 ,1,*, 石雷
,1,*, 石雷 ,1,*
,1,*Advances in Transcription Factors Regulating Plant Terpenoids Biosynthesis
Yanmei Dong1,2, Wenying Zhang1,2, Zhengyi Ling1,2, Jingrui Li1,2, Hongtong Bai1, Hui Li ,1,*, Lei Shi
,1,*, Lei Shi ,1,*
,1,*通讯作者:
责任编辑: 孙冬花
收稿日期:2019-09-19接受日期:2020-02-17网络出版日期:2020-05-01
| 基金资助: |
Corresponding authors:
Received:2019-09-19Accepted:2020-02-17Online:2020-05-01

摘要
关键词:
Abstract
Keywords:
PDF (1375KB)摘要页面多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
董燕梅, 张文颖, 凌正一, 李靖锐, 白红彤, 李慧, 石雷. 转录因子调控植物萜类化合物生物合成研究进展. 植物学报, 2020, 55(3): 340-350 doi:10.11983/CBB19186
Dong Yanmei, Zhang Wenying, Ling Zhengyi, Li Jingrui, Bai Hongtong, Li Hui, Shi Lei.
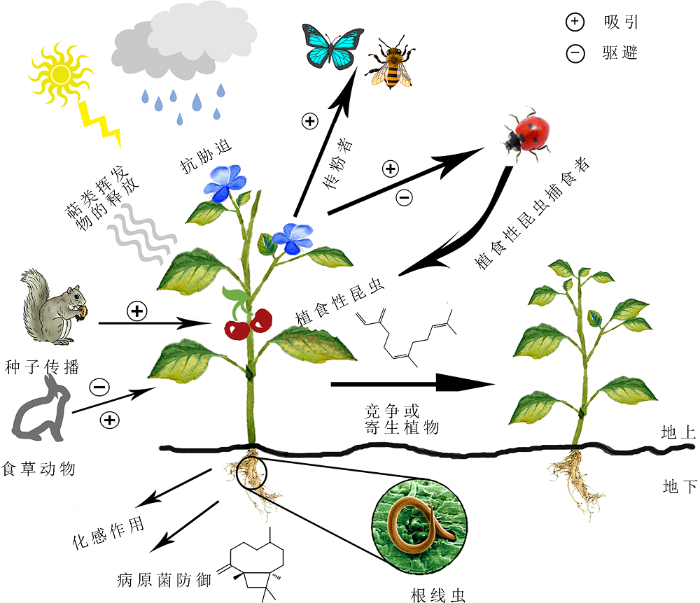
代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(孙俊聪等, 2019)。萜类化合物是由异戊二烯(C5)以头头或头尾方式连接而成的一类天然化合物的统称, 是植物次生代谢物中结构和数量最多的一类化合物(牟玉兰等, 2018)。自然界中已有60 000多种萜类化合物及其衍生物被分离和鉴定出来(Brandt et al., 2009)。按照植物萜类化合物的作用, 其可分为初生代谢产物和次生代谢产物两大类。初生代谢产物主要包含广泛参与植物生长发育的植物激素, 如赤霉素(gibberellin, GA)、脱落酸(abscisic acid, ABA)和油菜素内酯(brassinolide, BR), 以及参与植物光合作用的类胡萝卜素和叶绿素(Chappell, 2003; Roberts, 2007; Vranová et al., 2012)。植物中大部分萜类化合物属于次生代谢产物, 它们在植物与外界环境相互作用中发挥重要作用(图1), 如吸引昆虫传粉、趋避食草动物、抵抗逆境胁迫以及参与植物对微生物的防御(Gershenzon and Dudareva, 2007; Ben-Yehoshua et al., 2008; Moses et al., 2013)。萜类化合物除具天然作用外, 还因其独特的香气和风味, 被广泛用于化妆品及香水工业和作为食品添加剂等(Schwab et al., 2008; Caputi and Aprea, 2011)。
图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1挥发物介导的植物与周围环境的相互作用(改自Abbas et al., 2017)
Figure 1A summary of volatile-mediated interactions between plants and their surrounding environment (modified from Abbas et al., 2017)
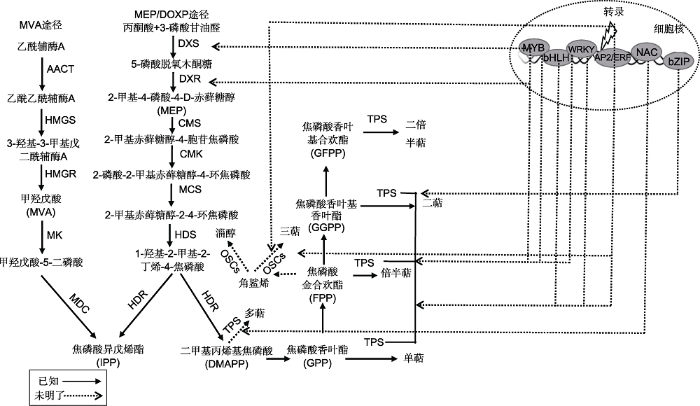
众所周知, 植物萜类化合物的合成不是单一过程, 不仅受到甲羟戊酸(mevalonic acid, MVA)途径和磷酸赤藓糖(methylerythritol phosphate, MEP)途径多种酶促反应和关键酶基因表达的影响(图2), 还受到外源诱导子(如茉莉酸(jasmonic acid, JA)和水杨酸(salicylic acid, SA))的诱导及转录因子的调控(岳跃冲和范燕萍, 2011)。
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2植物萜类化合物的生物合成途径(图中相近的转录因子可互作共同行使生物功能)
AACT: 乙酰乙酰辅酶A硫解酶; HMGS: 3-羟基-3-甲基戊二酰辅酶A合酶; HMGR: 羟甲基戊二酰辅酶A还原酶; MK: 甲羟戊酸激酶; MDC: 甲羟戊酸5-二磷酸脱羧酶; DXS: 1-脱氧-D-木酮糖-5-磷酸合酶; DXR: 1-脱氧-D-木酮糖-5-磷酸还原酶; CMS: 4-二磷酸胞苷- 2-C-甲基-D-赤藓醇合酶; CMK: 4-二磷酸胞苷-2-C-甲基-D-赤藓糖激酶; MCS: 2-C-甲基-D-赤藓糖-2,4-环二磷酸合酶; HDS: 1-羟基- 2-甲基-2-(E)-丁烯基-4-二磷酸合酶; HDR: 1-羟基-2-甲基-2-(E)-丁烯基-4-焦磷酸还原酶; TPS: 萜类合酶总称; OSCs: 氧化鲨烯环化酶
Figure 2Biosynthetic pathways of terpenoids in plants (close transcription factors could interact with each other to perform biological functions)
AACT: Acetoacetyl-CoA thiolase; HMGS: 3-hydroxy-3-methylglutaryl-CoA synthase; HMGR: 3-hydroxy-3-methylglutaryl-CoA reductase; MK: Mevalonate kinase; MDC: Mevalonate diphosphate decarboxylase; DXS: 1-deoxy-D-xylulose 5-phosphate synthase; DXR: 1-deoxy-D-xylulose 5-phosphate reductase; CMS: 4-diphosphocytidyl-2-C-methyl-D-erythritol synthase; CMK: 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; MCS: 2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase; HDS: 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate synthase; HDR: 1-hydroxy-2-methyl-2-(E)-butenyl-4-pyrophosphate reductase; TPS: Terpenoid synthases; OSCs: Oxidosqualene cyclase
转录因子(transcription factors, TFs)又称反式作用分子, 通过与启动子结合来调控目的基因的转录水平, 进而调节次生代谢物质含量, 是目前常用的一种有效的基因工程工具(孙璐, 2018)。迄今为止, 尽管已有许多关于克隆、分离和对转录因子进行功能验证的报道, 但相关工作基本只在拟南芥(Arabidopsis thaliana)、青蒿(Artemisia annua)、丹参(Salvia miltiorrhiza)、番茄(Solanum lycopersicum)和水稻(Oryza sativa)等少数物种中展开。萜类化合物种类繁多, 且代谢调控过程复杂, 转录因子可同时参与萜类代谢相关基因簇中多个关键基因的表达调控(Zhou et al., 2016)。因此, 鉴定相关萜类化合物合成过程中的关键转录因子将有助于植物萜类相关合成生物学研究。
1 转录因子的结构与分类
1.1 转录因子的结构
真核生物中通常多种蛋白质因子协同完成转录。植物典型的转录因子包括转录调控区(transcription regulation domain, TRD)、核定位信号区(nuclear localization signal, NLS)、寡聚化位点(oligomerization site, OS)和DNA结合区(DNA binding domain, DBD)四部分(张凯伦等, 2017)。转录因子包括AP2/EREBP、bHLH、MYB、bZIP、MADS和Homeo等不同的结构域, 根据氨基酸残基的数量和位置, 其中一些结构域又可划分为几个亚类(刘强等, 2000)。1.2 转录因子的分类
转录因子通过其DNA结合域与顺式因子互作来实现调控基因的表达(起始抑制或增强)。根据其作用特性, 植物中的转录因子主要分为2类, 一类称为普通转录因子, 它们与RNA聚合酶II形成转录起始复合物, 非选择性地调控基因的转录; 另一类称为特异转录因子, 它们在特异的组织细胞中选择性地调控某种或某些基因的转录起始, 或者在受到一些类固醇激素/生长因子(或其它)刺激后, 开始表达的某些特异性转录因子。例如, SlEOT1在番茄腺毛中特异表达并且能够特异性激活SlTPS5的启动子(Spyropoulou et al., 2014a, 2014b)。由于转录因子必须在细胞核内作用才可行使调控表达的功能, 因此多数转录因子都具有核定位序列。转录因子中一般含有1个或多个核定位序列, 无核定位序列的转录因子须与其它转录因子结合后才可进入到细胞核内发挥作用。因此, 除以上2类, 我们通常把辅助特异转录因子行使和完成功能的一类转录因子称为辅助转录因子。根据其作用结果, 转录因子通常又可分为激活型和抑制型。例如, 丹参SmMYB9b为激活型转录因子, 其通过促进SmDXS2、SmDXR、SmGGPPS以及SmKSL1的表达进而提高丹参酮的合成(Zhang et al., 2017); 留兰香(Mentha spicata) MsMYB转录因子通过抑制GPPS的表达, 减少倍半萜(香柑油烯、吉玛烯和依兰油烯)和单萜(松萜)的含量(Reddy et al., 2017), 为抑制型转录因子。行使转录激活和抑制功能的部位主要是转录因子的转录激活区(transcription activation domain)和转录抑制区(transcription repression domain)。同一家族不同转录因子的主要区别在于转录激活区和抑制区存在差异。
2 参与萜类合成的主要转录因子
萜类化合物种类繁多且应用广泛, 但含量低。转录因子能调节次级代谢产物合成途径基因的转录水平。迄今为止, 植物中主要有6个TFs家族被报道(AP2/ ERF、bHLH、MYB、NAC、WRKY和bZIP) (表1)与萜类化合物的代谢相关(Xu et al., 2019)。Table 1
表1
表1植物中已分离和鉴定的参与调控萜类代谢合成途径的转录因子
Table 1
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | Yu et al., 2012 |
| AaERF2 | AEQ93555.1 | Yu et al., 2012 | ||
| TAR1 | EZ159016.1 | Tan et al., 2015 | ||
| AaORA | AGB07586.1 | Lu et al., 2013 | ||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | 张慧敏等, 2014 | |
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | Ji et al., 2014 |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | Hong et al., 2012 | |
| PIF5 | AT3G59060 | Mannen et al., 2014 | ||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | Zhou et al., 2016 | |
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | Chuang et al., 2018 | |
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | Tamura et al., 2018 | |
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | Yin et al., 2017 | |
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | Matías-Hernández et al., 2017 |
| PAP1 | AT4G04020 | Zvi et al., 2012 | ||
| AtMYB21 | AT3G27810 | Reeves et al., 2012 | ||
| AtMYB24 | AT5G40350 | Reeves et al., 2012 | ||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | Reddy et al., 2017 | |
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | 孙璐, 2018 | |
| BpMYB61 | KT344120 | 孙璐, 2018 | ||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | Lv et al., 2016 |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | Zhu et al., 2014 | |
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | Nieuwenhuizen et al., 2015 | |
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | Zhou et al., 2015 |
| bZIP1 | PWA69369.1 | Zhang et al., 2015 | ||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | Okada et al., 2009 | |
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | Wang et al., 2016 |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | Tang et al., 2018 | |
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 | Nieuwenhuizen et al., 2015 |
新窗口打开|下载CSV
2.1 AP2/ERF转录因子
AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (魏海超等, 2015)。根据结构域的数目和结构特点, AP2/ERF可划分为ERF、DREB、AP2和RAV四个亚族和1个soloist单独成员(Sakuma et al., 2002; Zhuang et al., 2009)。AP2/ERF家族转录因子AaERF1、AaERF2和AaTAR1参与调控青蒿中青蒿素的合成, 即AaERF1和AaERF2通过与ADS和CYP71AV1的启动子中CBF2 (CRTDREHVCBF2)及RAA (RAV1AAT)基序结合, 激活其启动子, 从而调节其表达, 参与青蒿素的合成(Yu et al., 2012); TAR1转录因子则是通过参与青蒿叶毛状体的起始发生来调控青蒿素的合成(Tan et al., 2015)。转录因子CitERF71与催化甜橙(Citrus sinensis)中一种重要挥发性单萜香叶醇的CitTPS16萜类合酶有类似的表达模式。此外, 酵母单杂交和双荧光素酶瞬时表达分析结果显示, CitERF71能激活CitTPS16的启动子(Li et al., 2017), 即CitERF71通过激活CitTPS16的启动子来调节CitTPS16香叶醇合酶的表达; 且AP2/ERF转录因子CitAP2.10的表达模式与催化合成甜橙烯的萜烯合成酶CsTPS1表达呈正相关, 乙烯可以增强CitAP2.10的表达, 而乙烯拮抗剂1-甲基环丙烯可消除这种作用(Shen et al., 2016)。因此, 可外施乙烯来调控转录因子CitAP2.10的表达从而调节甜橙烯的合成。张慧敏等(2014)在黄瓜(Cucumis sativus)中也发现了转录因子CsERF, 可结合到黄瓜苦味形成关键基因Bi的启动子并激活其转录。2.2 bHLH转录因子
bHLH类转录因子是真核生物蛋白质中的一个大家族, 在生物的生长发育和次生代谢中起重要作用(张全琪等, 2011; 李欣等, 2017)。其中MYC类转录因子是目前研究最多的一类。张凯伦等(2017)研究发现, MYC类转录因子参与调控茉莉酸信号途径, 且证明其与多种植物中萜类生物合成基因的调控有关。拟南芥MYC2转录因子能与催化形成倍半萜的TPS21和TPS11合酶基因的启动子区结合, 激活其表达, 从而增加倍半萜的释放量(Hong et al., 2012)。将转录因子AabHLH1在青蒿叶中过表达, 青蒿素合成途径中关键酶基因ADS以及CYP71AV1的表达量显著增加, AabHLH1通过激活ADS和CYP71AV1的启动子来调节青蒿素的生物合成(Ji et al., 2014)。水稻bHLH转录因子DPF通过与CPS2和CYP99A2启动子区的顺式作用元件(N-box)结合, 激活CPS2以及CYP99A2基因的转录, 从而促进二萜类抗毒素(diterpenoid phytoalexins, DPs)的合成(Yamamura et al., 2015)。Shang等(2014)在黄瓜叶和果实中分别得到2个特异性表达的bHLH转录因子Bl和Bt, 二者均与黄瓜三萜类化合物葫芦素C (cucurbitacin C, CuC)的合成有关。在拟南芥T87悬浮培养细胞中过表达bHLH转录因子PIF5, 发现四萜类化合物类胡萝卜素的含量增加(Mannen et al., 2014)。
2.3 MYB转录因子
MYB转录因子在动植物中普遍存在。植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(Paz-Ares et al., 1987; De Geyter et al., 2012)。其中, 部分MYB类转录因子促进萜类化合物的合成, 另一部分则起抑制作用(苏文炳等, 2019)。留兰香腺毛中的R2R3-MYB成员MsMYB直接与GPP合成酶大亚基(MsGPPS. LSU)的顺式作用元件结合, 抑制其基因的表达, 负调控萜类的合成(Reddy et al., 2017)。MYB36/MYB9b以及MYB1转录因子分别参与丹参中丹参酮(Ding et al., 2017; Zhang et al., 2017)和青蒿中青蒿素的生物合成调控(Matías-Hernández et al., 2017), 且均发挥促进作用。孙璐(2018)在过表达BpMYB21和BpMYB61白桦(Betula platyphylla)株系中, 发现2个基因的调控方式存在显著差异, 即BpMYB21促进三萜化合物的合成, BpMYB61抑制三萜化合物的合成。2.4 NAC转录因子
NAC是植物特异性转录因子(Olsen et al., 2005)。其结构域通常可被细分为A、B、C、D和E五个亚结构域, 其中A、C和D在不同物种中高度保守, 核定位信号一般在C和D亚结构域中, B和E亚结构域的保守性相对较弱(Duval et al., 2002; Ooka et al., 2003)。Lv等(2016)研究发现, AaNAC1转录因子不仅响应水杨酸、干旱及茉莉酸的诱导, 而且可激活青蒿素合成途径中ADS、DBR2和ALDH1等基因的表达, 促进青蒿素的积累, 转基因青蒿的耐旱及抗灰霉菌能力增强。猕猴桃中的NAC可激活单萜合酶TPS1的转录, 中华猕猴桃(Actinidia chinensis)与软枣猕猴桃(A. arguta) 2个种间单萜含量差异显著, 原因是AcTPS1发生自然突变, NAC无法与AcTPS1的启动子结合, 进而造成其转录水平差异所致(Nieuwenhuizen et al., 2015)。2.5 WRKY转录因子
WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构。根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(Eulgem et al., 2000; Zhang and Wang, 2005; Rushton et al., 2010, 2012)。植物体内的WRKY转录因子通过参与多种信号通路(如SA、ABA、JA和ET)来调控植物的生理生化过程, 从而参与植物的生长发育和胁迫应答(李笑等, 2017; 郭倩倩和周文彬, 2019)。Suttipanta等(2011)(Catharanthus roseus)中发现, CrWRKY1过表达可下调ORCA2/3、CrMYC2以及ZCTs的表达水平, 进而调控单萜类的合成。GaWRKY1在棉花(Gossypium arboreum)萼片、柱头、花药和发育的种子等器官中显示出与倍半萜烯合成关键酶CAD1- A相同的时间和空间表达模式, 即GaWRKY1可能通过正向调节CAD1-A的表达来调控棉花中倍半萜的合成(Xu et al., 2004)。AaWRKY1和ADS在青蒿腺毛中高度表达, 并且二者可被茉莉酸甲酯(methyl jasmonate, MeJA)诱导, AaWRKY1可结合ADS启动子的W-box顺式作用元件, 从而促进青蒿中青蒿素的合成(Jiang et al., 2016)。2.6 bZIP转录因子
bZIP类转录因子在真核生物中分布广泛且高度保守, 包含负责结合DNA的碱性氨基酸区域和1个亮氨酸拉链区(Hurst, 1994)。bZIP成员通常参与植物的抗逆响应和次生代谢调控。bZIP转录因子TGAP1被证实为水稻中二萜类植物抗毒素生物合成簇状基因和MEP途径基因协同表达的关键调节因子(Yamane, 2013), 且OsTGAP1的敲除突变体株系中5个簇基因(OsCPS4、OsKSL4、CYP99A2、CYP99A3和OsMAS)几乎不表达(Okada et al., 2009)。体外pull-down实验表明, OsTGAP1和OsbZIP79表现出异二聚以及同二聚相互作用。双分子荧光互补分析显示, 体内OsTGAP1和OsbZIP79之间存在相互作用(Miyamoto et al., 2014), 而OsbZIP79对水稻中双萜植物抗毒素的合成有抑制效果(Miyamoto et al., 2015)。ABA处理、干旱和盐胁迫可强烈诱导AabZIP1的表达, 酵母单杂交和EMSA实验表明, AabZIP1能与青蒿素生物合成途径的关键结构基因ADS和CYP71AV1启动子区的ABA反应元件(ABRE)结合, AabZIP1基因缺失突变体丧失了激活ADS和CYP71AV1启动子的能力, 青蒿AabZIP1的过表达株系中青蒿素合成显著增加, 即ABA可能通过AabZIP激活青蒿中ADS和CYP71AV1的表达来促进青蒿素的生物合成(Zhang et al., 2015)。Zhang等(2018)通过酵母双杂交、双分子荧光互补以及pull-down等方法证实青蒿中AaAPK1与AabZIP1相互作用, AaAPK1通过磷酸化来增强AabZIP1对青蒿素生物合成基因的反式激活活性(Zhang et al., 2018)。2.7 其它转录因子
除以上6个TFs家族外, 还有其它转录因子家族成员被发现与萜类化合物的代谢相关。YABBY5基因主要参与器官的发育和形态建成, 而Wang等(2016)发现, 优先在留兰香盾状腺毛中表达的MsYABBY5基因可抑制萜烯类化合物的合成。在青蒿中, 响应乙烯信号的EIN3可抑制青蒿素合成基因的表达, 最终降低青蒿素的水平(Tang et al., 2018)。此外, 丹参中JA信号转导抑制因子SmJAZ1/2/3/9过表达均显著促进丹参须根中丹参酮的积累(Shi et al., 2016; Zhou et al., 2017)。转录因子不仅可单独调控植物中的基因转录, 还可与其它转录因子形成复合物共同调节植物的次生代谢。研究发现, 在植物中MYB转录因子主要与IIId、IIIe和IIIf家族的bHLH转录因子形成转录复合物发挥作用(Albert et al., 2011; 赵英, 2019)。SmMYB36与SmMYC2在丹参中的表达模式一致, 过表达SmMYB36可提高丹参酮的含量, 且酵母双杂交结果显示两者能相互作用, 即SmMYB36与SmMYC2结合, 形成转录复合物调控丹参酮的合成代谢(赵英, 2019)。除了MYB和bHLH转录因子, WRKY类转录因子也是典型的多基因形成复合物, 进而行使生物学功能。研究表明, 植物体内WRKY转录因子并非组成型表达(李笑等, 2017)。在长春花中过表达CrWRKY1可下调ORCA2/3、CrMYC2及ZCTs的表达水平, 即CrWRKY1通过与CrMYC2等转录因子互作, 抑制其转录水平来调控单萜合酶的活性, 进而调控单萜类的合成(Suttipanta et al., 2011)。除了与其它家族的转录因子互作来发挥生物学作用外, 同一家族的转录因子也可形成复合体, 共同调控萜类的合成。例如, 水稻中bZIP家族的OsTGAP1和OsbZIP79相互作用, 共同调控二萜类化合物的合成。
植物萜类的合成是一个复杂的过程, 结构基因编码的一系列酶参与合成各种萜类途径相关的化合物, 调节基因则独自或通过相互作用形成复合物, 与结构基因启动子中可识别的顺式元件特异结合, 通过上调或下调结构基因的表达, 最终调控萜类化合物的合成(图2)。
3 展望
随着测序技术的快速发展, 许多植物的全基因组和转录组数据被公布, 如茶树(Camellia sinensis) (Wei et al., 2018)、薰衣草(Lavandula angustifolia) (Li et al., 2019)、白花百合(Lilium candidum) (唐彪等, 2018)和桂花(Osmanthus fragrans) (张雪松等, 2016)。参与萜类代谢途径的关键基因被预测, 并逐渐被分离和验证。研究发现, 通过转基因手段在植物组织或细胞中同源或异源表达, 均可增加代谢产物的含量。但由于多方面原因, 想要达到预期的效果, 需要同时表达多个基因。而转录因子可从基因表达的初期阶段(转录水平)进行调控, 因此转录因子成为科研工作者关注的热点。萜类化合物在植物与外界环境互作中发挥重要作用, 而理解萜类化合物的合成和调控过程有助于高效获取并利用这些物质。目前, 相关研究主要集中在植物转录因子的克隆、表达及基本功能等方面。由于很多物种中尚无成熟的遗传转化体系, 因此难以在本体鉴定特异萜类化合物相关基因的功能。对于研究基础薄弱的物种, 鉴定单个关键转录因子比研究大量结构基因更加高效, 可为萜类植物的分子育种、优质栽培和病虫害生物防治等提供新的思路与方法。
随着转基因技术(如CRISPR和VIGS)和蛋白互作方法(如染色体免疫共沉淀、双分子荧光互补技术和酵母单杂交)的不断发展与完善, 更容易获取对植物有用的转录因子及其复合体, 从而进一步阐释植物萜类的合成途径, 丰富植物次生代谢调控机理, 进而助益于品质改良及药物/化妆品(或食品研发)等领域的发展。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
硕士论文.
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
硕士论文.
[本文引用: 2]
[本文引用: 2]
URLPMID:21235651 [本文引用: 1]
[本文引用: 1]
URLPMID:19878958 [本文引用: 1]
URLPMID:21114471 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:10785665 [本文引用: 1]
DOI:10.1038/nchembio.2007.5URLPMID:17576428 [本文引用: 1]

As the largest class of natural products, terpenes have a variety of roles in mediating antagonistic and beneficial interactions among organisms. They defend many species of plants, animals and microorganisms against predators, pathogens and competitors, and they are involved in conveying messages to conspecifics and mutualists regarding the presence of food, mates and enemies. Despite the diversity of terpenes known, it is striking how phylogenetically distant organisms have come to use similar structures for common purposes. New natural roles undoubtedly remain to be discovered for this large class of compounds, given that such a small percentage of terpenes has been investigated so far.
DOI:10.1105/tpc.112.098749URLPMID:22669881 [本文引用: 2]

Arabidopsis thaliana flowers emit volatile terpenes, which may function in plant-insect interactions. Here, we report that Arabidopsis MYC2, a basic helix-loop-helix transcription factor, directly binds to promoters of the sesquiterpene synthase genes TPS21 and TPS11 and activates their expression. Expression of TPS21 and TPS11 can be induced by the phytohormones gibberellin (GA) and jasmonate (JA), and both inductions require MYC2. The induction of TPS21 and TPS11 results in increased emission of sesquiterpene, especially (E)-beta-caryophyllene. DELLAs, the GA signaling repressors, negatively affect sesquiterpene biosynthesis, as the sesquiterpene synthase genes were repressed in plants overaccumulating REPRESSOR OF GA1-3 (RGA), one of the Arabidopsis DELLAs, and upregulated in a penta DELLA-deficient mutant. Yeast two-hybrid and coimmunoprecipitation assays demonstrated that DELLAs, represented by RGA, directly interact with MYC2. In yeast cells, the N terminus of MYC2 was responsible for binding to RGA. MYC2 has been proposed as a major mediator of JA signaling and crosstalk with abscisic acid, ethylene, and light signaling pathways. Our results demonstrate that MYC2 is also connected to GA signaling in regulating a subset of genes. In Arabidopsis inflorescences, it integrates both GA and JA signals into transcriptional regulation of sesquiterpene synthase genes and promotes sesquiterpene production.
[本文引用: 1]
DOI:10.1093/pcp/pcu090URLPMID:24969234 [本文引用: 2]

Amorpha-4,11-diene synthase (ADS) and Cyt P450 monooxygenase (CYP71AV1) in Artemisia annua L. are two key enzymes involved in the biosynthesis of artemisinin. The promoters of ADS and CYP71AV1 contain E-box elements, which are putative binding sites for basic helix-loop-helix (bHLH) transcription factors. This study successfully isolated a bHLH transcription factor gene from A. annua, designated as AabHLH1, from a cDNA library of the glandular secretory trichomes (GSTs) in which artemisinin is synthesized and sequestered. AabHLH1 encodes a protein of 650 amino acids containing one putative bHLH domain. AabHLH1 and ADS genes were strongly induced by ABA and the fungal elicitor, chitosan. The transient expression analysis of the AabHLH1-green fluorescent protein (GFP) reporter gene revealed that AabHLH1 was targeted to nuclei. Biochemical analysis demonstrated that the AabHLH1 protein was capable of binding to the E-box cis-elements, present in both ADS and CYP71AV1 promoters, and possessed transactivation activity in yeast. In addition, transient co-transformation of AabHLH1 and CYP71AV1Pro::GUS in A. annua leaves showed a significant activation of the expression of the GUS (beta-glucuronidase) gene in transformed A. annua, but mutation of the E-boxes resulted in abolition of activation, suggesting that the E-box is important for the CYP71AV1 promoter activity. Furthermore, transient expression of AabHLH1 in A. annua leaves increased transcript levels of the genes involved in artemisinin biosynthesis, such as ADS, CYP71AV1 and HMGR. These results suggest that AabHLH1 can positively regulate the biosynthesis of artemisinin.
[本文引用: 1]
URLPMID:31307374 [本文引用: 1]
DOI:10.1093/jxb/erx316URLPMID:28992329 [本文引用: 1]

The unique flavor of Citrus fruit depends on complex combinations of soluble sugars, organic acids, and volatile compounds. The monoterpene E-geraniol is an important volatile, contributing to flavor in sweet orange (Citrus sinensis Osbeck). Moreover, antifungal activity of E-geraniol has also been observed. However, the terpene synthase (TPS) responsible for its synthesis has not been identified in sweet orange. Terpene synthase 16 (CitTPS16) was shown to catalyze synthesis of E-geraniol in vitro, and transient overexpression of CitTPS16 in fruits and leaves of Newhall sweet orange resulted in E-geraniol accumulation in vivo. Having identified the responsible enzyme, we next examined transcriptional regulation of CitTPS16 in the fruit. Among cloned members of the AP2/ERF transcription factor gene family, CitERF71 showed a similar expression pattern to CitTPS16. Moreover, CitERF71 was able to activate the CitTPS16 promoter based on results from transient dual-luciferase assays and yeast one-hybrid assays. EMSAs showed that CitERF71 directly binds to ACCCGCC and GGCGGG motifs in the CitTPS16 promoter. These results indicate an important role for CitERF71 in transcriptional regulation of CitTP16 and, therefore, in controlling production of E-geraniol in Citrus fruit.
URLPMID:23448426 [本文引用: 1]
URLPMID:27388340 [本文引用: 2]
URLPMID:24342623 [本文引用: 2]
DOI:10.1111/tpj.13509URLPMID:28207974 [本文引用: 2]

The effective anti-malarial drug artemisinin (AN) isolated from Artemisia annua is relatively expensive due to the low AN content in the plant as AN is only synthesized within the glandular trichomes. Therefore, genetic engineering of A. annua is one of the most promising approaches for improving the yield of AN. In this work, the AaMYB1 transcription factor has been identified and characterized. When AaMYB1 is overexpressed in A. annua, either exclusively in trichomes or in the whole plant, essential AN biosynthetic genes are also overexpressed and consequently the amount of AN is significantly increased. Artemisia AaMYB1 constitutively overexpressing plants displayed a greater number of trichomes. In order to study the role of AaMYB1 on trichome development and other possibly connected biological processes, AaMYB1 was overexpressed in Arabidopsis thaliana. To support our findings in Arabidopsis thaliana, an AaMYB1 orthologue from this model plant, AtMYB61, was identified and atmyb61 mutants characterized. Both AaMYB1 and AtMYB61 affected trichome initiation, root development and stomatal aperture in A. thaliana. Molecular analyses indicated that two crucial trichome activator genes are misexpressed in atmyb61 mutant plants and in plants overexpressing AaMYB1. Furthermore, AaMYB1 and AtMYB61 are also essential for gibberellin (GA) biosynthesis and degradation in both species by positively affecting the expression of the enzymes that convert GA9 into the bioactive GA4 as well as the enzymes involved in the degradation of GA4 . Overall, these results identify AaMYB1/AtMYB61 as a key component of the molecular network that connects important biosynthetic processes, and reveal its potential value for AN production through genetic engineering.
[本文引用: 1]
DOI:10.1016/j.jplph.2014.09.001URLPMID:25462074 [本文引用: 1]

Phytoalexins are antimicrobial specialised metabolites that are produced by plants in response to pathogen attack. Momilactones and phytocassanes are major diterpenoid phytoalexins in rice that are synthesised from geranylgeranyl diphosphate that is derived from the methylerythritol phosphate (MEP) pathway. We have previously reported that rice cells overexpressing the basic leucine zipper (bZIP) transcription factor OsTGAP1 exhibit a hyperaccumulation of momilactones and phytocassanes, with hyperinductive expression of momilactone and phytocassane biosynthetic genes and MEP pathway genes, upon response to a chitin oligosaccharide elicitor. For a better understanding of OsTGAP1-mediated regulation of diterpenoid phytoalexin production, we identified OsTGAP1-interacting proteins using yeast two-hybrid screening. Among the OsTGAP1-interacting protein candidates, a TGA factor OsbZIP79 was investigated to verify its physical interaction with OsTGAP1 and involvement in the regulation of phytoalexin production. An in vitro pull-down assay demonstrated that OsTGAP1 and OsbZIP79 exhibited a heterodimeric as well as a homodimeric interaction. A bimolecular fluorescence complementation analysis also showed the interaction between OsTGAP1 and OsbZIP79 in vivo. Intriguingly, whereas OsbZIP79 transactivation activity was observed in a transient reporter assay, the overexpression of OsbZIP79 resulted in suppression of the elicitor-inducible expression of diterpenoid phytoalexin biosynthetic genes, and thus caused a decrease in the accumulation of phytoalexin in rice cells. These results suggest that OsbZIP79 functions as a negative regulator of phytoalexin production triggered by a chitin oligosaccharide elicitor in rice cells, although it remains open under which conditions OsbZIP79 can work with OsTGAP1.
DOI:10.1111/nph.12325URLPMID:23668256 [本文引用: 1]

Terpenoids constitute a large and diverse class of natural products that serve many functions in nature. Most of the tens of thousands of the discovered terpenoids are synthesized by plants, where they function as primary metabolites involved in growth and development, or as secondary metabolites that optimize the interaction between the plant and its environment. Several plant terpenoids are economically important molecules that serve many applications as pharmaceuticals, pesticides, etc. Major challenges for the commercialization of plant-derived terpenoids include their low production levels in planta and the continuous demand of industry for novel molecules with new or superior biological activities. Here, we highlight several synthetic biology methods to enhance and diversify the production of plant terpenoids, with a foresight towards triterpenoid engineering, the least engineered class of bioactive terpenoids. Increased or cheaper production of valuable triterpenoids may be obtained by 'classic' metabolic engineering of plants or by heterologous production of the compounds in other plants or microbes. Novel triterpenoid structures can be generated through combinatorial biosynthesis or directed enzyme evolution approaches. In its ultimate form, synthetic biology may lead to the production of large amounts of plant triterpenoids in in vitro systems or custom-designed artificial biological systems.
URLPMID:25649633 [本文引用: 3]
URLPMID:19635799 [本文引用: 2]
[本文引用: 1]
URLPMID:15029955 [本文引用: 1]
URLPMID:3428265 [本文引用: 1]

The structure of the wild-type c1 locus of Zea mays was determined by sequence analysis of one genomic and two cDNA clones. The coding region is composed of three exons (150 bp, 129 bp and one, at least 720 bp) and two small introns (88 bp and 145 bp). Transcription of the mRNAs corresponding to the two cDNA clones cLC6 (1.1 kb) and cLC28 (2.1 kb) starts from the same promoter. Both cDNAs are identical except that cLC28 extends further at its 3' end. A putative protein, 273 amino acids in length was deduced from the sequence of both transcripts. It contains two domains, one basic and the other acidic and might function as a transcriptional activator. The basic domain of this c1-encoded protein shows 40% sequence homology to the protein products of animal myb proto-oncogenes.
DOI:10.1105/tpc.113.121731URLPMID:24659329

Integration of diverse environmental and endogenous signals to coordinately regulate growth, development, and defense is essential for plants to survive in their natural habitat. The hormonal signals gibberellin (GA) and jasmonate (JA) antagonistically and synergistically regulate diverse aspects of plant growth, development, and defense. GA and JA synergistically induce initiation of trichomes, which assist seed dispersal and act as barriers to protect plants against insect attack, pathogen infection, excessive water loss, and UV irradiation. However, the molecular mechanism underlying such synergism between GA and JA signaling remains unclear. In this study, we revealed a mechanism for GA and JA signaling synergy and identified a signaling complex of the GA pathway in regulation of trichome initiation. Molecular, biochemical, and genetic evidence showed that the WD-repeat/bHLH/MYB complex acts as a direct target of DELLAs in the GA pathway and that both DELLAs and JAZs interacted with the WD-repeat/bHLH/MYB complex to mediate synergism between GA and JA signaling in regulating trichome development. GA and JA induce degradation of DELLAs and JASMONATE ZIM-domain proteins to coordinately activate the WD-repeat/bHLH/MYB complex and synergistically and mutually dependently induce trichome initiation. This study provides deep insights into the molecular mechanisms for integration of different hormonal signals to synergistically regulate plant development.
[本文引用: 3]
[本文引用: 2]
DOI:10.1038/nchembio.2007.8URLPMID:17576426 [本文引用: 1]

Terpenoids are a diverse class of natural products that have many functions in the plant kingdom and in human health and nutrition. Their chemical diversity has led to the discovery of over 40,000 different structures, with several classes serving as important pharmaceutical agents, including the anticancer agents paclitaxel (Taxol) and terpenoid-derived indole alkaloids. Many terpenoid compounds are found in low yield from natural sources, so plant cell cultures have been investigated as an alternate production strategy. Metabolic engineering of whole plants and plant cell cultures is an effective tool to both increase terpenoid yield and alter terpenoid distribution for desired properties such as enhanced flavor, fragrance or color. Recent advances in defining terpenoid metabolic pathways, particularly in secondary metabolism, enhanced knowledge concerning regulation of terpenoid accumulation, and application of emerging plant systems biology approaches, have enabled metabolic engineering of terpenoid production. This paper reviews the current state of knowledge of terpenoid metabolism, with a special focus on production of important pharmaceutically active secondary metabolic terpenoids in plant cell cultures. Strategies for defining pathways and uncovering rate-influencing steps in global metabolism, and applying this information for successful terpenoid metabolic engineering, are emphasized.
URLPMID:21696534 [本文引用: 1]
URLPMID:20304701 [本文引用: 1]
[本文引用: 1]
DOI:10.1111/j.1365-313X.2008.03446.xURLPMID:18476874 [本文引用: 1]

Plants have the capacity to synthesize, accumulate and emit volatiles that may act as aroma and flavor molecules due to interactions with human receptors. These low-molecular-weight substances derived from the fatty acid, amino acid and carbohydrate pools constitute a heterogenous group of molecules with saturated and unsaturated, straight-chain, branched-chain and cyclic structures bearing various functional groups (e.g. alcohols, aldehydes, ketones, esters and ethers) and also nitrogen and sulfur. They are commercially important for the food, pharmaceutical, agricultural and chemical industries as flavorants, drugs, pesticides and industrial feedstocks. Due to the low abundance of the volatiles in their plant sources, many of the natural products had been replaced by their synthetic analogues by the end of the last century. However, the foreseeable shortage of the crude oil that is the source for many of the artificial flavors and fragrances has prompted recent interest in understanding the formation of these compounds and engineering their biosynthesis. Although many of the volatile constituents of flavors and aromas have been identified, many of the enzymes and genes involved in their biosynthesis are still not known. However, modification of flavor by genetic engineering is dependent on the knowledge and availability of genes that encode enzymes of key reactions that influence or divert the biosynthetic pathways of plant-derived volatiles. Major progress has resulted from the use of molecular and biochemical techniques, and a large number of genes encoding enzymes of volatile biosynthesis have recently been reported.
DOI:10.1126/science.1259215URLPMID:25430763 [本文引用: 1]

Cucurbitacins are triterpenoids that confer a bitter taste in cucurbits such as cucumber, melon, watermelon, squash, and pumpkin. These compounds discourage most pests on the plant and have also been shown to have antitumor properties. With genomics and biochemistry, we identified nine cucumber genes in the pathway for biosynthesis of cucurbitacin C and elucidated four catalytic steps. We discovered transcription factors Bl (Bitter leaf) and Bt (Bitter fruit) that regulate this pathway in leaves and fruits, respectively. Traces in genomic signatures indicated that selection imposed on Bt during domestication led to derivation of nonbitter cucurbits from their bitter ancestors.
DOI:10.1093/jxb/erw189URLPMID:27194737 [本文引用: 1]

Aroma is a vital characteristic that determines the quality and commercial value of citrus fruits, and characteristic volatiles have been analyzed in different citrus species. In sweet orange, Citrus sinensis, the sesquiterpene (+)-valencene is a key volatile compound in the fruit peel. Valencene synthesis is catalyzed by the terpene synthase CsTPS1, but the transcriptional mechanisms controlling its gene expression are unknown. Here, the AP2/ERF (APETALA2/ethylene response factor) transcription factor, CitAP2.10, is characterized as a regulator of (+)-valencene synthesis. The expression pattern of CitAP2.10 was positively correlated with (+)-valencene content and CsTPS1 expression. Dual-luciferase assays indicated that CitAP2.10 could trans-activate the CsTPS1 promoter. Ethylene enhanced expression of CitAP2.10 and this effect was abolished by the ethylene antagonist 1-methylcyclopropene. The role and function of CitAP2.10 in (+)-valencene biosynthesis were confirmed using the Arabidopsis homolog (AtWRI1), which also transiently activated the CsTPS1 promoter. Furthermore, transient over-expression of CitAP2.10 triggered (+)-valencene biosynthesis in sweet orange fruit. These results indicate that CitAP2.10 regulates (+)-valencene synthesis via induction of CsTPS1 mRNA accumulation.
[本文引用: 1]
URLPMID:24142382 [本文引用: 1]
[本文引用: 1]
DOI:10.1104/pp.111.181834URLPMID:21988879 [本文引用: 2]

Catharanthus roseus produces a large array of terpenoid indole alkaloids (TIAs) that are an important source of natural or semisynthetic anticancer drugs. The biosynthesis of TIAs is tissue specific and induced by certain phytohormones and fungal elicitors, indicating the involvement of a complex transcriptional control network. However, the transcriptional regulation of the TIA pathway is poorly understood. Here, we describe a C. roseus WRKY transcription factor, CrWRKY1, that is preferentially expressed in roots and induced by the phytohormones jasmonate, gibberellic acid, and ethylene. The overexpression of CrWRKY1 in C. roseus hairy roots up-regulated several key TIA pathway genes, especially Tryptophan Decarboxylase (TDC), as well as the transcriptional repressors ZCT1 (for zinc-finger C. roseus transcription factor 1), ZCT2, and ZCT3. However, CrWRKY1 overexpression repressed the transcriptional activators ORCA2, ORCA3, and CrMYC2. Overexpression of a dominant-repressive form of CrWRKY1, created by fusing the SRDX repressor domain to CrWRKY1, resulted in the down-regulation of TDC and ZCTs but the up-regulation of ORCA3 and CrMYC2. CrWRKY1 bound to the W box elements of the TDC promoter in electrophoretic mobility shift, yeast one-hybrid, and C. roseus protoplast assays. Up-regulation of TDC increased TDC activity, tryptamine concentration, and resistance to 4-methyl tryptophan inhibition of CrWRKY1 hairy roots. Compared with control roots, CrWRKY1 hairy roots accumulated up to 3-fold higher levels of serpentine. The preferential expression of CrWRKY1 in roots and its interaction with transcription factors including ORCA3, CrMYC2, and ZCTs may play a key role in determining the root-specific accumulation of serpentine in C. roseus plants.
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1093/mp/sss015URLPMID:22442388 [本文引用: 1]

Isoprenoids are functionally and structurally the most diverse group of plant metabolites reported to date. They can function as primary metabolites, participating in essential plant cellular processes, and as secondary metabolites, of which many have substantial commercial, pharmacological, and agricultural value. Isoprenoid end products participate in plants in a wide range of physiological processes acting in them both synergistically, such as chlorophyll and carotenoids during photosynthesis, or antagonistically, such as gibberellic acid and abscisic acid during seed germination. It is therefore expected that fluxes via isoprenoid metabolic network are tightly controlled both temporally and spatially, and that this control occurs at different levels of regulation and in an orchestrated manner over the entire isoprenoid metabolic network. In this review, we summarize our current knowledge of the topology of the plant isoprenoid pathway network and its regulation at the gene expression level following diverse stimuli. We conclude by discussing agronomical and biotechnological applications emerging from the plant isoprenoid metabolism and provide an outlook on future directions in the systems analysis of the plant isoprenoid pathway network.
[本文引用: 2]
DOI:10.1073/pnas.1719622115URLPMID:29678829 [本文引用: 1]

Tea, one of the world's most important beverage crops, provides numerous secondary metabolites that account for its rich taste and health benefits. Here we present a high-quality sequence of the genome of tea, Camellia sinensis var. sinensis (CSS), using both Illumina and PacBio sequencing technologies. At least 64% of the 3.1-Gb genome assembly consists of repetitive sequences, and the rest yields 33,932 high-confidence predictions of encoded proteins. Divergence between two major lineages, CSS and Camellia sinensis var. assamica (CSA), is calculated to approximately 0.38 to 1.54 million years ago (Mya). Analysis of genic collinearity reveals that the tea genome is the product of two rounds of whole-genome duplications (WGDs) that occurred approximately 30 to 40 and approximately 90 to 100 Mya. We provide evidence that these WGD events, and subsequent paralogous duplications, had major impacts on the copy numbers of secondary metabolite genes, particularly genes critical to producing three key quality compounds: catechins, theanine, and caffeine. Analyses of transcriptome and phytochemistry data show that amplification and transcriptional divergence of genes encoding a large acyltransferase family and leucoanthocyanidin reductases are associated with the characteristic young leaf accumulation of monomeric galloylated catechins in tea, while functional divergence of a single member of the glutamine synthetase gene family yielded theanine synthetase. This genome sequence will facilitate understanding of tea genome evolution and tea metabolite pathways, and will promote germplasm utilization for breeding improved tea varieties.
DOI:10.1104/pp.104.038612URLPMID:15133151 [本文引用: 1]

The cotton (+)-delta-cadinene synthase (CAD1), a sesquiterpene cyclase, catalyzes a branch-point step leading to biosynthesis of sesquiterpene phytoalexins, including gossypol. CAD1-A is a member of CAD1 gene family, and its promoter contains a W-box palindrome with two reversely oriented TGAC repeats, which are the proposed binding sites of WRKY transcription factors. We isolated several WRKY cDNAs from Gossypium arboreum. One of them, GaWRKY1, encodes a protein containing a single WRKY domain and a putative N-terminal Leu zipper. Similar to genes encoding enzymes of cotton sesquiterpene pathway, GaWRKY1 was down-regulated in a glandless cotton cultivar that contained much less gossypol. GaWRKY1 showed a temporal and spatial pattern of expression comparable to that of CAD1-A in various aerial organs examined, including sepal, stigma, anther, and developing seeds. In suspension cells, expression of both GaWRKY1 and CAD1-A genes and biosynthesis of sesquiterpene aldehydes were strongly induced by a fungal elicitor preparation and methyl jasmonate. GaWRKY1 interacted with the 3x W-box derived from CAD1-A promoter in yeast (Saccharomyces cerevisiae) one-hybrid system and in vitro. Furthermore, in transgenic Arabidopsis plants, overexpression of GaWRKY1 highly activated the CAD1-A promoter, and transient assay in tobacco (Nicotiana tabacum) leaves demonstrated that W-box was required for this activation. These results suggest that GaWRKY1 participates in regulation of sesquiterpene biosynthesis in cotton, and CAD1-A is a target gene of this transcription factor.
DOI:10.3390/molecules24142564URL [本文引用: 1]
[本文引用: 1]
DOI:10.1271/bbb.130109URLPMID:23748776 [本文引用: 1]

We performed extensive functional characterization of diterpenoid phytoalexin biosynthetic genes in rice, and found that the genes for the biosynthesis of the major diterpenoid phytoalexins, phytocassanes and momilactones, are clustered on chromosomes 2 and 4, and that their expression is coordinately induced in rice cells after elicitation. Isopentenyl diphosphate, an early precursor of diterpenoid phytoalexins, was found to be synthesized through the plastidic methylerythritol phosphate pathway. We also found that chitin elicitor receptor kinase OsCERK1 and a mitogen-activated protein kinase cascade, the OsMKK4-OsMPK6 cascade, play essential roles in the elicitor-induced production of diterpenoid phytoalexins. In addition, a basic leucine zipper transcription factor, OsTGAP1, was identified as a key regulator of the coordinated expression of the clustered genes and the methylerythritol phosphate pathway genes. Naringenin 7-O-methyltransferase (OsNOMT) was also identified as a key enzyme in the biosynthesis of another major rice phytoalexin, sakuranetin.
URLPMID:29162040 [本文引用: 1]
DOI:10.1093/mp/ssr087URL [本文引用: 2]

Plants of Artemisia annua produce artemisinin, a sesquiterpene lactone widely used in malaria treatment. Amorpha-4,11-diene synthase (ADS), a sesquiterpene synthase, and CYP71AV1, a P450 monooxygenase, are two key enzymes of the artemisinin biosynthesis pathway. Accumulation of artemisinin can be induced by the phytohormone jasmonate (JA). Here, we report the characterization of two JA-responsive AP2 family transcription factors-AaERF1 and AaERF2-from A. annua L. Both genes were highly expressed in inflorescences and strongly induced by JA. Yeast one-hybrid and electrophoretic mobility shift assay (EMSA) showed that they were able to bind to the CRTDREHVCBF2 (CBF2) and RAV1AAT (RAA) motifs present in both ADS and CYP71AV1 promoters. Transient expression of either AaERF1 or AaERF2 in tobacco induced the promoter activities of ADS or CYP71AV1, and the transgenic A. annua plants overexpressing either transcription factor showed elevated transcript levels of both ADS and CYP71AV1, resulting in increased accumulation of artemisinin and artemisinic acid. By contrast, the contents of these two metabolites were reduced in the RNAi transgenic lines in which expression of AaERF1 or AaERF2 was suppressed. These results demonstrate that AaERF1 and AaERF2 are two positive regulators of artemisinin biosynthesis and are of great value in genetic engineering of artemisinin production.
DOI:10.1016/j.molp.2014.12.004URLPMID:25578280 [本文引用: 2]

Artemisinin is a sesquiterpenoid especially synthesized in the Chinese herbal plant, Artemisia annua, which is widely used in the treatment of malaria. Artemisinin accumulation can be enhanced by exogenous abscisic acid (ABA) treatment. However, it is not known how ABA signaling regulates artemisinin biosynthesis. A global expression profile and phylogenetic analysis as well as the dual-LUC screening revealed that a basic leucine zipper family transcription factor from A. annua (namely AabZIP1) was involved in ABA signaling to regulate artemisinin biosynthesis. AabZIP1 had a higher expression level in the inflorescences than in other tissues; ABA treatment, drought, and salt stress strongly induced the expression of AabZIP1. Yeast one-hybrid assay and electrophoretic mobility shift assay (EMSA) showed that AabZIP1 bound to the ABA-responsive elements (ABRE) in the promoter regions of the amorpha-4,11-diene synthase (ADS) gene and CYP71AV1, which are two key structural genes of the artemisinin biosynthetic pathway. A mutagenesis assay showed that the C1 domain in the N-terminus of AabZIP1 was important for its transactivation activity. Furthermore, the activation of ADS and CYP71AV1 promoters by AabZIP1 was enhanced by ABA treatment in transient dual-LUC analysis. The AabZIP1 variant with C1 domain deletion lost the ability to activate ADS and CYP71AV1 promoters regardless of ABA treatment. Notably, overexpression of AabZIP1 in A. annua resulted in significantly increased accumulation of artemisinin. Our results indicate that ABA promotes artemisinin biosynthesis, likely through 1 activation of ADS and CYP71AV1 expression by AabZIP in A. annua. Meanwhile, our findings reveal the potential value of AabZIP1 in genetic engineering of artemisinin production.
DOI:10.1093/jxb/erx444URLPMID:29301032 [本文引用: 2]

The plant Artemisia annua produces the anti-malarial compound artemisinin. Although the transcriptional regulation of artemisinin biosynthesis has been extensively studied, its post-translational regulatory mechanisms, especially that of protein phosphorylation, remain unknown. Here, we report that an ABA-responsive kinase (AaAPK1), a member of the SnRK2 family, is involved in regulating artemisinin biosynthesis. The physical interaction of AaAPK1 with AabZIP1 was confirmed by multiple assays, including yeast two-hybrid, bimolecular fluorescence complementation, and pull-down. AaAPK1, mainly expressed in flower buds and leaves, could be induced by ABA, drought, and NaCl treatments. Phos-tag mobility shift assays indicated that AaAPK1 phosphorylated both itself and AabZIP1. As a result, the phosphorylated AaAPK1 significantly enhanced the transactivational activity of AabZIP1 on the artemisinin biosynthesis genes. Substituting the Ser37 with Ala37 of AabZIP1 significantly suppressed its phosphorylation, which inhibited the transactivational activity of AabZIP1. Consistent overexpression of AaAPK1 significantly increased the production of artemisinin, as well as the expression levels of the artemisinin biosynthesis genes. Our study opens a window into the regulatory network underlying artemisinin biosynthesis at the post-translational level. Importantly, and for the first time, we provide evidence for why the kinase gene AaAPK1 is a key candidate for the metabolic engineering of artemisinin biosynthesis.
DOI:10.1007/s00299-017-2154-8URLPMID:28508121 [本文引用: 2]

KEY MESSAGE: A Salvia miltiorrhiza R2R3-MYB gene, SmMYB9b , has been cloned and characterized. Overexpression of SmMYB9b resulted in a significant improvement of tanshinones, the lipophilic active ingredients in danshen hairy roots. Plant R2R3-MYB transcription factors play important roles in various physiological and biochemical processes. Danshen (Salvia miltiorrhiza bunge) is a valuable medicinal herb with tanshinones and salvianolic acids as the principal bioactive ingredients. A number of putative R2R3-MYB transcription factors have been identified in the plant, but their function remains to be studied. Here, we report the cloning of SmMYB9b, an S20 R2R3-MYB member and its regulatory properties. SmMYB9b contains an open reading frame of 792 bp in length and encodes a 264-amino acid protein. Its transcripts were most abundant in blooming flowers (except for calyces) and increased with flower development. Exogenous abscisic acid strongly activated its transcription. Gibberellins and methyl jasmonate also showed a time-dependent activation effect on its transcription, but to a weaker degree. Overexpression of SmMYB9b in danshen hairy roots enhanced tanshinone concentration to 2.16 +/- 0.39 mg/g DW, a 2.2-fold improvement over the control. In addition to increased tanshinone concentration, the hairy root growth and lateral hairy root formation were also suppressed. KEGG pathway enrichment analysis with de novo RNAseq data indicated that stress-response-related metabolic pathways, such as the terpenoid and plant hormone signal transduction pathways, were significantly enriched, implying possible implication of SmMYB9b in such processes. Quantitative RT-PCR analysis showed that the transcription of terpenoid biosynthetic genes SmDXS2, SmDXR, SmGGPPS, and SmKSL1 was significantly up-regulated in danshen hairy roots over expressing SmMYB9b. These data suggest that overexpression of SmMYB9b results in enhanced tanshinone concentration through stimulation of the MEP pathway. The present findings shed new light on elucidating the roles of R2R3-MYB in the biosynthesis of diterpenoids in S. miltiorrhiza.
DOI:10.1186/1471-2148-5-1URLPMID:15629062 [本文引用: 1]

BACKGROUND: WRKY proteins are newly identified transcription factors involved in many plant processes including plant responses to biotic and abiotic stresses. To date, genes encoding WRKY proteins have been identified only from plants. Comprehensive search for WRKY genes in non-plant organisms and phylogenetic analysis would provide invaluable information about the origin and expansion of the WRKY family. RESULTS: We searched all publicly available sequence data for WRKY genes. A single copy of the WRKY gene encoding two WRKY domains was identified from Giardia lamblia, a primitive eukaryote, Dictyostelium discoideum, a slime mold closely related to the lineage of animals and fungi, and the green alga Chlamydomonas reinhardtii, an early branching of plants. This ancestral WRKY gene seems to have duplicated many times during the evolution of plants, resulting in a large family in evolutionarily advanced flowering plants. In rice, the WRKY gene family consists of over 100 members. Analyses suggest that the C-terminal domain of the two-WRKY-domain encoding gene appears to be the ancestor of the single-WRKY-domain encoding genes, and that the WRKY domains may be phylogenetically classified into five groups. We propose a model to explain the WRKY family's origin in eukaryotes and expansion in plants. CONCLUSIONS: WRKY genes seem to have originated in early eukaryotes and greatly expanded in plants. The elucidation of the evolution and duplicative expansion of the WRKY genes should provide valuable information on their functions.
DOI:10.3389/fpls.2015.00304URLPMID:25983739 [本文引用: 1]

The Artemisia annua L. beta-pinene synthase QH6 was previously determined to be circadian-regulated at the transcriptional level, showing a rhythmic fluctuation of steady-state transcript abundances. Here we isolated both the genomic sequence and upstream promoter region of QH6. Different regulatory elements, such as G-box (TGACACGTGGCA, -421 bp from the translation initiation site) which might have effects on rhythmic gene expression, were found. Using the yeast one-hybrid and electrophoretic mobility shift assay (EMSA), we confirmed that the bZIP transcription factor HY5 binds to this motif of QH6. Studies with promoter truncations before and after this motif suggested that this G-box was important for the diurnal fluctuation of the transgenic beta-glucuronidase gene (GUS) transcript abundance in Arabidopsis thaliana. GUS gene driven by the promoter region immediately after G-box showed an arrhythmic expression in both light/dark (LD) and constant dark (DD) conditions, whereas the control with G-box retained its fluctuation in both LD and DD. We further transformed A. thaliana with the luciferase gene (LUC) driven by an 1400 bp fragment upstream QH6 with its G-box intact or mutated, respectively. The luciferase activity assay showed that a peak in the early morning disappeared in the mutant. Gene expression analysis also demonstrated that the rhythmic expression of LUC was abolished in the hy5-1 mutant.
DOI:10.1038/nplants.2016.183URLPMID:27892922 [本文引用: 2]

Differentiation of secondary metabolite profiles in closely related plant species provides clues for unravelling biosynthetic pathways and regulatory circuits, an area that is still underinvestigated. Cucurbitacins, a group of bitter and highly oxygenated tetracyclic triterpenes, are mainly produced by the plant family Cucurbitaceae. These compounds have similar structures, but differ in their antitumour activities and ecophysiological roles. By comparative analyses of the genomes of cucumber, melon and watermelon, we uncovered conserved syntenic loci encoding metabolic genes for distinct cucurbitacins. Characterization of the cytochrome P450s (CYPs) identified from these loci enabled us to unveil a novel multi-oxidation CYP for the tailoring of the cucurbitacin core skeleton as well as two other CYPs responsible for the key structural variations among cucurbitacins C, B and E. We also discovered a syntenic gene cluster of transcription factors that regulates the tissue-specific biosynthesis of cucurbitacins and may confer the loss of bitterness phenotypes associated with convergent domestication of wild cucurbits. This study illustrates the potential to exploit comparative genomics to identify enzymes and transcription factors that control the biosynthesis of structurally related yet unique natural products.
DOI:10.1002/bab.1454URLPMID:28218974 [本文引用: 1]

Production of major effective metabolites, tanshinones and lithospermic acid B (LAB), was dramatically enhanced by exogenous jasmonate (JA) treatment in Salvia miltiorrhiza. However, the molecular mechanism of such metabolic activation in S. miltiorrhiza has not been elucidated yet. Here, we focused on jasmonate ZIM-domain (JAZ) proteins that act as repressors of JA signaling. Open reading frames of two novel genes, SmJAZ1 and SmJAZ2, from S. miltiorrhiza were amplified according to the annotation of S. miltiorrhiza transcriptome. Compared to plant JAZs, SmJAZ1 and SmJAZ2 were clustered into different groups by phylogenetic analysis. Organ expression pattern was studied by real-time quantitative PCR (RT-qPCR), showing higher transcription level of both genes in stems than roots and leaves. The two SmJAZs responded to methyl jasmonate at early stage and the transcriptional level significantly increased at 4 H. Our experimental results indicate that SmJAZ1 and SmJAZ2 are JA responsive and presented similar expression trend in JA response. The whole research will certainly facilitate further characterization of JAs effect on effective metabolites and help to ultimately achieve high yield of target compounds (tanshinones and LAB).
DOI:10.1093/pcp/pct162URLPMID:24265273 [本文引用: 1]

Fruit ripening in tomato (Solanum lycopersicum) is a complicated development process affected by both endogenous hormonal and genetic regulators and external signals. Although the role of NOR, a member of the NAC domain family, in mediating tomato fruit ripening has been established, its underlying molecular mechanisms remain unclear. To explore further the role of NAC transcription factors in fruit ripening, we characterized a new tomato NAC domain protein, named SlNAC4, which shows high accumulation in sepal and at the onset of fruit ripening. Various stress treatments including wounding, NaCl, dehydration and low temperature significantly increased the expression of SlNAC4. Reduced expression of SlNAC4 by RNA interference (RNAi) in tomato resulted in delayed fruit ripening, suppressed Chl breakdown and decreased ethylene synthesis mediated mainly through reduced expression of ethylene biosynthesis genes of system-2, and reduced carotenoids by alteration of the carotenoid pathway flux. Transgenic tomato fruits also displayed significant down-regulation of multiple ripening-associated genes, indicating that SlNAC4 functions as a positive regulator of fruit ripening by affecting ethylene synthesis and carotenoid accumulation. Moreover, we also noted that SlNAC4 could not be induced by ethylene and may function upstream of the ripening regulator RIN and positively regulate its expression. Yeast two-hybrid assay further revealed that SlNAC4 could interact with both RIN and NOR protein. These results suggested that ethylene-dependent and -independent processes are regulated by SlNAC4 in the fruit ripening regulatory network.
[本文引用: 1]
DOI:10.1111/j.1469-8137.2012.04161.xURLPMID:22548501 [本文引用: 1]

* Floral scent is a complex trait of biological and applied significance. To evaluate whether scent production originating from diverse metabolic pathways (e.g. phenylpropanoids and isoprenoids) can be affected by transcriptional regulators, Arabidopsis PRODUCTION OF ANTHOCYANIN PIGMENT1 (PAP1) transcription factor was introduced into Rosa hybrida. * Color and scent profiles of PAP1-transgenic and control (beta-glucuronidase-expressing) rose flowers and the expression of key genes involved in the production of secondary metabolites were analyzed. To evaluate the significance of the scent modification, olfactory trials were conducted with both humans and honeybees. * In addition to increased levels of phenylpropanoid-derived color and scent compounds when compared with control flowers, PAP1-transgenic rose lines also emitted up to 6.5 times higher levels of terpenoid scent compounds. Olfactory assay revealed that bees and humans could discriminate between the floral scents of PAP1-transgenic and control flowers. * The increase in volatile production in PAP1 transgenes was not caused solely by transcriptional activation of their respective biosynthetic genes, but probably also resulted from enhanced metabolic flux in both the phenylpropanoid and isoprenoid pathways. The mechanism(s) governing the interactions in these metabolic pathways that are responsible for the production of specialized metabolites remains to be elucidated.
植物响应联合胁迫机制的研究进展
1
2019
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
椒样薄荷、薄荷和苏格兰留兰香精油与抗生素的协同抑菌功能
2011
茄科植物WRKY转录因子的研究进展
2
2017
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
... 转录因子不仅可单独调控植物中的基因转录, 还可与其它转录因子形成复合物共同调节植物的次生代谢.研究发现, 在植物中MYB转录因子主要与IIId、IIIe和IIIf家族的bHLH转录因子形成转录复合物发挥作用(
bHLH转录因子在茉莉酸信号诱导植物次生产物合成中的作用及分子机制
1
2017
... bHLH类转录因子是真核生物蛋白质中的一个大家族, 在生物的生长发育和次生代谢中起重要作用(
植物转录因子的结构与调控作用
1
2000
... 真核生物中通常多种蛋白质因子协同完成转录.植物典型的转录因子包括转录调控区(transcription regulation domain, TRD)、核定位信号区(nuclear localization signal, NLS)、寡聚化位点(oligomerization site, OS)和DNA结合区(DNA binding domain, DBD)四部分(
萜类化合物的研究概况
1
2018
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
转录因子调控植物萜类化合物生物合成研究进展
1
2019
... MYB转录因子在动植物中普遍存在.植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(
新中国成立70年来我国植物代谢领域的重要进展
1
2019
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
白桦BpMYB21和BpMYB61基因的克隆、表达特性及功能研究
4
2018
... 转录因子(transcription factors, TFs)又称反式作用分子, 通过与启动子结合来调控目的基因的转录水平, 进而调节次生代谢物质含量, 是目前常用的一种有效的基因工程工具(
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
...
... MYB转录因子在动植物中普遍存在.植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(
不同花期‘西伯利亚’百合花瓣单萜合成途径转录组分析
1
2018
... 随着测序技术的快速发展, 许多植物的全基因组和转录组数据被公布, 如茶树(Camellia sinensis) (
大豆AP2/ERF基因家族的分子进化分析
1
2015
... AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (
植物萜类合成酶及其代谢调控的研究进展
1
2011
... 众所周知, 植物萜类化合物的合成不是单一过程, 不仅受到甲羟戊酸(mevalonic acid, MVA)途径和磷酸赤藓糖(methylerythritol phosphate, MEP)途径多种酶促反应和关键酶基因表达的影响(
调控黄瓜苦味基因Bi的AP2/ERF家族转录因子
2
2014
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (
bHLH转录因子调控药用植物萜类化合物生物合成的研究进展
2
2017
... 真核生物中通常多种蛋白质因子协同完成转录.植物典型的转录因子包括转录调控区(transcription regulation domain, TRD)、核定位信号区(nuclear localization signal, NLS)、寡聚化位点(oligomerization site, OS)和DNA结合区(DNA binding domain, DBD)四部分(
... bHLH类转录因子是真核生物蛋白质中的一个大家族, 在生物的生长发育和次生代谢中起重要作用(
植物bHLH转录因子的结构特点及其生物学功能
1
2011
... bHLH类转录因子是真核生物蛋白质中的一个大家族, 在生物的生长发育和次生代谢中起重要作用(
不同品种桂花转录组分析及桂花精油成分差异的初步探讨
1
2016
... 随着测序技术的快速发展, 许多植物的全基因组和转录组数据被公布, 如茶树(Camellia sinensis) (
丹参SmMYB36基因对萜类和苯丙烷代谢途径调控的研究
2
2019
... 转录因子不仅可单独调控植物中的基因转录, 还可与其它转录因子形成复合物共同调节植物的次生代谢.研究发现, 在植物中MYB转录因子主要与IIId、IIIe和IIIf家族的bHLH转录因子形成转录复合物发挥作用(
... ).SmMYB36与SmMYC2在丹参中的表达模式一致, 过表达SmMYB36可提高丹参酮的含量, 且酵母双杂交结果显示两者能相互作用, 即SmMYB36与SmMYC2结合, 形成转录复合物调控丹参酮的合成代谢(
Volatile terpenoids: multiple functions, biosynthesis, modulation and manipulation by genetic engineering
2
2017
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
... A summary of volatile-mediated interactions between plants and their surrounding environment (modified from
Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning
1
2011
... 转录因子不仅可单独调控植物中的基因转录, 还可与其它转录因子形成复合物共同调节植物的次生代谢.研究发现, 在植物中MYB转录因子主要与IIId、IIIe和IIIf家族的bHLH转录因子形成转录复合物发挥作用(
Involvement of limonene hydroperoxides formed after oil gland injury in the induction of defense response against Penicillium digitatum in lemon fruit
1
2008
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
Molecular and structural basis of metabolic diversity mediated by prenyldiphosphate converting enzymes
1
2009
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
Use of terpenoids as natural flavouring compounds in food industry
1
2011
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants
1
2003
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
PbbHLH4 regulates floral monoterpene biosynthesis in Phalaenopsis orchids
1
2018
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
Transcriptional machineries in jasmonate-elicited plant secondary metabolism
1
2012
... MYB转录因子在动植物中普遍存在.植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(
SmMYB36, a novel R2R3-MYB transcription factor, enhances tanshinone accumulation and decreases phenolic acid content in Salvia miltiorrhiza hairy roots
1
2017
... MYB转录因子在动植物中普遍存在.植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(
Molecular characterization of AtNAM: a member of the Arabidopsis NAC domain superfamily
1
2002
... NAC是植物特异性转录因子(
The WRKY superfamily of plant transcription factors
1
2000
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
The function of terpene natural products in the natural world
1
2007
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression
2
2012
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... bHLH类转录因子是真核生物蛋白质中的一个大家族, 在生物的生长发育和次生代谢中起重要作用(
Transcription factors 1: bZIP proteins
1
1994
... bZIP类转录因子在真核生物中分布广泛且高度保守, 包含负责结合DNA的碱性氨基酸区域和1个亮氨酸拉链区(
Cloning and characterization of AabHLH1, a bHLH transcription factor that positively regulates artemisinin biosynthesis in Artemisia annua
2
2014
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... bHLH类转录因子是真核生物蛋白质中的一个大家族, 在生物的生长发育和次生代谢中起重要作用(
Overexpression of AaWRKY1 leads to an enhanced content of artemisinin in Artemisia annua
1
2016
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
Time-series transcriptome provides insights into the gene regulation network involved in the volatile terpenoid metabolism during the flower development of lavender
1
2019
... 随着测序技术的快速发展, 许多植物的全基因组和转录组数据被公布, 如茶树(Camellia sinensis) (
Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit
1
2017
... AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (
AaORA, a trichome- specific AP2/ERF transcription factor of Artemisia annua, is a positive regulator in the artemisinin biosynthetic pathway and in disease resistance to Botrytis cinerea
1
2013
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
Overexpression of a novel NAC domain-containing transcription factor gene (AaNAC1) enhances the content of artemisinin and increases tolerance to drought and Botrytis cinerea in Artemisia annua
2
2016
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... NAC是植物特异性转录因子(
Coordinated transcriptional regulation of isopentenyl diphosphate biosynthetic pathway enzymes in plastids by phytochrome-interacting factor 5
2
2014
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... 水稻bHLH转录因子DPF通过与CPS2和CYP99A2启动子区的顺式作用元件(N-box)结合, 激活CPS2以及CYP99A2基因的转录, 从而促进二萜类抗毒素(diterpenoid phytoalexins, DPs)的合成(
AaMYB1 and its orthologue AtMYB61 affect terpene metabolism and trichome development in Artemisia annua and Arabidopsis thaliana
2
2017
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... MYB转录因子在动植物中普遍存在.植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(
Identification of target genes of the bZIP transcription factor OsTGAP1, whose overexpression causes elicitor-induced hyperaccumulation of diterpenoid phytoalexins in rice cells
1
2014
... bZIP类转录因子在真核生物中分布广泛且高度保守, 包含负责结合DNA的碱性氨基酸区域和1个亮氨酸拉链区(
Overexpression of the bZIP transcription factor OsbZIP79 suppresses the production of diterpenoid phytoalexin in rice cells
1
2015
... bZIP类转录因子在真核生物中分布广泛且高度保守, 包含负责结合DNA的碱性氨基酸区域和1个亮氨酸拉链区(
Bioengineering of plant (tri)terpenoids: from metabolic engineering of plants to synthetic biology in vivo and in vitro
1
2013
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
Natural variation in monoterpene synthesis in kiwifruit: transcriptional regulation of terpene synthases by NAC and ETHYLENE-INSENSITIVE3-Like transcription factors
3
2015
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
...
... NAC是植物特异性转录因子(
OsTGAP1, a bZIP transcription factor, coordinately regulates the inductive production of diterpenoid phytoalexins in rice
2
2009
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... bZIP类转录因子在真核生物中分布广泛且高度保守, 包含负责结合DNA的碱性氨基酸区域和1个亮氨酸拉链区(
NAC transcription factors: structurally distinct, functionally diverse
1
2005
... NAC是植物特异性转录因子(
Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana
1
2003
... NAC是植物特异性转录因子(
The regulatory c1 locus of Zea mays encodes a protein with homology to MYB proto-oncogene products and with structural similarities to transcriptional activators
1
1987
... MYB转录因子在动植物中普遍存在.植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(
Arabidopsis DELLA and JAZ proteins bind the WD-repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy
2014
Spearmint R2R3-MYB transcription factor MsMYB negatively regulates monoterpene production and suppresses the expression of geranyl diphosphate synthase large subunit (MsGPPS. LSU)
3
2017
... 根据其作用结果, 转录因子通常又可分为激活型和抑制型.例如, 丹参SmMYB9b为激活型转录因子, 其通过促进SmDXS2、SmDXR、SmGGPPS以及SmKSL1的表达进而提高丹参酮的合成(
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... MYB转录因子在动植物中普遍存在.植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(
A regulatory network for coordinated flower maturation
2
2012
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
...
Production and engineering of terpenoids in plant cell culture
1
2007
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
WRKY transcription factors: key components in abscisic acid signaling
1
2012
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
WRKY transcription factors
1
2010
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression
1
2002
... AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (
Biosynthesis of plant-derived flavor compounds
1
2008
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
Biosynthesis, regulation, and domestication of bitterness in cucumber
1
2014
... 水稻bHLH转录因子DPF通过与CPS2和CYP99A2启动子区的顺式作用元件(N-box)结合, 激活CPS2以及CYP99A2基因的转录, 从而促进二萜类抗毒素(diterpenoid phytoalexins, DPs)的合成(
CitAP2.10 activation of the terpene synthase CsTPS1 is associated with the synthesis of (+)-valencene in ‘Newhall’ orange
1
2016
... AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (
Methyl jasmonate induction of tanshinone biosynthesis in Salvia miltiorrhiza hairy roots is mediated by JASMONATE ZIM-DOMAIN repressor proteins
1
2016
... 除以上6个TFs家族外, 还有其它转录因子家族成员被发现与萜类化合物的代谢相关.YABBY5基因主要参与器官的发育和形态建成, 而
Expression of terpenoids 1, a glandular trichome-specific transcription factor from tomato that activates the terpene synthase 5 promoter
1
2014
... 转录因子通过其DNA结合域与顺式因子互作来实现调控基因的表达(起始抑制或增强).根据其作用特性, 植物中的转录因子主要分为2类, 一类称为普通转录因子, 它们与RNA聚合酶II形成转录起始复合物, 非选择性地调控基因的转录; 另一类称为特异转录因子, 它们在特异的组织细胞中选择性地调控某种或某些基因的转录起始, 或者在受到一些类固醇激素/生长因子(或其它)刺激后, 开始表达的某些特异性转录因子.例如, SlEOT1在番茄腺毛中特异表达并且能够特异性激活SlTPS5的启动子(
RNA sequencing on Solanum lycopersicum trichomes identifies transcription factors that activate terpene synthase promoters
1
2014
... 转录因子通过其DNA结合域与顺式因子互作来实现调控基因的表达(起始抑制或增强).根据其作用特性, 植物中的转录因子主要分为2类, 一类称为普通转录因子, 它们与RNA聚合酶II形成转录起始复合物, 非选择性地调控基因的转录; 另一类称为特异转录因子, 它们在特异的组织细胞中选择性地调控某种或某些基因的转录起始, 或者在受到一些类固醇激素/生长因子(或其它)刺激后, 开始表达的某些特异性转录因子.例如, SlEOT1在番茄腺毛中特异表达并且能够特异性激活SlTPS5的启动子(
The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus
2
2011
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
... 转录因子不仅可单独调控植物中的基因转录, 还可与其它转录因子形成复合物共同调节植物的次生代谢.研究发现, 在植物中MYB转录因子主要与IIId、IIIe和IIIf家族的bHLH转录因子形成转录复合物发挥作用(
The basic helix-loop-helix transcription factor GubHLH3 positively regulates soyasaponin biosynthetic genes in Glycyrrhiza uralensis
1
2018
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
TRICHOME and ARTEMISININ REGULATOR 1 is required for trichome development and artemisinin biosynthesis in Artemisia annua
2
2015
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (
AaEIN3 mediates the downregulation of artemisinin biosynthesis by ethylene signaling through promoting leaf senescence in Artemisia annua
2
2018
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... 除以上6个TFs家族外, 还有其它转录因子家族成员被发现与萜类化合物的代谢相关.YABBY5基因主要参与器官的发育和形态建成, 而
Structure and dynamics of the isoprenoid pathway network
1
2012
... 代谢物在维持植物正常生长发育和抵御各种逆境胁迫中发挥重要作用(
Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor MsYABBY5 from spearmint (Mentha spicata)
2
2016
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... 除以上6个TFs家族外, 还有其它转录因子家族成员被发现与萜类化合物的代谢相关.YABBY5基因主要参与器官的发育和形态建成, 而
Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality
1
2018
... 随着测序技术的快速发展, 许多植物的全基因组和转录组数据被公布, 如茶树(Camellia sinensis) (
Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-A1
1
2004
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
Integration of metabolite profiling and transcriptome analysis reveals genes related to volatile terpenoid metabolism in finger citron (C. medica var. sarcodactylis)
1
2019
... 萜类化合物种类繁多且应用广泛, 但含量低.转录因子能调节次级代谢产物合成途径基因的转录水平.迄今为止, 植物中主要有6个TFs家族被报道(AP2/ ERF、bHLH、MYB、NAC、WRKY和bZIP) (
Diterpenoid phytoalexin factor, a bHLH transcription factor, plays a central role in the biosynthesis of diterpenoid phytoalexins in rice
1
2015
... 水稻bHLH转录因子DPF通过与CPS2和CYP99A2启动子区的顺式作用元件(N-box)结合, 激活CPS2以及CYP99A2基因的转录, 从而促进二萜类抗毒素(diterpenoid phytoalexins, DPs)的合成(
Biosynthesis of phytoalexins and regulatory mechanisms of it in rice
1
2013
... bZIP类转录因子在真核生物中分布广泛且高度保守, 包含负责结合DNA的碱性氨基酸区域和1个亮氨酸拉链区(
Cloning and expression of BpMYC4 and BpbHLH9 genes and the role of BpbHLH9 in triterpenoid synthesis in birch
1
2017
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
Jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L
2
2012
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (
A basic leucine zipper transcription factor, AabZIP1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua
2
2015
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
... bZIP类转录因子在真核生物中分布广泛且高度保守, 包含负责结合DNA的碱性氨基酸区域和1个亮氨酸拉链区(
ARTEMISININ BIOSYNTHESIS PROMOTING KINASE 1 positively regulates artemisinin biosynthesis through phosphorylating AabZIP1
2
2018
... bZIP类转录因子在真核生物中分布广泛且高度保守, 包含负责结合DNA的碱性氨基酸区域和1个亮氨酸拉链区(
... 通过酵母双杂交、双分子荧光互补以及pull-down等方法证实青蒿中AaAPK1与AabZIP1相互作用, AaAPK1通过磷酸化来增强AabZIP1对青蒿素生物合成基因的反式激活活性(
Overexpression of SmMYB9b enhances tanshinone concentration in Salvia miltiorrhiza hairy roots
2
2017
... 根据其作用结果, 转录因子通常又可分为激活型和抑制型.例如, 丹参SmMYB9b为激活型转录因子, 其通过促进SmDXS2、SmDXR、SmGGPPS以及SmKSL1的表达进而提高丹参酮的合成(
... MYB转录因子在动植物中普遍存在.植物MYB转录因子家族包括4个亚类: 1R-MYB、R2R3-MYB、R1R2R3-MYB和4R-MYB, 其中R2R3-MYB是最大的一类, 不仅参与植物抗逆胁迫的生理过程, 还响应JA信号, 是调控黄酮和萜类化合物合成的重要转录因子(
The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants
1
2005
... WRKY类转录因子是植物中一大类转录调控蛋白, 其典型特征是特殊的DNA结合域及不规则的锌指结构.根据WRKY的锌指结构类型和保守域个数, 可将WRKY蛋白分为Group I-III: 类型I一般有2个WRKY功能域和1个C2H2型锌指基序; 类型II一般含有1个WRKY功能域和1个C2H2型锌指基序; 类型III一般含有1个WRKY功能域和1个C2HC型锌指基序(
The bZIP transcription factor HY5 interacts with the promoter of the monoterpene synthase gene QH6 in modulating its rhythmic expression
1
2015
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
Convergence and divergence of bitterness biosynthesis and regulation in Cucurbitaceae
2
2016
... 转录因子(transcription factors, TFs)又称反式作用分子, 通过与启动子结合来调控目的基因的转录水平, 进而调节次生代谢物质含量, 是目前常用的一种有效的基因工程工具(
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
Molecular cloning, bioinformatics analysis, and transcriptional profiling of JAZ1 and JAZ2 from Salvia miltiorrhiza
1
2017
... 除以上6个TFs家族外, 还有其它转录因子家族成员被发现与萜类化合物的代谢相关.YABBY5基因主要参与器官的发育和形态建成, 而
A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation
1
2014
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
Genome-wide analysis of the putative ap2/erf family genes in Vitis vinifera
1
2009
... AP2/ERF转录因子仅存在于植物中, 占植物转录因子的6%-7% (
PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers
1
2012
... Transcription factors involved in the regulation of the terpenoids biosynthetic pathway identified in some plant species
| 类型 | 植物 | TFs名称 | 登录号 | 参考文献 |
|---|---|---|---|---|
| AP2/ERF | 青蒿(Artemisia annua) | AaERF1 | AEQ93554.1 | |
| AaERF2 | AEQ93555.1 | |||
| TAR1 | EZ159016.1 | |||
| AaORA | AGB07586.1 | |||
| 黄瓜(Cucumis sativus) | CsERF | AAV66332.1 | ||
| bHLH | 青蒿(A. annua) | AabHLH1 | A0A3S9XA60 | |
| 拟南芥(Arabidopsis thaliana) | AtMYC2 | AT1G32640 | ||
| PIF5 | AT3G59060 | |||
| 丹参(Salvia miltiorrhiza) | SmMYC2a | KJ945636.1 | ||
| 蝴蝶兰(Phalaenopsis amabilis) | bHLH4 | AVZ23987.1 | ||
| 乌拉尔甘草(Glycyrrhiza uralensis) | bHLH3 | A0A2Z6BDF1 | ||
| 白桦(Betula platyphylla) | bHLH9 | A0A1X9RU20 | ||
| MYB | 拟南芥(A. thaliana) | MYB61 | AT1G09540 | |
| PAP1 | AT4G04020 | |||
| AtMYB21 | AT3G27810 | |||
| AtMYB24 | AT5G40350 | |||
| 留兰香(Mentha spicata) | MsMYB | AQR58379.1 | ||
| 白桦(B. platyphylla) | BpMYB21 | XP_018851905.1 | ||
| BpMYB61 | KT344120 | |||
| NAC | 青蒿(A. annua) | AaNAC1 | AQU15092.1 | |
| 番茄(Solanum lycopersicum) | SlNAC4 | AGH20612.1 | ||
| 软枣猕猴桃(Actinidia arguta) | NAC3 | KF319052 | ||
| bZIP | 青蒿(A. annua) | HY5 | JAT51023.1 | |
| bZIP1 | PWA69369.1 | |||
| 水稻(Oryza sativa) | TGAP1 | AK073715 | ||
| 其它 | 留兰香(M. spicata) | MsYABBY5 | AT2G26580 | |
| 青蒿(A. annua) | EIN3 | PWA84782.1 | ||
| 软枣猕猴桃(A. arguta) | AaEIL1 | KF319041 |
备案号: 京ICP备16067583号-21
版权所有 © 2021 《植物学报》编辑部
地址:北京香山南辛村20号 邮编:100093
电话:010-62836135 010-62836131 E-mail:cbb@ibcas.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
