0 引言
【研究意义】番茄褪绿病毒(Tomato chlorosis virus,ToCV)是一种隶属于长线形病毒科(Closteroviridae)毛形病毒属(Crinivirus)的RNA病毒,可侵染番茄、辣椒、烟草等多种作物[1]。ToCV是一种世界性病毒,1998年美国佛罗里达州对该病毒进行了首次报道[2],此后在南美洲、非洲、欧洲、亚洲相继被发现[3,4,5,6];国内自2012年ToCV在北京地区辣椒上首次被发现后[7],相继在山东、天津、河南、江苏、内蒙古、广东等多个地区发现其危害,给番茄等蔬菜生产造成了严重的损失[8,9,10,11,12,13,14]。作为一种仅能依靠虫媒传播的半持久性病毒,ToCV的迅速扩散离不开其传播媒介——粉虱,而烟粉虱(Bemisia tabaci)作为中国番茄产区的主要粉虱种群,有调查研究发现ToCV的扩散同其具有较为密切的关系[8,15]。而目前针对植物病毒病,早期检测、监测和预警是有效防止其蔓延的重要手段之一,因此建立一种可以准确、灵敏、快速检测田间植株样品和媒介昆虫体内ToCV的检测方法,对于该病毒的有效防控具有重要意义。【前人研究进展】目前,针对ToCV的检测,早期有血清学检测方法[16],但其检测灵敏度有限,而分子生物学检测技术能较好弥补这一缺陷。ToCV的分子生物学检测技术比较常见的有反转录PCR(reverse transcription-polymerase chain reaction,RT-PCR)检测法[6,17]和逆转录环介导等温扩增(reverse transcription loop-mediated isothermal amplification,RT-LAMP)检测法[18]等。上述两种方法均能够特异检测出样品中所包含的ToCV,灵敏度相对较高,但对于低丰度病毒样品中ToCV的检测仍然略显不足,并且无法测定样品中病毒含量。而实时荧光定量PCR技术(real-time fluorescent quantitative polymerase chain reaction,RT-qPCR)因其自身高灵敏度、高特异性以及可定量等优点,成为许多现有植物病毒检测的重要技术手段之一[19,20]。针对ToCV的实时荧光定量检测,ORFANIDOU等[21]运用TaqMan探针法对番茄、龙葵、苦苣菜、反枝苋、藜等寄主植物感染ToCV情况及带毒量进行检测。【本研究切入点】目前有关ToCV的RT-qPCR检测技术报道相对较少,虽然已有TaqMan探针检测ToCV的方法,但其检测成本较高,而基于SYBR Green I荧光染料的RT-qPCR成本较低,因此构建一种准确性好、灵敏度高、可定量并且成本低能够满足高通量应用的RT-qPCR检测技术体系尤为必要。【拟解决的关键问题】选择ToCV基因组特定区域设计特异性强的RT-qPCR检测引物,制备ToCV质粒标准品,建立合格标准曲线,摸索成熟完整的RT-qPCR检测技术体系,实现植物样品中和传毒媒介烟粉虱体内ToCV的准确、有效检测,为ToCV的早期监测、预警提供技术支撑。1 材料与方法
试验于2015—2016年在青岛农业大学山东省植物病虫害综合防控重点实验室完成。1.1 供试毒源和虫源
原始感染ToCV番茄毒株系于2014年采自青岛市上马区,并通过实验室内长期饲养的Q烟粉虱(即Q型烟粉虱)进行接种,接种成功后的番茄植株置于人工气候室(温度:(27±1)℃;相对湿度:(60±5)%;光周期:16L﹕8D)内培养,作为供试毒源。试验所用Q烟粉虱同样长期饲养在人工气候室中(条件同上)。
1.2 主要试剂
Trizol(Thermo Fisher Scientific公司)、反转录试剂盒PrimeScript RT Reagent Kit(Perfect real-time)(TaKaRa公司)、rTaq酶(TaKaRa公司)、胶回收试剂盒(TaKaRa Mini BEST Agarose GEL DNA Extraction Kit,TaKaRa公司)、质粒提取试剂盒(TIANprep Mini Plasmid Kit,天根生化科技有限公司)、pMDTM 18-T Vector(TaKaRa公司)、感受态细胞(Trans 5α,北京全式金生物技术有限公司)。1.3 引物设计
根据NCBI数据库中ToCV次要外壳蛋白(minor coat protein,CPm)基因(GenBank登录号:AGN91010.1)的保守序列,运用Primer 3.0在线软件(http://bioinfo.ut.ee/primer3-0.4.0/)设计ToCV的实时荧光定量PCR检测特异引物,并通过NCBI中的Primer-BLAST进行比对,保证所设计引物的特异性。上游引物ToCVqF:CTTTCTGGATGGTTTGCGGC;下游引物ToCVqR:TCCCCAACCAATGGTCGTTT。引物由英潍捷基(上海)贸易有限公司进行合成。1.4 番茄和单头烟粉虱总RNA的提取与RT-PCR
剪取0.05 g番茄叶片置于去除RNase的研钵中,加入液氮充分研磨后,采用Trizol试剂并按照说明书步骤完成番茄叶片总RNA的提取;将单头烟粉虱置于RNase free的1.5 mL离心管中,液氮冷冻后充分研磨,加入200 μL Trizol试剂充分裂解,并按照说明书同比例减少后续所需试剂的用量,最终使用8 μL RNase free溶解单头烟粉虱的总RNA。结合琼脂糖凝胶电泳检测提取RNA样品的完整性,并运用核酸蛋白浓度测定仪检测RNA样品纯度和浓度,选取优质合格的总RNA样品(A260/A280在1.8—2.1)进行后续试验。取质量合格的总RNA样品,按照反转录试剂盒说明书进行RT-PCR完成第一链cDNA的合成,并置于-20℃保存以备后续试验使用。
1.5 ToCV基因片段克隆与鉴定
将上述RT-PCR获得的cDNA作为模板,进行PCR扩增,总反应体系为25 μL:10×PCR Buffer(Mg2+ plus) 2.5 μL、dNTP Mixture 2 μL、ToCVqF和ToCVqR各1 μL、cDNA模板1 μL、r Taq 0.25 μL、ddH2O 17.25 μL。PCR反应程序:94℃预变性3 min;94℃变性30 s,60℃退火30 s,72℃延伸30 s,35个循环;72℃后延伸10 min。将上述PCR产物进行琼脂糖凝胶电泳后,切下目的条带,采用胶回收试剂盒进行纯化回收,连接至pMDTM 18-T Vector,并转化进入感受态细胞Trans 5α,37℃过夜培养后经蓝白斑筛选,挑取白色单菌落置于含有氨苄青霉素的LB液体培养基中振荡培养(37℃,220 r/min,6 h)。将菌液经特异引物ToCVqF和ToCVqR检测后,将阳性克隆送至英潍捷基(上海)贸易有限公司进行测序。
1.6 ToCV质粒标准品的制备
选择测序结果中序列完全正确的阳性克隆样品,继续放入含有氨苄青霉素的LB液体培养基中扩大培养(37℃,220 r/min,过夜)后,使用质粒提取试剂盒完成ToCV质粒的提取,使用核酸蛋白浓度测定仪测定质粒浓度后保存至-20℃。运用公式:C = A/B×6.02×1014(其中A代表质粒浓度ng·μL-1,B代表质粒DNA分子量,C代表copies/μL)计算出质粒浓度拷贝数,将其作为ToCV质粒标准品使用。1.7 标准曲线的建立
将质粒标准品用DNase/RNase free water(购自天根生化科技有限公司)按照10倍梯度进行稀释,获得终浓度为2.7×103—2.7×109 copies/μL的7个质粒样品作为模板,在实时荧光定量PCR仪(qTower 2.2 real-time PCR Thermal Cycler,Analytikjena公司)上进行RT-qPCR,每个浓度进行两次技术重复,仪器自动生成标准曲线。反应体系为20 μL:SYBR Premix Ex TaqTM II 10 μL、ToCVqF和ToCVqR各1 μL、模板1 μL、ddH2O 7 μL。1.8 实时荧光定量PCR的灵敏度检测
将质粒标准品按照10倍梯度稀释成2.7×1010—2.7×103 copies/μL的8个样品,并以此作为模板分别进行RT-qPCR和常规RT-PCR,比较这两种方法的检测灵敏度。1.9 田间番茄样品的ToCV检测
对田间采集的8份疑似ToCV感染的番茄样品进行总RNA提取,通过RT-qPCR进行病毒检测,根据扩增曲线、熔解曲线和Ct值确定其带毒情况,并根据标准曲线计算出病毒感染样品的携毒量。1.10 单头烟粉虱体内的ToCV毒量测定
用吸虫管吸取45头烟粉虱成虫,分成3组分别用微虫笼夹在ToCV番茄毒株的叶片上,获毒24 h后,从每个微虫笼中随机吸取8头烟粉虱,共计24头,单头分装于RNase free的1.5 mL离心管中,液氮冷冻后用于总RNA提取;同样,将20头烟粉虱置于健康番茄植株上取食24 h后,随机取6头进行总RNA提取,作为阴性对照。通过RT-qPCR方法检测单头烟粉虱体内携带ToCV的情况,并计算携毒量。2 结果
2.1 ToCV基因片段的克隆与鉴定
将感染ToCV番茄提取总RNA后经RT-PCR合成的第一链cDNA作为模板,运用ToCVqF和ToCVqR进行常规PCR扩增,其中实验室保存ToCV样品的cDNA作为阳性对照,番茄黄化曲叶病毒(Tomato yellow leaf curl virus,TYLCV)样品的DNA、番茄斑萎病毒(Tomato spotted wilt virus,TSWV)样品的cDNA及健康番茄的cDNA作为阴性对照,ddH2O作为空白对照。PCR扩增产物经1%的琼脂糖凝胶电泳,结果表明(图1),感染ToCV番茄样品出现特异单一条带,位置在230 bp,同阳性对照条带大小一致,而阴性对照和空白对照则无特异条带出现。将目的条带切胶回收,经连接克隆送至英潍捷基(上海)贸易有限公司进行测序,将测序结果分析并在NCBI数据库中进行BLASTn比对,结果证实经ToCVqF和ToCVqR扩增所获序列为ToCV CPm基因片段。 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图1常规RT-PCR扩增ToCV目的片段
-->Fig. 1The amplification of ToCV fragment by RT-PCR
-->
2.2 标准曲线的建立
以10倍梯度进行稀释的7个质粒样品作为模板,进行RT-qPCR,结果表明(图2),随着阳性质粒模板浓度逐渐降低,Ct值出现递增趋势,并且在质粒标准品浓度2.7×103—2.7×109 copies/μL范围内,Ct值同质粒标准品浓度之间线性关系良好,标准曲线斜率为-3.32,决定系数R2=0.9911,扩增效率为100%,直线方程为y=-3.32×lgx+40.06(y代表循环阈值即Ct值,x代表质粒初始浓度)。该标准曲线将用于ToCV含量的测定。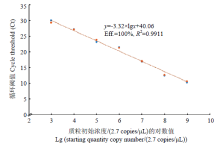 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图2ToCV的RT-qPCR标准曲线图
-->Fig. 2The standard curve of RT-qPCR for ToCV
-->
2.3 实时荧光定量PCR的灵敏度检测
RT-qPCR和常规RT-PCR的灵敏度检测比对结果表明,运用基于RT-qPCR的ToCV检测方法能够最低检测出2.7×103 copies/μL的病毒样品(图3),而常规RT-PCR仅能够检测到2.7×105 copies/μL的病毒样品(图4),表明RT-qPCR检测ToCV的灵敏度比常规RT-PCR检测提高了100倍。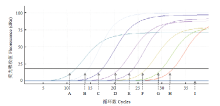 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图3RT-qPCR检测ToCV的灵敏度
-->Fig. 3The detection sensitivity of ToCV by RT-qPCR
-->
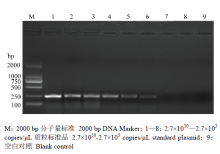 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图4常规RT-PCR检测ToCV的灵敏度
-->Fig. 4The detection sensitivity of ToCV by RT-PCR
-->
2.4 田间番茄样品的ToCV检测
运用RT-qPCR方法对采自田间的8份疑似ToCV感染症状的番茄样品进行检测,结果表明6份田间采集样品(样品1、2、4、5、7、8)均和阳性对照(实验室保存ToCV样品)一样,扩增曲线良好(图5-I);并且此6份田间样品和阳性对照在熔解曲线中均出现单一熔解峰,位置相同(图5-II);而其余两份样品(样品3和6)同阴性对照(健康番茄样品)和空白对照(ddH2O)一样均未出现扩增(图5-I),并且熔解曲线图中无单一峰出现(图5-II)。说明这8份疑似ToCV感染的样品中有6份携带ToCV。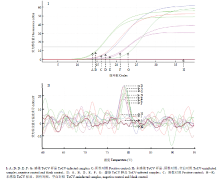 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图5田间样品的RT-qPCR检测结果曲线图
-->Fig. 5The amplification and melting curves of RT-qPCR for the ToCV detection of field samples
-->
6份感染ToCV样品的Ct值分别为23.58、23.75、25.22、16.35、16.50和20.10,结合ToCV质粒标准品的标准曲线,计算出6份样品中ToCV的含量为2.48×105、2.21×105、7.97×104、3.74×107、3.37×107、2.78×106 copies/μL。
此外对上述样品进行常规RT-PCR检测,结果发现样品1、2、4、5、7、8和阳性对照一样,均在相同位置(230 bp)出现了特异单一条带;而样品3和6同阴性对照、空白对照一样,无特异条带出现(图6)。常规RT-PCR进一步证实了RT-qPCR方法的检测结果。
 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图6田间样品的常规RT-PCR检测
-->Fig. 6The ToCV detection of field samples by RT-PCR
-->
2.5 单头烟粉虱体内的ToCV含量测定
运用RT-qPCR方法对获毒24 h后单头烟粉虱体内的ToCV进行定量检测,试验分3组进行,结果发现(表1),烟粉虱在感染ToCV的番茄毒株上获毒24 h后,带毒率为100%,单头烟粉虱最高携带ToCV量为2.55×104 copies/μL,最低为6.46× 102 copies/μL。Table 1
表1
表1实时荧光定量PCR检测单头烟粉虱体内ToCV含量
Table 1Detection and quantitation of ToCV in single B. tabaci by RT-qPCR
| 样品号 Sample number | 组1 Group 1 | 组2 Group 2 | 组3 Group 3 | |||
|---|---|---|---|---|---|---|
| Ct值 Ct value | ToCV含量 Concentration of ToCV (copies/μL) | Ct值 Ct value | ToCV含量 Concentration of ToCV (copies/μL) | Ct值 Ct value | ToCV含量 Concentration of ToCV (copies·μL-1) | |
| 1 | 30.37 | 2238 | 30.35 | 2270 | 31.35 | 1134 |
| 2 | 28.55 | 7910 | 31.3 | 1174 | 32.16 | 646 |
| 3 | 30.90 | 1550 | 27.69 | 14363 | 29.65 | 3688 |
| 4 | 30.53 | 2003 | 29.84 | 3233 | 29.76 | 3418 |
| 5 | 29.85 | 3211 | 30.68 | 1805 | 28.61 | 7588 |
| 6 | 29.95 | 2996 | 28.68 | 7228 | 26.86 | 25542 |
| 7 | 27.59 | 15395 | 28.34 | 9151 | 30.4 | 2192 |
| 8 | 31.02 | 1426 | 31.12 | 1330 | 27.83 | 13034 |
| Nc1 | No Ct | — | No Ct | — | No Ct | — |
| Nc2 | No Ct | — | No Ct | — | No Ct | — |
新窗口打开
3 讨论
ToCV是一种重要的世界性虫传病毒,近年来对于ToCV检测的相关报道也逐渐增多。目前来看,针对ToCV检测使用最为普遍的方法是RT-PCR法,其检测步骤较为繁琐,整体流程耗时较长;KIL等[18]将LAMP法进行改进,建立了一种快速、相对灵敏并有效避免携带污染的ToCV检测方法。但上述两种方法都存在检测灵敏度有限,且不能准确定量的缺点,而RT-qPCR检测方法能够很好地弥补上述缺陷,并广泛应用于多种植物病毒的检测和研究中,例如番茄黄化曲叶病毒[22]、玉米褪绿斑驳病毒(Maize chlorotic mottle virus,MCMV)[23]等。故本研究基于SYBR Green I,建立一种准确、灵敏且成本较低的RT-qPCR检测ToCV的方法,能够成功运用于植株样品及媒介昆虫带毒情况及病毒含量检测。针对ToCV检测引物的设计区域,研究者多集中于外壳蛋白(coat protein,CP)[7,24]和热激蛋白(heat shock protein,HSP)[25],本研究则选取ToCV CPm的保守区域设计出用于ToCV的RT-qPCR检测的特异引物ToCVqF/R,通过常规PCR扩增发现ToCV样品在目的大小处条带单一,并且不能扩增出其他两种番茄常见病毒片段,说明该对引物特异性较强,能够满足后续RT-qPCR验证试验;RT-qPCR结果发现该对引物扩增曲线良好,且具备特异熔解峰,结合本研究摸索的反应体系和条件,能够最大程度保证RT-qPCR过程中ToCV的有效扩增,增强反应特异性。本检测方法涉及样品病毒含量的绝对定量,因此标准品的选择尤为关键,笔者选择纯化的重组质粒DNA作为标准品,较许多体外反转录而成的cRNA标准品,具有稳定易保存,方便标准化等优点[26]。以ToCV重组质粒DNA作为标准品构建而成的标准曲线,质粒标准品中ToCV拷贝数的对数值同Ct值之间呈现良好的线性关系,且技术重复间差异性小,决定系数达0.9911;并且扩增效率为100%,完全满足样品中ToCV含量测定的需求。通过比较常规PCR和RT-qPCR检测ToCV的灵敏度,结果发现RT-qPCR方法能最低检测到2.7×103 copies/μL的病毒样品,比常规RT-PCR的检测灵敏度高出100倍,因此对田间低丰度病毒样品以及微小媒介昆虫体内ToCV的准确有效检测成为现实。高利利[27]基于ToCV的HSP70基因保守区建立了ToCV SYBR Green I RT-qPCR检测方法,该方法中同样选取质粒标准品建立标准曲线,扩增效率高达99.8%,进一步暗示了重组化质粒DNA可作为标准品的较优选择;该研究选取瓜类褪绿黄化病毒(Cucurbit chlorotic yellows virus,CCYV)、黄瓜花叶病毒(Cucumber mosaic virus,CMV)、番茄花叶病毒(Tomato masaic virus,ToMV)和TSWV进行特异性试验,均无交叉反应,特异性好,而本研究仅选取TYLCV和TSWV作为阴性对照,尚需在后续验证中扩大检测范围;研究者同样应用该体系进行了大规模感病植株的有效检测,但对于单头媒介昆虫体内ToCV的检测适用性研究尚未涉及。
在本研究中,对田间采集的8份疑似携带ToCV样品进行检测,通过分析扩增曲线、熔解曲线并与阳性、阴性和空白对照进行对比,表明其中有6份样品携带ToCV,结合常规RT-PCR验证,证实了该方法对于植株病毒样品的检测适用性。烟粉虱在中国属于优势粉虱种群,多种病毒的扩散均同其具有密切关系,最为典型的属番茄黄化曲叶病毒[28];并且有调查研究发现ToCV发病株率随烟粉虱种群数量增加而升高[15]。烟粉虱作为ToCV的“收集器”和“注射器”,通过对其携毒情况的检测,对田间ToCV的早期预警以及流行趋势预测具有重要的意义。因此本研究运用该方法对单头烟粉虱体内携毒情况和带毒量进行检测,结果发现,烟粉虱获毒24 h后,带毒率达到100%,携毒量主要集中在103数量级,占据带毒烟粉虱总数的79.17%。褐色橘蚜(Toxoptera citricida)同样作为半持久性病毒——柑橘衰退病毒(Citrus tristeza virus,CTV)的有效传播媒介之一[29],李玲娣等[30]对单头褐色橘蚜获毒24 h后虫体带毒量进行研究,发现多数带毒量集中在104—105数量级,也暗示不同媒介昆虫获毒能力差异较大。PAPAYIANNIS等[31]运用TaqMan探针法对感染ToCV番茄上的烟粉虱和温室白粉虱(Trialeurodes vaporariorium)体内ToCV进行检测,其Ct值约为25,略低于本研究测定的烟粉虱带毒量的Ct值(26.86—32.16),此二者研究的差异可能由于粉虱来源不同,感染ToCV番茄上的烟粉虱种群获毒时间相对较长,故其带毒量稍大于烟粉虱24 h的获毒量。此外,检测方法的不同也是造成结果差异的原因之一。
4 结论
基于SYBR Green I建立的RT-qPCR检测ToCV的方法,特异性强、灵敏度高,能够满足针对植物样品和媒介昆虫中ToCV的检测和带毒量测定,且成本较低,适用于批量样品的集中、快速检测,该技术体系可用于ToCV的长期监测预警和流行趋势研究。The authors have declared that no competing interests exist.
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | |
| [2] | . |
| [3] | |
| [4] | In March 2011, interveinal yellowing and necrosis symptoms on middle and lower leaves were observed in tomato (cv. Castle Rock) plants grown in 3 adjacent greenhouses of the Agricultural Research Corporation at Wad Medani (Gezira State, Sudan). These symptoms resembled those caused by (ToCV) and Tomato infectious chlorosis virus (TICV). Whitefly () was also observed in these greenhouses. The highest nucleotide sequence identity of the CP gene fragment obtained (GenBank Accession No. JN411685) was 99.2% related with North American ToCV isolates from Florida (DQ234674), Colorado (DQ2346675), and Georgia (HQ879842), while the RdRp gene fragment (JN411686) was more closely related (99.0%) to the Spanish AT80/99 isolate (DQ983480). This is thought to be the first report of ToCV infecting tomato in Sudan. |
| [5] | |
| [6] | |
| [7] | In 2012, 57 symptomless samples of , a common weed known as Asian copperleaf, were randomly collected from 7 tomato fields in Nanjing and Lianyungang, Jiangsu Province, from July to September. Total DNA of each sample was extracted and PCR was performed. The complete genome sequence (GenBank Accession No. JX910534) was 2,781 nucleotides in length and had 99 to 100% sequence identity with TYLCV accessions (GU434142, GU111505). The dot immunobinding assay using monoclonal antibody against TYLCV confirmed the 27 weed samples positive by PCR were infected by TYLCV. These results demonstrated that is a host of TYLCV that might play an important role in viral epidemics in tomato fields in China. This is thought to be the first report of as a host of TYLCV in China. |
| [8] | . 番茄褪绿病毒是最近暴发的新病害,发病初期容易与缺素症状混淆,常常因误诊而延误防治,给番茄生产造成严重的经济损失。目前该病毒在北京、河北、山东等地区已经处于大暴发的趋势,必须引起足够的重视。 . 番茄褪绿病毒是最近暴发的新病害,发病初期容易与缺素症状混淆,常常因误诊而延误防治,给番茄生产造成严重的经济损失。目前该病毒在北京、河北、山东等地区已经处于大暴发的趋势,必须引起足够的重视。 |
| [9] | . 为明确2014年夏天在天津番茄种植区采集到疑似感染番茄褪绿病毒的番茄样品的侵染病原,利用To CV外壳蛋白和热激蛋白的特异引物进行反转录PCR(RT-PCR)检测,分别扩增得到特异核苷酸片段。序列分析表明,HSP70核苷酸序列与已登录的番茄褪绿病毒日本分离物Tochigi(AB513442)相似性为99.5%,CP氨基酸和核苷酸序列与已登录的番茄褪绿病毒日本分离物Tochigi(AB513443)相似性分别为99.2%和99.3%,表明天津地区的番茄受到To CV侵染,且天津与日本To CV分离物序列相似性最高。由于调查地区番茄黄化曲叶病毒发生普遍,针对To CV阳性样品利用TYLCV特异性引物进行了分子检测,扩增产物序列与TYLCV以色列株系分离物的相似性均在99.0%以上,表明2种病毒在田间发生复合侵染,该研究首次明确天津地区的番茄受到To CV侵染和To CV与TYLCV的复合侵染。 为明确2014年夏天在天津番茄种植区采集到疑似感染番茄褪绿病毒的番茄样品的侵染病原,利用To CV外壳蛋白和热激蛋白的特异引物进行反转录PCR(RT-PCR)检测,分别扩增得到特异核苷酸片段。序列分析表明,HSP70核苷酸序列与已登录的番茄褪绿病毒日本分离物Tochigi(AB513442)相似性为99.5%,CP氨基酸和核苷酸序列与已登录的番茄褪绿病毒日本分离物Tochigi(AB513443)相似性分别为99.2%和99.3%,表明天津地区的番茄受到To CV侵染,且天津与日本To CV分离物序列相似性最高。由于调查地区番茄黄化曲叶病毒发生普遍,针对To CV阳性样品利用TYLCV特异性引物进行了分子检测,扩增产物序列与TYLCV以色列株系分离物的相似性均在99.0%以上,表明2种病毒在田间发生复合侵染,该研究首次明确天津地区的番茄受到To CV侵染和To CV与TYLCV的复合侵染。 |
| [10] | . . |
| [11] | . In 2014,a routine survey of tomato yellow leaf curl disease in Jiangsu Province was carried out. Besides whitefly-transmitted geminiviruses,a whitefly-transmitted crinivirus was also detected in 17 tomato samples collected from Nanjing with symptoms of reduced leaf size or leaf curling and yellowing in the upper leaves and interveinal chlorosis and upward rolling and thickening in the lower leaves. Sequence analysis showed that the whitefly-transmitted geminivirus was an isolate of Tomato yellow leaf curl virus(TYLCV)and the whitefly-transmitted crinivirus was an isolate of Tomato chlorosis virus(ToCV). The two viruses were also detected in Bemisia tabaci collected from the greenhouses. In 2014,a routine survey of tomato yellow leaf curl disease in Jiangsu Province was carried out. Besides whitefly-transmitted geminiviruses,a whitefly-transmitted crinivirus was also detected in 17 tomato samples collected from Nanjing with symptoms of reduced leaf size or leaf curling and yellowing in the upper leaves and interveinal chlorosis and upward rolling and thickening in the lower leaves. Sequence analysis showed that the whitefly-transmitted geminivirus was an isolate of Tomato yellow leaf curl virus(TYLCV)and the whitefly-transmitted crinivirus was an isolate of Tomato chlorosis virus(ToCV). The two viruses were also detected in Bemisia tabaci collected from the greenhouses. |
| [12] | . . |
| [13] | . 在广东番茄上发现一种新病害,病株表现为叶片褪绿,叶脉颜色变深及叶片增厚等症状。利用番茄褪绿病毒Tomato chlorosis virus(ToCV)HSP70基因的两对特异引物对番茄病样进行RT-PCR检测,结果表明,从所采集的6份病样中均扩增到预期大小的DNA特异片段。对其中1份样品的扩增片段进行克隆与序列分析,结果表明,扩增片段包括1个长度为1 665个核苷酸的完整病毒基因,其核苷酸序列与已报道的ToCV HSP70基因有较高的同源性,表明广东番茄受到了ToCV的侵染。但ToCV广东番茄分离物的HSP70序列与国内外已报道的各分离物的同源性均低于82%,存在较大差异,其中与塞浦路斯tomato、约旦JU_20分离物的同源性最高,为81.8%。这是ToCV在广东发生的首次报道,也是该病毒HSP70基因序列存在显著差异分离物的首次发现。 在广东番茄上发现一种新病害,病株表现为叶片褪绿,叶脉颜色变深及叶片增厚等症状。利用番茄褪绿病毒Tomato chlorosis virus(ToCV)HSP70基因的两对特异引物对番茄病样进行RT-PCR检测,结果表明,从所采集的6份病样中均扩增到预期大小的DNA特异片段。对其中1份样品的扩增片段进行克隆与序列分析,结果表明,扩增片段包括1个长度为1 665个核苷酸的完整病毒基因,其核苷酸序列与已报道的ToCV HSP70基因有较高的同源性,表明广东番茄受到了ToCV的侵染。但ToCV广东番茄分离物的HSP70序列与国内外已报道的各分离物的同源性均低于82%,存在较大差异,其中与塞浦路斯tomato、约旦JU_20分离物的同源性最高,为81.8%。这是ToCV在广东发生的首次报道,也是该病毒HSP70基因序列存在显著差异分离物的首次发现。 |
| [14] | . 番茄褪绿病毒(Tomato chlorosis virus,ToCV)是一种由粉虱传播的植物RNA 病毒,近年来在中国的为害逐年加重,具有危害严重、为害症状难以鉴别、寄主范围广、媒介昆虫种类多等特点。本文综述了当前ToCV 在国内外的分布、与其症状相似病毒——番茄侵染性褪绿病毒(Tomato infectious chlorosis virus,TICV)的鉴别方法、粉虱传播ToCV 的特性等方面的最新研究进展,并对该病毒的研究进行了展望。 . 番茄褪绿病毒(Tomato chlorosis virus,ToCV)是一种由粉虱传播的植物RNA 病毒,近年来在中国的为害逐年加重,具有危害严重、为害症状难以鉴别、寄主范围广、媒介昆虫种类多等特点。本文综述了当前ToCV 在国内外的分布、与其症状相似病毒——番茄侵染性褪绿病毒(Tomato infectious chlorosis virus,TICV)的鉴别方法、粉虱传播ToCV 的特性等方面的最新研究进展,并对该病毒的研究进行了展望。 |
| [15] | . 为明确山东寿光地区Q型烟粉虱对番茄褪绿病毒(Tomato chlorosis virus,ToCV)感病流行的影响及其传毒特性,于2014年调查了该地区设施番茄上烟粉虱种群动态与ToCV发病情况,利用特异引物对烟粉虱体内ToCV进行了RT-PCR检测;并在室内测定了带毒Q型烟粉虱取食时间和种群数量对ToCV感病株率的影响.结果表明,在番茄发病植株上采集的烟粉虱种群体内可检测到ToCV;春茬番茄ToCV发病株率随烟粉虱种群数量增加而逐渐升高,4-6月是ToCV发生高峰期,6月22日发病株率达100%;秋茬番茄烟粉虱种群数量从10月下旬明显下降,而ToCV发病株率升高,11月12日发病株率达100%;室内试验表明,ToCV感病株率随着带毒Q型烟粉虱数量与取食时间的增加而明显升高.研究表明,Q型烟粉虱能有效传播ToCV,且其种群数量对ToCV发病株率存在显著影响,可通过防控烟粉虱以控制ToCV的危害. . 为明确山东寿光地区Q型烟粉虱对番茄褪绿病毒(Tomato chlorosis virus,ToCV)感病流行的影响及其传毒特性,于2014年调查了该地区设施番茄上烟粉虱种群动态与ToCV发病情况,利用特异引物对烟粉虱体内ToCV进行了RT-PCR检测;并在室内测定了带毒Q型烟粉虱取食时间和种群数量对ToCV感病株率的影响.结果表明,在番茄发病植株上采集的烟粉虱种群体内可检测到ToCV;春茬番茄ToCV发病株率随烟粉虱种群数量增加而逐渐升高,4-6月是ToCV发生高峰期,6月22日发病株率达100%;秋茬番茄烟粉虱种群数量从10月下旬明显下降,而ToCV发病株率升高,11月12日发病株率达100%;室内试验表明,ToCV感病株率随着带毒Q型烟粉虱数量与取食时间的增加而明显升高.研究表明,Q型烟粉虱能有效传播ToCV,且其种群数量对ToCV发病株率存在显著影响,可通过防控烟粉虱以控制ToCV的危害. |
| [16] | |
| [17] | Interveinal leaf chlorosis, brittleness, limited necrotic flecking or bronzing developed on greenhouse-grown tobacco and tomato plants at Nanjing Agricultural University from 2010 to 2013. A positive RT-PCR using a pair of degenerate primers for Crinivirus confirmed the diseased plants were infected with Tomato chlorosis virus (ToCV). The complete RNA 1 genomic sequence of this ToCV isolate was determined; it comprises of 8596 nucleotides with four open reading frames. Phylogenetic analysis of ToCV isolates from diverse geographical regions categorized the ToCV isolates into two main groups. Group one consisted of Chinese, American-Florida, Greek and Brazilian isolates, while Group two contained only the Spanish isolate. The first group had two subgroups, one of Chinese and American-Florida isolates, while the other subgroup had Greek and Brazilian isolates. This is the first study of the complete nucleotide sequence of the RNA 1 of ToCV isolated from China. |
| [18] | In 2013, Tomato chlorosis virus (ToCV) was identified in symptomatic tomato plants in Korea. In the present study, a loop-mediated isothermal amplification (LAMP) method was developed using four specific primers designed against ORF6 in ToCV RNA2 to detect ToCV rapidly and with high sensitivity. The optimized reaction involved incubation of a reaction mixture containing 2U Bst DNA polymerase and 4mM MgSO4 for 1h at 60鈥62掳C. Although specific and rapid detection of ToCV by LAMP was confirmed, false-positive reactions caused by carry-over contamination sometimes occurred because of the high sensitivity of LAMP compared with other detection methods. To prevent false-positive reactions, dUTP was substituted for dTTP and uracil-DNA glycosylase (UDG) was added to the LAMP reaction. First, the LAMP reaction was conducted successfully with substitution of dUTP for dTTP. Before the next reaction, LAMP products with incorporated dUTP were cleaved selectively by UDG without any effect on thymine-containing DNA (template DNA). This modified LAMP method complemented with UDG treatment to prevent carry-over contamination offers a potentially powerful method for detecting plant viruses. |
| [19] | A rapid and sensitive real time reverse transcription-PCR (RT-PCR) assay based on SYBR Green I chemistry was developed for the quantitative detection of Citrus viroid III (CVd-III) in citrus samples. CVd-III titre was determined at different times in green bark of sour orange, Troyer citrange, trifoliate orange and alemow seedlings inoculated with a CVd-IIIb source. Ten weeks after inoculation the viroid was detected in the four species, without substantial differences in viroid titre among them. Nine weeks later an overall increase of viroid titre was observed. The copy number of CVd-III in sour orange and Troyer citrange was monitored up to 52 weeks after inoculation and a further increase of viroid titre was observed at 35 weeks. For validation purposes, field samples were tested from 58 citrus trees with mixed infections of CVd-III, Citrus exocortis viroid (CEVd) and Hop stunt viroid (HSVd), as well as from healthy controls. Based on the sensitivity (100%), specificity (96路7%), accuracy (99路2%) and repeatability (Cohen's kappa index 0路98) of the assay, it is suggested that its employment in breeding programmes would be helpful in the evaluation of host resistance and viroid accumulation in plants. |
| [20] | . 柑橘碎叶病是由橘碎叶病毒 (Citrus tatter leaf virus,CTLV)引起的一种重要的柑橘病害,为更快、更准确的检测CTLV,合成了一对特异性引物ASG-Pf和ASG-Pr,建立了运用 SYBRGreenI荧光染料法检测CTLV的实时荧光RT-PCR体系,并对该体系的特异性、灵敏性和适用性进行了测试。结果表明,该检测体系能特异的 检出CTLV,对测试的衰退病毒、温州蜜柑萎缩病毒和鳞皮病毒都不能检出;灵敏度比常规PCR高100倍;适用性广,可检测出多种柑橘类植物中的 CTLV。实时荧光RT-PCR检测整个过程完全闭管,无需PCR后处理,且SYBR GreenI荧光染料成本较低,适用于检测柑橘体内含量较低的CTLV病毒。 . 柑橘碎叶病是由橘碎叶病毒 (Citrus tatter leaf virus,CTLV)引起的一种重要的柑橘病害,为更快、更准确的检测CTLV,合成了一对特异性引物ASG-Pf和ASG-Pr,建立了运用 SYBRGreenI荧光染料法检测CTLV的实时荧光RT-PCR体系,并对该体系的特异性、灵敏性和适用性进行了测试。结果表明,该检测体系能特异的 检出CTLV,对测试的衰退病毒、温州蜜柑萎缩病毒和鳞皮病毒都不能检出;灵敏度比常规PCR高100倍;适用性广,可检测出多种柑橘类植物中的 CTLV。实时荧光RT-PCR检测整个过程完全闭管,无需PCR后处理,且SYBR GreenI荧光染料成本较低,适用于检测柑橘体内含量较低的CTLV病毒。 |
| [21] | Tomato chlorosis virus (ToCV) is implicated in tomato yellows disease in many countries worldwide. It has a wide host range, including cultivated species as well as arable weeds, and it is transmitted in a semipersistent manner by at least five whitefly species or biotypes of the genera Trialeurodes and Bemisia. ToCV is not seed transmitted and more than 36 weed species have been recorded as natural reservoirs, acting as unique sources both for the virus and its vectors when susceptible crops are harvested. In this study, experiments were conducted to determine the transmission parameters of ToCV by biotype Q, the most abundant biotype of Bemisia tabaci in Greece. Results showed that biotype Q is an efficient vector of ToCV and it is able to retain the virus for at least 6 days. This vector was then used for the evaluation of four widespread weed species (Solanum nigrum, Sonchus oleraceus, Amaranthus retroflexus, and Chenopodium album) as ToCV sources through transmission experiments. Solanum nigrum was shown to be the most significant viral source among the tested weeds, followed by Sonchus oleraceus, A. retroflexus, and, lastly, C. album. Nevertheless, none of them was as efficient a ToCV source as tomato. This variation could be attributed to differences in virus concentration in each plant species or possible host preference by the whitefly vector. |
| [22] | . |
| [23] | . 玉米褪绿斑驳病毒( Maize chlorotic mottle virus, MCMV)是我国对外公布的检疫性有害生物。本研究根据该病毒外壳蛋白基因的保守序列,设计得到特异性引物及Taqman荧光探针,建立了MCMV的实时荧光RT—PCR方法,并对其灵敏度与特异性进行了研究。该方法针对2个不同来源的毒株均能得到典型扩增曲线,而没有从小麦线条花叶病毒、玉米粗缩病毒和玉米矮花叶病毒的RNA得到扩增曲线,表明引物与荧光探针具有良好的特异性。针对玉米褪绿斑驳病毒RNA不同稀释度样品,实时荧光RT—PCR检测低限达到10。稀释度,检测灵敏度要比普通RT~PCR高出100倍。因此,本研究建立的MCMV实时荧光方法具有特异性强、灵敏度高和快速有效的优点。 玉米褪绿斑驳病毒( Maize chlorotic mottle virus, MCMV)是我国对外公布的检疫性有害生物。本研究根据该病毒外壳蛋白基因的保守序列,设计得到特异性引物及Taqman荧光探针,建立了MCMV的实时荧光RT—PCR方法,并对其灵敏度与特异性进行了研究。该方法针对2个不同来源的毒株均能得到典型扩增曲线,而没有从小麦线条花叶病毒、玉米粗缩病毒和玉米矮花叶病毒的RNA得到扩增曲线,表明引物与荧光探针具有良好的特异性。针对玉米褪绿斑驳病毒RNA不同稀释度样品,实时荧光RT—PCR检测低限达到10。稀释度,检测灵敏度要比普通RT~PCR高出100倍。因此,本研究建立的MCMV实时荧光方法具有特异性强、灵敏度高和快速有效的优点。 |
| [24] | . |
| [25] | |
| [26] | . 菠萝凋萎相关病毒(Pineapple mealybug wilt associated virus-2,PMWaV-2)是引起的菠萝凋萎病(Mealybug wilt of pineapple,MWP)的重要病原。本研究中根据PMWaV-2外壳蛋白基因序列设计特异性引物和探针,建立基于TaqMan探针的实时荧光定量RT-PCR检测方法。该方法能高灵敏检测出阳性样品,对阴性样品及空白对照均无荧光反应;灵敏度比普通PCR高100倍;重复性试验表明批内和批间变异系数均在1.85%以内,表明本方法是一种操作简便、特异性强、灵敏度高、重复性较好的PMWaV-2定量检测方法。 菠萝凋萎相关病毒(Pineapple mealybug wilt associated virus-2,PMWaV-2)是引起的菠萝凋萎病(Mealybug wilt of pineapple,MWP)的重要病原。本研究中根据PMWaV-2外壳蛋白基因序列设计特异性引物和探针,建立基于TaqMan探针的实时荧光定量RT-PCR检测方法。该方法能高灵敏检测出阳性样品,对阴性样品及空白对照均无荧光反应;灵敏度比普通PCR高100倍;重复性试验表明批内和批间变异系数均在1.85%以内,表明本方法是一种操作简便、特异性强、灵敏度高、重复性较好的PMWaV-2定量检测方法。 |
| [27] | . . |
| [28] | . |
| [29] | |
| [30] | . 【Objective】The objective of this study is to develop a SYBR Green I real-time RT-PCR assay to detect the Citrus tristeza virus in Toxoptera citricida (Kirkaldy). 【Method】A pair of primers HD-F/R were designed within highly conservative region of CP25, and the SYBR Green I real-time RT-PCR detection system was established with optimized reaction condition. Analytical sensitivity and reproducibility were evaluated, respectively. Finally, the method was used to quantify CTV in single T. citricida. 【Result】The assay had a detection limit of 9.0 copies/μL and the sensitivity was 100 times higher than the conventional RT-PCR. The standard curve established by cRNA showed a fine linear relationship between threshold cycle and template concentration. The correlation coefficient of the standard curve was 0.998 and amplification efficiency was 104.7%. The variation coefficient of Ct value of diluted standard cRNA was less than 3.24%, indicating a good reproducibility. After 24 h acquisition access period, the estimate number of CTV targets in single T. citricida ranged from 2.5×103 to 1.24×106 copies. 【Conclusion】 The quantitative method was used for accurate determination of Citrus tristeza virus in T. citricida and could be a potential tool for studying the aphids-CTV-host interaction and CTV epidemiology. 【Objective】The objective of this study is to develop a SYBR Green I real-time RT-PCR assay to detect the Citrus tristeza virus in Toxoptera citricida (Kirkaldy). 【Method】A pair of primers HD-F/R were designed within highly conservative region of CP25, and the SYBR Green I real-time RT-PCR detection system was established with optimized reaction condition. Analytical sensitivity and reproducibility were evaluated, respectively. Finally, the method was used to quantify CTV in single T. citricida. 【Result】The assay had a detection limit of 9.0 copies/μL and the sensitivity was 100 times higher than the conventional RT-PCR. The standard curve established by cRNA showed a fine linear relationship between threshold cycle and template concentration. The correlation coefficient of the standard curve was 0.998 and amplification efficiency was 104.7%. The variation coefficient of Ct value of diluted standard cRNA was less than 3.24%, indicating a good reproducibility. After 24 h acquisition access period, the estimate number of CTV targets in single T. citricida ranged from 2.5×103 to 1.24×106 copies. 【Conclusion】 The quantitative method was used for accurate determination of Citrus tristeza virus in T. citricida and could be a potential tool for studying the aphids-CTV-host interaction and CTV epidemiology. |
| [31] |
