 ,, 曹新南京医科大学医学遗传学系,南京 211166
,, 曹新南京医科大学医学遗传学系,南京 211166G protein-coupled receptor-associated sorting proteins: function and relevant disorders
Min Zhang, Lihong Liang, Yajie Lu ,, Xin CaoDepartment of Medical Genetics, Nanjing Medical University, Nanjing 211166, China
,, Xin CaoDepartment of Medical Genetics, Nanjing Medical University, Nanjing 211166, China通讯作者: 鲁雅洁,在读博士,高级实验师,研究方向:疾病的分子遗传学。E-mail:luyajie@njmu.edu.cn
编委: 史岸冰
收稿日期:2020-02-19修回日期:2020-04-16网络出版日期:2020-08-20
| 基金资助: |
Received:2020-02-19Revised:2020-04-16Online:2020-08-20
| Fund supported: |
作者简介 About authors
张敏,在读硕士研究生,专业方向:遗传学。E-mail:

摘要
G蛋白偶联受体(G protein-coupled receptors, GPCRs)作为最大的一类膜蛋白受体家族,可被多种配体激活并发挥相应的信号转导功能,参与生物体内重要的生理过程。G蛋白偶联受体相关分选蛋白(G protein-coupled receptors associated sorting proteins, GASPs)则对内吞后的GPCRs分选过程发挥着重要的作用,并介导受体进入降解或再循环途径,进而调控细胞的信号转导等过程。研究发现GASPs的功能缺陷与多种疾病相关,包括神经系统疾病、肿瘤和耳聋等。本文重点介绍了G蛋白偶联受体相关分选蛋白的功能特征及其相关信号通路,描述了GASPs功能缺陷与疾病的关联性及家族蛋白与GPCRs的相互作用、GASPs分选途径的发现、参与的信号通路及对基因转录调控,以期为GASPs相关多种疾病的治疗提供新的思路和策略。
关键词:
Abstract
G protein-coupled receptors (GPCRs), the largest family of membrane protein receptors, can be activated by a variety of ligands and participate in signaling transduction, and they are essential in the physiologic process in vivo. GPCR-associated sorting proteins (GASPs) play an important role in the post-endocytic sorting of GPCRs. They mediate the degradation or recycling pathway, and regulate cell signaling transduction and other biological processes. The functional defects of GASPs have been reported to be implicated in pathogenesis of some neurological diseases, tumors and deafness and so on. In this review, we summarize the GASPs’ function, GPCR-GASP interactions, GPCR sorting pathway and GASP-related signaling pathways implicated in the transcriptional regulation. It could help to understand the potential linkage between GASPs’ dysfunction and diseases, and provide a new approach and strategy for the treatment of GASP-related diseases.
Keywords:
PDF (771KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张敏, 梁丽鸿, 鲁雅洁, 曹新. G蛋白偶联受体相关分选蛋白功能特征与相关疾病研究进展. 遗传[J], 2020, 42(8): 713-724 doi:10.16288/j.yczz.20-020
Min Zhang.
G蛋白偶联受体(G protein-coupled receptors, GPCRs)是哺乳动物中最大且用途最为广泛的信号受体家族[1]。它们可以被各种外界物质如气体、光子、蛋白质、肽、化学物质、激素、脂质和糖类等信号激活,从而将细胞外信号转导至细胞内[2],影响个体心血管、免疫及神经等多系统的生理及病理作用。不仅如此,GPCRs还是细胞内的一类关键调节剂,是人体最大的一类治疗药物靶标,据分析已超过1/3的药物作用于GPCRs的家族成员[3,4]。与GPCRs相互作用蛋白如G蛋白偶联受体相关分选蛋白(GPCR associated sorting proteins, GASPs)、Na+/H+交换调节因子/埃兹蛋白结合蛋白50(Na+/H+ exchanger regulatory factor, NHERF/ezrin radixin moesin-binding protein 50 kDa, EBP50)、β-arrestin等在GPCRs信号转导、内吞及内吞后分选过程中发挥着重要的作用[5,6,7,8]。GASPs家族作为GPCRs分选转运的重要因子,主要通过与受体C末端保守序列相互结合,通过磷酸化或泛素化的作用对内吞后的GPCRs进行分选,介导受体发生复敏或脱敏,参与调节GPCRs活性、GPCRs转运过程、信号转导以及相关基因转录[9,10],进而影响相关疾病的发生或靶向GPCRs药物的效果。因此深入研究GASPs分子作用机制和功能特征,揭示GASPs功能缺陷与疾病的关系,将为以GASPs为靶标的生物医学的应用提供理论依据。本文对GASPs家族的结构特征、分选作用机制、相关信号转导、基因转录方面起的重要作用及其与疾病发生的关系进行系统的综述。
1 GPCRs与GASPs
1.1 GPCRs及其信号转导
GPCRs作为哺乳动物体内最大的跨膜受体家族,不仅种类众多,且其家族成员具有相同的结构特征:7次跨膜α螺旋,在其肽链的羧基端和连接第5及第6个跨膜螺旋的胞内环(即第3个胞内环)上都有鸟苷酸结合蛋白的结合位点,但其氨基酸的序列在进化上缺乏保守性,因而也使GPCRs的功能呈现复杂多样性[11,12]。GPCRs均具有与异三聚Gαβγ蛋白相互作用的能力,βγ二聚体通过共价键结合于膜上起稳定α亚基的作用,而α亚基本身具有GTP酶活性,它们作为GTPase活性中心从而启动下游信号的转导。按其序列的相似度主要可分为谷氨酸(glutamate, G)、视紫红质(rhodopsin, R)、粘附力(adhesion, A)、Frizzled/ Tastes (F)、分泌素(secretin, S)5个家族,简称为GRAFS[13]。其中,G家族成员较少,主要包括代谢型谷氨酸受体和钙受体样受体等,其氨基末端有500~600个氨基酸残基,ICL3很短且高度保守,羧基末端无棕榈酰化位点,不形成ICL4[14],其家族成员功能异常将导致慢性癫痫病、阿尔兹海默病和帕金森病等神经系统疾病[15]。R家族是视紫红质样受体和肾上腺素受体,该亚家族包含了绝大多数种类的G蛋白偶联受体,构成基础多肽链的结构具有氨基末端氨基酸残基数少的特点,第三胞内环(ICL3)较长,羧基末端有棕榈酰化的半胱氨酸,形成第四胞内环(ICL4)[14],其功能缺失主要与心血管疾病有关,如高血压、急性心肌梗塞和心力衰竭[16]。A家族受体具有一些GPCRs非经典特征,包括一个较长的细胞外氨基末端和一个自催化GPCR蛋白水解位点(GPCR proteolysis site, GPS),其主要与血管生成、炎症、男性不育及某些遗传病有关[13]。F家族包括10个frizzled受体(hFZD1-10)和一个平滑受体(hSmo),以及25个Taste2或苦味受体,而Frizzled/Tastes类受体缺乏R家族GPCRs中大多数保守性特征,但是Smo结构依旧显示出保守的7次跨膜结构,该家族成员的功能异常将导致慢性鼻窦炎、哮喘、囊性纤维化和癌症等疾病[17]。S家族包括15种不同类型的受体,其在体内的天然配体多为多肽类激素,它们在氨基末端的氨基酸残基多达100多个,ICL3较长,羧基末端无棕榈酰化位点,不形成ICL4[14];该家族成员的功能缺陷会导致肥胖、癌症及心脑血管疾病,因此它们也是临床相关疾病治疗的重要靶标[18]。GPCRs配体的多样性决定GPCRs涉及细胞内多种信号通路,因此GPCRs信号通路是细胞内比较重要的一类信号通路。目前认为GPCRs介导和调控生理功能主要经由两条途径:受体激活的G蛋白途径和配体激活的β-arrestin途径[19]。前者,G 蛋白偶联受体(GPCRs)若被各种不同的外界信号激活,激活G蛋白信号途径,包括cAMP通路、磷脂酰肌醇信号通路和MAPK/Erk信号通路等[20,21,22,23]。由于持续的GPCRs信号将会影响细胞存活和发生不良应激反应,因此需要严格调控GPCRs信号的终止。后者,β-arrestin途径不仅能阻断G蛋白的信号转导,同时还与网格蛋白和网格蛋白衔接蛋白相互作用,从而促进了活化受体的内化,在GPCRs的脱敏、复敏、细胞增殖和基因转录的过程中起重要作用[24]。受体内吞可能发生两种变化:(1)在衣被小泡内发生去磷酸化,通过再循环回到质膜表面;(2)经过内体分选,有些非泛素化的受体蛋白再循环至细胞膜表面,有些受体蛋白则在溶酶体中发生降解。
1.2 GASPs结构特征与表达分布
GASPs是在酵母双杂交筛选中利用δ-阿片受体(DOR)胞质的羧基尾作为诱饵而首次筛选出的新型分选蛋白,并通过与GPCRs的羧基末端相互作用介导了DOR的降解,该靶蛋白被命名为GASP-1[25]。GASP-1已被证明可以在体内和体外调节GPCRs的转运特性[25,26,27],后续与GASP-1结构与功能相似成员被鉴定,迄今共有10个家族成员,即GASP-1~GASP-10[8]。除GASP-8外,所有GASP成员均定位于X染色体的q22.1~q22.2区域,人类有两个GASP-8基因拷贝,分别定位于人类7号染色体(含有7个外显子,可产生4种剪切变体)和3号染色体(只含有一个编码蛋白质的外显子,一种剪切变体)上[27]。GASP-1~GASP-10具有从249~1395个不等的氨基酸,各成员之间有明显的序列相似性,在近氨基端都存在含250个保守的氨基酸残基的羧基末端区(犰狳类重复)的跨膜区[27]。GASP-1具有最长的氨基酸序列,其羧基末端与其它的GASPs家族成员的羧基末端之间约有20%~77%的序列相似性(图1),而GASP-10具有最短的氨基酸序列[28]。根据GASPs的序列特点将家族成员可分为两个亚家族,亚家族I包括GASP-1到GASP-5,它们在羧基末端之外都含有15个高度保守的氨基酸残基组成的重复基序,称为“GASP基序”,“GASP基序”中含有4个高度保守的氨基酸残基SWFW,其是与GPCRs的C末端发生相互作用的关键部位。GASP-1含有22个GASP基序,从GASP-2到GASP-5都含有2个GASP基序。而亚家族II包括GASP-6到GASP-10,它们均不含有GASP基序[28]。
图1
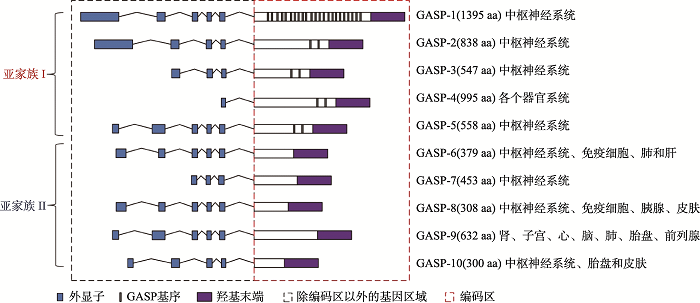 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1GASPs家族成员的比较
Fig. 1The comparison of GASP family members
尽管GASPs家族成员在生物体内普遍表达[29],但大部分成员主要在中枢神经系统(CNS)中高度表达,而GASP-6主要分布于免疫细胞、肺和肝组织中;GASP-8和GASP-10主要分布于胰和皮肤中;GASP-9在肾、子宫、心脏、脑、肺、胎盘以及前列腺等器官高度表达[30]。与此同时,超过90%的GPCRs也在CNS中表达,这种表达模式与GASPs家族参与调节这些受体的活性以及GASPs家族和GPCRs之间的相互作用相一致。
1.3 GASPs及其相互作用伴侣
GASPs根据其结构特征,其主要的相互作用伴侣是GPCRs(表1)。由于亚家族I成员的结构域主要是由氨基端跨膜域、GASP基序和羧基端犰狳类重复序列3个部分构成,而人们分别研究了这3个区域与GPCRs的结合作用。Bornert等[28]通过前期GASP-1蛋白的截短体共免疫沉淀实验结果表明,GASP-1的氨基端跨膜域与GPCRs之间不存在结合作用;只含有GASP-1保守的羧基末端区域与GPCRs(ADRB1和M1)几乎无相互作用,而与DOR受体仅有微弱的结合;相反的,含有GASP-1的中央结构域保留有70%与GPCRs(ADRB1和M1)结合作用,与DOR受体结合作用留有30%;而含有GASP-1的中央结构区域和羧基末端两个区域与GPCRs(DOR、ADRB1和M1)结合作用很强,与完整的GASP-1的结合能力相似。因此,研究发现该亚家族I成员GASP-1与GPCRs存在两种类型的相互作用关系:一类GPCRs靶向GASP-1的中间部位(GASP基序)相结合;而另一些GPCRs与GASP-1的中间部位和羧基端都能发生相互作用[28]。这表明GASP-1中央结构域的GASP基序是与GPCRs相互作用的关键部位。该中央结构域基序也存在于GASP-2到GASP-5的相应中央部分,因此提示在GPCRs与这些GASP亚家族成员之间的相互作用中GASP基序可能也发挥着重要作用。GASP-1不仅能与GPCRs发生结合,还能与接头蛋白(Gab1)[31]及微管(microtubules)[32]发生相互作用,从而对细胞骨架的形成与解聚进行动态调控。在GASP家族中GASP-2与GASP-1的序列同源性最为相似,因此GASP-2也同样具有类似GASP-1的功能,其可以与亨廷顿蛋白(Huntingtin, HTT)[33]、多巴胺D2[34]受体以及病毒趋化因子受体US28(由人巨细胞病毒HCMV所编码的G蛋白偶联受体[35,36])等受体相结合,将它们靶向至再循环或降解途径。据报道[28],GASP-3及GASP-5也能与GPCRs发生强结合。由于GASP亚家族II的结构域主要由两部分构成:N端跨膜域和C端犰狳类重复序列,不包含GASP基序,因此与GASP亚家族I相比,亚家族II与GPCRs的羧基末端作用比较弱[28]。尽管亚家族II的成员与GPCRs的作用较弱,但是它们仍然能与部分GPCRs发生作用,如GASP-6、GASP-7与肾上腺素能受体(ADRB1)、降钙素受体(CALCR)、血栓素A2受体(TXA2)存在一定的相互作用[28],而GASP-8虽不与GPCRs发生相互作用,但是P53可以作为其相互作用伴侣[37],从而参与肿瘤发生发展过程。因此可见,GASP家族的羧基端保守区域对其与GPCRs相互作用的过程发挥着重要作用。Table 1
表1
表1GASPs及其相互作用伴侣
Table 1
| GASPs | 别名 | 互作伴侣 | 结合位点 | 主要功能 | 参考文献 | ||
|---|---|---|---|---|---|---|---|
| 氨基末端 | 羧基末端 | GASP基序 | |||||
| GASP-1 | PIPS/KIAA0443 | GPCRs | √ | √ | 参与转运和降解 | [25] | |
| Period 1 | √ | √ | 与昼夜节律调控相关 | [38] | |||
| Gab 1 | 结合位点尚未明确 | 与生长发育相关 | [39] | ||||
| Microtubules | √ | √ | 维持细胞骨架相关 | [32] | |||
| GASP-2 | HIP15 | GPCRs | √ | √ | 参与转运和降解 | [30] | |
| Htt | √ | 与亨廷顿舞蹈症相关 | [40] | ||||
| Smoothened | √ | 参与Hedgehog信号通路 | [41] | ||||
| mGluR5 | √ | 与自闭症有关 | [42] | ||||
| GASP-3 | P60TRP/BHLHB9 | GPCRs | √ | √ | 参与转运和降解 | [28] | |
| PPP2R5E | 结合位点尚未明确 | 与神经元的生长调节有关 | [43] | ||||
| IPO5 | 结合位点尚未明确 | 与神经元的生长调节有关 | [43] | ||||
| GASP-5 | ARMCX5 | GPCRs | √ | √ | 参与转运和降解 | [28] | |
| GASP-6 | ALEX3/ARMCX3 | Sox10 | √ | 与转录有关 | [44] | ||
| GASP-7 | ALEX1/ARMCX1 | GPCRs | √ | 参与转运和降解 | [28] | ||
| PP110 | 结合位点尚未明确 | 与肿瘤的发生和发展有关 | [29] | ||||
| GASP-8 | SVH | P53 | √ | 与肿瘤的发生和发展有关 | [37] | ||
| GASP-9 | ALEX2/ARMCX2 | VIM | 结合位点尚未明确 | 维持细胞骨架相关 | [33] | ||
新窗口打开|下载CSV
2 GASPs分子功能
2.1 GASPs调控GPCRs内吞后的分选过程
GPCRs激活后被靶细胞内吞,其后面临两个分选途径:(1)去磷酸化进入再循环到胞膜;(2)受体经过内体分选,有些非泛素化的受体蛋白再循环至细胞膜表面,有些受体蛋白则在溶酶体中发生降解(图2)。当配体与GPCRs结合后,G蛋白偶联受体激酶将磷酸化细胞膜上的蛋白受体,一旦磷酸化,在β-arrestins作用下GPCRs与G蛋白解偶联,并通过网格蛋白有被小泡内化至分选内体[45],实现受体内吞作用。受体内吞后的分选途径的选择主要取决于内吞后受体的磷酸化以及泛素化过程,当受到激动剂的作用后,GPCRs发生磷酸化,这对受体的内吞以及受体再循环至质膜途径是至关重要的[46],通过快速再循环途径将内化的GPCRs运送质膜上,以恢复受体的功能,并促进受体介导的信号转导的重新致敏性。相反的,当GPCRs羧基端的赖氨酸残基与泛素化蛋白相互作用时,将促进受体的内吞和受体进入溶酶体中降解途径[47],促进了受体蛋白的水解和下调,从而导致细胞信号转导的衰减效应延长[48]。目前,已经发现多个受体内吞后再循环途径和受体内吞后降解途径相关的蛋白,而这些相关蛋白通常可以通过与GPCRs的羧基末端结构域相互作用,从而特异性靶向GPCRs,它们可以再循环利用受体也可以降解受体[5]。在此过程中,GASPs在调控GPCRs内吞后的分选过程中发挥了重要的作用。GASP-1作为该家族的较早发现的成员已被广泛地研究,GASP-1主要通过其羧基末端与受体的Helix-8相互作用[30],从而介导受体进入溶酶体中,如GASP-1可以与内吞作用后的δ-阿片类受体、多巴胺D2受体、多巴胺D3受体、大麻素受体等多个GPCRs的降解有关[49,50,51]。因此其羧基端区域的破坏,有可能抑制受体的降解,从而促进受体的再循环。但是,GASP-1并不能与所有内吞后的GPCRs进行相互作用,如μ-阿片类受体和多巴胺D1受体不能与GASP-1相互作用,从而它们会被“再循环蛋白”运送回细胞膜上。GASP-1不仅在细胞上发挥重要的作用,而且其可以将病毒趋化因子受体US28靶向至晚期核内体和溶酶体内,这一过程对病毒的外包是至关重要的[36]。最近,Edfawy等[42]研究发现GASP-2可以利用其内吞分选作用,调节代谢型谷氨酸受体的表面有效性,而I型代谢型谷氨酸受体在小脑和海马结构突触可塑性中起重要作用,它们的激活导致α-氨基-3-羟基-5-甲基-4-异恶唑丙酸(AMPA)受体的内化。当急性过表达GASP-2时,细胞膜表面的代谢型谷氨酸受体5(mGluR5)的信号降低20%左右;相反的,当敲除GASP-2时,在细胞膜表面的mGluR5聚集增加,这揭示GASP-2功能的异常干扰了受体内化后正常的分选机制[42]。GASPs参与的GPCRs分选保证细胞外信号向细胞内传递具有适度的强度和时空上的特异性,以及精确调控细胞膜上GPCRs的数量、活性及分布。若GPCRs结构异常或功能失调或者GASPs功能缺陷均会影响GPCRs内吞分选机制的正常调控,从而导致人类多疾病的发生,如阿尔兹海默病、肿瘤和耳聋等。因此,GASPs的正常调控对于维持细胞功能具有及其重要的作用。图2
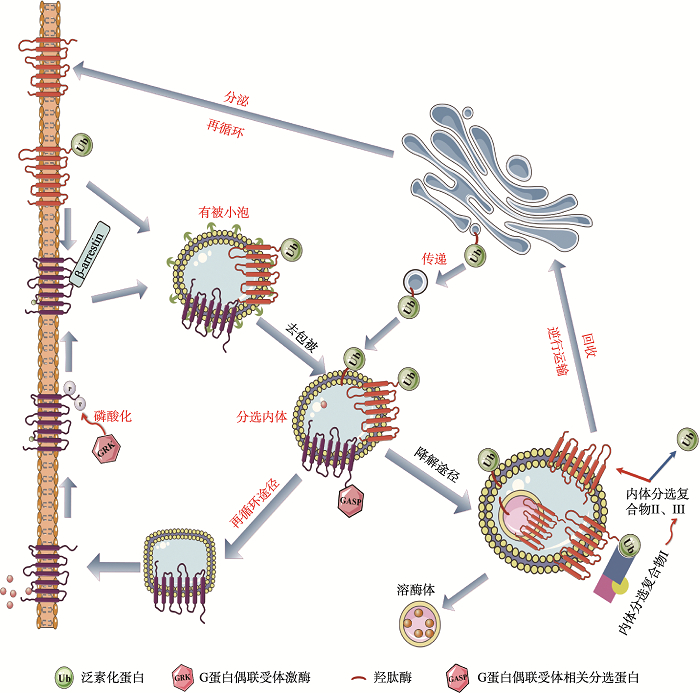 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2GASPs参与内吞后的GPCRs分选
Fig. 2GASPs participate in GPCR sorting after endocytosis
2.2 GASPs调控GPCRs相关的信号转导
GASPs家族蛋白的重要功能一方面是在GPCRs内吞后分选过程中发挥作用;另一方面,GASP家族对GPCRs的信号转导同样起到重要作用,而且GASP家族可能是连接这两个过程的重要调控因子。在HEK293细胞中敲除内源性的GASP-1会破坏US28介导的Gαq/PLC/inositol phosphate(IP)的累积,但当GASP-1过表达增强了IP的累积,与此同时,US28介导的相关核转录因子NF-?B、cAMP应答元件结合蛋白(CREB)也会随着GASP-1的过表达或敲除而表现出相应的上调或者下调[36],因此,GASP-1不仅调节了US28的内吞后运输,而且还调节了US28的信号转导能力。目前推测GASP-1的过表达上调US28的信号转导,主要可能由两个方面造成:一是US28受体与GASP-1结合后会呈现出一种更有活性的构象状态,从而增强下游信号的转导;二是GASP-1靶向US28至细胞内特定区域,进而增强信号转导。通过GST融合实验发现[30],R家族(即视紫红质类受体家族)的阿片类受体的羧基末端可以与GASP-1结合;不仅如此,还发现虽然S家族(分泌素家族)的降钙素受体的羧基末端区域与R亚家族成员没有序列同源性,但是同样也可以与GASP-1结合[30]。而G家族(即谷氨酸家族)的G蛋白偶联受体mGluR5在可塑性、神经棘形态、突触通讯[52,53,54,55,56]和神经突伸展[57]中起到关键作用,Heydorn等[26]提出GPRASPs可以调节代谢型谷氨酸受体(metabotropic glutamate receptor, mGluRs),研究者利用在转染后的HT-22细胞中证实了GPRASP2和mGluR5的相互作用,且缺失GPRASP2基因会干扰受体内吞后的正常机制,表现出异常的mGluR信号转导[42]。Jung等[41]研究发现,GASP-2蛋白可以与Smo蛋白和Pitchfork蛋白形成纤毛靶向复合体,能够调节Smo向初级纤毛的易位,从而参与Hedgehog通路的正常调控。Hedgehog(Hh)信号通路是一条从无脊椎动物到脊椎动物都高度保守的信号通路,在胚胎期多种组织器官的发育、纤毛形成及肿瘤发生发展中都发挥着重要的作用[58]。Hh信号传导通路核心组成部分包括分泌型糖蛋白配体(Hh)、膜蛋白受体复合物、核转录因子及下游目的基因4个部分。膜蛋白受体Ptch由肿瘤抑制基因Patched编码,Ptch是一种12次跨膜受体;而受体Smo作为Ptch蛋白的下游分子,其由原癌基因Smoothened编码,它属于G蛋白偶联受体相关蛋白,由7个跨膜区的单一肽链构成,N端位于细胞外,C端位于细胞内,其磷酸化部位位于羧基末端的丝氨酸和苏氨酸残基上,该蛋白家族成员只有维持蛋白全长时才具有转录启动活性,从而启动下游靶基因的转录[59,60]。而当GASP-2蛋白缺失时,将导致Smo蛋白易位的失败和抑制Hh靶基因的激活,从而抑制Hedgehog信号通路的激活。因此,GASPs家族成员的作用不仅仅体现在GPCRs内吞后的分选过程,还体现在参与相关的信号转导过程,且GASPs可能是连接两个过程的重要调控因子。
2.3 GASPs与GPCR相互作用的机制
经配体激活后,大多数GPCRs将受到一系列涉及脱敏和内吞作用的调节。内化后的受体会被再循环至质膜中或者靶向至溶酶体中降解。有文献报道[26,61],GASP-1可以与许多GPCRs的羧基末端进行相互作用,而在大部分GPCRs的羧基末端存在helix-8,其是参与GASP和GPCRs相互作用的主要区域[30]。而受体再循环至质膜主要取决于一些受体的羧基末端含有“回收序列”,此“回收序列”可以与NHERF/EPB50等再循环蛋白进行相互作用,从而促进受体迅速再循环[34]。Thompson等[34]在人类HEK293细胞中发现,MOR受体羧基末端的“回收序列”决定了MOR不与GASP-1结合,但当截短MOR受体的末尾17个氨基酸时,经免疫沉淀结果显示MOR可以与GASP-1进行结合,进而将受体运送至溶酶体中降解。因此,可以通过破坏与再循环蛋白的相互作用,或通过突变增强受体对GASP的亲和力来促进受体与GASP的相互作用,从而改变受体内吞后的命运。
2.4 GASPs调控基因转录表达
研究表明GASPs家族成员参与基因转录表达调控。Matsuki等[62]关于GASP-1的研究表明,GASP-1可以与Period-1(与哺乳动物昼夜节律调控相关的重要蛋白并且在转录调节过程中发挥着重要的作用)发生相互作用,并且在同一细胞中共表达这两种蛋白质可以促进GASP-1靶向细胞核,在神经生长因子(nerve growth factor, NGF)处理后的PC12细胞中进一步发现GASP-1发生了核转位。此外,在用GASP-1 siRNA预处理的细胞中,NGF可以使PC12细胞免于细胞凋亡或死亡。这些结果表明,在NGF介导作用下,GASP-1通过核转位调节相关靶基因的转录,从而保护细胞免于凋亡[38,39]。不仅是GASP-1,GASP-3主要定位在细胞质中,有些则在细胞核中。GASP-3的序列中存在bHLH转录因子家族特有的碱性螺旋-环-螺旋基序,这表明GASP-3可能参与基因调控转录。另一方面,在血清饥饿诱导条件下用siRNA干扰GASP-3处理PC12细胞的研究中,发现GASP-3对神经元细胞的存活和增殖起到重要的调控作用,可能参与了相关基因的转录调节[27]。据报道,GASPs亚家族II中的GASP-6定位于线粒体的外膜中,其可与转录因子Sox10相互作用。Mou等[44]外源性过表达GASP-6后,发现线粒体中Sox10的数量增加。另外,尽管GASP-6不具有固有的转录活性,但它确实增强了Sox10对烟碱乙酰胆碱受体(nACh)α3和β4亚基的基因启动子的反式激活,Sox10与GASP-6能够通过直接相互作用增加转录功能及细胞核与线粒体之间进行信号转导级联的可能性。而GASP-6与GASP-8均能通过与转录因子相互作用调节转录[37,63]:在QSG-1701(正常肝细胞系)中发现过表达GASP-8 能加速细胞生长,而抑制或干扰GASP-8表达则诱导肝癌细胞发生凋亡。由此,GASPs家族成员可以通过参与胞内基因的转录调控从而影响细胞的存活与增殖。
3 GASPs功能缺陷与疾病
3.1GASPs功能异常与肿瘤越来越多研究表明GASP家族成员的功能异常与癌症发生和发展存在密切关系,Tuszynski等[64]研究发现在早期乳腺癌患者的血清中存在GASP-1的特定蛋白片段,而正常患者的血清中则没有;同时在7例2期和3期乳腺癌的肿瘤提取物中也检测到GASP-1表达,但在相邻的正常组织中未检测到。而且GASP-1在多种癌症病人的血清中都显著过表达[65],包括乳腺癌、脑癌和肺癌等,因此GASP-1可以作为一类潜在的肿瘤生物标记物。在几种结肠直肠癌细胞系和原发性肿瘤中都发现了GASP-3基因启动子CpG岛的高甲基化,并且这几类肿瘤都与GASP-3的表达缺陷相关[66],结果表明GASP-3参与了细胞的恶性转化。此外,Rickman等[67]对经手术治疗发生转移和未发生转移的186例原发性头颈部鳞状细胞癌进行分析,研究发现与手术后未发生转移的患者相比,这些最初接受手术后发生转移的患者中其GASP-2和GASP-3在头颈部鳞状细胞癌中的表达水平也显著上调,这表明GASP-2和GASP-3可能参与了肿瘤的转移和恶化。还有研究发现GASP-5可以作为ZNF217的下游靶基因,而ZNF217广泛分布在各种肿瘤细胞中,其在肿瘤转化过程和基因转录调控中起关键作用,该基因水平下调时可能导致与分化相关的GASP-5基因下调,导致细胞去分化并促使细胞加速增殖,进而诱发癌症的发生[68]。在肺癌、前列腺癌、结肠癌、胰腺癌和卵巢癌中以及在不同人癌细胞系中都发现GASP-7和GASP-9转录物的表达缺失或下调[30],而且在肝细胞癌中GASP-8的亚型2表达上调,过表达这种亚型的人肝癌细胞系生长比率增高,促进肿瘤的发生发展,而干扰其RNA表达能够诱导肝癌细胞凋亡[63]。这些结果均揭示GASPs家族蛋白可以作为肿瘤发展早期的生物标志物,可能代表癌症治疗的新靶标。
3.2GASPs功能异常与神经系统疾病
GASPs家族成员广泛分布于中枢神经系统,GASP家族成员大部分位于X染色体q22.1~q22.2,该区域与人类的精神疾病有着很大的关联。尤其是GASP-1和GASP-2。研究表明[69],GASP-1基因敲除的小鼠表现出程序性的记忆丧失,小鼠缺乏获得性反应和复杂反应的能力。Horn等[40]研究发现HTT和GASP-2之间可以发生相互作用,从而影响受体的再循环和降解途径。由于HTT出现延长的polyQ序列[70],影响了GASP2和HTT之间亲和力,从而可能直接影响患者神经元受体的表面密度,这一过程与亨廷顿舞蹈症有很大的关联性。此外,GASP-2可以与7次跨膜受体Smo以及Pitchfork(Pifo)形成复合物,在Smo转运至初级纤毛的过程中起到重要的作用,GASP-2或者Pifo的缺失会导致Hedgehog信号通路的异常,从而无法激活Hh靶基因,进而会引起小脑发育异常、颅面缺陷、共济失调等症状[41]。特别是Xq22.1区域内的0.35 Mb的缺失,会导致个体表现出呼吸障碍、腭裂和癫痫等症状[71]。此外,GASP-3可能通过与两个重要的细胞内信号激酶RanBP5(importin-β样运输受体)和PP2A(调控tau蛋白磷酸化的磷酸酶)发生相互作用,从而参与调控神经元细胞的存活和生长。而在AD患者的大脑组织中发现GASP-3 mRNA水平下降,同时在阿尔茨海默症中观察到多种神经元的退化和死亡[43],这揭示了GASP-3在神经元细胞正常的生长和发育中发挥着重要作用,并且研究者还将GASP-2和GASP-4列入帕金森病患者大脑组织中892个高度失调的优先基因列表中,这些失调的优先基因可能位于疾病过程的核心,并且可以作为治疗PD疾病的新靶标[72]。这些研究表明,GASPs与神经系统性疾病密切相连。
3.3 GASPs功能异常与耳聋
在内耳的发育过程中,许多GPCRs,包括Vlgr1、Lgr5和腺苷酸受体等在内耳中都有表达。其中,Vlgr1蛋白是目前已知最大的G蛋白偶联受体,其主要表达在毛细胞的纤毛根部,参与纤毛踝连接的形成[73]。同时,Vlgr1基因的突变可以引起Usher综合征[74,75],实验表明腺苷酸环化酶AC6在纤毛上与Vlgr1共定位,从而推测可能通过G蛋白激活腺苷酸酶AC6,从而参与cAMP介导的信号传递途径[76],其异常导致静纤毛形态的异常,从而影响听觉转导和内耳发育。Lgr5,又称为GPR49,研究发现当内耳毛细胞受损伤刺激,激活Wnt信号通路可以促进Lgr5阳性细胞的增殖,而抑制Notch信号通路可以显著地促进Lgr5阳性细胞分化成毛细胞,从而显著地促进毛细胞的再生[77],这为在损伤模型中通过前体细胞再生来恢复耳蜗结构和功能奠定了理论和实验基础。而最新研究表明,能够靶向GPCRs的GASPs家族也与听觉功能密切相关。本实验室在一个中国人遗传性耳聋大家系中发现了GASP-2突变的遗传学效应[78],该突变造成蛋白质侧链的位阻和极性的改变,导致蛋白质的结构变化,进而患者出现耳廓畸形、内耳道闭锁(狭窄)、先天性传导性或混合性听力丧失、双侧上睑下垂伴随面部畸形等临床表现。随后,Liu等[79]利用原位杂交和逆转录聚合酶链反应揭示了GASP-2主要在斑马鱼的内耳和神经系统中表达,GASP-2的分子致聋机制正在研究中,有望对GASPs家族相关的致聋机制提供理论与实验依据。4 结语与展望
GPCRs作为哺乳动物体内分布最广、种类最多的一类膜表面受体[80],广泛参与体内体外多种生理过程,如免疫系统的调节、自主神经系统的调节和维持机体稳态等,它一直是人们研究的热点以及药物研究的靶点。如今,近一半的临床使用药物通过靶向不同种类的GPCRs而发挥作用[81]。GASPs作为重要的GPCRs互作蛋白,在调控GPCRs的活性、内吞分选及信号转导等过程发挥重要的作用,如通过对δ-阿片类受体、mGluR5、HTT和Smo等受体蛋白的靶向结合作用,参与多种疾病的发生、发展,如神经系统疾病、肿瘤、耳聋形成等。随着人们对GASPs家族的具体分选机制功能及调节过程的研究不断深入,有助于更好地理解这些GASPs的精确的分子功能,研究靶向GASPs及其相互作用伴侣,期望作为神经退行性疾病\癌症及耳聋等疾病新的药物治疗靶点,进而为其相关疾病的诊治或干预策略提供新的思路与方向。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:28931490 [本文引用: 1]
DOI:10.1210/en.2009-0760URLPMID:19887567 [本文引用: 1]

Through in silico subtraction and microarray analysis, we identified mouse Gpr149, a novel, oocyte-enriched transcript that encodes a predicted orphan G-protein-coupled receptor (GPR). Phylogenetic analysis of GPR149 from fish to mammals suggests that it is widely conserved in vertebrates. By multitissue RT-PCR analysis, we found that Gpr149 is highly expressed in the ovary and also in the brain and the digestive tract at low levels. Gpr149 levels are low in newborn ovaries but increase throughout folliculogenesis. In the ovary, we found that granulosa cells did not express Gpr149, whereas germinal vesicle and meiosis II stage oocytes showed high levels of Gpr149 expression. After fertilization, Gpr149 expression declined, becoming undetectable by the two-cell stage. To study the function of GPR149 in oocyte growth and maturation, we generated Gpr149 null mice. Surprisingly, Gpr149 null mice are viable and have normal folliculogenesis, but demonstrate increased fertility, enhanced ovulation, increased oocyte Gdf9 mRNA levels, and increased levels of FSH receptor and cyclin D2 mRNA levels in granulosa cells. Thus, Gpr149 null mice are one of the few models with enhanced fertility, and GPR149 could be a target for small molecules to enhance fertility in the assisted reproductive technology clinic.
DOI:10.1038/nrd4052URLPMID:23903222 [本文引用: 1]

Allosteric ligands bind to G protein-coupled receptors (GPCRs; also known as seven-transmembrane receptors) at sites that are distinct from the sites to which endogenous ligands bind. The existence of allosteric ligands has enriched the ways in which the functions of GPCRs can be manipulated for potential therapeutic benefit, yet the complexity of their actions provides both challenges and opportunities for drug screening and development. Converging avenues of research in areas such as biased signalling by allosteric ligands and the mechanisms by which allosteric ligands modulate the effects of diverse endogenous ligands have provided new insights into how interactions between allosteric ligands and GPCRs could be exploited for drug discovery. These new findings have the potential to alter how screening for allosteric drugs is performed and may increase the chances of success in the development of allosteric modulators as clinical lead compounds.
DOI:10.1016/j.bcp.2012.09.001URLPMID:22975406 [本文引用: 1]

Their ubiquitous nature, wide cellular distribution and versatile molecular recognition and signalling help make G-protein binding receptors (GPCRs) the most important class of membrane proteins in clinical medicine, accounting for approximately 40% of all current therapeutics. A large percentage of current drugs target the endogenous ligand binding (orthosteric) site, which are structurally and evolutionarily conserved, particularly among members of the same GPCR subfamily. With the recent advances in GPCR X-ray crystallography, new opportunities for developing novel subtype selective drugs have emerged. Given the increasing recognition that the extracellular surface conformation changes in response to ligand binding, it is likely that all GPCRs possess an allosteric site(s) capable of regulating GPCR signalling. Allosteric sites are less structurally conserved than their corresponding orthosteric site and thus provide new opportunities for the development of more selective drugs. Constitutive oligomerisation (dimerisation) identified in many of the GPCRs investigated, adds another dimension to the structural and functional complexity of GPCRs. In this review, we compare 60 crystal structures of nine GPCR subtypes (rhodopsin, ss(2)-AR, ss(1)-AR, A(2a)-AR, CXCR4, D(3)R, H(1)R, M(2)R, M(3)R) across four subfamilies of Class A GPCRs, and discuss mechanisms involved in receptor activation and potential allosteric binding sites across the highly variable extracellular surface of these GPCRs. This analysis has identified a new extracellular salt bridge (ESB-2) that might be exploited in the design of allosteric modulators.
DOI:10.1146/annurev.pharmtox.48.113006.094830URLPMID:18184106 [本文引用: 2]

The endocytic pathway tightly controls the activity of G protein-coupled receptors (GPCRs). Ligand-induced endocytosis can drive receptors into divergent lysosomal and recycling pathways, producing essentially opposite effects on the strength and duration of cellular signaling via heterotrimeric G proteins, and may also promote distinct signaling events from intracellular membranes. This chapter reviews recent developments toward understanding the molecular machinery and functional implications of GPCR sorting in the endocytic pathway, focusing on mammalian GPCRs whose ligand-induced endocytosis is mediated primarily by clathrin-coated pits. Lysosomal sorting of a number of GPCRs occurs via a highly conserved mechanism requiring covalent tagging of receptors with ubiquitin. There is increasing evidence that additional, noncovalent mechanisms control the sorting of endocytosed GPCRs to lysosomes in mammalian cells. Recycling of several GPCRs to the plasma membrane is also specifically sorted, via a mechanism requiring both receptor-specific and shared sorting proteins. The current data reveal an unprecedented degree of specificity and plasticity in the cellular regulation of mammalian GPCRs by endocytic membrane trafficking. These developments have fundamental implications for GPCR pharmacology, and suggest new mechanisms that could be exploited in GPCR-directed pharmacotherapy.
DOI:10.1038/nrn987URLPMID:12461549 [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
URLPMID:29288293 [本文引用: 1]
DOI:10.1038/srep22540URLPMID:26931153 [本文引用: 1]

Trafficking of the G protein-coupled receptor (GPCR) Smoothened (Smo) to the primary cilium (PC) is a potential target to inhibit oncogenic Hh pathway activation in a large number of tumors. One drawback is the appearance of Smo mutations that resist drug treatment, which is a common reason for cancer treatment failure. Here, we undertook a high content screen with compounds in preclinical or clinical development and identified ten small molecules that prevent constitutive active mutant SmoM2 transport into PC for subsequent Hh pathway activation. Eight of the ten small molecules act through direct interference with the G protein-coupled receptor associated sorting protein 2 (Gprasp2)-SmoM2 ciliary targeting complex, whereas one antagonist of ionotropic receptors prevents intracellular trafficking of Smo to the PC. Together, these findings identify several compounds with the potential to treat drug-resistant SmoM2-driven cancer forms, but also reveal off-target effects of established drugs in the clinics.
DOI:10.1016/j.bcp.2012.04.013URLPMID:22554564 [本文引用: 1]

Group-III metabotropic glutamate receptors (mGluRs) comprise four structurally related brain and retinal G protein-coupled receptors (GPCRs), mGluR4, mGluR6, mGluR7 and mGluR8, which receive much attention as promising targets for nervous system drugs. In particular, activation of mGluR4 is a major focus for the development of new therapeutics in Parkinson's disease, while mGluR7 activation is considered a potential approach for future treatments of specific psychiatric conditions. The first generation group-III mGluR agonists, e.g.l-AP4 and l-SOP, are characterized by an essential phosphonate functional group, which became a major limitation for the development of systemically active, potent and receptor subtype-selective drugs. Recently however, two approaches emerged in parallel providing resolution to this constraint: in silico high-throughput screening of chemical libraries against a 3D-model of the mGluR4 extracellular domain identified a hit that was optimized into a series of potent and subtype-selective orthosteric agonists with drug-like properties and novel chemotype structures; secondly, high-throughput random screening of chemical libraries against recombinantly expressed group-III receptors identified diverse chemical sets of allosteric agonists and positive modulators, which are drug-like, display selectivity for mGluR4, mGluR7, or mGluR8 and act via novel pharmacological sites. Here, we illustrate new scientific insights obtained via the use of those strategies. Also, we compare advantages and disadvantages of both approaches to identify the desired group-III mGluR activators and we conclude with suggestions how to employ those discovery strategies with success for the identification, optimization, and development of clinical drug candidates; this may have important implications for the entire field of GPCR research.
DOI:10.1210/me.2010-0217URLPMID:21084381 [本文引用: 1]

Melanocortin 1 receptor (MC1R), a Gs protein-coupled receptor expressed in melanocytes, is a major determinant of skin pigmentation, phototype and cancer risk. Upon stimulation by alphaMSH, MC1R triggers the cAMP and ERK1/ERK2 MAPK pathways. In mouse melanocytes, ERK activation by alphaMSH binding to Mc1r depends on cAMP, and melanocytes are considered a paradigm for cAMP-dependent ERK activation. However, human MC1R variants associated with red hair, fair skin [red hair color (RHC) phenotype], and increased skin cancer risk display reduced cAMP signaling but activate ERKs as efficiently as wild type in heterologous cells, suggesting independent signaling to ERKs and cAMP in human melanocytes. We show that MC1R signaling activated the ERK pathway in normal human melanocytes and melanoma cells expressing physiological levels of endogenous RHC variants. ERK activation was comparable for wild-type and mutant MC1R and was independent on cAMP because it was neither triggered by stimulation of cAMP synthesis with forskolin nor blocked by the adenylyl cyclase inhibitor 2',5'-dideoxyadenosine. Stimulation of MC1R with alphaMSH did not lead to protein kinase C activation and ERK activation was unaffected by protein kinase C inhibitors. Conversely, pharmacological interference, small interfering RNA studies, expression profiles, and functional reconstitution experiments showed that alphaMSH-induced ERK activation resulted from Src tyrosine kinase-mediated transactivation of the stem cell factor receptor, a receptor tyrosine kinase essential for proliferation, differentiation, and survival of melanocyte precursors, thus demonstrating a functional link between the stem cell factor receptor and MC1R. Moreover, this transactivation phenomenon is unique because it is unaffected by natural mutations impairing canonical MC1R signaling through the cAMP pathway.
[本文引用: 2]
DOI:10.1073/pnas.0230374100URLPMID:12679517 [本文引用: 3]

Diverse members of the G protein-coupled receptor (GPCR) superfamily participate in a variety of physiological functions and are major targets of pharmaceutical drugs. Here we report that the repertoire of GPCRs for endogenous ligands consists of 367 receptors in humans and 392 in mice. Included here are 26 human and 83 mouse GPCRs not previously identified. A direct comparison of GPCRs in the two species reveals an unexpected level of orthology. The evolutionary preservation of these molecules argues against functional redundancy among highly related receptors. Phylogenetic analyses cluster 60% of GPCRs according to ligand preference, allowing prediction of ligand types for dozens of orphan receptors. Expression profiling of 100 GPCRs demonstrates that most are expressed in multiple tissues and that individual tissues express multiple GPCRs. Over 90% of GPCRs are expressed in the brain. Strikingly, however, the profiles of most GPCRs are unique, yielding thousands of tissue- and cell-specific receptor combinations for the modulation of physiological processes.
DOI:10.3389/fphar.2019.01282URLPMID:31719824 [本文引用: 1]

Alzheimer's disease (AD), particularly its sporadic or late-onset form (SAD/LOAD), is the most prevalent (96-98% of cases) neurodegenerative dementia in aged people. AD's neuropathology hallmarks are intrabrain accumulation of amyloid-beta peptides (Abetas) and of hyperphosphorylated Tau (p-Tau) proteins, diffuse neuroinflammation, and progressive death of neurons and oligodendrocytes. Mounting evidences suggest that family C G-protein-coupled receptors (GPCRs), which include gamma-aminobutyric acid B receptors (GABABRs), metabotropic glutamate receptors (mGluR1-8), and the calcium-sensing receptor (CaSR), are involved in many neurotransmitter systems that dysfunction in AD. This review updates the available knowledge about the roles of GPCRs, particularly but not exclusively those expressed by brain astrocytes, in SAD/LOAD onset and progression, taking stock of their respective mechanisms of action and of their potential as anti-AD therapeutic targets. In particular, GABABRs prevent Abetas synthesis and neuronal hyperexcitability and group I mGluRs play important pathogenetic roles in transgenic AD-model animals. Moreover, the specific binding of Abetas to the CaSRs of human cortical astrocytes and neurons cultured in vitro engenders a pathological signaling that crucially promotes the surplus synthesis and release of Abetas and hyperphosphorylated Tau proteins, and also of nitric oxide, vascular endothelial growth factor-A, and proinflammatory agents. Concurrently, Abetas*CaSR signaling hinders the release of soluble (s)APP-alpha peptide, a neurotrophic agent and GABABR1a agonist. Altogether these effects progressively kill human cortical neurons in vitro and likely also in vivo. Several CaSR's negative allosteric modulators suppress all the noxious effects elicited by Abetas*CaSR signaling in human cortical astrocytes and neurons thus safeguarding neurons' viability in vitro and raising hopes about their potential therapeutic benefits in AD patients. Further basic and clinical investigations on these hot topics are needed taking always heed that activation of the several brain family C GPCRs may elicit divergent upshots according to the models studied.
DOI:10.1161/CIRCRESAHA.118.311403URLPMID:30355236 [本文引用: 1]

GPCRs (G-protein [guanine nucleotide-binding protein]-coupled receptors) play a central physiological role in the regulation of cardiac function in both health and disease and thus represent one of the largest class of surface receptors targeted by drugs. Several antagonists of GPCRs, such as betaARs (beta-adrenergic receptors) and Ang II (angiotensin II) receptors, are now considered standard of therapy for a wide range of cardiovascular disease, such as hypertension, coronary artery disease, and heart failure. Although the mechanism of action for GPCRs was thought to be largely worked out in the 80s and 90s, recent discoveries have brought to the fore new and previously unappreciated mechanisms for GPCR activation and subsequent downstream signaling. In this review, we focus on GPCRs most relevant to the cardiovascular system and discuss traditional components of GPCR signaling and highlight evolving concepts in the field, such as ligand bias, beta-arrestin-mediated signaling, and conformational heterogeneity.
DOI:10.1016/j.sbi.2019.02.009URLPMID:30933747 [本文引用: 1]

Recently, molecular dynamics simulations, from all atom and coarse grained to hybrid methods bridging the two scales, have provided exciting functional insights into class F (Frizzled and Taste2) GPCRs (about 40 members in humans). Findings include: (i) The activation of one member of the Frizzled receptors (FZD4) involves a bending of transmembrane helix TM7 far larger than that in class A GPCRs. (ii) The affinity of an anticancer drug targeting another member (Smoothened receptor) decreases in a specific drug-resistant variant, because the mutation ultimately disrupts the binding cavity and affects TM6. (iii) A novel two-state recognition mechanism explains the very large agonist diversity for at least one member of the Taste2 GPCRs, hTAS2R46.
[本文引用: 1]
[本文引用: 1]
URLPMID:25017568 [本文引用: 1]
DOI:10.1016/j.tips.2014.03.004URLPMID:24746475 [本文引用: 1]

G-protein-coupled receptors (GPCRs) that recognize the lysophospholipids (LPLs) are grouped into two phylogenetically distinct families: the endothelial differentiation gene (Edg) and non-Edg GPCRs. Owing to their more recent identification, and hindered by a lack of selective pharmacological tools, our understanding of the functions and signaling pathways of the non-Edg GPCRs is still in its infancy. Targeting the non-conserved allosteric binding sites of the LPL GPCRs shows particular promise for the development of selective modulators by structure-based drug design. However, only one Edg GPCR (S1PR1) structure has been determined to date, and it has low sequence identity with the non-Edg GPCRs (<20%). Thus, a representative structure of a non-Edg GPCR remains a pressing objective for selective structure-based drug design. Obtaining selective modulators targeting the non-Edg receptors would help to unravel the biology behind these novel GPCRs and potentially will support therapeutic treatment of diseases such as cancer, inflammation, and neuropsychiatric disorders.
DOI:10.1016/s0163-7827(99)00016-8URLPMID:10729607 [本文引用: 1]

Platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a biologically active phospholipid mediator. Although PAF was initially recognized for its potential to induce platelet aggregation and secretion, intense investigations have elucidated potent biological actions of PAF in a broad range of cell types and tissues, many of which also produce the molecule. PAF acts by binding to a unique G-protein-coupled seven transmembrane receptor. PAF receptor is linked to intracellular signal transduction pathways, including turnover of phosphatidylinositol, elevation in intracellular calcium concentration, and activation of kinases, resulting in versatile bioactions. On the basis of numerous pharmacological reports, PAF is thought to have many pathophysiological and physiological functions. Recently advanced molecular technics enable us not only to clone PAF receptor cDNAs and genes, but also generate PAF receptor mutant animals, i.e., PAF receptor-overexpressing mouse and PAF receptor-deficient mouse. These mutant mice gave us a novel and specific approach for identifying the pathophysiological and physiological functions of PAF. This review also describes the phenotypes of these mutant mice and discusses them by referring to previously reported pharmacological and genetical data.
DOI:10.1517/14728222.11.5.661URLPMID:17465724 [本文引用: 1]

G-protein-coupled receptors (GPCRs) are key regulators of several physiological functions. Their roles in cellular signal transduction have made them the target for majority of all currently prescribed drugs. Additionally, there are many orphan GPCRs that provide potential novel therapeutic targets. Several GPCRs are involved in metabolic regulation and glucose homeostasis such as GLP-1 receptor, glucagon receptor, adiponectin receptor and so on. Recently, free fatty acids (FFAs) have been demonstrated as ligands for orphan GPCRs and have been proposed to play a critical role in physiological glucose homeostasis. GPR40 and GPR120 are activated by medium and long-chain FFAs, whereas GPR41 and GPR43 can be activated by short-chain FFAs. GPR40, which is preferentially expressed in pancreatic beta-cells, mediates the majority of the effects of FFAs on insulin secretion. In this review, these findings and also critical analysis of these GPCRs as novel targets for diabetes are discussed.
DOI:10.1016/j.tips.2007.06.006URLPMID:17644195 [本文引用: 1]

Seven-transmembrane receptors (7TMRs), the most common molecular targets of modern drug therapy, are critically regulated by beta-arrestins, which both inhibit classic G-protein signaling and initiate distinct beta-arrestin signaling. The interplay of G-protein and beta-arrestin signals largely determines the cellular consequences of 7TMR-targeted drugs. Until recently, a drug's efficacy for beta-arrestin recruitment was believed to be proportional to its efficacy for G-protein activities. This paradigm restricts 7TMR drug effects to a linear spectrum of responses, ranging from inhibition of all responses to stimulation of all responses. However, it is now clear that 'biased ligands' can selectively activate G-protein or beta-arrestin functions and thus elicit novel biological effects from even well-studied 7TMRs. Here, we discuss the current state of beta-arrestin-biased ligand research and the prospects for beta-arrestin bias as a therapeutic target. Consideration of ligand bias might have profound influences on the way scientists approach 7TMR-targeted drug discovery.
DOI:10.1126/science.1073308URLPMID:12142540 [本文引用: 3]

Recycling of the mu opioid receptor to the plasma membrane after endocytosis promotes rapid resensitization of signal transduction, whereas targeting of the delta opioid receptor (DOR) to lysosomes causes proteolytic down-regulation. We identified a protein that binds preferentially to the cytoplasmic tail of the DOR as a candidate heterotrimeric GTP-binding protein (G protein)-coupled receptor-associated sorting protein (GASP). Disruption of the DOR-GASP interaction through receptor mutation or overexpression of a dominant negative fragment of GASP inhibited receptor trafficking to lysosomes and promoted recycling. The GASP family of proteins may modulate lysosomal sorting and functional down-regulation of a variety of G protein-coupled receptors.
URLPMID:15452121 [本文引用: 3]
DOI:10.1016/j.pharmthera.2010.03.004URLPMID:20394773 [本文引用: 4]

G protein-coupled receptors (GPCRs) represent one of the most abundant protein families encoded by the human genome. They are involved in the modulation of numerous physiological functions and represent major drug targets. Their activity is tightly controlled by a vast array of interacting partners that modulate their membrane targeting, intracellular trafficking and signalling properties. Among them, several proteins from the same family, G protein-coupled receptor associated sorting proteins (GASP), have been shown to display a broad spectrum of interactions with GPCRs. In addition to their postulated role in the modulation of the post-endocytic sorting of these receptors, recent data indicate that several GASPs may modulate the transcriptional activity of the cell through their interaction with transcription factors. However, no clear molecular function has been assigned yet to this protein family. In this review, we describe the discovery of GASPs, their major features, interacting partners, functions and possible involvement in pathological situations including neurodegenerative diseases and cancer.
DOI:10.1371/journal.pone.0056336URLPMID:23441177 [本文引用: 10]

GPCR desensitization and down-regulation are considered key molecular events underlying the development of tolerance in vivo. Among the many regulatory proteins that are involved in these complex processes, GASP-1 have been shown to participate to the sorting of several receptors toward the degradation pathway. This protein belongs to the recently identified GPCR-associated sorting proteins (GASPs) family that comprises ten members for which structural and functional details are poorly documented. We present here a detailed structure-function relationship analysis of the molecular interaction between GASPs and a panel of GPCRs. In a first step, GST-pull down experiments revealed that all the tested GASPs display significant interactions with a wide range of GPCRs. Importantly, the different GASP members exhibiting the strongest interaction properties were also characterized by the presence of a small, highly conserved and repeated
DOI:10.1006/bbrc.2000.4125URLPMID:11162520 [本文引用: 2]

Members of the armadillo (arm) repeat family of proteins are implicated in tumorigenesis, embryonic development, and maintenance of tissue integrity. We have cloned cDNA for a novel human arm repeat protein, ALEX1, encoding 453 amino acids. ALEX1 shares significant homology with uncharacterized ORF KIAA0512 and putative protein product of unknown mRNA (GenBank AF211175), designated here as ALEX2 and ALEX3, respectively. The genes encoding ALEX1, ALEX2 and ALEX3 co-localize to the same region in Xq21.33-q22.2. ALEX1 and ALEX2 transcripts are found in all human tissues examined except peripheral blood leukocytes. Expression of ALEX1 and ALEX2 mRNA is lost or significantly reduced in human lung, prostate, colon, pancreas, and ovarian carcinomas and also in cell lines established from different human carcinomas. These genes are, however, normally expressed in cell lines derived from other types of tumors, e.g., sarcomas, neuroblastomas, and gliomas. We speculate that ALEX genes may play a role in suppression of tumors originating from epithelial tissue, i.e., carcinomas.
DOI:10.1111/j.1471-4159.2004.02411.xURLPMID:15086532 [本文引用: 7]

During the past few years several new interacting partners for G protein-coupled receptors (GPCRs) have been discovered, suggesting that the activity of these receptors is more complex than previously anticipated. Recently, candidate G protein-coupled receptor associated sorting protein (GASP-1) has been identified as a novel interacting partner for the delta opioid receptor and has been proposed to determine the degradative fate of this receptor. We show here that GASP-1 associates in vitro with other opioid receptors and that the interaction domain in these receptors is restricted to a small portion of the carboxyl-terminal tail, corresponding to helix 8 in the three-dimensional structure of rhodopsin. In addition, we show that GASP-1 interacts with COOH-terminus of several other GPCRs from subfamilies A and B and that two conserved residues within the putative helix 8 of these receptors are critical for the interaction with GASP-1. In situ hybridization and northern blot analysis indicate that GASP-1 mRNA is mainly distributed throughout the central nervous system, consistent with a potential interaction with numerous GPCRs in vivo. Finally, we show that GASP-1 is a member of a novel family comprising at least 10 members, whose genes are clustered on chromosome X. Another member of the family, GASP-2, also interacts with the carboxyl-terminal tail of several GPCRs. Therefore, GASP proteins may represent an important protein family regulating GPCR physiology.
DOI:10.1016/j.bbrc.2005.11.036URLPMID:16298334 [本文引用: 1]

We previously identified Per1-interacting protein of the suprachiasmatic nucleus (PIPS) in rats. To reveal its role, its tissue distribution was examined by immunoblotting. PIPS-like immunoreactive substance (PIPSLS) was observed in the brain, adrenal gland, and PC12 cells. Since PIPS, which has no nuclear localization signal (NLS), is translocated into nuclei of COS-7 cells in the presence of mPer1, the effect of NGF on nuclear localization of PIPS was examined using PC12 cells. NGF caused nuclear translocation of either PIPSLS or GFP-PIPS. NGF mediated nuclear translocation of PIPSLS was blocked by K252a, a TrkA-inhibitor, or wortmannin, a PI3K-inhibitor. Gab1, which is implicated in TrkA signaling and has NLS, co-immunoprecipitated with PIPSLS from PC12 cells using an anti-PIPS antibody. Inhibition of PIPS expression by RNAi increased levels of apoptosis in PC12 cells. These findings suggest that nuclear translocation of PIPS is involved in NGF mediated neuronal survival via TrkA, PI3K, and Gab1 signaling pathway.
DOI:10.1111/j.1365-2443.2008.01175.xURLPMID:18363962 [本文引用: 2]

Microtubules (MTs) play crucial roles in a variety of cell functions, such as mitosis, vesicle transport and cell motility. MTs also compose specialized structures, such as centrosomes, spindles and cilia. However, molecular mechanisms of these MT-based functions and structures are not fully understood. Here, we analyzed MT co-sedimented proteins from rat brain by tandem mass spectrometry (MS) upon ion exchange column chromatography. We identified a total of 391 proteins. These proteins were grouped into 12 categories: 57 MT cytoskeletal proteins, including MT-associated proteins (MAPs) and motor proteins; 66 other cytoskeletal proteins; 4 centrosomal proteins; 10 chaperons; 5 Golgi proteins; 7 mitochondrial proteins; 62 nucleic acid-binding proteins; 14 nuclear proteins; 13 ribosomal proteins; 28 vesicle transport proteins; 83 proteins with diverse function and/or localization; and 42 uncharacterized proteins. Of these uncharacterized proteins, six proteins were expressed in cultured cells, resulting in the identification of three novel components of centrosomes and cilia. Our present method is not specific for MAPs, but is useful for identifying low abundant novel MAPs and components of MT-based structures. Our analysis provides an extensive list of potential candidates for future study of the molecular mechanisms of MT-based functions and structures.
DOI:10.1016/j.molcel.2004.09.016URLPMID:15383276 [本文引用: 2]

Analysis of protein-protein interactions (PPIs) is a valuable approach for characterizing proteins of unknown function. Here, we have developed a strategy combining library and matrix yeast two-hybrid screens to generate a highly connected PPI network for Huntington's disease (HD). The network contains 186 PPIs among 35 bait and 51 prey proteins. It revealed 165 new potential interactions, 32 of which were confirmed by independent binding experiments. The network also permitted the functional annotation of 16 uncharacterized proteins and facilitated the discovery of GIT1, a G protein-coupled receptor kinase-interacting protein, which enhances huntingtin aggregation by recruitment of the protein into membranous vesicles. Coimmunoprecipitations and immunofluorescence studies revealed that GIT1 and huntingtin associate in mammalian cells under physiological conditions. Moreover, GIT1 localizes to neuronal inclusions, and is selectively cleaved in HD brains, indicating that its distribution and function is altered during disease pathogenesis.
URLPMID:17635908 [本文引用: 3]
URLPMID:26973417 [本文引用: 1]
DOI:10.1111/j.1600-0854.2010.1045.xURLPMID:20102549 [本文引用: 3]

Human cytomegalovirus (HCMV) encodes the seven transmembrane(7TM)/G-protein coupled receptor (GPCR) US28, which signals and endocytoses in a constitutive, ligand-independent manner. Here we show that, following endocytosis, US28 is targeted to the lysosomes for degradation as a consequence of its interaction with the GPCR-associated sorting protein-1 (GASP-1). We find that GASP-1 binds to US28 in vitro and that disruption of the GASP-1/US28 interaction by either (i) overexpression of dominant negative cGASP-1 or by (ii) shRNA knock-down of endogenous GASP-1 is sufficient to inhibit the lysosomal targeting of US28 and slow its post-endocytic degradation. Furthermore, we found that GASP-1 affects US28-mediated signalling. The knock-down of endogenous GASP-1 impairs the US28-mediated Galphaq/PLC/inositol phosphate (IP) accumulation as well as the activation of the transcription factors Nuclear Factor-kappaB (NF-kappaB) and cyclic AMP responsive element binding protein (CREB). Overexpression of GASP-1 enhances both IP accumulation and transcription factor activity. Thus, GASP-1 is an important cellular determinant that not only regulates the post-endocytic trafficking of US28, but also regulates the signalling capacities of US28.
URLPMID:12839973 [本文引用: 3]

Hepatocellular carcinoma (HCC) is one of the most common malignant tumors with poor prognosis. By representational difference analysis (RDA), a novel human gene designated SVH, up-regulated in the clinical HCC sample, was identified. The deduced SVH protein consisted of 343 amino acids with a transmembrane domain and an armadillo repeat. Northern blot revealed that SVH was expressed in most human adult tissues. Four variants of SVH, SVH-A, -B, -C, and -D, resulting from alternative splicing in the coding region of the SVH transcript, were observed and were all localized in endoplasmic reticulum (ER). Up-regulation of SVH-B, but not the other variants, was evident in about 60% (28 of 46) of HCC samples, detected by quantitative real-time PCR. Human liver cell line QSG-7701, transfected with SVH-B, acquired an accelerated growth rate and tumorigenicity in nude mice, whereas inhibition of SVH-B in hepatoma cell line BEL-7404, using antisense oligodeoxynucleotides, induced apoptosis. It is suggested that the splicing variants of SVH have distinct biological functions, and SVH-B may play an important role in hepatocarcinogenesis.
DOI:10.1016/s0006-8993(01)02857-8URLPMID:11597585 [本文引用: 2]

Mammalian Per proteins are thought to be important in the mechanism of circadian rhythm. We identified a novel protein PIPS (Per1 interacting protein of the suprachiasmatic nucleus) with the yeast two-hybrid system using PAS domain of rat Per1 (rPer1) as a bait. PIPS is about a 180-kDa protein and expressed mainly in the brain, especially in the hypothalamus including the suprachiasmatic nuclei (SCN). PIPS interacts with mouse Per1 (mPer1) in vitro and in cultured cells transfected with both molecules. Furthermore, it was found that mPer1 translocated PIPS into the nuclei in the cultured cells. Thus, these findings suggest a possibility that PIPS is involved in the feedback loop or output mechanism of circadian rhythm through interacting with Per1 in the SCN.
DOI:10.1016/j.bbrc.2005.11.036URLPMID:16298334 [本文引用: 2]

We previously identified Per1-interacting protein of the suprachiasmatic nucleus (PIPS) in rats. To reveal its role, its tissue distribution was examined by immunoblotting. PIPS-like immunoreactive substance (PIPSLS) was observed in the brain, adrenal gland, and PC12 cells. Since PIPS, which has no nuclear localization signal (NLS), is translocated into nuclei of COS-7 cells in the presence of mPer1, the effect of NGF on nuclear localization of PIPS was examined using PC12 cells. NGF caused nuclear translocation of either PIPSLS or GFP-PIPS. NGF mediated nuclear translocation of PIPSLS was blocked by K252a, a TrkA-inhibitor, or wortmannin, a PI3K-inhibitor. Gab1, which is implicated in TrkA signaling and has NLS, co-immunoprecipitated with PIPSLS from PC12 cells using an anti-PIPS antibody. Inhibition of PIPS expression by RNAi increased levels of apoptosis in PC12 cells. These findings suggest that nuclear translocation of PIPS is involved in NGF mediated neuronal survival via TrkA, PI3K, and Gab1 signaling pathway.
[本文引用: 2]
DOI:10.1371/journal.pone.0149477URLPMID:26901434 [本文引用: 3]

The seven-transmembrane receptor Smoothened (Smo) activates all Hedgehog (Hh) signaling by translocation into the primary cilia (PC), but how this is regulated is not well understood. Here we show that Pitchfork (Pifo) and the G protein-coupled receptor associated sorting protein 2 (Gprasp2) are essential components of an Hh induced ciliary targeting complex able to regulate Smo translocation to the PC. Depletion of Pifo or Gprasp2 leads to failure of Smo translocation to the PC and lack of Hh target gene activation. Together, our results identify a novel protein complex that is regulated by Hh signaling and required for Smo ciliary trafficking and Hh pathway activation.
DOI:10.1038/s41467-019-09382-9URLPMID:30926797 [本文引用: 4]

Autism spectrum disorder (ASD) is characterized by dysfunction in social interactions, stereotypical behaviours and high co-morbidity with intellectual disability. A variety of syndromic and non-syndromic neurodevelopmental disorders have been connected to alterations in metabotropic glutamate receptor (mGluR) signalling. These receptors contribute to synaptic plasticity, spine maturation and circuit development. Here, we investigate the physiological role of Gprasp2, a gene linked to neurodevelopmental disabilities and involved in the postendocytic sorting of G-protein-coupled receptors. We show that Gprasp2 deletion leads to ASD-like behaviour in mice and alterations in synaptic communication. Manipulating the levels of Gprasp2 bidirectionally modulates the surface availability of mGluR5 and produces alterations in dendritic complexity, spine density and synaptic maturation. Loss of Gprasp2 leads to enhanced hippocampal long-term depression, consistent with facilitated mGluR-dependent activation. These findings demonstrate a role for Gprasp2 in glutamatergic synapses and suggest a possible mechanism by which this gene is linked to neurodevelopmental diseases.
URLPMID:15034937 [本文引用: 3]
DOI:10.1074/jbc.M901177200URLPMID:19304657 [本文引用: 2]

Sox10 is a member of the group E Sox transcription factor family and plays key roles in neural crest development and subsequent cellular differentiation. Sox10 binds to regulatory sequences in target genes via its conserved high mobility group domain. In most cases, Sox10 exerts its transcriptional effects in concert with other DNA-binding factors, adaptor proteins, and nuclear import proteins. These interactions can lead to synergistic gene activation and can be cell type-specific. In earlier work, we demonstrated that Sox10 transactivates the nicotinic acetylcholine receptor alpha3 and beta4 subunit genes and does so only in neuronal-like cell lines, raising the possibility that Sox10 mediates its effects via interactions with co-regulatory factors. Here we describe the identification of the armadillo repeat-containing protein, ARMCX3, as a Sox10-interacting protein. Biochemical analyses indicate that ARMCX3 is an integral membrane protein of the mitochondrial outer membrane. Others have shown that Sox10 is a nucleocytoplasmic shuttling protein. We extend this observation and demonstrate that, in the cytoplasm, Sox10 is peripherally associated with the mitochondrial outer membrane. Both Sox10 and ARMCX3 are expressed in mouse brain and spinal cord as well as several cell lines. Overexpression of ARMCX3 increased the amount of mitochondrially associated Sox10. In addition, although ARMCX3 does not possess intrinsic transcriptional activity, it does enhance transactivation of the nicotinic acetylcholine receptor alpha3 and beta4 subunit gene promoters by Sox10. These results suggest that Sox10 is a membrane-associated factor whose transcriptional function is increased by direct interactions with ARMCX3 and raise the possibility of a signal transduction cascade between the nucleus and mitochondria through Sox10/ARMCX3 interactions.
DOI:10.1083/jcb.200110132URLPMID:11839771 [本文引用: 1]

Nonvisual arrestins (arr) modulate G protein-coupled receptor (GPCR) desensitization and internalization and bind to both clathrin (CL) and AP-2 components of the endocytic coated pit (CP). This raises the possibility that endocytosis of some GPCRs may be a consequence of arr-induced de novo CP formation. To directly test this hypothesis, we examined the behavior of green fluorescent protein (GFP)-arr3 in live cells expressing beta2-adrenergic receptors and fluorescent CL. After agonist stimulation, the diffuse GFP-arr3 signal rapidly became punctate and colocalized virtually completely with preexisting CP spots, demonstrating that activated complexes accumulate in previously formed CPs rather than nucleating new CP formation. After arr3 recruitment, CP appeared larger: electron microscopy analysis revealed an increase in both CP number and in the occurrence of clustered CPs. Mutant arr3 proteins with impaired binding to CL or AP-2 displayed reduced recruitment to CPs, but were still capable of inducing CP clustering. In contrast, though constitutively present in CPs, the COOH-terminal moiety of arr3, which contains CP binding sites but lacks receptor binding, did not induce CP clustering. Together, these results indicate that recruitment of functional arr3-GPCR complexes to CP is necessary to induce clustering. Latrunculin B or 16 degrees C blocked CP rearrangements without affecting arr3 recruitment to CP. These results and earlier studies suggest that discrete CP zones exist on cell surfaces, each capable of supporting adjacent CPs, and that the cortical actin membrane skeleton is intimately involved with both the maintenance of existing CPs and the generation of new structures.
DOI:10.1074/jbc.C100527200URLPMID:11641392 [本文引用: 1]

Ligand-induced trafficking plays an important role in the physiologic regulation of many G protein-coupled receptors (GPCRs). Although numerous GPCRs are sorted to a degradative pathway upon prolonged stimulation, the molecular events leading to degradation are poorly understood. Here we report that the human immunodeficiency virus co-receptor CXCR4 undergoes rapid agonist-promoted degradation by a process involving endocytosis via clathrin-coated pits and subsequent sorting to lysosomes. Studies analyzing the sorting of various CXCR4 mutants revealed the presence of a degradation motif (SSLKILSKGK) in the carboxyl terminus of CXCR4. The first two serines as well as the dileucine motif were critical for agonist-induced endocytosis, whereas all three serines but not the dileucine were important in mediating degradation. Mutation of the three lysine residues had no effect on CXCR4 endocytosis yet completely inhibited receptor degradation. Because lysine residues represent potential sites of ubiquitination, we also examined the ubiquitination of CXCR4. Interestingly, CXCR4 was shown to undergo rapid agonist-promoted ubiquitination that was attenuated by mutation of the lysine residues within the degradation motif. These studies implicate a specific role for ubiquitination in sorting endocytosed GPCRs to lysosomes.
DOI:10.1126/science.1127085URLPMID:17218518 [本文引用: 1]

Ubiquitination is a reversible posttranslational modification of cellular proteins, in which a 76-amino acid polypeptide, ubiquitin, is primarily attached to the epsilon-amino group of lysines in target proteins. Ubiquitination is a major player in regulating a broad host of cellular processes, including cell division, differentiation, signal transduction, protein trafficking, and quality control. Aberrations in the ubiquitination system are implicated in pathogenesis of some diseases, certain malignancies, neurodegenerative disorders, and pathologies of the inflammatory immune response. Here, we discuss the proteasome-independent roles of ubiquitination in signaling and endocytosis.
DOI:10.1016/s0165-6147(00)01620-5URLPMID:11166853 [本文引用: 1]

Many G-protein-coupled receptors (GPCRs) undergo agonist-induced endocytosis. Such endocytosis has been implicated in diverse processes of receptor regulation, including reversible sequestration of receptors in endosomes and proteolytic downregulation of receptors in lysosomes. The precise relationships between membrane pathways that mediate receptor sequestration and downregulation remain controversial. Recent studies suggest that GPCRs can be segregated within distinct microdomains of the plasma membrane before endocytosis occurs, and others suggest that certain GPCRs are sorted between divergent membrane pathways after endocytosis by clathrin-coated pits. Furthermore, emerging data implicate a specific role of the actin cytoskeleton and receptor phosphorylation in controlling endocytic sorting of a particular GPCR. In this article, recent research into endocytosis of GPCRs will be discussed together with some important and unresolved questions regarding the diversity and specificity of mechanisms that mediate downregulation of GPCRs.
DOI:10.1074/jbc.M605398200URLPMID:17138565 [本文引用: 1]

Plasma membrane recycling of G protein-coupled receptors can occur by at least two distinct mechanisms as follows: a
DOI:10.1111/bph.12025URLPMID:23082996 [本文引用: 1]

BACKGROUND AND PURPOSE: GPCRs undergo both homologous and heterologous regulatory processes in which receptor phosphorylation plays a critical role. The protein kinases responsible for each pathway are well established; however, other molecular details that characterize each pathway remain unclear. In this study, the molecular mechanisms that determine the differences in the functional roles and intracellular trafficking between homologous and PKC-mediated heterologous internalization pathways for the dopamine D(2) receptor were investigated. EXPERIMENTAL APPROACH: All of the S/T residues located within the intracellular loops of D(2) receptor were mutated, and the residues responsible for GRK- and PKC-mediated internalization were determined in HEK-293 cells and SH-SY5Y cells. The functional role of receptor internalization and the cellular components that determine the post-endocytic fate of internalized D(2) receptors were investigated in the transfected cells. KEY RESULTS: T134, T225/S228/S229 and S325 were involved in PKC-mediated D(2) receptor desensitization. S229 and adjacent S/T residues mediated the PKC-dependent internalization of D(2) receptors, which induced down-regulation and desensitization. S/T residues within the second intracellular loop and T225 were the major residues involved in GRK-mediated internalization of D(2) receptors, which induced receptor resensitization. ARF6 mediated the recycling of D(2) receptors internalized in response to agonist stimulation. In contrast, GASP-1 mediated the down-regulation of D(2) receptors internalized in a PKC-dependent manner. CONCLUSIONS AND IMPLICATIONS: GRK- and PKC-mediated internalizations of D(2) receptors occur through different intracellular trafficking pathways and mediate distinct functional roles. Distinct S/T residues within D(2) receptors and different sorting proteins are involved in the dissimilar regulation of D(2) receptors by GRK2 and PKC.
DOI:10.1074/jbc.M110.158345URLPMID:21030592 [本文引用: 1]

The D(3) dopamine receptor is endocytosed through a heterologous mechanism mediated by phorbol esters. Here, we show that following this endocytosis the D(3) dopamine receptors fail to recycle and are instead targeted for degradation through an interaction with the G protein-coupled receptor (GPCR)-associated sorting protein-1 (GASP-1). Furthermore, we identified a specific binding motif in the C terminus common to the D(3) and D(2) that confers GASP-1 binding. shRNA knockdown of GASP-1 delayed post-endocytic degradation of both the D(2) and D(3) dopamine receptors. In addition, mutation of the D(2) and D(3) receptor C termini to resemble the D(4), which does not interact with GASP-1, not only inhibited GASP-1 binding but slowed degradation after endocytosis. Conversely, mutation of the C terminus of the D(4) to resemble that of the D(2) and D(3) facilitated GASP-1 binding and promoted post-endocytic degradation of the mutant D(4) receptor. Thus, we have identified a motif that is both necessary and sufficient to promote GASP-1 binding and receptor degradation. In addition, these data demonstrated that GASP-1 can mediate post-endocytic degradation of dopamine receptors that have been endocytosed not only as a consequence of dopamine activation but also as a consequence of activation by phorbol esters.
DOI:10.1038/372237a0URLPMID:7969468 [本文引用: 1]

Metabotropic glutamate receptor 1 (mGluR1) is a member of a large family of G-protein-coupled glutamate receptors, the physiological functions of which are largely unknown. Mice deficient in mGluR1 have severe motor coordination and spatial learning deficits. They have no gross anatomical or basic electrophysiological abnormalities in either the cerebellum or hippocampus, but they show impaired cerebellar long-term depression and hippocampal mossy fibre long-term potentiation. mGluR1-deficient mice should therefore be valuable models for studying synaptic plasticity.
DOI:10.1038/nn.2309URLPMID:19377472 [本文引用: 1]

In contrast with conventional NMDA receptor-dependent synaptic plasticity, the synaptic events controlling the plasticity of GluR2-lacking Ca(2+)-permeable AMPA receptors (CP-AMPARs) remain unclear. At parallel fiber synapses onto cerebellar stellate cells, Ca(2+) influx through AMPARs triggers a switch in AMPAR subunit composition, resulting in loss of Ca(2+) permeabilty. Paradoxically, synaptically induced depolarization will suppress this Ca(2+) entry by promoting polyamine block of CP-AMPARs. We therefore examined other mechanisms that may control this receptor regulation under physiological conditions. We found that activation of both mGluRs and CP-AMPARs is necessary and sufficient to drive an AMPAR subunit switch and that by enhancing mGluR activity, GABA(B)R activation promotes this plasticity. Furthermore, we found that mGluRs and GABA(B)Rs are tonically activated, thus setting the basal tone for EPSC amplitude and rectification. Regulation by both excitatory and inhibitory inputs provides an unexpected mechanism that determines the potential of these synapses to show dynamic changes in AMPAR Ca(2+) permeability.
DOI:10.1126/science.288.5469.1254URL [本文引用: 1]
DOI:10.1038/nn746URLPMID:11687813 [本文引用: 1]

Activation of group 1 metabotropic glutamate receptors (mGluRs) stimulates dendritic protein synthesis and long-term synaptic depression (LTD), but it remains unclear how these effects are related. Here we provide evidence that a consequence of mGluR activation in the hippocampus is the rapid loss of both AMPA and NMDA receptors from synapses. Like mGluR-LTD, the stable expression of this change requires protein synthesis. These data suggest that expression of mGluR-LTD is at least partly postsynaptic, and that a functional consequence of dendritic protein synthesis is the regulation of glutamate receptor trafficking.
DOI:10.1073/pnas.1214705110URLPMID:23269840 [本文引用: 1]

Refinement of neural circuits in the mammalian cerebral cortex shapes brain function during development and in the adult. However, the signaling mechanisms underlying the synapse-specific shrinkage and loss of spiny synapses when neural circuits are remodeled remain poorly defined. Here, we show that low-frequency glutamatergic activity at individual dendritic spines leads to synapse-specific synaptic weakening and spine shrinkage on CA1 neurons in the hippocampus. We found that shrinkage of individual spines in response to low-frequency glutamate uncaging is saturable, reversible, and requires NMDA receptor activation. Notably, shrinkage of large spines additionally requires signaling through metabotropic glutamate receptors (mGluRs) and inositol 1,4,5-trisphosphate receptors (IP(3)Rs), supported by higher levels of mGluR signaling activity in large spines. Our results support a model in which signaling through both NMDA receptors and mGluRs is required to drive activity-dependent synaptic weakening and spine shrinkage at large, mature dendritic spines when neural circuits undergo experience-dependent modification.
DOI:10.1006/mcne.2001.0993URLPMID:11414786 [本文引用: 1]

Alternative splicing in the mGluR5 gene generates two different receptor isoforms, of which expression is developmentally regulated. However, little is known about the functional significance of mGluR5 splice variants. We have examined the functional coupling, subcellular targeting, and effect on neuronal differentiation of epitope-tagged mGluR5 isoforms by expression in neuroblastoma NG108-15 cells. We found that both mGluR5 splice variants give rise to comparable [Ca2+]i transients and have similar pharmacological profile. Tagged receptors were shown by immunofluorescence to be inserted in the plasma membrane. In undifferentiated cells the subcellular localization of the two mGluR5 isoforms was partially segregated, whereas in differentiated cells the labeling largely redistributed to the newly formed neurites. Interestingly, we demonstrate that mGluR5 splice variants dramatically influence the formation and maturation of neurites; mGluR5a hinders the acquisition of mature neuronal traits and mGluR5b fosters the elaboration and extension of neurites. These effects are partly inhibited by MPEP.
DOI:10.1186/s12964-018-0220-7URLPMID:29558958 [本文引用: 1]

Hedgehog (Hh) signaling pathway plays an essential role during vertebrate embryonic development and tumorigenesis. It is already known that Sonic hedgehog (Shh) pathway is important for the evolution of radio and chemo-resistance of several types of tumors. Most of the brain tumors are resistant to chemotherapeutic drugs, consequently, they have a poor prognosis. So, a better knowledge of the Shh pathway opens an opportunity for targeted therapies against brain tumors considering a multi-factorial molecular overview. Therefore, emerging studies are being conducted in order to find new inhibitors for Shh signaling pathway, which could be safely used in clinical trials. Shh can signal through a canonical and non-canonical way, and it also has important points of interaction with other pathways during brain tumorigenesis. So, a better knowledge of Shh signaling pathway opens an avenue of possibilities for the treatment of not only for brain tumors but also for other types of cancers. In this review, we will also highlight some clinical trials that use the Shh pathway as a target for treating brain cancer.
DOI:10.1038/nrg2774URLPMID:20395968 [本文引用: 1]

The primary cilium has recently stepped into the spotlight, as a flood of data show that this organelle has crucial roles in vertebrate development and human genetic diseases. Cilia are required for the response to developmental signals, and evidence is accumulating that the primary cilium is specialized for hedgehog signal transduction. The formation of cilia, in turn, is regulated by other signalling pathways, possibly including the planar cell polarity pathway. The cilium therefore represents a nexus for signalling pathways during development. The connections between cilia and developmental signalling have begun to clarify the basis of human diseases associated with ciliary dysfunction.
DOI:10.1016/j.bbrc.2016.10.093URLPMID:27793670 [本文引用: 1]

Hedgehog (Hh) signaling plays key roles in animal development and tissue homeostasis. Binding of the secreted ligand to its Ptch1 receptor triggers Hh signaling through distinct canonical or noncanonical signaling pathways. Canonical Hh signaling leads to the activation of Gli transcription factors to induce Hh target-gene expression. In contrast, noncanonical Hh signaling regulates cytoskeleton rearrangement and apoptosis. Recently, it has been shown that primary cilia are important for canonical Hh signaling, but the ciliary role for signaling through the noncanonical pathway remains unresolved. Here, we examine the role of primary cilia in noncanonical Hh signaling in cultured mammalian cells. We found that Hh pathway activation in mouse embryonic fibroblast cells (MEFs) increases microtubule acetylation via smoothened (Smo), and suppression of Hh signaling by a Smo antagonist abrogates the microtubule acetylation. Using genetically engineered MEFs, we revealed that the increase in microtubule acetylation by Hh is dependent on Smo, but not on Sufu or Gli. In Kif3a(-/-) MEFs, which cannot form primary cilia, we observed that primary cilia were required for transducing noncanonical Hh signaling. Furthermore, we revealed that an increase in intracellular calcium is important for Hh-dependent tubulin acetylation at the downstream of Smo. Collectively, these findings suggest that Smo and primary cilia-dependent noncanonical Hh signaling leads to post-translational regulation of microtubules and may be important for modulating cell behaviors.
DOI:10.1111/j.1476-5381.2011.01562.xURLPMID:21718301 [本文引用: 1]

BACKGROUND AND PURPOSE: Many GPCRs, including the CB(1) cannabinoid receptor, are down-regulated following prolonged agonist exposure by interacting with the GPCR-associated sorting protein-1 (GASP-1). The CB(1) receptor antagonist rimonabant has also recently been described to be an agonist at GPR55, a cannabinoid-related receptor. Here we investigated the post-endocytic properties of GPR55 after agonist exposure and tested whether GASP-1 is involved in this process. EXPERIMENTAL APPROACH: We evaluated the direct protein-protein interaction of GPR55 with GASP-1 using (i) GST-binding assays and (ii) co-immunoprecipitation assays in GPR55-HEK293 cells with endogenous GASP-1 expression. We further tested the internalization, recycling and degradation of GPR55 using confocal fluorescence microscopy and biotinylation assays in the presence and absence of GASP-1 (lentiviral small hairpin RNA knockdown of GASP-1) under prolonged agonist [rimonabant (RIM), lysophosphatidylinositol (LPI)] stimulation. KEY RESULTS: We showed that the prolonged activation of GPR55 with rimonabant or LPI down-regulates GPR55 via GASP-1. GASP-1 binds to GPR55 in vitro, and this interaction was required for targeting GPR55 for degradation. Disrupting the GPR55-GASP-1 interaction prevented post-endocytic receptor degradation, and thereby allowed receptor recycling. CONCLUSION AND IMPLICATIONS: These data implicate GASP-1 as an important regulator of ligand-mediated down-regulation of GPR55. By identifying GASP-1 as a key regulator of the trafficking and, by extension, functional expression of GPR55, we may be one step closer to gaining a better understanding of this receptor in response to cannabinoid drugs. LINKED ARTICLES: This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7.
DOI:10.1038/s41598-019-54654-5URLPMID:31797953 [本文引用: 1]

Circadian rhythms in Per1, PER2 expression and intracellular Ca(2+) were measured from a solitary SCN neuron or glial cell which was physically isolated from other cells. Dispersed cells were cultured on a platform of microisland (100-200 mum in diameter) in a culture dish. Significant circadian rhythms were detected in 57.1% for Per1 and 70.0% for PER2 expression. When two neurons were located on the same island, the circadian rhythms showed desynchronization, indicating a lack of oscillatory coupling. Circadian rhythms were also detected in intracellular Ca(2+) of solitary SCN neurons. The ratio of circadian positive neurons was significantly larger without co-habitant of glial cells (84.4%) than with it (25.0%). A relatively large fraction of SCN neurons generates the intrinsic circadian oscillation without neural or humoral networks. In addition, glial cells seem to interrupt the expression of the circadian rhythmicity of intracellular Ca(2+) under these conditions.
DOI:10.1016/j.febslet.2007.09.025URLPMID:17904127 [本文引用: 2]

We previously reported that inhibition of SVH-B, a specific splicing variant of SVH, results in apoptotic cell death. In this study, we reveal that this apoptosis may be dependent on the presence of p53. Co-immunoprecipitation and GST pull-down assays have demonstrated that SVH-B directly interacts with p53. In both BEL-7404 cells and p53-null Saos-2 cells transfected with a temperature-sensitive mutant of p53, V143A, ectopically expressed SVH-B suppresses the transcriptional activity of p53, and suppression of SVH by RNA interference increases the transcriptional activity of p53. Our results suggested the function of SVH-B in accelerating growth and inhibition of apoptosis is related to its inhibitory binding to p53.
DOI:10.1016/j.yexmp.2011.06.015URLPMID:21791203 [本文引用: 1]

An innovative "2-D high performance liquid electrophoresis" (2-D HPLE) technology was used to identify serum biomarkers associated with the early stage of breast cancer in addition to other more advanced stages. 2-D HPLE is a newly developed electrophoretic technology that separates 100s of serum albumin complexes on a polyvinyl membrane based on their surface charges. Association of cancer proteins or their fragments (biomarkers) with pre-existing albumin complexes in the blood of cancer patients results in altered mobility on the membrane. Using 2-D HPLE we identified that a specific fragment of G-protein coupled receptor-associated sorting protein 1 (GASP-1) was present in the sera of patients with early stage disease but absent in sera of normal patients. GASP-1 has been shown to modulate lysosomal sorting and functional down-regulation of a variety of G-protein coupled receptors in neuronal cells. However, no reports have linked GASP-1 to breast cancer pathogenesis. GASP-1 was detected in tumor extracts of 7 cases of Stage 2 and Stage 3 breast cancers, but not in adjacent normal tissue as revealed by western blot analysis using an antibody developed against a GASP-1 peptide identified by our 2-D HPLE technology. Using this antibody, we immunohistochemically detected over-expression of GASP-1 in all of 107 cases of archived ductal breast carcinoma tumor samples, while normal adjacent breast tissue from 12 cases of ductal carcinoma showed little or no staining. Additionally, all 10 cases of metastatic breast carcinoma present in lymph nodes were positive. Strong positive GASP-1 staining was observed in all tumor tissue including ductal carcinoma in situ (DCIS) and invasive ductal carcinoma. Additionally, we observed a wide spectrum of enhanced staining of premalignant ductal epithelial cells present in benign ducts and those found in atypical ductal hyperplasia (ADH). These studies identify GASP-1 as a potential new serum and tumor biomarker for breast cancer and suggest that GASP-1 may be a novel target for the development of breast cancer therapeutics. (C) 2011 Elsevier Inc.
DOI:10.1158/0008-5472.CAN-06-0157URLPMID:16778180 [本文引用: 1]

Aberrant methylation of promoter CpG islands (CGI) is involved in silencing of tumor suppressor genes and is also a potential cancer biomarker. Here, to identify CGIs aberrantly methylated in human melanomas, we did a genome-wide search using methylation-sensitive representational difference analysis. CGIs in putative promoter regions of 34 genes (ABHD9, BARHL1, CLIC5, CNNM1, COL2A1, CPT1C, DDIT4L, DERL3, DHRS3, DPYS, EFEMP2, FAM62C, FAM78A, FLJ33790, GBX2, GPR10, GPRASP1, HOXA9, HOXD11, HOXD12, HOXD13, p14ARF, PAX6, PRDX2, PTPRG, RASD1, RAX, REC8L1, SLC27A3, TGFB2, TLX2, TMEM22, TMEM30B, and UNC5C) were found to be methylated in at least 1 of 13 melanoma cell lines but not in two cultured normal melanocytes. Among these genes, Peroxiredoxin 2 (PRDX2) was expressed in normal melanocytes, and its expression was lost in melanomas with methylation. The loss of expression was restored by treatment of melanomas with a demethylating agent 5-aza-2'-deoxycytidine. In surgical melanoma specimens, methylation of PRDX2 was detected in 3 of 36 (8%). Furthermore, immunohistochemical analysis of PRDX2 showed that disappearance of immunoreactivity tends to associate with its methylation. PRDX2 was recently reported to be a negative regulator of platelet-derived growth factor signaling, and its silencing was suggested to be involved in melanomas. On the other hand, 12 CGIs were methylated in >or=9 of the 13 melanoma cell lines and are considered as candidate melanoma biomarkers.
DOI:10.1158/0008-5472.CAN-07-2687URLPMID:18089774 [本文引用: 1]

CpG island promoter hypermethylation of tumor suppressor genes is a common hallmark of human cancer, and new large-scale epigenomic technologies might be useful in our attempts to define the complete DNA hypermethylome of tumor cells. Here, we report a functional search for hypermethylated CpG islands using the colorectal cancer cell line HCT-116, in which two major DNA methyltransferases, DNMT1 and DNMT3b, have been genetically disrupted (DKO cells). Using methylated DNA immunoprecipitation methodology in conjunction with promoter microarray analyses, we found that DKO cells experience a significant loss of hypermethylated CpG islands. Further characterization of these candidate sequences shows CpG island promoter hypermethylation and silencing of genes with potentially important roles in tumorigenesis, such as the Ras guanine nucleotide-releasing factor (RASGRF2), the apoptosis-associated basic helix-loop transcription factor (BHLHB9), and the homeobox gene (HOXD1). Hypermethylation of these genes occurs in premalignant lesions and accumulates during tumorigenesis. Thus, our results show the usefulness of DNMT genetic disruption strategies combined with methylated DNA immunoprecipitation in searching for unknown hypermethylated candidate genes in human cancer that might aid our understanding of the biology of the disease and be of potential translational use.
DOI:10.1038/onc.2008.251URLPMID:18679425 [本文引用: 1]

Propensity for subsequent distant metastasis in head and neck squamous-cell carcinoma (HNSCC) was analysed using 186 primary tumours from patients initially treated by surgery that developed (M) or did not develop (NM) metastases as the first recurrent event. Transcriptome (Affymetrix HGU133_Plus2, QRT-PCR) and array-comparative genomic hybridization data were collected. Non-supervised hierarchical clustering based on Affymetrix data distinguished tumours differing in pathological differentiation, and identified associated functional changes. Propensity for metastasis was not associated with these subgroups. Using QRT-PCR data we identified a four-gene model (PSMD10, HSD17B12, FLOT2 and KRT17) that predicts M/NM status with 77% success in a separate 79-sample validation group of HNSCC samples. This prediction is independent of clinical criteria (age, lymph node status, stage, differentiation and localization). The most significantly altered transcripts in M versus NM were significantly associated to metastasis-related functions, including adhesion, mobility and cell survival. Several genomic modifications were significantly associated with M/NM status (most notably gains at 4q11-22 and Xq12-28; losses at 11q14-24 and 17q11 losses) and partly linked to transcription modifications. This work yields a basis for the development of prognostic molecular signatures, markers and therapeutic targets for HNSCC metastasis.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.ejmg.2016.03.004URLPMID:26995686 [本文引用: 1]

Here we report the first male case of a novel Xq22.1 deletion. An 8-week-old boy with multiple congenital abnormalities and respiratory failure was referred to the Mayo Clinic Cytogenetics laboratory for testing. Chromosomal microarray analysis identified a novel 1.1 Mb deletion at Xq22.1. A similar deletion has only been described once in the literature in a female patient and her mother; both have intellectual disability and dysmorphic facial features. In addition, the mother had a son who died at 15 days due to breathing failure. Recently, a mouse model revealed that a 0.35 Mb sub-region, containing 4 genes, is sufficient to cause majority of the Xq22.1 deletion phenotypes. The deleted intervals in our male patient and the female patients contain 15 common genes, including the four described in the 0.35 Mb sub-region. Male mice with deletion of the 0.35 Mb sub-region died perinatally from respiratory failure due to pulmonary hypoplasia, consistent with the breathing problem and potential neonatal fatality in male patients. The phenotypes of the mouse models and the patients are strikingly similar; therefore, the deletion of these five genes (ARMCX5, ARMCX5-GPRASP2, GPRASP1, GPRASP2, and BHLHB9) is likely responsible for the novel Xq22.1 deletion syndrome.
DOI:10.1007/s10048-007-0116-yURLPMID:18196299 [本文引用: 1]

We have previously established a first whole genome transcriptomic profile of sporadic Parkinson's disease (PD). After extensive brain tissue-based validation combined with cycles of iterative data analysis and by focusing on the most comparable cases of the cohort, we have refined our analysis and established a list of 892 highly dysregulated priority genes that are considered to form the core of the diseased Parkinsonian metabolic network. The substantia nigra pathways, now under scrutiny, contain more than 100 genes whose association with PD is known from the literature. Of those, more than 40 genes belong to the highly significantly dysregulated group identified in our dataset. Apart from the complete list of 892 priority genes, we present pathways revealing PD 'hub' as well as 'peripheral' network genes. The latter include Lewy body components or interact with known PD genes. Biological associations of PD with cancer, diabetes and inflammation are discussed and interactions of the priority genes with several drugs are provided. Our study illustrates the value of rigorous clinico-pathological correlation when analysing high-throughput data to make optimal use of the histopathological phenome, or morphonome which currently serves as the key diagnostic reference for most human diseases. The need for systematic human tissue banking, following the highest possible professional and ethical standard to enable sustainability, becomes evident.
DOI:10.1007/s12031-012-9911-5URLPMID:23180093 [本文引用: 1]

The very large G protein coupled receptor (Vlgr1) is a member of adhesion receptors or large N-terminal family B-7 transmembrane helixes (LNB7TM) receptors within the seven trans-membrane receptor superfamily. Vlgr1 is the largest GPCR identified to date; its mRNA spans 19 kb and encodes 6,300 amino acids. Vlgr1 is a core component of ankle-link complex in inner ear hair cells. Knock-out and mutation mouse models show that loss of Vlgr1 function leads to abnormal stereociliary development and hearing loss, indicating crucial roles of Vlgr1 in hearing transduction or auditory system development. Over the past 10 or so years, human genetics data suggested that Vlgr1 mutations cause Usher syndromes and seizures. Although significant progresses have been made, the details of Vlgr1's function in hair cells, its signaling cascade, and the mechanisms underlying causative effects of Vlgr1 mutations in human diseases remain elusive and ask for further investigation.
DOI:10.1086/381685URLPMID:14740321 [本文引用: 1]

Usher syndrome type II (USH2) is a genetically heterogeneous autosomal recessive disorder with at least three genetic subtypes (USH2A, USH2B, and USH2C) and is classified phenotypically as congenital hearing loss and progressive retinitis pigmentosa. The VLGR1 (MASS1) gene in the 5q14.3-q21.1 USH2C locus was considered a likely candidate on the basis of its protein motif structure and expressed-sequence-tag representation from both cochlear and retinal subtracted libraries. Denaturing high-performance liquid chromatography and direct sequencing of polymerase-chain-reaction products amplified from 10 genetically independent patients with USH2C and 156 other patients with USH2 identified four isoform-specific VLGR1 mutations (Q2301X, I2906FS, M2931FS, and T6244X) from three families with USH2C, as well as two sporadic cases. All patients with VLGR1 mutations are female, a significant deviation from random expectations. The ligand(s) for the VLGR1 protein is unknown, but on the basis of its potential extracellular and intracellular protein-protein interaction domains and its wide mRNA expression profile, it is probable that VLGR1 serves diverse cellular and signaling processes. VLGR1 mutations have been previously identified in both humans and mice and are associated with a reflex-seizure phenotype in both species. The identification of additional VLGR1 mutations to test whether a phenotype/genotype correlation exists, akin to that shown for other Usher syndrome disease genes, is warranted.
DOI:10.1136/jmg.2008.059626URLPMID:19357117 [本文引用: 1]

Mutations in the large GPR98 gene underlie Usher syndrome type 2C (USH2C), and all patients described to date have been female. It was speculated that GPR98 mutations cause a more severe, and eventually lethal, phenotype in males. We describe for the first time two male patients with USH2 with novel GPR98 mutations. Clinical characterization of a male patient and his affected sister revealed a typical USH2 phenotype in both. GPR98 may have been excluded from systematic investigation in previous studies, and the proportion of patients with USH2C probably underestimated. GPR98 should be considered in patients with USH2 of both sexes.
DOI:10.1523/JNEUROSCI.0342-07.2007URLPMID:17567809 [本文引用: 1]

Several lines of evidence indicate that very large G-protein-coupled receptor 1 (Vlgr1) makes up the ankle links that connect the stereocilia of hair cells at their base. Here, we show that the transmembrane protein usherin, the putative transmembrane protein vezatin, and the PDZ (postsynaptic density-95/Discs large/zona occludens-1) domain-containing submembrane protein whirlin are colocalized with Vlgr1 at the stereocilia base in developing cochlear hair cells and are absent in Vlgr1-/- mice that lack the ankle links. Direct in vitro interactions between these four proteins further support their involvement in a molecular complex associated with the ankle links and scaffolded by whirlin. In addition, the delocalization of these proteins in myosin VIIa defective mutant mice as well as the myosin VIIa tail direct interactions with vezatin, whirlin, and, we show, Vlgr1 and usherin, suggest that myosin VIIa conveys proteins of the ankle-link complex to the stereocilia. Adenylyl cyclase 6, which was found at the base of stereocilia, was both overexpressed and mislocated in Vlgr1-/- mice. In postnatal day 7 Vlgr1-/- mice, mechanoelectrical transduction currents evoked by displacements of the hair bundle toward the tallest stereocilia (i.e., in the excitatory direction) were reduced in outer but not inner hair cells. In both cell types, stimulation of the hair bundle in the opposite direction paradoxically resulted in significant transduction currents. The absence of ankle-link-mediated cohesive forces within hair bundles lacking Vlgr1 may account for the electrophysiological results. However, because some long cadherin-23 isoforms could no longer be detected in Vlgr1-/- mice shortly after birth, the loss of some apical links could be involved too. The premature disappearance of these cadherin isoforms in the Vlgr1-/- mutant argues in favor of a signaling function of the ankle links in hair bundle differentiation.
DOI:10.3389/fnmol.2017.00426URLPMID:29311816 [本文引用: 1]

Hair cell (HC) loss is the major cause of permanent sensorineural hearing loss in mammals. Unlike lower vertebrates, mammalian cochlear HCs cannot regenerate spontaneously after damage, although the vestibular system does maintain limited HC regeneration capacity. Thus HC regeneration from the damaged sensory epithelium has been one of the main areas of research in the field of hearing restoration. Hedgehog signaling plays important roles during the embryonic development of the inner ear, and it is involved in progenitor cell proliferation and differentiation as well as the cell fate decision. In this study, we show that recombinant Sonic Hedgehog (Shh) protein effectively promotes sphere formation, proliferation, and differentiation of Lgr5+ progenitor cells isolated from the neonatal mouse cochlea. To further explore this, we determined the effect of Hedgehog signaling on cell proliferation and HC regeneration in cultured cochlear explant from transgenic R26-SmoM2 mice that constitutively activate Hedgehog signaling in the supporting cells of the cochlea. Without neomycin treatment, up-regulation of Hedgehog signaling did not significantly promote cell proliferation or new HC formation. However, after injury to the sensory epithelium by neomycin treatment, the over-activation of Hedgehog signaling led to significant supporting cell proliferation and HC regeneration in the cochlear epithelium explants. RNA sequencing and real-time PCR were used to compare the transcripts of the cochleae from control mice and R26-SmoM2 mice, and multiple genes involved in the proliferation and differentiation processes were identified. This study has important implications for the treatment of sensorineural hearing loss by manipulating the Hedgehog signaling pathway.
[本文引用: 1]
DOI:10.3892/mmr.2017.7357URLPMID:28849214 [本文引用: 1]

G proteincoupled receptorassociated sorting protein 2 (GPRASP2), a member of the GASP family, has been reported to be involved in the modulation of transcription. However, few studies have revealed the role of GPRASP2 in the development and progression of diseases. As a model organism, zebrafish have been widely used to investigate human diseases. In the present study, zebrafish armadillo repeatcontaining 10 (armc10), an orthologous gene of human GPRASP2 was identified, and the spatial and temporal expression patterns of armc10 in zebrafish during early embryonic development were revealed. Bioinformatics analyses showed that ARMC10 protein sequences were highly conserved. Reverse transcription polymerase chain reaction analysis and whole mount in situ hybridization revealed that zebrafish armc10 was maternally expressed and was detected at a weak level up to 12 h postfertilization (hpf), however, its expression increased to a high level at 24 hpf. At the 75% epiboly stage and 12 hpf, armc10 was widely expressed in the embryo. At 24 hpf, armc10 mRNA was expressed in the nervous system of the zebrafish head. When the embryo was 2 days old, the wide expression of armc10 was maintained in the nervous system of the zebrafish head. At 72 hpf, the mRNA expression of armc10 was located specifically in the otic vesicles in addition to the nervous system of the head. At 96 hpf, the expression of armc10 was maintained in the otic vesicles and the nervous system of the head. The results of the present study provided novel insight into the spatial and temporal mRNA expression of armc10 in zebrafish, for the further investigation of nervous system diseases.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
