

引用本文
贡献者
基金资助
接受日期:2017-11-19网络出版日期:2018-11-1
-->Copyright
2018《植物学报》编辑部
Contributors
History
Received:Online:
摘要:
Abstract:
Key words:
在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(Har- berd et al., 2009; Ueguchi-Tanaka and Matsuoka, 2010; Sun, 2010)。作为植物特有的转录因子, DEL- LA蛋白属于GRAS (GAI、RGA和SCARECROW)超家族中的一类(Pysh et al., 1999)。DELLA基因在不同植物中高度保守, 目前已在多种植物中克隆了该基因(图1A)。SLN1 (SLENDER1)和SLR1 (SLENDER RICE1)分别是大麦(Hordeum vulgare)和水稻(Oryza sativa)中唯一的DELLA基因(Ikeda et al., 2001; Fu et al., 2002)。已知拟南芥(Arabidopsis thaliana)中存在5个DELLA基因, 即GAI (GA-INSENSITIVE)、RGA (REPRESSOR OF GA1-3)、RGL1 (RGA-LIKE1)、RGL2和RGL3 (Peng et al., 1997; Dill et al., 2001; Wen and Chang, 2002; Lee et al., 2002)。研究表明DELLA广泛参与植物营养生长、抗逆、花器官发育和种子萌发等生命过程(Fleet and Sun, 2005; 姚涛等, 2011)。
图 1
Figure 1
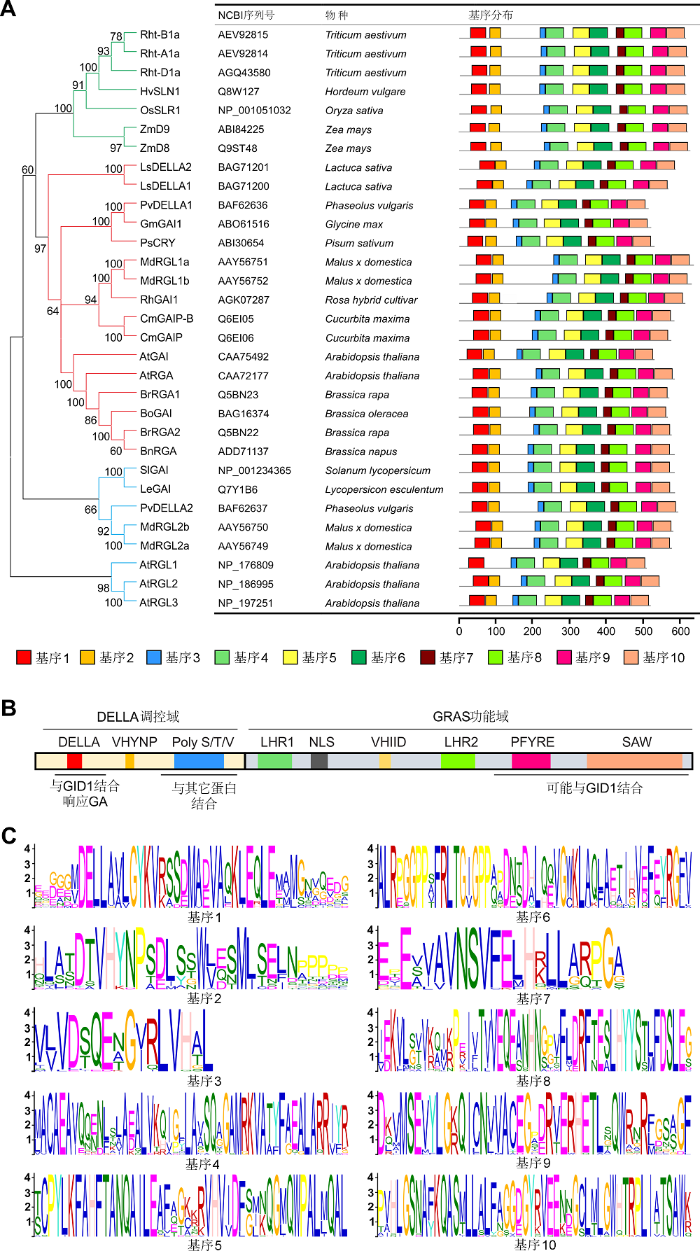
下载原图ZIP
生成PPT
图 1
已报道的植物DELLA蛋白功能域、家族进化树和保守基序分布
(A) DELLA蛋白的结构域和相关功能示意图。(B) DELLA蛋白家族进化树和保守基序分布。DELLA蛋白分为3类, 其中单子叶植物一类, 双子叶植物两类。使用Mega 5.1软件中的邻接法分析构建进化树。使用在线工具MEME (http://meme.nbcr.net)完成基序分析。(C) 通过MEME分析发现10个保守基序。DELLA蛋白保守域均包括在内: DELLA、LHRs、VHIID、PFYRE和SAW。
Figure 1
Functional domains, phylogenetic tree and conversed motif location of DELLAs reported in plant
(A) Diagram of the structural domain and relative functions of DELLA protein. (B) DELLAs are classified into 3 categories: one clade is monocot, the other two are dicots. The tree is calculated with Mega 5.1 software using the Neighbor-joining method. Motifs in DELLA protein sequences were identified with the MEME tool (http://meme.nbcr.net). (C) 10 discovered motifs of DELLA protein via MEME tool. DELLAs include all the conserved motifs: DELLA, LHRs, VHIID, PFYRE and SAW.
DELLA蛋白作为转录因子, 在靶基因的转录激活或抑制过程中起调控作用(黄先忠等, 2006; Thomas et al., 2016)。DELLA通常需要与其它调控因子共同行使调控功能, 主要有以下3种具体方式 (Thomas et al., 2016)。(1) 竞争性结合复合体中的其它成员, 进而阻止转录激活; (2) 竞争性结合复合体中的DELLA蛋白, 进而解除转录抑制; (3) 促进DELLA蛋白更好地结合到转录复合体中, 促进转录。在GA-DELLA降解模型中, GA结合到其受体GID1 (GA-INSENSITIVE DWARF1)上与DELLA互作, 然后通过26S蛋白酶途径降解DELLA蛋白(Sun, 2011)。此外, Conti等(2014)还发现了通过翻译后修饰调节DELLA蛋白水平的途径。
被子植物的有性生殖过程极其复杂且受到精确调控以保证传宗接代。各种信号分子参与调节其中的重要发育过程。大量的研究表明, GA参与从雌雄配子体发育、花粉管萌发及生长、双受精完成到胚胎发育整个过程。DELLA作为GA信号转导路径中的主要转录调节因子, 在GA参与调节的有性生殖过程中起着不可或缺的作用。本文概述并讨论了近10年来DELLA在植物有性生殖过程中的表达与功能, 旨在梳理共识, 开启思路。
1 DELLA蛋白的结构和特性DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(Silverstone et al., 1998; Hedden and Thomas, 2012)。该结构域突变会导致DELLA的抑制作用消失, 进而使植株长得更高且更加细长。在不同的DELLA突变体(如大麦sln1和水稻slr1)中, 都可观察到该突变表型(Ikeda et al., 2001; Itoh et al., 2002; Chandler et al., 2002)。DELLA蛋白中部有1个核定位信号结构域NLS (Nuclear Localization Signal)、1个保守氨基酸结构域(VHIID)和2个亮氨酸重复序列(LHR1和LHR2)。C端的PFYRE和SAW结构域(Bolle, 2004)与动物的STAT转录因子同源(Richards et al., 2000; Levy and Darnell, 2002)。大多数GRAS蛋白间接调控基因转录, 而豆科中的NSP1 (NODULATION SIGNALING PATHWAY1)可直接结合DNA (Hirsch et al., 2009)。最近, 对水稻SCL7-SCL7 (SCARECROW-LIKE7)同源二聚体晶体结构的解析, 发现其具有DNA结合功能(Li et al., 2016)。这暗示了该类蛋白不仅存在一种调控方式。N端的DELLA调控域(图1B, C)包括DELLA、VHYNP (或TVHYNP)和poly- S/T/V基序。这些结构域的缺失导致DELLA对GA的应答消失, 从而增强了其抑制作用以及出现半矮化表型(Itoh et al., 2002)。拟南芥突变体gai-1的DELLA/ LExLE域缺失了17个氨基酸, 导致其对GA不敏感和矮化等性状(Koornneef et al., 1985; Peng et al., 1997)。在小麦(Triticum aestivum)和玉米(Zea mays)中也发现了GAI和RGA突变体有类似的矮化表型(Silverstone et al., 1997; Peng et al., 1997, 1999)。该突变体的矮化性状能增加小麦和玉米的产量, “绿色革命”也曾利用矮化表型赋予的抗倒伏性状提高了水稻产量(Allan, 1986)。
对DELLA的鉴定一般采用以下3个标准: (1) 氨基酸序列特征为N端具DELLA结构域和C端有GRAS; (2) 对GA响应, 即用GA处理后, DELLA蛋白降解; (3) 依赖于GA与GID1的结合。但这些标准并非绝对。例如, AtGAI和AtRGL1用GA处理后不降解(Fleck and Harberd, 2002; Wen and Chang, 2002)。基于上述特征, DELLA长期以来被用作显示GA的标记, 即没有DELLA蛋白的组织有GA存在(Dorcey et al., 2009)。该方法的局限性在于DELLA本身在不同组织中的转录水平差异极大, 且DELLA在蛋白水平上也受到调控, 因而, 无法准确示踪活性GA。加之GA广泛存在于植物的各种组织中, 这也给分析DELLA蛋白的表达模式带来困难。最近, Rizza等(2017)利用光生物传感方法, 基于荧光共振能量转移(Fluores- cence Resonance Energy Transfer, FRET)技术与GID1-GA-DELLA互作产生构象变化, 开发了一种高分辨率量化且具有生物活性GA时空分布的生物传感器GPS1 (GIBBERELLIN PERCEPTION SENSOR 1)。利用GPS1能活体示踪不同组织中的GA分布, 并利于进一步探究DELLA蛋白在GA信号转导途径中的精确调控作用。
2 DELLA在雄性生殖器官中的作用赤霉素在雄蕊发育过程中有3个显著作用, 即调控花药中花粉的发育、花药的开裂和花粉管的伸长(Cheng et al., 2004)。单子叶植物大麦sln1和水稻slr1突变体表现为雄性育性缺陷。大麦sln1无花粉产生(Foster, 1977)。水稻SLR功能获得性突变体Slr1-d3抑制GA的作用(Chhun et al., 2007)。与野生型相比, Slr1-d3花粉形态近乎正常, 但花粉活力较低。授粉后, Slr1-d3花粉萌发率较低, 但花粉管生长不受影响, 仍能穿过柱头抵达胚珠。说明Slr1-d3育性缺陷表现为花粉活性降低, 而非影响花粉管的伸长(Chhun et al., 2007)。目前, 水稻中关于GA信号途径影响花药绒粘层细胞程序性死亡(programmed cell death, PCD)和花粉外壁形成的研究已非常深入。GA诱导SLR1降解, 解除其对转录因子GAMYB (GA- and ABA-regulated MYB)的抑制。一方面, GAMYB激活CYP703A3 (cytochrome P450 hydroxylase)、KAR (B-ketoacyl reductase)和其它合成孢粉素(花粉外壁和乌氏体的必要组成物质)的基因; 另一方面, GAMYB激活GA上调基因, 如花药绒粘层PCD相关的蛋白酶和蛋白酶抑制剂基因(Aya et al., 2009)。拟南芥Ler (Landsberg erecta)生态型的global-DELLA (gai-t6/rga-t2/rgl1-1/rgl2-1/rgl3-4)突变体对雄性育性几乎无影响(Fuentes et al., 2012)。值得一提的是, 在拟南芥Col (Columbia)背景下, rga-28/gai-td1双突变体则表现为完全雄性不育, 花粉母细胞减数分裂后异常且不能产生可育花粉。这可能是拟南芥2种生态型DELLA蛋白调控不同的下游或DELLA表达模式不同所致(Plackett et al., 2014)。
拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(Cheng et al., 2004; Yu et al., 2004)。拟南芥中RGA下调基因分布在花器官的各个部分, 但超过1/3在雄蕊中特异表达或优势表达(Hou et al., 2008)。其中一些下调基因的同源基因与雄蕊发育相关。例如, MS2 (MALE STERILITY2)影响花粉壁的形成, MS5影响减数分裂(Aarts et al., 1997; Glover et al., 1998)。在体外用GA处理后, 拟南芥花粉管生长受到抑制, 过量GA信号不利于其生长(Singh et al., 2002)。与其它细胞的顶端极性生长机制不同, GA调节的花粉管生长依赖于DELLA介导的信号转导(Hepler et al., 2001; Swain et al., 2004)。
最近有研究表明, 在GA处理下小孢子母细胞减数分裂中胞质分离受到影响, 花粉体积变大并产生二倍体花粉。rga-24/gai-t6突变体小孢子母细胞减数分裂过程中表现出相同的表型(Liu et al., 2017)。因为突变体rga-24/gai-t6花粉变大的频率仅为3.38%, 所以对育性并无影响。该突变体小孢子母细胞减数分裂的染色体行为正常, 但减数分裂MII (meiosis II)末期形成类成膜体的辐射微管阵列(Radial Microtubule Arrays, RMAs)的空间排布时受到影响。DELLA下调基因MYB33和MYB65参与RMAs的形成和减数分裂中的胞质分离。之前的研究发现, 因为GA-DELLA信号途径影响微管的排布, 所以GA-DELLA-GAYMB信号途径调节小孢子母细胞减数分裂MII的RMA排布和胞质分离(Locascio et al., 2013)。由于尚未对DELLA调控周质微管稳定性的机制进行更深入的研究, 目前仍未明确DELLA是否通过相同的调控方式影响减数分裂中RMAs的形成。在GA处理的植株及rga-24/gai-t6突变体中并未观察到有丝分裂细胞骨架异常引起的细胞分裂缺陷现象(Liu et al., 2017)。因此, 在体细胞和减数分裂期的花粉母细胞中微管可能存在不同的应答机制。值得一提的是, RGA和GAMYB在花药体细胞组织中(包括绒粘层)的表达水平很高, 在小孢子母细胞和小孢子中不表达或者表达水平很低。这表明绒毡层不仅影响花粉壁的形成, 而且影响减数分裂末期的微管形态。
3 DELLA在雌性生殖器官中的作用植物的雌蕊结构复杂, 完成双受精后形成果实。雌蕊包括柱头、花柱和子房。雌蕊的正常发育和形成保证了花粉与柱头识别后萌发, 及花粉管顺利进入胚珠、完成双受精和形成种子。用GA处理会改变雌蕊的形态, 说明GA参与调控雌蕊的发育。Fukazawa等(2017)的研究表明, DELLA参与GA合成的正向调控, DELLA缺失会导致体内GA含量降低(Hadden and Phillips, 2000)。Fuentes等(2012)研究发现, 拟南芥global-DELLA突变体的花柱长度和柱头宽度增加, 用GA处理野生型去雄后的柱头也能得到与global- DELLA类似的表型, 说明GA-DELLA信号途径调控柱头和花柱的生长发育。此外, 拟南芥quadruple- DELLA (gai-t6/rga-t2/rgl1-1/rgl2-1)突变体会发生不依赖于受精的子房膨大, 且rgl3-4不引起子房生长缺陷, 说明RGL3在决定子房长度的功能上与其它DEL- LA功能冗余(Dorcey et al., 2009)。番茄(Solanum lycopersicum)单一DELLA基因突变体 pro (procera)也有类似的表型。pro花柱变长, 柱头凸起, 无法完成自然授粉。另外, 与GA处理后的表型类似, 人工去雄后pro子房自发膨大, 这是由于细胞数目更多, 体积更大(Martl et al., 2007)。然而, 并非所有植物DELLA突变都会导致雌蕊发育缺陷。例如, 水稻Slr1-d3虽表现出育性缺陷, 但柱头和花柱形态正常(Chhun et al., 2007)。
被子植物的子房包裹着1个或多个胚珠。胚珠中胚囊的合点-珠孔轴具有明显的极性。典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞。目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用。对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(Yu et al., 2005; Schmidt et al., 2011)。另外, 无论用激光切割关键的胚囊细胞, 还是胚囊缺失突变体的转录谱, 均暗示GA合成和信号转导途径在其中起作用(Yu et al., 2005; Johnston et al., 2007; Jones-Rhoades et al., 2007; Steffan et al., 2007; Wuest et al., 2010)。原位杂交证明, RGL1在未成熟胚珠的外珠被中表达(Wen and Chang, 2002)。Gomez等(2016)发现, 拟南芥GAI、RGA、RGL1和RGL2在胚珠的发育过程中表达且具有不同的表达模式。GAI主要在珠柄表达, 在珠被原基和珠心组织的中心也有表达; RGA和RGL1在珠柄和珠被原基表达; RGL2在珠心组织优势表达, 在珠柄和珠被原基也有表达。相应地, RGA和GAI通过与转录因子ATS (ABERRANT TESTA SHAPE)互作, 调控拟南芥珠被的发育(Gomez et al., 2016)。GA-DELLA信号途径在胚囊的发育过程中具体起何种作用尚有待进一步探索。
4 DELLA在植物双受精过程中的作用花粉管穿过助细胞进入胚珠, 释放2个精子, 分别与中央细胞以及卵细胞结合形成胚乳和胚。Smyth等(1990)对拟南芥角果各个发育时期进行了划分。Fuentes等(2012)检测了受精前到角果非伸长期间(11-17b期) DELLA的转录水平, 发现在该过程中, DELLA具有不同的表达模式, 大致可分为以下3类。(1) RGA在各个时期都维持在较高的表达水平; (2) GAI、RGL1和RGL2在受精之前(11-12期)和角果伸长(15-17a)期表达水平相对较高, 角果不再伸长时(17b期)迅速下降; (3) RGL3在受精前表达量较高(11-12期), 受精后迅速下降, 角果成熟时(17b期)再次升高。尽管转录水平的调节只是DELLA蛋白活性众多调控机制中的一种, 但在受精前后可能起重要作用(Achard and Genschik, 2009)。Figueiredo等(2016)利用RGA::GFP:RGA示踪活性GA, 发现受精前珠被GFP信号很强, 受精后迅速消失。以上说明胚珠在受精后很可能激活GA的合成, GA参与珠被的发育, 但是否还参与其它生命活动尚不明了。拟南芥中花粉管接触到助细胞时, 助细胞会产生Ga2+的波动; 且在花粉管破裂后精细胞与中央细胞和卵细胞进行胞质融合时, 中央细胞和卵细胞中会产生一个短时间的Ga2+峰值, 卵细胞完成受精后又会出现1次Ga2+峰值(Denninger et al., 2014; Hamamura et al., 2014)。然而在玉米体外受精实验中, 精卵融合后的50分钟内并未产生第2次Ga2+峰值, 说明植物体内存在某种机制诱发卵细胞产生第2次Ga2+浓度升高。Okada等(2017)研究表明, Ga2+作为第二信使, 细胞内诱发其浓度升高的因素有多种。用GA处理细胞1分钟后液泡中Ga2+浓度显著升高, 而胚珠GA的合成又在受精后被激活。GA信号通路与双受精之间的潜在联系值得深入探究。
5 DELLA在胚胎发育中的作用卵细胞受精形成合子后, 经过1次不均等分裂形成顶、基细胞。随后顶细胞经过多次分裂和分化形成胚的主体, 而基细胞经过几次分裂形成胚柄。在合子的极性建立过程中, 细胞骨架微管的动态变化起着重要作用。卵细胞受精时, 微管的排列模式被打乱, 受精后会重新排布, 在合子接近顶端的位置形成新的横向环状微管(Kimata et al., 2016)。Locascio等(2013)研究发现, 赤霉素通过DELLA蛋白调控前折叠蛋白复合体(Prefoldin, PFD)来影响微管的行为。无GA的情况下, DELLA在细胞核中与PFD直接作用, 抑制PFD定位到胞质中促进微管的成束作用; GA存在的情况下, GA促使DELLA蛋白降解, 释放PFD复合物到胞质中, 促进微管蛋白二聚体的产生, 利于形成横向周质微管和细胞扩张。而受精后激活GA的合成, 是否对合子特定的微管排布起调控作用仍然未知。
拟南芥Ler生态型不同DELLA多突变体的确存在不同程度的胚珠败育(Gomez et al., 2016)。然而, rga-24/rgl1-1/rgl2-1、gai-t6/rgl1-1/rgl2-1和gai-t6/ rga-24/rgl2-1突变体并未出现胚珠败育。gai-t6/rga- 24/rgl1-1三突变体胚珠少部分变白。quadruple- DELLA突变体胚珠异常进一步增加。global-DELLA突变体胚珠异常率高达60%。异常胚珠白色且体积较小, 其珠被仅有3或4层细胞, 而野生型有5层珠被细胞(外珠被2层, 内珠被3层)。虽然体积较小的种子仍能萌发, 但是global-DELLA中胚胎发育模式是否正常尚未见报道。DELLA在胚胎发育中的作用是否类似于花药中不同生态型之间存在差异尚待进一步探索。
6 结语DELLA蛋白作为GA应答调控途径中的关键分子参与植物的多种发育过程。关于营养生长过程中GA- DELLA信号调控途径的蛋白质结构基础和分子调控机制已研究得十分深入(Murase et al., 2008)。在水稻和小麦等谷类作物中相关研究甚至对作物改良起到了明显的推动作用(Van De Velde et al., 2017)。近10年来, DELLA在雌雄生殖器官发育过程中的生物学功能研究取得了丰硕成果(图2), GA调控植物有性生殖的分子机制开始备受重视。与在植物营养生长中的研究相比, GA-DELLA信号调控途径在有性生殖中的作用研究仍很薄弱, 特别是对雌雄配子体发育、受精和胚胎发生过程的研究仍非常少。在这些有性生殖过程的关键发育环节, 对DELLA的精细表达模式和生物学功能了解几近空白。主要原因可能有以下4个方面。(1) 这些重要发育过程发生在层层体细胞组织之中, 难以观察。(2) 配子体乃至幼胚细胞数量少且难以获得, 相关研究必须基于少量的细胞展开。这对于检测GA含量限制极大, 难以获得可信数据。(3) 上述生殖发育过程快速且精巧, 故GA或DELLA的分布与含量必然呈现高度动态变化。而长期以来, 一直缺乏有效的实时观测系统, 无法精细把握其动态变化过程, 也就无法评估GA-DELLA信号调控途径在这些关键发育节点的瞬时作用。(4) 体内研究GA对DELLA蛋白的降解作用, 必须结合转录水平才能确定DELLA蛋白真正起功能的组织和细胞。
图 2
Figure 2
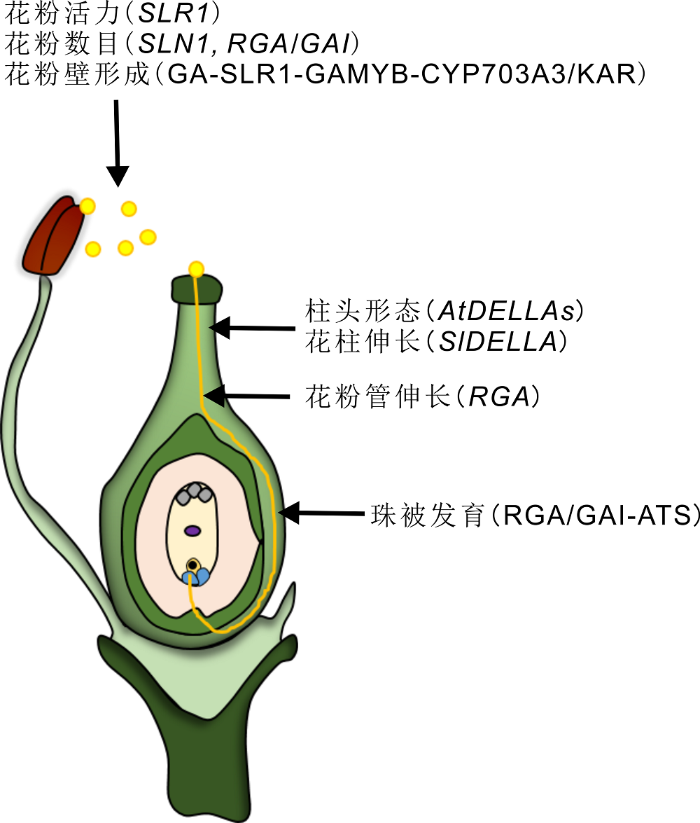
下载原图ZIP
生成PPT
图 2
DELLA蛋白在被子植物有性生殖中的作用
DELLA蛋白在植物有性生殖过程中起着至关重要的作用, 包括: 花粉活力、数目、花粉壁的形成以及花粉管的伸长, 柱头的形态建成和花柱伸长, 珠被的发育。
Figure 2
The role of DELLA protein in sexual reproduction of angiosperm
DELLAs play a key role in plant sexual reproduction including pollen viability and quantity, pollen wall formation, the growth of pollen tube, stigma morphogenesis, style elongation and integument development.
尽管如此, 近年的研究表明, GA-DELLA信号途径在绒毡层与小孢子母细胞的胞间通讯、胚囊形成过程中孢子体组织与配子体间的互作、受精过程中钙动态的调控、合子分裂面的选择与细胞命运决定等过程中的作用可能是未来的研究方向。GPS1的出现促进了对DELLA的深入研究。相信未来几年上述问题将会受到越来越多的关注。
参考文献
文献选项
原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | DOI:10.3969/j.issn.1674-3466.2006.05.006URLThe phytohormone gibberellins (GA) play an important role in controlling and modulating diverse developmental processes, such as seed germination, hypocotyls elongation, leaf expansion and flowering time.In recent years, there are significant progresses in understanding of GA biosynthesis pathway and GA signaling in Arabidopsis and rice. This review highlights GA biosynthesis pathway and their regulation, including a new pathway identified in rice, and molecular model of“ De-repress” in GA signaling. GA promotes plant growth via 26S proteasome-dependent proteolysis of DELLA proteins repressors, one of the key component in GAsignaling, and also depends on GA-mediated interaction between GA receptor and DELLA proteins. Finally, this paper discussed the cross-talking between GA and other hormones, and modulation of adaptation to environments. [本文引用: 1] |
| [2] | DOI:10.3724/SP.J.1259.2011.00481URLFive members of DELLAs, important plant growth repressors in the gibberellin pathway, have been described in Arabidopsis. DELLAs play an important role in stress tolerance. A low concentration of nitric oxide (NO) can promote plant growth and development, and a high concentration can inhibit plant growth, even cause cell death. We studied the effect of NO at a high concentration in Arabidopsis seedlings by treatment with the NO donor sodium nitroprusside (SNP), and the results indicate that the cell death caused by NO is correlated with HO accumulation. Study of phenotypes of serially DELLA-deleted mutants and DELLA gene expression in response to NO revealed that DELLAs contribute greatly to tolerance to NO stress in Arabidopsis seedlings. Thus, we reveal the relationship between DELLA and NO stress in such seedlings, which can contribute to agricultural production. [本文引用: 1] |
| [3] | DOI:10.1046/j.1365-313X.1997.d01-8.xPMID:9351246URLThe Arabidopsis thaliana MALE STERILITY 2 ( MS2 ) gene product is involved in male gametogenesis. The first abnormalities in pollen development of ms2 mutants are seen at the stage in microsporogenesis when microspores are released from tetrads. Expression of the MS2 gene is observed in tapetum of wild-type flowers at, and shortly after, the release of microspores from tetrads. The MS2 promoter controls GUS expression at a comparable stage in the tapetum of transgenic tobacco containing an MS2 promoter鈥揋US fusion. The occasional pollen grains produced by mutant ms2 plants have very thin pollen walls. They are also sensitive to acetolysis treatment, which is a test for the presence of an exine layer. The MS2 gene product shows sequence similarity to a jojoba protein that converts wax fatty acids to fatty alcohols. A possible function of the MS2 protein as a fatty acyl reductase in the formation of pollen wall substances is discussed. [本文引用: 1] |
| [4] | DOI:10.1093/jxb/ern301PMID:19043067URLBioactive gibberellins (GAs) are tetracyclic diterpenoid plant hormones that promote important processes of plant growth and development, such as seed germination, growth through elongation, and floral transition. Thus, mutant plants that are affected in GA biosynthesis or signalling exhibit altered seed germination and, at the adult stage, are dwarf and dark green and also show delayed flowering. The components of the GA metabolism and signalling pathways are reviewed here and recent findings regarding the regulation and possible mode of action of DELLA proteins are discussed. [本文引用: 1] |
| [5] | DOI:10.2135/cropsci1986.0011183X002600040014xURLThe two most commonly used reduced-height genes of wheat (Triticum aestivum L.) are Rht1, and Rht2. The Rht3, gene is known to be a potent height-reducing gene. Information is limited as to the comparative effects of these three genes on agronomic traits, especially when placed in a common genetic background. This study made multiple site-year comparisons in Washington for grain yield, plant height, volume weight, kernel weight, and tiller number among five reduced-height, near-isogenic lines and their recurrent parent, ‘Burt’. One line had the Rht3, gene. One line each had the Rht1, and Rht2, genes combined from ‘Norin 10’ and from ‘Suwon 92’. One line each had either the Rht1 or Rht2, gene separately from these two sources. Plant heights of lines with either Rht1, or Rht2, alone, Rht1, and Rht2, combined, and with Rht3, were reduced 24, 52, and 64%, respectively. The Rht3, line had the lowest (P<0.05) across-test mean yield of 3360 kg ha–1. The two lines with both Rht1, and Rht2, exceeded (P<0.05) the yield of the Rht3, line with means of 3660 and 3840 kg ha–1; these values were less (P<0.05) than the Burt mean yield of 4440 kg ha–1 as well as the yields (4300 and 4900 kg ha–1) of lines with either Rht1 or Rht2. The Suwon 92 derived line with either Rht1 or Rht2, exceeded (P<0.05) the mean yields of other lines and Burt by 10 to 46%. The Rht3 line had significantly lower (P<0.05) mean volume weight, kernel weight, and tiller number than the other lines and Burt. Reductions in these traits of the Rht3 line compared to Burt were 11, 31, and 11% respectively. Lines with one or both Rht1 and Rht2, genes had lower (P<0.05) kernel weights than Burt. All Rht genes reduced volume weight. Although Rht3, is an effective height-reducing gene, it caused negative effects on yield, volume weight, kernel weight, and tiller number. Single dosages of Rht1, or Rht2, caused neutral or positive effects on yield and tiller number, and warrant use in breeding. [本文引用: 1] |
| [6] | DOI:10.1105/tpc.108.062935URL [本文引用: 1] |
| [7] | DOI:10.1007/s00425-004-1203-zPMID:14760535URLGRAS proteins are a recently discovered family of plant-specific proteins named after GAI, RGA and SCR, the first three of its members isolated. Although the Arabidopsis genome encodes at least 33 GRAS protein family members only a few GRAS proteins have been characterized so far. However, it is becoming clear that GRAS proteins exert important roles in very diverse processes such as signal transduction, meristem maintenance and development. Here we present a survey of the different GRAS proteins and review the current knowledge of the function of individual members of this protein family. [本文引用: 1] |
| [8] | DOI:10.1104/pp.010917URLA dominant dwarf mutant of barley (Hordeum vulgare) that resembles dominant gibberellin (GA) “-insensitive” or “-nonresponsive” mutants in other species is described. α-Amylase production by endosperm half-grains of the mutant required GA3 at concentrations about 100 times that of the WT. The mutant showed only a slight growth response to GA3, even at very high concentrations. However, when additionally dwarfed, growth rate responded to GA3over the normal concentration range, although only back to the original (dwarf) elongation rate. Genetic studies indicated that the dominant dwarf locus was either closely linked or identical to theSln1 (Slender1) locus. A barley sequence related to Arabidopsis GAI/RGA was isolated, and shown to represent the Sln1 locus by the analysis ofsln1 mutants. The dominant dwarf mutant was also altered in this sequence, indicating that it too is an allele atSln1. Thus, mutations at Sln1 generate plants of radically different phenotypes; either dwarfs that are largely dominant and GA “-insensitive/-nonresponsive,” or the recessive slender types in which GA responses appear to be constitutive. Immunoblotting studies showed that in growing leaves, SLN1 protein localized almost exclusively to the leaf elongation zone. In mutants at the Sln1 locus, there were differences in both the abundance and distribution of SLN1 protein, and large changes in the amounts of bioactive GAs, and of their metabolic precursors and catabolites. These results suggest that there are dynamic interactions between SLN1 protein and GA content in determining leaf elongation rate. [本文引用: 1] |
| [9] | DOI:10.1242/dev.00992URL [本文引用: 2] |
| [10] | DOI:10.1105/tpc.107.054759URL [本文引用: 3] |
| [11] | DOI:10.1016/j.devcel.2013.12.004URL |
| [12] | DOI:10.1038/ncomms5645PMID:25145880URLAbstract Cell-cell communication and interaction is critical during fertilization and triggers free cytosolic calcium ([Ca2+]cyto) as a key signal for egg activation and a polyspermy block in animal oocytes. Fertilization in flowering plants is more complex, involving interaction of a pollen tube with egg adjoining synergid cells, culminating in release of two sperm cells and their fusion with the egg and central cell, respectively. Here, we report the occurrence and role of [Ca2+]cyto signals during the entire double fertilization process in Arabidopsis. [Ca2+]cyto oscillations are initiated in synergid cells after physical contact with the pollen tube apex. In egg and central cells, a short [Ca2+]cyto transient is associated with pollen tube burst and sperm cell arrival. A second extended [Ca2+]cyto transient solely in the egg cell is correlated with successful fertilization. Thus, each female cell type involved in double fertilization displays a characteristic [Ca2+]cyto signature differing by timing and behaviour from [Ca2+]cyto waves reported in mammals. [本文引用: 1] |
| [13] | DOI:10.1073/pnas.251534098URL [本文引用: 1] |
| [14] | DOI:10.1111/j.1365-313X.2008.03781.xPMID:19207215URLAbstract Fruit development is usually triggered by ovule fertilization, and it requires coordination between seed development and the growth and differentiation of the ovary to host the seeds. Hormones are known to synchronize these two processes, but the role of each hormone, and the mechanism by which they interact, are still unknown. Here we show that auxin and gibberellins (GAs) act in a hierarchical scheme. The synthetic reporter construct DR5:GFP showed that fertilization triggered an increase in auxin response in the ovules, which could be mimicked by blocking polar auxin transport. As the application of GAs did not affect auxin response, the most likely sequence of events after fertilization involves auxin-mediated activation of GA synthesis. We have confirmed this, and have shown that GA biosynthesis upon fertilization is localized specifically in the fertilized ovules. Furthermore, auxin treatment caused changes in the expression of GA biosynthetic genes similar to those triggered by fertilization, and also restricted to the ovules. Finally, GA signaling was activated in ovules and valves, as shown by the rapid downregulation of the fusion protein RGA-GFP after pollination and auxin treatment. Taken together, this evidence suggests a model in which fertilization would trigger an auxin-mediated promotion of GA synthesis specifically in the ovule. The GAs synthesized in the ovules would be then transported to the valves to promote GA signaling and thus coordinate growth of the silique. [本文引用: 2] |
| [15] | DOI:10.7554/eLife.20542PMID:27848912URL10.7554/eLife.20542.001In flowering plants, seed development is initiated by the fusion of the maternal egg and central cells with two paternal sperm cells, leading to the formation of embryo and endosperm, respectively. The fertilization products are surrounded by the maternally derived seed coat, whose development prior to fertilization is blocked by epigenetic regulators belonging to the Polycomb Group (PcG) protein family. Here we show that fertilization of the central cell results in the production of auxin and most likely its export to the maternal tissues, which drives seed coat development by removing PcG function. We furthermore show that mutants for the MADS-box transcription factor AGL62 have an impaired transport of auxin from the endosperm to the integuments, which results in seed abortion. We propose that AGL62 regulates auxin transport from the endosperm to the integuments, leading to the removal of the PcG block on seed coat development.DOI: http://dx.doi.org/10.7554/eLife.20542.001 [本文引用: 1] |
| [16] | DOI:10.1046/j.1365-313X.2002.01478.xPMID:12492836URLSummary Plant growth is regulated by bioactive gibberellin (GA), although there is an unexplained diversity in the magnitude of the GA responses exhibited by different plant species. GA acts via a group of orthologous proteins known as the DELLA proteins. The Arabidopsis genome contains genes encoding five different DELLA proteins, the best known of which are GAI and RGA. The DELLA proteins are thought to act as repressors of GA-regulated processes, whilst GA is thought to act as a negative regulator of DELLA protein function. Recent experiments have shown that GA induces rapid disappearance of nuclear RGA, SLR1 and SLN1 (DELLA proteins from rice and barley), suggesting that GA signalling and degradation of DELLA proteins are coupled. However, RGL1, another Arabidopsis DELLA protein, does not disappear from the nucleus in response to GA treatment. Here, we present evidence suggesting that GAI, like RGL1, is stable in response to GA treatment, and show that transgenic Arabidopsis plants containing constructs that enable high-level expression of GAI exhibit a dwarf, GA non-responsive phenotype. Thus, GAI appears to be less affected by GA than RGA, SLR1 or SLN1. We also show that neither of the two putative nuclear localisation signals contained in DELLA proteins are individually necessary for nuclear localisation of GAI. The various DELLA proteins have different properties, and we suggest that this functional diversity may explain, at least in part, why plant species differ widely in their GA response magnitudes. [本文引用: 1] |
| [17] | DOI:10.1016/j.pbi.2004.11.015PMID:15653404URLThe importance of gibberellin (GA) in vegetative and reproductive development has been known for some time. Recent studies have uncovered new roles of GA in leaf differentiation, photomorphogenesis and pollen-tube growth. Significant contributions to our understanding of GA-regulated morphogenesis include the identification of upstream regulators of GA biosynthesis, the elucidation of the function of GA signaling components, and the isolation of downstream targets. In addition, the mechanisms of interactions between GA and other hormone pathways are beginning to be revealed at the molecular level. [本文引用: 1] |
| [18] | [本文引用: 1] |
| [19] | DOI:10.1105/tpc.006197URL [本文引用: 1] |
| [20] | DOI:10.1105/tpc.112.103192URL [本文引用: 2] |
| [21] | DOI:10.1104/pp.17.00282URL |
| [22] | DOI:10.1046/j.1365-313X.1998.00216.xPMID:9750346URLSummary In this paper, we describe the cloning of the MS5 gene, a gene essential for male fertility in Arabidopsis . We previously defined the MS5 locus by characterizing an EMS‐induced allele, ms5–1 . We identified a new allele of MS5 ( ms5–2 ) that was T‐DNA‐generated and used the T‐DNA tag to clone the gene. Sequencing of mutant and wild‐type alleles together with complementation of the ms5–1 mutant phenotype with a wild‐type genomic clone confirmed the identity of the gene. Differences between the phenotypes of the two mutant alleles could be attributed to differences in mutant gene structure. The semi‐dominant and dominant negative phenotypes of the ms5–2 mutant probably result from production of a truncated polypeptide. An unknown locus in Landsberg erecta can counteract the dominant negative phenotype of ms5–2 . Mutations in MS5 cause the formation of ‘polyads’– tetrads with more than four pools of chromosomes after male meiosis. Similarities between the MS5 sequence and that of a number of proteins were found; two that may be significant were with a synaptonemal complex protein and with a regulatory subunit of a cyclin‐dependent kinase. The MS5 gene is a member of a small gene family highly conserved amongst plant species. [本文引用: 1] |
| [23] | DOI:10.1104/pp.16.01231PMID:27794102URLAbstract Gibberellins (GAs) are plant hormones that regulate most plant life cycle aspects, including flowering and fruit development. Here we demonstrate the implication of GAs in ovule development. DELLA proteins, negative GA response regulators, act as positive factors for ovule integument development in a mechanism that involves transcription factor ATS. The seeds of the della global mutant, a complete loss-of function of DELLA, and the ats-1 mutant are remarkably similar, with a round shape, a disorganized testa, and viviparism. These defects are the result of an alteration in integuments that fail to fully develop and are shorter than in WT plants. ats-1 also shows some GA-related phenotypes, e.g. higher germination rates and early flowering. In fact, ats-1 has elevated GA levels due to the activation of GA biosynthesis genes, which indicates that ATS inhibits GA biosynthesis. Moreover, DELLAs and ATS proteins interact, which suggests the formation of a transcriptional complex that regulates the expression of genes involved in integument growth. Therefore, the repression of GA biosynthesis by ATS would result in the stabilization of DELLAs to ensure correct ATS-DELLA complex formation. The requirement of both activities to coordinate proper ovule development strongly argues that the ATS-DELLA complex acts as a key molecular factor. This work provides the first evidence for a role of GAs in ovule and seed development. {copyright, serif} 2016 American Society of Plant Biologists. All rights reserved. [本文引用: 3] |
| [24] | DOI:10.1016/S1360-1385(00)01790-8PMID:11120474URLThe identification of most of the genes involved in the metabolic pathways for gibberellin hormones has helped us to understand these pathways and their regulation. Many of these enzymes are multifunctional and therefore fewer enzymes than might be expected are required to synthesize the various gibberellin structures. However, several of the enzymes are encoded by multiple genes that are regulated differently, adding unexpected genetic complexity. Several endogenous and environmental factors modify the expression of gibberellin biosynthesis genes, including developmental stage, hormonal status and light. A future challenge will be to dissect the complex, interacting pathways that mediate the regulation of gibberellin metabolism. [本文引用: 1] |
| [25] | DOI:10.1038/ncomms5722PMID:25146889URLCawaves and oscillation are key signalling elements during the fertilization process of animals, and are involved, for example, in egg activation. In the unique double fertilization process in flowering plants, both the egg cell and the neighbouring central cell fuse with a sperm cell each. Here we succeeded in imaging cytosolic Cain these two cells, and in the two synergid cells that accompany the gametes during semi-in vivo double fertilization. Following pollen tube discharge and plasmogamy, the egg and central cells displayed transient Caspikes, but not oscillations. Only the events in the egg cell correlated with the plasmogamy. In contrast, the synergid cells displayed Caoscillations on pollen tube arrival. The two synergid cells showed distinct Cadynamics depending on their respective roles in tube reception. These Cadynamics in the female gametophyte seem to represent highly specific signatures that coordinate successful double fertilization in the flowering plants. [本文引用: 1] |
| [26] | DOI:10.1105/tpc.109.066969PMID:19470587URLAbstract The phytohormone gibberellin (GA) has long been known to regulate the growth, development, and life cycle progression of flowering plants. However, the molecular GA-GID1-DELLA mechanism that enables plants to respond to GA has only recently been discovered. In addition, studies published in the last few years have highlighted previously unsuspected roles for the GA-GID1-DELLA mechanism in regulating growth response to environmental variables. Here, we review these advances within a general plant biology context and speculate on the answers to some remaining questions. We also discuss the hypothesis that the GA-GID1-DELLA mechanism enables flowering plants to maintain transient growth arrest, giving them the flexibility to survive periods of adversity. [本文引用: 1] |
| [27] | DOI:10.1042/BJ20120245URL [本文引用: 1] |
| [28] | DOI:10.1146/annurev.cellbio.17.1.159URL [本文引用: 1] |
| [29] | DOI:10.1105/tpc.108.064501PMID:2660633URLThe symbiotic association of legumes with rhizobia involves bacterially derived Nod factor, which is sufficient to activate the formation of nodules on the roots of the host plant. Perception of Nod factor by root hair cells induces calcium oscillations that are a component of the Nod factor signal transduction pathway. Perception of the calcium oscillations is a function of a calcium-and calmodulin-dependent protein kinase, and this activates nodulation gene expression via two GRAS domain transcriptional regulators, Nodulation Signaling Pathway1 (NSP1) and NSP2, and an ERF transcription factor required for nodulation. Here, we show that NSP1 and NSP2 form a complex that is associated with the promoters of early nodulin genes. We show that NSP1 binds directly to ENOD promoters through the novel c/s-element AATTT. While NSP1 shows direct binding to the ENOD11 promoter in vitro, this association in vivo requires NSP2. The NSP1-NSP2 association with the ENOD11 promoter is enhanced following Nod factor elicitation. Mutations in the domain of NSP2 responsible for its interaction with NSP1 highlight the significance of the NSP1-NSP2 heteropolymer for nodulation signaling. Our work reveals direct binding of a GRAS protein complex to DNA and highlights the importance of the NSP1-NSP2 complex for efficient nodulation in the model legume Medicago truncatula. [本文引用: 1] |
| [30] | DOI:10.1104/pp.108.121301URL [本文引用: 1] |
| [31] | DOI:10.2307/3871359PMID:11340177URLThe rice slender mutant (slr1-1) is caused by a single recessive mutation and results in a constitutive gibberellin (GA) response phenotype. The mutant elongates as if saturated with GAs. In this mutant, (1) elongation was unaffected by an inhibitor of GA biosynthesis, (2) GA-inducible -amylase was produced by the aleurone layers without gibberellic acid application, and (3) endogenous GA content was lower than in the wild-type plant. These results indicate that the product of the SLR1 gene is an intermediate of the GA signal transduction pathway. SLR1 maps to OsGAI in rice and has significant homology with height-regulating genes, such as RHT-1Da in wheat, D8 in maize, and GAI and RGA in Arabidopsis. The GAI gene family is likely to encode transcriptional factors belonging to the GRAS gene superfamily. DNA sequence analysis revealed that the slr1-1 mutation is a single basepair deletion of the nuclear localization signal domain, resulting in a frameshift mutation that abolishes protein production. Furthermore, introduction of a 6-kb genomic DNA fragment containing the wild-type SLR1 gene into the slr1-1 mutant restored GA sensitivity to normal. These results indicate that the slr1-1 mutant is caused by a loss-of-function mutation of the SLR1 gene, which is an ortholog of GAI, RGA, RHT, and D8. We also succeeded in producing GA-insensitive dwarf rice by transforming wild-type rice with a modified SLR1 gene construct that has a 17-amino acid deletion affecting the DELLA region. Thus, we demonstrate opposite GA response phenotypes depending on the type of mutations in SLR1. [本文引用: 2] |
| [32] | [本文引用: 2] |
| [33] | DOI:10.1186/gb-2007-8-10-r204URL [本文引用: 1] |
| [34] | DOI:10.1371/journal.pgen.0030171PMID:2014789URLThe female gametophyte of flowering plants, the embryo sac, develops within the diploid (sporophytic) tissue of the ovule. While embryo sac-expressed genes are known to be required at multiple stages of the fertilization process, the set of embryo sac-expressed genes has remained poorly defined. In particular, the set of genes responsible for mediating intracellular communication between the embryo sac and the male gametophyte, the pollen grain, is unknown. We used high-throughput cDNA sequencing and whole-genome tiling arrays to compare gene expression in wild-type ovules to that in dif1 ovules, which entirely lack embryo sacs, and myb98 ovules, which are impaired in pollen tube attraction. We identified nearly 400 genes that are downregulated in dif1 ovules. Seventy-eight percent of these embryo sac-dependent genes were predicted to encode for secreted proteins, and 60% belonged to multigenic families. Our results define a large number of candidate extracellular signaling molecules that may act during embryo sac development or fertilization; less than half of these are represented on the widely used ATH1 expression array. In particular, we found that 37 out of 40 genes encoding Domain of Unknown Function 784 (DUF784) domains require the synergid-specific transcription factor MYB98 for expression. Several DUF784 genes were transcribed in synergid cells of the embryo sac, implicating the DUF784 gene family in mediating late stages of embryo sac development or interactions with pollen tubes. The coexpression of highly similar proteins suggests a high degree of functional redundancy among embryo sac genes. [本文引用: 1] |
| [35] | DOI:10.1073/pnas.1613979113PMID:27911812URLThe asymmetric cell division of the zygote is the initial and crucial developmental step in most multicellular organisms. In flowering plants, whether zygote polarity is inherited from the preexisting organization in the egg cell or reestablished after fertilization has remained elusive. How dynamically the intracellular organization is generated during zygote... [本文引用: 1] |
| [36] | [本文引用: 1] |
| [37] | DOI:10.1101/gad.969002PMID:11877383URLAbstract The germination of Arabidopsis seeds is promoted by gibberellin (GA). Arabidopsis GAI, and RGA are genes encoding key GA signal-transduction components (GAI and RGA) that mediate GA regulation of stem elongation. The Arabidopsis genome contains two further genes, RGL1 and RGL2, that encode proteins (RGL1 and RGL2) that are closely related to GAI and RGA. Here, we show that RGL2 regulates seed germination in response to GA, and that RGL1, GAI, and RGA do not. In addition, we show that RGL2 transcript levels rise rapidly following seed imbibition, and then decline rapidly as germination proceeds. In situ GUS staining revealed that RGL2 expression in imbibed seeds is restricted to elongating regions of pre-emergent and recently emerged radicles. These observations indicate that RGL2 is a negative regulator of GA responses that acts specifically to control seed germination rather than stem elongation. Furthermore, as RGL2 expression is imbibition inducible, RGL2 may function as an integrator of environmental and endogenous cues to control seed germination. [本文引用: 1] |
| [38] | DOI:10.1038/nrm909PMID:12209125URLExtracellular proteins bound to cell-surface receptors can change nuclear gene expression patterns in minutes, with far-reaching consequences for development, cell growth and homeostasis. The signal transducer and activator of transcription (STAT) proteins are among the most well studied of the latent cytoplasmic signal-dependent transcription-factor pathways. In addition to several roles in normal cell decisions, dysregulation of STAT function contributes to human disease, making the study of these proteins an important topic of current research. [本文引用: 1] |
| [39] | [本文引用: 1] |
| [40] | DOI:10.1104/pp.16.00480PMID:27621423URL@article{8200648, author = {Liu, Bing and De Storme, Nico and Geelen, Danny}, issn = {0032-0889}, journal = {PLANT PHYSIOLOGY}, language = {eng}, number = {1}, pages = {338--353}, title = {Gibberellin induces diploid pollen formation by interfering with meiotic cytokinesis}, url = {http://dx.doi.org/10.1104/pp.16.00480}, volume = {173}, year = {2017}, } [本文引用: 2] |
| [41] | DOI:10.1016/j.cub.2013.03.053PMID:23583555URL78 The nuclear-localized DELLA proteins interact with α-subunits of the prefoldin complex. 78 Prefoldin accumulates in the nucleus in a DELLA-dependent manner. 78 Prefoldin nuclear localization reduces the pool of available α/B-tubulin heterodimers. 78 Prefoldin localization and microtubule orientation oscillate with a daily pattern [本文引用: 2] |
| [42] | URL [本文引用: 1] |
| [43] | DOI:10.1038/nature07519PMID:19037309URLGibberellins control a range of growth and developmental processes in higher plants and have been widely used in the agricultural industry. By binding to a nuclear receptor, GIBBERELLIN INSENSITIVE DWARF1 (GID1), gibberellins regulate gene expression by promoting degradation of the transcriptional regulator DELLA proteins, including GIBBERELLIN INSENSITIVE (GAI). The precise manner in which GID1 discriminates and becomes activated by bioactive gibberellins for specific binding to DELLA proteins remains unclear. Here we present the crystal structure of a ternary complex of Arabidopsis thaliana GID1A, a bioactive gibberellin and the amino-terminal DELLA domain of GAI. In this complex, GID1A occludes gibberellin in a deep binding pocket covered by its N-terminal helical switch region, which in turn interacts with the DELLA domain containing DELLA, VHYNP and LExLE motifs. Our results establish a structural model of a plant hormone receptor that is distinct from the mechanism of the hormone perception and effector recognition of the known auxin receptors. [本文引用: 1] |
| [44] | DOI:10.1104/pp.17.01433URL |
| [45] | DOI:10.1101/gad.11.23.3194PMID:9389651URLThe Arabidopsis gai mutant allele confers a reduction in gibberellin (GA) responsiveness. Here we report the molecular cloning of GAI and a closely related gene GRS. The predicted GAI (wild-type) and gai (mutant) proteins differ only by the deletion of a 17-amino-acid segment from within the amino-terminal region. GAI and GRS contain nuclear localization signals, a region of homology to a putative transcription factor, and motifs characteristic of transcriptional coactivators. Genetic analysis indicates that GAI is a repressor of GA responses, that GA can release this repression, and that gai is a mutant repressor that is relatively resistant to the effects of GA. Mutations at SPY and GAR2 suppress the gai phenotype, indicating the involvement of GAI, SPY, and GAR2 in a signaling pathway that regulates GA responses negatively. The existence of this pathway suggests that GA modulates plant growth through derepression rather than through simple stimulation. [本文引用: 3] |
| [46] | DOI:10.1038/22307URL [本文引用: 1] |
| [47] | DOI:10.1111/nph.12571PMID:24400898URLExcessive gibberellin (GA) signalling, mediated through the DELLA proteins, has a negative impact on plant fertility. Loss of DELLA activity in the monocot rice (Oryza sativa) causes complete male sterility, but not in the dicot model Arabidopsis (Arabidopsis thaliana) ecotype Landsberg erecta (Ler), in which DELLA function has been studied most extensively, leading to the assumption that DELLA activity is not essential for Arabidopsis pollen development. A novel DELLA fertility phenotype was identified in the Columbia (Col-0) ecotype that necessitates re-evaluation of the general conclusions drawn from Ler.Fertility phenotypes were compared between the Col-0 and Ler ecotypes under conditions of chemical and genetic GA overdose, including mutants in both ecotypes lacking the DELLA paralogues REPRESSOR OF ga1-3 (RGA) and GA INSENSITIVE (GAI).Ler displays a less severe fertility phenotype than Col-0 under GA treatment. Col-0 rga gai mutants, in contrast with the equivalent Ler phenotype, were entirely male sterile, caused by post-meiotic defects in pollen development, which were rescued by the reintroduction of DELLA into either the tapetum or developing pollen.We conclude that DELLA activity is essential for Arabidopsis pollen development. Differences between the fertility responses of Col-0 and Ler might be caused by differences in downstream signalling pathways or altered DELLA expression. [本文引用: 1] |
| [48] | [本文引用: 1] |
| [49] | DOI:10.1002/(SICI)1521-1878(200006)22:6<573::AID-BIES10>3.0.CO;2-HPMID:10842311URLAbstract GRAS is a recently discovered family of plant-specific proteins that play important regulatory roles in diverse aspects of plant development. Several of the motifs present in the GRAS proteins suggest that they function as transcription factors, although homology-searching programs have revealed no significant similarity to any non-plant proteins. Here we propose that the GRAS proteins are related to the Signal Transducers and Activators of Transcription (STAT) family of proteins. STATs are known in many non-plant species, and act as intracellular intermediaries between extracellular ligands and the transcription and activation of genes. Our hypothesis is that the GRAS proteins perform this function in plants, with mechanisms similar to those of the animal STATs. If true, this hypothesis has important implications for the evolution of phosphotyrosine based signal transduction systems in eukaryotic organisms. BioEssays 22:573 577, 2000. 2000 John Wiley & Sons, Inc. [本文引用: 1] |
| [50] | DOI:10.1038/s41477-017-0021-9PMID:28970478URLAbstract The phytohormone gibberellin (GA) is a key regulator of plant growth and development. Although the upstream regulation and downstream responses to GA vary across cells and tissues, developmental stages and environmental conditions, the spatiotemporal distribution of GA in vivo remains unclear. Using a combinatorial screen in yeast, we engineered an optogenetic biosensor, GIBBERELLIN PERCEPTION SENSOR 1 (GPS1), that senses nanomolar levels of bioactive GAs. Arabidopsis thaliana plants expressing a nuclear localized GPS1 report on GAs at the cellular level. GA gradients were correlated with gradients of cell length in rapidly elongating roots and dark-grown hypocotyls. In roots, accumulation of exogenously applied GA also correlated with cell length, intimating that a root GA gradient can be established independently of GA biosynthesis. In hypocotyls, GA levels were reduced in a phytochrome interacting factor (pif) quadruple mutant in the dark and increased in a phytochrome double mutant in the light, indicating that PIFs elevate GA in the dark and that phytochrome inhibition of PIFs could lower GA in the light. As GA signalling directs hypocotyl elongation largely through promoting PIF activity, PIF promotion of GA accumulation represents a positive feedback loop within the molecular framework driving rapid hypocotyl growth.Gibberellin (GA) is a plant hormone that plays an irreplaceable role in regulating growth and development. Now, the first fluorescent GA biosensor has been developed to visualize GA distributions at cellular resolution in Arabidopsis. |
| [51] | DOI:10.1371/journal.pbio.1001155PMID:21949639URLAuthor Summary Germline specification is a key step in sexual reproduction. In plants, the reproductive lineage or 鈥済ermline鈥 doesn't arise early in development, as it does in animals; rather, the germline is specified during flower development. In the female reproductive organs of the flower, a single sporophytic cell in each ovule is selected and differentiates into a megaspore mother cell (MMC), which will undergo meiosis. Despite the importance of the specification of the MMC as the first committed cell of the germline lineage, little is known about the genetic and molecular bases of this process. We performed a cell-type-specific transcriptome analysis of Arabidopsis MMCs using laser-assisted microdissection and microarrays. Statistical data analysis comparing these results with the transcriptomes of 71 other types of cells and tissues revealed the importance of translational control pathways and RNA helicases for plant germline development, a feature reminiscent of the animal germline. We further characterized a novel MMC-enriched RNA helicase, called MNEME, and showed that it plays important roles in MMC differentiation and the restriction of the plant germline to only one cell per ovule. This example illustrates the usefulness of our transcriptome dataset for the identification of novel candidate genes involved in this crucial step of plant reproduction. [本文引用: 1] |
| [52] | DOI:10.2307/3870695PMID:9490740URLThe recessive rga mutation is able to partially suppress phenotypic defects of the Arabidopsis gibberellin (GA) biosynthetic mutant ga1-3. Defects in stem elongation, flowering time, and leaf abaxial trichome initiation are suppressed by rga. This indicates that RGA is a negative regulator of the GA signal transduction pathway. We have identified 10 additional alleles of rga from a fast-neutron mutagenized ga1-3 population and used them to isolate the RGA gene by genomic subtraction. Our data suggest that RGA may be functioning as a transcriptional regulator. RGA was found to be a member of the VHIID regulatory family, which includes the radial root organizing gene SCARECROW and another GA signal transduction repressor, GAI. RGA and GAI proteins share a high degree of homology, but their N termini are more divergent. The presence of several structural features, including homopolymeric serine and threonine residues, a putative nuclear localization signal, leucine heptad repeats, and an LXXLL motif, indicates that the RGA protein may be a transcriptional regulator that represses the GA response. In support of the putative nuclear localization signal, we demonstrated that a transiently expressed green fluorescent protein-RGA fusion protein is localized to the nucleus in onion epidermal cells. Because the rga mutation abolished the high level of expression of the GA biosynthetic gene GA4 in the ga1-3 mutant background, we conclude that RGA may also play a role in controlling GA biosynthesis. [本文引用: 1] |
| [53] | [本文引用: 1] |
| [54] | DOI:10.1105/tpc.003046URL [本文引用: 1] |
| [55] | DOI:10.1105/tpc.2.8.755URL |
| [56] | DOI:10.1111/j.1365-313X.2007.03137.xPMID:17559508URLThe angiosperm female gametophyte typically consists of one egg cell, two synergid cells, one central cell, and three antipodal cells. Each of these four cell types has unique structural features and performs unique functions that are essential for the reproductive process. The gene regulatory networks conferring these four phenotypic states are largely uncharacterized. As a first step towards dissecting the gene regulatory networks of the female gametophyte, we have identified a large collection of genes expressed in specific cells of the Arabidopsis thaliana female gametophyte. We identified these genes using a differential expression screen based on reduced expression in determinant infertile1 ( dif1 ) ovules, which lack female gametophytes. We hybridized ovule RNA probes with Affymetrix ATH1 genome arrays and validated the identified genes using real-time RT-PCR. These assays identified 71 genes exhibiting reduced expression in dif1 ovules. We further validated 45 of these genes using promoter::GFP fusions and 43 were expressed in the female gametophyte. In the context of the ovule, 11 genes were expressed exclusively in the antipodal cells, 11 genes were expressed exclusively or predominantly in the central cell, 17 genes were expressed exclusively or predominantly in the synergid cells, one gene was expressed exclusively in the egg cell, and three genes were expressed strongly in multiple cells of the female gametophyte. These genes provide insights into the molecular processes functioning in the female gametophyte and can be used as starting points to dissect the gene regulatory networks functioning during differentiation of the four female gametophyte cell types. [本文引用: 1] |
| [57] | DOI:10.1104/pp.110.161554URL [本文引用: 1] |
| [58] | DOI:10.1016/j.cub.2011.02.036PMID:21549956URLBioactive gibberellins (GAs) are diterpene phytohormones that modulate growth and development throughout the whole life cycle of the flowering plant. Impressive advances have been made in elucidating the GA pathway with the cloning and characterization of genes encoding most GA biosynthesis and catabolism enzymes, GA receptors (GIBBERELLIN INSENSITIVE DWARF1, GID1) and early GA signaling components. Recent biochemical, genetic and structural analyses demonstrate that GA de-represses its signaling pathway by GID1-induced degradation of DELLA proteins, which are master growth repressors, via a ubiquitin–proteasome pathway. Multiple endogenous signals and environmental cues also interact with the GA–GID1–DELLA regulatory module by affecting the expression of GA metabolism genes, and hence GA content and DELLA levels. Importantly, DELLA integrates different signaling activities by direct protein–protein interaction with multiple key regulatory proteins from other pathways. Comparative studies suggest that the functional GA–GID1–DELLA module is highly conserved among vascular plants, but not in the bryophytes. Interestingly, differentiation of the moss Physcomitrella patens is regulated by as yet unidentified ent-kaurene-derived diterpenes, which are distinct from the common active GAs in vascular plants. [本文引用: 1] |
| [59] | [本文引用: 1] |
| [60] | DOI:10.1002/9781119210436.ch7URLreact-text: 213 The hormone gibberellin (GA) is a key regulator of plant growth. Many of the components of the gibberellin signal transduction [e.g., GIBBERELLIN INSENSITIVE DWARF 1 (GID1) and DELLA], biosynthesis [e.g., GA 20-oxidase (GA20ox) and GA3ox], and deactivation pathways have been identified. Gibberellin binds its receptor, GID1, to form a complex that mediates the degradation of DELLA proteins. In... /react-text react-text: 214 /react-text [Show full abstract] [本文引用: 2] |
| [61] | DOI:10.1016/j.pbi.2009.12.001PMID:20047852URLBergmann DC, Fleming AJ. [本文引用: 1] |
| [62] | DOI:10.1016/j.tplants.2017.07.010PMID:28843766URLAbstract The spectacular yield increases in rice and wheat during the green revolution were partly realized by reduced gibberellin (GA) synthesis or sensitivity, both causing the accumulation of DELLA proteins. Although insights into the regulation of plant growth and development by DELLA proteins advanced rapidly in arabidopsis (Arabidopsis thaliana), DELLA-mediated regulation of downstream responses in cereals has received little attention to date. Furthermore, translating this research from arabidopsis to cereals is challenging given their different growth patterns and our phylogenetic analysis which reveals that DELLA-related DGLLA proteins exist in cereals but not in arabidopsis. Therefore, understanding the molecular basis of DELLA function in cereals holds great potential to improve yield. In this review, we propose to extend the focus of DELLA functional research to cereals, and highlight the appropriate tools that are now available to achieve this. Copyright 2017 Elsevier Ltd. All rights reserved. [本文引用: 1] |
| [63] | DOI:10.1105/tpc.010325PMID:11826301URLIn Arabidopsis, the DELLA subfamily of GRAS regulatory genes consists of GAI, RGA, RGA-LIKE1 (RGL1), RGL2, and RGL3. GAI and RGA are known to be negative regulators of gibberellin (GA) responses. We found that RGL1 is a similar repressor of GA responses, as revealed by RGL1 gain-of-function and loss-of-function phenotypes. Repression of GA responses in Arabidopsis was conferred by a dominant 35S-rgl1 transgene carrying a DELLA domain deletion analogous to the GA-insensitive gai-1 mutation. As in GA-deficient Arabidopsis, the transgenic plants were dark green dwarfs with underdeveloped trichomes and flowers. Expression levels of GA4, a feedback-regulated GA biosynthetic gene, were increased correspondingly. Conversely, a loss-of-function rgl1 line had reduced GA4 expression and exhibited GA-independent activation of seed germination, leaf expansion, flowering, stem elongation, and floral development, as detected by resistance to the GA biosynthesis inhibitor paclobutrazol. RGL1 plays a greater role in seed germination than do GAI and RGA. The expression profile of RGL1 differed from those of the four other DELLA homologs. RGL1 message levels were predominant in flowers, with transcripts detected in developing ovules and anthers. As with RGA, green fluorescent protein (GFP)-tagged RGL1 protein was localized to the nucleus, but unlike GFP-RGA, there was no degradation after GA treatment. These findings indicate that RGL1 is a partially redundant, but distinct, negative regulator of GA responses and suggest that all DELLA subfamily members might possess separate as well as overlapping roles in GA signaling. [本文引用: 3] |
| [64] | DOI:10.1016/j.cub.2010.01.051PMID:20226671URLThe development of multicellular organisms is controlled by differential gene expression whereby cells adopt distinct fates. A spatially resolved view of gene expression allows the elucidation of transcriptional networks that are linked to cellular identity and function. The haploid female gametophyte of flowering plants is a highly reduced organism: at maturity, it often consists of as few as three cell types derived from a common precursor []. However, because of its inaccessibility and small size, we know little about the molecular basis of cell specification and differentiation in the female gametophyte. Here we report expression profiles of all cell types in the mature 3]. A comparison of human and Highlights? Transcriptomes of the cell types in the mature Arabidopsis female gametophyte ? Egg cell enrichment of PAZ/Piwi-domain genes indicates epigenetic regulation by siRNA ? Overrepresentation of three transcription factor families in the female gametophyte ? Human and Arabidopsis egg cells are enriched in similar functional groups [本文引用: 1] |
| [65] | DOI:10.1104/pp.105.067314PMID:16299181URLThe extensive data on the transcription of the plant genome are derived primarily from the sporophytic generation. There currently is little information on genes that are expressed during female gametophyte development in angiosperms, and it is not known whether the female gametophyte transcriptome contains a major set of genes that are not expressed in the sporophyte or whether it is primarily a subset of the sporophytic transcriptome. Because the embryo sac is embedded within the maternal ovule tissue, we have utilized the Arabidopsis (Arabidopsis thaliana) mutant sporocyteless that produces ovules without embryo sacs, together with the ATH1 Arabidopsis whole-genome oligonucleotide array, to identify genes that are preferentially or specifically expressed in female gametophyte development. From analysis of the datasets, 225 genes are identified as female gametophyte genes, likely a lower limit as stringent criteria were used for the analysis, eliminating many low expressed genes. Nearly 45% of the identified genes were not previously detected by sporophytic expression profiling, suggesting that the embryo sac transcriptome may contain a significant fraction of transcripts restricted to the gametophyte. Validation of six candidate genes was performed using promoter: -glucuronidase fusions, and all of these showed embryo sac-specific expression in the ovule. The unfiltered expression data from this study can be used to evaluate the possibility of female gametophytic expression for any gene in the ATH1 array, and contribute to identification of the functions of the component of the Arabidopsis genome not represented in studies of sporophytic expression and function. [本文引用: 2] |
| [66] | DOI:10.1073/pnas.0402377101URL [本文引用: 1] |
赤霉素作用机理的分子基础与调控模式研究进展
1
2006
... DELLA蛋白作为转录因子, 在靶基因的转录激活或抑制过程中起调控作用(
DELLA蛋白参与拟南芥幼苗对一氧化氮逆境的抵抗
1
2011
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
1
1997
... 拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(
1
2009
... 花粉管穿过助细胞进入胚珠, 释放2个精子, 分别与中央细胞以及卵细胞结合形成胚乳和胚.
1
1986
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
2009
... 赤霉素在雄蕊发育过程中有3个显著作用, 即调控花药中花粉的发育、花药的开裂和花粉管的伸长(
1
2004
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
2002
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
2
2004
... 赤霉素在雄蕊发育过程中有3个显著作用, 即调控花药中花粉的发育、花药的开裂和花粉管的伸长(
... 拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(
3
2007
... 赤霉素在雄蕊发育过程中有3个显著作用, 即调控花药中花粉的发育、花药的开裂和花粉管的伸长(
... 育性缺陷表现为花粉活性降低, 而非影响花粉管的伸长(
... 植物的雌蕊结构复杂, 完成双受精后形成果实.雌蕊包括柱头、花柱和子房.雌蕊的正常发育和形成保证了花粉与柱头识别后萌发, 及花粉管顺利进入胚珠、完成双受精和形成种子.用GA处理会改变雌蕊的形态, 说明GA参与调控雌蕊的发育.Fukazawa等(2017)的研究表明, DELLA参与GA合成的正向调控, DELLA缺失会导致体内GA含量降低(
2014
1
2014
... 花粉管穿过助细胞进入胚珠, 释放2个精子, 分别与中央细胞以及卵细胞结合形成胚乳和胚.
1
2001
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
2
2009
... 对DELLA的鉴定一般采用以下3个标准: (1) 氨基酸序列特征为N端具DELLA结构域和C端有GRAS; (2) 对GA响应, 即用GA处理后, DELLA蛋白降解; (3) 依赖于GA与GID1的结合.但这些标准并非绝对.例如, AtGAI和AtRGL1用GA处理后不降解(
... 植物的雌蕊结构复杂, 完成双受精后形成果实.雌蕊包括柱头、花柱和子房.雌蕊的正常发育和形成保证了花粉与柱头识别后萌发, 及花粉管顺利进入胚珠、完成双受精和形成种子.用GA处理会改变雌蕊的形态, 说明GA参与调控雌蕊的发育.Fukazawa等(2017)的研究表明, DELLA参与GA合成的正向调控, DELLA缺失会导致体内GA含量降低(
1
2016
... 花粉管穿过助细胞进入胚珠, 释放2个精子, 分别与中央细胞以及卵细胞结合形成胚乳和胚.
1
2002
... 对DELLA的鉴定一般采用以下3个标准: (1) 氨基酸序列特征为N端具DELLA结构域和C端有GRAS; (2) 对GA响应, 即用GA处理后, DELLA蛋白降解; (3) 依赖于GA与GID1的结合.但这些标准并非绝对.例如, AtGAI和AtRGL1用GA处理后不降解(
1
2005
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
1
1977
... 赤霉素在雄蕊发育过程中有3个显著作用, 即调控花药中花粉的发育、花药的开裂和花粉管的伸长(
1
2002
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
2
2012
... 赤霉素在雄蕊发育过程中有3个显著作用, 即调控花药中花粉的发育、花药的开裂和花粉管的伸长(
... 植物的雌蕊结构复杂, 完成双受精后形成果实.雌蕊包括柱头、花柱和子房.雌蕊的正常发育和形成保证了花粉与柱头识别后萌发, 及花粉管顺利进入胚珠、完成双受精和形成种子.用GA处理会改变雌蕊的形态, 说明GA参与调控雌蕊的发育.Fukazawa等(2017)的研究表明, DELLA参与GA合成的正向调控, DELLA缺失会导致体内GA含量降低(
2017
1
1998
... 拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(
3
2016
... 被子植物的子房包裹着1个或多个胚珠.胚珠中胚囊的合点-珠孔轴具有明显的极性.典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞.目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用.对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(
... 在珠心组织优势表达, 在珠柄和珠被原基也有表达.相应地, RGA和GAI通过与转录因子ATS (ABERRANT TESTA SHAPE)互作, 调控拟南芥珠被的发育(
... 拟南芥Ler生态型不同DELLA多突变体的确存在不同程度的胚珠败育(
1
2000
... 植物的雌蕊结构复杂, 完成双受精后形成果实.雌蕊包括柱头、花柱和子房.雌蕊的正常发育和形成保证了花粉与柱头识别后萌发, 及花粉管顺利进入胚珠、完成双受精和形成种子.用GA处理会改变雌蕊的形态, 说明GA参与调控雌蕊的发育.Fukazawa等(2017)的研究表明, DELLA参与GA合成的正向调控, DELLA缺失会导致体内GA含量降低(
1
2014
... 花粉管穿过助细胞进入胚珠, 释放2个精子, 分别与中央细胞以及卵细胞结合形成胚乳和胚.
1
2009
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
1
2012
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
2001
... 拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(
1
2009
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
2008
... 拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(
2
2001
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
2
2002
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
... , C)包括DELLA、VHYNP (或TVHYNP)和poly- S/T/V基序.这些结构域的缺失导致DELLA对GA的应答消失, 从而增强了其抑制作用以及出现半矮化表型(
1
2007
... 被子植物的子房包裹着1个或多个胚珠.胚珠中胚囊的合点-珠孔轴具有明显的极性.典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞.目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用.对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(
1
2007
... 被子植物的子房包裹着1个或多个胚珠.胚珠中胚囊的合点-珠孔轴具有明显的极性.典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞.目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用.对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(
1
2016
... 卵细胞受精形成合子后, 经过1次不均等分裂形成顶、基细胞.随后顶细胞经过多次分裂和分化形成胚的主体, 而基细胞经过几次分裂形成胚柄.在合子的极性建立过程中, 细胞骨架微管的动态变化起着重要作用.卵细胞受精时, 微管的排列模式被打乱, 受精后会重新排布, 在合子接近顶端的位置形成新的横向环状微管(
1
1985
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
2002
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
1
2002
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
2016
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
2
2017
... 最近有研究表明, 在GA处理下小孢子母细胞减数分裂中胞质分离受到影响, 花粉体积变大并产生二倍体花粉.rga-24/gai-t6突变体小孢子母细胞减数分裂过程中表现出相同的表型(
... 突变体中并未观察到有丝分裂细胞骨架异常引起的细胞分裂缺陷现象(
2
2013
... 最近有研究表明, 在GA处理下小孢子母细胞减数分裂中胞质分离受到影响, 花粉体积变大并产生二倍体花粉.rga-24/gai-t6突变体小孢子母细胞减数分裂过程中表现出相同的表型(
... 卵细胞受精形成合子后, 经过1次不均等分裂形成顶、基细胞.随后顶细胞经过多次分裂和分化形成胚的主体, 而基细胞经过几次分裂形成胚柄.在合子的极性建立过程中, 细胞骨架微管的动态变化起着重要作用.卵细胞受精时, 微管的排列模式被打乱, 受精后会重新排布, 在合子接近顶端的位置形成新的横向环状微管(
1
2007
... 植物的雌蕊结构复杂, 完成双受精后形成果实.雌蕊包括柱头、花柱和子房.雌蕊的正常发育和形成保证了花粉与柱头识别后萌发, 及花粉管顺利进入胚珠、完成双受精和形成种子.用GA处理会改变雌蕊的形态, 说明GA参与调控雌蕊的发育.Fukazawa等(2017)的研究表明, DELLA参与GA合成的正向调控, DELLA缺失会导致体内GA含量降低(
1
2008
... DELLA蛋白作为GA应答调控途径中的关键分子参与植物的多种发育过程.关于营养生长过程中GA- DELLA信号调控途径的蛋白质结构基础和分子调控机制已研究得十分深入(
2017
3
1997
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
... ;
1
1999
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
2014
... 赤霉素在雄蕊发育过程中有3个显著作用, 即调控花药中花粉的发育、花药的开裂和花粉管的伸长(
1
1999
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
1
2000
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
2017
1
2011
... 被子植物的子房包裹着1个或多个胚珠.胚珠中胚囊的合点-珠孔轴具有明显的极性.典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞.目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用.对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(
1
1998
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
1997
... DELLA蛋白的氨基酸序列中, C端具有保守的GRAS功能域(
1
2002
... 拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(
1990
1
2007
... 被子植物的子房包裹着1个或多个胚珠.胚珠中胚囊的合点-珠孔轴具有明显的极性.典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞.目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用.对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(
1
2010
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
1
2011
... DELLA蛋白作为转录因子, 在靶基因的转录激活或抑制过程中起调控作用(
1
2004
... 拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(
2
2016
... DELLA蛋白作为转录因子, 在靶基因的转录激活或抑制过程中起调控作用(
... ).DELLA通常需要与其它调控因子共同行使调控功能, 主要有以下3种具体方式 (
1
2010
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
1
2017
... DELLA蛋白作为GA应答调控途径中的关键分子参与植物的多种发育过程.关于营养生长过程中GA- DELLA信号调控途径的蛋白质结构基础和分子调控机制已研究得十分深入(
3
2002
... 在被子植物中, DELLA蛋白是赤霉素(GA)介导的生长发育抑制应答通路中的主要转录调节因子(
... 对DELLA的鉴定一般采用以下3个标准: (1) 氨基酸序列特征为N端具DELLA结构域和C端有GRAS; (2) 对GA响应, 即用GA处理后, DELLA蛋白降解; (3) 依赖于GA与GID1的结合.但这些标准并非绝对.例如, AtGAI和AtRGL1用GA处理后不降解(
... 被子植物的子房包裹着1个或多个胚珠.胚珠中胚囊的合点-珠孔轴具有明显的极性.典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞.目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用.对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(
1
2010
... 被子植物的子房包裹着1个或多个胚珠.胚珠中胚囊的合点-珠孔轴具有明显的极性.典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞.目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用.对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(
2
2005
... 被子植物的子房包裹着1个或多个胚珠.胚珠中胚囊的合点-珠孔轴具有明显的极性.典型的寥型胚囊结构形式是由7个细胞组成, 珠孔端有1个卵细胞和2个助细胞, 合点端有3个反足细胞, 且在这两群细胞之间有1个大的含2核的中央细胞.目前已有一些证据表明, GA-DELLA信号途径在雌配子体的发育过程中起作用.对拟南芥未成熟胚珠芯片的分析表明, GA合成和信号转导途径相关基因在该组织中表达(
... ).另外, 无论用激光切割关键的胚囊细胞, 还是胚囊缺失突变体的转录谱, 均暗示GA合成和信号转导途径在其中起作用(
1
2004
... 拟南芥RGA比其它DELLA在花的形态建成中起着更显著的作用(
