 ,1, 李增强1, 唐美琼1, 罗登杰1, 曹珊1, 岳娇1, 胡亚丽1, 黄震1, 陈涛2, 陈鹏
,1, 李增强1, 唐美琼1, 罗登杰1, 曹珊1, 岳娇1, 胡亚丽1, 黄震1, 陈涛2, 陈鹏 ,1,*
,1,*DNA methylation in response to cadmium stress and expression of different methylated genes in kenaf
LU Hai ,1, LI Zeng-Qiang1, TANG Mei-Qiong1, LUO Deng-Jie1, CAO Shan1, YUE Jiao1, HU Ya-Li1, HUANG Zhen1, CHEN Tao2, CHEN Peng
,1, LI Zeng-Qiang1, TANG Mei-Qiong1, LUO Deng-Jie1, CAO Shan1, YUE Jiao1, HU Ya-Li1, HUANG Zhen1, CHEN Tao2, CHEN Peng ,1,*
,1,*通讯作者: * 陈鹏, E-mail:hustwell@gmail.com
收稿日期:2020-12-9接受日期:2021-04-14网络出版日期:2021-05-14
| 基金资助: |
Corresponding authors: * E-mail:hustwell@gmail.com
Received:2020-12-9Accepted:2021-04-14Published online:2021-05-14
| Fund supported: |
作者简介 About authors
E-mail:294856020@qq.com

摘要
DNA甲基化是植物重要的表观遗传修饰方式之一, 在响应逆境胁迫中具有重要作用, 但是有关镉胁迫下植物DNA甲基化水平变化的报道甚少。本研究以红麻P3A为材料, 采用水培法对幼苗进行300 μmol L-1的CdCl2处理, 测定幼苗农艺性状及镉含量; 利用甲基化敏感扩增多态性技术(methylation-sensitive amplification polymorphism, MSAP)分析镉胁迫下根系DNA甲基化水平变化; 回收甲基化差异片段并克隆测序, 采用qRT-PCR技术对DNA甲基化差异基因的表达量进行分析。结果表明, CdCl2胁迫显著抑制红麻幼苗的株高、茎粗、根长、根表面积以及全鲜重。对照及镉处理下幼苗根系的DNA甲基化率分别为62.78%、68.23%, 其中全甲基化率分别为37.50%、36.36%, 半甲基化率分别为25.28%、31.87%, 表明镉胁迫显著提高红麻幼苗根系的DNA甲基化水平。qRT-PCR分析表明, 7个与抗性密切相关的DNA甲基化差异基因也存在表达量的差异, 推测DNA甲基化水平变化在响应红麻镉胁迫中发挥重要作用。本结果为深入探索DNA甲基化响应植物镉胁迫的潜在机制提供了理论基础。
关键词:
Abstract
DNA methylation is one of the important epigenetic modifications in plants and plays an important role in response to stress. However, there are few reports of the changes of DNA methylation levels in plants under cadmium stress. The seedlings of kenaf P3A were treated with 300 μmol L-1 CdCl2 by hydroponics, the agronomic traits and cadmium contents were investigated. The changes of DNA methylation level of root under cadmium stress were analyzed by using methylation sensitive amplified polymorphism (MSAP) method. In addition, the differential methylation fragments were recovered, cloned, and sequenced. The relative expression levels of differential methylated genes revealed that Cd stress significantly inhibited the plant height, stem diameter, root length, root surface area, and total fresh weight of kenaf seedlings. The whole DNA methylation ratio in root was 68.23% and 62.78% under cadmium and control treatments, respectively. Among them the total methylation ratio was 37.50% and 36.36%, and the semi-methylation ratio was 25.28% and 31.87%, respectively. These results indicated that cadmium stress significantly increased the DNA methylation level of roots DNA. Seven differential methylated genes which involved in stress resistance were characterized differentially expressed under cadmium stress, suggesting that the change of DNA methylation played an important role in response to cadmium stress. This study provides a theoretical basis for further exploring the potential mechanism of DNA methylation in response to cadmium stress in plants.
Keywords:
PDF (376KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
卢海, 李增强, 唐美琼, 罗登杰, 曹珊, 岳娇, 胡亚丽, 黄震, 陈涛, 陈鹏. 红麻DNA甲基化响应镉胁迫及甲基化差异基因的表达分析. 作物学报, 2021, 47(12): 2324-2334 DOI:10.3724/SP.J.1006.2021.04269
LU Hai, LI Zeng-Qiang, TANG Mei-Qiong, LUO Deng-Jie, CAO Shan, YUE Jiao, HU Ya-Li, HUANG Zhen, CHEN Tao, CHEN Peng.
镉是植物生长发育过程中的非必需微量元素, 不仅影响植物生长发育, 过量的镉还会造成植物死亡, 并且通过在植物体内积累被食用后会威胁人类的生命健康。因此, 植物对重金属镉的吸收是环境污染研究领域的一个热点问题[1]。红麻(Hibiscus cannabinus L.)是世界上重要的韧皮纤维作物, 主要用于纺织、造纸、建材和饲料等方面, 是一种多用途作物[2]。红麻生长速度快、生物量大、对重金属具有极强的吸附力和耐受性, 是重金属污染土壤良好的替代种植材料[3]。
DNA甲基化在维持基因组稳定性、调控基因表达和响应生物及非生物胁迫等方面具有重要作用[4,5]。甲基化敏感扩增多态性(methylation sensitive amplified polymorphism, MSAP)技术是在扩增片段长度多态性(amplified fragment length polymorphism, AFLP)技术的基础上发展而来的, 它与AFLP同样基于PCR扩增多态性, 不同的是MSAP技术利用2种识别CCGG序列的同裂酶Hpa II和Msp I, 替换了AFLP分析中识别4碱基的内切酶Mse I, 并且MSAP技术操作简单、多态性高, 已成功应用于检测多种植物基因组DNA甲基化水平变化中[6,7]。张凯凯等[8]利用MSAP技术检测不同浓度镉胁迫对孔雀草基因组DNA甲基化水平的变化发现, 其甲基化水平随着镉浓度的提高而上升, 甲基化模式变化主要以重新甲基化为主, 并推测孔雀草受到环境中重金属胁迫时, 植物体通过改变DNA甲基化来调控基因的表达, 以应对不良环境对自身的影响, 从而达到抵御重金属胁迫的作用。李照令等[9]运用MSAP技术发现, 随着镉胁迫浓度的升高, 拟南芥基因组DNA甲基化水平逐渐降低, 但均高于对照, 且基因组去甲基化位点增多。有研究表明, 镉胁迫能够引起DNA总甲基化水平提高[10], 也有研究发现镉胁迫处理后DNA甲基化水平降低[11]。上述研究结果表明DNA甲基化水平变化和响应非生物胁迫机制的复杂性。
红麻响应镉胁迫的相关研究报道主要集中在农艺性状及多组学研究上, 但有关DNA甲基化响应红麻镉胁迫的研究尚未见报道。本试验前期对红麻幼苗P3A进行不同浓度(0、100、200、300、600 μmol L-1)的CdCl2胁迫表明, 幼苗在300 μmol L-1浓度下的株高和生物量显著降低, 但其生长发育无显著影响, 结合红麻修复重金属土壤的目的, 因此本研究选用300 μmol L-1的CdCl2对红麻材料P3A幼苗进行胁迫处理并详细分析, 测定幼苗的生理生化指标, 利用MSAP技术分析镉胁迫下基因组DNA甲基化水平变化, 对甲基化差异片段进行测序比对, 并对响应逆境的甲基化差异基因进行qRT-PCR分析, 系统分析镉胁迫下红麻幼苗的生理生化响应、基因组DNA甲基化水平变化以及响应镉胁迫相关基因的DNA甲基化变化与表达量的关系, 旨在为进一步研究DNA甲基化响应红麻镉胁迫的潜在机制提供重要参考。
1 材料与方法
1.1 试验材料及处理
红麻材料P3A由广西大学农学院周瑞阳教授提供。挑选籽粒饱满且均匀一致的种子, 37℃蒸馏水浸泡1 h, 3%过氧化氢(成都金山化学试剂有限公司, 20170616)消毒10 min, 蒸馏水冲洗3~5次, 之后将种子均匀地摆放在铺有2层纸巾的保鲜盒(27 cm× 18 cm×9 cm)中, 添加100 mL蒸馏水, 放置于恒温光照培养箱(光/暗周期为10 h/14 h, 温度为白天27℃/夜晚25℃, 下同)中培养(注意定期定量地添加蒸馏水, 始终保持纸巾湿润)。培养4 d后, 挑选长势一致的幼苗210株, 随机分成6组, 每组35株, 即为2个处理, 每个处理3次生物学重复, 移栽于6个底部含有托盘的育苗盘(35孔花卉育苗盘)中。向托盘中分别添加含有不同CdCl2浓度(0、300 μmol L-1)的1/4 Hoagland处理液, 注意每天定时定量地更换处理液。1.2 农艺性状及相对抑制率的测定
胁迫处理7 d后, 使用直尺测量红麻各单株的株高, 使用游标卡尺测量各单株的茎粗, 使用电子天平称量各组全鲜重。将根部用蒸馏水清洗干净并用吸水纸吸干水分, 利用根系扫描分析仪(EPSON EXPRESSION 11000XL)对根系进行扫描分析。之后将材料用液氮速冻后保存于-80℃冰箱, 用于后续试验。采用相对抑制率表示红麻耐镉性的强弱, 相对抑制率的公式为; 相对抑制率(%) = (目标性状对照值-目标性状处理值)/目标性状对照值×100 [12]。1.3 镉含量的测定
将根系用20 mmol L-1 EDTA-Na2浸泡1 h, 去除表面的镉离子, 再用去离子水洗涤3次, 并将根、茎、叶置于烘箱150℃杀青30 min, 之后70℃烘干至恒重, 称取各部分组织干重后再用粉碎机将其粉碎用于Cd2+含量测定。本次试验采用微波消解法处理样品, 使用PerkinElmer PinAAcle 900石墨炉原子吸收分光光度计测定Cd2+ 含量[13]。各部分组织粉碎成粉末后进行消解、赶酸、过滤、定容、制作标准曲线, 最后测定样品的镉含量。1.4 甲基化敏感扩增多态性(MSAP)分析
首先采用改良的CTAB法[14]提取上述材料根系的基因组DNA, 之后进行1%琼脂糖凝胶电泳检测DNA的质量, 利用超微量紫外线分光光度计测定DNA的浓度, 保存于-20℃冰箱备用。参照李增强等[15]报道的方法并加以改良, 对2个处理的(0、300 μmol L-1)根系进行MSAP分析。选用EcoR I+Hpa II和EcoR I+Msp I两种酶组合分别对基因组DNA进行双酶切, 之后进行连接、预扩增、选择性扩增。酶切体系为: CutSmart buffer 2 μL、Hpa II或Msp I (NEB公司) 0.5 μL、EcoR I 0.5 μL、模板DNA (100 ng μL-1) 5 μL、ddH2O 12 μL; 反应程序为: 37℃ 6 h, 80℃ (EcoR I/Hpa II)或65℃ (EcoR I/Msp I) 20 min。连接体系为: T4 DNA Ligase (350 U μL-1, TaKaRa公司) 2 μL、10×T4 DNA Ligase buffer 2 μL、正反向引物(10 μmol L-1)各1 μL、酶切产物14 μL; 反应程序为: 16℃ 14 h, 65℃ 20 min。预扩增体系为: 连接产物5 μL (将连接产物稀释20倍)、正反向引物(10 μmol L-1)各1 μL、2×Rapid Taq Master Mix (南京诺唯赞生物科技有限公司, 下同) 10 μL、ddH2O 3 μL; 反应程序为: 94℃预变性3 min; 94℃ 15 s, 58℃ 15 s, 72℃ 30 s, 32个循环; 72℃延伸5 min, 12℃保存。选择性扩增体系为: 预扩增产物5 μL (预扩增产物稀释25倍)、正反向引物(10 μmol L-1)各1 μL、2×Rapid Taq Master Mix 10 μL、ddH2O 3 μL; 反应程序同预扩增PCR。选择性扩增产物经95℃变性10 min后, 进行6%聚丙烯酰胺凝胶电泳, 银染、显色, 照相保存并统计MSAP多态性片段。接头序列、预扩增和选择性扩增引物序列见表1。Table 1
表1
表1接头和引物序列
Table 1
| 引物类型 Primer type | 引物名称及序列 Primer name and sequence (5′-3′) | |||
|---|---|---|---|---|
| EcoR I (E) | Hpa II/Msp I (HM) | |||
| 接头 Adapter | EA1 | CTCGTAGACTGCGTACC | HMA1 | GACGATGAGTCTAGAA |
| EA2 | AATTGGTACGCAGTC | HMA2 | CGTTCTAGACTCATC | |
| 预扩增 | E0 | GACTGCGTACCAATTCA | HM0 | GATGAGTCTAGAACGGT |
| Pre-amplification | E1 | GACTGCGTACCAATTCAAC | HM1 | GATGAGTCTAGAACGGTAG |
| E2 | GACTGCGTACCAATTCAAG | HM2 | GATGAGTCTAGAACGGTAC | |
| E3 | GACTGCGTACCAATTCACA | HM3 | GATGAGTCTAGAACGGTTG | |
| 选择性扩增 | E4 | GACTGCGTACCAATTCACT | HM4 | GATGAGTCTAGAACGGTTC |
| Selective amplification | E5 | GACTGCGTACCAATTCACC | HM5 | GATGAGTCTAGAACGGTGT |
| E6 | GACTGCGTACCAATTCACG | HM6 | GATGAGTCTAGAACGGTGC | |
| E7 | GACTGCGTACCAATTCAGC | HM7 | GATGAGTCTAGAACGGTCT | |
| E8 | GACTGCGTACCAATTCAGG | HM8 | GATGAGTCTAGAACGGTCG | |
新窗口打开|下载CSV
1.5 差异片段的回收、克隆和测序分析
将6%聚丙烯酰胺凝胶中的差异性片段用刀片切下, 放入1.5 mL EP管中, 用吸头捣碎, 加入25 μL无菌水, 95℃水浴10 min, 离心30 s后取5 μL上清用相应的选择性扩增引物进行PCR扩增。扩增体系为DNA模板5 μL、正反向引物(10 μmol L-1) 2.5 μL、2×Rapid Taq Master Mix 25 μL、ddH2O 15 μL; 反应程序同选择性扩增。利用1%琼脂糖凝胶电泳检测扩增产物, 并进行回收纯化(南京诺唯赞生物科技有限公司)。将回收产物连入pEASY-T1载体(北京全式金生物技术有限公司), 之后转入DH5α感受态细胞中, 挑选阳性单克隆, 进行菌液PCR检测, 之后送广州华大基因科技服务有限公司测序。测序所得序列在NCBI (1.6 实时荧光定量 PCR (qRT-PCR)分析
使用异硫氰酸胍法[17]并稍作改良提取相应根系材料(0、300 μmol L-1)的RNA, 并检测RNA的质量和浓度, 使用诺唯赞反转录试剂盒(货号R223-01)逆转录成cDNA, 并以此为模板进行qRT-PCR分析。以组蛋白基因His3为内参基因, 采用2-ΔΔCT方法[2]计算基因的相对表达量。qRT-PCR引物序列见表2。Table 2
表2
表2实时荧光定量PCR引物序列
Table 2
| 引物名称 Primer name | 正向引物 Forward sequence (5′-3′) | 反向引物 Reverse sequence (5′-3′) |
|---|---|---|
| GH3.1 | GACCGCCGTCTGTAACTCG | GGTTCCACAACTCCGCCC |
| GrKCS1 | GCCGCCAGTGTGATGGATAT | CGGTGCTCGGTTCTCCATT |
| NADP-ME | TTCATCTTCCCTTCGCCTT | CATCCCAACAACACCTTCCT |
| ABCG26 | ATTGGTACGCAGTCACGATG | TTCACCTTCATTTGACACGG |
| L-AAOh | AAGCCTTTCCGATTGTTCTGC | GGAGTTAGATTTTTGTTGTTGGGAG |
| LRR-RLK | GCATATACACGATAAGAAACCGC | CATGAAGATATTCCAAGCCCC |
| GaTPPD | CCTTCCTCCCTTGGGTTAGT | TTCTGTTCGCCTGTGGTTTT |
| XlCGF8.2DB | GACTGCGTACCAATTCAAGGC | AATTGTTGCCATATTTCTCCCAT |
| His3 (reference gene) | GTGGAGTCAAGAAGCCTCACAG | ATGGCTCTGGAAACGCAAA |
新窗口打开|下载CSV
2 结果与分析
2.1 镉胁迫对红麻幼苗农艺性状的影响
由表3和表4可知, 300 μmol L-1的CdCl2胁迫显著抑制红麻幼苗的株高、茎粗、全鲜重、根鲜重、根长和根表面积, 抑制率分别为21.52%、21.07%、54.93%、40.67%、64.27%和49.66%。表明300 μmol L-1的CdCl2 胁迫显著抑制了红麻幼苗的生长。Table 3
表3
表3CdCl2胁迫对红麻幼苗农艺性状的影响
Table 3
| CdCl2浓度 Concentration of CdCl2 (μmol L-1) | 株高 Plant height (cm) | 茎粗 Stem diameter (mm) | 全鲜重 Fresh weight (g) | 根鲜重 Root fresh weight (g) | 根长 Root length (cm) | 根表面积 Root surface area (cm2) |
|---|---|---|---|---|---|---|
| 0 | 29.24 a | 2.49 a | 21.19 a | 9.27 a | 275.32 a | 23.68 a |
| 300 | 19.94 b | 1.70 b | 9.54 b | 5.50 b | 186.74 b | 8.98 b |
新窗口打开|下载CSV
Table 4
表4
表4CdCl2胁迫对红麻幼苗农艺性状的相对抑制率
Table 4
| CdCl2浓度 Concentration of CdCl2 (μmol L-1) | 株高 Plant height | 茎粗 Stem diameter | 全鲜重 Fresh weight | 根鲜重 Root fresh weight | 根长 Root length | 根表面积 Root surface area |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 300 | 21.52 | 21.07 | 54.93 | 40.67 | 64.27 | 49.66 |
新窗口打开|下载CSV
2.2 红麻幼苗不同部位镉含量的比较
由图1可知, 红麻幼苗在300 μmol L-1的CdCl2 胁迫下, 根系的镉含量最高, 为2555.47 μg L-1; 茎部的镉含量次之, 为1363.41 μg L-1; 叶片的镉含量最低, 为963.06 μg L-1; 并且各部位的镉含量均存在显著性差异。结合对红麻幼苗农艺性状的测定结果得出, 300 μmol L-1的CdCl2胁迫显著抑制了红麻幼苗的生长, 且植株体内尤其是根系积累了大量的镉元素。图1
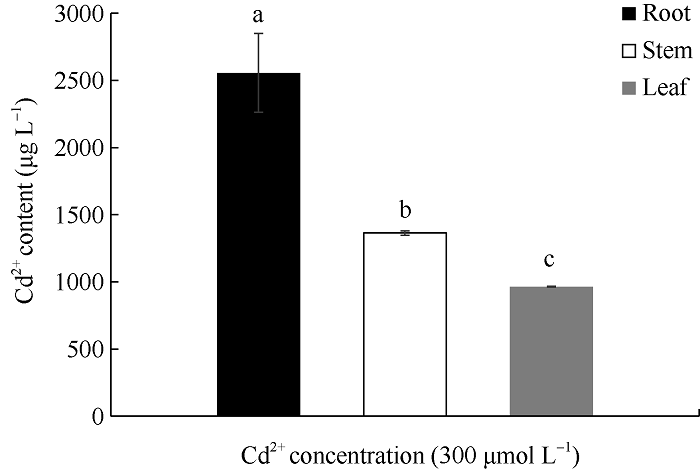 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1CdCl2胁迫对红麻不同部位镉含量的影响
不同小写字母表示在0.05水平差异显著。
Fig. 1Effects of CdCl2 stress on cadmium content of different parts in kenaf
Bars marked with different lowercase letters indicate significantly different at the 0.05 probability level.
2.3 镉胁迫对红麻幼苗根系DNA甲基化水平的影响
运用MSAP技术分析300 μmol L-1 CdCl2 胁迫下红麻幼苗根系的DNA甲基化水平变化情况, 部分代表性聚丙烯酰胺凝胶电泳结果见图2, 统计结果见表5。对照条件下, 幼苗根系的甲基化率、全甲基化率和半甲基化率分别为62.78%、37.5%和25.28%; 300 μmol L-1的CdCl2胁迫下幼苗根系的甲基化率、全甲基化率和半甲基化率分别为68.19%、36.37%和31.82%, 即该浓度的CdCl2胁迫使幼苗根系的甲基化率和半甲基化率升高, 全甲基化率变化较小。表明在整体水平上, 300 μmol L-1的CdCl2胁迫提高了红麻幼苗根系的DNA甲基化水平。图2
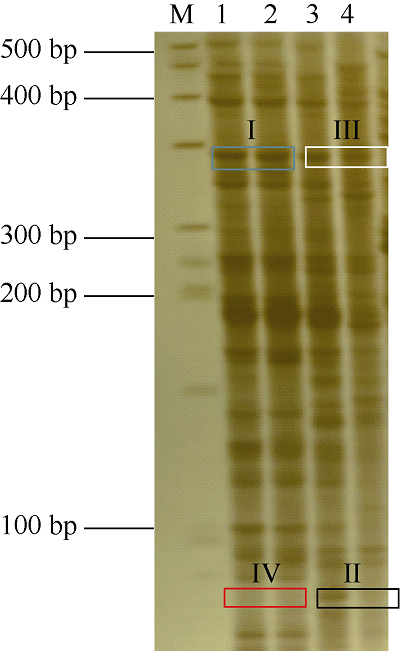 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2MSAP聚丙烯酰胺凝胶电泳图
泳道M代表BM50 DNA marker; 泳道1和3代表EcoR I/Hpa II酶切; 泳道2和4代表EcoR I/Msp I酶切。1和2: 0 μmol L-1 CdCl2; 3和4: 300 μmol L-1 CdCl2。蓝色方框; I型(无甲基化); 黑色方框; II型(半甲基化); 白色方框; III型(全甲基化); 红色方框; IV型(全甲基化)。
Fig. 2MSAP acrylamide gel detection
Lane M: BM50 DNA marker; Lanes 1 and 3 represent digestion with EcoR I/Hpa II; Lanes 2 and 4 represent digestion with EcoR I/Msp I. Lanes 1, 2 and 3, 4 represent CdCl2 concentration of 0 μmol L-1 and 300 μmol L-1, respectively. The blue frame, black frame, white frame, and red frame represent the type I (no methylation), type II (hemi-methylation), type III (full methylation), and type IV (full methylation), respectively.
Table 5
表5
表5DNA甲基化水平统计分析
Table 5
| 甲基化类型 Methylation type | 各类型的条带数和比率 Band numbers and ratio of each type | 变化比率(上升↑, 下降↓) Exchange rate (up↑, down↓) | |
|---|---|---|---|
| 0 μmol L-1 | 300 μmol L-1 | ||
| 类型I (无甲基化) Type I (Unmethylation) | 356 | 304 | ↓ 14.61 |
| 类型II (半甲基化) Type II (Hemi-methylation) | 242 | 305 | ↑ 26.03 |
| 类型III, IV (全甲基化) Type III and IV (Full methylation) | 359 | 348 | ↓ 3.06 |
| 半甲基化率Hemi-methylated ratio (%) | 25.28 | 31.87 | ↑ 26.07 |
| 全甲基化率Fully methylated ratio (%) | 37.50 | 36.36 | ↓ 3.04 |
| 甲基化率/MSAP Total methylated ratio/MSAP (%) | 62.78 | 68.23 | ↑ 8.68 |
新窗口打开|下载CSV
2.4 甲基化差异片段的测序及功能注释
本试验回收到157条甲基化差异条带, 测序比对到40条具有功能的DNA甲基化差异序列, 与植物抗逆性相关的甲基化差异序列见表6。其中16条DNA甲基化差异片段与响应植物抗逆性密切相关, 例如GH3.1 (Gretchen Hagen)基因通过调节植物体内激素的动态平衡, 参与调控植物的生长发育过程[18], NADP-ME (NADP-dependent malic enzyme)通过碳代谢来平衡细胞内的pH来防御生物和非生物胁迫[19], ABCG26 (ABC transporter G family member26)基因在八仙花根尖发生铝胁迫后, 参与响应铝胁迫[20], L-AAOh (L-ascorbate oxidase homolog)基因在调节植物发育过程和应激反应方面发挥重要作用[21], LRR-RLK (leucine-rich repeat receptor-like kinases)家族基因、GaTPPD (trehalose- phosphate phosphatase D)等在植物生长和胁迫反应中均起着重要作用[22]。Table 6
表6
表6甲基化差异片段比对分析
Table 6
| 序列编号 Sequence number | 甲基化差异片段比对结果 Comparisons of differentially methylated sequences | 功能注释 Functional annotation |
|---|---|---|
| 1 | Leucine-rich repeat receptor-like kinases, (LRR-RLK) At5g15730, Gossypium | 充当胞外信号的受体, 参与各种环境及发育信号的感知和传递[22]。 As the receptor of extracellular signal, it participates in the perception and transmission of various environmental and developmental signals[22]. |
| 2 | Zinc finger protein XlCGF8.2DB-like (XlCGF8.2DB), Bemisia tabaci gastrula | 参与一些重要的调控过程, 如; 形态建成、花粉发育、胚发育、胁迫反应等[23]。 Widely involved in regulatory of important processes, such as morphogenesis, pollen development, embryo development, stress response, and so on[23]. |
| 3 | Ankyrin-3-like, Gossypium arboreum | 尚未见报道。 Has not been reported. |
| 4 | Trehalose-phosphate phosphatase D (GaTPPD), Gossypium arboreum | 海藻糖磷酸磷酸酶在植物生长和胁迫反应中起重要作用[24]。 Trehalose phosphatase plays an important role in plant growth and stress response[24]. |
| 5 | Serine/threonine-protein kinase, Gossypium raimondii | 丝氨酸/苏氨酸蛋白激酶, 催化多种功能蛋白的磷酸化, 调控植物非生物胁迫[25]。 Serine/threonine protein kinases, catalyze the phosphorylation of various functional proteins and regulate abiotic stress in plants[25]. |
| 6 | Peroxidasin (Pxdn) transcript variant X6, Castor canadensis | 过氧化物酶, 植物抗逆过程中的关键酶之一[26]。 Peroxidase is one of the key enzymes in plant stress resistance[26]. |
| 7 | Protein NRT1/ PTR FAMILY 2.7 (NPF2.7), Gossypium hirsutum | NRT1/PTR家族蛋白参与转运植物激素及次生代谢物合成过程。 NRT1/PTR family proteins is involved in the transport of plant hormones and the synthesis of secondary metabolites. |
| 8 | 60S ribosomal protein L16(RPL16), Gossypium hirsutum | 参与细胞中蛋白质合成及调控基因表达[27]。 It is involved in protein synthesis and gene expression regulation[27]. |
| 9 | Receptor-like protein kinase At2g46850, Durio zibethinus | 类受体激酶通过接收和传递胞外信号调控细胞的生理反应, 参与植物生长发育过程。 Receptor like kinases are involved in plant growth and development by receiving and transmitting extracellular signals to regulate cell physiological responses. |
| 10 | Transcript variant X2, Gossypium raimondii | 转录变异体X2, 参与细胞增殖及细胞周期活动的调节。 Transcription variant X2 is involved in the regulation of cell proliferation and cell cycle activity. |
| 11 | 3-ketoacyl-CoA synthase 1 (GrKCS1), Gossypium raimondii | 3-酮脂酰辅酶A合成酶, 在抵抗干旱和盐害等非生物胁迫过程中起着重要的作用。 3-ketoacyl coenzyme A synthetase plays an important role in abiotic stresses such as drought and salt stress. |
| 12 | L-ascorbate oxidase homolog (L-AAOh), Gossypium hirsutum | 抗坏血酸氧化酶, 在调节植物发育过程和应激反应方面发挥重要作用[21]。 L-Ascorbic acid oxidase plays an important role in regulating plant development and stress response[21]. |
| 13 | Indole-3-acetic acid-amido synthetase (GH3.1), Camellia sinensis | 吲哚-3醋酸-酰胺合成酶, 通过调节植物体内激素的动态平衡, 参与调控植物的生长发育过程[18]。 Indole-3 acetate amide synthetase is involved in the regulation of plant growth and development by regulating the dynamic balance of hormones in plants[18]. |
| 14 | Acyl-CoA-binding domain-containing protein 4 (ACBD4) | 酰基辅酶A结合域蛋白, 在植物的生长发育、生物和非生物胁迫应答中起重要的作用。 Acyl-CoA-binding domain proteins play an important role in plant growth and development, biological and abiotic stress response. |
| 序列编号 Sequence number | 甲基化差异片段比对结果 Comparisons of differentially methylated sequences | 功能注释 Functional annotation |
| 15 | ABC transporter G family member 26 (ABCG26), Gossypium arboreum | ABC 转运蛋白G家族参与植物信号转导、次生代谢物运输和非生物胁迫响应等过程[20]。 ABC transporter G family are involved in plant signal transduction, secondary metabolite transport and abiotic stress response[20]. |
| 16 | NADP-dependent malic enzyme (NADP-ME) At1G65930, Gossypium hirsutum | NADP依赖苹果酸酶, 通过苹果酸代谢来平衡细胞内的pH来防御生物和非生物胁迫[19]。 NADP relies on malic acid enzyme to balance intracellular pH through malate metabolism to protect against biotic and abiotic stresses[19]. |
| 17 | NAC domain-containing protein 71 (GrNAC71) | NAC转录因子, 在逆境胁迫信号转导过程中发挥重要作用[28]。 NAC transcription factor plays an important role in stress signal transduction[28]. |
| 18 | F-box protein At3g07870, Gossypium raimondii | 参与调控胞内蛋白降解、受体识别和信号传导[29]。 It is involved in the regulation of intracellular protein degradation, receptor recognition and signal transduction[29]. |
| 19 | Pentatricopeptide repeat-containing protein (PPRC) At5g55740, Gossypium arboreum | 从RNA剪切、编辑、降解和翻译等多个方面影响细胞器中RNA的新陈代谢[30]。 RNA metabolism in organelles is affected by RNA splicing, editing, degradation, and translation[30]. |
| 20 | Ethylene-responsive transcription factor 2 (ERF2) | 乙烯转录因子(AP2/ERFs)调控激素响应非生物胁迫[31]。 Ethylene transcription factors (AP2/ERFs), widely involved in plant hormone regulation and thus response to abiotic stress[31]. |
新窗口打开|下载CSV
2.5 甲基化差异基因qRT-PCR分析
为分析DNA甲基化变化对基因表达的影响, 挑选9个响应植物非生物胁迫的甲基化差异基因进行qRT-PCR分析。由图3可知, 7个基因在镉胁迫下的表达量与对照呈显著性差异, 其中基因GH3.1、GrKCS1、NADP-ME、L-AAOh、TPP-D、NPF2.7的表达量在CdCl2胁迫下分别显著升高8.80、1.46、0.94、5.22、1.37、1.85倍, LRR-RLKs的表达量显著降低0.59倍。说明DNA甲基化的变化与基因表达水平的改变密切相关。图3
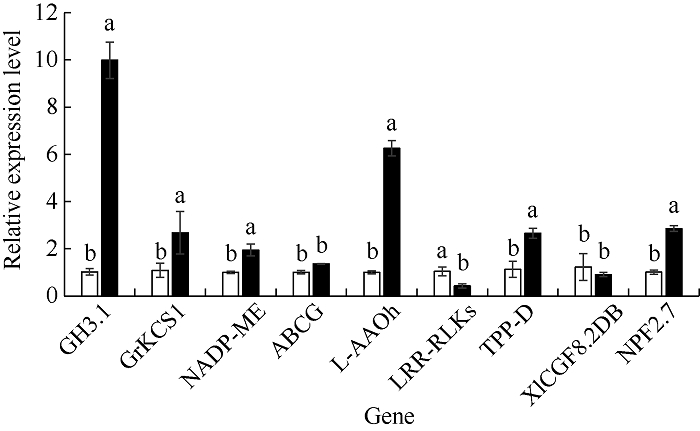 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3甲基化差异基因的qRT-PCR分析
不同小写字母表示处理在0.05水平差异显著。
Fig. 3qRT-PCR detection of the differentially methylated genes
Bars marked with different lowercase letters indicate significantly different at the 0.05 probability level among treatments.
3 讨论
3.1 红麻可以作为修复镉污染土壤的潜在作物
本研究结果表明, 300 μmol L-1镉胁迫虽然显著抑制了红麻幼苗的生长, 但是并不致死[32], 而且镉积累能力远超水稻、玉米、木薯[33,34,35]等其他作物。根据本试验中测得的红麻对镉的吸附量, 以及本课题组之前对国内外众多常规品种研究得出, 每公顷红麻对镉吸附量为102.9~125.8 g, 并且对其生长无显著影响。Sarra等[36]在镉和锌含量高的淤泥里进行红麻和玉米的修复试验发现, 红麻茎中镉和锌的含量分别2.49 mg kg-1和82.50 mg kg-1, 玉米茎中镉和锌的含量分别为2.10 mg kg-1和10.19 mg kg-1, 两者都积累了可观的镉和锌, 红麻中的锌含量是玉米的8倍多, 并且对其产量影响更小, 说明红麻具有更强的重金属修复和安全利用价值。栗原宏幸等[37]发现, 红麻对镉污染土壤具有明显的修复效果, 并且对红麻的产量无显著影响。因此可以在生产上利用红麻极强的镉耐性及吸附能力改良重金属污染土壤。3.2 300 µmol L-1的CdCl2胁迫使红麻幼苗的DNA甲基化水平提高
植物遭受非生物胁迫后, 通过激活胁迫响应机制或终止某些转录因子的表达来调控植物的生长, DNA甲基化在植物的整个生命周期中起着非常重要的作用, 例如参与逆境胁迫的响应, 应对不良环境带来的影响, 抵御非生物胁迫的毒害等[38]。在重金属、高盐和干旱等逆境胁迫中, 植物都能通过DNA甲基化的水平变化来参与调控相关基因的表达, 提高植物抗逆性, 维持植物的正常生长[39]。本结果表明, 红麻幼苗在300 μmol L-1的CdCl2 胁迫下根系的DNA甲基化率、半甲基化率较对照分别提高8.68%和26.07%, 全甲基化率降低3.04%, 在整体水平上, 300 μmol L-1的CdCl2胁迫提高了红麻幼苗根系的DNA甲基化水平, 这与何玲莉等[40]和王丙莲等[41]研究结果一致。殷欣[42]研究发现, 大豆DNA甲基化水平变化在镉胁迫下升高, 并且与镉胁迫浓度正相关, 某些功能蛋白还广泛参与大豆响应逆境胁迫的过程。
3.3 DNA甲基化参与响应逆境胁迫相关基因的表达
本研究对与植物抗逆性密切相关的基因进行qRT-PCR分析发现, 在镉胁迫下有7个甲基化差异基因的表达水平存在显著差异, 推测植物受到镉胁迫后, DNA甲基化水平变化参与调控基因的表达, 从而参与红麻对逆境的响应。NADP-ME酶通过苹果酸代谢平衡细胞内的pH来防御生物和非生物胁迫[19]。Fu等[43]研究发现, 小麦植株中的NADP-ME酶在NaCl和PEG胁迫处理下的酶活性升高, 表达量下降, 并且该参与了对逆境胁迫的响应。本研究中NADP-ME酶在镉胁迫下基因表达量升高了0.94倍, 并且甲基化水平发生了改变。推测甲基化水平的变化参与到了该基因的表达量变化中, 并最终在响应镉胁迫中起到了重要的作用。吲哚-3醋酸-酰胺合成酶家族能够维持植物内源激素的动态平衡, 参与调控植物的生长发育过程[18]。Park等[44]研究中发现, GH3基因在低温胁迫中基因表达量降低, 但仍被强烈诱导, 抑制自由态的生长素含量增加, 提高拟南芥抗逆性。本研究中本吲哚-3醋酸-酰胺合成酶基因GH3.1在镉胁迫下发生了去甲基化, 基因表达量显著升高了8.8倍, 因此推测其通过甲基化模式的变化参与调控基因表达、维持激素动态平衡, 并最终在响应镉胁迫中起到重要作用。抗坏血酸氧化酶(L-ascorbate oxidase, L-AAO)在植物生长发育过程和调节应激反应方面具有重要作用[21]。Parihar等[45]研究发现, 在多种逆境胁迫下, 抗坏血酸含量升高, 可以提高抗逆性。本研究中抗坏血酸氧化酶家族L-AAOh基因在镉胁迫下基因表达量显著升高了5.2倍, 并且甲基化水平发生了变化。推测甲基化水平的变化参与到了该基因的表达量变化中, 并最终在响应镉胁迫中起到了重要作用。
NRT1/PTR家族蛋白在植物转运硝酸盐、氨基酸和植物激素过程中起重要的作用[46]。在本研究中, 甲基化差异NRT1/PTR家族基因NPF2.7的表达量在镉胁迫下显著升高了1.8倍, 推测该基因可能参与到了植物的逆境胁迫响应中。海藻糖(trehalose- phosphate phosphatase)在逆境胁迫中可以起到抵抗保护作用, 提高植物的抗逆性[47]。丁泽红等[48]研究发现, 木薯在干旱胁迫下, MeTPP6基因的表达量显著上升, 并参与了对干旱胁迫的响应。本研究中GaTPPD基因在镉胁迫下的表达量显著升高了1.35倍, 并且其甲基化水平发生了变化, 推测DNA甲基化调控该基因表达水平, 并最终在响应镉胁迫中起到了重要作用。
4 结论
红麻对镉胁迫具有较强的耐受性, 在300 μmol L-1的镉胁迫下, 红麻幼苗的长势受到抑制, 但并没有严重影响其生长, 并且镉富集量大, 说明红麻具有较强的土壤修复潜力, 因此可以利用红麻来修复重金属镉污染农田。红麻幼苗在300 μmol L-1 CdCl2胁迫下, 根系DNA甲基化水平升高, 与响应逆境胁迫相关的GH3.1基因、ABC转运蛋白家族基因、NADP-ME基因、结合转运蛋白G家族基因、抗坏血酸氧化酶基因、LRR-RLKs家族基因、海藻糖磷酸磷酸酶基因、NRT1/PTR家族蛋白的DNA甲基化和基因表达水平都发生了显著变化。DNA甲基化水平变化可能参与了响应逆境胁迫相关基因的表达调控, 并最终在红麻响应镉胁迫中起到了重要的作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOIPMID [本文引用: 1]

A new adsorbent, polyhydroxybutyrate-b-polyethyleneglycol, was used for the separation and preconcentration of copper(II) and lead(II) ions prior to their flame atomic absorption spectrometric detections. The influences of parameters such as pH, amount of adsorbent, flow rates and sample volumes were investigated. The polymer does not interact with alkaline, alkaline-earth metals and transition metals. The enrichment factor was 50. The detection limits were 0.32 μg L(-1) and 1.82 μg L(-1) for copper and lead, respectively. The recovery values were found >95%. The relative standard deviations were found to be less than 6%. The validation of the procedure was performed by analysing certified reference materials; NIST SRM 1515 Apple leaves, IAEA-336 Lichen and GBW-07605 Tea. The method was successfully applied for the analysis of analytes in water and food samples.Copyright © 2013 Elsevier Ltd. All rights reserved.
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

Gene expression driven by developmental and stress cues often depends on nucleosome histone post-translational modifications and sometimes on DNA methylation. A number of studies have shown that these DNA and histone modifications play a key role in gene expression and plant development under stress. Most of these stress-induced modifications are reset to the basal level once the stress is relieved, while some of the modifications may be stable, that is, may be carried forward as 'stress memory' and may be inherited across mitotic or even meiotic cell divisions. Epigenetic stress memory may help plants more effectively cope with subsequent stresses. Comparative studies on stress-responsive epigenomes and transcriptomes will enhance our understanding of stress adaptation of plants.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 1]

Kenaf is an annual crop that is widely cultivated as a source of bast (phloem) fibres, the phytoremediation of heavy metal-contaminated farmlands and textile-relevant compounds. Leaf shape played a unique role in kenaf improvement, due to the inheritance as a single locus and the association with fibre development in typical lobed-leaf varieties. Here we report a high-quality genome assembly and annotation for var. 'Fuhong 952' with 1078 Mbp genome and 66 004 protein-coding genes integrating single-molecule real-time sequencing, a high-density genetic map and high-throughput chromosome conformation capture techniques. Gene mapping assists the identification of a homeobox transcription factor LATE MERISTEM IDENTITY 1 (HcLMI1) gene controlling lobed-leaf. Virus-induced gene silencing (VIGS) of HcLMI1 in a lobed-leaf variety was critical to induce round (entire)-like leaf formation. Candidate genes involved in cell wall formation were found in quantitative trait loci (QTL) for fibre yield and quality-related traits. Comparative genomic and transcriptome analyses revealed key genes involved in bast fibre formation, among which there are twice as many cellulose synthase A (CesA) genes due to a recent whole-genome duplication after divergence from Gossypium. Population genomic analysis showed two recent population bottlenecks in kenaf, suggesting domestication and improvement process have led to an increase in fibre biogenesis and yield. This chromosome-scale genome provides an important framework and toolkit for sequence-directed genetic improvement of fibre crops.© 2020 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
[本文引用: 4]
DOIURL [本文引用: 4]
URL [本文引用: 3]
URL [本文引用: 3]
URL [本文引用: 4]
URL [本文引用: 4]
[本文引用: 3]
[本文引用: 3]
[本文引用: 2]
[本文引用: 2]
DOIURL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOIPMID [本文引用: 2]

Long-chain alk(a/e)nes represent the major constituents of conventional transportation fuels. Biosynthesis of alkanes is ubiquitous in many kinds of organisms. Cyanobacteria possess two enzymes, acyl-acyl carrier protein (acyl-ACP) reductase (AAR) and aldehyde-deformylating oxygenase (ADO), which function in a two-step alkane biosynthesis pathway. These two enzymes act in series and possibly form a complex that efficiently converts long chain fatty acyl-ACP/fatty acyl-CoA into hydrocarbon. While the structure of ADO has been previously described, structures of both AAR and AAR-ADO complex have not been solved, preventing deeper understanding of this pathway. Here, we report a ligand-free AAR structure, and three AAR-ADO complex structures in which AARs bind various ligands. Our results reveal the binding pattern of AAR with its substrate/cofactor, and suggest a potential aldehyde-transferring channel from AAR to ADO. Based on our structural and biochemical data, we proposed a model for the complete catalytic cycle of AAR.
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

Plant growth and development are tightly controlled in response to environmental conditions that influence the availability of photosynthetic carbon in the form of sucrose. Trehalose-6-phosphate (T6P), the precursor of trehalose in the biosynthetic pathway, is an important signaling metabolite that is involved in the regulation of plant growth and development in response to carbon availability. In addition to the plant's own pathway for trehalose synthesis, formation of T6P or trehalose by pathogens can result in the reprogramming of plant metabolism and development. Developmental processes that are regulated by T6P range from embryo development to leaf senescence. Some of these processes are regulated in interaction with phytohormones, such as auxin. A key interacting factor of T6P signaling in response to the environment is the protein kinase sucrose non-fermenting related kinase-1 (SnRK1), whose catalytic activity is inhibited by T6P. SnRK1 is most likely involved in the adjustment of metabolism and growth in response to starvation. The transcription factor bZIP11 has recently been identified as a new player in the T6P/SnRK1 regulatory pathway. By inhibiting SnRK1, T6P promotes biosynthetic reactions. This regulation has important consequences for crop production, for example, in the developing wheat grain and during the growth of potato tubers.
[本文引用: 1]
[本文引用: 1]
