甘莉#, 祝艺然#, 卢亦杰, 孙静, 陈中炜, 程昌勇

 , 宋厚辉
, 宋厚辉

浙江农林大学动物科技学院动物医学院, 浙江省畜禽绿色生态健康养殖应用技术研究重点实验室, 浙江 杭州 311300
收稿日期:2020-02-09;修回日期:2020-05-19;网络出版日期:2020-07-08
基金项目:国家自然科学基金(31872620,31770040);浙江省自然科学基金(LZ19C180001,LQ19C180002,LQ20C010001);浙江农林大学学生科研训练项目(KX20180237)
*通信作者:程昌勇, Tel:+86-571-61083275, E-mail:lamge@zafu.edu.cn;
宋厚辉, E-mail:songhh@zafu.edu.cn.
#并列第一作者
摘要:[目的] 本试验旨在构建单核细胞增多性李斯特菌谷胱甘肽合成酶基因gshF缺失株和互补株并研究其在细菌运动和鞭毛形成中的调控作用。[方法] 采用同源重组的方法构建得到gshF缺失株后,测定野生株及缺失株的运动性和体外生长能力;同时利用荧光定量PCR方法检测gshF缺失株中鞭毛相关基因转录水平的变化。[结果] 缺失gshF后细菌在体外培养基中的生长能力未受影响,但缺失株的运动性及鞭毛形成能力显著降低。此外,缺失株中鞭毛形成重要调控基因gmaR以及鞭毛结构元件基因flaA的转录水平显著低于野生株,而其他鞭毛相关基因的转录水平变化不明显。[结论] 研究首次表明单增李斯特菌谷胱甘肽合成酶通过调控鞭毛重要基因的转录进而影响细菌的运动性和鞭毛形成,研究有助于深入理解重要食源性胞内菌通过精确调控鞭毛形成以适应外界和宿主环境的分子机制。
关键词:单增李斯特菌谷胱甘肽合成酶鞭毛形成运动性转录调控
Regulatory role of Listeria monocytogenes glutathione synthetase in bacterial motility and flagellar production
Li Gan#, Yiran Zhu#, Yijie Lu, Jing Sun, Zhongwei Chen, Changyong Cheng

 , Houhui Song
, Houhui Song

Key Laboratory of Applied Technology on Green-Eco-Healthy Animal Husbandry of Zhejiang Province, College of Animal Science and Technology & College of Veterinary Medicine, Zhejiang A & F University, Hangzhou 311300, Zhejiang Province, China
Received: 9 February 2020; Revised: 19 May 2020; Published online: 8 July 2020
*Corresponding author: Changyong Cheng, Tel:+86-571-61083275, E-mail:lamge@zafu.edu.cn;
Houhui Song, E-mail:songhh@zafu.edu.cn.
Foundation item: Supported by the National Natural Science Foundation of China (31872620, 31770040), by the Natural Science Foundation of Zhejiang Province (LZ19C180001, LQ19C180002, LQ20C010001) and by the Student Research and Training Program of Zhejiang A & F University (KX20180237)
#Theses authors contributed equally to this work
Abstract: [Objective] The foodborne pathogen Listeria monocytogenes is well-adapted outside of the environmental conditions and inside of the host niche by delicately controlling flagellar production. We here aimed to explore the regulatory roles of L. monocytogenes glutathione synthetase (encoded by gshF) in bacterial motility and flagella production. [Methods] The gene knock-out mutant and complemented strains (ΔgshF and CΔgshF) were constructed and then subjected to in vitro bacterial growth and motility assays compared with the wild-type strain EGD-e. In addition, transcriptional changes of the flagella-associated genes were determined for the wild-type and gshF mutant strains using the Real-time Quantitative Reverse Transcription PCR (qRT-PCR) method. [Results] The data showed that in vitro growth ability was not significantly affected by deletion of gshF while the swarming ability of ΔgshF was markedly impaired. More importantly, the transcriptional levels of the flagellar-associated factors GmaR, a protein thermometer that controls temperature-dependent transcription of flagellar genes, and FlaA, encoding flagellin, were most significantly down-regulated at 30℃ in the absence of gshF. [Conclusion] We in this study showed that the glutathione synthetase of L. monocytogenes plays a vital role in controlling transcription of flagellar genes and contributes to bacterial swarming motility and flagellar production. These data would be helpful to better understand the regulatory mechanisms employed by this intracellular pathogen to favor environmental adaption and host infection.
Keywords: Listeria monocytogenesglutathione synthetaseflagellar productionmotilitytranscriptional regulation
单核细胞增多性李斯特菌(Listeria monocytogenes),简称单增李斯特菌,是李斯特菌属中唯一具有致病性的菌种[1]。其易感人群为免疫低下的孕妇、老人、儿童,主要发病症状为败血症、单核细胞增多[2-3]。单增李斯特菌广泛存在于自然界中,生鲜牛奶、蔬菜水果等食品中均可检测到单增李斯特菌,感染单增李斯特菌的主要途径为误食被其污染的食物[4]。单增李斯特菌对不良环境的耐力极强,即使处于高渗透压、酸性(pH 4.5)、碱性(pH 9)、高温(45 ℃)等极端环境中都可生长[5-6]。单增李斯特菌不仅在外界环境中面临温度、pH、氧化、渗透压等多种应激,其在入侵宿主消化道以及巨噬细胞时也面临多种应激[7-8]。在单增李斯特菌入侵宿主细胞时,吞噬体将分泌大量的超氧离子,以破坏细菌蛋白等生物大分子的二硫键结构[9-10]。蛋白质二硫键的主要作用是维持蛋白质等生物大分子的高级构象,从而保证蛋白的正确折叠。还原反应引起的蛋白质二硫键的断裂,会使得蛋白质的高级构象发生改变从而影响其生物学活性,而氧化反应会使得蛋白质二硫键重新形成[10]。单增李斯特菌通过细胞内的硫氧还蛋白(Thioredoxin,Trx)[11]和谷氧还蛋白(Glutaredoxin,Grx)[12]这两套氧化还原稳态系统来应对宿主释放的超氧化物并修复被破坏的二硫键[13]。谷胱甘肽GSH是Grx系统中的重要组成之一[14],GSH由谷胱甘肽合成酶GshF合成。在氧化应激环境中,细菌中的GSH能利用其自身的半胱氨酸残基(Cys)与含有Cys的蛋白质相结合,形成可逆的二硫键,这一过程为谷胱甘肽化[15],此修饰可调节蛋白质活性且在氧化应激中对蛋白质起保护作用[12, 16]。
细菌在环境中的生存能力于细菌的运动能力密不可分,能够合成鞭毛的细菌可通过鞭毛从恶劣环境迁移入适合其生长的环境中[17]。细菌鞭毛还具有很强的抗原性,其在感染宿主过程中也起到举足轻重的作用[18]。单增李斯特菌的鞭毛结构主要分为三部分:鞭毛丝、基座和胞内游离鞭毛相关蛋白。单增李斯特菌特有的鞭毛相关蛋白主要存在于基座和胞质中。鞭毛基座的主要组件有MotA、FliE、FlgB、FlgC等,胞质内的鞭毛相关蛋白是FliS,其主要作用是协助细菌效应蛋白及鞭毛结构蛋白的转运[19]。
近年来,越来越多的研究表明GshF合成的GSH与含有Cys的蛋白质如PrfA、LLO等结合,形成可逆的翻译后修饰—S-谷胱甘肽化,从而调节蛋白的活性[20-21]。有相关研究表明缺失GshF后影响铜绿假单胞菌鞭毛的形成[22],但GshF是否影响单增李斯特菌鞭毛的形成尚未明确。因此,本试验通过同源重组的方法,利用单增李斯特菌野生型EGD-e构建gshF的缺失株及回补株,初步探究GshF对单增李斯特菌鞭毛形成的影响。
1 材料和方法 1.1 材料
1.1.1 菌株、质粒及培养条件: 单增李斯特菌EGD-e、大肠杆菌DH5α、温敏型穿梭质粒pKSV7和整合型回补质粒pIMK2,以上质粒及菌株均来自本实验室。单增李斯特菌培养于BHI (Brain Heart Infusion)培养基,大肠杆菌DH5α菌株接种于LB培养基。本试验所涉菌株培养条件为37 ℃振荡或30 ℃静置培养。
1.1.2 引物: 本试验所用引物见表 1。
表 1. 试验所用引物 Table 1. Primers used in this study
| Primers | Sequences(5′→3′) | Product/bp |
| ΔgshF-up-F | CGCGGATCCCAACTGTCTGTACACTGTATGTATTAAAATGGTTCAT | 536 |
| ΔgshF-up-R | ATCTAAAATCTTATCTTCTAAACCAAAATGTCCAGAAAATAAAAGCTT | |
| ΔgshF-down-F | CATTTTGGTTTAGAAGATAAGATTTTAGATTTCTTATTTGACTAAAAAAACCCA | 500 |
| ΔgshF-down-R | TTGGTTCTGCAGGGCTGAAAATCCTTATTTAAAAACAAATGATTG | |
| CΔgshF-F | TCCCCCGGGTTAGTCAAATAAGAAATCTAAAATCTTATCACCAATTTTTT | 2531 |
| CΔgshF-R | CGAGCTCCCATCTTTTTAGTTAAGTTCCGAATTTTCATTATTC | |
| RT-gmaR-F | AGCAAGTTCCATCAACCAAAAG | 79 |
| RT-gmaR-R | GTTGAGTTGTCATCGAAAGTAAGC | |
| RT-flaA-F | TGCTTTAGTTGCGATGGATTG | 100 |
| RT--flaA-R | AACGGTCGTGCGCTTCTAG | |
| RT-mogR-F | TCTGAAATGCTCAGCCTAAA | 105 |
| RT- mogR-R | TCGGAATATCTTCTACTTGGA | |
| RT-motB-F | GAGGCAAACAGAACGATAAATAGAG | 82 |
| RT- motB-R | GAGGCAAACAGAACGATAAATAGAG | |
| RT-flgG-F | TTGAATCGCTTCTCCAGTCG | 62 |
| RT- flgG-R | CCAGTTTCATTCCGATCAGTTT | |
| RT-flhB-F | CGGTGTAACTAATGAGCGTATCTAA | 69 |
| RT- flhB-R | GAGGTCGTGAAAGCGTTATTGT | |
| RT-fliE-F | CTATCCAGCATTTGCGTGAAG | 96 |
| RT- fliE-R | ATGCAGCCAGCGTCTTACC | |
| RT-fliH-F | CGCCGTAATCCAGTCGCATAT | 96 |
| RT- fliH-R | ACGTCACCACCCACCCAGAA | |
| RT-flgC-F | GGCGTGATTTCGGATAGGAC | 117 |
| RT- flgC-R | ACAAGCGGCTCTGCGTTAA | |
| RT- flgB-F | GTTTCGTCTATGTCGGTTCCAG | 71 |
| RT- flgB-R | TTTGCGAGCATCCGGTCAG | |
| RT- flgL-F | TTCGGTTGTCCCGTTATAGATG | 87 |
| RT- flgL-R | TAACTCAGAAGACGATGGCAGAT | |
| RT- flgK-F | GCGGATTGTTTGGCGATTT | 107 |
| RT- flgK-R | TTACGGTGACGGTGGTCTACTTG | |
| RT- flgE-F | CGAACCGGAACCGAAACT | 114 |
| RT- flgE-R | GCCAATGCCAACACGACA | |
| RT- flgD-F | AGTCATCTTTGCCAAGGGTTT | 61 |
| RT- flgD-R | ATTAGTAGTTTATCAGGAGCGAGTCAG | |
| RT-fliM-F | TGCTCTGTCCGCCCTGTAT | 87 |
| RT-fliM-R | CACTCAAAGGCTATGTCACCG | |
| RT-fliR-F | AGCGTCGTAATACCAGAAACATCCA | 65 |
| RT-fliR-R | GAACATTCCGAACAGTGTCAAGG | |
| RT-fliQ-F | ATCCACGGTCCAAGAATAAAGA | 96 |
| RT-fliQ-R | TGGTAGTGATTGTGGTTGCGATTT | |
| RT-fliP-F | CCAAGACCCTGCCTCGTTA | 74 |
| RT-fliP-R | TACGGTTCTTTCTTTGTCTGCA | |
| RT-fliD-F | CACGCCTGGGATGTAGTTGG | 123 |
| RT-fliD-R | AAGGTGATTCGGCTGTTCTCG | |
| RT-fliF-F | GATGACGGCGAAGAGTAAACC | 107 |
| RT-fliF-R | TCACCATCATGCCTATCCAGTT | |
| RT-fliG-F | CGCCGTCCATTTCTTTCATT | 97 |
| RT-fliG-R | CGCTTATTATTTGGAGCCTTGA | |
| RT-fliI-F | CGTAACGCCAAAGCAGACAT | 111 |
| RT-fliI-R | TCCGAGGTAGCAGCAACAAT | |
| RT-flhA-F | GGTTTCCATATTCAAGTAAGCACGT | 109 |
| RT-flhA-R | CAGCGGAGGTAGCAGCAAGA | |
| The restriction enzyme sites are underlined. | ||
表选项
1.1.3 主要试剂与仪器: BHI培养基(Oxoid公司,英国);PCR产物纯化试剂盒(上海莱枫生物科技有限公司);本试验所用T4连接酶于Thermo公司购买;脱脂奶粉(BD公司,德国);多功能摇床(Thermo公司,美国);卧式恒温振荡器(CRYSTAL公司,美国);Golden View于北京艾德莱生物科技有限公司购买;柱式细菌总RNA抽提纯化试剂盒于上海生工公司购买;Trizol Reagent (Invitrogen公司,美国)。
1.2 ΔgshF重组质粒的构建和筛选 于NCBI获得gshF基因序列后,针对gshF基因上游片段536 bp处与下游片段500 bp处设计相应的引物ΔgshF-up-F、ΔgshF-up-R、ΔgshF-down-F和ΔgshF-down-R。以EGD-e基因组为模板,利用上述引物扩增得到大小为536 bp、500 bp的片段。以上述片段为模版,引物为ΔgshF-up-F、ΔgshF-down-R,扩增得到大小为1036 bp的片段。利用BamH I和Pst I双酶切上述基因片段及温敏型穿梭质粒pKSV7,将酶切后的基因片段及温敏型穿梭质粒pKSV7酶连后,进行热激转化。利用PCR筛选获得含重组质粒(命名为pSL1628)的大肠杆菌。测序验证正确后,获得用于构建gshF基因缺失株的重组质粒。
1.3 CΔgshF重组质粒的构建和筛选 于NCBI获得gshF基因序列,针对gshF基因设计相应的引物CΔgshF-F、CΔgshF-R。以EGD-e基因组为模板,利用上述引物扩增得到大小为2531 bp的片段。利用Sac I和Sma I双酶切上述基因片段及整合型回补质粒pIMK2,将酶切后的整合型回补质粒pIMK2与gshF基因片段酶连后进行热激转化。利用PCR筛选获得含自身启动子Pnative的重组质粒(命名为pSL1843)的大肠杆菌,测序验证正确后,获得用于构建gshF基因回补株的重组质粒。
1.4 ΔgshF缺失株与CΔgshF回补株的构建 根据同源重组原理,将携带gshF上下游同源臂的重组质粒电转入EGD-e的感受态细胞中,从而获得携带重组质粒的菌株。然后,利用双重选择压力:温度(42 ℃)及抗生素(Cm,10 mg/mL),使重组质粒上的同源臂序列与单增李斯特菌基因组发生双交换,并在低温(30 ℃)及无抗生素的无选择压力环境中丢失pKSV7质粒,经测序验证正确后获得gshF的基因缺失株。回补株在gshF缺失株的基础上构建,将构建成功的携带gshF自身启动子及其完整阅读框的重组质粒电转入ΔgshF感受态细胞,PCR验证单克隆菌落,经比对测序正确后即得到CΔgshF回补株。
1.5 检测生长曲线 37 ℃过夜振荡培养EGD-e,ΔgshF,CΔgshF的单克隆菌落。收集菌体,调节菌液的OD600为0.6后,1:100转接菌液,分别放于37 ℃振荡培养,30 ℃静置培养。每隔1 h检测样品,连续检测12 h。利用Excel及GraphPad Prism 7软件对所得数据进行分析并绘制生长曲线。
1.6 运动性分析 37 ℃过夜振荡培养EGD-e,ΔgshF,CΔgshF的单克隆菌落。调节菌液的OD600至0.6。使用灭菌牙签将EGD-e,ΔgshF,CΔgshF的菌液穿刺于0.25%的TSA半固体平板(0.25% Agar、2% NaCl和1.5% Tryptone)。TSA半固体平板于37 ℃,30 ℃静置培养,24 h后观察细菌的运动圈大小。
1.7 荧光定量PCR方法检测EGD-e、ΔgshF鞭毛基因的转录水平 将过夜培养的EGD-e,ΔgshF的菌液接入BHI培养基中,放于30 ℃培养箱中静置培养。当菌液的OD600在0.4–0.5时,取5 mL菌液离心后弃上清。加入500 μL Trizol Reagent后,使用均质仪破碎菌体,收集上清。利用细菌总RNA快速抽提试剂盒提取EGD-e、ΔgshF的总RNA,以EGD-e、ΔgshF的总RNA为模版,反转录得到相应的cDNA。采用荧光定量PCR方法研究gshF对鞭毛基因的转录调控。每个样品每对引物均设置2个平行,试验所用引物如表 1,利用GraphPad Prism 7软件对所得数据进行处理分析。
2 结果和分析 2.1 ΔgshF重组质粒的构建 引物如表 1,扩增后获得gshF基因片段上下游各536 bp、500 bp处的基因片段。温敏型穿梭质粒pKSV7与PCR扩增后获得的gshF上下游同源臂经酶切、酶连后热激转化,然后利用PCR进行筛选。结果如图 1-A,阳性克隆经PCR验证后,其条带大小为1036 bp。将该阳性克隆送测序,比对测序结果正确,表明获得重组缺失质粒。
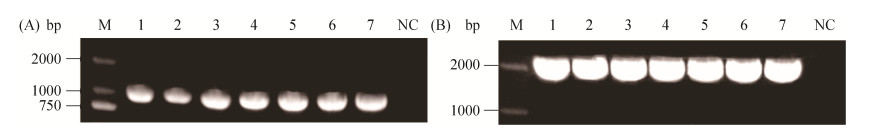 |
| 图 1 缺失株和回补株重组质粒的构建 Figure 1 PCR Verification of the recombinant plasmids used to construct the ΔgshF (A) and CΔgshF (B) strains. The numbers (1-7) indicate the positive colonies. M: DNA ladder; NC: Negative control. |
| 图选项 |
2.2 CΔgshF重组质粒的构建 利用CΔgshF-F,CΔgshF-R引物扩增得到大小为2531 bp的gshF基因片段。整合型回补质粒pIMK2和PCR扩增所得gshF基因片段经酶切、酶连后热激转化。然后利用PCR进行筛选,结果如图 1-B。阳性克隆经PCR验证后,其条带大小为2531 bp。将该阳性克隆送测序,比对测序结果正确,表明获得重组回补质粒。
2.3 ΔgshF和CΔgshF的构建 为验证gshF的缺失对鞭毛形成是否产生影响,构建ΔgshF和CΔgshF。以EGD-e为阳性对照,利用PCR、荧光定量PCR及测序方法鉴定ΔgshF和CΔgshF构建成功与否。结果如图 2-A,阳性对照的PCR片段大小为3440 bp,缺失gshF基因后,PCR片段大小为1205 bp,与预期片段大小一致;回补株阳性克隆经PCR验证后,其条带大小为2531 bp,与目的片段大小一致。利用荧光定量PCR验证ΔgshF,CΔgshF中gshF基因的转录水平,结果如图 2-B,ΔgshF中gshF基因的转录水平远低于EGD-e,CΔgshF中gshF基因的转录水平略高于EGD-e。测序结果如图 2-C,ΔgshF中gshF基因敲除成功,CΔgshF中gshF基因回补成功。上述结果表明ΔgshF与CΔgshF构建成功。
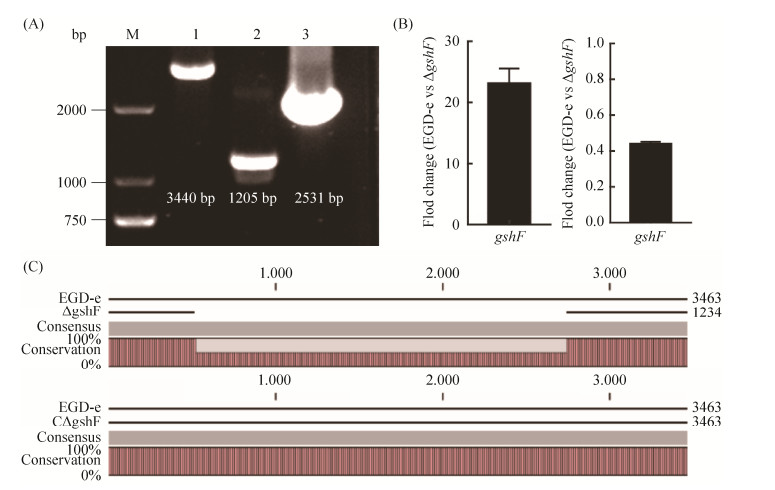 |
| 图 2 gshF缺失株和回补株的验证 Figure 2 Verification of the ΔgshF mutant and CΔgshF strains by PCR (A), RT-PCR (B), and sequencing (C). M: marker; lane 1–3: PCR products using genomic templates from EGD-e, ΔgshF, and CΔgshF, respectively. |
| 图选项 |
2.4 检测生长曲线 EGD-e,ΔgshF,CΔgshF于37 ℃振荡,30 ℃静置培养后,在不同时期检测OD600值,结果如图 3,ΔgshF的生长曲线几乎与EGD-e、CΔgshF一致,生长速率无明显差异,试验结果表明缺失gshF后并不影响菌株的生长状况。
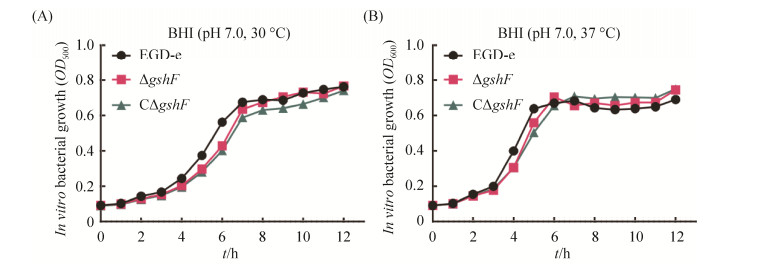 |
| 图 3 EGD-e及其突变株ΔgshF和CΔgshF的体外生长能力 Figure 3 In vitro growth of the wild-type EGD-e, ΔgshF, and CΔgshF in BHI at 30 ℃ (A) or 37 ℃ (B). |
| 图选项 |
2.5 gshF突变株体外运动能力测定 测定EGD-e,ΔgshF,CΔgshF在37 ℃,30 ℃时的体外运动能力。结果如图 4所示,当培养条件为37 ℃时,EGD-e,ΔgshF,CΔgshF均无运动性。在30 ℃条件下,CΔgshF的运动圈与EGD-e相比有所差异,由于回补株中gshF的转录水平略高于野生株,而细菌中gshF的表达受到严谨调控,过表达可能也会影响细菌胞质内的氧化还原平衡进而影响细菌的生物学功能。ΔgshF的运动圈明显小于EGD-e和CΔgshF的运动圈,该结果表明gshF与李斯特菌体外运动能力相关。
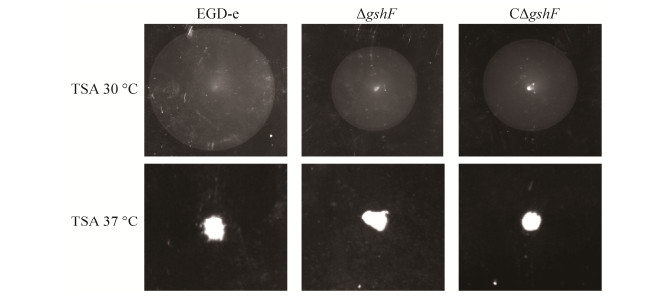 |
| 图 4 gshF缺失对李斯特菌运动性的影响 Figure 4 Swarming ability of the wild-type EGD-e, ΔgshF and CΔgshF at 30 ℃ or 37 ℃. |
| 图选项 |
2.6 分析gshF对鞭毛基因的转录调控 提取EGD-e,ΔgshF的总RNA后,利用荧光定量PCR方法研究gshF对鞭毛基因的转录调控。结果如图 5、表 2,缺失gshF基因后,鞭毛马达蛋白基因motB、fliM、fliF,基座蛋白基因flgB、flgE、flgG、fliE、flhA、flhB,鞭毛钩蛋白基因flgD、flgE、flgK、flgL等鞭毛基因的转录水平下降,其中以鞭毛温度调控蛋白基因gmaR、鞭毛丝蛋白基因flaA的转录水平的下降最为显著;mogR、基座蛋白基因fliI、fliP、fliQ转录水平上调;基座蛋白基因fliG、flgC、fliH、fliR转录水平无差异。
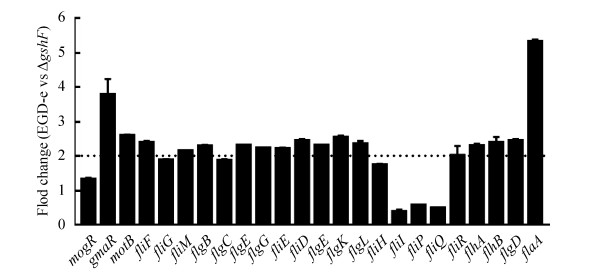 |
| 图 5 gshF缺失对李斯特菌部分鞭毛相关基因转录的影响 Figure 5 Effects of gshF on transcriptional changes of the flagella-associated genes. |
| 图选项 |
表 2. ΔgshF中鞭毛相关基因转录水平的变化 Table 2. Transcriptional changes of the flagellar genes in ΔgshF at 30 ℃
| Transcriptional change (EGD-e/ΔgshF) | Gene name |
| Down | mogR, fliI, fliP, fliQ |
| Up | motB, fliM, fliF, flgB, flgE, flgG, fliE, flhA, flhB, flgD, flgE, flgK, flgL, gmaR, flaA, fliD |
| No significant change | fliG, flgC, fliH, fliR |
表选项
3 讨论 本研究结果表明gshF的缺失影响鞭毛温度调控蛋白gmaR、鞭毛丝蛋白flaA等相关鞭毛基因的转录,从而影响单增李斯特菌鞭毛的形成。细菌的鞭毛在生物膜形成和细胞侵袭等过程中起着极其重要的作用[23-25]。然而,细菌入侵宿主时,鞭毛的持续产生可刺激机体的先天性免疫应答,从而抑制细菌的侵袭[26-27]。因此,为躲避宿主机体免疫系统对细菌鞭毛的识别,李斯特菌鞭毛形成的过程受温度严格调控[28]。当细菌进入宿主细胞(37 ℃)后,其鞭毛基因的转录受到抑制,并伴随着依赖于温度的毒力基因的转录上调;而当李斯特菌处于30 ℃环境时,鞭毛基因的转录被激活,从而产生细菌鞭毛[29-30]。李斯特菌鞭毛基因的转录受MogR转录阻遏物和双功能鞭毛抗阻遏物/糖基转移酶GmaR的双重调控[31]。当环境温度低于37 ℃时,抗阻遏物GmaR与MogR结合,从而拮抗MogR的抑制作用,细菌鞭毛基因转录被激活[32]。GmaR抗阻遏物对温度敏感,当环境温度处于宿主生理温度(37 ℃)时,GmaR的构象发生改变,MogR/GmaR复合物不稳定,释放MogR从而抑制gmaR及其他鞭毛运动基因的转录[29-30]。
低温下GmaR缓解MogR的抑制作用,这一过程还需要DegU的参与[33]。DegU结合于fliN-gmaR启动区,特异性的激活gmaR的转录[34]。本试验中gshF突变株的鞭毛运动受到影响且gmaR基因的转录水平明显降低。gmaR调控鞭毛基因的转录,而DegU调控gmaR的转录,因此我们推测DegU调控gmaR转录的过程中需要GshF的参与。研究表明许多蛋白功能的发挥需要还原环境[21, 35],GshF可能通过合成抗氧化剂GSH来为DegU调控gmaR转录的过程提供还原环境,使得DegU更易与fliN-gmaR启动区结合;也可能通过合成的GSH与DegU的Cys结合,改变DegU的结构,从而影响DegU对gmaR转录的调控。在此基础上,我们下一步将探究GshF参与DegU调控gmaR转录及影响鞭毛形成的具体作用机制,并研究GshF在李斯特菌感染过程中可能发挥的生物学功能,为深入理解以李斯特菌为模型的胞内菌利用氧化还原修饰机制介导细菌在环境适应以及宿主内感染过程中的分子调控机理。
References
| [1] | Pizarro-Cerda J, Cossart P. Listeria monocytogenes membrane trafficking and lifestyle:the exception or the rule?. Annual Review of Cell and Developmental Biology, 2009, 25: 649-670. DOI:10.1146/annurev.cellbio.042308.113331 |
| [2] | Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infection and Immunity, 2000, 68(2): 999-1003. DOI:10.1128/IAI.68.2.999-1003.2000 |
| [3] | K?ster S, van Pee K, Hudel M, Leustik M, Rhinow D, Kühlbrandt W, Chakraborty T, Yildiz ?. Crystal structure of listeriolysin O reveals molecular details of oligomerization and pore formation. Nature Communications, 2014, 5: 3690. DOI:10.1038/ncomms4690 |
| [4] | Chen BY, Wang CY, Wang CL, Fan YC, Weng IT, Chou CH. Prevalence and persistence of Listeria monocytogenes in ready-to-eat tilapia sashimi processing plants. Journal of Food Protection, 2016, 79(11): 1898-1903. DOI:10.4315/0362-028X.JFP-16-149 |
| [5] | Gahan CGM, Hill C. Listeria monocytogenes:survival and adaptation in the gastrointestinal tract. Frontiers in Cellular and Infection Microbiology, 2014, 4: 9. |
| [6] | Milillo SR, Friedly EC, Saldivar JC, Muthaiyan A, O'Bryan C, Crandall PG, Johnson MG, Ricke SC. A review of the ecology, genomics, and stress response of Listeria innocua and Listeria monocytogenes. Critical Reviews in Food Science and Nutrition, 2012, 52(8): 712-725. DOI:10.1080/10408398.2010.507909 |
| [7] | Smith JL, Liu YH, Paoli GC. How does Listeria monocytogenes combat acid conditions?. Canadian Journal of Microbiology, 2013, 59(3): 141-152. DOI:10.1139/cjm-2012-0392 |
| [8] | Camargo AC, Todorov SD, Chihib NE, Drider D, Nero LA. Lactic Acid Bacteria (LAB) and their bacteriocins as alternative biotechnological tools to control Listeria monocytogenes biofilms in food processing facilities. Molecular Biotechnology, 2018, 60(9): 712-726. DOI:10.1007/s12033-018-0108-1 |
| [9] | Bekendam RH, Bendapudi PK, Lin L, Nag PP, Pu J, Kennedy DR, Feldenzer A, Chiu J, Cook KM, Furie B, Huang MD, Hogg PJ, Flaumenhaft R. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nature Communications, 2016, 7: 12579. DOI:10.1038/ncomms12579 |
| [10] | Cataldo G, Conte MP, Chiarini F, Seganti L, Ammendolia MG, Superti F, Longhi C. Acid adaptation and survival of Listeria monocytogenes in Italian-style soft cheeses. Journal of Applied Microbiology, 2007, 103(1): 185-193. |
| [11] | Cheng CY, Dong ZM, Han X, Wang H, Jiang L, Sun J, Yang YC, Ma TT, Shao CY, Wang XD, Chen ZW, Fang WH, Freitag NE, Huang HR, Song HH. Thioredoxin a is essential for motility and contributes to host infection of Listeria monocytogenes via redox interactions. Frontiers in Cellular and Infection Microbiology, 2017, 7: 287. DOI:10.3389/fcimb.2017.00287 |
| [12] | Begas P, Liedgens L, Moseler A, Meyer AJ, Deponte M. Glutaredoxin catalysis requires two distinct glutathione interaction sites. Nature Communications, 2017, 8: 14835. DOI:10.1038/ncomms14835 |
| [13] | Allen EMG, Mieyal JJ. Protein-thiol oxidation and cell death:regulatory role of glutaredoxins. Antioxidants & Redox Signaling, 2012, 17(12): 1748-1763. |
| [14] | López-Grueso MJ, González-Ojeda R, Requejo-Aguilar R, McDonagh B, Fuentes-Almagro CA, Muntane J, Bárcena JA, Padilla CA. Thioredoxin and glutaredoxin regulate metabolism through different multiplex thiol switches. Redox Biology, 2019, 21: 101049. DOI:10.1016/j.redox.2018.11.007 |
| [15] | Karimi M, Crossett B, Cordwell SJ, Pattison DI, Davies MJ. Characterization of disulfide (cystine) oxidation by HOCl in a model peptide:evidence for oxygen addition, disulfide bond cleavage and adduct formation with thiols. Free Radical Biology and Medicine, 2020, 154: 62-74. DOI:10.1016/j.freeradbiomed.2020.04.023 |
| [16] | Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin:role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxidants & Redox Signaling, 2005, 7(3-4): 348-366. |
| [17] | Inoue Y, Morimoto YV, Namba K, Minamino T. Novel insights into the mechanism of well-ordered assembly of bacterial flagellar proteins in Salmonella. Scientific Reports, 2018, 8: 1787. DOI:10.1038/s41598-018-20209-3 |
| [18] | Papaneophytou CP, Papi RM, Pantazaki AA, Kyriakidis DA. Flagellin gene (fliC) of Thermus thermophilus HB8:characterization of its product and involvement to flagella assembly and microbial motility. Applied Microbiology and Biotechnology, 2012, 94(5): 1265-1277. |
| [19] | Desvaux M, Hébraud M. The protein secretion systems in Listeria:inside out bacterial virulence. FEMS Microbiology Reviews, 2006, 30(5): 774-805. DOI:10.1111/j.1574-6976.2006.00035.x |
| [20] | Portman JL, Dubensky SB, Peterson BN, Whiteley AT, Portnoy DA. Activation of the Listeria monocytogenes virulence program by a reducing environment. MBio, 2017, 8(5): e01595-17. DOI:10.1128/mBio.01595-17 |
| [21] | Portman JL, Huang QY, Reniere ML, Iavarone AT, Portnoy DA. Activity of the pore-forming virulence factor listeriolysin o is reversibly inhibited by naturally occurring s-glutathionylation. Infection and Immunity, 2017, 85(4): e00959-16. DOI:10.1128/IAI.00959-16 |
| [22] | Zhang YN, Zhang C, Du X, Zhou Y, Kong WN, Lau GW, Chen GK, Kohli GS, Yang L, Wang TT, Liang HH. Glutathione activates type Ⅲ secretion system through Vfr in Pseudomonas aeruginosa. Frontiers in Cellular and Infection Microbiology, 2019, 9: 164. DOI:10.3389/fcimb.2019.00164 |
| [23] | Duan QD, Zhou MX, Zhu LQ, Zhu GQ. Flagella and bacterial pathogenicity. Journal of Basic Microbiology, 2013, 53(1): 1-8. |
| [24] | Subramanian S, Kearns DB. Functional regulators of bacterial flagella. Annual Review of Microbiology, 2019, 73: 225-246. DOI:10.1146/annurev-micro-020518-115725 |
| [25] | Nakamura S, Minamino T. Flagella-driven motility of bacteria. Biomolecules, 2019, 9(7): 279. DOI:10.3390/biom9070279 |
| [26] | Horstmann JA, Lunelli M, Cazzola H, Heidemann J, Kuhne C, Steffen P, Szefs S, Rossi C, Lokareddy RK, Wang C, Lemaire L, Hughes KT, Uetrecht C, Schlüter H, Grassl GA, Stradal TEB, Rossez Y, Kolbe M, Erhardt M. Methylation of Salmonella typhimurium flagella promotes bacterial adhesion and host cell invasion. Nature Communications, 2020, 11(1): 2013. DOI:10.1038/s41467-020-15738-3 |
| [27] | Salehi S, Howe K, Lawrence ML, Brooks JP, Bailey RH, Karsi A. Salmonella enterica serovar kentucky flagella are required for broiler skin adhesion and caco-2 cell invasion. Applied and Environmental Microbiology, 2017, 83(2): e02115-16. |
| [28] | Kamp HD, Higgins DE. A protein thermometer controls temperature-dependent transcription of flagellar motility genes in Listeria monocytogenes. PLoS Pathogens, 2011, 7(8): 1002153. DOI:10.1371/journal.ppat.1002153 |
| [29] | Kamp HD, Higgins DE. Transcriptional and post-transcriptional regulation of the GmaR antirepressor governs temperature-dependent control of flagellar motility in Listeria monocytogenes. Molecular Microbiology, 2009, 74(2): 421-435. |
| [30] | Shen A, Higgins DE. The MogR transcriptional repressor regulates nonhierarchal expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathogens, 2006, 2(4): 30. DOI:10.1371/journal.ppat.0020030 |
| [31] | Knudsen GM, Olsen JE, Dons L. Characterization of DegU, a response regulator in Listeria monocytogenes, involved in regulation of motility and contributes to virulence. FEMS Microbiology Letters, 2004, 240(2): 171-179. DOI:10.1016/j.femsle.2004.09.039 |
| [32] | Gründling A, Burrack LS, Bouwer HGA, Higgins DE. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(33): 12318-12323. DOI:10.1073/pnas.0404924101 |
| [33] | Mauder N, Williams T, Fritsch F, Kuhn M, Beier D. Response regulator DegU of Listeria monocytogenes controls temperature-responsive flagellar gene expression in its unphosphorylated state. Journal of Bacteriology, 2008, 190(13): 4777-4781. DOI:10.1128/JB.00258-08 |
| [34] | Gueriri I, Cyncynatus C, Dubrac S, Arana AT, Dussurget O, Msadek T. The DegU orphan response regulator of Listeria monocytogenes autorepresses its own synthesis and is required for bacterial motility, virulence and biofilm formation. Microbiology, 2008, 154(8): 2251-2264. DOI:10.1099/mic.0.2008/017590-0 |
| [35] | Ku JWK, Gan YH. Modulation of bacterial virulence and fitness by host glutathione. Current Opinion in Microbiology, 2019, 47: 8-13. DOI:10.1016/j.mib.2018.10.004 |
