 ,*河北师范大学生命科学学院, 河北石家庄 050024
,*河北师范大学生命科学学院, 河北石家庄 050024Effects of OsRPK1 gene overexpression and RNAi on the salt-tolerance at seedling stage in rice
LI Jing-Lan**, CHEN Xin-Xin**, SHI Cui-Cui, LIU Fang-Hui, SUN Jing, GE Rong-Chao ,*College of Life Science, Hebei Normal University, Shijiazhuang 050024, Hebei, China
,*College of Life Science, Hebei Normal University, Shijiazhuang 050024, Hebei, China通讯作者:
收稿日期:2019-11-25接受日期:2020-03-24网络出版日期:2020-08-12
| 基金资助: |
Received:2019-11-25Accepted:2020-03-24Online:2020-08-12
| Fund supported: |
作者简介 About authors
李晶岚,E-mail:
陈鑫欣,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (2560KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李晶岚, 陈鑫欣, 石翠翠, 刘方惠, 孙静, 葛荣朝. OsRPK1基因过表达和RNA干涉对水稻苗期耐盐性的影响[J]. 作物学报, 2020, 46(8): 1217-1224. doi:10.3724/SP.J.1006.2020.92060
LI Jing-Lan, CHEN Xin-Xin, SHI Cui-Cui, LIU Fang-Hui, SUN Jing, GE Rong-Chao.
目前土壤盐渍化在全世界范围严重影响着农业生产[1]。我国在东北、西北、华北及滨海地区也分布着大面积的盐碱地, 盐碱化耕地面积达6.7×106 hm2, 占全国耕地总面积的25%左右[2,3]。因此, 在我国农作物育种工作中选育耐盐碱的优良品种显得尤为重要。其中探究耐盐相关基因、研究其耐盐内在生理机制, 成为耐盐碱农作物选育的重要研究方向之一。植物对逆境胁迫应答反应是一个涉及多基因、多信号传导途径及多基因表达产物的复杂过程, 这一过程中蛋白激酶是植物体内一类重要的调节因子, 通过膜受体蛋白激酶感知外界环境胁迫信号, 导致细胞内Ca2+、Na+/K+、ABA (脱落酸)等离子和分子浓度改变, 进而激活不同蛋白磷酸化途径, 调控下游抗逆基因的转录表达, 启动相应的生理生化等适应性反应来降低或消除危害[4,5,6]。
类受体蛋白激酶(receptor-like kinase, RLK)属于丝氨酸/苏氨酸蛋白激酶, 通过Ser/Thr残基的磷酸化和脱磷酸化, 控制下游靶蛋白的开启和关闭, 将胞外信号转换为胞质信号进而诱导特定功能基因的表达[7,8]。目前, 人们已经从玉米、拟南芥、水稻、小麦等多种高等植物基因组中克隆出大量的RLK基因。类受体蛋白激酶参与了植物体一系列的发育过程, 如调节原韧皮部和分生组织的生长[9]、影响茎和导管的生长发育[10]、抑制器官脱落[11]、调控花药的生长发育[12]以及维持植物细胞壁的完整性[13]。大量的RLK家族成员也被证实参与了植物对病原体入侵[14,15,16]、非生物胁迫的防御反应[17,18,19,20,21,22,23]。富含亮氨酸重复序列的类受体蛋白激酶(leucine-rich repeat receptor-like kinase, LRR-RLK)是在植物中广泛存在的、与抗逆性密切相关的类受体激酶, 其LRR单元的氨基酸序列一般为LXXLXXLXXLXLXXNXLXG XIPXX(X为任意氨基酸)[24]。AtRPK1具有2个胞外LRR结构, 在ABA、脱水、高盐和低温等胁迫下, AtRPK1基因转录水平明显增加[25]。AtRPK1在拟南芥对ABA的早期识别中发挥重要作用, 在发芽、生长和气孔关闭期间, AtRPK1基因的抑制表达将降低植物对脱落酸识别的敏感性[26]。在野生型拟南芥中过表达AtRPK1增加了植株对ABA的敏感性[19]。水稻抗白叶枯病基因Xa21、番茄的抗病基因Cf9和Cf2在结构上均具有胞外LRR区, Cf9蛋白有28个LRR单元, Cf2蛋白有38个LRR单元[22,23]。
水稻基因Os07g0602700(OsRPK1)与拟南芥基因AtRPK1序列具有高度的同源性。OsRPK1蛋白质长度为1084个氨基酸, 约为AtRPK1蛋白质(540个氨基酸)的2倍, 其胞外部分明显具有2个LRR单元的聚集区域, 1~355位氨基酸区域具有7个LRR单元, 355~685位氨基酸区域具有6个LRR单元, 其后685~801位氨基酸区域为跨膜区(MSD)。前期实验表明, AtRPK1、OsRPK1基因过表达拟南芥与对照植株相比表现出对NaCl和ABA的耐受性明显降低。AtRPK1-RNAi拟南芥与野生型拟南芥相比, 对NaCl和ABA的耐受性得到明显提高[27]。本文拟通过对基因OsRPK1在水稻中实现过量表达及RNA干涉, 进一步研究OsRPK1的抗逆性功能。
1 材料与方法
1.1 实验材料
供试水稻品种为日本晴(Oryza sativa L. cv. Nipponbare)、水稻RNAi载体pTCK303由本室保存; RNA提取试剂、DNA限制性内切酶、PrimerSTAR DNA聚合酶、T4-DNA连接酶、qRT-PCR荧光酶、反转录试剂盒均购自TaKaRa公司; PCR引物均由生工生物工程(上海)股份有限公司合成。1.2 实验方法
1.2.1 OsRPK1基因在盐胁迫下的表达模式分析将10日龄水稻幼苗移栽到140 mmol L-1 NaCl溶液中处理0、1、6、24 h后, 剪取幼苗叶片, 提取总RNA, 反转录获得cDNA, 利用Actin基因调整cDNA浓度使其一致, 然后利用LP-q: 5'-GCTAT GGTGTTGTACTGATGGAGT-3'、RP-q: 5'-GTGCAC ATCACTGCTAAATGCAATG-3'对不同处理的cDNA进行qRT-PCR检测, 重复检测3次确定OsRPK1基因在盐胁迫后的表达模式。
1.2.2 OsRPK1基因过量表达与RNAi表达载体的构建 提取水稻总RNA, 反转录获得cDNA, 利用引物PU: 5'-GCTCTAGACTAGCTAGCTCCCACC- 3' (酶切位点Xba I)和PD: 5'-GCTCTAGAGTTGAA TGTGTAATCATCGGT-3' (酶切位点Xba I)扩增获得基因OsRPK1, 构建到表达载体pCAMBIA1300上, 获得过量表达载体p1300:35S:OsRPK1。
选取OsRPK1基因距转录起始点69~419 bp区域和2043~2371 bp区域作为RNAi的干涉序列, 分别命名为OsRPK1-RNAi-1和OsRPK1-RNAi-2。以p1300:35S:OsRPK1质粒为模板, 用引物LP-1: 5'- GACTAGTGGTACCATCACTCTCTTCCTCCTCCTG-3' (酶切位点Spe I、Kpn I)和RP-1: 5'-GGAGCTC GGATCCCGGCGGAGCGACCAGAT-3' (酶切位点Sac I、BamH I), 采用PrimerSTAR DNA聚合酶进行PCR扩增, 得到350 bp目的片段, 回收、连接到pMD18-T载体pMD18-OsRPK1-RNAi-1, 转化到DH5α进行测序。提取pTCK303和pMD18-T- OsRPK1-RNAi-1的质粒, 首先采用内侧酶Kpn I/BamH I进行双酶切, 电泳、回收、连接、转化到大肠杆菌, 酶切鉴定获得正连载体pTCK303-OsRPK1- RNAi-antisense-1; 提取pTCK303-OsRPK1-RNAi- antisense-1和pMD18-T-OsRPK1-RNAi-1质粒, 采用外侧酶Spe I/Sac I进行双酶切, 回收、连接、构建获得RNAi表达载体pTCK303-OsRPK1-RNAi-sense-1, 即pTCK303-OsRPK1-RNAi-1载体。
利用引物LP-2: 5'-GACTAGTGGTACCGTTCG GTGTGATAGCGTTATTGG-3' (酶切位点Spe I、Kpn I)和RP-2: 5'-GGAGCTCGGATCCTGATTGGCACT CCGATGTCTTG-3'(酶切位点Sac I、BamH I), 用相同的方法构建获得RNAi表达载体pTCK303- OsRPK1-RNAi-sense-2, 即pTCK303-OsRPK1-RNAi-2载体。
1.2.3 水稻愈伤组织的遗传转化及鉴定 对日本晴水稻种子消毒后, 均匀铺于诱导培养基(NB培养基+2 mg L-1 2,4-D)诱导愈伤组织, 28℃暗培养25~ 28 d。挑选光滑、淡黄色、较硬的胚性愈伤组织, 均匀铺放在新鲜NB培养基上继代培养3~4 d。然后将胚性愈伤组织转移到无菌培养皿中, 倒入适量的农杆菌悬浮液, 室温放置10~20 min, 并不时晃动。取出愈伤组织用无菌滤纸吸去多余的菌液, 接种到固体共培养培养基(NB培养基+100 μmol L-1乙酰丁香酮, pH 5.5), 28℃暗培养2~3 d。随后, 将经过共培养的愈伤组织转移到无菌培养皿, 用无菌水冲洗2~3次。将愈伤组织转入含潮霉素的选择性培养基(NB培养基+羧苄青霉素500 mg L-1+潮霉素50 mg L-1), 28℃避光培养7 d。第2轮筛选培养基为NB培养基+羧苄青霉素250 mg L-1 +潮霉素50 mg L-1, 在28℃培养箱避光继续培养2周。然后, 选择生长旺盛的抗性愈伤组织转移到分化培养基(NB培养基+2.5 mg L-1 KT+0.5 mg L-1 NAA+羧苄青霉素250 mg L-1+潮霉素50 mg L-1), 于28℃、12 h光照/12 h黑暗条件下培养。分化的幼苗长到2 cm左右时, 将其移到壮苗培养基(1/2 MS +1/2 B5维生素+10 g L-1蔗糖+ 50 mg L-1潮霉素)。继续培养幼苗长到瓶口时, 打开瓶盖、加入少量水, 将其于自然状态下炼苗1周左右。然后将幼苗移入营养土中, 将成活的幼苗移栽至大田, 培养、收取种子。
利用上述方法, 将已构建好的过量表达载体p1300:35S:OsRPK1、RNAi表达载体pTCK303- OsRPK1-RNAi-1和pTCK303-OsRPK1-RNAi-2转入农杆菌EHA105后, 对水稻愈伤组织进行遗传转化, 最终得到35S:OsRPK1、OsRPK1-RNAi-1和OsRPK1- RNAi-2水稻种子。用RT-PCR检测35S:OsRPK1和RNAi水稻的基因表达。
质粒pTCK303携带GUS报告基因, 因此在转化水稻愈伤组织过程中, 可以对愈伤组织及分化幼苗进行GUS基因表达的组织化学染色进行转基因初步鉴定。具体方法是将准备好的愈伤组织、幼苗用蒸馏水冲洗干净后放入EP管中, 加入染色液(50 μL X-Gluc母液+950 μL基液)浸没样品, 封好盖子。37℃培养箱中避光温育1~12 h。将浸染过的样品转入95%乙醇中脱色2~3次, 至阴性对照材料呈白色为止, 在体视显微镜下观察拍照。
1.2.4 转基因水稻的耐盐性检测 选取p1300:35S:OsRPK1、pTCK303-OsRPK1-RNAi-1和pTCK303-OsRPK1-RNAi-2转基因水稻T1代株系, 在潮霉素水(50 mg L-1)中浸泡, 筛选出生根长芽的阳性苗, 挑选生长状态基本一致的转基因水稻移栽入微孔板中, 用含有0 mmol L-1、140 mmol L-1 NaCl水稻营养液培养7~10 d, 并以生长状态一致的野生型水稻作为对照进行耐盐性分析。
1.2.5 转基因水稻的生理指标检测 对正常培养7 d的野生型和转基因水稻幼苗分别用清水和140 mmol L-1 NaCl溶液浇灌6~10 d, 测定其脯氨酸含量、MDA含量。
选取叶龄相似的对照日本晴水稻与转基因水稻的叶片, 剪成小块, 称取0.2 g, 将叶片压入10 mL蒸馏水中, 真空抽气1 h后重新充入空气。将样品于室温搅动浸提1 h后, 用电导仪测定样品电导率。再将同样的叶片样品放入100℃沸水浴15 min, 转入清水中冷却10 min, 测定其煮沸电导率。相对电导率(%) = (处理电导率 - 空白电导率)/(煮沸电导率 - 空白电导率) × 100%。
2 结果与分析
2.1 盐胁迫后OsRPK1基因在水稻中的表达模式
对10日龄水稻幼苗用140 mmol L-1 NaCl溶液处理0、1、6、24 h, 分别取叶片提取总RNA, 反转录获得cDNA。qRT-PCR检测表明, 水稻中OsRPK1基因在盐胁迫后1 h出现暂时的应激性增加, 在胁迫6 h时表达量出现持续下降, 胁迫24 h时, 表达量比对照显著下降(图1)。图1
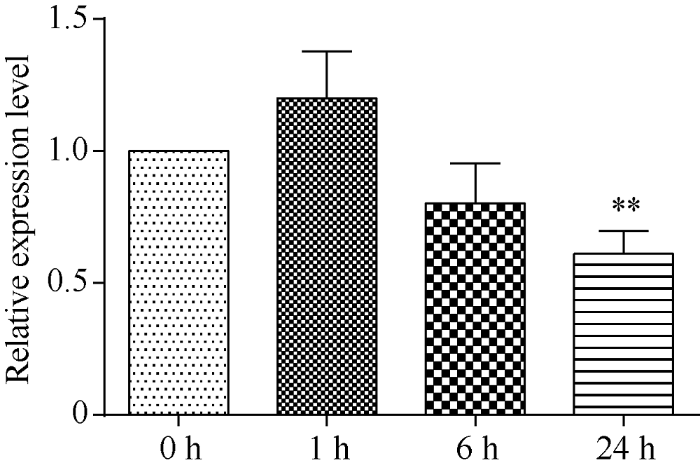 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1OsRPK1基因在140 mmol L-1 NaCl胁迫的水稻中表达量的变化
差异显著性分析采用t-test方法, *P < 0.05、**P < 0.01、***P < 0.001。
Fig. 1Changes of the expression of OsRPK1 gene in rice under 140 mmol L-1 NaCl stress
The significant difference was evaluated by the Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
2.2 OsRPK1过表达和RNAi转基因水稻的获得
2.2.1 OsRPK1过表达载体的构建 以水稻日本晴基因组cDNA为模板, 扩增得到OsRPK1基因3317 bp的目的条带。测序正确后, 对pMD18-T- OsRPK1质粒进行Xba I单酶切, 电泳后回收、纯化3317 bp目的DNA条带。将该基因与p1300表达载体重组融合, 对阳性克隆进行酶切鉴定后, 测序判断插入方向正确, 获得过量表达载体p1300:35S: OsRPK1。2.2.2 OsRPK1-RNAi表达载体的构建 利用设计的LP-1、RP-1和LP-2、RP-2两对引物, PCR扩增得到两段长度分别为350 bp、328 bp的OsRPK1特异性序列, 对PCR产物电泳、回收、连接到pMD18-T载体上, 进行双酶切鉴定、测序。测序正确的质粒分别命名为pMD18-T-OsRPK1-RNAi-1、pMD18-T-OsRPK1-RNAi-2。用内侧酶Kpn I/BamH I分别酶切两种RNAi T载体和pTCK303载体, 回收目标片段进行连接、转化、酶切鉴定, 分别命名为新构建的载体pTCK303-OsRPK1-RNAi-antisense-1、pTCK303-OsRPK1-RNAi-antisense-2。然后用外侧酶Sac I/Spe I双酶切两种RNAi T载体质粒和两种pTCK303-OsRPK1-RNAi-antisense质粒, 回收目的片段进行连接转化, 用BamH I单酶切鉴定分别得到1199 bp、1155 bp的DNA片段, 鉴定表明连接正确, 分别命名质粒为pTCK303-OsRPK1-RNAi-sense-1、pTCK303-OsRPK1-RNAi-sense-2, 成功构建获得水稻pTCK303-OsRPK1-RNAi-1、 pTCK303-OsRPK1- RNAi-2载体。
2.2.3 水稻愈伤组织的培养与转化 将消毒好的水稻种子在诱导培养基培养得到愈伤组织, 选择光滑、淡黄色、较硬、大小为2~3 mm的胚性愈伤组织, 转移到继代培养基上继续培养3~4 d; 利用含有p1300:35S:OsRPK1、pTCK303-OsRPK1-RNAi-1和pTCK303-OsRPK1-RNAi-2的农杆菌EHA105转化水稻愈伤组织, 平铺于带有滤纸的固体共培养培养基上暗培养3 d; 然后将愈伤组织转移至含有潮霉素的第1次选择培养基上, 7 d后将淡黄色、生长状态良好的愈伤组织转移到第2次选择培养基上进行筛选; 培养2周后, 将黄色、生长活性良好的愈伤组织转移至分化培养基上; 分化培养至发绿、生长出幼苗; 最后将生长出的幼苗进行壮苗、炼苗; 然后移栽到营养土中, 等其适应外部环境后再栽种到大田, 培养、收获种子。将RNAi转基因后的水稻愈伤组织和幼苗在GUS染液中染色, 初步确定RNAi质粒对水稻的成功转化。
2.3 水稻OsRPK1转基因植株的鉴定和耐盐性检测
2.3.1 水稻OsRPK1转基因植株的鉴定 分别选取T1代p1300:35S:OsRPK1、pTCK303-OsRPK1- RNAi-1和pTCK303-OsRPK1-RNAi-2转基因水稻的OX1、OX2、OX3三个株系和对照日本晴水稻, 提取总RNA, 反转录获得cDNA, 根据OsRPK1序列3′端设计的引物LP-q和RP-q, 进行qRT-PCR扩增检测表明, 在模板量相同的条件下, 35S:OsRPK1水稻相对于对照水稻日本晴表达量显著上升; OsRPK1-RNAi转基因水稻相对于对照水稻日本晴表达量则显著减弱, 其中利用基因编码区5′区域作为RNAi干涉序列的OsRPK1-RNAi-1表达量下降更为显著(图2)。图2
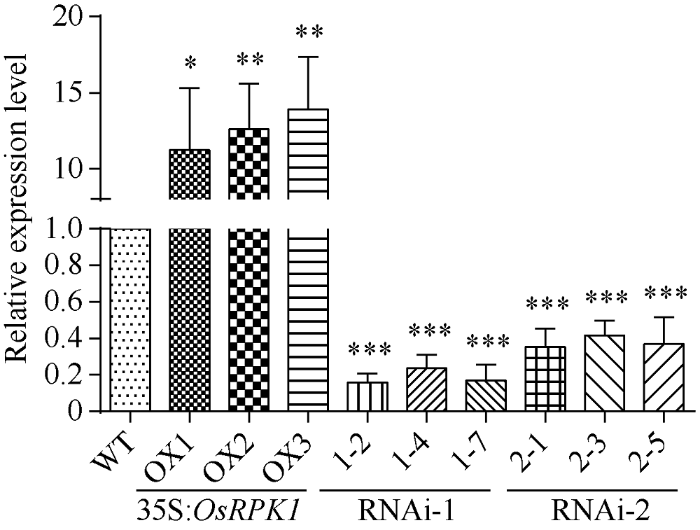 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图235S:OsRPK1植株与OsRPK1-RNAi植株的qRT-PCR检测
差异显著性分析采用t-test方法, *P < 0.05、**P < 0.01、 ***P < 0.001。
Fig. 2qRT-PCR identify of the 35S:OsRPK1 plants and OsRPK1-RNAi plants
The significant difference was evaluated by the Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
2.3.2 水稻OsRPK1转基因植株的耐盐性检测 分别选取p1300:35S:OsRPK1、pTCK303- OsRPK1-RNAi-1和pTCK303-OsRPK1-RNAi-2 T1代转基因水稻的不同株系, 以水稻日本晴作为对照进行幼苗耐盐性分析。
正常水培条件下, OsRPK1过表达水稻与对照日本晴的长势无明显差异(图3-A)。140 mmol L-1 NaCl胁迫处理6 d后, OsRPK1过表达水稻幼苗叶片出现明显干枯萎蔫现象, 而对照日本晴水稻受盐胁迫损伤较轻, 依旧有很多绿色叶片(图3-B), 恢复培养9 d后, 过表达植株绝大多数都干枯萎蔫, 出现失绿现象, 而对照水稻依旧有部分植株存活(图3-C)。存活率统计表明, 野生型水稻约为61%, 而OsRPK1过表达水稻OX1、OX2、OX3株系则分别仅有5.57%、6.67%、4.43%, 耐盐性明显下降(图3-D)。
图3
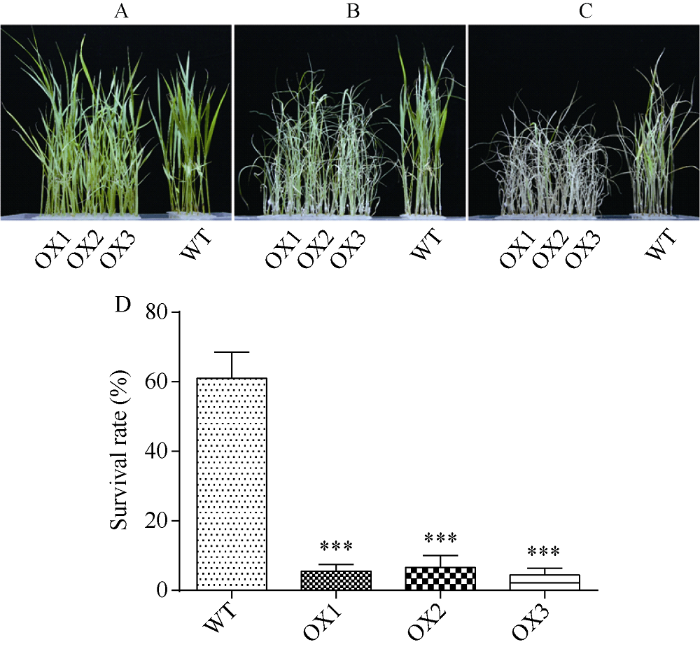 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3OsRPK1过表达水稻在盐胁迫下的生长情况
A: NaCl胁迫前; B: 140 mmol L-1 NaCl胁迫6 d; C: 140 mmol L-1 NaCl胁迫后恢复9 d; D: 盐胁迫恢复培养后存活率统计。差异显著性分析采用t-test方法, *P < 0.05、**P < 0.01、***P < 0.001。
Fig. 3Salt tolerance of the OsRPK1 overexpressing plants
A: rice seedling without NaCl stress; B: treated with 140 mmol L-1 NaCl for 6 days; C: recovery for nine days after 140 mmol L-1 NaCl stress; D: survival rate after renewing culture under salt stress. The significant difference was evaluated by the Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
在正常水培条件下, OsRPK1-RNAi-1水稻与对照日本晴的长势均无明显差异(图4-A)。在140 mmol L-1 NaCl胁迫10 d后, OsRPK1-RNAi-1转基因水稻与对照水稻相比存活绿叶更多, 生长状态较好; OsRPK1-RNAi-2转基因水稻在盐胁迫后也具有比对照野生型水稻更多的绿叶(图4-B)。恢复培养7 d后, OsRPK1-RNAi-1转基因水稻表现仍较葱绿, 但是对照日本晴已经全部干枯, 无法存活; 其中OsRPK1-RNAi-2转基因水稻在盐胁迫后的抗逆性与对照植株差异并不显著, 幼苗也基本枯萎(图4-C)。最终野生型水稻存活率为1%左右, 而OsRPK1- RNAi水稻株系存活率均明显较高, 其中表达量抑制效果更好的OsRPK1-RNAi-1株系1-2、1-4、1-7存活率更高, 达到50%~77%; 而RNA干涉区域设计在开放阅读框3′下游区域的OsRPK1-RNAi-2株系2-1、2-3、2-5存活率约为27%~32% (图4-D, E)。
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4OsRPK1-RNAi水稻在胁迫下生长情况
A: NaCl胁迫前; B: 140 mmol L-1 NaCl胁迫10 d; C: 140 mmol L-1 NaCl胁迫后恢复7 d; D, E: 盐胁迫恢复培养后存活率统计。差异显著性分析采用t-test方法, *P < 0.05、**P < 0.01、***P < 0.001。
Fig. 4Salt stress tolerance of the OsRPK1-RNAi rice plants
A: rice seedling without NaCl stress; B: treated with 140 mmol L-1 NaCl for 10 days; C: recovery for seven days after 140 mmol L-1 NaCl stress; D, E: survival rate after renewing culture under salt stress. The significant difference was evaluated by the Student’s t-test. * P < 0.05, ** P < 0.01, *** P < 0.001.
OsRPK1在水稻中的过量表达降低了植株对盐胁迫的耐受性; 而OsRPK1的表达被干涉后, OsRPK1-RNAi水稻对盐的耐受性有所提高, 与过表达植株的表型正好相反。
2.4 水稻OsRPK1转基因植株的生理指标检测
2.4.1 脯氨酸含量的检测 选取p1300:35S: OsRPK1、两种OsRPK1-RNAi转基因水稻和对照水稻日本晴植株, 分别用清水和140 mmol L-1 NaCl溶液浇灌, 然后测定其脯氨酸含量表明, 盐胁迫前, OsRPK1过表达水稻、两种OsRPK1-RNAi水稻和野生型水稻植株的脯氨酸含量均无明显差异; 盐胁迫后, OsRPK1过表达植株脯氨酸含量显著低于对照水稻, 而OsRPK1-RNAi水稻的脯氨酸含量显著高于对照植株(图5)。图5
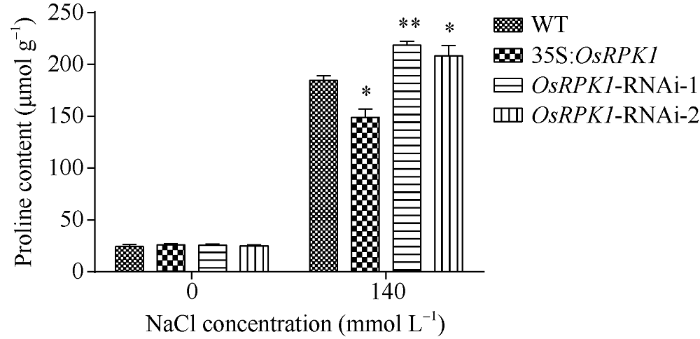 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5140 mmol L-1 NaCl盐胁迫后转基因水稻脯氨酸含量
差异显著性分析采用t-test方法, *P < 0.05、**P < 0.01、***P < 0.001。
Fig. 5Proline content of transgenic rice plants after 140 mmol L-1 NaCl treatment
The significant difference was evaluated by the Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
OsRPK1过表达植株体内脯氨酸合成不足可能是造成植株盐敏感的原因之一, 而OsRPK1-RNAi植株体内有更多的脯氨酸从而提高了水稻植株对盐的耐受性。
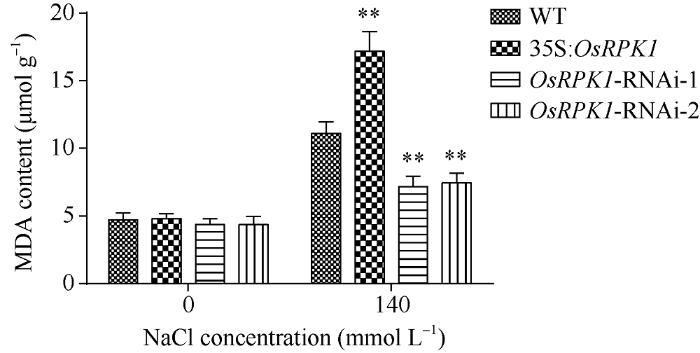
2.4.2 MDA含量的检测 对p1300:35S: OsRPK1、两种OsRPK1-RNAi转基因水稻和对照水稻植株, 分别用清水和140 mmol L-1 NaCl溶液浇灌培养, 然后测定其MDA含量。表明, 胁迫前OsRPK1过表达水稻、OsRPK1-RNAi水稻和野生型日本晴水稻的MDA含量无明显差异; 胁迫后所有水稻植株的MDA含量均显著升高, 其中OsRPK1过表达水稻MDA含量显著高于野生型日本晴水稻, OsRPK1- RNAi水稻的MDA含量均显著低于野生型日本晴水稻植株(图6)。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6140 mmol L-1 NaCl盐胁迫后转基因水稻株系中MDA含量
差异显著性分析采用t-test方法, *P < 0.05、**P < 0.01、***P<0.001。
Fig. 6MDA content of transgenic rice plants after 140 mmol L-1 NaCl treatment
The significant difference was evaluated by the Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
在盐胁迫条件下, OsRPK1过表达水稻植株细胞受损程度比野生型日本晴更高, OsRPK1-RNAi水稻植株细胞受破损程度比野生型日本晴低。
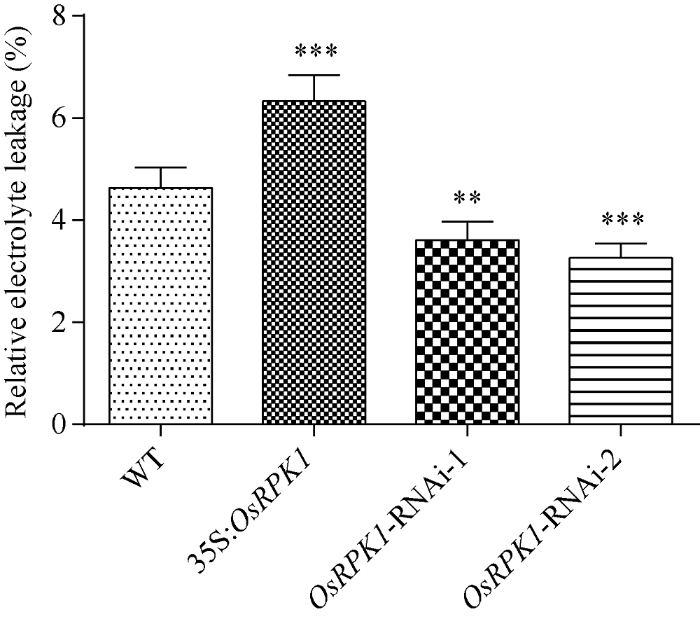
2.4.3 相对电导率的检测 OsRPK1过表达水稻的相对电导率显著高于野生型日本晴水稻; 而 OsRPK1-RNAi水稻植株的相对电导率则均显著低于野生型日本晴水稻(图7)。
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7转基因水稻的相对电导率
差异显著性分析采用t-test方法, *P < 0.05、**P < 0.01、***P < 0.001。
Fig. 7Relative electrical conductivity of transgenic rice plants
The significant difference was evaluated by the Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
OsRPK1基因过表达水稻在受到盐胁迫时, 可能会有更多的Na+离子进入细胞之中, 从而表现出盐敏感表型; 而OsRPK1-RNAi水稻植株则由于较低的质膜透性, 盐胁迫时, 进入细胞的Na+离子较少, 从而植株表现出对盐胁迫的耐受性。
3 讨论
类受体蛋白激酶在植物体内能够参与信号转导、抗逆反应和病原反应等途径, 对植物的抗逆信号传导具有重要意义。类受体激酶的表达量上升往往会提高植物体的抗逆性, 如PnLRR-RLK27的过量表达提高了拟南芥对盐、干旱、H2O2和ABA的耐受性[28]; 在拟南芥中类受体丝氨酸/苏氨酸蛋白激酶基因GsRLCK的过量表达同样提高了转基因植株对盐、干旱和ABA的耐受性[29]; An等[30]从辣椒中分离获得的CAMRP基因的表达使拟南芥提高了耐盐性和抗病性。同时, 一些类受体激酶的表达量上升也会造成植物抗逆性的下降, 如水稻类受体激酶SIT1可以被NaCl诱导表达量明显上升, 进而SIT1磷酸化MAPK3/6, 介导细胞中的乙烯传导信号通路, 导致植物活性氧的产生和积累, 造成细胞生长受抑和死亡[31]。在苔藓中, 凝集素受体样激酶PnLec RLK1在低温、盐胁迫、干旱胁迫、外源ABA和茉莉酸甲酯胁迫下表达量迅速上升。过表达PnLec RLK1的转基因拟南芥对低温胁迫的耐受性增强, 而对ABA的耐受性明显减弱[32]。AtRPK1是Hong等在1997年从拟南芥中克隆到的受干旱、高盐及低温诱导的受体蛋白激酶基因。AtRPK1在脱落酸诱导、脱水、高盐和低温等胁迫环境下转录水平明显增加[11]。AtRPK1基因的过量表达造成了拟南芥的耐盐性明显下降, 而AtRPK1基因的表达受到抑制时, 拟南芥植株的耐盐性则明显得到提高。OsRPK1与AtRPK1基因具有高度同源性, 前期研究表明, OsRPK1基因过表达拟南芥与对照拟南芥相比, 其种子萌发、幼苗生长阶段对NaCl和ABA的耐性明显降低[27]。
本实验对OsRPK1基因在盐胁迫下的表达模式检测表明, 水稻受到盐胁迫时, OsRPK1的表达量暂时升高后, 明显下降。在水稻中实现OsRPK1的过量表达和RNA干涉, 结果表明OsRPK1基因表达量增加时, 水稻植株对盐胁迫表现为抗逆性明显降低; 在RNA干涉使其表达量下调后, 水稻植株则表现为耐盐性明显增加。通过生理检测发现, OsRPK1过表达造成拟南芥质膜透性增加, 在受到盐胁迫时对细胞造成更大的损伤。前期亚细胞定位检测实验表明, OsRPK1蛋白分布于细胞膜上, 且明显呈簇状分布, 这可能是过表达植株细胞膜通透性增加的原因之一。同时OsRPK1过表达拟南芥细胞内脯氨酸含量的减少, 使其在遭受盐胁迫时受到了更严重的渗透胁迫。无论在抗逆表型还是在生理指标的变化趋势方面, OsRPK1基因在水稻中的表现与在转基因拟南芥中的表现一致[27]。
总之, 通过本实验证实, 在水稻中OsRPK1基因的过表达会明显降低植株的耐盐性, 而对OsRPK1基因表达抑制后, 减弱了盐胁迫对早期细胞质膜的损伤, 提高了水稻植株的耐盐性。在农作物育种方面, 对相应的RPK1基因敲除, 可能会获得耐盐性提高的品系, 为培育优良耐盐作物品种奠定了基础。
4 结论
水稻类受体蛋白激酶基因OsRPK1在盐胁迫下其表达量暂时升高后出现明显下降。OsRPK1基因的表达量对水稻耐盐性具有明显影响, 其表达量增加时, 水稻幼苗耐盐性下降; OsRPK1基因的RNA干涉则使水稻幼苗耐盐性增加。生理指标检测结果表明, 脯氨酸含量及细胞质膜受破损程度的改变可能是造成转基因水稻耐盐性变化的内在原因。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:21451255 [本文引用: 1]
URLPMID:21399949 [本文引用: 1]
URLPMID:30209320 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1073/pnas.1222314110URLPMID:23569225 [本文引用: 1]

Peptide signaling presumably occupies a central role in plant development, yet only few concrete examples of receptor-ligand pairs that act in the context of specific differentiation processes have been described. Here we report that second-site null mutations in the Arabidopsis leucine-rich repeat receptor-like kinase gene barely any meristem 3 (BAM3) perfectly suppress the postembryonic root meristem growth defect and the associated perturbed protophloem development of the brevis radix (brx) mutant. The roots of bam3 mutants specifically resist growth inhibition by the CLAVATA3/ENDOSPERM SURROUNDING REGION 45 (CLE45) peptide ligand. WT plants transformed with a construct for ectopic overexpression of CLE45 could not be recovered, with the exception of a single severely dwarfed and sterile plant that eventually died. By contrast, we obtained numerous transgenic bam3 mutants transformed with the same construct. These transgenic plants displayed a WT phenotype, however, supporting the notion that CLE45 is the likely BAM3 ligand. The results correlate with the observation that external CLE45 application represses protophloem differentiation in WT, but not in bam3 mutants. BAM3, BRX, and CLE45 are expressed in a similar spatiotemporal trend along the developing protophloem, up to the end of the transition zone. Induction of BAM3 expression upon CLE45 application, ectopic overexpression of BAM3 in brx root meristems, and laser ablation experiments suggest that intertwined regulatory activity of BRX, BAM3, and CLE45 could be involved in the proper transition of protophloem cells from proliferation to differentiation, thereby impinging on postembryonic growth capacity of the root meristem.
URLPMID:21853254 [本文引用: 1]
DOI:10.1104/pp.111.175224URLPMID:21628627 [本文引用: 2]

Receptor-like kinase-mediated cell signaling pathways play fundamental roles in many aspects of plant growth and development. A pair of Arabidopsis (Arabidopsis thaliana) leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA (HAE) and HAESA-LIKE2 (HSL2), have been shown to activate the cell separation process that leads to organ abscission. Another pair of LRR-RLKs, EVERSHED (EVR) and SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1, act as inhibitors of abscission, potentially by modulating HAE/HSL2 activity. Cycling of these RLKs to and from the cell surface may be regulated by NEVERSHED (NEV), a membrane trafficking regulator that is essential for organ abscission. We report here the characterization of CAST AWAY (CST), a receptor-like cytoplasmic kinase that acts as a spatial inhibitor of cell separation. Disruption of CST suppresses the abscission defects of nev mutant flowers and restores the discrete identity of the trans-Golgi network in nev abscission zones. After organ shedding, enlarged abscission zones with obscured boundaries are found in nev cst flowers. We show that CST is a dual-specificity kinase in vitro and that myristoylation at its amino terminus promotes association with the plasma membrane. Using the bimolecular fluorescence complementation assay, we have detected interactions of CST with HAE and EVR at the plasma membrane of Arabidopsis protoplasts and hypothesize that CST negatively regulates cell separation signaling directly and indirectly. A model integrating the potential roles of receptor-like kinase signaling and membrane trafficking during organ separation is presented.
URLPMID:17419837 [本文引用: 1]
DOI:10.1093/aob/mcu043URLPMID:24723447 [本文引用: 1]

BACKGROUND: Plant cell walls form the interface between the cells and their environment. They perform different functions, such as protecting cells from biotic and abiotic stress and providing structural support during development. Maintenance of the functional integrity of cell walls during these different processes is a prerequisite that enables the walls to perform their particular functions. The available evidence suggests that an integrity maintenance mechanism exists in plants that is capable of both detecting wall integrity impairment caused by cell wall damage and initiating compensatory responses to maintain functional integrity. The responses involve 1-aminocyclopropane-1-carboxylic acid (ACC), jasmonic acid, reactive oxygen species and calcium-based signal transduction cascades as well as the production of lignin and other cell wall components. Experimental evidence implicates clearly different signalling molecules, but knowledge regarding contributions of receptor-like kinases to this process is less clear. Different receptor-like kinase families have been considered as possible sensors for perception of cell wall damage; however, strong experimental evidence that provides insights into functioning exists for very few kinases. SCOPE AND CONCLUSIONS: This review examines the involvement of cell wall integrity maintenance in different biological processes, defines what constitutes plant cell wall damage that impairs functional integrity, clarifies which stimulus perception and signal transduction mechanisms are required for integrity maintenance and assesses the available evidence regarding the functions of receptor-like kinases during cell wall integrity maintenance. The review concludes by discussing how the plant cell wall integrity maintenance mechanism could form an essential component of biotic stress responses and of plant development, functions that have not been fully recognized to date.
DOI:10.1111/jipb.12123URLPMID:24308571 [本文引用: 1]

Plants employ a highly effective surveillance system to detect potential pathogens, which is critical for the success of land plants in an environment surrounded by numerous microbes. Recent efforts have led to the identification of a number of immune receptors and components of immune receptor complexes. It is now clear that receptor-like kinases (RLKs) and receptor-like proteins (RLPs) are key pattern-recognition receptors (PRRs) for microbe- and plant-derived molecular patterns that are associated with pathogen invasion. RLKs and RLPs involved in immune signaling belong to large gene families in plants and have undergone lineage specific expansion. Molecular evolution and population studies on phytopathogenic molecular signatures and their receptors have provided crucial insight into the co-evolution between plants and pathogens. [Figure: see text] Jian-Min Zhou (Corresponding author).
[本文引用: 1]
URLPMID:22427336 [本文引用: 1]
[本文引用: 1]
DOI:10.1105/tpc.112.100107URLPMID:22730405 [本文引用: 1]

The plant hormone abscisic acid (ABA) regulates stomatal movement under drought stress, and this regulation requires hydrogen peroxide (H2O2). We isolated GUARD CELL HYDROGEN PEROXIDE-RESISTANT1 (GHR1), which encodes a receptor-like kinase localized on the plasma membrane in Arabidopsis thaliana. ghr1 mutants were defective ABA and H2O2 induction of stomatal closure. Genetic analysis indicates that GHR1 is a critical early component in ABA signaling. The ghr1 mutation impaired ABA- and H2O2-regulated activation of S-type anion currents in guard cells. Furthermore, GHR1 physically interacted with, phosphorylated, and activated the S-type anion channel SLOW ANION CHANNEL-ASSOCIATED1 when coexpressed in Xenopus laevis oocytes, and this activation was inhibited by ABA-INSENSITIVE2 (ABI2) but not ABI1. Our study identifies a critical component in ABA and H2O2 signaling that is involved in stomatal movement and resolves a long-standing mystery about the differential functions of ABI1 and ABI2 in this process.
DOI:10.1074/jbc.M109.051938URLPMID:20089852 [本文引用: 2]

RPK1 (receptor-like protein kinase 1) localizes to the plasma membrane and functions as a regulator of abscisic acid (ABA) signaling in Arabidopsis. In our current study, we investigated the effect of RPK1 disruption and overproduction upon plant responses to drought stress. Transgenic Arabidopsis overexpressing the RPK1 protein showed increased ABA sensitivity in their root growth and stomatal closure and also displayed less transpirational water loss. In contrast, a mutant lacking RPK1 function, rpk1-1, was found to be resistant to ABA during these processes and showed increased water loss. RPK1 overproduction in these transgenic plants thus increased their tolerance to drought stress. We performed microarray analysis of RPK1 transgenic plants and observed enhanced expression of several stress-responsive genes, such as Cor15a, Cor15b, and rd29A, in addition to H(2)O(2)-responsive genes. Consistently, the expression levels of ABA/stress-responsive genes in rpk1-1 had decreased compared with wild type. The results suggest that the overproduction of RPK1 enhances both the ABA and drought stress signaling pathways. Furthermore, the leaves of the rpk1-1 plants exhibit higher sensitivity to oxidative stress upon ABA-pretreatment, whereas transgenic plants overproducing RPK1 manifest increased tolerance to this stress. Our current data suggest therefore that RPK1 overproduction controls reactive oxygen species homeostasis and enhances both water and oxidative stress tolerance in Arabidopsis.
DOI:10.1074/jbc.M109.035659URLPMID:20026608 [本文引用: 1]

Cold is a limiting environmental factor that adversely affects plant growth and productivity. Calcium/calmodulin-mediated signaling is believed to play a pivotal role in plant response to cold stress, but its exact role is not clearly understood. Here, we report that CRLK1, a novel calcium/calmodulin-regulated receptor-like kinase, is crucial for cold tolerance in plants. CRLK1 has two calmodulin-binding sites with different affinities as follows: one located at residues 369-390 with a K(d) of 25 nm, and the other located at residues 28-112 with a K(d) of 160 nm. Calcium/calmodulin stimulated the kinase activity, but the addition of chlorpromazine, a calmodulin antagonist, blocked its stimulation. CRLK1 is mainly localized in the plasma membrane, and its expression is stimulated by cold and hydrogen peroxide treatments. Under normal growth conditions, there is no noticeable phenotypic difference between wild-type and crlk1 knock-out mutant plants. However, as compared with wild-type plants, the crlk1 knock-out mutants exhibited an increased sensitivity to chilling and freezing temperatures. Northern analysis showed that the induction of cold-responsive genes, including CBF1, RD29A, COR15a, and KIN1 in crlk1 mutants, is delayed as compared with wild-type plants. These results indicate that CRLK1 is a positive regulator of cold tolerance in plants. Furthermore, our results suggest that CRLK1 plays a role in bridging calcium/calmodulin signaling and cold signaling.
DOI:10.1186/s12870-019-1964-yURLPMID:31416418 [本文引用: 1]

BACKGROUND: To compensate for the lack of information about the molecular mechanism involved in Arundo donax L. response to salt stress, we de novo sequenced, assembled and analyzed the A. donax leaf transcriptome subjected to two levels of long-term salt stress (namely, S3 severe and S4 extreme). RESULTS: The picture that emerges from the identification of differentially expressed genes is consistent with a salt dose-dependent response. Hence, a deeper re-programming of the gene expression occurs in those plants grew at extreme salt level than in those subjected to severe salt stress, probably representing for them an
DOI:10.1126/science.270.5243.1804URLPMID:8525370 [本文引用: 2]

The rice Xa21 gene, which confers resistance to Xanthomonas oryzae pv. oryzae race 6, was isolated by positional cloning. Fifty transgenic rice plants carrying the cloned Xa21 gene display high levels of resistance to the pathogen. The sequence of the predicted protein, which carries both a leucine-rich repeat motif and a serine-threonine kinase-like domain, suggests a role in cell surface recognition of a pathogen ligand and subsequent activation of an intracellular defense response. Characterization of Xa21 should facilitate understanding of plant disease resistance and lead to engineered resistance in rice.
DOI:10.1016/0968-0004(94)90090-6URLPMID:7817399 [本文引用: 2]

Leucine-rich repeats are short sequence motifs present in a number of proteins with diverse functions and cellular locations. All proteins containing these repeats are thought to be involved in protein-protein interactions. The crystal structure of ribonuclease inhibitor protein has revealed that leucine-rich repeats correspond to beta-alpha structural units. These units are arranged so that they form a parallel beta-sheet with one surface exposed to solvent, so that the protein acquires an unusual, nonglobular shape. These two features may be responsible for the protein-binding functions of proteins containing leucine-rich repeats.
[本文引用: 1]
DOI:10.1104/pp.113.4.1203URLPMID:9112773 [本文引用: 1]

A cDNA clone for a receptor-like protein kinase gene (RPK1) was isolated from Arabidopsis thaliana. The clone is 1952 bp long with 1623 bp of an open reading frame encoding a peptide of 540 amino acids. The deduced peptide (RPK1) contains four distinctive domains characteristic of receptor kinases: (a) a putative amino-terminal signal sequence domain; (b) a domain with five extracellular leucine-rich repeat sequences; (c) a membrane-spanning domain; and (d) a cytoplasmic protein kinase domain that contains all of the 11 subdomains conserved among protein kinases. The RPK1 gene is expressed in flowers, stems, leaves, and roots. Expression of the RPK1 gene is induced within 1 h after treatment with abscisic acid (ABA). The gene is also rapidly induced by several environmental stresses such as dehydration, high salt, and low temperature, suggesting that the gene is involved in a general stress response. The dehydration-induced expression is not impaired in aba-1, abi1-1, abi2-1, and abi3-1 mutants, suggesting that the dehydration-induced expression of the RPK1 gene is ABA-independent. A possible role of this gene in the signal transduction pathway of ABA and the environmental stresses is discussed.
DOI:10.1105/tpc.104.027474URLPMID:15772289 [本文引用: 1]

Abscisic acid (ABA) is important in seed maturation, seed dormancy, stomatal closure, and stress response. Many genes that function in ABA signal transduction pathways have been identified. However, most important signaling molecules involved in the perception of the ABA signal or with ABA receptors have not been identified yet. Receptor-like kinase1 (RPK1), a Leu-rich repeat (LRR) receptor kinase in the plasma membrane, is upregulated by ABA in Arabidopsis thaliana. Here, we show the phenotypes of T-DNA insertion mutants and RPK1-antisense plants. Repression of RPK1 expression in Arabidopsis decreased sensitivity to ABA during germination, growth, and stomatal closure; microarray and RNA gel analysis showed that many ABA-inducible genes are downregulated in these plants. Furthermore, overexpression of the RPK1 LRR domain alone or fused with the Brassinosteroid-insensitive1 kinase domain in plants resulted in phenotypes indicating ABA sensitivity. RPK1 is involved in the main ABA signaling pathway and in early ABA perception in Arabidopsis.
DOI:10.1016/j.plantsci.2013.12.002URLPMID:24467897 [本文引用: 3]

AtRPK1 (AT1G69270) is a leucine-rich repeat receptor-like protein kinase (LRR-RLK) gene in Arabidopsis thaliana. The rice gene Os07g0602700 (OsRPK1) is the homolog of AtRPK1. AtRPK1 and OsRPK1 were overexpressed and the expression of AtRPK1 was inhibited by RNAi in A. thaliana. The functional results showed that the degrees of salt tolerance of the 35S:RPK1 A. thaliana plants were significantly lower than that of the control plants. The AtRPK1-RNAi A. thaliana plants exhibited higher salt tolerance than the wild-type plants (Col). The subcellular localisation results showed that the RPK1 proteins were mainly distributed on the cell membrane and that the overexpressed AtRPK1 proteins exhibited a significantly clustered distribution. The physiological analyses revealed that the overexpression of the RPK1 genes increased the membrane permeability in the transgenic A. thaliana plants. In response to salt stress, these plants exhibited an increased Na(+) flux into the cell, which caused greater damage to the cell. The real-time quantitative PCR analysis showed that the expression of the P5CS1 gene was inhibited and the SOS signalling pathway was blocked in the 35S:AtRPK1 A. thaliana plants. These effects at least partially contribute to the salt-sensitive phenotype of the 35S:RPK1 plants.
[本文引用: 1]
DOI:10.1007/s00425-013-1864-6URLPMID:23494614 [本文引用: 1]

Receptor such as protein kinases are proposed to work as sensors to initiate signaling cascades in higher plants. However, little is known about the precise functions of receptor such as protein kinases in abiotic stress response in plants, especially in wild soybean. Here, we focused on characterization of the biological functions of a receptor-like cytoplasmic serine/threonine protein kinase gene, GsRLCK, which was previously identified as a putative salt-alkali stress-related gene from the transcriptome profiles of Glycine soja. Bioinformatic analysis showed that GsRLCK protein contained a conserved kinase catalytic domain and two transmembrane domains at the N-terminus, but no typical extracellular domain. Consistently, GsRLCK-eGFP fusion protein was observed on the plasma membrane, but eGFP alone was distributing throughout the cytoplasm in onion epidermal cells. Quantitative real-time PCR analysis revealed the induced expression of GsRLCK by ABA, salt, alkali, and drought stresses. However, the expression levels of GsRLCK seemed to be similar in different tissues, except soybean pod. Phenotypic assays demonstrated that GsRLCK overexpression decreased ABA sensitivity and altered expression levels of ABA-responsive genes. Furthermore, we also found that GsRLCK conferred increased tolerance to salt and drought stresses and increased expression levels of a handful of stress-responsive genes, when overexpressing in Arabidopsis. In a word, we gave exact evidence that GsRLCK was a novel receptor-like cytoplasmic protein kinase and played a crucial role in plant responses to ABA, salt, and drought stresses.
DOI:10.1007/s11103-008-9337-1URLPMID:18427932 [本文引用: 1]

Plant receptor proteins are involved in the signaling networks required for defense against pathogens. The novel pepper pathogen-induced gene CaMRP1 was isolated from pepper leaves infected with Xanthomonas campestris pv. vesicatoria (Xcv). This gene is predicted to encode a membrane-located receptor-like protein that has an N-terminal signal peptide and a C-terminal transmembrane helix. A CaMRP1-GFP fusion protein localized primarily to the plasma membrane of plant cells. Strong and early induction of CaMRP1 expression occurred following exposure of pepper plants to Xcv, Colletotricum coccodes, methyl jasmonate (MeJA) and wounding stress. Virus-induced gene silencing (VIGS) of CaMRP1 in pepper conferred enhanced basal resistance to Xcv infection, accompanied by induction of genes encoding basic PR1 (CaBPR1), defensin (CaDEF1) and SAR8.2 (CaSAR82A). In contrast, CaMRP1 overexpression (OX) in transgenic Arabidopsis plants resulted in increased disease susceptibility to Hyaloperonospora parasitica infection. Arabidopsis plants overexpressing CaMRP1 exhibited insensitivity to MeJA by causing reduced expression of MeJA-responsive genes. Overexpression also resulted in tolerance to NaCl and during salt stress, the expression of several abscisic acid-responsive genes was induced. Together, these results suggest that pepper CaMRP1 may belong to a new subfamily of membrane-located receptor-like proteins that regulate disease susceptibility, MeJA-insensitivity and salt tolerance.
DOI:10.1105/tpc.114.125187URL [本文引用: 1]

High salinity causes growth inhibition and shoot bleaching in plants that do not tolerate high salt (glycophytes), including most crops. The molecules affected directly by salt and linking the extracellular stimulus to intracellular responses remain largely unknown. Here, we demonstrate that rice (Oryza sativa) Salt Intolerance 1 (SIT1), a lectin receptor-like kinase expressed mainly in root epidermal cells, mediates salt sensitivity. NaCl rapidly activates SIT1, and in the presence of salt, as SIT1 kinase activity increased, plant survival decreased. Rice MPK3 and MPK6 function as the downstream effectors of SIT1. SIT1 phosphorylates MPK3 and 6, and their activation by salt requires SIT1. SIT1 mediates ethylene production and salt-induced ethylene signaling. SIT1 promotes accumulation of reactive oxygen species (ROS), leading to growth inhibition and plant death under salt stress, which occurred in an MPK3/6- and ethylene signaling-dependent manner in Arabidopsis thaliana. Our findings demonstrate the existence of a SIT1-MPK3/6 cascade that mediates salt sensitivity by affecting ROS and ethylene homeostasis and signaling. These results provide important information for engineering salt-tolerant crops.
DOI:10.1007/s10725-016-0217-4URL [本文引用: 1]
