 ,1, 王刘艳1, 雷维1,2, 吴家怡1, 史红松1, 李晨阳1, 唐章林1,2, 李加纳1,2, 周清元
,1, 王刘艳1, 雷维1,2, 吴家怡1, 史红松1, 李晨阳1, 唐章林1,2, 李加纳1,2, 周清元 ,1,2,*, 崔翠
,1,2,*, 崔翠 ,1,*
,1,*Screening candidate genes related to aluminum toxicity stress at germination stage via RNA-seq and QTL mapping in Brassica napus L.
WANG Rui-Li ,1, WANG Liu-Yan1, LEI Wei1,2, WU Jia-Yi1, SHI Hong-Song1, LI Chen-Yang1, TANG Zhang-Lin1,2, LI Jia-Na1,2, ZHOU Qing-Yuan
,1, WANG Liu-Yan1, LEI Wei1,2, WU Jia-Yi1, SHI Hong-Song1, LI Chen-Yang1, TANG Zhang-Lin1,2, LI Jia-Na1,2, ZHOU Qing-Yuan ,1,2,*, CUI Cui
,1,2,*, CUI Cui ,1,*
,1,*通讯作者: * 周清元, E-mail:qingyuan@swu.edu.cn;崔翠, E-mail:cuicui@swu.edu.cn
收稿日期:2020-10-27接受日期:2021-05-17网络出版日期:2021-06-09
| 基金资助: |
Corresponding authors: * E-mail:qingyuan@swu.edu.cn;E-mail:cuicui@swu.edu.cn
Received:2020-10-27Accepted:2021-05-17Published online:2021-06-09
| Fund supported: |
作者简介 About authors
E-mail:1525731297@qq.com

摘要
随着土壤酸化程度的加剧, 铝毒害已成为影响种子萌发质量和作物产量的重要胁迫因子之一。为研究铝毒对油菜种子萌发过程影响的分子机理, 本文采用RNA-seq技术对耐铝品系18D300和敏铝品系27011进行转录组分析, 共检测到9344个显著差异表达基因[|log2(fold change)|≥1和FDR≤0.05], 其中4406个DEGs基因上调, 4938个DEGs下调。GO富集分析发现, 差异表达基因主要与氧化反应、细胞碳水化合物代谢过程、转运蛋白活性等相关。KEGG富集分析表明, 差异表达基因主要富集于苯丙烷生物合成、淀粉和蔗糖代谢、MAPK信号通路-植物、植物-病原体相互作用、植物激素信号转导等途径。此外, 通过整合RNA转录组测序和铝毒胁迫下油菜萌发期根系相关性状的QTL定位结果, 共筛出44个差异表达基因(10个下调和34个上调), 这些基因主要与氧化应激、渗透调节、细胞壁修饰、转运蛋白、激素信号传导等功能有关。
关键词:
Abstract
With the aggravation of soil acidification, aluminum toxicity has become one of the important stress factors affecting seed germination quality and crop yield. In order to study the molecular mechanism of the effect of aluminum toxicity on rapeseed seed germination, a total of 9344 significantly differentially expressed genes [log2 (fold change) ≥ 1 and FDR ≤ 0.05] were detected in the transcriptome analysis of aluminum-tolerant strain 18D300 and aluminum-sensitive strain 27011 by RNA-seq technology, among which 4406 DEGs (differentially expressed genes) were up-regulated and 4938 DEGs were down-regulated. GO enrichment showed that DEGs were mainly related to oxidation reaction, carbohydrate metabolism, and transporter activity. KEGG enrichment revealed that DEGs were mainly concentrated in phenylpropane biosynthesis, starch and sucrose metabolism, MAPK signal pathway-plant, plant-pathogen interaction, plant hormone signal transduction and so on. In addition, 44 DEGs (10 down-regulated and 34 up-regulated) were screened by integrating the results of RNA transcriptome sequencing and QTL mapping of root-related traits at germination stage under aluminum toxicity stress in rapeseed, which were mainly related to oxidative stress, osmotic regulation, cell wall modification, transporter, and hormone signal transduction.
Keywords:
PDF (4714KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王瑞莉, 王刘艳, 雷维, 吴家怡, 史红松, 李晨阳, 唐章林, 李加纳, 周清元, 崔翠. 结合RNA-seq分析和QTL定位筛选甘蓝型油菜萌发期与铝毒胁迫相关的候选基因. 作物学报, 2021, 47(12): 2407-2422 DOI:10.3724/SP.J.1006.2021.04231
WANG Rui-Li, WANG Liu-Yan, LEI Wei, WU Jia-Yi, SHI Hong-Song, LI Chen-Yang, TANG Zhang-Lin, LI Jia-Na, ZHOU Qing-Yuan, CUI Cui.
铝是自然界中较丰富的金属元素, 在地壳中的含量仅次于硅和氧而居于第3位[1]。在酸性土壤条件中(pH<5), 结合态铝解离成有毒害作用的活性铝[2], 而活性铝是制约作物生长的重要因素之一[3]。研究表明, 铝离子会破坏根分生组织的细胞活性, 抑制细胞有丝分裂和根系生长, 影响营养吸收, 植物会表现出萎蔫矮小, 叶片黄化卷曲、老化或坏死等形态特征, 最终限制作物生长和产量[4]。油菜是重要的油料作物, 我国长江以南酸性土壤种植区每年因铝毒危害造成的油菜减产相当严重, 直接影响到我国油菜籽的产量和品质[5]。
生物对各种胁迫因子的应答, 本质上都是基因差异表达的结果。比较同一类细胞或不同类细胞在不同生理状态下的基因表达差异, 对研究生物生长发育调控及代谢机制具有重要意义。自然界中, 植物为适应和抵御铝毒胁迫, 部分植物进化出一系列的形态、生理和分子适应机制。铝毒胁迫下, 植物可以通过减轻氧化损伤、调节激素水平、分泌有机酸、启动酶保护系统、修饰细胞壁、增加逆境蛋白及脯氨酸等渗透调节物质等来阻止或减轻植物铝毒害[6]。近年来, 高通量测序技术发展迅速, 研究人员利用遗传学和基因组学在多种作物中筛选出上百个耐铝毒胁迫基因, 部分基因已被证实在作物抗性改良中具有潜在应用价值[7,8,9,10]。在拟南芥中超量表达ABC家族的蛋白复合体STAR1基因是耐铝性相关的最重要的转录因子之一[7]。基因TaALMT1在小麦根细胞中表达, 可以打通有机酸阴离子的通道, 有利于根尖分泌出苹果酸, 增强植物耐铝性[8]。Chen等[9]研究发现, 转基因烟草叶片中过表达的苹果酸脱氢酶可通过增强苹果酸的合成从而增强作物的耐铝性。还有如谷胱甘肽S转移酶(GST)、过氧化氢酶(CAT)细胞色素P450抗氧化基因的表达在防御铝毒害的机制中起重要作用[10]。目前转录组测序技术获得转录信息比较广泛, 但差异基因数据较大, 很难筛选出关键的基因。而数量性状位点(quantitative trait locus, QTL)是通过提供基因组区域来确定基因的筛选[11,12], 可以减少筛选基因的时间和成本。因此, 部分研究将QTL定位和转录组分析相结合对植物的农艺性状和抗逆性进行分析, 从而找出与农艺性状或胁迫相关的候选基因及关键的代谢通路[13,14,15]。李明等[13]对甜玉米自交系耐铝亲本T56和铝敏亲本T49构建的F2:3家系苗期经铝处理后根尖组织的5个性状进行了QTL定位, 并对亲本T49和T56进行转录组分析发现, 亲本之间存在3532个差异表达基因, 其中1901个上调基因, 1631个下调基因。Wang等[14]通过整合的QTL作图和RNA测序分析, 筛选了编码热击蛋白和磷酸果糖激酶等7个基因作为参与种子衰老的候选基因。Jian等[15]通过QTL定位和RNA-Seq技术鉴定了33个与甘蓝型油菜叶片形态性状相关的候选基因。这些研究为人们了解植物的抗逆机制、生长发育机制及分子辅助育种提供了可靠依据。尽管利用QTL定位和转录组技术等方法解析了多种作物的农艺性状或逆境胁迫的应答机制, 但尚未发现有关于基于QTL和转录组分析方法对甘蓝型油菜耐铝毒机制的研究。
本研究从甘蓝型油菜重组自交系(recombinant inbred lines, RILs)群体中筛选出的耐铝品系18D300和敏铝品系27011作为材料, 通过转录组测序和QTL定位相结合, 筛选油菜萌发期耐铝毒相关基因, 并结合分子水平和生理来探讨油菜对铝毒胁迫的响应机制, 旨在为筛选和培育耐铝毒油菜种质资源奠定基础。
1 材料与方法
1.1 试验材料和生长条件
由母本10D130 (敏铝品系)和父本中双11号(耐铝品系, 简称ZS11)进行杂交, 采用单粒传法连续自交7代获得含有182份株系的重组自交系(RILs)群体。其中, ZS11是中国农业科学院油料作物研究所选育的常规优质油菜品种, 10D130是由西南大学油菜工程技术研究中心通过羽衣甘蓝和芥菜型油菜进行种间杂种, 后代选育中获得的衍生品系。2017—2018年在重庆市北碚区歇马镇油菜试验基地种植该重组自交系群体, 在油菜初花期进行套袋自交, 种子完全成熟后每株系收取5株正常植株的种子, 自然风干后保存。收获的种子进行铝毒胁迫发芽试验, 7 d后, 测量各株系相对根长值, 在RILs中选取耐铝品系18D300 (R系)和敏铝品系27011 (S系)为试验材料。所有材料均由重庆市油菜工程技术研究中心提供。1.2 试验处理
甘蓝型油菜耐铝品系18D300 (R系)和敏铝品系27011 (S系)分别在蒸馏水和由试剂A1Cl3·6H2O配置成80 µg mL-1铝毒溶液中培养7 d, 第7天时将发芽种子的根部组织切下装入离心管中, 立刻放进液氮中, 并在-80℃下保存进行生理指标测定和转录组测序。R系和S系对照组和处理组分别命名S0、ST、R0、RT。1.3 RNA测序和差异表达基因筛选
取S0、ST、R0、RT根部组织0.5 g左右用于构建转录组文库。由上海派森诺生物科技有限公司完成RNA-seq测序和原始数据分析。由表1可知, 4个样品中共获得197,533,456个原始读数, 其中181,472,174个干净读数, 27,220,826,100碱基。97%以上和93%以上的干净读数的质量分数分别为Q20和Q30 (碱基识别准确率在99.0%和99.9%的碱基所占百分比), 表明该测序数据质量高, 可用于后续的研究分析。按照生物过程(biological process, BP)、细胞组分(cellular component, CC)和分子功能(molecular function, MF)对S0 vs R0、ST vs RT、S0 vs ST和R0 vs RT四组的差异表达基因进行GO富集分析, 找出差异基因显著富集的GO term, 确定差异基因行使的主要生物学功能。利用KEGG (Kyoto Encyclopedia of Genes and Genomes)途径富集分析, 找出与整个基因组背景中, 在差异表达基因中显著富集的通路。结合油菜QTL置信区间鉴定的所有基因[16], 进一步筛选出与油菜铝毒胁迫相关的基因。筛选差异表达基因条件为: 表达差异倍数|log2(fold change)|≥1, 显著性P- value<0.05。Table 1
表1
表1测序质量分析
Table 1
| 样品名称 Sample name | S0 | R0 | ST | RT | 总数 Total |
|---|---|---|---|---|---|
| 原始读数Raw reads | 48,585,792 | 55,778,592 | 45,860,428 | 47,308,644 | 197,533,456 |
| 干净读数Clean reads | 44,642,424 | 51,491,214 | 41,819,768 | 43,518,768 | 181,472,174 |
| 干净碱基Clean data (bp) | 6,696,363,600 | 7,723,682,100 | 6,272,965,200 | 6,527,815,200 | 27,220,826,100 |
| N (%) | 0.001385 | 0.00138 | 0.001418 | 0.00137 | |
| Q20 (%) | 97.57 | 97.59 | 97.72 | 97.43 | |
| Q30 (%) | 94.14 | 94.22 | 94.43 | 93.74 |
新窗口打开|下载CSV
1.4 实时定量PCR验证
根据整合转录组与QTL定位结果获得候选基因的基因序列, 选择6个候选基因, 利用Vector NTI软件设计出基因扩增引物(表2), 然后由生工生物公司合成相关引物, 以BraACTIN7基因为内参基因[17]。利用SYBR染料法在CFX96 Real-time PCR Detection System上进行实时荧光定量PCR检测。由Vazyme公司的ChamQ Universal SYBR qPCR Master Mix试剂盒开展RT-PCR试验。Table 2
表2
表2用于qRT-PCR的候选差异表达基因引物
Table 2
| 基因名称 Gene name | 正向引物 Forward primer (5°-3°) | 反向引物 Reverse primer (5°-3°) |
|---|---|---|
| BraACTIN7 | GGAGCTGAGAGATTCCGTTG | GAACCACCACTGAGGACGAT |
| BnaA03g47720D | ACAAAGCAATGCACTCTTTAGG | GTTGTTGAAGACTCTGCAGTTT |
| BnaC03g62130D | AACAAAGACAAACCTGGGACTA | CTTGTTGATCGAACGGTAATCG |
| BnaA09g02030D | CTTCTGCTCAAGGCTCT | GGGTTTCCCAATGTTAG |
| BnaA03g03740D | GAAGTATGTGAGGATGGAGAGG | GATCTTCTCCAAATCAGCGTTC |
| BnaA03g53200D | GCCATTGTTGTATATTGGGCAT | TGATGTGTATGAAACAAGCAGC |
| BnaC03g65590D | ACGTTGAGCCAAACAGAGTG | CTATTATCGTAGACACCGGACG |
新窗口打开|下载CSV
1.5 生理生化指标测定
分别称取R0、RT、S0、ST根部组织各0.1 g左右, 采用上海优选生物科技有限公司的试剂盒测定其过氧化氢酶(peroxidase, POD)、过氧化氢酶(catalase, CAT)超氧化物歧化酶(superoxidedismutase, SOD)的活性, 以及脯氨酸(proline, Pro)、丙二醛(malondialdehyde, MDA)、苯丙氨酸(phenylalanine, PAL)及可溶性糖含量(soluble sugar, SUG)的含量。采用愈创木酚法测定POD活性; 采用紫外吸收法测定CAT活性; 采用氮蓝四唑(NBT)还原法测定SOD活性。采用茚三酮加热反应法测定Pr含量, 采用蒽酮比色法测定SUG含量, 以上指标测定均参照《植物生理学实验技术》[18]进行。采用硫代巴比妥酸(TBA)反应法[19]测定MDA含量, 采用Nairp法[20]测定PAL活性。每处理中每个指标测3次重复。1.6 数据处理
采用Microsoft Excel 2010整理数据; 运用SAS 9.0对处理后的数据进行相关性分析。2 结果与分析
2.1 根系长度
由图1可知, 80 µg mL-1铝毒胁迫下, R系与S系的处理较对照相比根系长度均下降, 都受铝毒的抑制。但是RT较R0下降13.24%, 未达到显著水平, 而ST较S0显著下降24.64%, 说明铝毒更能抑制S系的根长。图1
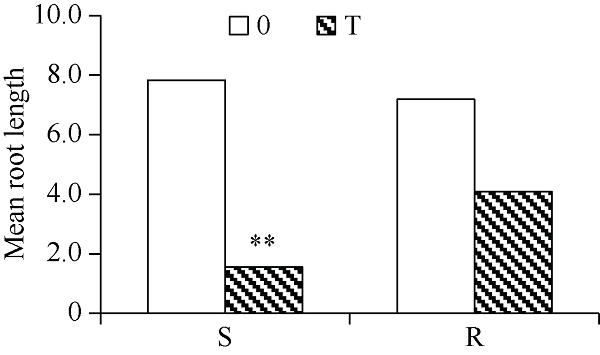 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1不同耐性材料的根长均值
0代表对照; T代表处理; S代表敏铝品系; R代表耐铝品系。**表示在0.01水平差异显著。
Fig. 1Mean root length of different resistant materials
0 represents the control; T represents the treatments; S represents the sensitive strain to aluminum; R represents the resistant strain to aluminum. **: P < 0.01.
2.2 DEG分析
由图2可知, 分别在S0与R0中识别2770个DEGs (1606个上调, 1164个下调), R0与RT中识别1447个DEGs (485个上调, 962个下调), S0与ST中识别2358个DEGs (591个上调, 1767个下调), ST与RT中识别2769个DEGs (1724个上调, 1045个下调)。总体来看, R0 vs RT和S0 vs ST材料的下调表达的基因数量明显多于上调表达的基因数量, 说明在铝毒胁迫下, 植物主要通过基因的负调控提高对逆境的抵抗能力。图2
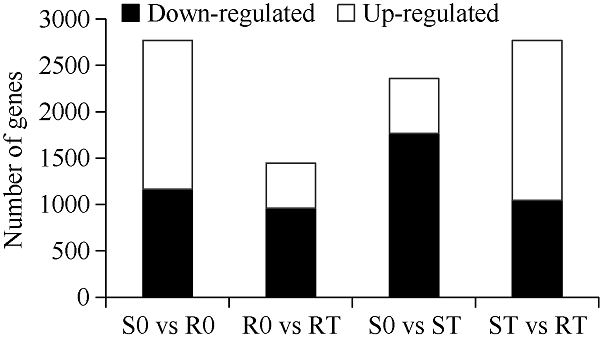 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图24个文库中所有差异基因的比较结果
样品名称缩写同
Fig. 2Differentially expressed genes in four libraries
Abbreviations of sample name are the same as those given in
应用Venny在线软件(
图3
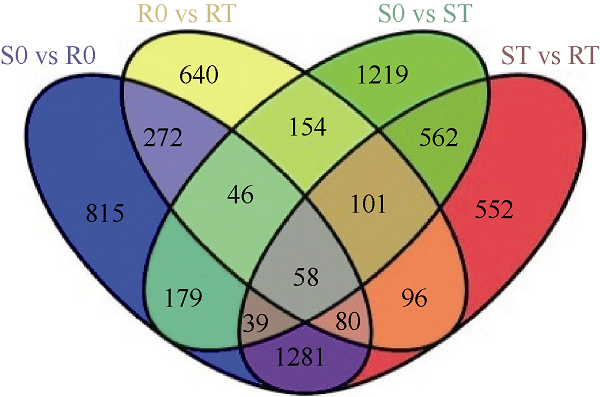 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3差异基因的维恩分析结果
样品名称缩写同
Fig. 3Venn analysis of differentially expressed genes
Abbreviations of sample name are the same as those given in
2.3 通过GO和KEGG途径分析对DEG进行功能分类
2.3.1 R0 vs RT和S0 vs ST的DEGs分析 由图4可知, 对S0 vs ST及R0 vs RT中的DEGs进行了GO功能注释, 结果2358个DEGs和1447个DEGs分别被分配给59个和60个GO条目。其中, 涉及生物过程主要包括跨膜运输过程、胁迫反应、氧化应激反应、细胞壁组织或生物合成、细胞碳水化合物代谢过程等; 分子功能中DEGs主要被注释到催化活性、氧化还原酶活性、血红素结合、四吡咯结合、活性跨膜转运蛋白活性等; 细胞成分中, DEGs主要富集在细胞膜和细胞膜固有成分等途径。为进一步了解基因的生物学功能, 对DEGs进行KEGG途径富集分析, 结果在R0 vs RT和S0 vs ST中的显著富集项分别有13个和8个, 包含5个是共同代谢途径, 以及8个和3个特殊途径(表3)。共同代谢途径涉及苯丙烷生物合成、MAPK信号通路-植物、氮素代谢、氰基氨基酸代谢、植物激素信号转导, 其中, 苯丙烷生物合成是这2组差异基因主要富集的通路(R0 vs RT中有55个DEG, S0 vs ST中有97个DEG), 说明不同铝毒耐性油菜品系既有共同路径, 也有独特途径来应对铝毒胁迫。图4
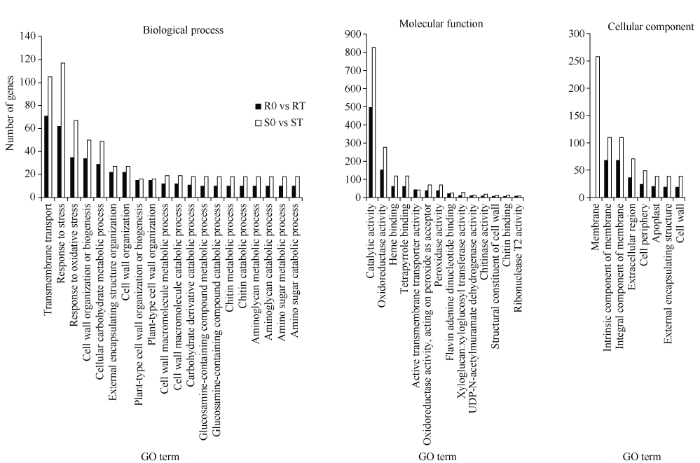 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4R0 vs RT和S0 vs ST中差异表达基因的GO分类
样品名称缩写同
Fig. 4GO classification of differentially expressed genes in S0 vs R0 and S0 vs ST
Abbreviations of sample name are the same as those given in
Table 3
表3
表3R0 vs RT和S0 vs ST中的KEGG显著富集分析
Table 3
| KEGG编号KEGG ID | KEGG术语 KEGG term | 基因数目 Genes number | 比值 Ratio (%) | 矫正后的P值 P-value | ||||
|---|---|---|---|---|---|---|---|---|
| R0 vs RT | ||||||||
| bna00940 | 苯丙烷生物合成Phenylpropanoid biosynthesis | 55 | 21.32 | 3.81844E-21 | ||||
| bna00500 | 淀粉和蔗糖代谢Starch and sucrose metabolism | 27 | 10.47 | 2.29236E-06 | ||||
| bna00910 | 氮素代谢Nitrogen metabolism | 13 | 5.04 | 1.81952E-05 | ||||
| bna00270 | 半胱氨酸和蛋氨酸代谢CySteine and methionine metabolism | 21 | 8.14 | 0.000110181 | ||||
| bna04016 | MAPK信号通路-植物MAPK signaling pathway-plant | 23 | 8.91 | 0.000143365 | ||||
| bna00010 | 糖酵解/糖异生Glycolysis/Gluconeogenesis | 21 | 8.14 | 0.000268893 | ||||
| bna00130 | 泛醌和其他萜类醌生物合成 Ubiquinone and other terpenoid-quinone biosynthesis | 11 | 4.26 | 0.001478368 | ||||
| bna00380 | 色氨酸代谢Tryptophan metabolism | 12 | 4.65 | 0.003486812 | ||||
| bna00360 | 苯丙氨酸代谢Phenylalanine metabolism | 10 | 3.88 | 0.006641539 | ||||
| KEGG编号KEGG ID | KEGG术语 KEGG term | 基因数目 Genes number | 比值 Ratio (%) | 矫正后的P值 P-value | ||||
| bna00460 | 氰基氨基酸代谢Cyanoamino acid metabolism | 12 | 4.65 | 0.006641539 | ||||
| bna00350 | 酪氨酸代谢Tyrosine metabolism | 9 | 3.49 | 0.007588613 | ||||
| bna04075 | 植物激素信号转导Plant hormone signal transduction | 34 | 13.18 | 0.007588613 | ||||
| bna00052 | 半乳糖代谢Galactose metabolism | 10 | 3.88 | 0.009316627 | ||||
| S0 vs ST | ||||||||
| bna00940 | 苯丙烷生物合成Phenylpropanoid biosynthesis | 97 | 31.80 | 5.89634E-43 | ||||
| bna04016 | MAPK信号通路-植物MAPK signaling pathway-plant | 38 | 12.46 | 9.09024E-08 | ||||
| bna00910 | 氮素代谢Nitrogen metabolism | 16 | 5.25 | 2.123E-05 | ||||
| bna00460 | 氰基氨基酸代谢Cyanoamino acid metabolism | 19 | 6.23 | 0.000428636 | ||||
| bna04075 | 植物激素信号转导Plant hormone signal transduction | 52 | 17.05 | 0.001727744 | ||||
| bna00520 | 氨基糖和核苷酸糖代谢 Amino sugar and nucleotide sugar metabolism | 27 | 8.85 | 0.004361711 | ||||
| bna04626 | 植物-病原体相互作用Plant-pathogen interaction | 35 | 11.48 | 0.004455032 | ||||
| bna00040 | 戊糖和葡糖醛酸相互转化 Pentose and glucuronate interconversions | 21 | 6.89 | 0.005458802 | ||||
新窗口打开|下载CSV
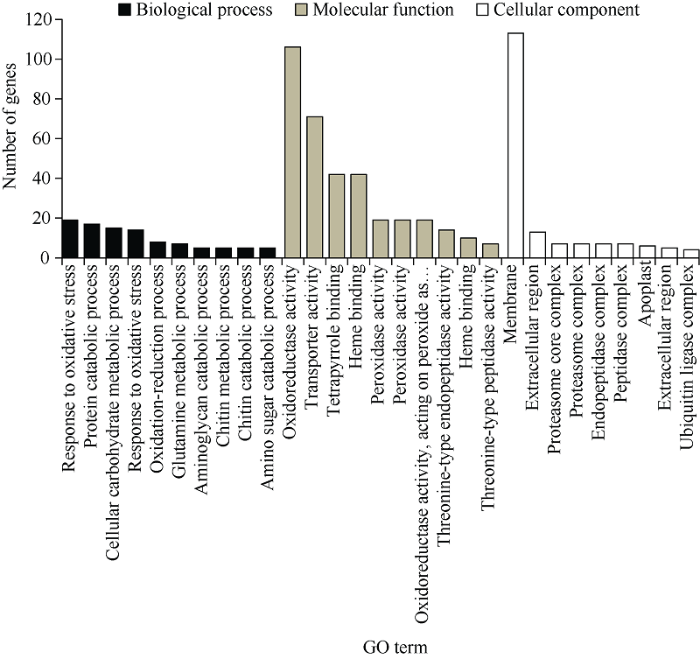
2.3.2 铝毒处理下不同品系DEGs分析 为阐明R品系和S品系受到铝毒胁迫后产生共同的耐铝毒机制, 故在RT vs ST组去除R0 vs S0组后的显著差异基因进行GO和KEGG分析, 共有618个差异基因被划分到29个GO显著条目中(图5)。生物过程中主要涉及氧化应激反应、蛋白质分解过程、细胞碳水化合物代谢过程、氧化还原过程、谷氨酰胺代谢等过程; 在分子过程中, 差异基因主要被注释到氧化还原酶活性、转运蛋白活性、四吡咯结合、血红素结合、过氧化物酶活性等方面; 细胞组分中则主要涉及细胞膜、胞外区、蛋白酶体复合体、质外体、泛素连接酶复合物等方面。此外, 2组共鉴定出11条KEGG富集通路(图6), 分别是关于苯丙烷生物合成、蛋白酶体、半乳糖代谢、氮素代谢、内质网中的蛋白质加工、糖酵解/糖异生、淀粉和蔗糖代谢、精氨酸生物合成、托烷、哌啶和吡啶生物碱生物合成、维生素B6代谢、α-亚麻酸代谢途径。其中差异基因主要富集于苯丙烷生物合成代谢途径。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5S0 vs R0和ST vs RT中差异基因的GO分类
样品名称缩写同
Fig. 5GO classification of differentially expressed genes in S0 vs R0 and ST vs RT
Abbreviations of sample name are the same as those given in
图6
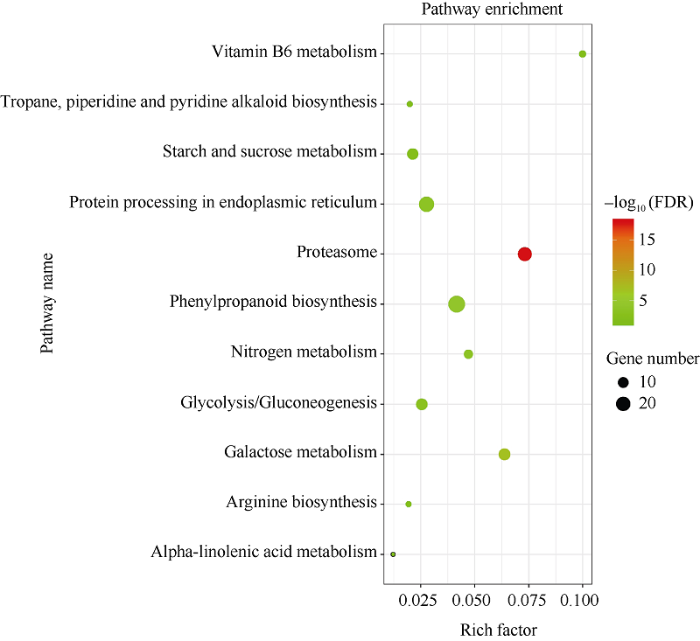 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6S0 vs R0和ST vs RT中前11个重要KEGG途径的富集因子散点图
横轴代表富集因子, 纵轴代表代谢通路。气泡所在的位置代表着富集的条目, 气泡的大小表示差异基因的数量, 气泡的颜色代表富集显著的程度。样品名称缩写同
Fig. 6Enrichment factor scatter plot of the top 11 significant KEGG pathways in S0 vs R0 and ST vs RT
The horizontal axis represents enrichment factors, and the vertical axis represents metabolic pathways. The location of bubbles represents the enrichment item, the size of bubbles represents the number of differential genes, and the color of bubbles represents the significant degree of enrichment. Abbreviations of sample name are the same as those given in
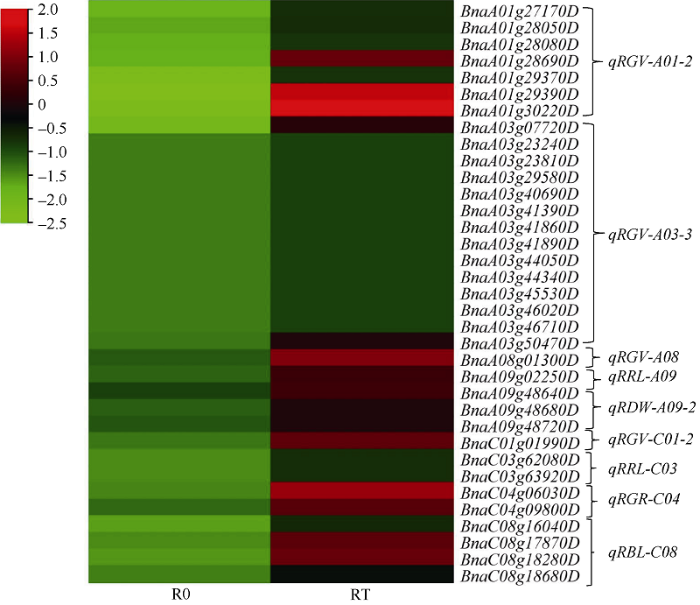
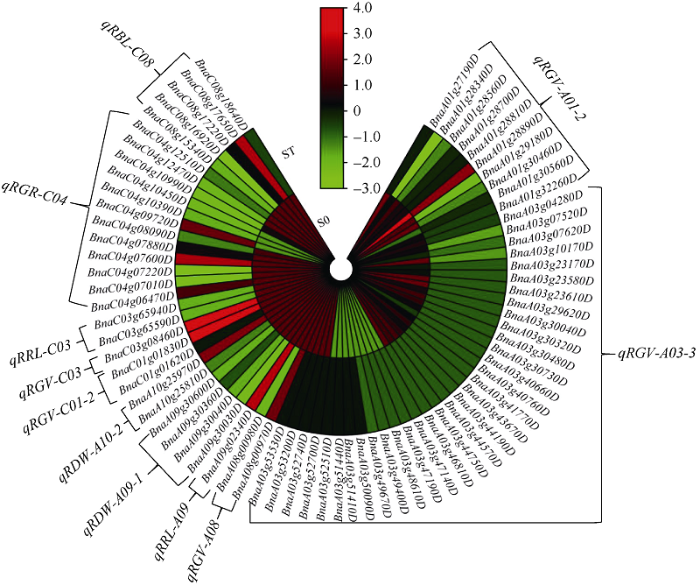
2.4 QTL定位与RNA测序结合后筛选的候选基因
将铝毒胁迫下油菜萌发期相关性状的QTL定位与转录组测序结果相结合, 根据拟南芥中同源基因注释,在R0 vs RT中筛出35个候选基因, 这些基因在R0中均表现为下调, 主要分布在qRGV-A01-2、qRGV- A03-3、qRGV-C03、qRBL-C08等8个QTL上(图7); 在S0 vs ST中筛出74个候选基因, 下调基因主要集中在ST中, 分布于qRGV-A01-2、qRGV-A03-3、qRGV-A08等11个QTL (图8); 而在ST vs RT中筛选出与铝毒胁迫相关的基因44个(11个下调和33个上调), 主要分布在qRGV-A01-2、qRGV-A03-1、qRGV-A03-2、qRGV-C03、qRGR-C04、qRRL-A03-2、qRRL-A09、qRRL-C03、qRBL-C08、qRDW-A09-1、qRDW-A10-2 11个QTL上(表4)。我们将这些主要基因归了五大类, 其中与氧化反应有关的基因有6个(13.64%), 分别是2-氧代戊二酸和铁依赖性加氧酶超家族蛋白基因BnaA03g09460D、过氧化物酶超家族蛋白基因BnaC03g08610D和BnaA03g47720D、细胞色素P450家族基因BnaC04g10740D、氧化还原反应转录因子1 (RRTF1)基因BnaA03g52830D、醛脱氢酶2 B4 (ALDPH 4)基因BnaA03g50730D; 与植物激素相关的基因有3个(6.82%), 分别是生长素诱导的根生长(AIR1)基因BnaA09g02030D和BnaA09g02040D、乙烯响应因子104 BnaA03g40380D; 与酶活性有关的基因有7个 (15.91%), 主要与转移酶/水解酶类相关, 如编码的nudix水解酶同源物4 (NUDT4)的基因BnaC08g17880D、植物转化酶/果胶甲基酯酶抑制剂超家族基因BnaA01g31480D和BnaA03g47250D、α/β-水解酶超家族蛋白基因BnaA03g42860D和BnaA03g42310D以及在RT中下调的UDP-糖基转移酶超家族蛋白基因BnaC04g08480D; 与蛋白质相关的基因有26个(59.09%), 分别是一些编码转运蛋白、逆境蛋白和跨膜蛋白相关的基因, 如编码的伴侣蛋白RbcX基因BnaA03g07790D、硝酸盐转运蛋白(NRT1.8)基因BnaA03g44820D、硫酸盐转运蛋白3基因BnaA03g07660D、磷酸盐转运蛋白1基因BnaC04g06480D均是在RT中的下调基因, 而编码YGL和LRDR结构的铝诱导蛋白质基因BnaA01g28900D、胚胎后期丰富性蛋白(LEA)基因BnaA03g08870D、编码的热击蛋白的基因BnaA03g03740D和BnaA01g30490D、还有编码的锌指蛋白的基因BnaA03g09400D等均在RT中上调的逆境蛋白; 与质膜相关的基因有2个(4.54%), 分别是编码阳离子/H+交换剂17 (CHX17)基因BnaC03g62130D和冷调节413-质膜2 (COR413-PM2)基因BnaA03g40990D (图9)。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7候选基因在R0与RT中的热图
样品名称缩写同
Fig. 7Heat map of candidate genes in R0 and RT
Abbreviations of samples names are the same as those given in
图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8候选基因在S0与ST中的热图
样品名称缩写同
Fig. 8Heat map of candidate genes in S0 and ST
Abbreviations of samples names are the same as those given in
Table 4
表4
表4QTL和转录组结合后筛出可能与铝毒胁迫相关的候选基因
Table 4
| 性状 Trait | 位点 QTL | 甘蓝型油菜基因编号 Gene ID in B. napus | 基因登录号 Gene accession | 调节 Regulated | RT:fpkm | ST:fpkm | 基因注释 Gene annotations |
|---|---|---|---|---|---|---|---|
| 相对发芽势RGV | Up | 19.6 | 0.87 | ||||
| BnaA01g31480D | AT3G10720 | Up | 58.3 | 16.8 | 植物转化酶/果胶甲基酯酶抑制剂超家族 Plant invertase/Pectin methyl esterase inhibitor superfamily | ||
| BnaA01g30490D | AT3G12580 | Up | 176 | 56.5 | 热激蛋白70heatshockprotein70,hsp70 | ||
| BnaA01g28900D | AT3G15450 | Up | 264 | 30 | Al具有YGL和LRDR图案的铝诱导蛋白质 Al-induced protein with YGL and LRDR patterns | ||
| BnaA01g27670D | AT3G16690 | Up | 11.7 | 0.47 | 结蛋白MtN3家族蛋白Nodin MtN3 family protein | ||
| qRGV-A03-1 | BnaA03g50730D | AT3G48000 | Down | 0.08 | 13.5 | 乙醛脱氢酶2B4Acetaldehyde dehydrogenase 2B4 | |
| BnaA03g50130D | AT4G30420 | Up | 20.9 | 4.05 | 结蛋白MtN21 /EamA样转运蛋白家族蛋白 Nodin MtN21 /EamA-like transporter family protein | ||
| BnaA03g50050D | AT4G30270 | Up | 36.2 | 9.65 | 木葡聚糖内葡聚糖酰化酶/水解酶 (XTH) Xyloglucan endoglucanase/hydrolase (XTH) | ||
| BnaA03g47720D | AT4G26010 | Up | 6.4 | 0.7 | 过氧化物酶超家族蛋白Peroxidase superfamily protein | ||
| BnaA03g47250D | AT4G25260 | Up | 36.6 | 4.89 | 植物转化酶/果胶甲基酯酶抑制剂超家族 Plant invertase/Pectin methyl esterase inhibitor superfamily | ||
| BnaA03g46060D | AT4G23590 | Up | 11 | 0.16 | 酪氨酸转氨酶家族蛋白Tyrosine transaminase family protein | ||
| BnaA03g45950D | AT4G00430 | Up | 18.7 | 2.9 | 跨膜蛋白CTransmembrane protein c | ||
| BnaA03g44820D | AT4G21680 | Down | 3.21 | 4.06 | 硝酸盐转运体Nitrate transporter | ||
| BnaA03g43140D | AT4G17550 | Up | 10.3 | 0.05 | 主要促进因子超家族蛋白质Major promoter superfamily proteins | ||
| BnaA03g42860D | AT4G16820 | Up | 6.25 | 0.62 | α/β-水解酶超家族蛋白质α/β-hydrolase superfamily protein | ||
| BnaA03g42310D | AT4G15960 | Up | 17.6 | 4.18 | α/β-水解酶超家族蛋白质α/β-hydrolase superfamily protein | ||
| BnaA03g40990D | AT3G50830 | Down | 0.31 | 17.7 | 冷调节413等离子体Cold conditioning 413 plasma | ||
| BnaA03g40380D | AT5G61600 | Up | 173 | 31.6 | 乙烯响应因子104 (ERF104) Ethylene response factor 104 (ERF104) | ||
| qRGV-A03-2 | BnaA03g09460D | AT5G59530 | Down | 1.94 | 14.8 | 2-氧代戊二酸和铁依赖性加氧酶超家族蛋白 2-oxoglutarate and iron-dependent oxygenase superfamily proteins | |
| BnaA03g09400D | AT5G59550 | Up | 42.4 | 10.6 | 锌指(C3HC4型无名指)家族蛋白 Zinc finger (C3HC4 ring finger) family protein | ||
| BnaA03g09290D | AT5G59730 | Up | 27.3 | 5.38 | 外胚层亚单位exo70家族蛋白 Exoderm subunit exo70 family protein | ||
| BnaA03g08870D | AT5G60530 | Up | 10.7 | 0.26 | 晚期胚胎发生丰富蛋白/ LEA蛋白 Late embryogenesis rich protein/LEA protein | ||
| BnaA03g08820D | AT5G60660 | Up | 44.5 | 0.84 | 质膜内在蛋白2 Plasma membrane intrinsic protein 2 | ||
| BnaA03g07790D | AT5G19855 | Down | 3.53 | 6.86 | 伴侣蛋白RbcX Companion protein RbcX | ||
| BnaA03g07660D | AT5G19600 | Down | 2.83 | 21.8 | 硫酸盐转运蛋白Sulfate transporter | ||
| BnaA03g03740D | AT5G12020 | Up | 99.8 | 18.3 | 17.6 kD二类热激蛋白(HSP17.6II) 17.6 kD class ii heat shock protein (HSP17.6II) | ||
| qRGV-C03 | BnaC03g08610D | AT5G17820 | Up | 14.7 | 0.78 | 过氧化物酶超家族蛋白Peroxidase superfamily protein | |
| 相对发芽率RGR | qRGR-C04 | BnaC04g10740D | AT2G34500 | Up | 55.9 | 12.3 | 细胞色素P450,家族710,亚家族A,多肽1 (CYP710) Cytochrome P450, family 710, subfamily A, polypeptide 1 (CYP710) |
| BnaC04g08480D | AT2G36780 | Down | 0.9 | 23.3 | UDP-糖基转移酶超家族蛋白 UDP-glycosyltransferase superfamily protein | ||
| BnaC04g07650D | AT2G37820 | Up | 10.1 | 0.22 | 富含半胱氨酸/组氨酸结构域家族蛋白 Protein rich in cysteine/histidine domain family | ||
| BnaC04g06480D | AT2G38940 | Down | 3.11 | 182 | 磷酸盐转运蛋白Phosphate transporter | ||
| BnaC04g06140D | AT2G39430 | Up | 33.4 | 5.44 | 抗病反应(定向蛋白样)家族蛋白 Disease resistance response (directed protein-like) family protein | ||
| 相对根长RRL | qRRL-A03-2 | BnaA03g53200D | AT4G35180 | Down | 0.46 | 0.31 | 赖氨酸/组氨酸转运蛋白7 (LHT7) Lysine/histidine transporter 7 (LHT7) |
| BnaA03g52830D | AT4G34410 | Up | 17.2 | 2.6 | 氧化还原反应转录因子1 (RRTF1) Redox transcription factor 1 (RRTF1) | ||
| qRRL-A09 | BnaA09g02040D | AT4G12550 | Up | 37.1 | 0 | 生长素诱导的根培养Root culture induced by auxin | |
| BnaA09g02030D | AT4G12550 | Up | 470 | 34.5 | 生长素诱导的根培养1Auxin-induced root culture 1 | ||
| qRRL-C03 | BnaC03g65590D | AT4G35060 | Down | 4.57 | 6.67 | 重金属转运/解毒超家族蛋白 Heavy metal transport/detoxification superfamily protein | |
| BnaC03g65200D | AT4G23550 | Up | 13.6 | 1.28 | WRKY29家族WRKY29 familyWRKY29 family wrky 29 family | ||
| BnaC03g62130D | AT4G23700 | Up | 256 | 70.6 | 阳离子/氢离子交换器17 (CHX17) Cation/hydrogen ion exchanger 17 (CHX17) | ||
| 相对芽长RBL | qRBL-C08 | BnaC08g17880D | AT1G18300 | Up | 115 | 28.1 | nudix水解酶同源物(NUDT) Nudix hydrolase homologue (NUDT) |
| BnaC08g16940D | AT1G16390 | Up | 1.89 | 0.05 | 有机阳离子/肉碱转运蛋白Organic cation/carnitine transporter | ||
| 相对干重RDW | qRDW-A09-1 | BnaA09g30120D | AT1G23090 | Up | 0.04 | 0.66 | 硫酸盐转运蛋白(AST) Sulfate transporter (AST) |
| BnaA09g29940D | AT4G13390 | Up | 38.4 | 3.17 | 富含脯氨酸的伸展蛋白样家族蛋白 Proline-rich extension-like family proteins | ||
| qRDW-A10-2 | BnaA10g25970D | AT5G04250 | Down | 7.13 | 22.2 | 半胱氨酸蛋白酶超家族蛋白 Cysteine protease superfamily protein |
新窗口打开|下载CSV
图9
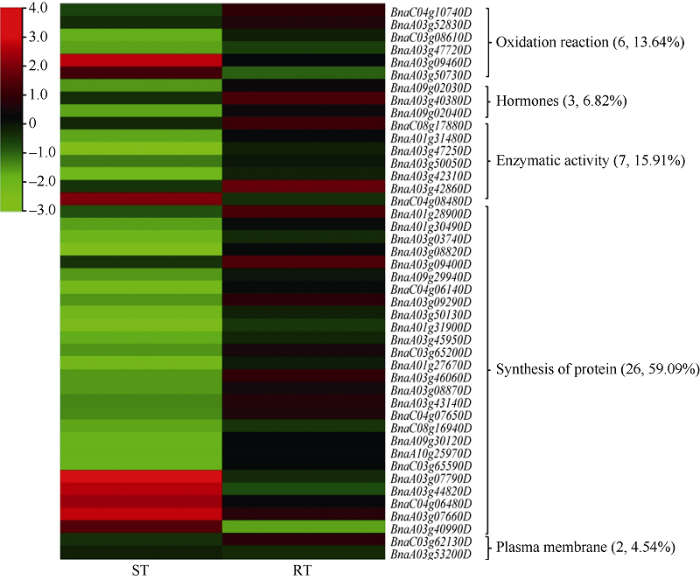 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9候选基因在RT与ST中的热图
样品名称缩写同
Fig. 9Heat map of candidate genes in RT and ST
Abbreviations of samples names are the same as those given in
2.5 对候选基因进行qRT-PCR验证
从表4中找出编码氧化反应相关的基因BnaA03g47720D、激素相关的基因BnaA09g02030D、逆境蛋白的基因BnaA03g03740D、跨膜运输相关的基因BnaC03g62130D以及与转运蛋白相关的基因BnaA03g53200D和BnaC03g65590D进行实时荧光定量PCR验证。RT与ST相比较, 铝毒胁迫处理后油菜根系中差异表达基因BnaA03g47720D、BnaC03g62130D、BnaA09g02030D、BnaA03g03740D上调, BnaA03g53200D和BnaC03g65590D下调, qPCR验证结果与RNA-Seq表达结果一致(图10)。图10
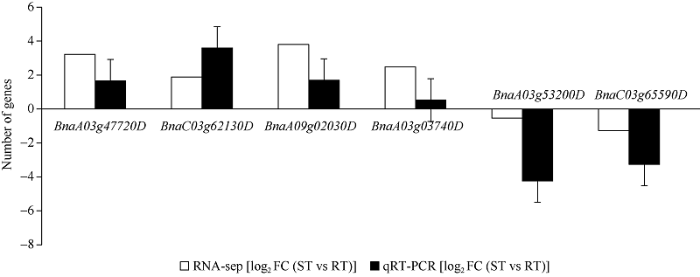 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10qRT-PCR和RNA-seq结果的相关性分析
Fig. 10Correlation analysis between qRT-PCR and RNA-seq
2.6 铝毒胁迫处理对萌发期油菜根部组织的生理指标
植物在逆境胁迫下遭受的伤害与活性氧积累诱发的膜脂过氧化作用密切相关, 其中丙二醛(malondialdehyde, MDA)是膜脂过氧化最重要的产物之一。由表5可知, 铝毒处理后, 油菜R系和S系根部组织MDA的含量均升高, 其中, ST相对于S0显著增加9.48%, 而RT相对于R0则增加不显, 说明ST较RT品系膜氧化损伤严重。另外, 超氧化物歧化(superoxidedismutase, SOD)、过氧化氢酶(catalase, CAT)、过氧化物酶(peroxidase, POD)属于膜保护酶, 与植物的抗逆性有关。结果显示, R系和S系品种SOD活性均较对照显著上升, 其中, RT和ST的SOD活性分别增加57.6%和12.8%, 而CAT和POD活性则均较对照显著下降, 因此, SOD活性在油菜抗铝毒过程中起着重要的作用。此外, 逆境胁迫下, 植物通过增加某些代谢物含量(如Pro、SUG、PAL)抵抗逆境胁迫, Pro和SUG均可以调节及维持细胞渗透压来增加植物抗逆性; PAL属于一种诱导酶, 其活性高低控制着植物体内多种抗菌物质的合成, 因此PAL可以作为植物抗逆的一种指标。本研究发现, 油菜在铝毒处理后, R系和S系的Pro和PAL含量均升高, 且R系的Pro和PAL含量均高于S系, 但R系和S系的SUG的含量均显著性下降。说明R系可以通过积累较多的Pro和PAL含量来抵抗铝毒害。Table 5
表5
表5铝毒胁迫后油菜根系的生理指标
Table 5
| 生理指标 Physiological index | R系 R strain | S系 S strain | ||||
|---|---|---|---|---|---|---|
| R0 | RT | 增幅 Range of increase (%) | S0 | ST | 增幅 Range of increase (%) | |
| 丙二醛 MDA (nmol g-1) | 4.26 | 4.44 | 0.04 | 5.63 | 6.16 | 0.10** |
| 苯丙氨酸解氨酶 PAL (U g-1) | 4.54 | 6.53 | 0.44 | 5.06 | 6.45 | 0.27 |
| 脯氨酸 Pro (µg g-1) | 51.26 | 56.70 | 0.11** | 52.39 | 57.20 | 0.09** |
| 超氧化物歧化酶 SOD (U g-1) | 54.89 | 86.50 | 0.58** | 58.63 | 66.13 | 0.13** |
| 过氧化氢酶 CAT (U g-1) | 281.71 | 261.39 | -0.07** | 376.24 | 282.13 | -0.25** |
| 过氧化物酶 POD (U g-1) | 14,521.79 | 10,566.33 | -0.27 | 13,381.75 | 10,337.50 | -0.23** |
| 可溶性糖 SUG (mg g-1) | 2.18 | 1.80 | -0.17** | 3.12 | 2.85 | -0.09** |
新窗口打开|下载CSV
3 讨论
铝毒是酸性土壤中限制植物生长和作物产量的主要因素。研究表明, 它会诱导氧化应激和根细胞死亡[21]。本研究中, 油菜在铝毒胁迫响应下受到多个基因的调控, 对这些基因进行GO分析和功能注释分类发现, S0 vs R0、ST vs RT、S0 vs ST与R0 vs RT这4组文库DEGs中有大量基因显著富集于氧化应激反应、抗氧化活性、过氧化物酶活性和氧化还原酶活性等途径。其中过氧化物酶超家族蛋白基因BnaC03g08610D和BnaA03g47720D在R系中表现为上调, 该家族蛋白基因被证明可有效清除ROS, 缓解植物生长抑制, 减轻铝诱导的氧化应激, 激活抗氧化酶系统[22,23]。结合本研究中生理指标测定结果, RT中过氧化物酶(POD)和过氧化氢酶(CAT)的活性较ST相比均较高, 丙二醛(MDA)和可溶性糖(SUG)含量较低, 使其RT根中的活性氧积累较低, 表明RT具有较高水平的抗氧化能力, 这也可以解释为编码氧化还原反应转录因子BnaA03g52830D等上调基因的过表达赋予RT品系氧化应激的抗性。因此, 从转录组分析及生理分析来看, 铝毒可以诱导油菜根部组织产生过量的ROS, 从而引发油菜根部细胞的氧化应激反应。这些与氧化反应相关的基因在其他作物中过表达同样起到减轻植物铝毒害作用。如拟南芥过氧化物酶基因(AtPrx64)在烟草中的过表达提高了植物对铝胁迫的耐受性[24], 铝诱导下过氧化氢酶的产生和根尖抗氧化系统的变化在小麦适应铝毒害中起重要作用[25], 还有铁超氧化物歧化酶在水稻耐铝品系中高表达减轻了水稻的铝毒害[26]。通过KEGG分析发现上述4组文库中还有大量DEGs显著富集于苯丙烷类生物的合成。基因咖啡酸-O-甲基转移酶(COMT)、肉桂醇脱氢酶(CAD)、阿魏酸-5-羟化酶(F5H1)均在RT中上调, 与木质素合成有关。而且生理测定结果显示RT与ST根部组织的苯丙氨酸(PAL)含量均上升, 这表明该途径可能参与了油菜对铝毒胁迫的响应。还有报道称, 杉木[27]和玉米[28]经过铝毒处理后, 也有大量的差异基因均参与苯丙烷类的相关途径。脯氨酸作为渗透调节剂具有清除ROS, 平衡细胞内氧化还原稳态和促进细胞信号传导的作用[29,30], 对提高植物抗逆性有重要作用。脯氨酸由∆1-吡咯啉-5-羧酸合成酶(P5CS)催化, 然后由∆1-吡咯啉-5-羧酸还原酶(P5CR)还原而成[31]。本研究在RT中找出了编码富含脯氨酸的延伸蛋白样家族的上调基因BnaA09g29940D, 并发现油菜品种经过铝毒处理后, RT中的Pro含量增幅高于ST根中的Pro含量。由此可以看出, 耐铝毒油菜可以更好的调控基因的表达, 使其根部脯氨酸含量上升, 从而避免遭受铝毒害。还有研究表明, 小麦根中NADPH氧化酶的过表达会使脯氨酸含量的增加, 也会减轻盐胁迫诱导的氧化损伤[32], 中药材射干体内脯氨酸生物量的增加可以保护植物细胞免受铜伤害[33]。此外, 本研究还筛选出编码生长素类的基因BnaA02g22420D和BnaA09g02040D以及编码乙烯反应因子基因BnaA03g40380D均在RT中表现上调。有研究表明植物激素(ABA、生长素和乙烯)信号途径相互作用, 影响小麦、紫花苜蓿等植物的发育及抵抗逆境胁迫的能力[34,35,36]。目前已有研究表明, 细胞壁组成的改变在抵抗铝毒害中发挥着重要作用[37]。本研究中, 在RT中发现了与细胞壁松弛相关的基因, 如编码的木葡聚糖内转葡糖基化酶/水解酶的基因BnaA03g50050D以及编码的植物转化酶/果胶甲酯酶抑制剂超家族蛋白基因BnaA01g31480D和BnaA03g47250D均表现为上调。木葡聚糖内转移葡萄糖基酶/水解酶在植物细胞壁中生成, 调控着细胞的生长与分化, 对细胞壁通透性具有调节作用, 进而影响到植物的抗逆性[38]。植物转化酶/果胶甲酯酶抑制剂超家族蛋白能够抑制果胶甲酯酶活性, 而果胶甲酯酶对细胞壁强度及细胞生长均具有调节作用, 所以该酶在植物的生长发育和逆境胁迫响应过程中具有重要作用[39]。因此, 细胞壁中相关基因的表达对于保护植物细胞免受逆境胁迫至关重要[40]
本研究筛选的基因BnaA03g08870D编码的植物胚胎晚期富集蛋白(LEA)以及BnaA03g03740D和BnaA03g09400D编码的锌指蛋白均是参与植物抗逆的重要逆境蛋白。编码这2类蛋白的基因在干旱、低温、高盐胁迫等逆境条件下表达水平提高[41,42]。LEA蛋白在耐铝毒胁迫的研究上相对较少, 其作用机理仍然不太完善。但是Mowla等[43]发现LEA蛋白在植物中通过清除细胞内的活性氧而具有抗氧化能力。同时, Rizhsky等[44]发现编码锌指蛋白的基因Zat12是拟南芥氧化应激反应信号转导网络的重要组成部分。还有编码热激蛋白基因BnaA01g30490D和BnaA03g03740D也参与了植物的非生物胁迫(包括铝毒害)。因此, LEA蛋白、锌指蛋白还有热激蛋白可能通过氧化应激反应途径起到油菜耐铝毒的作用。此外, 植物根部还可能会通过转录蛋白和转录因子分泌等方式来阻止 Al3+进入细胞内, 从而避免植物遭受铝毒害。如MATE转运蛋白基因和ALMT基因均与小分子有机酸的分泌紧密相关[45]。ABC通道蛋白家族中的ALS3和ALS1基因以及转运蛋白Nrat1, 均能使铝在细胞内形成复合物完成区室化, 阻止Al3+进入细胞内[46]。BnaC03g65200D编码的WRKY转录因子参与MAPK植物信号通路和植物-病原体相互作用代谢途径, 在耐铝品种中其表达量上调, 有研究表明WRKY家族基因的过表达可以增强植物的耐受性和根系生长调节[47], 这意味着该转录因子对于铝的富集和解毒具有重要的耐受机制。本文还找出如基因BnaA09g30120D、BnaA03g44820D、BnaC04g06480D分别编码的硫酸盐转运蛋白91 (AST91)、硝酸盐转运蛋白1.8 (NRT1.8)、磷酸盐转运蛋白等许多转运蛋白相关的基因, 说明油菜可能通过磷酸盐、硫酸盐、硝酸盐等转运方式来解除铝毒伤害。这些方面的研究在荞麦中已有报道[48]。此外, 本文还找出在耐铝品种中表达量较高的MtN21转运家族蛋白, 该家族基因在小分子跨膜运输、信号转导和抗逆性等方面发挥作用[49]。故MtN21转运家族蛋白在油菜中可能具有跨膜转运Al3+功能的作用。
4 结论
通过对差异表达基因GO和KEGG功能注释分析发现, 这些基因参与了氧化应激过程、苯丙烷生物合成、植物病原体相互作用、MAPK信号传导、淀粉和蔗糖代谢、细胞碳水化合物代谢、植物激素信号转导等多种代谢途径。结合QTL定位和RNA测序数据, 共筛出与氧化应激、渗透调节、细胞壁修饰、转运蛋白、激素信号传导相关的基因44个(10个下调和34个上调)。这些基因并非单独起作用的, 而是相互交叉、协同作用。从生理分析来看, 脯氨酸、苯丙氨酸解氨酶、可溶性糖、丙二醛含量和抗氧化酶活性在不同耐铝品种升降幅度不太一致, 但是相对于敏铝品种, 耐铝品种通过可以更好的调控基因表达以避免氧化损伤。最后我们将筛出具有代表性的6个基因被用于qRT-PCR检测, 其表达模式与RNA-Seq分析结果对一步研究甘蓝型油菜根部组织的抗铝毒机制具有重要的参考价值。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 2]
DOIPMID [本文引用: 2]

Acid soils restrict plant production around the world. One of the major limitations to plant growth on acid soils is the prevalence of soluble aluminium (Al(3+)) ions which can inhibit root growth at micromolar concentrations. Species that show a natural resistance to Al(3+) toxicity perform better on acid soils. Our understanding of the physiology of Al(3+) resistance in important crop plants has increased greatly over the past 20 years, largely due to the application of genetics and molecular biology. Fourteen genes from seven different species are known to contribute to Al(3+) tolerance and resistance and several additional candidates have been identified. Some of these genes account for genotypic variation within species and others do not. One mechanism of resistance which has now been identified in a range of species relies on the efflux of organic anions such as malate and citrate from roots. The genes controlling this trait are members of the ALMT and MATE families which encode membrane proteins that facilitate organic anion efflux across the plasma membrane. Identification of these and other resistance genes provides opportunities for enhancing the Al(3+) resistance of plants by marker-assisted breeding and through biotechnology. Most attempts to enhance Al(3+) resistance in plants with genetic engineering have targeted genes that are induced by Al(3+) stress or that are likely to increase organic anion efflux. In the latter case, studies have either enhanced organic anion synthesis or increased organic anion transport across the plasma membrane. Recent developments in this area are summarized and the structure-function of the TaALMT1 protein from wheat is discussed.
[本文引用: 2]
DOIURL [本文引用: 2]
DOIPMID [本文引用: 1]

Seeds per silique (SS), seed weight (SW), and silique length (SL) are important determinant traits of seed yield potential in rapeseed (Brassica napus L.), and are controlled by naturally occurring quantitative trait loci (QTLs). Mapping QTLs to narrow chromosomal regions provides an effective means of characterizing the genetic basis of these complex traits. Orychophragmus violaceus is a crucifer with long siliques, many SS, and heavy seeds. A novel B. napus introgression line with many SS was previously selected from multiple crosses (B. rapa ssp. chinesis x O. violaceus) x B. napus. In present study, a doubled haploid (DH) population with 167 lines was established from a cross between the introgression line and a line with far fewer SS, in order to detect QTLs for silique-related traits. By screening with a Brassica 60K single nucleotide polymorphism (SNP) array, a high-density linkage map consisting of 1,153 bins and spanning a cumulative length of 2,209.1 cM was constructed, using 12,602 high-quality polymorphic SNPs in the DH population. The average recombination bin densities of the A and C subgenomes were 1.7 and 2.4 cM, respectively. 45 QTLs were identified for the three traits in all, which explained 4.0-34.4% of the total phenotypic variation; 20 of them were integrated into three unique QTLs by meta-analysis. These unique QTLs revealed a significant positive correlation between SS and SL and a significant negative correlation between SW and SS, and were mapped onto the linkage groups A05, C08, and C09. A trait-by-trait meta-analysis revealed eight, four, and seven consensus QTLs for SS, SW, and SL, respectively, and five major QTLs (cqSS.A09b, cqSS.C09, cqSW.A05, cqSW.C09, and cqSL.C09) were identified. Five, three, and four QTLs for SS, SW, and SL, respectively, might be novel QTLs because of the existence of alien genetic loci for these traits in the alien introgression. Thirty-eight candidate genes underlying nine QTLs for silique-related traits were identified.
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
PMID [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
PMID [本文引用: 1]

Aluminium (Al) toxicity is widely considered to be the most important growth-limiting factor for plants in strongly acid soils (pH<5.0). The inhibition of root elongation in three varieties of maize (Zea mays L. vars Clavito, HS701b and Sikuani) was followed over the first 48 h of Al treatment, and during the initial 10 h elongation was determined on an hourly basis. The silicon (Si)-induced amelioration of Al toxicity was investigated by pre-treating seedlings for 72 h in nutrient solutions with 1000 microM Si before transfer into solutions with 0, 20 or 50 microM Al (without Si). Plants were either grown in complete low ionic strength nutrient solutions (CNS) or in low salt solutions of 0.4 mM CaCl2 (LSS). In addition, the role of root exudation of organic compounds as a mechanism of Si-induced alleviation of Al toxicity was investigated. Aluminium-induced inhibition of root elongation in the maize var. HS701b was observed within 1 h of Al exposure. After a lag time of at least 8 h, Si-induced alleviation of Al toxicity was observed in this variety when grown in LSS. In the Al-resistant var. Sikuani, Al-resistance was only observed after exposure to 50 microM Al, and not after exposure to 20 microM Al, suggesting that there exists a threshold Al concentration before the mechanisms of Al resistance are activated. Aluminium stimulated root exudation of oxalic acid in all three varieties, but exudate concentrations did not increase with either Al resistance or with Si pretreatment. Aluminium and Si triggered release of catechol and of the flavonoid-type phenolics: catechin, and quercetin. In the Al-resistant variety, Sikuani, Al-exposed plants pretreated with Si exuded up to 15 times more phenolics than those plants not pretreated with Si. The flavonoid-type phenolics, to date unconsidered, appear to play a role in the mechanism(s) of Si-induced amelioration of Al toxicity.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 1]

Growth of petal cells is a basis for expansion and morphogenesis (outward bending) of petals during opening of carnation flowers (Dianthus caryophyllus L.). Petal growth progressed through elongation in the early stage, expansion with outward bending in the middle stage, and expansion of the whole area in the late stage of flower opening. In the present study, four cDNAs encoding xyloglucan endotransglucosylase/hydrolase (XTH) (DcXTH1-DcXTH4) and three cDNAs encoding expansin (DcEXPA1-DcEXPA3) were cloned from petals of opening carnation flowers and characterized. Real-time reverse transcription-PCR analyses showed that transcript levels of XTH and expansin genes accumulated differently in floral and vegetative tissues of carnation plants with opening flowers, indicating regulated expression of these genes. DcXTH2 and DcXTH3 transcripts were detected in large quantities in petals as compared with other tissues. DcEXPA1 and DcEXPA2 transcripts were markedly accumulated in petals of opening flowers. The action of XTH in growing petal tissues was confirmed by in situ staining of xyloglucan endotransglucosylase (XET) activity using a rhodamine-labelled xyloglucan nonasaccharide as a substrate. Based on the present findings, it is suggested that two XTH genes (DcXTH2 and DcXTH3) and two expansin genes (DcEXPA1 and DcEXPA2) are associated with petal growth and development during carnation flower opening.
DOIPMID [本文引用: 1]

An intact cell wall is critical for cellular interactions with the environment and protecting the cell from environmental challenges. Signaling mechanisms are necessary to monitor cell wall integrity and to regulate cell wall production and remodeling during growth and division cycles. The green alga, Chlamydomonas, has a proteinaceous cell wall of defined structure that is readily removed by gametolysin (g-lysin), a metalloprotease released during sexual mating. Naked cells treated with g-lysin induce the mRNA accumulation of >100 cell wall-related genes within an hour, offering a system to study signaling and regulatory mechanisms for de novo cell wall assembly. Combining quantitative RT-PCR and luciferase reporter assays to probe transcript accumulation and promoter activity, we revealed that up to 500-fold upregulation of cell wall-related genes was driven at least partly by transcriptional activation upon g-lysin treatment. To investigate how naked cells trigger this rapid transcriptional activation, we tested whether osmotic stress and cell wall integrity are involved in this process. Under a constant hypotonic condition, comparable levels of cell wall-gene activation were observed by g-lysin treatment. In contrast, cells in an iso- or hypertonic condition showed up to 80% reduction in the g-lysin-induced gene activation, suggesting that osmotic stress is required for full-scale responses to g-lysin treatment. To test whether mechanical perturbation of cell walls is involved, we isolated and examined a new set of cell wall mutants with defective or little cell walls. All cell wall mutants examined showed a constitutive upregulation of cell wall-related genes at a level that is only achieved by treatment with g-lysin in wild-type cells. Our study suggests a cell wall integrity monitoring mechanism that senses both osmotic stress and mechanical defects of cell walls and regulates cell wall-gene expression in Chlamydomonas, which may relate to cell wall integrity signaling mechanisms in other organisms.
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

The phytohormone abscisic acid (ABA) induces accumulation of reactive oxygen species (ROS), which can disrupt seed dormancy and plant development. Here, we report the isolation and characterization of an Arabidopsis thaliana mutant called ars1 (aba and ros sensitive 1) that showed hypersensitivity to ABA during seed germination and to methyl viologen (MV) at the seedling stage. ARS1 encodes a nuclear protein with one zinc finger domain, two nuclear localization signal (NLS) domains, and one nuclear export signal (NES). The ars1 mutants showed reduced expression of a gene for superoxide dismutase (CSD3) and enhanced accumulation of ROS after ABA treatment. Transient expression of ARS1 in Arabidopsis protoplasts strongly suppressed ABA-mediated ROS production. Interestingly, nuclear localized ARS1 translocated to the cytoplasm in response to treatment with ABA, H2O2, or MV. Taken together, these results suggest that ARS1 modulates seed germination and ROS homeostasis in response to ABA and oxidative stress in plants.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

Buckwheat (Fagopyrum esculentum Moench) is a species with high aluminum (Al) tolerance and accumulation. Although the physiological mechanisms for external and internal detoxification of Al have been well studied, the molecular mechanisms responsible are poorly understood. Here, we conducted a genome-wide transcriptome analysis of Al-responsive genes in the roots and leaves using RNA sequencing (RNA-Seq) technology. RNA-Seq generated reads ranging from 56×10(6) to 93×10(6). A total of 148,734 transcript contigs with an average length of 1,014 bp were assembled, generating 84,516 unigenes. Among them, 31,730 and 23,853 unigenes were annotated, respectively, in the NCBI plant database and TAIR database for Arabidopsis. Of the annotated genes, 4,067 genes in the roots and 2,663 genes in the leaves were up-regulated (>2-fold) by Al exposure, while 2,456 genes in the roots and 2,426 genes in the leaves were down-regulated (<2-fold) A few STOP1/ART1 (SENSITIVE TO PROTON RHIZOTOXICITY1/AL RESISTANCE TRANSCRIPTION FACTOR1)-regulated gene homologs including FeSTAR1, FeALS3 (ALUMINUM SENSITIVE3), FeALS1 (ALUMINUM SENSITIVE1), FeMATE1 and FeMATE2 (MULTIDRUG AND TOXIC COMPOUND EXTRUSION1 and 2) were also up-regulated in buckwheat, indicating some common Al tolerance mechanism across the species, although most STOP1/ART1-regulated gene homologs were not changed. Most genes involved in citric and oxalic acid biosynthesis were not significantly altered. Some transporter genes were highly expressed in the roots and leaves and responded to Al stress, implicating their role in Al tolerance and accumulation. Overall, our data provide a platform for further characterizing the functions of genes involved in Al tolerance and accumulation in buckwheat. © The Author 2014. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For permissions, please email: journals.permissions@oup.com.
[本文引用: 1]
