

引用本文
贡献者
接受日期:2017-10-18网络出版日期:2018-11-1
-->Copyright
2018《植物学报》编辑部
Contributors
History
Received:Online:
摘要:
Abstract:
Key words:
脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制。能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫。积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚。研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(Szabados and Savouré, 2010)。胁迫条件下, 脯氨酸积累主要通过合成和降解完成。脯氨酸合成主要在叶绿体和细胞质中进行, 底物谷氨酸在吡咯啉-5-羧酸合成酶(P5CS)和吡咯啉-5-羧酸还原酶(P5CR)的催化下被还原为脯氨酸(Hu et al., 1992; Savouré et al., 1995)。脯氨酸的降解过程发生在线粒体中。在脯氨酸脱氢酶(PDH)和吡咯啉-5-羧酸脱氢酶(P5CDH)的催化下, 脯氨酸转化为谷氨酸(Kiyosue et al., 1996; Verbruggen et al., 1996)。目前, 国内外****已经对脯氨酸代谢的调节进行了较多的总结(全先庆等, 2007; 赵瑞雪等, 2008; Verbruggen and Hermans, 2008; Lehmann et al., 2010; Szabados and Savouré, 2010)。虽然在多数逆境条件下, 脯氨酸积累对植物抗逆起积极作用, 但拟南芥(Arabidopsis thaliana)在受到高温胁迫时, 过量积累脯氨酸的转基因植株的抗热性反而减弱(Lv et al., 2011)。也有研究表明, 正常生长条件下过量的内源或外源脯氨酸会抑制植物的生长, 且抑制过程通常与通过诱导线粒体中活性氧(ROS)的产生, 继而引起细胞程序性死亡有关(Deuschle et al., 2004; Chen et al., 2011)。
早期的研究表明, 胁迫诱导的脯氨酸积累也会受到吸收和转运的影响。例如, 低水势条件下, 脯氨酸在玉米(Zea mays)根系伸长区的积累主要来源于脯氨酸的运输, 而不是合成增加(Verslues and Sharp, 1999)。即使在脯氨酸代谢起主导作用的情况下, 脯氨酸转运也可能会影响其在各器官的分布。在拟南芥、水稻(Oryza sativa)和大麦(Hordeum vulgare)等物种中已经发现了能够运输脯氨酸的氨基酸转运蛋白, 如AtAAP1、AtProTs和HvProT等(Ueda et al., 2001; Grallath et al., 2005; Lee et al., 2007)。它们在植物生长、种子发育及抵抗逆境中起重要作用。本文对脯氨酸吸收和转运的分子机制进行了综述, 旨在全面了解有关脯氨酸运输的研究现状, 为其在植物抗逆方面的研究提供参考。
1 氨基酸转运蛋白家族成员及相互关系氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(Fischer et al., 1998; Ortiz-Lopez et al., 2000; Su et al., 2004)。在拟南芥中, APC超级家族共有14个成员, 其中9个成员为阳离子氨基酸转运蛋白(cationic amino acid transporter, CAT) (不能转运脯氨酸) (Su et al., 2004); ATF超级家族中至少有5个氨基酸转运蛋白家族, 分别为氨基酸通透酶(amino acid permease, AAPs)、赖氨酸组氨酸转运蛋白(lysine histidine transporter, LHTs)、脯氨酸转运蛋白(proline transporter, ProTs)、γ-氨基丁酸转运蛋白(γ-aminobutyric acid transporter, GATs)和生长素转运蛋白(auxin-resistant, AUXs) (Liu and Bush, 2006)。其中, AAP、LHT和ProT家族的成员能够运输脯氨酸(Rentsch et al., 2007)。AAP和LHT家族成员都是广谱氨基酸转运蛋白, 但AAP家族成员主要介导谷氨酸盐、天冬氨酸盐和中性氨基酸的吸收, 而LHT家族成员主要介导中性和碱性氨基酸的吸收(Frommer et al., 1993; Fischer et al., 1995; Chen and Bush, 1997; Fischer et al., 2002; Okumoto et al., 2002; Lee and Tegeder, 2004; Hirner et al., 2006; Lee et al., 2007; Schmidt et al., 2007)。ProT是最早发现的能够转运脯氨酸的蛋白家族(不转运其它蛋白质氨基酸) (Rentsch et al., 1996)。进一步研究表明, ProT也转运甘氨酸甜菜碱和γ-氨基丁酸(GABA) (Breitkreuz et al., 1999; Schwacke et al., 1999; Waditee et al., 2002; Grallath et al., 2005)。
在真菌、细菌和动物中也存在转运脯氨酸的蛋白。酿酒酵母(Saccharomyces cerevisiae)中一般是氨基酸通透酶Gap1p和脯氨酸转运蛋白Put4p一起介导大部分脯氨酸的吸收(Lasko and Brandriss, 1981)。大肠杆菌(Escherichia coli)中, H+/proline同向转运蛋白ProP和依赖结合蛋白的ABC转运蛋白ProU也能介导脯氨酸、甘氨酸甜菜碱及其它相关基质的吸收(Csonka, 1989; Wood et al., 2001); 哺乳动物中, BGT1作为神经递质转运蛋白家族中的一员同样能转运脯氨酸(Matskevitch et al., 1999; Chen et al., 2004)。
2 脯氨酸转运蛋白对脯氨酸的亲和性酵母和爪蟾(Xenopus laeivs)卵母细胞是检测氨基酸转运蛋白转运能力常用的异源表达系统。异源表达的氨基酸转运蛋白对脯氨酸的亲和力不同, 说明它们对脯氨酸转运发挥不同的作用。AAP家族成员对脯氨酸的亲和力较低, Km值介于60-500 μmol·L-1之间(Lehmann et al., 2010)。而LHTs对包括脯氨酸在内的中性和酸性氨基酸的亲和力高, Km值约为10 μmol·L-1 (Chen and Bush, 1997; Lee et al., 2004; Hirner et al., 2006)。AtProTs对甘氨酸甜菜碱的亲和性最高, 对脯氨酸的亲和性次之, 对GABA的亲和性最低(Grallath et al., 2005)。大麦HvProT比其它植物ProTs的脯氨酸亲和性更高, 可能是脯氨酸运输的主要蛋白(Igarashi et al., 2000; Ueda et al., 2001)。表1列出了拟南芥中氨基酸转运蛋白在异源表达系统中对脯氨酸的亲和力。
表 1
Table 1
表 1
| 氨基酸转运蛋白 | 基因表达位置 | 对脯氨酸的亲和力 | 参考文献 |
|---|---|---|---|
| AtAAP1 | 根, 主茎, 花, 胚 | 60 μmol·L-1 | Lee and Tegeder, 2004; Sanders et al., 2009; Perchlik et al., 2014 |
| AtAAP2 | 韧皮部 | (140±20) μmol·L-1 | Okumoto et al., 2002 |
| AtAAP3 | 根 | (250±25) μmol·L-1 | Okumoto et al., 2004; Svennerstam et al., 2008 |
| AtAAP5 | 根 | (500±25) μmol·L-1 | Okumoto et al., 2004; Svennerstam et al., 2008 |
| AtAAP6 | 根, 库叶, 茎叶 | (67±21) μmol·L-1 | Hunt et al., 2010 |
| AtAAP8 | 花蕾, 角果 | - | Okumoto et al., 2002; Schmidt et al., 2007 |
| AtLHT1 | 根, 花, 叶 | (10±0.5) μmol·L-1 | Hirner et al., 2006 |
| AtLHT2 | 花 | (13±3) μmol·L-1 | Lee and Tegeder, 2004 |
| AtProT1 | 根, 茎, 花 | (427±17) μmol·L-1 | Rentsch et al., 1996; Grallath et al., 2005 |
| AtProT2 | 根 | (500±5) μmol·L-1 | Grallath et al., 2005 |
| AtProT3 | 叶, 花和角果 | (999±36) μmol·L-1 | Grallath et al., 2005 |
表 1
拟南芥中脯氨酸转运蛋白基因表达位置及转运蛋白对脯氨酸的亲和力
Table 1
The tissue-specific expression of proline transporters genes and affinity to proline of proline transporters in Arabidopsis
在酵母和动物体内, 氨基酸转运蛋白对脯氨酸的亲和力也有差异。酿酒酵母中脯氨酸转运蛋白Put4p能以高亲和性转运脯氨酸, 但也能转运GABA、丙氨酸和甘氨酸(Grenson et al., 1970; Lasko and Brandriss, 1981; Jauniaux et al., 1987; Regenberg et al., 1999)。氨基酸透过酶Agp1对脯氨酸的吸收为低亲和性(Andréasson et al., 2004)。哺乳动物中BGT1对脯氨酸转运的亲和性较低(Matskevitch et al., 1999; Chen et al., 2004)。
3 植物脯氨酸转运蛋白的表达和生理作用3.1 AAP基因家族AAP基因家族的成员编码广谱性氨基酸转运蛋白, 对脯氨酸无特异性, 被认为在植物总氮的获得和分配中起作用。在拟南芥中, AAP基因家族有8个成员, 分别在不同的组织中表达。
有研究表明, AtAAP1在根尖和侧根的表皮细胞中表达, 在拟南芥根际吸收脯氨酸方面起作用(Lee et al., 2007; Perchlik et al., 2014)。AtAAP1在胚中也有表达, 参与种子发育过程中的氨基酸转运(Sanders et al., 2009)。在aap1突变体中, 种皮和胚乳中的氨基酸总量显著增加, 这可能是由于AtAAP1功能缺失, 无法向胚运输氨基酸, 导致胚附近的组织中氨基酸含量增加。在幼苗期, aap1表现出对脯氨酸不敏感。本实验室近期的研究表明, AtAAP1在花萼和开放花的雄蕊中表达。盐胁迫条件下, AtAAP1对花中脯氨酸的积累具有十分重要的作用(未发表数据)。AtAAP2在拟南芥整个植株的韧皮部中表达, 在韧皮部装载和向胚运输
氨基酸方面起作用(Okumoto et al., 2002)。在aap2突变体叶片中, 脯氨酸、异亮氨酸和缬氨酸等中性氨基酸的含量显著减少。此外, 与野生型相比aap2的侧茎数和角果数均显著增加。这可能是由于AtAAP2功能缺失, 导致向种子运输的氨基酸减少, 留在营养组织中的氨基酸量增加, 因而促进了侧生茎的生长(Zhang et al., 2010)。AtAAP6是一种高亲和力的氨基酸转运蛋白, 主要在根和叶中起作用(Hunt et al., 2010)。对AtAAP6进行组织定位, 发现其主要在木质部薄壁细胞中表达, 说明AtAAP6主要参与氨基酸从木质部向韧皮部的运输(Okumoto et al., 2002)。AtAAP6的T-DNA插入突变体aap6的莲座叶、茎生叶和种子都比野生型显著增大(Hunt et al., 2010)。在筛管细胞中, aap6中的总氨基酸含量显著低于野生型, 其中赖氨酸、苯丙氨酸、亮氨酸和天冬氨酸浓度降低最显著, 但是脯氨酸含量却未发生显著变化。AtAAP8与AtAAP1的核苷酸序列相似度最高, 异源表达结果表明, AtAA- P8能够运输脯氨酸(Okumoto et al., 2002)。AtAAP8主要在花蕾期的花萼和发育中的角果表达。角果进一步成熟后, 即授粉时间超过10天, AtAAP8在角果中的表达停止(Okumoto et al., 2002; Schmidt et al., 2007)。aap8突变体种子数目相比野生型减少了约50%, 且停止发育的种子比例(48%)显著高于野生型(1%) (Schmidt et al., 2007)。aap8幼小角果(4-8 mm)的总氨基酸含量比野生型降低了18%, 减少的主要为天冬氨酸和谷氨酸, 脯氨酸含量则显著增加。这可能是由于脯氨酸在种子发育过程中非常重要, 拟南芥中其它的氨基酸转运蛋白也参与运输脯氨酸。Santiago和Tegeder (2016)研究表明, AtAAP8在脯氨酸韧皮部装载中也起重要作用。
AtAAP3和AtAAP5主要在根中表达, 其中At- AAP3在根韧皮部表达; 它们编码的蛋白主要负责运输精氨酸和赖氨酸, 而并不运输脯氨酸(Okumoto et al., 2004; Svennerstam et al., 2008)。
除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (Miranda et al., 2001; Koch et al., 2003; Tan et al., 2008; Couturier et al., 2010; Peng et al., 2014; Zhang et al., 2015)。水稻的AAP家族有19个成员, OsAAP6在根、叶枕、节间、种子和胚中表达, 亚细胞定位于内质网, 对籽粒蛋白质含量(grain protein content, GPC)具正调节作用。过表达OsAAP6的转基因植株根际吸收脯氨酸、苏氨酸和丝氨酸等氨基酸的量增加, 而RNAi植株中这些氨基酸的含量减少(Peng et al., 2014)。说明OsAAP6对脯氨酸等氨基酸的根际吸收起作用。根据基因组测序结果, 三角叶杨的基因组中有14个AAP基因模型(Tuskan et al., 2006)。其中, PtAAP11在茎尖发育的早期、根伸长和次生生长阶段都有较高水平的表达。PtAAP11在酵母中异源表达的结果表明, PtAAP11能以很高的亲和力(Km值为(4.4±0.3) μmol·L-1)运输脯氨酸(Couturier et al., 2010)。陆地植物中, 木质部细胞的细胞壁所含的结构蛋白包括脯氨酸/羟基脯氨酸富集的糖蛋白(或者伸展蛋白)、阿拉伯半乳糖蛋白、甘氨酸富集的蛋白以及脯氨酸富集的蛋白(Cassab, 1998)。PtAAP11对脯氨酸的高亲和力表明, 其可能在木质部细胞分化时运输脯氨酸, 以保证细胞分裂时对脯氨酸的需求(Couturier et al., 2010)。
3.2 LHT基因家族在拟南芥中, LHT基因家族共有11个成员, 它们也都编码广谱性的氨基酸转运蛋白。
AtLHT1在根被皮、萼片、花梗和叶中表达, 作用于氨基酸的根际吸收和由木质部向叶肉细胞的运输。在土壤中生长的lht1突变体明显小于野生型, 这一表型可被AtLHT1基因功能回补(Hirner et al., 2006)。异源表达的AtLHT1能够作用于脯氨酸的吸收, 并对脯氨酸有较高的亲和力, Km值为10 μmol·L-1 (Hirner et al., 2006)。Northern印染分析显示, AtLHT2在花蕾中高表达。进一步研究发现, AtLHT2在花粉的绒毡层中表达, 作用于氨基酸在花粉粒中的分配。AtLHT2对脯氨酸和天冬氨酸有很高的亲和性, 然而中性和酸性氨基酸对AtLHT2吸收脯氨酸或天冬氨酸具有强烈的抑制作用, 表明AtLHT2转运中性和酸性氨基酸(Lee and Tegeder, 2004)。RT-PCR分析结果表明, At- LHT4、5和6在花中表达(Foster et al., 2008), 但对其功能的研究较少。
3.3 ProT基因家族在拟南芥中, ProT基因家族有3个成员, 分别为At- ProT1、AtProT2和AtProT3, 它们在拟南芥多个组织中均有表达(Rentsch et al., 1996; Grallath et al., 2005 )。AtProT1在根、茎和花中表达量较高, 在花中主要在进入心皮的花梗韧皮部表达(Rentsch et al., 1996)。AtProT2在根的表皮和皮层细胞中表达, AtProT3则仅在地上部分(如叶、花和角果)检测到表达(Rentsch et al., 1996)。ProTs对脯氨酸和其它相容性溶质的选择性表明, 在胁迫和非胁迫条件下其对维持脯氨酸的内稳态具重要意义。对ProTs的表达分析结果支持了这一推测, 因为ProTs的表达一般与低水势或高脯氨酸(或甘氨酸甜菜碱)含量相关。同样, 花粉中高水平的转录本AtProT1和LeProT1, 表皮中高水平的转录本AtProT3与脯氨酸含量增加相关联(Schwa- cke et al., 1999; Lehmann et al., 2010)。AtProT1转录本在除花粉以外的其它器官中也有发现, 但其表达局限于韧皮部, 表明它可能在氨基酸的长距离转运中起作用(Grallath et al., 2005)。在非生物胁迫或正常条件下, 拟南芥敲除突变体atprot1、atprot2或atprot3均未显示出表型上的差异或脯氨酸含量的变化, 暗示有其它转运蛋白的互补或脯氨酸代谢发生了改变(Lehmann et al., 2010)。然而, 在拟南芥中过表达HvProT导致地上部分的生物量和脯氨酸水平下降, 外施低浓度的脯氨酸可互补这种效应(Ueda et al., 2008)。HvProT过表达植株的PDH mRNA含量和活性均增加, 暗示转运的改变可能诱导了脯氨酸的降解, 致使脯氨酸含量降低(Ueda et al., 2008)。此外, 在拟南芥中过表达根冠特异表达的HvProT可导致根尖更高水平的脯氨酸积累和根伸长的增加(Ueda et al., 2008), 这再次印证了脯氨酸在器官发育中起作用。番茄(Lycopersicon esculentum)中LeProT1在成熟和萌发的花粉中强烈表达, 暗示其可能与花粉营养的供给有关(Schwacke et al., 1999)。
在拟南芥中, 编码脯氨酸转运蛋白涉及多个基因家族和成员, 但是它们的表达模式及对脯氨酸的亲和力有差异, 并在不同的发育阶段和组织中起作用。图1为不同氨基酸转运蛋白对脯氨酸转运作用的示意图。
图 1
Figure 1
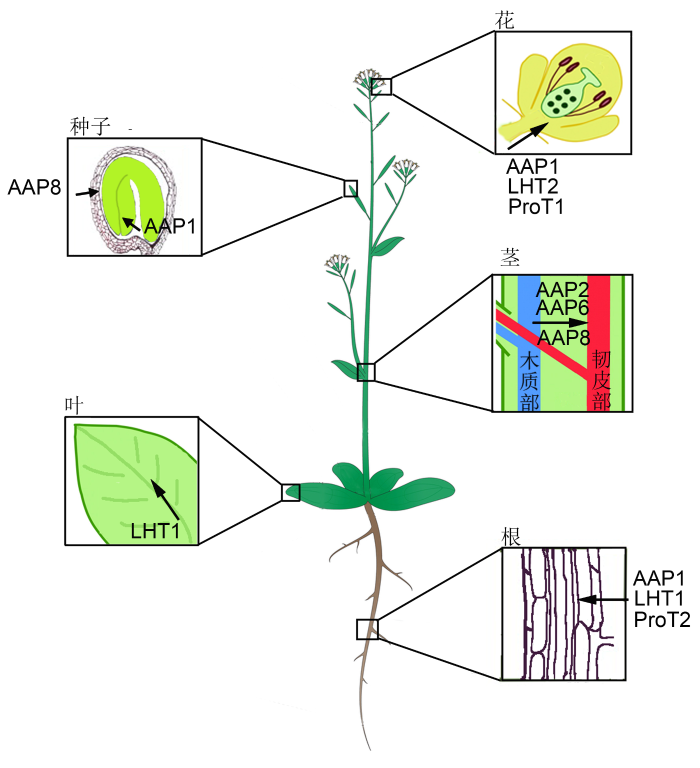
下载原图ZIP
生成PPT
图 1
拟南芥各器官中脯氨酸转运蛋白分布示意图
Figure 1
Proline transporters in different organs in Arabidopsis
4 启动子分析越来越多的证据表明, 植物氨基酸转运蛋白基因受到多种环境和发育信号的转录调控。但目前对这些调控表达的启动子顺式元件了解尚少。对AtAAP1启动子的序列分析表明, 其含有NIT-2识别的GATA元件和富含AT的元件(Chang, 1998)。这两个顺式作用元件可能与氮调节的AtAAP1的表达有关(Guo, 2004)。Liu和Bush (2006)用PlantCare在线工具(http://intra.psb. ugent.be:8080/PlantCARE/) (Lescot et al., 2002)对22个植物氨基酸转运蛋白基因的1 kb启动子序列进行了分析, 发现其中7个基因(AtAAP1、AtAAP3、AtAA- P4、LHT1、LHT2、AtCAT1和AtCAT5)启动子区含有种子特异的氮响应(类似GCN4/RY)元件(Liu and Bu- sh, 2006)。植物中, GCN4元件在一些储存蛋白基因中起增强子的作用, 在氨基酸生物合成和非储存蛋白基因中起沉默子的作用。类似GCN4的RY元件和其它顺式作用元件在种子特异的基因调节中起关键作用(Bäumlein et al., 1992)。
一些植物中, ProTs的表达能被渗透胁迫诱导上调。PlantCare分析结果表明, 在AtAAP3、AtLHT2和AtProT2启动子中有1个重要的干旱响应元件(DRE) (Liu and Bush, 2006)。AtAAPs和AtProTs基因家族成员在它们的启动子区有推测的植物激素(生长素、脱落酸或茉莉酸)响应元件(Liu and Bush, 2006)。这些数据显示, 植物氨基酸转运蛋白基因的表达由一批复杂的涉及糖、氮和植物激素信号途径交叉的顺式元件与转录因子调节。
5 转运蛋白转录水平的调节据报道, 生物因素(如线虫伤害(Hammes et al., 2006; Marella et al., 2013)、菌根真菌互作(Guether et al., 2011))和非生物因素(如光(Guo, 2004)、代谢物(Guo, 2004; Liu and Bush, 2006)和高盐胁迫(Ueda et al., 2001)等)会导致大多数编码氨基酸转运蛋白基因的转录水平发生变化。下面我们重点介绍非生物胁迫条件下转运蛋白转录调节研究现状。
环境信号(如光(Guo, 2004)和渗透变化(Girousse et al., 1996; Rentsch et al., 1996; Ueda et al., 2001))高度调节氨基酸的转运。处于黑暗中的植物暴露于光下, AtAAP1的表达会在6小时内被诱导(Ortiz-Lopez et al., 2000)。用光合成抑制剂DCMU处理表明, 光至少是AtAAP1表达被诱导的一部分信号(Guo, 2004)。响应水和盐胁迫时脯氨酸转运蛋白基因表达上调, 这在许多种植物中已有报道。例如, 拟南芥中的At- ProT2, 大麦中的HvProT, 红树(Rhizophora api- culata)中的AmT1、AmT2和AmT3 (Rentsch et al., 1996; Ueda et al., 2001; Waditee et al., 2002)。在高盐胁迫下, 冰叶日中花(Mesembryanthemum crystallinum)根和叶组织中的脯氨酸含量增加, 且氨基酸转运蛋白基因McAAT1和McAAT2的表达调控也有差异。无胁迫时, McAAT1仅在叶中表达, 而McAAT2仅在根中表达。植物受盐胁迫6小时后, McAAT1在根中的表达短暂上调, McAAT2的表达下调(Popova et al., 2003)。这与Waditee等(2002)的研究结果一致。此外, 有研究表明, 水稻氨基酸转运蛋白家族成员的表达也受环境因素影响(Schwacke et al., 1999; Ueda et al., 2008; Zhao et al., 2012)。
另外, 碳氮代谢物对氨基酸转运蛋白的表达也有影响。用高浓度NO3处理植物, 其体内属于不同亚家族的AtAAP1、AtProT2和AtLHT1的表达量升高(Liu and Bush, 2006)。AAP家族成员的表达还可被代谢物影响。例如, 葡萄糖、蔗糖、NH4和氨基酸能诱导AtAAP1的表达, 谷氨酸能诱导AtAAP2的表达, 但抑制AtAAP6的表达(Guo, 2004)。AtAAP1的这些表达变化与Ortiz-Lopez等(2000)的研究结果一致。高氮条件下, 油菜(Brassica napus)花中的BnAAP1、BnAAP2和BnAAP6的表达上调(Tilsner et al., 2005)。然而高浓度谷氨酰胺、蔗糖以及半胱氨酸(1 mmol·L-1)使VfAAP1的表达下调(Miranda et al., 2001)。这些结果暗示植物体内存在调节碳氮平衡的综合机制。Zhang等(2013)以人参(Panax ginseng)为材料进行研究, 发现植物激素可能影响氨基酸转运蛋白的表达, 响应ABA、水杨酸和茉莉酸甲酯时人参中PgLHT1的表达量增加(Zhang et al., 2013)。
一些中等活性的转运蛋白(如大肠杆菌H+/proline同向转运蛋白ProP和依赖结合蛋白的ABC转运蛋白), 在渗透胁迫下可能通过增加自身基因的表达量和提高自身活性来应对渗透胁迫(Wood et al., 2001)。此外, 有些转运蛋白行使渗透感应器的功能(Morbach and Krämer, 2002; Wood, 2006)。
无内源脯氨酸时, PutP的表达被三功能性的PutA蛋白(在同1个蛋白上结合PDH、P5CDH和发挥调节功能)抑制; 在脯氨酸降解期间, 一旦PutA蛋白被转运到质膜上, PutP立刻被激活(Tanner, 2008; Zhou et al., 2008)。值得一提的是, 哺乳动物中可依靠氯化钠的神经递质转运蛋白家族中的BGT1转运GABA、甘氨酸甜菜碱和脯氨酸(Matskevitch et al., 1999)。与细菌及植物中的脯氨酸和甘氨酸甜菜碱转运蛋白一样, BGT1的表达也可被渗透变化调节, 推测其可能在肾的渗透调节方面起作用(Chen et al., 2004)。
综上, 在响应基因变化时, 氨基酸转运蛋白的表达可能被改变。与野生型相比, 在aap1敲除突变体的种子中AtCAT6以及AtAAP8的转录水平发生改变(Sanders et al., 2009)。在AtAAP2的T-DNA插入突变体中, AtAAP8、AtCAT和AtANT1的表达下调(Zhang et al., 2010), 暗示存在一种以综合方式调整氨基酸转运蛋白表达和活性的调节机制。
6 氨基酸转运蛋白可能在蛋白水平被调节磷酸化是一种蛋白翻译后修饰, 能够改变蛋白的活性。植物中氨基酸转运蛋白磷酸化的证据来自Phos- phAt数据库(Heazlewood et al., 2008), 在这个数据库中, 4个转运蛋白的磷酸化位点已有报道(CAT4、CAT8、LHT4和VAAT4) (Pratelli and Pilot, 2014)。研究人员通过蛋白组学的方法发现了这些位点被磷酸化, 但对其作用尚未进行深入研究。
泛素化是另一种广泛存在的蛋白翻译后调控机制。泛素化过程通常需要ATP提供能量以及E1、E2和E3三种泛素化酶协同作用。泛素化能调节蛋白的活性、丰度、位置和功能。在动物、真菌和细菌中, 泛素化对氨基酸转运蛋白调节的报道较少。例如, 在哺乳动物中, 通过连接酶Nedd4聚泛素化后, 依赖钠的中性氨基酸转运蛋白ATA2表达下调(Hatanaka et al., 2006)。在酵母中, 两个色氨酸透过酶ScTat1以及ScTat2在高压下可被泛素连接酶ScRsp5调节, 触发细胞内吞作用的蛋白质降解(Suzuki et al., 2013)。但在植物中, 尚未见关于泛素化对植物氨基酸转运蛋白活性调节的报道。
7 研究展望随着基因组学研究的快速发展, 越来越多植物中的转运蛋白基因家族被鉴定出来。尽管我们对植物中氨基酸转运蛋白的数目和类型有了更清楚的了解, 但对这些转运蛋白在脯氨酸转运方面的功能了解仍然有限。过表达或异位表达是研究转运蛋白功能的有效方法。Guo (2004)研究发现, 过表达AtAAP1的转基因植物有明显的表型变化, 如变形的叶子、短角果和晚花。这暗示了AtAAP1在拟南芥发育方面起重要作用, 但具体机理还有待进一步研究。截至目前, 大多数已报道的转运蛋白对底物氨基酸的选择具广谱性。考虑到预测的编码氨基酸转运蛋白基因的数目、表达模式以及蛋白表达的共定位, 我们还需深入揭示这些转运蛋白的功能和调控机制。
鉴于脯氨酸的合成与降解发生在不同的细胞器中, 脯氨酸在胞内的转运不可或缺。然而脯氨酸在细胞器间转运的信息尚知之甚少。目前, 仅在小麦(Triti- cum aestivum)中发现2个转运系统能介导脯氨酸向线粒体运输(Di Martino et al., 2006)。故细胞内脯氨酸转运及区室化内容尚需进一步探讨。
参考文献
文献选项
原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | DOI:10.3969/j.issn.1002-5464.2007.01.004URL很多植物在胁迫条件下可以通过增加合成、减少降解而在体内累积大量脯氨酸,这对于调节渗透平衡、防止渗透胁迫对植物造成伤害、清除自由基、保护细胞结构具有重要意义.脯氨酸合成、降解相关酶的编码基因大都已经克隆到,但对脯氨酸在植物发育中的具体作用、胁迫条件下脯氨酸累积的分子机理了解还比较少.概述了植物控制脯氨酸合成、降解相关酶的编码基因的研究进展情况. [本文引用: 1] |
| [2] | URL脯氨酸在植物渗透调节中起着举足轻重的作用,本文就植物在胁迫逆境条件下脯氨酸的自然分布、合成、积累过程及合成酶分子生物学研究现状进行综述,并对已在GenBank中登录的脯氨酸基因家族成员进、行了汇集整理。 [本文引用: 1] |
| [3] | DOI:10.1002/yea.1052PMID:14968425URLAbstract We have found that proline and the toxic proline analogue azetidine-2-carboxylate (AzC) are efficiently imported into Saccharomyces cerevisiae cells by four amino acid permeases, including two nitrogen-regulated permeases ( PUT4 and GAP1 ) and two permeases that are regulated by the SPS sensor of extracellular amino acids ( AGP1 and GNP1 ). In contrast to Agp1p, Gnp1p is not functionally expressed when cells are grown on media containing proline as sole nitrogen source. These findings have implications for the interpretation of studies using AzC to characterize nitrogen source-dependent regulation of amino acid uptake and of post-Golgi targeting and localization of amino acid permeases in yeast. Copyright 2004 John Wiley & Sons, Ltd. [本文引用: 1] |
| [4] | [本文引用: 1] |
| [5] | DOI:10.1016/S0014-5793(99)00516-5PMID:10359089URLArabidopsis thaliana grows efficiently on GABA as the sole nitrogen source, thereby providing evidence for the existence of GABA transporters in plants. Heterologous complementation of a GABA uptake-deficient yeast mutant identified two previously known plant amino acid transporters, AAP3 and ProT2, as GABA transporters with Michaelis constants of 12.9 +/- 1.7 and 1.7 +/- 0.3 mM at pH 4, respectively. The simultaneous transport of [1-14C]GABA and [2,3-3H]proline by ProT2 as a function of pH, provided evidence that the zwitterionic state of GABA is an important parameter in substrate recognition. ProT2-mediated [1-14C]GABA transport was inhibited by proline and quaternary ammonium compounds. [本文引用: 1] |
| [6] | DOI:10.1146/annurev.arplant.49.1.281URL [本文引用: 1] |
| [7] | URLA genomic clone of NAT2 was isolated and sequenced. The 5' -flanking region contains several consensus recognition sites for putative transcription factors. I have now shown that NAT2 expression is nitrate and light inducible, but not circadian-regulated. Since the nitrate assimilatory enzymes are also induced by nitrate, the nitrate-inducible expression of NAT2 provides the first evidence connecting nitrate assimilation and amino acid transport. This observation supports the notion that NAT2 may be involved in phloem loading of newly synthesized amino acids in leaves. In addition, carbohydrate is able to enhance NAT2 expression in dark-adapted plants, suggesting the light induction may be mediated by production of carbohydrate, at least in part. These results provide new insight of environmental regulation of plant amino acid transporters. [本文引用: 1] |
| [8] | DOI:10.1007/s00726-010-0757-2PMID:20890619URLAbstractAlthough free proline accumulation is a well-documented phenomenon in many plants in response to a variety of environmental stresses, and is proposed to play protective roles, high intracellular proline content, by either exogenous application or endogenous over-production, in the absence of stresses, is found to be inhibitory to plant growth. We have shown here that exogenous application of proline significantly induced intracellular Ca accumulation in tobacco and calcium-dependent ROS production in Arabidopsis seedlings, which subsequently enhanced salicylic acid (SA) synthesis and genes expression. This suggested that proline can promote a reaction similar to hypersensitive response during pathogen infection. Other amino acids, such as glutamate, but not arginine and phenylalanine, were also found to be capable of inducing gene expression. In addition, proline at concentration as low as 0.5 mM could induce gene expression. However, proline could not induce the expression of [本文引用: 1] |
| [9] | DOI:10.1104/pp.115.3.1127PMID:9390441URLWe have identified a new amino acid transporter from the Arabidopsis thaliana expressed sequence tag cDNA collection by functional complementation of a yeast amino acid transport mutant. Transport analysis of the expressed protein in yeast shows that it is a high-affinity transporter for both lysine (Lys) and histidine with Michaelis constant values of 175 and 400 M, respectively. This transporter (LHT1, lysine histidine transporter) has little affinity for arginine when measured directly in uptake experiments or indirectly with substrate competition. The cDNA is 1.7 kb with an open reading frame that codes for a protein with 446 amino acids and a calculated molecular mass of 50.5 kD. Hydropathy analysis shows that LHT1 is an integral membrane protein with 9 to 10 putative membrane-spanning domains. Southern-blot analysis suggests that LHT1 is a single-copy gene in the Arabidopsis genome. RNA gel-blot analysis shows that this transporter is present in all tissues, with the strongest expression in young leaves, flowers, and siliques. Wholemount, in situ hybridization revealed that expression is further localized on the surface of roots in young seedlings and in pollen. Overall, LHT1 belongs to a new class of amino acid transporter that is specific for Lys and histidine, and, given its substrate specificity, it has significant promise as a tool for improving the Lys content of Lys-deficient grains. [本文引用: 2] |
| [10] | DOI:10.1007/s00424-003-1064-5PMID:12719981URLAbstract The SLC6 family is a diverse set of transporters that mediate solute translocation across cell plasma membranes by coupling solute transport to the cotransport of sodium and chloride down their electrochemical gradients. These transporters probably have 12 transmembrane domains, with cytoplasmic N- and C-terminal tails, and at least some may function as homo-oligomers. Family members include the transporters for the inhibitory neurotransmitters GABA and glycine, the aminergic transmitters norepinephrine, serotonin, and dopamine, the osmolytes betaine and taurine, the amino acid proline, and the metabolic compound creatine. In addition, this family includes a system B(0+) cationic and neutral amino acid transporter, and two transporters for which the solutes are unknown. In general, SLC6 transporters act to regulate the level of extracellular solute concentrations. In the central and the peripheral nervous system, these transporters can regulate signaling among neurons, are the sites of action of various drugs of abuse, and naturally occurring mutations in several of these proteins are associated with a variety of neurological disorders. For example, transgenic animals lacking specific aminergic transporters show profoundly disturbed behavioral phenotypes and probably represent excellent systems for investigating psychiatric disease. SLC6 transporters are also found in many non-neural tissues, including kidney, intestine, and testis, consistent with their diverse physiological roles. Transporters in this family represent attractive therapeutic targets because they are subject to multiple forms of regulation by many different signaling cascades, and because a number of pharmacological agents have been identified that act specifically on these proteins. [本文引用: 3] |
| [11] | DOI:10.1093/jxb/erq036PMID:20190041URLAmino acids are the currency of nitrogen exchange between source and sink tissues in plants and constitute a major source of the components used for cellular growth and differentiation. The characterization of a new amino acid transporter belonging to the amino acid permease (AAP) family, AAP11, expressed in the perennial species Populus trichocarpa is reported here. PtAAP11 expression analysis was performed by semi-quantitative RT-PCR and GUS activity after poplar transformation. PtAAP11 function was studied in detail by heterologous expression in yeast. The poplar genome contains 14 putative AAPs which is quite similar to other species analysed except Arabidopsis. PtAAP11 was mostly expressed in differentiating xylem cells in different organs. Functional characterization demonstrated that PtAAP11 was a high affinity amino acid transporter, more particularly for proline. Compared with other plant amino acid transporters, PtAAP11 represents a novel high-affinity system for proline. Thus, the functional characterization and expression studies suggest that PtAAP11 may play a major role in xylogenesis by providing proline required for xylem cell wall proteins. The present study provides important information highlighting the role of a specific amino acid transporter in xylogenesis in poplar. [本文引用: 3] |
| [12] | DOI:10.1007/BF02722627PMID:2651863URLThe capacity of organisms to respond to fluctuations in their osmotic environments is an important physiological process that determines their abilities to thrive in a variety of habitats. The primary response of bacteria to exposure to a high osmotic environment is the accumulation of certain solutes, K+, glutamate, trehalose, proline, and glycinebetaine, at concentrations that are proportional to the osmolarity of the medium. The supposed function of these solutes is to maintain the osmolarity of the cytoplasm at a value greater than the osmolarity of the medium and thus provide turgor pressure within the cells. Accumulation of these metabolites is accomplished by de novo synthesis or by uptake from the medium. Production of proteins that mediate accumulation or uptake of these metabolites is under osmotic control. This review is an account of the processes that mediate adaptation of bacteria to changes in their osmotic environment. [本文引用: 1] |
| [13] | DOI:10.1105/tpc.104.023622URL [本文引用: 1] |
| [14] | DOI:10.1007/s00425-005-0166-zPMID:16322984URLAbiotic stresses, such as high salinity or drought, can cause proline accumulation in plants. Such an accumulation involves proline transport into mitochondria where proline catabolism occurs. By using durum wheat seedlings as a plant model system, we investigated how proline enters isolated coupled mitochondria. The occurrence of two separate translocators for proline, namely a carrier solely for proline and a proline/glutamate antiporter, is shown in a functional study in which we found the following: (1) Mitochondria undergo passive swelling in isotonic proline solutions in a stereospecific manner. (2) Externally added L-proline (Pro) generates a mitochondrial membrane potential with a rate depending on the transport of Pro across the mitochondrial inner membrane. (3) The dependence of the rate of generation of on increasing Pro concentrations exhibits hyperbolic kinetics. Proline transport is inhibited in a competitive manner by the non-penetrant thiol reagent mersalyl, but it is insensitive to the penetrant thiol reagent N-ethylmaleimide (NEM). (4) No accumulation of proline occurs inside the mitochondria as a result of the addition of proline externally, whereas the content of glutamate increases both in mitochondria and in the extramitochondrial phase. (5) Glutamate efflux from mitochondria occurs at a rate which depends on the mitochondrial transport, and it is inhibited in a non-competitive manner by NEM. The dependence of the rate of glutamate efflux on increasing proline concentration shows saturation kinetics. The physiological role of carrier-mediated transport in the regulation of proline catabolism, as well as the possible occurrence of a proline/glutamate shuttle in durum wheat seedlings mitochondria, are discussed. [本文引用: 1] |
| [15] | DOI:10.1016/S1360-1385(98)01231-XURLAmino acids are transported between different organs through both xylem and phloem. This redistribution of nitrogen and carbon requires the activity of amino acid transporters in the plasma membrane. In addition, amino acids can be taken up directly by the roots. Amino acid transport has been well characterized in the yeast Saccharomyces cerevisiae, and functional complementation has served as an excellent tool for identifying and characterizing amino acid transporters from plants. The transporters from yeast and plants are related and can be grouped into two large superfamilies. Based on substrate specificity and affinity, as well as expression patterns in plants, different functions have been assigned to some of the individual transporters. Plant mutants for amino acid transporter genes are now being used to study the physiological functions of many of the cloned genes. [本文引用: 1] |
| [16] | DOI:10.1074/jbc.270.27.16315PMID:7608199URLAbstract Three amino acid transporter genes (AAP3-5) were isolated from Arabidopsis by complementation of a yeast mutant defective in histidine uptake. Transport is driven against a concentration gradient and sensitive to protonophores. Analysis of the substrate specificity demonstrates that the carriers have a broad substrate specificity covering the major transport forms of reduced nitrogen, i.e. glutamine and glutamate. The transporters have similar affinities for glutamate, glutamine, and alanine but differ with respect to valine, phenylalanine, histidine, arginine, and lysine. AAP3 and AAP5 efficiently transport arginine and lysine and are involved in basic amino acid transport. The predicted polypeptides of 53 kDa are highly hydrophobic with 12 putative membrane-spanning regions and show significant homologies to Arabidopsis amino acid transporters AAP1 and AAP2. Each of the genes has a different organ-specific expression in the plant. AAP3 is exclusively expressed in roots and AAP4 mainly in source leaves, stems, and flowers, whereas AAP5 is found in all tissues. The specific distribution in the plant and the different substrate specificities of AAP transporters may indicate that tissues differ both qualitatively and quantitatively regarding import or export of amino acids. [本文引用: 1] |
| [17] | DOI:10.1046/j.1365-313X.2002.01248.xURL [本文引用: 1] |
| [18] | DOI:10.1007/s00497-008-0074-zURLSexual plant reproduction necessitates proper development of pollen, pollen germination and tube growth through various tissues of the pistil, the female organ of the flower. Finally, sperm cells are released to fertilize the female gametophyte. These processes require high metabolic activities of all tissues involved and rely on the delivery of nitrogen assimilates for success. However, transporters mediating nitrogen fluxes are mostly unknown. The presented work provides an expression analysis of members of the LHT amino acid transporter family in relation to pollen development and pollen istil interaction. Expression of Arabidopsis LHT s was analyzed during flower development and the location of LHT function resolved by transporter-GFP and promoter- GUS studies. GFP-LHT localization in onion cells indicates that all LHTs analyzed are targeted to the plasma membrane. We further showed that LHT s are expressed in anthers and male gametophytes where they are proposed to function in transport of amino acids for pollen development and maturation. Expression in germinating pollen, pollen tubes and transmitting tissue of the pistil points to a role of LHTs in support of the fertilization process. Overall, our study suggests that LHT function in flowers is cell or tissue specific, developmentally regulated and highly coordinated between male and female tissue. [本文引用: 1] |
| [19] | [本文引用: 1] |
| [20] | DOI:10.1104/pp.111.1.109PMID:12226278URLChanges in amino acid composition of alfalfa (Medicago sativa L.) phloem sap were studied in response to a water deficit. Sap was collected by stylectomy. As the leaf water potential (ψ) decreased from -0.4 to -2.0 MPa, there was a significant increase of the total amino acid concentration, due to that of some amino acids: proline, valine, isoleucine, leucine, glutamic acid, aspartic acid, and threonine. Asparagine concentration, which is the main amino acid assayed in the phloem sap of alfalfa (it accounts for 70% of the total content), did not vary with the plant water status. The other amino acid concentrations remained stable as ψ varied; in particular, γ-amino butyric acid concentration remained unchanged, whereas it varied in response to wounding. The more striking change in the sieve tubes was the accumulation of proline, which was observed below a ψ threshold value of about -0.9 MPa (concentration ×60 for a decrease of ψ from -0.9 to -2.0 MPa). The role of such changes in phloem sap amino acid concentration in osmotic adjustment of growing tissues is discussed. [本文引用: 1] |
| [21] | DOI:10.1104/pp.104.055079PMID:15618414URLProline transporters (ProTs) mediate transport of the compatible solutes Pro, glycine betaine, and the stress-induced compound -aminobutyric acid. A new member of this gene family, AtProT3, was isolated from Arabidopsis (Arabidopsis thaliana), and its properties were compared to AtProT1 and AtProT2. Transient expression of fusions of AtProT and the green fluorescent protein in tobacco (Nicotiana tabacum) protoplasts revealed that all three AtProTs were localized at the plasma membrane. Expression in a yeast (Saccharomyces cerevisiae) mutant demonstrated that the affinity of all three AtProTs was highest for glycine betaine (Km= 0.1-0.3 mM), lower for Pro (Km= 0.4-1 mM), and lowest for -aminobutyric acid (Km= 4-5 mM). Relative quantification of the mRNA level using real-time PCR and analyses of transgenic plants expressing the -glucuronidase (uidA) gene under control of individual AtProT promoters showed that the expression pattern of AtProTs are complementary. AtProT1 expression was found in the phloem or phloem parenchyma cells throughout the whole plant, indicative of a role in long-distance transport of compatible solutes. 尾-Glucuronidase activity under the control of the AtProT2 promoter was restricted to the epidermis and the cortex cells in roots, whereas in leaves, staining could be demonstrated only after wounding. In contrast, AtProT3 expression was restricted to the above-ground parts of the plant and could be localized to the epidermal cells in leaves. These results showed that, although intracellular localization, substrate specificity, and affinity are very similar, the transporters fulfill different roles in planta. [本文引用: 4] |
| [22] | [本文引用: 1] |
| [23] | [本文引用: 1] |
| [24] | [本文引用: 6] |
| [25] | DOI:10.1111/j.1365-313X.2006.02880.xPMID:17052324URLAmino acids represent the major form of reduced nitrogen that is transported in plants. Amino acid transporters in plants often show tissue-specific expression patterns and are used by plants to transport these metabolites from source to sink during development and under changing environmental conditions. We identified one amino acid transporter, AtCAT6, which is expressed in sink tissues such as lateral root primordia, flowers and seeds. Additionally AtCAT6 was induced during infestation of roots by the plant-parasitic root-knot nematode, Meloidogyne incognita . Quantitative reverse-transcriptase PCR revealed nematode inducibility throughout the duration of nematode infestation and in nematode-induced feeding sites. Promoter analyses confirmed expression in endogenous sink tissues and nematode-induced feeding sites. In Xenopus oocytes, AtCAT6 mediated electrogenic transport of proteinogenic as well as non-proteinogenic amino acids with moderate affinity. AtCAT6 transported large, neutral and cationic amino acids in preference to other amino acids. Knockout mutants of this transporter failed to grow on medium containing l -glutamine as the sole nitrogen source. Our data suggest that AtCAT6 plays a role in supplying amino acids to sink tissues of plants and nematode-induced feeding structures. [本文引用: 1] |
| [26] | DOI:10.1074/jbc.M606577200PMID:17003038URLAbstract We report here that ubiquitin ligase Nedd4-2 regulates amino acid transporter ATA2 activity on the cell surface. We first found that a proteasome inhibitor MG132 increased the uptake of alpha-(methylamino)isobutyric acid, a model substrate for amino acid transport system A, in 3T3-L1 adipocytes as well as the preadipocytes. Transient expression of Nedd4-2 in Xenopus oocytes and Chinese hamster ovary cells down-regulated the ATA2 transport activity induced by injected cRNA and transfected cDNA, respectively. Neither the Nedd4-2 mutant with defective catalytic domain nor c-Cbl affected the ATA2 activity significantly. RNA-mediated interference of Nedd4-2 increased the ATA2 activity in the cells, and this was associated with decreased polyubiquitination of ATA2 on the cell surface membrane. Immunofluorescent analysis of Nedd4-2 in the adipocytes stably transfected with the enhanced green fluorescent protein (EGFP)-tagged ATA2 showed the co-localization of Nedd4-2 and EGFP-ATA2 in the plasma membrane but not in the perinuclear ATA2 storage site, supporting the idea that the primary site for the ubiquitination of ATA2 is the plasma membrane. These data suggest that ATA2 on the plasma membrane is subject to polyubiquitination by Nedd4-2 with consequent endocytotic sequestration and proteasomal degradation and that this process is an important determinant of the density of ATA2 functioning on the cell surface. [本文引用: 1] |
| [27] | [本文引用: 1] |
| [28] | DOI:10.1105/tpc.106.041012PMID:16816136URLAmino acid transport in plants is mediated by at least two large families of plasma membrane transporters. Arabidopsis thaliana, a nonmycorrhizal species, is able to grow on media containing amino acids as the sole nitrogen source. Arabidopsis amino acid permease (AAP) subfamily genes are preferentially expressed in the vascular tissue, suggesting roles in long-distance transport between organs. We show that the broad-specificity, high-affinity amino acid transporter LYSINE HISTIDINE TRANSPORTER1 (LHT1), an AAP homolog, is expressed in both the rhizodermis and mesophyll of Arabidopsis. Seedlings deficient in LHT1 cannot use Glu or Asp as sole nitrogen sources because of the severe inhibition of amino acid uptake from the medium, and uptake of amino acids into mesophyll protoplasts is inhibited. Interestingly, Iht1 mutants, which show growth defects on fertilized soil, can be rescued when LHT1 is reexpressed in green tissue. These findings are consistent with two major LHT1 functions: uptake in roots and supply of leaf mesophyll with xylem-derived amino acids. The capacity for amino acid uptake, and thus nitrogen use efficiency under limited inorganic N supply, is increased severalfold by LHT1 overexpression. These results suggest that LHT1 overexpression may improve the N efficiency of plant growth under limiting nitrogen, and the mutant analyses may enhance our understanding of N cycling in plants. [本文引用: 4] |
| [29] | DOI:10.1073/pnas.89.19.9354PMID:1384052URLMany plants synthesize and accumulate proline in response to osmotic stress. Despite the importance of this pathway, however, the exact metabolic route and enzymes involved in the synthesis of proline in plants have not been unequivocally identified. We report here the isolation of a mothbean (Vigna aconitifolia) cDNA clone encoding a bifunctional enzyme, Δ1-pyrroline-5-carboxylate synthetase (P5CS), with both γ-glutamyl kinase and glutamic-γ-semialdehyde dehydrogenase activities that catalyzes the first two steps in proline biosynthesis. The two enzymatic domains of P5CS correspond to the ProB and ProA proteins of Escherichia coli and contain a leucine zipper in each domain, which may facilitate inter- or intramolecular interaction of this protein. The Vigna P5CS enzyme activity is feedback regulated by proline but is less sensitive to end-product inhibition than is the E. coli γ-glutamyl kinase. The P5CS gene is expressed at high levels in Vigna leaves and is inducible in roots subjected to salt stress, suggesting that P5CS plays a key role in proline biosynthesis, leading to osmoregulation in plants. [本文引用: 1] |
| [30] | DOI:10.1093/jxb/erp274PMID:19755569URLThe aim of this study was to investigate the role of the amino acid permease geneAAP6in regulating phloem amino acid composition and then to determine the effects of this altered diet on aphid performance. A genotype ofArabidopsis thaliana(L.) was produced in which the function of the amino acid permease geneAAP6(At5g49630) was abolished. Plants homozygous for the insertionally inactivatedAAP6gene had a significantly larger mean rosette width than the wild type and a greater number of cauline leaves. Seeds from theaap6mutant were also significantly larger than those from the wild-type plants. Sieve element (SE) sap was collected by aphid stylectomy and the amino acids derivatized, separated, and quantified using Capillary Electrophoresis with Laser Induced Fluorescence (CE-LIF). In spite of the large variation across samples, the total amino acid concentration of SE sap of theaap6mutant plants was significantly lower than that of the wild-type plants. The concentrations of lysine, phenylalanine, leucine, and aspartic acid were all significantly lower in concentration in theaap6mutant plants compared with wild-type plants. This is the first direct demonstration of a physiological role for an amino acid transporter in regulating SE compositionin vivo. The amino acid availability in sieve element sap is thought to be the major limiting factor for aphid growth and reproduction. Despite the changes in their diet, the aphidMyzus persicae(Sulzer) displayed only small changes in feeding behaviour on mutant plants when measured using the Electronic Penetration Graph (EPG) technique. Salivation by the aphid into the SE (E1 phase) was increased on mutant plants but there was no significant effect on other feeding EPG behaviours, or in the rate of honeydew production. Consistent with the small effect on aphid feeding behaviour, there was only a small effect of reduced sieve element amino acid concentration on aphid reproduction. The data are discussed in relation to the regulation of phloem composition and the role of phloem amino acids in regulating aphid performance. [本文引用: 2] |
| [31] | DOI:10.1093/pcp/41.6.750PMID:10945345URLA cDNA encoding a proline (Pro) transporter (ProT) was isolated and characterized from a cDNA library prepared from 14-d-old seedlings of Oryza sativa cv. Akibare. The deduced amino acid sequence of the rice ProT protein (OsProT) had 68.8% homology to the ProT protein 1 from Arabidopsis thaliana and 59.6% homology to that from Lycopersicon esculentum. Northern blot analysis revealed that the gene for OsProT (OsProT) was expressed in all organs examined, comparatively strongly in leaf sheath and stem. Salt treatment did not induce expression of OsProT but strongly induced expression of the gene for delta1-pyrroline-5-carboxylate synthetase (P5CS), a key enzyme in Pro biosynthesis. Southern blot analysis revealed that OsProT has a gene family. OsProT specifically transported L-Pro in a transport assay using Xenopus laevis oocytes. [本文引用: 1] |
| [32] | [本文引用: 1] |
| [33] | DOI:10.1105/tpc.8.8.1323PMID:8776899URLProline is one of the most common compatible osmolytes in water-stressed plants. The accumulation of proline in dehydrated plants is caused both by the activation of proline biosynthesis and by the inactivation of proline degradation; a decrease in the level of accumulated proline in rehydrated plants is caused both by the inhibition of proline biosynthesis and by the activation of proline degradation. The proline biosynthetic pathway has been well characterized, but the degradation of proline is poorly understood. Sequence analysis of an Arabidopsis cDNA clone, ERD5 (for early responsive to dehydration stress), isolated from plants dehydrated for 1 hr. revealed that it encodes a protein with identity to products of the yeast PUT1 (for proline utilization) gene (23.6% over 364 amino acids) and the Drosophila sluggish-A gene (34.5% over 255 amino acids). Their gene products are precursors of proline oxidases (dehydrogenase) (EC 1.5.99.8), which are the first enzymes involved in the conversion of proline to glutamic acid. Proline oxidase is localized in mitochondria. RNA gel blot analysis demonstrated that transcripts of the ERD5 gene were undetectable when plants had been dehydrated for 10 hr, but large amounts of the transcript accumulated when plants subsequently were rehydrated. Elevated levels of the transcript were also found in plants that had been incubated in a medium that contained proline. Immunologically, we showed that the product of ERD5 is localized in the mitochondrial fraction and accumulates in response to proline in cultured cells. Fusion genes for ERD5 and PUT1 complemented a put1 mutant of yeast, allowing put1 to grow with proline as the source of nitrogen. These results suggest that ERD5 encodes a precursor of proline dehydrogenase (oxidase), which is regulated at the level of mRNA accumulation in both dehydrated and rehydrated plants. [本文引用: 1] |
| [34] | DOI:10.1046/j.1365-313X.2003.01618.xURL [本文引用: 1] |
| [35] | [本文引用: 3] |
| [36] | DOI:10.1111/j.1365-313X.2007.03045.xPMID:17419840URLAmino acids are available to plants in some soils in significant amounts, and plants frequently make use of these nitrogen sources. The goal of this study was to identify transporters involved in the uptake of amino acids into root cells. Based on the fact that high concentrations of amino acids inhibit plant growth, we hypothesized that mutants tolerating toxic levels of amino acids might be deficient in the uptake of amino acids from the environment. To test this hypothesis, we employed a forward genetic screen for Arabidopsis thaliana mutants tolerating toxic concentrations of amino acids in the media. We identified an Arabidopsis mutant that is deficient in the amino acid permease 1 (AAP1, At1g58360) and resistant to 10 m m phenylalanine and a range of other amino acids. The transporter was localized to the plasma membrane of root epidermal cells, root hairs, and throughout the root tip of Arabidopsis. Feeding experiments with [ 14 C]-labeled neutral, acidic and basic amino acids showed significantly reduced uptake of amino acids in the mutant, underscoring that increased tolerance of aap1 to high levels of amino acids is coupled with reduced uptake by the root. The growth and uptake studies identified glutamate, histidine and neutral amino acids, including phenylalanine, as physiological substrates for AAP1, whereas aspartate, lysine and arginine are not. We also demonstrate that AAP1 imports amino acids into root cells when these are supplied at ecologically relevant concentrations. Together, our data indicate an important role of AAP1 for efficient use of nitrogen sources present in the rhizosphere. [本文引用: 3] |
| [37] | DOI:10.1111/j.1365-313X.2004.02186.xPMID:155001URLWithin the flower, microsporogenesis represents a major sink for nitrogen, but knowledge on how the imported nitrogen is transferred from the anther cell layers to developing pollen is lacking. Here, we provide information on characterization of a transporter (AtLHT2) that might play an important role in partitioning of amino acids for microspore development. Biochemical analysis in yeast showed that AtLHT2 transports proline and aspartate with high affinity. However, other neutral and acidic amino acids act as strong competitors for proline and aspartate uptake indicating that AtLHT2 generally transports uncharged and negatively charged amino acids. Comparison of the apparent K m values of AtLHT2 with previously characterized amino acid transporters clearly demonstrated that AtLHT2 represents a novel high-affinity system for neutral and acidic amino acids. Northern blot analysis showed strong expression of the amino acid transporter in flower buds. Cellular expression could be resolved by using RNA in situ hybridization and in situ RT-PCR methods, which localized AtLHT2 specifically to the tapetum tissue of the anthers. Developing pollen grains are symplasmically isolated from the sporophytic tissue and rely on the nutrients and other compounds secreted from the tapetum cells. Thus, the functional characterization of AtLHT2, together with our expression and localization studies, strongly suggest that in Arabidopsis flowers, AtLHT2 has a critical function in import of neutral and acidic amino acids into the tapetum cells for synthesis of compounds important for microspore structure and in transfer of organic nitrogen to the locule for pollen development. [本文引用: 3] |
| [38] | DOI:10.1007/s00726-010-0525-3PMID:20204435URLProline fulfils diverse functions in plants. As amino acid it is a structural component of proteins, but it also plays a role as compatible solute under environmental stress conditions. Proline metabolism involves several subcellular compartments and contributes to the redox balance of the cell. Proline synthesis has been associated with tissues undergoing rapid cell divisions, such as shoot apical meristems, and appears to be involved in floral transition and embryo development. High levels of proline can be found in pollen and seeds, where it serves as compatible solute, protecting cellular structures during dehydration. The proline concentrations of cells, tissues and plant organs are regulated by the interplay of biosynthesis, degradation and intra- as well as intercellular transport processes. Among the proline transport proteins characterized so far, both general amino acid permeases and selective compatible solute transporters were identified, reflecting the versatile role of proline under stress and non-stress situations. The review summarizes our current knowledge on proline metabolism and transport in view of plant development, discussing regulatory aspects such as the influence of metabolites and hormones. Additional information from animals, fungi and bacteria is included, showing similarities and differences to proline metabolism and transport in plants. [本文引用: 4] |
| [39] | DOI:10.1007/s00726-005-0248-zPMID:16525755URLRecent studies have shown that there are more than 50 amino acid transporter genes in the Arabidopsis genome. This abundance of amino acid transporters implies that they play a multitude of fundamental roles in plant growth and development. Current research on the expression and regulation (i.e., tissue-specific expression and regulation of expression in response to nutrient and environmental changes) of these genes has provided useful information about the functional significance of plant amino acid transport systems. [本文引用: 6] |
| [40] | DOI:10.1104/pp.111.175810URL [本文引用: 1] |
| [41] | DOI:10.1094/MPMI-05-12-0123-FIPMID:23194341URLThe root-knot nematode, Meloidogyne incognita, is an obligate parasite which depends entirely on the host plant for its nutrition. Root-knot nematodes induce the formation of a highly specialized feeding site consisting of several giant cells surrounded by a network of vascular tissues. Nutrients, including amino acids and sugars, are transferred apoplastically from the vascular tissues to the feeding site. Using Arabidopsis thaliana lacking the vascular-expressed amino acid permeases (AAP) AAP3 or AAP6, we demonstrate that disruption of amino acid transport can affect nematode parasitism. Nematode infestation levels are significantly reduced on the aap3 and aap6 mutants. AAP3 and AAP6 act distinctly in the transport of amino acids to the feeding site, as demonstrated by differences in their carrying capacity profiles. Furthermore, analyses of promoter:beta-glucuronidase lines show different expression patterns for AAP3 and AAP6 in infected roots. In the aap3-3 mutant, part of the decrease in infestation is connected to a defect in early infection, where juveniles enter but then leave the root. Both aap3-3 and aap6-1 produce fewer females and produce more adult male nematodes. Additionally, detrimental effects are observed in the nematodes harvested from aap3-3 and aap6-1 mutants, including decreased egg hatching and infectivity and lower levels of lipid reserves. The transport of amino acids by AAP3 and AAP6 is important for nematode infection and success of the progeny. [本文引用: 1] |
| [42] | DOI:10.1074/jbc.274.24.16709PMID:10358010URLBetaine is an osmolyte accumulated in cells during osmotic cell shrinkage. The canine transporter mediating cellular accumulation of the osmolyte betaine and the neurotransmitter gamma-aminobutyric acid (BGT-1) was expressed in Xenopus oocytes and analyzed by two-electrode voltage clamp and tracer flux studies. Exposure of oocytes expressing BGT-1 to betaine or gamma-aminobutyric acid (GABA) depolarized the cell membrane in the current clamp mode and induced an inward current under voltage clamp conditions. At 1 mM substrate the induced currents decreased in the following order: betaine = GABA > diaminobutyric acid = beta-alanine > proline = quinidine > dimethylglycine > glycine > sarcosine. Both the Vmax and Km of GABA- and betaine-induced currents were voltage-dependent, and GABA- and betaine-induced currents and radioactive tracer uptake were strictly Na+-dependent but only partially dependent on the presence of Cl-. The apparent affinity of GABA decreased with decreasing Na+ concentrations. The Km of Na+ also depended on the GABA and Cl- concentration. A decrease of the Cl- concentration reduced the apparent affinity for Na+ and GABA, and a decrease of the Na+ concentration reduced the apparent affinity for Cl- and GABA. A comparison of 22Na+-, 36Cl--, and 14C-labeled GABA and 14C-labeled betaine fluxes and GABA- and betaine-induced currents yielded a coupling ratio of Na+/Cl-/organic substrate of 3:1:1 or 3:2:1. Based on the data, a transport model of ordered binding is proposed in which GABA binds first, Na+ second, and Cl- third. In conclusion, BGT-1 displays significant functional differences from the other members of the GABA transporter family. [本文引用: 3] |
| [43] | DOI:10.1046/j.1365-313X.2001.01129.xPMID:11696187URLSummary Full length cDNAs encoding three amino acid permeases were isolated from seed-specific libraries of Vicia faba . The predicted proteins VfAAP1, VfAAP3 and VfAAP4 share up to 66% identity among themselves. Functional characterization of VfAAP1 and VfAAP3 in a yeast mutant showed that these permeases transport a broad range of amino acids. However, VfAAP1 had a preference for cysteine and VfAAP3 for lysine and arginine. VfAAP1 was highly expressed in cotyledons at early developmental stages and moderately in other sink tissues. Its peak of expression in cotyledons corresponded to the appearance of storage protein transcripts, suggesting that this transporter fulfills an important role in providing amino acids for storage protein biosynthesis. VfAAP3 was expressed most abundantly in maternal tissues, that is in roots, stems, gynoecia, pods and seed coats at different developmental stages. VfAAP4 transcripts could not be detected by northern hybridization. In situ hybridization showed that VfAAP1 mRNA is distributed throughout cotyledon storage parenchyma cells, but could not be detected in the abaxial epidermal cell layer. It also accumulate in the chlorenchyma and thin-walled parenchyma cells of seed coats. VfAAP1 mRNA levels were lower in cotyledons cultured in the presence of glutamine, whereas expression of a vicilin storage protein gene was up-regulated under similar conditions. Cysteine repressed the expression of the GUS reporter gene under control of the VfAAP1 promoter, suggesting that this transporter is modulated at the transcriptional level. Regulation of amino acid transport in relation to storage protein accumulation is discussed. [本文引用: 2] |
| [44] | DOI:10.1002/chin.200229289PMID:12007171URLChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option. [本文引用: 1] |
| [45] | DOI:10.1074/jbc.M207730200URL [本文引用: 6] |
| [46] | DOI:10.1016/S0005-2736(00)00144-9PMID:10748260URLAmino acid transporters are essential participants in the resource allocation processes that support plant growth and development. Recent results have identified several new transporters that contribute to a wide array of physiological activities, and detailed molecular analysis has provided fundamental insights into the structure, function and regulation of these integral membrane proteins. [本文引用: 2] |
| [47] | [本文引用: 2] |
| [48] | DOI:10.1093/jxb/eru278PMID:4157705URLPlants use soil amino acids as a nitrogen source. Plasma membrane-localized transport proteins are essential for the import of a broad spectrum of amino acids into the root cells. Plants acquire nitrogen in the form of amino acids from the soil, and transport proteins located in the plasma membrane of root cells are required for this process. It was found that the Arabidopsis lysine-histidine-like transporter LHT6 is expressed in root cells important for amino acid uptake, including the epidermis, root hairs, and cortex. Transport studies with lht6 mutants using high levels of amino acids demonstrated that LHT6 is in fact involved in amino acid uptake. To determine if LHT6 plays a role in nitrogen acquisition at soil amino acid concentrations, growth and uptake studies were performed with low levels of toxic amino acid analogues and radiolabelled amino acids, respectively. In addition, mutants of AAP1, another root amino acid transporter, and lht6/aap1 double mutants were examined. The results showed that LHT6 is involved in uptake of acidic amino acids, glutamine and alanine, and probably phenylalanine. LHT6 seems not to transport basic or other neutral amino acids, or, alternatively, other transporters might compensate for eliminated LHT6 function. Previous studies suggested that AAP1 only takes up amino acids at high concentrations; however, here it is demonstrated that the transporter functions in acquisition of glutamate and neutral amino acids when present at soil concentrations. When comparing the characterized root uptake systems, it appears that transporters both with overlapping substrate specificity and with preference for specific substrates are required to access the soil amino acid pool. [本文引用: 1] |
| [49] | [本文引用: 1] |
| [50] | DOI:10.1093/jxb/eru320PMID:25114014URLAmino acids play several critical roles in plants, from providing the building blocks of proteins to being essential metabolites interacting with many branches of metabolism. They are also important molecules that shuttle organic nitrogen through the plant. Because of this central role in nitrogen metabolism, amino acid biosynthesis, degradation, and transport are tightly regulated to meet demand in response to nitrogen and carbon availability. While much is known about the feedback regulation of the branched biosynthesis pathways by the amino acids themselves, the regulation mechanisms at the transcriptional, post-transcriptional, and protein levels remain to be identified. This review focuses mainly on the current state of our understanding of the regulation of the enzymes and transporters at the transcript level. Current results describing the effect of transcription factors and protein modifications lead to a fragmental picture that hints at multiple, complex levels of regulation that control and coordinate transport and enzyme activities. It also appears that amino acid metabolism, amino acid transport, and stress signal integration can influence each other in a so-far unpredictable fashion. [本文引用: 1] |
| [51] | [本文引用: 1] |
| [52] | DOI:10.1105/tpc.8.8.1437PMID:8776904URLAbstract A yeast mutant lacking SHR3, a protein specifically required for correct targeting of plasma membrane amino acid permeases, was used to study the targeting of plant transporters and as a tool to isolate new SHR3-independent amino acid transporters. For this purpose, an shr3 mutant was transformed with an Arabidopsis cDNA library. Thirty-four clones were capable of growth under selective conditions, but none showed homology with SHR3. However, genes encoding eight different amino acid transporters belonging to three different transporter families were isolated. Five of these are members of the general amino acid permease (AAP) gene family, one is a member of the NTR family, encoding an oligopeptide transporter, and two belong to a new class of transporter genes. A functional analysis of the latter two genes revealed that they encode specific proline transporters (ProT) that are distantly related to the AAP gene family. ProT1 was found to be expressed in all organs, but highest levels were found in roots, stems, and flowers. Expression in flowers was highest in the floral stalk phloem that enters the carpels and was downregulated after fertilization, indicating a specific role in supplying the ovules with proline. ProT2 transcripts were found ubiquitously throughout the plant, but expression was strongly induced under water or salt stress, implying that ProT2 plays an important role in nitrogen distribution during water stress, unlike members of the AAP gene family whose expression was repressed under the same conditions. These results corroborate the general finding that under water stress, amino acid export is impaired whereas proline export is increased. [本文引用: 6] |
| [53] | DOI:10.1016/j.febslet.2007.04.013PMID:17466985URLNitrogen is an essential macronutrient for plant growth. Following uptake from the soil or assimilation within the plant, organic nitrogen compounds are transported between organelles, from cell to cell and over long distances in support of plant metabolism and development. These translocation processes require the function of integral membrane transporters. The review summarizes our current understanding of the molecular mechanisms of organic nitrogen transport processes, with a focus on amino acid, ureide and peptide transporters. [本文引用: 1] |
| [54] | DOI:10.1111/j.1365-313X.2009.03890.xPMID:19392706URLThe embryo of Arabidopsis seeds is symplasmically isolated from the surrounding seed coat and endosperm, and uptake of nutrients from the seed apoplast is required for embryo growth and storage reserve accumulation. With the aim of understanding the importance of nitrogen (N) uptake into developing embryos, we analysed two mutants of AAP1 (At1g58360), an amino acid transporter that was localized to Arabidopsis embryos. In mature and desiccated aap1 seeds the total N and carbon content was reduced while the total free amino acid levels were strongly increased. Separately analysed embryos and seed coats/endosperm of mature seeds showed that the elevated amounts in amino acids were caused by an accumulation in the seed coat/endosperm, demonstrating that a decrease in uptake of amino acids by the aap1 embryo affects the N pool in the seed coat/endosperm. Also, the number of protein bodies was increased in the aap1 endosperm, suggesting that the accumulation of free amino acids triggered protein synthesis. Analysis of seed storage compounds revealed that the total fatty acid content was unchanged in aap1 seeds, but storage protein levels were decreased. Expression analysis of genes of seed N transport, metabolism and storage was in agreement with the biochemical data. In addition, seed weight, as well as total silique and seed number, was reduced in the mutants. Together, these results demonstrate that seed protein synthesis and seed weight is dependent on N availability and that AAP1-mediated uptake of amino acids by the embryo is important for storage protein synthesis and seed yield. [本文引用: 2] |
| [55] | DOI:10.1104/pp.16.00244PMID:27016446URLAllocation of large amounts of nitrogen to developing organs occurs in the phloem and is essential for plant growth and seed development. In Arabidopsis (Arabidopsis thaliana) and many other plant species, amino acids represent the dominant nitrogen transport forms in the phloem, and they are mainly synthesized in photosynthetically active source leaves. Following their synthesis, a broad spectrum of the amino nitrogen is actively loaded into the phloem of leaf minor veins and transported within the phloem sap to sinks such as developing leaves, fruits, or seeds. Controlled regulation of the source-to-sink transport of amino acids has long been postulated; however, the molecular mechanism of amino acid phloem loading was still unknown. In this study, Arabidopsis AMINO ACID PERMEASE8 (AAP8) was shown to be expressed in the source leaf phloem and localized to the plasma membrane, suggesting its function in phloem loading. This was further supported by transport studies with aap8 mutants fed with radiolabeled amino acids and by leaf exudate analyses. In addition, biochemical and molecular analyses revealed alterations in leaf nitrogen pools and metabolism dependent on the developmental stage of the mutants. Decreased amino acid phloem loading and partitioning to sinks led to decreased silique and seed numbers, but seed protein levels were unchanged, demonstrating the importance of AAP8 function for sink development rather than seed quality. Overall, these results show that AAP8 plays an important role in source-to-sink partitioning of nitrogen and that its function affects source leaf physiology and seed yield. |
| [56] | [本文引用: 1] |
| [57] | [本文引用: 3] |
| [58] | DOI:10.2307/3870867PMID:10072398URLDuring maturation, pollen undergoes a period of dehydration accompanied by the accumulation of compatible solutes. Solute import across the pollen plasma membrane, which occurs via proteinaceous transporters, is required to support pollen development and also for subsequent germination and pollen tube growth. Analysis of the free amino acid composition of various tissues in tomato revealed that the proline content in flowers was 60 times higher than in any other organ analyzed. Within the floral organs, proline was confined predominantly to pollen, where it represented >70% of total free amino acids. Uptake experiments demonstrated that mature as well as germinated pollen rapidly take up proline. To identify proline transporters in tomato pollen, we isolated genes homologous to Arabidopsis proline transporters. LeProT1 was specifically expressed both in mature and germinating pollen, as demonstrated by RNA in situ hybridization. Expression in a yeast mutant demonstrated that LeProT1 transports proline and -amino butyric acid with low affinity and glycine betaine with high affinity. Direct uptake and competition studies demonstrate that LeProT1 constitutes a general transporter for compatible solutes. [本文引用: 4] |
| [59] | DOI:10.1104/pp.104.045278PMID:15377779URLMore than 50 distinct amino acid transporter genes have been identified in the genome of Arabidopsis, indicating that transport of amino acids across membranes is a highly complex feature in plants. Based on sequence similarity, these transporters can be divided into two major superfamilies: the amino acid transporter family and the amino acid polyamine choline transporter family. Currently, mainly transporters of the amino acid transporter family have been characterized. Here, a molecular and functional characterization of amino acid polyamine choline transporters is presented, namely the cationic amino acid transporter (CAT) subfamily. CAT5 functions as a high-affinity, basic amino acid transporter at the plasma membrane. Uptake of toxic amino acid analogs implies that neutral or acidic amino acids are preferentially transported by CAT3, CAT6, and CAT8. The expression profiles suggest that CAT5 may function in reuptake of leaking amino acids at the leaf margin, while CAT8 is expressed in young and rapidly dividing tissues such as young leaves and root apical meristem. CAT2 is localized to the tonoplast in transformed Arabidopsis protoplasts and thus may encode the long-sought vacuolar amino acid transporter. [本文引用: 2] |
| [60] | DOI:10.1128/EC.00049-13PMID:3697464URLCells of Saccharomyces cerevisiae express two tryptophan permeases, Tat1 and Tat2, which have different characteristics in terms of their affinity for tryptophan and intracellular localization. Although the high-affinity permease Tat2 has been well documented in terms of its ubiquitin-dependent degradation, the low-affinity permease Tat1 has not yet been characterized fully. Here we show that a high hydrostatic pressure of 25 MPa triggers a degradation of Tat1 which depends on Rsp5 ubiquitin ligase and the EH domain-containing protein End3. Tat1 was resistant to a 3-h cycloheximide treatment, suggesting that it is highly stable under normal growth conditions. The ubiquitination of Tat1 most likely occurs at N-terminal lysines 29 and 31. Simultaneous substitution of arginine for the two lysines prevented Tat1 degradation, but substitution of either of them alone did not, indicating that the roles of lysines 29 and 31 are redundant. When cells were exposed to high pressure, Tat1-GFP was completely lost from the plasma membrane, while substantial amounts of Tat1(K29R-K31R)-GFP remained. The HPG1-1 (Rsp5(P514T)) and rsp5-ww3 mutations stabilized Tat1 under high pressure, but any one of the rsp5-ww1, rsp5-ww2, and bul1 Delta bul2 Delta mutations or single deletions of genes encoding arrestin-related trafficking adaptors did not. However, simultaneous loss of 9-arrestins and Bul1/Bul2 prevented Tat1 degradation at 25 MPa. The results suggest that multiple PPxY motif proteins share some essential roles in regulating Tat1 ubiquitination in response to high hydrostatic pressure. [本文引用: 1] |
| [61] | DOI:10.1111/j.1469-8137.2008.02589.xPMID:18681934URL090004 Specific transporters mediate uptake of amino acids by plant roots. Earlier studies have indicated that the lysine histidine transporter 1 and amino acid permease 1 participate in this process, but although plant roots have been shown to absorb cationic amino acids with high affinity, neither of these transporters seems to mediate transport of L-arginine (L-Arg) or L-lysine (L-Lys). 090004 Here, a collection of T-DNA knockout mutants were screened for alterations in Arabidopsis root uptake rates of L-Arg and it was found that only the AAP5 mutant displayed clear phenotypic divergence on high concentrations of L-Arg. A second screen using low concentrations of 15 N-labelled L-Arg in the growth media also identified AAP5 as being involved in L-Arg acquisition. 090004 Momentaneous root uptake of basic amino acids was strongly affected in AAP5 mutant lines, but their uptake of other types of amino acids was only marginally affected. Comparisons of the root uptake characteristics of AAP5 and LHT1 mutants corroborated the hypothesis that the two transporters have distinct affinity spectra in planta . 090004 Root uptake of all tested amino acids, except L-aspartic acid (L-Asp), was significantly affected in double AAP5 * LHT1 mutants, suggesting that these two transporters account for a major proportion of roots' uptake of amino acids at low concentrations. [本文引用: 1] |
| [62] | DOI:10.1016/j.tplants.2009.11.009PMID:20036181URLProline accumulates in many plant species in response to environmental stress. Although much is now known about proline metabolism, some aspects of its biological functions are still unclear. Here, we discuss the compartmentalization of proline biosynthesis, accumulation and degradation in the cytosol, chloroplast and mitochondria. We also describe the role of proline in cellular homeostasis, including redox balance and energy status. Proline can act as a signaling molecule to modulate mitochondrial functions, influence cell proliferation or cell death and trigger specific gene expression, which can be essential for plant recovery from stress. Although the regulation and function of proline accumulation are not yet completely understood, the engineering of proline metabolism could lead to new opportunities to improve plant tolerance of environmental stresses. [本文引用: 2] |
| [63] | DOI:10.1016/j.plantsci.2007.12.008URLNitrogen (N) is essential for plant growth and development. In tropical legumes amino acids and ureides are the main N-transport forms within plants and transporters are necessary to accommodate partitioning of the organic nitrogen. Here, we describe the isolation and functional characterization of an amino acid transporter, PvAAP1 from French bean ( Phaseolus vulgaris) and its expression and localization within the plant. Functional complementation of yeast mutants deficient in amino acid transport with a newly constructed bean cDNA expression library from RNA of developing cotyledons resulted in the isolation of PvAAP1. Heterologous expression of PvAAP1 in yeast mutants showed that the transporter mediates cell growth on a broad spectrum of amino acids. RNA expression analysis demonstrated that PvAAP1 is found in all plant organs, and using RNA in situ hybridization experiments PvAAP1 transcripts were localized to the phloem throughout the plant and to stem xylem parenchyma cells. In seeds, PvAAP1 was detected in the outer-epidermal cells of the developing cotyledons (embryos). Together, the yeast experiments and RNA expression and localization studies suggest a role of PvAAP1 in xylem鈥損hloem transfer and phloem loading for amino acid transport to sinks as well as in amino-N import into the cotyledons for seed development and storage compound accumulation. [本文引用: 1] |
| [64] | DOI:10.1007/s00726-008-0062-5PMID:2664619URLThe proline catabolic enzymes proline dehydrogenase and 1 -pyrroline-5-carboxylate dehydrogenase catalyze the 4-electron oxidation of proline to glutamate. These enzymes play important roles in cellular redox control, superoxide generation, apoptosis and cancer. In some bacteria, the two enzymes are fused into the bifunctional enzyme, proline utilization A. Here we review the three-dimensional structural information that is currently available for proline catabolic enzymes. Crystal structures have been determined for bacterial monofunctional proline dehydrogenase and 螖 1 -pyrroline-5-carboxylate dehydrogenase, as well as the proline dehydrogenase and DNA-binding domains of proline utilization A. Some of the functional insights provided by analyses of these structures are discussed, including substrate recognition, catalytic mechanism, biochemical basis of inherited proline catabolic disorders and DNA recognition by proline utilization A. [本文引用: 1] |
| [65] | DOI:10.1007/s00425-004-1446-8PMID:15599760URLOilseed rape (Brassica napus L.) needs very high nitrogen fertilizer inputs. Significant amounts of this nitrogen are lost during early leaf shedding and are a source of environmental and economic concern. The objective of this study was to investigate whether the remobilization of leaf amino acids could be limiting for nitrogen use efficiency. Therefore, amino acid concentrations were analyzed in subcellular compartments of leaf mesophyll cells of plants grown under low (0.5 mM ${\mathrm{N}\mathrm{O}}_{3}^{-}$) and high (4 mM ${\mathrm{N}\mathrm{O}}_{3}^{-}$) nitrogen supply. With high nitrogen supply, young leaves showed an elevated amino acid content, mainly in vacuoles. In old leaves, however, subcellular concentrations were similar under high and low nitrogen conditions, showing that the excess nitrogen had been exported during leaf development. The phloem sap contained up to 650 mM amino acids, more than four times as much than the cytosol of mesophyll cells, indicating a very efficient phloem-loading process. Three amino acid permeases, BnAAP1, BnAAP2, and BnAAP6, were identified and characterized. BnAAP1 and BnAAP6 mediated uptake of neutral and acidic amino acids into Xenopus laevis oocytes at the actual apoplastic substrate concentrations. All three transporters were expressed in leaves and the expression was still detectable during leaf senescence, with BnAAP1 and BnAAP2 mRNA levels increasing from mature to old leaves. We conclude that phloem loading of amino acids is not limiting for nitrogen remobilization from senescing leaves in oilseed rape. [本文引用: 1] |
| [66] | [本文引用: 1] |
| [67] | DOI:10.1093/pcp/pce166PMID:11726714URLAbstract We cloned a cDNA encoding Hordeum vulgare Proline Transporter (HvProT) from salt-stressed barley roots by differential display. HvProT was 2,161 bp long and had an open reading frame encoding 450 amino acids. The deduced amino acid sequence of HvProT was similar to those of proline transporter proteins of rice (65.7%), Arabidopsis (57.7%) and tomato (42.0%). Northern blot analysis showed that the transcript level of HvProT was induced in roots at 30 min after 200 mM NaCl treatment and its peak was observed at 3 h. However, the transcript level was very low in leaves and did not increase by salt stress. The expression level of Delta(1)-pyrroline-5-carboxylate synthetase (P5CS), encoding a key enzyme of proline synthesis, was induced later than HvProT by salt stress. A transport assay using a yeast with mutation in proline uptake revealed that HvProT was a transporter with high affinity for L-proline (K(m) = 25 microM). HvProT was found to be a unique transporter with high affinity for L-proline. Since its transport activity was dependent on the pH gradient, HvProT was suggested to be a H(+)/amino acid symporter. In situ hybridization analysis showed that the HvProT mRNA was strongly expressed in root cap cells under salt stress. HvProT might play an important role in the transport of proline to root tip region urgently upon salt stress. [本文引用: 5] |
| [68] | DOI:10.1007/s00425-007-0615-yPMID:17828417URLA compatible solute, proline is accumulated in various kinds of plants and microorganisms under environmental stresses. The function of proline is thought to be an osmotic regulator under water stress, and its transport into cells is mediated by a proline transporter. Here, we report the effects of expressing the barley proline transporter (HvProT) under the control of either the CaMV35S promoter (35Sp) or a root cap promoter (RCp), on Arabidopsis growth. In Arabidopsis, transformed HvProT functions in the plasma membrane, like other amino acid transporters. Reduction in biomass production was observed in aerial parts of 35Sp-HvProT plants, and it was accompanied with decreased proline accumulation in leaves. Impaired growth of 35Sp-HvProT plants was restored by exogenously adding L-proline. These results suggested that growth reduction was caused by a deficiency of endogenous proline. In 35Sp-HvProT plants, the amount of proline dehydrogenase (PDH) transcript was increased compared to wild type (WT) plants, with a consequent enhancement of the activity of PDH. On the other hand, the transgenic RCp-HvProT plants accumulated 2- to 3-fold more proline in the root tip region compared to WT, and root elongation was enhanced at the same time. Thus, different physiological responses were caused by the altered location in accumulation of proline using two different promoters for heterologous expression of HvProT. These results indicate the importance of proline distribution at the tissue level during vegetative development. [本文引用: 4] |
| [69] | DOI:10.1007/s00726-008-0061-6PMID:18379856URLProline (Pro) accumulation is a common physiological response in many plants in response to a wide range of biotic and abiotic stresses. Controversy has surrounded the possible role(s) of proline accumulation. In this review, knowledge on the regulation of Pro metabolism during development and stress, results of genetic manipulation of Pro metabolism and current debate on Pro toxicity in plants are presented. [本文引用: 1] |
| [70] | DOI:10.1073/pnas.93.16.8787PMID:8710950URLIn many plants, osmotic stress induces a rapid accumulation of proline through de novo synthesis from glutamate. This response is thought to play a pivotal role in osmotic stress tolerance [Kishor, P. B. K., Hong, Z., Miao, G.-H., Hu, C.-A. A. and Verma, D. P. S. (1995) Plant Physiol. 108, 1387-1394]. During recovery from osmotic stress, accumulated proline is rapidly oxidized to glutamate and the first step of this process is catalyzed by proline oxidase. We have isolated a full-length cDNA from Arabidopsis thaliana, At-POX, which maps to a single locus on chromosome 3 and that encodes a predicted polypeptide of 499 amino acids showing significant similarity with proline oxidase sequences from Drosophila and Saccharomyces cerevisiae (55.5% and 45.1%, respectively). The predicted location of the encoded polypeptide is the inner mitochondrial membrane. RNA gel blot analysis revealed that At-POX mRNA levels declined rapidly upon osmotic stress and this decline preceded proline accumulation. On the other hand, At-POX mRNA levels rapidly increased during recovery. Free proline, exogenously added to plants, was found to be an effective inducer of At-POX expression; indeed, At-POX was highly expressed in flowers and mature seeds where the proline level is higher relative to other organs of Arabidopsis. Our results indicate that stress- and developmentally derived signals interact to determine proline homeostasis in Arabidopsis. [本文引用: 1] |
| [71] | [本文引用: 1] |
| [72] | DOI:10.1074/jbc.M112012200PMID:11907031URLBetaine is an important osmoprotectant in many plants, but its transport activity has only been demonstrated using a proline transporter from tomato, a betaine-nonaccumulating plant. In this study, two full-length and one partial transporter genes were isolated from betaine-accumulating mangrove Avicennia marina. Their homologies to betaine transporters from bacteria and betaine/4-aminobutyrate transporters from mammalian cells were low but were high to proline transporters from Arabidopsis and tomato. Two full-length transporters could complement the Na(+)-sensitive phenotype of the Escherichia coli mutant deficient in betT, putPA, proP, and proU. Both transporters could efficiently take up betaine and proline with similar affinities (K(m), 0.32-0.43 mm) and maximum velocities (1.9-3.6 nmol/min/mg of protein). The uptakes of betaine and proline were significantly inhibited by mono- and dimethylglycine but only partially inhibited by betaine aldehyde, choline, and 4-aminobutyrate. Sodium and potassium chloride markedly enhanced betaine uptake rates with optimum concentrations at 0.5 m, whereas sucrose showed only modest activation. The change of amino acids Thr(290)-Thr-Ser(292) in a putative periplasmic loop to Arg(290)-Gly-Arg(292) yielded the active transporter independent of salts, suggesting the positive charge induced a conformational change to the active form. These data clearly indicate that the betaine-accumulating mangrove contains betaine/proline transporters whose properties are distinct from betaine transporters of bacteria and mammalian cells. [本文引用: 2] |
| [73] | [本文引用: 1] |
| [74] | DOI:10.1016/S1095-6433(01)00442-1PMID:11913457URLBacteria inhabit natural and artificial environments with diverse and fluctuating osmolalities, salinities and temperatures. Many maintain cytoplasmic hydration, growth and survival most effectively by accumulating kosmotropic organic solutes (compatible solutes) when medium osmolality is high or temperature is low (above freezing). They release these solutes into their environment when the medium osmolality drops. Solutes accumulate either by synthesis or by transport from the extracellular medium. Responses to growth in high osmolality medium, including biosynthetic accumulation of trehalose, also protect Salmonella typhimurium from heat shock. Osmotically regulated transporters and mechanosensitive channels modulate cytoplasmic solute levels in Bacillus subtilis, Corynebacterium glutamicum, Escherichia coli, Lactobacillus plantarum, Lactococcus lactis, Listeria monocytogenes and Salmonella typhimurium. Each organism harbours multiple osmoregulatory transporters with overlapping substrate specificities. Membrane proteins that can act as both osmosensors and osmoregulatory transporters have been identified (secondary transporters ProP of E. coli and BetP of C. glutamicum as well as ABC transporter OpuA of L. lactis). The molecular bases for the modulation of gene expression and transport activity by temperature and medium osmolality are under intensive investigation with emphasis on the role of the membrane as an antenna for osmo- and/or thermosensors. [本文引用: 2] |
| [75] | DOI:10.1111/tpj.12716PMID:25353986URLSummary The development of sink organs such as fruits and seeds strongly depends on the amount of nitrogen that is moved within the phloem from photosynthetic-active source leaves to the reproductive sinks. In many plant species nitrogen is transported as amino acids. In pea ( Pisum sativum L.), source to sink partitioning of amino acids requires at least two active transport events mediated by plasma membrane-localized proteins, and these are: (i) amino acid phloem loading; and (ii) import of amino acids into the seed cotyledons via epidermal transfer cells. As each of these transport steps might potentially be limiting to efficient nitrogen delivery to the pea embryo, we manipulated both simultaneously. Additional copies of the pea amino acid permease PsAAP1 were introduced into the pea genome and expression of the transporter was targeted to the sieve element-companion cell complexes of the leaf phloem and to the epidermis of the seed cotyledons. The transgenic pea plants showed increased phloem loading and embryo loading of amino acids resulting in improved long distance transport of nitrogen, sink development and seed protein accumulation. Analyses of root and leaf tissues further revealed that genetic manipulation positively affected root nitrogen uptake, as well as primary source and sink metabolism. Overall, the results suggest that amino acid phloem loading exerts regulatory control over pea biomass production and seed yield, and that import of amino acids into the cotyledons limits seed protein levels. [本文引用: 1] |
| [76] | DOI:10.1105/tpc.110.073833PMID:21075769URLSeed development and nitrogen (N) storage depend on delivery of amino acids to seed sinks. For efficient translocation to seeds, amino acids are loaded into the phloem in source leaves and along the long distance transport pathway through xylem-phloem transfer. We demonstrate that Arabidopsis thaliana AMINO ACID PERMEASE2 (AAP2) localizes to the phloem throughout the plant. AAP2 T-DNA insertion lines showed changes in source-sink translocation of amino acids and a decrease in the amount of seed total N and storage proteins, supporting AAP2 function in phloem loading and amino acid distribution to the embryo. Interestingly, in aap2 seeds, total carbon (C) levels were unchanged, while fatty acid levels were elevated. Moreover, branch and silique numbers per plant and seed yield were strongly increased. This suggests changes in N and delivery to sinks and subsequent modulations of sink development and seed metabolism. This is supported by tracer experiments, expression studies of genes of N/C transport and metabolism in source and sink, and by phenotypic and metabolite analyses of aap2 plants. Thus, AAP2 is key for xylem to phloem transfer and sink N and supply; moreover, modifications of N allocation can positively affect assimilation and source-sink transport and benefit sink development and oil yield. [本文引用: 2] |
| [77] | DOI:10.5142/jgr.2013.37.361PMID:24198663URLA lysine histidine transporter (LHT) cDNA was isolated and characterized from the roots ofPanax ginseng, designatedPgLHT. The cDNA is 1,865 bp with an open reading frame that codes for a protein with 449 amino acids and a calculated molecular mass of 50.6 kDa with a predicted isoelectric point of 8.87. Hydropathy analysis shows thatPgLHTis an integral membrane protein with 9 putative membrane-spanning domains. Multiple sequence alignments show thatPgLHTshares a high homology with other plant LHTs. The expression profile of the gene was investigated by real-time quantitative polymerase chain reaction during various chemical treatments.PgLHTwas up-regulated in the presence of abscisic acid, salicylic acid, methyl jasmonate, NaCl, and amino acids. To further explore the function ofPgLHTgene, full-length cDNA ofPgLHTwas introduced intoP. ginsengbyAgrobacterium rhizogenesA4. The overexpression ofPgLHTin the hairy roots led to an obviously increase of biomass compared to the controls, and after addition of the amino acids, the overexpressed-PgLHThairy roots grew more rapidly than untreated controls during early stage of the culture cycle. The results suggested that thePgLHTisolated from ginseng might have role in the environmental stresses and growth response. [本文引用: 1] |
| [78] | DOI:10.1371/journal.pone.0049210PMID:3499563URLAmino acid transporters (AATs) that transport amino acids across cellular membranes are essential for plant growth and development. To date, a genome-wide overview of the AAT gene family in rice is not yet available. In this study, a total of 85 AAT genes were identified in rice genome and were classified into eleven distinct subfamilies based upon their sequence composition and phylogenetic relationship. A large number of OsAAT genes were expanded via gene duplication, 23 and 24 OsAAT genes were tandemly and segmentally duplicated, respectively. Comprehensive analyses were performed to investigate the expression profiles of OsAAT genes in various stages of vegetative and reproductive development by using data from EST, Microarrays, MPSS and Real-time PCR. Many OsAAT genes exhibited abundant and tissue-specific expression patterns. Moreover, 21 OsAAT genes were found to be differentially expressed under the treatments of abiotic stresses. Comparative analysis indicates that 26 AAT genes with close evolutionary relationships between rice and Arabidopsis exhibited similar expression patterns. This study will facilitate further studies on OsAAT family and provide useful clues for functional validation of OsAATs. [本文引用: 1] |
| [79] | DOI:10.1007/s00726-008-0053-6PMID:2666929URLThe control of gene expression by enzymes provides a direct pathway for cells to respond to fluctuations in metabolites and nutrients. One example is the proline utilization A (PutA) protein from Escherichia coli . PutA is a membrane-associated enzyme that catalyzes the oxidation of l -proline to glutamate using a flavin containing proline dehydrogenase domain and a NAD + dependent Δ 1 -pyrroline-5-carboxylate dehydrogenase domain. In some Gram-negative bacteria such as E. coli , PutA is also endowed with a ribbon–helix–helix DNA-binding domain and acts as a transcriptional repressor of the proline utilization genes. PutA switches between transcriptional repressor and enzymatic functions in response to proline availability. Molecular insights into the redox-based mechanism of PutA functional switching from recent studies are reviewed. In addition, new results from cell-based transcription assays are presented which correlate PutA membrane localization with put gene expression levels. General membrane localization of PutA, however, is not sufficient to activate the put genes. [本文引用: 1] |
高等植物脯氨酸代谢研究进展
1
2007
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
植物脯氨酸及其合成酶系研究进展
1
2008
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
1
2004
... 在酵母和动物体内, 氨基酸转运蛋白对脯氨酸的亲和力也有差异.酿酒酵母中脯氨酸转运蛋白Put4p能以高亲和性转运脯氨酸, 但也能转运GABA、丙氨酸和甘氨酸(
1
1992
... 越来越多的证据表明, 植物氨基酸转运蛋白基因受到多种环境和发育信号的转录调控.但目前对这些调控表达的启动子顺式元件了解尚少.对AtAAP1启动子的序列分析表明, 其含有NIT-2识别的GATA元件和富含AT的元件(
1
1999
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
1
1998
... 除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (
1
1998
... 越来越多的证据表明, 植物氨基酸转运蛋白基因受到多种环境和发育信号的转录调控.但目前对这些调控表达的启动子顺式元件了解尚少.对AtAAP1启动子的序列分析表明, 其含有NIT-2识别的GATA元件和富含AT的元件(
1
2011
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
2
1997
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 酵母和爪蟾(Xenopus laeivs)卵母细胞是检测氨基酸转运蛋白转运能力常用的异源表达系统.异源表达的氨基酸转运蛋白对脯氨酸的亲和力不同, 说明它们对脯氨酸转运发挥不同的作用.AAP家族成员对脯氨酸的亲和力较低, Km值介于60-500 μmol·L-1之间(
3
2004
... 在真菌、细菌和动物中也存在转运脯氨酸的蛋白.酿酒酵母(Saccharomyces cerevisiae)中一般是氨基酸通透酶Gap1p和脯氨酸转运蛋白Put4p一起介导大部分脯氨酸的吸收(
... 在酵母和动物体内, 氨基酸转运蛋白对脯氨酸的亲和力也有差异.酿酒酵母中脯氨酸转运蛋白Put4p能以高亲和性转运脯氨酸, 但也能转运GABA、丙氨酸和甘氨酸(
... 无内源脯氨酸时, PutP的表达被三功能性的PutA蛋白(在同1个蛋白上结合PDH、P5CDH和发挥调节功能)抑制; 在脯氨酸降解期间, 一旦PutA蛋白被转运到质膜上, PutP立刻被激活(
3
2010
... 除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (
... )运输脯氨酸(
... ).PtAAP11对脯氨酸的高亲和力表明, 其可能在木质部细胞分化时运输脯氨酸, 以保证细胞分裂时对脯氨酸的需求(
1
1989
... 在真菌、细菌和动物中也存在转运脯氨酸的蛋白.酿酒酵母(Saccharomyces cerevisiae)中一般是氨基酸通透酶Gap1p和脯氨酸转运蛋白Put4p一起介导大部分脯氨酸的吸收(
1
2004
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
1
2006
... 鉴于脯氨酸的合成与降解发生在不同的细胞器中, 脯氨酸在胞内的转运不可或缺.然而脯氨酸在细胞器间转运的信息尚知之甚少.目前, 仅在小麦(Triti- cum aestivum)中发现2个转运系统能介导脯氨酸向线粒体运输(
1
1998
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
1
1995
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
1
2002
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
1
2008
... AtLHT1在根被皮、萼片、花梗和叶中表达, 作用于氨基酸的根际吸收和由木质部向叶肉细胞的运输.在土壤中生长的lht1突变体明显小于野生型, 这一表型可被AtLHT1基因功能回补(
1
1993
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
1
1996
... 环境信号(如光(
4
2005
... 早期的研究表明, 胁迫诱导的脯氨酸积累也会受到吸收和转运的影响.例如, 低水势条件下, 脯氨酸在玉米(Zea mays)根系伸长区的积累主要来源于脯氨酸的运输, 而不是合成增加(
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 酵母和爪蟾(Xenopus laeivs)卵母细胞是检测氨基酸转运蛋白转运能力常用的异源表达系统.异源表达的氨基酸转运蛋白对脯氨酸的亲和力不同, 说明它们对脯氨酸转运发挥不同的作用.AAP家族成员对脯氨酸的亲和力较低, Km值介于60-500 μmol·L-1之间(
... 在拟南芥中, ProT基因家族有3个成员, 分别为At- ProT1、AtProT2和AtProT3, 它们在拟南芥多个组织中均有表达(
1
1970
... 在酵母和动物体内, 氨基酸转运蛋白对脯氨酸的亲和力也有差异.酿酒酵母中脯氨酸转运蛋白Put4p能以高亲和性转运脯氨酸, 但也能转运GABA、丙氨酸和甘氨酸(
1
2011
... 据报道, 生物因素(如线虫伤害(
6
2004
... 越来越多的证据表明, 植物氨基酸转运蛋白基因受到多种环境和发育信号的转录调控.但目前对这些调控表达的启动子顺式元件了解尚少.对AtAAP1启动子的序列分析表明, 其含有NIT-2识别的GATA元件和富含AT的元件(
... 据报道, 生物因素(如线虫伤害(
... )、代谢物(
... 环境信号(如光(
... 表达被诱导的一部分信号(
... 另外, 碳氮代谢物对氨基酸转运蛋白的表达也有影响.用高浓度NO3处理植物, 其体内属于不同亚家族的AtAAP1、AtProT2和AtLHT1的表达量升高(
1
2006
... 据报道, 生物因素(如线虫伤害(
1
2006
... 泛素化是另一种广泛存在的蛋白翻译后调控机制.泛素化过程通常需要ATP提供能量以及E1、E2和E3三种泛素化酶协同作用.泛素化能调节蛋白的活性、丰度、位置和功能.在动物、真菌和细菌中, 泛素化对氨基酸转运蛋白调节的报道较少.例如, 在哺乳动物中, 通过连接酶Nedd4聚泛素化后, 依赖钠的中性氨基酸转运蛋白ATA2表达下调(
1
2008
... 磷酸化是一种蛋白翻译后修饰, 能够改变蛋白的活性.植物中氨基酸转运蛋白磷酸化的证据来自Phos- phAt数据库(
4
2006
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 酵母和爪蟾(Xenopus laeivs)卵母细胞是检测氨基酸转运蛋白转运能力常用的异源表达系统.异源表达的氨基酸转运蛋白对脯氨酸的亲和力不同, 说明它们对脯氨酸转运发挥不同的作用.AAP家族成员对脯氨酸的亲和力较低, Km值介于60-500 μmol·L-1之间(
... AtLHT1在根被皮、萼片、花梗和叶中表达, 作用于氨基酸的根际吸收和由木质部向叶肉细胞的运输.在土壤中生长的lht1突变体明显小于野生型, 这一表型可被AtLHT1基因功能回补(
... (
1
1992
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
2
2010
... 氨基酸方面起作用(
... 的莲座叶、茎生叶和种子都比野生型显著增大(
1
2000
... 酵母和爪蟾(Xenopus laeivs)卵母细胞是检测氨基酸转运蛋白转运能力常用的异源表达系统.异源表达的氨基酸转运蛋白对脯氨酸的亲和力不同, 说明它们对脯氨酸转运发挥不同的作用.AAP家族成员对脯氨酸的亲和力较低, Km值介于60-500 μmol·L-1之间(
1
1987
... 在酵母和动物体内, 氨基酸转运蛋白对脯氨酸的亲和力也有差异.酿酒酵母中脯氨酸转运蛋白Put4p能以高亲和性转运脯氨酸, 但也能转运GABA、丙氨酸和甘氨酸(
1
1996
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
1
2003
... 除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (
3
1981
... 在真菌、细菌和动物中也存在转运脯氨酸的蛋白.酿酒酵母(Saccharomyces cerevisiae)中一般是氨基酸通透酶Gap1p和脯氨酸转运蛋白Put4p一起介导大部分脯氨酸的吸收(
... 在酵母和动物体内, 氨基酸转运蛋白对脯氨酸的亲和力也有差异.酿酒酵母中脯氨酸转运蛋白Put4p能以高亲和性转运脯氨酸, 但也能转运GABA、丙氨酸和甘氨酸(
... 越来越多的证据表明, 植物氨基酸转运蛋白基因受到多种环境和发育信号的转录调控.但目前对这些调控表达的启动子顺式元件了解尚少.对AtAAP1启动子的序列分析表明, 其含有NIT-2识别的GATA元件和富含AT的元件(
3
2007
... 早期的研究表明, 胁迫诱导的脯氨酸积累也会受到吸收和转运的影响.例如, 低水势条件下, 脯氨酸在玉米(Zea mays)根系伸长区的积累主要来源于脯氨酸的运输, 而不是合成增加(
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 有研究表明, AtAAP1在根尖和侧根的表皮细胞中表达, 在拟南芥根际吸收脯氨酸方面起作用(
3
2004
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 酵母和爪蟾(Xenopus laeivs)卵母细胞是检测氨基酸转运蛋白转运能力常用的异源表达系统.异源表达的氨基酸转运蛋白对脯氨酸的亲和力不同, 说明它们对脯氨酸转运发挥不同的作用.AAP家族成员对脯氨酸的亲和力较低, Km值介于60-500 μmol·L-1之间(
... AtLHT1在根被皮、萼片、花梗和叶中表达, 作用于氨基酸的根际吸收和由木质部向叶肉细胞的运输.在土壤中生长的lht1突变体明显小于野生型, 这一表型可被AtLHT1基因功能回补(
4
2010
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
... 酵母和爪蟾(Xenopus laeivs)卵母细胞是检测氨基酸转运蛋白转运能力常用的异源表达系统.异源表达的氨基酸转运蛋白对脯氨酸的亲和力不同, 说明它们对脯氨酸转运发挥不同的作用.AAP家族成员对脯氨酸的亲和力较低, Km值介于60-500 μmol·L-1之间(
... 在拟南芥中, ProT基因家族有3个成员, 分别为At- ProT1、AtProT2和AtProT3, 它们在拟南芥多个组织中均有表达(
... 均未显示出表型上的差异或脯氨酸含量的变化, 暗示有其它转运蛋白的互补或脯氨酸代谢发生了改变(
6
2006
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 越来越多的证据表明, 植物氨基酸转运蛋白基因受到多种环境和发育信号的转录调控.但目前对这些调控表达的启动子顺式元件了解尚少.对AtAAP1启动子的序列分析表明, 其含有NIT-2识别的GATA元件和富含AT的元件(
... 一些植物中, ProTs的表达能被渗透胁迫诱导上调.PlantCare分析结果表明, 在AtAAP3、AtLHT2和AtProT2启动子中有1个重要的干旱响应元件(DRE) (
... 基因家族成员在它们的启动子区有推测的植物激素(生长素、脱落酸或茉莉酸)响应元件(
... 据报道, 生物因素(如线虫伤害(
... 另外, 碳氮代谢物对氨基酸转运蛋白的表达也有影响.用高浓度NO3处理植物, 其体内属于不同亚家族的AtAAP1、AtProT2和AtLHT1的表达量升高(
1
2011
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
1
2013
... 据报道, 生物因素(如线虫伤害(
3
1999
... 在真菌、细菌和动物中也存在转运脯氨酸的蛋白.酿酒酵母(Saccharomyces cerevisiae)中一般是氨基酸通透酶Gap1p和脯氨酸转运蛋白Put4p一起介导大部分脯氨酸的吸收(
... 在酵母和动物体内, 氨基酸转运蛋白对脯氨酸的亲和力也有差异.酿酒酵母中脯氨酸转运蛋白Put4p能以高亲和性转运脯氨酸, 但也能转运GABA、丙氨酸和甘氨酸(
... 无内源脯氨酸时, PutP的表达被三功能性的PutA蛋白(在同1个蛋白上结合PDH、P5CDH和发挥调节功能)抑制; 在脯氨酸降解期间, 一旦PutA蛋白被转运到质膜上, PutP立刻被激活(
2
2001
... 除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (
... 另外, 碳氮代谢物对氨基酸转运蛋白的表达也有影响.用高浓度NO3处理植物, 其体内属于不同亚家族的AtAAP1、AtProT2和AtLHT1的表达量升高(
1
2002
... 一些中等活性的转运蛋白(如大肠杆菌H+/proline同向转运蛋白ProP和依赖结合蛋白的ABC转运蛋白), 在渗透胁迫下可能通过增加自身基因的表达量和提高自身活性来应对渗透胁迫(
6
2002
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 氨基酸方面起作用(
... 进行组织定位, 发现其主要在木质部薄壁细胞中表达, 说明AtAAP6主要参与氨基酸从木质部向韧皮部的运输(
... 的核苷酸序列相似度最高, 异源表达结果表明, AtAA- P8能够运输脯氨酸(
... 在角果中的表达停止(
... AtAAP3和AtAAP5主要在根中表达, 其中At- AAP3在根韧皮部表达; 它们编码的蛋白主要负责运输精氨酸和赖氨酸, 而并不运输脯氨酸(
2
2000
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 环境信号(如光(
2
2014
... 除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (
... 的转基因植株根际吸收脯氨酸、苏氨酸和丝氨酸等氨基酸的量增加, 而RNAi植株中这些氨基酸的含量减少(
1
2014
... 有研究表明, AtAAP1在根尖和侧根的表皮细胞中表达, 在拟南芥根际吸收脯氨酸方面起作用(
1
2003
... 环境信号(如光(
1
2014
... 磷酸化是一种蛋白翻译后修饰, 能够改变蛋白的活性.植物中氨基酸转运蛋白磷酸化的证据来自Phos- phAt数据库(
1
1999
... 在酵母和动物体内, 氨基酸转运蛋白对脯氨酸的亲和力也有差异.酿酒酵母中脯氨酸转运蛋白Put4p能以高亲和性转运脯氨酸, 但也能转运GABA、丙氨酸和甘氨酸(
6
1996
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 在拟南芥中, ProT基因家族有3个成员, 分别为At- ProT1、AtProT2和AtProT3, 它们在拟南芥多个组织中均有表达(
... 在根、茎和花中表达量较高, 在花中主要在进入心皮的花梗韧皮部表达(
... 则仅在地上部分(如叶、花和角果)检测到表达(
... 环境信号(如光(
... (
1
2007
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
2
2009
... 有研究表明, AtAAP1在根尖和侧根的表皮细胞中表达, 在拟南芥根际吸收脯氨酸方面起作用(
... 综上, 在响应基因变化时, 氨基酸转运蛋白的表达可能被改变.与野生型相比, 在aap1敲除突变体的种子中AtCAT6以及AtAAP8的转录水平发生改变(
2016
1
1995
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
3
2007
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 氨基酸方面起作用(
... 突变体种子数目相比野生型减少了约50%, 且停止发育的种子比例(48%)显著高于野生型(1%) (
4
1999
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 在拟南芥中, ProT基因家族有3个成员, 分别为At- ProT1、AtProT2和AtProT3, 它们在拟南芥多个组织中均有表达(
... 在成熟和萌发的花粉中强烈表达, 暗示其可能与花粉营养的供给有关(
... 环境信号(如光(
2
2004
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... ).在拟南芥中, APC超级家族共有14个成员, 其中9个成员为阳离子氨基酸转运蛋白(cationic amino acid transporter, CAT) (不能转运脯氨酸) (
1
2013
... 泛素化是另一种广泛存在的蛋白翻译后调控机制.泛素化过程通常需要ATP提供能量以及E1、E2和E3三种泛素化酶协同作用.泛素化能调节蛋白的活性、丰度、位置和功能.在动物、真菌和细菌中, 泛素化对氨基酸转运蛋白调节的报道较少.例如, 在哺乳动物中, 通过连接酶Nedd4聚泛素化后, 依赖钠的中性氨基酸转运蛋白ATA2表达下调(
1
2008
... AtAAP3和AtAAP5主要在根中表达, 其中At- AAP3在根韧皮部表达; 它们编码的蛋白主要负责运输精氨酸和赖氨酸, 而并不运输脯氨酸(
2
2010
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
... ;
1
2008
... 除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (
1
2008
... 无内源脯氨酸时, PutP的表达被三功能性的PutA蛋白(在同1个蛋白上结合PDH、P5CDH和发挥调节功能)抑制; 在脯氨酸降解期间, 一旦PutA蛋白被转运到质膜上, PutP立刻被激活(
1
2005
... 另外, 碳氮代谢物对氨基酸转运蛋白的表达也有影响.用高浓度NO3处理植物, 其体内属于不同亚家族的AtAAP1、AtProT2和AtLHT1的表达量升高(
1
2006
... 除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (
5
2001
... 早期的研究表明, 胁迫诱导的脯氨酸积累也会受到吸收和转运的影响.例如, 低水势条件下, 脯氨酸在玉米(Zea mays)根系伸长区的积累主要来源于脯氨酸的运输, 而不是合成增加(
... 酵母和爪蟾(Xenopus laeivs)卵母细胞是检测氨基酸转运蛋白转运能力常用的异源表达系统.异源表达的氨基酸转运蛋白对脯氨酸的亲和力不同, 说明它们对脯氨酸转运发挥不同的作用.AAP家族成员对脯氨酸的亲和力较低, Km值介于60-500 μmol·L-1之间(
... 据报道, 生物因素(如线虫伤害(
... 环境信号(如光(
... ;
4
2008
... 在拟南芥中, ProT基因家族有3个成员, 分别为At- ProT1、AtProT2和AtProT3, 它们在拟南芥多个组织中均有表达(
... 过表达植株的PDH mRNA含量和活性均增加, 暗示转运的改变可能诱导了脯氨酸的降解, 致使脯氨酸含量降低(
... 可导致根尖更高水平的脯氨酸积累和根伸长的增加(
... 环境信号(如光(
1
2008
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
1
1996
... 脯氨酸积累是许多植物在不同环境胁迫下的一种代谢适应机制.能够诱导脯氨酸积累的胁迫包括干旱、高盐、低温、重金属、紫外线、氧化和生物胁迫.积累的脯氨酸对植物具有保护作用, 但确切机制尚不清楚.研究人员提出了一些假说, 如作为渗透调节剂、自由基清除剂、大分子结构保护剂以及pH缓冲剂等来解释其保护作用(
1
1999
... 早期的研究表明, 胁迫诱导的脯氨酸积累也会受到吸收和转运的影响.例如, 低水势条件下, 脯氨酸在玉米(Zea mays)根系伸长区的积累主要来源于脯氨酸的运输, 而不是合成增加(
2
2002
... 氨基酸-多胺-胆碱转运蛋白超级家族(amino acid polyamine choline transporters, APC)和氨基酸转运蛋白家族(amino acid transporter family, ATF)包含了植物中能转运脯氨酸的主要转运蛋白(
... 环境信号(如光(
1
2006
... 一些中等活性的转运蛋白(如大肠杆菌H+/proline同向转运蛋白ProP和依赖结合蛋白的ABC转运蛋白), 在渗透胁迫下可能通过增加自身基因的表达量和提高自身活性来应对渗透胁迫(
2
2001
... 在真菌、细菌和动物中也存在转运脯氨酸的蛋白.酿酒酵母(Saccharomyces cerevisiae)中一般是氨基酸通透酶Gap1p和脯氨酸转运蛋白Put4p一起介导大部分脯氨酸的吸收(
... 一些中等活性的转运蛋白(如大肠杆菌H+/proline同向转运蛋白ProP和依赖结合蛋白的ABC转运蛋白), 在渗透胁迫下可能通过增加自身基因的表达量和提高自身活性来应对渗透胁迫(
1
2015
... 除拟南芥外, 其它物种的AAP基因家族成员也陆续被报道, 如单子叶植物中的水稻, 双子叶植物中的马铃薯(Solanum tuberosum)、菜豆(Phaseolus vulgaris)、蚕豆(Vicia faba)、豌豆(Pisum sativum)和三角叶杨(Populus trichocarpa) (
2
2010
... 氨基酸方面起作用(
... 综上, 在响应基因变化时, 氨基酸转运蛋白的表达可能被改变.与野生型相比, 在aap1敲除突变体的种子中AtCAT6以及AtAAP8的转录水平发生改变(
1
2013
... 另外, 碳氮代谢物对氨基酸转运蛋白的表达也有影响.用高浓度NO3处理植物, 其体内属于不同亚家族的AtAAP1、AtProT2和AtLHT1的表达量升高(
1
2012
... 环境信号(如光(
1
2008
... 无内源脯氨酸时, PutP的表达被三功能性的PutA蛋白(在同1个蛋白上结合PDH、P5CDH和发挥调节功能)抑制; 在脯氨酸降解期间, 一旦PutA蛋白被转运到质膜上, PutP立刻被激活(
