型分子检测的准确性[10,11,12,13,14]。催乳激素受体基因(PRLR)与精子鞭毛蛋白2(SPEF2)是影响快慢羽的候选基因[15,16],但后续的****研究结论不一,如PRLR在一日龄杏花鸡和文昌鸡的慢羽翅膀皮肤中表达显著高于快羽[15],但此结论未在苏禽绿壳蛋鸡中得到支持[16];同样,SPEF2 在苏禽绿壳蛋慢羽鸡中表达显著高于快羽[16],但使用微阵列法分析认为SPEF2在一日龄杏花鸡翅膀皮肤中不表达[15]。【本研究切入点】慢羽公鸡杂合子基因分型与测交判型比对其准确率,慢羽各亚型、分子分型与生产性能关系,快慢羽表型形成的候选基因和调控机制尚不清晰。【拟解决的关键问题】测交验证慢羽公鸡杂合子分子分型准确率,慢羽各表型、基因型与生产性能的关系;利用荧光定量PCR(RT-PCR)初步筛选快慢羽的候选基因。1 材料与方法
1.1 材料
本试验在广东天农食品有限公司原种场进行,包括核心群慢羽纯系M系和祖代慢羽公鸡,饲养方式按照公司种鸡饲养标准实施;按照《家禽生产性能名词术语和度量统计方法》在2014—2015年期间测定了105日龄的公鸡冠高、体重、胫长、胫围和通管值[17]。选取一日龄快羽型(R2)、慢羽中主翼羽与覆主翼羽均未长出羽毛的未出型(L4)和倒长型(L2)各6只鸡,在佛山科学技术学院通过荧光定量PCR(RT-PCR)分析SPEF2和PRLR的mRNA表达量。1.2 慢羽公鸡基因分型
在翅静脉采集血样,柠檬酸葡萄糖溶液(ACD)抗凝。采用北京艾德来生物科技有限公司提供的小量全血基因组DNA快速提取试剂盒提取DNA后进行PCR扩增,采用IRAQI[7]等设计的引物和程序进行扩增,扩增产物用限制性内切酶(Hae Ⅲ)进行酶切,酶切结果用1.5% 的琼脂糖电泳进行检测。1.3 慢羽候选基因定量分析
采集翅膀皮肤,液氮研磨样品,使用Trizol 法根据说明书提取翅膀皮肤总RNA。使用宝生物工程(大连)有限公司反转录试剂盒PrimeScriptTM RT reagent Kit、荧光定量试剂盒SYBR? Premix Ex TaqTM Ⅱ,分别进行反转录和定量。其中内参β-actin引物为:FGAG AAATTGTGCGTGACATCA,R为CCTGAACCTCTC ATTGCCA,PRLR定量引物为F:GCCCAGACTACAG AACATCA,R:GAGGATCCGAGCTGTTACTT,SPEF2定量引物为F:ACACACCAGAACAGTGAAGC,R:A GGTCTGTAAAGGGCTGAAC,使用ABI 7500定量PCR 仪进行定量,退火温度为57℃。1.4 统计分析
试验数据用Excel进行初步整理后,运用SPSS 18.0软件进行组别间的单因素方差分析的LSD检验,并对两两样本间的百分率进行t检验,基因的定量数据使用2- ΔΔCt方法进行分析。2 结果
2.1 慢羽公鸡酶切分型
清远麻鸡成年公鸡的酶切分型结果表明(图1):纯合子只有一条带(1 450 bp),ev21缺失体有两条带(1 068 bp、382 bp)、杂合子有3条带(1 450 bp、1 068 bp和382 bp)。由表1可知,检测的568只慢羽公鸡中纯合子、缺失体和杂合子分别占总数的8.80%、41.73%和49.47%,其中慢羽纯系中纯合子10.09%、缺失体43.86%和杂合子46.05%;祖代纯合子、缺失体和杂合子分别占比7.94%、40.29%和51.77%。总体来看,缺失体高达41.73%、纯合子与杂合子占比分别为8.8%和49.74%。图1
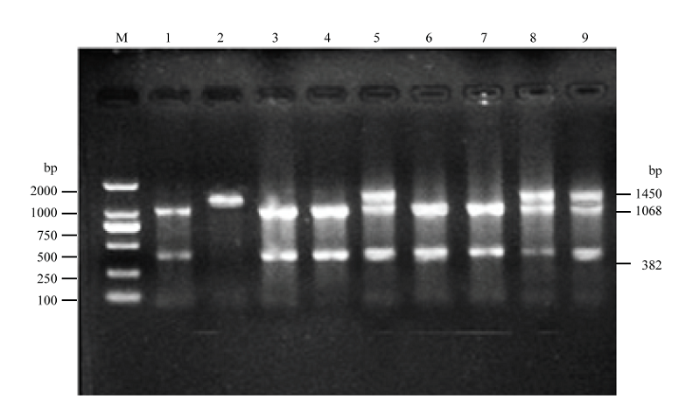 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1慢羽公鸡的酶切分型
M为Marker DL2 000;2为纯合个体;5、8、9为杂合个体;1、3、4、6、7为ev21缺失个体
Fig. 1PCR-RFLP results of late-feathering Qingyuan Partridge cocks
M represents Marker DL2 000; 2 is homozygote, 5, 8 and 9 are heterozygotes, while 1, 3, 4, 6 and 7 are deletants
Table 1
表1
表1慢羽系公鸡分子分型
Table 1
| 群体 Population | 纯合子Homozygote | 缺失体Deletant | 杂合子Heterozygote | 合计Total | |||
|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | No | |
| 纯系Pure line | 23 | 10.09 | 100 | 43.86 | 105 | 46.05 | 228 |
| 祖代Progenitor | 27 | 7.94 | 137 | 40.29 | 176 | 51.77 | 340 |
| 合计Total | 50 | 8.80 | 237 | 41.73 | 281 | 49.47 | 568 |
新窗口打开|下载CSV
2.2 现场测交与基因分型比对
通过性能比较和家系分析,筛选了79只公鸡进行继代并测交,验证分子检测的准确性,核心群慢羽纯系结果表明(表2):出苗883只鸡全部为慢羽,其中微长型11.78%(104只)、倒长型为62.74%(554只)、等长型7.70%(68只)和未出型17.78%(157只)。测交验证基因分型为杂合子的42只公鸡其后代无一表现为快羽,分子分型判型错认为的杂合子实际为纯合子(53.17%),慢羽纯合子和缺失体基因分型准确率为46.83%;缺失体组(ev21-)占比高达41.77%,可以组建抗病品系。合并杂合子组和纯合子组为有ev21插入组(ev21+),与缺失体组(ev21-)进行比较,仅ev21-组的等长型比率显著低于ev21+ 组(0.01<P≤0.05),其余组间差异不显著(P>0.05)。Table 2
表2
表2慢羽公鸡测交的表型与分子分型比较
Table 2
| 基因分型组 Genotyping group | 数量 Count | 出苗Seedlings | 慢羽表型Phenotyes (%) | ||||
|---|---|---|---|---|---|---|---|
| 只 | % | 只 | 微长型L1 | 倒长型L2 | 等长型L3 | 未出型L4 | |
| 纯合子Ⅰ HomozygoteⅠ | 4 | 5.06 | 34 | 5.89 | 70.60 | 11.76 | 11.76 |
| 杂合子Ⅱ HeterozygoteⅡ | 42 | 53.17 | 453 | 13.02 | 61.59 | 9.49 | 15.90 |
| Ⅰ+Ⅱ (ev21+) | 46 | 58.23 | 487 | 12.52 | 62.22 | 9.65b | 15.61 |
| 缺失体(ev21-) Deletant | 33 | 41.77 | 396 | 10.86 | 63.39 | 5.30a | 20.45 |
| 合计Total | 79 | 100 | 883 | 11.78 | 62.74 | 7.70 | 17.78 |
新窗口打开|下载CSV
2.3 慢羽公鸡不同基因分型间生产性能的比较
对慢羽系核心群后备留种成年的慢羽公鸡,分别检测105日龄的平均体重、冠高、胫长和胫围(表3),结果表明ev21- 组通管性能优于ev21+ 组,达到差异极显著水平(P≤0.01),其余指标间差异均不显著(P>0.05)。Table 3
表3
表3105日龄慢羽公鸡生产性能的比较
Table 3
| 基因分型组 Genotyping group | 体重 Weight (g) | 冠高 Crown height (mm) | 胫长 Shank length (mm) | 胫围 Shank circumstance (cm) | 通管值 Feather maturity |
|---|---|---|---|---|---|
| ev21+ n=123 | 1302.6±159.7 | 43.1±5.4 | 81.7±2.8 | 3.9±0.2 | 40.3±15.7c |
| 缺失体Deletant n=94 | 1268.5±129.6 | 42.8±5.6 | 81.7±3.3 | 3.9±0.1 | 47.5±22.5a |
| 合计Total n=217 | 1287.8±144.7 | 42.9±5.7 | 81.7±3.1 | 3.9±0.1 | 43.4±20.6 |
新窗口打开|下载CSV
2.4 候选基因定量表达分析
以一日龄慢羽型(L2+L4)为对照,SPEF2 在快羽R2 型中极显著下调(P≤0.01),而PRLR 表达差异不显著(P>0.05)。在快慢羽各亚型比较中,以未出型(L4)作为对照,PRLR 和SPEF2 在倒长型(L2)中均表达差异不显著(P>0.05),PRLR 和SPEF2 均分别在快羽R2型中显著低表达,分别达到显著(0.01<P≤0.05)和极显著差异水平(P≤0.01);与慢羽L2 型相比,SPEF2 在快羽R2型中极显著低表达(P≤0.01,图2)。图2
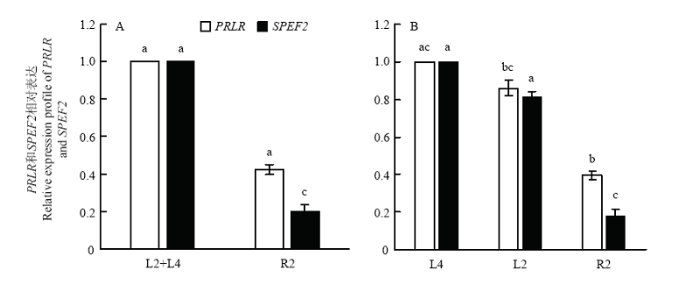 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2RT-PCR法定量分析PRLR与SPEF2的表达
A以慢羽型(L2+L4)为对照;B以未出型(L4)为对照;同基因标字母相同表示差异不显著(P>0.05),标字母相邻差异显著(0.01<P≤0.05),标字母相间差异极显著(P≤0.01)
Fig. 2Expression of PRLR and SPEF2 by RT-PCR
A indicating the control group is (L2+L4); B indicating the control group is L4; Gene expression with the same letter indicate not significant difference(P>0.05); Gene expression with adjacent letters was significant difference (0.01<P≤0.05); Gene expression with interphase letters was highly significant difference (P≤0.01)
3 讨论
3.1 分子检测分型的准确性
本试验所有慢羽系的公鸡已经通过多个世代的现场测交早已纯化[7, 9],但基因分型结果与现场测交选育并不吻合,基因分型鉴定出为杂合子的公鸡,现场测交验证为纯合子,因此,基因分型准确率仅为46.83%,本试验首次通过分子分型结合测交验证种公鸡杂合子的准确率。李竞一等[10]运用相同的方法对大午种禽有限公司商品代慢羽公鸡进行鉴别,准确率达到95.45%,由于只是商品代,并未进行测交验证其杂合性;李珊珊等[13]对麒麟公鸡同理鉴别了高比例的慢羽杂合公鸡,并通过短片段的测序支持分子分型的结果,但未通过测交验证。近期TAKENOUCHI等[12]通过多品种的研究,表明ev21插入并非是导致慢羽的原因。因此,只有探明慢羽形成的真实基因,才能破解理论与实践脱节的难题。清远麻鸡慢羽缺失型高比例支持了课题组前几个世代的报道[11],这为组建高免疫应答的抗病性品系打下了基础。现场选育中,慢羽四种表型一般选留倒长型和等长型,剔除主翼羽和覆主翼羽均未长出的未出型和相对难以区分的微长型。本试验首次将慢羽不同基因分型与慢羽4种亚表型结合深度解析,表明无ev21组插入的慢羽公鸡的后代依然以倒长型最多,未出型和等长型次之,微长型最少,这与朱庆等的结果相一致[18]。ev21+组与ev21-组比较,仅ev21-组的等长型比率显著低于ev21+组(P≤0.05),这表明了ev21插入对等长型羽速生长有一定影响,但其他亚型影响却不显著,究竟是什么分子调控机制造成这种差异,值得进一步研究。ELFERINK等[19]对K座位的分子结构研究表明了K座位中的ev21结合位点不是和K座位连锁,而是ev21插入其中间的非翻译区,本研究所找到的ev21缺失个体,是2个URa所在的区域发生了缺失,还是K座位内部发生了重组,还有待测序进一步研究。李珊珊[13]通过短片段的杂合子与纯合子测序发现有明显差异,但测交结果已经否定了基于此方法建立的杂合子分型的准确性。鸡Z染色体上慢羽基因位点大致在9 607 480—10 607 757 bp处,且9 966 364—10 142 688 bp 基因组的复制导致了慢羽表型,值得进一步从大范围的测序和功能分析去洞悉[20,21,22]。基于近期慢羽的新分型方法的研究,有待于进一步在各快慢羽各亚型中实施和验证[14]。值得注意的是,谢后清等[23]1985年最早报道慢羽微长型细分为主微长型和覆微长型,除了PRLR、dPRLR、SPEF2、dSPEF2互作调控以及与ev21基因插入有关外[15-16,21,24],通过对慢羽的未出型与倒长型分别与快羽的R2型的研究结果表明,快慢羽的调控与常染色体基因、miRNA和LncRNA等调控有关[22,25-26],今后尚需要进一步从胚胎和生后阶段从不同亚型和组学探讨快慢羽差异[27,28,29],设计更为严密的试验阐述羽毛的发育与调控。
3.2 慢羽公鸡不同分型对生产性能的影响
早期的研究主要集中在不同日龄的快慢羽系对生产性能的影响[2],本试验首次揭示了不同慢羽公鸡基因分型对105日龄的体重、冠高、胫长和胫围生产性能无影响(P>0.05),但ev21- 组通管性能优于ev21+ 组,达到差异极显著水平(P≤0.01),说明建立ev21-品系利于通管,尚需要进一步探讨其通管机制的差异[26]。此外,早前报道ev21的存在与禽白血病病毒(ALV)的免疫应答的降低有关,与慢羽鸡感染外源性白血病毒后,其病毒血症的发生率升高,引起产蛋下降和高死亡率有关[27]。因此,育成无ev21 基因的慢羽品系利于免疫和羽毛早熟,意义重大。3.3 候选基因定量表达分析
PRLR和SPEF2位于快慢羽等位基因座K上,被认为是快慢表型形成的候选基因。在慢羽的杏花鸡和文昌鸡中,其PRLR表达显著高于快羽,SPEF2 在快慢羽中表达无差异[15]。本研究却发现初生时R2与L4型中的PRLR基因表达差异显著(0.01<P≤0.05),但以一日龄慢羽型(L2+L4)为对照时,PRLR 快慢羽表达差异却不显著(P>0.05)。表明PRLR参与了羽速发育[15],但在快慢羽不同亚型中由于表达量差异而有所不同,尚需要针对不同快慢羽亚型进行具体分析。SPEF2 在慢羽各亚型中表达极显著高于快羽鸡,这与在苏秦绿壳蛋鸡中结论相似[16]。因此,认为SPEF2和PRLR均可作为快慢羽不同表型的各亚型在一日龄具有显著差异的候选基因。鉴于鸡不同部位的羽毛其进化和发育不一致,且具有胚胎期和生后期的时空表达差异,对快慢羽分子作用机制尚待进一步深入解析[24,28-32]。4 结论
慢羽杂合子公鸡基因分型目前不能替代测交,杂合子公鸡选育判别必须借鉴现场测交,但慢羽公鸡基因分型可为组建无ev21基因抗病品系打下基础。本试验仅ev21-组的等长型比率显著低于ev21+ 组(0.01<P≤0.05),ev21- 组通管性能优于ev21+ 组,达到差异极显著水平(P≤0.01),其余组间差异均不显著(P>0.05)。一日龄的SPEF2和PRLR均可作为鉴别快慢羽表型的候选基因,但其在不同慢羽亚型和生理阶段的作用还需进一步解析。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.3382/ps.0730939URLPMID:7937481 [本文引用: 3]

The dominant sex-linked late-feathering (LF) gene, K, is of commercial importance for sex determination at hatch. Knowledge of the zygosity of sequences associated with K would enable breeders to more efficiently select homozygous grandparent LF males on the maternal side of the standard feather-sex cross because all of their progeny would be LF, whereas only half of the progeny from heterozygous grandparent males would be LF. A specific, polymerase chain reaction (PCR) assay is described that distinguishes White Leghorn K/K males from K/k+ males and obviates the need to raise all LF grandparent males to sexual maturity. Because the Z chromosome of some LF broiler breeders have, in addition to the endogenous virus gene, ev21, the wild type allele, which is termed the unoccupied repeat b (URb), this approach may not be applicable to some broiler lines.
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.3382/ps/pex345URLPMID:29253229 [本文引用: 2]

The late-feathering (LF) gene K on the Z chromosome is an important gene in the chicken industry, which is frequently utilized for the feather sexing, a type of autosexing, of neonatal chicks. The K gene is closely associated with the endogenous ev21 gene from an avian leukosis virus and the incomplete duplication (ID) of prolactin receptor (PRLR) and sperm flagellar protein 2 (SPEF2) genes, and ev21 has been used as a molecular marker to detect LF birds. In the present study, a comprehensive survey for the presence or absence of ev21 and ID across 1,994 birds from 52 chicken breeds, three commercial hybrid groups, and the Red Jungle Fowl revealed that almost all LF breeds have both ev21 and ID. However, only one LF breed (Ingie) has only ID and no ev21. Moreover, this study revealed that almost all early (normal)-feathering (EF) breeds lack both ev21 and ID, but only one breed (White Plymouth Rock) included EF birds with ev21 but no ID. Therefore, regarding LF expression, the results indicated that ID is responsible, but ev21 is not required. Henceforth, ID should be used as a molecular marker to detect LF birds instead of ev21. Because ev21 contains the full genome of an avian leukosis virus, there is a risk of disease development in breeds with this gene. Therefore, the Ingie breed, which has no ev21 at the K locus, represents excellent material for the establishment of new LF stocks.
[本文引用: 3]
[本文引用: 3]
DOI:10.3382/ps/pey231URLPMID:29924355 [本文引用: 2]

In the current study, we sought to determine whether or not the endogenous retroviral ev21 influences feathering type of chickens, and if one mutation locus in the unoccupied repeat (UR) region can be used to predict the corresponding feathering type and genotype. The distribution of ev21 as well as the mutation locus in UR and occupied site (OR) regions was detected in HY-line gray progenitor (HYGP) 4 lines, HY-line brown (HYB) and Taihang chickens (TH). Furthermore, a detection method for the genotype resulting in late feathering (LF) phenotype was developed by double PCR using C line of HYGP, C line of Dawu progenitor, commercial line of HY-line gray (HYG) males, LF males of TH and Bashang long-tail chickens (BS). Results indicated that a product of 7590?bp from the long fragment amplification was observed to be a partial segment of ev21, and was linked with the LF phenotype in HYGP but not in HYB and TH chickens. A total of 2 of 35 males and 10 of 29 females of TH LF chickens were found to be ev21 negative. HaeIII RFLP mutations of 1450?bp of UR, 1440?bp of OR, and 538?bp in the UR and OR common region were analyzed, and genotypic features at the locus correlated with the feathering type phenotype in HYGP, but exhibited no significant effects in HYB and TH chickens. The cut-off of relative intensity of 857 and 1305?bp from the double PCR for distinction between homozygous and heterozygous LF males was 1.37. In conclusion, ev21 and the HaeIII RFLP patterns within the locus in UR cannot be used for prediction of feathering type phenotypes in Chinese heritage chickens. However, the partial duplication of PRLR and SPEF2 were able to predict the LF phenotype. Therefore, the double PCR detecting products of 857 and 1305?bp described herein could be used for the accurate identification of genotypes influencing feathering type.
DOI:10.1007/s11033-012-1449-7URLPMID:22297689 [本文引用: 6]

One duplicated segment on chicken Z chromosome is a causal mutation to the late-feathering phenotype. However, understanding biological process of the late-feathering formation is also of interest to chicken breeding and feather development theory. One hundred and thirty-seven valid single nucleotide polymorphisms (SNPs) from an SNP database were used to perform an association study of the Z chromosome in Xinghua chickens. Two SNPs, which were respectively on 9607480 bp and 10607757 bp, were significantly associated with feathering phenotypes. This result indicated the causal mutation of the late-feathering formation in Xinghua chickens was consistent with the previous report which showed the late-feathering locus ranged 9966364–10142688 bp on Z chromosome. Microarray expressions were implemented for six 1-day-old female Xinghua chicks. Compared to the early-feathering chicks, there were 249 and 83 upregulated and downregulated known genes in the late-feathering chicks. Forty-one genes were expressed in late-feathering chicks, but not in early-feathering ones. At least 14 significantly differentially expressed genes were directly related to keratin. In the region of the sex-linked feathering gene, only prolactin receptor (PRLR) gene was a significantly differentially expressed gene. Expression of PRLR in late-feathering chicks was 1.78-fold as that in early-feathering chicks. Late-feathering Wenchang chicks also had higher expression level of PRLR than early-feathering ones. This study suggested that increasing PRLR expression that resulted from the special variant on chicken Z chromosome caused the late-feathering phenotype.
DOI:10.3382/ps/pew131URLPMID:27081197 [本文引用: 5]

Previous studies suggest that prolactin receptor (Prlr) is a potential causative gene for chicken early- (EF) and late-feathering (LF) phenotypes. In this study, we evaluated candidate genes for this trait and determined the expression of 3 genes, including Prlr, sperm flagellar protein 2 (Spef2), and their fusion gene, in the skins of one-day-old EF and LF chicks using RT-qPCR. Data indicated that Prlr expression in the skin did not show significant difference between EF and LF chicks, suggesting Prlr may not be a suitable candidate gene. In contrast, Spef2 expression in the skin displayed a significant difference between EF and LF chicks (P?&lt;?0.01), suggesting that Spef2 may be a good candidate gene for chicken feathering. Moreover, dPrlr/dSpef2, the fusion gene, was also a good candidate gene as it was expressed only in LF chicks. However, the expression of the fusion gene was much lower than that of Prlr Additionally, using strand-specific primers, we found that the fusion gene was transcribed in 2 directions (one from dPrlr promoter, another from dSpef2 promoter), which could result in the formation of a double strand RNA. In conclusion, both Spef2 and the fusion gene are good candidate genes for chicken feathering, but Prlr is not. The research on the function and regulation of the candidate genes will help elucidate the molecular basis of the chicken feathering trait.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1186/1471-2164-9-391URLPMID:18713476 [本文引用: 1]

One of the loci responsible for feather development in chickens is K. The K allele is partially dominant to the k+ allele and causes a retard in the emergence of flight feathers at hatch. The K locus is sex linked and located on the Z chromosome. Therefore, the locus can be utilized to produce phenotypes that identify the sexes of chicks at hatch. Previous studies on the organization of the K allele concluded the integration of endogenous retrovirus 21 (ev21) into one of two large homologous segments located on the Z chromosome of late feathering chickens. In this study, a detailed molecular analysis of the K locus and a DNA test to distinguish between homozygous and heterozygous late feathering males are presented.
DOI:10.1530/JME-13-0068URLPMID:23940279 [本文引用: 1]

A partial duplication of the prolactin (PRL) receptor gene (designated as dPRLR) has been identified at the late-feathering (LF) K locus on chromosome Z of some chicken strains recently, implying that dPRLR is probably a candidate gene associated with LF development in chickens. However, little is known about the structure, functionality, and spatiotemporal expression of the dPRLR gene in chickens. In this study, using 3'-RACE and RT-PCR, the full-length cDNA of the dPRLR obtained from the kidneys of male Lohmann layer chickens carrying a K allele was cloned. The cloned dPRLR is predicted to encode a membrane-spanning receptor of 683 amino acids, which is nearly identical to the original PRLR, except for its lack of a 149-amino acid C-terminal tail. Using a 5× STAT5-Luciferase reporter system and western blot analysis, we demonstrated that dPRLR expressed in HepG2 cells could be potently activated by chicken PRL and functionally coupled to the intracellular STAT5 signaling pathway, suggesting that dPRLR may function as a novel receptor for PRL. RT-PCR assays revealed that similar to the original PRLR gene, dPRLR mRNA is widely expressed in all embryonic and adult tissues examined including the skin of male Lohmann chickens with a K allele. These findings, together with the expression of PRL mRNA detected in the skin of embryos at embryonic day 20 and 1-week-old chicks, suggest that skin-expressed dPRLR and PRLR, together with plasma and skin-derived PRL, may be involved in the control of the LF development of chicks at hatching. Moreover, the wide tissue expression of dPRLR implies that dPRLR may regulate other physiological processes of chickens carrying the K allele.
[本文引用: 2]
DOI:10.1186/s12864-018-4773-zURLPMID:29801437 [本文引用: 2]

Early feathering and late feathering in chickens are sex-linked phenotypes, which have commercial application in the poultry industry for sexing chicks at hatch and have important impacts on performance traits. However, the genetic mechanism controlling feather development and feathering patterns is unclear. Here, miRNA and mRNA expression profiles in chicken wing skin tissues were analysed through high-throughput transcriptomic sequencing, aiming to understand the biological process of follicle development and the formation of different feathering phenotypes.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.ygcen.2018.12.011URLPMID:30594591 [本文引用: 2]

Chicken early (EF) and late feathering (LF) are sex-linked phenotypes conferred by wild-type k+ and dominant K alleles on chromosome Z, respectively. Besides prolactin (PRL) receptor (PRLR) and sperm flagellar 2 (SPEF2) genes, the K allele contains a fusion gene in which partially duplicated PRLR (dPRLR) and SPEF2 (dSPEF2) genes are linked in a tail-to-tail manner. The causative dPRLR gene encodes a C-terminal truncated receptor. LF chickens have short or no primaries at hatching; however, their feather growth rate is higher than that of EF chickens. This study aimed to elucidate the molecular basis of the K allele's biphasic effect on feather development. By 3'RACE and RT-PCR analyses, we demonstrated that dSPEF2 gene transcription occurred beyond all coding exons of the dPRLR gene on the opposite strand and that dPRLR mRNA was less abundant than PRLR mRNA. In addition, a 5'UTR splice variant (SPV) of PRL receptor mRNAs was increased in LF chickens. In vitro expression analysis of 5'UTR linked to the luciferase reporter gene revealed higher translation efficiency of SPV. RT-qPCR showed that the dPRLR mRNA level was higher in embryos; conversely, SPV was higher in hatched chickens, as was dSPEF2 mRNA. These findings suggest that the K allele inhibits feather development at the fetal stage by expressing dPRLR to attenuate PRLR function and promotes feather growth after hatching by increasing PRLR through dSPEF2 mRNA expression. Increased SPV may cause greater feather growth than that in EF chickens by increasing the availability of PRLR homodimers and enhancing PRL signaling.
DOI:10.1093/nar/gku1338URLPMID:25541195 [本文引用: 1]

When messenger RNA splicing occurs co-transcriptionally, the potential for kinetic control based on transcription dynamics is widely recognized. Indeed, perturbation studies have reported that when transcription kinetics are perturbed genetically or pharmacologically splice patterns may change. However, whether kinetic control is contributing to the control of splicing within the normal range of physiological conditions remains unknown. We examined if the kinetic determinants for co-transcriptional splicing (CTS) might be reflected in the structure and expression patterns of the genome and epigenome. To identify and then quantitatively relate multiple, simultaneous CTS determinants, we constructed a scalable mathematical model of the kinetic interplay of RNA synthesis and CTS and parameterized it with diverse next generation sequencing (NGS) data. We thus found a variety of CTS determinants encoded in vertebrate genomes and epigenomes, and that these combine variously for different groups of genes such as housekeeping versus regulated genes. Together, our findings indicate that the kinetic basis of splicing is functionally and physiologically relevant, and may meaningfully inform the analysis of genomic and epigenomic data to provide insights that are missed when relying on statistical approaches alone.
DOI:10.1242/dev.162388URLPMID:30327322 [本文引用: 2]

Long non-coding RNAs (lncRNAs) are non-protein coding transcripts that are involved in a broad range of biological processes. Here, we examine the functional role of lncRNAs in feather regeneration. RNA-seq profiling of the regenerating feather blastema revealed that Wnt signaling is among the most active pathways during feather regeneration, with Wnt ligands and their inhibitors showing distinct expression patterns. Co-expression analysis identified hundreds of lncRNAs with similar expression patterns to either the Wnt ligands (the Lwnt group) or their downstream target genes (the Twnt group). Among these, we randomly picked two lncRNAs in the Lwnt group and three lncRNAs in the Twnt group to validate their expression and function. Members in the Twnt group regulated feather regeneration and axis formation, whereas members in the Lwnt group showed no obvious phenotype. Further analysis confirmed that the three Twnt group members inhibit Wnt signal transduction and, at the same time, are downstream target genes of this pathway. Our results suggest that the feather regeneration model can be utilized to systematically annotate the functions of lncRNAs in the chicken genome.
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.3864/j.issn.0578-1752.2015.04.20URL [本文引用: 1]

【Objective】The Objective of this study is to analyzes the features and curve process of frizzled feather, the development of frizzled feather follice, sequence features and expression rules of candidate gene KRT75 in frizzled feather follicle development. 【Method】 The frizzled feathers of 360 frizzled chicks from 1 to 300 days were observedto know the curve process, and the microstructure of rachis, barbules, anf hooks between frizzled and contour feather were compared. The development of feather follicle between frizzled feather and contour feather at 8th in embryonic period was compared by Microscopic section technology. The total RNA from the embryonic 13 day skin of frizzled feather and contour feather was extracted, respectively, reverse transcripted cDNA,connected with the PMD-18T vector,the products were transformed into DH5α competent cells, coated plates, monoclonal colony was selected for PCR, and the positive plasmid was chosen for sequencing. In addition, 15 frizzled and contour feather chicks at 300 days were randomly selected to collect vein blood under the wing, blood genome pillar extraction kit was used to extract DNA, the PCR products were also sequenced after purification, and the DNAMAN software was used to analyze genetic characteristics of KRT75 gene. The total RNA was also extracted from embryonic 8 to 17 day skin of frizzled feather and contour feather; respectively, reverse transcripted cDNA, and the SYBR kit was used to analyze KRT75 gene expression during the formation of feather follicle. 【Result】 The down feather appeared disorder and curved in newborn frizzle chicks, the largest curvature of feather was observed at 6 weeks. The frizzled feather rachis curved outward, barbules could not hooked together to form a closed feather; the development of frizzled feather follicle and contour feather follicle was both from E8 to E16, there were some differences in the medulla and barb ridge between frizzled feather and normal one from E12 to E15, in the E12 to E14 day, the medulla of frizzled feather were bigger than contour feather, but the barbule plates were smaller than contour feather. The CDS of KRT75 gene was 1 569 bp both in frizzled feather and contour feather chickens, no 69 bp deletion mutation was detected in CDS region of frizzled chicken KRT75 gene. Three SNPs ( 954bp: T>C; 967bp: T>C; 978bp: C>T) were found compared frizzled chicken with Princess chicken. The expression of KRT75 was significantly different between frizzled feather and contour feather, KRT75 was low expression in contour feather follice but was highly expressed in frizzled feather follicle from E9 to E17, especially in E9,E10 and E12 to E15, the KRT75 of frizzled feather was highly significant than contour feather(P<0.01). 【Conclusion】 Frizzled feather of KiRin Chicken was not caused by 69 bp detection mutation of KRT75 gene. The three SNPs (955bp: T>C; 967bp: T>C; 978bp: C>T) could be used as molecular markers in distinguishing frizzled and contour feathers.
DOI:10.3864/j.issn.0578-1752.2015.04.20URL [本文引用: 1]

【Objective】The Objective of this study is to analyzes the features and curve process of frizzled feather, the development of frizzled feather follice, sequence features and expression rules of candidate gene KRT75 in frizzled feather follicle development. 【Method】 The frizzled feathers of 360 frizzled chicks from 1 to 300 days were observedto know the curve process, and the microstructure of rachis, barbules, anf hooks between frizzled and contour feather were compared. The development of feather follicle between frizzled feather and contour feather at 8th in embryonic period was compared by Microscopic section technology. The total RNA from the embryonic 13 day skin of frizzled feather and contour feather was extracted, respectively, reverse transcripted cDNA,connected with the PMD-18T vector,the products were transformed into DH5α competent cells, coated plates, monoclonal colony was selected for PCR, and the positive plasmid was chosen for sequencing. In addition, 15 frizzled and contour feather chicks at 300 days were randomly selected to collect vein blood under the wing, blood genome pillar extraction kit was used to extract DNA, the PCR products were also sequenced after purification, and the DNAMAN software was used to analyze genetic characteristics of KRT75 gene. The total RNA was also extracted from embryonic 8 to 17 day skin of frizzled feather and contour feather; respectively, reverse transcripted cDNA, and the SYBR kit was used to analyze KRT75 gene expression during the formation of feather follicle. 【Result】 The down feather appeared disorder and curved in newborn frizzle chicks, the largest curvature of feather was observed at 6 weeks. The frizzled feather rachis curved outward, barbules could not hooked together to form a closed feather; the development of frizzled feather follicle and contour feather follicle was both from E8 to E16, there were some differences in the medulla and barb ridge between frizzled feather and normal one from E12 to E15, in the E12 to E14 day, the medulla of frizzled feather were bigger than contour feather, but the barbule plates were smaller than contour feather. The CDS of KRT75 gene was 1 569 bp both in frizzled feather and contour feather chickens, no 69 bp deletion mutation was detected in CDS region of frizzled chicken KRT75 gene. Three SNPs ( 954bp: T>C; 967bp: T>C; 978bp: C>T) were found compared frizzled chicken with Princess chicken. The expression of KRT75 was significantly different between frizzled feather and contour feather, KRT75 was low expression in contour feather follice but was highly expressed in frizzled feather follicle from E9 to E17, especially in E9,E10 and E12 to E15, the KRT75 of frizzled feather was highly significant than contour feather(P<0.01). 【Conclusion】 Frizzled feather of KiRin Chicken was not caused by 69 bp detection mutation of KRT75 gene. The three SNPs (955bp: T>C; 967bp: T>C; 978bp: C>T) could be used as molecular markers in distinguishing frizzled and contour feathers.
DOI:10.1016/j.ceb.2006.10.009URLPMID:17049829

The development and regeneration of feathers have gained much attention recently because of progress in the following areas. First, pattern formation. The exquisite spatial arrangement provides a simple model for decoding the rules of morphogenesis. Second, stem cell biology. In every molting, a few stem cells have to rebuild the entire epithelial organ, providing much to learn on how to regenerate an organ physiologically. Third, evolution and development ('Evo-Devo'). The discovery of feathered dinosaur fossils in China prompted enthusiastic inquiries about the origin and evolution of feathers. Progress has been made in elucidating feather morphogenesis in five successive phases: macro-patterning, micro-patterning, intra-bud morphogenesis, follicle morphogenesis and regenerative cycling.
DOI:10.1016/j.gene.2016.06.027URLPMID:27320726

MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression at the post-transcriptional level. Previous studies have shown that miRNA regulation contributes to a diverse set of processes including cellular differentiation and morphogenesis which leads to the creation of different cell types in multicellular organisms and is thus key to animal development. Feathers are one of the most distinctive features of extant birds and are important for multiple functions including flight, thermal regulation, and sexual selection. However, the role of miRNAs in feather development has been woefully understudied despite the identification of cell signaling pathways, cell adhesion molecules and structural genes involved in feather development. In this study, we performed a microarray experiment comparing the expression of miRNAs and mRNAs among three embryonic stages of development and two tissues (scutate scale and feather) of the chicken. We combined this expression data with miRNA target prediction tools and a curated list of feather related genes to produce a set of 19 miRNA-mRNA duplexes. These targeted mRNAs have been previously identified as important cell signaling and cell adhesion genes as well as structural genes involved in feather and scale morphogenesis. Interestingly, the miRNA target site of the cell signaling pathway gene, Aldehyde Dehydrogenase 1 Family, Member A3 (ALDH1A3), is unique to birds indicating a novel role in Aves. The identified miRNA target site of the cell adhesion gene, Tenascin C (TNC), is only found in specific chicken TNC splice variants that are differentially expressed in developing scutate scale and feather tissue indicating an important role of miRNA regulation in epidermal differentiation. Additionally, we found that β-keratins, a major structural component of avian and reptilian epidermal appendages, are targeted by multiple miRNA genes. In conclusion, our work provides quantitative expression data on miRNAs and mRNAs during feather and scale development and has produced a highly diverse, but manageable list of miRNA-mRNA duplexes for future validation experiments.
URLPMID:10331970 [本文引用: 1]

How do vertebrate epithelial appendages form from the flat epithelia? Following the formation of feather placodes, the previously radially symmetrical primordia become anterior-posterior (A-P) asymmetrical and develop a proximo-distal (P-D) axis. Analysis of the molecular heterogeneity revealed a surprising parallel of molecular profiles in the A-P feather buds and the ventral-dorsal (V-D) Drosophila appendage imaginal discs. The functional significance was tested with an in vitro feather reconstitution model. Wnt-7a expression initiated all over the feather tract epithelium, intensifying as it became restricted first to the primordia domain, then to an accentuated ring pattern within the primordia border, and finally to the posterior bud. In contrast, sonic hedgehog expression was induced later as a dot within the primordia. RCAS was used to overexpress Wnt-7a in reconstituted feather explants derived from stage 29 dorsal skin to further test its function in feather formation. Control skin formed normal elongated, slender buds with A-P orientation, but Wnt-7a overexpression led to plateau-like skin appendages lacking an A-P axis. Feathers in the Wnt-7a overexpressing skin also had inhibited elongation of the P-D axes. This was not due to a lack of cell proliferation, which actually was increased although randomly distributed. While morphogenesis was perturbed, differentiation proceeded as indicated by the formation of barb ridges. Wnt-7a buds have reduced expression of anterior (Tenascin) bud markers. Middle (Notch-1) and posterior bud markers including Delta-1 and Serrate-1 were diffusely expressed. The results showed that ectopic Wnt-7a expression enhanced properties characteristic of the middle and posterior feather buds and suggest that P-D elongation of vertebrate skin appendages requires balanced interactions between the anterior and posterior buds.
