 ,, 祁静静, 窦万福, 陈善春, 何永睿
,, 祁静静, 窦万福, 陈善春, 何永睿 ,, 李强
,, 李强 ,西南大学/中国农业科学院柑桔研究所,重庆 400712
,西南大学/中国农业科学院柑桔研究所,重庆 400712Identification of Rboh Family and the Response to Hormone and Citrus Bacterial Canker in Citrus
QIN XiuJuan ,, QI JingJing, DOU WanFu, CHEN ShanChun, HE YongRui
,, QI JingJing, DOU WanFu, CHEN ShanChun, HE YongRui ,, LI Qiang
,, LI Qiang ,Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712
,Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712通讯作者:
责任编辑: 岳梅
收稿日期:2020-03-7接受日期:2020-04-3网络出版日期:2020-10-16
| 基金资助: |
Received:2020-03-7Accepted:2020-04-3Online:2020-10-16
作者简介 About authors
秦秀娟,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (4171KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
秦秀娟, 祁静静, 窦万福, 陈善春, 何永睿, 李强. 柑橘Rboh家族鉴定及其对激素和柑橘溃疡病的响应[J]. 中国农业科学, 2020, 53(20): 4189-4203 doi:10.3864/j.issn.0578-1752.2020.20.008
QIN XiuJuan, QI JingJing, DOU WanFu, CHEN ShanChun, HE YongRui, LI Qiang.
0 引言
【研究意义】柑橘溃疡病(citrus bacterial canker,CBC)是由柑橘黄单胞杆菌柑橘亚种(Xanthomonas citri subsp. citri,Xcc)引起的检疫性病害,会导致柑橘树木落叶、落果、产量和品质下降,严重影响柑橘产业的健康发展[1,2,3]。传统防治方法难以根治,会造成巨大的人力、物力投入并影响环境[4]。随着柑橘转基因体系的日趋成熟,通过分子育种的方法高效获得抗溃疡病品种成为可能。通过将抗病基因利用农杆菌介导转入甜橙(Citrus sinensis)增加其抗溃疡病的能力[5]。分子育种相比传统杂交育种具有准确、高效的特点,近年来得到快速发展,运用分子生物学技术培育抗溃疡病柑橘品种也成为防治溃疡病侵害最根本、最有效的方式,而准确筛选候选基因是分子育种的关键[6]。植物应对生物胁迫时会产生大量活性氧(reactive oxygen species,ROS)以抑制病原体生长,或作为植物早期防御反应中的信号分子诱导下游抗性基因表达[7]。活性氧作为植物体内参与胁迫反应和调控植物生长发育的重要物质,其化学性质活泼、具备较强的氧化活性[8]。在植物体内产生机制多样,主要由植物呼吸爆发氧化酶(respiratory burst oxidase homologue,Rboh,又称NADPH氧化酶)介导生成[9,10]。研究呼吸爆发相关基因的功能与调控机理是发掘潜在抗溃疡病候选基因的重要途径。Rboh是参与植物活性氧产生的关键酶,因此,以柑橘为材料,基于全基因组数据,对Rboh家族进行鉴定、信息学和诱导表达分析,挖掘抗柑橘溃疡病相关成员,对于抗病育种具有重要意义。【前人研究进展】植物Rboh蛋白N端区域含有保守的Ca2+结合EF手性模体结构,在调节NADPH氧化酶活性产生活性氧中起到重要作用[11]。当细胞受到激素、病原等刺激时,细胞产生Ca2+与Rboh的EF结构结合后激活该酶,使之催化产生大量活性氧。植物Rboh以多基因家族形式存在,因Rboh在抵御胁迫方面的重要功能,越来越多的研究聚焦该家族。从第一个Rboh基因在水稻中被克隆出来后[12],Rboh家族陆续在拟南芥[13]、番茄[14,15]、烟草[16,17,18,19]、马铃薯[20,21]、玉米[22]、西瓜[23]、大麦[24,25]、苜蓿[26]和豌豆[27]等植物中得以鉴定和分析,揭示了不同物种该家族的进化和表达特性,为功能研究和机理探索提供了可能。当前,也有很多关于Rboh在抵御胁迫中的功能研究。在拟南芥和烟草等模式植物中研究发现,该酶介导产生的活性氧参与了植物的逆境胁迫[10];YOSHIOKA等研究发现,NbRbohA和NbRbohB在烟草抗病反应中参与了H2O2的生成和抵抗致病霉菌(Phytophthora infestans)[18];花叶病毒处理烟草叶片后可增加Rboh酶活性,从而经一系列反应促进H2O2的产生,且水杨酸(salicylic acid,SA)处理可增加NbRbohB的转录水平,最终诱导H2O2的大量积累[28];拟南芥中茉莉酸(jasmonic acid,JA)可诱导AtRbohD和AtRbohF表达升高,并伴随H2O2的积累;茉莉酸处理AtRbohD与AtRbohF双突变体后,H2O2并未明显上升[29];李业等分别利用水杨酸、茉莉酸甲酯(MeJA)和稻黄单胞菌稻致病变种(Xanthomonas oryzae pv. oryzae,Xoo)PX099处理水稻秧苗,均提高了OsRboh家族部分成员表达量,并伴随H2O2的积累[30];脱落酸(abscisic acid,ABA)处理玉米幼苗,ZmRbohA-D的表达量和活性氧产量均显著升高,表明玉米Rboh参与了脱落酸诱导的信号途径[31]。【本研究切入点】植物Rboh受病原体和植物抗病相关激素的诱导,重建活性氧的平衡,进而调控植物对胁迫的抗性。前期对溃疡病菌诱导易感与抗病柑橘品种后的差异表达基因(differentially expressed gene,DEG)研究发现,部分Rboh家族基因差异明显,这为深入挖掘和研究该家族与柑橘溃疡病抗性提供了依据。【拟解决的关键问题】鉴定并分析柑橘Rboh家族,探索各成员在生物胁迫信号途径相关激素和柑橘溃疡病菌诱导下的应答模式,分析其与柑橘溃疡病发生的相关性,挖掘有潜力的候选基因用于抗溃疡病分子育种。1 材料与方法
1.1 植物材料与菌体
2019年6月在西南大学温网室和国家柑桔品种改良中心育种圃(坐标:19°51′N,106°37′E)选取晚锦橙(Wanjincheng)和四季橘(Calamondin)叶片为材料进行诱导表达分析。供试溃疡病菌为西南大学柑桔研究所保存的柑橘黄单胞杆菌柑橘亚种A株系[2]。1.2 CsRboh家族鉴定
甜橙基因组和蛋白组数据下载于Phytozome(1.3 CsRboh信息学分析
使用ExPASy Proteomics Server(1.4 植物激素和柑橘溃疡病菌诱导处理
根据LI等[45]的方法对晚锦橙和四季橘叶片进行处理,简述如下:采集成熟叶片(约3月龄)用75%的乙醇消毒后置于无菌培养皿;注射接种溃疡病菌菌液(OD600=0.5);于28℃光照培养;分别于0、12、24、36和48 h切取接种部位。叶圆片(直径7 mm)分别浸泡于茉莉酸(100 μmol?L-1)、脱落酸(100 μmol?L-1)、水杨酸(10 μmol?L-1)溶液和无菌水(对照)置于28℃光照培养,0、12、24、36和48 h取样。上述样品使用RNA提取试剂盒提取总RNA(艾德莱,北京),使用反转录试剂盒(TaKaRa,日本)反转录为cDNA,利用实时荧光定量PCR(qRT-PCR)检测各样品中基因的相对表达量。
1.5 qRT-PCR与统计分析
使用NCBI Primer Blast根据CsRboh和柑橘内参基因CsActin(GenBank:GU911361.1)特异性区域设计qRT-PCR引物(表1)。利用ChamQTM UniversaL SYBR qPCR Master Mix(诺唯赞,南京)将溃疡病菌、激素处理的叶片cDNA分别进行实时定量检测。每个处理进行3次生物学重复和3次技术重复;相对表达量采用2-ΔΔCt法计算(ΔCt=CtCsKat01-CtCsActin)[46];使用SPSS V25(IBM,美国)进行差异显著性分析。Table 1
表1
表1本研究使用的实时荧光定量PCR引物
Table 1
| 基因Gene | 上游引物Forward primer | 下游引物Reverse primer |
|---|---|---|
| CsRboh01 | GCTCTCGGCTTCAACTTCCT | TCAAAGCTCGAGCCGCTAAA |
| CsRboh02 | ATGGAGCACCAGCTCAAGAC | AGTAGCCCCAATTCCAAGCC |
| CsRboh03 | TTGGCCAGCAGATTGGAGAG | CTGCGAAACTTGCCTCAACC |
| CsRboh04 | ACGCCCGTCTTCAGATTTTCT | GCCATAAGTGACTGACACGCT |
| CsRboh05 | CCGGCGCAATCTGTTAAAGG | TGTAGGCTTCAGCTTCTGGC |
| CsRboh06 | CGCATGAAAGGGACACCGAAT | CAAATGAGCCTTGTTCCCTTGTT |
| CsRboh07 | AACCGGTGATCAAGTGGTCG | GCATGGCTACAAAATTTGAGGGAA |
| CsActin | CATCCCTCAGCACCTTCC | CCAACCTTAGCACTTCTCC |
新窗口打开|下载CSV
2 结果
2.1 CsRboh家族鉴定和理化性质
对柑橘全基因组范围内Rboh家族进行了鉴定和理化性质分析,最终从柑橘基因组中鉴定到7个Rboh成员。这些基因编码蛋白的长度在784(CsRboh07)—946(CsRboh01)aa;其分子量变化范围为89 837.0—106 370.8 Da;等电点(PI)分布在8.67(CsRboh05)—9.40(CsRboh01),均为碱性蛋白;该家族基因编码的蛋白质亲水性平均值分布在-0.015(CsRboh07)—-0.299(CsRboh02),除CsRboh07外,其他成员均为亲水性蛋白。CsRboh04编码的氨基酸脂肪指数最低(82.61),CsRboh07的最高(89.06),其余脂肪指数分布在83.84—87.68。蛋白质不稳定系数分布在40.42—50.16,其中CsRboh01最不稳定。经CELLO分析,柑橘CsRboh在细胞内膜(CsRboh04、CsRboh06和CsRboh07)、细胞外膜(CsRboh01和CsRboh03)、细胞质(CsRboh02和CsRboh05)均有分布(表2)。Table 2
表2
表2CsRboh家族成员信息
Table 2
| 名称 Name | CAP号 CAP ID | 大小 Size (aa) | 分子量 Molecular weight (Da) | 等电点 Isoelectric point (PI) | 亲水性平均值 Grand average of hydropathicity | 脂肪指数 Aliphatic index | 不稳定系数 Instability index | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|
| CsRboh01 | Cs5g02940.1 | 946 | 106370.8 | 9.40 | -0.247 | 87.68 | 50.16 | 细胞外膜Outer membrane |
| CsRboh02 | Cs8g12000.1 | 912 | 103139.3 | 9.05 | -0.299 | 83.84 | 42.57 | 细胞质Cytoplasmic |
| CsRboh03 | Cs3g14240.1 | 889 | 101017.9 | 9.10 | -0.265 | 85.43 | 40.96 | 细胞外膜Outer membrane |
| CsRboh04 | Cs4g06920.1 | 875 | 99488.8 | 9.31 | -0.252 | 82.61 | 48.38 | 细胞内膜Inner membrane |
| CsRboh05 | Cs7g19320.1 | 811 | 91648.2 | 8.67 | -0.269 | 86.94 | 40.91 | 细胞质Cytoplasmic |
| CsRboh06 | Cs8g17640.1 | 844 | 96907.0 | 8.99 | -0.173 | 84.69 | 40.42 | 细胞内膜Inner membrane |
| CsRboh07 | Cs5g11890.1 | 784 | 89837.0 | 9.09 | -0.015 | 89.06 | 44.93 | 细胞内膜Inner membrane |
新窗口打开|下载CSV
二级结构分析发现,这些蛋白均由α-螺旋、β-转角、伸展链和不规则卷曲构成,且主要以α-螺旋为主(41.83%—44.61%),其次是不规则卷曲(34.87%—38.29%),占比较少的为伸展链(13.85%—16.28%)和β-转角(4.19%—5.48%)(表3)。柑橘Rboh家族各序列信息已提交至RedOxiBase数据库[9],其登录号为13472—13476、13487—13488。
Table 3
表3
表3CsRboh的二级结构
Table 3
| 名称 Name | α-螺旋 Alpha helix (%) | β-转角 Beta turn (%) | 不规则卷曲 Random coil (%) | 伸展链 Extended strand (%) |
|---|---|---|---|---|
| CsRboh01 | 44.61 | 4.76 | 36.79 | 13.85 |
| CsRboh02 | 43.75 | 5.26 | 34.87 | 16.12 |
| CsRboh03 | 44.43 | 5.29 | 35.32 | 14.96 |
| CsRboh04 | 41.83 | 4.57 | 38.29 | 15.31 |
| CsRboh05 | 42.17 | 4.19 | 37.36 | 16.28 |
| CsRboh06 | 43.60 | 5.21 | 36.85 | 14.34 |
| CsRboh07 | 43.24 | 5.48 | 36.48 | 14.80 |
新窗口打开|下载CSV
2.2 CsRboh家族系统进化分析
为了研究Rboh家族在多个物种中的进化关系,使用最大似然法构建了包含柑橘、拟南芥和杨树3个物种Rboh家族的系统发育树(图1)。根据系统进化树,3个物种的27个Rboh蛋白(柑橘7个、拟南芥10个、杨树10个)可划分为I—IV 4个亚家族,其中I亚家族又可以分为A、B两个分支。4个亚家族分别包含3、2、1和1个柑橘Rboh成员。值得注意的是第IV亚家族为柑橘所特有,且只含有1个Rboh成员(CsRboh07),表明CsRboh07是进化过程形成的柑橘特有的酶,可能具有特殊功能。图1
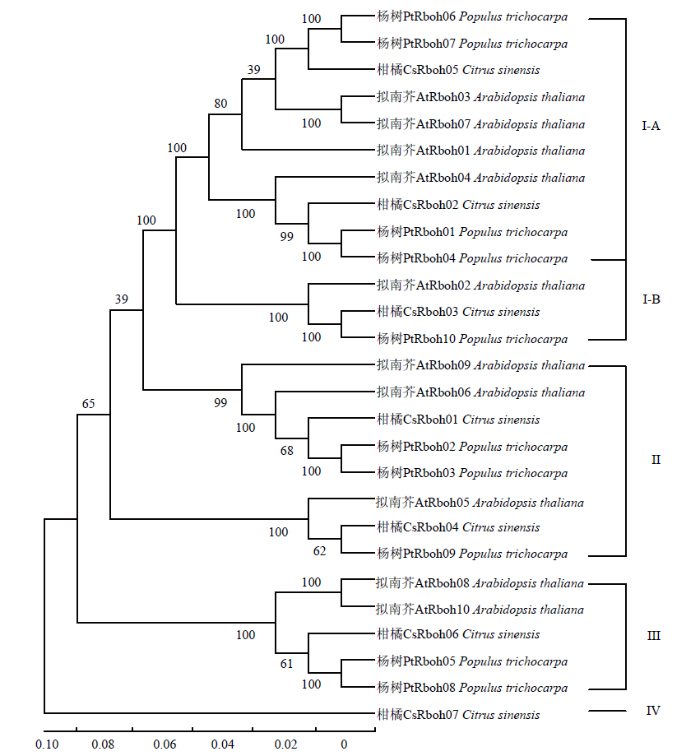 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1多物种Rboh家族系统进化树
PtRboh01-10:杨树Rboh成员The Rbohs of Populus trichocarpa;AtRboh01-10:拟南芥Rboh成员The Rbohs of Arabidopsis thaliana;CsRboh01-07:柑橘Rboh成员The Rbohs of C. sinensis
拟南芥和杨树Rboh蛋白序列获取自RedoxiBase数据库,比对采用蛋白的全长序列进行,进化树采用最大似然法和泊松模型The sequences of P. trichocarpa and A. thaliana are retrieved from RedoxiBase (http://peroxibase.toulouse.inra.fr). The full-length of protein sequences are used for the maximum likelihood phylogeny with Poisson model
Fig. 1The phylogenetic tree of Rboh family in several organisms
2.3 CsRboh家族染色体定位和基因结构
对CsRboh在染色体上的定位进行分析和展示,发现CsRboh不均匀地分布于柑橘的5条染色体上(图2),其中,CsRboh03定位于3号染色体,CsRboh04位于4号染色体,CsRboh01和CsRboh07定位于5号染色体,CsRboh05位于7号染色体,CsRboh02和CsRboh06定位于8号染色体。为更好地了解CsRboh的结构特点,利用GSDS[43]对CsRboh的基因结构进行了分析和展示(图2),发现7个基因均含11—13个外显子;结合系统进化树,发现相同亚家族基因具有相似基因结构,但内含子并非完全一致,其长度上有较大变异;柑橘Rboh家族第I亚家族中的基因均含11个外显子,CsRboh03与CsRboh02、CsRboh05相比,基因的内含子长度变异较大;第II亚家族中的基因具有12个外显子;第III亚家族中CsRboh06有12个外显子;第IV亚家族中的CsRboh07有13个外显子;III和IV亚家族中两个基因均无UTR区域。图2
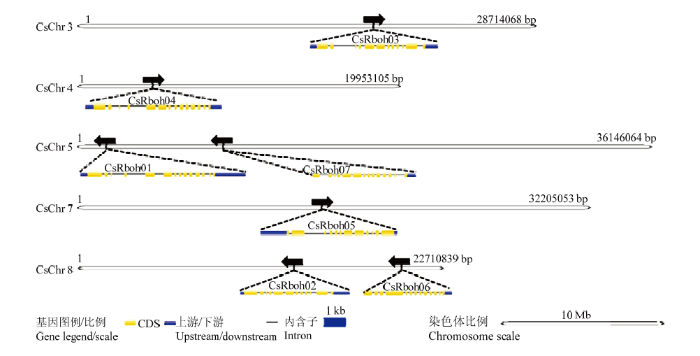 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2CsRboh家族的染色体定位和基因结构
染色体定位由软件Mapchart展示,基因结构由GSDS分析;黑色箭头表示基因的方向;基因长度和染色体长度均成比例;染色体上方的数字表示该染色体的大小
Fig. 2The chromosomal loci and gene structures of CsRbohs
The chromosomal loci were visualized by Mapchart, gene structures were displayed by GSDS. The sizes of chromosomes and genes are on the scales. Black arrows represent the gene directions. The number above each chromosome represents the chromosome size
2.4 CsRboh家族多序列比对及功能结构域
利用Pfam对7个CsRboh进行功能结构域分析,结果表明CsRboh家族成员均含有典型的4个功能结构域,从N端起分别为呼吸爆发NADPH氧化酶结构域(respiratory burst NADPH oxidase,NADPH_Ox,PF08414.10)、铁还原酶跨膜组件(ferric reductase like transmembrane component,Ferric_reduct,PF01794.19)、FAD结合结构域(FAD-binding domain,FAD_binding,PF08022.12)和铁还原酶NAD结合结构域(ferric reductase NAD binding domain,NAD_binding,PF08030.12)(图3-A)。蛋白序列的比对发现NADPH氧化酶结构域为207—308号氨基酸、铁还原酶跨膜组件为514—677号氨基酸、FAD结合结构域为720—851号氨基酸、铁还原酶NAD结合结构域为856—1 070号氨基酸,这些结构域高度保守(图3-B)。图3
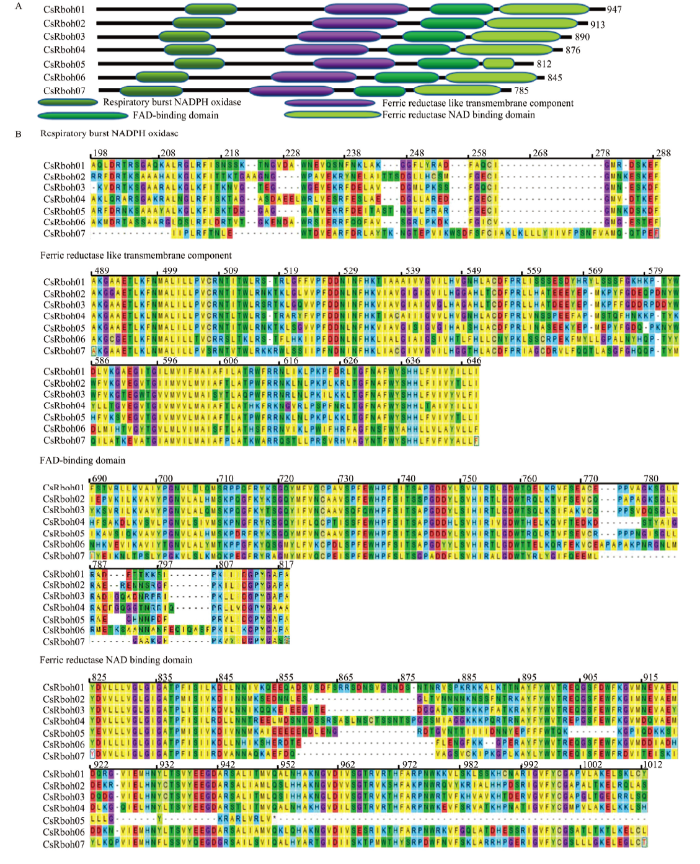 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3CsRboh家族的功能结构域
A:柑橘Rboh家族的功能结构域,使用在线软件Pfam预测The functional domains of CsRbohs predicted by Pfam;B:4个功能结构域比对The alignments of functional domains of CsRbohs
Fig. 3The functional domains of CsRbohs
2.5 CsRboh家族保守基序分析
利用MEME对CsRboh家族保守基序进行预测,得到15个相关性最高的保守元件(图4-A),并整合基序的Logo(图4-B)。CsRboh01、CsRboh03和CsRboh04均包含15个保守基序,CsRboh05和CsRboh06包含14个,CsRboh02、CsRboh07包含12个;除CsRboh02没有Motif 5、Motif 9、Motif 10,其他6个成员均含有;Motif 1、Motif 2、Motif 3、Motif 6、Motif 8、Motif 12和Motif 13共7个保守基序为7个CsRboh共有,且分布几乎一致;结合Pfam分析的功能结构域(图3),发现这7个共有基序对应CsRboh的功能结构域。图4
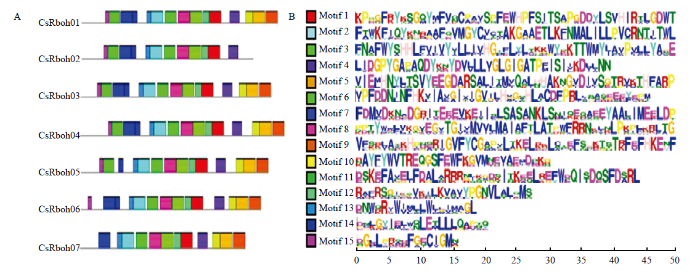 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4CsRboh家族蛋白保守基序
A:柑橘Rboh家族蛋白保守基序及分布,使用MEME软件进行分析Conserved protein motifs in CsRboh family analyzed by MEME;B:15个保守基序的Logo展示Logo display of the 15 conserved motifs
Fig. 4Conserved protein motifs in CsRboh family
2.6 CsRboh家族启动子顺式作用元件分析
为深入了解CsRboh家族基因对逆境信号的胁迫机制以及基因的诱导表达模式,对柑橘Rboh的核心启动子顺式作用元件进行分析,发现植物抗病信号有关的3种植物激素响应元件茉莉酸响应元件(CGTCA-motif)、脱落酸响应元件(ABRE)和水杨酸响应元件(TCA-element)在7个基因中呈现不均匀分布(表4)。除CsRboh05外,其余6个基因启动子均含有茉莉酸响应元件,数量在1—3个。水杨酸响应元件在该基因家族中较少,仅在CsRboh01、CsRboh02、CsRboh06中各包含1个。除CsRboh07外,其余基因均包含1—7个脱落酸响应元件。Table 4
表4
表4CsRboh上游2 kb顺式作用元件分布
Table 4
| 基因 Gene | 茉莉酸响应元件 CGTCA-motif | 水杨酸响应元件 TCA-element | 脱落酸响应元件 ABRE |
|---|---|---|---|
| Csrboh01 | 3 | 1 | 7 |
| Csrboh02 | 1 | 1 | 1 |
| Csrboh03 | 2 | - | 5 |
| Csrboh04 | 3 | - | 5 |
| Csrboh05 | - | - | 6 |
| Csrboh06 Csrboh07 | 2 1 | 1 - | 1 - |
新窗口打开|下载CSV
2.7 植物激素诱导下CsRboh表达模式
在晚锦橙和四季橘叶片中,7个CsRboh表达均受脱落酸、茉莉酸和水杨酸诱导,但其诱导模式存在差异(图5—图7)。图5
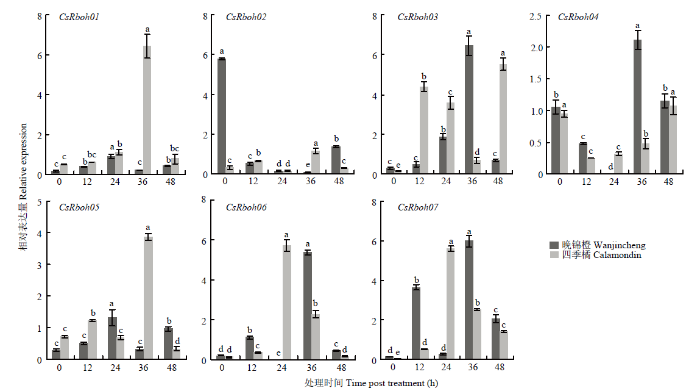 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5脱落酸诱导下CsRboh的相对表达量
柱上不同小写字母表示差异显著(P<0.05)。下同
Fig. 5The relative expression of CsRbohs induced by ABA
Different lowercases on the bars indicate significant differences (P<0.05). The same as below
图6
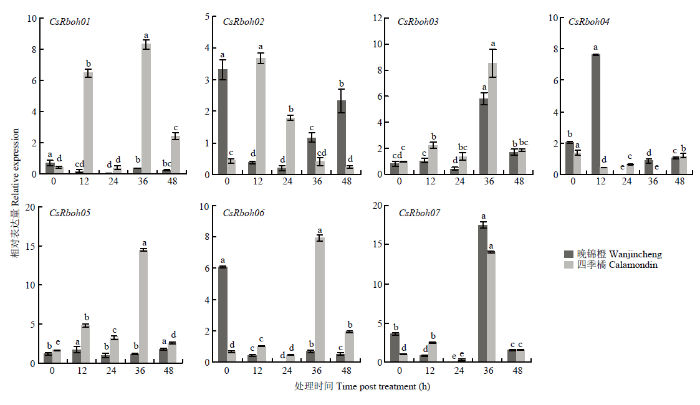 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6茉莉酸诱导下CsRboh的相对表达量
Fig. 6The relative expression of CsRbohs induced by JA
图7
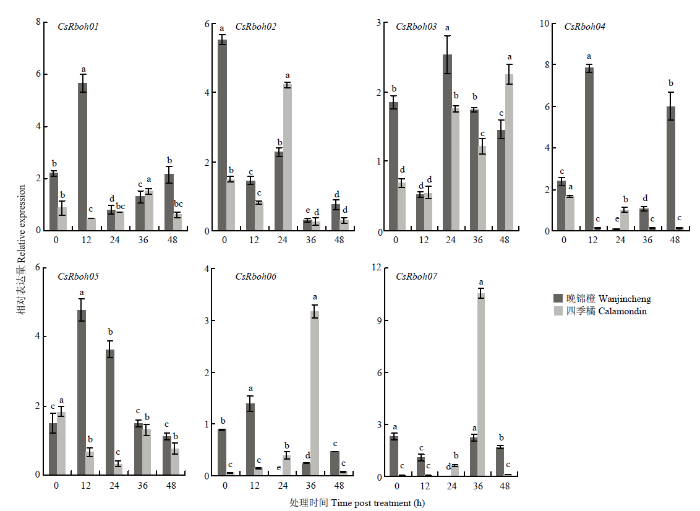 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7水杨酸诱导下CsRboh的相对表达量
Fig. 7The relative expression of CsRbohs induced by SA
脱落酸诱导时,CsRboh03、CsRboh04、CsRboh07在两个品种中诱导表达模式不同。晚锦橙中,CsRboh03在0—36 h内表达量先上升,后急剧下降;四季橘中,0—12 h内上调表达,12—36 h表达量下降,之后上升至最高水平。CsRboh04在两个品种中表达模式类似,0—24 h表达量逐步下降,之后上升。两个品种中CsRboh07也对脱落酸有明显响应,晚锦橙中,0—12 h内上调表达,后期呈下降、上升、下降的趋势;四季橘中则是在处理前24 h一直上调表达,之后表达量下降。其他基因虽响应脱落酸诱导,但效果并不明显(图5)。
茉莉酸诱导时,CsRboh01、CsRboh03、CsRboh05、CsRboh06和CsRboh07在四季橘中表达均先上升后下降,36 h达到最高,而在晚锦橙中CsRboh03和CsRboh07表达模式相似,CsRboh01、CsRboh05和CsRboh06无明显诱导。CsRboh02受茉莉酸诱导表达模式相反,晚锦橙中CsRboh02表达量先下降再上升,四季橘中CsRboh02表达量则先上升后下降(图6)。
水杨酸诱导时,晚锦橙中CsRboh01、CsRboh04、CsRboh05和CsRboh06表达量总体呈先升后降趋势,四季橘中这4个基因受水杨酸诱导不明显;CsRboh02、CsRboh03和CsRboh07在两个品种中受水杨酸诱导趋势相似(图7)。
2.8 柑橘溃疡病菌诱导下CsRboh表达模式
溃疡病菌侵染后,7个基因在两个品种中均有不同程度的响应(图8)。晚锦橙中,CsRboh01、CsRboh04表达量变化总体呈现先升后降趋势,CsRboh02、CsRboh06、CsRboh07表达量呈现出先升、后降、再升的趋势,CsRboh03、CsRboh05表达量变化为先降、后升、再降的趋势。四季橘中,CsRboh01、CsRboh05和CsRboh06表达呈现先降、后升、再降的趋势,CsRboh05则在36—48 h内表达量再次升高。CsRboh03、CsRboh07表达量先升后降,CsRboh02、CsRboh04则是先升、后降、再升的趋势,CsRboh04在最后一个时间段内表达量出现下降。CsRboh02、CsRboh05和CsRboh07在两个品种中具有相似诱导趋势。除CsRboh07外,其他CsRboh表达量均在24 h达到最高。CsRboh04、CsRboh06在晚锦橙和四季橘两个品种中诱导总体趋势基本相反。图8
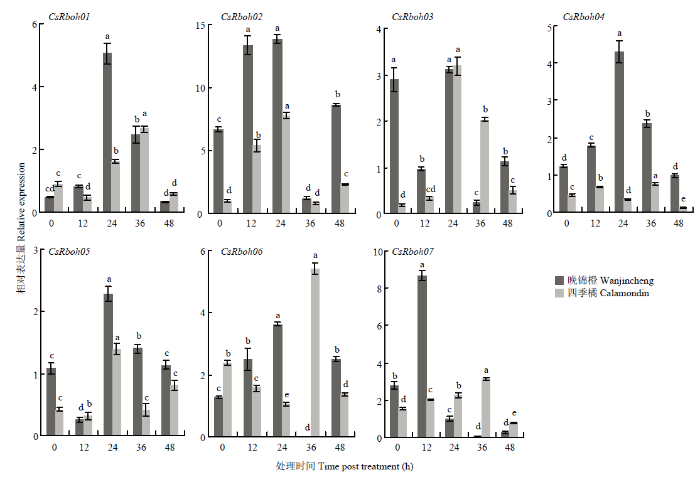 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8柑橘溃疡病菌侵染诱导CsRboh的相对表达量
Fig. 8The relative expression of CsRbohs induced by Xcc infection
3 讨论
植物体在应对多种生物胁迫时产生大量的活性氧以对抗外界病原菌并激活信号转导途径,而Rboh可控制植物体内活性氧产生来影响植物抗病性[47]。因此,越来越多的研究聚焦于Rboh的结构和功能分析。Rboh在不同物种中基因家族数目不同,在水稻[30]、拟南芥[28]、小麦[48]和烟草[49]中Rboh家族分别有9、10、5和20个基因成员,而本试验发现柑橘中有7个基因编码CsRboh蛋白(CsRboh01-07)。基因家族大小的不同与家族中基因复制事件相关,在烟草中含有较多的复制事件,使该家族得以扩张[49],而柑橘中并没有基因复制。各物种中Rboh家族均包含4个功能结构域,分别为呼吸爆发NADPH氧化酶结构域、铁还原酶跨膜组件、FAD结合结构域和铁还原酶NAD结合结构域。这些结构在进化上高度保守,是植物Rboh的显著特征,也是发挥功能产生活性氧的前提。Rboh在不同物种中基因家族编码产物大多位于细胞膜,拟南芥和柑橘中分别有8和5个基因编码蛋白位于细胞膜,但葡萄中的一些Rboh预测发现其位于叶绿体的类囊体膜上[50],这类酶亚细胞定位的多样性可能与该酶参与不同的胁迫应答有关。有研究发现将水杨酸喷施到拟南芥上,不仅诱导病原相关蛋白(PR)基因的上调表达,而且能获得系统性抗性,从而使植物对病原菌的抵抗能力普遍增强[51]。植物激素与植物抗病密切相关,但不同激素的信号途径交叉,有时相互拮抗[52]。本研究检测了7个CsRboh在3种与生物胁迫应答相关的植物激素和溃疡病菌诱导下的表达情况,证实了某些成员在不同激素和溃疡病菌诱导下的表达模式,供试qRT-PCR引物根据Rboh家族基因保守结构域设计,确保两个品种qRT-PCR结果可以真实反映CsRboh在溃疡病抗、感品种中的表达模式。其中,CsRboh02均可响应柑橘溃疡病菌及3种植物激素的诱导。顺式作用调控元件常在应激条件下对基因转录进行关键的分子调控[53],CsRboh02响应激素诱导可能与其启动子元件中含有茉莉酸响应元件CGTCA-motif、水杨酸响应元件TCA-element和脱落酸响应元件ABRE有关,推测CsRboh02可能由于启动子区域含有激素响应元件响应脱落酸、茉莉酸和水杨酸,通过调节激素变化响应溃疡病菌诱导从而参与柑橘抗病过程。通过蛋白质序列比对发现CsRboh02与拟南芥AtRbohD、烟草NbRbohD具有较高同源性。AtRbohD可参与病原体诱导的活性氧产生和寡半乳糖苷诱导的植物免疫,从而抵御外界病原菌侵染[54]。另外,AtRbohD在活性氧依赖的脱落酸信号传导中起着重要作用,并参与植物防御反应期间活性氧中间体的积累[55]。YOSHIOKA等[18]研究表明NbRbohD、NbRbohA和NbRbohB也直接参与对真菌病原的抗性。CsRboh02在其他物种中的同源基因多与植物抗病性相关,且本研究发现CsRboh02在柑橘溃疡病菌诱导下表达水平明显高于家族其他基因,在溃疡病菌侵染的前24 h内,在抗病品种四季橘中CsRboh02上调表达,表明该基因参与柑橘的基础免疫。不过,CsRboh02表达量增长幅度高于晚锦橙,可能在抗病品种中经溃疡病菌诱导产生更多的H2O2,从而激活植物过敏反应。综上,该基因可能在柑橘抗溃疡病发生的早期发挥着关键的基础免疫作用,可在后续对其功能进行深入研究。
溃疡病菌侵染后,CsRboh04表达量在感病品种晚锦橙和抗病品种四季橘中明显不同,在晚锦橙中CsRboh04表达量受溃疡病菌诱导明显,表达量明显高于四季橘(图8)。且前期通过CAP甜橙数据库(http://citrus.hzau.edu.cn/orange/)对CsRboh04表达量预测时发现其在叶片中的表达量仅为0.3,其真实表达量较低,这与溃疡病菌诱导后CsRboh04在四季橘中表达量较低的情况相一致。系统进化树中聚类关系相近的基因之间可能具有类似的功能[56],结合系统进化树及其蛋白质同源序列比对发现拟南芥中AtRbohE为CsRboh04的同源基因,尽管尚未阐明AtRbohE的作用,但有研究表明,在生物胁迫期间,其启动子区域中存在伤口反应元件(W-box)[57]。W-box可与WRKY转录因子家族的成员相互作用,在生物胁迫中发挥关键作用[51]。大量研究证明WRKY家族大部分基因都参与植物抗病反应[58]。由此可推断CsRboh04在柑橘中可能参与抗病。
本试验中,溃疡病菌诱导24 h内,CsRboh06在感病品种晚锦橙中表达量逐渐上调,在抗病品种四季橘中逐渐下调,在抗、感品种中表达趋势基本相反,且顺式作用元件分析发现该基因在启动子区域均包含脱落酸、水杨酸、茉莉酸响应元件,CsRboh06也受到相应激素不同程度的诱导表达,推断CsRboh06可能通过响应植物激素信号代谢通路进一步参与植物抗病过程。系统进化树聚类分析发现,AtRboh08(H)、AtRboh10(J)、PtRboh05、PtRboh08与CsRboh06同属第3组。目前对杨树中Rboh的功能研究相对较少,仅有部分研究发现拟南芥中NADPH氧化酶RbohH和RbohJ在花粉中特异表达,共同参与植物花粉管的生长发育,是重要的花粉基因[59]。CsRboh06在抗、感品种中所表现出的完全不同的表达情况及其与植物抗病反应的相关性均有必要在后续试验中进行功能的深入挖掘,不仅可为其他物种中相关基因的功能研究提供参考,也能为柑橘抗溃疡病分子育种抗病基因的筛选提供依据。相关研究表明,受柑橘溃疡病菌诱导后基因的表达差异性可能与抗病过程相关,比如CsBZIP40[3]、CsXTH04[60]和CsAP2-09[6]等在抗、感品种中以相反模式响应溃疡病菌侵染的基因,经功能验证均与溃疡病发生有紧密关系。综合以上分析,推断CsRboh02、CsRboh04、CsRboh06为有潜力的抗溃疡病候选基因。
4 结论
基于柑橘全基因组数据鉴定到7个Rboh基因;定位在柑橘5条染色体上,编码蛋白均含有Rboh保守结构域;CsRboh02、CsRboh04和CsRboh06在抗、感品种中受柑橘溃疡病菌诱导表现出不同的表达模式,推测其可能与柑橘对溃疡病抗性相关。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1038/hortres.2015.42URLPMID:26504581 [本文引用: 1]

Citrus canker, caused by the bacterial pathogen Xanthomonas citri ssp. citri (Xcc), has been attributed to millions of dollars in loss or damage to commercial citrus crops in subtropical production areas of the world. Since identification of resistant plants is one of the most effective methods of disease management, the ability to screen for resistant seedlings plays a key role in the production of a long-term solution to canker. Here, an inverse correlation between reactive oxygen species (ROS) production by the plant and the ability of Xcc to grow and form lesions on infected plants is reported. Based on this information, a novel screening method that can rapidly identify citrus seedlings that are less susceptible to early infection by Xcc was devised by measuring ROS accumulation triggered by a 22-amino acid sequence of the conserved N-terminal part of flagellin (flg22) from X. citri ssp. citri (Xcc-flg22). In addition to limiting disease symptoms, ROS production was also correlated with the expression of basal defense-related genes such as the pattern recognition receptors LRR8 and FLS2, the leucine-rich repeat receptor-like protein RLP12, and the defense-related gene PR1, indicating an important role for pathogen-associated molecular pattern-triggered immunity (PTI) in determining resistance to citrus canker. Moreover, the differential expression patterns observed amongst the citrus seedlings demonstrated the existence of genetic variations in the PTI response among citrus species/varieties.
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1080/10715762.2018.1473572URLPMID:29732902 [本文引用: 1]

Plant respiratory burst oxidase homologs (Rbohs), which are also named nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs), are the homologs of mammalian phagocyte gp91(phox). As a unique among other reactive oxygen species (ROS) production mechanisms in plants, NADPH oxidases can integrate different signal transduction pathways, such as calcium, protein phosphorylation catalysed by protein kinases, nitric oxide, and lipid messengers. Coupling with genetic studies, the ability of plant NADPH oxidases to integrate different signal transduction pathways with ROS production demonstrates their involvement in many important biological processes in cells, such as morphogenesis and development, and stress responses. Here, we focus on several current studies concerning the role of plant NADPH oxidases in stress responses.
[本文引用: 1]
[本文引用: 3]
[本文引用: 2]
.
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URLPMID:9628030 [本文引用: 1]
URLPMID:14973161 [本文引用: 1]
URLPMID:11158538 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
DOI:10.1046/j.1365-313x.2002.01342.xURLPMID:12121444 [本文引用: 1]

A cDNA encoding a protein, NtrbohD, located on the plasma membrane and homologue to the flavocytochrome of the neutrophil NADPH oxidase, was cloned in tobacco. The corresponding mRNA was accumulated when tobacco leaves and cells were treated with the fungal elicitor cryptogein. After elicitation with cryptogein, tobacco cells transformed with antisense constructs of NtrbohD showed the same extracellular alkalinization as the control, but no longer produced active oxygen species (AOS). This work represents the first demonstration of the function of a homologue of gp91-phox in AOS production in elicited tobacco cells.
URLPMID:11386368 [本文引用: 1]
[本文引用: 1]
URLPMID:19592501 [本文引用: 1]
URLPMID:20181664 [本文引用: 1]
URLPMID:17046982 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/jxb/ers284URLPMID:23095998 [本文引用: 1]

Reactive oxygen species are increasingly perceived as players in plant development and plant hormone signalling pathways. One of these species, superoxide, is produced in the apoplast by respiratory burst oxidase homologues (rbohs), a family of proteins that is conserved throughout the plant kingdom. Because of the availability of mutants, the focus of research into plant rbohs has been on Arabidopsis thaliana, mainly on AtrbohD and AtrbohF. This study investigates: (i) a different member of the Atrboh family, AtrbohB, and (ii) several rbohs from the close relative of A. thaliana, Lepidium sativum ('cress'). Five cress rbohs (Lesarbohs) were sequenced and it was found that their expression patterns were similar to their Arabidopsis orthologues throughout the life cycle. Cress plants in which LesarbohB expression was knocked down showed a strong seedling root phenotype that resembles phenotypes associated with defective auxin-related genes. These transgenic plants further displayed altered expression of auxin marker genes including those encoding the auxin responsive proteins 14 and 5 (IAA14 and IAA5), and LBD16 (LATERAL ORGAN BOUNDARIES DOMAIN16), an auxin-responsive protein implicated in lateral root initiation. It is speculated that ROS produced by rbohs play a role in root development via auxin signalling.
URLPMID:16760484 [本文引用: 2]
URLPMID:18650210 [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
URLPMID:12355163 [本文引用: 1]
URLPMID:24908277 [本文引用: 1]
DOI:10.1038/ng.2472URLPMID:23179022 [本文引用: 1]

Oranges are an important nutritional source for human health and have immense economic value. Here we present a comprehensive analysis of the draft genome of sweet orange (Citrus sinensis). The assembled sequence covers 87.3% of the estimated orange genome, which is relatively compact, as 20% is composed of repetitive elements. We predicted 29,445 protein-coding genes, half of which are in the heterozygous state. With additional sequencing of two more citrus species and comparative analyses of seven citrus genomes, we present evidence to suggest that sweet orange originated from a backcross hybrid between pummelo and mandarin. Focused analysis on genes involved in vitamin C metabolism showed that GalUR, encoding the rate-limiting enzyme of the galacturonate pathway, is significantly upregulated in orange fruit, and the recent expansion of this gene family may provide a genomic basis. This draft genome represents a valuable resource for understanding and improving many important citrus traits in the future.
URLPMID:24489955 [本文引用: 1]
URLPMID:25017189 [本文引用: 1]
URLPMID:23180785 [本文引用: 1]
URLPMID:18554390 [本文引用: 1]
[本文引用: 2]
DOI:10.1038/s41438-019-0234-3URLPMID:32025315 [本文引用: 1]

Citrus is one of the most important commercial fruit crops worldwide. With the vast genomic data currently available for citrus fruit, genetic relationships, and molecular markers can be assessed for the development of molecular breeding and genomic selection strategies. In this study, to permit the ease of access to these data, a web-based database, the citrus genomic variation database (CitGVD, http://citgvd.cric.cn/home) was developed as the first citrus-specific comprehensive database dedicated to genome-wide variations including single nucleotide polymorphisms (SNPs) and insertions/deletions (INDELs). The current version (V1.0.0) of CitGVD is an open-access resource centered on 1,493,258,964 high-quality genomic variations and 84 phenotypes of 346 organisms curated from in-house projects and public resources. CitGVD integrates closely related information on genomic variation annotations, related gene annotations, and details regarding the organisms, incorporating a variety of built-in tools for data accession and analysis. As an example, CitGWAS can be used for genome-wide association studies (GWASs) with SNPs and phenotypic data, while CitEVOL can be used for genetic structure analysis. These features make CitGVD a comprehensive web portal and bioinformatics platform for citrus-related studies. It also provides a model for analyzing genome-wide variations for a wide range of crop varieties.
DOI:10.1093/nar/gks400URLPMID:22661580 [本文引用: 1]

ExPASy (http://www.expasy.org) has worldwide reputation as one of the main bioinformatics resources for proteomics. It has now evolved, becoming an extensible and integrative portal accessing many scientific resources, databases and software tools in different areas of life sciences. Scientists can henceforth access seamlessly a wide range of resources in many different domains, such as proteomics, genomics, phylogeny/evolution, systems biology, population genetics, transcriptomics, etc. The individual resources (databases, web-based and downloadable software tools) are hosted in a 'decentralized' way by different groups of the SIB Swiss Institute of Bioinformatics and partner institutions. Specifically, a single web portal provides a common entry point to a wide range of resources developed and operated by different SIB groups and external institutions. The portal features a search function across 'selected' resources. Additionally, the availability and usage of resources are monitored. The portal is aimed for both expert users and people who are not familiar with a specific domain in life sciences. The new web interface provides, in particular, visual guidance for newcomers to ExPASy.
[本文引用: 1]
DOI:10.1093/molbev/msw054URLPMID:27004904 [本文引用: 1]

We present the latest version of the Molecular Evolutionary Genetics Analysis (Mega) software, which contains many sophisticated methods and tools for phylogenomics and phylomedicine. In this major upgrade, Mega has been optimized for use on 64-bit computing systems for analyzing larger datasets. Researchers can now explore and analyze tens of thousands of sequences in Mega The new version also provides an advanced wizard for building timetrees and includes a new functionality to automatically predict gene duplication events in gene family trees. The 64-bit Mega is made available in two interfaces: graphical and command line. The graphical user interface (GUI) is a native Microsoft Windows application that can also be used on Mac OS X. The command line Mega is available as native applications for Windows, Linux, and Mac OS X. They are intended for use in high-throughput and scripted analysis. Both versions are available from www.megasoftware.net free of charge.
[本文引用: 2]
[本文引用: 2]
URLPMID:19458158 [本文引用: 1]
URLPMID:32089529 [本文引用: 1]

Class III peroxidase (CIII prx) is a plant-specific multigene family that regulates the physiological and stress responses. This research aimed to exhaustively annotate and analyse the CIII prx family in sweet orange and to explore the regulated expression profiles by Xanthomonas citri subsp. citri (Xcc) and plant hormones. We further assessed the relationship between CIII prxs and citrus bacterial canker. The phylogeny, gene structure, conserved motifs, gene duplications and microsynteny of the CIII prx family were analysed. Expression profiles of specific CsPrxs induced by Xanthomonas citri subsp. citri and plant hormones were detected by quantitative reverse transcription-polymerase chain reaction. Subcellular localization was analysed through transient expression assessments. A total of 72 CIII prx members were identified from the genomes of sweet orange. In all chromosomes of sweet orange, the CsPrxs could be detected except in chromosome 8. In addition, three segmental duplications, four tandem duplications and 11 whole-genome duplications occurred among the CsPrxs, contributing to the family size expansion. From the Ka/Ks ratios, 15 of 18 duplicated CsPrxs pairs have experienced purifying selection process. A total of 15 conserved motifs were detected in CsPrxs, four of which were detected in all complete CsPrxs. A total of 12 expressed genes were identified from the EST database. The expression trends of 12 CsPrxs were differently expressed at different stages of infection by Xcc, five of which were potential candidate genes involved in Xcc resistance. These genes could be induced by salicylic acidand methyl jasmonate, and were extracellular proteins. These results further support our understanding of CIII prxs in citrus, particularly incitrus bacterial canker studies.
URLPMID:11846609 [本文引用: 1]
URLPMID:26113854 [本文引用: 1]
DOI:10.1007/s13258-019-00821-xURLPMID:31140145 [本文引用: 1]

BACKGROUND: Membrane-bound NADPH oxidases (Nicotinamide adenine ainucleotide phosphate oxidase) also called respiratory burst oxidase homologs (Rboh) play an essential role in ROS production under normal as well as environmental stress conditions in plants. OBJECTIVE: To identify and study respiratory burst homologs (Rboh) from the wheat genome as well as characterize their role in various biological and molecular processes along with expression in response to biotic and abiotic stresses. METHODS: The Rboh homologs in the wheat genome were predicted based on data processing, alignment of sequences and phylogenetic analysis of sequences in numerous plant species and wheat. The conserved motifs were known followed by domain design study. The 3-D structure prediction and similarity modeling were administered for NADPH enzyme domain. Gene ontology and a functional study were done in addition to expression analysis of Triticum aestivum respiratory burst oxidase (TaRboh) gene family in response to biotic as well as abiotic stress. RESULTS: Phylogenetic analysis of Rboh gene family members among seven plant species including wheat, classified the family into four subfamilies. Rboh genes are mainly involved in various biological processes such as Response to oxidative stress, Superoxide anion generation, Hydrogen peroxide biosynthetic process. Among the molecular functions, calcium ion binding, peroxidase activity, oxidoreductase activity, superoxide-generating NADPH oxidase activity are essential. Enzyme annotation of the family and superfamily revealed that it encodes to five structural clusters and coding to enzymes NAD(P)H oxidase (H2O2-forming) (EC:1.6.3.1), Ferric-chelate reductase (NADH) (EC: 1.16.1.7), Peroxidase (EC: 1.11.1.7), Ribose-phosphate diphosphokinase (EC: 2.7.6.1). The enzymes contain six membrane-spanning domains, two hemes, and conserved motifs associated with NADPH, EF-hand and FAD binding. The outcomes additionally reflect a distinct role of this enzyme in different molecular functions which are responsible for the stress signaling. Further, the transcripts of TaRboh found expressed in various plant parts such as stem, leaves, spike, seed, and roots. We also observed expression of these gene family members under drought/combination of drought + heat and important wheat pathogens such as Puccinia striformis, Blumeria graminis f.sp. tritici, Fusarium graminiarum, F. pseudograminiarum, and Zymoseptoria tritici. CONCLUSIONS: The investigation demonstrated that identified respiratory burst homologs (Rboh) in T. aestivum were involved in pathogen activated ROS production and have regulatory functions in cell death and defense responses.
DOI:10.1016/j.ygeno.2019.08.010URLPMID:31430516 [本文引用: 2]

Plant respiratory burst oxidase homolog (Rboh) gene family encodes the key enzymatic subunits of reactive oxygen species (ROS) production pathways, and play crucial role in plant signaling, development and stress responses. In present work, twenty genes were identified in Nicotiana tabacum Rboh family (NtabRboh) and classified into four phylogenetic groups (I-IV). Fourteen NtabRboh genes were positioned on ten chromosomes (i.e., Ch1, 2, 4, 7-11, 14 and 21), and six scaffolds. Synteny and evolutionary analysis showed that most of the NtabRboh genes have evolved from the genomes of the ancestor species (N.tomentosiformis and N.sylvestris), which afterwards expanded through duplication events. The promoter regions of the NtabRboh genes contained numerous cis-acting regulatory elements for hormones, plant growth, and different biotic and abiotic factors. The NtabRbohF gene transcript comprised target sites for wounding and stress responsive microRNAs: nta-miR166a-d, g and h. The transcript abundance of NtabRboh genes in different tissues reflected their important for plant growth and organ development in tobacco. RT-qPCR-assays demonstrated that the expression of NtabRboh genes are regulated by viral and bacterial pathogens, drought, cold and cadmium stress. The expression levels NtabRbohA, B and C were significantly up-regulated in
URLPMID:24351809 [本文引用: 1]
[本文引用: 2]
DOI:10.1073/pnas.0708139104URLPMID:17998535 [本文引用: 1]

Plants activate distinct defense responses depending on the lifestyle of the attacker encountered. In these responses, salicylic acid (SA) and jasmonic acid (JA) play important signaling roles. SA induces defense against biotrophic pathogens that feed and reproduce on live host cells, whereas JA activates defense against necrotrophic pathogens that kill host cells for nutrition and reproduction. Cross-talk between these defense signaling pathways has been shown to optimize the response against a single attacker. However, its role in defense against multiple pathogens with distinct lifestyles is unknown. Here we show that infection with biotrophic Pseudomonas syringae, which induces SA-mediated defense, rendered plants more susceptible to the necrotrophic pathogen Alternaria brassicicola by suppression of the JA signaling pathway. This process was partly dependent on the cross-talk modulator NPR1. Surprisingly, this tradeoff was restricted to tissues adjacent to the site of initial infection; A. brassicicola infection in systemic tissue was not affected. Even more surprisingly, tradeoff occurred only with the virulent Pseudomonas strain. Avirulent strains that induced programmed cell death (PCD), an effective plant-resistance mechanism against biotrophs, did not cause suppression of JA-dependent defense. This result might be advantageous to the plant by preventing necrotrophic pathogen growth in tissues undergoing PCD. Our findings show that plants tightly control cross-talk between SA- and JA-dependent defenses in a previously unrecognized spatial and pathogen type-specific fashion. This process allows them to prevent unfavorable signal interactions and maximize their ability to concomitantly fend off multiple pathogens.
DOI:10.1104/pp.108.129791URLPMID:19126699 [本文引用: 1]
DOI:10.1016/j.biotechadv.2014.02.002URL [本文引用: 1]

NADPH oxidase (NOX) is a key player in the network of reactive oxygen species (ROS) producing enzymes. It catalyzes the production of superoxide (O-2(-)), that in turn regulates a wide range of biological functions in a broad range of organisms. Plant Noxes are known as respiratory burst oxidase homologs (Rbohs) and are homologs of catalytic subunit of mammalian phagocyte gp91(phox). They are unique among other ROS producing mechanisms in plants as they integrate different signal transduction pathways in plants. In recent years, there has been addition of knowledge on various aspects related to its structure, regulatory components and associated mechanisms, and its plethora of biological functions. This update highlights some of the recent developments in the field with particular reference to important members of the plant kingdom. (C) 2014 Elsevier Inc.
DOI:10.1073/pnas.012452499URLPMID:11756663 [本文引用: 1]

Reactive oxygen intermediates (ROI) are strongly associated with plant defense responses. The origin of these ROI has been controversial. Arabidopsis respiratory burst oxidase homologues (rboh genes) have been proposed to play a role in ROI generation. We analyzed lines carrying dSpm insertions in the highly expressed AtrbohD and AtrbohF genes. Both are required for full ROI production observed during incompatible interactions with the bacterial pathogen Pseudomonas syringae pv. tomato DC3000(avrRpm1) and the oomycete parasite Peronospora parasitica. We also observed reduced cell death, visualized by trypan blue stain and reduced electrolyte leakage, in the Atrboh mutants after DC3000(avrRpm1) inoculation. However, enhanced cell death is observed after infection of mutant lines with P. parasitica. Paradoxically, although atrbohD mutation eliminated the majority of total ROI production, atrbohF mutation exhibited the strongest effect on cell death.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.compbiolchem.2016.04.002URLPMID:27111707 [本文引用: 1]

NADPH oxidase (NOX) is a critical enzyme in the production of reactive oxygen species (ROS). It catalyzes the production of apoplastic superoxide (O2(-)), that regulates a wide array of biological functions in different organisms. Plant Noxes are homologs of catalytic subunit of mammalian NADPH oxidase and are well-known as Respiratory burst oxidase homologs (Rbohs). In recent years, there has been growing interest to study plant Noxes due to their versatile roles in plant systems. In the present work, comprehensive analysis on upstream regions from 10 Rbohs from Arabidopsis thaliana and 9 from Oryza sativa japonica was conducted. The distribution of various cis-elements, CpG islands and tandem repeats were analyzed to uncover the 5' regulatory region in wide array of functions from Rbohs. Information retrieved from cis-elements analysis was also correlated with the microarray data. Present study which involves uncovering transcription regulatory elements provided vital clues for diverse functions of plant Rbohs.
[本文引用: 1]
DOI:10.1111/j.1469-8137.2007.02042.xURLPMID:17504458 [本文引用: 1]

Tip-localized reactive oxygen species (ROS) were detected in growing pollen tubes by chloromethyl dichlorodihydrofluorescein diacetate oxidation, while tip-localized extracellular superoxide production was detected by nitroblue tetrazolium (NBT) reduction. To investigate the origin of the ROS we cloned a fragment of pollen specific tobacco NADPH oxidase (NOX) closely related to a pollen specific NOX from Arabidopsis. Transfection of tobacco pollen tubes with NOX-specific antisense oligodeoxynucleotides (ODNs) resulted in decreased amount of NtNOX mRNA, lower NOX activity and pollen tube growth inhibition. The ROS scavengers and the NOX inhibitor diphenylene iodonium chloride (DPI) inhibited growth and ROS formation in tobacco pollen tube cultures. Exogenous hydrogen peroxide (H2O2) rescued the growth inhibition caused by NOX antisense ODNs. Exogenous CaCl2 increased NBT reduction at the pollen tube tip, suggesting that Ca2+ increases the activity of pollen NOX in vivo. The results show that tip-localized ROS produced by a NOX enzyme is needed to sustain the normal rate of pollen tube growth and that this is likely to be a general mechanism in the control of tip growth of polarized plant cells.
DOI:10.3389/fpls.2019.01109URLPMID:31611887 [本文引用: 1]

In this study, we performed a comprehensive survey of xyloglucan endotransglucosylase/hydrolase (XTH) and a functional validation of Citrus sinensis (Cs) XTH genes to provide new insights into the involvement of XTHs in Xanthomonas citri subsp. citri (Xcc) infection. From the genome of sweet orange, 34 CsXTH genes with XTH characteristic domains were identified and clustered into groups I/II, IIIA, and IIIB. Except for chromosome 9, the CsXTH genes were unevenly distributed and duplicated among all chromosomes, identifying a CsXTH duplication hot spot on chromosome 4. With Xcc induction, a group of citrus canker-related CsXTHs were detected. CsXTH04 was identified as a putative candidate gene, which is up-regulated in citrus bacterial canker (CBC)-resistant varieties and induced by exogenous treatment with salicylic acid (SA) and methyl jasmonate (MeJA). CsXTH04 overexpression conferred CBC susceptibility to transgenic citrus, while CsXTH04 silencing conferred CBC resistance. Taken together, the annotation of the CsXTH family provides an initial basis for the functional and evolutionary study of this family as potential CBC-susceptible genes. CsXTH04, validated in this study, can be used in citrus breeding to improve CBC resistance.
