 ,1
,1Molecular regulation mechanism of Myomaker and Myomerger in myoblast fusion
Yan Zhu1, Jinwei Zhang1, Jing Qi1, Xuewei Li1, Lei Chen2, Mingzhou Li1, Jideng Ma ,1
,1通讯作者: 马继登,博士,副教授,研究方向:动物遗传育种与繁殖。E-mail:jideng.ma@sicau.edu.cn
编委: 赵要风
收稿日期:2019-08-8修回日期:2019-10-25网络出版日期:2019-12-20
| 基金资助: |
Editorial board:
Received:2019-08-8Revised:2019-10-25Online:2019-12-20
| Fund supported: |
作者简介 About authors
朱艳,硕士研究生,专业方向:动物遗传育种与繁殖。E-mail:zhuyan_494062079@163.com。

摘要
骨骼肌形成是一个复杂的生理过程,主要涉及肌源性干细胞增殖形成成肌细胞,进而分化、融合形成多核肌管。研究发现,有多种蛋白参与成肌细胞融合过程,但它们均不具有肌肉特异性。近年来,两种肌肉特异性膜蛋白Myomaker和Myomerger先后被发现和鉴定,它们能协调促进成肌细胞融合,从而参与骨骼肌形成过程。本文对成肌过程中Myomaker和Myomerger的表达模式、功能域等研究现状及其参与成肌细胞的融合机制进行了综述,旨在为深入研究骨骼肌形成过程及治疗肌细胞融合相关疾病提供参考信息。
关键词:
Abstract
Myogenesis is a complex physiological process that is mainly involved in the proliferation of myogenic stem cells to form myoblasts, which then differentiated and fused to form multinucleated myotubes. Many proteins have been found to be involved in myoblast fusion, but none of them are muscle-specific fusion proteins. In recent years, two muscle-specific transmembrane proteins, i.e. Myomaker and Myomerger, have been discovered and identified, which can coordinate and promote the fusion of myoblasts and thus participate in the process of myogenesis. In this review, we summarize the research progress of Myomaker and Myomerger in myogenesis, including their expression patterns and functional domains, as well as their participation in myoblast fusion mechanisms, aiming to provide relevant ideas for in-depth study of the myogenesis process and treatment of diseases related to myoblast fusion.
Keywords:
PDF (928KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
朱艳, 张进威, 齐婧, 李学伟, 陈磊, 李明洲, 马继登. Myomaker和Myomerger调控成肌细胞融合的分子机制. 遗传[J], 2019, 41(12): 1110-1118 doi:10.16288/j.yczz.19-232
Yan Zhu.
骨骼肌形成(myogenesis)是一个复杂的生理过程,包括胚胎和幼年的肌肉发育以及成年肌肉损伤后再生过程[1]。成肌调控因子(myogenic regulatory factors, MRFs)的时序性表达促进了肌肉结构和功能的完善,对成肌命运决定和肌管分化至关重要[2]。单核成肌细胞或肌原性祖细胞被激活进而融合形成多核肌管是成肌的关键步骤[3,4],研究表明这一过程受多种蛋白的协作调控,但很少涉及肌肉特异性的调控蛋白。直到2013年,美国德克萨斯大学达拉斯西南医学中心的Millay团队[5]发现TMEM8c (并命名为Myomaker)是一种能直接调控成肌细胞融合的肌肉特异性膜蛋白。Myomaker含有7个跨膜域[6]且在骨骼肌生成和损伤修复过程中瞬时高丰度表达,能有效促进成肌细胞融合[5,7]。2017年,另一种肌肉特异性融合蛋白GM7325被发现和鉴定,并命名为Myomerger (也称Minion或Myomixer)。Myomerger能促进融合性细胞 (如成肌细胞) 间发生融合,当与Myomaker共同表达时,也能诱导非融合性细胞间(如成纤维细胞)发生融合[1,8,9]。然而目前Myomaker和Myomerger协同调控细胞融合的机制还有待深入研究。本文综述了Myomaker和Myomerger在成肌细胞融合方面的最新进展,为研究骨骼肌形成的分子机制提供理论依据,同时也为治疗细胞融合相关疾病提供一定思路。
1 骨骼肌形成过程
1.1 骨骼肌形成
骨骼肌是机体的重要组成部分[10],由多核肌纤维彼此紧密排列构成。骨骼肌形成包括前体细胞招募、成肌细胞分化、单核肌细胞融合等。在胚胎骨骼肉发育过程中,PAX7+前体细胞 (肌源性干细胞) 被招募为成肌细胞,然后增殖和分化,最后彼此融合或与现存肌纤维融合形成多核成熟肌管[11];同样,当骨骼肌受到外源损伤后,依附于肌纤维基部的静息肌卫星细胞会被激活进而增殖、分化,最终融合形成新肌管以修复肌肉创伤[12],因此成肌细胞融合是骨骼肌形成的关键步骤。1.2 成肌细胞融合
成肌细胞融合是一个受到高度调控的动态过程,主要涉及细胞骨架重排以及在细胞膜上形成融合孔并交换细胞质成分,最终完成细胞融合[13]。Yafe等[14]研究表明,许多调控因子参与了细胞融合的调控过程,如MRFs家族(主要包括Myf5、MyoD、MyoG和Myf4),该家族可以与E-box元件结合,调控大多数成肌相关基因的转录。Myf5和MyoD被认为是肌形成的决定因子,而MyoG和Myf4在分化后期高度表达,触发成肌细胞融合最终形成肌管[15]。目前研究表明,部分细胞粘附蛋白参与了成肌细胞融合过程[16,17,18],如Myoferlin是一种在融合细胞膜上高表达的跨膜蛋白,由6个C2结构域(8个β链形成的折叠结构)和一个羧基端跨膜域构成[19,20],依赖于钙离子与磷脂结合[21]。Myoferlin氨基末端的C2结构域(C2 domain of the amino terminus, C2A)突变可以破坏这种结合,从而降低肌管的融合效率[22]。然而,上述蛋白均是非肌肉特异性蛋白,并且许多蛋白在成肌细胞融合过程中并未发挥直接调控作用。因此,研究和揭示直接参与成肌细胞融合的肌特异性膜蛋白成为当前研究热点。近年来,随着对Myomaker和 Myomerger的研究,肌细胞融合过程的调控机制逐渐清晰(图1)。图1
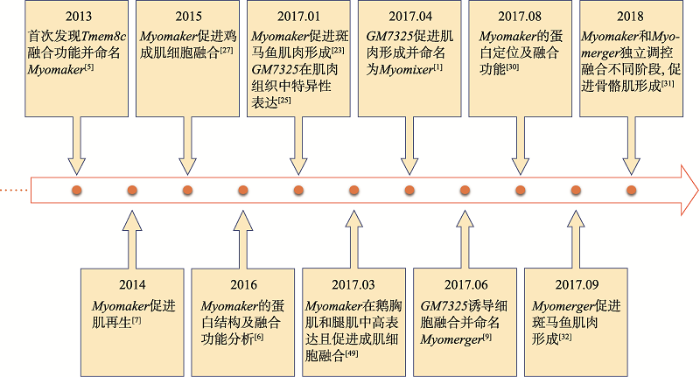 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1Myomaker和Myomerger研究进展
Fig. 1Research progress in Myomaker and Myomerger
2 成肌过程中Myomaker和Myomerger表达模式
2013年以前,对Tmem8c基因的研究几乎是空白,直到Millay团队[5]首次发现Tmem8c的融合功能并命名为Myomaker。该团队通过对小鼠(Mus musculus)胚胎进行原位杂交,发现Myomaker主要在肌肉组织中特异性表达,提示Myomaker对骨骼肌发育的重要性。同时,Zhang等[23]和Landemaine等[24]研究发现Myomaker在斑马鱼(Danio rerio)快肌(融合能力较强)中高表达,在慢肌(融合能力较弱)中表达量显著降低;另外,通过qPCR发现Myomaker的表达模式类似于MyoD和MyoG,即在胚胎骨骼肌形成过程中的表达水平明显高于骨骼肌发育完成后。2017年,Takei等[25]发现了另一种膜蛋白GM7325的表达量在激活的肌卫星细胞中显著上调,暗示对骨骼肌形成的重要作用。同年,Quinn等[9]将GM7325命名为Myomerger,发现Myomerger也在肌肉组织中特异性表达,并且在小鼠胚胎发育过程中Myomerger和Myomaker的表达模式类似[5]。骨骼肌生长通常发生在胚胎期和成年肌肉再生期两个阶段。对此,Millay等[7]为了研究Myomaker在骨骼肌再生过程中的表达情况,向成年小鼠骨骼肌注射心脏毒素(cardiotoxin, CTX)使之损伤再生,结果显示当骨骼肌受到损伤刺激时,处于静息期的肌卫星细胞被迅速激活,同时Myomaker的表达量显著上升进而促进细胞融合、修复损伤;在C2C12细胞水平,Myomaker和Myomerger在细胞分化和融合过程中的表达量逐渐上升,在分化末期则迅速降低。另外,He等[26]通过免疫印迹分析,发现Myomaker和Myomerger蛋白表达模式与其mRNA相似,该结果在斑马鱼[23,24]和鸡(Gallus gallus)[27]中再次得到了验证。虽然Myomaker和Myomerger的功能性表达在小鼠、斑马鱼和鸡中相似,但是其物种保守性仍有待在更多物种中研究确定。3 Myomaker和Myomerger的功能域
3.1 Myomaker蛋白定位及结构
随着基因注释研究的不断完善,目前一些常见物种,包括人(Homo sapiens)[28]、小鼠[5-9,25,29]、斑马鱼[23]、鸡[27]的Myomaker和Myomerger基因结构已经得到解析(表1),但对于蛋白功能研究还相对滞后。Gamage等[30]通过设计两种不同定位的Myomaker抗体,发现Myomaker不仅定位于细胞膜,也存在于高尔基体和囊泡,这揭示Myomaker在细胞中的精确定位及其潜在的细胞间转运功能。另外,Millay等[6]通过免疫荧光染色(immunofluorescence, IF)发现Myomaker具有7个跨膜结构域 (含胞外N端和胞内C端),且Myomaker最后7个氨基酸(氨基酸215~221)的缺失会抑制细胞融合;Gamage等[30]通过对Myomaker C端分析,发现Myomaker蛋白含有3个保守的棕榈酰化半胱氨酸,其可能参与调控高尔基体转运(图2A)。综上所述,这些研究将有利于进一步解析和阐释成肌细胞的融合机制。Table 1
表1
表1常见物种Myomaker和Myomerger基因结构
Table 1
| 基因 | 物种 | 染色体位置 | 可变剪 接体数 | 转录ID | 外显 子数 | 转录长度 (bp) | 翻译长度 (aa) | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Myomaker | 小鼠 (M. musculus) | Chr.2:27061636-27072179 | 2 | ENSMUST00000009358.2 | 5 | 1382 | 221 | [5~7,29] |
| ENSMUST00000163967.1 | 5 | 1210 | 180 | |||||
| 人 (H. sapiens) | Chr.9:136379708-136393734 | 2 | ENST00000339996.3 | 5 | 818 | 221 | [28] | |
| ENST00000413714.1 | 0 | 531 | - | |||||
| 斑马鱼 (D. rerio) | Chr.5:68934948-68940186 | 2 | ENSDART00000033962.6 | 5 | 663 | 220 | [23] | |
| ENSDART00000138519.1 | 1 | 258 | 45 | |||||
| 鸡 (G. gallus) | Chr.17:6958313-6965254 | 1 | ENSGALT00000034784.3 | 5 | 1078 | 220 | [27] | |
| Myomerger | 小鼠 (M. musculus) | Chr.17: 45600967-45602102 | 3 | ENSMUST00000113529.2 | 1 | 811 | 84 | [8,9,25] |
| ENSMUST00000169137.1 | 2 | 852 | 18 | |||||
| ENSMUST00000178858.1 | 1 | 808 | 84 | |||||
| 大鼠 (R. norvegicus) | Chr.9: 16670994-16671263 | 1 | ENSRNOT00000064497.2 | 1 | 270 | 89 | - | |
| 人 (H. sapiens) | Chr.6: 44216939-44218236 | 2 | ENST00000573382.2 | 1 | 817 | 84 | - | |
| ENST00000576476.1 | 1 | 682 | 84 |
新窗口打开|下载CSV
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2常见物种Myomaker和Myomerger蛋白序列对比
A:常见物种Myomaker蛋白序列对比。TM1-7:7个跨膜区域;红色序列:具有促进细胞融合功能;红色下划线序列:棕榈酰化半胱氨酸;小鼠、人、斑马鱼和鸡在UNIPROT中的ID号分别为:Q9D1N4、A6NI61、Q6IQ69和G3RFK8。B:常见物种Myomerger蛋白序列对比。小鼠、大鼠、人、斑马鱼和鸡在UNIPROT中的ID号分别为Q2Q5T5、D4A557、A0A1B0GTQ4、P0DP88和G3RUY6。
Fig. 2Protein sequence alignment of Myomaker and Myomerger between common species
3.2 Myomerger蛋白结构
相比于Myomaker,目前对Myomerger的结构研究还较少。研究发现,Myomerger含有多重螺旋区 (图2B),其中N端疏水区域(氨基酸5~25)可能具有跨膜结构。Bi等[1]通过免疫共沉淀(Co-immunoprecipitation, Co-IP)分析表明,Myomerger N端第一个α-螺旋域内带正电的精氨酸能显著影响细胞融合能力。同时,Leikina等[31]通过免疫印迹(Western blot)证实Myomerger在细胞表面表达,且通过异源融合实验表明Myomerger能显著促进成肌细胞与成纤维细胞完全融合,但具体作用仍需进一步研究。4 Myomaker和Myomerger对细胞融合的影响
4.1 Myomaker和Myomerger促进细胞完全融合
在成肌细胞分化过程中,Myomaker和Myomerger的表达量逐渐增加,在分化末期,其表达量显著降低,这种峰值表达模式暗示Myomaker和Myomerger可能在细胞分化过程中发挥着重要作用。对此,Millay等[6]和Quinn等[9]利用CRISPR/Cas9基因编辑技术分别将小鼠Myomaker和Myomerger敲除后,发现这两种蛋白的缺失能显著抑制成肌细胞融合,该结果同样在鸡[27]和斑马鱼[32]中也得到了验证。然而,Myomaker和Myomerger敲除后均检测到Myosin (分化标志基因)的表达,说明Myomaker和Myomerger并不直接影响细胞分化,而是调控细胞融合。一直以来,Myomaker被认为能有效促进肌源性细胞融合。Myomaker能赋予非融合细胞融合能力,即Myomaker也能促进非融合细胞如成纤维细胞与成肌细胞融合。但Myomaker不能促进非肌细胞间的融合,即使过表达Myomaker后,成纤维细胞间不能发生融合,由此暗示其他因子可能参与非肌细胞间发生融合。近来研究发现,共表达Myomaker和Myomerger的成纤维细胞在数小时内开始融合,最终形成巨大的合胞体[1,8],表明Myomaker和Myomerger能协同作用促进非肌细胞完全融合。4.2 Myomaker和Myomerger独立调控成肌细胞融合的不同阶段
近年来,Myomaker和Myomerger协同促进细胞融合的机制逐渐得到解析。Bi[1]和Millay等[5]研究发现,Myomaker和Myomerger 敲除的小鼠胚胎表现出相似表型,但这些表型却存在细微差异,例如在胚胎17.5天,Myomerger敲除小鼠的肌细胞排列更紧密,暗示Myomaker和Myomerger在融合过程中可能发挥着不同功能。Leikina等[31]发现Myomaker和Myomerger调控成肌细胞融合和骨骼肌形成,但其结构却与传统的融合蛋白不同;免疫印迹结果证明Myomerger主要在细胞表面表达,并作用于胞外应力膜;异源融合实验表明Myomerger能独立于Myomaker促进融合完成,并且Myomaker和Myomerger间的物理交互作用并不是其功能所必需的;通过双标记探针发现Myomaker主要参与膜半融合 (即双层膜中仅一层发生融合),而Myomerger主要促进融合孔形成 (即双层膜均发生融合,为胞质交换提供有利条件)。总之,成肌细胞融合是一个逐步完成的过程,其中Myomaker和Myomerger独立调控成肌细胞融合的两个不同阶段[31]:第一阶段,Myomaker促进半融合形成,而Myomerger并未发挥调控作用;第二阶段,Myomerger作用于细胞膜,产生膜应力,以独立于Myomaker的方式促进完全融合,最后形成多核的合胞体。另外,该研究还发现一些因子(如:Octyl glucopyranoside和magainin 2)能弥补Myomerger的缺失进而促进细胞融合[31],这将为治疗肌肉相关疾病提供一定参考,但Myomaker和Myomerger是如何驱动半融合向完全融合转变仍有待进一步研究。5 Myomaker、Myomerger及其相关因子对成肌细胞融合过程的调控机制
5.1 参与成肌细胞融合的调控因子
成肌细胞融合主要涉及3个关键步骤:首先成肌细胞彼此识别并粘附在一起,其次细胞膜的脂质双层发生重排形成融合孔,最后融合孔扩张形成合胞体并出现多核肌管。在这些复杂的过程中,除了Myomaker和Myomerger这两种肌肉特异性蛋白参与调控外,还有许多其他因子参与其中(图3)。例如一些免疫球蛋白超家族(immunoglobulin superfamily, Ig superfamily)被证实参与了成肌细胞融合过程[33]。Srinivas等[34]利用吗啉代(morpholino)介导的敲除实验,发现斑马鱼胚胎中Kirrel和Nephrin基因缺失会导致更短的肌管形成,暗示它们在成肌细胞融合过程发挥着重要作用。Powell等[35]研究表明,细胞黏附分子Jamb和Jamc突变会抑制斑马鱼胚胎中成肌细胞融合,从而形成单核的胚胎肌纤维。同样,另一种蛋白Cadherins被认为在细胞融合中发挥着黏附分子的作用[36,37],但具体的重要作用还有待进一步研究。在C2C12细胞分化过程中,Zhang等[8]对Myomerger进行免疫沉淀质谱分析(IP-mass spectrometry)发现了许多具有注释功能的蛋白。例如Ferlin家族成员Dysferlin,该基因突变会导致遗传性肌营养不良。Cárdenas等[38]发现Dysferlin在与细胞融合相关的过程中(如膜修复、囊泡转运和胞吐)具有调控作用。De等[39]研究表明Dysferlin基因缺失能明显抑制成肌细胞融合,但具体的调控机制仍有待深入研究。近年来还发现了另一个调控成肌细胞融合的潜在途径,即磷脂酰丝氨酸(phosphatidylserine, PS)与其受体BAI1[40]或Stability-2[41]结合,BAI1或Stability-2通过ELMO/Dock1通路激活Rac1,进而调控细胞膜重排。Kim等[42]和Jeong等[43]研究发现,PS可转移到胞外且可以作为直接促进细胞融合的信号。BAI1和Stability-2作为一种膜蛋白,其作用机制与Myomaker和Myomerger类似,但敲除Myomaker和Myomerger具有胚胎致死性,而BAI1或Stabilin-2缺失对肌肉损伤则相对温和[40,41],但具体机制还需进一步研究。
5.2 Myomaker和Myomerger对成肌细胞融合的转录调控机制
研究表明,参与调控成肌细胞融合的Myomaker和Myomerger主要受MRFs和一些非编码RNA调控。Luo等[27]对鸟类成肌细胞研究发现,在分化过程中,MyoD和MyoG可直接与Myomaker启动子结合,从而调控Myomaker转录。研究表明,miRNAs在骨骼肌分化中发挥着关键作用[44,45,46],例如:miR-1能促进骨骼肌成肌细胞分化[47];miR-133促进细胞增殖但抑制肌源性基因的表达[47];miR-206在骨骼肌中高表达,但其作用还有待研究[48]。Luo等[27]研究表明,miR-140-3p与Myomaker 3′-UTR靶向结合从而抑制鸟类Myomaker表达及成肌细胞融合,暗示Myomaker可能是miRNAs调节骨骼肌分化的关键因子。另外,He等[26]对小鼠的研究发现,miR-491能与Myomaker 3′-UTR特异性结合使Myomaker表达下调。过表达miR-491能显著抑制肌肉细胞分化和成年肌肉损伤后再生,而抑制miR-491表达则能促进肌管分化,因此,miR-491可作为一种新的肌源性分化负调控因子通过靶向Myomaker影响成肌过程。Ke等[49]研究发现,在鹅(Anser cygnoides)的胸肌和腿肌中与Myomaker表达具有负相关的miRNAs有4个,包括miR-125b-5p、miR-15a、miR-16-1和miR-23。双荧光素酶报告基因检测(dual-Luciferase Reporter Gene Assay)发现只有miR-16-1能直接靶向Myomaker,表明miR-16-1可能是影响Myomaker的潜在因素,但进一步的功能研究有待进一步被验证。总之,Myomaker和Myomerger是控制肌细胞融合的重要蛋白,同样受到MRFs和一些非编码RNA调控,因此,进一步完善Myomaker和Myomerger的上下游调控网络有利于深入解析肌细胞融合过程及潜在机制。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3Myomaker、Myomerger及其相关因子对成肌细胞融合过程的调控机制
A:多种调控因子参与细胞膜的黏附及并排过程;B:Myomaker促进半融合发生;C:Myomerger促进融合孔形成;D:细胞融合形成多核的合胞体。?:代表潜在调控机制。
Fig. 3Schematic diagram of the regulatory roles of Myomaker, Myomerger and related genes at each stage of myoblast fusion
6 结语与展望
近年来,Myomaker和Myomerger已成为研究成肌细胞融合的“明星分子”。Fineman-Ziter综合征[28]和杜氏肌肉营养不良症 (Duchenne muscular dystrophy, DMD) 是两种由成肌细胞融合受阻导致的遗传性肌肉疾病。Myomaker和Myomerger能使受损肌纤维的成肌细胞再次发生融合从而修复骨骼肌损伤,这不仅为研究骨骼肌再生的分子机制提供理论依据,也可为临床上治疗骨骼肌萎缩等相关疾病提供思路。虽然治疗肌营养不良症可通过外源细胞融合来促进新肌纤维形成[50,51],但这种方法所形成的肌纤维融合率极低,恢复肌肉功能也是难以实现。近年来Myomaker和Myomerger的发现为提高肌纤维融合率提供了新思路。Myomaker和Myomerger在细胞水平上能显著提高融合能力,从而诱导多核肌纤维形成。另外,Myomaker过表达可以促进一些非肌源性细胞(如成纤维细胞)与骨骼肌在体内发生融合[52],由此为治疗骨骼肌损伤相关疾病提供新策略。对于肉用动物,其肌肉的良好生长发育关系到其本身健康和经济价值。由于肌纤维的组织学特征是决定肉质性状指标的关键因素[53],因此Myomaker和Myomerger促进多核肌纤维形成的潜能也有助于改善畜禽肉用性能。目前已发现Myomaker在鸡、鹅等农业动物骨骼肌发育中发挥着重要作用,对此通过研究Myomaker和Myomerger对成肌细胞融合的影响机制,可为研究肌肉品质提供一定程度的理论依据,从而推动畜禽肉用品质的改善。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1126/science.aam9361URLPMID:28386024 [本文引用: 5]

Skeletal muscle formation occurs through fusion of myoblasts to form multinucleated myofibers. From a genome-wide clustered regularly interspaced short palindromic repeats (CRISPR) loss-of-function screen for genes required for myoblast fusion and myogenesis, we discovered an 84-amino acid muscle-specific peptide that we call Myomixer. Myomixer expression coincides with myoblast differentiation and is essential for fusion and skeletal muscle formation during embryogenesis. Myomixer localizes to the plasma membrane, where it promotes myoblast fusion and associates with Myomaker, a fusogenic membrane protein. Myomixer together with Myomaker can also induce fibroblast-fibroblast fusion and fibroblast-myoblast fusion. We conclude that the Myomixer-Myomaker pair controls the critical step in myofiber formation during muscle development.
DOI:10.18632/aging.101904URLPMID:30996129 [本文引用: 1]

Optimization of resistance exercise (RE) remains a hotbed of research for muscle building and maintenance. However, the interactions between the contractile components of RE (i.e. concentric (CON) and eccentric (ECC)) and age, are poorly defined. We used transcriptomics to compare age-related molecular responses to acute CON and ECC exercise. Eight young (21±1 y) and eight older (70±1 y) exercise-na?ve male volunteers had vastus lateralis biopsies collected at baseline and 5 h post unilateral CON and contralateral ECC exercise. RNA was subjected to next-generation sequencing and differentially expressed (DE) genes tested for pathway enrichment using Gene Ontology (GO). The young transcriptional response to CON and ECC was highly similar and older adults displayed moderate contraction-specific profiles, with no GO enrichment. Age-specific responses to ECC revealed 104 DE genes unique to young, and 170 DE genes in older muscle, with no GO enrichment. Following CON, 15 DE genes were young muscle-specific, whereas older muscle uniquely expressed 147 up-regulated genes enriched for cell adhesion and blood vessel development, and 28 down-regulated genes involved in mitochondrial respiration, amino acid and lipid metabolism. Thus, older age is associated with contraction-specific regulation often without clear functional relevance, perhaps reflecting a degree of stochastic age-related dysregulation.
DOI:10.1016/j.ydbio.2009.10.024URLPMID:19932206 [本文引用: 1]

Cell-cell fusion is a crucial and highly regulated event in the genesis of both form and function of many tissues. One particular type of cell fusion, myoblast fusion, is a key cellular process that shapes the formation and repair of muscle. Despite its importance for human health, the mechanisms underlying this process are still not well understood. The purpose of this review is to highlight the recent literature pertaining to myoblast fusion and to focus on a comparison of these studies across several model systems, particularly the fly, zebrafish and mouse. Advances in technical analysis and imaging have allowed identification of new fusion genes and propelled further characterization of previously identified genes in each of these systems. Among the cellular steps identified as critical for myoblast fusion are migration, recognition, adhesion, membrane alignment and membrane pore formation and resolution. Importantly, striking new evidence indicates that orthologous genes govern several of these steps across these species. Taken together, comparisons across three model systems are illuminating a once elusive process, providing exciting new insights and a useful framework of genes and mechanisms.
DOI:10.1016/j.gde.2015.06.006URLPMID:26189082 [本文引用: 1]

Release of muscle stem cells from quiescence involves the coordinated effort of transcription, mRNA stability, and translation. We focus this review on post-transcriptional regulation of muscle stem cells and highlight the impact of deregulated mRNA homeostasis on muscle aging and muscle disease pathogenesis.
DOI:10.1038/nature12343URLPMID:23868259 [本文引用: 7]

Fusion of myoblasts is essential for the formation of multi-nucleated muscle fibres. However, the identity of muscle-specific proteins that directly govern this fusion process in mammals has remained elusive. Here we identify a muscle-specific membrane protein, named myomaker, that controls myoblast fusion. Myomaker is expressed on the cell surface of myoblasts during fusion and is downregulated thereafter. Overexpression of myomaker in myoblasts markedly enhances fusion, and genetic disruption of myomaker in mice causes perinatal death due to an absence of multi-nucleated muscle fibres. Remarkably, forced expression of myomaker in fibroblasts promotes fusion with myoblasts, demonstrating the direct participation of this protein in the fusion process. Pharmacological perturbation of the actin cytoskeleton abolishes the activity of myomaker, consistent with previous studies implicating actin dynamics in myoblast fusion. These findings reveal a long-sought myogenic fusion protein that controls mammalian myoblast fusion and provide new insights into the molecular underpinnings of muscle formation.
DOI:10.1073/pnas.1600101113URLPMID:26858401 [本文引用: 4]

During skeletal muscle development, myoblasts fuse to form multinucleated myofibers. Myomaker [Transmembrane protein 8c (TMEM8c)] is a muscle-specific protein that is essential for myoblast fusion and sufficient to promote fusion of fibroblasts with muscle cells; however, the structure and biochemical properties of this membrane protein have not been explored. Here, we used CRISPR/Cas9 mutagenesis to disrupt myomaker expression in the C2C12 muscle cell line, which resulted in complete blockade to fusion. To define the functional domains of myomaker required to direct fusion, we established a heterologous cell-cell fusion system, in which fibroblasts expressing mutant versions of myomaker were mixed with WT myoblasts. Our data indicate that the majority of myomaker is embedded in the plasma membrane with seven membrane-spanning regions and a required intracellular C-terminal tail. We show that myomaker function is conserved in other mammalian orthologs; however, related family members (TMEM8a and TMEM8b) do not exhibit fusogenic activity. These findings represent an important step toward deciphering the cellular components and mechanisms that control myoblast fusion and muscle formation.
DOI:10.1101/gad.247205.114URLPMID:25085416 [本文引用: 3]

Regeneration of injured adult skeletal muscle involves fusion of activated satellite cells to form new myofibers. Myomaker is a muscle-specific membrane protein required for fusion of embryonic myoblasts, but its potential involvement in adult muscle regeneration has not been explored. We show that myogenic basic helix-loop-helix (bHLH) transcription factors induce myomaker expression in satellite cells during acute and chronic muscle regeneration. Moreover, genetic deletion of myomaker in adult satellite cells completely abolishes muscle regeneration, resulting in severe muscle destruction after injury. Myomaker is the only muscle-specific protein known to be absolutely essential for fusion of embryonic and adult myoblasts.
DOI:10.1038/ncomms15664URLPMID:28569745 [本文引用: 4]

Although recent evidence has pointed to the existence of small open reading frame (smORF)-encoded microproteins in mammals, their function remains to be determined. Skeletal muscle development requires fusion of mononuclear progenitors to form multinucleated myotubes, a critical but poorly understood process. Here we report the identification of Minion (microprotein inducer of fusion), a smORF encoding an essential skeletal muscle specific microprotein. Myogenic progenitors lacking Minion differentiate normally but fail to form syncytial myotubes, and Minion-deficient mice die perinatally and demonstrate a marked reduction in fused muscle fibres. The fusogenic activity of Minion is conserved in the human orthologue, and co-expression of Minion and the transmembrane protein Myomaker is sufficient to induce cellular fusion accompanied by rapid cytoskeletal rearrangement, even in non-muscle cells. These findings establish Minion as a novel microprotein required for muscle development, and define a two-component programme for the induction of mammalian cell fusion. Moreover, these data also significantly expand the known functions of smORF-encoded microproteins.
DOI:10.1038/ncomms15665URLPMID:28569755 [本文引用: 5]

Despite the importance of cell fusion for mammalian development and physiology, the factors critical for this process remain to be fully defined, which has severely limited our ability to reconstitute cell fusion. Myomaker (Tmem8c) is a muscle-specific protein required for myoblast fusion. Expression of myomaker in fibroblasts drives their fusion with myoblasts, but not with other myomaker-expressing fibroblasts, highlighting the requirement of additional myoblast-derived factors for fusion. Here we show that Gm7325, which we name myomerger, induces the fusion of myomaker-expressing fibroblasts. Thus, myomaker and myomerger together confer fusogenic activity to otherwise non-fusogenic cells. Myomerger is skeletal muscle-specific and genetic deletion in mice results in a paucity of muscle fibres demonstrating its requirement for normal muscle formation. Myomerger deficient myocytes differentiate and harbour organized sarcomeres but are fusion-incompetent. Our findings identify myomerger as a fundamental myoblast fusion protein and establish a system that begins to reconstitute mammalian cell fusion.
URLPMID:12963831 [本文引用: 1]

Skeletal muscle formation and growth require the fusion of myoblasts to form multinucleated myofibers or myotubes. Studies of the calcium activated transcription factor NFATC2 demonstrate that cell fusion during myogenesis occurs in two distinctly regulated phases. NFATC2 controls myoblast fusion after the initial formation of a myotube and is necessary for further cell growth. Recently we have shown that following myotube formation, myotubes recruit myoblast fusion by secretion of IL-4 and prostaglandin F2alpha. Molecules that control muscle cell fusion are of great interest from a therapeutic standpoint to enhance growth of muscle after injury or to alleviate the loss of muscle mass found in disease or aging.
DOI:10.1242/dev.02517URLPMID:16936075 [本文引用: 1]

Canonical Wnt/beta-catenin signaling regulates the activation of the myogenic determination gene Myf5 at the onset of myogenesis, but the underlying molecular mechanism is unknown. Here, we report that the Wnt signal is transduced in muscle progenitor cells by at least two Frizzled (Fz) receptors (Fz1 and/or Fz6), through the canonical beta-catenin pathway, in the epaxial domain of newly formed somites. We show that Myf5 activation is dramatically reduced by blocking the Wnt/beta-catenin pathway in somite progenitor cells, whereas expression of activated beta-catenin is sufficient to activate Myf5 in somites but not in the presomitic mesoderm. In addition, we identified Tcf/Lef sequences immediately 5' to the Myf5 early epaxial enhancer. These sites determine the correct spatiotemporal expression of Myf5 in the epaxial domain of the somite, mediating the synergistic action of the Wnt/beta-catenin and the Shh/Gli pathways. Taken together, these results demonstrate that Myf5 is a direct target of Wnt/beta-catenin, and that its full activation requires a cooperative interaction between the canonical Wnt and the Shh/Gli pathways in muscle progenitor cells.
DOI:10.1152/physrev.00019.2003URLPMID:14715915 [本文引用: 1]

Under normal circumstances, mammalian adult skeletal muscle is a stable tissue with very little turnover of nuclei. However, upon injury, skeletal muscle has the remarkable ability to initiate a rapid and extensive repair process preventing the loss of muscle mass. Skeletal muscle repair is a highly synchronized process involving the activation of various cellular responses. The initial phase of muscle repair is characterized by necrosis of the damaged tissue and activation of an inflammatory response. This phase is rapidly followed by activation of myogenic cells to proliferate, differentiate, and fuse leading to new myofiber formation and reconstitution of a functional contractile apparatus. Activation of adult muscle satellite cells is a key element in this process. Muscle satellite cell activation resembles embryonic myogenesis in several ways including the de novo induction of the myogenic regulatory factors. Signaling factors released during the regenerating process have been identified, but their functions remain to be fully defined. In addition, recent evidence supports the possible contribution of adult stem cells in the muscle regeneration process. In particular, bone marrow-derived and muscle-derived stem cells contribute to new myofiber formation and to the satellite cell pool after injury.
DOI:10.1007/s10126-018-9865-xURLPMID:30467785 [本文引用: 1]

Myoblast fusion is a vital step for skeletal muscle development, growth, and regeneration. Loss of Jamb, Jamc, or Myomaker (Mymk) function impaired myoblast fusion in zebrafish embryos. In addition, mymk mutation hampered fish muscle growth. However, the effect of Jamb and Jamc deficiency on fish muscle growth is not clear. Moreover, whether jamb;jamc and jamb;mymk double mutations have stronger effects on myoblast fusion and muscle growth remains to be investigated. Here, we characterized the muscle development and growth in jamb, jamc, and mymk single and double mutants in zebrafish. We found that although myoblast fusion was compromised in jamb and jamc single or jamb;jamc double mutants, these mutant fish showed no defect in muscle cell fusion during muscle growth. The mutant fish were able to grow into adults that were indistinguishable from the wild-type sibling. In contrast, the jamb;mymk double mutants exhibited a stronger muscle phenotype compared to the jamb and jamc single and double mutants. The jamb;mymk double mutant showed reduced growth and partial lethality, similar to a mymk single mutant. Single fiber analysis of adult skeletal myofibers revealed that jamb, jamc, or jamb;jamc mutants contained mainly multinucleated myofibers, whereas jamb;mymk double mutants contained mostly mononucleated fibers. Significant intramuscular adipocyte infiltration was found in skeletal muscles of the jamb;mymk mutant. Collectively, these studies demonstrate that although Jamb, Jamc, and Mymk are all involved in myoblast fusion during early myogenesis, they have distinct roles in myoblast fusion during muscle growth. While Mymk is essential for myoblast fusion during both muscle development and growth, Jamb and Jamc are dispensable for myoblast fusion during muscle growth.
DOI:10.1093/nar/gkn340URLPMID:18511462 [本文引用: 1]

Four myogenic regulatory factors (MRFs); MyoD, Myf-5, MRF4 and Myogenin direct muscle tissue differentiation. Heterodimers of MRFs with E-proteins activate muscle-specific gene expression by binding to E-box motifs d(CANNTG) in their promoters or enhancers. We showed previously that in contrast to the favored binding of E-box by MyoD-E47 heterodimers, homodimeric MyoD associated preferentially with quadruplex structures of regulatory sequences of muscle-specific genes. To inquire whether other MRFs shared the DNA binding preferences of MyoD, the DNA affinities of hetero- and homo-dimeric MyoD, MRF4 and Myogenin were compared. Similarly to MyoD, heterodimers with E47 of MRF4 or Myogenin bound E-box more tightly than quadruplex DNA. However, unlike homodimeric MyoD or MRF4, Myogenin homodimers associated weakly and nonpreferentially with quadruplex DNA. By reciprocally switching basic regions between MyoD and Myogenin we demonstrated dominance of MyoD in determining the quadruplex DNA-binding affinity. Thus, Myogenin with an implanted MyoD basic region bound quadruplex DNA nearly as tightly as MyoD. However, a grafted Myogenin basic region did not diminish the high affinity of homodimeric MyoD for quadruplex DNA. We speculate that the dissimilar interaction of MyoD and Myogenin with tetrahelical domains in muscle gene promoters may differently regulate their myogenic activities.
DOI:10.1038/s41419-019-1993-3URLPMID:31601787 [本文引用: 1]

Adult skeletal muscle regeneration after injury depends on normal myoblast function. However, the intrinsic mechanisms for the control of myoblast behaviors are not well defined. Herein, we identified Pim1 kinase as a novel positive regulator of myoblast behaviors in vitro and muscle regeneration in vivo. Specifically, knockdown of Pim1 significantly restrains the proliferation and accelerates the apoptosis of myoblasts in vitro, indicating that Pim1 is critical for myoblast survival and amplification. Meanwhile, we found that Pim1 kinase is increased and translocated from cytoplasm into nucleus during myogenic differentiation. By using Pim1 kinase inhibitor, we proved that inhibition of Pim1 activity prevents myoblast differentiation and fusion, suggesting the necessity of Pim1 kinase activity for proper myogenesis. Mechanistic studies demonstrated that Pim1 kinase interacts with myogenic regulator MyoD and controls its transcriptional activity, inducing the expression of muscle-specific genes, which consequently promotes myogenic differentiation. Additionally, in skeletal muscle injury mouse model, deletion of Pim1 hinders the regeneration of muscle fibers and the recovery of muscle strength. Taken together, our study provides a potential target for the manipulation of myoblast behaviors in vitro and the myoblast-based therapeutics of skeletal muscle injury.
DOI:10.1091/mbc.e06-08-0766URLPMID:17332503 [本文引用: 1]

Cadherins are transmembrane glycoproteins that mediate Ca(2+)-dependent homophilic cell-cell adhesion and play crucial role during skeletal myogenesis. M-cadherin is required for myoblast fusion into myotubes, but its mechanisms of action remain unknown. The goal of this study was to cast some light on the nature of the M-cadherin-mediated signals involved in myoblast fusion into myotubes. We found that the Rac1 GTPase activity is increased at the time of myoblast fusion and it is required for this process. Moreover, we showed that M-cadherin-dependent adhesion activates Rac1 and demonstrated the formation of a multiproteic complex containing M-cadherin, the Rho-GEF Trio, and Rac1 at the onset of myoblast fusion. Interestingly, Trio knockdown efficiently blocked both the increase in Rac1-GTP levels, observed after M-cadherin-dependent contact formation, and myoblast fusion. We conclude that M-cadherin-dependent adhesion can activate Rac1 via the Rho-GEF Trio at the time of myoblast fusion.
DOI:10.1083/jcb.200202034URLPMID:12213839 [本文引用: 1]

N-cadherin, a member of the Ca(2+)-dependent cell-cell adhesion molecule family, plays an essential role in skeletal muscle cell differentiation. We show that inhibition of N-cadherin-dependent adhesion impairs the upregulation of the two cyclin-dependent kinase inhibitors p21 and p27, the expression of the muscle-specific genes myogenin and troponin T, and C2C12 myoblast fusion. To determine the nature of N-cadherin-mediated signals involved in myogenesis, we investigated whether N-cadherin-dependent adhesion regulates the activity of Rac1, Cdc42Hs, and RhoA. N-cadherin-dependent adhesion decreases Rac1 and Cdc42Hs activity, and as a consequence, c-jun NH2-terminal kinase (JNK) MAPK activity but not that of the p38 MAPK pathway. On the other hand, N-cadherin-mediated adhesion increases RhoA activity and activates three skeletal muscle-specific promoters. Furthermore, RhoA activity is required for beta-catenin accumulation at cell-cell contact sites. We propose that cell-cell contacts formed via N-cadherin trigger signaling events that promote the commitment to myogenesis through the positive regulation of RhoA and negative regulation of Rac1, Cdc42Hs, and JNK activities.
DOI:10.1016/s1534-5807(03)00118-7URLPMID:12737803 [本文引用: 1]

The mechanisms that regulate the formation of multinucleated muscle fibers from mononucleated myoblasts are not well understood. We show here that extracellular matrix (ECM) receptors of the beta1 integrin family regulate myoblast fusion. beta1-deficient myoblasts adhere to each other, but plasma membrane breakdown is defective. The integrin-associated tetraspanin CD9 that regulates cell fusion is no longer expressed at the cell surface of beta1-deficient myoblasts, suggesting that beta1 integrins regulate the formation of a protein complex important for fusion. Subsequent to fusion, beta1 integrins are required for the assembly of sarcomeres. Other ECM receptors such as the dystrophin glycoprotein complex are still expressed but cannot compensate for the loss of beta1 integrins, providing evidence that different ECM receptors have nonredundant functions in skeletal muscle fibers.
DOI:10.1126/science.273.5272.248URLPMID:8662510 [本文引用: 1]

C2 domains are found in many proteins involved in membrane traffic or signal transduction. Although C2 domains are thought to bind calcium ions, the structural basis for calcium binding is unclear. Analysis of calcium binding to C2 domains of synaptotagmin I and protein kinase C-beta by nuclear magnetic resonance spectroscopy revealed a bipartite calcium-binding motif that involves the coordination of two calcium ions by five aspartate residues located on two separate loops. Sequence comparisons indicated that this may be a widely used calcium-binding motif, designated here as the C2 motif.
DOI:10.1016/0092-8674(95)90296-1URLPMID:7697723 [本文引用: 1]

C2 domains are regulatory sequence motifs that occur widely in nature. Synaptotagmin I, a synaptic vesicle protein involved in the Ca2+ regulation of exocytosis, contains two C2 domains, the first of which acts as a Ca2+ sensor. We now describe the three-dimensional structure of this C2 domain at 1.9 A resolution in both the Ca(2+)-bound and Ca(2+)-free forms. The C2 polypeptide forms an eight-stranded beta sandwich constructed around a conserved four-stranded motif designated as a C2 key. Ca2+ binds in a cup-shaped depression between two polypeptide loops located at the N- and C-termini of the C2-key motif.
DOI:10.1074/jbc.M201858200URLPMID:11959863 [本文引用: 1]

Mutations in dysferlin, a novel membrane protein of unknown function, lead to muscular dystrophy. Myoferlin is highly homologous to dysferlin and like dysferlin is a plasma membrane protein with six C2 domains highly expressed in muscle. C2 domains are found in a variety of membrane-associated proteins where they have been implicated in calcium, phospholipid, and protein-binding. We investigated the pattern of dysferlin and myoferlin expression in a cell culture model of muscle development and found that dysferlin is expressed in mature myotubes. In contrast, myoferlin is highly expressed in elongated "prefusion" myoblasts and is decreased in mature myotubes where dysferlin expression is greatest. We tested ferlin C2 domains for their ability to bind phospholipid in a calcium-sensitive manner. We found that C2A, the first C2 domain of dysferlin and myoferlin, bound 50% phosphatidylserine and that phospholipid binding was regulated by calcium concentration. A dysferlin point mutation responsible for muscular dystrophy was engineered into the dysferlin C2A domain and demonstrated reduced calcium-sensitive phospholipid binding. Based on these data, we propose a mechanism for muscular dystrophy in which calcium-regulated phospholipid binding is abnormal, leading to defective maintenance and repair of muscle membranes.
DOI:10.1242/dev.02155URLPMID:16280346 [本文引用: 1]

Muscle growth occurs during embryonic development and continues in adult life as regeneration. During embryonic muscle growth and regeneration in mature muscle, singly nucleated myoblasts fuse to each other to form myotubes. In muscle growth, singly nucleated myoblasts can also fuse to existing large, syncytial myofibers as a mechanism of increasing muscle mass without increasing myofiber number. Myoblast fusion requires the alignment and fusion of two apposed lipid bilayers. The repair of muscle plasma membrane disruptions also relies on the fusion of two apposed lipid bilayers. The protein dysferlin, the product of the Limb Girdle Muscular Dystrophy type 2 locus, has been shown to be necessary for efficient, calcium-sensitive, membrane resealing. We now show that the related protein myoferlin is highly expressed in myoblasts undergoing fusion, and is expressed at the site of myoblasts fusing to myotubes. Like dysferlin, we found that myoferlin binds phospholipids in a calcium-sensitive manner that requires the first C2A domain. We generated mice with a null allele of myoferlin. Myoferlin null myoblasts undergo initial fusion events, but they form large myotubes less efficiently in vitro, consistent with a defect in a later stage of myogenesis. In vivo, myoferlin null mice have smaller muscles than controls do, and myoferlin null muscle lacks large diameter myofibers. Additionally, myoferlin null muscle does not regenerate as well as wild-type muscle does, and instead displays a dystrophic phenotype. These data support a role for myoferlin in the maturation of myotubes and the formation of large myotubes that arise from the fusion of myoblasts to multinucleate myotubes.
DOI:10.1016/j.ydbio.2017.01.019URLPMID:28161523 [本文引用: 4]

During skeletal muscle development, myocytes aggregate and fuse to form multinucleated muscle fibers. Inhibition of myocyte fusion is thought to significantly derail the differentiation of functional muscle fibers. Despite the purported importance of fusion in myogenesis, in vivo studies of this process in vertebrates are rather limited. Myomaker, a multipass transmembrane protein, has been shown to be the first muscle-specific fusion protein essential for myocyte fusion in the mouse. We have generated loss-of-function alleles in zebrafish myomaker, and found that fusion of myocytes into syncytial fast-twitch muscles was significantly compromised. However, mutant myocytes could be recruited to fuse with wild-type myocytes in chimeric embryos, albeit rather inefficiently. Conversely, overexpression of Myomaker was sufficient to induce hyperfusion among fast-twitch myocytes, and it also induced fusion among slow-twitch myocytes that are normally fusion-incompetent. In line with this, Myomaker overexpression also triggered fusion in another myocyte fusion mutant compromised in the function of the junctional cell adhesion molecule, Jam2a. We also provide evidence that Rac, a regulator of actin cytoskeleton, requires Myomaker activity to induce fusion, and that an approximately 3kb of myomaker promoter sequence, with multiple E-box motifs, is sufficient to direct expression within the fast-twitch muscle lineage. Taken together, our findings underscore a conserved role for Myomaker in vertebrate myocyte fusion. Strikingly, and in contrast to the mouse, homozygous myomaker mutants are viable and do not exhibit discernible locomotory defects. Thus, in the zebrafish, myocyte fusion is not an absolute requirement for skeletal muscle morphogenesis and function.
DOI:10.1016/j.bbrc.2014.07.093URLPMID:25078621 [本文引用: 2]

Myomaker (also called Tmem8c), a new membrane activator of myocyte fusion was recently discovered in mice. Using whole mount in situ hybridization on zebrafish embryos at different stages of embryonic development, we show that myomaker is transiently expressed in fast myocytes forming the bulk of zebrafish myotome. Zebrafish embryos injected with morpholino targeted against myomaker were alive after yolk resorption and appeared morphologically normal, but they were unable to swim, even under effect of a tactile stimulation. Confocal observations showed a marked phenotype characterized by the persistence of mononucleated muscle cells in the fast myotome at developmental stages where these cells normally fuse to form multinucleated myotubes. This indicates that myomaker is essential for myocyte fusion in zebrafish. Thus, there is an evolutionary conservation of myomaker expression and function among Teleostomi.
DOI:10.2220/biomedres.38.215URLPMID:28637957 [本文引用: 3]

The Gm7325 gene, bioinformatically identified in the mouse genome, encodes a small protein but has not been characterized until recently. Our gene expression analysis revealed that Gm7325 transcription is remarkably upregulated in injured skeletal muscle tissues. Activated satellite cells and immature myotubes were densely decorated with positive signals for Gm7325 mRNA in in situ hybridization analysis, while no obvious signals were observed in quiescent satellite cells and mature myofibers. In the 5'-flanking regions of mouse Gm7325 and its human homologue, conserved E-box motifs for helix-loop-helix transcription factors are repeatedly arranged around the putative promoter regions. Reporter gene assays suggested that MyoD, a master transcription factor for myogenesis, binds to the conserved E-box motifs to activate Gm7325 expression. Therefore, Gm7325, as a novel MyoD-target gene, is specifically induced in activated satellite cells, and may have an important role in skeletal myogenesis.
DOI:10.1016/j.abb.2017.05.020URLPMID:28579197 [本文引用: 2]

The myogenesis of skeletal muscle has several stages, including satellite cell proliferation, differentiation, fusion and specific muscle formation. Recent studies have shown that myomaker, a muscle-specific transmembrane protein, was critical for myoblasts fusion. However, the regulatory mechanism of myomaker and its effects on myogenesis remain elusive. In this study, miR-491 was identified as a post-transcriptional regulator of myomaker, which binds specifically to its 3' untranslated region leading to its down-regulation. At the end of myotube differentiation, the expression levels of miR-491 increased drastically, while myomaker was significantly down-regulated, which indicated that miR-491 shut down the expression of myomaker. Functional studies showed that miR-491 overexpression suppressed muscle cell differentiation and adult muscle regeneration, while the inhibition of miR-491 promoted myotube differentiation. Taken together, our findings identified miR-491 as a novel negative regulator of myogenic differentiation through targeting myomaker.
DOI:10.3390/ijms161125946URLPMID:26540045 [本文引用: 6]

The fusion of myoblasts is an important step during skeletal muscle differentiation. A recent study in mice found that a transmembrane protein called Myomaker, which is specifically expressed in muscle, is critical for myoblast fusion. However, the cellular mechanism of its roles and the regulatory mechanism of its expression remain unclear. Chicken not only plays an important role in meat production but is also an ideal model organism for muscle development research. Here, we report that Myomaker is also essential for chicken myoblast fusion. Forced expression of Myomaker in chicken primary myoblasts promotes myoblast fusion, whereas knockdown of Myomaker by siRNA inhibits myoblast fusion. MYOD and MYOG, which belong to the family of myogenic regulatory factors, can bind to a conserved E-box located proximal to the Myomaker transcription start site and induce Myomaker transcription. Additionally, miR-140-3p can inhibit Myomaker expression and myoblast fusion, at least in part, by binding to the 3' UTR of Myomaker in vitro. These findings confirm the essential roles of Myomaker in avian myoblast fusion and show that MYOD, MYOG and miR-140-3p can regulate Myomaker expression.
DOI:10.1038/ncomms16077URLPMID:28681861 [本文引用: 3]

Multinucleate cellular syncytial formation is a hallmark of skeletal muscle differentiation. Myomaker, encoded by Mymk (Tmem8c), is a well-conserved plasma membrane protein required for myoblast fusion to form multinucleated myotubes in mouse, chick, and zebrafish. Here, we report that autosomal recessive mutations in MYMK (OMIM 615345) cause Carey-Fineman-Ziter syndrome in humans (CFZS; OMIM 254940) by reducing but not eliminating MYMK function. We characterize MYMK-CFZS as a congenital myopathy with marked facial weakness and additional clinical and pathologic features that distinguish it from other congenital neuromuscular syndromes. We show that a heterologous cell fusion assay in vitro and allelic complementation experiments in mymk knockdown and mymkinsT/insT zebrafish in vivo can differentiate between MYMK wild type, hypomorphic and null alleles. Collectively, these data establish that MYMK activity is necessary for normal muscle development and maintenance in humans, and expand the spectrum of congenital myopathies to include cell-cell fusion deficits.
DOI:10.1074/jbc.M609368200URLPMID:17289669 [本文引用: 2]

Calcineurin (Cn) is a Ca(2+)/calmodulin-dependent serine/threonine phosphatase that regulates differentiation-specific gene expression in diverse tissues, including the control of fiber-type switching in skeletal muscle. Recent studies have implicated Cn signaling in diminishing skeletal muscle pathogenesis associated with muscle injury or disease-related muscle degeneration. For example, use of the Cn inhibitor cyclosporine A has been shown to delay muscle regeneration following toxin-induced injury and inhibit regeneration in the dystrophin-deficient mdx mouse model of Duchenne muscular dystrophy. In contrast, transgenic expression of an activated mutant of Cn in skeletal muscle was shown to increase utrophin expression and reduce overall disease pathology in mdx mice. Here we examine the effect of altered Cn activation in the context of the delta-sarcoglycan-null (scgd(-/-)) mouse model of limb-girdle muscular dystrophy. In contrast to results discussed in mdx mice, genetic deletion of a loxP-targeted calcineurin B1 (CnB1) gene using a skeletal muscle-specific cre allele in the scgd(-/-) background substantially reduced skeletal muscle degeneration and histopathology compared with the scgd(-/-) genotype alone. A similar regression in scgd-dependent disease manifestation was also observed in calcineurin Abeta (CnAbeta) gene-targeted mice in both skeletal muscle and heart. Conversely, increased Cn expression using a muscle-specific transgene increased cardiac fibrosis, decreased cardiac ventricular shortening, and increased muscle fiber loss in the quadriceps. Our results suggest that inhibition of Cn may benefit select types of muscular dystrophy.
DOI:10.1074/jbc.M117.811372URLPMID:28860190 [本文引用: 2]

Multinucleated skeletal muscle fibers form through the fusion of myoblasts during development and regeneration. Previous studies identified myomaker (Tmem8c) as a muscle-specific membrane protein essential for fusion. However, the specific function of myomaker and how its function is regulated are unknown. To explore these questions, we first examined the cellular localization of endogenous myomaker. Two independent antibodies showed that whereas myomaker does localize to the plasma membrane in cultured myoblasts, the protein also resides in the Golgi and post-Golgi vesicles. These results raised questions regarding the precise cellular location of myomaker function and mechanisms that govern myomaker trafficking between these cellular compartments. Using a synchronized fusion assay, we demonstrated that myomaker functions at the plasma membrane to drive fusion. Trafficking of myomaker is regulated by palmitoylation of C-terminal cysteine residues that allows Golgi localization. Moreover, dissection of the C terminus revealed that palmitoylation was not sufficient for complete fusogenic activity suggesting a function for other amino acids within this C-terminal region. Indeed, C-terminal mutagenesis analysis highlighted the importance of a C-terminal leucine for function. These data reveal that myoblast fusion requires myomaker activity at the plasma membrane and is potentially regulated by proper myomaker trafficking.
DOI:10.1016/j.devcel.2018.08.006URLPMID:30197239 [本文引用: 4]

Classic mechanisms for membrane fusion involve transmembrane proteins that assemble into complexes and dynamically alter their conformation to bend membranes, leading to mixing of membrane lipids (hemifusion) and fusion pore formation. Myomaker and Myomerger govern myoblast fusion and muscle formation but are structurally divergent from traditional fusogenic proteins. Here, we show that Myomaker and Myomerger independently mediate distinct steps in the fusion pathway, where Myomaker is involved in membrane hemifusion and Myomerger is necessary for fusion pore formation. Mechanistically, we demonstrate that Myomerger is required on the cell surface where its ectodomains stress membranes. Moreover, we show that Myomerger drives fusion completion in a heterologous system independent?of Myomaker and that a Myomaker-Myomerger?physical interaction is not required for function. Collectively, our data identify a stepwise cell fusion mechanism in myoblasts where different proteins are delegated to perform unique membrane functions essential for membrane coalescence.
DOI:10.1073/pnas.1715229114URLPMID:29078404 [本文引用: 1]

Skeletal muscle formation requires fusion of mononucleated myoblasts to form multinucleated myofibers. The muscle-specific membrane proteins myomaker and myomixer cooperate to drive mammalian myoblast fusion. Whereas myomaker is highly conserved across diverse vertebrate species, myomixer is a micropeptide that shows relatively weak cross-species conservation. To explore the functional conservation of myomixer, we investigated the expression and function of the zebrafish myomixer ortholog. Here we show that myomixer expression during zebrafish embryogenesis coincides with myoblast fusion, and genetic deletion of myomixer using CRISPR/Cas9 mutagenesis abolishes myoblast fusion in vivo. We also identify myomixer orthologs in other species of fish and reptiles, which can cooperate with myomaker and substitute for the fusogenic activity of mammalian myomixer. Sequence comparison of these diverse myomixer orthologs reveals key amino acid residues and a minimal fusogenic peptide motif that is necessary for promoting cell-cell fusion with myomaker. Our findings highlight the evolutionary conservation of the myomaker-myomixer partnership and provide insights into the molecular basis of myoblast fusion.
DOI:10.1101/cshperspect.a029298URLPMID:28062562 [本文引用: 1]

Development of skeletal muscle is a multistage process that includes lineage commitment of multipotent progenitor cells, differentiation and fusion of myoblasts into multinucleated myofibers, and maturation of myofibers into distinct types. Lineage-specific transcriptional regulation lies at the core of this process, but myogenesis is also regulated by extracellular cues. Some of these cues are initiated by direct cell-cell contact between muscle precursor cells themselves or between muscle precursors and cells of other lineages. Examples of the latter include interaction of migrating neural crest cells with multipotent muscle progenitor cells, muscle interstitial cells with myoblasts, and neurons with myofibers. Among the signaling factors involved are Notch ligands and receptors, cadherins, Ig superfamily members, and Ephrins and Eph receptors. In this article we describe recent progress in this area and highlight open questions raised by the findings.
DOI:10.1038/ng2055URLPMID:17529975 [本文引用: 1]

Skeletal muscles arise by fusion of precursor cells, myoblasts, into multinucleated fibers. In vertebrates, mechanisms controlling this essential step in myogenesis remain poorly understood. Here we provide evidence that Kirrel, a homolog of receptor proteins that organize myoblast fusion in Drosophila melanogaster, is necessary for muscle precursor fusion in zebrafish. Within developing somites, Kirrel expression localized to membranes of fusion-competent myoblasts of the fast-twitch lineage. Unlike wild-type myoblasts that form spatially arrayed syncytial (multinucleated) fast myofibers, those deficient in Kirrel showed a significant reduction in fusion capacity. Inhibition of Rac, a GTPase and the most downstream intracellular transducer of the fusion signal in D. melanogaster, also compromised fast-muscle precursor fusion in zebrafish. However, unlike in D. melanogaster, constitutive Rac activation in zebrafish led to hyperfused giant syncytia, highlighting an entirely new function for this protein in zebrafish for gating the number and polarity of fusion events. These findings uncover a substantial degree of evolutionary conservation in the genetic regulation of myoblast fusion.
DOI:10.1371/journal.pbio.1001216URLPMID:22180726 [本文引用: 1]

Cellular fusion is required in the development of several tissues, including skeletal muscle. In vertebrates, this process is poorly understood and lacks an in vivo-validated cell surface heterophilic receptor pair that is necessary for fusion. Identification of essential cell surface interactions between fusing cells is an important step in elucidating the molecular mechanism of cellular fusion. We show here that the zebrafish orthologues of JAM-B and JAM-C receptors are essential for fusion of myocyte precursors to form syncytial muscle fibres. Both jamb and jamc are dynamically co-expressed in developing muscles and encode receptors that physically interact. Heritable mutations in either gene prevent myocyte fusion in vivo, resulting in an overabundance of mononuclear, but otherwise overtly normal, functional fast-twitch muscle fibres. Transplantation experiments show that the Jamb and Jamc receptors must interact between neighbouring cells (in trans) for fusion to occur. We also show that jamc is ectopically expressed in prdm1a mutant slow muscle precursors, which inappropriately fuse with other myocytes, suggesting that control of myocyte fusion through regulation of jamc expression has important implications for the growth and patterning of muscles. Our discovery of a receptor-ligand pair critical for fusion in vivo has important implications for understanding the molecular mechanisms responsible for myocyte fusion and its regulation in vertebrate myogenesis.
DOI:10.1128/mcb.22.13.4760-4770.2002URLPMID:12052883 [本文引用: 1]

M-cadherin is a classical calcium-dependent cell adhesion molecule that is highly expressed in developing skeletal muscle, satellite cells, and cerebellum. Based on its expression pattern and observations in cell culture, it has been postulated that M-cadherin may be important for the fusion of myoblasts to form myotubes, the correct localization and function of satellite cells during muscle regeneration, and the specialized architecture of adhering junctions in granule cells of cerebellar glomeruli. In order to investigate the potential roles of M-cadherin in vivo, we generated a null mutation in mice. Mutant mice were viable and fertile and showed no gross developmental defects. In particular, the skeletal musculature appeared essentially normal. Moreover, muscle lesions induced by necrosis were efficiently repaired in mutant mice, suggesting that satellite cells are present, can be activated, and are able to form new myofibers. This was also confirmed by normal growth and fusion potential of mutant satellite cells cultured in vitro. In the cerebellum of M-cadherin-lacking mutants, typical contactus adherens junctions were present and similar in size and numbers to the equivalent junctions in wild-type animals. However, the adhesion plaques in the cerebellum of these mutants appeared to contain elevated levels of N-cadherin compared to wild-type animals. Taken together, these observations suggest that M-cadherin in the mouse serves no absolutely required function during muscle development and regeneration and is not essential for the formation of specialized cell contacts in the cerebellum. It seems that N-cadherin or other cadherins can largely compensate for the lack of M-cadherin.
DOI:10.1083/jcb.138.2.331URLPMID:9230075 [本文引用: 1]

Myoblast fusion is essential to muscle tissue development yet remains poorly understood. N-cadherin, like other cell surface adhesion molecules, has been implicated by others in muscle formation based on its pattern of expression and on inhibition of myoblast aggregation and fusion by antibodies or peptide mimics. Mice rendered homozygous null for N-cadherin revealed the general importance of the molecule in early development, but did not test a role in skeletal myogenesis, since the embryos died before muscle formation. To test genetically the proposed role of N-cadherin in myoblast fusion, we successfully obtained N-cadherin null primary myoblasts in culture. Fusion of myoblasts expressing or lacking N-cadherin was found to be equivalent, both in vitro by intracistronic complementation of lacZ and in vivo by injection into the muscles of adult mice. An essential role for N-cadherin in mediating the effects of basic fibroblast growth factor was also excluded. These methods for obtaining genetically homozygous null somatic cells from adult tissues should have broad applications. Here, they demonstrate clearly that the putative fusion molecule, N-cadherin, is not essential for myoblast fusion.
DOI:10.1016/j.expneurol.2016.06.026URLPMID:27349407 [本文引用: 1]

Mutations in the dysferlin gene are linked to a group of muscular dystrophies known as dysferlinopathies. These myopathies are characterized by progressive atrophy. Studies in muscle tissue from dysferlinopathy patients or dysferlin-deficient mice point out its importance in membrane repair. However, expression of dysferlin homologous proteins that restore sarcolemma repair function in dysferlinopathy animal models fail to arrest muscle wasting, therefore suggesting that dysferlin plays other critical roles in muscle function. In the present review, we discuss dysferlin functions in the skeletal muscle, as well as pathological mechanisms related to dysferlin mutations. Particular focus is presented related the effect of dysferlin on cell membrane related function, which affect its repair, vesicle trafficking, as well as Ca(2+) homeostasis. Such mechanisms could provide accessible targets for pharmacological therapies.
DOI:10.1074/jbc.M601885200URLPMID:16608842 [本文引用: 1]

Mutations in dysferlin cause a type of muscular dystrophy known as dysferlinopathy. Dysferlin may be involved in muscle repair and differentiation. We compared normal human skeletal muscle cultures expressing dysferlin with muscle cultures from dysferlinopathy patients. We quantified the fusion index of myoblasts as a measure of muscle development and conducted optic and electronic microscopy, immunofluorescence, Western blot, flow cytometry, and real-time PCR at different developmental stages. Short interference RNA was used to corroborate the results obtained in dysferlin-deficient cultures. A luciferase reporter assay was performed to study myogenin activity in dysferlin-deficient cultures. Myoblasts fusion was consistently delayed as compared with controls whereas the proliferation rate did not change. Electron microscopy showed that control cultured cells at 10 days were fusiform, whereas dysferlin-deficient cells were star-shaped and large. After 15 days the normal multinucleated appearance and structured myofibrils were not present in dysferlin-deficient cells. Strikingly, myogenin was not detected in myotubes from dysferlin-deficient cultures using Western blot, and mRNA analysis showed low levels (p < 0.05) compared with controls. Flow cytometry and immunofluorescence also showed reduced levels of myogenin in dysferlin-deficient cultures. When the dysferlin gene was knocked down ( approximately 80%), myogenin mRNA leveled down to approximately 70%. MyoD and desmin mRNA levels in controls and dysferlin-deficient cultures were similar. The reporter luciferase assay demonstrated a low myogenin activity in dysferlin-deficient cultures. These results point to a functional link between dysferlin and myogenin, and both proteins may share a new signaling pathway involved in differentiation of skeletal muscle in vitro.
DOI:10.1038/nature12135URLPMID:23615608 [本文引用: 2]

Skeletal muscle arises from the fusion of precursor myoblasts into multinucleated myofibres. Although conserved transcription factors and signalling proteins involved in myogenesis have been identified, upstream regulators are less well understood. Here we report an unexpected discovery that the membrane protein BAI1, previously linked to recognition of apoptotic cells by phagocytes, promotes myoblast fusion. Endogenous BAI1 expression increased during myoblast fusion, and BAI1 overexpression enhanced myoblast fusion by means of signalling through ELMO/Dock180/Rac1 proteins. During myoblast fusion, a fraction of myoblasts within the population underwent apoptosis and exposed phosphatidylserine, an established ligand for BAI1 (ref. 3). Blocking apoptosis potently impaired myoblast fusion, and adding back apoptotic myoblasts restored fusion. Furthermore, primary human myoblasts could be induced to form myotubes by adding apoptotic myoblasts, even under normal growth conditions. Mechanistically, apoptotic cells did not directly fuse with the healthy myoblasts, rather the apoptotic cells induced a contact-dependent signalling with neighbours to promote fusion among the healthy myoblasts. In vivo, myofibres from Bai1(-/-) mice are smaller than those from wild-type littermates. Muscle regeneration after injury was also impaired in Bai1(-/-)mice, highlighting a role for BAI1 in mammalian myogenesis. Collectively, these data identify apoptotic cells as a new type of cue that induces signalling via the phosphatidylserine receptor BAI1 to promote fusion of healthy myoblasts, with important implications for muscle development and repair.
DOI:10.1038/ncomms10871URLPMID:26972991 [本文引用: 2]

Myoblast fusion is essential for the formation of skeletal muscle myofibres. Studies have shown that phosphatidylserine is necessary for myoblast fusion, but the underlying mechanism is not known. Here we show that the phosphatidylserine receptor stabilin-2 acts as a membrane protein for myoblast fusion during myogenic differentiation and muscle regeneration. Stabilin-2 expression is induced during myogenic differentiation, and is regulated by calcineurin/NFAT signalling in myoblasts. Forced expression of stabilin-2 in myoblasts is associated with increased myotube formation, whereas deficiency of stabilin-2 results in the formation of small, thin myotubes. Stab2-deficient mice have myofibres with small cross-sectional area and few myonuclei and impaired muscle regeneration after injury. Importantly, myoblasts lacking stabilin-2 have reduced phosphatidylserine-dependent fusion. Collectively, our results show that stabilin-2 contributes to phosphatidylserine-dependent myoblast fusion and provide new insights into the molecular mechanism by which phosphatidylserine mediates myoblast fusion during muscle growth and regeneration.
DOI:10.1111/febs.14261URLPMID:28881496 [本文引用: 1]

Xk-related protein 8 (Xkr8) is a scramblase and responsible for phosphatidylserine (PS) exposure on the cell surface in a caspase-dependent manner. Although PS exposure is found to be important for myotube formation during myoblast differentiation, the role of Xkr8 during myogenesis has not been elucidated. Here we show that Xkr8 contributes to myoblast differentiation. Xkr8 overexpression induced the formation of large myotubes during early differentiation, but this phenotype was not related to caspase-dependent cleavage of Xkr8. Furthermore, forced Xkr8 expression accelerated myoblast differentiation and conferred cell-death resistance after the induction of differentiation. Consistent with these results, Xkr8-knocked-down myoblasts exhibited impaired differentiation and more apoptotic cells during differentiation, implying the involvements of Xkr8 in the survival and proliferation of myoblasts. Taken together, the study shows Xkr8 influences myogenesis by acting as a positive regulator of terminal differentiation and myoblast survival.
DOI:10.1016/j.bbrc.2011.08.128URLPMID:21910971 [本文引用: 1]

Cell membrane consists of various lipids such as phosphatidylserine (PS), phosphatidylcholine (PC), and phosphatidylethanolamine (PE). Among them, PS is a molecular marker of apoptosis, because it is located to the inner leaflet of plasma membrane generally but it is moved to the outer leaflet during programmed cell death. The process of apoptosis has been implicated in the fusion of muscle progenitor cells, myoblasts, into myotubes. However, it remained unclear whether PS regulates muscle cell differentiation directly. In this paper, localization of PS to the outer leaflet of plasma membrane in proliferating primary myoblasts and during fusion of these myoblasts into myotubes is validated using Annexin V. Moreover, we show the presence of PS clusters at the cell-cell contact points, suggesting the importance of membrane ruffling and PS exposure for the myogenic cell fusion. Confirming this conclusion, experimentally constructed PS, but not PC liposomes dramatically enhance the formation of myotubes from myoblasts, thus demonstrating a direct positive effect of PS on the muscle cell fusion. In contrast, myoblasts exposed to PC liposomes produce long myotubes with low numbers of myonuclei. Moreover, pharmacological masking of PS on the myoblast surface inhibits fusion of these cells into myotubes in a dose-dependent manner.
DOI:10.1016/j.ceb.2009.01.029URL [本文引用: 1]

Cardiac and skeletal muscle development are controlled by evolutionarily conserved networks of transcription factors that coordinate the expression of genes involved in muscle growth, morphogenesis, differentiation, and contractility. In addition to regulating the expression of protein-coding genes, recent studies have revealed that myogenic transcription factors control the expression of a collection of microRNAs, which act through multiple mechanisms to modulate muscle development and function. In some cases, microRNAs fine-tune the expression of target mRNAs, whereas in other cases they function as ‘on–off’ switches. MicroRNA control of gene expression appears to be especially important during cardiovascular and skeletal muscle diseases, in which microRNAs participate in stress-dependent remodeling of striated muscle tissues. We review findings that point to the importance of microRNA-mediated control of gene expression during muscle development and disease, and consider the potential of microRNAs as therapeutic targets.
DOI:10.1042/CS20110634URLPMID:22888971 [本文引用: 1]

miRNAs (microRNAs) are novel post-transcriptional regulators of gene expression. Several miRNAs, expressed exclusively in muscle, play important roles during muscle development, growth and regeneration; other ubiquitously expressed miRNAs are also essential for muscle function. In the present review, we outline the miRNAs involved in embryonic muscle development and those that have been found to be dysregulated in diseases associated with skeletal muscle or are changed during muscle adaptation. miRNAs are promising biomarkers and candidates for potential therapeutic intervention. We discuss the strategies that aim to develop novel therapies through modulating miRNA activity. In time, some of these approaches may become available to treat muscle-associated diseases.
DOI:10.16288/j.yczz.17-112URLPMID:29254922 [本文引用: 1]

MicroRNA (miRNA) is a class of short non-coding RNA, which is about 22 bp in length. In mammals, miRNA exerts its funtion through binding with the 3°-UTR region of target genes and inhibiting their translation. Skeletal muscle development is a complex event, including: proliferation, migration and differentiation of skeletal muscle stem cells; proliferation, differentiation and fusion of myocytes; as well as hypertrophy, energy metabolism and conversion of muscle fiber types. The miRNA plays important roles in all processes of skeletal muscle development through targeting the key factors of different stages. Herein we summarize the miRNA related to muscle development, providing a better understanding of the skeletal muscle development.
DOI:10.16288/j.yczz.17-112URLPMID:29254922 [本文引用: 1]

MicroRNA (miRNA) is a class of short non-coding RNA, which is about 22 bp in length. In mammals, miRNA exerts its funtion through binding with the 3°-UTR region of target genes and inhibiting their translation. Skeletal muscle development is a complex event, including: proliferation, migration and differentiation of skeletal muscle stem cells; proliferation, differentiation and fusion of myocytes; as well as hypertrophy, energy metabolism and conversion of muscle fiber types. The miRNA plays important roles in all processes of skeletal muscle development through targeting the key factors of different stages. Herein we summarize the miRNA related to muscle development, providing a better understanding of the skeletal muscle development.
DOI:10.1038/ng1725URLPMID:16380711 [本文引用: 2]

Understanding the molecular mechanisms that regulate cellular proliferation and differentiation is a central theme of developmental biology. MicroRNAs (miRNAs) are a class of regulatory RNAs of approximately 22 nucleotides that post-transcriptionally regulate gene expression. Increasing evidence points to the potential role of miRNAs in various biological processes. Here we show that miRNA-1 (miR-1) and miRNA-133 (miR-133), which are clustered on the same chromosomal loci, are transcribed together in a tissue-specific manner during development. miR-1 and miR-133 have distinct roles in modulating skeletal muscle proliferation and differentiation in cultured myoblasts in vitro and in Xenopus laevis embryos in vivo. miR-1 promotes myogenesis by targeting histone deacetylase 4 (HDAC4), a transcriptional repressor of muscle gene expression. By contrast, miR-133 enhances myoblast proliferation by repressing serum response factor (SRF). Our results show that two mature miRNAs, derived from the same miRNA polycistron and transcribed together, can carry out distinct biological functions. Together, our studies suggest a molecular mechanism in which miRNAs participate in transcriptional circuits that control skeletal muscle gene expression and embryonic development.
DOI:10.1126/science.1139089URLPMID:17379774 [本文引用: 1]

The heart responds to diverse forms of stress by hypertrophic growth accompanied by fibrosis and eventual diminution of contractility, which results from down-regulation of alpha-myosin heavy chain (alphaMHC) and up-regulation of betaMHC, the primary contractile proteins of the heart. We found that a cardiac-specific microRNA (miR-208) encoded by an intron of the alphaMHC gene is required for cardiomyocyte hypertrophy, fibrosis, and expression of betaMHC in response to stress and hypothyroidism. Thus, the alphaMHC gene, in addition to encoding a major cardiac contractile protein, regulates cardiac growth and gene expression in response to stress and hormonal signaling through miR-208.
DOI:10.1007/s12041-016-0737-8URLPMID:28360388 [本文引用: 1]

Transmembrane protein 8C (Tmem8C) is a muscle-specific membrane protein that controls myoblast fusion, which is essential for the formation of multinucleated muscle fibres. As most of the birds can fly, they have enormous requirement for the muscle, but there are only a few studies of Tmem8C in birds. In this study, we obtained the coding sequence (CDS) of Tmem8C in goose, predicted miRNAs that can act on the 3'UTR, analysed expression profiles of this gene in breast and leg muscles (BM and LM) during the embryonic period and neonatal stages, and identified miRNAs that might affect the targeted gene. The results revealed a high homology between Tmem8C in goose and other animals (indicated by sequence comparisons and phylogenetic trees), some conservative characteristics (e.g., six transmembrane domains and two E-boxes in the 5'UTR might be the potential binding sites of muscle regulatory factors (MRFs)), and the dN/dS ratio indicated purifying selection acting on this gene, facilitating conservatism in vertebrates. Q-PCR indicated Tmem8C had a peak expression pattern, reaching its highest expression levels in stage E15 in LM and E19 in BM, and then dropping transiently in E23 (P < 0.05). We examined 13 candidate miRNAs, and negative relationships were detected both in BM and LM (mir-125b-5p, mir-15a, mir-16-1 and mir-n23). Notably, mir-16-1 significantly decreased luciferase activity in dual luciferase reporter gene (LRG) assay, suggesting that it can be identified as potential factors affecting Tmem8C. This study investigated Tmem8C in water bird for the first time, and provided useful information about this gene and its candidate miRNAs in goose.
URLPMID:7738097 [本文引用: 1]

Duchenne muscular dystrophy is a primary muscle disease that manifests itself in young boys as a result of a defect in a gene located on the X-chromosome. This gene codes for dystrophin, a normal muscle protein that is located beneath the sarcolemma of muscle fibres. Therapies to alleviate this disease have centred on implanting normal muscle precursor cells into dystrophic fibres to compensate for the lack of this gene and its product. To date, donor cells for implantation in such therapy have been of myogenic origin, derived from paternal biopsies. Success in human muscle, however, has been limited and may reflect immune rejection problems. To overcome this problem the patient's own myogenic cells, with the dystrophin gene inserted, could be used, but this could lead to other problems, since these cells are those that are functionally compromised by the disease. Here, we report the presence of high numbers of dystrophin-positive fibres after implanting dermal fibroblasts from normal mice into the muscle of the mdx mouse-the genetic homologue of Duchenne muscular dystrophy. Dystrophin-positive fibres were also abundant in mdx muscle following the implantation of cloned dermal fibroblasts from the normal mouse. Our results suggest the in vivo conversion of these non-myogenic cells to the myogenic pathway resulting in the formation of dystrophin-positive muscle fibres in the deficient host. The use of dermal fibroblasts may provide an alternative approach to the previously attempted myoblast transfer therapy, which in human trials has yielded disappointing results.
DOI:10.1038/43919URLPMID:10517639 [本文引用: 1]

The development of cell or gene therapies for diseases involving cells that are widely distributed throughout the body has been severely hampered by the inability to achieve the disseminated delivery of cells or genes to the affected tissues or organ. Here we report the results of bone marrow transplantation studies in the mdx mouse, an animal model of Duchenne's muscular dystrophy, which indicate that the intravenous injection of either normal haematopoietic stem cells or a novel population of muscle-derived stem cells into irradiated animals results in the reconstitution of the haematopoietic compartment of the transplanted recipients, the incorporation of donor-derived nuclei into muscle, and the partial restoration of dystrophin expression in the affected muscle. These results suggest that the transplantation of different stem cell populations, using the procedures of bone marrow transplantation, might provide an unanticipated avenue for treating muscular dystrophy as well as other diseases where the systemic delivery of therapeutic cells to sites throughout the body is critical. Our studies also suggest that the inherent developmental potential of stem cells isolated from diverse tissues or organs may be more similar than previously anticipated.
DOI:10.1096/fj.201600945RURLPMID:27825107 [本文引用: 1]

Knowledge regarding cellular fusion and nuclear reprogramming may aid in cell therapy strategies for skeletal muscle diseases. An issue with cell therapy approaches to restore dystrophin expression in muscular dystrophy is obtaining a sufficient quantity of cells that normally fuse with muscle. Here we conferred fusogenic activity without transdifferentiation to multiple non-muscle cell types and tested dystrophin restoration in mouse models of muscular dystrophy. We previously demonstrated that myomaker, a skeletal muscle-specific transmembrane protein necessary for myoblast fusion, is sufficient to fuse 10T 1/2 fibroblasts to myoblasts in vitro. Whether myomaker-mediated heterologous fusion is functional in vivo and whether the newly introduced nonmuscle nuclei undergoes nuclear reprogramming has not been investigated. We showed that mesenchymal stromal cells, cortical bone stem cells, and tail-tip fibroblasts fuse to skeletal muscle when they express myomaker. These cells restored dystrophin expression in a fraction of dystrophin-deficient myotubes after fusion in vitro. However, dystrophin restoration was not detected in vivo although nuclear reprogramming of the muscle-specific myosin light chain promoter did occur. Despite the lack of detectable dystrophin reprogramming by immunostaining, this study indicated that myomaker could be used in nonmuscle cells to induce fusion with muscle in vivo, thereby providing a platform to deliver therapeutic material.-Mitani, Y., Vagnozzi, R. J., Millay, D. P. In vivo myomaker-mediated heterologous fusion and nuclear reprogramming.
DOI:10.3724/SP.J.1005.2013.01081URL [本文引用: 1]

Satellite cell is a kind of myogenic stem cells, which plays an important role in muscle development and injury repair. Through proliferation, differentiation and fusion of muscle fiber can satellite cells make new myonuclear, leading to the hypertrophy of skeletal muscle and fiber type transformation, and this would further affect the meat quality. Here, we review the relationship between muscle fiber development and meat quality attributes as well as the influence of the satellite cell differentiation on muscle fiber character. Besides, we also summarize the classical signaling pathway (i.e., Notch etc.) and influence of epigenetic regulation (i.e. miRNA) on muscle quality.
DOI:10.3724/SP.J.1005.2013.01081URL [本文引用: 1]

Satellite cell is a kind of myogenic stem cells, which plays an important role in muscle development and injury repair. Through proliferation, differentiation and fusion of muscle fiber can satellite cells make new myonuclear, leading to the hypertrophy of skeletal muscle and fiber type transformation, and this would further affect the meat quality. Here, we review the relationship between muscle fiber development and meat quality attributes as well as the influence of the satellite cell differentiation on muscle fiber character. Besides, we also summarize the classical signaling pathway (i.e., Notch etc.) and influence of epigenetic regulation (i.e. miRNA) on muscle quality.
