0 引言
【研究意义】猪瘟(classical swine fever,CSF)是一种由猪瘟病毒(classical swine fever virus,CSFV)引起的猪的高致病性、高死亡率的急性传染病,对很多国家养猪业造成重大经济损失[1,2]。CSFV属于黄病毒科瘟病毒属成员,病毒粒子有囊膜,基因组是一条长约12.3 kb的单股正链RNA[3,4]。CSFV的复制周期包括吸附、内化、膜融合、早期蛋白质的合成、基因组复制以及病毒粒子的装配和释放等,其中病毒的侵入作为其复制周期的初始环节,对病毒的繁殖发挥决定性作用。瘟病毒属成员大都利用受体介导的内吞作用侵入宿主细胞[5,6,7],最近报道,CSFV利用受体介导的网格蛋白依赖的内吞作用侵入宿主细胞,该进程在低pH环境下进行,依赖动力蛋白和胆固醇,同时需要Rab5和Rab7的参与[8]。在侵入过程中,CSFV利用自身囊膜蛋白与细胞膜表面受体分子发生相互作用,目前,在吸附受体方面的研究已经取得一定进展,但内化受体仍有待鉴定。总之,对侵入机制的研究不仅能够明确病毒的感染和发病机理,更为抗病毒治疗提供新型的药物靶标,因此,研究CSFV的侵入机制具有重要的科学意义。【前人研究进展】Erns、E1和E2是位于CSFV表面的3种囊膜蛋白[9],Erns通过与吸附受体作用介导CSFV的吸附,已知的CSFV吸附受体包括硫酸乙酰肝素(heparan sulfate,HS)[10]和层粘连蛋白受体(laminin receptor,LamR)[11],CD46分子被鉴定为CSFV的吸附因子[12]。E2蛋白位于病毒粒子表面,与E1蛋白形成异源二聚体,是CSFV重要的抗原蛋白,可诱导机体产生中和抗体[13]。在伪型病毒侵入试验中,证明E1和E2蛋白足以介导CSFV侵入易感细胞[14]。CSFV的侵入包含吸附和内化过程,在吸附过程中,通过吸附受体与囊膜蛋白结合使病毒粒子富集在宿主表面,随之病毒粒子与宿主细胞的空间距离和细胞内部环境发生变化,使病毒囊膜蛋白发生变构,进一步与内化受体结合,从而介导病毒的内化[15]。【本研究切入点】目前,明确参与CSFV侵入的囊膜蛋白包括Erns、E1和E2,但具体哪种蛋白、如何介导CSFV的吸附和内化仍然未知。【拟解决的关键问题】通过探究重组E2蛋白对CSFV吸附和内化的影响,明确E2蛋白是否参与CSFV吸附和内化,为发现CSFV细胞受体提供理论基础。1 材料与方法
1.1 主要材料
研究在中国农业科学院哈尔滨兽医研究所兽医生物技术国家重点实验室完成。PK-15细胞和CSFV石门株由本团队保存,悬浮293细胞购自上海源培生物科技股份有限公司,针对CSFV E2蛋白的单克隆抗体WH303[16]由英国动植物卫生机构(APHA)Trevor Drew教授提供,pMD19-T Simple载体购自宝生物工程有限公司,pLVX-IRES-ZsGreen1载体购自Clontech公司,psPAX2和pMD2.G慢病毒包装辅助质粒购自Addgene。1.2 引物设计与合成
为了构建重组E2蛋白的表达载体,根据CSFV E2基因全长序列设计引物,在E2基因5°端引入EcoR I酶切位点,3°端去除编码跨膜区的基因片段,引入Flag和Strep双标签(两个标签之间以TEV酶切位点连接)以及Xho I酶切位点,并由上海英骏生物技术有限公司合成,序列如下(斜体字分别为EcoR I和Xho I酶切位点,下划线处分别为Strep标签、TEV酶切位点和Flag标签):SME2-F:5'-CGGAATTCGCCACCATGG TATTAAGAGGGCAGATCGTGC-3';SME2-R(FTS):5'-CCCTCGAGCTACTTCTCGAACTGGGGGTGGGACCAACCCTGAAAATAAAGATTCTCCTTGTCGTCATCGTCTTTGTAGTCTTCTGCGAAGTAATCTGAGTGG-3'。1.3 重组E2蛋白真核表达载体的构建
以CSFV石门株cDNA为模板,利用Ex-Taq酶扩增E2基因片段。回收PCR产物,连接至pMD19-T simple载体。测序正确后,以该重组质粒为模板,利用PrimeSTAR高保真酶扩增包含Flag和Strep双标签的E2基因片段,将其插入pLVX-IRES-ZsGreen1载体中,得到重组质粒pLVX-SM-E2-FTS。1.4 包含E2基因的慢病毒的包装
将HEK-293T细胞铺入六孔板,待细胞汇合度达到80%后进行转染。首先,把细胞培养液换成含10% FBS的DMEM,同时将重组质粒pLVX-SM-E2-FTS、辅助质粒psPAX2和pMD2.G与X-tremeGENE HP DNA转染试剂(罗氏公司)配成转染复合物,静置20 min后加到细胞上清中,培养48 h后收获细胞上清,5 000×g离心去除细胞碎片,分装后贮存于-80℃冰箱中,得到慢病毒Lenti-SM-E2-FTS。1.5 表达E2蛋白的悬浮293细胞系的构建
将悬浮293细胞培养传代至细胞稳定生长之后,按感染复数(multiplicity of infection, MOI)为1用重组慢病毒Lenti-SM-E2-FTS进行转导,得到悬浮细胞系293-SM-E2。37℃培养48 h后,换成新鲜的培养基。待细胞生长状态稳定后,倒置荧光显微镜下观察细胞转导水平。1.6 重组E2蛋白的纯化
将细胞悬液倒入50 mL离心管,500×g离心5 min后,收取上清到新管,用10 kD超滤管(Merck Millipore公司)将含有重组蛋白的上清液从100 mL浓缩至10 mL。将高亲和力Strep标签树脂(GE公司)填入层析柱中,用预冷的PBS洗5次,之后加入浓缩的蛋白,于4℃条件下翻转结合过夜,弃去穿流液后,用PBS洗5次以除去未结合的蛋白,用脱硫生物素(Sigma公司)于4℃条件下洗脱。1.7 SDS-PAGE电泳及Western blotting
将纯化的蛋白加入5×loading buffer后煮沸5 min,进行SDS-PAGE电泳,之后转印到硝酸纤维素薄膜上,经5%脱脂乳封闭1 h后,用抗E2蛋白单克隆抗体WH303于室温孵育2 h,用PBS洗5次,加入IRDye800CW山羊抗小鼠IgG抗体(LI-COR Bioscience公司)避光孵育1 h,再用PBS洗5次,利用近红外荧光成像系统(Odyssey)扫描并读取结果。1.8 抗E2蛋白多克隆抗体的制备及检测
将20 μg纯化的重组E2蛋白与QuickAntibody- Mouse3W免疫佐剂(北京博奥龙公司)混合后,通过肌肉注射免疫BALB/c小鼠,一免后2 w以相同的剂量进行二次免疫,二免后1 w采血并分离血清,利用猪瘟抗体检测试剂盒(IDEXX公司)检测E2蛋白特异性抗体水平,最后摘除眼球采血,分离血清,得到抗E2蛋白多克隆抗体(抗E2多抗),保存于-20℃备用。利用中和试验检测抗E2多抗的中和滴度,具体操作步骤参照欧盟猪瘟诊断手册有关抗体检测部分。1.9 细胞总RNA的提取及荧光定量RT-PCR
RNA的提取步骤参照Life Technologies公司的TRIzol试剂说明书进行,利用反转录酶(宝生物工程有限公司)将RNA反转录为cDNA,通过荧光定量RT-PCR(RT-qPCR)检测CSFV基因组拷贝数,操作参照ZHAO等的报道进行[17]。1.10 蛋白阻断试验
重组E2蛋白对CSFV感染的影响:将不同浓度重组E2蛋白(0、10、20和40 μg·mL-1)与MOI为0.1的CSFV混合后加入到PK-15细胞中,37℃感染48 h后检测病毒基因组拷贝数和病毒滴度。重组E2蛋白对CSFV侵入的影响:将不同浓度重组E2蛋白(0、10、20和40 μg·mL-1)与MOI为0.1的CSFV混合后加入到PK-15细胞中,37℃培养2 h,弃掉上清后换成含2% FBS的DMEM,37℃感染48 h后检测病毒基因组拷贝数和病毒滴度。
重组E2蛋白对CSFV吸附的影响:将不同浓度重组E2蛋白(0、10、20和40μg·mL-1)与MOI为1的CSFV混合后加入到PK-15细胞中,4℃吸附PK-15细胞2 h,弃去上清后用PBS洗细胞3次,以去掉未吸附的病毒粒子,加入含2% FBS的DMEM,37℃感染48 h后检测病毒基因组拷贝数和病毒滴度。
1.11 吸附和内化试验
将PK-15细胞铺入24孔板,待细胞生长48 h后进行吸附和内化试验。在吸附试验中,将阴性血清和不同稀释度的抗E2多抗(1﹕50、1﹕100、1﹕200、1﹕400和1﹕800)与MOI为1的CSFV在4℃感作2 h,同时将PK-15细胞在4℃条件下预冷15 min,再将处理后的病毒加到PK-15细胞中,4℃吸附2 h,弃掉上清后,用PBS洗细胞3次去掉未吸附的病毒粒子,用TRIzol试剂裂解细胞提取总RNA,RT-qPCR检测病毒基因组拷贝数。在内化试验中,将不同浓度重组E2蛋白(0、10、20和40 μg·mL-1)与MOI为1的CSFV混合后加入到PK-15细胞中,4℃吸附2 h,然后转至37℃培养2 h以完成内化,或将MOI为1的CSFV接种于PK-15细胞,在4℃吸附2 h,用PBS洗细胞3次以去掉未吸附的病毒粒子,再加入阴性血清或不同稀释度的抗E2多抗(1﹕50、1﹕100、1﹕200、1﹕400和1﹕800)在4℃感作1 h,然后转至37℃培养2 h以完成内化,将以上的细胞用PBS洗3次后,依次用0.05%的胰酶处理2 min、stripping buffer处理1 min、0.5 mg·mL-1的蛋白酶K处理2 min,以除去吸附到细胞表面但未内化的病毒粒子[18,19],用TRIzol试剂裂解细胞提取总RNA,RT-qPCR检测病毒基因组拷贝数。
1.12 抗体封闭试验
将PK-15细胞铺24孔板,待细胞汇和度达到80%后进行试验,将阴性血清和不同稀释度的抗E2多抗(1﹕50、1﹕100、1﹕200、1﹕400和1﹕800)与MOI为0.1的CSFV在37℃感作2 h,再加入PK-15细胞中,感染48 h后,收取上清测定病毒滴度,利用间接免疫荧光试验检测细胞内病毒感染量[20]。2 结果
2.1 重组CSFV E2蛋白的表达与鉴定
2.1.1 表达重组E2蛋白的悬浮293细胞系建立 通过慢病毒转导的方法建立悬浮293细胞系,倒置荧光显微镜下可见明显的绿色荧光(图1-a),表明慢病毒转导成功。利用Strep标签树脂通过亲和层析的方法纯化目的蛋白,经SDS-PAGE和Western blotting检测(图1-b、c),与对照组相比,在还原和非还原条件下分别在55—70 kD之间以及130 kD处各呈现一条特异性条带,为E2蛋白单体和二聚体形式,表明构建的悬浮细胞系可稳定表达重组E2蛋白。2.1.2 重组E2蛋白表达条件的优化 慢病毒载体携带ZsGreen1荧光标记,因此,采用流式细胞分选系统进行阳性细胞的分选以增加蛋白表达量(结果未显示)。为了优化重组蛋白表达,将悬浮293细胞系传至125 mL的细胞瓶,在相同的培养条件下,分别在第2、4、6、8、10和12天收集培养上清,经Strep标签树脂纯化后测定蛋白浓度,结果显示,在培养至第8 天时重组蛋白表达量最高,上清中的浓度约为5.84 μg·mL-1(图1-d)。
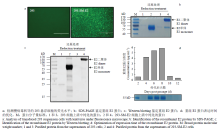 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图1重组E2蛋白的表达及其鉴定
-->Fig. 1Expression and identification of the recombinant E2 protein
-->
2.2 重组E2蛋白抑制CSFV感染
为了验证重组E2蛋白对CSFV感染的影响,用重组E2蛋白预处理细胞30 min,接种CSFV后培养2 h,换成含2% FBS的DMEM,48 h后检测病毒基因组拷贝数和病毒滴度,结果显示,重组E2蛋白预处理细胞对CSFV感染没有影响(结果未显示)。已有研究显示,CSFV E2蛋白与宿主细胞表面受体结合力不强[21],因此,将不同浓度的重组E2蛋白和CSFV混合后加入到PK-15细胞中,48 h后检测重组E2蛋白对CSFV感染的影响,结果显示,与对照组相比,病毒基因组复制水平和病毒滴度均显著下降(图2-a、b)。以上结果表明,制备的重组E2蛋白具有阻断CSFV感染的活性。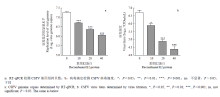 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图2重组E2蛋白对CSFV感染的抑制活性
-->Fig. 2The inhibitory activity of the recombinant E2 protein against CSFV
-->
2.3 E2蛋白参与CSFV的吸附
CSFV侵入过程包含吸附和内化阶段,利用囊膜蛋白与吸附受体互作后,进一步通过内化受体将病毒内吞到宿主细胞内,然后起始病毒基因组的翻译、转录及复制等功能。先利用蛋白封闭试验验证E2蛋白对CSFV侵入的影响,结果显示,与对照组相比,病毒基因组复制水平和病毒滴度均显著下降(图3-a、b),表明E2蛋白参与CSFV的侵入。进一步验证E2蛋白对CSFV吸附的影响,结果显示,与对照组相比,病毒基因组复制水平和病毒滴度均无显著差异(图3-c、d)。这表明,重组E2蛋白不能阻断CSFV对细胞的吸附。 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图3在侵入或吸附过程中添加重组E2蛋白对CSFV感染的影响为了进一步明确E2蛋白对CSFV吸附的影响,免疫BALB/c小鼠制备抗E2多抗,经阻断ELISA试剂盒检测,血清均发生阳转(结果未显示);中和试验结果显示,抗E2多抗的中和滴度可达1 600—5 120(
-->Fig. 3Effects of addition of the recombinant E2 protein during the entry or attachment on CSFV infection
-->
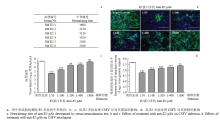 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图4抗E2多抗处理CSFV对其感染和吸附的影响
-->Fig. 4The effects of treatment of CSFV with the anti-E2 pAb on its infection and attachment
-->
2.4 E2蛋白介导CSFV的内化
为了探究E2蛋白对CSFV内化的作用,首先,利用重组E2蛋白进行内化试验,结果显示,重组E2蛋白能够显著抑制CSFV的内化水平,处理浓度为2.5 μg·mL-1时抑制效率可达50%左右,且随着蛋白浓度的增加,其抑制水平显著增强(图5-a)。氯丙嗪(chlorpromazine,CPZ)是一种通过抑制网格蛋白形成从而抑制病毒内化的药物抑制剂[22],利用该试剂作为对照,结果显示,其对CSFV内化有明显的抑制作用(图5-b)。随后,为了佐证E2蛋白参与CSFV的内化,利用抗E2多抗进行内化试验,结果显示,与对照组相比,CSFV的内化水平无显著差异(图5-c),表明抗E2多抗不能通过封闭吸附到宿主细胞上的CSFV而抑制CSFV的内化。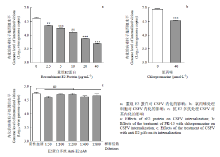 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图5E2蛋白对CSFV内化的影响
-->Fig. 5The effects of the E2 protein on CSFV internalization
-->
3 讨论
E2蛋白是CSFV的主要保护性抗原之一,不仅可以诱导机体产生中和抗体,而且能激活机体的细胞免疫应答[23,24]。该蛋白是CSFV重要的毒力决定因子,与病毒的致弱机制相关[25]。通过表达CSFV囊膜蛋白的伪型病毒研究病毒感染的机制,证明E2蛋白是参与CSFV侵入的关键蛋白,但与其互作的细胞受体仍然未知[14]。近些年,相继有研究人员尝试了CSFV受体筛选相关的研究,但始终没有突破性进展,分析可能受病毒粒子与宿主特定的作用关系的限制,因此,解析参与CSFV侵入的关键囊膜蛋白和宿主的作用机制,可以推进这一进程的发展。黄病毒科代表成员丙型肝炎病毒(hepatitis C virus,HCV)的侵入是由病毒粒子表面多个囊膜蛋白与宿主蛋白协同互作,从而引发的一系列反应[26,27]。CSFV是否也与同科病毒具有类似的侵入机制?由Erns蛋白与宿主相互作用起始侵入环节,之后引发其他囊膜蛋白与一个甚至多个宿主分子作用后完成整个侵入进程?在这其中还需要哪些宿主分子的参与?这都是未来亟待解决的问题。在本研究中,笔者初步探索了E2蛋白在CSFV吸附和内化中的作用,而没有涉及参与CSFV侵入的其他蛋白分子,包括Erns蛋白是否参与CSFV内化的研究,或者E1和E2蛋白之间是否通过二聚体形式与宿主细胞发生相互作用,从而引发网格蛋白介导的内吞作用等。
本研究通过慢病毒系统构建了以分泌形式表达重组E2蛋白的悬浮293细胞系,同时制备了针对重组E2蛋白的多抗,抗体封闭试验证明,E2蛋白参与CSFV吸附(图4-d),利用重组E2蛋白进行内化试验证明,E2蛋白参与CSFV的内化(图5-a),有趣的是,当CSFV吸附到细胞表面之后再加入抗体进行内化试验,则对CSFV内化无显著影响(图5-c)。据此推测,介导CSFV吸附的细胞受体也能将其内吞进细胞内。另外,用重组E2蛋白预处理PK-15细胞后,无法阻断CSFV的感染,然而,在细胞培养上清中添加重组E2蛋白却显著抑制CSFV的感染。由此推测,重组E2蛋白与宿主细胞的结合力较弱,但是能够通过关键位点的“位阻效应”阻断CSFV的感染。这表明,全长E2蛋白并不适合CSFV受体的筛选。尽管如此,能够结合宿主细胞且阻断病毒感染的肽段具有一定的应用价值[28,29]。
4 结论
4.1 通过慢病毒系统成功构建了稳定表达重组E2蛋白的悬浮293细胞系;
4.2 通过蛋白阻断和抗体封闭试验证明,E2蛋白参与CSFV的吸附和内化。
The authors have declared that no competing interests exist.参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | . Classical swine fever (CSF) is a highly contagious and often fatal disease that affects domestic pigs and wild boars. Outbreak of CSF can cause heavy economic losses to the pig industry. The strategies to prevent, control and eradicate CSF disease are based on containing the disease through a systematic prophylactic vaccination policy and a non-vaccination stamping-out policy. The quest for prevention, control and eradication of CSF has moved research forward in academia and industry, and has produced noticeable advances in understanding fundamental aspects of the virus replication mechanisms, virulence, and led to the development of new vaccines. In this review we summarize recent progress in CSFV epidemiology, molecular features of the genome and proteome, the molecular basis of virulence, and the development of anti-virus technologies. |
| [2] | . Classical swine fever (CSF), caused by Classical swine fever virus (CSFV), is an OIE-listed, highly contagious, often fatal disease of swine worldwide. Currently, the disease is controlled by prophylactic vaccination in China and many other countries using the modified live vaccines derived from C-strain, which was developed in China in the mid-1950s. This minireview summarizes the epidemiology, diagnostic assays, control and challenges of CSF in China. Though CSF is essentially under control, complete eradication of CSF in China remains a challenging task and needs long-term, joint efforts of stakeholders. (C) 2014 Elsevier B.V. All rights reserved. |
| [3] | . Pestiviruses, which include economically important animal pathogens such as bovine viral diarrhea virus and classical swine fever virus, possess three envelope glycoproteins, namely Erns, E1, and E2. This article discusses the structures and functions of these glycoproteins and their effects on viral pathogenicity in cells in culture and in animal hosts. E2 is the most important structural protein as it interacts with cell surface receptors that determine cell tropism and induces neutralizing antibody and cytotoxic T-lymphocyte responses. All three glycoproteins are involved in virus attachment and entry into target cells. E1-E2 heterodimers are essential for viral entry and infectivity. Erns is unique because it possesses intrinsic ribonuclease (RNase) activity that can inhibit the production of type I interferons and assist in the development of persistent infections. These glycoproteins are localized to the virion surface; however, variations in amino acids and antigenic structures, disulfide bond formation, glycosylation, and RNase activity can ultimately affect the virulence of pestiviruses in animals. Along with mutations that are driven by selection pressure, antigenic differences in glycoproteins influence the efficacy of vaccines and determine the appropriateness of the vaccines that are currently being used in the field. |
| [4] | A cDNA clone derived from genomic RNA of hog cholera virus (HCV) was identified using an oligonucleotide complementary to the RNA encoding a hexapeptide from the putative RNA-dependent RNA polymerase of the closely related bovine viral diarrhea virus (BVDV). This clone served as a probe for screening different size-selected cDNA libraries. After molecular cloning and nucleotide sequencing the HCV genome was shown to consist of 12,284 nucleotides containing one long open reading frame. Sequence comparison revealed a high degree of homology between HCV and BVDV genomic RNAs. With respect to HCV the genome of BVDV contains an insertion coding for 90 amino acids. |
| [5] | . We have used non-cytopathic (ncp) and cytopathic (cp) bovine viral diarrhoea viruses (BVDV) to determine how the two biotypes affect mannose receptor (MR)-mediated endocytosis and fluid phase uptake in bovine monocytes. We have demonstrated that endocytosis in uninfected monocytes after 1 h of culture was mediated by the MR and fluid phase uptake, and after 24 h of culture it was mediated via fluid phase uptake only. Both cp and ncp BVDV affected the mechanisms of antigen uptake in monocytes. Endocytosis in BVDV infected monocytes, unlike in uninfected cells, was MR-independent and mediated by fluid phase uptake after 1 h of infection. The 24-h-BVDV infection changed the antigen uptake mechanisms to become MR- and fluid phase uptake-dependent. We conclude that antigen uptake, an important antigen presenting cell (APC) function, is affected in the early stage of BVDV infection during the first 24 h, with both BVDV biotypes, cp and ncp, having similar effects on monocyte antigen uptake in cattle. By influencing the early antigen uptake function of APC, BVDV might disrupt the function of monocytes as professional APC and contribute to the specific immunotolerance to BVDV. |
| [6] | . Pestiviruses initiate infection of susceptible cells by receptor-mediated endocytosis. Cellular plasma membrane or endosomal molecules involved in translocation of these viruses into the cytosol have not been unequivocally identified. We reported previously that a mutant cell line derived from Madin-Darby bovine kidney (MDBK) cells, termed CRIB-1, was resistant to infection with bovine viral diarrhoea virus. CRIB-1 cells were also resistant to infection with classical swine fever virus and border disease virus of sheep, suggesting that entry of these three different pestiviruses into bovine cells requires a common cell membrane function. The resistance is pestivirus-specific: CRIB-1 cells were as susceptible as the parental MDBK cells to 14 other viruses of cattle and swine belonging to unrelated families. The resistance of CRIB-1 cells to pestivirus infection involves a block in virus entry since transfection of virus RNA or virus inoculation in the presence of PEG resulted in productive infection. Furthermore, quantitative analyses of the outcome of PEG-mediated infection of CRIB-1 cells indicated that the intracellular milieu was fully permissive for pestivirus replication. Binding studies revealed that virus attachment to CRIB-1 cells was not completely abrogated. These results indicate that entry of pestiviruses into MDBK cells depends on a common plasma membrane or endosomal function, which is lacking in CRIB-1 cells. |
| [7] | . Bovine viral diarrhoea virus (BVDV) is a pestivirus within the family Flaviviridae. In contrast to the members of the genus flavivirus, nothing is known about the viral entry route for pestiviruses. In this study, the process of BVDV infection following attachment to the cell surface was examined. BVDV clearly co-localizes with clathrin, with early endosome antigen-1 (EEA-1), an early endosome marker, and also with lysosomal-associated membrane protein-2 (LAMP-2), a lysosomal marker. BVDV internalization is inhibited by compounds that block clathrin- but not caveolae-dependent endocytosis. These findings demonstrate that BVDV enters the cells via the clathrin-coated pit pathway. |
| [8] | . |
| [9] | . Genomic RNA of hog cholera virus (HCV) strain Brescia was cloned and sequenced. The nucleotide sequence was deduced from overlapping cDNA clones and comprises 12,283 nucleotides. We cloned the complete 3′ end of the HCV genome, but could not unequivocally prove that the cDNA sequence also completely covers HCV RNA at the 5′ end. The HCV genome contained one large open reading frame, which spans the viral plus strand RNA and encodes an amino acid sequence of 3898 residues with a calculated molecular weight of 438,300. To identify structural HCV glycoproteins, we prepared rabbit antisera against three synthetic peptides deduced from the sequence. Because one of these antisera reacted with a 51- to 54-kDa glycoprotein (envelope protein Et of HCV) on Western blot, the genomic position of the sequence encoding gp51–54 could be clearly established. The amino acid sequence of Brescia was compared with that of HCV strain Alfort and that of BVDV strains NADL and Osloss. The degree of homology between the two HCV strains was 93%, and between Brescia and the BVDV strains about 70%. NADL contained an inserted sequence of 90 amino acids that was absent from the sequences of Brescia, Alfort, and Osloss, whereas Osloss contained an inserted sequence of 76 amino acids that was absent from the sequences of Brescia, Alfort, and NADL. Sequences in p80, the most homologous protein among pestiviruses, showed similarity to six sequence motifs found conserved in helicase-like proteins represented by elF-4A. Furthermore, a trypsin-like serine protease domain detected in p80 of BVDV was also found conserved in HCV, suggesting that pestivirus p80 may be bifunctional. |
| [10] | . Abstract Infection of cells with Classical swine fever virus (CSFV) is mediated by the interaction of envelope glycoprotein E(rns) and E2 with the cell surface. In this report we studied the role of the cell surface glycoaminoglycans (GAGs), chondroitin sulfates A, B, and C (CS-A, -B, and -C), and heparan sulfate (HS) in the initial binding of CSFV strain Brescia to cells. Removal of HS from the surface of swine kidney cells (SK6) by heparinase I treatment almost completely abolished infection of these cells with virus that was extensively passaged in swine kidney cells before it was cloned (clone C1.1.1). Infection with C1.1.1 was inhibited completely by heparin (a GAG chemically related to HS but sulfated to a higher extent) and by dextran sulfate (an artificial highly sulfated polysaccharide), whereas HS and CS-A, -B, and -C were unable to inhibit infection. Bound C1.1.1 virus particles were released from the cell surface by treatment with heparin. Furthermore, C1.1.1 virus particles and CSFV E(rns) purified from insect cells bound to immobilized heparin, whereas purified CSFV E2 did not. These results indicate that initial binding of this virus clone is accomplished by the interaction of E(rns) with cell surface HS. In contrast, infection of SK6 cells with virus clones isolated from the blood of an infected pig and minimally passaged in SK6 cells was not affected by heparinase I treatment of cells and the addition of heparin to the medium. However, after one additional round of amplification in SK6 cells, infection with these virus clones was affected by heparinase I treatment and heparin. Sequence analysis of the E(rns) genes of these virus clones before and after amplification in SK6 cells showed that passage in SK6 cells resulted in a change of an Ser residue to an Arg residue in the C terminus of E(rns) (amino acid 476 in the polyprotein of CSFV). Replacement of the E(rns) gene of an infectious DNA copy of C1.1.1 with the E(rns) genes of these virus variants proved that acquisition of this Arg was sufficient to alter an HS-independent virus to a virus that uses HS as an E(rns) receptor. |
| [11] | . Abstract Classical swine fever virus (CSFV) is the causative agent of classical swine fever (CSF), a highly contagious, economically important viral disease in many countries. The E(rns) and E2 envelope glycoproteins are responsible for the binding to and entry into the host cell by CSFV. To date, only one cellular receptor, heparan sulfate (HS), has been identified as being involved in CSFV attachment. HS is also present on the surface of various cells that are nonpermissive to CSFV. Hence, there must be another receptor(s) that has been unidentified to date. In this study, we used a set of small interfering RNAs (siRNAs) against a number of porcine cell membrane protein genes to screen cellular proteins involved in CSFV infection. This approach resulted in the identification of several proteins, and of these, the laminin receptor (LamR) has been demonstrated to be a cellular receptor for several viruses. Confocal analysis showed that LamR is colocalized with CSFV virions on the membrane, and a coimmunoprecipitation assay indicated that LamR interacts with the CSFV E(rns) protein. In inhibition assays, anti-LamR antibodies, soluble laminin, or LamR protein significantly inhibited CSFV infection in a dose-dependent manner. Transduction of PK-15 cells with a recombinant lentivirus expressing LamR yielded higher viral titers. Moreover, an attachment assay demonstrated that LamR functions during virus attachment. We also demonstrate that LamR acts as an alternative attachment receptor, especially in SK6 cells. These results indicate that LamR is a cellular attachment receptor for CSFV. IMPORTANCE: Classical swine fever virus (CSFV) is the causative agent of classical swine fever (CSF), an economically important viral disease affecting the pig industry in many countries. To date, only heparan sulfate (HS) has been identified to be an attachment receptor for CSFV. Here, using RNA interference screening with small interfering RNAs (siRNAs) against a number of porcine membrane protein genes, we identified the laminin receptor (LamR) to be another attachment receptor. We demonstrate the involvement of LamR together with HS in virus attachment, and we elucidate the relationship between LamR and HS. LamR also serves as an attachment receptor for many viral pathogens, including dengue virus, a fatal human flavivirus. The study will help to enhance our understanding of the life cycle of flaviviruses and the development of antiviral strategies for flaviviruses. Copyright 脗漏 2015, American Society for Microbiology. All Rights Reserved. |
| [12] | Abstract Classical swine fever virus (CSFV) is the causative agent of a severe multi-systemic disease of pigs. While several aspects of virus-host-interaction are known, the early steps of infection remain unclear. For the closely related bovine viral diarrhea virus (BVDV), a cellular receptor is known: bovine complement regulatory protein CD46. Given that these two pestiviruses are closely related, porcine CD46 is also a candidate receptor for CSFV. In addition to CD46, cell-culture-adapted CSFV strains have been shown to use heparan sulfates as an additional cellular factor. In the present study, the interaction of field-type and cell-culture-adapted CSFV with a permanent porcine cell line or primary macrophages was assessed using anti-porcine CD46 monoclonal antibodies and a heparan-sulfate-blocking compound, DSTP-27. The influence of receptor blocking was assessed using virus titration and quantitative PCR. Treatment of cells with monoclonal antibodies against porcine CD46 led to a reduction of viral growth in both cell types. The effect was most pronounced with field-type CSFV. The blocking could be enhanced by addition of DSTP-27, especially for cell-culture-adapted CSFV. The combined use of both blocking agents led to a significant reduction of viral growth but was also not able to abolish infection completely. The results obtained in this study showed that both porcine CD46 and heparan sulfates play a major role in the initial steps of CSFV infection. Additional receptors might also play a role for attachment and entry; however, their impact is obviously limited in vitro in comparison to CD46 and heparan sulfates. |
| [13] | . The glycoproteins E(rns) of classical swine fever virus (CSFV) and E(rns) and E2 of bovine viral diarrhoea virus (BVDV) are shown to be located at the surface of infected cells by the use of indirect immunofluorescence and by cytofluorometric analysis. The positive immunostaining of the cell surface was further analysed by immunogold electron microscopy and it could be shown that only extracellular virions were labelled. Gold granules were not seen at the cellular plasma membrane. In contrast to BVDV E2, the CSFV E2 of virions sticking to the plasma membrane was not accessible to the respective monoclonal antibodies. However, CSFV particles isolated from culture supernatant were able to bind both monoclonal anti-E(rns) and anti-E2 antibodies. For CSFV and BVDV, binding of anti-E(rns) antibodies to the virions was more pronounced than that of anti-E2. This finding was unexpected since E2 is considered to be the immunodominant glycoprotein. |
| [14] | . Classical swine fever virus (CSFV) is the causative agent of classical swine fever. Its envelope comprises glycoproteins E rns, E1, and E2. In this study, we showed that the unmodified CSFV glycoproteins could incorporate into the HIV core to generate an infectious CSFV pseudotyped virus. The infection was specific to several porcine cell lines, and could be neutralized by anti-E2 monoclonal antibodies (mAbs) completely and by anti-E rns mAbs partially, indicating that this pseudotyped virus can mimic the early infection steps of parental CSFV. To investigate the specific role of each envelope protein involved in viral entry, a series of pseudotyped viruses were generated bearing CSFV glycoproteins in various combinations. It was found that specific infectivity was also achieved with non-E rns pseudotyped virus carrying E1 and E2 glycoproteins. This indicated that E1 and E2 are sufficient to mediate CSFV entry, and E rns is not indispensable in this process. |
| [15] | . Stuart and colleagues have determined the atomic structure of the ectodomain of bovine viral diarrhea virus E2 glycoprotein, the major, antigenically dominant protein on the virus surface. The structure was expected to resemble the fusion molecules found on the surface of viruses such as dengue virus, but it is unlike anything previously seen. E2 itself is not, in fact, the fusion protein but binds the cell receptor and directs fusion via a pH-dependent conformational switch. |
| [16] | . Nineteen monoclonal antibodies (MAbs) with specificity for hog cholera virus (HCV) were prepared. They were used in an immune binding (peroxidase linked) assay to determine the reaction patterns of HCV isolates from Europe, Brazil, USA, Japan and Malaysia, as well as laboratory reference strains of the virus. A further panel of 17 MAbs raised against bovine virus diarrhoea virus (BVDV) was included in the study, together with 5 MAbs raised against a non-HCV pestivirus of porcine origin. All the MAbs were also tested against representative strains of BVDV and border disease virus. Six MAbs were HCV-specific, reacting with all isolates of HCV and none of the ruminant viruses. Among the other HCV MAbs geographical variation in reaction patterns was observed. There was evidence of antigenic distinction between recent European isolates, and archive material originally isolated more than 10 years ago. |
| [17] | . |
| [18] | . Epstein-Barr virus (EBV) is implicated as an aetiological factor in B lymphomas and nasopharyngeal carcinoma. The mechanisms of cell-free EBV infection of nasopharyngeal epithelial cells remain elusive. EBV glycoprotein B (gB) is the critical fusion protein for infection of both B and epithelial cells, and determines EBV susceptibility of non-B cells. Here we show that neuropilin 1 (NRP1) directly interacts with EBV gB 23-431. Either knockdown of NRP1 or pretreatment of EBV with soluble NRP1 suppresses EBV infection. Upregulation of NRP1 by overexpression or EGF treatment enhances EBV infection. However, NRP2, the homologue of NRP1, impairs EBV infection. EBV enters nasopharyngeal epithelial cells through NRP1-facilitated internalization and fusion, and through macropinocytosis and lipid raft-dependent endocytosis. NRP1 partially mediates EBV-activated EGFR/RAS/ERK signalling, and NRP1-dependent receptor tyrosine kinase (RTK) signalling promotes EBV infection. Taken together, NRP1 is identified as an EBV entry factor that cooperatively activates RTK signalling, which subsequently promotes EBV infection in nasopharyngeal epithelial cells. 漏 2014 Macmillan Publishers Limited. All rights reserved. |
| [19] | . Abstract The hepatitis E virus (HEV) causes large outbreaks and sporadic cases of acute viral hepatitis in developing countries. In the developed world, HEV occurrence has increased as a result of zoonotic transmission from swine. The cellular aspects of HEV infection, especially the determinants of entry, are poorly understood. In the absence of a robust in vitro culture system for HEV, it is not possible to produce high titre infectious virus that can be labeled for tracking its internalization. We have therefore used an Escherichia coli expressed HEV-like particle (HEV-LP) to study HEV entry. Following internalization, the HEV-LP initially trafficks to Rab5-positive compartments en route to acidic lysosomal compartments where it is degraded. Using pharmacological inhibitors, dominant negative and constitutively active mutants, and siRNA-mediated perturbations, we show that HEV entry requires dynamin-2, clathrin, membrane cholesterol and actin, but is independent of factors associated with macropinocytosis. The HEV-LP results were further validated through infection of liver cells with virus from the stool of an infected patient. The comparative analysis also showed involvement of the phosphatidylinositol-3-kinase/Akt pathway in an early post-entry step of viral replication. This report provides a detailed description of endocytic processes associated with HEV infection. |
| [20] | . |
| [21] | . Abstract Pure preparations of envelope glycoproteins E(rns) and E2 of classical swine fever virus (CSFV) synthesized in insect cells were used to study infection of porcine and bovine cells with the pestiviruses CSFV and bovine viral diarrhoea virus (BVDV). Almost 100% inhibition of infection of porcine kidney cells with CSFV was produced by 100 microg/ml E(rns). After removal of the virus no E(rns) was needed in the overlay medium (growth medium) to maintain this level of inhibition. In contrast, 100% inhibition of infection of porcine kidney cells with CSFV by 10 microg/ml E2 was only achieved when E2 was added to the overlay medium. When E2 was omitted, a maximum of 50% inhibition was achieved. This indicated that after the virus and E2 were removed from the cells, infection still occurred, by virus particles which were still bound to the cell surface. Treatment with 100 microg/ml E(rns) released these particles from the cell surface. Furthermore, E(rns) bound irreversibly to the surface of cells susceptible or unsusceptible to pestivirus infection and cell-to-cell spread of CSFV was completely inhibited by E2 but not by E(rns). These results demonstrated that E(rns) and E2 interacted with different cell surface receptors. Inhibition of BVDV infection of porcine and bovine cells by CSFV E2 suggested that CSFV E2 and BVDV E2 share an identical receptor. BVDV strain 5250 isolated from pigs was efficiently inhibited by CSFV E(rns), whereas several BVDV strains isolated from cattle were not, suggesting that the conformation of E(rns) plays a role in host tropism. |
| [22] | . The lack of a suitable hepatitis B virus (HBV) infectivity model has limited examination of the early stages of the virus-cell interaction. In this study, we used an immortalized cell line derived from human primary hepatocytes, HuS-E/2, to study the mechanism of HBV infection. HBV infection efficiency was markedly increased after dimethyl sulfoxide (DMSO)-induced differentiation of the cells. Transmission electron microscopy demonstrated the presence of intact HBV particles in DMSO-treated HBV-infected HuS-E/2 cells, which could be infected with HBV for up to at least 50 passages. The pre-S1 domain of the large HBsAg (LHBsAg) protein specifically interacted with clathrin heavy chain (CHC) and clathrin adaptor protein AP-2. Short hairpin RNA knockdown of CHC or AP-2 in HuS-E/2 cells significantly reduced their susceptibility to HBV, indicating that both are necessary for HBV infection. Furthermore, HBV entry was inhibited by chlorpromazine, an inhibitor of clathrin-mediated endocytosis. LHBsAg also interfered with the clathrin-mediated endocytosis of transferrin by human hepatocytes. This infection system using an immortalized human primary hepatocyte cell line will facilitate investigations into HBV entry and in devising therapeutic strategies for manipulating HBV-associated liver disorders. |
| [23] | . Abstract Vaccination of pigs against Classical swine fever virus (CSFV) by using live-virus vaccines induces early protection before detectable humoral immune responses. Immunological analyses indicate that this is associated with T-cell activation, underlining the importance of targeting cytotoxic T-lymphocyte (CTL) responses for vaccine improvement. Antigen-presenting cells (APCs) transfected with mRNA encoding structural protein E2 or non-structural viral proteins NS3-NS4A were used to identify viral genes encoding CTL epitopes. Monocyte-derived dendritic cells (DCs) and fibrocytes served as the APCs. In vitro translation of the mRNA and microscopic analysis of transfected cells demonstrated that E2 and NS3-NS4A could be identified. APCs transfected with either of the mRNA molecules restimulated CSFV-specific T cells to produce gamma interferon and specific cytotoxic activity against CSFV-infected target cells. The presence of CTL epitopes on E2 was confirmed by using d/d-haplotype MAX cells expressing E2 constitutively as target cells in d/d-haplotype CTL assays. A potent CTL activity against E2 was detected early (1-3 weeks) after CSFV challenge. This work corroborates the existence of CTL epitopes within the non-structural protein domain NS3-NS4A of CSFV. Furthermore, epitopes on the E2 protein can also now be classified as targets for CTLs, having important implications for vaccine design, especially subunit vaccines. As for the use of mRNA-transfected APCs, this represents a simple and efficient method to identify viral genes encoding CTL epitopes in outbred populations. |
| [24] | . Immunization of domestic pigs with a DNA vaccine expressing the complete E2 protein of classical swine fever virus (CSFV) conferred total protection against a severe viral challenge. Immunization with three doses of plasmid pcDNA3.1/E2 elicited a consistent and specific, MHC class II restricted T cell response in the three domestic pigs analyzed, in the absence of detectable anti-CSFV antibodies in serum. Upon challenge specific T cell responses were boosted in the three vaccinated pigs, and a rapid rise in the titers of CSFV neutralizing antibodies was noticed in two of them, which correlated with a total protection. In these two pigs, neither disease symptoms were observed nor was virus detected at any time after CSFV infection. Neutralizing antibody titers were lower in the third vaccine, which developed a mild and transient peak of pyrexia. As expected, similar analyses in three control pigs (injected with the empty vector or PBS) did not reveal the induction of specific T cells or viral antibodies and, upon challenge, animals developed severe symptoms of the disease, including high titers of viremia, hyperthermia and virus spread to different organs. Control pigs developed, also, a marked leucopenia, resulting in SWC3+ (myelomonocytic cells) being the major PBMC population, and a drastic decrease CD3+ T cells. This T cell depletion was prevented in animals immunized with pcDNA3.1/E2. The total protection achieved, in the absence of CSFV antibodies before challenge, supports the relevance in the antiviral response observed of specific T cell responses primed by pcDNA3.1/E2 vaccine, which, upon challenge, led to a rapid induction of neutralizing antibodies. The observation that CSFV antibodies could only be detected in protected animals after viral challenge opens the possibility of exploring the potential of the DNA vaccine approach used to develop marker vaccines against CSF. |
| [25] | . Transposon linker insertion mutagenesis of a full-length infectious clone (IC) (pBIC) of the pathogenic classical swine fever virus (CSFV) strain Brescia was used to identify genetic determinants of CSFV virulence and host range. Here, we characterize a virus mutant, RB-C22v, possessing a 19-residue insertion at the carboxyl terminus of E1 glycoprotein. Although RB-C22v exhibited normal growth characteristics in primary porcine macrophage cell cultures, the major target cell of CSFV in vivo, it was markedly attenuated in swine. All RB-C22v-infected pigs survived infection remaining clinically normal in contrast to the 100% mortality observed for BICv-infected animals. Comparative pathogenesis studies demonstrated a delay in RB-C22v spread to, and decreased replication in the tonsils, a 10 2 to 10 7 log 10 reduction in virus titers in lymphoid tissues and blood, and an overall delay in generalization of infection relative to BICv. Notably, RB-C22v-infected animals were protected from clinical disease when challenged with pathogenic BICv at 3, 5, 7, and 21 days post-RB-C22v inoculation. Viremia, viral replication in tissues, and oronasal shedding were reduced in animals challenged at 7 and 21 DPI. Notably BICv-specific RNA was not detected in tonsils of challenged animals. These results indicate that a carboxyl-terminal domain of E1 glycoprotein affects virulence of CSFV in swine, and they demonstrate that mutation of this domain provides the basis for a rationally designed and efficacious live-attenuated CSF vaccine. |
| [26] | . Hepatitis C virus (HCV) is an enveloped, positive strand RNA virus classified within the Flaviviridae family and is a major cause of liver disease worldwide. HCV life cycle and propagation are tightly linked to several aspects of lipid metabolism. HCV propagation depends on and also shapes several aspects of lipid metabolism such as cholesterol uptake and efflux through different lipoprotein receptors during its entry into cells, lipid metabolism modulating HCV genome replication, lipid droplets acting as a platform for recruitment of viral components, and very low density lipoprotein assembly pathway resulting in incorporation of neutral lipids and apolipoproteins into viral particles. During the first steps of infection, HCV enters hepatocytes through a multistep and slow process. The initial capture of HCV particles by glycosaminoglycans and/or lipoprotein receptors is followed by coordinated interactions with the scavenger receptor class B type I, a major receptor of high-density lipoprotein, the CD81 tetraspanin, and the tight junction proteins Claudin-1 and Occludin. This tight concert of receptor interactions ultimately leads to uptake and cellular internalization of HCV through a process of clathrin-dependent endocytosis. Over the years, the identification of the HCV entry receptors and cofactors has led to a better understanding of HCV entry and of the narrow tropism of HCV for the liver. Yet, the role of the two HCV envelope glycoproteins, E1 and E2, remains ill-defined, particularly concerning their involvement in the membrane fusion process. Here, we review the current knowledge and advances addressing the mechanism of HCV cell entry within hepatocytes and we highlight the challenges that remain to be addressed. |
| [27] | . Transposon linker insertion mutagenesis of a full-length infectious clone (IC) (pBIC) of the pathogenic classical swine fever virus (CSFV) strain Brescia was used to identify genetic determinants of CSFV virulence and host range. Here, we characterize a virus mutant, RB-C22v, possessing a 19-residue insertion at the carboxyl terminus of E1 glycoprotein. Although RB-C22v exhibited normal growth characteristics in primary porcine macrophage cell cultures, the major target cell of CSFV in vivo, it was markedly attenuated in swine. All RB-C22v-infected pigs survived infection remaining clinically normal in contrast to the 100% mortality observed for BICv-infected animals. Comparative pathogenesis studies demonstrated a delay in RB-C22v spread to, and decreased replication in the tonsils, a 10 2 to 10 7 log 10 reduction in virus titers in lymphoid tissues and blood, and an overall delay in generalization of infection relative to BICv. Notably, RB-C22v-infected animals were protected from clinical disease when challenged with pathogenic BICv at 3, 5, 7, and 21 days post-RB-C22v inoculation. Viremia, viral replication in tissues, and oronasal shedding were reduced in animals challenged at 7 and 21 DPI. Notably BICv-specific RNA was not detected in tonsils of challenged animals. These results indicate that a carboxyl-terminal domain of E1 glycoprotein affects virulence of CSFV in swine, and they demonstrate that mutation of this domain provides the basis for a rationally designed and efficacious live-attenuated CSF vaccine. |
| [28] | . The envelope proteins of classical swine fever virus (CSFV) mediate the binding of CSFV to cell surface molecules and allow CSFV subsequent to enter host cells. However, the proteins binding to host cells and their binding sequences are uncertain. The results showed that the protein E1, E2, and Erns were displayed on the surfaces of T7 phages. The E2 and Erns phage clones showed high binding affinity to host cells, in which the E2 phage clone interacted more specifically with host cells than with other cells, while the Erns phage clone interacted with all tested cells. A 30-mer phage displaying peptide library was constructed and screened against immobilized host cells, in which each peptide was overlapped 10aa to another peptide and spanned all amino acid sequences of Erns and E2. Fifty-eight clones with specific binding to host cells were isolated. Amino acid sequence analyses for two phage clones (P2 and P6) demonstrated the strongest binding positions were at 101–130 (S2) in Erns, and 141–170 (S6) in E2, respectively. The synthetic peptides (S2 and S6) could inhibit the binding of phage clones (P2 and P6) and CSFV to cell. About 86.74 and 74.24% inhibition rates of CSFV infection were achieved at 5502μM of the synthetic peptides S2 and S6. The results also indicated that the S2 (LAEGPPVKECAVTCRYDKDADINVVTQARN) and S6 (AVSPTTLRTEVVKTFRRDKPFPHRMDCVTT) from CSFV were host cell binding peptides, and both of them had potential for research of CSFV entering host cells. |
| [29] | . Importance: Mitogen-activated protein kinase kinase 2 (MEK2) is a kinase that operates immediately upstream of extracellular regulated kinase 1/2 (ERK1/2) and links to Raf and ERK via phosphorylation. Currently, little is known about the role of MEK2 in the replication of classical swine fever virus (CSFV), a devastating porcine pestivirus. Here, we investigate the roles of MEK2 and the... [Show full abstract] |
