 ,*, 戴思兰
,*, 戴思兰 ,*北京林业大学园林学院, 国家花卉工程技术研究中心, 花卉种质创新与分子育种北京市重点实验室, 城乡生态环境北京实验室, 林木花卉遗传育种教育部重点实验室, 北京 100083
,*北京林业大学园林学院, 国家花卉工程技术研究中心, 花卉种质创新与分子育种北京市重点实验室, 城乡生态环境北京实验室, 林木花卉遗传育种教育部重点实验室, 北京 100083Advances in Photoreceptor-mediated Signaling Transduction in Flowering Time Regulation
Chaofeng Ma ,*, Silan Dai
,*, Silan Dai ,*College of Landscape Architecture, Beijing Forestry University, Beijing 100083, China
,*College of Landscape Architecture, Beijing Forestry University, Beijing 100083, China通讯作者:
收稿日期:2018-06-25接受日期:2018-09-17网络出版日期:2019-01-30
| 基金资助: |
Corresponding authors:
Received:2018-06-25Accepted:2018-09-17Online:2019-01-30

摘要
关键词:
Abstract
Keywords:
PDF (1616KB)摘要页面多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
马朝峰, 戴思兰. 光受体介导信号转导调控植物开花研究进展. 植物学报, 2019, 54(1): 9-22 doi:10.11983/CBB18147
Ma Chaofeng, Dai Silan.
高等植物从营养生长向生殖生长的成花转变和花器官的形成在物种发育、繁殖和进化中发挥核心作用(Teotia and Tang, 2015)。在长日照(LD)植物拟南芥(Arabidopsis thaliana)中已探明至少有6种开花调控途径: 光周期途径(photoperiod pathway)、春化途径(vernalization pathway)、温度途径(thermorespon- sive pathway)、赤霉素途径(gibberellin pathway)、年龄途径(age pathway)和自主途径(autonomous pathway) (Blümel et al., 2015)。在这些途径中, 植物通过光受体等感受外部环境信号, 历经一系列基因调控的信号转导, 将信号传递到CO (CONSTANS)等成花整合子基因, 进而激活成花素基因FT (FLOWERING LOCUS T)的转录并最终实现开花(Andrés and Coupland, 2012)。CO属于B-box锌指蛋白家族(BBX)成员, 其N端具有B-box B1和B2结构域, C端可结合CCT (CO、CO-like及TOC1)结构域。CO在叶片韧皮部伴胞中激活FT的转录和TSF (TWIN SISTERS OF FT)的微弱表达(Samach et al., 2000; Yamaguchi et al., 2005)。FT是1个RAF (Rapidly Accelerated Fibrosarcoma)相关的激酶抑制蛋白, 通过韧皮部运输到茎尖分生组织, 与分生组织特异性的bZIP转录因子FD (FLOWERING LOCUS D)和FD PARALO- GUE相互作用, 激活成花整合子基因SOC1 (SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1)、花分生组织特征基因LFY (LEAFY)和花器官特征基因AP1 (APETALA 1)的表达, 并引起下游基因的级联反应, 进而调控花发育过程(Corbesier et al., 2007)。本文重点介绍模式植物拟南芥中光受体的结构特征和开花调控等生理功能的研究进展, 并对光敏色素(phytochromes, PHYA-PHYE)、隐花色素(cryptochromes, CRY1/CRY2)和ZTL (zeitlupe)/ FKF1 (Flavin binding, Kelch repeat, F-box protein 1)/LKP2 (Lov Kelch Protein 2)等通过光周期途径或整合其它成花途径调控植物开花的分子机制进行了综述。同时, 指明光受体调控植物开花机制研究中存在的问题, 并对未来的研究方向进行了展望。
1 光受体的种类、结构特征与生理功能
1.1 光受体的种类
植物通过3类光受体(photoreceptor)来感知红光、蓝光和紫外光UV-B并进行信号转导, 包括5个红光/远红光受体光敏色素(Chen and Chory, 2011); 7个蓝光受体, 其中2个隐花色素(Chaves et al., 2011)、3个LOV/F-box/Kelch结构域蛋白即ZTLs家族成员FKF1、ZTL及LKP2 (Suetsugu and Wada, 2013), 2个向光素(phototropins, PHOT1-2) (Christie, 2007)和1个UV-B受体UVR8 (UVB-resistance locus 8) (Rizzini et al., 2011)。1.2 光受体的结构特征
光敏色素是主要的红光/远红光感受器, 是植物自身合成的水溶性色素蛋白。功能性光敏色素由2个单体组成, 每个单体又含有2个结构域, 其中N端的光感受结构域可共价结合生色团, 与光敏色素的光化学特性相关, 而C端的光调节结构域主要参与光敏色素二聚体的形成及下游信号转导过程(Matsushita et al., 2003; Rockwell et al., 2006)。光敏色素N端功能域又分为4个亚功能域: P1、P2/PAS、P3/GAF和P4/PHY; C端功能域可分为PAS (包括PAS-A和PAS-B)和HKRD (histidine kinase-related domain)两个亚功能域(Bae and Choi, 2008) (图1)。光照会触发光敏色素蛋白在具有生物活性的远红光吸收型Pfr (phytochrome FR-absorbing isomer)和无活性的红光吸收型Pr (phytochrome R-absorbing form)之间相互转化。一般认为, 光敏色素生色团与高度保守的GAF区域结合, 以Pr形式存在于细胞质中。接收红光刺激后, 生色团的线性四吡咯环发生光质异构化, Pr转变为Pfr, 并从细胞质转移到细胞核中, 直接与PIFs (Phytochrome Interacting Factors)或其它信号转导组分互作, 进行光信号的放大和传递(Leivar and Monte, 2014)。在远红光下, Pfr又转变为Pr构象。隐花色素是一种类光解酶(photolyase)的蓝光受体, 在动植物中均有存在(Cashmore, 2003), 但植物中隐花色素不具有催化紫外光损伤DNA修复的功能(Chaves et al., 2011)。CRYs具有2个重要的结构域, N端为非共价结合生色团黄素FAD (Flavin Adenine Dinucleotide)和叶酸MTHF (methenyltetrahydrofolate)的光裂解酶相关PHR (photolyase-homologous region)结构域, C端为不具有光裂解酶活性, 但对蛋白互作和信号转导十分重要的CCE (Cryptochrome C-terminal Extension)结构域(Cashmore et al., 1999; Yang et al., 2000) (图1)。
向光素(phototropins)是光活化的丝氨酸/苏氨酸蛋白激酶。目前, 在拟南芥中仅发现2种向光素, 即PHOT1和PHOT2 (Briggs and Christie, 2002)。PHOT1和PHOT2通过2个N端的LOV (Light, Oxygen, Voltage)结构域(LOV1和LOV2)结合生色团黄素单核苷酸FMN (Flavin Mononucleotide)来感知光信号, 并通过LOV结构域与辅因子结合或与其它蛋白发生互作(Christie, 2007) (图1)。LOV结构域最早来自PAS大家族的一个亚家族(Ito et al., 2012a), 该结构域不仅存在于向光素中, 还存在于植物、真菌和细菌的其它蓝光感受器中。
ZTLs是一类新发现的蓝光受体蛋白。拟南芥中已知的ZTLs家族包括FKF1、ZTL和LKP2。这3个蛋白都含有3个重要的功能保守结构域: N端的LOV结构域、中间的F-box基序和C端的Kelch重复序列(Somers et al., 2000; Schultz et al., 2001; Ito et al., 2012a)。值得注意的是, 拟南芥中ZTLs家族仅有1个LOV结构域, 而向光素有2个LOV结构域。ZTLs家族成员均以LOV结构域结合生色团黄素单核苷酸FMN (Nelson et al., 2000; Schultz et al., 2001)。Kelch结构域则与蛋白质的相互作用相关(Ito et al., 2012a) (图1)。
图1
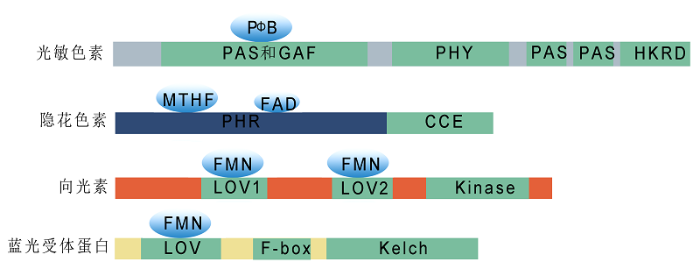 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1植物光受体结构示意图
光敏色素N端为可共价结合生色团的光感受结构域, 包括PAS、GAF和PHY等亚功能域; C端为光调节结构域, 主要参与光敏色素二聚体的形成及下游的信号转导过程, 包括PAS和HKRD两个亚功能域。隐花色素N端为非共价结合生色团黄素(FAD)和叶酸(MTHF)的光裂解酶相关PHR结构域, C端是对蛋白互作和信号转导十分重要的CCE结构域。向光素通过2个N端的LOV结构域(LOV1和LOV2)结合生色团黄素单核苷酸FMN来感知光照信号, 并通过LOV结构域与辅因子结合或与其它蛋白发生互作。蓝光受体蛋白ZTLs含有3个重要的功能保守结构域: N端的LOV结构域、中间的F-box基序和C端的Kelch重复序列。ZTLs以LOV结构域结合生色团黄素单核苷酸FMN, Kelch结构域则与蛋白质的相互作用相关。
Figure 1Schematic structural diagrams of plant photoreceptors
The N-terminus of phytochromes is a photosensory region (containing PAS, GAF, and PHY), which covalently binds chromophores. The C-terminal light regulatory domains, including PAS and HKRD, are involved in phytochrome dimer formation and downstream signaling transduction. The N-terminus of cryptochromes is a PHR domain, which non-covalently binds FAD and MTHF, and the C-terminal CCE domain is important for protein-protein interactions and signaling transduction. The two N-terminal LOV domains (LOV1 and LOV2) of phototropins bind FMN to perceive light signals and are required for interaction with other proteins. ZTL proteins contain three important functional conserved domains: the N-terminal LOV domain, the intermediate F-box motif, and the C-terminal Kelch repeat region. The LOV domain binds to FMN, and the Kelch domain mediates protein-protein interactions.
UVR8参与调控植物对UV-B辐射的响应。拟南芥UVR8编码440个氨基酸残基, 分子量为47 kDa。目前已获得在N端缺少11个氨基酸且在C端缺少59个氨基酸的UVR8蛋白核心的高分辨率晶体结构, 其由7个片状的β螺旋构成, 但C端和N端的位置、功能结构域特征以及UVR8蛋白从二聚体到单体如何变化尚不清楚(Jenkins, 2014)。
1.3 光受体的生理功能
拟南芥光敏色素基因家族有5个成员: PHYA、PHYB、PHYC、PHYD和PHYE, 不同成员间既相互独立, 又存在功能冗余。PHYA是一类在光照下迅速分解的光不稳定型蛋白, 参与幼苗的远红光感受及介导早期的红光反应; PHYB、PHYC、PHYD和PHYE属于光稳定型蛋白, 在持续红光或白光下起主要作用(Tepper- man et al., 2006; Kami et al., 2010)。PHYA可促进拟南芥开花(Johnson et al., 1994)。PHYB抑制开花(Guo et al., 1998)。PHYC在短日照(SD)下抑制开花, 而在长日照下与PHYA共同促进开花(Monte et al., 2003)。PHYD和PHYE抑制开花, PHYB、PHYD和PHYE存在功能冗余(Devlin and Kay, 2000) (表1)。拟南芥隐花色素基因家族由3个成员组成: CRY1、CRY2和CRY3。CRY1和CRY2与植物昼夜节律钟相关, 参与调控幼苗去黄化和成花诱导过程(Chaves et al., 2011)。CRY1和CRY2在拟南芥中均可促进开花(Koornneef et al., 1991; Ahmad and Cashmore, 1993; Guo et al., 1998) (表1)。CRY3属于cry-DASH (Drosophila、Arabidopsis、Synecho- cystis及Homo)分支, 从进化上看CRY3是介于隐花色素和光裂解酶之间的中间体(Brudler et al., 2003), 其功能有待进一步研究。
作为昼夜节律钟的重要组成因子, ZTLs参与植物昼夜节律信号的输入, 调控下游的成花诱导和花器官形成基因的表达(Baudry et al., 2010)。FKF1是一种E3泛素化连接酶, 通过调控蛋白稳定性, 影响下游基因的转录(Imaizumi et al., 2005)。FKF1对开花具有促进作用(Imaizumi et al., 2005), ZTL和LKP2对开花具有抑制作用, LKP2和ZTL均可以与FKF1互作进而抑制其蛋白积累(Somers et al., 2000; Más et al., 2003; Takase et al., 2011)。ZTL可以在体外与PHYB和CRY1相互作用, 可能具有将信号输入到昼夜节律钟的功能(Jarillo et al., 2001) (表1)。
Table 1
表1
表1拟南芥光受体类型及生理功能
Table 1
| 光受体 | 基因 | 生理功能 | 主要参考文献 |
|---|---|---|---|
| 光敏色素 | PHYA | 促进开花, 幼苗去黄化, 种子萌发, 避阴反应 | Johnson et al., 1994; Sch?fer and Bowler, 2002; Tepperman et al., 2006; Heschel et al., 2007 |
| PHYB | 抑制开花, 幼苗去黄化, 种子萌发, 气孔发育, 避阴反应 | Guo et al., 1998; Sch?fer and Bowler, 2002; Heschel et al., 2007; Wang et al., 2010; Kami et al., 2010 | |
| PHYC | 短日照下抑制开花, 长日照下促进开花 | Monte et al., 2003 | |
| PHYD | 抑制开花, 种子萌发, 避阴反应 | Devlin and Kay, 2000; Sch?fer and Bowler, 2002; Heschel et al., 2007 | |
| PHYE | 抑制开花, 种子萌发, 避阴反应 | Devlin and Kay, 2000; Sch?fer and Bowler, 2002; Heschel et al., 2007 | |
| 隐花色素 | CRY1 | 促进开花, 气孔发育, 幼苗去黄化 | Ahmad and Cashmore, 1993; Mao et al., 2005 |
| CRY2 | 促进开花, 幼苗去黄化, 昼夜节律调控 | Koornneef et al., 1991; Guo et al., 1998 | |
| 向光素 | PHOT1 | 向光性, 光诱导的气孔开放和叶绿体运动, 抑制下胚轴伸长 | Briggs and Christie, 2002; Celaya and Liscum, 2005 |
| PHOT2 | 向光性, 光诱导的气孔开放和叶绿体运动, 抑制下胚轴伸长 | Briggs and Christie, 2002; Celaya and Liscum, 2005 | |
| ZTLs | FKF1 | 促进开花, 昼夜节律信号输入 | Imaizumi et al., 2005; Baudry et al., 2010 |
| ZTL | 长日照下抑制开花, 昼夜节律信号输入 | Somers et al., 2000; Baudry et al., 2010 | |
| LKP2 | 抑制开花 | Takase et al., 2011 | |
| 紫外受体 | UVR8 | 抑制下胚轴伸长, 光形态建成, 抑制开花 | Favory et al., 2009; Gruber et al., 2010; Yang et al., 2018; Liang et al., 2018 |
新窗口打开|下载CSV
向光素是植物中与细胞质膜相关的蓝光受体, 参与调控植物的向光性生长、光诱导的气孔开放和叶绿体运动及下胚轴伸长等生物学过程(Briggs and Christie, 2002; Celaya and Liscum, 2005) (表1)。
UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能。在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(Rizzini et al., 2011; Christie et al., 2012; Wu et al., 2012; Qian et al., 2016)。目前, 已陆续鉴定出多个与UVR8相互作用的蛋白, 包括COP1 (CONSTITUTIVE PHOTOMORPHOGENIC 1)、WRKY36、BES1 (BRI1-EMS-SUPPRESSOR 1)和BIM1 (BES1-INTERACTING MYC-LIKE 1)等, 其在植物体内的生理功能主要包括抑制下胚轴伸长、调控光形态建成及开花等(Favory et al., 2009; Gruber et al., 2010; Yang et al., 2018; Liang et al., 2018; Dotto et al., 2018) (表1)。
2 光受体在光周期成花途径中的调控作用
在许多植物中, 光周期是影响开花的重要环境因子, 光周期途径是调控植物开花的途径之一(Johansson and Staiger, 2015)。拟南芥是一种典型的长日照植物, 长日照促进其开花, 短日照则抑制其开花。CO的转录及转录后水平调控和FT的转录水平调控是关键调控节点, 而这种机制受到上游光受体输入信号的影响。2.1 光受体对CO转录水平的调控
隐花色素、光敏色素和FKF1/ZTL/LKP2均参与CO基因转录水平的调控。CO的转录主要受到CDF1 (CY- CLING ODF FACTOR 1)和FKF1-GI (GIGANTEA)复合体的调控(Imaizumi et al., 2005; Fornara et al., 2009)。CDFs家族是一类转录因子, 早晨CDF1可直接抑制CO的转录(Sawa et al., 2007)。FKF1是一种E3泛素化连接酶, GI是植物特有的核蛋白(Mizoguchi et al., 2005)。FKF1和GI呈现出振荡表达, 以蓝光依赖的形式相互作用形成FKF1-GI复合体, 该复合体使转录抑制因子CDF1在下午发生降解, 进而激活CO的转录(Sawa et al., 2007; Fornara et al., 2009)。除FKF1外, ZTL和LKP2也均能与GI形成蛋白复合体, 进而作用于相同的靶蛋白(如CDF1) (图2)。然而, 与FKF1不同, 过表达ZTL和LKP2的转基因植株在长日照条件下强烈抑制CO的表达而表现出晚花表型(Schultz et al., 2001; Somers et al., 2004), 这是由于LKP2和/或ZTL会影响光照初期FKF1蛋白的积累(Takase et al., 2011)。CRY2在CO的转录调控中行使重要功能。光通过诱导CRY2形成同源二聚体的方式来激活CRY2, 而BIC1 (BLUE-LIGHTINHIBITOR OF CRYPTOCHR- OMES 1)可与CRY2直接互作阻断CRY2的二聚化反应和后续的信号转导过程(Wang et al., 2016)。COP1 是含有Ring结构域的E3泛素化连接酶, ELF3 (EA- RLY FLOWERING 3)是无保守结构域的植物特异性蛋白, 也是植物昼夜节律晚间复合体的组成部分(Bendix et al., 2015)。黑暗状态下, COP1与ELF3形成COP1/ELF3复合体并降解GI蛋白, 蓝光激活的CRY2能够抑制COP1/ELF3复合体的活性, 促进GI蛋白的积累, 形成FKF1-GI复合体(Yu et al., 2008) (图2)。
图2
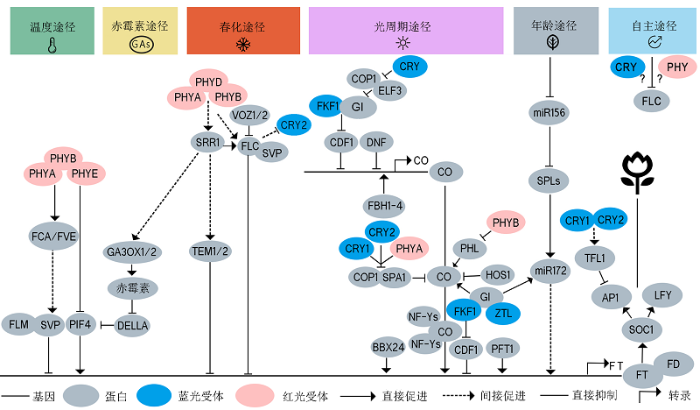 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2光受体介导环境信号调控拟南芥开花示意图
植物中已探明至少有6种开花调控途径: 光周期途径、春化途径、温度途径、自主途径、赤霉素途径和年龄途径。在叶片中, 光敏色素、隐花色素和ZTL/FKF1/LKP2等光受体介导光信号并将信号传递给昼夜节律钟, 经过多种开花途径的信号整合, 最终直接或间接调控CO、FT和FLC的表达和蛋白稳定性。温度途径中光敏色素通过FCA/FVE间接调控FLM、SVP和PIF4进而调控FT的转录; 赤霉素途径和春化途径由SRR1进行信号整合, 通过GA生物合成途径GA3OX1/2以及DELLA蛋白调控FT的转录, 春化途径通过VOZ1/2和光敏色素调控FLC、SVP和TEM1/2进而调控FT的表达; 自主途径通过抑制开花阻遏物和春化途径关键基因FLC促进开花; 光受体在光周期途径中发挥重要作用, GI、CDF1、DNF、FBH1-4、COP1、SPA1、HOS1和NF-Ys等蛋白直接或间接与光受体互作, 对CO转录、转录后水平以及FT的转录水平等关键调控节点进行调控。年龄途径通过miR156/172和SPLs调控FT的表达。不同的开花途径之间存在信号整合机制, 最终开花信号被整合到FT、SOC1和LFY, 激活AP1和LFY, 进而完成开花起始。实线箭头表示直接促进, 虚线箭头表示间接促进, 钝化线表示直接抑制, 红色表示红光/远红光受体, 蓝色表示蓝光受体, 灰色表示信号蛋白。
Figure 2A schematic diagram of flowering time regulation by photoreceptor-mediated environmental signaling in Arabidopsis
Six flowering regulatory pathways have been identified in plants, including photoperiodic pathway, vernalization pathway, temperature pathway, autonomous pathway, gibberellin pathway, and age pathway. Phytochromes, cryptochromes, and ZTL/FKF1/ LKP2 perceive light signals in leaves, and transmit the signals to the circadian clock. After signaling integration through multiple flowering pathways, the photoreceptors eventually directly or indirectly regulate CO, FT and FLC expression and protein stability. In the ambient temperature pathway, the phytochromes indirectly regulate the transcription of FT by FLM, SVP and PIF4 through FCA/FVE. SRR1 integrates signals from the gibberellin pathway and vernalization pathway and regulate FT transcription through GA3OX1/2 and DELLA proteins in the GA pathway. In the vernalization pathway, VOZ1/2 and phytochromes regulates FT expression through FLC, SVP, and TEM1/2. The autonomous pathway promotes flowering by repressing FLC. Photoreceptors play an important role in the photoperiodic pathway. GI, CDF1, DNF, FBH1-4, COP1, SPA1, HOS1, and NF-Ys directly or indirectly interact with photoreceptors to regulate CO transcription, CO stability, and FT transcription. The age pathway regulates FT expression through miR156/172 and SPLs. There are signal integration mechanisms between different flowering pathways. The signals are integrated into FT, SOC1, and LFY, leading to the activation of AP1 and LFY and eventually the initiation of flowering. Solid arrows indicate direct promotion; dotted arrows indicate indirect promotion; blunted lines indicate direct inhibition; red ovals indicate red/far-red light photoreceptors; blue ovals indicate blue light photoreceptors, and gray ovals indicates signaling intermediate proteins.
此外, 人们还鉴定出其它CO转录的调节因子。ELF4 (EARLY FLOWERING 4)可在夜间限制GI蛋白结合CO启动子的能力, 进而影响其转录(Kim et al., 2013)。E3泛素化连接酶DNF (DAY NEUTRAL FLOWERING)在短日照下抑制CO的表达, 在维持CO的低水平表达中起重要作用(Morris et al., 2010)。FLOWERING BHLH蛋白FBH1、FBH2、FBH3和FBH4以及中介体复合物PFT1 (PHYTOCHROME AND FLOWERING TIME)/MED25可促进CO的表达(I?igo et al., 2012; Ito et al., 2012b)。此外, MSI1 (MULTICOPY SUPPRESSOR OF IRA 1)可结合组蛋白, 在光周期途径中对于CO的转录激活是必需的(Steinbach and Hennig, 2014)。
2.2 光受体对CO蛋白稳定性的调控
除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性。在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(Song et al., 2012)。COP1-SPA1 (SUPPRESSOR OF PHYTOCHROME A)泛素连接酶复合体参与CO蛋白丰度的动态调节, 可在夜间降解CO蛋白(Laubinger et al., 2006; Jang et al., 2008)。光活化的CRY降低了COP1-SPA1复合物的活性, 但CRY1和CRY2对COP1-SPA1复合体活性的抑制机制不同。CRY1与SPA1的C端结合阻止SPA1-COP1复合体的形成(Liu et al., 2011), 而CRY2通过结合SPA1的N端进而促进SPA1与COP1的结合, 但抑制SPA1-COP1复合体的活性, 促进CO蛋白的积累(Zuo et al., 2011)。此外, 光敏色素PHYA可稳定CO蛋白进而促进开花; 而PHYB促进CO的降解从而抑制开花。与CRY2机制类似, PHYA也能够抑制COP1-SPA1复合体的形成(Valverde et al., 2004; Sheerin et al., 2015)。PHYB则在早晨以独立于COP1的途径降解CO蛋白(Jang et al., 2008)。还有研究表明, PHL (PHYTOCHROME- DEPENDENT LATE-FLOWERING)参与PHYB依赖的开花调控过程, PHL有可能通过形成PHYB-PHL- CO复合体从而拮抗PHYB对CO的抑制作用(Endo et al., 2013)。除了COP1, 另一种E3泛素连接酶HOS1 (HIGH EXPRESSION OF OSMOTICALLY RES- PONSIVE GENE1)也参与CO蛋白的去稳定性过程(Lazaro et al., 2012) (图2)。此外, FKF1能够通过LOV结构域与CO互作从而稳定CO蛋白活性, 蓝光能增强它们之间的相互作用(Song et al., 2012)。有研究推测, UVR8可能与光敏色素和/或隐花色素共同竞争COP1 (Favory et al., 2009)。自然状态下的UVR8二聚体在UV-B辐射下解聚成单体, 快速在细胞核中积累, 其单体直接与COP1结合, 推测二者结合进而释放HY5 (ELONGATED HYPOCOTYL 5)转录因子, 打开了UV-B信号通路(Rizzini et al., 2011; Jenkins, 2014)。COP1和HY5均是光信号转导的重要下游信号分子, 这暗示UV-B信号与可见光信号可能存在相互干扰机制。但是目前UVR8/COP1作用如何影响COP1对下游响应基因的激活并不清楚。
2.3 光受体对FT转录水平的调控
FT的表达丰度决定了开花时间。CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(Tiwari et al., 2010)。NF-YA、NF-YB和NF-YC形成NF-Y (NUCLEAR FACTOR Y)复合物, 结合在FT启动子上的CCAAT-box可进一步增强CO介导的FT转录激活(Cai et al., 2007; Kumimoto et al., 2008; Cao et al., 2014)。此外, 光周期途径B-box家族成员STO (SALT TOLERANT)或BBX24可激活FT的表达(Li et al., 2014), BBX19则被确定为长日照下开花的负调节因子(Wang et al., 2014)。隐花色素、光敏色素以及FKF1等光受体都参与FT的转录调控。在蓝光下转录因子CIB1 (CRYPTOCHROME INTERACTING BASIC HELIX-LOOP-HELIX1)与CR- Y2互作形成CRY2-CIB1复合物并结合FT启动子E- box元件(CANNTG), 进而激活FT的转录(Liu et al., 2008)。此外, ZTL和LKP2 (不包括FKF1)是CIB1蛋白积累所必需的(Liu et al., 2013)。CO的转录抑制因子CDF蛋白也可与FT启动子结合抑制其转录(Song et al., 2012), 而FKF1-GI复合体可以移除FT转录起始位点附近的CDF1, 从而促进FT的表达(Song et al., 2012)。GI是PHYB信号转导的重要组分, 可直接激活FT的转录(Huq et al., 2000; Sawa and Kay, 2011)。PHYB与GI之间的相互作用已经在体内和酵母双杂交分析中得到证实, 这暗示红光参与FT的表达调控(Yeom et al., 2014)。此外, 在红光下PHYB也可与PFT1互作抑制其活性, 进而抑制FT的表达(图2)。3 光受体在其它成花途径中的调控作用
3.1 光受体与春化途径
某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(Kim et al., 2009)。转录因子FRI (FRIGIDA)和FLC (FLOWERING LOCUS C)在其中发挥重要作用。开花抑制因子FLC与SVP (SHORT VEGETATIVE PHASE)形成蛋白复合体, 进而抑制SOC1、FT和FD的转录(Amasino, 2010) (图2)。持续低温会下调FLC的表达及蛋白积累(Helliwell et al., 2006)。拟南芥在营养生长阶段响应低温, 通过一类B3转录因子VAL1 (VERNALIZATION 1)或VAL2等沉默FLC的表达, 形成“低温记忆”(Yuan et al., 2016b)。而在开花后的胚胎发育早期, FLC会被1个种子特有的“先驱”转录因子LEC1 (LEAFY COTYLEDON 1)重新激活(Tao et al., 2017; 许淑娟和种康, 2018)。此外, FLC也被自主途径的内源性调节因子下调表达, 包括染色质修饰因子FVE以及RNA结合蛋白FCA、FPA和FLK (Rataj and Simpson, 2014)。已证明FLC和自主途径中的几个组分影响植物昼夜节律振荡周期(Salathia et al., 2006)。有研究表明, 光敏色素介导的光信号可以被整合到春化途径中, 参与调控FLC的转录(Strasser et al., 2009)。PHYB的互作蛋白VOZ1 (VASCULAR PLANT ONE-ZINC FINGER 1)和VOZ2可抑制FLC的表达, 从而促进开花, voz1/voz2双突变体表现晚花表型(Yasui et al., 2012; Yasui and Kohchi, 2014)。
3.2 光受体与温度途径
适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(Samach and Wigge, 2005)。高温不仅会导致提早开花, 而且还会导致下胚轴和叶柄变长(Balasubramanian et al., 2006)。SVP通过抑制FT的转录在开花的温度途径中起重要作用(Lee et al., 2007)。另一类MADS-box蛋白FLM (FLOWE- RING LOCUS M)可在低温下形成SVP-FLM-β阻遏物, 抑制FT的转录; 而在高温下形成的SVP-FLM-δ复合物没有结合DNA的能力, 从而促进开花(Posé et al., 2013)。此外, SVP的表达呈现昼夜节律性振荡, 表明温度途径与昼夜节律钟之间存在整合机制(Fujiwara et al., 2008)。拟南芥phyB突变体在其最适温度(22°C)下开花提前, 而在16°C该突变体与WT具有相似的开花时间。在phyA/phyB/phyD三重突变背景下, phyE的功能突变表现显著的早花表型, 表明16°C时PHYE在开花调控中起主要作用(Halliday et al., 2003); cry1、cry2和phyA/cry2双突变体在16°C时开花显著推迟, 表明隐花色素和光敏色素也在温度途径中发挥调控开花的功能(Blázquez et al., 2003)。TFL1 (TERMINAL FLOWER 1)和FT二者同属磷脂酰乙醇胺结合蛋白家族(phosphatidylethanola-minebinding protein, PEBP), 尽管其氨基酸序列同源性高达60%, 但TFL1抑制开花, 而FT促进开花(Coelho et al., 2014)。tfl1突变可消除crys突变体中的温度反应, 昼夜节律组分elf3的突变可以抑制phyB突变体的温度反应, 表明至少存在2条路径可以将光信号整合到温度途径中(Strasser et al., 2009)。bHLH类转录因子PIF4 (PHYTOCHROME-INTERACTING FACTOR 4)是连接光受体和环境温度信号转导的重要因子, 通过与FT启动子结合介导高温开花, 过表达PIF4的株系表现出早花表型(Kumar et al., 2012), 而PIF4蛋白积累受到PHYB的负调控(de Lucas et al., 2008) (图2)。3.3 光受体与赤霉素途径
赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(Galv?o et al., 2012)。GA生物合成途径受阻则会抑制高温下提早开花(Balas- ubramanian et al., 2006)。转录因子TEM (TEM-RANILLO)可能具有整合光周期信号和GA途径的功能(Osnato et al., 2012)。TEM1和TEM2可抑制GA生物合成途径中GA3OX1 (GA3OXIDASE 1)和GA3OX2基因的表达, 过表达TEM基因的植物具有类似GA缺失型突变体的表型(Osnato et al., 2012)。此外, TEM1和TEM2也是FT的直接抑制因子, 二者可与CO竞争结合FT启动子, CO和TEM之间相对蛋白量的高低决定了FT的转录水平(Castillejo and Pelaz, 2008)。此外, SRR1 (SENSITIVITY TO RED LIGHT REDUCED)蛋白在调节昼夜节律钟和PHYB介导的信号转导中发挥重要作用(Staiger et al., 2003)。srr1突变体和phyB突变体表现出许多相似的表型, 表明SRR1是进行正常的PHYB信号转导所必需的。SRR1通过激活CDF1、TEM1、TEM2和FLC等FT转录抑制因子的表达, 从而抑制拟南芥在短日照条件下开花(Johansson and Staiger, 2014) (图2)。此外, PIF4也可以进行光信号和GA信号的整合(de Lucas and Prat, 2014)。GA信号途径中关键的DELLA蛋白是PIF4的转录抑制因子, DELLA蛋白在长日照条件下以独立于CO和GI的方式调控FT的表达。GA含量增加可降低DELLA蛋白的稳定性, 进而使PIF4在细胞核中积累(de Lucas et al., 2008)。3.4 光受体与年龄途径
植物必须完成一定程度的营养生长才能进入生殖生长。miR156和miR172在拟南芥开花的年龄途径中发挥重要作用。成花抑制因子miR156的表达在幼龄期高于成年期, 而成花促进因子miR172的表达在幼龄期低于成年期(Wu et al., 2009)。miR156是植物进化上最为保守的miRNA之一, 其靶基因是SPLs (SQUAMOSA PROMOTER BINDING LIKEs)转录因子家族基因(Reinhart et al., 2002)。拟南芥基因组中有11个由miR156靶向的SPL基因, 可以分为SPL3 (SPL3、SPL4和SPL5)和SPL9 (SPL2、SPL6、SPL9、SPL10、SPL11、SPL13、SPL13-like及SPL15)两组(Xing et al., 2010)。SPL9直接结合miR172启动子促进其表达(Wu et al., 2009)。miR172可调节AP2-like转录因子家族基因的转录, 包括AP2、SMZ (SCHLA- FMUTZE)、SNZ (SCHNARCHZAPFEN)、TOE1 (TARGET OFEAT 1)、TOE2和TOE3等FT基因的转录抑制因子基因(Chen, 2004; Wu et al., 2009)。年龄途径和光周期途径的整合发生在2个层面上。首先, 通过光周期途径中的GI参与miR172丰度的调控(图2)。其次, miR156的靶基因SPL在光周期途径的下游 起作用, 当拟南芥从短日照变为长日照时, SPL被 快速诱导转录, 这一过程可能由2个MADS-box基因SOC1和FUL (FRUITFULL)介导(Wang, 2014)。此 外, UV-B可通过调控拟南芥体内miR156水平从而调 控开花, UVR8在其中发挥关键作用(Dotto et al., 2018)。4 总结与展望
在过去的几十年中, 高等植物开花的调控网络一直是植物学研究的前沿和热点。目前, 不同光受体的分子结构和生理功能已逐渐得到解析, 发现了一大批与光受体互作的信号蛋白, 人们对光周期途径、温度途径及GA途径的信号转导及整合机制也有了一定的认识(图2), 但是还存在一些问题有待深入研究。例如: (1) 植物内源性昼夜节律钟的组分ELF3和GI被整合到光周期途径, 而温度和激素等外界信号与昼夜节律钟的关系尚未阐明; (2) 有研究表明低氮会激活CRY1的表达, 而蓝光信号可以逆转高氮引起的开花延迟(Yuan et al., 2016a), 这为研究氮和磷等营养信号与开花机制的关系提供了思路; (3) 关键信号蛋白的亚细胞定位、磷酸化和泛素化的作用机制有待探究; (4) 除了年龄途径的miR156和miR172, 其它的表观遗传调控, 如DNA甲基化和组蛋白修饰等在开花调控中的作用尚待揭示; (5) 自主途径通过抑制开花阻遏物和春化途径关键基因FLC而促进开花(Yan et al., 2010), 在春化途径中光敏色素参与FLC的转录调控, 但是自主途径中FLC是否受到光受体的调控尚不清楚。此外, 光受体在水稻(Oryza sativa)等重要农作物和园艺作物中的功能研究也取得了一定进展(Takano et al., 2005; Yang et al., 2017), 但下游关键调控因子的功能鉴定及其调控机制亟待研究。功能基因组学时代的到来以及转录组学、蛋白质组学、代谢组学和表型组学等组学技术的进步也可能为破解高等植物成花之谜带来全新的视角。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1046/j.1365-313X.2003.01674.xURLPMID:12609029 [本文引用: 1]

Summary In Arabidopsis flowering is accelerated by reduced red:far-red (R:FR) ratio which signals the presence of neighbouring vegetation. Hastened flowering is one component of the shade-avoidance syndrome of responses, which alter many aspects of development in response to the threat of potential competition. Of the red/far-red-absorbing photoreceptors it is phyB that plays the most prominent role in shade-avoidance, although other related phytochromes act redundantly with phyB . It is well established that the phyB mutant has a constitutively early flowering phenotype. However, we have shown that the early flowering phenotype of phyB is temperature-dependent. We have established that this temperature-sensitive flowering response defines a pathway that appears to be independent of the autonomous-FLC pathway. Furthermore, we have demonstrated that the phytochromes control the expression of the floral promoter FT . We have also shown that other phyB-controlled responses, including petiole elongation, are not sensitive to the same temperature change. This suggests that discrete pathways control flowering and petiole elongation, components of the shade-avoidance response. This work provides an insight into the phytochrome and temperature interactions that maintain flowering control.
.
DOI:10.1016/j.ctcp.2009.09.009URLPMID:16623882 [本文引用: 1]

Abstract The Arabidopsis Flowering Locus C (FLC) protein is a repressor of flowering regulated by genes in the autonomous and vernalization pathways. Previous genetic and transgenic data have suggested that FLC acts by repressing expression of the floral integrator genes SOC1 and FT. We have taken an in vivo approach to determine whether the FLC protein interacts directly with potential DNA targets. Using chromatin immunoprecipitation, we have shown that FLC binds to a region of the first intron of FT that contains a putative CArG box, and have confirmed that FLC binds to a CArG box in the promoter of the SOC1 gene. MADS box proteins are thought to bind their DNA targets as dimers or higher-order multimers. We have shown that FLC is a component of a multimeric protein complex in vivo and that more than one FLC polypeptides can be present in the complex.
DOI:10.1111/j.1469-8137.2007.02044.xURLPMID:17504457

090004 Germination timing is a fundamental life-history trait, as seedling establishment predicates realized fitness in the wild. Light and temperature are two important cues by which seeds sense the proper season of germination. Using Arabidopsis thaliana , we provide evidence that phytochrome-mediated germination pathways simultaneously respond to light and temperature cues in ways that affect germination. 090004 Phytochrome mutant seeds were sown on agar plates and allowed to germinate in lit, growth chambers across a range of temperatures (700°C to 2800°C). 090004 phyA had an important role in promoting germination at warmer temperatures, phyE was important to germination at colder temperatures and phyB was important to germination across a range of temperatures. 090004 Different phytochromes were required for germination at different temperatures, indicating a restriction or even a potential specialization of individual phytochrome activity as a function of temperature. This temperature-dependent activity of particular phytochromes reveals a potentially novel role for phytochrome pathways in regulating the seasonal timing of germination.
DOI:10.1073/pnas.170283997URLPMID:10920210 [本文引用: 1]

In a genetic screen of available T-DNA-mutagenized Arabidopsis populations for loci potentially involved in phytochrome (phy) signaling, we identified a mutant that displayed reduced seedling deetiolation under continuous red light, but little if any change in responsiveness to continuous far-red light. This behavior suggests disruption of phyB, but not phyA signaling. We have cloned the mutant locus by using the T-DNA insertion and found that the disrupted gene is identical to the recently described GIGANTEA (GI) gene identified as being involved in control of flowering time. The encoded GI polypeptide has no sequence similarity to any known proteins in the database. However, by using glucuronidase-GI and green fluorescent protein-GI fusion constructs, we have shown that GI is constitutively targeted to the nucleus in transient transfection assays. Optical sectioning by using the green fluorescent protein-GI fusion protein showed green fluorescence throughout the nucleoplasm. Thus, contrary to previous computer-based predictions that GI would be an integral plasmamembrane-localized polypeptide, the data here indicate that it is a nucleoplasmically localized protein. This result is consistent with the proposed role in phyB signaling, given recent evidence that early phy signaling events are nuclear localized.
.
[本文引用: 3]
[本文引用: 1]
DOI:10.1093/mp/sss013URLPMID:22402262 [本文引用: 3]

Plants constantly survey the surrounding environment using several sets of photoreceptors. They can sense changes in the quantity (=intensity) and quality (=wavelength) of light and use this information to adjust their physiological responses, growth, and developmental patterns. In addition to the classical photoreceptors, such as phytochromes, cryptochromes, and phototropins, ZEITLUPE (ZTL), FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1), and LOV KELCH PROTEIN 2 (LKP2) proteins have been recently identified as blue-light photoreceptors that are important for regulation of the circadian clock and photoperiodic flowering. The ZTL/FKF1/LKP2 protein family possesses a unique combination of domains: a blue-light-absorbing LOV (Light, Oxygen, or Voltage) domain along with domains involved in protein degradation. Here, we summarize recent advances in our understanding of the function of the Arabidopsis ZTL/FKF1/LKP2 proteins. We summarize the distinct photochemical properties of their LOV domains and discuss the molecular mechanisms by which the ZTL/FKF1/LKP2 proteins regulate the circadian clock and photoperiodic flowering by controlling blue-light-dependent protein degradation.
DOI:10.1073/pnas.1118876109URLPMID:22334645 [本文引用: 1]

Many plants monitor day-length changes throughout the year and use the information to precisely regulate the timing of seasonal flowering for maximum reproductive success. In Arabidopsis thaliana. transcriptional regulation of the CONSTANS (CO) gene and posttranslational regulation of CO protein are crucial mechanisms for proper day-length measurement in photoperiodic flowering. Currently, the CYCLING DOF FACTOR proteins are the only transcription factors known to directly regulate CO gene expression, and the mechanisms that directly activate CO transcription have remained unknown. Here we report the identification of four CO transcriptional activators, named FLOWERING BHLH 1 (FBH1), FBH2, FBH3, and FBH4. All FBH proteins are related basic helix-loop-helix-type transcription factors that preferentially bind to the E-box c/s-elements in the CO promoter. Overexpression of all FBH genes drastically elevated CO levels and caused early flowering regardless of photoperiod, whereas CO levels were reduced in the fbh quadruple mutants. In addition, FBH1 is expressed in the vascular tissue and bound near the transcription start site of the CO promoter in vivo. Furthermore, FBH homologs in poplar and rice induced CO expression in Arabidopsis. These results indicate that FBH proteins positively regulate CO transcription for photoperiodic flowering and that this mechanism may be conserved in diverse plant species. Our results suggest that the diurnal CO expression pattern is generated by a concert of redundant functions of positive and negative transcriptional regulators.
[本文引用: 2]
DOI:10.1038/35068589URLPMID:11260718 [本文引用: 1]

Most organisms, from cyanobacteria to mammals, use circadian clocks to coordinate their activities with the natural 24-h light/dark cycle. The clock proteins of Drosophila and mammals exhibit striking homology but do not show similarity with clock proteins found so far from either cyanobacteria or Neurospora. Each of these organisms uses a transcriptionally regulated negative feedback loop in which the messenger RNA levels of the clock components cycle over a 24-h period. Proteins containing PAS domains are invariably found in at least one component of the characterized eukaryotic clocks. Here we describe ADAGIO1 (ADO1), a gene of Arabidopsis thaliana that encodes a protein containing a PAS domain. We found that a loss-of-function ado1 mutant is altered in both gene expression and cotyledon movement in circadian rhythmicity. Under constant white or blue light, the ado1 mutant exhibits a longer period than that of wild-type Arabidopsis seedlings, whereas under red light cotyledon movement and stem elongation are arrhythmic. Both yeast two-hybrid and in vitro binding studies show that there is a physical interaction between ADO1 and the photoreceptors CRY1 and phyB. We propose that ADO1 is an important component of the Arabidopsis circadian system.
DOI:10.1105/tpc.113.119446URLPMID:24481075 [本文引用: 2]

Low doses of UV-B light (280 to 315 nm) elicit photomorphogenic responses in that modify biochemical composition, photosynthetic competence, , and defense. UV RESISTANCE LOCUS8 (UVR8) mediates photomorphogenic -B by regulating of a set of target genes. UVR8 differs from other known photoreceptors in that it uses specific amino acids instead of a prosthetic for light absorption during UV-B photoreception. Absorption of UV-B dissociates the UVR8 dimer into monomers, initiating signal through interaction with CONSTITUTIVELY PHOTOMORPHOGENIC1. However, much remains to be learned about the physiological role of UVR8 and its interaction with other signaling pathways, the molecular mechanism of UVR8 photoreception, how the UVR8 protein initiates signaling, how it is regulated, and how UVR8 regulates of its target genes.
.
[本文引用: 1]
DOI:10.1093/jxb/eru441URLPMID:25371508 [本文引用: 1]

Plants precisely time the onset of flowering to ensure reproductive success. A major factor in seasonal control of flowering time is the photoperiod. The length of the daily light period is measured by the circadian clock in leaves, and a signal is conveyed to the shoot apex to initiate floral transition accordingly. In the last two decades, the molecular players in the photoperiodic pathway have been identified in Arabidopsis thaliana. Moreover, the intricate connections between the circadian clockwork and components of the photoperiodic pathway have been unravelled. In particular, the molecular basis of time-of-day-dependent sensitivity to floral stimuli, as predicted by B眉nning and Pittendrigh, has been elucidated. This review covers recent insights into the molecular mechanisms underlying clock regulation of photoperiodic responses and the integration of the photoperiodic pathway into the flowering time network in Arabidopsis. Furthermore, examples of conservation and divergence in photoperiodic flower induction in other plant species are discussed.
.
DOI:10.1104/pp.105.1.141URLPMID:12232194 [本文引用: 1]

Several aspects of the photophysiology of wild-type Arabidopsis thaliana seedlings were compared with those of a phytochrome A null mutant, phyA-1, and a mutant, fhy1, that is putatively involved in the transduction of light signals from phytochrome A. Although phyA seedlings display a near wild-type phenotype when grown in white light (W), they nevertheless display several photomorphogenic abnormalities. Thus, whereas the germination of wild-type and fhy1 seeds is almost fully promoted by a pulse of red light (R) or by continuous far-red light (FR), phyA seed germination is responsive only to R. Following growth under day/night cycles, but not under continuous W, the hypocotyls of light-grown phyA and fhy1 seedlings are more elongated than those of wild-type seedlings. For seedlings grown under low red/far-red (R/FR) ratio light conditions, phyA and fhy1 seedlings display a more marked promotion of hypocotyl elongation than wild-type seedlings. Similarly, seedlings that are doubly null for phytochrome A and phytochrome B(phyA phyB) also have more elongated hypocotyls under low R/FR ratio conditions than phyB seedlings. This indicates that phytochrome A action in light-grown seedlings is antagonistic to the action of phytochrome B. Although wild-type, fhy1, and phyA seedlings flower at essentially the same time under both short-day and long-day conditions, an obvious consequence of phytochrome A deficiency is a pronounced late flowering under conditions where a short day of 8 h of fluorescent W is extended by 8 h of low-fluence-rate incandescent light. The evidence thus indicates that phytochrome A plays a role in seed germination, in the control of elongation growth of light-grown seedlings, and in the perception of daylength.
DOI:10.1016/S0070-2153(10)91002-8URL [本文引用: 1]

Plants are sessile and photo-autotrophic; their entire life cycle is thus strongly influenced by the ever-changing light environment. In order to sense and respond to those fluctuating conditions higher plants possess several families of photoreceptors that can monitor light from UV-B to the near infrared (far-red). The molecular nature of UV-B sensors remains unknown, red (R) and far-red (FR) light is sensed by the phytochromes (phyA hyE in Arabidopsis) while three classes of UV-A/blue photoreceptors have been identified: cryptochromes, phototropins, and members of the Zeitlupe family (cry1, cry2, phot1, phot2, ZTL, FKF1, and LKP2 in Arabidopsis). Functional specialization within photoreceptor families gave rise to members optimized for a wide range of light intensities. Genetic and photobiological studies performed in Arabidopsis have shown that these light sensors mediate numerous adaptive responses (e.g., phototropism and shade avoidance) and developmental transitions (e.g., germination and flowering). Some physiological responses are specifically triggered by a single photoreceptor but in many cases multiple light sensors ensure a coordinated response. Recent studies also provide examples of crosstalk between the responses of Arabidopsis to different external factors, in particular among light, temperature, and pathogens. Although the different photoreceptors are unrelated in structure, in many cases they trigger similar signaling mechanisms including light-regulated protein rotein interactions or light-regulated stability of several transcription factors. The breath and complexity of this topic forced us to concentrate on specific aspects of photomorphogenesis and we point the readers to recent reviews for some aspects of light-mediated signaling (e.g., transition to flowering).
DOI:10.1146/annurev.cellbio.042308.113411URLPMID:19575660 [本文引用: 1]

Plants have evolved many systems to sense their environment and to modify their growth and development accordingly. One example is vernalization, the process by which flowering is promoted as plants sense exposure to the cold temperatures of winter. A requirement for vernalization is an adaptive trait that helps prevent flowering before winter and permits flowering in the favorable conditions of spring. In Arabidopsis and cereals, vernalization results in the suppression of genes that repress flowering. We describe recent progress in understanding the molecular basis of this suppression. In Arabidopsis, vernalization involves the recruitment of chromatin-modifying complexes to a clade of flowering repressors that are silenced epigenetically via histone modifications. We also discuss the
DOI:10.1242/dev.096651URLPMID:24004949 [本文引用: 1]

Nucleocytoplasmic partitioning of core clock components is essential for the proper operation of the circadian system. Previous work has shown that the F-box protein ZEITLUPE (ZTL) and clock element GIGANTEA (GI) heterodimerize in the cytosol, thereby stabilizing ZTL. Here, we report that ZTL post-translationally and reciprocally regulates protein levels and nucleocytoplasmic distribution of GI in Arabidopsis. We use ectopic expression of the N-terminus of ZTL, which contains the novel, light-absorbing region of ZTL (the LOV domain), transient expression assays and ztl mutants to establish that the levels of ZTL, a cytosolic protein, help govern the abundance and distribution of GI in the cytosol and nucleus. Ectopic expression of the ZTL N-terminus lengthens period, delays flowering time and alters hypocotyl length. We demonstrate that these phenotypes can be explained by the competitive interference of the LOV domain with endogenous GI-ZTL interactions. A complex of the ZTL N-terminus polypeptide with endogenous GI (LOV-GI) blocks normal GI function, causing degradation of endogenous ZTL and inhibition of other GI-related phenotypes. Increased cytosolic retention of GI by the LOV-GI complex additionally inhibits nuclear roles of GI, thereby lengthening flowering time. Hence, we conclude that under endogenous conditions, GI stabilization and cytoplasmic retention occurs naturally through a LOV domainmediated GI-ZTL interaction, and that ZTL indirectly regulates GI nuclear pools by sequestering GI to the cytosol. As the absence of either GI or ZTL compromises clock function and diminishes the protein abundance of the other, our results highlight how their reciprocal co-stabilization is essential for robust circadian oscillations.
.
[本文引用: 1]
DOI:10.1038/nature10928URLPMID:22437497 [本文引用: 1]

Plant growth and development are strongly affected by small differences in temperature. Current climate change has already altered global plant phenology and distribution, and projected increases in temperature pose a significant challenge to agriculture. Despite the important role of temperature on plant development, the underlying pathways are unknown. It has previously been shown that thermal acceleration of flowering is dependent on the florigen, FLOWERING LOCUS T (FT). How this occurs is, however, not understood, because the major pathway known to upregulate FT, the photoperiod pathway, is not required for thermal acceleration of flowering. Here we demonstrate a direct mechanism by which increasing temperature causes the bHLH transcription factor PHYTOCHROME INTERACTING FACTOR4 (PIF4) to activate FT. Our findings provide a new understanding of how plants control their timing of reproduction in response to temperature. Flowering time is an important trait in crops as well as affecting the life cycles of pollinator species. A molecular understanding of how temperature affects flowering will be important for mitigating the effects of climate change. 2012 Macmillan Publishers Limited. All rights reserved.
DOI:10.1007/s00425-008-0773-6URLPMID:18600346 [本文引用: 1]

Accumulating evidence supports a role for members of the plant Nuclear Factor Y (NF-Y) family of CCAAT-box binding transcription factors in the regulation of flowering time. In this study we have used a genetic approach to show that the homologous proteins NF-YB3 and NF-YB2 have comparable activities and play additive roles in the promotion of flowering, specifically under inductive photoperiodic conditions. We demonstrate that NF-YB2 and NF-YB3 are both essential for the normal induction of flowering by long-days and act through regulation of the expression of FLOWERING LOCUS T (FT). Using an ELISA-based in-vitro assay, we provide a novel demonstration that plant NF-YB subunits are capable of directly binding to a CCAAT-box containing region of the FLOWERING LOCUS T promoter as part of an NF-Y trimer in combination with the yeast HAP2 and HAP5 subunits. These results support an emerging model in which NF-Y complexes provide a component of the DNA target specificity for transcriptional regulators such as CONSTANS.
DOI:10.1242/dev.02481URLPMID:16854975 [本文引用: 1]

Abstract The four-member SPA protein family of Arabidopsis acts in concert with the E3 ubiquitin ligase COP1 to suppress photomorphogenesis in dark-grown seedlings. Here, we demonstrate that SPA proteins are, moreover, essential for photoperiodic flowering. Mutations in SPA1 cause phyA-independent early flowering under short day (SD) but not long day (LD) conditions, and this phenotype is enhanced by additional loss of SPA3 and SPA4 function. These spa1 spa3 spa4 triple mutants flower at the same time in LD and SD, indicating that the SPA gene family is essential for the inhibition of flowering under non-inductive SD. Among the four SPA genes, SPA1 is necessary and sufficient for normal photoperiodic flowering. Early flowering of SD-grown spa mutant correlates with strongly increased FT transcript levels, whereas CO transcript levels are not altered. Epistasis analysis demonstrates that both early flowering and FT induction in spa1 mutants is fully dependent on CO. Consistent with this finding, SPA proteins interact physically with CO in vitro and in vivo, suggesting that SPA proteins regulate CO protein function. Domain mapping shows that the SPA1-CO interaction requires the CCT-domain of CO, but is independent of the B-box type Zn fingers of CO. We further show that spa1 spa3 spa4 mutants exhibit strongly increased CO protein levels, which are not caused by a change in CO gene expression. Taken together, our results suggest, that SPA proteins regulate photoperiodic flowering by controlling the stability of the floral inducer CO.
DOI:10.1105/tpc.110.081885URLPMID:22408073 [本文引用: 1]

The Arabidopsis thaliana early in short days6 (esd6) mutant was isolated in a screen for mutations that accelerate flowering time. Among other developmental alterations, esd6 displays early flowering in both long-and short-day conditions. Fine mapping of the mutation showed that the esd6 phenotype is caused by a lesion in the HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENESI (HOS1) locus, which encodes a RING finger-containing E3 ubiquitin ligase. The esd6/hos1 mutation causes decreased FLOWERING LOCUS expression and requires CONSTANS (CO) protein for its early flowering phenotype under long days. Moreover, CO and HOS1 physically interact in vitro and in planta, and HOS1 regulates CO abundance, particularly during the daylight period. Accordingly, hos1 causes a shift in the regular long-day pattern of expression of FLOWERING LOCUS T (FT) transcript, starting to rise 4 h after dawn in the mutant. In addition, HOS1 interacts synergistically with CONSTITUTIVE PHOTOMORPHOGENIC1, another regulator of CO protein stability, in the regulation of flowering time. Taken together, these results indicate that HOS1 is involved in the control of CO abundance, ensuring that CO activation of FT occurs only when the light period reaches a certain length and preventing precocious flowering in Arabidopsis.
.
DOI:10.1101/gad.1518407URLPMID:17322399 [本文引用: 1]

Abstract Plants must perceive and rapidly respond to changes in ambient temperature for their successful reproduction. Here we demonstrate that Arabidopsis SHORT VEGETATIVE PHASE (SVP) plays an important role in the response of plants to ambient temperature changes. The loss of SVP function elicited insensitivity to ambient temperature changes. SVP mediates the temperature-dependent functions of FCA and FVE within the thermosensory pathway. SVP controls flowering time by negatively regulating the expression of a floral integrator, FLOWERING LOCUS T (FT), via direct binding to the CArG motifs in the FT sequence. We propose that this is one of the molecular mechanisms that modulate flowering time under fluctuating temperature conditions.
DOI:10.1105/tpc.113.120857URLPMID:24481072 [本文引用: 1]

Phytochrome-interacting factors (PIFs) are members of the Arabidopsis thaliana basic helix-loop-helix family of transcriptional regulators that interact specifically with the active Pfr conformer of phytochrome (phy) photoreceptors. PIFs are central regulators of photomorphogenic development that act to promote stem growth, and this activity is reversed upon interaction with phy in response to light. Recently, significant progress has been made in defining the transcriptional networks directly regulated by PIFs, as well as the convergence of other signaling pathways on the PIFs to modulate growth. Here, we summarize and highlight these findings in the context of PIFs acting as integrators of light and other signals. We discuss progress in our understanding of the transcriptional and posttranslational regulation of PIFs that illustrates the integration of light with hormonal pathways and the circadian clock, and we review seedling hypocotyl growth as a paradigm of PIFs acting at the interface of these signals. Based on these advances, PIFs are emerging as required factors for growth, acting as central components of a regulatory node that integrates multiple internal and external signals to optimize plant development.
URL [本文引用: 1]
.
[本文引用: 1]
.
[本文引用: 1]
DOI:10.1016/j.devcel.2017.12.028URLPMID:29398622 [本文引用: 1]

UV-B light (UV-B radiation) is known to inhibit plant growth, but the mechanism is not well understood. UVR8 (UV RESISTANCE LOCUS 8) is a UV-B light photoreceptor that mediates UV-B light responses in plants. We report here that UV-B inhibits plant growth by repressing plant steroid hormone brassinosteroid (BR)-promoted plant growth. UVR8 physically interacts with the functional dephosphorylated BES1 (BRI1-EMS-SUPPRESSOR1) and BIM1 (BES1-INTERACTING MYC-LIKE 1) transcription factors that mediate BR-regulated gene expression and plant growth to inhibit their activities. Genome-wide gene expression analysis defined a BES1-dependent UV-B-regulated transcriptome, which is enriched with genes involved in cell elongation and plant growth. We further showed that UV-B-activated and nucleus-localized UVR8 inhibited the DNA-bindingactivities of BES1/BIM1 to directly regulate transcription of growth-related genes. Our results therefore establish that UVR8-BES1/BIM1 interaction represents an early photoreceptor signaling mechanism in plants and serves as an important module integrating light and BR signaling.
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/j.1365-313X.2010.04148.xURLPMID:20409274 [本文引用: 1]

The coordination of the timing of flowering with seasonal and development cues is a critical life-history trait that has been shaped by evolution to maximize reproductive success. Decades of studying many plant species have revealed several of the fascinating systems that plants have evolved to control flowering time: such as the perception of day length in leaves, which leads to the production of a mobile signal, florigen, that promotes flowering at the shoot apical meristem; the vernalization process in which exposure to prolonged cold results in meristem competence to flower; and the juvenile to adult phase transition. Arabidopsis research has contributed greatly to understanding these systems at a molecular level.
[本文引用: 1]
DOI:10.1126/science.1163927URLPMID:1898880912 [本文引用: 1]

Cryptochromes (CRY) are photolyase-like blue-light receptors that mediate light responses in plants and animals. How plant cryptochromes act in response to blue light is not well understood. We report here the identification and characterization of the Arabidopsis CIB1 (cryptochrome-interacting basic-helix-loop-helix) protein. CIB1 interacts with CRY2 (cryptochrome 2) in a blue light-specific manner in yeast and Arabidopsis cells, and it acts together with additional CIB1-related proteins to promote CRY2-dependent floral initiation. CIB1 binds to G box (CACGTG) in vitro with a higher affinity than its interaction with other E-box elements (CANNTG). However, CIB1 stimulates FT messenger RNA expression, and it interacts with chromatin DNA of the FT gene that possesses various E-box elements except G box. We propose that the blue light-dependent interaction of cryptochrome(s) with CIB1 and CIB1-related proteins represents an early photoreceptor signaling mechanism in plants.
DOI:10.1016/S0264-410X(02)00484-XURLPMID:18257712 [本文引用: 1]

Phytochromes are red/far-red light photoreceptors that convert theinformation contained in external light into biological signals. Thedecoding process starts with the perception of red light, which occursthrough photoisomerization of a chromophore located within thephytochrome, leading to structural changes that include the disrup-tion of intramolecular interactions between the N- and C-terminaldomains of the phytochrome. This disruption exposes surfaces re-quired for interactions with other proteins. In contrast, the percep-tion of far-red light reverses the photoisomerization, restores theintramolecular interaction, and closes the interacting surfaces. Lightinformation represented by the concentration of opened interactingsurfaces is converted into biological signals through the modulat-ing activity of interacting proteins. This review summarizes plantphytochromes, phytochrome-interacting proteins, and signal trans-mission from phytochromes to their interacting proteins.
.
[本文引用: 2]
.
[本文引用: 1]
DOI:10.1038/nature01837URLPMID:12891362 [本文引用: 1]

A plant modulates its developmental processes in response to light by several informational photoreceptors such as phytochrome. Phytochrome is a dimeric chromoprotein which regulates various aspects of plant development from seed germination to flowering. Upon absorption of red light, phytochrome translocates from the cytoplasm to the nucleus, and regulates gene expression through interaction with transcription factors such as PIF3 (refs 5-7). The phytochrome polypeptide has two domains: the amino-terminal photosensory domain with a chromophore and the carboxy-terminal domain which contains signalling motifs such as a kinase domain. The latter is widely believed to transduce the signal to downstream components. Here we show that the C-terminal domain of Arabidopsis phytochrome B (phyB), which is known as the most important member of the phytochrome family, is not directly involved in signal transduction. The N-terminal domain isolated from phyB, when dimerized and localized in the nucleus, triggered full phyB responses with much higher photosensitivity than the full-length phyB. These findings indicate that the C-terminal domain attenuates the activity of phyB rather than positively transducing the signal.
[本文引用: 1]
DOI:10.1016/j.molp.2015.03.003URLPMID:25772379 [本文引用: 1]

Circadian clocks are endogenous timers that enable plants to synchronize central processes, such as growth, development, and reproduction, with daily and seasonal environmental conditions. This review examines the roles that alleles of three groups of circadian clock genes have played in domestication and improvement of crop plants. Emerging insights into circadian clock regulation of other fundamental plant processes are also discussed to highlight promising avenues for further crop improvement.
.
DOI:10.1105/tpc.105.033464URLPMID:16006578 [本文引用: 1]

The circadian clock acts as the timekeeping mechanism in photoperiodism. In Arabidopsis thaliana, a circadian clock-controlled flowering pathway comprising the genes GIGANTEA (GI), CONSTANS (CO), and FLOWERING LOCUS T (FT) promotes flowering specifically under long days. Within this pathway, GI regulates circadian rhythms and flowering and acts earlier in the hierarchy than CO and FT, suggesting that GI might regulate flowering indirectly by affecting the control of circadian rhythms. We studied the relationship between the roles of GI in flowering and the circadian clock using late elongated hypocotyl circadian clock associated1 double mutants, which are impaired in circadian clock function, plants overexpressing GI (35S:GI), and gi mutants. These experiments demonstrated that GI acts between the circadian oscillator and CO to promote flowering by increasing CO and FT mRNA abundance. In addition, circadian rhythms in expression of genes that do not control flowering are altered in 35S:GI and gi mutant plants under continuous light and continuous darkness, and the phase of expression of these genes is changed under diurnal cycles. Therefore, GI plays a general role in controlling circadian rhythms, and this is different from its effect on the amplitude of expression of CO and FT. Functional GI:green fluorescent protein is localized to the nucleus in transgenic Arabidopsis plants, supporting the idea that GI regulates flowering in the nucleus. We propose that the effect of GI on flowering is not an indirect effect of its role in circadian clock regulation, but rather that GI also acts in the nucleus to more directly promote the expression of flowering-time genes.
.
[本文引用: 1]
.
[本文引用: 1]
DOI:10.1016/j.copbio.2014.11.023URLPMID:25553537 [本文引用: 1]

The change from vegetative to reproductive growth is a key developmental switch in flowering plants. In agriculture, flowering is a prerequisite for crop production whenever seeds or fruits are harvested. An intricate network with various (epi-) genetic regulators responding to environmental and endogenous triggers controls the timely onset of flowering. Changes in the expression of a single flowering time (FTi) regulator can suffice to drastically alter FTi. FTi regulation is of utmost importance for genetic improvement of crops. We summarize recent discoveries on FTi regulators in crop species emphasizing crop-specific genes lacking homologs in Arabidopsis thaliana. We highlight pleiotropic effects on agronomically important characters, impact on adaptation to new geographical/climate conditions and future perspectives for crop improvement.
.
[本文引用: 1]
DOI:10.1016/S0092-8674(00)80842-9URLPMID:10847687 [本文引用: 1]

Plant reproduction requires precise control of flowering in response to environmental cues. We isolated a late-flowering Arabidopsis mutant, fkf1, that is rescued by vernalization or gibberellin treatment. We positionally cloned FKF1, which encodes a novel protein with a PAS domain similar to the flavin-binding region of certain photoreceptors, an F box characteristic of proteins that direct ubiquitin-mediated degradation, and six kelch repeats predicted to fold into a propeller. FKF1 mRNA levels oscillate with a circadian rhythm, and deletion of FKF1 alters the waveform of rhythmic expression of two clock-controlled genes, implicating FKF1 in modulating the Arabidopsis circadian clock.
DOI:10.1016/S1360-1385(02)02245-8URLPMID:11992825 [本文引用: 2]

Novel family of blue-light receptors, exclusive to plants-photoreceptors that mediate phototropism, chloroplast movement, stomatal opening and probably other plant blue-light responses.
DOI:10.1016/S1097-2765(03)00008-XURLPMID:12535521 [本文引用: 1]

Cryptochrome flavoproteins, which share sequence homology with light-dependent DNA repair photolyases, function as photoreceptors in plants and circadian clock components in animals. Here, we coupled sequencing of an Arabidopsis cryptochrome gene with phylogenetic, structural, and functional analyses to identify a new cryptochrome class (cryptochrome DASH) in bacteria and plants, suggesting that cryptochromes evolved before the divergence of eukaryotes and prokaryotes. The cryptochrome crystallographic structure, reported here for Synechocystis cryptochrome DASH, reveals commonalities with photolyases in DNA binding and redox-dependent function, despite distinct active-site and interaction surface features. Whole genome transcriptional profiling together with experimental confirmation of DNA binding indicated that Synechocystis cryptochrome DASH functions as a transcriptional repressor.
DOI:10.1038/ncomms1810URLPMID:22549837 [本文引用: 2]

In Arabidopsis, FLOWERING LOCUS T (FT) promotes flowering in response to long days in the photoperiod pathway, while signalling downstream gibberellin (GA) perception is critical for flowering under short days. Previously we have established that the TEMPRANILLO (TEM) genes have a pivotal role in the direct repression of FT. Here we show that TEM genes directly regulate the expression of the GA(4) biosynthetic genes GA 3-oxidase1 and 2 (GA3OX1 and GA3OX2). Plants overexpressing TEM genes resemble GA-deficient mutants, and conversely, TEM downregulation give rise to elongated hypocotyls perhaps as a result of an increase in GA content. We consistently find that TEM1 represses GA3OX1 and GA3OX2 by directly binding a regulatory region positioned in the first exon. Our results indicate that TEM genes seem to link the photoperiod and GA-dependent flowering pathways, controlling floral transition under inductive and non-inductive day lengths through the regulation of the floral integrators.
DOI:10.1038/nature12633URLPMID:24067612 [本文引用: 1]

The appropriate timing of flowering is crucial for plant reproductive success. It is therefore not surprising that intricate genetic networks have evolved to perceive and integrate both endogenous and environmental signals, such as carbohydrate and hormonal status, photoperiod and temperature(1,2). In contrast to our detailed understanding of the vernalization pathway, little is known about how flowering time is controlled in response to changes in the ambient growth temperature. In Arabidopsis thaliana, the MADS-box transcription factor genes FLOWERING LOCUS M (FLM) and SHORT VEGETATIVE PHASE (SVP) have key roles in this process(3,4). FLM is subject to temperature-dependent alternative splicing(3). Here we report that the two main FLM protein splice variants, FLM-beta and FLM-delta, compete for interaction with the floral repressor SVP. The SVP-FLM-beta complex is predominately formed at low temperatures and prevents precocious flowering. By contrast, the competing SVP-FLM-delta complex is impaired in DNA binding and acts as a dominant-negative activator of flowering at higher temperatures. Our results show a new mechanism that controls the timing of the floral transition in response to changes in ambient temperature. A better understanding of how temperature controls the molecular mechanisms of flowering will be important to cope with current changes in global climate(5,6).
DOI:10.1104/pp.107.102079URLPMID:17631525 [本文引用: 1]

Flowering at the appropriate time of year is essential for successful reproduction in plants. We found that HAP3b in Arabidopsis (Arabidopsis thaliana), a putative CCAAT-binding transcription factor gene, is involved in controlling flowering time. Over-expression of HAP3b promotes early flowering while hap3b, a null mutant of HAP3b, is delayed in flowering under a long-day photoperiod. Under short-day conditions, however, hap3b did not show a delayed flowering compared to wild type based on the leaf number, suggesting that HAP3b may normally be involved in the photoperiod-regulated flowering pathway Mutant hap3b plants showed earlier flowering upon gibberellic acid or vernalization treatment, which means that HAP3b is not involved in flowering promoted by gibberellin or vernalization. Further transcript profiling and gene expression analysis suggests that HAP3b can promote flowering by enhancing expression of key flowering time genes such as FLOWERING LOCUS T and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1. Our results provide strong evidence supporting a role of HAP3b in regulating flowering in plants grown under long-day conditions.
.
[本文引用: 1]
DOI:10.1016/j.molp.2016.10.005URLPMID:27756574 [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.cell.2003.08.004URLPMID:13678578 [本文引用: 1]

Cryptochromes are flavin-containing blue light photoreceptors related to photolyases—they are found in both plants and animals and have recently been described for bacteria. In plants, cryptochromes perform a variety of functions including the entrainment of circadian rhythms. They serve a similar role in Drosophila and mammals, where the cryptochromes also perform an additional function as an essential component of the circadian clock.
[本文引用: 1]
[本文引用: 1]
DOI:10.1126/science.1200660URLPMID:21454788 [本文引用: 3]

To optimize their growth and survival, plants perceive and respond to ultraviolet-B (UV-B) radiation. However, neither the molecular identity of the UV-B photoreceptor nor the photoperception mechanism is known. Here we show that dimers of the UVR8 protein perceive UV-B, probably by a tryptophan-based mechanism. Absorption of UV-B induces instant monomerization of the photoreceptor and interaction with COP1, the central regulator of light signaling. Thereby this signaling cascade controlled by UVR8 mediates UV-B photomorphogenic responses securing plant acclimation and thus promotes survival in sunlight.
.
DOI:10.1016/j.cub.2008.07.075URLPMID:18718758 [本文引用: 1]

Seasonal changes in day length influence flowering time in many plant species. In Arabidopsis, flowering is accelerated by exposure to long day (LD). Those inductive photoperiods are perceived in leaves [1] and initiate a long-distance signaling mediated by CO and FT. CO is expressed in the phloem according to a circadian rhythm [2-4]. Only under LD does CO induce FT expression as high levels of CO in the evening coincide with the external light that stabilizes CO protein [4, 5]. Subsequently, FT protein travels through the phloem to the shoot apex where, together with FD, it initiates flowering [6-12]. Despite the photoperiodic induction, a mechanism of floral repression is needed to avoid precocious flowering. We show that TEMPRANILLO genes ( TEM1 and TEM2) act as novel direct FT repressors. Molecular and genetic analyses suggest that a quantitative balance between the activator CO and the repressor TEM determines FT levels. Moreover, developmental TEM downregulation marks the timing of flowering, as it shifts the CO/TEM balance in favor of CO activity, allowing FT transcript to reach the threshold level required to trigger flowering. We envision that this might be a general mechanism between long-day plants to ensure a tight regulation of flowering time.
[本文引用: 1]
DOI:10.1146/annurev.arplant.56.032604.144208URLPMID:16669784 [本文引用: 1]

Abstract Phytochromes are a widespread family of red/far-red responsive photoreceptors first discovered in plants, where they constitute one of the three main classes of photomorphogenesis regulators. All phytochromes utilize covalently attached bilin chromophores that enable photoconversion between red-absorbing (P(r)) and far-red-absorbing (P(fr)) forms. Phytochromes are thus photoswitchable photosensors; canonical phytochromes have a conserved N-terminal photosensory core and a C-terminal regulatory region, which typically includes a histidine-kinase-related domain. The discovery of new bacterial and cyanobacterial members of the phytochrome family within the last decade has greatly aided biochemical and structural characterization of this family, with the first crystal structure of a bacteriophytochrome photosensory core appearing in 2005. This structure and other recent biochemical studies have provided exciting new insights into the structure of phytochrome, the photoconversion process that is central to light sensing, and the mechanism of signal transfer by this important family of photoreceptors.
[本文引用: 1]
DOI:10.1126/science.288.5471.1613URLPMID:10834834 [本文引用: 1]

In plants, flowering is triggered by endogenous and environmental signals. CONSTANS (CO) promotes flowering of Arabidopsis in response to day length. Four early target genes of CO were identified using a steroid-inducible version of the protein. Two of these genes, SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1) and FLOWERING LOCUS T (FT), are required for CO to promote flowering; the others are involved in proline or ethylene biosynthesis. The SOC1 and FT genes are also regulated by a second flowering-time pathway that acts independently of CO. Thus, early target genes of CO define common components of distinct flowering-time pathways.
DOI:10.1016/j.pbi.2005.07.011URLPMID:16054430 [本文引用: 1]

Since plants are sessile they must be able to sense and rapidly respond to changes in ambient temperature. Key aspects of plant development, including the transition to flowering and the circadian clock, have important inputs from ambient temperature. In the model system Arabidopsis thaliana, molecular candidates for mediating these roles have recently been uncovered, which will be critical for obtaining an understanding of the mechanisms involved.
DOI:10.1146/annurev-arplant-042110-103759URLPMID:21526969 [本文引用: 3]

Abstract Cryptochromes are flavoprotein photoreceptors first identified in Arabidopsis thaliana, where they play key roles in growth and development. Subsequently identified in prokaryotes, archaea, and many eukaryotes, cryptochromes function in the animal circadian clock and are proposed as magnetoreceptors in migratory birds. Cryptochromes are closely structurally related to photolyases, evolutionarily ancient flavoproteins that catalyze light-dependent DNA repair. Here, we review the structural, photochemical, and molecular properties of cry-DASH, plant, and animal cryptochromes in relation to biological signaling mechanisms and uncover common features that may contribute to better understanding the function of cryptochromes in diverse systems including in man.
DOI:10.1016/j.tcb.2011.07.002URLPMID:21852137 [本文引用: 1]

http://linkinghub.elsevier.com/retrieve/pii/S096289241100136X
.
[本文引用: 1]
[本文引用: 2]
.
DOI:10.1126/science.1088060URLPMID:5127708 [本文引用: 1]

Plant microRNAs (miRNAs) show a high degree of sequence complementarity to, and are believed to guide the cleavage of, their target messenger RNAs. Here, I show that miRNA172, which can base-pair with the messenger RNA of a floral homeotic gene, APETALA2, regulates APETALA2 expression primarily through translational inhibition. Elevated miRNA172 accumulation results in floral organ identity defects similar to those in loss-of-function apetala2 mutants. Elevated levels of mutant APETALA2 RNA with disrupted miRNA172 base pairing, but not wild-type APETALA2 RNA, result in elevated levels of APETALA2 protein and severe floral patterning defects. Therefore, miRNA172 likely acts in cell-fate specification as a translational repressor of APETALA2 in Arabidopsis flower development.
[本文引用: 2]
DOI:10.1093/embo-reports/kvf222URL

Light provides a major source of information from the environment during plant growth and development. Light perception is mediated through the action of several photoreceptors, including the phytochromes. Recent results demonstrate that light responses involve the regulation of several thousand genes. Some of the key events controlling this gene expression are the translocation of the phytochrome photoreceptors into the nucleus followed by their binding to transcription factors. Coupled with these events, the degradation of positively acting intermediates appears to be an important process whereby photomorphogenesis is repressed in darkness. This review summarizes our current knowledge of these processes.
[本文引用: 3]
DOI:10.1126/science.1218091URLPMID:3505452 [本文引用: 1]

Abstract The recently identified plant photoreceptor UVR8 (UV RESISTANCE LOCUS 8) triggers regulatory changes in gene expression in response to ultraviolet-B (UV-B) light through an unknown mechanism. Here, crystallographic and solution structures of the UVR8 homodimer, together with mutagenesis and far-UV circular dichroism spectroscopy, reveal its mechanisms for UV-B perception and signal transduction. propeller subunits form a remarkable, tryptophan-dominated, dimer interface stitched together by a complex salt-bridge network. Salt-bridging arginines flank the excitonically coupled cross-dimer tryptophan "pyramid" responsible for UV-B sensing. Photoreception reversibly disrupts salt bridges, triggering dimer dissociation and signal initiation. Mutation of a single tryptophan to phenylalanine retunes the photoreceptor to detect UV-C wavelengths. Our analyses establish how UVR8 functions as a photoreceptor without a prosthetic chromophore to promote plant development and survival in sunlight.
.
DOI:10.3389/fpls.2014.00221URLPMID:24904616 [本文引用: 1]

Agriculturally important grasses such as rice, maize and sugarcane are evolutionarily distant from Arabidopsis, yet some components of the floral induction process are highly conserved. Flowering in sugarcane is an important factor that negatively affects cane yield and reduces sugar/ethanol production from this important perennial bioenergy crop. Comparative studies have facilitated the identification and characterization of putative orthologs of key flowering time genes in sugarcane, a complex polyploid plant whose genome has yet to be sequenced completely. Using this approach we identified phosphatidylethanolamine-binding protein (PEBP) gene family members in sugarcane that are similar to the archetypical FT and TFL1 genes of Arabidopsis that play an essential role in controlling the transition from vegetative to reproductive growth. Expression analysis of ScTFL1, which falls into the TFL1-clade of floral repressors, showed transcripts in developing leaves surrounding the shoot apex but not at the apex itself. ScFT1 was detected in immature leaves and apical regions of vegetatively growing plants and, after the floral transition, expression also occurred in mature leaves. Ectopic over-expression of ScTFL1 in Arabidopsis caused delayed flowering in Arabidopsis, as might be expected for a gene related to TFL1. In addition, lines with the latest flowering phenotype exhibited aerial rosette formation. Unexpectedly, over-expression of ScFT1, which has greatest similarity to the florigen-encoding FT, also caused a delay in flowering. This preliminary analysis of divergent sugarcane FT and TFL1 gene family members from Saccharum spp. suggests that their expression patterns and roles in the floral transition has diverged from the predicted role of similar PEBP family members.
DOI:10.1105/tpc.114.134775URLPMID:25627066 [本文引用: 1]

Phytochromes function as red/far-red photoreceptors in plants and are essential for light-regulated growth and development. Photomorphogenesis, the developmental program in light, is the default program in seed plants. In dark-grown seedlings, photomorphogenic growth is suppressed by the action of the CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1)/SUPPRESSOR OF phyA-105 (SPA) complex, which targets positive regulators of photomorphogenic growth for degradation by the proteasome. Phytochromes inhibit the COP1/SPA complex, leading to the accumulation of transcription factors promoting photomorphogenesis; yet, the mechanism by which they inactivate COP1/SPA is still unknown. Here, we show that light-activated phytochrome A (phyA) and phytochrome B (phyB) interact with SPA1 and other SPA proteins. Fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy analyses show that SPAs and phytochromes colocalize and interact in nuclear bodies. Furthermore, light-activated phyA and phyB disrupt the interaction between COP1 and SPAs, resulting in reorganization of the COP1/SPA complex in planta. The light-induced stabilization of HFR1, a photomorphogenic factor targeted for degradation by COP1/SPA, correlates temporally with the accumulation of phyA in the nucleus and localization of phyA to nuclear bodies. Overall, these data provide a molecular mechanism for the inactivation of the COP1/SPA complex by phyA- and phyB-mediated light perception.
[本文引用: 1]
DOI:10.1016/S0092-8674(00)80841-7URLPMID:10847686 [本文引用: 2]

We have conducted genetic screens for period length mutants in Arabidopsis using a transgenic bioluminescence phenotype. This screen identified mutations at a locus, ZEITLUPE ( ZTL), that lengthen the free-running period of clock-controlled gene transcription and cell expansion, and alter the timing of the daylength-dependent transition from vegetative to floral development. Map-based cloning of ZTL identified a novel 609 amino acid polypeptide consisting of an amino-terminal PAS domain, an F box and six carboxy-terminal kelch repeats. The PAS region is highly similar to the PAS domain of the Arabidopsis blue-light receptor NPH1, and the Neurospora circadian-associated protein WHITE COLLAR-1 (WC-1). The striking fluence rate-dependent effect of the ztl mutations suggests that ZTL plays a primary role in the photocontrol of circadian period in higher plants.
DOI:10.1126/science.1219644URLPMID:22628657 [本文引用: 4]

Abstract Plants use day-length information to coordinate flowering time with the appropriate season to maximize reproduction. In Arabidopsis, the long day-specific expression of CONSTANS (CO) protein is crucial for flowering induction. Although light signaling regulates CO protein stability, the mechanism by which CO is stabilized in the long-day afternoon has remained elusive. Here, we demonstrate that FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1) protein stabilizes CO protein in the afternoon in long days. FKF1 interacts with CO through its LOV domain, and blue light enhances this interaction. In addition, FKF1 simultaneously removes CYCLING DOF FACTOR 1 (CDF1), which represses CO and FLOWERING LOCUS T (FT) transcription. Together with CO transcriptional regulation, FKF1 protein controls robust FT mRNA induction through multiple feedforward mechanisms that accurately control flowering timing.
DOI:10.1126/science.1141752URLPMID:17446353 [本文引用: 1]

In plants, seasonal changes in day length are perceived in leaves, which initiate long-distance signaling that induces flowering at the shoot apex. The identity of the long-distance signal has yet to be determined. In Arabidopsis, activation of FLOWERING LOCUS T (FT) transcription in leaf vascular tissue (phloem) induces flowering. We found that FT messenger RNA is required only transiently in the leaf. In addition, FT fusion proteins expressed specifically in phloem cells move to the apex and move long distances between grafted plants. Finally, we provide evidence that FT does not activate an intermediate messenger in leaves. We conclude that FT protein acts as a long-distance signal that induces Arabidopsis flowering.
[本文引用: 2]
.
[本文引用: 1]
DOI:10.3389/fpls.2014.00077URLPMID:3945484 [本文引用: 1]

Appropriate timing of flowering is crucial for crop yield and the reproductive success of plants. Flowering can be induced by a number of molecular pathways that respond to internal and external signals such as photoperiod, vernalization or light quality, ambient temperature and biotic as well as abiotic stresses. The key florigenic signal FLOWERING LOCUS T (FT) is regulated by several flowering activators, such as CONSTANS (CO), and repressors, such as FLOWERING LOCUS C (FLC). Chromatin modifications are essential for regulated gene expression, which often involves the well conserved MULTICOPY SUPRESSOR OF IRA 1 (MSI1)-like protein family. MSI1-like proteins are ubiquitous partners of various complexes, such as POLYCOMB REPRESSIVE COMPLEX2 or CHROMATIN ASSEMBLY FACTOR 1. In Arabidopsis, one of the functions of MSI1 is to control the switch to flowering. Arabidopsis MSI1 is needed for the correct expression of the floral integrator gene SUPPRESSOR OF CO 1 (SOC1). Here, we show that the histone-binding protein MSI1 acts in the photoperiod pathway to regulate normal expression of CO in long day (LD) photoperiods. Reduced expression of CO in msi1-mutants leads to failure of FT and SOC1 activation and to delayed flowering. MSI1 is needed for normal sensitivity of Arabidopsis to photoperiod, because msi1-mutants responded less than wild type to an intermittent LD treatment of plants grown in short days. Finally, genetic analysis demonstrated that MSI1 acts upstream of the CO-FT pathway to enable an efficient photoperiodic response and to induce flowering.
DOI:10.1111/nph.12725URLPMID:24571056 [本文引用: 1]

'Summary'1126I.1126II.1127III.1128IV.1128V.1129VI.1132VII.1135VIII.1136IX.1136 1137 References1137SummaryLight and temperature, in coordination with the endogenous clock and the hormones gibberellin (GA) and brassinosteroids (BRs), modulate plant growth and development by affecting the expression of multiple cell wall- and auxin-related genes. PHYTOCHROME INTERACTING FACTORS (PIFs) play a central role in the activation of these genes, the activity of these factors being regulated by the circadian clock and phytochrome-mediated protein destabilization. GA signaling is also integrated at the level of PIFs; the DELLA repressors are found to bind these factors and impair their DNA-binding ability. The recent finding that PIFs are co-activated by BES1 and BZR1 highlights a further role of these regulators in BR signal integration, and reveals that PIFs act in a concerted manner with the BR-related BES1/BZR1 factors to activate auxin synthesis and transport at the gene expression level, and synergistically activate several genes with a role in cell expansion. Auxins feed back into this growth regulatory module by inducing GA biosynthesis and BES1/BZR1 gene expression, in addition to directly regulating several of these growth pathway gene targets. An exciting challenge in the future will be to understand how this growth program is dynamically regulated in time and space to orchestrate differential organ expansion and to provide plants with adaptation flexibility.
DOI:10.2307/3871244URL [本文引用: 1]

The circadian clock is entrained to the daily cycle of day and night by light signals at dawn and dusk. Plants make use of both the phytochrome (phy) and cryptochrome (cry) families of photoreceptors in gathering information about the light environment for setting the clock. We demonstrate that the phytochromes phyA, phyB, phyD, and phyE act as photoreceptors in red light input to the clock and that phyA and the cryptochromes cry1 and cry2 act as photoreceptors in blue light input. phyA and phyB act additively in red light input to the clock, whereas cry1 and cry2 act redundantly in blue light input. In addition to the action of cry1 as a photoreceptor that modiates blue light input into the clock, we demonstrate a requirement of cry1 for phyA signaling to the clock in both red and blue light. Importantly, Arabidopsis cry1 cry2 double mutants still show robust rhythmicity, indicating that cryptochromes do not form a part of the central circadian oscillator in plants as they do in mammals.
DOI:10.1111/j.1365-313X.2009.03811.xURLPMID:19187043 [本文引用: 2]

Summary Plants regulate their time to flowering by gathering information from the environment. Photoperiod and temperature are among the most important environmental variables. Sub-optimal, but not near-freezing, temperatures regulate flowering through the thermosensory pathway, which overlaps with the autonomous pathway. Here we show that ambient temperature regulates flowering by two genetically distinguishable pathways, one requiring TFL1 and another requiring ELF3. The delay in flowering time observed at lower temperatures was partially suppressed in single elf3 and tfl1 mutants, whereas double elf3 tfl1 mutants were insensitive to temperature. tfl1 mutations abolished the temperature response in cryptochrome mutants that are deficient in photoperiod perception, but not in phyB mutants, which have a constitutive photoperiodic response. In contrast to tfl1, elf3 mutations were able to suppress the temperature response in phyB mutants, but not in cryptochrome mutants. Gene expression profiles revealed that the tfl1 and elf3 effects are due to the activation of different sets of genes, and identified CCA1 and SOC1/AGL20 as being important cross-talk points. Finally, genome-wide gene expression analysis strongly suggests a general and complementary role for ELF3 and TFL1 in temperature signalling.
DOI:10.1093/pcp/pcs165URLPMID:23220691 [本文引用: 1]

Many organisms, including bacteria, fungi, animal, plants and algae, utilize blue light to adapt to a fluctuating light environment. Plants and algae, and photosynthetic stramenopiles in particular, require light energy for photosynthesis and have thus evolved a range of sophisticated light-sensing systems to utilize light information efficiently for growth, development and physiological responses. LOV (light, oxygen or voltage) domain photoreceptors are widely distributed among prokaryotic and eukaryotic organisms, and the number of specific LOV photoreceptors are increased in certain taxa. In this review, we summarize the molecular basis and physiological functions of three different families of LOV blue light receptors specific to green plants and photosynthetic stramenopiles: phototropin, ZEITLUPE/FLAVIN-BINDING, KELCH REPEAT, F-BOX 1/LOV KELCH PROTEIN 2 (ZTL/FKF1/LKP2) and aureochrome.
.
DOI:10.1111/pce.13166URLPMID:29447428 [本文引用: 2]

Abstract UV-B is a high-energy component of the solar radiation perceived by the plant and induces a number of modifications in plant growth and development, including changes in flowering time. However, the molecular mechanisms underlying these changes are largely unknown. In the present work, we demonstrate that Arabidopsis plants grown under white light supplemented with UV-B show a delay in flowering time, and this developmental reprogramming is mediated by the UVR8 photoreceptor. Using a combination of gene expression analyses and UV-B irradiation of different flowering mutants, we gained insight into the pathways involved in the observed flowering time delay in UV-B exposed Arabidopsis plants. We provide evidence that UV-B light downregulates the expression of MSI1 and CLF , two of the components of the Polycomb Repressive Complex 2 (PRC2), which in consequence drives a decrease in H3K27me3 histone methylation of MIR156 and FLC genes. Modification in the expression of several flowering time genes as a consequence of the decrease in the PRC2 complex activity was also determined. UV-B exposure of flowering mutants supports the involvement of this complex in the observed delay in flowering time, mostly through the age pathway.
DOI:10.1073/pnas.1310631110URL [本文引用: 1]

In flowering plants, light is one of the major environmental stimuli that determine the timing of the transition from the vegetative to reproductive phase. In Arabidopsis, phytochrome B (phyB); phyA; cryptochrome 2; and FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 are major photoreceptors that regulate flowering. Unlike phyA; cryptochrome 2; and FLAVIN-BINDING, KELCH REPEAT, F-BOX 1, phyB delays flowering mainly by destabilizing the CONSTANS (CO) protein, whose reduction leads to decreased expression of a florigen gene, FLOWERING LOCUS T. However, it remains unclear how the phyB-mediated CO destabilization is mechanistically regulated. Here, we identify a unique PHYTOCHROME-DEPENDENT LATEFLOWERING (PHL) gene, which is mainly involved in the phyBdependent regulation of flowering. Plants with mutant phl exhibited a late-flowering phenotype, especially under long-day conditions. The late-flowering phenotype of the phl mutant was completely overridden by a phyB mutation, indicating that PHL normally accelerates flowering by countering the inhibitory effect of phyB on flowering. Accordingly, PHL physically interacted with phyB both in vitro and in vivo in a red light-dependent manner. Furthermore, in the presence of phyB under red light, PHL interacted with CO as well. Taken together, we propose that PHL regulates photoperiodic flowering by forming a phyB-PHL-CO tripartite complex.
[本文引用: 2]
.
DOI:10.1016/j.devcel.2009.06.015URLPMID:19619493 [本文引用: 2]

Flowering of Arabidopsis is induced by long summer days (LDs). The transcriptional regulator CONSTANS (CO) promotes flowering, and its transcription is increased under LDs. We systematically misexpressed transcription factors in companion cells and identified several DOF proteins that delay flowering by repressing CO transcription. Combining mutations in four of these, including CYCLING DOF FACTOR 2 (CDF2), caused photoperiod-insensitive early flowering by increasing CO mRNA levels. CO transcription is promoted to differing extents by GIGANTEA (GI) and the F-box protein FKF1. We show that GI stabilizes FKF1, thereby reducing CDF2 abundance and allowing transcription of CO. Despite the crucial function of GI in wild-type plants, introducing mutations in the four DOF-encoding genes into gi mutants restored the diurnal rhythm and light inducibility of CO. Thus, antagonism between GI and DOF transcription factors contributes to photoperiodic flowering by modulating an underlying diurnal rhythm in CO transcript levels.
[本文引用: 1]
.
[本文引用: 1]
[本文引用: 1]
.
[本文引用: 2]
DOI:10.1073/pnas.0914532107URLPMID:21041653 [本文引用: 1]

Plants respond to low levels of UV-B radiation with a coordinated photomorphogenic response that allows acclimation to this environmental stress factor. The key players in this UV-B response are COP1 (an E3 ubiquitin ligase), UVR8 (a 尾-propeller protein), and HY5 (a bZIP transcription factor). We have shown previously that an elevated UV-B-specific response is associated with dwarf growth, indicating the importance of balancing UV-B-specific signaling. Negative regulators of this pathway are not known, however. Here, we describe two highly related WD40-repeat proteins, REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and RUP2, that interact directly with UVR8 as potent repressors of UV-B signaling. Both genes were transcriptionally activated by UV-B in a COP1-, UVR8-, and HY5-dependent manner. rup1 rup2 double mutants showed an enhanced response to UV-B and elevated UV-B tolerance after acclimation. Overexpression of RUP2 resulted in reduced UV-B-induced photomorphogenesis and impaired acclimation, leading to hypersensitivity to UV-B stress. These results are consistent with an important regulatory role for RUP1 and RUP2, which act downstream of UVR8-COP1 in a negative feedback loop impinging on UVR8 function, balancing UV-B defense measures and plant growth.
[本文引用: 2]
DOI:10.1038/nature24300URLPMID:29072296 [本文引用: 1]

Epigenetic modifications, including chromatin modifications and DNA methylation, have a central role in the regulation of gene expression in plants and animals. The transmission of epigenetic marks is crucial for certain genes to retain cell lineage-specific expression patterns and maintain cell fate. However, the marks that have accumulated at regulatory loci during growth and development or in response to environmental stimuli need to be deleted in gametes or embryos, particularly in organisms such as plants that do not set aside a germ line, to ensure the proper development of offspring. In Arabidopsis thaliana, prolonged exposure to cold temperatures (winter cold), in a process known as vernalization, triggers the mitotically stable epigenetic silencing of the potent floral repressor FLOWERING LOCUS C (FLC), and renders plants competent to flower in the spring; however, this silencing is reset during each generation. Here we show that the seed-specific transcription factor LEAFY COTYLEDON1 (LEC1) promotes the initial establishment of an active chromatin state at FLC and activates its expression de novo in the pro-embryo, thus reversing the silenced state inherited from gametes. This active chromatin state is passed on from the pro-embryo to post-embryonic life, and leads to transmission of the embryonic memory of FLC activation to post-embryonic stages. Our findings reveal a mechanism for the reprogramming of embryonic chromatin states in plants, and provide insights into the epigenetic memory of embryonic active gene expression in post-embryonic phases, through which an embryonic factor acts to 'control' post-embryonic development processes that are distinct from embryogenesis in plants.
DOI:10.1016/j.molp.2014.12.018URLPMID:25737467 [本文引用: 1]

This review discusses different types of flower-timing miRNAs and their target genes as key negative and positive timers in regulating plant vegetative and reproductive development. The inter- and intra-regulation of these miRNA targets, their feedback effects, and crosstalks with other miRNA pathways are thoroughly discussed. Different miRNA target modules are presented along with their interactions with environmental, biochemical, and genetic factors in coordinating flowering time.
DOI:10.1111/j.1365-313X.2006.02914.xURLPMID:17076805 [本文引用: 1]

<P>Contrary to expectations based on the visible phenotypic behavior of seedlings undergoing de-etiolation in response to continuous red light (Rc), previous gene expression profiling showed that one or more of the five-membered phytochrome (phy) family of Arabidopsis, other than phyB, is predominantly responsible for transducing the Rc signals to light-responsive genes. To begin to identify which phys are involved, and to define potential primary targets of phy signaling, we have examined the genome-wide expression profiles of genes responding to Rc within 1 h (early response genes) of initial exposure of dark-grown wild-type, phyA , phyB and phyAphyB double mutant seedlings to the light signal. The data show that phyA has a quantitatively dominant role in Rc-induced expression of these early response genes, that phyB has minimal detectable regulatory activity in the presence of phyA, but assumes a quantitatively larger role in its absence, and that phyA and phyB combined are responsible for the full extent of Rc responsiveness of 96% of these genes. No evidence was obtained of a significant role for the remaining family members, phyC, phyD or phyE, in this process. In striking contrast, Rc-imposed repression of early response gene expression remains quantitatively strong in the phyAphyB double mutant, as well as the monogenic mutants, suggesting a significant role for one or more of the other three phys in this response. Examination of the established or predicted functional roles of the early response genes indicates that genes encoding transcription factors represent the largest single category, at a frequency three times their prevalence genome-wide. This dominance is particularly striking among those genes responding most robustly to the Rc signal, where >50% are classified as involved in transcriptional regulation, suggesting that these may have potentially primary regulatory roles at the interface between phy signaling and the light-responsive transcriptional network. Integration of the present data with those of a previous genome-scale transcriptional analysis of a pif3 mutant, suggests a complex network involving perception and transduction of inductive Rc signals by both phyA and phyB through both PIF3 and other undefined signaling partners to early response genes.</P>
.
[本文引用: 1]
[本文引用: 1]
.
DOI:10.1105/tpc.114.130252URLPMID:25228341 [本文引用: 2]

Abstract The timely transition of vegetative to reproductive development is coordinated through quantitative regulation of floral pathway genes in response to physiological and environmental cues. Here, we show that the circadian-controlled expression of the Arabidopsis thaliana floral transition regulators FLOWERING LOCUS T (FT) and CONSTANS (CO) is antiphasic to that of BBX19, a transcription factor with two B-Box motifs. Diminished expression of BBX19 by RNA interference accelerates flowering, and constitutive expression of BBX19 delays flowering under inductive photoperiods. This delay is not accompanied by the alteration of CO expression levels but rather by a reduction of transcript levels of FT and the FT-regulated genes SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1, LEAFY, and FRUITFUL. Similar to CO, BBX19 is expressed in vasculature. BBX19 and CO colocalize in the nucleus and interact physically in vivo. In transient assays, coinfiltration of 10-fold more CO overcomes the BBX19-mediated repression of FT activation. Substitution of the conserved Cys-25 to Ser in the BBX19 Box1 motif abolishes the BBX19-CO interaction and eliminates the negative regulation of flowering time, while the analogous C76S substitution in the Box2 motif is ineffective. Together, these results implicate BBX19 as a circadian clock output that depletes the active CO pool to accurately monitor daylength and precisely time FT expression. 2014 American Society of Plant Biologists. All rights reserved.
.
DOI:10.1093/jxb/eru246URLPMID:24958896

Precise flowering time is critical to reproductive success. In response to diverse exogenous and endogenous cues including age, hormones, photoperiod, and temperature, the floral transition is controlled by a complex regulatory network, which involves extensive crosstalks, feedback, or feedforward loops between the components within flowering time pathways. The newly identified age pathway, which is controlled by microRNA156 (miR156) and its target SQUAMOSA PROMOTER BINDING-LIKE (SPL) transcription factors, ensures plants flower under non-inductive conditions. In this review, I summarize the recent advance in understanding of the age pathway, focusing on the regulatory basis of the developmental decline in miR156 level by age and the molecular mechanism by which the age pathway is integrated into other flowering time pathways.
DOI:10.1126/science.aaf9030URLPMID:27846570 [本文引用: 1]

Abstract Cryptochromes are blue-light receptors that regulate development and the circadian clock in plants and animals. We found that Arabidopsis cryptochrome 2 (CRY2) undergoes blue light-dependent homodimerization to become physiologically active. We identified BIC1 (blue-light inhibitor of cryptochromes 1) as an inhibitor of plant cryptochromes that binds to CRY2 to suppress the blue light-dependent dimerization, photobody formation, phosphorylation, degradation, and physiological activities of CRY2. We hypothesize that regulated dimerization governs homeostasis of the active cryptochromes in plants and other evolutionary lineages. Copyright 2016, American Association for the Advancement of Science.
DOI:10.1038/nature10931URLPMID:22388820 [本文引用: 1]

The Arabidopsis thaliana protein UVR8 is a photoreceptor for ultraviolet-B. Upon ultraviolet-B irradiation, UVR8 undergoes an immediate switch from homodimer to monomer, which triggers a signalling pathway for ultraviolet protection. The mechanism by which UVR8 senses ultraviolet-B remains largely unknown. Here we report the crystal structure of UVR8 at 1.86503 resolution, revealing a symmetric homodimer of seven-bladed β-propeller that is devoid of any external cofactor as the chromophore. Arginine residues that stabilize the homodimeric interface, principally Arg65286 and Arg65338, make elaborate intramolecular cation-π interactions with surrounding tryptophan amino acids. Two of these tryptophans, Trp65285 and Trp65233, collectively serve as the ultraviolet-B chromophore. Our structural and biochemical analyses identify the molecular mechanism for UVR8-mediated ultraviolet-B perception, in which ultraviolet-B radiation results in destabilization of the intramolecular cation-π interactions, causing disruption of the critical intermolecular hydrogen bonds mediated by Arg65286 and Arg65338 and subsequent dissociation of the UVR8 homodimer.
DOI:10.1016/j.cell.2009.06.031URLPMID:19703400 [本文引用: 3]

The transition from the juvenile to the adult phase of shoot development in plants is accompanied by changes in vegetative morphology and an increase in reproductive potential. Here, we describe the regulatory mechanism of this transition. We show that miR156 is necessary and sufficient for the expression of the juvenile phase, and regulates the timing of the juvenile-to-adult transition by coordinating the expression of several pathways that control different aspects of this process. miR156 acts by repressing the expression of functionally distinct SPL transcription factors. miR172 acts downstream of miR156 to promote adult epidermal identity. miR156 regulates the expression of miR172 via SPL9 which, redundantly with SPL10, directly promotes the transcription of miR172b. Thus, like the larval-to-adult transition in Caenorhabditis elegans, the juvenile-to-adult transition in Arabidopsis is mediated by sequentially operating miRNAs. miR156 and miR172 are positively regulated by the transcription factors they target, suggesting that negative feedback loops contribute to the stability of the juvenile and adult phases.
[本文引用: 1]
.
[本文引用: 1]
DOI:10.1134/S1021443710020020URL [本文引用: 1]

The timing of floral transition has significant consequences for reproductive success in plants. The molecular genetic dissection of flowering time control in Arabidopsis identified an integrated network of pathways that quantitatively control this developmental switch. A central player in this process is the FLOWERING LOCUS C gene ( FLC ), which blocks flowering by inhibiting the genes required to switch the meristem from vegetative to floral development. Three systems (the FRIGIDA gene, vernalization, and the autonomous pathway) all influence the state of FLC . Last years many new genes have been identified that regulate FLC expression, and most of them are involved in the modification of FLC chromatin. This review focuses on recent insights in FLC regulation.
DOI:10.1016/S0092-8674(00)00184-7URLPMID:11114337 [本文引用: 1]

Cryptochrome blue light photoreceptors share sequence similarity to photolyases, flavoproteins that mediate light-dependent DNA repair. However, cryptochromes lack photolyase activity and are characterized by distinguishing C-terminal domains. Here we show that the signaling mechanism of Arabidopsis cryptochrome is mediated through the C terminus. On fusion with 尾-glucuronidase (GUS), both the Arabidopsis CRY1 C-terminal domain (CCT1) and the CRY2 C-terminal domain (CCT2) mediate a constitutive light response. This constitutive photomorphogenic (COP) phenotype was not observed for mutants of cct1 corresponding to previously described cry1 alleles. We propose that the C-terminal domain of Arabidopsis cryptochrome is maintained in an inactive state in the dark. Irradiation with blue light relieves this repression, presumably through an intra- or intermolecular redox reaction mediated through the flavin bound to the N-terminal photolyase-like domain.
.
[本文引用: 1]
DOI:10.1038/s41477-017-0099-0URLPMID:29379156 [本文引用: 1]

UVR8 is a UV-B photoreceptor that employs specific tryptophans in its primary sequence as chromophores in photoreception. UV-B absorption causes dissociation of the dimeric photoreceptor by neutralizing interactions between monomers. The monomeric form initiates signalling through interaction with the COP1 protein, leading to transcriptional responses. This article discusses the structural... [Show full abstract]
.
DOI:10.1080/09168451.2014.932670URL [本文引用: 1]

Floral transition is regulated by environmental and endogenous signals. Previously, we identified VASCULAR PLANT ONE-ZINC FINGER1 (VOZ1) and VOZ2 as phytochrome B-interacting factors. VOZ1 and VOZ2 redundantly promote flowering and have pivotal roles in the downregulation of FLOWERING LOCUS C (FLC), a central repressor of flowering in Arabidopsis. Here, we showed that the late-flowering phenotypes of the voz1 voz2 mutant were suppressed by vernalization in the Columbia and FRIGIDA (FRI)-containing accessions, which indicates that the late-flowering phenotype of voz1 voz2 mutants was caused by upregulation of FLC. We also showed that the other FLC clade members, MADS AFFECTING FLOWERING (MAF) genes, were also a downstream target of VOZ1 and VOZ2 as their expression levels were also increased in the voz1 voz2 mutant. Our results suggest that the FLC clade genes integrate signals from VOZ1/VOZ2 and vernalization to regulate flowering.
.
[本文引用: 1]
DOI:10.1093/mp/ssu086URLPMID:25095795 [本文引用: 1]
DOI:10.1016/j.molcel.2008.09.026URLPMID:19061637 [本文引用: 1]

Seasonal changes in day length are perceived by plant photoreceptors and transmitted to the circadian clock to modulate developmental responses such as flowering time. Blue-light-sensing cryptochromes, the E3 ubiquitin-ligase COP1, and clock-associated proteins ELF3 and GI regulate this process, although the regulatory link between them is unclear. Here we present data showing that COP1 acts with ELF3 to mediate day length signaling from CRY2 to GI within the photoperiod flowering pathway. We found that COP1 and ELF3 interact in02vivo and show that ELF3 allows COP1 to interact with GI in02vivo, leading to GI02degradation in planta. Accordingly, mutation of COP1 or ELF3 disturbs the pattern of GI cyclic accumulation. We propose a model in which ELF3 acts as a substrate adaptor, enabling COP1 to modulate light input signal to the circadian clock through targeted destabilization of GI.
DOI:10.1073/pnas.1602004113URLPMID:27325772

Abstract The phenomenon of delayed flowering after the application of nitrogen (N) fertilizer has long been known in agriculture, but the detailed molecular basis for this phenomenon is largely unclear. Here we used a modified method of suppression-subtractive hybridization to identify two key factors involved in N-regulated flowering time control in Arabidopsis thaliana, namely ferredoxin-NADP + -oxidoreductase and the blue-light receptor cryptochrome 1 (CRY1). The expression of both genes is induced by low N levels, and their loss-of-function mutants are insensitive to altered N concentration. Low-N conditions increase both NADPH/NADP + and ATP/AMP ratios, which in turn affect adenosine monophosphate-activated protein kinase (AMPK) activity. Moreover, our results show that the AMPK activity and nuclear localization are rhythmic and inversely correlated with nuclear CRY1 protein abundance. Low-N conditions increase but high-N conditions decrease the expression of several key components of the central oscillator (e.g., CCA1, LHY, and TOC1) and the flowering output genes (e.g., GI and CO). Taken together, our results suggest that N signaling functions as a modulator of nuclear CRY1 protein abundance, as well as the input signal for the central circadian clock to interfere with the normal flowering process.
.
DOI:10.1038/ng.3712URLPMID:27819666 [本文引用: 2]

Some plants acquire competence to flower in spring after experiencing a seasonal temperature drop-winter cold, in a process termed vernalization. In Arabidopsis thaliana, prolonged exposure to cold induces epigenetic silencing of the potent floral repressor locus FLOWERING LOCUS C (FLC) by Polycomb group (PcG) proteins, and this silencing is stably maintained in subsequent growth and development upon return to warm temperatures. Here we show that a cis-regulatory DNA element in the nucleation region for PcG silencing at FLC and two homologous trans-acting epigenome readers, VAL1 and VAL2, control vernalization-mediated FLC silencing. The sequence-specific readers recognize both the cis element (termed the cold memory element) and a repressive mark, trimethylation of histone H3 at lysine 27 (H3K27me3), and directly associate with LIKE HETEROCHROMATIN PROTEIN 1 (LHP1), leading to establishment of the H3K27me3 peak in the nucleation region at FLC during vernalization. Thus, our work describes a mechanism for PcG-mediated silencing by a DNA sequence-specific epigenome reader.
DOI:10.1016/j.cub.2011.03.048URLPMID:21514160 [本文引用: 1]

Cryptochromes are blue light receptors that mediate light regulation of gene expression in all major evolution lineages, but the molecular mechanism underlying cryptochrome signal transduction remains not fully understood []. It has been reported that cryptochromes suppress activity of the multifunctional E3 ubiquitin ligase ]. But how plant cryptochromes mediate light suppression of COP1 activity remains unclear. We report here that ]. We demonstrate that SPA1 acts genetically downstream from CRY2 to mediate blue light suppression of the COP1-dependent proteolysis of the flowering-time regulator ]. We further show that blue light-dependent CRY2-SPA1 interaction stimulates CRY2-COP1 interaction. These results reveal for the first time a wavelength-specific mechanism by which a cryptochrome photoreceptor mediates light regulation of protein degradation to modulate developmental timing in Graphical AbstractView high quality image (204K)
Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT
1
2003
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex
1
2006
... 某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(
A new role for phytochromes in temperature-dependent germination
0
2007
GIGANTEA is a nuclear protein involved in phytochrome signaling in Ara- bidopsis
1
2000
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis
3
2005
... 作为昼夜节律钟的重要组成因子, ZTLs参与植物昼夜节律信号的输入, 调控下游的成花诱导和花器官形成基因的表达(
... 对开花具有促进作用(
... 隐花色素、光敏色素和FKF1/ZTL/LKP2均参与CO基因转录水平的调控.CO的转录主要受到CDF1 (CY- CLING ODF FACTOR 1)和FKF1-GI (GIGANTEA)复合体的调控(
PFT1, the MED25 subunit of the plant mediator complex, promotes flowering through CONSTANS depen- dent and independent mechanisms in Arabidopsis
1
2012
... 此外, 人们还鉴定出其它CO转录的调节因子.ELF4 (EARLY FLOWERING 4)可在夜间限制GI蛋白结合CO启动子的能力, 进而影响其转录(
a). LOV domain-contai- ning F-box proteins: light-dependent protein degradation modules in Arabidopsis
3
2012
... 向光素(phototropins)是光活化的丝氨酸/苏氨酸蛋白激酶.目前, 在拟南芥中仅发现2种向光素, 即PHOT1和PHOT2 (
... ZTLs是一类新发现的蓝光受体蛋白.拟南芥中已知的ZTLs家族包括FKF1、ZTL和LKP2.这3个蛋白都含有3个重要的功能保守结构域: N端的LOV结构域、中间的F-box基序和C端的Kelch重复序列(
... ).Kelch结构域则与蛋白质的相互作用相关(
b). Flowering BHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis
1
2012
... 此外, 人们还鉴定出其它CO转录的调节因子.ELF4 (EARLY FLOWERING 4)可在夜间限制GI蛋白结合CO启动子的能力, 进而影响其转录(
Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response
2
2008
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
... ).PHYB则在早晨以独立于COP1的途径降解CO蛋白(
An Arabidopsis circadian clock component interacts with both CRY1 and phyB
1
2001
... 作为昼夜节律钟的重要组成因子, ZTLs参与植物昼夜节律信号的输入, 调控下游的成花诱导和花器官形成基因的表达(
The UV-B photoreceptor UVR8: from structure to physiology
2
2014
... UVR8参与调控植物对UV-B辐射的响应.拟南芥UVR8编码440个氨基酸残基, 分子量为47 kDa.目前已获得在N端缺少11个氨基酸且在C端缺少59个氨基酸的UVR8蛋白核心的高分辨率晶体结构, 其由7个片状的β螺旋构成, 但C端和N端的位置、功能结构域特征以及UVR8蛋白从二聚体到单体如何变化尚不清楚(
... 此外, FKF1能够通过LOV结构域与CO互作从而稳定CO蛋白活性, 蓝光能增强它们之间的相互作用(
SRR1 is essential to repress flowering in non-inductive conditions in Arabidopsis thaliana
1
2014
... 赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(
Time to flower: interplay between photoperiod and the circadian clock
1
2015
... 在许多植物中, 光周期是影响开花的重要环境因子, 光周期途径是调控植物开花的途径之一(
Photoresponses of light-grown phyA mutants of Arabidopsis (Phytochrome A is required for the perception of daylength extensions)
1
1994
... 拟南芥光敏色素基因家族有5个成员: PHYA、PHYB、PHYC、PHYD和PHYE, 不同成员间既相互独立, 又存在功能冗余.PHYA是一类在光照下迅速分解的光不稳定型蛋白, 参与幼苗的远红光感受及介导早期的红光反应; PHYB、PHYC、PHYD和PHYE属于光稳定型蛋白, 在持续红光或白光下起主要作用(
Chapter two—light-regulated plant growth and development
1
2010
... 拟南芥光敏色素基因家族有5个成员: PHYA、PHYB、PHYC、PHYD和PHYE, 不同成员间既相互独立, 又存在功能冗余.PHYA是一类在光照下迅速分解的光不稳定型蛋白, 参与幼苗的远红光感受及介导早期的红光反应; PHYB、PHYC、PHYD和PHYE属于光稳定型蛋白, 在持续红光或白光下起主要作用(
Vernalization: winter and the timing of flowering in plants
1
2009
... 某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(
The F-box protein ZEITLUPE controls stability and nucleocytoplasmic partitioning of GIGANTEA
1
2013
... 此外, 人们还鉴定出其它CO转录的调节因子.ELF4 (EARLY FLOWERING 4)可在夜间限制GI蛋白结合CO启动子的能力, 进而影响其转录(
A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana
1
1991
... 拟南芥隐花色素基因家族由3个成员组成: CRY1、CRY2和CRY3.CRY1和CRY2与植物昼夜节律钟相关, 参与调控幼苗去黄化和成花诱导过程(
Transcription factor PIF4 controls the thermosensory activation of flowering
1
2012
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long- day photoperiods in Arabidopsis
1
2008
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability
1
2006
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
The Arabidopsis E3 ubiquitin ligase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering
1
2012
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
Role of SVP in the control of flowering time by ambient temperature in Arabidopsis
1
2007
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
PIFs: systems integrators in plant development
1
2014
... 光敏色素是主要的红光/远红光感受器, 是植物自身合成的水溶性色素蛋白.功能性光敏色素由2个单体组成, 每个单体又含有2个结构域, 其中N端的光感受结构域可共价结合生色团, 与光敏色素的光化学特性相关, 而C端的光调节结构域主要参与光敏色素二聚体的形成及下游信号转导过程(
“先驱”转录因子LEC1在早期胚胎重置春化状态的机制
1
2018
... 某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(
HY4 gene of A.thaliana encodes a protein with characteristics of a blue-light photoreceptor.
1
1993
... 拟南芥隐花色素基因家族由3个成员组成: CRY1、CRY2和CRY3.CRY1和CRY2与植物昼夜节律钟相关, 参与调控幼苗去黄化和成花诱导过程(
The B-Box Family Gene STO (BBX24) in Arabidopsis tha- liana regulates flowering time in different pathways
1
2014
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
UVR8 interacts with BES1 and BIM1 to regulate transcription and photomorphogenesis in Arabidopsis
1
2018
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light
1
2011
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
Arabidopsis CRY2 and ZTL mediate blue-light regulation of the transcription factor CIB1 by distinct mechanisms
1
2013
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
Seasonal and developmental timing of flowering
1
2010
... 某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(
The genetic basis of flowe- ring responses to seasonal cues
1
2012
... 高等植物从营养生长向生殖生长的成花转变和花器官的形成在物种发育、繁殖和进化中发挥核心作用(
Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis
1
2008
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
From the cover: a role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening
0
2005
Decoding of light signals by plant phytochromes and their interacting proteins
1
2008
... 光敏色素是主要的红光/远红光感受器, 是植物自身合成的水溶性色素蛋白.功能性光敏色素由2个单体组成, 每个单体又含有2个结构域, 其中N端的光感受结构域可共价结合生色团, 与光敏色素的光化学特性相关, 而C端的光调节结构域主要参与光敏色素二聚体的形成及下游信号转导过程(
Potent induction of Arabidopsis thaliana flowering by elevated growth temperature
2
2006
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
... 赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(
Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana
1
2003
... 作为昼夜节律钟的重要组成因子, ZTLs参与植物昼夜节律信号的输入, 调控下游的成花诱导和花器官形成基因的表达(
Dimers of the N-terminal domain of phytochrome B are functional in the nucleus
1
2003
... 光敏色素是主要的红光/远红光感受器, 是植物自身合成的水溶性色素蛋白.功能性光敏色素由2个单体组成, 每个单体又含有2个结构域, 其中N端的光感受结构域可共价结合生色团, 与光敏色素的光化学特性相关, 而C端的光调节结构域主要参与光敏色素二聚体的形成及下游信号转导过程(
F-box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression
1
2010
... 作为昼夜节律钟的重要组成因子, ZTLs参与植物昼夜节律信号的输入, 调控下游的成花诱导和花器官形成基因的表达(
Circadian clock genes universally control key agricultural traits
1
2015
... CRY2在CO的转录调控中行使重要功能.光通过诱导CRY2形成同源二聚体的方式来激活CRY2, 而BIC1 (BLUE-LIGHTINHIBITOR OF CRYPTOCHR- OMES 1)可与CRY2直接互作阻断CRY2的二聚化反应和后续的信号转导过程(
Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis
1
2005
... 隐花色素、光敏色素和FKF1/ZTL/LKP2均参与CO基因转录水平的调控.CO的转录主要受到CDF1 (CY- CLING ODF FACTOR 1)和FKF1-GI (GIGANTEA)复合体的调控(
Isolation and characterization of phyC mutants in Arabidopsis reveals complex crosstalk between phytochrome signaling pathways
1
2003
... 拟南芥光敏色素基因家族有5个成员: PHYA、PHYB、PHYC、PHYD和PHYE, 不同成员间既相互独立, 又存在功能冗余.PHYA是一类在光照下迅速分解的光不稳定型蛋白, 参与幼苗的远红光感受及介导早期的红光反应; PHYB、PHYC、PHYD和PHYE属于光稳定型蛋白, 在持续红光或白光下起主要作用(
A thermosensory pathway controlling flowering time in Arabidopsis thaliana
1
2003
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
Flowering time regulation in crops-what did we learn from Arabidopsis?
1
2015
... 高等植物从营养生长向生殖生长的成花转变和花器官的形成在物种发育、繁殖和进化中发挥核心作用(
DAY NEUTRAL FLOWERING represses CONSTANS to prevent Arabidopsis flowering early in short days
1
2010
... 此外, 人们还鉴定出其它CO转录的调节因子.ELF4 (EARLY FLOWERING 4)可在夜间限制GI蛋白结合CO启动子的能力, 进而影响其转录(
FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis
1
2000
... ZTLs是一类新发现的蓝光受体蛋白.拟南芥中已知的ZTLs家族包括FKF1、ZTL和LKP2.这3个蛋白都含有3个重要的功能保守结构域: N端的LOV结构域、中间的F-box基序和C端的Kelch重复序列(
Phototropins 1 and 2: versatile plant blue-light receptors
2
2002
... 向光素(phototropins)是光活化的丝氨酸/苏氨酸蛋白激酶.目前, 在拟南芥中仅发现2种向光素, 即PHOT1和PHOT2 (
... 向光素是植物中与细胞质膜相关的蓝光受体, 参与调控植物的向光性生长、光诱导的气孔开放和叶绿体运动及下胚轴伸长等生物学过程(
Identification of a new cryptochrome class: structure, function, and evolution
1
2003
... 拟南芥隐花色素基因家族由3个成员组成: CRY1、CRY2和CRY3.CRY1和CRY2与植物昼夜节律钟相关, 参与调控幼苗去黄化和成花诱导过程(
TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis
2
2012
... 赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(
... 基因的植物具有类似GA缺失型突变体的表型(
Temperature- dependent regulation of flowering by antagonistic FLM variants
1
2013
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
A putative CCAAT-binding transcription factor is a regulator of flowe- ring timing in Arabidopsis
1
2007
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
A distal CCAAT/NUCLEAR FACTOR Y complex promotes chromatin looping at the FLOWERING LOCUS T promoter and regulates the timing of flowering in Arabidopsis
1
2014
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
Dual-source nuclear monomers of UV-B light receptor direct photomorphogenesis in Arabidopsis
1
2016
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
Message ends: RNA 3' pro- cessing and flowering time control
1
2014
... 某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(
Cryptochromes: enabling plants and animals to determine circadian time
1
2003
... 隐花色素是一种类光解酶(photolyase)的蓝光受体, 在动植物中均有存在(
Cryptochromes: blue light receptors for plants and animals
1
1999
... 隐花色素是一种类光解酶(photolyase)的蓝光受体, 在动植物中均有存在(
MicroRNAs in plants
1
2002
... 植物必须完成一定程度的营养生长才能进入生殖生长.miR156和miR172在拟南芥开花的年龄途径中发挥重要作用.成花抑制因子miR156的表达在幼龄期高于成年期, 而成花促进因子miR172的表达在幼龄期低于成年期(
Perception of UV-B by the Arabidopsis UVR8 protein
3
2011
... 植物通过3类光受体(photoreceptor)来感知红光、蓝光和紫外光UV-B并进行信号转导, 包括5个红光/远红光受体光敏色素(
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
... 此外, FKF1能够通过LOV结构域与CO互作从而稳定CO蛋白活性, 蓝光能增强它们之间的相互作用(
The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering
1
2008
... 赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(
Phototropins and associated signaling: providing the power of movement in higher plants
1
2005
... 向光素是植物中与细胞质膜相关的蓝光受体, 参与调控植物的向光性生长、光诱导的气孔开放和叶绿体运动及下胚轴伸长等生物学过程(
Phytochrome structure and signaling mechanisms
1
2006
... 光敏色素是主要的红光/远红光感受器, 是植物自身合成的水溶性色素蛋白.功能性光敏色素由2个单体组成, 每个单体又含有2个结构域, 其中N端的光感受结构域可共价结合生色团, 与光敏色素的光化学特性相关, 而C端的光调节结构域主要参与光敏色素二聚体的形成及下游信号转导过程(
FLOWERING LOCUS C-dependent and -independent regulation of the circadian clock by the autonomous and vernalization pathways
1
2006
... 某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(
Distinct roles of CONSTANS target genes in reproductive deve- lopment of Arabidopsis
1
2000
... 高等植物从营养生长向生殖生长的成花转变和花器官的形成在物种发育、繁殖和进化中发挥核心作用(
Ambient temperature perception in plants
1
2005
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
The cryptochromes: blue light photoreceptors in plants and animals
3
2011
... 植物通过3类光受体(photoreceptor)来感知红光、蓝光和紫外光UV-B并进行信号转导, 包括5个红光/远红光受体光敏色素(
... 隐花色素是一种类光解酶(photolyase)的蓝光受体, 在动植物中均有存在(
... 拟南芥隐花色素基因家族由3个成员组成: CRY1、CRY2和CRY3.CRY1和CRY2与植物昼夜节律钟相关, 参与调控幼苗去黄化和成花诱导过程(
Phytochrome signaling mechanisms and the control of plant development
1
2011
... 植物通过3类光受体(photoreceptor)来感知红光、蓝光和紫外光UV-B并进行信号转导, 包括5个红光/远红光受体光敏色素(
GIGANTEA directly activates Flowering Locus T in Arabidopsis thaliana
1
2011
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
FKF1 and GIGANTEA complex formation is required for day- length measurement in Arabidopsis
2
2007
... 隐花色素、光敏色素和FKF1/ZTL/LKP2均参与CO基因转录水平的调控.CO的转录主要受到CDF1 (CY- CLING ODF FACTOR 1)和FKF1-GI (GIGANTEA)复合体的调控(
... 的转录(
A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development
1
2004
... 植物必须完成一定程度的营养生长才能进入生殖生长.miR156和miR172在拟南芥开花的年龄途径中发挥重要作用.成花抑制因子miR156的表达在幼龄期高于成年期, 而成花促进因子miR172的表达在幼龄期低于成年期(
Phototropin blue-light receptors
2
2007
... 植物通过3类光受体(photoreceptor)来感知红光、蓝光和紫外光UV-B并进行信号转导, 包括5个红光/远红光受体光敏色素(
... 向光素(phototropins)是光活化的丝氨酸/苏氨酸蛋白激酶.目前, 在拟南芥中仅发现2种向光素, 即PHOT1和PHOT2 (
Phytochrome-mediated photoperception and signal transduction in higher plants
0
2002
A role for LKP2 in the circadian clock of Arabidopsis
3
2001
... ZTLs是一类新发现的蓝光受体蛋白.拟南芥中已知的ZTLs家族包括FKF1、ZTL和LKP2.这3个蛋白都含有3个重要的功能保守结构域: N端的LOV结构域、中间的F-box基序和C端的Kelch重复序列(
... ;
... 隐花色素、光敏色素和FKF1/ZTL/LKP2均参与CO基因转录水平的调控.CO的转录主要受到CDF1 (CY- CLING ODF FACTOR 1)和FKF1-GI (GIGANTEA)复合体的调控(
Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges
1
2012
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
Putative sugarcane FT/TFL1 genes delay flowe- ring time and alter reproductive architecture in Arabidopsis
1
2014
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex
1
2015
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
The F-box protein ZEITLUPE confers dosage-dependent control on the circadian clock, photomorphogenesis, and flowering time
1
2004
... 隐花色素、光敏色素和FKF1/ZTL/LKP2均参与CO基因转录水平的调控.CO的转录主要受到CDF1 (CY- CLING ODF FACTOR 1)和FKF1-GI (GIGANTEA)复合体的调控(
ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis
2
2000
... ZTLs是一类新发现的蓝光受体蛋白.拟南芥中已知的ZTLs家族包括FKF1、ZTL和LKP2.这3个蛋白都含有3个重要的功能保守结构域: N端的LOV结构域、中间的F-box基序和C端的Kelch重复序列(
... 作为昼夜节律钟的重要组成因子, ZTLs参与植物昼夜节律信号的输入, 调控下游的成花诱导和花器官形成基因的表达(
FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering
4
2012
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
... 此外, FKF1能够通过LOV结构域与CO互作从而稳定CO蛋白活性, 蓝光能增强它们之间的相互作用(
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
... 的表达(
FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis
1
2007
... 高等植物从营养生长向生殖生长的成花转变和花器官的形成在物种发育、繁殖和进化中发挥核心作用(
A molecular framework for light and gibberellin control of cell elongation
2
2008
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
... 赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(
The Arabidopsis SRR1 gene mediates phyB signaling and is required for normal circadian clock function
1
2003
... 赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(
Arabidopsis MSI1 functions in photoperiodic flowering time control
1
2014
... 此外, 人们还鉴定出其它CO转录的调节因子.ELF4 (EARLY FLOWERING 4)可在夜间限制GI蛋白结合CO启动子的能力, 进而影响其转录(
PIFs get BRright: phytochrome interacting factors as integrators of light and hormonal signals
1
2014
... 赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(
Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity
1
2000
... 拟南芥光敏色素基因家族有5个成员: PHYA、PHYB、PHYC、PHYD和PHYE, 不同成员间既相互独立, 又存在功能冗余.PHYA是一类在光照下迅速分解的光不稳定型蛋白, 参与幼苗的远红光感受及介导早期的红光反应; PHYB、PHYC、PHYD和PHYE属于光稳定型蛋白, 在持续红光或白光下起主要作用(
A complementary role for ELF3 and TFL1 in the regulation of flowering time by ambient temperature
2
2009
... 有研究表明, 光敏色素介导的光信号可以被整合到春化途径中, 参与调控FLC的转录(
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: phototropin, ZTL/FKF1/LKP2 and aureochrome
1
2013
... 植物通过3类光受体(photoreceptor)来感知红光、蓝光和紫外光UV-B并进行信号转导, 包括5个红光/远红光受体光敏色素(
UV-B radiation delays flowering time through changes in the PRC2 complex activity and miR156 levels in Arabidopsis tha-liana
2
2018
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
... 植物必须完成一定程度的营养生长才能进入生殖生长.miR156和miR172在拟南芥开花的年龄途径中发挥重要作用.成花抑制因子miR156的表达在幼龄期高于成年期, 而成花促进因子miR172的表达在幼龄期低于成年期(
PHYTOCHROME-DEPENDENT LATE-FLOWE- RING accelerates flowering through physical interactions with phytochrome B and CONSTANS
1
2013
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis
2
2009
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
... 此外, FKF1能够通过LOV结构域与CO互作从而稳定CO蛋白活性, 蓝光能增强它们之间的相互作用(
Arabidopsis DOF trans- cription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response
2
2009
... 隐花色素、光敏色素和FKF1/ZTL/LKP2均参与CO基因转录水平的调控.CO的转录主要受到CDF1 (CY- CLING ODF FACTOR 1)和FKF1-GI (GIGANTEA)复合体的调控(
... ;
Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowe- ring in Arabidopsis
1
2008
... 适当提高植物营养生长阶段的环境温度可促进开花, 而低温则会抑制开花(
Spatial control of flowering by DELLA proteins in Arabidopsis thaliana
1
2012
... 赤霉素(gibberellins, GA)可通过降解转录抑制因子DELLA蛋白促进植物开花(
Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice
1
2005
... 在过去的几十年中, 高等植物开花的调控网络一直是植物学研究的前沿和热点.目前, 不同光受体的分子结构和生理功能已逐渐得到解析, 发现了一大批与光受体互作的信号蛋白, 人们对光周期途径、温度途径及GA途径的信号转导及整合机制也有了一定的认识(
LOV KELCH PROTEIN2 and ZEITLUPE repress Arabidopsis photoperiodic flowe- ring under non-inductive conditions, dependent on FLAVIN- BINDING KELCH REPEAT F-BOX1
2
2011
... 作为昼夜节律钟的重要组成因子, ZTLs参与植物昼夜节律信号的输入, 调控下游的成花诱导和花器官形成基因的表达(
... 隐花色素、光敏色素和FKF1/ZTL/LKP2均参与CO基因转录水平的调控.CO的转录主要受到CDF1 (CY- CLING ODF FACTOR 1)和FKF1-GI (GIGANTEA)复合体的调控(
Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis
1
2010
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
Regulation of flowering time by Arabidopsis photoreceptors
2
1998
... 拟南芥光敏色素基因家族有5个成员: PHYA、PHYB、PHYC、PHYD和PHYE, 不同成员间既相互独立, 又存在功能冗余.PHYA是一类在光照下迅速分解的光不稳定型蛋白, 参与幼苗的远红光感受及介导早期的红光反应; PHYB、PHYC、PHYD和PHYE属于光稳定型蛋白, 在持续红光或白光下起主要作用(
... 拟南芥隐花色素基因家族由3个成员组成: CRY1、CRY2和CRY3.CRY1和CRY2与植物昼夜节律钟相关, 参与调控幼苗去黄化和成花诱导过程(
Embryonic epigenetic reprogramming by a pioneer transcription factor in plants
1
2017
... 某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(
To bloom or not to bloom: role of microRNAs in plant flowering
1
2015
... 高等植物从营养生长向生殖生长的成花转变和花器官的形成在物种发育、繁殖和进化中发挥核心作用(
phyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of Arabidopsis seedling de-etiolation
1
2006
... 拟南芥光敏色素基因家族有5个成员: PHYA、PHYB、PHYC、PHYD和PHYE, 不同成员间既相互独立, 又存在功能冗余.PHYA是一类在光照下迅速分解的光不稳定型蛋白, 参与幼苗的远红光感受及介导早期的红光反应; PHYB、PHYC、PHYD和PHYE属于光稳定型蛋白, 在持续红光或白光下起主要作用(
The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-ele- ment
1
2010
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
Photoreceptor regulation of CONSTANS protein in photoperiodic flowering
1
2004
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
BBX19 interacts with CONSTANS to repress FLOWE- RING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis
2
2014
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
... 植物必须完成一定程度的营养生长才能进入生殖生长.miR156和miR172在拟南芥开花的年龄途径中发挥重要作用.成花抑制因子miR156的表达在幼龄期高于成年期, 而成花促进因子miR172的表达在幼龄期低于成年期(
Phytochrome B is involved in mediating red light-induced sto- matal opening in Arabidopsis thaliana
0
2010
Regulation of flowering time by the miR156-mediated age pathway
0
2014
Photoactivation and inactivation of Arabidopsis cryptochrome 2
1
2016
... CRY2在CO的转录调控中行使重要功能.光通过诱导CRY2形成同源二聚体的方式来激活CRY2, 而BIC1 (BLUE-LIGHTINHIBITOR OF CRYPTOCHR- OMES 1)可与CRY2直接互作阻断CRY2的二聚化反应和后续的信号转导过程(
Structural basis of ultraviolet-B perception by UVR8
1
2012
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis
3
2009
... 植物必须完成一定程度的营养生长才能进入生殖生长.miR156和miR172在拟南芥开花的年龄途径中发挥重要作用.成花抑制因子miR156的表达在幼龄期高于成年期, 而成花促进因子miR172的表达在幼龄期低于成年期(
... ).SPL9直接结合miR172启动子促进其表达(
... ;
miR156-targeted and nontargeted SBP-box transcription factors act in concert to secure male fertility in Arabidopsis
1
2010
... 植物必须完成一定程度的营养生长才能进入生殖生长.miR156和miR172在拟南芥开花的年龄途径中发挥重要作用.成花抑制因子miR156的表达在幼龄期高于成年期, 而成花促进因子miR172的表达在幼龄期低于成年期(
TWIN SISTER OF FT ( TSF) acts as a floral pathway integrator redundantly with FT
1
2005
... 高等植物从营养生长向生殖生长的成花转变和花器官的形成在物种发育、繁殖和进化中发挥核心作用(
FLC: a key regulator of flowering time in Arabidopsis
1
2010
... 在过去的几十年中, 高等植物开花的调控网络一直是植物学研究的前沿和热点.目前, 不同光受体的分子结构和生理功能已逐渐得到解析, 发现了一大批与光受体互作的信号蛋白, 人们对光周期途径、温度途径及GA途径的信号转导及整合机制也有了一定的认识(
The C termini of Arabidopsis cryptochromes mediate a constitutive light response
1
2000
... 隐花色素是一种类光解酶(photolyase)的蓝光受体, 在动植物中均有存在(
Molecular cloning and function analysis of ClCRY1a and ClCRY1b, two genes in Chrysanthemum lavandulifolium that play vital roles in promoting floral transition
1
2017
... 在过去的几十年中, 高等植物开花的调控网络一直是植物学研究的前沿和热点.目前, 不同光受体的分子结构和生理功能已逐渐得到解析, 发现了一大批与光受体互作的信号蛋白, 人们对光周期途径、温度途径及GA途径的信号转导及整合机制也有了一定的认识(
UVR8 interacts with WRKY36 to regulate HY5 transcription and hypocotyl elongation in Arabidopsis
1
2018
... UVR8是拟南芥中吸收紫外线UV-B的植物特有的色素蛋白, 在没有UV-B的情况下, UVR8形成对称的同源二聚体, 其表面上的色氨酸残基可行使生色团功能.在UV-B辐射下, 细胞质中的UVR8发生单体化并迁移入细胞核内积累, 维持核内UV-B的信号转导(
VASCULAR PLANT ONE-ZINC FINGER1 and VOZ2 repress the FLOWERING LOCUS C clade members to control flowering time in Arabidopsis
1
2014
... 有研究表明, 光敏色素介导的光信号可以被整合到春化途径中, 参与调控FLC的转录(
The phytochrome-interac-ting VASCULAR PLANT ONE-ZINC FINGER 1 and VOZ2 redundantly regulate flowering in Arabidopsis
1
2012
... 有研究表明, 光敏色素介导的光信号可以被整合到春化途径中, 参与调控FLC的转录(
How do phytochromes transmit the light quality information to the circadian clock in Arabidopsis?
1
2014
... FT的表达丰度决定了开花时间.CO是FT基因主要的转录激活因子之一, CO可以通过其CCT结构域结合FT启动子中2个CO应答元件(CO-responsive elements, CORE), 并可能通过富含谷氨酸的区域激活FT的转录(
COP1 and ELF3 control circadian function and photoperiodic flowering by regula- ting GI stability
1
2008
... CRY2在CO的转录调控中行使重要功能.光通过诱导CRY2形成同源二聚体的方式来激活CRY2, 而BIC1 (BLUE-LIGHTINHIBITOR OF CRYPTOCHR- OMES 1)可与CRY2直接互作阻断CRY2的二聚化反应和后续的信号转导过程(
a). Arabidopsis cryptochrome 1 functions in nitrogen regulation of flowering
0
2016
A cis cold memory element and a trans epigenome reader mediate Polycomb silencing of FLC by vernalization in Arabidopsis
2
2016
... 某些植物需要经历一段低温才能克服开花障碍的现象被称为春化, 春化作用是植物适应温带气候, 防止冬季开花并在春季有利条件下开花的重要保障(
... 在过去的几十年中, 高等植物开花的调控网络一直是植物学研究的前沿和热点.目前, 不同光受体的分子结构和生理功能已逐渐得到解析, 发现了一大批与光受体互作的信号蛋白, 人们对光周期途径、温度途径及GA途径的信号转导及整合机制也有了一定的认识(
Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis
1
2011
... 除CO的转录水平调控外, 隐花色素、光敏色素和FKF1可以调控CO蛋白的稳定性.在长日照条件下, CO蛋白在早晨丰度很低, 而在下午出现积累(
备案号: 京ICP备16067583号-21
版权所有 © 2021 《植物学报》编辑部
地址:北京香山南辛村20号 邮编:100093
电话:010-62836135 010-62836131 E-mail:cbb@ibcas.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
