 ,, 李培源, 李安琪, 余文艳, 郭绰, 杨曦, 郭玉蓉
,, 李培源, 李安琪, 余文艳, 郭绰, 杨曦, 郭玉蓉 ,陕西师范大学食品工程与营养科学学院,西安710119
,陕西师范大学食品工程与营养科学学院,西安710119Effects of Xanthan Addition on the Gel Properties and Gel Mechanism of Alkaline-Induced Konjac Glucomannan Gels
LI XiaoFei ,, LI PeiYuan, LI AnQi, YU WenYan, GUO Chuo, YANG Xi, GUO YuRong
,, LI PeiYuan, LI AnQi, YU WenYan, GUO Chuo, YANG Xi, GUO YuRong ,College of Food Engineering and Nutritional Science, Shaanxi Normal University, Xi'an 710119
,College of Food Engineering and Nutritional Science, Shaanxi Normal University, Xi'an 710119通讯作者:
责任编辑: 赵伶俐
收稿日期:2019-12-2接受日期:2020-04-29网络出版日期:2020-07-16
| 基金资助: |
Received:2019-12-2Accepted:2020-04-29Online:2020-07-16
作者简介 About authors
李晓飞,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (8118KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李晓飞, 李培源, 李安琪, 余文艳, 郭绰, 杨曦, 郭玉蓉. 黄原胶添加对碱法诱导魔芋胶凝胶特性及凝胶机制的影响[J]. 中国农业科学, 2020, 53(14): 2941-2955 doi:10.3864/j.issn.0578-1752.2020.14.017
LI XiaoFei, LI PeiYuan, LI AnQi, YU WenYan, GUO Chuo, YANG Xi, GUO YuRong.
0 引言
【研究意义】魔芋(Amorphophallus konjac)属天南星科多年生草本植物,在我国西南部地区和中西部地区有着悠久的种植历史,魔芋胶(KGM)的主要成分是魔芋葡甘聚多糖,存在于球状块茎中[1,2,3],是一种低热能、低蛋白质、高膳食纤维的物质,并且富含人体所需的十几种氨基酸和微量元素,对高血压、肥胖症、糖尿病、便秘等有一定疗效,可作为功能性食品食用[3,4,5,6]。魔芋胶还具有良好的水溶、增稠、稳定、悬浮、凝胶、成膜、粘结等多种优良的理化特性,可作为食品添加剂[1-2,7]。魔芋胶属于非凝胶多糖,但在强碱性加热条件下,魔芋胶会发生脱乙酰基反应形成热不可逆凝胶[8,9]。然而,该凝胶存在凝胶强度低、析水明显等缺点,一定程度上限制了魔芋胶食品的开发应用[10]。黄原胶(Xanthan)又称黄胶、汉生胶,是由甘蓝黑腐病野油菜黄单胞菌以碳水化合物为主要原料,经过好氧发酵产生的一种高黏度水溶性微生物胞外多糖[3,11]。在冷水和热水中,黄原胶均可溶解[12],黄原胶溶液黏度很高,具有显著的触变性,且耐高温、对pH不敏感等,在食品工业中常作为增稠剂、稳定剂,被广泛应用[3,13-14]。黄原胶也属于非凝胶多糖,但有研究表明[3,8],黄原胶与魔芋胶能形成协同凝胶,这种凝胶在室温下有较好的粘弹性,但是在加热条件下会由凝胶转变为溶胶,是热可逆凝胶,从而限制了该凝胶的进一步应用。本研究基于魔芋胶碱法诱导的凝胶特性以及魔芋胶与黄原胶协同凝胶化原理,制备出将两种凝胶网络贯穿为一体的凝胶。这种凝胶不仅具有热不可逆的特性,而且兼具魔芋胶与黄原胶协同凝胶的优势,可有效解决碱法诱导的魔芋胶凝胶在室温下凝胶强度较低、析水明显等问题,以期为魔芋胶相关食品的开发利用提供理论依据。【前人研究进展】游东宏等[15]研究发现,在pH为9.0、温度75℃、以碳酸氢钠为胶凝剂的条件下,魔芋葡甘聚糖凝胶的凝胶强度较大。GENEVRO等[16]研究表明,冷冻速度是影响魔芋胶凝胶最终物理性质的重要因素,冷冻速率缓慢时,凝胶网络呈各向同性,且孔径较大;冷冻速率较快时,网络孔径较小且网孔排列规整。此外,较高温度条件下(-8℃)冷冻的水凝胶比低温(-28℃)冷冻的水凝胶具有更高的穿透模量。ZHOU等[17]研究表明,与Na2CO3混合后,KGM链立即发生脱乙酰反应,诱导凝胶化,当温度上升到70℃以上时,凝胶化才能完成,这表明KGM链的分子聚集不仅与氢键有关,而且与疏水相互作用有关。王元兰等[11]将魔芋胶与黄原胶复配,发现在魔芋胶与黄原胶质量比为3﹕7时,复配凝胶表现出最高的黏度,且黏度随温度升高而下降。倪俊杰等[18]研究表明,魔芋胶-黄原胶复配体系具有假塑性,当魔芋胶的添加比例逐渐增大时,复配体系黏度系数K增大,流体系数n减小,当魔芋胶与黄原胶质量比为6﹕4时,复配体系的K值达到最大、n值最小,具有最强的假塑性及黏弹性。YANG等[19]测定了总胶浓度为1%的条件下,去离子水中魔芋胶与黄原胶的最佳协同比,发现当两者比例为魔芋胶﹕黄原胶=6﹕4时,凝胶强度最大,这与倪俊杰等[18]研究结果一致。此外,YANG等[20]基于混合多糖体系的一般原理,研究了魔芋胶和海藻酸钠的兼容性,并以魔芋胶和海藻酸钠的混合体系为基质,制备出一类具有粘弹性、触变性和涂抹性的乳液凝胶。上述结果表明,混合多糖体系可以改善单一多糖的缺点,扩大多糖在凝胶特性改良方面的应用范围。【本研究切入点】虽然前人对魔芋胶凝胶形成条件以及魔芋胶-黄原胶协同凝胶方面进行了深入研究,但尚未解决魔芋胶凝胶强度较低、冻融稳定性差等缺点,也未能将魔芋胶-黄原胶协同凝胶的特性拓展应用于魔芋胶凝胶改性方面。本研究根据食品胶复配原理,首次将魔芋胶凝胶网络与魔芋胶-黄原胶协同凝胶网络贯穿在一起,制备出兼具两种凝胶网络优点的复合网络凝胶,具有较深理论基础及良好的应用前景。【拟解决的关键问题】基于流变学、电子显微学、X-射线衍射以及热重分析等手段,探究魔芋胶-黄原胶混合多糖体系在热碱处理过程中凝胶网络的形成过程,揭示两层网络之间的相互作用。1 材料与方法
试验于2019年8—11月在陕西师范大学食品工程与营养科学学院进行。1.1 材料与试剂
魔芋胶、黄原胶,上海源叶生物科技有限公司;无水碳酸钠,天津市天力化学试剂有限公司;柠檬酸,天津市科密欧化学试剂有限公司;以上均为分析纯。1.2 仪器与设备
PL203电子天平;EYELA OSB-2100恒温水浴锅;TA. XT. Plus质构仪,英国stable micro system公司;FD-1A-50真空冷冻干燥机,北京博医康实验仪器有限公司;Quanta 200环境扫描电子显微镜,FEI公司;AR-G2流变仪,美国TA公司;D8 Advance粉末X射线衍射仪,德国布鲁克公司;Q1000DSC+LNCS+ FACS Q600SDT热分析系统,美国TA公司。1.3 方法
1.3.1 样品制备 母液配置:用分析天平准确称取30.00 g魔芋胶粉末溶解于1 L去离子水中,用去离子水溶解时一边添加魔芋胶粉末一边快速搅拌,随后置于90℃的水浴锅中加热,不断搅拌至完全水化,在室温下平衡12 h,配置成3%的魔芋胶母液,备用。用分析天平准确称取50.00 g黄原胶粉末溶解于1 L的去离子水中,用去离子水溶解时一边添加黄原胶粉末一边快速搅拌,随后置于90℃的水浴锅中加热,不断搅拌至完全水化,在室温下平衡12 h,配置成5%的黄原胶母液,备用。水化过程中,用去离子水补偿魔芋胶和黄原胶溶液因水分蒸发造成的损失。取7个干净的250 mL烧杯,编号为0—6号,按表1配方添加配置好的3% KGM母液、5% Xanthan母液和去离子水,置于90℃的水浴锅中加热,用玻璃棒搅拌至完全混合均匀,另外准确称取4.00 g Na2CO3用10 mL去离子水溶解,缓慢加入到0—5号烧杯中,快速搅拌1 min,搅拌均匀后倒入100 mL烧杯中,用保鲜膜密封,在90℃水浴锅加热2 h后取出,在室温下平衡12 h,制备的凝胶为圆柱形,大小和100 mL烧杯一致。同时,准确称取4.00 g Na2CO3用6.7 mL去离子水溶解,缓慢加入到6号烧杯中,快速搅拌1 min,搅拌均匀后倒入100 mL烧杯中,用保鲜膜密封,在90℃水浴锅中加热2 h后取出,室温下平衡12 h。
Table 1
表1
表1样品制备各组分添加量
Table 1
| 编号 No. | 3%魔芋胶 3% KGM (g) | 魔芋胶干重 KGM dry weight (g) | 魔芋胶浓度 KGM concentration (w?v-1) | 5%黄原胶 5% Xanthan (g) | 黄原胶干重 Xanthan dry weight (g) | 黄原胶浓度 Xanthan concentration (w?v-1) | 去离子水 Deionized water (mL) |
|---|---|---|---|---|---|---|---|
| 0 | 133.3 | 4 | 2% | 0 | 0 | 0 | 56.7 |
| 1 | 133.3 | 4 | 2% | 10 | 0.5 | 0.25% | 46.7 |
| 2 | 133.3 | 4 | 2% | 20 | 1 | 0.5% | 36.7 |
| 3 | 133.3 | 4 | 2% | 30 | 1.5 | 0.75% | 26.7 |
| 4 | 133.3 | 4 | 2% | 40 | 2 | 1.0% | 16.7 |
| 5 | 133.3 | 4 | 2% | 50 | 2.5 | 1.25% | 6.7 |
| 6 | 133.3 | 4 | 2% | 60 | 3 | 1.5% | 0 |
新窗口打开|下载CSV
1.3.2 凝胶强度测定 在90℃的条件下,将制备好的复合凝胶样品用质构仪进行凝胶强度的测定[19,21]。参数设定:TPA压缩模式,探头P/0.5(直径0.5英寸的圆柱状平头探头),测试前速度:1.5 mm·s-1,测试速度:1.0 mm·s-1,测试后速度:1.0 mm·s-1,感应力:2 g,凝胶破裂点的力为凝胶强度,每个样品重复3次。
此外,将制备好的样品在室温下平衡12 h,按照上述方法测定凝胶强度。
1.3.3 微观结构观察 将所有凝胶切割成边长约为2 cm的立方块,立刻将凝胶小块浸没于液氮中速冻。液氮浸没约3 min后,将冻住的凝胶立刻转移至真空冷冻干燥机中,在-50℃条件下冻干48 h。将冻干的凝胶折断后,用导电双面胶固定到样品台上,断面朝上,喷金,采用Quanta 200环境扫描电镜观察凝胶断面形貌,观察时选择高真空模式,高压为20.0 kV,放大倍数800×[19,22]。
1.3.4 冻融稳定性 将制备好的凝胶在-18℃冷冻12 h,然后在室温下解冻6 h后[5],参照1.3.2中的方法测定凝胶强度,并参照1.3.3中的方法观察微观结构。
此外,凝胶冻融后出现明显的析水现象,因此也测定凝胶的析水率。具体操作如下:用分析天平准确称取冻融前凝胶的质量记为m1,准确称取冻融处理后凝胶的质量记为m2。每个样品重复3次。按照以下公式计算样品的析水率:
$析水率(\%)=\frac{m_{1}-m_{2}}{m_{1}}\times 100$
1.3.5 溶胀特性 将制备好的复合凝胶样品分别浸泡在去离子水和2%柠檬酸溶液中12 h[8],参照1.3.2中的方法测定凝胶强度,并参照1.3.3中的方法观察微观结构。
1.3.6 流变学特性测定 时间扫描:按照表1添加配置好的3% KGM母液、5% Xanthan母液和去离子水,在室温下搅拌均匀,准确称取4.00 g的Na2CO3用10 mL去离子水溶解,加入烧杯中快速搅拌至均匀,移取3 mL混合样品置于已经升温至90℃的样品台上,立刻进行时间扫描,监测储能模量G'与损耗模量G"的变化趋势[23,24]。采用直径为20 mm的转子,平行板间隙设置为1 mm,应变为1%,频率为1 Hz,时间为2 h,在转子边缘涂抹低密度硅油,防止试验过程中样品水分蒸发。
降温扫描:时间扫描过程结束后,立刻进行降温扫描,监测G'与G"的变化趋势。采用直径为20 mm的转子,平行板间隙设置为1 mm,应变为1%,频率为1 Hz,温度从90℃降低到20℃,降温速率为2 ℃·min-1,在转子边缘涂抹低密度硅油,防止试验过程中水的蒸发[3,18]。
频率扫描:当样品温度降低至20℃后,在该温度条件下平衡10 min,然后在1—100 rad·s-1频率范围内进行频率扫描,应变为1%[18]。
方程拟合:为了进一步分析凝胶在相同的碱浓度(2%)、温度(90℃)条件下的凝胶化过程,本研究把上述时间扫描的G'汇总,并采用如下方程进行拟合:
$y=G'sat-G'sat*{{e}^{(-k*x)}}$
其中,G'sat为在无限的加热时间下,G'的极限值;k为凝胶化速率参数;x为时间;y为储能模量G'。
1.3.7 X-射线衍射测定 将经过真空冷冻干燥的样品置于60℃的烘箱中保持干燥,取干燥样品进行X-射线衍射测定,XRD扫描范围为2θ=5-80°[22,25-26]。
1.3.8 热重分析 取大约10mg样品,以氮气作为保护气体,从室温(20℃)加热到600℃,加热速率为10 ℃·min-1。结果分析采用TA Universal 2000 software[25]。
1.3.9 数据处理 上述所有测定均重复3次,每次测试均需更换样品。所有图表均使用Origin 2018软件进行绘制,利用IBM SPSS22.0软件进行差异显著性分析(P<0.05为差异显著)。
2 结果
2.1 90℃和室温下的凝胶强度
凝胶强度是反映凝胶内部网络结构的一个重要参数。如图1所示,在90℃下,所有样品均表现出较低的凝胶强度,并且随着黄原胶添加量的增加,样品的凝胶强度呈下降趋势,可能是因为黄原胶添加不利于魔芋胶凝胶网络形成。冷却至室温后,样品的凝胶强度急剧增加,随着黄原胶添加量的增加,样品的凝胶强度呈上升趋势,一方面是因为总多糖浓度增加,另一方面是因为随黄原胶添加量的增加,更多的黄原胶可以与魔芋胶分子形成协同凝胶。由于浓度高于5%的黄原胶母液较难制备,因此本研究并未进一步增加黄原胶添加量。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1样品在90℃和室温下的凝胶强度
相同处理中,标注不同字母表示样品间差异显著(P<0.05)。下同
Fig. 1Gel strength of samples at 90℃ and room temperature
In the same treatment, different letters indicate significant differences between samples (P<0.05). The same as below
2.2 微观结构
凝胶微观网络结构的致密程度与凝胶强度密切相关。如图2所示,在黄原胶添加量为0的情况下,魔芋胶凝胶呈现出层状结构;当黄原胶添加量为0.25%时,复合凝胶层状结构更加明显;随黄原胶添加量进一步增加,复合凝胶层状结构逐渐转变为有交联的网络结构;随着黄原胶添加量持续增加,复合凝胶网络更加致密,表明黄原胶的添加能够增大整个凝胶体系的网络致密度,从而增强凝胶强度,一方面是因为总多糖浓度增加,另一方面是因为随黄原胶添加量的增加,更多的黄原胶可以与魔芋胶分子形成协同凝胶。这与凝胶在室温下测定的凝胶强度结果一致。图2
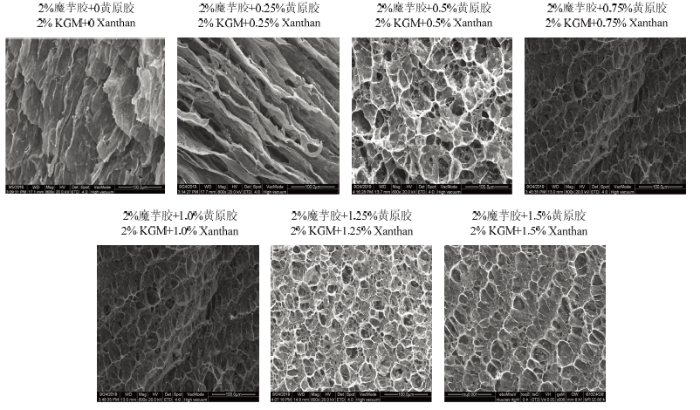 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2样品的微观结构
Fig. 2Microstructure of samples
2.3 冻融稳定性
由图3-A、B可知,在黄原胶添加量为0—1.0%时,冻融后凝胶样品的强度增大,这是因为冻融导致水分析出,造成凝胶体积收缩,致使凝胶网络致密度增加。然而当黄原胶添加量大于1.0%时,样品冻融后凝胶强度显著降低(P<0.05)。此外,由图3-C可看出,随黄原胶添加量增加,冻融后样品的析水率显著降低(P<0.05),说明添加黄原胶可改善魔芋胶凝胶的析水行为。由图3-D可以看出,在黄原胶添加量为0的情况下,魔芋胶凝胶的层状结构更加紧密,当黄原胶添加量为0.25%时,复合凝胶层状结构转变为有交联的网络结构,相较于未经过冻融处理的样品凝胶网络结构更加致密。当黄原胶添加量大于1.0%时,复合凝胶网络网孔明显增大。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3样品在冻融处理前后凝胶强度、析水率及微观结构
Fig. 3Gel strength before and after freezing and thawing, the syneresis rate and microstructure of samples after freeze-thawing
2.4 去离子水与柠檬酸溶液浸泡对凝胶强度和微观形貌的影响
由图4可以看出,在去离子水和2%柠檬酸溶液中浸泡后,样品的凝胶强度均呈现降低趋势;浸泡在2%柠檬酸溶液中的样品的凝胶强度更低,可能是因为制样过程中添加了Na2CO3,浸泡过程中柠檬酸与Na2CO3反应生成CO2气体,引起凝胶网络结构被轻微扰乱。当黄原胶添加量为0时,魔芋胶凝胶网络结构不明显,以片层状结构为主,当黄原胶添加量为0.25%时,复合凝胶开始呈现网络结构,黄原胶添加量越高,网络结构越密集。此外,与去离子水相比,2%柠檬酸溶液浸泡后的复合凝胶网络孔径略大。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4样品在去离子水和2%柠檬酸溶液中浸泡后的凝胶强度以及微观结构
Fig. 4Gel strength and microstructure of samples after immersion in deionized water and 2% citric acid solution
2.5 凝胶化过程监测及凝胶流变学特性
2.5.1 时间扫描 魔芋胶凝胶化过程是时间依赖型的过程,在相同的碱浓度(2%)、温度(90℃)条件下,随时间的变化,魔芋胶的凝胶强度也会随之变化。因此,本研究对2% KGM(对照)、2% KGM+0.25% Xanthan、2% KGM+0.5% Xanthan、2% KGM+0.75% Xanthan、2% KGM+1.0% Xanthan和2% Xanthan进行90℃下的时间扫描,如图5所示。可以看出,在最初的1 200 s内,G'和G''迅速增加,表明在此阶段随着时间的延长,凝胶网络迅速形成;在2 400 s后,G'和G''虽然仍有增加,但增加趋势明显变缓,说明凝胶网络基本形成;且只有2.0% KGM和2.0% KGM+0.25% Xanthan监测到G'与G''的交点,其他混合体系未监测到,可能是因为黄原胶的存在,使整个复合体系表现出G'>G''的特点。此外,从图5-F中可以看出,2% Xanthan在相同的碱浓度(2%)、温度(90℃)条件下,G'总是大于G",说明黄原胶表现出类似弱凝胶的结构,但随着时间的延长,其模量逐渐降低,这可能是因为黄原胶分子在碱性条件下加热发生降解,导致黄原胶分子量减小[27]。图5
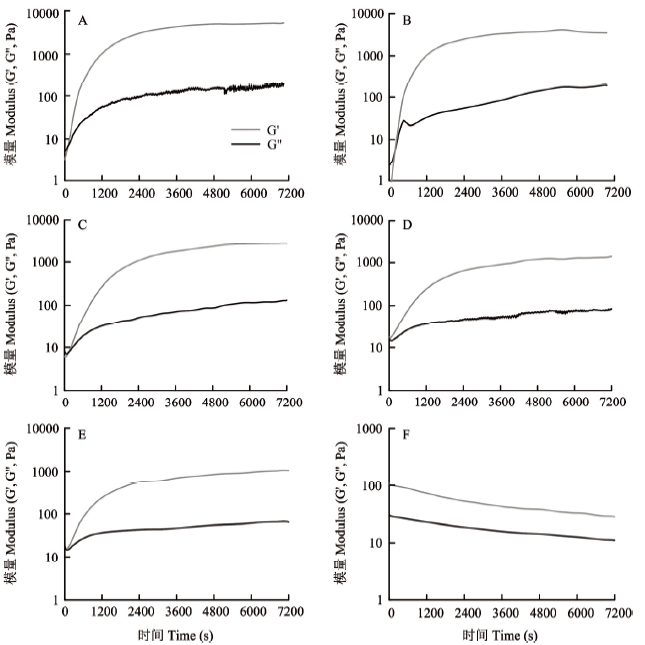 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5样品在2%的碱浓度、90℃条件下的时间扫描结果
A:2.0%魔芋胶;B:2.0%魔芋胶+0.25%黄原胶;C:2.0%魔芋胶+0.5%黄原胶;D:2.0%魔芋胶+0.75%黄原胶;E:2.0%魔芋胶+1.0%黄原胶;F:2.0% 黄原胶
Fig. 5Time sweep curves of sample at 2% alkali concentration and 90℃
A: 2.0% KGM; B: 2.0% KGM+0.25% Xanthan; C: 2.0% KGM+0.5% Xanthan; D: 2.0% KGM+0.75% Xanthan; E: 2.0% KGM+1.0% Xanthan; F: 2.0% Xanthan
2.5.2 黄原胶添加量对复合体系中魔芋胶凝胶化过程的影响 对样品的G'进行拟合,发现拟合度R2均在0.98以上,表现出较高的拟合精度。拟合的G'sat和k值见表2。
Table 2
表2
表2不同黄原胶添加量的复合凝胶90℃加热时凝胶网络G'变化趋势一级动力学拟合参数
Table 2
| 样品Sample | G'sat | k | R2 |
|---|---|---|---|
| 2% KGM (Control) | 5944.73 | 3.58×10-4 | 0.9800 |
| 2% KGM+0.25% Xanthan | 4613.90 | 2.42×10-4 | 0.9918 |
| 2% KGM+0.5% Xanthan | 3853.96 | 1.14×10-4 | 0.9829 |
| 2% KGM+0.75% Xanthan | 1760.91 | 0.78×10-4 | 0.9915 |
| 2% KGM+1.0% Xanthan | 1247.19 | 0.54×10-4 | 0.9963 |
新窗口打开|下载CSV
从表2和图6可以看出,2% KGM(对照)的G'sat和k最大,随着黄原胶添加量的增多,G'sat和k都降低,说明黄原胶的添加,不仅降低了魔芋胶凝胶最终的凝胶强度,而且减缓了魔芋胶凝胶网络的形成速率,这与在90℃下测得的凝胶强度一致。
图6
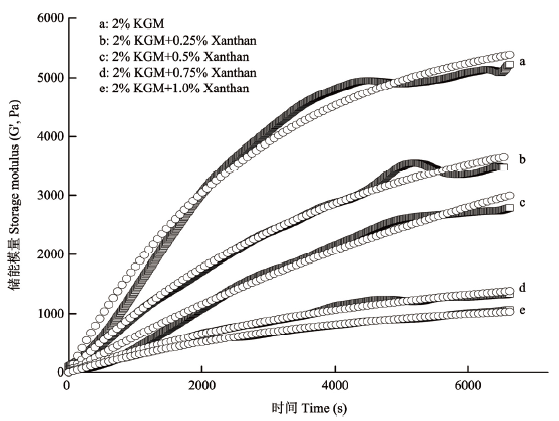 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6样品时间扫描的储能模量以及拟合曲线
Fig. 6Storage modulus and fitting curve of sample during time sweep
2.5.3 降温扫描 魔芋胶-黄原胶协同凝胶的形成与温度有关,体系在90℃加热2 h后,对其进行自然冷却降温,在此过程中,黄原胶分子可能与魔芋胶网络结合,起到加固凝胶体系的作用。如图7所示,2% KGM+0.5% Xanthan和2% KGM+1.0% Xanthan在整个温度范围内都表现出G'>G",说明整个体系为较强的凝胶;随着温度的降低,体系的储能模量(G')表现出先下降后上升的趋势,上升的拐点约为60℃。下降的原因可能是在降温过程中,魔芋胶凝胶网络疏水相互作用力减弱,降低了魔芋胶凝胶网络的缚束力,这与在高温下魔芋胶凝胶强度较大,在低温下魔芋胶凝胶强度较小这一性质相一致。当温度降低至60℃左右时,黄原胶分子与魔芋胶凝胶网络发生协同作用,开始加固整个凝胶体系,G'不断增大,随着温度进一步降低,这种加固作用更明显,这与在室温下测得的整个体系的凝胶强度较大相一致。从图7还可以看出,2% KGM+1.0% Xanthan体系的储能模量(G')大于2% KGM+0.5% Xanthan体系,说明随着黄原胶添加量增多,体系的凝胶强度越大。此外,无论黄原胶添加量为0.5%还是1.0%,拐点对应的温度均约为60℃,表明黄原胶分子与魔芋胶凝胶网络的协同结合温度不随黄原胶添加量的变化而改变。
图7
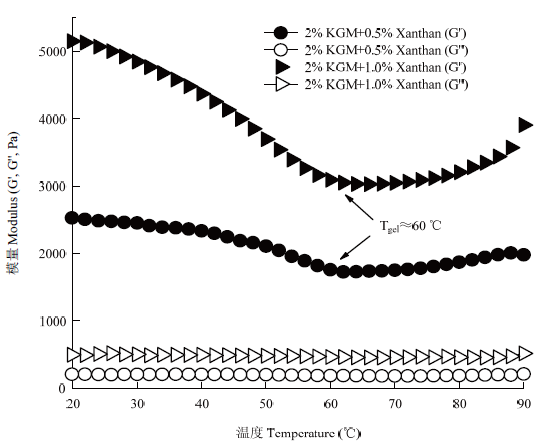 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7样品降温扫描过程中储能模量变化趋势
Fig. 7Change trend of storage modulus of samples during decreasing temperature sweep
2.5.4 频率扫描 当整个体系温度降低到20℃时,对其进行频率扫描,频率范围为1—100 rad·s-1,扫描结果如图8所示。由图8可知,在整个频率范围内,样品都表现出G'>G"的特点,并且有明显的平台区出现,说明所有的复合体系均形成了较强的凝胶。由图8-F可知,黄原胶也表现出G'>G",说明黄原胶也有类似凝胶的特性,但随着频率增加,黄原胶体系的G'和G"都呈现出轻微增加的趋势,说明黄原胶体系是一种弱凝胶。
图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8样品的频率扫描结果
A:2.0%魔芋胶;B:2.0%魔芋胶+0.25%黄原胶;C:2.0%魔芋胶+0.5%黄原胶;D:2.0%魔芋胶+0.75%黄原胶;E:2.0%魔芋胶+1.0%黄原胶;F:2.0% 黄原胶
Fig. 8Frequency sweep curves of sample
A: 2.0% KGM; B: 2.0% KGM+0.25% Xanthan; C: 2.0% KGM+0.5% Xanthan; D: 2.0% KGM+0.75% Xanthan; E: 2.0% KGM+1.0% Xanthan; F: 2.0% Xanthan
2.6 X-射线衍射测定
X-射线衍射是表征聚合物结晶情况的常用手段。如图9所示,在2θ=20—80°的衍射角度范围内,Na2CO3有若干尖锐的散射峰,表明Na2CO3以高度结晶化的晶体结构存在,这是典型无机化合物的特点。2% KGM凝胶的散射峰在35°左右,但其并不明显,说明该体系以无定型状态以及由若干微晶区存在。2% Xanthan体系的散射峰非常小;不同量黄原胶的添加,体系的散射峰仍然存在,说明黄原胶的添加并没有改变2% KGM体系微晶区的结构。图9
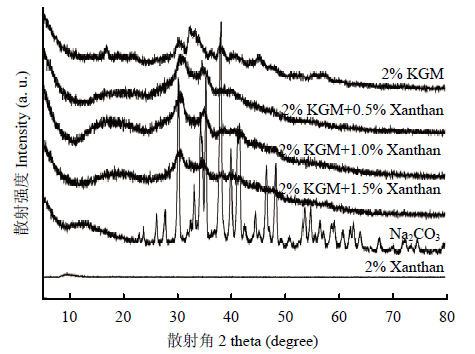 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9样品X-射线衍射
Fig. 9X-ray diffraction of samples
2.7 热重分析
冻干后凝胶样品的热稳定性见图10。从图10-A可以看出,Na2CO3在100℃以内出现一个明显的重量降低阶段,很可能是由于水分丧失而引起。其余5个样品均出现2—3个明显的重量降低阶段,在100℃内的重量损失可能是样品游离水丧失而引起,在150℃左右出现的重量损失可能是由于结合水丧失而引起,在200—300℃,所有凝胶样品有一个明显的重量降低趋势,这可能是多糖分子的热降解所致。随着黄原胶添加量的增加,多糖分子热降解的温度轻微增大,说明整个凝胶体系的热稳定性增加,进一步表明凝胶网络结构更加致密。图10
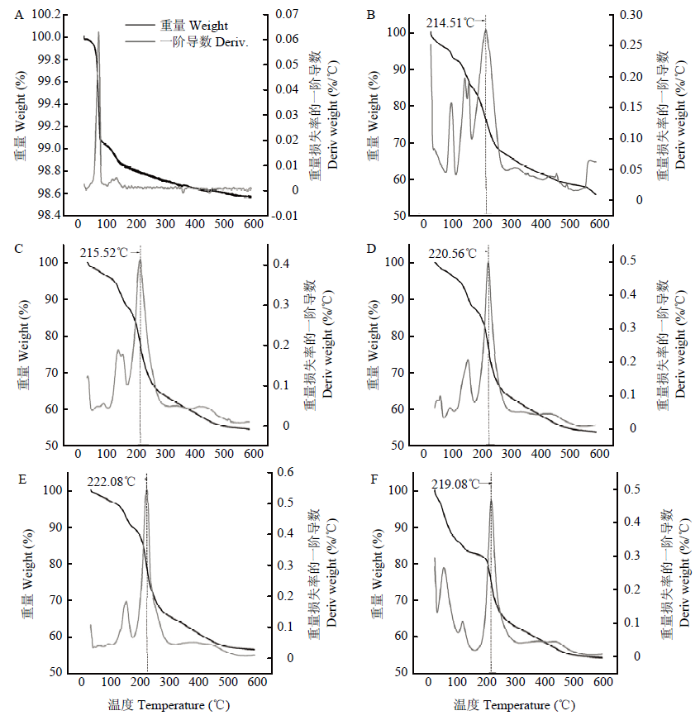 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10样品的热重分析
A:2.0%魔芋胶;B:2.0%魔芋胶+0.25%黄原胶;C:2.0%魔芋胶+0.5%黄原胶;D:2.0%魔芋胶+0.75%黄原胶;E:2.0%魔芋胶+1.0%黄原胶;F:2.0% 黄原胶
Fig. 10Thermogravimetric analysis of samples
A: 2.0% KGM; B: 2.0% KGM+0.25% Xanthan; C: 2.0% KGM+0.5% Xanthan; D: 2.0% KGM+0.75% Xanthan; E: 2.0% KGM+1.0% Xanthan; F: 2.0% Xanthan
3 讨论
3.1 碱法诱导的魔芋胶-黄原胶混合体系凝胶化的理论基础
在碱性条件下加热魔芋胶溶液即可获得魔芋胶凝胶,这种方式是魔芋胶凝胶的传统制备方式[28]。近年来,有研究表明[3],混合魔芋胶和黄原胶也可制得凝胶,凝胶化原理在于魔芋胶分子和黄原胶分子之间存在协同结合作用。然而,这两种方式制得的魔芋胶凝胶各有优缺点。本试验基于对上述两种凝胶形成过程及机制的理解,提出以下假设:在碱性条件下加热魔芋胶和黄原胶的混合体系时,魔芋胶因为去乙酰基作用而首先发生分子间交联,形成三维网络结构,随后冷却混合体系时,黄原胶分子可能和魔芋胶网络进一步结合,从而加固整个凝胶网络体系。通过这种方法制备的凝胶理论上应具有上述两种凝胶网络的优点,即该凝胶既是热不可逆凝胶,而且在室温下可以克服热碱法诱导的魔芋胶凝胶强度较差、析水严重等缺点。基于此设想,本研究成功制备出了复合凝胶。此外,虽然魔芋胶和kappa-卡拉胶也具有明显的协同效应[29,30],但由于魔芋胶凝胶制备时需要在碱性条件下长时间加热,kappa-卡拉胶会发生明显的分子降解而极大降低与魔芋胶协同结合的能力。因此,本试验未采用kappa-卡拉胶和魔芋胶的混合体系制备凝胶,而是选择具备优良热稳定性以及酸碱稳定性的黄原胶。3.2 碱法诱导的魔芋胶-黄原胶复合凝胶体系的形成过程
当在碱性条件下加热混合溶胶体系时,魔芋胶分子聚集速率(k值)及聚集程度(G′sat)明显受到抑制。原因很可能是由于碱性条件下加热时,一部分碳酸钠参与了黄原胶分子的降解,因此只有一部分碳酸钠诱导魔芋胶发生凝胶化,意味着该复合体系中诱导魔芋胶分子发生凝胶化的碳酸钠有效浓度降低了。有研究表明[31],魔芋胶在热碱性条件下的凝胶化速率和魔芋胶浓度、魔芋胶乙酰化程度、碱浓度以及加热温度等因素密切相关,即魔芋胶浓度越高、碱浓度越大、加热温度越高,凝胶化速率越快。在碱性条件下加热魔芋胶和黄原胶的复合体系2 h后,立刻测定了各个混合凝胶体系的凝胶强度,发现随黄原胶添加量增加,复合体系的凝胶强度呈显著降低趋势(P<0.05),进一步说明黄原胶的添加抑制了魔芋胶分子聚集速率及聚集程度。碱性条件下加热魔芋胶和黄原胶混合溶胶体系2 h后,冷却凝胶体系至室温,发现室温条件下,该凝胶体系的强度极大增加,且黄原胶添加量越多,凝胶强度越高。例如,当黄原胶添加量增加至1.5%时,室温下测得的凝胶强度高达2 000 g,是该凝胶体系在90℃条件下的20倍以上。通过对魔芋胶和黄原胶混合凝胶体系进行降温扫描,发现在90℃至20℃的温度范围内,所有凝胶的弹性模量总是高于黏性模量,没有发现两者的交点,这是由于在整个温度范围内,魔芋胶和黄原胶混合体系均表现出凝胶的特性,因此其弹性模量占主导。此外,发现当凝胶体系的温度从90℃降低至60℃时,凝胶弹性模量呈明显降低趋势,这可能是由于疏水作用力是维持魔芋胶三维网络结构的主要因素,温度降低时,疏水作用力减弱,导致魔芋胶三维网络松散[28];然而,当温度降低至60℃时,凝胶弹性模量开始呈现明显的增加趋势,温度越低,弹性模量越高,这是因为当温度降低至60℃时,黄原胶分子开始与魔芋胶凝胶网络发生协同结合作用,氢键是维持黄原胶分子与魔芋胶凝胶网络的主要作用力,随温度降低,协同结合作用越明显,使整个凝胶体系在室温条件下表现出较大的凝胶强度。此外,研究还发现,魔芋胶和黄原胶协同结合的温度与黄原胶添加量无关,对于黄原胶添加量为0.5%和1.0%的混合体系,协同作用的起始温度均为60℃。
3.3 复合凝胶体系的冻融稳定性
魔芋胶分子上的乙酰基是影响魔芋胶水溶解性的关键因素[28]。乙酰基含量越高,魔芋胶分子水化越容易。碱性条件下,魔芋胶分子上的乙酰基逐渐脱除,虽然可以诱导魔芋胶分子发生聚集,形成三维凝胶网络结构,但是也降低了魔芋胶分子的亲水性。尤其是冻融处理后,魔芋胶凝胶出现明显的析水现象,原因是冷冻过程中魔芋胶分子在冰晶体的挤压作用下形成分子间结合区。当冷冻的凝胶解冻后,形成的分子间交联能够很好地保留,虽然一定程度上增加了冻融后的凝胶强度。但冰晶体仍然会转变为液态水从凝胶网络中流出。单纯魔芋胶凝胶析水率可达50%,然而,当魔芋胶凝胶体系中含有黄原胶后,该凝胶体系的析水作用显著降低,这是因为黄原胶表现出较强的亲水性和冻融稳定性,当黄原胶加入魔芋胶凝胶体系时,黄原胶对水分子的亲和力更强,有效减弱凝胶在冷冻过程中的水分子结晶现象。此外,当黄原胶添加量在0—1.0%的情况下,冻融后复合凝胶均出现凝胶强度增加的现象,原因可能是冷冻过程中,不断长大的冰晶体迫使魔芋胶分子彼此靠近,形成局部的高浓度,有利于魔芋胶分子间形成规整有序的结合区。当凝胶解冻后,这样的结合区也不会消失,因此引起凝胶强度增加。然而,当黄原胶添加量大于1.0%的情况下,冻融后复合凝胶出现凝胶强度降低的趋势,原因可能是在黄原胶添加量大于1.0%的情况下,复合凝胶冷冻过程中冰晶体生成破坏了凝胶原有的网络结构,且这种破坏作用大于冷冻过程对凝胶的促进作用,从而使整个凝胶体系解冻后凝胶强度降低。4 结论
以碳酸钠作为碱性调节剂,采用高温条件下加热魔芋胶和黄原胶混合体系的方式,制备出双重凝胶网络的复合凝胶,该凝胶兼具魔芋胶凝胶网络、魔芋胶与黄原胶协同凝胶网络二者的优点,不仅保持了魔芋胶凝胶的热不可逆性,也改善了魔芋胶凝胶强度低、冻融稳定性差等缺点。随着黄原胶浓度增加,复合凝胶的强度不仅表现出增加趋势,且凝胶冻融后析水现象明显降低。本研究提出的魔芋胶凝胶改性方法简单可行、可操作性强,能够将魔芋胶凝胶机制和魔芋胶与黄原胶协同结合机制合二为一,可为魔芋胶凝胶相关食品的设计提供理论依据和技术参考。致谢:
感谢陕西师范大学化学与化工学院大型仪器共享平台的仪器支持及管理老师在测样过程中的指导和帮助。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 7]
[本文引用: 7]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
DOI:10.1021/bm034302fURLPMID:14715024 [本文引用: 1]

Effect of the degree of acetylation (DA) on the gelation behaviors on addition of sodium carbonate for native and acetylated konjac glucomannan (KGM) samples with a DA range from 1.38 to 10.1 wt % synthesized using acetic anhydride in the presence of pyridine as catalyst was studied by dynamic viscoelastic measurements. At a fixed alkaline concentration (CNa), both the critical gelation times (tcr) and the plateau values of storage moduli (G'sat) of the KGM gels increased with increasing DA, while at a fixed ratio of alkaline concentrations to values of DA (CNa/DA), similar tcr and values independent of DA were observed. On the whole, increasing KGM concentration or temperature shortened the gelation time and enhanced the elastic modulus for KGM gel. The effect of deacetylation rate related to the CNa/DA on the gelation kinetics of the KGM samples was discussed.
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
URLPMID:29103488 [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1016/j.ijbiomac.2019.03.052URLPMID:30880058 [本文引用: 1]

Pasting and functional properties of water chestnut starch (WCS) alone and mixture of WCS and xanthan gum (XG) were determined by addition of NaCl (0.5, 1, and 2%) at fixed water chestnut starch (5%) and xanthan gum (0.3%) concentration. Results indicated that presence of NaCl had a significant impact on functional and pasting properties of both WCS alone and WCS - XG mixture. Pasting temperature of WCS and WCS - XG mixture increased linearly with increasing salt content, whereas a reverse trend was observed in peak viscosity and sets back in case of WCS alone. It was found that addition of NaCl decreased the swelling power of WCS alone, while it increased in case of WCS - XG mixture. The water absorption of WCS - XG improved drastically by the addition of NaCl while a rapid decline in syneresis was observed with WCS - XG mixture. The transparency of both WCS and WCS - XG mixture were found to be increased after the addition of NaCl.
[本文引用: 1]
[本文引用: 1]
URLPMID:30684579 [本文引用: 1]
DOI:10.1016/j.foodchem.2018.05.116URLPMID:30100487 [本文引用: 1]

A molecular-level mechanism of alkali induced konjac glucomannan (KGM) hydrogel gelation processing is considered with the application of nuclear magnetic resonance (NMR) spectroscopy and atomic force microscopy (AFM) as complementary methods to diffusive wave spectroscopy (DWS) microrheology and thermoanalysis. It is shown that deacetylation of KGM chains occurs immediately upon mixing with Na2CO3, inducing self-packaging. Partial unfolding of the packed loose structure of dehydrated KGM is observed upon heating. The configuration transition from random coils to self-assembling filament networks takes place before KGM aggregating to form large irreversible bundles with a lower degree of cross-linking. The gelation is not fulfilled until the temperature is increased to above 70 degrees C when the significant agglomeration is initiated among transitional fibrils to form junction zones essentially composed of acetyl-free portions. This suggests the intermolecular aggregation of KGM chains not simply regarding to hydrogen bonds, but essentially relating to hydrophobic interactions.
[本文引用: 4]
[本文引用: 4]
DOI:10.1016/j.carbpol.2019.115278URLPMID:31582087 [本文引用: 3]

In the study, a facile methodology for fabricating high viscoelastic emulsion gels has been proposed, which is based on the synergistic gelation mechanism of xanthan and konjac glucomannan (KGM). The effects of preparation strategies, oil contents and simulated sterilization conditions (121 degrees C, 30min) on the emulsion gels were systemically investigated. All emulsion gels exhibited similar gelation temperature (Tgel) and gel melting temperature (Tmelt), indicating that the gelling continuous phase played a predominant role in the emulsion gel formation. However, the viscoelasticity of the emulsion gels was different depending on preparation strategies and oil phase fractions. When a low concentration of tween-80 (<1.0 %, v/v) was incorporated during emulsification process, the resultant emulsion gels showed satisfactory thermal stability and could undergo simulated sterilization conditions without discernible oil droplet coalescence observed. Conclusively, these results demonstrate the potentials of co-utilization of xanthan and KGM in designing novel food products.
DOI:10.1016/j.carbpol.2019.115468URLPMID:31826449 [本文引用: 1]

In this study, the compatibility of alginate (Alg) and konjac glucomannan (KGM) in aqueous solutions was evaluated by dilute solution viscometry (DSV). It was found that when Alg: KGM ratio was lower than 6:4 (w/w), Alg and KGM were compatible, which was subsequently confirmed by SEM, AFM and TEM. Moreover, by dispersing emulsified oil droplets into Alg gel matrix, followed by addition of KGM to thicken the system, where the ratio of Alg: KGM was 5:5, a class of emulsion gels with significant thixotropy and viscoelasticity could be obtained. The prepared emulsion gels displayed good thermal stability and freeze-thaw stability, with no oil droplet coalescence observed after heating at 100 degrees C for 30 min or freezing the gels at -18 degrees C for 24 h. Overall, the mixed Alg/KGM system is expected to provide a template for designing low-fat mayonnaise-like food emulsions.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 2]
URLPMID:31100393 [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
DOI:10.1016/j.ijbiomac.2019.03.137URLPMID:30910680 [本文引用: 1]

In this study, the rheological properties and gelation mechanism of high acyl gellan (HAG) were investigated. The critical overlap concentration (C()) of HAG was determined to be 0.045%, and the specific viscosity (etasp) of HAG solutions linearly varied as the concentration of C(1.42) and C(3.51) before and after the concentration, respectively. The transition concentration was found to shift toward a lower value (0.045% vs. 0.08%) when compared with the generality of random coil polysaccharide solutions, indicating the occurrence of intermolecular associations. At sufficiently high concentrations (i.e., above 0.125%), HAG molecules associated with each other to form a heterogeneous three-dimensional network, thus leading to gelation. The gelling temperature depended on HAG concentration, and there was no significant thermal hysteresis observed when heating the formed gels. Furthermore, it was found that acyl substituents allowed HAG molecules to form hydrophobic regions during the gelation process, which was attributed to the intermolecular aggregations driven by hydrophobic interactions. Our findings may deepen the understanding on HAG gelation and thus broaden the application of HAG in the food and biomaterial fields.
DOI:10.1016/j.carbpol.2018.09.034URLPMID:30318193 [本文引用: 1]

Xanthan gum solutions are used in the oil industry for flooding, drilling and completion operations. The stabilization of the structure of xanthan gum solutions in presence of salts increases the value of both the order-disorder transition temperature and the gel strength. This effect is very important in order to design drilling and completion fluids since not only density and viscosity of the fluid can be improved by increasing the concentration of salts but also the range of temperature where the solution shows viscoelastic behaviour can be extended. This paper presents results from a study on the rheological behaviour of xanthan gum solutions in different saturated brines. Chloride and formate potassium brines not only increase the viscosity of the solution but also extend the shear thinning behaviour to temperatures near 200 degrees C, maintaining a simple relaxation mechanism over the whole range of temperature where the ordered conformation dominates the rheological behaviour.
DOI:10.1016/j.carbpol.2017.02.078URLPMID:28363561 [本文引用: 3]

The gelation of konjac glucomannan (KGM) often involves an induction period featured by an initial drop in modulus. The gelation kinetics recorded as a time-dependent modulus change is composed of an initial drop followed by a steady increase in modulus until an equilibrium stage is reached. In this work, the time-dependent modulus curve is described by a kinetic model which incorporates the cascade theory with the kinetic equations consisting of sequential reactions of deacetylation and cross-link formation. The proposed model is capable of predicting the presence of the induction period and the kinetic parameters can be extracted from the concentration dependence of the initial drop. A rheological experiment of KGM gelation using sodium carbonate as deacetylation agent was conducted to illustrate the feasibility of the proposed fitting approach, which yields kinetic curves agreeing satisfactorily with the experimental results.
DOI:10.7506/spkx1002-6630-201423067URL [本文引用: 1]

Food gums play a significant role in the food industry. For more efficient, economical and scientific use offood gums, it is essential to have a thorough understanding of these food ingredients. Based on this statement, this papersummarizes the gelling mechanism and synergistic interaction of gelatin, carrageenan, sodium alginate, and xanthan gumwith the aim to offer some guidance for the application in food processing and production.
DOI:10.7506/spkx1002-6630-201423067URL [本文引用: 1]

Food gums play a significant role in the food industry. For more efficient, economical and scientific use offood gums, it is essential to have a thorough understanding of these food ingredients. Based on this statement, this papersummarizes the gelling mechanism and synergistic interaction of gelatin, carrageenan, sodium alginate, and xanthan gumwith the aim to offer some guidance for the application in food processing and production.
[本文引用: 1]
DOI:10.1021/bm0255995URLPMID:12425668 [本文引用: 1]

Gelation kinetics of native and acetylated konjac glucomannan (KGM) samples in the presence of alkali (sodium carbonate) was studied by dynamic viscoelastic measurements. Molecular weight and other molecular parameters of KGM were determined by static light scattering and viscosity measurements. It was found that KGM molecules were degraded during acetylation treatment, but the molecular weights of acetylated samples were almost independent of the degree of acetylation (DA) and were about a half of that of a native sample. At a fixed alkaline concentration, increasing concentration of KGM or temperature shortened the gelation time, but increasing DA delayed it. The deacetylation reaction and subsequent aggregation process of acetylated samples needed longer time than that of native sample, and acetylated samples formed finally more elastic gels. It implied that the presence of acetyl groups exerts a strong influence on gelation behavior of KGM. It was suggested that the gelation rate of acetylated KGM and native KGM, which depends on the alkaline concentration and temperature, is an important factor that determines the elastic modulus of gels. This was supported by the experimental finding that the saturated elastic modulus tends to the same value when the ratio of alkali concentration to acetylated groups was kept constant. In slower gelation processes, junction zones are more homogeneously distributed and more numerous, leading to the more elastic gels.
