Role of Post-translational Modification of Proteins in ABA Signaling Transduction
Jing Zhang, Suiwen Hou*Key Laboratory of Cell Activities and Stress Adaptations, Ministry of Education, School of Life Sciences, Lanzhou University, Lanzhou 730000, China通讯作者:
收稿日期:2018-10-18接受日期:2019-01-2网络出版日期:2019-07-01
| 基金资助: |
Corresponding authors:
Received:2018-10-18Accepted:2019-01-2Online:2019-07-01

摘要
关键词:
Abstract
Keywords:
PDF (1731KB)摘要页面多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
张静, 侯岁稳. 蛋白质翻译后修饰在ABA信号转导中的作用. 植物学报, 2019, 54(3): 300-315 doi:10.11983/CBB18217
Zhang Jing, Hou Suiwen.
脱落酸(abscisic acid, ABA)作为经典植物激素之一, 不仅参与植物的生长发育过程, 如种子休眠与萌发、根系统发育、叶片衰老和成花转变, 还在植物逆境响应中起着非常重要的作用(Dong et al., 2015; Vishwakarma et al., 2017; 伍静辉等, 2018)。拟南芥 (Arabidopsis thaliana)细胞通过类胡萝卜素途径以β-胡萝卜素作为前体起始ABA的生物合成, 其早期非特异反应在质体中进行, 由玉米黄质环氧化酶(zeaxanthin epoxidase, ZEP)和9-顺式-环氧类胡萝卜素双加氧酶(9-cis-epoxycarotenoid dioxygenases, NCEDs)等一系列酶催化产生黄氧素(xanthoxin), 然后转移到细胞质中进行一系列ABA合成的特异性反应, 最终由ABA醛氧化酶(abscisic aldehyde oxidases, AAOs)及其辅酶ABA3催化产生有活性的ABA (Finkelstein, 2013)。ABA通过2条代谢途径失活: (1) 通过细胞色素氧化酶(CYP707As)等氧化产生红花菜豆酸(phaseic acid, PA)和二氢红花菜豆酸(dihydrophaseic acid, DPA) (Finkelstein, 2013; Weng et al., 2016); (2)通过葡糖基转移酶将ABA转化成脱落酸葡糖酯(ABA-GE), 该产物在植物逆境响应中起重要作用(Lee et al., 2006; Dong et al., 2015)。ABA转运蛋白(如输出载体ABCG25)将ABA及其代谢物运出细胞, 再将ABA导入维管束进行长距离运输; 或(如输入载体ABCG22/40)将ABA重新载入需要的细胞(如气孔保卫细胞)。这种运输机制是植物响应胁迫的重要方式(Dong et al., 2015; Merilo et al., 2015)。
ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(Umezawa et al., 2010)。无ABA信号时, PP2Cs结合并去磷酸化SnRK2s, 抑制SnRK2s激酶活性, 使SnRK2s不能激活其底物; 当受体RCAR/PYR/PYLs感知到ABA存在时, 便相互结合, 进一步与PP2Cs结合, 形成三元复合体, 抑制PP2Cs的酶活性, 同时使PP2Cs-SnRK2s复合体解离, SnRK2s发生自磷酸化, 随后通过磷酸化激活转录因子或离子通道等下游底物, 诱导ABA响应基因表达或气孔关闭(Fujii et al., 2007; Fujita et al., 2009; Ma et al., 2009; Park et al., 2009; Umezawa et al., 2009)。SnRK2s下游底物包括转录因子(主要是ABRE-binding proteins/ABRE- binding factors, AREBs/ABFs)、钾离子通道蛋白KAT1、阴离子通道蛋白SLAC1 (slow anion channel 1)及其它功能蛋白(TOR激酶、NADPH氧化酶和DNA解旋酶BRAHMA等) (Fujii et al., 2007; Sirichandra et al., 2009; Han et al., 2012; Finkelstein, 2013; Fujita et al., 2013; Dong et al., 2015; Wang et al., 2018b)。
蛋白质翻译后修饰(post-translational modifications, PTMs)能调节蛋白质结构、动态变化和生物学功能等, 是真核细胞生命活动中的重要调节方式, 其中常见的有磷酸化、糖基化、甲基化、酰基化、泛素化和硫酸化等(Jensen, 2006)。植物细胞中, PTMs参与许多重要生理过程。 例如, 蛋白质磷酸化调节植物激素油菜素内酯(brassinosteroids, BRs)信号通路(Belkhadir and Jaillais, 2015); 糖基化调控蛋白质合成以及内质网压力响应(Nagashima et al., 2018); 磷酸化、糖基化和泛素化协同参与植物免疫过程(Withers and Dong, 2017)。本文重点阐述磷酸化、泛素化、类泛素化和氧化还原修饰在ABA信号转导中的作用及其最新研究进展。
1 磷酸化/去磷酸化
蛋白的磷酸化和去磷酸化(phosphorylation/dephosphorylation)过程由蛋白激酶和蛋白磷酸酶分别完成, 是蛋白最主要的翻译后修饰之一(Cohen, 2002; Humphrey et al., 2015)。在植物中, 蛋白的磷酸化修饰调控是ABA信号中非常关键的调节手段。RCAR/PYR/PYLs是最主要的ABA受体, 但有关其磷酸化的研究一直没有进展。直到最近, 朱健康实验室利用磷酸化组学方法研究发现受体PYL4的114位丝氨酸残基(PYL4 Ser114)、PYL1 Ser119和PYL9 Ser94存在磷酸化修饰, 且这些位点的磷酸化都会被外源ABA抑制(Wang et al., 2018b)。PYL4 Ser114和PYL1 Ser119位点的模拟持续磷酸化突变(丝氨酸突变为天冬氨酸, PYL4S114D、PYL1S119D)会抑制受体与ABA或PP2Cs的结合, 失去抑制PP2Cs酶活性的能力; 超表达突变基因PYL1S119D不能恢复突变体pyr1/pyl1/2/4对ABA不敏感的表型。这些证据表明, 磷酸化PYL1/4会抑制受体的活性及功能(Wang et al., 2018b)。进一步研究发现, 在没有ABA或逆境信号条件下, 能量代谢关键组分TOR (target of rapamycin)激酶复合体磷酸化PYL1/4, 使ABA信号被阻断; 当受到逆境胁迫后, SnRK2s被激活并磷酸化TOR的调节亚基RaptorB, 导致TOR的激酶活性被抑制, 使之不能激活能量调节, 从而抑制植物生长及促进植物逆境生存。这些结果表明, TOR激酶复合体和ABA信号相互拮抗调节植物生长与逆境生存(Rosenberger and Chen, 2018; Wang et al., 2018b) (图1)。拟南芥酪蛋白激酶AELs (Arabidopsis EL1-like proteins, AEL1-AEL4)磷酸化PYL1 Ser136和PYR1 Ser109, AELs缺失会降低PYL1/PYR1的泛素化, 导致PYR/PYLs降解变慢, 说明PYL1/PYR1存在一条磷酸化介导的泛素化降解途径(Chen et al., 2018)。有趣的是, 受体不同位点的磷酸化可能起着相反的作用, CARK1 (cytosolic ABA receptor kinase 1)磷酸化PYL8/PYR1的77/78位苏氨酸残基(T77/T78), 导致PYL8/PYR1的稳定性增强, 对PP2Cs的抑制作用加强, 从而促进ABA信号转导(Zhang et al., 2018)。当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(Belin et al., 2006; Umezawa et al., 2009; Ng et al., 2011)。SnRK2s除自磷酸化外, BIN2 (brassinosteroid insensitive 2)、BAK1 (BRI1-associated receptor kinase 1)、HT1 (high leaf temperature 1)和酪蛋白激酶CK2 (casein kinase 2)等多个激酶都能磷酸化SnRK2s (Cai et al., 2014; Tian et al., 2015; Vilela et al., 2015; Shang et al., 2016)。BIN2属于GSK3 (glycogen synthase kinase 3)激酶家族, 调节多种信号过程: 包括抑制BR信号转导、抑制生长素信号转导和调节气孔运动等, 是BR信号通路中的关键组分之一(He et al., 2002; Vert et al., 2008; Kim et al., 2012b; Youn and Kim, 2015)。在ABA信号通路中, BIN2磷酸化激活SnRK2.2/2.3 (Cai et al., 2014), 同时BIN2也磷酸化激活ABI5 (Hu and Yu, 2014)。最新研究表明, 作为ABA通路中2个关键的PP2Cs—— ABI1/2 (ABA-insensitive 1/2), 通过去磷酸化BIN2 抑制BIN2激酶活性(Wang et al., 2018a)。这些结果表明, BIN2是整合BR和ABA两大激素信号通路的关键因子(Wang and Wang, 2018) (图1)。另一个BR信号通路中的激酶BAK1也磷酸化SnRK2.6/OST1 (open stomata 1), 并与ABI1拮抗调节OST1的磷酸化以及气孔运动(Shang et al., 2016)。CK2是一类高度保守的蛋白激酶, 由催化亚基和调节亚基组成异源二聚体或四聚体(Mulekar and Huq, 2014)。玉米(Zea mays) ZmCK2磷酸化ZmOST1碳端结构域中的氨基酸残基, 促进ZmOST1与PP2Cs的结合, 从而抑制OST1的活性和ABA信号转导(Vilela et al., 2015)。HT1是CO2诱导气孔关闭的一个负调控因子, 通过磷酸化抑制OST1的激酶活性, 进而抑制气孔关闭(Tian et al., 2015)。此外, MPKs等激酶和PP2A、PP1等磷酸酶都影响SnRK2s的磷酸化水平与激酶活性, 但是否直接调控SnRK2s的磷酸化还需进一步验证(Saruhashi et al., 2015; Waadt et al., 2015; Hou et al., 2016)。
图1
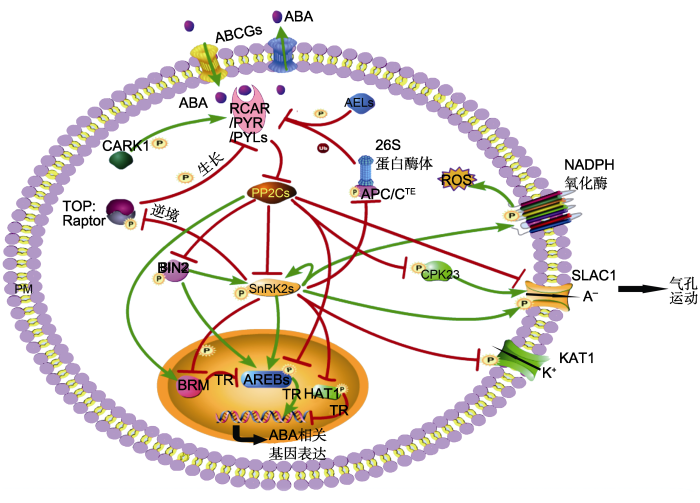 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1磷酸化修饰在ABA信号中的作用
ABA的转运由运输载体如AtABCGs完成, 其转导途径由受体RCAR/PYR/PYLs、磷酸酶PP2Cs、激酶SnRK2s以及SnRK2s的底物等组成。转录因子(AREBs和HAT1)、膜蛋白(SLAC1、KAT1和NADPH氧化酶)、DNA解旋酶BRM、TOR激酶复合体和APC/C泛素复合体被SnRK2s磷酸化, 其中AREBs、BRM和SLAC1被PP2Cs去磷酸化。激酶BIN2是整合BR信号通路和ABA信号通路的关键因子。TOR激酶复合体与ABA信号相互拮抗调节植物生长与逆境响应。钙调激酶CPK23独立于SnRK2s参与气孔运动。激酶CARK1和AEL1磷酸化ABA受体PYR1的不同位点引起相反结果。绿色箭头表示促进作用; 红色T型线表示抑制作用。PM: 细胞膜; P: 磷酸化; Ub: 泛素化; A-: 阴离子; ROS: 活性氧; TR: 转录调控
Figure 1The regulatory roles of protein phosphorylation in core
ABA signaling ABA transport is performed by transporters, such as AtABCGs. The core ABA signaling pathway is composed of RCAR/PYR/PYLs, PP2Cs, SnRK2s, and the substrates of SnRK2s. The substrates of SnRK2s include AREBs, HAT1, SLAC1, KAT1, NADPH oxidases, BRM, TOR complex, and APC/C complex. AREBs, BRM and SLAC1 can be dephosphorylated by PP2Cs. BIN2 is a key factor that integrates BR and ABA signaling pathway. The TOR complex and ABA signaling antagonistically regulates plant growth and stress response. CPK23 is independent of SnRK2s in stomatal movement. Different phosphorylation sites of ABA receptor PYR1 by CARK1 and AEL1 give opposite results. Green arrows represent promotion; Red T-shaped bars represent repression. PM: Plasma membrane; P: Phosphorylation; Ub: Ubiquitination; A-: Negative ions; ROS: Reactive oxygen species; TR: Transcriptional regulation
AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(Fujita et al., 2013)。AREBs/ABFs被SnRK2s磷酸化, 并被ABI1/2直接去磷酸化(Fujii and Zhu, 2009; Antoni et al., 2012; Fujita et al., 2013; Yoshida et al., 2015; Bhatnagar et al., 2017)。大部分转录因子被SnRK2s磷酸化后激活其转录活性, 如水稻(Oryza sativa)中OsbZIP23被SAPK2 (SnRK2s在水稻中的同源蛋白, 参与ABA信号转导)磷酸化后激活其转录活性, 然后促进OsNCED4的表达和ABA的合成(Kim et al., 2012a; Zong et al., 2016)。最近研究表明, ABFs被SnRK2s磷酸化后, 促进ABI1/2的表达, 随后ABI1/2蛋白量增加, 对ABFs的去磷酸化加强, 转而抑制ABFs的转录活性, 从而形成一条精确调控ABA信号及对ABA进行脱敏反应的反馈通路(Wang et al., 2018d)。有部分转录因子被SnRK2s磷酸化后转录活性受到抑制。例如, 正常条件下, 没有磷酸化的HAT1 (homeodomain-leucine zipper protein 1)结合在NCED3的启动子上, 抑制NCED3的表达和ABA合成; 在干旱条件下, HAT1被SnRK2s磷酸化, 其转录抑制活性被抑制, 导致植物体内ABA含量上升, 以响应干旱胁迫(Tan et al., 2018)。除SnRK2s和PP2Cs外, 还有多种激酶或磷酸酶参与调控AREBs/ABFs磷酸化修饰。例如, 苹果(Malus domestica) MdMKK1-MdMPK1级联通路磷酸化MdABI5 (Wang et al., 2010b); BIN2和PSK5 (SOS2-like protein kinase 5)磷酸化激活ABI5 (Hu and Yu, 2014; Zhou et al., 2015); 磷酸酶PP6和PP2A去磷酸化ABI5 (Dai et al., 2013; Hu et al., 2014); 苹果中钙依赖蛋白激酶MdCIPK22磷酸化MdAREB2 (Ma et al., 2017)。
在ABA促进气孔关闭的过程中, SnRK2s磷酸化激活SLAC1, 促进阴离子(A-)外排; ABI1直接去磷酸化SLAC1, 抑制气孔关闭。同时SnRK2s磷酸化抑制KAT1的活性, 阻止钾离子(K+)内流(Geiger et al., 2009; Sato et al., 2009)。SnRK2s还磷酸化bHLH (basic helix-loop-helix)类转录因子AKS1 (ABA-re-
sponsive kinase substrate 1), 促使AKS1解聚成单体形式, 失去结合靶基因KAT1的能力, 从而抑制KAT1的表达(Takahashi et al., 2016, 2017b)。除此通路外, ABI1还通过去磷酸化钙蛋白激酶CPK23, 抑制CPK23对SLAC1的磷酸化, 形成一条独立于SnRK2s的调节气孔运动的通路(Geiger et al., 2010) (图1); CPK6也磷酸化SLAC1, 部分取代OST1的功能(Brandt et al., 2012)。激酶GHR1 (guard cell hydrogen peroxide-resistant 1)磷酸化激活SLAC1, 参与ABA调节的气孔关闭, 该过程被ABI2抑制, 但不被ABI1抑制(Hua et al., 2012)。另外, 在CO2诱导的气孔关闭过程中, HT1不仅抑制OST1, 还通过磷酸化抑制GHR1和SLAC1。而CO2能促进MPK4/MPK12磷酸化抑制HT1的激酶活性(Tian et al., 2015; H?rak et al., 2016)。
SnRK2s还磷酸化其它的功能蛋白, 使植物感应ABA信号后出现多种生理变化。 例如, 磷酸化NADPH氧化酶AtrbohF, 促进ABA诱发的活性氧爆发(Sirichandra et al., 2009); 磷酸化TOR激酶的调节亚基RaptorB, 抑制植物生长(Wang et al., 2018b)。 BRM (BRAHMA)是SWI/SNF染色体重组复合体中的关键组分, 结合ABI5的基因序列并抑制ABI5表达。SnRK2s (或ABI1)对BRM的磷酸化(或去磷酸化)抑制(或稳定) BRM与ABI5基因的结合(Han et al., 2012; Peirats-Llobet et al., 2016)。
2 泛素化
泛素是真核生物中高度保守的一类小肽, 由76个氨基酸残基组成, 它通过共价连接的方式, 即泛素化(ubiquitination)修饰蛋白的赖氨酸残基。泛素化过程通常经一系列连续的催化反应, 由E1泛素激活酶、E2泛素结合酶和E3泛素连接酶将泛素连接到靶蛋白(Yu et al., 2016b)。在拟南芥中, E1和E2两种酶类编码基因较少, E3连接酶的编码基因约有1 500个, 根据E3结构和与E2互作的特异性, 可将E3连接酶分为4大类: HECT (homology to E6-AP C-terminus)类、RING (really interesting new gene)类、U-box类以及CRL (cullin-ring)类(Yu et al., 2016b; Miricescu et al., 2018)。泛素化修饰根据连接泛素的数量和方式可分为单泛素化、多泛素化和多聚泛素化。单泛素化或多泛素化主要起修饰蛋白功能和调节蛋白定位等作用; 多聚泛素化通常偶联蛋白酶体(ubiquitin/26S proteasome, Ub/26S体系)进行蛋白质的选择性降解(Miricescu et al., 2018)。目前发现的与ABA信号转导有关的泛素化修饰几乎都是多聚泛素化。最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(Kurup et al., 2000; Zhang et al., 2005)。KEG (keep on going)是一个重要的调控ABA信号转导的RING类E3, 定位在细胞质中的反面内质网和早期内体(trans-Golgi network/early endosome, TGN/EE)上, 将核定位的ABI5、ABF1和ABF3招募到TGN/EE进行降解, 同时KEG泛素化ABI5的激酶CIPK26 (Stone et al., 2006; Chen et al., 2013b; Lyzenga et al., 2013)。当植物感知ABA后, 会促进KEG的自泛素化和降解, 从而减少由KEG介导的ABI5降解, 促进ABA信号响应(Liu and Stone, 2010)。当CIPK26被激活后磷酸化KEG, 随后也会增加KEG的自泛素化和降解(Lyzenga et al., 2017)。从这些结果可以推测, 当ABA或逆境胁迫激活CIPK26后, CIPK26磷酸化KEG和ABI5, 激活ABI5的转录活性, 促进KEG自泛素化和降解, 进一步导致KEG介导的CIPK26和ABI5降解减少, 从而增强植物对ABA的响应(图2)。RSL1 (single-subunit ring-type E3 ubiquitin ligase)泛素化并促进降解PYL4和PYR1 (Bueso et al., 2014)。ABA促进RGLG1/5 (ring domain ligase 1/5)对PP2CA的泛素化(Wu et al., 2016); 辣椒(Capsicum annuum)中RING类E3泛素酶CaAIRF1降解CaADIP1 (一个ABA信号途径中的PP2C) (Lim et al., 2017); AtAIRP3 (ABA-insensitive ring protein 3)通过促进RD21 (responsive to dehydration 21)的降解来调节植物对ABA介导的干旱胁迫响应(Kim and Kim, 2013)。玉米中的RING类E3连接酶ZmXERICO1泛素化调控ZmABA8ox3a (拟南芥CYP707A的同源蛋白)的蛋白稳定性, 从而调节干旱胁迫下植株体内ABA的平衡, 而其在拟南芥中的同源蛋白XERICO也参与ABA的合成(Ko et al., 2006; Brugière et al., 2017)。
图2
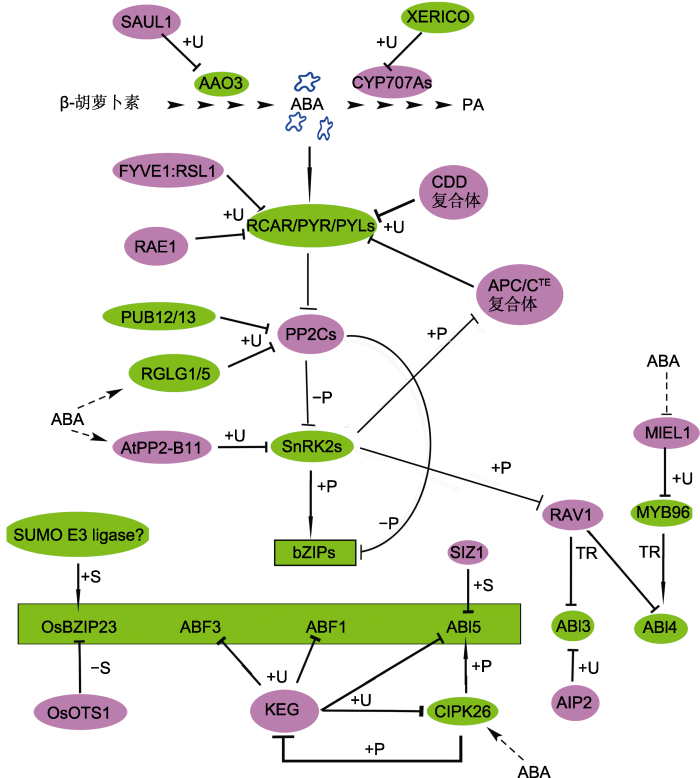 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2蛋白泛素化和SUMO化修饰调节ABA合成代谢、识别、转导和响应
AAO3和CYP707A分别是ABA的合成酶和氧化酶。ABA中心转导途径由RCAR/PYR/PYLs-PP2Cs-SnRK2s-bZIPs组成。图中bZIPs代表OsBZIP23、ABF3、ABF1和ABI5 (绿色背景方框内)。红色背景中蛋白是ABA信号的负调节因子; 绿色背景中蛋白是ABA信号的正调节因子。箭头表示促进作用; T型线表示抑制作用; 实线表示有直接的互作关系; 虚线和?表示具体过程未知。+/-P: 磷酸化/去磷酸化; +U: 泛素化; +/-S: SUMO/去SUMO化; TR: 转录调控
Figure 2Protein ubiquitination and sumoylation regulate ABA biosynthesis and catabolism, ABA perception, signal transduction and responses
AAO3 and CYP707A are ABA synthase and oxidase enzyme, respectively. The core ABA signaling pathway is composed of RCAR/PYR/PYLs-PP2Cs-SnRK2s-bZIPs. In this figure, bZIPs represent OsBZIP23, ABF3, ABF1 and ABI5 (inside the box on the green background). The proteins in the red (green) background are negative (positive) factors in ABA signaling. Arrows represent promotion; T-shaped bars represent repression; The solid line indicates direct interaction; Dotted lines and ? represent uncon?rmed. +/-P: Phosphorylation/dephosphorylation; +U: Ubiquitination; +/-S: SUMOylation/de-SUMOylation; TR: Transcriptional regulation
CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(Hua and Vierstra, 2011)。BPMs (Meprin and TRAF homology/Brica- brac-tramtrak-broad complex, MATH/BTB)是与CUL3相连的接头蛋白, 通过泛素化促进ABA信号的负调节转录因子ATHB6降解(Himmelbach et al., 2002; Lechner et al., 2011)。随后又发现BPM与另一个转录因子RAV1 (related to ABI3/VP1)互作; 而RAV1被SnRK2s磷酸化后会失去对ABI3/4/5的转录抑制能力(Chen et al., 2013a; Feng et al., 2014)。DWA1/2 (DWD hypersensitive to ABA 1/2)是2个同源的CUL4-DDB1 (damaged DNA binding1)-DWD (DDB1 binding WD40)复合体的接头蛋白, 与ABI5互作, 调节ABI5的积累和ABA响应(Lee et al., 2010)。另一个CUL4-DDB1复合体的接头蛋白ABD1 (ABA- hypersensitive DCAF1)也通过泛素化调节ABI5的蛋白积累。abd1突变体与dwa1-1/dwa2-1双突变体表型相似, 在种子萌发和幼苗生长阶段都对外源ABA超敏感(Seo et al., 2014)。由DDB1、COP10 (constitutive photomorphogenic 10)、DET1 (deetiolated 1)、DDA1 (DDB1-associated 1)和CUL4组成的CDD复合体通过Ub/26S体系降解PYL8, 参与ABA响应(Irigoyen et al., 2014)。最新报道显示CUL4-DDB1-RAE1 (RNA export factor 1 in Arabidopsis)复合体可降解PLY9, 从而参与植物对ABA和干旱胁迫的响应(Li et al., 2018)。AtPP2-B11是一个F-box蛋白, 与CUL1和SKP1 (S-phase kinase-associated protein 1)组成SCF (SKP1-Cul1-F-box ligases)泛素复合体, 利用Ub/26S体系促进SnRK2.3的降解, 但不会影响SnRK2.2/2.6的稳定性, 说明AtPP2-B11特异性地招募底物SnRK2.3 (Cheng et al., 2017)。RIFP1 (RCAR3 interacting F-box protein 1)与ASKs (Arabidopsis SKP1-like proteins)组成SCF复合体, 通过泛素化RCAR3/PYL8, 促进PYL8的降解, 抑制ABA信号转导(Li et al., 2016)。TE (tiller enhancer)是水稻APC/CTE (anaphase promoting complex/cyclosome)泛素复合体中的激活子, 参与调控水稻株型发育(Lin et al., 2012)。在ABA信号途径中, SAPKs被ABA激活后磷酸化TE, 抑制APC/CTE复合体的泛素化活性, 导致APC/CTE复合体不能泛素化降解OsPYL/RCAR10, 进一步增强ABA响应(Lin et al., 2015)。CSN (COP9 signalosome)复合体可解除CULLIN蛋白的NEDD修饰(de-neddylation), 调节CRL复合体的活性, CSN5A促进ABI5的降解, 参与调节种子休眠和萌发, 但具体机制还不清楚(Lyapina et al., 2001; Jin et al., 2018)。
SAUL1 (senescence-associated E3 ubiquitin ligase 1)是U-box类E3连接酶, 通过Ub/26S体系降解AAO3, 参与调节叶片衰老和ABA合成(Raab et al., 2009)。多个PUBs (plant U-box E3 ligases)直接调控ABA中心组分的降解。PUB22/23泛素化PYL9, 并与多个PYLs互作(Kong et al., 2015)。ABA促进PUB12/13对ABI1的泛素化及降解(Zhao et al., 2017)。AFPs (ABI5-binding proteins)与ABI5和ABFs互作, 促进ABI5与E3泛素连接酶互作及降解(Lopez- Molina et al., 2003; Garcia et al., 2008)。在水稻中, AFPs的同源蛋白MODD (mediator of OsbZIP46 deactivation and degradation)与ABI5的同源蛋白OsbZIP46互作, 增强E3连接酶OsPUB70对OsbZIP46的泛素化修饰, 促进OsbZIP46降解, 抑制ABA或逆境信号过度响应(Tang et al., 2016)。
此外, RING类泛素酶SDIR1、U-box类泛素酶CHIP、PUB18多种泛素连接酶参与调控ABA响应, 但其底物并非ABA中心转导途径组分(Luo et al., 2006; Zhang et al., 2015a; Seo et al., 2016; Yu et al., 2016b)。例如, RING类E3连接酶MIEL1 (MYB30- interacting E3 ligase 1)降解MYB类转录因子MYB96和MYB30, 该过程被ABA抑制。而ABA激活MYB96, 促进其靶基因ABI4的表达(Lee et al., 2015; Lee and Seo, 2016) (图2)。Exo70B1是泡外复合体(exocyst complex)的一个亚基, 参与细胞的胞吞、胞吐和囊泡运输等过程, 是ABA信号中的正调节因子, PUB18通过促进Exo70B1的降解参与调控ABA和逆境胁迫响应(Kulich et al., 2013; Seo et al., 2016)。
泛素化后的降解途径还有几条不依赖于26S蛋白酶体, 称之为非26S蛋白酶体内膜转运体系, 包括胞内体转运(endosomal traf?cking pathway)和自噬泡途径。这里简要介绍一下这类降解途径在ABA信号中的作用, 详情可以参阅文献(Yu and Xie, 2017)。RSL1和PYL4共定位在细胞质膜和TGN/EE上, 当用囊泡运输抑制剂BFA处理后, PYL4在微粒体中的积累增加, 表明被RSL1泛素化的PYL4不仅通过Ub/26S体系降解, 还可能存在其它降解途径(Bueso et al., 2014)。ESCRTs (endosomal sorting complex required for transport proteins)是胞内体转运途径中关键的复合体, 其组成成分包括FYVE1和VPS23A (Zhuang et al., 2015)。FYVE1和VPS23A都能与PYL4在胞内体上互作, 在fyve1和vps23a突变体中可观察到被多聚泛素化标记的PYL4在内吞泡中积累, 说明ESCRT-1复合体通过筛选和运输被RSL1泛素化的PYL4到胞内体转运途径进行降解(Belda-Palazon et al., 2016; Yu et al., 2016a)。ABCG25是ABA的输出载体, 其在质膜上的定位受到ABA和逆境胁迫的调节。在逆境胁迫下, ABCG25通过胞吞作用从质膜转运到胞内体; 外源ABA处理后, ABCG25从胞内体转移到质膜, 该循环过程依赖于网格蛋白和AP-2 (adaptor protein complex-2)复合体, 其中ABCG25蛋白积累水平的变化可能依于液泡降解途径(Kuro-mori et al., 2016)。
3 类泛素化
SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成。SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等。蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(Castro et al., 2012; Augustine and Vierstra, 2018; 韩丹璐等, 2018)。拟南芥AtSIZ1是较早报道的参与ABA信号和植物逆境响应的SUMO E3连接酶。生化实验证明, SIZ1能SUMO化ABI5的391位赖氨酸(K391)残基, 增加ABI5的蛋白稳定性。从生化结果推测, siz1突变体对ABA的响应与abi5突变体相似, 但siz1对ABA敏感性增强, 应该与abi5-4的表型相反(Catala et al., 2007; Miura et al., 2009)。由于生化结果不符合生理表型, 因此推测被ABI5的SUMO化不仅影响它的泛素化, 还可能影响ABI5的其它修饰(如磷酸化), 导致ABI5不能被激活, 所以SIZ1最终抑制ABI5响应ABA的能力(Miura et al., 2009; Yu et al., 2015)。MYB30是ABA响应的负调节因子, 与ABI5协同调节ABA信号。SIZ1还能SUMO修饰MYB30, 增强MYB30的稳定性(Zheng et al., 2012)。OsOTS1 (overly tolerant to salt 1)是水稻中的SUMO蛋白酶, 在响应ABA和干旱胁迫过程中催化靶蛋白OsbZIP23去SUMO修饰。ots1突变体的干旱耐受性增强, 植株体内OsbZIP23的SUMO修饰增强, 蛋白积累增加。上述结果表明, OsbZIP23的SUMO修饰有利于增强蛋白的稳定性和转录活性(Srivastava et al., 2016, 2017)。此外, 还有多个SUMO修饰相关酶类参与ABA信号, 但其具体机制尚不清楚, 如SUMO E2结合酶AtSCE1a, E3连接酶ASP1和MMS21, SIZ1在水稻、番茄(Solanum lycopersicum)、石斛兰(Dendrobium)和苹果等植物中的同源蛋白, 以及去SUMO修饰酶ULP1c (Lois et al., 2003; Park et al., 2010; Zhang et al., 2013, 2016, 2017; Liu et al., 2015; Castro et al., 2016; Wang et al., 2018c)。4 氧化还原修饰
ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(Kim et al., 2010; Finkelstein, 2013; Qi et al., 2018)。过氧化氢(H2O2)等活性氧分子和一氧化氮(NO)等活性氮分子也是蛋白调节子, 通过调节蛋白的半胱氨酸残基(Cys)氧化还原状态, 即氧化还原修饰(redox)参与多种生理过程。例如, 植物SnRK1激酶复合体活性受到H2O2或还原性谷胱甘肽(GSH)的调节(Wurzinger et al., 2017); 被氧化或过度磷酸化的MPK4会发生聚集和失活(Zhang et al., 2015b); 被H2O2激活的MPK6磷酸化硝酸还原酶NIA2促进NO合成(Wang et al., 2010a)。在ABA信号通路中, 谷胱甘肽过氧化物酶AtGPX3 (glutathione peroxidase 3)调节H2O2的内平衡, 同时H2O2氧化AtGPX3, 而氧化态AtGPX3促进ABI2从还原态转变为氧化态, 从而抑制ABI2的酶活性(Miao et al., 2006)。最新研究发现, 欧洲油菜(Brassica napus)中的OST1同源蛋白BnSnRK2.6-2C的半胱氨酸残基被氧化成亚磺酸或磺酸, 导致其自磷酸化活性被抑制(Ma et al., 2018)。NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用。半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(Vandelle and Delledonne, 2011; Mur et al., 2013; 王宇和何奕騉, 2017; Begara-Morales et al., 2018)。在ABA信号中, NO是负调节因子(Lozano-Juste and León, 2010; Arc et al., 2013)。受ABA诱导的活性氮硝基化多个ABA受体PYR/PYL/RCARs, 而被硝化的受体再被多聚泛素化后降解(Castillo et al., 2015)。NO的供体GSNO和亚硝基半胱氨酸Cys-NO以一种剂量依赖性的方式亚硝基化OST1的Cys137, 并抑制OST1的激酶活性。ABA促进OST1亚硝基化(Wang et al., 2015)。ABI5的表达受ABA和NO的清除剂cPTIO强烈诱导, 被NO的供体SNAP抑制。同时GSNO和SNAP促进ABI5通过26S蛋白酶体途径的降解。ABI5的Cys153被亚硝基化, 导致其被KEG和CUL4泛素复合体偶联的蛋白酶体降解(Albertos et al., 2015)。由以上研究结果可以推测, ABA诱导的活性氮促进受体的硝基化, 增加OST1和ABI5的亚硝基化, 从而抑制这些蛋白的活性和ABA信号的过度响应, 由此形成一种反馈调节机制, 精确地调节ABA信号。
5 总结与展望
ABA信号转导过程是一个复杂的协同作用的交互网络, 除上文详细阐述的关键组分外, 还有多种重要组分参与ABA信号转导和响应, 包括由质体定位的受体CHLH (H subunit of the Mg2+ Cheletase)或质膜定位的受体GTG1 (G-protein coupled receptor-type G-proteins)介导的2条不依赖于受体PYR/PYL/RCARs的ABA信号转导途径(Shen et al., 2006; Pandey et al., 2009); 由钙离子依赖蛋白激酶CDPKs、MPKs和SnRK2s等激酶组成的磷酸化调控网络(Umezawa et al., 2014); 由ABF/AREB、DREB (dehydration-responsive element binding protein)、NAC、WRKY和MYB/MYC等转录因子组成的转录调控网络(Fujita et al., 2011; Singh and Laxmi, 2015)。这些组分之间通过相互作用来调控ABA信号。例如, ABF4的转录活性依赖于与钙依赖蛋白激酶AtCPK32的互作(Choi et al., 2005); 棉花(Gossypium hirsutum)响应干旱过程中GhMAP3K15-GhMKK4-GhMPK6级联通路磷酸化GhWRKY59 (Li et al., 2017); RGLG1/2泛素化转录因子AtERF53负调节干旱响应(Cheng et al., 2012); 在ABA诱导气孔关闭过程中, CHLH被磷酸化, 并与SnRK2.6/OST1存在互作(Liang et al., 2015)。
随着技术的进步, 目前已经鉴定到200多种不同类型的PTMs, 已报道多种PTMs参与ABA信号途径。例如, 半胱氨酸残基的酰化(Batisti? et al., 2012)及法尼基修饰(Brady et al., 2003; Huizinga et al., 2010), 氨基酸残基的乙酰化修饰(Linster et al., 2015)。但这些修饰参与调控ABA信号的分子机制并不清楚。虽然磷酸化和泛素化在ABA信号转导中的作用研究得比较深入, 但也存在诸多问题和挑战。例如, 泛素化和SUMO修饰都是可逆的过程, 但去泛素酶或去SUMO酶的报道都很少; 对ABA信号转导的研究主要集中在中心转导途径, 对其它途径尤其是这些组分的翻译后修饰研究较少, 也需要深入探索。多种修饰会作用于同一个靶蛋白, 那么这些修饰之间如何协同作用? 这些修饰是否与蛋白的定位改变或响应不同的环境信号有关? ABI5的几种修饰之间如何协同作用? 根据已有文献的报道结果可以假设: 没有ABA或逆境刺激时, ABI5被SUMO修饰, 处于稳定的非活性状态, 而当ABA或逆境激活其激酶后, ABI5被迅速去SUMO化, 同时被磷酸化, 导致其转录活性被激活, 从而促进ABA有关基因表达, 使植物适应逆境; 当逆境信号消失后, ABI5被泛素化随后转运到TGN/EE被降解, 使植物对ABA脱敏感, 转为生长状态。一些蛋白的同一种修饰会引起相反的效应, 如ABA受体PYR1的2个磷酸化位点(T78和S136), 那么植物如何感知不同位点修饰引起的效应? 靶蛋白不同位点的修饰是否会引起蛋白构象的不同变化, 从而引起不同的效应? 通过分析靶蛋白在响应ABA过程中的翻译后修饰变化, 发现植物可以快速精准地启动或抑制ABA信号, 从而适应逆境或迅速恢复生长, 但这些过程是否需要其它因子的帮助, 如磷脂肌醇信号(Takahashi et al., 2017a), 还有待探明。回答上述问题可以让我们更全面地理解多种蛋白质翻译后修饰之间的关系, 更深入地揭示植物响应ABA或逆境胁迫的机制, 以及更清晰地认识植物生长与逆境生存之间的微妙平衡。
(责任编辑: 白羽红)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URL [本文引用: 1]

SUMO化是真核生物中一种重要的蛋白质翻译后修饰。SUMO E3连接酶具有对底物特异的识别功能,可以促进SUMO化反应,是SUMO化修饰过程中的重要组成部分。目前,在植物中已经鉴定出多种SUMO E3连接酶,它们参与植物重要器官的发育调控。该文对植物SUMO E3连接酶在根系发育、开花途径、配子发育和光形态建成中的作用及其调节机制进行综述。
DOI:10.11983/CBB17177URL [本文引用: 1]

一氧化氮(NO)作为一种具有活性的小分子物质参与众多动植物生理活动。在蛋白转录后修饰方面,NO主要以S-亚硝基化(S-nitrosylation)的形式参与。而甲基化作为另一种蛋白翻译后修饰,在DNA损伤及m RNA翻译方面具有重要作用。虽然近年来有关这2种蛋白翻译后修饰方面的研究成果较多,但是2种途径之间是否存在相互作用却报道较少。近期,我国科学家发现NO可以通过S-亚硝基化修饰PRMT5的第125位半胱氨酸,正向调节该精氨酸甲基转移酶活性。prmt5-1突变体表现出严重的发育障碍且对非生物胁迫敏感。通过互补第125位半胱氨酸点突变PRMT5基因,使之转化为不可被S-亚硝基化修饰的氨基酸后,拟南芥(Arabidopsis thaliana)植株可恢复突变体的发育障碍,但无法恢复其非生物胁迫敏感表型。实验同时证明,PRMT5蛋白第125位半胱氨酸的S-亚硝基化修饰参与调节NaCl诱导的精氨酸对二甲基化。该研究引领了蛋白S-亚硝基化和蛋白甲基化修饰新方向,开辟了新的研究领域,同时为相关研究树立了新的榜样。
DOI:10.11983/CBB18080URL [本文引用: 1]

脱落酸(ABA)是调控种子休眠和萌发过程的主要植物激素。种子内源ABA含量和种胚对ABA敏感性共同调控种子休眠和萌发过程,确保植物种子以休眠状态在逆境中保持其自身繁衍能力,并在适宜的环境下启动萌发程序。种子ABA合成代谢和ABA信号转导途径涉及许多重要基因家族,它们通过复杂的调控网络精确地控制着种胚发生、种子成熟、休眠及萌发进程。该文对ABA调控种子休眠和萌发的分子机制最新研究进展进行综述,并展望了今后的研究方向。
DOI:10.1038/ncomms9669URLPMID:26493030 [本文引用: 1]

Plant survival depends on seed germination and progression through post-germinative developmental checkpoints. These processes are controlled by the stress phytohormone abscisic acid (ABA). ABA regulates the basic leucine zipper transcriptional factor ABI5, a central hub of growth repression, while the reactive nitrogen molecule nitric oxide (NO) counteracts ABA during seed germination. However, the molecular mechanisms by which seeds sense more favourable conditions and start germinating have remained elusive. Here we show that ABI5 promotes growth via NO, and that ABI5 accumulation is altered in genetic backgrounds with impaired NO homeostasis.S-nitrosylation of ABI5 at cysteine-153 facilitates its degradation through CULLIN4-based and KEEP ON GOING E3 ligases, and promotes seed germination. Conversely, mutation of ABI5 at cysteine-153 deregulates protein stability and inhibition of seed germination by NO depletion. These findings suggest an inverse molecular link between NO and ABA hormone signalling through distinct posttranslational modifications of ABI5 during early seedling development. Nitric oxide counteracts the inhibitory effects of the plant hormone ABA during seed germination and seedling growth. Here, Albertoset al. show that nitric oxide can act antagonistically to ABA by inducing theS-nitrosylation and degradation of the ABI5 transcription factor involved in ABA signalling.
DOI:10.1104/pp.111.188623URLPMID:22198272 [本文引用: 1]

Clade A protein phosphatases type 2C (PP2Cs) are negative regulators of abscisic acid (ABA) signaling that are inhibited in an ABA-dependent manner by PYRABACTIN RESISTANCE1 (PYR1)/PYR1-LIKE (PYL)/REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR) intracellular receptors. We provide genetic evidence that a previously uncharacterized member of this PP2C family in Arabidopsis (Arabidopsis thaliana), At5g59220, is a negative regulator of osmotic stress and ABA signaling and that this function was only apparent when double loss-of-function mutants with pp2ca-1/ahg3 were generated. At5g59220-green fluorescent protein and its close relative PP2CA-green fluorescent protein showed a predominant nuclear localization; however, hemagglutinin-tagged versions were also localized to cytosol and microsomal pellets. At5g59220 was selectively inhibited by some PYR/PYL ABA receptors, and close relatives of this PP2C, such as PP2CA/ABA-HYPERSENSITIVE GERMINATION3 (AHG3) and AHG1, showed a contrasting sensitivity to PYR/PYL inhibition. Interestingly, AHG1 was resistant to inhibition by the PYR/PYL receptors tested, which suggests that this seed-specific phosphatase is still able to regulate ABA signaling in the presence of ABA and PYR/PYL receptors and therefore to control the highly active ABA signaling pathway that operates during seed development. Moreover, the differential sensitivity of the phosphatases At5g59220 and PP2CA to inhibition by ABA receptors reveals a functional specialization of PYR/PYL ABA receptors to preferentially inhibit certain PP2Cs.
DOI:10.3389/fpls.2013.00063URLPMID:23531630 [本文引用: 1]

Abstract Dormancy is an adaptive trait that enables seed germination to coincide with favorable environmental conditions. It has been clearly demonstrated that dormancy is induced by abscisic acid (ABA) during seed development on the mother plant. After seed dispersal, germination is preceded by a decline in ABA in imbibed seeds, which results from ABA catabolism through 8'-hydroxylation. The hormonal balance between ABA and gibberellins (GAs) has been shown to act as an integrator of environmental cues to maintain dormancy or activate germination. The interplay of ABA with other endogenous signals is however less documented. In numerous species, ethylene counteracts ABA signaling pathways and induces germination. In Brassicaceae seeds, ethylene prevents the inhibitory effects of ABA on endosperm cap weakening, thereby facilitating endosperm rupture and radicle emergence. Moreover, enhanced seed dormancy in Arabidopsis ethylene-insensitive mutants results from greater ABA sensitivity. Conversely, ABA limits ethylene action by down-regulating its biosynthesis. Nitric oxide (NO) has been proposed as a common actor in the ABA and ethylene crosstalk in seed. Indeed, convergent evidence indicates that NO is produced rapidly after seed imbibition and promotes germination by inducing the expression of the ABA 8'-hydroxylase gene, CYP707A2, and stimulating ethylene production. The role of NO and other nitrogen-containing compounds, such as nitrate, in seed dormancy breakage and germination stimulation has been reported in several species. This review will describe our current knowledge of ABA crosstalk with ethylene and NO, both volatile compounds that have been shown to counteract ABA action in seeds and to improve dormancy release and germination.
DOI:10.1016/j.pbi.2018.06.006URLPMID:30014889 [本文引用: 1]

The small ubiquitin-like modifier (SUMO) pathway in eukaryotes is an essential post-translational modification required for a variety of cellular processes, development and organelle biogenesis. SUMO-conjugating enzyme (Ubc9) is an important conjunction enzyme in the SUMO pathway. SUMO-1 and Ubc9 have been found in vertebrates; however, their expression in crustaceans was poorly characterized.... [Show full abstract]
DOI:10.1038/cr.2012.71URLPMID:22547024 [本文引用: 1]

Calcineurin B-like (CBL) proteins contribute to decoding calcium signals by interacting with CBL-interacting protein kinases (CIPKs). Currently, there is still very little information about the function and specific targeting mechanisms of CBL proteins that are localized at the vacuolar membrane. In this study, we focus on CBL2, an abundant vacuolar membrane-localized calcium sensor of unknown function from Arabidopsis thaliana. We show that vacuolar targeting of CBL2 is specifically brought about by S-acylation of three cysteine residues in its N-terminus and that CBL2 S-acylation and targeting occur by a Brefeldin A-insensitive pathway. Loss of CBL2 function renders plants hypersensitive to the phytohormone abscisic acid (ABA) during seed germination and only fully S-acylated and properly vacuolar-targeted CBL2 proteins can complement this mutant phenotype. These findings define an S-acylation-dependent vacuolar membrane targeting pathway for proteins and uncover a crucial role of vacuolar calcium sensors in ABA responses.
DOI:10.1093/jxb/ery072URL [本文引用: 1]
DOI:10.1105/tpc.16.00178URLPMID:27495812 [本文引用: 1]

Abstract Recently, we described the ubiquitylation of PYL4 and PYR1 by the RING E3 ubiquitin ligase RSL1 at the plasma membrane of Arabidopsis thaliana. This suggested that ubiquitylated ABA receptors might be targeted to the vacuolar degradation pathway because such ubiquitylation is usually an internalization signal for the endocytic route. Here, we show that FYVE1 (previously termed FREE1), a recently described component of the endosomal sorting complex required for transport (ESCRT) machinery, interacted with RSL1-receptor complexes and recruited PYL4 to endosomal compartments. Although the ESCRT pathway has been assumed to be reserved for integral membrane proteins, we show the involvement of this pathway in the degradation of ABA receptors, which can be associated with membranes but are not integral membrane proteins. Knock-down fyve1 alleles are hypersensitive to ABA, illustrating the biological relevance of the ESCRT pathway for the modulation of ABA signaling. In addition, fyve1 mutants are impaired in the targeting of ABA receptors for vacuolar degradation, leading to increased accumulation of PYL4 and an enhanced response to ABA. Pharmacological and genetic approaches revealed a dynamic turnover of ABA receptors from the plasma membrane to the endosomal/vacuolar degradation pathway, which was mediated by FYVE1 and was dependent on RSL1. This process involves clathrin-mediated endocytosis and trafficking of PYL4 through the ESCRT pathway, which helps to regulate the turnover of ABA receptors and attenuate ABA signaling. 2016 American Society of Plant Biologists. All rights reserved.
DOI:10.1104/pp.106.079327URLPMID:16766677 [本文引用: 1]

The phytohormone abscisic acid (ABA) mediates drought responses in plants and, in particular, triggers stomatal closure. Snf1-related kinase 2 (SnRK2) proteins from several plant species have been implicated in ABA-signaling pathways. In Arabidopsis (Arabidopsis thaliana) guard cells, OPEN STOMATA 1 (OST1)/SRK2E/SnRK2-6 is a critical positive regulator of ABA signal transduction. A better understanding of the mechanisms responsible for SnRK2 protein kinase activation is thus a major goal toward understanding ABA signal transduction. Here, we report successful purification of OST1 produced in Escherichia coli: The protein is active and autophosphorylates. Using mass spectrometry, we identified five target residues of autophosphorylation in recombinant OST1. Sequence analysis delineates two conserved boxes located in the carboxy-terminal moiety of OST1 after the catalytic domain: the SnRK2-specific box (glutamine-303 to proline-318) and the ABA-specific box (leucine-333 to methionine-362). Site-directed mutagenesis and serial deletions reveal that serine (Ser)-175 in the activation loop and the SnRK2-specific box are critical for the activity of recombinant OST1 kinase. Targeted expression of variants of OST1 kinase in guard cells uncovered additional features that are critical for OST1 function in ABA signaling, although not required for OST1 kinase activity: Ser-7, Ser-18, and Ser-29 and the ABA-specific box. Ser-7, Ser-18, Ser-29, and Ser-43 represent putative targets for regulatory phosphorylation and the ABA-specific box may be a target for the binding of signaling partners in guard cells.
DOI:10.1111/nph.13269URLPMID:25615890 [本文引用: 1]

Abstract Because they are tethered in space, plants have to make the most of their local growth environment. In order to grow in an ever-changing environment, plants constantly remodel their shapes. This adaptive attribute requires the orchestration of complex environmental signals at the cellular and organismal levels. A battery of small molecules, classically known as phytohormones, allows plants to change their body plan by using highly integrated signaling networks and transcriptional cascades. Amongst these hormones, brassinosteroids (BRs), the polyhydroxylated steroid of plants, influence plant responsiveness to the local environment and exquisitely promote, or interfere with, many aspects of plant development. The molecular circuits that wire steroid signals at the cell surface to the promoters of thousands of genes in the nucleus have been defined in the past decade. This review recapitulates how the transduction of BR signals impacts the temporally unfolding programs of plant growth. First, we summarize the paradigmatic BR signaling pathway acting primarily in cellular expansion. Secondly, we describe the current wiring diagram and the temporal dynamics of the BR signal transduction network. And finally we provide an overview of how key players in BR signaling act as molecular gates to transduce BR signals onto other signaling pathways. 2015 The Authors. New Phytologist 2015 New Phytologist Trust.
DOI:10.1007/s11103-016-0568-2URLPMID:28000033 [本文引用: 1]

Protein phosphatase 2C clade A members are major signaling components in the ABA-dependent signaling cascade that regulates seed germination. To elucidate the role of PP2CA genes in germination of rice seed, we selected OsPP2C51 , which shows highly specific expression in the embryo compared with other protein phosphatases based on microarray data. GUS histochemical assay confirmed that OsPP2C51 is expressed in the seed embryo and that this expression pattern is unique compared with those of other OsPP2CA genes. Data obtained from germination assays and alpha-amylase assays of OsPP2C51 knockout and overexpression lines suggest that OsPP2C51 positively regulates seed germination in rice. The expression of alpha-amylase synthesizing genes was high in OsPP2C51 overexpressing plants, suggesting that elevated levels of OsPP2C51 might enhance gene expression related to higher rates of seed germination. Analysis of protein interactions between ABA signaling components showed that OsPP2C51 interacts with OsPYL/RCAR5 in an ABA-dependent manner. Furthermore, interactions were observed between OsPP2C51 and SAPK2, and between OsPP2C51 and OsbZIP10 and we found out that OsPP2C51 can dephosphorylates OsbZIP10. These findings suggest the existence of a new branch in ABA signaling pathway consisting of OsPYL/RCAR-OsPP2C-bZIP apart from the previously reported OsPYL/RCAR-OsPP2C-SAPK-bZIP. Overall, our result suggests that OsPP2C51 is a positive regulator of seed germination by directly suppressing active phosphorylated OsbZIP10 .
[本文引用: 1]
DOI:10.1073/pnas.1116590109URLPMID:22689970 [本文引用: 1]

The plant hormone abscisic acid (ABA) is produced in response to abiotic stresses and mediates stomatal closure in response to drought via recently identified ABA receptors (pyrabactin resistance/regulatory component of ABA receptor; PYR/RCAR). SLAC1 encodes a central guard cell S-type anion channel that mediates ABA-induced stomatal closure. Coexpression of the calcium-dependent protein kinase 21 (CPK21), CPK23, or the Open Stomata 1 kinase (OST1) activates SLAC1 anion currents. However, reconstitution of ABA activation of any plant ion channel has not yet been attained. Whether the known core ABA signaling components are sufficient for ABA activation of SLAC1 anion channels or whether additional components are required remains unknown. The Ca(2+)-dependent protein kinase CPK6 is known to function in vivo in ABA-induced stomatal closure. Here we show that CPK6 robustly activates SLAC1-mediated currents and phosphorylates the SLAC1 N terminus. A phosphorylation site (S59) in SLAC1, crucial for CPK6 activation, was identified. The group A PP2Cs ABI1, ABI2, and PP2CA down-regulated CPK6-mediated SLAC1 activity in oocytes. Unexpectedly, ABI1 directly dephosphorylated the N terminus of SLAC1, indicating an alternate branched early ABA signaling core in which ABI1 targets SLAC1 directly (down-regulation). Furthermore, here we have successfully reconstituted ABA-induced activation of SLAC1 channels in oocytes using the ABA receptor pyrabactin resistant 1 (PYR1) and PP2C phosphatases with two alternate signaling cores including either CPK6 or OST1. Point mutations in ABI1 disrupting PYR1-ABI1 interaction abolished ABA signal transduction. Moreover, by addition of CPK6, a functional ABA signal transduction core from ABA receptors to ion channel activation was reconstituted without a SnRK2 kinase.
DOI:10.1104/pp.17.01072URLPMID:28899960 [本文引用: 1]

Abstract Drought stress is one of the main environmental problems encountered by crop growers. Reduction in arable land area and reduced water availability make it paramount to identify and develop strategies to allow crops to be more resilient in water limiting environments. The plant hormone abscisic acid (ABA) plays an important role in the plants' response to drought stress through its control of stomatal aperture and water transpiration; and transgenic modulation of ABA levels therefore represents an attractive avenue to improve the drought tolerance of crops. Several steps in the ABA signaling pathway are controlled by ubiquitination involving RING domain containing proteins. We characterized the maize RING protein family and identified two novel RING-H2 genes called ZmXerico1 and ZmXerico2. Expression of ZmXerico genes is induced by drought stress and we show that overexpression of ZmXerico1 and ZmXerico2 in Arabidopsis and maize confers ABA hypersensitivity and improved water use efficiency which can lead to enhanced maize yield performance in a controlled drought stress environment. Overexpression of ZmXerico1 and ZmXerico2 in maize results in increased ABA levels and decreased levels of ABA degradation products diphaseic acid and phaseic acid. We show that ZmXerico1 is localized in the endoplasmic reticulum, where ABA 8'-hydroxylases have been shown to be localized, and that it functions as an E3 ubiquitin ligase. We demonstrate that ZmXerico1 plays a role in the control of ABA homeostasis through regulation of ABA 8'-hydroxylase protein stability, representing a novel control point in the regulation of the ABA pathway. {copyright, serif} 2017 American Society of Plant Biologists. All rights reserved.
DOI:10.1111/tpj.12708URLPMID:25330042 [本文引用: 2]

Summary Membrane-delimited events play a crucial role for ABA signaling and PYR/PYL/RCAR ABA receptors, clade A PP2Cs and SnRK2/CPK kinases modulate the activity of different plasma membrane components involved in ABA action. Therefore, the turnover of PYR/PYL/RCARs in the proximity of plasma membrane might be a step that affects receptor function and downstream signaling. In this study we describe a single-subunit RING-type E3 ubiquitin ligase RSL1 that interacts with the PYL4 and PYR1 ABA receptors at the plasma membrane. Overexpression of RSL1 reduces ABA sensitivity and rsl1 RNAi lines that impair expression of several members of the RSL1/RFA gene family show enhanced sensitivity to ABA. RSL1 bears a C-terminal transmembrane domain that targets the E3 ligase to plasma membrane. Accordingly, bimolecular fluorescent complementation (BiFC) studies showed the RSL1鈥揚YL4 and RSL1鈥揚YR1 interaction is localized to plasma membrane. RSL1 promoted PYL4 and PYR1 degradation in vivo and mediated in vitro ubiquitylation of the receptors. Taken together, these results suggest ubiquitylation of ABA receptors at plasma membrane is a process that might affect their function via effect on their half-life, protein interactions or trafficking.
DOI:10.1073/pnas.1316717111URLPMID:24928519 [本文引用: 2]

Arabidopsis glycogen synthase kinase 3 (GSK3)-like kinases have versatile functions in plant development and in responding to abiotic stresses. Although physiological evidence suggested a potential role of GSK3-like kinases in abscisic acid (ABA) signaling, the underlying molecular mechanism was largely unknown. Here we identified members of Snf1-related kinase 2s (SnRK2s), SnRK2.2 and SnRK2.3, that can interact with and be phosphorylated by a GSK3-like kinase, brassinosteroid insensitive 2 (BIN2). bin2-3 bil1 bil2, a loss-of-function mutant of BIN2 and its two closest homologs, BIN2 like 1 (BIL1) and BIN2 like 2 (BIL2), was hyposensitive to ABA in primary root inhibition, ABA-responsive gene expression, and phosphorylating ABA Response Element Binding Factor (ABF) 2 fragment by in-gel kinase assays, whereas bin2-1, a gain-of-function mutation of BIN2, was hypersensitive to ABA, suggesting that these GSK3-like kinases function as positive regulators in ABA signaling. Furthermore, BIN2 phosphorylated SnRK2.3 on T180, and SnRK2.3(T180A) had decreased kinase activity in both autophosphorylation and phosphorylating ABFs. Bikinin, a GSK3 kinase inhibitor, inhibited the SnRK2.3 kinase activity and its T180 phosphorylation in vivo. Our genetic analysis further demonstrated that BIN2 regulates ABA signaling downstream of the PYRABACTIN RESISTANCE1/PYR1-LIKE/REGULATORY COMPONENTS OF ABA RECEPTORS receptors and clade A protein phosphatase 2C but relies on SnRK2.2 and SnRK2.3. These findings provide significant insight into the modulation of ABA signaling by Arabidopsis GSK3-like kinases.
DOI:10.1126/scisignal.aaa7981PMID:26329583 [本文引用: 1]

Abscisic acid (ABA) is a phytohormone that inhibits growth and enhances adaptation to stress in plants. ABA perception and signaling rely on its binding to receptors of the pyrabactin resistance1/PYR1-like/regulatory components of ABA receptors (PYR/PYL/RCAR) family, the subsequent inhibition of clade A type 2C protein phosphatases (PP2Cs), and the phosphorylation of ion channels and transcription factors by protein kinases of the SnRK2 family. Nitric oxide (NO) may inhibit ABA signaling because NO-deficient plants are hypersensitive to ABA. Regulation by NO often involves posttranslational modification of proteins. Mass spectrometry analysis of ABA receptors expressed in plants and recombinant receptors modified in vitro revealed that the receptors were nitrated at tyrosine residues and S-nitrosylated at cysteine residues. In an in vitro ABA-induced, PP2C inhibition assay, tyrosine nitration reduced receptor activity, whereas S-nitrosylated receptors were fully capable of ABA-induced inhibition of the phosphatase. PYR/PYL/RCAR proteins with nitrated tyrosine, which is an irreversible covalent modification, were polyubiquitylated and underwent proteasome-mediated degradation. We propose that tyrosine nitration, which requires NO and superoxide anions, is a rapid mechanism by which NO limits ABA signaling under conditions in which NO and reactive oxygen species are both produced.
DOI:10.1007/s11103-016-0500-9URLPMID:27325215 [本文引用: 1]

Sumoylation is an essential post-translational regulator of plant development and the response to environmental stimuli. SUMO conjugation occurs via an E1-E2-E3 cascade, and can be removed by SUMO proteases (ULPs). ULPs are numerous and likely to function as sources of specificity within the pathway, yet most ULPs remain functionally unresolved. In this report we used loss-of-function reverse genetics and transcriptomics to functionally characterize Arabidopsis thaliana ULP1c and ULP1d SUMO proteases. GUS reporter assays implicated ULP1c/d in various developmental stages, and subsequent defects in growth and germination were uncovered using loss-of-function mutants. Microarray analysis evidenced not only a deregulation of genes involved in development, but also in genes controlled by various drought-associated transcriptional regulators. We demonstrated that ulp1c ulp1d displayed diminished in vitro root growth under low water potential and higher stomatal aperture, yet leaf transpirational water loss and whole drought tolerance were not significantly altered. Generation of a triple siz1 ulp1c ulp1d mutant suggests that ULP1c/d and the SUMO E3 ligase SIZ1 may display separate functions in development yet operate epistatically in response to water deficit. We provide experimental evidence that Arabidopsis ULP1c and ULP1d proteases act redundantly as positive regulators of growth, and operate mainly as isopeptidases downstream of SIZ1 in the control of water deficit responses.
DOI:10.1007/s00018-012-1094-2URLPMID:22903295 [本文引用: 1]

Abstract Protein post-translational modifications diversify the proteome and install new regulatory levels that are crucial for the maintenance of cellular homeostasis. Over the last decade, the ubiquitin-like modifying peptide small ubiquitin-like modifier (SUMO) has been shown to regulate various nuclear processes, including transcriptional control. In plants, the sumoylation pathway has been significantly implicated in the response to environmental stimuli, including heat, cold, drought, and salt stresses, modulation of abscisic acid and other hormones, and nutrient homeostasis. This review focuses on the emerging importance of SUMO in the abiotic stress response, summarizing the molecular implications of sumoylation and emphasizing how high-throughput approaches aimed at identifying the full set of SUMO targets will greatly enhance our understanding of the SUMO-abiotic stress association.
DOI:10.1105/tpc.106.049981URL [本文引用: 1]

Posttranslational modifications of proteins by small ubiquitin-like modifiers (SUMOs) regulate protein degradation and localization, protein-protein interaction, and transcriptional activity. SUMO E3 ligase functions are executed by SIZ1/SIZ2 and Mms21 in yeast, the PIAS family members RanBP2, and Pc2 in human. The Arabidopsis thaliana genome contains only one gene, SIZ1, that is orthologous to the yeast SIZ1/SIZ2. Here, we show that Arabidopsis SIZ1 is expressed in all plant tissues. Compared with the wild type, the null mutant siz1-3 is smaller in stature because of reduced expression of genes involved in brassinosteroid biosynthesis and signaling. Drought stress induces the accumulation of SUMO-protein conjugates, which is in part dependent on SIZ1 but not on abscisic acid (ABA). Mutant plants of siz1-3 have significantly lower tolerance to drought stress. A genome-wide expression analysis identified 鈭1700 Arabidopsis genes that are induced by drought, with SIZ1 mediating the expression of 300 of them by a pathway independent of DREB2A and ABA. SIZ1-dependent, drought-responsive genes include those encoding enzymes of the anthocyanin synthesis pathway and jasmonate response. From these results, we conclude that SIZ1 regulates Arabidopsis growth and that this SUMO E3 ligase plays a role in drought stress response likely through the regulation of gene expression.
DOI:10.1016/j.molp.2018.02.012URLPMID:29505832 [本文引用: 1]

Unveiling the signal transduction of phytohormone abscisic acid (ABA) and its regulatory mechanisms is critical for developing the strategies toward improving plant responses to stressful environments.ABA signaling is perceived and mediated by multiple PYR/PYL receptors,whose post-translational modifications,especially phosphorylation,remain largely unknown.In this study,we demonstrate that Arabidopsis EL1-like (AEL) protein,a casein kinase that regulates various physiological processes,phosphorylate PYR/PYLs to promote their ubiquitination and degradation,resulting in suppressed ABA responses.Arabidopsis ael triple mutants display hypersensitive responses to ABA treatment,which is consistent with the suppressed degradation of PYR/PYL proteins.PYR/PYLs are phosphorylated in vivo and mutation of the conserved AEL phosphorylation sites results in reduced phosphorylation,ubiquitination,and degradation of PYR/PYLs,and hence enhanced ABA responses.Taken together,these results demonstrate that AEL-mediated phosphorylation plays crucial roles in regulating the stability and function of PYR/ PYLs,providing significant insights into the post-translational regulation of PYR/PYL receptors and ABA signaling.
DOI:10.1105/tpc.112.107292URLPMID:23792371 [本文引用: 1]

Regulation of transcriptional processes is a critical mechanism that enables efficient coordination of the synthesis of required proteins in response to environmental and cellular changes. Transcription factors require accurate activity regulation because they play a critical role as key mediators assuring specific expression of target genes. In this work, we show that CULLIN3-based E3 ligases have the potential to interact with a broad range of ETHYLENE RESPONSE FACTOR (ERF)/APETALA2 (AP2) transcription factors, mediated by MATH-BTB/POZ (for Meprin and TRAF [tumor necrosis factor receptor associated factor] homolog)-Broad complex, Tramtrack, Bric-a-brac/Pox virus and Zinc finger) proteins. The assembly with an E3 ligase causes degradation of their substrates via the 26S proteasome, as demonstrated for the WRINKLED1 ERF/AP2 protein. Furthermore, loss of MATH-BTB/POZ proteins widely affects plant development and causes altered fatty acid contents in mutant seeds. Overall, this work demonstrates a link between fatty acid metabolism and E3 ligase activities in plants and establishes CUL3-based E3 ligases as key regulators in transcriptional processes that involve ERF/AP2 family members.
DOI:10.1111/tpj.12259URLPMID:23742014 [本文引用: 1]

The ABA Binding Factor/ABA-Responsive Element Binding Proteins (ABF/AREB) subfamily of bZIP-type transcription factors are positive effectors of ABA responses. Here, we examine the proteolytic regulation of two members: Arabidopsis thaliana ABF1 and ABF3. Both transcription factors are unstable in seedlings, and their degradation is sensitive to proteasome inhibition. ABA treatment of seedlings leads to their rapid accumulation, the result of slowed proteolysis. Deletion of the conserved C-terminal region required for 14-3-3 interaction destabilizes the proteins. The degradation of ABF1 and ABF3 are slower in vivo in seedlings lacking the ubiquitin E3 ligase KEEP ON GOING (KEG), and in vitro in extracts from keg seedlings, implicating KEG in their degradation. ABF1 and ABF3 are ubiquitylation substrates of KEG in vitro, and in vitro pull-down assays document their direct interaction. In contrast to ABI5, another KEG substrate, the degradation of ABFs and proteolytic regulation of ABFs by ABA still occurs in keg seedlings, suggesting that additional E3s participate in ABF1 and ABF3 proteolysis. Loss of ABF1 or ABF3 in the keg background has a phenotypic effect similar to the loss of ABI5, and there is no additional rescue of the keg phenotype in abf1 abf3 abi5 keg seedlings. This result suggests that the abundance of other substrates is altered in keg seedlings, affecting growth. In conclusion, ABF1 and ABF3 abundance is affected by ABA and KEG, and the conserved C4 region serves as a stabilizing element.
[本文引用: 1]
DOI:10.1104/pp.111.189738URLPMID:22095047 [本文引用: 1]

Transcriptional activities of plants play important roles in responses to environmental stresses. ETHYLENE RESPONSE FACTOR53 (AtERF53) is a drought-induced transcription factor that belongs to the AP2/ERF superfamily and has a highly conserved AP2 domain. It can regulate drought-responsive gene expression by binding to the GCC box and/or the dehydration-responsive element in the promoter of downstream genes. Overexpression of AtERF53 driven by the cauliflower mosaic virus 35S promoter resulted in an unstable drought-tolerant phenotype in T2 transgenic Arabidopsis (Arabidopsis thaliana) plants. Using a yeast two-hybrid screen, we identified a RING domain ubiquitin E3 ligase, RGLG2, which interacts with AtERF53 in the nucleus. The copine domain of RGLG2 exhibited the strongest interacting activity. We also demonstrated that RGLG2 could move from the plasma membrane to the nucleus under stress treatment. Using an in vitro ubiquitination assay, RGLG2 and its closest sequelog, RGLG1, were shown to have E3 ligase activity and mediated AtERF53 ubiquitination for proteasome degradation. The rglg1rglg2 double mutant but not the rglgl or rglg2 single mutant exhibited a drought-tolerant phenotype when compared with wild-type plants. AtERF53-green fluorescent proteins expressed in the rglglrglgl double mutants were stable. The 35S: AtERF53-green fluorescent protein/rglg1rglg2 showed enhanced AtERF53-regulated gene expression and had greater tolerance to drought stress than the rglg1rglg2 double mutant. In conclusion, RGLG2 negatively regulates the drought stress response by mediating AtERF53 transcriptional activity in Arabidopsis.
DOI:10.1104/pp.105.069757URL [本文引用: 1]
DOI:10.1038/ncb0502-e127URLPMID:11988757 [本文引用: 1]

Abstract The reversible phosphorylation of proteins is central to the regulation of most aspects of cell function but, even after the first protein kinase was identified, the general significance of this discovery was slow to be appreciated. Here I review the discovery of protein phosphorylation and give a personal view of the key findings that have helped to shape the field as we know it today.
DOI:10.1105/tpc.112.105767URLPMID:23404889 [本文引用: 1]

The basic Leucine zipper transcription factor ABSCISIC ACID INSENSITIVE5 (ABI5) is a key regulator of abscisic acid (ABA)-mediated seed germination and postgermination seedling growth. While a family of SUCROSE NONFERMENTING1-related protein kinase2s (SnRK2s) is responsible for ABA-induced phosphorylation and stabilization of ABI5, the phosphatase(s) responsible for dephosphorylating ABI5 is still unknown. Here, we demonstrate that mutations in FyPP1 (for Phytochrome-associated serine/threonine protein phosphatase1) and FyPP3, two homologous genes encoding the catalytic subunits of Ser/Thr PROTEIN PHOSPHATASE6 (PP6), cause an ABA hypersensitive phenotype in Arabidopsis thaliana, including ABA-mediated inhibition of seed germination and seedling growth. Conversely, overexpression of FyPP causes reduced sensitivity to ABA. The ABA hypersensitive phenotype of FyPP loss-of-function mutants is ABI5 dependent, and the amount of phosphorylated and total ABI5 proteins inversely correlates with the levels of FyPP proteins. Moreover, FyPP proteins physically interact with ABI5 in vitro and in vivo, and the strength of the interaction depends on the ABI5 phosphorylation status. In vitro phosphorylation assays show that FyPP proteins directly dephosphorylate ABI5. Furthermore, genetic and biochemical assays show that FyPP proteins act antagonistically with SnRK2 kinases to regulate ABI5 phosphorylation and ABA responses. Thus, Arabidopsis PP6 phosphatase regulates ABA signaling through dephosphorylation and destabilization of ABI5.
DOI:10.1042/bse0580029URLPMID:26374885 [本文引用: 4]

Abstract The phytohormone abscisic acid (ABA) plays crucial roles in numerous physiological processes during plant growth and abiotic stress responses. The endogenous ABA level is controlled by complex regulatory mechanisms involving biosynthesis, catabolism, transport and signal transduction pathways. This complex regulatory network may target multiple levels, including transcription, translation and post-translational regulation of genes involved in ABA responses. Most of the genes involved in ABA biosynthesis, catabolism and transport have been characterized. The local ABA concentration is critical for initiating ABA-mediated signalling during plant development and in response to environmental changes. In this chapter we discuss the mechanisms that regulate ABA biosynthesis, catabolism, transport and homoeostasis. We also present the findings of recent research on ABA perception by cellular receptors, and ABA signalling in response to cellular and environmental conditions. 2015 Authors; published by Portland Press Limited.
DOI:10.1111/tpj.12670URL [本文引用: 1]
DOI:10.1199/tab.0166URLPMID:22303212 [本文引用: 4]

ABSTRACT Abscisic acid (ABA) is one of the “classical” plant hormones, i.e. discovered at least 50 years ago, that regulates many aspects of plant growth and development. This chapter reviews our current understanding of ABA synthesis, metabolism, transport, and signal transduction, emphasizing knowledge gained from studies of Arabidopsis. A combination of genetic, molecular and biochemical studies has identified nearly all of the enzymes involved in ABA metabolism, almost 200 loci regulating ABA response, and thousands of genes regulated by ABA in various contexts. Some of these regulators are implicated in cross-talk with other developmental, environmental or hormonal signals. Specific details of the ABA signaling mechanisms vary among tissues or developmental stages; these are discussed in the context of ABA effects on seed maturation, germination, seedling growth, vegetative stress responses, stomatal regulation, pathogen response, flowering, and senescence.
DOI:10.1105/tpc.106.048538URLPMID:17307925 [本文引用: 2]

Abscisic acid (ABA) is an important phytohormone regulating various plant processes, including seed germination. Although phosphorylation has been suggested to be important, the protein kinases required for ABA signaling during seed germination and seedling growth remain elusive. Here, we show that two protein kinases, SNF1-RELATED PROTEIN KINASE2.2 (SnRK2.2) and SnRK2.3, control responses to ABA in seed germination, dormancy, and seedling growth in Arabidopsis thaliana. A snrk2.2 snrk2.3 double mutant, but not snrk2.2 or snrk2.3 single mutants, showed strong ABA-insensitive phenotypes in seed germination and root growth inhibition. Changes in seed dormancy and ABA-induced Pro accumulation consistent with ABA insensitivity were also observed. The snrk2.2 snrk2.3 double mutant had a greatly reduced level of a 42-kD kinase activity capable of phosphorylating peptides from ABF (for ABA Response Element Binding Factor) transcription factors. ABA-induced expression of several genes whose promoters contain an ABA response element (ABRE) was reduced in snrk2.2 snrk2.3, suggesting that the mechanism of SnRK2.2 and SnRK2.3 action in ABA signaling involves the activation of ABRE-driven gene expression through the phosphorylation of ABFs. Together, these results demonstrate that SnRK2.2 and SnRK2.3 are redundant but key protein kinases that mediate a major part of ABA signaling in Arabidopsis.
DOI:10.1073/pnas.0903144106URLPMID:19420218 [本文引用: 1]

Abscisic acid (ABA) is an important phytohormone regulating seed dormancy, germination, seedling growth, and plant transpiration. We report here an Arabidopsis triple mutant that is disrupted in 3 SNF1-related protein kinase subfamily 2 (SnRK2s) and nearly completely insensitive to ABA. These SnRK2s, SnRK2.2, SnRK2.3, and SnRK2.6 (also known as OST1), are activated by ABA and can phosphorylate the ABA-responsive element binding factor family of b-ZIP transcription factors, which are important for the activation of ABA-responsive genes. Although stomatal regulation of snrk2.6 and seed germination and seedling growth of the snrk2.2/2.3 double mutant are insensitive to ABA, ABA responses are still present in these mutants, and the growth and reproduction of these mutants are not very different from those of the WT. In contrast, the snrk2.2/2.3/2.6 triple mutant grows poorly and produces few seeds. The triple mutant plants lose water extremely fast when ambient humidity is not high. Even on 50 碌M ABA, the triple mutant can germinate and grow, whereas the most insensitive known mutants cannot develop on 10 碌M ABA. In-gel kinase assays showed that all ABA-act隆vated protein kinase activities are eliminated in the triple mutant. Also, the expression of ABA-induced genes examined is completely blocked in the triple mutant. These results demonstrate that the protein kinases SnRK2.2, SnRK2.3, and SnRK2.6 have redundant functions, and suggest that ABA signaling is critical for plant growth and reproduction.
DOI:10.1007/s10265-011-0412-3URL [本文引用: 1]
DOI:10.1093/pcp/pcp147URLPMID:19880399 [本文引用: 1]

Abstract Responses to water stress are thought to be mediated by transcriptional regulation of gene expression via reversible protein phosphorylation events. Previously, we reported that bZIP (basic-domain leucine zipper)-type AREB/ABF (ABA-responsive element-binding protein/factor) transcription factors are involved in ABA signaling under water stress conditions in Arabidopsis. The AREB1 protein is phosphorylated in vitro by ABA-activated SNF1-related protein kinase 2s (SnRK2s) such as SRK2D/SnRK2.2, SRK2E/SnRK2.6 and SRK2I/SnRK2.3 (SRK2D/E/I). Consistent with this, we now show that SRK2D/E/I and AREB1 co-localize and interact in nuclei in planta. Our results show that unlike srk2d, srk2e and srk2i single and double mutants, srk2d srk2e srk2i (srk2d/e/i) triple mutants exhibit greatly reduced tolerance to drought stress and highly enhanced insensitivity to ABA. Under water stress conditions, ABA- and water stress-dependent gene expression, including that of transcription factors, is globally and drastically impaired, and jasmonic acid (JA)-responsive and flowering genes are up-regulated in srk2d/e/i triple mutants, but not in other single and double mutants. The down-regulated genes in srk2d/e/i and areb/abf triple mutants largely overlap in ABA-dependent expression, supporting the view that SRK2D/E/I regulate AREB/ABFs in ABA signaling in response to water stress. Almost all dehydration-responsive LEA (late embryogenesis abundant) protein genes and group-A PP2C (protein phosphatase 2C) genes are strongly down-regulated in the srk2d/e/i triple mutants. Further, our data show that these group-A PP2Cs, such as HAI1 and ABI1, interact with SRK2D. Together, our results indicate that SRK2D/E/I function as main positive regulators, and suggest that ABA signaling is controlled by the dual modulation of SRK2D/E/I and group-A PP2Cs.
DOI:10.1111/j.1399-3054.2012.01635.xURLPMID:22519646 [本文引用: 3]

Water availability is one of the main limiting factors for plant growth and development. The phytohormone abscisic acid (ABA) fulfills a critical role in coordinating the responses to reduced water availability as well as in multiple developmental processes. Endogenous ABA levels increase in response to osmotic stresses such as drought and high salinity, and ABA activates the expression of many genes via ABA-responsive elements (ABREs) in their promoter regions. ABRE-binding protein/ABRE-binding factor (AREB/ABF) transcription factors (TFs) regulate the ABRE-mediated transcription of downstream target genes. Three subclass III sucrose non-fermenting-1 related protein kinase 2 (SnRK2) protein kinases (SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3) phosphorylate and positively control the AREB/ABF TFs. Substantial progress has been made in our understanding of the ABA-sensing system mediated by Pyrabactin resistance1/PYR1-like/regulatory components of ABA receptor (PYR/PYL/RCAR)-protein phosphatase 2C complexes. In addition to PP2C-PYR/PYL/RCAR ABAreceptor complex, the AREB/ABF-SnRK2 pathway, which is well conserved in land plants, was recently shown to play a major role as a positive regulator of ABA/stress signaling through ABRE-mediated transcription of target genes implicated in the osmotic stress response. This review focuses on current progress in the study of the AREB/ABF-SnRK2 positive regulatory pathway in plants and describes additional signaling factors implicated in the AREB/ABF-SnRK2 pathway. Moreover, to help promote the link between basic and applied studies, the nomenclature and phylogenetic relationships between the AREB/ABFs and SnRK2s are summarized and discussed.
DOI:10.1007/s11103-008-9344-2URLPMID:18484180 [本文引用: 1]

http://link.springer.com/article/10.1007%2Fs11103-008-9344-2
DOI:10.1073/pnas.0912030107URLPMID:20385816 [本文引用: 1]

In response to drought stress, the phytohormone abscisic acid (ABA) induces stomatal closure. Thereby the stress hormone activates guard cell anion channels in a calcium-dependent, as well as —independent, manner. Open stomata 1 protein kinase (OST1) and ABI1 protein phosphatase (ABA insensitive 1) represent key components of calcium-independent ABA signaling. Recently, the guard cell anion channel SLAC1 was identified. When expressed heterologously SLAC1 remained electrically silent. Upon coexpression with Ca0562-independent OST1, however, SLAC1 anion channels appear activated in an ABI1-dependent manner. Mutants lacking distinct calcium-dependent protein kinases (CPKs) appeared impaired in ABA stimulation of guard cell ion channels, too. To study SLAC1 activation via the calcium-dependent ABA pathway, we studied the SLAC1 response to CPKs in the Xenopus laevis oocyte system. Split YFP-based protein—protein interaction assays, using SLAC1 as the bait, identified guard cell expressed CPK21 and 23 as major interacting partners. Upon coexpression of SLAC1 with CPK21 and 23, anion currents document SLAC1 stimulation by these guard cell protein kinases. Ca0562-sensitive activation of SLAC1, however, could be assigned to the CPK21 pathway only because CPK23 turned out to be rather Ca0562-insensitive. In line with activation by OST1, CPK activation of the guard cell anion channel was suppressed by ABI1. Thus the CPK and OST1 branch of ABA signal transduction in guard cells seem to converge on the level of SLAC1 under the control of the ABI1/ABA-receptor complex.
DOI:10.1073/pnas.0912021106URL [本文引用: 1]

In response to drought stress the phytohormone ABA (abscisic acid) induces stomatal closure and, therein, activates guard cell anion channels in a calcium-dependent as well as-independent manner. Two key components of the ABA signaling pathway are the protein kinase OST1 (open stomata 1) and the protein phosphatase ABI1 (ABA insensitive 1). The recently identified guard cell anion channel SLAC1 appeared to be the key ion channel in this signaling pathway but remained electrically silent when expressed heterologously. Using split YFP assays, we identified OST1 as an interaction partner of SLAC1 and ABI1. Upon coexpression of SLAC1 with OST1 in Xenopus oocytes, SLAC1-related anion currents appeared similar to those observed in guard cells. Integration of ABI1 into the SLAC1/OST1 complex, however, prevented SLAC1 activation. Our studies demonstrate that SLAC1 represents the slow, deactivating, weak voltage-dependent anion channel of guard cells controlled by phosphorylation/dephosphorylation.
DOI:10.1105/tpc.112.105114URLPMID:23209114 [本文引用: 2]

The survival of plants as sessile organisms depends on their ability to cope with environmental challenges. Of key importance in this regard is the phytohormone abscisic acid (ABA). ABA not only promotes seed dormancy but also triggers growth arrest in postgermination embryos that encounter water stress. This is accompanied by increased desiccation tolerance. Postgermination ABA responses in Arabidopsis thaliana are mediated in large part by the ABA-induced basic domain/leucine zipper transcription factor ABA INSENSITIVE5 (ABI5). Here, we show that loss of function of the SWI2/SNF2 chromatin remodeling ATPase BRAHMA (BRM) causes ABA hypersensitivity during postgermination growth arrest. ABI5 expression was derepressed in brm mutants in the absence of exogenous ABA and accumulated to high levels upon ABA sensing. This effect was likely direct; chromatin immunoprecipitation revealed BRM binding to the ABI5 locus. Moreover, loss of BRM activity led to destabilization of a nucleosome likely to repress ABI5 transcription. Finally, the abi5 null mutant was epistatic to BRM in postgermination growth arrest. In addition, vegetative growth defects typical of brm mutants in the absence of ABA treatment could be partially overcome by reduction of ABA responses, and brm mutants displayed increased drought tolerance. We propose a role for BRM in the balance between growth or stress responses.
DOI:10.1073/pnas.152342599URL [本文引用: 1]
[本文引用: 1]
DOI:10.1105/tpc.16.00131URL [本文引用: 1]
DOI:10.1371/journal.pgen.1005835URLPMID:26943172 [本文引用: 1]

The phytohormone abscisic acid (ABA) regulates plant growth, development and responses to biotic and abiotic stresses. The core ABA signaling pathway consists of three major components: ABA receptor (PYR1/PYLs), type 2C Protein Phosphatase (PP2C) andSNF1-related proteinkinase 2 (SnRK2). Nevertheless, the complexity of ABA signaling remains to be explored. To uncover new components of ABA signal transduction pathways, we performed a yeast two-hybrid screen for SnRK2-interacting proteins. We found thatTypeOneProteinPhosphatase 1 (TOPP1) and its regulatory protein,At Inhibitor-2 (AtI-2), physically interact with SnRK2s and also with PYLs. TOPP1 inhibited the kinase activity of SnRK2.6, and this inhibition could be enhanced by AtI-2. Transactivation assays showed that TOPP1 and AtI-2 negatively regulated the SnRK2.2/3/6-mediated activation of the ABA responsive reporter geneRD29B, supporting a negative role of TOPP1 and AtI-2 in ABA signaling. Consistent with these findings,topp1andati-2mutant plants displayed hypersensitivities to ABA and salt treatments, and transcriptome analysis ofTOPP1andAtI-2knockout plants revealed an increased expression of multiple ABA-responsive genes in the mutants. Taken together, our results uncover TOPP1 and AtI-2 as negative regulators of ABA signaling. The phytohormone abscisic acid (ABA) regulates multiple developmental processes such as seed dormancy, germination, root/shoot growth, flowering and senescence in plants. Although the core ABA perception and signaling pathway has been elucidated, the complexity of the pathway remains to be exploited. In the present work, we uncovered two new proteins, TOPP1 and its regulatory protein AtI-2, interact with both ABA receptor PYLs and their downstream positive regulator SnRK2s. In addition to their physical interaction, TOPP1 could inhibit the kinase activity of SnRK2s and this inhibition could be further enhanced by AtI-2, which is likely due to a promotion of the interaction between TOPP1 and SnRK2s by AtI-2.topp1andati-2mutants exhibited hypersensitivity to ABA and salt treatments; and transcriptome studies revealed multiple ABA-responsive genes were up-regulated in the mutants. In summary, our work identified two new components, TOPP1 and AtI-2, and characterized their negative roles in ABA signaling.
[本文引用: 1]
DOI:10.1105/tpc.114.130849URLPMID:25415975 [本文引用: 2]

Abstract Seed germination and postgerminative growth are regulated by a delicate hormonal balance. Abscisic acid (ABA) represses Arabidopsis thaliana seed germination and postgerminative growth, while brassinosteroids (BRs) antagonize ABA-mediated inhibition and promote these processes. However, the molecular mechanism underlying BR-repressed ABA signaling remains largely unknown. Here, we show that the Glycogen Synthase Kinase 3-like kinase BRASSINOSTEROID INSENSITIVE2 (BIN2), a critical repressor of BR signaling, positively regulates ABA responses during seed germination and postgerminative growth. Mechanistic investigation revealed that BIN2 physically interacts with ABSCISIC ACID INSENSITIVE5 (ABI5), a bZIP transcription factor. Further genetic analysis demonstrated that the ABA-hypersensitive phenotype of BIN2-overexpressing plants requires ABI5. BIN2 was found to phosphorylate and stabilize ABI5 in the presence of ABA, while application of epibrassinolide (the active form of BRs) inhibited the regulation of ABI5 by BIN2. Consistently, the ABA-induced accumulation of ABI5 was affected in BIN2-related mutants. Moreover, mutations of the BIN2 phosphorylation sites on ABI5 made the mutant protein respond to ABA improperly. Additionally, the expression of several ABI5 regulons was positively modulated by BIN2. These results provide evidence that BIN2 phosphorylates and stabilizes ABI5 to mediate ABA response during seed germination, while BRs repress the BIN2-ABI5 cascade to antagonize ABA-mediated inhibition. 2014 American Society of Plant Biologists. All rights reserved.
DOI:10.1105/tpc.112.100107URLPMID:22730405 [本文引用: 1]

The plant hormone abscisic acid (ABA) regulates stomatal movement under drought stress, and this regulation requires hydrogen peroxide (H 2 O 2 ). We isolated GUARD CELL HYDROGEN PEROXIDE-RESISTANT1 (GHR1), which encodes a receptor-like kinase localized on the plasma membrane in Arabidopsis thaliana. ghr1 mutants were defective ABA and H 2 O 2 induction of stomatal closure. Genetic analysis indicates that GHR1 is a critical early component in ABA signaling. The ghr1 mutation impaired ABA- and H 2 O 2 -regulated activation of S-type anion currents in guard cells. Furthermore, GHR1 physically interacted with, phosphorylated, and activated the S-type anion channel SLOW ANION CHANNEL-ASSOCIATED1 when coexpressed in Xenopus laevis oocytes, and this activation was inhibited by ABA-INSENSITIVE2 (ABI2) but not ABI1. Our study identifies a critical component in ABA and H 2 O 2 signaling that is involved in stomatal movement and resolves a long-standing mystery about the differential functions of ABI1 and ABI2 in this process.
DOI:10.1146/annurev-arplant-042809-112256URL [本文引用: 1]
DOI:10.1093/mp/ssp091URLPMID:2807925 [本文引用: 1]

The Arabidopsis FCLY gene encodes a specific farnesylcysteine (FC) lyase, which is responsible for the oxidative metabolism of FC to farnesal and cysteine. In addition, fcly mutants with quantitative decreases in FC lyase activity exhibit an enhanced response to ABA. However, the enzymological properties of the FCLY-encoded enzyme and its precise role in ABA signaling remain unclear. Here, we show that recombinant Arabidopsis FC lyase expressed in insect cells exhibits high selectivity for FC as a substrate and requires FAD and molecular oxygen for activity. Arabidopsis FC lyase is also shown to undergo post-translational N-glycosylation. FC, which is a competitive inhibitor of isoprenylcysteine methyltransferase (ICMT), accumulates in fcly mutants. Moreover, the enhanced response of fcly mutants to ABA is reversed by ICMT overexpression. These observations support the hypothesis that the ABA hypersensitive phenotype of fcly plants is the result of FC accumulation and inhibition of ICMT.
DOI:10.1016/j.tem.2015.09.013URL [本文引用: 1]

Tremendous advances in MS-based phosphoproteomics have uncovered tens of thousands of phosphorylation sites on the majority of cellular proteins. Well-studied kinases and their substrates represent only a small fraction of the regulated phosphoproteome, suggesting that many important regulatory nodes remain unexplored. Metabolism is regulated by and regulates signal transduction in an intricate network of cellular and organismal regulation. Recent developments aimed at simplifying the phosphoproteomics workflow allow for many larger andin vivostudies, and promise to make the technology accessible to a wider audience.
[本文引用: 1]
DOI:10.1038/nrm1939URLPMID:16723975 [本文引用: 1]

Post-translational modifications define the functional and structural plasticity of proteins in archaea, prokaryotes and eukaryotes. Multi-site protein modification modulates protein activity and macromolecular interactions and is involved in a range of fundamental molecular processes. Combining state-of-the-art technologies in molecular cell biology, protein mass spectrometry and bioinformatics, it is now feasible to discover and study the structural and functional roles of distinct protein post-translational modifications.
DOI:10.1371/journal.pgen.1007237URL [本文引用: 1]

The control of seed germination and seed dormancy are critical for the successful propagation of plant species, and are important agricultural traits. Seed germination is tightly controlled by the balance of gibberellin (GA) and abscisic acid (ABA), and is influenced by environmental factors. The COP9 Signalosome (CSN) is a conserved multi-subunit protein complex that is best known as a regulator of the Cullin-RING family of ubiquitin E3 ligases (CRLs). Multiple viable mutants of the CSN showed poor germination, except for csn5b-1. Detailed analyses showed that csn1-10 has a stronger seed dormancy, while csn5a-1 mutants exhibit retarded seed germination in addition to hyperdormancy. Both csn5a-1 and csn1-10 plants show defects in the timely removal of the germination inhibitors: RGL2, a repressor of GA signaling, and ABI5, an effector of ABA responses. We provide genetic evidence to demonstrate that the germination phenotype of csn1-10 is caused by over-accumulation of RGL2, a substrate of the SCF (CRL1) ubiquitin E3 ligase, while the csn5a-1 phenotype is caused by over-accumulation of RGL2 as well as ABI5. The genetic data are consistent with the hypothesis that CSN5A regulates ABI5 by a mechanism that may not involve CSN1. Transcriptome analyses suggest that CSN1 has a more prominent role than CSN5A during seed maturation, but CSN5A plays a more important role than CSN1 during seed germination, further supporting the functional distinction of these two CSN genes. Our study delineates the molecular targets of the CSN complex in seed germination, and reveals that CSN5 has additional functions in regulating ABI5, thus the ABA signaling pathway.
DOI:10.1093/jxb/err338URLPMID:22071266 [本文引用: 1]

Abstract Abscisic acid (ABA) is a phytohormone that positively regulates seed dormancy and stress tolerance. PYL/RCARs were identified an intracellular ABA receptors regulating ABA-dependent gene expression in Arabidopsis thaliana. However, their function in monocot species has not been characterized yet. Herein, it is demonstrated that PYL/RCAR orthologues in Oryza sativa function as a positive regulator of the ABA signal transduction pathway. Transgenic rice plants expressing OsPYL/RCAR5, a PYL/RCAR orthologue of rice, were found to be hypersensitive to ABA during seed germination and early seedling growth. A rice ABA signalling unit composed of OsPYL/RCAR5, OsPP2C30, SAPK2, and OREB1 for ABA-dependent gene regulation was further identified, via interaction assays and a transient gene expression assay. Thus, a core signalling unit for ABA-responsive gene expression modulating seed germination and early seedling growth in rice has been unravelled. This study provides substantial contributions toward understanding the ABA signal transduction pathway in rice.
DOI:10.1104/pp.113.220103URL [本文引用: 1]

Really Interesting New Gene (RING) E3 ubiquitin ligases have been implicated in cellular responses to the stress hormone abscisic acid (ABA) as well as to environmental stresses in higher plants. Here, an ABA-insensitive RING protein3 (atairp3) loss-of-function mutant line in Arabidopsis (Arabidopsis thaliana) was isolated due to its hyposensitivity to ABA during its germination stage as compared with wildtype plants. AtAIRP3 contains a single C3HC4-type RING motif, a putative myristoylation site, and a domain associated with RING2 (DAR2) domain. Unexpectedly, AtAIRP3 was identified as LOSS OF GDU2 (LOG2), which was recently shown to participate in an amino acid export system via interaction with GLUTAMINE DUMPER1. Thus, AtAIRP3 was renamed as AtAIRP3/LOG2. Transcript levels of AtAIRP3/LOG2 were up-regulated by drought, high salinity, and ABA, suggesting a role for this factor in abiotic stress responses. The atairp3/log2-2 knockout mutant and 35S:AtAIRP3-RNAi knockdown transgenic plants displayed impaired ABA-mediated seed germination and stomata closure. Cosuppression and complementation studies further supported a positive role for AtAIRP3/LOG2 in ABA responses. Suppression of AtAIRP3/LOG2 resulted in marked hypersensitive phenotypes toward high salinity and water deficit relative to wild-type plants. These results suggest that Arabidopsis RING E3 AtAIRP3/LOG2 is a positive regulator of the ABA-mediated drought and salt stress tolerance mechanism. Using yeast (Saccharomyces cerevisiae) two-hybrid, in vitro, and in vivo immunoprecipitation, cell-free protein degradation, and in vitro ubiquitination assays, RESPONSIVE TO DEHYDRATION21 was identified as a substrate protein of AtAIRP3/LOG2. Collectively, our data suggest that AtAIRP3/LOG2 plays dual functions in ABA-mediated drought stress responses and in an amino acid export pathway in Arabidopsis.
DOI:10.1146/annurev-arplant-042809-112226URL [本文引用: 1]
DOI:10.1038/nature10794URLPMID:3292258 [本文引用: 1]

Plants must coordinate the regulation of biochemistry and anatomy to optimize photosynthesis and water-use efficiency. The formation of stomata, epidermal pores that facilitate gas exchange, is highly coordinated with other aspects of photosynthetic development. The signalling pathways controlling stomata development are not fully understood, although mitogen-activated protein kinase (MAPK) signalling is known to have key roles. Here we demonstrate in Arabidopsis that brassinosteroid regulates stomatal development by activating the MAPK kinase kinase (MAPKKK) YDA (also known as YODA). Genetic analyses indicate that receptor kinase-mediated brassinosteroid signalling inhibits stomatal development through the glycogen synthase kinase 3 (GSK3)-like kinase BIN2, and BIN2 acts upstream of YDA but downstream of the ERECTA family of receptor kinases. Complementary in vitro and in vivo assays show that BIN2 phosphorylates YDA to inhibit YDA phosphorylation of its substrate MKK4, and that activities of downstream MAPKs are reduced in brassinosteroid-deficient mutants but increased by treatment with either brassinosteroid or GSK3-kinase inhibitor. Our results indicate that brassinosteroid inhibits stomatal development by alleviating GSK3-mediated inhibition of this MAPK module, providing two key links; that of a plant MAPKKK to its upstream regulators and of brassinosteroid to a specific developmental output.
DOI:10.1111/j.1365-313X.2006.02782.xURLPMID:16792696 [本文引用: 1]

RING (really interesting new gene) zinc-finger proteins have important regulatory roles in the development of a variety of organisms. The XERICO gene encodes a small protein (162 amino acids) with an N-terminal trans -membrane domain and a RING-H2 zinc-finger motif located at the C-terminus. In silico gene-expression analysis indicated that XERICO is induced by salt and osmotic stress. Compared with wild-type (WT) Arabidopsis plants, transgenic plants overexpressing XERICO ( 35S::XERICO ) exhibited hypersensitivity to salt and osmotic stress and exogenous abscisic acid (ABA) during germination and early seedling growth. When subjected to a drought treatment, transcriptional upregulation of a key ABA-biosynthesis gene, AtNCED3 , was much faster and stronger in 35S::XERICO plants compared with WT plants. Further, upregulation of XERICO substantially increased cellular ABA levels. The adult 35S::XERICO plants, in contrast to early seedling growth, showed a marked increase in their tolerance to drought stress. Yeast two-hybrid screening indicated that XERICO interacts with an E2 ubiquitin-conjugating enzyme (AtUBC8) and ASK1-interacting F-box protein (AtTLP9), which is involved in the ABA-signaling pathway. Affymetrix GeneChip array analysis showed that the expressions of many of the genes involved in the biosynthesis of plant hormones (e.g. ethylene, brassinosteroid, gibberellic acid) were significantly changed in the 35S::XERICO plants. These results suggest that the homeostasis of various plant hormones might be altered in 35S::XERICO plants, possibly by overaccumulation of ABA.
DOI:10.1038/ncomms9630URLPMID:4667695 [本文引用: 1]

http://www.nature.com/ncomms/2015/151020/ncomms9630/full/ncomms9630.html
DOI:10.1111/tra.12101URLPMID:23944713 [本文引用: 1]

Autophagic transport to the vacuole represents an endomembrane trafficking route, which is widely used in plants, not only during stress situations, but also for vacuole biogenesis and during developmental processes. Here we report a role in autophagic membrane transport for EXO70B1one of 23 paralogs of ArabidopsisEXO70 exocyst subunits. EXO70B1 positive compartments are internalized into the central vacuole and co-localize with autophagosomal marker ATG8f. This internalization is boosted by induction of autophagy. Loss of function (LOF) mutations in exo70B1 cause reduction of internalized autopagic bodies in the vacuole. Mutant plants also show ectopic hypersensitive response (HR) mediated by salicylic acid (SA) accumulation, increased nitrogen starvation susceptibility and anthocyanin accumulation defects. Anthocyanin accumulation defect persists in npr1x exo70B1 double mutants with SA signaling compromised, while ectopic HR is suppressed. EXO70B1 interacts with SEC5 and EXO84 and forms an exocyst subcomplex involved in autophagy-related, Golgi-independent membrane traffic to the vacuole. We show that EXO70B1 is functionally completely different from EXO70A1 exocyst subunit and adopted a specific role in autophagic transport.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.devcel.2011.10.018URLPMID:22172674 [本文引用: 1]

78 CUL3BPM ubiquitin ligases regulate abscisic acid (ABA) signaling 78 CUL3BPM targets the ABA-regulatory transcription factor ATHB6 for proteolysis 78 ATHB6 turnover is important for stomatal behavior
DOI:10.1038/ncomms12525URLPMID:27615387 [本文引用: 1]

The phytohormone abscisic acid (ABA) regulates plant responses to various environmental challenges. Controlled protein turnover is an important component of ABA signalling. Here we show that the RING-type E3 ligase MYB30-INTERACTING E3 LIGASE 1 (MIEL1) regulates ABA sensitivity by promoting MYB96 turnover inArabidopsis. Germination ofMIEL1-deficient mutant seeds is hypersensitive to ABA, whereasMIEL1-overexpressing transgenic seeds are less sensitive. MIEL1 can interact with MYB96, a regulator of ABA signalling, and stimulate its ubiquitination and degradation. Genetic analysis shows thatMYB96is epistatic toMIEL1in the control of ABA sensitivity in seeds. While MIEL1 acts primarily via MYB96 in seed germination, MIEL1 regulates protein turnover of both MYB96 and MYB30 in vegetative tissues. We find that ABA regulates the expression of MYB30-responsive genes during pathogen infection and this regulation is partly dependent on MIEL1. These results suggest that MIEL1 may facilitate crosstalk between ABA and biotic stress signalling. The phytohormone abscisic acid controls plant responses to environmental stress, partly by regulating protein turnover. Here the authors propose that abscisic acid regulates seed germination by promoting degradation of the MYB96 transcription factor via the MIEL1 E3 ubiquitin (Ub) ligase.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.cell.2006.07.034URLPMID:16990135 [本文引用: 1]

Abscisic acid (ABA) is a phytohormone critical for plant growth, development, and adaptation to various stress conditions. Plants have to adjust ABA levels constantly to respond to changing physiological and environmental conditions. To date, the mechanisms for fine-tuning ABA levels remain elusive. Here we report that AtBG1, a 尾-glucosidase, hydrolyzes glucose-conjugated, biologically inactive ABA to produce active ABA. Loss of AtBG1 causes defective stomatal movement, early germination, abiotic stress-sensitive phenotypes, and lower ABA levels, whereas plants with ectopic AtBG1 accumulate higher ABA levels and display enhanced tolerance to abiotic stress. Dehydration rapidly induces polymerization of AtBG1, resulting in a 4-fold increase in enzymatic activity. Furthermore, diurnal increases in ABA levels are attributable to polymerization-mediated AtBG1 activation. We propose that the activation of inactive ABA pools by polymerized AtBG1 is a mechanism by which plants rapidly adjust ABA levels and respond to changing environmental cues.
DOI:10.1111/pce.13086URLPMID:29044697 [本文引用: 1]

Abstract The phytohormone abscisic acid (ABA) plays an important role in regulating plant growth, development and adaption to various environmental stresses. Regulatory components of ABA receptors (RCARs, also known as PYR/PYLs) sense ABA and initiate ABA signaling through inhibiting the activities of protein phosphatase 2C (PP2C) in Arabidopsis. However, the way in which ABA receptors are regulated is not well known. A DWD protein AtRAE1 (for RNA export factor 1 in Arabidopsis), which may act as a substrate receptor of CUL4-DDB1 E3 ligase, is an interacting partner of RCAR1/PYL9. The physical interaction between RCAR1 and AtRAE1 is confirmed in vitro and in vivo . Overexpression of AtRAE1 in Arabidopsis causes reduced sensitivity of plants to ABA, while suppression of AtRAE1 causes increased sensitivity to ABA. Analysis of protein stability demonstrates that RCAR1 is ubiquitinated and degraded in plant cells and AtRAE1 regulates the degradation speed of RCAR1. Our findings indicate that AtRAE1 likely participates in ABA signaling through regulating the degradation of ABA receptor RCAR1.
DOI:10.1111/nph.14680URLPMID:28700082 [本文引用: 1]

Abstract Drought is a key limiting factor for cotton (Gossypium spp.) production, as more than half of the global cotton supply is grown in regions with high water shortage. However, the underlying mechanism of the response of cotton to drought stress remains elusive. By combining genome-wide transcriptome profiling and a loss-of-function screen using virus-induced gene silencing, we identified Gossypium0002hirsutum GhWRKY59 as an important transcription factor that regulates the drought stress response in cotton. Biochemical and genetic analyses revealed a drought stress-activated mitogen-activated protein (MAP) kinase cascade consisting of GhMAP3K15-Mitogen-activated Protein Kinase Kinase 4 (GhMKK4)-Mitogen-activated Protein Kinase 6 (GhMPK6) that directly phosphorylates GhWRKY59 at residue serine 221. Interestingly, GhWRKY59 is required for dehydration-induced expression of GhMAPK3K15, constituting a positive feedback loop of GhWRKY59-regulated MAP kinase activation in response to drought stress. Moreover, GhWRKY59 directly binds to the W-boxes of DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN 2 (GhDREB2), which encodes a dehydration-inducible transcription factor regulating the plant hormone abscisic acid (ABA)-independent drought response. Our study identified a complete MAP kinase cascade that phosphorylates and activates a key WRKY transcription factor, and elucidated a regulatory module, consisting of GhMAP3K15-GhMKK4-GhMPK6-GhWRKY59-GhDREB2, that is involved in controlling the cotton drought response. 0008 2017 The Authors. New Phytologist 0008 2017 New Phytologist Trust.
DOI:10.1111/pce.12639URLPMID:26386272 [本文引用: 1]

Abstract The phytohormone abscisic acid (ABA) plays a vital role in plant growth and development. The function of ABA is mediated by a group of newly discovered ABA receptors, named PYRABACTIN RESISTANCE 1/PYR-LIKE/REGULATORY COMPONENTS OF ABA RECEPTORs (PYR1/PYLs/RCARs). Here, we report that an Arabidopsis thaliana F-box protein RCAR3 INTERACTING F-BOX PROTEIN 1 (RIFP1) interacts with ABA receptor (RCAR3) and SCF E3 ligase complex subunits Arabidopsis SKP1-LIKE PROTEINs (ASKs) in vitro and in vivo . The rifp1 mutant plants displayed increased ABA-mediated inhibition of seed germination and water loss of detached leaves, while the overexpression of RIFP1 in Arabidopsis led to plants being insensitive to ABA. Meanwhile, the rifp1 mutant plants showed greater tolerance to water deficit. In addition, the RCAR3 protein level was more stable in the rifp1 mutant plants than in the wild-type plants, indicating that RIFP1 facilitates the proteasome degradation of RCAR3. Accordingly, the loss of RIFP1 increased the transcript levels of several ABA-responsive genes. Taken together, these data indicate that RIFP1 plays a negative role in the RCAR3-mediated ABA signalling pathway and likely functions as an adaptor subunit of the SCF ubiquitin ligase complex to regulate ABA receptor RCAR3 stability.
DOI:10.1093/jxb/erv341URLPMID:4588886 [本文引用: 1]

A sucrose nonfermenting 1 (SNF1)-related protein kinase 2, SnRK2.6/ open stomata 1 (OST1), which plays critical role in abscisic acid (ABA) signalling in Arabidopsis guard cells, interacts directly with, and functions downstream of, the magnesium-chelatase H subunit in guard cell signalling in response to ABA. Magnesium-chelatase H subunit [CHLH/putative abscisic acid (ABA) receptor ABAR] positively regulates guard cell signalling in response to ABA, but the molecular mechanism remains largely unknown. A member of the sucrose nonfermenting 1 (SNF1)-related protein kinase 2 family, SnRK2.6/open stomata 1 (OST1)/SRK2E, which plays a critical role in ABA signalling in Arabidopsis guard cells, interacts with ABAR/CHLH. Neither mutation nor over-expression of the ABAR gene affects significantly ABA-insensitive phenotypes of stomatal movement in the OST1 knockout mutant allele srk2e. However, OST1 over-expression suppresses ABA-insensitive phenotypes of the ABAR mutant allele cch in stomatal movement. These genetic data support that OST1 functions downstream of ABAR in ABA signalling in guard cells. Consistent with this, ABAR protein is phosphorylated, but independently of the OST1 protein kinase. Two ABAR mutant alleles, cch and rtl1, show ABA insensitivity in ABA-induced reactive oxygen species and nitric oxide production, as well as in ABA-activated phosphorylation of a K+ inward channel KAT1 in guard cells, which is consistent with that observed in the pyr1 pyl1 pyl2 pyl4 quadruple mutant of the well-characterized ABA receptor PYR/PYL/RCAR family acting upstream of OST1. These findings suggest that ABAR shares, at least in part, downstream signalling components with PYR/PYL/RCAR receptors for ABA in guard cells; though cch and rtl1 show strong ABA-insensitive phenotypes in both ABA-induced stomatal closure and inhibition of stomatal opening, while the pyr1 pyl1 pyl2 pyl4 quadruple mutant shows strong ABA insensitivity only in ABA-induced stomatal closure. These data establish a link between ABAR/CHLH and SnRK2.6/OST1 in guard cell signalling in response to ABA.
DOI:10.1104/pp.16.01817URLPMID:28184010 [本文引用: 1]

Ubiquitin-mediated protein modification occurs at multiple steps of abscisic acid (ABA) signaling. Here, we sought proteins responsible for degradation of the pepper type 2C protein phosphatase CaADIP1 via the 26S proteasome system. We showed that the RING type E3 ligase CaAIRF1 (Capsicum annuum ADIP1 Interacting RING Finger Protein 1) interacts with and ubiquitinates CaADIP1. CaADIP1 degradation was slower in crude proteins from CaAIRF1-silenced peppers than in those from control plants. CaAIRF1-silenced pepper plants displayed reduced ABA sensitivity and decreased drought tolerance characterized by delayed stomatal closure and suppressed induction of ABA- and drought-responsive marker genes. In contrast, CaAIRF1-overexpressing Arabidopsis plants exhibited ABA-hypersensitive and drought-tolerant phenotypes. Moreover, in these plants, CaADIP1-induced ABA hyposensitivity was strongly suppressed by CaAIRF1 overexpression. Our findings highlight a potential new route for fine-tune regulation of ABA signaling in pepper via CaAIRF1 and CaADIP1.
DOI:10.1038/ncomms1716URLPMID:3316886 [本文引用: 1]

The formation of axillary meristems in leaf axils is a prerequisite for the development of lateral shoots, which largely contribute to plant architecture. Several transcription factor-encoding genes, including CUC3, RAX, LAS, LOF1, and ROX, have been cloned by screening for axillary meristem mutants in Arabidopsis thaliana. These genes will facilitate our understanding of the mechanisms... [Show full abstract]
DOI:10.1038/ncomms8981URL [本文引用: 1]

Abscisic acid (ABA) and gibberellic acid (GA) antagonistically regulate many developmental processes and responses to biotic or abiotic stresses in higher plants. However, the molecular mechanism underlying this antagonism is still poorly understood. Here, we show that loss-of-function mutation in rice Tiller Enhancer (TE), an activator of the APC/CTE complex, causes hypersensitivity and hyposensitivity to ABA and GA, respectively. We find that TE physically interacts with ABA receptor OsPYL/RCARs and promotes their degradation by the proteasome. Genetic analysis also shows OsPYL/RCARs act downstream of TE in mediating ABA responses. Conversely, ABA inhibits APC/CTE activity by phosphorylating TE through activating the SNF1-related protein kinases (SnRK2s), which may interrupt the interaction between TE and OsPYL/RCARs and subsequently stabilize OsPYL/RCARs. In contrast, GA can reduce the level of SnRK2s and may promote APC/CTE-mediated degradation of OsPYL/RCARs. Thus, we propose that the SnRK2-APC/CTE regulatory module represents a regulatory hub underlying the antagonistic action of GA and ABA in plants. The hormones abscisic acid and gibberellins act antagonistically in plant development and stress responses. Here Lin et al. show that the rice Tiller Enhancer protein is required for gibberellin-induced degradation of abscisic acid signalling components, uncovering mechanistic insights into hormone signalling crosstalk.
DOI:10.1038/ncomms8640URLPMID:26184543 [本文引用: 1]

Abstract N-terminal acetylation (NTA) catalysed by N-terminal acetyltransferases (Nats) is among the most common protein modifications in eukaryotes, but its significance is still enigmatic. Here we characterize the plant NatA complex and reveal evolutionary conservation of NatA biochemical properties in higher eukaryotes and uncover specific and essential functions of NatA for development, biosynthetic pathways and stress responses in plants. We show that NTA decreases significantly after drought stress, and NatA abundance is rapidly downregulated by the phytohormone abscisic acid. Accordingly, transgenic downregulation of NatA induces the drought stress response and results in strikingly drought resistant plants. Thus, we propose that NTA by the NatA complex acts as a cellular surveillance mechanism during stress and that imprinting of the proteome by NatA is an important switch for the control of metabolism, development and cellular stress responses downstream of abscisic acid.
Dendrobium.
DOI:10.1186/s12870-015-0613-3URLPMID:4574183 [本文引用: 1]

SUMOylation is an important post-translational modification of eukaryotic proteins that involves the reversible conjugation of a small ubiquitin-related modifier (SUMO) polypeptide to its specific protein substrates, thereby regulating numerous complex cellular processes. The PIAS (protein inhibitor of activated signal transducers and activators of transcription [STAT]) and SIZ (scaffold attachment factor A/B/acinus/PIAS [SAP] and MIZ) proteins are SUMO E3 ligases that modulate SUMO conjugation. The characteristic features and SUMOylation mechanisms of SIZ1 protein in monocotyledon are poorly understood. Here, we examined the functions of a homolog of Arabidopsis SIZ1, a functional SIZ/PIAS-type SUMO E3 ligase from Dendrobium. In Dendrobium, the predicted DnSIZ1 protein has domains that are highly conserved among SIZ/PIAS-type proteins. DnSIZ1 is widely expressed in Dendrobium organs and has a up-regulated trend by treatment with cold, high temperature and wounding. The DnSIZ1 protein localizes to the nucleus and shows SUMO E3 ligase activity when expressed in an Escherichia coli reconstitution system. Moreover, ectopic expression of DnSIZ1 in the Arabidopsis siz1-2 mutant partially complements several phenotypes and results in enhanced levels of SUMO conjugates in plants exposed to heat shock conditions. We observed that DnSIZ1 acts as a negative regulator of flowering transition which may be via a vernalization-induced pathway. In addition, ABA-hypersensitivity of siz1-2 seed germination can be partially suppressed by DnSIZ1. Our results suggest that DnSIZ1 is a functional homolog of the Arabidopsis SIZ1 with SUMO E3 ligase activity and may play an important role in the regulation of Dendrobium stress responses, flowering and development.
[本文引用: 1]
[本文引用: 1]
DOI:10.1101/gad.1055803URL [本文引用: 1]

Plants have evolved protective mechanisms to ensure their survival when threatened by adverse environmental conditions during their transition to autotrophic growth. During germination, there is a 2- to 3-d period during which a plant can execute growth arrest when challenged by water deficit. This postgermination developmental checkpoint is signaled by the stress hormone abscisic acid (ABA), which induces the expression of the bZIP transcription activator ABI5. The growth arrest efficiency depends on ABI5 levels, and mutants are ABA-insensitive and unable to execute the ABA-mediated growth arrest. Here we show that a novel ABI5-interacting protein, designated as AFP, can form high molecular weight (Mr) complexes with ABI5 in embryo-derived extracts. Like ,() mRNA and protein levels are induced by ABA during seed germination. Two different mutant alleles (and ) are hypersensitive to ABA, whereas transgenic plants overexpressing AFP are resistant; in these plants, AFP and ABI5 protein levels are inversely correlated. Genetic analysis shows that is epistatic to, indicating the ABA hypersensitivity of mutants requires ABI5. Proteasome inhibitor studies show that ABI5 stability is regulated by ABA through ubiquitin-related events. When expressed together, AFP and ABI5 are colocalized in nuclear bodies, which also contain COP1, a RING motif protein. Our results suggest that AFP attenuates ABA signals by targeting ABI5 for ubiquitin-mediated degradation in nuclear bodies.
DOI:10.4161/psb.5.3.11235URLPMID:20007448 [本文引用: 1]

Nitric oxide (NO) is a gas with crucial signaling functions in plant defence and development. As demonstrated by generating a triple nia1nia2noa1-2 mutant with extremely low levels of NO (February 2010 issue of Plant Physiology), NO is synthesized in plants through mainly two different pathways involving nitrate reductase (NR/NIA) and NO Associated 1 (AtNOA1) proteins. Depletion of basal NO levels leads to a priming of ABA-triggered responses that causes hypersensitivity to this hormone and results in enhanced seed dormancy and decreased seed germination and seedling establishment in the triple mutant. NO produced under non-stressed conditions represses inhibition of seed developmental transitions by ABA. Moreover, NO plays a positive role in post-germinative vegetative development and also exerts a critical control of ABA-related functions on stomata closure. The triple nia1nia2noa1-2 mutant is hypersensitive to ABA in stomatal closure thus resulting in a extreme phenotype of resistance to drought. In the light of the recent discovery of PYR/PYL/RCAR as a family of potential ABA receptors, regulation of ABA sensitivity by NO may be exerted either directly on ABA receptors or on downstream signalling components; both two aspects that deserve our present and future attention.
DOI:10.1111/j.1365-313X.2006.02730.xURLPMID:16640601 [本文引用: 1]

CHIP proteins are E3 ubiquitin ligases that promote degradation of Hsp70 and Hsp90 substrate proteins through the 26S proteasome in animal systems. A CHIP-like protein in Arabidopsis, AtCHIP, also has E3 ubiquitin ligase activity and has important roles to play under conditions of abiotic stress. In an effort to study the mode of action of AtCHIP in plant cells, proteins that physically interact with it were identified. Like its animal orthologs, AtCHIP interacts with a unique class of ubiquitin-conjugating enzymes (UBC or E2) that belongs to the stress-inducible UBC4/5 class in yeast. AtCHIP also interacts with other proteins, including an A subunit of protein phosphatase 2A (PP2A). This PP2A subunit appears to be a substrate of AtCHIP, because it can be ubiquitylated by AtCHIP in vitro and because the activity of PP2A is increased in AtCHIP -overexpressing plants in the dark or under low-temperature conditions. Unlike the rcn1 mutant, that has reduced PP2A activity due to a mutation in one of the A subunit genes of PP2A, AtCHIP -overexpressing plants are more sensitive to ABA treatment. Since PP2A was previously shown to be involved in low-temperature responses in plants, the low-temperature-sensitive phenotype observed in AtCHIP -overexpressing plants might be partly due to the change in PP2A activity. These data suggest that the E3 ubiquitin ligase AtCHIP may function upstream of PP2A in stress-responsive signal transduction pathways under conditions of low temperature or in the dark.
DOI:10.1126/science.1059780URLPMID:11337588 [本文引用: 1]

SCF ubiquitin ligases control various processes by marking regulatory proteins for ubiquitin-dependent proteolysis. To illuminate how SCF complexes are regulated, we sought proteins that interact with the human SCF component CUL1. The COP9 signalosome (CSN), a suppressor of plant photomorphogenesis, associated with multiple cullins and promoted cleavage of the ubiquitin-like protein NEDD8 from Schizosaccharomyces pombe CUL1 in vivo and in vitro. Multiple NEDD8-modified proteins uniquely accumulated in CSN-deficient S. pombe cells. We propose that the broad spectrum of activities previously attributed to CSN subunits-including repression of photomorphogenesis, activation of JUN, and activation of p27 nuclear export-underscores the importance of dynamic cycles of NEDD8 attachment and removal in biological regulation.
DOI:10.1093/jxb/ert123URLPMID:36979547 [本文引用: 1]

The RING-type E3 ligase, Keep on Going (KEG), is required for early seedling establishment in Arabidopsis thaliana. Post-germination, KEG negatively regulates abscisic acid (ABA) signalling by targeting Abscisic Acid Insensitive 5 (ABI5) for ubiquitination and subsequent degradation. Previous reports suggest that the role of KEG during early seedling development is not limited to regulation of ABI5 abundance. Using a yeast two-hybrid screen, this study identified Calcineurin B-like Interacting Protein Kinase (CIPK) 26 as a KEG-interacting protein. In vitro pull-down and in planta bimolecular fluorescence complementation assays confirmed the interactions between CIPK26 and KEG. In planta experiments demonstrated that CIPK26 was ubiquitinated and degraded via the 26S proteasome. It was also found that turnover of CIPK26 was increased when KEG protein levels were elevated, suggesting that the RING-type E3 ligase is involved in targeting CIPK26 for degradation. CIPK26 was found to interact with the ABA signalling components ABI1, ABI2, and ABI5. In addition, CIPK26 was capable of phosphorylating ABI5 in vitro. Consistent with a role in ABA signalling, overexpression of CIPK26 increased the sensitivity of germinating seeds to the inhibitory effects of ABA. The data presented in this report suggest that KEG mediates the proteasomal degradation of CIPK26 and that CIPK26 is part of the ABA signalling network.
DOI:10.3389/fpls.2017.00502URLPMID:5385374 [本文引用: 1]

The Really Interesting New Gene (RING)-type E3 ligase, Keep on Going (KEG) plays a critical role in Arabidopsis growth after germination and the connections between KEG and hormone signaling pathways are expanding. With regards to abscisic acid (ABA) signaling, KEG targets ABA-responsive transcription factors abscisic acid insensitive 5, ABF1 and ABF3 for ubiquitination and subsequent degradation through the 26S proteasome. Regulation of E3 ligases through self-ubiquitination is common to RING-type E3 ligases and ABA promotes KEG self-ubiquitination and degradation. ABA-mediated degradation of KEG is phosphorylation-dependent; however, upstream signaling proteins that may regulate KEG stability have not been characterized. In this report, we show that CBL-Interacting Protein Kinase (CIPK) 26 can phosphorylate KEGin vitro.Using bothin vitroandin plantadegradation assays we provide evidence which suggests that the kinase activity of CIPK26 promotes the degradation of KEG. Furthermore, we found that the kinase activity of CIPK26 also influences its own stability; a constitutively active version is more stable than a wild type or a kinase dead version. Our results suggest a reciprocal regulation model wherein an activated and stable CIPK26 phosphorylates KEG to promote degradation of the E3.
DOI:10.1111/pce.13013URLPMID:28667821 [本文引用: 1]

Phytohormone abscisic acid (ABA) regulates many important processes in plants. It is a major molecule facilitating signal transduction during the abiotic stress response. In this study, an ABA‐inducible transcription factor gene, MdAREB2, was identified in apple. Transgenic analysis was performed to characterize its function in ABA sensitivity. Overexpression of the MdAREB2 gene increased ABA sensitivity in the transgenic apple compared with the WT control. In addition, it was found that the protein MdAREB2 was phosphorylated at a novel site Thrin response to ABA. A yeast two‐hybridization screen of an apple cDNA library demonstrated that a protein kinase, MdCIPK22, interacted with MdAREB2. Their interaction was further verified with Pull Down and Co‐IP assays. A series of transgenic analyses in apple calli and plantlets showed that MdCIPK22 was required for ABA‐induced phosphorylation at Throf the MdAREB2 protein and enhanced its stability and transcriptional activity. Finally, it was found that MdCIPK22 increased ABA sensitivity in an MdAREB2‐dependent manner. Our findings indicate a novel phosphorylation site in CIPK‐AREB regulatory module for the ABA signaling pathway, which would be helpful for researchers to identify the functions of uncharacterized homologs in the future.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.molp.2015.06.006URLPMID:26099923 [本文引用: 1]
DOI:10.1105/tpc.106.044230URLPMID:16998070 [本文引用: 1]

We isolated two T-DNA insertion mutants of Arabidopsis thaliana GLUTATHIONE PEROXIDASE3 (ATGPX3) that exhibited a higher rate of water loss under drought stress, higher sensitivity to H60O60 treatment during seed germination and seedling development, and enhanced production of H60O60 in guard cells. By contrast, lines engineered to overexpress ATGPX3 were less sensitive to drought stress than the wild type and displayed less transpirational water loss, which resulted in higher leaf surface temperature. The atgpx3 mutation also disrupted abscisic acid (ABA) activation of calcium channels and the expression of ABA- and stress-responsive genes. ATGPX3 physically interacted with the 2C-type protein phosphatase ABA INSENSITIVE2 (ABI2) and, to a lesser extent, with ABU. In addition, the redox states of both ATGPX3 and ABI2 were found to be regulated by H60O60. The phosphatase activity of ABI2, measured in vitro, was reduced approximately fivefold by the addition of oxidized ATGPX3. The reduced form of ABI2 was converted to the oxidized form by the addition of oxidized ATGPX3 in vitro, which might mediate ABA and oxidative signaling. These results suggest that ATGPX3 might play dual and distinctive roles in H60O60 homeostasis, acting as a general scavenger and specifically relaying the H60O60 signal as an oxidative signal transducer in ABA and drought stress signaling.
DOI:10.1093/jxb/ery165URL [本文引用: 2]
DOI:10.1073/pnas.0811088106URL [本文引用: 2]

SUMO (small ubiquitin-related modifier) conjugation (i.e., sumoylation) to protein substrates is a reversible posttranslational modification that regulates signaling by modulating transcription factor activity. This paper presents evidence that the SUMO E3 ligase SIZ1 negatively regulates abscisic acid (ABA) signaling, which is dependent on the bZIP transcription factor ABI5. Loss-of-function T-DNA insertion siz1-2 and siz1-3 mutations caused ABA hypersensitivity for seed germination arrest and seedling primary root growth inhibition. Furthermore, expression of genes that are ABA-responsive through ABI5-dependent signaling (e.g., RD29A, Rd29B, AtEm6, RAB18, ADM) was hyperinduced by the hormone in siz1 seedlings. abi5-4 suppressed ABA hypersensitivity caused by siz1 (siz1-2 abiS-4), demonstrating an epistatic genetic interaction between SIZ1 and ABI5. A K391R substitution in ABI5 [ABI5(K391R)] blocked SIZ1-mediated sumoylation of the transcription factor in vitro and in Arabidopsis protoplasts, indicating that ABI5 is sumoylated through SIZ1 and that K391 is the principal site for SUMO conjugation. In abi5-4 plants, ABI5(K391R) expression caused greater ABA hypersensitivity (gene expression, seed germination arrest and primary root growth inhibition) compared with ABI5 expression. Together, these results establish that SIZ1-dependent sumoylation of ABI5 attenuates ABA signaling. The double mutant siz1-2 afp-1 exhibited even greater ABA sensitivity than the single mutant siz1, suggesting that SIZ1 represses ABI5 signaling function independent of AFP1.
DOI:10.1093/jxb/ert401URLPMID:24307718 [本文引用: 1]

Protein kinase CK2 (formerly known as casein kinase II) is a ubiquitious Ser/Thr kinase present in all eukaryotes. The α (catalytic) and β (regulatory) subunits of CK2 exist both as a tetrameric...
DOI:10.1093/aobpla/pls052URLPMID:23372921 [本文引用: 1]

Background and aims After a series of seminal works during the last decade of the 20th century, nitric oxide (NO) is now firmly placed in the pantheon of plant signals. Nitric oxide acts in plant???microbe interactions, responses to abiotic stress, stomatal regulation and a range of developmental processes. By considering the recent advances in plant NO biology, this review will highlight certain key aspects that require further attention.<br>Scope and conclusions The following questions will be considered. While cytosolic nitrate reductase is an important source of NO, the contributions of other mechanisms, including a poorly defined arginine oxidizing activity, need to be characterized at the molecular level. Other oxidative pathways utilizing polyamine and hydroxylamine also need further attention. Nitric oxide action is dependent on its concentration and spatial generation patterns. However, no single technology currently available is able to provide accurate in planta measurements of spatio-temporal patterns of NO production. It is also the case that pharmaceutical NO donors are used in studies, sometimes with little consideration of the kinetics of NO production. We here include in planta assessments of NO production from diethylamine nitric oxide, S-nitrosoglutathione and sodium nitroprusside following infiltration of tobacco leaves, which could aid workers in their experiments. Further, based on current data it is difficult to define a bespoke plant NO signalling pathway, but rather NO appears to act as a modifier of other signalling pathways. Thus, early reports that NO signalling involves cGMP???as in animal systems???require revisiting. Finally, as plants are exposed to NO from a number of external sources, investigations into the control of NO scavenging by such as non-symbiotic haemoglobins and other sinks for NO should feature more highly. By crystallizing these questions the authors encourage their resolution through the concerted efforts of the plant NO community.<br>
DOI:10.1016/j.plantsci.2018.05.007URL [本文引用: 1]

Protein N- glycosylation is one of the major post-translational modifications in eukaryotic cells. In lower unicellular eukaryotes, the known functions of N- glycans are predominantly in protein folding and quality control within the lumen of the endoplasmic reticulum (ER). In multicellular organisms, complex N- glycans are important for developmental programs and immune responses. However, little is known about the functions of complex N- glycans in plants. Formed in the Golgi apparatus, plant complex N- glycans have structures distinct from their animal counterparts due to a set of glycosyltransferases unique to plants. Severe basal underglycosylation in the ER lumen induces misfolding of newly synthesized proteins, which elicits the unfolded protein response (UPR) and ER protein quality control (ERQC) pathways. The former promotes higher capacity of proper protein folding and the latter degradation of misfolded proteins to clear the ER. Although our knowledge on plant complex N- glycan functions is limited, genetic studies revealed the importance of complex N- glycans in cellulose biosynthesis and growth under stress.
DOI:10.1073/pnas.1118651109URLPMID:22160701 [本文引用: 1]

Abscisic acid (ABA) is an essential hormone that controls plant growth, development, and responses to abiotic stresses. Central for ABA signaling is the ABA-mediated autoactivation of three monomeric Snf1-related kinases (SnRK2.2, -2.3, and -2.6). In the absence of ABA, SnRK2s are kept in an inactive state by forming physical complexes with type 2C protein phosphatases (PP2Cs). Upon relief of this inhibition, SnRK2 kinases can autoactivate through unknown mechanisms. Here, we report the crystal structures of full-length Arabidopsis thaliana SnRK2.3 and SnRK2.6 at 1.9- and 2.3-脜 resolution, respectively. The structures, in combination with biochemical studies, reveal a two-step mechanism of intramolecular kinase activation that resembles the intermolecular activation of cyclin-dependent kinases. First, release of inhibition by PP2C allows the SnRK2s to become partially active because of an intramolecular stabilization of the catalytic domain by a conserved helix in the kinase regulatory domain. This stabilization enables SnRK2s to gain full activity by activation loop autophosphorylation. Autophosphorylation is more efficient in SnRK2.6, which has higher stability than SnRK2.3 and has well-structured activation loop phosphate acceptor sites that are positioned next to the catalytic site. Together, these data provide a structural framework that links ABA-mediated release of PP2C inhibition to activation of SnRK2 kinases.
DOI:10.1016/j.cell.2008.12.026URLPMID:19135895 [本文引用: 1]

Abstract In plants, G proteins modulate signaling by the stress hormone, abscisic acid (ABA). We identify and characterize two novel Arabidopsis proteins that show homology to an orphan vertebrate GPCR (GPR89) and interact with the sole Arabidopsis G protein alpha subunit, GPA1, but also have intrinsic GTP-binding and GTPase activity. We have named these proteins GPCR-type G proteins (GTG1 and GTG2). Arabidopsis mutants lacking both GTG1 and GTG2 exhibit ABA hyposensitivity. GTG1 and GTG2 bind ABA specifically. The GDP-bound form of the GTGs exhibits greater ABA binding than the GTP-bound form, the GTPase activity of the GTGs is inhibited by GPA1, and gpa1 null mutants exhibit ABA-hypersensitive phenotypes. These results predict that, unusually, it is the GDP-bound, not the GTP-bound, form of the GTGs that actively relays the signal. We propose that GTG proteins function both as a new type of G protein and as a class of membrane-localized ABA receptors.
DOI:10.1111/j.1365-3040.2010.02195.xURLPMID:20561251 [本文引用: 1]

Sumoylation is a post-translational regulatory process in diverse cellular processes in eukaryotes, involving conjugation/deconjugation of small ubiquitin-like modifier (SUMO) proteins to other proteins thus modifying their function. The PIAS [protein inhibitor of activated signal transducers and activators of transcription (STAT)] and SAP (scaffold attachment factor A/B/acinus/PIAS)/MIZ (SIZ) proteins exhibit SUMO E3-ligase activity that facilitates the conjugation of SUMO proteins to target substrates. Here, we report the isolation and molecular characterization of Oryza sativa SIZ1 (OsSIZ1) and SIZ2 (OsSIZ2), rice homologs of Arabidopsis SIZ1. The rice SIZ proteins are localized to the nucleus and showed sumoylation activities in a tobacco system. Our analysis showed increased amounts of SUMO conjugates associated with environmental stresses such as high and low temperature, NaCl and abscisic acid (ABA) in rice plants. The expression of OsSIZ1 and OsSIZ2 in siz1-2 Arabidopsis plants partially complemented the morphological mutant phenotype and enhanced levels of SUMO conjugates under heat shock conditions. In addition, ABA-hypersensitivity of siz1-2 seed germination was partially suppressed by OsSIZ1 and OsSIZ2. The results suggest that rice SIZ1 and SIZ2 are able to functionally complement Arabidopsis SIZ1 in the SUMO conjugation pathway. Their effects on the Arabidopsis mutant suggest a function for these genes related to stress responses and stress adaptation.
[本文引用: 1]
DOI:10.1016/j.molp.2015.10.003URLPMID:26499068 [本文引用: 1]

TheArabidopsisSWI/SNF chromatin-remodeling ATPase BRAHMA (BRM) represses abscisic acid (ABA) response during germination and establishment. Genetic and biochemical studies reveal an ABA-dependent phosphorylation-based switch to control BRM activity. Thus, SnRK2-dependent phosphorylation of BRM leads to its inhibition, and PP2CA-mediated dephosphorylation of BRM restores its ability to repress ABA response.
DOI:10.1111/jipb.12654URLPMID:29660240 [本文引用: 1]

Cold stress is a major environmental factor that limits plant growth and development. The C-repeat-binding factor (CBF)-dependent cold signaling pathway is extensively studied in Arabidopsis; however, the specific protein kinases involved in this pathway remain elusive. Here we report that OST1 (OPEN STOMATA 1), a well-known Ser/Thr protein kinase in ABA signaling, acts upstream of CBFs to... [Show full abstract]
DOI:10.1111/j.1365-313X.2009.03846.xURLPMID:19309463 [本文引用: 1]

During leaf senescence, resources are recycled by redistribution to younger leaves and reproductive organs. Candidate pathways for the regulation of onset and progression of leaf senescence include ubiquitin-dependent turnover of key proteins. Here, we identified a novel plant U-box E3 ubiquitin ligase that prevents premature senescence in Arabidopsis plants, and named it SENESCENCE-ASSOCIATED E3 UBIQUITIN LIGASE 1 (SAUL1). Using in vitro ubiquitination assays, we show that SAUL1 has E3 ubiquitin ligase activity. We isolated two alleles of saul1 mutants that show premature senescence under low light conditions. The visible yellowing of leaves is accompanied by reduced chlorophyll content, decreased photochemical efficiency of photosystem II and increased expression of senescence genes. In addition, saul1 mutants exhibit enhanced abscisic acid (ABA) biosynthesis. We show that application of ABA to Arabidopsis is sufficient to trigger leaf senescence, and that this response is abolished in the ABA-insensitive mutants abi1-1 and abi2-1 , but enhanced in the ABA-hypersensitive mutant era1-3 . We found that increased ABA levels coincide with enhanced activity of Arabidopsis aldehyde oxidase 3 (AAO3) and accumulation of AAO3 protein in saul1 mutants. Using label transfer experiments, we showed that interactions between SAUL1 and AAO3 occur. This suggests that SAUL1 participates in targeting AAO3 for ubiquitin-dependent degradation via the 26S proteasome to prevent premature senescence.
DOI:10.1016/j.molcel.2017.12.013URL [本文引用: 1]

For optimal growth, plants must tightly control the switch between stress responses and regrowth upon restoration of favorable conditions. In this issue of,reveal that reciprocal regulation of growth-promoting TOR and stress-activated SnRK2 facilitates plant adaptation to environmental variations.
DOI:10.1073/pnas.1511238112URLPMID:26540727 [本文引用: 1]

Plant response to drought and hyperosmosis is mediated by the phytohormone abscisic acid (ABA), a sesquiterpene compound widely distributed in various embryophyte groups. Exogenous ABA as well as hyperosmosis activates the sucrose nonfermenting 1 (SNF1)-related protein kinase2 (SnRK2), which plays a central role in cellular responses against drought and dehydration, although the details of the activation mechanism are not understood. Analysis of a mutant of the moss Physcomitrella patens with reduced ABA sensitivity and reduced hyperosmosis tolerance revealed that a protein kinase designated "ARK" (for "ABA and abiotic stress-responsive Raf-like kinase") plays an essential role in the activation of SnRK2. ARK encoded by a single gene in P. patens belongs to the family of group B3 Raf-like MAP kinase kinase kinases (B3-MAPKKKs) mediating ethylene, disease resistance, and salt and sugar responses in angiosperms. Our findings indicate that ARK, as a novel regulatory component integrating ABA and hyperosmosis signals, represents the ancestral B3-MAPKKKs, which multiplied, diversified, and came to have specific functions in angiosperms.
DOI:10.1042/BJ20091221URL [本文引用: 1]
[本文引用: 2]
DOI:10.1105/tpc.113.119974URLPMID:24563203 [本文引用: 1]

Members of the DDB1-CUL4鈥揳ssociated factors (DCAFs) family directly bind to DAMAGED DNA BINDING PROTEIN1 (DDB1) and function as the substrate receptors in CULLIN4-based E3 (CUL4) ubiquitin ligases, which regulate the selective ubiquitination of proteins. Here, we describe a DCAF protein, ABD1 (for ABA-hypersensitive DCAF1), that negatively regulates abscisic acid (ABA) signaling in Arabidopsis thaliana. ABD1 interacts with DDB1 in vitro and in vivo, indicating that it likely functions as a CUL4 E3 ligase substrate receptor. ABD1 expression is induced by ABA, and mutations in ABD1 result in ABA- and NaCl-hypersensitive phenotypes. Loss of ABD1 leads to hyperinduction of ABA-responsive genes and higher accumulation of the ABA-responsive transcription factor ABA INSENSITIVE5 (ABI5), hypersensitivity to ABA during seed germination and seedling growth, enhanced stomatal closure, reduced water loss, and, ultimately, increased drought tolerance. ABD1 directly interacts with ABI5 in yeast two-hybrid assays and associates with ABI5 in vivo by coimmunoprecipitation, and the interaction was found in the nucleus by bimolecular fluorescence complementation. Furthermore, loss of ABD1 results in a retardation of ABI5 degradation by the 26S proteasome. Taken together, these data suggest that the DCAF-CUL4 E3 ubiquitin ligase assembled with ABD1 is a negative regulator of ABA responses by directly binding to and affecting the stability of ABI5 in the nucleus.
DOI:10.1016/j.molp.2015.12.014URLPMID:26724418 [本文引用: 2]

有气孔的运动在为在植物纸巾和空气之间的光合作用和水平衡调整煤气的交换是批评的。植物荷尔蒙 abscisic 酸(骆驼毛的织物) 戏在在各种各样的不能生活的压力下面调整有气孔的闭合给角色调音。在这研究,我们在警卫房间骆驼毛的织物发信号揭示了 BAK1 的一个新奇角色。我们发现表明变异的 bak1 的 brassinosteroid (BR ) 比野类型的植物失去了更多的水并且在有气孔的闭合显示出骆驼毛的织物 insensitivity。导致骆驼毛的织物的 OST1 表示和生产也是的反应的氧种类(ROS ) 在 bak1 损害了。有 H 2 OST1 的 O 2 , overexpression 完全没救 bak1 的 insensitivity 到骆驼毛的织物。我们证明 BAK1 在血浆膜附近与 OST1 形成建筑群并且 BAK1/OST1 建筑群在 planta 响应骆驼毛的织物被增加。Brassinolide,最活跃的 BR,在 BAK1/OST1 建筑群和 OST1 表示的导致骆驼毛的织物的形成上施加了否定效果。而且,我们发现 BAK1 和 ABI1 相对地调整 OST1 phosphorylation invitro,和那 ABI1 与 BAK1 交往并且禁止 BAK1 和 OST1 的相互作用。一起拿,我们的结果建议 BAK1 在警卫房间调整导致骆驼毛的织物的有气孔的闭合。
DOI:10.1038/nature05176URLPMID:17051210 [本文引用: 1]

Abscisic acid (ABA) is a vital phytohormone that regulates mainly stomatal aperture and seed development, but ABA receptors involved in these processes have yet to be determined. We previously identified from broad bean an ABA-binding protein (ABAR) potentially involved in stomatal signalling, the gene for which encodes the H subunit of Mg-chelatase (CHLH), which is a key component in both chlorophyll biosynthesis and plastid-to-nucleus signalling. Here we show that Arabidopsis ABAR/CHLH specifically binds ABA, and mediates ABA signalling as a positive regulator in seed germination, post-germination growth and stomatal movement, showing that ABAR/CHLH is an ABA receptor. We show also that ABAR/CHLH is a ubiquitous protein expressed in both green and non-green tissues, indicating that it might be able to perceive the ABA signal at the whole-plant level.
DOI:10.3389/fpls.2015.00895URLPMID:4625044 [本文引用: 1]

Drought is one of the leading factors responsible for the reduction in crop yield worldwide. Due to climate change, in future, more areas are going to be affected by drought and for prolonged periods. Therefore, understanding the mechanisms underlying the drought response is one of the major scientific concerns for improving crop yield. Plants deploy diverse strategies and mechanisms to respond and tolerate drought stress. Expression of numerous genes is modulated in different plants under drought stress that help them to optimize their growth and development. Plant hormone abscisic acid (ABA) plays a major role in plant response and tolerance by regulating the expression of many genes under drought stress. Transcription factors being the major regulator of gene expression play a crucial role in stress response. ABA regulates the expression of most of the target genes through ABA-responsive element (ABRE) binding protein/ABRE binding factor (AREB/ABF) transcription factors. Genes regulated by AREB/ABFs constitute a regulon termed as AREB/ABF regulon. In addition to this, drought responsive genes are also regulated by ABA-independent mechanisms. In ABA-independent regulation, dehydration-responsive element binding protein (DREB), NAM, ATAF, and CUC regulons play an important role by regulating many drought-responsive genes. Apart from these major regulons, MYB/MYC, WRKY, and nuclear factor-Y (NF-Y) transcription factors are also involved in drought response and tolerance. Our understanding about transcriptional regulation of drought is still evolving. Recent reports have suggested the existence of crosstalk between different transcription factors operating under drought stress. In this article, we have reviewed various regulons working under drought stress and their crosstalk with each other.
DOI:10.1016/j.febslet.2009.08.033URLPMID:19716822 [本文引用: 2]

The plant hormone abscisic acid (ABA) triggers production of reactive oxygen species (ROS) in guard cells via the AtrbohD and AtrbohF NADPH oxidases, leading to stomatal closure. The ABA-activated SnRK2 protein kinase open stomata 1 (OST1) (SRK2E/SnRK2.6) acts upstream of ROS in guard cell ABA signaling. Here, we report that OST1 phosphorylates Ser13 and Ser174 on AtrbohF. In addition, substitution of Ser174 to Ala results in a 鈭40% reduction in the phosphorylation of AtrbohF by OST1. We also show that OST1 physically interacts with AtrbohF. These results provide biochemical evidence suggesting that OST1 regulates AtrbohF activity. MINT- 7260179, MINT- 7260147, MINT- 7260165: OST1 (uniprotkb: Q940H6) phosphorylates (MI: 0217) ATRBOHF (uniprotkb: O48538) by protein kinase assay (MI: 0424) MINT- 7260208: OST1 (uniprotkb: Q940H6) and ATRBOHF (uniprotkb: O48538) physically interact (MI: 0915) by bimolecular fluorescence complementation (MI: 0809)
DOI:10.1111/tpj.13739URLPMID:29024118 [本文引用: 1]

Abstract Conjugation of SUMO (Small Ubiquitin-like Modifier) protein to cellular targets is emerging as a very influential protein modification system. Once covalently bound SUMO conjugation can change the stability or functionality of its cognate target proteins. SUMO protease can rapidly reverse SUMO conjugation making this modification system highly dynamic. A major factor in the variation of SUMO-target function is the balance between the conjugated/de-conjugated forms. The mechanistic role of these regulatory SUMO proteases in mediating stress responses has not been defined in any crops. In this study, we reveal the role of the SUMO protease, OsOTS1 in mediating tolerance to drought in rice. OsOTS1 depleted transgenic plants accumulate more ABA and exhibit more productive agronomic traits during drought whilst OsOTS1 overexpressing lines are drought sensitive but ABA insensitive. Drought and ABA treatment stimulates the degradation of OsOTS1 protein indicating that SUMO conjugation is an important response to drought stress in rice achieved through down-regulation of OTS1/2 activity. We reveal that OsOTS1 SUMO protease directly targets the ABA and drought responsive transcription factor OsbZIP23 for de-SUMOylation affecting its stability. OsOTS-RNAi lines show increased abundance of OsbZIP23 and increased drought responsive gene expression while OsOTS1 overexpressing lines show reduced levels of OsbZIP23 leading to suppressed drought responsive gene expression. Our data reveals a mechanism where rice plants govern ABA dependant drought responsive gene expression by controlling the stability of OsbZIP23 by SUMO conjugation through manipulating specific SUMO protease levels. This article is protected by copyright. All rights reserved. This article is protected by copyright. All rights reserved.
DOI:10.1104/pp.15.01530URLPMID:26869703 [本文引用: 1]

SUMO (Small Ubiquitin-like Modifier) conjugation onto target proteins has emerged as a very influential class of protein modification system. SUMO1/2 double mutant plants are non viable underlining the importance of SUMO conjugation to plant survival. Once covalently bound SUMO can alter a conjugated protein's stability and/or functionality. SUMO conjugation is a highly dynamic process that can be rapidly reversed by the action of SUMO proteases. The balance between the conjugated / de-conjugated forms is a major determinant in the modulation of SUMO-target function. Given the important mechanistic role of SUMO proteases in model plants, till now the identity or the function of these regulatory enzymes has not been defined in any crop plant. In this report, we reveal the Ubiquitin-like Protease (ULP) class of SUMO protease gene family in rice and demonstrate a critical role for OsOTS1 SUMO protease in salt stress. OsOTS-RNAi rice plants accumulate high levels of SUMO1 conjugated proteins during salt stress and are highly salt sensitive however, in non-salt conditions they are developmentally indistinguishable from wild type plants. Transgenic rice plants overexpressing OsOTS1 have increased salt tolerance and a concomitant reduction in the levels of SUMOylated proteins. We demonstrate that OsOTS1 confers salt tolerance in rice by increasing root biomass. High salinity triggers OsOTS1 degradation indicating that increased SUMO conjugation in rice plants during salt stress is in part achieved by down-regulation of OTS1/2 activity. OsOTS1 is nuclear localised indicating a direct requirement of OsOTS1 dependent deSUMOylation activity in rice nuclei for salt tolerance.
DOI:10.1105/tpc.106.046532URLPMID:17194765 [本文引用: 1]

Analysis of the Arabidopsis thaliana RING-ANK (for Really Interesting New Gene-Ankyrin) family, a subgroup of RING-type E3 ligases, identified KEEP ON GOING (KEG) as essential for growth and development. In addition to the RING-HCa and ankyrin repeats, KEG contains a kinase domain and 12 HERC2-like repeats. The RING-HCa and kinase domains were functional in in vitro ubiquitylation and phosphorylation assays, respectively. Seedlings homozygous for T-DNA insertions in KEG undergo growth arrest immediately after germination, suggestive of increased abscisic acid (ABA) signaling, a major phytohormone that plays a key role in plant development and survival under unfavorable conditions. Here, we show that KEG is a negative regulator of ABA signaling, keg roots are extremely sensitive to the inhibitory effects of ABA and exhibit hypersensitivity to exogenous glucose, consistent with the known interaction between glucose and ABA signaling. The observations that KEG accumulates high levels of ABSCISIC ACID-INSENSITIVE5 (ABI5) without exogenous ABA, interacts with ABI5 in vitro, and that loss of ABI5 rescues the growth-arrest phenotype of keg mutant seedlings indicate that KEG is required for ABI5 degradation. In this capacity, KEG is central to ABA signaling by maintaining low levels of ABI5 in the absence of stress.
DOI:10.3389/fpls.2017.00677URLPMID:5410623 [本文引用: 1]

Specific cellular components including products of phosphatidylinositol (PI) metabolism play an important role as signaling molecules in stomatal responses to environmental signals. In this study, pharmacological inhibitors of a set of cellular components, including PI4-kinase (PI4K) and PI3K, were used to investigate stomatal closure in response to CO2, darkness, and abscisic acid (ABA). Treatment with PAO, a specific inhibitor of PI4K, specifically inhibited the stomatal response to CO2compared with that to darkness and ABA. In contrast, treatment with LY294002, a PI3K-specific inhibitor, specifically inhibited the stomatal response to darkness compared with that to CO2and ABA. The specific inhibitory effects of PAO and LY294002 were also observed as changes in the spatial density of dot-like structures labeled by green fluorescent protein-tagged PATROL1, a protein that controls stomatal aperture possibly via regulation of H+-ATPase amount in guard cell plasma membranes. Our results suggest an important role for PI4K and PI3K in the CO2and darkness signal transduction pathways, respectively, that mediate PATROL1 dynamics.
DOI:10.1104/pp.16.01825URLPMID:28438792 [本文引用: 1]

The plant hormone abscisic acid (ABA) confers drought tolerance in plants through stomatal closure and regulation of gene expression. The complex consisting of the ABA receptor PYRABACTIN RESISTANCE/REGULATORY COMPONENTS OF ABA RECEPTOR (PYR/RCAR), type 2C protein phosphatase (PP2C), and SNF1-related protein kinase 2 (SnRK2) has a key role in ABA signaling. Basic helix-loop-helix (bHLH) transcriptional activator ABA-RESPONSIVE KINASE SUBSTRATE1 (AKS1, also known as FBH3) is released from DNA by phosphorylation-induced monomerization in response to ABA in guard cells. Here we reconstituted the release of AKS1 from DNA via the ABA signaling core complex in vitro. We first obtained evidence to confirm that AKS1 is an endogenous substrate for SnRK2s. Phosphorylation of AKS1 and activation of SnRK2 showed the same time course in response to ABA in guard cells. AKS1 was bound to SnRK2.6 in vivo. Three ABA-responsive SnRK2s (SnRK2.2/SRK2D, SnRK2.3/SRK2I, and SnRK2.6/SRK2E/OST1) phosphorylated AKS1 in vitro, and the phosphorylation was eliminated by the triple mutation of SnRK2s in plants. We reconstituted the AKS1 phosphorylation in vitro via the signaling complex containing the ABA receptor PYR1, a PP2C, HYPERSENSITIVE TO ABA1 (HAB1), and a protein kinase, SnRK2.6 in response to ABA. We further reconstituted the release of AKS1 from the target gene of POTASSIUM CHANNEL IN ARABIDOPSIS THALIANA 1 (KAT1) via the complex in response to ABA. These results demonstrate that AKS1 provides a link between the signaling complex and ABA-responsive genes and furnish evidence for a minimal signaling mechanism from ABA perception to DNA.
DOI:10.1111/tpj.13217URLPMID:27227462 [本文引用: 1]

Summary We have demonstrated that the Arabidopsis basic helix–loop–helix (bHLH) transcription factor, ABA-responsive kinase substrate 1 (AKS1; also known as FLOWERING BHLH 3, FBH3), enhances K+ channel expression in guard cells leading to stomatal opening. The expression is suppressed by ABA-induced phosphorylation of AKS1. Here we show that the phosphorylation results in the monomerization of AKS1 multimers and inhibits AKS1 binding to DNA. AKS1 forms homo-multimers which dissociate following phosphorylation. Replacement of a critical amino acid in the bHLH domain inhibited multimer formation and decreased the binding of AKS1 to DNA. The monomerization was elicited via phosphorylation at three serine residues, which is mediated by SNF1-related protein kinase 2.6 (SnRK2.6), in the vicinity of bHLH domain. Furthermore, ABA induced the phosphorylation-dependent release of AKS1 from DNA, thereby suppressing transcriptional activity in02vivo . Our results document a mechanism that inhibits gene expression by phosphorylation of a bHLH transcription factor.
DOI:10.1371/journal.pgen.1007336URLPMID:29659577 [本文引用: 1]

Author summary Drought stress is a key environmental factor that severely reduces crop yield all over the world. The phytohormone abscisic acid (ABA) is known to mediate drought responses through regulating drought-responsive genes expression and stomatal closure, but the mechanisms that negatively regulate this process and prevent the adverse effects of excess drought responses on plant growth is less well studied. Here, we show that a HD-ZIP II transcription factor HAT1 negatively regulates ABA-mediated drought responses through suppressing ABA biosynthesis and signaling. The hat1hat3 mutant showed ABA-hypersensitive and drought-tolerant phenotypes, whereas the HAT1-overexpressing lines were insensitive to ABA and less tolerant to drought. Furthermore, we found SnRK2.3 kinase, a positive component of ABA signaling, interacts with and phosphorylates HAT1 to destabilize and suppress its binding activity. Overexpression of SnRK2.3 reduces HAT1 protein level and inhibits HAT1OX phenotypes in ABA and drought responses. Our results revealed a HAT1-mediated negative regulatory mechanism in attenuating the ABA signaling and drought response.
DOI:10.1105/tpc.16.00171URLPMID:27468891 [本文引用: 1]

Cold stress is one of the major limiting factors for rice productivity. Several MYB transcriptional factors have been reported as important regulators in cold response, but the molecular mechanisms are largely unknown. In this study, we characterized a cold-responsive R2R3-type MYB gene OsMYB30 for its regulatory function in cold tolerance in rice (Oryza sativa). Functional analysis revealed... [Show full abstract]
[本文引用: 3]
DOI:10.1093/pcp/pcq156URLPMID:20980270 [本文引用: 1]

ABA is a major phytohormone that regulates a broad range of plant traits and is especially important for adaptation to environmental conditions. Our understanding of the molecular basis of ABA responses in plants improved dramatically in 2009 and 2010, banner years for ABA research. There are three major components; PYR/PYL/ RCAR (an ABA receptor), type 2C protein phosphatase (PP2C; a negative regulator) and SNF1-related protein kinase 2 (SnRK2; a positive regulator), and they offer a double negative regulatory system, [PYR/PYL/RCAR—| PP2C—| SnRK2]. In the absence of ABA, PP2C inactivates SnRK2 by direct dephosphorylation. In response to environmental or developmental cues, ABA promotes the interaction of PYR/PYL/RCAR and PP2C, resulting in PP2C inhibition and SnRK2 activation. This signaling complex can work in both the nucleus and cytosol, as it has been shown that SnRK2 phosphorylates basic-domain leucine zipper (bZIP) transcription factors or membrane proteins. Several structural analyses of PYR/PYL/RCAR have provided the mechanistic basis for this ‘core signaling’ model, by elucidating the mechanism of ABA binding of receptors, or the ‘gate–latch–lock’ mechanism of interaction with PP2C in inhibiting activity. On the other hand, intercellular ABA transport had remained a major issue, as had intracellular ABA signaling. Recently, two plasma membrane-type ABC transporters were identified and shed light on the influx/efflux system of ABA, resolving how ABA is transported from cell to cell in plants. Our knowledge of ABA responses in plants has been greatly expanded from intracellular signaling to intercellular transport of ABA.
[本文引用: 2]
DOI:10.1016/B978-0-12-801922-1.00002-6URL [本文引用: 1]
DOI:10.1016/j.plantsci.2011.05.002URLPMID:21893249 [本文引用: 1]

Peroxynitrite (ONOO 61) is a reactive nitrogen species formed when nitric oxide (NO) reacts with the superoxide anion (O 2 61). It was first identified as a mediator of cell death in animals but was later shown to act as a positive regulator of cell signaling, mainly through the posttranslational modification of proteins by tyrosine nitration. In plants, peroxynitrite is not involved in NO-mediated cell death and its physiological function is poorly understood. However, it is emerging as a potential signaling molecule during the induction of defense responses against pathogens and this could be mediated by the selective nitration of tyrosine residues in a small number of proteins. In this review we discuss the general role of tyrosine nitration in plants and evaluate recent evidence suggesting that peroxynitrite is an effector of NO-mediated signaling following pathogen infection.
DOI:10.1073/pnas.0803996105URLPMID:18599455 [本文引用: 1]

Plant form is shaped by a complex network of intrinsic and extrinsic signals. Light-directed growth of seedlings (photomorphogenesis) depends on the coordination of several hormone signals, including brassinosteroids (BRs) and auxin. Although the close relationship between BRs and auxin has been widely reported, the molecular mechanism for combinatorial control of shared target genes has remained elusive. Here we demonstrate that BRs synergistically increase seedling sensitivity to auxin and show that combined treatment with both hormones can increase the magnitude and duration of gene expression. Moreover, we describe a direct connection between the BR-regulated BIN2 kinase and ARF2, a member of the Auxin Response Factor family of transcriptional regulators. Phosphorylation by BIN2 results in loss of ARF2 DNA binding and repression activities. arf2 mutants are less sensitive to changes in endogenous BR levels, whereas a large proportion of genes affected in an arf2 background are returned to near wild-type levels by altering BR biosynthesis. Together, these data suggest a model where BIN2 increases expression of auxin-induced genes by directly inactivating repressor ARFs, leading to synergistic increases in transcription.
DOI:10.1016/j.molp.2014.12.012URLPMID:25744360 [本文引用: 2]

SnRK2 kinases, PP2C 磷酸酶和 PYR/PYL/RCAR 受体组成表明被认为内在的性质包含所有到的模块的核心 abscisic 酸(骆驼毛的织物) 自我调整荷尔蒙信号产量。这里,我们识别酷蛋白 Kinase (CK ) 2 作为 SnRK2 的一个新奇否定管理者。CK2 phosphorylates 在 SnRK2 的 ABA 框的保存丝氨酸的簇,增加它的绑定到 PP2C 和被触发的蛋白质降级。因而, CK2 行动在到不能生活的刺激的 SnRK2 蛋白质层次,以及 kinase 活动和它的反应上有含意。
DOI:10.3389/fpls.2017.00161URLPMID:5316533 [本文引用: 1]

Abiotic stress is one of the severe stresses of environment that lowers the growth and yield of any crop even on irrigated land throughout the world. A major phytohormone abscisic acid (ABA) plays an essential part in acting toward varied range of stresses like heavy metal stress, drought, thermal or heat stress, high level of salinity, low temperature, and radiation stress. Its role is also elaborated in various developmental processes including seed germination, seed dormancy, and closure of stomata. ABA acts by modifying the expression level of gene and subsequent analysis ofcis- andtrans-acting regulatory elements of responsive promoters. It also interacts with the signaling molecules of processes involved in stress response and development of seeds. On the whole, the stress to a plant can be susceptible or tolerant by taking into account the coordinated activities of various stress-responsive genes. Numbers of transcription factor are involved in regulating the expression of ABA responsive genes by acting together with their respectivecis-acting elements. Hence, for improvement in stress-tolerance capacity of plants, it is necessary to understand the mechanism behind it. On this ground, this article enlightens the importance and role of ABA signaling with regard to various stresses as well as regulation of ABA biosynthetic pathway along with the transcription factors for stress tolerance.
DOI:10.1104/pp.15.00575URLPMID:26175513 [本文引用: 1]

The plant hormone abscisic acid (ABA) controls growth and development and regulates plant water status through an established signaling pathway. In the presence of ABA, pyrabactin resistance/regulatory component of ABA receptor proteins inhibit type 2C protein phosphatases (PP2Cs). This, in turn, enables the activation of Sucrose Nonfermenting1-Related Protein Kinases2 (SnRK2). Open Stomata1 (OST1)/SnRK2.6/SRK2E is a major SnRK2-type protein kinase responsible for mediating ABA responses. Arabidopsis (Arabidopsis thaliana) expressing an epitope-tagged OST1 in the recessive ost1-3 mutant background was used for the copurification and identification of OST1-interacting proteins after osmotic stress and ABA treatments. These analyses, which were confirmed using bimolecular fluorescence complementation and coimmunoprecipitation, unexpectedly revealed homo- and heteromerization of OST1 with SnRK2.2, SnRK2.3, OST1, and SnRK2.8. Furthermore, several OST1-complexed proteins were identified as type 2A protein phosphatase (PP2A) subunits and as proteins involved in lipid and galactolipid metabolism. More detailed analyses suggested an interaction network between ABA-activated SnRK2-type protein kinases and several PP2A-type protein phosphatase regulatory subunits. pp2a double mutants exhibited a reduced sensitivity to ABA during seed germination and stomatal closure and an enhanced ABA sensitivity in root growth regulation. These analyses add PP2A-type protein phosphatases as another class of protein phosphatases to the interaction network of SnRK2-type protein kinases.
DOI:10.1016/j.molp.2017.12.013URLPMID:29275167 [本文引用: 1]

Abscisic acid (ABA) and brassinosteroid (BR) antagonistically regulate many aspects of plant growth and development. A physiological study revealed that the inhibition of BR signaling by ABA is largely dependent on ABI1 and ABI2, but genetic and molecular evidence is lacking. In the BR signaling, their crosstalk occurs after BR receptor complex, but upstream of BIN2. However, which components act as the hub to directly mediate their crosstalk has remains a big mystery. Here, we found that ABI1 and ABI2 interact with BIN2, and dephosphorylate BIN2 to regulate its activity on the phosphorylation of BES1. By in vitro mimicking ABA signal transduction, we confirmed that ABA can promote BIN2 phosphorylation by inhibiting ABI2 through ABA receptors. RNA-seq analysis further supports that ABA inhibits BR signaling through the ABA primary signaling components, including its receptors and ABI2; and GSK3s co-regulate significant number of stress responsive genes together with ABA. Because BIN2 can interact with and phosphorylate SnRK2s to activate its kinase, this study also discovers a novel module of PP2Cs-BIN2-SnRK2s in the ABA signaling pathway. These findings provide significant insights into how plants balance growth and survival by coordinately regulating the growth promoting signaling pathway and stress responses under abiotic stresses.
DOI:10.1016/j.molp.2018.03.019URLPMID:29625191 [本文引用: 6]

Brassinosteroids (BRs) play essential roles in regulating various aspects of plant growth and development and in responding to diverse environmental cues, and their metabolism is an important way to regulate their homeostasis in plants. Here, we identified a dominant mutant, dwarf and round leaf-1 (drl1-D), which exhibits a weak BR-deficient or BR-insensitive mutant phenotypes, including short... [Show full abstract]
DOI:10.1073/pnas.1423481112URLPMID:25550508 [本文引用: 1]

Significance Drought stress induces the accumulation of the plant stress hormone abscisic acid (ABA). ABA then quickly activates the protein kinase OST1/SnRK2.6 to phosphorylate a number of proteins in guard cells, resulting in stomatal closure to reduce transpirational water loss. How SnRK2.6 is deactivated and how ABA signaling may be desensitized are unclear. This study found that nitric oxide (NO) resulting from ABA signaling causes S-nitrosylation of SnRK2.6 at a cysteine residue close to the kinase catalytic site, which blocks the kinase activity. Dysfunction of S-nitrosoglutathione (GSNO) reductase causes GSNO overaccumulation in guard cells and ABA insensitivity in stomatal regulation. This work thus reveals how ABA-induced NO functions in guard cells to inactivate SnRK2.6 to negatively feedback regulate ABA signaling.
DOI:10.1105/tpc.109.072959URLPMID:20870959 [本文引用: 1]

Nitric oxide (NO) is a bioactive molecule that functions in numerous physiological and developmental processes in plants, including lateral root development. In this study, we used biochemical and genetic approaches to analyze the function of Arabidopsis thaliana mitogen-activated protein kinase 6 (MPK6) in the regulation of NO synthesis in response to hydrogen peroxide (H2O2) during lateral ro...
DOI:10.1016/j.molcel.2017.12.002URLPMID:29290610 [本文引用: 1]

Abstract As sessile organisms, plants must adapt to variations in the environment. Environmental stress triggers various responses, including growth inhibition, mediated by the plant hormone abscisic acid (ABA). The mechanisms that integrate stress responses with growth are poorly understood. Here, we discovered that the Target of Rapamycin (TOR) kinase phosphorylates PYL ABA receptors at a conserved serine residue to prevent activation of the stress response in unstressed plants. This phosphorylation0002disrupts PYL association with ABA and with PP2C phosphatase effectors, leading to inactivation0002of SnRK2 kinases. Under stress, ABA-activated SnRK2s phosphorylate Raptor, a component of the TOR complex, triggering TOR complex dissociation0002and inhibition. Thus, TOR signaling represses ABA signaling and stress responses in unstressed conditions, whereas ABA signaling represses TOR signaling and growth during times of stress. Plants utilize this conserved phospho-regulatory feedback mechanism to optimize the balance of growth and stress responses.
URL [本文引用: 1]

The small ubiquitin-related modifier(SUMO)modification plays an important role in the regulation of abscisic acid(ABA)signaling,but the function of the SUMO protease,in ABA signaling,remains largely unknown.Here,we show that the SUMO protease,ASP1 positively regulates ABA signaling.Mutations in ASP1 resulted in an ABA-insensitive phenotype,during early seedling development.Wild-type ASP1 successfully rescued,whereas an ASP1 mutant(C577S),defective in SUMO protease activity,failed to rescue,the ABA-insensitive phenotype of asp1-1.Expression of ABI5 and MYB30 target genes was attenuated in asp1-1 and our genetic analyses revealed that ASP1 may function upstream of ABI5 and MYB30.Interestingly,ASP1 accumulated upon ABA treatment,and ABA-induced accumulation of ABI5(a positive regulator of ABA signaling)was abolished,whereas ABA-induced accumulation of MYB30(a negative regulator of ABA signaling)was increased in asp1-1.These findings support the hypothesis that increased levels of ASP1,upon ABA treatment,tilt the balance between ABI5 and MYB30towards ABI5-mediated ABA signaling.
DOI:10.1111/nph.15345URL
DOI:10.1093/pcp/pcq037URLPMID:20360020 [本文引用: 1]

Abstract Plant mitogen-activated protein kinase (MAPK) cascades are involved in a range of biotic and abiotic stress responses, but many members of the MAPK family involved in signal transduction of the stress-related hormone ABA remain to be identified and how they regulate ABA signaling is still unclear. Here we characterized biochemically an apple MAPK signaling cascade MdMKK1-MdMPK1, which is transiently activated by ABA. Expression of MdMKK1 or MdMPK1 in the reference plant Arabidopsis (Arabidopsis thaliana) confers ABA hypersensitivity in both seed germination and seedling growth, showing that MdMKK1 and MdMPK1 are positively involved in ABA signaling. Expression of MdMKK1 or MdMPK1 up-regulates expression of several ABA-responsive transcription factor-encoding genes including ABI5. Furthermore, MdMPK1 phosphorylates the Arabidopsis ABI5 protein through the unique residue Ser314, showing that ABI5 is a potential direct downstream component of MAPK in ABA signaling. These findings indicate that the apple MdMKK1-MdMPK1-coupled signaling cascade may function in ABA signaling by regulating both expression and the phosphorylation status of the important ABA signaling component ABI5 or ABI5-like transcription factors.
DOI:10.1016/j.cell.2016.06.027URLPMID:27518563 [本文引用: 1]

Plant hormone signals have a broad range of activity that can be made more specific via conversion to more narrowly acting molecule, as revealed by the ability ofArabidopsisto convert the highly pleiotropic abscisic acid to the more selectively acting phaseic acid in seeds expanding the adaptive plasticity of signaling outcomes.
DOI:10.1016/j.pbi.2017.05.004URLPMID:28538164 [本文引用: 1]

Abstract Plants have evolved multi-layered molecular defense strategies to protect against pathogens. Plant immune signaling largely relies on post-translational modifications (PTMs) to induce rapid alterations of signaling pathways to achieve a response that is appropriate to the type of pathogen and infection pressure. In host cells, dynamic PTMs have emerged as powerful regulatory mechanisms that cells use to adjust their immune response. PTM is also a virulence strategy used by pathogens to subvert host immunity through the activities of effector proteins secreted into the host cell. Recent studies focusing on deciphering post-translational mechanisms underlying plant immunity have offered an in-depth view of how PTMs facilitate efficient immune responses and have provided a more dynamic and holistic view of plant immunity. Copyright 漏 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
DOI:10.1105/tpc.16.00364URLPMID:27577789 [本文引用: 1]

[EN] Abscisic acid (ABA) is an essential hormone for plant development and stress responses. ABA signaling is suppressed by clade A PP2C phosphatases, which function as key repressors of this pathway through inhibiting ABA-activated SnRK2s (SNF1-related protein kinases). Upon ABA perception, the PYR/PYL/RCAR ABA receptors bind to PP2Cs with high affinity and biochemically inhibit their activity. While thismechanismhas been extensively studied, how PP2Cs are regulated at the protein level is only starting to be explored. Arabidopsis thaliana RING DOMAIN LIGASE5 (RGLG5) belongs to a five-member E3 ubiquitin ligase family whose target proteins remain unknown. We report that RGLG5, together with RGLG1, releases the PP2C blockade of ABA signaling by mediating PP2CA protein degradation. ABA promotes the interaction of PP2CA with both E3 ligases, which mediate ubiquitination of PP2CA and are required for ABA-dependent PP2CA turnover. Downregulation of RGLG1 and RGLG5 stabilizes endogenous PP2CA and diminishes ABA-mediated responses. Moreover, the reduced response to ABA in germination assays is suppressed in the rglg1 amiR (artificial microRNA)-rglg5 pp2ca-1 triple mutant, supporting a functional link among these loci. Overall, our data indicate that RGLG1 and RGLG5 are important modulators of ABA signaling, and they unveil amechanismfor activation of the ABA pathway by controlling PP2C half-life.
DOI:10.1002/1873-3468.12852URLPMID:28940407 [本文引用: 1]

The evolutionarily highly conserved SNF1-related protein kinase (SnRK1) protein kinase is a metabolic master regulator in plants, balancing the critical energy consumption between growth- and stress response-related metabolic pathways. While the regulation of the mammalian [AMP-activated protein kinase (AMPK)] and yeast (SNF1) orthologues of SnRK1 is well-characterised, the regulation of SnRK1 kinase activity in plants is still an open question. Here we report that the activity and T-loop phosphorylation of AKIN10, the kinase subunit of the SnRK1 complex, is regulated by the redox status. Although this regulation is dependent on a conserved cysteine residue, the underlying mechanism is different to the redox regulation of animal AMPK and has functional implications for the regulation of the kinase complex in plants under stress conditions.
DOI:10.1111/pce.12351URLPMID:247386452 [本文引用: 1]

Abstract Under osmotic stress conditions such as drought and high salinity, the plant hormone abscisic acid (ABA) plays important roles in stress-responsive gene expression mainly through three bZIP transcription factors, AREB1/ABF2, AREB2/ABF4 and ABF3, which are activated by SNF1-related kinase 2s (SnRK2s) such as SRK2D/SnRK2.2, SRK2E/SnRK2.6 and SRK2I/SnRK2.3 (SRK2D/E/I). However, since the three AREB/ABFs are crucial, but not exclusive, for the SnRK2-mediated gene expression, transcriptional pathways governed by SRK2D/E/I are not fully understood. Here, we show that a bZIP transcription factor, ABF1, is a functional homolog of AREB1, AREB2 and ABF3 in ABA-dependent gene expression in Arabidopsis . Despite lower expression levels of ABF1 than those of the three AREB / ABF s, the areb1 areb2 abf3 abf1 mutant plants displayed increased sensitivity to drought and decreased sensitivity to ABA in primary root growth compared with the areb1 areb2 abf3 mutant. Genome-wide transcriptome analyses revealed that expression of downstream genes of SRK2D/E/I, which include many genes functioning in osmotic stress responses and tolerance such as transcription factors and LEA proteins, was mostly impaired in the quadruple mutant. Thus, these results indicate that the four AREB/ABFs are the predominant transcription factors downstream of SRK2D/E/I in ABA signalling in response to osmotic stress during vegetative growth.
DOI:10.1016/j.molp.2014.12.006URLPMID:25655825 [本文引用: 1]

Recent progress indicates that GSK3-like kinases have versatile functions in the growth and development of plants. Various cellular signaling events mediated by GSK3-like kinases are reviewed based on newly identified substrates of GSK3-like kinases in Arabidopsis and rice.
DOI:10.1016/j.molp.2016.11.002URLPMID:27856401 [本文引用: 1]

The reverse-genetic screening of variousE2orUEVmutant lines in response to ABA treatment identified E2-like VPS23A as an ABA negative regulator. The ESCRT-I component VPS23A affects PYR1/PYL4 via vacuole-mediated degradation besides 26S proteasome system, which strengthens our understanding of both the turnover of ABA receptors and ESCRTs in plant hormone signaling.
DOI:10.1016/j.tplants.2015.05.004URLPMID:26044742 [本文引用: 1]

Abscisic acid-insensitive 5 (ABI5), a plant basic leucine zipper (bZIP) transcription factor, has been revealed to be the key regulator in the abscisic acid (ABA) signaling pathway controlling seed dormancy, germination, plant growth, and flowering time. Recently, new evidence has come to light that a combination of different post-translational modifications (PTMs) might together control the stability and activity of ABI5. In this review, we highlight three types of PTM (protein phosphorylation/dephosphorylation, ubiquitination, and sumoylation) and their interactions that precisely regulate ABI5 signaling. ABI5 is the best-studied key molecule in the ABA signaling pathway with respect to PTMs; therefore, this review could serve as a model to guide post-translational studies of important regulators in other plant hormone signaling pathways.
DOI:10.1016/j.molp.2015.09.015URLPMID:26455462 [本文引用: 3]

This article focuses on the action of ubiquitination-proteasome system in ABA signaling. Most of key factors in the classic ABA pathway, from perception to action, are found to be precisely modulated by ubiquitination, a very important protein post-translational modification. The studies of ubiquitination in ABA signaling may provide a model for researches on key factors of other hormones signaling modulated by ubiquitination or other protein post-translational modifications.
DOI:10.1016/j.tplants.2017.08.009URLPMID:28919033 [本文引用: 1]

Abstract The phytohormone abscisic acid (ABA) is a vital endogenous messenger that regulates diverse physiological processes in plants. The regulation of ABA signaling has been well studied at both the transcriptional and translational levels. Post-translational modification of key regulators in ABA signaling by the 26S ubiquitin proteasome pathway is well known. Recently, increasing evidence demonstrates that atypical turnover of key regulators by the endocytic trafficking pathway and autophagy also play vital roles in ABA perception, signaling, and action. We summarize and synthesize here recent findings in the field of ABA signaling. Copyright 2017 Elsevier Ltd. All rights reserved.
DOI:10.1105/tpc.114.134163URLPMID:25616872 [本文引用: 1]

Abstract The plant hormone abscisic acid (ABA) regulates many aspects of plant development and the stress response. The intracellular E3 ligase SDIR1 (SALT- AND DROUGHT-INDUCED REALLY INTERESTING NEW GENE FINGER1) plays a key role in ABA signaling, regulating ABA-related seed germination and the stress response. In this study, we found that SDIR1 is localized on the endoplasmic reticulum membrane in Arabidopsis thaliana. Using cell biology, molecular biology, and biochemistry approaches, we demonstrated that SDIR1 interacts with and ubiquitinates its substrate, SDIRIP1 (SDIR1-INTERACTING PROTEIN1), to modulate SDIRIP1 stability through the 26S proteasome pathway. SDIRIP1 acts genetically downstream of SDIR1 in ABA and salt stress signaling. In detail, SDIRIP1 selectively regulates the expression of the downstream basic region/leucine zipper motif transcription factor gene ABA-INSENSITIVE5, rather than ABA-RESPONSIVE ELEMENTS BINDING FACTOR3 (ABF3) or ABF4, to regulate ABA-mediated seed germination and the plant salt response. Overall, the SDIR1/SDIRIP1 complex plays a vital role in ABA signaling through the ubiquitination pathway. 2015 American Society of Plant Biologists. All rights reserved.
DOI:10.1038/s41421-018-0029-yURLPMID:29928509 [本文引用: 1]
DOI:10.1016/j.jplph.2016.04.007URLPMID:27152458 [本文引用: 1]

SUMOylation, the conjugation of target proteins with SUMO (small ubiquitin-related modifier), is a type of post-translational modification in eukaryotes and involves the sequential action of activation (E1), conjugation (E2) and ligation (E3) enzymes. InArabidopsis, the AtSIZ1 protein is a SUMO E3 ligase that promotes the conjugation of SUMO proteins to target substrates. Here, we isolated and identified a SUMO E3 ligase, MdSIZ1, in apple, which was similar to AtSIZ1. SUMOylation analysis showed that MdSIZ1 had SUMO E3 ligase activityin vitroandin vivo. SUMO conjugation was increased by high temperatures, low temperatures, and abscisic acid (ABA). The ectopic expression ofMdSIZ1inArabidopsis siz1-2mutant plants partially complemented the morphological mutant phenotype and enhanced the levels of SUMO conjugation. Taken together, these results suggest that MdSIZ1-mediated SUMO conjugation of target proteins is an important process that regulates the adaptation of apple plants to various environmental stresses.
[本文引用: 1]
DOI:10.1111/jipb.12514URLPMID:27995772

SUMOylation is an important post-translational modification process that regulates different cellular functions in eukaryotes.SIZ/PIAS-type SAP and Miz1(SIZ_1) proteins exhibit SUMO E_3 ligase activity,which modulates SUMOylation.However,SIZ_1 in tomato has been rarely investigated.In this study,a tomato SIZ_1 gene(Sl SIZ_1)was isolated and its molecular characteristics and role in tolerance to drought stress are described.Sl SIZ_1 was upregulated by cold,sodium chloride(Na Cl),polyethylene glycol(PEG),hydrogen peroxide(H_2O_2) and abscisic acid(ABA),and the corresponding proteins were localized in the nucleus.The expression of Sl SIZ_1 in Arabidopsis thaliana(Arabidopsis) siz1-2 mutants partially complemented the phenotypes of dwarf,cold sensitivity and ABA hypersensitivity.Sl SIZ_1 also exhibited the activity of SUMO E_3 ligase to promote the accumulation of SUMO conjugates.Under drought stress,the ectopic expression of Sl SIZ_1 in transgenic tobacco lines enhanced seed germination and reduced the accumulation of reactive oxygen species.SlSIZ_1 overexpression conferred the plants with improved growth,high free proline content,minimal malondialdehyde accumulation and increased accumulation of SUMO conjugates.Sl SIZ_1 is a functional homolog of Arabidopsis SIZ_1 with SUMO E_3 ligase activity.Therefore,overexpression of Sl SIZ_1 enhanced the tolerance of transgenic tobacco to drought stress.
DOI:10.1016/j.bbapap.2014.11.006URLPMID:254332644 [本文引用: 1]

61MPK4 is activated by stress, and active MPK4 promotes ROS production.61MPK4 phosphorylation sites were mapped.61MPK4 autophosphorylation led to protein aggregation in the presence of H2O2in vitro.
DOI:10.1101/gad.1318705URLPMID:15998807 [本文引用: 1]

The phytohormone abscisic acid (ABA) mediates many complex aspects of plant development including seed maturation, dormancy, and germination as well as root growth. The B3-domain transcription factor abscisic acid-insensitive 3 (ABI3) is a central regulator in ABA signaling, but little is known of how this factor is regulated. Here, we show that ABI3 is an unstable protein and that an ABI3-interacting protein (AIP2), which contains a RING motif, can polyubiquitinate ABI3 in vitro. The AIP2 E3 ligase activity is abolished by mutations (C230S; C231S) in the RING motif and the AIP2 (C/S) mutant functions in a dominant-negative manner. AIP2 has a stronger binding affinity for the B2 + B3 domain of ABI3 than the A1 + B1 domain, but only ubiquitinates the latter. In double-transgenic plants, induced AIP2 expression leads to a decrease in ABI3 protein levels. In contrast, ABI3 levels are elevated upon induced expression of the AIP2 RING mutant, which interferes with the endogenous AIP2 E3 activity. An aip2-1-null mutant shows higher ABI3 protein levels compared with wild type after seed stratification, and is hypersensitive to ABA, mimicking the ABI3-overexpression phenotype, whereas AIP2-overexpression plants contain lower levels of ABI3 protein than wild type and are more resistant to ABA, phenocopying abi3. Our results indicate that AIP2 negatively regulates ABA signaling by targeting ABI3 for post-translational destruction.
DOI:10.3390/ijms18091841URLPMID:5618490 [本文引用: 1]

Several kinesins, the ATPヾriven microtubule (MT)‐based motor proteins, have been reported to be involved in many basic processes of plant development. However, little is known about the biological relevance of their ATPase activity. Here, we characterized the rice (Oryza sativa) stemless dwarf1 (std1) mutant showing a severely dwarfed phenotype with no differentiation of the node and... [Show full abstract]
DOI:10.1073/pnas.1202630109URL [本文引用: 1]

The phytohormone abscisic acid (ABA) plays an essential role in plant development and during the response of the plant to abiotic stress. In this study, we report that the R2R3-type transcription factor MYB30 is involved in the regulation of ABA signaling. Arabidopsis mutants lacking MYB30 are hypersensitive to ABA during germination and seedling growth. A K283R substitution in MYB30 blocks its SUMO E3 ligase SIZ1-mediated sumoylation in Arabidopsis protoplasts, indicating that MYB30 is sumoylated by SIZ1 and that K283 is the principal site for small ubiquitin-like modifier conjugation. Expression of MYB30K283R in myb30 partially rescues the mutant ABA-hypersensitive. phenotype, but expression of wild-type MYB30 complements the mutant phenotype. Overexpression of MYB30 in wild-type results in an ABA-insensitive phenotype, whereas overexpression of MYB30 in the siz1 mutant does not alter siz1 hypersensitivity to ABA. The siz1-2 myb30-2 double-mutant exhibits greater ABA sensitivity than either single mutant but a mutation in the SIZ1-sumoylated ABI5 transcription factor suppresses the ABA hypersensitivity of myb30-2 to wild-type levels. Our results suggest that coordination of ABI5 and MYB30 sumoylation by SIZ1 may balance gene expression, which is required for regulation of ABA signaling during seed germination.
[本文引用: 1]
DOI:10.1016/j.pbi.2015.08.010URLPMID:26453966 [本文引用: 1]

Highlights 61 ESCRT machinery and Rab GTPases play conserved and unique roles in mediating MVB/PVC to vacuole protein trafficking. 61 SH3P2 mediates autophagic degradation via association with the autophagy machinery. 61 The ESCRT component FREE1 interacts with SH3P2 to crosstalk with the autophagy pathway and regulates autophagic degradation. The vacuole is the central site for both storage and metabolism in plant cells and mediates multiple cellular events during plant development and growth. Cargo proteins are usually sequestered into membrane-bound organelles and delivered into the vacuole upon membrane fusion. Two major organelles are responsible for the recognition and transport of cargos targeted to the vacuole: the single-membrane multivesicular body (MVB) or prevacuolar compartment (PVC) and the double-membrane autophagosome. Here, we will highlight recent discoveries about MVB/PVC-mediated and autophagosome-mediated protein trafficking and degradation, and will pay special attention to a possible interplay between the endocytic and autophagic pathways in regulating vacuolar degradation in plants.
DOI:10.1104/pp.16.00469URLPMID:27325665 [本文引用: 1]

The OsbZIP23 transcription factor has been characterized for its essential role in drought resistance in rice (Oryza sativa), but the mechanism is unknown. In this study, we first investigated the transcriptional activation of OsbZIP23. A homolog of SnRK2 protein kinase (SAPK2) was found to interact with and phosphorylate OsbZIP23 for its transcriptional activation. SAPK2 also interacted with OsPP2C49, an ABI1 homolog, which deactivated the SAPK2 to inhibit the transcriptional activation activity of OsbZIP23. Next, we performed genome-wide identification of OsbZIP23 targets by immunoprecipitation sequencing and RNA sequencing analyses in the OsbZIP23-overexpression, osbzip23 mutant, and wild-type rice under normal and drought stress conditions. OsbZIP23 directly regulates a large number of reported genes that function in stress response, hormone signaling, and developmental processes. Among these targets, we found that OsbZIP23 could positively regulate OsPP2C49, and overexpression of OsPP2C49 in rice resulted in significantly decreased sensitivity of the abscisic acid (ABA) response and rapid dehydration. Moreover, OsNCED4 (9-cis-epoxycarotenoid dioxygenase4), a key gene in ABA biosynthesis, was also positively regulated by OsbZIP23. Together, our results suggest that OsbZIP23 acts as a central regulator in ABA signaling and biosynthesis, and drought resistance in rice.
SUMO E3连接酶在植物生长发育中的功能研究进展
1
2018
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
一氧化氮介导蛋白质亚硝基化与甲基化协调植物非生物胁迫的分子机制
1
2017
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
脱落酸调控种子休眠和萌发的分子机制
1
2018
... 脱落酸(abscisic acid, ABA)作为经典植物激素之一, 不仅参与植物的生长发育过程, 如种子休眠与萌发、根系统发育、叶片衰老和成花转变, 还在植物逆境响应中起着非常重要的作用(
S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth
1
2015
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
Selective inhibition of clade A phosphatases type 2C by PYR/PYL/RCAR abscisic acid receptors
1
2012
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination
1
2013
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
SUMOylation: re-wiring the plant nucleus during stress and development
1
2018
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
S-acylation- dependent association of the calcium sensor CBL2 with the vacuolar membrane is essential for proper abscisic acid responses
1
2012
... 随着技术的进步, 目前已经鉴定到200多种不同类型的PTMs, 已报道多种PTMs参与ABA信号途径.例如, 半胱氨酸残基的酰化(
Nitric oxide buffering and conditional nitric oxide release in stress response
1
2018
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
FYVE1/FREE1 interacts with the PYL4 ABA receptor and mediates its delivery to the vacuolar degradation pathway
1
2016
... 泛素化后的降解途径还有几条不依赖于26S蛋白酶体, 称之为非26S蛋白酶体内膜转运体系, 包括胞内体转运(endosomal traf?cking pathway)和自噬泡途径.这里简要介绍一下这类降解途径在ABA信号中的作用, 详情可以参阅文献(
Identification of features regulating OST1 kinase activity and OST1 function in guard cells
1
2006
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
The molecular circuitry of brassinosteroid signaling
1
2015
... 蛋白质翻译后修饰(post-translational modifications, PTMs)能调节蛋白质结构、动态变化和生物学功能等, 是真核细胞生命活动中的重要调节方式, 其中常见的有磷酸化、糖基化、甲基化、酰基化、泛素化和硫酸化等(
The protein phosphatase 2C clade A protein OsPP2C51 positively regulates seed germination by directly inactivating OsbZIP10
1
2017
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
The
1
2003
... 随着技术的进步, 目前已经鉴定到200多种不同类型的PTMs, 已报道多种PTMs参与ABA信号途径.例如, 半胱氨酸残基的酰化(
Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action
1
2012
... sponsive kinase substrate 1), 促使AKS1解聚成单体形式, 失去结合靶基因KAT1的能力, 从而抑制KAT1的表达(
Overexpression of RING domain E3 ligase ZmXERICO1 confers drought tolerance through regulation of ABA homeostasis
1
2017
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
The single-subunit RING-type E3 ubiquitin ligase RSL1 targets PYL4 and PYR1 ABA receptors in plasma membrane to modulate abscisic acid signaling
2
2014
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
... 泛素化后的降解途径还有几条不依赖于26S蛋白酶体, 称之为非26S蛋白酶体内膜转运体系, 包括胞内体转运(endosomal traf?cking pathway)和自噬泡途径.这里简要介绍一下这类降解途径在ABA信号中的作用, 详情可以参阅文献(
GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis
2
2014
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
... ).在ABA信号通路中, BIN2磷酸化激活SnRK2.2/2.3 (
Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants
1
2015
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
SUMO proteases ULP1c and ULP1d are required for development and osmotic stress responses in Arabidopsis thaliana
1
2016
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
SUMO, a heavyweight player in plant abiotic stress responses
1
2012
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses
1
2007
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
EL1-like casein kinases suppress ABA signaling and responses by phosphorylating and destabilizing the ABA receptors PYR/PYLs in Arabidopsis
1
2018
... 蛋白的磷酸化和去磷酸化(phosphorylation/dephosphorylation)过程由蛋白激酶和蛋白磷酸酶分别完成, 是蛋白最主要的翻译后修饰之一(
. Arabidopsis BPM proteins function as substrate adaptors to a cullin3-based E3 ligase to affect fatty acid metabolism in plants
1
2013a
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
ABA and the ubiquitin E3 ligase KEEP ON GOING affect proteolysis of the Arabidopsis thaliana transcription factors ABF1 and ABF3
1
2013b
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
SCFAtPP2-B11 modulates ABA signaling by facilitating SnRK2.3 degradation in Arabidopsis thaliana
1
2017
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response
1
2012
... responsive element binding protein)、NAC、WRKY和MYB/MYC等转录因子组成的转录调控网络(
Arabidopsis calcium- dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity
1
2005
... responsive element binding protein)、NAC、WRKY和MYB/MYC等转录因子组成的转录调控网络(
The origins of protein phosphorylation
1
2002
... 蛋白的磷酸化和去磷酸化(phosphorylation/dephosphorylation)过程由蛋白激酶和蛋白磷酸酶分别完成, 是蛋白最主要的翻译后修饰之一(
The PP6 phosphatase regulates ABI5 phosphorylation and abscisic acid signaling in Arabidopsis
1
2013
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
Abscisic acid: biosynthesis, inactivation, homoeostasis and signaling
4
2015
... 脱落酸(abscisic acid, ABA)作为经典植物激素之一, 不仅参与植物的生长发育过程, 如种子休眠与萌发、根系统发育、叶片衰老和成花转变, 还在植物逆境响应中起着非常重要的作用(
... ;
... ).ABA转运蛋白(如输出载体ABCG25)将ABA及其代谢物运出细胞, 再将ABA导入维管束进行长距离运输; 或(如输入载体ABCG22/40)将ABA重新载入需要的细胞(如气孔保卫细胞).这种运输机制是植物响应胁迫的重要方式(
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
Arabidopsis RAV1 transcription factor, phosphory- lated by SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development
1
2014
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
Abscisic acid synthesis and response
4
2013
... 脱落酸(abscisic acid, ABA)作为经典植物激素之一, 不仅参与植物的生长发育过程, 如种子休眠与萌发、根系统发育、叶片衰老和成花转变, 还在植物逆境响应中起着非常重要的作用(
... ).ABA通过2条代谢途径失活: (1) 通过细胞色素氧化酶(CYP707As)等氧化产生红花菜豆酸(phaseic acid, PA)和二氢红花菜豆酸(dihydrophaseic acid, DPA) (
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
... ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(
Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis
2
2007
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
... ).SnRK2s下游底物包括转录因子(主要是ABRE-binding proteins/ABRE- binding factors, AREBs/ABFs)、钾离子通道蛋白KAT1、阴离子通道蛋白SLAC1 (slow anion channel 1)及其它功能蛋白(TOR激酶、NADPH氧化酶和DNA解旋酶BRAHMA等) (
Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress
1
2009
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
ABA-mediated transcriptional regulation in response to osmotic stress in plants
1
2011
... responsive element binding protein)、NAC、WRKY和MYB/MYC等转录因子组成的转录调控网络(
Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis
1
2009
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants
3
2013
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
... ;
A small plant-specific protein family of ABI five binding proteins (AFPs) regulates stress response in germinating Arabidopsis seeds and seedlings
1
2008
... SAUL1 (senescence-associated E3 ubiquitin ligase 1)是U-box类E3连接酶, 通过Ub/26S体系降解AAO3, 参与调节叶片衰老和ABA合成(
Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca 2+ affinities
1
2010
... sponsive kinase substrate 1), 促使AKS1解聚成单体形式, 失去结合靶基因KAT1的能力, 从而抑制KAT1的表达(
Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair
1
2009
... 在ABA促进气孔关闭的过程中, SnRK2s磷酸化激活SLAC1, 促进阴离子(A-)外排; ABI1直接去磷酸化SLAC1, 抑制气孔关闭.同时SnRK2s磷酸化抑制KAT1的活性, 阻止钾离子(K+)内流(
The SWI2/SNF2 chromatin remodeling ATPase BRAHMA represses abscisic acid responses in the absence of the stress stimulus in Arabidopsis
2
2012
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
... SnRK2s还磷酸化其它的功能蛋白, 使植物感应ABA信号后出现多种生理变化. 例如, 磷酸化NADPH氧化酶AtrbohF, 促进ABA诱发的活性氧爆发(
The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis
1
2002
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis
1
2002
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure
1
2016
... sponsive kinase substrate 1), 促使AKS1解聚成单体形式, 失去结合靶基因KAT1的能力, 从而抑制KAT1的表达(
Type one protein phosphatase 1 and its regulatory protein inhibitor 2 negatively regulate ABA signaling
1
2016
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
TAP46 plays a positive role in the ABSCISIC ACID INSENSITIVE 5-regulated gene expression in Arabidopsis
1
2014
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
BRASSINOSTEROID INSENSITIVE 2 interacts with ABSCISIC ACID INSENSITIVE 5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis
2
2014
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis
1
2012
... sponsive kinase substrate 1), 促使AKS1解聚成单体形式, 失去结合靶基因KAT1的能力, 从而抑制KAT1的表达(
The cullin-RING ubiquitin- protein ligases
1
2011
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
Farnesylcysteine lyase is involved in negative regulation of abscisic acid signaling in Arabidopsis
1
2010
... 随着技术的进步, 目前已经鉴定到200多种不同类型的PTMs, 已报道多种PTMs参与ABA信号途径.例如, 半胱氨酸残基的酰化(
Protein phosphorylation: a major switch mechanism for metabolic regulation
1
2015
... 蛋白的磷酸化和去磷酸化(phosphorylation/dephosphorylation)过程由蛋白激酶和蛋白磷酸酶分别完成, 是蛋白最主要的翻译后修饰之一(
Targeted degradation of abscisic acid receptors is mediated by the ubiquitin ligase substrate adaptor DDA1 in Arabidopsis
1
2014
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
Interpreting the protein language using proteomics
1
2006
... 蛋白质翻译后修饰(post-translational modifications, PTMs)能调节蛋白质结构、动态变化和生物学功能等, 是真核细胞生命活动中的重要调节方式, 其中常见的有磷酸化、糖基化、甲基化、酰基化、泛素化和硫酸化等(
The COP9 signalosome regulates seed germination by facilitating protein degradation of RGL2 and ABI5
1
2018
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
A rice orthologue of the ABA receptor, OsPYL/RCAR5, is a positive regulator of the ABA signal transduction pathway in seed germination and early seedling growth
1
2012a
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
The Arabidopsis RING E3 ubiquitin ligase AtAIRP3/LOG2 participates in positive regulation of high-salt and drought stress responses
1
2013
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca 2+ signaling
1
2010
... ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(
Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway
1
2012b
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis
1
2006
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases
1
2015
... SAUL1 (senescence-associated E3 ubiquitin ligase 1)是U-box类E3连接酶, 通过Ub/26S体系降解AAO3, 参与调节叶片衰老和ABA合成(
Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole
1
2013
... 此外, RING类泛素酶SDIR1、U-box类泛素酶CHIP、PUB18多种泛素连接酶参与调控ABA响应, 但其底物并非ABA中心转导途径组分(
Overexpression of AtABCG25 enhan-ces the abscisic acid signal in guard cells and improves plant water use efficiency
1
2016
... 泛素化后的降解途径还有几条不依赖于26S蛋白酶体, 称之为非26S蛋白酶体内膜转运体系, 包括胞内体转运(endosomal traf?cking pathway)和自噬泡途径.这里简要介绍一下这类降解途径在ABA信号中的作用, 详情可以参阅文献(
Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds
1
2000
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
MATH/BTB CRL3 receptors target the homeodomain-leucine zipper ATHB6 to modulate abscisic acid signaling
1
2011
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
The Arabidopsis MIEL1 E3 ligase negatively regulates ABA signaling by promoting protein turnover of MYB96
1
2016
... 此外, RING类泛素酶SDIR1、U-box类泛素酶CHIP、PUB18多种泛素连接酶参与调控ABA响应, 但其底物并非ABA中心转导途径组分(
DWA1 and DWA2, two Arabidopsis DWD protein components of CUL4-based E3 ligases, act together as negative regulators in ABA signal transduction
1
2010
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
The Arabidopsis MYB96 transcription factor is a positive regulator of ABSCISIC ACID-INSENSITIVE 4 in the control of seed germination
1
2015
... 此外, RING类泛素酶SDIR1、U-box类泛素酶CHIP、PUB18多种泛素连接酶参与调控ABA响应, 但其底物并非ABA中心转导途径组分(
Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid
1
2006
... 脱落酸(abscisic acid, ABA)作为经典植物激素之一, 不仅参与植物的生长发育过程, 如种子休眠与萌发、根系统发育、叶片衰老和成花转变, 还在植物逆境响应中起着非常重要的作用(
AtRAE1 is involved in degradation of ABA receptor RCAR1 and negatively regulates ABA signaling in Arabidopsis
1
2018
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
Regulation of cotton ( Gossypium hirsutum) drought responses by mitogen-activated protein (MAP) kinase cascade-mediated phosphorylation of GhWRKY59.
1
2017
... responsive element binding protein)、NAC、WRKY和MYB/MYC等转录因子组成的转录调控网络(
The Arabidopsis F-box E3 ligase RIFP1 plays a negative role in abscisic acid signaling by facilitating ABA receptor RCAR3 degradation
1
2016
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
A link between magnesium-chelatase H subunit and sucrose nonfermenting 1 (SNF1)related protein kinase SnRK2.6/OST1 in Arabidopsis guard cell signaling in response to abscisic acid
1
2015
... responsive element binding protein)、NAC、WRKY和MYB/MYC等转录因子组成的转录调控网络(
The pepper RING-type E3 ligase CaAIRF1 regulates ABA and drought signaling via CaADIP1 protein phosphatase degradation
1
2017
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
Rice APC/C(TE) controls tillering by mediating the degradation of MONOCULM 1
1
2012
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
The SnRK2-APC/C (TE) regulatory module mediates the antagonistic action of gibberellic acid and abscisic acid pathways
1
2015
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
Downregulation of N-terminal acetylation triggers ABA-mediated drought responses in Arabidopsis
1
2015
... 随着技术的进步, 目前已经鉴定到200多种不同类型的PTMs, 已报道多种PTMs参与ABA信号途径.例如, 半胱氨酸残基的酰化(
Functional characterization of DnSIZ1, a SIZ/PIAS-type SUMO E3 ligase from
1
2015
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
Abscisic acid increases Arabidopsis ABI5 transcription factor levels by promoting KEG E3 ligase self-ubiquitination and proteasomal degradation
1
2010
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
Small ubiquitin-like modifier modulates abscisic acid signaling in Arabidopsis
1
2003
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation
1
2003
... SAUL1 (senescence-associated E3 ubiquitin ligase 1)是U-box类E3连接酶, 通过Ub/26S体系降解AAO3, 参与调节叶片衰老和ABA合成(
Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis
1
2010
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment
1
2006
... 此外, RING类泛素酶SDIR1、U-box类泛素酶CHIP、PUB18多种泛素连接酶参与调控ABA响应, 但其底物并非ABA中心转导途径组分(
Promotion of NEDD8-CUL1 conjugate cleavage by COP9 signalosome
1
2001
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
Arabidopsis CIPK26 interacts with KEG, components of the ABA signaling network and is degraded by the ubiquitin-proteasome system
1
2013
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
The kinase activity of calcineurin B-like interacting protein kinase 26 (CIPK26) influences its own stability and that of the ABA-regulated ubiquitin ligase, keep on going (KEG)
1
2017
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
An apple CIPK protein kinase targets a novel residue of AREB transcription factor for ABA-dependent phosphorylation
1
2017
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
Characterization of thiol-based redox modifications of Brassica napus SNF1-related protein kinase 2.6-2C
1
2018
... ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(
Regulators of PP2C phosphatase activity function as abscisic acid sensors
1
2009
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
Abscisic acid transport and homeostasis in the context of stomatal regulation
1
2015
... 脱落酸(abscisic acid, ABA)作为经典植物激素之一, 不仅参与植物的生长发育过程, 如种子休眠与萌发、根系统发育、叶片衰老和成花转变, 还在植物逆境响应中起着非常重要的作用(
An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses
1
2006
... ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(
Ubiquitylation in plants: signaling hub for the integration of environmental signals
2
2018
... 泛素是真核生物中高度保守的一类小肽, 由76个氨基酸残基组成, 它通过共价连接的方式, 即泛素化(ubiquitination)修饰蛋白的赖氨酸残基.泛素化过程通常经一系列连续的催化反应, 由E1泛素激活酶、E2泛素结合酶和E3泛素连接酶将泛素连接到靶蛋白(
... ).泛素化修饰根据连接泛素的数量和方式可分为单泛素化、多泛素化和多聚泛素化.单泛素化或多泛素化主要起修饰蛋白功能和调节蛋白定位等作用; 多聚泛素化通常偶联蛋白酶体(ubiquitin/26S proteasome, Ub/26S体系)进行蛋白质的选择性降解(
Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling
2
2009
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
... ).由于生化结果不符合生理表型, 因此推测被ABI5的SUMO化不仅影响它的泛素化, 还可能影响ABI5的其它修饰(如磷酸化), 导致ABI5不能被激活, 所以SIZ1最终抑制ABI5响应ABA的能力(
Expanding roles of protein kinase CK2 in regulating plant growth and development
1
2014
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
Nitric oxide in plants: an assessment of the current state of knowledge
1
2013
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
Function of N-glycosylation in plants
1
2018
... 蛋白质翻译后修饰(post-translational modifications, PTMs)能调节蛋白质结构、动态变化和生物学功能等, 是真核细胞生命活动中的重要调节方式, 其中常见的有磷酸化、糖基化、甲基化、酰基化、泛素化和硫酸化等(
Structural basis for basal activity and autoactivation of abscisic acid (ABA) signaling SnRK2 kinases
1
2011
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis
1
2009
... ABA信号转导过程是一个复杂的协同作用的交互网络, 除上文详细阐述的关键组分外, 还有多种重要组分参与ABA信号转导和响应, 包括由质体定位的受体CHLH (H subunit of the Mg2+ Cheletase)或质膜定位的受体GTG1 (G-protein coupled receptor-type G-proteins)介导的2条不依赖于受体PYR/PYL/RCARs的ABA信号转导途径(
Functional characterization of the SIZ/PIAS-type SUMO E3 ligases, OsSIZ1 and OsSIZ2 in rice
1
2010
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins
1
2009
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
A direct link between abscisic acid sensing and the chromatin-remodeling ATPase BRAHMA via core ABA signaling pathway components
1
2016
... SnRK2s还磷酸化其它的功能蛋白, 使植物感应ABA信号后出现多种生理变化. 例如, 磷酸化NADPH氧化酶AtrbohF, 促进ABA诱发的活性氧爆发(
Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack
1
2018
... ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(
Identification of a novel E3 ubiquitin ligase that is required for suppression of pre mature senescence in Arabidopsis
1
2009
... SAUL1 (senescence-associated E3 ubiquitin ligase 1)是U-box类E3连接酶, 通过Ub/26S体系降解AAO3, 参与调节叶片衰老和ABA合成(
To grow or not to grow: TOR and SnRK2 coordinate growth and stress response in Arabidopsis
1
2018
... 蛋白的磷酸化和去磷酸化(phosphorylation/dephosphorylation)过程由蛋白激酶和蛋白磷酸酶分别完成, 是蛋白最主要的翻译后修饰之一(
Plant Raf-like kinase integrates abscisic acid and hyperosmotic stress signaling upstream of SNF1-related protein kinase2
1
2015
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase
1
2009
... 在ABA促进气孔关闭的过程中, SnRK2s磷酸化激活SLAC1, 促进阴离子(A-)外排; ABI1直接去磷酸化SLAC1, 抑制气孔关闭.同时SnRK2s磷酸化抑制KAT1的活性, 阻止钾离子(K+)内流(
The N-terminal UND motif of the Arabidopsis U-box E3 ligase PUB18 is critical for the negative regulation of ABA- mediated stomatal movement and determines its ubiquitination specificity for exocyst subunit Exo70B1
2
2016
... 此外, RING类泛素酶SDIR1、U-box类泛素酶CHIP、PUB18多种泛素连接酶参与调控ABA响应, 但其底物并非ABA中心转导途径组分(
... ;
ABD1 is an Arabidopsis DCAF substrate receptor for CUL4-DDB1-based E3 ligases that acts as a negative regulator of abscisic acid signaling
1
2014
... CRL类E3连接酶复合体均由1个支架蛋白CULs (Cullins)和负责底物招募的接头蛋白组成, 是拟南芥中最大的一类泛素化修饰酶(
BRI1-associated receptor kinase 1 regulates guard cell ABA signaling mediated by open stomata 1 in Arabidopsis
2
2016
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
... ).另一个BR信号通路中的激酶BAK1也磷酸化SnRK2.6/OST1 (open stomata 1), 并与ABI1拮抗调节OST1的磷酸化以及气孔运动(
The Mg-chelatase H subunit is an abscisic acid receptor
1
2006
... ABA信号转导过程是一个复杂的协同作用的交互网络, 除上文详细阐述的关键组分外, 还有多种重要组分参与ABA信号转导和响应, 包括由质体定位的受体CHLH (H subunit of the Mg2+ Cheletase)或质膜定位的受体GTG1 (G-protein coupled receptor-type G-proteins)介导的2条不依赖于受体PYR/PYL/RCARs的ABA信号转导途径(
Transcriptional regulation of drought response: a tortuous network of transcriptional factors
1
2015
... responsive element binding protein)、NAC、WRKY和MYB/MYC等转录因子组成的转录调控网络(
Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase
2
2009
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
... SnRK2s还磷酸化其它的功能蛋白, 使植物感应ABA信号后出现多种生理变化. 例如, 磷酸化NADPH氧化酶AtrbohF, 促进ABA诱发的活性氧爆发(
Rice SUMO protease Overly Tolerant to Salt 1 targets the transcription factor, OsbZIP23 to promote drought tolerance in rice
1
2017
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
SUMO is a critical regulator of salt stress responses in rice
1
2016
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling
1
2006
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
Differential effects of phosphatidylinositol 4-kinase (PI4K) and 3-kinase (PI3K) inhibitors on stomatal responses to environmental signals
1
2017a
... 随着技术的进步, 目前已经鉴定到200多种不同类型的PTMs, 已报道多种PTMs参与ABA信号途径.例如, 半胱氨酸残基的酰化(
Reconstitution of abscisic acid signaling from the receptor to DNA via bHLH transcription factors
1
2017b
... sponsive kinase substrate 1), 促使AKS1解聚成单体形式, 失去结合靶基因KAT1的能力, 从而抑制KAT1的表达(
Inhibition of the Arabidopsis bHLH transcription factor by monomerization through abscisic acid-induced phosphorylation
1
2016
... sponsive kinase substrate 1), 促使AKS1解聚成单体形式, 失去结合靶基因KAT1的能力, 从而抑制KAT1的表达(
Transcription factor HAT1 is a substrate of SnRK2.3 kinase and negatively regulates ABA synthesis and signaling in Arabidopsis responding to drought
1
2018
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate ABA signaling and drought resistance in rice
1
2016
... SAUL1 (senescence-associated E3 ubiquitin ligase 1)是U-box类E3连接酶, 通过Ub/26S体系降解AAO3, 参与调节叶片衰老和ABA合成(
A molecular pathway for CO2 response in Arabidopsis guard cells
3
2015
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
... 诱导气孔关闭的一个负调控因子, 通过磷酸化抑制OST1的激酶活性, 进而抑制气孔关闭(
... sponsive kinase substrate 1), 促使AKS1解聚成单体形式, 失去结合靶基因KAT1的能力, 从而抑制KAT1的表达(
Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport
1
2010
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
Type 2C protein phosphatases directly regulate abscisic acid-activated pro-tein kinases in Arabidopsis
2
2009
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
Phosphory- lation networks in the abscisic acid signaling pathway
1
2014
... ABA信号转导过程是一个复杂的协同作用的交互网络, 除上文详细阐述的关键组分外, 还有多种重要组分参与ABA信号转导和响应, 包括由质体定位的受体CHLH (H subunit of the Mg2+ Cheletase)或质膜定位的受体GTG1 (G-protein coupled receptor-type G-proteins)介导的2条不依赖于受体PYR/PYL/RCARs的ABA信号转导途径(
Peroxynitrite formation and function in plants
1
2011
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2
1
2008
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
Casein kinase 2 negatively regulates abscisic acid-activated SnRK2s in the core abscisic acid-signaling module
2
2015
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
... ) ZmCK2磷酸化ZmOST1碳端结构域中的氨基酸残基, 促进ZmOST1与PP2Cs的结合, 从而抑制OST1的活性和ABA信号转导(
Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects
1
2017
... 脱落酸(abscisic acid, ABA)作为经典植物激素之一, 不仅参与植物的生长发育过程, 如种子休眠与萌发、根系统发育、叶片衰老和成花转变, 还在植物逆境响应中起着非常重要的作用(
Identification of open stomata1- interacting proteins reveals interactions with sucrose non- fermenting1-related protein kinases 2 and with type 2A protein phosphatases that function in abscisic acid responses
1
2015
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2
1
2018a
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
GSK3-like kinases are a class of positive components in the core ABA signaling pathway
6
2018
... ABA信号转导中心通路由受体RCAR/PYR/ PYLs、磷酸酶PP2Cs、激酶SnRK2s (SnRK2.2/2.3/ 2.6)和SnRK2s的底物构成, 是一个双抑制系统(
... 蛋白的磷酸化和去磷酸化(phosphorylation/dephosphorylation)过程由蛋白激酶和蛋白磷酸酶分别完成, 是蛋白最主要的翻译后修饰之一(
... 对ABA不敏感的表型.这些证据表明, 磷酸化PYL1/4会抑制受体的活性及功能(
... ;
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
... SnRK2s还磷酸化其它的功能蛋白, 使植物感应ABA信号后出现多种生理变化. 例如, 磷酸化NADPH氧化酶AtrbohF, 促进ABA诱发的活性氧爆发(
Nitric oxide negatively regulates abscisic acid signaling in guard cells by S- nitrosylation of OST1
1
2015
... NO作为调节子通常通过蛋白的氧化还原修饰(由过氧亚硝基介导的酪氨酸残基硝化(nitration)和由S-亚硝基谷胱甘肽(GSNO)介导的半胱氨酸残基亚硝基化(nitrosylation))在生物体内发挥作用.半胱氨酸的亚硝基化是可逆的翻译后修饰; 而酪氨酸的硝化会导致蛋白结构不可逆改变或使蛋白降解(
Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis
1
2010a
... ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(
Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response
1
2018b
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
Arabidopsis small ubiquitin-related modifier protease ASP1 positively regulates abscisic acid signaling during early seedling development
1
2018c
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
ABRE-BINDING FACTORS play a role in the feedback regulation of ABA signaling by mediating rapid ABA induction of ABA co-receptor genes
0
2018d
Two coupled components of the mitogen- activated protein kinase cascade MdMPK1 and MdMKK1 from apple function in ABA signal transduction
1
2010b
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
Co-evolution of hormone metabolism and signaling networks expands plant adaptive plasticity
1
2016
... 脱落酸(abscisic acid, ABA)作为经典植物激素之一, 不仅参与植物的生长发育过程, 如种子休眠与萌发、根系统发育、叶片衰老和成花转变, 还在植物逆境响应中起着非常重要的作用(
Post-translational regulation of plant immunity
1
2017
... 蛋白质翻译后修饰(post-translational modifications, PTMs)能调节蛋白质结构、动态变化和生物学功能等, 是真核细胞生命活动中的重要调节方式, 其中常见的有磷酸化、糖基化、甲基化、酰基化、泛素化和硫酸化等(
Ubiquitin ligases RGLG1 and RGLG5 regulate abscisic acid signaling by controlling the turnover of phosphatase PP2CA
1
2016
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
Redox state-dependent modulation of plant SnRK1 kinase activity differs from AMPK regulation in animals
1
2017
... ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(
Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 ki- nases in abscisic acid signaling in response to osmotic stress
1
2015
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
Functional insights of plant GSK3-like kinases: multi-taskers in diverse cellular signal transduction pathways
1
2015
... 当植物感知ABA信号后, SnRK2s的抑制被解除, SnRK2s通过激酶催化域和调节域的分子内互作恢复部分活性, 然后自磷酸化活性环中的多个氨基酸残基, 恢复全部的激酶活性, 从而磷酸化下游底物(
ESCRT-I component VPS23A affects ABA signaling by recognizing ABA receptors for endosomal degradation
1
2016a
... 泛素化后的降解途径还有几条不依赖于26S蛋白酶体, 称之为非26S蛋白酶体内膜转运体系, 包括胞内体转运(endosomal traf?cking pathway)和自噬泡途径.这里简要介绍一下这类降解途径在ABA信号中的作用, 详情可以参阅文献(
Precise protein post-translational modifications modulate ABI5 activity
1
2015
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
Ubiquitin-proteasome system in ABA signaling: from perception to action
3
2016b
... 泛素是真核生物中高度保守的一类小肽, 由76个氨基酸残基组成, 它通过共价连接的方式, 即泛素化(ubiquitination)修饰蛋白的赖氨酸残基.泛素化过程通常经一系列连续的催化反应, 由E1泛素激活酶、E2泛素结合酶和E3泛素连接酶将泛素连接到靶蛋白(
... ).在拟南芥中, E1和E2两种酶类编码基因较少, E3连接酶的编码基因约有1 500个, 根据E3结构和与E2互作的特异性, 可将E3连接酶分为4大类: HECT (homology to E6-AP C-terminus)类、RING (really interesting new gene)类、U-box类以及CRL (cullin-ring)类(
... 此外, RING类泛素酶SDIR1、U-box类泛素酶CHIP、PUB18多种泛素连接酶参与调控ABA响应, 但其底物并非ABA中心转导途径组分(
Non-26S proteasome endomembrane trafficking pathways in ABA signaling
1
2017
... 泛素化后的降解途径还有几条不依赖于26S蛋白酶体, 称之为非26S蛋白酶体内膜转运体系, 包括胞内体转运(endosomal traf?cking pathway)和自噬泡途径.这里简要介绍一下这类降解途径在ABA信号中的作用, 详情可以参阅文献(
The RING finger ubiquitin E3 ligase SDIR1 targets SDIR1-INTERACTING PROTEIN 1 for degradation to modulate the salt stress response and ABA signaling in Arabidopsis
1
2015a
... 此外, RING类泛素酶SDIR1、U-box类泛素酶CHIP、PUB18多种泛素连接酶参与调控ABA响应, 但其底物并非ABA中心转导途径组分(
CARK1 mediates ABA signaling by phosphorylation of ABA receptors
1
2018
... 蛋白的磷酸化和去磷酸化(phosphorylation/dephosphorylation)过程由蛋白激酶和蛋白磷酸酶分别完成, 是蛋白最主要的翻译后修饰之一(
Functional identification of MdSIZ1 as a SUMO E3 ligase in apple
1
2016
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
SUMO E3 ligase AtMMS21 regulates drought tolerance in Arabidopsis thaliana
1
2013
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
A novel tomato SUMO E3 ligase, SlSIZ1, confers drought tolerance in transgenic tobacco
0
2017
Oxidation and phosphorylation of MAP kinase 4 cause protein aggregation
1
2015b
... ABA促发包括活性氧、活性氮及钙离子在内的多种信号分子, 以响应多种生理过程(
The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation
1
2005
... 最早报道参与ABA信号调控的RING类E3泛素连接酶是AIP2 (ABI3-interacting protein 2), 其通过泛素化ABI3参与胚胎发育和ABA信号转导(
Arabidopsis E3 ubiquitin ligases PUB22 and PUB23 negatively regulate drought tolerance by targeting ABA receptor PYL9 for degradation
1
2017
... SAUL1 (senescence-associated E3 ubiquitin ligase 1)是U-box类E3连接酶, 通过Ub/26S体系降解AAO3, 参与调节叶片衰老和ABA合成(
Sumoylation of transcription factor MYB30 by the small ubiquitin-like modifier E3 ligase SIZ1 mediates abscisic acid response in Arabidopsis thaliana
1
2012
... SUMO是一类由110个氨基酸残基组成的小肽, 与泛素化过程类似, 其连接过程由SUMO E1激活酶、SUMO E2结合酶和SUMO E3连接酶级联催化完成.SUMO修饰(类泛素化, SUMOylation)后会改变靶蛋白的亚细胞定位、表面结构和催化活性以及抑制靶蛋白的泛素化修饰等.蛋白的SUMO修饰参与植物多种生命活动, 包括逆境适应、胚胎发育和植株形态建成等重要过程(
SOS2-LIKE PROTEIN KINASE 5, an SNF1-RELATED PROTEIN KINASE 3-type protein kinase, is important for abscisic acid responses in Arabidopsis through phosphorylation of ABSCISIC ACID-INSENSITIVE 5
1
2015
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
Endocytic and autophagic pathways crosstalk in plants
1
2015
... 泛素化后的降解途径还有几条不依赖于26S蛋白酶体, 称之为非26S蛋白酶体内膜转运体系, 包括胞内体转运(endosomal traf?cking pathway)和自噬泡途径.这里简要介绍一下这类降解途径在ABA信号中的作用, 详情可以参阅文献(
Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought resistance related genes
1
2016
... AREBs/ABFs属于亮氨酸拉链类(bZIP)转录因子, 包括ABI5、ABF1-ABF4、OsbZIP23和OsbZIP46等, 结合在ABA响应元件(ABA response element, ABRE)上, 激活或抑制相关基因的表达(
备案号: 京ICP备16067583号-21
版权所有 © 2021 《植物学报》编辑部
地址:北京香山南辛村20号 邮编:100093
电话:010-62836135 010-62836131 E-mail:cbb@ibcas.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
