 ,1, 唐美玲
,1, 唐美玲 ,2, 杨庆山1,3, 高雅超1, 刘万好1,2, 程杰山1, 张洪霞1, 宋志忠
,2, 杨庆山1,3, 高雅超1, 刘万好1,2, 程杰山1, 张洪霞1, 宋志忠 ,1,4
,1,4Cloning, Expression and Electrophysiological Function Analysis of Potassium Channel Gene VviSKOR in Grape
SHEN JingYuan ,1, TANG MeiLing
,1, TANG MeiLing ,2, YANG QingShan1,3, GAO YaChao1, LIU WanHao1,2, CHENG JieShan1, ZHANG HongXia1, SONG ZhiZhong
,2, YANG QingShan1,3, GAO YaChao1, LIU WanHao1,2, CHENG JieShan1, ZHANG HongXia1, SONG ZhiZhong ,1,4
,1,4通讯作者:
责任编辑: 赵伶俐
收稿日期:2019-12-30接受日期:2020-02-23网络出版日期:2020-08-01
| 基金资助: |
Received:2019-12-30Accepted:2020-02-23Online:2020-08-01
作者简介 About authors
沈静沅,Tel:0535-6664662;E-mail:
唐美玲,Tel:0535-6352051;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (7553KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
沈静沅, 唐美玲, 杨庆山, 高雅超, 刘万好, 程杰山, 张洪霞, 宋志忠. 葡萄钾离子通道基因VviSKOR的克隆、表达及电生理功能[J]. 中国农业科学, 2020, 53(15): 3158-3168 doi:10.3864/j.issn.0578-1752.2020.15.015
SHEN JingYuan, TANG MeiLing, YANG QingShan, GAO YaChao, LIU WanHao, CHENG JieShan, ZHANG HongXia, SONG ZhiZhong.
0 引言
【研究意义】钾离子(K+)是作物生长发育所必需的关键矿质营养元素[1,2,3]。植物为了满足正常生长,需要通过根部从土壤中有效地吸收所需的钾素,然后分配到不同器官部位,或者在某些组织富集积累[4,5,6,7]。SKOR编码一类外向整流型Shaker类钾离子通道,负责将韧皮部的K+分泌至木质部,介导K+向根系输导组织的释放,属于典型的电压依赖型钾外排过程,在植物生长发育过程中起重要作用[4,8]。近20年来,模式作物拟南芥中SKOR通道生理功能的分子机制研究较为详尽。然而,果树中Shaker类钾离子通道的功能依然未知,有关SKOR蛋白的研究仍然空白。【前人研究进展】钾离子是植物细胞中最为重要的金属元素之一,参与信号传导、气孔运动、光合作用、蒸腾作用和胁迫抗逆等生命过程,是作物生长发育所必需的关键营养元素,缺钾不仅会导致作物产量下降,同时也会影响作物品质[1-2,7-8]。植物钾吸收动力学研究表明植物钾营养吸收存在2种机制:机制Ⅰ,即主动吸钾过程,指外界钾浓度小于0.2 mmol?L-1时起主要作用的高亲和性钾吸收系统;机制Ⅱ,即被动吸钾过程,指外界钾浓度大于1 mmol?L-1时起主要作用的低亲和性钾吸收系统[9,10]。植物体内钾离子的动态平衡及分配主要由定位于细胞质膜的各类钾离子通道来介导完成,其中,Shaker类钾通道研究最为透彻,在植物钾素营养高效中起至关重要的作用。Shaker类钾通道对底物K+的亲和常数约在数十mmol?L-1,属于典型的低亲和、高通量的钾离子通道。1992年,ANDERSON等[11]和SENTENAC等[12]分别在模式植物拟南芥中报道了第一个植物Shaker类型钾离子通道KAT1和AKT1,此后20年内,国内外****陆续从不同植物中发现了30多个的钾离子通道[13,14,15]。另外,拟南芥AthSKOR[4]和AthGORK[16]是两类外向整流型钾离子通道,其中,AthGORK主要在叶片保卫细胞中表达,该通道功能缺失后导致气孔关闭受抑制,AthGORK也可以被蛋白激酶CPK33调节[15,16,17];AthSKOR主要定位于拟南芥根的中柱鞘和中柱薄壁细胞,负责将韧皮部的钾离子分泌至木质部,进而实现钾离子经由木质部的根-茎长距离运输,介导典型的电压依赖型钾外排过程,该通道功能缺失后导致地上部含钾量降低约50%[4]。此后,在水稻[18]、小花碱茅[19]、霸王[20]、甜瓜[14]、黑果枸杞[21]等多种植物中陆续报道了SKOR型钾离子通道,发现这些钾通道具有相似的蛋白结构特征,并包含一段极保守的标签序列GYGD。【本研究切入点】果树中,有关Shaker类钾离子通道的研究未见报道,SKOR的生物学功能有待研究。【拟解决的关键问题】本研究以‘马瑟兰’葡萄为材料,克隆VviSKOR,明确其表达模式与电生理功能,为研究果树钾素营养高效机制提供理论基础和基因材料。1 材料与方法
1.1 试验时间、地点
试验于2017年1月至2019年11月在鲁东大学农林作物遗传改良中心、国家现代葡萄产业技术体系烟台综合试验站和剑桥大学植物系离子运输研究室进行。1.2 试验设计与方法
1.2.1 试验材料与胁迫处理 供试材料为国家现代葡萄产业技术体系烟台综合试验站的7年生‘马瑟兰’葡萄树,树形采用单干单臂,直立叶幕,露天篱架栽培,株行距为1 m×2 m,树体健壮,常规田间管理。样品采集参照王壮伟等[22]方法,分别于2019年不同日期采集7年生‘马瑟兰’的显露期花序(4月21号)、新生叶片(5月10号)、韧皮部(5月18号)、盛开期的花(5月18号,花序中部)、新生根(5月28号)、幼果(5月28号)和熟果(9月29号)等组织材料,液氮充分冷冻后保存于超低温冰箱。非生物胁迫处理所用材料为国家现代葡萄产业技术体系烟台综合试验站提供的‘马瑟兰’组培幼苗,根据王壮伟等[22]方法,在人工气候箱中利用MS营养液预培养3 d,然后分别进行缺钾(MS配方中的KNO3和KH2PO4分别被NaNO3和NaH2PO3代替)、高钾(40 mmol·L-1 KCl)、脱落酸(200 μmol·L-1 ABA)和盐胁迫(160 mmol·L-1 NaCl)等处理,每个处理进行3组生物学重复,每组处理9株幼苗,处理48 h后,分别采集幼苗根、茎和叶片等材料,液氮冷冻后备用。1.2.2 葡萄VviSKOR克隆 以拟南芥AthSKOR(AT3G02850)氨基酸序列为参考,在Phytozome葡萄基因组(
Table 1
表1
表1本研究所用特异性引物
Table 1
| 目的 Intention | 引物 Primer (5′-3′) | 扩增产物大小 Amplicon size (bp) |
|---|---|---|
| VviSKOR CDS扩增 Amplication of VviSKOR CDS | F: ATGATGAATGATTGGTTCTCTG R: CTAAATATCTAGTGTTTCACAT | 2385 |
| SKOR特异性表达引物 Specific expression primers of SKOR | F: GTGGTTTTCTGTGTGCGACC R: GCCACATGAAGTGGGGTTCT | 118 |
| Ubiquitin特异性表达引物 Specific expression primers of Ubiquitin | F: CCTCATCTTCGCTGGCAAAC R: GGTGTAGGTCTTCTTCTTGCG | 133 |
| pTracer-CMV3-SKOR质粒构建 Construction of plasmid of pTracer-CMV3-SKOR vector | F: GAGAGAATTCCATGATGAATGATTGGTTC R: GAGAGCGGCCGCCTAAATATCTAGTGTTTC | 2385 |
新窗口打开|下载CSV
1.2.3 葡萄VviSKOR生物信息学分析 在Phytozome葡萄基因组数据库中下载VviSKOR的CDS序列、基因组DNA序列、启动子区域序列和编码氨基酸序列。根据王壮伟等[22]的方法,分别利用Gene Structure Display、PSORT和Plant CARE在线服务器预测VviSKOR的基因结构、亚细胞定位和cis-顺式作用元件;运用TMpredict和ProtParam在线软件分析VviSKOR蛋白的跨膜结构域、理论等电点、稳定性、亲水性等理化特征;利用ClustalX 2.0软件对葡萄、拟南芥、水稻、玉米、高粱、短柄草、大豆、番茄、黄瓜、白杨、桃、梨、草莓、苹果、木瓜、柑橘、香蕉、凤梨等18种不同科属植物的SKOR同源蛋白进行氨基酸序列一致性分析,借助MEGA 7.0软件对上述18种植物的SKOR同源蛋白构建系统进化树;利用Phytozome葡萄基因组数据库表达谱信息分析VviSKOR的表达模式。
1.2.4 实时荧光定量PCR分析 利用RNA提取试剂盒MiniBEST Plant RNA Extraction Kit(TaKaRa,大连)分别提取组培幼苗和7年生葡萄树不同组织材料的总RNA;借助反转录试剂盒PrimeScriptTM RT reagent Kit(TaKaRa,大连)合成第一链cDNA;通过NCBI/Primer-BLAST在线服务器设计VviSKOR的特异性表达引物(表1),以葡萄Ubiquitin(GenBank No. MH114011)作为内参基因,荧光染料使用SYBR Green(TaKaRa,大连),参照王壮伟等[22]的方法在ABI 7500荧光定量PCR仪分析VviSKOR的表达特征,在ABI 7500 PCR仪获得相应反应的Ct值,经Ubiquitin内参基因均一化后,利用2-ΔΔCt法计算相对表达量[23]。每个样品设置3个生物学重复,每个生物学重复进行3次技术重复。
1.2.5 pTracer-CMV3-SKOR表达载体构建 设计构建表达载体pTracer-CMV3-SKOR的特异引物对,上游引物添加酶切位点EcoR I,下游引物添加酶切位点Not I(表1,下划线已标注),由生工生物工程(上海)股份有限公司合成,扩增产物通过限制性内切酶EcoR I/Not I(New England Biolabs,美国)双酶切作用后,利用T4 DNA连接酶(New England Biolabs,美国)构建到同样双酶切的pTracer-CMV3载体多克隆位点,获得重组表达载体pTracer-CMV3-SKOR,转化大肠杆菌感受态DH5α,挑选阳性克隆,经EcoR I/Not I双酶切验证后,再次送往生工生物工程(上海)股份有限公司测序验证。
1.2.6 膜片钳电生理研究 按照SU等[24]的方法,通过膜片钳电生理系统进行SKOR的功能研究:纯化浓缩后的pTracer-CMV3-SKOR质粒转染到HEK293-T细胞(ATCC公司,美国),同时转染pTracer-CMV3空载体作为对照试验,挑取带绿色荧光的细胞,利用pCLAMP 10.0(Axon,美国)采集通道基因的电流信号,借助pCLAMP 10.0和SigmaPlot 10.0软件进行图像采集及数据分析。每种KCl浓度下选择5个细胞进行重复性研究。
1.2.7 数据显著性分析 利用SPSS 13.0软件(SPSS Chicago,美国)开展数据的显著性分析,在胁迫处理与对照条件两个独立样品间进行t-检验(检验水平0.01<*P<0.05;**P<0.01)。
2 结果
2.1 葡萄VviSKOR的克隆
以拟南芥AthSKOR氨基酸序列为参考序列,在Phytozome葡萄基因组数据库中检索得到1个同源基因,命名为VviSKOR(Gene ID:GSVIVT01030667001),在Pfam在线服务器预测到环核苷酸结合域(PF00027)、离子通道跨膜域(PF00520)、Ankyrin重复序列(PF12796)和KHA(PF11834)等功能结构域(图1-A),并检测到极保守的标签序列GYGD,表明葡萄VviSKOR属于典型的外排型钾离子通道。扩增片段经测序验证,获得‘马瑟兰’葡萄的VviSKOR的CDS序列,共2 385 bp,编码794个氨基酸。图1
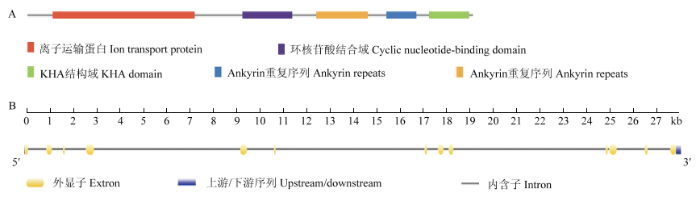 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1VvSKOR结构域预测及VviSKOR结构
A:结构域预测;B:基因结构分析
Fig. 1Domain of VvSKOR and gene structure of VviSKOR gene
A: Domain prediction; B: Gene structure analysis
2.2 VviSKOR定位、编码蛋白特征
由数据库信息可知,VviSKOR定位于第14号染色体上,含有12个长度不一的内含子(图1-B);VviSKOR蛋白含有6个跨膜区,等电点PI为6.24,表明其含有的酸性氨基酸较多;VviSKOR蛋白的不稳定指数为39.85,属于稳定蛋白;VviSKOR的GRAVY值为-0.108,表明其为亲水性蛋白质。2.3 18种不同植物SKOR同源蛋白的系统进化树
不同科属植物SKOR同源蛋白之间具有较高的同源性,两两物种之间同源蛋白的氨基酸序列一致性均高于78%,18种植物SKOR同源成员在氨基酸水平依然具有58.92%的一致性(表2),尤其在第250位至700位的氨基酸序列一致性最为显著(图2),在核苷酸水平具有62.03%的一致性(数据未展示)。Table 2
表2
表219种已测序植物SKOR蛋白信息
Table 2
| 物种 Species | 蛋白 Protein | GenBank登录号 GenBank No. | 编码区 CDS (bp) | 氨基酸数目 Amino acid No. |
|---|---|---|---|---|
| 葡萄Vitis vinifera | VviSKOR | GSVIVT01030667001 | 2385 | 794 |
| 拟南芥Arabidopsis thaliana | AthSKOR | AT3G02850 | 2487 | 828 |
| 水稻Oryza sativa | OsaSKOR | LOC_Os06g14030 | 2577 | 858 |
| 玉米Zea mays | ZmaSKOR | GRMZM2G310569_T01 | 2640 | 879 |
| 高粱Sorghum bicolor | SbiSKOR | Sobic.010G102800 | 2565 | 854 |
| 短柄草Brachypodium distachyon | BdiSKOR | Bradi1g44317 | 2502 | 833 |
| 大豆Glycine max | GmaSKOR | Glyma.02G243400 | 2550 | 849 |
| 番茄Solanum lycopersicum | SlySKOR | Solyc11g011500 | 2490 | 829 |
| 黄瓜Cucumis sativus | CsaSKOR | Cucsa.034670 | 2487 | 828 |
| 白杨Populus trichocarpa | PtrSKOR | Potri.017G135400 | 2526 | 841 |
| 桃Prunus persica | PpeSKOR | Prupe.3G164900 | 2493 | 830 |
| 梨Pyrus bretschneideri | PbrSKOR | Pbr022827 | 2521 | 839 |
| 草莓Fragaria vesca | FveSKOR | mrna30492.1-v1.0-hybrid | 3795 | 1264 |
| 苹果Malus domestica | MdoSKOR | MDP0000263295 | 2523 | 840 |
| 木瓜Carica papaya | CpaSKOR | evm.model.supercontig_116.82 | 2376 | 791 |
| 柑橘Citrus sinensis | CsiSKOR | orange1.1g003425m | 2466 | 821 |
| 香蕉Musa acuminata | MacSKOR | GSMUA_Achr9T15840_001 | 2538 | 845 |
| 凤梨Ananas comosus | AcoSKOR | Aco002953 | 2457 | 818 |
新窗口打开|下载CSV
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图218种植物SKOR高保守氨基酸序列区域一致性分析
Fig. 2Identity analysis of highly conservative regions of SKOR proteins from 18 plants
系统进化树建构结果表明,18种不同科属植物SKOR同源蛋白在遗传进化关系上有较大差异:其中,葡萄和黄瓜同为双子叶植物,葡萄VviSKOR和黄瓜CsaSKOR在系统进化关系上紧密聚在一起,在遗传距离上可能是相近的(图3);水稻、玉米、高粱和短柄草同为禾本科植物,其SKOR同源蛋白在进化关系上更倾向于聚在一起,苹果、梨、桃和草莓同属蔷薇科植物,其SKOR同源蛋白更倾向于聚在一起,在遗传距离上更为相近,而蔷薇科的木瓜却与拟南芥、番茄和柑橘等不同属的双子叶植物在遗传距离上相近,其SKOR同源蛋白在系统进化树上紧密聚在一起(图3)。
图3
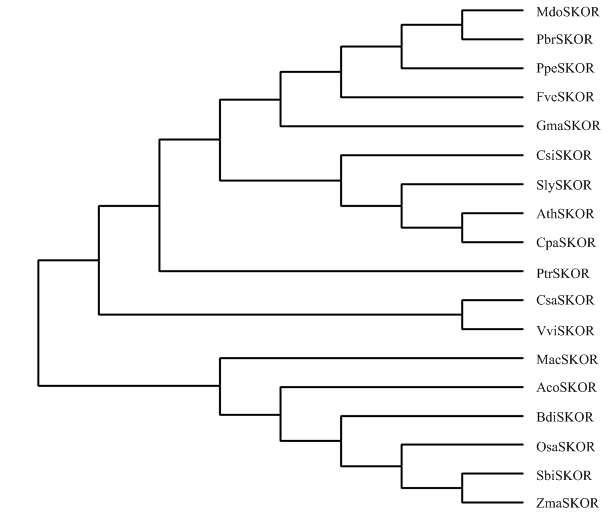 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图318种植物SKOR蛋白的系统进化树
Fig. 3The phylogenetic tree of SKOR proteins from 18 plants
2.4 VviSKOR亚细胞定位及启动子顺式作用元件预测
如图4所示,亚细胞定位预测表明VviSKOR主要定位于细胞膜(约占33%)和线粒体内膜(29%),其次是高尔基体(22%)和内质网膜(22%)。图4
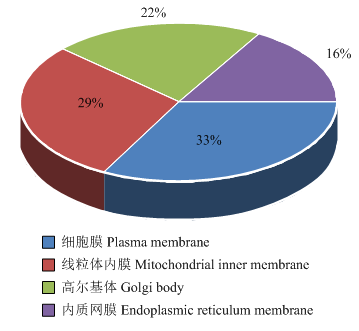 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4VviSKOR亚细胞定位预测
Fig. 4Subcellular localization prediction of VviSKOR
cis-顺式作用元件预测结果表明,VviSKOR启动子区域鉴定到至少12种顺式作用元件(表3),包括启动子和增强子区域、胁迫响应(低温感应、光感应、厌氧诱导等)、激素响应(茉莉酮酸甲酯MeJ-A、脱落酸ABA等)、细胞周期调控等不同生命活动相关的调控元件(表3)。
Table 3
表3
表3VviSKOR启动子顺式作用元件分析
Table 3
| 顺式作用元件 cis-regulatory element | 特征序列 Characteristic sequence | 潜在调控途径 Putative regulatory pathway |
|---|---|---|
| CAAT-box | CAAT | 启动子和增强子区域 Promoter and enhancer regions |
| GATA-motif | GATAGGG | 光感应 Light response |
| Sp1 | GGGCGG | 光感应 Light response |
| Box 4 | ATTAAT | 光感应 Light response |
| G-Box | CACGTT | 光感应 Light response |
| TCT-motif | TCTTAC | 光感应 Light response |
| ARE | AAACCA | 厌氧诱导 Anaerobic induction |
| MSA-like | TCAAACGGT | 细胞周期调控 Cell cycle regulation |
| TGACG-motif | TGACG | 茉莉酮酸甲酯响应 Methyl jasmonate response |
| CGTCA-motif | CGTCA | 茉莉酮酸甲酯响应 Methyl jasmonate response |
| ABRE | ACGTG | 脱落酸响应 Abscisic acid response |
| LTR | CCGAAA | 低温感应Low temperature |
新窗口打开|下载CSV
2.5 VviSKOR表达分析
Phytozome数据库表达谱分析表明,VviSKOR在根部的表达水平最高(约占65%),其次是叶(13%),此外,在木质部(6%)、果实(5%)、果皮(4%),雌蕊(4%)、花瓣(3%)和花粉(1%)中也有表达(图5)。图5
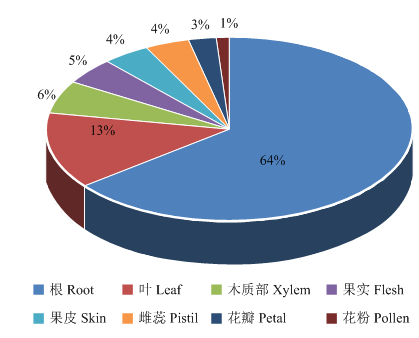 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5VviSKOR表达谱分析
Fig. 5Expression profiles analysis of VviSKOR
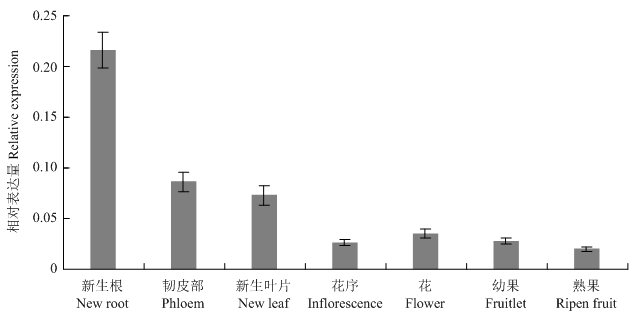
实时荧光定量PCR验证结果表明,VviSKOR在7年生‘马瑟兰’葡萄树不同组织、器官中的表达水平有差异:在根部的表达水平最高,其次是一年生韧皮部和新叶,在花序、花和果实(幼果、熟果)中的表达水平较低(图6)。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6实时荧光定量PCR分析VviSKOR在7年生葡萄树不同组织的表达特征
Fig. 6Real time quantitative PCR expression analysis of VviSKOR in different tissues in 7-year-old grape tree
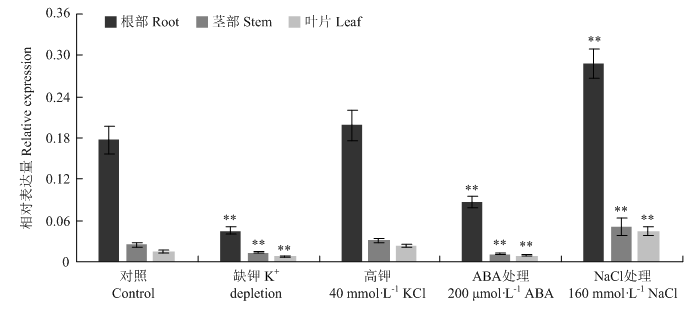
2.6 幼苗中VviSKOR对胁迫处理的响应
以‘马瑟兰’幼苗为材料,设置不同非生物胁迫处理,并进行实时荧光定量PCR分析。结果表明,VviSKOR主要在幼苗根部表达,在叶片和茎部表达量较低,再次证实该基因主要定位于葡萄根部并发挥作用。此外,VviSKOR在转录水平对不同胁迫处理的响应有差异,VviSKOR对缺钾、ABA和NaCl处理最为敏感,而对高钾胁迫没有响应,其中,缺钾和ABA处理分别显著抑制VviSKOR在幼苗根部、茎部和叶片中的表达水平,而NaCl处理则显著诱导VviSKOR在所有检测组织中的表达水平(图7)。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7幼苗中VviSKOR对缺钾、高钾、ABA和NaCl处理的响应
**表示差异极显著(P<0.01)
Fig. 7Response of VviSKOR gene under K+ depletion, high K+, ABA and NaCl stresses in seedlings
**indicates statistically extremely significant differences (P<0.01)
2.7 VviSKOR的电生理功能
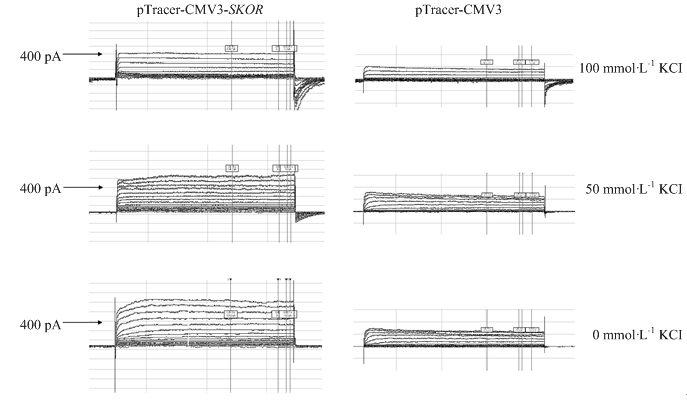
以转染pTracer-CMV3空载体的HEK293-T细胞作为空白对照,通过pCLAMP 10.0膜片钳系统记录pTracer-CMV3-SKOR在外界不同K+浓度条件下电流特征曲线。结果表明,表达pTracer-CMV3-SKOR的细胞记录到大量的外向整流电流,且外向电流随细胞外钾离子浓度的增加而降低,初步证实VviSKOR是一个典型的外流型钾离子通道(图8);此外,记录到的电流随着电压的增加而增加,说明VviSKOR也是电压依赖型钾离子通道。图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8利用膜片钳技术研究VviSKOR电生理功能
Fig. 8Physiological function of VviSKOR gene by using patch clamping
3 讨论
钾素营养与果树的生长发育、果实的品质和产量密切相关[1-3,25]。然而,大量报道主要体现在生理生化层面,果树钾素营养高效的分子机制研究很少。植物SKOR蛋白是一类外向整流型的Shaker类钾离子通道[4,8],在植物生长发育过程中起重要作用。21世纪以来,基因组测序技术迅猛发展,为植物科学研究提供了便利。本研究生物信息学分析表明,葡萄VviSKOR蛋白含有相同的植物Shaker类钾离子通道的功能结构域,包括6个跨膜区和GYGD通道标签序列,且多重序列比对显示,这些结构相似的通道属于同一家族,在其核心区域具有高度的序列一致性[2,3]。系统进化树分析表明不同物种SKOR通道成员在遗传进化关系上存在差异,禾本科植物(水稻、玉米、高粱和短柄草)SKOR同源蛋白一致性更高,在进化关系上更为紧密,相似地,蔷薇科植物(苹果、梨、桃和草莓)SKOR同源蛋白在遗传距离上更为相近。葡萄VviSKOR和黄瓜CsaSKOR在所检测植物的同源蛋白中紧密聚在一起,推测其生理功能可能具有相似性。前人研究表明拟南芥AtSKOR和AtGORK等Shaker类离子通道基因主要定位于细胞膜上[2,3,4],本研究亚细胞定位预测也揭示葡萄VviSKOR主要定位在细胞膜和线粒体内膜上,仍需后续试验进一步验证。数据库表达谱表明基因VviSKOR主要在葡萄根部表达,与前人报道基本吻合[4,14,16-21];实时荧光定量PCR结果与表达谱分析结果一致,进一步验证VviSKOR在‘马瑟兰’成年树根部或幼苗根部的表达量均最高,推测该通道基因主要在葡萄根部钾素营养动态中发挥重要作用。
植物SKOR通道在K+动态平衡、渗透调节和质子调控中起重要作用,且在转录水平易受外界K+供应水平和干旱、ABA、NaCl等非生物胁迫的调控[14,16-21]。荧光定量PCR结果表明葡萄幼苗中VviSKOR缺钾显著下调VviSKOR的表达,与拟南芥[3]、甜瓜[14]、霸王[20]的报道一致,推测缺钾条件下SKOR表达量的下降,可能反映了植物本身缺钾时,没有过多的钾往地上部运输,所以减少了SKOR的需求量(表达丰度);作为一种抑制生长的植物激素,ABA显著抑制VviSKOR在转录水平的表达,与此同时,在VviSKOR启动子区域鉴定到ABA激素响应的顺式作用元件,暗示SKOR钾通道的活性易受ABA调控,具体机制需要进一步研究;相反地,NaCl显著上调VviSKOR的表达,与黄瓜[14]、霸王[20]和黑果枸杞[21]中的报道一致,充分证实SKOR钾通道的活性易受NaCl调控,且参与调控Na+/K+离子平衡和渗透势,具体机制亟待深入解析。此外,本研究发现VviSKOR对高钾胁迫没有响应,暗示葡萄树体中SKOR的丰度够多,足以维持其在高钾环境中正常发挥作用。
在生理功能水平上,植物SKOR蛋白是典型的外向整流型钾离子通道,属于钾离子选择性的Shaker类家族通道,在模式作物拟南芥和甜瓜中均已利用非洲爪蛙卵和双电极电压钳技术证明[3,14]。本研究利用膜片钳技术证实表达VviSKOR的HEK293-T细胞展现出外向整流型通道的特点:K+外排型电流,且通道活性(外向电流激活阈值)依赖于K+浓度,与拟南芥和甜瓜中K+电流特征的报道类似[3,14],但其在葡萄中运输K+的特点和具体调控机理还未清晰,与其他模式作物SKOR同源基因的功能差异尚无法进行比较。综上可知,VviSKOR是葡萄根中一个具有K+通透性的Shaker类外向整流型钾通道,K+可以调节VviSKOR的功能。
4 结论
从葡萄中鉴定并克隆了钾离子通道基因VviSKOR;葡萄VviSKOR与黄瓜CsaSKOR成员在遗传距离上较近;VviSKOR主要在葡萄根部表达,并在转录水平受缺钾、ABA和NaCl胁迫的调控;膜片钳研究表明VviSKOR是葡萄中一个电压门控的外排型钾离子通道。研究结果为解析果树内部离子动态平衡和钾素营养高效机制奠定了技术支撑,并为园艺作物的遗传改良与分子育种提供了基因资源和理论指导。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1146/annurev.arplant.54.031902.134831URL [本文引用: 3]
URLPMID:24666983 [本文引用: 4]
URLPMID:17495982 [本文引用: 7]
DOI:10.1016/s0092-8674(00)81606-2URLPMID:9741629 [本文引用: 7]

SKOR, a K+ channel identified in Arabidopsis, displays the typical hydrophobic core of the Shaker channel superfamily, a cyclic nucleotide-binding domain, and an ankyrin domain. Expression in Xenopus oocytes identified SKOR as the first member of the Shaker family in plants to be endowed with outwardly rectifying properties. SKOR expression is localized in root stelar tissues. A knockout mutant shows both lower shoot K+ content and lower xylem sap K+ concentration, indicating that SKOR is involved in K+ release into the xylem sap toward the shoots. SKOR expression is strongly inhibited by the stress phytohormone abscisic acid, supporting the hypothesis that control of K+ translocation toward the shoots is part of the plant response to water stress.
URLPMID:17397836 [本文引用: 1]
[本文引用: 1]
DOI:10.1146/annurev.physiol.010908.163204URLPMID:18842100 [本文引用: 2]

Distinct potassium, anion, and calcium channels in the plasma membrane and vacuolar membrane of plant cells have been identified and characterized by patch clamping. Primarily owing to advances in Arabidopsis genetics and genomics, and yeast functional complementation, many of the corresponding genes have been identified. Recent advances in our understanding of ion channel genes that mediate signal transduction and ion transport are discussed here. Some plant ion channels, for example, ALMT and SLAC anion channel subunits, are unique. The majority of plant ion channel families exhibit homology to animal genes; such families include both hyperpolarization- and depolarization-activated Shaker-type potassium channels, CLC chloride transporters/channels, cyclic nucleotide-gated channels, and ionotropic glutamate receptor homologs. These plant ion channels offer unique opportunities to analyze the structural mechanisms and functions of ion channels. Here we review gene families of selected plant ion channel classes and discuss unique structure-function aspects and their physiological roles in plant cell signaling and transport.
URLPMID:28710919 [本文引用: 3]
URLPMID:16662752 [本文引用: 1]
URLPMID:16591089 [本文引用: 1]
URLPMID:1570292 [本文引用: 1]
DOI:10.1126/science.1585180URLPMID:1585180 [本文引用: 1]

A membrane polypeptide involved in K+ transport in a higher plant was cloned by complementation of a yeast mutant defective in K+ uptake with a complementary DNA library from Arabidopsis thaliana. A 2.65-kilobase complementary DNA conferred ability to grow on media with K+ concentration in the micromolar range and to absorb K+ (or 86Rb+) at rates similar to those in wild-type yeast. The predicted amino acid sequence (838 amino acids) has three domains: a channel-forming region homologous to animal K+ channels, a cyclic nucleotide-binding site, and an ankyrin-like region.
[本文引用: 1]
URLPMID:30080639 [本文引用: 8]
URLPMID:28543075 [本文引用: 2]
URLPMID:11113445 [本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s10725-016-0157-zURL [本文引用: 3]
[本文引用: 4]
[本文引用: 4]
DOI:10.3864/j.issn.0578-1752.2018.23.011URL [本文引用: 4]

【Objective】 Isolation and characterization of KEA family genes from grape. Analysis of the tissue-specific expression patterns of KEA family genes and response to K + depletion, ABA, NaCl and sorbitol treatments. Screen the potential major KEA genes in grape. 【Method】 By carrying out homology-based cloning, putative KEA family genes were isolated and characterized from grape. A phylogenetic tree was constructed by multiple alignment of KEA family proteins from 9 known plants (grape, Arabidopsis, rice, maize, sorghum, slender false brome, polar, pear, and apple) using the neighbor-joining method via MEGA7.0 software. Details of grape KEA family genes and encoded proteins were analyzed with the help of bioinformatical analysis softwares. By screening the EST database, electrical expression profiles of grape KEA genes were determined. Quantitative real-time PCR (qRT-PCR) was carried out to analyze the expression patterns of KEA family genes and response to K + depletion, ABA, NaCl, and sorbitol treatments, and obtained the major genes. 【Result】 Four KEA family genes were isolated from grape, entitled by VvKEA1—VvKEA4, which were all containing the K/H exchanger and TrkA-N functional domains that belonging to the classic plant KEA family antiporters. The amino acid sequences of KEA proteins from 9 plants shared an overall identity of 33.10%. These KEA members were classified into 2 major groups (Groups Ⅰ and Ⅱ), and VvKEA1and VvKEA2 belong to Group Ⅰ that containing 7 Motifs, while VvKEA3 and VvKEA4 belong to GroupⅡ that just containing 4 Motifs. Phylogenetic tree analysis showed that VvKEA1, VvKEA 2 and VvKEA 4 of grape were closely clustered with AtKEA2, AtKEA 3 and AtKEA 5 of Arabidopsis, respectively, and VvKEA3 was clustered with PbrKEA5 of pear and MdoKEA7 of apple. KEA members of 4 grass family plants (rice, maize, sorghum and slender false brome) were prone to clustered together, while three woody plants (polar, apple and pear) KEA members were prone to clustered together. Mainly localized in plasma membrane, all predicted VvKEA proteins possessed similar tertiary structures, contained 12 or 13 transmembrane domains (TMs), and the theoretical isoelectric point (pI) were all less than 7.0. In particular, only VvKEA3 possessed the signal peptide. Fifteen cis-acting regulatory elements, including the stress response, nutrition and development, hormone response and circadian rhythm regulations, et al., were identified in the promoter region of VvKEA genes. Expression profile analysis showed that VvKEA family genes were expressed in different tissues or organs in grape, and the highest percentage was predicted in fruit, followed by leaf, seed, root and pistil. qRT-PCR analysis showed that VvKEA3 was the most abundant expressed gene during different parts of 8-year-old ‘Rosario Bianco’ on the whole, especially in fruitlet, and the other 3 genes were less expressed with similar amount. In grape seedlings, VvKEA1—VvKEA4 genes were more sensitive to ABA treatment, whose expression were all induced in both tested shoots and roots, but had no response to NaCl treatment. The expression of VvKEA3 in both shoots and roots and VvKEA1 in shoots were up-regulated by K + depletion treatment, and the expression of VvKEA3 in both shoots and roots and VvKEA4 in roots were increased by sorbitol treatment. 【Conclusion】 Four predicted KEA family genes were cloned and characterized from grape, which were majorly expressed in fruit, leaf and seed. Notably, VvKEA3 was the most abundant gene in 8-year old grape tree, especially in fruitlet, whose expression was prone to be regulated by K + depletion, ABA, and sorbitol osmotic stress. VvKEA3 may be a crucial K +/H + antiporter during grape fruit development.
DOI:10.3864/j.issn.0578-1752.2018.23.011URL [本文引用: 4]

【Objective】 Isolation and characterization of KEA family genes from grape. Analysis of the tissue-specific expression patterns of KEA family genes and response to K + depletion, ABA, NaCl and sorbitol treatments. Screen the potential major KEA genes in grape. 【Method】 By carrying out homology-based cloning, putative KEA family genes were isolated and characterized from grape. A phylogenetic tree was constructed by multiple alignment of KEA family proteins from 9 known plants (grape, Arabidopsis, rice, maize, sorghum, slender false brome, polar, pear, and apple) using the neighbor-joining method via MEGA7.0 software. Details of grape KEA family genes and encoded proteins were analyzed with the help of bioinformatical analysis softwares. By screening the EST database, electrical expression profiles of grape KEA genes were determined. Quantitative real-time PCR (qRT-PCR) was carried out to analyze the expression patterns of KEA family genes and response to K + depletion, ABA, NaCl, and sorbitol treatments, and obtained the major genes. 【Result】 Four KEA family genes were isolated from grape, entitled by VvKEA1—VvKEA4, which were all containing the K/H exchanger and TrkA-N functional domains that belonging to the classic plant KEA family antiporters. The amino acid sequences of KEA proteins from 9 plants shared an overall identity of 33.10%. These KEA members were classified into 2 major groups (Groups Ⅰ and Ⅱ), and VvKEA1and VvKEA2 belong to Group Ⅰ that containing 7 Motifs, while VvKEA3 and VvKEA4 belong to GroupⅡ that just containing 4 Motifs. Phylogenetic tree analysis showed that VvKEA1, VvKEA 2 and VvKEA 4 of grape were closely clustered with AtKEA2, AtKEA 3 and AtKEA 5 of Arabidopsis, respectively, and VvKEA3 was clustered with PbrKEA5 of pear and MdoKEA7 of apple. KEA members of 4 grass family plants (rice, maize, sorghum and slender false brome) were prone to clustered together, while three woody plants (polar, apple and pear) KEA members were prone to clustered together. Mainly localized in plasma membrane, all predicted VvKEA proteins possessed similar tertiary structures, contained 12 or 13 transmembrane domains (TMs), and the theoretical isoelectric point (pI) were all less than 7.0. In particular, only VvKEA3 possessed the signal peptide. Fifteen cis-acting regulatory elements, including the stress response, nutrition and development, hormone response and circadian rhythm regulations, et al., were identified in the promoter region of VvKEA genes. Expression profile analysis showed that VvKEA family genes were expressed in different tissues or organs in grape, and the highest percentage was predicted in fruit, followed by leaf, seed, root and pistil. qRT-PCR analysis showed that VvKEA3 was the most abundant expressed gene during different parts of 8-year-old ‘Rosario Bianco’ on the whole, especially in fruitlet, and the other 3 genes were less expressed with similar amount. In grape seedlings, VvKEA1—VvKEA4 genes were more sensitive to ABA treatment, whose expression were all induced in both tested shoots and roots, but had no response to NaCl treatment. The expression of VvKEA3 in both shoots and roots and VvKEA1 in shoots were up-regulated by K + depletion treatment, and the expression of VvKEA3 in both shoots and roots and VvKEA4 in roots were increased by sorbitol treatment. 【Conclusion】 Four predicted KEA family genes were cloned and characterized from grape, which were majorly expressed in fruit, leaf and seed. Notably, VvKEA3 was the most abundant gene in 8-year old grape tree, especially in fruitlet, whose expression was prone to be regulated by K + depletion, ABA, and sorbitol osmotic stress. VvKEA3 may be a crucial K +/H + antiporter during grape fruit development.
URLPMID:11846609 [本文引用: 1]
DOI:10.1105/tpc.104.030551URLPMID:15805483 [本文引用: 1]

An inward Shaker K(+) channel identified in Zea mays (maize), ZmK2.1, displays strong regulation by external K(+) when expressed in Xenopus laevis (African clawed frog) oocytes or COS cells. ZmK2.1 is specifically activated by K(+) with an apparent K(m) close to 15 mM independent of the membrane hyperpolarization level. In the absence of K(+), ZmK2.1 appears to enter a nonconducting state. Thus, whatever the membrane potential, this maize channel cannot mediate K(+) influx in the submillimolar concentration range, unlike its relatives in Arabidopsis thaliana. Its expression is restricted to the shoots, the strongest signal (RT-PCR) being associated with vascular/bundle sheath strands. Based on sequence and gene structure, the closest relatives of ZmK2.1 in Arabidopsis are K(+) Arabidopsis Transporter 1 (KAT1) (expressed in guard cells) and KAT2 (expressed in guard cells and leaf phloem). Patch-clamp analyses of guard cell protoplasts reveal a higher functional diversity of K(+) channels in maize than in Arabidopsis. Channels endowed with regulation by external K(+) similar to that of ZmK2.1 (channel activity regulated by external K(+) with a K(m) close to 15 mM, regulation independent of external Ca(2+)) constitute a major component of the maize guard cell inward K(+) channel population. The presence of such channels in maize might reflect physiological traits of C4 and/or monocotyledonous plants.
DOI:10.3864/j.issn.0578-1752.2015.06.13URL [本文引用: 1]

【Objective】 The objective of this study is to analyze the transcriptional expression of KT/HAK/KUP family genes and response characteristics to potassium fertilizer application during different flowering stages, and key K+ transporter genes and its function. A close relationship between K+ nutritional status and flower development was discussed, thus providing a theoretical basis for K fertilization in orchards and genetic improvement and breeding of efficient horticulture crops.【Method】The K fertilizer treatment experiment was conducted via applying 834 g KCl (K2O content 60%) to each ‘Xiahui6’ peach tree at flower bud expanding stage. The effect of K fertilizer application on flower development, K+ nutritional status and blooming date of peach trees was analyzed. The K+ concentration of peach flowers at different developmental stages was determined by using ICP-AES apparatus with HNO3-HClO4 digestion method. Quantitative real-time PCR was used to analyze the expression profiles of KT/HAK/KUP family genes during the whole flowering process, and the key K+ transporter genes were identified. The response of KT/HAK/KUP family genes to K fertilizer application at different developmental stages was also revealed. Functions of the key genes were validated by using heterologous complementation of bacterial function lossing mutant. Coding sequence of KUP11 was cloned into pPAB404 vector to obtain the recombinant expression vector pPAB404-KUP11. Sequencing verified recombinant vector was then transformed into functional mutant E. coli strain TK2420. Whether the recombinant vector pPAB404-KUP11 can restore the ability of TK2420 mutant strain to uptake external K+, supplied with either KCl or K2SO4, was determined.【Result】Application of K fertilizer treatment favorably induced ‘Xiahui6’ flowers to bloom 2-day earlier, and specifically contributed to flower development at full bloom stage, with an increase of 21.5% in fresh weight. The highest K+ accumulation occurred at full-bloom stage, which was followed by begin bloom, bud period and petal fall stages. Potasium application significantly enhanced the K+ nutritional status, with an increase of 24.3%, 27.4%, 29.1% and 26.3% of K+ concentration, respectively, during four flowering stages. Genes of KUP1-13 were differentially expressed during the four stages, and the highest expression level appeared especially in full-bloom stage. The KT/HAK/KUP family genes were differentially regulated by K, i.e., KUP1 and KUP5 were most sensitive to Kapplication, whose expression was consistently induced from bud swell stage to full-bloom stage. KUP11 was the most expressed gene throughout the whole flowering process, which was highly up-regulated at bud swell stage but greatly reduced at petal fall stage by K treatment. The recombinant expression vector pPAB404-KUP11 can restore the K+ uptake capacity in TK2420 bacterial mutant, and can utilize either external KCl or K2SO4, indicating that the expression level of KUP11 was positively correlated with the K+ uptake of bacterial cells.【Conclusion】Application of K fertilizer favorably promotes peach flower development, improves the K+ nutritional status, makes peach flower early to open, and differentially regulated KT/HAK/KUP family genes at different flowering stages. KUP11 transporter possesses the capacity to uptake external K+, which may play an important role in peach flowering.
DOI:10.3864/j.issn.0578-1752.2015.06.13URL [本文引用: 1]

【Objective】 The objective of this study is to analyze the transcriptional expression of KT/HAK/KUP family genes and response characteristics to potassium fertilizer application during different flowering stages, and key K+ transporter genes and its function. A close relationship between K+ nutritional status and flower development was discussed, thus providing a theoretical basis for K fertilization in orchards and genetic improvement and breeding of efficient horticulture crops.【Method】The K fertilizer treatment experiment was conducted via applying 834 g KCl (K2O content 60%) to each ‘Xiahui6’ peach tree at flower bud expanding stage. The effect of K fertilizer application on flower development, K+ nutritional status and blooming date of peach trees was analyzed. The K+ concentration of peach flowers at different developmental stages was determined by using ICP-AES apparatus with HNO3-HClO4 digestion method. Quantitative real-time PCR was used to analyze the expression profiles of KT/HAK/KUP family genes during the whole flowering process, and the key K+ transporter genes were identified. The response of KT/HAK/KUP family genes to K fertilizer application at different developmental stages was also revealed. Functions of the key genes were validated by using heterologous complementation of bacterial function lossing mutant. Coding sequence of KUP11 was cloned into pPAB404 vector to obtain the recombinant expression vector pPAB404-KUP11. Sequencing verified recombinant vector was then transformed into functional mutant E. coli strain TK2420. Whether the recombinant vector pPAB404-KUP11 can restore the ability of TK2420 mutant strain to uptake external K+, supplied with either KCl or K2SO4, was determined.【Result】Application of K fertilizer treatment favorably induced ‘Xiahui6’ flowers to bloom 2-day earlier, and specifically contributed to flower development at full bloom stage, with an increase of 21.5% in fresh weight. The highest K+ accumulation occurred at full-bloom stage, which was followed by begin bloom, bud period and petal fall stages. Potasium application significantly enhanced the K+ nutritional status, with an increase of 24.3%, 27.4%, 29.1% and 26.3% of K+ concentration, respectively, during four flowering stages. Genes of KUP1-13 were differentially expressed during the four stages, and the highest expression level appeared especially in full-bloom stage. The KT/HAK/KUP family genes were differentially regulated by K, i.e., KUP1 and KUP5 were most sensitive to Kapplication, whose expression was consistently induced from bud swell stage to full-bloom stage. KUP11 was the most expressed gene throughout the whole flowering process, which was highly up-regulated at bud swell stage but greatly reduced at petal fall stage by K treatment. The recombinant expression vector pPAB404-KUP11 can restore the K+ uptake capacity in TK2420 bacterial mutant, and can utilize either external KCl or K2SO4, indicating that the expression level of KUP11 was positively correlated with the K+ uptake of bacterial cells.【Conclusion】Application of K fertilizer favorably promotes peach flower development, improves the K+ nutritional status, makes peach flower early to open, and differentially regulated KT/HAK/KUP family genes at different flowering stages. KUP11 transporter possesses the capacity to uptake external K+, which may play an important role in peach flowering.
