 ,1,2, 楚品品2, 宋帅2, 蔡汝健2, 杨冬霞2, 卞志标2, 勾红潮2, 李艳2, 蒋智勇2, 李春玲
,1,2, 楚品品2, 宋帅2, 蔡汝健2, 杨冬霞2, 卞志标2, 勾红潮2, 李艳2, 蒋智勇2, 李春玲 ,2, 闫鹤
,2, 闫鹤 ,1
,1Construction of lpxM Gene Deletion Strain of Haemophilus parasuis and It's Some Biological Characteristics
YANG Jun ,1,2, CHU PinPin2, SONG Shuai2, CAI RuJian2, YANG DongXia2, BIAN ZhiBiao2, GOU HongChao2, LI Yan2, JIANG ZhiYong2, LI ChunLing
,1,2, CHU PinPin2, SONG Shuai2, CAI RuJian2, YANG DongXia2, BIAN ZhiBiao2, GOU HongChao2, LI Yan2, JIANG ZhiYong2, LI ChunLing ,2, YAN He
,2, YAN He ,1
,1通讯作者:
责任编辑: 林鉴非
收稿日期:2019-09-3接受日期:2019-12-5网络出版日期:2020-08-16
| 基金资助: |
Received:2019-09-3Accepted:2019-12-5Online:2020-08-16
作者简介 About authors
杨君,Tel:15626090558;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (796KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
杨君, 楚品品, 宋帅, 蔡汝健, 杨冬霞, 卞志标, 勾红潮, 李艳, 蒋智勇, 李春玲, 闫鹤. 副猪嗜血杆菌lpxM基因缺失株构建及生物学特性分析[J]. 中国农业科学, 2020, 53(16): 3394-3403 doi:10.3864/j.issn.0578-1752.2020.16.016
YANG Jun, CHU PinPin, SONG Shuai, CAI RuJian, YANG DongXia, BIAN ZhiBiao, GOU HongChao, LI Yan, JIANG ZhiYong, LI ChunLing, YAN He.
0 引言
【研究意义】副猪嗜血杆菌(Haemophilus parasuis,HPS)属于巴斯德菌科、嗜血杆菌属,短杆状的革兰氏阴性(G-)菌[1]。HPS感染者的症状是一系列炎症反应,如多发性浆膜炎、关节炎、脑膜炎等[2,3],使猪致病甚至死亡,死亡率可高达50%[4],每年给养猪业造成巨大损失。基于目前的血清分型方法可将HPS分成15个血清型,其中5型最普遍且表现高毒力[5,6,7,8],证实HPS的毒力因子有外膜蛋白(Omp)、荚膜多糖(Cps)、脂多糖(LPS)、转铁蛋白、菌毛等[9]。LPS被证实是一种内毒素,能致使机体发炎,严重时可导致败血症[10]。LPS结构由三部分组成,即类脂A、核心多糖和O-抗原多糖[11,12],HPS合成的LPS因缺少O-抗原多糖部分,也被称为脂寡糖(LOS)[13]。而类脂A属于LOS的活性部位,是以葡萄糖胺二糖为主体,上面含有多条酰基链,lpxM基因编码形成豆蔻酰酰基链的转移酶,属于其潜在的毒力因子。虽然目前对HPS的毒力基因有一些研究,但对于其致病机制研究的还不够透彻,进一步挖掘HPS的毒力基因对深入研究其致病机制进而防控HPS感染具有重要意义。【前人研究进展】ANDREY[14]等研究对鼠疫杆菌lpxM基因缺失对其毒力和疫苗潜力影响,该基因缺失后阻断了其合成六酰基化的类脂A,在小鼠模型中验证毒力和疫苗保护效力时发现,缺失株的LD50无变化,但其毒力有2.5—1.6倍的降低,免疫保护效力有明显提高。HAUTEVILLE[15]等发现弗氏志贺菌的两个msbB(lpxM)基因对其介导的炎症反应和破坏肠道的上皮细胞具有重要作用,将该基因钝化后能减少刺激人单核细胞TNF-α的分泌和削弱对上皮屏障的炎症破坏作用。XU[16]等研究了lpxM基因对禽致病性大肠杆菌毒力等一些生物学特性的影响,结果lpxM缺失对生长速率和对抗鸡血清杀菌能力无影响,但对鸡巨噬细胞系的入侵及存活能力显著降低,动物试验中也发现了缺失对动物器官的入侵能力降低,以上结果表明lpxM基因对细胞的毒力及其它生物活性具有一定的影响。目前,对HPS的LOS合成相关基因研究有很多,包括多糖合成相关基因galU和galE[4]、rfaD和rfaF[17]、lgtB 和lex-1[13]、lgtF[18]、rfaE[19]等,这些基因的缺失对HPS的毒力、生长、生物被膜形成、血清抗性及对细胞的炎症作用具有一定的影响。上述研究为lpxM基因缺失对HPS生物学功能影响奠定了基础。【本研究切入点】虽然lpxM基因在对多数G-菌生物学特性特别是毒力具有一定影响,但该基因对HPS影响的研究未见报道,lpxM基因对HPS的生长、毒力等生物学特性的影响还未知,故需要对此展开研究。【拟解决的关键问题】验证lpxM基因缺失对HPS的生长情况,生物被膜形成能力、抗血清杀菌能力、对RAW264.7细胞毒力及对临床上常用的13种抗生素和多粘菌素B敏感性部分生物学特性的影响,为研究lpxM基因的功能,揭示HPS的致病机制及开发HPS的基因工程弱毒疫苗奠定一定理论基础。1 材料与方法
1.1 菌种和载体
5型地方分离株副猪嗜血杆菌H45由广东省农业科学院动物卫生研究所猪病研究室分离保存;大肠杆菌DH5α感受态细胞购自北京鼎国生物科技有限公司;RAW264.7细胞、自杀载体PK18mobsacB和辅助质粒pCas由广东省农业科学院动物卫生研究所猪病研究室保存。本研究于广东省农业科学院动物卫生研究所2019年上半年完成。1.2 试剂、培养基和试剂盒
胰蛋白胨大豆琼脂(TSA)培养基、胰蛋白胨大豆肉汤(TSB)培养基和结晶紫购至广东环凯微生物科技有限公司;2×TaqPCRMaster Mix、T4DNA连接酶、DNA限制性核酸内切酶购于大连宝生物工程有限公司;卡那霉素(KanR)购自北京鼎国生物科技有限公司;细菌基因组提取试剂盒、质粒小提试剂盒、胶回收试剂盒及纯化试剂盒购自美国OMEGA公司;细胞毒性LDH检测试剂盒购自美国Promega公司;抗生素药敏纸片购自杭州微生物试剂有限公司。1.3 引物
本研究所用引物均由上海生工生物工程有限公司合成(表1)。Table 1
表1
表1引物序列
Table 1
| 引物Primers | 序列(5′→3′) Sequence | 长度 Length |
|---|---|---|
| kan-F | CATTGCACAAGATAAAAATATAT | 909 bp |
| kan-R | CAATTAACCAATTCTGATTAG | |
| lpxM-up-EcoRI-F | CCGGAATTCACCGCTTGT CCTACAACCTGTTTCGCC | 489 bp |
| lpxM-up-kan-R | TATTTTTATCTTGTGCAATG CAACATTGCCAGGGCTAG | |
| lpxM-kan-down -F | CTAATCAGAATTGGTTAATTG AGGTCGCCAGCTTATCAC | 593 bp |
| lpxM-down-PstI-R | TAACTGCAGACAAGCGGT CCGTATTCCGATTTATGTG | |
| HPS-F | F1:TATCGGGAGATGAAAGAC F2:GTAATGTCTAAGGACTAG | 1090 bp |
| HPS-R | CCTCGCGGCTTCGTC | |
| sacB-F | GCAGGAGGCGCAACTCAA | 986 bp |
| sacB-R | ATTTTAAAGACGTTCGCGC |
新窗口打开|下载CSV
1.4 副猪嗜血杆菌缺失株的构建与鉴定
1.4.1 重组片段的制备 以副猪嗜血杆菌SH0165(GenBank登录CP001321.1)在NCBI上公布基因序列为参考,设计lpxM基因上下游同源臂引物,并在上下游同源臂加上酶切位点和USS序列,以H45基因组为模板通过PCR扩增上下游同源臂片段,质粒pCas为模板扩增卡那抗性表达盒,以这3个片段为模板通过重叠延伸PCR得到同源重组片段M-UKD。1.4.2 重组质粒的构建 将同源重组片段M-UKD和载体PK18mobsacB双酶切,纯化后用T4DNA连接酶过夜连接。将连接好的质粒热击转化进大肠杆菌DH5α感受态细胞,并涂布于含有卡那霉素抗性平板的TSA平板(50ng·μL-1,下同)上,PCR和双酶切测序鉴定重组质粒是否构建成功。
1.4.3 自然转化法构建缺失株 利用构建好的重组质粒PKM-UKD自然转化进H45构建lpxM基因缺失株,转化方法参考文献[4, 20-21]中构建HPS自然转化法。其步骤大致如下:将转化用的受体菌H45接种于TSA(含10%新鲜牛血清和0.1%NAD,下同)平板活化,37℃恒温烘箱过夜培养。将过夜培养的菌用3 mL TSB洗下,调整其OD600为0.9—1.0。取20 μL菌液于1.5 mL EP管中,加入20 μL cAMP(8 mmol·L-1)作用10 min,然后加10 μL(约1μg)质粒,混匀后作用10 min;同时以10 μL无菌水与细菌混匀,作为阴性对照。把作用后的菌与质粒混合物转移到TSA平板上,直径约1 cm,平皿正置于37℃培养5 h后用200 μL TSB洗涤菌体并涂布于含卡那霉素的TSA抗性平板上,37℃倒置培养得到转化子,PCR鉴别是否发生了lpxM基因的缺失。
1.5 缺失株与野生株生长情况比较
挑取活化的基因缺失株H45-△lpxM和野生株H45于5 mL含相应抗性的TSB培养基中,37℃,220 r/min培养12 h左右,利用紫外分光光度计调节两株菌的OD600值保持一致;按1﹕100的比例转接至200 mL含相应抗性的TSB培养基中,连续培养16 h,每间隔1 h测定其OD600值,绘制时间t-OD600的曲线。1.6 缺失株与野生株生物被膜形成能力比较
生物被膜形成能力比较参考文献[1, 4],做一定修改。准备洁净玻璃试管分成3组,1组空白组和2组试验组,每组3根试管,每支玻璃试管中加入3 mL TSB,试验组中每个试管中各加入60μL的H45或H45-△lpxM,空白组只加培养基,将试管静置于37℃培养箱中静置培养24h,移除菌液并用蒸馏水轻轻冲掉游离菌体,室温下用3 mL 1%的结晶紫溶液染色5—10 min后用流水轻缓冲洗至少3次,至冲洗液变为无色为止,将试管倒置晾干后观察结果(拍照),每根试管用1 mL 33%(v/v)醋酸溶解,并630 nm波长下测定吸光度。1.7 缺失株与野生株抗血清杀菌能力比较
抗血清杀菌试验参考文献[22],方法如下:血清来源于3—4周龄未感染HPS的健康小猪,血清过0.22 μm滤膜除菌,置于-80℃保存。取部分血清于56℃水浴锅灭活补体30 min,得到灭活血清。取100 μL灭活或没灭活血清分别与100 μL菌液(107—108cfu·mL-1)混合(菌液﹕血清=1﹕1),37℃,150 r/min振荡培养1 h,梯度稀释涂布平板进行计数,每个样本设置3个平行。1.8 缺失株与野生株对RAW264.7细胞毒性比较
RAW264.7细胞培养至良好状态后转移到96孔板中(105Cells/孔)培养过夜,第二天分别用H45或H45-△lpxM(MOI=10, 106cfu/孔)刺激,不加菌作为阴性对照,作用时间为6、12和24 h,每个时间点收取细胞上清液并使用LDH检测试剂盒进行细胞毒性检验,每组做3个平行。1.9 缺失株和野生株对抗生素敏感性比较
使用 Kirby-Bauer 纸片扩散法(K-B法)进行抗生素敏感性试验[23,24]。本试验使用的抗生素为临床上常用的13种抗生素及多粘菌素B,分别为氨苄西林、阿莫西林克拉维酸、头孢噻吩、头孢氨苄、头孢唑林、头孢拉定、头孢噻肟、庆大霉素、多西环素、磺胺二甲异噁唑、氟苯尼考、阿莫西林、恩诺沙星。实验步骤为,菌液复苏和传代,涂板、贴药敏片,37℃倒置18—24 h,读取抑菌圈直径,每个药敏片做3个平行,耐药结果根据药敏标准判定。1.10 数据处理与分析
Word和Excel软件用于数据的初步统计以及作图,统计学分析和作图使用GraphPad Prism 7。2 结果
2.1 重组片段的扩增
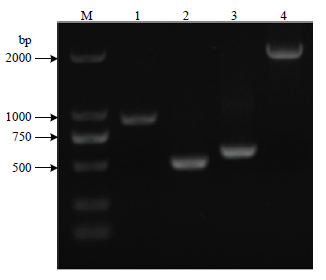
以H45基因组为模板,PCR扩增lpxM基因上游同源臂(489 bp)、下游同源臂(593 bp),以pCas为模板,PCR扩增卡那抗性表达盒(909 bp),重叠延伸PCR将上游、卡那抗性表达盒、下游同源臂连接得到重组片段M-UKD(1 991 bp,图1)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1重组片段的构建
M:DL2000 DNA ;1:kanR抗性表达盒(909 bp);2:lpxM-up上游同源臂(489 bp);3:lpxM-down 下游同源臂(593 bp);4:M-UKD 重组片段(1991 bp)
Fig. 1Construction of recombinant fragments
marker;1: kanR resistance expression cassette (909 bp); 2: lpxM-up upstream homology arm (489 bp); 3: lpxM-down downstream homology arm (593 bp); 4: M-UKD recombinant fragment (1991 bp)
2.2 重组质粒的构建
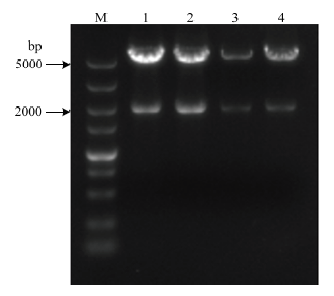
将M-UKD和PK18mobsacB双酶切后过夜连接,转化DH5α,挑取抗性平板上长出的单菌落摇菌,小提质粒进行双酶切鉴定,得到1 991 bp的重组片段M-UKD和5 719 bp的载体片段(图2),将酶切的片段进行测序,将测序结果与目的序列进行比对无误后确定lpxM基因的重组质粒构建成功,命名为PKM-UD。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2重组质粒PKM-UKD双酶切鉴定
M:DL5000 DNA ;1,2,3,4:双酶切载体(5719bp)和片段(1991bp)
Fig. 2Identification of recombinant plasmid PKM-UKD by double enzyme digestion
marker;1,2,3,4:Double digestion vector (5719 bp) and fragment(1991 bp)
2.3 缺失株的鉴定
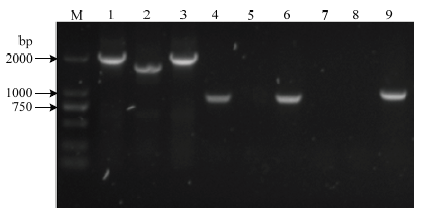
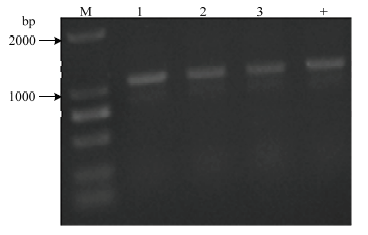
利用3对引物对抗性平板上长出的转化子进行PCR鉴定,以构建好的重组质粒为阳性对照,鉴别其是否发生了目的片段的缺失(图3)。第1对引物lpxM-up-EcoRI-F/ lpxM-down-PstI-R进行扩增,H45扩增得到1 622 bp的条带,H45-△lpxM扩增得到1 991 bp的条带,说明909 bp的卡那抗性表达盒替代了lpxM基因的540 bp部分片段,H45发生了lpxM基因部分片段的缺失;第2对引物kanR-F/R扩增,H45扩增不到条带,而H45-△lpxM扩增得到909 bp的条带,说明卡那抗性表达盒整合到染色体上;第3对引物sacB-F/R进行鉴定,两者均不能扩增到条带,说明质粒在缺失株内发生消除。最后,用引物HPS-F/R鉴定该缺失株是否为HPS,结果显示可扩到条带(图4),说明在卡那抗性平板上长出的转化子是HPS。结果表明关于H45的lpxM基因部分片段缺失的缺失株H45-△lpxM已经构建成功。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3lpxM基因缺失株的鉴定
M:DL2000 DNA marker;1-3:鉴别缺失片段,分别是缺失株、野生株和阳性对照,引物lpxM-up-EcoRI-F/ lpxM-down-PstI-R;4-6:鉴别卡那抗性表达盒,顺序同上,引物kanR-F/R;7-9:鉴别质粒是否消除,顺序同上,引物sacB-F/R
Fig. 3Identification of the lpxM gene deletion strain
M: DL2000 DNA marker; 1-3: Identification of the deletion fragments, the deletion strain, wild strain and positive control, primer lpxM-up-EcoRI-F/ lpxM-down-PstI-R ; 4-6: Identification of the kanR resistance expression cassette, the deletion strain ,wild strain and positive control, primer kanR-F/R; 7-9: Identification of plasmid elimination, the deletion strain, wild strain and positive control, primer sacB-F/R
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4鉴定是否为HPS
M:DL2000 DNA marker;1-3:缺失菌株;+:野生菌株,引物HPS-F/R
Fig. 4Identification of the HPS
M: DL2000 DNA marker; 1-3: the deletion strain; +: wild strain, primer HPS-F/R
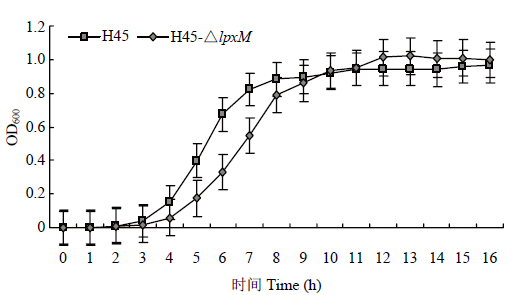
2.4 lpxM基因缺失对HPS生长的影响
H45和H45-△lpxM生长曲线图t-OD600如图5所示,8 h之前前者生长情况慢于后者,但在8 h后两者的生长情况保持一致,说明缺失lpxM基因后对H45的生长情况造成一定的影响。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5野生株和缺失株的生长曲线
Fig. 5Growth curves of wild and deletion strains
2.5 lpxM基因缺失对HPS生物被膜形成的影响
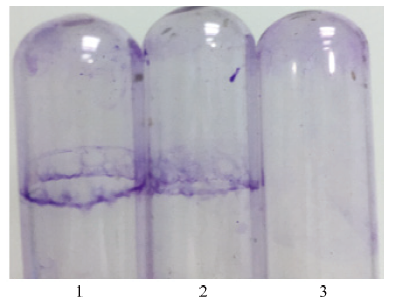
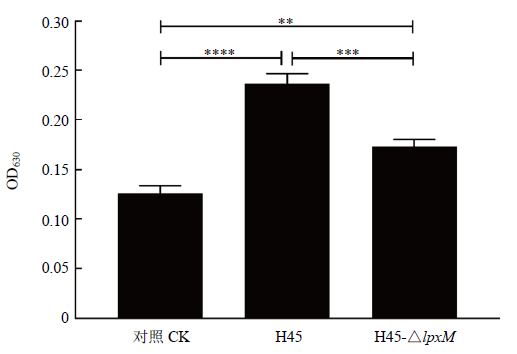
生物被膜形成能力的结果如图6,从左到右依次是H45、H45-△lpxM和空白对照静置培养24 h后生物被膜产生的情况,图6中显示缺失株产生的生物被膜弱于野生株H45。测定OD630值(图7),H45的OD630值为0.237,而H45-△lpxM只有0.173,说明lpxM基因的缺失使得H45生物被膜形成的能力减弱。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6生物被膜形成
1-3:野生株、缺失株和空白对照
Fig. 6Biofilm formation
1-3: wild strains、deletion strains and blank control
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7生物被膜OD630值
Fig. 7Biofilm OD630 value
2.6 lpxM基因缺失对HPS血清抗性的影响
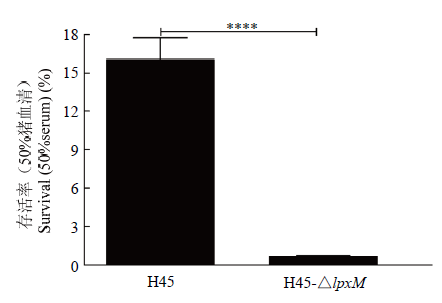
如图8,H45在缺失lpxM基因后在50%的猪血清中存活能力显著降低,由16.1%降到了0.71%,说明缺失该基因后导致H45抵抗血清补体杀菌能力降低,lpxM基因可能与HPS抗血清杀菌的能力相关。图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8血清存活能力
Fig. 8Survival of HPS treated with porcine serum
2.7 lpxM基因缺失对HPS致细胞毒性的影响
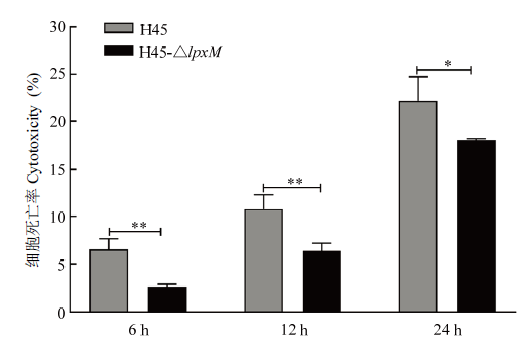
结果如图9,H45和H45-△lpxM刺激RAW264.7细胞后均能引起细胞死亡,作用时间为6、12和24 h,H45对细胞的致死率分别为6.63%、10.86%和22.17%,H45-△lpxM对细胞的致死率分别为2.62%、6.35%和18.01%,随着作用时间的延长,两者对细胞毒性越来越大,但在3个时间点H45-△lpxM对细胞的毒性均低于H45,说明lpxM基因可能与HPS毒力相关。图9
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9细胞毒性试验
Fig. 9Cell cytotoxicity test
2.8 lpxM基因缺失对抗生素敏感性影响
H45和H45-△lpxM对13种临床上常用抗生素和多粘菌素B的药敏结果如表2,两者均对头孢噻吩等10种抗生素和多粘菌素B表现敏感,但是抑菌圈直径存在一定的差异,大多数抗生素后者的抑菌圈要大于前者,说明后者对抗生素的敏感性更高,对氨苄西林、阿莫西林克拉维酸和磺胺二甲异噁唑这3种抗生素,两者的敏感性有较大差异,缺失lpxM基因后,菌株对氨苄西林的耐药性由中介(I)变成敏感(S),对阿莫西林克拉维酸和磺胺二甲异噁唑由耐药(R)变成了敏感(S),说明lpxM基因对H45的抗生素耐药性具有一定的影响,但具体机制还需要进一步研究。Table 2
表2
表2药物敏感性结果(抑菌圈直径mm)
Table 2
| 抗生素名称 Antimicrobial agents | H45 | H45-△lpxM | 抗生素敏感性判断标准 Standard for antibiotic sensitivity |
|---|---|---|---|
| 氨苄西林ampicillin | 20.6(I) | 26(S) | R:<=18;I:19-21;S:>22 |
| 阿莫西林克拉维酸amoxicillin clavulantic acid | 16.8(R) | 22.2(S) | R:<=19;I:--;S:>20 |
| 头孢噻吩cephalothin | 25.5(S) | 37(S) | R:<=14;I:15-17;S:>=18 |
| 头孢氨苄cephalexin | 19(S) | 25(S) | R:<=14;I:15-17;S:>=18 |
| 头孢唑啉cefazolin | 23(S) | 30(S) | R:<=14;I:15-17;S:>=18 |
| 头孢拉定cefradine | 20(S) | 27.5(S) | R:<=14;I:15-17;S:>=18 |
| 头孢噻肟cefotaxime | 35(S) | 26(S) | R:<=14;I:15-17;S:>=18 |
| 庆大霉素gentamicin | 22(S) | 26.8(S) | R:<=12;I:13-14;S:>=15 |
| 多西环素doxycycline | 20(S) | 22.4(S) | R:<=12;I:13-15;S:>=16 |
| 磺胺二甲异噁唑sulfisoxazole | 10(R) | 19(S) | R:<=12;I:13-16;S:>=17 |
| 氟苯尼考florfenicol | 29(S) | 35(S) | R:<=12;I:13-17;S:>=18 |
| 阿莫西林amoxicillin | 20(S) | 23(S) | R:--;I:--;S:19-25 |
| 恩诺沙星enrofloxacin | 16(R) | 21(R) | R:--;I:--;S:28-36 |
| 多粘菌素B polymyxin B | 18.9(S) | 23.4(S) | R:<=8;I:8-11;S:>=12 |
新窗口打开|下载CSV
3 讨论
血清5型的HPS属于强毒株,能引起猪产生一系列炎症反应甚至死亡。LOS是HPS的一个毒力因子,而类脂A是LPS活性部位,其结构影响着LOS的毒力[13]。类脂A是以葡萄糖胺二糖为主体,含有多条酰基链和磷酸基团,而lpxM基因编码的酰基转移酶LpxM负责类脂A合成的最后一步,即将豆蔻酰(C14)酰基链连接到葡萄糖胺二糖3′-位点上,得到完整结构的类脂A[16, 25]。类脂A上酰基链的数量影响LOS的结构,继而影响其毒力。本研究以自杀性质粒PK18mobsacB为载体构建了关于lpxM基因缺失的重组质粒,通过将重组质粒自然转化进HPS得到基因缺失株,继而比较了缺失株和野生株的生长情况、生物被膜形成能力、抗血清杀菌能力、对RAW264.7细胞毒力作用和对部分抗生素的敏感性。通过生长曲线的测定,发现8 h前缺失株生长速度明显慢于野生株,7 h时野生株的OD600为0.8223,H45-△lpxM只有0.5473,8 h之后野生株和缺失株生长情况达到一致。前期明显慢于野生株,后期达到一致,说明lpxM基因的缺失对H45的生长情况具有一定影响,该基因的缺失影响LOS合成继而导致HPS生长变慢,这也与大肠杆菌lpxM基因缺失后生长情况变化情况一致[25]。
生物被膜形成的能力能反映菌株毒力,生物被膜产生能力越强可认为菌株毒力越强,生物被膜能帮助细菌抵御不良的环境的危害,使其逃逸于宿主的免疫系统和抗生素的作用,最终导致其抵抗能力增强[26]。目前的研究证明影响HPS生物被膜形成能力的基因有vacJ[27]、galU和galE[4]等,本研究首次证实lpxM基因与HPS生物被膜形成的能力相关,该基因的缺失导致能力减弱。
血清抗性在HPS先天性免疫防御中发挥巨大作用,被认为是HPS的一个毒力机制,LOS和多糖的合成蛋白CapD参与其对血清补体的抗性[28,29]。根据之前的研究证实HPS的lgtB和lex-1[13]、galU和galE[4]等基因对其抗血清杀菌具有一定影响,本研究也初步证实lpxM基因对HPS在50%猪血清中的抗性具有一定影响,该基因缺失能降低其在血清中的存活能力。
细胞毒性可能不是HPS用来增加血脑屏障通透性的机制,HPS和其分泌的LOS均不能对猪脑微血管内皮细胞(PBMEC)产生毒力[30,31]。而本研究结果表明HPS对RAW264.7细胞具有一定的毒力,并且缺失LOS合成的相关基因lpxM后,对细胞的毒力降低,说明LOS合成的相关基因lpxM对HPS的毒力具有一定影响,但具体机制还需要进一步研究。
lpxM基因是关于类脂A合成的最后一步基因,而类脂A是细胞膜的主要组成部分,多粘菌素B的作用机制是破坏细菌细胞膜的磷脂结构[25]。HPS防治的过程使用大量抗生素,导致耐药性产生。本研究展示lpxM基因缺失对临床上常用的13种抗生素和多粘菌素B的影响,缺失该基因对各种抗生素敏感性提高,部分抗生素从耐药变成敏感,结果与文献[16, 25]中lpxM基因缺失导致大肠杆菌对一些抗生素敏感性提高的结果一致,说明lpxM基因可能与菌株对部分抗生素的敏感性相关。
4 结论
本研究成功构建HPS的5型地方分离株H45的lpxM基因缺失株,通过部分生物学特性比较结果显示,lpxM基因缺失对 HPS 生长速度具有一定影响,生长前期抑制其生长,降低其生物被膜形成能力、抗血清杀菌能力和对RAW264.7细胞毒力,提高对氨苄西林等多种临床上常用抗生素和多粘菌素B的敏感性,对少量的抗生素敏感性甚至发生变化,从耐药变成敏感,这些研究结果对进一步探究lpxM基因功能及揭示HPS的致病机制和相关基因的弱毒疫苗开发提供一定的科学试验依据。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.vetmic.2012.08.006URL [本文引用: 4]
URLPMID:30036124 [本文引用: 1]
[本文引用: 1]
URLPMID:26993332 [本文引用: 1]
URLPMID:25704800 [本文引用: 1]
DOI:10.1016/j.vetmic.2013.07.027URLPMID:23972951 [本文引用: 1]

Haemophilus parasuis is the causative agent of Glasser's disease in pigs, a severe systemic disease that has led to increasing economic losses in the pig industry worldwide. The H. parasuis genome sequence has been completed, but the function and essentiality of the annotated genes remain largely unknown, especially virulence factors. The recent developments in the efficient genetic manipulation of H. parasuis have greatly facilitated the study of gene function, pathogenesis mechanisms and virulence factors. In this review, we provided update information regarding that (i) how the pathogen overcome host immune responses and cell barriers which were tightly associated with the pathogenesis, and (ii) the several recent identification of virulence factors were involved in evading the immune responses and cell barriers in H. parasuis.
URLPMID:19013513 [本文引用: 1]
DOI:10.1146/annurev-biochem-060713-035600URLPMID:24580642 [本文引用: 1]

Lipopolysaccharide molecules represent a unique family of glycolipids based on a highly conserved lipid moiety known as lipid A. These molecules are produced by most gram-negative bacteria, in which they play important roles in the integrity of the outer-membrane permeability barrier and participate extensively in host-pathogen interplay. Few bacteria contain lipopolysaccharide molecules composed only of lipid A. In most forms, lipid A is glycosylated by addition of the core oligosaccharide that, in some bacteria, provides an attachment site for a long-chain O-antigenic polysaccharide. The complexity of lipopolysaccharide structures is reflected in the processes used for their biosynthesis and export. Rapid growth and cell division depend on the bacterial cell's capacity to synthesize and export lipopolysaccharide efficiently and in large amounts. We review recent advances in those processes, emphasizing the reactions that are essential for viability.
[本文引用: 1]
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
URLPMID:23856328 [本文引用: 2]
URLPMID:29628479 [本文引用: 1]
DOI:10.1016/j.micpath.2017.06.035URLPMID:28716662 [本文引用: 1]

The lgtF gene encodes a glucosyltransferase responsible for adding a glucose to the first sugar of heptose I in the synthesis of lipooligosaccharides (LOS). To study the function of lgtF, we constructed an lgtF mutant (DeltalgtF) from Haemophilus parasuis SC096 using a natural transformation system. A highly purified preparation of LOS from DeltalgtF (DeltalgtF-LOS) exhibited an obvious truncation in structure compared to the LOS of the wild-type SC096 strain (WT-LOS). The DeltalgtF-LOS also displayed a significantly reduced ability to induce inflammatory cytokine mRNA expression of tumor necrosis factor alpha (TNF-alpha), interleukin-1alpha (IL-1alpha), IL-1beta, IL-6 and IL-8 in porcine alveolar macrophages (PAMs) in comparison with the WT-LOS. Furthermore, we also found that DeltalgtF-LOS-treated cells had significantly decreased phospho-p65 and phospho-p38, and inhibited IkappaBalpha degradation. These findings suggested that the lgtF gene mediated LOS induction of pro-inflammatory cytokines in PAMs by regulating the NF-kappaB and MAPKs signaling pathways during H. parasuis infection.
URLPMID:25078003 [本文引用: 1]
URLPMID:27733782
URLPMID:30003899
URLPMID:22221379 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:29932223 [本文引用: 3]
URLPMID:24129258 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.vetmic.2013.01.037URLPMID:23434184 [本文引用: 1]

The capD gene, encoding a polysaccharide biosynthesis protein, was identified previously as a differential gene between Haemophilus parasuis virulent strain Nagasaki and avirulent strain SW114; however, the characteristics of this gene associating with the pathogenicity of H. parasuis remain unclear. Here, the capD deletion mutant (DeltacapD) and its complement strain (C-capD) were generated in H. parasuis virulent strain SH0165. The deletion of capD gene significantly attenuated the pathogenicity of the SH0165 strain, while the complementation of this gene largely recovered the pathogenicity to piglets. Additionally, the DeltacapD strain could not be recovered from piglets after challenge, while both SH0165 and C-capD strains were recovered from most systemic sites. Moreover, the DeltacapD strain exhibited an extreme sensitivity to the complement-mediated killing compared with SH0165 strain, while its serum-resistance ability largely restored with the capD gene complementation. These data present the evidence that the capD gene is a novel pathogenicity-associated determinant and involved in serum-resistance ability of H. parasuis.
URLPMID:22857892 [本文引用: 1]
[本文引用: 1]
URLPMID:18387279 [本文引用: 1]
