 ,, 别同德, 王慧, 赵仁慧, 范金平, 张伯桥, 吴素兰, 王玲, 汪尊杰, 高德荣
,, 别同德, 王慧, 赵仁慧, 范金平, 张伯桥, 吴素兰, 王玲, 汪尊杰, 高德荣 ,*江苏里下河地区农业科学研究所 / 农业农村部长江中下游小麦生物学与遗传育种重点实验室, 江苏扬州 225007
,*江苏里下河地区农业科学研究所 / 农业农村部长江中下游小麦生物学与遗传育种重点实验室, 江苏扬州 225007Evaluation and molecular detection of three major diseases resistance of new bred wheat varieties (lines) from the lower reaches of the Yangtze River
LYU Guo-Feng ,, BIE Tong-De, WANG Hui, ZHAO Ren-Hui, FAN Jin-Ping, ZHANG Bo-Qiao, WU Su-Lan, WANG Ling, WANG Zun-Jie, GAO De-Rong
,, BIE Tong-De, WANG Hui, ZHAO Ren-Hui, FAN Jin-Ping, ZHANG Bo-Qiao, WU Su-Lan, WANG Ling, WANG Zun-Jie, GAO De-Rong ,*Lixiahe Institute of Agricultural Sciences / Key Laboratory of Wheat Biology and Genetic Improvement for Lower & Middle Yangtze Valley, Ministry of Agriculture and Rural Affairs, Yangzhou 225007, Jiangsu, China
,*Lixiahe Institute of Agricultural Sciences / Key Laboratory of Wheat Biology and Genetic Improvement for Lower & Middle Yangtze Valley, Ministry of Agriculture and Rural Affairs, Yangzhou 225007, Jiangsu, China通讯作者: * 高德荣, E-mail:gdr@wheat.org.cn
收稿日期:2020-12-15接受日期:2021-04-26网络出版日期:2021-06-02
| 基金资助: |
Corresponding authors: * E-mail:gdr@wheat.org.cn
Received:2020-12-15Accepted:2021-04-26Published online:2021-06-02
| Fund supported: |
作者简介 About authors
E-mail:lgf@wheat.org.cn

摘要
小麦赤霉病、白粉病和黄花叶病是长江下游麦区小麦生产的主要病害。本研究对长江下游麦区新育成49个品种(系)的上述3种病害进行抗性鉴定, 同时利用与抗赤霉病主效QTL Fhb1和QFhs.crc-2D、抗白粉病基因Pm21以及抗黄花叶病主效QTL QYm.nau-5A.1和QYm.nau-2D连锁的分子标记检测试验品种3种病害抗病基因/QTL组成。结果显示, 49.0%的品种赤霉病抗性达中抗以上, 32.6%的品种对白粉病免疫或抗, 44.9%的品种抗黄花叶病。30.6%和73.6%的试验品种分别含有抗赤霉病主效QTL Fhb1和QFhs.crc-2D, 宁麦9号和扬麦158及其衍生品种分别是Fhb1和QFhs.crc-2D的主要载体品种; 28.6%的品种含抗白粉病基因Pm21, 镇麦9号和扬麦18及其衍生品种为Pm21的主要载体品种; 分子检测含抗黄花叶病主效QTL QYm.nau-5A.1和QYm.nau-2D的品种比例均为24.5%, 宁麦9号和苏麦6号及其衍生品种分别是QYm.nau-5A.1和QYm.nau-2D的主要载体品种。宁麦9号和扬麦158衍生品种在小麦抗赤霉病和黄花叶病基因/QTL的组成存在分化。结果为长江下游麦区小麦抗病分子育种提供了重要参考。
关键词:
Abstract
Fusarium head blight (FHB), powdery mildew (PM), and wheat yellow mosaic virus (WYMV) are three major diseases of wheat in the lower reaches of Yangtze River. The resistance to FHB, PM, and WYMV of 49 new bred wheat varieties (advanced lines) from the lower reaches of the Yangtze River were evaluated, and the molecular markers linked to FHB resistance QTL Fhb1 and QFhs.crc-2D, PM gene Pm21 and WYMV resistance QTL QYm.nau-5A.1 and QYm.nau-2D were used to detect the correspond resistance genes/QTLs. 49.0% of the varieties were above moderate resistance to FHB, 32.6% varieties were immune or resistant to PM, and 44.9% varieties were resistant to WYMV. 30.6% and 73.6% of the tested varieties contained Fhb1 and QFhs.crc-2D, respectively. Ningmai 9 and Yangmai 158 as well as their derived varieties were the main carriers of Fhb1 and QFhs.crc-2D, respectively. Pm21, a powdery mildew resistant gene, was detected in 28.6% of the tested varieties, Zhenmai 9 and Yangmai 18 were two major donors of Pm21. QYm.nau-5A.1 and QYm.nau-2D, two MYMV resistant QTL, were detected in 24.5% of the tested varieties. Ningmai 9 and Zhenmai 9 were two major QTL donors. The composition of resistance genes/QTLs to FHB and WYMV in Yangmai 158 and their derived varieties diverged from Ningmai 9 and its derived varieties. These results provide the important information for molecular breeding for wheat disease resistance in lower reaches of Yangtze River.
Keywords:
PDF (2377KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
吕国锋, 别同德, 王慧, 赵仁慧, 范金平, 张伯桥, 吴素兰, 王玲, 汪尊杰, 高德荣. 长江下游麦区新育成品种(系) 3种主要病害的抗性鉴定及抗病基因/ QTL的分子检测. 作物学报, 2021, 47(12): 2335-2347 DOI:10.3724/SP.J.1006.2021.01097
LYU Guo-Feng, BIE Tong-De, WANG Hui, ZHAO Ren-Hui, FAN Jin-Ping, ZHANG Bo-Qiao, WU Su-Lan, WANG Ling, WANG Zun-Jie, GAO De-Rong.
长江中下游麦区小麦生长后期多雨潮湿, 是小麦赤霉病和白粉病的重发区, 近年来黄花叶病有蔓延趋势。小麦赤霉病 (Fusarium head blight, FHB)是一种世界性真菌病害, 病原为禾谷镰刀菌, 主要侵染小麦穗部, 严重影响小麦产量和品质[1]。培育抗赤霉病品种是本麦区抗病育种的首要目标。小麦赤霉病是多基因控制的复杂性状, 目前已命名的小麦抗赤霉病基因有7个[2], 其中以Fhb1效应最大, 抗性最稳定, 在国内外得到广泛应用[3,4,5]。Zhu等[6]利用全基因组关联分析对240个中国小麦品种(系)赤霉病抗性的遗传研究表明, 在1AS、2DL、5AS、5AL和7DS共关联到5个QTL, 以2DL上的QTL效应最大, 其主要存在于长江中下游麦区江苏省和湖北省的品种中, 而Fhb1在中国小麦品种中总体频率很低。长江下游的苏麦3号和宁麦9号是中国小麦品种Fhb1的主要供体, 并以后者为主[7]。扬麦系列和宁麦系列品种是长江下游小麦生产的主体品种, 多数品种的赤霉病为中抗。宁麦系列品种中的宁麦9号衍生品种多含Fhb1[8], 扬麦系列品种(系)中含Fhb1的品种(系)均为宁麦9号衍生品种, 而扬麦158衍生品种大多不含Fhb1, 其赤霉病抗性由非Fhb1的抗性控制[9]。对扬麦158及其衍生品种扬麦16的遗传研究表明, 两品种的赤霉病抗性都由Fhb1外的多个QTL控制, 其中位于2DL上的QTL是两品种所共有[10,11]。2DL上的QTL不仅是Wuhan-1[12]、VA00W-38[13]、Shanghai-3/Catbird[14]、Soru#1 [15]、Kenyon[16]和C615[17]等不含Fhb1品种的主要抗赤霉病位点之一, 也是苏麦3号衍生品种DH181[18]和CJ9306[19]的主效抗性位点。
小麦白粉病是由禾布氏白粉病菌(Blumeria graminis f. sp. tritici)引起的真菌性病害, 是我国长江中下游麦区仅次于赤霉病的第二大病害。小麦-黑麦易位系1B/1R所携带的Pm8是我国20世纪70—80年代中期应用最广泛的抗白粉病基因, 其抗性已在全国各地丧失[20], 该基因在长江下游麦区应用较少。本世纪初长江中下游麦区利用Pm4a和Pm2相继育成扬麦10号(含Pm4a)、扬麦11 (含Pm4a)、扬麦12 (含Pm2)、扬麦13 (含Pm2)等抗白粉病品种[21], 并在生产上大面积应用, 近年来这些品种在本麦区出现抗性丧失的现象。小麦-簇毛麦易位系6AL/6VS所含的Pm21是目前抗谱最广、抗性最强的抗白粉病基因[22,23], 长江中下游麦区利用该基因育成了扬麦18、扬麦21、镇麦9号等多个抗白粉病品种[24]。
小麦黄花叶病是由禾谷多黏菌(Polymyxa graminis)为小麦黄花叶病毒(Wheat yellow mosaic virus, WYMV)传播介体的春季病害, 在我国长江流域和黄淮麦区都有发生[25]。小麦发病后一般产量损失10%~30% [26]。小麦黄花叶病是土传性病害, 常规药剂防治方法难以控制。由于长江中下游麦区推广品种中抗病品种较少, 该病害呈逐渐蔓延态势。对扬辐9311[27]、Ibis[28]、Yumechikara[29]、仪宁小麦[30]等抗源的遗传研究表明, 这些材料的主效黄花叶病抗病QTL均定位于2DL染色体上。在5AL上还定位到来自小麦品种西风的抗黄花叶病主效QTL QYm.nau-5A.1[31]。Zhu等[31]对34个抗黄花叶病品种的检测表明, 10个品种只含有QYm.nau-5A.1, 17个品种只含有QYm.nau-2D, 2个品种同时含有上述2个QTL, 品种的抗性QTL组成存在分化。长江下游麦区利用西风育成了抗黄花叶病品种宁麦9号和苏麦5号[32], 利用扬辐9311育成了抗黄花叶病品种扬辐麦5号[33]。
近年来随着小麦播期的推迟, 小麦赤霉病的流行频率和为害程度增加, 同时小麦白粉病和黄花叶病的发病面积扩大, 需要进一步提高生产推广品种对上述病害的抗性, 保证小麦的高产稳产。目前小麦抗病育种还主要依赖表型选择, 选择效率受环境影响大, 易在选择过程中发生抗病基因丢失的现象。小麦抗赤霉病、白粉病和黄花叶病基因/QTL发掘和紧密连锁标记开发, 为抗病分子育种提供材料和方法。扬麦158和宁麦9号及其衍生品种是长江下游麦区的主要应用品种, 亲缘系数分析表明两类品种间存在一定的遗传差异[34], 但对其主要病害抗病基因/QTL的组成和分布还缺少了解, 限制了抗病基因在育种中应用。本研究对长江下游麦区当前推广品种、新育成品种(系)的小麦赤霉病、白粉病和黄花叶病进行鉴定, 同时利用相关抗病基因/QTL紧密连锁标记对其所含的抗病基因/QTL进行分子鉴定, 追溯抗病基因来源、组成及分布频率, 以期为本麦区抗病分子育种提供参考。
1 材料与方法
1.1 试验材料
试验品种共49个, 包括长江下游麦区当前推广品种、2000年后新育成品种和区试中表现突出的品系以及扬麦5号、扬麦158和宁麦9号3个长江下游麦区当前育成品种的骨干亲本(附表1)。以苏麦3号为含小麦抗赤霉病基因Fhb1和QFhs.crc-2D的对照品种, 镇麦9号为含抗白粉病基因Pm21的对照品种, 以西风和苏麦6号分别为含小麦黄花叶病主效抗性QTL QYm.nau-5A.1和QYm.nau-2D的对照品种。Table S1
附表1
附表1 试验品种、系谱、抗病性及抗性基因/QTL组成
Table S1
| 序号 Order number | 品种名称 Variety name | 系谱 Pedigree | 赤霉病 FHB | 黄花叶病 WYMV | 白粉病 PM | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 抗性等级resistance type | Fbh1 | QFhs.crc-2D | 抗性等级resistance type | QYm.nau-5A.1 | QYm.nau-2D | 抗性等级resistance type | Pm21/ Pmv | |||
| 1 | 苏麦3号 Sumai 3 | 阿夫/台湾小麦 Funo/Taiwan wheat | R | + | + | S | – | – | HS | – |
| 2 | 镇麦9号 Zhenmai 9 | 苏麦6号/扬97G59 Sumai 6/Yang 97G59 | MS | – | + | R | – | + | IM | + |
| 3 | 苏麦6号 Sumai 6 | 6698白/扬麦5号 6698 Bai/Yangmai 5 | MR | – | + | R | – | + | HS | – |
| 4 | 西风 Xifeng | 日本品种 Japanese Variety | MS | + | – | R | + | – | HS | – |
| 5 | 扬麦5号 Yangmai 5 | 9-16/ST1472/506 | MS | – | + | S | – | – | HS | – |
| 6 | 扬麦158 Yangmai 158 | 扬麦4号//ST1472/506 Yangmai 4//ST1472/506 | MR | – | + | S | – | – | HS | – |
| 7 | 宁麦9号 Ningmai 9 | 扬麦6号/西风 Yangmai 6/Xifeng | MR | + | – | R | + | – | HS | – |
| 8 | 宁麦13 Ningmai 13 | 宁麦9号选系 Selection from Ningmai 9 | MR | + | + | S | – | – | HS | – |
| 9 | 宁麦18 Ningmai 18 | 宁麦9号3/扬麦10号 Ningmai 93/Yangmai 10 | MR | + | – | R | + | – | HS | – |
| 10 | 宁麦21 Ningmai 21 | 宁麦9号/扬麦158//宁麦9号 Ningmai 9/Yangmai 158// Ningmai 9 | MS | + | – | S | – | – | HS | – |
| 11 | 宁麦26 Ningmai 26 | 宁9351/宁麦9号 Ning 9351/Ningmai 9 | MR | + | – | R | + | – | HS | – |
| 12 | 扬辐麦4号Yangfumai 4 | 宁麦8号/宁麦9号 Ningmai 8/Ningmai 9 | MS | + | + | R | + | – | HS | – |
| 13 | 扬麦18 Yangmai 18 | 宁麦9号4/3/扬麦1586//88-128/南农P045 Ningmai 94/3/Yangmai 1586//88-128/Nannong P045 | MR | + | + | R | + | – | IM | + |
| 14 | 扬14-122 Yang 14-122 | 镇麦9号/邯6172 Zhenmai 9/Han 6172 | MS | + | + | S | – | – | IM | + |
| 15 | 扬15-65 Yang 15-65 | 扬麦18//扬02-60/Baldus Yangmai 18//Yang 02-60/Baldus | MS | + | + | S | – | – | IM | + |
| 16 | 扬16-157 Yang 16-157 | 苏麦6号/扬97G59//扬麦18 Sumai 6/Yang 97G59//Yangmai 18 | R | + | – | S | – | – | IM | + |
| 17 | 扬麦9号 Yangmai 9 | 扬鉴三/扬麦5号 Yangjiansan/Yangmai5 | MS | – | + | S | – | – | HS | – |
| 18 | 扬麦11 Yangmai 11 | 扬1583/3/Y.C/扬鉴二//扬85-85 Yangmai 1583/3/Y.C/Yangjian 2//Yang 85-85 | MR | – | + | S | – | – | MS | – |
| 19 | 扬麦16 Yangmai 16 | 91F138/扬90-30 91F138/Yang 90-30 | MR | – | + | S | – | – | HS | – |
| 20 | 扬麦20 Yangmai 20 | 扬麦10/扬麦9号 Yangmai 10/Yangmai 9 | MS | – | + | S | – | – | MS | – |
| 21 | 扬麦22 Yangmai 22 | 扬麦9号3/97033-2 Yangmai 93/97033-2 | MS | – | + | S | – | – | IM | + |
| 22 | 扬麦30 Yangmai 30 | 扬09纹1009/扬麦18 Yang 09 Wen 1009/Yangmai 18 | MR | – | + | S | – | – | IM | + |
| 23 | 扬13-32 Yang 13-32 | 扬麦9号/扬麦18 Yangmai 9/Yangmai 18 | MR | – | + | R | + | – | IM | + |
| 24 | 扬14-214 Yang 14-214 | 扬麦162/92R137 Yangmai 162/92R137 | MR | – | + | S | – | – | IM | + |
| 25 | 扬麦23 Yangmai 23 | 扬麦16/扬辐9311 Yangmai 16/Yangfumai 9311 | MR | – | + | R | – | + | HS | – |
| 26 | 镇麦168 Zhenmai 168 | 苏麦6号/扬97G59 Sumai 6/Yang 97G59 | MS | – | + | R | – | + | HS | – |
| 27 | 扬15-126 Yang 15-126 | 扬麦22/镇麦9号 Yangmai 22/Zhenmai 9 | MS | – | + | R | – | + | IM | + |
| 28 | 扬14-179 Yang 14-179 | 宁麦9号/扬麦152//镇麦9号2 Ningmai 9/Yangmai 152//Zhenmai 92 | MR | – | – | R | + | + | IM | + |
| 29 | 安农1124 Annong 1124 | 02P67/安农95081-8 02P67/Annong 95081-8 | MS | – | + | R | + | – | IM | + |
| 30 | 明麦133 Mingmai 133 | 郑麦9023/扬麦11 Zhengmai 9023/Yangmai 11 | MS | – | + | S | – | + | HS | – |
| 31 | 扬麦13 Yangmai 13 | 扬88-84// (Maris Dove/扬麦3号) Yang 88-84//Maris Dove/Yangmai 3 | S | – | + | S | – | – | HS | – |
| 32 | 扬麦14 Yangmai 14 | 扬麦158/扬麦6号 Yangmai 158/Yangmai 6 | MR | – | + | S | – | – | HS | – |
| 33 | 扬麦15 Yangmai 15 | 扬89-40/川育21526 Yang 89-40/Chuanyu 21526 | S | – | + | S | – | – | HS | – |
| 34 | 扬麦19 Yangmai 19 | 扬麦9号6/4/1584/3/扬85-854//扬麦5号/ Y.C Yangmai 96/4/Yangmai 1584/3/Yang 85-854//Yangmai 5/Y.C | MS | – | + | S | – | – | MS | – |
| 35 | 扬麦24 Yangmai 24 | 扬麦17//扬麦11/豫麦18 Yangmai 17//Yangmai 11/Yumai 18 | MS | – | + | S | – | – | HS | – |
| 36 | 扬麦25 Yangmai 25 | 扬麦172//扬麦11/豫麦18 Yangmai 172//Yangmai 11/Yumai 18 | MS | – | + | S | – | – | HS | – |
| 37 | 扬麦27 Yangmai 27 | 扬麦19/扬07纹5418 Yangmai 18/扬07 Wen 5418 | MR | – | + | S | – | – | HS | – |
| 38 | 扬15G35 Yang 15 G35 | 镇麦9号2/5/扬麦152/4/宁麦14/3/扬麦17//扬麦15/扬99G56 Zhenmai 92/5/Yangmai 152/4/Ningmai 14/3/Yangmai 17//Yangmai 15/Yang 99G56 | MS | – | + | R | – | + | IM | + |
| 39 | 扬辐麦6号 Yangfumai 6 | 扬辐麦4号/扬麦14M1 Yangfumai 4/Yangmai 14M1 | MR | – | + | R | + | – | HS | – |
| 40 | 扬辐麦9号 Yangfumai 9 | 1-扬辐麦4号/扬麦19 1-Yangfumai 4/ Yangmai 19 | MR | + | – | R | + | – | HS | – |
| 41 | 扬辐麦10号 Yangfumai 10 | (扬辐麦4号/扬麦19)F1辐照 (Yangfumai 4/Yangmai 19) M1 | MR | – | + | R | – | + | HS | – |
| 42 | 镇麦10号 Zhenmai 10 | 苏麦6号/扬97G59 Sumai 6/Yang 97G59 | MR | – | + | R | – | + | IM | + |
| 43 | 宁麦19 Ningmai 19 | 宁麦8号/扬麦158//扬麦158 Ningmai 8/Yangmai 158//Yangmai 158 | MS | – | – | S | – | – | HS | – |
| 44 | 宁麦20 Ningmai 20 | 宁麦8号/宁麦9号 Ningmai 8/Ningmai 9 | MR | + | – | R | + | – | HS | – |
| 45 | 苏麦899 Sumai 899 | 宁麦9号/宁麦8号 Ningmai 9/Ningmai 8 | MR | + | – | S | – | – | HS | – |
| 46 | 华麦6号 Huamai 6 | 扬麦13/苏麦6号 Yangmai 13/Sumai 6 | MS | – | – | R | – | + | HS | – |
| 47 | 华麦1028 Huamai 1028 | 扬麦11/华麦6号 Yangmai 11/Huamai 6 | MS | – | – | R | – | + | HS | – |
| 48 | 金丰0515 Jinfeng 0515 | 郑麦9023/镇麦168 Zhengmai 9023/Zhenmai 168 | MR | + | + | S | – | – | R | – |
| 49 | 国红6号 Guohong 6 | 扬麦11/扬麦18 Yangmai 11/Yangmai 18 | MR | – | + | R | – | + | HS | – |
| 50 | 国红9号 Guohong 9 | 扬辐麦2号/罗麦10号 Yangfumai 2/Luomai 10 | MR | + | – | R | + | – | HS | – |
| 51 | 鄂麦170 Emai 170 | 济麦19/豫麦34 Jimai 19/Yumai 34 | S | – | + | S | – | – | HS | – |
| 52 | 襄麦25 Xiangmai 25 | Tai1062/鄂麦19 TAI1062/Emai 19 | S | – | + | S | – | – | R | – |
新窗口打开|下载CSV
1.2 试验品种(系)的抗病性鉴定
1.2.1 小麦白粉病 2018年在江苏里下河地区农业科学研究所万福试验基地(江苏扬州)进行成株期抗病性鉴定。每品种2行, 行长1.4 m, 行距0.23 m, 每行50粒, 每50行种植1组抗、感病对照品种镇麦9号和扬麦15。当扬麦15充分发病时, 调查试验品种的病害发生情况。抗性等级按盛宝钦[35]的0~4级分类方法记录, 0级为免疫(IM), 植株无病斑; 1级为高抗(HR), 病斑直径小l mm, 菌丝层稀薄, 可见绿色叶面, 偶见较大病斑, 但仍透绿, 产孢量极少; 2级为中抗(MR), 叶片病斑直径小于l mm, 但菌丝层较厚, 不透绿, 能产生一定量孢子; 3级为中感(MS), 叶片病斑多, 且直径大于l mm, 菌丝层厚, 产孢量大, 但病斑不连片; 4级为高感(HS), 叶片病斑直径大于l mm, 菌丝层厚, 产孢量多, 病斑连片。1.2.2 小麦赤霉病 2017年和2018年在江苏里下河地区农业科学研究所万福试验基地采用单花滴注进行赤霉病抗性鉴定。于小麦开花初期, 用注射器吸取10 µL孢子液注入麦穗自顶部小穗开始自上而下第6个小穗的任意一个小花中, 每个品种接种20个单穗, 并用弥雾装置定时喷水保湿, 接种后21 d调查赤霉病发生数据, 计算平均病小穗数及严重度。抗性等级按胡文静等[36]的4级分类方法记录。
1.2.3 小麦黄花叶病 2017年和2018年在江苏里下河地区农业科学研究所万福试验基地小麦黄花叶病圃中鉴定。每品种2行, 行长1.4 m, 行距0.23 m, 每行50粒, 每50行种植1组抗、感病对照品种宁麦9号和扬麦15。于春季2月下旬发病盛期进行发病程度调查, 采用0~4级的方法记录。0级: 无病症; 1级: 部分叶片轻度黄化, 无明显条纹坏死斑和黄化现象; 2级: 花叶和条纹斑明显, 部分叶片黄化, 少数枯死, 植株轻度矮缩; 3级: 心叶严重花叶, 或是扭曲或是缩顶状, 全部黄化矮缩, 部分叶片和分蘖和整株枯死。0级为抗病类型, 1、2、3级为感病类型。
1.3 抗病基因的分子标记检测
采用CTAB法提取小麦基因组DNA。利用与小麦抗赤霉病、白粉病和黄花叶病基因/QTL紧密连锁标记对试验品种进行扩增(表1)。采用8%聚丙烯酰胺凝胶电泳及银染技术检测PCR扩增产物。品种(系)具有与对照品种一致的抗性扩增条带, 记为“+”, 反之记为“-”。Table 1
表1
表1试验品种抗病基因/QTL分型所用的标记
Table 1
| 性状 Trait | 基因/QTL名称 Name of genes/QTLs | 染色体 Chr. | 标记名称 Marker name | 参考文献 References |
|---|---|---|---|---|
| 小麦赤霉病Fusarium head blight | Fhb1 | 3BS | His3B-4 | Zhu et al. [7] |
| QFhb.crc-2D | 2DL | gwm539 | Somers et al. [12] | |
| 小麦白粉病Powdery mildew | Pm21 | 6V | MBH1 | Bie et al. [24] |
| 小麦黄花叶病Wheat yellow mosaic virus | QYm.nau-5A.1 | 5AL | wmc415 | Zhu et al. [31] |
| QYm.nau-2D | 2DL | wmc41 | Xiao et al. [30] |
新窗口打开|下载CSV
2 结果与分析
2.1 试验品种抗赤霉病抗性及抗病QTL组成
试验品种的抗性存在较大差异。仅有扬16-157为抗(R); 23个品种为中抗(MR), 占46.9%; 21个品种为中感(MS), 占42.9%; 4个品种为感(S)。利用Fhb1诊断标记His3B-4对试验品种进行扩增显示, 15个品种扩增出与对照品种苏麦3号相同的抗性条带(图1), 表明这些品种均含有抗赤霉病基因Fhb1, 占试验品种的30.6%, 这其中12个为宁麦9号及其衍生品种(图2), 推测这些品种的Fhb1来自宁麦9号。此外, 扬14-122、金丰0515和国红9号系谱上与宁麦9号无亲缘关系, 但也检测到含有Fhb1。15个含有Fhb1的品种(系)中扬16-157的抗性与抗赤霉病对照品种苏麦3号相当, 也超过其双亲的抗性水平, 10个品种为MR, 4个品种为MS, 无感病品种。
图1
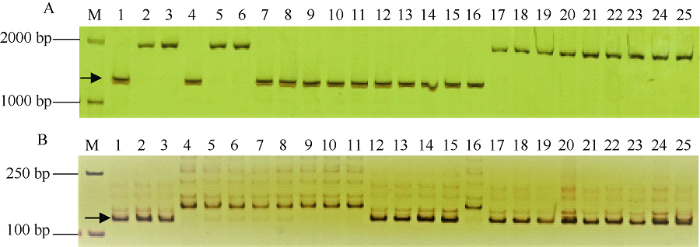 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1His3B-4 (A)和gwm539 (B)分别对部分品种的Fhb1和QFhs.crc-2D扩增结果
M: DNA ladder; 1~25: 为附表1中相应序号品种; 箭头分别表示Fhb1和QFhs.crc-2D的特异扩增产物。
Fig. 1PCR amplifications of the tested partial varieties using markers His3B-4 (A) and gwm539 (B) linked with Fhb1 and QFhs.crc-2D, respectively
M: DNA ladder. 1-25 refer to the tested varieties with corresponding serial number in Table S1. Arrows indicate the specific polymorphic bands for Fhb1 and QFhs.crc-2D, respectively.
图2
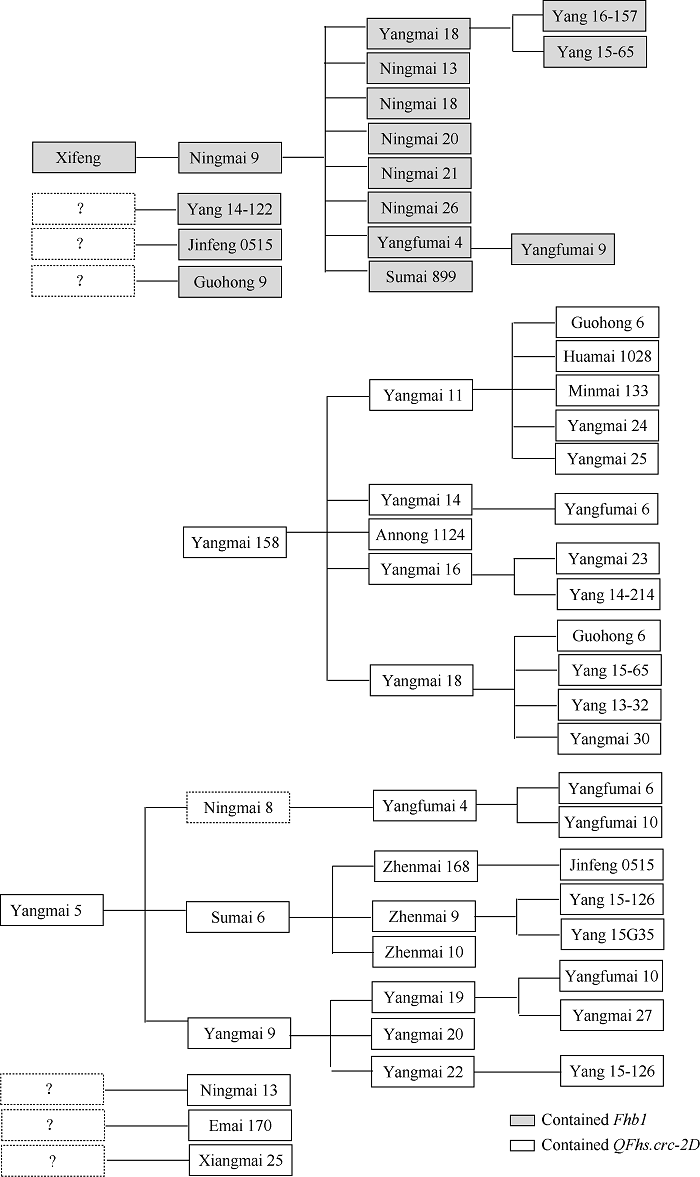 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2Fhb1和QFhs.crc-2D在49个试验品种中的传递路径
“?”表示抗性来源未知。
Fig. 2Transmission of Fhb1 and QFhs.crc-2D in 49 tested varieties
‘?’ indicates an unknown resistance source.
国红6号(扬麦11/扬麦18)、扬麦30 (扬09纹1009/扬麦18)、扬13-32 (扬麦9号/扬麦18)、扬辐麦6号(扬辐麦4号/扬麦14M1)和扬辐麦10号(扬辐麦4号/扬麦19F1辐照)等宁麦9号衍生品种未能检测出Fhb1抗性条带, 表明这些品种不含Fhb1, 占宁麦9号衍生品种的31.2%, 这些品种的赤霉病抗性为MS~MR。
利用gwm539对试验品种进行扩增, 结果显示有36个品种扩增出QFhs.crc-2D抗性条带(图1), 占试验品种的73.5%, 这其中32个为扬麦158或扬麦5号衍生品种, 宁麦9号衍生品种宁麦13、扬麦18和扬辐麦4号也含有QFhs.crc-2D (图2)。扬麦158和宁麦8号分别是扬麦18和扬辐麦4号的亲本之一, 宁麦8号 (扬麦5号/扬麦6号)为扬麦5号衍生品种, 这2个品种的QFhs.crc-2D来自于分别扬麦158和扬麦5号。此外宁麦13、襄麦25、鄂麦170与扬麦158和扬麦5号无亲缘关系, 但也含有QFhs.crc-2D。含QFhs.crc-2D的品种 (系)中无抗赤霉病品种, 15个品种为MR, 17个品种为MS, 4个品种为S。
试验品种中还有宁麦13、扬麦18和金丰0515同时含有Fhb1和QFhs.crc-2D, 这3个品种为中抗赤霉病。
2.2 试验品种的小麦白粉病抗性及Pm21组成
试验品种中有16个品种表现为白粉病免疫(IM)或抗(R), 占32.6%, 扬麦11、扬麦19和扬麦20共3个品种表现中感(MS), 其余品种都为高感白粉病(HS)。以Pm21的功能标记MBH1对试验品种扩增, 14个品种能扩增出Pm21的特异片段(图3), 表明这些品种含有Pm21, 占试验品种的28.6%, 这其中5个为扬麦18及其衍生品种, 6个为镇麦9号及其衍生品种(图4), 推测这些品种的Pm21来自镇麦9号和扬麦18。镇麦9号(系谱为苏麦6号/扬97G59)的亲本之一苏麦6号不含Pm21, 扬麦18 (系谱为宁麦9号4/3/扬麦1586//扬88-128/南农P045)的2个轮回亲本扬麦158和宁麦9号均不含Pm21, 另一亲本扬88-128为感白粉病品种, 因此镇麦9号和扬麦18的Pm21应分别来自于扬97G59和南农P045。此外安农1124 (系谱为02P67/安农95081-8), 其双亲02P67 (系谱为92R91/扬麦158//扬麦158)和安农95081-8 (系谱为豫麦18/扬麦158)的亲本豫麦18和扬麦158都为感白粉病品种, 因此安农1124的Pm21应来自92R91。扬14-214 (系谱为扬麦162/92R137)和扬麦22 (系谱为扬麦9号3/97033-2)的轮回亲本分别为扬麦16和扬麦9号, 这2个品种都不抗白粉病, 其抗病基因应分别来自92R137和97033-2。此外金丰0515和襄麦25白粉病为抗(R), 分子检测不含Pm21, 其白粉病抗性有待进一步研究。扬麦11、扬麦19和扬麦20其白粉病抗性仅达中感(MS), 表明其所含抗白粉病基因Pm4a在本麦区已失去抗性。试验品种中镇麦168和国红6号虽然其亲本含有白粉病抗源扬97G59和扬麦18, 但表现为高感白粉病, 分子检测也不含有Pm21。
图3
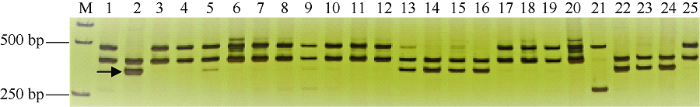 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3MBH1对部分品种Pm21的扩增结果
M: DNA ladder; 1~25: 为附表1中相应序号品种; 箭头分别表示Pm21的特异扩增产物。
Fig. 3PCR amplifications of the tested partial varieties using marker MBH1 of Pm21
M: DNA ladder; 1-25 refer to the tested varieties with corresponding serial number in Table S1; Arrow indicates the specific polymorphic bands for Pm21.
图4
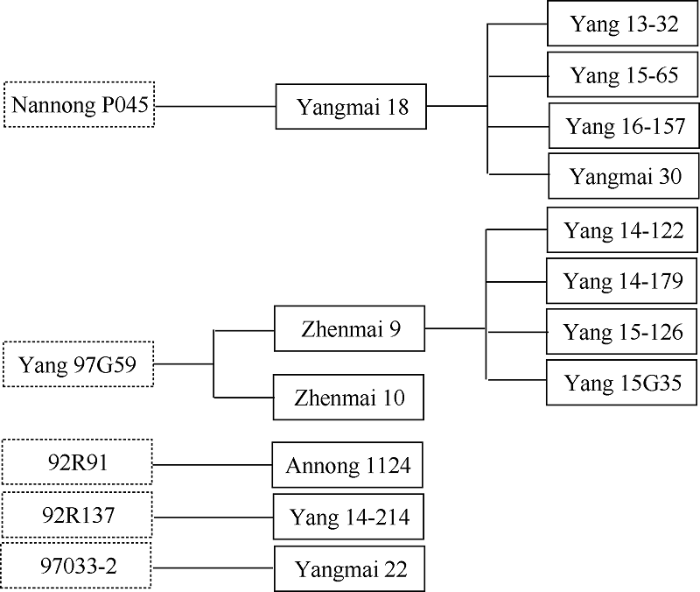 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4Pm21在14个抗白粉病品种中的传递路径
Fig.4Transmission of powdery mildew resistant gene Pm21 in 14 tested varieties
2.3 小麦抗黄花叶病抗性及QTL组成
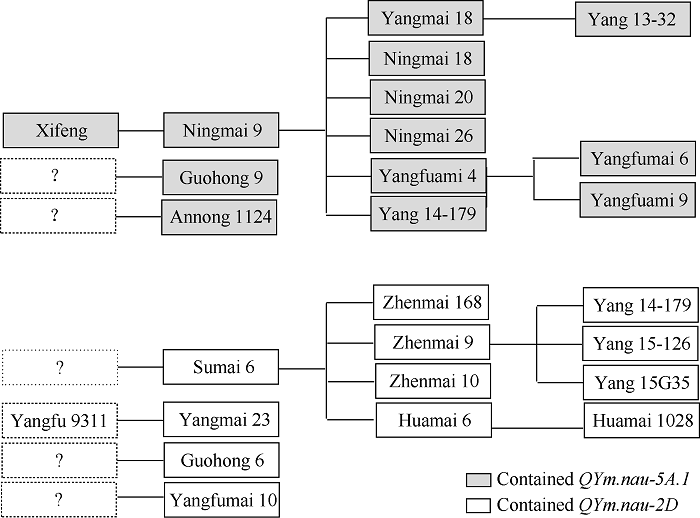
试验品种中有23个品种抗小麦黄花叶病, 占46.9%, 感病品种的感病程度也存在一定差异, 宁麦13和苏麦899虽然表现感病, 但感病等级为1级, 而扬麦158、扬麦16等品种的感病等级为3级。用wmc415对试验品种扩增, 12个品种能扩增出与对照西风相同的抗病条带(图5), 表明这些品种含QYm.nau-5A.1, 均表现为抗小麦黄花叶病, 占试验品种的24.5%, 这其中10个为宁麦9号及其衍生品种(图6), 占含QYm.nau-5A.1品种的83.3%。此外安农1124和国红9号分子检测也含QYm.nau-5A.1, 但其系谱上与宁麦9号无亲缘关系, 可能是QYm.nau-5A.1新来源。此外试验品种中还有9个宁麦9号衍生品种标记检测不含QYm.nau-5A.1, 占宁麦9号衍生品种的47.4%, 这些品种也不抗小麦黄花叶病。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5wmc415 (A)和wmc41 (B)分别对部分品种小麦抗黄花叶病QTL QYm.nau-5A.1和QYm.nau-2D的扩增结果
M: DNA ladder; 1~25: 为附表1中相应序号品种; 箭头分别表示QYm.nau-5A.1和QYm.nau-2D的特异扩增产物。
Fig. 5PCR amplifications of the tested partial cultivars using the markers wmc415 (A) and wmc41 (B) closely linked with QYm.nau-5A.1 and QYm.nau-2D, respectively
M: DNA ladder; 1-25 refer to the tested varieties with corresponding serial number in Table S1; Arrows indicate the specific polymorphic bands for QYm.nau-5A.1 and QYm.nau-2D, respectively.
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6QYm.nau-5A.1和QYm.nau-2D在22个抗小麦黄花叶病品种(系)中的传递路径
“?”表示抗性来源未知。
Fig. 6Transmission of QYm.nau-5A.1 and QYm.nau-2D in 22 WYMV resistant varieties
‘?’ indicates an unknown resistance source.
用wmc41对试验品种扩增, 12个品种能扩增出对照苏麦6号相同的抗病条带(图5), 占试验品种的24.5%, 除明麦133外, 其余11个分子检测阳性品种都表现为抗病, 表明这些品种含QYm.nau-2D, 这其中8个为苏麦6号及其衍生品种(图6), 占含QYm.nau-2D品种的72.7%。扬麦23 (扬麦16/扬辐9311)的亲本之一扬麦16为感病品种, 因此其所含的QYm.nau-2D应来自扬辐9311。扬辐麦10和国红6号所含的QYm.nau-2D还需研究。试验品种还有扬14-122和金丰0515等苏麦6号衍生品种标记检测不含QYm.nau-2D, 占苏麦6号衍生品种的21.4%, 这些品种均为感病(S)。试验品种含QYm.nau-5A.1的多数为宁麦9号及其衍生品种, 含QYm.nau-2D的多数为苏麦6号及其衍生品种, 只有扬14-179同时含有2个QTL, 其余品种只含有其中一个QTL, 表明试验品种在小麦黄花叶病抗性QTL组成上存在分化。
3 讨论
Pm4a、Pm2和Pm21是长江下游麦区应用最多的抗白粉病基因, 试验品种中扬麦11 (Pm4a)、扬麦19 (Pm4a)和扬麦20 (Pm4a)的白粉病抗性为中感(MS), 扬麦13 (含Pm2)为高感白粉病, 表明这2个基因在本麦区已失去抗性, 这与Bie等[24]结果一致。长江下游麦区当前主栽品种扬麦16、宁麦13、扬辐麦4号、扬麦23均不含上述任何抗白粉病基因, 田间表现为感白粉病。近年来, 长江下游麦区育成扬麦18、扬麦22、镇麦9号和镇麦10号等一些含有Pm21的抗白粉病品种, 这些品种的应用面积不大, 小麦生产的主导品种仍是扬麦16等本世纪初育成的品种, 未能改变当前小麦生产应用品种白粉病抗性整体偏弱的局面。新育成品系中含Pm21的品系比例明显上升, 其基因供体主要为扬麦18和镇麦9号。与Pm21的原始供体92R系列、P045等材料相比, 扬麦18和镇麦9号的综合农艺性状和产量水平得到大幅提升, 因此以其为亲本育成品种的能力显著上升, 使得Pm21在新品系中快速扩散。本研究中含Pm21的新品系多数为江苏里下河地区农业科学研究所育成, 由于其选种环境白粉病自然选择压大, 使得Pm21在后代选择中容易保留。因此, 在白粉病发生充分的环境下, 通过表型选择可以实现对抗病基因的选择。扬麦18和镇麦9号的Pm21都来自于小麦-簇毛麦6AL/6VS易位系[24], 安农1124和扬14-214所含Pm21分别来自于92R91和92R137, 这2个材料也是含Pm21的小麦-簇毛麦6AL/6VS易位系[22], 扬麦22的抗白粉病基因来自小麦-簇毛麦T6DL/6VS易位系PmV[24]。江苏里下河地区农业科学研究所近期研究表明, Pm21和PmV高度同源, 均为具有CC- NBS-LRR结构域的典型RGA类基因。当前长江下游麦区抗病品种的抗白粉病基因单一, 存在着Pm21加速抗性丧失的潜在风险。同时, Pm21供体品种扬麦18和镇麦9号在育种中高频率使用, 也使新品种(系)的遗传多样性过于狭窄, 未来品种的综合农艺性状难以突破。因此, 亟需引入新的白粉病后备抗源(或基因)。此外本研究中金丰0515和襄麦25在扬州表现抗白粉病, 具有较好的农艺性状, 是Pm21外新的抗白粉病材料, 要加强其所含白粉病基因研究和利用。近20年来, 大批新的Pm基因相继发现[37], 加快这些基因的利用, 可有效解决抗白粉病基因单一化的局面, 同时对延长抗病品种和Pm21的利用周期有重要意义。
本研究结果表明, 试验品种中QYm.nau-5A.1和QYm.nau-2D的载体品种可清晰的分成宁麦9号和苏麦6号衍生品种两类, 小麦抗黄花叶病品种的抗病QTL组成的分化现象也存在其他研究结果中[30,31]。本研究试验品种的QYm.nau-5A.1多来自于宁麦9号(扬麦6号/西风), QYm.nau-5A.1是西风的抗小麦黄花叶病主效QTL[31], 因此宁麦9号及其衍生品种的QYm.nau-5A.1应都来自于西风。试验品种中QYm.nau-2D多来自苏麦6号(6698白/扬麦5号), 扬麦5号为感病品种, 分子检测也不含QYm.nau-2D, 苏麦6号的抗性应来自于另一亲本6698白(安徽11/毛颖阿夫//丰产3号///大丰1087)。苏麦6号与已报道的含位于2D上的抗病QTL中国抗源扬辐9311 (扬麦3号/高加索)、仪宁小麦(江都1号/ST1472/506γ辐射)、郑麦9023 ({(小偃6号×西农65)×[83(2)3-3× 84(14)43]}F3×陕213)无系谱关系, 说明小麦2D上的小麦抗黄花叶病主效QTL广泛存在于我国不同麦区的抗病品种中。本研究中有47.4%的宁麦9号衍生品种不含QYm.nau-5A.1, 21.4%的苏麦6号衍生品种不含QYm.nau-2D, 表明这些品种的抗病基因在选择过程中丢失。本研究所用的2个小麦抗黄花叶病QTL效应较大, 单个QTL足以应对黄花叶病为害, 因此标记辅助选择(MAS)的效率较高。在育种早代可优先农艺性状选择, 待主要农艺性状基本稳定后, 辅之抗病基因的标记选择, 这样不仅可解决抗性基因丢失的问题, 还可较好解决抗病性和农艺性状结合的问题, 提高抗病品种的育成效率。
扬麦和宁麦系列品种是我国长江中下游麦区生产应用的主要中抗赤霉病品种, 系谱上与我国抗赤霉病品种苏麦3号无亲缘关系。宁麦9号及其衍生品种是中国小麦品种Fhb1的主要载体品种[7], 本研究中含Fhb1品种也多为宁麦9号及其衍生品种。对扬麦158和扬麦16的赤霉病抗性遗传研究表明, 两品种的抗性遗传机制与苏麦3号不同, 扬麦158的赤霉病抗性主要由2DL、2DS、3AL和7AS 上的QTL提供[10], 而扬麦16的抗性QTL位于2DL、3BL、4DS、5BL和6AS上[11], 2DL上的抗赤霉病QTL是2品种所共有的。本研究中多数扬麦158或扬麦5号衍生品种不含Fhb1, 但含此抗病QTL。2D上的抗赤霉病QTL也是Wuhan-1[12]、VA00W-38[13]、Shanghai-3/Catbird[14]、Soru#1[15]、Kenyon[16]、C615[17]和扬麦13[38]等不含Fhb1材料赤霉病抗性的主要来源之一, 这都表明此QTL是除Fhb1外的另一个广泛存在于小麦品种中的重要赤霉病抗性位点。
赤霉病抗性和丰产性结合一直是小麦抗赤霉病育种的难题[39]。扬麦158和宁麦9号及其衍生品种一直是长江下游麦区的主栽品种, 本研究表明两类品种在抗赤霉病主效位点Fhb1和QFhs.crc-2D组成上存在分化。因此, 聚合这两类品种的主效抗性位点更易取得后代品系的丰产性和赤霉病抗性在更高水平上的结合, 进而实现小麦抗赤霉病育种的突破。Fhb1的抗性效应已在我国黄淮麦区的抗赤霉病育种中得到验证[5], 而QFhs.crc-2D的效应在不同环境表现稳定性略显不足[10-12,16,19,38], 其MAS育种效率仍需要进一步评价。因此, 将Fhb1导入扬麦主体品种中, 聚合Fhb1和原本抗性优异的扬麦系列品种的本底抗性是当前值得期待的提高长江中下游麦区品种赤霉病总体抗性的方法。
小麦基因组中的基因在染色体上不是均等分布的, 存在基因富集区域[40]。小麦第1、2、3群的短臂和5、6群的长臂是抗病基因的热点区域[41]。小麦3BS上存在抗赤霉病基因Fhb1[42], 还存在抗秆锈病基因Sr2和抗叶锈病基因Lr27[43], Sr2与Fhb1呈互斥相连锁, 与Fhb1连锁标记gwm533的遗传距离仅为0.4 cM[44]。此外在3BS上还定位到与小麦生育后期叶片延绿性[45,46,47,48]、灌浆速率[49]和千粒重[47,49]相关的QTL, 且都与gwm533连锁。由于控制性状的基因或QTL位置靠近, 对某一基因的强烈选择, 就会对附近区域的其他基因产生选择牵连效应, 并降低基因组这一区域的遗传多样性[50]。叶片的延绿性对提高小麦产量和抗逆性有重要作用, 是小麦生育后期的重要选择性状。宁麦9号及其衍生品种生育后期叶片延绿性高于扬麦158衍生品种[51], 宁麦9号衍生品种是Fhb1的载体品种, 育种者对叶片延绿性的选择偏好, 就会产生对Fhb1的选择牵连, 使得叶片延绿QTL和Fhb1作为1个单倍型向后代遗传, 这可能是扬麦158和宁麦9号及其衍生品种在延绿性和Fhb1组成分化的重要原因。
小麦5AL上存在抗黄花叶病主效QTL QYm.nau-5A.1[31]。江苏里下河地区农业科学研究所在宁麦9号系选品种宁麦13的5AL上(物理位置为650.0 Mb)定位到与小麦基部小穗结实性的QTL, 此QTL与QYm.nau-5A.1 (共分离标记wmc415物理位置为535.1 Mb)存在一定程度的连锁。穗部结实性是宁麦系列品种产量改良的重要选择性状[52], 宁麦系列品种含有较多的结实性有利等位变异, 其顶部和基部结实率也高于扬麦系列品种[53]。宁麦系列品种对结实性的强烈选择, 牵连对QYm.nau-5A.1抗病单倍型的选择, 这可能是扬麦158和宁麦9号及其衍生品种在结实性和QYm.nau-5A.1组成分化的重要原因。
小麦2DL上存在小麦抗黄花叶病主效QTL QYm.nau-2D[27,28,29,30], 在2DL上还存在控制小麦多酚氧化酶(PPO)活性基因Ppo-2DL[54], 其与QYm.nau-2D的诊断标记wmc41遗传距离仅有0.8 cM。QYm.nau-2D也是本研究中苏麦6号及其衍生品种小麦黄花叶病的主要抗性来源, 用Ppo-D1的STS标记[55]对试验品种检测, 含QYm.nau-2D的小麦抗黄花叶病的11个试验品种均为高PPO活性的Ppo-D1b单倍型, 表明QYm.nau-2D与Ppo-D1b存在连锁。在小麦2DL上还存在抗赤霉病QTL QFhs.crc- 2D[10-12,14-17], 并与SSR标记gwm539 (物理位置为515.2 Mb)连锁, 其与QYm.nau-2D (共分离标记wmc41物理位置为577.4 Mb)仅相距62.2 Mb。姜朋等[56]以宁麦9号衍生系群体为材料, 在2DL的gwm539位点关联到与小麦蛋白质含量相关的QTL。蛋白质含量是小麦品种分类的重要指标, 宁麦9号衍生品种多为蛋白质含量较低类型, 与扬麦158衍生品种有明显差异, 两类品种品质类型的差异与小麦赤霉病和黄花叶病抗性位点组成上差异是否存在关联需进一步研究。
同一染色体区段的基因呈相斥相连锁在小麦中较为普遍, 如位于小麦2BL上抗白粉病基因Pm64和抗叶锈病基因Lr5[57], 3BS上抗赤霉病基因Fhb1和抗秆锈病基因Sr2[44]。这些呈互斥相连锁的基因必须通过遗传重组打破连锁, 构建2个基因新的单倍型组合, 才能使2个基因得到有效利用。通过这一方法将QYm.nau-2D和Ppo-D1a连锁在一起成为1个单倍型, 进而创制QFhs.crc-2D+QYm.nau- 2D+Ppo-D1a性状聚合的单倍型, 不仅可减轻由于镇麦9号应用带来的面粉色泽较差的不利效应, 同时还可提高其育种价值。本研究试验品种中镇麦9号和扬麦18均含有小麦赤霉病、白粉病和黄花叶病多个抗病基因, 因而对上述病害均具有较好的抗性, 这也是其在育种中得到大量应用的重要原因。
4 结论
长江下游麦区当前推广品种和新育成品种(系)在小麦抗赤霉病和黄花叶病主效QTL组成上存在分化, 根据2种病害抗性QTL组成分为扬麦158和宁麦9号衍生品种两类。聚合扬麦和宁麦系列品种的赤霉病抗性, 是进一步提高长江下游麦区小麦品种的赤霉病抗性重要途径。Pm21是当前长江下游唯一有效的抗白粉病基因, 应加快后备抗源(基因)的利用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
URL [本文引用: 1]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 4]
[本文引用: 2]
[本文引用: 2]
PMID [本文引用: 5]

Fusarium head blight of wheat is an extremely damaging disease, causing severe losses in seed yield and quality. The objective of the current study was to examine and characterize alternate sources of resistance to Fusarium head blight (FHB). Ninety-one F1-derived doubled haploid lines from the cross Triticum aestivum 'Wuhan-1' x Triticum aestivum 'Maringa' were examined for disease reaction to Fusarium graminearum by single-floret injection in replicated greenhouse trials and by spray inoculation in replicated field trials. Field and greenhouse experiments were also used to collect agronomic and spike morphology characteristics. Seed samples from field plots were used for deoxynivalenol (DON) determination. A total of 328 polymorphic microsatellite loci were used to construct a genetic linkage map in this population and together these data were used to identify QTL controlling FHB resistance, accumulation of DON, and agronomic and spike morphology traits. The analysis identified QTL for different types of FHB resistance in four intervals on chromosomes 2DL, 3BS, and 4B. The QTLs on 4B and 3BS proximal to the centromere are novel and not reported elsewhere. QTL controlling accumulation of DON independent of FHB resistance were located on chromosomes 2DS and 5AS. Lines carrying FHB resistance alleles on 2DL and 3BS showed a 32% decrease in disease spread after single-floret injection. Lines carrying FHB resistance alleles on 3BS and 4B showed a 27% decrease from the mean in field infection. Finally, lines carrying favourable alleles on 3BS and 5AS, showed a 17% reduction in DON accumulation. The results support a polygenic and quantitative mode of inheritance and report novel FHB resistance loci. The data also suggest that resistance to FHB infection and DON accumulation may be controlled, in part, by independent loci and (or) genes.
DOIURL [本文引用: 2]
DOIURL [本文引用: 3]
URL [本文引用: 2]
PMID [本文引用: 3]

Fusarium head blight (FHB), caused by, is a very important disease of wheat globally. Damage caused by includes reduced grain yield, reduced grain functional quality, and results in the presence of the trichothecene mycotoxin deoxynivalenol in Fusarium-damaged kernels. The development of FHB resistant wheat cultivars is an important component of integrated management. The objective of this study was to identify QTL for FHB resistance in a recombinant inbred line (RIL) population of the spring wheat cross Kenyon/86ISMN 2137. Kenyon is a Canadian spring wheat, while 86ISMN 2137 is an unrelated spring wheat. The RIL population was evaluated for FHB resistance in six FHB nurseries. Nine additive effect QTL for FHB resistance were identified, six from Kenyon and three from 86ISMN 2137. and co-located with two FHB resistance QTL on chromosome arm 2DS. A major QTL for FHB resistance from Kenyon () was identified on chromosome 7D. The QTL from Kenyon mapped to the same region as a FHB resistance QTL from Wuhan-1 on chromosome arm 2DL. This result was unexpected since Kenyon does not share common ancestry with Wuhan-1. Other FHB resistance QTL on chromosomes 4A, 4D, and 5B also mapped to known locations of FHB resistance. Four digenic epistatic interactions were detected for FHB resistance, which involved eight QTL. None of these QTL were significant based upon additive effect QTL analysis. This study provides insight into the genetic basis of native FHB resistance in Canadian spring wheat.
URL [本文引用: 3]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 2]

Several Triticum aestivum L.-Haynaldia villosa disomic 6VS/6AL translocation lines with powdery mildew resistance were developed from the hybridization between common wheat cultivar Yangmai 5 and alien substitution line 6V(6A). Mitotic and meiotic C-banding analysis, aneuploid analysis with double ditelosomic stocks, in situ hybridization, as well as the phenotypic assessment of powdery mildew resistance, were used to characterize these lines. The same translocated chromosome, with breakpoints near the centromere, appears to be present in all the lines, despite variation among the lines in their morphology and agronomic characteristics. The resistance gene, conferred by H. villosa and designated as Pm21, is a new and promising source of powdery mildew resistance in wheat breeding.
DOIURL [本文引用: 1]
DOIURL [本文引用: 5]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 4]
DOIURL [本文引用: 6]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
PMID [本文引用: 1]

The objectives of this study were to isolate and physically localize expressed resistance (R) genes on wheat chromosomes. Irrespective of the host or pest type, most of the 46 cloned R genes from 12 plant species share a strong sequence similarity, especially for protein domains and motifs. By utilizing this structural similarity to perform modified RNA fingerprinting and data mining, we identified 184 putative expressed R genes of wheat. These include 87 NB/LRR types, 16 receptor-like kinases, and 13 Pto-like kinases. The remaining were seven Hm1 and two Hs1(pro-1) homologs, 17 pathogenicity related, and 42 unique NB/kinases. About 76% of the expressed R-gene candidates were rare transcripts, including 42 novel sequences. Physical mapping of 121 candidate R-gene sequences using 339 deletion lines localized 310 loci to 26 chromosomal regions encompassing approximately 16% of the wheat genome. Five major R-gene clusters that spanned only approximately 3% of the wheat genome but contained approximately 47% of the candidate R genes were observed. Comparative mapping localized 91% (82 of 90) of the phenotypically characterized R genes to 18 regions where 118 of the R-gene sequences mapped.
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

Sr2 is the only known durable, race non-specific adult plant stem rust resistance gene in wheat. The Sr2 gene was shown to be tightly linked to the leaf rust resistance gene Lr27 and to powdery mildew resistance. An analysis of recombinants and mutants suggests that a single gene on chromosome arm 3BS may be responsible for resistance to these three fungal pathogens. The resistance functions of the Sr2 locus are compared and contrasted with those of the adult plant resistance gene Lr34.
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 2]

A set of 142 winter wheat recombinant inbred lines (RILs) deriving from the cross Heshangmai x Yu8679 were tried in four ecological environments during the seasons 2006 and 2007. Nine agronomic traits comprising mean grain filling rate (GFR(mean)), maximum grain filling rate (GFR(max)), grain filling duration (GFD), grain number per ear (GNE), grain weight per ear (GWE), flowering time (FT), maturation time (MT), plant height (PHT) and thousand grain weight (TGW) were evaluated in Beijing (2006 and 2007), Chengdu (2007) and Hefei (2007). A genetic map comprising 173 SSR markers and two EST markers was generated. Based on the genetic map and phenotypic data, quantitative trait loci (QTL) were mapped for these agronomic traits. A total of 99 putative QTLs were identified for the nine traits over four environments except GFD, PHT and MT, measured in two environments (BJ07 and CD07), respectively. Of the QTL detected, 17 for GFR(mean), 16 for GFR(max), 21 for TGW and 10 for GWE involving the chromosomes 1A, 1B, 2A, 2D, 3A, 3B, 3D, 4A, 4D, 5A, 5B, 6D and 7D were identified. Moreover, 13 genomic regions showing pleiotropic effects were detected in chromosomes 1A, 1B, 1D, 2A, 2B, 2D, 3A, 3B, 4B, 4D, 5B, 6D and 7D; these QTL revealing pleiotropic effects may be informative for a better understanding of the genetic basis of grain filling rate and other yield-related traits, and represent potential targets for multi-trait marker aided selection in wheat.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
