 ,1,*, 李昕
,1,*, 李昕 ,2,*1.
,2,*1. 2.
Open a Door of Defenses: Plant Resistosome
Shitou Xia ,1,*, Xin Li
,1,*, Xin Li ,2,*1.
,2,*1. 2.
通讯作者:
收稿日期:2019-02-23接受日期:2019-04-3网络出版日期:2019-07-01
Corresponding authors:
Received:2019-02-23Accepted:2019-04-3Online:2019-07-01

摘要
关键词:
Abstract
Keywords:
PDF (1041KB)摘要页面多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
夏石头, 李昕. 开启防御之门: 植物抗病小体. 植物学报, 2019, 54(3): 288-292 doi:10.11983/CBB19035
Xia Shitou, Li Xin.
自人类开始应用育种技术改良农作物以来, 培育抗病品种一直备受重视。抗病基因及其编码蛋白(R蛋白)的发现和应用, 无疑对作物的抗病性改良与环保高效生产具有重要意义。二十多年前, 人们应用分子生物学技术成功克隆了第一批抗病蛋白基因, 其编码的蛋白大部分属于具有核苷酸结合域并富含亮氨酸重复序列(nucleotide-binding domain, leucine-rich repeat)的NLR家族。过去20年间, 尽管科学家们付出了巨大努力, 但迄今仍未解析出一个完整的植物NLR蛋白结构。最近, 清华大学柴继杰研究组和王宏伟研究组以及中国科学院遗传与发育生物学研究所周俭民研究组合作对该问题进行了探索, 并取得了突破性进展。
NLR受体蛋白是存在于动植物细胞中的一个免疫受体大家族(Jones and Dangl, 2006; Maekawa et al., 2011)。植物中的NLR可以直接或间接地感知进入细胞的病原效应蛋白, 在植物免疫信号调控中起关键作用(Dodds and Rathjen, 2010; Duxbury et al., 2016)。NLR蛋白为具有多结构域的信号转导ATP酶(signal transduction ATPases with numerous domains, STAND)的成员, 包含1个非保守的N端域、1个位于序列中间的核苷酸结合的寡聚结构域(nucleoti-
de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(Lukasik and Takken, 2009)。由于位于序列中间的结构域保守存在于最初发现的3个NLR成员(Apaf-1、抗性(R)蛋白和Ced-4)中, 故被称为NB-ARC域(Jones and Dangl, 2006; Maekawa et al., 2011; Duxbury et al., 2016)。ZAR1 (HOPZ-ACTIVATED RESISTANCE 1)是拟南芥(Arabidopsis thaliana) (Lewis et al., 2010)和烟草(Nicotiana benthamiana) (Schultink et al., 2019)共有的典型CC-NLR (CC (coiled-coil)-NLR)蛋白, 其通过与受体样胞质激酶(RLCKs)亚家族XII-2的多个成员互作形成特异响应的免疫受体复合物, 每个复合物特异响应不同的效应蛋白并触发ETI (effector-trigge-red immunity)抗病反应(Lewis et al., 2013; Wang et al., 2015; Seto et al., 2017)。例如, ZAR1与RLCK XII家族成员RKS1形成的复合物, 特异识别野油菜黄单胞菌(Xanthomonas campestris)的效应蛋白AvrAC。这一识别过程中, 首先需要AvrAC对PBL2 (拟南芥的另一个激酶)进行尿苷酸修饰形成PBL2UMP, 之后PBL2UMP通过与RKS1相互作用被ZAR1-RKS1招募, 完成拟南芥对黄单胞菌AvrAC的识别, 进而激活免疫反应(Wang et al., 2015)。激活后的NLR通过诱导超敏反应(hypersensitive response, HR)等多种防御机制阻止病原体增殖, 从而将病原体限制在感染部位(Chisholm et al., 2006; Cui et al., 2015)。
NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用。然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚。此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(Rairdan and Moffett, 2006; Qi et al., 2012)的支持, 但在很大程度上也是根据人体APAF-1 (Riedl et al., 2005; Reubold et al., 2011; Zhou et al., 2015)和动物NLR (Hu et al., 2013; Hu et al., 2015; Zhang et al., 2015; Maekawa et al., 2016)中NOD的结构提出的。植物NLR中的NB-ARC被认为是一种具有ADP-和ATP-结合形式, 指示NLR信号“关闭”和“打开”状态的分子开关(Tameling et al., 2002; Williams et al., 2011; Bernoux et al., 2016), 但其内在分子机制仍然未知。柴继杰、周俭民和王宏伟团队通过结构生物学、生化、遗传以及生物学功能研究, 发现尿苷酰化的PBL2UMP作为配体结合ZAR1-RKS1复合物后诱导ZAR1-NB结构域的构象变化, 促进ADP释放, 进入中间状态; 中间状态的ZAR1结合ATP后诱发ZAR1寡聚结构域暴露, 致使ZAR1-RKS1-PBL2UMP形成轮状五聚体免疫抗病小体; 该抗病小体通过新形成的漏斗状结构定位于质膜, 激活超敏反应和抗病性(Wang et al., 2019a, 2019b)。
三位科学家团队利用冷冻电镜手段(cryo-elect- ron microscopy), 在体外组装并解析了3个ZAR1复合物结构(Wang et al., 2019a, 2019b)。正常生长条件下, 植物NLR受到严格调控, 以免因免疫失控导致植物生长受阻和受损。通过解析ZAR1与假激酶RKS1形成的复合物结构, 他们一方面揭示了NLR被维持在非激活状态的机制(Wang et al., 2019a); 另一方面揭示了ZAR1与不同RLCK XII成员结合的机制。在ZAR1-RKS1复合物中, ZAR1的LRR结构域通过分子内互作, 遮盖了ZAR1中的寡聚化结构域, ADP的结合进一步稳定了非激活状态。当病原物入侵时, 植物NLR则转换为激活状态。他们对第2个复合物的结构以及功能进行解析, 发现了ZAR1-RKS1特异招募PBL2UMP的分子机制(Wang et al., 2019a)。ZAR1- RKS1中RKS1蛋白的独特基序与PBL2UMP中的UMP基团相互作用, 形成ZAR1-RKS1-PBL2UMP复合物。 PBL2UMP的结合使本来松散的RKS1激活片段稳定下来, 后者与ZAR1中的NBD结构域发生空间碰撞, 导致ZAR1NBD向外旋转约60度, ADP从ZAR1中释放出来, 从而使得ZAR1-RKS1-PBL2UMP复合物进入随时可结合ATP的中间状态(Wang et al., 2019a) (图1)。由配体结合导致的NLRNBD结构域构象变化, 对理解植物中其它NLR受体的激活机制具有重大意义。
在后续研究中, 他们将ZAR1-RKS1-PBL2UMP复合物(中间状态)与dATP(或者ATP)共同孵育, 获得了分子量约为900 kDa的寡聚复合物, 称之为“ZAR1抗病小体”。进一步通过冷冻电镜分析, 他们获得了该抗病小体的高分辨率(3.4 ?)结构。ZAR1抗性小体为轮状五聚体, 其寡聚化由ZAR1介导。ZAR1的所有结构域(包括CC、NB结构域(NBD)、螺旋结构域1 (HD1)、翼螺旋结构域(WHD)和LRR结构域)均参与了ZAR1抗性小体的五聚体化, 并被dATP进一步稳定(Wang et al., 2019b) (图1)。该结构与NLRC4炎症体(或APAF-1凋亡体)有较大差异。突变分析和功能研究证实, 抗病小体的形成导致了ZAR1的激活、HR细胞死亡和抗性启动。此外, 抗病小体还形成了一个由CC结构域组成的新功能结构。ZAR1寡聚化后, CC域的氮端α-螺旋组成了一个双性的漏斗状结构, 在五聚轮状结构的平面上形成一个突出结构(Wang et al., 2019b)。进一步的生化和功能分析表明, 该漏斗状结构使得激活状态的抗病小体与质膜(plasma membrane, PM)结合, 而这一功能对细胞死亡和抗病性不可或缺, 暗示抗病小体很可能通过质膜穿孔或形成离子通道发挥作用。
图1
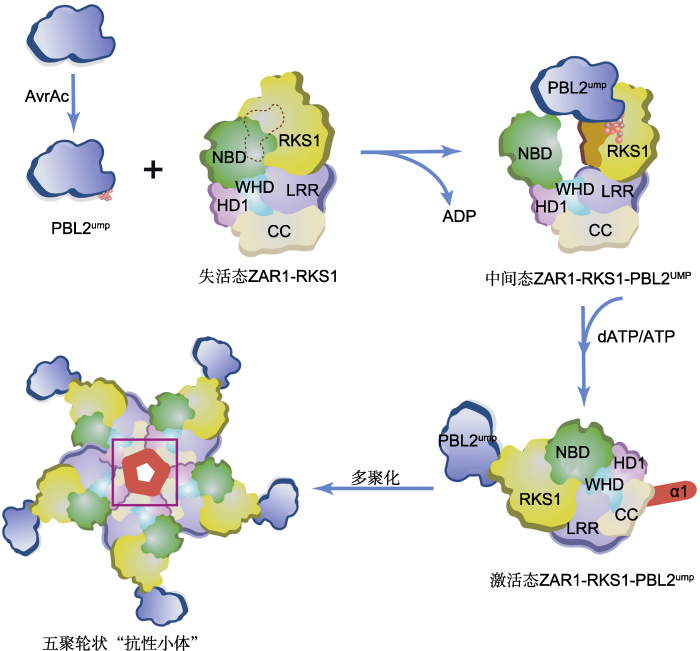 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1PBL2UMP诱导的ZAR1抗性小体的激活与装配
野油菜黄单胞菌的效应蛋白AvrAC以尿苷酰化修饰拟南芥PBL2激酶, 尿苷酰化的PBL2UMP作为配体通过与RKS1互作而被ZAR1-RKS1复合物招募。RKS1的激活片段在与PBL2UMP的2个尿苷基部分(球形)相互作用后变得稳定(橙色表面), 并与ZAR1NBD结构域发生立体碰撞, 导致后者向外旋转, 从而释放ADP, 形成可与dATP/ATP结合的中间状态ZAR1-RKS1-PBL2UMP复合体。该复合物结合dATP/ATP后, 诱导ZAR1结构重塑和折叠转换, 隐藏在非活性ZAR1-RKS1复合物中的ZAR1的N顶末端α-螺旋(α1, 红色)暴露在溶剂中, 导致ZAR1完全激活(激活态ZAR1-RKS1-PBL2UMP), 继而通过多聚化形成五聚轮状结构的ZAR1抗性小体(紫色方框内突出显示形成的漏斗状结构)。CC、NBD、HD1、WHD和LRR为ZAR1的不同结构域。
Figure 1PBL2UMP-induced activation and assembly of the ZAR1 resistosome
Arabidopsis PBL2 is modified by uridylyl transferase AvrAC, which is an effector protein from Xanthomonas campestris. The uridylylated PBL2 (PBL2UMP) as a ligand is then recruited by the ZAR1-RKS1 complex through interaction with the pseudokinase RKS1. The activation segment of RKS1 becomes stabilized (orange surface) after interacting with the two uridylyl moieties (in sphere) of PBL2UMP, and sterically clashes with ZAR1NBD, causing the latter to rotate outward and consequently release ADP, forming an intermediate ZAR1-RKS1-PBL2UMP complex which allows it to bind dATP/ATP. Binding of dATP/ATP induces structural remodeling and fold switching of ZAR1. The very N-terminal helix (α1, red) of ZAR1 buried in the inactive ZAR1-RKS1 complex becomes solvent-exposed in the activated ZAR1-RKS1-PBL2UMP complex, forming a ZAR1 resistosome pentameric structure through polymerization (a funnel-shaped structure highlighted within the purple frame). CC, NBD, HD1, WHD and LRR are different structural domains of ZAR1.
综上所述, 柴继杰、周俭民和王宏伟团队基于结构、生化、遗传和功能多重数据, 首次完成了植物NLR蛋白复合物组装、结构和功能分析, 揭示了NLR作用的关键分子机制。该研究是植物免疫领域的里程碑发现, 也是继国内****近年在多个领域取得突破(施怡婷和杨淑华, 2016; 于倩倩等, 2018)后又一项重要原创性成果。这两项工作不仅为理解植物中其它NLR蛋白作用方式提供了结构模型, 还为人工改造NLR和发展广谱抗病新技术打下基础。
植物体内的另一大类NLR, 其N端为TIR (Toll/interleukin receptor)域。TIR-NLR是否也通过类似CC-NLR蛋白的方式介导免疫信号目前尚不清楚。已有研究表明, 有几种CC-NLR作用于TIR-NLR的免疫信号下游(Castel et al., 2018; Qi et al., 2018; Wu et al., 2018)。例如, CC-NLR NRG1 (N requirement gene 1) (Peart et al., 2005)和ADR1 (activated disease resistance 1) (Bonardi et al., 2011; Dong et al., 2016)属于RPW8分支族, 对拟南芥和烟草多种TIR-NLR的抗性有重要调控作用。由此推测, 这种新的免疫分子开关机制可为解释TIR-NLR免疫信号转导提供参考模型。但是TIR-NLR免疫信号转导与CC-NLR是否有所不同? 植物体内NLR是否还存在其它的调控机制? 对上述问题的回答将有助于我们深入理解免疫信号转导的分子机理。
(责任编辑: 孙冬花)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.str.2011.05.013URLPMID:21827944 [本文引用: 1]

The apoptotic protease-activating factor 1 (Apaf-1) relays the death signal in the mitochondrial pathway of apoptosis. Apaf-1 oligomerizes on binding of mitochondrially released cytochrome into the heptameric apoptosome complex to ignite the downstream cascade of caspases. Here, we present the 3.0 03 crystal structure of full-length murine Apaf-1 in the absence of cytochrome . The structure shows how the mammalian death switch is kept in its “off” position. By comparing the off state with a recent cryo-electron microscopy derived model of Apaf-1 in its apoptosomal conformation, we depict the molecular events that transform Apaf-1 from autoinhibited monomer to a building block of the caspase-activating apoptosome. Moreover, we have solved the crystal structure of the R265S mutant of full-length murine Apaf-1 in the absence of cytochrome to 3.55 03 resolution and we show that proper function of Apaf-1 relies on R265 in the vicinity of the bound nucleotide.Highlights? Full-length structure of Apaf-1 shows the location of the regulatory domain ? Depiction of the molecular events that lead to apoptosome formation ? Identification of a residue essential for Apaf-1 function ? Explanation of how cytochrome binding releases autoinhibition of Apaf-1
DOI:10.1038/nature03465URLPMID:15829969 [本文引用: 1]

Apoptosis is executed by caspases, which undergo proteolytic activation in response to cell death stimuli. The apoptotic protease-activating factor 1 (Apaf-1) controls caspase activation downstream of mitochondria. During apoptosis, Apaf-1 binds to cytochrome c and in the presence of ATP/dATP forms an apoptosome, leading to the recruitment and activation of the initiator caspase, caspase-9 (ref. 2). The mechanisms underlying Apaf-1 function are largely unknown. Here we report the 2.2-01…crystal structure of an ADP-bound, WD40-deleted Apaf-1, which reveals the molecular mechanism by which Apaf-1 exists in an inactive state before ATP binding. The amino-terminal caspase recruitment domain packs against a three-layereda/0100fold, a short helical motif and a winged-helix domain, resulting in the burial of the caspase-9-binding interface. The deeply buried ADP molecule serves as an organizing centre to strengthen interactions between these four adjoining domains, thus locking Apaf-1 in an inactive conformation. Apaf-1 binds to and hydrolyses ATP/dATP and their analogues. The binding and hydrolysis of nucleotides seem to drive conformational changes that are essential for the formation of the apoptosome and the activation of caspase-9.
[本文引用: 1]
DOI:10.1038/nplants.2017.27URLPMID:28288096 [本文引用: 1]

Plants evolved NLR sensors to detect bacterial effectors or their activity. A genetic analysis in Arabidopsis expands the role of the ZAR1 NLR protein.
[本文引用: 1]
DOI:10.1016/j.chom.2015.08.004URLPMID:26355215 [本文引用: 2]

A plant pathogen effector AvrAC uridylylates several host kinases to promote virulence. Wang et al. find that plants have evolved a decoy substrate which upon uridylylation by AvrAC is recognized by a pseudokinase-immune receptor complex to trigger immunity. Thus, decoy substrates and pseudokinases specify and expand immune capacity in plants.
DOI:10.1094/MPMI-03-11-0052URL [本文引用: 1]
DOI:10.1111/nph.15665URL [本文引用: 1]
DOI:10.1126/science.aac5789URLPMID:26449474 [本文引用: 1]

The NLR family apoptosis inhibitory proteins (NAIPs) bind conserved bacterial ligands, such as the bacterial rod protein PrgJ, and recruit NLR family CARD-containing protein 4 (NLRC4) as the inflammasome adapter to activate innate immunity. We found that the PrgJ-NAIP2-NLRC4 inflammasome is assembled into multisubunit disk-like structures through a unidirectional adenosine triphosphatase polymerization, primed with a single PrgJ-activated NAIP2 per disk. Cryo-electron microscopy (cryo-EM) reconstruction at subnanometer resolution revealed a ~90掳 hinge rotation accompanying NLRC4 activation. Unlike in the related heptameric Apaf-1 apoptosome, in which each subunit needs to be conformationally activated by its ligand before assembly, a single PrgJ-activated NAIP2 initiates NLRC4 polymerization in a domino-like reaction to promote the disk assembly. These insights reveal the mechanism of signal amplification in NAIP-NLRC4 inflammasomes.
DOI:10.1101/gad.272278.115URLPMID:26543158 [本文引用: 1]

The apoptotic protease-activating factor 1 (Apaf-1) controls the onset of many known forms of intrinsic apoptosis in mammals. Apaf-1 exists in normal cells as an autoinhibited monomer. Upon binding to cytochrome c and dATP, Apaf-1 oligomerizes into a heptameric complex known as the apoptosome, which recruits and activates cell-killing caspases. Here we present an atomic structure of an intact mammalian apoptosome at 3.8 脜 resolution, determined by single-particle, cryo-electron microscopy (cryo-EM). Structural analysis, together with structure-guided biochemical characterization, uncovered how cytochrome c releases the autoinhibition of Apaf-1 through specific interactions with the WD40 repeats. Structural comparison with autoinhibited Apaf-1 revealed how dATP binding triggers a set of conformational changes that results in the formation of the apoptosome. Together, these results constitute the molecular mechanism of cytochrome c- and dATP-mediated activation of Apaf-1.
DOI:10.11983/CBB15191URL [本文引用: 1]

乙烯是一种气态植物激素,在植物生长发育的各个阶段发挥着非常重要的作用。最近,中国科学家在乙烯信号转导的分子机制研究中取得了突破性进展。
DOI:10.11983/CBB15115URL [本文引用: 1]

Auxin is one of the most important phytohormones and participates in a variety of growth and development processes in plants. Recently, Chinese scientists have made breakthrough advances toward our understanding on the molecular mechanisms of auxin signaling transduction.
DOI:10.1105/tpc.15.00303URLPMID:26744216 [本文引用: 1]

Abstract NOD-like receptors (NLRs) are central components of the plant immune system. L6 is a Toll/interleukin-1 receptor (TIR) domain-containing NLR from flax (Linum usitatissimum) conferring immunity to the flax rust fungus. Comparison of L6 to the weaker allele L7 identified two polymorphic regions in the TIR and the nucleotide binding (NB) domains that regulate both effector ligand-dependent and -independent cell death signaling as well as nucleotide binding to the receptor. This suggests that a negative functional interaction between the TIR and NB domains holds L7 in an inactive/ADP-bound state more tightly than L6, hence decreasing its capacity to adopt the active/ATP-bound state and explaining its weaker activity in planta. L6 and L7 variants with a more stable ADP-bound state failed to bind to AvrL567 in yeast two-hybrid assays, while binding was detected to the signaling active variants. This contrasts with current models predicting that effectors bind to inactive receptors to trigger activation. Based on the correlation between nucleotide binding, effector interaction, and immune signaling properties of L6/L7 variants, we propose that NLRs exist in an equilibrium between ON and OFF states and that effector binding to the ON state stabilizes this conformation, thereby shifting the equilibrium toward the active form of the receptor to trigger defense signaling. 2016 American Society of Plant Biologists. All rights reserved.
DOI:10.1073/pnas.1113726108URLPMID:21911370 [本文引用: 1]

Abstract Plants and animals deploy intracellular immune receptors that perceive specific pathogen effector proteins and microbial products delivered into the host cell. We demonstrate that the ADR1 family of Arabidopsis nucleotide-binding leucine-rich repeat (NB-LRR) receptors regulates accumulation of the defense hormone salicylic acid during three different types of immune response: (i) ADRs are required as "helper NB-LRRs" to transduce signals downstream of specific NB-LRR receptor activation during effector-triggered immunity; (ii) ADRs are required for basal defense against virulent pathogens; and (iii) ADRs regulate microbial-associated molecular pattern-dependent salicylic acid accumulation induced by infection with a disarmed pathogen. Remarkably, these functions do not require an intact P-loop motif for at least one ADR1 family member. Our results suggest that some NB-LRR proteins can serve additional functions beyond canonical, P-loop-dependent activation by specific virulence effectors, extending analogies between intracellular innate immune receptor function from plants and animals.
[本文引用: 1]
DOI:10.1016/j.cell.2006.02.008URLPMID:16497589 [本文引用: 1]

The evolution of the plant immune response has culminated in a highly effective defense system that is able to resist potential attack by microbial pathogens. The primary immune response is referred to as PAMP-triggered immunity (PTI) and has evolved to recognize common features of microbial pathogens. In the coevolution of host-microbe interactions, pathogens acquired the ability to deliver effector proteins to the plant cell to suppress PTI, allowing pathogen growth and disease. In response to the delivery of pathogen effector proteins, plants acquired surveillance proteins (R proteins) to either directly or indirectly monitor the presence of the pathogen effector proteins. In this review, taking an evolutionary perspective, we highlight important discoveries over the last decade about the plant immune response.
DOI:10.1146/annurev-arplant-050213-040012URLPMID:25494461 [本文引用: 1]

In plant innate immunity, individual cells have the capacity to sense and respond to pathogen attack. Intracellular recognition mechanisms have evolved to intercept perturbations by pathogen virulence factors (effectors) early in host infection and convert it to rapid defense. One key to resistance success is a polymorphic family of intracellular nucleotide-binding/leucine-rich-repeat (NLR) receptors that detect effector interference in different parts of the cell. Effector-activated NLRs connect, in various ways, to a conserved basal resistance network in order to transcriptionally boost defense programs. Effector-triggered immunity displays remarkable robustness against pathogen disturbance, in part by employing compensatory mechanisms within the defense network. Also, the mobility of some NLRs and coordination of resistance pathways across cell compartments provides flexibility to fine-tune immune outputs. Furthermore, a number of NLRs function close to the nuclear chromatin by balancing actions of defense-repressing and defense-activating transcription factors to program cells dynamically for effective disease resistance.
DOI:10.1038/nrg2812URLPMID:20585331 [本文引用: 1]

Plants are engaged in a continuous co-evolutionary struggle for dominance with their pathogens. The outcomes of these interactions are of particular importance to human activities, as they can have dramatic effects on agricultural systems. The recent convergence of molecular studies of plant immunity and pathogen infection strategies is revealing an integrated picture of the plant09“pathogen interaction from the perspective of both organisms. Plants have an amazing capacity to recognize pathogens through strategies involving both conserved and variable pathogen elicitors, and pathogens manipulate the defence response through secretion of virulence effector molecules. These insights suggest novel biotechnological approaches to crop protection.
DOI:10.1111/nph.13821URLPMID:27074399 [本文引用: 1]

Summary Nucleotide-binding leucine-rich repeat proteins (NLRs) serve as intracellular immune receptors in animals and plants. Sensor NLRs perceive pathogen-derived effector molecules and trigger robust host defense. Recent studies revealed the role of three coiled-coil-type NLRs (CNLs) of the ADR1 family 鈥 ADR1, ADR1-L1 and ADR1-L2 鈥 as redundant helper NLRs, whose function is required for defense mediated by multiple sensor NLRs. From a mutant snc1 -enhancing (MUSE) forward genetic screen in Arabidopsis targeted to identify negative regulators of snc1 that encodes a TIR-type NLR (TNL), we isolated two alleles of muse15 , both carrying mutations in ADR1-L1 . Interestingly, loss of ADR1-L1 also enhances immunity-related phenotypes in other autoimmune mutants including cpr1 , bal and lsd1 . This immunity-enhancing effect is not mediated by increased SNC1 protein stability, nor is it fully dependent on the accumulation of the defense hormone salicylic acid (SA). Transcriptional analysis revealed an upregulation of ADR1 and ADR1-L2 in the adr1-L1 background, which may overcompensate the loss of ADR1-L1 , resulting in enhanced immunity. Interestingly, autoimmunity of snc1 and chs2 , which encode typical TNLs, is fully suppressed by the adr1 triple mutant, suggesting that the ADRs are required for TNL downstream signaling. This study extends our knowledge on the interplay among ADRs and reveals their complexity in defense regulation.
DOI:10.1002/bies.201600046URLPMID:27339076 [本文引用: 2]

Abstract Intracellular NLR ( N ucleotide-binding domain and L eucine-rich R epeat-containing) receptors are sensitive monitors that detect pathogen invasion of both plant and animal cells. NLRs confer recognition of diverse molecules associated with pathogen invasion. NLRs must exhibit strict intramolecular controls to avoid harmful ectopic activation in the absence of pathogens. Recent discoveries have elucidated the assembly and structure of oligomeric NLR signalling complexes in animals, and provided insights into how these complexes act as scaffolds for signal transduction. In plants, recent advances have provided novel insights into signalling-competent NLRs, and into the myriad strategies that diverse plant NLRs use to recognise pathogens. Here, we review recent insights into the NLR biology of both animals and plants. By assessing commonalities and differences between kingdoms, we are able to develop a more complete understanding of NLR function.
DOI:10.1126/science.1236381URLPMID:23765277 [本文引用: 1]

Nucleotide-binding and oligomerization domain-like receptor (NLR) proteins oligomerize into multiprotein complexes termed inflammasomes when activated. Their autoinhibition mechanism remains poorly defined. Here, we report the crystal structure of mouse NLRC4 in a closed form. The adenosine diphosphate-mediated interaction between the central nucleotide-binding domain (NBD) and the winged-helix domain (WHD) was critical for stabilizing the closed conformation of NLRC4. The helical domain HD2 repressively contacted a conserved and functionally important alpha-helix of the NBD. The C-terminal leucine-rich repeat (LRR) domain is positioned to sterically occlude one side of the NBD domain and consequently sequester NLRC4 in a monomeric state. Disruption of ADP-mediated NBD-WHD or NBD-HD2/NBD-LRR interactions resulted in constitutive activation of NLRC4. Together, our data reveal the NBD-organized cooperative autoinhibition mechanism of NLRC4 and provide insight into its activation.
DOI:10.1126/science.aac5489URLPMID:26449475 [本文引用: 1]

Abstract Responding to stimuli, nucleotide-binding domain and leucine-rich repeat-containing proteins (NLRs) oligomerize into multiprotein complexes, termed inflammasomes, mediating innate immunity. Recognition of bacterial pathogens by NLR apoptosis inhibitory proteins (NAIPs) induces NLR family CARD domain-containing protein 4 (NLRC4) activation and formation of NAIP-NLRC4 inflammasomes. The wheel-like structure of a PrgJ-NAIP2-NLRC4 complex determined by cryogenic electron microscopy at 6.6 angstrom reveals that NLRC4 activation involves substantial structural reorganization that creates one oligomerization surface (catalytic surface). Once activated, NLRC4 uses this surface to catalyze the activation of an inactive NLRC4, self-propagating its active conformation to form the wheel-like architecture. NAIP proteins possess a catalytic surface matching the other oligomerization surface (receptor surface) of NLRC4 but not those of their own, ensuring that one NAIP is sufficient to initiate NLRC4 oligomerization. Copyright 2015, American Association for the Advancement of Science.
DOI:10.1038/nature05286 [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.pbi.2009.03.001URLPMID:19394891 [本文引用: 1]

Resistance (R) proteins are involved in specific pathogen recognition and subsequent initiation of host defence. Most R proteins are nucleotide binding – leucine rich repeat (NB–LRR) proteins, which form a subgroup within the STAND (signal transduction ATPases with numerous domains) family. Activity of these multi-domain proteins depends on their ability to bind and hydrolyse nucleotides. Since R protein activation often triggers cell-death tight regulation of activation is essential. Autoinhibition, which seems to be accomplished by intramolecular interactions between the various domains, is important to retain R proteins inactive. This review summarizes recent data on intra- and intermolecular interactions that support a model in which pathogen perception triggers a series of conformational changes, allowing the newly exposed NB domain to interact with downstream signalling partners and activate defence signalling.
DOI:10.1038/ncomms11813URLPMID:27283905 [本文引用: 1]

Nucleotide-binding oligomerization domain-containing protein 2 (NOD2), a member of the NOD-like receptors family, are crucial for innate immune responses. Mutations of NOD2 have been associated with chronic inflammatory disorders such as Crohn's disease (CD), Blau syndrome (BS) and early-onset sarcoidosis (EOS), but little is known about its signalling mechanism and the role it plays in these diseases. Here, we report the crystal structure of rabbit NOD2 in an ADP-bound state. The structure reveals an inactive closed conformation in which the subdomains in the NOD domain are closely packed by ADP-mediated and inter-domain interactions. Mapping of the BS- or EOS-associated gain-of-function mutations reveals that most of these mutations are located in the NOD subdomain interfaces, and are likely to disrupt the inner domain interactions, facilitating a conformational change to the active form. Conversely, mutations associated with CD are distributed throughout the protein, some of which may affect oligomer formation and ligand binding. NOD2 has a role in host innate immune responses, activating the NF-魏B signalling pathway and mutations have been associated with chronic inflammatory disorders. Here, Maekawaet al. solved the structure of NOD2 in its inactive form, suggesting a mechanism for autoinhibition.
DOI:10.1038/ni.2083URLPMID:21852785 [本文引用: 2]

In plants and animals, the NLR family of receptors perceives non-self and modified-self molecules inside host cells and mediates innate immune responses to microbial pathogens. Despite their similar biological functions and protein architecture, animal NLRs are normally activated by conserved microbe- or damage-associated molecular patterns, whereas plant NLRs typically detect strain-specific pathogen effectors. Plant NLRs recognize either the effector structure or effector-mediated modifications of host proteins. The latter indirect mechanism for the perception of non-self, as well as the within-species diversification of plant NLRs, maximize the capacity to recognize non-self through the use of a finite number of innate immunoreceptors. We discuss recent insights into NLR activation, signal initiation through the homotypic association of N-terminal domains and subcellular receptor dynamics in plants and compare those with NLR functions in animals.
DOI:10.1016/j.cub.2005.04.053URLPMID:15916955 [本文引用: 1]

In animals and plants, innate immunity is regulated by nucleotide binding domain and leucine-rich repeat (NB-LRR) proteins that mediate pathogen recognition and that activate host-cell defense responses [ 1, 2]. Plant NB-LRR proteins, referred to as R proteins, have amino-terminal domains that contain a coiled coil (CC) or that share similarity with animal Toll and interleukin 1 receptors (TIR) [ 3]. To investigate R protein function, we are using the TIR-NB-LRR protein N that mediates resistance against tobacco mosaic virus (TMV) [ 4] through recognition of the TMV p50 protein. Here, we describe N requirement gene 1 ( NRG1), a novel N-resistance component that was identified by a virus-induced gene silencing (VIGS) screen of a cDNA library [ 5]. Surprisingly, NRG1 encodes an NB-LRR type R protein that, in contrast to N, contains a CC rather than a TIR domain. Our findings support emerging evidence that many disease-resistance pathways each recruit more than a single NB-LRR protein. The results also indicate that, in addition to the previously recognized role in elicitor recognition, NB-LRR proteins may also function in downstream signaling pathways.
DOI:10.1104/pp.112.194035URL [本文引用: 1]

The Arabidopsis (Arabidopsis thaliana) RESISTANCE TO PSEUDOMONAS SYRINGAE5 (RPS5) disease resistance protein mediates recognition of the Pseudomonas syringae effector protein AvrPphB. RPS5 belongs to the coiled-coil-nucleotide-binding site-leucine-rich repeat (CC-NBS-LRR) family and is activated by AvrPphB-mediated cleavage of the protein kinase PBS1. Here, we present a structure-function analysis of the CC and LRR domains of RPS5 using transient expression assays in Nicotiana benthamiana. We found that substituting the CC domain of RPS2 for the RPS5 CC domain did not alter RPS5 specificity and only moderately reduced its ability to activate programmed cell death, suggesting that the CC domain does not play a direct role in the recognition of PBS1 cleavage. Analysis of an RPS5-super Yellow Fluorescent Protein fusion revealed that RPS5 localizes to the plasma membrane (PM). Alanine substitutions of predicted myristoylation (glycine-2) and palmitoylation (cysteine-4) residues affected RPS5 PM localization, protein stability, and function in an additive manner, indicating that PM localization is essential to RPS5 function. The first 20 amino acids of RPS5 were sufficient for directing super Yellow Fluorescent Protein to the PM. C-terminal truncations of RPS5 revealed that the first four LRR repeats are sufficient for inhibiting RPS5 autoactivation; however, the complete LRR domain was required for the recognition of PBS1 cleavage. Substitution of the RPS2 LRR domain resulted in the autoactivation of RPS5, indicating that the LRR domain must coevolve with the NBS domain. We conclude that the RPS5 LRR domain functions to suppress RPS5 activation in the absence of PBS1 cleavage and promotes RPS5 activation in its presence.
[本文引用: 1]
DOI:10.1105/tpc.106.042747URLPMID:16844906 [本文引用: 1]

Plant nucleotide binding and leucine-rich repeat (NB-LRR) proteins contain a region of homology known as the ARC domain located between the NB and LRR domains. Structural modeling suggests that the ARC region can be subdivided into ARC1 and ARC2 domains. We have used the potato (Solanum tuberosum) Rx protein, which confers resistance to Potato virus X (PVX), to investigate the function of the ARC region. We demonstrate that the ARC1 domain is required for binding of the Rx N terminus to the LRR domain. Domain-swap experiments with Rx and a homologous disease resistance gene, Gpa2, showed that PVX recognition localized to the C-terminal half of the LRR domain. However, inappropriate pairings of LRR and ARC2 domains resulted in autoactive molecules. Thus, the ARC2 domain is required to condition an autoinhibited state in the absence of elicitor as well as for the subsequent elicitor-induced activation. Our data suggest that the ARC region, through its interaction with the LRR, translates elicitor-induced modulations of the C terminus into a signal initiation event. Furthermore, we demonstrate that physical disruption of the LRR--ARC interaction is not required for signal initiation. We propose instead that this activity can lead to multiple rounds of elicitor recognition, providing a means of signal amplification.
Crystal structure of full-length Apaf-1: how the death signal is relayed in the mitochondrial pathway of apoptosis
1
2011
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
Structure of the apoptotic protease-activating factor 1 bound to ADP
1
2005
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
Using forward genetics in
1
2019
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
Expanded type III effector recognition by the ZAR1 NLR protein using ZED1-related 5 kinases
1
2017
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity
1
2002
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
The decoy substrate of a pathogen effector and a pseudokinase specify pathogen-induced modified-self recognition and immunity in plants
2
2015
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
... 通过与RKS1相互作用被ZAR1-RKS1招募, 完成拟南芥对黄单胞菌AvrAC的识别, 进而激活免疫反应(
Ligand-triggered allosteric ADP release primes a plant NLR complex
0
2019a
Reconstitution and structure of a plant NLR resistosome conferring immunity
0
2019b
An autoactive mutant of the M flax rust resistance protein has a preference for binding ATP, whereas wild-type M protein binds ADP
1
2011
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
Differential regulation of TNL-mediated immune signaling by redundant helper CNLs
1
2018
... 植物体内的另一大类NLR, 其N端为TIR (Toll/interleukin receptor)域.TIR-NLR是否也通过类似CC-NLR蛋白的方式介导免疫信号目前尚不清楚.已有研究表明, 有几种CC-NLR作用于TIR-NLR的免疫信号下游(
Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization
1
2015
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
Atomic structure of the apoptosome: mechanism of cytochrome c- and 25 dATP-mediated activation of Apaf-1
1
2015
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
中国科学家在乙烯信号转导领域取得突破性进展
1
2016
... 综上所述, 柴继杰、周俭民和王宏伟团队基于结构、生化、遗传和功能多重数据, 首次完成了植物NLR蛋白复合物组装、结构和功能分析, 揭示了NLR作用的关键分子机制.该研究是植物免疫领域的里程碑发现, 也是继国内****近年在多个领域取得突破(
中国科学家在生长素信号转导领域取得突破性研究进展
1
2018
... 综上所述, 柴继杰、周俭民和王宏伟团队基于结构、生化、遗传和功能多重数据, 首次完成了植物NLR蛋白复合物组装、结构和功能分析, 揭示了NLR作用的关键分子机制.该研究是植物免疫领域的里程碑发现, 也是继国内****近年在多个领域取得突破(
Comparative analysis of the flax immune receptors L6 and L7 suggests an equilibrium-ba-sed switch activation model
1
2016
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors
1
2011
... 植物体内的另一大类NLR, 其N端为TIR (Toll/interleukin receptor)域.TIR-NLR是否也通过类似CC-NLR蛋白的方式介导免疫信号目前尚不清楚.已有研究表明, 有几种CC-NLR作用于TIR-NLR的免疫信号下游(
Diverse NLR immune receptors activate defence via the RPW8-NLR NRG1
1
2018
... 植物体内的另一大类NLR, 其N端为TIR (Toll/interleukin receptor)域.TIR-NLR是否也通过类似CC-NLR蛋白的方式介导免疫信号目前尚不清楚.已有研究表明, 有几种CC-NLR作用于TIR-NLR的免疫信号下游(
Host-microbe interactions: shaping the evolution of the plant immune response
1
2006
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
Effector-triggered immunity: from pathogen perception to robust defense
1
2015
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
Plant immunity: towards an integrated view of plant-pathogen interactions
1
2010
... NLR受体蛋白是存在于动植物细胞中的一个免疫受体大家族(
TNL-mediated immunity in Arabidopsis requires complex regulation of the redundant ADR1 gene family
1
2016
... 植物体内的另一大类NLR, 其N端为TIR (Toll/interleukin receptor)域.TIR-NLR是否也通过类似CC-NLR蛋白的方式介导免疫信号目前尚不清楚.已有研究表明, 有几种CC-NLR作用于TIR-NLR的免疫信号下游(
Pathogen perception by NLRs in plants and animals: parallel worlds
2
2016
... NLR受体蛋白是存在于动植物细胞中的一个免疫受体大家族(
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
Crystal structure of NLRC4 reveals its autoinhibition mechanism
1
2013
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
Structural and biochemical basis for induced self-propagation of NLRC4
1
2015
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
The plant immune system
2
2006
... NLR受体蛋白是存在于动植物细胞中的一个免疫受体大家族(
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
The Arabidopsis ZED1 pseudokinase is required for ZAR1-mediated immunity induced by the
1
2013
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
Allele-specific virulence attenuation of the
1
2010
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
STANDing strong, resistance proteins instigators of plant defence
1
2009
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
Crystal structure of NOD2 and 35 its implications in human disease
1
2016
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
NLR functions in plant and animal immune systems: so far and yet so close
2
2011
... NLR受体蛋白是存在于动植物细胞中的一个免疫受体大家族(
... de-binding and oligomerization domain, NOD)以及C端LRR (Leucine-rich repeat)结构域(
NRG1, a CC-NB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus
1
2005
... 植物体内的另一大类NLR, 其N端为TIR (Toll/interleukin receptor)域.TIR-NLR是否也通过类似CC-NLR蛋白的方式介导免疫信号目前尚不清楚.已有研究表明, 有几种CC-NLR作用于TIR-NLR的免疫信号下游(
Structure-function analysis of the coiled-coil and leucine-rich repeat domains of the RPS5 disease resistance protein
1
2012
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
NRG1 functions downstream of EDS1 to regulate TIR-NLR-me-diated plant immunity in
1
2018
... 植物体内的另一大类NLR, 其N端为TIR (Toll/interleukin receptor)域.TIR-NLR是否也通过类似CC-NLR蛋白的方式介导免疫信号目前尚不清楚.已有研究表明, 有几种CC-NLR作用于TIR-NLR的免疫信号下游(
Distinct domains in the ARC region of the potato resistance protein Rx mediate LRR binding and inhibition of activation
1
2006
... NLR作为AAA+ATPase的成员, 常被认为通过寡聚方式发挥作用.然而, 植物NLR在活化后是否也通过寡聚方式形成类似人体和动物凋亡小体(apopotosome)及炎症小体(inflammasomes)的大蛋白复合物仍不清楚.此外, 尽管已经进行了多年的深入研究, 但人们对植物NLR的生化功能仍知之甚少, 有关其自抑制、病原效应子识别和免疫激活模型虽然已得到植物实验结果(
备案号: 京ICP备16067583号-21
版权所有 © 2021 《植物学报》编辑部
地址:北京香山南辛村20号 邮编:100093
电话:010-62836135 010-62836131 E-mail:cbb@ibcas.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
