 ,*云南省农业科学院茶叶研究所 / 云南省茶学重点实验室, 云南勐海 666201
,*云南省农业科学院茶叶研究所 / 云南省茶学重点实验室, 云南勐海 666201Biological characteristics and cytological studies on anther abortion of male sterile Camellia crassocolumna
JIANG Hui-Bing, YANG Sheng-Mei, LIU Yu-Fei, TIAN Yi-Ping, SUN Yun-Nan, CHEN Lin-Bo, TANG Yi-Chun ,*Tea Research Institute, Yunnan Academy of Agricultural Sciences / Yunnan Provincial Key Laboratory of Tea Science, Menghai 666201, Yunnan, China
,*Tea Research Institute, Yunnan Academy of Agricultural Sciences / Yunnan Provincial Key Laboratory of Tea Science, Menghai 666201, Yunnan, China通讯作者:
收稿日期:2019-10-24接受日期:2020-03-24网络出版日期:2020-07-12
| 基金资助: |
Received:2019-10-24Accepted:2020-03-24Online:2020-07-12
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (16191KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
蒋会兵, 杨盛美, 刘玉飞, 田易萍, 孙云南, 陈林波, 唐一春. 厚轴茶雄性不育株花药败育的生物学特性和细胞学研究[J]. 作物学报, 2020, 46(7): 1076-1086. doi:10.3724/SP.J.1006.2020.94159
JIANG Hui-Bing, YANG Sheng-Mei, LIU Yu-Fei, TIAN Yi-Ping, SUN Yun-Nan, CHEN Lin-Bo, TANG Yi-Chun.
野生茶树为山茶属茶组(Section Thea)植物, 是栽培茶树的野生近缘种, 可制作茶叶直接饮用, 或用于茶树杂交育种。野生茶树具有独特的适应性[1]和遗传基因多样性[2,3], 利用其蕴涵的丰富基因资源创造育种新材料、研制茶叶新产品将成为国内外茶学科技发展的新领域。2014年, 课题组从国家种质勐海茶树分圃保存的野生茶树厚轴茶(Camellia crassocolumna H. T. Chang)群体中首次发现天然雄性不育种质, 这为开展茶树不育性育种和杂种优势利用提供了重要的材料基础, 同时对深入研究茶树的繁衍进化和遗传变异等理论问题具有重要意义。
植物雄性不育的研究一直是植物杂种优势利用和生殖发育研究的热点内容之一。国内外研究者从植物雄性不育的形态学、遗传学、细胞学、生理生化特性和分子生物学等领域进行了大量的研究工作, 取得了一系列新进展。我国已在水稻、拟南芥等多种作物上开展了雄性不育基因定位及功能分析研究, 克隆了一些与雄性不育有关基因, 阐明了这些基因在减数分裂[4]、绒毡层形成[5]、小孢子营养供应[6]和花粉外壁发育[7]等方面的作用, 提高了人们对雄性不育机理的认识。不同的植物雄性不育材料, 其败育的时期、方式和原因不尽相同。植物花药败育的细胞生物学研究是联系分子水平与个体水平研究的中间环节, 是全面了解雄性不育花药和花粉败育的基础[8]。目前已在禾本科[9]、茄科[10]和十字花科[11]等多种作物上开展了花药结构与功能的细胞学研究, 阐明了这些植物雄性不育发生败育的时期和细胞学特征, 探索了植物雄性不育花药的败育过程。
茶树生殖发育体系的研究已从外部形态发展到分子水平, 茶树花发育相关基因表达研究也取得了进展[12,13]。然而, 有关茶树花发育的细胞形态学研究仍较缺乏, 江昌俊等[14]和施雁飞等[15]分别对茶树胚胎和花芽的形成与发育过程进行了解剖观察, 但都未涉及到雄性不育现象。茶树雄性不育机制的基础研究, 国内外文献还未见报道。本研究从形态学和细胞学方面比较观察不同遗传背景下的厚轴茶雄性不育株花器发育进程和不育类型, 拟阐明厚轴茶雄性不育株花药和花粉发生败育的时期及其细胞学特点, 完善茶树生殖生物学研究, 为深入研究茶树雄性不育机理和杂种优势利用奠定基础。
1 材料与方法
1.1 试验材料
以国家种质勐海茶树分圃保存的厚轴茶(C. crassocolumna)雄性不育株(圃编号为M350)和可育株(圃编号为M352)为试验材料。该厚轴茶种群为1984年征集入圃, 有性繁殖, 种植行距1.5 m、株距0.33 m, 树龄30余年, 常规管理, 观测期间不修剪。试验地位于100°25'E, 21°55'N, 海拔高度1150 m, 属亚热带西南季风气候。年平均温度18.1℃, 活动积温6600℃, 年平均降水量1400 mm, 年平均相对湿度82%。1.2 取材与固定
2018年, 从厚轴茶现蕾至花朵盛开, 上午9:00-10:00时, 随机采集雄性不育株和可育株不同发育时期花蕾。根据花蕾形态特征, 以花蕾横径长度为标准, 结合花药压片镜检法确定不同发育时期, 将其分为以下6级。(1) 1级花蕾(横径≥7.00 mm), 花粉母细胞及之前时期;
(2) 2级花蕾(横径7.10~7.90 mm), 减数分裂期为主;
(3) 3级花蕾(横径8.00~8.50 mm), 四分体时期为主;
(4) 4级花蕾(横径8.60~9.50 mm), 单核花粉期为主;
(5) 5级花蕾(横径9.60~10.50 mm), 二核花粉期为主;
(6) 6级花蕾(横径≥11.00 mm), 完全成熟花粉期。
将各级花蕾分别用卡诺氏固定液(95%乙醇:冰醋酸=3:1)室温固定24 h, 梯度乙醇(95%、85%、70%)清洗, 转入70%乙醇中, 4℃保存备用。
1.3 花部形态特征观察
田间观察厚轴茶雄性不育株和正常可育株的花器形态及发育情况。随机采集不育株和可育株上部、中部和下部的花蕾和开放花朵, 用游标卡尺测量花蕾横径, 直尺测量花冠大小、花瓣长、花瓣宽、花丝长度和花柱长度。用体视显微镜(LEICA M165C/ DMC4500)观察花药、花丝和花柱形态并拍照。观察每级10个花蕾, 重复3次。1.4 花药石蜡切片观察
采用常规石蜡切片程序制片[16]。取固定好的花蕾, 经逐级乙醇(70%、85%、95%、100%)脱水, 二甲苯透明, 石蜡浸蜡和包埋, SLEE CUT5062切片机横切, 切片厚度8 μm, 郝伯特配方粘片剂粘片, 38℃恒温箱烤片, 番红-固绿染色液染片, 梯度乙醇再次退水、二甲苯透明, 中性树胶封片。用LEICA DM4000荧光显微镜观察并照相。观察每级10个花蕾, 重复3次。1.5 花粉母细胞减数分裂观察
采用改良苯酚品红压片法制片[17]。取固定好的花蕾, 经蒸馏水漂洗2~3次, 转入1 mol L-1 HCl溶液, 60℃水解10 min, 蒸馏水洗净。挑出2~3枚花药置载玻片上, 加1滴改良苯酚品红染液, 用镊子挑破花药并捣碎, 染色2~3 min, 盖上盖玻片、滤纸, 均匀压片。用LEICA DM4000荧光显微镜观察并照相。观察每级10个花蕾, 重复3次。1.6 小孢子发育过程荧光显微观察
采用4’,6-二脒基-2-苯基吲哚(4’,6-diamidino- 2-phenylindole, DAPI)荧光染色法[18], 观察小孢子发育过程。取固定好的花药(四分体至成熟花粉期), 转入1 mol L-1 HCl溶液, 60℃水解15 min, 用PBS缓冲液洗涤3次(10 min 次-1), 并保存于PBS缓冲液中。挑出2~3枚花药置载玻片上, 捣碎, 加1滴2 μg mL-1 DAPI染色液, 迅速盖片, 适度轻压, 黑暗中染色8~10 min。用LEICA DM4000荧光显微镜观察并拍照。观察每级10个花蕾, 重复3次。2 结果与分析
2.1 厚轴茶花器外部形态特征
厚轴茶每朵花均由花梗、花托、花萼、花瓣、雄蕊和雌蕊6部分组成, 属完全花(图1-A)。花梗较粗, 通常下弯, 花梗顶端为碟状圆平花托, 着生雄蕊群。花萼5片、绿色、外侧被茸毛、宿存, 花瓣9~12枚、白色。雄蕊由花丝和花药组成, 为多体雄蕊, 分内外2轮, 外轮花丝较长, 花丝基部与花瓣连生。雌蕊1枚, 由子房、花柱和柱头3部分组成, 柱头3~4裂、外翻、被茸毛。子房上位、3~4室、密被茸毛, 具花盘蜜腺。雄性不育株的花器官组成与可育花相同, 但表现出明显的雄性不育特征: 花丝弯曲, 花药合生, 相互黏连, 聚合成一团锥体, 环绕花柱, 类似于菊科植物中的“聚药雄蕊”, 且花药干瘪、变形、呈坏死色泽, 不裂药, 无花粉散出, 但花冠正常展开, 花瓣完整, 雌蕊发育良好, 花柱长柱型, 柱头外露, 能异交结实(图1-B)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1可育株M352和雄性不育株M350的花器官形态
A: 可育株M352; B: 雄性不育株M350。
Fig. 1Flower morphology of fertile plant M352 and male sterile plant M350
A: fertile plant M352; B: male sterile plant M350.
表1表明, 可育花和雄性不育花在花冠大小、花瓣大小、花柱长度和雄蕊数量等指标上的差异没有统计学意义(P>0.05), 仅花丝长度差异显著(P< 0.05)。可育花和不育花的花冠直径分别为7.28 cm和6.94 cm, 花瓣长分别为3.53 cm和3.20 cm, 花瓣宽分别为2.80 cm和3.07 cm, 外轮花丝长分别为1.66 cm和1.37 cm, 内轮花丝长分别为0.94 cm和0.65 cm, 花柱长分别为1.61 cm和1.73 cm, 雄蕊数量分别为190.62个和184.85个。
Table 1
表1
表1可育株M352和雄性不育株M350开花期花器性状比较
Table 1
| 植株 Plant | 花冠直径 Expansion of corolla (cm) | 花瓣长 Length of petal (cm) | 花瓣宽 Width of petal (cm) | 外轮花丝长 Length of out thrum (cm) | 内轮花丝长 Length of inside thrum (cm) | 花柱长 Length of style (cm) | 雄蕊数量 Number of stamen (No.) |
|---|---|---|---|---|---|---|---|
| 可育株M352 Fertile plant M352 | 7.28±0.57 a | 3.53±0.30 a | 2.80±0.24 a | 1.66±0.18 a | 0.94±0.18 a | 1.61±0.18 a | 190.62±16.37 a |
| 不育株M350 Sterile plant M350 | 6.94±0.51 a | 3.20±0.28 a | 3.07±0.34 a | 1.37±0.13 b | 0.65±0.14 b | 1.73±0.21 a | 184.85±18.52 a |
新窗口打开|下载CSV
不育株与可育株的花蕾发育进程基本一致。花蕾横径约≤7. 00 mm时, 花药白色透明, 为花粉母细胞及之前时期(图2-A)。花蕾横径约7.10~7.90 mm时, 花药米黄色, 为减数分裂期(图2-B)。花蕾横径约8.60~9.50 mm时, 花药淡黄色, 为单核花粉期(图2-C)。花蕾横径约9.60~10.50 mm时, 花药鲜黄色, 为二核花粉期(图2-D)。花蕾横径约≥11.00 mm时, 花药橘黄色, 为成熟花粉期(图2-E)。这表明花蕾大小及花药色泽与花蕾发育进程有密切的相关性, 可作为判断花蕾所处发育时期的外部形态指标, 为取样提供确切依据。
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2可育株M352和雄性不育株M350花蕾发育过程
A~E: 可育株M352; F~J: 不育株M350。A和F: 花粉母细胞期; B和G: 减数分裂期; C和H: 单核期; D和I: 二核期; E和J: 展花期。
Fig. 2Comparison of buds development between fertile plant M352 and male sterile plant M350
A-E: fertile plant M352; F-J: sterile plant M350. A and F: pollen mother cell stage; B and G: meiotic stage; C and H: uninucleate stage; D and I: binucleate stage; E and J: flowering stage.
在花蕾发育过程中, 可育株花药饱满, 色泽鲜艳, 展花后, 花丝伸直, 花药彼此分离, 有大量花粉散出(图2-E)。不育株花蕾发育早期(减数分裂及之前时期), 其雄蕊发育正常, 花丝、花药形状规则, 与可育株无明显差异(图2-F, G)。但在花蕾发育后期(单核期以后), 不育株的花药排列紧密, 无间隙(图2-H, I)。展花后, 花丝弯曲, 无法伸直, 花药彼此粘连, 呈褐色、灰白色, 与可育株存在明显差异(图2-J)。从花器官表型来看, 该厚轴茶雄性不育花器官形态属雄蕊萎缩型和花药异常型。
2.2 厚轴茶花药的发育过程
切片观察显示, 厚轴茶花药发育主要经历了造孢细胞、花粉母细胞、减数分裂、四分体、单核花粉、二核花粉和成熟花粉等7个时期。造孢细胞期, 药室表皮、内壁、中层、绒毡层和造孢细胞均已形成, 造胞细胞位于药室内侧中央(图3-A)。花粉母细胞期, 花粉母细胞被胼胝质包绕, 细胞核大, 绒毡层排列整齐, 细胞质浓厚(图3-B)。减数分裂期, 花粉母细胞体积变大, 开始减数分裂, 绒毡层细胞充分发育, 具双核(图3-C)。四分体时期, 可见胼胝质包裹的四面体型小孢子, 绒毡层收缩, 形成分泌型, 开始退化(图3-D)。单核期, 花粉外壁开始形成, 绒毡层液泡化, 降解加速(图3-E)。双核期, 单核花粉发育成二核花粉, 绒毡层充分降解, 药室内壁纵向增厚(图3-F)。成熟花粉期, 花粉粒椭圆形、胞质饱满、染色深, 绒毡层完全解体, 药室内壁呈“U”型进一步增厚, 两花粉囊交界处细胞层变薄、破裂消失、纵行开裂, 完成散粉(图3-G, H)。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3可育株M352花药发育过程
A: 造孢细胞期; B: 花粉母细胞期; C: 减数分裂期; D: 四分体时期; E: 单核期; F: 双核期; G和H: 成熟期。Ep: 表皮层; En: 内皮层; ML: 中层; T: 绒毡层; Sc: 造孢细胞; Pmc: 花粉母细胞; Tds: 四分体; Mpg: 单核花粉; Bpg: 双核花粉; Pg: 成熟花粉粒。
Fig. 3Anther pollen development process of fertile plant M352
A: spore-forming cell stage; B: pollen mother cell stage; C: meiosis stage; D: tetrad stage; E: uninucleate stage; F: binucleate stage; G and H: mature stage. Ep: epidermis; En: endothecium; ML: middle layer; T: tapetum; Sc: sporogenous cell; Pmc: pollen mother cell; Tds: tetrads; Mpg: mononuclear pollen grain; Bpg: binuclear pollen grains; Pg: mature pollen grain.
与正常可育花药的发育相比较, 败育花药在造孢细胞和花粉母细胞时期花药壁各层发育良好, 与可育花无明显差异(图4-A, B)。但在减数分裂期, 败育花药绒毡层细胞异常加厚、增生, 排列混乱, 脱离药室壁, 包围、挤压花粉母细胞, 花粉母细胞原生质体异常收缩, 细胞壁、细胞膜解体消失, 细胞粘连和胞质融合、核仁消失, 处于解体状态, 不能进行减数分裂(图4-C)。四分体时期, 败育花药绒毡层仍呈现出细胞质浓厚, 细胞核大的特征, 未形成分泌型, 四分体形状不规则、或解体破碎(图4-D)。单核花粉期, 败育花药绒毡层胞质凝集, 异常凋亡, 花粉粒皱缩, 胞质稀薄, 内含物降解(图4-E)。双核花粉期, 败育花药绒毡层液泡化, 延迟降解, 花粉粒形态异常, 细胞质和核仁消失, 成为空瘪花粉(图4-F)。成熟花粉期, 败育花药药室扭曲、不规则, 花粉囊内含有空瘪、畸形花粉粒(图4-G)或残留有解体的花粉母细胞残留物(图4-H)。从花药结构组织切片来看, 厚轴茶雄性不育株花药在减数分裂至成熟花粉期均能观察到异常现象, 且同一花蕾及同一花药内败育进程并不完全一致, 但最终完全败育。
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4雄性不育株M350花粉败育过程
A: 造孢细胞期; B: 花粉母细胞期; C: 减数分裂期; D: 四分体时期; E: 单核期; F: 双核期; G和H: 成熟期。Ep: 表皮层; En: 内皮层; ML: 中层; T: 绒毡层; Sc: 造孢细胞; Pmc: 花粉母细胞; Mc: 减数分裂体; Tds: 四分体; Mpg: 单核花粉; Bpg: 双核花粉。
Fig. 4Anther abortion process of male sterile plant M350
A: spore-forming cell stage; B: pollen mother cell stage; C: meiosis stage; D: tetrad stage; E: uninucleate stage; F: binucleate stage; G and H: mature stage. Ep: epidermis; En: endothecium; ML: middle layer; T: tapetum; Sc: sporogenous cell; Pmc: pollen mother cell; Mc: meiotic cell; Tds: tetrads; Mpg: mononuclear pollen grain; Bpg: binuclear pollen grains.
2.3 厚轴茶花粉母细胞减数分裂观察
花粉母细胞经过减数分裂I、减数分裂Ⅱ和胞质分裂后形成四分体。间期I, 染色质凝结成团, 位于细胞中央(图5-A)。前期I细线期, 染色质浓缩形成细长如线的染色体, 细胞核增大(图5-B)。前期I偶线期, 同源染色体开始联会, 可见多处形成的联接点, 仍为细线状(图5-C)。前期I粗线期, 同源染色体完成配对, 染色体相互缠绕, 明显缩短变粗(图5-D)。前期I双线期, 染色体进一步缩短、互相排斥、彼此分离、交叉存在(图5-E)。前期I终变期, 染色体螺旋化, 每对二价体均匀分散于细胞内, 可清晰地观察到15对二价体(图5-F)。中期I, 核膜、核仁消失, 染色体整齐、集中排列在赤道板中央(图5-G)。后期I, 染色体受纺锤体牵引, 向两极移动, 染色体数目减半(图5-H)。末期I, 染色体到达细胞两级, 逐渐形成细胞板(图5-I)。前期II, 细胞两极的染色体松散变细, 形成2个子核(图5-J)。中期II, 染色体平行排列在分裂细胞的赤道板上(图5-K)。后期II, 姊妹染色单体分离, 由纺锤丝牵引移向两极(图5-L)。末期II, 到达两极的染色体浓缩在4个极区成4个团块(图5-M)。四分体时期, 此时进行胞质分裂, 各孢子形成胼胝质, 4个小孢子又被共同的胼胝质壁所包围, 形成四分体(图5-N)。在同一花蕾或同一花药内绝大多数细胞分裂具同步性, 但在各个分裂时期均存在少数不同步分裂相, 最大跨度达4个分裂相(图5-O)。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5可育株M352花粉母细胞正常减数分裂过程
A: 间期I; B: 前期I细线期; C: 前期I偶线期; D: 前期I粗线期; E: 前期I双线期; F: 前期I终变期; G: 中期I; H: 后期I; I: 末期I; J: 前期II; K: 中期II; L: 后期II; M: 末期II; N: 四分体时期; O: 不同步分裂相。
Fig. 5Normal meiosis of pollen mother cells of fertile plant M352
A: interphase I ; B: prophase I leptotene; C: prophase I zygotene; D: prophase I pachytene; E: prophase I diplotene; F: prophase I diakinesis; G: metaphase I ; H: anaphase I ; I: telophase I; J: prophase II; K: metaphase II; L: anaphase II; M: tetrahedral II; N: tetrad stage; O: asynchronism meiotic.
败育花药花粉母细胞减数分裂中存在环状单价体、染色体桥、滞后染色体, 不对称、不均等分离, 染色体断片、缺失, 微核、三分体、异常四分体、五核四分体、六核四分体等诸多异常现象。前期I, 染色体异常联会, 非同源染色体发生易位, 形成环状单价染色体(图6-A~C)。在后期I和后期II, 染色体向两极移动时, 出现单桥、双桥和多桥等染色体桥(图6-D~G)。在中期I、后期I和末期I等时期, 存在染色体不同步移动, 成为落后染色体(图6-H~K)。在前期I、后期I、末期I和后期II等时期, 一些细胞中染色体明显减少、缺失, 染色体散乱排列或处于凝集状态(图6-L~P)。在中期II和后期II, 染色体分裂不同步, 不对称, 不等分(图6-Q~S)。在减数分裂的前期I、前期II等分裂时期均观察到微核存在(图6-T~V)。在四分体形成时期, 4个子细胞大小不同, 形态异常, 细胞质解体, 同时还观察到有三分体、多核四分体的存在(图6-W~Y)。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6雄性不育株M350花粉母细胞异常减数分裂过程
A~C: 环状、棒状单价体; D~G: 染色体单桥、多桥; H~K: 落后染色体; L: 染色体缺失; M~P: 染色体散乱排列; Q和R: 分裂不同步; S: 不对称排列; T~V: 微核; W: 三分体; X和Y: 多核四分体。
Fig. 6Abnormal meiosis of pollen mother cells of male sterile plant M350
A-C: annular and clavate univalent; D-G: multi-chromosome bridges and single chromosome bridge; H-K: laggard chromosomes; L: deletion chromosomes; M-P: scattered chromosomes; Q and R: meiotic asynchronous; S: mis-orientated chromosomes; T-V: micronucleus; W: trisomy; X and Y: polynuclear tetrad.
对厚轴茶雄性不育株和可育株花粉母细胞减数分裂过程及染色体行为统计分析表明(表2), 可育株绝大多数花粉母细胞减数分裂过程正常, 仅有不同步分裂相异常频率较高。可育株花粉母细胞减数分裂过程中染色体平均异常频率为13.65%。雄性不育株花粉母细胞减数分裂的细线期、偶线期和粗线期未能观察到染色体异常, 但从双线期开始, 直到四分体时期, 均能观察到各种异常现象。雄性不育株花粉母细胞减数分裂过程中染色体平均异常频率为38.40%, 其中后期I、前期II和四分体时期异常频率较高。可见, 雄性不育株花粉母细胞减数分裂过程中染色体异常频率高于正常可育株, 说明雄性不育株花粉母细胞减数分裂过程中染色体异常行为可能是导致其花药和花粉败育的原因之一。
Table 2
表2
表2可育株M352和不育株M350花粉母细胞减数分裂中异常染色体的频率
Table 2
| 减数分裂时期 Meiosis stage | 可育株M352 Fertile plant M352 | 雄性不育株M350 Male sterile plant M350 | |||||
|---|---|---|---|---|---|---|---|
| 统计细胞数 Number of cells examined | 异常细胞数量 Number of abnormal cells | 频率 Frequency (%) | 统计细胞数 Number of cells examined | 异常细胞数量 Number of abnormal cells | 频率 Frequency (%) | ||
| 前期I Prophase I | 177 | 20 | 11.30 | 205 | 47 | 22.93 | |
| 中期I Metaphase I | 90 | 16 | 17.78 | 97 | 36 | 37.11 | |
| 后期I Anaphase I | 87 | 13 | 14.94 | 92 | 44 | 47.83 | |
| 末期I Telophase I | 113 | 16 | 14.16 | 124 | 52 | 41.94 | |
| 前期II Prophase II | 98 | 12 | 12.24 | 108 | 48 | 44.44 | |
| 中期II Metaphase II | 72 | 11 | 15.28 | 93 | 33 | 35.48 | |
| 后期II Anaphase II | 93 | 12 | 12.90 | 105 | 40 | 38.10 | |
| 末期II Telophase II | 85 | 9 | 10.59 | 95 | 32 | 33.68 | |
| 四分体时期 Tetrad | 198 | 27 | 13.64 | 220 | 97 | 44.09 | |
新窗口打开|下载CSV
2.4 厚轴茶小孢子发育过程观察
对四分体至成熟花粉期花药细胞的DAPI染色显示, 正常可育株花药内的四分体形态规则, 着色深, 细胞核明亮(图7-A)。小孢子呈三角形, 细胞质浓稠, 细胞核大、位居中央, 为单核花粉早期(图7-B)。随着小孢子体积增大, 花粉粒外壁开始形成, 细胞壁呈收缩状态, 为单核居中期(图7-C)。之后, 细胞膨大变圆, 花粉壁加厚, 可见花粉壁上3个萌发沟, 细胞核靠边, 为单核靠边期(图7-D)。随着花粉的发育, 细胞质充满整个细胞, 出现一个圆形的营养核和一个长形的精核, 为二核花粉期(图7-E)。图7
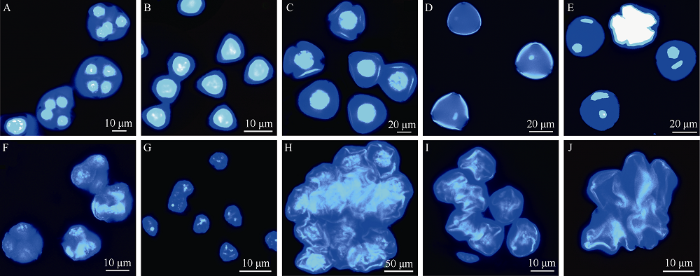 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7可育株M352和不育株M350小孢子发育过程比较
A~E: 可育株M352; F~J: 不育株M350。A和F: 四分体时期; B和G: 单核早期; C和H: 单核期; D和I: 单核晚期; E和J: 双核期。
Fig. 7Comparison of microspores development between fertile plant M352 and male sterile plant M350
A-E: fertile plant M352; F-J: sterile plant M350. A and F: tetrad stage; B and G: early uninucleate stage; C and H: uninucleate stage; D and I: later uninucleate stage; E and J: binucleate stage.
雄性不育株大部分花粉母细胞能经减数分裂形成四分体, 并发育至单核花粉期, 但表现不育特征, 四分体和游离小孢子形状不规则, 细胞核散乱模糊, 染色浅, 出现拟四分孢子、拟小孢子现象(图7-F, G)。单核花粉期花粉壁皱缩, 花粉粒相互黏连变形、花粉内细胞质稀薄、染色浅(图7-H)。单核靠边期花粉壁未能进一步加厚, 花粉内含物降解, 未见萌发孔或萌发孔皱缩闭合(图7-I)。二核花粉期, 花粉内含物消失, 细胞空胞化, 花粉壁进一步皱缩、变形, 成为败育花粉(图7-J)。单核花粉期是该厚轴茶雄性不育株花药和花粉败育的主要时期。
3 讨论
3.1 厚轴茶雄性不育株花药败育的形态学表现
植物雄性不育花器形态特征存在多种表型和育性程度的变异, 可将其大致分为花冠退化型、雄蕊萎缩型、花药异常型、小孢子退化型和花粉功能不育型等[19]。本研究首次在厚轴茶中发现雄性不育现象, 对其花器官发育过程调查表明, 雄性不育花和可育花的发育进程基本一致, 经历了花芽分化、花蕾形成、花蕾生长、花蕾膨大、花蕾露白、花冠展开和花冠脱落7个阶段。花蕾发育早期, 雄性不育花雄蕊发育正常, 花器形状及大小与可育花无明显差异。花蕾发育后期, 雄性不育花花药排列异常, 花药发育开始受阻。展花后, 雄性不育花花丝弯曲, 花药粘连、干瘪、畸形、呈坏死色泽, 不裂药, 无花粉散出。雄性不育花在花冠大小、花瓣大小和花柱长度等指标上与可育花差异不显著, 但在花丝长度、花药形状及花药色泽等方面与可育花存在明显差异。说明该厚轴茶花瓣和花柱等组织的发育与其雄性不育性没有明确的关系, 而花药的发育及花药外部形态与其雄性不育性密切相关。从花器官表型来看, 本研究中获得的厚轴茶雄性不育材料花器形态属于雄蕊萎缩型和花药异常型。3.2 厚轴茶雄性不育株花药败育时期和特点
植物雄性不育发生时期具有多样性, 不同植物、不同类型及不同来源的雄性不育材料的败育时期不一。如花椰菜温敏雄性不育系花药发育受阻于花粉母细胞到四分体时期[20], D2型细胞质雄性不育小麦[21]和杨树雄性不育品种‘泗杨1号’[22]等不育材料的败育主要发生在单核时期。本研究表明, 雄性不育株花药发生败育的时期和细胞学特征较复杂, 同一花蕾及同一花药内败育进程并不完全一致, 花药和花粉发育受阻于多个时期。部分内轮花药在花粉母细胞减数分裂期发育受阻, 形成花粉母细胞败育型, 多数外轮花药发育受阻于单核花粉期, 形成单核败育型, 其败育发生的时期可大体划分为减数分裂期、小孢子发育期和单核花粉期3个时期, 单核花粉期是该厚轴茶雄性不育株花药和花粉发生败育的主要时期。3.3 厚轴茶雄性不育株花药败育原因
植物雄性不育细胞生物学研究表明, 引起花药和花粉败育的原因复杂, 涉及绒毡层细胞结构与功能、Ca2+浓度、ATP酶的分布特征、细胞骨架的排列方式和细胞程序性死亡等[8]。本研究观察到厚轴茶雄性不育株的花器官外部形态发育异常, 花药粘连, 药室扭曲变形。进一步对花药切片观察表明, 在花粉母细胞及之前时期, 败育花药绒毡层发育正常, 与可育花无明显差异。在减数分裂时期, 败育花药绒毡层细胞异常增生、排列混乱, 包围、挤压花粉母细胞。四分体时期, 败育花药绒毡层仍充分发育, 未形成分泌型。单核至双核花粉期, 败育花药绒毡层表现出延迟降解现象。这说明厚轴茶雄性不育株花药和花粉败育可能是由于绒毡层细胞发育紊乱和延迟降解引起的, 而绒毡层细胞发育异常可能受到花粉囊形态扭曲、变形的影响, 具体原因还需进一步研究。花粉母细胞减数分裂是维持花粉正常发育的重要过程。前人对水稻[23]、小麦[24]、百合[25]等不育系材料的研究结果表明, 花粉母细胞减数分裂过程出现问题, 会导致花粉败育。本研究通过染色体压片法, 观察到厚轴茶雄性不育株花粉母细胞减数分裂过程中存在环状单价体、滞后染色体、染色体桥、染色体缺失、不均等分离、微核和多分体等较多异常现象, 其染色体异常频率达38.40%, 明显高于正常可育株的异常频率。说明厚轴茶雄性不育株花粉母细胞减数分裂过程中染色体行为异常可能是导致其花粉败育的原因之一。
另外, 小孢子和花粉壁发育异常均会引起花粉败育[26]。本研究观察到厚轴茶雄性不育株四分体分离不完整, 小孢子形状不规则, 胞质紊乱模糊, 单核期花粉壁皱缩黏连, 成熟花粉生殖核与营养核消失, 花粉粒空瘪凹陷。一方面可能是由于绒毡层细胞异常发育和延迟降解, 不能为小孢子发育提供营养物质, 引起小孢子发育不良; 另一方面也可能是小孢子和花粉壁自身发育异常, 不能有效转运和吸收所需营养物质和能量, 而出现花粉畸形, 最终导致花粉败育[27]。本研究鉴于荧光显微观察发现厚轴茶雄性不育株花粉细胞结构存在差异, 但其败育的具体原因还有待从花粉超微结构方面观察确认。总之, 厚轴茶雄性不育株花药和花粉败育的可能影响因素主要是绒毡层细胞结构异常、花粉母细胞减数分裂染色体行为异常、小孢子和花粉发育异常及核质互作影响, 原因较复杂, 还需要多方面深入探讨和研究。
4 结论
厚轴茶雄性不育花器形态属雄蕊萎缩型和花药异常型。花药发育受阻于减数分裂至单核花粉期, 存在花粉母细胞败育型和单核败育型。单核花粉期是其花药败育的主要时期。花药绒毡层异常发育和延迟降解, 花粉母细胞减数分裂染色体行为异常, 小孢子和花粉粒发育异常可能是厚轴茶雄性不育株花药败育的主要原因。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1186/1471-2229-14-14URLPMID:24405939 [本文引用: 1]

BACKGROUND: Tea is one of the most popular beverages in the world. Many species in the Thea section of the Camellia genus can be processed for drinking and have been domesticated. However, few investigations have focused on the genetic consequence of domestication and geographic origin of landraces on tea plants using credible wild and planted populations of a single species. Here, C. taliensis provides us with a unique opportunity to explore these issues. RESULTS: Fourteen nuclear microsatellite loci were employed to determine the genetic diversity and domestication origin of C. taliensis, which were represented by 587 individuals from 25 wild, planted and recently domesticated populations. C. taliensis showed a moderate high level of overall genetic diversity. The greater reduction of genetic diversity and stronger genetic drift were detected in the wild group than in the recently domesticated group, indicating the loss of genetic diversity of wild populations due to overexploitation and habitat fragmentation. Instead of the endangered wild trees, recently domesticated individuals were used to compare with the planted trees for detecting the genetic consequence of domestication. A little and non-significant reduction in genetic diversity was found during domestication. The long life cycle, selection for leaf traits and gene flow between populations will delay the emergence of bottleneck in planted trees. Both phylogenetic and assignment analyses suggested that planted trees may have been domesticated from the adjacent central forest of western Yunnan and dispersed artificially to distant places. CONCLUSIONS: This study contributes to the knowledge about levels and distribution of genetic diversity of C. taliensis and provides new insights into genetic consequence of domestication and geographic origin of planted trees of this species. As an endemic tea source plant, wild, planted and recently domesticated C. taliensis trees should all be protected for their unique genetic characteristics, which are valuable for tea breeding.
DOI:10.1186/1471-2148-14-151URLPMID:25001059 [本文引用: 1]

BACKGROUND: Camellia is an economically and phylogenetically important genus in the family Theaceae. Owing to numerous hybridization and polyploidization, it is taxonomically and phylogenetically ranked as one of the most challengingly difficult taxa in plants. Sequence comparisons of chloroplast (cp) genomes are of great interest to provide a robust evidence for taxonomic studies, species identification and understanding mechanisms that underlie the evolution of the Camellia species. RESULTS: The eight complete cp genomes and five draft cp genome sequences of Camellia species were determined using Illumina sequencing technology via a combined strategy of de novo and reference-guided assembly. The Camellia cp genomes exhibited typical circular structure that was rather conserved in genomic structure and the synteny of gene order. Differences of repeat sequences, simple sequence repeats, indels and substitutions were further examined among five complete cp genomes, representing a wide phylogenetic diversity in the genus. A total of fifteen molecular markers were identified with more than 1.5% sequence divergence that may be useful for further phylogenetic analysis and species identification of Camellia. Our results showed that, rather than functional constrains, it is the regional constraints that strongly affect sequence evolution of the cp genomes. In a substantial improvement over prior studies, evolutionary relationships of the section Thea were determined on basis of phylogenomic analyses of cp genome sequences. CONCLUSIONS: Despite a high degree of conservation between the Camellia cp genomes, sequence variation among species could still be detected, representing a wide phylogenetic diversity in the genus. Furthermore, phylogenomic analysis was conducted using 18 complete cp genomes and 5 draft cp genome sequences of Camellia species. Our results support Chang's taxonomical treatment that C. pubicosta may be classified into sect. Thea, and indicate that taxonomical value of the number of ovaries should be reconsidered when classifying the Camellia species. The availability of these cp genomes provides valuable genetic information for accurately identifying species, clarifying taxonomy and reconstructing the phylogeny of the genus Camellia.
DOI:10.1371/journal.pone.0151424URLPMID:26962860 [本文引用: 1]

Tea is one of the most popular beverages across the world and is made exclusively from cultivars of Camellia sinensis. Many wild relatives of the genus Camellia that are closely related to C. sinensis are native to Southwest China. In this study, we first identified the distinct genetic divergence between C. sinensis and its wild relatives and provided a glimpse into the artificial selection of tea plants at a genome-wide level by analyzing 15,444 genomic SNPs that were identified from 18 cultivated and wild tea accessions using a high-throughput genome-wide restriction site-associated DNA sequencing (RAD-Seq) approach. Six distinct clusters were detected by phylogeny inferrence and principal component and genetic structural analyses, and these clusters corresponded to six Camellia species/varieties. Genetic divergence apparently indicated that C. taliensis var. bangwei is a semi-wild or transient landrace occupying a phylogenetic position between those wild and cultivated tea plants. Cultivated accessions exhibited greater heterozygosity than wild accessions, with the exception of C. taliensis var. bangwei. Thirteen genes with non-synonymous SNPs exhibited strong selective signals that were suggestive of putative artificial selective footprints for tea plants during domestication. The genome-wide SNPs provide a fundamental data resource for assessing genetic relationships, characterizing complex traits, comparing heterozygosity and analyzing putatitve artificial selection in tea plants.
[本文引用: 1]
[本文引用: 1]
URLPMID:24313845 [本文引用: 1]
DOI:10.1105/tpc.114.127282URLPMID:25035401 [本文引用: 1]

Tapetal programmed cell death (PCD) is a prerequisite for pollen grain development in angiosperms, and cysteine proteases are the most ubiquitous hydrolases involved in plant PCD. We identified a papain-like cysteine protease, CEP1, which is involved in tapetal PCD and pollen development in Arabidopsis thaliana. CEP1 is expressed specifically in the tapetum from stages 5 to 11 of anther development. The CEP1 protein first appears as a proenzyme in precursor protease vesicles and is then transported to the vacuole and transformed into the mature enzyme before rupture of the vacuole. cep1 mutants exhibited aborted tapetal PCD and decreased pollen fertility with abnormal pollen exine. A transcriptomic analysis revealed that 872 genes showed significantly altered expression in the cep1 mutants, and most of them are important for tapetal cell wall organization, tapetal secretory structure formation, and pollen development. CEP1 overexpression caused premature tapetal PCD and pollen infertility. ELISA and quantitative RT-PCR analyses confirmed that the CEP1 expression level showed a strong relationship to the degree of tapetal PCD and pollen fertility. Our results reveal that CEP1 is a crucial executor during tapetal PCD and that proper CEP1 expression is necessary for timely degeneration of tapetal cells and functional pollen formation.
DOI:10.1104/pp.113.233387URLPMID:24567187 [本文引用: 1]

During angiosperm microsporogenesis, callose serves as a temporary wall to separate microsporocytes and newly formed microspores in the tetrad. Abnormal callose deposition and dissolution can lead to degeneration of developing microspores. However, genes and their regulation in callose metabolism during microsporogenesis still remain largely unclear. Here, we demonstrated that the Arabidopsis (Arabidopsis thaliana) CALLOSE DEFECTIVE MICROSPORE1 (CDM1) gene, encoding a tandem CCCH-type zinc finger protein, plays an important role in regulation of callose metabolism in male meiocytes and in integrity of newly formed microspores. First, quantitative reverse transcription PCR and in situ hybridization analyses showed that the CDM1 gene was highly expressed in meiocytes and the tapetum from anther stages 4 to 7. In addition, a transfer DNA insertional cdm1 mutant was completely male sterile. Moreover, light microscopy of anther sections revealed that microspores in the mutant anther were initiated, and then degenerated soon afterward with callose deposition defects, eventually leading to male sterility. Furthermore, transmission electron microscopy demonstrated that pollen exine formation was severely affected in the cdm1 mutant. Finally, we found that the cdm1 mutation affected the expression of callose synthesis genes (CALLOSE SYNTHASE5 and CALLOSE SYNTHASE12) and potential callase-related genes (A6 and MYB80), as well as three other putative beta-1,3-glucanase genes. Therefore, we propose that the CDM1 gene regulates callose metabolism during microsporogenesis, thereby promoting Arabidopsis male fertility.
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
