 ,*河北农业大学农学院, 河北保定071001
,*河北农业大学农学院, 河北保定071001Functional characteristics of TaPYR1, an abscisic acid receptor family gene in mediating wheat tolerance to drought stress
HAN Le, DU Ping-Ping, XIAO Kai ,*College of Agronomy, Hebei Agricultural University, Baoding 071001, Hebei, China
,*College of Agronomy, Hebei Agricultural University, Baoding 071001, Hebei, China通讯作者:
收稿日期:2019-11-9接受日期:2020-01-15网络出版日期:2020-06-12
| 基金资助: |
Received:2019-11-9Accepted:2020-01-15Online:2020-06-12
| Fund supported: |
作者简介 About authors
E-mail:hanle95@163.com。

摘要
关键词:
Abstract
Keywords:
PDF (2376KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
韩乐, 杜萍萍, 肖凯. 小麦脱落酸受体基因TaPYR1介导植株抵御干旱逆境功能研究[J]. 作物学报, 2020, 46(6): 809-818. doi:10.3724/SP.J.1006.2020.91067
HAN Le, DU Ping-Ping, XIAO Kai.
干旱是作物生长、发育和产量形成的重要限制因素, 增强小麦等麦类作物水资源利用效率对于保障我国粮食安全及农业可持续发展具有重要实践意义[1]。研究表明, 植株在长期的演化过程中, 已形成系统的抵御干旱等渗透胁迫逆境信号转导和相关生化途径[2,3]。其中, 脱落酸(abscisic acid, ABA)依赖途径通过介导干旱诱导的ABA信号传递, 在转录水平上激活下游渗透胁迫防御、初级和次级代谢、气孔开度调节等相关基因表达, 在增强植株适应和抵御干旱逆境中发挥着重要生物学功能[4,5,6]。
近年来研究证实, ABA依赖的信号传递途径与感受渗透胁迫逆境诱发的ABA受体蛋白密切相关[7,8,9]。干旱等渗透胁迫诱导的ABA首先被特定ABA受体家族成员感知, 该家族成员识别和结合干旱诱导的ABA后形成受体-ABA复合体, 进一步与信号组分磷酸酶(PP2Cs)互作, 形成受体-ABA- PP2Cs三聚体, 由此解除PP2Cs与SnRK转录因子互作造成的对SnRK转录和功能的限制。活化的SnRK转录因子进而调控下游干旱响应关键基因转录, 增强植株对干旱胁迫的适应和抵御能力[10]。近年来, 有关模式植物拟南芥中ABA受体基因及其功能的鉴定研究已有较多报道, 证实Pyrabactin 1 (PYR 1)/PYR 1和PYL蛋白具有结合ABA及将ABA信号向下游传递的功能[11]。通过上述ABA受体与ABA和PP2Cs核心ABA信号元件互作激活SnRK 2家族成员, 调控干旱逆境下生理生化过程, 改善植株响应干旱等渗透胁迫逆境的相关生理生化过程[12,13]。
植物种属中ABA受体PYR (PYRABACTINRE SISTANCE)以家族的形式存在。迄今在模式植物拟南芥中鉴定了PYR1及其他13个同源基因(PYR1- Like), 分别命名为PYL1~PYL13。对上述拟南芥ABA受体编码基因应答干旱逆境模式和基因突变体研究表明, 部分PYR家族成员呈干旱诱导表达模式, 基因失活的突变体对干旱胁迫的耐受性与野生型对照相比发生明显改变[14]。表明PYR家族成员通过介导ABA信号传递, 在植株适应和抵御干旱逆境中发挥着重要生物学功能。
小麦是我国北方重要粮食作物, 增强小麦抗旱能力对于节省水资源开发及促进我国粮食可持续发展具有重要实践意义。作者前期利用高通量测序分析鉴定了1个对干旱显著应答的小麦ABA受体基因TaPYR1。基于此, 本研究针对迄今有关小麦种属PYR家族成员研究尚少的现状, 对该小麦PYR家族成员分子特征、应答干旱表达模式及介导植株抵御干旱逆境的能力进行了研究, 旨在丰富小麦响应干旱逆境分子机理的认识, 为今后小麦抗旱遗传改良提供理论。
1 材料与方法
1.1 材料及处理
以小麦品种济麦22为材料, 采用试验室建立的Hoagland培养法培养小麦幼苗。培养条件为温度25℃/20℃ (白天/夜间), 光周期12 h/12 h (光期/暗期), 空气湿度65%~75%。期间每3 d更换一次营养液。三叶期时, 将幼苗移至含有5%聚乙二醇(PEG-6000)的营养液内, 处理6、12、24和48 h后收获根叶样本。此外, 将部分干旱处理48 h后幼苗转移至正常Hoagland营养液, 处理24 h和48 h收获根叶样本。以干旱处理前(0 h)根叶作对照。收获各处理时间点样本经液氮速冻后置?80℃冰箱备用。1.2 TaPYR1分子特征研究
以TaPYR1蛋白序列为基础查找GenBank同源蛋白, 采用Mega软件建立TaPYR1与其植物种属同源蛋白系统进化树。利用Protparam软件, 在线分析TaPYR1分子量和等电点; 运用NCBI的CDD (Conserved Domain Database)在线工具, 鉴定TaPYR1保守域; 采用TaPYR1与报告基因GFP融合蛋白(TaPYR1-GFP)在细胞内定位试验, 明确TaPYR1亚细胞定位特征。方法如下: 采用DNA重组技术构建表达载体 pCAMBIA1300-TaPYR1- GFP。扩增TaPYR1 CDS的正向引物为5'-TGCTCTA GAATGGAGCAGCAGCCTGTG-3', 反向引物为5'- CGGACTAGTTTATTCTGCCGGCGGCGC-3'。用将重组质粒农杆菌用针头注射入烟草表皮细胞, 48 h后用激光共聚焦显微镜(FV10-ASW, OLYMPUS)于激发光波长 480 nm 和发射光波长510 nm下观察GFP信号, 确定供试基因编码蛋白的亚细胞位置。1.3 TaPYR1应答干旱逆境的表达模式研究
参照试剂说明书采用TRIzol提取样本根、叶总RNA。参照Guo等[15]的方法采用qRT-PCR技术, 鉴定TaPYR1在各处理时间点的转录本丰度。以小麦组成型基因Tatubulin作为目标基因转录本均一化内标, 扩增该内标基因正向引物为5'-CATGCTATCCCTC GTCTCGACCT-3', 反向引物为5'-CGCACTTCATG ATGGAGTTGTAT-3'。1.4 转基因烟草株系建立
参照Sun等[16]的方法, 以干旱处理48 h小麦根系cDNA为模板, 采用RT-PCR技术扩增TaPYR1正、反义序列。其中, 扩增正义序列正向引物为5'-AAACCATGGAGCAGCAGCCTGTG-3', 反向引物为5'- GCAAAGAGTATCACAGCATCCGG-3'; 扩增反义序列正向引物为5'-AAACCATGGCAAAGAGTA TCACAGCA-3', 反向引物为5'-AAAGGTAACCGC GTCGAGTCGAGTCCA-3'。采用农杆菌介导转化法将正、反义表达载体分别转化烟草(cv. Wiscosin 35), 构建正、反义表达TaPYR1转化株系。1.5 干旱处理下转化株系植株形态和干质量测定
以TaPYR1正义株系Sen 1、Sen 2和反义株系Anti 1、Anti 2及野生型对照(WT)为材料, 采用与上述培养小麦幼苗相似的溶液培养法培养供试材料, 三叶期将供试材料分为两组条件, 一组用正常Hoaland营养液培养(对照), 另一组转入含有5% PEG的Hoagland营养液进行干旱处理。4周后用数码相机记录植株长势。此外, 选取各处理代表性植株3株, 置烘箱内烘干后称重, 获得植株干质量。1.6 干旱处理下转化株系生理生化参数测定
以上述不同处理4周后供试材料叶片为样本, 参照Guo等[15]方法, 测定不同处理下转化株系光合速率(Pn)、胞间CO2浓度(Ci)、光系统II光化学活性(ФPSII)和叶绿体光化学淬灭系数(NPQ)。此外, 参照Huang等[17]的方法测定超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)活性和丙二醛(MDA)含量; 参照Wang等[18]的方法, 测定脯氨酸含量和可溶性糖含量。1.7 干旱处理下转化株系细胞保护酶编码基因表达模式研究
以干旱处理4周后供试材料叶片为样本, 对4个编码SOD家族基因(NtSOD1、NtFeSOD、NtSOD2、NtMnSOD2)、6个编码CAT蛋白基因(NtCAT、NtCAT1、NtCAT1;1、NtCAT1;2、NtCAT1;3、NtCAT3)和10个编码POD蛋白基因(NtPOD1;1、NtPOD1;2、NtPOD1;3、NtPOD1;4、NtPOD1;5、NtPOD1;6、NtPOD1;7、NtPOD4、NtPOD9、NtPOD2;1)的表达模式进行了研究。上述细胞保护酶基因的GenBank登录号和用于扩增上述基因扩增的引物见附表1。参照Sun等[16]的方法检测上述细胞保护酶各家族基因的转录丰度进行。以烟草组成型表达基因Nttubulin为目标基因表达丰度均一化内参。扩增该内参基因正向引物为5'-TACACAGGGGAAGG AATGG-3', 反向引物为5'-CTCGAAACCAACGGTA TC-3'。Supplementary table 1
附表1
附表1NtSOD、NtCAT和NtPOD家族基因特异性扩增引物
Supplementary table 1
| 目的基因 Gene | 登录号 Accession number | 上游引物 Forward primer (5'-3') | 下游引物 Reverse primer (5'-3') |
|---|---|---|---|
| NtSOD1 | KJ874395 | GTGGACATGTCGTGTCAAGG | TTCTCACCAACTCCTGCACTT |
| NtFeSOD | KF724056 | CATCACAGAGCTTATGTCGACA | CTAGAACTGACTGCTTCCCA |
| NtSOD2 | EU123521 | ATGTCACGGGACCACATTAC | AACCCTTCCACCAGCATTTC |
| NtMnSOD2 | AB093097 | GACGGACCTTAGCAACAGGG | ACCAATGGGTCCTGATTAGCAG |
| NtCAT | NTU07627 | GTCTCAGGCTGACAAGTCTT | ACGGAAGACAGAGTAGCAGC |
| NtCAT1 | EF532799 | CAAGGATCTCTACGACTCGATT | CTTGAGGGCAAATAATCCACCT |
| NtCAT1;1 | NTU93244 | TCCTGCTAATGCTCCAAAGTGT | AATGCATATGTATTAGGAATGCTC |
| NtCAT1;2 | HF564632 | GGTATCGACTTGGACCAAACTA | GGTCTCACATTAAGCCTAGAAG |
| NtCAT1;3 | HF564631 | TTGCAGCCGGTGGGAAGATT | GGTCTCACATTAAGCCTAGAAG |
| NtCAT3 | HF564633 | GTCTTGGGCCAAACTATCTGCA | TCAGCTTCACATTGTGGGCC |
| NtPOD1;1 | L02124 | GGAATTTGTCCTCAAGGTGGAA | CTTATTGGAATTGCCATTTCAGC |
| NtPOD1;2 | AB044154 | CTGACATGGTCTGTGCCTAC | TCAGTTGATAGCAGAGCAAACTT |
| NtPOD1;3 | AB044153 | AAGATCTTGTCGCTCTTACTGG | AATTGGATTTTCCAGCTTGCG |
| NtPOD1;4 | D11396 | TGCTGGTAGTCAAAGTCAGTTTT | CCCATGTTGAACACGTTCTTACC |
| NtPOD1;5 | AB178953 | AACAGCAACAACGTTAACCCAGC | TTAATTTTGGACCACATTCAGGA |
| NtPOD1;6 | AB027753 | TCAACTCCACTGGTGGCCCT | AATTCGATTTTGCAGCTTGCGC |
| NtPOD1;7 | AB027752 | GCCCAAGAAGTTCAGGCTCA | ATACAAATACAGTCCTTTACTCG |
| NtPOD2;1 | AB178954 | AGGGGAAAAGACCTCACCAC | AGTTTCCCATCTTGATCATAGCA |
| NtPOD4 | AY032675 | AGACTCAAAGATAGCAAACCTCA | CTTCCTGATGTCACCCTTGA |
| NtPOD9 | AY032674 | CACCACCTTCATTCAACGCTA | ACATCTCAGACAAAACACTTGTC |
新窗口打开|下载CSV
1.8 数据统计学分析
采用Microsoft Excel 2003和DPS 7.05软件进行数据处理和统计分析。各测定数据均源于4次测试重复结果。2 结果与分析
2.1 TaPYR1的分子特征
TaPYR1 cDNA全长 840 bp, 开放读码框长度为636 bp, 编码211个氨基酸。TaPYR1蛋白分子量为 22.621 kD, 等电点(pI)为4.97。蛋白结构预测分析表明, TaPYR1含有植物种属PYR家族成员保守的SRPBCC超家族保守区域(图1-A); 蛋白多重比对结果表明, TaPYR1在44~192氨基酸区段与其同源蛋白高度保守, 和其他物种同源蛋白具有相似的保守域结构(图1-B)。系统进化分析表明, 在氨基酸水平上, TaPYR1与单子叶植物种属如节节麦、大麦、水稻和玉米等同源蛋白在序列组成上高度相似(图1-C), 表明上述成员可能具有相似的进化祖先。采用蛋白定位检测试验鉴定TaPYR1-GFP融合蛋白的亚细胞位置表明, TaPYR1-GFP融合蛋白的荧光信号出现在细胞质膜。说明TaPYR1经内质网分选后定位于质膜, 在此处参与结合ABA及特定ABA信号的传递。图1
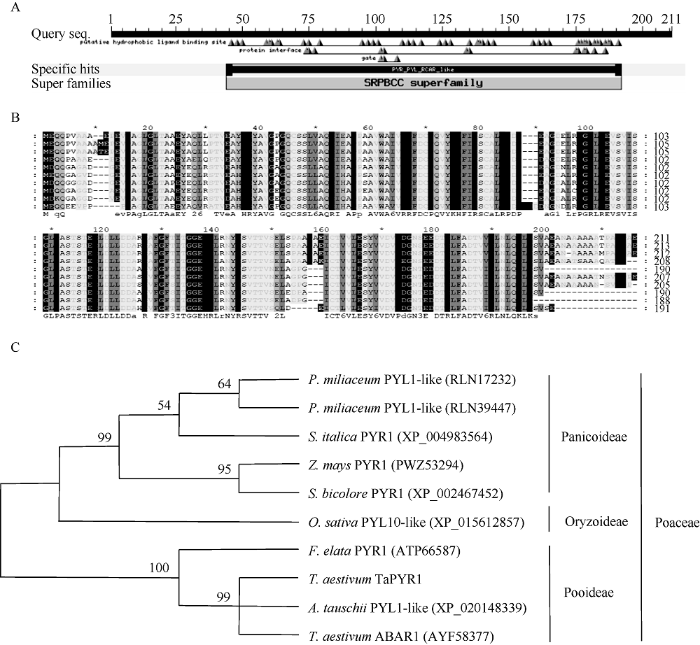 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1TaPYR1蛋白的分子特征
A: TaPYR1蛋白结构预测; B: TaPYR1蛋白多重序列比对; C: TaPYR1氨基酸水平系统进化分析。
Fig. 1Molecular characteristics of the TaPYR1 protein
A: TaPYR1 protein structure predicted; B: alignment results among TaPYR1 protein and its homolougous proteins; C: phylogenetic relations among TaPYR1 and its counterparts in plant species.
2.2 TaPYR1应答干旱胁迫逆境的表达模式
正常生长条件下, 小麦根、叶片内TaPYR1转录本数量均较低; 干旱处理下, TaPYR1表达上调, 表现在48 h处理时间内, 随处理进程转录本数量不断增多。此外, 将经48 h干旱处理植株转移至正常条件后, 根、叶内TaPYR1表达水平逐渐下调, 大致恢复至处理前水平(图2)。表明TaPYR1呈典型干旱应答表达模式, 通过转录调节参与植株应答干旱胁迫过程。图2
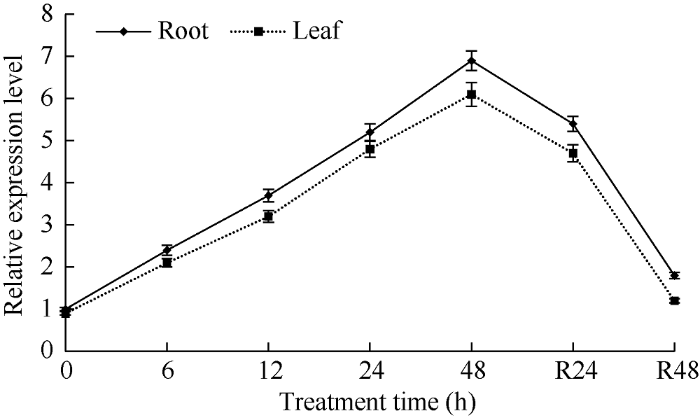 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2根叶中TaPYR1应答干旱胁迫逆境表达模式
0 h, 处理前对照; 6、12、24和48 h, 干旱处理时间点; R24 h和R48 h, 恢复处理时间点。
Fig. 2Expression patterns of TaPYR1 in both roots and leaves under drought stress
0 h, control before treatment; 6, 12, 24, and 48 hours, time points under drought treatment; R24 h and R48 h, time points of recovery treatment after drought treatment.
2.3 干旱胁迫下转化株系的植株生长特征
正常生长条件下, 供试转化株系和与野生型(wild type, WT)植株长势一致(图3-A)。干旱处理下, 与WT相比, 正义转化TaPYR1烟草株系(Sen 1和Sen 2)植株长势明显增强(图3-A), 植株干质量显著增多(图3-B); 与此相反, 反义转化TaPYR1的烟草株系(Anti 1和Anti 2)植株长势明显变差(图3-A), 植株干质量显著降低(图3-B)。说明TaPYR1转录丰度改变能诱发干旱逆境下植株长势和干物质积累能力的明显改变, 该基因在介导植株抵御干旱胁迫过程中发挥着重要生物学作用。图3
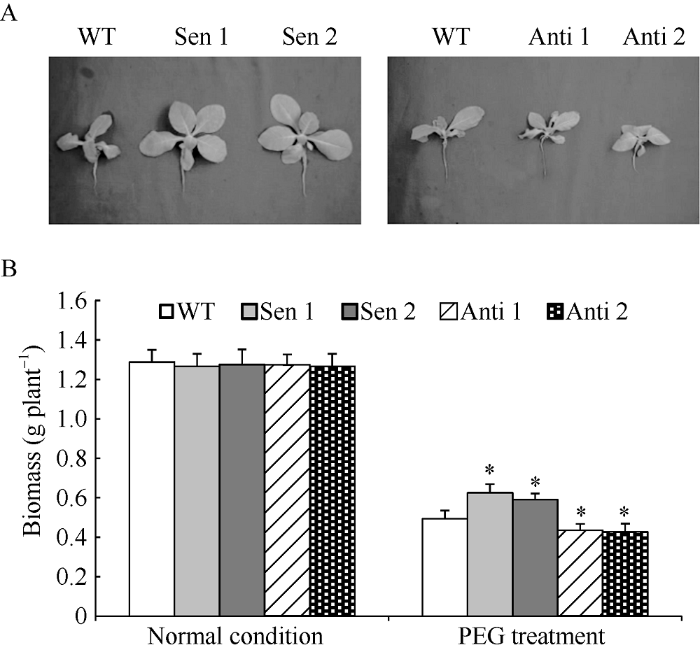 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3干旱胁迫下转化株系的植株生长特征
A: 干旱胁迫下TaPYR1转化株系植株生长情况; B: 干旱胁迫下TaPYR1转化株系干质量。WT, 野生型; Sen 1和Sen 2, 正义表达TaPYR1株系; Anti 1和Anti 2, 反义表达TaPYR1株系。误差线表示标准差, 符号*表示相对于WT的差异显著(P < 0.05)。
Fig. 3Growth characteristics of the transgenic plants under drought stress
A: Plant growth characteristics of transgenic lines overexpressing TaPYR1 under drought stress; B: Plant biomass of transgenic lines overexpressing TaPYR1 under drought stress. WT, wild type; Sen 1 and Sen 2, lines overexpressing TaPYR1; Anti 1 and Anti 2, lines with TaPYR1 knockdown expression. Error bars indicate SE and symbol * represents significant difference relative to WT (P < 0.05).
2.4 干旱胁迫下转化株系的光合特性
与前述的植株长势一致, 正常生长条件下, 转化株系和WT的光合参数无明显差异(图4-A~D)。干旱处理下, 与WT相比, 超表达TaPYR1烟草株系(Sen 1和Sen 2)叶片光合速率(Pn)和光系统II光化学效率(ФPSII)显著增高, 胞间CO2浓度(Ci)叶绿体光化学淬灭系数(NPQ)降低; 而反义表达TaPYR1烟草株系(Anti 1和Anti 2) Pn和ФPSII显著降低, Ci和NPQ增大(图4-A~D)。表明TaPYR1具有显著增强干旱胁迫下植株光合作用的能力。图4
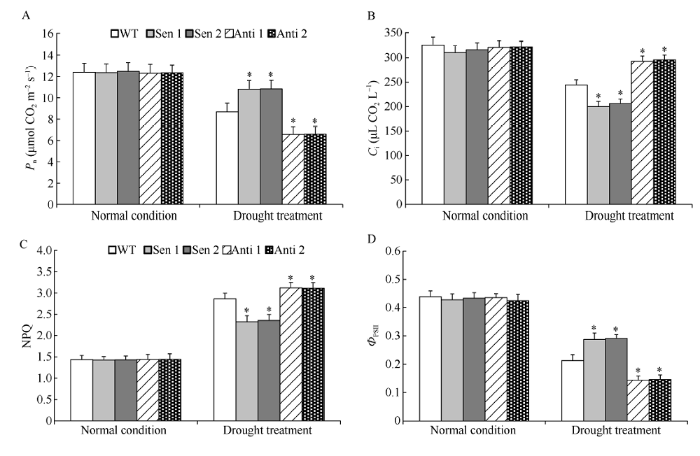 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4干旱胁迫下转化株系的光合特性
A: 光合速率(Pn); B: 胞间CO2浓度(Ci); C: 叶绿体光化学淬灭系数 (NPQ); D: 光系统II光化学效率(ФPSII)。误差线表示标准差, 符号*表示相对于WT的差异显著(P < 0.05)。
Fig. 4Photosynthetic characteristic of transgenic lines under drought stress
A: Photosynthetic rate (Pn); B: Intercellular CO2 concentration (Ci); C: Photochemical quenching absorption of chloroplast (NPQ); D: Photochemical efficiency of photo system II (ФPSII). Error bars indicate SE and symbol * represents significant difference relative to WT (P < 0.05).
2.5 干旱胁迫下转化株系的气孔关闭及持水特征
以干旱处理60 min内不同时间点(处理前0、0.25、0.5和1 h)转化株系(Sen 1和Anti 1)和WT为材料, 对TaPYR1调控气孔开闭特性和叶片持水力进行了研究。随干旱处理进程, 转化株系和WT气孔开度均不断变小。但与WT相比, 正义烟草株系(Sen 1)气孔关闭速率加快, 而反义株系(Anti 1)气孔关闭速率变慢(图5-A)。与气孔关闭速率表现相反, Sen 1在干旱各处理时间点的叶片持水力均高于WT, 而Anti 1则相反, 各处理时间点的叶片持水力均低于WT (图5-B)。上述结果表明, TaPYR1能调控干旱处理下的植株叶片气孔加速关闭, 减少植株水分散失, 一定程度上增强植株持水和抵御干旱逆境能力。图5
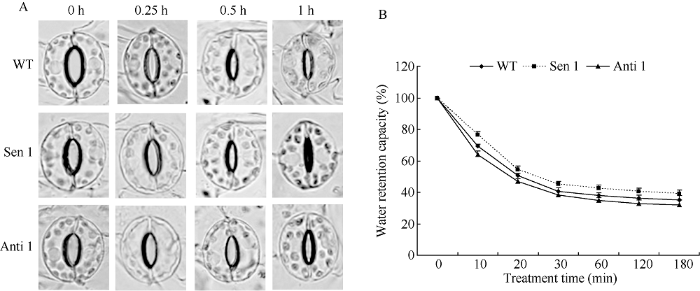 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5干旱胁迫下转化株系的气孔关闭及叶片持水特征
A: 气孔关闭特征; B: 叶片持水力。
Fig. 5Stomatal closure properties and water retention capacities of transgenic lines under drought stress
A: stomatal closure characteristic; B: water retention capacity of leaves.
2.6 干旱胁迫下转化株系的细胞保护酶活性、渗透物质含量及保护酶基因表达模式
干旱处理下, 与WT相比, Sen 1株系SOD、CAT和POD活性增高, MDA含量降低; 与此相反, Anti 1株系上述细胞保护酶活性降低, MDA含量增高(图6-A~D)。此外, 与WT相比, 干旱处理下Sen 1株系可溶性糖与脯氨酸含量增高, Anti 1株系可溶性糖与脯氨酸含量降低(图6-E, F)。表明TaPYR1介导植株抵御干旱逆境能力的增强, 与其改善逆境胁迫下的细胞活性氧稳态和增强渗透物质生化合成代谢有关。图6
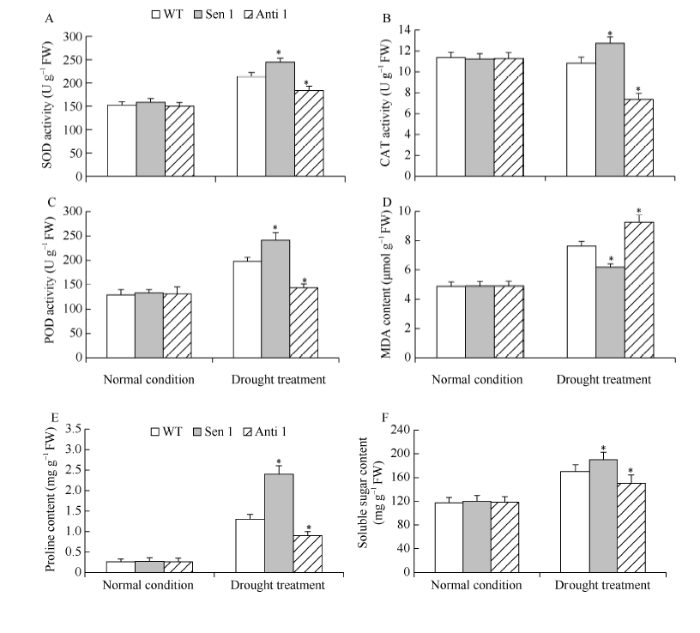 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6干旱胁迫下转化株系的细胞保护酶活性及渗透物质含量
A: SOD活性; B: CAT活性; C: POD活性; D: MDA含量; E: 脯氨酸含量; F: 可溶性糖含量。误差线表示标准差, 符号*表示相对于WT的差异显著(P < 0.05)。
Fig. 6Activities of the cellular protection enzymes and osmolyte contents in transgenic lines under drought stress
A: SOD activities; B: POD activities; C: CAT activities; D: MDA contents; E: proline contents; D: soluble sugar contents. Error bars indicate SE and symbol * represents significant difference relative to WT (P < 0.05).
对干旱胁迫下转化株系和WT编码细胞保护酶家族基因的表达模式研究发现, 与WT相比, Sen 1株系NtSOD2、NtCAT和NtPOD1;3、NtPOD1;4及NtPOD4的表达显著上调; 而Anti 1株系上述基因的表达则显著下调(图7)。上述结果表明, TaPYR1对特定细胞保护酶编码基因进行转录调控, 由此提高植株干旱逆境下的细胞保护酶活性, 改善干旱胁迫下的细胞胞活性氧相对稳态, 增强植株干旱抵御能力。
图7
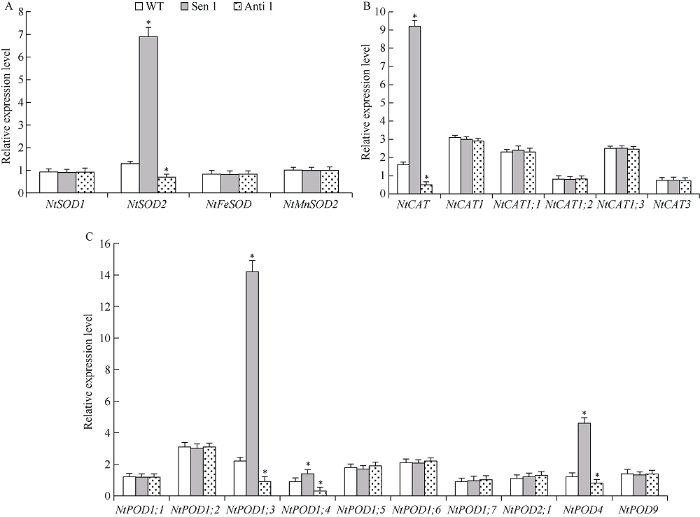 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7干旱胁迫下转化株系的保护酶基因表达模式
A: SOD家族基因; B: CAT家族基因C: POD家族基因。误差线表示标准差, 符号*表示相对于WT的差异显著(P < 0.05)。
Fig. 7Expression patterns of protective enzyme genes in transformed lines under drought stress
A: SOD family genes; B: CAT family genes; C: POD family genes. Error bars indicate SE and symbol * represents significant difference relative to WT (P < 0.05).
3 讨论
ABA信号通路诱发一系列逆境防御相关生理生化过程改变, 在介导植株抵御干旱、盐分等渗透胁迫逆境过程中发挥着重要生物学功能[19,20,21]。其中, ABA受体蛋白通过与逆境诱发的ABA结合, 介导逆境信号传递, 是特定渗透胁迫诱导的ABA信号传递通路重要组分[22]。本研究对小麦种属TaPYR1分子特征研究表明, 该基因编码蛋白与植物种属部分同源蛋白在序列组成上高度相似, 其编码蛋白含有PYR家族蛋白的SRPBCC超家族保守区域(44~192 aa)。此外, 对该基因编码蛋白的亚细胞定位试验结果显示, TaPYR1 蛋白经内质网分选后定位于细胞质膜。上述基因及其编码蛋白特征表明, TaPYR1是小麦种属中ABA受体家族成员, 其编码蛋白通过在胞质膜上结合ABA, 参与植株特定逆境信号的转导过程。部分ABA信号通路组分编码基因, 通过在转录水平上对渗透胁迫逆境产生应答, 对特定逆境信号进行传递[23]。如拟南芥PYR家族成员AtPYL的转录本丰度在干旱和盐分逆境下显著增大, 通过基因转录效率改变, 改善与ABA结合能力, 对参与植株逆境响应的ABA信号通路产生调控[24]。本研究发现, TaPYR1在植株根叶组织内呈典型的干旱诱导表达模式, 该基因在上述组织内的表达随干旱处理进程不断增强, 且其干旱诱导表达随正常生长恢复处理不断减弱。表明该基因转录受到干旱逆境的调节。进一步揭示该基因转录机制, 将深入对小麦等麦类种属ABA信号转导通路及适应干旱逆境分子机理的认识。
气孔调节是植物抵御干旱胁迫和适应环境的重要机制之一。植物通过控制气孔运动特征影响气孔开闭程度, 进而调控叶片蒸腾速率和水分利用效率(WUE)[25]。同时, 气孔开闭程度通过影响光合作用底物CO2向光合羧化位点的传输, 对干旱等渗透胁迫逆境下的光合碳同化能力产生重要影响[26]。本研究对遗传转化TaPYR1烟草株系干旱逆境下植株长势和干物质积累特征研究表明, 正反义表达供试基因的烟草株系, 植株抵御干旱逆境的能力发生明显改变。表现为与对照 (WT) 相比, 正义株系的长势增强, 干质量增大; 反义株系的长势减弱, 干质量降低。对上述株系抵御干旱逆境相关的气孔开闭及光合生理研究表明, TaPYR1对干旱逆境下植株长势和干质量的调控, 与其对气孔运动和光合碳同化能力的调节密切相关。因此, 该小麦ABA受体基因通过对气孔关闭和光合碳同化正向调控, 改善干旱逆境下的植株保水和干物质累积能力。
干旱等非生物逆境下, 植株体内通过增强细胞保护酶如SOD、CAT和POD等生化活性, 增强对逆境诱导增多的活性氧 (超氧阴离子、过氧化氢和羟基自由基等) 清除能力, 减轻细胞的膜质过氧化程度, 在增强植株抵御逆境胁迫过程中发挥着重要生物学功能[27]。本研究表明, 与WT相比, 干旱胁迫下正义株系上述细胞保护酶活性均明显增强, 膜质过氧化产物丙二醛含量降低。与此相反, 干旱处理下反义株系细胞保护酶活性降低, 丙二醛含量增多。表明TaPYR1通过对干旱逆境下细胞活性氧内稳态的调节, 对植株适应和抵御干旱逆境的能力也产生重要影响。本研究通过对编码上述保护酶的基因家族成员表达分析表明, 特定细胞保护酶家族基因包括NtSOD2、NtCAT和NtPOD1;3、NtPOD1;4和NtPOD4转录受到PYR的调控。与WT相比, 干旱逆境下正、反义株系中上述保护酶基因分别呈典型的上、下调表达特征, 表明上述保护酶基因通过转录调节, 影响转化株系干旱处理下的细胞保护功能, 进而影响植株抵御干旱逆境的能力。进一步解析特定应答干旱逆境细胞保护酶编码基因的转录机制及耐逆功能, 将丰富对作物维持逆境下细胞活性氧稳态及抗逆分子机制的认识。
4 结论
小麦ABA受体基因TaPYR1编码蛋白含有PYR/PYL/RCAR家族保守结构域, 翻译蛋白经内质网分选后定位于细胞质膜。TaPYR1呈典型干旱诱导表达模式, 遗传转化该小麦ABA受体基因对植株抵御干旱逆境能力产生重要影响。该基因通过对气孔关闭速率、光合碳同化、细胞保护能力和渗透物质正向调控, 增强植株抵御干旱逆境能力。TaPYR1对细胞保护能力的调节, 与其对细胞保护酶家族基因NtSOD2、NtCAT和NtPOD1;3、NtPOD1;4和NtPOD4的转录调控有关。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.bbrc.2018.08.146URL [本文引用: 1]
DOI:10.3389/fpls.2017.01613URL [本文引用: 1]
DOI:10.1111/jipb.12605URL [本文引用: 1]

Environmental stresses that perturb plant water relations influence abscisic acid (ABA) concentrations, but it is unclear whether long-distance ABA transport contributes to changes in local ABA levels. To determine the physiological relevance of ABA transport, we made reciprocal- and self-grafts of ABA-deficient flacca mutant and wild-type (WT) tomato plants, in which low phosphorus (P) conditions decreased ABA concentrations while salinity increased ABA concentrations. Whereas foliar ABA concentrations in the WT scions were rootstock independent under conditions, salinity resulted in long-distance transport of ABA: flacca scions had approximately twice as much ABA when grafted on WT rootstocks compared to flacca rootstocks. Root ABA concentrations were scion dependent: both WT and flacca rootstocks had less ABA with the flacca mutant scion than with the WT scion under conditions. In WT scions, whereas rootstock genotype had limited effects on stomatal conductance under conditions, a flacca rootstock decreased leaf area of stressed plants, presumably due to attenuated root-to-shoot ABA transport. In flacca scions, a WT rootstock decreased stomatal conductance but increased leaf area of stressed plants, likely due to enhanced root-to-shoot ABA transport. Thus, long-distance ABA transport can affect responses in distal tissues by changing local ABA concentrations.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.molp.2017.08.003URL [本文引用: 1]
DOI:10.1007/s00299-013-1418-1URL [本文引用: 1]

We review the recent progress on ABA signaling, especially ABA signaling for ABA-dependent gene expression, including the AREB/ABF regulon, SnRK2 protein kinase, 2C-type protein phosphatases and ABA receptors.
Drought negatively impacts plant growth and the productivity of crops. Drought causes osmotic stress to organisms, and the osmotic stress causes dehydration in plant cells. Abscisic acid (ABA) is produced under osmotic stress conditions, and it plays an important role in the stress response and tolerance of plants. ABA regulates many genes under osmotic stress conditions. It also regulates gene expression during seed development and germination. The ABA-responsive element (ABRE) is the major cis-element for ABA-responsive gene expression. ABRE-binding protein (AREB)/ABRE-binding factor (ABF) transcription factors (TFs) regulate ABRE-dependent gene expression. Other TFs are also involved in ABA-responsive gene expression. SNF1-related protein kinases 2 are the key regulators of ABA signaling including the AREB/ABF regulon. Recently, ABA receptors and group A 2C-type protein phosphatases were shown to govern the ABA signaling pathway. Moreover, recent studies have suggested that there are interactions between the major ABA signaling pathway and other signaling factors in stress-response and seed development. The control of the expression of ABA signaling factors may improve tolerance to environmental stresses.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1186/1471-2229-10-230URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
.
DOI:10.1007/s11103-019-00862-6URL [本文引用: 1]
DOI:10.1073/pnas.1706593114URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/S2095-3119(17)61879-3URL [本文引用: 1]
