 ,1,2, 刘玉汇1,2, 孙超1,2, 毕真真1,2, 李安一3, 许德蓉1,2, 王一好1,2, 张俊莲1, 白江平
,1,2, 刘玉汇1,2, 孙超1,2, 毕真真1,2, 李安一3, 许德蓉1,2, 王一好1,2, 张俊莲1, 白江平 ,1,2,*
,1,2,*Identification of StIgt gene family and expression profile analysis of response to drought stress in potato
QIN Tian-Yuan ,1,2, LIU Yu-Hui1,2, SUN Chao1,2, BI Zhen-Zhen1,2, LI An-Yi3, XU De-Rong1,2, WANG Yi-Hao1,2, ZHANG Jun-Lian1, BAI Jiang-Ping
,1,2, LIU Yu-Hui1,2, SUN Chao1,2, BI Zhen-Zhen1,2, LI An-Yi3, XU De-Rong1,2, WANG Yi-Hao1,2, ZHANG Jun-Lian1, BAI Jiang-Ping ,1,2,*
,1,2,*通讯作者:
收稿日期:2020-06-4接受日期:2020-10-14网络出版日期:2021-04-12
| 基金资助: |
Received:2020-06-4Accepted:2020-10-14Online:2021-04-12
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (531KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
秦天元, 刘玉汇, 孙超, 毕真真, 李安一, 许德蓉, 王一好, 张俊莲, 白江平. 马铃薯StIgt基因家族的鉴定及其对干旱胁迫的响应分析[J]. 作物学报, 2021, 47(4): 780-786. doi:10.3724/SP.J.1006.2021.04122
QIN Tian-Yuan, LIU Yu-Hui, SUN Chao, BI Zhen-Zhen, LI An-Yi, XU De-Rong, WANG Yi-Hao, ZHANG Jun-Lian, BAI Jiang-Ping.
马铃薯(Solanum tuberosum L.), 属茄科(Solanaceae)龙葵亚属(Subg. Solanum), 一年生植物, 是继玉米、小麦和水稻之后的世界第四大粮食作物[1]。马铃薯属于块茎类作物, 在块茎发育过程中, 干旱胁迫会改变马铃薯的库源关系, 从而影响块茎的形成, 降低产量和品质[2,3,4]。植物为了避免在干旱胁迫下的不利影响, 在进化过程中建立了一套自我防卫机制[5,6,7,8], 其中Igt是普遍存在于植物中的一类与根系发育和抗逆相关的功能性基因, 主要包括Dro1、Tac1和Lazy1 3种不同的亚簇[9]。其中, Dro1 (Igt1)在作物中的研究相对深入。水稻中的研究发现, Dro1受到生长素信号下游关键转录因子的直接调控, 主要控制植物根细胞的伸长, 影响根系的向地性生长, 可通过减小根系的垂直角度而造成深根效应, 进而提高水稻抗旱性[9,10]; 在小麦、拟南芥和李属植物中也发现, Dro1可通过改变侧根角度和初生根长度来影响根系构型[11]。Tac1主要调控植物分蘖角度[12,13,14]。Lazy1同样主要参与植物根系角度的调控[15,16]。此外, 还有研究表明, Igt家族在作物应对高盐和干旱等非生物胁迫中也发挥着重要作用[10,11,12]。
目前为止, Igt基因家族仅在模式植物拟南芥和禾本科的少数作物中有所研究, 在马铃薯中StIgt基因家族成员尚未被鉴定和克隆, 其在植物地上部分和根系的形态建成以及抵御干旱胁迫过程中的功能尚有待阐明。随着马铃薯全基因组序列的测序完成, 为研究者更充分地研究马铃薯StIgt基因家族提供了可能。因此本研究首先通马铃薯全基因组范围内鉴定了该基因家族成员的10个成员, 并对其序列特征、染色体位置、系统发育关系、顺式元件和干旱胁迫响应的表达谱等方面进行了综合分析。这些结果为进一步研究马铃薯StIgt基因家族的分化历程及生物学功能奠定了基础, 也为马铃薯抗逆基因挖掘和种质创新提供了理论参考。
1 材料与方法
1.1 马铃薯StIgt基因家族成员的鉴定
从Ensemble数据库下载马铃薯参考基因组序列(PGSC,1.2 马铃薯StIgt基因的生物信息学分析
将最终鉴定的StIgts进行系统发育分析。所有获得的序列首先由ClustalX (版本1.83)软件[20] (默认参数)进行对齐, 再利用MEGA7软件[21]采用邻接法构建系统进化树, bootstrap测试1000次, 根据系统发育树的拓扑结构将马铃薯StIgt基因家族成员分为不同的类群。所有高置信度StIgt序列均提交至ExPASy网站(1.3 马铃薯StIgt的基因表达模式分析
利用课题组已有的马铃薯二代Illumina的RNA-seq数据, 进一步分析StIgt基因基因家族响应干旱的表达谱。将供试马铃薯品系C119 (CIP398098.119)的无菌试管苗培养于含有3%蔗糖和0.8%琼脂的Murashige和Skoog (MS)培养基上, pH 5.9 [23]。C119保存在人工气候室中, 光照时间为16 h光照/8 h黑暗, 培养温度为 (22±1)℃。待C119长至25 d时, 将幼苗重新移入含有150 mmol L-1甘露醇的MS液体培养基中进行干旱胁迫, 分别于0、2、6、12和24 h时采集植株根部, 用于转录组测序。从上述RNA-seq数据中提取出StIgts在这5个时间点的FPKM值, 运用R软件中的Heatmap包来绘制其响应干旱胁迫的表达模式热图。2 结果与分析
2.1 马铃薯StIgt基因家族的成员鉴定和聚类分析
利用生物信息学方法通过双向BLAST分析, 从马铃薯‘DM-v4.03’全基因组中共筛选到了21个StIgt 备选序列, 通过局部BLASTP并删除重复序列后, 保留15个序列, 提交CDD、Pfam确认蛋白结构域。再将这15条序列与已在拟南芥、李属植物、水稻、玉米等其他作物中报道的Igt基因序列进行同源比对(表1), 最后在马铃薯中共确定了10个StIgt家族成员(表2)。由表2可知, 马铃薯中StIgt家族蛋白长度范围从110 (StIgt10)到283个氨基酸(StIgt1)不等, 分子量介于13.136 kD (StIgt10)和32.542 kD (StIgt1)之间, 预测等电点为3.82 (StIgt4)~9.86 (StIgt10)。大多数StIgt蛋白(90%)的等电点小于7.0, 推测其可能是一类酸性蛋白。为进一步了解StIgt基因家族成员之间的同源关系, 本研究利用拟南芥、辣椒、水稻和玉米中已研究的Igt基因共同构建了Igts的系统进化树, 聚类为3个亚族: Lazy1 (I)、Tac1 (II)和Dro1 (III), 分别含有4、4、2个StIgt基因(图1)。Table 1
表1
表1拟南芥、桃、玉米与水稻Igt基因登录号
Table 1
| 基因名称 Gene name | 基因登录号 GenBank No. | 基因名称 Gene name | 基因登录号 GenBank No. |
|---|---|---|---|
| AtDro1 | AT1G72490 | AtLazy6 | AT3G27025 |
| AtDro2 | AT1G19115 | PpeLazy1 | LOC18782538 |
| AtDro3 | AT1G17400 | PpeLazy2 | LOC18790006 |
| OsDro1 | BAN59748.1 | ZmLazy | LOC100193776 |
| PpeDro1 | LOC103327608 | OsLazy1 | LOC4350543 |
| PpeDro2 | LOC18770522 | PpeTac1 | LOC18773917 |
| AtLazy1 | AT5G14090 | Ostac1 | LOC4347655 |
| AtLazy5 | AT3G24750 | AtTac1 | AT2G46640 |
新窗口打开|下载CSV
Table 2
表2
表2马铃薯StIgt基因家族信息
Table 2
| 基因名称 Gene name | 基因ID Gene ID | 亚组 Group | 染色体位置 Chromosome location | 大小 Size | 分子量 MW (kD) | 等电点 pI |
|---|---|---|---|---|---|---|
| StIgt10 | PGSC0003DMG400042006 | I | chr11: 19822270-19822602 | 110 | 13,136.1 | 9.86 |
| StIgt9 | PGSC0003DMG400035280 | I | chr01: 12153224-12154281 | 139 | 15,371.1 | 4.61 |
| StIgt8 | PGSC0003DMG400020205 | I | chr02: 48071799-48076548 | 256 | 29,350.3 | 6.41 |
| StIgt7 | PGSC0003DMG401019811 | I | chr05: 15169941-15172623 | 255 | 27,797.7 | 5.41 |
| StIgt6 | PGSC0003DMG400041371 | II | chr07: 26993146-27002422 | 275 | 31,382.8 | 4.38 |
| StIgt5 | PGSC0003DMG400005529 | II | chr10: 5159202-5164672 | 281 | 32,338.2 | 5.15 |
| StIgt4 | PGSC0003DMG400022847 | II | chr01: 59885567-59885980 | 137 | 15,989.9 | 3.82 |
| StIgt3 | PGSC0003DMG400000126 | II | chr01: 73826302-73827911 | 221 | 24,499.6 | 5.38 |
| StIgt2 | PGSC0003DMG400016036 | III | chr07: 39417955-39420740 | 228 | 26,123 | 6.17 |
| StIgt1 | StDro1 | III | 未知Unknown | 283 | 32,542.3 | 6.68 |
新窗口打开|下载CSV
图1
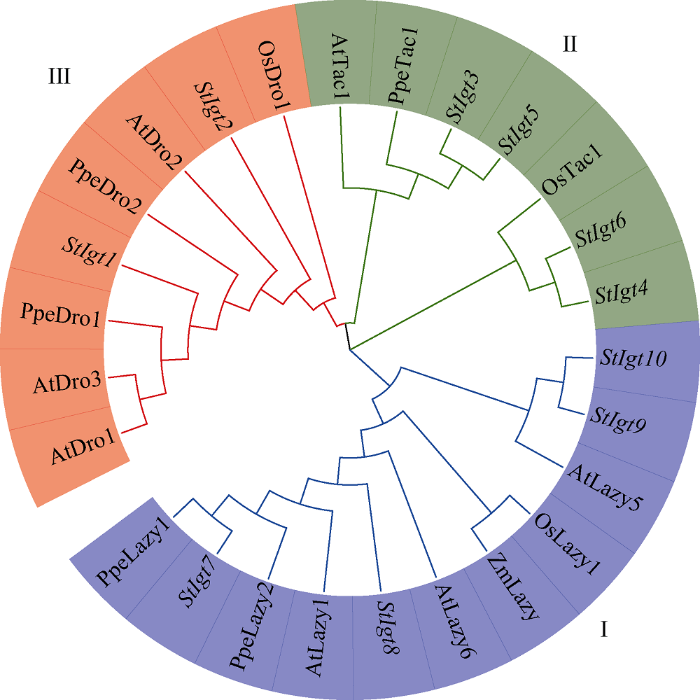 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1基于马铃薯和其他物种Igt基因家族系统进化树
Fig. 1Phylogenetic tree of Igt gene family in potato and other species
2.2 马铃薯StIgt基因家族的结构特征分析
对马铃薯StIgt基因家族蛋白保守结构域进行分析, 共鉴定出10个保守基序(Motif)。其中, 第I亚族中包含Motif 1、Motif 2、Motif 3、Motif 5、Motif 8、Motif 9和Motif 10, 第II亚族中包含Motif 1、Motif 3、Motif 4、Motif 6、Motif 7、Motif 9和Motif 10, 第III亚族中包含Motif 1、Motif 3、Motif 4和Motif 10 (图2)。I亚族中的基因StIgt8相对于其他成员拥有更多的蛋白保守序列, 推测I亚族基因结构可能较II和III亚族更为复杂。此外, 在StIgt中普遍存在的保守结构域为Motif 1、Motif 3和Motif 10。从3个亚族的结构来看, I亚族包含除Motif 3、Motif 6和Motif 7之外的其他所有结构域; II亚族主要包含Motif 1、Motif 3、Motif 6和Motif 7, 且Motif多集中于序列前半段和后半段; III亚族则主要含有Motif 1和Motif 3结构域, 其中III亚族基因StIgt1相对于同亚族StIgt2多了一个Motif 3和Motif 4, 且Motif 1和Motif 3之间的内含子长度不同, 说明这些基因在遗传进化过程中可能存在遗传信息的遗失(图2-A)。同时, 基因结构分析表明, StIgts 3个亚族的基因结构具有显著差别, 整体看来, I亚族外显子数目为1~4个, II亚族为1~6个, III亚族为2个。同亚族内基因一般都具有类似的外显子结构, 但从基因全长来看, 相似的CDS长度却有不同的基因全长, 说明内含子长度具有较大的差异性(图2-B)。图2
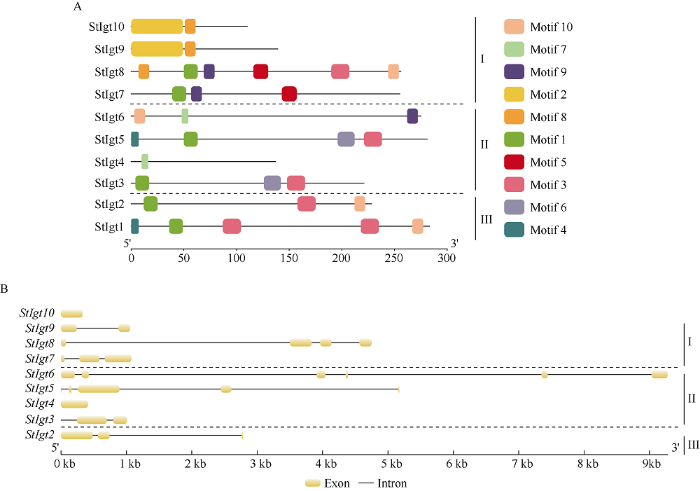 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2马铃薯StIgt基因家族成员的蛋白保守域和基因结构
A: 蛋白结构域特征; B: 基因结构。
Fig. 2Protein conserved domains and structural characteristics of StIgt gene family members in potato
A: protein structure; B: gene structure.
2.3 马铃薯StIgt基因家族的染色体定位分析与胁迫相关的顺式元件分析
马铃薯StIgt基因家族共鉴定到10个成员, 其中StIgt1可能由于参考基因组组装完整度的问题, 并没有找到染色体位置信息, 其余9条基因分别分布在1、2、5、7、10和11号染色体上, 其中1号染色体上有3个基因, 7号染色体上分有2个, 其余染色体各有1个。2号染色体上的基因分布在其下端, 7号和11号染色体的基因分布在其中间部位, 5号和10号染色体上基因分布在其上端。通过比较3个亚族基因在染色体上分布情况, 发现I亚族基因分布于1、2、5和11号染色体上, II亚族基因分布在1、7和10号染色体上, III亚族基因在7号染色体上(图3-A)。为进一步研究StIgt基因是否响应非生物胁迫, 本研究提取StIgt基因翻译起始位点上游1.5 kb的序列提交到PlantCare在线网站进行顺式元件检测。结果如图3-B所示, 共检测到6个非生物胁迫响应元件, 分别为脱落酸响应元件(ABRE)、脱水反应元件(MBS), 参与防御和应激反应的作用元件(ERE)、胁迫响应元件(W-box)、水杨酸响应元件(TCA- element)和生长素响应元件(TGA-element)。通过3个亚族基因的顺式元件分布情况比较发现, 马铃薯StIgt基因家族均含有ERE顺式作用元件, 其中II亚族基因相对于Ⅲ亚族富含更多逆境响应元件如ABRE、MBS、TCA-element和W-box, 而I亚族基因相对于II亚族富含更多生长素响应的顺式作用元件如TGA-element。这表明, StIgt基因家族与逆境响应, 尤其是非生物胁迫的响应密切相关。图3
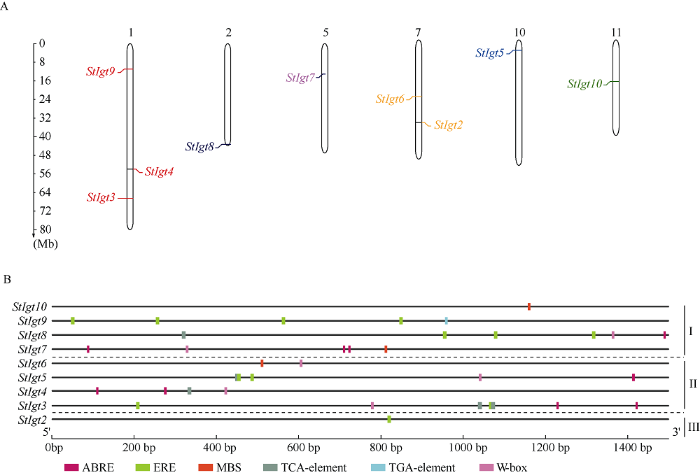 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3马铃薯StIgts的染色体定位(A)和顺式作用元件分析(B)
ABRE: 脱落酸作用元件; ERE: 参与防御和应激反应的作用元件; MBS: 干旱胁迫反应元件; TCA-element: 水杨酸作用元件; TGA-element: 生长素响应元件; W-box: 胁迫响应元件。
Fig. 3Chromosome location and cis-elements analysis of the StIgt genes in potato
ABRE: cis-acting element involved in the abscisic acid responsiveness; ERE: elements involved in defense and stress response; MBS: MYB binding site involved in drought-inducibility; TCA-element: cis-acting element involved in salicylic acid responsiveness; TGA-element: cis-acting element involved in auxin responsiveness; W-box: cis-acting element involved in the stress responsiveness.
2.4 马铃薯StIgt基因家族在响应干旱胁迫的表达谱分析
基于前述生物信息分析结果, StIgt 基因家族可能响应非生物胁迫。因此, 本研究利用课题组前期的对四倍体马铃薯品系C119的转录组测序数据, 提取不同干旱处理时间下StIgts的FPKM值, 对其响应干旱胁迫的表达谱进行了分析, 并对其中3个基因进行了qPCR验证。结果显示, 该家族的3个亚族间的表达趋势各不相同, 其中亚族I中的StIgt7、StIgt9和StIgt10均在干旱胁迫2 h时基因表达量升高至最高, 在6 h和24 h时表达量下降; StIgt8的表达量也呈先升高后降低的趋势, 但是于6 h时达到峰值。亚族II的StIgt4和StIgt5在干旱胁迫2 h时表达量开始降低, 随后保持较低水平, 直至在胁迫24 h的表达量降至最低。亚族III的StIgt1和StIgt2在干旱胁迫早期2 h时表达量呈下降趋势, 到6 h时降至最低, 随后在胁迫12 h时基因的表达量开始上升, 到24 h时又表达量升至最高, 这与亚族I和II呈现相反的表达趋势。综合来看, StIgt6、StIgt7、StIgt9和StIgt10基因在干旱胁迫2 h时表达量均发生上调且达到峰值, 说明这部分基因可能迅速响应早期干旱胁迫(图4)。随后我们重新处理了材料, 选取了3个基因StIgt1、StIgt3和StIgt10进行qPCR验证, 它们分别为拟南芥的AtDro1、AtTac1和AtLazy5的直系同源基因。qPCR结果表明, 这3个基因的表达趋势与转录组结果相吻合, 印证了转录组数据的可靠性, 其中StIgt1基因表达量在6 h和12 h时相对于对照0 h时差异极显著, StIgt3基因表达量在12 h时相对于对照0 h时差异极显著, StIgt10基因表达量在2 h和12 h时相对于对照0 h时差异极显著(图5)。表明, StIgt基因家族可响应干旱胁迫, 且3个亚族的在不同的胁迫条件下响应模式也不同。图4
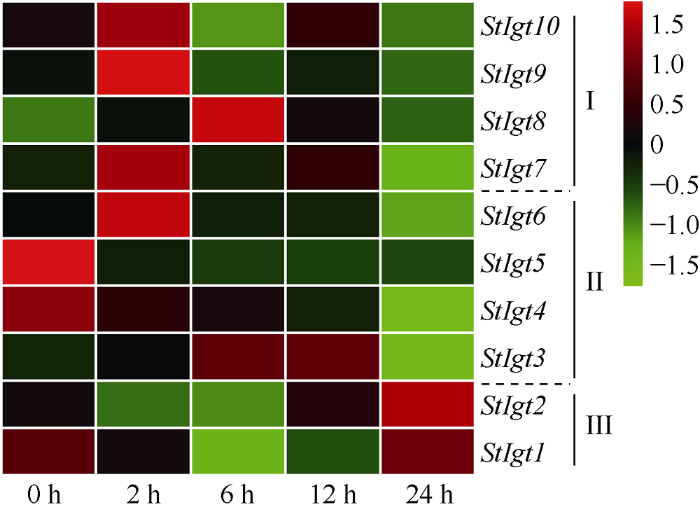 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4马铃薯StIgt基因在不同干旱胁迫时间下的表达模式分析
Fig. 4Expression pattern of StIgt genes in different drought stress conditions in potato
图5
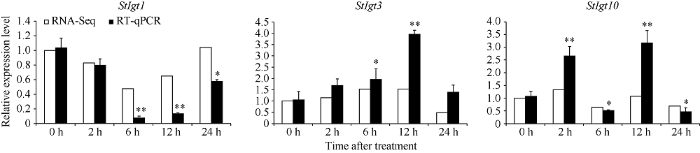 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5干旱胁迫时间下马铃薯StIgt1、StIgt3和StIgt10基因的表达
*, **分别表示在0.05和0.01水平上显著差异。
Fig. 5Gene expression of StIgt1, StIgt3 and StIgt10 under drought stress in potato
* and ** mean significant difference at the 0.05 and 0.01 probability levels, respectively.
3 讨论
目前我国60%左右的马铃薯种植于干旱和半干旱地区(年平均降水量小于500 mm, 干燥度在1.5以上)。长期或季节性的干旱胁迫会严重影响马铃薯的植株长势、块茎产量和商品性质量[24,25]。在禾本科作物中的研究表明, 较深的根系可以明显提高作物在极端或者季节性干旱环境下的产量, 且可作为耐旱作物育种评鉴与筛选的指标之一[26,27]。在块根块茎类作物中根系构型也是重要的产量构成因素[28]。深根性状除了可由主根系的长度增加造成外, 还可由侧根系或不定根系的向地性增强造成。而目前在多种植物中的研究发现, Igt基因家族与重力反应相关的向性调控如地上部分支角度、垂直根系角度等密切相关[15-16,29-30]。例如, Tac1和Lazy1, 已被证明可以调控单子叶(水稻和玉米)和双子叶(拟南芥和李属植物)物种的各种侧枝器官的角度, 包括分蘖、分枝、花梗、叶柄和果柄, 以及垂直根系角度[10,11], 此外, 功能研究较为深入的OsDro1(OsIgt1)已被证实可通过增加根系的向地性生长, 形成深根效应, 进而提高水稻的耐旱性[10]。而在马铃薯中, 关于根系构型影响抗旱性的基因调控研究还少见报道。本课题组前期克隆了马铃薯StDro1(StIgt1), 并在近期的研究中发现, StDro1响应干旱胁迫和生长素处理, 并在拟南芥中验证了其参与调控侧根角度和抵御干旱胁迫。因此, Igt基因家族在调控马铃薯根系构型和抗旱方面的研究值得深入。为进一步研究StIgts的其他成员, 本研究利用马铃薯‘DM-v4.03’参考基因组, 经过Fgenesh[31]和blast[32]方法对马铃薯StIgt基因序列进行手动矫正和重新注释后共鉴定获得10个StIgts成员, 并对其结构、染色体定位、系统发育和逆境相关顺式元件等进行了分析。系统发育树显示, StIgt基因家族可分为3个不同的亚族, 不同亚族间的蛋白结构域和基因结构差异显著, 暗示其进化地位的差异和功能的多样性。此外, 在StIgts的启动子区域发现了多种植物激素和非生物胁迫相关的顺式作用元件, 说明StIgts的上游可能受到生长素、胁迫相关激素(脱落酸和水杨酸)以及胁迫信号的协同调控, 从而发挥调节植物向地性生长和抵御逆境的功能。
鉴于此, 我们利用本课题组前期的四倍体马铃薯品系在不同干旱处理条件下的转录组数据, 提取FPKM值, 分析了StIgts各个成员响应干旱胁迫的表达谱。结果表明, 不同的StIgt亚族对干旱的响应模式不同, 同一亚族内则有一定的规律性趋势。亚族I和亚族II的StIgt6、StIgt7、StIgt9和StIgt10可在早期(2 h)迅速响应干旱胁迫, 说明可能直接受到干旱信号的诱导, 但具体的机理有待于进一步研究。而StDro1所在的亚族III与其他2个亚族的表达模式差异较大, 对干旱胁迫响应相对滞后, 前人在禾本科作物中的研究也发现, Dro1受到生长素的直接调控, 这2种信号通路可能在上游通过未知的机制互作来协同调控Dro1的表达。同时, 本研究也利用q-PCR验证了转录组表达谱的可靠性。综上所述, 马铃薯StIgts的10个成员可能在马铃薯地上部和根系的分支角度以及响应干旱胁迫中发挥不同的作用。本研究对StIgt基因家族提供了较全面的信息, 为进一步研究马铃薯StIgts的功能和作用机理提供了基础支持。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1007/s12230-019-09755-2URL [本文引用: 1]
[本文引用: 1]
DOI:10.1017/S001447970001721XURL [本文引用: 1]
[本文引用: 1]
DOI:10.1051/agro:2008021URL [本文引用: 1]

2 assimilation by leaves is reduced mainly by stomatal closure, membrane damage and disturbed activity of various enzymes, especially those of CO2 fixation and adenosine triphosphate synthesis. Enhanced metabolite flux through the photorespiratory pathway increases the oxidative load on the tissues as both processes generate reactive oxygen species. Injury caused by reactive oxygen species to biological macromolecules under drought stress is among the major deterrents to growth. Plants display a range of mechanisms to withstand drought stress. The major mechanisms include curtailed water loss by increased diffusive resistance, enhanced water uptake with prolific and deep root systems and its efficient use, and smaller and succulent leaves to reduce the transpirational loss. Among the nutrients, potassium ions help in osmotic adjustment; silicon increases root endodermal silicification and improves the cell water balance. Low-molecular-weight osmolytes, including glycinebetaine, proline and other amino acids, organic acids, and polyols, are crucial to sustain cellular functions under drought. Plant growth substances such as salicylic acid, auxins, gibberrellins, cytokinin and abscisic acid modulate the plant responses towards drought. Polyamines, citrulline and several enzymes act as antioxidants and reduce the adverse effects of water deficit. At molecular levels several drought-responsive genes and transcription factors have been identified, such as the dehydration-responsive element-binding gene, aquaporin, late embryogenesis abundant proteins and dehydrins. Plant drought tolerance can be managed by adopting strategies such as mass screening and breeding, marker-assisted selection and exogenous application of hormones and osmoprotectants to seed or growing plants, as well as engineering for drought resistance.]]>
[本文引用: 1]
DOI:10.3390/ijms160510214URLPMID:25950766 [本文引用: 1]

Drought stress is one of the major abiotic stresses that are a threat to crop production worldwide. Drought stress impairs the plants growth and yield. Therefore, the aim of the present experiment was to select the tolerant genotype/s on the basis of moprpho-physiological and biochemical characteristics of 10 Vicia faba genotypes (Zafar 1, Zafar 2, Shebam, Makamora, Espan, Giza Blanka, Giza 3, C4, C5 and G853) under drought stress. We studied the effect of different levels of drought stress i.e., (i) normal irrigation (ii) mild stress (iii) moderate stress, and (iv) severe stress on plant height (PH) plant-1, fresh weight (FW) and dry weight (DW) plant-1, area leaf-1, leaf relative water content (RWC), proline (Pro) content, total chlorophyll (Total Chl) content, electrolyte leakage (EL), malondialdehyde (MDA), hydrogen peroxide (H2O2) content, and activities of catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD) of genotypes of faba bean. Drought stress reduced all growth parameters and Total Chl content of all genotypes. However, the deteriorating effect of drought stress on the growth performance of genotypes
DOI:10.1111/nph.13204URLPMID:25483362 [本文引用: 1]

The architecture of trees greatly impacts the productivity of orchards and forestry plantations. Amassing greater knowledge on the molecular genetics that underlie tree form can benefit these industries, as well as contribute to basic knowledge of plant developmental biology. This review describes the fundamental components of branch architecture, a prominent aspect of tree structure, as well as genetic and hormonal influences inferred from studies in model plant systems and from trees with non-standard architectures. The bulk of the molecular and genetic data described here is from studies of fruit trees and poplar, as these species have been the primary subjects of investigation in this field of science.
DOI:10.1038/ng.2725URLPMID:23913002 [本文引用: 2]

The genetic improvement of drought resistance is essential for stable and adequate crop production in drought-prone areas. Here we demonstrate that alteration of root system architecture improves drought avoidance through the cloning and characterization of DEEPER ROOTING 1 (DRO1), a rice quantitative trait locus controlling root growth angle. DRO1 is negatively regulated by auxin and is involved in cell elongation in the root tip that causes asymmetric root growth and downward bending of the root in response to gravity. Higher expression of DRO1 increases the root growth angle, whereby roots grow in a more downward direction. Introducing DRO1 into a shallow-rooting rice cultivar by backcrossing enabled the resulting line to avoid drought by increasing deep rooting, which maintained high yield performance under drought conditions relative to the recipient cultivar. Our experiments suggest that control of root system architecture will contribute to drought avoidance in crops.
[本文引用: 4]
URLPMID:28029738 [本文引用: 3]
DOI:10.1111/tpj.12234URLPMID:23663106 [本文引用: 2]

Trees are capable of tremendous architectural plasticity, allowing them to maximize their light exposure under highly competitive environments. One key component of tree architecture is the branch angle, yet little is known about the molecular basis for the spatial patterning of branches in trees. Here, we report the identification of a candidate gene for the br mutation in Prunus persica (peach) associated with vertically oriented growth of branches, referred to as 'pillar' or 'broomy'. Ppa010082, annotated as hypothetical protein in the peach genome sequence, was identified as a candidate gene for br using a next generation sequence-based mapping approach. Sequence similarity searches identified rice TAC1 (tiller angle control 1) as a putative ortholog, and we thus named it PpeTAC1. In monocots, TAC1 is known to lead to less compact growth by increasing the tiller angle. In Arabidopsis, an attac1 mutant showed more vertical branch growth angles, suggesting that the gene functions universally to promote the horizontal growth of branches. TAC1 genes belong to a gene family (here named IGT for a shared conserved motif) found in all plant genomes, consisting of two clades: one containing TAC1-like genes; the other containing LAZY1, which contains an EAR motif, and promotes vertical shoot growth in Oryza sativa (rice) and Arabidopsis through influencing polar auxin transport. The data suggest that IGT genes are ancient, and play conserved roles in determining shoot growth angles in plants. Understanding how IGT genes modulate branch angles will provide insights into how different architectural growth habits evolved in terrestrial plants.
[本文引用: 1]
URLPMID:17908158 [本文引用: 1]
[本文引用: 2]
URLPMID:24089437 [本文引用: 2]
URLPMID:9254694 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:24630073 [本文引用: 1]
[本文引用: 1]
DOI:10.1104/pp.17.00942URLPMID:28821594 [本文引用: 1]

A rice (Oryza sativa) mutant led to the discovery of a plant-specific LAZY1 protein that controls the orientation of shoots. Arabidopsis (Arabidopsis thaliana) possesses six LAZY genes having spatially distinct expression patterns. Branch angle phenotypes previously associated with single LAZY genes were here studied in roots and shoots of single and higher-order atlazy mutants. The results identify the major contributors to root and shoot branch angles and gravitropic behavior of seedling hypocotyls and primary roots. AtLAZY1 is the principal determinant of inflorescence branch angle. The weeping inflorescence phenotype of atlazy1,2,4 mutants may be due at least in part to a reversal in the gravitropism mechanism. AtLAZY2 and AtLAZY4 determined lateral root branch angle. Lateral roots of the atlazy2,4 double mutant emerged slightly upward, approximately 10 degrees greater than perpendicular to the primary root axis, and they were agravitropic. Etiolated hypocotyls of the quadruple atlazy1,2,3,4 mutant were essentially agravitropic, but their phototropic response was robust. In light-grown seedlings, the root of the atlazy2,3,4 mutant was also agravitropic but when adapted to dim red light it displayed a reversed gravitropic response. A reversed auxin gradient across the root visualized by a fluorescent signaling reporter explained the reversed, upward bending response. We propose that AtLAZY proteins control plant architecture by coupling gravity sensing to the formation of auxin gradients that override a LAZY-independent mechanism that creates an opposing gravity-induced auxin gradient.
[本文引用: 1]
[本文引用: 1]
