 ,*山东大学生命科学学院, 植物发育与环境适应教育部重点实验室, 青岛 266237
,*山东大学生命科学学院, 植物发育与环境适应教育部重点实验室, 青岛 266237A TIR1-independent Auxin Signaling Module
Kongqin Hu, Zhaojun Ding ,*Key Laboratory of Plant Development and Environmental Adaptation Biology, Ministry of Education, College of Life Sciences, Shandong University, Qingdao 266237, China
,*Key Laboratory of Plant Development and Environmental Adaptation Biology, Ministry of Education, College of Life Sciences, Shandong University, Qingdao 266237, China通讯作者:
收稿日期:2019-03-29接受日期:2019-03-31网络出版日期:2019-07-01
Corresponding authors:
Received:2019-03-29Accepted:2019-03-31Online:2019-07-01

摘要
关键词:
Abstract
Keywords:
PDF (802KB)摘要页面多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
胡孔琴, 丁兆军. 非TIR1受体依赖型激活生长素信号的新机制. 植物学报, 2019, 54(3): 293-295 doi:10.11983/CBB19063
Hu Kongqin, Ding Zhaojun.
生长素(auxin, indole acetic acid, IAA)是最早被发现的植物激素之一。近百余年来, 其调控植物生长发育和生长反应的研究备受广大植物学家关注。然而, 21世纪之前, 人们对生长素调控植物生长发育的认识主要停留在生理及生化水平, 关于其信号传递机制的研究多年来进展缓慢。伴随着拟南芥(Arabidopsis thaliana)作为模式植物的广泛应用, Estelle和Leyser两个团队在2005年同时独立发现F-box蛋白TIR1 (Transport Inhibitor Response 1)是生长素的受体, 使生长素信号转导通路的研究取得飞速发展。后续研究表明, 生长素受体TIR1及其同源受体蛋白AFBs (Auxin Signaling F-box Proteins)能够与生长素信号的抑制因子Aux/IAA蛋白互作, 而生长素与其受体TIR1/AFBs结合后, 能够稳定其受体-Aux/IAA蛋白的互作, 且能够泛素化修饰及降解Aux/IAA蛋白, 从而释放出被Aux/IAA蛋白抑制的生长素响应因子ARFs (Auxin Response Factors), 介导生长素信号的传递(Dharmasiri et al., 2005a, 2005b; Kepinski and Leyser, 2005)。拟南芥有23个核定位ARFs, 大部分ARFs包括N端的DNA结合结构域(DNA-binding domain)、中间的激活或抑制结构域(activation domain (AD) or repression domain (RD))以及C端的蛋白互作结构域(carboxy-terminal dimerization domain (CTD) (Guilfoyle and Hagen, 2007)。拟南芥有29个Aux/IAAs蛋白, 大部分Aux/IAAs含有N端的抑制结构域I、中间能够被受体TIR1识别调控其蛋白稳定性的结构域II以及C端参与蛋白互作的结构域III和IV。ARFs的C端蛋白互作结构域及Aux/IAAs的结构域III和IV介导ARFs蛋白之间、Aux/IAAs蛋白之间及ARFs蛋白与Aux/IAAs蛋白之间的互作, 从而调控生长素信号转导以及参与植物多样化生长发育的调控(Peer, 2013)。
除了上述经典的Aux/IAAs蛋白外, 拟南芥IAA30-34蛋白没有典型的N端结构域I和II, 被称为非经典Aux/IAAs, 其是否参与生长素信号的传递尚无定论(Mutte et al., 2018)。此外, 据报道, 一类跨膜蛋白激酶家族TMKs (transmembrane kinases)也参与生长素的信号传递, 但具体调控机理并不清楚(Xu et al., 2014)。徐通达课题组最新发现了植物中该类受体蛋白激酶家族成员之一TMK1响应生长素信号调控顶端弯钩(apical hook)发育的新机制(Cao et al., 2019)。TMK1缺失突变体tmk1-3具有明显的顶端弯钩发育缺陷的表型。与外源施加生长素能够恢复生长素合成突变体wei8/tar2表型不同, tmk1-3突变体顶端弯钩发育缺陷的表型不能被外源施加的生长素恢复, 表明TMK1缺失突变体的表型是由于生长素信号被阻断所致。通过GFP荧光标记TMK1和蛋白印迹杂交实验证明, 生长素能促进TMK1蛋白剪切形成TMK1 C末端片段并从细胞膜转运到细胞质和细胞核。为了证明进入细胞核的TMK1 C末端片段TMK1C参与生长素信号的传递并调控植物顶端弯钩发育, 他们通过酵母双杂交筛选发现拟南芥所有的Aux/IAAs蛋白家族中, 只有非经典的Aux/IAA家族成员IAA32和IAA34能够与TMK1C互作。与之对应, GUS报告基因染色分析表明, pIAA32::GUS和pIAA34::GUS主要在拟南芥顶端弯钩表达, iaa32/34突变体也具有明显的顶端弯钩缺陷表型。进一步分析表明, TMK1C能够特异地磷酸化IAA32/34蛋白。与TMK1C互作的IAA32/34并不具有与TIR1互作的结构域, 不能与TIR1受体结合, 表明TIR1-介导的生长素信号途径和TMK1-介导的生长素信号途径通过选择与不同的Aux/IAA蛋白互作来区分下游信号途径(Cao et al., 2019)。
为进一步阐明非经典的IAA32/34调控生长素信号响应基因的机制, 徐通达课题组验证了拟南芥ARFs和IAA32/34蛋白的互作关系, 发现IAA32和IAA34蛋白既能够与ARF5及ARF7等生长素信号的激活子互作, 也能够与ARF1及ARF2等抑制子互作。他们还发现, 与之前报道的TIR1/AFB介导的生长素对Aux/IAA蛋白泛素化降解过程相反, 生长素通过TMK1剪切后形成的TMK1C磷酸化修饰IAA32和IAA34蛋白, 使IAA32/34蛋白更加稳定。上述结果表明, 生长素通过TMK1剪切后形成的TMK1C来稳定IAA32/34蛋白, 最终依然通过ARF转录因子调控基因表达, 在生长素聚集的部位抑制细胞生长, 从而导致顶端弯钩内外侧的差异性生长(Cao et al., 2019)。
综上所述, 该项研究阐明了一条新的生长素TMK1-IAA32/34-ARFs信号通路(图1), 该信号通路独立于经典的受体TIR1介导的生长素信号转导通路。 TMK1介导的生长素信号利用非经典的Aux/IAA32/34蛋白与生长素响应因子ARFs互作调节下游基因的表达, 参与植物顶端弯钩维持阶段内外侧差异性生长的分子调控。这一发现是生长素信号领域的突破性进展。此外, 复旦大学杨洪全团队和李琳团队发现光受体CRY1、PHYB和PHYA能够通过磷酸化修饰Aux/IAAs蛋白, 影响其蛋白稳定性及生长素信号的传递, 从而参与植物生长发育和生长反应的调控(Xu et al., 2018; Yang et al., 2018)。上述研究表明, Aux/IAAs蛋白的翻译后修饰在生长素信号转导途径中发挥重要作用。然而, 这种被磷酸化修饰的经典或非经典Aux/IAA, 是否影响其与ARFs的互作? 生长素如何调控TMK1的剪切及核质穿梭? TMKs是否与光信号的光受体激酶PHYA/B及CRY1之间存在互作? 其它非经典Aux/IAAs是否参与生长素信号响应? TMK1C如何特异地识别并与IAA32/34互作? 磷酸化后的IAA32/34为什么更加稳定? 其生物学意义是什么? 对上述问题的回答将有助于深入解析生长素信号转导的分子机理, 进一步加深我们对生长素调控植物生长发育与生长反应的认识。
图1
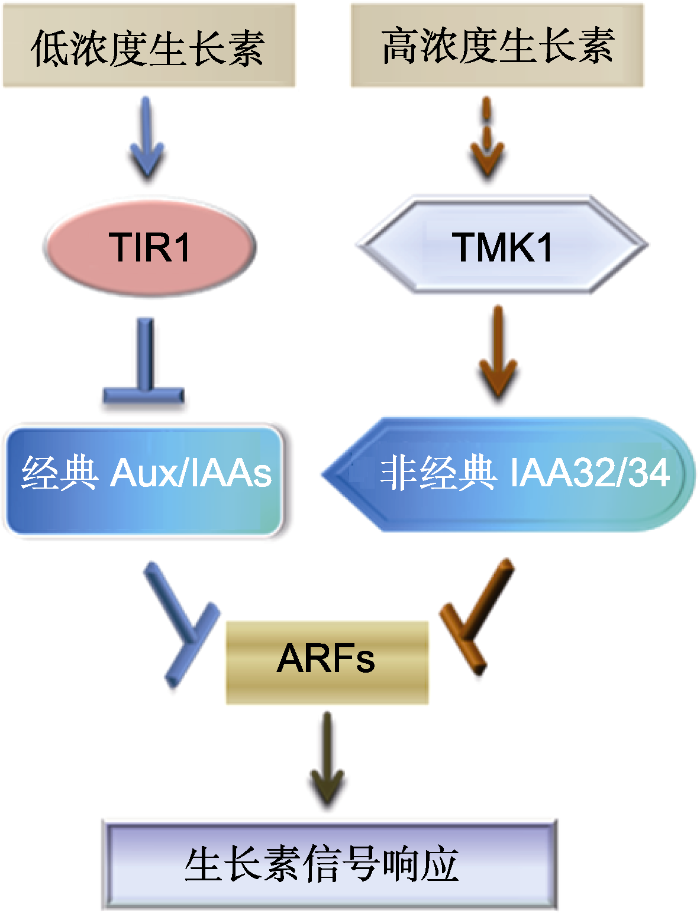 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1生长素信号转导途径
低浓度生长素主要通过受体TIR1泛素化并降解经典的Aux/IAAs蛋白, 解除对生长素响应因子ARFs的抑制, 启动对下游基因的调控及生长素信号响应。高浓度生长素主要通过TMK1蛋白激酶磷酸化并稳定非经典Aux/IAA32/34, 抑制ARFs的活性。TMK1-IAA32/34-ARFs模型解释了植物发育过程中生长素积累调控顶端弯钩内外侧的差异性生长。
Figure 1Pathway of auxin signal transduction
The perception of low levels of auxin is mainly mediated through the TIR1 receptor, which ubiquitinates and degrades Aux/IAAs proteins, and thus de-represses ARFs to activate downstream gene transcription and trigger auxin responses. Higher levels of auxin activate TMK1, which phosphorylates and stabilizes non-canonical IAA32/34, and repress ARFs activities. The TMK1-IAA32/34-ARFs module interprets how the local auxin accumulation modulates asymmetric growth during apical hook development.
(责任编辑: 白羽红)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1038/s41586-019-1069-7 [本文引用: 3]
DOI:10.1038/nature03543 [本文引用: 1]
DOI:10.1016/j.devcel.2005.05.014URLPMID:15992545 [本文引用: 1]

The plant hormone auxin has been implicated in virtually every aspect of plant growth and development. Auxin acts by promoting the degradation of transcriptional regulators called Aux/IAA proteins. Aux/IAA degradation requires TIR1, an F box protein that has been shown to function as an auxin receptor. However, loss of TIR1 has a modest effect on auxin response and plant development. Here we show that three additional F box proteins, called AFB1, 2, and 3, also regulate auxin response. Like TIR1, these proteins interact with the Aux/IAA proteins in an auxin-dependent manner. Plants that are deficient in all four proteins are auxin insensitive and exhibit a severe embryonic phenotype similar to the mp/arf5 and bdl/iaa12 mutants. Correspondingly, all TIR1/AFB proteins interact with BDL, and BDL is stabilized in triple mutant plants. Our results indicate that TIR1 and the AFB proteins collectively mediate auxin responses throughout plant development.
DOI:10.1016/j.pbi.2007.08.014URL [本文引用: 1]
[本文引用: 1]
DOI:10.7554/elife.33399URL [本文引用: 1]

The small signaling molecule auxin controls numerous developmental processes in land plants, acting mostly by regulating gene expression. Auxin response proteins are represented by large families of diverse functions, but neither their origin nor their evolution is understood. Here, we use a deep phylogenomics approach to reconstruct both the origin and the evolutionary trajectory of all nuclear auxin response protein families. We found that, while all subdomains are ancient, a complete auxin response mechanism is limited to land plants. Functional phylogenomics predicts defined steps in the evolution of response system properties, and comparative transcriptomics across six ancient lineages revealed how these innovations shaped a sophisticated response mechanism. Genetic analysis in a basal land plant revealed unexpected contributions of ancient non-canonical proteins in auxin response as well as auxin-unrelated function of core transcription factors. Our study provides a functional evolutionary framework for understanding diverse functions of the auxin signal. Across all kingdoms of life, signaling molecules like hormones, for example, control many aspects of the lives of organisms, including how they grow and develop. Cells have dedicated proteins that can recognize the signaling molecules, relay the information, and respond to the signal, for example by switching genes on or off. Such response systems usually consist of multiple components, and, throughout evolution, these response components have regularly been copied such that many species have multiple different versions of each one. Auxin is a plant hormone that controls virtually all growth and developmental processes in plants, including many yield traits in crops. However, no one knows why it is involved in so many processes. This is partly because it is not clear how the response system for this central signaling molecule was first born, or how it has increased in its complexity. To address this, Mutte, Kato et al. explored the genetic information of more than a thousand plant species, including algae, which span more than 700 million years of evolution. Their analysis showed that all auxin response components were assembled from pieces of much older genes, but that they first came together when plants conquered land. Indeed, the auxin response appears to have developed on top of a pre-existing genetic regulator that is still present in modern-day algae. Mutte, Kato et al. then used experiments to show how stepwise increases in the number and types of auxin response components have shaped sophisticated, complex responses in land plants, and to demonstrate how ancient components control auxin response. Together these findings provide a framework for understanding the many functions of auxin in plants, and how this came to be. They also show how complexity can be accomplished in a signal response pathway, and how diversity evolves in gene families. Similar studies on other response systems in plants and beyond are likely to help reveal common principles of hormone response evolution and diversification of gene regulation systems.
DOI:10.1016/j.pbi.2013.08.003URLPMID:24004572 [本文引用: 1]

The plant hormone auxin is essential for growth, development, and responses to environmental factors. Recently, Auxin Binding Protein 1 was shown to mediate non-transcriptional auxin signalling at the cell periphery. This has provoked reexamination of the paradigm that all auxin perception is intracellular and is mediated by the TIR1/AFB-Aux/IAA co-receptors for which auxin functions as a concentration-dependent molecular glue. Further, another F-box protein, SKP2a, was shown to bind auxin in the same way as TIR1/AFB, which provides a link to the role of auxin in the cell cycle. New work on auxin signalling and homeostasis include D6 PROTEIN KINASE activation of PINFORMED (PIN) auxin carriers, ROP-GTPase mediation of PIN localization, endoplasmic reticulum localization PIN and PIN-LIKES auxin carriers, and auxin biosynthesis and metabolism.
DOI:10.1016/j.molp.2017.12.003URLPMID:29269022 [本文引用: 1]

Abstract Light is a key environmental cue that inhibits hypocotyl cell elongation through the blue and red/far-red light photoreceptors cryptochrome- and phytochrome-mediated pathways in Arabidopsis. In contrast, as a pivotal endogenous phytohormone auxin promotes hypocotyl elongation through the auxin receptors TIR1/AFBs-mediated degradation of AUX/IAA proteins (AUX/IAAs). However, the molecular mechanisms underlying the antagonistic interaction of light and auxin signaling remain unclear. Here, we report that light inhibits auxin signaling through stabilization of AUX/IAAs by blue and red light-dependent interactions of cryptochrome 1 (CRY1) and phytochrome B with AUX/IAAs, respectively. Blue light-triggered interactions of CRY1 with AUX/IAAs inhibit the associations of TIR1 with AUX/IAAs, leading to the repression of auxin-induced degradation of these proteins. Our results indicate that photoreceptors share AUX/IAAs with auxin receptors as the same direct downstream signaling components. We propose that antagonistic regulation of AUX/IAA protein stability by photoreceptors and auxin receptors allows plants to balance light and auxin signals to optimize their growth.
DOI:10.1126/science.1245125URLPMID:24578577 [本文引用: 1]

Auxin-binding protein 1 (ABP1) was discovered nearly 40 years ago and was shown to be essential for plant development and morphogenesis, but its mode of action remains unclear. Here, we report that the plasma membrane-localized transmembrane kinase (TMK) receptor-like kinases interact with ABP1 and transduce auxin signal to activate plasma membrane-associated ROPs [Rho-like guanosine triphosphatases (GTPase) from plants], leading to changes in the cytoskeleton and the shape of leaf pavement cells in Arabidopsis. The interaction between ABP1 and TMK at the cell surface is induced by auxin and requires ABP1 sensing of auxin. These findings show that TMK proteins and ABP1 form a cell surface auxin perception complex that activates ROP signaling pathways, regulating nontranscriptional cytoplasmic responses and associated fundamental processes.
DOI:10.1016/j.devcel.2017.11.017URLPMID:29275991 [本文引用: 1]

The reduction in the red to far-red light ratio (R/FR) and photosynthetically active radiation caused by dense planting initiates shade avoidance responses (SARs) to help plants compete against their neighbors. However, deep shade attenuates shade-induced stem elongation to suppress excessive reversion toward skotomorphogenic development, in which photoreceptor phytochrome A (PHYA) has been known to play the major role. However, the molecular mechanism underlying PHYA function in deep shade is poorly understood. Here, we report that shade-accumulated PHYA can release auxin/indole-3-acetic acid (AUX/IAA), suppressors in the auxin signaling pathway, from SCF TIR1 , an auxin receptor, to weaken auxin signaling and negatively regulate shade response. Corroborating this, phyA mutants display an enhanced auxin response to deep shade and auxin treatment. Specifically, PHYA competes with TIR1 by directly binding and stabilizing AUX/IAA. Our findings illustrate a mechanistic model of how plants sense different shade levels to fine-tune auxin signaling and generate appropriate SAR.
TMK1-mediated auxin signaling regulates differential growth of the apical hook
3
2019
... 除了上述经典的Aux/IAAs蛋白外, 拟南芥IAA30-34蛋白没有典型的N端结构域I和II, 被称为非经典Aux/IAAs, 其是否参与生长素信号的传递尚无定论(
... 突变体也具有明显的顶端弯钩缺陷表型.进一步分析表明, TMK1C能够特异地磷酸化IAA32/34蛋白.与TMK1C互作的IAA32/34并不具有与TIR1互作的结构域, 不能与TIR1受体结合, 表明TIR1-介导的生长素信号途径和TMK1-介导的生长素信号途径通过选择与不同的Aux/IAA蛋白互作来区分下游信号途径(
... 为进一步阐明非经典的IAA32/34调控生长素信号响应基因的机制, 徐通达课题组验证了拟南芥ARFs和IAA32/34蛋白的互作关系, 发现IAA32和IAA34蛋白既能够与ARF5及ARF7等生长素信号的激活子互作, 也能够与ARF1及ARF2等抑制子互作.他们还发现, 与之前报道的TIR1/AFB介导的生长素对Aux/IAA蛋白泛素化降解过程相反, 生长素通过TMK1剪切后形成的TMK1C磷酸化修饰IAA32和IAA34蛋白, 使IAA32/34蛋白更加稳定.上述结果表明, 生长素通过TMK1剪切后形成的TMK1C来稳定IAA32/34蛋白, 最终依然通过ARF转录因子调控基因表达, 在生长素聚集的部位抑制细胞生长, 从而导致顶端弯钩内外侧的差异性生长(
. The F-box protein TIR1 is an auxin receptor
1
(2005a)
... 生长素(auxin, indole acetic acid, IAA)是最早被发现的植物激素之一.近百余年来, 其调控植物生长发育和生长反应的研究备受广大植物学家关注.然而, 21世纪之前, 人们对生长素调控植物生长发育的认识主要停留在生理及生化水平, 关于其信号传递机制的研究多年来进展缓慢.伴随着拟南芥(Arabidopsis thaliana)作为模式植物的广泛应用, Estelle和Leyser两个团队在2005年同时独立发现F-box蛋白TIR1 (Transport Inhibitor Response 1)是生长素的受体, 使生长素信号转导通路的研究取得飞速发展.后续研究表明, 生长素受体TIR1及其同源受体蛋白AFBs (Auxin Signaling F-box Proteins)能够与生长素信号的抑制因子Aux/IAA蛋白互作, 而生长素与其受体TIR1/AFBs结合后, 能够稳定其受体-Aux/IAA蛋白的互作, 且能够泛素化修饰及降解Aux/IAA蛋白, 从而释放出被Aux/IAA蛋白抑制的生长素响应因子ARFs (Auxin Response Factors), 介导生长素信号的传递(
. Plant development is regulated by a family of auxin receptor F box proteins
1
(2005b)
... 生长素(auxin, indole acetic acid, IAA)是最早被发现的植物激素之一.近百余年来, 其调控植物生长发育和生长反应的研究备受广大植物学家关注.然而, 21世纪之前, 人们对生长素调控植物生长发育的认识主要停留在生理及生化水平, 关于其信号传递机制的研究多年来进展缓慢.伴随着拟南芥(Arabidopsis thaliana)作为模式植物的广泛应用, Estelle和Leyser两个团队在2005年同时独立发现F-box蛋白TIR1 (Transport Inhibitor Response 1)是生长素的受体, 使生长素信号转导通路的研究取得飞速发展.后续研究表明, 生长素受体TIR1及其同源受体蛋白AFBs (Auxin Signaling F-box Proteins)能够与生长素信号的抑制因子Aux/IAA蛋白互作, 而生长素与其受体TIR1/AFBs结合后, 能够稳定其受体-Aux/IAA蛋白的互作, 且能够泛素化修饰及降解Aux/IAA蛋白, 从而释放出被Aux/IAA蛋白抑制的生长素响应因子ARFs (Auxin Response Factors), 介导生长素信号的传递(
Auxin response factors
1
2007
... 生长素(auxin, indole acetic acid, IAA)是最早被发现的植物激素之一.近百余年来, 其调控植物生长发育和生长反应的研究备受广大植物学家关注.然而, 21世纪之前, 人们对生长素调控植物生长发育的认识主要停留在生理及生化水平, 关于其信号传递机制的研究多年来进展缓慢.伴随着拟南芥(Arabidopsis thaliana)作为模式植物的广泛应用, Estelle和Leyser两个团队在2005年同时独立发现F-box蛋白TIR1 (Transport Inhibitor Response 1)是生长素的受体, 使生长素信号转导通路的研究取得飞速发展.后续研究表明, 生长素受体TIR1及其同源受体蛋白AFBs (Auxin Signaling F-box Proteins)能够与生长素信号的抑制因子Aux/IAA蛋白互作, 而生长素与其受体TIR1/AFBs结合后, 能够稳定其受体-Aux/IAA蛋白的互作, 且能够泛素化修饰及降解Aux/IAA蛋白, 从而释放出被Aux/IAA蛋白抑制的生长素响应因子ARFs (Auxin Response Factors), 介导生长素信号的传递(
The Arabidopsis F-box protein TIR1 is an auxin receptor
1
2005
... 生长素(auxin, indole acetic acid, IAA)是最早被发现的植物激素之一.近百余年来, 其调控植物生长发育和生长反应的研究备受广大植物学家关注.然而, 21世纪之前, 人们对生长素调控植物生长发育的认识主要停留在生理及生化水平, 关于其信号传递机制的研究多年来进展缓慢.伴随着拟南芥(Arabidopsis thaliana)作为模式植物的广泛应用, Estelle和Leyser两个团队在2005年同时独立发现F-box蛋白TIR1 (Transport Inhibitor Response 1)是生长素的受体, 使生长素信号转导通路的研究取得飞速发展.后续研究表明, 生长素受体TIR1及其同源受体蛋白AFBs (Auxin Signaling F-box Proteins)能够与生长素信号的抑制因子Aux/IAA蛋白互作, 而生长素与其受体TIR1/AFBs结合后, 能够稳定其受体-Aux/IAA蛋白的互作, 且能够泛素化修饰及降解Aux/IAA蛋白, 从而释放出被Aux/IAA蛋白抑制的生长素响应因子ARFs (Auxin Response Factors), 介导生长素信号的传递(
Origin and evolution of the nuclear auxin response system
1
2018
... 除了上述经典的Aux/IAAs蛋白外, 拟南芥IAA30-34蛋白没有典型的N端结构域I和II, 被称为非经典Aux/IAAs, 其是否参与生长素信号的传递尚无定论(
From perception to attenuation: auxin signaling and responses
1
2013
... 生长素(auxin, indole acetic acid, IAA)是最早被发现的植物激素之一.近百余年来, 其调控植物生长发育和生长反应的研究备受广大植物学家关注.然而, 21世纪之前, 人们对生长素调控植物生长发育的认识主要停留在生理及生化水平, 关于其信号传递机制的研究多年来进展缓慢.伴随着拟南芥(Arabidopsis thaliana)作为模式植物的广泛应用, Estelle和Leyser两个团队在2005年同时独立发现F-box蛋白TIR1 (Transport Inhibitor Response 1)是生长素的受体, 使生长素信号转导通路的研究取得飞速发展.后续研究表明, 生长素受体TIR1及其同源受体蛋白AFBs (Auxin Signaling F-box Proteins)能够与生长素信号的抑制因子Aux/IAA蛋白互作, 而生长素与其受体TIR1/AFBs结合后, 能够稳定其受体-Aux/IAA蛋白的互作, 且能够泛素化修饰及降解Aux/IAA蛋白, 从而释放出被Aux/IAA蛋白抑制的生长素响应因子ARFs (Auxin Response Factors), 介导生长素信号的传递(
Photoactivated CRY1 and phyB interact directly with Aux/IAA proteins to inhibit auxin signaling in Arabidopsis
1
2018
... 综上所述, 该项研究阐明了一条新的生长素TMK1-IAA32/34-ARFs信号通路(
Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling
1
2014
... 除了上述经典的Aux/IAAs蛋白外, 拟南芥IAA30-34蛋白没有典型的N端结构域I和II, 被称为非经典Aux/IAAs, 其是否参与生长素信号的传递尚无定论(
Phytochrome a negatively regulates the shade avoidance response by increasing auxin/indole acidic acid protein stability
1
2018
... 综上所述, 该项研究阐明了一条新的生长素TMK1-IAA32/34-ARFs信号通路(
备案号: 京ICP备16067583号-21
版权所有 © 2021 《植物学报》编辑部
地址:北京香山南辛村20号 邮编:100093
电话:010-62836135 010-62836131 E-mail:cbb@ibcas.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
