 ,浙江大学生命科学学院遗传与再生生物学研究所,浙江省细胞与基因工程重点研究实验室,杭州 310058
,浙江大学生命科学学院遗传与再生生物学研究所,浙江省细胞与基因工程重点研究实验室,杭州 310058Molecular mechanism of microRNA in regulating cochlear hair cell development
Lin Rao, Feilong Meng, Ran Fang, Chenyi Cai, Xiaoli Zhao ,Key Laboratory for Cell and Gene Engineering of Zhejiang Province, Institute of Genetics and Regenerative Biology, College of Life Sciences, Zhejiang University, Hangzhou 310058, China
,Key Laboratory for Cell and Gene Engineering of Zhejiang Province, Institute of Genetics and Regenerative Biology, College of Life Sciences, Zhejiang University, Hangzhou 310058, China通讯作者: 赵小立,副教授,硕士生导师,研究方向:干细胞分化。E-mail:zhaoxiaoli@zju.edu.cn
编委: 袁慧军
收稿日期:2019-07-20修回日期:2019-09-26网络出版日期:2019-11-20
| 基金资助: |
Editorial board:
Received:2019-07-20Revised:2019-09-26Online:2019-11-20
| Fund supported: |
作者简介 About authors
饶琳,硕士研究生,专业方向:干细胞分化。E-mail:

摘要
耳聋是严重影响人类生活质量的全球重大健康问题之一。目前,因耳蜗毛细胞损伤而导致的耳聋疾病尚未有成功的治疗方法。MicroRNA (miRNA)作为一类高度保守的内源性非编码小RNA,在耳蜗以及毛细胞发育过程中发挥着重要作用。本文介绍了miRNA在耳蜗毛细胞产生过程中的时空表达,揭示了其不可或缺的重要作用;同时阐述了miRNA参与调控耳蜗毛细胞发育中相关转录因子的分子机制,为耳聋的毛细胞移植治疗和毛细胞再生研究提供理论参考。
关键词:
Abstract
Deafness has become one of the most frequent health problems worldwide, and affects almost every age group. Hair cell damage or absence is the main cause of hearing loss, but there is no successful treatment to heal deafness. MicroRNA (miRNA), as a highly conserved endogenous non-coding small RNA, plays an important role in inner ear cochlea and hair cell development. In this review, we elaborate on the expression and function of miRNAs in cochlear hair cell development, and reveal its indispensable important role. We summarize the molecular mechanism of miRNA in regulating transcription factors involved in cochlear hair cell development, which may provide references and insights for hair cell regeneration in vivo and cellular transplantation therapy of deafness.
Keywords:
PDF (870KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
饶琳, 孟飞龙, 房冉, 蔡晨依, 赵小立. MicroRNA调控耳蜗毛细胞发育的分子机制. 遗传[J], 2019, 41(11): 994-1008 doi:10.16288/j.yczz.19-119
Lin Rao.
miRNA是一类高度保守的内源性非编码小RNA,通过抑制mRNA转录负调控靶基因的表达水平,从而参与调控细胞的生长发育、细胞信号转导、增殖分化、细胞凋亡、脂类代谢、蛋白质降解等过程[1]。1993年,在秀丽隐杆线虫(Caenorhabditis elegans)中最早发现miRNA基因lin-4[2]。它与lin-14 mRNA 3?-UTR的碱基序列部分互补,通过降解靶基因lin-14参与调控线虫的生长发育[3]。随后越来越多的miRNA在植物、无脊椎动物和脊椎动物的组织中被发现[4]。近几年的研究发现miRNA在动物耳蜗的各类细胞中表达丰富[5],已有研究表明miR-183家族在内耳毛细胞发育功能的调控中发挥了重要作用[6]。本文归纳总结了耳蜗毛细胞中主要miRNA的详细表达分布情况,并以miR-183家族的3个成员miR-96、miR-182和miR-183为主,分别阐述miRNA在内耳中的时空表达以及在内耳和毛细胞发育过程中参与调控的相关机制,旨在为进一步探索内耳毛细胞的发育分化、体外诱导及原位再生提供理论依据。
1 耳蜗中各类型细胞表达的miRNA
1.1 内耳的结构与功能
哺乳动物的耳是由外耳、中耳和内耳3个部分组成,内耳由负责感受声音的耳蜗和感受位置及运动觉的前庭器官组成[7]。耳蜗螺旋器(Corti器)坐落在基膜上,由感觉上皮(毛细胞)和支持细胞以及其他一些附属结构组成[8]。Corti器有3排外毛细胞(outer hair cell)和1排内毛细胞(inner ear hair cells)[9]。外毛细胞被称为“耳蜗放大器”,增强感觉上皮细胞对不同声音频率的响应能力,形成“机械—电—机械”的正反馈环路[10]。内毛细胞受到声音刺激,纤毛向外侧摆动,触发神经递质谷氨酸的释放,促使听神经传入冲动产生。声音冲动穿过传入神经到达耳蜗螺旋神经节(spiral ganglion),进一步传到听觉中枢,传达到大脑产生听觉[11]。耳蜗膜性结构包括基膜、前庭膜和盖膜3个部分。基膜是上皮组织基底面与深部结缔组织之间的一层薄膜,给耳蜗部分提供韧性和质量,基膜与耳蜗螺旋韧带的蜗管相连形成一定的功能联系[12]。前庭膜起始于蜗轴侧的螺旋缘,与基底膜成45°,由两层细胞组成的一层薄膜,该膜可调节离子和液体平衡的作用[13]。1.2 miRNA在耳蜗中的表达
miRNA与听觉功能密切相关,在耳蜗各类型的细胞中已经检测出超过100种miRNA[14],如miR- 183、miR-96、miR-182、miR-124、miR-34a、miR-376和miR-135b等[15]。其中,miR-96、miR-182和miR-183等在小鼠和人的基因组中成簇排列,并且都是朝向同一方向转录生成,所以将这3种miRNA统称为miR-183基因簇或miR-183家族[16]。在毛细胞和螺旋神经节中的miRNA种类较多,已被证实的有miR-183家族、miR-15a、miR-30b、miR-99a、miR-18a、miR-140和miR-194等[17]。在内螺旋沟也检测到miR-96、miR-182和miR-183共3个miRNA,而在螺旋缘除了检测到miR-183家族的3个miRNA成员,还检测到miR-205表达[18]。同时,miR-205也存在于前庭膜和耳蜗螺旋韧带上[19]。基膜上除了存在miR-205a,此外还高表达miR-15a、miR-30b和miR-99a等miRNA[20]。但是支持细胞只有miR-15a、miR-30b和miR-99a表达[21]。边缘细胞中存在miR-376a和miR-376b,这些miRNA在内耳的其他部位中没有检测出来[22]。除了上述提及的表达水平较高的miRNA,在已知成熟miRNA中有102种在耳蜗中表达,占全身miRNA总量的1/3[23]。组成耳蜗的细胞种类丰富,从miRNA的表达情况中可以看出一些组织和细胞存在着相同的miRNA[24],比如毛细胞、螺旋神经节、螺旋缘、内螺旋沟等组织都有miR-96、miR-182和miR- 183的存在,前庭膜、螺旋缘、耳蜗螺旋韧带、基膜等组织则都表达了miR-205a[25]。这些结果为进一步掌握耳蜗的发育过程以及不同细胞组织之间的协同作用提供了研究依据[26]。在耳蜗中不同细胞和组织中主要高度表达的miRNA的表达情况如图1所示。
图1
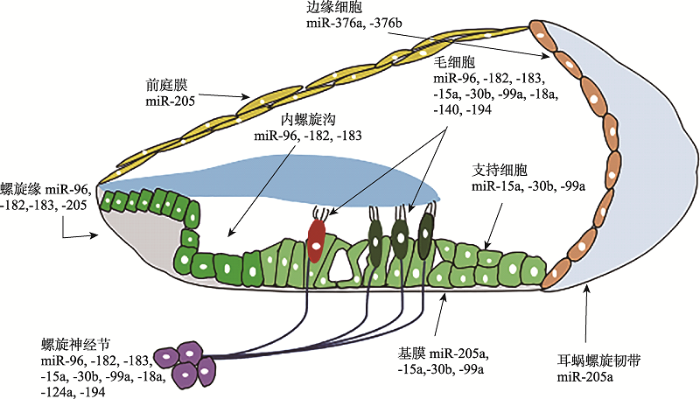 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1miRNA在耳蜗各类细胞中的表达
Fig. 1Expression of miRNA in the inner ear cochlea cells
2 miRNA在耳蜗发育过程中的时空表达
2.1 内耳的发育过程
脊椎动物的内耳发育起源于胚胎的外胚层[27]。听泡(otic vesicle)又称耳囊(otic capsule),起源于外胚层的听基板,在外胚层表面接近于神经板[28]。内耳的始基听泡发育产生于小鼠胚胎第8天(embryonic day 8, E8)至第11天,而人类在胚胎第4周末期才发育产生听泡[29]。在此发育阶段,内耳的平衡和听觉神经节也开始发育,该神经节是由内耳原始听泡的前腹内侧细胞从听泡分离并融合形成[30]。小鼠在E10.5~E14开始形成前庭和耳蜗,听泡脱离表面外胚层沉降到下方间充质内形成了听囊,听囊背侧发育为前庭部,而听囊腹侧发育为耳蜗部[31]。而感觉细胞的分化期,小鼠约在E13~E19,耳蜗上皮逐渐分化为感觉上皮,已有可分辨出的支持细胞和毛细胞[32]。出生时,前庭感觉器官发育已经接近于成熟,耳蜗已成型但体积比成熟期的耳蜗小[33]。出生后,前庭感觉器官、耳蜗逐渐发育成熟[34],小鼠出生后第30天(postnatal day 30, P30)左右内耳器官完全发育成熟[35]。2.2 miRNA在动物模型耳蜗中的时空表达
miRNAs的表达呈现时间、空间及组织细胞的特异性[36],表明其参与了组织的形态形成和细胞分化的过程[37]。由于人类的耳蜗组织不易获取,关于耳蜗miRNA的时空表达研究多局限于模式生物,再利用外推法来理解其在人类耳蜗中的具体功能[38]。在耳蜗领域最早进行研究的动物模型是小鼠,通过表达谱芯片分析小鼠耳蜗发育过程中不同时间点miRNA表达的状况[39]。在小鼠胚胎的整个发育过程中,miR-183和miR-182最早在胚胎期E9.5于听泡中表达。随着内耳在胚胎期的进一步发育,miR-183家族的3个成员在E11.5时出现表达差异,miR-182只有miR-182-5p表达,而在E12时miR-96、miR-182和miR-183呈现无差异表达,这可能反映了不同种类miRNA在内耳发育中的微小差异[40]。胚胎发育前期在miR-96、miR-182、miR-183听囊和螺旋神经节均有表达,E17.5时开始仅在毛细胞及其神经元中表达[41]。出生时(P0),耳蜗毛细胞中检测到了miR-183家族、miR-15a*、miR-18a*、miR-30a*、miR-99a*、miR-199a*、miR-200*等诸多miRNAs的表达[42]。其中miR-183家族在小鼠出生后4-5天还存在于感觉前体细胞中,随后集中在耳蜗毛细胞呈现高度表达状态[43]。在P30时小鼠耳蜗已完全发育,此时在毛细胞中仍然可以检测到miR-183家族的表达[44]。从新生小鼠的耳蜗检测出的miRNA表达谱开始,经过听觉功能的发育和成熟,miRNA并没有发生实质性的改变,这表明miRNA的表达在很大程度上是在胚胎发育过程中建立起来的。从耳蜗发育的整个过程上看,miR-183、miR-96和miR-182的表达呈现出了时空组织的特异性,这种时间和空间上的表达与耳蜗的功能成熟密切相关[45]。miRNA家族时空表达的特异性见图2所示。图2
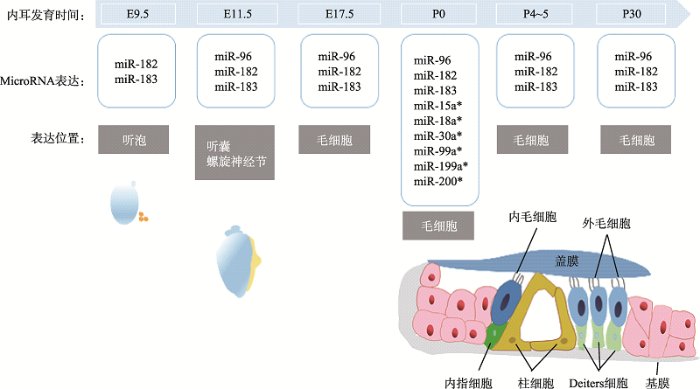 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2miR-183家族在小鼠耳蜗发育过程中表达的时间图
E为胚胎期,P为出生后。
Fig. 2Time diagram of miR-183 family expression during mouse inner ear cochlear development
3 miR-183家族与毛细胞发育
3.1 毛细胞概述
人类内耳约有15 000个毛细胞,其中作为听觉感受器的耳蜗毛细胞约有3000个[46]。耳蜗毛细胞是分化成熟、高度特异性的终末细胞,哺乳动物毛细胞在出生后再生能力非常有限,听觉毛细胞损伤后很难分化形成新的毛细胞[47]。遗传或者获得性因素如年龄增长、耳毒性药物、病毒感染、噪音和外伤等都会使毛细胞受到损伤[48],从而造成感音神经性耳聋(sensorineural hearing loss, SNHL)[49]。长期以来,感音神经性耳聋患者改善听力的选择仅仅限于助听器、人工耳蜗等设备,但这些方法无法从根本上解决问题[50]。因此,研究毛细胞的发育和再生的机制,可用于指导体外诱导干细胞分化为类毛细胞的研究,并通过细胞移植替换受损毛细胞,为治疗耳聋疾病带来新曙光[51]。3.2 miR-183家族
目前在耳蜗毛细胞的miRNA研究中,miR-183家族的研究比较深入[52]。这个家族在进化过程中具有高度保守性,在结构上具有高度同源性(图3)。miR-183和miR-96之间有约1 kb的间隔区,miR-96和miR-182之间有约2.7~3.5 kb的间隔区。尽管3者之间的序列具有高度的相似性,但是其中微小的序列差异导致它们拥有不同的mRNA靶标。miR-183家族是最先被报道参与了纤毛化的感觉上皮细胞和神经纤毛细胞的器官发生和发育功能的基因簇[53],它们在某些器官如眼睛、鼻子和内耳中有特殊的表达,对动物感觉器官的发育和功能的形成至关重要[54]。图3
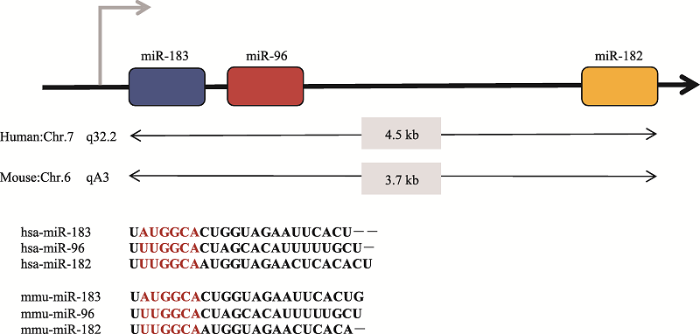 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3miR-183家族基因簇在人和小鼠中的染色体位置及种子序列
红色部分为microRNA种子系列。
Fig. 3Chromosome positions and seed sequences of miR-183 family gene clusters in human and mouse
3.2.1 miR-96
miR-96首先在人类癌细胞中被检测到,是miR-183家族中第一个被发现的miRNA成员[55]。miR-96是一种感觉器官特异性的miRNA,在哺乳动物耳蜗发育期间表达,可导致Gfi1、Ptprq和Tmc1等重要发育基因表达下调。miR-96的种子区域的点突变会引起DNA序列多态性,导致人和小鼠常染色体显性非综合征性耳聋(non-syndromic hearing loss, NSHL)[56]。miR-96的种子序列第4个碱基G>A的突变,是第一个被发现的与遗传性耳聋相关的miRNA突变。Mencia等[57]从遗传性耳聋家系中证实+13G>A和+14C>A两个种子区域点突变也会影响成熟的miR-96与靶基因的结合效率,从而导致其对耳蜗毛细胞的调节失衡,最终引起了耳聋产生。Lewis等[58]利用强致癌剂N-亚硝基-N-乙基脲(N-ethyl-N-nitrosourea, ENU)致小鼠听力损失,进一步对miR-96的种子区域点突变进行研究,发现有的突变体小鼠完全听力丧失并且毛细胞纤毛束不规则。Kuhn等[59]利用ENU小鼠突变体来探索miR-96在听觉器官发育至成熟过程中的作用机制,发现miR-96种子区域的突变影响了Slc26a5、Ocm、Gfi1、Ptprq和Pitpnm1等内耳毛细胞相关靶基因的正常表达,毛细胞静纤毛束的成熟和耳蜗内听觉神经连接的重塑都会受到影响,进一步阐明了这一种子区域与听力损失有关[60],miR-96可能与内耳毛细胞的静纤毛束的成熟和耳蜗神经的发育密切联系[61]。因此,了解miR-96的作用机制有助于进一步解释维持耳蜗正常活动所需基因的有序表达,并有助于深入研究非综合征性聋病发生的机制[62]。
3.2.2 miR-182
miR-182活性可能与靶基因Tbx1有关,Tbx1是一种参与毛细胞发育和分化的转录因子[63]。顺铂(cisplatin, CDDP)诱导的毛细胞凋亡前过表达miR-182,可抑制内源性凋亡途径的3个关键基因Bax、Apaf-1和caspase,从而保护耳蜗毛细胞免于细胞凋亡[64]。miR-182过表达会导致耳蜗毛细胞数量增加,在支持细胞中miR-182的低表达可抑制该细胞转分化为毛细胞。因此,在感觉细胞中过表达miR-182可以促进毛细胞再生,有望治疗由毛细胞丢失引起的感音神经性耳聋。Hildebrand等[65]利用隐性常染色体非综合征性耳聋人类家系,在RDX(DFNB24)基因的3?-UTR中发现了miR-182结合位点的C>A的纯合子突变。Wang等[66]将小鼠耳蜗干/祖细胞进行体外培养,发现过表达miR-182促进耳蜗干/祖细胞分化成毛细胞,此外,miR-182还与神经感觉器官、视觉感觉器官等器官的发育调控有关。在针对自闭症的全基因组研究中,Schellenberg等[67]在接近miR-182染色体位点的地方发现了这种疾病的易感基因,miR-182缺陷会导致自闭症的发生。Xu等[51]体外研究表明MITF是miR-96和miR-182的直接靶点,MITF是建立和维持视网膜发育和维持所必需的转录因子,miR-182的异常导致感觉器官发育程序的缺陷。
3.2.3 miR-183
miR-183能够调控耳蜗内毛细胞的发育分化及成熟的生理过程,miR-183可通过负调控其下游靶基因,使毛细胞的细胞骨架发生改变[68]。内耳在暴露于噪声28 d后miR-183、miR-96和miR-182的表达水平降低, 这与噪声导致外毛细胞的减少有关。在强烈的噪声刺激导致耳蜗毛细胞损伤后,miR-183可以通过抑制Taok1的表达来保护强刺激后受到损伤的耳蜗[69]。在体外培养的耳蜗螺旋器中,用吗啡反义寡核苷酸抑制miR-183的表达可导致Taok1蛋白增加并伴随耳蜗毛细胞的凋亡,说明miR-183在调节听觉创伤的耳蜗反应方面具有潜在的作用。Kim等[70]发现在新霉素诱导耳毒性斑马鱼中抑制miR-183表达,会降低毛细胞的再生,反之在斑马鱼胚胎中人工注射miR-183可以促进毛细胞正常发育。miR-183表达的变化先于动物形态学和功能的变化,在小鼠耳蜗发育的过程中,促进细胞增殖和分化的miR-183呈上调趋势,而在小鼠衰老时miR-183下调,促凋亡通路的调控因子miR-29家族和miR-34家族成员上调。
4 miRNA调控耳蜗发育的分子机制
4.1 miRNA与靶基因
人们对miRNA如何控制耳蜗发育的理解始于对Dicer1 突变体动物的研究。在斑马鱼模型中,Dicer1幼体突变体的听觉器官严重畸形[71]。在小鼠中,Dicer1基因在耳部早期发育时缺失,会导致内耳的整体尺寸减小,耳蜗生长受到严重阻碍[72]。Dicer1基因在pre-miRNA加工成为成熟miRNA过程中至关重要,Dicer1缺失严重影响了内耳的发育,间接地说明了miRNAs对耳蜗的重要性。miRNAs在耳蜗发育过程中参与调控重要基因的表达水平,从而参与了调控耳蜗细胞的增殖、迁移、发育和凋亡等过程。Sox2作为感觉前体细胞区域较早出现的标志之一,在人类耳蜗发育过程中Sox2的缺失引起了感音神经性耳聋,Tbx1是内耳发育和毛细胞命运有关的转录因子,miR-182参与了靶基因Sox2和Tbx1的表达调控[66]。miR-96的靶基因是Slc26a5、Ocm、Ptprq和Pitpnm1,其中Ptprq是毛细胞成熟的重要基因[59]。此外,miR-96的靶基因还包括了渐进性耳聋的2个关键基因EGFR(表皮生长因子受体)和TRK(神经营养因子受体)[55]。C1ic5在其3'-UTR中包含一个高度保守的miR-96/-182结合位点,被认为是miR-96和miR-182的共同靶基因。Gu等[73]研究证实C1ic5基因突变小鼠与ENU突变小鼠具有相似的立体纤毛形态,利用脂质体将miR-96和miR-182转染到耳蜗毛细胞中,可导致C1ic5在mRNA水平和蛋白水平的表达量下降,进一步研究结果表明C1ic5是由miR-96和miR-182直接调控的,确认靶序列位于C1ic5 3?-UTR内的核苷760~766 bp之间。miR-183以ltgA3为靶基因,通过抑制整合素α3的表达来控制耳蜗发育中的细胞增殖[71]。除了上述的miR-183家族参与耳蜗发育的重要靶基因的调控,其他miRNA也在耳蜗发育过程中发挥重要作用。COL9A1是负责产生透明软骨组分的基因,miR-9是COL9A1的调控因子[72]。miR-124在耳蜗中的靶基因是Wnt信号通路的两个抑制因子Sfrp4和Sfrp5。miR-124于耳囊的神经上皮中高水平表达,促进神经细胞分化和轮廓形成[74]。miR-135b调控耳蜗中的转录激活因子PSIP1-P75[75]。miR-194在耳蜗神经元和毛细胞中高度表达,通过调控Fgf4和RhoB基因影响耳蜗神经细胞的分化[76]。内耳形态发生的关键调节因子是miR-200,在耳蜗和前庭上皮细胞中选择性表达,通过转录沉默Zeb1和Zeb2基因调控上皮-间质转化[77]。磷酸核糖焦磷酸合成酶1(PRPS1)的突变与一系列非综合征到综合征性听力损失有关,PRPS1表达水平受miR-376的调控[78]。总之,这些miRNA以及其下游靶基因在耳蜗中组成了复杂的调控网络,共同调控耳蜗的发育过程[79]。有关miRNA调控耳蜗发育的靶基因见表1。
Table 1
表1
表1miRNA在耳蜗中的靶基因
Table 1
| miRNA | 靶基因 | 参考文献 |
|---|---|---|
| miR-9 | COL9A1 | [72] |
| miR-29 | SIRT1 | [80] |
| miR-34a | SIRT1、bcl-2和E2F-3 | [80] |
| miR-96 | TRK和EGFR | [52] |
| Slc26a5、Ocm、Gfi1、Ptprq和Pitpnm1 | [59] | |
| miR-96/-182 | CLIC5 | [73] |
| miR-124 | Sfrp4和Sfrp5 | [74] |
| miR-135b | PSIP1-P75 | [82] |
| miR-140 | NR2F1和Klf9 | [83] |
| miR-182 | Sox2和Tbx1 | [84] |
| miR-183 | Taok1和ltgA3 | [70] |
| miR-194 | Fgf4和RhoB | [76] |
| miR-200 | Zeb1和Zeb2 | [85] |
| miR-204 | TMPRSS3 | [86] |
| miR-224 | Ptx3 | [87] |
| miR-376 | PRPS1 | [78] |
新窗口打开|下载CSV
4.2 miRNA参与的信号通路
耳蜗前体细胞在耳蜗分化的过程中主要产生3种谱系的细胞,分别是神经前体细胞、感觉前体细胞和其它细胞[88]。神经细胞产生所必需的细胞因子是Sox2和Ngn1,Tbx1可以抑制Ngn1和神经元的分化,而miR-182抑制Tbx1的表达[89]。感觉细胞的产生时需要Jagged1、Notch1、SOX2、BMP-4、FGF和IGF-1等基因参与调控,细胞周期蛋白依赖性激酶(Cyclin-dependent kinase)抑制剂p27kip1,p19Ink4d和Rb抑制感觉细胞进入细胞周期,促进感觉前体细胞分化成毛细胞和支持细胞[90]。毛细胞的形成和成熟需要MyosinVII、Atoh1、Espin、Brn3c、Gfi1和Barhl1等细胞因子的调控[91]。Wnt信号通路[92]、Notch信号通路[93]、Shh信号通路[94]、FGF信号通路[95]和TGF信号通路[96]等信号通路参与了耳蜗的发育过程。其中,经典Wnt/β-catenin信号通路作用于耳蜗发育的最初阶段, 主要负责调控听囊和听基板的特化;而Wnt/PCP信号通路在哺乳动物的毛细胞静纤毛的生长排列和蜗管的延伸过程中发挥着重要作用[97]。miR-183家族可以通过抑制LRP6的表达,调控Wnt/β-catenin信号通路的传导[98],而糖原合成酶激酶GSK3β通过Wnt/β-catenin /TCF/LEF-1信号通路影响miR-183家族的表达[99]。在哺乳动物发育过程中,Notch信号通路参与耳蜗感觉上皮的发育与分化过程,通过侧向抑制作用调控耳蜗感觉前体细胞向毛细胞的分化,从而确保内毛细胞至外毛细胞的正常分化顺序[100]。miR-384-5p转染细胞后,Notch1的表达水平显著下调[101],miR-183通过抑制基因NICD3和NICD4从而抑制 Notch信号通路,参与毛细胞的分化和再生[102]。在耳蜗发育的早期阶段,FGF信号通路调控早期听基板的形成,在耳蜗发育后期,FGF信号分子主要参与毛细胞的发育,然而miRNA参与FGF信号通路调节的报道目前尚未见报道[103]。miRNA在耳蜗发育过程中调节细胞凋亡方面还发挥了重要作用[104]。在电离辐射诱导的毛细胞死亡模型中,作为促凋亡因子的miR-207通过靶向基因Akt3(Akt是PI3K/AKT途径等信号通路的关键基因)发挥了重要作用[105]。miR-182通过抑制PI3K AKT信号通路的直接靶点FOXO3a (促凋亡转录因子)的翻译来抑制细胞凋亡通路,可减轻毛细胞死亡[106]。miR-183通过抑制PDCD4的表达,抑制TGFβ1诱导的细胞凋亡,调控TGF通路参与支持细胞和毛细胞的分化[107]。因此,通过下调和上调miRNA的表达来精准调控耳蜗干细胞的发育进程并减少毛细胞的凋亡是一种体内原位毛细胞再生的可行策略[108]。miRNA调控耳蜗发育的分子机制示意图见图4。
图4
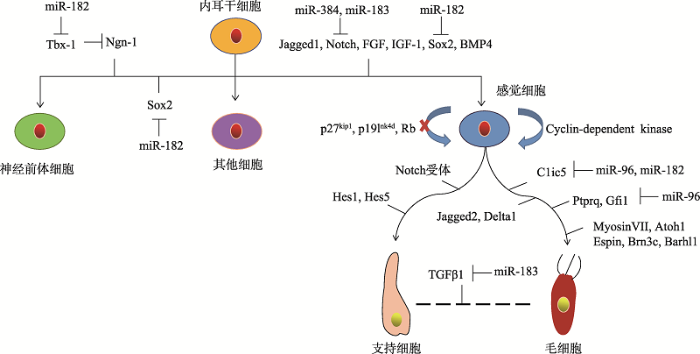 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4miRNA调控耳蜗发育的分子机制示意图
∣表示正调控;⊥表示负调控。
Fig. 4Molecular mechanism of miRNA in regulating cochlear development
5 miRNA在治疗聋病方面应用前景
目前已有6000余个miRNA被找到,这些miRNA与生物体中约1/3的蛋白编码基因的调控密切相关[109]。miRNA已被证实是参与诸多内耳相关的病理发生过程的关键因素,如渐进性感音神经性耳聋、老年化耳聋、噪声性耳聋和内耳炎症等[110]。miRNA还参与了感觉毛细胞束发育、肌动蛋白重组、细胞粘附和内耳形态发生[111]。目前感音神经性耳聋治疗寄希望于毛细胞的移植治疗,细胞移植的关键是获得符合要求的毛细胞[90]。而获得毛细胞的唯一途径是来自于干细胞的体外诱导,所谓利用干细胞治疗感音神经性耳聋的最终目标是将干细胞诱导分化,再移植到毛细胞受损伤的部位作为替代细胞,达到重建损伤耳蜗并修复听力功能[112]。近年来一系列的研究表明胚胎干细胞、间充质干细胞、神经干细胞、内耳干细胞、iPS细胞等都可以在体外诱导分化为耳蜗类毛细胞[113]。然而,干细胞体外诱导获得的耳蜗类毛细胞虽然可以表达毛细胞相关的标志性蛋白,如Brn3c、Aoth1和MyosinⅦ等,但是扫描电镜观测到的类毛细胞的静纤毛和动纤毛仍与正常毛细胞的纤毛束有差距、神经电生理也有差异[114]。miRNA已经在毛囊细胞移植[115]、肝脏细胞体外分化[116]、心肌细胞体外分化[117]等方面有成功的案例。为此,本实验室构建过表达miR-183、miR-182和miR-96的载体导入到胚胎干细胞,利用这种胚胎干细胞研究体外诱导分化为毛细胞的机理,希望获得功能形态更加完整的毛细胞用于细胞移植治疗[37]。6 结语与展望
耳聋是全球性的疾病问题之一,世界上有5亿人遭受听力丧失的困扰,其中包括了3200万名儿童[118]。根据中国残联的最新数据显示:中国听力残疾的人数已达2780万人,听力残疾仅次于肢体残疾,是中国第二大致残疾病[119]。miRNA与耳蜗及毛细胞发育调控密切相关[120] ,耳蜗中miRNA数量庞大,且一个miRNA可调控多个靶基因,多个miRNA也可协同调控一个靶基因,需要进一步明确与耳聋相关联的miRNA种类及生物特性。目前,miRNA在耳蜗中的具体分子机制尚未完全清楚,miRNA的成熟体究竟是在内耳的单个细胞内参与调控还是以外泌体等方式分泌到细胞外产生作用?内耳中表达了相同miRNA的细胞之间具有何种联系?miRNA参与调控内耳毛细胞纤毛束的具体作用方式是什么?这些问题都值得人们深入探讨。另外,在耳蜗miRNA作用机理研究的基础上,将来可用小分子化合物和关键的miRNA共同导入到耳蜗诱导毛细胞的原位再生,也可以用外泌体作为载体负载miRNA或者使用miRNA拮抗剂,移植耳蜗诱导毛细胞的原位再生。这些以miRNA为基础的新技术,将为耳聋的治疗提供新的思路。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.3390/molecules23071500URLPMID:29933586 [本文引用: 1]

Targeted gene delivery relies on the ability to limit the expression of a transgene within a defined cell/tissue population. MicroRNAs represent a class of highly powerful and effective regulators of gene expression that act by binding to a specific sequence present in the corresponding messenger RNA. Involved in almost every aspect of cellular function, many miRNAs have been discovered with expression patterns specific to developmental stage, lineage, cell-type, or disease stage. Exploiting the binding sites of these miRNAs allows for construction of targeted gene delivery platforms with a diverse range of applications. Here, we summarize studies that have utilized miRNA-regulated systems to achieve targeted gene delivery for both research and therapeutic purposes. Additionally, we identify criteria that are important for the effectiveness of a particular miRNA for such applications and we also discuss factors that have to be taken into consideration when designing miRNA-regulated expression cassettes.
DOI:10.1126/science.1065062URLPMID:11679671 [本文引用: 1]

Two small temporal RNAs (stRNAs), lin-4 and let-7, control developmental timing in Caenorhabditis elegans. We find that these two regulatory RNAs are members of a large class of 21- to 24-nucleotide noncoding RNAs, called microRNAs (miRNAs). We report on 55 previously unknown miRNAs in C. elegans. The miRNAs have diverse expression patterns during development: a let-7 paralog is temporally coexpressed with let-7; miRNAs encoded in a single genomic cluster are coexpressed during embryogenesis; and still other miRNAs are expressed constitutively throughout development. Potential orthologs of several of these miRNA genes were identified in Drosophila and human genomes. The abundance of these tiny RNAs, their expression patterns, and their evolutionary conservation imply that, as a class, miRNAs have broad regulatory functions in animals.
DOI:10.1016/j.jmb.2004.03.065URLPMID:15136036 [本文引用: 1]

Many of the known microRNAs are encoded in polycistronic transcripts. Here, we reconstruct the evolutionary history of the mir17 microRNA clusters which consist of miR-17, miR-18, miR-19a, miR-19b, miR-20, miR-25, miR-92, miR-93, miR-106a, and miR-106b. The history of this cluster is governed by an initial phase of local (tandem) duplications, a series of duplications of entire clusters and subsequent loss of individual microRNAs from the resulting paralogous clusters. The complex history of the mir17 microRNA family appears to be closely linked to the early evolution of the vertebrate lineage.
DOI:10.1261/rna.2146903URLPMID:12554859 [本文引用: 1]

MicroRNAs (miRNAs) represent a new class of noncoding RNAs encoded in the genomes of plants, invertebrates, and vertebrates. MicroRNAs regulate translation and stability of target mRNAs based on (partial) sequence complementarity. Although the number of newly identified miRNAs is still increasing, target mRNAs of animal miRNAs remain to be identified. Here we describe 31 novel miRNAs that were identified by cloning from mouse tissues and the human Saos-2 cell line. Fifty-three percent of all known mouse and human miRNAs have homologs in Fugu rubripes (pufferfish) or Danio rerio (zebrafish), of which almost half also have a homolog in Caenorhabditis elegans or Drosophila melanogaster. Because of the recurring identification of already known miRNAs and the unavoidable background of ribosomal RNA breakdown products, it is believed that not many more miRNAs may be identified by cloning. A comprehensive collection of miRNAs is important for assisting bioinformatics target mRNA identification and comprehensive genome annotation.
DOI:10.1016/j.gene.2018.10.075URLPMID:30389561 [本文引用: 1]

The etiology of hearing loss tends to be multi-factorial and affects a significant proportion of the global population. Despite the differences in etiology, a common physical pathological change that leads to hearing loss is damage to the mechanosensory hair cells of the inner ear. MicroRNAs (miRNAs) have been shown to play a role in inner ear development and thus, may play a role in the development or prevention of hearing loss. In this paper, we review the mechanism of action of miRNAs in the auditory system. We present an overview about the role of miRNAs in inner ear development, summarize the current research on the role of miRNAs in gene regulation, and discuss the effects of both miRNA mutations as well as overexpression. We discuss the crucial role of miRNAs in ensuring normal physiological development of the inner ear. Any deviation from the proper function of miRNA in the cochlea seems to contribute to deleterious damage to the structure of the auditory system and subsequently results in hearing loss. As interest for miRNA research increases, this paper serves as a platform to review current understandings and postulate future avenues for research. A better knowledge about the role of miRNA in the auditory system will help in developing novel treatment modalities for restoring hearing function based on regeneration of damaged inner ear hair cells.
DOI:10.7874/jao.2016.20.3.131URLPMID:27942598 [本文引用: 1]

miRNAs are essential factors of an extensively conserved post-transcriptional process controlling gene expression at mRNA level. Varoius biological processes such as growth and differentiation are regulated by miRNAs. Web of Science and PubMed databases were searched using the Endnote software for the publications about the role miRNA-183 family in inner ear: hair cell development and deafness published from 2000 to 2016. A triplet of these miRNAs particularly the miR-183 family is highly expressed in vertebrate hair cells, as with some of the peripheral neurosensory cells. Point mutations in one member of this family, miR-96, underlie DFNA50 autosomal deafness in humans and lead to abnormal hair cell development and survival in mice. In zebrafish, overexpression of the miR-183 family induces extra and ectopic hair cells, while knockdown decreases the number of hair cell. The miR-183 family (miR-183, miR-96 and miR-182) is expressed abundantly in some types of sensory cell in the eye, nose and inner ear. In the inner ear, mechanosensory hair cells have a robust expression level. Despite much similarity of these miRs sequences, small differences lead to distinct targeting of messenger RNAs targets. In the near future, miRNAs are likely to be explored as potential therapeutic agents to repair or regenerate hair cells, cell reprogramming and regenerative medicine applications in animal models because they can simultaneously down-regulate dozens or even hundreds of transcripts.
DOI:10.1016/j.ydbio.2017.08.034URLPMID:28866362 [本文引用: 1]

We review the development and evolution of the ear neurosensory cells, the aggregation of neurosensory cells into an otic placode, the evolution of novel neurosensory structures dedicated to hearing and the evolution of novel nuclei in the brain and their input dedicated to processing those novel auditory stimuli. The evolution of the apparently novel auditory system lies in duplication and diversification of cell fate transcription regulation that allows variation at the cellular level [transforming a single neurosensory cell into a sensory cell connected to its targets by a sensory neuron as well as diversifying hair cells], organ level [duplication of organ development followed by diversification and novel stimulus acquisition] and brain nuclear level [multiplication of transcription factors to regulate various neuron and neuron aggregate fate to transform the spinal cord into the unique hindbrain organization]. Tying cell fate changes driven by bHLH and other transcription factors into cell and organ changes is at the moment tentative as not all relevant factors are known and their gene regulatory network is only rudimentary understood. Future research can use the blueprint proposed here to provide both the deeper molecular evolutionary understanding as well as a more detailed appreciation of developmental networks. This understanding can reveal how an auditory system evolved through transformation of existing cell fate determining networks and thus how neurosensory evolution occurred through molecular changes affecting cell fate decision processes. Appreciating the evolutionary cascade of developmental program changes could allow identifying essential steps needed to restore cells and organs in the future.
DOI:10.1016/j.heares.2016.12.003URLPMID:27986594 [本文引用: 1]

The frequency selectivity of a gerbil cochlea, unlike other mammals, does not depend on varying thickness and width of its basilar membrane from the basal to the apical end. We model the gerbil arched basilar membrane focusing on the radial tension, embedded fiber thickness, and the membrane arch, which replace the functionality of the variation in thickness and width. The model is verified with the previous gerbil cochlea model which estimated the equivalent basilar membrane thickness and is shown to be more accurate than the flat sandwiched basilar membrane model. The simple sinusoidal-shaped bending mode assumption in previous models is found to be valid in the present model with <12% error. Parametric study on the present model shows that fiber thickness contribution to the membrane stiffness is close to the 3rd order, higher than the 1st order estimation of previous models. We found that the effective Young's modulus of the fiber bundle is at least 6 orders higher than the shear modulus of the soft-cells and the membrane radial bending stiffness is more sensitive to the membrane arch and the shear modulus of the soft-cells near the apical end.
DOI:10.1046/j.1365-201X.1996.563313000.xURLPMID:8931771 [本文引用: 1]

In the inner ear, the Reissner's membrane separates the scala vestibuli from the scala media and is thus of importance for maintaining a positive endocochlear potential. The motion of the membrane is thought to be driven by the vibrations of the underlying hearing organ caused by a hydromechanical coupling between the structures. Since the Reissner's membrane is relatively easily accessible in the cochlea its vibratory response has been used as a measure of the micromechanical behaviour of the hearing organ. To determine whether this indirect measure revealed the true characteristics of the hearing organ, experiments were performed using laser heterodyne interferometry in an in vitro preparation of the guinea-pig temporal bone. Interferometric measurements at the Reissner's membrane and at the surface of the hearing organ directly beneath made it possible to compare the mechanical tuning characteristics of both structures. It was found that the mechanical response characteristics of the Reissner's membrane differed considerably from the hearing organ. The tuning frequency was different and only minor changes in the maximal vibration amplitude were seen when measuring at different radial locations. However, the shape of the response curve changes with location. The Reissner's membrane response appeared to be affected by the mechanical vibrations originating both at the middle ear ossicles and at the hearing organ. It is concluded that the Reissner's membrane response is a poor indicator of cochlear mechanics and that investigations of cochlear micromechanics should be performed directly at the level of the hearing organ.
DOI:10.1007/s00405-017-4470-6URLPMID:28224282 [本文引用: 1]

miRNAs are important factors for post-transcriptional process that controls gene expression at mRNA level. Various biological processes, including growth and differentiation, are regulated by miRNAs. miRNAs have been demonstrated to play an essential role in development and progression of hearing loss. Nowadays, miRNAs are known as critical factors involved in different physiological, biological, and pathological processes, such as gene expression, progressive sensorineural hearing loss, age-related hearing loss, noise-induced hearing loss, cholesteatoma, schwannomas, and inner ear inflammation. The miR-183 family (miR-183, miR-96 and miR-182) is expressed abundantly in some types of sensory cells in inner ear specially mechanosensory hair cells that exhibit a great expression level of this family. The plasma levels of miR-24-3p, miR-16-5p, miR-185-5p, and miR-451a were upregulated during noise exposures, and increased levels of miR-21 have been found in vestibular schwannomas and human cholesteatoma. In addition, upregulation of pro-apoptotic miRNAs and downregulation of miRNAs which promote differentiation and proliferation in age-related degeneration of the organ of Corti may potentially serve as a helpful biomarker for the early detection of age-related hearing loss. This knowledge represents miRNAs as promising diagnostic and therapeutic tools in the near future.
DOI:10.1016/j.heares.2015.08.013URLPMID:26341477 [本文引用: 1]

The mammalian inner ear consists of the cochlea and the vestibular labyrinth (utricle, saccule, and semicircular canals), which participate in both hearing and balance. Proper development and life-long function of these structures involves a highly complex coordinated system of spatial and temporal gene expression. The characterization of the inner ear transcriptome is likely important for the functional study of auditory and vestibular components, yet, primarily due to tissue unavailability, detailed expression catalogues of the human inner ear remain largely incomplete. We report here, for the first time, comprehensive transcriptome characterization of the adult human cochlea, ampulla, saccule and utricle of the vestibule obtained from patients without hearing abnormalities. Using RNA-Seq, we measured the expression of >50,000 predicted genes corresponding to approximately 200,000 transcripts, in the adult inner ear and compared it to 32 other human tissues. First, we identified genes preferentially expressed in the inner ear, and unique either to the vestibule or cochlea. Next, we examined expression levels of specific groups of potentially interesting RNAs, such as genes implicated in hearing loss, long non-coding RNAs, pseudogenes and transcripts subject to nonsense mediated decay (NMD). We uncover the spatial specificity of expression of these RNAs in the hearing/balance system, and reveal evidence of tissue specific NMD. Lastly, we investigated the non-syndromic deafness loci to which no gene has been mapped, and narrow the list of potential candidates for each locus. These data represent the first high-resolution transcriptome catalogue of the adult human inner ear. A comprehensive identification of coding and non-coding RNAs in the inner ear will enable pathways of auditory and vestibular function to be further defined in the study of hearing and balance. Expression data are freely accessible at https://www.tgen.org/home/research/research-divisions/neurogenomics/supplementary-data/inner-ear-transcriptome.aspx.
DOI:10.1016/j.joto.2017.03.003URLPMID:29937838 [本文引用: 1]

miRNA-183 family, in normal biology, is expressed in a harmonious and stable manner in the neurosensory organs and cells. Studies have also shown that miRNA-183 family, in different pathways, affects the neurosensory development, maintenance, survival and function. In addition, it has potential neuroprotective effects in response to neurosensory destructive stimulations. miRNA-96 mutation causes hereditary deafness in humans and mice, and therefore affects the inner ear activity and its maintenance. Certain roles have been identified for miR-96 in the maintenance and function of the inner ear. The comparison of the target genes of family-183 in transcriptomes of newborn and adult hair cells shows that hundreds of target genes in this family may affect development and maintenance of the ears. Identifying the genes that are regulated by miRNA-183 family provides researchers with important information about the complex development and environmental regulation of the inner ear, and can offer new approaches to the maintenance and regeneration of hair cells and auditory nerve.
DOI:10.1007/s40495-016-0064-zURLPMID:28758056 [本文引用: 1]

The spiral ganglion neurons (SGNs) of the cochlea are essential for our ability to hear. SGN loss after exposure to ototoxic drugs or loud noise results in hearing loss. Pluripotent stem cell-derived and endogenous progenitor cell types have the potential to become SGNs and are cellular foundations for replacement therapies. Repurposing transcriptional regulatory networks to promote SGN differentiation from progenitor cells is a strategy for regeneration. Advances in the Fludigm C1 workflow or Drop-seq allow sequencing of single cell transcriptomes to reveal variability between cells. During differentiation, the individual transcriptomes obtained from single-cell RNA-seq can be exploited to identify different cellular states. Pseudotemporal ordering of transcriptomes describes the differentiation trajectory, allows monitoring of transcriptional changes and determines molecular barriers that prevent the progression of progenitors into SGNs. Analysis of single cell transcriptomes will help develop novel strategies for guiding efficient SGN regeneration.
DOI:10.1080/15419060600631805URLPMID:16613783 [本文引用: 1]

To elucidate the role of the spiral limbus in glucose transport in the cochlea, we analyzed the expression and localization of GLUT1, connexin26, connexin30, and occludin in the spiral limbus of the rat cochlea. GLUT1 and occludin were detected in blood vessels. GLUT1, connexin26, connexin30, and occludin were also expressed in fibrocytes just basal to the supralimbal lining cells. Connexin26 and connexin30 were present among not only these GLUT1-positive fibrocytes but also GLUT1-negative fibrocytes. In vivo glucose imaging using 6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-6-deoxyglucose (6-NBDG, MW 342) together with Evans Blue Albumin (EBA, MW 68,000) showed that 6-NBDG was rapidly distributed throughout the spiral limbus, whereas EBA was localized only in the vessels. Moreover, the gap junctional uncoupler heptanol inhibited the distribution of 6-NBDG. These findings suggest that gap junctions play an important role in glucose transport in the spiral limbus, i.e., that gap junctions mediate glucose transport from GLUT1-positive fibrocytes to GLUT1-negative fibrocytes in the spiral limbus.
DOI:10.3390/ijms19092836URLPMID:30235835 [本文引用: 1]

To confirm levels and detection timing of circulating microRNAs (miRNAs) in the serum of a mouse model for diagnosis of ototoxicity, circulating miR-205 in the serum was evaluated to reflect damages in the cochlear microstructure and compared to a kidney injury model.
DOI:10.7554/eLife.37625URLPMID:30183615 [本文引用: 1]

Auditory sensory outer hair cells are thought to amplify sound-induced basilar membrane vibration through a feedback mechanism to enhance hearing sensitivity. For optimal amplification, the outer hair cell-generated force must act on the basilar membrane at an appropriate time at every cycle. However, the temporal relationship between the outer hair cell-driven reticular lamina vibration and the basilar membrane vibration remains unclear. By measuring sub-nanometer vibrations directly from outer hair cells using a custom-built heterodyne low-coherence interferometer, we demonstrate in living gerbil cochleae that the reticular lamina vibration occurs after, not before, the basilar membrane vibration. Both tone- and click-induced responses indicate that the reticular lamina and basilar membrane vibrate in opposite directions at the cochlear base and they oscillate in phase near the best-frequency location. Our results suggest that outer hair cells enhance hearing sensitivity through a global hydromechanical mechanism, rather than through a local mechanical feedback as commonly supposed.
DOI:10.1016/j.heares.2013.01.008URLPMID:23347917 [本文引用: 1]

Many studies that aim to investigate the underlying mechanisms of hearing loss or balance disorders focus on the hair cells and spiral ganglion neurons of the inner ear. Fewer studies have examined the supporting cells that contact both of these cell types in the cochlea and vestibular end organs. While the roles of supporting cells are still being elucidated, emerging evidence indicates that they serve many functions vital to maintaining healthy populations of hair cells and spiral ganglion neurons. Here we review recent studies that highlight the critical roles supporting cells play in the development, function, survival, death, phagocytosis, and regeneration of other cell types within the inner ear. Many of these roles have also been described for glial cells in other parts of the nervous system, and lessons from these other systems continue to inform our understanding of supporting cell functions. This article is part of a Special Issue entitled "Annual Reviews 2013".
DOI:10.1038/srep20903URLPMID:26864824 [本文引用: 1]

We confirmed that ATP is released from cochlear marginal cells in the stria vascular but the cell organelle in which ATP stores was not identified until now. Thus, we studied the ATP-containing cell organelles and suggest that these are lysosomes. Primary cultures of marginal cells of Sprague-Dawley rats aged 1-3 days was established. Vesicles within marginal cells stained with markers were identified under confocal laser scanning microscope and transmission electron microscope (TEM). Then ATP release from marginal cells was measured after glycyl-L-phenylalanine-?- naphthylamide (GPN) treatment using a bioluminescent assay. Quinacrine-stained granules within marginal cells were labeled with LysoTracker, a lysosome tracer, and lysosomal-associated membrane protein 1(LAMP1), but not labeled with the mitochondrial tracer MitoTracker. Furthermore, LysoTracker-labelled puncta showed accumulation of Mant-ATP, an ATP analog. Treatment with 200?μM GPN quenched fluorescently labeled puncta after incubation with LysoTracker or quinacrine, but not MitoTracker. Quinacrine-labeled organelles observed by TEM were lysosomes, and an average 27.7 percent increase in ATP luminescence was observed in marginal cells extracellular fluid after GPN treatment. ATP-containing vesicles in cochlear marginal cells of the stria vascular from neonatal rats are likely lysosomes. ATP release from marginal cells may be via Ca(2+)-dependent lysosomal exocytosis.
DOI:10.1016/j.heares.2012.03.008URLPMID:22484222 [本文引用: 1]

MicroRNAs (miRNA) are a group of small noncoding RNAs that regulate gene expression. The discovery of these small RNAs has added a new layer of complexity to molecular biology. Every day, new advances are being made in understanding the biochemistry and genetics of miRNAs and their roles in cellular function and homeostasis. Studies indicate diverse roles for miRNAs in inner ear biology and pathogenesis. This article reviews recent developments in miRNA research in the field of inner ear biology. A brief history of miRNA discovery is discussed, and their genomics and functional roles are described. Advances in the understanding of miRNA involvement in inner ear development in the zebrafish and the mouse are presented. Finally, this review highlights the potential roles of miRNAs in genetic hearing loss, hair cell regeneration, and inner ear pathogenesis resulting from various pathological insults.
DOI:10.1387/ijdb.150124sfURLPMID:26864490 [本文引用: 1]

MicroRNAs (miRNAs) comprise a class of approximately 22 nucleotide regulatory non-coding RNAs that play several roles in diverse biological processes. Recent reports suggest that embryonic development in mammals is accompanied by dynamic changes in miRNA expression; however, there is no information regarding the role of miRNAs in the development of the external ear. The aim of this study was to determine the stage-specific expression of miRNAs during mouse external ear development in order to identify potentially implicated miRNAs along with their possible targets. miRNA expression profiles from fetal mice pinnae and back skin tissues at 13.5 dpc and 14.5 dpc were obtained using an Affymetrix GeneChip miRNA 3.0 array. Biological triplicates for both tissues, each collected from a litter averaging 16 fetuses, were analyzed. The results were analyzed with Affymetrix's Transcriptome Analysis Console software to identify differentially expressed miRNAs. We observed differential expression of 40 miRNAs including some predicted to target genes implicated in external ear development, such as mmu-miR-10a, an miRNA known to modulate Hoxa1 mRNA levels, and mmu-miR-200c and mmu-miR-205. To our knowledge, this is the first miRNA expression profiling study of external ear development in mammals. These data could set the basis to understand the implications of miRNAs in normal external ear development.
DOI:10.1007/978-1-4939-3615-1_5URLPMID:27259922 [本文引用: 1]

The amphibian Xenopus offers a unique model system for uncovering the genetic basis of auditory and vestibular function in an organism that is well-suited for experimental manipulation during animal development. However, many procedures for analyzing gene expression in the peripheral auditory and vestibular systems mandate the ability to isolate intact RNA from inner ear tissue. Methods presented here facilitate preparation of high-quality inner ear RNA from larval and post-metamorphic Xenopus specimens that can be used for a variety of purposes. We demonstrate that RNA isolated with these protocols is suitable for microarray analysis and Illumina-Solexa sequencing (RNA-Seq) of inner ear organs, and for cloning of large transcripts, such as those for ion channels. Genetic sequences cloned with these procedures can be used for transient transfection of Xenopus kidney cell lines with fluorescent protein fusion constructs.
DOI:10.1016/j.heares.2014.12.003URLPMID:25528492 [本文引用: 1]

Bone conduction (BC) hearing relies on sound vibration transmission in the skull bone. Several clinical findings indicate that in the human, the skull vibration of the inner ear dominates the response for BC sound. Two phenomena transform the vibrations of the skull surrounding the inner ear to an excitation of the basilar membrane, (1) inertia of the inner ear fluid and (2) compression and expansion of the inner ear space. The relative importance of these two contributors were investigated using an impedance lumped element model. By dividing the motion of the inner ear boundary in common and differential motion it was found that the common motion dominated at frequencies below 7 kHz but above this frequency differential motion was greatest. When these motions were used to excite the model it was found that for the normal ear, the fluid inertia response was up to 20 dB greater than the compression response. This changed in the pathological ear where, for example, otosclerosis of the stapes depressed the fluid inertia response and improved the compression response so that inner ear compression dominated BC hearing at frequencies above 400 Hz. The model was also able to predict experimental and clinical findings of BC sensitivity in the literature, for example the so called Carhart notch in otosclerosis, increased BC sensitivity in superior semicircular canal dehiscence, and altered BC sensitivity following a vestibular fenestration and RW atresia.
DOI:10.1016/j.gde.2015.02.006URLPMID:25796080 [本文引用: 1]

The vertebrate inner ear is a sensory organ of exquisite design and sensitivity. It responds to sound, gravity and movement, serving both auditory (hearing) and vestibular (balance) functions. Almost all cell types of the inner ear, including sensory hair cells, sensory neurons, secretory cells and supporting cells, derive from the otic placode, one of the several ectodermal thickenings that arise around the edge of the anterior neural plate in the early embryo. The developmental patterning mechanisms that underlie formation of the inner ear from the otic placode are varied and complex, involving the reiterative use of familiar signalling pathways, together with roles for transcription factors, transmembrane proteins, and extracellular matrix components. In this review, I have selected highlights that illustrate just a few of the many recent discoveries relating to the development of this fascinating organ system.
DOI:10.1002/dneu.22242URLPMID:25363666 [本文引用: 1]

Studies on the formation of neuronal structures of the human cochlea are rare, presumptively, due to the difficult accessibility of specimens, so that most investigations are performed on mouse models. By means of immunohistochemical and transmission electron microscopic techniques, we investigated an uninterrupted series of unique specimens from gestational week 8 to week 12. We were able to demonstrate the presence of nerve fibers in the prosensory domain at gestational week 8, followed by afferent synaptogenesis at week 11. We identified PAX2 as an early marker for hair cell differentiation. Glutamine synthetase-positive peripheral glial cells occurred at the beginning of week 8. Transcription factor MAF B was used to demonstrate maturation of the spiral ganglion neurons. The early expression of tyrosine hydroxylase could be assessed. This study provides insights in the early assembly of the neural circuit and organization in humans.
DOI:10.1371/journal.pone.0191452URLPMID:29373586 [本文引用: 1]

Due to the extreme inaccessibility of fetal human inner ear tissue, defining of the microRNAs (miRNAs) that regulate development of the inner ear has relied on animal tissue. In the present study, we performed the first miRNA sequencing of otic precursors in human specimens. Using HTG miRNA Whole Transcriptome assays, we examined miRNA expression in the cochleovestibular ganglion (CVG), neural crest (NC), and otic vesicle (OV) from paraffin embedded (FFPE) human specimens in the Carnegie developmental stages 13-15. We found that in human embryonic tissues, there are different patterns of miRNA expression in the CVG, NC and OV. In particular, members of the miR-183 family (miR-96, miR-182, and miR-183) are differentially expressed in the CVG compared to NC and OV at Carnegie developmental stage 13. We further identified transcription factors that are differentially targeted in the CVG compared to the other tissues from stages 13-15, and we performed gene set enrichment analyses to determine differentially regulated pathways that are relevant to CVG development in humans. These findings not only provide insight into the mechanisms governing the development of the human inner ear, but also identify potential signaling pathways for promoting regeneration of the spiral ganglion and other components of the inner ear.
DOI:10.3389/fphar.2015.00322URLPMID:26924983 [本文引用: 1]

The Human Toxome Project is part of a long-term vision to modernize toxicity testing for the 21st century. In the initial phase of the project, a consortium of six academic, commercial, and government organizations has partnered to map pathways of toxicity, using endocrine disruption as a model hazard. Experimental data is generated at multiple sites, and analyzed using a range of computational tools. While effectively gathering, managing, and analyzing the data for high-content experiments is a challenge in its own right, doing so for a growing number of -omics technologies, with larger data sets, across multiple institutions complicates the process. Interestingly, one of the most difficult, ongoing challenges has been the computational collaboration between the geographically separate institutions. Existing solutions cannot handle the growing heterogeneous data, provide a computational environment for consistent analysis, accommodate different workflows, and adapt to the constantly evolving methods and goals of a research project. To meet the needs of the project, we have created and managed The Human Toxome Collaboratorium, a shared computational environment hosted on third-party cloud services. The Collaboratorium provides a familiar virtual desktop, with a mix of commercial, open-source, and custom-built applications. It shares some of the challenges of traditional information technology, but with unique and unexpected constraints that emerge from the cloud. Here we describe the problems we faced, the current architecture of the solution, an example of its use, the major lessons we learned, and the future potential of the concept. In particular, the Collaboratorium represents a novel distribution method that could increase the reproducibility and reusability of results from similar large, multi-omic studies.
DOI:10.1242/dev.139840URLPMID:27789624 [本文引用: 1]

Disorders of hearing and balance are most commonly associated with damage to cochlear and vestibular hair cells or neurons. Although these cells are not capable of spontaneous regeneration, progenitor cells in the hearing and balance organs of the neonatal mammalian inner ear have the capacity to generate new hair cells after damage. To investigate whether these cells are restricted in their differentiation capacity, we assessed the phenotypes of differentiated progenitor cells isolated from three compartments of the mouse inner ear - the vestibular and cochlear sensory epithelia and the spiral ganglion - by measuring electrophysiological properties and gene expression. Lgr5+ progenitor cells from the sensory epithelia gave rise to hair cell-like cells, but not neurons or glial cells. Newly created hair cell-like cells had hair bundle proteins, synaptic proteins and membrane proteins characteristic of the compartment of origin. PLP1+ glial cells from the spiral ganglion were identified as neural progenitors, which gave rise to neurons, astrocytes and oligodendrocytes, but not hair cells. Thus, distinct progenitor populations from the neonatal inner ear differentiate to cell types associated with their organ of origin.
DOI:10.1002/neu.20310URLPMID:17013931 [本文引用: 1]

Hearing loss in mammals is irreversible because cochlear neurons and hair cells do not regenerate. To determine whether we could replace neurons lost to primary neuronal degeneration, we injected EYFP-expressing embryonic stem cell-derived mouse neural progenitor cells into the cochlear nerve trunk in immunosuppressed animals 1 week after destroying the cochlear nerve (spiral ganglion) cells while leaving hair cells intact by ouabain application to the round window at the base of the cochlea in gerbils. At 3 days post transplantation, small grafts were seen that expressed endogenous EYFP and could be immunolabeled for neuron-specific markers. Twelve days after transplantation, the grafts had neurons that extended processes from the nerve core toward the denervated organ of Corti. By 64-98 days, the grafts had sent out abundant processes that occupied a significant portion of the space formerly occupied by the cochlear nerve. The neurites grew in fasciculating bundles projecting through Rosenthal's canal, the former site of spiral ganglion cells, into the osseous spiral lamina and ultimately into the organ of Corti, where they contacted hair cells. Neuronal counts showed a significant increase in neuronal processes near the sensory epithelium, compared to animals that were denervated without subsequent stem cell transplantation. The regeneration of these neurons shows that neurons differentiated from stem cells have the capacity to grow to a specific target in an animal model of neuronal degeneration.
DOI:10.1016/j.heares.2019.01.014URLPMID:30711386 [本文引用: 1]

The development of the inner ear complex cytoarchitecture and functional geometry requires the exquisite coordination of a variety of cellular processes in a temporal manner. At early stages of inner ear development several rounds of cell proliferation in the otocyst promote the growth of the structure. The apoptotic program is initiated in exceeding cells to adjust cell type numbers. Apoptotic cells are cleared by phagocytic cells that recognize the phosphatidylserine residues exposed in the cell membrane thanks to the energy supplied by autophagy. Specific molecular programs determine hair and supporting cell fate, these populations are responsible for the functions of the adult sensory organ: detection of sound, position and acceleration. The neurons that transmit auditory and balance information to the brain are also born at the otocyst by neurogenesis facilitated by autophagy. Cellular senescence participates in tissue repair, cancer and aging, situations in which cells enter a permanent cell cycle arrest and acquire a highly secretory phenotype that modulates their microenvironment. More recently, senescence has also been proposed to take place during vertebrate development in a limited number of transitory structures and organs; among the later, the endolymphatic duct in the inner ear. Here, we review these cellular processes during the early development of the inner ear, focusing on how the most recently described cellular senescence participates and cooperates with proliferation, apoptosis and autophagy to achieve otic morphogenesis and differentiation.
DOI:10.1152/physrev.2001.81.3.1305URLPMID:11427697 [本文引用: 1]

In mammals, environmental sounds stimulate the auditory receptor, the cochlea, via vibrations of the stapes, the innermost of the middle ear ossicles. These vibrations produce displacement waves that travel on the elongated and spirally wound basilar membrane (BM). As they travel, waves grow in amplitude, reaching a maximum and then dying out. The location of maximum BM motion is a function of stimulus frequency, with high-frequency waves being localized to the "base" of the cochlea (near the stapes) and low-frequency waves approaching the "apex" of the cochlea. Thus each cochlear site has a characteristic frequency (CF), to which it responds maximally. BM vibrations produce motion of hair cell stereocilia, which gates stereociliar transduction channels leading to the generation of hair cell receptor potentials and the excitation of afferent auditory nerve fibers. At the base of the cochlea, BM motion exhibits a CF-specific and level-dependent compressive nonlinearity such that responses to low-level, near-CF stimuli are sensitive and sharply frequency-tuned and responses to intense stimuli are insensitive and poorly tuned. The high sensitivity and sharp-frequency tuning, as well as compression and other nonlinearities (two-tone suppression and intermodulation distortion), are highly labile, indicating the presence in normal cochleae of a positive feedback from the organ of Corti, the "cochlear amplifier." This mechanism involves forces generated by the outer hair cells and controlled, directly or indirectly, by their transduction currents. At the apex of the cochlea, nonlinearities appear to be less prominent than at the base, perhaps implying that the cochlear amplifier plays a lesser role in determining apical mechanical responses to sound. Whether at the base or the apex, the properties of BM vibration adequately account for most frequency-specific properties of the responses to sound of auditory nerve fibers.
DOI:10.1016/j.heares.2011.08.005URLPMID:21875659 [本文引用: 1]

Heterozygous mutations in the gene encoding chromodomain-DNA-binding-protein 7 (CHD7) cause CHARGE syndrome, a multiple anomaly condition which includes vestibular dysfunction and hearing loss. Mice with heterozygous Chd7 mutations exhibit semicircular canal dysgenesis and abnormal inner ear neurogenesis, and are an excellent model of CHARGE syndrome. Here we characterized Chd7 expression in mature middle and inner ears, analyzed morphological features of mutant ears and tested whether Chd7 mutant mice have altered responses to noise exposure and correlated those responses to inner and middle ear structure. We found that Chd7 is highly expressed in mature inner and outer hair cells, spiral ganglion neurons, vestibular sensory epithelia and middle ear ossicles. There were no obvious defects in individual hair cell morphology by prestin immunostaining or scanning electron microscopy, and cochlear innervation appeared normal in Chd7(Gt)(/+) mice. Hearing thresholds by auditory brainstem response (ABR) testing were elevated at 4 and 16 kHz in Chd7(Gt)(/+) mice, and there were reduced distortion product otoacoustic emissions (DPOAE). Exposure of Chd7(Gt)(/+) mice to broadband noise resulted in variable degrees of hair cell loss which inversely correlated with severity of stapedial defects. The degrees of hair cell loss and threshold shifts after noise exposure were more severe in wild type mice than in mutants. Together, these data indicate that Chd7(Gt)(/+) mice have combined conductive and sensorineural hearing loss, correlating with changes in both middle and inner ears.
DOI:10.1016/S0960-9822(02)00809-6URL [本文引用: 1]

Abstract
MicroRNAs (miRNAs) are a new class of noncoding RNAs, which are encoded as short inverted repeats in the genomes of invertebrates and vertebrates [1] and [2]. It is believed that miRNAs are modulators of target mRNA translation and stability, although most target mRNAs remain to be identified. Here we describe the identification of 34 novel miRNAs by tissue-specific cloning of approximately 21-nucleotide RNAs from mouse. Almost all identified miRNAs are conserved in the human genome and are also frequently found in nonmammalian vertebrate genomes, such as pufferfish. In heart, liver, or brain, it is found that a single, tissue-specifically expressed miRNA dominates the population of expressed miRNAs and suggests a role for these miRNAs in tissue specification or cell lineage decisions. Finally, a miRNA was identified that appears to be the fruitfly and mammalian ortholog of C. elegans lin-4 stRNA.DOI:10.1055/s-0035-1567886URLPMID:26667630 [本文引用: 1]

The reconstruction of ear deformities has been challenging plastic surgeons since centuries. However, it is only in the 19th century that reports on partial and total ear reconstruction start increasing. In the quest for an aesthetically pleasing and natural-looking result, surgeons worked on the perfect framework and skin coverage. Different materials and flap techniques have evolved. Some were abandoned out of frustration, while others kept evolving over the years. In this article, we discuss the milestones in ear reconstruction-from ancient times to early attempts in Western civilization to the key chapters of ear reconstruction in the 20th century leading to the current techniques.
DOI:10.1007/s00441-017-2650-8URLPMID:28687930 [本文引用: 1]

Clusterin (CLU) is an extracellular chaperone protein that is implicated in diverse physiological and pathophysiological cellular processes. CLU expression is upregulated in response to cellular stress and under certain conditions, such as neurodegenerative disease and cancer. CLU primarily functions as a chaperone that exerts cytoprotective effects by removing cellular debris and misfolded proteins and also acts as a signaling molecule that regulates pro-survival pathways. Deafness is caused by genetic factors and various extrinsic insults, including ototoxic drugs, exposure to loud sounds and aging. Considering its cytoprotectivity, CLU may also mediate cellular defense mechanisms against hearing loss due to cellular stresses. To understand the function of CLU in the inner ear, we analyze CLU expression patterns in the mouse inner ear during development and in the adult stage. Results of quantitative real-time polymerase chain reaction analysis showed that Clu mRNA levels in the inner ear were increased during embryogenesis and were constantly expressed in the adult. Detailed spatial expression patterns of Clu both in the mRNA and protein levels were analyzed throughout various developmental stages via in situ hybridization and immunofluorescence staining. Clu expression was found in specific domains of developing inner ear starting from the otocyst stage, mainly adjacent to the prosensory domain of the cochlear epithelium. In the mature inner ear, Clu expression was observed in Deiter's cells and pillar cells of the organ of Corti, outer sulcus and in basal cells of the stria vascularis in the cochlea. These specific spatiotemporal expression patterns suggest the possible roles of CLU in inner ear development and in maintaining proper hearing function.
DOI:10.1016/j.omtn.2018.01.005URLPMID:29858093 [本文引用: 1]

Parkinson's disease (PD) is the second-most-frequent neurodegenerative disorder worldwide. One major hallmark of PD is the degeneration of dopaminergic (DA) neurons in the substantia nigra. Glial cell line-derived neurotrophic factor (GDNF) potently increases DA neuron survival in models of PD; however, the underlying mechanisms are incompletely understood. MicroRNAs (miRNAs) are small, non-coding RNAs that are important for post-transcriptional regulation of gene expression. Using small RNA sequencing, we show that GDNF specifically increases the expression of miR-182-5p and miR-183-5p in primary midbrain neurons (PMNs). Transfection of synthetic miR-182-5p and miR-183-5p mimics leads to increased neurite outgrowth and mediates neuroprotection of DA neurons in?vitro and in?vivo, mimicking GDNF?effects. This is accompanied by decreased expression of FOXO3 and FOXO1 transcription factors and increased PI3K-Akt signaling. Inhibition of endogenous miR-182-5p or miR-183-5p in GDNF-treated PMNs attenuated the pro-DA effects of GDNF. These findings unveil an unknown miR-mediated mechanism of GDNF action and suggest that targeting miRNAs is a new therapeutic avenue to PD phenotypes.
DOI:10.3724/SP.J.1005.2013.01153URL [本文引用: 1]

Embryonic stem cells (ESCs) are pluripotent stem cells characterized by their ability to self-renew and their pluripotency to differentiate into all cell types. MicroRNA (miRNA) is a small non-coding RNA molecule which can regulate transcriptional and post-transcriptional gene expression, and may also play significant roles in regulating proliferation and differentiation of ESCs. The maintenance of pluripotency in ESCs may involve a regulatory network of many factors and pathways regulated by miRNA, which includes ESCs transcription factors, cell cycle regulation, epigenetic modifications as well as intracelluar signal transduction. This review mainly elaborates the biogenesis of miRNA, the miRNA families regulating the pluripotency of ESCs, and the effect of miRNA on the regulatory network of pluripotency in ESCs.
DOI:10.3724/SP.J.1005.2013.01153URL [本文引用: 1]

Embryonic stem cells (ESCs) are pluripotent stem cells characterized by their ability to self-renew and their pluripotency to differentiate into all cell types. MicroRNA (miRNA) is a small non-coding RNA molecule which can regulate transcriptional and post-transcriptional gene expression, and may also play significant roles in regulating proliferation and differentiation of ESCs. The maintenance of pluripotency in ESCs may involve a regulatory network of many factors and pathways regulated by miRNA, which includes ESCs transcription factors, cell cycle regulation, epigenetic modifications as well as intracelluar signal transduction. This review mainly elaborates the biogenesis of miRNA, the miRNA families regulating the pluripotency of ESCs, and the effect of miRNA on the regulatory network of pluripotency in ESCs.
DOI:10.1038/s41598-018-21811-1URLPMID:29476110 [本文引用: 2]

Germline mutations in Mir96, one of three co-expressed polycistronic miRNA genes (Mir96, Mir182, Mir183), cause hereditary hearing loss in humans and mice. Transgenic FVB/NCrl- Tg(GFAP-Mir183,Mir96,Mir182)MDW1 mice (Tg1MDW), which overexpress this neurosensory-specific miRNA cluster in the inner ear, were developed as a model system to identify, in the aggregate, target genes and biologic processes regulated by the miR-183 cluster. Histological assessments demonstrate Tg1MDW/1MDW homozygotes have a modest increase in cochlear inner hair cells (IHCs). Affymetrix mRNA microarray data analysis revealed that downregulated genes in P5 Tg1MDW/1MDW cochlea are statistically enriched for evolutionarily conserved predicted miR-96, miR-182 or miR-183 target sites. ABR and DPOAE tests from 18 days to 3 months of age revealed that Tg1MDW/1MDW homozygotes develop progressive neurosensory hearing loss that correlates with histologic assessments showing massive losses of both IHCs and outer hair cells (OHCs). This mammalian miRNA misexpression model demonstrates a potency and specificity of cochlear homeostasis for one of the dozens of endogenously co-expressed, evolutionally conserved, small non-protein coding miRNA families. It should be a valuable tool to predict and elucidate miRNA-regulated genes and integrated functional gene expression networks that significantly influence neurosensory cell differentiation, maturation and homeostasis.
DOI:10.1016/j.brainres.2006.07.006URLPMID:16904081 [本文引用: 1]

MicroRNAs (miRNAs) are small non-coding RNAs that function through the RNA interference (RNAi) pathway and post-transcriptionally regulate gene expression in eukaryotic organisms. While miRNAs are known to affect cellular proliferation, differentiation, and morphological development, neither their expression nor roles in mammalian inner ear development have been characterized. We have investigated the extent of miRNA expression at various time points throughout maturation of the postnatal mouse inner ear by microarray analysis. Approximately one third of known miRNAs are detected in the inner ear, and their expression persists to adulthood. Expression of such miRNAs is validated by quantitative PCR and northern blot analysis. Further analysis by in situ hybridization demonstrates that certain miRNAs exhibit cell-specific expression patterns in the mouse inner ear. Notably, we demonstrate that miRNAs previously associated with mechanosensory cells in zebrafish are also expressed in hair cells of the auditory and vestibular endorgans. Our results demonstrate that miRNA expression is abundant in the mammalian inner ear and that certain miRNAs are evolutionarily associated with mechanosensory cell development and/or function. The data suggest that miRNAs contribute substantially to genetic programs intrinsic to development and function of the mammalian inner ear and that specific miRNAs might influence formation of sensory epithelia from the primitive otic neuroepithelium.
DOI:10.1523/JNEUROSCI.4948-09.2010URLPMID:20203184 [本文引用: 1]

Members of the microRNA (miRNA) 183 family (miR-183, miR-96, and miR-182) are expressed abundantly in specific sensory cell types in the eye, nose, and inner ear. In the inner ear, expression is robust in the mechanosensory hair cells and weak in the associated statoacoustic ganglion (SAG) neurons; both cell types can share a common lineage during development. Recently, dominant-progressive hearing loss in humans and mice was linked to mutations in the seed region of miR-96, with associated defects in both development and maintenance of hair cells in the mutant mice. To understand how the entire triplet functions in the development of mechanosensory hair cells and neurons of the inner ear, we manipulated the levels of these miRNAs in zebrafish embryos using synthesized miRNAs and antisense morpholino oligonucleotides (MOs). Overexpression of miR-96 or miR-182 induces duplicated otocysts, ectopic or expanded sensory patches, and extra hair cells, whereas morphogenesis of the SAG is adversely affected to different degrees. In contrast, knockdown of miR-183, miR-96, and miR-182 causes reduced numbers of hair cells in the inner ear, smaller SAGs, defects in semicircular canals, and abnormal neuromasts on the posterior lateral line. However, the prosensory region of the posterior macula, where the number of hair cells is reduced by approximately 50%, is not significantly impaired. Our findings suggest both distinct and common roles for the three miRNAs in cell-fate determination in the inner ear, and these principles might apply to development of other sensory organs.
DOI:10.1073/pnas.1618757114URLPMID:28559309 [本文引用: 1]

MicroRNAs (miRNAs) are known to be essential for retinal maturation and functionality; however, the role of the most abundant miRNAs, the miR-183/96/182 cluster (miR-183 cluster), in photoreceptor cells remains unclear. Here we demonstrate that ablation of two components of the miR-183 cluster, miR-183 and miR-96, significantly affects photoreceptor maturation and maintenance in mice. Morphologically, early-onset dislocated cone nuclei, shortened outer segments and thinned outer nuclear layers are observed in the miR-183/96 double-knockout (DKO) mice. Abnormal photoreceptor responses, including abolished photopic electroretinography (ERG) responses and compromised scotopic ERG responses, reflect the functional changes in the degenerated retina. We further identify Slc6a6 as the cotarget of miR-183 and miR-96. The expression level of Slc6a6 is significantly higher in the DKO mice than in the wild-type mice. In contrast, Slc6a6 is down-regulated by adeno-associated virus-mediated overexpression of either miR-183 or miR-96 in wild-type mice. Remarkably, both silencing and overexpression of Slc6a6 in the retina are detrimental to the electrophysiological activity of the photoreceptors in response to dim light stimuli. We demonstrate that miR-183/96-mediated fine-tuning of Slc6a6 expression is indispensable for photoreceptor maturation and maintenance, thereby providing insight into the epigenetic regulation of photoreceptors in mice.
DOI:10.1016/j.gep.2009.01.003URLPMID:19602392 [本文引用: 1]

MicroRNAs (miRNAs) constitute a class of small non-coding endogenous RNAs that downregulate gene expression by binding to 3' untranslated region (UTR) of target messenger RNAs. Although they have been found to regulate developmental and physiological processes in several organs and tissues, their role in the regulation of the inner ear transcriptome remains unknown. In this report, we have performed systematic in situ hybridization to analyze the temporal and spatial distribution of three miRNAs (miR-96, mR-182, and mR-183) that are likely to arise from a single precursor RNA during the development and the maturation of the cochlea. Strikingly we found that the expression of mR-96, mR-182 and mR-183 was highly dynamic during the development of the cochlea, from the patterning to the differentiation of the main cochlear structures.
DOI:10.1002/cphy.c160049URLPMID:28915323 [本文引用: 1]

Sound pressure fluctuations striking the ear are conveyed to the cochlea, where they vibrate the basilar membrane on which sit hair cells, the mechanoreceptors of the inner ear. Recordings of hair cell electrical responses have shown that they transduce sound via submicrometer deflections of their hair bundles, which are arrays of interconnected stereocilia containing the mechanoelectrical transducer (MET) channels. MET channels are activated by tension in extracellular tip links bridging adjacent stereocilia, and they can respond within microseconds to nanometer displacements of the bundle, facilitated by multiple processes of Ca2+-dependent adaptation. Studies of mouse mutants have produced much detail about the molecular organization of the stereocilia, the tip links and their attachment sites, and the MET channels localized to the lower end of each tip link. The mammalian cochlea contains two categories of hair cells. Inner hair cells relay acoustic information via multiple ribbon synapses that transmit rapidly without rundown. Outer hair cells are important for amplifying sound-evoked vibrations. The amplification mechanism primarily involves contractions of the outer hair cells, which are driven by changes in membrane potential and mediated by prestin, a motor protein in the outer hair cell lateral membrane. Different sound frequencies are separated along the cochlea, with each hair cell being tuned to a narrow frequency range; amplification sharpens the frequency resolution and augments sensitivity 100-fold around the cell's characteristic frequency. Genetic mutations and environmental factors such as acoustic overstimulation cause hearing loss through irreversible damage to the hair cells or degeneration of inner hair cell synapses. ? 2017 American Physiological Society. Compr Physiol 7:1197-1227, 2017.
DOI:10.1056/NEJMra1616601URLPMID:29262274 [本文引用: 1]
DOI:10.1371/journal.pone.0162726URLPMID:27618300 [本文引用: 1]

Recent work suggests that hair cells are not the most vulnerable elements in the inner ear; rather, it is the synapses between hair cells and cochlear nerve terminals that degenerate first in the aging or noise-exposed ear. This primary neural degeneration does not affect hearing thresholds, but likely contributes to problems understanding speech in difficult listening environments, and may be important in the generation of tinnitus and/or hyperacusis. To look for signs of cochlear synaptopathy in humans, we recruited college students and divided them into low-risk and high-risk groups based on self-report of noise exposure and use of hearing protection. Cochlear function was assessed by otoacoustic emissions and click-evoked electrocochleography; hearing was assessed by behavioral audiometry and word recognition with or without noise or time compression and reverberation. Both groups had normal thresholds at standard audiometric frequencies, however, the high-risk group showed significant threshold elevation at high frequencies (10-16 kHz), consistent with early stages of noise damage. Electrocochleography showed a significant difference in the ratio between the waveform peaks generated by hair cells (Summating Potential; SP) vs. cochlear neurons (Action Potential; AP), i.e. the SP/AP ratio, consistent with selective neural loss. The high-risk group also showed significantly poorer performance on word recognition in noise or with time compression and reverberation, and reported heightened reactions to sound consistent with hyperacusis. These results suggest that the SP/AP ratio may be useful in the diagnosis of "hidden hearing loss" and that, as suggested by animal models, the noise-induced loss of cochlear nerve synapses leads to deficits in hearing abilities in difficult listening situations, despite the presence of normal thresholds at standard audiometric frequencies.
DOI:10.14639/0392-100X-587URLPMID:27196076 [本文引用: 1]

A retrospective chart review was used for 31 patients with sudden, progressive or fluctuating sensorineural hearing loss (SHL) in the only hearing ear who had been consecutively evaluated at the ENT, Audiology and Phoniatrics Unit of the University of Pisa. The group of patients was evaluated with a complete history review, clinical evaluation, imaging exam (MRI, CT), audiologic tests (tone and speech audiometry, tympanometry, study of stapedial reflexes, ABR and otoacoustic emission) evaluation. In order to exclude genetic causes, patients were screened for CX 26 and CX30 mutations and for mitochondrial DNA mutation A1555G. Patients with sudden or rapidly progressive SHL in the only hearing ear were treated with osmotic diuretics and corticosteroids. In patients who did not respond to intravenous therapy we performed intratympanic injections of corticosteroid. Hearing aids were fitted when indicated and patients who developed severe to profound SHL were scheduled for cochlear implant surgery. The aim of this study is to report and discuss the epidemiology, aetiopathogenesis, therapy and clinical characteristic of patients affected by SHL in the only hearing hear and to discuss the issues related to the cochlear implant procedure in some of these patients, with regard to indications, choice of the ear to implant and results.
URLPMID:27413627 [本文引用: 1]

The primary care physician's role in recognizing sudden sensorineural hearing (SSNHL) loss and delivering initial treatment is critical in the management of the syndrome. This role involves recognizing its clinical symptoms, distinguishing it from conductive hearing loss with the Weber tuning fork or the Rauch hum test, and urgent administration of high dose oral corticosteroids. Diagnosis and treatment should not be delayed for audiometric testing or referral to otolaryngology. This paper provides an update on the initial evaluation and treatment of this syndrome based on the literature and clinical guideline recommendations.
DOI:10.1016/j.neuron.2011.07.015URL [本文引用: 1]

Vision, olfaction, hearing, and balance are mediated by receptors that reside in specialized sensory epithelial organs. Age-related degeneration of the photoreceptors in the retina and the hair cells in the cochlea, caused by macular degeneration and sensorineural hearing loss, respectively, affect a growing number of individuals. Although sensory receptor cells in the mammalian retina and inner ear show only limited or no regeneration, in many nonmammalian vertebrates, these sensory epithelia show remarkable regenerative potential. We summarize the current state of knowledge of regeneration in the specialized sense organs in both nonmammalian vertebrates and mammals and discuss possible areas where new advances in regenerative medicine might provide approaches to successfully stimulate sensory receptor cell regeneration. The field of regenerative medicine is still in its infancy, but new approaches using stem cells and reprogramming suggest ways in which the potential for regeneration may be restored in individuals suffering from sensory loss.
DOI:10.1159/000479724URLPMID:28968605 [本文引用: 1]

The molecular mechanisms underlying age-related hearing loss are unknown, and currently, there is no treatment for this condition. Recent studies have shown that microRNAs (miRNAs) and age-related diseases are intimately linked, suggesting that some miRNAs may present attractive therapeutic targets. In this study, we obtained 8 human temporal bones from 8 elderly subjects at brain autopsy in order to investigate the expression profile of miRNAs in the inner ear with miRNA arrays. A mean of 478 different miRNAs were expressed in the samples, of which 348 were commonly expressed in all 8 samples. Of these, levels of 16 miRNAs significantly differed between young elderly and old elderly subjects. miRNAs, which play important roles in inner ear development, were detected in all samples, i.e., in both young and old elderly subjects, whether with or without hearing loss. Our results suggest that these miRNAs play important roles not only in development, but also in the maintenance of inner ear homeostasis.
DOI:10.1111/j.1525-142X.2007.00217.xURLPMID:18184361 [本文引用: 1]

MicroRNAs (miRNAs) are an integral component of the metazoan genome and affect posttranscriptional repression of target messenger RNAs. The extreme phylogenetic conservation of certain miRNAs suggests their ancient origin and crucial function in conserved developmental processes. We demonstrate that highly conserved miRNA-183 orthologs exist in both deuterostomes and protostomes and their expression is predominant in ciliated ectodermal cells and organs. The miRNA-183 family members are expressed in vertebrate sensory hair cells, in innervated regions of invertebrate deuterostomes, and in sensilla of Drosophila and C. elegans. Thus, miRNA-183 family member expression is conserved in possibly homologous but morphologically distinct sensory cells and organs. The results suggest that miR-183 family members contribute specifically to neurosensory development or function, and that extant metazoan sensory organs are derived from cells that share genetic programs of common evolutionary origin.
DOI:10.1038/s41598-017-07979-yURLPMID:28794468 [本文引用: 1]

The miR-183 cluster, which is comprised of paralogous miRs-183, -96 and -182, is overexpressed in many cancers, including prostate adenocarcinoma (PCa). Prior studies showed that overexpression of individual pre-miRs-182, -96 and -183 in prostate cells decreased zinc import, which is a characteristic feature of PCa tumours. Zinc is concentrated in healthy prostate 10-fold higher than any other tissue, and an >80% decrease in zinc is observed in PCa specimens. Here, we studied the effect of overexpression of the entire 4.8?kb miR-183 family cluster, including the intergenic region which contains highly conserved genomic regions, in prostate cells. This resulted in overexpression of mature miR-183 family miRs at levels that mimic cancer-related changes. Overexpression of the miR-183 cluster reduced zinc transporter and intracellular zinc levels in benign prostate cells, PCa xenografts and fresh prostate epithelial organoids. Microarray analysis of miR-183 family cluster overexpression in prostate cells showed an enrichment for cancer-related pathways including adhesion, migration and wound healing. An active secondary transcription start site was identified within the intergenic region of the miR-183 cluster, which may regulate expression of miR-182. Taken together, this study shows that physiologically relevant expression of the miR-183 family regulates zinc levels and carcinogenic pathways in prostate cells.
DOI:10.1074/jbc.M700501200URLPMID:17597072 [本文引用: 2]

Although microRNAs (miRNAs) provide a newly recognized level of regulation of gene expression, the miRNA transcriptome of the retina and the contributions of miRNAs to retinal development and function are largely unknown. To begin to understand the functions of miRNAs in retina, we compared miRNA expression profiles in adult mouse retina, brain, and heart by microarray analysis. Our results show that at least 78 miRNAs are expressed in adult mouse retina, 21 of which are potentially retina-specific. Among these, we identified a polycistronic, sensory organ-specific paralogous miRNA cluster that includes miR-96, miR-182, and miR-183 on mouse chromosome 6qA3 with conservation of synteny to human chromosome 7q32.2. In situ hybridization showed that members of this cluster are expressed in photoreceptors, retinal bipolar and amacrine cells. Consistent with their genomic organization, these miRNAs have a similar expression pattern during development with abundance increasing postnatally and peaking in adult retina. Target prediction and in vitro functional studies showed that MITF, a transcription factor required for the establishment and maintenance of retinal pigmented epithelium, is a direct target of miR-96 and miR-182. Additionally, to identify miRNAs potentially involved in circadian rhythm regulation of the retina, we performed miRNA expression profiling with retinal RNA harvested at noon (Zeitgeber time 5) and midnight (Zeitgeber time 17) and identified a subgroup of 12 miRNAs, including members of the miR-183/96/182 cluster with diurnal variation in expression pattern. Our results suggest that miR-96 and miR-182 are involved in circadian rhythm regulation, perhaps by modulating the expression of adenylyl cyclase VI (ADCY6).
DOI:10.1016/j.chemosphere.2018.09.118URLPMID:30265930 [本文引用: 2]

Chronic ototoxicity of β-diketone antibiotics (DKAs) to zebrafish (Danio rerio) was explored in detail by following abnormal expressions of two hearing-related miRNAs. Dose-dependent down-regulation of miR-96 and miR-184 was observed in otoliths during embryonic-larval development. Continuous DKA exposure to 120-hpf larva decreased sensitivity to acoustic stimulation. Development of otolith was delayed in treatment groups, showing unclear boundaries and vacuolization at 72-hpf, and utricular enlargement as well as decreased saccular volume in 96-hpf or latter larval otoliths. If one miRNA was knocked-down and another over-expressed, only a slight influence on morphological development of the otic vesicle occurred, but knocked-down or over-expressed miRNA both significantly affected zebrafish normal development. Injection of miR-96, miR-184 or both micRNA mimics to yolk sac resulted in marked improvement of otic vesicle phenotype. However, hair cell staining showed that only the injected miR-96 mimic restored hair cell numbers after DKA exposure, demonstrating that miR-96 played an important role in otic vesicle development and formation of hearing, while miR-184 was only involved in otic vesicle construction during embryonic development. These observations advance our understanding of hearing loss owing to acute antibiotic exposure and provide theoretical guidance for early intervention and gene therapy for drug-induced diseases.
DOI:10.1016/j.ijporl.2019.05.038URLPMID:31163360 [本文引用: 1]

Approximately 60% of congenital pediatric hearing loss is of genetic etiology. To evaluate non-syndromic sensorineural hearing loss (NSSNHL), guidelines emphasize the use of comprehensive genetic testing (CGT) with next generation sequencing (NGS), yet these tests have limited accessibility, and potential CGT results may not be well understood. Thus, our objective was to analyze genetic testing practices and results for pediatric patients with NSSNHL.
DOI:10.1097/MOO.0b013e32833e0601URLPMID:20717030 [本文引用: 1]

The identification of transcriptional activators and repressors of hair cell fates has recently been augmented by the discovery of microRNAs (miRNAs) that can function as post-transcriptional repressors in sensory hair cells.
DOI:10.1111/ejn.12484URL [本文引用: 2]

miR-96 is a microRNA, a non-coding RNA gene which regulates a wide array of downstream genes. The miR-96 mouse mutant diminuendo exhibits deafness and arrested hair cell functional and morphological differentiation. We have previously shown that several genes are markedly downregulated in the diminuendo organ of Corti; one of these is Ptprq, a gene known to be important for maturation and maintenance of hair cells. In order to study the contribution that downregulation of Ptprq makes to the diminuendo phenotype, we carried out microarrays, scanning electron microscopy and single hair cell electrophysiology to compare diminuendo mutants (heterozygous and homozygous) with mice homozygous for a functional null allele of Ptprq. In terms of both morphology and electrophysiology, the auditory phenotype of mice lacking Ptprq resembles that of diminuendo heterozygotes, while diminuendo homozygotes are more severely affected. A comparison of transcriptomes indicates there is a broad similarity between diminuendo homozygotes and Ptprq-null mice. The reduction in Ptprq observed in diminuendo mice appears to be a major contributor to the morphological, transcriptional and electrophysiological phenotype, but does not account for the complete diminuendo phenotype.
DOI:10.1016/j.euroneuro.2013.07.002URLPMID:23906647 [本文引用: 1]

Attention deficit-hyperactivity disorder (ADHD) is a neuropsychiatric disorder characterized by inappropriate and impaired levels of hyperactivity, impulsivity and inattention. Around 75% of adults with ADHD show comorbidity with other psychiatric disorders such as disruptive behavior disorders or substance use disorders (SUDs). Recently, there has been growing interest in studying the role of microRNAs (miRNAs) in the susceptibility to complex disorders. Interestingly, converging evidence suggests that single nucleotide polymorphisms (SNPs) within miRNAs or miRNA target sites may modulate the miRNA-mediated regulation of gene expression through the alteration of the miRNA maturation, structure or expression pattern as well as the silencing mechanisms of target genes. Genetic studies and animal models support the involvement of the serotonin receptor (HTR1B) in ADHD. We evaluated the contribution of one SNP in the miR-96 target site at HTR1B and eight tagSNPs within the genomic region containing this miRNA in 695 adults with ADHD (266 and 396 subjects with and without comorbid SUD, respectively), 403 subjects with SUD without life-time diagnosis of ADHD and 485 sex-matched controls from Spain. Single and multiple marker analyses revealed association between two SNPs located at the 3' region of miR-96 (rs2402959 and rs6965643) and ADHD without SUD. Our results provide preliminary evidence for the contribution of two sequence variants at the miR-183-96-182 cluster to ADHD without comorbid SUD, and emphasize the need to take comorbidities into account in genetic studies to minimize the effect of heterogeneity and to clarify these complex phenotypes.
DOI:10.1038/ng.355URLPMID:19363479 [本文引用: 1]

MicroRNAs (miRNAs) bind to complementary sites in their target mRNAs to mediate post-transcriptional repression, with the specificity of target recognition being crucially dependent on the miRNA seed region. Impaired miRNA target binding resulting from SNPs within mRNA target sites has been shown to lead to pathologies associated with dysregulated gene expression. However, no pathogenic mutations within the mature sequence of a miRNA have been reported so far. Here we show that point mutations in the seed region of miR-96, a miRNA expressed in hair cells of the inner ear, result in autosomal dominant, progressive hearing loss. This is the first study implicating a miRNA in a mendelian disorder. The identified mutations have a strong impact on miR-96 biogenesis and result in a significant reduction of mRNA targeting. We propose that these mutations alter the regulatory role of miR-96 in maintaining gene expression profiles in hair cells required for their normal function.
DOI:10.1038/ng.369URLPMID:19363478 [本文引用: 1]

Progressive hearing loss is common in the human population, but little is known about the molecular basis. We report a new N-ethyl-N-nitrosurea (ENU)-induced mouse mutant, diminuendo, with a single base change in the seed region of Mirn96. Heterozygotes show progressive loss of hearing and hair cell anomalies, whereas homozygotes have no cochlear responses. Most microRNAs are believed to downregulate target genes by binding to specific sites on their mRNAs, so mutation of the seed should lead to target gene upregulation. Microarray analysis revealed 96 transcripts with significantly altered expression in homozygotes; notably, Slc26a5, Ocm, Gfi1, Ptprq and Pitpnm1 were downregulated. Hypergeometric P-value analysis showed that hundreds of genes were upregulated in mutants. Different genes, with target sites complementary to the mutant seed, were downregulated. This is the first microRNA found associated with deafness, and diminuendo represents a model for understanding and potentially moderating progressive hair cell degeneration in hearing loss more generally.
DOI:10.1073/pnas.1016646108URLPMID:21245307 [本文引用: 3]

MicroRNAs (miRNAs) are small noncoding RNAs able to regulate a broad range of protein-coding genes involved in many biological processes. miR-96 is a sensory organ-specific miRNA expressed in the mammalian cochlea during development. Mutations in miR-96 cause nonsyndromic progressive hearing loss in humans and mice. The mouse mutant diminuendo has a single base change in the seed region of the Mir96 gene leading to widespread changes in the expression of many genes. We have used this mutant to explore the role of miR-96 in the maturation of the auditory organ. We found that the physiological development of mutant sensory hair cells is arrested at around the day of birth, before their biophysical differentiation into inner and outer hair cells. Moreover, maturation of the hair cell stereocilia bundle and remodelling of auditory nerve connections within the cochlea fail to occur in miR-96 mutants. We conclude that miR-96 regulates the progression of the physiological and morphological differentiation of cochlear hair cells and, as such, coordinates one of the most distinctive functional refinements of the mammalian auditory system.
DOI:10.1038/cddis.2016.246URLPMID:27607577 [本文引用: 1]

Cisplatin is widely used for chemotherapy of a variety of malignancies. However, the clinical application of cisplatin is hampered by the resultant irreversible hearing loss due to hair cell apoptosis. To date, no practical regimen to resolve this has been developed. Meanwhile, the role of microRNA in protecting hair cells from cisplatin-induced apoptosis in the inner ear has not been extensively investigated. In this study, we monitored miR-183, -96, and -182 turnover in the cochlea during cisplatin treatment in vitro. We found that overexpression of miR-182, but not miR-183 and -96, improved hair cell survival after 3?μM cisplatin treatment in vitro. We demonstrated that overexpression of miR-182 repressed the intrinsic apoptotic pathway by inhibiting the translation of FOXO3a. Our study offers a new therapeutic target for alleviating cisplatin-induced hair cell apoptosis in a rapid and tissue-specific manner.
DOI:10.1371/journal.pone.0058471URLPMID:23472202 [本文引用: 1]

Acoustic trauma, one of the leading causes of sensorineural hearing loss, induces sensory hair cell damage in the cochlea. Identifying the molecular mechanisms involved in regulating sensory hair cell death is critical towards developing effective treatments for preventing hair cell damage. Recently, microRNAs (miRNAs) have been shown to participate in the regulatory mechanisms of inner ear development and homeostasis. However, their involvement in cochlear sensory cell degeneration following acoustic trauma is unknown. Here, we profiled the expression pattern of miRNAs in the cochlear sensory epithelium, defined miRNA responses to acoustic overstimulation, and explored potential mRNA targets of miRNAs that may be responsible for the stress responses of the cochlea. Expression analysis of miRNAs in the cochlear sensory epithelium revealed constitutive expression of 176 miRNAs, many of which have not been previously reported in cochlear tissue. Exposure to intense noise caused significant threshold shift and apoptotic activity in the cochleae. Gene expression analysis of noise-traumatized cochleae revealed time-dependent transcriptional changes in the expression of miRNAs. Target prediction analysis revealed potential target genes of the significantly downregulated miRNAs, many of which had cell death- and apoptosis-related functions. Verification of the predicted targets revealed a significant upregulation of Taok1, a target of miRNA-183. Moreover, inhibition of miR-183 with morpholino antisense oligos in cochlear organotypic cultures revealed a negative correlation between the expression levels of miR-183 and Taok1, suggesting the presence of a miR-183/Taok1 target pair. Together, miRNA profiling as well as the target analysis and validation suggest the involvement of miRNAs in the regulation of the degenerative process of the cochlea following acoustic overstimulation. The miR-183/Taok1 target pair is likely to play a role in this regulatory process.
DOI:10.2174/138920210793175895URLPMID:21532838 [本文引用: 1]

MicroRNAs are small, highly conserved non-coding RNA molecules involved in the regulation of gene expression. MicroRNAs are transcribed by RNA polymerases II and III, generating precursors that undergo a series of cleavage events to form mature microRNA. The conventional biogenesis pathway consists of two cleavage events, one nuclear and one cytoplasmic. However, alternative biogenesis pathways exist that differ in the number of cleavage events and enzymes responsible. How microRNA precursors are sorted to the different pathways is unclear but appears to be determined by the site of origin of the microRNA, its sequence and thermodynamic stability. The regulatory functions of microRNAs are accomplished through the RNA-induced silencing complex (RISC). MicroRNA assembles into RISC, activating the complex to target messenger RNA (mRNA) specified by the microRNA. Various RISC assembly models have been proposed and research continues to explore the mechanism(s) of RISC loading and activation. The degree and nature of the complementarity between the microRNA and target determine the gene silencing mechanism, slicer-dependent mRNA degradation or slicer-independent translation inhibition. Recent evidence indicates that P-bodies are essential for microRNA-mediated gene silencing and that RISC assembly and silencing occurs primarily within P-bodies. The P-body model outlines microRNA sorting and shuttling between specialized P-body compartments that house enzymes required for slicer -dependent and -independent silencing, addressing the reversibility of these silencing mechanisms. Detailed knowledge of the microRNA pathways is essential for understanding their physiological role and the implications associated with dysfunction and dysregulation.
DOI:10.1126/science.1109020URLPMID:15774722 [本文引用: 1]

MicroRNAs (miRNAs) are small RNAs that regulate gene expression posttranscriptionally. To block all miRNA formation in zebrafish, we generated maternal-zygotic dicer (MZdicer) mutants that disrupt the Dicer ribonuclease III and double-stranded RNA-binding domains. Mutant embryos do not process precursor miRNAs into mature miRNAs, but injection of preprocessed miRNAs restores gene silencing, indicating that the disrupted domains are dispensable for later steps in silencing. MZdicer mutants undergo axis formation and differentiate multiple cell types but display abnormal morphogenesis during gastrulation, brain formation, somitogenesis, and heart development. Injection of miR-430 miRNAs rescues the brain defects in MZdicer mutants, revealing essential roles for miRNAs during morphogenesis.
DOI:10.1002/dvdy.22591URLPMID:21360794 [本文引用: 1]

MicroRNAs (miRNAs) post-transcriptionally repress complementary target gene expression and can contribute to cell differentiation. The coordinate expression of miRNA-183 family members (miR-183, miR-96, and miR-182) has been demonstrated in sensory cells of the mouse inner ear and other vertebrate sensory organs. To further examine hair cell miRNA expression in the mouse inner ear, we have analyzed miR-183 family expression in wild type animals and various mutants with defects in neurosensory development. miR-183 family member expression follows neurosensory cell specification, exhibits longitudinal (basal-apical) gradients in maturating cochlear hair cells, and is maintained in sensory neurons and most hair cells into adulthood. Depletion of hair cell miRNAs resulting from Dicer1 conditional knockout (CKO) in Atoh1-Cre transgenic mice leads to more disparate basal-apical gene expression profiles and eventual hair cell loss. Results suggest that hair cell miRNAs subdue cochlear gradient gene expression and are required for hair cell maintenance and survival.
DOI:10.1002/ajmg.a.33299URLPMID:20186779 [本文引用: 1]

Mutations in miRNA genes have been implicated in hearing loss in human families and mice. It is also possible that mutations in miRNA binding sites of inner ear targets alter gene expression levels and lead to hearing loss. To investigate these possibilities we screened predicted target genes of the miR-183 miRNA cluster known to be expressed in the inner ear sensory epithelium. In one Iranian family segregating autosomal recessive non-syndromic hearing loss (ARNSHL), we identified a homozygous variant in a predicted miR-96/182 binding site in the 3'UTR of the RDX (DFNB24) gene. However, in vitro functional studies showed that this site is not a functional target for miR-96/182. We extended our study to include the miR-183 genes themselves and 24 additional predicted target genes of the miRNA-183 cluster. Screening these miRNAs and target sequences in numerous families segregating either autosomal dominant non-syndromic deafness (ADNSHL) or ARNSHL did not identify any potential deafness-causing mutations. These results suggest that mutations disrupting gene regulation by the miR-183 cluster are not a common cause of human hearing loss.
DOI:10.1016/j.heares.2012.02.005URLPMID:22381690 [本文引用: 2]

Recently, in?vitro and in?vivo models have identified that microRNAs (miRNAs), which are extensively expressed in the inner ear, play important roles in inner ear development and function. However, the function of miRNA in vertebrate tissue is not well understood.
DOI:10.1038/sj.mp.4001874URLPMID:16880825 [本文引用: 1]

We performed a genome-wide linkage scan using highly polymorphic microsatellite markers. To minimize genetic heterogeneity, we focused on sibpairs meeting the strict diagnosis of autism. In our primary analyses, we observed a strong linkage signal (P=0.0006, 133.16 cM) on chromosome 7q at a location coincident with other linkage studies. When a more relaxed diagnostic criteria was used, linkage evidence at this location was weaker (P=0.01). The sample was stratified into families with only male affected subjects (MO) and families with at least one female affected subject (FC). The strongest signal unique to the MO group was on chromosome 11 (P=0.0009, 83.82 cM), and for the FC group on chromosome 4 (P=0.002, 111.41 cM). We also divided the sample into regression positive and regression negative families. The regression-positive group showed modest linkage signals on chromosomes 10 (P=0.003, 0 cM) and 14 (P=0.005, 104.2 cM). More significant peaks were seen in the regression negative group on chromosomes 3 (P=0.0002, 140.06 cM) and 4 (P=0.0005, 111.41 cM). Finally, we used language acquisition data as a quantitative trait in our linkage analysis and observed a chromosome 9 signal (149.01 cM) of P=0.00006 and an empirical P-value of 0.0008 at the same location. Our work provides strong conformation for an autism locus on 7q and suggestive evidence for several other chromosomal locations. Diagnostic specificity and detailed analysis of the autism phenotype is critical for identifying autism loci.
DOI:10.1016/j.ydbio.2009.01.037URLPMID:19389351 [本文引用: 1]

Inner ear development requires coordinated transformation of a uniform sheet of cells into a labyrinth with multiple cell types. While numerous regulatory proteins have been shown to play critical roles in this process, the regulatory functions of microRNAs (miRNAs) have not been explored. To demonstrate the importance of miRNAs in inner ear development, we generated conditional Dicer knockout mice by the expression of Cre recombinase in the otic placode at E8.5. Otocyst-derived ganglia exhibit rapid neuron-specific miR-124 depletion by E11.5, degeneration by E12.5, and profound defects in subsequent sensory epithelial innervations by E17.5. However, the small and malformed inner ear at E17.5 exhibits residual and graded hair cell-specific miR-183 expression in the three remaining sensory epithelia (posterior crista, utricle, and cochlea) that closely corresponds to the degree of hair cell and sensory epithelium differentiation, and Fgf10 expression required for morphohistogenesis. The highest miR-183 expression is observed in near-normal hair cells of the posterior crista, whereas the reduced utricular macula demonstrates weak miR-183 expression and develops presumptive hair cells with numerous disorganized microvilli instead of ordered stereocilia. The correlation of differential and delayed depletion of mature miRNAs with the derailment of inner ear development demonstrates that miRNAs are crucial for inner ear neurosensory development and neurosensory-dependent morphogenesis.
DOI:10.1007/s12038-015-9560-2URLPMID:26564979 [本文引用: 1]

MicroRNAs are a class of important post-transcriptional regulators. Genetic and somatic mutations in miRNAs, especially those in the seed regions, have profound and broad impacts on gene expression and physiological and pathological processes. Over 500 SNPs were mapped to the miRNA seeds, which are located at position 2-8 of the mature miRNA sequences. We found that the central positions of the miRNA seeds contain fewer genetic variants and therefore are more evolutionary conserved than the peripheral positions in the seeds. We developed a knowledgebased method to analyse the functional impacts of mutations in miRNA seed regions. We computed the gene ontology-based similarity score GOSS and the GOSS percentile score for all 517 SNPs in miRNA seeds. In addition to the annotation of SNPs for their functional effects, in the present article we also present a detailed analysis pipeline for finding the key functional changes for seed SNPs. We performed a detailed gene ontology graph-based analysis of enriched functional categories for miRNA target gene sets. In the analysis of a SNP in the seed region of hsa-miR-96 we found that two key biological processes for progressive hearing loss 'Neurotrophin TRK receptor signaling pathway' and 'Epidermal growth factor receptor signaling pathway' were significantly and differentially enriched by the two sets of allele-specific target genes of miRNA hsa-miR-96.
DOI:10.3349/ymj.2018.59.1.141URLPMID:29214789 [本文引用: 2]

microRNAs (miRNAs) are non-coding RNAs composed of 20 to 22 nucleotides that regulate development and differentiation in various organs by silencing specific RNAs and regulating gene expression. In the present study, we show that the microRNA (miR)-183 cluster is upregulated during hair cell regeneration and that its inhibition reduces hair cell regeneration following neomycin-induced ototoxicity in zebrafish.
DOI:10.1038/cdd.2017.127URLPMID:28777373 [本文引用: 2]

MicroRNAs are important regulators of gene expression and are involved in cellular processes such as proliferation or differentiation, particularly during development of numerous organs including the inner ear. However, it remains unknown if miRNAs are required during the earliest stages of otocyst and cochlear duct development. Here, we report that a conditional loss of Dicer expression in the otocyst impairs the early development of the inner ear as a result of the accumulation of DNA damage that trigger p53-mediated apoptosis. Moreover, cochlear progenitors in the prosensory domain do not exit the cell cycle. Our unbiased approach identified ItgA3 as a target of miR-183, which are both enriched in the otic vesicle. We observed that the repression of integrin alpha 3 by miR-183 controls cell proliferation in the developing cochlea. Collectively, our results reveal that Dicer and miRNAs play essential roles in the regulation of early inner ear development.
DOI:10.1007/s10162-006-0032-0URL [本文引用: 3]

EST N66408 represents one of several large unique clusters expressed in the Morton human fetal cochlear cDNA library. N66408 is 575bp in size and initial BLAST analysis of this sequence showed no homology to any known genes or expressed sequence tags (ESTs) from other organs or tissues. Sequence of the original cochlear clone from which N66408 was derived revealed that the corresponding cDNA was about 700bp in size, including 125bp at its 5′ end with homology to the 3′ end of COL9A1 in addition to 575bp of novel sequence. RT-PCR analysis using primers specific to COL9A1 isoforms 1 and 2 detected expression of both isoforms in human fetal cochlea. Tissue in situ hybridization using the novel 3′ UTR sequence as probe showed abundant expression in spiral limbus and spiral ligament, and a moderate level of expression in the organ of Corti. dbEST analysis of ESTs specific to the 3′ UTR of COL9A1 showed 19 ESTs derived from various tissues; three polyadenylation sites were identified and the majority of these ESTs were derived from overlapping polyadenylation signals at the second site (position 749–758). Comparison of the 3′ UTR of human COL9A1 with its orthologs as well as with dbEST uncovered a highly conserved region around the overlapping polyadenylation signals at position 749–758 in mammals. A search of the microRNA database revealed a highly conserved target sequence for miR-9 immediately preceding the overlapping polyadenylation signals in the novel 3′ UTR of COL9A1, suggesting its role in posttranscriptional regulation of COL9A1.
DOI:10.1016/j.tiv.2012.07.008URLPMID:22889583 [本文引用: 2]

The roles of miRNAs in the onset of hearing and deafness are beginning to be revealed. Although there has been no reported link between chloride intracellular channel 5 (CLIC5) and the miR-183 family to date, we here present evidence that they are co-expressed in the inner ear and have functions that are related to stereocilia. Moreover, CLIC5 contains a single predicted and highly conserved miR-96/-182 binding site within its 3'-UTR. Our current results further show that miR-96/-182 and CLIC5 are co-expressed in HEI-OC1 cells, in which two isoforms of the CLIC5 protein exist. Furthermore, miR-96 and miR-182 were found to be specifically overexpressed in HEI-OC1 cells into which mimics of these molecules had been transfected by liposomes causing the downregulation of CLIC5 at both the mRNA and protein levels. Finally, miR-96/-182 specifically downregulate the expression of the luciferase reporter gene which was cloned into a mouse CLIC5 3'-UTR fragment containing the wild-type miR-96/-182 target sequence. Our findings thus suggest that CLIC5 is directly regulated by miR-96 and miR-182 and that the target sequence in this regard is located between nucleotides 760-766 within the CLIC5 3'-UTR.
DOI:10.3892/ijmm.2016.2751URLPMID:28025992 [本文引用: 2]

MicroRNAs (miRNAs or miRs) act as key regulators in neuronal development, synaptic morphogenesis and plasticity. However, their role in the neuronal differentiation of inner ear neural stem cells?(NSCs) remains unclear. In this study, 6?miRNAs were selected and their expression patterns during the neuronal differentiation of inner ear NSCs were examined by RT-qPCR. We demonstrated that the culture of spiral ganglion stem cells present in the inner ears of newborn mice gave rise to neurons in?vitro. The expression patterns of miR?124, miR?132, miR?134, miR?20a, miR?17-5p and miR?30a-5p were examined during a 14-day neuronal differentiation period. We found that miR?124 promoted the neuronal differentiation of and neurite outgrowth in mouse inner ear NSCs, and that the changes in the expression of tropomyosin receptor kinase?B?(TrkB) and cell division control protein?42 homolog?(Cdc42) during inner ear NSC differentiation were associated with miR?124 expression. Our findings indicate that miR?124 plays a role in the neuronal differentiation of inner ear NSCs. This finding may lead to the development of novel strategies for restoring hearing in neurodegenerative diseases.
DOI:10.1186/1471-2164-15-484URLPMID:24942165 [本文引用: 1]

The mammalian inner ear contains sensory organs, the organ of Corti in the cochlea and cristae and maculae in the vestibule, with each comprised of patterned sensory epithelia that are responsible for hearing and balance. The development, cell fate, patterning, and innervation of both the sensory and nonsensory regions of the inner ear are governed by tight regulation involving, among others, transcription factors and microRNAs (miRNAs). In humans, mutations in specific miRNA genes are associated with hearing loss. In mice, experimental reduction or mutations of miRNAs in the inner ear leads to severe developmental and structural abnormalities. A comprehensive identification of miRNAs in the sensory epithelia and their gene targets will enable pathways of auditory and vestibular function to be defined.
DOI:10.1016/j.ijdevneu.2017.09.004URLPMID:28941704 [本文引用: 2]

Many microRNAs participate in the development, differentiation and function preservation of the embryonic and adult inner ear, but many details still need to be elucidated regarding the numerous microRNAs in the inner ear. Based on previous investigations on the microRNA profile in the inner ear, we confirmed that several microRNAs are expressed in the inner ear, and we detected the spatial expression of these microRNAs in the neonatal mouse inner ear. Then we focused on miR-194 for its specific expression with a dynamic spatiotemporal pattern during inner ear development. Overexpression of miR-194 in cultured spiral ganglion cells significantly affected the dendrites of differentiated neurons, with more branching and obviously dispersed nerve fibres. Furthermore, the cytoskeleton of cultured cells was markedly affected, as disordered actin filaments resulting from miR-194 overexpression and enhanced filaments resulting from miR-194 knockdown were observed. Together with the bioinformatic methods, the RT-qPCR and western blot results showed that RhoB is a candidate target of miR-194 in the morphogenesis of spiral ganglion neurons. Additionally, the double luciferase reporter system was used to identify RhoB as a novel target of miR-194. Finally, the inhibition of RhoB activation by Clostridium difficile toxin B disturbed the organization of the actin filament, similar to the effects of miR-194 overexpression. In summary, we investigated microRNA expression in the mouse inner ear, and demonstrated that miR-194 is dynamically expressed during inner ear development; importantly, we found that miR-194 affects neuron morphogenesis positively through Rho B-mediated F-actin rearrangement.
DOI:10.1101/gad.1640608URLPMID:18381893 [本文引用: 1]

Cancer progression has similarities with the process of epithelial-to-mesenchymal transition (EMT) found during embryonic development, during which cells down-regulate E-cadherin and up-regulate Vimentin expression. By evaluating the expression of 207 microRNAs (miRNAs) in the 60 cell lines of the drug screening panel maintained by the Nation Cancer Institute, we identified the miR-200 miRNA family as an extraordinary marker for cells that express E-cadherin but lack expression of Vimentin. These findings were extended to primary ovarian cancer specimens. miR-200 was found to directly target the mRNA of the E-cadherin transcriptional repressors ZEB1 (TCF8/deltaEF1) and ZEB2 (SMAD-interacting protein 1 [SIP1]/ZFXH1B). Ectopic expression of miR-200 caused up-regulation of E-cadherin in cancer cell lines and reduced their motility. Conversely, inhibition of miR-200 reduced E-cadherin expression, increased expression of Vimentin, and induced EMT. Our data identify miR-200 as a powerful marker and determining factor of the epithelial phenotype of cancer cells.
DOI:10.1111/j.1365-2613.2012.00840.xURL [本文引用: 2]

Mutations in phosphoribosyl pyrophosphate synthetase 1 (PRPS1) are associated with a spectrum of non-syndromic to syndromic hearing loss. PRPS1 transcript levels have been shown to be regulated by the microRNA-376 genes. The long primary RNA transcript of the miR-376 RNA cluster members undergo extensive and simultaneous A ? I editing at one or both of two specific sites (+4 and +44) in particular human and mouse tissues. The PRPS1 gene, which contains target sites for the edited version of miR-376a-5p within its 3'UTR, has been shown to be repressed in a tissue-specific manner. To investigate whether the transcription of Prps1 is regulated by miR-376 cluster members in the mouse inner ear, we first quantified the expression of the mature miR-376 RNAs by quantitative real-time-PCR. The spatio-temporal patterns of miR-376 expression were assessed by in situ hybridization. Finally, we examined whether A ?I editing of pri-miR-376 RNAs occurs in mouse inner ear by direct sequencing. Our data showed that the miR-376a-3p, b-3p, c-3p are present in mouse embryonic inner ears and intensive expression of miR-376a-3p/b-3p was detected in the sensory epithelia and ganglia of both auditory and vestibular portions of the inner ear. In adult inner ear, the expression of miR-376a-3p/b-3p is restricted within ganglion neurons of auditory and vestibular systems as well as the cells in the stria vascularis. Only unedited pri-miR-376 RNAs were detected in the cochlea suggesting that the activity of PRPS1 in the inner ear may not be regulated through the editing of miR-376 cluster.
DOI:10.1371/journal.pone.0018195URLPMID:21483685 [本文引用: 1]

We have employed a novel approach for the identification of functionally important microRNA (miRNA)-target interactions, integrating miRNA, transcriptome and proteome profiles and advanced in silico analysis using the FAME algorithm. Since miRNAs play a crucial role in the inner ear, demonstrated by the discovery of mutations in a miRNA leading to human and mouse deafness, we applied this approach to microdissected auditory and vestibular sensory epithelia. We detected the expression of 157 miRNAs in the inner ear sensory epithelia, with 53 miRNAs differentially expressed between the cochlea and vestibule. Functionally important miRNAs were determined by searching for enriched or depleted targets in the transcript and protein datasets with an expression consistent with the dogma of miRNA regulation. Importantly, quite a few of the targets were detected only in the protein datasets, attributable to regulation by translational suppression. We identified and experimentally validated the regulation of PSIP1-P75, a transcriptional co-activator previously unknown in the inner ear, by miR-135b, in vestibular hair cells. Our findings suggest that miR-135b serves as a cellular effector, involved in regulating some of the differences between the cochlear and vestibular hair cells.
DOI:10.1038/srep12911URLPMID:26246194 [本文引用: 2]

MicroRNAs (miRNAs) are important regulators and potential therapeutic targets of metabolic disease. In this study we show by in vivo administration of locked nucleic acid (LNA) inhibitors that suppression of endogenous miR-29 lowers plasma cholesterol levels by ~40%, commensurate with the effect of statins, and reduces fatty acid content in the liver by ~20%. Whole transcriptome sequencing of the liver reveals 883 genes dysregulated (612 down, 271 up) by inhibition of miR-29. The set of 612 down-regulated genes are most significantly over-represented in lipid synthesis pathways. Among the up-regulated genes are the anti-lipogenic deacetylase sirtuin 1 (Sirt1) and the anti-lipogenic transcription factor aryl hydrocarbon receptor (Ahr), the latter of which we demonstrate is a direct target of miR-29. In vitro radiolabeled acetate incorporation assays confirm that pharmacologic inhibition of miR-29 significantly reduces de novo cholesterol and fatty acid synthesis. Our findings indicate that miR-29 controls hepatic lipogenic programs, likely in part through regulation of Ahr and Sirt1, and therefore may represent a candidate therapeutic target for metabolic disorders such as dyslipidemia.
DOI:10.1016/j.exger.2016.01.009URLPMID:26802970

Age-related hearing loss (AHL) is a progressive neurodegenerative disease that is largely silent in its initial stages. There is no sensitive blood biomarker for diagnosis or early detection of AHL. MicroRNAs (miRNAs or miRs) are abundant and highly stable in blood, and have been recently described as powerful circulating biomarkers in a wide range of diseases. In the present study, we identified concordant increases in miR-34a levels in the cochlea, auditory cortex, and plasma of C57BL/6 mice during aging. These increases were accompanied by elevated hearing thresholds and greater loss of hair cells. Levels of miR-34a targets, silent information regulator 1 (SIRT1), B-cell lymphoma-2 (Bcl-2), and E2F transcription factor 3 (E2F3), in the cochlea, auditory cortex, and plasma decreased with aging inversely to miR-34a. Moreover, plasma miR-34a levels were significantly higher in patients with AHL compared with controls who had normal hearing and had a receiver-operating characteristic curve that distinguished AHL patients from controls. However, SIRT1, Bcl-2, and E2F3 showed no correlation with AHL in humans. In summary, circulating miR-34a level may potentially serve as a useful biomarker for early detection of AHL.
DOI:10.1038/nrd4140URL [本文引用: 1]

The first cancer-targeted microRNA (miRNA) drug - MRX34, a liposome-based miR-34 mimic - entered Phase I clinical trials in patients with advanced hepatocellular carcinoma in April 2013, and miRNA therapeutics are attracting special attention from both academia and biotechnology companies. Although miRNAs are the most studied non-coding RNAs (ncRNAs) to date, the importance of long non-coding RNAs (lncRNAs) is increasingly being recognized. Here, we summarize the roles of miRNAs and lncRNAs in cancer, with a focus on the recently identified novel mechanisms of action, and discuss the current strategies in designing ncRNA-targeting therapeutics, as well as the associated challenges.
DOI:10.1371/journal.pone.0083358URLPMID:24349493 [本文引用: 1]

Both nuclear receptor subfamily 2 group F member 1 (NR2F1) and microRNAs (miRNAs) have been shown to play critical roles in the developing and functional inner ear. Based on previous studies suggesting interplay between NR2F1 and miRNAs, we investigated the coregulation between NR2F1 and miRNAs to better understand the regulatory mechanisms of inner ear development and functional maturation.
DOI:10.3389/fnmol.2018.00022URLPMID:29440989 [本文引用: 1]

Hibernating 13-lined ground squirrels (Ictidomys tridecemlineatus; TLGS) rank among the most brain hypoperfusion-tolerant mammals known. Herein we provide some evidence of cycling between an epithelial phenotype and a hybrid epithelial/mesenchymal (E/M) phenotype (partial EMT) within the brains of TLGS during each bout of hibernation torpor. During hibernation torpor, expression of the epithelial marker E-cadherin (E-CDH) was reduced, while expression of the well-known mesenchymal markers vimentin and Sox2 were increased. P-cadherin (P-CDH), which has recently been proposed as a marker of intermediate/partial EMT, also increased during torpor, suggesting that a partial EMT may be taking place during hibernation torpor. Members of the miR-200 family and miR-182 cluster and Akt isoforms (Akt1, Akt2), well-known EMT regulators, were also differentially regulated in the TLGS brain during hibernation bouts. Using SHSY5Y cells, we also demonstrate that the Akt1/Akt2 ratio determined the expression levels of miR-200/miR-182 miRNA family members, and that these miRNAs controlled the expression of EMT-related proteins. Accordingly, we propose that such cell state transitions (EMT/MET) may be one of the mechanisms underlying the extraordinary ischemic tolerance of the TLGS brain during hibernation bouts; hibernator brain cells appear to enter reversible states that confer the stress survival characteristics of cancer cells without the risk of neoplastic transformation.
DOI:10.1038/embor.2008.74URLPMID:18483486 [本文引用: 1]

The embryonic programme 'epithelial-mesenchymal transition' (EMT) is thought to promote malignant tumour progression. The transcriptional repressor zinc-finger E-box binding homeobox 1 (ZEB1) is a crucial inducer of EMT in various human tumours, and was recently shown to promote invasion and metastasis of tumour cells. Here, we report that ZEB1 directly suppresses transcription of microRNA-200 family members miR-141 and miR-200c, which strongly activate epithelial differentiation in pancreatic, colorectal and breast cancer cells. Notably, the EMT activators transforming growth factor beta2 and ZEB1 are the predominant targets downregulated by these microRNAs. These results indicate that ZEB1 triggers an microRNA-mediated feedforward loop that stabilizes EMT and promotes invasion of cancer cells. Alternatively, depending on the environmental trigger, this loop might switch and induce epithelial differentiation, and thus explain the strong intratumorous heterogeneity observed in many human cancers.
DOI:10.1016/j.heares.2014.05.002URLPMID:24924414 [本文引用: 1]

Sensorineural hearing loss (SNHL) is the most common cause of hearing impairment. One of the essential steps to prevent progressive hearing loss is to protect spiral ganglion neurons (SGNs) from ongoing degeneration. MicroRNAs and TMPRSS3 (transmembrane protease, serine 3) have been reported to be involved in development of SGNs and genesis of SNHL. The aim of this study was to investigate the role of miR-204 and TMPRSS3 in SGNs. Effect of miR-204 on cell viability of SGNs was first examined using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay. Expression of TMPRSS3 in SGNs with or without addition of miR-204 was assessed by real-time PCR and western blot further. A luciferase reporter activity assay was conducted to confirm target association between miR-204 and 3'-UTR of TMPRSS3. Finally, role of TMPRSS3 on cell viability of SGNs was evaluated by transfection of TMPRSS3 siRNA. Cell viability of SGNs was suppressed by miR-204 in a concentration-dependent manner. Overexpression of miR-204 reduced expression of TMPRSS3 in SGNs at both mRNA and protein levels. Binding to the 3'-UTR of TMPRSS3 by miR-204 was identified by luciferase assay. Knockdown of TMPRSS3 by siRNA significantly inhibits cell viability of SGNs. miR-204 could be a potential therapeutic target in sensorineural hearing loss.
DOI:10.1093/hmg/ddu023URL [本文引用: 1]

microRNAs (miRNAs) are regulators of differentiation and development of inner ear cells. Mutations in miRNAs lead to deafness in humans and mice. Among inner ear pathologies, inflammation may lead to structural and neuronal defects and eventually to hearing loss and vestibular dysfunction. While the genetic factors of these pathways have not been defined, autoimmunity participates in these processes. We report that inflammatory stimuli in the inner ear induce activation of the innate immune system via miR-224 and pentraxin 3 (Ptx3). miR-224 is a transcriptional target of nuclear factor kappa B, a key mediator of innate immunity. Ptx3 is a regulator of the immune response. It is released in response to inflammation and regulated by nuclear factor kappa B. We show that miR-224 and Ptx3 are expressed in the inner ear and we demonstrate that miR-224 targets Ptx3. As a model of the innate immune response, we injected lipopolysaccharide into the scala tympani of mouse inner ears. This resulted in changes in the levels of miR-224 and Ptx3, in addition to activation of the complement system, as measured by immune cell infiltration and activated C3. This suggests that while miR-224 regulates Ptx3 under normal conditions, upon inflammation, both are recruited to offer a front line of defense in acting as responders to inflammation in the inner ear. miR-224 diminishes the innate immune response by down-regulating Ptx3 expression, while Ptx3 stimulates the innate immune response. An understanding of the molecular components of the inflammatory pathway may help develop therapeutics for reducing inflammation associated with inner ear injury.
DOI:10.1371/journal.pgen.0020004URLPMID:16410827 [本文引用: 1]

In mammals, six separate sensory regions in the inner ear are essential for hearing and balance function. Each sensory region is made up of hair cells, which are the sensory cells, and their associated supporting cells, both arising from a common progenitor. Little is known about the molecular mechanisms that govern the development of these sensory organs. Notch signaling plays a pivotal role in the differentiation of hair cells and supporting cells by mediating lateral inhibition via the ligands Delta-like 1 and Jagged (JAG) 2. However, another Notch ligand, JAG1, is expressed early in the sensory patches prior to cell differentiation, indicating that there may be an earlier role for Notch signaling in sensory development in the ear. Here, using conditional gene targeting, we show that the Jag1 gene is required for the normal development of all six sensory organs within the inner ear. Cristae are completely lacking in Jag1-conditional knockout (cko) mutant inner ears, whereas the cochlea and utricle show partial sensory development. The saccular macula is present but malformed. Using SOX2 and p27kip1 as molecular markers of the prosensory domain, we show that JAG1 is initially expressed in all the prosensory regions of the ear, but becomes down-regulated in the nascent organ of Corti by embryonic day 14.5, when the cells exit the cell cycle and differentiate. We also show that both SOX2 and p27kip1 are down-regulated in Jag1-cko inner ears. Taken together, these data demonstrate that JAG1 is expressed early in the prosensory domains of both the cochlear and vestibular regions, and is required to maintain the normal expression levels of both SOX2 and p27kip1. These data demonstrate that JAG1-mediated Notch signaling is essential during early development for establishing the prosensory regions of the inner ear.
DOI:10.1016/j.neuron.2018.07.033URLPMID:30138589 [本文引用: 1]

The proteins that form the permeation pathway of mechanosensory transduction channels in inner-ear?hair cells have not been definitively identified. Genetic, anatomical, and physiological evidence support a role for transmembrane channel-like protein (TMC) 1 in hair cell sensory transduction, yet the molecular function of TMC proteins remains unclear. Here, we provide biochemical evidence suggesting TMC1 assembles as a dimer, along with structural and sequence analyses suggesting similarity to dimeric TMEM16 channels. To identify the pore region of TMC1, we used cysteine mutagenesis and expressed mutant TMC1 in hair cells of Tmc1/2-null mice. Cysteine-modification reagents rapidly and irreversibly altered permeation properties of mechanosensory transduction. We propose that TMC1 is structurally similar to TMEM16 channels and includes ten transmembrane domains with four domains, S4-S7, that line the channel pore. The data provide compelling evidence that TMC1 is a pore-forming component of sensory transduction channels in auditory and vestibular hair cells.
DOI:10.1038/nrd4533URLPMID:25792261 [本文引用: 2]

Hearing loss is the most common form of sensory impairment in humans and affects more than 40 million people in the United States alone. No drug-based therapy has been approved by the Food and Drug Administration, and treatment mostly relies on devices such as hearing aids and cochlear implants. Over recent years, more than 100 genetic loci have been linked to hearing loss and many of the affected genes have been identified. This understanding of the genetic pathways that regulate auditory function has revealed new targets for pharmacological treatment of the disease. Moreover, approaches that are based on stem cells and gene therapy, which may have the potential to restore or maintain auditory function, are beginning to emerge.
DOI:10.1126/science.284.5421.1837URLPMID:10364557 [本文引用: 1]

The mammalian inner ear contains the cochlea and vestibular organs, which are responsible for hearing and balance, respectively. The epithelia of these sensory organs contain hair cells that function as mechanoreceptors to transduce sound and head motion. The molecular mechanisms underlying hair cell development and differentiation are poorly understood. Math1, a mouse homolog of the Drosophila proneural gene atonal, is expressed in inner ear sensory epithelia. Embryonic Math1-null mice failed to generate cochlear and vestibular hair cells. This gene is thus required for the genesis of hair cells.
DOI:10.16288/j.yczz.17-037URLPMID:29070485 [本文引用: 1]

Wnt signaling pathway plays important roles in the development and homeostasis of multicellular organisms. Through their bindings with the Frizzled receptors, the Wnt ligands regulate a wide range of developmental processes, such as axis patterning, cell division, and cell fate specification. Wnt signaling plays vital roles in the development of inner ear of the mouse. In the early stages of inner ear development, Wnt signaling specifies the size of the placode and the formation of the otic vesicle. In later stages, Wnt signaling mediates hair cell specification and orients the stereociliary bundles in a uniform direction. In this review, we summarize the current knowledge on the roles of Wnt signaling in hair cell differentiation and regeneration, which may provide references and insights for investigators in the field.
DOI:10.16288/j.yczz.17-037URLPMID:29070485 [本文引用: 1]

Wnt signaling pathway plays important roles in the development and homeostasis of multicellular organisms. Through their bindings with the Frizzled receptors, the Wnt ligands regulate a wide range of developmental processes, such as axis patterning, cell division, and cell fate specification. Wnt signaling plays vital roles in the development of inner ear of the mouse. In the early stages of inner ear development, Wnt signaling specifies the size of the placode and the formation of the otic vesicle. In later stages, Wnt signaling mediates hair cell specification and orients the stereociliary bundles in a uniform direction. In this review, we summarize the current knowledge on the roles of Wnt signaling in hair cell differentiation and regeneration, which may provide references and insights for investigators in the field.
DOI:10.1007/s10162-013-0414-zURL [本文引用: 1]

Balance disorders caused by hair cell loss in the sensory organs of the vestibular system pose a significant health problem worldwide, particularly in the elderly. Currently, this hair cell loss is permanent as there is no effective treatment. This is in stark contrast to nonmammalian vertebrates who robustly regenerate hair cells after damage. This disparity in regenerative potential highlights the need for further manipulation in order to stimulate more robust hair cell regeneration in mammals. In the utricle, Notch signaling is required for maintaining the striolar support cell phenotype into the second postnatal week. Notch signaling has further been implicated in hair cell regeneration after damage in the mature utricle. Here, we investigate the role of Notch signaling in the mature mammalian cristae in order to characterize the Notch-mediated regenerative potential of these sensory organs. For these studies, we used the gamma-secretase inhibitor, N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT), in conjunction with a method we developed to culture cristae in vitro. In postnatal and adult cristae, we found that 5 days of DAPT treatment resulted in a downregulation of the Notch effectors Hes1 and Hes5 and also an increase in the total number of Gfi1(+) hair cells. Hes5, as reported by Hes5-GFP, was downregulated specifically in peripheral support cells. Using lineage tracing with proteolipid protein (PLP)/CreER;mTmG mice, we found that these hair cells arose through transdifferentiation of support cells in cristae explanted from mice up to 10 weeks of age. These transdifferentiated cells arose without proliferation and were capable of taking on a hair cell morphology, migrating to the correct cell layer, and assembling what appears to be a stereocilia bundle with a long kinocilium. Overall, these data show that Notch signaling is active in the mature cristae and suggest that it may be important in maintaining the support cell fate in a subset of peripheral support cells.
DOI:10.1242/dev.095398URL [本文引用: 1]

Mechanosensory hair cells and supporting cells develop from common precursors located in the prosensory domain of the developing cochlear epithelium. Prosensory cell differentiation into hair cells or supporting cells proceeds from the basal to the apical region of the cochleae, but the mechanism and significance of this basal-to-apical wave of differentiation remain to be elucidated. Here, we investigated the role of Hedgehog (Hh) signaling in cochlear development by examining the effects of up-and downregulation of Hh signaling in vivo. The Hh effector smoothened (Smo) was genetically activated or inactivated specifically in the developing cochlear epithelium after prosensory domain formation. Cochleae expressing a constitutively active allele of Smo showed only one row of inner hair cells with no outer hair cells (OHCs); abnormal undifferentiated prosensory-like cells were present in the lateral compartment instead of OHCs and their adjacent supporting cells. This suggests that Hh signaling inhibits prosensory cell differentiation into hair cells or supporting cells and maintains their properties as prosensory cells. Conversely, in cochlea with the Smo conditional knockout (Smo CKO), hair cell differentiation was preferentially accelerated in the apical region. Smo CKO mice survived after birth, and exhibited hair cell disarrangement in the apical region, a decrease in hair cell number, and hearing impairment. These results indicate that Hh signaling delays hair cell and supporting cell differentiation in the apical region, which forms the basal-to-apical wave of development, and is required for the proper differentiation, arrangement and survival of hair cells and for hearing ability.
DOI:10.1096/fj.09-1201ufmURLPMID:19948522 [本文引用: 1]

The gamma isoform of phosphoinositide 3-kinase (PI3Kgamma) has been viewed as restricted to leukocytes mediating the regulation of chemokine-induced migration and recruitment of neutrophils, monocytes, and macrophages. In line with the observation that PI3Kgamma-deficient mice display defects in adaptive immunity, inhibition of PI3Kgamma reduces synovial inflammation in the collagen-induced arthritis mouse model of inflammatory arthritis [rheumatoid arthritis (RA)], which has been attributed to reduced influx of inflammatory cells. Challenging the concept of leukocyte-restricted PI3Kgamma function, we report here a novel, nonredundant function of PI3Kgamma as an important regulator of fibroblast-induced cartilage destruction during chronic destructive arthritis. We show that in human tumor necrosis factor transgenic mice, the loss of PI3Kgamma leads to a milder inflammatory arthritis. Interestingly, PI3Kgamma deficiency does not alter the recruitment of inflammatory cells, but significantly reduces cartilage damage through reduced expression of matrix metalloproteinases in fibroblasts and chondrocytes. In vitro analyses demonstrate that the decreased invasiveness of fibroblasts is mediated by reduced phosphorylation of Akt and extracellular signal-regulated kinase. Using a PI3Kgamma specific inhibitor, these data are confirmed in human synovial fibroblasts from patients with RA who exhibit a disease-specific up-regulation of PI3Kgamma. Our data indicate that in addition to mediating the recruitment of inflammatory cells, PI3Kgamma is an important regulator of fibroblast-mediated joint destruction in RA and suggest that specific inhibitors of PI3Kgamma will interfere with the activation of RA synovial fibroblasts and reduce cartilage destruction in RA.
DOI:10.3346/jkms.2006.21.1.136URLPMID:16479080 [本文引用: 1]

In order to investigate the expression patterns of the transforming growth factor (TGF)beta isoforms in the internal ear, an immunohistochemical study of rat embryos was performed. Rat embryos were taken on the 13th, 15th, 17th, and 19th day after conception and their internal ears were immunohistochemically stained against TGF beta1, beta2, and beta3. As a result, the 13-day-old embryo showed a very weak positivity to TGF beta1. After the 15th day of pregnancy, no reactivity to TGF beta1 was defected. Immunoreactivity to TGF beta2 was observed from the 15th day of pregnancy throughout the rest of the period. The ampulla of the semicircular canal and the cochlear duct showed a notably strong immunohistochemical reaction. A strong reaction to TGF beta3 was observed on the 15th day of pregnancy. However, no positive reactions were observed thereafter. A strong immunoreactivity was observed especially on the apical cytoplasms, the surfaces of the epithelial cells, and basement membranes of the cochlear duct, as well as the semicircular canals of the developing internal ear of rat embryo.
DOI:10.1371/journal.pone.0148339URLPMID:26859490 [本文引用: 1]

In the inner ear Wnt signaling is necessary for proliferation, cell fate determination, growth of the cochlear duct, polarized orientation of stereociliary bundles, differentiation of the periotic mesenchyme, and homeostasis of the stria vascularis. In neonatal tissue Wnt signaling can drive proliferation of cells in the sensory region, suggesting that Wnt signaling could be used to regenerate the sensory epithelium in the damaged adult inner ear. Manipulation of Wnt signaling for regeneration will require an understanding of the dynamics of Wnt pathway gene expression in the ear. We present a comprehensive screen for 84 Wnt signaling related genes across four developmental and postnatal time points.
DOI:10.1016/j.cellsig.2014.02.003URL [本文引用: 1]

Differentiation of preadipocytes into adipocytes and the formation of the subsequent adipose tissue are critical for mammalian growth and development. The molecular mechanism relating to preadipocyte differentiation and adipogenesis from the perspective of miRNAs is not yet completely understood. Here we investigated whether miR-183 functioned in the differentiation process. Both gain-of-function and loss-of-function assays demonstrated that miR-183 positively regulated 3T3-L1 differentiation by enhancing the expression of adipogenic marker genes such as CCAAT/enhancer binding protein a (C/EBP alpha), peroxisome proliferator-activated receptor gamma(PPAR gamma), adiponectin and fatty acid synthase (FAS), as well as the triglyceride content and accumulation of lipid droplets. Meanwhile, low-density lipoprotein receptor-related protein 6 (LRP6) was known to impair the canonical Wnt/beta-catenin signaling pathway and thereafter reduce c-myc and nuclear beta-catenin protein. We showed that the inhibition of LRP6 by siRNA promoted 3T3-L1 adipogenic differentiation and adipogenesis. Further analysis showed that mouse miR-183 gene had its own transcription unit containing CpG islands, transcription start site (TSS), coding sequence (CDS) and polyA signal within the flanking sequences 2500 nt upstream and downstream of mouse miR-183 in genome. The core promoter of miR-183 gene was identified and transcription factor GATA3 (GATA binding protein 3) significantly inhibited the expression of mature miR-183 by binding to its core promoter in vivo, as indicated by thechromatin immunoprecipitation (ChIP) assay. These results suggest that miR-183, though negatively regulated by transcription factor GATA3, enhances 3T3-L1 preadipocyte differentiation and adipogenesis through the inhibition of the canonical Wnt/beta-catenin signaling pathway by targeting LRP6. (C) 2014 Elsevier Inc
DOI:10.1093/nar/gkt1275URLPMID:24335145 [本文引用: 1]

Glycogen synthase kinase 3 beta (GSK3β) is a critical protein kinase that phosphorylates numerous proteins in cells and thereby impacts multiple pathways including the β-Catenin/TCF/LEF-1 pathway. MicroRNAs (miRs) are a class of noncoding small RNAs of ~22 nucleotides in length. Both GSK3β and miR play myriad roles in cell functions including stem cell development, apoptosis, embryogenesis and tumorigenesis. Here we show that GSK3β inhibits the expression of miR-96, miR-182 and miR-183 through the β-Catenin/TCF/LEF-1 pathway. Knockout of GSK3β in mouse embryonic fibroblast cells increases expression of miR-96, miR-182 and miR-183, coinciding with increases in the protein level and nuclear translocation of β-Catenin. In addition, overexpression of β-Catenin enhances the expression of miR-96, miR-182 and miR-183 in human gastric cancer AGS cells. GSK3β protein levels are decreased in human gastric cancer tissue compared with surrounding normal gastric tissue, coinciding with increases of β-Catenin protein, miR-96, miR-182, miR-183 and primary miR-183-96-182 cluster (pri-miR-183). Furthermore, suppression of miR-183-96-182 cluster with miRCURY LNA miR inhibitors decreases the proliferation and migration of AGS cells. Knockdown of GSK3β with siRNA increases the proliferation of AGS cells. Mechanistically, we show that β-Catenin/TCF/LEF-1 binds to the promoter of miR-183-96-182 cluster gene and thereby activates the transcription of the cluster. In summary, our findings identify a novel role for GSK3β in the regulation of miR-183-96-182 biogenesis through β-Catenin/TCF/LEF-1 pathway in gastric cancer cells.
DOI:10.1073/pnas.1002827107URLPMID:20798046 [本文引用: 1]

During inner ear morphogenesis, the process of prosensory specification defines the specific regions of the otic epithelium that will give rise to the six separate inner ear organs essential for hearing and balance. The mechanism of prosensory specification is not fully understood, but there is evidence that the Notch intercellular signaling pathway plays a critical role. The Notch ligand Jagged1 (Jag1) is expressed in the prosensory domains, and mutation of Jag1 impairs sensory formation. Furthermore, pharmacological inhibition of Notch in vitro during prosensory specification disrupts the prosensory process. Additionally, activation of Notch by cDNA electroporation in chick otocysts results in formation of ectopic sensory patches. Here we test whether Notch activity is sufficient for prosensory specification in the mouse, using a Cre-/loxP approach to conditionally activate the Notch pathway in nonsensory regions of the inner ear epithelia during different stages of otic vesicle morphogenesis. We find that broad ectopic activation of Notch at very early developmental stages causes induction of prosensory markers throughout the entire otic epithelium. At later stages of development, activation of Notch in nonsensory regions leads to induction of sensory patches that later differentiate to form complete ectopic sensory structures. Activation of Notch in isolated nonsensory cells results in lateral induction of Jag1 expression in neighboring cells and spreading of prosensory specification to the adjacent cells through an intercellular mechanism. These results support a model where activation of Notch and propagation through lateral induction promote prosensory character in specific regions of the developing otocyst.
DOI:10.3760/cma.j.issn.1673-0860.2018.11.007URLPMID:30453402 [本文引用: 1]

Objective: To screen the key microRNAs targeting Notch signaling pathway in inner ear and investigate its potential regulating function. Methods: The interaction network and the Core-Notch network, involved with key genes in Notch signal pathway and differential-expressed microRNAs in inner ear, were constructed by bioinformatics methods. The important microRNAs in regulating Notch signaling pathway were screened via topological and GO analysis, followed by in vivo and in vitro investigation. Results: MiRNA-384-5p was identified as a key regulator specifically expressed in mouse brain and inner ear, which could down-regulate Notch1. The Notch1 expression was found significantly down-regulated in miRNA-384-5p-mimic-transfected HeLa cells. The dual-luciferase reporter gene assay further confirmed the effect of miRNA-384-5p on the down-regulation of Notch1 and Dll4 in Notch signaling pathway. Conclusions: The Core-Notch network is constructed to screen microRNAs implicated in inner ear development, and miRNA-384-5p is screened and verified to be target-regulating the Notch signaling pathway, which could be the potential target in the regeneration of impaired hair cells.
DOI:10.3892/mmr.2018.9127URLPMID:29901127 [本文引用: 1]

Auditory hair cell regeneration following injury is critical to hearing restoration. The Notch signaling pathway participates in the regulation of inner ear development and cell differentiation. Recent evidence suggests that microRNA (miR)?183 has a similar role in the inner ear. However, it is unclear how Notch signaling functions in hair cell regeneration in mammals and if there is cross?talk between Notch signaling and miR?183. The present study used a gentamicin?induced cochlear injury mouse model. Gentamicin?induced damage of the hair cells activated the Notch signaling pathway and downregulated miR?183 expression. Notch signaling inhibition by the γ?secretase inhibitor, 24?diamino?5?phenylthiazole (DAPT), attenuated gentamicin?induced hair cell loss and reversed the downregulation of miR?183 expression. Further investigation revealed that the novel hair cells produced, induced by DAPT, were derived from transdifferentiated supporting cells. Additionally, myosin VI?positive hair cell numbers were increased by Notch signaling inhibition in in?vitro experiments with cultured neonatal mouse inner ear precursor cells. This effect was reversed by miR?183 inhibition. These findings indicate that the Notch signaling pathway served a repressing role during the regeneration of hair cells. Inhibiting this signal improved hair cell regeneration in the gentamicin?damaged cochlear model. miR?183 was demonstrated to be involved in hair cell differentiation and regeneration, and was required for the differentiation of the Notch?inhibited hair cells.
DOI:10.16288/j.yczz.17-407URLPMID:30021715 [本文引用: 1]

The inner ear is a complex sensory organ that detects sound and mediates balance. During inner ear development, fibroblast growth factor (FGF) signaling pathway is involved in the induction of otic placode, cell fate determination of statoacoustic ganglion (SAG) neurons, and epithelial differentiation of the Corti organ. FGF signaling initiates the regulatory network of otic genes in the early development of inner ear, and induces the formation of pre-placodal region and the otic placode. The specification of the neuroblast ventral to the otic vesicle could be promoted by the normally-expressed FGF, and inhibited by excessive FGF5 secreted by mature SAG neurons, which could form a negative feedback loop and stabilize the SAG cell identity. The expression of FGF20 is regulated by the Notch signaling pathway and implicated in the differentiation of hair cells and supporting cells in the prosensory epithelium. FGF8 secreted by hair cells could regulate the differentiation of partial supporting cells into pillar cells. Abnormal FGF signaling in humans could lead to different kinds of deafness-related genetic diseases. In addition, it is noteworthy that FGF signaling pathway plays an important role in hair cell regeneration and induction from stem cells in lower vertebrates. In this review, we summarize recent advancements on roles of the FGF signaling pathway in inner ear development and hair cell regeneration, and lay a theoretical foundation for elucidating the regulatory mechanisms of FGF signal pathway in hair cell regeneration.
DOI:10.16288/j.yczz.17-407URLPMID:30021715 [本文引用: 1]

The inner ear is a complex sensory organ that detects sound and mediates balance. During inner ear development, fibroblast growth factor (FGF) signaling pathway is involved in the induction of otic placode, cell fate determination of statoacoustic ganglion (SAG) neurons, and epithelial differentiation of the Corti organ. FGF signaling initiates the regulatory network of otic genes in the early development of inner ear, and induces the formation of pre-placodal region and the otic placode. The specification of the neuroblast ventral to the otic vesicle could be promoted by the normally-expressed FGF, and inhibited by excessive FGF5 secreted by mature SAG neurons, which could form a negative feedback loop and stabilize the SAG cell identity. The expression of FGF20 is regulated by the Notch signaling pathway and implicated in the differentiation of hair cells and supporting cells in the prosensory epithelium. FGF8 secreted by hair cells could regulate the differentiation of partial supporting cells into pillar cells. Abnormal FGF signaling in humans could lead to different kinds of deafness-related genetic diseases. In addition, it is noteworthy that FGF signaling pathway plays an important role in hair cell regeneration and induction from stem cells in lower vertebrates. In this review, we summarize recent advancements on roles of the FGF signaling pathway in inner ear development and hair cell regeneration, and lay a theoretical foundation for elucidating the regulatory mechanisms of FGF signal pathway in hair cell regeneration.
DOI:10.1016/s0092-8674(03)00231-9URLPMID:12679032 [本文引用: 1]

Cell proliferation, cell death, and pattern formation are coordinated in animal development. Although many proteins that control cell proliferation and apoptosis have been identified, the means by which these effectors are linked to the patterning machinery remain poorly understood. Here, we report that the bantam gene of Drosophila encodes a 21 nucleotide microRNA that promotes tissue growth. bantam expression is temporally and spatially regulated in response to patterning cues. bantam microRNA simultaneously stimulates cell proliferation and prevents apoptosis. We identify the pro-apoptotic gene hid as a target for regulation by bantam miRNA, providing an explanation for bantam's anti-apoptotic activity.
DOI:10.1038/cddis.2014.407URLPMID:25275594 [本文引用: 1]

MicroRNAs (miRNAs) have important roles in various types of cellular biological processes. Our study aimed to determine whether miRNAs function in the regulation of ionizing radiation (IR)-induced cell death in auditory cells and to determine how they affect the cellular response to IR. Microarray and qRT-PCR were performed to identify and confirm the differential expression of miRNAs in the cochlea hair cell line HEI-OC1 and in vivo after IR. Upregulation or downregulation of miRNAs using miRNA mimics or inhibitor were detected to characterize the biological effects of the indicated miRNAs. Bioinformatic analyses, luciferase reporter assays and mRNA knockdown were performed to identify a miRNA target gene. We determined that miR-207 was significantly upregulated after IR. MiR-207 enhances IR-induced apoptosis and DNA damage in HEI-OC1 cells. Furthermore, Akt3 was confirmed to be a direct target of miR-207. Downregulation of Akt3 mimics the effects of miR-207. MiR-207 enhances IR-induced apoptosis by directly targeting Akt3 and anti-miR-207 may have a potential role in protecting cochlea hair cells from IR.
DOI:10.1007/bf00179956URLPMID:8950550 [本文引用: 1]

The expression of epidermal growth factor (EGF), EGF receptor and transforming growth factor (TGF)-alpha was analyzed in the human fetal inner ear using immuno-histochemical methods. EGF receptor was observed only in 9.5-week-old fetal vestibular epithelia. In 14- and 16-week-old fetuses, EGF receptor could not be detected. TGF-alpha was observed strongly in the 9- and 11-week-old vestibular epithelia, whereas only trace amounts were detectable in the 14- and 16-week-old vestibular epithelia. These findings suggest that EGF and TGF-alpha probably have a mitogenic effect in the sensory epithelia of the fetal inner ear, especially at early stages of development.
DOI:10.1016/j.biopha.2015.01.016URLPMID:25776494 [本文引用: 1]

The aim of this study was to investigate the function of miR-183 in the SW1990 cancer cell line, and the mechanisms regulating these processes. miRNAs are known to play important roles in cancer cell development. However, the pattern and biological role of miR-183 in pancreatic cancer remain largely unknown. Here, we have reported the reduction in pancreatic cancer cell growth in vitro by miR-183 intervention, by inducing apoptosis and decreasing the Bcl-2 expression. Moreover, miR-183 was observed to enhance pancreatic cancer cell migration and invasion, whereas inhibition of miR-183 caused an opposite effect. miR-183 inhibition was shown to increase E-cadherin expression and decrease N-cadherin expression. These regulatory actions play an important role in the cancer epithelial-mesenchymal transition (EMT). Mechanistically, we demonstrated that the overexpression of miR-183 decreased the expression of PDCD4 (programmed cell death 4) mRNA and protein, and vice versa. This helped to identify PDCD4 as the target genes in pancreatic cancer. In conclusion, our analyses indicated miR-183 to be an important contributor to cell migration. This could also be used as a potential therapeutic target for pancreatic cancer treatment.
DOI:10.1016/j.cell.2012.02.005URL [本文引用: 1]

Disease is often the result of an aberrant or inadequate response to physiologic and pathophysiologic stress. Studies over the last 10 years have uncovered a recurring paradigm in which microRNAs (miRNAs) regulate cellular behavior under these conditions, suggesting an especially significant role for these small RNAs in pathologic settings. Here, we review emerging principles of miRNA regulation of stress signaling pathways and apply these concepts to our understanding of the roles of miRNAs in disease. These discussions further highlight the unique challenges and opportunities associated with the mechanistic dissection of miRNA functions and the development of miRNA-based therapeutics.
DOI:10.3945/an.116.013839URLPMID:28096131 [本文引用: 1]

MicroRNAs (miRs) hybridize with complementary sequences in mRNA and silence genes by destabilizing mRNA or preventing translation of mRNA. Over 60% of human protein-coding genes are regulated by miRs, and 1881 high-confidence miRs are encoded in the human genome. Evidence suggests that miRs not only are synthesized endogenously, but also might be obtained from dietary sources, and that food compounds alter the expression of endogenous miR genes. The main food matrices for studies of biological activity of dietary miRs include plant foods and cow milk. Encapsulation of miRs in exosomes and exosome-like particles confers protection against RNA degradation and creates a pathway for intestinal and vascular endothelial transport by endocytosis, as well as delivery to peripheral tissues. Evidence suggests that the amount of miRs absorbed from nutritionally relevant quantities of foods is sufficient to elicit biological effects, and that endogenous synthesis of miRs is insufficient to compensate for dietary miR depletion and rescue wild-type phenotypes. In addition, nutrition alters the expression of endogenous miR genes, thereby compounding the effects of nutrition-miR interactions in gene regulation and disease diagnosis in liquid biopsies. For example, food components and dietary preferences may modulate serum miR profiles that may influence biological processes. The complex crosstalk between nutrition, miRs, and gene targets poses a challenge to gene network analysis and studies of human disease. Novel pipelines and databases have been developed recently, including a dietary miR database for archiving reported miRs in 15 dietary resources. miRs derived from diet and endogenous synthesis have been implicated in physiologic and pathologic conditions, including those linked with nutrition and metabolism. In fact, several miRs are actively regulated in response to overnutrition and tissue inflammation, and are involved in facilitating the development of chronic inflammation by modulating tissue-infiltrated immune cell function.
DOI:10.1089/ars.2017.7175URLPMID:28562070 [本文引用: 1]

MicroRNAs (miRNAs) are important regulators of gene expression and define part of the epigenetic signature. Their influence on every realm of biomedicine is established and progressively increasing. The impact of environment on human health is enormous. Among environmental risk factors impinging on quality of life are those of chemical nature (toxic chemicals, heavy metals, pollutants, and pesticides) as well as those related to everyday life such as exposure to noise or mental and psychosocial stress. Recent Advances: This review elaborates on the relationship between miRNAs and these environmental risk factors.
DOI:10.3389/fphar.2018.01113URLPMID:30349480 [本文引用: 1]

Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and remains the most common life-shortening diseases affecting the exocrine organs. The absence of this channel results in an imbalance of ion concentrations across the cell membrane and results in more abnormal secretion and mucus plugging in the gastrointestinal tract and in the lungs of CF patients. The direct introduction of fully functional CFTR by gene therapy has long been pursued as a therapeutical option to restore CFTR function independent of the specific CFTR mutation, but the different clinical trials failed to propose persuasive evidence of this strategy. The last ten years has led to the development of new pharmacotherapies which can activate CFTR function in a mutation-specific manner. Although approximately 2,000 different disease-associated mutations have been identified, a single codon deletion, F508del, is by far the most common and is present on at least one allele in approximately 70% of the patients in CF populations. This strategy is limited by chemistry, the knowledge on CFTR and the heterogenicity of the patients. New research efforts in CF aim to develop other therapeutical approaches to combine different strategies. Targeting RNA appears as a new and an important opportunity to modulate dysregulated biological processes. Abnormal miRNA activity has been linked to numerous diseases, and over the last decade, the critical role of miRNA in regulating biological processes has fostered interest in how miRNA binds to and interacts explicitly with the target protein. Herein, this review describes the different strategies to identify dysregulated miRNA opens up a new concept and new opportunities to correct CFTR deficiency. This review describes therapeutic applications of antisense techniques currently under investigation in CF.
DOI:10.1016/j.tins.2018.06.008URLPMID:30033182 [本文引用: 1]

Hearing loss in mammals is an irreversible process caused by degeneration of the hair cells of the inner ear. Current therapies for hearing loss include hearing aids and cochlear implants that provide substantial benefits to most patients, but also have several shortcomings. There is great interest in the development of regenerative therapies to treat deafness in the future. Cell-based therapies, based either on adult, multipotent stem, or other types of pluripotent cells, offer promise for generating differentiated cell types to replace lost or damaged hair cells of the inner ear. In this review, we focus on the methods proposed and avenues for research that seem the most promising for stem cell-based auditory sensory cell regeneration, from work collected over the past 15 years.
DOI:10.1016/j.jcyt.2017.04.008URLPMID:28532627 [本文引用: 1]

Hearing loss, or deafness, affects 360 million people worldwide of which about 32 million are children. Deafness is irreversible when it involves sensory hair cell death because the regenerative ability of these cells is lost in mammals after embryo development. The therapeutic strategies for deafness include hearing aids and/or implantable devices. However, not all patients are eligible or truly benefit from these medical devices. Regenerative medicine based on stem cell application could play a role in both improvement of extant medical devices and in vivo recovery of auditory function by regeneration of inner ear cells and neurons. A review of recent literature on the subject indicates that two promising approaches to renewal and differentiation of cochlear tissues are transplantation of stem cells and in situ administration of growth factors. Rather than directly regenerating dead cells, these procedures apparently induce, through various pathways, differentiation of resident cochlear cells. More studies on the possible adverse effects of transplanted cells and the recovery of tonotopic sensorineural activity or required. To date, no reliable clinical results have been obtained in the field of cochlear regeneration.
DOI:10.1016/j.cell.2018.07.007URLPMID:30078709 [本文引用: 1]

In the auditory system, type I spiral ganglion neurons (SGNs) convey complex acoustic information from inner hair cells (IHCs) to the brainstem. Although SGNs exhibit variation in physiological and anatomical properties, it is unclear which features are endogenous and which reflect input from synaptic partners. Using single-cell RNA sequencing, we derived a molecular classification of mouse type I SGNs comprising three subtypes that express unique combinations of Ca2+ binding proteins, ion channel regulators, guidance molecules, and transcription factors. Based on connectivity and susceptibility to age-related loss, these subtypes correspond to those defined physiologically. Additional intrinsic differences among subtypes and across the tonotopic axis highlight an unexpectedly active role for SGNs in auditory processing. SGN identities emerge postnatally and are disrupted in a mouse model of deafness that lacks IHC-driven activity. These results elucidate the range, nature, and origins of SGN diversity, with implications for treatment of congenital deafness.
DOI:10.1016/j.semcdb.2012.08.011URLPMID:22960356 [本文引用: 1]

Embryonic hair follicle induction and formation are regulated by mesenchymal-epithelial interactions between specialized dermal cells and epidermal stem cells that switch to a hair fate. Similarly, during postnatal hair growth, communication between mesenchymal dermal papilla cells and surrounding epithelial matrix cells coordinates hair shaft production. Adult hair follicle regeneration in the hair cycle again is thought to be controlled by activating signals originating from the mesenchymal compartment and acting on hair follicle stem cells. Although many signaling pathways are implicated in hair follicle formation and growth, the precise nature, timing, and intersection of these inductive and regulatory signals remains elusive. The goal of this review is to summarize our current understanding and to discuss recent new insights into mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling.
DOI:10.1371/journal.pone.0020238URLPMID:21655280 [本文引用: 1]

MicroRNAs are a class of small regulatory RNAs that modulate a variety of biological processes, including cellular differentiation, apoptosis, metabolism and proliferation. This study aims to explore the effect of miR-34a in hepatocyte proliferation and its potential role in liver regeneration termination.
DOI:10.1016/j.ijcard.2015.06.163URLPMID:26298346 [本文引用: 1]

In mammals, the heart grows by hypertrophy but not proliferation of cardiomyocytes after birth. The paucity of cardiomyocyte proliferation limits cardiac regeneration in a variety of heart diseases. To explore the efficient strategies that drive cardiomyocyte proliferation, we employed in vitro and in vivo models to investigate the function of miRNA-204, which was demonstrated to regulate the proliferation and differentiation of human cardiac progenitor cells in our previous study.
DOI:10.1016/S0140-6736(17)32154-2URLPMID:28919117 [本文引用: 1]

As mortality rates decline, life expectancy increases, and populations age, non-fatal outcomes of diseases and injuries are becoming a larger component of the global burden of disease. The Global Burden of Diseases, Injuries, and Risk Factors Study 2016 (GBD 2016) provides a comprehensive assessment of prevalence, incidence, and years lived with disability (YLDs) for 328 causes in 195 countries and territories from 1990 to 2016.
DOI:10.1371/journal.pone.0195227URLPMID:29596478 [本文引用: 1]

Hearing loss is the most common sensory impairment, but limited studies focused on the association of socioeconomic status (SES) with hearing loss among adults of working age. This paper aimed to fill this gap among Chinese adults. We obtained data from Ear and Hearing Disorder Survey conducted in four provinces of China in 2014-2015. The survey was based on WHO Ear and Hearing Disorders Survey Protocol and 25,860 adults aged 25 to 59 years were selected in this study. Trained local examiners performed pure tone audiometry to screen people with hearing loss, and those who were screened positively for hearing loss were referred to audiologists to make final diagnosis. SES was measured by occupation, education and income. Results show after adjusting for SES measures and covariates, in urban areas, compared with white-collar workers, blue-collar workers and the unemployed were more likely to have hearing loss, with an odds ratio of 1.2 (95%CI: 1.0, 1.3) and 1.2 (95%CI: 1.0, 1.4), respectively. Compared with people with education of senior high school or above, those with junior high school, primary school and illiteracy had 1.6 (95%CI: 1.4, 1.8), 2.1(95%CI: 1.7, 2.5) and 2.6 (95%CI: 1.9, 3.7) times as likely to have hearing loss, respectively. In rural areas, the unemployed had 1.5 (95%CI: 1.0, 2.3) times the risk of hearing loss compared with white-collar workers, and illiterates had 1.6 (95%CI: 1.6, 2.1) times the risk of hearing loss compared with people with education of senior high school or above, after SES variables and covariates were taken into considerations. Income was not significantly associated with hearing loss in urban and rural areas. In conclusion, SES, in the form of occupation and education, was associated with hearing loss among working-aged population, and further studies are needed to explore the mechanism of such association.
DOI:10.1016/j.brainres.2009.02.027URLPMID:19245798 [本文引用: 1]

The impact of small RNA function has resonated throughout nearly every aspect of eukaryotic biology and captured the varied interests of researchers, whether they are endeavoring to understand the basis of development and disease or seeking novel therapeutic targets and tools. The genetic regulatory roles of microRNAs (miRNAs) are particularly interesting given that these often highly conserved factors post-transcriptionally silence many complementary target genes by inhibiting messenger RNA translation. In this regard, miRNAs can be considered as counterparts to transcription factors, the ensemble of which establishes the set of expressed genes that define the characteristics of a specific cell type. In this review, evidence supporting a resounding role for small RNAs in development and maturation of sensory epithelia in the mouse inner ear will be considered with an emphasis on the contribution of one hair cell miRNA family (miR-183, miR-96, and miR-182). Although there is much yet to be explored in this fledgling aspect of ear biology, the breadth of miRNA expression and functional requirement for ear development are already sounding off.
