 ,1, 郭俊1,2, 张学尧1, 马恩波1, 张建珍1
,1, 郭俊1,2, 张学尧1, 马恩波1, 张建珍1Molecular Characteristics and Function Analysis of Nuclear Receptor Gene LmE75 in Locusta migratoria
LIU XiaoJian ,1, GUO Jun1,2, ZHANG XueYao1, MA EnBo1, ZHANG JianZhen1
,1, GUO Jun1,2, ZHANG XueYao1, MA EnBo1, ZHANG JianZhen1通讯作者:
责任编辑: 岳梅
收稿日期:2019-10-30接受日期:2019-12-10网络出版日期:2020-06-01
| 基金资助: |
Received:2019-10-30Accepted:2019-12-10Online:2020-06-01
作者简介 About authors
刘晓健

摘要
关键词:
Abstract
Keywords:
PDF (3362KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
刘晓健, 郭俊, 张学尧, 马恩波, 张建珍. 飞蝗核受体基因LmE75的分子特性和功能分析[J]. 中国农业科学, 2020, 53(11): 2219-2231 doi:10.3864/j.issn.0578-1752.2020.11.008
LIU XiaoJian, GUO Jun, ZHANG XueYao, MA EnBo, ZHANG JianZhen.
0 引言
【研究意义】核受体(nuclear receptor,NR)是一类在生物体中广泛分布的配体依赖型的转录因子[1],可通过多个信号途径调控机体的胚胎及胚后发育、衰老、昼夜节律等生命过程[2]。飞蝗(Locusta migratoria)是重要的农业害虫,围绕其核受体基因LmE75开展研究工作,对阐明核受体在飞蝗蜕皮过程中的作用机制以及基于RNA干扰(RNAi)的飞蝗防治具有重要意义。【前人研究进展】昆虫经过变态发育获得飞翔和生殖能力[3]。前胸腺分泌的蜕皮激素(20-hydroxyecdysone,20E)与咽侧体分泌的保幼激素(juvenile hormone,JH)是昆虫变态的主要因素[4]。蜕皮激素20E调控昆虫蜕皮的分子机制已在完全变态昆虫中有较多研究,20E与蜕皮激素受体(EcR)和超气门蛋白(USP)的复合物结合成为三聚体,启动一系列下游转录因子的表达,进而调控昆虫蜕皮等生理活动[5]。参与20E信号传导的基因包括转录因子E74、Broad和一些核受体。核受体含有5个典型结构域:A/B域(转录激活域,trans-activation domain)、C域(DNA结合域,DNA binding domain,DBD)、D域(铰链域,hinge domain)、E域(配体结合域,ligand binding domain,LBD)和F域。可分为7个亚家族:NR0、NR1、NR2、NR3、NR4、NR5和NR6[6]。E75核受体属于NR1亚家族,命名为NR1D3。在黑腹果蝇(Drosophila melanogaster)中研究发现,E75有4个亚型,是20E信号传导通路的早期响应基因,其纯合的E75缺失品系致使1龄幼虫期发育延迟,不能蜕皮为2龄幼虫而死亡[7]。迄今为止,E75已在埃及伊蚊(Aedes aegypti)[8]、烟草天蛾(Manduca sexta)[9]、大蜡螟(Galleria mellonella)[10]、云杉芽卷蛾(Choristoneura fumiferana)[11]、家蚕(Bombyx mori)[12]、印度谷螟(Plodia interpunctella)[13]、西方蜜蜂(Apis mellifera)[14]、赤拟谷盗(Tribolium castaneum)[15]、马铃薯甲虫(Leptinotarsa decemlineata)[16]、德国小蠊(Blattella germanica)[17]等多种昆虫中克隆获得。已有研究表明E75在昆虫生长发育过程中发挥重要作用,如德国小蠊中有5个BgE75亚型,注射BgE75共同区域的dsRNA后大部分个体无法正常蜕皮而死亡[17]。马铃薯甲虫中有3个E75亚型,各亚型均受20E调控,利用RNAi技术将LdE75沉默后可影响蜕皮激素20E和保幼激素JH的滴度从而调控蜕皮激素和保幼激素信号途径,最终导致马铃薯甲虫化蛹困难而死亡[16]。【本研究切入点】目前E75在完全变态昆虫的研究中取得了较大进展[7,8,9,10,11,12,13,14,15,16],但该基因在不完全变态昆虫飞蝗中的作用机制有待深入解析。【拟解决的关键问题】通过RNAi结合H&E染色方法分析飞蝗核受体基因LmE75的生物学功能,丰富蜕皮激素调控昆虫蜕皮的理论体系,为基于RNAi的害虫控制提供新的靶标基因。1 材料与方法
试验于2018—2019年在山西大学应用生物学研究所完成。1.1 供试昆虫
飞蝗虫卵由河北省沧州市建民虫业公司提供。将虫卵置于孵化盒中进行孵化,期间喷洒适量水以保持沙土潮湿,孵化出的1龄若虫转移至25 cm×25 cm×25 cm的纱笼中,置于温度为(30±2)℃,相对湿度为(40±10)%,光照14 h、黑暗10 h的养虫室中,饲喂新鲜的小麦苗,待其生长发育至3龄若虫期开始辅以麦麸饲喂。1.2 LmE75 cDNA序列分析
1.2.1 LmE75 cDNA序列搜索及分析 通过搜索飞蝗转录组数据库获得3条LmE75的cDNA序列。利用NCBI中的Open Reading Frame Finder(ORF Finder)(http://cms.ncbi.nlm.nih.gov/orf finder/)软件找到LmE75的全长开放阅读框。利用ExPASy网站中(https://www.expasy.org/)相关软件对各个基因的开放阅读框序列进行翻译、预测各蛋白分子量(Mw)及等电点(pI)。SMART(http://smart. embl-heidelberg. de/)分析预测其蛋白结构域。运用GENEDOC软件将LmE75A、LmE75B和LmE75C特异性的A/B域核苷酸进行比对,分析三者的核苷酸一致度;运用GENEDOC软件将LmE75A、LmE75B和LmE75C氨基酸序列与马铃薯甲虫(LdE75A、LdE75B 和LdE75C)和德国小蠊(BgE75A、BgE75C和BgE75E)氨基酸序列进行比对,分析其DNA结合域(DBD)和配体结合域(LBD),而两个结构域通过不保守的绞链结构域连接。1.2.2 昆虫E75系统发育分析 在NCBI网站上下载其他物种E75蛋白的同源序列,运用MEGA 6.0软件中NJ(neighbor-joining)法构建不同昆虫E75的系统进化树。不同昆虫E75的GenBank登录号见表1。
Table 1
表1
表1本研究中用于构建系统进化树的物种和E75的 GenBank 登录号
Table 1
| 目 Order | 物种 Species | 基因 Gene | GenBank 登录号 GenBank accession number |
|---|---|---|---|
| 半翅目 Hemiptera | 绿盲蝽Apolygus lucorum | AlE75A、AlE75B、AlE75C | ATN39778.1、ATN39779.1、ATN39780.1 |
| 温带臭虫Cimex lectularius | ClE75X1、ClE75X2、ClE75X3 | XP_014240379.1、XP_014240380.1、XP_014240381.1 | |
| 褐飞虱Nilaparvata lugens | NlE75X1、NlE75X2 | XP_022197917.1、XP_022197918.1 | |
| 膜翅目 Hymenoptera | 哥伦比亚芭切叶蚁Atta colombica | AcE75 | KYM84307.1 |
| 黑毛蚁Lasius niger | LnE75 | KMQ93219.1 | |
| 红胡须蚁Pogonomyrmex barbatus | PbE75X1 | XP_011640767.1 | |
| 小黄家蚁Monomorium pharaonis | MpE75X1、MpE75X2、MpE75X4 | XP_012534660.1、XP_028049966.1、XP_028049973.1 | |
| 西方蜜蜂Apis mellifera | AmE75X1 | XP_006564380.1 | |
| 丽蝇蛹集金小蜂Nasonia vitripennis | NvE75 | XP_008204671.1 | |
| 双翅目 Diptera | 黑腹果蝇Drosophila melanogaster | DmE75A、DmE75B、DmE75C、DmE75D | AAN11688.1、AAF49282.3、AAN11687.1、AAN11689.1 |
| 埃及伊蚊Aedes aegypti | AaE75X1、AaE75X2、AaE75X3 | XP_021704146.1、XP_011493380.2、XP_001652743.3 | |
| 橘小实蝇Bactrocera dorsalis | BdE75 | AGF44569.1 | |
| 鞘翅目 Coleoptera | 马铃薯甲虫Leptinotarsa decemlineata | LdE75X1、LdE75X2、LdE75X3 | AKN56577.1、AKN56578.1、ALU57795.1 |
| 赤拟谷盗Tribolium castaneum | TcE75X1、TcE75X2、TcE75X3、TcE75X4、TcE75X5 | XP_008197843.1、XP_008197844.1、XP_971362.2、XP_008197845、XP_015838722.1 | |
| 蜚蠊目 Blattodea | 德国小蠊Blattella germanica | BgE75A、BgE75B、BgE75C、BgE75D、BgE75E | CAJ87513.1、CAJ87514.1、CAM97373.1、CAM97374.1、CAM97375.1 |
| 湿木白蚁Zootermopsis nevadensis | ZnE75X1、ZnE75X2、ZnE75X3 | XP_021938067.1、XP_021938068.1、XP_021938069.1 | |
| 鳞翅目 Lepidoptera | 家蚕Bombyx mori | BmE75A、BmE75B、BmE75C、BmE75D | NP_001106079.1、NP_001106080.1、NP_001037042.1、BAR88292.1 |
| 直翅目Orthoptera | 飞蝗Locusta migratoria | LmE75A、LmE75B、LmE75C | MN584732、MN584733、MN584734 |
新窗口打开|下载CSV
1.3 LmE75亚型在不同组织及发育时期的表达特性分析
1.3.1 样品收集 为了研究LmE75亚型在不同组织和发育时间的表达特性,选用5龄第2天(N5D2)飞蝗若虫,在体视显微镜下解剖触角、脑、前肠、胃盲囊、中肠、后肠、精巢、卵巢、体壁、脂肪体、肌肉和翅12个组织;选取4—5龄每天(N4D1—N4D5和N5D1—N5D7)若虫第2—3腹节处体壁立即冻存于液氮中,-80℃保存备用。以上所有样品均取4个生物学重复,每个生物学重复至少4头若虫。1.3.2 总RNA提取、cDNA合成及RT-qPCR 提取上述组织与发育时间的样品总RNA,具体步骤均按照 RNAisoTM Plus(TaKaRa)试剂说明书操作,用1.5%的琼脂糖凝胶电泳检测所提取RNA的质量,利用NanoDrop 2000定量各样品的RNA浓度,并检验A260/A280和A260/A230的值。以1 μg总RNA为模板,根据Reverse Transcriptase M-MLV(RNase H-)(TaKaRa)反转录试剂盒说明书,合成cDNA。运用Primer 3.0软件设计3个LmE75亚型的特异性表达引物,以β-actin作为内参[18,19],引物信息见表2。利用RT-qPCR方法检测LmE75A、LmE75B和LmE75C在不同组织和发育时间的表达特性。在Applied Biosystems 7300 real-time PCR system上进行RT-qPCR,PCR反应体系按照SYBR ? Green real-time PCR Master Mix(TaKaRa)说明书进行配置,扩增程序第一步为预变性10 min,第二阶段为95℃ 15 s,退火温度60℃ 1 min,40个循环;DNA熔解曲线为65—95℃,每步上升1℃。每个样品均设置3个生物学重复和2个技术重复,基因相对表达量采用2-ΔCt法[20]进行分析,每个组织与发育时间点的数据以平均数±标准差表示。采用SPSS中的 Tukey’s HSD 进行差异显著性检验,柱形图上不同字母代表基因表达差异显著。
Table 2
表2
表2所用RT-qPCR和RNAi的引物信息
Table 2
| 基因Gene | 引物序列 Primer sequence (5′-3′) | 用途 Application | 产物长度 Length of product (bp) |
|---|---|---|---|
| β-actin-F/R | CGAAGCACAGTCAAAGAGAGGTA/GCTTCAGTCAAGAGAACAGGATG | RT-qPCR | 156 |
| LmE75A-F/R | TTCCGTGTCCTCCAAGCTAG/ACCTTGCACGGGATGATCTC | 111 | |
| LmE75B-F/R | ATGATCCTGTCCGACGGC/CCCGTCGAACTCTATGTGGA | 150 | |
| LmE75C-F/R | ATGACTGTGAAGGACCCGG/ACTCTTCTAGAACCCTGCCT | 133 | |
| LmE75-F/R | CTGCTCGGTGGTGGTGAT/GTCATCTTGAAGGCGAGCAG | 85 | |
| LmEcR-F/R | GACAAACTGCTACGGGAAGA/CTATCGTTTCTCCCATACCAG | 172 | |
| dsLmE75-F/R | taatacgactcactatagggAGCTGGAGGACCAGTCTGC/taatacgactcactatagggGCGAAGTCGAACATGGAGTC | RNAi | 460 |
| dsLmEcR-F/R | taatacgactcactatagggCAGCAACGCCGCACCCT/taatacgactcactatagggCACTGGTACACGGCATTT | 408 | |
| dsGFP-F/R | taatacgactcactatagggTGGAGAGGGTGAAGG/taatacgactcactatagggGGCAGATTGTGTGGAC | 712 |
新窗口打开|下载CSV
1.4 20E诱导表达分析
1.4.1 20E诱导 为了检测LmE75对20E诱导的响应,配置10%的乙醇并将20E(Sigma)稀释至浓度为5 μmol·L-1保存备用,具体参考文献[21]的方法;挑取健康的5龄第2天(N5D2)若虫注射10 μg 20E,同时注射等体积的10%的乙醇作为对照组,分别在注射3、6、12 h后取飞蝗第2—3体节的体壁提取RNA,并反转录为cDNA。每个时间点的对照组和处理组均设置5个生物学重复,4头试虫为1个生物学重复,共计20头试虫。采用RT-qPCR方法检测LmE75在对照组和20E处理组各时间点中的表达量。1.4.2 干扰20E核受体LmEcR后LmE75的表达变化 为了进一步检测LmE75响应20E诱导表达情况,首先合成LmEcR的dsRNA,具体参考文献[22]的方法。在N5D2若虫注射10 μg LmEcR双链 RNA(dsLmEcR),以注射GFP的双链RNA(dsGFP)作为对照组。注射48 h后取飞蝗第2—3体节的体壁提取RNA,并反转录为cDNA。对照和注射dsLmEcR组均设置5个生物学重复,4头试虫为1个生物学重复,共计20头试虫。采用RT-qPCR方法检测LmEcR和LmE75的表达量。1.4.1和1.4.2的数据采用student t-test方法进行差异表达分析,*、**和***分别表示在P<0.05、P<0.01、P<0.001水平差异显著。
1.5 基于RNAi的LmE75功能分析
1.5.1 dsRNA合成与注射 根据3个LmE75亚型共同区域的核苷酸序列,在E-RNAi(http://www.dkfz.de/ signaling/e-rnai3/idseq.php)网站设计dsRNA引物,引物信息见表2。以稀释后的LmE75和GFP质粒DNA分别为模板进行PCR扩增以获得用于体外转录dsRNA的模板,按照2×Taq PCR MasterMix(TIANGEN)说明书进行PCR反应体系的配置及反应程序的设置。PCR产物的回收纯化按照FastPure Gel DNA Extraction Mini Kit(Vazyme)说明书操作,之后用1.5%的琼脂糖凝胶电泳检测扩增条带的单一性。利用纯化后的DNA产物为模板,参照T7 RiboMAXTM Express RNAi System(Promega)试剂盒说明书步骤分别合成LmE75和GFP的dsRNA,利用NanoDrop 2000定量各样品的dsRNA浓度和纯度。用ddH2O将合成的dsRNA稀释至终浓度为2 μg·μL-1,置于-80℃冰箱保存备用。挑取N5D1的若虫进行dsLmE75和dsGFP的注射。每头试虫的dsRNA注射量均为10 μg,设置3个生物学重复,20头若虫为1个生物学重复,共计60头试虫。注射完毕后,将所有试虫放置于养虫室内细心饲养用于后续试验。1.5.2 RNAi后LmE75表达量检测及表型观察 注射dsLmE75和dsGFP 48 h后分别收集对照组和处理组的第2—3体节体壁提取RNA,反转录为cDNA,采用 RT-qPCR方法检测LmE75的沉默效果,设置3个生物学重复,每组3头试虫,2个技术重复,RT-qPCR方法与1.3的检测方法相同,采用2-ΔCt法对LmE75的相对表达量进行计算,数据处理及统计方法参考1.4,剩余试虫用来观察表型并拍照。
1.5.3 H&E 染色 为了观察干扰LmE75后对飞蝗表皮形成的影响,分别解剖对照组脊线开裂时若虫和处理组相应时间点以及最终死亡时的飞蝗体壁组织。取其第4—5腹节体壁,用3%戊二醛固定,4℃过夜以制备石蜡切片。固定后的体壁样品经过脱水和石蜡包埋后进行5 μm切片,苏木精和伊红(H&E)进行染色,具体方法参照文献[23],封片后利用Olympus BX51显微镜(Japan)观察注射dsLmE75和dsGFP的体壁结构。
2 结果
2.1 LmE75序列特性
根据其结构特征,将获得的3个飞蝗LmE75命名为LmE75A、LmE75B和LmE75C,GenBank登录号分别为MN584732、MN584733和MN584734。LmE75A、LmE75B和LmE75C的cDNA序列长度分别为2 238、2 280和2 271 bp,编码745、759和756个氨基酸;使用ExPASy网站上的相关软件预测蛋白分子量及等电点,LmE75A、LmE75B和LmE75C的相对分子量分别为81.60、82.55和82.37 kD,其pI均为碱性,分别为8.43、8.08和8.32。3个LmE75亚型具有典型的核受体结构域:A/B域、C域(DBD)、D域、E域(LBD)和F域(图1-A)。飞蝗3个E75亚型N端A/B域有较小的差异,LmE75A和LmE75B以及LmE75C的A/B域核苷酸一致度为45.0%和47.7%,LmE75B和LmE75C的A/B域核苷酸一致度为64.1%(图1-B)。LmE75A、LmE75B和LmE75C的DBD和LBD结构域与其他昆虫的DBD和LBD结构域具有高度一致度,其中DBD结构域包含两个锌指区域,每一个具有4个半胱氨酸残基,LBD结构域中包含4个参与血红素结合的重要氨基酸残基(图1-C)。图1
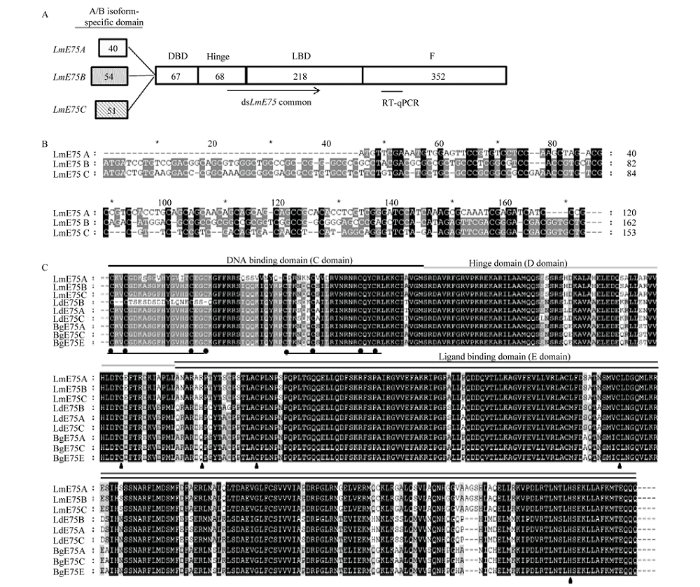 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图13个LmE75亚型序列分析及不同昆虫E75氨基酸序列多重比对
A:3个LmE75亚型结构域分析 Domain structure analysis of the three LmE75 nuclear receptor isoforms;B:3个LmE75亚型A/B域核苷酸序列比对 Nucleotide alignment of the A/B domain of three LmE75 isoforms;C:LmE75亚型氨基酸序列与马铃薯甲虫和德国小蠊E75多重比对 Multiple alignment of LmE75 isoforms with E75 of L. decemlineata and B. germanica。在序列的上方标记DNA结合域、铰链域和配体结合域。序列下方的圆圈指示锌指中的4个Cys残基,三角形标记参与血红素结合的重要氨基酸残基The DNA binding domain, hinge domain and ligand binding domain are tagged above the sequence, the circles indicate that there are four Cys residues in the zinc finger, and the important amino acid residues involved in heme binding are marked with triangles
Fig. 1Sequence analysis of three LmE75 isoforms and amino acid multiple alignment with E75 in different insects
2.2 系统发育分析
E75的系统发育分析表明,发育树分别形成了鞘翅目类群、半翅目类群、蜚蠊目类群、膜翅目类群、直翅目类群、鳞翅目类群和双翅目类群,其中半翅目类群分为两支,绿盲蝽(Apolygus lucorum)和温带臭虫(Cimex lectularius)的E75聚为一支,褐飞虱(Nilaparvata lugens)两个E75亚型单独聚为一支,鳞翅目类群和双翅目类群进而形成长翅亚群总支,飞蝗中的3个LmE75亚型属于直翅目类群(图2)。图2
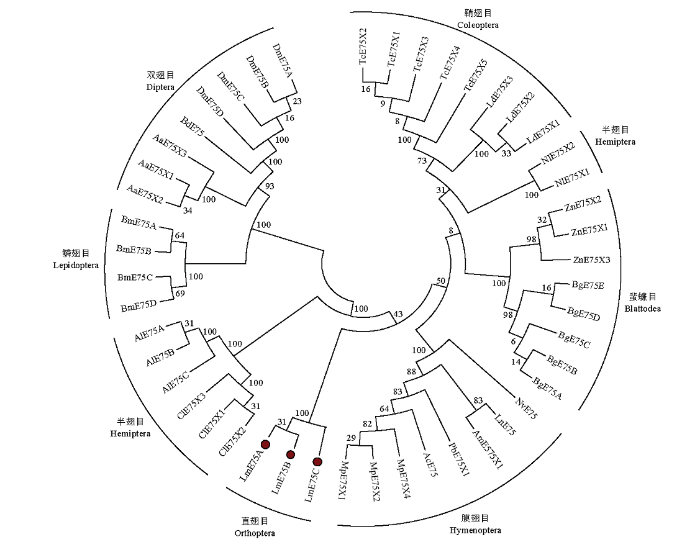 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2昆虫E75系统发育分析
Fig. 2Phylogenetic analysis of E75 from different insect species
2.3 LmE75亚型基因组织和时期表达特性
RT-qPCR分析结果表明3个LmE75亚型具有不同的组织和发育表达特性。LmE75A在体壁和肌肉中高表达;LmE75B在体壁中高表达,其次是前肠;LmE75C特异性在体壁高表达;LmE75A在4龄和5龄均呈现先升高后降低的表达趋势,在N4D3—N4D4以及N5D4—N5D6表达量最高;LmE75B在4龄和5龄的表达趋势一致,均在龄期中一天表达量最高(N4D3和N5D5),蜕皮前后表达量均较低;LmE75C在4龄和5龄均呈现逐渐升高的表达趋势,在蜕皮前表达最高(图3)。图3
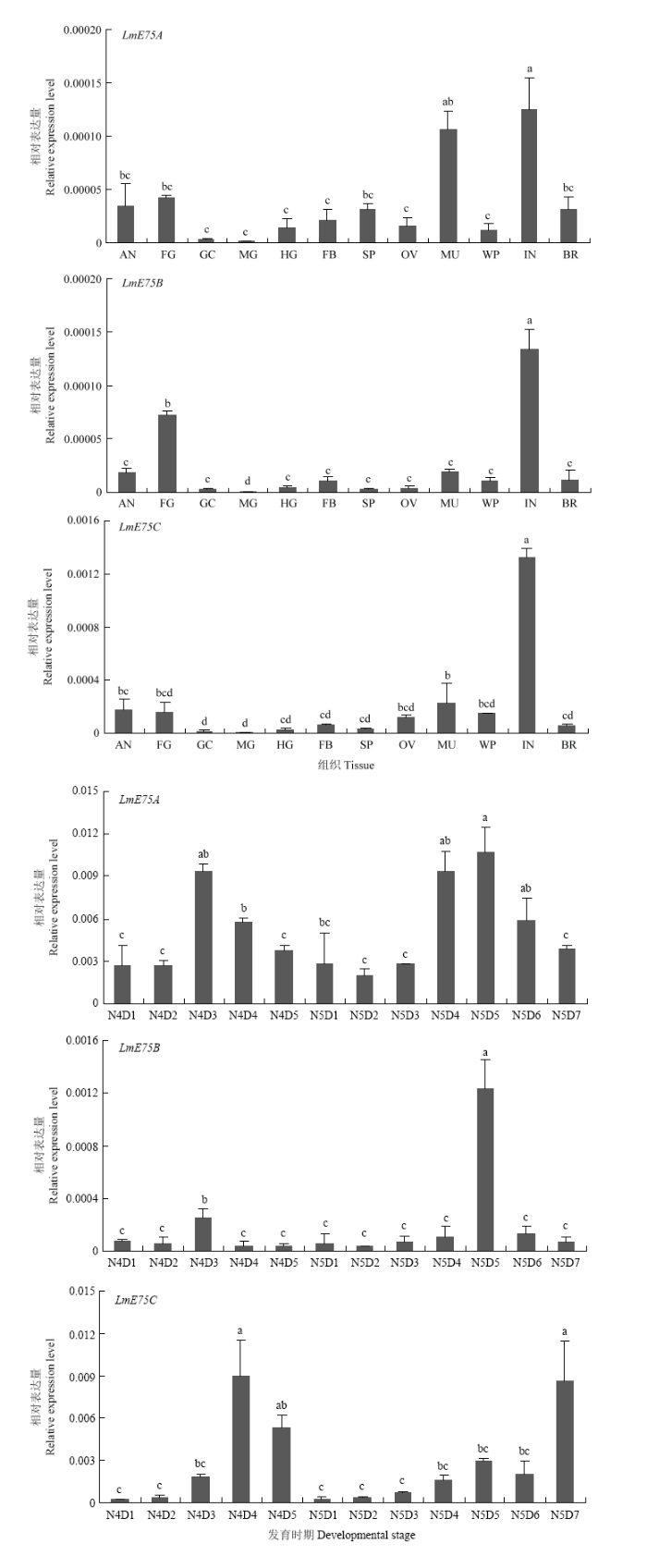 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3LmE75在5龄飞蝗不同组织和4—5龄不同发育天数中的mRNA表达
AN:触角Antennae;FG:前肠Foregut;GC:胃盲囊Gastric caeca;MG:中肠 Midgut;HG:后肠 Hindgut;FB:脂肪体 Fat body;SP:精巢 Sperm;OV:卵巢 Ovary;MU:肌肉 Muscle;WP:翅 Wing;IN:体壁 Integument;BR:脑Brain
Fig. 3The mRNA expression of LmE75 in different tissues and days from 4th-5th instar nymphs of L. migratoria
2.4 20E诱导表达及LmEcR RNAi后LmE75的表达变化
为了检测LmE75是否受20E诱导,选取N5D2的飞蝗若虫进行了20E和dsLmEcR注射,RT-qPCR结果如图所示,与对照组相比,LmE75在20E诱导3 h和6 h后表达量显著上调,分别上调了4.2倍和4.5倍(图4-A);注射dsLmEcR 48 h后LmEcR的沉默效率为83%,LmE75的表达量亦显著被抑制,与对照组相比其表达量下调了59%(图4-B)。图4
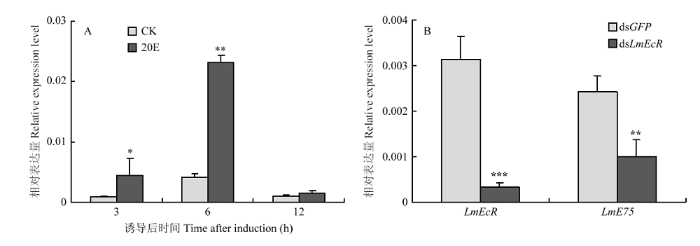 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图420E诱导及干扰LmEcR后LmE75的表达
Fig. 4The relative expression of LmE75 after injection with 20E and dsLmEcR
2.5 基于RNAi的LmE75的生物学功能
RT-qPCR的结果显示,注射dsLmE75的飞蝗LmE75与注射dsGFP对照组相比被显著沉默(图5-A)。对照组7 d羽化为成虫,而注射dsLmE75组7 d后飞蝗进食较对照组明显减少,活动力下降,且100%出现严重的发育延迟现象,5龄若虫期长达13—18 d,并未出现蜕皮迹象,死亡时外形无显著变化(图5-B)。体壁H&E染色的结果显示,对照组脊线开裂时有皮层溶离现象,旧表皮降解,新表皮明显形成。dsLmE75处理组则未观察到皮层溶离,旧表皮不降解,亦无新表皮的形成(图5-C)。图5
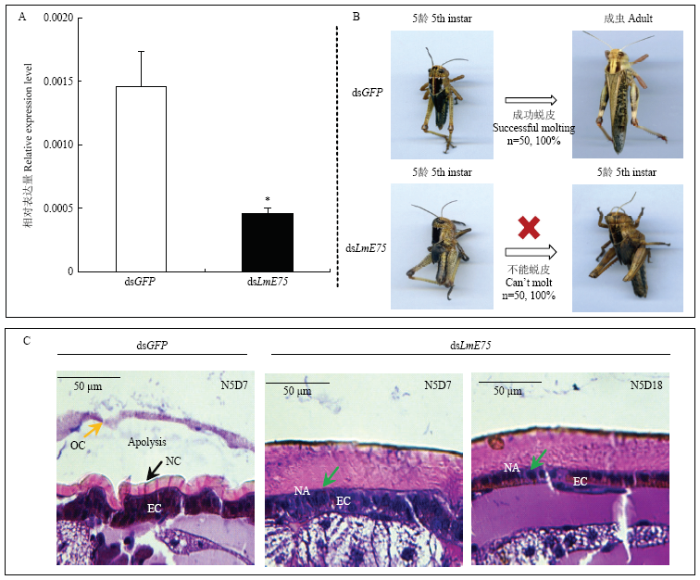 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5注射dsLmE75后表型分析与飞蝗体壁组织学观察
OC:旧表皮Old cuticle;NC:新表皮New cuticle;EC:皮细胞层 Epidermal cell;NA:无皮层溶离 No apolysis
A:注射dsGFP和dsLmE75后LmE75的表达量The expression of LmE75 after dsGFP and dsLmE75 injection;B:注射dsLmE75后的表型Representative phenotypes of the nymphs after dsLmE75 injection;C:注射dsLmE75后的体壁切片图,dsGFP为对照组Histological observation of the integument after dsLmE75 injection, dsGFP is the control
Fig. 5Phenotypic analysis and histological observation of the integument of 5th instar nymphs of L. migratoria after injected with dsLmE75
3 讨论
本研究基于飞蝗转录组数据库搜索获得E75的3条cDNA序列,根据其结构特征分别命名为LmE75A、LmE75B和LmE75C。在黑腹果蝇[7]、家蚕[24]、赤拟谷盗[15]、马铃薯甲虫[16]和德国小蠊[17]等昆虫中也发现E75具有不同数量的亚型,如马铃薯甲虫中有3个E75亚型,黑腹果蝇有4个E75亚型,德国小蠊有5个E75亚型。LmE75的3个亚型具有典型的核受体结构域:一个非依赖配体的激活结构域(A/B结构域)、DNA结合域(DBD,C结构域)、铰链连接域(D结构域)、配体结合域(LBD、E结构域)和F结构域。飞蝗LmE75A、LmE75B和LmE75C中,A/B结构域分别由40、54和51个氨基酸组成,A/B结构域在核受体成员间高度可变,表明其来源于同一个基因的不同剪切方式。飞蝗LmE75的3个亚型与其他昆虫的DBD和LBD结构域具有高度一致度,DBD结构域中包含两个锌指区域,有研究报道其中的4个半胱氨酸残基可参与单个锌原子与激素响应原件DNA序列上的结合[25],也有一些昆虫如马铃薯甲虫LdE75A中只有一个锌指区域。果蝇E75是血红素传感器,位于LBD结构域的血红素中心能与双原子分子(CO或NO)结合[26],并且含有4个参与血红素结合的重要氨基酸残基,其中第1和第3个半胱氨酸残基以及第4个组氨酸在飞蝗中是保守的。本文采用RT-qPCR技术研究了3个LmE75亚型在5龄飞蝗不同组织中的表达特性,结果表明3个LmE75亚型具有不同的组织表达特性。LmE75A在体壁和肌肉中高表达;LmE75B在体壁中高表达,其次是前肠;LmE75C特异性在体壁高表达;LmE75在体壁中特异性高表达,推测该基因可能参与了飞蝗表皮的发育过程。E75的多组织分布特性在家蚕[24]、黑腹果蝇[27]、烟草天蛾[28]和马铃薯甲虫[16]中也有报道,但不同昆虫E75亚型的表达特性有所不同,如马铃薯甲虫中3个E75亚型均在体壁低表达,而在马氏管、中肠、腹神经节、脑-心侧体-咽侧体复合体等多个组织高表达[16]。时期表达分析发现,3个LmE75亚型在飞蝗若虫期的表达量并不一致,LmE75A和LmE75B在4龄和5龄均为蜕皮前后表达量均较低,但LmE75A表达量高于LmE75B;LmE75C在4龄和5龄蜕皮前表达量最高。家蚕中3个E75亚型也表现出不同的发育表达模式[24],而在马铃薯甲虫中,3个E75亚型具有相似的发育表达特性[16],表明不同昆虫E75转录本在各龄期可能发挥着不同的功能,从而共同完成E75的功能。LmE75在5龄若虫中期表达量较高,这一表达模式与20E滴度[22]正相关,推测LmE75的表达可能受20E的调控。事实上,体内注射20E诱导3 h和6 h后LmE75均显著上调,沉默20E受体基因LmEcR后LmE75表达显著下调。类似结果在其他昆虫中也有报道,如家蚕中3个E75亚型均在mRNA和蛋白水平表现出对20E的响应[24]。马铃薯甲虫中,体内注射20E和体外中肠培养均表明20E可直接诱导LdE75A和LdE75B表达[16]。德国小蠊中,解剖脂肪体进行体外组织培养,加入20E后可诱导BgE75的4个亚型(BgE75A、BgE75B、BgE75C、BgE75E)表达[17]。
目前仅在黑腹果蝇[7]、德国小蠊[17]和褐飞虱[29]中报道了E75亚型的功能,结果发现E75亚型功能存在冗余现象,如德国小蠊不同转录本RNAi均能下调各自靶基因的表达量,但其他转录本上调,最终没有可见表型出现。因此本文选择3个LmE75亚型共同区域设计dsRNA,以全部沉默3个LmE75转录本进一步研究LmE75在飞蝗中的生物学功能,dsLmE75注射至5龄第1天后飞蝗出现发育延迟现象,死亡率达100%,且体壁无皮层溶离现象。类似表型也见于其他昆虫,如赤拟谷盗在幼虫阶段沉默TcE75后,一部分幼虫在静息阶段死亡,另一些幼虫在蜕皮时死亡[15]。在德国小蠊中,注射dsBgE75的6龄若虫可存活长达80 d,最终不能蜕皮为成虫而死亡[17]。马铃薯甲虫中LdE75通过调节20E和JH的滴度影响其信号传导通路,沉默LdE75后导致个体不能正常化蛹,在入土18 d后,大多数个体死亡[16]。以上研究表明E75功能在完全变态和不完全变态昆虫中是保守的。
蜕皮是昆虫等特有的生命现象,表皮是昆虫体壁的典型结构,脂类、蛋白质以及几丁质是其主要成分,并且昆虫蜕皮受激素调控。解析昆虫蜕皮发育关键基因的功能是RNAi技术应用于害虫防治的关键环节,目前关于飞蝗蜕皮发育相关基因的研究主要集中在以下几个方面:(1)蜕皮激素和保幼激素信号通路相关基因,如蜕皮激素受体EcR[22]、核受体HR3[30]和HR39[31]等;(2)表皮脂代谢关键基因,如表皮碳氢化合物合成的基因CYP4G102[32]、参与表皮脂转运的基因ABCH-9C[33]等;(3)几丁质代谢以及排布关键基因,如几丁质合成酶1[34]、几丁质酶5[21]、几丁质脱乙酰基酶基因[35]等;(4)表皮蛋白基因,如ACP7[36]等。本文研究的E75是蜕皮激素信号通路中的关键核受体基因,基因沉默可导致飞蝗100%不蜕皮死亡,比较这些基因作用机制以及RNAi效果的差异可为筛选更合适的害虫控制靶标提供基础数据。
4 结论
飞蝗核受体LmE75有3个亚型,均具有典型的核受体结构域。3个LmE75亚型基因具有不同的组织和发育表达特性。体内20E诱导及LmEcR RNAi试验表明,LmE75受LmEcR介导的20E信号通路调控。基于RNAi功能分析发现,显著沉默LmE75后飞蝗发育延迟导致100%不蜕皮死亡。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1152/physrev.2001.81.3.1269URL [本文引用: 1]
DOI:10.1016/j.mce.2013.09.009URL [本文引用: 1]

While the structures of the DNA- and ligand-binding domains of many nuclear receptors have been determined in great detail; the mechanisms by which these domains interact and possibly 'communicate' is still under debate. The first crystal structures of receptor dimers bound to ligand, DNA and coactivator peptides provided new insights in this matter. The observed binding modes revealed exciting new interaction surfaces between the different nuclear receptor domains. Such interfaces are proposed to be the route through which allosteric signals from the DNA are passed on to the ligand-binding domain and the activating functions of the receptor. The structural determinations of DNA-bound receptor dimers in solution, however, revealed an extended structure of the receptors. Here, we discuss these apparent contradictory structural data and their possible implications for the functioning of nuclear receptors. (C) 2013 Elsevier Ireland Ltd.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/S0965-1748(97)00075-1URL [本文引用: 1]
DOI:10.1146/annurev-ento-120710-100607URL [本文引用: 1]

The nuclear receptors (NRs) of metazoans are an ancient family of transcription factors defined by conserved DNA- and ligand-binding domains (DBDs and LBDs, respectively). The Drosophila melanogaster genome project revealed 18 canonical NRs (with DBDs and LBDs both present) and 3 receptors with the DBD only. Annotation of subsequently sequenced insect genomes revealed only minor deviations from this pattern. A renewed focus on functional analysis of the isoforms of insect NRs is therefore required to understand the diverse roles of these transcription factors in embryogenesis, metamorphosis, reproduction, and homeostasis. One insect NR, ecdysone receptor (EcR), functions as a receptor for the ecdysteroid molting hormones of insects. Researchers have developed nonsteroidal ecdysteroid agonists for EcR that disrupt molting and can be used as safe pesticides. An exciting new technology allows EcR to be used in chimeric, ligand-inducible gene-switch systems with applications in pest management and medicine.
DOI:10.1016/S1534-5807(02)00204-6URL [本文引用: 4]

Abstract
Isoform-specific null mutations were used to define the functions of three orphan members of the nuclear receptor superfamily, E75A, E75B, and E75C, encoded by the E75 early ecdysteroid-inducible gene. E75B mutants are viable and fertile, while E75C mutants die as adults. In contrast, E75A mutants have a reduced ecdysteroid titer during larval development, resulting in developmental delays, developmental arrests, and molting defects. Remarkably, some E75A mutant second instar larvae display a heterochronic phenotype in which they induce genes specific to the third instar and pupariate without undergoing a molt. We propose that ecdysteroid-induced E75A expression defines a feed-forward pathway that amplifies or maintains the ecdysteroid titer during larval development, ensuring proper temporal progression through the life cycle.DOI:10.1016/S0303-7207(99)00022-2URL [本文引用: 2]
DOI:10.1016/j.ydbio.2004.04.028URL [本文引用: 2]
DOI:10.1111/ejb.1994.221.issue-2URL [本文引用: 2]
[本文引用: 2]
DOI:10.1074/jbc.M203581200URL [本文引用: 2]
DOI:10.1111/(ISSN)1432-1033URL [本文引用: 2]
DOI:10.2108/zsj.23.1085URL [本文引用: 2]
[本文引用: 4]
DOI:10.1111/imb.12197URL [本文引用: 9]
DOI:10.1016/j.ydbio.2007.12.015URL [本文引用: 6]
DOI:10.1371/journal.pone.0098164URL [本文引用: 1]
DOI:10.1371/journal.pone.0071970URL [本文引用: 1]
DOI:10.1006/meth.2001.1262URL [本文引用: 1]
DOI:10.1016/j.ibmb.2015.01.004URL [本文引用: 2]
DOI:10.1111/1744-7917.12406URL [本文引用: 3]
DOI:10.1111/1744-7917.12342URL [本文引用: 1]
DOI:10.1038/srep12114URL [本文引用: 4]
DOI:10.1042/bse0400011URL [本文引用: 1]
DOI:10.1101/gad.2064111URL [本文引用: 1]
DOI:10.1016/j.molcel.2011.07.033URL [本文引用: 1]

The Drosophila ecdysone receptor (EcR/Usp) is thought to activate or repress gene transcription depending on the presence or absence, respectively, of the hormone ecdysone. Unexpectedly, we found an alternative mechanism at work in salivary glands during the ecdysone-dependent transition from larvae to pupae. In the absense of ecdysone, both ecdysone receptor subunits localize to the cytoplasm, and the heme-binding nuclear receptor E75A replaces EcR/Usp at common target sequences in several genes. During the larval-pupal transition, a switch from gene activation by EcR/Usp to gene repression by E75A is triggered by a decrease in ecdysone concentration and by direct repression of the EcR gene by E75A. Additional control is provided by developmentally timed modulation of E75A activity by NO, which inhibits recruitment of the corepressor SMRTER. These results suggest a mechanism for sequential modulation of gene expression during development by competing nuclear receptors and their effector molecules, ecdysone and NO.
DOI:10.1016/j.ydbio.2006.03.049URL [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1016/j.ibmb.2017.11.001URL [本文引用: 1]
[本文引用: 1]
DOI:10.1038/srep29980URL [本文引用: 1]
.
DOI:10.1016/j.ibmb.2017.06.005URL [本文引用: 1]
DOI:10.1016/j.ibmb.2010.08.001URL [本文引用: 1]

Abstract
Chitin synthases are crucial enzymes responsible for chitin biosynthesis in fungi, nematodes and arthropods. We characterized two alternative splicing-derived variants of chitin synthase 1 gene (LmCHS1) from the oriental migratory locust, Locusta migratoria manilensis (Meyen). Each cDNA of the two variants (LmCHS1A and LmCHS1B) consists of 5116 nucleotides that include a 4728-nucleotide open reading frame (ORF) encoding 1576 amino acid residues, and 67- and 321-bp non-coding regions at the5′- and 3′-ends of the cDNA, respectively. The two variants differ only in one exon consisting of 177 nucleotides that encode 59 amino acid residues. The amino acid sequences within this alternative splicing region are 75% identical between the two variants. Both variants were expressed in all the developmental stages. However, LmCHS1A was predominately expressed in the integument whereas LmCHS1B was mainly expressed in the trachea. Our RNAi-based gene silencing study resulted in a dramatic reduction in the levels of the corresponding mRNA in the locust nymphs injected with dsRNA of LmCHS1, or either of its two variants, LmCHS1A and LmCHS1B. Consequentially, 95, 88 and 51% of mortalities were observed in the locusts injected with the LmCHS1, LmCHS1A and LmCHS1B dsRNA, respectively. The phenotypes resulted from the injection of LmCHS1A dsRNA were similar to those from the injection of LmCHS1 dsRNA, whereas the locusts injected with LmCHS1B dsRNA exhibited crimpled cuticle phenotype. Our results suggest that both variants of chitin synthase 1 are essential for insect growth and development.Graphical abstract

View high quality image (130K)
[本文引用: 1]
[本文引用: 1]
