 ,1,2,**, 张晓莉
,1,2,**, 张晓莉 ,1,2,**, 孟晓静1,2, 姚梦楠1,2, 缪文杰1,2, 袁大双1,2, 朱冬鸣1,2, 曲存民1,2, 卢坤1,2, 李加纳
,1,2,**, 孟晓静1,2, 姚梦楠1,2, 缪文杰1,2, 袁大双1,2, 朱冬鸣1,2, 曲存民1,2, 卢坤1,2, 李加纳 ,1,2,*, 梁颖
,1,2,*, 梁颖 ,1,2,*
,1,2,*Identification of upstream regulators for mitogen-activated protein kinase 7 gene (BnMAPK7) in rapeseed (Brassica napus L.)
WANG Zhen ,1,2,**, ZHANG Xiao-Li
,1,2,**, ZHANG Xiao-Li ,1,2,**, MENG Xiao-Jing1,2, YAO Meng-Nan1,2, MIU Wen-Jie1,2, YUAN Da-Shuang1,2, ZHU Dong-Ming1,2, QU Cun-Min1,2, LU Kun1,2, LI Jia-Na
,1,2,**, MENG Xiao-Jing1,2, YAO Meng-Nan1,2, MIU Wen-Jie1,2, YUAN Da-Shuang1,2, ZHU Dong-Ming1,2, QU Cun-Min1,2, LU Kun1,2, LI Jia-Na ,1,2,*, LIANG Ying
,1,2,*, LIANG Ying ,1,2,*
,1,2,*通讯作者: * 梁颖, E-mail:yliang@swu.edu.cn;李加纳, E-mail:ljn1950@swu.edu.cn
第一联系人:
收稿日期:2020-12-21接受日期:2021-04-14网络出版日期:2021-06-09
| 基金资助: |
Corresponding authors: * E-mail:yliang@swu.edu.cn;E-mail:ljn1950@swu.edu.cn
First author contact:
Received:2020-12-21Accepted:2021-04-14Published online:2021-06-09
| Fund supported: |
作者简介 About authors
王珍,E-mail:wangzhencq@swu.edu.cn;
张晓莉,E-mail:zxl19930619@email.swu.edu.cn

摘要
丝裂原活化蛋白激酶(Mitogen-activated protein kinases, MAPKs)级联途径在植物的生长发育、分裂分化、细胞凋亡以及抗逆等多种生命过程中发挥着极其重要的作用。本研究从甘蓝型油菜中分离克隆了1个C族BnMAPK7基因的启动子, 序列长度为1612 bp, 命名为ProBnMAPK7。启动子分析工具PlantCARE预测结果表明, ProBnMAPK7含有大量响应光照、激素、防御和损伤等相关顺式作用元件, 包括ACE、MRE、ABRE、TGACG-motif和TC-rich repeats等。同时, 我们对拟南芥及甘蓝型油菜MAPK7基因的表达模式进行分析发现, MAPK7对生长发育以及生物和非生物胁迫应答过程具有重要调控意义。将ProBnMAPK7逐步分段连接至pCambia1305.1-GUS表达载体筛选启动子的核心区段, GUS组织化学染色分析显示该启动子的核心区段定位于-467~ -239 bp (ProBnMAPK7-rPE)。将核心区段3拷贝串联重复后整合至Y1H gold基因组, 并进行AbA背景测试, 结果显示AbA浓度为500 ng mL-1时, ProBnMAPK7-rPE×3在酵母细胞中的本底表达被完全抑制。利用酵母单杂交技术对BnMAPK7的上游调控因子进行文库筛选, 获得3个候选因子BnNAD1B (NADH dehydrogenase 1B)、BnERD6 (early response to dehydration 6)和BnPIG3 (quinone oxidoreductase PIG3-like)。表明BnNAD1B、BnERD6及BnPIG3蛋白可能通过结合ProBnMAPK7-rPE区段调控BnMAPK7的转录, 使得BnMAPK7参与光合作用以及逆境胁迫应答等生物学过程, 为进一步阐明甘蓝型油菜BnMAPK7基因的功能奠定了基础, 也为MAPKs级联的研究提供了新的思路。
关键词:
Abstract
Mitogen-activated protein kinases (MAPKs) cascade plays a key role in plant growth and development, division, differentiation, apoptosis, and stress resistance. In this study, a 1612 bp promoter of C group BnMAPK7 gene, designated ProBnMAPK7, was cloned from Brassica napus. Promoter structure prediction by PlantCARE revealed that ProBnMAPK7 contained a lot of ACE, MRE, ABRE, TGACG-motif, and TC-rich repeats cis-acting elements, which involved in light, hormones, defense, and wounding responsiveness. At the same time, we analyzed the expression patterns of MAPK7 genes in Arabidopsis and B. napus, and found that MAPK7 played an important regulatory role in growth and development process and responding to biotic and abiotic stresses. Different lengths of ProBnMAPK7 were gradually ligated to the pCambia1305.1-GUS expression vector to identify the core fragment. GUS histochemical staining analysis showed that the core fragment of ProBnMAPK7 was located in the -467 to -239 bp (ProBnMAPK7-rPE) region. Three copies of the promoter core fragment were integrated into the genome of Y1H gold to test the AbA background. The data demonstrated that the expression background of ProBnMAPK7-rPE in yeast cells was completely inhibited by 500 ng mL-1 AbA. Using yeast one-hybrid, we screened the library of the upstream regulatory factors of BnMAPK7, and obtained three candidates, including BnNAD1B (NADH dehydrogenase 1B), BnERD6 (early response to dehydration 6), and BnPIG3 (quinone oxidoreductase PIG3-like). Taken together, these results suggested that BnNAD1B, BnERD6, and BnPIG3 might bind to ProBnMAPK7-rPE to regulate the transcription of BnMAPK7, to further involve in photosynthesis and responding to stresses. This study lays a foundation for further elucidating the function of BnMAPK7 in rapeseed, and provides a new perspective for research into MAPKs cascade.
Keywords:
PDF (12120KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王珍, 张晓莉, 孟晓静, 姚梦楠, 缪文杰, 袁大双, 朱冬鸣, 曲存民, 卢坤, 李加纳, 梁颖. 甘蓝型油菜丝裂原活化蛋白激酶7基因(BnMAPK7)上游调控因子的鉴定. 作物学报, 2021, 47(12): 2379-2393 DOI:10.3724/SP.J.1006.2021.04280
WANG Zhen, ZHANG Xiao-Li, MENG Xiao-Jing, YAO Meng-Nan, MIU Wen-Jie, YUAN Da-Shuang, ZHU Dong-Ming, QU Cun-Min, LU Kun, LI Jia-Na, LIANG Ying.
油菜是世界上重要的农作物之一, 在种植面积、产量、植物油供给、蛋白来源等方面位于我国油料作物之首, 在农业生产上占据着举足轻重的地位[1]。甘蓝型油菜(Brassica napus)作为我国广泛种植的栽培种, 较白菜型油菜及芥菜型油菜, 在植株抗性及产量等方面存在明显优势[2]。作为一种异源四倍体植物, 其基因组相对复杂, 在驯化栽培中具有广泛的变异, 在进化研究中也具有重要的科学价值。我国幅员辽阔、地形复杂、气候多变、环境特殊, 生物及非生物胁迫等问题直接影响国民的增收及油料产业的发展, 包括核盘菌(Sclerotinia sclerotiorum)、霜霉病(Peronospora parasitica)、干旱、渍害和涝害等[3,4]。近年来, 对抗逆基因进行功能研究、并对抗性表现良好的基因加以利用, 已经成为培育油菜抗性新品种的一条新途径。
为了应对逆境, 植物进化出一些特殊机制, 通过调控酶活性、转录、翻译及修饰等, 感知并适应生理、生长及发育。其中, 通过信号级联中的蛋白激酶进行蛋白磷酸化调控是所有生物体中最常见的一种翻译后修饰[5]。丝裂原活化蛋白激酶(mitogen- activated protein kinases, MAPKs)级联是进化上非常保守的信号通路, 是多种信号跨膜传递的交汇点或共同通路[6,7]。植物感受到外源刺激信号后, MAPKs级联通过磷酸化过程激活信号并将其转入胞内, 活化细胞骨架蛋白、转录因子、磷脂酶、微管相关蛋白等[8,9,10], 进而调控植物的生长发育以及生物和非生物胁迫应答过程, 包括病原菌侵害、冷、热、活性氧、紫外线、干旱等[11,12,13,14,15]。常见的MAPKs级联包含由不同基因家族编码的3种激酶, 从上至下分别由MAPKKKs (MAPKs kinases kinases)、MKKs (MAPKs kinases)和MAPKs组成[6]。活化的MAPKKKs首先磷酸化MKKs的Ser/Thr残基, 随后活化的MKKs对下游MAPKs保守结构域T-X-Y进行双重磷酸化, 触发MAPKs对下游底物的活化[16]。拟南芥中大约有20个MAPKs, 分为A~D四个亚族, 其中C亚族共有4个基因: AtMAPK1、AtMAPK2、AtMAPK7和AtMAPK14[13, 17]。研究报道, 在拟南芥中, AtMAPK7受到H2O2和丁香假单胞菌(Pseudomonas syringae)的诱导与激活, 参与调控下游病原菌防御相关基因的表达[18]。AtMAPKKK17/18-AtMKK3- AtMAPK7级联被报道与脱落酸(abscisic acid, ABA)信号途径交联, 参与ABA的应答过程[19]。与拟南芥AtMAPK7的功能相似, Zong等[20]在玉米中发现, ZmMAPK7基因也受到ABA与H2O2的诱导, 并正向调控ABA和活性氧(reactive oxygen species, ROS)介导的渗透胁迫应答。此外, 棉花GhMAPK7基因响应H2O2、盐、损伤、水杨酸(salicylic acid, SA)和茉莉酸甲酯(methyl jasmonate, MeJA)的非生物胁迫应答和立枯病(Rhizoctonia solani)、炭疽病(Colletotrichum gossypii)、枯萎病(Fusarium oxysporum f. sp. vasinfectum)的防御应答过程[21]。GhMKK3-GhMAPK7级联途径被报道受到ABA及干旱诱导, 并通过调控棉花根系的生长和失水率在抗旱性中发挥重要作用[22]。本课题组前期克隆了甘蓝型油菜BnMAPK7基因, 发现其受到ABA、H2O2、MeJA、SA、热、损伤及核盘菌的诱导[23]。这些结果表明, MAPK7基因在植物的生物和非生物胁迫过程中具有重要的作用, 同时, 以MAPK7为底物的MAPKs级联通过蛋白磷酸化修饰参与调控胁迫应答。然而, 上游因子是如何调控植物MAPK7基因转录表达的分子网络尚未见报道。
本试验参考拟南芥和甘蓝型油菜数据库, 克隆了BnMAPK7 (BnaA09g09520D)启动子序列, 经过分析ProBnMAPK7的顺式作用元件及MAPK7的表达模式, 通过构建BnMAPK7启动子核心区段的酵母单杂交体系筛选与其相互作用的上游调控因子等深入探索, 解析了BnMAPK7可能参与的生物学过程及其潜在的上游调控因子, 旨在为逐步深入完善甘蓝型油菜BnMAPK7的基因功能提供新的内容, 并为MAPKs级联途径的研究提供新的思路。
1 材料与方法
1.1 供试材料
甘蓝型油菜中油821单倍体加倍(doubled haploid, DH)系与本氏烟草(Nicotiana benthamiana)由重庆市油菜工程技术研究中心提供, 并种植于人工气候光照培养箱, 生长条件为16 h光照(25℃)/8 h黑暗(16℃), 湿度50%。利用甘蓝型油菜中油821克隆BnMAPK7基因的启动子, 本氏烟草用于启动子的核心区段筛选。大肠杆菌(Escherichia coli) DH5α、根癌农杆菌(Agrobacterium tumefaciens) GV3101及pCambia1305.1-GUS表达载体均由本研究室保存, 用于启动子的克隆、转化及瞬时表达研究。酿酒酵母菌(Saccharomyces cerevisiae) Y1H gold、pAbAi表达载体及pGADT7-Rec-p53对照载体购自Takara公司, 用于启动子及其上游的功能研究。植物DNA提取试剂盒(Plant Genomic DNA Kit)购自天根生化科技(北京)有限公司, KOD neo plus高保真酶购自Toyobo公司, LB/YEB培养基所用试剂购自生工生物工程(上海)股份有限公司, 抗生素AbA (Aureobasidin A)、酵母培养及转化试剂购自Clontech公司, 引物合成和测序由英潍捷基(上海)贸易有限公司完成。1.2 启动子的克隆及核心元件分析
参照植物DNA提取试剂盒说明书, 提取苗期(20~25 d)甘蓝型油菜植株幼嫩叶片基因组DNA。利用分光光度计NanoDrop 2000c (Thermo Scientific)检测DNA浓度及纯度, 并用1.0%琼脂糖凝胶电泳检测其完整性。根据TAIR10数据库(Table 1
表1
表1本研究所用PCR引物
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence (5°-3°) | 用途 Usage | ||
|---|---|---|---|---|
| ProBnMAPK7-PA | F: GACGTCGACAATATAATGTCTAATGAGTGAACCAAAC R: GACCCATGGGCTTTCTTGTCCCTAAACTCAACC | ProBnMAPK7-PA (1612 bp)全长启动子表达载体的构建 Construction of promoter expression vector of full-length ProBnMAPK7-PA (1612 bp) | ||
| ProBnMAPK7-lPB | F: GACGTCGACAATATAATGTCTAATGAGTGAACCAAAC R: GACCCATGGTATAAAAAACATGTTTATCTTTTAGGTTG | ProBnMAPK7-lPB (539 bp)启动子表达载体的构建 Construction of promoter expression vector of ProBnMAPK7-lPB (539 bp) | ||
| ProBnMAPK7-rPB | F: GACGTCGACGTTTGGATGAGAGAGTATTATGGATCCCGG R: GACCCATGGGCTTTCTTGTCCCTAAACTCAACC | ProBnMAPK7-rPB (1073 bp)启动子表达载体的构建 Construction of promoter expression vector of ProBnMAPK7-rPB (1073 bp) | ||
| ProBnMAPK7-lPC | F: GACGTCGACGTTTGGATGAGAGAGTATTATGGATCCCGG R: GACCCATGGAATATGACACGTGGCTTACG | ProBnMAPK7-lPC (212 bp)启动子表达载体的构建 Construction of promoter expression vector of ProBnMAPK7-lPC (212 bp) | ||
| ProBnMAPK7-rPC | F: GACGTCGACCCACTGTACCCACGTGCTTAGCCGGTTGC R: GACCCATGGGCTTTCTTGTCCCTAAACTCAACC | ProBnMAPK7-rPC (861 bp)启动子表达载体的构建 Construction of promoter expression vector of ProBnMAPK7-rPC (861 bp) | ||
| ProBnMAPK7-lPD | F: GACGTCGACCCACTGTACCCACGTGCTTAGCCGGTTGC R: GACCCATGGCCAGATTGTAATTACAGAAGCTG | ProBnMAPK7-lPD (623 bp)启动子表达载体的构建 Construction of promoter expression vector of ProBnMAPK7-lPD (623 bp) | ||
| ProBnMAPK7-rPD | F: GACGTCGACGTATGTCGAAATCTTCTTTTCTTTGC R: GACCCATGGGCTTTCTTGTCCCTAAACTCAACC | ProBnMAPK7-rPD (238 bp)启动子表达载体的构建 Construction of promoter expression vector of ProBnMAPK7-rPD (238 bp) | ||
| ProBnMAPK7-lPE | F: GACGTCGACCCACTGTACCCACGTGCTTAGCCGGTTGC R: GACCCATGGTTTCTTACTTCTTTTCTTTTTTCAGAA | ProBnMAPK7-lPE (394 bp)启动子表达载体的构建 Construction of promoter expression vector of ProBnMAPK7-lPE (394 bp) | ||
| 引物名称 Primer name | 引物序列 Primer sequences (5°-3°) | 用途 Usage | ||
| ProBnMAPK7-rPE | F: GACGTCGACGGCTTCTTCATCAAACATAACGTATTGCTTC R: GACCCATGGCCAGATTGTAATTACAGAAGCTG | ProBnMAPK7-rPE (229 bp)启动子表达载体的构建 Construction of promoter expression vector of ProBnMAPK7-rPE (229 bp) | ||
| p1305.1 | F: GATTCATTAATGCAGCTGGCACGACAGG R: GTTGATCGGGTACAGACTAGTTCGTCGG | 启动子表达载体的插入检测 Insertion detection of promoter expression vector | ||
| GUS | F: CAACTCGCTGCGTGATGGCATG R: GATAGGAGTGTCCTCATGTTTGCC | 启动子表达载体的GUS基因检测 Detection of GUS gene in promoter expression vector | ||
| Hyg | F: GAACTCACCGCGACGTCTGT R: GGCGTCGGTTTCCACTATCG | 启动子表达载体的Hyg基因检测 Detection of Hyg gene in promoter expression vector | ||
| pAbAi | F: CTTCCTTCTGTTCGGAGATTACCGAATC R: TATACATACAGAGCACATGCCTCG | pAbAi-ProBnMAPK7-rPE×3表达载体的检测 Detection of expression vector of pAbAi- ProBnMAPK7-rPE×3 | ||
| Bait | F: GGCGGATAATGCCTTTAGCGGCTTAACTG R: CCCGGAATGCAGTGAAGGAAAAGCACCG | ProBnMAPK7-rPE×3整合到Y1Hgold染色体的检测 Detection of ProBnMAPK7-rPE×3 integration in to Y1Hgold chromosome | ||
| Y1H | F: CTATCTATTCGATGATGAAGATACCCC R: GCACAGTTGAAGTGAACTTGCGGGGTTTTTC | ProBnMAPK7-rPE的候选上游调控因子检测 Detection of candidate upstream regulators of ProBnMAPK7-rPE | ||
| AD | F: CTATTCGATGATGAAGATACCCCACCAAACCC R: AGTGAACTTGCGGGGTTTTTCAGTATCTACGAT | 文库筛选Prey酵母菌落的检测 Detection of Prey yeast cells from library screening | ||
新窗口打开|下载CSV
1.3 MAPK7基因在拟南芥及甘蓝型油菜中的RNA-seq分析
利用Arabidopsis RNA-seq Database (1.4 启动子表达载体的构建及本氏烟草的遗传转化
1.4.1 植物表达载体pCambia1305.1-ProBnMAPK7- GUS的构建 利用限制性内切酶Nco I和Sal I对pGEM-T-ProBnMAPK7重组载体以及pCambia1305.1-GUS空白表达载体分别进行双酶切, 并将回收纯化的ProBnMAPK7片段连接至pCambia1305.1-GUS骨架以替换35S启动子, 转化E. coli DH5α感受态细胞, 分别采用p1305.1-F+ p1305.1-R、GUS-F+GUS-R以及Hyg-F+Hyg-R (引物序列见表1) 3对引物进行PCR鉴定, 测序后获得阳性pCambia1305.1-ProBnMAPK7-GUS重组质粒。将验证后的重组质粒转化至农杆菌GV3101菌株中, 同时使用利福平Rif、链霉素Str及卡那霉素Kan三种抗生素进行筛选, 并采用p1305.1-F+p1305.1-R、GUS-F+GUS-R和Hyg-F+Hyg-R三对引物检测重组菌株的完整性, 阳性菌株保存备用。1.4.2 表达载体转化本氏烟草 参照本研究室前述方法[28]收集菌体, GV3101-pCambia1305.1- ProBnMAPK7-GUS阳性菌株先以1:1000 (保存菌液:液体培养基)比例进行活化培养1~2 d, 再按照1:100 (活化菌液:液体培养基)比例扩大培养至OD600为0.8~1.0。将菌液收集至离心管中, 室温条件下6000×g离心5 min, 收集菌体。根据靳义荣等[29]方法改进制备重悬菌液, 加入适量的Injection buffer (10 mmol L-1 MgCl2, 10 mmol L-1 MES, 200 μmol L-1 AS)将菌体重悬至OD600为0.4~0.5, 28℃避光静置3~6 h以提高重悬菌液的活性。根据Leuzinger等[30]方法改进, 将重悬菌液注射至1月龄的本氏烟草幼嫩叶片, 注射后的植株置于25℃黑暗培养36~ 48 h, 用于ProBnMAPK7的GUS组织化学染色及信号观察。
1.4.3 启动子的核心区段筛选 根据预测的核心元件位置, 首先将全长启动子截短为左右2个片段, 命名为ProBnMAPK7-lPB和ProBnMAPK7-rPB。分别采用特异引物(表1)进行PCR扩增, 加dA连接至pGEM-T easy载体, 菌液PCR及测序检测后, 使用限制性内切酶Nco I和Sal I将截短后的片段分别连接至空白表达载体pCambia1305.1-GUS骨架。采用与pCambia1305.1-ProBnMAPK7-GUS重组菌株相同的引物进行PCR检测, 测序后的阳性重组表达质粒转化至农杆菌GV3101感受态细胞。重组菌株同样用于本氏烟草的瞬时遗传转化及其GUS组织化学染色及信号观察。根据GUS结果, 选择ProBnMAPK7-lPB和ProBnMAPK7-rPB中具有GUS信号或信号更强的一段序列, 再次截短为2个片段并构建重组表达载体, 瞬时转化本氏烟草后检测其GUS表达信号, 直至筛选到<250 bp大小的核心区段。ProBnMAPK7的核心区段用于酵母单杂交的Bait毒性和自激活活性检测以及文库筛选。
1.5 GUS组织化学染色
分别对全长启动子及逐步截短的不同长度的启动子片段进行GUS信号检测, 以确定ProBnMAPK7的核心区段。阳性对照为空白表达载体pCambia1305.1-GUS农杆菌菌株, 阴性对照为空Injection buffer。将暗培养36~48 h的本氏烟草叶片浸没于GUS Staining buffer (50 mmol L-1 NaH2PO4, 50 mmol L-1 Na2HPO4, 10 mmol L-1 Na2EDTA, 0.5 mmol L-1 K3[Fe(CN)6], 0.5 mol L-1 K4[Fe(CN)6], 0.1% Triton-X100, 0.5 mg mL-1 X-Gluc, pH 7.2)中, 并将其置于37℃黑暗条件下反应8 h以上。反应结束后, 弃GUS Staining buffer, 使用蒸馏水清洗反应后的叶片3次以去除残留的GUS Staining buffer。而后, 向叶片中加入95%乙醇初步脱色10 min, 再使用75%乙醇进行脱色, 直至脱色完全。在体视显微镜(Olympus DP80)下观察GUS信号并照相。1.6 ProBnMAPK7的酵母单杂交文库筛选
1.6.1 pAbAi-ProBnMAPK7-rPE×3的Bait载体及菌株的构建 委托深圳华大基因股份有限公司合成ProBnMAPK7-rPE核心区段的3拷贝串联重复序列ProBnMAPK7-rPE×3。分别采用限制性内切酶Hind III和Sal I对T-ProBnMAPK7-rPE×3以及pAbAi质粒进行双酶切, 将ProBnMAPK7-rPE×3连接至pAbAi骨架, 转化E. coli DH5α感受态细胞。采用pAbAi-F+pAbAi-R引物对pAbAi-ProBnMAPK7- rPE×3 (Bait)菌液进行PCR检测及测序(引物序列见表1)。使用BstB I线性化Bait及阳性对照pAbAi- p53质粒, 1.0%琼脂糖凝胶电泳检测后, 分别回收线性化的Bait及pAbAi-p53骨架。参照Clontech Yeastmaker Yeast Transformation System说明, 制备Y1H gold感受态细胞, 并将线性化的Bait及pAbAi-p53质粒分别转化至Y1H gold细胞, 涂布于SD/-Ura培养基, 置于30℃恒温培养箱内倒置培养1~3 d。分别挑取Y1H-Bait及Y1H-p53单克隆, 加入适量0.9% NaCl稀释菌体, 置于98℃加热10 min后, 采用KOD neo plus高保真酶进行PCR检测, 引物为Bait-F+Bait-R (表1), 阳性菌落保存备用。1.6.2 Y1H-pAbAi-ProBnMAPK7-rPE×3 (Y1H-Bait)的AbA背景检测 首先取适量Y1H gold、Y1H-Bait及Y1H-p53菌液分别涂布至不含AbA和100 ng mL-1 AbA的SD/-Ura平板上, 30℃倒置培养3~5 d, 观察比较菌落的生长情况, 以测试抗生素AbA的有效性。随后, 沾取适量Y1H gold、阳性Y1H-Bait和Y1H-p53菌落至100 μL 0.9% NaCl溶液重悬, 将重悬菌液分别按照10-1、10-2及10-3 3个梯度进行稀释。各取10 μL稀释菌液置于含有不同浓度AbA的SD/-Ura平板上, AbA浓度分别为0、100、200、300、500 ng mL-1, 30℃倒置培养3~5 d后, 观察比较菌落的生长情况, 以确定能够完全抑制Y1H-Bait自激活的最低AbA浓度。阳性对照为Y1H-p53, 阴性对照为Y1H gold空白菌株。
1.6.3 pAbAi-ProBnMAPK7-rPE×3的酵母单杂交文库的筛选及测序 采用Matchmaker Gold Yeast One-Hybrid Library Screening System, 参照Yeastmaker Transformation System 2操作手册, 制备Y1H-p53及Y1H-Bait的酵母感受态细胞。按照本实验室前期酵母双杂交的小量转化法[28]将pGADT7- Rec-p53质粒转化至Y1H-p53感受态细胞, 取100~200 μL转化液涂布于80 mm SD/-Leu/AbA500平板。文库筛选则采用本研究室前期制备的甘蓝型油菜苗期(3~4片真叶)的根系、茎(茎尖2~3 cm)和叶片(芯芽叶及幼叶)的混合cDNA酵母文库[28], 按照文库量转化法进行Y1H-Bait的酵母单杂交文库筛选。将Carrier DNA置于100℃加热5 min, 立即取出置于冰上2 min使其变性。向15 mL无菌螺口离心管内加入20 μL变性Carrier DNA与10 μg文库质粒均匀混合后, 加入600 μL Y1H-Bait酵母感受态细胞, 温和混匀; 加入2.5 mL PEG/LiAc buffer (2 mL 40% PEG-3350, 250 μL 10×TE buffer, 250 μL 1 mol L-1 LiAc), 轻柔涡旋混匀, 30℃孵育45 min, 每间隔15 min温和混匀一次。随后, 加入160 μL DMSO, 轻柔涡旋混匀, 42℃孵育20 min, 每间隔10 min温和混匀一次。取出后2000×g室温离心5 min, 枪头吸除上清, 加入3 mL YPD Plus Medium重悬酵母菌体, 置于30℃摇床复苏90 min。取出后2000×g室温离心5 min, 枪头吸除上清, 加入15 mL 0.9% NaCl 重悬酵母菌体, 涂布于80 mm SD/-Leu/AbA500平板, 每个平板100~200 μL, 直至所有重悬转化液涂布完全。平板置于30℃倒置培养3~5 d后, 观察菌落的生长状况。将文库筛选过程中的所有SD/-Leu/AbA500平板上的酵母菌落划线分离纯化后, 采用AD-F+AD-R引物进行PCR扩增, 并对PCR产物进行测序及序列比对分析。提取分离纯化酵母中的Prey质粒, 转化至E. coli DH5α感受态细胞扩繁质粒后, 分别在AbA浓度为500、800 ng mL-1条件下与Y1H-Bait进行点对点Y1H回转验证。
2 结果与分析
2.1 ProBnMAPK7的克隆及顺式作用元件分析
以甘蓝型油菜中油821基因组DNA为模板, 参考TAIR10和Genoscope数据库设计特异引物扩增BnMAPK7 (BnaA09g09520D)基因上游的启动子序列。经克隆、测序得到BnMAPK7基因起始密码子ATG上游1612 bp长度的启动子片段, 命名为ProBnMAPK7。其中, 腺嘌呤(A) 527个, 占32.69%; 鸟嘌呤(G) 230个, 占14.27%; 胞嘧啶(C) 260个, 占16.13%; 胸腺嘧啶(T) 595个, 占36.91%。利用PlantCARE数据库分析ProBnMAPK7序列所含的关键顺式作用调控DNA元件发现, 该启动子中含有多种响应植物外在信号的顺式作用元件(表2)。如光照响应元件ACE、Box 4、G-box、MRE、TCT-motif等; 感知厌氧诱导、干旱、防御及损伤的响应元件ARE、MBS、TC-rich repeats、WUN-motif等; 激素类(ABA、GA、IAA、MeJA)响应元件ABRE、GARE-motif、TGA-element、CGTCA-motif、TGACG-motif等。表明, BnMAPK7基因的启动子具备多种调控作用元件, 可能通过光合作用、逆境和激素等相关信号途径参与调控甘蓝型油菜的生长发育和胁迫应答过程。
Table 2
表2
表2甘蓝型油菜ProBnMAPK7的主要顺式作用元件预测
Table 2
| 元件名称 Element name | 序列 Sequence (5°-3°) | 功能 Function |
|---|---|---|
| ABRE | ACGTGGC | 响应ABA Abscisic acid responsiveness |
| ACE | CTAACGTATT | 响应光照 Light responsiveness |
| ARE | TGGTTT | 厌氧诱导 Anaerobic induction |
| Box 4 | ATTAAT | 光响应的保守DNA模块 A conserved DNA module involved in light responsiveness |
| CGTCA-motif | CGTCA | 响应MeJA MeJA responsiveness |
| G-box | ACACGTGGC | 响应光照 Light responsiveness |
| GARE-motif | TCTGTTG | 响应GA Gibberellin responsiveness |
| MBS | CGGTCA | 干旱诱导的MYB结合位点 MYB binding site involved in drought-inducibility |
| MRE | AACCTAA | 响应光照的MYB结合位点 MYB binding site involved in light responsiveness |
| TC-rich repeats | GTTTTCTTAC | 响应防御和胁迫 Defense and stress responsiveness |
| TCT-motif | TCTTAC | 响应光照 Light responsiveness |
| TGA-element | AACGAC | 响应IAA Auxin responsiveness |
| TGACG-motif | TGACG | 响应MeJA MeJA responsiveness |
| WUN-motif | TCATTACGAA | 响应损伤 Wound responsiveness |
新窗口打开|下载CSV
2.2 AtMAPK7 (At2g18170)及BnMAPK7 (Bna A09g09520D)基因在RNA-seq中的表达模式分析
本研究从Arabidopsis RNA-seq Database (图1
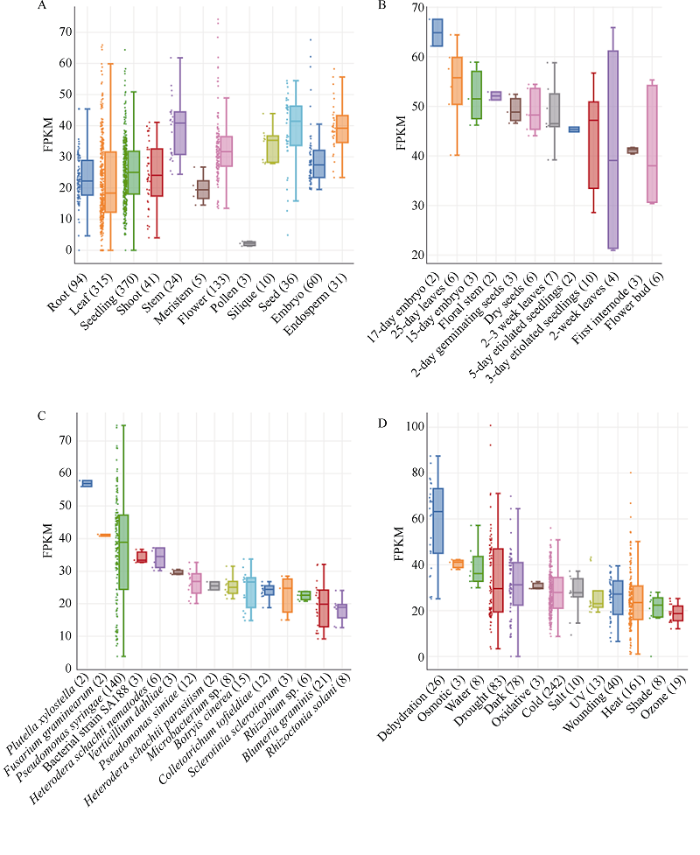 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1AtMAPK7 (At2g18170)在拟南芥的不同组织器官(A)、不同生长时期(B)以及生物(C)和非生物(D)胁迫过程中的表达模式分析
横坐标表示不同的组织器官、发育时期或胁迫处理, 括号内的数字表示富集到的文库数量; 纵坐标表示AtMAPK7基因在均一化后的各个文库中的FPKM值。
Fig. 1Relative expression patterns of AtMAPK7 (At2g18170) in different tissues (A), at different stages (B), biotic (C), and abiotic (D) stresses in Arabidopsis
The abscissa represents different tissues, developmental stages, or stress treatments, the number in parentheses represents the number of enriched libraries; the ordinate represents the FPKM value of AtMAPK7 gene in each normalization library.
根据本研究室甘蓝型油菜中双11植株不同组织器官及不同生长时期的RNA-seq数据对BnMAPK7 (BnaA09g09520D)基因的时空表达模式进行分析发现, BnMAPK7 (BnaA09g09520D)基因在甘蓝型油菜整个生长发育期的不同组织器官中均有表达(图2-A), 表明该基因的组织表达不具备时空特异性。此外, 根据本课题组前期对甘蓝型油菜中油821植株在冷、干旱、热以及弱光胁迫处理的RNA-seq数据, 分析BnMAPK7 (BnaA09g09520D)基因在4种胁迫处理下的响应程度。结果显示, BnMAPK7基因在上述4种胁迫环境下的基因表达量均上调, 分别是正常环境(Mock)下该基因表达量的4.51、4.34、1.30、4.44倍(图2-B), 表明BnMAPK7对冷、干旱、热及弱光胁迫具有正向调控作用。因此, 我们推测, MAPK7基因的表达模式具有保守性, 在植物的生长发育过程以及生物和非生物胁迫等逆境响应过程中具有重要的调控功能。
图2
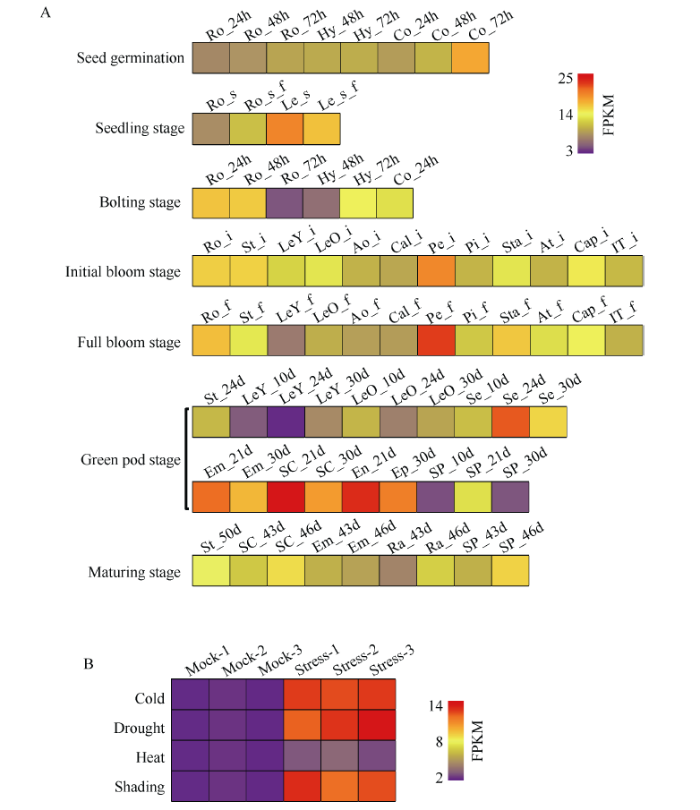 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2BnMAPK7 (BnaA09g09520D)在甘蓝型油菜不同时期不同组织中的表达模式(A)和逆境响应模式(B)分析
Ro_24h、Ro_48h、Ro_72h分别表示种子萌发24 h、48 h、72 h的胚根; Hy_48h、Hy_72h分别表示种子萌发48 h、72h的下胚轴; Co_24h、Co_48h、Co_72h分别表示种子萌发24 h、48 h、72 h的子叶。Ro_s、Ro_s_f分别表示苗期的温室植株根系、大田植株根系; Le_s、Le_s_f分别表示苗期的温室植株叶片、大田植株叶片。Ro_b、St_b、LeY_b、LeO_b、Bu_b、IT_b分别表示蕾薹期的根系、茎、幼叶、成熟叶片、花蕾、主序顶端。Ro_i、St_i、LeY_i、LeO_i、Ao_i、Cal_i、Pe_i、Pi_i、Sta_i、At_i、Cap_i、IT_i分别表示初花期的根系、茎、幼叶、成熟叶片、花柄、萼片、花瓣、雌蕊、雄蕊、花药、花丝、主序顶端。Ro_f、St_f、LeY_f、LeO_f、Ao_f、Cal_f、Pe_f、Pi_f、Sta_f、At_f、Cap_f、IT_ f分别表示盛花期的根系、茎、幼叶、成熟叶片、花柄、萼片、花瓣、雌蕊、雄蕊、花药、花丝、主序顶端。St_24d表示青荚期花后24 d的茎; LeY_10d、LeY_24d、LeY_30d分别表示青荚期花后10 d、24 d、30 d的幼叶; LeO_10d、LeO_24d、LeO_30d分别表示青荚期花后10 d、24 d、30 d的成熟叶片; Se_10d、Se_24d、Se_30d分别表示青荚期花后10 d、24 d、30 d的种子; Em_21d、Em_30d分别表示青荚期花后21 d、30 d的种胚; SC_21d、SC_30d分别表示青荚期花后21 d、30 d的种皮; En_21d表示青荚期花后21 d的内种皮; Ep_30d表示青荚期花后30 d的外种皮; SP_10d、SP_24d、SP_30d表示青荚期花后10 d、24 d、30 d的荚果皮。St_50d表示成熟期花后50 d的茎; SC_43d、SC_46d分别表示成熟期花后43 d、46 d的种皮; Em_43d、Em_46d分别表示成熟期花后43 d、46 d的种胚; Ra_43d、Ra_46d分别表示成熟期花后43 d、46 d的胚芽; SP_43d、SP_46d分别表示成熟期花后43 d、46 d的荚果皮。
Fig. 2Relative expression patterns of BnMAPK7 (BnaA09g09520D) in different tissues at different stages (A) and stresses responses (B) in Brassica napus
Ro_24h, Ro_48h, and Ro_72h represent the root at 24, 48, and 72 hours after seed germination, respectively; Hy_48h and Hy_72h represent the hypocotyl at 48 hours and 72 hours after seed germination, respectively; Co_24h, Co_48h, and Co_72h represent the cotyledon at 24, 48, and 72 hours after seed germination, respectively. Ro_s, Ro_s_f represent the root from seedlings planted in greenhouse and field at the seedling stage, respectively; Le_s, Le_s_f represent the leaf from seedlings planted in greenhouse and field at the seedling stage, respectively. Ro_b, St_b, LeY_b, LeO_b, Bu_b, and IT_b represent the root, stems, young leaf, mature leaf, bud, and the tip of main inflorescence at the bolting stage, respectively. Ro_i, St_i, LeY_i, LeO_i, Ao_i, Cal_i, Pe_i, Pi_i, Sta_i, At_i, Cap_i, and IT_i represent the root, stem, young leaf, mature leaf, anthocallus, calyx, petal, pistil, stamen, anther, capillament, and the tip of main inflorescence at the initial bloom stage, respectively. Ro_f, St_f, LeY_f, LeO_f, Ao_f, Cal_f, Pe_f, Pi_f, Sta_f, At_f, and Cap_f, IT_f represent the root, stem, young leaf, mature leaf, anthocallus, calyx, petal, pistil, stamen, anther, capillament, and the tip of main inflorescence at the full bloom stage, respectively. St_24d represents the stem at 24 days after flowering at the green pod stage; LeY_10d, LeY_24d, and LeY_30d represent the young leaf at 10, 24, and 30 days after flowering at the green pod stage, respectively; LeO_10d, LeO_24d, and LeO_30d represent the mature leaf at 10, 24, and 30 days after flowering at the green pod stage, respectively; Se_10d, Se_24d, and Se_30d represent the seed at 10, 24, and 30 days after flowering at the green pod stage, respectively; Em_21d and Em_30d represent the embryo at 21 days and 30 days after flowering at the green pod stage, respectively; SC_21d and SC_30d represent the seed coat at 21 days and 30 days after flowering at the green pod stage, respectively; En_21d represents the endotesta at 21 days after flowering at the green pod stage; Ep_30d represents the episperm at 30 days after flowering at the green pod stage; SP_10d, SP_24d, and SP_30d represent the silique pericarp at 10, 21, and 30 days after flowering at the green pod stage, respectively. St_50d represents the stem at 50 days after flowering at the mature stage; SC_43d and SC_46d represent the seed coat at 43 days and 46 days after flowering at the mature stage, respectively; Em_43d and Em_46d represent the embryo at 43 days and 46 days after flowering at the mature stage, respectively; Ra_43d and Ra_46d represent the radicle at 43 days and 46 days after flowering at the mature stage, respectively; SP_43d and SP_46d represent the silique pericarp at 43 days and 46 days after flowering at the mature stage, respectively.
2.3 ProBnMAPK7的核心区段定位于-467~ -239 bp区域
为查找BnMAPK7启动子的主要活性区域, 本研究将ProBnMAPK7全长序列(ProBnMAPK7-PA)连接至pCambia1305.1表达载体中, 通过农杆菌介导瞬时转化至本氏烟草叶片。根据顺式作用元件的预测结果, 将1612 bp的全长ProBnMAPK7-PA片段首先截短为539 bp长度的ProBnMAPK7-lPB片段和1073 bp长度的ProBnMAPK7-rPB片段(图3)。GUS染色结果显示, ProBnMAPK7-PA和ProBnMAPK7- rPB均能够启动GUS表达, 而ProBnMAPK7-lPB转化至烟草叶片后未检测到GUS信号(图4)。将ProBnMAPK7-rPB片段截短为212 bp长度的ProBn MAPK7-lPC片段和861 bp长度的ProBnMAPK7- rPC片段(图3), 仅在ProBnMAPK7-rPC叶片中检测到GUS信号(图4)。随后, 将ProBnMAPK7-rPC片段截短为623 bp长度的ProBnMAPK7-lPD和238 bp长度的ProBnMAPK7-rPD片段(图3), ProBnMAPK7- lPD叶片中具有较强的GUS信号, 而ProBnMAPK7- rPD叶片中无GUS表达(图4)。再将ProBnMAPK7- lPD截短为394 bp长度的ProBnMAPK7-lPE片段和229 bp长度的ProBnMAPK7-rPE片段(图3), 2个片段均能启动GUS表达, 但ProBnMAPK7-lPE叶片中的GUS信号非常弱, 而ProBnMAPK7-rPE叶片中有较强的GUS表达(图4)。因此, 我们推测, ProBn MAPK7的核心区段为-467~ -239 bp位置的ProBn MAPK7-rPE区段, 长度为229 bp, 可用于酵母单杂交的文库筛选。图3
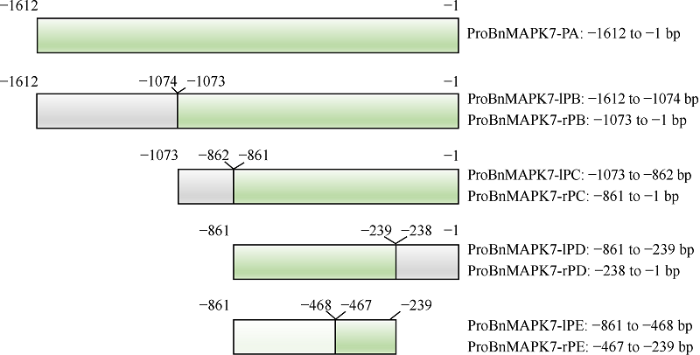 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3ProBnMAPK7不同长度片段示意图
Fig. 3Schematic diagram of different lengths of ProBnMAPK7 regions
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4瞬时表达筛选ProBnMAPK7核心区段的GUS组织化学染色
Fig. 4GUS histochemical staining of transient expression screening the core fragment of ProBnMAPK7
2.4 pAbAi-ProBnMAPK7-rPE×3在Y1H gold酵母细胞中的毒性及自激活检测
将3个拷贝的ProBnMAPK7-rPE片段进行串联重复, 连接至pAbAi表达载体。将线性化后的pAbAi-ProBnMAPK7-rPE×3整合至Y1H gold基因组中, 成功获得Y1H-Bait重组酵母菌株。同时, 将线性化的pAbAi-p53整合至Y1H gold基因组中, 成功获得Y1H-p53阳性对照酵母菌株。使用不同浓度梯度的AbA抗生素对Y1H-Bait的本底表达背景进行检测发现, 阴性对照Y1H gold在不同筛选压的培养基上均不能生长; 在低浓度AbA的筛选培养基上, 阳性对照Y1H-p53及试验组Y1H-Bait能够生长, 表明Bait对Y1H gold细胞无毒性。高浓度的AbA对Y1H-p53及Y1H-Bait具有明显的抑制效应, 在AbA浓度为100 ng mL-1时, Y1H-p53及Y1H-Bait受到明显的抑制; AbA浓度达到300 ng mL-1时, 二者几乎不再生长; AbA浓度为500 ng mL-1时, Y1H-p53及Y1H-Bait的生长均被完全抑制(图5)。表明, Y1H-Bait具有自激活活性, 可使用500 ng mL-1浓度的AbA抑制其本底表达, 进行酵母单杂交文库筛选。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5Y1H-Bait酵母菌株的毒性与自激活活性检测(A)以及Y1H文库筛选回转验证(B)
Fig. 5Toxicity and autoactivation test of Y1H-Bait in yeast cells (A) and retransformation validation in Y1H screening assays (B)
2.5 甘蓝型油菜ProBnMAPK7-rPE核心区段的Y1H文库筛选获得3个候选上游调控因子
为查找调控BnMAPK7基因转录表达的上游因子, 本研究利用酵母单杂交技术对ProBnMAPK7- rPE核心区段进行文库筛选, 分离纯化后, 采用高保真酶对酵母单菌落进行PCR扩增, 共获得5个互作菌落。BLAST结果显示, 共获得3个候选上游调控因子; 根据拟南芥同源蛋白信息, 将其分别命名为BnNAD1B (NADH dehydrogenase 1B)、BnERD6 (Early response to dehydration 6)和BnPIG3 (quinone oxidoreductase PIG3-like), 出现的次数分别为2、2和1次。提取Y1H文库筛选获得的酵母质粒, 并分别与Y1H-Bait进行点对点回转验证(图5-B), BnNAD1B、BnERD6和BnPIG3在AbA浓度为500 ng mL-1甚至800 ng mL-1时, 均能够与ProBnMAPK7-rPE结合。拟南芥TAIR10数据库的注释信息显示, NAD1B编码线粒体NAD(P)H脱氢酶的亚基NAD1B, 主要参与细胞呼吸、氧化还原过程及光呼吸过程; ERD6编码蔗糖转运蛋白, 主要参与碳水化合物的跨膜转运, 响应ABA、几丁质、冷胁迫、盐胁迫以及缺水胁迫等; PIG3编码醌氧化还原酶, 属于锌结合脱氢酶家族, 主要参与氧化还原过程等(表3)。这些结果与BnMAPK7启动子顺式作用元件预测结果基本一致, 表明甘蓝型油菜BnNAD1B、BnERD6和BnPIG3蛋白可能通过与核心区段ProBnMAPK7-rPE相结合, 调控BnMAPK7的转录表达, 进而参与光合作用和逆境胁迫应答等过程。Table 3
表3
表3甘蓝型油菜ProBnMAPK7酵母单杂交文库筛选的上游调控因子注释信息
Table 3
| 基因名称 Gene name | 基因编号(甘蓝型油菜/拟南芥) Gene ID (B. napus/A. thaliana) | 出现次数 Frequency | 基因注解 Gene description | 基因功能 Gene function |
|---|---|---|---|---|
| BnNAD1B | chrUn_random ATMG01120 | 2 | 编码从3个前体NAD1A、NAD1B和NAD1C反式剪接的线粒体NAD(P)H脱氢酶的亚基。 Encodes subunit of mitochondrial NAD(P)H dehydrogenase that is trans-spliced from three precursors, NAD1A, NAD1B, and NAD1C. | 参与细胞呼吸、氧化还原过程、光呼吸。 Involved in cellular respiration, oxidation-reduction process, photorespiration. |
| BnERD6 | BnaC08g42900D BnaA09g48640D AT1G08930 | 2 | 编码假定的蔗糖转运蛋白, 其基因表达受到脱水和冷诱导。 Encodes a putative sucrose transporter whose gene expression is induced by dehydration and cold. | 参与碳水化合物的跨膜转运, 响应ABA和几丁质, 响应冷、盐、缺水胁迫。 Involved in carbohydrate transmembrane transport, response to abscisic acid, response to chitin, response to cold, response to salt stress, response to water deprivation. |
| BnPIG3 | BnaA01g11430D AT4G21580 | 1 | 氧化还原酶, 锌结合脱氢酶家族蛋白。 Oxidoreductase, zinc-binding dehydrogenase family protein. | 参与氧化还原过程。 Involved in oxidation-reduction process |
新窗口打开|下载CSV
3 讨论
MAPKs级联在进化上是一种高度保守的信号通路。当植物感受到外源刺激时, MAPKs级联被快速激活并将信号转入胞内, 通过调控特定的基因簇[6]或翻译后修饰[10], 引起下游基因表达、磷酸化和去磷酸化、磷脂代谢、钙调节、蛋白降解等生物学途径发生变化[8]。位于MAPKs级联模块最下游的MAPKs基因被报道, 参与调控植物生长、发育、细胞凋亡等过程, 在应对多种生物及非生物胁迫, 包括冷、热、氧化胁迫、紫外线、干旱、病原菌侵害等方面具有重要作用[13,14,15]。本研究室前期根据白菜(Brassica rapa)基因组序列, 获得了甘蓝型油菜中AtMAPK7和Bra037234垂直同源基因的启动子JX827381.1, 大小为1058 bp[23]。近年来, 得益于测序技术的飞速发展, 甘蓝型油菜的测序工作得到了极大的推进, 我们结合拟南芥和甘蓝型油菜数据库, 对甘蓝型油菜BnMAPK7 (BnaA09g09520D)的启动子序列及其潜在的顺式作用元件信息进行了完善, ProBnMAPK7大小为1612 bp。启动子的顺式作用元件种类和数量的多样性直接影响基因的表达模式[31]。JX827381.1和ProBnMAPK7序列中, 均具有响应ABA的ABRE元件。研究报道, 拟南芥AtMKK3-AtMAPK7复合体可保护AtMAPK7免受降解[18], 且AtMAPKKK17/ 18-AtMKK3-AtMAPK7级联与ABA信号途径交联, 参与调控ABA介导的植物逆境胁迫应答[19]。玉米ZmMAPK7受到ABA的诱导后, 正向调控ABA介导的渗透胁迫应答[20]。棉花GhMKK3-GhMAPK7途径受到ABA及干旱诱导, 并通过调控根系和气孔的水分运输响应干旱胁迫应答[22]。水稻OsMAPKKK62- OsMKK3-OsMAPK7级联被报道通过磷酸化修饰调控下游基因的转录表达进而负调控种子的休眠过程以及种子萌发期和成熟后期对ABA的敏感性[32]。我们前期对甘蓝型油菜进行胁迫处理发现, BnMAPK7基因能够快速响应ABA, 且转录水平明显上调[23]。表明MAPK7在不同物种中对ABA的响应具有保守性。因此, 我们推测ABRE元件在BnMAPK7响应ABA介导的逆境胁迫应答过程中具有重要作用。另外, JX827381.1和ProBnMAPK7中均存在防御相关的TC-rich repeats元件。拟南芥中, AtMAPK7受到多种生物胁迫的诱导与激活, 包括小菜蛾、谷禾镰刀菌、黄萎病(Verticillium dahliae)、立枯病、核盘菌等(图1-C)。棉花GhMAPK7基因被报道也参与调控病原菌的防御过程, 包括立枯病、炭疽病和枯萎病[21]。本课题组前期对甘蓝型油菜进行核盘菌胁迫处理发现, BnMAPK7能够快速响应核盘菌, 转录水平在24 h内迅速上调[23]。这些证据表明, ProBnMAPK7中TC-rich repeats元件可能在甘蓝型油菜防御相关的过程中扮演着重要角色。值得注意的是, JX827381.1和ProBnMAPK7中还有大量的光照响应元件ACE、Box4、G-box、TCT-motif等。本研究在甘蓝型油菜RNA-seq数据中发现, BnMAPK7基因在弱光胁迫处理条件下的转录水平显著上调, 这可能与ProBnMAPK7中存在的光照响应元件相关; 对甘蓝型油菜ProBnMAPK7的顺式作用元件和BnMAPK7时空表达及逆境响应模式的分析证实了BnMAPK7具有转录表达保守性和功能多样性, 我们推测该基因在生长发育、生物和非生物胁迫应答过程中均具有重要的调控功能。
在MAPKs级联中, MKK3通过磷酸化激活下游MAPK7, 使得MAPK7参与调控植物的生长发育和抗逆等多种生物学过程。然而, 不依赖于MAPKs级联的上游因子对MAPK7转录调控的分子网络还很空白。本研究通过农杆菌介导的本氏烟草瞬时表达发现, ProBnMAPK7的核心区段定位于-467~-239 bp, 并通过酵母单杂交技术对BnMAPK7的上游调控因子进行文库筛选, 获得了3个潜在的候选因子BnNAD1B、BnERD6和BnPIG3。研究报道, 拟南芥NAD1由NAD1A、NAD1B和NAD1C 3个亚基构成, 是线粒体呼吸链NADH脱氢酶复合体(Complex I)的重要组成部分, NAD1的生物活性直接影响线粒体的活性和光呼吸, 参与调控植物的生长发育和初级代谢过程[33,34], 并维持电子传递、ATP合成和NADH氧化等过程的正常进行[35,36]。本研究在ProBnMAPK7中发现了大量的光响应顺式作用元件, 且BnMAPK7基因在弱光胁迫下的转录水平显著上调, 我们推测, 甘蓝型油菜BnNAD1B通过与ProBnMAPK7-rPE核心区段结合, 调节线粒体的生物活性和光呼吸过程, 进而响应植物的非生物胁迫应答。ERD6是一种脱水诱导早期应答转录因子基因, 存在于细菌、酵母、植物和哺乳动物中, 编码蔗糖转运蛋白, 受到缺水和冷胁迫的诱导[37]。植物中ERD6还被报道响应几丁质、ABA、盐胁迫等, 可能通过与液泡转化酶协同作用, 参与调控植物细胞的蔗糖运输和胞内渗透压[38]。本研究发现, BnERD6能够与ProBnMAPK7结合, 并且BnMAPK7基因能够迅速响应ABA和干旱胁迫处理, 表明BnERD6可能是BnMAPK7在转录水平上响应ABA和干旱的重要上游调控因子。PIG3编码醌氧化还原酶类蛋白, 与人p53诱导基因PIG3同源, 研究表明, PIG3在人的肿瘤及癌症中参与平衡氧化还原过程及DNA损伤[39,40]。对拟南芥防御信号的研究过程中发现, AtPIG3具有锌结合脱氢酶活性, 可能在防御过程中增强植株的抗氧化能力[41,42]。本试验结果显示, BnPIG3蛋白能够与ProBnMAPK7-rPE结合, 表明甘蓝型油菜BnPIG3和BnMAPK7可能在氧化还原过程中具有一定的生物学意义。基于ProBnMAPK7的酵母单杂交文库筛选, 挖掘了不依赖于MAPKs级联途径的BnMAPK7的上游调控因子, 进一步证实了ProBnMAPK7中的顺式作用元件在生长发育和逆境胁迫应答过程中的调控作用。因此, 对BnMAPK7功能的深入研究必将大大推动植物MAPKs级联途径在提高甘蓝型油菜生长发育和抗逆等机制中的应用, 为改良甘蓝型油菜品质、培养抗逆新品种提供重要的理论依据。
4 结论
从甘蓝型油菜中油821品种基因组DNA中克隆了BnMAPK7启动子ProBnMAPK7, 全长1612 bp, 包含响应光照、激素及逆境胁迫等顺式作用元件ACE、MRE、ABRE、CGTCA-motif和TC-rich repeats等。MAPK7在拟南芥和甘蓝型油菜中, 在各个组织器官及各个时期中均有表达, 并响应生物及非生物胁迫, 证实了启动子顺式作用元件的功能。此外, ProBnMAPK7的核心区段定位于-467~-239 bp (ProBnMAPK7-rPE)区段。Y1H文库筛选到3个上游候选调控因子: BnNAD1B、BnERD6和BnPIG3。本研究为植物MAPKs级联途径的功能研究奠定了基础, 也为合理利用MAPKs基因启动子以改良作物的生长发育和抗逆性提供了依据。附图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT附图1甘蓝型油菜ProBnMAPK7的序列及其顺式作用元件
Fig. S1Sequence of ProBnMAPK7 and its cis-acting elements in Brassica napus
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 1]

The c-Jun NH(2)-terminal kinase (JNK) group of mitogen-activated protein kinases (MAPKs) is activated in response to the treatment of cells with inflammatory cytokines and by exposure to environmental stress. JNK activation is mediated by a protein kinase cascade composed of a MAPK kinase and a MAPK kinase kinase. Here we describe the molecular cloning of a putative molecular scaffold protein, JIP3, that binds the protein kinase components of a JNK signaling module and facilitates JNK activation in cultured cells. JIP3 is expressed in the brain and at lower levels in the heart and other tissues. Immunofluorescence analysis demonstrated that JIP3 was present in the cytoplasm and accumulated in the growth cones of developing neurites. JIP3 is a member of a novel class of putative MAPK scaffold proteins that may regulate signal transduction by the JNK pathway.
DOIURL [本文引用: 3]
DOIPMID [本文引用: 1]

The sensing of stress signals and their transduction into appropriate responses is crucial for the adaptation and survival of plants. Kinase cascades of the mitogen-activated protein kinase (MAPK) class play a remarkably important role in plant signalling of a variety of abiotic and biotic stresses. MAPK cascade-mediated signalling is an essential step in the establishment of resistance to pathogens. Here, we describe the most recent insights into MAPK-mediated pathogen defence response regulation with a particular focus on the cascades involving MPK3, MPK4 and MPK6. We also discuss the strategies developed by plant pathogens to circumvent, inactivate or even 'hijack' MAPK-mediated defence responses.
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 2]

Although mitogen-activated protein kinase (MAPK) signal transduction cascades are known regulators of various aspects of plant biology, our knowledge of these systems has been largely restricted to a small subset of the MAPKs. However, global analyses are now revealing that many more of these kinases are probably engaged in modulating developmental and fitness adaptation processes in the plant kingdom. In this review, we show how these new findings are beginning to define the overall architecture of plant MAPK signaling, with a particular focus on the interplay between the terminal MPKs and their activators, inactivators and cellular targets.Copyright 2009 Elsevier Ltd. All rights reserved.
DOIPMID [本文引用: 1]

Plants have evolved with complex signaling circuits that operate under multiple conditions and govern numerous cellular functions. Stress signaling in plant cells is a sophisticated network composed of interacting proteins organized into tiered cascades where the function of a molecule is dependent on the interaction and the activation of another. In a linear scheme, the receptors of cell surface sense the stimuli and convey stress signals through specific pathways and downstream phosphorylation events controlled by mitogen-activated protein (MAP) kinases and second messengers, leading to appropriate adaptive responses. The specificity of the pathway is guided by scaffolding proteins and docking domains inside the interacting partners with distinctive structures and functions. The flexibility and the fine-tuned organization of the signaling molecules drive the activated MAP kinases into the appropriate location and connection to control and integrate the information flow. Here, we overview recent findings of the involvement of MAP kinases in major abiotic stresses (drought, cold and temperature fluctuations) and we shed light on the complexity and the specificity of MAP kinase signaling modules.
DOIURL [本文引用: 1]
DOIPMID [本文引用: 3]

Many changes in environmental conditions and hormones are mediated by MAPK (mitogen-activated protein kinase) cascades in all eukaryotes, including plants. Studies of MAPK pathways in genetic model organisms are especially informative in revealing the molecular mechanisms by means of which MAPK cascades are controlled and modulate cellular processes. The present review highlights recent insights into MAPK-based signalling in Arabidopsis thaliana (thale cress), revealing the complexity and future challenges to understanding signal-transduction networks on a global scale.
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
PMID [本文引用: 1]

Mitogen-activated protein kinase (MAPK) cascades play an important role in mediating stress responses in plants. In Arabidopsis, 20 MAPKs have been identified and classified into four major groups (A-D). Little is known about the role of group C MAPKs. We have studied the activation of Arabidopsis subgroup C1 MAPKs (AtMPK1/AtMPK2) in response to mechanical injury. An increase in their kinase activity was detected in response to wounding that was blocked by cycloheximide. Jasmonic acid (JA) activated AtMPK1/AtMPK2 in the absence of wounding. Wound and JA-induction of AtMPK1/2 kinase activity was not prevented in the JA-insensitive coi1 mutant. Other stress signals, such as abscisic acid (ABA) and hydrogen peroxide, activated AtMPK1/2. This report shows for the first time that regulation of AtMPK1/2 kinase activity in Arabidopsis might be under the control of signals involved in different kinds of stress.
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
[本文引用: 4]
[本文引用: 4]
DOIPMID [本文引用: 1]

Designing PCR primers for amplifying regions of eukaryotic genomes is a complicated task because the genomes contain a large number of repeat sequences and other regions unsuitable for amplification by PCR. We have developed a novel k-mer based masking method that uses a statistical model to detect and mask failure-prone regions on the DNA template prior to primer design. We implemented the software as a standalone software primer3_masker and integrated it into the primer design program Primer3.The standalone version of primer3_masker is implemented in C. The source code is freely available at https://github.com/bioinfo-ut/primer3_masker/ (standalone version for Linux and macOS) and at https://github.com/primer3-org/primer3/ (integrated version). Primer3 web application that allows masking sequences of 196 animal and plant genomes is available at http://primer3.ut.ee/.maido.remm@ut.ee.Supplementary data are available at Bioinformatics online.
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
PMID [本文引用: 1]

Pathogen-inducible plant promoters contain multiple cis-acting elements, only some of which may contribute to pathogen inducibility. Therefore, we made defined synthetic promoters containing tetramers of only a single type of element and present evidence that a range of cis-acting elements (boxes W1, W2, GCC, JERE, S, Gst1, and D) can mediate local gene expression in planta after pathogen attack. The expression patterns of the promoters were monitored during interactions with a number of pathogens, including compatible, incompatible, and nonhost interactions. Interestingly, there were major differences in the inducibilities of the various promoters with the pathogens tested as well as differences in the speed of induction and in the basal expression levels. We also show that defense signaling is largely conserved across species boundaries at the cis-acting element level. Many of these promoters also direct local wound-induced expression, and this provides evidence for the convergence of resistance gene, nonhost, and wound responses at the level of the promoter elements. We have used these cis-acting elements to construct improved synthetic promoters and show the effects of varying the number, order, and spacing of such elements. These promoters are valuable additions to the study of signaling and transcriptional activation during plant-pathogen interactions.
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
PMID [本文引用: 1]

The mitochondrial NADH:ubiquinone oxidoreductase complex (Complex I) is a large protein complex formed from both nuclearly and mitochondrially encoded subunits. Subunit ND1 is encoded by a mitochondrial gene comprising five exons, and the mature transcript requires four RNA splicing events, two of which involve trans-splicing independently transcribed RNAs. We have identified a nuclear gene (OTP43) absolutely required for trans-splicing of intron 1 (and only intron 1) of Arabidopsis thaliana nad1 transcripts. This gene encodes a previously uncharacterized pentatricopeptide repeat protein. Mutant Arabidopsis plants with a disrupted OTP43 gene do not present detectable mitochondrial Complex I activity and show severe defects in seed development, germination, and to a lesser extent in plant growth. The alternative respiratory pathway involving alternative oxidase is significantly induced in the mutant.
DOIURL [本文引用: 1]
PMID [本文引用: 1]

Previously, we constructed a cDNA library from Arabidopsis plants that were exposed to dehydration stress for 1 h and obtained the ERD6 clone. Here we report that the ERD6 cDNA consists of 1741 bp and encodes a polypeptide of 496 amino acids having a predicted molecular weight of 54,354. The putative polypeptide of ERD6 is related to those of sugar transporters of bacteria, yeasts, plants and mammals. Hydropathy analysis revealed that ERD6 protein has 12 putative transmembrane domains and a central hydrophilic region. Sequences that are conserved at the ends of the 6th and 12th membrane-spanning domains of sugar transporters are also present in ERD6. These data suggest that ERD6 encodes a sugar transporter. Genomic Southern blots indicate that the ERD6 gene is a member of a multigene family in the Arabidopsis genome. The expression of the ERD6 gene was induced not only by dehydration but also by cold treatment.Copyright 1998 Elsevier Science B.V.
DOIPMID [本文引用: 1]

Sugars play indispensable roles in biological reactions and are distributed into various tissues or organelles via transporters in plants. Under abiotic stress conditions, plants accumulate sugars as a means to increase stress tolerance. Here, we report an abiotic stress-inducible transporter for monosaccharides from Arabidopsis thaliana that is termed ESL1 (ERD six-like 1). Expression of ESL1 was induced under drought and high salinity conditions and with exogenous application of abscisic acid. Promoter analyses using beta-glucuronidase and green fluorescent protein reporters revealed that ESL1 is mainly expressed in pericycle and xylem parenchyma cells. The fluorescence of ESL1-green fluorescent protein-fused protein was detected at tonoplast in transgenic Arabidopsis plants and tobacco BY-2 cells. Furthermore, alanine-scanning mutagenesis revealed that an N-terminal LXXXLL motif in ESL1 was essential for its localization at the tonoplast. Transgenic BY-2 cells expressing mutated ESL1, which was localized at the plasma membrane, showed an uptake ability for monosaccharides. Moreover, the value of K(m) for glucose uptake activity of mutated ESL1 in the transgenic BY-2 cells was extraordinarily high, and the transport activity was independent from a proton gradient. These results indicate that ESL1 is a low affinity facilitated diffusion transporter. Finally, we detected that vacuolar invertase activity was increased under abiotic stress conditions, and the expression patterns of vacuolar invertase genes were similar to that of ESL1. Under abiotic stress conditions, ESL1 might function coordinately with the vacuolar invertase to regulate osmotic pressure by affecting the accumulation of sugar in plant cells.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
