 ,1,*
,1,*Heterologous Overexpression of Rice OsSAPP3 Gene Promotes Leaf Senescence in Transgenic Arabidopsis
Shengnan Cui1,2, Yihan Zhang1, Fan Xu ,1,*
,1,*通讯作者:
收稿日期:2018-02-12接受日期:2018-05-21网络出版日期:2019-01-30
| 基金资助: |
Corresponding authors:
Received:2018-02-12Accepted:2018-05-21Online:2019-01-30

摘要
关键词:
Abstract
Keywords:
PDF (27559KB)摘要页面多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
崔胜男, 张艺函, 徐凡. 异源过表达水稻OsSAPP3基因促进拟南芥叶片衰老. 植物学报, 2019, 54(1): 46-57 doi:10.11983/CBB18045
Cui Shengnan, Zhang Yihan, Xu Fan.
生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(肖冬等, 2014)。植物进入衰老阶段后, 自动激活自毁程序, 将组织细胞的结构退化, 在衰老叶中储存的营养物质会被重新利用, 从而为植株做最后的贡献(Quirino et al., 2000; 杨同文和李成伟, 2014); 其主要特征为叶片泛黄、叶绿素含量显著减少、叶片光合作用潜力下降、叶绿体结构被破坏以及营养物质转移到植物其它组织, 从而最终导致叶片衰老死亡(徐凡等, 2010; Wang et al., 2016)。叶片衰老受众多内外因素协同调控, 其中叶龄是最主要的调控因子, 同时叶片衰老的起始和进展受到各种环境与内源因素的调节(Lim et al., 2003; Buchananwollaston et al., 2005; Lim and Hong, 2007; Zhao et al., 2015)。
水稻(Oryza sativa)叶片衰老相关研究显示, 水稻衰老过程中会产生大量的水解酶类(郑建敏等, 2009)。蛋白激酶(protein kinase, PK)和蛋白磷酸酶(protein phosphatase, PP)所催化的蛋白质可逆磷酸化反应是细胞信号识别与转导的重要环节(Xu et al., 2011)。Menges等(2002)研究表明, 蛋白磷酸酶通过去磷酸化调控蛋白激酶活性来参与细胞周期的调节。
PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(翁华等, 2003)。目前已经在多种植物中鉴定到PP2C的编码基因, 它们大多参与植物对非生物胁迫的响应。例如, 拟南芥(Arabidopsis thaliana) AtbZIP19/23 (Castro et al., 2017)、玉米(Zea mays) ABP9 (Wang et al., 2017)和水稻OsPP2C51 (Bhatnagar et al., 2017)作为ABA信号转导的辅助受体, 在干旱、寒冷和盐胁迫的ABA依赖性应激反应中起重要作用; 大豆(Glycine max)中GsAPD的表达水平明显受盐碱胁迫的影响(Chen et al., 2018); PP2C型蛋白磷酸酶编码基因AP2C1在植物组织发生创伤或病原体胁迫时, 与MPK4或者MPK6协同抑制MAPK的活性(Schweighofer et al., 2007); PacPP2C1协同SnRK2蛋白激酶在甜樱桃(Prunus avium)果实成熟和改善植物对干旱胁迫的耐受性中起关键作用(Shen et al., 2017); 拟南芥中的SAG113与AtNAP协同调控ABA诱导的叶片衰老进程(Zhang and Gan, 2012); 拟南芥PP2C中的SSPP通过直接去磷酸化AtSARK来抑制SARK介导的衰老信号转导, 从而负向调节拟南芥叶片的衰老进程(Xiao et al., 2015)。
本研究中, 我们克隆并鉴定了1个水稻的PP2C型蛋白磷酸酶编码基因(LOC_10g39780; Os10g- 0544900), 将该基因命名为OsSAPP3 (Oryza sativa Senescence-Associated Protein Phosphatase, OsSAPP3)。根据Xue等(2008)对水稻中PP2Cs的聚类分析, 水稻中PP2Cs共有11个亚家族, OsSAPP3基因是水稻PP2C亚家族D的成员。OsSAPP3基因CDS全长1 182 bp, 编码394个氨基酸残基, 经SMART、NCBI和Pfam等生物信息学软件预测, 该蛋白具有明显的PP2C结构域, 位于第53-329位氨基酸之间, 预测为丝/苏氨酸蛋白磷酸酶。我们还克隆了OsSAPP3基因ATG上游2 063 bp的启动子序列, 利用PlantCare网站进行在线分析, 预测OsSAPP3启动子区域不仅含有核心元件TATA-box, 还具有脱落酸响应元件(ABRE)、光响应元件(AE-box和G-box)及MBS结合位点等其它调控元件; 构建了ProOsSAPP3- GUS双元表达载体, 并获得相应的转基因拟南芥株系。研究发现组成型异源过表达OsSAPP3基因导致转基因拟南芥发育严重受阻, 因此后续实验中使用可诱导型启动子驱动OsSAPP3基因异源过表达。在转基因拟南芥中外源诱导OsSAPP3基因异源过表达会导致转基因拟南芥叶片黄化、叶绿素含量降低及植株提前进入衰老程序。各衰老标志基因的表达水平也随着外源诱导OsSAPP3的上调表达而上调。以上结果表明, OsSAPP3基因参与叶片衰老进程的调控, 是水稻叶片衰老的正调控因子。
1 材料与方法
1.1 基因克隆、双元表达载体构建与植物材料 培养
设计引物(表1)扩增OsSAPP3的CDS序列, 分别在上游引物CDSOsSAPP3-F和cGVG-SAPP3-F中引入Sma I和Sal I的酶切位点; 分别在下游引物CDSOsSAPP3-R和cGVG-SAPP3-R中引入Sac I和Cla I的酶切位点。以水稻(Oryza sativa L.) (沈农9816) cDNA为模板, 用高保真聚合酶进行PCR反应, 克隆产物长度为1 182 bp, 然后将PCR产物分别连入T载体(pGEM-T Easy Vector), 完成TA克隆; 将所获得的TA克隆分别与pBI121基本载体以及pTA7002载体用Sma I/Sac I以及Xho I/Cla I进行双酶切, 分别回收小片段和载体片段, 连接后完成35S-OsSAPP3双元表达载体以及GVG-OsSAPP3双元表达载体的构建。Table 1
表1
表1引物序列
Table 1
| Primer name | Primer sequence (5'-3') | Annotation |
|---|---|---|
| CDSOsSAPP3-F | CCCGGGATGCTATCTGCTGCGATGGAATACTT | PCR |
| CDSOsSAPP3-R | GAACTCTATGTAGGAACTGAGAATGGAGCAAGG | PCR |
| cGVG-SAPP3-F | GTCGACATGCTATCTGCTGCGATGGAATAC | PCR |
| cGVG-SAPP3-R | ATCGATCTATGTAGGAACTGAGAATGGAGCAA | PCR |
| ProOsSAPP3-F | AAGCTTTCATATCCGTTGTTCCGTAGCG | PCR |
| ProOsSAPP3-R | TCTAGACCTTCATCTCCGACACCTCCC | PCR |
新窗口打开|下载CSV
设计引物(表1)扩增OsSAPP3基因初始密码子ATG上游2 063 bp的序列, 并在上游引物ProOsSAPP3- F中引入EcoR I酶切位点、在下游引物ProOsSAPP3-R中引入Nco I酶切位点。以水稻(沈农9816)基因组DNA为模板, 用高保真聚合酶进行PCR反应, 然后将PCR产物连入T载体(pGEM-T Easy Vector), 完成启动子的TA克隆; 将所获得的TA克隆和pCAMBIA1301基本载体用EcoR I/Nco I双酶切, 分别回收小片段和载体片段, 连接完成ProOsSAPP3-GUS双元表达载体的构建。
ProOsSAPP3-GUS转基因拟南芥、35S-OsSAPP3转基因拟南芥、GVG-OsSAPP3转基因拟南芥和对照转基因拟南芥(转化空载体, 以下简称CK)均为Colu- mbia-0生态型背景, 且均在沈阳农业大学水稻研究所拟南芥培养室中进行培养。培养条件: 光周期为16小时光照/8小时黑暗, 温度为(21±1)°C, 光照强度为90 μmol·m-2·s-1。在拟南芥不同生育时期(stage)对其进行表型观察, 各生育时期的表示方法参照已发表文献(Boyes et al., 2001), 并使用相机(Nikon D3200)和扫描仪(EPSON Perfection v33)记录表型。
1.2 生物试剂
实验中所使用的Sma I等所有快切酶均购自NEB公司。pBI121载体、pCAMBIA1301载体和pTA7002载体均购自质粒载体菌种细胞基因保藏中心(Bio- vector)。T载体(pGEM-T Easy Vector)和反转录试剂盒(PrimeScriptTM RT Master Mix)购自Promega公司。荧光定量试剂盒(SYBR? Premix Ex TaqTM)购自大连宝生物公司(TaKaRa)。抗性筛选剂潮霉素(Hyg)购自Roche公司。GUS组织化学染色所用GUS荧光染剂(X-GLUC, sodium salt)购自上海生工生物工程公司。地塞米松诱导剂(Dexamethasone, DEX)和各植物激素购自Simga公司。荧光实时定量RT-PCR引物和克隆引物在Invitrogen公司合成。转基因植物检测引物在苏州金唯智生物科技有限公司合成。1.3 RT-PCR分析
采用Eastep Super总RNA提取试剂盒(Promega, Cat No.LS1040)提取不同叶龄(从移苗第2天开始计算)的GVG-OsSAPP3转基因拟南芥和CK第5和6片莲座叶总RNA。采用PrimeScriptTM RT Master Mix试剂盒(TaKaRa, Cat No.RR036A)将总RNA反转录成cDNA。以TIP41-like作为内参基因, 采用SYBR? Premix Ex TaqTM试剂盒(TaKaRa, Cat No.RR420A)进行实时定量RT-PCR分析。1.4 叶绿素含量检测
将经过DEX诱导处理后正常生长的GVG-OsSAPP3转基因拟南芥和CK第5和6片叶子剪下, 立即称取0.5-1 g, 迅速剪碎置于2.0 mL EP管中, 随后快速向EP管中加入2 mL 95%无水乙醇。用锡箔纸包裹好, 遮光条件下放置1-2天, 浸泡至叶片绿色褪去, 使用多功能酶标仪(购自基因有限公司)测量叶绿素含量。数据分析参照李合生(2000)的方法。1.5 植物激素/地塞米松诱导处理
将苗龄Stage 1.04 (Boyes et al., 2001)的ProOsSAPP3- GUS转基因拟南芥幼苗从基本培养基上转移至含有不同植物激素的1/2MS培养液中处理24小时, 同时转移至只含有10 μmol?L-1无水乙醇(植物激素溶剂)的1/2MS培养液中处理24小时作为MOCK对照。培养条件同1.1节所述, 所用激素处理浓度为10 μmol?L-1 ABA和6-BA。将0.781 6 g地塞米松粉末溶于2 mL无水乙醇溶剂中。将GVG-OsSAPP3转基因拟南芥和转基因拟南芥对照的种子铺于含有10 μmol?L-1地塞米松的诱导培养基上水平培养4天, 使用扫描仪对各相应时间点的表型进行记录。连续3天每天对GVG-OsSAPP3转基因拟南芥和CK的成熟苗喷施含有30 μmol?L-1 DEX/ MOCK诱导剂和0.01%吐温-20的溶液, 使用相机记录转基因成熟苗的表型变化(Aoyama and Chua, 1997)。
1.6 GUS组织化学染色
将在1/2MS潮霉素抗性培养基上培养48小时的ProOsSAPP3-GUS转基因拟南芥种子、培养7天的ProOsSAPP3-GUS转基因拟南芥幼苗(Stage 1.0)和培养14天的ProOsSAPP3-GUS转基因拟南芥幼苗(Stage 1.04)完整取出, 用扫描仪记录染色前形态, 将整株苗浸入GUS染色液中避光染色, 然后对其进行脱色, 用扫描仪记录染色结果。在营养土中培养14天的ProOsSAPP3-GUS转基因拟南芥(Stage 1.10)、培养45天的ProOsSAPP3-GUS转基因拟南芥花序和ProOsSAPP3- GUS转基因拟南芥的成熟果荚染色步骤同上。染色和脱色方法均参考已发表文献(Liu et al., 2010; 徐凡, 2012)。2 结果与讨论
2.1 ProOsSAPP3-GUS转基因拟南芥时空表达模式分析
通过GUS组织化学染色对ProOsSAPP3-GUS转基因拟南芥植株中OsSAPP3启动子的活性进行分析(图1A-E)。我们分别在Stage 1.0和Stage 1.04的ProOs- SAPP3-GUS转基因拟南芥幼苗的子叶、真叶和下胚轴及Stage 1.10的转基因拟南芥莲座叶中检测到Pro- OsSAPP3-GUS的表达活性(图1B), 并且其表达水平随叶龄的增加而逐渐升高, 说明OsSAPP3启动子的活性具有随衰老程度加剧上调的特点。外源施加10 μmol?L-1 ABA和10 μmol?L-1 6-BA处理结果显示, 10 μmol?L-1 ABA处理显著增加ProOsSAPP3-GUS转基因拟南芥幼苗中的GUS活性, 尤其子叶中的GUS活性增加更为明显(图1D); 10 μmol?L-1 6-BA处理显著抑制ProOsSAPP3-GUS转基因拟南芥幼苗中的GUS活性(图1E)。图1
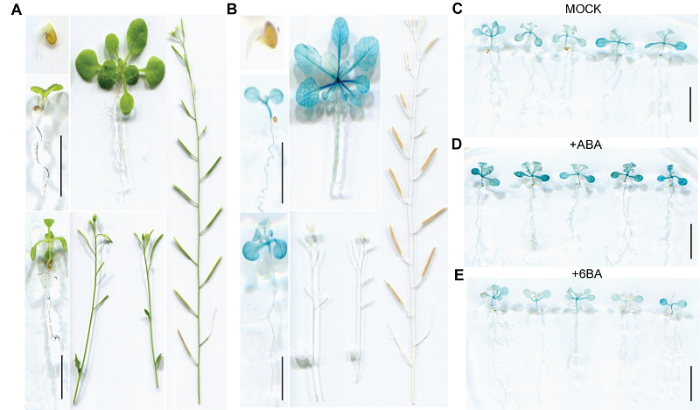 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1ProOsSAPP3-GUS转基因拟南芥不同发育时期GUS组织化学染色
(A) 染色前的转基因拟南芥, 从上到下, 从左到右, 依次为Stage 0.5、Stage 1.02、Stage 1.04、Stage 1.10、Stage 6.0、Stage 6.9; (B) 染色后的转基因拟南芥, 从上到下, 从左到右苗龄同染色前; (C) 经MOCK溶液(激素溶剂)处理的转基因拟南芥; (D) 经10 μmol?L-1 ABA溶液处理的转基因拟南芥; (E) 经10 μmol?L-1 6-BA溶液处理的转基因拟南芥。Bars=5 mm
Figure 1GUS histochemical staining of ProOsSAPP3-GUS transgenic Arabidopsis thaliana at different developmental stages
(A) From top to bottom, from left to right, the transgenic Arabidopsis thaliana before stain: Stage 0.5, Stage 1.02, Stage 1.04, Stage 1.10, Stage 6.0, Stage 6.9; (B) From top to bottom, from left to right, the transgenic Arabidopsis thaliana was stained in turn, and the seedling age was identical to that before staining; (C) Transgenic Arabidopsis thaliana treated with MOCK solution (hormone solvent); (D) Transgenic Arabidopsis thaliana treated with 10 μmol?L-1 ABA solution; (E) Transgenic Arabidopsis thaliana treated with 10 μmol?L-1 6-BA solution. Bars=5 mm
2.2 组成型异源过表达OsSAPP3对转基因拟南芥生长发育的影响
在35S-OsSAPP3转基因拟南芥培养过程中, 发现组成型异源过表达OsSAPP3基因(图2A)导致转基因拟南芥植株生长受到严重抑制, 叶片极小且逐渐出现坏死斑症状, 虽有果荚出现但是无法正常结种(图2B)。研究结果表明, 超量组成型异源过表达OsSAPP3会导致拟南芥植株生长严重受阻, 不能正常发育。图2
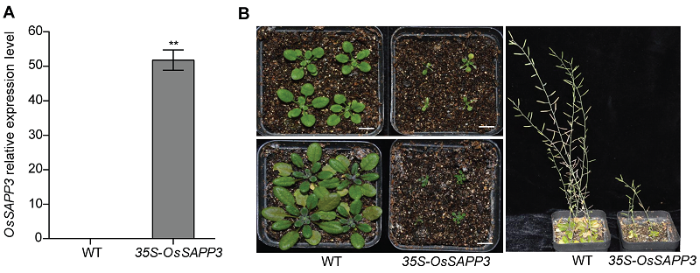 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2组成型异源过表达OsSAPP3基因导致转基因拟南芥生长发育异常
(A) 35S-OsSAPP3转基因与野生型拟南芥的基因表达水平; (B) WT为野生型拟南芥植株; 35S-OsSAPP3为35S-OsSAPP3转基因拟南芥株系; 图左上为营养土中生长14天的幼苗, 图左下为营养土中生长21天的植株, 图右为营养土中培养75天的植株。图中数据为平均值±标准差, n=10, 实验经3次生物学重复。** P<0.01。Bars=1 cm
Figure 2Heterologous expression of OsSAPP3 gene lead to abnormal growth and development of transgenic Arabidopsis
(A) 35S-OsSAPP3 transgenic Arabidopsis and wild-type Arabidopsis gene expression levels; (B) Wild-type Arabidopsis plant (WT) and 35S-OsSAPP3 transgenic Arabidopsis line (35S-OsSAPP3). The top left panel was the 14-day-old seedling growing in the nutrient soil, the bottom left is the 21-day-old plant growing in the nutrient soil, and the right panel was the plants cultured in nutrient soil for 25 days. Values are means±SD of one representative biological replicate (n=10) out of three, ** P<0.01. Bars=1 cm
2.3 可诱导型启动子GVG系统驱动OsSAPP3在拟南芥中异源过表达
为进一步研究OsSAPP3的基因功能, 我们采用可诱导型启动子GVG系统驱动OsSAPP3基因异源过表达, 并获得相应的转基因拟南芥株系。外源DEX诱导后检测OsSAPP3基因的表达情况, 最终获得6个纯合株系(图3L)。在后续实验中选择line2、line14和line20为典型代表株系。我们将3个纯合株系(line2、line14和line20)和CK的种子铺于含有10 μmol?L-1DEX和MOCK溶液的1/2MS固体培养基上进行培养。发现CK的种子正常萌发且幼苗生长正常, 但line2、line14和line20株系的种子萌发率低且缓慢, 已经萌发的幼苗子叶无法张开, 下胚轴无法伸长到正常程度。培养96小时line14株系的幼苗开始死亡; 而line2和line20株系幼苗, 畸形生长, 移入土中后无法存活并最终致死(图3A-I)。图3
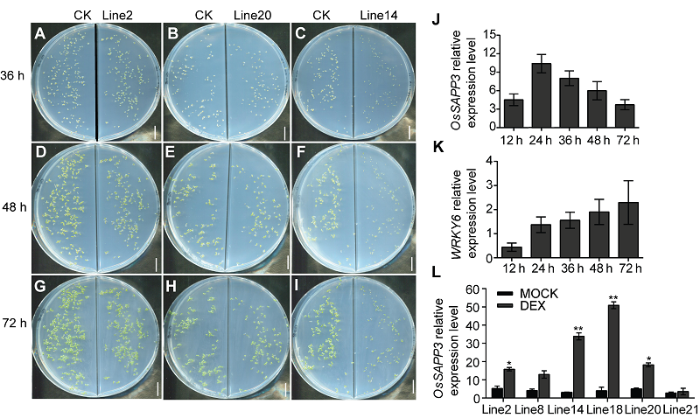 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3外源诱导OsSAPP3异源过表达导致转基因拟南芥无法正常萌发
(A)-(C) DEX诱导36小时, 各转基因种子与对照种子萌发情况; (D)-(F) DEX诱导48小时, 各转基因种子与对照种子萌发情况; (G)-(I) DEX诱导72小时, 各转基因种子与对照种子萌发情况; (J) 外源诱导不同时长, OsSAPP3基因的表达变化情况; (K) 外源诱导不同时长, WRKY6基因的表达变化情况; (L) GVG-OsSAPP3转基因拟南芥纯合株系的基因表达水平。数据为平均值±标准差, n=10, 实验经3次生物学重复。* P<0.05, ** P<0.01。Bars=1 cm
Figure 3Exogenously induced OsSAPP3 heterologous over expression resulted in the inability of germination of transgenic Arabidopsis
(A)-(C) DEX induced 36 h, the germination of transgenic and control seeds; (D)-(F) DEX induced 48 h, the germination of transgenic and control seeds; (G)-(I) DEX induced 72 h, the germination of transgenic and control seeds; (J) The expression of OsSAPP3 induced by exogenous factors at different times; (K) Exogenous induction of WRKY6 expression changes at different times; (L) Gene expression levels of GVG-OsSAPP3 transgenic Arabidopsis homozygous lines. Values are means±SD of one representative biological replicate (n=10) out of three. * P<0.05, ** P<0.01. Bars=1 cm
将正常生长5天的line14幼苗转入到含10 μmol?L-1 DEX的1/2MS固体培养基上进行诱导处理, 利用荧光定量RT-PCR方法分别检测处理12、24、36、48和72小时的幼苗中目的基因OsSAPP3和衰老标志基因WRKY6的表达量。我们发现诱导处理24小时左右, OsSAPP3的表达水平最高, 之后随着诱导时间的延长缓慢下降(图3J); WRKY6的表达量随着诱导时间的延长而上升(图3K)。
2.4 外源诱导OsSAPP3基因异源过表达促进转基因拟南芥早衰
为研究异源过表达OsSAPP3基因对转基因拟南芥生长发育的影响, 我们对正常培养15天的转基因拟南芥喷施30 μmol?L-1 DEX溶液, 连续喷施3天后, 对其进行表型观察。结果显示, GVG-OsSAPP3转基因植株比对照植株莲座叶数目多、叶片小, 且差异显著或极显著(图4A; 表2); 比对照植株提前抽薹、提早开花2-4天(图4B-E)。Table 2
Table 2Phenotypic data of Arabidopsis GVG-OsSAPP3 transgenic lines and control (CK)
| Number of rosette leavesa | 5% significant level | 1% very significant level | Rosette leaf sizeb | 5% significant level | 1% very significant level | |
|---|---|---|---|---|---|---|
| CK | 9.4 | c | A | 2.84 | d | C |
| Line2 | 10.2 | bc | A | 2.3 | ab | BC |
| Line20 | 11.8 | abc | A | 1.9 | bc | AB |
| Line14 | 12.6 | ab | A | 1.5 | cd | A |
新窗口打开|下载CSV
图4
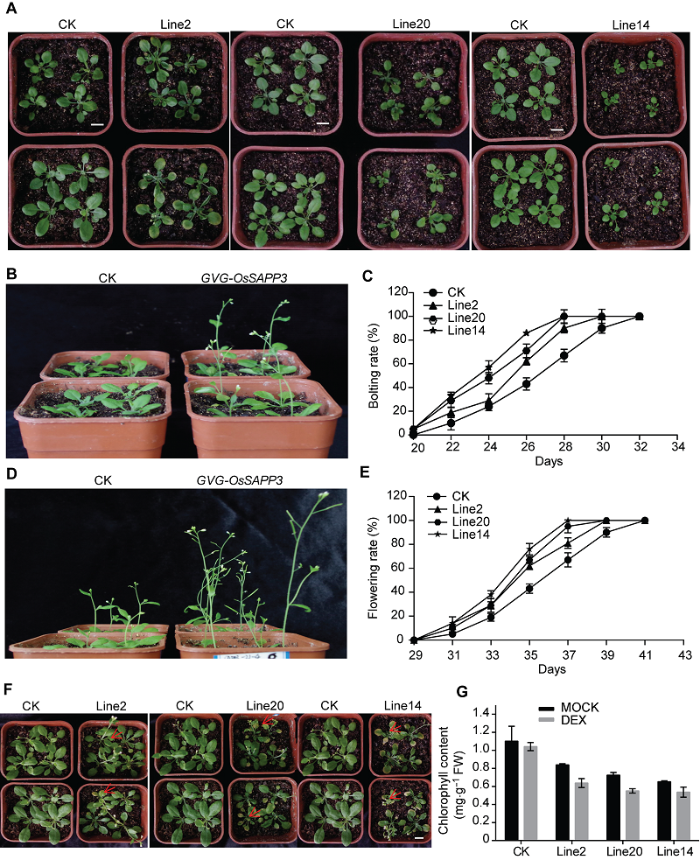 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4外源诱导OsSAPP3基因异源过表达促进转基因拟南芥成熟苗提前衰老
(A) 喷施处理5天后的转化对照(CK)和GVG-OsSAPP3转基因拟南芥; (B) 苗龄24天的CK和GVG-OsSAPP3转基因拟南芥的代表植株; (C) CK和GVG-OsSAPP3转基因拟南芥的抽薹进程; (D) 苗龄34天的CK和GVG-OsSAPP3转基因拟南芥植株; (E) CK和GVG-OsSAPP3转基因拟南芥的开花进程; (F) 喷施处理后21天的CK和GVG-OsSAPP3转基因拟南芥; (G) 苗龄26天的CK和GVG-OsSAPP3转基因拟南芥第4、5和6片叶的叶绿素含量。数据为平均值±标准差, n=10, 实验经3次生物学重复。Bars=1 cm
Figure 4Exogenous-induced OsSAPP3 heterologous over expression promotes premature aging of transgenic Arabidopsis mature seedlings
(A) Arabidopsis control (CK) and GVG-OsSAPP3 transgenic Arabidopsis after spray treatment for 5 days; (B) 24-day-old CK and GVG-OsSAPP3 transgenic Arabidopsis plants; (C) The CK and GVG-OsSAPP3 transgenic Arabidopsis bolting process; (D) 34-day-old CK and GVG-OsSAPP3 transgenic Arabidopsis plants; (E) The CK and GVG-OsSAPP3 transgenic Arabidopsis flowering process; (F) CK and GVG-OsSAPP3 transgenic Arabidopsis after spray treatment for 21 days; (G) The chlorophyll contents of the fourth, fifth and sixth leaf of 26-day-old CK and GVG-OsSAPP3 transgenic Arabidopsis. Values are means±SD of one representative biological replicate (n=10) out of three. Bars=1 cm
诱导处理21天后, GVG-OsSAPP3转基因拟南芥出现明显的衰老表型(图4F), 转基因植株叶片边缘变黄, 且由边缘向中心蔓延, 由老叶向新叶蔓延; 部分老叶已完全衰老黄化, 并且衰老程度与DEX诱导后OsSAPP3表达程度相对应, 即line14株系的衰老程度最高。叶绿素含量测定结果表明, line14、line20与line2的叶绿素含量分别比CK降低50%、47.27%和36.36% (图4G)。
2.5 外源诱导OsSAPP3基因异源过表达影响衰老相关标志基因表达
为进一步研究外源诱导OsSAPP3基因异源过表达对转基因拟南芥中各衰老相关基因和衰老转录因子表达量的影响, 我们利用荧光定量PCR检测外源诱导OsSAPP3基因异源过表达后, 衰老标志基因SAG12, 衰老关键转录因子基因NAC2、NAP和WRKY6, 光合作用相关基因RbcL和RbcS, 以及叶绿素降解相关基因ACD1的表达变化情况(图5A-G)。与对照相比, 异源表达OsSAPP3导致SAG12、NAC2、NAP、WRKY6和ACD1的表达量上升; 而RbcL和RbcS的表达量下降。图5
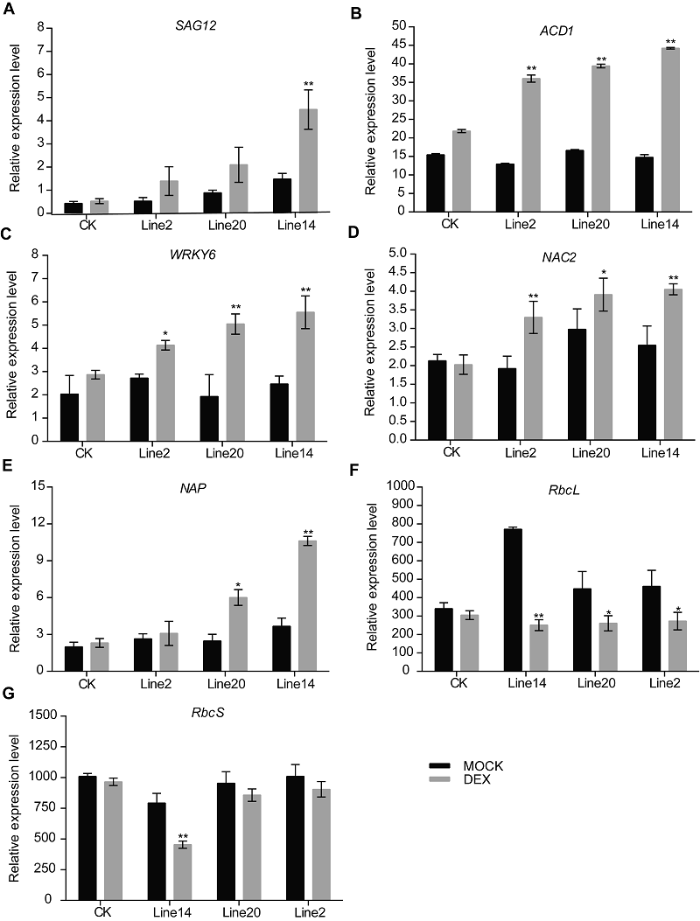 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图530 μmol?L-1 DEX与MOCK溶液处理24小时后, 转化对照(CK)和GVG-OsSAPP3转基因拟南芥第5和6片叶的衰老标志基因表达变化
Figure 5Changes of senescence marker gene expression in the fifth and sixth leaves of control (CK) and GVG-OsSAPP3 transgenic Arabidopsis after 30 μmol?L-1 DEX and MOCK solution treatment for 24 hoursValues are means±SD of one representative biological replicate (n=10) out of three. * P<0.05, ** P<0.01.
数据为平均值±标准差, n=10, 实验经3次生物学重复。* P<0.05, ** P<0.01。
2.6 讨论
牟少亮等(2011)分离出水稻PP2C家族中ABI2的启动子, 并发现OsABI2启动子驱动的GUS报告基因在水稻根、茎和叶中均有表达, 且在根中表达量高、叶中表达量低。本研究中, 我们分离了OsSAPP3的启动子, 发现ProOsSAPP3-GUS在转基因拟南芥不同时期的叶中均有活性, 且活性依赖叶龄方式增加(图1B), 表明OsSAPP3基因参与调控植物叶片的自然衰老过程。虽然PP2C在植物多个生物学过程中起调节作用, 但主要参与ABA信号通路的调节过程。本研究显示, 外源ABA可以显著促进ProOsSAPP3-GUS的表达, 外源6-BA则显著抑制ProOsSAPP3-GUS的表达(图1D, E), 表明ABA是OsSAPP3基因表达的一个正调控因子, OsSAPP3可能在ABA信号通路的下游发挥作用。由此我们推测, ABA与6-BA都可以通过对OsSAPP3表达的协同调控来参与水稻自然衰老和外源激素应答反应的过程。这为深入研究不同激素对增强作物叶片功能和延缓叶片衰老的协同调控作用提供了线索。叶片由绿变黄是叶片衰老终末阶段最显著的形态特征之一, 其根本原因是叶绿素的降解。有研究表明, 玉米叶片中叶绿素降解导致功能叶的叶面积减小, 光合效率和光合能力降低, 从而使玉米的生育周期提前, 并最终影响产量(孙高阳等, 2017)。在拟南芥中, SGR1通过与叶绿体降解酶(CCEs)以及捕光色素复合体结合调控叶片衰老过程(Sakuraba et al., 2012)。本研究表明, 外源诱导OsSAPP3异源过表达导致GVG-OsSAPP3转基因拟南芥成熟苗莲座叶面积极小且数量增多(图4A; 表1); 莲座叶提前黄化(图4F), 叶绿素含量下降(图4G); 抽薹开花均提前2-4天(图4B-E)。我们发现转基因株系叶片形态发育畸形、变黄以及抽薹开花提前等现象的严重程度均与其OsSAPP3基因表达量相对应。以上结果表明, GVG-OsSAPP3转基因拟南芥提前进入衰老程序的终末阶段, 正常的营养生长被破坏, 转基因植株提前从营养生长进入生殖生长阶段。叶片作为水稻重要的源器官, 具有为作物提供各种营养物质的功能; 叶片早衰造成源器官功能期缩短, 导致营养物质缺乏并严重影响籽粒发育和水稻产量(徐娜等, 2017)。本研究显示, OsSAPP3表达量升高使叶片形态发育畸形、萎蔫黄化、叶绿素含量下降显著, 出现叶片早衰表型, 这些功能叶片光合作用能力的丧失将会对产量和品质产生影响。对于叶片衰老进程及调控机制的研究不仅可以使我们了解其生物学过程, 也可以服务于农业生产, 用于改良作物品质和提高作物产量等。例如, 在明恢63水稻品系的剑叶衰老过程中, W-box和G-box顺式作用元件是早期叶片衰老调控的重要正向因子(Liu et al., 2016)。在后续的水稻育种中, 可以通过精确调控OsSAPP3的表达量来提高水稻叶片的光合能力, 减少叶片中叶绿素的降解, 从而提高水稻产量和籽粒品质。
在植物叶片衰老过程中, NAC和WRKY家族中的一些转录因子不可或缺。WRKY转录因子家族中的AtWRKY4、6、11和57是一类重要的SAGs, 也受衰老诱导表达(Jiang et al., 2014)。Robatzek和Somssich (2002)研究发现, 钙调素相关基因和各种激酶(如SIRK)与拟南芥AtWRKY6相互作用从而调节植物的衰老进程。NAC家族中AtNAP的转录水平随着拟南芥叶片衰老而逐渐升高。本研究中, 利用实时荧光定量PCR检测到, GVG-OsSAPP3转基因拟南芥幼苗与成熟苗经地塞米松外源诱导OsSAPP3异源过表达后, SAG12、WRKY6、NAP和NAC2等衰老标志基因均上调表达(图3J, K; 图5)。以上结果表明, 异源过表达OsSAPP3影响其它衰老相关基因的表达, 从而协同调控衰老进程。我们可以通过调控OsSAPP3编码的PP2C蛋白磷酸酶来调节作物的适时衰老, 从而深入研究植物衰老调控过程。同时, GVG-OsSAPP3转基因成熟苗的光合作用相关基因RbcL和RbcS表达下调、叶绿素降解相关基因ACD1表达上调。我们推测异源过表达OsSAPP3影响叶绿体的结构和功能, 导致植物叶片进入衰老阶段, 使转基因植物叶片光合作用减弱, 叶绿素降解加快, 最终表现出叶片提前变黄等早衰表型。以上结果也证实, OsSAPP3编码的PP2C蛋白磷酸酶在植物衰老的生物学过程中起重要调节作用。因此, 我们认为OsSAPP3是植物叶片衰老的正向调控因子, 可促进植物衰老。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.3969/j.issn.1000-2561.2011.12.019URL [本文引用: 1]

分离了水稻PP2C家族中的一个成员OsA BI2的启动子,测序鉴定后构建其GUS融合表达载体,通过农杆菌介导的方法获得水稻转基因植株.GUS蛋白组织化学染色结果表明,OsA BI2启动子驱动的GUS基因在水稻根茎叶中都有表达,在根中表达量最高,叶中表达量最低,而且根部分生组织区明显高于根部其他组织.另外在萌发的芽中也 有明显表达.OsA BI2启动子在T1代转基因植株的诱导表达的GUS蛋白活性分析结果表明,在机械损伤、稻瘟病、干旱逆境和ABA、MeJA和SA处理条件下,OsA BI2启动子驱动的GUS活性都出现了明显上调.上述研究结果说明,OsABI2很可能参与了发育,生物和非生物逆境胁迫的调控.
URL [本文引用: 1]

以玉米杂交种先玉335和郑单958及其亲本为材料,分析了叶片和子粒在授粉后不同时期衰老相关基因表达的变化规律。结果表明,2个叶绿体绿色保持基因SGR1和SGR2在杂交种先玉335和郑单958及其亲本穗三叶中的表达随着授粉后时间的延长整体上呈现上升趋势,但是先玉335的基因表达量要高于郑单958。叶片衰老诱导基因SAP1在叶片发育过程中上调表达,SAP2则表现出先增后减的趋势。以不同生长发育时期的子粒为材料,分析了子粒发育中衰老相关基因SAG1,SAG2和SAG3的表达规律。结果表明,SAG1基因的表达水平随着子粒的发育而降低,而SAG2和SAG3基因则在子粒的成熟过程中表达量呈现上升的趋势。
DOI:10.3969/j.issn.1674-3466.2003.05.013URL [本文引用: 1]

With the discovery of many types of protein phosphatases in plants, recent advances has also been made in elucidating the functions of protein phosphatases modulating plant''s cellular processes. In this paper, the authors present a brief outline of the types and the traits of protein phosphatases, review the progress in identifying and characterizing protein phosphatases, and discuss in detail the protein phosphatase''s roles in many environmental stress events.
URL [本文引用: 1]

衰老是受遗传程序严格控制的植物个体发育过程中的一个必经阶段,由特殊发育信号通过一定的信号传导路径来启动和控制。研究发现,蛋白激酶和蛋白磷酸酶所介导的可逆磷酸化反应在叶片衰老信号传递和衰老的启动和进程控制过程中发挥了重要作用。本文对近年参与叶片衰老调控的蛋白激酶和蛋白磷酸酶基因的分离鉴定及功能研究进行了综述。
URL [本文引用: 1]

叶片衰老是受遗传程序严格控制的、植物个体发育过程中的一个必经阶段,与其它发育过程一样,是由特殊发育信号通过一定的信号传导路径来启动和控制的。植物LRR型类受体蛋白激酶(LRR-RLKs)已被证明参与多种生命活动,并在其中发挥着重要功能。 我们实验室前期研究发现,一个大豆的LRR-RLK,GmSARK,具有双底物特异性,它可以在体外自磷酸化丝/苏氨酸残基和酪氨酸残基;同时发现GmSARK参与大豆叶片衰老的调控,利用RNA干扰技术敲减GmSARK表达可以明显延缓转基因大豆衰老,而利用CaMV35S启动子驱动GmSARK组成型过表达则导致转基因大豆、番茄和拟南芥早衰致死;由于GmSARK-RNAi转基因大豆的花发育异常,具有一个细弯的柱头和不开放的花瓣而导致不育,所以不能使用上述两种转基因材料对GmSARK的功能进行具体分析。为了进一步研究GmSARK的功能及其参与衰老信号调控的分子机制,本文在以前研究的基础上,以GVG系统可诱导型启动子(诱导剂为地塞米松dexamethasone,DEX)控制GmSARK表达的转基因拟南芥为实验材料,对GmSARK所介导的叶片衰老尤其是几种不同的植物激素在其中的调控作用进行了更为深入的分析。 研究发现,外源诱导GmSARK过表达可以导致转基因拟南芥幼苗产生早衰、弯曲根表型;导致成熟转基因拟南芥叶片衰老加速、产生与组成型乙烯超响应突变体类似花等表型;并导致一些衰老关键转录因子AtNAP、NAC1、NAC2和WRKY6等的表达量上升。 在叶片衰老过程中,叶绿体结构与功能的破坏和叶绿素含量的下降是该过程的标志性事件;通过透射电镜观察发现,外源诱导GmSARK过表达可以导致转基因拟南芥叶绿体结构严重破坏,造成其内部简单、类囊体数目明显减少并积累大量淀粉颗粒;利用启动子-报告基因β-葡萄糖醛酸酶(β-glucuronidase,GUS)系统,对三种叶绿体功能缺陷型突变体背景下GmSARK-GUS转基因拟南芥进行分析,发现叶绿体功能缺陷又反馈促进GmSARK-GUS的活性,形成一个滚动环使叶片衰老进入不可逆转的阶段。 利用基因芯片技术进行分析,发现在过表达GmSARK的转基因拟南芥中,有大量激素相关基因的表达发生变化,其中变化最明显的是生长素和乙烯。我们研究了细胞分裂素、生长素和乙烯与GmSARK之间发挥作用的关系。 外源施加细胞分裂素可以有效抑制GmSARK-GUS转基因拟南芥中GmSARK启动子活性,外源施加生长素和乙烯前体ACC都可以促进GmSARK-GUS转基因拟南芥中GmSARK启动子活性;另外,外源诱导GmSARK过表达可以有效降低细胞分裂素合成和信号响应相关基因的表达,可以促进生长素和乙烯的合成和响应相关基因的表达。同时,过表达GmSARK导致转基因拟南芥对外源生长素的敏感性增强;通过检测人工合成的生长素响应元件DR5的表达情况,发现过表达GmSARK不仅改变根中生长素的浓度,还改变了其分布情况。 通过外源激素处理与药物抑制剂结合实验发现,虽然外源细胞分裂素能够强烈抑制GmSARK启动子的活性,但只能部分恢复由GmSARK过表达所造成的幼苗早衰表型,而外源施加生长素作用抑制剂PCIB和乙烯合成抑制剂AVG能够有效恢复由GmSARK过表达造成的早衰表型;PCIB的衰老恢复作用可以被外源ACC所逆转,而AVG的衰老恢复作用不能被外源IAA逆转;同时,我们发现在生长素输入载体突变体aux1-7和乙烯不敏感突变体ein2-1中过表达GmSARK不仅不能引起转基因拟南芥早衰,还可以恢复由GmSARK过表达造成的花发育异常。以上结果表明,生长素和乙烯直接参与GmSARK介导的叶片衰老信号,并在其中发挥十分重要的作用,尤其是生长素促进的乙烯合成增加和信号响应增强是导致这个过程中衰老事件发生和衰老过程加速的关键环节。我们据此提出GmSARK和GmSARK类基因参与叶片衰老调控的模型,并对细胞分裂素、生长素和乙烯三种激素在GmSARK所介导的叶片衰老过程中的协同作用进行了分析和讨论。 我们首次在拟南芥中分离鉴定了GmSARK的同功能基因AtSARK。实验室同期生化实验证明,AtSARK也是一个新的、定位在质膜的、具有双底物特异性的LRR-RLK。定量RT-PCR分析发现,无论是在拟南芥自然衰老还是由GmSARK过表达造成的早衰过程中,AtSARK的表达量都显著上升。通过对AtSARK T-DNA插入突变体和过表达AtSARK转基因拟南芥系统的表型分析发现,敲减AtSARK表达可以有效延缓叶片衰老,而外源诱导AtSARK过表达则可以导致转基因拟南芥早衰并产生异常的、与过表达GmSARK类似的花;同时外源抑制剂处理实验也表明生长素和乙烯直接参与由AtSARK介导的衰老信号通路。因此,我们认为由SARKs介导的衰老信号通路在高等植物中普遍存在。 最后,我们又从拟南芥中分离和鉴定了一个参与叶片衰老负调控的蛋白磷酸酶编码基因,我们将其命名为SSPP。定量RT-PCR分析发现,无论是在拟南芥自然衰老还是由SARKs过表达造成的早衰过程中,SSPP的表达都显著下降。对组成型过表达SSPP的转基因拟南芥进行表型分析,发现其叶片叶绿素含量升高,具有晚花、晚衰、生活史延长等表型;令人兴奋的是,组成型过表达SSPP可以有效逆转由AtSARK过表达造成的转基因拟南芥早衰。此外,我们还发现过表达SSPP可以导致转基因拟南芥的抗干旱能力和对高温敏感性增强。 综上所述,本课题研究了大豆叶片衰老相关的LRR-RLK基因GmSARK及其在拟南芥中同功能基因AtSARK参与叶片衰老调控的分子机制,结合叶片衰老负调控因子、功能未知蛋白磷酸酶编码基因SSPP的初步研究,对揭示高等植物叶片衰老的分子机制和该过程中的信号转导机制具有重要的理论意义。
DOI:10.3969/j.issn.1674-3466.2010.02.002URL [本文引用: 1]

植物LRR型类受体蛋白激酶在 植物生命活动中发挥着重要作用。前期研究发现,大豆(Glycine max)LRR型类受体蛋白激酶基因GmSARK可能参与调控大豆叶片的衰老过程。利用CaMV35S启动子驱动组成型过表达GmSARK基因可导致转基 因植株出现致死表型,据此构建了可诱导型启动子GVG驱动GmSARK基因过表达的双元表达载体,转化野生型拟南芥(Arabidopsis thaliana)并获得了多株转基因植株。研究结果表明,外源施加诱导物地塞米松可引起GmSARK基因在转基因植株中过表达,并导致转基因植株出现叶 片变黄下卷和生长受抑制等表型;外源细胞分裂素处理可以抑制GmSARK的表达,但是不能逆转GmSARK过表达所引起的上述变化。
DOI:10.11983/CBB16222URL [本文引用: 1]

植物叶片衰老是叶片发育的最终阶段,也是植物在长期进化过程中形成的适应性机制。水稻(Oryza sativa)叶片的衰老对其产量和品质影响极大,相关研究主要集中在早衰。该文综述了水稻早衰及其调控基因的研究进展,尤其对水稻叶片早衰的形成原因、发生过程、生理变化及防治措施进行了阐述,以期为深入解析水稻早衰的分子机制奠定理论基础,同时为水稻育种提供参考。
DOI:10.3724/SP.J.1259.2014.00729URL [本文引用: 1]

Leaves are important organs for plant photosynthesis. Their senescence is triggered by external environmental stimuli and internal developing signals, and precisely controlled by complex gene regulatory networks. Recent studies have shown that the ageing process is adjusted at epigenetic level through reprogramming of gene expression. In this review, the epigenetic molecular mechanisms were briefly introduced nd the recent advances in leaf senescence regulation through histone modification, chromatin remodeling and small RNAs pathway were addressed, while the present existing problems and future directions in the field were also discussed.
DOI:10.3969/gab.028.001010URL [本文引用: 1]

水稻叶片的衰老是制约杂交稻产量提高的主要因素之一,有数据表明水稻籽粒灌浆所需营养物质的60%~80%来自叶片的光合作用,实践证明叶片每推迟1天衰老,产量可提高产1%左右。因此,对叶片衰老的形态、生理生化及其相关分子机理等进行研究具有重要的现实意义。近年来水稻叶片衰老的相关研究表明,叶片的衰老是一个受众多因素影响的复杂过程,在这个过程中叶片发生了巨大的形态与生理生化变化,而这些变化均离不开基因的调控作用。大量实验结果表明:在衰老过程中,叶片细胞有选择地启动或增强某些基因(叶片衰老相关基因)的表达,而关闭或减弱另一些基因(衰老下调基因)的表达,由此来调控叶片衰老的进程。目前研究者已在研究衰老突变体等相关的材料中发现了许多与水稻叶片衰老有关的基因。本文重点概述了近年来水稻叶片衰老相关基因的研究状况,并对未来研究方向等问题做了思考与探讨,以期能为开展进一步的研究工作提供参考。
DOI:10.1046/j.1365-313X.1997.11030605.xURLPMID:9107046 [本文引用: 1]

A novel chemical induction system for transcription in plants has been developed, taking advantage of the regulatory mechanism of vertebrate steroid hormone receptors. A chimeric transcription factor, designated GVG was constructed, consisting of the DNA-binding domain of the yeast transcription factor GAL4, the transactivating domain of the herpes viral protein VP16, and the receptor domain of the rat glucocorticoid receptor (GR). The GVG gene was introduced into transgenic tobacco and Arabidopsis together with a luciferase ( Luc ) gene which was transcribed from a promoter containing six tandem copies of the GAL4 upstream activating sequence. Induction of luciferase activity was observed when the transgenic tobacco plants were grown on an agar medium containing dexamethasone (DEX), a strong synthetic glucocorticoid. Induction levels of the luciferase activity were well correlated with DEX concentrations in the range from 0.1 to 10 M and the maximum expression level was over 100 times that of the basal level. Analysis of the induction kinetics by Northern blot analysis showed that the Luc mRNA was first detected 1 h after DEX treatment and increased to the maximum level in 4 h. The stationary induction level and the duration of the induction varied with the glucocorticoid derivative used. The GVG gene activity can also be regulated by DEX in transgenic Arabidopsis plants. The results indicate that a stringent chemical control of transcription can be achieved in plants with the GVG system. Advantages and potential uses of this system are also discussed.
.
DOI:10.1007/s11103-016-0568-2URLPMID:28000033 [本文引用: 1]

Protein phosphatase 2C clade A members are major signaling components in the ABA-dependent signaling cascade that regulates seed germination. To elucidate the role of PP2CA genes in germination of rice seed, we selected OsPP2C51 , which shows highly specific expression in the embryo compared with other protein phosphatases based on microarray data. GUS histochemical assay confirmed that OsPP2C51 is expressed in the seed embryo and that this expression pattern is unique compared with those of other OsPP2CA genes. Data obtained from germination assays and alpha-amylase assays of OsPP2C51 knockout and overexpression lines suggest that OsPP2C51 positively regulates seed germination in rice. The expression of alpha-amylase synthesizing genes was high in OsPP2C51 overexpressing plants, suggesting that elevated levels of OsPP2C51 might enhance gene expression related to higher rates of seed germination. Analysis of protein interactions between ABA signaling components showed that OsPP2C51 interacts with OsPYL/RCAR5 in an ABA-dependent manner. Furthermore, interactions were observed between OsPP2C51 and SAPK2, and between OsPP2C51 and OsbZIP10 and we found out that OsPP2C51 can dephosphorylates OsbZIP10. These findings suggest the existence of a new branch in ABA signaling pathway consisting of OsPYL/RCAR-OsPP2C-bZIP apart from the previously reported OsPYL/RCAR-OsPP2C-SAPK-bZIP. Overall, our result suggests that OsPP2C51 is a positive regulator of seed germination by directly suppressing active phosphorylated OsbZIP10 .
DOI:10.2307/3871382URLPMID:11449047 [本文引用: 2]

With the completion of the Arabidopsis genome sequencing project, the next major challenge is the large-scale determination of gene function. As a model organism for agricultural biotechnology, Arabidopsis presents the opportunity to provide key insights into the way that gene function can affect commercial crop production. In an attempt to aid in the rapid discovery of gene function, we have established a high throughput phenotypic analysis process based on a series of defined growth stages that serve both as developmental landmarks and as triggers for the collection of morphological data. The data collection process has been divided into two complementary platforms to ensure the capture of detailed data describing Arabidopsis growth and development over the entire life of the plant. The first platform characterizes early seedling growth on vertical plates for a period of 2 weeks. The second platform consists of an extensive set of measurements from plants grown on soil for a period of 2 months. When combined with parallel processes for metabolic and gene expression profiling, these platforms constitute a core technology in the high throughput determination of gene function. We present here analyses of the development of wild-type Columbia (Col-0) plants and selected mutants to illustrate a framework methodology that can be used to identify and interpret phenotypic differences in plants resulting from genetic variation and/or environmental stress.
DOI:10.1111/j.1365-313X.2005.02399.xURLPMID:15860015 [本文引用: 1]

An analysis of changes in global gene expression patterns during developmental leaf senescence in Arabidopsis has identified more than 800 genes that show a reproducible increase in transcript abundance. This extensive change illustrates the dramatic alterations in cell metabolism that underpin the developmental transition from a photosynthetically active leaf to a senescing organ which functions as a source of mobilizable nutrients. Comparison of changes in gene expression patterns during natural leaf senescence with those identified, when senescence is artificially induced in leaves induced to senesce by darkness or during sucrose starvation-induced senescence in cell suspension cultures, has shown not only similarities but also considerable differences. The data suggest that alternative pathways for essential metabolic processes such as nitrogen mobilization are used in different senescent systems. Gene expression patterns in the senescent cell suspension cultures are more similar to those for dark-induced senescence and this may be a consequence of sugar starvation in both tissues. Gene expression analysis in senescing leaves of plant lines defective in signalling pathways involving salicylic acid (SA), jasmonic acid (JA) and ethylene has shown that these three pathways are all required for expression of many genes during developmental senescence. The JA/ethylene pathways also appear to operate in regulating gene expression in dark-induced and cell suspension senescence whereas the SA pathway is not involved. The importance of the SA pathway in the senescence process is illustrated by the discovery that developmental leaf senescence, but not dark-induced senescence, is delayed in plants defective in the SA pathway.
DOI:10.1038/s41598-017-03903-6URLPMID:28630437 [本文引用: 1]

Zinc (Zn) depletion adversely affects plant growth. To avoid the lethal depletion of cellular Zn, plants have evolved mechanisms to adjust the expression of genes associated with Zn homeostasis, the details of which are poorly understood. In the present study, we isolated an Arabidopsis thaliana T-DNA insertion mutant that exhibited hypersensitivity to Zn depletion. By monitoring root... [Show full abstract]
DOI:10.1007/s00709-017-1172-2URLPMID:29052008 [本文引用: 1]

Abstract Protein phosphatase 2Cs (PP2Cs) belong to the largest protein phosphatase family in plants. Some members have been described as being negative modulators of plant growth and development, as well as responses to hormones and environmental stimuli. However, little is known about the members of PP2C clade D, which may be involved in the regulation of signaling pathways, especially in response to saline and alkali stresses. Here, we identified 13 PP2C orthologs from the wild soybean (Glycine soja) genome. We examined the sequence characteristics, chromosome locations and duplications, gene structures, and promoter cis-elements of the PP2C clade D genes in Arabidopsis and wild soybean. Our results showed that GsPP2C clade D (GsAPD) genes exhibit more gene duplications than AtPP2C clade D genes. Plant hormone and abiotic stress-responsive elements were identified in the promoter regions of most PP2C genes. Moreover, we investigated their expression patterns in roots, stems, and leaves. Quantitative real-time PCR analyses revealed that the expression levels of representative GsPP2C and AtPP2C clade D genes were significantly influenced by alkali and salt stresses, suggesting that these genes might be associated with or directly involved in the relevant stress signaling pathways. Our results established a foundation for further functional characterization of PP2C clade D genes in the future.
DOI:10.1105/tpc.113.117838URLPMID:24424094 [本文引用: 1]

Abstract Leaf senescence is regulated by diverse developmental and environmental factors. Exogenous jasmonic acid (JA) can induce leaf senescence, whereas auxin suppresses this physiological process. Crosstalk between JA and auxin signaling has been well studied, but not during JA-induced leaf senescence. Here, we found that upon methyl jasmonate treatment, Arabidopsis thaliana wrky57 mutants produced typical leaf senescence symptoms, such as yellowing leaves, low chlorophyll content, and high cell death rates. Further investigation suggested that senescence-associated genes were upregulated in the wrky57 mutants. Chromatin immunoprecipitation experiments revealed that WRKY57 directly binds to the promoters of SENESCENCE4 and SENESCENCE-ASSOCIATED GENE12 and represses their transcription. In vivo and in vitro experiments suggested that WRKY57 interacts with JASMONATE ZIM-DOMAIN4/8 (JAZ4/8) and the AUX/IAA protein IAA29, repressors of the JA and auxin signaling pathways, respectively. Consistent with the opposing functions of JA and auxin in JA-induced leaf senescence, JAZ4/8 and IAA29 also displayed opposite functions in JA-induced leaf senescence and competitively interacted with WRKY57. Our results suggested that the JA-induced leaf senescence process can be antagonized by auxin via WRKY57. Moreover, WRKY57 protein levels were downregulated by JA but upregulated by auxin. Therefore, as a repressor in JA-induced leaf senescence, WRKY57 is a common component of the JA- and auxin-mediated signaling pathways.
DOI:10.1007/BF03030657URL [本文引用: 1]

Leaf senescence is a sequence of biochemical and physiological events comprising the final stage in leaf development. It encompasses the period from a fully expanded mature state up to the death, thereby limiting longevity. The changes occurring during leaf senescence are very complex but highly regulated, and are genetically programmed with actions coordinated at the cellular, tissue, organ, and organism levels. A major breakthrough in our molecular understanding of this phenomenon has been achieved through the characterization of various mutants and senescence-associated genes, including regulatory genes. In particular, a genetic screening and assay system for leaf senescence has been well established in Arabidopsis , which led leaf senescence into the realm of genetic subject along with the rich genetic and genomic resources in this model plant. These advances have not only revealed the existence of a complex regulatory network of senescence-associated signaling pathways, but have also allowed us to postulate the molecular mechanisms for signal perception, execution, and regulation. The key regulatory genes identified to date encode a variety of proteins, including transcription regulators and signal-transduction proteins, regulators of protein degradation, proteins associated with phytohormone pathways, and regulators of metabolism. Elucidation of their roles in leaf senescence and analyses of senescence regulatory pathways, including systems-level approaches, will increase our knowledge of the networks involved in senescence activity.
DOI:10.1016/S1360-1385(03)00103-1URLPMID:12818661 [本文引用: 1]

Leaf senescence is a developmentally programmed degeneration process that constitutes the final step of leaf development and is controlled by multiple developmental and environmental signals. In addition to the information obtained from other plants, Arabidopsis has, as a model system, contributed to our understanding of this complex phenomenon in molecular genetic terms. Recent discoveries have identified several genetic mutants and potential regulatory components in Arabidopsis. Identifying further mutants that exploit novel biological resources, screening Scheme and a global functional analysis of senescence-associated genes in Arabidopsis should increase our understanding of the complex regulatory networks.
DOI:10.1093/jxb/erq033URLPMID:20194926 [本文引用: 1]

The endosymbiont-derived Sec-dependent protein sorting pathway is essential for protein import into the thylakoid lumen and is important for the proper functioning of the chloroplast. Two loss-of-function mutants of cpSecA, the ATPase subunit of the chloroplast Sec translocation machinery, were analysed in Arabidopsis. The homozygous mutants were albino and seedling lethal under autotrophic conditions and remained dwarf and infertile with an exogenous carbon supply. They were subject to oxidative stress and accumulated superoxide under normal lighting conditions. Electron microscopy revealed that the chloroplast of the mutants had underdeveloped thylakoid structures. Histochemical GUS assay of the AtcpSecA::GUS transgenic plants confirmed that AtcpSecA was expressed in green organs in a light-inducible way. Real-time RT-PCR and microarray analysis revealed repressed transcription of nucleus- and chloroplast- encoded subunits of photosynthetic complexes, and induced transcription of chloroplast protein translocation machinery and mitochondrion-encoded respiratory complexes in the mutants. It is inferred that AtcpSecA plays an essential role in chloroplast biogenesis, the absence of which triggered a retrograde signal, eventually leading to a reprogramming of chloroplast and mitochondrial gene expression.
DOI:10.1038/srep20881URLPMID:26864250 [本文引用: 1]

Abstract Plant cis-elements play important roles in global regulation of gene expression. Based on microarray data from rice flag leaves during early senescence, we identified W-box and G-box cis-elements as positive regulators of senescence in the important rice variety Minghui 63. Both cis-elements were bound by leaf senescence-specific proteins in vitro and influenced senescence in vivo. Furthermore, combination of the two elements drove enhanced expression during leaf senescence, and copy numbers of the cis-elements significantly affected the levels of expression. The W-box is the cognate cis-element for WRKY proteins, while the G-box is the cognate cis-element for bZIP, bHLH and NAC proteins. Consistent with this, WRKY, bZIP, bHLH and NAC family members were overrepresented among transcription factor genes up-regulated according during senescence. Crosstalk between ABA, CTK, BR, auxin, GA and JA during senescence was uncovered by comparing expression patterns of senescence up-regulated transcription factors. Together, our results indicate that hormone-mediated signaling could converge on leaf senescence at the transcriptional level through W-box and G-box elements. Considering that there are very few documented early senescence-related cis-elements, our results significantly contribute to understanding the regulation of flag leaf senescence and provide prioritized targets for stay-green trait improvement.
DOI:10.1016/S1360-1385(00)01655-1URLPMID:10871899 [本文引用: 1]

Senescence is the last stage of leaf development and one type of programmed cell death that occurs in plants. The relationships among senescence programs that are induced by a variety of factors have been addressed at a molecular level in recent studies. Furthermore, an overlap between the pathogen-response and senescence programs is beginning to be characterized. The complexity of the senescence program is also evident in studies of senescence-specific gene regulation and the role of photosynthesis and plant hormones in senescence regulation. New molecular-genetic approaches are expected to be useful in unraveling the molecular mechanisms of the leaf senescence program.
.
DOI:10.1101/gad.222702URLPMID:12000796

Abstract In Arabidopsis, WRKY factors comprise a large gene family of plant-specific transcriptional regulators controlling several types of plant stress responses. To understand the regulatory role of WRKY proteins during such processes, we identified targets of the senescence- and defense-associated WRKY6 factor. WRKY6 was found to suppress its own promoter activity as well as that of a closely related WRKY family member, indicating negative autoregulation. On the other hand, WRKY6 positively influenced the senescence- and pathogen defense-associated PR1 promoter activity, most likely involving NPR1 function. One novel identified target gene, SIRK, encodes a receptor-like protein kinase, whose developmental expression is strongly induced specifically during leaf senescence. The transcriptional activation of SIRK is dependent on WRKY6 function. Senescing leaves of wrky6 knockout mutants showed a drastic reduction, and green leaves of WRKY6 overexpression lines showed clearly elevated SIRK transcript levels. Furthermore, the SIRK gene promoter was specifically activated by WRKY6 in vivo, functioning very likely through direct W-box interactions.
[本文引用: 1]
DOI:10.1105/tpc.106.049585URLPMID:17630279 [本文引用: 1]

Wound signaling pathways in plants are mediated by mitogen-activated protein kinases (MAPKs) and stress hormones, such as ethylene and jasmonates. In Arabidopsis thaliana, the transmission of wound signals by MAPKs has been the subject of detailed investigations; however, the involvement of specific phosphatases in wound signaling is not known. Here, we show that AP2C1, an Arabidopsis Ser/Thr phosphatase of type 2C, is a novel stress signal regulator that inactivates the stress-responsive MAPKs MPK4 and MPK6. Mutant ap2c1 plants produce significantly higher amounts of jasmonate upon wounding and are more resistant to phytophagous mites (Tetranychus urticae). Plants with increased AP2C1 levels display lower wound activation of MAPKs, reduced ethylene production, and compromised innate immunity against the necrotrophic pathogen Botrytis cinerea. Our results demonstrate a key role for the AP2C1 phosphatase in regulating stress hormone levels, defense responses, and MAPK activities in Arabidopsis and provide evidence that the activity of AP2C1 might control the plant's response to B. cinerea.
.
DOI:10.1016/j.plaphy.2017.08.025URLPMID:28926798 [本文引用: 1]

Plant SNF1-related protein kinase 2 (SnRK2) and protein phosphatase 2C (PP2C) family members are core components of the ABA signal transduction pathway. SnRK2 and PP2C proteins have been suggested to play crucial roles in fruit ripening and improving plant tolerance to drought stress, but supporting genetic information has been lacking in sweet cherry ( Prunus avium L.). Here, we cloned six full-length SnRK2 genes and three full-length PP2C genes from sweet cherry cv. Hong Deng. Quantitative PCR analysis revealed that PacSnRK2.2 , PacSnRK2.3 , PacSnRK2.6 , and PacPP2C13 were negatively regulated in fruits in response to exogenous ABA treatment, PacSnRK2.4 and PacSnRK2.5 were upregulated, and PacSnRK2.1 expression was not affected. The ABA treatment also significantly promoted the accumulation of anthocyanins in sweet cherry fruit. The expression of all PacSnRK2 and PacPP2C genes was induced by dehydration stress, which also promoted the accumulation of drought stress signaling molecules in the sweet cherry fruits, including ABA, soluble sugars, and anthocyanin. Furthermore, a yeast two-hybrid analysis demonstrated that PacPP2C1 interacts with all six PacSnRK2s, while PacPP2C3 does not interact with PacSnRK2.5. PacPP2C2 does not interact with PacSnRK2.1 or PacSnRK2.4. These results indicate that PacSnRK2s and PacPP2Cs may play a variety of roles in the sweet cherry ABA signaling pathway and the fruit response to drought stress.
DOI:10.1007/s00425-017-2704-xURLPMID:28474114 [本文引用: 1]

ABP9, encoding a bZIP transcription factor from maize, enhances tolerance to multiple stresses and may participate in the ABA signaling pathway in transgenic cotton by altering physiological and bioch
DOI:10.1371/journal.pone.0161203URLPMID:27532299 [本文引用: 1]

D1 protein in the PSII reaction center is the major target of photodamage, and it exhibits the highest turnover rate among all the thylakoid proteins. In this paper, ricepsf(premature senescence of flag leaves) mutant and its wild type were used to investigate the genotype-dependent alteration in PSII photo-damage and D1 protein turnover during leaf senescence and its relation to ABA accumulation in senescent leaves. The symptom and extent of leaf senescence of thepsfmutant appeared to be sunlight-dependent under natural field condition. Thepsfalso displayed significantly higher levels of ABA accumulation in senescent leaves than the wild type. However, the premature senescence lesion ofpsfleaves could be alleviated by shaded treatment, concomitantly with the strikingly suppressed ABA level in the shaded areas of flag leaves. The change in ABA concentration contributed to the regulation of shade-delayed leaf senescence. The participation of ABA in the timing of senescence initiation and in the subsequent rate of leaf senescence was closely associated with PSII photodamage and D1 protein turnover during leaf senescence, in which the transcriptional expression of several key genes (psbA,psbB,psbCandOsFtsH2) involved in D1 protein biosynthesis and PSII repair cycle was seriously suppressed by the significantly increased ABA level. This response resulted in the low rate of D1 protein synthesis and impaired repair recovery in the presence of ABA. Thepsfshowed evidently decreased D1 protein amount in the senescent leaves. Both the inhibition of de novo synthesized D1 protein and the slow rate of proteolytic removal for the photodamaged D1 protein was among the most crucial steps for the linkage between light-dependent leaf senescence and the varying ABA concentration inpsfmutant leaves.OsFtsH2transcriptional expression possibly played an important role in the regulation of D1 protein turnover and PSII repair cycle in relation to ABA mediated leaf senescence.
DOI:10.1104/pp.15.01112URLPMID:26304848 [本文引用: 1]

Abstract Reversible protein phosphorylation mediated by protein kinases and phosphatases plays an important role in the regulation of leaf senescence. We previously reported that the leucine-rich repeat receptor-like kinase SENESCENCE-ASSOCIATED RECEPTOR-LIKE KINASE (AtSARK) positively regulates leaf senescence in Arabidopsis (Arabidopsis thaliana). Here, we report the involvement of a protein serine/threonine phosphatase 2C-type protein phosphatase, SENESCENCE-SUPPRESSED PROTEIN PHOSPHATASE (SSPP), in the negative regulation of Arabidopsis leaf senescence. SSPP transcript levels decreased greatly during both natural senescence and SARK-induced precocious senescence. Overexpression of SSPP significantly delayed leaf senescence in Arabidopsis. Protein pull-down and bimolecular fluorescence complementation assays demonstrated that the cytosol-localized SSPP could interact with the cytoplasmic domain of the plasma membrane-localized AtSARK. In vitro assays showed that SSPP has protein phosphatase function and can dephosphorylate the cytosolic domain of AtSARK. Consistent with these observations, overexpression of SSPP effectively rescued AtSARK-induced precocious leaf senescence and changes in hormonal responses. All our results suggested that SSPP functions in sustaining proper leaf longevity and preventing early senescence by suppressing or perturbing SARK-mediated senescence signal transduction. 2015 American Society of Plant Biologists. All Rights Reserved.
DOI:10.1104/pp.111.182899URLPMID:3327223 [本文引用: 1]

As the last stage of leaf development, senescence is a fine-tuned process regulated by interplays of multiple signaling pathways. We have previously identified soybean (Glycine max) SENESCENCE-ASSOCIATED RECEPTOR-LIKE KINASE (SARK), a leucine-rich repeat-receptor-like protein kinase from soybean, as a positive regulator of leaf senescence. Here, we report the elucidation of the molecular mechanism of GmSARK-mediated leaf senescence, especially its specific roles in senescence-inducing hormonal pathways. A glucocorticoid-inducible transcription system was used to produce transgenic Arabidopsis (Arabidopsis thaliana) plants for inducible overexpression of GmSARK, which led to early leaf senescence, chloroplast destruction, and abnormal flower morphology in Arabidopsis. Transcript analyses of the GmSARK-o ver expressing seedlings revealed a multitude of changes in phytohormone synthesis and signaling, specifically the repression of cytokinin functions and the induction of auxin and ethylene pathways. Inhibition of either auxin action or ethylene biosynthesis alleviated the senescence induced by GmSARK. Consistently, mutation of either AUXIN RESISTANT1 or ETHYLENE INSENSITIVE2 completely reversed the GraSARK-induced senescence. We further identified a homolog of GmSARK with a similar expression pattern in Arabidopsis and named it AtSARK. Inducible overexpression of AtSARK caused precocious senescence and abnormal floral organ development nearly identical to the GmSARK-overexpressing plants, whereas a T-DNA insertion mutant of AtSARK showed significantly delayed senescence. A kinase assay on recombinant catalytic domains of GmSARK and AtSARK revealed that these two leucine-rich repeat-receptor-like protein kinases autophosphorylate on both serine/threonine and tyrosine residues. We inferred that the SARK-mediated pathway may be a widespread mechanism in regulating leaf senescence.
DOI:10.1186/1471-2164-9-550URLPMID:2612031

pAbstract/p pBackground/p pThe protein phosphatase 2Cs (PP2Cs) from various organisms have been implicated to act as negative modulators of protein kinase pathways involved in diverse environmental stress responses and developmental processes. A genome-wide overview of the PP2C gene family in plants is not yet available./p pResults/p pA comprehensive computational analysis identified 80 and 78 PP2C genes in itArabidopsis thaliana /it(AtPP2Cs) and itOryza sativa /it(OsPP2Cs), respectively, which denotes the PP2C gene family as one of the largest families identified in plants. Phylogenic analysis divided PP2Cs in Arabidopsis and rice into 13 and 11 subfamilies, respectively, which are supported by the analyses of gene structures and protein motifs. Comparative analysis between the PP2C genes in Arabidopsis and rice identified common and lineage-specific subfamilies and potential gene birth-and-death events. Gene duplication analysis reveals that whole genome and chromosomal segment duplications mainly contributed to the expansion of both OsPP2Cs and AtPP2Cs, but tandem or local duplication occurred less frequently in Arabidopsis than rice. Some protein motifs are widespread among the PP2C proteins, whereas some other motifs are specific to only one or two subfamilies. Expression pattern analysis suggests that 1) most PP2C genes play functional roles in multiple tissues in both species, 2) the induced expression of most genes in subfamily A by diverse stimuli indicates their primary role in stress tolerance, especially ABA response, and 3) the expression pattern of subfamily D members suggests that they may constitute positive regulators in ABA-mediated signaling pathways. The analyses of putative upstream regulatory elements by two approaches further support the functions of subfamily A in ABA signaling, and provide insights into the shared and different transcriptional regulation machineries in dicots and monocots./p pConclusion/p pThis comparative genome-wide overview of the PP2C family in Arabidopsis and rice provides insights into the functions and regulatory mechanisms, as well as the evolution and divergence of the PP2C genes in dicots and monocots. Bioinformatics analyses suggest that plant PP2C proteins from different subfamilies participate in distinct signaling pathways. Our results have established a solid foundation for future studies on the functional divergence in different PP2C subfamilies./p
[本文引用: 1]
DOI:10.1073/pnas.1522840113URLPMID:26831097 [本文引用: 1]

Drought stress is an important environmental factor limiting plant productivity. In this study, we screened drought-resistant transgenic plants from 65 promoter-pyrabactin resistance 1-like (PYL) abscisic acid (ABA) receptor gene combinations and discovered that pRD29A::PYL9 transgenic lines showed dramatically increased drought resistance and drought-induced leaf senescence in both Arabidopsis and rice. Previous studies suggested that ABA promotes senescence by causing ethylene production. However, we found that ABA promotes leaf senescence in an ethylene-independent manner by activating sucrose nonfermenting 1-related protein kinase 2s (SnRK2s), which subsequently phosphorylate ABA-responsive element-binding factors (ABFs) and Related to ABA-Insensitive 3/VP1 (RAV1) transcription factors. The phosphorylated ABFs and RAV1 up-regulate the expression of senescence-associated genes, partly by up-regulating the expression of Oresara 1. The pyl9 and ABA-insensitive 1-1 single mutants, pyl8-1pyl9 double mutant, and snrk2.2/3/6 triple mutant showed reduced ABA-induced leaf senescence relative to the WT, whereas pRD29A::PYL9 transgenic plants showed enhanced ABA-induced leaf senescence. We found that leaf senescence may benefit drought resistance by helping to generate an osmotic potential gradient, which is increased in pRD29A::PYL9 transgenic plants and causes water to preferentially flow to developing tissues. Our results uncover the molecular mechanism of ABA-induced leaf senescence and suggest an important role of PYL9 and leaf senescence in promoting resistance to extreme drought stress.
0
水稻蛋白磷酸酶ABI2基因启动子的克隆和功能分析
1
2011
...
玉米衰老相关基因在2个杂交种及其亲本中的表达分析
1
2017
... 叶片由绿变黄是叶片衰老终末阶段最显著的形态特征之一, 其根本原因是叶绿素的降解.有研究表明, 玉米叶片中叶绿素降解导致功能叶的叶面积减小, 光合效率和光合能力降低, 从而使玉米的生育周期提前, 并最终影响产量(
植物蛋白磷酸酶及其在植物抗逆中的作用
1
2003
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
叶片衰老过程中的蛋白激酶和蛋白磷酸酶
1
2014
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
GmSARK和AtSARK基因调控叶片衰老分子机制的研究
1
2012
... 将在1/2MS潮霉素抗性培养基上培养48小时的ProOsSAPP3-GUS转基因拟南芥种子、培养7天的ProOsSAPP3-GUS转基因拟南芥幼苗(Stage 1.0)和培养14天的ProOsSAPP3-GUS转基因拟南芥幼苗(Stage 1.04)完整取出, 用扫描仪记录染色前形态, 将整株苗浸入GUS染色液中避光染色, 然后对其进行脱色, 用扫描仪记录染色结果.在营养土中培养14天的ProOsSAPP3-GUS转基因拟南芥(Stage 1.10)、培养45天的ProOsSAPP3-GUS转基因拟南芥花序和ProOsSAPP3- GUS转基因拟南芥的成熟果荚染色步骤同上.染色和脱色方法均参考已发表文献(
大豆诱导型启动子驱动类受体蛋白激酶GmSARK转基因植物分析
1
2010
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
水稻叶片早衰成因及分子机理研究进展
1
2017
... 叶片由绿变黄是叶片衰老终末阶段最显著的形态特征之一, 其根本原因是叶绿素的降解.有研究表明, 玉米叶片中叶绿素降解导致功能叶的叶面积减小, 光合效率和光合能力降低, 从而使玉米的生育周期提前, 并最终影响产量(
植物叶片衰老的表观遗传调控
1
2014
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
水稻叶片衰老相关基因的研究进展
1
2009
... 水稻(Oryza sativa)叶片衰老相关研究显示, 水稻衰老过程中会产生大量的水解酶类(
A glucocorticoid-mediated transcriptional induction system in transgenic plants
1
1997
... 将0.781 6 g地塞米松粉末溶于2 mL无水乙醇溶剂中.将GVG-OsSAPP3转基因拟南芥和转基因拟南芥对照的种子铺于含有10 μmol?L-1地塞米松的诱导培养基上水平培养4天, 使用扫描仪对各相应时间点的表型进行记录.连续3天每天对GVG-OsSAPP3转基因拟南芥和CK的成熟苗喷施含有30 μmol?L-1 DEX/ MOCK诱导剂和0.01%吐温-20的溶液, 使用相机记录转基因成熟苗的表型变化(
The protein phosphatase 2C clade a protein OsPP2C51 positively regulates seed germination by directly inactivating OsbZIP10
1
2017
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants
2
2001
... ProOsSAPP3-GUS转基因拟南芥、35S-OsSAPP3转基因拟南芥、GVG-OsSAPP3转基因拟南芥和对照转基因拟南芥(转化空载体, 以下简称CK)均为Colu- mbia-0生态型背景, 且均在沈阳农业大学水稻研究所拟南芥培养室中进行培养.培养条件: 光周期为16小时光照/8小时黑暗, 温度为(21±1)°C, 光照强度为90 μmol·m-2·s-1.在拟南芥不同生育时期(stage)对其进行表型观察, 各生育时期的表示方法参照已发表文献(
... 将苗龄Stage 1.04 (
Comparative transcriptome analysis reveals significant differences in gene expression and signaling pathways between developmental and dark/ starvation-induced senescence in Arabidopsis
1
2005
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
Phylogenetic analysis of F-bZIP transcription factors indicates conservation of the zinc deficiency response across land plants
1
2017
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
Genome-wide analysis and expression profiling of PP2C clade D under saline and alkali stresses in wild soybean and Arabidopsis
1
2018
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid- induced leaf senescence
1
2014
... 在植物叶片衰老过程中, NAC和WRKY家族中的一些转录因子不可或缺.WRKY转录因子家族中的AtWRKY4、6、11和57是一类重要的SAGs, 也受衰老诱导表达(
Aging and senescence of the leaf organ
1
2007
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
Molecular genetics of leaf senescence in Arabidopsis
1
2003
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
cpSecA, a thylakoid protein translocase subunit, is essential for photosynthetic development in Arabidopsis
1
2010
... 将在1/2MS潮霉素抗性培养基上培养48小时的ProOsSAPP3-GUS转基因拟南芥种子、培养7天的ProOsSAPP3-GUS转基因拟南芥幼苗(Stage 1.0)和培养14天的ProOsSAPP3-GUS转基因拟南芥幼苗(Stage 1.04)完整取出, 用扫描仪记录染色前形态, 将整株苗浸入GUS染色液中避光染色, 然后对其进行脱色, 用扫描仪记录染色结果.在营养土中培养14天的ProOsSAPP3-GUS转基因拟南芥(Stage 1.10)、培养45天的ProOsSAPP3-GUS转基因拟南芥花序和ProOsSAPP3- GUS转基因拟南芥的成熟果荚染色步骤同上.染色和脱色方法均参考已发表文献(
W-box and G-box elements play important roles in early senescence of rice flag leaf
1
2016
... 叶片由绿变黄是叶片衰老终末阶段最显著的形态特征之一, 其根本原因是叶绿素的降解.有研究表明, 玉米叶片中叶绿素降解导致功能叶的叶面积减小, 光合效率和光合能力降低, 从而使玉米的生育周期提前, 并最终影响产量(
Cell cycle-regulated gene expression in Arabidopsis
0
2002
Molecular aspects of leaf senescence
1
2000
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
Targets of AtWRKY6 regulation during plant senescence and pathogen defense
0
2002
STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex ii for chlorophyll detoxification during leaf senescence in Arabidopsis
1
2012
... 叶片由绿变黄是叶片衰老终末阶段最显著的形态特征之一, 其根本原因是叶绿素的降解.有研究表明, 玉米叶片中叶绿素降解导致功能叶的叶面积减小, 光合效率和光合能力降低, 从而使玉米的生育周期提前, 并最终影响产量(
The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis
1
2007
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
Cloning and expression profiling of the PacSnRK2 and PacPP2C gene families during fruit development, ABA treatment, and dehydration stress in sweet cherry
1
2017
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
ABP9, a maize bZIP transcription factor, enhances tolerance to salt and drought in transgenic cotton
1
2017
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
Involvement of abscisic acid in PSII photodamage and D1 protein turnover for light-induced premature senescence of rice flag leaves
1
2016
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
Senescence-suppressed protein phosphatase directly interacts with the cytoplasmic domain of senescence-associated receptor-like kinase and negatively regulates leaf senescence in Arabidopsis
1
2015
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
A soybean dual-specificity kinase, GmSARK, and its Arabidopsis homolog, AtSARK, regulate leaf senescence through synergistic actions of auxin and ethylene
1
2011
... 水稻(Oryza sativa)叶片衰老相关研究显示, 水稻衰老过程中会产生大量的水解酶类(
Genome-wide and expression analysis of protein phosphatase 2C in rice and Ara- bidopsis
0
2008
An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves
1
2012
... PP2C磷酸酶是高等植物中存在的最大蛋白磷酸酶家族, 它是1种丝氨酸/苏氨酸残基蛋白磷酸酶(
ABA receptor PYL9 promotes drought resistance and leaf senescence
1
2016
... 生理和遗传学研究表明, 植物叶片衰老是一个受调控的细胞程序性死亡过程(PCD), 包含诸多有序事件的发生(
备案号: 京ICP备16067583号-21
版权所有 © 2021 《植物学报》编辑部
地址:北京香山南辛村20号 邮编:100093
电话:010-62836135 010-62836131 E-mail:cbb@ibcas.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
