 ,1,2, 王美1, 刘建阳2, 林伟2, 张成省1, 赵栋霖
,1,2, 王美1, 刘建阳2, 林伟2, 张成省1, 赵栋霖 ,1
,1Secondary Metabolites from a Marine-Derived Fungus Aspergillus versicolor and Their Anti-Phytopathogenic Bacterial Activity
FU Bing ,1,2, WANG Mei1, LIU JianYang2, LIN Wei2, ZHANG ChengSheng1, ZHAO DongLin
,1,2, WANG Mei1, LIU JianYang2, LIN Wei2, ZHANG ChengSheng1, ZHAO DongLin ,1
,1通讯作者:
责任编辑: 岳梅
收稿日期:2020-02-29接受日期:2020-05-8网络出版日期:2020-10-01
| 基金资助: |
Received:2020-02-29Accepted:2020-05-8Online:2020-10-01
作者简介 About authors
付兵,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (534KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
付兵, 王美, 刘建阳, 林伟, 张成省, 赵栋霖. 海洋来源杂色曲霉次级代谢产物及其抗植物病原细菌活性[J]. 中国农业科学, 2020, 53(19): 3964-3974 doi:10.3864/j.issn.0578-1752.2020.19.010
FU Bing, WANG Mei, LIU JianYang, LIN Wei, ZHANG ChengSheng, ZHAO DongLin.
0 引言
【研究意义】随着世界人口数量的不断增长,避免饥饿、保障粮食安全显得尤为重要,因此,需要控制植物病害的发生,增加和保证粮食的产量[1,2]。植物病原细菌在世界农业生产历史上曾经多次造成重大影响,作物细菌病害是导致农作物减产、人口数量下降和社会动荡的客观因素之一[3]。已知的植物病原细菌有200多个属,其中最重要的包括假单胞菌属(Pseudomonas)、雷尔氏菌属(Ralstonia)、农杆菌属(Agrobacterium)、黄单胞菌属(Xanthomonas)、欧文氏菌属(Erwinia)、木质部小菌属(Xylella)和果胶杆菌属(Pectobacterium)[4,5,6]。目前防治植物细菌病害的方法主要是采用抗病品种、加强田间管理和轮作等方式,但效果有限[4];防治的药剂主要为化学农药,容易造成环境污染、食品安全和病原抗性等一系列问题[4,7]。一直以来,农用硫酸链霉素都是我国农作物细菌病害防治的主力军,而随着我国停止使用农用硫酸链霉素,植物细菌病害的防治面临巨大挑战。随着国家大力推行发展绿色农业,更加注重环境友好,研发新型高效低毒的抗细菌生物农药迫在眉睫。【前人研究进展】众所周知,海洋真菌由于生活在高盐、高压、低温、低氧、少光和寡营养的海洋环境中,拥有独特的生存策略,经过长期的进化演变形成了不同于陆地真菌的特殊代谢机制,能够产生种类繁多、结构新颖、生物活性显著的次级代谢产物,因而近年来成为研究的热点[8]。许多海洋真菌次级代谢产物具有明显的抗细菌活性,显示出了作为新型抗细菌农药的巨大开发潜力[9]。然而,对于这些化合物的抗细菌活性研究大多集中在人类医学领域,关于农业领域的研究和应用还很少,但仅有的少量研究展现了极其可观的研究价值和前景。UCHIDA等[10]从一株未鉴定的海洋真菌发酵粗提物中分离获得了两个聚酮类化合物chlorogentisylquinone和gentisylquinone,它们在10 μg/disk的浓度下对水稻黄单胞菌(Xanthomonas oryzae)的抑菌圈分别为9和10 mm,表现出了较强的抑菌活性;SWATHI等[11]对海洋来源的一株曲霉(Aspergillus sp.)发酵提取物进行抗菌活性研究,发现其对野油菜黄单胞菌(Xanthomonas campestris)具有较强的抑菌活性,在100 μg·mL-1的浓度下抑菌圈达到12 mm,有望从中发现强活性抗菌化合物;SILBER等从一株海水来源的真菌Calcarisporium sp. KF525发酵液中分离获得了一个二十四元大环内酯类化合物calcaride A,其对野油菜黄单胞菌显示了中等强度的抑菌活性,最小抑菌浓度(MIC)为5.5 μmol·L-1[12];随后,该小组发现分离自波罗的海和南极海域的两株Lindgomycetaceae所产聚酮类化合物lindgomycin和ascosetin,对野油菜黄单胞菌的IC50分别为17.8和14.8 μmol·L-1[13];HENRIQUEZ等[14]从南极海域采集的海绵中分离获得了101株真菌,发现其中11株真菌的发酵提取物对密执安棒形杆菌(Clavibacter michiganensis)具有抑菌活性,22株真菌的提取物对野油菜黄单胞菌表现出了明显的抑菌活性;NIU等[15]对分离自深海来源真菌Spiromastix sp. MCCC 3A00308的多酚类化合物进行研究,发现spiromastols A和C表现出极强的抗植物病原细菌活性,对野油菜黄单胞菌、假单胞菌(Pseudomonas lachrymans)、根癌农杆菌(Agrobacterium tumefaciens)和青枯雷尔氏菌(Ralstonia solanacearum)的MIC达到0.25—0.5 μmol·L-1,活性强于阳性药氯霉素,具有开发成为抗细菌农药的潜力;WANG等[16]对一株海胆来源的亮白曲霉(Aspergillus candidus)HDf2进行研究,从中获得了两个具有抗菌活性的刺孢青霉酸类化合物spiculisporic acid F和G,它们对青枯雷尔氏菌显示了中等强度的抑菌活性;CHEN等[17]对一株海藻来源的格孢腔菌(Pleosporales sp.)所产混源萜类次级代谢产物进行研究,发现pleosporallins D和E对密执安棒形杆菌显示了中等活性的抑菌活性,MIC为9.48 μg·mL-1。【本研究切入点】笔者课题组在前期研究中,从山东沿海和海南海洋特殊红树林生境中分离纯化了200余株海洋真菌,通过初步的形态学排重后,对其进行了种属鉴定。对其中近80株真菌进行小规模发酵,测试其发酵产物乙酸乙酯提取物的抗植物病原细菌活性,筛选得到多株抗菌活性显著的海洋真菌[18,19]。其中,一株海藻来源的杂色曲霉(Aspergillus versicolor)D5,其发酵提取物对青枯雷尔氏菌等植物病原细菌表现出了明显的抗菌活性。HPLC指纹图谱分析发现,D5次级代谢产物丰富,结构类型多样,产量较大,有望从中发现抗菌活性化合物。【拟解决的关键问题】从杂色曲霉D5发酵产物中分离、纯化单体化合物,并采用多种谱学方法对其进行结构鉴定,进而对单体化合物进行抗植物病原细菌活性测试并分析构效关系,阐明目标菌株的活性成分及活性化合物的关键作用基团,为海洋真菌来源高效低毒生物农药的研发提供化合物基础。1 材料与方法
试验于2017—2019年在中国农业科学院烟草研究所海洋农业研究中心/滩涂生物资源保护利用创新团队完成。1.1 仪器与试剂
核磁共振波谱仪Agilent DD2 500(美国安捷伦公司);质谱仪ESI-MS Q-TOF Ultima Global GAA076(美国Waters公司)和Q-TOF maXis(美国Bruker公司);高效液相色谱仪(日本日立公司);C18柱:(5 μm,10 mm×250 mm,美国Waters公司);旋转蒸发仪Hei-VAP Industrial-glassware set R(德国Heidoph公司);LRH-250生化培养箱(上海一恒科学仪器有限公司);正相柱层析硅胶100—200、200—300目(青岛海洋化工厂);凝胶Sephadex LH-20(美国GE公司);正相TLC预制板(烟台汇有硅胶开发有限公司);马铃薯葡萄糖水培养基(青岛海博生物技术有限公司);马铃薯葡萄糖琼脂培养基(青岛海博生物技术有限公司);HPLC用甲醇和乙腈为色谱纯(国药集团),其他试剂均为分析纯。1.2 菌株发酵与培养
1.2.1 菌株来源 海洋真菌分离自山东青岛沿海一种未鉴定的海藻,通过形态学及分子生物学鉴定方法确定为杂色曲霉D5。菌种于中国微生物菌种保藏管理委员会普通微生物中心保藏,保藏编号为CGMCC NO. 15386。1.2.2 菌株发酵与培养 500 mL的发酵瓶中倒入250 mL马铃薯葡萄糖水(PDW)培养基,灭菌。挑取生长于马铃薯葡萄糖琼脂(PDA)培养基上的目标菌株加入其中,共发酵10瓶。摇床(180 r/min,28℃)振荡培养72 h,获得种子液。将400 mL PDW培养基加入容量为1 L的发酵瓶中,加入粗海盐使其浓度为3%,灭菌,共发酵80 L。取种子液5 mL分别加入400 mL PDW培养基中,于28℃静置培养30 d。
发酵结束后,用纱布将菌体和发酵液过滤分开,发酵液采用乙酸乙酯萃取两遍,浓缩至干;菌体采用乙酸乙酯浸泡菌体一次,后采用二氯甲烷﹕甲醇=1﹕1浸泡一遍,浓缩后,将未干的溶液用乙酸乙酯萃取3遍,浓缩至干,与菌液提取物合并,得到发酵提取物26.8 g。
1.3 化合物分离纯化
将发酵提取物进行减压硅胶柱层析,以乙酸乙酯-石油醚(0—100%)、甲醇-乙酸乙酯(0—100%)为流动相进行梯度洗脱,分为7个组分(Fr.1—Fr.7)。Fr.2(1.6 g)经ODS反相硅胶柱层析梯度洗脱(甲醇-水,60%—100%),后经过Sephadex LH-20凝胶柱层析(二氯甲烷-甲醇,1/1)得到Fr.2-1—Fr.2-5。Fr.2-5经反相HPLC(70%乙腈-水加0.1%三氟乙酸,2 mL·min-1)纯化制备后得到11(28.1 mg)和12(11.5 mg)。Fr.3(4.5 g)经正相硅胶柱层析(乙酸乙酯-石油醚,5%—100%)分离,后经过ODS反相硅胶柱层析梯度洗脱,流动相采用30%—100%甲醇-水,得到Fr.3-1—Fr.3-6。Fr.3-2经过Sephadex LH-20凝胶柱层析(二氯甲烷-甲醇,1/1),得到6(236.3 mg)。Fr.3-4经过与Fr.3-2相同的分离步骤后,得到1(38.1 mg)。Fr.3-5经过Sephadex LH-20凝胶柱层析(二氯甲烷-甲醇,1/1),后经过100﹕1二氯甲烷/甲醇正相硅胶柱层析纯化,得到Fr.3-5-1—Fr.3-5-3。Fr.3-5-1经HPLC(60%甲醇-水,2 mL·min-1)分离纯化后得到7(46.7 mg)。Fr.3-5-2和Fr.3-5-3分别在二氯甲烷-甲醇=1/1溶液中重结晶,得到2(13.6 mg)和3(5.4 mg)。Fr.3-6经过反复的正相硅胶柱层析,最后通过HPLC制备,流动相为75%甲醇-水,得到8(14.0 mg)。Fr.4(3.3 g)经过30%—90%甲醇-水ODS反相硅胶柱梯度洗脱,后经过Sephadex LH-20凝胶柱层析(二氯甲烷-甲醇,1/1)纯化,得到Fr.4-1—Fr.4-4。Fr.4-2在二氯甲烷-甲醇混合液中重结晶,得到5(118.4 mg)。Fr.4-3经过HPLC纯化,流动相为45%甲醇-(水+0.1%三氟乙酸),得到4(5.1 mg)。Fr.4-3经HPLC 50%甲醇-水制备,得到10(35.8 mg)。Fr.5(2.4 g)经过ODS反相硅胶柱层析梯度洗脱(甲醇-水,30%—90%),然后通过Sephadex LH-20凝胶柱层析(二氯甲烷-甲醇,1/1)分离,最后经反相HPLC(55%甲醇-水,2 mL·min-1)制备,得到9(48.0 mg)。1.4 抗菌活性测定
病原细菌:燕麦食酸菌(Acidovorax avenae)、胡萝卜软腐欧文氏菌(Erwinia carotovora)、密执安棒形杆菌、丁香假单胞菌(Pseudomonas syringae)、青枯雷尔氏菌和野油菜黄单胞菌。采用微量稀释法,测试化合物对几种植物病原细菌的MIC [20]。病原菌振荡培养至对数期,并稀释至106 cfu/mL。将待测化合物用DMSO配成10 mg·mL-1母液。每种病原菌采用单独的96孔板进行测试。在96孔板第1列的第1孔加198 μL菌液,余下2—8孔各加100 μL菌液。吸取2 μL化合物母液加入第1孔,混合均匀。采用二倍稀释法将1—8孔依次稀释,即从第一孔中吸取100 μL含药菌液加入第2孔混匀后,从第2孔吸取100 μL含药菌液加入第3孔,并混匀,以此类推。到第8孔混匀后,移除100 μL含药菌液,使每孔都含有100 μL含药菌液,后再每孔加入100 μL菌液,使每孔体积达到200 μL。每个化合物重复3列。用无菌培养基作空白对照,并设立未加化合物的菌液对照,DMSO作阴性对照,空白对照和阴性对照分别做3个孔,硫酸链霉素做阳性对照,测试方法与化合物相同。将96孔板放入生物培养箱中,37℃培养24 h,酶标仪600 nm波长下测量吸光度,并结合肉眼观察,以吸光度出现大幅度变化,且最后一个澄清的孔对应的浓度,作为化合物MIC值。
2 结果
2.1 结构鉴定
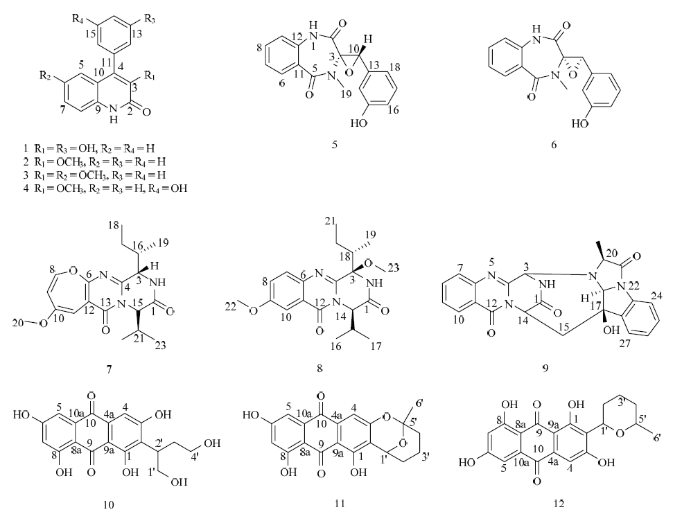
化合物1为白色晶体,质谱ESIMS给出分子离子峰m/z 254.1 [M + H]+,结合其氢谱和碳谱,推测其分子式为C15H11NO3,不饱和度为11。在1H NMR中给出了8个芳香氢信号,提示分子中存在2个苯环。在13C NMR中,低场区给出了14个芳香碳和烯碳信号以及1个酰胺碳信号δC 160.5,其中包括2个连氧的sp2杂化碳信号δC 158.7和143.0,结合其分子式,提示分子中存在2个羟基。以上数据与文献报道的化合物viridicatol数据一致[21],故鉴定化合物1为 viridicatol(图1)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1化合物1—12的化学结构图
Fig. 1The chemical structure of compounds 1-12
核磁波谱数据:1H-NMR(500 MHz,CD3OD):δH 7.34(2H,m,H-7,H-8),7.31(1H,d,J=8.0 Hz,H-15),7.24(1H,d,J=8.0 Hz,H-6),7.11(1H,m,H-5),6.87(1H,m,H-14),6.80—6.82(2H,m,H-12,H-16);13C-NMR(125 MHz,CD3OD):δC 160.5(C-2),158.7(C-13),143.0(C-3),136.1(C-4),134.3(C-9),130.6(C-11),128.0(C-15),127.3(C-7),126.3(C-5),123.8(C-6),123.0(C-10),122.1(C-16),117.9(C-12),116.5(C-8),116.0(C-14);ESIMS:m/z 254.1 [M + H]+。
化合物2为白色晶体,ESIMS:m/z 252.1 [M + H]+,结合其氢谱和碳谱,推测分子式为C16H13NO2,不饱和度为11。1H NMR谱δH 12.66处给出1个酰胺氢特征信号,低场区给出了9个芳香氢信号,以上信息结合TLC特征,推测2与1为同系列化合物。在13C-NMR中,δC 161.1提示酰胺碳的存在;在低场区给出了14个碳信号,包括5个季碳和9个叔碳;δC 60.4处的信号提示分子中存在1个甲氧基。以上数据经过与文献对比,确定化合物2为3-O- methylviridicatin[22](图1)。
核磁波谱数据:1H-NMR(500 MHz,CDCl3):δH 12.66(1H,s,NH-1),7.53(2H,t,J=7.5 Hz,H-13,H-15),7.49(1H,d,J=7.5 Hz,H-14),7.45(1H,t,J=7.5 Hz,H-7),7.38(2H,d,J=7.5 Hz,H-12,H-16),7.23(1H,d,J=7.5 Hz,H-5),7.12(1H,t,J=7.5 Hz,H-6),3.85(3H,s,3-OCH3);13C-NMR(125 MHz,CDCl3):δC 161.1(C-2),145.2(C-3),139.4(C-4),135.5(C-9),133.5(C-11),129.4(C-12,C-16),128.8(C-14),128.4(C-13,C-15),128.2(C-7),126.4(C-5),122.6(C-6),120.9(C-10),116.0(C-8),60.4(3-OCH3);ESIMS:m/z 252.1 [M + H]+。
化合物3为白色晶体,ESIMS质谱中在m/z 282.1 [M + H]+处给出分子离子峰,结合氢谱和碳谱,推测分子式为C17H15NO3,不饱和度为11。仔细分析其1H-NMR和13C NMR数据,发现3的核磁数据与2十分相似。与2相比,3少了1个芳香氢信号,同时增加了1个甲氧基信号δH 3.67,δC 55.5,提示该分子中的1个芳香氢被甲氧基取代。经过与文献数据比对,确定化合物3为3,6-O-dimethylviridicatin[23](图1)。
核磁波谱数据:1H-NMR(500 MHz,CDCl3):δH 12.11(1H,s,NH-1),7.53(2H,t,J=7.5 Hz,H-13,H-15),7.47(1H,t,J=7.5 Hz,H-14),7.41(1H,d,J=8.5 Hz,H-8),7.37(2H,m,H-12,H-16),7.07(1H,dd,J=8.5,2.5 Hz,H-7),6.65(1H,d,J=2.5 Hz,H-5),3.83(3H,s,3-OCH3),3.67(3H,s,6-OCH3);13C-NMR(125 MHz,CDCl3):δC 160.3(C-2),155.2(C-6),145.7(C-3),138.9(C-4),133.5(C-11),129.9(C-9),129.3(C-12,C-16),128.5(C-13,C-15),128.3(C-14),121.7(C-10),117.5(C-7),117.0(C-8),108.6(C-5),60.3(3-OCH3),55.5(6-OCH3);ESIMS:m/z 282.1 [M + H]+。
化合物4为白色粉末,ESIMS:m/z 268.1 [M + H]+,结合氢谱和碳谱,推测其分子式为C16H13NO3。1H-NMR中δH 12.07和9.64处给出2个活泼氢信号,分别对应酰胺氢和羟基氢;低场区给出8个芳香氢信号;δH 3.69信号提示分子中存在1个甲氧基。1C-NMR中δC 158.5和157.3证明了酰胺碳和连氧芳香碳的存在;δC 59.5证明了甲氧基的存在。化合物4与2十分相似,不同之处在于多了1个苯环上的羟基。经过与文献数据比对,确定化合物4为3-O-methylviridicatol[23](图1)。
核磁波谱数据:1H-NMR(500 MHz,DMSO-d6):δH 12.07(1H,s,NH-1),9.64(1H,s,15-OH),7.41(1H,t,J=7.5 Hz,H-7),7.34(1H,d,J=7.5 Hz,H-8),7.30(1H,t,J=7.5 Hz,H-13),7.09(1H,t,J=7.5 Hz,H-6),7.04(1H,d,J=7.5 Hz,H-5),6.85(1H,d,J=7.5 Hz,H-14),6.71(1H,d,J=7.5 Hz,H-12),6.68(1H,s,H-16),3.69(3H,s,3-OCH3);13C-NMR(125 MHz,DMSO-d6):δC 158.5(C-2),157.3(C-15),144.9(C-3),137.6(C-4),135.7(C-9),134.7(C-11),129.5(C-13),128.6(C-7),125.8(C-5),121.9(C-6),119.9(C-10),119.7(C-12),116.0(C-16),115.1(C-8),115.0(C-14),59.5(3-OCH3);ESIMS:m/z 268.1 [M + H]+。
化合物5为白色晶体,ESIMS:m/z 311.1 [M + H]+,结合化合物1H和13C NMR,推断分子式为C17H14N2O4,不饱和度为12。1H-NMR中给出了8个芳香氢信号,1个连氧次甲基δH 4.07和1个氮甲基信号δH 3.19;13C-NMR中给出12个芳香碳信号包括1个连氧芳香碳信号,提示分子中存在2个苯环;另外还给出2个酰胺碳信号δC 168.6和168.4以及1个氮甲基碳信号δC 31.7。以上数据表明化合物5为dioxopiperazine类衍生物,经过与文献进行比对,确定化合物5为(+)-cyclopenol[24](图1)。
核磁波谱数据:1H-NMR(500 MHz,CD3OD):δH 7.55(1H,m,H-8),7.15(3H,m,H-6,H-7,H-9),7.01(1H,t,J=7.5 Hz,H-17),6.71(1H,d,J=7.5 Hz,H-16),6.12(2H,m,H-14,H-18),5.48(1H,s,15-OH),4.07(1H,s,H-10),3.19(3H,s,H-19);13C-NMR(125 MHz,CD3OD):δC 168.6(C-2),168.4(C-5),158.4(C-15),136.4(C-11),134.0(C-8),133.5(C-13),132.2(C-6),130.2(C-17),128.0(C-12),126.1(C-7),122. 3(C-9),118.5(C-18),117.0(C-16),113.9(C-14),71.6(C-3),65.9(C-10),31.7(C-19);ESIMS:m/z 311.1 [M + H]+。
化合物6为白色晶体,HRESIMS:m/z 311.1028 [M + H]+,分子式为C17H14N2O4,不饱和度为12。1H NMR中δH 7.52(1H,dt,J=8.0,1.5 Hz),7.14(1H,d,J=8.0 Hz),7.09(1H,dt,J=8.0,1.0 Hz),7.01(1H,dd,J=8.0,1.0 Hz)提示分子中存在1,2-二取代苯环;δH 6.97(1H,t,J=8.0 Hz),6.66(1H,dd,J=8.0,1.5 Hz),6.11(1H,m),6.00(1H,d,J=8.0 Hz)提示分子中存在1,3-二取代苯环;13C NMR中给出2个酰胺碳信号δC 166.0和165.4,1个氮甲基信号即与氢谱对应的芳香碳信号。以上数据与化合物5极为相似,且分子量相同,仔细与文献比对其核磁数据,确定化合物6为(-)-cyclopenol[23](图1)。
核磁波谱数据:1H-NMR(500 MHz,DMSO-d6):δH 7.52(1H,dt,J=8.0,1.5 Hz,H-8),7.14(1H,d,J=8.0 Hz,H-9),7.09(1H,dt,J=8.0,1.0 Hz,H-7),7.01(1H,dd,J=8.0,1.0 Hz,H-6),6.97(1H,t,J=8.0 Hz,H-17),6.66(1H,dd,J=8.0,1.5 Hz,H-16),6.11(1H,m,H-14),6.00(1H,d,J=8.0 Hz,H-18),4.23(1H,s,H-10),3.06(3H,s,H-19);13C-NMR(125 MHz,DMSO-d6):δC 166.0(C-2),165.4(C-5),156.9(C-15),135.1(C-11),132.4(C-8),132.3(C-13),130.5(C-6),128.9(C-17),126.5(C-12),124.3(C-7),121.1(C-9),117.0(C-18),115.7(C-16),112.8(C-14),70.1(C-3),63.8(C-10),30.9(C-19);HRESIMS:m/z 311.1028 [M + H]+,333.0849 [M + Na]+。
化合物7为黄色粉末,结合其ESIMS、1H和13C NMR,判断分子式为C19H25N3O4,不饱和度为9。1H NMR中δH 7.54(1H,s)给出1个NH信号,6.16(1H,d,J=6.0 Hz,H-8),5.77(1H,s,H-11),5.49(1H,d,J=6.0 Hz,H-9),给出3个芳香氢信号;δH 5.10给出1个连氮氢信号;δH 3.69给出1个甲氧基氢信号;δH 1.13、1.11、0.98和0.84处给出4个甲基信号。13C-NMR给出2个酰胺碳信号δC 167.3和161.4,4个季碳信号,7个次甲基碳信号,1个亚甲基碳信号,1个甲氧基碳信号和4个甲基碳信号。以上数据与文献报道的versicoloid A极为相似,仔细对比核磁数据,确定化合物7为versicoloid A[23](图1)。
核磁波谱数据:1H-NMR(500 MHz,CDCl3):δH 7.54(1H,s,NH-2),6.16(1H,d,J=6.0 Hz,H-8),5.77(1H,s,H-11),5.49(1H,d,J=6.0 Hz,H-9),5.10(1H,d,J=5.5 Hz,H-15),4.46(1H,s,H-3),3.69(3H,s,H-20),2.62(1H,m,H-16),2.26(1H,m,H-21),1.20(2H,m,H-17),1.13(3H,d,J=7.5 Hz,H-19),1.11(3H,d,J=7.0 Hz,H-23),0.98(3H,d,J=7.0 Hz,H-22),0.84(3H,t,J=7.0 Hz,H-18);13C-NMR(125 MHz,CDCl3):δC 167.3(C-1),161.4(C-13),159.6(C-6),156.8(C-10),152.9(C-4),144.3(C-8),115.5(C-9),109.3(C-12),94.8(C-11),60.6(C-15),59.2(C-3),55.1(C-20),36.9(C-16),32.4(C-21),23.0(C-17),20.0(C-22),17.8(C-23),15.8(C-19),12.0(C-18);ESIMS:m/z 360.2 [M + H]+。
化合物8为白色粉末,根据HRESIMS数据,判断分子式为C20H27N3O4。化合物8的核磁数据与7很相似,主要的区别在于2个化合物的1,2,4-三取代芳香环(8:δC/H C/H-7,C/H-8,C/H-10,129.6/7.65, 125.1/7.38,106.4/7.65 vs 7:C/H-8,C/H-9,C/H-11,144.3/6.16,115.5/5.49,94.8/5.77)。同时,化合物8中多了1个甲氧基信号δC/H C/H-7,51.1/3.92。进一步仔细分析其核磁数据,并与文献进行比对,确定化合物8为chrysopiperazine C[25](图1)。
核磁波谱数据:1H-NMR(500 MHz,CDCl3):δH 7.65(2H,d,J=9.0 Hz,H-7,H-10),7.38(1H,d,J=9.0 Hz,H-8),6.99(1H,s,NH-2),5.23(1H,d,J=9.0 Hz,H-14),3.92(3H,s,H-22),3.27(3H,s,H-23),2.96(1H,m,H-18),2.53(1H,m,H-15),1.27(1H,m,H-20b),1.23(3H,m,H-17),1.10(3H,d,J = 7.0 Hz,H-19),1.04(1H,m,H-20a),0.95(6H,m,H-16,H-21);13C-NMR(125 MHz,CDCl3):δC 170.3(C-1),161.5(C-12),159.2(C-9),144.9(C-4),140.6(C-6),129.6(C-7),125.1(C-8),121.5(C-11),106.4(C-10),88.7(C-3),60.9(C-14),56.0(C-22),51.1(C-23),36.4(C-18),33.6(C-15),25.3(C-20),20.2(C-17),19.8(C-16),12.6(C-21),11.1(C-19)。HRESIMS:m/z 374.2078 [M + H]+,396.1897 [M + Na]+。
化合物9为淡黄色粉末,ESIMS:m/z 430.2 [M + H]+,结合其1H-NMR和13C-NMR数据,推断其分子式为C23H19N5O4。1H-NMR在δH 8.10(1H,d,J=8.0 Hz),7.83(1H,t,J=7.5 Hz),7.72(1H,d,J=8.0 Hz),7.52(1H,t,J=7.0 Hz)给出信号,以及7.39(1H,d,J=7.5 Hz),7.27(2H,m),7.07(1H,dd,J=7.0,4.5 Hz)给出信号,提示分子中存在2个1,2-二取代苯环。13C-NMR中δC 167.8、165.6和159.4给出3个酰胺碳信号;低场区给出13个芳香碳信号;此外还给出了4个次甲基信号,1个亚甲基信号和1个甲基信号。以上数据经过与文献进行比对,确定化合物9为cottoquinazoline A[26](图1)。
核磁波谱数据:1H-NMR(500 MHz,DMSO-d6):δH 9.05(1H,d,J=4.0 Hz,NH-2),8.10(1H,d,J=8.0 Hz,H-10),7.83(1H,t,J=7.5 Hz,H-8),7.72(1H,d,J=8.0 Hz,H-7),7.52(1H,t,J=7.0 Hz,H-9),7.39(1H,d,J=7.5 Hz,H-27),7.27(2H,m,H-24,H-25),7.07(1H,dd,J=7.0,4.5 Hz,H-26),5.57(1H,s,17-OH),5.24(2H,m,H-3,H-14),4.85(1H,s,H-18),4.07(1H,q,J=6.5 Hz,H-20),3.05(1H,dd,J=15.0,5.0 Hz,H-15b),2.40(1H,d,J=15.0 Hz,H-15a),1.47(3H,d,J=6.5 Hz,H-29);13C-NMR(125 MHz,DMSO-d6):δC 167.8(C-1),165.6(C-21),159.4(C-12),147.5(C-4),146.7(C-6),139.8(C-28),136.0(C-23),134.5(C-8),129.3(C-25,C-26),127.3(C-7),127.2(C-9),126.2(C-10),124.4(C-27),120.7(C-11),113.8(C-24),79.8(C-18),74.1(C-17),65.5(C-3),63.4(C-20),53.9(C-14),36.1(C-15a),14.8(C-29);ESIMS:m/z 430.2 [M + H]+。
化合物10为橘红色粉末,质谱中在m/z 361.1处给出 [M + H]+分子离子峰,结合1H-NMR和13C-NMR数据,推断其分子式为C18H16O8,不饱和度为11。其HPLC吸收与TLC特征提示该10为蒽醌类化合物。1H-NMR中低场区给出3个芳香氢信号,高场区给出1个亚甲基、1个次甲基信号和1个甲基信号,同时给出2个连氧亚甲基信号。13C-NMR中给出2个羰基碳信号,12个芳香碳信号,2个连氧碳信号。以上数据经过与文献进行比对,确定化合物10为versiconol[27](图1)。
核磁波谱数据:1H-NMR(500 MHz,DMSO-d6):δH 12.74(1H,s,1-OH),12.09(1H,s,8-OH),11.17(2H,s,3-OH,6-OH),7.14(1H,s,H-5),7.02(1H,s,H-4),6.50(1H,s,H-7),3.67(2H,m,H-1′),3.45(1H,m,H-2′),3.28(2H,m,H-4′),1.90(2H,s,H-3′);13C-NMR(125 MHz,DMSO-d6):δC 189.0(C-9),181.3(C-10),165.0(C-6),164.2(C-8),163.5(C-3),163.1(C-1),135.0(C-11),132.2(C-14),122.9(C-2),108.8(C-4),108.6(C-5,C-8a),108.2(C-9a),108.1(C-7),62.8(C-1′),60.1(C-4′),35.3(C-2′),32.6(C-3′);ESIMS:m/z 361.1 [M + H]+。
化合物11为橘红色粉末,通过HRESIMS:m/z 367.0819 [M - H]-,判断分子式为C20H16O7,不饱和度为13。其HPLC吸收提示11与10为同系列蒽醌化合物,但不饱和度和碳个数均多了2。1H-和3C-NMR显示2个化合物蒽醌骨架一致,区别仅在于11的分子中多了2个环结构(δC 101.1(C-5′),66.1(C-1′),35.2(C-2′),27.4(C-4′),26.9(C-6′),15.4(C-3′)δH 5.15(H-1′),1.40—1.92(H-2′,3′,4′),1.50(H-6′))。仔细分析其核磁数据,确定化合物11为averufin[27](图1)。
核磁波谱数据:1H-NMR(500 MHz,DMSO-d6):δH 12.29(1H,s,1-OH),11.92(1H,s,8-OH),11.30(1H,s,6-OH),6.95(1H,s,H-5),6.83(1H,s,H-4),6.46(1H,s,H-7),5.15(1H,s,H-1′),1.40—1.92(6H,m,H-2′,3′,4′),1.50(3H,s,H-6′);13C-NMR(125 MHz,DMSO-d6):δC 188.6(C-9),180.5(C-10),165.2(C-8),164.2(C-1),159.8(C-6),158.1(C-3),134.6(C-4a),132.9(C-10a),115.9(C-2),108.9(C-5),108.4(C-9a),108.2(C-8a),107.9(C-7),107.1(C-4),101.1(C-5′),66.1(C-1′),35.2(C-2′),27.4(C-4′),26.9(C-6′),15.4(C-3′)。HRESIMS:m/z 367.0819 [M - H]-。
化合物12为橘红色粉末,通过高分辨质谱,推断其分子式为C20H18O7,不饱和度为12。仔细分析12的1H-NMR和13C-NMR数据,发现其也是一个蒽醌类化合物,比10多了1个不饱和度,碳个数比10多了2个,提示10比12多了1个环结构。化合物12蒽醌骨架的核磁数据与10十分相似,不同之处在于两个化合物的高场区。经过与文献进行比对,确定化合物12为noraverufanin[28](图1)。
核磁波谱数据:1H-NMR(500 MHz,DMSO-d6):δH 12.78(1H,s,1-OH),12.10(1H,s,8-OH),11.27(1H,s,6-OH),10.72(1H,s,3-OH),7.11(1H,s,H-5),7.09(1H,s,H-4),6.58(1H,s,H-7),4.96(1H,d,J=14.0 Hz,H-1′),3.61(1H,m,H-5′),1.30—1.97(6H,m,H-2′,3′,4′),1.16(3H,d,J=7.5 Hz,H-6′);13C-NMR(125 MHz,DMSO-d6):δC 188.8(C-9),181.1(C-10),165.1(C-6),164.2(C-8),162.7(C-3),161.6(C-1),134.9(C-10a),133.3 (C-4a),120.0(C-2),108.8(C-5),108.7(C-9a),108.7(C-8a),108.4(C-4),108.1(C-7),74.6(C-1′),73.0(C-5′),32.4(C-2′),28.2(C-4′),23.4(C-3′),21.9(C-6′)。HRESIMS:m/z 369.0987 [M - H]-。
2.2 抗菌活性
对化合物1—12进行抗燕麦食酸菌、胡萝卜软腐欧文氏菌、密执安棒形杆菌、丁香假单胞菌、青枯雷尔氏菌和野油菜黄单胞菌活性测试,发现在初筛浓度为200和100 μg·mL-1浓度下,仅3,6-O-dimethylviridicatin(3)对青枯雷尔氏菌和野油菜黄单胞菌表现出了显著的抗植物病原细菌活性。进一步测试其MIC,化合物3对青枯雷尔氏菌和野油菜黄单胞菌的MIC分别为50和100 μg·mL-1(表1),阳性药硫酸链霉素的MIC为25 μg·mL-1。3,6-O-dimethylviridicatin(3)对其余4种植物病原细菌没有明显的抑菌活性。Table 1
表1
表1化合物3对青枯雷尔氏菌和野油菜黄单胞菌抑菌数据
Table 1
| 化合物浓度 Concentration (μg·mL-1) | 加药Dose青枯雷尔氏菌 R. solanacearum OD600值OD600 value | 加药Dose野油菜黄单胞菌 X. campestris OD600值OD600 value |
|---|---|---|
| 200 | 0.0917 | — |
| 0.0885 | — | |
| 0.0835 | — | |
| 100 | 0.0902 | 0.0891 |
| 0.0947 | 0.0790 | |
| 0.0958 | 0.0788 | |
| 50 | 0.1364 | 1.0253 |
| 0.1504 | 1.0450 | |
| 0.1448 | 1.0277 | |
| 25 | 0.2496 | 1.0800 |
| 0.2457 | 1.0953 | |
| 0.2458 | 1.0898 | |
| 12.5 | 0.4333 | 1.0911 |
| 0.4246 | 1.0891 | |
| 0.4340 | 1.1001 | |
| 6.25 | — | 1.1058 |
| — | 1.0913 | |
| — | 1.0943 |
新窗口打开|下载CSV
3 讨论
本研究从一株海洋杂色曲霉D5发酵提取物中分离鉴定了12个化合物,包括4个2-羰基-4-苯基喹啉生物碱(1—4)、2个双氧代哌嗪生物碱(5—6)、3个喹唑啉fumiquinazolines生物碱及其衍生物(7—9)和3个蒽醌类化合物(10—12)。曲霉是重要的海洋真菌类群,在海洋真菌中占有很大比例,天然产物研究最多的海洋真菌是海洋曲霉。海洋曲霉源天然产物的研究始于1992年,NUMATA等[29]报道了首例海洋曲霉来源的新天然产物fumiquinazolines A-C,至2014年8月,已报道512个海洋曲霉来源的新天然产物。此后,越来越多的化合物从海洋曲霉中分离出来。2015—2018年,共报道了389个海洋曲霉来源新化合物,这不包括红树林来源的海洋曲霉,这些化合物结构类型多样,包括聚酮、生物碱、萜类、甾体、肽类等,且有多种生物活性,包括抗菌、细胞毒、抗氧化等[8,30-32]。本文所获得的4种结构类型化合物在海洋曲霉中均有过报道。自然界中的2-羰基-4-苯基喹啉生物碱只由真菌产生,生源途径是由邻氨基苯甲酸衍生而来,目前发现的该类化合物大部分来自于青霉菌,亚热带、温带、寒带环境的青霉中均发现了该类化合物的存在,其中也包括海洋青霉[33]。2-羰基-4-苯基喹啉生物碱的生物活性报道较少,多数研究没有发现其明显的生物活性,但该类化合物抗肿瘤活性显著。WEI等[21]从一株红树来源的青霉Penicillium sp.发酵提取物中分离鉴定了一个新的2-羰基-4-苯基喹啉生物碱viridicatol,通过核磁及单晶衍射确定了其结构,并发现该化合物对人非小细胞肺癌细胞A549、人肝癌细胞HepG2、人乳腺癌细胞MCF7、人白血病细胞K56具有显著的抑制活性,IC50在16.5—80.0 μg·mL-1;HE等[34]从一株海水来源的杨奇青霉(Penicillium janczewskii)中获得了4种化合物,测试了它们对8种肿瘤细胞的细胞毒活性,其中3R*,4R*-dihydroxy-4-(4′-methoxyphenyl)- 3,4-dihydro-2(1H)- quinolinone对人卵巢癌细胞SKOV-3表现出了中等的毒性,quinolinonepeniprequinolone表现出了强的非选择性细胞毒活性。也有研究报道了2-羰基-4-苯基喹啉生物碱对革兰氏阳性菌中等的抑制活性,PAN等[35]从螃蟹来源的杂色曲霉XZ-4发酵物中分离鉴定出3,6-Odimethylviridicatin和9-hydroxy-3- methoxyviridicatin,发现它们对大肠杆菌(Escherichia coli)具有中等的抗菌活性,MIC为32 μg·mL-1。在本研究中所获得的4个2-羰基-4-苯基喹啉生物碱中,只有3,6-O-dimethylviridicatin(3)表现了显著的抗植物病原细菌活性。分析其结构特征发现,3与1、2、4结构上的不同之处主要体现在C-6多了1个甲氧基,该甲氧基可能是化合物发挥抗植物病原细菌作用的关键基团。同时,文献中报道了9-hydroxy-3- methoxyviridicatin对大肠杆菌的抑菌作用,表明8-OH可能也是该类化合物发挥抗菌作用的重要基团。
喹唑啉fumiquinazolines生物碱是一类结构新颖独特的化合物,结构特征为包含一个pyrazino [2,1-b] quinazoline-3,6-dione母核,并连接一个吲哚。自1992年首次发现以来,目前共发现了80多个该类化合物,主要由陆地和海洋真菌代谢产生,这类化合物及其前体具有十分显著的生物活性,包括抗肿瘤、抗菌,尤其是在化疗药物方面具有显著疗效[36]。本研究获得了一个喹唑啉fumiquinazolines生物碱(9)和两个未连吲哚的喹唑啉生物碱(7、8),据文献报道,其中8和9的生物活性显著,而并未发现7明显的生物活性。FREMLIN等[26]从澳大利亚海沙来源的杂色曲霉中分离获得化合物9,通过核磁及Marfey反应确定了它的结构,活性测试发现了其抗菌作用;WANG等[23]从一株深海来源的杂色曲霉发酵液中获得了化合物8和9,对几种植物病原菌尖孢炭疽菌(Colletotrichum acutatum)、稻瘟病菌(Magnaporthe oryzae)和尖镰孢(Fusarium oxysporum)表现出了显著的抑菌活性,特别是化合物8对尖孢炭疽菌的抑制效果强于阳性药放线菌酮。本研究发现,所获得的喹唑啉fumiquinazoline生物碱及其衍生物均没有抗植物病原细菌活性,该类化合物结构复杂,新颖度高,且文献报道了它们的抗植物病原真菌活性。因此,值得继续挖掘该类化合物的结构多样性,从中发现抗真菌生物农药先导化合物。
4 结论
从一株海藻来源的杂色曲霉D5发酵培养基中分离鉴定了9个生物碱类化合物(1—9)和3个蒽醌类化合物(10—12)。其中,2-羰基-4-苯基喹啉生物碱3,6-O-dimethylviridicatin(3)对青枯雷尔氏菌和野油菜黄单胞菌等植物病原细菌具有显著的抗菌活性,6-OCH3可能是该类化合物抗菌作用的关键基团。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1126/science.1185383URLPMID:20110467 [本文引用: 1]

Continuing population and consumption growth will mean that the global demand for food will increase for at least another 40 years. Growing competition for land, water, and energy, in addition to the overexploitation of fisheries, will affect our ability to produce food, as will the urgent requirement to reduce the impact of the food system on the environment. The effects of climate change are a further threat. But the world can produce more food and can ensure that it is used more efficiently and equitably. A multifaceted and linked global strategy is needed to ensure sustainable and equitable food security, different components of which are explored here.
URLPMID:23950530 [本文引用: 1]
URL [本文引用: 1]

基于《微生物学通报》创刊40年来发表的植物相关细菌研究论文的数量与内容,对该领域研究在国际和国内的发展进行了初步总结和对比。提出了学术期刊与研究方向相互合作,共同提高的可能方式。
URL [本文引用: 1]

基于《微生物学通报》创刊40年来发表的植物相关细菌研究论文的数量与内容,对该领域研究在国际和国内的发展进行了初步总结和对比。提出了学术期刊与研究方向相互合作,共同提高的可能方式。
DOI:10.1111/mpp.12436URLPMID:27238249 [本文引用: 3]

Plant diseases caused by bacterial pathogens place major constraints on crop production and cause significant annual losses on a global scale. The attainment of consistent effective management of these diseases can be extremely difficult, and management potential is often affected by grower reliance on highly disease-susceptible cultivars because of consumer preferences, and by environmental conditions favouring pathogen development. New and emerging bacterial disease problems (e.g. zebra chip of potato) and established problems in new geographical regions (e.g. bacterial canker of kiwifruit in New Zealand) grab the headlines, but the list of bacterial disease problems with few effective management options is long. The ever-increasing global human population requires the continued stable production of a safe food supply with greater yields because of the shrinking areas of arable land. One major facet in the maintenance of the sustainability of crop production systems with predictable yields involves the identification and deployment of sustainable disease management solutions for bacterial diseases. In addition, the identification of novel management tactics has also come to the fore because of the increasing evolution of resistance to existing bactericides. A number of central research foci, involving basic research to identify critical pathogen targets for control, novel methodologies and methods of delivery, are emerging that will provide a strong basis for bacterial disease management into the future. Near-term solutions are desperately needed. Are there replacement materials for existing bactericides that can provide effective disease management under field conditions? Experience should inform the future. With prior knowledge of bactericide resistance issues evolving in pathogens, how will this affect the deployment of newer compounds and biological controls? Knowledge is critical. A comprehensive understanding of bacterial pathosystems is required to not only identify optimal targets in the pathogens, but also optimal seasonal timings for deployment. Host resistance to effectors must be exploited, carefully and correctly. Are there other candidate genes that could be targeted in transgenic approaches? How can new technologies (CRISPR, TALEN, etc.) be most effectively used to add sustainable disease resistance to existing commercially desirable plant cultivars? We need an insider's perspective on the management of systemic pathogens. In addition to host resistance or reduced sensitivity, are there other methods that can be used to target these pathogen groups? Biological systems are variable. Can biological control strategies be improved for bacterial disease management and be made more predictable in function? The answers to the research foci outlined above are not all available, as will become apparent in this article, but we are heading in the right direction. In this article, we summarize the contributions from past experiences in bacterial disease management, and also describe how advances in bacterial genetics, genomics and host-pathogen interactions are informing novel strategies in virulence inhibition and in host resistance. We also outline potential innovations that could be exploited as the pressures to maximize a safe and productive food supply continue to become more numerous and more complex.
DOI:10.3389/fmicb.2017.00034URLPMID:28163700 [本文引用: 1]

Losses in crop yields due to disease need to be reduced in order to meet increasing global food demands associated with growth in the human population. There is a well-recognized need to develop new environmentally friendly control strategies to combat bacterial crop disease. Current control measures involving the use of traditional chemicals or antibiotics are losing their efficacy due to the natural development of bacterial resistance to these agents. In addition, there is an increasing awareness that their use is environmentally unfriendly. Bacteriophages, the viruses of bacteria, have received increased research interest in recent years as a realistic environmentally friendly means of controlling bacterial diseases. Their use presents a viable control measure for a number of destructive bacterial crop diseases, with some phage-based products already becoming available on the market. Phage biocontrol possesses advantages over chemical controls in that tailor-made phage cocktails can be adapted to target specific disease-causing bacteria. Unlike chemical control measures, phage mixtures can be easily adapted for bacterial resistance which may develop over time. In this review, we will examine the progress and challenges for phage-based disease biocontrol in food crops.
DOI:10.3864/j.issn.0578-1752.2017.12.011URL [本文引用: 1]

With the deepening of the research on plant pathogenic bacteria, taxonomy on plant pathogenic bacteria has been in a changing process. In addition to the traditional genera were divided into several genera, new genus has been also increasing to nearly 40 genera. Four genera were separated from Erwinia in gram-negative bacteria, such as Pectobacterium, Dickeya, Pantoea and Brenneria. Pectobacterium carotovorum and P. atrosepticum causing soft rot diseases of several crop plants, were originally classified as Erwinia carotovora subsp. carotovora and subsp. atroseptica, respectively. The genera of Ralstonia, Burkholderia and Acidovorax were separated from Pseudomonas. The naming of new species is also emerging one after another. Now, there are 8 species in Dickeya, the bacteria causing maize bacterial stalk rot and rice bacterial foot rot were reclassified as D. zeae. Some subspecies or pathovar have been upgraded to species. Acidovorax citrulli causing bacterial fruit blotch were originally classified as A. avenae subsp. citrulli. TheLatin names of many pathogenic bacteria had experienced several changes. Xanthomonas axonopodis pv. malvacearum causing cotton angular leaf spot were originally classified as X. campestris pv. malvacearum or X. citri pv. malvacearum. Members of the gram-positive bacteria family are also growing. Domestic scholars exist outdated and nonstandard phenomenon in usingLatin names. The purpose of this review is topresenttaxonomy and recent changes of plant pathogenic bacteria, and show the changing process of the old and new Latin names. The focus on plant pathogenic bacteria taxonomy will help to in line with international convention.
DOI:10.3864/j.issn.0578-1752.2017.12.011URL [本文引用: 1]

With the deepening of the research on plant pathogenic bacteria, taxonomy on plant pathogenic bacteria has been in a changing process. In addition to the traditional genera were divided into several genera, new genus has been also increasing to nearly 40 genera. Four genera were separated from Erwinia in gram-negative bacteria, such as Pectobacterium, Dickeya, Pantoea and Brenneria. Pectobacterium carotovorum and P. atrosepticum causing soft rot diseases of several crop plants, were originally classified as Erwinia carotovora subsp. carotovora and subsp. atroseptica, respectively. The genera of Ralstonia, Burkholderia and Acidovorax were separated from Pseudomonas. The naming of new species is also emerging one after another. Now, there are 8 species in Dickeya, the bacteria causing maize bacterial stalk rot and rice bacterial foot rot were reclassified as D. zeae. Some subspecies or pathovar have been upgraded to species. Acidovorax citrulli causing bacterial fruit blotch were originally classified as A. avenae subsp. citrulli. TheLatin names of many pathogenic bacteria had experienced several changes. Xanthomonas axonopodis pv. malvacearum causing cotton angular leaf spot were originally classified as X. campestris pv. malvacearum or X. citri pv. malvacearum. Members of the gram-positive bacteria family are also growing. Domestic scholars exist outdated and nonstandard phenomenon in usingLatin names. The purpose of this review is topresenttaxonomy and recent changes of plant pathogenic bacteria, and show the changing process of the old and new Latin names. The focus on plant pathogenic bacteria taxonomy will help to in line with international convention.
DOI:10.1021/cr4005605URLPMID:24869800 [本文引用: 1]
DOI:10.1039/c9np00069kURLPMID:32025684 [本文引用: 2]

This review covers the literature published between January and December in 2018 for marine natural products (MNPs), with 717 citations (706 for the period January to December 2018) referring to compounds isolated from marine microorganisms and phytoplankton, green, brown and red algae, sponges, cnidarians, bryozoans, molluscs, tunicates, echinoderms, mangroves and other intertidal plants and microorganisms. The emphasis is on new compounds (1554 in 469 papers for 2018), together with the relevant biological activities, source organisms and country of origin. Reviews, biosynthetic studies, first syntheses, and syntheses that led to the revision of structures or stereochemistries, have been included. The proportion of MNPs assigned absolute configuration over the last decade is also surveyed.
DOI:10.1002/ddr.21482URLPMID:30370576 [本文引用: 1]

Natural products and derivatives thereof are of considerable importance in the discovery of new pharmaceuticals, for example, for the treatment of cancer, diabetes, inflammation diseases, and infection diseases caused by bacteria, fungi, viruses, or parasites. The great biodiversity of marine microorganisms is reflected in their huge chemical diversity, which provides a rich source of biologically active compounds. An increasing interest in marine microorganisms as promising producers of new compounds with potential medical applications has raised increasing interest in the sustainable exploration of marine microbial resources for the discovery of new antibiotics, which is highlighted. The bottlenecks in the development of drugs using the large marine natural product pipeline are also discussed.
DOI:10.7164/antibiotics.54.882URLPMID:11827029 [本文引用: 1]

Chlorogentisylquinone, a new inhibitor of neutral sphingomyelinase activity, was purified from the culture broth of a fungal strain FOM-8108 isolated from a marine environment by solvent extraction, silica gel chromatography and Sephadex LH-20 chromatography. Its chemical structure was elucidated by spectroscopic studies including 1H, 13C, DEPT, HMQC and HMBC NMR experiments. Chlorogentisylquinone inhibited neutral sphingomyelinase activity of rat brain membranes with an IC50 value of 1.2 microM.
DOI:10.1016/j.jopr.2013.04.052URL [本文引用: 1]
DOI:10.3390/md11093309URL [本文引用: 1]

Bioactive compounds were detected in crude extracts of the fungus, Calcarisporium sp. KF525, which was isolated from German Wadden Sea water samples. Purification of the metabolites from the extracts yielded the five known polyesters, 15G256, -2, , -2 and (1-5), and five new derivatives thereof, named calcarides A-E (6-10). The chemical structures of the isolated compounds were elucidated on the basis of one- and two-dimensional NMR spectroscopy supported by UV and HRESIMS data. The compounds exhibited inhibitory activities against Staphylococcus epidermidis, Xanthomonas campestris and Propionibacterium acnes. As the antibacterial activities were highly specific with regard to compound and test strain, a tight structure-activity relationship is assumed.
DOI:10.3390/md13084617URLPMID:26225984 [本文引用: 1]

An unusual polyketide with a new carbon skeleton, lindgomycin (1), and the recently described ascosetin (2) were extracted from mycelia and culture broth of different Lindgomycetaceae strains, which were isolated from a sponge of the Kiel Fjord in the Baltic Sea (Germany) and from the Antarctic. Their structures were established by spectroscopic means. In the new polyketide, two distinct domains, a bicyclic hydrocarbon and a tetramic acid, are connected by a bridging carbonyl. The tetramic acid substructure of compound 1 was proved to possess a unique 5-benzylpyrrolidine-2,4-dione unit. The combination of 5-benzylpyrrolidine-2,4-dione of compound 1 in its tetramic acid half and 3-methylbut-3-enoic acid pendant in its decalin half allow the assignment of a new carbon skeleton. The new compound 1 and ascosetin showed antibiotic activities with IC50 value of 5.1 (+/-0.2) microM and 3.2 (+/-0.4) muM, respectively, against methicillin-resistant Staphylococcus aureus.
DOI:10.1007/s11274-013-1418-xURLPMID:23824664 [本文引用: 1]

The diversity of sponge-associated fungi has been poorly investigated in remote geographical areas like Antarctica. In this study, 101 phenotypically different fungal isolates were obtained from 11 sponge samples collected in King George Island, Antarctica. The analysis of ITS sequences revealed that they belong to the phylum Ascomycota. Sixty-five isolates belong to the genera Geomyces, Penicillium, Epicoccum, Pseudeurotium, Thelebolus, Cladosporium, Aspergillus, Aureobasidium, Phoma, and Trichocladium but 36 isolates could not be identified at genus level. In order to estimate the potential of these isolates as producers of interesting bioactivities, antimicrobial, antitumoral and antioxidant activities of fungal culture extracts were assayed. Around 51% of the extracts, mainly from the genus Geomyces and non identified relatives, showed antimicrobial activity against some of the bacteria tested. On the other hand, around 42% of the extracts showed potent antitumoral activity, Geomyces sp. having the best performance. Finally, the potential of the isolated fungi as producers of antioxidant activity seems to be moderate. Our results suggest that fungi associated with Antarctic sponges, particularly Geomyces, would be valuable sources of antimicrobial and antitumoral compounds. To our knowledge, this is the first report describing the biodiversity and the metabolic potential of fungi associated with Antarctic marine sponges.
URLPMID:25913707 [本文引用: 1]
DOI:10.1007/s10482-015-0462-yURLPMID:25912731 [本文引用: 1]

Two novel antibiotic spiculisporic acid analogues, named as spiculisporic acid F (1) and G (2), and two known compounds, (-)-spiculisporic acid (3) and secospiculisporic acid B (4), were isolated by bioactivity-guided fractionation from the fermentation broth of the sea urchin-derived Aspergillus candidus strain HDf2. Their structures were unambiguously established by comprehensive analysis of 1D and 2D NMR, and high-resolution MS spectra, and by comparison with known compounds. Biological experiments demonstrated that compounds 1 and 2 displayed antibacterial activity against Gram-negative Pseudomonas solanacearum and Gram-positive Staphylococcus aureus, but showed no cytotoxicity against SGC-7901 human gastric adenocarcinoma and SPC-A-1 human lung adenocarcinoma tumor cell lines. This is the first critical evidence identifying spiculisporic acid derivatives as a potential bio-control agent for the soil borne pathogen P. solanacearum (E. F. Smith) Smith. These findings provide further insight into the chemical and biological activity diversity of this class of compounds.
[本文引用: 1]
[本文引用: 1]
DOI:10.1039/C8RA08047JURL [本文引用: 1]
DOI:10.3390/md18020073URL [本文引用: 1]
URL [本文引用: 2]
DOI:10.1021/np50055a008URLPMID:3373229 [本文引用: 1]

Verrucofortine [8], an alkaloid derived from tryptophan and leucine, has been isolated from the fungus Penicillium verrucosum var. cyclopium. The structure and absolute configuration have been established by a combination of spectroscopic and chemical techniques. Its structure is unrelated to that of other major metabolite of the organism, the highly toxic pyrone-type polyketide verrucosidin [1], which was previously reported to be a tremorgen. A second novel metabolite, normethylverrucosidin [3], has also been isolated and identified. Small quantities of several other secondary metabolites, ergosterol, cyclopenin [4], cyclopenol [5], and 3-O-methylviridicatin [6], were isolated. They are known fungal metabolites but had not previously been obtained from this fungus. Studies of verrucofortine toxicity in mice showed no apparent toxic effects at doses as high at 160 mg/kg ip.
DOI:10.1021/acs.jafc.6b00527URLPMID:26998701 [本文引用: 5]

Phytopathogenic fungi remain a continuous and huge threat in the agricultural fields. The agrochemical industry has made great development of the use of microbial natural products, which has been regarded as an effective strategy against phytopathogenic fungi. Antifungal bioassay-directed fractionation was used to isolate two new oxepine-containing alkaloids (1 and 2), two new 4-aryl-quinolin-2-one alkaloids (3 and 4), and four new prenylated xanthones (5-8) from the deep-sea-derived fungus Aspergillus versicolor SCSIO 05879. Extensive NMR spectroscopic analysis, quantum mechanical calculations, and X-ray single-crystal diffraction were used to elucidate their structures, including their absolute configurations. Versicoloids A and B, versicone A, and cottoquinazoline A showed antifungal activities against three phytopathogenic fungi. The antifungal activities of these bioactive compounds strongly depend on the fungal species. Especially versicoloids A and B showed strong fungicidal effect (MIC of 1.6 mug/mL) against Colletotrichum acutatum, compared with that of the positive control cycloheximide (MIC of 6.4 mug/mL). The results of antifungal experiments indicated that versicoloids A and B may be regarded as candidate agents of antifungal agrochemicals.
DOI:10.1080/10286020.2014.911290URLPMID:24773150 [本文引用: 1]

One new naturally occurring 7-membered 2,5-dioxopiperazine alkaloid named (+)-cyclopenol (1), along with nine known compounds including viridicatol (2), 3-(dimethylaminomethyl)-1-(1,1-dimethyl-2-propenyl)indole (3), anacine (4), aurantiomide C (5), viridicatin (6), 3-O-methylviridicatin (7), verrucosidin (8), ergosterol (9), and ergosterol peroxide (10), was isolated from the EtOAc extract of fungus Penicillium sclerotiorum, an endophytic fungal strain isolated from Chinese mangrove Bruguiera gymnorrhiza. The chemical structure of the new compound 1 was elucidated on the basis of detailed spectroscopic analysis. The absolute configuration of 1 was determined by single-crystal X-ray analysis with Cu Kalpha radiation (lambda = 1.54178 A). To our knowledge, (+)-cyclopenol (1) represents the first example of 7-membered 2,5-dioxopiperazine isolated from mangrove endophytic fungus.
[本文引用: 1]
DOI:10.1021/np800777fURLPMID:19245260 [本文引用: 2]

An Australian marine-derived isolate of Aspergillus versicolor (MST-MF495) yielded the known fungal metabolites sterigmatocystin, violaceol I, violaceol II, diorcinol, (-)-cyclopenol, and viridicatol, along with a new alkaloid, cottoquinazoline A (1), and two new cyclopentapeptides, cotteslosins A (2) and B (3). Structures for 1-3 and the known compounds were determined by spectroscopic analysis. The absolute configurations of 1-3 were addressed by chemical degradation and application of the C(3) Marfey's method. The use of
DOI:10.7164/antibiotics.48.199URLPMID:7730152 [本文引用: 2]

Paeciloquinones A to F and versiconol have been isolated as inhibitors of protein tyrosine kinases from the culture broth of the fungus Paecilomyces carneus P-177. The structures of the new anthraquinones were determined by spectroscopic methods, mainly 1H NMR and 13C NMR. The substitution pattern was established by investigation of the respective methylated derivatives.
DOI:10.1021/np9905259URLPMID:10869191 [本文引用: 1]

An undescribed fungus of the genus Microsphaeropsis, isolated from the Mediterranean sponge Aplysina aerophoba, produces two new betaenone derivatives (1, 2) and three new 1,3,6, 8-tetrahydroxyanthraquinone congeners (5-7). The structures of the compounds were established on the basis of NMR spectroscopic and mass spectrometric data and by CD spectroscopy. This is the first report wherein the (1)H and (13)C NMR data of the betaenone congeners are fully and unambiguously assigned on the basis of two-dimensional NMR spectroscopy. Furthermore, we describe the first elucidation of the absolute configuration of 1-(2'-anthraquinonyl)ethanols such as 5 and 6, by quantum chemical calculation of their circular dichroism (CD) and comparison with experimentally measured spectra. Moreover, it was shown that compounds 1, 5, 6, and 7 are inhibitors of PKC-epsilon, CDK4, and EGF receptor tyrosine kinases.
DOI:10.1016/S0040-4039(00)91690-3URL [本文引用: 1]
DOI:10.1039/c8np00092aURLPMID:30663727 [本文引用: 1]

Covering: January to December 2017This review covers the literature published in 2017 for marine natural products (MNPs), with 740 citations (723 for the period January to December 2017) referring to compounds isolated from marine microorganisms and phytoplankton, green, brown and red algae, sponges, cnidarians, bryozoans, molluscs, tunicates, echinoderms, mangroves and other intertidal plants and microorganisms. The emphasis is on new compounds (1490 in 477 papers for 2017), together with the relevant biological activities, source organisms and country of origin. Reviews, biosynthetic studies, first syntheses, and syntheses that led to the revision of structures or stereochemistries, have been included. Geographic distributions of MNPs at a phylogenetic level are reported.
DOI:10.1039/c7np00052aURLPMID:29335692

Covering: 2016. Previous review: Nat. Prod. Rep., 2017, 34, 235-294This review covers the literature published in 2016 for marine natural products (MNPs), with 757 citations (643 for the period January to December 2016) referring to compounds isolated from marine microorganisms and phytoplankton, green, brown and red algae, sponges, cnidarians, bryozoans, molluscs, tunicates, echinoderms, mangroves and other intertidal plants and microorganisms. The emphasis is on new compounds (1277 in 432 papers for 2016), together with the relevant biological activities, source organisms and country of origin. Reviews, biosynthetic studies, first syntheses, and syntheses that led to the revision of structures or stereochemistries, have been included.
DOI:10.1039/c6np00124fURLPMID:28290569 [本文引用: 1]

Covering: 2015. Previous review: Nat. Prod. Rep., 2016, 33, 382-431This review covers the literature published in 2015 for marine natural products (MNPs), with 1220 citations (792 for the period January to December 2015) referring to compounds isolated from marine microorganisms and phytoplankton, green, brown and red algae, sponges, cnidarians, bryozoans, molluscs, tunicates, echinoderms, mangroves and other intertidal plants and microorganisms. The emphasis is on new compounds (1340 in 429 papers for 2015), together with the relevant biological activities, source organisms and country of origin. Reviews, biosynthetic studies, first syntheses, and syntheses that lead to the revision of structures or stereochemistries, have been included.
DOI:10.1039/b612168nURLPMID:18250901 [本文引用: 1]

This review covers the isolation, structure determination, synthesis and biological activity of quinoline, quinazoline and acridone alkaloids from plant, microbial and animal sources; 115 references are cited.
DOI:10.1021/np058018gURLPMID:16180822 [本文引用: 1]

From Penicillium janczewskii, obtained from a marine sample, two new diastereomeric quinolinones, 3S,4R-dihydroxy-4-(4'-methoxyphenyl)-3,4-dihydro-2(1H)-quinolinone (1) and 3R,4R-dihydroxy-4-(4'-methoxyphenyl)-3,4-dihydro-2(1H)-quinolinone (2), were identified, along with two known alkaloids, peniprequinolone (3) and 3-methoxy-4-hydroxy-4-(4'-methoxyphenyl)-3,4-dihydro-2(1H)-quinolinone (4). Cytotoxicity testing on eight tumor cell lines revealed a moderate specificity of 2 on SKOV-3 cells.
DOI:10.1039/c6ob02374fURLPMID:28074949 [本文引用: 1]

Three new quinazoline derivatives (1-3), one new oxepin-containing natural product (4) and four new cyclopenin derivatives (5-7 and 9) have been isolated from an EtOAc extract of the Taiwan Kueishantao hydrothermal vent crab-associated fungus Aspergillus versicolor XZ-4. Their planar structures were established by HRMS, 1D and 2D NMR spectroscopic data analyses. The absolute configurations for compounds 1 and 4 were determined by chiral phase HPLC analysis of their hydrolysis products. The absolute configurations of 2, 3 and 7 were defined mainly by comparison of the quantum chemical TDDFT calculated and the experimental ECD spectra, and the absolute configuration of 5 was deduced from comparison of the optical rotation values reported in the literature. The presence of two atropisomers of 5 was established by NOE analyses. The Ile & Val units in compounds 1-3 allowed the assignment of a new quinazoline skeleton and it's the first time the configuration of isoleucine in the quinazoline skeleton was defined. A series of 7-methoxy cyclopenin derivatives were reported for the first time in this study. The bioevaluation of compounds 5, 7, 8 and 9 revealed inhibitory activities against E. coli at MIC values around 32 mug mL(-1).
DOI:10.1039/c8np00043cURLPMID:30091435 [本文引用: 1]

Covering: this review covers the literature from 1992 to 2018To date, approximately 80 naturally-occurring secondary metabolites which are structurally-related to fumiquinazolines have been isolated, mainly from marine sources. These alkaloids can be classified into twelve different groups and exhibit different structure motifs depending on the amino acids from which they are derived. This review is focused on isolation, structure elucidation, biological activities, biosynthetic pathways, and synthetic studies of these natural products.
