 ,1,2,3,*
,1,2,3,*Transcriptional Regulatory Network of Secondary Cell Wall Biosynthesis in Plants
Yu Zhang1,2,3, Mingjie Zhao1,2,3, Wei Zhang ,1,2,3,*
,1,2,3,*通讯作者:
责任编辑: 朱亚娜
收稿日期:2019-07-15接受日期:2020-03-23网络出版日期:2020-05-01
| 基金资助: |
Corresponding authors:
Received:2019-07-15Accepted:2020-03-23Online:2020-05-01

摘要
关键词:
Abstract
Keywords:
PDF (1135KB)摘要页面多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
张雨, 赵明洁, 张蔚. 植物次生细胞壁生物合成的转录调控网络. 植物学报, 2020, 55(3): 351-368 doi:10.11983/CBB19135
Zhang Yu, Zhao Mingjie, Zhang Wei.
细胞壁是位于植物细胞膜外的一层较厚、较坚韧并且略具弹性的结构, 为植物细胞所特有, 是区别于动物细胞的主要特征之一。植物不同组织的细胞具有不同类型的细胞壁, 根据其成分及其在生长过程中是否延伸可分为2种类型: 初生细胞壁(primary cell wall, PCW)和次生细胞壁(secondary cell wall, SCW) (以下简称次生壁)。PCW是指细胞分裂后期细胞板形成后, 由原生质体分泌物质在中层的表面最初阶段所沉积的壁, 弹性较大, 普遍存在于所有植物细胞中。SCW比PCW更厚, 沉积在PCW与细胞膜之间, 主要成分包括纤维素、半纤维素和木质素。SCW只沉积于特殊类型的细胞, 如管状分子(tracheary elements, TEs)和纤维细胞的内部(Cosgrove and Jarvis, 2012)。SCW在特化细胞中具有特殊的重要性, 如具有支撑作用的细胞和参与水分输导的细胞。研究表明, 以NAC (NAM、ATAF1/2和CUC2)和MYB为核心成员的转录因子对植物次生壁的形成发挥关键的调控作用。此外, 这2类转录因子对次生壁的调控不仅存在于双子叶植物如陆地棉(Gossypium hirsutum)中, 也存在于单子叶植物如水稻(Oryza sativa)和二穗短柄草(Brachypodium distachyon)中; 不仅存在于草本植物如拟南芥(Arabidopsis thaliana)和紫花苜蓿(Medicago sativa)中, 也存在于木本植物如毛果杨(Populus trichocarpa)、白桦(Betula platyphylla)和桉树(Eucalyptus robusta)中, 表明NAC和MYB转录因子在调控次生壁生物合成方面具有功能保守性。本文综述了调控植物次生壁生物合成的一级开关和二级开关, 以及其它对次生壁生物合成起调控作用的转录因子的研究进展, 旨在进一步厘清次生壁合成过程中的转录因子在调控网络中的层级关系, 并深化对调控网络的整体认识。
1 调控植物次生壁生物合成的一级开关
NAC转录因子是植物一类特有的转录因子, 其家族成员众多。NAC一词源于最早发表的3个具有NAC结构域转录因子的首字母缩写, 分别是矮牵牛(Petunia hybrida)中的NAM (NO APICAL MERISTEM), 以及拟南芥中的ATAF1/2 (Arabidopsis thaliana ACTIVATION FACTOR 1/2)和CUC2 (CUP-SHAPED COTYLEDON 2) (Souer et al., 1996; Aida et al., 1997)。NAC转录因子的N端为高度保守的功能结构域, 与核定位、DNA结合以及蛋白互作二聚体的形成有关; 而C端为转录激活域(Hao et al., 2010), 其序列组成和长度具有高度变异性, 能够激活或抑制靶基因的转录活性(Ernst et al., 2004)。自第1个NAC转录因子从矮牵牛中克隆后, 相继在模式植物(拟南芥、水稻和毛果杨等)、农作物(玉米(Zea mays)、小麦(Triticum aestivum)和大豆(Glycine max))以及园艺作物(葡萄(Vitis vinifera)、番茄(Lycopersicon esculentum)和草莓(Fragaria × ananassa)等)中发现多个NAC转录因子。研究表明, NAC转录因子在植物生长发育(Olsen et al., 2005)、胁迫应答(Christianson et al., 2010; Tran et al., 2010; Nakashima et al., 2012; Puranik et al., 2012; Shao et al., 2015)以及激素信号转导(Yang et al., 2011)等过程中均发挥重要调控作用。
1.1 拟南芥AtVNS家族
VNS (VND、NST/SND和SMB (SOMBRERO))基因家族在次生壁形成中发挥关键调控作用, 为次生壁合成调控网络的转录因子开关。自首次从百日草(Zinnia elegans)中发现与植物次生壁形成相关的NAC转录因子以来(Demura et al., 2002), 已获得一系列与次生壁合成相关的NAC转录因子, 将其依次命名为VND1-7 (VASCULAR-RELATED NAC DOMAIN 1-7) (Kubo et al., 2005)。其中, VND6和VND7是调控木质部导管形成的核心开关。在拟南芥中超量表达VND6引起后生木质部加厚, 而超量表达VND7则导致原生木质部加厚(Kubo et al., 2005)。VND1-5正向调控纤维细胞次生壁的沉积(Zhou et al., 2014)。此外, VND1-3在子叶木质部导管元件分化中也起关键作用。在拟南芥vnd1/vnd2/vnd3三突变体中, 拟南芥幼苗子叶的木质部导管元件分化受到强烈抑制(Tan et al., 2018)。综上, VND蛋白是木质部导管细胞分化的关键调控因子。NST1 (NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1)、NST2和SND1 (NST3/SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN 1)为次生壁合成的关键转录因子(Zhong et al., 2006; Mitsuda et al., 2007; Zhong and Ye, 2015)。NST1和NST2调控花药开裂所必需的花药内皮层细胞的次生壁增厚(Mitsuda et al., 2005)。同时, NST2也参与茎秆纤维细胞次生壁合成的调控(Zhong and Ye, 2015)。NST2在维管束间纤维细胞和木质部纤维细胞中高度表达, 当NST1、NST2和NST3/SND1三者同时发生突变时, 纤维细胞次生壁完全缺失, 表明NST2、NST1以及NST3/SND1协同调控纤维次生壁的合成(Zhong and Ye, 2015b)。
NST3 (又称SND1或ANAC012) (Arabidopsis NAC DOMAIN CONTAINING PROTEIN 012)是1个可以双向调控次生壁形成的NAC转录因子。SND1在茎维管束间纤维细胞和木质纤维细胞中特异表达, 通过显性抑制SND1导致纤维细胞次生壁增厚显著下降(Zhong et al., 2006)。研究表明, NST3/SND1是NST1的同源基因, NST3/SND1和NST1在调控拟南芥果实中瓣膜边缘次生壁的形成和促进植物次生壁增厚方面功能冗余(Zhong et al., 2007a, 2008; Mitsuda et al., 2007; Mitsuda and Ohme-Takagi, 2008)。SND1基因过量表达能够激活与次生壁合成相关基因的表达, 从而引起木质部导管细胞次生壁的大量沉积(Zhong et al., 2006; Ko et al., 2007; Mitsuda et al., 2007)。综上, NST3/SND1可以正向调控植物纤维细胞和木质部导管细胞次生壁的合成。然而, SND1超量表达植株中纤维细胞次生壁的增厚却被强烈抑制。具体表现为维管束间纤维细胞的细胞壁非常薄; 而木质纤维细胞几乎没有次生壁的形成(Zhong et al., 2006; Ko et al., 2007), 表明SND1基因的适度表达对纤维细胞次生壁的正常沉积至关重要(Zhong et al., 2006)。而SND1基因的超量表达会引起木质部导管细胞次生壁的大量沉积, 推测这是纤维细胞次生壁受到抑制后的一种弥补机制(Ko et al., 2007)。此外, Tan等(2018)利用体外植物激素KDB诱导系统培养nst1/nst3突变体的子叶, 结果发现, 相比野生型, nst1/nst3双突变体中木质部导管分化程度更高, 表明NST1和NST3对木质部导管分化发挥负向调控作用。总之, SND1/ NST3/ANAC012可以负向调控维管束间纤维和木质纤维细胞的次生壁增厚。由此推测, NSTs的异位表达因细胞类型的不同而引起次生壁增厚的模式不同, 同一基因对于不同组织部位所起到的作用有所差别。综上所述, NST1、NST2和NST3为调控植物次生壁合成中不可或缺的转录因子。
此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余。对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(Willemsen et al., 2008; Bennett et al., 2012), 表明其与VND和NST转录因子的功能相似。在毛果杨基因组中含有16个VNS基因, 也称WNDs (WOOD ASSOCIATED NAC DOMAIN TRANSCRIPTION FACTORs)基因(Zhong et al., 2010a)。其中, 8个VNS基因(VNS01-08)属于AtVND亚族, 4个VNS基因(VNS09-12)属于AtNST亚族, 4个VNS基因(VNS13-16)属于AtSMB亚族(Ohtani et al., 2011)。上调表达VNS10和VNS11引起纤维素、半纤维素和木质素的异位沉积, 使木质部和韧皮部组织中纤维细胞次生壁增厚(Zhong et al., 2011; Zhao and Bartley, 2014)。杂交杨(P. tremula × P. tremuloides) vns09/vns10/vns11/vns12四突变体中木质部木纤维、木质部射线薄壁细胞和韧皮部纤维中的SCW出现缺失, 只有靠近导管细胞的一些木质纤维出现了次生壁的沉积(Takata et al., 2019), 说明杨树中VNS基因协同调控次生壁的形成。上述结果表明, VNS蛋白诱导次生细胞壁合成的作用是保守的, 并且它们在进化上可能来源于同一祖先(Nakano et al., 2015)。
1.2 NAC转录因子的调控机制
1.2.1 转录及转录后水平的调控研究发现, VNS转录因子通过与其下游基因启动子上特殊位点结合的方式来调控下游基因的表达, 进而调控细胞次生壁合成(Zhong et al., 2010a, 2010b; Ohashi-Ito et al., 2010; McCarthy et al., 2011; Endo et al., 2015)。VND6/7和NST1-3等转录因子的靶基因启动子序列均含有SNBE (secondary wall NAC- binding element)基序, 为19 bp的核心序列(T/A)NN (C/T)(T/C/G)TNNNNNNNA(A/C)GN(A/C/T)(A/T) (Zhong et al., 2010b)。所有AtVNDs基因都能直接结合到AtVND7启动子上的SNBE基序激活其表达(Zhou et al., 2014; Endo et al., 2015)。VND6也能够结合其下游基因启动子上的TERE (tracheary element-regulating cis-element)序列, 调控木质部管状分子的分化(Ohashi-Ito et al., 2010)。TERE (CTTGAAAGCAA)序列对TE的特异分化具有重要作用(Pyo et al., 2007; Ohashi-Ito et al., 2010)。
其它物种中也有类似结合元件。棉花中1个NAC转录因子GhFSN1 (fiber secondary cell wall-related NAC1)通过激活其下游与棉花纤维次生壁合成相关基因的表达, 正向调控棉花纤维次生壁发育(Zhang et al., 2018b)。酵母单杂交实验显示, GhFSN1能够与其自身及其下游基因GhKNL1、GhMYBL1、GhGUT1、GhDUF231L1和GhIRX12的启动子结合。凝胶迁移率实验表明, GhFSN1能直接结合到其下游基因启动子内的SNBE基序上。进一步通过定点突变的方法, 鉴定出SNBE基序包含1个13 bp的核心序列, 即(C/T) (C/G/T)TN(A/T)(G/T)(A/C/G)(A/G)(A/T/G)(A/T/G) AAG (Zhang et al., 2018b)。因此, VNS转录因子通过与其下游基因启动子上SNBE/TERE基序结合来调控次生壁的形成。
转录后调控对调节VNS转录因子的活性也发挥重要作用。毛果杨VNS基因PtrWND1B/PtVNS11/ PtrSND1-A2含有选择性剪接变异体, 且在不同组织中的表达丰度不同(Li et al., 2012b; Zhao and Bartley, 2014)。这类小变异体的蛋白产物缺乏C端结构域, 但能够结合到PtVNS的全长蛋白上。研究发现, 截短的PtrWND1B/PtVNS11/PtrSND1-A2通过PtVNS蛋白抑制其自身的转录激活活性(Li et al., 2012b), 从而抑制毛果杨中纤维细胞的次生壁加厚(Zhao and Bartley, 2014)。这种选择性剪接完全依赖于PtrWND1B/PtVNS11/PtrSND1-A2的内含子序列, 能够特异性调控毛果杨纤维细胞次生壁的形成。对VNS基因的深入研究将有助于更好地理解可变剪接对VNS活性的调控。
1.2.2 蛋白质相互作用与翻译后修饰
研究发现, NAC转录因子可与自身或其它蛋白形成同源或异源二聚体(Olsen et al., 2005; Weiner et al., 2012)。例如, VND和NST蛋白可以相互结合形成异源二聚体, 也可以形成同源二聚体(Yamaguchi et al., 2008; Li et al., 2012)。NAC转录因子通过形成二聚体来调节其转录活性。VND7与其互作蛋白VNI2结合形成二聚体后, VND7的转录激活活性受到抑制, 最终导致木质部导管细胞的分化受到抑制。VNI1及其同源基因ANAC103不仅能够与VND7相互作用, 也能和VND1/2/3互作(Yamaguchi et al., 2015)。但与VNI2的负向调控作用不同, VNI1和ANAC103具有转录激活活性, 通过调节多种NAC转录因子的转录活性促进各种类型细胞的分化(Yamaguchi et al., 2015)。研究表明, 当多个VND和NST基因同时表达时, 应考虑到二聚体之间可能产生的效应(Nakano et al., 2015)。目前普遍的认识是, 二聚体的形成能够极大地提高NAC转录因子对次生壁调控网络中下游基因的调控效率, 从而更好地满足植物的需求。
棉花NAC转录因子GhFSN1不仅能够与其自身形成同源二聚体, 也可与同家族的其它成员(GhFSN2)以及泛素结合酶E2形成异源二聚体, 由此推测GhFSN1可能存在蛋白酶体介导的泛素化调控途径(Zhang et al., 2018b)。从mRNA和蛋白质水平对不同处理条件下GhFSN1的表达量进行检测, 结果表明, 在棉花纤维发育过程中, GhFSN1转录因子的活性存在由蛋白酶体介导的潜在调控机制, 其降解可能通过蛋白酶体介导的泛素化途径实现(Zhang et al., 2018b)。
1.2.3 植物激素及环境条件的调控路径
植物激素尤其是生长素, 对维管组织的分化具有重要作用(Fukuda, 2004)。LBD15/ASL11 (Lateral Organ Boundaries Domain 15/Asymmetric Leaves2-like11)、LBD18/ASL20和LBD30/ASL19已被证明参与木质部管状分子的形成(Soyano et al., 2008; Ohashi- Ito et al., 2010; Zhong et al., 2010b; Yamaguchi et al., 2011)。LBD15/ASL11为AtVND7和AtSND1的直接靶基因(Zhong et al., 2010b), LBD30/ASL19和LBD18/ASL20是AtVND6和AtVND7的直接靶基因(Soyano et al., 2008; Yamaguchi et al., 2011), 能被AtVND6和AtVND7诱导上调表达, 从而诱导不同类型的细胞产生次生壁的异位沉积。此外, 超量表达LBD18/ASL20也能够引起AtVND7的异位表达(Soyano et al., 2008)。因此, ASL基因与AtVND6和AtVND7形成一条反馈通路, 协同调控管状分子的分化。此外, LBD18/ASL20也能够被生长素上调(Soyano et al., 2008)。由此暗示在VND和LBD/ASL之间可能存在由生长素介导的反馈调控(Nakano et al., 2015)。
此外, 赤霉素对次生壁中的主要组成部分纤维素的合成发挥重要作用。纤维素的合成受到一系列CESAs基因调控(Taylor et al., 1999, 2000, 2003; Doblin et al., 2002; Williamson et al., 2002)。Huang等(2015)发现了水稻中由GA-SLR1 (SLENDER RICE 1)介导的连接一级开关NAC转录因子和二级开关MYB转录因子的复合调控网络, 即NAC29/31- MYB61-CESA调控通路。赤霉素能够激活CESAs基因的表达, 进而促进纤维素的合成, GA和GA信号抑制子slr1介导的信号通路是纤维素合成所必需的。NAC29/31也是CESAs基因的调控元件, NAC29/31通过与MYB61启动子的SNBE基序结合促进MYB61的表达, 进而激活CESAs基因的转录。而在GA和NAC-MYB转录因子之间, SLR1起到了桥梁作用。SLR1能够结合NAC29/31的DNA结合域, 从而阻碍三者(NAC-MYB-CESA)的级联调控路径, 通过抑制纤维素的合成抑制次生壁的形成(Huang et al., 2015)。
综上, 在次生壁合成的转录调控网络中, 一级开关转录因子NAC作为网络中枢, 连接下游转录因子和植物激素等内部和外部因素, 协同调控次生壁合成。
2 调控植物次生壁生物合成的二级开关
MYB转录因子广泛存在于高等植物中, 其主要的结构特征为N端具有高度保守的DNA结合结构域(MYB结构域)。根据MYB蛋白含有的MYB结构域数量可将其分为4类: 1R-MYB/MYB-related、R2R3-MYB、3R-MYB和4R-MYB (4个R1/R2的重复)。其中, R2R3- MYB转录因子的数目最多, 其功能和调控机理的研究也更为深入。已有研究表明, MYB转录因子在植物次生壁生物合成途径中扮演着重要角色(Liu et al., 2015a)。2.1 拟南芥AtMYB46和AtMYB83
拟南芥AtMYB46和AtMYB83是2个功能冗余的R2R3-MYB转录因子。AtMYB46和AtMYB83位于次生壁生物合成调控网络中的第2级, 是SND1的直接靶基因, 也是调控拟南芥次生壁形成的节点基因(Zhong et al., 2007a; Ko et al., 2009, 2012; McCarthy et al., 2009)。不仅SND1, SND1的同源基因NST1/2和VND6/7也能够直接调控AtMYB46和AtMYB83的表达(Zhong et al., 2007a, 2010c; McCarthy et al., 2009; Ohashi-Ito et al., 2010; Yamaguchi et al., 2011)。AtMYB46和AtMYB83在花序茎的纤维细胞和导管细胞中特异表达。将其在拟南芥中超量表达, 转基因植株中纤维素、木质素和木聚糖的生物合成途径被激活, 导致非厚壁细胞中次生壁的异位沉积; 而AtMYB46或AtMYB83的显性抑制植株表现为纤维细胞和导管细胞的次生壁增厚显著减弱(Zhong et al., 2007a; McCarthy et al., 2009)。此外, 在myb46/ myb83双突变体中, 拟南芥导管细胞中的次生壁沉积受到严重影响, 导致突变株幼苗生长停滞(McCarthy et al., 2009)。
一级开关基因VNS在维管束植物中十分保守(Nakano et al., 2015)。与此类似, 调控网络的第2级开关MYB46/MYB83在维管植物中也非常保守。例如, PtrMYB3和PtrMYB20是拟南芥AtMYB46/AtMYB83的同源基因, 参与毛果杨次生壁的合成与调控。当其在拟南芥中超量表达时, 能够同时激活纤维素、木聚糖和木质素的生物合成途径, 也能够激活与次生壁合成相关基因启动子的表达(McCarthy et al., 2010)。此外, 水稻OsMYB46和玉米ZmMYB46是拟南芥AtMYB46/AtMYB83的直系同源基因。在拟南芥中超量表达OsMYB46或ZmMYB46能够激活整个次生壁的合成途径(Zhong et al., 2011)。OsMYB46和ZmMYB46作为OsSWNs与ZmSWNs下游的直接靶基因, 其启动子上也含有SNBE位点(Zhong et al., 2011)。
2.2 拟南芥AtMYB46和AtMYB83调控的下游基因
AtMYB46与AtMYB83作为二级调控开关, 具有承上启下的关键作用, 其上游既受到NAC转录因子一级开关的调控, 也能调控一系列位于其下游与次生壁合成相关基因的表达。由于AtMYB46和AtMYB83功能冗余, 二者调控的下游转录因子基因也有所重叠(McCarthy et al., 2009; Ko et al., 2009)。2.2.1 拟南芥AtMYB46和AtMYB83调控的转录因子基因
前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(Zhong et al., 2007b, 2008; Ko et al., 2009, 2014; Nakano et al., 2010)。其中, AtMYB4、AtMYB7和AtMYB32在二级结构上具有相似的抑制元件, 被认为在次生壁生物合成中起负调控作用(Jin et al., 2000; Preston et al., 2004; Ko et al., 2009; Wang and Dixon, 2012)。AtMYB4特异性地抑制木质素单体合成相关基因C4H的表达(Jin et al., 2000), 而AtMYB7和AtMYB32能够负调控木质素合成相关基因的表达(Preston et al., 2004)。AtMYB4在毛果杨中的同源基因PdMYB4, 在次生壁合成过程中也发挥负调控作用, 表明其功能保守(Tang et al., 2015)。此外, 超量表达PdMYB4还能够抑制与纤维素和木聚糖合成相关基因的表达(Tang et al., 2015), 这在一定程度上反映出不同物种中基因功能的进化与分化。此外, 拟南芥AtMYB58、AtMYB63和AtMYB85及其在其它物种中的直系同源基因较特异地调控木质素的生物合成(Bomal et al., 2008; Zhong et al., 2008; Cassan-Wang et al., 2013), 三者在调控次生壁形成过程中的木质素合成方面可能存在功能冗余(Zhou et al., 2009)。
AtMYB42、AtMYB43、AtMYB52和AtMYB54均在木质部组织中优势表达(Zhong et al., 2008), 然而至今人们对这些基因在次生壁形成过程中的功能还存在争议。通过使用嵌合抑制子沉默技术(chimeric repressor gene silencing technology, CRES-T)显著抑制AtMYB52或AtMYB54, 花序茎维管束间纤维细胞和木质纤维细胞的次生壁增厚受到强烈抑制, 说明AtMYB52和AtMYB54参与植物次生壁增厚; 然而, AtMYB52和AtMYB54的超量表达却对次生壁合成无显著影响(Zhong et al., 2008)。这表明个别基因的高表达不足以引起次生壁的异位沉积, 但其正常表达对于次生壁的形成具有重要作用(Zhong et al., 2008)。另有关于AtMYB52的研究却认为, AtMYB52对次生壁的合成起负调控作用, 原因是拟南芥突变体myb52中出现了木质素的异位沉积; 而且与次生壁合成相关基因的表达均大幅提升(Cassan-Wang et al., 2013)。Cassan-Wang等(2013)给出了比较合理的解释: AtMYB52编码转录抑制因子, 因此当其与EAR基序形成嵌合蛋白时, AtMYB52转变为高效的负调控因子, 表现出更强烈的转录抑制活性, 从而抑制纤维细胞次生壁的增厚。此外, 在AtMYB46/AtMYB83的调控下, AtMYB43的表达水平上调, 但AtMYB43基因具体的生物学功能还有待进一步探究(Nakano et al., 2010)。
AtMYB103主要在维管束间纤维细胞和木质部组织中表达, 超量表达AtMYB103可显著增加转基因株系中木质部纤维细胞和维管束间纤维细胞次生壁的厚度。而且AtMYB103可以在体外激活纤维素合酶CESA8基因的启动子, 因此其最初被认为是特异性调控纤维素生物合成的调控因子(Zhong et al., 2008)。研究证实AtMYB103是AtMYB46/83的靶基因(Nakano et al., 2010; Yamaguchi and Demura, 2010; Yamaguchi et al., 2011), 同时也是受SND1、NST1/2以及VND6/7直接调控的靶基因(Zhong et al., 2008)。?hman等(2013)发现myb103突变体中1个编码细胞色素P450亚酶的基因F5H (FERULATE-5-HYDROXYLASE)的表达量显著下降, 导致紫丁香基木质素(syringyl lignin, S-木质素)含量大幅降低。这说明AtMYB103是F5H表达以及合成S-型木质素所必需的, 该转录因子不仅对次生壁的生长结构有影响, 也能调控木质素单体组分的合成过程。
此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因。KNAT7既是AtMYB46和AtMYB83的靶基因(Zhong et al., 2007b, 2008; McCarthy et al., 2009; Ko et al., 2014), 也是受SND1、NST1、VND6和VND7直接调控的靶基因(Zhong et al., 2008)。最早研究发现, KNAT7作为转录抑制因子发挥作用(Brown et al., 2005; Li et al., 2011, 2012), 且其抑制活性可通过与OFP4 (OVATE FAMILY PROTEIN 4)和OFP1蛋白的互作而增强(Li et al., 2011)。超量表达KNAT7引起维管束间纤维细胞壁厚度下降(Li et al., 2012)。knat7功能缺失突变体木质部不规则, 且表现出严重的木质部塌陷(Brown et al., 2005; Li et al., 2012)。然而, 其纤维细胞的次生壁厚度却有所增加, 并且伴有木质素含量的增加以及纤维素、木质素和木聚糖生物合成基因表达量的上升(Li et al., 2012)。银腺杂种杨(P. alba × P. glandulosa)的KNAT2/6b通过调控一级开关NAC转录因子抑制木质部导管的细胞分化及次生壁沉积, 从而抑制次生壁合成(Zhao et al., 2020)。此外, KNAT7还能正向调控木聚糖的生物合成(He et al., 2018)。KNAT7能够激活木聚糖生物合成基因的启动子, 包括IRX9 (IRREGULAR XYLEM 9)、IRX10、IRX14L (IRREGULAR XYLEM 14- LIKE)和FRA8 (FRAGILE FIBER 8)。综上, KNAT7既能作为转录抑制子也能作为转录激活因子调控次生壁的合成, 这取决于不同组织和细胞中的转录因子组分(He et al., 2018)。
KNAT7能与多种转录因子发生互作, 形成复合体参与次生壁的合成。有研究证明, KNAT7能与AtMYB75在体外发生相互作用(Bhargava et al., 2010), 也能在体内互作形成功能复合体, 调控拟南芥花序茎的维管组织和种皮, 从而调控次生壁的形成(Bhargava et al., 2013)。研究表明, BLH6 (BELL1-LIKE HOMEODOMAIN)蛋白能够与KNAT7特异结合, 负向调控次生壁的形成(Liu et al., 2015b)。BLH6是转录抑制子, BLH6-KNAT7复合体能够增强KNAT7和BLH6的抑制活性。超量表达KNAT7和BLH6引起维管束纤维次生壁厚度下降(Liu et al., 2015b)。进一步研究表明, OFP1和OFP4能够增强BLH6的抑制活性。因此, OFP可能作为BLH6-KNAT7复合物的组成部分形成多蛋白转录调控复合体, 特异性地调控某个细胞类型或发育阶段的次生壁合成(Liu and Douglas, 2015)。由于KNAT7存在多个互作蛋白, 研究者认为KNAT7可以根据不同的细胞类型, 通过与不同的蛋白发生相互作用, 进而调控次生壁的合成(Li et al., 2012; Liu and Douglas, 2015)。此外, KNAT7还受到AtMYB61的调控。AtMYB61调控木质部分化, 通过某个特定的基序AC元件结合到KNAT7的启动子上, 共同调控拟南芥子叶维管系统、木质部和种皮形成(Romano et al., 2012)。最近在毛果杨中发现的1个MYB6转录因子也能够与KNAT7相互作用, 通过负调控拟南芥和毛白杨(P. tometosa)中木质素生物合成的代谢通路抑制次生壁的合成(Wang et al., 2019)。上述研究表明, KNAT7能够通过调控纤维细胞和木质部导管细胞次生壁增厚调控次生壁的形成。KNAT7可能通过靶向不同的基因调控不同细胞类型次生细胞壁沉积的不同方面。由于KNAT7存在多个互作蛋白, KNAT7可能通过与不同的互作蛋白形成功能复合体, 在不同类型的细胞中发挥不一样的功能, 最终共同形成反馈调控环, 协同调控次生壁的形成。
植物特异性串联CCCH锌指蛋白基因处于MYB转录因子的下游, 参与次生壁合成。拟南芥AtC3H14是AtSND1以及AtMYB46的直接靶基因(Ko et al., 2009), 能够激活与纤维素、半纤维素和木质素合成相关基因的表达(Ko et al., 2009; Kim et al., 2014b)。AtC3H14既能直接结合到纤维素与木质素合成相关基因的启动子上, 也能结合到聚半乳糖醛酸酶ADPG1的RNA上。因此, AtC3H14可能参与次生壁生物合成基因的转录和转录后调控(Kim et al., 2014b)。Chai等(2014)在白杨(P. deltoides)中也鉴定出2个C3H锌指蛋白基因(PdC3H17/18), 能够激活与纤维素、木聚糖和木质素合成相关基因的表达。其上游转录因子PdMYB3/21通过与PdC3H17/18的启动子结合, 调控其表达水平。水稻中1个非典型的C3H锌指蛋白IIP4能够与次生壁合成网络中的一级调控因子及二级调控因子发生相互作用, 进而抑制次生壁的合成(Zhang et al., 2018a)。由此表明, C3H锌指蛋白在次生壁合成中起桥梁作用, 深入探究其作用机制将有助于进一步完善次生壁生物合成的调控网络。
2.2.2 拟南芥AtMYB46和AtMYB83的调控机制
AtMYB46和AtMYB83不仅能调控转录因子基因, 也能调控一系列与次生壁合成相关的基因。且二者的靶基因启动子上都具有7个核苷酸(ACC[A/T]A[A/C][T/ C])的特异结合元件, 即SMRE (secondary wall MYB- responsive element)基序。作为调控植物次生壁生物合成的二级开关, AtMYB46和AtMYB83均通过与下游基因的SMRE基序相结合激活下游基因的表达, 实现对次生壁生物合成的转录调控(Zhong and Ye, 2012)。例如, AtMYB46的下游转录因子基因, 包括AtMYB43、AtMYB58、AtMYB63和KNAT7均含有AtMYB46结合的SMRE基序(Kim et al., 2012; Zhong and Ye, 2012)。此外, AtMYB46和AtMYB83的毛果杨同源基因PtrMYB2/3/20/21、桉树EgMYB2以及松树(Pinus taeda) PtMYB4均能结合到其下游靶基因的SMRE基序, 进而激活下游基因的表达(Zhong et al., 2013)。由此表明, 在草本植物拟南芥和木本植物中, MYB46及其同源基因均通过结合SMRE基序来激活其下游基因的表达, 暗示MYB46及其同源基因在草本和木本植物中功能保守。
Kim等(2012)通过分析AtC3H14基因的启动子区域, 鉴定出1个AtMYB46特异识别的顺式作用元件M46RE (MYB46-responsive cis-regulatory element), 该元件为含有8个核苷酸([A/G][G/T]T[A/T]GGT[A/ G])的核心基序, 是AtMYB46实现转录调控的必要和充分条件。例如, 3种纤维素合酶CESA4、CESA7和CESA8的启动子上均含有M46RE基序, 若该基序发生突变, AtMYB46便无法有效地与这3个基因的启动子结合, 说明AtMYB46与其启动子的结合依赖于完整的M46RE基序(Kim et al., 2013a, 2013b)。另一个纤维素类合酶A9 (CELLULOSE SYNTHASE-LIKE A9, CSLA9)为参与拟南芥花序轴内初生细胞壁和次生细胞壁葡甘露聚糖的主要合酶(Liepman et al., 2005; Goubet et al., 2009)。AtMYB46通过与M46RE基序相互作用, 结合到CSLA9基因的启动子上, 当超量表达AtMYB46时, 甘露聚糖的含量显著增加(Kim et al., 2014c)。此外, MYB46也能直接激活木聚糖和木质素生物合成基因的表达, 超量表达MYB46能增加木聚糖和木质素的含量(Kim et al., 2014a)。上述研究表明, AtMYB46/83通过与其下游基因启动子上特定的SMRE/M46RE序列结合, 调控次生壁的形成。
3 其它转录因子对次生壁生物合成的调控作用
除了前文所述的一级开关NAC转录因子、二级开关AtMYB46/AtMYB83及其下游调控因子外, 还有VNS蛋白调控的下游转录因子AtSND2和AtSND3等, 以独立于AtMYB46/AtMYB83的方式调控次生壁生物合成的MYB转录因子(包括AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99等), WRKY和bHLH (basic helix loop-helix)类转录因子以及HD-ZIPIII转录因子和miR165/166。3.1 正向调控因子AtSND2和AtSND3
AtSND2和AtSND3为SND1的下游转录因子(Zhong et al., 2006, 2007a)。AtSND3除了是SND1的直接靶基因外, 也是SND1的同源基因NST1、NST2、VND6和VND7的直接靶基因(Zhong et al., 2008)。AtSND2参与几乎所有与次生壁形成相关的调控进程。例如, 纤维素、木聚糖和甘露聚糖的生物合成, 木质素聚合和细胞壁修饰(Hussey et al., 2011)。在桉树中过量表达AtSND2也能增加桉树纤维细胞次生壁的厚度, 表明AtSND2的功能在草本和木本植物中比较保守(Hussey et al., 2011)。但是, 将AtSND2的毛果杨同源基因PopNAC154在毛果杨中超量表达, 转基因植株的木质部次生壁厚度并未发生明显变化(Grant et al., 2010)。最近, 在水稻中分离了1个AtSND2的同源基因OsSND2, 该基因能正向调控水稻的次生壁形成。OsSND2还能与OsMYB61等多个调控次生壁生物合成的MYB基因启动子直接结合。简而言之, OsSND2是一个调控次生壁生物合成的开关因子(Ye et al., 2018)。上述研究表明, AtSND2及其同源基因在不同物种中作用的重要程度有所不同, 这可能取决于不同物种中其它调控因子的作用效果。3.2 负向调控因子AtXND1和VNI2
与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (Zhong et al., 2010a; Zhong and Ye, 2014)中, 既有正向调控因子(如VNS蛋白), 也有负向调控因子, 如XND1 (XYLEM NAC DOMAIN 1)和VNI2 (VND-INTERACTING 2)。XND1也称为ANAC104, 最初从拟南芥中鉴定出来(Ooka et al., 2003), 由于其在木质部中特异表达, 又将其命名为XND1 (Zhao et al., 2005)。XND1起初被认为与拟南芥叶片衰老有关(Guo et al., 2004), 但之后的研究发现XND1作为负调控因子, 通过特异调控木质部导管的分化进而调控植物细胞次生壁的形成(Zhao et al., 2008, 2017; Tang et al., 2018)。研究者在其它物种中鉴定出AtXND1的同源基因, 均以负向调控的方式参与细胞次生壁的合成, 说明其功能在不同类型的物种中保守性较高(Grant et al., 2010; Li et al., 2014)。XND1受VND7直接调控(Zhong et al., 2010b); 反之, 在导管分子分化期间, XND1也能抑制VND7的表达(Zhao et al., 2017)。研究发现, XND1拥有高度保守的C端, 其内部存在4个能与细胞周期和分化调控因子RBR (RETINOBLASTOMA-RELATED)发生相互作用的基序, 分别为CKII-acidic、LXCXE、E2FTD- LIKE和LXCXE-mimic。其中, LXCXE或LXCXE-mimic基序的完整性对于XND1和RBR的互作十分关键, 直接决定了XND1调控木质部管状分子分化作用的强弱。当LXCXE或LXCXE-mimic基序出现碱基突变, 或者LXCXE基序缺失时, XND1对木质部管状分子的抑制作用则会下降甚至消失, 导致相应的超量表达表型也减弱或消失。XND1的C端所含基序能与RBR发生相互作用, 从而特异地抑制木质部细胞的分化(Zhao et al., 2017)。
VNI2在根和花茎的木质部以及韧皮部细胞中都有表达。VNI2能与VND家族蛋白VND7、VND1-5以及其它NAC蛋白发生相互作用, 但VNI2与VND7结合的亲和性高于其它NAC蛋白。组成型超量表达VNI2的植株, 其幼苗期根中木质部导管的正常发育受到抑制。此外, 在VND7启动子的控制下, C-端截短的VNI2 能抑制根部和地上部分木质部导管的正常发育。VNI2与VND7结合后, 抑制了VND7的转录激活活性, 从而抑制VND7下游靶基因的表达, 最终抑制木质部的合成(Yamaguchi et al., 2010c)。上述结果表明, VNI2 作为1个转录抑制子调控木质部细胞特异化, 是VND的负向调控因子(Yamaguchi et al., 2010c)。
3.3 调控次生壁形成的其它MYB转录因子
AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控。其中, AtMYB20和AtMYB69在木质部细胞中优势表达(Zhong et al., 2006, 2007a, 2007b, 2008)。显性抑制分析表明, AtMYB69参与调控次生壁的形成(Zhong et al., 2008), 但是具体的调控路径还不十分清楚。AtMYB75最初被鉴定为花青素合成的正向调控因子, 因此被命名为PAP1 (PRODUCTION OF ANTHOCYANIN PIGMENT 1) (Borevitz et al., 2000)。之后发现AtMYB75还能负调控木质部纤维和维管束间纤维的次生壁形成, 作为抑制因子调控木质素的合成(Bhargava et al., 2010)。Nakano等(2010)发现在木质部导管细胞的体外分化过程中, AtMYB99基因的表达上调, 并且早期就能在木质部导管细胞中检测到AtMYB99的表达, 这暗示其可能参与导管次生壁的形成。AtMYB26是1个调控花药内壁次生壁形成的转录因子。拟南芥myb26突变体中花药内壁的次生壁不能正常形成, 导致花药开裂失败, 最终引起雄性不育(Dawson et al., 1999; Steiner- Lange et al., 2003)。超量表达AtMYB26能诱导次生壁的异位沉积(Yang et al., 2007)。这2个表型分别与nst1/nst2双突变体及超量表达AtNST1/AtNST2植株的表型类似(Mitsuda et al., 2005)。需要指出的是, AtNST1的过表达能诱导AtMYB26的表达(Mitsuda et al., 2005), 且AtMYB26的过表达也能引起AtNST1和AtNST2表达上调(Yang et al., 2007)。上述研究表明, 就花药内壁而言, NAC与MYB之间形成了一种正向的反馈调控回路, 而不是转录调控的级联反应。3.4 参与次生壁合成的WRKY和bHLH类转录因子
研究表明, WRKY和bHLH类转录因子也参与对木质素生物合成的调控。在拟南芥中, WRKY12以负向调控的方式参与茎髓组织薄壁细胞的次生壁加厚(Wang et al., 2010)。WRKY12在拟南芥的髓组织中特异表达, WRKY12的功能缺失会引起次生壁异常加厚, 同时伴随着木质素、木聚糖和纤维素的异位沉积, 说明WRKY12发挥抑制茎髓组织薄壁细胞次生壁加厚的作用(Wang et al., 2010)。此外, 在苜蓿中发现了WRKY12的同源基因MtSTP。苜蓿mtstp-1突变体髓细胞的木质化程度显著高于野生型, 随后发现MtSTP编码1个WRKY转录因子(Wang et al., 2010)。WRKY12基因突变导致NST2及其它与次生壁合成相关转录因子的表达量上升。凝胶迁移实验表明, WRKY12与NST2的启动子结合从而抑制其表达。由此表明, WRKY12能够负向调控次生壁的合成(Wang et al., 2010)。此外, 蛋白质与DNA互作分析表明, 在真核生物中十分保守的转录因子E2Fc负向调控植物体内的核内复制, 可能是次生壁合成的1个关键转录因子(Taylor-Teeples et al., 2015)。在不同情况下, E2Fc能对AtVND6和AtVND7起到激活或抑制作用, 而且E2Fc能够结合到除AtVND外其它在木质部中特异表达的转录因子基因的启动子上(Taylor-Teeples et al., 2015)。bHLH转录因子能分别与NAC和MYB转录因子发生相互作用, 调控次生壁合成。Yan等(2013)利用高粱(Sorghum bicolor) bmr (brown midrib)突变体与野生型构建了1个差减文库, 从中分离到1个bHLH类转录因子SbbHLH1。进一步将其在拟南芥中进行超量表达, SbbHLH1与MYB转录因子竞争性地结合到与木质素合成相关基因的启动子上, 进而抑制木质素的合成。或者, SbbHLH1与MYB转录因子形成复合体, 抑制其活性从而抑制木质素合成相关基因的表达, 进而抑制木质素的合成。当木质素含量过低时, SbbHLH1又作为信号激活MYB转录因子的表达, 说明其对木质素的合成起负调控作用(Yan et al., 2013)。拟南芥中另外2个bHLH转录因子MYC2/4与一级调控转录因子NST1调控次生壁的形成(Zhang et al., 2018d)。在蓝光信号条件下, 蓝光受体CRY1 (CRYPTOCHROME1)能够激活其下游基因MYC2/4的表达, 使其结合到NST1基因的启动子上, 从而激活一系列与次生壁合成相关转录因子的表达, 促进次生壁细胞的增厚(Zhang et al., 2018d)。综上, bHLH转录因子与NAC-MYB转录因子形成了层级路径调控次生壁的合成。
3.5 参与次生壁调控的HD-ZIPIII转录因子和miR165/166
拟南芥中HD-ZIPIIITFs (Class III homeodomain leucine zipper transcription factors)参与维管束分化和次生壁合成(Baima et al., 2001; Ohashi-Ito and Fukuda, 2003; Ohashi-Ito et al., 2005)。HD-ZIPIIITFs包括5个成员, 分别是REV/IFL1 (REVOLUTA/INTERFASCICULAR FIBERLESS 1)、PHB (PHABULOSA)、PHV (PHAVOLUTA)、CORONA (CAN/AtHB15)和AtHB8 (Du and Wang, 2015)。其中, REV/IFL1、PHB和PHV在维管束的分化和形成中存在功能冗余。CAN/AtHB15负向调控次生壁的形成, 在athb15tu突变体中, 2个NAC关键转录因子SND1和NST2表达上调(Du et al., 2015)。而AtHB8作为正向调控因子与生长素信号互作调控维管组织的发育, 超量表达AtHB8可以促进木质部的分化(Baima et al., 2001)。此外, 从HD-ZIPIIITFs同源基因在百日草和水稻中所起的作用可以看出, 在不同物种中其对维管束组织分化和形成的功能保守(Ohashi-Ito and Fukuda, 2003; Ohashi-Ito et al., 2005; Itoh et al., 2008)。激活标记突变体(activation tagged mutants)的鉴定和分析明确了miR156/166在维管发育中的作用(Du and Wang, 2015)。miR156/166通过与HD-ZIPIIITFs中的START (steroidogenic acute regulatory protein-related lipid transfer)结构域上的特定序列结合, 进而调控其表达水平(Mallory et al., 2004)。MiR165b、miR166a和miR166g的激活降低了PHB、PHV和AtHB15的转录本水平。因此, 当START结构域发生无义突变或是点突变时, miR156/166无法正常与其结合, HD-ZIPIIITFs的转录本水平上升, 进而影响维管束组织的发育进程(Kim et al., 2005; Williams et al., 2005; Du and Wang, 2015)。
4 总结与展望
近20年来, 得益于遗传学和分子生物学的迅猛发展, 植物次生壁生物合成的转录调控研究取得了空前巨大的进展。尤其在模式植物拟南芥中, 结合突变体筛选和全基因组信息, 已获得多个植物细胞壁合成相关基因, 明确了NAC和MYB类转录因子在维管束组织的木质部导管、纤维细胞和花药皮层次生细胞壁加厚等过程中的核心作用, 以及其它转录因子在此过程中的调控作用, 并解析了这些调控因子之间的层级关系(Zhong and Ye, 2014; Nakano et al., 2015; Yang and Wang, 2016), 由此植物次生细胞壁生物合成的调控网络逐渐清晰和明朗。本文综述了以拟南芥为代表的植物中细胞壁合成转录调控的研究进展, 并基于此, 绘制了次生壁合成的调控网络(图1A, B)。NAC转录因子作为调控次生壁合成的一级转录开关, 不同的成员所起作用不同。VNDs主要调控导管元件的分化与形成, 而NSTs则主要调控纤维细胞次生壁的形成。然而, 这两类转录因子是否有功能上的重叠还需进一步研究。虽然在导管分子分化过程中, VNDs基因活性处于动态变化状态, 而NSTs则无明显变化。然而, 在分化为维管束导管的细胞中却检测出了NSTs启动子的活性(Mitsuda et al., 2005, 2007), 表明NSTs可能在维管束导管次生壁的形成中具有一定作用。二级开关转录因子MYB46/83在纤维细胞和导管细胞中均发挥作用, 且两者所调控的下游转录因子基因和次生壁合成相关合酶基因主要通过调控木质素和纤维素的合成调控次生壁的沉积。其它转录因子通过与一级和二级开关转录因子相互作用, 形成一个错综复杂的反馈调控网络, 共同调控次生壁的形成。其中, 植物激素(如生长素和赤霉素)以及外界环境(如蓝光)对于次生壁的合成也起到了一定的作用, 作为响应因子促进次生壁的合成。值得思考的是, 同一转录因子在不同的细胞类型中可能发挥不同功能, 甚至功能截然相反。由此说明次生壁合成调控网络非常复杂。图1
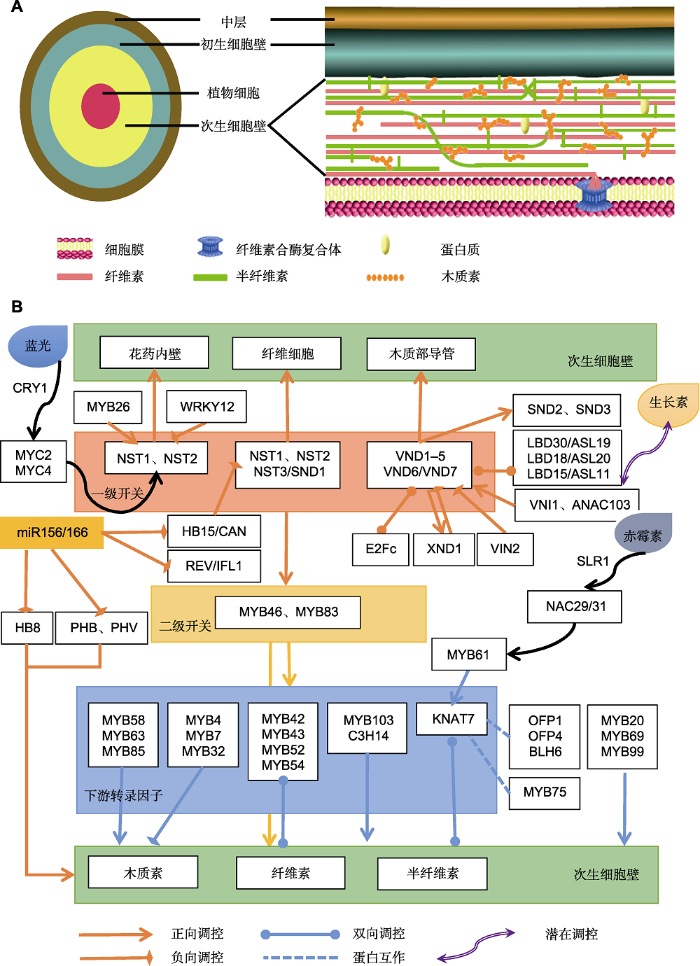 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1拟南芥次生细胞壁结构及其生物合成转录调控网络
(A) 次生细胞壁结构示意图; (B) 次生细胞壁合成转录调控网络图
Figure 1Structure of secondary cell wall and its biosynthetic transcriptional regulatory network in Arabidopsis thaliana
(A) Structural sketch of the secondary cell wall; (B) Transcriptional regulatory network of the secondary cell wall biosynthesis
研究表明, 次生壁加厚现象除了在维管束中导管和纤维细胞中存在, 在树叶、种皮、花药以及果实中石细胞的皮层细胞里也有发生(Mitsuda and Ohme- Takagi, 2008)。此外, 除了拟南芥, 在其它物种(如水稻、棉花)甚至是非维管束植物(如小立碗藓(Physcomitrella patens))中也存在类似的转录调控途径(Xu et al., 2014)。在木本植物中也发现了一系列与拟南芥调控网络中一级开关、二级开关以及其它调控因子的同源基因, 其中, 一级和二级转录因子功能的保守性相对较高(Zhang et al., 2018c)。由此可见, 由NAC-MYB转录因子介导的次生壁合成调控网络在大多数物种中均比较保守。当然, 除了转录因子功能的相似性外, 不同物种不同组织的不同结构也会存在一定的差异。例如, 禾本科植物中次生壁的结构和形成模式与双子叶植物拟南芥有所不同, 这也暗示两者的次生壁合成调控网络存在差异(Handakumbura and Hazen, 2012; Rao and Dixon, 2018)。
由于次生细胞壁含有较多的纤维素、半纤维素及木质素, 因而是植物生物量的主要来源之一。例如, 水稻、玉米和小麦等农作物的秸秆就属于农业生态系统中十分宝贵的生物质能资源。然而, 农作物生产首先需要满足人类的食品需求。相较之下, 由于木本植物能产生大量的木质纤维素, 因此木材生物量作为一种可再生的、成本效益高的生物能源和工业资源, 预计将成为下一代生物燃料的原材料之一。但是, 来源于木质纤维素的生物乙醇要比来源于粮食作物的昂贵许多(Mosier et al., 2005)。为了降低生物燃料转换的成本, 利用转基因技术改善和提高木材的质量和数量显得尤为重要。已有研究提出并验证了人工重建次生细胞壁的可能性, 这将为生产生物乙醇和其它化学品的新原料提供理论依据(Sakamoto and Mitsuda, 2014)。Sakamoto等(2016)利用拟南芥NST3/SND1基因的启动子驱动水稻中NST3/SND1的同源基因, 发现其能增加杂交杨的生物量且不影响其生长发育。进一步通过组织化学法染色表明其在杂交杨次生木本组织中具有依赖性表达模式(Takata et al., 2017)。这表明AtNST3/SND1基因的启动子将成为表达特定效应基因以修饰木材次生细胞壁组分和生物量的有效工具。多年生草本柳枝稷(Panicum virgatum)也被认为是生物燃料的主要可再生和可持续原料作物之一。PvSWNs和PvMYB46A为拟南芥中SWNs和MYB46/83的同源基因, 作为转录开关因子调控次生壁合成(Zhong et al., 2015)。另一项研究中, 通过调控WRKY基因在玉米、柳枝稷和苜蓿中的表达实现了作物生物量质量和数量的显著提高(Gallego-Giraldo et al., 2016)。因此, 明确次生壁合成途径中的关键调控因子, 解析次生细胞壁合成途径, 可为植物生物量的遗传改良及生产应用提供理论依据。
此外, 阐明次生壁生物合成的调控网络, 对于经济树种如桉树和洋槐(Robinia pseudoacacia), 经济作物如棉花、苎麻(Boehmeria nivea)和亚麻(Linum usitatissimum), 以及观赏植物如月季(Rosa chinensis)、玫瑰(R. rugosa)和野蔷薇(R. multiflora)的品质性状改良具有十分重要的意义。以棉花为例, 棉纤维是由胚珠外珠被表皮细胞在受精前后经分化突起、伸长和细胞壁增厚而形成。棉纤维次生壁的最大特点是组成简单, 主要由纤维素构成, 没有木质素沉积。而次生壁合成时期决定了棉纤维的强度。月季花色丰富, 花型多变, 但茎秆多皮刺, 使得其在栽种管理和切花釆摘、包装过程中存在诸多不便, 并带来安全隐患。而采用机械法去除皮刺又会对花枝造成伤害, 从而缩短瓶插寿命。有研究表明, 月季皮刺的主要成分为木质素、纤维素、半纤维素及木栓质(李慧等, 2012)。通过观察木质素沉积部位, 发现木质素的转移方向为刺顶部向刺基部沉积, 表明皮刺的硬化与木质素的积累有关(Asano et al., 2008)。因此, 将有望通过生物技术手段调控皮刺中木质素的合成, 从而调控皮刺的生长与硬化过程。
不同物种中的次生壁合成调控网络存在保守的途径, 也可能还存在特异性的调控途径。随着研究的不断深入和系统化, 通过蛋白质相互作用分析、共表达分析, 并综合运用基因组、转录组以及蛋白质组等分析方法, 将更好地揭示不同物种中各层级调控因子在次生壁生物合成过程中的功能。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
URLPMID:9212461 [本文引用: 1]
[本文引用: 1]
URLPMID:11402194 [本文引用: 2]
DOI:10.1105/tpc.109.072272URLPMID:20197506 [本文引用: 1]

The root cap has a central role in root growth, determining the growth trajectory and facilitating penetration into the soil. Root cap cells have specialized functions and morphologies, and border cells are released into the rhizosphere by specific cell wall modifications. Here, we demonstrate that the cellular maturation of root cap is redundantly regulated by three genes, SOMBRERO (SMB), BEARSKIN1 (BRN1), and BRN2, which are members of the Class IIB NAC transcription factor family, together with the VASCULAR NAC DOMAIN (VND) and NAC SECONDARY WALL THICKENING PROMOTING FACTOR (NST) genes that regulate secondary cell wall synthesis in specialized cell types. Lateral cap cells in smb-3 mutants continue to divide and fail to detach from the root, phenotypes that are independent of FEZ upregulation in smb-3. In brn1-1 brn2-1 double mutants, columella cells fail to detach, while in triple mutants, cells fail to mature in all parts of the cap. This complex genetic redundancy involves differences in expression, protein activity, and target specificity. All three genes have very similar overexpression phenotypes to the VND/NST genes, indicating that members of this family are largely functionally equivalent. Our results suggest that Class IIB NAC proteins regulate cell maturation in cells that undergo terminal differentiation with strong cell wall modifications.
DOI:10.1007/s00425-012-1821-9URLPMID:23328896 [本文引用: 1]

The Arabidopsis thaliana KNAT7 (KNOX family) and MYB75 (MYB family) transcription factors were each shown earlier to interact in yeast two-hybrid assays, and to modulate secondary cell wall formation in inflorescence stems. We demonstrate here that their interaction also occurs in vivo, and that specific domains of each protein mediate this process. The participation of these interacting transcription factors in secondary cell wall formation was then extended to the developing seed coat through the use of targeted transcript analysis and SEM in single loss-of-function mutants. Novel genetic and protein-protein interactions of MYB75 and KNAT7 with other transcription factors known to be involved in seed coat regulation were also identified. We propose that a MYB75-associated protein complex is likely to be involved in modulating secondary cell wall biosynthesis in both the Arabidopsis inflorescence stem and seed coat, and that at least some parts of the transcriptional regulatory network in the two tissues are functionally conserved.
DOI:10.1104/pp.110.162735URLPMID:20807862 [本文引用: 2]

Deposition of lignified secondary cell walls in plants involves a major commitment of carbon skeletons in both the form of polysaccharides and phenylpropanoid constituents. This process is spatially and temporally regulated by transcription factors, including a number of MYB family transcription factors. MYB75, also called PRODUCTION OF ANTHOCYANIN PIGMENT1, is a known regulator of the anthocyanin branch of the phenylpropanoid pathway in Arabidopsis (Arabidopsis thaliana), but how this regulation might impact other aspects of carbon metabolism is unclear. We established that a loss-of-function mutation in MYB75 (myb75-1) results in increased cell wall thickness in xylary and interfascicular fibers within the inflorescence stem. The total lignin content and S/G ratio of the lignin monomers were also affected. Transcript profiles from the myb75-1 inflorescence stem revealed marked up-regulation in the expression of a suite of genes associated with lignin biosynthesis and cellulose deposition, as well as cell wall modifying proteins and genes involved in photosynthesis and carbon assimilation. These patterns suggest that MYB75 acts as a repressor of the lignin branch of the phenylpropanoid pathway. Since MYB75 physically interacts with another secondary cell wall regulator, the KNOX transcription factor KNAT7, these regulatory proteins may form functional complexes that contribute to the regulation of secondary cell wall deposition in the Arabidopsis inflorescence stem and that integrate the metabolic flux through the lignin, flavonoid, and polysaccharide pathways.
DOI:10.1093/jxb/ern234URLPMID:18805909 [本文引用: 1]

The involvement of two R2R3-MYB genes from Pinus taeda L., PtMYB1 and PtMYB8, in phenylpropanoid metabolism and secondary cell wall biogenesis was investigated in planta. These pine MYBs were constitutively overexpressed (OE) in Picea glauca (Moench) Voss, used as a heterologous conifer expression system. Morphological, histological, chemical (lignin and soluble phenols), and transcriptional analyses, i.e. microarray and reverse transcription quantitative PCR (RT-qPCR) were used for extensive phenotyping of MYB-overexpressing spruce plantlets. Upon germination of somatic embryos, root growth was reduced in both transgenics. Enhanced lignin deposition was also a common feature but ectopic secondary cell wall deposition was more strongly associated with PtMYB8-OE. Microarray and RT-qPCR data showed that overexpression of each MYB led to an overlapping up-regulation of many genes encoding phenylpropanoid enzymes involved in lignin monomer synthesis, while misregulation of several cell wall-related genes and other MYB transcription factors was specifically associated with PtMYB8-OE. Together, the results suggest that MYB1 and MYB8 may be part of a conserved transcriptional network involved in secondary cell wall deposition in conifers.
DOI:10.1105/tpc.12.12.2383URLPMID:11148285 [本文引用: 1]

Plants produce a wide array of natural products, many of which are likely to be useful bioactive structures. Unfortunately, these complex natural products usually occur at very low abundance and with restricted tissue distribution, thereby hindering their evaluation. Here, we report a novel approach for enhancing the accumulation of natural products based on activation tagging by Agrobacterium-mediated transformation with a T-DNA that carries cauliflower mosaic virus 35S enhancer sequences at its right border. Among approximately 5000 Arabidopsis activation-tagged lines, we found a plant that exhibited intense purple pigmentation in many vegetative organs throughout development. This upregulation of pigmentation reflected a dominant mutation that resulted in massive activation of phenylpropanoid biosynthetic genes and enhanced accumulation of lignin, hydroxycinnamic acid esters, and flavonoids, including various anthocyanins that were responsible for the purple color. These phenotypes, caused by insertion of the viral enhancer sequences adjacent to an MYB transcription factor gene, indicate that activation tagging can overcome the stringent genetic controls regulating the accumulation of specific natural products during plant development. Our findings suggest a functional genomics approach to the biotechnological evaluation of phytochemical biodiversity through the generation of massively enriched tissue sources for drug screening and for isolating underlying regulatory and biosynthetic genes.
DOI:10.1105/tpc.105.031542URLPMID:15980264 [本文引用: 2]

Forward genetic screens have led to the isolation of several genes involved in secondary cell wall formation. A variety of evidence, however, suggests that the list of genes identified is not exhaustive. To address this problem, microarray data have been generated from tissue undergoing secondary cell wall formation and used to identify genes that exhibit a similar expression pattern to the secondary cell wall-specific cellulose synthase genes IRREGULAR XYLEM1 (IRX1) and IRX3. Cross-referencing this analysis with publicly available microarray data resulted in the selection of 16 genes for reverse genetic analysis. Lines containing an insertion in seven of these genes exhibited a clear irx phenotype characteristic of a secondary cell wall defect. Only one line, containing an insertion in a member of the COBRA gene family, exhibited a large decrease in cellulose content. Five of the genes identified as being essential for secondary cell wall biosynthesis have not been previously characterized. These genes are likely to define entirely novel processes in secondary cell wall formation and illustrate the success of combining expression data with reverse genetics to address gene function.
DOI:10.3389/fpls.2013.00189URLPMID:23781226 [本文引用: 3]

The presence of lignin in secondary cell walls (SCW) is a major factor preventing hydrolytic enzymes from gaining access to cellulose, thereby limiting the saccharification potential of plant biomass. To understand how lignification is regulated is a prerequisite for selecting plant biomass better adapted to bioethanol production. Because transcriptional regulation is a major mechanism controlling the expression of genes involved in lignin biosynthesis, our aim was to identify novel transcription factors (TFs) dictating lignin profiles in the model plant Arabidopsis. To this end, we have developed a post-genomic approach by combining four independent in-house SCW-related transcriptome datasets obtained from (1) the fiber cell wall-deficient wat1 Arabidopsis mutant, (2) Arabidopsis lines over-expressing either the master regulatory activator EgMYB2 or (3) the repressor EgMYB1 and finally (4) Arabidopsis orthologs of Eucalyptus xylem-expressed genes. This allowed us to identify 502 up- or down-regulated TFs. We preferentially selected those present in more than one dataset and further analyzed their in silico expression patterns as an additional selection criteria. This selection process led to 80 candidates. Notably, 16 of them were already proven to regulate SCW formation, thereby validating the overall strategy. Then, we phenotyped 43 corresponding mutant lines focusing on histological observations of xylem and interfascicular fibers. This phenotypic screen revealed six mutant lines exhibiting altered lignification patterns. Two of them [Bel-like HomeoBox6 (blh6) and a zinc finger TF] presented hypolignified SCW. Three others (myb52, myb-like TF, hb5) showed hyperlignified SCW whereas the last one (hb15) showed ectopic lignification. In addition, our meta-analyses highlighted a reservoir of new potential regulators adding to the gene network regulating SCW but also opening new avenues to ultimately improve SCW composition for biofuel production.
DOI:10.1111/nph.12825URLPMID:24786865 [本文引用: 1]

Wood biomass is mainly made of secondary cell walls, whose formation is controlled by a multilevel network. The tandem CCCH zinc finger (TZF) proteins involved in plant secondary wall formation are poorly understood. Two TZF genes, PdC3H17 and PdC3H18, were isolated from Populus deltoides and functionally characterized in Escherichia coli, tobacco, Arabidopsis and poplar. PdC3H17 and PdC3H18 are predominantly expressed in cells of developing wood, and the proteins they encode are targeted to cytoplasmic foci. Transcriptional activation assays showed that PdMYB2/3/20/21 individually activated the PdC3H17 and PdC3H18 promoters, but PdMYB3/21 were most significant. Electrophoretic mobility shift assays revealed that PdMYB3/21 bound directly to the PdC3H17/18 promoters. Overexpression of PdC3H17/18 in poplar increased secondary xylem width and secondary wall thickening in stems, whereas dominant repressors of them had the opposite effects on these traits. Similar alteration in secondary wall thickening was observed in their transgenic Arabidopsis plants. qRT-PCR results showed that PdC3H17/18 regulated the expression of cellulose, xylan and lignin biosynthetic genes, and several wood-associated MYB genes. These results demonstrate that PdC3H17 and PdC3H18 are the targets of PdMYB3 and PdMYB21 and are an additional two components in the regulatory network of secondary xylem formation in poplar.
DOI:10.4161/psb.5.4.10847URLPMID:20118664 [本文引用: 1]
DOI:10.3389/fpls.2012.00204URLPMID:22936943 [本文引用: 1]

Recent insights into the physical biology of plant cell walls are reviewed, summarizing the essential differences between primary and secondary cell walls and identifying crucial gaps in our knowledge of their structure and biomechanics. Unexpected parallels are identified between the mechanism of expansion of primary cell walls during growth and the mechanisms by which hydrated wood deforms under external tension. There is a particular need to revise current
[本文引用: 1]
URLPMID:12438691 [本文引用: 1]
DOI:10.1093/pcp/pcf164URLPMID:12514238 [本文引用: 1]

Modern techniques of gene cloning have identified the CesA genes as encoding the probable catalytic subunits of the plant CelS, the cellulose synthase enzyme complex visualized in the plasma membrane as rosettes. At least 10 CesA isoforms exist in Arabidopsis and have been shown by mutant analyses to play distinct role/s in the cellulose synthesis process. Functional specialization within this family includes differences in gene expression, regulation and, possibly, catalytic function. Current data points towards some CesA isoforms potentially being responsible for initiation or elongation of the recently identified sterol beta-glucoside primer within different cell types, e.g. those undergoing either primary or secondary wall cellulose synthesis. Different CesA isoforms may also play distinct roles within the rosette, and there is some circumstantial evidence that CesA genes may encode the catalytic subunit of the mixed linkage glucan synthase or callose synthase. Various other proteins such as the Korrigan endocellulase, sucrose synthase, cytoskeletal components, Rac13, redox proteins and a lipid transfer protein have been implicated to be involved in synthesizing cellulose but, apart from CesAs, only Korrigan has been definitively linked with cellulose synthesis. These proteins should prove valuable in identifying additional CelS components.
DOI:10.1111/tpj.12897URLPMID:26043238 [本文引用: 1]

Secondary cell-wall thickening takes place in sclerenchyma cells, but not in surrounding parenchyma cells. The molecular mechanism of switching on and off secondary wall synthesis in various cell types is still elusive. Here, we report the identification of a dominant mutant stp-2d showing secondary wall thickening in pith cells (STP). Immunohistochemistry assays confirmed accumulation of secondary cell walls in the pith cells of the stp-2d mutant. Activation of microRNA 165b (miR165b) expression is responsible for the STP phenotype, as demonstrated by transgenic over-expression experiments. The expression of three class III HD-ZIP transcription factor genes, including AtHB15, was repressed in the stp-2d mutant. Transgenic over-expression of a mutant form of AtHB15 that is resistant to miR165-mediated cleavage reversed the stp-2d mutant phenotype to wild-type, indicating that AtHB15 represses secondary wall development in pith. Characterization of two athb15 mutant alleles further confirmed that functional AtHB15 is necessary for retaining primary walls in parenchyma pith cells. Expression analyses of cell-wall synthetic genes and wall-related transcription factors indicated that a transcriptional pathway is involved in AtHB15 function. These results provide insight into the molecular mechanism of secondary cell-wall development.
DOI:10.1080/15592324.2015.1078955URLPMID:26340415 [本文引用: 3]

The Arabidopsis vascular system is composed of xylem and phloem, which form a well-defined collateral pattern in vascular bundles. Xylary element and fibers develop secondary cell walls (SCWs) that provide mechanical strength to support plant growth and to transport water and minerals to all above ground organs. SCWs also constitute the majority of terrestrial biomass for biofuel production. The biosynthesis of secondary cell walls are known to be under transcriptional regulation. Transcription factors, such as NAC (NAM, ATAF1/2 and CUC2) and MYB domain proteins, serve as master regulators in SCW development. Recent studies indicated that Class III homeodomain leucine zipper transcription factors (HD-ZIP III TFs) and microRNA 165/166 (miR165/166) may play important roles in SCW formation. Here we discuss the diverse functions of miR165/166 and HD-ZIPIII in vascular development and their interaction with the regulatory pathways of SCW biosynthesis.
DOI:10.1093/pcp/pcu134URLPMID:25265867 [本文引用: 2]

The secondary cell walls of xylem cells, including vessel elements, provide mechanical strength and contribute to the conduction of water and minerals. VASCULAR-RELATED NAC-DOMAIN7 (VND7) is a NAC-domain transcription factor that regulates the expression of genes required for xylem vessel element formation. Transient expression assays using 68 transcription factors that are expressed during xylem vessel differentiation showed that 14 transcription factors, including VND1-VND7, are putative positive regulators of VND7 expression. Electrophoretic mobility shift assays revealed that all seven VND proteins bound to the VND7 promoter region at its SMBE/TERE motif, indicating that VND7 is a direct target of all of the VND transcription factors. Overexpression of VND1-VND5, GATA12 and ANAC075, newly identified transcription factors that function upstream of VND7, resulted in ectopic xylem vessel element formation. These data suggest that VND7 transcription is a regulatory target of multiple classes of transcription factors.
DOI:10.1038/sj.embor.7400093URLPMID:15083810 [本文引用: 1]

The structure of the DNA-binding NAC domain of Arabidopsis ANAC (abscisic-acid-responsive NAC) has been determined by X-ray crystallography to 1.9A resolution (Protein Data Bank codes 1UT4 and 1UT7). This is the first structure determined for a member of the NAC family of plant-specific transcriptional regulators. NAC proteins are characterized by their conserved N-terminal NAC domains that can bind both DNA and other proteins. NAC proteins are involved in developmental processes, including formation of the shoot apical meristem, floral organs and lateral shoots, as well as in plant hormonal control and defence. The NAC domain does not possess a classical helix-turn-helix motif; instead it reveals a new transcription factor fold consisting of a twisted beta-sheet surrounded by a few helical elements. The functional dimer formed by the NAC domain was identified in the structure, which will serve as a structural template for understanding NAC protein function at the molecular level.
DOI:10.1038/nrm1364URLPMID:15122351 [本文引用: 1]
DOI:10.1111/pbi.12439URLPMID:26190611 [本文引用: 1]

To generate a forage crop with increased biomass density that retains forage quality, we have genetically transformed lines of alfalfa (Medicago sativa L.) expressing antisense constructs targeting two different lignin pathway biosynthetic genes with a construct for down-regulation of a WRKY family transcription factor that acts as a repressor of secondary cell wall formation in pith tissues. Plants with low-level expression of the WRKY dominant repressor construct produced lignified cell walls in pith tissues and exhibited enhanced biomass and biomass density, with an increase in total sugars in the cell wall fraction; however, lines with high expression of the WRKY dominant repressor construct exhibited a very different phenotype, with loss of interfascicular fibres associated with repression of the NST1 transcription factor. This latter phenotype was not observed in transgenic lines in which the WRKY transcription factor was down-regulated by RNA interference. Enhanced and/or ectopic deposition of secondary cell walls was also seen in corn and switchgrass expressing WRKY dominant repressor constructs, with enhanced biomass in corn but reduced biomass in switchgrass. Neutral detergent fibre digestibility was not impacted by WRKY expression in corn. Cell walls from WRKY-DR-expressing alfalfa plants with enhanced secondary cell wall formation exhibited increased sugar release efficiency, and WRKY dominant repressor expression further increased sugar release in alfalfa down-regulated in the COMT, but not the HCT, genes of lignin biosynthesis. These results suggest that significant enhancements in forage biomass and quality can be achieved through engineering WRKY transcription factors in both monocots and dicots.
DOI:10.1111/j.1365-313X.2009.03977.xURLPMID:19619156 [本文引用: 1]

Mannans are hemicellulosic polysaccharides that have previously been implicated as structural constituents of cell walls and as storage reserves but which may serve other functions during plant growth and development. Several members of the Arabidopsis cellulose synthase-like A (CSLA) family have previously been shown to synthesise mannan polysaccharides in vitro when heterologously expressed. It has also been found that CSLA7 is essential for embryogenesis, suggesting a role for the CSLA7 product in development. To determine whether the CSLA proteins are responsible for glucomannan synthesis in vivo, we characterised insertion mutants in each of the nine Arabidopsis CSLA genes and several double and triple mutant combinations. csla9 mutants showed substantially reduced glucomannan, and triple csla2csla3csla9 mutants lacked detectable glucomannan in stems. Nevertheless, these mutants showed no alteration in stem development or strength. Overexpression of CSLA2, CSLA7 and CSLA9 increased the glucomannan content in stems. Increased glucomannan synthesis also caused defective embryogenesis, leading to delayed development and occasional embryo death. The embryo lethality of csla7 was complemented by overexpression of CSLA9, suggesting that the glucomannan products are similar. We conclude that CSLA2, CSLA3 and CSLA9 are responsible for the synthesis of all detectable glucomannan in Arabidopsis stems, and that CSLA7 synthesises glucomannan in embryos. These results are inconsistent with a substantial role for glucomannan in wall strength in Arabidopsis stems, but indicate that glucomannan levels affect embryogenesis. Together with earlier heterologous expression studies, the glucomannan deficiency observed in csla mutant plants demonstrates that the CSLA family encodes glucomannan synthases.
DOI:10.1007/s00425-010-1181-2URLPMID:20458494 [本文引用: 2]

Wood has a wide variety of uses and is arguably the most important renewable raw material. The composition of xylem cell types in wood determines the utility of different types of wood for distinct commercial applications. Using expression profiling and phylogenetic analysis, we identified many xylem-associated regulatory genes that may control the differentiation of cells involved in wood formation in Arabidopsis and poplar. Prominent among these are NAC domain transcription factors (NACs). We studied NACs with putative involvement as negative (XND1 from Arabidopsis and its poplar orthologs PopNAC118, PopNAC122, PopNAC128, PopNAC129), or positive (SND2 and SND3 from Arabidopsis and their poplar orthologs PopNAC105, PopNAC154, PopNAC156, PopNAC157) regulators of secondary cell wall synthesis. Using quantitative PCR and in situ hybridization, we evaluated expression of these Populus NACs in a developmental gradient and in association with reaction wood and found that representatives from both groups were associated with wood-forming tissue and phloem fibers. Additionally, XND1 orthologs were expressed in mesophyll cells of developing leaves. We prepared transgenic Arabidopsis and poplar plants for overexpression of selected NACs. XND1 overexpression in poplar resulted in severe stunting. Additionally, poplar XND1 overexpressors lacked phloem fibers and showed reductions in cell size and number, vessel number, and frequency of rays in the xylem. Overexpression of PopNAC122, an XND1 ortholog, yielded an analogous phenotype in Arabidopsis. Overexpression of PopNAC154 in poplar reduced height growth and increased the relative proportion of bark versus xylem.
[本文引用: 1]
DOI:10.3389/fpls.2012.00074URLPMID:22639662 [本文引用: 1]

Secondary cell wall synthesis occurs in specialized cell types following completion of cell enlargement. By virtue of mechanical strength provided by a wall thickened with cellulose, hemicelluloses, and lignin, these cells can function as water-conducting vessels and provide structural support. Several transcription factor families regulate genes encoding wall synthesis enzymes. Certain NAC and MYB proteins directly bind to the SNBE and AC elements upstream of structural genes and other transcription factors. The most detailed model of this regulatory network is established predominantly for a eudicot, Arabidopsis thaliana. In grasses, both the patterning and the composition of secondary cell walls are distinct from that of eudicots. These differences suggest transcriptional regulation is similarly distinct. Putative rice and maize orthologs of several eudicot cell wall regulators genetically complement mutants of A. thaliana or result in wall defects when constitutively overexpressed; nevertheless, aside from a maize, ZmMYB31, and a switchgrass protein, PvMYB4, function has not been tested in a grass. Similar to the seminal work conducted in A. thaliana, gene expression profiling in maize, rice, and other grasses implicates additional genes as regulators. Characterization of these genes will continue to elucidate the relationship between the transcription regulatory networks of eudicots and grasses.
DOI:10.1007/s00425-010-1238-2URLPMID:20683728 [本文引用: 1]

Plant-specific transcription factor NAC proteins play essential roles in many biological processes such as development, senescence, morphogenesis, and stress signal transduction pathways. In the NAC family, some members function as transcription activators while others act as repressors. In the present study we found that though the full-length GmNAC20 from soybean did not have transcriptional activation activity, the carboxy-terminal activation domain of GmNAC20 had high transcriptional activation activity in the yeast assay system. Deletion experiments revealed an active repression domain with 35 amino acids, named NARD (NAC Repression Domain), in the d subdomain of NAC DNA-binding domain. NARD can reduce the transcriptional activation ability of diverse transcription factors when fused to either the amino-terminal or the carboxy-terminal of the transcription factors. NARD-like sequences are also present in other NAC family members and they are functional repression domain when fused to VP16 in plant protoplast assay system. Mutation analysis of conserved amino acid residues in NARD showed that the hydrophobic LVFY motif may partially contribute to the repression function. It is hypothesized that the interactions between the repression domain NARD and the carboxy-terminal activation domain may finally determine the ability of NAC family proteins to regulate downstream gene expressions.
DOI:10.1111/jipb.12638URL [本文引用: 2]

Xylan is the major plant hemicellulosic polysaccharide in the secondary cell wall. The transcription factor KNOTTED‐LIKE HOMEOBOX OF ARABIDOPSIS THALIANA 7 (KNAT7) regulates secondary cell wall biosynthesis, but its exact role in regulating xylan biosynthesis remains unclear. Using transactivation analyses, we demonstrate that KNAT7 activates the promoters of the xylan biosynthetic genes, IRREGULAR XYLEM 9 (IRX9), IRX10, IRREGULAR XYLEM 14‐LIKE (IRX14L), and FRAGILE FIBER 8 (FRA8). The knat7 T‐DNA insertion mutants have thinner vessel element walls and xylary fibers, and thicker interfascicular fiber walls in inflorescence stems, relative to wild‐type (WT). KNAT7 overexpression plants exhibited opposite effects. Glycosyl linkage and sugar composition analyses revealed lower xylan levels in knat7 inflorescence stems, relative to WT; a finding supported by labeling of inflorescence walls with xylan‐specific antibodies. The knat7 loss‐of‐function mutants had lower transcript levels of the xylan biosynthetic genes IRX9, IRX10, and FRA8, whereas KNAT7 overexpression plants had higher mRNA levels for IRX9, IRX10, IRX14L, and FRA8. Electrophoretic mobility shift assays indicated that KNAT7 binds to the IRX9 promoter. These results support the hypothesis that KNAT7 positively regulates xylan biosynthesis.
DOI:10.1105/tpc.15.00015URLPMID:26002868 [本文引用: 2]

Cellulose, which can be converted into numerous industrial products, has important impacts on the global economy. It has long been known that cellulose synthesis in plants is tightly regulated by various phytohormones. However, the underlying mechanism of cellulose synthesis regulation remains elusive. Here, we show that in rice (Oryza sativa), gibberellin (GA) signals promote cellulose synthesis by relieving the interaction between SLENDER RICE1 (SLR1), a DELLA repressor of GA signaling, and NACs, the top-layer transcription factors for secondary wall formation. Mutations in GA-related genes and physiological treatments altered the transcription of CELLULOSE SYNTHASE genes (CESAs) and the cellulose level. Multiple experiments demonstrated that transcription factors NAC29/31 and MYB61 are CESA regulators in rice; NAC29/31 directly regulates MYB61, which in turn activates CESA expression. This hierarchical regulation pathway is blocked by SLR1-NAC29/31 interactions. Based on the results of anatomical analysis and GA content examination in developing rice internodes, this signaling cascade was found to be modulated by varied endogenous GA levels and to be required for internode development. Genetic and gene expression analyses were further performed in Arabidopsis thaliana GA-related mutants. Altogether, our findings reveal a conserved mechanism by which GA regulates secondary wall cellulose synthesis in land plants and provide a strategy for manipulating cellulose production and plant growth.
DOI:10.1186/1471-2229-11-173URLPMID:22133261 [本文引用: 2]

BACKGROUND: NAC domain transcription factors initiate secondary cell wall biosynthesis in Arabidopsis fibres and vessels by activating numerous transcriptional regulators and biosynthetic genes. NAC family member SND2 is an indirect target of a principal regulator of fibre secondary cell wall formation, SND1. A previous study showed that overexpression of SND2 produced a fibre cell-specific increase in secondary cell wall thickness in Arabidopsis stems, and that the protein was able to transactivate the cellulose synthase8 (CesA8) promoter. However, the full repertoire of genes regulated by SND2 is unknown, and the effect of its overexpression on cell wall chemistry remains unexplored. RESULTS: We overexpressed SND2 in Arabidopsis and analyzed homozygous lines with regards to stem chemistry, biomass and fibre secondary cell wall thickness. A line showing upregulation of CesA8 was selected for transcriptome-wide gene expression profiling. We found evidence for upregulation of biosynthetic genes associated with cellulose, xylan, mannan and lignin polymerization in this line, in agreement with significant co-expression of these genes with native SND2 transcripts according to public microarray repositories. Only minor alterations in cell wall chemistry were detected. Transcription factor MYB103, in addition to SND1, was upregulated in SND2-overexpressing plants, and we detected upregulation of genes encoding components of a signal transduction machinery recently proposed to initiate secondary cell wall formation. Several homozygous T4 and hemizygous T1 transgenic lines with pronounced SND2 overexpression levels revealed a negative impact on fibre wall deposition, which may be indirectly attributable to excessive overexpression rather than co-suppression. Conversely, overexpression of SND2 in Eucalyptus stems led to increased fibre cross-sectional cell area. CONCLUSIONS: This study supports a function for SND2 in the regulation of cellulose and hemicellulose biosynthetic genes in addition of those involved in lignin polymerization and signalling. SND2 seems to occupy a subordinate but central tier in the secondary cell wall transcriptional network. Our results reveal phenotypic differences in the effect of SND2 overexpression between woody and herbaceous stems and emphasize the importance of expression thresholds in transcription factor studies.
DOI:10.1104/pp.108.118679URLPMID:18567825 [本文引用: 1]

Members of the Class III homeodomain leucine zipper (Class III HD-Zip) gene family are central regulators of crucial aspects of plant development. To better understand the roles of five Class III HD-Zip genes in rice (Oryza sativa) development, we investigated their expression patterns, ectopic expression phenotypes, and auxin responsiveness. Four genes, OSHB1 to OSHB4, were expressed in a localized domain of the shoot apical meristem (SAM), the adaxial cells of leaf primordia, the leaf margins, and the xylem tissue of vascular bundles. In contrast, expression of OSHB5 was observed only in phloem tissue. Plants ectopically expressing microRNA166-resistant versions of the OSHB3 gene exhibited severe defects, including the ectopic production of leaf margins, shoots, and radialized leaves. The treatment of seedlings with auxin quickly induced ectopic OSHB3 expression in the entire region of the SAM, but not in other tissues. Furthermore, this ectopic expression of OSHB3 was correlated with leaf initiation defects. Our findings suggest that rice Class III HD-Zip genes have conserved functions with their homologs in Arabidopsis (Arabidopsis thaliana), but have also acquired specific developmental roles in grasses or monocots. In addition, some Class III HD-Zip genes may regulate the leaf initiation process in the SAM in an auxin-dependent manner.
DOI:10.1093/emboj/19.22.6150URLPMID:11080161 [本文引用: 2]

An Arabidopsis thaliana line that is mutant for the R2R3 MYB gene, AtMYB4, shows enhanced levels of sinapate esters in its leaves. The mutant line is more tolerant of UV-B irradiation than wild type. The increase in sinapate ester accumulation in the mutant is associated with an enhanced expression of the gene encoding cinnamate 4-hydroxylase, which appears to be the principal target of AtMYB4 and an effective rate limiting step in the synthesis of sinapate ester sunscreens. AtMYB4 expression is downregulated by exposure to UV-B light, indicating that derepression is an important mechanism for acclimation to UV-B in A.thaliana. The response of target genes to AtMYB4 repression is dose dependent, a feature that operates under physiological conditions to reinforce the silencing effect of AtMYB4 at high activity. AtMYB4 works as a repressor of target gene expression and includes a repression domain. It belongs to a novel group of plant R2R3 MYB proteins involved in transcriptional silencing. The balance between MYB activators and repressors on common target promoters may provide extra flexibility in transcriptional control.
DOI:10.1111/j.1365-313X.2005.02354.xURLPMID:15773855 [本文引用: 1]

Class III homeodomain-leucine zipper proteins regulate critical aspects of plant development, including lateral organ polarity, apical and lateral meristem formation, and vascular development. ATHB15, a member of this transcription factor family, is exclusively expressed in vascular tissues. Recently, a microRNA (miRNA) binding sequence has been identified in ATHB15 mRNA, suggesting that a molecular mechanism governed by miRNA binding may direct vascular development through ATHB15. Here, we show that miR166-mediated ATHB15 mRNA cleavage is a principal mechanism for the regulation of vascular development. In a gain-of-function MIR166a mutant, the decreased transcript level of ATHB15 was accompanied by an altered vascular system with expanded xylem tissue and interfascicular region, indicative of accelerated vascular cell differentiation from cambial/procambial cells. A similar phenotype was observed in Arabidopsis plants with reduced ATHB15 expression but reversed in transgenic plants overexpressing an miR166-resistant ATHB15. ATHB15 mRNA cleavage occurred in standard wheat germ extracts and in Arabidopsis and was mediated by miR166 in Nicotiana benthamiana cells. miR166-assisted ATHB15 repression is likely to be a conserved mechanism that regulates vascular development in all vascular plants.
DOI:10.1007/s11103-014-0205-xURLPMID:24879533 [本文引用: 1]

Secondary wall formation requires coordinated transcriptional regulation of the genes involved in the biosynthesis of the components of secondary wall. Transcription factor (TF) MYB46 (At5g12870) has been shown to function as a central regulator for secondary wall formation in Arabidopsis thaliana, activating biosynthetic genes as well as the TFs involved in the pathways. Recently, we reported that MYB46 directly regulates secondary wall-associated cellulose synthase (CESA4, CESA7, and CESA8) and a mannan synthase (CSLA9) genes. However, it is not known whether MYB46 directly activates the biosynthetic genes for hemicellulose and lignin, which are the other two major components of secondary wall. Based on the observations that the promoter regions of many of the secondary wall biosynthetic genes contain MYB46-binding cis-regulatory motif(s), we hypothesized that MYB46 directly regulates the genes involved in the biosynthesis of the secondary wall components. In this report, we describe several lines of experimental evidence in support of the hypothesis. Electrophoretic mobility shift assay and chromatin immunoprecipitation analysis showed that MYB46 directly binds to the promoters of 13 genes involved in lignin and xylan biosynthesis. We then used steroid receptor-based inducible activation system to confirm that MYB46 directly activates the transcription of the xylan and lignin biosynthetic genes. Furthermore, ectopic up-regulation of MYB46 resulted in a significant increase in xylose and a small increase in lignin content based on acetyl bromide soluble lignin measurements in Arabidopsis. Taken together, we conclude that MYB46 function as a central and direct regulator of the genes involved in the biosynthesis of all three major secondary wall components.
DOI:10.1111/tpj.12667URLPMID:25228083 [本文引用: 2]

AtC3H14 (At1 g66810) is a plant-specific tandem CCCH zinc-finger (TZF) protein that belongs to the 68-member CCCH family in Arabidopsis thaliana. In animals, TZFs have been shown to bind and recruit target mRNAs to the cytoplasmic foci where mRNA decay enzymes are active. However, it is not known whether plant TZF proteins such as AtC3H14 function. So far, no mRNA targets of plant TZFs have been identified. We have obtained several lines of experimental evidence in support of our hypothesis that AtC3H14 is involved in post-transcriptional regulation of its target genes. Nucleic acid binding assays using [(35) S]-labeled AtC3H14 protein showed that AtC3H14 could bind to ssDNA, dsDNA, and ribohomopolymers, suggesting its RNA-binding activity. RNA immunoprecipitation (RIP) assay identified several putative target RNAs of AtC3H14, including a polygalacturonase, a well-known cell wall modifying gene. RNA electrophoretic mobility shift assays (RNA-EMSA) were used to confirm the RIP results and demonstrate that the TZF domain of AtC3H14 is required for the target RNA binding. Microarray analysis of 35S::AtC3H14 plants revealed that many of the cell wall elongation and/or modification-associated genes were differentially expressed, which is consistent with the cell elongation defect phenotype and the changes in the cell wall monosaccharide composition. In addition, yeast activation assay showed that AtC3H14 also function as a transcriptional activator, which is consistent with the previous finding that AtC3H14 activate the secondary wall biosynthesis genes. Taken together, we conclude that AtC3H14 may play a key role in both transcriptional and post-transcriptional regulation.
DOI:10.1016/j.jplph.2013.04.012URLPMID:23726771 [本文引用: 1]

Cellulose, the most abundant biopolymer on Earth, is a central component in plant cell walls and highly abundant (up to 50%) in the secondary walls. In Arabidopsis thaliana, the cellulose biosynthesis in the secondary walls is catalyzed by three cellulose synthases CESA4, CESA7 and CESA8. The transcription factor MYB46 and its close homolog MYB83 directly regulate the expression of the three secondary wall cellulose synthases (CESAs). However, it is not known whether MYB46 is the necessary regulator for functional expression of the secondary wall CESAs or one of the multiple transcriptional factors involved in the transcriptional regulatory program. To address this question, we used a series of genetic complementation experiments of the cesa knock-out mutants with the CESA coding sequence driven by either native- or mutated promoter of the genes. The mutant promoters have two nucleotide point mutations in the MYB46 binding cis element (M46RE) such that MYB46 cannot bind to the promoter, while the binding of other known secondary wall transcription factors is not affected. The mutant complementation results showed that MYB46 is essential to restore normal phenotype from the cesa mutants. We conclude that MYB46 is an obligate component of the transcriptional regulatory complex toward the commitment of secondary wall cellulose synthesis in Arabidopsis.
DOI:10.1007/s11103-012-9880-7URLPMID:22271306 [本文引用: 2]

While many aspects of primary cell wall have been extensively elucidated, our current understanding of secondary wall biosynthesis is limited. Recently, transcription factor MYB46 has been identified as a master regulator of secondary wall biosynthesis in Arabidopsis thaliana. To gain better understanding of this MYB46-mediated transcriptional regulation, we analyzed the promoter region of a direct target gene, AtC3H14, of MYB46 and identified a cis-acting regulatory motif that is recognized by MYB46. This MYB46-responsive cis-regulatory element (M46RE) was further characterized and shown to have an eight-nucleotide core motif, RKTWGGTR. We used electrophoretic mobility shift assay, transient transcriptional activation assay and chromatin immunoprecipitation analysis to show that the M46RE was necessary and sufficient for MYB46-responsive transcription. Genome-wide analysis identified that the frequency of M46RE in the promoters were highly enriched among the genes upregulated by MYB46, especially in the group of genes involved in secondary wall biosynthesis.
DOI:10.1111/j.1365-313x.2012.05124.xURLPMID:26011122 [本文引用: 1]

Cellulose is the most abundant biopolymer on Earth. Three cellulose synthases (CESA4, CESA7 and CESA8) are necessary for cellulose production in the secondary cell walls of Arabidopsis. Little is known about how expression of these CESA genes is regulated. We recently identified a cis-regulatory element (M46RE) that is recognized by MYB46, which is a master switch for secondary wall formation in Arabidopsis. A genome-wide survey of promoter sequences for the presence of M46REs led to the hypothesis that MYB46 may function as a direct regulator of all three secondary wall-associated cellulose synthase genes: CESA4, CESA7 and CESA8. We tested this hypothesis using several lines of experimental evidence. All three CESA genes are highly up-regulated by both constitutive and inducible over-expression of MYB46 in planta. Using a steroid receptor-based inducible activation system, we show that MYB46 directly activates transcription of the three CESA genes. We then used an electrophoretic mobility shift assay and chromatin immunoprecipitation analysis to confirm that MYB46 protein directly binds to the promoters of the three CESA genes both in vitro and in vivo. Furthermore, ectopic up-regulation of MYB46 resulted in a significant increase of crystalline cellulose content in Arabidopsis. Taken together, we have identified MYB46 as a transcription factor that directly regulates all three secondary wall-associated CESA genes. Yeast one-hybrid screening identified additional transcription factors that regulate the CESA genes. However, none of the putative regulators appears to be regulated by MYB46, suggesting the multi-faceted nature of transcriptional regulation of secondary wall cellulose biosynthesis.
DOI:10.1007/s11103-013-0154-9URLPMID:24243147 [本文引用: 1]

Mannans are hemicellulosic polysaccharides that have a structural role and serve as storage reserves during plant growth and development. Previous studies led to the conclusion that mannan synthase enzymes in several plant species are encoded by members of the cellulose synthase-like A (CSLA) gene family. Arabidopsis has nine members of the CSLA gene family. Earlier work has shown that CSLA9 is responsible for the majority of glucomannan synthesis in both primary and secondary cell walls of Arabidopsis inflorescence stems. Little is known about how expression of the CLSA9 gene is regulated. Sequence analysis of the CSLA9 promoter region revealed the presence of multiple copies of a cis-regulatory motif (M46RE) recognized by transcription factor MYB46, leading to the hypothesis that MYB46 (At5g12870) is a direct regulator of the mannan synthase CLSA9. We obtained several lines of experimental evidence in support of this hypothesis. First, the expression of CSLA9 was substantially upregulated by MYB46 overexpression. Second, electrophoretic mobility shift assay (EMSA) was used to demonstrate the direct binding of MYB46 to the promoter of CSLA9 in vitro. This interaction was further confirmed in vivo by a chromatin immunoprecipitation assay. Finally, over-expression of MYB46 resulted in a significant increase in mannan content. Considering the multifaceted nature of MYB46-mediated transcriptional regulation of secondary wall biosynthesis, we reasoned that additional transcription factors are involved in the CSLA9 regulation. This hypothesis was tested by carrying out yeast-one hybrid screening, which identified ANAC041 and bZIP1 as direct regulators of CSLA9. Transcriptional activation assays and EMSA were used to confirm the yeast-one hybrid results. Taken together, we report that transcription factors ANAC041, bZIP1 and MYB46 directly regulate the expression of CSLA9.
DOI:10.1093/aob/mcu126URLPMID:24984711 [本文引用: 2]

BACKGROUND: The secondary cell wall is a defining feature of xylem cells and allows them to resist both gravitational forces and the tension forces associated with the transpirational pull on their internal columns of water. Secondary walls also constitute the majority of plant biomass. Formation of secondary walls requires co-ordinated transcriptional regulation of the genes involved in the biosynthesis of cellulose, hemicellulose and lignin. This co-ordinated control appears to involve a multifaceted and multilayered transcriptional regulatory programme. SCOPE: Transcription factor MYB46 (At5g12870) has been shown to function as a master regulator in secondary wall formation in Arabidopsis thaliana. Recent studies show that MYB46 not only regulates the transcription factors but also the biosynthesis genes for all of the three major components (i.e. cellulose, hemicellulose and lignin) of secondary walls. This review considers our current understanding of the MYB46-mediated transcriptional regulatory network, including upstream regulators, downstream targets and negative regulators of MYB46. CONCLUSIONS AND OUTLOOK: MYB46 is a unique transcription factor in that it directly regulates the biosynthesis genes for all of the three major components of the secondary wall as well as the transcription factors in the biosynthesis pathway. As such, MYB46 may offer a useful means for pathway-specific manipulation of secondary wall biosynthesis. However, realization of this potential requires additional information on the 'MYB46-mediated transcriptional regulatory programme', such as downstream direct targets, upstream regulators and interacting partners of MYB46.
DOI:10.1111/j.1365-313X.2009.03989.xURLPMID:19674407 [本文引用: 6]

MYB46 functions as a transcriptional switch that turns on the genes necessary for secondary wall biosynthesis. Elucidating the transcriptional regulatory network immediately downstream of MYB46 is crucial to our understanding of the molecular and biochemical processes involved in the biosynthesis and deposition of secondary walls in plants. To gain insights into MYB46-mediated transcriptional regulation, we first established an inducible secondary wall thickening system in Arabidopsis by expressing MYB46 under the control of dexamethasone-inducible promoter. Then, we used an ATH1 GeneChip microarray and Illumina digital gene expression system to obtain a series of transcriptome profiles with regard to the induction of secondary wall development. These analyses allowed us to identify a group of transcription factors whose expression coincided with or preceded the induction of secondary wall biosynthetic genes. A transient transcriptional activation assay was used to confirm the hierarchical relationships among the transcription factors in the network. The in vivo assay showed that MYB46 transcriptionally activates downstream target transcription factors, three of which (AtC3H14, MYB52 and MYB63) were shown to be able to activate secondary wall biosynthesis genes. AtC3H14 activated the transcription of all of the secondary wall biosynthesis genes tested, suggesting that AtC3H14 may be another master regulator of secondary wall biosynthesis. The transcription factors identified here may include direct activators of secondary wall biosynthesis genes. The present study discovered novel hierarchical relationships among the transcription factors involved in the transcriptional regulation of secondary wall biosynthesis, and generated several testable hypotheses.
DOI:10.1093/mp/sss076URLPMID:22914575 [本文引用: 1]
DOI:10.1111/j.1365-313X.2007.03109.xURLPMID:17565617 [本文引用: 3]

Vascular plants evolved to have xylem that provides physical support for their growing body and serves as a conduit for water and nutrient transport. In a previous study, we used comparative-transcriptome analyses to select a group of genes that were upregulated in xylem of Arabidopsis plants undergoing secondary growth. Subsequent analyses identified a plant-specific NAC-domain transcription factor gene (ANAC012) as a candidate for genetic regulation of xylem formation. Promoter-GUS analyses showed that ANAC012 expression was preferentially localized in the (pro)cambium region of inflorescence stem and root. Using yeast transactivation analyses, we confirmed the function of ANAC012 as a transcriptional activator, and identified an activation domain in the C terminus. Ectopic overexpression of ANAC012 in Arabidopsis (35S::ANAC012 plants) dramatically suppressed secondary wall deposition in the xylary fiber and slightly increased cell-wall thickness in the xylem vessels. Cellulose compositions of the cell wall were decreased in the inflorescent stems and roots of 35S::ANAC012 plants, probably resulting from defects in xylary fiber formation. Our data suggest that ANAC012 may act as a negative regulator of secondary wall thickening in xylary fibers.
URLPMID:16103214 [本文引用: 2]
DOI:10.1111/j.1469-8137.2011.04016.xURLPMID:22236040 [本文引用: 8]

* The formation of secondary cell walls in cell types such as tracheary elements and fibers is a defining characteristic of vascular plants. The Arabidopsis transcription factor KNAT7 is a component of a transcription network that regulates secondary cell wall biosynthesis, but its function has remained unclear. * We conducted anatomical, biochemical and molecular phenotypic analyses of Arabidopsis knat7 loss-of-function alleles, KNAT7 over-expression lines and knat7 lines expressing poplar KNAT7. * KNAT7 was strongly expressed in concert with secondary wall formation in Arabidopsis and poplar. Arabidopsis knat7 loss-of-function alleles exhibited irregular xylem phenotypes, but also showed increased secondary cell wall thickness in fibers. Increased commitment to secondary cell wall biosynthesis was accompanied by increased lignin content and elevated expression of secondary cell wall biosynthetic genes. KNAT7 over-expression resulted in thinner interfascicular fiber cell walls. * Taken together with data demonstrating that KNAT7 is a transcriptional repressor, we hypothesize that KNAT7 is a negative regulator of secondary wall biosynthesis, and functions in a negative feedback loop that represses metabolically inappropriate commitment to secondary wall formation, thereby maintaining metabolic homeostasis. The conservation of the KNAT7 regulatory module in poplar suggests new ways to manipulate secondary cell wall deposition for improvement of bioenergy traits in this tree.
DOI:10.1111/j.1365-313X.2011.04595.xURLPMID:21457372 [本文引用: 2]

The homeodomain transcription factor KNAT7 has been reported to be involved in the regulation of secondary cell wall biosynthesis. Previous work suggested that KNAT7 can interact with members of the Ovate Family Protein (OFP) transcription co-regulators. However, it remains unknown whether such an OFP-KNAT7 complex could be involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. We re-tested OFP1 and OFP4 for their abilities to intact with KNAT7 using yeast two-hybrid assays, and verified KNAT7-OFP4 interaction but found only weak interaction between KNAT7 and OFP1. Further, the interaction of KNAT7 with OFP4 appears to be mediated by the KNAT7 homeodomain. We used bimolecular fluorescence complementation to confirm interactions and found that OFP1 and OFP4 both interact with KNAT7 in planta. Using a protoplast transient expression system we showed that KNAT7 as well as OFP1 and OFP4 act as transcriptional repressors. Furthermore, in planta interactions between KNAT7 and both OFP1 and OFP4 enhance KNAT7's transcriptional repression activity. An ofp4 mutant exhibited similar irx and fiber cell wall phenotypes as knat7, and the phenotype of a double ofp4 knat7mutant was similar to those of the single mutants, consistent with the view that KNAT7 and OFP function in a common pathway or complex. Furthermore, the pleiotropic OFP1 and OFP4 overexpression phenotype was suppressed in a knat7 mutant background, suggesting that OFP1 and OFP4 functions depend at least partially on KNAT7 function. We propose that KNAT7 forms a functional complex with OFP proteins to regulate aspects of secondary cell wall formation.
DOI:10.1016/j.plaphy.2014.07.022URLPMID:25137291 [本文引用: 1]

NAC proteins that compose of one large family of plant specific transcription factors (TF) play the important roles in many biological processes (such as morphogenesis, development, senescence and stress signal transduction). In this study, a gene (designated as GhXND1) encoding a NAC transcription factor was identified in cotton. Sequence analysis indicated that GhXND1 gene contains two introns inserted in its open reading frame (ORF). GhXND1 protein is localized in the cell nucleus, and displays the transactivation activity. GhXND1 transcripts were mainly detected in cotyledons, petals, roots, hypocotyls and stems, but little or no signals of GhXND1 expression were found in the other tissues. Ectopic expression of GhXND1 in Arabidopsis resulted in a reduction in number of xylem vessel cells and cell wall thickness of interfascicular fibers in the transgenic plants, compared with those of wild type. And expression of some cell wall biosynthesis-related genes was down-regulated in the GhXND1 transgenic plants. Collectively, the data presented in this study suggested that GhXND1 may be involved in regulation of plant xylem development.
DOI:10.1073/pnas.0409179102URLPMID:15647349 [本文引用: 1]

Glucuronoarabinoxylan, xyloglucan, and galactomannan are noncellulosic polysaccharides found in plant cell walls. All consist of beta-linked glycan backbones substituted with sugar side chains. Although considerable progress has been made in characterizing the structure of these polysaccharides, little is known about the biosynthetic enzymes that produce them. Cellulose synthase-like (Csl) genes are hypothesized to encode Golgi-localized beta-glycan synthases that polymerize the backbones of noncellulosic polysaccharides. To investigate this hypothesis, we used heterologous expression in Drosophila Schneider 2 (S2) cells to systematically analyze the functions of the gene products of a group of Csl genes from Arabidopsis and rice (Oryza sativa L.), including members from five Csl gene families (CslA, CslC, CslD, CslE, and CslH). Our analyses indicate that several members of the CslA gene family encode beta-mannan synthases. Recombinant CslA proteins produce beta-linked mannan polymers when supplied GDP-mannose. The same proteins can produce beta-linked glucomannan heteropolymers when supplied both GDP-mannose and GDP-glucose. One CslA protein also produced beta-linked glucan polymers when supplied GDP-glucose alone. Heterologous expression studies of additional candidate glycan synthases in insect cells or other systems may help identify other noncellulosic polysaccharide biosynthetic enzymes.
DOI:10.1016/j.molp.2015.03.012URLPMID:25840349 [本文引用: 1]

Phenylpropanoid-derived compounds represent a diverse family of secondary metabolites that originate from phenylalanine. These compounds have roles in plant growth and development, and in defense against biotic and abiotic stress. Many of these compounds are also beneficial to human health and welfare. V-myb myeloblastosis viral oncogene homolog (MYB) proteins belong to a large family of transcription factors and are key regulators of the synthesis of phenylpropanoid-derived compounds. This review summarizes the current understanding of MYB proteins and their roles in the regulation of phenylpropanoid metabolism in plants.
DOI:10.1080/15592324.2015.1033126URLPMID:26107719 [本文引用: 2]

Formation of secondary walls is a complex process that requires the coordinated and developmentally regulated expression of secondary wall biosynthetic genes. In Arabidopsis thaliana, a transcriptional network orchestrates the biosynthesis and deposition of the main SCW components in xylem and fiber cells. It was recently reported that interacting TALE homeodomain proteins BEL-LIKE HOMEODOMAIN6 (BLH6) and KNOTTED ARABIDOPSIS THALIANA7 (KNAT7) negatively regulate secondary cell wall formation in the interfascicular fibers of Arabidopsis inflorescence stems. Members of the Arabidopsis OVATE FAMILY PROTEIN (OFP) family of transcriptional regulators have been shown to physically interact in yeast with various KNAT and BLH proteins, forming a proposed TALE-OFP protein interaction network. This study presents molecular and genetic data indicating that OFP1 and OFP4, previously reported to interact with TALE homeodomain proteins, enhance the repression activity of BLH6, supporting a role for these OFPs as components of a putative multi-protein transcription regulatory complex containing BLH6 and KNAT7.
DOI:10.1105/tpc.114.128322URLPMID:25490916 [本文引用: 2]

The TALE homeodomain transcription factor KNOTTED ARABIDOPSIS THALIANA7 (KNAT7) is part of a regulatory network governing the commitment to secondary cell wall biosynthesis of Arabidopsis thaliana, where it contributes to negative regulation of this process. Here, we report that BLH6, a BELL1-LIKE HOMEODOMAIN protein, specifically interacts with KNAT7, and this interaction influences secondary cell wall development. BLH6 is a transcriptional repressor, and BLH6-KNAT7 physical interaction enhances KNAT7 and BLH6 repression activities. The overlapping expression patterns of BLH6 and KNAT7 and phenotypes of blh6, knat7, and blh6 knat7 loss-of-function mutants are consistent with the existence of a BLH6-KNAT7 heterodimer that represses commitment to secondary cell wall biosynthesis in interfascicular fibers. BLH6 and KNAT7 overexpression results in thinner interfascicular fiber secondary cell walls, phenotypes that are dependent on the interacting partner. A major impact of the loss of BLH6 and KNAT7 function is enhanced expression of the homeodomain-leucine zipper transcription factor REVOLUTA/INTERFASCICULAR FIBERLESS1 (REV/IFL1). BLH6 and KNAT7 bind to the REV promoter and repress REV expression, while blh6 and knat7 interfascicular fiber secondary cell wall phenotypes are suppressed in blh6 rev and knat7 rev double mutants, suggesting that BLH6/KNAT7 signaling acts through REV as a direct target.
DOI:10.1038/sj.emboj.7600340URLPMID:15282547 [本文引用: 1]

MicroRNAs (miRNAs) are approximately 22-nucleotide noncoding RNAs that can regulate gene expression by directing mRNA degradation or inhibiting productive translation. Dominant mutations in PHABULOSA (PHB) and PHAVOLUTA (PHV) map to a miR165/166 complementary site and impair miRNA-guided cleavage of these mRNAs in vitro. Here, we confirm that disrupted miRNA pairing, not changes in PHB protein sequence, causes the developmental defects in phb-d mutants. In planta, disrupting miRNA pairing near the center of the miRNA complementary site had far milder developmental consequences than more distal mismatches. These differences correlated with differences in miRNA-directed cleavage efficiency in vitro, where mismatch scanning revealed more tolerance for mismatches at the center and 3' end of the miRNA compared to mismatches to the miRNA 5' region. In this respect, miR165/166 resembles animal miRNAs in its pairing requirements. Pairing to the 5' portion of the small silencing RNA appears crucial regardless of the mode of post-transcriptional repression or whether it occurs in plants or animals, supporting a model in which this region of the silencing RNA nucleates pairing to its target.
DOI:10.1093/pcp/pcq064URLPMID:20427511 [本文引用: 1]

Dicot wood is mainly composed of cellulose, xylan and lignin, and its formation requires the coordinated regulation of their biosynthesis. In this report, we demonstrate that the poplar wood-associated MYB transcriptional activators, PtrMYB3 and PtrMYB20, activate the biosynthetic pathways of cellulose, xylan and lignin when overexpressed in Arabidopsis and they are also able to activate the promoter activities of poplar wood biosynthetic genes. We also show that PtrMYB3 and PtrMYB20 are functional orthologs of Arabidopsis MYB46 and MYB83, and their expression is directly activated by poplar PtrWND2, suggesting their involvement in the regulation of wood formation in poplar.
DOI:10.1093/pcp/pcp139URLPMID:19808805 [本文引用: 6]

It has been proposed that the transcriptional regulation of secondary wall biosynthesis in Arabidopsis is controlled by a transcriptional network mediated by SND1 and its close homologs. Uncovering all the transcription factors and deciphering their interrelationships in the network are essential for our understanding of the molecular mechanisms underlying the transcriptional regulation of biosynthesis of secondary walls, the major constituent of wood and fibers. Here, we present functional evidence that the MYB83 transcription factor is another molecular switch in the SND1-mediated transcriptional network regulating secondary wall biosynthesis. MYB83 is specifically expressed in fibers and vessels where secondary wall thickening occurs. Its expression is directly activated by SND1 and its close homologs, including NST1, NST2, VND6 and VND7, indicating that MYB83 is their direct target. MYB83 overexpression is able to activate a number of the biosynthetic genes of cellulose, xylan and lignin and concomitantly induce ectopic secondary wall deposition. In addition, its overexpression upregulates the expression of several transcription factors involved in regulation of secondary wall biosynthesis. Dominant repression of MYB83 functions or simultaneous RNAi inhibition of MYB83 and MYB46 results in a reduction in secondary wall thickening in fibers and vessels and a deformation of vessels. Furthermore, double T-DNA knockout mutations of MYB83 and MYB46 cause a lack of secondary walls in vessels and an arrest in plant growth. Together, these results demonstrate that MYB83 and MYB46, both of which are SND1 direct targets, function redundantly in the transcriptional regulatory cascade leading to secondary wall formation in fibers and vessels.
DOI:10.4161/psb.6.9.16402URLPMID:21847026 [本文引用: 1]

The biosynthesis of secondary walls in vascular plants requires the coordinated regulation of a suite of biosynthetic genes, and this coordination has recently been shown to be executed by the secondary wall NAC (SWN)-mediated transcriptional network. In Arabidopsis, five SWNs, including SND1, NST1/2 and VND6/7, function as master transcriptional switches to activate their common targets and consequently the secondary wall biosynthetic program. A recent report by Zhong et al. revealed that SWNs bind to a common cis-acting element, namely secondary wall NAC binding element (SNBE), which is composed of an imperfect palindromic 19-bp consensus sequence, (T/A)NN(C/T)(T/C/G)TNNNNNNNA(A/C)GN(A/C/T) (A/T). Genome-wide analysis of direct targets of SWNs showed that SWNs directly activate the expression of not only many transcription factors but also a battery of genes involved in secondary wall biosynthesis, cell wall modification and programmed cell death, the promoters of which all contain multiple SNBE sites. The functional significance of the SNBE sites is further substantiated by our current in planta expression study demonstrating that representative SNBE sequences from several SWN direct target promoters are sufficient to drive the expression of the GUS reporter gene in secondary wall-forming cells. The identification of the SWN DNA binding element (SNBE) and the SWN direct targets marks an important step forward toward the dissection of the transcriptional network regulating the biosynthesis of secondary walls, the most abundant biomass produced by land plants.
DOI:10.1105/tpc.106.047043URLPMID:17237351 [本文引用: 4]

Wood is formed by the successive addition of secondary xylem, which consists of cells with a conspicuously thickened secondary wall composed mainly of lignin and cellulose. Several genes involved in lignin and cellulose biosynthesis have been characterized, but the factors that regulate the formation of secondary walls in woody tissues remain to be identified. In this study, we show that plant-specific transcription factors, designated NAC SECONDARY WALL THICKENING PROMOTING FACTOR1 (NST1) and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis thaliana. In nst1-1 nst3-1 double knockout plants, the secondary wall thickenings in interfascicular fibers and secondary xylem, except for vascular vessels, were completely suppressed without affecting formation of cells destined to be woody tissues. Conversely, as shown previously for NST1, overexpression of NST3 induced ectopic secondary wall thickenings in various aboveground tissues. Furthermore, the expression of chimeric repressors derived from NST1 and NST3 suppressed secondary wall thickenings in the presumptive interfascicular fibers. Because putative orthologs of NST1 and NST3 are present in the genome of poplar, our results suggest that they are also key regulators of the formation of secondary walls in woody plants and could be used as a tool for the genetic engineering of wood and its derivatives.
DOI:10.1111/j.1365-313X.2008.03633.xURLPMID:18657234 [本文引用: 2]

Three distinct pattern elements of the silique are thought to contribute to its dehiscence: a separation layer, cells with a secondary wall adjacent to the separation layer, and a valve endocarp layer with secondary wall. However, the role of the secondary wall has not been proven, and the factors that regulate its formation in siliques remain to be characterized. We show here that secondary wall formation in siliques is necessary for dehiscence, and that two plant-specific transcription factors, NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1 and 3 (NST1 and NST3), regulate its formation in siliques of Arabidopsis. The promoters of the NST1 and NST3 genes were active in the valve endocarp layer and in cells surrounding vascular vessels in the replum, and NST1 promoter activity only was faintly detectable at valve margins. In nst1 mutants, specific loss of secondary walls was evident at valve margins, while nst1 nst3 double mutants lacked secondary walls in all parts of the siliques, with the exception of vascular vessels. These siliques were similarly indehiscent. The promoters of two tissue-identity genes, INDEHISCENT (IND) and SHATTERPROOF2 (SHP2), were as active in the nst1 nst3 mutant as in the wild-type. Moreover, the ectopic secondary wall formation that occurs in the fruitfull (ful) mutant was absent in the ful nst1 double mutant. We propose that secondary walls in valve margins are required for dehiscence, and that NST1 and NST3 regulate their formation in siliques in a partially redundant manner after the establishment of tissue identity.
DOI:10.1105/tpc.105.036004URLPMID:16214898 [本文引用: 4]

In plants, secondary wall thickenings play important roles in various biological processes, although the factors regulating these processes remain to be characterized. We show that expression of chimeric repressors derived from NAC SECONDARY WALL THICKENING PROMOTING FACTOR1 (NST1) and NST2 in Arabidopsis thaliana resulted in an anther dehiscence defect due to loss of secondary wall thickening in anther endothecium. Plants with double, but not single, T-DNA-tagged lines for NST1 and NST2 had the same anther-indehiscent phenotype as transgenic plants that expressed the individual chimeric repressors, indicating that NST1 and NST2 are redundant in regulating secondary wall thickening in anther walls. The activity of the NST2 promoter was particularly strong in anther tissue, while that of the NST1 promoter was detected in various tissues in which lignified secondary walls develop. Ectopic expression of NST1 or NST2 induced ectopic thickening of secondary walls in various aboveground tissues. Epidermal cells with ectopic thickening of secondary walls had structural features similar to those of tracheary elements. However, among genes involved in the differentiation of tracheary elements, only those related to secondary wall synthesis were clearly upregulated. None of the genes involved in programmed cell death were similarly affected. Our results suggest NAC transcription factors as possible regulators of secondary wall thickening in various tissues.
DOI:10.1016/j.biortech.2004.06.025URLPMID:15588770 [本文引用: 1]

Cellulosic plant material represents an as-of-yet untapped source of fermentable sugars for significant industrial use. Many physio-chemical structural and compositional factors hinder the enzymatic digestibility of cellulose present in lignocellulosic biomass. The goal of any pretreatment technology is to alter or remove structural and compositional impediments to hydrolysis in order to improve the rate of enzyme hydrolysis and increase yields of fermentable sugars from cellulose or hemicellulose. These methods cause physical and/or chemical changes in the plant biomass in order to achieve this result. Experimental investigation of physical changes and chemical reactions that occur during pretreatment is required for the development of effective and mechanistic models that can be used for the rational design of pretreatment processes. Furthermore, pretreatment processing conditions must be tailored to the specific chemical and structural composition of the various, and variable, sources of lignocellulosic biomass. This paper reviews process parameters and their fundamental modes of action for promising pretreatment methods.
[本文引用: 4]
DOI:10.3389/fpls.2015.00288URLPMID:25999964 [本文引用: 5]

Plant cells biosynthesize primary cell walls (PCW) in all cells and produce secondary cell walls (SCWs) in specific cell types that conduct water and/or provide mechanical support, such as xylem vessels and fibers. The characteristic mechanical stiffness, chemical recalcitrance, and hydrophobic nature of SCWs result from the organization of SCW-specific biopolymers, i.e., highly ordered cellulose, hemicellulose, and lignin. Synthesis of these SCW-specific biopolymers requires SCW-specific enzymes that are regulated by SCW-specific transcription factors. In this review, we summarize our current knowledge of the transcriptional regulation of SCW formation in plant cells. Advances in research on SCW biosynthesis during the past decade have expanded our understanding of the transcriptional regulation of SCW formation, particularly the functions of the NAC and MYB transcription factors. Focusing on the NAC-MYB-based transcriptional network, we discuss the regulatory systems that evolved in land plants to modify the cell wall to serve as a key component of structures that conduct water and provide mechanical support.
DOI:10.1016/j.bbagrm.2011.10.005URLPMID:22037288 [本文引用: 1]

Abiotic stresses such as drought and high salinity adversely affect the growth and productivity of plants, including crops. The development of stress-tolerant crops will be greatly advantageous for modern agriculture in areas that are prone to such stresses. In recent years, several advances have been made towards identifying potential stress related genes which are capable of increasing the tolerance of plants to abiotic stress. NAC proteins are plant-specific transcription factors and more than 100 NAC genes have been identified in Arabidopsis and rice to date. Phylogenetic analyses indicate that the six major groups were already established at least in an ancient moss lineage. NAC transcription factors have a variety of important functions not only in plant development but also in abiotic stress responses. Stress-inducible NAC genes have been shown to be involved in abiotic stress tolerance. Transgenic Arabidopsis and rice plants overexpressing stress-responsive NAC (SNAC) genes have exhibited improved drought tolerance. These studies indicate that SNAC factors have important roles for the control of abiotic stress tolerance and that their overexpression can improve stress tolerance via biotechnological approaches. Although these transcription factors can bind to the same core NAC recognition sequence, recent studies have demonstrated that the effects of NAC factors for growth are different. Moreover, the NAC proteins are capable of functioning as homo- or hetero-dimer forms. Thus, SNAC factors can be useful for improving stress tolerance in transgenic plants, although the mechanism for mediating the stress tolerance of these homologous factors is complex in plants. Recent studies also suggest that crosstalk may exist between stress responses and plant growth. This article is part of a Special Issue entitled: Plant gene regulation in response to abiotic stress.
DOI:10.1093/pcp/pcg164URLPMID:14701930 [本文引用: 2]

HD-Zip III homeobox genes are known to be essential transcriptional factors for vascular development. To further understand the relation of HD-Zip III genes in vascular differentiation, we isolated a new member of the HD-Zip III genes, ZeHB-13, as a Zinnia homolog of ATHB-15, and then characterized the expression profile using a Zinnia xylogenic cell culture and Zinnia plants. We compared the accumulation pattern of transcripts for ZeHB-13 and other HD-Zip III genes and suggested that the expression of ZeHB-13 was restricted to the procambium and was not severely suppressed by brassinazole, an inhibitor of brassinosteroid biosynthesis, unlike other HD-Zip III genes. We also characterized its Arabidopsis counterpart, ATHB-15. A histochemical promoter analysis using ATHB-15::GUS transgenic Arabidopsis plants indicated that ATHB-15 was active specifically in the procambium. These results strongly suggest that ZeHB-13/ATHB-15 is a pivotal transcriptional regulator responsible for early vascular development. Based on these results, we will discuss the regulation of xylem development in light of the functions of HD-Zip III members and brassinosteroids.
DOI:10.1093/pcp/pci180URLPMID:16081527 [本文引用: 2]

Although it has been suggested that class III homeodomain leucine-zipper proteins (HD-Zip III) are involved in vascular development, details of the function of individual HD-Zip III proteins in vascular differentiation have not been resolved. To understand the function of each HD-Zip III protein in vascular differentiation precisely, we analyzed the in vitro transcriptional activity and in vivo function of Zinnia HD-Zip III genes, ZeHB-10, ZeHB-11 and ZeHB-12, which show xylem-related expression. Transgenic Arabidopsis plants harboring cauliflower mosaic virus 35S-driven ZeHB-10 and ZeHB-12 with a mutation in the START domain (mtZeHB-10, mtZeHB-12) showed a higher production of tracheary elements (TEs) and xylem precursor cells, respectively. A systematic analysis with Genechip arrays revealed that overexpression of mtZeHB-12 rapidly induced various genes, including brassinosteroid-signaling pathway-related genes and genes for transcription factors that are expressed specifically in vascular tissues in situ. Furthermore, mtZeHB-12 overexpression did not induce TE-specific genes, including genes related to programmed cell death and lignin polymerization, but did induce lignin monomer synthesis-related genes, which are expressed in xylem parenchyma cells. These results suggest that ZeHB-12 is involved in the differentiation of xylem parenchyma cells, but not of TEs.
DOI:10.1105/tpc.110.075036URLPMID:20952636 [本文引用: 5]

Xylem consists of three types of cells: tracheary elements (TEs), parenchyma cells, and fiber cells. TE differentiation includes two essential processes, programmed cell death (PCD) and secondary cell wall formation. These two processes are tightly coupled. However, little is known about the molecular mechanisms underlying these processes. Here, we show that VASCULAR-RELATED NAC-DOMAIN6 (VND6), a master regulator of TEs, regulates some of the downstream genes involved in these processes in a coordinated manner. We first identified genes that are expressed downstream of VND6 but not downstream of SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN1 (SND1), a master regulator of xylem fiber cells, using transformed suspension culture cells in microarray experiments. We found that VND6 and SND1 governed distinct aspects of xylem formation, whereas they regulated a number of genes in common, specifically those related to secondary cell wall formation. Genes involved in TE-specific PCD were upregulated only by VND6. Moreover, we revealed that VND6 directly regulated genes that harbor a TE-specific cis-element, TERE, in their promoters. Thus, we found that VND6 is a direct regulator of genes related to PCD as well as to secondary wall formation.
DOI:10.1111/tpj.12018URL [本文引用: 1]

The transcription factor MYB103 was previously identified as a member of the transcriptional network regulating secondary wall biosynthesis in xylem tissues of Arabidopsis, and was proposed to act on cellulose biosynthesis. It is a direct transcriptional target of the transcription factor SECONDARY WALL ASSOCIATED NAC DOMAIN PROTEIN 1 (SND1), and 35S-driven dominant repression or over-expression of MYB103 modifies secondary wall thickness. We identified two myb103 T-DNA insertion mutants and chemically characterized their lignocellulose by pyrolysis/GC/MS, 2D NMR, FT-IR microspectroscopy and wet chemistry. The mutants developed normally but exhibited a 70-75% decrease in syringyl (S) lignin. The level of guaiacyl (G) lignin was co-ordinately increased, so that total Klason lignin was not affected. The transcript abundance of FERULATE-5-HYDROXYLASE (F5H), the key gene in biosynthesis of S lignin, was strongly decreased in the myb103 mutants, and the metabolomes of the myb103 mutant and an F5H null mutant were very similar. Other than modification of the lignin S to G ratio, there were only very minor changes in the composition of secondary cell-wall polymers in the inflorescence stem. In conclusion, we demonstrate that F5H expression and hence biosynthesis of S lignin are dependent on MYB103.
DOI:10.1111/j.1365-313X.2011.04614.xURL [本文引用: 1]

Wood harvested from trees is one of the most widely utilized natural materials on our planet. Recent environmental issues have prompted an increase in the demand for wood, especially as a cost-effective and renewable resource for industry and energy, so it is important to understand the process of wood formation. In the present study, we focused on poplar (Populus trichocarpa) NAC domain protein genes which are homologous to well-known Arabidopsis transcription factors regulating the differentiation of xylem vessels and fiber cells. From phylogenetic analysis, we isolated 16 poplar NAC domain protein genes, and named them PtVNS (VND-, NST/SND- and SMB-related proteins) genes. Expression analysis revealed that 12 PtVNS (also called PtrWND) genes including both VND and NST groups were expressed in developing xylem tissue and phloem fiber, whereas in primary xylem vessels, only PtVNS/PtrWND genes of the VND group were expressed. By using the post-translational induction system of Arabidopsis VND7, a master regulator of xylem vessel element differentiation, many poplar genes functioning in xylem vessel differentiation downstream from NAC domain protein genes were identified. Transient expression assays showed the variation in PtVNS/PtrWND transactivation activity toward downstream genes, even between duplicate gene pairs. Furthermore, overexpression of PtVNS/PtrWND genes induced ectopic secondary wall thickening in poplar leaves as well as in Arabidopsis seedlings with different levels of induction efficiency according to the gene. These results suggest that wood formation in poplar is regulated by cooperative functions of the NAC domain proteins.
DOI:10.1016/j.tplants.2004.12.010URLPMID:15708345 [本文引用: 2]

NAC proteins constitute one of the largest families of plant-specific transcription factors, and the family is present in a wide range of land plants. Here, we summarize the biological and molecular functions of the NAC family, paying particular attention to the intricate regulation of NAC protein level and localization, and to the first indications of NAC participation in transcription factor networks. The recent determination of the DNA and protein binding NAC domain structure offers insight into the molecular functions of the protein family. Research into NAC transcription factors has demonstrated the importance of this protein family in the biology of plants and the need for further studies.
DOI:10.1093/dnares/10.6.239URLPMID:15029955

The NAC domain was originally characterized from consensus sequences from petunia NAM and from Arabidopsis ATAF1, ATAF2, and CUC2. Genes containing the NAC domain (NAC family genes) are plant-specific transcriptional regulators and are expressed in various developmental stages and tissues. We performed a comprehensive analysis of NAC family genes in Oryza sativa (a monocot) and Arabidopsis thaliana (a dicot). We found 75 predicted NAC proteins in full-length cDNA data sets of O. sativa (28,469 clones) and 105 in putative genes (28,581 sequences) from the A. thaliana genome. NAC domains from both predicted and known NAC family proteins were classified into two groups and 18 subgroups by sequence similarity. There were a few differences in amino acid sequences in the NAC domains between O. sativa and A. thaliana. In addition, we found 13 common sequence motifs from transcriptional activation regions in the C-terminal regions of predicted NAC proteins. These motifs probably diverged having correlations with NAC domain structures. We discuss the relationship between the structure and function of the NAC family proteins in light of our results and the published data. Our results will aid further functional analysis of NAC family genes.
DOI:10.1111/j.1365-313X.2004.02280.xURLPMID:15584962 [本文引用: 2]

AtMYB32 gene is a member of the R2R3 MYB gene family coding for transcription factors in Arabidopsis thaliana. Its expression pattern was analysed using Northern blotting, in situ hybridization and promoter-GUS fusions. AtMYB32 is expressed in many tissues, but most strongly in the anther tapetum, stigma papillae and lateral root primordia. AtMYB32-GUS was induced in leaves and stems following wounding, and in root primordia by auxin. T-DNA insertion populations were screened and two insertion mutants were identified, both of which were partially male sterile, more than 50% of the pollen grains being distorted in shape and lacking cytoplasm. AtMYB4 is closely related to AtMYB32 and represses the CINNAMATE 4-HYDROXYLASE gene. Distorted pollen grains were produced in both AtMYB4 insertion mutant and overexpression lines. In an AtMYB32 insertion mutant, the transcript levels of the DIHYDROFLAVONOL 4-REDUCTASE and ANTHOCYANIDIN SYNTHASE genes decreased while the level of the CAFFEIC ACID 0-METHYLTRANSFERASE transcript increased. Change in the levels of AtMYB32 and AtMYB4 expression may influence pollen development by changing the flux along the phenylpropanoid pathways, affecting the composition of the pollen wall.
DOI:10.1016/j.tplants.2012.02.004URLPMID:22445067 [本文引用: 1]

The plant-specific NAC (NAM, ATAF1,2 and CUC2) proteins constitute a major transcription factor family renowned for their roles in several developmental programs. Despite their highly conserved DNA-binding domains, their remarkable diversification across plants reflects their numerous functions. Lately, they have received much attention as regulators in various stress signaling pathways which may include interplay of phytohormones. This review summarizes the recent progress in research on NACs highlighting the proteins' potential for engineering stress tolerance against various abiotic and biotic challenges. We discuss regulatory components and targets of NAC proteins in the context of their prospective role for crop improvement strategies via biotechnological intervention.
DOI:10.1111/j.1365-313X.2007.03180.xURLPMID:17683474 [本文引用: 1]

The differentiation of water-conducting tracheary elements (TEs) is the result of the orchestrated construction of secondary wall structure, including lignification, and programmed cell death (PCD), including cellular autolysis. To understand the orchestrated regulation of differentiation of TEs, we investigated the regulatory mechanism of gene expression directing TE differentiation. Detailed loss-of-function and gain-of-function analyses of the ZCP4 (Zinniacysteine protease 4) promoter, which confers TE-specific expression, demonstrated that a novel 11-bp cis-element is necessary and sufficient for the immature TE-specific promoter activity. The 11-bp cis-element-like sequences were found in promoters of many Arabidopsis TE differentiation-related genes. A gain-of-function analysis with similar putative cis-elements from secondary wall formation or modification-related genes as well as PCD-related genes indicated that the cis-elements are also sufficient for TE-specific expression of genes. These results demonstrate that a common sequence, designated as the tracheary-element-regulating cis-element, confers TE-specific expression to both genes related to secondary wall formation or modification and PCD.
DOI:10.3389/fpls.2018.00399URLPMID:29670638 [本文引用: 1]

Secondary cell walls mediate many crucial biological processes in plants including mechanical support, water and nutrient transport and stress management. They also provide an abundant resource of renewable feed, fiber, and fuel. The grass family contains the most important food, forage, and biofuel crops. Understanding the regulatory mechanism of secondary wall formation in grasses is necessary for exploiting these plants for agriculture and industry. Previous research has established a detailed model of the secondary wall regulatory network in the dicot model species Arabidopsis thaliana. Grasses, branching off from the dicot ancestor 140-150 million years ago, display distinct cell wall morphology and composition, suggesting potential for a different secondary wall regulation program from that established for dicots. Recently, combined application of molecular, genetic and bioinformatics approaches have revealed more transcription factors involved in secondary cell wall biosynthesis in grasses. Compared with the dicots, grasses exhibit a relatively conserved but nevertheless divergent transcriptional regulatory program to activate their secondary cell wall development and to coordinate secondary wall biosynthesis with other physiological processes.
DOI:10.1111/j.1469-8137.2012.04201.xURLPMID:22708996 [本文引用: 1]

Throughout their lifetimes, plants must coordinate the regulation of various facets of growth and development. Previous evidence has suggested that the Arabidopsis thaliana R2R3-MYB, AtMYB61, might function as a coordinate regulator of multiple aspects of plant resource allocation. Using a combination of cell biology, transcriptome analysis and biochemistry, in conjunction with gain-of-function and loss-of-function genetics, the role of AtMYB61 in conditioning resource allocation throughout the plant life cycle was explored. In keeping with its role as a regulator of resource allocation, AtMYB61 is expressed in sink tissues, notably xylem, roots and developing seeds. Loss of AtMYB61 function decreases xylem formation, induces qualitative changes in xylem cell structure and decreases lateral root formation; in contrast, gain of AtMYB61 function has the opposite effect on these traits. AtMYB61 coordinates a small network of downstream target genes, which contain a motif in their upstream regulatory regions that is bound by AtMYB61, and AtMYB61 activates transcription from this same motif. Loss-of-function analysis supports the hypothesis that AtMYB61 targets play roles in shaping subsets of AtMYB61-related phenotypes. Taken together, these findings suggest that AtMYB61 links the transcriptional control of multiple aspects of plant resource allocation.
DOI:10.1093/pcp/pcu208URLPMID:25535195 [本文引用: 1]

The secondary cell wall constitutes a rigid frame of cells in plant tissues where rigidity is required. Deposition of the secondary cell wall in fiber cells contributes to the production of wood in woody plants. The secondary cell wall is assembled through co-operative activities of many enzymes, and their gene expression is precisely regulated by a pyramidal cascade of transcription factors. Deposition of a transmuted secondary cell wall in empty fiber cells by expressing selected gene(s) in this cascade has not been attempted previously. In this proof-of-concept study, we expressed chimeric activators of 24 transcription factors that are preferentially expressed in the stem, in empty fiber cells of the Arabidopsis nst1-1 nst3-1 double mutant, which lacks a secondary cell wall in fiber cells, under the control of the NST3 promoter. The chimeric activators of MYB46, SND2 and ANAC075, as well as NST3, reconstituted a secondary cell wall with different characteristics from those of the wild type in terms of its composition. The transgenic lines expressing the SND2 or ANAC075 chimeric activator showed increased glucose and xylose, and lower lignin content, whereas the transgenic line expressing the MYB46 chimeric activator showed increased mannose content. The expression profile of downstream genes in each transgenic line was also different from that of the wild type. This study proposed a new screening strategy to identify factors of secondary wall formation and also suggested the potential of the artificially reconstituted secondary cell walls as a novel raw material for production of bioethanol and other chemicals.
DOI:10.1038/srep19925URLPMID:26812961 [本文引用: 1]

Lignocellulose, composed of cellulose, hemicellulose, and lignin, in the secondary cell wall constitutes wood and is the most abundant form of biomass on Earth. Enhancement of wood accumulation may be an effective strategy to increase biomass as well as wood strength, but currently only limited research has been undertaken. Here, we demonstrated that OsSWN1, the orthologue of the rice NAC Secondary-wall Thickening factor (NST) transcription factor, effectively enhanced secondary cell wall formation in the Arabidopsis inflorescence stem and poplar (Populus tremulaxPopulus tremuloides) stem when expressed by the Arabidopsis NST3 promoter. Interestingly, in transgenic Arabidopsis and poplar, ectopic secondary cell wall deposition in the pith area was observed in addition to densification of the secondary cell wall in fiber cells. The cell wall content or density of the stem increased on average by up to 38% and 39% in Arabidopsis and poplar, respectively, without causing growth inhibition. As a result, physical strength of the stem increased by up to 57% in poplar. Collectively, these data suggest that the reinforcement of wood by NST3pro:OsSWN1 is a promising strategy to enhance wood-biomass production in dicotyledonous plant species.
DOI:10.3389/fpls.2015.00902URLPMID:26579152 [本文引用: 1]

Abiotic stresses adversely affect plant growth and agricultural productivity. According to the current climate prediction models, crop plants will face a greater number of environmental stresses, which are likely to occur simultaneously in the future. So it is very urgent to breed broad-spectrum tolerant crops in order to meet an increasing demand for food productivity due to global population increase. As one of the largest families of transcription factors (TFs) in plants, NAC TFs play vital roles in regulating plant growth and development processes including abiotic stress responses. Lots of studies indicated that many stress-responsive NAC TFs had been used to improve stress tolerance in crop plants by genetic engineering. In this review, the recent progress in NAC TFs was summarized, and the potential utilization of NAC TFs in breeding abiotic stress tolerant transgenic crops was also be discussed. In view of the complexity of field conditions and the specificity in multiple stress responses, we suggest that the NAC TFs commonly induced by multiple stresses should be promising candidates to produce plants with enhanced multiple stress tolerance. Furthermore, the field evaluation of transgenic crops harboring NAC genes, as well as the suitable promoters for minimizing the negative effects caused by over-expressing some NAC genes, should be considered.
DOI:10.1016/s0092-8674(00)81093-4URLPMID:8612269 [本文引用: 1]

Petunia embryos carrying the no apical meristem (nam) mutation fail to develop a shoot apical meristem. Occasional shoots on nam- seedlings bear flowers that develop ten instead of five primordia in the second whorl. Double mutants with the homeotic gene green petals show that nam acts independently of organ identify in whorl 2 and now also affects primordium number in whorl 3. The nam gene was isolated by transposon tagging. The encoded protein shares a conserved N-terminal domain with several other proteins of unknown function and thus represents a novel class of proteins. Strikingly, nam mRNA accumulates in cells at the boundaries of meristems and primordia. These data indicate a role for nam in determining positions of meristems and primordia.
DOI:10.1105/tpc.108.061796URLPMID:19088331 [本文引用: 4]

ASYMMETRIC LEAVES2 (AS2)/LATERAL ORGAN BOUNDARIES DOMAIN (LBD) family proteins are plant-specific nuclear proteins, and genes encoding several family members have been implicated in plant development. We investigated the function of two members of the Arabidopsis thaliana AS2/LBD family, AS2-LIKE19 (ASL19)/LBD30 and ASL20/LBD18, which encode homologous proteins. Both ASL19 and ASL20 were expressed in immature tracheary elements (TEs), and the expression was dependent on VASCULAR-RELATED NAC-DOMAIN PROTEIN6 (VND6) and VND7, which are transcription factors required for TE differentiation. Overexpression of ASL19 and ASL20 induced transdifferentiation of cells from nonvascular tissues into TE-like cells, similar to those induced upon VND6/7 overexpression. By contrast, aberrant TEs were formed when a cDNA encoding a fusion protein of ASL20 with an artificial repressor domain (ASL20-SRDX) was expressed from its native promoter. These results provide evidence that ASL proteins positively regulate TE differentiation. In transgenic plants overexpressing both ASL19 and ASL20, the xylem-deficient phenotype caused by the expression of dominant-negative versions of VND6/7 proteins was not rescued. However, ectopic expression of VND7 was detected in plants overexpressing ASL20. Moreover, VND genes and their downstream targets were downregulated in ASL20-SRDX plants. Therefore, ASL20 appears to be involved in a positive feedback loop for VND7 expression that regulates TE differentiation-related genes.
DOI:10.1046/j.1365-313x.2003.01745.xURLPMID:12753590 [本文引用: 1]

A male sterile mutant with a defect in anther dehiscence was identified in an Arabidopsis thaliana population mutagenized with the Zea mays transposon En-1/Spm. Mutants produce viable pollen that can fertilize when released mechanically from the anthers. Mutant stamens are of normal size and shape, but lack cell wall fortifications in the endothecial cell layer of the anther, which are required for the dehiscence process. The mutant phenotype was shown to be caused by a transposon insertion in AtMYB26, disrupting the putative DNA-binding domain of this R2R3-type MYB transcription factor. RT-PCR revealed that expression of AtMYB26 is restricted to inflorescences. Sterility was shown to be stable under several environmental conditions. The high stability of the sterile phenotype, together with the fact that pollen is functional, makes AtMYB26 and its orthologs a valuable tool for manipulating male fertility in higher plants.
DOI:10.1093/treephys/tpz004URLPMID:30806711 [本文引用: 1]

Wood fibers form thick secondary cell wall (SCW) in xylem tissues to give mechanical support to trees. NAC SECONDARY WALL THICKENING PROMOTING FACTOR3/SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN 1 (NST3/SND1) and NST1 were identified as master regulators of SCW formation in xylem fiber cells in the model plant Arabidopsis thaliana. In Populus species, four NST/SND orthologs have been conserved and coordinately control SCW formation in wood fibers and phloem fibers. However, it remains to be elucidated whether SCW formation in other xylem cells, such as ray parenchyma cells and vessel elements, is regulated by NST/SND orthologs in poplar. We knocked out all NST/SND genes in hybrid aspen using the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 nuclease (Cas9) system and investigated the detailed histological appearance of stem tissues in the knockout mutants. Observation by light microscopy and transmission electron microscopy showed that SCW was severely suppressed in wood fibers, phloem fibers and xylem ray parenchyma cells in the knockout mutants. Although almost all wood fibers lacked SCW, some fiber cells formed thick cell walls. The irregularly cell wall-forming fibers retained primary wall and SCW, and were mainly located in the vicinity of vessel elements. Field emission-scanning electron microscope observation showed that there were no apparent differences in the structural features of pits such as the shape and size between irregularly SCW-forming wood fibers in the knockout mutants and normal wood fibers in wild-type. Cell wall components such as cellulose, hemicellulose and lignin were deposited in the cell wall of irregularly SCW-forming wood fibers in quadruple mutants. Our results indicate that four NST/SND orthologs are master switches for SCW formation in wood fibers, xylem ray parenchyma cells and phloem fibers in poplar, while SCW is still formed in limited wood fibers, which are located at the region adjacent to vessel elements in the knockout mutants.
DOI:10.1007/s10086-017-1627-2URL [本文引用: 1]
DOI:10.1104/pp.17.00461URLPMID:29133368 [本文引用: 2]

Arabidopsis (Arabidopsis thaliana) VASCULAR-RELATED NAC-DOMAIN1 (VND1) to VND7 encode a group of NAC domain transcription factors that function as master regulators of xylem vessel element differentiation. These transcription factors activate the transcription of genes required for secondary cell wall formation and programmed cell death, key events in xylem vessel element differentiation. Because constitutive overexpression of VND6 and VND7 induces ectopic xylem vessel element differentiation, functional studies of VND proteins have largely focused on these two proteins. Here, we report the roles of VND1, VND2, and VND3 in xylem vessel formation in cotyledons. Using our newly established in vitro system in which excised Arabidopsis cotyledons are stimulated to undergo xylem cell differentiation by cytokinin, auxin, and brassinosteroid treatment, we found that ectopic xylem vessel element differentiation required VND1, VND2, and VND3 but not VND6 or VND7. The importance of VND1, VND2, and VND3 also was indicated in vivo; in the vnd1 vnd2 vnd3 seedlings, xylem vessel element differentiation of secondary veins in cotyledons was inhibited under dark conditions. Furthermore, the light responsiveness of VND gene expression was disturbed in the vnd1 vnd2 vnd3 mutant, and vnd1 vnd2 vnd3 failed to recover lateral root development in response to the change of light conditions. These findings suggest that VND1 to VND3 have specific molecular functions, possibly linking light conditions to xylem vessel formation, during seedling development.
DOI:10.1038/s41467-018-06430-8URLPMID:30250259 [本文引用: 1]

Soil water uptake by roots is a key component of plant performance and adaptation to adverse environments. Here, we use a genome-wide association analysis to identify the XYLEM NAC DOMAIN 1 (XND1) transcription factor as a negative regulator of Arabidopsis root hydraulic conductivity (Lpr). The distinct functionalities of a series of natural XND1 variants and a single nucleotide polymorphism that determines XND1 translation efficiency demonstrate the significance of XND1 natural variation at species-wide level. Phenotyping of xnd1 mutants and natural XND1 variants show that XND1 modulates Lpr through action on xylem formation and potential indirect effects on aquaporin function and that it diminishes drought stress tolerance. XND1 also mediates the inhibition of xylem formation by the bacterial elicitor flagellin and counteracts plant infection by the root pathogen Ralstonia solanacearum. Thus, genetic variation at XND1, and xylem differentiation contribute to resolving the major trade-off between abiotic and biotic stress resistance in Arabidopsis.
DOI:10.1038/srep12240URLPMID:26179205 [本文引用: 2]

Wood is formed by the successive addition of secondary xylem, which consists of cells with a conspicuously thickened secondary wall composed mainly of cellulose, xylan and lignin. Currently, few transcription factors involved in the direct regulation of secondary wall biosynthesis have been characterized in tree species. Here, we show that PdMYB221, a poplar ortholog of the Arabidopsis R2R3-MYB transcription factor AtMYB4, directly regulates secondary wall biosynthesis during wood formation. PdMYB221 is predominantly expressed in cells of developing wood, and the protein it encodes localizes to the nucleus and acts as a transcriptional repressor. Ectopic expression of PdMYB221 resulted in reduced cell wall thicknesses of fibers and vessels in Arabidopsis inflorescence stems. The amounts of cellulose, xylose, and lignin were decreased and the expression of key genes synthesizing the three components was suppressed in PdMYB221 overexpression plants. Transcriptional activation assays showed that PdMYB221 repressed the promoters of poplar PdCESA7/8, PdGT47C, PdCOMT2 and PdCCR1. Electrophoretic mobility shift assays revealed that PdMYB221 bound directly to the PdCESA8, PdGT47C, and PdCOMT2 promoters. Together, our results suggest that PdMYB221 may be involved in the negative regulation of secondary wall formation through the direct and indirect suppression of the gene expression of secondary wall biosynthesis.
DOI:10.1073/pnas.0337628100URLPMID:12538856 [本文引用: 1]

In a screen to identify novel cellulose deficient mutants, three lines were shown to be allelic and define a novel complementation group, irregular xylem5 (irx5). IRX5 was cloned and encodes a member of the CesA family of cellulose synthase catalytic subunits (AtCesA4). irx5 plants have an identical phenotype to previously described mutations in two other members of this gene family (IRX1 and IRX3). IRX5, IRX3, and IRX1 are coexpressed in exactly the same cells, and all three proteins interact in detergent solubilized extracts, suggesting that three members of this gene family are required for cellulose synthesis in secondary cell walls. The association of IRX1 and IRX3 was reduced to undetectable levels in the absence of IRX5. Consequently, these data suggest that IRX5, IRX3, and IRX1 are all essential components of the cellulose synthesizing complex and the presence of all three subunits is required for the correct assembly of this complex.
DOI:10.1105/tpc.12.12.2529URLPMID:11148295 [本文引用: 1]

The irregular xylem 1 (irx1) mutant of Arabidopsis has a severe deficiency in the deposition of cellulose in secondary cell walls, which results in collapsed xylem cells. This mutation has been mapped to a 140-kb region of chromosome 4. A cellulose synthase catalytic subunit was found to be located in this region, and genomic clones containing this gene complemented the irx1 mutation. IRX1 shows homology to a previously described cellulose synthase (IRX3). Analysis of the irx1 and irx3 mutant phenotypes demonstrates that both IRX1 and IRX3 are essential for the production of cellulose in the same cell. Thus, IRX1 and IRX3 define distinct classes of catalytic subunits that are both essential for cellulose synthesis in plants. This finding is supported by coprecipitation of IRX1 with IRX3, suggesting that IRX1 and IRX3 are part of the same complex.
DOI:10.1105/tpc.11.5.769URLPMID:10330464 [本文引用: 1]

The irregular xylem3 (irx3) mutant of Arabidopsis has a severe deficiency in secondary cell wall cellulose deposition that leads to collapsed xylem cells. The irx3 mutation has been mapped to the top arm of chromosome V near the marker nga106. Expressed sequence tag clone 75G11, which exhibits sequence similarity to cellulose synthase, was found to be tightly linked to irx3, and genomic clones containing the gene corresponding to clone 75G11 complemented the irx3 mutation. Thus, the IRX3 gene encodes a cellulose synthase component that is specifically required for the synthesis of cellulose in the secondary cell wall. The irx3 mutant allele contains a stop codon that truncates the gene product by 168 amino acids, suggesting that this allele is null. Furthermore, in contrast to radial swelling1 (rsw1) plants, irx3 plants show no increase in the accumulation of beta-1,4-linked glucose in the noncrystalline cell wall fraction. IRX3 and RSW1 fall into a distinct subgroup (Csa) of Arabidopsis genes showing homology to bacterial cellulose synthases.
DOI:10.1038/nature14099URLPMID:25533953 [本文引用: 2]

The plant cell wall is an important factor for determining cell shape, function and response to the environment. Secondary cell walls, such as those found in xylem, are composed of cellulose, hemicelluloses and lignin and account for the bulk of plant biomass. The coordination between transcriptional regulation of synthesis for each polymer is complex and vital to cell function. A regulatory hierarchy of developmental switches has been proposed, although the full complement of regulators remains unknown. Here we present a protein-DNA network between Arabidopsis thaliana transcription factors and secondary cell wall metabolic genes with gene expression regulated by a series of feed-forward loops. This model allowed us to develop and validate new hypotheses about secondary wall gene regulation under abiotic stress. Distinct stresses are able to perturb targeted genes to potentially promote functional adaptation. These interactions will serve as a foundation for understanding the regulation of a complex, integral plant component.
DOI:10.4161/gmcr.1.1.10569URLPMID:21912210 [本文引用: 1]

Abiotic stresses such as extreme temperature, drought, high salinity, cold and waterlogging often result in significant losses to the yields of economically important crops. Plants constantly exposed to capricious conditions have adapted at the molecular, cellular, physiological and biochemical level, enabling them to survive and cope with adverse environmental stresses. NAC (NAM, ATAF and CUC) transcription factors (TFs), which constitute one of the largest families of plant-specific TFs, have been reported to enhance tolerance against various stresses, such as drought, high salinity and cold, in a number of plants. In this review the NAC TF family will be described and the potential use of NAC TFs in development of improved stress tolerant transgenic crops will be discussed.
DOI:10.1073/pnas.1016436107URLPMID:21135241 [本文引用: 4]

Stems of dicotyledonous plants consist of an outer epidermis, a cortex, a ring of secondarily thickened vascular bundles and interfascicular cells, and inner pith parenchyma cells with thin primary walls. It is unclear how the different cell layers attain and retain their identities. Here, we show that WRKY transcription factors are in part responsible for the parenchymatous nature of the pith cells in dicotyledonous plants. We isolated mutants of Medicago truncatula and Arabidopsis thaliana with secondary cell wall thickening in pith cells associated with ectopic deposition of lignin, xylan, and cellulose, leading to an approximately 50% increase in biomass density in stem tissue of the Arabidopsis mutants. The mutations are caused by disruption of stem-expressed WRKY transcription factor (TF) genes, which consequently up-regulate downstream genes encoding the NAM, ATAF1/2, and CUC2 (NAC) and CCCH type (C3H) zinc finger TFs that activate secondary wall synthesis. Direct binding of WRKY to the NAC gene promoter and repression of three downstream TFs were confirmed by in vitro assays and in planta transgenic experiments. Secondary wall-bearing cells form lignocellulosic biomass that is the source for second generation biofuel production. The discovery of negative regulators of secondary wall formation in pith opens up the possibility of significantly increasing the mass of fermentable cell wall components in bioenergy crops.
DOI:10.1093/mp/ssr098URLPMID:22138968 [本文引用: 1]

Secondary cell walls provide plants with rigidity and strength to support their body weight and ensure water and nutrient transport. They also provide textiles, timber, and potentially second-generation biofuels for human use. Genes responsible for synthesis of the different cell wall components, namely cellulose, hemicelluloses, and lignin, are coordinately expressed and under transcriptional regulation. In the past several years, cell wall-related NAC and MYB transcription factors have been intensively investigated in different species and shown to be master switches of secondary cell wall biosynthesis. Positive and negative regulators, which function upstream of NAC master switches, have also been identified in different plant tissues. Further elucidation of the regulatory mechanisms of cell wall synthesis will facilitate the engineering of plant feedstocks suitable for biofuel production.
DOI:10.1111/tpj.14364URLPMID:31021017 [本文引用: 1]

The secondary cell wall is an important carbon sink in higher plants and its biosynthesis requires coordination of metabolic fluxes in the phenylpropanoid pathway. In Arabidopsis (Arabidopsis thaliana), MYB75 and the KNOX transcription factor KNAT7 form functional complexes to regulate secondary cell wall formation in the inflorescence stem. However, the molecular mechanism by which these transcription factors control different branches of the phenylpropanoid pathway remains poorly understood in woody species. We isolated an R2R3-MYB transcription factor MYB6 from Populus tomentosa and determined that it was expressed predominately in young leaves. Overexpression of MYB6 in transgenic poplar upregulated flavonoid biosynthetic gene expression, resulting in significantly increased accumulation of anthocyanin and proanthocyanidins. MYB6-overexpression plants showed reduced secondary cell wall deposition, accompanied by repressed expression of secondary cell wall biosynthetic genes. We further showed that MYB6 interacted physically with KNAT7 and formed functional complexes that acted to repress secondary cell wall development in poplar and Arabidopsis. The results provide an insight into the transcriptional mechanisms involved in the regulation of the metabolic fluxes between the flavonoid and lignin biosynthetic pathways in poplar.
DOI:10.1042/BJ20111742URLPMID:22455904 [本文引用: 1]

NAC (NAM/ATAF/CUC) plant transcription factors regulate essential processes in development, stress responses and nutrient distribution in important crop and model plants (rice, Populus, Arabidopsis), which makes them highly relevant in the context of crop optimization and bioenergy production. The structure of the DNA-binding NAC domain of ANAC019 has previously been determined by X-ray crystallography, revealing a dimeric and predominantly beta-fold structure, but the mode of binding to cognate DNA has remained elusive. In the present study, information from low resolution X-ray structures and small angle X-ray scattering on complexes with oligonucleotides, mutagenesis and (DNase I and uranyl photo-) footprinting, is combined to form a structural view of DNA-binding, and for the first time provide experimental evidence for the speculated relationship between plant-specific NAC proteins, WRKY transcription factors and the mammalian GCM (Glial cell missing) transcription factors, which all use a beta-strand motif for DNA-binding. The structure shows that the NAC domain inserts the edge of its core beta-sheet into the major groove, while leaving the DNA largely undistorted. The structure of the NAC-DNA complex and a new crystal form of the unbound NAC also indicate limited flexibility of the NAC dimer arrangement, which could be important in recognizing suboptimal binding sites.
DOI:10.1016/j.devcel.2008.09.019URLPMID:19081078 [本文引用: 1]

Because plant cells do not migrate, cell division planes are crucial determinants of plant cellular architecture. In Arabidopsis roots, stringent control of cell divisions leads to a virtually invariant division pattern, including those that create new tissue layers. However, the mechanisms that control oriented cell divisions are hitherto poorly understood. Here, we reveal one such mechanism in which FEZ and SOMBRERO (SMB), two plant-specific NAC-domain transcription factors, control the delicately tuned reorientation and timing of cell division in a subset of stem cells. FEZ is expressed in root cap stem cells, where it promotes periclinal, root cap-forming cell divisions. In contrast, SMB negatively regulates FEZ activity, repressing stem cell-like divisions in the root cap daughter cells. FEZ becomes expressed in predivision stem cells, induces oriented cell division, and activates expression of its negative regulator, SMB, thus generating a feedback loop for controlled switches in cell division plane.
DOI:10.1242/dev.01942URLPMID:16033795 [本文引用: 1]

Plant development is characterized by precise control of gene regulation, leading to the correct spatial and temporal tissue patterning. We have characterized the Arabidopsis jabba-1D (jba-1D) mutant, which displays multiple enlarged shoot meristems, radialized leaves, reduced gynoecia and vascular defects. The jba-1D meristem phenotypes require WUSCHEL (WUS) activity, and correlate with a dramatic increase in WUS expression levels. We demonstrate that the jba-1D phenotypes are caused by over-expression of miR166g, and require the activity of the RNase III helicase DCL1. miR166g over-expression in jba-1D plants affects the transcripts of several class III homeodomain-leucine zipper (AtHD-ZIP) family target genes. The expression of PHABULOSA (PHB), PHAVOLUTA (PHV) and CORONA (CNA) is significantly reduced in a jba-1D background, while REVOLUTA (REV) expression is elevated and ATHB8 is unchanged. In addition, we show that miR166 has a dynamic expression pattern in wild-type and jba-1D embryos. Our analysis demonstrates an indirect role for miRNAs in controlling meristem formation via regulation of WUS expression, and reveals complex regulation of the class III AtHD-ZIP gene family.
DOI:10.1016/s1360-1385(02)02335-xURLPMID:12399182 [本文引用: 1]

Recent research has provided insights into how plants make cellulose - the major structural material of their cell walls and the basis of the cotton and wood fibre industries. Arabidopsis thaliana mutants impaired in cellulose production are defective in genes encoding membrane-bound glycosyltransferases, an endo-1,4-beta-glucanase and several enzymes involved in the N-glycosylation and quality-control pathways of the endoplasmic reticulum. The glycosyltransferases form the rosette terminal complexes seen in plasma membranes making cellulose. Synthesis might start by making lipoglucans, which, in turn, might form the substrate for the endo-1,4-beta-glucanase, before being elongated to form the long, crystalline microfibrils that assemble in the cell wall.
DOI:10.1126/science.1248417URLPMID:24652936 [本文引用: 1]

The development of cells specialized for water conduction or support is a striking innovation of plants that has enabled them to colonize land. The NAC transcription factors regulate the differentiation of these cells in vascular plants. However, the path by which plants with these cells have evolved from their nonvascular ancestors is unclear. We investigated genes of the moss Physcomitrella patens that encode NAC proteins. Loss-of-function mutants formed abnormal water-conducting and supporting cells, as well as malformed sporophyte cells, and overexpression induced ectopic differentiation of water-conducting-like cells. Our results show conservation of transcriptional regulation and cellular function between moss and Arabidopsis thaliana water-conducting cells. The conserved genetic basis suggests roles for NAC proteins in the adaptation of plants to land.
DOI:10.5511/plantbiotechnology.27.237URL [本文引用: 1]
DOI:10.1111/j.1365-313X.2008.03533.xURLPMID:18445131 [本文引用: 1]

SUMMARY: The Arabidopsis thaliana NAC domain transcription factor, vascular-related NAC-DOMAIN7 (VND7), plays a pivotal role in regulating the differentiation of root protoxylem vessels. In order to understand the mechanisms underscoring the function of VND7 in vessel differentiation in more detail, we conducted extensive molecular analyses in yeast (Saccharomyces cerevisiae), Arabidopsis, and Nicotiana tabacum L. cv. Bright Yellow 2 (tobacco BY-2) cells. The transcriptional activation activity of VND7 was confirmed in yeast and Arabidopsis, and the C-terminal region was shown to be required for VND7 transcriptional activation. Expression of the C-terminus-truncated VND7 protein under the control of the native VND7 promoter resulted in inhibition of the normal development of metaxylem vessels in roots and vessels in aerial organs, as well as protoxylem vessels in roots. The expression pattern of VND7 overlapped that of VND2 to VND5 in most of the differentiating vessels. Furthermore, a yeast two-hybrid assay revealed the ability of VND7 to form homodimers and heterodimers with other VND proteins via their N-termini, which include the NAC domain. The heterologous expression of VND7 in tobacco BY-2 cells demonstrated that the stability of VND7 could be regulated by proteasome-mediated degradation. Together these data suggest that VND7 regulates the differentiation of all types of vessels in roots and shoots, possibly in cooperation with VND2 to VND5 and other regulatory proteins.
DOI:10.1111/j.1365-313X.2011.04514.xURLPMID:21284754 [本文引用: 4]

The Arabidopsis thaliana NAC domain transcription factor, VASCULAR-RELATED NAC-DOMAIN7 (VND7), acts as a key regulator of xylem vessel differentiation. In order to identify direct target genes of VND7, we performed global transcriptome analysis using Arabidopsis transgenic lines in which VND7 activity could be induced post-translationally. This analysis identified 63 putative direct target genes of VND7, which encode a broad range of proteins, such as transcription factors, IRREGULAR XYLEM proteins and proteolytic enzymes, known to be closely associated with xylem vessel formation. Recombinant VND7 protein binds to several promoter sequences present in candidate direct target genes: specifically, in the promoter of XYLEM CYSTEINE PEPTIDASE1, two distinct regions were demonstrated to be responsible for VND7 binding. We also found that expression of VND7 restores secondary cell wall formation in the fiber cells of inflorescence stems of nst1 nst3 double mutants, as well as expression of NAC SECONDARY WALL THICKENING PROMOTING FACTOR3 (NST3, however, the vessel-type secondary wall deposition was observed only as a result of VND7 expression. These findings indicated that VND7 upregulates, directly and/or indirectly, many genes involved in a wide range of processes in xylem vessel differentiation, and that its target genes are partially different from those of NSTs.
DOI:10.5511/plantbiotechnology.15.0208aURL [本文引用: 2]
DOI:10.1105/tpc.108.064048URLPMID:20388856 [本文引用: 2]

The Arabidopsis thaliana NAC domain transcription factor VASCULAR-RELATED NAC-DOMAIN7 (VND7) acts as a master regulator of xylem vessel differentiation. To understand the mechanism by which VND7 regulates xylem vessel differentiation, we used a yeast two-hybrid system to screen for proteins that interact with VND7 and identified cDNAs encoding two NAC domain proteins, VND-INTERACTING1 (VNI1) and VNI2. Binding assays demonstrated that VNI2 effectively interacts with VND7 and the VND family proteins, VND1-5, as well as with other NAC domain proteins at lower affinity. VNI2 is expressed in both xylem and phloem cells in roots and inflorescence stems. The expression of VNI2 overlaps with that of VND7 in elongating vessel precursors in roots. VNI2 contains a predicted PEST motif and a C-terminally truncated VNI2 protein, which lacks part of the PEST motif, is more stable than full-length VNI2. Transient reporter assays showed that VNI2 is a transcriptional repressor and can repress the expression of vessel-specific genes regulated by VND7. Expression of C-terminally truncated VNI2 under the control of the VND7 promoter inhibited the normal development of xylem vessels in roots and aerial organs. These data suggest that VNI2 regulates xylem cell specification as a transcriptional repressor that interacts with VND proteins and possibly also with other NAC domain proteins.
DOI:10.1093/jxb/ert150URLPMID:23698629 [本文引用: 2]

Basic helix-loop-helix (bHLH) genes are important regulators of development in plants. SbbHLH1, a Sorghum bicolor bHLH sequence, was isolated from a suppression subtractive hybridization library constructed using 13 independent brown midrib (bmr) mutants as the tester and wild-type sorghum as the driver. The gene was upregulated in at least five of the mutants at the five- to seven-leaf stage. Using a yeast expression system, the N-terminal portion of SbbHLH1 was shown to be required for proper transactivation. Its heterologous expression in Arabidopsis thaliana markedly reduced the plant's lignin content. It downregulated the lignin synthesis genes 4CL1, HCT, COMT, PAL1, and CCR1, and upregulated the transcription factors MYB83, MYB46, and MYB63. The hypothesis is proposed that SbbHLH1 has stronger effect on the regulation of lignin synthesis than the various MYB transcription factors, with a possible feedback mechanism acting on the MYB transcriptional regulators.
DOI:10.1105/tpc.106.046391URLPMID:17329564 [本文引用: 2]

The Arabidopsis thaliana MYB26/MALE STERILE35 (MS35) gene is critical for the development of secondary thickening in the anther endothecium and subsequent dehiscence. MYB26 is localized to the nucleus and regulates endothecial development and secondary thickening in a cell-specific manner in the anther. MYB26 expression is seen in anthers and also in the style and nectaries, although there is no effect on female fertility in the ms35 mutant. MYB26 expression in anthers occurs early during endothecial development, with maximal expression during pollen mitosis I and bicellular stages, indicating a regulatory role in specifying early endothecial cell development. Overexpression of MYB26 results in ectopic secondary thickening in both Arabidopsis and tobacco (Nicotiana tabacum) plants, predominantly within the epidermal tissues. MYB26 regulates a number of genes linked to secondary thickening, including IRREGULAR XYLEM1 (IRX1), IRX3, IRX8, and IRX12. Changes in expression were also detected in two NAC domain genes, NAC SECONDARY WALL-PROMOTING FACTOR1 (NST1) and NST2, which have been linked to secondary thickening in the anther endothecium. These data indicate that MYB26 regulates NST1 and NST2 expression and in turn controls the process of secondary thickening. Therefore, MYB26 appears to function in a regulatory role involved in determining endothecial cell development within the anther and acts upstream of the lignin biosynthesis pathway.
DOI:10.3389/fpls.2016.00356URLPMID:27047525 [本文引用: 1]

Vascular tissues are important for transporting water and nutrients throughout the plant and as physical support of upright growth. The primary constituents of vascular tissues, xylem, and phloem, are derived from the meristematic vascular procambium and cambium. Xylem cells develop secondary cell walls (SCWs) that form the largest part of plant lignocellulosic biomass that serve as a renewable feedstock for biofuel production. For the last decade, research on vascular development and SCW biosynthesis has seen rapid progress due to the importance of these processes to plant biology and to the biofuel industry. Plant hormones, transcriptional regulators and peptide signaling regulate procambium/cambium proliferation, vascular patterning, and xylem differentiation. Transcriptional regulatory pathways play a pivot role in SCW biosynthesis. Although most of these discoveries are derived from research in Arabidopsis, many genes have shown conserved functions in biofuel feedstock species. Here, we review the recent advances in our understanding of vascular development and SCW formation and discuss potential biotechnological uses.
DOI:10.1105/tpc.111.084913URLPMID:21673078 [本文引用: 1]

Leaf aging is a highly regulated developmental process, which is also influenced profoundly by diverse environmental conditions. Accumulating evidence in recent years supports that plant responsiveness to abiotic stress is intimately related with leaf longevity. However, molecular mechanisms underlying the signaling crosstalks and regulatory schemes are yet unknown. In this work, we demonstrate that an abscisic acid (ABA)-responsive NAC transcription factor VND-INTERACTING2 (VNI2) integrates ABA-mediated abiotic stress signals into leaf aging by regulating a subset of COLD-REGULATED (COR) and RESPONSIVE TO DEHYDRATION (RD) genes. The VNI2 gene was induced by high salinity in an ABA-dependent manner. In addition, spatial and temporal expression patterns of the VNI2 gene are correlated with leaf aging and senescence. Accordingly, leaf aging was delayed in transgenic plants overexpressing the VNI2 gene but significantly accelerated in a VNI2-deficient mutant. The VNI2 transcription factor regulates the COR and RD genes by binding directly to their promoters. Notably, transgenic plants overexpressing the COR or RD genes exhibited prolonged leaf longevity. These observations indicate that the VNI2 transcription factor serves as a molecular link that integrates plant responses to environmental stresses into modulation of leaf longevity.
DOI:10.1186/s12284-018-0228-zURLPMID:29855737 [本文引用: 1]

BACKGROUND: As one of the most important staple food crops, rice produces huge agronomic biomass residues that contain lots of secondary cell walls (SCWs) comprising cellulose, hemicelluloses and lignin. The transcriptional regulation mechanism underlying SCWs biosynthesis remains elusive. RESULTS: In this study, we isolated a NAC family transcription factor (TF), OsSND2 through yeast one-hybrid screening using the secondary wall NAC-binding element (SNBE) on the promoter region of OsMYB61 which is known transcription factor for regulation of SCWs biosynthesis as bait. We used an electrophoretic mobility shift assay (EMSA) and chromatin immunoprecipitation analysis (ChIP) to further confirm that OsSND2 can directly bind to the promoter of OsMYB61 both in vitro and in vivo. OsSND2, a close homolog of AtSND2, is localized in the nucleus and has transcriptional activation activity. Expression pattern analysis indicated that OsSND2 was mainly expressed in internodes and panicles. Overexpression of OsSND2 resulted in rolled leaf, increased cellulose content and up-regulated expression of SCWs related genes. The knockout of OsSND2 using CRISPR/Cas9 system decreased cellulose content and down-regulated the expression of SCWs related genes. Furthermore, OsSND2 can also directly bind to the promoters of other MYB family TFs by transactivation analysis in yeast cells and rice protoplasts. Altogether, our findings suggest that OsSND2 may function as a master regulator to mediate SCWs biosynthesis. CONCLUSION: OsSND2 was identified as a positive regulator of cellulose biosynthesis in rice. An increase in the expression level of this gene can improve the SCWs cellulose content. Therefore, the study of the function of OsSND2 can provide a strategy for manipulating plant biomass production.
URLPMID:29175437 [本文引用: 1]
DOI:10.1111/nph.14864URLPMID:29105766 [本文引用: 4]

Cotton (Gossypium hirsutum) fibers are the highly elongated and thickened single-cell trichomes on the seed epidermis. However, little is known about the molecular base of fiber cell wall thickening in detail. In this study, a cotton NAC transcription factor (GhFSN1) that is specifically expressed in secondary cell wall (SCW) thickening fibers was functionally characterized. The GhFSN1 transgenic cotton plants were generated to study how FSN1 regulates fiber SCW formation. Up-regulation of GhFSN1 expression in cotton resulted in an increase in SCW thickness of fibers but a decrease in fiber length. Transcriptomic analysis revealed that GhFSN1 activates or represses numerous downstream genes. GhFSN1 has the ability to form homodimers, binds to its promoter to activate itself, and might be degraded by the ubiquitin-mediated proteasome pathway. The direct targets of GhFSN1 include the fiber SCW-related GhDUF231L1, GhKNL1, GhMYBL1, GhGUT1 and GhIRX12 genes. GhFSN1 binds directly to a consensus sequence (GhNBS), (C/T)(C/G/T)TN(A/T)(G/T)(A/C/G)(A/G)(A/T/G)(A/T/G)AAG, which exists in the promoters of these SCW-related genes. Our data demonstrate that GhFSN1 acts as a positive regulator in controlling SCW formation of cotton fibers by activating its downstream SCW-related genes. Thus, these findings give us novel insights into comprehensive understanding of GhFSN1 function in fiber development.
DOI:10.3389/fpls.2018.01535URLPMID:30405670 [本文引用: 1]

Plant cell walls provide structural support for growth and serve as a barrier for pathogen attack. Plant cell walls are also a source of renewable biomass for conversion to biofuels and bioproducts. Understanding plant cell wall biosynthesis and its regulation is of critical importance for the genetic modification of plant feedstocks for cost-effective biofuels and bioproducts conversion and production. Great progress has been made in identifying enzymes involved in plant cell wall biosynthesis, and in Arabidopsis it is generally recognized that the regulation of genes encoding these enzymes is under a transcriptional regulatory network with coherent feedforward and feedback loops. However, less is known about the transcriptional regulation of plant secondary cell wall (SCW) biosynthesis in woody species despite of its high relevance to biofuels and bioproducts conversion and production. In this article, we synthesize recent progress on the transcriptional regulation of SCW biosynthesis in Arabidopsis and contrast to what is known in woody species. Furthermore, we evaluate progress in related emerging regulatory machineries targeting transcription factors in this complex regulatory network of SCW biosynthesis.
DOI:10.1105/tpc.18.00315URLPMID:30242037 [本文引用: 2]

Secondary cell walls (SCWs) are formed in some specific types of plant cells, providing plants with mechanical strength. During plant growth and development, formation of secondary cell walls is regulated by various developmental and environmental signals. The underlying molecular mechanisms are poorly understood. In this study, we analyzed the blue light receptor cryptochrome1 (cry1) mutant of Arabidopsis thaliana for its SCW phenotypes. During inflorescence stem growth, SCW thickening in the vasculature was significantly affected by blue light. cry1 plants displayed a decline of SCW thickening in fiber cells, while CRY1 overexpression led to enhanced SCW formation. Transcriptome analysis indicated that the reduced SCW thickening was associated with repression of the NST1-directed transcription regulatory networks. Further analyses revealed that the expression of MYC2/MYC4 that is induced by blue light activates the transcriptional network underlying SCW thickening. The activation is caused by direct binding of MYC2/MYC4 to the NST1 promoter. This study demonstrates that SCW thickening in fiber cells is regulated by a blue light signal that is mediated through MYC2/MYC4 activation of NST1-directed SCW formation in Arabidopsis.
DOI:10.1111/j.1365-313X.2007.03350.xURLPMID:18069942 [本文引用: 1]

Members of the large Arabidopsis NAC domain transcription factor family are regulators of meristem development, organ elongation and separation, and deposition of patterned secondary cell walls. XYLEM NAC DOMAIN 1 (XND1) is highly expressed in xylem. Changes observed for XND1 knockout plants compared with wild-type Arabidopsis thaliana included a reduction in both plant height and tracheary element length and an increase in metaxylem relative to protoxylem in roots of plants treated with the proteasome inhibitor MG132. Overexpression of XND1 resulted in extreme dwarfism associated with the absence of xylem vessels and little or no expression of tracheary element marker genes. In contrast, phloem marker-gene expression was not altered and phloem-type cells still formed. Transmission electron microscopy showed that parenchyma-like cells in the incipient xylem zone in hypocotyls of XND1 overexpressors lacked secondary wall thickenings and retained their cytoplasmic content. Considered together, these findings suggest that XND1 affects tracheary element growth through regulation of secondary wall synthesis and programmed cell death.
URLPMID:15923329 [本文引用: 1]
DOI:10.1111/nph.14704URLPMID:28742236 [本文引用: 3]

The Arabidopsis thaliana gene XYLEM NAC DOMAIN1 (XND1) is upregulated in xylem tracheary elements. Yet overexpression of XND1 blocks differentiation of tracheary elements. The molecular mechanism of XND1 action was investigated. Phylogenetic and motif analyses indicated that XND1 and its homologs are present only in angiosperms and possess a highly conserved C-terminal region containing linear motifs (CKII-acidic, LXCXE, E2F(TD) -like and LXCXE-mimic) predicted to interact with the cell cycle and differentiation regulator RETINOBLASTOMA-RELATED (RBR). Protein-protein interaction and functional analyses of XND1 deletion mutants were used to test the importance of RBR-interaction motifs. Deletion of either the LXCXE or the LXCXE-mimic motif reduced both the XND1-RBR interaction and XND1 efficacy as a repressor of differentiation, with loss of the LXCXE motif having the strongest negative impacts. The function of the XND1 C-terminal domain could be partially replaced by RBR fused to the N-terminal domain of XND1. XND1 also transactivated gene expression in yeast and plants. The properties of XND1, a transactivator that depends on multiple linear RBR-interaction motifs to inhibit differentiation, have not previously been described for a plant protein. XND1 harbors an apparently angiosperm-specific combination of interaction motifs potentially linking the general differentiation regulator RBR with a xylem-specific pathway for inhibition of differentiation.
DOI:10.1186/1471-2229-14-135URLPMID:24885077 [本文引用: 3]

BACKGROUND: R2R3 MYB proteins constitute one of the largest plant transcription factor clades and regulate diverse plant-specific processes. Several R2R3 MYB proteins act as regulators of secondary cell wall (SCW) biosynthesis in Arabidopsis thaliana (At), a dicotyledenous plant. Relatively few studies have examined SCW R2R3 MYB function in grasses, which may have diverged from dicots in terms of SCW regulatory mechanisms, as they have in cell wall composition and patterning. Understanding cell wall regulation is especially important for improving lignocellulosic bioenergy crops, such as switchgrass. RESULTS: Here, we describe the results of applying phylogenic, OrthoMCL, and sequence identity analyses to classify the R2R3 MYB family proteins from the annotated proteomes of Arabidposis, poplar, rice, maize and the initial genome (v0.0) and translated transcriptome of switchgrass (Panicum virgatum). We find that the R2R3 MYB proteins of the five species fall into 48 subgroups, including three dicot-specific, six grass-specific, and two panicoid grass-expanded subgroups. We observe four classes of phylogenetic relationships within the subgroups of known SCW-regulating MYB proteins between Arabidopsis and rice, ranging from likely one-to-one orthology (for AtMYB26, AtMYB103, AtMYB69) to no homologs identifiable (for AtMYB75). Microarray data for putative switchgrass SCW MYBs indicate that many maintain similar expression patterns with the Arabidopsis SCW regulators. However, some of the switchgrass-expanded candidate SCW MYBs exhibit differences in gene expression patterns among paralogs, consistent with subfunctionalization. Furthermore, some switchgrass representatives of grass-expanded clades have gene expression patterns consistent with regulating SCW development. CONCLUSIONS: Our analysis suggests that no single comparative genomics tool is able to provide a complete picture of the R2R3 MYB protein family without leaving ambiguities, and establishing likely false-negative and -positive relationships, but that used together a relatively clear view emerges. Generally, we find that most R2R3 MYBs that regulate SCW in Arabidopsis are likely conserved in the grasses. This comparative analysis of the R2R3 MYB family will facilitate transfer of understanding of regulatory mechanisms among species and enable control of SCW biosynthesis in switchgrass toward improving its biomass quality.
URLPMID:31257603 [本文引用: 1]
DOI:10.1105/tpc.106.047399URLPMID:17114348 [本文引用: 7]

Secondary walls in fibers and tracheary elements constitute the most abundant biomass produced by plants. Although a number of genes involved in the biosynthesis of secondary wall components have been characterized, little is known about the molecular mechanisms underlying the coordinated expression of these genes. Here, we demonstrate that the Arabidopsis thaliana NAC (for NAM, ATAF1/2, and CUC2) domain transcription factor, SND1 (for secondary wall-associated NAC domain protein), is a key transcriptional switch regulating secondary wall synthesis in fibers. We show that SND1 is expressed specifically in interfascicular fibers and xylary fibers in stems and that dominant repression of SND1 causes a drastic reduction in the secondary wall thickening of fibers. Ectopic overexpression of SND1 results in activation of the expression of secondary wall biosynthetic genes, leading to massive deposition of secondary walls in cells that are normally nonsclerenchymatous. In addition, we have found that SND1 upregulates the expression of several transcription factors that are highly expressed in fibers during secondary wall synthesis. Together, our results reveal that SND1 is a key transcriptional activator involved in secondary wall biosynthesis in fibers.
DOI:10.1093/pcp/pcr123URLPMID:21908441 [本文引用: 3]

The bulk of grass biomass potentially useful for cellulose-based biofuel production is the remains of secondary wall-containing sclerenchymatous fibers. Hence, it is important to uncover the molecular mechanisms underlying the regulation of secondary wall thickening in grass species. So far, little is known about the transcriptional regulatory switches responsible for the activation of the secondary wall biosynthetic program in grass species. Here, we report the roles of a group of rice and maize NAC and MYB transcription factors in the regulation of secondary wall biosynthesis. The rice and maize secondary wall-associated NACs (namely OsSWNs and ZmSWNs) were able to complement the Arabidopsis snd1 nst1 double mutant defective in secondary wall thickening. When overexpressed in Arabidopsis, OsSWNs and ZmSWNs were sufficient to activate a number of secondary wall-associated transcription factors and secondary wall biosynthetic genes, and concomitantly result in the ectopic deposition of cellulose, xylan and lignin. It was also found that the rice and maize MYB transcription factors, OsMYB46 and ZmMYB46, are functional orthologs of Arabidopsis MYB46/MYB83 and, when overexpressed in Arabidopsis, they were able to activate the entire secondary wall biosynthetic program. Furthermore, the promoters of OsMYB46 and ZmMYB46 contain secondary wall NAC-binding elements (SNBEs), which can be bound and activated by OsSWNs and ZmSWNs. Together, our results indicate that the rice and maize SWNs and MYB46 are master transcriptional activators of the secondary wall biosynthetic program and that OsSWNs and ZmSWNs activate their direct target genes through binding to the SNBE sites.
DOI:10.1104/pp.109.148270URLPMID:19965968 [本文引用: 3]

Wood is the most abundant biomass produced by land plants. Dissection of the molecular mechanisms underlying the transcriptional regulation of wood formation is a fundamental issue in plant biology and has important implications in tree biotechnology. Although a number of transcription factors in tree species have been shown to be associated with wood formation and some of them are implicated in lignin biosynthesis, none of them have been demonstrated to be key regulators of the biosynthesis of all three major components of wood. In this report, we have identified a group of NAC domain transcription factors, PtrWNDs, that are preferentially expressed in developing wood of poplar (Populus trichocarpa). Expression of PtrWNDs in the Arabidopsis (Arabidopsis thaliana) snd1 nst1 double mutant effectively complemented the secondary wall defects in fibers, indicating that PtrWNDs are capable of activating the entire secondary wall biosynthetic program. Overexpression of PtrWND2B and PtrWND6B in Arabidopsis induced the expression of secondary wall-associated transcription factors and secondary wall biosynthetic genes and, concomitantly, the ectopic deposition of cellulose, xylan, and lignin. Furthermore, PtrWND2B and PtrWND6B were able to activate the promoter activities of a number of poplar wood-associated transcription factors and wood biosynthetic genes. Together, these results demonstrate that PtrWNDs are functional orthologs of SND1 and suggest that PtrWNDs together with their downstream transcription factors form a transcriptional network involved in the regulation of wood formation in poplar.
DOI:10.1093/mp/ssq062URLPMID:20935069 [本文引用: 5]

We report the genome-wide analysis of direct target genes of SND1 and VND7, two Arabidopsis thaliana NAC domain transcription factors that are master regulators of secondary wall biosynthesis in fibers and vessels, respectively. Systematic mapping of the SND1 binding sequence using electrophoretic mobility shift assay and transactivation analysis demonstrated that SND1 together with other secondary wall NACs (SWNs), including VND6, VND7, NST1, and NST2, bind to an imperfect palindromic 19-bp consensus sequence designated as secondary wall NAC binding element (SNBE), (T/A)NN(C/T) (T/C/G)TNNNNNNNA(A/C)GN(A/C/T) (A/T), in the promoters of their direct targets. Genome-wide analysis of direct targets of SND1 and VND7 revealed that they directly activate the expression of not only downstream transcription factors, but also a number of non-transcription factor genes involved in secondary wall biosynthesis, cell wall modification, and programmed cell death, the promoters of which all contain multiple SNBE sites. SND1 and VND7 directly regulate the expression of a set of common targets but each of them also preferentially induces a distinct set of direct targets, which is likely attributed to their differential activation strength toward SNBE sites. Complementation study showed that the SWNs were able to rescue the secondary wall defect in the snd1 nst1 mutant, indicating that they are functionally interchangeable. Together, our results provide important insight into the complex transcriptional program and the evolutionary mechanism underlying secondary wall biosynthesis, cell wall modification, and programmed cell death in secondary wall-containing cell types.
DOI:10.1016/j.tplants.2010.08.007URLPMID:20833576 [本文引用: 1]

The ability to make secondary cell walls was a pivotal step for vascular plants in their conquest of dry land. Here, we review recent molecular and genetic studies that reveal that a group of Arabidopsis (Arabidopsis thaliana) secondary wall-associated NAC domain transcription factors are master switches regulating a cascade of downstream transcription factors, leading to activation of the secondary wall biosynthetic program. Close homologs of the Arabidopsis secondary wall NACs and their downstream transcription factors exist in diverse taxa of vascular plants and some are functional orthologs of their Arabidopsis counterparts. There is evidence to suggest that the secondary wall NAC-mediated transcriptional regulation of secondary wall biosynthesis is a conserved mechanism throughout vascular plants.
DOI:10.1105/tpc.108.061325URLPMID:18952777 [本文引用: 13]

SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN1 (SND1) is a master transcriptional switch activating the developmental program of secondary wall biosynthesis. Here, we demonstrate that a battery of SND1-regulated transcription factors is required for normal secondary wall biosynthesis in Arabidopsis thaliana. The expression of 11 SND1-regulated transcription factors, namely, SND2, SND3, MYB103, MYB85, MYB52, MYB54, MYB69, MYB42, MYB43, MYB20, and KNAT7 (a Knotted1-like homeodomain protein), was developmentally associated with cells undergoing secondary wall thickening. Of these, dominant repression of SND2, SND3, MYB103, MYB85, MYB52, MYB54, and KNAT7 significantly reduced secondary wall thickening in fiber cells. Overexpression of SND2, SND3, and MYB103 increased secondary wall thickening in fibers, and overexpression of MYB85 led to ectopic deposition of lignin in epidermal and cortical cells in stems. Furthermore, SND2, SND3, MYB103, MYB85, MYB52, and MYB54 were able to induce secondary wall biosynthetic genes. Direct target analysis using the estrogen-inducible system revealed that MYB46, SND3, MYB103, and KNAT7 were direct targets of SND1 and also of its close homologs, NST1, NST2, and vessel-specific VND6 and VND7. Together, these results demonstrate that a transcriptional network consisting of SND1 and its downstream targets is involved in regulating secondary wall biosynthesis in fibers and that NST1, NST2, VND6, and VND7 are functional homologs of SND1 that regulate the same downstream targets in different cell types.
DOI:10.1371/journal.pone.0069219URLPMID:23922694 [本文引用: 1]

Wood is mainly composed of secondary walls, which constitute the most abundant stored carbon produced by vascular plants. Understanding the molecular mechanisms controlling secondary wall deposition during wood formation is not only an important issue in plant biology but also critical for providing molecular tools to custom-design wood composition suited for diverse end uses. Past molecular and genetic studies have revealed a transcriptional network encompassing a group of wood-associated NAC and MYB transcription factors that are involved in the regulation of the secondary wall biosynthetic program during wood formation in poplar trees. Here, we report the functional characterization of poplar orthologs of MYB46 and MYB83 that are known to be master switches of secondary wall biosynthesis in Arabidopsis. In addition to the two previously-described PtrMYB3 and PtrMYB20, two other MYBs, PtrMYB2 and PtrMYB21, were shown to be MYB46/MYB83 orthologs by complementation and overexpression studies in Arabidopsis. The functional roles of these PtrMYBs in regulating secondary wall biosynthesis were further demonstrated in transgenic poplar plants showing an ectopic deposition of secondary walls in PtrMYB overexpressors and a reduction of secondary wall thickening in their dominant repressors. Furthermore, PtrMYB2/3/20/21 together with two other tree MYBs, the Eucalyptus EgMYB2 and the pine PtMYB4, were shown to differentially bind to and activate the eight variants of the 7-bp SMRE consensus sequence, composed of ACC(A/T)A(A/C)(T/C). Together, our results indicate that the tree MYBs, PtrMYB2/3/20/21, EgMYB2 and PtMYB4, are master transcriptional switches that activate the SMRE sites in the promoters of target genes and thereby regulate secondary wall biosynthesis during wood formation.
DOI:10.1007/s00425-007-0498-yURLPMID:17333250 [本文引用: 7]

Secondary walls are the major component of wood, and studies of the mechanisms regulating secondary wall synthesis is important for understanding the process of wood formation. We have previously shown that the NAC domain transcription factor SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN1 (SND1) is a key regulator of secondary wall synthesis in fibers of Arabidopsis thaliana stems and dominant repression of SND1 leads to a reduction in secondary wall thickening in fibers. However, T-DNA knockout of the SND1 gene did not cause an alteration in secondary wall thickness, suggesting that other SND1 homologs may compensate for the loss of SND1 expression. Here, we studied the effects of simultaneous inhibition of SND1 and its homolog, NAC SECONDARY WALL THICKENING PROMOTING FACTOR1 (NST1), on secondary wall synthesis in fibers. We show that simultaneous RNA interference (RNAi) inhibition of the expression of both SND1 and NST1 genes results in loss of secondary wall formation in fibers of stems. The fiber cells in the stems of SND1/NST1-RNAi plants lack all three major secondary wall components, including cellulose, xylan, and lignin, which is accompanied by a severe reduction in the expression of genes involved in their biosynthesis. In addition, inhibition of SND1 and NST1 leads to down-regulation of several fiber-associated transcription factor genes. Double T-DNA knockout mutations of SND1 and NST1 genes cause the same effects, as does simultaneous RNAi inhibition of SND1 and NST1. Our results provide first line evidence demonstrating that SND1 and NST1 function redundantly in the regulation of secondary wall synthesis in fibers.
DOI:10.1105/tpc.107.053678URLPMID:17890373 [本文引用: 3]

We demonstrate that the Arabidopsis thaliana MYB46 transcription factor is a direct target of SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN1 (SND1), which is a key transcriptional activator regulating the developmental program of secondary wall biosynthesis. The MYB46 gene is expressed predominantly in fibers and vessels in stems, and its encoded protein is targeted to the nucleus and can activate transcription. MYB46 gene expression was shown to be regulated by SND1, and transactivation analysis demonstrated that SND1 as well as its close homologs were able to activate the MYB46 promoter. Electrophoretic mobility shift assays and chromatin immunoprecipitation experiments revealed that SND1 binds to the MYB46 promoter. Dominant repression of MYB46 caused a drastic reduction in the secondary wall thickening of fibers and vessels. Overexpression of MYB46 resulted in an activation of the biosynthetic pathways of cellulose, xylan, and lignin and concomitantly led to ectopic deposition of secondary walls in cells that are normally nonsclerenchymatous. In addition, the expression of two secondary wall-associated transcription factors, MYB85 and KNAT7, was highly upregulated by MYB46 overexpression. These results demonstrate that MYB46 is a direct target of SND1 and is another key player in the transcriptional network involved in the regulation of secondary wall biosynthesis in Arabidopsis.
DOI:10.1093/pcp/pcr185URLPMID:22197883 [本文引用: 2]

MYB46 and MYB83 are two functionally redundant Arabidopsis thaliana MYB transcription factors that act as master switches regulating secondary wall biosynthesis. Here, we report the identification of the transcriptional responsive elements and global analysis of the direct targets of MYB46 and MYB83. Using the estrogen-inducible direct activation system, we found that a number of previously identified MYB46 downstream transcription factors, including MYB43, MYB52, MYB54, MYB58, MYB63 and KNAT7, are direct targets of MYB46. Promoter deletion coupled with transactivation analysis of the MYB63 promoter led to the identification of a 7 bp sequence that is sufficient to be responsive to MYB46 activation, and therefore this sequence is designated as the secondary wall MYB-responsive element (SMRE). Further single nucleotide mutation together with electrophoretic mobility shift assay mapped the SMRE consensus sequence as ACC(A/T)A(A/C)(T/C). Genome-wide analysis of direct targets of MYB46 demonstrated that it directly regulates the expression of not only a number of downstream transcription factors, but also a suite of secondary wall biosynthetic genes, some of which are also directly activated by secondary wall NAC (SWN) master switches or by MYB46 direct targets. Furthermore, MYB83 was found to bind to the same SMRE consensus sequence and activate the same set of direct targets as MYB46. Our study has revealed that the transcription program regulating secondary wall biosynthesis involves a multileveled feed-forward loop regulatory structure in which MYB46/MYB83 together with their regulators SWNs and their direct targets regulate an array of downstream genes thereby activating the secondary wall biosynthetic program.
URLPMID:25294860 [本文引用: 2]
DOI:10.4161/15592324.2014.989746URLPMID:25751728 [本文引用: 4]

Transcriptional regulation of secondary wall biosynthesis in Arabidopsis thaliana has been shown to be mediated by a group of secondary wall NAC master switches, including NST1, NST2, SND1 and VND1 to VND7. It has been shown that VND1 to VND7 regulate secondary wall biosynthesis in vessels, NST1 and NST2 function redundantly in anther endothecium, and SND1 and NST1 are required for secondary wall thickening in fibers of stems. However, it is unknown whether NST2 is involved in regulating secondary wall biosynthesis in fibers of stems. In this report, we demonstrated that similar to SND1, NST2 together with NST1 were highly expressed in interfascicular fibers and xylary fibers but not in vessels of stems. Although simultaneous mutations of SND1 and NST1 have been shown to result in a significant impairment of secondary wall thickening in fibers, a small amount of secondary walls was deposited in fibers during the late stage of stem development. In contrast, simultaneous mutations of SND1, NST1 and NST2 led to a complete loss of secondary wall thickening in fibers. These results demonstrate that NST2 together with SND1 and NST1 regulate secondary wall biosynthesis in fibers of stems.
DOI:10.1371/journal.pone.0134611URLPMID:26248336

Switchgrass is a promising biofuel feedstock due to its high biomass production and low agronomic input requirements. Because the bulk of switchgrass biomass used for biofuel production is lignocellulosic secondary walls, studies on secondary wall biosynthesis and its transcriptional regulation are imperative for designing strategies for genetic improvement of biomass production in switchgrass. Here, we report the identification and functional characterization of a group of switchgrass transcription factors, including several NACs (PvSWNs) and a MYB (PvMYB46A), for their involvement in regulating secondary wall biosynthesis. PvSWNs and PvMYB46A were found to be highly expressed in stems and their expression was closely associated with sclerenchyma cells. Overexpression of PvSWNs and PvMYB46A in Arabidopsis was shown to result in activation of the biosynthetic genes for cellulose, xylan and lignin and ectopic deposition of secondary walls in normally parenchymatous cells. Transactivation and complementation studies demonstrated that PvSWNs were able to activate the SNBE-driven GUS reporter gene and effectively rescue the secondary wall defects in the Arabidopsis snd1 nst1 double mutant, indicating that they are functional orthologs of Arabidopsis SWNs. Furthermore, we showed that PvMYB46A could activate the SMRE-driven GUS reporter gene and complement the Arabidopsis myb46 myb83 double mutant, suggesting that it is a functional ortholog of Arabidopsis MYB46/MYB83. Together, these results indicate that PvSWNs and PvMYB46A are transcriptional switches involved in regulating secondary wall biosynthesis, which provides molecular tools for genetic manipulation of biomass production in switchgrass.
DOI:10.1105/tpc.108.063321URLPMID:19122102 [本文引用: 1]

It has previously been shown that SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN1 (SND1) is a key transcription factor regulating secondary cell wall formation, including the biosynthesis of cellulose, xylan, and lignin. In this study, we show that two closely related SND1-regulated MYB transcription factors, MYB58 and MYB63, are transcriptional regulators specifically activating lignin biosynthetic genes during secondary wall formation in Arabidopsis thaliana. MYB58 and MYB63 are phylogenetically distinct from previously characterized MYBs shown to be associated with secondary wall formation or phenylpropanoid metabolism. Expression studies showed that MYB58 and MYB63 are specifically expressed in fibers and vessels undergoing secondary wall thickening. Dominant repression of their functions led to a reduction in secondary wall thickening and lignin content. Overexpression of MYB58 and MYB63 resulted in specific activation of lignin biosynthetic genes and concomitant ectopic deposition of lignin in cells that are normally unlignified. MYB58 was able to activate directly the expression of lignin biosynthetic genes and a secondary wall-associated laccase (LAC4) gene. Furthermore, the expression of MYB58 and MYB63 was shown to be regulated by the SND1 close homologs NST1, NST2, VND6, and VND7 and their downstream target MYB46. Together, our results indicate that MYB58 and MYB63 are specific transcriptional activators of lignin biosynthesis in the SND1-mediated transcriptional network regulating secondary wall formation.
DOI:10.1371/journal.pone.0105726URLPMID:25148240 [本文引用: 2]

One of the most prominent features of xylem conducting cells is the deposition of secondary walls. In Arabidopsis, secondary wall biosynthesis in the xylem conducting cells, vessels, has been shown to be regulated by two VASCULAR-RELATED NAC-DOMAIN (VND) genes, VND6 and VND7. In this report, we have investigated the roles of five additional Arabidopsis VND genes, VND1 to VND5, in regulating secondary wall biosynthesis in vessels. The VND1 to VND5 genes were shown to be specifically expressed in vessels but not in interfascicular fibers in stems. The expression of VND4 and VND5 was also seen specifically in vessels in the secondary xylem of the root-hypocotyl region. When overexpressed, VND1 to VND5 were able to activate the expression of secondary wall-associated transcription factors and genes involved in secondary wall biosynthesis and programmed cell death. As a result, many normally parenchymatous cells in leaves and stems acquired thickened secondary walls in the VND1 to VND5 overexpressors. In contrast, dominant repression of VND3 function resulted in reduced secondary wall thickening in vessels and a collapsed vessel phenotype. In addition, VND1 to VND5 were shown to be capable of rescuing the secondary wall defects in the fibers of the snd1 nst1 double mutant when expressed under the SND1 promoter. Furthermore, transactivation analysis revealed that VND1 to VND5 could activate expression of the GUS reporter gene driven by the secondary wall NAC binding element (SNBE). Together, these results demonstrate that VND1 to VND5 possess functions similar to that of the SND1 secondary wall NAC and are transcriptional regulators of secondary wall biosynthesis in vessels.
月季皮刺的组织结构与化学组成
1
2012
... 此外, 阐明次生壁生物合成的调控网络, 对于经济树种如桉树和洋槐(Robinia pseudoacacia), 经济作物如棉花、苎麻(Boehmeria nivea)和亚麻(Linum usitatissimum), 以及观赏植物如月季(Rosa chinensis)、玫瑰(R. rugosa)和野蔷薇(R. multiflora)的品质性状改良具有十分重要的意义.以棉花为例, 棉纤维是由胚珠外珠被表皮细胞在受精前后经分化突起、伸长和细胞壁增厚而形成.棉纤维次生壁的最大特点是组成简单, 主要由纤维素构成, 没有木质素沉积.而次生壁合成时期决定了棉纤维的强度.月季花色丰富, 花型多变, 但茎秆多皮刺, 使得其在栽种管理和切花釆摘、包装过程中存在诸多不便, 并带来安全隐患.而采用机械法去除皮刺又会对花枝造成伤害, 从而缩短瓶插寿命.有研究表明, 月季皮刺的主要成分为木质素、纤维素、半纤维素及木栓质(
Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant
1
1997
... NAC转录因子是植物一类特有的转录因子, 其家族成员众多.NAC一词源于最早发表的3个具有NAC结构域转录因子的首字母缩写, 分别是矮牵牛(Petunia hybrida)中的NAM (NO APICAL MERISTEM), 以及拟南芥中的ATAF1/2 (Arabidopsis thaliana ACTIVATION FACTOR 1/2)和CUC2 (CUP-SHAPED COTYLEDON 2) (
Growth, structure and lignin localization in rose prickle
1
2008
... 此外, 阐明次生壁生物合成的调控网络, 对于经济树种如桉树和洋槐(Robinia pseudoacacia), 经济作物如棉花、苎麻(Boehmeria nivea)和亚麻(Linum usitatissimum), 以及观赏植物如月季(Rosa chinensis)、玫瑰(R. rugosa)和野蔷薇(R. multiflora)的品质性状改良具有十分重要的意义.以棉花为例, 棉纤维是由胚珠外珠被表皮细胞在受精前后经分化突起、伸长和细胞壁增厚而形成.棉纤维次生壁的最大特点是组成简单, 主要由纤维素构成, 没有木质素沉积.而次生壁合成时期决定了棉纤维的强度.月季花色丰富, 花型多变, 但茎秆多皮刺, 使得其在栽种管理和切花釆摘、包装过程中存在诸多不便, 并带来安全隐患.而采用机械法去除皮刺又会对花枝造成伤害, 从而缩短瓶插寿命.有研究表明, 月季皮刺的主要成分为木质素、纤维素、半纤维素及木栓质(
The Arabidopsis ATHB- 8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems
2
2001
... 拟南芥中HD-ZIPIIITFs (Class III homeodomain leucine zipper transcription factors)参与维管束分化和次生壁合成(
... 可以促进木质部的分化(
SOMBRERO, BEARSKIN1, and BEARSKIN2 regulate root cap maturation in Arabidopsis
1
2012
... 此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余.对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(
The interacting MYB75 and KNAT7 transcription factors modulate secondary cell wall deposition both in stems and seed coat in Arabidopsis
1
2013
... KNAT7能与多种转录因子发生互作, 形成复合体参与次生壁的合成.有研究证明, KNAT7能与AtMYB75在体外发生相互作用(
MYB75 functions in regulation of secondary cell wall formation in the Arabidopsis inflorescence stem
2
2010
... KNAT7能与多种转录因子发生互作, 形成复合体参与次生壁的合成.有研究证明, KNAT7能与AtMYB75在体外发生相互作用(
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
Involvement of Pinus taeda MYB1 and MYB8 in phenylpropanoid metabolism and secondary cell wall biogenesis: a comparative in planta analysis
1
2008
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis
1
2000
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics
2
2005
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
... 功能缺失突变体木质部不规则, 且表现出严重的木质部塌陷(
Identification of novel transcription factors regulating secondary cell wall formation in Arabidopsis
3
2013
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... AtMYB42、AtMYB43、AtMYB52和AtMYB54均在木质部组织中优势表达(
... ).
Poplar PdC3H17 and PdC3H18 are direct targets of PdMYB3 and PdMYB21, and positively regulate secondary wall formation in Arabidopsis and poplar
1
2014
... 植物特异性串联CCCH锌指蛋白基因处于MYB转录因子的下游, 参与次生壁合成.拟南芥AtC3H14是AtSND1以及AtMYB46的直接靶基因(
ATAF NAC transcription factors: regulators of plant stress signaling
1
2010
... 自第1个NAC转录因子从矮牵牛中克隆后, 相继在模式植物(拟南芥、水稻和毛果杨等)、农作物(玉米(Zea mays)、小麦(Triticum aestivum)和大豆(Glycine max))以及园艺作物(葡萄(Vitis vinifera)、番茄(Lycopersicon esculentum)和草莓(Fragaria × ananassa)等)中发现多个NAC转录因子.研究表明, NAC转录因子在植物生长发育(
Comparative structure and biomechanics of plant primary and secondary cell walls
1
2012
... 细胞壁是位于植物细胞膜外的一层较厚、较坚韧并且略具弹性的结构, 为植物细胞所特有, 是区别于动物细胞的主要特征之一.植物不同组织的细胞具有不同类型的细胞壁, 根据其成分及其在生长过程中是否延伸可分为2种类型: 初生细胞壁(primary cell wall, PCW)和次生细胞壁(secondary cell wall, SCW) (以下简称次生壁).PCW是指细胞分裂后期细胞板形成后, 由原生质体分泌物质在中层的表面最初阶段所沉积的壁, 弹性较大, 普遍存在于所有植物细胞中.SCW比PCW更厚, 沉积在PCW与细胞膜之间, 主要成分包括纤维素、半纤维素和木质素.SCW只沉积于特殊类型的细胞, 如管状分子(tracheary elements, TEs)和纤维细胞的内部(
Characterization and genetic mapping of a mutation (ms35) which prevents anther dehiscence in Arabidopsis thaliana by affecting secondary wall thickening in the endothecium
1
1999
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells
1
2002
... VNS (VND、NST/SND和SMB (SOMBRERO))基因家族在次生壁形成中发挥关键调控作用, 为次生壁合成调控网络的转录因子开关.自首次从百日草(Zinnia elegans)中发现与植物次生壁形成相关的NAC转录因子以来(
Cellulose biosynthesis in plants: from genes to rosettes
1
2002
... 此外, 赤霉素对次生壁中的主要组成部分纤维素的合成发挥重要作用.纤维素的合成受到一系列CESAs基因调控(
Activation of miR165b represses AtHB15 expression and induces pith secondary wall development in Arabidopsis
1
2015
... 拟南芥中HD-ZIPIIITFs (Class III homeodomain leucine zipper transcription factors)参与维管束分化和次生壁合成(
The role of HD-ZIP III transcription factors and miR165/166 in vascular development and secondary cell wall formation
3
2015
... 拟南芥中HD-ZIPIIITFs (Class III homeodomain leucine zipper transcription factors)参与维管束分化和次生壁合成(
... 激活标记突变体(activation tagged mutants)的鉴定和分析明确了miR156/166在维管发育中的作用(
... ;
Multiple classes of transcription factors regulate the expression of VASCULAR- RELATED NAC-DOMAIN 7, a master switch of xylem vessel differentiation
2
2015
... 研究发现, VNS转录因子通过与其下游基因启动子上特殊位点结合的方式来调控下游基因的表达, 进而调控细胞次生壁合成(
... ;
Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors
1
2004
... NAC转录因子是植物一类特有的转录因子, 其家族成员众多.NAC一词源于最早发表的3个具有NAC结构域转录因子的首字母缩写, 分别是矮牵牛(Petunia hybrida)中的NAM (NO APICAL MERISTEM), 以及拟南芥中的ATAF1/2 (Arabidopsis thaliana ACTIVATION FACTOR 1/2)和CUC2 (CUP-SHAPED COTYLEDON 2) (
Signals that control plant vascular cell differentiation
1
2004
... 植物激素尤其是生长素, 对维管组织的分化具有重要作用(
Combining enhanced biomass density with reduced lignin level for improved forage quality
1
2016
... 由于次生细胞壁含有较多的纤维素、半纤维素及木质素, 因而是植物生物量的主要来源之一.例如, 水稻、玉米和小麦等农作物的秸秆就属于农业生态系统中十分宝贵的生物质能资源.然而, 农作物生产首先需要满足人类的食品需求.相较之下, 由于木本植物能产生大量的木质纤维素, 因此木材生物量作为一种可再生的、成本效益高的生物能源和工业资源, 预计将成为下一代生物燃料的原材料之一.但是, 来源于木质纤维素的生物乙醇要比来源于粮食作物的昂贵许多(
Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis
1
2009
...
Characterization of NAC domain transcription factors implicated in control of vascular cell differentiation in Arabidopsis and Populus
2
2010
... AtSND2和AtSND3为SND1的下游转录因子(
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
Transcriptome of Arabidopsis leaf senescence
1
2004
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
Transcriptional regulation of grass secondary cell wall biosynthesis: playing catch-up with Arabidopsis thaliana
1
2012
... 研究表明, 次生壁加厚现象除了在维管束中导管和纤维细胞中存在, 在树叶、种皮、花药以及果实中石细胞的皮层细胞里也有发生(
Plant NAC-type transcription factor proteins contain a NARD domain for repression of transcriptional activation
1
2010
... NAC转录因子是植物一类特有的转录因子, 其家族成员众多.NAC一词源于最早发表的3个具有NAC结构域转录因子的首字母缩写, 分别是矮牵牛(Petunia hybrida)中的NAM (NO APICAL MERISTEM), 以及拟南芥中的ATAF1/2 (Arabidopsis thaliana ACTIVATION FACTOR 1/2)和CUC2 (CUP-SHAPED COTYLEDON 2) (
KNAT7 positively regulates xylan biosynthesis by directly activating IRX9 expression in Arabidopsis
2
2018
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
... ).综上, KNAT7既能作为转录抑制子也能作为转录激活因子调控次生壁的合成, 这取决于不同组织和细胞中的转录因子组分(
A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice
2
2015
... 此外, 赤霉素对次生壁中的主要组成部分纤维素的合成发挥重要作用.纤维素的合成受到一系列CESAs基因调控(
... 基因的转录.而在GA和NAC-MYB转录因子之间, SLR1起到了桥梁作用.SLR1能够结合NAC29/31的DNA结合域, 从而阻碍三者(NAC-MYB-CESA)的级联调控路径, 通过抑制纤维素的合成抑制次生壁的形成(
SND2, a NAC transcription factor gene, regulates genes involved in secondary cell wall development in Arabidopsis fibres and increases fibre cell area in Eucalyptus
2
2011
... AtSND2和AtSND3为SND1的下游转录因子(
... 的功能在草本和木本植物中比较保守(
Developmental role and auxin responsiveness of class III homeodomain leucine zipper gene family members in rice
1
2008
... 拟南芥中HD-ZIPIIITFs (Class III homeodomain leucine zipper transcription factors)参与维管束分化和次生壁合成(
Transcriptional repression by AtMYB4 controls production of UV- protecting sunscreens in Arabidopsis
2
2000
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... 的表达(
MicroRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems
1
2005
... 激活标记突变体(activation tagged mutants)的鉴定和分析明确了miR156/166在维管发育中的作用(
Identification of direct targets of transcription factor MYB46 provides insights into the transcriptional regulation of secondary wall biosynthesis
1
2014
...
AtC3H14, a plant-specific tandem CCCH zinc-finger protein, binds to its target mRNAs in a sequence-specific manner and affects cell elongation in Arabidopsis thaliana
2
2014
... 植物特异性串联CCCH锌指蛋白基因处于MYB转录因子的下游, 参与次生壁合成.拟南芥AtC3H14是AtSND1以及AtMYB46的直接靶基因(
... ).AtC3H14既能直接结合到纤维素与木质素合成相关基因的启动子上, 也能结合到聚半乳糖醛酸酶ADPG1的RNA上.因此, AtC3H14可能参与次生壁生物合成基因的转录和转录后调控(
Transcription factor MYB46 is an obligate component of the transcriptional regulatory complex for functional expression of secondary wall-associated cellulose synthases in Arabidopsis thaliana
1
2013
...
Identification of a cis- acting regulatory motif recognized by MYB46, a master transcriptional regulator of secondary wall biosynthesis
2
2012
... AtMYB46和AtMYB83不仅能调控转录因子基因, 也能调控一系列与次生壁合成相关的基因.且二者的靶基因启动子上都具有7个核苷酸(ACC[A/T]A[A/C][T/ C])的特异结合元件, 即SMRE (secondary wall MYB- responsive element)基序.作为调控植物次生壁生物合成的二级开关, AtMYB46和AtMYB83均通过与下游基因的SMRE基序相结合激活下游基因的表达, 实现对次生壁生物合成的转录调控(
...
MYB46 directly regulates the gene expression of secondary wall-associated cellulose synthases in Arabidopsis
1
2013
...
Transcription factors that directly regulate the expression of CSLA9 encoding mannan synthase in Arabidopsis thaliana
1
2014
...
The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis
2
2014
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
Ectopic expression of MYB46 identifies transcriptional regulatory genes involved in secondary wall biosynthesis in Arabidopsis
6
2009
... 拟南芥AtMYB46和AtMYB83是2个功能冗余的R2R3-MYB转录因子.AtMYB46和AtMYB83位于次生壁生物合成调控网络中的第2级, 是SND1的直接靶基因, 也是调控拟南芥次生壁形成的节点基因(
... AtMYB46与AtMYB83作为二级调控开关, 具有承上启下的关键作用, 其上游既受到NAC转录因子一级开关的调控, 也能调控一系列位于其下游与次生壁合成相关基因的表达.由于AtMYB46和AtMYB83功能冗余, 二者调控的下游转录因子基因也有所重叠(
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... ;
... 植物特异性串联CCCH锌指蛋白基因处于MYB转录因子的下游, 参与次生壁合成.拟南芥AtC3H14是AtSND1以及AtMYB46的直接靶基因(
... ), 能够激活与纤维素、半纤维素和木质素合成相关基因的表达(
MYB46-mediated transcriptional regulation of secondary wall biosynthesis
1
2012
... 拟南芥AtMYB46和AtMYB83是2个功能冗余的R2R3-MYB转录因子.AtMYB46和AtMYB83位于次生壁生物合成调控网络中的第2级, 是SND1的直接靶基因, 也是调控拟南芥次生壁形成的节点基因(
ANAC012, a member of the plant-specific NAC transcription factor family, negatively regulates xylary fiber development in Arabidopsis thaliana
3
2007
... NST3 (又称SND1或ANAC012) (Arabidopsis NAC DOMAIN CONTAINING PROTEIN 012)是1个可以双向调控次生壁形成的NAC转录因子.SND1在茎维管束间纤维细胞和木质纤维细胞中特异表达, 通过显性抑制SND1导致纤维细胞次生壁增厚显著下降(
... ;
... 基因的超量表达会引起木质部导管细胞次生壁的大量沉积, 推测这是纤维细胞次生壁受到抑制后的一种弥补机制(
Transcription switches for protoxylem and metaxylem vessel formation
2
2005
... VNS (VND、NST/SND和SMB (SOMBRERO))基因家族在次生壁形成中发挥关键调控作用, 为次生壁合成调控网络的转录因子开关.自首次从百日草(Zinnia elegans)中发现与植物次生壁形成相关的NAC转录因子以来(
... 则导致原生木质部加厚(
The Class II KNOX gene KNAT7 negatively regulates secondary wall formation in Arabidopsis and is functionally conserved in Populus
8
2012
... 转录后调控对调节VNS转录因子的活性也发挥重要作用.毛果杨VNS基因PtrWND1B/PtVNS11/ PtrSND1-A2含有选择性剪接变异体, 且在不同组织中的表达丰度不同(
... ).这类小变异体的蛋白产物缺乏C端结构域, 但能够结合到PtVNS的全长蛋白上.研究发现, 截短的PtrWND1B/PtVNS11/PtrSND1-A2通过PtVNS蛋白抑制其自身的转录激活活性(
... 研究发现, NAC转录因子可与自身或其它蛋白形成同源或异源二聚体(
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
... ).超量表达KNAT7引起维管束间纤维细胞壁厚度下降(
... ;
... ).然而, 其纤维细胞的次生壁厚度却有所增加, 并且伴有木质素含量的增加以及纤维素、木质素和木聚糖生物合成基因表达量的上升(
... KNAT7能与多种转录因子发生互作, 形成复合体参与次生壁的合成.有研究证明, KNAT7能与AtMYB75在体外发生相互作用(
OVATE FAMILY PROTEIN 4 (OFP4) interaction with KNAT7 regulates secondary cell wall formation in Arabidopsis thaliana
2
2011
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
... ), 且其抑制活性可通过与OFP4 (OVATE FAMILY PROTEIN 4)和OFP1蛋白的互作而增强(
A cotton (Gossypium hirsutum) gene encoding a NAC transcription factor is involved in negative regulation of plant xylem development
1
2014
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases
1
2005
...
MYB transcription factors as regulators of phenylpropanoid metabolism in plants
1
2015
... MYB转录因子广泛存在于高等植物中, 其主要的结构特征为N端具有高度保守的DNA结合结构域(MYB结构域).根据MYB蛋白含有的MYB结构域数量可将其分为4类: 1R-MYB/MYB-related、R2R3-MYB、3R-MYB和4R-MYB (4个R1/R2的重复).其中, R2R3- MYB转录因子的数目最多, 其功能和调控机理的研究也更为深入.已有研究表明, MYB转录因子在植物次生壁生物合成途径中扮演着重要角色(
A role for OVATE FAMILY PROTEIN 1 (OFP1) and OFP4 in a BLH6-KNAT7 multi- protein complex regulating secondary cell wall formation in Arabidopsis thaliana
2
2015
... KNAT7能与多种转录因子发生互作, 形成复合体参与次生壁的合成.有研究证明, KNAT7能与AtMYB75在体外发生相互作用(
... ;
BEL1-LIKE HOMEODOMAIN6 and KNOTTED ARABIDOPSIS THALIANA7 interact and regulate secondary cell wall formation via repression of REVOLUTA
2
2015
... KNAT7能与多种转录因子发生互作, 形成复合体参与次生壁的合成.有研究证明, KNAT7能与AtMYB75在体外发生相互作用(
... ).BLH6是转录抑制子, BLH6-KNAT7复合体能够增强KNAT7和BLH6的抑制活性.超量表达KNAT7和BLH6引起维管束纤维次生壁厚度下降(
MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5' region
1
2004
... 激活标记突变体(activation tagged mutants)的鉴定和分析明确了miR156/166在维管发育中的作用(
The poplar MYB transcription factors, PtrMYB3 and PtrMYB20, are involved in the regulation of secondary wall biosynthesis
1
2010
... 一级开关基因VNS在维管束植物中十分保守(
MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis
6
2009
... 拟南芥AtMYB46和AtMYB83是2个功能冗余的R2R3-MYB转录因子.AtMYB46和AtMYB83位于次生壁生物合成调控网络中的第2级, 是SND1的直接靶基因, 也是调控拟南芥次生壁形成的节点基因(
... ;
... AtMYB46和AtMYB83在花序茎的纤维细胞和导管细胞中特异表达.将其在拟南芥中超量表达, 转基因植株中纤维素、木质素和木聚糖的生物合成途径被激活, 导致非厚壁细胞中次生壁的异位沉积; 而AtMYB46或AtMYB83的显性抑制植株表现为纤维细胞和导管细胞的次生壁增厚显著减弱(
... 双突变体中, 拟南芥导管细胞中的次生壁沉积受到严重影响, 导致突变株幼苗生长停滞(
... AtMYB46与AtMYB83作为二级调控开关, 具有承上启下的关键作用, 其上游既受到NAC转录因子一级开关的调控, 也能调控一系列位于其下游与次生壁合成相关基因的表达.由于AtMYB46和AtMYB83功能冗余, 二者调控的下游转录因子基因也有所重叠(
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
Secondary wall NAC binding element (SNBE), a key cis-acting element required for target gene activation by secondary wall NAC master switches
1
2011
... 研究发现, VNS转录因子通过与其下游基因启动子上特殊位点结合的方式来调控下游基因的表达, 进而调控细胞次生壁合成(
NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis
4
2007
... NST1 (NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1)、NST2和SND1 (NST3/SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN 1)为次生壁合成的关键转录因子(
... NST3 (又称SND1或ANAC012) (Arabidopsis NAC DOMAIN CONTAINING PROTEIN 012)是1个可以双向调控次生壁形成的NAC转录因子.SND1在茎维管束间纤维细胞和木质纤维细胞中特异表达, 通过显性抑制SND1导致纤维细胞次生壁增厚显著下降(
... ;
... 近20年来, 得益于遗传学和分子生物学的迅猛发展, 植物次生壁生物合成的转录调控研究取得了空前巨大的进展.尤其在模式植物拟南芥中, 结合突变体筛选和全基因组信息, 已获得多个植物细胞壁合成相关基因, 明确了NAC和MYB类转录因子在维管束组织的木质部导管、纤维细胞和花药皮层次生细胞壁加厚等过程中的核心作用, 以及其它转录因子在此过程中的调控作用, 并解析了这些调控因子之间的层级关系(
NAC transcription factors NST1 and NST3 regulate pod shattering in a partially redundant manner by promoting secondary wall formation after the establishment of tissue identity
2
2008
... NST3 (又称SND1或ANAC012) (Arabidopsis NAC DOMAIN CONTAINING PROTEIN 012)是1个可以双向调控次生壁形成的NAC转录因子.SND1在茎维管束间纤维细胞和木质纤维细胞中特异表达, 通过显性抑制SND1导致纤维细胞次生壁增厚显著下降(
... 研究表明, 次生壁加厚现象除了在维管束中导管和纤维细胞中存在, 在树叶、种皮、花药以及果实中石细胞的皮层细胞里也有发生(
The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence
4
2005
... NST1 (NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1)、NST2和SND1 (NST3/SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN 1)为次生壁合成的关键转录因子(
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
... 的表达(
... 近20年来, 得益于遗传学和分子生物学的迅猛发展, 植物次生壁生物合成的转录调控研究取得了空前巨大的进展.尤其在模式植物拟南芥中, 结合突变体筛选和全基因组信息, 已获得多个植物细胞壁合成相关基因, 明确了NAC和MYB类转录因子在维管束组织的木质部导管、纤维细胞和花药皮层次生细胞壁加厚等过程中的核心作用, 以及其它转录因子在此过程中的调控作用, 并解析了这些调控因子之间的层级关系(
Features of promising technologies for pretreatment of lignocellulosic biomass
1
2005
... 由于次生细胞壁含有较多的纤维素、半纤维素及木质素, 因而是植物生物量的主要来源之一.例如, 水稻、玉米和小麦等农作物的秸秆就属于农业生态系统中十分宝贵的生物质能资源.然而, 农作物生产首先需要满足人类的食品需求.相较之下, 由于木本植物能产生大量的木质纤维素, 因此木材生物量作为一种可再生的、成本效益高的生物能源和工业资源, 预计将成为下一代生物燃料的原材料之一.但是, 来源于木质纤维素的生物乙醇要比来源于粮食作物的昂贵许多(
MYB transcription factors orchestrating the developmental program of xylem vessels in Arabidopsis roots
4
2010
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... AtMYB42、AtMYB43、AtMYB52和AtMYB54均在木质部组织中优势表达(
... AtMYB103主要在维管束间纤维细胞和木质部组织中表达, 超量表达AtMYB103可显著增加转基因株系中木质部纤维细胞和维管束间纤维细胞次生壁的厚度.而且AtMYB103可以在体外激活纤维素合酶CESA8基因的启动子, 因此其最初被认为是特异性调控纤维素生物合成的调控因子(
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants
5
2015
... 此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余.对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(
... 研究发现, NAC转录因子可与自身或其它蛋白形成同源或异源二聚体(
... 植物激素尤其是生长素, 对维管组织的分化具有重要作用(
... 一级开关基因VNS在维管束植物中十分保守(
... 近20年来, 得益于遗传学和分子生物学的迅猛发展, 植物次生壁生物合成的转录调控研究取得了空前巨大的进展.尤其在模式植物拟南芥中, 结合突变体筛选和全基因组信息, 已获得多个植物细胞壁合成相关基因, 明确了NAC和MYB类转录因子在维管束组织的木质部导管、纤维细胞和花药皮层次生细胞壁加厚等过程中的核心作用, 以及其它转录因子在此过程中的调控作用, 并解析了这些调控因子之间的层级关系(
NAC transcription factors in plant abiotic stress responses
1
2012
... 自第1个NAC转录因子从矮牵牛中克隆后, 相继在模式植物(拟南芥、水稻和毛果杨等)、农作物(玉米(Zea mays)、小麦(Triticum aestivum)和大豆(Glycine max))以及园艺作物(葡萄(Vitis vinifera)、番茄(Lycopersicon esculentum)和草莓(Fragaria × ananassa)等)中发现多个NAC转录因子.研究表明, NAC转录因子在植物生长发育(
HD-ZIP III homeobox genes that include a novel member, ZeHB-13 (Zinnia)/ATHB-15 (Arabidopsis), are involved in procambium and xylem cell differentiation
2
2003
... 拟南芥中HD-ZIPIIITFs (Class III homeodomain leucine zipper transcription factors)参与维管束分化和次生壁合成(
... ).此外, 从HD-ZIPIIITFs同源基因在百日草和水稻中所起的作用可以看出, 在不同物种中其对维管束组织分化和形成的功能保守(
Class III homeodomain leucine-zipper proteins regulate xylem cell differentiation
2
2005
... 拟南芥中HD-ZIPIIITFs (Class III homeodomain leucine zipper transcription factors)参与维管束分化和次生壁合成(
... ;
Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation
5
2010
... 研究发现, VNS转录因子通过与其下游基因启动子上特殊位点结合的方式来调控下游基因的表达, 进而调控细胞次生壁合成(
... -element)序列, 调控木质部管状分子的分化(
... ;
... 植物激素尤其是生长素, 对维管组织的分化具有重要作用(
... 拟南芥AtMYB46和AtMYB83是2个功能冗余的R2R3-MYB转录因子.AtMYB46和AtMYB83位于次生壁生物合成调控网络中的第2级, 是SND1的直接靶基因, 也是调控拟南芥次生壁形成的节点基因(
MYB103 is required for FERULATE- 5-HYDROXYLASE expression and syringyl lignin biosynthesis in Arabidopsis stems
1
2013
... AtMYB103主要在维管束间纤维细胞和木质部组织中表达, 超量表达AtMYB103可显著增加转基因株系中木质部纤维细胞和维管束间纤维细胞次生壁的厚度.而且AtMYB103可以在体外激活纤维素合酶CESA8基因的启动子, 因此其最初被认为是特异性调控纤维素生物合成的调控因子(
A NAC domain protein family contributing to the regulation of wood formation in poplar
1
2011
... 此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余.对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(
NAC transcription factors: structurally distinct, functionally diverse
2
2005
... 自第1个NAC转录因子从矮牵牛中克隆后, 相继在模式植物(拟南芥、水稻和毛果杨等)、农作物(玉米(Zea mays)、小麦(Triticum aestivum)和大豆(Glycine max))以及园艺作物(葡萄(Vitis vinifera)、番茄(Lycopersicon esculentum)和草莓(Fragaria × ananassa)等)中发现多个NAC转录因子.研究表明, NAC转录因子在植物生长发育(
... 研究发现, NAC转录因子可与自身或其它蛋白形成同源或异源二聚体(
Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana
2003
AtMYB32 is required for normal pollen development in Arabidopsis thaliana
2
2004
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... ), 而AtMYB7和AtMYB32能够负调控木质素合成相关基因的表达(
NAC proteins: regulation and role in stress tolerance
1
2012
... 自第1个NAC转录因子从矮牵牛中克隆后, 相继在模式植物(拟南芥、水稻和毛果杨等)、农作物(玉米(Zea mays)、小麦(Triticum aestivum)和大豆(Glycine max))以及园艺作物(葡萄(Vitis vinifera)、番茄(Lycopersicon esculentum)和草莓(Fragaria × ananassa)等)中发现多个NAC转录因子.研究表明, NAC转录因子在植物生长发育(
TERE; a novel cis- element responsible for a coordinated expression of genes related to programmed cell death and secondary wall formation during differentiation of tracheary elements
1
2007
... 研究发现, VNS转录因子通过与其下游基因启动子上特殊位点结合的方式来调控下游基因的表达, 进而调控细胞次生壁合成(
Current models for transcriptional regulation of secondary cell wall biosynthesis in grasses
1
2018
... 研究表明, 次生壁加厚现象除了在维管束中导管和纤维细胞中存在, 在树叶、种皮、花药以及果实中石细胞的皮层细胞里也有发生(
AtMYB61, an R2R3-MYB transcription factor, functions as a pleiotropic regulator via a small gene network
1
2012
... KNAT7能与多种转录因子发生互作, 形成复合体参与次生壁的合成.有研究证明, KNAT7能与AtMYB75在体外发生相互作用(
Reconstitution of a secondary cell wall in a secondary cell wall-deficient Arabidopsis mutant
1
2014
... 由于次生细胞壁含有较多的纤维素、半纤维素及木质素, 因而是植物生物量的主要来源之一.例如, 水稻、玉米和小麦等农作物的秸秆就属于农业生态系统中十分宝贵的生物质能资源.然而, 农作物生产首先需要满足人类的食品需求.相较之下, 由于木本植物能产生大量的木质纤维素, 因此木材生物量作为一种可再生的、成本效益高的生物能源和工业资源, 预计将成为下一代生物燃料的原材料之一.但是, 来源于木质纤维素的生物乙醇要比来源于粮食作物的昂贵许多(
Wood reinforcement of poplar by rice NAC transcription factor
1
2016
... 由于次生细胞壁含有较多的纤维素、半纤维素及木质素, 因而是植物生物量的主要来源之一.例如, 水稻、玉米和小麦等农作物的秸秆就属于农业生态系统中十分宝贵的生物质能资源.然而, 农作物生产首先需要满足人类的食品需求.相较之下, 由于木本植物能产生大量的木质纤维素, 因此木材生物量作为一种可再生的、成本效益高的生物能源和工业资源, 预计将成为下一代生物燃料的原材料之一.但是, 来源于木质纤维素的生物乙醇要比来源于粮食作物的昂贵许多(
NAC transcription factors in plant multiple abiotic stress responses: progress and prospects
1
2015
... 自第1个NAC转录因子从矮牵牛中克隆后, 相继在模式植物(拟南芥、水稻和毛果杨等)、农作物(玉米(Zea mays)、小麦(Triticum aestivum)和大豆(Glycine max))以及园艺作物(葡萄(Vitis vinifera)、番茄(Lycopersicon esculentum)和草莓(Fragaria × ananassa)等)中发现多个NAC转录因子.研究表明, NAC转录因子在植物生长发育(
The no apical meristem gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries
1
1996
... NAC转录因子是植物一类特有的转录因子, 其家族成员众多.NAC一词源于最早发表的3个具有NAC结构域转录因子的首字母缩写, 分别是矮牵牛(Petunia hybrida)中的NAM (NO APICAL MERISTEM), 以及拟南芥中的ATAF1/2 (Arabidopsis thaliana ACTIVATION FACTOR 1/2)和CUC2 (CUP-SHAPED COTYLEDON 2) (
ASYMMETRIC LEAVES2-LIKE19/LATERAL ORGAN BOUNDARIES DOMAIN30 and ASL20/LBD18 regulate tracheary element differentiation in Arabidopsis
4
2008
... 植物激素尤其是生长素, 对维管组织的分化具有重要作用(
... 是AtVND6和AtVND7的直接靶基因(
... 也能够引起AtVND7的异位表达(
... 也能够被生长素上调(
Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers
1
2003
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
Populus NST/SND orthologs are key regulators of secondary cell wall formation in wood fibers, phloem fibers and xylem ray parenchyma cells
1
2019
... 此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余.对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(
The Arabidopsis NST3/SND1 promoter is active in secondary woody tissue in poplar
1
2017
... 由于次生细胞壁含有较多的纤维素、半纤维素及木质素, 因而是植物生物量的主要来源之一.例如, 水稻、玉米和小麦等农作物的秸秆就属于农业生态系统中十分宝贵的生物质能资源.然而, 农作物生产首先需要满足人类的食品需求.相较之下, 由于木本植物能产生大量的木质纤维素, 因此木材生物量作为一种可再生的、成本效益高的生物能源和工业资源, 预计将成为下一代生物燃料的原材料之一.但是, 来源于木质纤维素的生物乙醇要比来源于粮食作物的昂贵许多(
Transcription factors VND1-VND3 contribute to cotyledon xylem vessel formation
2
2018
... VNS (VND、NST/SND和SMB (SOMBRERO))基因家族在次生壁形成中发挥关键调控作用, 为次生壁合成调控网络的转录因子开关.自首次从百日草(Zinnia elegans)中发现与植物次生壁形成相关的NAC转录因子以来(
... NST3 (又称SND1或ANAC012) (Arabidopsis NAC DOMAIN CONTAINING PROTEIN 012)是1个可以双向调控次生壁形成的NAC转录因子.SND1在茎维管束间纤维细胞和木质纤维细胞中特异表达, 通过显性抑制SND1导致纤维细胞次生壁增厚显著下降(
Natural variation at XND1 impacts root hydraulics and trade-off for stress responses in Arabidopsis
1
2018
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
Poplar PdMYB221 is involved in the direct and indirect regulation of secondary wall biosynthesis during wood formation
2
2015
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... 还能够抑制与纤维素和木聚糖合成相关基因的表达(
Interactions among three distinct CesA proteins essential for cellulose synthesis
1
2003
... 此外, 赤霉素对次生壁中的主要组成部分纤维素的合成发挥重要作用.纤维素的合成受到一系列CESAs基因调控(
Multiple cellulose synthase catalytic subunits are required for cellulose synthesis in Arabidopsis
1
2000
... 此外, 赤霉素对次生壁中的主要组成部分纤维素的合成发挥重要作用.纤维素的合成受到一系列CESAs基因调控(
The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis
1
1999
... 此外, 赤霉素对次生壁中的主要组成部分纤维素的合成发挥重要作用.纤维素的合成受到一系列CESAs基因调控(
An Arabidopsis gene regulatory network for secondary cell wall synthesis
2
2015
... 研究表明, WRKY和bHLH类转录因子也参与对木质素生物合成的调控.在拟南芥中, WRKY12以负向调控的方式参与茎髓组织薄壁细胞的次生壁加厚(
... 外其它在木质部中特异表达的转录因子基因的启动子上(
Potential utilization of NAC transcription factors to enhance abiotic stress tolerance in plants by biotechnological approach
1
2010
... 自第1个NAC转录因子从矮牵牛中克隆后, 相继在模式植物(拟南芥、水稻和毛果杨等)、农作物(玉米(Zea mays)、小麦(Triticum aestivum)和大豆(Glycine max))以及园艺作物(葡萄(Vitis vinifera)、番茄(Lycopersicon esculentum)和草莓(Fragaria × ananassa)等)中发现多个NAC转录因子.研究表明, NAC转录因子在植物生长发育(
Mutation of WRKY transcription factors initiates pith secondary wall formation and increases stem biomass in dicotyledonous plants
4
2010
... 研究表明, WRKY和bHLH类转录因子也参与对木质素生物合成的调控.在拟南芥中, WRKY12以负向调控的方式参与茎髓组织薄壁细胞的次生壁加厚(
... 发挥抑制茎髓组织薄壁细胞次生壁加厚的作用(
... 编码1个WRKY转录因子(
... 的启动子结合从而抑制其表达.由此表明, WRKY12能够负向调控次生壁的合成(
On-off switches for secondary cell wall biosynthesis
1
2012
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
R2R3-MYB transcription factor MYB6 promotes anthocyanin and proanthocyanidin biosynthesis but inhibits secondary cell wall formation in Populus tomentosa
1
2019
... KNAT7能与多种转录因子发生互作, 形成复合体参与次生壁的合成.有研究证明, KNAT7能与AtMYB75在体外发生相互作用(
DNA binding by the plant-specific NAC transcription factors in crystal and solution: a firm link to WRKY and GCM transcription factors
1
2012
... 研究发现, NAC转录因子可与自身或其它蛋白形成同源或异源二聚体(
The NAC domain transcription factors FEZ and SOMBRERO control the orientation of cell division plane in Arabidopsis root stem cells
1
2008
... 此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余.对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(
Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes
1
2005
... 激活标记突变体(activation tagged mutants)的鉴定和分析明确了miR156/166在维管发育中的作用(
Towards the mechanism of cellulose synthesis
1
2002
... 此外, 赤霉素对次生壁中的主要组成部分纤维素的合成发挥重要作用.纤维素的合成受到一系列CESAs基因调控(
Contribution of NAC transcription factors to plant adaptation to land
1
2014
... 研究表明, 次生壁加厚现象除了在维管束中导管和纤维细胞中存在, 在树叶、种皮、花药以及果实中石细胞的皮层细胞里也有发生(
Transcriptional regulation of secondary wall formation controlled by NAC domain proteins
1
2010
... AtMYB103主要在维管束间纤维细胞和木质部组织中表达, 超量表达AtMYB103可显著增加转基因株系中木质部纤维细胞和维管束间纤维细胞次生壁的厚度.而且AtMYB103可以在体外激活纤维素合酶CESA8基因的启动子, 因此其最初被认为是特异性调控纤维素生物合成的调控因子(
VASCULAR-RELATED NAC-DOMAIN 7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots
1
2008
... 研究发现, NAC转录因子可与自身或其它蛋白形成同源或异源二聚体(
VASCULAR-RELATED NAC- DOMAIN 7 directly regulates the expression of a broad range of genes for xylem vessel formation
4
2011
... 植物激素尤其是生长素, 对维管组织的分化具有重要作用(
... ;
... 拟南芥AtMYB46和AtMYB83是2个功能冗余的R2R3-MYB转录因子.AtMYB46和AtMYB83位于次生壁生物合成调控网络中的第2级, 是SND1的直接靶基因, 也是调控拟南芥次生壁形成的节点基因(
... AtMYB103主要在维管束间纤维细胞和木质部组织中表达, 超量表达AtMYB103可显著增加转基因株系中木质部纤维细胞和维管束间纤维细胞次生壁的厚度.而且AtMYB103可以在体外激活纤维素合酶CESA8基因的启动子, 因此其最初被认为是特异性调控纤维素生物合成的调控因子(
Arabidopsis NAC domain proteins VND-INTERACTING1 and ANAC103 interact with multiple NAC domain proteins
2
2015
... 研究发现, NAC转录因子可与自身或其它蛋白形成同源或异源二聚体(
... 具有转录激活活性, 通过调节多种NAC转录因子的转录活性促进各种类型细胞的分化(
VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis
2
2010
... VNI2在根和花茎的木质部以及韧皮部细胞中都有表达.VNI2能与VND家族蛋白VND7、VND1-5以及其它NAC蛋白发生相互作用, 但VNI2与VND7结合的亲和性高于其它NAC蛋白.组成型超量表达VNI2的植株, 其幼苗期根中木质部导管的正常发育受到抑制.此外, 在VND7启动子的控制下, C-端截短的VNI2 能抑制根部和地上部分木质部导管的正常发育.VNI2与VND7结合后, 抑制了VND7的转录激活活性, 从而抑制VND7下游靶基因的表达, 最终抑制木质部的合成(
... ).上述结果表明, VNI2 作为1个转录抑制子调控木质部细胞特异化, 是VND的负向调控因子(
The heterologous expression in Arabidopsis thaliana of sorghum transcription factor SbbHLH1 downregulates lignin synthesis
2
2013
... bHLH转录因子能分别与NAC和MYB转录因子发生相互作用, 调控次生壁合成.
... )突变体与野生型构建了1个差减文库, 从中分离到1个bHLH类转录因子SbbHLH1.进一步将其在拟南芥中进行超量表达, SbbHLH1与MYB转录因子竞争性地结合到与木质素合成相关基因的启动子上, 进而抑制木质素的合成.或者, SbbHLH1与MYB转录因子形成复合体, 抑制其活性从而抑制木质素合成相关基因的表达, 进而抑制木质素的合成.当木质素含量过低时, SbbHLH1又作为信号激活MYB转录因子的表达, 说明其对木质素的合成起负调控作用(
Arabidopsis MYB26/MALESTERILE35 regulates secondary thickening in the endothecium and is essential for anther dehiscence
2
2007
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
... 表达上调(
Molecular mechanisms for vascular development and secondary cell wall formation
1
2016
... 近20年来, 得益于遗传学和分子生物学的迅猛发展, 植物次生壁生物合成的转录调控研究取得了空前巨大的进展.尤其在模式植物拟南芥中, 结合突变体筛选和全基因组信息, 已获得多个植物细胞壁合成相关基因, 明确了NAC和MYB类转录因子在维管束组织的木质部导管、纤维细胞和花药皮层次生细胞壁加厚等过程中的核心作用, 以及其它转录因子在此过程中的调控作用, 并解析了这些调控因子之间的层级关系(
The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes
1
2011
... 自第1个NAC转录因子从矮牵牛中克隆后, 相继在模式植物(拟南芥、水稻和毛果杨等)、农作物(玉米(Zea mays)、小麦(Triticum aestivum)和大豆(Glycine max))以及园艺作物(葡萄(Vitis vinifera)、番茄(Lycopersicon esculentum)和草莓(Fragaria × ananassa)等)中发现多个NAC转录因子.研究表明, NAC转录因子在植物生长发育(
OsSND2, a NAC family transcription factor, is involved in secondary cell wall biosynthesis through regulating MYBs expression in rice
1
2018
... AtSND2和AtSND3为SND1的下游转录因子(
An uncanonical CCCH-tandem zinc-finger protein represses secondary wall synthesis and controls mechanical strength in rice
1
2018
... 植物特异性串联CCCH锌指蛋白基因处于MYB转录因子的下游, 参与次生壁合成.拟南芥AtC3H14是AtSND1以及AtMYB46的直接靶基因(
The cotton (Gossypium hirsutum) NAC transcription factor (FSN1) as a positive regulator participates in controlling secondary cell wall biosynthesis and modification of fibers
4
2018
... 其它物种中也有类似结合元件.棉花中1个NAC转录因子GhFSN1 (fiber secondary cell wall-related NAC1)通过激活其下游与棉花纤维次生壁合成相关基因的表达, 正向调控棉花纤维次生壁发育(
... 能直接结合到其下游基因启动子内的SNBE基序上.进一步通过定点突变的方法, 鉴定出SNBE基序包含1个13 bp的核心序列, 即(C/T) (C/G/T)TN(A/T)(G/T)(A/C/G)(A/G)(A/T/G)(A/T/G) AAG (
... 棉花NAC转录因子GhFSN1不仅能够与其自身形成同源二聚体, 也可与同家族的其它成员(GhFSN2)以及泛素结合酶E2形成异源二聚体, 由此推测GhFSN1可能存在蛋白酶体介导的泛素化调控途径(
... 的表达量进行检测, 结果表明, 在棉花纤维发育过程中, GhFSN1转录因子的活性存在由蛋白酶体介导的潜在调控机制, 其降解可能通过蛋白酶体介导的泛素化途径实现(
Recent advances in the transcriptional regulation of secondary cell wall biosynthesis in the woody plants
1
2018
... 研究表明, 次生壁加厚现象除了在维管束中导管和纤维细胞中存在, 在树叶、种皮、花药以及果实中石细胞的皮层细胞里也有发生(
Blue light regulates secondary cell wall thickening via MYC2/MYC4 activation of the NST1-directed transcriptional network in Arabidopsis
2
2018
... bHLH转录因子能分别与NAC和MYB转录因子发生相互作用, 调控次生壁合成.
... 基因的启动子上, 从而激活一系列与次生壁合成相关转录因子的表达, 促进次生壁细胞的增厚(
XND1, a member of the NAC domain family in Arabidopsis thaliana, negatively regulates lignocellulose synthesis and programmed cell death in xylem
1
2008
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
The xylem and phloem transcriptomes from secondary tissues of the Arabidopsis root-hypocotyl
1
2005
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
XYLEM NAC DOMAIN 1, an angiosperm NAC transcription factor, inhibits xylem differentiation through conserved motifs that interact with RETINOBLASTOMA-RELATED
3
2017
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
... XND1受VND7直接调控(
... - LIKE和LXCXE-mimic.其中, LXCXE或LXCXE-mimic基序的完整性对于XND1和RBR的互作十分关键, 直接决定了XND1调控木质部管状分子分化作用的强弱.当LXCXE或LXCXE-mimic基序出现碱基突变, 或者LXCXE基序缺失时, XND1对木质部管状分子的抑制作用则会下降甚至消失, 导致相应的超量表达表型也减弱或消失.XND1的C端所含基序能与RBR发生相互作用, 从而特异地抑制木质部细胞的分化(
Comparative genomic analysis of the R2R3 MYB secondary cell wall regulators of Arabidopsis, poplar, rice, maize, and switchgrass
3
2014
... 此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余.对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(
... 转录后调控对调节VNS转录因子的活性也发挥重要作用.毛果杨VNS基因PtrWND1B/PtVNS11/ PtrSND1-A2含有选择性剪接变异体, 且在不同组织中的表达丰度不同(
... ), 从而抑制毛果杨中纤维细胞的次生壁加厚(
KNAT2/6b, a class I KNOX gene, impedes xylem differentiation by regulating NAC domain transcription factors in poplar
1
2020
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis
7
2006
... NST1 (NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1)、NST2和SND1 (NST3/SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN 1)为次生壁合成的关键转录因子(
... NST3 (又称SND1或ANAC012) (Arabidopsis NAC DOMAIN CONTAINING PROTEIN 012)是1个可以双向调控次生壁形成的NAC转录因子.SND1在茎维管束间纤维细胞和木质纤维细胞中特异表达, 通过显性抑制SND1导致纤维细胞次生壁增厚显著下降(
... 基因过量表达能够激活与次生壁合成相关基因的表达, 从而引起木质部导管细胞次生壁的大量沉积(
... 超量表达植株中纤维细胞次生壁的增厚却被强烈抑制.具体表现为维管束间纤维细胞的细胞壁非常薄; 而木质纤维细胞几乎没有次生壁的形成(
... 基因的适度表达对纤维细胞次生壁的正常沉积至关重要(
... AtSND2和AtSND3为SND1的下游转录因子(
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
Transcriptional activation of secondary wall biosynthesis by rice and maize NAC and MYB transcription factors
3
2011
... 此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余.对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(
... 一级开关基因VNS在维管束植物中十分保守(
... 作为OsSWNs与ZmSWNs下游的直接靶基因, 其启动子上也含有SNBE位点(
Functional characterization of poplar wood-associated NAC domain transcription factors
3
2010
... 此外, NAC转录因子家族IIB分支蛋白SMB (SOMBRERO)、BRN1 (BEARSKINI1)和BRN2在促进细胞分化、根冠成熟以及产生功能性根冠所需的细胞壁分离等方面功能冗余.对其进行超量表达时, 也能诱导植物次生细胞壁的异位沉积(
... 研究发现, VNS转录因子通过与其下游基因启动子上特殊位点结合的方式来调控下游基因的表达, 进而调控细胞次生壁合成(
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis
5
2010
... 研究发现, VNS转录因子通过与其下游基因启动子上特殊位点结合的方式来调控下游基因的表达, 进而调控细胞次生壁合成(
... ).VND6/7和NST1-3等转录因子的靶基因启动子序列均含有SNBE (secondary wall NAC- binding element)基序, 为19 bp的核心序列(T/A)NN (C/T)(T/C/G)TNNNNNNNA(A/C)GN(A/C/T)(A/T) (
... 植物激素尤其是生长素, 对维管组织的分化具有重要作用(
... 为AtVND7和AtSND1的直接靶基因(
... XND1受VND7直接调控(
Evolutionary conservation of the transcriptional network regulating secondary cell wall biosynthesis
1
2010
... 拟南芥AtMYB46和AtMYB83是2个功能冗余的R2R3-MYB转录因子.AtMYB46和AtMYB83位于次生壁生物合成调控网络中的第2级, 是SND1的直接靶基因, 也是调控拟南芥次生壁形成的节点基因(
A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis
13
2008
... NST3 (又称SND1或ANAC012) (Arabidopsis NAC DOMAIN CONTAINING PROTEIN 012)是1个可以双向调控次生壁形成的NAC转录因子.SND1在茎维管束间纤维细胞和木质纤维细胞中特异表达, 通过显性抑制SND1导致纤维细胞次生壁增厚显著下降(
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... ;
... AtMYB42、AtMYB43、AtMYB52和AtMYB54均在木质部组织中优势表达(
... 的超量表达却对次生壁合成无显著影响(
... ).这表明个别基因的高表达不足以引起次生壁的异位沉积, 但其正常表达对于次生壁的形成具有重要作用(
... AtMYB103主要在维管束间纤维细胞和木质部组织中表达, 超量表达AtMYB103可显著增加转基因株系中木质部纤维细胞和维管束间纤维细胞次生壁的厚度.而且AtMYB103可以在体外激活纤维素合酶CESA8基因的启动子, 因此其最初被认为是特异性调控纤维素生物合成的调控因子(
... ), 同时也是受SND1、NST1/2以及VND6/7直接调控的靶基因(
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
... 直接调控的靶基因(
... AtSND2和AtSND3为SND1的下游转录因子(
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
... ).显性抑制分析表明, AtMYB69参与调控次生壁的形成(
The poplar MYB master switches bind to the SMRE site and activate the secondary wall biosynthetic program during wood formation
1
2013
... AtMYB46和AtMYB83不仅能调控转录因子基因, 也能调控一系列与次生壁合成相关的基因.且二者的靶基因启动子上都具有7个核苷酸(ACC[A/T]A[A/C][T/ C])的特异结合元件, 即SMRE (secondary wall MYB- responsive element)基序.作为调控植物次生壁生物合成的二级开关, AtMYB46和AtMYB83均通过与下游基因的SMRE基序相结合激活下游基因的表达, 实现对次生壁生物合成的转录调控(
Two NAC domain transcription factors, SND1 and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis
7
2007
... NST3 (又称SND1或ANAC012) (Arabidopsis NAC DOMAIN CONTAINING PROTEIN 012)是1个可以双向调控次生壁形成的NAC转录因子.SND1在茎维管束间纤维细胞和木质纤维细胞中特异表达, 通过显性抑制SND1导致纤维细胞次生壁增厚显著下降(
... 拟南芥AtMYB46和AtMYB83是2个功能冗余的R2R3-MYB转录因子.AtMYB46和AtMYB83位于次生壁生物合成调控网络中的第2级, 是SND1的直接靶基因, 也是调控拟南芥次生壁形成的节点基因(
... 的表达(
... AtMYB46和AtMYB83在花序茎的纤维细胞和导管细胞中特异表达.将其在拟南芥中超量表达, 转基因植株中纤维素、木质素和木聚糖的生物合成途径被激活, 导致非厚壁细胞中次生壁的异位沉积; 而AtMYB46或AtMYB83的显性抑制植株表现为纤维细胞和导管细胞的次生壁增厚显著减弱(
... AtSND2和AtSND3为SND1的下游转录因子(
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis
3
2007
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
... 此外, AtMYB46和AtMYB83还能调控KNOX家族的KNAT7基因和具有C3H锌指结构域的AtC3H14基因.KNAT7既是AtMYB46和AtMYB83的靶基因(
... AtMYB20、AtMYB26、AtMYB69、AtMYB75和AtMYB99转录因子不受二级转录开关AtMYB46/ AtMYB83的调控.其中, AtMYB20和AtMYB69在木质部细胞中优势表达(
MYB46 and MYB83 bind to the SMRE sites and directly activate a suite of transcription factors and secondary wall biosynthetic genes
2
2012
... AtMYB46和AtMYB83不仅能调控转录因子基因, 也能调控一系列与次生壁合成相关的基因.且二者的靶基因启动子上都具有7个核苷酸(ACC[A/T]A[A/C][T/ C])的特异结合元件, 即SMRE (secondary wall MYB- responsive element)基序.作为调控植物次生壁生物合成的二级开关, AtMYB46和AtMYB83均通过与下游基因的SMRE基序相结合激活下游基因的表达, 实现对次生壁生物合成的转录调控(
... ;
Secondary cell walls: biosynthesis, patterned deposition and transcriptional regulation
2
2014
... 与植物次生壁合成相关的NAC转录因子SWNs (secondary wall-related NAC transcription factors) (
... 近20年来, 得益于遗传学和分子生物学的迅猛发展, 植物次生壁生物合成的转录调控研究取得了空前巨大的进展.尤其在模式植物拟南芥中, 结合突变体筛选和全基因组信息, 已获得多个植物细胞壁合成相关基因, 明确了NAC和MYB类转录因子在维管束组织的木质部导管、纤维细胞和花药皮层次生细胞壁加厚等过程中的核心作用, 以及其它转录因子在此过程中的调控作用, 并解析了这些调控因子之间的层级关系(
The Arabidopsis NAC transcription factor NST2 functions together with SND1 and NST1 to regulate secondary wall biosynthesis in fibers of inflorescence stems
4
2015
... NST1 (NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1)、NST2和SND1 (NST3/SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN 1)为次生壁合成的关键转录因子(
... ).同时, NST2也参与茎秆纤维细胞次生壁合成的调控(
... ).NST2在维管束间纤维细胞和木质部纤维细胞中高度表达, 当NST1、NST2和NST3/SND1三者同时发生突变时, 纤维细胞次生壁完全缺失, 表明NST2、NST1以及NST3/SND1协同调控纤维次生壁的合成(
... 由于次生细胞壁含有较多的纤维素、半纤维素及木质素, 因而是植物生物量的主要来源之一.例如, 水稻、玉米和小麦等农作物的秸秆就属于农业生态系统中十分宝贵的生物质能资源.然而, 农作物生产首先需要满足人类的食品需求.相较之下, 由于木本植物能产生大量的木质纤维素, 因此木材生物量作为一种可再生的、成本效益高的生物能源和工业资源, 预计将成为下一代生物燃料的原材料之一.但是, 来源于木质纤维素的生物乙醇要比来源于粮食作物的昂贵许多(
Functional characterization of NAC and MYB transcription factors involved in regulation of biomass production in Switchgrass (Panicum virgatum)
2015
MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis
1
2009
... 前期已鉴定出多个位于AtMYB46和AtMYB83下游的转录因子基因, 包括AtMYB4、AtMYB7、AtMYB32、AtMYB42、AtMYB43、AtMYB52、AtMYB54、AtMYB85、AtMYB58、AtMYB63和AtMYB103等一系列MYB基因, 以及其它类型的转录因子, 如TZF (Tandem CCCH Zinc Finger)锌指蛋白基因和KNAT7 (KNOTTED Arabidopsis THALIANA 7)基因(
Arabidopsis NAC domain proteins, VND1 to VND5, are transcriptional regulators of secondary wall biosynthesis in vessels
2
2014
... VNS (VND、NST/SND和SMB (SOMBRERO))基因家族在次生壁形成中发挥关键调控作用, 为次生壁合成调控网络的转录因子开关.自首次从百日草(Zinnia elegans)中发现与植物次生壁形成相关的NAC转录因子以来(
... 研究发现, VNS转录因子通过与其下游基因启动子上特殊位点结合的方式来调控下游基因的表达, 进而调控细胞次生壁合成(
备案号: 京ICP备16067583号-21
版权所有 © 2021 《植物学报》编辑部
地址:北京香山南辛村20号 邮编:100093
电话:010-62836135 010-62836131 E-mail:cbb@ibcas.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
