 ,1, 吴思逢2, 李程勋1, 郑开斌
,1, 吴思逢2, 李程勋1, 郑开斌 ,1
,1Composition Analysis of Gas Phase and Liquid Phase of Fresh Floral Water Extract and Fresh Flowers Cell Sap from Gardenia jasminoides
XU XiaoYu1, LI AiPing ,1, WU SiFeng2, LI ChengXun1, ZHENG KaiBin
,1, WU SiFeng2, LI ChengXun1, ZHENG KaiBin ,1
,1通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-03-25接受日期:2020-06-10网络出版日期:2020-11-16
| 基金资助: |
Received:2020-03-25Accepted:2020-06-10Online:2020-11-16
作者简介 About authors
徐晓俞,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (537KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
徐晓俞, 李爱萍, 吴思逢, 李程勋, 郑开斌. 栀子鲜花花水和鲜花细胞液气相和液相的成分分析[J]. 中国农业科学, 2020, 53(22): 4710-4726 doi:10.3864/j.issn.0578-1752.2020.22.017
XU XiaoYu, LI AiPing, WU SiFeng, LI ChengXun, ZHENG KaiBin.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】栀子(Gardenia jasminoides Ellis)为茜草科栀子属灌木状植物,又名水横枝、黄果子、黄叶下、山黄枝、黄栀子、山栀子等。栀子花气味芳香,甜美优雅,可用于提取挥发油,已应用于多种香型化妆品、香皂香精以及高级香水香精的调香剂[1,2,3]。栀子纯露也称栀子花水,是水蒸气蒸馏提取栀子鲜花精油过程中得到的副产物,其饱和了一部分的精油成分,具有与栀子精油类似的香气,目前在市场上已被作为护肤品等使用。栀子鲜花细胞液是在低温条件下利用低温抽湿工艺冷凝收集的栀子鲜花细胞原液[4,5],含有一定的栀子花精油成分,具有栀子鲜花的自然花香。近年来栀子鲜花细胞液越来越多地被应用于化妆品、香精香料、功能保健品等领域,但是关于细胞液的成分组成还未知,而且人们对栀子鲜花细胞液的评价褒贬不一。栀子鲜花花水和细胞液不同于栀子花精油,但却具有栀子的花香,在功能化妆品、香精香料、保健品中有一定的应用。因此,对栀子花花水与细胞液进行液相与气相的全组分分析,对科学评价栀子花花水与栀子花细胞液的异同优劣及相关产品开发具有重要意义。【前人研究进展】目前对于栀子花香气成分的研究多集中在对栀子鲜花头香成分的分析[6,7,8,9,10]、栀子花挥发油成分的分析[11,12,13]、栀子花挥发油提取工艺的优化研究[14,15]等方面。但关于花水和细胞液的液相成分分析方法以及气相成分分析未见相关报道。精油是指从植物的花、叶、茎、根或果实中,通过水蒸气蒸馏法提取而得的挥发性芳香物质[16],是挥发性化合物的聚集,浓度大,刺激性强,往往不能直接用于皮肤,而花水和细胞液除了有精油中所具有的大部分挥发性成分外,其液相部分也含有许多具有不同功效的化合物,具有精油无可比拟的功效,在化妆品市场已广泛应用,因此栀子鲜花花水和鲜花细胞液也同样具有栀子花精油无法具有的独特优势,应用价值突出。液质联用分析具有分析时间短、检测限低、灵敏度高的特点,广泛用于植物提取物的成分分析,特别是对于微量成分具有快速检出的优势,刘田园[17]认为对于金银花水这类含有挥发性成分的提取物,快速地检测其中所含的微量水溶性成分才能更准确地反映植物提取物的成分组成,可为提取物功效研究奠定基础。因此,液质联用技术有助于分析栀子鲜花花水和鲜花细胞液的液相成分,从而更全面地分析栀子鲜花花水和鲜花细胞液的功效。【本研究切入点】目前关于栀子鲜花花水和鲜花细胞液的气相成分以及液相成分的分析未见报道。本研究首次从气相和液相两个方面全面分析栀子鲜花花水、鲜花细胞液的气味和功效的差异与联系。【拟解决的关键问题】利用顶空固相微萃取/气相色谱—质谱联用技术(HS-SPME/ GC-MS)分析栀子鲜花花水和鲜花细胞液中的挥发性成分,利用超高效液相色谱—质谱联用技术(UPLC-ESI-QTOF-MS/MS)分析栀子鲜花花水和鲜花细胞液的化学成分,通过对栀子鲜花花水和栀子鲜花细胞液香气成分、相对含量及其液相化学成分、相对含量的分析评价,探明栀子鲜花花水和鲜花细胞液差异的本质,为香气修饰、应用配方及它们在特定功能化妆品、香精香料、保健品开发上的应用提供数据支撑和理论依据。1 材料与方法
试验于2018年在福建省农业科学院作物研究所进行。1.1 试验材料与仪器
试验所用栀子鲜花于2018年6月1日采自福建省福鼎市前岐镇‘分关1号’栀子种植基地,鲜花为当天开放,不发黄,且花瓣完全展开。GCMS-TQ8040三重四极杆型气相色谱质谱联用仪,日本岛津公司;手动SPME迸样器、65 μm PDMS/DVB萃取头,美国Supelco公司;超高效液相色谱UPLC-TripleTOF串联飞行时间质谱仪,美国AB SCIEX公司。
1.2 样品制备
(1)常压水蒸汽蒸馏提取栀子鲜花花水(FFWE):称取栀子花5 kg,置于100 L蒸馏罐中,按料液比为1﹕4加水,进行水蒸汽蒸馏,馏出液的收集完全处于封闭状态,按鲜花﹕花水=1﹕1收集5 kg花水[18]。(2)栀子鲜花细胞液(FFCS):称取栀子鲜花5 kg,置于烘干箱中,28—32℃常温烘干,鼓风机转速为2 000 r/min,压缩机制冷运行频率为30 Hz,通过冷凝器冷凝收集蒸发液体,冷凝及蒸发液收集过程处于完全封闭状态,脱水8 h直至鲜花脱水率达80%左右,收集2 kg细胞液。
1.3 试验方法
1.3.1 HS-SPME取样 取样前先将固相微萃取头在丙酮中浸泡30 min,紧接着插入色谱仪进样口进行老化30 min,老化温度250℃。用移液枪分别吸取5 mL栀子鲜花花水和细胞液样品,置于20 mL顶空瓶中,将老化好的固相微萃取头插入顶空瓶中,25℃吸附40 min。1.3.2 GC-MS分析 样品吸附完成后将固相微萃取头抽出,插入气相色谱仪进样口中,250℃解吸3 min,同时进行数据采集。色谱条件:HP-5MS标准色谱柱;进样量1 μL,载气为He(99.99%),流量1 mL·min-1,分流比10﹕1;程序升温,进样口250℃,柱温起始温度50℃保持2 min,以5℃·min-1升温至180℃,再以20℃·min-1升温至280℃保持2 min。总程序时间40 min。质谱条件:GC-MS接口温度280℃;离子源温度200℃,电离方式EI,电子能量70 eV;扫描质量范围35—550 amu。
1.3.3 UPLC-MS/MS分析 分别移取5 mL栀子花样品,真空浓缩抽干,加入提取试剂(乙腈﹕甲醇﹕水=2﹕2﹕1)500 μL进行超声提取15 min,重复3次,提取液在1 200 g·min-1、4℃下离心10 min,取上清液,真空抽干,再用100 μL复溶液复溶(乙腈﹕水=1﹕1),转移至2 mL带有内衬管的进样小瓶上机进行LC-MS检测。
色谱条件:色谱柱为BEH C18柱(100 mm×2.1 mm i.d., 1.7 μm; Waters, Milford, USA);流动相A为水(含0.1%甲酸),流动相B为乙腈/异丙醇=1﹕1(含0.1%甲酸);梯度洗脱程序为0—3 min:5%—25% B,3—9 min:25%—95% B,9—13 min:95—95% B,13—13.1 min:95%—5% B,5%B保持3 min。流速为0.40 mL·min-1,进样量为10 μL,柱温为45℃。
质谱条件:样品质谱信号采集分别采用正负离子扫描模式,电喷雾毛细管电压,进样电压和碰撞电压分别为:1.0 kV、40 V和6 eV。离子源温度和去溶剂温度分别为:120℃和500℃,载气流量:900 L·h-1,质谱扫描范围:50—1 000 m/z,扫描时间和间隔时间分别为:0.1 s和0.02 s。
1.4 数据处理与分析
GC-MS数据分析根据已有标样(C9-C22正构烷烃)的色谱保留时间,计算各样品中每个成分的保留指数RI(retention index),根据NIST 2014标准谱库中信息进行比对,同时结合文献中相应的参考物质RI值进行定性分析。每个成分的物质含量以相对含量表示,运用峰面积归一化法,求得各成分的相对含量[19]。每个样品数据重复3次。UPLC-MS/MS数据分析中的原始数据经代谢组学处理软件QI(Waters, Milford, USA)进行基线过滤、峰识别、积分、保留时间校正、峰对齐和归一化,最终得到一个保留时间、质荷比和峰强度的数据矩阵,并与metlin数据库和HMDB数据库进行比对,对各代谢物质进行定性。运用峰面积归一化法,求得各代谢物质的相对含量。每个样品数据重复3次。
2 结果
2.1 栀子鲜花花水和细胞液的香气成分比较
从表1可以看出,栀子鲜花花水中的香气成分以芳樟醇和惕各酸顺-3-己烯酯为主,相对含量分别达到63.00%和13.24%,栀子鲜花细胞液中以芳樟醇和反式-橙花叔醇为主要香气成分,相对含量分别高达69.37%和19.08%。栀子鲜花花水中的反式-橙花叔醇相对含量仅为0.05%,而栀子鲜花细胞液中惕各酸顺-3-己烯酯的相对含量只有0.88%。栀子鲜花花水中的香叶醇相对含量达到1.71%,而在栀子鲜花细胞液中未检测到。Table 1
表1
表1栀子鲜花花水和细胞液的香气成分及其相对含量(高于0.1%)
Table 1
| 编号 No. | 文献保留指数 Reported retention index | 测定保留指数 Tested retention index | 化合物名称 Compound name | 相对含量 Relative content (%) | |
|---|---|---|---|---|---|
| 鲜花花水 FFWE | 鲜花细胞液FFCS | ||||
| 醇类 Alcohols | |||||
| 1 | 844[20] | 841 | (Z)-3-己烯-1-醇3-Hexen-1-ol, (Z)- | 1.95±0.06** | 1.69±0.04 |
| 2 | 860[21] | 855 | 正己醇 1-Hexanol | 1.09±0.02** | 0.35±0.01 |
| 3 | 969[22] | 965 | 正庚醇 1-Heptanol | 0.13±0.00** | - |
| 4 | 1035[23] | 1031 | 二氢香芹醇 Dihydrocarveol | 0.14±0.00** | - |
| 5 | 1038[24] | 1034 | 桉叶油醇 Eucalyptol | 0.13±0.00** | - |
| 6 | 1078[24] | 1080 | 顺式-芳樟醇氧化物 cis-Linalool oxide | 0.18±0.01** | - |
| 7 | 1085[22] | 1083 | 正辛醇 1-Octanol | 0.17±0.01** | - |
| 8 | 1099[24] | 1098 | 反式芳樟醇氧化物(呋喃) trans-Linalool oxide (furanoid) | 0.16±0.00** | 0.13±0.00 |
| 9 | 1114[25] | 1120 | 芳樟醇 Linalool | 63.00±1.89 | 69.37±1.39** |
| 10 | 1118[26] | 1122 | 二氢芳樟醇 1,5,7-Octatrien-3-ol, 3,7-dimethyl- | 0.48±0.01** | - |
| 11 | 1200[27] | 1202 | 2-莰醇 Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, (1S-endo)- | 0.12±0.00** | - |
| 12 | 1201[28] | 1204 | 1-壬醇 1-Nonanol | 0.39±0.01** | - |
| 13 | 1265[29] | 1269 | 橙花醇 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- | 0.80±0.02** | - |
| 14 | 1301[30] | 1302 | 香叶醇 Geraniol | 1.71±0.05** | - |
| 15 | 1583[31] | 1594 | 反式-橙花叔醇 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | 0.05±0.00 | 19.08±0.38** |
| 16 | 1639[32] | 1639 | T-杜松醇 .tau.-Cadinol | 0.25±0.01 | 0.28±0.01* |
| 17 | 1655[33] | 1657 | (Z,E)-法呢醇 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, (Z,E)- | - | 0.13±0.00** |
| 18 | 1660[30] | 1661 | 反式-香叶基香叶醇 trans-Geranylgeraniol | - | 0.20±0.01** |
| 19 | 1662[34] | 1663 | 法呢醇 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl- | - | 2.11±0.04** |
| 萜烯类 Terpenes | |||||
| 20 | 1510[35] | 1512 | 9-去甲-10-脱氧二氢青蒿素 9-Nor-10-deoxydihydroartemisinin | - | 0.88±0.03** |
| 21 | 1513[36] | 1517 | (E)-β-法呢烯 (E)-.beta.-Famesene | - | 0.29±0.01** |
| 酯类 Esters | |||||
| 22 | 855[37] | 850 | 惕各酸甲酯 Methyl tiglate | 0.36±0.01** | 0.05±0.00 |
| 23 | 923[26] | 927 | 惕各酸乙酯 Ethyl tiglate | 0.19±0.01** | - |
| 24 | 1103[38] | 1105 | (E)-2-甲基-2-丁酸-2-甲丙酯 2-Butenoic acid, 2-methyl-, 2-methylpropyl ester, (E)- | 0.16±0.00** | - |
| 25 | 1228[39] | 1232 | (E)-2-甲基巴豆酸异戊酯 2-Butenoic acid, 2-methyl-, 3-methylbutyl ester, (E)- | 4.64±0.09** | 0.16±0.00 |
| 26 | 1273[22] | 1276 | 2-甲基丁酸叶醇酯 cis-3-Hexenyl-.alpha.-methylbutyrate | 0.15±0.00** | - |
| 27 | 1275[22] | 1279 | 2Z-戊烯惕各酸酯 Pentenyl tiglate, 2Z- | 0.12±0.00** | - |
| 28 | 1285[22] | 1288 | 1-戊烯-3-惕各酸酯 Penten-3-yl tiglate, 1- | 3.94±0.08** | 1.15±0.03 |
| 29 | 1390[27] | 1392 | 惕各酸顺-3-己烯酯 Tiglate <3(Z)-hexenyl-> | 13.24±0.40** | 0.88±0.03 |
| 30 | 1399[27] | 1400 | 惕各酸己酯 Hexyl tiglate | 1.51±0.05** | 0.02±0.00 |
| 31 | 1510[40] | 1513 | 丙酮香叶酯 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | 0.11±0.00** | - |
| 32 | 1539[41] | 1542 | 茉莉内酯 2H-Pyran-2-one, tetrahydro-6-(2-pentenyl)-, (Z)- | - | 0.25±0.01** |
| 33 | 1600[24] | 1607 | 惕各酸苯乙酯 2-Phenylethyl tiglate | 0.42±0.01** | 0.07±0.00 |
| 醛类 Aldehydes | |||||
| 34 | 1664[38] | 1668 | 法呢醛 Farnesal | - | 0.45±0.01** |
| 酮类 Ketones | |||||
| 35 | 1254[42] | 1260 | 4,7,7-三甲基双环[4.1.0]-3-庚烯-2-酮 4,7,7-Trimethylbicyclo[4.1.0]hept-3-en-2-one | - | 0.40±0.01** |
| 36 | 1289[43] | 1292 | (+)-香芹酮 D-Carvone | 1.15±0.03** | - |
| 酚类 Phenols | |||||
| 37 | 1543[44] | 1548 | 甲基异丁香酚 Benzene, 1,2-dimethoxy-4-(1-propenyl)- | - | 0.11±0.00** |
| 其他类 Others | |||||
| 38 | 1000[45] | 1004 | 2-乙烯基-2-甲基-5-(1-甲基乙烯基)四氢呋喃 (2R,5R)-2-Methyl-5-(prop-1-en-2-yl)-2-vinyltetrahydrofuran | 0.12±0.00** | - |
| 39 | 1350[46] | 1353 | 吲哚 Benzo-2,3-pyrrole | - | 0.30±0.01** |
新窗口打开|下载CSV
从栀子鲜花花水和鲜花细胞液中共检测出香气成分111种,其中栀子鲜花花水检测出香气成分79种,而鲜花细胞液中检测出香气成分56种,仅占检测出的挥发性成分总量的一半。从图1和图2可知,检测出的香气成分包含醇类化合物、萜烯类化合物、酯类化合物、醛类化合物、酮类化合物、酚类化合物等8个种类的化合物。栀子鲜花花水中的醇类化合物、酮类化合物、酯类化合物的种类分别为32、5、26种,高于鲜花细胞液,但萜烯类化合物仅检测到5种,远远低于鲜花细胞液(12种)。栀子鲜花细胞液中醇类化合物的相对含量占94.17%,而栀子鲜花花水中仅有71.54%;萜烯类化合物的相对含量达到1.54%,而栀子鲜花花水仅占0.46%。栀子鲜花细胞液中酯类化合物和酮类化合物的相对含量分别仅为2.91%和0.51%,而栀子鲜花花水中分别达到26.06%和1.33%。表明栀子鲜花细胞液中的挥发性成分以醇类和萜烯类为主,较为单一;而栀子鲜花花水中除了醇类成分,酯类、酮类成分也占有较大比例。
图1
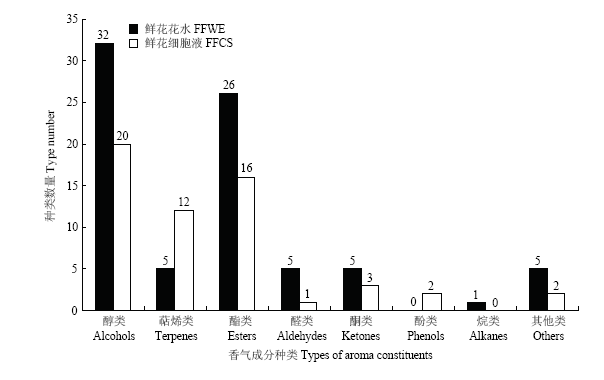 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1栀子鲜花花水和细胞液的香气成分种类数量
Fig. 1Type number of aroma constituents of floral water extract and flowers cell sap from Gardenia jasminoides
检测发现栀子鲜花花水和鲜花细胞液的酯类化合物中存在着多种惕各酸酯类成分,其中栀子鲜花花水中的惕各酸酯类成分有10种,数量高于鲜花细胞液的7种。栀子鲜花花水中各惕各酸酯类化合物相对含量总和为19.96%,其中惕各酸顺-3-己烯酯相对含量达13.24%,而鲜花细胞液中各惕各酸酯类成分的含量仅为2.30%。
2.2 栀子鲜花花水和细胞液的化学成分比较
正、负离子模式下栀子鲜花花水和细胞液的化学成分及其相对含量分析结果见表2。从表2可以看出,栀子鲜花花水和栀子鲜花细胞液在正、负离子模式下同时进行UPLC-QTOF-MS/MS分析,分别鉴定出200个、212个和46个、54个成分。可见栀子鲜花花水和栀子鲜花细胞液中的化学成分在正离子模式下响应较为灵敏,栀子鲜花细胞液在正、负离子模式下检出的成分数量均多于栀子鲜花花水。
图2
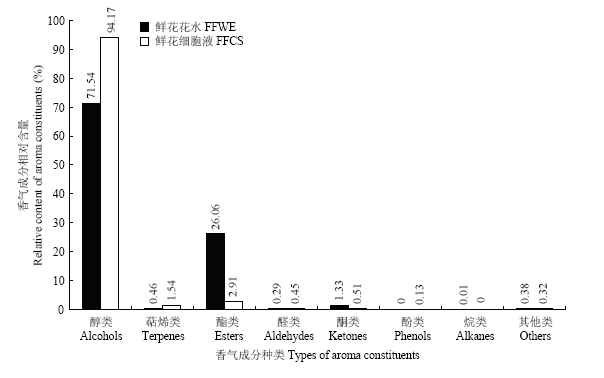 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2栀子鲜花花水和细胞液的香气成分相对含量
Fig. 2Relative contents of aroma constituents of floral water extract and flowers cell sap from Gardenia jasminoides
Table 2
表2
表2栀子鲜花花水和细胞液的化学成分及其相对含量(高于0.1%)
Table 2
| 编号 No. | 保留 时间 Retention time (min) | 加和离子 模式 Additive ion mode | m/z Mass charge ratio | 分子式 Molecular formula | 成分名称 Compound name | 相对含量 Relative content (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| 鲜花花水 FFWE | 鲜花细胞液 FFCS | ||||||||
| 正离子模式 Positive ion mode | 负离子模式 Negative ion mode | 正离子模式 Positive ion mode | 负离子模式 Negative ion mode | ||||||
| 氨基酸类 Amino acids | |||||||||
| 1 | 2.44 | M+K-2H | 152.0111 | C5H9NO2 | L-脯氨酸 L-Proline | - | 0.59±0.02 | - | 3.05±0.06** |
| 2 | 3.10 | M-H | 300.1221 | C17H19NO4 | 3-(3,4-二羟苯基)-N-[2-(4-羟苯基)乙基]丙氨酸3-(3,4-Dihydroxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]propanimidic acid | - | - | - | 0.17±0.01** |
| 单萜类 Monoterpeniods | |||||||||
| 3 | 3.50 | M+H | 153.1271 | C10H16O | 桧醇 Sabinol | 0.48±0.01** | - | 0.23±0.01 | - |
| 4 | 6.16 | M+H-H2O, M+H | 135.1168 | C10H16O | (S)-(-)-紫苏醇 (S)-(-)-Perillyl alcohol | 13.73±0.27** | - | 5.11±0.15 | - |
| 5 | 3.09 | M-H | 183.0657 | C9H12O4 | 京尼平酸 Genipic acid | - | 1.76±0.05** | - | 0.18±0.01 |
| 6 | 4.61 | M-H, M+Na-2H | 315.1801 | C16H28O6 | 薄荷醇-葡萄糖醛酸 Menthol-glucoronide | - | 0.83±0.02** | - | 0.18±0.01 |
| 倍半萜类 Sesquiterpenoids | |||||||||
| 7 | 3.60 | M+ACN+Na | 494.2162 | C24H30O7 | 蜜环菌醛H Melleolide H | - | - | 0.23±0.01** | - |
| 8 | 5.61 | M+H-2H2O, M+Na, M+H-H2O | 249.1470 | C15H22O4 | 青蒿琥酯 Artemin | 0.06±0.00 | - | 0.19±0.01** | - |
| 9 | 5.65 | M+H-H2O, M+H | 203.1790 | C15H24O | 氧化石竹烯 Caryophyllene epoxide | 0.38±0.01 | - | 1.77±0.05** | - |
| 10 | 5.76 | M+H | 205.1947 | C15H24 | α-佛手柑油烯 alpha-Bergamotene | - | - | 0.11±0.00** | - |
| 11 | 6.03 | M+H | 205.1948 | C15H24 | (+)-长叶环烯 Longicyclene | - | - | 0.12±0.00** | - |
| 12 | 7.61 | M+H | 205.1949 | C15H24 | α-石竹烯 α-Humulene | 0.06±0.00 | - | 0.39±0.01** | - |
| 二萜类 Diterpenoids | |||||||||
| 13 | 5.69 | 2M+H | 661.2964 | C19H22O5 | 赤霉素A62 Gibberellin A62 | - | - | 0.10±0.00** | - |
| 14 | 7.53 | M+Na-2H | 421.1863 | C20H32O8 | 辛卡西醇B Cincassiol B | - | 1.56±0.05** | - | 0.30±0.01 |
| 三萜类 Triterpenoids | |||||||||
| 15 | 6.37 | M+H, M+Na, M+H-H2O | 489.3558 | C30H48O5 | 山茶皂苷元B Camelliagenin B | 2.16±0.06** | - | 0.02±0.00 | - |
| 16 | 6.63 | M+H-H2O, M+H | 491.3712 | C30H50O5 | 山茶皂苷元C Camelliagenin C | 0.18±0.01** | - | - | - |
| 17 | 3.86 | M+Cl | 541.1830 | C26H34O10 | 异柠檬苦素酸 Isolimonic acid | - | 0.20±0.01 | - | 0.64±0.02** |
| 18 | 4.25 | M+Na-2H | 803.4226 | C41H66O14 | 麦德龙苷D Madlongiside D | - | 4.35±0.09** | - | 0.23±0.01 |
| 19 | 5.22 | M-H, M+Cl | 503.3367 | C30H48O6 | 羟基积雪草苷 Madecassic acid | - | 20.20±0.61** | - | 0.02±0.00 |
| 黄酮类 Flavonoids | |||||||||
| 20 | 4.72 | M+H | 823.3062 | C39H50O19 | 朝藿定D Hexandraside D | - | - | 0.11±0.00** | - |
| 香豆素类 Coumarins | |||||||||
| 21 | 4.38 | M+H, M+ CH3OH+H | 179.0699 | C9H6O2 | 香豆素 Coumarin | - | - | 0.74±0.02** | - |
| 22 | 0.66 | M+H | 141.1134 | C6H12N4 | 六亚甲基四胺 Methenamine | 0.33±0.01** | - | 0.11±0.00 | - |
| 23 | 1.90 | M+H, 2M+H | 217.0974 | C12H12N2O2 | L-1,2,3,4-四氢-β-咔啉-3-羧酸 L-1,2,3,4-Tetrahydro-beta-carboline-3- carboxylic acid | 0.35±0.01 | - | 3.17±0.06** | - |
| 24 | 2.39 | M+H | 476.3055 | C21H41N5O7 | 乙基紫苏霉素 Netilmicin | 0.17±0.00 | - | 0.18±0.01** | - |
| 25 | 2.86 | M+H-H2O, M+H | 120.0446 | C7H7NO2 | 氨茴酸 Anthranilic acid | 1.15±0.03 | - | 2.02±0.06** | - |
| 26 | 4.38 | M+H-H2O | 172.0752 | C11H11NO2 | 吲哚-3-丙酸 Indole-3-propionic acid | - | - | 0.44±0.01** | - |
| 27 | 5.19 | 2M+ACN+H | 706.3567 | C14H24N2O7 | 大观霉素 Spectinomycin | 0.29±0.01** | - | 18.16±0.36** | - |
| 28 | 7.50 | M+H | 270.3152 | C18H39N | 十八胺 Octadecylamine | 0.14±0.00 | - | 0.15±0.00** | - |
| 29 | 9.68 | M+H, M+Na, 2M+H, 2M+Na | 338.3415 | C22H43NO | 芥酸酰胺 13Z-Docosenamide | 43.66±1.31** | - | 19.41±0.58 | - |
| 30 | 1.92 | M-H2O-H | 171.0926 | C11H14N2O | 艾胺 Alline | - | - | - | 0.57±0.02** |
| 31 | 2.78 | M-H | 164.0353 | C8H7NO3 | 2-甲酰胺基苯甲酸 N-formylanthranilic acid | - | - | - | 0.26±0.01 |
| 32 | 2.99 | M-H2O-H | 146.0248 | C8H7NO3 | (R)-2-羟基-2H-1,4-苯并恶嗪-3(4H)-酮 (R)-2-Hydroxy-2H-1,4-benzoxazin-3 (4H)-one | - | - | - | 0.31±0.01 |
| 33 | 3.11 | M-H | 136.0405 | C7H7NO2 | 葫芦巴碱 Trigonelline | - | - | - | 1.58±0.05** |
| 34 | 3.72 | M+FA-H | 462.1899 | C21H27N3O6 | (E)-卡西定 (E)-Casimiroedine | - | - | - | 0.22±0.01** |
| 35 | 3.93 | M+Cl | 296.0910 | C11H19NO6 | 百脉根苷 Lotaustralin | - | - | - | 0.23±0.01** |
| 36 | 3.97 | M+FA-H | 302.1028 | C8H20NO6P | 甘油磷酰胆碱 Glycerophosphocholine | - | 1.54±0.05** | - | - |
| 37 | 4.16 | M-H2O-H | 322.1069 | C19H19NO5 | 4-羟基降南天竹宁 4-Hydroxynornantenine | - | - | - | 0.58±0.02** |
| 38 | 4.42 | M-H2O-H | 742.2463 | C39H43N3O11S | 曲贝替定 Trabectedin | - | - | - | 3.30±0.07** |
| 39 | 4.43 | M-H | 357.3149 | C17H19NO5 | 3-(3,4-二羟苯基)-N-[2-(5-羟苯基)乙基] 丙酮酸3-(3,4-Dihydroxyphenyl)-N-[2-(5- hydroxyphenyl)ethyl]propanimidic acid | - | 0.40±0.01 | - | 3.33±0.10** |
| 40 | 4.46 | 3M-H | 482.1357 | C9H7NO2 | 喹啉-4,8-二醇 Quinoline-4,8-diol | - | - | - | 0.30±0.01** |
| 41 | 5.26 | M+Cl | 700.2743 | C33H47NO13 | 纳他霉素 Natamycin | - | - | - | 2.61±0.05** |
| 42 | 5.97 | M+FA-H | 236.1049 | C10H13N3O | 4-羟基异喹胍 4-Hydroxydebrisoquine | - | 1.25±0.04** | - | - |
| 甾体类 Steroids | |||||||||
| 43 | 5.61 | M+H | 305.2095 | C19H28O3 | 16-氧雄甾烯二醇 16-Oxoandrostenediol | 0.12±0.00** | - | - | - |
| 芳香类 Aromatics | |||||||||
| 44 | 4.24 | M+H | 133.1012 | C10H12 | 对-孟-1,3,5,8-四烯 p-Mentha-1,3,5,8-tetraene | 8.46±0.25 | - | 8.03±0.24 | - |
| 45 | 4.25 | M+H | 107.0858 | C8H10 | 间二甲苯 m-Xylene | 1.31±0.04** | - | 1.11±0.03 | - |
| 46 | 9.22 | M+H, M+Na, M+K, 2M+Na | 391.2835 | C24H38O4 | 邻苯二甲酸二(2-乙基己)酯 Bis(2-ethylhexyl) phthalate | 10.41±0.31** | - | 4.21±0.13 | - |
| 47 | 4.60 | M-H | 549.1998 | C27H34O12 | 络石苷 Tracheloside | - | - | - | 0.43±0.01** |
| 醇类 Alcohols | |||||||||
| 48 | 3.19 | M+H-H2O, 2M+Na | 361.1640 | C20H26O7 | 氨茶醇 Carinol | - | - | 0.19±0.01** | - |
| 49 | 3.75 | M+H-H2O, M+H, M+Na | 171.1491 | C10H18O2 | 反式-对-孟-2-烯-1,4-二醇 trans-p-Menth-2-ene-1,4-diol | 5.78±0.17** | - | 4.45±0.13 | - |
| 50 | 5.24 | M+ACN+H | 158.1538 | C7H16O | 正庚醇 1-Heptanol | 0.22±0.01** | - | 0.15±0.00 | - |
| 51 | 5.30 | M+ACN+Na | 482.2169 | C23H30O7 | 星形曲霉毒素 Asteltoxin | - | - | 0.11±0.00** | - |
| 52 | 0.76 | M-H | 349.1855 | C16H30O8 | 反式-对-孟-1,7,8-三醇 8-葡萄糖苷 trans-p-Menthane-1,7,8-triol 8-glucoside | - | 1.78±0.05** | - | - |
| 53 | 3.40 | M+FA-H | 351.1796 | C18H26O4 | 5'-羧基-γ-色原烷醇 5'-Carboxy-gamma-chromanol | - | 0.96±0.03** | - | - |
| 54 | 3.95 | M-H | 375.1431 | C20H24O7 | 加利烷醇 Carissanol | - | - | - | 0.53±0.02** |
| 55 | 4.45 | M-H | 356.9991 | C16H30O9 | 反式-对-孟-1,7,8-三醇 9-葡萄糖苷 trans-p-Menthane-1,7,8-triol 9-glucoside | - | 0.38±0.01 | - | 3.39±0.07** |
| 56 | 4.48 | M+FA-H | 356.3675 | C18H26O5 | 6'-羧基-γ-色原烷醇 6'-Carboxy-gamma-chromanol | - | 0.35±0.01 | - | 3.49±0.07** |
| 57 | 7.55 | M+Na-2H | 303.1568 | C16H26O4 | 乳聚醇 CLactapiperanol C | - | - | - | 0.41±0.01** |
| 酚类 Phenols | |||||||||
| 58 | 2.73 | M+H | 153.0544 | C8H8O3 | 香草醛 Vanillin | 0.49±0.02** | - | 0.28±0.01 | - |
| M-H | 151.0400 | - | 12.15±0.24** | - | 3.60±0.11 | ||||
| 59 | 6.45 | M+H-H2O, 2M+H | 653.3095 | C20H22O4 | (+)-加利恩 (+)-Galeon | - | - | 1.01±0.03** | - |
| 60 | 8.88 | M+H | 413.3017 | C27H40O3 | 4-甲氧基-3-牛龙牛儿基牛龙牛儿酯-1,2-二羟基苯4-Methoxy-3-geranylgeranyl-1,2- dihydroxybenzene | - | - | 0.31±0.01** | - |
| 61 | 4.27 | M+K-3H | 360.4726 | C27H36O11 | 杨梅醇 6-葡萄糖苷 Myricanol 6-glucoside | - | 0.54±0.02 | - | 2.81±0.06** |
| 酸类 Acids | |||||||||
| 62 | 6.13 | M+H-2H2O | 337.1317 | C17H24O9 | 3,4,5-三羟基-6-[4-(3-羟丁基)-2-甲氧苯基]噁烷-2-羧酸3,4,5-Trihydroxy-6-[4-(3-hydroxybutyl)-2-methoxyphenoxy]oxane-2-carboxylic acid | 0.16±0.00** | - | 0.13±0.00 | - |
| 63 | 7.15 | M+Na | 317.2084 | C18H30O3 | 9顺,11反-共轭亚油酸 13-OxoODE | 0.19±0.01** | - | - | - |
| 64 | 0.26 | M-H | 112.9861 | C2HF3O2 | 三氟乙酸 Trifluoroacetic acid | - | 10.30±0.31** | - | 2.90±0.06 |
| 65 | 2.15 | M-H2O-H | 311.0781 | C14H18O9 | 6-(5-乙基-2,3-二羟苯氧基)-3,4,5-三羟基噁烷-2-羧酸6-(5-Ethyl-2,3-dihydroxyphenoxy)-3,4,5-trihydroxyoxane-2-carboxylic acid | - | 1.32±0.04** | - | - |
| 66 | 3.39 | M-H2O-H | 157.0506 | C7H12O5 | 2-异丙基苹果酸 2-Isopropylmalic acid | - | - | - | 0.15±0.00** |
| 67 | 4.14 | M-H | 173.1181 | C9H18O3 | (±)-3-羟基壬酸 (±)-3-Hydroxynonanoic acid | - | 0.32±0.01** | - | 0.27±0.00 |
| 68 | 4.28 | M+Cl, M+K-3H | 360.1568 | C36H32O20S | (5-{8-[5,7-二羟基-2-(4-羟苯基)-4-氧-3,4-二氢-2H-1-苯并吡喃-3-烷基]-5-羟基-4-氧-7-{[3,4,5-三羟基-6-(羟甲基)噁烷-2-烷基]氧}-3,4-二氢-2H-1-苯并吡喃-2-烷基}-3-羟苯基)氧化磺酸(5-{8-[5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2H-1-benzopyran-3-yl]-5-hydroxy-4-oxo-7-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydro-2H-1-benzopyran-2-yl}-3- hydroxyphenyl)oxidanesulfonic acid | - | 0.53±0.02 | - | 2.86±0.06** |
| 69 | 4.38 | M-H | 358.2622 | C9H18O4 | (±)-4-羟基壬酸 (±)-4-Hydroxynonanoic acid | - | 0.44±0.01 | - | 3.18±0.06** |
| 70 | 4.50 | M-H3O-H | 356.0518 | C7H12O6 | 3-异丙基苹果酸 3-Isopropylmalic acid | - | 0.34±0.01 | - | 3.54±0.11** |
| 71 | 4.79 | M+Cl, M+K-2H | 835.1021 | C36H32O19S | (5-{8-[5,7-二羟基-2-(4-羟基苯基)-4-氧-3,4-二氢-2H-1-苯并吡喃-3-烷基]-5-羟基-4-氧-7-{[3,4,5-三羟基-6-(羟甲基)噁烷-2-烷基]氧}-3,4-二氢-2H-1-苯并吡喃-2-烷基}-2-羟基苯基)氧化磺酸(5-{8-[5,7-Dihydroxy-2-(4-hydroxyphenyl) -4-oxo-3,4-dihydro-2H-1-benzopyran-3-yl]-5-hydroxy-4-oxo-7-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4- dihydro-2H-1-benzopyran-2-yl}-2- hydroxyphenyl)oxidanesulfonic acid | - | - | - | 0.94±0.03** |
| 72 | 6.94 | M-H | 319.2267 | C20H32O3 | 11(S)-羟基二十碳-5Z,8Z,12E,14Z-四烯酸 11(S)-HETE | - | 1.65±0.05** | - | 0.63±0.02 |
| 73 | 7.48 | M-H | 277.1435 | C16H22O4 | 2,5,7,8-四甲基-6-羟基苯并二氢吡喃基-2-丙酸 α-CEHC | - | 2.42±0.07** | - | 0.22±0.01 |
| 74 | 8.94 | M-H | 279.2322 | C18H32O2 | 17-十八炔酸 17-Octadecynoic Acid | - | 13.46±0.27** | - | 0.80±0.02 |
| 75 | 9.14 | M-H | 305.2469 | C20H34O2 | 5(Z),11(Z),14(Z)-二十碳三烯酸 5(Z),11(Z),14(Z)-Eicosatrienoic acid | - | 0.60±0.02** | - | - |
| 76 | 9.32 | M-H | 307.2627 | C20H36O2 | 5(Z),14(Z)-二十碳二烯酸 5(Z),14(Z)-Eicosadienoic Acid | - | 2.83±0.08** | - | - |
| 77 | 9.47 | M-H | 333.2788 | C22H38O2 | 顺13,16,19-二十二碳三烯酸 Docosatrienoic Acid | - | 1.19±0.04** | - | - |
| 酮类 Ketones | |||||||||
| 78 | 2.76 | M+CH3OH+H | 361.1639 | C19H20O5 | 1-(3,4-二羟苯基)-7-(4-羟苯基)庚烷- 3,5-二酮1-(3,4-Dihydroxyphenyl)-7-(4- hydroxyphenyl)heptane-3,5-dione | - | - | 0.14±0.00** | - |
| 79 | 3.49 | M+ACN+Na | 448.1750 | C22H24O6 | 12-去氢波森 12-Dehydroporson | - | - | 0.16±0.00** | - |
| 80 | 4.23 | M+H-H2O, M+H | 93.0704 | C7H10O | 3-甲基-2-环己烯-1-酮 3-Methyl-2-cyclohexen-1-one | 0.28±0.01** | - | 0.24±0.01 | - |
| 81 | 4.76 | M+H-H2O, M+Na | 313.1425 | C19H22O5 | 6-(1-羟基-2-甲基丁基-3-烯-2-烷基)- 2-(2-羟丙基-2-烷基)-2H,3H,7H-呋喃[3,2-g]苯并吡喃-7-酮6-(1-Hydroxy-2-methylbut-3-en-2-yl)-2-(2-hydroxypropan-2-yl)-2H,3H,7H-furo [3,2-g]chromen-7-one | 0.16±0.00** | - | - | - |
| 82 | 2.44 | 3M-H | 545.1655 | C9H10O4 | 1-(2,6-二羟基-4-甲氧基苯基)乙酮 2',6'-Dihydroxy-4'-methoxyacetophenone | - | 0.09±0.00 | - | 3.49±0.10** |
| 83 | 4.35 | M-H3O-H | 358.8937 | C11H12O4 | 4-(3,4-亚甲基二氧苯基)-3-丁酮 4-(3,4-Methylenedioxyphenyl)-3-butanone | - | 0.47±0.01 | - | 3.07±0.06** |
| 84 | 4.78 | M-H2O-H | 173.0605 | C11H12O3 | 胡椒基丙酮 4-(3,4-Methylenedioxyphenyl)-2-butanone | - | 1.48±0.05** | - | - |
| 85 | 4.80 | M-H, M+FA-H | 329.1381 | C19H22O5 | 3-羟基-2-(2-羟丙基-2-烷基)-6-(2-甲丁基-3-烯-2-烷基)-2H,3H,7H-呋喃[3,2-g]色原烷-7-酮3-Hydroxy-2-(2-hydroxypropan-2-yl)-6-(2-methylbut-3-en-2-yl)-2H,3H,7H-furo[3,2-g]chromen-7-one | - | 0.02±0.00 | - | 23.98±0.48** |
| 酯类 Esters | |||||||||
| 86 | 4.75 | M+Na, 2M+Na, M+H-H2O | 327.1589 | C20H24O5 | 异千叶蓍酯二烯 Isoachifolidiene | 0.58±0.02 | - | 16.76±0.50** | - |
| 87 | 5.67 | M+ACN+H | 390.2268 | C20H28O5 | 蜂斗菜内酯C Bakkenolide C | 0.21±0.01 | - | 0.52±0.02** | - |
| 88 | 3.48 | M-H | 171.1027 | C9H16O3 | 乙酰乙酸异戊酯 3-Methylbutyl 3-oxobutanoate | - | 9.00±0.18** | - | 7.83±0.16 |
| 89 | 4.01 | M+FA-H | 399.1330 | C17H22O8 | 蒽酸甲酯 F 葡萄糖苷 Methyl helianthenoate F glucoside | - | - | - | 2.51±0.08** |
| 90 | 4.52 | M-H | 355.7360 | C9H16O4 | 3-甲丁基 4-氧丁酸酯 3-Methylbutyl 4-oxobutanoate | - | 0.33±0.01 | - | 3.60±0.07** |
| 肽类 Peptides | |||||||||
| 91 | 5.00 | M+H | 640.2524 | C27H37N5O13 | 苏氨酸-苯丙氨酸-葡萄糖-葡萄糖-天冬氨酸 Thr Phe Glu Glu Asp | - | - | 0.42±0.01** | - |
| 92 | 5.27 | M+H | 446.2743 | C23H35N5O4 | 赖氨酸-异亮氨酸-色氨酸 Lys Ile Trp | 0.20±0.01** | - | - | - |
| 93 | 5.37 | M+H | 473.1920 | C22H28N6O4S | 色氨酸-蛋氨酸-组氨酸 Trp Met His | - | - | 0.25±0.01** | |
| 94 | 6.49 | M+H | 474.3047 | C20H39N7O6 | 谷氨酰胺-丙氨酸-赖氨酸-赖氨酸 Gln Ala Lys Lys | 0.77±0.02** | - | - | - |
| 95 | 5.18 | M-H2O-H | 283.1539 | C11H22N6O4 | 精氨酸-谷氨酰胺 Arginyl-Glutamine | - | 0.96±0.03** | - | 0.24±0.01 |
| 96 | 6.39 | M-H | 592.2326 | C28H39N3O9S | 4-羟基-17β-雌二醇-2-S-谷胱甘肽 4-Hydroxy-17beta-estradiol-2-S- glutathione | - | - | - | 0.51±0.02** |
| 胺类 Amines | |||||||||
| 97 | 7.20 | M+H-H2O | 444.3312 | C24H47NO7 | 葡萄糖鞘氨醇半乳糖苷 Glucosylsphingosine | 0.24±0.01** | - | - | - |
| 其他类 Others | |||||||||
| 98 | 2.92 | M+H-2H2O | 343.1540 | C20H26O7 | 15-羟基勒普妥卡品 15-Hydroxyleptocarpin | 0.01±0.00 | - | 0.27±0.01** | - |
| M-H | 377.1591 | - | 0.11±0.00 | - | 2.80±0.08** | ||||
| 99 | 2.49 | 2M+FA-H | 505.2998 | C12H22O4 | 塔罗霉素A Talaromycin A | - | 0.65±0.02** | - | - |
新窗口打开|下载CSV
在正离子模式下,栀子鲜花花水和栀子鲜花细胞液中的化学成分以氨基酸、萜类、生物碱、肽类等为主要成分,其中以生物碱的数量最多,在栀子鲜花花水和栀子鲜花细胞液中分别为30种和39种。从各成分相对含量上看(图3),栀子鲜花花水中相对含量较高的成分种类有生物碱类、芳香类、单萜类,相对含量分别达到47.10%、20.74%、14.24%,而栀子鲜花细胞液中的主要成分有生物碱类、酯类、芳香类,相对含量分别为45.21%、18.01%、14.68%。由此可见,生物碱类成分是栀子鲜花花水和栀子鲜花细胞液中的主要活性物质,不仅含量高,而且成分种类多样。
图3
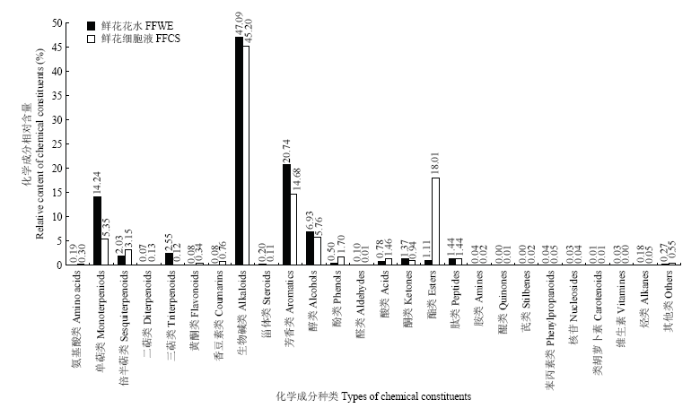 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3正离子模式下的栀子鲜花花水和细胞液的化学成分种类及其相对含量
Fig. 3Types of chemical constituents and their relative contents of floral water extract and flowers cell sap from Gardenia jasminoides in positive ion mode
在负离子模式下,栀子鲜花花水和栀子鲜花细胞液中的化学成分以生物碱、酸类成分为主,它们在栀子鲜花花水和栀子鲜花细胞液中的数量分别为5种、14种和12种、12种。从各成分相对含量上看(图4),栀子鲜花花水中相对含量较高的成分有酸类、三萜类、酯类,相对含量分别达到37.87%、24.75%、9.37%,而栀子鲜花细胞液以酮类、酸类、酯类、生物碱类为主,相对含量分别为31.29%、15.64%、14.19%、13.45%。可以看出,酸类和酯类成分均是栀子鲜花花水和栀子鲜花细胞液中大量存在的物质。
图4
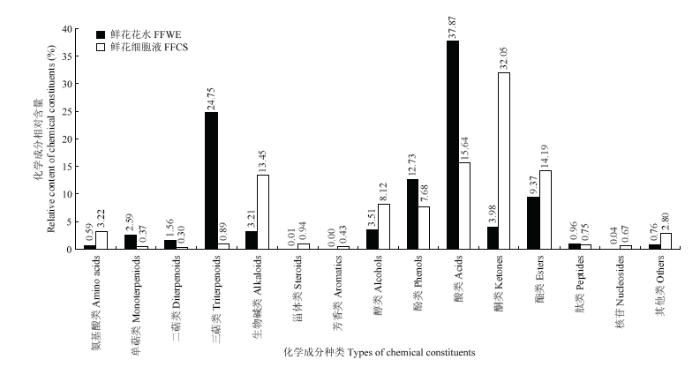 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4负离子模式下的栀子鲜花花水和细胞液的化学成分种类及其相对含量
Fig. 4Types of chemical constituents and their relative contents of floral water extract and flowers cell sap from Gardenia jasminoides in negative ion mode
2.2.1 氨基酸类成分的鉴定 氨基酸是栀子鲜花花水和鲜花细胞液中的一类主要成分。在正离子模式下,从栀子鲜花花水和鲜花细胞液中鉴定出的氨基酸成分有9种,如L-组氨酸、L-脯氨酸等,相对含量均小于0.1%。而在负离子模式下,仅检测出2种氨基酸,分别为L-脯氨酸和3-(3,4-二羟苯基)-N-[2-(4-羟苯基)乙基]丙氨酸,其中L-脯氨酸在栀子鲜花细胞液中的相对含量高达3.05%,在栀子鲜花花水中的相对含量为0.59%,也是相对含量最高的氨基酸成分。
2.2.2 萜类成分的鉴定 栀子鲜花花水和鲜花细胞液中的萜类成分较为丰富,不仅体现在数量上,在相对含量上也占有重要比例,包括单萜、倍半萜、二萜和三萜成分。
2.2.2.1 单萜成分的鉴定 栀子鲜花花水和鲜花细胞液中检测出的单萜成分不多。正离子模式下鉴定的成分有二氢黄蒿萜酮、桧醇和(S)-(-)-紫苏醇,其中栀子鲜花花水中的(S)-(-)-紫苏醇的相对含量高达13.73%,在鲜花细胞液中也有5.11%。在负离子模式下,发现了薄荷醇-葡萄糖醛酸和栀子中特有的活性成分京尼平酸,其中栀子鲜花花水中的京尼平酸相对含量较高,达到1.76%。
2.2.2.2 倍半萜成分的鉴定 负离子模式下没有发现倍半萜成分,而在正离子模式下响应灵敏,检测出了19种倍半萜成分。相对含量较高的有蜜环菌醛H、青蒿琥酯、氧化石竹烯、α-佛手柑油烯、(+)-长叶环烯、α-石竹烯等,其中相对含量最高的氧化石竹烯在栀子鲜花花水和鲜花细胞液中的相对含量分别达到1.77%和0.38%。
2.2.2.3 二萜成分的鉴定 栀子鲜花花水和鲜花细胞液中检出的二萜类成分不多。正离子模式下共检测到4种二萜类成分,如西红花苷II、赤霉素A62等,相对含量均在0.1%以下,而负离子模式下仅检测到辛卡西醇B这一种二萜成分,在栀子鲜花花水中的相对含量为1.56%,而在栀子鲜花细胞液中仅为0.30%。
2.2.2.4 三萜成分的鉴定 栀子鲜花花水和鲜花细胞液中检测到的三萜类成分虽然也不多,但是有些三萜化合物的相对含量较高。正离子模式下检测到的4种三萜成分中,山茶皂苷元B在栀子鲜花花水中的相对含量达到2.16%,而鲜花细胞液中只有0.02%。负离子模式下检测到的三萜成分有异柠檬苦素酸、麦德龙苷D和羟基积雪草苷,其中麦德龙苷D和羟基积雪草苷在栀子鲜花花水中的相对含量分别达到4.35%和20.20%。
2.2.3 生物碱成分的鉴定 生物碱成分是栀子鲜花花水和鲜花细胞液中成分数量最多,也是相对含量最高的一类成分,相对含量分别达到47.09%和45.20%。生物碱成分在正离子模式下更容易响应,检测到的成分数量要远高于负离子模式。正离子模式下检测到的44种生物碱成分中,芥酸酰胺、大观霉素、氨茴酸、L-1,2,3,4-四氢-β-咔啉-3-羧酸的相对含量均较高。芥酸酰胺在栀子鲜花花水中的相对含量达到43.66%,而在鲜花细胞液中的相对含量为19.41%,均是相对含量最高的生物碱成分,是栀子鲜花花水和鲜花细胞液中的重要功效成分。大观霉素在栀子鲜花细胞液中的相对含量为18.16%,而在栀子鲜花花水中仅为0.29%。负离子模式下检测到的14种生物碱成分中,鲜花细胞液中相对含量较高的成分有曲贝替定、3-(3,4-二羟苯基)-N-[2-(5-羟苯基)乙基]丙酮酸、纳他霉素和葫芦巴碱,相对含量分别达到3.30%、3.33%、2.61%和1.58%,而栀子鲜花花水中的3-(3,4-二羟苯基)-N-[2-(5-羟苯基)乙基]丙酮酸相对含量仅有0.40%。鲜花细胞液未检测到的甘油磷酰胆碱和4-羟基异喹胍在栀子鲜花花水中的含量则达到1.54%和1.25%。
3 讨论
由于栀子鲜花细胞液中醇类化合物和萜烯类化合物的相对含量总和达到95.71%,而其中芳樟醇及其氧化物的含量高达69.52%,反式-橙花叔醇和橙花叔醇的含量高达19.12%,可见醇类化合物对栀子鲜花细胞液的香气品质影响最大,起到了决定性作用,而水蒸气蒸馏提取的栀子鲜花花水中醇类化合物和萜烯类化合物的相对含量总和为72.00%,其中芳樟醇及其氧化物的含量达63.82%,反式-橙花叔醇仅有0.05%。由于萜烯类化合物具有新鲜的头香,芳樟醇及其氧化物使香气偏轻[47],反式-橙花叔醇带有类似于玫瑰、铃兰和苹果花的气息[48],因此,栀子鲜花细胞液较栀子鲜花花水具有充足的头香,但香气简单,层次不丰富。而栀子鲜花花水除了醇类化合物和萜烯类化合物外,还含有相对含量高达26.06%的酯类化合物,主要以惕各酸酯类化合物存在,各惕各酸酯类化合物相对含量总和为19.47%,其中惕各酸顺-3-己烯酯相对含量达13.24%,与报道的栀子鲜花中的主要成分结果相一致[10-11,13-14]。通过文献检索,发现惕各酸酯类成分仅能在栀子花[8,10,14]、香叶天竺葵[49]、百合[50]等少数花卉中有报道,尤以栀子花中的相对含量较高,数量最多,而其他花卉相对含量较低,是栀子花的特征性香气成分。其中,惕各酸顺-3-己烯酯有优雅的花香气[51],使栀子鲜花花水具有很好的底香。因此,栀子鲜花花水更具有栀子花特有的香气,而且香气更悠长,层次更丰富。与张银华等[12]制备的栀子花精油的成分进行比较,发现栀子鲜花花水中惕各酸顺-3-己烯酯的比例很高,而栀子花精油中非常低,表明栀子鲜花花水比稀释后的栀子花精油更能显现“栀子香型”。L-脯氨酸是一种重要的五元环状氨基酸,是人体合成蛋白质的十八种氨基酸之一,味微甜,可作为营养增补剂[52],在栀子鲜花花水和鲜花细胞液中均是相对含量最高的氨基酸成分,但栀子鲜花细胞液中的氨基酸类种类多于栀子鲜花花水,营养成分更全面。可见栀子鲜花细胞液的营养功效较栀子鲜花花水全面。(S)-(-)-紫苏醇是一种单环单萜烯醇,具有较好的抗癌功效,在国外已作为防癌保健品推广,或作为新型抗癌药物进行临床试验[53],(S)-(-)-紫苏醇是栀子鲜花花水和鲜花细胞液中相对含量最高的单萜类成分。通过分析,发现栀子鲜花花水中存在较多的抗菌、抗炎活性物质,如倍半萜成分双氢青蒿素、三萜成分山茶皂苷元B、三萜成分羟基积雪草苷等,而栀子鲜花细胞液中仅含有山茶皂苷元B、羟基积雪草苷,抗菌、抗炎成分种类较少而且相对含量极低。双氢青蒿素对大肠杆菌有抗菌活性[54],且具有较好的抗炎活性[55],山茶皂苷元B对病原真菌和酵母菌具有较强的抑制活性[56],羟基积雪草苷具有抗炎药理作用,已在临床上得到应用[57]。此外,栀子鲜花花水中还存在双环孢素、塔罗霉素A等抗生素,而鲜花细胞液中仅含有塔罗霉素A。栀子鲜花细胞液中含有相对含量较高的大观霉素,栀子鲜花花水中也含有大观霉素,其具有抗淋球菌活性[58]。由此可见,栀子鲜花花水中的抗菌、抗炎成分更为多样,在抗菌、抗炎功效上更为突出。京尼平酸是栀子中存在的特征性活性成分之一,是评价栀子药效的主要化学成分[59],具有抗氧化、抗应激等药理作用[60],负离子模式下,在栀子鲜花花水中的相对含量达1.76%,占其单萜类成分的比例为67.95%;而在栀子鲜花细胞液中的相对含量仅有0.18%,占其单萜类成分的比例为48.65%,可见京尼平酸在栀子鲜花花水抗氧化功效的表现上更突出。芥酸酰胺作为芥酸的重要衍生物,是一种应用范围广泛的生物活性物质,有研究表明,芥酸酰胺对小鼠具有一定的抗焦虑样作用[61]。芥酸酰胺作为栀子鲜花花水和鲜花细胞液中相对含量最高的一种生物碱成分,赋予了栀子鲜花花水和鲜花细胞液具有一定的抗焦虑生理功能。
4 结论
通过气相色谱—质谱联用技术分析了栀子鲜花花水和鲜花细胞液的香气成分,表明芳樟醇是栀子鲜花细胞液和栀子鲜花花水的主要香气成分。栀子鲜花细胞液较栀子鲜花花水具有充足的头香,但香气简单,留香时间短;而栀子鲜花花水含有更多的代表栀子花特征香气成分的惕各酸酯类化合物,香气成分较栀子鲜花细胞液丰富,各类化合物含量分布较均衡,使栀子鲜花花水除了具有良好的头香,而且具有浓郁的底香,持香时间长,香气层次更丰富。通过超高效液相色谱—质谱联用技术(UPLC-ESI-QTOF-MS/MS)分析了栀子鲜花花水和鲜花细胞液液相化学成分,表明生物碱类成分是栀子鲜花花水和鲜花细胞液中的主要特征性物质,可作为栀子鲜花花水和鲜花细胞液品质的评价指标。栀子鲜花花水含有丰富的萜类成分及生物碱成分,而栀子鲜花细胞液在营养功效上更显著。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.5650/jos.62.175URLPMID:23470445 [本文引用: 1]

The components of the essential oil from the roots of Pueraria mirifica were analyzed by capillary gas chromatography-mass spectrometry (GC-MS). Eighty-two components, representing 88.5% of the total oil, were identified by GC-MS. The main component of the oil was 2-pentylfuran, followed by hexanal and hexadecanol. With regard to the odor components from the essential oil of P. mirifica as determined by gas chromatography-olfactometry and aroma extract dilution analysis, it was revealed that phenylacetaldehyde and (2E)-nonenal imparted the green odor of the oil, and geraniol contributed to the sweet odor.
DOI:10.5650/jos.62.631URLPMID:23985493 [本文引用: 1]

The aim of the present study was to investigate the essential oils isolated from flower and leaf in order to get insight into similarities and differences as to their aroma-active composition. The essential oil obtained from the two parts were analyzed by gas chromatography-mass spectrometry and gas chromatography olfactometry (GC-O). Flower and leaf oils, 38 and 36 constituents, representing 96.4 and 91.0% of the total oil composition, respectively, were identified. The main compounds in flower oil were camphor (47.64%), bornyl acetate (11.87%), and nojigiku alcohol (6.29%), whereas those in leaf oil were camphor (39.14%), nojigiku alcohol (10.76%) and gamma-muurolene (7.02%). 13 Aroma-active compounds were identified by GC-O analysis in flower oil and 12 in leaf oil. The main aroma-active compounds in flower oil were camphor (camphor, FD (flavor dilution) = 7, OAV (odor active value) = 136913), bornyl acetate (camphor, FD = 6, OAV = 113711), and beta-caryophyllene (spicy, FD = 5, OAV = 116480). In leaf oil, the main aroma-active compounds were camphor (camphor, FD = 7, OAV = 106784), nojigiku alcohol (camphor, FD = 5, OAV = not determined), and beta-caryophyllene (spicy, FD = 6, OAV = 526267).
DOI:10.5650/jos.ess13125URL [本文引用: 1]

The chemical composition of volatile oil obtained from aerial parts of Pavonia odorata were investigated using gas chromatography-mass spectrometry (GC-MS). Its aroma-active compounds were identified using gas chromatography-olfactometry (GC-O) and aroma extraction dilution analysis (AEDA). In order to determine the relative contribution of each compound to the aroma of P odorata, relative flavour activity (RFA) was calculated. The hydrodistillation of P odorata afforded yellowish oil and the yield was 0.009% (w/w) with a spicy, sweet, and green odour. Eighty-five compounds were identified in the oil by GC-MS; the major constituents of the volatile oil were ageratochromene (11.95%), palmitic acid (9.95%), hexahydrofarnesyl acetone (5.96%), beta-eudesmol (4.53%) and beta-caryophyllene oxide (3.08%). The most characteristic aroma compounds in the volatile oil were identified for p-caryophyllene oxide (FD-factor = 128, spicy), (E)-pinocarveol (FD-factor = 64, sweet), 3-butylpyridine (FD-factor = 64, spicy), and 2-nonanone (FD-factor = 32, green) by GC-MS, GC-O and AEDA. It seems that these compounds are responsible for the spicy, sweet and green odour of the aerial parts of P odorata. The antioxidant activity of the volatile oil was also investigated by the oxygen radical absorbance capacity (ORAC) assay using fluorescein (FL) as the fluorescent probe. The ORAC value of the oil was 594.2 +/- 25.9 mu M TE/g. The results indicated that the volatile oil from the aerial parts of P odorata could be considered as a natural antioxidant effect agent.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:12541711 [本文引用: 1]

The headspace constituents of fresh Gardenia flower were investigated by GC/MS. The headspace volatiles were sampled by solid-phase microextraction (SPME) and dynamic headspace sampling (DHS). SPME sampling was conducted with 100 microns PDMS fiber at 28 degrees C for 60 min. In DHS sampling, purified nitrogen was used as purging gas with a flow rate at 80 mL/min for 120 min. Tenax GR(20 mesh-40 mesh) was used as adsorbent and the volatiles were eluted by ether, and concentrated to 0.5 mL for GC/MS analysis. A Supelco-wax capillary column (30 m x 0.25 mm i.d. x 0.25 micron df) was employed in GC/MS analysis. Initial oven temperature was kept at 45 degrees C for 2 min, then raised to 250 degrees C at 4 degrees C/min, and kept at 250 degrees C for 10 min. According to SPME-GC/MS analysis, the main compounds in headspace of fresh Gardenia flower included farnesene(64.86%), cis-ocimene(29.33%), linalool(2.74%), cis-3-hexenyl tiglate(1.34%), methyl benzoate(0.25%). Results obtained from SPME and DHS sampling were also compared. In this study, SPME afforded a simpler and more sensitive sampling method, and much more accurate information about headspace volatiles of Gardenia flower.
URLPMID:12541711 [本文引用: 1]

The headspace constituents of fresh Gardenia flower were investigated by GC/MS. The headspace volatiles were sampled by solid-phase microextraction (SPME) and dynamic headspace sampling (DHS). SPME sampling was conducted with 100 microns PDMS fiber at 28 degrees C for 60 min. In DHS sampling, purified nitrogen was used as purging gas with a flow rate at 80 mL/min for 120 min. Tenax GR(20 mesh-40 mesh) was used as adsorbent and the volatiles were eluted by ether, and concentrated to 0.5 mL for GC/MS analysis. A Supelco-wax capillary column (30 m x 0.25 mm i.d. x 0.25 micron df) was employed in GC/MS analysis. Initial oven temperature was kept at 45 degrees C for 2 min, then raised to 250 degrees C at 4 degrees C/min, and kept at 250 degrees C for 10 min. According to SPME-GC/MS analysis, the main compounds in headspace of fresh Gardenia flower included farnesene(64.86%), cis-ocimene(29.33%), linalool(2.74%), cis-3-hexenyl tiglate(1.34%), methyl benzoate(0.25%). Results obtained from SPME and DHS sampling were also compared. In this study, SPME afforded a simpler and more sensitive sampling method, and much more accurate information about headspace volatiles of Gardenia flower.
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 3]

In order to under the aroma composition and release regularity of Gardenia jasminoides during the flowering period, the aroma-active components of Gardenia jasminoides at the early, full and late flowering stages were determined by solid phase microextraction (SPME) and gas chromatograph-mass spectrophotometry (GC-MS). A total of 36 aroma-active components were detected throughout the whole flowering stages, which were mainly esters, terpenoids and hydrocarbons and together accounted for 85.25%, 85.29% and 94.37% of total volatile compounds at the early, full and late flowering stages, respectively. The relative contents of esters and hydrocarbons were low at the early flowering stage and then increased as the flowering period proceeded; however, the relative content of terpenoids showed a trend of decreasing initially and then increasing. The main compounds responsible for the aroma of Gardenia jasminoides flowers were ocimene, α-farnesene, benzoic acid methyl ester, isopropyl cyclohexane and 3,7-dimethyl 1,6-octadiene-3-alcohol propionate.
URL [本文引用: 3]

In order to under the aroma composition and release regularity of Gardenia jasminoides during the flowering period, the aroma-active components of Gardenia jasminoides at the early, full and late flowering stages were determined by solid phase microextraction (SPME) and gas chromatograph-mass spectrophotometry (GC-MS). A total of 36 aroma-active components were detected throughout the whole flowering stages, which were mainly esters, terpenoids and hydrocarbons and together accounted for 85.25%, 85.29% and 94.37% of total volatile compounds at the early, full and late flowering stages, respectively. The relative contents of esters and hydrocarbons were low at the early flowering stage and then increased as the flowering period proceeded; however, the relative content of terpenoids showed a trend of decreasing initially and then increasing. The main compounds responsible for the aroma of Gardenia jasminoides flowers were ocimene, α-farnesene, benzoic acid methyl ester, isopropyl cyclohexane and 3,7-dimethyl 1,6-octadiene-3-alcohol propionate.
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
URL [本文引用: 2]
URL [本文引用: 2]
[本文引用: 3]
[本文引用: 3]
URLPMID:27125012 [本文引用: 1]

The volatile profiles of aroma extracts prepared from the flower of Gardenia jasminoides by different methods were investigated using gas chromatography-mass spectrometry. The enfleurage extraction using spermaceti wax and palm oil afforded the best aroma extract with a preference that was significantly (p < 0.05) better than those from solvent extractions, as sensorially evaluated in 43 volunteers. The odor quality of the absolute de enfleurage was similar to the floral scent of fresh gardenia, as confirmed in 152 volunteers. Although female volunteers were insignificantly (p > 0.05) better sensed than male volunteers, age was significant (p < 0.05). The nuance gardenia floral scent was contributed by farnesene, Z-3-hexenyl tiglate, Z-3-hexenyl benzoate, and indole. The relaxing and refreshing sensations of the gardenia odor suggest its applications in body care, cleansing products, and perfume. This study addresses the increasing interest in floral fragrances. The aroma profile and sensory property of this sweet and elegant scent flower will strengthen and expand the applications of gardenia from traditional medicine to those of perfumery and the field of phytochemistry.
DOI:10.11869/j.issn.100-8551.2014.11.2079URL [本文引用: 1]

Plant essential oils, as a important secondary metabolites in plants, can be used as alternative way of chemical method in food preservation for their broad spectrum of antibacterial properties, which has become a current hot spot in food preservation. This paper reviewed the characteristics of plant oils, and emphasize on the inhibitory effect and antibacterial mechanism of plant essential oils on animal derived foods, as well as the factors influencing the antibacterial ability. Furthermore, the application methods and preservation effects of plant oils in the field of animal food preservation are also discussed. This review may provide a theoretical basis and technical reference for commercial application of plant essential oils in animal product foods.
DOI:10.11869/j.issn.100-8551.2014.11.2079URL [本文引用: 1]

Plant essential oils, as a important secondary metabolites in plants, can be used as alternative way of chemical method in food preservation for their broad spectrum of antibacterial properties, which has become a current hot spot in food preservation. This paper reviewed the characteristics of plant oils, and emphasize on the inhibitory effect and antibacterial mechanism of plant essential oils on animal derived foods, as well as the factors influencing the antibacterial ability. Furthermore, the application methods and preservation effects of plant oils in the field of animal food preservation are also discussed. This review may provide a theoretical basis and technical reference for commercial application of plant essential oils in animal product foods.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.3864/j.issn.0578-1752.2017.04.012URL [本文引用: 1]

【Objective】 The objective of this experiment is to clarify the impacts of different extraction processes (water steam distillation and coeno-water distillation) and collecting periods on the aroma constituents of Rosa damascena flower water, and to provide reference for the reasonable processing and utilization of rose water. 【Method】 The dominant aroma compositions of R. damascena flower water from different extraction processes and collecting periods were identified and quantified by using the following methods: sampled by solid phase micro extraction first, then the constituent was isolated by gas chromatography- mass spectrometry (GC-MS), and each constituent was retrieved and analyzed by the computer spectral database (NIST/WILEY), finally confirmed artificially referring to data. 【Result】 Types and relative percentage of aroma constituents of R. damascena flower water extracted by water steam distillation were all higher than that extracted by coeno-water distillation, as well as the relative percentage of geraniol and benzyl alcohol. Therefore, R. damascena flower water extracted by water steam distillation was rich in aroma and sweet smell, and the quality was superior to that extracted by coeno-water distillation. Whether R. damascena flower water extracted by coeno-water distillation or water steam distillation had the maximum quantity and content of alcohols in the second period. However, the main aroma of R. damascena flower water extracted by coeno-water distillation and collected in the second period was the strongest, while the main aroma of R. damascene rose water extracted by water steam distillation and collected in the first period was as strong as that collected in the second period. Therefore, R. damascena flower water extracted by coeno-water distillation and collected in the second period (the second weight which is equal to flowers) had the best quality, and the first (the first weight which is equal to flowers) and second periods were suitable for collecting in the production of rose water. While R. damascena flower water from the first period (the first weight which is equal to flowers) extracted by water steam distillation had the best quality, and the first, second and third periods were suitable for collecting in the production of rose water. 【Conclusion】 The aroma of R. damascene rose water extracted by water steam distillation is superior to that extracted by coeno-water distillation. R. damascena flower water extracted by coeno-water distillation and collected in the first two periods is applicable in the production, while rose water extracted by water steam distillation and collected in the first three periods is applicable.
DOI:10.3864/j.issn.0578-1752.2017.04.012URL [本文引用: 1]

【Objective】 The objective of this experiment is to clarify the impacts of different extraction processes (water steam distillation and coeno-water distillation) and collecting periods on the aroma constituents of Rosa damascena flower water, and to provide reference for the reasonable processing and utilization of rose water. 【Method】 The dominant aroma compositions of R. damascena flower water from different extraction processes and collecting periods were identified and quantified by using the following methods: sampled by solid phase micro extraction first, then the constituent was isolated by gas chromatography- mass spectrometry (GC-MS), and each constituent was retrieved and analyzed by the computer spectral database (NIST/WILEY), finally confirmed artificially referring to data. 【Result】 Types and relative percentage of aroma constituents of R. damascena flower water extracted by water steam distillation were all higher than that extracted by coeno-water distillation, as well as the relative percentage of geraniol and benzyl alcohol. Therefore, R. damascena flower water extracted by water steam distillation was rich in aroma and sweet smell, and the quality was superior to that extracted by coeno-water distillation. Whether R. damascena flower water extracted by coeno-water distillation or water steam distillation had the maximum quantity and content of alcohols in the second period. However, the main aroma of R. damascena flower water extracted by coeno-water distillation and collected in the second period was the strongest, while the main aroma of R. damascene rose water extracted by water steam distillation and collected in the first period was as strong as that collected in the second period. Therefore, R. damascena flower water extracted by coeno-water distillation and collected in the second period (the second weight which is equal to flowers) had the best quality, and the first (the first weight which is equal to flowers) and second periods were suitable for collecting in the production of rose water. While R. damascena flower water from the first period (the first weight which is equal to flowers) extracted by water steam distillation had the best quality, and the first, second and third periods were suitable for collecting in the production of rose water. 【Conclusion】 The aroma of R. damascene rose water extracted by water steam distillation is superior to that extracted by coeno-water distillation. R. damascena flower water extracted by coeno-water distillation and collected in the first two periods is applicable in the production, while rose water extracted by water steam distillation and collected in the first three periods is applicable.
DOI:10.3864/j.issn.0578-1752.2019.13.010URL [本文引用: 1]

【Objective】 Fruit volatiles of day-neutral strawberries harvested in autumn and winter were compared to clarify the effects of seasonal changes on volatile composition and odor activity value (OAV) of volatiles, aiming to provide theoretical basis for research on strawberry aroma and utilization of day-neutral cultivars. 【Method】 Matured fruits of day-neutral cultivars (including Albion, Monterey, Portola, and San Andreas) were harvested from high-tunnel in autumn and greenhouse in winter, respectively. Volatiles were extracted by using solid-phase microextraction (SPME), detected by gas chromatograph-mass spectrometer (GC-MS) and analyzed qualitatively and quantitatively. The numbers, contents, percentages and OAVs of volatiles were calculated. Principal component analysis (PCA) was employed to analyze the differences in volatiles content between autumn and winter strawberries. Clustered heatmap was used to classify the samples depending on the volatiles with OVA higher than 1. 【Result】 A total of 88 individual volatiles were identified and the OAVs of 57 volatiles were calculated. It was found that there were 30 components with OAV higher than 1. Effects of seasonal changes on the number of each chemical category and the total content of volatiles varied among cultivars. Compared to the autumn fruits, the content and percentage of esters in winter fruits were significantly higher, while terpenes were remarkably lower. The result of PCA indicated that, compared with other cultivars, volatiles pattern of Portola showed less variation, whereas San Andreas showed the highest variation between two seasons. Ethyl butanoate was very important for these tested cultivars since its average OAV was the highest in all volatiles. The OAVs of butyl acetate and methyl butanoate in winter fruits were noticeably higher than that in autumn fruits. However, compared with autumn fruits, the winter fruits of each cultivar had a lower sum of terpene OAVs that were higher than 1. Ethyl butanoate, butyl butanoate, 4-methoxy-2, 5-dimethyl- 3(2H)-furanone, ethyl hexanoate and linalool played an important role in the aroma differences between fruits of different seasons. The eight tested samples were classified into 3 groups using clustered heatmap. The group I included Albion autumn, Albion winter and Monterey autumn, of which total OAVs and the OAVs of ethyl butanoate were significantly higher than other groups. 【Conclusion】 Seasonal change had a remarkable impact on esters and terpenes in fruit volatiles of day-neutral strawberry cultivars. It was showed that the content and percentage of esters in winter fruits were significantly higher than autumn fruits, while the percentage of terpenes was remarkably lower in winter fruits. Among the 4 tested cultivars, Portola showed the least variation in volatile content between autumn and winter. Albion was the cultivar with intense aroma and its aroma performance presented the least changes when season changed, however, San Andreas was the one with the largest volatile variation both in volatile content and fruit aroma.
DOI:10.3864/j.issn.0578-1752.2019.13.010URL [本文引用: 1]

【Objective】 Fruit volatiles of day-neutral strawberries harvested in autumn and winter were compared to clarify the effects of seasonal changes on volatile composition and odor activity value (OAV) of volatiles, aiming to provide theoretical basis for research on strawberry aroma and utilization of day-neutral cultivars. 【Method】 Matured fruits of day-neutral cultivars (including Albion, Monterey, Portola, and San Andreas) were harvested from high-tunnel in autumn and greenhouse in winter, respectively. Volatiles were extracted by using solid-phase microextraction (SPME), detected by gas chromatograph-mass spectrometer (GC-MS) and analyzed qualitatively and quantitatively. The numbers, contents, percentages and OAVs of volatiles were calculated. Principal component analysis (PCA) was employed to analyze the differences in volatiles content between autumn and winter strawberries. Clustered heatmap was used to classify the samples depending on the volatiles with OVA higher than 1. 【Result】 A total of 88 individual volatiles were identified and the OAVs of 57 volatiles were calculated. It was found that there were 30 components with OAV higher than 1. Effects of seasonal changes on the number of each chemical category and the total content of volatiles varied among cultivars. Compared to the autumn fruits, the content and percentage of esters in winter fruits were significantly higher, while terpenes were remarkably lower. The result of PCA indicated that, compared with other cultivars, volatiles pattern of Portola showed less variation, whereas San Andreas showed the highest variation between two seasons. Ethyl butanoate was very important for these tested cultivars since its average OAV was the highest in all volatiles. The OAVs of butyl acetate and methyl butanoate in winter fruits were noticeably higher than that in autumn fruits. However, compared with autumn fruits, the winter fruits of each cultivar had a lower sum of terpene OAVs that were higher than 1. Ethyl butanoate, butyl butanoate, 4-methoxy-2, 5-dimethyl- 3(2H)-furanone, ethyl hexanoate and linalool played an important role in the aroma differences between fruits of different seasons. The eight tested samples were classified into 3 groups using clustered heatmap. The group I included Albion autumn, Albion winter and Monterey autumn, of which total OAVs and the OAVs of ethyl butanoate were significantly higher than other groups. 【Conclusion】 Seasonal change had a remarkable impact on esters and terpenes in fruit volatiles of day-neutral strawberry cultivars. It was showed that the content and percentage of esters in winter fruits were significantly higher than autumn fruits, while the percentage of terpenes was remarkably lower in winter fruits. Among the 4 tested cultivars, Portola showed the least variation in volatile content between autumn and winter. Albion was the cultivar with intense aroma and its aroma performance presented the least changes when season changed, however, San Andreas was the one with the largest volatile variation both in volatile content and fruit aroma.
DOI:10.1080/10412905.2010.9700290URL [本文引用: 1]
[本文引用: 1]
DOI:10.1021/jf0402633URLPMID:15769159 [本文引用: 5]

The volatile components of 20 mango cultivars were investigated by means of simultaneous distillation-extraction, GC, and GC-MS. Three hundred and seventy-two compounds were identified, of which 180 were found for the first time in mango fruit. The total concentration of volatiles was approximately 18-123 mg/kg of fresh fruit. Terpene hydrocarbons were the major volatiles of all cultivars, the dominant terpenes being delta-3-carene (cvs. Haden, Manga amarilla, Macho, Manga blanca, San Diego, Manzano, Smith, Florida, Keitt, and Kent), limonene (cvs. Delicioso, Super Haden, Ordonez, Filipino, and La Paz), both terpenes (cv. Delicia), terpinolene (cvs. Obispo, Corazon, and Huevo de toro), and alpha-phellandrene (cv. Minin). Other qualitative and quantitative differences among the cultivars could be demonstrated.
URL [本文引用: 1]

A method for the preparation of volatile compounds in Changyu XO brandy was established. The volatile compounds were extracted using liquid-liquid extraction and then were separated into two fractions, namely, the acidic/water-soluble fraction and the neutral/basic fraction. The neutral/basic fraction was furthermore separated into 4 fractions using silica gel normal phase chromatography, and each fraction was then concentrated and analyzed using gas chromatography-mass spectrometry (GC-MS). In comparison with the pure standards and the retention indices (RIs) reported in the literature, a total of 302 volatile compounds were identified in Changyu XO brandy, including 30 alcohols, 35 aldehydes and ketones, 20 carboxylic acids, 104 esters, 24 substituted benzenes and derivatives, 14 phenolic derivatives, 14 acetals, 16 furan derivatives, 22 terpenic and norisoprenoidic derivatives and 23 others. It was demonstrated that this method of preparation was effective for the separation and concentration of volatile compounds in Changyu XO brandy.
DOI:10.1016/j.chroma.2006.02.034URLPMID:16545828 [本文引用: 4]

The essential oil components of geranium oil cultivated in center of Iran were identified and determined using gas chromatography-mass spectrometry data combined with the chemometric resolution techniques. A total of 61 components accounting for 91.51% were identified using similarity searches between the mass spectra and MS database. This number was extended to 85 components using chemometric techniques. Various chemometric methods such as morphological scores, simplified Borgen method (SBM) and fixed size moving window evolving factor analysis (FSMWEFA) were used for determining the number of components, pure variables, zero concentration and selective regions. Then the overlapping peak clusters were resolved into pure chromatograms and pure mass spectra using heuristic evolving latent projections (HELP) method. A characteristic feature of the Iranian geranium oil is the absence of 10-epi-gamma-eudesmol in its constituents compared with the oil from northern and southern parts of India. The results of this work show that combination of hyphenated chromatographic methods and resolution techniques provide a complementary method for accurate analysis of essential oils.
DOI:10.1021/jf801070rURLPMID:19061307 [本文引用: 1]

The aroma-active compounds of Pontianak orange peel oil ( Citrus nobilis Lour. var. microcarpa Hassk.) were characterized by using gas chromatography-olfactometry (GC-O) and aroma extract dilution analysis (AEDA) techniques. Forty-one compounds were found to be aroma-active, which were mainly dominated by saturated and unsaturated aldehydes. The flavor dilution (FD) factor was within the range of 2-2048, and compounds having the highest FD factor were alpha-pinene, beta-pinene, linalool, and 2-methoxy-3-(2-methylpropyl) pyrazine, including a few unknown compounds. On the basis of GC-O results, odor activity value (OAV) and relative flavor activity (RFA) were determined for aroma model reconstitution. These resembled the original aroma of the peel oil for the green, fatty, fresh, peely, floral, and tarry attributes, with the model solution derived from OAV being the closest to Pontianak oil. Omission tests were carried out to verify the significance of (Z)-5-dodecenal and 1-phenylethyl mercaptan as key compounds in the aroma of Pontianak orange peel oil.
DOI:10.1016/j.bse.2003.09.002URL [本文引用: 2]

Abstract
The composition of the essential oils of 16 individual plants of Artemisia herba-alba Asso ssp. valentina (Lam.) Marcl. (at the full bloom stage) growing wild in four different locations from southern Spain were investigated by capillary GC and GC–MS in combination with retention indices. Among the 60 identified constituents (accounting for 80.6–95.0% of the oils), 33 have been reported for the first time in Spanish A. herba-alba oil and 17 of them have not been previously described in A. herba-alba oil. From the analysis of the oil samples, it could be deduced that a noticeable chemical polymorphism typified this taxon. Four groups of essential oils exhibited a single compound with percentages near 30% or higher: davanone, 1,8-cineole, chrysanthenone and cis-chrysanthenol. Two further oil types showed p-cymene and cis-chrysanthenyl acetate as major components in moderate amounts (ca. 20%). All of these types of essential oils have not been previously found in A. herba-alba from Spain and the appearance of such considerable amount of p-cymene is described here for the first time in A. herba-alba.DOI:10.1016/j.chroma.2005.11.087URLPMID:16359681 [本文引用: 3]

The volatile composition of eight Anthemis species has been studied. The essential oils were obtained by hydrodistillation in a modified Clevenger-type apparatus, and their analyses were performed by GC and GC-MS. Identification of the substances was made by comparison of mass spectra and retention indices with literature records. A total of 284 different compounds were identified and significant qualitative and quantitative differences and similarities were observed among the samples. The main constituents of the investigated populations of each taxon have been revealed as follows: A. altissima: (-)-linalool, trans-caryophyllene, cis-chrysanthenyl acetate; A. auriculata: spathulenol, trans-caryophyllene, beta-eudesmol; A. chia: cis-chrysanthenyl acetate, trans-caryophyllene, germacrene-d; A. cotula: germacrene-d, spathulenol A. tinctoria: spathulenol, (-)-caryophyllene oxide, T-cadinol; A. melanolepis: p-cymene, chrysanthenone, trans-verbenol, (-)-caryophyllene oxide; A. tomentosa: (-)-linalool, 1,8-cineole; A. werneri subsp. werneri: nopol, terpineol-4, trans-caryophyllene. Sesquiterpene hydrocarbons were shown to be the main group of constituents of all taxa.
DOI:10.1021/jf071442yURLPMID:17824662 [本文引用: 1]

Thyme honey is the most appreciated unifloral Greek honey in Greece as well as around the world. In an effort to investigate the headspace composition of this type of honey, 28 samples were analyzed by means of solid-phase microextraction coupled to a gas chromatography-mass spectrometry system. The botanical origin of the samples was ascertained by pollen analysis, and samples displayed relative frequencies of thyme pollen between 18 and 41%. A total of 62 compounds were isolated, and phenylacetaldehyde was the most abundant (32.9% of the total peak area). Possible botanical markers are 1-phenyl-2,3-butanedione (13.4%), 3-hydroxy-4-phenyl-2-butanone, 3-hydroxy-1-phenyl-2-butanone (14.7%), phenylacetonitrile (4.8%), and carvacrol (0.9%), since these compounds are found only in thyme honey. Additionally, high proportions of phenylacetaldehyde are also characteristic ( F = 12.282, p < 0.001). The average concentrations of seven compounds were significantly different ( p < 0.05), namely phenylacetaldehyde, acetophenone, octanoic acid, carvacrol, phenylethyl alcohol, nonanal, and hexadecane. Applying principal component analysis to the data, six components were extracted, explaining 85.4% of the total variance. The first component explained 46.2% of the total variance and was positively correlated to phenylacetaldehyde, nonanoic acid, acetophenone, decanoic acid, benzaldehyde, phenylacetonitrile, isophorone, and nonanal. The extracted components were used as variables to the discriminant analysis, which showed good discrimination, especially for samples from Crete. A leave-one-out classification showed 85.7% of cross-validated grouped cases correctly classified. These results are promising to establish a discrimination model for these geographical regions. This is crucial for local beekeeper corporations on their effort to produce honey with geographical origin label.
DOI:10.1016/S0021-9673(01)01382-6URL [本文引用: 1]
DOI:10.1016/j.jfoodeng.2004.04.015URL [本文引用: 2]
DOI:10.1016/j.bse.2007.07.001URL [本文引用: 1]

Abstract
The chemical composition of the essential oil extracted from fresh leaves of Lippia citriodora (Verbenaceae) was analyzed by GC-FID and GC–MS in May, when growth rates are maximal, and in September, in full bloom. In both samples the main constituents were geranial, neral and limonene constituting 66.3% of the total essential oil yield in May and increasing to 69% in September. Their individual percentage values, however, changed considerably for geranial and neral decreasing from 38.7 to 26.8% and from 24.5 to 21.8%, respectively, and for limonene increasing from 5.8 to 17.7%. All other components remained more or less unchanged both qualitatively and quantitatively. FT-IR spectrometry was also applied for the qualitative determination of the main components.DOI:10.1021/jf0606104URLPMID:16910725 [本文引用: 1]

The composition of the essential oils of Origanum and Thymus species restricted to Algeria and the North Africa region was determined. Antioxidant and antibacterial activities of the isolated essential oils were also determined. The oils of oregano plants were strongly characterized by p-cymene (16.8-24.9%), gamma-terpinene (16.8-24.9%), thymol (8.4-36.0%), and carvacrol (1.1-29.7%), a thymol chemotype for Origanum floribundum and a alpha-terpineol chemotype for Thymus numidicus being described for the first time. The strains of Listeria monocytogenes tested were relatively resistant to the action of essential oils of either Origanum or Thymus species. All essential oils possessed antioxidant activity, but this was dependent on the specific chemical composition and the method employed to determine such activity.
[本文引用: 1]
DOI:10.1021/jf0303316URLPMID:14664540 [本文引用: 1]

The influence of nitrogen fertilizers on the yield of crop, as well as on the production and composition of the essential oil and some other chemical characteristics of thyme, was investigated. Different levels of fertilizers (N = 0, 45, 90, and 135 kg x ha(-)(1)) were applied. It was found that fertilizers increase thyme crop, but differences in the yield of essential oil were not remarkable. However, the use of certain amounts of nitrogen fertilizers resulted in higher yields of essential oil obtainable from the cultivation area unit (dm(3) ha(-)(1)). Totally, 61 constituents were identified in thyme essential oil by capillary GC and GC-MS. Thymol was the dominating compound in the all analyzed oils (44.4-58.1%), followed by p-cymene (9.1-18.5%), gamma-terpinene (6.9-18.9%), and carvacrol (2.4-4.2%). Differences in the percentage of these and other compounds in thyme herb cultivated under different fertilization doses were not significant; very slight changes in the percentage composition were detected after drying. Some variations in the amount of individual constituents expressed in arbitrary units per kilogram of herb (which is almost equivalent to mg x kg(-)(1)) were observed. The highest amounts of sugars and sucrose, in particular, were determined in the second year of thyme cultivation. Differences in the content of dry soluble substances were not meaningful, and there was no effect of nitrogen fertilizers on this chemical characteristic. Some effect of fertilization on the content of vitamin C and carotenes was observed in the first year of thyme cultivation. It was determined that nitrogen fertilizers influence the amount of nitrates, which was highest in the second-year-first-harvest.
DOI:10.1016/j.phymed.2009.07.016URL [本文引用: 1]

Abstract
Artemisia species are one of the many traditional medicinal plants of Ethiopia used for the treatment of infectious and non-infectious health problems. In the present study, eight extracts prepared from leaves and aerial parts of four Artemisia species (Artemisia absinthium, A. abyssinica, A. afra, and A. annua) growing in Ethiopia were tested in vitro against bloodstream forms of Trypanosoma brucei brucei. The most active extract was the dichloromethane extract from aerial parts of A. abyssinica with an IC50 value of 19.13 μg/ml. A selectivity index (SI) of 8.24 was obtained with HL-60 cells treated with the same extract. Artemisinin, the best known antimalarial compound from A. annua showed antitrypanosomal activity with an IC50 value of 35.91 μg/ml and with a selectivity index of 2.44. The dichloromethane extracts of the four species were further investigated for their volatile components using GLC/MS. Camphor was detected in the four species and was found to be the principal compound (38.73%) of A. absinthium extract. Octa-3,5-diene-2,7-dione, 4,5-dihydroxy was detected in three species except in A. afra and was present as the main volatile component (54.95%) of A. abyssinica. Epoxylinalool was detected only in A. afra and was the principal component (29.10%) of dichloromethane extract of the plant. Deoxyqinghaosu was only present in A. annua and absent in the other three Artemisia species. Deoxyqinghaosu was the principal volatile component (20.44%) of the dichloromethane extract of A. annua. In conclusion, the dichloromethane extract from aerial part of A. abyssinica should be considered for further study for the treatment of trypanosomiasis.DOI:10.1016/j.bse.2004.06.005URL [本文引用: 1]

Abstract
The composition of the essential oils of Lomatium dasycarpum ssp. dasycarpum, Lomatium lucidum, Lomatium macrocarpum var. macrocarpum and Lomatium utriculatum is described. Identification of components was determined from their GC, GC/MS data and many were confirmed by coinjections with authentic samples. Several components were isolated by liquid and gas chromatographic techniques and their structures confirmed from their 1H and 13C NMR spectral data. 2-Methyl and 3-methylbutanoates were the major components of L. dasycarpum fruits as well as stems and leaves oils. β-Phellandrene/limonene, decanal, dodecanal, bornyl acetate, germacrene D, α-humulene and bicyclogermacrene were the major components of the corresponding L. lucidum oils. α-Pinene and β-pinene were the major components of the fruit oil of L. macrocarpum. Its stem and leaf oil was rich in peucenin 7-methyl ether, β-caryophyllene, (Z)-3-hexenol, palmitic acid, linoleic acid and (E)-2-hexenal. Sabinene, (Z)-ligustilide, terpinen-4-ol, β-phellandrene/limonene, β-caryophyllene, myrcene, α-pinene and β-pinene were the major compounds in L. utriculatum fruit oil, while its stem and leaf oil was rich in (Z)-ligustilide, palmitic acid, terpinen-4-ol, linoleic acid and germacrene D. (Z)-Falcarinol was a major component of all the four root oils.DOI:10.1016/S0045-6535(02)00074-7URL [本文引用: 1]

Abstract
Qualitative composition of volatile emissions of litter of five species of deciduous trees was investigated by GC-MS. The list of identified substances contains more than 70 organic compounds of various classes. It was established that the composition of components emitted by the litter into the gas phase greatly differs from that of essential oils extracted by hydrodistillation from turned leaves collected from trees during fall. It is suggested that most compounds found in litter emissions are products of vital activity of microorganisms decomposing it. The reported data indicate that after the vegetative period is over the decomposition processes of litter are important seasonal sources of reactive organic compounds under the forest canopy.[本文引用: 2]
DOI:10.1021/jf040107wURLPMID:15373407 [本文引用: 1]

The volatile constituents of 10 clones (4 parents with different flavors and 6 hybrids from selected crossings among these parents) of pepino fruit (Solanum muricatum) were isolated by simultaneous distillation-extraction and analyzed by gas chromatography-mass spectrometry (GC-MS). Odor-contributing volatiles (OCVs) were detected by GC-olfactometry-MS analyses and included 24 esters (acetates, 3-methylbutanoates, and 3-methylbut-2-enoates), 7 aldehydes (especially hexenals and nonenals), 6 ketones, 9 alcohols, 3 lactones, 2 terpenes, beta-damascenone, and mesifurane. Among these compounds, 17, of which 5 had not been reported previously in pepino, were found to contribute significantly to pepino aroma. OCVs can be assigned to three groups according to their odor quality: fruity fresh (acetates and prenol), green vegetable (C6 and C9 aldehydes), and exotic (lactones, mesifuran, and beta-damascenone). Quantitative and qualitative differences between clones for these compounds are clearly related to differences in their overall flavor impression. The positive value found for the hybrid-midparent regression coefficient for volatile composition indicates that an important fraction of the variation observed is inheritable, which has important implications in breeding for improving aroma. Significant and positive correlations were found between OCVs having common precursors or related pathways.
DOI:10.1016/j.biortech.2007.08.058URLPMID:17920880 [本文引用: 1]

This study is designed to examine the chemical composition and in vitro antioxidant activity of the essential oil and sub-fractions of the methanol extract of Marrubium globosum subsp. globosum. The GC and GC-MS analysis of the essential oil were resulted in the determination of 84 components representing 88.2% of the oil. The major constituents of the oil were spathulenol (15.8%), beta-caryophyllene (9.0%), caryophyllene oxide (7.9%), germacrene D (6.5%), and bicyclogermacrene (3.1%). Antioxidant activities of the samples were determined by three different test systems namely DPPH, beta-carotene/linoleic acid and reducing power assay. In DPPH system, the weakest radical scavenging activity was exhibited by the essential oil (1203.38+/-7.18 microg ml(-1)). Antioxidant activity of the polar sub-fraction of methanol extract was superior to the all samples tested with an EC(50) value of 157.26+/-1.12 microg ml(-1). In the second case, the inhibition capacity (%) of the polar sub-fraction of methanol extract (97.39%+/-0.84) was found the strongest one, which is almost equal to the inhibition capacity of positive control BHT (97.44%+/-0.74). In the case of reducing power assay, a similar activity pattern was observed as given in the first two systems. Polar sub-fraction was the strongest radical reducer when compared with the non-polar one, with an EC(50) value of 625.63+/-1.02 microg ml(-1). The amount of the total phenolics was highest in polar sub-fraction (25.60+/-0.74 microg/mg). A positive correlation was observed between the antioxidant activity potential and total phenolic level of the extracts. On the other hand, total flavonoid content was found equal for the both sub-fractions.
DOI:10.1021/jf00081a036URL [本文引用: 1]
DOI:10.1021/jf9705781URL [本文引用: 1]
DOI:10.1021/jf991001hURLPMID:10956168 [本文引用: 1]

Volatiles play a large role in governing the behavior of boll weevils (Anthonomus grandis Boheman). They are attracted to cotton plants, and the female is sexually attracted to the male. The attracting compounds in both instances are terpenoids. Primarily in the fall of the year, boll weevils seek hibernation sites in leaf trash, where they remain until the following spring or summer. In the present study, essential oils were prepared by steam distillation from several leaf samples known to be prevalent at hibernation sites, and the oils were analyzed by GLC-MS. On the basis of the resulting presumptive identifications by comparison with those of standards, a number of mixtures were formulated and were field tested, as were the essential oils. The field tests failed to support unambiguously the premise that boll weevils select hibernation sites on the basis of leaf odor alone. However, in the presence of the sex pheromone, beta-caryophyllene (P > T = 0.08), or a mixture of three sesquiterpene hydrocarbons (P > T = 0.10), or a mixture of alkyl alcohols (P > T = 0.15) increased captures. The response to formulations of the sex pheromone with beta-caryophyllene may be primarily sexual, based on its presence in female boll weevils.
DOI:10.1016/j.chroma.2004.10.005URL [本文引用: 1]
DOI:10.1021/jf010414rURLPMID:11743779 [本文引用: 1]

Volatile compounds were isolated from strawberry guava fruit by simultaneous steam distillation-solvent extraction according to Likens-Nickerson. Compounds were identified by capillary GC-MS and sensorially characterized by sniffing GC. Two hundred and four compounds were identified in the aroma concentrate, of which ethanol, alpha-pinene, (Z)-3-hexenol, (E)-beta-caryophyllene, and hexadecanoic acid were found to be the major constituents. The presence of many aliphatic esters and terpenic compounds is thought to contribute to the unique flavor of the strawberry guava fruit.
DOI:10.1016/j.biortech.2005.11.003URLPMID:16406607 [本文引用: 1]

Investigations were carried out to evaluate the therapeutic properties of the seeds and leaves of Moringa oleifera Lam as herbal medicines. Ethanol extracts showed anti-fungal activities in vitro against dermatophytes such as Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis. GC-MS analysis of the chemical composition of the essential oil from leaves showed a total of 44 compounds. Isolated extracts could be of use for the future development of anti-skin disease agents.
[本文引用: 1]
[本文引用: 1]
DOI:10.7506/spkx1002-6630-200908037URL [本文引用: 1]

The aroma constituents of Oriental Beauty tea and Tieguanyin tea were determined by HS-SPME/GC-MS. Results showed that there are many differences in aroma constitutes between oriental-beauty tea and Tieguanyin tea. The aromatic compounds of Oriental Beauty tea are much richer than those of Tieguanyin tea, and the contents of alcohol, ketones and phenolic compounds are higher but the contents of ester and hydrocarbon compounds are lower than those of Tieguanyin tea. The major aromatic compounds of Oriental Beauty tea are geraniol, butylated hydroxytoluene, β-linalool, trans-linaloloxide, linalool oxide I, and 3,7-octadiene-2,6-diol-2,6-dimethyl, while the major aromatic compounds of Tieguanyin tea are trans-nerolidol, α-farnesene, indole, butanoic acid, 2-methyl-2-phenylethyl ester, and phenylethyl butyrate.
DOI:10.7506/spkx1002-6630-200908037URL [本文引用: 1]

The aroma constituents of Oriental Beauty tea and Tieguanyin tea were determined by HS-SPME/GC-MS. Results showed that there are many differences in aroma constitutes between oriental-beauty tea and Tieguanyin tea. The aromatic compounds of Oriental Beauty tea are much richer than those of Tieguanyin tea, and the contents of alcohol, ketones and phenolic compounds are higher but the contents of ester and hydrocarbon compounds are lower than those of Tieguanyin tea. The major aromatic compounds of Oriental Beauty tea are geraniol, butylated hydroxytoluene, β-linalool, trans-linaloloxide, linalool oxide I, and 3,7-octadiene-2,6-diol-2,6-dimethyl, while the major aromatic compounds of Tieguanyin tea are trans-nerolidol, α-farnesene, indole, butanoic acid, 2-methyl-2-phenylethyl ester, and phenylethyl butyrate.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1155/S1110724302207020URLPMID:12488578 [本文引用: 1]

The monoterpene d-limonene exhibits chemotherapeutic and chemopreventive potential in breast cancer patients. D-limonene and its related compounds, perillyl alcohol and perillyl aldehyde, were chosen as candidate drugs for application in a screen for nontoxic inhibitors of cell migration. Using the nontumorigenic human breast cell line MCF-10A, we delineated the toxicity as greatest for the perillyl aldehyde, intermediate for perillyl alcohol, and least for limonene. A noncytotoxic concentration of 0.5 mmol/L perillyl alcohol inhibited the migration, while the same concentration of limonene failed to do so. Adhesion of the MCF-10A cell line and the human breast cancer cell line MDA-MB 435 to fibronectin was unaffected by 1.5 mmol/L perillyl alcohol. 0.4 mmol/L perillyl alcohol inhibited the growth of MDA-MB 435 cells. All migration-inhibiting concentrations of perillyl alcohol for MDA-MB 435 cells proved to be toxic. These results suggest that subtoxic doses of perillyl alcohol may have prophylactic potential in the treatment of breast cancer.
DOI:10.19540/j.cnki.cjcmm.20190131.001URLPMID:31342725 [本文引用: 1]

In this study,in order to detect the antimicrobial activity of artemisinin and its derivatives artesunate and dihydroartemisinin,two methods including broth dilution and plate punching method were used to detect the antibacterial activity against gram-negative bacteria(Escherichia coli)and gram-positive bacteria(Staphylococcus aureus)of artemisinin,dihydroartemisinin and artesunate at various concentrations within 5 mmol.L~(-1)and at four time points(8,16,24,32 h).Two antibacterial positive drugs,streptomycin against E.coli and penicillin against S.aureus,were used as positive controls.Plate punching method showed that,unlike the results of 5 mmol.L~(-1)dihydroartemisinin or artesunate,no inhibition zone was detected at the same concentration of artemisinin after 24 h-treatment against E.coli.Broth dilution method showed that,the antibacterial activity of dihydroartemisinin against E.coli.was stronger than those of both artesunate and artemisinin;IC_(50)at24 h-treatment was 155.9mumol.L~(-1)for dihydroartemisinin,370.0mumol.L~(-1)for artesunate and none for artemisinin.Interestingly,dihydroartemisinin and artesunate showed the strongest antibacterial activity between 16-24 h,while artemisinin showed relatively stronger antibacterial activity between 8-16 h.Dihydroartermisinin showed no antibacterial activity against S.aureus.Above all,the antibacterial activity of artemisinins against E.coli is dihydroartemisinin>artesunate>artemisinin.Artemisinin and its derivatives have showed different antibacterial kinetics,and no antibacterial activity against S.aureus.has been detected with dihydroartemisinin.
DOI:10.19540/j.cnki.cjcmm.20190131.001URLPMID:31342725 [本文引用: 1]

In this study,in order to detect the antimicrobial activity of artemisinin and its derivatives artesunate and dihydroartemisinin,two methods including broth dilution and plate punching method were used to detect the antibacterial activity against gram-negative bacteria(Escherichia coli)and gram-positive bacteria(Staphylococcus aureus)of artemisinin,dihydroartemisinin and artesunate at various concentrations within 5 mmol.L~(-1)and at four time points(8,16,24,32 h).Two antibacterial positive drugs,streptomycin against E.coli and penicillin against S.aureus,were used as positive controls.Plate punching method showed that,unlike the results of 5 mmol.L~(-1)dihydroartemisinin or artesunate,no inhibition zone was detected at the same concentration of artemisinin after 24 h-treatment against E.coli.Broth dilution method showed that,the antibacterial activity of dihydroartemisinin against E.coli.was stronger than those of both artesunate and artemisinin;IC_(50)at24 h-treatment was 155.9mumol.L~(-1)for dihydroartemisinin,370.0mumol.L~(-1)for artesunate and none for artemisinin.Interestingly,dihydroartemisinin and artesunate showed the strongest antibacterial activity between 16-24 h,while artemisinin showed relatively stronger antibacterial activity between 8-16 h.Dihydroartermisinin showed no antibacterial activity against S.aureus.Above all,the antibacterial activity of artemisinins against E.coli is dihydroartemisinin>artesunate>artemisinin.Artemisinin and its derivatives have showed different antibacterial kinetics,and no antibacterial activity against S.aureus.has been detected with dihydroartemisinin.
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
