 ,, 阳小胡, 艾亮霞, 潘燕平, 胡勇
,, 阳小胡, 艾亮霞, 潘燕平, 胡勇 ,中国科学院深圳先进技术研究院,合成生物研究所,深圳 518055
,中国科学院深圳先进技术研究院,合成生物研究所,深圳 518055Bioinformatics analysis of differentially expressed genes on alopecia
Hong Xiang ,, Xiaohu Yang, Liangxia Ai, Yanping Pan, Yong Hu
,, Xiaohu Yang, Liangxia Ai, Yanping Pan, Yong Hu ,Institute for Synthetic Biology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China
,Institute for Synthetic Biology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China通讯作者: 胡勇,博士,副教授,研究方向:基因治疗。E-mail:yong.hu@siat.ac.cn
第一联系人:
编委: 赵方庆
收稿日期:2019-10-2修回日期:2019-12-13网络出版日期:2019-12-20
| 基金资助: |
Received:2019-10-2Revised:2019-12-13Online:2019-12-20
| Fund supported: |

摘要
利用生物信息学方法分析脱发相关差异表达基因,有望帮助了解脱发发生发展的分子机制。本研究从NCBI的子数据库GEO中选择基因表达谱GSE45512和GSE45513数据集,利用R语言limma工具包,筛选出两个物种斑秃样本与正常样本的共同显著差异表达基因。对这部分基因进行功能注释和蛋白互作网络分析,同时对全部差异表达基因进行基因集富集分析。结果发现,人头皮斑秃样本共筛选出225个差异表达基因;C3H/HeJ小鼠自发斑秃皮肤样本共筛选出337个差异表达基因;两个物种的共同显著差异表达基因有23个。GO功能富集分析和蛋白互作网络分析显示,这部分差异基因显著富集于免疫相关功能,并且彼此间存在蛋白互作关系。基因集富集分析显示两个物种的差异基因都能显著富集到趋化因子信号通路、细胞因子受体相互作用、金葡菌感染及抗原加工与呈递通路;而且人的下调差异基因不仅映射到了人类表型数据库的脱发表型,也映射到皮肤附属物病理相关表型。综上所述,本研究通过生物信息方法分析脱发皮肤组织与正常皮肤组织的差异表达基因,最终筛选出23个在人和小鼠中共同存在的显著差异表达基因;此外,分析发现脱发与免疫过程及皮肤附属物病变密切相关,这些结果为脱发的诊断和治疗提供了新思路。
关键词:
Abstract
The molecular mechanism of alopecia areata (AA) is still elusive and here we utilized bioinformatics methods to analyze AA-related differentially expressed genes. In this study, GSE45512 and GSE45513 were downloaded from the NCBI sub-database Gene Expression Omnibus (GEO). The gene expressions of AA and normal samples were analyzed using the R package limma, which showed significant differences between AA and normal samples in two species. These genes were subject to functional annotation and protein interaction networks. At the same time, gene set enrichment analysis was conducted for all differentially expressed genes. The study revealed that a total of 225 differentially expressed genes were screened from human AA samples, and a total of 337 differentially expressed genes were screened from spontaneous AA skin samples in C3H/HeJ mice. There are 23 differentially expressed genes in the two species. GO and protein interaction network analysis shown gene enrichment in immune-related functions, and these proteins interact with each other. Gene set enrichment analysis showed that differential genes from both species were significantly enriched to chemokine signaling pathways, cytokine-cytokine receptor interactions, staphylococcus aureus infection, and antigen processing and presentation. Moreover, the human down-regulated differential gene not only maps to the alopecia in human phenotype ontology, but also maps to the pathologically relevant phenotype of the skin appendage. In brief, 23 significant differentially expressed genes were screened out coexisting in AA human and mouse by bioinformatics methods. In addition, the result demonstrated that AA is closely related to the immune process and skin appendage lesions. These results provide new ideas for the diagnosis and treatment of AA.
Keywords:
PDF (1695KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
向虹, 阳小胡, 艾亮霞, 潘燕平, 胡勇. 脱发相关差异表达基因的生物信息学分析. 遗传[J], 2020, 42(2): 172-182 doi:10.16288/j.yczz.19-214
Hong Xiang.
斑秃(alopecia areata, AA)是一种常见的脱发症状,AA患者一般表现为斑秃部位无疤痕、斑片状脱发,并且头皮附近的毛干变窄。AA作为一种自身免疫性疾病,具有高度变异的表型和基因型,是由遗传和环境因素共同决定的复杂遗传病,终生患病率约为2%[1]。尽管对AA患者和小鼠模型的遗传学研究表明AA是一种复杂的多基因疾病,然而毛囊免疫豁免功能的破坏也被认为是AA形成的重要诱因[2]。AA传统上被分为斑片状斑秃(alopecia patchy)、全秃(alopecia totalis, AT)和普秃(alopecia universalis, AU)[2]。AA症状最常发生在头皮,但也可能发生在眉毛和睫毛(AT患者)或体毛(AU患者)。基于疾病的持续时间和斑状秃发的程度,AA还可被分为隐秃(alopecia incognita)、蛇形斑秃(ophiasis)、马蹄形斑秃(sisaipho)和玛丽·安托瓦内特综合征(Marie Antoinette syndrome)[2]。
AA也是一种对生活质量有重大影响的疾病。AA患者表现出较差的健康相关生活质量(Health- related quality of life, HRQoL)评分,头皮受累程度越大,HRQoL评分越低[3]。由于AA的可见性、缺乏治疗和慢性病的性质,AA及其衍生症状可能会给患者造成严重的心理负担,更会导致包括高度焦虑和抑郁在内的心理疾病,尤其是青少年患者更易引发社交障碍[4,5]。此外,有调查分析显示,年轻的男性患者具有更高的心理压力和自杀风险[6]。因此,有效的治疗方法将会对AA患者的生活质量和身心健康产生深远和积极的影响。脱发影响着全球数千万人,但目前缺乏一种安全的、合理的和有针对性的用于治疗头发再生的方法。现代常用的治疗方法包括局部使用米诺地尔(2%和5%),口服非那雄胺和植发手术等。由于存在药物的副作用、停药后复发、新毛囊存活率和患者个体差异等问题,对AA的治疗富有挑战性,并且治疗效果并不令人满意。
生物信息学是生命科学领域前沿交叉学科,可为脱发或斑秃的形成揭示潜在的分子机制,从而帮助阐明和理解AA独特的病理生理学。使用生物信息学技术对高通量数据进行联合分析不仅为科学研究提供可行的思路和方案,也为该类疾病的治疗提供更有针对性和合理选择的疗法。本文通过生物信息学相关方法对Xing等[7]构建的人类AA头皮表达谱芯片和C3H/HeJ小鼠自发AA皮肤表达谱芯片数据进行生物信息学分析,以探讨影响AA在人和小鼠中保守的差异表达基因以及由脱发引起的一系列生理病理过程的改变,为AA分子机制的进一步研究提供生物信息学依据。
1 材料与方法
1.1 数据来源
在美国国家生物技术信息中心(National Center for Biotechnology Information, NCBI)的基因表达综合数据库(Gene Expression Omnibus, GEO)下载GEO数据集,包括GSE45512和GSE45513[7]。其中,GSE45512是基于GPL570 Affymetrix Human Genome U133 Plus 2.0 Array的人AA皮肤表达谱,含有5例AA患者人头皮样本和5例健康人头皮样本,而GSE45513是基于GPL1261 Affymetrix Mouse Genome 430 2.0 Array的C3H/HeJ小鼠自发性AA皮肤表达谱,含有3例自发小鼠自发AA皮肤样本和3例正常小鼠皮肤样本。
1.2 基因芯片的处理与差异表达基因的筛选
芯片的处理使用R语言“affy”包对GSE45512和GSE45513数据中的每个样本探针表达值进行背景校正归一化处理。通过“limma”包对实验组AA皮肤和对照组正常皮肤中每个表达值进行t检验。GSE45512差异表达基因使用Bioconductor的“hgu133plus2.db”进行注释,GSE45513差异表达基因使用Bioconductor的“mouse4302.db”进行注释。差异表达基因(differently expressed genes, DEGs)的筛选标准为|log2FC|≥1 & P value≤0.05。利用venn图获得两个数据集中共同出现的差异表达基因名称。利用“pheatmap”工具包对差异表达基因绘制热图。1.3 DEGs的注释和富集分析
采用Gene Ontology Resource数据库[8],对前面筛选出的两物种中共有的DEGs进行GO (Gene Ontology)富集分析和KEGG (Kyoto Encyclopedia of Gene and Genome)信号通路分析。显著性富集的筛选标准为P<0.01和FDR<0.05。分析的结果利用R语言“REVIGO”进行可视化呈现。对两物种中单独存在的差异表达基因使用DAVID数据库[9]进行GO注释分析、KEGG通路富集分析和组织表达分析。1.4 蛋白互作网络的构建
将前面筛选的DEGs导入STRING (version 11.0)数据库,得到一个蛋白质相互作用网络(protein- protein interaction, PPI)图。利用软件参数将免疫相关功能蛋白标注出。1.5 基因富集分析
基因集富集分析(gene set enrichment analysis, GSEA)使用WEB-based GEne SeT AnaLysis Toolkit数据库。对两个物种校正后的基因集进行富集分析,Redundancy reduction参数设定为Weighted set cover。2 结果与分析
2.1 获得DEGs
对GSE45512和GSE45513两个数据集进行分析,分别获得差异倍数在2倍以上的显著上、下调差异表达基因。GSE45512人头皮AA样本共筛选出225个DEGs (图1A),其中上调基因79个,下调基因146个(图2A)。GSE45513 C3H/HeJ小鼠自发AA皮肤样本共筛选出337个DEGs (图1B),其中上调基因280个,下调基因57个(图2A)。两个物种共有的DEGs有23个(图2A),并对这23个在人和小鼠中相对保守的DEGs进行聚类热图分析(图2,B和C)。可见两类样本基因表达具有显著差异,上调基因主要包括CXCL9、CXCL10、CCL2和IRF1等19个,下调基因有CHAC1、FGF18、LYPD6和BMP2 (图1和附表1)。图1
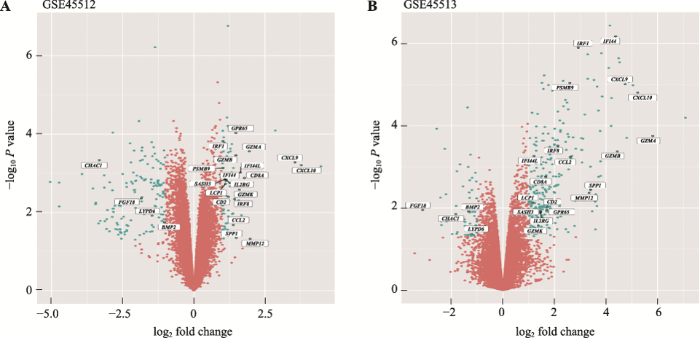 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1差异表达基因火山图
A:GSE45512人头皮AA样本差异表达基因火山图;B:GSE45513 C3H/HeJ小鼠自发AA皮肤样本差异表达基因火山图。火山图横轴表示差异倍数,数值显示为log2fold change。纵轴表示P值,数值显示为-log10P value。深青色的点代表显著差异表达基因,玫瑰红色的点代表非显著差异表达基因。人和小鼠中共有的差异表达基因在图中显示基因名称。
Fig. 1Volcano plots of DEGs
图2
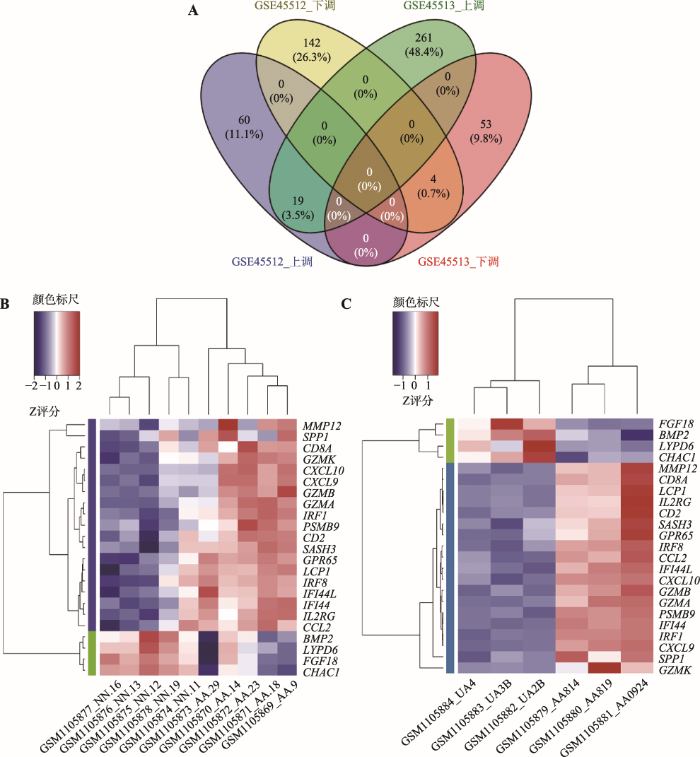 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2人和小鼠AA样本中共同差异表达的基因
A:人和小鼠AA样本差异表达基因的韦恩图;B:人AA样本中共同差异表达基因的聚类热图;C:小鼠AA样本中共同差异表达基因的聚类热图。
Fig. 2DEGs both in human and mice alopecia areata samples
2.2 DEGs的注释富集分析和蛋白互作分析
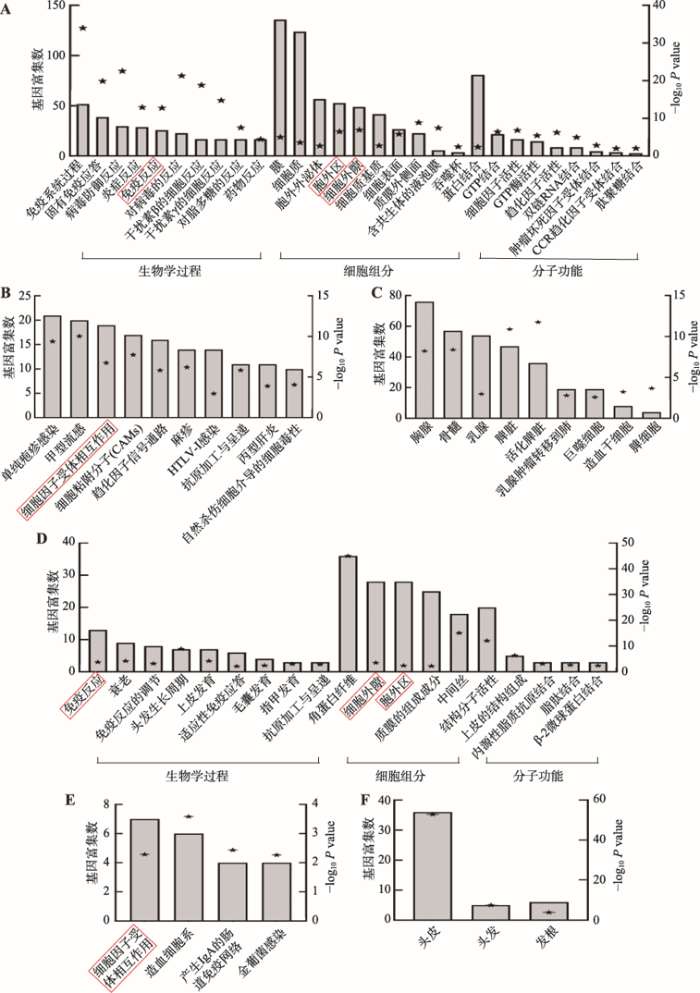
DEGs的GO富集分析可分为生物学过程(biological process)、细胞组分(cellular component)和分子功能(molecular function) 3个方面。对于两物种共有的DEGs,GO富集分析图显示,生物学过程主要富集在免疫相关的一些过程,包括免疫反应(immune response),免疫效应过程(immune effector process),防御反应(defense response),T细胞活化(T cell activation)和细胞因子介导的信号通路(cytokine-mediated signaling pathway)等(图3A)。这部分DEGs的细胞组分条目较少,主要涉及一些细胞表面的组分包括质膜外侧面(external side of plasma membrane)、膜侧(side of membrane)、细胞表面(cell surface)、免疫突触(immunological synapse)、细胞外隙(extracellular space)和BMP受体复合物(BMP receptor complex) (图3A)。而分子功能主要富集在细胞因子活性(cytokine activity)、受体结合(receptor binding)和内肽酶活性(endopeptidase activity)等过程(图3A)。对于两物种中单独存在的DEGs,GO富集分析显示,免疫相关的生物学过程仍然是两物种最主要的生物学过程,并且免疫反应(immune response)在人和小鼠中都出现了(附图1,A和D)。在细胞组分方面,小鼠的DEGs主要富集在膜(membrane)和细胞质(cytoplasm)范围较广的细胞组分,而人的DEGs主要富集在角蛋白纤维(keratin filament)专有的与皮肤附属物相关的组分。此外,胞外区(extracellular region)和细胞外隙(extracellular space)在人和小鼠中都出现了(附图1,A和D),这与前面提到的两物种共有DEGs富集在细胞表面的组分相关联。在分子功能方面,人的主要富集在结构分子功能相关的条目,小鼠的主要富集在结合功能相关的条目。在KEGG信号通路富集分析中,人和小鼠都出现了一个免疫相关的条目细胞因子受体相互作用(Cytokine-cytokine receptor interaction) (附图1,B和E)。然而,在组织富集方面,人的这些非共有DEGs主要富集在头皮、头发和发根,小鼠的主要富集在免疫器官中(胸腺和骨髓) (附图1,C和F)。
利用STRING数据库对在人和小鼠AA样本中共有的差异表达基因编码的蛋白分析发现,除了CHAC1、LYPD6和GPR65,其他20个蛋白之间都存在互作网络。结合之前的GO富集分析结果,发现处于该蛋白网络节点位置的蛋白都多与免疫相关(如:IRF1、CCL2、CXCL9、GZMA和GZMB等),如图3B所示标记出红色的蛋白表示参与免疫系统过程(GO:0002376, immune system process),标记出蓝色的蛋白表示参与免疫反应过程(GO:0006955, immune response)。说明AA症状在人和小鼠中都引起了一系列的免疫响应事件,如显著地增加了一些免疫相关基因和因子的表达。
图3
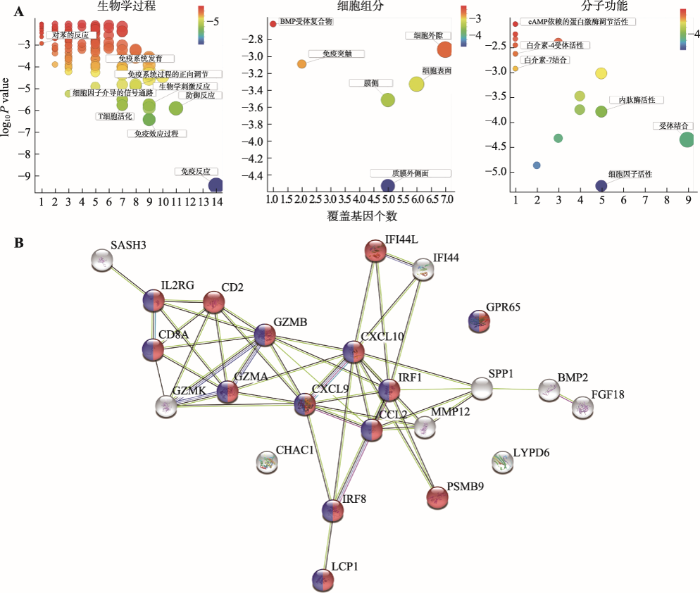 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3差异表达基因的GO富集分析和蛋白互作分析
A:差异表达基因的GO富集分析,包括生物学过程、细胞组分和分子功能。纵坐标代表P值,颜色变化从红色到蓝色代表P值从高到低;横坐标代表包含的基因数,圆圈越大代表包含的基因数越多。B:差异表达基因的蛋白互作分析。红色代表参与免疫系统过程(GO:0002376),蓝色代表参与免疫反应过程(GO:0006955)。
Fig. 3GO enrichment analysis and protein interaction analysis of DEGs
2.3 DEGs的GSEA分析
对人头皮AA样本(GSE45512)的DEGs基因集和C3H/HeJ小鼠自发AA皮肤样本(GSE45513)的DEGs基因集分别进行信号通路富集分析后,发现上调或者下调基因都富集在一些信号通路上。如同共同出现在人和小鼠中的23个DEGs一样,两个物种的DEGs基因集也共同地富集在一些信号通路上(图4),包括:趋化因子信号通路(chemokine signaling pathway)、细胞因子受体相互作用(cytokine-cytokine receptor interaction)、金葡菌感染(Staphylococcus aureus infection)和抗原加工与呈递(antigen processing and presentation)。比较巧合的是,除了金葡菌感染(Staphylococcus aureus infection)这个条目外,其他3个条目与前面提到的23个DEGs一样,是与免疫学过程存在直接相关的。这也进一步表明了斑秃在人和小鼠中会触发一系列的免疫学事件。图4
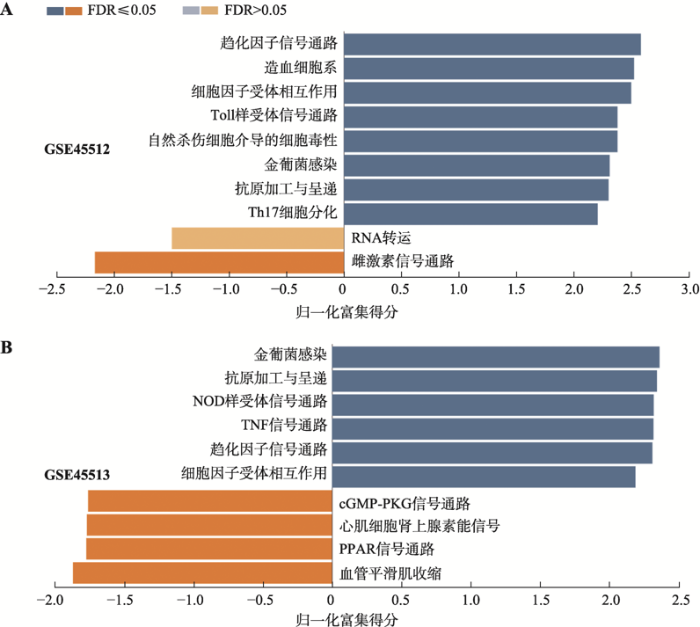 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4参与信号通路的基因集富集分析
A:人斑秃样本(GSE45512)的基因集富集结果;B:小鼠斑秃样本(GSE45513)的基因集富集结果。
Fig. 4Gene set enrichment analysis (GSEA) in signaling pathways
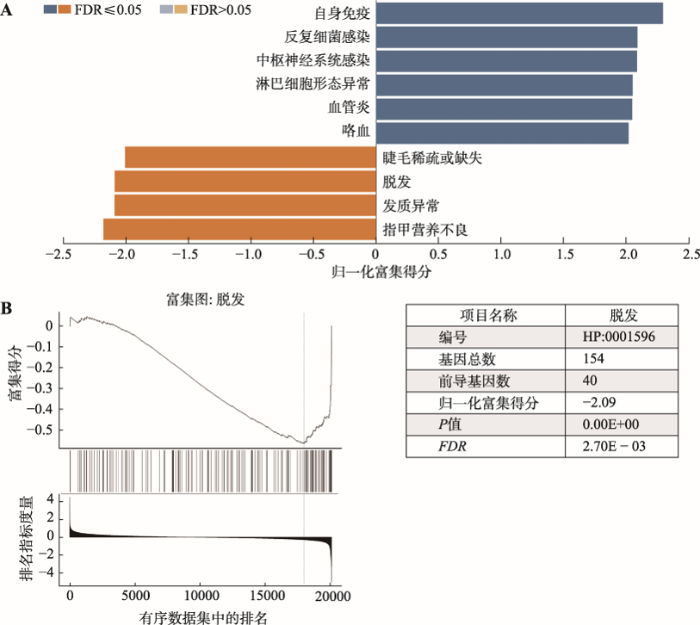
将人头皮AA样本(GSE45512)的基因集富集到人类表型数据库发现,上调基因集富集的条目有自身免疫(autoimmunity)、反复细菌感染(recurrent bacterial infections)、中枢神经系统感染(CNS infection)、淋巴细胞形态异常(abnormal lymphocyte morphology)、血管炎(vasculitis)和咯血(hemoptysis)。下调基因集富集的条目有指甲营养不良(nail dystrophy)、发质异常(abnormality of hair texture)、脱发(alopecia)和睫毛稀疏或缺失(sparse or absent eyelashes)。有意思的是,上调的基因集仍然多富集在免疫、细菌感染和一些炎症表型,而下调的基因集 都富集在脱发和皮肤附属物(指甲和睫毛)发育异常的表型上,这与本研究的样本表型相关,也与预期相符。
3 讨论
脱发影响着全球数千万人。几十年来,对于脱发的治疗渐渐从广泛和非特异性疗法发展到现在更有针对性和合理选择的疗法。这需要通过临床医生、基础科学家和生物信息学家等多个领域之间的密切合作和交流来实现。关于脱发的治疗方法多种多样, 包括植发手术[10]、低能量激光治疗[11]、光化学疗法(PUVA)[12]、针灸疗法[13]和药物治疗[14,15,16]等。然而,目前没有有效的疗法能医治所有受到脱发困扰的患者,且部分疗法除了具有较大的副作用外也会在停止治疗之后出现严重的复发情况,这主要与脱发独特的病理生理学和复杂的病因有关。因此,对于AA发生发展机制的研究特别重要,既可以帮助深入了解AA的发病机制,也有助于AA治疗药物的研发和治疗方法的开发。本研究通过生物信息学方法分析了人头皮AA样本和C3H/HeJ小鼠自发性AA皮肤样本,筛选出23个在两个物种AA样本与正常样本中共同显著差异表达基因。并且基于GO和STRING数据库对23个基因进行功能注释分析和蛋白互作网络分析。同时为了对所有数据集有一个全面的了解,进一步将校正后的差异表达基因集进行了基因集富集分析。除了人AA头皮样本数据,本研究也选用了C3H/HeJ小鼠自发性AA皮肤样本数据。C3H/HeJ小鼠自发脱发的发展过程和人的AA病理特征非常相似[17],因而能够作为一种良好、经济的模型用于研究脱发相关的疾病。本研究将两个物种的数据集联合分析,期望探究出在人和小鼠两个物种中相对保守的AA相关基因以及病理学和生理学过程。
人和小鼠中共有的DEGs有23个,比较有意思的是,其中部分基因也曾出现在AA相关的其他研究中。在一项关于AA的转录组测序研究中,FGF18也是差异表达基因,并参与细胞增殖的有丝分裂过程[18]。FGF18在毛囊休止期强表达,有研究表明,FGF18可以作为辐射诱导脱发的辐射保护剂[19]。DNA宏阵列分析显示BMP2与雄激素性脱发有关[20]。BMP信号参与调控头发的生长周期[21]。Anti-IL5能减少嗜酸性粒细胞的数量并导致脱发,加重红斑的严重程度,同时增加MMP12的表达[22]。利用免疫组化,在分级毛囊的球茎、小叶、峡部和漏斗部计数GZMB阳性细胞,发现AA小鼠中的阳性细胞显著高于对照组,而膳食维生素A可改变GZMB阳性细胞的数量和定位,从而影响AA的发展进程[23]。CXCL9,CXCL10作为T淋巴细胞的招募者,在AA患者血清中CXCL9和CXCL10表达水平明显升高,表明它们可能参与了T淋巴细胞募集到发炎组织的过程[24,25,26]。此外在C3H/HeJ小鼠自发形成AA过程中,CXCL9和CXCL10的表达水平逐渐增加[27]。另一项芯片和RT-PCR表达谱数据分析也显示CXCL9和CXCL10在AA组显著上调[28]。此外,通过阻断ifn诱导的趋化因子(CXCL9和CXCL10)可以预防AA的发生[29]。毛囊中CXCL10能增加T细胞的趋化性,而抑制T细胞的趋化性可有效治疗AA[30]。上述已有研究报道表明了这些在人和小鼠中共有的差异表达基因不仅在本文中显示出显著的差异,而且在脱发相关的其他样本中也显示出类似的差异,并且机制研究显示部分基因参与AA发生发展进程。同时,后续也应关注那些挖掘出来尚未报道的DEGs,对它们深入细致的研究可能有助于丰富AA的病理学知识,进而帮助治疗AA病症。
GO富集分析显示两物种共有的23个基因主要富集在免疫反应(immune response),细胞因子介导的信号通路(cytokine-mediated signaling pathway)和T细胞活化(T cell activation)等一些免疫相关的过程。比较有意思的是,单独存在于两个物种的非共有DEGs的生物学过程也都富集在免疫相关的生物学过程中。而且,对这些非共有DEGs进行GO富集分析和KEGG通路分析发现,在人和小鼠中,除了都涉及免疫事件外,小鼠所涉及的生物学过程、细胞组分、分子功能和信号通路的范围更广泛,而上 述与人相关的内容则涉及的更具体甚至更直接,如人的某些条目直接显示与皮肤附属物相关(附图1, 头发生长周期(GO:0042633~hair cycle),上皮发育(GO:0008544~ epidermis development),毛囊发育(GO:0001942~hair follicle development),指甲发育(GO:0035878~nail development),角蛋白纤维(GO:0045095~keratin filament),上皮的结构组成(GO:0030280~structural constituent of epidermis))。尤其是对这些非共有基因进行组织富集分析,发现这些在人中的非共有基因直接富集在头皮和头发相关的组织(头皮(Scalp),头发(Hair)和发根(Hair root))。这些结果可能表明,虽然在两个物种中,斑秃都与免疫事件相关,然而,在小鼠中斑秃可能是一类全身性的疾病,而在人中,由于毛发分布的原因,斑秃更倾向于是一个局部发生的疾病。当然,这些结果也反应了样品来源的不同,一个来自人类AA患者头皮,另一个来自小鼠自发性AA皮肤。PPI网络节点位置的一些差异表达基因编码的蛋白也显示与免疫事件相关。除此之外,GSEA分析结果显示在人和小鼠中相对保守的信号通路也都集中在免疫相关的过程。这些结果表明斑秃能够引起了一系列的免疫响应相关事件。本研究结果与之前的报道相一致,AA是一类人类自身免疫性疾病,AA的发生被认为是毛囊的天然免疫功能崩溃导致的,包括上皮细胞中MHC I class异位表达和自身抗原呈递给自身反应性CD8+ T细胞[31]。除了毛囊自身的免疫功能丧失,自身免疫会进一步破坏毛囊并引发一系列炎症反应[32]。近些年来,一类高效的免疫类药物JAK抑制剂(Janus kinase inhibitors)用于研究AA的治疗,具有比较好的疗效。在使用C3H/HeJ小鼠模型进行的临床前试验中,这些药物在局部和口服时均显示出预防和逆转AA的效果[7]。几个病例和个别报告已证明,不同类型斑秃患者在口服JAK抑制剂药物后,头发再生,这些药物包括托法替尼(tofacitinib)[33]、鲁索替尼(ruxolitinib)[34]和巴瑞克替尼(baricitinib)[35]。目前,美国FDA批准了几种JAK抑制剂用于治疗其他疾病,如ruxolitinib (JAK1/2抑制剂)用于治疗骨髓纤维化,tofacitinib (pan-JAK抑制剂)用于治疗类风湿性关节炎。JAK抑制剂等免疫类药物也提供了一种新的用于研究脱发治疗的策略,不过也应该意识到任何新药潜在副作用。
据报道,在2%~44%的AA患者中有指甲受累,并且这种情况在儿童(>40%)中比成人(<20%)更常见,此外,越是严重的斑秃患者也越容易出现在指甲受累[36,37]。指甲属于皮肤附属物,尽管GSEA(图5)显示上调基因集多富集在免疫相关的表型,然而下调基因集富集的条目都在皮肤附属物受损相关的表型,如指甲营养不良(nail dystrophy)、发质异常(abnormality of hair texture)、脱发(alopecia)和睫毛稀疏或缺失(sparse or absent eyelashes)。说明AA患者除了引起一些自身免疫相关的症状外,也会伴随出现自身皮肤附属物(如指甲、睫毛和眉毛等)生长异常的症状。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5涉及人类表型的基因集富集分析
A:人斑秃样本(GSE45512)基因集在人类表型上的富集结果;B:人斑秃样本基因集在秃头症中富集的结果。
Fig. 5Gene set enrichment analysis in human phenotype ontology
高通量分析技术的发展和测序成本的降低产生了大量与脱发相关的研究数据。本研究通过利用NCBI公共数据库中的数据和生物信息学分析工具整合分析了两个物种的AA数据,从基因层面分析了AA相关的分子机制,并获得了一些可信的结果,为脱发发生发展的机制和治疗提供有价值的参考信息。本文挖掘的23个在人和小鼠AA样本中共有的DEGs,其中部分已在AA相关的研究报道中得到证
实(MMP12[22],GZMB[23],CXCL9[29],CXCL10[29],IRF1[38],CD2[39],CCL2[40],IL2RG[41],FGF18[19,42]和BMP2[21,42]),然而,目前关于AA病症发生发展的机制以及AA与免疫成因的机理还有待完善。基于现有研究,对尚未报道这部分DEGs进行深入的分析和研究,有望对未来治疗斑秃提供了新的药物靶点。
附录
附图1和附表1详见文章电子版 www.chinagene.cn。附图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT附图1斑秃在人头皮样本和小鼠皮肤样本中的差异
利用DAVID功能注释工具分析人和小鼠中非共有差异表达基因。A:C3H/HeJ小鼠自发性AA皮肤非共有差异表达基因的GO注释分析,包括生物学过程(biological process),细胞组分(cellular component)和分子功能(molecular function)。B:C3H/HeJ小鼠自发性AA皮肤非共有差异表达基因所涉及的信号通路。C:C3H/HeJ小鼠自发性AA皮肤非共有差异表达基因的组织表达。D:人头皮非共有差异表达基因的GO注释分析。E:人头皮非共有差异表达基因所涉及的信号通路。F:人头皮非共有差异表达基因的组织表达。左侧Y轴代表所涉及的差异基因个数,用柱状图显示。右侧Y轴代表所涉及条目的显著性,用五角星显示,数据用-log10(P value)表示。A、B、D和E图的红色矩形框代表在人和鼠中共同出现的条目。
Fig. S1The differences of alopecia areata between human scalp samples and mouse skin samples
| 组别 | 组内基因数 | 组内基因名称 |
|---|---|---|
| GSE45512-up GSE45513-up | 19 | GPR65 IRF1 GZMA IFI44L SASH3 CXCL9 GZMB GZMK CXCL10 LCP1 CD8A SPP1 CD2 IRF8 CCL2 MMP12 IFI44 PSMB9 IL2RG |
| GSE45512-down GSE45513-down | 4 | FGF18 BMP2 CHAC1 LYPD6 |
| GSE45512-up | 60 | CRTAM GIMAP2 PRKAR2B CD1B PDGFRL C16orf54 PLAAT4 OGN TRIM22 CD33 LPAR4 FCGR2B ZFP14 ADAMDEC1 SELL EVI2A TNFSF13B IGDCC4 XIST MICB HLA-DRA EVI2B LRRK2 GPR171 MNDA HBB AHR TRAT1 GLYATL2 CA3 SLC19A3 TSLP CD1E CTSS RSAD2 ATP8B4 HLA-DPA1 CMA1 LYZ FDPSP2 FCER1G IL15 TRBC1 AGTR1 GALNT7 SRGN EPSTI1 PLP1 SLC7A10 STMN2 CCDC178 RGS18 IL2RB COL6A6 RNASE4 IL7 CSF2RB DACT1 CD1C CCL13 |
| GSE45512-down | 142 | DSG4 CAPN8 KRTAP4-12 LOC101928881 ZAR1 CST4 SLC1A6 S100P KRT82 UTY KRTAP9-3 PLA2G2F HOXC13 ADCK1 CSDC2 PADI3 KRTAP1-3 KRTAP4-5 CSNK1E TTTY15 KRT72 KRT73 SP6 LYG2 CALCB KRTAP9-8 KRTAP4-3 GPRC5D KRTAP3-1 DMBT1 KRTAP4-11 KRTAP2-1 MT4 KRTAP5-8 KRTAP4-6 KRTAP7-1 DDX3Y KRTAP19-1 HSPB3 FGG ERP27 PIRT KRTAP11-1 IL1F10 KRTAP3-2 EFHD1 KRTAP4-4 KRT16 CAPN14 PLEKHG4B ANGPTL7 DLX3 COMP BAMBI KRTAP4-9 KRTAP5-9 PINLYP TENM2 CAPN12 SLC10A4 CST1 CRNN KRTAP17-1 OXCT2 PRKY KRTAP3-3 SMTNL2 DLX4 MSX2 C1orf105 TTTY14 ODC1 ABCA4 LOC105374809 TXLNGY KRTAP4-1 FLRT3 KRTAP8-1 LSMEM1 RNF182 CABYR S100A3 EIF1AY PSORS1C2 SLN GLB1L2 FOXN1 LOC100505782 UNC5B-AS1 PCOTH KRTAP4-7 FRMPD1 USP9Y KRTAP1-5 KRT36 FAM49A KRT35 TGM3 LINC00868 KRT33A WNK4 KRT83 KRTAP4-2 KRTAP9-4 SLCO5A1 ATP8A2 SPINK7 SPTBN5 LRRC15 KDM5D KRT74 KLK12 KRTAP4-8 KRT84 LINGO1-AS2 SLC7A11 KRTAP9-9 KRT85 SERPINA3 KRT33B KRT31 RPS4Y1 ZFY PRSS53 BPY2 CYFIP2 ZNF503-AS1 CBLN2 KRT40 KRT81 TTTY10 KRTAP19-3 ERVW-1 KRTAP10-11 KRTAP1-1 KRT86 PARM1 FHOD3 KRT75 CREB5 KRT32 LAMP5 |
| GSE45513-up | 261 | XCL1 KIF26B C1S1 SOX7 CYBA EIF2AK2 IDO1 FLT1 MMP13 GM20559 COTL1 TRAV9D-3 AI467606 PML EXOC3L4 TMEM51 BATF2 IFITM3 SPN AI790276 OAS1B IGSF3 PARP12 GIMAP8 B4GALNT4 IRGM2 PTGER2 BST2 PTPN22 DUSP6 2010309G21RIK RAB19 SP100 HCST IRX4 A730054J21RIK SLFN2 9430051O21RIK ZMYND15 RAC2 SERPINB1A TRIM21 SAMHD1 OASL2 NLRC5 TAP2 LY75 PYY EFHD2 GBP7 TAPBPL AU015263 NPPB SERPINB9 APOBEC1 GM12185 AI662270 5830453J16RIK AI661384 A130071D04RIK TCAF2 UBA7 IIGP1 CLEC7A IFNG LAT IFIT1 TNFSF10 DTX3L GM9706 SELPLG TBC1D10C APOL11B AU020206 D17H6S56E-5 OAS2 TMEM173 CORO1A LIPG MGST2 CTSW NLRP10 CD52 MS4A4B FYB ICAM1 FCGR4 CD40 LGALS9 GBP6 LRRC4 SERPINB6B GM36723 GSTP3 ADGRG5 PMAIP1 ERAP1 C2 5930433N17RIK FCGBP 2610528A11RIK ALOX15 CIITA AKNA CCL1 CCL7 LPXN SOCS1 CXCL11 UBE2L6 AI504432 TAPBP BC023105 CD244A GIMAP3 RTP4 MS4A4C ADAR CCL17 SLC15A3 IFI27L2A PARP14 IL18BP ITGAX IRGM1 IFIT2 IGSF8 ITGB7 CD3G APOL7A NKG7 KLRC1 IL7R BCL2L15 SLFN4 HCK NMI IFI35 TFEC ALOX12E GBP8 TBX21 RRAD NDUFA4L2 APOL8 GM38448 PGLYRP2 GBP3 IL13RA2 VCAM1 STAT2 CCL5 ADAM8 ROBO2 MX1 LCK GLIPR1 ANG2 PGAM1 EGR2 4921509J17RIK MS4A6B TNFAIP2 NFKBIE LTB LY6E LGALS3BP PGLYRP1 IRF7 SAMD9L THY1 SLFN10-PS DDX60 H2-K1 DDX58 HELZ2 HERC6 CASP1 MPEG1 CCR5 RASSF10 SLAMF8 XAF1 MLKL MYO1G AA467197 STX11 CYP7B1 IFIH1 EGLN3 ACOD1 LY6A ICOS BCL3 HAS2 GBP2 TRIM30A CHIL1 GZMC CXCR6 CALHM6 IGTP USP18 H2-M3 KRT6B LCN2 LST1 GCH1 PLAC8 GPR68 H2-T24 ITGB8 CD69 LTA ITGB2 ITGAE CD3D PSMB8 KRT42 DHX58 CASP4 UBD IL18R1 CCL3 IRF9 RBP1 PARP9 CXCL16 KRT79 TIMP1 RBM43 PSMB10 KCNN4 TRIM25 ZBP1 AW112010 GM4951 AIF1 THEMIS2 CCL12 CD274 SERPINA3G STFA3 IFI47 CHIL3 GBP2B STAT1 SEMA4C CAR9 KLRD1 TLR3 MGARP JCHAIN SLC2A1 PSME1 CD8B1 ARHGAP8 HCLS1 IFIT3 CYTH4 PTPRC |
| GSE45513-down | 53 | ADH1 HOXD8 BB086117 PRG4 PLIN4 TTR NGEF HNMT SCGB1A1 DEFB1 FAM171B HAMP2 CLDN3 GDF10 UPB1 SEMA3E SLC38A3 AKAP12 MLF1 EGFL6 ABCA8A PDZRN4 SNCG IL31RA PCSK6 THSD1 TENT5B TMEM246 IL22RA2 BMP6 LRRC20 SERPINB2 TDRP KRT24 SULT1A1 WFDC3 RHPN2 NPR3 IL20RA ABLIM3 COL8A2 CRIP3 TIMP4 SERPINB6E ERRFI1 GREM1 LHX2 PCOLCE2 DEPP1 GPM6A TXNIP CYP4B1 ADCY1 |
新窗口打开|下载CSV
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.4065/70.7.628URLPMID:7791384 [本文引用: 1]

To assess the incidence and natural history of alopecia areata (AA) among unselected patients from a community.
DOI:10.1038/nrdp.2017.11URLPMID:28300084 [本文引用: 3]

Alopecia areata is an autoimmune disorder characterized by transient, non-scarring hair loss and preservation of the hair follicle. Hair loss can take many forms ranging from loss in well-defined patches to diffuse or total hair loss, which can affect all hair-bearing sites. Patchy alopecia areata affecting the scalp is the most common type. Alopecia areata affects nearly 2% of the general population at some point during their lifetime. Skin biopsies of affected skin show a lymphocytic infiltrate in and around the bulb or the lower part of the hair follicle in the anagen (hair growth) phase. A breakdown of immune privilege of the hair follicle is thought to be an important driver of alopecia areata. Genetic studies in patients and mouse models have shown that alopecia areata is a complex, polygenic disease. Several genetic susceptibility loci were identified to be associated with signalling pathways that are important to hair follicle cycling and development. Alopecia areata is usually diagnosed based on clinical manifestations, but dermoscopy and histopathology can be helpful. Alopecia areata is difficult to manage medically, but recent advances in understanding the molecular mechanisms have revealed new treatments and the possibility of remission in the near future.
[本文引用: 1]
DOI:10.1136/bmj.331.7522.951URLPMID:16239692 [本文引用: 1]
DOI:10.1016/j.jaad.2018.07.044URLPMID:30092332 [本文引用: 1]
DOI:10.1111/bjd.14497URLPMID:26914830 [本文引用: 1]

No systematic review has yet evaluated the available evidence on health-related quality of life (HRQOL) in alopecia areata (AA).
DOI:10.1038/nm.3645URLPMID:25129481 [本文引用: 3]

Alopecia areata (AA) is a common autoimmune disease resulting from damage of the hair follicle by T cells. The immune pathways required for autoreactive T cell activation in AA are not defined limiting clinical development of rational targeted therapies. Genome-wide association studies (GWAS) implicated ligands for the NKG2D receptor (product of the KLRK1 gene) in disease pathogenesis. Here, we show that cytotoxic CD8(+)NKG2D(+) T cells are both necessary and sufficient for the induction of AA in mouse models of disease. Global transcriptional profiling of mouse and human AA skin revealed gene expression signatures indicative of cytotoxic T cell infiltration, an interferon-γ (IFN-γ) response and upregulation of several γ-chain (γc) cytokines known to promote the activation and survival of IFN-γ-producing CD8(+)NKG2D(+) effector T cells. Therapeutically, antibody-mediated blockade of IFN-γ, interleukin-2 (IL-2) or interleukin-15 receptor β (IL-15Rβ) prevented disease development, reducing the accumulation of CD8(+)NKG2D(+) T cells in the skin and the dermal IFN response in a mouse model of AA. Systemically administered pharmacological inhibitors of Janus kinase (JAK) family protein tyrosine kinases, downstream effectors of the IFN-γ and γc cytokine receptors, eliminated the IFN signature and prevented the development of AA, while topical administration promoted hair regrowth and reversed established disease. Notably, three patients treated with oral ruxolitinib, an inhibitor of JAK1 and JAK2, achieved near-complete hair regrowth within 5 months of treatment, suggesting the potential clinical utility of JAK inhibition in human AA.
DOI:10.1093/nar/gky1055URLPMID:30395331 [本文引用: 1]

The Gene Ontology resource (GO; http://geneontology.org) provides structured, computable knowledge regarding the functions of genes and gene products. Founded in 1998, GO has become widely adopted in the life sciences, and its contents are under continual improvement, both in quantity and in quality. Here, we report the major developments of the GO resource during the past two years. Each monthly release of the GO resource is now packaged and given a unique identifier (DOI), enabling GO-based analyses on a specific release to be reproduced in the future. The molecular function ontology has been refactored to better represent the overall activities of gene products, with a focus on transcription regulator activities. Quality assurance efforts have been ramped up to address potentially out-of-date or inaccurate annotations. New evidence codes for high-throughput experiments now enable users to filter out annotations obtained from these sources. GO-CAM, a new framework for representing gene function that is more expressive than standard GO annotations, has been released, and users can now explore the growing repository of these models. We also provide the 'GO ribbon' widget for visualizing GO annotations to a gene; the widget can be easily embedded in any web page.
DOI:10.1093/nar/gkn923URLPMID:19033363 [本文引用: 1]

Functional analysis of large gene lists, derived in most cases from emerging high-throughput genomic, proteomic and bioinformatics scanning approaches, is still a challenging and daunting task. The gene-annotation enrichment analysis is a promising high-throughput strategy that increases the likelihood for investigators to identify biological processes most pertinent to their study. Approximately 68 bioinformatics enrichment tools that are currently available in the community are collected in this survey. Tools are uniquely categorized into three major classes, according to their underlying enrichment algorithms. The comprehensive collections, unique tool classifications and associated questions/issues will provide a more comprehensive and up-to-date view regarding the advantages, pitfalls and recent trends in a simpler tool-class level rather than by a tool-by-tool approach. Thus, the survey will help tool designers/developers and experienced end users understand the underlying algorithms and pertinent details of particular tool categories/tools, enabling them to make the best choices for their particular research interests.
DOI:10.1016/j.mehy.2019.05.018URLPMID:31203916 [本文引用: 1]

Higher risk of rapid progression in alopecia or male pattern baldness was observed in men who had family history. This could result from accumulation of DHT in hair follicles. Hair follicles on frontal region are more vulnerable to DHT. With development of minimal invasive hair transplantation surgery, hair follicles transplantation could be performed from frontal or occipital region to frontal region. However, limited hair follicles remained a problem. With development of technology of vitrification, we suggested extracting hair follicles from frontal region without affecting the appearance and preserving them with vitrification when the patient was young. When alopecia progressively developed, these extracted hair follicles would increase the donor number of hair follicles used for transplantation, which could extend longer dense hair appearance.
DOI:10.1002/lsm.22512URLPMID:27114071 [本文引用: 1]

Androgenetic alopecia (AGA) affects 50% of males by age 50 and 50% of females by age 80. Recently, the use of low-level laser therapy (LLLT) has been proposed as a treatment for hair loss and to stimulate hair regrowth in AGA. This paper aims to review the existing research studies to determine whether LLLT is an effective therapy for AGA based on objective measurements and patient satisfaction.
DOI:10.1016/j.jaad.2005.04.078URLPMID:16243155 [本文引用: 1]
DOI:10.1136/bmjopen-2015-008841URLPMID:26503391 [本文引用: 1]

Acupuncture is frequently used in dermatology for treating a number of skin disorders. There is no critically appraised evidence of the potential benefits and harm of acupuncture for alopecia areata (AA). This review aims to systematically evaluate the efficacy of acupuncture for the management of AA in randomised clinical trials (RCTs).
DOI:10.1111/jdv.15171URLPMID:29972712 [本文引用: 1]

The synergism of combined use between oral finasteride and topical minoxidil has been established in treating androgenetic alopecia among men. However, the concern regarding adverse effects of finasteride use has been rising.
DOI:10.1016/j.jaad.2015.01.008URLPMID:25653027 [本文引用: 1]

Some patients with chronic extensive alopecia areata (AA) may be refractory to topical immunotherapy. Combination therapy is recommended for such patients. Efficacy and safety of a combination therapy with diphenylcyclopropenone (DPCP) and anthralin in chronic extensive AA is unknown.
DOI:10.1016/j.jaad.2018.09.033URLPMID:30287324 [本文引用: 1]

Promising?results with platelet-rich plasma (PRP) in androgenetic alopecia that could be associated with platelet number and growth factor levels were described.
DOI:10.1038/jid.1995.38URLPMID:7738375 [本文引用: 1]
DOI:10.1016/j.ygeno.2010.05.002URLPMID:20546884 [本文引用: 1]

Alopecia areata (AA) is a non-scarring inflammatory hair loss disease with a complex autoimmune etiopathogenesis that is poorly understood. In order to investigate the pathogenesis of AA at the molecular level, we examined the gene expression profiles in skin samples from lesional (n=10) and non-lesional sites (n=10) of AA patients using Affymetrix Hu95A-v2 arrays. 363 genes were found to be differentially expressed in AA skin compared to non-lesional skin; 97 were up-regulated and 266 were down-regulated. Functional classification of the differentially expressed genes (DEGs) provides evidence for T-cell mediated immune response (CCL5, CXCL10, CD27, ICAM2, ICAM3, IL7R, and CX3CL1), and a possible humoral mechanism (IGHG3, IGHM, and CXCR5) in AA. We also find modulation in gene expression favoring cellular proliferation arrest at various levels (FGF5, FGF18, EREG, and FOXC2) with apoptotic dysregulation (LCK, TNF, TRAF2, and SFN) and decreased expression of hair follicle structural proteins. Further analysis of patients with AAT (&lt;1 year duration, n=4) and AAP (&gt;1 year duration, n=6) of disease revealed 262 DEGs distinctly separating the 2 groups, indicating the existence of gene profiles unique to the initial and later stages of disease.
DOI:10.1016/j.adro.2016.05.004URLPMID:28740887 [本文引用: 2]

Telogen (resting phase) hair follicles (HFs) are more radioresistant than their anagen (growth phase) counterparts. Fibroblast growth factor (FGF) 18 is strongly expressed in telogen HFs to maintain the telogen phase, whereas several other FGFs exert radioprotective effects; however, the role of FGF18 in the radioresistance of HFs remains unknown. This study focused on clarifying the role of FGF18 in the radioresistance of telogen HFs and its potential as a radioprotector.
DOI:10.1016/j.jdermsci.2004.05.001URLPMID:15488702 [本文引用: 1]

Androgenic alopecia (AGA) is the most common type of baldness in men. Although etiological studies have proved that androgen is one of the causes of this symptom, the defined molecular mechanism underlying androgen-related actions remains largely unknown.
DOI:10.1387/ijdb.072564mpURLPMID:19378257 [本文引用: 2]

The control of hair growth in the adult mammalian coat is a fascinating topic which has just begun to be explored with molecular genetic tools. Complex hair cycle domains and regenerative hair waves are present in normal adult (&gt; 2 month) mice, but more apparent in mutants with cyclic alopecia phenotypes. Each hair cycle domain consists of initiation site(s), a propagating wave and boundaries. By analyzing the dynamics of hair growth, time required for regeneration after plucking, in situ hybridization and reporter activity, we showed that there is oscillation of intra-follicular Wnt signaling which is synchronous with hair cycling, and there is oscillation of dermal bone morphogenetic protein (BMP) signaling which is asynchronous with hair cycling. The interactions of these two rhythms lead to the recognition of refractory and competent phases in the telogen, and autonomous and propagating phases in the anagen. Boundaries form when propagating anagen waves reach follicles which are in refractory telogen. Experiments showed that Krt14-Nog mice have shortened refractory telogen and simplified wave dynamics. Krt14-Nog skin grafts exhibit non-autonomous interactions with surrounding host skin. Implantation of BMP coated beads into competent telogen skin prevents hair wave propagation around the bead. Thus, we have developed a new molecular understanding of the classic early concepts of inhibitory &quot;chalone&quot;, suggesting that stem cells within the hair follicle micro-environment, or other organs, are subject to a higher level of macro-environmental regulation. Such a novel understanding has important implications in the field of regenerative medicine. The unexpected links with Bmp2 expression in subcutaneous adipocytes has implications for systems biology and Evo-Devo.
DOI:10.1111/j.1600-0625.2009.00944.xURLPMID:19650867 [本文引用: 2]

Sharpin-deficient (Sharpin(cpdm)) mutant mice develop a chronic eosinophilic dermatitis. To determine the efficacy of eosinophil-depletion in chronic inflammation, Sharpin(cpdm) mice were treated with anti-IL5 antibodies. Mice treated with anti-IL5 had a 90% reduction of circulating eosinophils and a 50% decrease in cutaneous eosinophils after 10 days compared with sham-treated littermates. Reducing the number of eosinophils resulted in increased severity of alopecia and erythema and a significant increase in epidermal thickness. Skin homogenates from mice treated with anti-IL5 had decreased mRNA expression of arylsulfatase B (Arsb), diamine oxidase (amiloride-binding protein 1, also called histaminase; Abp1) and Il10, which are mediators that eosinophils may release to quench inflammation. Skin homogenates from mice treated with anti-IL5 also had decreased mRNA expression of Il4, Il5, Ccl11, kit ligand (Kitl) and Tgfa; and increased mRNA expression of Tgfb1, Mmp12 and tenascin C (Tnc). In order to further decrease the accumulation of eosinophils, Sharpin(cpdm) mice were crossed with IL5 null mice. Il5(-/-), Sharpin(cpdm)/Sharpin(cpdm) mice had a 98% reduction of circulating eosinophils and a 95% decrease in cutaneous eosinophils compared with IL5-sufficient Sharpin(cpdm) mice. The severity of the lesions was similar between IL5-sufficient and IL5-deficient mice. Double mutant mice had a significant decrease in Abp1, and a significant increase in Tgfb1, Mmp12 and Tnc mRNA compared with controls. These data indicate that eosinophils are not essential for the development of dermatitis in Sharpin(cpdm) mice and suggest that eosinophils have both pro-inflammatory and anti-inflammatory roles in the skin of these mice.
DOI:10.1038/jid.2012.344URLPMID:23014334 [本文引用: 2]

Alopecia areata (AA) is an autoimmune disease that attacks anagen hair follicles. Gene array in graft-induced C3H/HeJ mice revealed that genes involved in retinoic acid (RA) synthesis were increased, whereas RA degradation genes were decreased in AA compared with sham controls. This was confirmed by immunohistochemistry in biopsies from patients with AA and both mouse and rat AA models. RA levels were also increased in C3H/HeJ mice with AA. C3H/HeJ mice were fed a purified diet containing one of the four levels of dietary vitamin A or an unpurified diet 2 weeks before grafting and disease progression followed. High vitamin A accelerated AA, whereas mice that were not fed vitamin A had more severe disease by the end of the study. More hair follicles were in anagen in mice fed high vitamin A. Both the number and localization of granzyme B-positive cells were altered by vitamin A. IFNγ was also the lowest and IL13 highest in mice fed high vitamin A. Other cytokines were reduced and chemokines increased as the disease progressed, but no additional effects of vitamin A were seen. Combined, these results suggest that vitamin A regulates both the hair cycle and immune response to alter the progression of AA.
URL [本文引用: 1]

Background: Alopecia Areata (AA) is a non-scarring, autoimmune disorder which causes hair loss. Inflammatory reactions are involved in hair loss of the scalp and/or body. The involvement of chemokine receptors in the pathogenesis of AA is well defined among which, CXCL1 acts on neutrophils and CXCL9, CXCL10 and CXCL12 and serve as T lymphocytes recruiters. Objective: To study the serum levels of ELR+ and ELR- CXCL1, CXCL9, CXCL10 and CXCL12 in the patients suffering from AA and healthy controls. Methods: The study population of consisted of 30 patients suffering from AA and 30 healthy controls. Serum concentrations of CXCL1, CXCL9, CXCL10 and CXCL12 were measured using enzyme-linked immunosorbent assay (ELISA). Results: Current results showed that AA patients had significantly elevated serum levels of CXCL9 and CXCL10 in comparison to controls (p<0.001). These results also demonstrated that serum levels of CXCL1 and CXCL12 were significantly decreased in AA patients compared to control (p<0.001). Conclusion: CXCL9 and CXCL10 are elevated in the AA patients and may be involved in the recruitment of T lymphocytes to the inflamed tissues. Moreover, due to the significant role played by these chemokines in angiogenesis/angiostatis phenomenon they could be considered as useful biomarkers in AA diagnosis and therapy.
DOI:10.1111/pcmr.12559URLPMID:27863059 [本文引用: 1]
DOI:10.3109/09546634.2015.1093591URLPMID:26367497 [本文引用: 1]

Most evidence supports the role of altered T cell-mediated immunity in the pathogenesis of alopecia areata (AA). Tough cytokines and chemokines play an important role in the immune process of AA, their expressions have been examined in limited studies.
DOI:10.1038/jid.2012.17URLPMID:22358057 [本文引用: 1]
DOI:10.1016/j.jaci.2015.06.032URLPMID:26316095 [本文引用: 1]

Alopecia areata (AA) is a common T cell-mediated disorder with limited therapeutics. A molecular profile of cytokine pathways in AA tissues is lacking. Although studies have focused on TH1/IFN-γ responses, several observations support a shared genetic background between AA and atopy.
DOI:10.4049/jimmunol.1501798URLPMID:27412416 [本文引用: 3]

Alopecia areata (AA) is an autoimmune disease of the hair follicle that results in hair loss of varying severity. Recently, we showed that IFN-γ-producing NKG2D(+)CD8(+) T cells actively infiltrate the hair follicle and are responsible for its destruction in C3H/HeJ AA mice. Our transcriptional profiling of human and mouse alopecic skin showed that the IFN pathway is the dominant signaling pathway involved in AA. We showed that IFN-inducible chemokines (CXCL9/10/11) are markedly upregulated in the skin of AA lesions, and further, that the IFN-inducible chemokine receptor, CXCR3, is upregulated on alopecic effector T cells. To demonstrate whether CXCL9/10/11 chemokines were required for development of AA, we treated mice with blocking Abs to CXCR3, which prevented the development of AA in the graft model, inhibiting the accumulation of NKG2D(+)CD8(+) T cells in the skin and cutaneous lymph nodes. These data demonstrate proof of concept that interfering with the Tc1 response in AA via blockade of IFN-inducible chemokines can prevent the onset of AA. CXCR3 blockade could be approached clinically in human AA with either biologic or small-molecule inhibition, the latter being particularly intriguing as a topical therapeutic.
DOI:10.1016/j.jdermsci.2012.12.003URLPMID:23312578 [本文引用: 1]

Alopecia areata (AA) is an organ-specific and cell-mediated autoimmune disease. T lymphocytes densely surround lesional hair bulbs, which is histologically referred to as &quot;swarm of bees&quot;. However, pathomechanisms of &quot;swarm of bees&quot; are still uncertain.
DOI:10.1016/j.autrev.2016.03.008URLPMID:26971464 [本文引用: 1]

One of the most common human autoimmune diseases, alopecia areata (AA), is characterized by sudden, often persisting and psychologically devastating hair loss. Animal models have helped greatly to elucidate critical cellular and molecular immune pathways in AA. The two most prominent ones are inbred C3H/HeJ mice which develop an AA-like hair phenotype spontaneously or after experimental induction, and healthy human scalp skin xenotransplanted onto SCID mice, in which a phenocopy of human AA is induced by injecting IL-2-stimulated PBMCs enriched for CD56+/NKG2D+ cells intradermally. The current review critically examines the pros and cons of the available AA animal models and how they have shaped our understanding of AA pathobiology, and the development of new therapeutic strategies. AA is thought to arise when the hair follicle's (HF) natural immune privilege (IP) collapses, inducing ectopic MHC class I expression in the HF epithelium and autoantigen presentation to autoreactive CD8+ T cells. In common with other autoimmune diseases, upregulation of IFN-γ and IL-15 is critically implicated in AA pathogenesis, as are NKG2D and its ligands, MICA, and ULBP3. The C3H/HeJ mouse model was used to identify key immune cell and molecular principles in murine AA, and proof-of-principle that Janus kinase (JAK) inhibitors are suitable agents for AA management in vivo, since both IFN-γ and IL-15 signal via the JAK pathway. Instead, the humanized mouse model of AA has been used to demonstrate the previously hypothesized key role of CD8+ T cells and NKG2D+ cells in AA pathogenesis and to discover human-specific pharmacologic targets like the potassium channel Kv1.3, and to show that the PDE4 inhibitor, apremilast, inhibits AA development in human skin. As such, AA provides a model disease, in which to contemplate general challenges, opportunities, and limitations one faces when selecting appropriate animal models in preclinical research for human autoimmune diseases.
DOI:10.1016/j.jaad.2017.04.1141URLPMID:29241771 [本文引用: 1]

Alopecia areata (AA) is a common, inflammatory, nonscarring type of hair loss. Significant variations in the clinical presentation of AA have been observed, ranging from small, well-circumscribed patches of hair loss to a complete absence of body and scalp hair. Patients affected by AA encompass all age groups, sexes, and ethnicities, and may experience frustration with the unpredictable nature of their disease for which there is currently no definitive treatment. The cause of AA remains incompletely understood, though it is believed to result-at least in part-from a loss of immune privilege in the hair follicle, autoimmune-mediated hair follicle destruction, and the upregulation of inflammatory pathways. Patients with AA frequently experience marked impairment in psychological well-being, self-esteem, and may be more likely to suffer from psychiatric comorbidities. Part one of this two-part continuing medical education series describes the epidemiology, clinical evaluation, prognosis, and recent advancements in the understanding of the pathogenesis of AA.
DOI:10.1016/j.jaad.2016.09.007URLPMID:27816293 [本文引用: 1]

Alopecia areata (AA) is a common autoimmune disorder. There are no reliably effective therapies for AA.
DOI:10.1172/jci.insight.89790URLPMID:27699253 [本文引用: 1]

BACKGROUND. Alopecia areata (AA) is a common autoimmune disease with a lifetime risk of 1.7%; there are no FDA-approved treatments for AA. We previously identified a dominant IFN-γ transcriptional signature in cytotoxic T lymphocytes (CTLs) in human and mouse AA skin and showed that treatment with JAK inhibitors induced durable hair regrowth in mice by targeting this pathway. Here, we investigated the use of the oral JAK1/2 inhibitor ruxolitinib in the treatment of patients with moderate-to-severe AA. METHODS. We initiated an open-label clinical trial of 12 patients with moderate-to-severe AA, using oral ruxolitinib, 20 mg twice per day, for 3-6 months of treatment followed by 3 months follow-up off drug. The primary endpoint was the proportion of subjects with 50% or greater hair regrowth from baseline to end of treatment. RESULTS. Nine of twelve patients (75%) demonstrated a remarkable response to treatment, with average hair regrowth of 92% at the end of treatment. Safety parameters remained largely within normal limits, and no serious adverse effects were reported. Gene expression profiling revealed treatment-related downregulation of inflammatory markers, including signatures for CTLs and IFN response genes and upregulation of hair-specific markers. CONCLUSION. In this pilot study, 9 of 12 patients (75%) treated with ruxolitinib showed significant scalp hair regrowth and improvement of AA. Larger randomized controlled trials are needed to further assess the safety and efficacy of ruxolitinib in the treatment of AA. TRIAL REGISTRATION. Clinicaltrials.gov NCT01950780. FUNDING. Locks of Love Foundation, the Alopecia Areata Initiative, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and the Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award (CUMC CTSA).
DOI:10.1016/j.ebiom.2015.02.015URLPMID:26137574 [本文引用: 1]

Alopecia areata (AA) is an autoimmune disease resulting in hair loss with devastating psychosocial consequences. Despite its high prevalence, there are no FDA-approved treatments for AA. Prior studies have identified a prominent interferon signature in AA, which signals through JAK molecules.
DOI:10.1111/ijd.13866URLPMID:29318582 [本文引用: 1]

Nail changes are a common feature of alopecia areata (AA) and are a significant source of cosmetic disfigurement and functional impairment. This review provides an update of the prevalence, clinical and histopathological features, pathogenesis, differential diagnosis, clinical course, prognosis, and management of nail changes in patients with AA. Searches for peer-reviewed journal articles were conducted using the PubMed/MEDLINE database with the search terms &quot;nail changes alopecia areata,&quot; &quot;alopecia areata nails,&quot; and specific searches on &quot;trachyonychia alopecia areata&quot; and &quot;pitting alopecia areata.&quot; Other sources of articles included the reference lists of retrieved articles. Nail changes are a common feature of AA, with an average prevalence of 30%, and can cause significant disfigurement and loss of function. Pitting and trachyonychia were by far the most common manifestations of AA, with an average prevalence of 20 and 8%, respectively. Red spotted lunulae, onycholysis, and punctate leukonychia were other reported findings. Other etiologies, such as onychomycosis or lichen planus, may coexist with or confound the diagnosis. There is limited published data on the clinical manifestations of AA-associated nail changes and therapeutic options. Larger controlled trials are necessary to guide treatment decisions.
DOI:10.1111/j.1468-3083.2008.02830.xURLPMID:18540984 [本文引用: 1]
DOI:10.3390/ijerph15122882URLPMID:30558329 [本文引用: 1]

Alopecia areata (AA) is associated with Interferon- γ (IFN-γ) mediated T-lymphocyte dysfunction and increased circulating Interleukine-17 (IL-17) levels. Epigallocatechin-3-gallate (EGCG) specifically inhibits IFN-γ pathways and unlike Janus Kinase 1 and 2 (JAK1/JAK2) inhibitors (tofacitinib, ruxolitinib), EGCG is safer, more cost-effective, and is a topically active agent. Our objective is to test the mode of action of EGCG in vitro and ex vivo using HaCat, Jurkat cell lines, and peripheral blood mononuclear cells (PBMCs) of AA patients and healthy controls (HCs), respectively.
DOI:10.5507/bp.2019.049URLPMID:31558844 [本文引用: 1]

Alopecia areata (AA) is mainly a T cell-medicated autoimmune disease with non-scarring hair loss and limited treatment options. Of these, the patchy-type alopecia areata (AAP) is the most common and relatively easy to treat due to smaller areas of the scalp affected. To understand the pathogenesis of AAP and explore the therapeutic target, we focus on the molecular signatures by comparing AAP and normal subjects.
DOI:10.1038/ni.2353URLPMID:22729248 [本文引用: 1]

Langerhans cells (LCs) are epidermal dendritic cells with incompletely understood origins that associate with hair follicles for unknown reasons. Here we show that in response to external stress, mouse hair follicles recruited Gr-1(hi) monocyte-derived precursors of LCs whose epidermal entry was dependent on the chemokine receptors CCR2 and CCR6, whereas the chemokine receptor CCR8 inhibited the recruitment of LCs. Distinct hair-follicle regions had differences in their expression of ligands for CCR2 and CCR6. The isthmus expressed the chemokine CCL2; the infundibulum expressed the chemokine CCL20; and keratinocytes in the bulge produced the chemokine CCL8, which is the ligand for CCR8. Thus, distinct hair-follicle keratinocyte subpopulations promoted or inhibited repopulation with LCs via differences in chemokine production, a feature also noted in humans. Pre-LCs failed to enter hairless skin in mice or humans, which establishes hair follicles as portals for LCs.
DOI:10.1111/1346-8138.15054URLPMID:31456262 [本文引用: 1]

Prominent dermal infiltration by Langerhans cells (LC) is a rare finding in patients with Omenn syndrome (OS). Here, we report the case study of a 7-month-old boy with OS and with prominent dermal infiltration by LC, which is a rare histological manifestation of the skin. Striking erythroderma appeared in the patient 2?weeks after birth. We also noted alopecia, lymphadenopathy, hepatosplenomegaly, eosinophilia and an elevated serum immunoglobulin E level with hypogammaglobulinemia. Peripheral blood flow cytometry showed the Tlow NK+ B+ immunophenotype and genetic analysis, a novel mutation in the IL2RG gene (c.337_339delTCT, p.Ser113del). The final diagnosis was that of OS. He responded well to an allograft umbilical cord blood transplantation that was performed when the patient was 8?months of age. We speculate that the LC accumulated in the dermis will eventually migrate to the regional lymph node, then stimulate autoreactive T cells by overpresenting antigens, thus causing OS-specific skin symptoms.
DOI:10.16288/j.yczz.14-440URLPMID:26351048 [本文引用: 2]

Recently, transcriptome sequencing technology has achieved significant progresses in gene network regulation of important economic traits in animals. As the derivative of mammalian skin, hair follicle is capable of self-renew. Its proliferation and differentiation result in hair formation. Researches have revealed that many growth factors and receptors coordinate genes and environment, as well as play an extremely important role during hair growth. In this review, we summarize the progresses that transcriptome sequencing technologies have achieved in researches of hair follicle development and renegeration in a variety of species, such as humans, mice, goats. We aim to provide theoretical mechanisms for the artificial interference of villus growth cycle, and new ideas for therapeutic treatment of skin hair follicle- related diseases.
DOI:10.16288/j.yczz.14-440URLPMID:26351048 [本文引用: 2]

Recently, transcriptome sequencing technology has achieved significant progresses in gene network regulation of important economic traits in animals. As the derivative of mammalian skin, hair follicle is capable of self-renew. Its proliferation and differentiation result in hair formation. Researches have revealed that many growth factors and receptors coordinate genes and environment, as well as play an extremely important role during hair growth. In this review, we summarize the progresses that transcriptome sequencing technologies have achieved in researches of hair follicle development and renegeration in a variety of species, such as humans, mice, goats. We aim to provide theoretical mechanisms for the artificial interference of villus growth cycle, and new ideas for therapeutic treatment of skin hair follicle- related diseases.
