 ,1,2
,1,2Isolation, Structural Characterization and Antioxidant Activity of Black Sesame Melanin
LI Jie1,2, JIA XuChao2, ZHANG RuiFen2, LIU Lei2, CHI JianWei2, HUANG Fei2, DONG LiHong2, ZHANG MingWei ,1,2
,1,2通讯作者:
责任编辑: 杨鑫浩
收稿日期:2019-10-27接受日期:2020-02-25网络出版日期:2020-06-16
| 基金资助: |
Received:2019-10-27Accepted:2020-02-25Online:2020-06-16
作者简介 About authors
李杰,E-mail:771906135@qq.com。

摘要
关键词:
Abstract
Keywords:
PDF (722KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李杰, 贾栩超, 张瑞芬, 刘磊, 池建伟, 黄菲, 董丽红, 张名位. 黑芝麻黑色素的分离纯化、结构表征及体外抗氧化活性[J]. 中国农业科学, 2020, 53(12): 2477-2492 doi:10.3864/j.issn.0578-1752.2020.12.014
LI Jie, JIA XuChao, ZHANG RuiFen, LIU Lei, CHI JianWei, HUANG Fei, DONG LiHong, ZHANG MingWei.
0 引言
【研究意义】黑芝麻(Sesamum indicum L.)又称胡麻、黑脂麻、巨胜等,是脂麻科(胡麻科)脂麻属植物脂麻的干燥成熟种子[1]。黑芝麻是中国传统的药食两用作物,古代医书记载黑芝麻具有补肝肾、益精血、养五脏、生津乌发、延缓衰老等功效[2]。现代营养学研究表明黑芝麻富含蛋白质、脂类、碳水化合物、维生素、矿物质等营养成分[3]。另外,黑芝麻中还含有一些如芝麻酚、芝麻素、黑色素等功能性成分,其中黑色素是黑芝麻的特征活性成分。研究报道黑芝麻黑色素具有多种药理保健活性,包括抗氧化、清除自由基、抗肿瘤、保肝、预防神经性疾病等[4,5,6,7,8,9];此外,还可作为天然色素添加到食品、医药和保健品等当中,具有广泛的应用前景。与黑豆、黑米中的花色苷类黑色素不同,黑芝麻黑色素是一种非均质的类多酚聚合物,结构十分复杂[10],目前人们对黑芝麻黑色素的化学结构仍缺乏深入认识。因此,对黑芝麻黑色素进行分离纯化和结构表征,明确其骨架结构信息,有助于推进对大分子黑色素结构的认知,也进一步为黑芝麻保健食品的开发提供可靠的科学支持,对黑芝麻精深加工与利用具有重要的科学意义和经济价值。【前人研究进展】已有****对天然黑色素进行了研究,发现黑色素是由酚类或吲哚类化合物氧化聚合而形成的大分子,不溶于水和常见的有机溶剂(如己烷、氯仿、乙酸乙酯、乙醇、甲醇或丙酮),溶于碱水[10,11,12],存在于动物[13,14]、植物[10]和微生物[15,16]中。黑色素根据结构特征可以分为真黑色素(黑色或深棕色)、棕黑色素(红色至黄棕色)和异黑色素(黑色或棕色)[14,17]。目前对黑芝麻黑色素的研究主要集中在提取纯化工艺[18,19,20,21,22,23,24]、理化性质[20,21,22,23]及生物活性评价[4,6,8-9]等方面,也有部分****对黑芝麻黑色素的结构进行了初步研究。前人研究表明碱提酸沉法是提取黑芝麻黑色素的有效手段[21,22]。徐利萍[21]通过研究得出黑芝麻黑色素可能是含有酚羟基、C=O、高度共轭的C=C、芳香羧基等官能团的真黑素,而不含棕黑素。有****采用红外光谱、紫外光谱、元素分析等手段对黑芝麻黑色素进行研究[22,25-27],结果表明黑芝麻黑色素结构中含有羟基、羧基等官能团,并且存在吲哚结构,不含噻嗪环。陆懋荪等[28]、尹佩玉等[29]采用元素分析、紫外光谱、碱熔降解GC-MS等试验表明黑芝麻黑色素可能属于儿茶酚型的生物大分子。PANZELLA等[6]使用碱性过氧化氢分解黑芝麻色素并对降解产物进行分析,推断黑芝麻色素和聚松伯醇在结构上有一定的相似性。而黑芝麻黑色素的精细结构仍有待进一步研究明确,且黑芝麻黑色素的空间结构特征和自由基特性也有待探究。黑芝麻黑色素被报道具有抗氧化、清除自由基、抗肿瘤等生物活性,单良等[5]、徐利萍[21]、白亮[23]研究发现黑芝麻黑色素具有良好的DPPH·、·OH、O2-·、ABTS+等自由基清除能力,刘晓芳等[8]研究表明,黑芝麻黑色提取物具有保肝作用,这可能与其抗氧化作用有关。此外,KAMEI等[7]研究发现黑芝麻黑色素对体内外肿瘤细胞的生长有抑制功能。CHU等[30]报道天然黑芝麻黑色素可用于淋巴结肿瘤光热治疗。【本研究切入点】黑色素是一种非均质类多酚聚合物,目前有关黑芝麻黑色素结构的研究多是直接对粗黑色素提取物进行结构表征,获得的波谱信号复杂、重叠严重,且非黑色素杂质对结构表征有较大干扰;因此,本研究对黑芝麻黑色素进行分离纯化,将其划分为不同级分而后进行结构表征,获得不同级分的纯度、含量和精细结构信息、进而明确黑芝麻黑色素的级分组成及结构特征,降低结构表征的难度。此外黑色素是具有顺磁性的多聚物大分子,结构中含有大量的自由基,而黑芝麻黑色素的自由基特性和空间结构特征仍然未知。本研究首次采用电子顺磁共振(EPR)和X-射线衍射(XRD)对黑芝麻黑色素进行研究,明确其不同级分的自由基特性和空间结构特征。【拟解决的关键问题】通过HW-40C尺寸排阻色谱柱对黑芝麻黑色素粗提物进行分离,采用紫外可见光谱(UV-Vis)、元素分析(EA)、傅里叶红外光谱(FT-IR)、核磁共振氢谱(1H-NMR)和碳谱(13C-NMR)、X-射线光电子能谱(XPS)、电子顺磁共振(EPR)、X-射线衍射(XRD)等手段表征黑芝麻黑色素不同级分的结构,旨在明确不同级分的色素纯度、精细结构信息、自由基特性和空间结构特征,推进对黑芝麻黑色素结构的认知,并通过DPPH、ABTS、FRAP和ORAC 4种方法评价黑芝麻黑色素不同级分的体外抗氧化活性,明确黑芝麻黑色素不同级分抗氧化活性的差异,为后期黑芝麻黑色素的深入科学研究和开发利用提供理论依据。1 材料与方法
试验于2019年1—7月在广东省农业科学院蚕业与农产品加工研究所/农业农村部功能食品重点实验室/广东省农产品加工重点实验室内进行。1.1 材料与仪器
1.1.1 试验材料 黑芝麻,2018年9月购自山东济南,品种为中芝33号。YOYOPEARL HW-40C尺寸排阻填料,日本东曹株式会社;DPPH(2,2-二苯基-1-苦基肼)、ABTS(2,2'-叠氮基-(3-乙基苯并噻唑啉-6-磺酸)、AAPH(2,2-偶氮二(2-甲基丙基咪)二盐酸盐)、Trolox(水溶性维生素E)、没食子酸(gallic acid,GA)、乌贼墨黑色素(sepia melanin,SM)、福林酚试剂均购自美国Sigma公司,FRAP试剂盒购自上海碧云天生物技术有限公司,其他试剂均为国产分析纯。
1.1.2 主要仪器 BS124S分析天平,赛多利斯科学仪器公司;FDU-2110真空冷冻干燥机,日本东京理化器械株式会社;EYELAN-1100旋转蒸发仪,东京理化器械株式会社;GZX-9420 MBE电热恒温鼓风干燥箱,上海博讯实业有限公司;UV-1800型紫外可见分光光度计,日本岛津有限公司;Agilent 1100型高效液相色谱仪(配备示差折光检测器),美国Agilent公司;Elementar Vario EL Cube元素分析仪,德国公司;JES-FA 200电子自旋共振波谱仪,日本东京理化器械株式会社;Axis Ultra DLD多功能X-射线光电子能谱仪,英国Kratos公司;锐影(Empyrean)X-射线粉末衍射仪,荷兰帕纳科公司;VERTEX70傅里叶变换红外光谱仪,德国公司;AVANCE AV 400型超导脉冲傅立叶变换核磁共振波谱仪,瑞士Bruker BioSpin公司;BioTek Gen5酶标仪,美国BioTek公司;TECAN Infinite 200酶标仪,瑞士Tecan公司。
1.2 黑芝麻黑色素原料的制备
1.2.1 黑芝麻黑色素粗提物的制备 黑芝麻黑色素的制备参考徐利萍[21]及康静静[22]的方法,稍作修改,取一定质量的黑芝麻皮,按料液比1 : 10(w/v)加入甲醇,常温下超声提取30 min,过滤,重复3次,所得黑芝麻皮于50℃烘箱中烘干。甲醇提取后的黑芝麻皮,按料液比1﹕40(w/v)加入30 g?L-1的氢氧化钠溶液,在60℃条件下水浴浸提90 min,过滤收集上清液,4 000 r/min离心5 min,合并上清液并抽滤,用6 mol·L-1 HCl调节pH=2,静置待其沉淀,离心弃上清,用水反复洗涤沉淀,分装,真空冷冻干燥后即得黑芝麻黑色素粗品。1.2.2 黑芝麻黑色素的分离 取一定量的黑芝麻黑色素粗品,用0.1 mol?L-1 NaOH溶液溶解后通过TOYOPEARL HW-40C尺寸排阻色谱柱分离,洗脱液为2%的乙醇水,分离得到两个级分,分别记为Fr1和Fr2,Fr1呈黑色,Fr2呈棕褐色。
1.3 测定指标及方法
1.3.1 黑芝麻黑色素不同级分色价的测定 精确称取不同级分黑色素0.01 g,加入少量0.1 mol?L-1 NaOH溶液使黑色素溶解,配成1 mg·mL-1的母液,再加入氢氧化钠溶液稀释一定的倍数,在λmax处测定其吸光值,色价计算公式如下:$E=\frac{A}{C}×\frac{1}{100}$
式中,E:试样液浓度为1%,用1 cm比色皿,在最大吸收波长λmax处测得吸光值;A:实际测得样品的吸光值;C:被测试样的浓度(g·mL-1)。
1.3.2 黑芝麻黑色素不同级分光谱定量测定 参考USACH等[31]的方法,稍作修改,将乌贼墨黑色素标准品配置成一系列不同浓度的溶液,分别测定不同浓度标准品溶液在470 nm下的吸光值,绘制标准曲线;将不同级分黑色素样品配制成一定浓度的溶液,进行吸光值测定,代入乌贼墨黑色素标曲计算黑色素含量,黑色素含量以每g不同级分黑色素(干重)中所含的乌贼墨黑色素当量(Sepia melanin equivalents/g dry weight)表示,简写为mg SME·g-1 DW。
1.3.3 黑芝麻黑色素不同级分的GPC测定 色谱条件:TSK-GEL G4000SW凝胶柱,TSK-GEL G3000SW凝胶柱;流动相为pH 6.8的磷酸盐缓冲溶液,流速0.8 mL·min-1;柱温40℃;进样10 μL;标样为已知分子量的葡聚糖标准品(Dextran,分子量为670 000、410 000、270 000、150 000、50 000、12 000、5 000和1 000 Da,Sigma公司)。
测定方法:将已知分子量的葡聚糖标准品用流动相分别配制成1.0 mg·mL-1的溶液,进样量为10 μL,记录色谱图,以标准葡聚糖的分子量对洗脱体积进行回归处理。将黑色素样品用流动相配制成一定浓度的溶液,进样10 μL,测得黑色素样品的保留时间,利用标准曲线求得黑色素样品分子量。
1.3.4 黑芝麻黑色素不同级分的紫外-可见光谱(UV-Vis) 按照1.3.1的方法将黑色素配制成一定的浓度,用UV-1800型紫外分光光度计扫描190–800 nm范围内不同波长下溶液的吸光值,绘制吸光值随波长变化的曲线图。
1.3.5 黑芝麻黑色素不同级分的元素分析(EA) 采用Elementar Vario EL III型元素分析仪测定黑芝麻黑色素不同级分的C、H、N、S含量,每个样品3个平行。
1.3.6 黑芝麻黑色素不同级分的傅里叶红外光谱(FT-IR) 将样品和溴化钾于105℃烘箱中干燥至衡重,除去样品中的游离水或结晶水,以消除水分子对吸收峰的干扰。分别称取2 mg样品置于研钵中,放入40 mg干燥的溴化钾粉末,在红外灯光附近研磨均匀,将粉末装入压片模具中抽真空压片成薄片。采用傅立叶红外光谱仪对样品进行扫描测定,扫描波长范围400—4 000 cm-1。
1.3.7 黑芝麻黑色素不同级分的核磁共振
1.3.7.1 核磁共振氢谱(1H-NMR) 1H共振频率为600.17 MHz,谱宽12 019.2 Hz,采样脉冲宽度为13.2 s,两次采样间隔时间为0,各样品采样次数为16次。样品溶于DMSO中。
1.3.7.2 固体核磁碳谱(13C-NMR) 固体核磁参考DUFF等[32]的方法,稍作修改,采用交叉极化/魔角旋转固体核磁共振。测试条件:共振频率为100.628 MHz,4 mm CP/MAS固体探头,场强9.4 T。
1.3.8 黑芝麻黑色素不同级分的XPS 参考XIAO等[33]的方法,稍作修改,采用XPS测定黑芝麻黑色素不同级分的表面化学成分。使用单色化的Al Kα X射线作为激发源(hν=1 486.60 eV),工作电压为15 kV,功率为150 W。对每个样品以160 eV的通能进行扫描(0—1 400 eV),然后以20 eV的通能进行Ti 2p、C 1s、N 1s和O 1s的高分辨率光谱扫描,根据灵敏度系数,从高分辨率光谱中得到各元素的原子百分比。将中性碳1s峰(C-C(H))设置为285.00 eV作为电荷校正的参考标准。
1.3.9 黑芝麻黑色素不同级分的EPR 参考YAO等[34]的方法稍作修改,取适量烘干后的黑色素样品于石英管中,插入样品孔,室温下进行波谱扫描。检测条件:工作频率为9.86 GHz(X波段),调制频率为100 kHz,调制幅度为1 G,微波功率为1.85 mW,扫描时间为20.48 s。中心归档:3 509.650 G,扫描宽度:50 G,静态场:3 484.65 G。
1.3.10 黑芝麻黑色素不同级分的X-射线衍射 将烘干后的黑色素样品压入直径为1 cm、厚度为1 mm的圆片中。使用锐影X射线衍射仪对样品进行扫描。检测条件:Cu靶Kα射线,电压40 kV,电流40 mA,发散射狭缝1/8°,防散射狭缝1/4°,发散射狭缝7.5 mm,2θ范围:3°—80°,步长0.02°,每步停留时间40 s。
参考CASADEVALL等[35]的方法,将散射强度作为散射角的函数,数据由角度坐标转换为Q标度,Q是X射线光子通过角度θ弹性散射时动量变化的大小。参数Q与散射角θ的关系式为:Q=(4πsinθ)/λ,λ为X-射线光子波长(λ=1.54 A)。将Q峰最大值与分子层之间的距离R联系起来,其中R=2π/Q,R代表距离的平均值,与Q空间中堆叠特征的宽度有关。
1.3.11 黑芝麻黑色素不同级分的体外抗氧化活性测定
1.3.11.1 DPPH自由基清除能力的测定 黑芝麻黑色素不同级分的DPPH自由基清除能力参考TU等[14]和P?O-LEóN等[36]的方法,并稍作修改,称取3.94 mg DPPH溶解于100 mL 95%的乙醇中配成0.1 mmol·L-1的DPPH工作液。将不同级分的黑色素配成不同浓度梯度的溶液,测定他们对DPPH自由基的清除能力。准确吸取1.0 mL不同浓度的样品溶液与现配制的2.0 mL DPPH工作液充分混合,室温避光反应30 min,测定其在517 nm吸光度A1,同时测定2 mL 95%乙醇溶液与1 mL样品溶液的吸光度A2以及2 mL DPPH工作液与1 mL样品溶剂的吸光度A0。每个样品每个浓度重复3次,取平均值,计算DPPH自由基清除率,同时求得清除率为50%时所需样品的浓度,即IC50值。按下列公式计算DPPH自由基清除率:
$清除率(\%)=[1-\frac{ A_1- A_2}{A_0}]×100$
式中,A0:空白对照,表示2 mL DPPH工作液与1 mL样品溶剂反应后的吸光值;A1:样品组,表示2 mL DPPH工作液与1 mL样品反应后的吸光值;A2:样品空白,表示2 mL 95%乙醇与1 mL样品反应后的吸光值。
1.3.11.2 ABTS自由基清除能力的测定 黑芝麻黑色素不同级分的ABTS自由基清除能力参考P?O-LEóN等[36]的方法,稍作修改,ABTS储备液的配制:称取66.23 mg过硫酸钾溶解于100 mL蒸馏水中配制成浓度为2.45 mmol·L-1的过硫酸钾溶液,再称取19.20 mg ABTS溶解于5 mL过硫酸钾溶液中配制成浓度为7 mmol·L-1的ABTS储备液,在室温、避光条件下放置13—16 h。ABTS待测液的配制:将ABTS储备液在波长λ=734 nm处以10 mmol·L-1、pH 8的磷酸盐缓冲液稀释至吸光度为0.70±0.05。准确吸取1 mL不同浓度梯度的黑色素样品溶液与4 mL ABTS待测液混合,涡旋震荡30 s,避光反应6 min,在734 nm波长处测定吸光度A1,同时测定4 mL样品溶剂与1 mL样品的吸光度A2以及4 mL ABTS待测液与1 mL样品溶剂的吸光度A0。每个样品每个浓度重复3次,取平均值,计算ABTS自由基清除率,同时求得清除率为50%时所需样品浓度,即IC50值。按下列公式计算ABTS自由基清除率:
$清除率(\%)=[1-\frac{ A_1- A_2}{A_0}]×100$
式中,A0:空白对照,表示4 mL ABTS待测液与1 mL样品溶剂反应后的吸光值;A1:样品组,表示4 mL ABTS待测液与1 mL样品溶液反应后的吸光值;A2:样品空白,表示4 mL样品溶剂与1 mL样品溶液反应后的吸光值。
1.3.11.3 铁离子还原力(FRAP)的测定 参照董丽红等[37]的方法,具体步骤参考FRAP测定试剂盒说明书。向96孔板的每个检测孔中加入现配的180 μL FRAP工作液。空白对照孔中加入5 μL蒸馏水,标准曲线检测孔内加入5 μL各种浓度的FeSO4-7H2O标准溶液。样品检测孔内加入5 μL待测样品溶液,37℃孵育1 min后,于593 nm下测定吸光值。FRAP结果以每g不同级分黑色素(干重)中所含的FeSO4-7H2O当量(FeSO4-7H2O equivalents/g dry weight)表示,简写为mmol FeE·g-1 DW。
1.3.11.4 ORAC抗氧化能力的测定 参考ZHANG等[38]的方法,并稍作修改。Trolox标准品及各种待测样品用75 mmol·L-1 pH 7.4磷酸盐缓冲液稀释到适当浓度,反应用黑色96孔板,除样品孔外,另设空白孔、对照孔、不同浓度的标准溶液孔(Trolox)和阳性对照孔(没食子酸),各处理均设3个复孔。先向空白孔加入20 μL磷酸盐缓冲液,其余孔分别加入样品溶液、不同浓度的Trolox标准溶液(6.25、12.5、25和50 μmol·L-1)以及17.5 μmol·L-1的没食子酸溶液,将96孔板放入提前调节温度至37℃的酶标仪中孵育10 min;然后每孔加入200 μL 0.96 μmol·L-1荧光素钠工作液,继续孵育20 min后,除对照孔外,每孔再加入20 μL新鲜配置的119 mmol·L-1 AAPH溶液。最后,将96孔板立即放入酶标仪中,在激发波长485 nm、发射波长520 nm下测定各孔荧光值,每4.5 min测定一次,共35个循环。计算各孔荧光强度曲线下的面积(AUG),减掉空白孔的AUG,即得到各孔的Net AUG,根据不同浓度Trolox的Net AUG做标准曲线,计算各样品的ORAC值。ORAC结果以每g不同级分黑色素样品(干重)中所含的μmol Trolox当量(Trolox equivalents/g dry weight)表示,简写为μmol TE·g-1 DW。
1.3.12 数据分析 采用Origin9.0软件和SPSS19.0软件进行数据分析及作图。试验结果用均数±标准差(Mean±SD)表示,每组试验重复3次。
2 结果
2.1 黑芝麻黑色素不同级分得率及色价
如表1所示,黑芝麻皮经碱提酸沉得到黑芝麻黑色素粗提物,粗黑色素经过HW-40C尺寸排阻色谱柱分离后得到Fr1(黑色)和Fr2(棕褐色)两个级分,得率分别为60%和24%,可知Fr1为黑色素的主要级分。从色价测定结果来看,Fr1的色价低于粗黑色素,Fr2的色价高于粗黑色素,表明Fr2的纯度高于Fr1。Table 1
表1
表1黑芝麻黑色素不同级分的得率及色价
Table 1
| 样品 Sample | 得率 Yield (%) | 色价 Color value (U·g-1) |
|---|---|---|
| 粗黑色素 Crude melanin | -- | 180.54±8.85b |
| Fr1 | 60.97±4.79b | 155.55±6.22a |
| Fr2 | 24.26±2.64a | 183.65±4.98b |
新窗口打开|下载CSV
2.2 黑芝麻黑色素不同级分光谱定量测定结果
以乌贼墨黑色素为标准品,对黑芝麻黑色素不同级分采用吸收光谱法进行测定,由表2可知,Fr1的含量为782.16 mg SME·g-1 DW,Fr2的含量为884.66 mg SME·g-1 DW,通过吸收光谱法测定的黑芝麻黑色素两个级分的纯度为Fr2较Fr1高。Table 2
表2
表2黑色素不同级分光谱定量结果
Table 2
| 样品 Sample | 乌贼墨黑色素当量 Sepia melanin equivalent (mg SME·g-1 DW) |
|---|---|
| 粗黑色素 Crude melanin | 578.18±1.15a |
| Fr1 | 782.16±3.33b |
| Fr2 | 884.66±2.93c |
新窗口打开|下载CSV
2.3 黑芝麻黑色素不同级分GPC结果
以标准葡聚糖的分子量对洗脱体积进行回归得到葡聚糖Dextran系列标准品的标准曲线方程,并由标准曲线求得Fr1和Fr2的重均分子量Mw分别为38 800和6 000 Da,数均分子量Mn分别为18 200和3 600 Da,分子量分布宽度分别为2.13和1.67(表3)。2.4 黑芝麻黑色素不同级分紫外可见吸收光谱
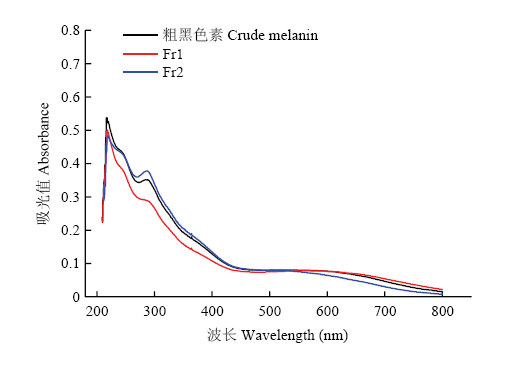
黑芝麻黑色素的两个级分与分离前粗黑色素的紫外吸收光谱如图1所示,Fr1和Fr2的紫外可见吸收光谱均为典型的黑色素吸收光谱,它在紫外区吸收强烈,随着波长的增加,吸收逐渐减弱,两个级分在210 nm左右均有一个明显的吸收峰,在270—280 nm处有一小的吸收峰,在可见光范围内没有最大吸收峰,且在270—280 nm处Fr1的吸收低于粗黑色素与Fr2。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1黑芝麻黑色素不同级分紫外可见吸收光谱
Fig. 1UV-visible absorption spectrum of different fractions of black sesame melanin
2.5 黑芝麻黑色素不同级分元素分析结果
黑芝麻黑色素不同级分的元素分析结果如表4所示,Fr1的C、N、H、S元素含量均高于Fr2,Fr1的N/C和H/C高于Fr2。Fr1的C、H、S元素含量与粗黑色素相当,但N元素含量高于粗黑色素,Fr2的C、H、N、S元素含量低于粗黑色素。Fr1、Fr2及粗黑色素的S元素与其他元素相比,含量很低,可忽略不计。Table 3
表3
表3黑色素不同级分GPC结果
Table 3
| 样品 Sample | 数均分子量 (Mn) Number average molecular weight (Da) | 重均分子量(Mw) Weight average molecular weight (Da) | 分布宽度(D) Wide distribution |
|---|---|---|---|
| Fr1 | 1.82×104 | 3.88×104 | 2.13 |
| Fr2 | 3.60×103 | 6.00×103 | 1.67 |
新窗口打开|下载CSV
Table 4
表4
表4黑芝麻黑色素不同级分元素分析
Table 4
| 样品 Sample | 元素组成 Elemental composition | 元素比例 Element ratio | |||||
|---|---|---|---|---|---|---|---|
| C% | N% | H% | S% | N/C | H/C | ||
| 粗黑色素 Crude melanin | 47.6525 | 3.6625 | 5.0225 | 0.2365 | 0.0769 | 0.1054 | |
| Fr1 | 46.1900 | 7.7750 | 6.0300 | 0.2080 | 0.1683 | 0.1305 | |
| Fr2 | 30.4900 | 1.1600 | 3.3400 | 0 | 0.0380 | 0.1095 | |
新窗口打开|下载CSV
2.6 黑芝麻黑色素不同级分的FT-IR
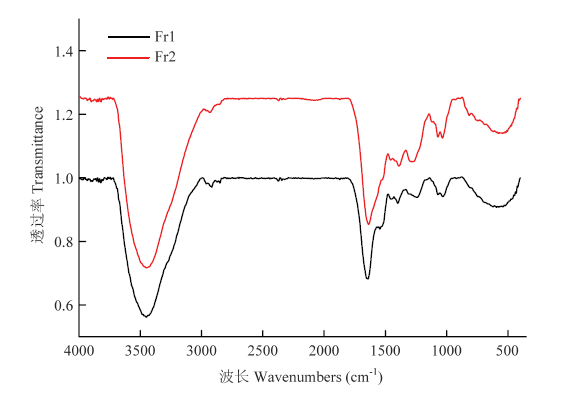
黑芝麻黑色素不同级分的红外光谱如图2所示,与文献报道[17,34,39]的乌贼墨、栗子壳及合成黑色素等的红外光谱相似,Fr1和Fr2在3 500—3 300 cm-1处均有一较宽的吸收峰,且吸收较强,该处为-OH、-NH2的伸缩振动;两个级分在2 900—2 800 cm-1处有两个较弱的吸收峰,属于脂肪族中饱和烃基C-H的伸缩振动;在1 650 cm-1处的吸收峰为芳香族C=C键的拉伸与-COOH的对称拉伸振动,1 450 cm-1处的吸收是脂肪族C-H变形引起,1 400 cm-1处的吸收峰是酚羟基的-OH变形和C-O伸缩以及COO-基团的对称伸展引起,但在1 650、1 450和1 400 cm-1处Fr2较Fr1的吸收变得更强,Fr1在1 540 cm-1左右有较弱吸收峰,Fr2在1 540 cm-1吸收峰消失,可能与N-H变形和C-N拉伸有关;在1 250 cm-1处的吸收峰是由于-COOH中C=O的拉伸和O-H的变形以及酚的C-O键的拉伸,1 070和1 030 cm-1处均有吸收峰,属于苯环上的C-H键弯曲振动引起,Fr2较Fr1的吸收变得更强;900—700 cm-1间有许多较弱的吸收峰,是芳环和芳杂环上氢的弯曲振动引起,700 cm-1以下是脂肪碳上氢的振动吸收峰。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2黑芝麻黑色素不同级分FT-IR图谱
Fig. 2FT-IR spectrum of different fractions of black sesame melanin
2.7 黑芝麻黑色素不同级分的核磁共振
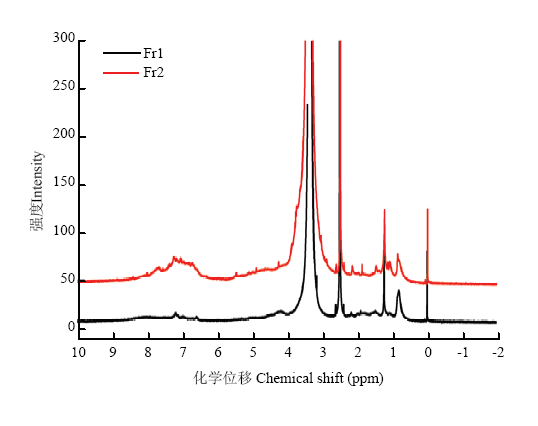
2.7.1 核磁共振氢谱(1H-NMR) 黑芝麻黑色素不同级分的核磁共振氢谱如图3所示,Fr1与Fr2的核磁共振氢谱脂肪区谱峰较为接近,芳香区谱峰有明显区别。高场区δ 0.82处吸收峰为脂肪烃末端甲基的特征峰;δ 1.23为中心的吸收峰主要是直链或支链烷烃基团中的亚甲基峰;δ 2.50处的峰为DMSO溶剂峰;δ 3.37处的谱峰是水在氘代二甲基亚砜中的残留吸收,化学位移在3.00—5.00 ppm的谱峰为与电负性较强的基团相连碳上的氢,例如连氧碳和连氮碳上的质子,两个级分在此区间内都有较弱的吸收峰;低场区δ 6.00—8.40区域内出现较弱的宽峰,属于芳香氢的吸收峰,部分活泼氢也在此区域内出现。芳香氢化学位移跨度大,说明各种芳香氢所处的化学环境差别较大,这些谱峰可能是芳环或芳杂环上的质子。从图谱峰面积区域可以看出,芳香氢和脂肪氢的比例为Fr1小于Fr2,表明Fr1中芳香结构单元少,以饱和结构单元为主,或者Fr1结构中芳环取代基较多。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3黑芝麻黑色素不同级分的1H-NMR谱图
Fig. 31H-NMR spectrum of different fractions of black sesame melanin
2.7.2 固体核磁(CP/MAS 13C-NMR) 黑芝麻黑色素不同级分的核磁共振碳谱如图4所示,在脂肪区(10—95 ppm)、芳香区(95—165 ppm)和羰基官能团区(165—220 ppm)都有谱峰出现。表明黑芝麻黑色素两个级分中除了含有芳香聚合物的骨架外,还连接有其他结构片段如脂肪酸、氨基酸和蛋白质等。化学位移在10—40 ppm的谱峰可能是脂肪链上的甲基、亚甲基或者次甲基。化学位移在55 ppm的谱峰可能是连氮的饱和碳或者甲氧基,化学位移在73 ppm处的谱峰是连氧的饱和碳。化学位移在100—140 ppm的谱峰是苯环或者双键上的不饱和碳,140—165 ppm为连接氧、氮等杂原子的芳香不饱和碳的吸收峰。165—190 ppm的谱峰为羧酸、羧酸酯、酰胺及醌式结构中的羰基吸收峰。Fr1中的羰基区域吸收峰较强,芳香碳区域(95—165 ppm)吸收峰面积小于脂肪碳区域吸收面积(10—95 ppm),表明Fr1结构中可能含有较多的饱和结构单元,可能是脂肪酸。Fr2的羰基区域吸收峰相对较小,芳香碳区域吸收峰面积与脂肪碳区域相当,表明结构中不饱和结构占比较高。两个级分相比,Fr1中羰基和饱和碳结构单元含量较高,可能是结构中有较多脂肪酸和蛋白质取代,而Fr2芳香结构单元(芳环、双键)占比较高。
图4
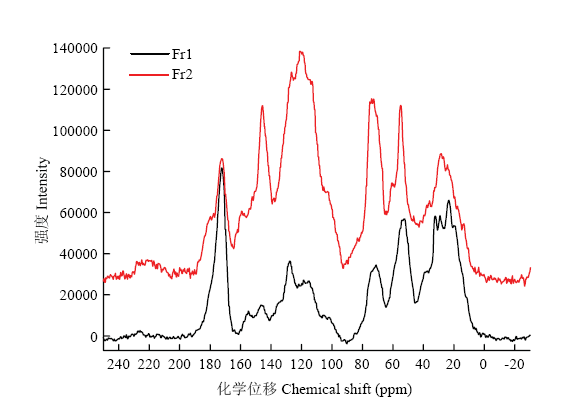 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4黑芝麻黑色素不同级分的CP/MAS 13C-NMR谱图
Fig. 4CP/MAS 13C-NMR spectrum of different fractions of black sesame melanin
2.8 黑芝麻黑色素不同级分的XPS
为了确定黑色素不同级分的官能团百分比,将C1s、O1s、N1s的高分辨率光谱曲线拟合如图5。将C1s扫描得到的原始数据中最大的峰移至285.00 eV对整个光谱进行校正,进行峰的拟合,C1s图谱有5个谱峰,分别为285.00 eV的C-C(H)、286.40 eV的C-OH/C-N、288.00 eV的C=O、289.00 eV的O-C=O和π→π键。N1s图谱有3个谱峰,分别对应400.30 eV的-C-NH、399.50 eV的芳香N及402.00 eV的C-NH3+ 3个峰。O1s图谱在531.50 eV和533.00 eV处有两个主要谱峰,分别来自C=O和C-OH。图5
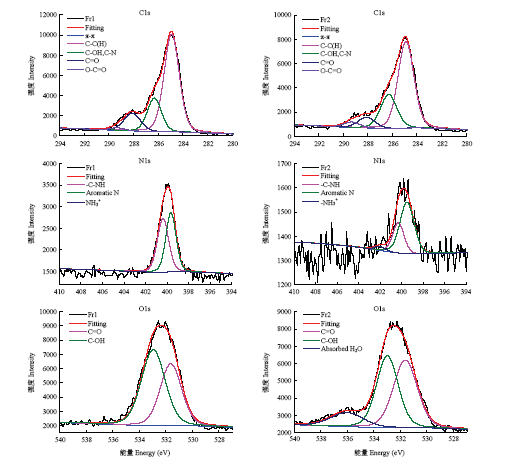 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5黑芝麻黑色素不同级分的C1s、N1s和O1s的XPS高分辨率光谱图
Fig. 5High-resolution spectrum of C1s, N1s and O1s of different fractions of black sesame melanin
不同级分官能团的比例如图6所示,C、N、O 3个元素分峰结果表明,Fr1和Fr2两个级分官能团的含量不同,在C1s谱中,Fr1的C-C(H)和C=O官能团的含量高于Fr2,而Fr1的C-OH/C-N和O-C=O官能团的含量低于Fr2,Fr1的C=O和O-C=O之间比例为7.90,Fr2的C=O和O-C=O之间比例为2.10;在N1s谱中,Fr1的C-NH官能团含量高于Fr2,而Fr1的芳香N含量低于Fr2,可能是Fr2比Fr1含有更多的杂环结构单元,且Fr1中不含质子化的C-NH3+;在O1s谱中,Fr1的C-OH官能团的含量高于Fr2,但Fr1的C=O官能团含量略低于Fr2,且Fr1中不含吸收的H2O。
图6
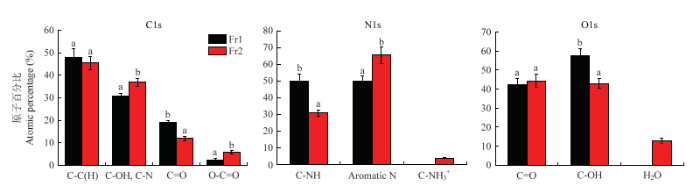 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6黑芝麻黑色素不同级分的官能团相对百分比
同一种官能团中,不同字母表示差异显著(P<0.05) Values not sharing a common letter are significantly different (P<0.05)
Fig. 6Relative percentage of different functional groups of different fractions of black sesame melanin
2.9 黑芝麻黑色素不同级分的EPR
黑色素因含有自由基而具有顺磁共振特性,在EPR光谱中g值表示光谱分裂因子,线宽表示磁共振弛豫的强弱,通常用一次微分曲线上两极值之间的距离表示(以G为单位),称“峰对峰宽度”,记作ΔHpp。由图7可知黑芝麻黑色素分离后的两个级分均显示出强烈的共振吸收,由单个对称线组成,没有超精细分裂,Fr1较Fr2显示出较强的共振吸收,类似于不同来源的其他合成黑色素和天然黑色素的EPR光谱。Fr1和Fr2的g值分别为2.0078和2.0085,Fr1和Fr2的ΔHpp分别为0.7430和0.6950 mT。图7
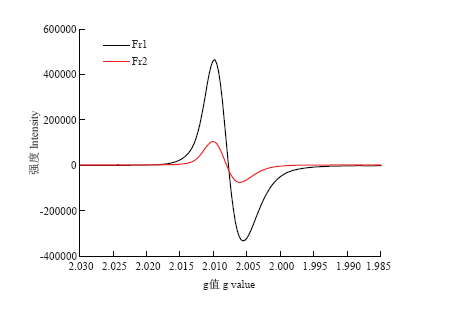 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7黑芝麻黑色素不同级分的EPR光谱
Fig. 7EPR spectrum of different fractions of black sesame melanin
2.10 黑芝麻黑色素不同级分的X-射线衍射
黑芝麻黑色素不同级分的X-射线衍射图谱如图8所示,由图可知Fr1和Fr2的X-射线衍射图谱有明显区别,Fr1的X-射线衍射图谱中出现了弥散的较弱的“隆峰”,而Fr2的X-衍射图谱中未发现“隆峰”,Fr1在2θ为22.52°处有较为突出的“隆峰”,由Q=(4πsinθ)/λ,Q值为1.59,由R=2π/Q,R值为3.94 ?。图8
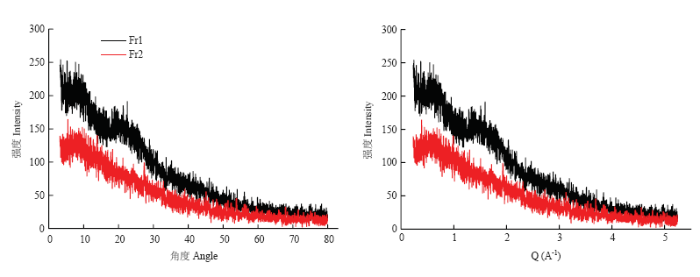 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8黑芝麻黑色素不同级分的X-射线衍射图谱
Fig. 8X-ray diffraction pattern of different fractions of black sesame melanin
2.11 黑芝麻黑色素不同级分的体外抗氧化活性
采用DPPH、ABTS、FRAP和ORAC四种方法评价黑芝麻黑色素不同级分的体外抗氧化活性,结果如表5所示,粗黑色素与Fr1和Fr2都具有DPPH和ABTS自由基清除能力,两种自由基清除率IC50值大小依次为Fr1>Fr2>粗黑色素>Vc,说明Fr1的DPPH和ABTS自由基清除能力弱于Fr2,且均弱于粗黑色素与天然抗氧化剂Vc。Fr1的FRAP值与粗黑色素差异不显著,Fr2的FRAP值显著高于Fr1与粗黑色素,说明Fr2的FRAP抗氧化能力较强。ORAC值大小依次为:Vc>Fr2>粗黑色素>Fr1,说明Fr2的ORAC抗氧化能力强于Fr1。以上结果表明,Fr2的DPPH和ABTS自由基清除能力、FRAP和ORAC抗氧化能力均高于Fr1,说明Fr2的体外抗氧化活性较Fr1好。Table 5
表5
表5黑芝麻黑色素不同级分的体外抗氧化活性
Table 5
| 样品 Sample | DPPH (IC50 μg·mL-1) | ABTS (IC50 μg·mL-1) | FRAP (mmol FeE·g-1 DW) | ORAC (μmol TE·g-1 DW) |
|---|---|---|---|---|
| Vc | 13.00±1.04a | 12.00±1.12a | 13.27±0.25c | 9 091.75±347.74d |
| 粗黑色素 Crude melanin | 45.00±1.40b | 23.00±2.60b | 1.22±0.09a | 3 730.19±191.38b |
| Fr1 | 83.00±2.14d | 53.00±1.41d | 1.05±0.15a | 3 141.80±195.74a |
| Fr2 | 54.00±2.13c | 30.00±1.04c | 1.62±0.05b | 4 143.76±99.96c |
新窗口打开|下载CSV
3 讨论
3.1 黑芝麻黑色素不同级分结构表征
黑芝麻黑色素经分离得到了黑色的Fr1和棕褐色的Fr2,两个级分的紫外可见吸收光谱与刘元法等[26]报道的黑芝麻黑色素的吸收特征一致,其他天然黑色素如鱿鱼墨、乌骨鸡黑色素也表现出同样的紫外吸收特征[14,40-41]。TU等[14]认为天然黑色素的紫外吸收光谱在270—280 nm处的平台吸收峰可能是因为与天然黑色素结合的蛋白质引起的,因此,可以推测本研究分离得到的两个黑芝麻黑色素级分中可能都含有蛋白质。由元素分析结果可知,Fr1含有C、H、N、S四种元素,Fr2含有C、H、N而不含S元素,ITO等[42]报道真黑色素N元素含量为6%—9%,不含S元素(0—1%);棕黑色素N元素含量为8%—11%,S元素含量为9%—12%;KAMEI等[7,43]报道异黑色素含有极少量的氮甚至不含氮,仅存在于高等植物和真菌中,属于酚类与蛋白质结合的聚合物。根据Fr1和Fr2的N元素和S元素含量可知,黑芝麻黑色素的两个级分均不是棕黑色素,Fr1可能为真黑色素,Fr2可能为异黑色素,与陆懋荪等[28]的元素分析结果基本一致。
黑芝麻黑色素的两个级分在3 500—3 300 cm-1、1 650 cm-1、500 cm-1附近均有较强的吸收峰,由此推测Fr1和Fr2中含有-OH、-NH2、-COOH、C=O等官能团,与陆懋荪等[28]的红外结果基本一致。与鱿鱼墨黑色素[40]相比,Fr1和Fr2在2 900—2 800 cm-1处存在较弱的吸收峰,在1 070 cm-1及1 030 cm-1处有吸收峰,而鱿鱼墨黑色素在该处均无吸收峰,可能是黑芝麻黑色素的两个级分含有较多的饱和结构单元,且芳香环上含有较少的取代物。
核磁共振氢谱结果表明,Fr2和Fr1在芳香区和脂肪区均有吸收,但Fr2比Fr1芳香区谱峰多,表明Fr2结构中可能含有更多的芳香结构单元,而Fr1结构中芳香骨架上有较多的饱和单元(脂肪酸、氨基酸和蛋白质)等取代。陆懋荪等[28]的核磁氢谱结果表明芝黑素I的芳香性低于芝黑素II,与本研究结果一致,而本研究中,Fr1和Fr2在δ 0—2 ppm处的吸收峰强度较陆懋荪的弱了许多,该处属于脂肪链烷烃的吸收区域,可能是Fr1和Fr2中脂肪酸的含量较少。KATRITZKY等[44]用核磁共振氢谱对乌贼墨黑色素进行了结构表征,乌贼墨黑色素氢谱中芳香区信号呈现精细裂分特征,而黑芝麻黑色素两个级分氢谱芳香区均是簇峰,究其原因是乌贼墨黑色素构成单元较为单一,以5,6-二羟基吲哚(DHI)和5,6-二羟基吲哚二羧酸(DHICA)为主;而黑芝麻黑色素的构成单元种类较多,连接方式多样,因而呈现典型的杂聚物核磁共振氢谱特征。本研究中Fr1中羰基和脂肪碳结构单元含量较Fr2高,但芳香区碳信号明显弱于Fr2,表明Fr1结构中可能有较多脂肪酸和蛋白质取代,而Fr2芳香结构单元(芳环、双键)占比较Fr1高。ADHYARU等[45]研究发现乌贼墨黑色素的碳谱在脂肪区(10—95 ppm)、芳香区(95—165 ppm)和羰基官能团区(165—220 ppm)都有谱峰出现,与黑芝麻黑色素两个级分的固体核磁类似。乌贼墨黑色素中连接杂原子的芳香碳信号(140—160 ppm)较黑芝麻黑色素的两个级分强,而黑芝麻黑色素两个级分在100—130 ppm处碳信号较强,表明乌贼墨黑色素芳香结构中有更多氧、氮等杂原子取代,而黑芝麻黑色素的两个级分结构中芳香碳上氢原子和碳原子取代较多。
高分辨率XPS为黑色素化学结构表征的有力工具[33,46],本研究首次采用XPS高分辨率光谱对黑芝麻黑色素的两个级分进行研究。由C1s分峰结果可知,Fr1结构中含有较多的酮羰基,而Fr2结构中含有更多的羧羰基,Fr1的羰基含量多于Fr2,与核磁共振碳谱结果一致。Fr1的C=O和O-C=O之间比例为7.9,Fr2的C=O和O-C=O之间比例为2.1,与XIAO等[33]报道的乌贼墨(7.0)和乌鸦(1.9)的结果类似,表明Fr2较Fr1含有更多的单体及其氧化物。N1s谱中,Fr1的C-NH官能团的含量高于Fr2,而Fr1的芳香氮的含量低于Fr2,且Fr1中不含质子化的C-NH3+,说明Fr2中的氮多是以芳香氮的形式存在,表明结构中可能存在更多的吲哚结构单元,Fr1中的氮多是脂肪氮,可能是一些蛋白质上的氨基。在O1s谱中,Fr1的C-OH官能团的含量高于Fr2,但Fr1的C=O官能团含量略低于Fr2,且Fr1中不含吸收的H2O,说明Fr1中的羟基含量高,结合核磁共振碳谱(60—90 ppm吸收峰较高)推测其可能更多的以醇羟基的形式存在。
黑芝麻黑色素的两个级分均显示出较强的共振吸收,与鱿鱼墨黑色素的EPR光谱类似。文献报道鱿鱼墨黑色素g值为2.0037,ΔHpp为0.5090 mT,Fr1和Fr2的g值分别为2.0078和2.0085,均略高于文献报道[34,47]的黑色素g值,这是由黑芝麻黑色素的结构决定的,Fr1和Fr2的ΔHpp分别为0.7430和0.6950 mT,均高于鱿鱼墨黑色素的ΔHpp,在0.4—0.8 mT天然黑色素范围内[34]。以上说明黑芝麻黑色素不同级分与鱿鱼墨黑色素具有相似的自由基性质。
晶体在X-射线衍射光谱中通常产生尖锐的峰,非晶态化合物如黑色素和多糖在衍射光谱中产生广泛的特征,称为非布拉格特征,这是由于无规则和重复结构的相干散射造成的。研究报道[35,48-50]黑色素都有一个基本的衍射单元,该单元类似平面石墨烯结构,在堆积距离上有所不同,堆积峰是黑色素的一个普遍特征。Fr1的X-射线衍射图谱中出现了弥散的较弱的“隆峰”,Q值为1.59,R值为3.94 ?,Fr2中并未出现类似的“隆峰”,表明二者空间结构不同。Fr1结构中存在平面共轭结构的堆叠,而Fr2中堆叠结构较少或者不存在。研究报道[35,48-49]乌贼墨黑色素的Q值为1.60,R值为3.46 ?,黑曲霉黑色素的Q值为1.41,R值为4.45 ?,鸡腿菇黑色素的Q值为1.53,R值为4.10 ?,由此发现不同的黑色素Q值与层堆叠距离会有所不同,本研究中Fr1与乌贼墨黑色素的Q值与R值最为接近。
3.2 黑芝麻黑色素不同级分体外抗氧化活性分析
本研究采用DPPH、ABTS、FRAP、ORAC四种方法评价黑芝麻黑色素不同级分的抗氧化活性, 其中,DPPH、ABTS、FRAP三种抗氧化均属于单电子转移(ET)机制,是体外评价活性物质抗氧化能力的常见方法,操作简单[51,52];而ORAC抗氧化属于经典的氢原子转移(HAT)机制,是目前国际上常用的一种评价食品抗氧化能力的方法,较一般的自由基清除法更准确灵敏[52,53]。单良等[5]研究表明黑芝麻黑色素具有清除DPPH·、O2-·、·OH等自由基的能力,黑色素的这种特性与其结构中含有的酚羟基和醌式结构有关。JACOBSON等[54]研究表明黑色素的抗氧化功能可能与其结构中含有的类似醌和对苯二酚的残基结构有关。金春英等[55]研究得出具有多酚羟基或易氧化成醌式共轭结构的化合物对自由基有显著的清除作用。以上研究均表明酚羟基和醌式结构与抗氧化活性密切相关。本研究4种抗氧化方法结果都表明Fr2的抗氧化活性较Fr1好,这与两个级分的结构密切相关。核磁共振碳谱表明Fr2含有更多的芳香结构,也意味着Fr2可能具有更多的酚羟基和醌式羰基,而Fr1结构中饱和结构单元占比大,说明脂肪酸和蛋白质取代较多,这些结构往往不具备清除自由基能力,导致Fr1的抗氧化活性不如Fr2。VATE等[56]研究发现鱿鱼墨黑色素具有DPPH、ABTS自由基清除能力与铁离子还原(FRAP)能力,抗氧化能力依次为(179.60±2.10)μmol TE·g-1蛋白质、(957.80 ±89.30)μmol TE·g-1蛋白质、(171.10±7.30)μmol TE·g-1蛋白质;TU等[14]研究得出乌鸡黑色素和合成黑色素的DPPH自由基清除率的IC50值分别为(37.30± 2.62)μg·mL-1和(49.20±2.48)μg·mL-1,均高于Fr1和Fr2的IC50值,低于天然抗氧化剂Vc的IC50值((13.00±1.04)μg·mL-1),说明Fr1与Fr2的DPPH自由基清除能力低于乌鸡黑色素。
本研究通过分离纯化结合多种波谱学手段对黑芝麻黑色素的结构特征、自由基特性和空间结构进行了表征,在前人研究基础上推进了对黑芝麻黑色素的认知。目前黑芝麻黑色素不同级分的构成单元及连接方式仍未明确,后续研究应尝试采用合适的降解手段和更加先进的波谱学表征技术(如傅立叶变换离子回旋共振质谱)对其结构进行研究,明确黑芝麻黑色素的精细结构。此外,本研究通过体外抗氧化手段明确了黑芝麻黑色素的抗氧化活性,其体内抗氧化作用有待后续研究,将通过动物试验进一步证实。
4 结论
本研究采用HW-40C尺寸排阻色谱柱对黑芝麻黑色素进行分离纯化,得到Fr1和Fr2两个级分,分子量分别为38 800 Da和6 000 Da,且Fr2的纯度较Fr1高。利用多种波谱学手段对两个级分的结构进行表征并评价它们的抗氧化活性,结果表明Fr1为黑芝麻黑色素的主要级分;结构分析表明Fr1主要是真黑色素,Fr2可能是异黑色素。Fr1和Fr2结构中均含有羰基、羟基、氨基、芳环和氮杂环等官能团,Fr2结构芳香性较Fr1高;Fr2的DDPH和ABTS自由基清除能力、FRAP和ORAC抗氧化能力均高于Fr1。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.foodchem.2004.05.051URL [本文引用: 2]
[本文引用: 3]
[本文引用: 3]
DOI:10.1021/jf2053096URL [本文引用: 3]

An improved purification procedure leading to black sesame (Sesamum Indicum L.) pigment was developed involving fat removal by treatment of ground black sesame seeds with dichloromethane followed by an optimized hydrolytic protocol with 6 M HCl, at 100 degrees C, overnight. The black pigment thus obtained displayed good antioxidant efficiency by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical assay (82% reduction at 0.5 mg/mL), good ferric ion-reducing capacity (61 mu M Trolox equivalent concentration at 0.5 mg/mL), and potent antinitrosating properties (74% inhibition of 2,3-diaminonaphthalene (DAN) nitrosation at gastric pH at 2.5 mg/mL). A synthetic pigment obtained by oxidative polymerization of coniferyl alcohol (polyconiferyl alcohol, PCA), the putative biosynthetic precursor to the sesame pigment, was characterized as a reference standard. FT IR spectra of the purified sesame pigment and PCA supported the structural similarity. HPLC analysis of degradation products by alkaline hydrogen peroxide of purified black sesame pigment showed the formation of vanillic acid (VA) as the main isolable fragment. Similar yields of VA were obtained by degradation of PCA. A positive correlation between VA yields and DPPH activity was determined in samples of different purities. It is suggested that VA is a structural marker of black sesame pigment, confirming the biosynthetic origin from coniferyl alcohol and pointing to the o-methoxyphenol motif as the key factor accounting for the potent antioxidant properties of the pigment.
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
DOI:10.3390/molecules23010001URL [本文引用: 2]
DOI:10.1016/j.phytochem.2016.07.002URLPMID:27427433 [本文引用: 3]

The brown to black coloration found in plants is due to the melanins, which have been relatively poorly investigated among the plant pigments. The aim of this work was to study the dark pigment extracted from the black oat hull with respect to composition and structure. Ultraviolet-visible (UV-Vis) spectroscopy, electron paramagnetic resonance (EPR) spectroscopy, matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) and Fourier transform infrared (FT-IR) spectroscopy were applied for the characterization of the pigment. UV-Vis spectroscopy revealed that the extracted material displays a broadband, structureless absorption profile a common feature of melanins. MALDI-TOF MS measurements demonstrated that oat melanin is a homopolymer built up from p-coumaric acid and consists mainly of low molecular weight (527-1499 Da) oligomers of 3-9 monomer units. The tetramer oligomer proved to be dominant. The results of the FT-IR analysis indicated that oat melanin is a fully conjugated aromatic system containing tetrasubstituted aromatic rings linked by CC coupling. The in vitro preparation of melanin from p-coumaric acid by horseradish peroxidase was performed for comparison. The resulting polymer consisted of oligomers of 4-9 monomer units similarly to those in oat melanin. However, the building blocks proved to be connected to each other via COC linkages in contrast with the CC linkages in oat melanin.
[本文引用: 1]
DOI:10.1016/j.lwt.2005.04.001URL [本文引用: 1]
DOI:10.1562/2005-03-02-RA-453URLPMID:15790301 [本文引用: 1]

Bovine iris and choroid melanosomes at two ages (<1 week and >2 years) were examined by inductively coupled plasma mass spectrometry (ICP-MS), elemental analysis, infrared spectrometry (IR) and X-ray photoelectron spectrometry (XPS). When iris and choroid melanosomes at the same age were compared, the quantification of metal elements by ICP-MS revealed that choroid melanosomes had a higher binding capacity for the carboxylate-binding metal ions (e.g. Na+ K+, Mg2+, Ca2+ and Zn2+). Elemental analysis showed a higher O:N ratio in choroid melanosomes. Both observations suggested that choroid melanosomes have a higher content of carboxylate-containing monomer than iris melanosomes. IR spectrometric analysis showed a red shift (approximately 8 cm(-1)) of the absorption peak of aromatic C=C, C=N and C=O at approximately 1630 cm(-1) in the IR spectrum of iris melanosomes relative to choroid melanosomes. Increased conjugation in the molecular structure of the pigment is proposed to contribute to this peak shift. It is also notable that although the elemental analysis showed different C, N and O contents in the two types of melanosomes, XPS showed almost the same elemental compositions on the surface of two types of iris and choroid melanosomes studied. When the melanosomes from the same tissues at different ages were compared, ICP-MS analysis suggested that the number of carboxylate groups in the melanosomes decreased with age. Both elemental analysis and XPS showed that C:N ratio decreased with age, which was proposed to be due to both a decrease in carboxylate groups in mature samples and to the fissure of phenol rings caused by age-associated oxidation. Such age-related oxidative damage diminishes conjugation and is manifested by blue shifts of absorption peaks for aromatic double bonds in the IR spectra of mature melanosomes. XPS analysis showed that the ratio of C-O:C=O decreased with age. These tissue-related and age-related chemical differences between samples affected the optic density and metal binding properties of melanosomes, which are believed to be closely associated with the biological functions of melanins.
DOI:10.1016/j.foodchem.2008.11.015URL [本文引用: 6]
DOI:10.1134/S0003683807010115URL [本文引用: 1]

Comparative studies of fungal melanin and two preparations of the high-molecular-weight humin-like substances formed during a solid-phase cultivation of the basidiomycete Cerrena maxima 0275 for 45 and 70 days were performed. The fungal melanin from Aspergillus niger and the humin-like substances synthesized by the basidiomycete C. maxima 0275 are similar in their physicochemical properties (elemental composition and behavior in acids and alkalis) and auxin-like activities. However, these biopolymers differ, essentially, at the structural level. According to IR spectroscopy data, the obtained humin-like substances display a higher similarity to natural humic acids and are more diverse in their functional groups compared with fungal melanins. Presumably, this is connected with the fact that laccase is involved in formation of humin-like substances; moreover, this enzyme is involved not only in the synthesis of these polymers, but also in their modification and degradation.
DOI:10.1002/jobm.200700366URLPMID:18506908 [本文引用: 1]

Melanin produced by Hypoxylon archeri, a companion fungus of Tremella fuciformis, was purified from the submerged culture medium and designated as HM. Ultraviolet-visible and FTIR spectra of the purified HM showed significant similarities with those of the synthesized melanin derived from tyrosine. Thus, the HM melanin was identified to be derived from the precursor molecule of tyrosine or dihydroxyphenylalanine (DOPA). The antioxidant activity of HM melanin, synthetic melanin and vitamin C were compared by inhibition of the oxidation of 5-thio-2-nitrobenzoic acid (TNB) caused by hypochlorous acid (HOCl) and hydrogen peroxide (H(2)O(2)). The HM melanin is likely to be more efficient eliminating oxygen free radicals generated by H(2)O(2) than by HOCl. At a concentration of 100 mg x l(-1), the HM melanin protected 80.95% of TNB oxidation by H(2)O(2), slightly higher than the synthetic melanin. Inhibition curves as a function of time also revealed the HM melanin was a more efficient inhibitor of H(2)O(2) oxidation with an average TNB (TNB(acr)) consumption rate of 0.0553 mmol l(-1 ) x min(-1 )during the inhibition phase. Therefore, HM melanin is an efficient scavenger of peroxide free radicals.
DOI:10.1016/j.lwt.2018.09.033URL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[D].
[本文引用: 6]
[D].
[本文引用: 6]
[D].
[本文引用: 5]
[D].
[本文引用: 5]
[D].
[本文引用: 3]
[D].
[本文引用: 3]
DOI:10.1016/j.lwt.2018.10.040URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 4]

The structure characteristics of sesame melanin was studied by elemental analysis, UV spectra, IR spectra, 1HNMR, 13CNMR and alkali fusion/GC-MS. The study shows that aromatic rings with the substitutes of aliphatic, phenolic, carboxy and amino form the bio-macromolecule with conjugation system.
URL [本文引用: 4]

The structure characteristics of sesame melanin was studied by elemental analysis, UV spectra, IR spectra, 1HNMR, 13CNMR and alkali fusion/GC-MS. The study shows that aromatic rings with the substitutes of aliphatic, phenolic, carboxy and amino form the bio-macromolecule with conjugation system.
URLPMID:12541815 [本文引用: 1]

Black sesame melanin, a kind of biopolymer was degraded by alkali fusion to study structure characterization. The degraded products were derivatized with bis-(trimethylsilyl) trifloroacetamide in a sealed tube at 125 degrees C for 30 min. The silylanization derivatives of degradation products were analyzed by GC/MS. Catechol, 1,4-dihydroxy benzene and catechuic acid were detected. This method can be used to characterize the structure type of black sesame melanin.
URLPMID:12541815 [本文引用: 1]

Black sesame melanin, a kind of biopolymer was degraded by alkali fusion to study structure characterization. The degraded products were derivatized with bis-(trimethylsilyl) trifloroacetamide in a sealed tube at 125 degrees C for 30 min. The silylanization derivatives of degradation products were analyzed by GC/MS. Catechol, 1,4-dihydroxy benzene and catechuic acid were detected. This method can be used to characterize the structure type of black sesame melanin.
DOI:10.1016/j.biomaterials.2016.03.018URLPMID:27031812 [本文引用: 1]

The use of non-toxic or low toxicity materials exhibiting dual functionality for use in sentinel lymph node (SLN) mapping and cancer therapy has attracted considerable attention during the past two decades. Herein, we report that the natural black sesame melanin (BSM) extracted from black sesame seeds (Sesamum indicum L.) shows exciting potential for SLN mapping and cancer photothermal therapy. Aqueous solutions of BSM under neutral and alkaline conditions can assemble into sheet-like nanoparticles ranging from 20 to 200 nm in size. The BSM nanoparticles were encapsulated by liposomes to improve their water solubility and the encapsulated and bare BSM nanoparticles were both non-toxic to cells. Furthermore, the liposome-encapsulated BSM nanoparticles (liposome-BSM) did not exhibit any long-term toxicity in mice. The liposome-BSM nanoparticles were subsequently used to passively target healthy and tumor-bearing mice SLNs, which were identified by the black color of the nanoparticles. BSM also strongly absorbed light in the near-infrared (NIR) range, which was rapidly converted to heat energy. Human esophagus carcinoma cells (Eca-109) were killed efficiently by liposome-BSM nanocomposites upon NIR laser irradiation. Furthermore, mouse tumor tissues grown from Eca-109 cells were seriously damaged by the photothermal effects of the liposome-BSM nanocomposites, with significant tumor growth suppression compared with controls. Given that BSM is a safe and nutritious biomaterial that can be easily obtained from black sesame seed, the results presented herein represent an important development in the use of natural biomaterials for clinical SLN mapping and cancer therapy.
DOI:10.1016/j.fct.2015.02.017URLPMID:25765751 [本文引用: 1]

One of the major sources of flavonoids for humans are citrus fruits, hesperidin being the predominant flavonoid. Hesperetin (HSP), the aglycon of hesperidin, has been reported to provide health benefits such as antioxidant, anti-inflammatory and anticarcinogenic effects. However, the effect of HSP on skin pigmentation is not clear. Some authors have found that HSP induces melanogenesis in murine B16-F10 melanoma cells, which, if extrapolated to in vivo conditions, might protect skin against photodamage. Since the effect of HSP on normal melanocytes could be different to that observed on melanoma cells, the described effect of HSP on murine melanoma cells has been compared to the effect obtained using normal human melanocytes. HSP concentrations of 25 and 50 microM induced melanin synthesis and tyrosinase activity in human melanocytes in a concentration-dependent manner. Compared to control melanocytes, 25 microM HSP increased melanin production and tyrosinase activity 1.4-fold (p < 0.01) and 1.1-fold (p < 0.01), respectively, and the corresponding increases in the case of 50 microM HSP were 1.9-fold (p < 0.001) and 1.3-fold (p < 0.001). Therefore, HSP could be considered a valuable photoprotective substance if its capacity to increase melanin production in human melanocyte cultures could be reproduced on human skin.
DOI:10.1021/bi00418a067URLPMID:3143409 [本文引用: 1]

The structures of one synthetic and two natural melanins are examined by solid-state NMR using cross polarization, magic angle sample spinning, and high-power proton decoupling. The structural features of synthetic dopa melanin are compared to those of melanin from malignant melanoma cells grown in culture and sepia melanin from squid ink. Natural abundance 13C and 15N spectra show resonances consistent with known pyrrolic and indolic structures within the heterogeneous biopolymer; 13C spectra indicate the presence of aliphatic residues in all three materials. These solid-phase experiments illustrate the promise of solid-phase NMR for elucidating structural information from insoluble biomaterials.
[本文引用: 3]
[本文引用: 4]
[本文引用: 3]
[本文引用: 2]
DOI:10.3864/j.issn.0578-1752.2016.20.014URL [本文引用: 1]

【Objective】The total phenolics, total flavoniods and tannin content and antioxidant activity of different phenolic compound fractions from litchi pulp were compared to clarify the effective phenolic compounds of antioxidant activity from litchi pulp. The results of research will provide a basis for revealing the main compounds from litchi pulp to human health.【Method】Litchi pulp polyphenol extracts were divided into four phenolic compound fractions (F1, F2, F3 and F4) by C18 silica gel column. Then the total phenolics, total flavonoids and tannin content and the compositions of free phenol of four compound fractions were determined. Also, the FRAP, DPPH, ORAC and CAA antioxidation indexes were adopted to evaluate the antioxidant capacities of four different compound fractions.【Result】The results showed that litchi pulp polyphenol extracts were well-divided into four phenolic compound fractions. Among them, the yield of F2 was as high as 36%, and others’ were 18.71%, 16.79% and 21.12%, respectively. Total phenolics, total flavonoids and tannin content of four phenolic compound fractions ranged from 218.86 to 499.78 mg GAE/g DW, 414.94 to 1 285.45 mg RE·g-1 DW and 83.35 to 483.43 mg CE·g-1 DW, respectively. Among the four compound fractions tested, F2 exhibited the highest total phenolics, total flavonoids and tannin content with their corresponding percentage contribution as high as 50.31%, 54.24% and 72.06% to litchi pulp polyphenol extracts, followed by F3, F4, and finally F1. Procyanidin B2 and epicatechin in F2, quercetin-3-O-rutinoside-7-O-α-L-rhamnoside in F3 and rutin in F4 were identified by HPLC. The results of antioxidation indexes showed that FRAP values of four compound fractions were F2>F3>F4>F1. The IC50 values of DPPH free radical scavenging were 27.00, 9.76, 19.41 and 16.25 μg·mL-1, respectively, then F2 exhibited the strongest DPPH free radical scavenging ability with minimum IC50 value among the four compound fractions, followed by F4, F3, and finally F1. Also, the ORAC values of them were F2>F3>F4>F1 and the CAA values were F2>F3>F4, F1. Among them, F2 exhibited the highest ORAC and CAA values as 8.36 mmol TE·g-1 DW and 190.71 μmol QE·g-1 DW with the percentage contribution as high as 50.42% and 84.91% to total ORAC and CAA values of litchi pulp polyphenol extracts, respectively.【Conclusion】These results indicated that there were significant differences in total phenolics, total flavonoids and tannin content and antioxidant activity among the four phenolic compounds fractions from litchi pulp. The compositions of each phenolic compound fraction were also different. What’s more, F2 exhibited the highest yield, total phenolics, total flavonoids and tannin content with the best antioxidant capacities. Interestingly, the phenolics in F2 may be the most main active compounds of antioxidant activity from litchi pulp.
DOI:10.3864/j.issn.0578-1752.2016.20.014URL [本文引用: 1]

【Objective】The total phenolics, total flavoniods and tannin content and antioxidant activity of different phenolic compound fractions from litchi pulp were compared to clarify the effective phenolic compounds of antioxidant activity from litchi pulp. The results of research will provide a basis for revealing the main compounds from litchi pulp to human health.【Method】Litchi pulp polyphenol extracts were divided into four phenolic compound fractions (F1, F2, F3 and F4) by C18 silica gel column. Then the total phenolics, total flavonoids and tannin content and the compositions of free phenol of four compound fractions were determined. Also, the FRAP, DPPH, ORAC and CAA antioxidation indexes were adopted to evaluate the antioxidant capacities of four different compound fractions.【Result】The results showed that litchi pulp polyphenol extracts were well-divided into four phenolic compound fractions. Among them, the yield of F2 was as high as 36%, and others’ were 18.71%, 16.79% and 21.12%, respectively. Total phenolics, total flavonoids and tannin content of four phenolic compound fractions ranged from 218.86 to 499.78 mg GAE/g DW, 414.94 to 1 285.45 mg RE·g-1 DW and 83.35 to 483.43 mg CE·g-1 DW, respectively. Among the four compound fractions tested, F2 exhibited the highest total phenolics, total flavonoids and tannin content with their corresponding percentage contribution as high as 50.31%, 54.24% and 72.06% to litchi pulp polyphenol extracts, followed by F3, F4, and finally F1. Procyanidin B2 and epicatechin in F2, quercetin-3-O-rutinoside-7-O-α-L-rhamnoside in F3 and rutin in F4 were identified by HPLC. The results of antioxidation indexes showed that FRAP values of four compound fractions were F2>F3>F4>F1. The IC50 values of DPPH free radical scavenging were 27.00, 9.76, 19.41 and 16.25 μg·mL-1, respectively, then F2 exhibited the strongest DPPH free radical scavenging ability with minimum IC50 value among the four compound fractions, followed by F4, F3, and finally F1. Also, the ORAC values of them were F2>F3>F4>F1 and the CAA values were F2>F3>F4, F1. Among them, F2 exhibited the highest ORAC and CAA values as 8.36 mmol TE·g-1 DW and 190.71 μmol QE·g-1 DW with the percentage contribution as high as 50.42% and 84.91% to total ORAC and CAA values of litchi pulp polyphenol extracts, respectively.【Conclusion】These results indicated that there were significant differences in total phenolics, total flavonoids and tannin content and antioxidant activity among the four phenolic compounds fractions from litchi pulp. The compositions of each phenolic compound fraction were also different. What’s more, F2 exhibited the highest yield, total phenolics, total flavonoids and tannin content with the best antioxidant capacities. Interestingly, the phenolics in F2 may be the most main active compounds of antioxidant activity from litchi pulp.
DOI:10.1021/jf201593nURL [本文引用: 1]

Phenolics in black soybean seed coat (BSSC) are considered to be responsible for the health benefits of black soybean. BSSCs of 60 Chinese varieties were examined for phenolic contents, anthocyanin profiles, and antioxidant activity. Total phenolic and condensed tannin contents ranged from 512.2 to 6057.9 mg gallic acid equivalents/100 g and from 137.2 to 1741.1 mg (+)-catechin equivalents/100 g, respectively. Six anthocyanins (delphinidin-3-glucoside, cyanidin-3-galactoside, cyanidin-3-glucoside, petunidin-3-glucoside, peonidin-3-glucoside, and malvidin-3-glucoside) were detected by HPLC. Total anthocyanin contents (TAC) were from 98.8 to 2132.5 mg/100 g, and cyanidin-3-glucoside was the most abundant anthocyanin in all varieties, with a distribution of 48.8-94.1% of TAC. Antioxidant properties detected by DPPH, FRAP, and ORAC methods all showed wide variations ranging from 4.8 to 65.3 mu g/100 mL (expressed as EC(50)), from 17.5 to 105.8 units/g, and from 42.5 to 1834.6 mu mol Trolox equivalent/g, respectively. Sixty varieties were classified into four groups by hierarchical clustering analysis, and group 4 consisting of nine varieties had the highest phytochemicals content and antioxidant activity.
DOI:10.1016/j.molstruc.2013.04.061URL [本文引用: 1]
URL [本文引用: 2]

In this work, the enzymatic hydrolysis of squid ink was investigated to prepare natural melanin. Squid ink was hydrolyzed by papain, neutral protease, acidic protease, pepsin and tyrpsin, respectively, and pepsin was found to have the strongest ability to hydrolyze squid ink. Based on one-factor-at-a-time experiments, an L9(34) orthogonal array design was used to optimize the hydrolysis of squid ink by pepsin. The degree of hydrolysis of squid ink was investigated with respect to pH, hydrolysis time, temperature and enzyme amount. The four conditions were optimized as follows: pH 1.5, temperature 37 ℃, reaction time 5 h and pepsin amount 1.5%. Under the optimized conditions, the degree of hydrolysis of squid ink exceeded 13.50%. Ultraviolet-visible spectral analysis revealed the characteristic absorption of melanin derived from squid ink at 220 nm wavelength. High-intensity absorption peaks at 3384.54 cm-1 and 1617.35 cm-1, assigned to the structure of indole ring were observed in the infrared spectrum.
URL [本文引用: 2]

In this work, the enzymatic hydrolysis of squid ink was investigated to prepare natural melanin. Squid ink was hydrolyzed by papain, neutral protease, acidic protease, pepsin and tyrpsin, respectively, and pepsin was found to have the strongest ability to hydrolyze squid ink. Based on one-factor-at-a-time experiments, an L9(34) orthogonal array design was used to optimize the hydrolysis of squid ink by pepsin. The degree of hydrolysis of squid ink was investigated with respect to pH, hydrolysis time, temperature and enzyme amount. The four conditions were optimized as follows: pH 1.5, temperature 37 ℃, reaction time 5 h and pepsin amount 1.5%. Under the optimized conditions, the degree of hydrolysis of squid ink exceeded 13.50%. Ultraviolet-visible spectral analysis revealed the characteristic absorption of melanin derived from squid ink at 220 nm wavelength. High-intensity absorption peaks at 3384.54 cm-1 and 1617.35 cm-1, assigned to the structure of indole ring were observed in the infrared spectrum.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/0003-2697(85)90150-2URLPMID:3993914 [本文引用: 1]

A method for the quantitative analysis of eumelanin and pheomelanin in tissues, e.g., hair and melanoma, is described. The method is simple and rapid because it does not require the isolation of melanins from the tissues. The rationale is that permanganate oxidation of eumelanin yields pyrrole-2,3,5-tricarboxylic acid (PTCA) which may serve as a quantitatively significant indicator of eumelanin, while hydriodic acid hydrolysis of pheomelanin yields aminohydroxyphenylalanine (AHP) as a specific indicator of pheomelanin. The degradation products, PTCA and AHP, can be readily analyzed by high-performance liquid chromatography. Chemical degradations of synthetic melanins, prepared from dopa, 5-S-cysteinyldopa, and their mixtures in various ratios, gave PTCA and AHP in yields that correlated with the dopa/5-S-cysteinyldopa ratio. The PTCA/AHP ratio as well as the contents of PTCA and AHP reflected well the type of melanogenesis in hair and melanomas. The amounts needed for each degradation were 0.5 mg of melanin, 2 mg of hair, and 5 mg of tissue samples. As many as 20 samples can be analyzed within 3 working days.
DOI:10.1089/cbr.1997.12.47URLPMID:10851446 [本文引用: 1]

Allomelanins, which belong to the melanins produced by higher plants and fungi, were studied with respect to their growth suppressive effects on cultured HCT-15 cells derived from human intestinal carcinoma and Meth/A cells derived from a Balb/C mouse lymphoma. Allomelanins were extracted from black soy beans and black sesame seeds with 0.2% NaOH in water. Proteins bonded to the allomelanins were removed by boiling the extracts for 20 hours in 30% NaOH. Although native allomelanins conjugated with protein did not suppress the growth of the cells, protein-free allomelanins did suppress it. That is, 50% suppression of HCT- 15 cell growth was found at a concentration between 100 and 200 micrograms/ml of the melanin prepared from the black soy beans, and between 25 and 100 micrograms/ml of that from the sesame seeds. For Meth/A cells, 50% suppression by allomelanin from the black beans was approximately 100 micrograms/ml; and that by the allomelanin preparation from sesame seeds, between 25 and 100 micrograms/ml. Thus, protein-free allomelanins seemingly suppress cultured mammalian and animal tumor cells.
DOI:10.1034/j.1600-0749.2002.1o062.xURLPMID:11936275 [本文引用: 1]

A 1H nuclear magnetic resonance study of Sepia melanin, Sepia melanin free acid (Sepia MFA) and human hair melanin was carried out in deuterium oxide solution at pH 10-11. The empirical formula of Sepia MFA was calculated and used to estimate the number of protons in the aromatic region of the Sepia MFA polymeric chain and to suggest a possible monomeric unit profile.
DOI:10.1002/(ISSN)1097-458XURL [本文引用: 1]
DOI:10.1021/la5026608URL [本文引用: 1]
DOI:10.1111/j.1600-0749.1989.tb00227.xURLPMID:2555809 [本文引用: 1]

The photoinduced radicals formed in eumelanin from hair at various excitation wavelengths, in KBr matrix, are compared with the photoinduced radicals observed with bovine eye iris and squid ink eumelanins. Qualitative similarities and quantitative differences are observed, specially on exposure at short wavelengths.
DOI:10.1111/j.1600-0749.1994.tb00060.xURLPMID:7855074 [本文引用: 2]

The intrinsic local structure characterization of natural sepia melanin and L-dopa and tyrosine synthetic melanin powder has been carried out by X-ray diffraction using synchrotron radiation. The derived structure factor, S(q), shows six significant diffuse peaks within the q-range from 0.3 A-1 to 16 A-1 in the reciprocal space (q = (4 pi sin theta)/lambda, 2 theta is the scattering angle). The Fourier transform of S(q), which yields the radial distribution function (RDF), gives us information in real space of a 1.42 A distance averaged over the C-C, C-O and C-N bond lengths as well as peaks at 2.40-2.41 A, 3.67-3.71 A and 4.67-4.70 A discrete neighbor distances. There is a great similarity in the scattering intensity profiles of the natural and synthetic melanins indicating that the synthetically prepared material may be essentially similar to
DOI:10.1073/pnas.0808768105URL [本文引用: 1]
DOI:10.1016/0005-2728(94)90241-0URLPMID:8180232 [本文引用: 1]

A series of antimycin A analogues was synthesized by modifying the salicylic acid moiety, whereas the portion of the molecule corresponding to the natural dilactone-ring moiety was fixed as di-n-octyl L-glutamate. To probe the structure of the antimycin A binding site, the structural factors of the salicylic acid moiety required for inhibitory action were examined by means of structure-activity studies with intact rat-liver mitochondria and the cytochrome bc1 complex isolated from bovine heart mitochondria. As suggested earlier (Rieske, J.S. (1976) Biochim. Biophys. Acta 456, 195-247), the phenolic OH was very important for inhibition. For the derivatives which do not possess a formylamino group in the 3-position (ortho to the phenolic OH), the inhibitory activity tended to increase as the electron-withdrawing property of the substituent increased, i.e., as the acidity of the phenolic OH group increased. This indicates that the acidity of the phenolic OH is an important factor governing inhibition. While the electron-withdrawing property of the formylamino group itself is rather poor, 3-formylamino derivatives elicited potent activity. The conformation of the 3-formylamino group was also found to be a very important factor in establishing inhibitory activity. In addition, the bulkier the moiety corresponding to the 3-formylamino group, the lower the activity. These results demonstrate that the presence of the 3-formylamino group, and its proper conformation, are needed for a close fitting of antimycin A to its binding domain. Although the inhibitors that lack a 3-formylamino group retained fairly potent activity, their effects on the reduction of cytochromes b and c1 were somewhat different from those of natural antimycin A, indicating that the 3-formylamino group is essential for inhibitor binding to the cytochrome bc1 complex in the same manner as natural antimycin A. It is concluded that both the 3-formylamino group and the phenolic OH of antimycin A make important contributions to specific interactions with the amino acid residues of the cytochrome b.
DOI:10.1016/j.jfca.2017.01.004URL [本文引用: 1]
DOI:10.1021/jf803011rURLPMID:19199445 [本文引用: 2]

Aqueous extracts of 30 plants were investigated for their antioxidant properties using DPPH and ABTS radical scavenging capacity assay, oxygen radical absorbance capacity (ORAC) assay, superoxide dismutase (SOD) assay, and ferric reducing antioxidant potential (FRAP) assay. Total phenolic content was also determined by the Folin-Ciocalteu method. Antioxidant properties and total phenolic content differed significantly among selected plants. It was found that oak (Quercus robur), pine (Pinus maritima), and cinnamon (Cinnamomum zeylanicum) aqueous extracts possessed the highest antioxidant capacities in most of the methods used, and thus could be potential rich sources of natural antioxidants. These extracts presented the highest phenolic content (300-400 mg GAE/g). Mate (Ilex paraguariensis) and clove (Eugenia caryophyllus clovis) aqueous extracts also showed strong antioxidant properties and a high phenolic content (about 200 mg GAE/g). A significant relationship between antioxidant capacity and total phenolic content was found, indicating that phenolic compounds are the major contributors to the antioxidant properties of these plants.
[本文引用: 1]
[本文引用: 1]
DOI:10.1128/jb.175.21.7102-7104.1993URLPMID:8226653 [本文引用: 1]

Polyphenols have been implicated in the virulence and oxidant resistance of Cryptococcus neoformans. Although monomeric polyphenols did not protect against the prooxidant, plumbagin, polymeric dopamine-melanin conferred resistance both to hypochlorite and to permanganate. The physiologic antioxidant capacity conferred by melanin was found to be 21.3 x 10(-15) mole-equivalents per cell, a value which approximates oxidant production by stimulated macrophages.
[本文引用: 1]
[本文引用: 1]
DOI:10.1186/2008-6970-5-3URL [本文引用: 1]

The aim of the present study was to determine species of the fungal genera Aspergillus, Fusarium, and Penicillium and fumonisin B1 (FB1), aflatoxin B1 (AFB1), and ochratoxin A (OTA) contamination from feed intended for fish farms. A total of 60 samples were sampled from tilapia farms in the Rio de Janeiro State, Brazil. The quantitative enumeration of fungi as colony-forming units per gram of feed (CFU/g) was performed using the surface spread method in different culture media. The results were expressed as fungal isolation frequency and relative density. Fungal total counts ranged from <1 × 102 to 4.7 × 104 CFU/g. Fusarium counts were not observed. Among toxigenic genera, Aspergillus (68%) was the most prevalent, followed by Penicillium species (60%). Aspergillus niger aggregate (36%), Aspergillus flavus (35%), and Penicillium citrinum (71%) were the most prevalent species. A high percentage of samples (98%) were contaminated with FB1 levels, while 55% and 3.3% were contaminated with AFB1 and OTA, respectively. The simultaneous occurrence of these mycotoxins emphasizes the need for further research in the area to better assess the risk to the health of fish farms and their implications for the health of consumers of this meat.
