 ,1, 杨晶文1, 沈建国
,1, 杨晶文1, 沈建国 ,2, 蔡伟3, 高芳銮
,2, 蔡伟3, 高芳銮 ,1
,1Multi-Gene-Based PCR Detection and Identification of Chilli veinal mottle virus
YANG HongKai ,1, YANG JingWen1, SHEN JianGuo
,1, YANG JingWen1, SHEN JianGuo ,2, CAI Wei3, GAO FangLuan
,2, CAI Wei3, GAO FangLuan ,1
,1通讯作者:
责任编辑: 岳梅
收稿日期:2019-10-27接受日期:2019-11-25网络出版日期:2020-08-16
| 基金资助: |
Received:2019-10-27Accepted:2019-11-25Online:2020-08-16
作者简介 About authors
杨宏凯,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1816KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
杨宏凯, 杨晶文, 沈建国, 蔡伟, 高芳銮. 辣椒脉斑驳病毒的多基因联合检测与鉴定[J]. 中国农业科学, 2020, 53(16): 3412-3420 doi:10.3864/j.issn.0578-1752.2020.16.018
YANG HongKai, YANG JingWen, SHEN JianGuo, CAI Wei, GAO FangLuan.
0 引言
【研究意义】辣椒(Capsicum annuum)属茄科、辣椒属一年或有限多年生草本植物,在世界范围内广泛种植。在我国,辣椒种植面积高达140万公顷以上,约占我国所有蔬菜种植面积的10%[1]。病毒病是辣椒作物上的一类重要病害,严重影响其品质和产量,目前世界各地陆续报道的辣椒病毒超过70种[2],而我国发现至少有37种[3],其中辣椒脉斑驳病毒(Chilli veinal mottle virus,ChiVMV)是辣椒上重要病毒之一,其传播速度快且危害大,侵染辣椒后能造成严重的经济损失。ChiVMV是一种正义单链RNA病毒,属马铃薯Y病毒科(Potyviridae)马铃薯Y病毒属(Potyvirus)成员,其基因组全长约9 711个核苷酸[4],粒体形态呈弯曲线状[5]。该病毒最早于1979年在马来西亚被报道[6],在我国最早发现于海南的黄灯笼椒上,随后在陕西、云南、福建、湖南等地区被发现并报道[7,8,9,10]。ChiVMV侵染辣椒的典型症状为叶脉呈暗绿色条纹,叶片皱缩、畸形,植株矮小,结果前落花落果,仅留下少量杂色畸形果[11]。该病毒的自然寄主主要为辣椒、番茄等茄科作物[12],自然条件下主要依靠蚜虫进行非持久性传播。此外,ChiVMV还可以通过机械传播和汁液传播,但不能通过种子传播。由于该病毒常与其他病毒复合侵染[13,14],且不同病毒侵染后症状也较为相似,因此亟需建立一种可靠、高效的检测鉴定方法。【前人研究进展】近年来,国内外关于ChiVMV的研究报道较多,且已有多种成熟的技术用于该病毒的检测与鉴定。RAVI等[5]利用电镜对提纯后的病毒进行观察,首次报道了该病毒粒体形态;SIRIWONG等[15]通过对该病毒泰国分离物的血清学研究,发现其与ChiVMV台湾分离物抗血清存在强烈的阳性反应;MOURY等[16]根据不同辣椒病毒的核内含体蛋白b(nuclear inclusion body protein,NIb)及外壳蛋白(coat protein,CP)基因序列相互比较并设计引物,通过RT-PCR的特异性片段区分了ChiVMV与其他辣椒病毒;唐前君等[17]通过建立能够同时检测包含ChiVMV等4种辣椒病毒的四重RT-PCR检测体系,提高了辣椒病毒病复合侵染的检测效率;汤亚飞等[18]依据CP设计了能检测ChiVMV的RT-LAMP引物,建立了ChiVMV的RT-LAMP检测体系,后又利用小RNA深度测序及RT-PCR的方法鉴定了包括ChiVMV在内侵染广东省辣椒的8种病毒[3]。由于马铃薯Y病毒属的不同病毒之间可能存在非常高的亲缘关系,无法实现ChiVMV的快速、准确检测与鉴定,如ChiVMV与甜椒脉斑驳病毒(Pepper veinal mottle virus,PVMV)之间在系统发育上亲缘关系非常近,且两者CP的C末端有一段长204个氨基酸的保守区,序列一致性超过90.2%[19],应用传统的血清学容易产生交叉反应。多基因联合检测是一种特殊的多重RT-PCR扩增方法[20],该方法通过选取两个或多个靶基因,分别设计引物,通过反应体系的构建、优化从而建立起针对两个或多个靶标基因的快速检测体系,多基因联合PCR检测相比于常规PCR具有更高的准确性和可靠性。多基因联合检测的方法现已广泛用于病原菌的快速检测与鉴定,谯天敏等[21]利用factor 1-α (tef1)和β-tubulin两个保守基因,建立了水稻叶鞘网斑病的双基因联合PCR检测技术;刘真真等[22]应用mecA、femB联合PCR扩增法提高了耐甲氧西林金黄色葡萄球菌(MRSA)的检出效率。在植物病毒的检测中,相对保守的CP是最为常用的分子标记,而ADAMS等[23]发现马铃薯Y病毒属的CI相对于CP更为保守,因此也可以用于区分不同病毒种。【本研究切入点】目前,尚未有多基因联合检测的方法应用于检测ChiVMV的研究报道。本研究针对ChiVMV已报道序列的CI与CP设计、筛选引物,建立ChiVMV的多基因检测体系。【拟解决的关键问题】通过设计、筛选引物,对建立的多基因联合检测体系进行反应条件和参数的优化,以提高ChiVMV的检测效率和准确率,为辣椒生产上以及口岸检疫中ChiVMV的快速、准确检测提供技术支持。1 材料与方法
1.1 材料
供试样品为2016年3月福建口岸截获的印度进境辣椒、2019年3月福州郊区田间采集的辣椒样品和2019年4月福清地区田间采集的辣椒样品,均由福州海关技术中心保存。同时,供试的辣椒环斑病毒(Chilli ringspot virus,ChiRSV)、黄瓜花叶病毒(Cucumber mosaic virus,CMV)、辣椒脉黄化病毒(Pepper vein yellows virus,PeVYV)、马铃薯Y病毒(Potato virus Y,PVY)病毒阳性样品均为福州海关技术中心保存。1.2 方法
1.2.1 引物设计 根据GenBank已登录的ChiVMV的CI和CP基因序列,应用Primer Premier 5设计用于RT-PCR检测以及多基因联合检测所需的引物(表1),引物由上海铂尚生物技术有限公司合成。Table 1
表1
表1本研究中辣椒样品病毒检测所用引物
Table 1
| 基因 Gene | 上下游引物序列 Upper and lower primer sequences (5′-3′) | 长度 Length (nt) | 退火温度 Tm (℃) | 目的片段 Expected size (bp) |
|---|---|---|---|---|
| CP | CP861-F: GCAGGAGAGAGTGTTGATGC CP861-R: CAATCCTCGAACGCCCAGCAG CP337-F: ACACCTTCTTGATTATGCTCC CP337-R: ATAAGGCTTCTCAGAATTGCG | 20 21 21 21 | 59.9 63.9 56.1 56.1 | 861a 337b |
| CI | CI655-F: ACAGCRCCAGATGCAAAATC | 20 | 58.0 | 655 |
| CI655-R: CCTGTGCCYTGYGCATCMA | 19 | 54.1 |
新窗口打开|下载CSV
1.2.2 印度进境辣椒样品的血清学和常规RT-PCR检测 每个辣椒样品称取0.1 g,参照病毒试剂盒说明书进行DAS-ELISA检测,用多功能酶标仪测定其405 nm下的光密度值(OD),当样品OD405 nm与阴性对照OD405 nm比值>2时,即判定为阳性。其中,设携带ChiVMV的样品材料为阳性对照,健康辣椒为阴性对照,提取缓冲液为空白对照。
采用Trizol 试剂法提取侵染ChiVMV的辣椒阳性样品,提取方法参考试剂盒说明书进行。取3 μL总RNA,加入1 μL随机引物(Random Primer)按照操作说明书进行反转录获得cDNA。PCR扩增采用25 μL反应体系:cDNA 2 μL、10 μmol·L-1 CP861-F/CP861-R上下游引物各1 μL、2×PCR Master Mix 12.5 μL、ddH2O 8.5 μL。PCR反应条件:94℃ 3 min;94℃ 30 s,55℃ 45 s,72℃ 1 min,共35个循环,72℃延伸10 min。反应结束后,取PCR产物5 μL用1.5%琼脂糖凝胶电泳进行检测。
1.2.3 多基因联合检测体系的建立及其优化 以含ChiVMV阳性样品cDNA为模板,取相同浓度的两个引物对 CP337-F/CP337-R、CI655-F/CI655-R进行反应,初步建立基于ChiVMV CP与CI两个基因的多基因联合检测体系。25 μL总反应体系:cDNA 2 μL、10 μmol·L-1两个引物对各1 μL、2×PCR Master Mix 12.5 μL、ddH2O 6.5 μL。反应条件:94℃ 3 min;94℃ 30 s,50℃ 45 s,72℃ 1 min,共35个循环,72℃延伸10 min。取PCR产物5 μL用1.5%琼脂糖凝胶电泳进行检测,观察凝胶电泳结果。
根据凝胶电泳结果对反应体系进行部分优化,根据两对引物的Tm值,对反应条件的退火温度进行优化,设置46、48、50、52、54、56℃共6个温度梯度,根据凝胶电泳结果,选取最优的退火温度。
在优化退火温度反应条件的基础上,为保证两对引物扩增条带亮度、效果基本一致,适当调整两对引物的用量,设置9个不同的引物用量组合(表2),根据凝胶电泳结果,寻找最优的两对引物用量组合,建立多基因联合检测体系。
Table 2
表2
表2不同梯度组合及引物用量
Table 2
| 引物对 Primer pair | 不同引物用量梯度 Different gradient combinations (μL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
| CP337-F/CP337-R | 1.5 | 1.375 | 1.25 | 1.125 | 1 | 0.875 | 0.75 | 0.625 | 0.5 |
| CI655-F/CI655-R | 0.5 | 0.625 | 0.75 | 0.875 | 1 | 1.125 | 1.25 | 1.375 | 1.5 |
新窗口打开|下载CSV
1.2.4 多基因联合检测特异性及其灵敏度 分别以ChiVMV、ChiRSV、CMV、PeVYV、PVY病毒阳性样品为材料,提取RNA后按照建立的多基因联合检测方法进行检测,同时采用单个基因进行常规RT-PCR检测,测定多基因联合检测方法的特异性。
将反转录后辣椒样品cDNA按10倍梯度稀释,原溶液依次稀释至10-1、10-2、10-3、10-4、10-5、10-6、10-7,按照建立的多基因联合检测方法进行检测,同时采用单个基因进行常规RT-PCR灵敏度检测,两者进行对比,以测定多基因联合检测方法的灵敏度。
1.2.5 多基因联合检测的实际应用 从福州郊区及福清地区田间采集疑似病毒感染的辣椒样品,利用建立的多基因联合检测方法对其是否携带ChiVMV进行检测,并与常规RT-PCR检测结果进行比较,验证ChiVMV多基因联合检测体系在实际生产中的应用效果。
2 结果
2.1 印度进境辣椒样品的常规RT-PCR检测
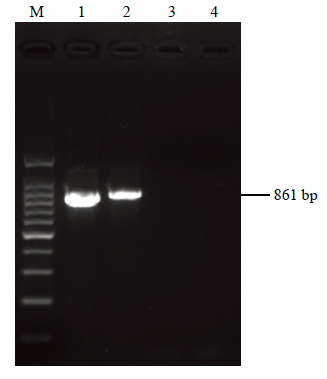
利用ChiVMV特异性引物(CP861-F/CP861-R)对血清学检测到ChiVMV的阳性样品进行常规RT-PCR检测,扩增到大小约861 bp的目的片段(图1),与预期相符,而阴性对照和空白对照中均未扩增出目的片段。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1印度进境辣椒常规RT-PCR扩增
M:DNA分子量标准;1:供试辣椒样品CP PCR产物;2—4:阳性对照、阴性对照和空白对照
Fig. 1Conventional RT-PCR amplification of chilli from India
Marker DNA (100 bp);1:PCR product of CP of ChiVMV isolate from India;2—4:Positive control (with known ChiVMV infected plant), negative control 1 (healthy plant) and negative control 2 (water), respectively
2.2 多基因联合检测体系的建立及其优化
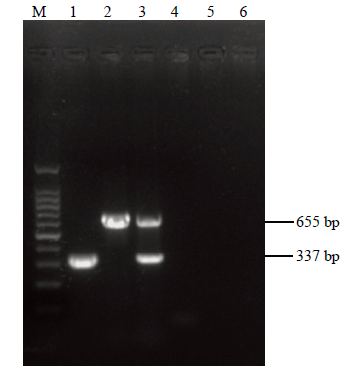
利用CP337-F/CP337-R、CI655-F/CI655-R两对引物分别进行RT-PCR扩增及多基因联合检测,结果显示多基因联合检测体系扩增出与预期大小一致的CP及CI两个片段,大小分别约为337和655 bp,与两个基因单独扩增效果一致,且无非特异性扩增(图2)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2多基因联合检测RT-PCR结果
M:DNA分子量标准;1:CP单独扩增产物RT-PCR;2:CI单独扩增产物RT-PCR;3:多基因联合检测RT-PCR;4:单独扩增CP的阴性对照;5:单独扩增CI的阴性对照;6:多基因联合检测的阴性对照
Fig. 2The results of RT-PCR by multi-gene-based PCR detection
Marker DNA (100 bp);1:amplification of CP;2:amplification of CI;3:amplifications by multi-gene-based PCR detection;4:Negative control for amplification of CP (healthy plant);5:Negative control for amplification of CI (healthy plant);6:Negative control by multi-gene-based PCR detection (healthy plant)
不同退火温度梯度下扩增结果表明,在6个温度梯度下均能获得清晰且特异的目的条带,其中50℃时相对较好(图3)。针对两对引物设置不同的用量组合,结果显示T8组合效果最佳,即CP337-F/CP337-R用量各 0.625 μL、CI655F/CI655R用量各1.375 μL时,扩增出两核酸片段亮度、大小基本一致(图4)。因此,该反应体系优化的各参数最终确定为cDNA 2 μL、CP337-F/CP337-R各0.625 μL(10 μmol·L-1)、CI655-F/CI655R 各1.375 μL(10 μmol·L-1)、2×PCR Master Mix 12.5 μL、ddH2O 6.5 μL,反应条件为94℃ 3 min;94℃ 30 s,50℃ 45 s,72℃ 1 min,共35个循环,72℃延伸10 min。
图3
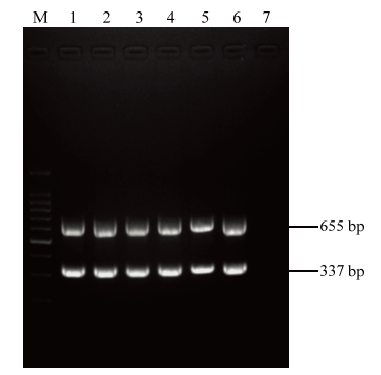 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3多基因联合检测体系退火温度的优化
M:DNA分子量标准;1—6:退火温度分别为46、48、50、52、54和56℃;7:阴性对照
Fig. 3Optimization of the annealing temperature in multi- gene-based PCR detection
Marker DNA (100 bp);1—6:Annealing temperature of 46, 48, 50, 52, 54 and 56℃, respectively;7:Negative control (healthy plant)
图4
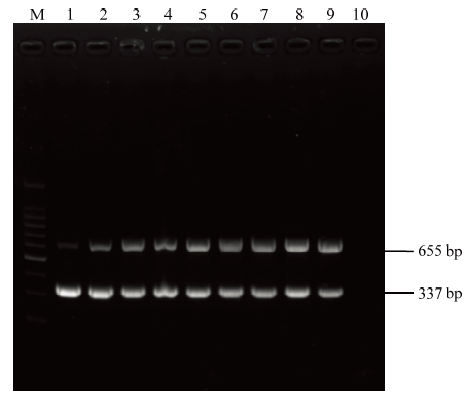 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4多基因联合检测体系引物用量的优化
M:DNA分子量标准;1:引物用量组合T1—T9;10:阴性对照
Fig. 4Optimization of primer dosage in multi-gene-based PCR detection
Marker DNA (100 bp);1:The combination of primer dosage T1 to T9, respectively;10:Negative control (healthy plant)
2.3 多基因联合检测的特异性
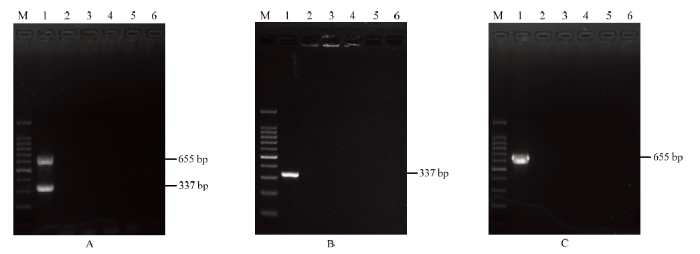
经优化之后的多基因联合检测体系能够从ChiVMV上同时扩增出CP及CI的目的片段,且未出现非特异性扩增,而其他辣椒主要病毒均未扩增出相应的条带(图5);同时,两个基因片段的PCR产物经回收纯化后进行克隆测序,所测的序列与已报道的ChiVMV序列一致性均高达98%以上,表明所扩增出的两条带均为特异性产物。因此,该多基因联合检测体系具有良好的特异性。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5多基因联合检测的特异性
A:多基因联合检测;B:基于CP的PCR检测PCR; C:基于CI的PCR检测PCR。M:DNA分子量标准;1:辣椒脉斑驳病毒;2—5:其他辣椒主要病毒;6:阴性对照
Fig. 5Specificity of the multi-gene-based PCR detection
A:Multi-gene-based PCR detection;B:assay based on CP; C:assay based on CI。Marker DNA (100 bp);1:ChiVMV;2—5:Other main viruses of chilli;6:Negative control (healthy plant)
2.4 多基因联合检测的灵敏度
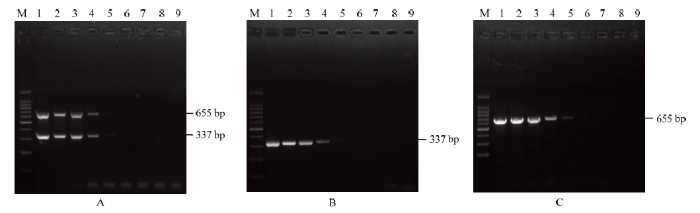
灵敏度测定结果表明,当ChiVMV阳性样品cDNA模板浓度稀释至10-4时,优化之后的多基因联合检测体系样品仍能扩增出大小为300和600 bp左右清晰可见的条带(图6-A),灵敏度与CP和CI单独RT-PCR的灵敏度相当(检测下限均为稀释至10-4倍的阳性样品cDNA),说明该多基因联合检测体系具有较高的灵敏度。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6多基因联合检测的灵敏度
A:多基因联合检测;B:基于CP的PCR检测PCR; C:基于CI的PCR检测PCR。M:DNA分子量标准;1—8:100、10-1、10-2、10-3、10-4、10-5、10-6和10-7 ;9:阴性对照
Fig. 6Sensitivity of the multi-gene-based PCR detection
A:Multi-gene-based PCR detection;B:assay based on CP; C:assay based on CI。Marker DNA (100 bp);1—8:Dilution concentration of 100, 10-1, 10-2, 10-3, 10-4, 10-5, 10-6, 10-7, respectively;9:Negative control (healthy plant)
2.5 多基因联合检测体系检测辣椒带毒样品
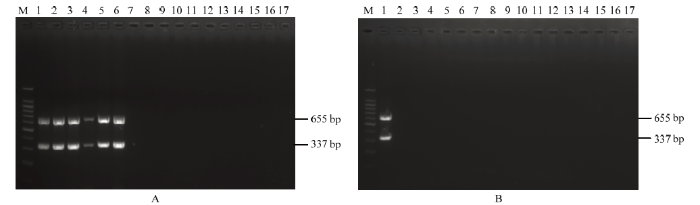
利用建立的多基因联合检测体系和常规RT-PCR对田间采集的辣椒样品进行检测,样品信息如表3所示。多基因联合检测方法扩增结果显示,福州郊区田间采集的5个样品均样品能够扩增出大小为300和600 bp左右的2个特异性条带,与预期的337和655 bp大小相吻合,福清地区采集的25个样品均未扩增出目的条带(图7)。常规RT-PCR方法验证结果与多基因联合检测体系检测结果完全一致,福州郊区田间采集的5个样品能够扩增出861 bp的条带,福清地区采集的25个样品均未扩增出条带(图8);上述结果表明该多基因联合检测体系能够准确用于ChiVMV的实际检测。Table 3
表3
表3用于多基因联合检测辣椒样品信息
Table 3
| 来源Origin | 数量Quantity | 阳性样品数量Number of positive samples | 采集日期Date of collection |
|---|---|---|---|
| 福州郊区Fuzhou suburb | 5 | 5 | 2019-03-17 |
| 福清地区Fuqing area | 25 | 0 | 2019-04-16 |
新窗口打开|下载CSV
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7多基因联合检测辣椒样品
M:DNA分子量标准;1:阳性对照;17:阴性对照。A:2—6为福州郊区辣椒样品,7—16为福清地区辣椒样品;B:2—16为福清地区辣椒样品。
Fig. 7PCR products of samples detected by multi-gene-based PCR
Marker DNA (100 bp);1:Positive control;17:Negative control (healthy plant)。A:2-6 are chilli samples from Fuzhou suburb, 7-16 are chilli samples from Fuqing area;B:2-16 are chilli samples from Fuqing area。The same as
图8
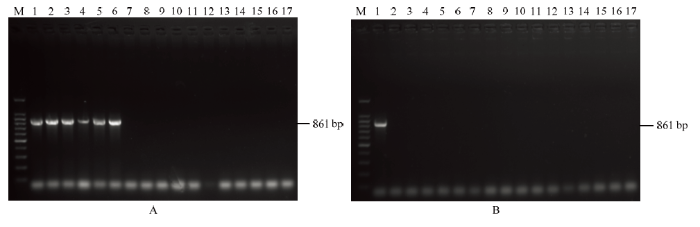 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8常规RT-PCR检测辣椒样品
Fig. 8PCR products of samples detected by conventional RT-PCR
3 讨论
ChiVMV对辣椒生产危害严重,且近年来研究表明其在我国乃至世界的侵染范围正逐步扩展,对我国辣椒生产造成严重威胁。目前,对该病毒的防治研究虽取得一定的进展[24,25,26],但传统的抗病育种因周期较长难以满足生产上的需要,而抗病基因工程等手段成本较高且存在生物安全性争议,加强检疫仍然是阻止该病毒传播的最有效手段之一。同时,该病毒与一些其他马铃薯Y病毒属成员存在密切的亲缘关系,病毒粒体、基因结构非常相似[27],应用常规的血清学检测、电镜观察及单一的RT-PCR方法进行检测、鉴定容易造成误检。因此,生产上迫切需要建立一种快速、准确、可靠的病毒检测技术。血清学检测具有高效、快捷的特点,适用于样品的批量检测,在病毒检测中通常用于样品的初步筛查,但准确鉴定需要结合其他方法进一步验证。相对于分子生物学方法,血清学方法检测灵敏度较低[28,29],对于亲缘关系较近的病原物容易因交叉反应而造成误检。RT-LAMP技术操作简便,灵敏度高,但其对引物要求相对较高,操作时易污染造成假阳性[30],在病毒检测应用中还存在一定局限性。RT-PCR方法目前在病毒检测中应用最为广泛。与常规RT-PCR不同,多基因联合RT-PCR检测方法一次可扩增多个目的基因片段,因此具有更高的准确性和可靠性,同时节约了试验成本,特别适合一些株系分化明显的植物病毒鉴定,如PVY的株系鉴定[31]。目前,国内外对于ChiVMV检测鉴定的报道较多,但其主要检测方法还是局限于血清学检测以及常规RT-PCR等检测方法。本研究根据ChiVMV的CP和CI两个基因设计引物,筛选了Tm值相近、片段大小差异显著的两对引物,通过优化退火温度、引物用量等反应条件,建立了能够同时检测扩增CP、CI两个基因片段的多基因联合检测方法。该方法与单一常规RT-PCR扩增结果一致,具有良好的特异性;灵敏度也与单一的RT-PCR灵敏度相同,均为10-4数量级,显示出良好的检测灵敏度。
带病植株的调运容易造成病毒的远距离传播,且不同地区间病毒存在很强的地区适应性[32],一旦传入易造成定殖和传播,为保护我国辣椒产业的健康发展,应实施严格的检疫,防止国外辣椒病毒的传入。本研究建立的多基因联合检测体系能在一个反应中同时检测出ChiVMV的CP及CI基因保守区片段,可以满足口岸进出境ChiVMV的快速、准确检测需求。
4 结论
通过引物设计、筛选和反应条件优化,建立了能够同时检测、扩增ChiVMV CP和CI两个基因的多基因联合检测体系,该多基因联合检测体系具有特异性好、灵敏度高、重复性好的特点,能够快速检测ChiVMV,可为辣椒生产和口岸ChiVMV的检测提供技术支持。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOI:10.3864/j.issn.0578-1752.2019.02.005URL [本文引用: 1]

【Objective】 The objective of this study is to determine the pathogens, dominant viruses, distributions of Solanaceae, Cucurbitaceae, Leguminosae, and Cruciferae vegetables viral diseases, and to analyze the expansion trend of some important viruses in China.【Method】 Viral disease survey and virus detection using a combination of serological and molecular biology methods on Solanaceae, Cucurbitaceae, Leguminosae, and Cruciferae vegetables were carried out in 31 provinces (municipalities, autonomous regions) of mainland China from 2013 to 2017.【Result】 A total of 41 653 suspected virus disease samples of Solanaceae, Cucurbitaceae, Leguminosae and Cruciferae vegetables were collected from the 31 provinces (municipalities, autonomous regions) across the country. A total of 63 viruses were detected, of which 40 viruses were detected in solanaceous vegetable crops, as well as 26 viruses in cucurbitaceous vegetable crops, 19 viruses in leguminous vegetable crops, and 14 viruses in cruciferous vegetable crops. Among them, 33 and 25 viruses were detected in pepper and tomato of solanaceous samples, respectively; 22 and 19 viruses were detected in pumpkin and cucumber of cucurbitaceous samples, respectively; 14, 12, 12 and 7 viruses were detected in cowpea, kidney beans, radish and Chinese cabbage, respectively. Mixed infections were common in the vegetable crops with two to six viruses respectively, most of the co-infected crops were infected with two viruses and some of the pepper, tomato and eggplant plants were infected with six viruses. Tomato mottle mosaic virus (ToMMV), Zucchini tigre mosaic virus (ZTMV), Pepper vein yellows virus (PeVYV), Pepper cryptic virus 1 (PCV-1) and Pepper cryptic virus 2 (PCV-2) were firstly recorded in China. ToMMV, Melon aphid-borne yellows virus (MABYV), Cucurbit aphid-borne yellows virus (CABYV) were firstly detected on pepper plants, ZTMV and Tobacco mild green mosaic virus (TMGMV) were firstly recorded on cucumber and pumpkin plants, respectively. While Tomato spotted wilt tospovirus (TSWV) was firstly detected on celery, Datura stramonium, cowpea, pea, Codonopsis pilosula, dahlia, Tropaeolum majus, Solanum indicum, and so on. Chilli veinal mottle virus (ChiVMV) was firstly found infecting Solanum yingjiangense. Meanwhile, pepper and tomato plants were found as the new natural hosts of Tobacco bushy top virus (TBTV). 【Conclusion】 Cucumber mosaic virus (CMV), Tobacco mosaic virus (TMV), Turnip mosaic virus (TuMV) and Broad bean wilt virus 2 (BBWV2) are the dominant viruses currently in China due to their widely distribution and serious damage on vegetable crops nationwide. Among them, CMV occurs in all of the 31 provinces (municipalities, autonomous regions), and is also the dominant virus in the all regions. CMV and TMV are the dominant viruses on solanaceous vegetable crops, while CMV, Zucchini yellow mosaic virus (ZYMV), Cucumber green mottle mosaic virus (CGMMV) and TMV are the dominant viruses on cucurbitaceous vegetable crops. CMV and BBWV2 are the dominant viruses of leguminous vegetable crops, and TuMV, CMV, TMV are the dominant viruses of cruciferous vegetable crops. Tomato chlorosis virus (ToCV), TSWV, CGMMV and ToMMV, which occurring seriously in some provinces and spreading rapidly, may have the risk of national epidemic.
DOI:10.3864/j.issn.0578-1752.2019.02.005URL [本文引用: 1]

【Objective】 The objective of this study is to determine the pathogens, dominant viruses, distributions of Solanaceae, Cucurbitaceae, Leguminosae, and Cruciferae vegetables viral diseases, and to analyze the expansion trend of some important viruses in China.【Method】 Viral disease survey and virus detection using a combination of serological and molecular biology methods on Solanaceae, Cucurbitaceae, Leguminosae, and Cruciferae vegetables were carried out in 31 provinces (municipalities, autonomous regions) of mainland China from 2013 to 2017.【Result】 A total of 41 653 suspected virus disease samples of Solanaceae, Cucurbitaceae, Leguminosae and Cruciferae vegetables were collected from the 31 provinces (municipalities, autonomous regions) across the country. A total of 63 viruses were detected, of which 40 viruses were detected in solanaceous vegetable crops, as well as 26 viruses in cucurbitaceous vegetable crops, 19 viruses in leguminous vegetable crops, and 14 viruses in cruciferous vegetable crops. Among them, 33 and 25 viruses were detected in pepper and tomato of solanaceous samples, respectively; 22 and 19 viruses were detected in pumpkin and cucumber of cucurbitaceous samples, respectively; 14, 12, 12 and 7 viruses were detected in cowpea, kidney beans, radish and Chinese cabbage, respectively. Mixed infections were common in the vegetable crops with two to six viruses respectively, most of the co-infected crops were infected with two viruses and some of the pepper, tomato and eggplant plants were infected with six viruses. Tomato mottle mosaic virus (ToMMV), Zucchini tigre mosaic virus (ZTMV), Pepper vein yellows virus (PeVYV), Pepper cryptic virus 1 (PCV-1) and Pepper cryptic virus 2 (PCV-2) were firstly recorded in China. ToMMV, Melon aphid-borne yellows virus (MABYV), Cucurbit aphid-borne yellows virus (CABYV) were firstly detected on pepper plants, ZTMV and Tobacco mild green mosaic virus (TMGMV) were firstly recorded on cucumber and pumpkin plants, respectively. While Tomato spotted wilt tospovirus (TSWV) was firstly detected on celery, Datura stramonium, cowpea, pea, Codonopsis pilosula, dahlia, Tropaeolum majus, Solanum indicum, and so on. Chilli veinal mottle virus (ChiVMV) was firstly found infecting Solanum yingjiangense. Meanwhile, pepper and tomato plants were found as the new natural hosts of Tobacco bushy top virus (TBTV). 【Conclusion】 Cucumber mosaic virus (CMV), Tobacco mosaic virus (TMV), Turnip mosaic virus (TuMV) and Broad bean wilt virus 2 (BBWV2) are the dominant viruses currently in China due to their widely distribution and serious damage on vegetable crops nationwide. Among them, CMV occurs in all of the 31 provinces (municipalities, autonomous regions), and is also the dominant virus in the all regions. CMV and TMV are the dominant viruses on solanaceous vegetable crops, while CMV, Zucchini yellow mosaic virus (ZYMV), Cucumber green mottle mosaic virus (CGMMV) and TMV are the dominant viruses on cucurbitaceous vegetable crops. CMV and BBWV2 are the dominant viruses of leguminous vegetable crops, and TuMV, CMV, TMV are the dominant viruses of cruciferous vegetable crops. Tomato chlorosis virus (ToCV), TSWV, CGMMV and ToMMV, which occurring seriously in some provinces and spreading rapidly, may have the risk of national epidemic.
[本文引用: 2]
[本文引用: 2]
URLPMID:14991448 [本文引用: 1]
URLPMID:30861857 [本文引用: 2]
[本文引用: 1]
URLPMID:30786570 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:30743508 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:30699808 [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/ppa.1995.44.issue-4URL [本文引用: 1]
URLPMID:18943114 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/j.1365-3059.2007.01780.xURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:15592889 [本文引用: 1]
URLPMID:30105182 [本文引用: 1]
DOI:10.1094/MPMI.1998.11.10.943URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:20025905 [本文引用: 1]
URLPMID:26831930 [本文引用: 1]
