 ,*重庆师范大学生命科学学院, 重庆 401331
,*重庆师范大学生命科学学院, 重庆 401331Cloning and function analysis of a type 2 diacylglycerol acyltransferase (DGAT2) from Perilla frutescens
LU Geng, TANG Xin, LU Jun-Xing, LI Dan, HU Qiu-Yun, HU Tian, ZHANG Tao ,*College of Life Sciences, Chongqing Normal University, Chongqing 401331, China
,*College of Life Sciences, Chongqing Normal University, Chongqing 401331, China通讯作者:
收稿日期:2019-12-11接受日期:2020-03-24网络出版日期:2020-08-12
| 基金资助: |
Received:2019-12-11Accepted:2020-03-24Online:2020-08-12
| Fund supported: |
作者简介 About authors
E-mail:778448973@qq.com。

摘要
关键词:
Abstract
Keywords:
PDF (1860KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
鲁庚, 唐鑫, 陆俊杏, 李丹, 胡秋芸, 胡田, 张涛. 紫苏二酰基甘油酰基转移酶2基因克隆与功能研究[J]. 作物学报, 2020, 46(8): 1283-1290. doi:10.3724/SP.J.1006.2020.94192
LU Geng, TANG Xin, LU Jun-Xing, LI Dan, HU Qiu-Yun, HU Tian, ZHANG Tao.
三酰基甘油(TAG)是植物种子中的主要储存脂质, 是植物油的主要形式。植物油是重要的可再生能源, 可用于食品和饲料, 并且正日益成为生物柴油和工业化学品的原料[1,2]。植物油含较高的不饱和脂肪酸, 可以降低动脉硬化发生的概率。其中, 多不饱和脂肪酸α-亚麻酸是人体组织细胞的主要组成成分, 人体缺乏α-亚麻酸会引起健忘、疲劳、视力减退、动脉硬化等症状[3]。随着对多不饱和脂肪酸需求的增加, 需要开发具有高含油量和高不饱和脂肪酸的油料作物。唇形科一年生草本植物紫苏[Perilla frutescens (L.) Britt.], 因其种子出油率达45%以及含大量多不饱和脂肪酸α-亚麻酸(60%以上)而成为人们重点关注的新型油料作物[4,5]。通过克隆紫苏PfDGAT2基因, 研究其表达模式及功能, 对提高植物含油率、促进不饱和脂肪酸合成积累具有重要经济价值和理论意义。
TAG的生物合成涉及质体中脂肪酸的生物合成和内质网中TAG的组装2个主要阶段。脂肪酸的生物合成主要是乙酰辅酶A羧化酶(ACCase)将乙酰辅酶A活化为丙二酰辅酶A, 然后将丙二酰基从CoA转移到酰基载体蛋白(ACP)作为酰基链载体, 合成的脂肪酸则输出到内质网(ER)[6]。在肯尼迪途径中, 三磷酸甘油酰基转移酶(GPAT)、溶血磷脂酰基转移酶(LPAAT)和二酰基甘油酰基转移酶(DGAT)催化三磷酸甘油骨架链上酰化反应, 最后生成三酰甘油[7,8]。其中DGAT是用于TAG合成的最后一种酶, 它将第3个酰基链添加到DAG并产生TAG。目前, 在植物中鉴定出DGAT1、DGAT2、DGAT3[8,9]和WS/ DGAT[10]4种类型的DGAT。DGAT1和DGAT2在真核生物中普遍存在, 它们是2个结构不同的膜结合酰基转移酶, 没有序列同源性[12]。另外, DGAT1和DGAT2在底物特异性和表达模式等方面也不同[12,13,14]。Saha等[8]在花生子叶中克隆出了第3类可溶性DGAT酶, 命名为DGAT3, 定位于细胞质中。第4种类型的DGAT酶是从醋酸钙不动杆菌ADP1鉴定的双功能膜相关酶, 同时具有WS和DGAT活性[15,16]。在拟南芥和矮牵牛中的WS/DGAT同源物主要是催化蜡酯的合成[16,17]。有研究表明, 拟南芥的二酰基甘油酰基转移酶1 (DGAT1)是油脂中正常TAG积累所必需的[18,19,20]。但是, 拟南芥中的AtDGAT2作用尚不清楚, 因为dgat1突变体与AtDGAT2敲除突变体杂交后种子的含油量并没有明显降低[12]。与拟南芥相比, 蓖麻胚乳中的DGAT2在种子中积累了大量的TAG (含90%以上蓖麻酸)[21]。此外, Shockey等[13]表明, DGAT2可能参与异常脂肪酸、桐油酸和桐油的选择性积累。随后Oelkers[22]表明, 蓖麻DGAT2能与蓖麻羟化酶共同表达, 在拟南芥种子中使羟基脂肪酸积累加倍(从~17%到~30%)。Li等[23]也证明了斑鸠菊、大戟和琉璃菊中的PDAT和DGAT2积累了更高的环氧和羟基脂肪酸。Yuan等[24]通过在拟南芥中过表达油棕EgDGAT2, 提高了拟南芥种子TAG中多不饱和脂肪酸亚油酸和亚麻酸的含量, 硬脂酸和花生酸的比例也相应降低。在最近的研究中, Zheng等[25]在拟南芥中过表达椰子CoDGAT2, 导致拟南芥种子的亚油酸含量显著增加, 二十碳一烯酸和花生酸的比例降低。因此, DGAT2在某些植物种子油中选择性积累不饱和脂肪酸方面所起的作用比DGAT1更为显著[12]。
α-亚麻酸能预防心血管疾病, 也可增强智力、保护视力、抑制衰老和血小板聚集[26,27]。紫苏是目前发现含α-亚麻酸最高的植物, 但迄今为止, 对紫苏PfDGAT2基因的研究非常少, 仅有梁倩等[28]克隆了该基因并进行了生物信息学及表达性分析, 而对PfDGAT2的表达模式及生物学功能等迄今未见报道。本研究克隆了PfDGAT2基因并利用生物信息学方法对其序列进行了分析, 在此基础上, 分析不同组织、种子不同发育时期的表达特性, 构建植物表达载体, 通过农杆菌介导法转化拟南芥, 研究过表达PfDGAT2拟南芥种子的含油率及脂肪酸组分的变化, 可为植物高含量不饱和脂肪酸积累的分子机理提供参考及理论依据。
1 材料与方法
1.1 材料
1.1.1 植物材料 紫苏、拟南芥种子由重庆师范大学油用牡丹种质资源创新与利用重点实验室保存。于2018年4月将紫苏种植于重庆师范大学植物园, 9月采集花、叶、茎、根以及花后5、10、15、20、25、30 d不同发育阶段的种子, 液氮冷冻运输, 然后在-80℃冰箱保存待用。所有拟南芥种子均经2%次氯酸钠表面消毒, 播种在1/2 MS培养基上, 将22℃长日条件下的2周龄植物移栽到土壤(营养土:细土:珍珠岩 = 1.0:3.0:0.5)中。1.1.2 菌株和试剂 大肠杆菌DH5α、Premix Taq酶、DL2000 Marker、T/A克隆载体pMD19-T、逆转录试剂盒均购于TaKaRa公司, 植物RNA快速提取试剂盒购于天根生化有限公司, 荧光定量试剂盒购于成都百乐公司, 根瘤农杆菌GV3101、表达载体pCAMBIA1303由本实验室保存。
1.2 方法
1.2.1 紫苏总RNA提取及cDNA合成 利用植物RNA快速提取试剂盒提取紫苏根、茎、叶、花、不同时期种子(5、10、15、20、25、30 d)的总RNA, 保存于-80℃备用。采用RT Reagent Kit with gDNA Eraser (Perfect Real-Time)试剂盒方法, 以提取的RNA为模板, 反转录为cDNA。1.2.2 PfDGAT2基因的克隆 从实验室前期紫苏转录组测序数据中挑选出功能注释为DGAT2的基因[29], 以拟南芥AtDGAT2为参照, 利用BioEdit软件比对分析, 获得紫苏PfDGAT2全长。采用Primer Premier 5.0软件分别设计5'端及3'端全长特异性引物。反应引物为PfDGAT2 F和PfDGAT2 R (表1)。根据设计的引物以反转录的cDNA为模板进行PCR扩增, 将扩增产物纯化后与载体pMD-T 19连接, 转化大肠杆菌DH5α感受态, 筛选蓝白斑阳性克隆, PCR鉴定挑选单克隆送测序, 引物及测序均由英潍捷基有限公司完成。
Table 1
表1
表1PCR引物
Table 1
| 引物名称 Primer name | 引物序列 Sequences (5'-3') |
|---|---|
| PfDGAT2 F | CTCGCTTACTGCTACTTCAATG |
| PfDGAT2 R | CGACAATTAGAGAATCCTGAGC |
| PfDGAT2-DL F | AGTCCGAGCCCAACGGCGATGTCAG |
| PfDGAT2-DL R | GGATGCCCAACCCTTGCTTTGTGCC |
| Actin F | AGACCTTCAATGTGCCAGCCA |
| Actin R | CACGACCAGCAAGATCCAACC |
| 18S RNAF | CGGCGACGCATCATTCAAA |
| DGAT2-BglII F | ACTCTTGACCATGGTAGATCTGGAGTCCGAGCCCAACGGCG |
| DGAT2-BglII R | GGACGTAAACTAGTCAGATCTAGAATCCTGAGCTCTAAGTCG |
| JD-F | TTTCATTTGGAGAGAACACGGGGGA |
| JD-R | CGCTGATCAATTCCACAGTTTTCGC |
新窗口打开|下载CSV
1.2.3 生物信息学分析 将得到的PfDGAT2用Vector NTI Advance 11进行序列比对和蛋白质翻译, 利用在线分析软件ProtParam (
1.2.4 PfDGAT2基因组织特异性表达分析 根据已克隆PfDGAT2的cDNA序列分析结果, 设计荧光定量PCR特异引物(表1), 以紫苏β-actin和18S RNA作为内参基因, 参照试剂盒SYBR Premix Ex Taq II在CFX96 Real-Time PCR Detection System仪器(Bio-Rad公司)上进行荧光定量PCR检测, 反应体系为10 μL, 包含SYBR Green supermix 5.0 μL、上下游引物各0.5 μL、Template 4.0 μL; 反应条件为95℃预变性60 s; 95℃变性10 s, 60℃退火20 s, 40个循环; 反应结束后再升温至95℃, 最后进行溶解曲线分析。每组实验设置3次生物学重复和3次技术重复。
1.2.5 植物表达载体构建及转化 用限制性内切酶Bgl II酶切植物表达载体pCAMBIA1303, 设计带有Bgl II酶切位点的1对引物DGAT2-BglII F和DGAT2-BglII R (表1), 采用In-Fusion无缝克隆得到过表达重组质粒 WT: Pro35SPfDGAT2, 将重组质粒转化到农杆菌中, 利用农杆菌介导的花序侵染法转化野生型拟南芥Col-0。农杆菌侵染的当代植株为T0代, 单株收的每代种子经1/2 MS培养基(含30 mg mL-1潮霉素)筛选后移栽至温室生长, 鉴定阳性苗使用分子鉴定, 分子鉴定引物为JD-F、JD-R (表1)。
1.2.6 拟南芥种子含油率、脂肪酸组分的测定 使用Li等[30]的方法, 并做了部分改动, 提取拟南芥种子总油。分别采收PD2-1至PD2-9转基因拟南芥株系的T2代成熟种子, 干燥后使用1/1000天平称取每个株系的种子0.05 g, 重复5~10次。研钵研磨后加入氯仿:甲醇(2:1)混匀, 离心取下层氯仿相至玻璃管, 用氮吹仪浓缩干至恒重后称量, 计算含油率。利用GC-MS (岛津Trace1310-ISQ单四级杆气质联用仪)测定脂肪酸组分, 参照付松等[31]的脂肪酸甲酯化方法, 有所改动。取油脂于10 mL玻璃试管中, 加异辛烷(含0.001% BHT)溶解, 再加氢氧化钾-甲醇溶液, 旋涡震荡2 min后静置10 min, 最后加8% NaCl离心取上清液过油系滤膜待用。GC-MS进样体积1 μL; 程序升温: 起始温度180℃, 保持1 min, 以15℃ min-1升至230℃, 保持6 min; 高纯氮气载气, 流速1.0 mL min-1; 进样口温度250℃; 脉冲分流进样, 分流比35:1, 脉冲压力120 kPa。传输线温度为230℃, 离子源温度230℃, 质量扫描范围(m/z) 40~400 amu。对每个株系总脂提取及脂肪酸甲酯化, 设置3次生物学重复和3次技术重复。
1.2.7 数据统计分析 采用Microsoft Excel统计分析PfDGAT2基因表达量、拟南芥种子含油率及脂肪酸组分等数据并作图。使用2-ΔΔCt法计算基因相对表达量, 利用DPS 7.05软件进行基因表达差异显著性分析、种子含油率和脂肪酸组分的显著性分析。
2 结果与分析
2.1 PfDGAT2克隆及生物信息学分析
以紫苏不同时期种子混样的cDNA为模板,用特异性引物进行PCR扩增, 获得全长为1200 bp的PfDGAT2 (图1-A), 经1%琼脂糖凝胶电泳检测, 结果与预测大小一致。生物信息学分析表明, PfDGAT2基因开放阅读框(open reading frame, ORF)长为990 bp, 编码329个氨基酸, 使用在线分析软件ProtParam分析表明, PfDGAT2基因蛋白质分子量为37.01 kD, 等电点为9.55, 不稳定系数为43.06, 为不稳定蛋白。SignaIP server预测该蛋白无信号肽, TMHMM Server v.2.0分析该蛋白有2个跨膜域。蛋白序列保守结构域分析表明, PfDGAT2属于LPLAT蛋白超家族(图1-B)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1PfDGAT2基因的克隆与分析
A: PfDGAT2的PCR扩增产物, M: DL2000 DNA marker。B: PfDGAT2 保守结构域的预测结果。
Fig. 1Cloning and analysis of the PfDGAT2 gene
A: PCR amplification product of PfDGAT2, M: DL2000 DNA marker. B: conserved domains analysis of PfDGAT2 protein.
2.2 系统进化分析
为了研究紫苏与拟南芥、烟草、玉米等植物间的亲缘关系, 利用MEGA4.0软件对紫苏Perilla frutescens、芝麻Sesamum indicum (XP_011098009.1)、蓖麻Ricinus com munis (NP_001310616.1)、拟南芥Arabidopsis thaliana (OAP06431.1)、大豆Glycine max (NP_001299586.1)、蒺藜苜蓿Medicago truncatula (XP_003612436.1)、麻疯树Jatropha curcas (NP_001292973.1)、可可Theobroma cacao (EOX90582.1)、亚麻荠Camelina sativa (XP_010426724)、烟草Nicotiana tabacum (AGL46984.1)、甘蓝型油菜Brassica napus (XP_013734399.2)、马铃薯Solanum tuberosum (XP_006365015.1)和玉米Zea mays (AQL03437.1)的DGAT2候选蛋白进行系统树分析表明(图2), 紫苏与油菜、蓖麻、可可等双子叶植物的DGAT2聚为一支, 与单子叶植物玉米亲缘关系最远, 其进化基本符合植物进化分类。其中, 紫苏与芝麻SiDGAT2亲缘关系最近, 其次与烟草、马铃薯蛋白亲缘关系较近; 与蓖麻、可可树等其他物种的DGAT2蛋白在进化上的亲缘关系较远, 推测紫苏PfDGAT2基因的功能可能与芝麻SiDGAT2基因相似。多序列比对分析(图3)发现, PfDGAT2编码的蛋白与其他植物DGAT2蛋白相似, 具有YFP、EPHS、GGVQE、VPVFCFG和VVGRPI五个典型的植物DGAT2酶的保守结构域。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2不同植物DGAT2氨基酸序列的系统进化树
分支上的数字表示Bootstrap验证中基于1000次重复该节点的可信度。
Fig. 2Phylogenetic tree of different plants based on amino acid of DGAT2
The number on the branches represents the reliability percent of bootstraps values based on 1000 replications.
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3不同植物DGAT2蛋白序列比对分析
PfDGAT2: 紫苏; SiDGAT2: 芝麻(XP_011098009.1); StDGAT2: 马铃薯(XP_006365015.1); AtDGAT2: 拟南芥(OAP06431.1); GmDGAT2: 大豆(NP_001299586.1); NtDGAT2: 烟草(AGL46984.1); CsDGAT2: 亚麻荠 Camelina sativa(XP_010426724)。黑色方框内依次为DGAT2蛋白的YFP、EPHS、GGVQE、RXGFX(K/R)XAXXXGXX(L/V)VPXXXFG(E/Q)和VVGRPI的保守结构域。
Fig. 3Multiple sequence alignment analysis of DGAT2 in different plants
PfDGAT2: Perilla frutescens; SiDGAT2: Sesamum indicum (XP_011098009.1); StDGAT2: Solanum tuberosum (XP_006365015.1); AtDGAT2: Arabidopsis thaliana (OAP06431.1); GmDGAT2: Glycine max (NP_001299586.1); NtDGAT2: Nicotiana tabacum (AGL46984.1); CsDGAT2: Camelina sativa (XP_010426724). In the black box there are the YFP, EPHS, GGVQE, RXGFX(K/R)XAXXXGXX(L/V) VPXXXFG(E/Q), and VVGRPI conserved domains in turn.
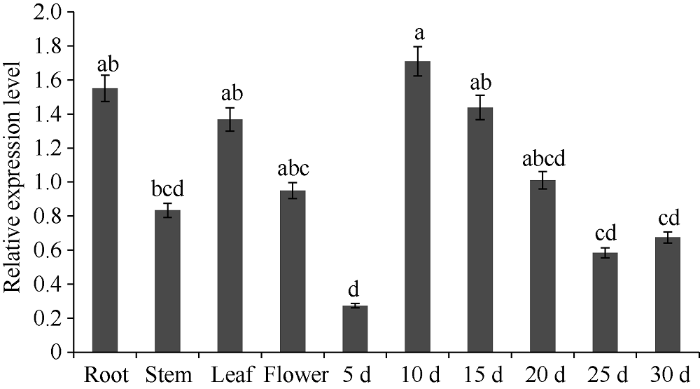
2.3 PfDGAT2基因组织特异性表达分析
实时荧光定量分析表明, PfDGAT2在紫苏不同组织中均有表达, 在发育初期10 d种子中表达量最高, 根中表达量次之, 但与其他组织相比, 5 d种子表达量最低, 且在10 d种子后, 随着种子发育成熟, PfDGAT2表达量呈降低趋势(图4)。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4PfDGAT2基因不同组织和种子不同时期的相对表达量
柱值标以不同字母表示在P < 0.05水平差异显著性。数据点为平均值±标准误(n = 3)。
Fig. 4Analysis of relative expression of PfDGAT2 gene in different tissues and different growth stages of seeds
Bars superscripted by different lowercase letters are significantly different at P < 0.05. Data points are means±SE (n = 3).
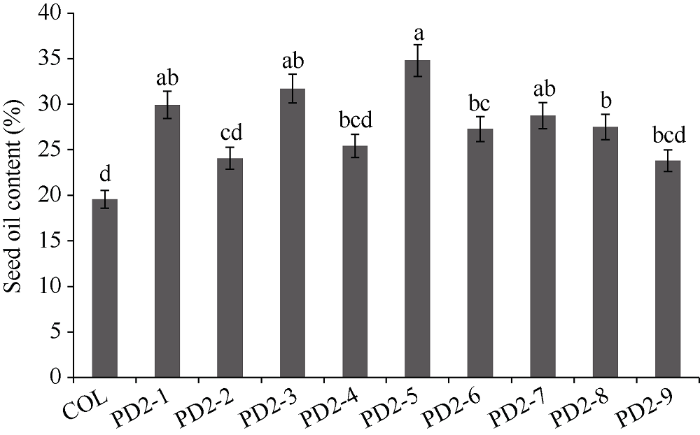
2.4 转基因拟南芥种子含油率、脂肪酸组分分析
经潮霉素筛选并分子鉴定后, 最终得到9个转基因株系, 命名为PD2-1至PD2-9。单株收取9个株系T2代种子测含油率, 选含油率最高的4个株系分析脂肪酸组分。由图5可知, 与野生型拟南芥相比, 转基因种子含油率提高了21.68%~77.89%, 其中PD2-1、PD2-3、PD2-5、PD2-8株系种子含油率增加最多, 分别增加了53.04%、62.10%、77.89%和47.01%。PD2-1、PD2-3、PD2-5、PD2-8四个株系的脂肪酸组分(图6), 与对照相比, 亚麻酸(C18:3)增加了4.57%, 花生一烯酸(C20:1)增加了7.44%, 花生二烯酸(C20:2)提高了5.4%, 而二十二一烯酸(C22:1)含量提高了10.37%。棕榈酸(C16:0)、硬脂酸(C18:0)和亚油酸(C18:2)显著降低, 分别降低了3.47%、6.64%和4.83%。油酸(C18:1)和花生酸(C20:0)呈降低趋势, 但变化不明显, 分别只降低了0.18%和1.91%。这说明, PfDGAT2所编码的DGAT2酶不仅显著提高种子含油率, 还提高亚麻酸等不饱和脂肪酸的含量, 显然, PfDGAT2在TAG组装过程中倾向于以不饱和脂肪酸亚麻酸、花生一烯酸、花生二烯酸和二十二一烯酸作为底物。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5转PfDGAT2拟南芥种子含油率
柱值标以不同字母表示在P < 0.05水平差异显著性。数据点为平均值±标准误(n = 3)。
Fig. 5Seeds oil content of transgenic PfDGAT2 Arabidopsis thaliana
Bars superscripted by different lowercase letters are significantly different at P < 0.05. Data are means±SE (n = 3).
图6
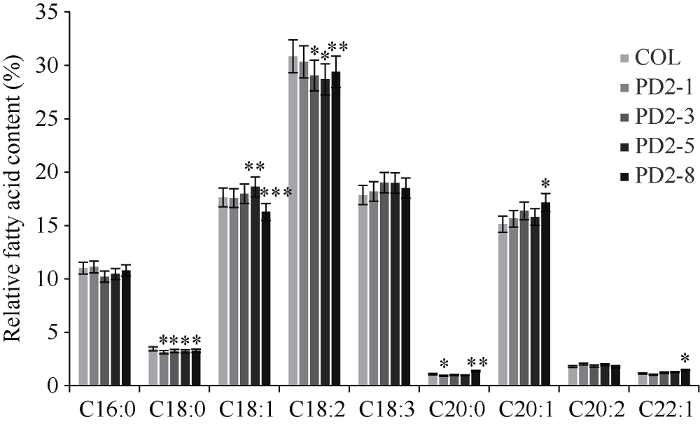 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6转 PfDGAT2 拟南芥种子脂肪酸相对含量分析
图中*为PD2-1、PD2-3、PD2-5、PD2-5与野生型Col-0之间各组分显著性分析。*代表在P < 0.05时显著性的差异, **代表在P < 0.01时显著性的差异, ***代表在P < 0.001时显著性的差异。数据点为平均值±标准误(n = 3)。
Fig. 6Relative fatty acid content in PfDGAT2 transgenic Arabidopsis thaliana seeds
In the figure, * shows significant difference of each component of PD2-1, PD2-3, PD2-5 with wild-type Col-0. * represents the significant difference at P < 0.05, ** represents significant difference at P < 0.01, *** represents significant difference at P < 0.001. Data are means±SE (n = 3).
3 讨论
TAG是植物油最重要的储存形式, 随着对TAG合成研究的不断深入, 作为催化TAG合成积累途径中的最后一步, DGAT2的表达模式及功能受到了极大的关注。本研究在紫苏中克隆得到编码329个氨基酸的PfDGAT2基因, PfDGAT2包含2个预测的跨膜结构域, 此外, 它还具有DGAT2的保守结构域(图3), 其中YFP和EPSH是DGAT2蛋白的关键酶活性位点。Stone等[32]在酿酒酵母中突变DGAT2基因发现, YFP是DGAT2的关键保守结构域, Liu等[33]发现, RXGFX(K/R)XAXXXGXX(L/V)VPXXXFG(E/ Q)是DGAT2同源蛋白中都保守的功能元件。与其他植物的DGAT2蛋白质序列比对表明, PfDGAT2蛋白属于DGAT2家族, 此外, 进化分析也支持这一假设, 因为PfDGAT2与其他植物物种的DGAT2蛋白聚在一起(图2)。PfDGAT2蛋白与芝麻SiDGAT2的亲缘关系最近, 其次与烟草、马铃薯DGAT2蛋白亲缘关系较近。前人研究中, 普遍认为DGAT2在营养组织中的表达和活性高于种子, 郑玲等[34]发现, 烟草不同器官中都表达了花生AhDGAT2a基因, 但在柱头和花药中显示出较强表达。He等[35]对蓖麻RcDGAT2基因表达分析表明, DGAT2在种子中的表达量比在营养组织中的更高, 因此还需从不同植物中分离DGAT2基因并研究其表达模式。前人对紫苏PfDGAT2仅研究了不同品种的种子表达模式, 没有做深入的研究, 本试验从紫苏不同组织以及种子不同发育过程2个阶段做了PfDGAT2的表达模式研究, 表明PfDGAT2在10 d种子中表达量最高, 在15 d种子中表现出较高的表达水平(图4), 验证了紫苏中DGAT2基因在种子的表达和活性高于其他组织。由于种子发育中早期是脂肪酸积累的关键时期, 中后期虽然PfDGAT2的表达量逐渐下调, 但TAG已经储存到了油体蛋白中, 所以转PfDGAT2基因拟南芥成熟种子含油率显著升高, 以上结果说明PfDGAT2在种子脂肪酸积累中发挥主导作用。有研究表明, 过表达DGAT2基因可以增加油的积累和不饱和脂肪酸的合成。Bourgis等[36]和Tranbarger等[37]发现, 过表达油棕EgDGAT2基因使大量的油沉积在中果皮中, 提高了油棕的含油量。本研究在拟南芥中过表达PfDGAT2后发现, 拟南芥种子油增加了21.68%~77.89% (图5), 说明PfDGAT2在种子油积累中起重要作用, 能提高种子含油量。TAG中不饱和脂肪酸的积累首先是将官能团插入酰基链中, 然后将不饱和的脂肪酸组装到TAG。在本实验中, 外源基因PfDGAT2在拟南芥中的过表达导致多不饱和脂肪酸亚麻酸、花生一烯酸、花生二烯酸和二十二一烯酸的增加, 棕榈酸、硬脂酸、油酸、亚油酸和花生酸降低(图6), 显然PfDGAT2对种子中亚麻酸等不饱和脂肪酸积累起着重要作用。Chen等[38]发现, GmDGAT2表现出了对不饱和脂肪酸的底物偏好性, 其中亚油酸的含量增加, 而亚麻酸的含量减少。Zheng等[27]在拟南芥中过表达椰子CoDGAT2, 导致拟南芥的种子亚油酸含量显著增加, 花生一烯酸和花生酸的比例降低, 表现出对亚油酸底物的偏爱。在这些研究中, 与本试验亚麻酸、二十二一烯酸等含量增加, 亚油酸等含量减少不同的是, 过表达大豆、椰子、油棕等植物的DGAT2基因都表现出了亚油酸含量增加, 其他不饱和脂肪酸含量减少, 例如亚麻酸或花生一烯酸减少。这可能是因为这些植物的脂肪酸组分不同, 例如油棕榈中果皮只有10%亚油酸, 不含亚麻酸[39], 而酿酒酵母不含亚油酸或不含亚麻酸[25]。紫苏含丰富的α-亚麻酸, 因此其PfDGAT2表现出对亚麻酸等组分的偏好性, 导致在TAG组装过程中, 有更多的亚麻酸、花生一烯酸、花生二烯酸和二十二一烯酸组装到TAG骨架上, 从而降低了棕榈酸、硬脂酸、油酸、亚油酸和花生酸含量。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1111/j.1365-313X.2008.03430.xURLPMID:18476869 [本文引用: 1]

The seed oils of domesticated oilseed crops are major agricultural commodities that are used primarily for nutritional applications, but in recent years there has been increasing use of these oils for production of biofuels and chemical feedstocks. This is being driven in part by the rapidly rising costs of petroleum, increased concern about the environmental impact of using fossil oil, and the need to develop renewable domestic sources of fuel and industrial raw materials. There is also a need to develop sustainable sources of nutritionally important fatty acids such as those that are typically derived from fish oil. Plant oils can provide renewable sources of high-value fatty acids for both the chemical and health-related industries. The value and application of an oil are determined largely by its fatty acid composition, and while most vegetable oils contain just five basic fatty acid structures, there is a rich diversity of fatty acids present in nature, many of which have potential usage in industry. In this review, we describe several areas where plant oils can have a significant impact on the emerging bioeconomy and the types of fatty acids that are required in these various applications. We also outline the current understanding of the underlying biochemical and molecular mechanisms of seed oil production, and the challenges and potential in translating this knowledge into the rational design and engineering of crop plants to produce high-value oils in plant seeds.
DOI:10.1146/annurev-arplant-043015-111641URLPMID:26845499 [本文引用: 1]

Oils in the form of triacylglycerols are the most abundant energy-dense storage compounds in eukaryotes, and their metabolism plays a key role in cellular energy balance, lipid homeostasis, growth, and maintenance. Plants accumulate oils primarily in seeds and fruits. Plant oils are used for food and feed and, increasingly, as feedstocks for biodiesel and industrial chemicals. Although plant vegetative tissues do not accumulate significant levels of triacylglycerols, they possess a high capacity for their synthesis, storage, and metabolism. The development of plants that accumulate oil in vegetative tissues presents an opportunity for expanded production of triacylglycerols as a renewable and sustainable bioenergy source. Here, we review recent progress in the understanding of triacylglycerol synthesis, turnover, storage, and function in leaves and discuss emerging genetic engineering strategies targeted at enhancing triacylglycerol accumulation in biomass crops. Such plants could potentially be modified to produce oleochemical feedstocks or nutraceuticals.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11746-010-1646-2URL [本文引用: 1]
DOI:10.1111/pbi.12590URLPMID:27307093 [本文引用: 1]

Potato tuber is a high yielding food crop known for its high levels of starch accumulation but only negligible levels of triacylglycerol (TAG). In this study, we evaluated the potential for lipid production in potato tubers by simultaneously introducing three transgenes, including WRINKLED 1 (WRI1), DIACYLGLYCEROL ACYLTRANSFERASE 1 (DGAT1) and OLEOSIN under the transcriptional control of tuber-specific (patatin) and constitutive (CaMV-35S) promoters. This coordinated metabolic engineering approach resulted in over a 100-fold increase in TAG accumulation to levels up to 3.3% of tuber dry weight (DW). Phospholipids and galactolipids were also found to be significantly increased in the potato tuber. The increase of lipids in these transgenic tubers was accompanied by a significant reduction in starch content and an increase in soluble sugars. Microscopic examination revealed that starch granules in the transgenic tubers had more irregular shapes and surface indentations when compared with the relatively smooth surfaces of wild-type starch granules. Ultrastructural examination of lipid droplets showed their close proximity to endoplasmic reticulum and mitochondria, which may indicate a dynamic interaction with these organelles during the processes of lipid biosynthesis and turnover. Increases in lipid levels were also observed in the transgenic potato leaves, likely due to the constitutive expression of DGAT1 and incomplete tuber specificity of the patatin promoter. This study represents an important proof-of-concept demonstration of oil increase in tubers and provides a model system to further study carbon reallocation during development of nonphotosynthetic underground storage organs.
DOI:10.1016/j.biotechadv.2009.07.001URL [本文引用: 1]
DOI:10.1046/j.1365-313x.1999.00555.xURLPMID:10571850 [本文引用: 3]

In Arabidopsis thaliana (ecotype Columbia) mutant line AS11, an EMS-induced mutation at a locus on chromosome II results in a reduced diacylglycerol acyltransferase (DGAT; EC 2.3.1.20) activity, reduced seed triacylglycerol, an altered seed fatty acid composition, and delayed seed development. A mutation has been identified in AS11 in a gene, which we designated as TAG1, that encodes a protein with an amino acid sequence which is similar to a recently reported mammalian DGAT, and, to a lesser extent, to acyl CoA:cholesterol acyltransferases. Molecular analysis revealed that the mutant allele in AS11 has a 147 bp insertion located at the central region of intron 2. At the RNA level, an 81 bp insertion composed entirely of an exon 2 repeat was found in the transcript. While the seed triacylglycerol content is reduced by the lesion in AS11, there is no apparent effect on sterol ester content in the mutant seed. The TAG1 cDNA was over-expressed in yeast, and its activity as a microsomal DGAT confirmed. Therefore, the TAG1 locus encodes a diacylglycerol acyltransferase, and the insertion mutation in the TAG1 gene in mutant AS11 results in its altered lipid phenotype.
DOI:10.1104/pp.106.082198URLPMID:16798944 [本文引用: 1]

Triacylglycerols (TAGs) are the most important storage form of energy for eukaryotic cells. TAG biosynthetic activity was identified in the cytosolic fraction of developing peanut (Arachis hypogaea) cotyledons. This activity was NaF insensitive and acyl-coenzyme A (CoA) dependent. Acyl-CoA:diacylglycerol acyltransferase (DGAT) catalyzes the final step in TAG biosynthesis that acylates diacylglycerol to TAG. Soluble DGAT was identified from immature peanuts and purified by conventional column chromatographic procedures. The enzyme has a molecular mass of 41 +/- 1.0 kD. Based on the partial peptide sequence, a degenerate probe was used to obtain the full-length cDNA. The isolated gene shared less than 10% identity with the previously identified DGAT1 and 2 families, but has 13% identity with the bacterial bifunctional wax ester/DGAT. To differentiate the unrelated families, we designate the peanut gene as AhDGAT. Expression of peanut cDNA in Escherichia coli resulted in the formation of labeled TAG and wax ester from [14C]acetate. The recombinant E. coli showed high levels of DGAT activity but no wax ester synthase activity. TAGs were localized in transformed cells with Nile blue A and oil red O staining. The recombinant and native DGAT was specific for 1,2-diacylglycerol and did not utilize hexadecanol, glycerol-3-phosphate, monoacylglycerol, lysophosphatidic acid, and lysophosphatidylcholine. Oleoyl-CoA was the preferred acyl donor as compared to palmitoyl- and stearoyl-CoAs. These data suggest that the cytosol is one of the sites for TAG biosynthesis in oilseeds. The identified pathway may present opportunities of bioengineering oil-yielding plants for increased oil production.
DOI:10.1016/0163-7827(96)00005-7URLPMID:8944226 [本文引用: 1]
DOI:10.1073/pnas.95.22.13018URLPMID:9789033

Triacylglycerols are quantitatively the most important storage form of energy for eukaryotic cells. Acyl CoA:diacylglycerol acyltransferase (DGAT, EC 2.3.1.20) catalyzes the terminal and only committed step in triacylglycerol synthesis, by using diacylglycerol and fatty acyl CoA as substrates. DGAT plays a fundamental role in the metabolism of cellular diacylglycerol and is important in higher eukaryotes for physiologic processes involving triacylglycerol metabolism such as intestinal fat absorption, lipoprotein assembly, adipose tissue formation, and lactation. DGAT is an integral membrane protein that has never been purified to homogeneity, nor has its gene been cloned. We identified an expressed sequence tag clone that shared regions of similarity with acyl CoA:cholesterol acyltransferase, an enzyme that also uses fatty acyl CoA as a substrate. Expression of a mouse cDNA for this expressed sequence tag in insect cells resulted in high levels of DGAT activity in cell membranes. No other acyltransferase activity was detected when a variety of substrates, including cholesterol, were used as acyl acceptors. The gene was expressed in all tissues examined; during differentiation of NIH 3T3-L1 cells into adipocytes, its expression increased markedly in parallel with increases in DGAT activity. The identification of this cDNA encoding a DGAT will greatly facilitate studies of cellular glycerolipid metabolism and its regulation.
DOI:10.1016/j.plipres.2012.06.001URLPMID:22705711 [本文引用: 4]

Triacylglycerol (TG) is a storage lipid which serves as an energy reservoir and a source of signalling molecules and substrates for membrane biogenesis. TG is essential for many physiological processes and its metabolism is widely conserved in nature. Acyl-CoA:diacylglycerol acyltransferase (DGAT, EC 2.3.1.20) catalyzes the final step in the sn-glycerol-3-phosphate pathway leading to TG. DGAT activity resides mainly in two distinct membrane bound polypeptides, known as DGAT1 and DGAT2 which have been identified in numerous organisms. In addition, a few other enzymes also hold DGAT activity, including the DGAT-related acyl-CoA:monoacylglycerol acyltransferases (MGAT). Progress on understanding structure/function in DGATs has been limited by the lack of detailed three-dimensional structural information due to the hydrophobic properties of theses enzymes and difficulties associated with purification. This review examines several aspects of DGAT and MGAT genes and enzymes, including current knowledge on their gene structure, expression pattern, biochemical properties, membrane topology, functional motifs and subcellular localization. Recent progress in probing structural and functional aspects of DGAT1 and DGAT2, using a combination of molecular and biochemical techniques, is emphasized. Biotechnological applications involving DGAT enzymes ranging from obesity therapeutics to oilseed engineering are also discussed.
DOI:10.1105/tpc.106.043695URLPMID:16920778 [本文引用: 2]

Seeds of the tung tree (Vernicia fordii) produce large quantities of triacylglycerols (TAGs) containing approximately 80% eleostearic acid, an unusual conjugated fatty acid. We present a comparative analysis of the genetic, functional, and cellular properties of tung type 1 and type 2 diacylglycerol acyltransferases (DGAT1 and DGAT2), two unrelated enzymes that catalyze the committed step in TAG biosynthesis. We show that both enzymes are encoded by single genes and that DGAT1 is expressed at similar levels in various organs, whereas DGAT2 is strongly induced in developing seeds at the onset of oil biosynthesis. Expression of DGAT1 and DGAT2 in yeast produced different types and proportions of TAGs containing eleostearic acid, with DGAT2 possessing an enhanced propensity for the synthesis of trieleostearin, the main component of tung oil. Both DGAT1 and DGAT2 are located in distinct, dynamic regions of the endoplasmic reticulum (ER), and surprisingly, these regions do not overlap. Furthermore, although both DGAT1 and DGAT2 contain a similar C-terminal pentapeptide ER retrieval motif, this motif alone is not sufficient for their localization to specific regions of the ER. These data suggest that DGAT1 and DGAT2 have nonredundant functions in plants and that the production of storage oils, including those containing unusual fatty acids, occurs in distinct ER subdomains.
URLPMID:23505340 [本文引用: 1]
DOI:10.1074/jbc.M210533200URLPMID:12502715 [本文引用: 1]

Triacylglycerols (TAGs) and wax esters are neutral lipids with considerable importance for dietetic, technical, cosmetic, and pharmaceutical applications. Acinetobacter calcoaceticus ADP1 accumulates wax esters and TAGs as intracellular storage lipids. We describe here the identification of a bifunctional enzyme from this bacterium exhibiting acyl-CoA:fatty alcohol acyltransferase (wax ester synthase, WS) as well as acyl-CoA:diacylglycerol acyltransferase (DGAT) activity. Experiments with a knock-out mutant demonstrated the key role of the bifunctional WS/DGAT for biosynthesis of both storage lipids in A. calcoaceticus. This novel type of long-chain acyl-CoA acyltransferase is not related to known acyltransferases including the WS from jojoba (Simmondsia chinensis), the DGAT1 or DGAT2 families present in yeast, plants, and animals, and the phospholipid:diacylglycerol acyltransferase catalyzing TAG formation in yeast and plants. A large number of WS/DGAT-related proteins were identified in Mycobacterium and Arabidopsis thaliana indicating an important function of these proteins. WS and DGAT activity was demonstrated for the translational product of one WS/DGAT homologous gene from M. smegmatis mc(2)155. The potential of WS/DGAT to establish novel processes for biotechnological production of jojoba-like wax esters was demonstrated by heterologous expression in recombinant Pseudomonas citronellolis. The potential of WS/DGAT as a selective therapeutic target of mycobacterial infections is discussed.
URLPMID:18621978 [本文引用: 2]
URLPMID:17323080 [本文引用: 1]
DOI:10.1104/pp.108.1.399URLPMID:7784510 [本文引用: 1]

In characterizing the enzymes involved in the formation of very long-chain fatty acids (VLCFAs) in the Brassicaceae, we have generated a series of mutants of Arabidopsis thaliana that have reduced VLCFA content. Here we report the characterization of a seed lipid mutant, AS11, which, in comparison to wild type (WT), has reduced levels of 20:1 and 18:1 and accumulates 18:3 as the major fatty acid in triacylglycerols. Proportions of 18:2 remain similar to WT. Genetic analyses indicate that the fatty acid phenotype is caused by a semidominant mutation in a single nuclear gene, designated TAG1, located on chromosome 2. Biochemical analyses have shown that the AS11 phenotype is not due to a deficiency in the capacity to elongate 18:1 or to an increase in the relative delta 15 or delta 12 desaturase activities. Indeed, the ratio of desaturase/elongase activities measured in vitro is virtually identical in developing WT and AS11 seed homogenates. Rather, the fatty acid phenotype of AS11 is the result of reduced diacylglycerol acyltransferase activity throughout development, such that triacylglycerol biosynthesis is reduced. This leads to a reduction in 20:1 biosynthesis during seed development, leaving more 18:1 available for desaturation. Thus, we have demonstrated that changes to triacylglycerol biosynthesis can result in dramatic changes in fatty acid composition and, in particular, in the accumulation of VLCFAs in seed storage lipids.
DOI:10.1104/pp.126.2.861URLPMID:11402213 [本文引用: 1]

We recently reported the cloning and characterization of an Arabidopsis (ecotype Columbia) diacylglycerol acyltransferase cDNA (Zou et al., 1999) and found that in Arabidopsis mutant line AS11, an ethyl methanesulfonate-induced mutation at a locus on chromosome II designated as Tag1 consists of a 147-bp insertion in the DNA, which results in a repeat of the 81-bp exon 2 in the Tag1 cDNA. This insertion mutation is correlated with an altered seed fatty acid composition, reduced diacylglycerol acyltransferase (DGAT; EC 2.3.1.20) activity, reduced seed triacylglycerol content, and delayed seed development in the AS11 mutant. The effect of the insertion mutation on microsomal acyl-coenzyme A-dependent DGAT is examined with respect to DGAT activity and its substrate specificity in the AS11 mutant relative to wild type. We demonstrate that transformation of mutant AS11 with a single copy of the wild-type Tag1 DGAT cDNA can complement the fatty acid and reduced oil phenotype of mutant AS11. More importantly, we show for the first time that seed-specific over-expression of the DGAT cDNA in wild-type Arabidopsis enhances oil deposition and average seed weight, which are correlated with DGAT transcript levels. The DGAT activity in developing seed of transgenic lines was enhanced by 10% to 70%. Thus, the current study confirms the important role of DGAT in regulating the quantity of seed triacylglycerols and the sink size in developing seeds.
DOI:10.1074/jbc.R111.290072URLPMID:22090025 [本文引用: 1]

Triacylglycerols from plants, familiar to most people as vegetable oils, supply 25% of dietary calories to the developed world and are increasingly a source for renewable biomaterials and fuels. Demand for vegetable oils will double by 2030, which can be met only by increased oil production. Triacylglycerol synthesis is accomplished through the coordinate action of multiple pathways in multiple subcellular compartments. Recent information has revealed an underappreciated complexity in pathways for synthesis and accumulation of this important energy-rich class of molecules.
DOI:10.1111/j.1467-7652.2008.00361.xURLPMID:18643899 [本文引用: 1]

SUMMARY: A central goal of green chemistry is to produce industrially useful fatty acids in oilseed crops. Although genes encoding suitable fatty acid-modifying enzymes are available from many wild species, progress has been limited because the expression of these genes in transgenic plants produces low yields of the desired products. For example, Ricinus communis fatty acid hydroxylase 12 (FAH12) produces a maximum of only 17% hydroxy fatty acids (HFAs) when expressed in Arabidopsis. cDNA clones encoding R. communis enzymes for additional steps in the seed oil biosynthetic pathway were identified. Expression of these cDNAs in FAH12 transgenic plants revealed that the R. communis type-2 acyl-coenzyme A:diacylglycerol acyltransferase (RcDGAT2) could increase HFAs from 17% to nearly 30%. Detailed comparisons of seed neutral lipids from the single- and double-transgenic lines indicated that RcDGAT2 substantially modified the triacylglycerol (TAG) pool, with significant increases in most of the major TAG species observed in native castor bean oil. These data suggest that RcDGAT2 prefers acyl-coenzyme A and diacylglycerol substrates containing HFAs, and biochemical analyses of RcDGAT2 expressed in yeast cells confirmed a strong preference for HFA-containing diacylglycerol substrates. Our results demonstrate that pathway engineering approaches can be used successfully to increase the yields of industrial feedstocks in plants, and that members of the DGAT2 gene family probably play a key role in this process.
DOI:10.1074/jbc.C000144200URLPMID:10747858 [本文引用: 1]

The terminal step in triglyceride biosynthesis is the esterification of diacylglycerol. To study this reaction in the model eukaryote, Saccharomyces cerevisiae, we investigated five candidate genes with sequence conservation to mammalian acyltransferases. Four of these genes are similar to the recently identified acyl-CoA diacylglycerol acyltransferase and, when deleted, resulted in little or no decrease in triglyceride synthesis as measured by incorporation of radiolabeled oleate or glycerol. By contrast, deletion of LRO1, a homolog of human lecithin cholesterol acyltransferase, resulted in a dramatic reduction in triglyceride synthesis, whereas overexpression of LRO1 yielded a significant increase in triglyceride production. In vitro microsomal assays determined that Lro1 mediated the esterification of diacylglycerol using phosphatidylcholine as the acyl donor. The residual triglyceride biosynthesis that persists in the LRO1 deletion strain is mainly acyl-CoA-dependent and mediated by a gene that is structurally distinct from the previously identified mammalian diacylglycerol acyltransferase. These mechanisms may also exist in mammalian cells.
DOI:10.1007/s11745-010-3385-4URLPMID:20101470 [本文引用: 1]

Triacylglycerol (TAG) is the main storage lipid in plants. Acyl-CoA: diacylglycerol acyltransferase (DGAT1 and DGAT2) and phospholipid: diacylglycerol acyltransferase (PDAT) can catalyze TAG synthesis. It is unclear how these three independent genes are regulated in developing seeds, and particularly if they have specific functions in the high accumulation of unusual fatty acids in seed oil. The expression patterns of DGAT1, DGAT2 and a PDAT in relation to the accumulation of oil and epoxy and hydroxy fatty acids in developing seeds of the plant species Vernonia galamensis, Euphorbia lagascae, Stokesia laevis and castor that accumulate high levels of these fatty acids in comparison with soybean and Arabidopsis were investigated. The expression patterns of DGAT1, DGAT2 and the PDAT are consistent with all three enzymes playing a role in the high epoxy or hydroxy fatty acid accumulation in developing seeds of these plants. PDAT and DGAT2 transcript levels are present at much higher levels in developing seeds of epoxy and hydroxy fatty acid accumulating plants than in soybeans or Arabidopsis. Moreover, PDAT, DGAT1 and DGAT2 are found to be expressed in many different plant tissues, suggesting that these enzymes may have other roles in addition to seed oil accumulation. DGAT1 appears to be a major enzyme for seed oil accumulation at least in Arabidopsis and soybeans. For the epoxy and hydroxy fatty acid accumulating plants, DGAT2 and PDAT also show expression patterns consistent with a role in the selective accumulation of these unusual fatty acids in seed oil.
DOI:10.3389/fpls.2017.01791URLPMID:29089956 [本文引用: 1]

Oil palm (Elaeis guineensis Jacq.) is the highest oil-yielding plant in the world, storing 90 and 60% (dry weight) oil in its mesocarp and kernel, respectively. To gain insights into the oil accumulation mechanism, one of the key enzymes involved in triacylglycerol (TAG) biosynthesis, a Type 2 diacylglycerol acyltransferase (DGAT2) from oil palm, was characterized for its in vivo activity. EgDGAT2 is highly expressed in mesocarp during the last two developmental stages while large amounts of oil are accumulated at the highest rate during ripening. Heterologous expression of EgDGAT2 in mutant yeast H1246 restored TAG biosynthesis with substrate preference toward unsaturated fatty acids (FAs) (16:1 and 18:1). Furthermore, seed-specific overexpression of EgDGAT2 in Arabidopsis thaliana enhanced the content of polyunsaturated FAs 18:2 and 18:3 (each by 6 mol%) in seed TAGs, when compared to that from wild-type Arabidopsis. In turn, the proportion of 18:0 and 20:0 FAs in seed TAGs from EgDGAT2 transgenic lines decreased accordingly. These results provide new insights into understanding the in vivo activity of EgDGAT2 from oil palm mesocarp, which will be of importance for metabolic enhancement of unsaturated FAs production.
DOI:10.1016/j.gene.2019.03.060URLPMID:30928362 [本文引用: 2]

Coconut (Cocos nucifera L.) is one of the most characteristic plants of tropical areas. Coconut oil and its derivatives have been widely used in various industries. In this paper, a type 2 diacylglycerol acyltransferase (DGAT2), which is one of the key enzymes involved in triacylglycerol (TAG) biosynthesis, was first characterized in coconut pulp (endosperm). The results indicated that CoDGAT2 was highly expressed in coconut pulp approximately 7months after pollination. The heterologous expression of CoDGAT2 in the mutant yeast H1246 restored TAG biosynthesis in the yeast, which exhibited substrate preference for two unsaturated fatty acids (UFAs), palmitoleic acid (C16:1) and oleic acid (C18:1). Moreover, the seed-specific overexpression of CoDGAT2 in Arabidopsis thaliana led to a significant increase in the linoleic acid (C18:2) content (approximately 6%) compared with that in the wild type. In contrast, the proportions of eicosadienoic acid (C20:1) and arachidic acid (C20:0) were decreased. These results offer new insights on the function of CoDGAT2 in coconut and provide a novel molecular target for lipid genetic modification to change the fatty acid (FA) composition of oils.
DOI:10.3177/jnsv.45.759URLPMID:10737229 [本文引用: 1]

Although important roles of dietary n-3 fatty acids in the prevention of coronary heart disease (CHD) have been suggested, long-term effects of dietary alpha-linolenic acid (ALA, 18:3n-3) have not yet been established under controlled conditions. We tested whether a moderate increase of dietary ALA affects fatty acids composition in serum and the risk factors of CHD. Oxidized LDL (OxLDL) was directly measured by ELISA using antibody specific to OxLDL. By merely replacing soybean cooking oil (SO) with perilla oil (PO) (i.e., increasing 3 g/d of ALA), the n-6/n-3 ratio in the diet was changed from 4:1 to 1:1. Twenty Japanese elderly subjects were initially given a SO diet for at least 6 mo (baseline period), a PO diet for 10 mo (intervention period), and then returned to the previous SO diet (washout period). ALA in the total serum lipid increased from 0.8 to 1.6% after 3 mo on the PO diet, but EPA and DHA increased in a later time, at 10 mo after the PO diet, from 2.5 to 3.6% and 5.3 to 6.4%, respectively (p<0.05), and then returned to baseline in the washout period. In spite of increases of serum n-3 fatty acids, the OxLDL concentration did not change significantly when given the PO diet. Body weight, total serum cholesterol, triacylglycerol, glucose, insulin and HbA1c concentrations, platelet count and aggregation function, prothrombin time, partial thromboplastin time, fibrinogen and PAI-1 concentration, and other routine blood analysis did not change significantly when given the PO diet. These data indicate that, even in elderly subjects, a 3 g/d increase of dietary ALA could increase serum EPA and DHA in 10 mo without any major adverse effects.
DOI:10.1007/s11883-002-0045-zURLPMID:12361488 [本文引用: 2]

The intake of saturated fat seems to be the main environmental factor for coronary heart disease (CHD). However, decreasing the intake of saturated fat and replacing it in part with linoleic acid in primary or secondary intervention trials did not satisfactorily reduce CHD clinical manifestations. It is only when omega-3 fatty acids, alpha-linolenic acid (ALA), or eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were added to the diet that sudden cardiac death (ALA, EPA plus DHA) and nonfatal myocardial infarction (only ALA) were significantly lowered. The protective effect of omega-3 fatty acids occurs rapidly, within weeks. The mechanism for preventing ventricular fibrillation seems to be through a direct effect on myocytes. The additional effect of ALA on nonfatal myocardial infarction may be through thrombosis, at least partly caused by an effect on platelets.
[本文引用: 1]
[本文引用: 1]
DOI:10.1186/s12864-018-4595-zURLPMID:29562889 [本文引用: 1]

BACKGROUND: Perilla frutescens is well known for its high alpha-linolenic acid (ALA) accumulation in seeds and medicinal values as well as a source of edible and general-purpose oils. However, the regulatory mechanisms of the biosynthesis of fatty acid in its seeds remain poorly understood due to the lacking of sequenced genome. For better understanding the regulation of lipid metabolism and further increase its oil content or modify oil composition, time-course transcriptome and lipid composition analyses were performed. RESULTS: Analysis of fatty acid content and composition showed that the alpha-linolenic acid and oleic acid accumulated rapidly from 5 DAF to 15 DAF and then kept relatively stable. However, the amount of palmitic acid and linoleic acid decreased quickly from 5 DAF to 15DAF. No significant variation of stearic acid content was observed from 5 DAF to 25DAF. Our transcriptome data analyses revealed that 110,176 unigenes were generated from six seed libraries at 5, 10, 20 DAF. Of these, 53 (31 up, 22 down) and 653 (259 up, 394 down) genes showed temporal and differentially expression during the seed development in 5 DAF vs 10 DAF, 20 vs 10 DAF, respectively. The differentially expressed genes were annotated and found to be involved in distinct functional categories and metabolic pathways. Deep mining of transcriptome data led to the identification of key genes involved in fatty acid and triacylglycerol biosynthesis and metabolism. Thirty seven members of transcription factor family AP2, B3 and NFYB putatively involved in oil synthesis and deposition were differentially expressed during seed development. The results of qRT-PCR for selected genes showed a strong positive correlation with the expression abundance measured in RNA-seq analysis. CONCLUSIONS: The present study provides valuable genomic resources for characterizing Perilla seed gene expression at the transcriptional level and will extend our understanding of the complex molecular and cellular events of oil biosynthesis and accumulation in oilseed crops.
DOI:10.1016/j.foodchem.2014.10.017URLPMID:25466004 [本文引用: 1]

Seeds from Paeonia ostii and Paeoniarockii have been recently identified as novel resources of alpha-linolenic acid (ALA) in China. To assess whether tree peony cultivars can be used as oil resource, fatty acids (FAs) in 60 cultivars were monitored and evaluated in this study. The results indicated that the composition and content of FAs varied dramatically among different cultivars, in which ALA, linoleic acid, oleic acid, palmitic acid, and stearic acid were the dominant. The 60 cultivars were classified into six clusters by hierarchical cluster analysis, and they were quite distinct from each other. Finally, six cultivars with high yield and high quality were screened out, comprising of 'Liuliguanzhu', 'Hongguanyupei', 'LSS-2', 'LSS-1', 'Jingshenhuanfa' and 'LSS-11'. These cultivars were appropriately applied in practical oil production. Overall, tree peony oil with abundant unsaturated fatty acids especially ALA was proved to be a top-grade source for edible oil and nutritional supplements.
[本文引用: 1]
[本文引用: 1]
DOI:10.1074/jbc.M607986200URLPMID:17035227 [本文引用: 1]

Triacylglycerols are the predominant molecules of energy storage in eukaryotes. However, excessive accumulation of triacylglycerols in adipose tissue leads to obesity and, in nonadipose tissues, is associated with tissue dysfunction. Hence, it is of great importance to have a better understanding of the molecular mechanisms of triacylglycerol synthesis. The final step in triacylglycerol synthesis is catalyzed by the acyl-CoA:diacylglycerol acyltransferase (DGAT) enzymes, DGAT1 and DGAT2. Although recent studies have shed light on metabolic functions of these enzymes, little is known about the molecular aspects of their structures or functions. Here we report the topology for murine DGAT2 and the identification of key amino acids that likely contribute to enzymatic function. Our data indicate that DGAT2 is an integral membrane protein with both the N and C termini oriented toward the cytosol. A long hydrophobic region spanning amino acids 66-115 likely comprises two transmembrane domains or, alternatively, a single domain that is embedded in the membrane bilayer. The bulk of the protein lies distal to the transmembrane domains. This region shares the highest degree of homology with other enzymes of the DGAT2 family and contains a sequence HPHG that is conserved in all family members. Mutagenesis of this sequence in DGAT2 demonstrated that it is required for full enzymatic function. Additionally, a neutral lipid-binding domain that is located in the putative first transmembrane domain was also required for full enzymatic function. Our findings provide the first insights into the topography and molecular aspects of DGAT2 and related enzymes.
DOI:10.1074/jbc.M110.204412URLPMID:21321129 [本文引用: 1]

Acyl-CoA:diacylglycerol acyltransferase (EC 2.3.1.20) is a membrane protein present mainly in the endoplasmic reticulum. It catalyzes the final and committed step in the biosynthesis of triacylglycerol, which is the principal repository of fatty acids for energy utilization and membrane formation. Two distinct family members of acyl-CoA:diacylglycerol acyltransferase, known as DGAT1 and DGAT2, have been characterized in different organisms, including mammals, fungi, and plants. In this study, we characterized the functional role and topological orientation of signature motifs in yeast (Saccharomyces cerevisiae) DGAT2 using mutagenesis in conjunction with chemical modification. Our data provide evidence that both the N and C termini are oriented toward the cytosol and have different catalytic roles. A highly conserved motif, (129)YFP(131), and a hydrophilic segment exclusive to yeast DGAT2 reside in a long endoplasmic reticulum luminal loop following the first transmembrane domain and play an essential role in enzyme catalysis. In addition, the strongly conserved His(195) within the motif HPHG, which may play a role in the active site of DGAT2, is likely embedded in the membrane. These results indicate some similarities to the topology model of murine DGAT2 but also reveal striking differences suggesting that the topological organization of DGAT2 is not ubiquitously conserved.
DOI:10.3724/SP.J.1006.2016.01094URL [本文引用: 1]

二酰甘油酰基转移酶(DGAT)是三酰甘油(TAG)合成途径的限速酶,对脂肪酸合成的调节具有关键作用。为了研究AhDGAT2a的表达调控,利用GenomeWalking方法从鲁花14基因组中克隆了AhDGAT2a上游5¢侧翼调控区1200 bp序列,即AhDGAT2a启动子(pAhDGAT2a)序列,并利用生物信息学软件分析其包含的调控元件,发现其含有多个TATA-box和CAAT-box、光调控元件、胁迫防御相关元件和激素响应元件。用pAhDGAT2a构建pAhDGAT2a:GUS植物表达载体并转化烟草品种SR1。利用组织染色法鉴定转基因烟草的GUS表达模式,发现在转基因烟草的各个器官均有GUS酶活,而在柱头、花药和幼嫩种子中表达量较高,说明pAhDGAT2a具有一定的组成型启动子活性。
DOI:10.3724/SP.J.1006.2016.01094URL [本文引用: 1]

二酰甘油酰基转移酶(DGAT)是三酰甘油(TAG)合成途径的限速酶,对脂肪酸合成的调节具有关键作用。为了研究AhDGAT2a的表达调控,利用GenomeWalking方法从鲁花14基因组中克隆了AhDGAT2a上游5¢侧翼调控区1200 bp序列,即AhDGAT2a启动子(pAhDGAT2a)序列,并利用生物信息学软件分析其包含的调控元件,发现其含有多个TATA-box和CAAT-box、光调控元件、胁迫防御相关元件和激素响应元件。用pAhDGAT2a构建pAhDGAT2a:GUS植物表达载体并转化烟草品种SR1。利用组织染色法鉴定转基因烟草的GUS表达模式,发现在转基因烟草的各个器官均有GUS酶活,而在柱头、花药和幼嫩种子中表达量较高,说明pAhDGAT2a具有一定的组成型启动子活性。
DOI:10.1007/s11745-004-1234-2URLPMID:15357018 [本文引用: 1]

The oil from castor seed (Ricinus communis) contains 90% ricinoleate, a hydroxy FA that is used in producing numerous industrial products. Castor diacylglycerol acyltransferase (RcDGAT) is a critical enzyme, as it catalyzes the terminal step in castor oil biosynthesis in which the products contain two or three ricinoleoyl moieties. We have isolated a cDNA encoding RcDGAT from developing castor seeds. Analysis of the sequence reveals that this cDNA encodes a protein of 521 amino acids with a molecular mass of 59.9 kDa. Although there are regions of high similarity to other plant DGAT coding sequences, there are sequences that distinguish it as well. Southern blot analysis suggests that the castor genome contains a single copy of RcDGAT. Analysis by reverse transcription-PCR reveals that the accumulation of the mRNA reaches its highest level at 19 d after pollination and declines thereafter. Expression of the full-length cDNA for RcDGAT in the yeast Saccharomyces cerevisiae, strain INVSc1 results in sevenfold higher DGAT activity compared with controls. When different molecular species of DAG were provided as substrates to the microsomal mixture, the RcDGAT showed a greater preference to catalyze the transfer of oleate from [14C]oleoyl-CoA to diricinolein than to diolein and dipalmitolein. With the addition of 0.25 mM substrates, diricinolein gave 318 pmol/mg/min diricinoleoyloleoylglycerol (RRO), while diolein and dipalmitolein gave only about 195 pmol/mg/min of triolein (OOO) and 120 pmol/mg/min dipalmitoyleoylglycerol (PoPoO), respectively. This work will facilitate investigation of the role of RcDGAT in castor oil biosynthesis.
DOI:10.1073/pnas.1106502108URLPMID:21709233 [本文引用: 1]

Oil palm can accumulate up to 90% oil in its mesocarp, the highest level observed in the plant kingdom. In contrast, the closely related date palm accumulates almost exclusively sugars. To gain insight into the mechanisms that lead to such an extreme difference in carbon partitioning, the transcriptome and metabolite content of oil palm and date palm were compared during mesocarp development. Compared with date palm, the high oil content in oil palm was associated with much higher transcript levels for all fatty acid synthesis enzymes, specific plastid transporters, and key enzymes of plastidial carbon metabolism, including phosphofructokinase, pyruvate kinase, and pyruvate dehydrogenase. Transcripts representing an ortholog of the WRI1 transcription factor were 57-fold higher in oil palm relative to date palm and displayed a temporal pattern similar to its target genes. Unexpectedly, despite more than a 100-fold difference in flux to lipids, most enzymes of triacylglycerol assembly were expressed at similar levels in oil palm and date palm. Similarly, transcript levels for all but one cytosolic enzyme of glycolysis were comparable in both species. Together, these data point to synthesis of fatty acids and supply of pyruvate in the plastid, rather than acyl assembly into triacylglycerol, as a major control over the storage of oil in the mesocarp of oil palm. In addition to greatly increasing molecular resources devoted to oil palm and date palm, the combination of temporal and comparative studies illustrates how deep sequencing can provide insights into gene expression patterns of two species that lack genome sequence information.
DOI:10.1104/pp.111.175141URLPMID:21487046 [本文引用: 1]

Fruit provide essential nutrients and vitamins for the human diet. Not only is the lipid-rich fleshy mesocarp tissue of the oil palm (Elaeis guineensis) fruit the main source of edible oil for the world, but it is also the richest dietary source of provitamin A. This study examines the transcriptional basis of these two outstanding metabolic characters in the oil palm mesocarp. Morphological, cellular, biochemical, and hormonal features defined key phases of mesocarp development. A 454 pyrosequencing-derived transcriptome was then assembled for the developmental phases preceding and during maturation and ripening, when high rates of lipid and carotenoid biosynthesis occur. A total of 2,629 contigs with differential representation revealed coordination of metabolic and regulatory components. Further analysis focused on the fatty acid and triacylglycerol assembly pathways and during carotenogenesis. Notably, a contig similar to the Arabidopsis (Arabidopsis thaliana) seed oil transcription factor WRINKLED1 was identified with a transcript profile coordinated with those of several fatty acid biosynthetic genes and the high rates of lipid accumulation, suggesting some common regulatory features between seeds and fruits. We also focused on transcriptional regulatory networks of the fruit, in particular those related to ethylene transcriptional and GLOBOSA/PISTILLATA-like proteins in the mesocarp and a central role for ethylene-coordinated transcriptional regulation of type VII ethylene response factors during ripening. Our results suggest that divergence has occurred in the regulatory components in thismonocot fruit compared with those identified in the dicot tomato (Solanum lycopersicum) fleshy fruit model.
DOI:10.1038/srep28541URLPMID:27345221 [本文引用: 1]

Diacylglycerol acyltransferases (DGATs) play a key role in plant triacylglycerol (TAG) biosynthesis. Two type 1 and 2 DGATs from soybean were characterized for their functions in TAG biosynthesis and physiological roles. GmDGAT1A is highly expressed in seeds while GmDGAT2D is mainly expressed in flower tissues. They showed different expression patterns in response to biotic and abiotic stresses. GmDGAT2D was up-regulated by cold and heat stress and ABA signaling, and repressed by insect biting and jasmonate, whereas GmDGAT1A show fewer responses. Both GmDGAT1A and GmDGAT2D were localized to the endoplasmic reticulum and complemented the TAG deficiency of a yeast mutant H1246. GmDGAT2D-transgenic hairy roots synthesized more 18:2- or 18:1-TAG, whereas GmDGAT1A prefers to use 18:3-acyl CoA for TAG synthesis. Overexpression of both GmDGATs in Arabidopsis seeds enhanced the TAG production; GmDGAT2D promoted 18:2-TAG in wild-type but enhanced 18:1-TAG production in rod1 mutant seeds, with a decreased 18:3-TAG. However, GmDGAT1A enhanced 18:3-TAG and reduced 20:1-TAG contents. The different substrate preferences of two DGATs may confer diverse fatty acid profiles in soybean oils. While GmDGAT1A may play a role in usual seed TAG production and GmDGAT2D is also involved in usual TAG biosynthesis in other tissues in responses to environmental and hormonal cues.
DOI:10.1016/s0163-7827(00)00015-1URLPMID:11106812 [本文引用: 1]
