 ,1, 张唯唯1, 马天怡1, 蔡敏1, 张怡帆1, 胡荣蓉2, 唐长波
,1, 张唯唯1, 马天怡1, 蔡敏1, 张怡帆1, 胡荣蓉2, 唐长波 ,2
,2Influence of Oxidative Modification by Malondialdehyde on Structure and Emulsifying Properties of Walnut Protein
WANG YaoSong ,1, ZHANG WeiWei1, MA TianYi1, CAI Min1, ZHANG YiFan1, HU RongRong2, TANG ChangBo
,1, ZHANG WeiWei1, MA TianYi1, CAI Min1, ZHANG YiFan1, HU RongRong2, TANG ChangBo ,2
,2通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-03-2接受日期:2020-04-21网络出版日期:2020-08-16
| 基金资助: |
Received:2020-03-2Accepted:2020-04-21Online:2020-08-16
作者简介 About authors
王耀松,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (830KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王耀松, 张唯唯, 马天怡, 蔡敏, 张怡帆, 胡荣蓉, 唐长波. 丙二醛氧化对核桃分离蛋白结构及乳化性的影响[J]. 中国农业科学, 2020, 53(16): 3372-3384 doi:10.3864/j.issn.0578-1752.2020.16.014
WANG YaoSong, ZHANG WeiWei, MA TianYi, CAI Min, ZHANG YiFan, HU RongRong, TANG ChangBo.
0 引言
【研究意义】核桃含有丰富的蛋白质、不饱和脂肪酸、维生素、矿物质和多酚类物质[1],具有极高的营养价值和生理活性[2]。在核桃这种多组分构成的食品物料体系中,随着食品物料生产、贮藏和加工过程中经历不同的环境(比如内源性因素中的脂氧合酶和外源性因素中的紫外线、氧气、温度、辐射和相对湿度等),而使各个组分内和组分间发生潜在的各类氧化反应[3]。一方面内源性脂肪酶启动氧化不饱和脂肪酸[4]及脂肪酸自动氧化[5],这些反应产生各类自由基及醛类等物质,进而在共组分体系中氧化蛋白[6];另一方面是蛋白质的自发氧化反应[7]。蛋白质相对于脂类物质更易被氧化[3],而对于核桃原料体系高不饱和脂类含量和蛋白作为乳状液重要功能组分的特点,在脂肪氧合酶作用下发生次级氧化反应[4]进而影响核桃组分的感官品质、营养性及加工功能性的下降,从而直接影响核桃蛋白的各类功能性;而蛋白原料承载食品的营养性和各类加工性能,特别是乳化功能性[8],它为核桃蛋白的应用和饮料制造提供了基础。中国是核桃生产大国,2017年核桃产量约为384.55万 t干果,种植面积仍不断在扩大,产量还有持续增产的趋势,可见它是大宗的林下经济产品。对其进行产品品质保藏的研究具有重要的理论价值和现实意义。【前人研究进展】目前对核桃中重要组分蛋白开展研究,采用不同氧化体系模拟核桃仁体系中发生的各类蛋白氧化,取得了一定的进展。孙领鸽等[9]采用丙烯醛模拟脂肪氧合酶诱导脂类氧化成小分子醛类物质,发现这类氧化修饰核桃蛋白的结构并引起溶解度、乳化性能的显著下降。王丹丹等[10]采用2,2'-盐酸脒基丙烷热降解形成烷过氧自由基模拟脂质过氧化反应过程中产生的脂质自由基对蛋白的氧化,发现这类氧化降低蛋白结构有序性,形成以疏水作用力、氢键和离子键等作用力为基础的高分子聚集体。该研究组[11,12]又采用脂肪氧合酶/亚油酸体系构建脂质氢过氧化物对核桃蛋白进行不同程度的氧化,发现氧化形成不同类蛋白聚集体,通过对蛋白结构的修饰,在低浓度氧化作用下促使蛋白结构展开,形成可溶性聚集体并提高蛋白乳化功能性;而高浓度氧化则增大蛋白粒径、形成不溶性聚集体进而降低蛋白乳化功能性。【本研究切入点】虽然采用不同氧化体系对核桃蛋白结构与功能的影响有一些研究,但作为脂质过氧化产物的MDA对核桃蛋白氧化的影响尚未见报道。【拟解决的关键问题】通过构建MDA氧化模拟多不饱和脂肪酸在脂肪氧合酶作用下及其自发氧化的产物对核桃蛋白的氧化,研究核桃蛋白物理化学性质、结构和乳化功能,从分子水平上探讨蛋白结构与其乳化功能性之间的内在关系,为揭示不饱和脂类氧化物质对蛋白功能性的影响机制提供参考。1 材料与方法
试验于2018年7月至2019年6月在南京林业大学轻工与食品学院进行。1.1 材料与试剂
核桃2018年3月购于南京玄武区锁金村菜市场(产地新疆);1,1,3,3-四乙基环丙烷(分析纯),购于美国Sigma Chemical公司;其他化学试剂均为分析纯,购于国药集团化学试剂有限公司。1.2 主要仪器与设备
FE28型pH计,梅特勒-托利多仪器(上海)有限公司;Avanti JXN-26高速冷冻离心机,美国贝克曼库尔特有限公司;SCIENTZ-18N冷冻干燥机,宁波新芝生物科技股份有限公司;UVmini-1240紫外可见分光光度计,岛津企业管理(中国)有限公司;SpectraMax? i3x多功能酶标仪,美谷分子仪器(上海)有限公司;Mos-45圆二色谱仪,法国Bio-Logic公司;Mini- Protean? Tetra Cell电泳仪,美国BIO-RAD公司;Nanoplus-2 Zeta电位仪,麦克默瑞提克(上海)仪器有限公司;BT-90纳米激光粒度分布仪,丹东百特仪器有限公司;H1-16KR台式高速微量冷冻离心机,湖南可成设备仪器有限公司。1.3 试验方法
1.3.1 核桃蛋白的提取 参考MAO等[13]的方法做适当修改。将核桃去壳、去种皮后50℃烘干,用高速粉碎机磨碎成浆糊状(时间60 s);为了减少油脂含量,在10℃下10 000 r/min离心50 min以得核桃粕。加入正己烷,按料液质量体积比1﹕10处理1 h以脱除核桃中的油脂;使用中速定性滤纸抽滤得到滤饼,将滤饼用正己烷再次处理,直到滤液呈无色为止。收集滤饼,并摊平置于通风橱内挥发干溶剂以备用(脱脂块状物);最后用高速粉碎机将脱脂块状物磨成粉,即得到脱脂粉。采用碱溶酸沉法制备核桃分离蛋白。将脱脂后的粗核桃粉加去离子水(质量/体积为1﹕60)溶解,调节核桃蛋白溶液pH至11,搅拌1 h后在10 000 r/min、4℃条件下离心20 min取上清液。将上清液pH调为4.5后搅拌1 h,再次在同等条件下离心取沉淀,将沉淀物水洗至中性;所提取核桃蛋白冷冻干燥后放置-20℃冰箱备用。1.3.2 MDA氧化体系的构建 参照ADAMS等[14]的方法。称取0.44 g 1,1,3,3-四乙基环丙烷溶于20 mL 0.1 mol·L-1 HCl溶液中,水浴50℃加热1 h,直至溶液颜色变为深黄色,冷却后置于4℃冰箱冷藏备用。MDA浓度可在267 nm波长处测定,摩尔吸光度系数为31 500 L·mol-1·cm-1。
1.3.3 MDA氧化核桃蛋白的制备 稀释MDA储备溶液,使其终浓度分别为0、0.1、1、5、10和20 mmol·L-1,将溶液pH调为8.0,加入核桃蛋白使蛋白浓度为10 mg·mL-1,在室温下反应24 h。之后将上述样品在4℃超纯水中透析72 h,真空冷冻干燥得到MDA氧化核桃蛋白,置于4℃下贮存备用。
1.3.4 浊度、溶解度的测定 将不同MDA氧化度的核桃蛋白样品溶解于去离子水中,使蛋白浓度为10 mg·mL-1,再用1 mol·L-1NaOH将pH均调至8.0后,在600 nm处直接测定其吸光度,其值表示为蛋白浊度。参考WANG等[15]方法测定溶解度,将浓度为10 mg·mL-1、pH 8.0的不同MDA氧化度溶液样品,在室温下12 000×g离心20 min。采用双缩脲法测定上清液中蛋白浓度,再与初始蛋白浓度之比表示为蛋白溶解度。
1.3.5 总巯基、二硫键的测定 参考LIU等[16]测定方法并略作修改。使用浓度为10 mmol?L-1 pH 8.0的磷酸盐缓冲液将氧化后的核桃蛋白溶液样品稀释到浓度为2 mg?mL-1,然后取0.5 mL稀释后的核桃蛋白溶液,加入2.0 mL的10 mmol?L-1的磷酸盐缓冲液(pH 8.0,含8 mol·L-1尿素、3% SDS);再加入50 μL 10 mmol?L-1 5,5'-二硫代二硝基苯甲酸盐,充分混合后在室温下放置15 min,最后在412 nm波长下测定其吸光值。在二硫键的测定中,取0.1 mL稀释液加入1.5 mL新鲜制备的2-硝基-5-硫代磺酸基苯甲酸(NTSB),避光环境中反应25 min,空白对照组用0.1 mL去离子水代替,在412 nm波长下进行比色。巯基和二硫键的含量使用摩尔吸光系数13 600 M-1?cm-1计算。
1.3.6 自由氨基的测定 参考WANG等[17]测定方法略作修改,将核桃蛋白溶液稀释到0.4%,取稀释后的蛋白溶液125 μL,加入2 mL 0.1 mol·L-1磷酸盐缓冲液(pH 8.0)和1 mL 0.01%(v/v)的三硝基苯磺酸(TNBS)溶液,充分振荡混匀后在50℃的水浴中避光反应30 min,再加入2 mL 0.1 mol?L-1 Na2SO3以终止反应;冷却15 min左右至室温,在420 nm处测定其吸光值。空白对照为相同操作下,用去离子水代替样液。自由氨基含量根据L-亮氨酸(L-Leu)含量的标准曲线来确定。
1.3.7 羰基的测定 参考LEVINE等[18]的测定方法,略作修改,取50 μL 1%的核桃蛋白溶液,加入2 mL 10 mmol·L-1 2,4,-二硝基苯肼(溶解于2 mol·L-1盐酸中),避光条件下反应1 h,每隔10 min使用涡旋仪混匀样品一次。之后加入2 mL 20%(w/v)三氯乙酸沉淀蛋白,5 000×g离心10 min,弃去上清液,将沉淀物搅碎后加入4 mL脱色液(乙醇/乙酸乙酯体积比为1﹕1),再静置10 min,按同样的方法反复清洗沉淀3次,最终弃去上清液,用空气吹干样品。吹干后的样品中加入1.5 mL的6 mol·L-1盐酸胍,混匀后在50℃水浴中反应30 min,在370 nm条件下测定吸光度。蛋白的浓度采用双缩脲法测定,标准曲线用BSA作为反应蛋白。羰基含量使用摩尔吸光系数22 400 M-1?cm-1计算。
1.3.8 聚丙烯酰胺凝胶电泳 参考LAEMMLI法[19]进行电泳。采用5%浓缩胶和10%分离胶;样品制备采用还原和非还原两种方式,还原剂β巯基乙醇(β-ME)浓度为5%(v/v),非还原剂采用加入1 mmol·L-1 N-乙基顺丁烯二酰亚胺来抑制在制备样品中可能生成的二硫键。上样量为15 μg;开始电泳时电流设定为20 mA,待样品越过浓缩胶和分离胶之间的界限后调为40 mA。用考马斯亮蓝R250染色3 h或者过夜染色,之后脱色直至无背景色。
1.3.9 荧光光谱的测定 根据1.3.4中测定的蛋白溶解度,并参考WANG等[20]的方法,用10 mmol·L-1pH 8.0的磷酸盐缓冲液将蛋白溶液稀释至0.5 mg·mL-1,在多功能酶标仪上设定激发波长λex为280 nm,发射波长λem为300—500 nm,扫描样品荧光强度。
1.3.10 蛋白二级结构的测定 采用远紫外区圆二色谱法测定核桃蛋白的二级结构含量。参照HARLEY等[21]测定方法略作修改。将上述冷冻干燥的核桃蛋白溶于10 mmol·L-1磷酸(pH 8.0)缓冲液中充分溶解后,在室温下10 000 ×g离心10 min,取上清液并调整浓度为0.1 mg·mL-1,室温下190—250 nm扫描;样品池光程长为2 mm,灵敏度为100 mdeg·cm-1,试验值为8次扫描的均值。采用曲线拟合软件CDpro计算蛋白质的二级结构组成。
1.3.11 疏水性的测定 参考KATO等的[22]测定方法,采用8-苯胺基-1-萘磺酸镁(ANS-Mg)荧光探针测定。将浓度为1%的核桃蛋白溶液在22℃、12 000×g条件下离心20 min,取1 mL上清液和4 mL双缩脲混匀后测定蛋白含量。用10 mmol·L-1 pH 8.0的磷酸盐缓冲液将蛋白溶液稀释到0.5—4.0 mg·mL-1,分别取不同浓度的样液2 mL与20 μL 8 mmol·L-1 ANS-Mg混合,充分振荡后采用多功能酶标仪测定其荧光强度。空白为不加核桃蛋白溶液的2 mL 0.01 mol·L-1磷酸盐缓冲液(pH 8.0),其余操作均相同。设定激发波长λex=365 nm,发射波长λem=484 nm,狭缝校正均为5 nm。以荧光强度对蛋白浓度作曲线,得到回归线方程的斜率即为蛋白的表面疏水性指数值。
1.3.12 ζ-电位和粒径的测定 参考WANG等[21]方法,使用10 mmol·L-1磷酸盐缓冲液(pH 8.0)稀释新鲜制备的氧化核桃蛋白溶液浓度至5 mg·mL-1,采用纳米激光粒度分布仪BT-90和Nanoplus 2 Zeta电位仪分别测定蛋白粒径和Zeta电位。
1.3.13 乳化活性和乳化稳定性的测定 参考WANG等[15]方法,取氧化后的蛋白溶液15 mL(浓度为10 mg·mL-1)和5 mL大豆油混合,在13 000 r/min速度下均质2 min,立即倒入25 mL烧杯中,在距离烧杯底部5 cm处取20 μL的乳状液与5 mL质量/体积为0.1% SDS溶液混合,测定其在500 nm处的吸光值,记为A0,乳状液静置30 min后采用相同的方法测定乳状液的吸光值,记为At。空白对照用0.1% SDS溶液。
$乳化活性(EAI)=2T×\frac{A_{0}×N}{C×\Phi×10000}$
$乳化稳定性(ESI)=\frac{A_{t}}{A_{0}}×100$
式中,T=2.303;N:稀释倍数为250;C:配置的蛋白质水溶液中的蛋白浓度(g?mL-1);Φ:乳化液中油的体积分数(25%);A0:0 min吸光值;At:30 min吸光值。
1.4 数据统计分析
所有数据至少平行3次,且试验重复了2次,结果表示为“平均值±标准差”。数据处理采用Statistix软件进行统计分析,显著性水平为α=0.05;用LSD检验数据间的差异显著性,用P<0.05表示。2 结果
2.1 MDA氧化对WPI溶解度和浊度的影响
从图1可以看出,核桃蛋白的溶解度随着MDA浓度提高而显著下降(P<0.05),其值从68.74%下降到11.88%。蛋白溶解度降低的趋势可分为两个主要下降阶段,在0.1—1 mmol·L-1 MDA对核桃蛋白的溶解度影响较大;当MDA浓度超过5 mmol·L-1时,氧化对核桃蛋白溶解度降低的影响则极为显著(P<0.05),且呈现近线性关系下降。对于不同MDA氧化度核桃蛋白的浊度,在0.1—20 mmol·L-1内的MDA氧化对核桃蛋白浊度的降低影响不显著;所有氧化蛋白样品的浊度在0.32左右,比对照组(0 mmol·L-1)下降约0.05;5 mmol·L-1MDA氧化度的蛋白溶液浊度最低。相比于0.1—5 mmol·L-1氧化度核桃蛋白的溶解度和浊度,基本呈现一致的变化规律;然而当MDA浓度超过5 mmol·L-1时,核桃蛋白的溶解度与浊度变化速度规律不再平行一致,核桃蛋白溶解性的下降速率显著加大,而其溶液的浊度变化则不显著。综上,MDA氧化显著影响核桃蛋白溶解度,其中最大氧化度的MDA致使核桃蛋白溶解度降低约82.8%,然而MDA对核桃蛋白溶液的浊度影响不显著。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1MDA氧化对核桃分离蛋白溶解度和浊度的影响
不同大、小写字母分别表示同一指标在不同处理间差异显著(P<0.05)。下同
Fig. 1Effect of MDA-induced protein oxidation on the solubility and turbidity of walnut protein isolate
Different large and lowercase letters indicate the same indexes are significantly different among different treatments (P<0.05). The same as below
2.2 MDA氧化对WPI分子基团的修饰
从分子结构上来分析核桃蛋白分子作为氧化标记性的基团,测定了总巯基、二硫键、羰基和自由氨基含量变化如图2所示。在0.1—5 mmol·L-1浓度内的MDA氧化对总巯基含量影响不显著,而当MDA浓度超过10 mmol·L-1则显著降低总巯基含量(P<0.05)。对应的二硫键在1 mmol·L-1 MDA以上浓度时,其含量即显著增加(P<0.05);在高于此浓度中,总巯基含量的下降、二硫键含量的上升,几乎呈现线性变化。在最高氧化度时,它们含量分别对应地降低了55.93%和上升了188.46%。对于不同氧化核桃蛋白的羰基含量,在低于5 mmol·L-1浓度时,MDA对其含量上升没有显著性影响(P>0.05),当MDA浓度在5—10 mmol·L-1则显著增加了羰基含量(P<0.05),之后随着MDA浓度的增加而趋于平稳。在0.1—5 mmol·L-1浓度内的MDA对自由氨基含量没有显著影响(P>0.05),而高于此浓度时,MDA显著降低自由氨基含量(P<0.05)。与未氧化核桃蛋白相比,蛋白中的羰基和氨基含量在MDA浓度20 mmol·L-1时分别上升了705.50%和下降了51.38%。以上这些代表性化学基团含量变化表示了MDA对核桃蛋白的氧化程度。图2
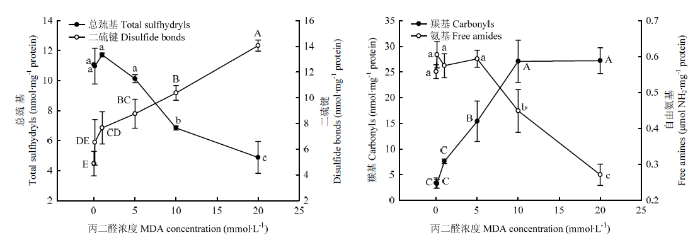 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2MDA氧化对核桃分离蛋白总巯基、二硫键、羰基及自由氨基含量的影响
Fig. 2Effect of MDA-induced protein oxidation on total sulfhydryls, disulfide bonds, carbonyls and free amines in walnut protein isolate
2.3 MDA氧化对WPI交联性的影响
蛋白交联是其氧化的一个重要结果。采用SDS-PAGE对MDA氧化核桃蛋白的分离结果如图3所示。在非还原条件下,随着氧化剂MDA浓度的上升,发现进入胶体的内蛋白量开始降低,特别是当MDA浓度为1 mmol·L-1时,进入胶体的蛋白总量开始显著减少;从胶体上可分离的核桃蛋白组分来看,每个条带的含量都在降低(非还原胶-βME,主要可分为14个组分),含量下降比较明显的组分是1、6、7、8、9、10,另外10和20 mmol·L-1 MDA氧化的组分4和5消失,而此浓度下组分11、12、13、14含量显著降低。为了验证这些条带中可能存在是的二硫键,在还原胶+βME中发现,样品中加入还原剂后出现在胶体上条带数明显增多,图3中将变化较为明显的组分作了标记,主要为组分b、d、e、f、g、h和i随着氧化浓度的升高而降低,组分k在氧化浓度超过5 mmol·L-1后消失;组分a和c则随着氧化剂浓度升高而较为明显的升高;在5—20 mmol·L-1浓度下的MDA氧化,出现新组分j。以上这些变化说明MDA氧化将蛋白分子间也引入了非还原性共价键,进一步验证了氧化对核桃蛋白的修饰。图3
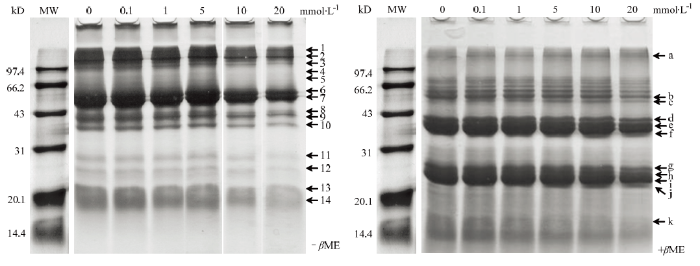 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3不同MDA氧化度的核桃分离蛋白的聚丙烯酰胺凝胶电泳图
Fig. 3Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) patterns of walnut protein isolate treated with MDA at various concentrations
2.4 MDA氧化WPI的荧光光谱
MDA氧化对核桃蛋白结构影响的荧光光谱见图4,可以看出总体上随着氧化度的增加,核桃蛋白强度不断下降。0.1 mmol·L-1 MDA氧化对核桃蛋白结构的影响不显著,而当MDA浓度为1 mmol·L-1时则较为明显地降低核桃蛋白的荧光强度;当被超过5 mmol·L-1浓度的MDA氧化,蛋白荧光强度显著降低,直到MDA浓度增加到20 mmol·L-1时,核桃蛋白的荧光强度趋于0。从蛋白二级结构数据(表1)可以看出,低浓度氧化(比如:0.1 mmol·L-1时)对核桃蛋白二级结构无显著影响(P>0.05),随着MDA浓度增加至较低浓度1 mmol·L-1时,则显著降低核桃蛋白二级结构α-螺旋、β-折叠和β-转角的相对含量(P<0.05),相应地显著增加无规则卷的相对含量(P<0.05)。较高浓度的MDA(>1 mmol·L-1)进一步显著降低有序结构α-螺旋、β-折叠和β-转角的相对含量(P<0.05),进一步显著提高无规则卷曲的含量(P<0.05)。这些变化体现了MDA氧化对核桃蛋白分子结构的修饰并进而影响蛋白功能性。图4
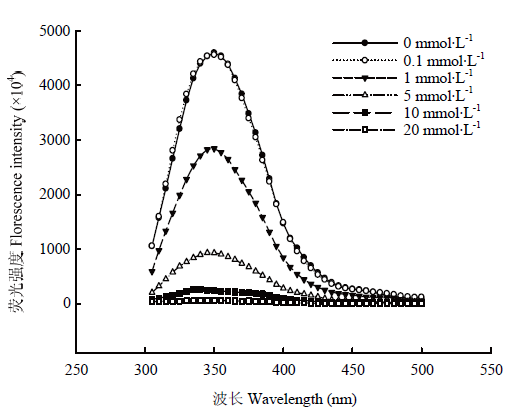 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4不同MDA氧化度的核桃分离蛋白的荧光光谱
Fig. 4Fluorescence spectrum of walnut protein isolate treated with different MDA oxidation degree
Table 1
表1
表1MDA氧化核桃分离蛋白的二级结构含量
Table 1
| 丙二醛浓度 MDA concentration (mmol·L-1) | 二级结构相对含量 Secondary structure content (%) | |||
|---|---|---|---|---|
| α-螺旋 α-helix | β-折叠 β-sheet | β-转角 β-turn | 无规则卷曲Random coil | |
| 0 | 2.78±0.02a | 49.73±0.38a | 22.68±0.18a | 25.36±0.20e |
| 0.1 | 2.79±0.02a | 49.28±0.31a | 22.90±0.15a | 25.49±0.16e |
| 1 | 2.00±0.00c | 48.45±0.07b | 22.02±0.03b | 27.43±0.04d |
| 5 | 2.05±0.07c | 45.7±0.57c | 21.85±0.07bc | 30.00±0.14c |
| 10 | 2.00±0.01c | 43.69±0.12d | 21.64±0.06cd | 32.47±0.09b |
| 20 | 2.31±0.01b | 43.03±0.18d | 21.56±0.09d | 32.80±0.14a |
新窗口打开|下载CSV
2.5 MDA氧化对WPI疏水性的影响
MDA氧化对核桃蛋白疏水性的影响如图5所示。MDA在较低浓度(<1 mmol·L-1)时对核桃蛋白疏水性的影响不显著(P>0.05);当MDA浓度超过1 mmol·L-1时,核桃蛋白的疏水性显著下降(P<0.05)。在10 mmol·L-1以下的浓度时,核桃蛋白疏水性要比在高浓度时(10—20 mmol·L-1)时的下降速率大。在MDA最高浓度为20 mmol·L-1时,疏水性指数为32.1,仅为对照组(即未氧化核桃蛋白,0 mmol·L-1)样品疏水性的1/10。疏水性的降低暗示着蛋白疏水性氨基酸可能被修饰或蛋白构象发生改变。图5
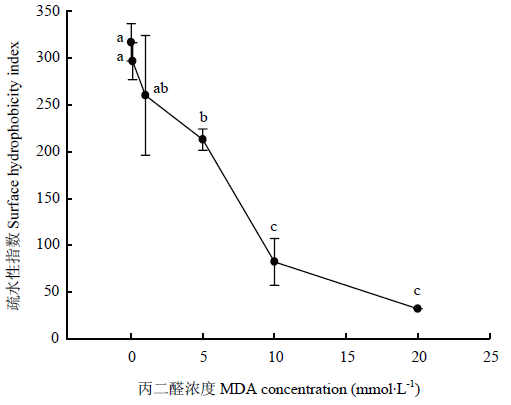 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5MDA氧化对核桃分离蛋白疏水性的影响
Fig. 5Effect of MDA-induced protein oxidation on surface hydrophobicity of walnut protein isolate
2.6 MDA氧化对WPI粒径和Zeta电位的影响
从图6可以看出,0.1 mmol·L-1以下浓度的MDA对核桃蛋白平均粒径无显著性影响(P>0.05),其大小约为790 nm;当MDA浓度在1—5 mmol·L-1时,氧化显著增加蛋白粒径(P<0.05),达到1 000 nm左右;随着MDA浓度增加到10 mmol·L-1时,蛋白粒径增大至约1 158 nm,但当MDA浓度增加到最大浓度20 mmol·L-1时,蛋白粒大小几乎不再增加。对于蛋白粒子带电量,0.1 mmol·L-1 MDA氧化没有造成蛋白Zeta电位的显著性变化(P>0.05),1—5 mmol·L-1 MDA浓度则显著降低蛋白Zeta电位(P<0.05),其值下降到-12 mv左右;当浓度从10 mmol·L-1增大到20 mmol·L-1时,核桃蛋白的Zeta电位从-11.75 mv降至-6.85 mv,仅为未被MDA氧化(0 mmol·L-1)核桃蛋白Zeta电位值的1/3,这大幅降低了蛋白的带电量(P<0.05)。在从低浓度到高浓度的MDA氧化过程中,核桃蛋白的带电性一直未改变,始终为负电但其绝对值在降低。图6
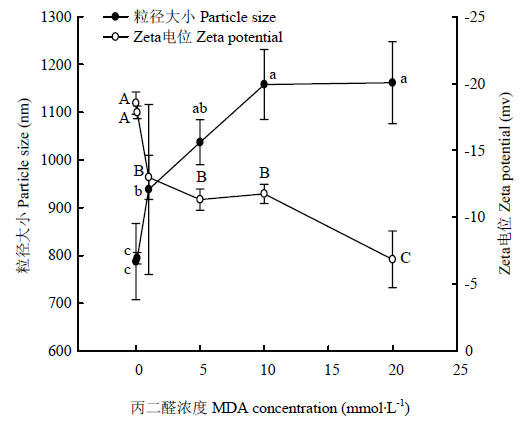 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6MDA氧化对核桃分离蛋白粒径和电位的影响
Fig. 6Effect of MDA-induced protein oxidation on particle size and Zeta potential of walnut protein isolate
2.7 MDA氧化对WPI乳化性的影响
图7显示了不同浓度MDA氧化对核桃蛋白乳化功能性的影响。低浓度MDA(0.1 mmol·L-1)使核桃蛋白乳化活性显著降低(P<0.05),但对核桃蛋白乳化稳定性没有显著影响(P>0.05)。在1—10 mmol?L-1内,核桃蛋白乳化活性几乎呈现直线显著性下降,而在10 mmol?L-1至最高浓度时,乳化活性降低速率变慢,最终乳化活性值为未氧化蛋白的1/3。MDA浓度在1 mmol?L-1以上时,核桃蛋白乳化稳定性也是保持降低的趋势,最终乳化稳定性值不及未氧化蛋白的1/2。说明低浓度MDA氧化对核桃蛋白乳化功能性影响不显著,而MDA浓度超过1 mmol·L-1时,极显著降低核桃蛋白乳化功能性。图7
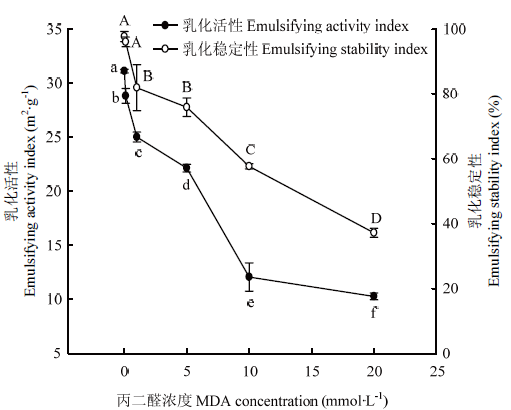 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7MDA氧化对核桃分离蛋白乳化性的影响
Fig. 7Effect of MDA-induced protein oxidation on the emulsifying properties of walnut protein isolate
3 讨论
3.1 MDA氧化对WPI修饰的分子基础
MDA是脂类氧化的产物,它的结构上存在两个醛基/羰基,这决定了它与核桃蛋白分子反应基础;另外从蛋白氨基酸残基组成与结构来看,它也有与MDA的醛基/羰基反应的基团,比如His、Lys、Arg、Glu及蛋白分子N-末端[15,23-24]。对于其他氨基酸,比较典型的是Cys,它有一个自由巯基,对氧化反应比较敏感,易形成对蛋白质物化性质和功能性有比较重要影响的二硫键和其他含硫的多种产物[25]。本研究发现随着MDA浓度的增加,巯基含量显著降低,这一方面是巯基基团与MDA反应过程中被消耗[26];另一方面是在氧化作用下转化为二硫键[27]及其他含硫产物[28]。在大豆蛋白[29,30]、米蛋白[31]和乳清蛋白[32]的MDA氧化体系中也发现巯基含量下降的现象;而二硫键含量变化不尽相同,在米蛋白MDA氧化体系中二硫键的含量也提高[31],然而在大豆蛋白体系中,MDA氧化却降低了二硫键的含量,认为是构象改变导致二硫键的断裂[29,30]。核桃蛋白分子中的氨基含量也在氧化后显著下降,这暗示着蛋白中来自氨基酸残基中的氨基在减少,比如Lys中的ε-氨基酸和N-末端的氨基;它们与来自MDA中的羰基相互作用,最为典型的是羰氨反应(美拉德反应)[33],或与组氨酸(His)、赖氨酸(Lys)和Cys形成Schiff碱[24,34]。作为蛋白氧化修饰最为代表性的反应是蛋白羰基化[18],在本试验中也得到了反映,发现核桃蛋白羰基含量随着MDA含量增加而显著增加。在肌纤维蛋白中,也发现有类似的现象[35]。MDA通过与蛋白亲和加成反应形成Schiff碱或Michael加成反应而使蛋白引入羰基[36],抑或蛋白直接氧化形成羰基[37]。相比于核桃蛋白中的羰基浓度与加入的MDA浓度值,显然是MDA对核桃蛋白羰基的主要贡献来自于它对蛋白的直接氧化。
以上这些MDA与核桃蛋白分子相互之间潜在的化学反应以及分子间的巯基/二硫键转化反应,通过测定它们的含量得到了验证;在SDS-PAGE试验中也得到了验证。加入还原剂β巯基乙醇(βME)后,位于非还原性胶体上端大分子量的大部分聚合物被还原,致使分子量显著降低;然而仍然有部分相对高分子量的聚合物无法被还原成低分子量的条带,这意味着MDA与核桃蛋白反应生成非可还原性共价键,比如MDA的两个醛基与核桃蛋白中的两个Lys反应而桥接分子[23],或通过其他加合物的方式[33]。
3.2 MDA氧化修饰WPI结构
以上潜在的分子间反应在核桃蛋白结构变化上得到进一步的反映和验证。采用荧光强度来表征氧化对蛋白结构的影响,而对荧光强度贡献最大的是色氨酸(Trp)[38]。有文献报道,MDA可与Trp残基的吲哚环反应[39]从而破坏Trp的含量,降低荧光强度。如果核桃蛋白与MDA形成加合物,那么加合物的荧光强度会随着MDA升高而显著升高[40];然而当前数据显示,MDA显著降低核桃蛋白的荧光强度,即MDA对核桃蛋白的修饰主要是破坏氨基酸残基结构而不是与其形成加合物质。在牛血清蛋白与MDA研究中,认为MDA与蛋白反应削减Trp荧光强度是因为Trp的破坏或其所在的微环境发生了改变[41],然而核桃蛋白羰基含量在增加,因此认为MDA是直接破坏Trp而降低样品的荧光强度。在核桃蛋白二级结构上,表现出有序结构的丧失及无序结构的提高。在丙烯醛氧化米蛋白研究中[31],发现α-螺旋结构含量随着氧化度的增加显著损失;在肌纤维蛋白中也发现此现象[42],然而β-折叠结构含量却在提高。本研究结果与孙领鸽等[9]利用丙烯醛氧化核桃蛋白所获得的二级结构含量变化类似。也就是说,不同蛋白可能对氧化应激反应不同。而蛋白二级结构的变化则影响蛋白氢键、疏水性作用和范德华力,进而为蛋白聚集提供了条件[43]。
蛋白在结构上的变化为其行为的变动提供了基础,特别是蛋白理化性质方面的变化[44]。疏水性的变化与蛋白结构、聚集行为有很大的内在联系。氧化使疏水性下降,可能正如前面所讨论,MDA与具有一定疏水性的Trp和Cys反应而破坏它的疏水性,或因为氧化形成的蛋白聚集体而包埋疏水性基团有关[45]。从氧化蛋白的溶解度下降和带电量的减少来看,对应着的是维系蛋白有序结构的氢键及静电作用力的弱化,进而未能提高蛋白疏水性,因此,蛋白疏水性的下降是氧化破坏Trp、形成聚集体造成的。维系蛋白二级结构的氢键弱化则降低蛋白结构的有序性,也就是说蛋白溶解度的下降与其二级结构含量减少有关[46]。
氧化诱导作用引起的蛋白分子交联[47]和聚集作用使其粒径变大。MDA氧化过程中产生的二硫键和非还原性共价键为交联提供了基础作用力,这可能很大程度上影响着蛋白分子粒径大小,在MDA氧化米蛋白、米糠蛋白过程中也有类似的现象[31,48]。在氧化过程中,也有溶解性差的聚集体产生,这部分聚集体贡献了蛋白分子粒径大小[40]。在测定蛋白质溶解度过程中,发现离心之后样品中的蛋白含量显著下降,这也验证了蛋白溶解度下降的事实。
蛋白分子的带电性是其在不同食品体系中稳定性的重要基础[49],比如凝结和聚集等失稳现象,通常使用Zeta电位来表示。它反映带电粒子表面电荷状态;在胶体体系中,一般其值绝对值越大表示体系越倾向稳定[50]。蛋白的带电性取决于所在体系的pH及自身氨基酸残基的电离特性。在MDA氧化过程中,通常涉及的氨基酸是Lys、His和Cys[51]以及Arg、Gln和Asn[24],特别对于碱性氨基酸Arg、Lys和His,它们遭到破坏则降低蛋白的电离和等电点进而降低蛋白的带电性。
3.3 MDA氧化修饰WPI乳化功能性
蛋白溶解度可作为蛋白变性或聚集的一个重要标志[52],也是作为蛋白的功能性之一及其他功能性的基础[53]。MDA氧化蛋白的结果虽然降低了本身的疏水性,然而氧化反应形成的微溶性聚合物,显著降低了聚合体的带电量,侧链修饰而改变蛋白的等电点等因素显著降低了蛋白溶解性。大豆蛋白[29]、米糠蛋白[48]、肌纤维蛋白[54]等蛋白在MDA氧化中均引起溶解度的显著下降。同时本试验中使用浊度方法对因氧化而导致蛋白聚集对其在溶液中的分散性影响进行了评估,反映了蛋白量和聚集体大小及其分布情况。从测定结果看,MDA氧化核桃蛋白的浊度呈现下降趋势,意味着蛋白发生了聚集或聚结并引起聚集体或聚结体溶解性能的下降。在一些由pH或热诱导的蛋白聚集体系中[55,56],用此法或能表征蛋白构象变化。蛋白乳化活性取决于蛋白分子的形状、带电量和疏水性以及偶极子中性、极性基团的水化作用以及乳状液制备加工工艺(比如温度、设备选型);而乳化稳定性取决于以上这些因素相互作用的程度[57]。球蛋白的溶解度、表面疏水性、分子柔性等因素对其乳化性行为的影响显得更为重要[58]。MDA氧化对蛋白分子修饰和结构的影响进而改变了其物理化学性质,特别是因氧化形成共价交联的聚集体粒径变大,降低了蛋白的溶解度和分子柔韧性,同时也显著降低了表面疏水性和带电量,这显然弱化了蛋白分子间的静电相互作用和乳状液界面膜与水的相互作用,在物理结构上也降低了乳状液界面膜的厚度且不能形成紧密结构。因此,上述因MDA氧化造成对乳化活性有重要影响的因素调低而显著降低了乳化能力。从蛋白聚集物尺度上看,氧化显著削弱了这些聚集体的溶解度和带电量,从而降低它们之间的相互作用,而倾向于聚集体间的分子聚结、絮凝等作用,这不仅仅降低了核桃蛋白的乳化性,也降低了其乳化稳定性。
MDA氧化虽然增加了蛋白中二硫键及其他非还原性共价键的含量,但这不足以补偿对乳化功能性起支配因素(比如带电量、疏水性)所产生的正面效应,为此总体上降低了核桃蛋白的乳化功能性。然而在一些氧化体系中发现,低浓度氧化能够通过促进蛋白疏水基团展开,结合高压均质处理诱导蛋白柔韧性可显著提高氧化蛋白的乳化活性[59,60]。在羟基氧化体系中,对氧化程度的控制可提高蛋白乳化功能性[61]。也就是说,通过对氧化剂浓度或对蛋白界面起支配性的作用力进行调节可获得蛋白乳化功能性的提升;从蛋白抗氧化角度来看,对一些蛋白,控制其氧化可避免乳化功能性的损失。
4 结论
丙二醛(MDA)氧化引起核桃蛋白分子中巯基和自由氨基含量的显著降低,二硫键和羰基含量增加;形成还原性和非还原性共价键,促进蛋白分子聚集和交联;从而显著提高蛋白分子粒径大小,降低分子带电性和疏水性;打乱蛋白分子有序的二级结构,提高了其无序性;进而造成核桃蛋白溶解性和乳化功能性的显著降低。尤其是5 mmol·L-1以上的MDA氧化度,对核桃蛋白分子修饰、结构改造和功能性损失等影响显著;而1 mmol·L-1以下的MDA氧化对核桃蛋白上述这些性质影响不显著。研究结果为核桃蛋白在加工和贮藏过程中,避免因MDA氧化而造成核桃蛋白功能性的损失提供重要参考。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
URLPMID:26396054 [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
URLPMID:11743765 [本文引用: 1]
URLPMID:16403681 [本文引用: 1]
[本文引用: 1]
URLPMID:26948632 [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:22408408 [本文引用: 1]
[J]
[本文引用: 1]
URLPMID:31174759 [本文引用: 3]
[本文引用: 1]
DOI:10.1016/j.foodres.2015.04.004URL [本文引用: 1]
URLPMID:1978225 [本文引用: 2]
DOI:10.1038/227680a0URLPMID:5432063 [本文引用: 1]
[本文引用: 1]
URLPMID:22396785 [本文引用: 2]
[本文引用: 1]
URLPMID:10391905 [本文引用: 2]
DOI:10.1021/jf3026277URLPMID:22946674 [本文引用: 3]

Antioxidative peptides in food systems are potential targets of lipid oxidation-generated reactive aldehydes, such as malonaldehyde (MDA) and 4-hydroxynonenal (HNE). In this study, covalent modifications on radical-scavenging peptides prepared from soy protein hydrolysate by MDA and HNE were characterized by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS/MS). MS/MS analyses detected the formation of Schiff base type adducts of MDA on the side-chain groups of lysine, histidine, arginine, glutamine, and asparagine residues as well as the N-termini of peptides. MDA also formed a fluorescent product with lysine residues. HNE adducted on lysine residues through Schiff base formation and on histidine, arginine, glutamine, and asparagine residues mainly through Michael addition. Despite the extensive MDA modification, peptide cross-linking by this potential mechanism was undetectable.
DOI:10.1002/anie.201814144URLPMID:30919556 [本文引用: 1]

Oxidation is one of the deterioration reactions of proteins in food, the importance of which is comparable to others such as Maillard, lipation, or protein-phenol reactions. While research on protein oxidation has led to a precise understanding of the processes and consequences in physiological systems, knowledge about the specific effects of protein oxidation in food or the role of
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 2]
[本文引用: 4]
URLPMID:31502294 [本文引用: 1]
DOI:10.1016/j.redox.2019.101277URLPMID:31352127 [本文引用: 2]

Carbonylation is one of the most remarkable expressions of the oxidative damage to proteins and the DNPH method the most common procedure to assess protein oxidation in biological samples. The present study was elicited by two hypotheses: i) is malondialdehyde, as a reactive dicarbonyl, able to induce the formation of allysine through a Maillard-type reaction? and ii) to which extent does the attachment of MDA to proteins interfere in the assessment of protein carbonyls using the DNPH method? Human serum albumin (HSA), human hemoglobin (HEM) and beta-lactoglobulin (LAC) (5mg/mL) were incubated with MDA (0.25mM) for 24hat 37 degrees C (HSA and HEM) or 80 degrees C (LAC). Results showed that MDA was unable to induce oxidative deamination of lysine residues and instead, formed stable and fluorescent adducts with proteins. Such adducts were tagged by the DNPH method, accounting for most of the protein hydrazones quantified. This interfering effect was observed in a wide range of MDA concentrations (0.05-1mM). Being aware of its limitations, protein scientists should accurately interpret results from the DNPH method, and apply, when required, other methodologies such as chromatographic methods to detect specific primary oxidation products such as allysine.
DOI:10.1021/jf404099yURLPMID:24236702 [本文引用: 1]

Soy protein isolate (SPI) was modified by lipid peroxidation product malondialdehyde (MDA), and the in vitro digestibility of modified SPI was investigated. Results indicated that incubation with increasing MDA concentration resulted in significant carbonyl group generation and loss of free amino groups of SPI. Fluorescence loss of natural tryptophan and formation of Schiff base were observed. Noncovalent interaction between molecules was enhanced and became the main force that led to the solubility reduction of MDA-modified SPI. Differential scanning calorimetry (DSC) indicated that SPI had higher thermal stability and lower total calorimetric enthalpy after MDA pretreatment. Electrophoresis showed that beta-conglycinin was more sensitive to MDA modification. In vitro digestion indicated that MDA could induce non-disulfide covalent polymer of SPI, which could not be digested by pepsin and pancreatin. beta subunits of beta-conglycinin became more resistant to digestion with increasing MDA concentration. Evaluation of the free amino acid profile in the digests indicated that MDA-modified SPI had deteriorating nutritive quality.
DOI:10.1016/j.foodchem.2019.02.126URLPMID:30902311 [本文引用: 1]

The effects of malondialdehyde (MDA) as a byproduct of lipid oxidation on myoglobin and myofibrillar proteins (MP) oxidations in muscle homogenates containing components native to rabbit muscle were investigated. For myoglobin, MDA could lead to increase in metmyoglobin percentage. For MP, MDA could promote protein carbonylation and loss of tryptophan fluorescence. In addition, MDA could affect reactive oxygen species (ROS)-generating system by promoting the formation of hypervalent myoglobin species and release of non-heme iron. The result of MDA-MP adducts fluorescence intensity indicated that ROS-generating systems may be the main reason for protein carbonylation at the later of incubation treatment. SDS-PAGE analysis revealed that the ability of ROS-generating systems to facilitate protein oxidation was enhanced with MDA, which was responsible for the formation of protein cross-linking throughout incubation treatment. Taken together, the ability of MDA on promoting the oxidation of MP in rabbit muscle homogenates may be relied on both adduct reactions and the influence on ROS-generating system.
DOI:10.1016/j.foodchem.2014.11.154URLPMID:25577084 [本文引用: 1]

Interactions between secondary lipid oxidation product malondialdehyde (MDA) and selected beta-lactoglobulin (beta-Lg) peptides were investigated. Selected tryptic peptides of beta-Lg (ALPMHIR, LIVTQTMK and VLVLDTDYK) were fractionated via preparative-HPLC and incubated with MDA at 37 degrees C and 60 degrees C for 7 days. Changes in samples were monitored with LC-ESI-MS coupled with UV and fluorescence detectors. Prominent modifications in peptide samples included formation of two distinct types of MDA adducts observed with mass increments of 54 and 134 amu, corresponding to Schiff base and dihydropyridine (DHP)-type adducts, respectively. Modified peptides with m/z +54 amu were more stable at 37 degrees C than at 60 degrees C but showed more rapid formation than compounds with m/z +134 amu. MDA-peptide adducts resulting in +134 amu mass increment displayed strong fluorescent characteristics and they were more stable than Schiff base adducts at 60 degrees C.
DOI:10.1016/j.meatsci.2011.04.025URLPMID:21621336 [本文引用: 1]

Protein oxidation (P-OX) is an innovative topic of increasing interest among meat researchers. Carbonylation is generally recognized as one of the most remarkable chemical modifications in oxidized proteins. In fact, the quantification of protein carbonyls by the dinitrophenylhydrazine (DNPH) method is the most common procedure for assessing P-OX in meat systems. Numerous studies have investigated the occurrence of protein carbonylation right after slaughter and during subsequent processing and cold storage of meat. However, the significance of protein carbonylation in meat systems is still poorly understood. Beyond their role as markers of protein oxidation, specific protein carbonyls such as alpha-aminoadipic and gamma-glutamic semialdehydes (AAS and GGS, respectively) are active compounds that may be implicated in several chemical reactions with relevant consequences on meat quality. The formation of protein carbonyls from particular amino acid side chains contribute to impair the conformation of myofibrillar proteins leading to denaturation and loss of functionality. Recent studies also highlight the potential impact of specific protein carbonyls in particular meat quality traits such as water-holding capacity (WHC), texture, flavor and its nutritional value. As a truly emerging topic, the results from current studies provide grounds from the development of further investigations. The present paper reviews the current knowledge on the mechanisms and consequences of protein carbonylation in meat systems and aims to encourage meat researchers to accomplish further investigations on this fascinating research topic.
[本文引用: 1]
[本文引用: 1]
DOI:10.1021/bc070001hURLPMID:17705413 [本文引用: 1]

A method for the selective modification of tryptophan residues based on the reaction of malondialdehyde with the indole nitrogen of the tryptophan side chain at acidic conditions is presented. The condensation reaction is quantitative and leads to a substituted acrolein moiety with a remaining reactive aldehyde group. As is shown, this group can be further converted to a hydrazone using hydrazide compounds, but if hydrazine or phenylhydrazine are used, release of the free indole group is observed upon cleavage of the substitution. Alternatively, secondary amines such as pyrrolidine may also act as cleavage reagents. This general reaction scheme has been adapted and optimized for the derivatization of tryptophan-containing peptides and small N-heterocyclic compounds. It serves as the basis of a reversible tagging scheme for Trp-peptides or molecules of interest carrying indole structures as it allows the specific attachment and removal of a reactive group that may be used for a variety of purposes such as affinity tagging.
DOI:10.1016/j.foodres.2017.11.001URLPMID:29433202 [本文引用: 2]

The interactions of soy protein isolate (SPI) and flavor compounds (hexanal, trans-2-hexenal, 1-octen-3-ol, trans-2-octenal, nonanal, and trans-2-nonenal) were investigated. The influence of SPI structure modified by malondialdehyde (MDA) and flavor compound structure on the interactions were determined by using headspace solid-phase microextraction (SPME) and gas chromatography (GC) combined with mass spectrometry (MS). The binding of native SPI to the flavor compounds decreased in the order trans-2-nonenal>nonanal>trans-2-octenal>trans-2-hexenal>hexanal>1-octen-3-ol. It might be attributed to that aldehydes are more hydrophobic than alcohols. The former is more conducive to hydrophobic binding with the SPI. Furthermore, the aldehydes, in particular trans-s-undecenal, could also react covalently. The effect of MDA modification on protein-flavor interactions depended on the structure of the flavor compound. Upon low concentration of MDA (/=2.5mM), more soluble and even insoluble aggregates formed, which reduced the binding of hexanal and nonanal to SPI. The other four flavors with double bond revealed little changes in binding (trans-2-octenal, and trans-2-nonenal) or even an increase in binding (trans-2-hexenal, and 1-octen-3-ol). The results suggested that hydrophobic interactions were weakened upon high extent of oxidation, whereas covalent interactions were enhanced.
DOI:10.1093/gerona/59.9.B890URL [本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pcbi.1002169URLPMID:22022239 [本文引用: 1]

Identifying the forces that drive proteins to misfold and aggregate, rather than to fold into their functional states, is fundamental to our understanding of living systems and to our ability to combat protein deposition disorders such as Alzheimer's disease and the spongiform encephalopathies. We report here the finding that the balance between hydrophobic and hydrogen bonding interactions is different for proteins in the processes of folding to their native states and misfolding to the alternative amyloid structures. We find that the minima of the protein free energy landscape for folding and misfolding tend to be respectively dominated by hydrophobic and by hydrogen bonding interactions. These results characterise the nature of the interactions that determine the competition between folding and misfolding of proteins by revealing that the stability of native proteins is primarily determined by hydrophobic interactions between side-chains, while the stability of amyloid fibrils depends more on backbone intermolecular hydrogen bonding interactions.
[本文引用: 1]
[本文引用: 1]
DOI:10.3390/molecules24234337URL [本文引用: 1]
DOI:10.1016/j.jfoodeng.2012.09.013URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1016/j.foodhyd.2019.03.031URL [本文引用: 1]
[本文引用: 1]
DOI:10.1081/DMR-100102336URL [本文引用: 1]
DOI:10.1111/1750-3841.12207URLPMID:23957418 [本文引用: 1]

Forming whey proteins into soluble aggregates is a modification shown to improve or expand the applications in foaming, emulsification, gelation, film-formation, and encapsulation. Whey protein soluble aggregates are defined as aggregates that are intermediates between monomer proteins and an insoluble gel network or precipitate. The conditions under which whey proteins denature and aggregate have been extensively studied and can be used as guiding principles of producing soluble aggregates. These conditions are reviewed for pH, ion type and concentration, cosolutes, and protein concentration, along with heating temperature and duration. Combinations of these conditions can be used to design soluble aggregates with desired physicochemical properties including surface charge, surface hydrophobicity, size, and shape. These properties in turn can be used to obtain target macroscopic properties, such as viscosity, clarity, and stability, of the final product. A proposed approach to designing soluble aggregates with improved thermal stability for beverage applications is presented.
[本文引用: 1]
URLPMID:10552619 [本文引用: 1]
DOI:10.1021/jf9710185URL [本文引用: 1]
[本文引用: 1]
DOI:10.1080/10408398.2015.1067594URLPMID:26463743 [本文引用: 1]

Soy proteins as important food ingredients exhibit a great potential to be widely applied in food formulations, due to their good nutrition, functional properties and health effects. The knowledge about the structure-function relationships of these proteins is crucial for their applications, but still very scant, especially that on their molecular mechanism of emulsification. The purpose of this review is to present a comprehensive summary of the knowledge about emulsifying and interfacial properties of soy proteins, achieved during the past decades, and particularly to provide an insight in understanding the role of conformational flexibility in their emulsifying properties. The interplays between the emulsifying and interfacial properties are also elucidated. For these proteins, the conformational flexibility rather than the surface hydrophobicity is the crucial parameter determining their emulsification performance. On the other hand, evidence is fast growing to indicate that because of the insoluble nature, soy proteins are a kind of unique materials to perform as food-grade Pickering stabilizers. The knowledge about the Pickering emulsion stabilization is distinctly different from that for conventional emulsions stabilized by soy proteins. Thus, different strategies should be employed to develop soy proteins into a kind of effective emulsifiers, depending on the preference of emulsification performance or emulsion stability.
[本文引用: 1]
DOI:10.1016/j.lwt.2011.12.001URL [本文引用: 1]
DOI:10.1016/j.foodres.2013.02.028URL [本文引用: 1]

Soy protein isolate (SPI) was oxidized by peroxyl radicals derived from 2,2'-azobis (2-amidinopropane) dihydrochloride (AAPH) and the structural and emulsifying properties of oxidized SPI were evaluated. Increasing extent of oxidation resulted in gradual carbonyl group generation, free sulfhydryl group degradation and dityrosine formation. Moderate oxidization could generate soluble protein aggregates with more flexible structure while over-oxidization would induce the formation of insoluble aggregates. Compared with the control, emulsions stabilized by moderately oxidized SPI had smaller droplet size and better thermal stability. Results from creaming index and microstructure measurement after 15 days indicated that emulsions stabilized by SPI of over-oxidation underwent severe droplet aggregation during storage while moderate oxidation had a positive effect on the emulsion stability. (c) 2013 Elsevier Ltd.
DOI:10.1007/s11947-011-0674-8URL [本文引用: 1]
