 ,1, 王学敏2, 马琳2, 崔苗苗2, 曹晓宇2, 赵威
,1, 王学敏2, 马琳2, 崔苗苗2, 曹晓宇2, 赵威 ,1
,1Isolation, Identification, and Response to Abiotic Stress of MsWRKY42 Gene from Medicago sativa L.
LIU JiaoJiao ,1, WANG XueMin2, MA Lin2, CUI MiaoMiao2, CAO XiaoYu2, ZHAO Wei
,1, WANG XueMin2, MA Lin2, CUI MiaoMiao2, CAO XiaoYu2, ZHAO Wei ,1
,1通讯作者:
责任编辑: 李莉
收稿日期:2019-10-30接受日期:2020-02-16网络出版日期:2020-09-01
| 基金资助: |
Received:2019-10-30Accepted:2020-02-16Online:2020-09-01
作者简介 About authors
刘佼佼,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3693KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
刘佼佼, 王学敏, 马琳, 崔苗苗, 曹晓宇, 赵威. 紫花苜蓿MsWRKY42的分离、鉴定及其对非生物胁迫的响应[J]. 中国农业科学, 2020, 53(17): 3455-3466 doi:10.3864/j.issn.0578-1752.2020.17.004
LIU JiaoJiao, WANG XueMin, MA Lin, CUI MiaoMiao, CAO XiaoYu, ZHAO Wei.
0 引言
【研究意义】紫花苜蓿(Medicago sativa L.)是多年生豆科牧草,其营养价值高、分布范围广,是中国北方干旱和半干旱地区促进农业生产和经济发展的关键草种之一[1]。随着生态环境的不断恶化,紫花苜蓿的产量和品质都面临严峻考验,苜蓿产业化发展受到遏制。发掘紫花苜蓿重要抗逆基因资源,了解其抗逆调控机制,对于指导苜蓿抗逆遗传育种和生产实际具有重要意义。【前人研究进展】植物在生长发育过程中不断适应环境变化,已经形成了一套复杂高效的信号传导网络,转录因子在其中起核心调控作用[2]。WRKY作为植物中十大转录因子家族之一,因其N端含有保守的WRKYGQK结构域而得名,其C末端包含独特的锌指基序——C2H2(C-X4-5-C-X22-23-H- X-H)或C2HC(C-X5-8-C-X25-28-H-X1-2-C)。一般情况下,根据N端结构域的数量和C端锌指基序的特征将WRKY家族分为三类:第一类(Ⅰ)有2个WRKY结构域和C2H2型锌指结构域;第二类(Ⅱ)有1个WRKY结构域和C2H2型锌指结构域,在此基础上根据其结构进一步分为5个亚类(Ⅱa、Ⅱb、Ⅱc、Ⅱd和Ⅱe);第三类(Ⅲ)有1个WRKY结构域和C2HC型锌指结构域。WRKY蛋白能够与靶基因启动子中的顺式作用元件W-box(TTGACC/T)发生特异性结合,从而调控目的基因的表达,进而广泛参与植物生长发育代谢以及各种生物、非生物胁迫(干旱胁迫、高盐胁迫、温度胁迫和营养胁迫)响应过程,被称为非生物胁迫响应的“中心调控因子”[3,4,5]。拟南芥(Arabidopsis thaliana)中有众多WRKY转录因子都和植物耐旱或耐盐性相关[6]。其中,AtWRKY1通过调节膜转运蛋白参与气孔关闭以保持水分[7];AtWRKY57直接与逆境诱导型基因RD29A和NCED3启动子中的W-box结合,提高拟南芥的耐旱和耐盐性[8]。玉米(Zea mays L.)中的ZmWRKY33可在干旱和高盐条件下被诱导,并且过表达ZmWRKY33可以激活RD29A等多个基因,从而增强植物对干旱和高盐胁迫的耐受性[9]。YANG等[10]研究表明核桃(Juglans regia)JrWRKY6和JrWRKY53通过脱落酸(abscisic acid,ABA)信号传导途径提高植物对盐、渗透等的耐受性。温度环境是影响植物生长发育的另一重要因素。ZOU等[11]研究发现,低温敏感性植物的成熟花粉对冷胁迫刺激的敏感度非常高,花粉中特异性表达的AtWRKY34能负调控低温应答途径。油菜(Brassica campestris)中的BcWRKY46能激活ABA信号途径中的相关基因提高植物的耐低温能力[12]。在热激蛋白HSP101启动子的控制下,过表达OsWRKY11增强了转基因水稻(Oryza sativa L.)幼苗的耐热性[13]。拟南芥的AtWRKY75是首个被报道的参与调节低磷胁迫的WRKY家族成员。在AtWRKY75沉默株系中,磷饥饿相关基因(如磷酸酶、Mt4/TPS1和高亲和力磷转运蛋白)的表达降低[14]。另有研究发现AtWRKY42和AtWRKY6可与AtPHO1(AtPHOSPHATE1)结合,通过抑制AtPHO1的表达能够负调控磷从地下部分向地上部分的转运[15];同时,磷的缺乏诱导26S蛋白酶体降解AtWRKY6和AtWRKY42转录因子[16]。低磷条件下,与野生型相比,过表达OsWRKY74的水稻植株低磷耐受性增强,同时OsWRKY74能够调节磷稳态与磷饥饿之间的潜在串扰[17]。【本研究切入点】目前,关于WRKY家族的研究多集中在一些模式植物中,在牧草中鲜有报道。紫花苜蓿作为牧草中的典型代表,WRKY蛋白在紫花苜蓿中的分子机理尚不明确。【拟解决的关键问题】本研究从紫花苜蓿中成功克隆WRKY基因MsWRKY42,对其进行生物信息学分析,并研究在不同组织中和非生物逆境胁迫下MsWRKY42的表达模式,同时进行亚细胞定位分析、构建超表达植物表达载体,为深入研究MsWRKY42的功能和抗逆调控机制奠定基础,同时为紫花苜蓿抗逆育种筛选优良的基因资源。1 材料与方法
1.1 试验材料
试验材料为紫花苜蓿中苜一号(Medicago sativa L.,Zhongmu No.1)品种,由中国农业科学院北京畜牧兽医研究所保存。PROMEGA总RNA提取试剂盒,购自普洛麦格公司,TransScript Green One-Step qRT-PCR SuperMix、pEASY-Blunt Simple Cloning Kit、DH5α Chemically Competent Cell、EasyPure Quick Gel Extraction Kit、DNA凝胶试剂盒,购自北京全式金生物技术有限公司。试验所用的内切酶、连接酶均购自NEB(北京)有限公司。1.2 紫花苜蓿MsWRKY42的克隆
使用Eastep? Super Total RNA Extraction Kit试剂盒(Promega)提取紫花苜蓿叶片总RNA,用紫外分光光度计测定RNA的纯度和浓度。利用TransScript Green One-Step qRT-PCR反转录体系合成cDNA。根据已公开的拟南芥AtWRKY42的cDNA序列,在紫花苜蓿二倍体水平CADL比对数据库(
Table 1
表1
表1PlantCARE 启动子预测结果
Table 1
| 顺式作用元件 Cis-elements | 序列 Sequence (5′-3′) | 功能 Function | 数量 No. |
|---|---|---|---|
| CGTCA-motif | CGTCA | 参与MeJA反应的顺式作用调节元件 Cis-acting regulatory element involved in the MeJA-responsiveness | 2 |
| GARE-motif | TCTGTTG | 赤霉素反应元件 Gibberellin-responsive element | 1 |
| I-box | GTATAAGGCC | 部分光响应元件 Part of a light responsive element | 1 |
| Box 4 | ATTAAT | 参与光响应的保守 DNA 模块的部分元件 Part of a conserved DNA module involved in light responsiveness | 5 |
| HD-Zip 3 | GTAAT(G/C)ATTAC | 蛋白质结合位点 Protein binding site | 1 |
| circadian | CAAAGATATC | 参与昼夜节律顺行调控元件 Cis-acting regulatory element involved in circadian control | 1 |
| TCCC-motif | TCTCCCT | 部分光响应元件 Part of a light responsive element | 1 |
| TGACG-motif | TGACG | 参与MeJA反应的顺式作用调节元件 Cis-acting regulatory element involved in the MeJA-responsiveness | 2 |
| 3-AF3 binding site | CACTATCTAAC | 保守DNA模块阵列的一部分(CMA3) Part of a conserved DNA module array (CMA3) | 1 |
| TATC-box | TATCCCA | 参与赤霉素反应的顺式作用元件 Cis-acting element involved in gibberellin-responsiveness | 1 |
| Sp1 | GGGCGG | 光响应元件 Light responsive element | 1 |
| TC-rich repeats | ATTCTCTAAC | 参与防御和胁迫反应的顺式作用元件 Cis-acting element involved in defense and stress responsiveness | 1 |
| GCN4_motif | TGAGTCA | 参与胚乳表达的顺式调控元件 Cis-regulatory element involved in endosperm expression | 1 |
| CAAT-box | CAAAT | 启动子和增强子区域中常见的顺式作用元件 Common cis-acting element in promoter and enhancer regions | 16 |
| G-Box | CACGTG | 参与光响应顺式作用元件 Cis-acting regulatory element involved in light responsiveness | 5 |
| GATA-motif | GATAGGG | 部分光响应元件 Part of a light responsive element | 1 |
| O2-site | GATGA(C/T)(A/G)TG(A/G) | 参与玉米醇溶蛋白代谢调节的顺式作用调节元件 Cis-acting regulatory element involved in zein metabolism regulation | 1 |
| GT1-motif | GGTTAAT | 光响应元件 Light responsive element | 2 |
| TATA-box | TATA | 在转录起始的-30 附近的核心启动子元件 Core promoter element around -30 of transcription start | 41 |
| ARE | AAACCA | 厌氧诱导必需元件 Cis-acting regulatory element essential for the anaerobic induction | 2 |
| GC-motif | CCCCCG | 参与缺氧特异性诱导的类似增强子元件 Enhancer-like element involved in anoxic specific inducibility | 1 |
| TCA-element | CCATCTTTTT | 参与水杨酸响应的顺式作用元件 Cis-acting element involved in salicylic acid responsiveness | 1 |
| TCT-motif | TCTTAC | 部分光响应元件 Part of a light responsive element | 2 |
| ATCT-motif | AATCTAATCC | 部分参与光响应的保守DNA区域 Part of a conserved DNA module involved in light responsiveness | 2 |
| ABRE | CACGTG | 参与脱落酸反应的顺式作用元件 Cis-acting element involved in the abscisic acid responsiveness | 5 |
新窗口打开|下载CSV
1.3 MsWRKY42的序列分析
利用SnapGene软件分析MsWRKY42开放阅读框及其氨基酸序列;通过Protparam(https://web.expasy. org/protparam/)分析MsWRKY42的理化性质;利用在线程序PSORT II Prediction(https://psort.hgc.jp/form2. html)对MsWRKY42蛋白进行亚细胞定位预测;利用SOPMA(http://www.sopma.org/)在线预测该蛋白的二级结构;利用在线软件SWISS-model(https:// swissmodel.expasy.org)预测该蛋白的三级结构。使用ClustalX软件与其他物种的同源基因进行多序列比对;将MsWRKY42与拟南芥WRKY基因家族多序列比对后,用MEGA-X软件选择最大似然法(ML)法构建系统发育树,Bootstrap值设定为500,通过Bootstrap对生成的系统进化树进行校正;利用PlantCARE(http://www.plantcarescience.com/)预测分析MsWRKY42启动子。
1.4 MsWRKY42组织特异性表达分析
分别提取中苜1号根、茎、叶、芽、花和荚果的总RNA,反转录成cDNA后,进行实时荧光定量PCR。根据得到的MsWRKY42序列设计特异引物Q42F/R(电子附表1),以紫花苜蓿Actin为内参基因,利用ABI7500 Real-Time PCR system(美国ABI公司),依照TaKaRa SYBY Premix Ex Taq说明书进行PCR扩增。3次生物学重复,2次技术重复。采用2-ΔΔCT方法[18]计算MsWRKY42的表达量。1.5 不同处理下MsWRKY42的表达分析
中苜一号种子经氯气消毒,平铺在有滤纸的培养皿中,用无菌水将滤纸浸湿,在光照培养箱(16 h光照/8 h黑暗)中培养至种子露白,发芽后的种子移至1/2 MS液体培养基中,培养4周后,分别移入0.3 mol·L-1NaCl、15% PEG、4℃、40℃、缺磷和0.1×10-3 mol·L-1ABA处理的环境中,分别在0、2、4、6、8、12、24和48 h取样,每个处理3个生物学重复。每个样品取样完成,液氮速冻,-80℃保存。1.6 MsWRKY42的亚细胞定位
设计亚细胞定位引物GFP-MsWRKY42F/R(电子附表1),在目的基因ORF两端(去掉终止密码子)加入酶切位点BamHⅠ和SacⅠ,以测序成功的阳性质粒T-blunt-MsWRKY42为模板进行扩增、回收。用BamHⅠ和SacⅠ双酶切质粒pCAMBIA1300-GFP,产物纯化。用连接酶将目的基因片段连接载体pCAMBIA1300-GFP,并转化大肠杆菌DH5α感受态细胞,经菌液PCR及测序验证,获得重组质粒pCAMBIA1300-MsWRKY42-GFP。将重组质粒和对照空载质粒pCAMBIA1300-GFP分别转入农杆菌GV3101菌株中,把菌液PCR检测为阳性的单克隆菌落于含有35 μg·mL-1利福平和50 μg·mL-1卡那霉素的LB液体培养基中培养,在28℃摇床以250 r/min培养14 h;收集、重悬菌体,调整菌体浓度使其OD600在0.5—1.0,静置3 h后选择5—7片长势一致的本氏烟草叶片(带有RFP标签的核定位蛋白的烟草),用去掉针头的注射器注射含有目的基因载体和空载体的菌液。注射后的烟草置于28℃温室中培养2 d,光照周期为16 h/8 h(光照/黑暗)。用LEICA TCS SP8共聚焦显微镜观察侵染的叶片,确定蛋白的亚细胞定位。1.7 MsWRKY42结合活性分析
根据W-box的序列(C/T)TGAC(C/T)设计能合成3个串联重复序列的W-box引物,在设计引物的同时加上EcoRⅠ/SacⅠ酶切位点(电子附表1),连接酵母载体pHISi,构建重组质粒pHISi-W- box。测序验证正确后将重组质粒转入酵母YM4271,挑取阳性克隆,摇菌后制作感受态细胞,同时菌液均匀涂于加有不同浓度 3-AT的SD/-His单缺陷平板上,观察转化子的生长情况,筛选出3-AT浓度以消除pHISi-W-box引起的HIS的本底表达。根据MsWRKY42的ORF序列(去掉终止密码子)设计带有BamHⅠ和SacⅠ双酶切位点的上下游引物PMsWRKY42F/R(电子附表1)。以含有MsWRKY42 CDS片段的T载体质粒为模板,扩增含有酶切位点的目的片段并回收,连接酵母表达载体pGADT7,构建重组质粒pGADT7- MsWRKY42,测序验证正确后将pGADT7-MsWRKY42、pHISi-W-box质粒转入酵母YM4271菌株中,挑取单克隆,摇菌后稀释成不同浓度,点涂在3-AT固体缺陷培养基SD/-His-Leu-ura上,同时分别将pHISi+pGADT7-42、pGADT7+pHISi、pGADT7+pHISi-W-box作为对照,观察转化子生长情况并拍照记录。2 结果
2.1 MsWRKY42的克隆
以紫花苜蓿总RNA反转录获得的cDNA为模板,MsWRKY42F/R为引物进行PCR扩增,获得反应产物。该产物的琼脂糖凝胶电泳结果显示,条带明亮单一,大小与基因组测序结果一致(图1),初步判定获得目的条带。图1
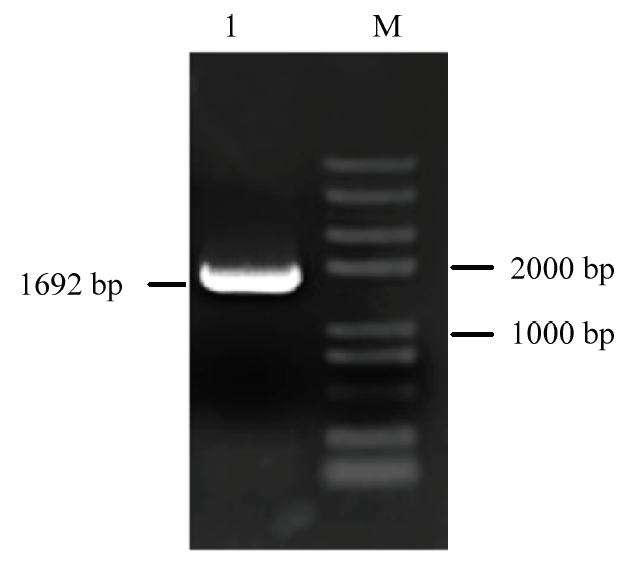 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1紫花苜蓿MsWRKY42的PCR扩增
M:Trans2K Plus II DNA分子标记;1:PCR扩增产物
Fig. 1PCR amplified product of MsWRKY42
M: Trans2K Plus II Marker; 1: PCR amplified product
将PCR产物纯化回收后,连接至Peasy-T1-Blunt载体上,再转化至DH5α大肠杆菌感受态中,挑取单菌落检验。菌液PCR结果显示,所选菌液能够扩增出与目的片段大小一致的DNA片段。将上述菌液送至生物公司测序,结果显示与参考序列一致,表明MsWRKY42克隆成功。
2.2 MsWRKY42的序列分析
利用SnapGene软件对序列进行分析,该基因片段包含一个1 692 bp的开放阅读框,共编码563个氨基酸。该序列编码的蛋白理论等电点为7.70,相对分子量60.74 kD,不稳定系数为42.49,属于不稳定蛋白,该蛋白平均亲水系数为-0.668,为亲水性蛋白。利用在线程序PSORT II Prediction对MsWRKY42蛋白进行亚细胞定位预测,结果显示该蛋白位于细胞核上的可能性最高,为56.15%。利用DNAMAN软件将MsWRKY42与其他物种氨基酸序列进行同源比对,发现该蛋白序列含有WRKY家族典型的WRKYGQK结构域,同时含有1个C2H2 锌指基序(图2)。氨基酸序列与豆科植物的相似度很高,其中,与蒺藜苜蓿的相似度为96.27%。MsWRKY42与拟南芥WRKY基因家族多序列比对后构建系统进化树(图3),参考拟南芥分组方法,MsWRKY42转录因子属于Ⅱb组。
图2
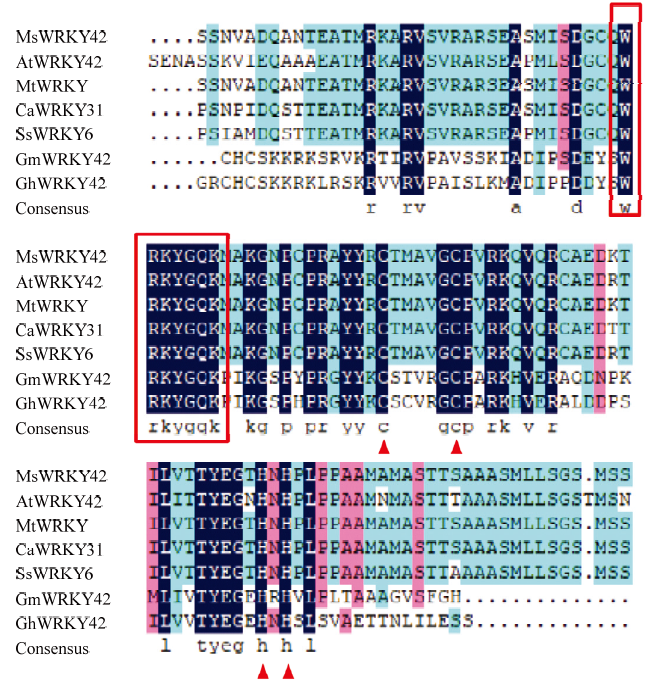 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2MsWRKY42与其他物种同源蛋白氨基酸序列比对
MsWRKY42;紫花苜蓿Medicago sativa;AtWRKY42:拟南芥Arabidopsis thaliana,NP_001328851.1;MtWRKY:蒺藜苜蓿Medicago truntula,XP_013462871.1;CaWRKY31:鹰嘴豆Cicer arietinum,XP_004486177.1;SsWRKY6:密花豆Spatholobus suberectus,TKY72179.1;GmWRKY42:大豆Glycine max,AJB84600.1;GhWRKY42:陆地棉Gossypium hirsutum,AIE43854.1。下同The same as below。方框表示WRKY结构域。三角表示C2H2基序The WRKY domain are represented in the boxes. The C2H2 motif are represented on the triangle
Fig. 2Amino acid sequence alignment of MsWRKY42 with other homologous proteins
图3
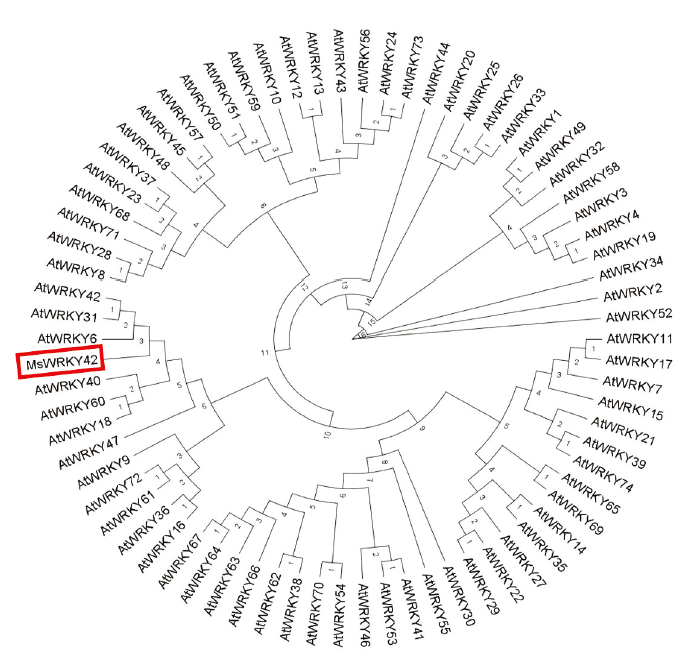 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3基于拟南芥和MsWRKY42构建的WRKY系统发育树
Fig. 3A phylogenetic tree constructed based on WRKYs of Arabidopsis thaliana and MsWRKY42
对MsWRKY42蛋白的二级结构预测结果显示(图4),该蛋白的主要二级结构元件中,无规则卷曲所占比例最高,为63.94%。α-螺旋和β-转角所占比例依次为22.91%和10.83%,没有β-折叠。通过在线程序SWISS-model对蛋白三维结构进行同源模拟预测发现(图5),MsWRKY42蛋白与PDB数据库(
图4
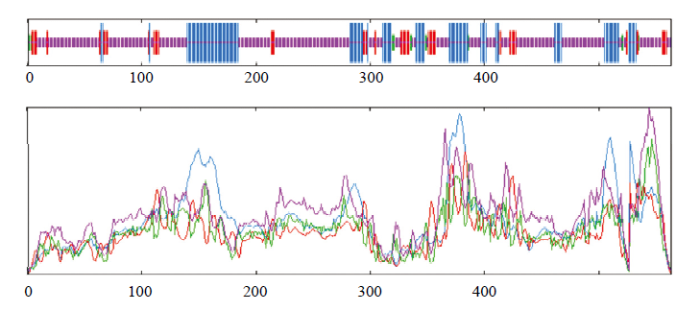 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4MsWRKY42蛋白二级结构的预测
红色线条表示延伸链;紫色线条表示无规则卷曲;蓝色线条表示α-螺旋
Fig. 4Prediction of second structure of MsWRKY42
The red line represents the extension chain; The purple line represents the random coil; The blue line represents the alphahelix
图5
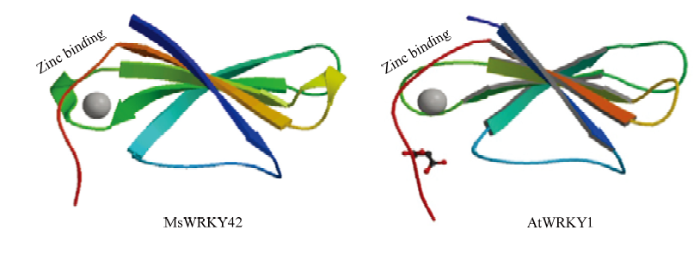 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5MsWRKY42蛋白三级结构的预测
Fig. 5Prediction of the tertiary structure of MsWRKY42
2.3 MsWRKY42启动子分析
通过PlantCARE启动子预测软件分析MsWRKY42启动子(表1)。结果显示,该基因启动子包含许多顺式作用元件,其中有参与逆境胁迫、参与ABA以及MeJA反应的元件,也存在光响应、昼夜节律响应等元件。暗示MsWRKY42可能受干旱、高温等与水分胁迫相关的逆境诱导。2.4 MsWRKY42蛋白的定位
通过进行亚细胞定位(图6),在注射空载体pCAMBIA1300-GFP的对照组中,绿色荧光在细胞膜、细胞核、细胞质中均有分布,而注射融合表达载体pCAMBIA1300-MsWRKY42-GFP的细胞中,仅在细胞核中发现有绿色荧光,在细胞膜和细胞质中均无荧光,且绿色荧光与核定位的marker蛋白的红色荧光完全重合。说明MsWRKY42蛋白定位且只定位在细胞核上,这一结果与亚细胞定位预测结果一致。图6
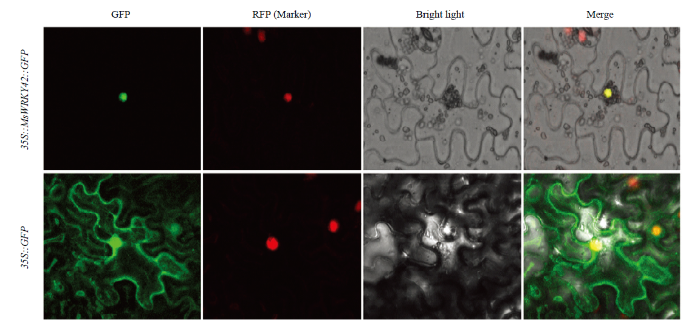 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6MsWRKY42在本氏烟草下表皮细胞中的亚细胞定位
图片采用绿色荧光、明场、绿色荧光和明场叠加3个视野拍摄。35S::GFP:携带空载pCAMBIA1300-GFP的农杆菌菌株。35S::MsWRKY42::GFP:携带重组载体pCAMBIA1300-MsWRKY42-GFP的农杆菌菌株。比例尺=25 μm
Fig. 6Subcellular localization of MsWRKY42 in lower epidermal cells of Nicotiana benthamiana
The images are taken by green fluorescence, visible light, merged green fluorescence and visible light. 35S::GFP: The Agrobacterium tumefaciens strain carrying the empty vector pCAMBIA1300-GFP. 35S::MsWRKY42::GFP: The A. tumefaciens strain carrying the recombinant vector pCAMBIA1300- MsWRKY42-GFP. Scale bar = 25 μm
2.5 MsWRKY42组织特异性表达分析
采用qRT-PCR技术分析MsWRKY42在紫花苜蓿各个组织中的相对表达量。结果(图7)表明,MsWRKY42在紫花苜蓿在各个组织中均有不同程度表达。其中在根、叶中的表达丰度较高,茎、花、荚果中次之,在芽中表达丰度最低。图7
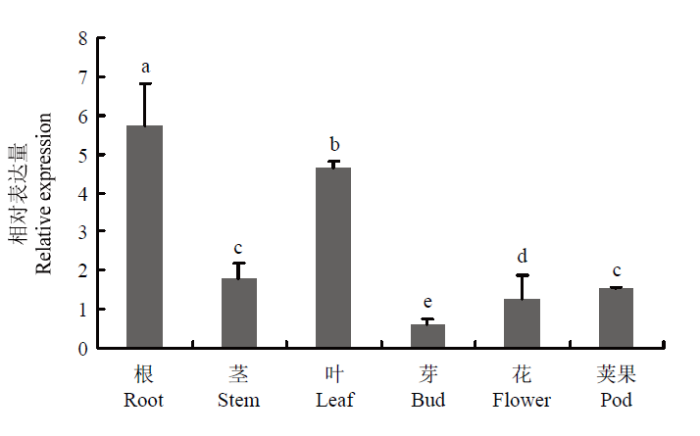 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7紫花苜蓿不同组织中MsWRKY42的表达模式
不同字母表示在P<0.05水平差异显著。下同
Fig. 7Expression pattern of MsWRKY42 in different tissues of alfalfa
Different letters indicate statistical difference (P<0.05). The same as below
2.6 MsWRKY42受多种逆境胁迫诱导
通过对材料进行非生物胁迫研究(图8),在300 mmol·L-1NaCl处理下,MsWRKY42在2 h和6 h出现峰值,在6 h之后逐渐降低,但处理48 h后表达量仍显著高于对照(0 h);用15%的PEG模拟干旱胁迫, MsWRKY42的表达量在2 h内迅速上调,随后下降,在12和48 h时表达量与对照差异不显著;4℃低温处理下,MsWRKY42表达量在2 h时被快速诱导,在4 h急剧下降,随后又逐渐上升;40℃高温处理下,MsWRKY42表达量整体趋势是升高的,且到处理的48 h时仍保持上升趋势;缺磷处理下,MsWRKY42在4 h时的表达量最高,为对照表达量的6.2倍,随后表达逐渐降低;0.1×10-3 mol·L-1ABA处理下,MsWRKY42表达量在2 h时剧增,随后表达丰度下降,整个处理过程中表达量始终显著高于对照。图8
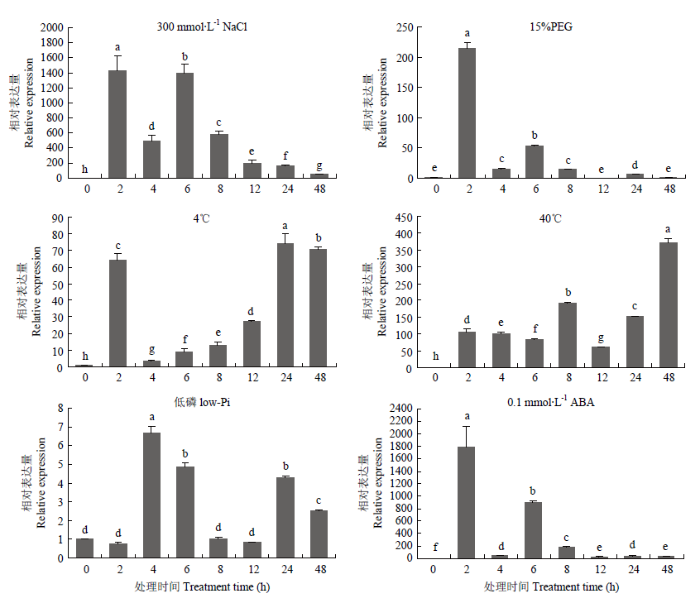 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8不同胁迫下MsWRKY42的表达模式
Fig. 8The expression of MsWRKY42 gene under different stress
2.7 MsWRKY42蛋白结合活性分析
为了明确MsWRKY42蛋白与W-box的结合活性,对其进行了体外结合活性分析。结果显示,抑制酵母报告载体pHISi和pHISi-W-box本底表达的3-AT浓度为150 mmol·L-1(图9-A)。将不同的酵母转化子点涂在含有150 mmol·L-1 3-AT的培养基上,结果显示,只有pGADT7-42+pHISi-W-box的酵母转化子能够生长,其他对照均没有生长(图9-B)。说明报告基因HIS在MsWRKY42的酵母中表达,证明MsWRKY42能够与W-box顺式作用元件特异性结合。图9
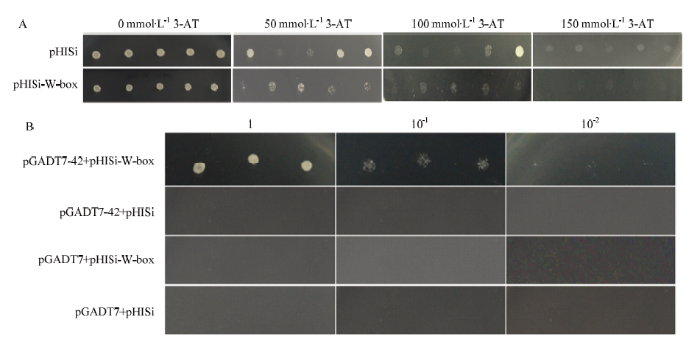 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9MsWRKY42蛋白的结合活性分析
A:3-AT浓度的筛选;B:稀释不同倍数的4种酵母转化子在SD/-His-Leu-ura培养基上的生长状态
Fig. 9Binding activity analysis of MsWRKY42 protein
A: 3-AT concentration screening; B: Growth state of four yeast transformants diluted in different times on SD/-His-Leu-ura medium
3 讨论
3.1 MsWRKY42蛋白结构与功能的关系
本研究从紫花苜蓿中克隆得到MsWRKY42的cDNA序列,该基因编码的蛋白可能为不稳定蛋白。系统进化树聚类结果表明MsWRKY42属于WRKY家族Ⅱb亚类(图3),该分支多个基因已证实与植物的非生物胁迫应答相关,例如AtWRKY61与AtWRKY9、AtWRKY72互作关联,对干旱、冷、ABA等多种非生物逆境应答相关[19];CmWRKY1能够通过ABA转导途径参与菊花(Chrysanthemum morifolium)对干旱胁迫的响应过程[20]。因此,推测MsWRKY42也可能参与了紫花苜蓿的非生物胁迫响应。本研究对MsWRKY42蛋白进行三级结构预测发现,该蛋白是由5条反平行β链和锌结合位点组成的球状结构,与AtWRKY1蛋白结构相似(图5)。DUAN等[21]研究发现转录因子AtWRKY1与DNA的结合能力是由β2链与β3链之间的β折叠区域决定的,推测MsWRKY42具有与AtWRKY1相似的与DNA的结合方式。亚细胞定位结果显示MsWRKY42蛋白定位在细胞核上,这与生物信息学预测结果相同,同时与拟南芥AtWRKY42[16]报道相同,也与其作为转录因子的转录调节功能一致。W-box大多存在于植物WRKY基因的启动子中,尤其是与胁迫相关的WRKY基因,其中,W-box的数量、排列方式等对结合活性都有很大影响。WRKY家族与顺式作用元件W-box的结合已有许多研究,AtWRKY57[8]、AtWRKY6[22]等转录因子均与W-box特异性结合,从而调控相关基因的表达,本研究中MsWRKY42与W-box能够特异性结合,这些结果不仅表明W-box在WRKY转录因子发挥其生物学功能中起重要且广泛的作用,而且,推测MsWRKY42与拟南芥中的AtWRKY57、AtWRKY6存在相似的调控模式。
3.2 非生物胁迫对MsWRKY42的影响
通过PlantCARE在线网站对MsWRKY42启动子内顺式作用元件进行预测,包括2个胁迫响应元件ARE、5个脱落酸(ABA)响应元件ABRE、2个茉莉酸甲酯响应元件TGACG-motif和2个CGTCA-motif,因此,推测MsWRKY42可能通过ABA信号途径参与多种胁迫反应。基于此,研究对多种逆境下基因的表达进行了分析。PEG是一种高分子渗透剂,能使植物细胞和组织处于类似于干旱胁迫的状态中[23]。WRKY 家族基因可通过ABA信号转导途径行使功能[24]。拟南芥中AtWRKY18、AtWRKY40和AtWRKY60三者能够协同互作,与ABA信号途径基因ABI4和ABI5启动子中的W-box结合,单独或协同调控这些基因的表达,进而影响拟南芥的耐旱和耐盐性[25]。本研究中,在干旱、ABA诱导下MsWRKY42的表达量在2 h处均显著上调,推测MsWRKY42可能通过ABA信号通路参与干旱胁迫反应。GRUBER等[26]研究显示,盐胁迫下WRKY转录因子家族被显著诱导,说明其广泛参与到植物的耐盐机制中。本研究中,盐胁迫下MsWRKY42的表达量显著增高,与GRUBER等[26]研究结果一致。拟南芥中的AtWRKY25、AtWRKY26和AtWRKY33能与一些热激转录因子的W-box结合从而参与调控高温胁迫反应[27]。本研究结果显示,在高温条件下,MsWRKY42的表达量在2 h升高后没有太大的变化,但在48 h时有一个显著的升高。资料表明,WRKY能够通过不同的信号转导途径参与植物的低温胁迫应答。例如花粉中特异性表达的AtWRKY34能负调控CBF介导的低温应答途径[11]。在低温下被诱导表达的OsWRKY71,通过激活下游基因OsTGFR、OsDREB1A、TPP1和WSI76的表达增强植物的耐寒性[28]。本研究中低温也显著诱导MsWRKY42的增强表达。以上的结果表明,MsWRKY42可能参与了多个逆境响应过程。
土壤有效磷的供应情况以及植物对磷的吸收能力是植物生长发育的关键制约因素[29]。以往的研究发现AtWRKY45可以与磷转运蛋白PHT1;1启动子区域中的两个W-box结合,上调PHT1;1表达,从而参与对低磷胁迫的反应[30]。本研究对MsWRKY42启动子区的序列分析发现,该启动子中含有W-box,可能其他的WRKY转录因子也能够调控WRKY42的表达,暗示WRKY42在抗逆调控中不是简单的线性关系,而是处于复杂但相互平衡的调节网络中。本研究中在低磷条件下MsWRKY42迅速被诱导,随后下降,在24 h开始上升,可能是因为WRKY蛋白和自身启动子中的W-box顺式作用元件相结合从而调控自身表达,也可能在此过程中存在其他转录因子的竞争,抑制WRKY对自身的调控。
从本研究的结果发现,紫花苜蓿的WRKY42对多种逆境胁迫均有响应,且基因诱导表达的丰度较高,暗示该基因可能参与了多种非生物逆境,包括磷营养胁迫的调控。前人对其他物种WRKY42的研究中发现,AtWRKY42通过调节PHO1和PHT1;1的表达调控低磷逆境[16];FvWRKY42通过ABA信号途径来提高野生草莓(Fragaria vesca)对盐和干旱的抗性[31]。但像MsWRKY42这样,在同一物种中参与多个逆境响应的还未见报道。虽然非生物胁迫下紫花苜蓿中MsWRKY42的生物学功能以及其调控机制还需要进一步验证,但本研究结果加深了对WRKY42参与植物逆境应答调控的认识,为紫花苜蓿WRKY转录因子调控非生物胁迫分子机制研究提供一定的依据。
4 结论
从紫花苜蓿中克隆获得一个WRKY转录因子基因,命名为MsWRKY42,该序列ORF为1 692 bp,编码563个氨基酸,为不稳定蛋白,该蛋白具有1个WRKY保守结构域和C2H2锌指结构域,属于WRKY转录因子家族的Ⅱb亚类,位于细胞核,可以与W-box特异性结合;MsWRKY42在紫花苜蓿具有组织特异性,推测其在紫花苜蓿适应多种非生物胁迫过程中起重要作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.11686/cyxb20120638URL [本文引用: 1]
DOI:10.11686/cyxb20120638URL [本文引用: 1]
DOI:10.1093/jxb/ert422URLPMID:24347464 [本文引用: 1]

Plant cells utilize mobile transcription factors to transmit intercellular signals when they perceive environmental stimuli or initiate developmental programmes. Studies on these novel cell-to-cell signals have accumulated multiple pieces of evidence showing that non-cell-autonomous transcription factors play pivotal roles in most processes related to the formation and development of plant organs. Recent studies have explored the evolution of mobile transcription factors and proposed mechanisms for their trafficking through plasmodesmata, where a selective system exists to facilitate this process. Mobile transcription factors contribute to the diversity of the intercellular signalling network, which is also established by peptides, hormones, and RNAs. Crosstalk between mobile transcription factors and other intercellular molecules leads to the development of complex biological signalling networks in plants. The regulation of plasmodesmata appears to have been another major step in controlling the intercellular trafficking of transcription factors based on studies of many plasmodesmal components. Furthermore, diverse omics approaches are being successfully applied to explore a large number of candidate transcription factors as mobile signals in plants. Here, we review these fascinating discoveries to integrate current knowledge of non-cell-autonomous transcription factors.
DOI:10.1016/j.pbi.2017.04.004URLPMID:28458046 [本文引用: 1]

Rapid and massive transcriptional reprogramming upon pathogen recognition is the decisive step in plant-phytopathogen interactions. Plant transcription factors (TFs) are key players in this process but they require a suite of other context-specific co-regulators to establish sensory transcription regulatory networks to bring about host immunity. Molecular, genetic and biochemical studies, particularly in the model plants Arabidopsis and rice, are continuously uncovering new components of the transcriptional machinery that can selectively impact host resistance toward a diverse range of pathogens. Moreover, detailed studies on key immune regulators, such as WRKY TFs and NPR1, are beginning to reveal the underlying mechanisms by which defense hormones influence the function of these factors. Here we provide a short update on such recent developments.
DOI:10.1111/jipb.12513URLPMID:27995748 [本文引用: 1]

The WRKY gene family is among the largest families of transcription factors (TFs) in higher plants. By regulating the plant hormone signal transduction pathway, these TFs play critical roles in some plant processes in response to biotic and abiotic stress. Various bodies of research have demonstrated the important biological functions of WRKY TFs in plant response to different kinds of biotic and abiotic stresses and working mechanisms. However, very little summarization has been done to review their research progress. Not just important TFs function in plant response to biotic and abiotic stresses, WRKY also participates in carbohydrate synthesis, senescence, development, and secondary metabolites synthesis. WRKY proteins can bind to W-box (TGACC (A/T)) in the promoter of its target genes and activate or repress the expression of downstream genes to regulate their stress response. Moreover, WRKY proteins can interact with other TFs to regulate plant defensive responses. In the present review, we focus on the structural characteristics of WRKY TFs and the research progress on their functions in plant responses to a variety of stresses.
DOI:10.3389/fpls.2016.00760URLPMID:27375634 [本文引用: 1]

Plants in their natural habitat have to face multiple stresses simultaneously. Evolutionary adaptation of developmental, physiological, and biochemical parameters give advantage over a single window of stress but not multiple. On the other hand transcription factors like WRKY can regulate diverse responses through a complicated network of genes. So molecular orchestration of WRKYs in plant may provide the most anticipated outcome of simultaneous multiple responses. Activation or repression through W-box and W-box like sequences is regulated at transcriptional, translational, and domain level. Because of the tight regulation involved in specific recognition and binding of WRKYs to downstream promoters, they have become promising candidate for crop improvement. Epigenetic, retrograde and proteasome mediated regulation enable WRKYs to attain the dynamic cellular homeostatic reprograming. Overexpression of several WRKYs face the paradox of having several beneficial affects but with some unwanted traits. These overexpression-associated undesirable phenotypes need to be identified and removed for proper growth, development and yeild. Taken together, we have highlighted the diverse regulation and multiple stress response of WRKYs in plants along with the future prospects in this field of research.
DOI:10.1111/j.1744-7909.2011.01081.xURLPMID:22040287 [本文引用: 1]

It is well-documented that phytochromes can control plant growth and development from germination to flowering. Additionally, these photoreceptors have been shown to modulate both biotic and abiotic stress. This has led to a series of studies exploring the molecular and biochemical basis by which phytochromes modulate stresses, such as salinity, drought, high light or herbivory. Evidence for a role of phytrochromes in plant stress tolerance is explored and reviewed.
URLPMID:26820136 [本文引用: 1]
DOI:10.1093/mp/sss080URLPMID:22930734 [本文引用: 2]

Drought is one of the most serious environmental factors that limit the productivity of agricultural crops worldwide. However, the mechanism underlying drought tolerance in plants is unclear. WRKY transcription factors are known to function in adaptation to abiotic stresses. By screening a pool of WRKY-associated T-DNA insertion mutants, we isolated a gain-of-function mutant, acquired drought tolerance (adt), showing improved drought tolerance. Under drought stress conditions, adt accumulated higher levels of ABA than wild-type plants. Stomatal aperture analysis indicated that adt was more sensitive to ABA than wild-type plants. Molecular genetic analysis revealed that a T-DNA insertion in adt led to activated expression of a WRKY gene that encodes the WRKR57 protein. Constitutive expression of WRKY57 also conferred similar drought tolerance. Consistently with the high ABA content and enhanced drought tolerance, three stress-responsive genes (RD29A, NCED3, and ABA3) were up-regulated in adt. ChIP assays demonstrated that WRKY57 can directly bind the W-box of RD29A and NCED3 promoter sequences. In addition, during ABA treatment, seed germination and early seedling growth of adt were inhibited, whereas, under high osmotic conditions, adt showed a higher seed germination frequency. In summary, our results suggested that the activated expression of WRKY57 improved drought tolerance of Arabidopsis by elevation of ABA levels. Establishment of the functions of WRKY57 will enable improvement of plant drought tolerance through gene manipulation approaches.
[本文引用: 1]
[本文引用: 1]
URLPMID:20643804 [本文引用: 2]
DOI:10.1007/s11033-011-1245-9URLPMID:21938429 [本文引用: 1]

WRKY TFs belong to one of the largest families of transcriptional regulators in plants and form integral parts of signaling webs that modulate many plant processes. BcWRKY46, a cDNA clone encoding a polypeptide of 284 amino acids and exhibited the structural features of group III of WRKY protein family, was isolated from the cold-treated leaves of Pak-choi (Brassica campestris ssp. chinensis Makino, syn. B. rapa ssp. chinensis) using the cDNA-AFLP technique. Expression of this gene was induced quickly and strongly in response to various environmental stresses, including low temperatures, ABA, salt and dehydration. Constitutive expression of BcWRKY46 in tobacco under the control of the CaMV35S promoter reduced the susceptibility of transgenic tobacco to freezing, ABA, salt and dehydration stresses. Our studies suggest that BcWRKY46 plays an important role in responding to ABA and abiotic stress.
URLPMID:18818929 [本文引用: 1]
DOI:10.1104/pp.106.093971URLPMID:17322336 [本文引用: 1]

Phosphate (Pi) deficiency limits plant growth and development, resulting in adaptive stress responses. Among the molecular determinants of Pi stress responses, transcription factors play a critical role in regulating adaptive mechanisms. WRKY75 is one of several transcription factors induced during Pi deprivation. In this study, we evaluated the role of the WRKY75 transcription factor in regulating Pi starvation responses in Arabidopsis (Arabidopsis thaliana). WRKY75 was found to be nuclear localized and induced differentially in the plant during Pi deficiency. Suppression of WRKY75 expression through RNAi silencing resulted in early accumulation of anthocyanin, indicating that the RNAi plants were more susceptible to Pi stress. Further analysis revealed that the expression of several genes involved in Pi starvation responses, including phosphatases, Mt4/TPS1-like genes, and high-affinity Pi transporters, was decreased when WRKY75 was suppressed. Consequently, Pi uptake of the mutant plant was also decreased during Pi starvation. In addition, when WRKY75 expression was suppressed, lateral root length and number, as well as root hair number, were significantly increased. However, changes in the root architecture were obvious under both Pi-sufficient and Pi-deficient conditions. This indicates that the regulatory effect of WRKY75 on root architecture could be independent of the Pi status of the plant. Together, these results suggest that WRKY75 is a modulator of Pi starvation responses as well as root development. WRKY75 is the first member of the WRKY transcription factor family reported to be involved in regulating a nutrient starvation response and root development.
DOI:10.1105/tpc.108.064980URLPMID:19934380 [本文引用: 1]

Arabidopsis thaliana WRKY family comprises 74 members and some of them are involved in plant responses to biotic and abiotic stresses. This study demonstrated that WRKY6 is involved in Arabidopsis responses to low-Pi stress through regulating PHOSPHATE1 (PHO1) expression. WRKY6 overexpression lines, similar to the pho1 mutant, were more sensitive to low Pi stress and had lower Pi contents in shoots compared with wild-type seedlings and the wrky6-1 mutant. Immunoprecipitation assays demonstrated that WRKY6 can bind to two W-boxes of the PHO1 promoter. RNA gel blot and beta-glucuronidase activity assays showed that PHO1 expression was repressed in WRKY6-overexpressing lines and enhanced in the wrky6-1 mutant. Low Pi treatment reduced WRKY6 binding to the PHO1 promoter, which indicates that PHO1 regulation by WRKY6 is Pi dependent and that low Pi treatment may release inhibition of PHO1 expression. Protein gel blot analysis showed that the decrease in WRKY6 protein induced by low Pi treatment was inhibited by a 26S proteosome inhibitor, MG132, suggesting that low Pi-induced release of PHO1 repression may result from 26S proteosome-mediated proteolysis. In addition, WRKY42 also showed binding to W-boxes of the PHO1 promoter and repressed PHO1 expression. Our results demonstrate that WRKY6 and WRKY42 are involved in Arabidopsis responses to low Pi stress by regulation of PHO1 expression.
DOI:10.1104/pp.114.253799URLPMID:25733771 [本文引用: 3]

The Arabidopsis (Arabidopsis thaliana) WRKY transcription factor family has more than 70 members, and some of them have been reported to play important roles in plant response to biotic and abiotic stresses. This study shows that WRKY42 regulated phosphate homeostasis in Arabidopsis. The WRKY42-overexpressing lines, similar to the phosphate1 (pho1) mutant, were more sensitive to low-inorganic phosphate (Pi) stress and had lower shoot Pi content compared with wild-type plants. The PHO1 expression was repressed in WRKY42-overexpressing lines and enhanced in the wrky42 wrky6 double mutant. The WRKY42 protein bound to the PHO1 promoter under Pi-sufficient condition, and this binding was abrogated during Pi starvation. These data indicate that WRKY42 modulated Pi translocation by regulating PHO1 expression. Furthermore, overexpression of WRKY42 increased root Pi content and Pi uptake, whereas the wrky42 mutant had lower root Pi content and Pi uptake rate compared with wild-type plants. Under Pi-sufficient condition, WRKY42 positively regulated PHOSPHATE TRANSPORTER1;1 (PHT1;1) expression by binding to the PHT1;1 promoter, and this binding was abolished by low-Pi stress. During Pi starvation, the WRKY42 protein was degraded through the 26S proteasome pathway. Our results showed that AtWRKY42 modulated Pi homeostasis by regulating the expression of PHO1 and PHT1;1 to adapt to environmental changes in Pi availability.
DOI:10.1093/jxb/erv515URLPMID:26663563 [本文引用: 1]

The WRKY transcription factor family has 109 members in the rice genome, and has been reported to be involved in the regulation of biotic and abiotic stress in plants. Here, we demonstrated that a rice OsWRKY74 belonging to group III of the WRKY transcription factor family was involved in tolerance to phosphate (Pi) starvation. OsWRKY74 was localized in the nucleus and mainly expressed in roots and leaves. Overexpression of OsWRKY74 significantly enhanced tolerance to Pi starvation, whereas transgenic lines with down-regulation of OsWRKY74 were sensitive to Pi starvation. Root and shoot biomass, and phosphorus (P) concentration in rice OsWRKY74-overexpressing plants were ~16% higher than those of wild-type (WT) plants in Pi-deficient hydroponic solution. In soil pot experiments, >24% increases in tiller number, grain weight and P concentration were observed in rice OsWRKY74-overexpressing plants compared to WT plants when grown in P-deficient medium. Furthermore, Pi starvation-induced changes in root system architecture were more profound in OsWRKY74-overexpressing plants than in WT plants. Expression patterns of a number of Pi-responsive genes were altered in the OsWRKY74-overexpressing and RNA interference lines. In addition, OsWRKY74 may also be involved in the response to deficiencies in iron (Fe) and nitrogen (N) as well as cold stress in rice. In Pi-deficient conditions, OsWRKY74-overexpressing plants exhibited greater accumulation of Fe and up-regulation of the cold-responsive genes than WT plants. These findings highlight the role of OsWRKY74 in modulation of Pi homeostasis and potential crosstalk between P starvation and Fe starvation, and cold stress in rice.
DOI:10.1006/meth.2001.1262URLPMID:11846609 [本文引用: 1]

The two most commonly used methods to analyze data from real-time, quantitative PCR experiments are absolute quantification and relative quantification. Absolute quantification determines the input copy number, usually by relating the PCR signal to a standard curve. Relative quantification relates the PCR signal of the target transcript in a treatment group to that of another sample such as an untreated control. The 2(-Delta Delta C(T)) method is a convenient way to analyze the relative changes in gene expression from real-time quantitative PCR experiments. The purpose of this report is to present the derivation, assumptions, and applications of the 2(-Delta Delta C(T)) method. In addition, we present the derivation and applications of two variations of the 2(-Delta Delta C(T)) method that may be useful in the analysis of real-time, quantitative PCR data.
[本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0150572URLPMID:26938878 [本文引用: 1]

WRKY transcription factors serve as antagonistic or synergistic regulators in a variety of abiotic stress responses in plants. Here, we show that CmWRKY1, a member of the group IIb WRKY family isolated from Chrysanthemum morifolium, exhibits no transcriptional activation in yeast cells. The subcellular localization examination showed that CmWRKY1 localizes to the nucleus in vivo. Furthermore, CmWRKY1-overexpressing transgenic lines exhibit enhanced dehydration tolerance in response to polyethylene glycol (PEG) treatment compared with wild-type plants. We further confirmed that the transgenic plants exhibit suppressed expression levels of genes negatively regulated by ABA, such as PP2C, ABI1 and ABI2, and activated expression levels of genes positively regulated by ABA, such as PYL2, SnRK2.2, ABF4, MYB2, RAB18, and DREB1A. Taken together, our results indicate that CmWRKY1 plays an important role in the response to drought in chrysanthemum through an ABA-mediated pathway.
DOI:10.1093/nar/gkm001URLPMID:17264121 [本文引用: 1]

WRKY proteins, defined by the conserved WRKYGQK sequence, are comprised of a large superfamily of transcription factors identified specifically from the plant kingdom. This superfamily plays important roles in plant disease resistance, abiotic stress, senescence as well as in some developmental processes. In this study, the Arabidopsis WRKY1 was shown to be involved in the salicylic acid signaling pathway and partially dependent on NPR1; a C-terminal domain of WRKY1, AtWRKY1-C, was constructed for structural studies. Previous investigations showed that DNA binding of the WRKY proteins was localized at the WRKY domains and these domains may define novel zinc-binding motifs. The crystal structure of the AtWRKY1-C determined at 1.6 A resolution has revealed that this domain is composed of a globular structure with five beta strands, forming an antiparallel beta-sheet. A novel zinc-binding site is situated at one end of the beta-sheet, between strands beta4 and beta5. Based on this high-resolution crystal structure and site-directed mutagenesis, we have defined and confirmed that the DNA-binding residues of AtWRKY1-C are located at beta2 and beta3 strands. These results provided us with structural information to understand the mechanism of transcriptional control and signal transduction events of the WRKY proteins.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.tplants.2016.04.004URLPMID:27143288 [本文引用: 1]

To respond to abiotic stresses, plants have developed specific mechanisms that allow them to rapidly perceive and respond to environmental changes. The phytohormone abscisic acid (ABA) was shown to be a pivotal regulator of abiotic stress responses in plants, triggering major changes in plant physiology. The ABA core signaling pathway largely relies on the activation of SnRK2 kinases to mediate several rapid responses, including gene regulation, stomatal closure, and plant growth modulation. Mitogen-activated protein kinases (MAPKs) have also been implicated in ABA signaling, but an entire ABA-activated MAPK module was uncovered only recently. In this review, we discuss the evidence for a role of MAPK modules in the context of different plant ABA signaling pathways.
[本文引用: 1]
DOI:10.1007/s00438-008-0392-8URLPMID:18987888 [本文引用: 2]

The root apex contains meristematic cells that determine root growth and architecture in the soil. Specific transcription factor (TF) genes in this region may integrate endogenous signals and external cues to achieve this. Early changes in transcriptional responses involving TF genes after a salt stress in Medicago truncatula (Mt) roots were analysed using two complementary transcriptomic approaches. Forty-six salt-regulated TF genes were identified using massive quantitative real-time RT-PCR TF profiling in whole roots. In parallel, Mt16K+ microarray analysis revealed 824 genes (including 84 TF sequences) showing significant changes (p < 0.001) in their expression in root apexes after a salt stress. Analysis of salt-stress regulation in root apexes versus whole roots showed that several TF genes have more than 30-fold expression differences including specific members of AP2/EREBP, HD-ZIP, and MYB TF families. Several salt-induced TF genes also respond to other abiotic stresses as osmotic stress, cold and heat, suggesting that they participate in a general stress response. Our work suggests that spatial differences of TF gene regulation by environmental stresses in various root regions may be crucial for the adaptation of their growth to specific soil environments.
DOI:10.1007/s00425-011-1375-2URLPMID:21336597 [本文引用: 1]

Limited information is available regarding the exact function of specific WRKY transcription factors in plant responses to heat stress. We analyzed the roles of WRKY25, WRKY26, and WRKY33, three types of group I WRKY proteins, in the regulation of resistance to heat stress. Expression of WRKY25 and WRKY26 was induced upon treatment with high temperature, whereas WRKY33 expression was repressed. Heat-treated WRKY single mutants exhibited small responses, while wrky25wrky26 and wrky25wrky33 double mutants and the wrky25wrky26wrky33 triple mutants showed substantially increased susceptibility to heat stress, showing reduced germination, decreased survival, and elevated electrolyte leakage, compared with wild-type plants. In contrast, constitutive expression of WRKY25, WRKY26, or WRKY33 enhanced resistance to heat stress. Expression studies of selected heat-defense genes in single, double, and triple mutants, as well as in over-expressing lines, were correlated with their thermotolerance phenotypes and demonstrated that the three WRKY transcription factors modulate transcriptional changes of heat-inducible genes in response to heat treatment. In addition, our findings provided evidence that WRKY25, WRKY26, and WRKY33 were involved in regulation of the heat-induced ethylene-dependent response and demonstrated positive cross-regulation within these three genes. Together, these results indicate that WRKY25, WRKY26, and WRKY33 positively regulate the cooperation between the ethylene-activated and heat shock proteins-related signaling pathways that mediate responses to heat stress; and that these three proteins interact functionally and play overlapping and synergetic roles in plant thermotolerance.
[本文引用: 1]
[本文引用: 1]
DOI:10.1104/pp.113.235077URLPMID:24586044 [本文引用: 1]

The WRKY transcription factor family has more than 70 members in the Arabidopsis (Arabidopsis thaliana) genome, and some of them are involved in plant responses to biotic and abiotic stresses. This study evaluated the role of WRKY45 in regulating phosphate (Pi) uptake in Arabidopsis. WRKY45 was localized in the nucleus and mainly expressed in roots. During Pi starvation, WRKY45 expression was markedly induced, typically in roots. WRKY45 overexpression in Arabidopsis increased Pi content and uptake, while RNA interference suppression of WRKY45 decreased Pi content and uptake. Furthermore, the WRKY45-overexpressing lines were more sensitive to arsenate, the analog of Pi, compared with wild-type seedlings. These results indicate that WRKY45 positively regulates Arabidopsis Pi uptake. Quantitative real-time polymerase chain reaction and beta-glucuronidase staining assays showed that PHOSPHATE TRANSPORTER1;1 (PHT1;1) expression was enhanced in the WRKY45-overexpressing lines and slightly repressed in the WRKY45 RNA interference line. Chromatin immunoprecipitation and electrophoretic mobility shift assay results indicated that WRKY45 can bind to two W-boxes within the PHT1;1 promoter, confirming the role of WRKY45 in directly up-regulating PHT1;1 expression. The pht1;1 mutant showed decreased Pi content and uptake, and overexpression of PHT1;1 resulted in enhanced Pi content and uptake. Furthermore, the PHT1;1-overexpressing line was much more sensitive to arsenate than WRKY45-overexpressing and wild-type seedlings, indicating that PHT1;1 overexpression can enhance Arabidopsis Pi uptake. Moreover, the enhanced Pi uptake and the increased arsenate sensitivity of the WRKY45-overexpressing line was impaired by pht1;1 (35S:WRKY45-18::pht1;1), demonstrating an epistatic genetic regulation between WRKY45 and PHT1;1. Together, our results demonstrate that WRKY45 is involved in Arabidopsis response to Pi starvation by direct up-regulation of PHT1;1 expression.
DOI:10.1016/j.plantsci.2018.07.010URLPMID:30107882 [本文引用: 1]

WRKY transcription factors play a critical role in biotic and abiotic stress responses in plants, but very few WRKYs have been reported in strawberry plants. Here, a multiple stress-inducible gene, FvWRKY42, was isolated from the wild diploid woodland strawberry (accession Heilongjiang-3). FvWRKY42 expression was induced by treatment with powdery mildew, salt, drought, salicylic acid (SA), methyl jasmonate (MeJA), abscisic acid (ABA), and ethylene. The protein interaction network analysis showed that the FvWRKY42 protein interacts with various stress-related proteins. Overexpression of FvWRKY42 in Arabidopsis resulted in cell death, sporulation, slow hypha growth, and enhanced resistance to powdery mildew that was concomitant with increased expression of PR1 genes in Arabidopsis. Overexpression also led to enhanced salt and drought stress tolerance, increased primary root length and germination rate, decreased water loss rate, reduced relative electrolyte leakage, and malondialdehyde accumulation, and upregulation of superoxide dismutase and catalase activity. Additionally, FvWRKY42-overexpressing Arabidopsis plants showed increased ABA sensitivity during seed germination and seedling growth, increased stomatal closure after ABA and drought treatment, and altered expression of ABA-responsive genes. Collectively, our data demonstrate that FvWRKY42 may play an important role in powdery mildew infection and the regulation of salt and drought stress responses in plants.
