 ,2,*, 史春余
,2,*, 史春余 ,1,*
,1,*Cloning and functional analysis of the sweet potato sucrose transporter IbSUT3
WANG Dan-Dan1,**, LIU Hong-Juan1,**, WANG Hong-Xia2, ZHANG Peng ,2,*, SHI Chun-Yu
,2,*, SHI Chun-Yu ,1,*
,1,*通讯作者:
第一联系人:
收稿日期:2019-11-11接受日期:2020-03-24网络出版日期:2020-07-12
| 基金资助: |
Received:2019-11-11Accepted:2020-03-24Online:2020-07-12
| Fund supported: |
作者简介 About authors
王丹丹,E-mail:wdd201712@126.com。
柳洪鹃,E-mail:liumei0535@126.com。

摘要
关键词:
Abstract
Keywords:
PDF (2616KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王丹丹, 柳洪鹃, 王红霞, 张鹏, 史春余. 甘薯蔗糖转运蛋白基因IbSUT3的克隆及功能分析[J]. 作物学报, 2020, 46(7): 1120-1127. doi:10.3724/SP.J.1006.2020.94173
WANG Dan-Dan, LIU Hong-Juan, WANG Hong-Xia, ZHANG Peng, SHI Chun-Yu.
在高等植物中, 光合同化物从源器官到库器官的长距离运输以蔗糖为主要形式[1]。蔗糖不仅可以作为碳源为植物生长发育提供营养物质, 而且可以作为信号物质参与植物体内的信号转导过程[2]。在源器官中, 蔗糖以共质体或质外体的途径被装载到韧皮部细胞, 通过筛管运输后再以共质体或质外体途径卸载到库器官中[3]。在质外体的装载或卸载过程中, 蔗糖转运蛋白(sucrose transporters or carriers, SUTs or SUCs)起重要作用[4]。
蔗糖转运蛋白是一种典型的膜结合蛋白, 属于MFS家族, 具有12个典型的跨膜结构域, 存在于植物的组织或细胞中, 利用胞内外的H+浓度对蔗糖进行跨膜运输[5]。迄今为止, 已经发现拟南芥、水稻、马铃薯、玉米等多种植物蔗糖转运蛋白基因[6,7,8,9]。反义抑制PtaSUT4、StSUT4、 NtSUT1的转基因植株表现出生长受阻, 源叶中蔗糖含量升高, 库器官中蔗糖含量降低, 生物量下降[10,11,12]。AtSUC2和AtSUC4突变体的种子和幼苗对干旱、高盐、冷胁迫和ABA处理超敏感, 并且叶片中的蔗糖含量较高, 而根中的蔗糖含量较低[13]。目前, 关于蔗糖转运蛋白的研究主要集中于其如何介导蔗糖在源库间的运输与分配以及如何参与逆境、糖信号的响应。
甘薯作为以地下器官为产品的双子叶作物, 其产量形成与光合产物的运输密切相关, 因此弄清关键性膜蛋白的运输机制对提高甘薯产量至关重要。李岩从甘薯中分离出IbSUT1和IbSUT2, 并在酵母中验证其转运功能, 免疫定位发现IbSUT2定位于筛管伴胞复合体; 同时发现IbSUT1x编码的蛋白定位于酵母细胞膜且不具有蔗糖转运功能[14,15]。上述报道指出, 虽然甘薯蔗糖转运蛋白的研究已取得部分进展, 但仍有大量信息值得深入挖掘。本研究首先采用RT-PCR结合RACE技术, 从甘薯中克隆得到IbSUT3基因, 并对其进行生物信息学分析; 利用酵母突变菌株SUSY7/ura3对其转运功能进行验证; 利用烟草原生质体进行亚细胞定位分析; 通过实时荧光定量PCR, 研究IbSUT3组织表达模式以及在非生物胁迫(干旱、高盐、低温)和外源ABA处理下的表达特性, 研究其在胁迫处理下的响应机制, 为深入研究IbSUT3基因的功能奠定基础。
1 材料与方法
1.1 试验材料
1.1.1 植物材料 选用“泰中6号(鉴定编号为国品鉴2013003)”为甘薯[Ipomoea batatas (L.) Lam.]试验材料。将甘薯组培苗单节点扩繁后种于组培瓶中, 每瓶3棵, 置光照培养箱中培养, 16 h光照/8 h黑暗, 温度25℃, 光照强度600 μmol m-2 s-1, 用于胁迫处理。用于亚细胞定位的烟草为本氏烟草(Nicotiana benthamiana Domin), 种子撒播于花盆中, 待烟草生长1个半月后, 用于原生质体的提取。1.1.2 菌种与质粒 大肠杆菌DH5α购自北京全式金生物有限公司。酵母菌株SUSY7/ura3、酵母表达载体P416、GFP表达载体PUC18均由中国科学院植物生理研究所张鹏老师实验室提供。
1.1.3 试剂 RNA提取试剂盒RNAprep Pure plant Kit购自TIANGEN公司(中国); SMARTer-RACE试剂盒购自Clontech公司; 逆转录试剂盒ReverTra Ace qPCR RT Master Mix购自TOYOBO公司; pMD18T载体、Marker、SYBR Premix Ex Tap荧光定量试剂盒均购自宝生物工程(大连)有限公司。脱落酸(ABA)购自Sigma公司(美国)。
1.2 材料处理
选择生长3个月且长势一致的大田甘薯植株5株, 分别取叶片(leaf, L; 即第3片展开叶)、叶柄(petiole, B)、茎(stem, J)、白须根(white root, WR)、发育根(developmental root, DR; 即指刚开始膨大的块根)和成熟根(mature root, MR; 即指膨大时间较长的块根), 用于荧光定量PCR的测定, 研究IbSUT3基因在不同组织中的表达情况。将甘薯组培苗培养到株高4 cm, 分别进行低温(4℃)、高盐(200 mmol L-1 NaCl)、干旱(300 mmol L-1 Mannitol)以及脱落酸(25 μmol L-1 ABA)胁迫处理, 分别于胁迫处理9、12和24 h取样, 每个时间点取3株植株地上部叶片, 液氮速冻。1.3 IbSUT3基因的克隆
从4 cm高的组培苗取约100 mg叶片, 液氮研磨至粉末状, 转至1.5 mL离心管中, 利用试剂盒提取总RNA。根据植物SUT基因的保守序列设计简并引物E3-F和E3-R (表1), 扩增IbSUT3基因的EST序列并测序。根据测序结果设计3'和5'端特异性引物(Sp-R1,2和Sp- F1,2)(表1), 参照RACE试剂盒SMART RACE cDNA Amplification Kit (Clontech)说明书进行IbSUT3基因的3'端和5'端扩增并测序。序列拼接并设计扩增全长的引物IbSUT3-F和IbSUT3-R (表1), 以叶片cDNA为模版进行PCR扩增, 程序为98℃预变性5 min; 98℃变性10 s, 55℃退火10 s, 72℃延伸15 s, 32个循环; 72℃延伸10 min。以1%琼脂糖凝胶电泳检测扩增结果。Table 1
表1
表1引物信息
Table 1
| 引物Primer | 序列 Sequence (5'-3') | 目的 Purpose |
|---|---|---|
| E3-F | AGATAGTAATGGTGGCCTCCATTGC | EST序列扩增 |
| E3-R | GCGAAGCCAATGAGGAAGACAGC | EST sequence amplification |
| Sp-F1 | AGATAGTAATGGTGGCGTCCATTGC | 3'端和5'端RACE扩增 |
| Sp-F2 | GCGTCCTTTATGTGGCTTTGTGG | 3' and 5'-RACE amplification |
| Sp-R1 | TGAGATTGGCGCAGTAAACG | |
| Sp-R2 | GCGGCGAAGCCAATGAGGAA | |
| IbSUT3-F | CCATTCAGGCTGCGCAACT | IbSUT3全长扩增 |
| IbSUT3-R | TTAAGAAATTAATTCCAAGGTCCAT | IbSUT3 full-length amplification |
| SUT3Q-F | CGGAAAATCCTCCTTGCAAGTG | 荧光定量PCR |
| SUT3Q-R | GACGCCACCATTACTATCTTCC | Real-time PCR |
| Actin-F | CTGGTGTTATGGTTGGGATGG | |
| Actin-R | GGGGTGCCTCGGTAAGAAG | |
| SUT3m-F | CATCGATATGGAGAGAGACTCCGTTAACG | 酵母功能验证 |
| SUT3m-R | CCCCGGGTCAGTGGAAACCACCACCAGAT | Yeast function verification |
| SUT3g-F | ACGCGTCGACATGGAGAGAGACTCCGTTAACGGAA | 亚细胞定位 |
| SUT3g-R | GGACTAGTACGTGGAAACCACCACCAGATAGCTCA | Subcellular localization |
新窗口打开|下载CSV
1.4 IbSUT3基因的生物信息学分析
经测序所得的氨基酸序列, 利用NCBI的ORF Finder, 参照Kozak法则分析该基因的开放阅读框。采用ProtParam (http://web.expasy.org/protparam) 分析蛋白质的理化性质、亲疏水性以及跨膜结构; DNAman进行氨基酸多序列比对; MEG5.2的NJ算法进行系统进化树的构建, 设bootstrap值为1000。1.5 qRT-PCR分析
胁迫处理前后的样品, 加液氮迅速研磨成粉末, 根据试剂盒的操作步骤提取组织RNA, 反转录为cDNA (统一浓度为100 ng μL-1)。运用Primers3Plus设计IbSUT3基因的实时荧光定量PCR引物(表1)。以甘薯Actin为内参基因, 每个样品3个平行, 3次生物学重复, 采用2-ΔΔCt方法分析试验结果。所用操作软件为Bio-Rad CFX Manager, 反应程序为95℃ 1 min; 95℃ 15 s, 55℃ 30 s, 72℃ 45 s, 共39个循环; 溶解曲线, 65℃到95℃, 每5 s增加0.5℃。1.6 酵母功能验证
前人常使用酿酒酵母突变株SUSY7/ura3对蔗糖转运功能进行验证, 该菌株只能够利用胞内蔗糖而不能利用胞外蔗糖, 只有在导入具有蔗糖转运功能的外源蛋白的情况下才能在以蔗糖为唯一碳源的培养基上正常生长[16]。运用PrimerPrimer 5.0设计带有酶切位点(Cal I和Sma I)的引物SUT3m-F和SUT3m-R (表1), 通过PCR扩增得到带有酶切位点的IbSUT3基因的开放阅读框(ORF)区。构建酵母表达载体P416-IbSUT3, 转化酵母菌株SUSY7/ura3。挑取正确表达的单克隆菌斑, 用0.9%NaCl调制OD~1, 然后稀释成10-1、10-2、10-3和10-4, 取10 μL分别点于含2%蔗糖和2%葡萄糖的酵母培养基中。培养基配方为1.7 g L-1酵母基础培养基YNB (Difco), 2%蔗糖(Difco)或2%葡萄糖(Difco), 5 g L-1硫酸铵, 20 mg L-1色氨酸和1.5%琼脂糖, 用HCl调节至pH为5.0。30℃培养3 d后, 观察菌斑生长情况。1.7 亚细胞定位分析
运用PrimerPrimer 5.0设计带有酶切位点(Sal I和Spe I)的引物SUT3g-F和SUT3g-R (表1), 通过PCR扩增得到带有酶切位点且不含有终止密码子的IbSUT3基因的ORF区。构建GFP表达载体CaMV35S:IbSUT3-GFP (PUC18), 转化烟草叶片原生质体。提取烟草叶片原生质体参考Drechsel的方法[17]; 采用PEG介导法, 将GFP融合蛋白转入原生质体参照Abel的方法[18]。用配备603/1.2 NA UPLSAPO油镜(Olympus)的Olympus FV1000共聚焦扫描显微镜观察烟草叶片原生质体。通过用473 nm激光激发并在487 nm和521 nm之间发射, 观察到GFP荧光。通过用559 nm激光激发并在606 nm至673 nm之间发射, 观察到叶绿体自发荧光。
1.8 数据处理
所有试验数据均由3次重复试验获得, 应用DPSS软件分析差异显著性, 运用最小显著性差数法(LSD)进行单因素方差分析, 差异显著性水平均为P<0.005。2 结果与分析
2.1 IbSUT3基因的获得及序列分析
2.1.1 IbSUT3基因的克隆 为了分析甘薯蔗糖转运蛋白的功能, 本研究克隆了一个基因(GenBank登录号为MN233361)并命名为IbSUT3。通过简并引物扩增得到IbSUT3基因的EST序列, 长度为267 bp。再根据NCBI和PrimerPrimer 5.0软件设计3'-RACE和5'-RACE的基因特异性引物, 进行5'-DNA和3'-DNA克隆, 得到的片段长度分别为426 bp和1425 bp, 拼接两者得到IbSUT3基因的全长。根据拼接序列设计引物扩增全长, 得到1607 bp的序列片段(图1)。图1
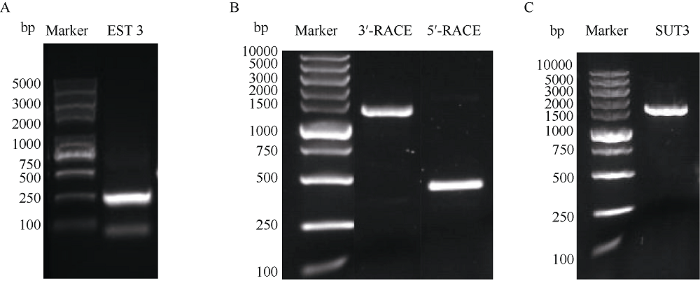 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1IbSUT3基因扩增结果
A: IbSUT3基因EST序列扩增结果; B: IbSUT3 3'-RACE, 5'-RACE扩增结果; C: IbSUT3全长扩增结果。
Fig. 1PCR amplified products of IbSUT3
A: the EST sequences amplification of IbSUT3; B: the 3'-RACE and 5'-RACE amplification of IbSUT3; C: the full length amplification of IbSUT3.
2.1.2 IbSUT3基因编码蛋白的理化性质 ORF大小为1518 bp, 编码的蛋白质有505个氨基酸。该蛋白的分子量为53.82 kD, 等电点(pI)为9.19; 正电荷残基(Asp + Glu)有31个, 负电荷残基(Arg + Lys)有41个; 总的亲水性平均系数为0.572, 预测IbSUT3基因编码的蛋白为疏水性蛋白。TMHMM软件预测蛋白跨膜结构域显示, IbSUT3基因编码的蛋白具有12个跨膜结构域(图2)。
图2
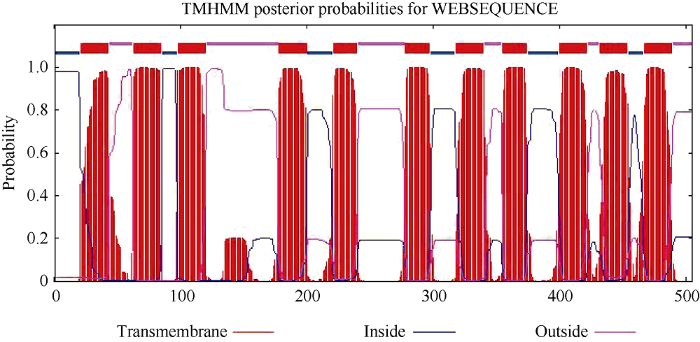 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2IbSUT3蛋白跨膜区预测
Fig. 2Putative transmembrane predication of IbSUT3
2.1.3 IbSUT3序列比对和进化树分析 将IbSUT3编码的氨基酸序列与拟南芥(Arabidopsis thaliala)、烟草(Nicotiana tabacum)、马铃薯(Solanum tuberosum) 3个代表性物种中的SUTs编码氨基酸进行序列多重比对(图3)显示, 该基因编码的蛋白与其他物种的SUTs蛋白的氨基酸序列具有较高同源性, 保守区域分析发现IbSUT3基因所编码的蛋白同属于MFS蛋白家族, 暗示其功能的高度保守。
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3IbSUT3编码的氨基酸序列与其他物种SUTs的多重比对
Fig. 3Multiple alignment of the amino acid sequences of IbSUT3 with other species
为进一步了解IbSUT3与其他物种基因之间的进化关系, 选取不同物种的SUT蛋白序列, 使用MEGA5.2软件, 采用Neighbor-Joining原理构建系统进化树(图4)。分析发现, IbSUT3编码的蛋白属于Group I亚族, 并且IbSUT3、IbSUT1y、IbSUT2y、NtSUT1以及NtSUT3处在同一进化支上, 说明它们的亲缘关系较近。
图4
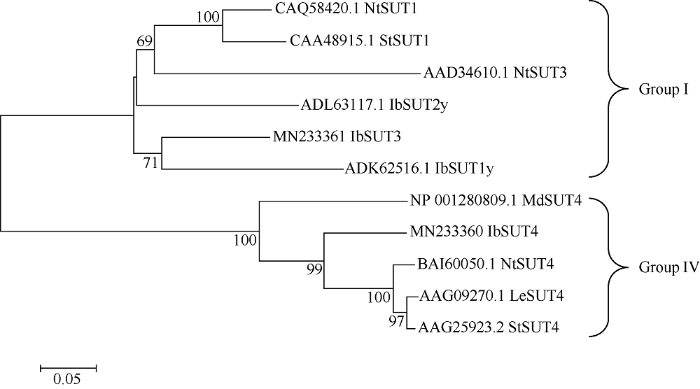 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4IbSUT3与其他植物SUTs蛋白的系统发生分析
Fig. 4Phylogenetic analysis between IbSUT3 and SUTs proteins from other plants species
Nt: Nicotiana tabacum; St: Solanum tuberosum; Ib: Ipomoea batatas; Md: Malus domestica; Le: Solanum lycopersicum.
2.2 IbSUT3酵母功能验证
为确认IbSUT3基因编码的蛋白是否具有转运蔗糖的功能, 构建酵母菌株表达载体, 并转化酵母。结果表明, 在含有2%蔗糖作为唯一碳源的培养基上, IbSUT3转化的SUSY7/ura3酵母菌株相比空载体转化的酵母菌株能更好地生长(图5-A); 但在含有2%葡萄糖为唯一碳源的培养基上, IbSUT3及空载体转化的SUSY7/ura3酵母菌株生长情况没有差异(图5-B)。IbSUT3的表达使SUSY7/ura3酵母菌株能够在蔗糖为唯一碳源的培养基上生长, 说明IbSUT3编码了一种具有转运蔗糖功能的蛋白。图5
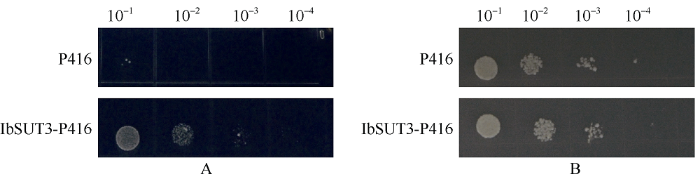 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5IbSUT3功能分析
A: 转化的酵母菌株在含2%蔗糖培养基上的生长情况; B: 转化的酵母菌株在含2%葡萄糖培养基上生长情况。
Fig. 5IbSUT3 function analysis
A: growth of transformed yeast strain on media of 2% sucrose; B: growth of transformed yeast strain on media of 2% glucose.
2.3 IbSUT3蛋白亚细胞定位
为探索IbSUT3蛋白在植物亚细胞结构中的分布, 构建了IbSUT3基因的GFP表达载体CaMV35S::IbSUT3 -GFP。将载体转化到烟草原生质体中, 借助绿色荧光蛋白信号确定目标蛋白在原生质体内的分布。PEG介导原生质体转化18 h后, 通过共聚焦激光扫描显微镜观察显示, 转CaMV35S-GFP载体的烟草原生质体内绿色荧光分布在整个原生质体膜、胞质和细胞核中, 转CaMV35S:: IbSUT3-GFP(图6)载体的烟草原生质体内绿色荧光蛋白均匀分布于质膜, 说明IbSUT3蛋白分布于质膜上。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6IbSUT3亚细胞定位分析
Fig. 6Analysis of IbSUT3 subcellular localization
2.4 IbSUT3基因的表达模式分析
2.4.1 IbSUT3基因在不同组织中的表达 分别提取甘薯植株不同部位的RNA进行qRT-PCR检测, 分析IbSUT3基因在不同组织中的表达模式表明, IbSUT3基因在整株植物中具有相对较高的表达, 其中在L (叶片)中相对表达最高, 在J (茎)中相对表达量最低(图7)。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7IbSUT3基因在不同组织中的表达
L: 叶片; B: 柄; J: 茎; WR: 白须根; DR: 发育根; MR: 成熟根。图柱上不同小写字母表示在0.05水平上显著差异。
Fig. 7Expression of IbSUT3 in different tissues
L: leaf; B: petiol; J: stem; WR: white root; DR: developmental root; MR: mature root. Bars indexed with different lowercase letters are significantly different at the 0.05 probability level.
2.4.2 IbSUT3基因在非生物胁迫及外源ABA处理下的表达模式 低温(4℃)、高盐(200 mmol L-1 NaCl)、干旱(300 mmol L-1 Mannitol)以及脱落酸(25 μmol L-1 ABA)处理均可诱导IbSUT3基因的表达(图8)。与对照处理(0 h)相比, 胁迫处理9~24 h时IbSUT3基因的表达均呈现上升的趋势, 仅在干旱处理12 h时出现下降趋势, 但下降不显著。在低温、高盐及干旱处理条件下, 与9 h和24 h相比, 处理12 h时IbSUT3基因的表达量较低, 这可能与该取样时间在夜间有关。
图8
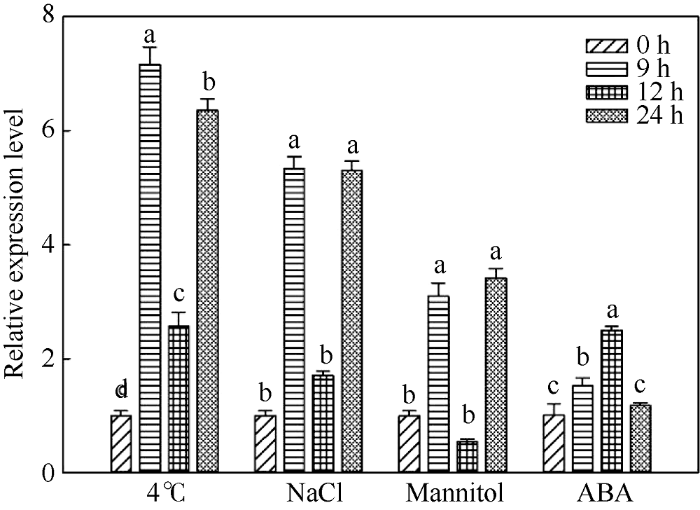 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8IbSUT3在胁迫处理下的表达模式
图柱上不同小写字母表示在0.05水平上显著差异。
Fig. 8Expression profiles of IbSUT3 under stresses
Bars indexed with different lowercase letters are significantly different at the 0.05 probability level.
3 讨论
由于蔗糖是光合作用的主要产物, 是植物贮藏、运输和利用糖分的主要形式, 其运输和分配的结果将直接影响作物的产量和品质[19], 所以分离、鉴定甘薯中的蔗糖转运蛋白, 深入了解其功能, 不仅有助于了解甘薯体内光合产物的运输、分配过程, 而且可以分析其对产量和品质的影响, 进而为实现甘薯高产优质提供理论依据。本研究填补了甘薯蔗糖转运蛋白研究的空缺, 首次成功克隆了甘薯IbSUT3基因, 该基因编码的蛋白属于Group I亚族, 定位于质膜且具有转运蔗糖的功能。IbSUT3基因在叶片中高表达且响应多种胁迫处理, 暗示IbSUT3可能参与蔗糖装载过程、参与植物抗逆反应。3.1 IbSUT3具有蔗糖转运功能
SUTs家族在植物中最典型的功能是将蔗糖转运到韧皮部中, 以实现光合同化物的长距离运输。因此对于SUTs的研究, 首先需要分析其是否具有转运功能。本研究从甘薯品种“泰中6号”中克隆得到的IbSUT3基因, 其编码的蛋白通过在缺陷酵母中进行异源表达, 发现可以互补缺陷酵母的蔗糖吸收, 说明其编码的蛋白具有蔗糖转运的功能(图5)。拟南芥AtSUC4和小麦的TaSUT2A、TaSUT2B和TaSUT2D等均通过此试验验证了蛋白的转运功能[20,21]。3.2 IbSUT3基因在叶片中表达较高
已有研究指出Group I亚族的蔗糖转运蛋白主要在源器官中表达, 并且对蔗糖的韧皮部装载和长距离运输起着非常关键的作用[22,23,24]。在反义抑制StSUT1的马铃薯植株中, SUT1基因的表达量下降, 植株生长迟缓, 而且在叶片中积累大量的可溶性糖和淀粉, 最终导致块茎产量降低[22]。反义抑制NtSUT1的烟草植株表现出植株生长减缓, 叶片中蔗糖的输出速率大大降低, 导致蔗糖在叶片中大量积累[25]。本研究发现, IbSUT3基因编码的蛋白属于Group I亚族(图4)且在源叶中高表达(图7), 这暗示着该基因编码的蛋白参与了蔗糖在韧皮部的装载。源叶中的蔗糖能够及时高效地装载到韧皮部, 对光合产物从源器官到库器官的运输至关重要, 对甘薯产量的形成意义重大。3.3 IbSUT3基因响应多种非生物胁迫
Xu等[26]通过分析5种双子叶植物(Arabidopsis thaliana、Glycine max、Solanum tuberosum、Solanum lycopersicum、Populus spp.)和4种单子叶植物(Zea mays、Oryza sativa、Triticum aestivum、Hordeum vulgare)中SUTs对逆境处理的响应情况, 证明SUTs在植物抵抗逆境过程中发挥重要作用。在盐胁迫下, 所有芹菜组织中的AgSUT1基因的表达均降低, 根系中降低更为显著, 这可能表明在盐胁迫下根系对蔗糖代谢需求降低[27]。AtSUC2和AtSUC4在低温、高盐、干旱及外源ABA处理下基因的表达发生明显变化, 且通过ABA-依赖的信号通路抵抗胁迫[13]。上述研究均证实SUTs作为重要的调控因子参与逆境响应, 但是关于甘薯IbSUT3基因是否参与逆境响应尚未明确。本研究发现非生物胁迫均可诱导IbSUT3基因的表达发生显著变化, 且在低温、高盐及干旱处理下的表达模式呈现相同规律(图8), 这意味着IbSUT3可能以相同的机制响应这3种胁迫处理。IbSUT3基因在非生物胁迫下的诱导表达, 表明该基因在甘薯抗逆中发挥重要作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1146/annurev.arplant.55.031903.141758URLPMID:15377224 [本文引用: 1]

Sugars and amino acids are generated in plants by assimilation from inorganic forms. Assimilated forms cross multiple membranes on their way from production sites to storage or use locations. Specific transport systems are responsible for vacuolar uptake and release, for efflux from the cells, and for uptake into the vasculature. Detailed phylogenetic analyses suggest that only proton-coupled cotransporters involved in phloem loading have been identified to date, whereas systems for vacuolar transport and efflux still await identification. Novel imaging approaches may provide the means to characterize the cellular events and elucidate whole plant control of assimilate partitioning and allocation.
URLPMID:29138971 [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.jplph.2011.02.005URLPMID:21444123 [本文引用: 1]

Sucrose transporters are crucial to carbon partitioning in higher plants. With their role in loading of sucrose into the phloem they control sucrose distribution throughout the whole plant and drive the osmotic flow system in the phloem. Recently, first insight was obtained on the coordination of sucrose transporter action with plant growth and development. The analysis of transgenic plants with reduced or enhanced expression of sucrose transporters helped to elucidate their physiological function and regulation in detail and connections to light and hormone signalling pathways were discovered. Whereas members of the SUT1 subfamily of sucrose transporters seem to be tightly controlled at the transcriptional and post-translational level in solanaceous plants, other family members show primarily post-transcriptional control of their mRNA stability. Post-translational regulation of sucrose transporters might be affected by direct protein-protein interactions or by recycling of sucrose transporters at the plasma membrane. A model is proposed showing cell-to-cell movement of both the SUT1 mRNA as well as the SUT1 protein via the desmotubule connecting companion cells where transcription of sucrose transporters occurs, and the neighbouring sieve elements. We provide an overview over sucrose transporter regulation in Solanum species at the transcriptional, post-transcriptional and post-translational level with emphasis on the many old and new questions surrounding the topic and how they could be answered.
DOI:10.1016/j.tibs.2013.01.003URLPMID:23403214 [本文引用: 1]

The major facilitator superfamily (MFS) is one of the largest groups of secondary active transporters conserved from bacteria to humans. MFS proteins selectively transport a wide spectrum of substrates across biomembranes and play a pivotal role in multiple physiological processes. Despite intense investigation, only seven MFS proteins from six subfamilies have been structurally elucidated. These structures were captured in distinct states during a transport cycle involving alternating access to binding sites from either side of the membrane. This review discusses recent progress in MFS structure analysis and focuses on the molecular basis for substrate binding, co-transport coupling, and alternating access.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:18083796 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:21261761 [本文引用: 1]
URLPMID:23429841 [本文引用: 1]
DOI:10.1104/pp.118.1.59URLPMID:9733526 [本文引用: 1]

In many species translocation of sucrose from the mesophyll to the phloem is carrier mediated. A sucrose/H+-symporter cDNA, NtSUT1, was isolated from tobacco (Nicotiana tabacum) and shown to be highly expressed in mature leaves and at low levels in other tissues, including floral organs. To study the in vivo function of NtSUT1, tobacco plants were transformed with a SUT1 antisense construct under control of the cauliflower mosaic virus 35S promoter. Upon maturation, leaves of transformants expressing reduced amounts of SUT1 mRNA curled downward, and strongly affected plants developed chloroses and necroses that led to death. The leaves exhibited impaired ability to export recently fixed 14CO2 and were unable to export transient starch during extended periods of darkness. As a consequence, soluble carbohydrates accumulated and photosynthesis was reduced. Autoradiographs of leaves show a heterogenous pattern of CO2 fixation even after a 24-h chase. The 14C pattern does not change with time, suggesting that movement of photosynthate between mesophyll cells may also be impaired. The affected lines show a reduction in the development of the root system and delayed or impaired flowering. Taken together, the effects observed in a seed plant (tobacco) demonstrate the importance of SUT1 for sucrose loading into the phloem via an apoplastic route and possibly for intermesophyll transport as well.
URLPMID:24814155 [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:1464305 [本文引用: 1]

Active loading of the phloem with sucrose in leaves is an essential part of the process of supplying non-photosynthetic tissues with carbon and energy. The transport is protein mediated and coupled to proton-symport, but so far no sucrose carrier gene has been identified. Using an engineered Saccharomyces cerevisiae strain, a cDNA from spinach encoding a sucrose carrier was identified by functional expression. Yeast strains that allow the phenotypic recognition of a sucrose carrier activity were constructed by expressing a cytoplasmic invertase from yeast, or the potato sucrose synthase gene, in a strain unable to transport or grow on sucrose due to a deletion in the SUC2 gene. A spinach cDNA expression library established from the poly(A)+ RNA from source leaves of spinach and cloned in a yeast expression vector yielded transformed yeast clones which were able to grow on media containing sucrose as the sole carbon source. This ability was strictly linked to the presence of the spinach cDNA clone pS21. Analysis of the sucrose uptake process in yeast strains transformed with this plasmid show a pH-dependent uptake of sucrose with a Km of 1.5 mM, which can be inhibited by maltose, alpha-phenylglucoside, carbonyl cyanide m-chlorophenylhydrazone and p-chloromercuribenzenesulfonic acid. These data are in accordance with measurements using both leaf discs and plasma membrane vesicles from leaves of higher plants. DNA sequence analysis of the pS21 clone reveals the presence of an open reading frame encoding a protein with a molecular mass of 55 kDa. The predicted protein contains several hydrophobic regions which could be assigned to 12 membrane-spanning regions.(ABSTRACT TRUNCATED AT 250 WORDS)
DOI:10.1093/jxb/erq313URL [本文引用: 1]
DOI:10.1111/j.1365-313x.1994.00421.xURLPMID:8180625 [本文引用: 1]

An improved protocol is reported to isolate and transiently transform mesophyll protoplasts of Arabidopsis thaliana. Transfected leaf protoplasts support high levels of expression of the bacterial reporter gene coding for beta-glucuronidase (GUS), under the control of the cauliflower mosaic virus (CaMV) 35S promoter. Transient expression of GUS activity was monitored spectrophotometrically and reached a maximum between 18 and 48 h after polyethylene glycol (PEG)-mediated DNA uptake. Histochemical staining for GUS activity revealed reproducible transformation frequencies between 40 and 60%, based on the number of protoplasts survived. To demonstrate the applicability of the transient expression system, the subcellular localization of GUS proteins tagged with different nuclear polypeptides was studied in transfected mesophyll protoplasts, revealing nuclear compartmentalization of the chimeric GUS enzymes. Furthermore, Arabidopsis mesophyll protoplasts support auxin-mediated induction of chloramphenicol acetyl-transferase (CAT) activity when transfected with a transcriptional fusion between the CAT reporter gene and the early auxin-inducible PS-IAA4/5 promoter. Hence, the method allows in vivo analysis of promoter activity and subcellular localization of fusion proteins in a homologous transformation system.
[本文引用: 1]
[本文引用: 1]
URLPMID:10948254 [本文引用: 1]
[本文引用: 1]
URLPMID:8306952 [本文引用: 2]

Sucrose is the principal transport form of assimilates in most plants. In many species, translocation of assimilates from the mesophyll into the phloem for long distance transport is assumed to be carrier mediated. A putative sucrose proton cotransporter cDNA has been isolated from potato and shown to be expressed mainly in the phloem of mature exporting leaves. To study the in vivo role and function of the protein, potato plants were transformed with an antisense construct of the sucrose transporter cDNA under control of the CaMV 35S promoter. Upon maturation of the leaves, five transformants that expressed reduced levels of sucrose transporter mRNA developed local bleaching and curling of leaves. These leaves contained > 20-fold higher concentrations of soluble carbohydrates and showed a 5-fold increase in starch content as compared with wild type plants, as expected from a block in export. Transgenic plants with a reduced amount of sucrose carrier mRNA show a dramatic reduction in root development and tuber yield. Maximal photosynthetic activity was reduced at least in the strongly affected transformants. The effects observed in the antisense plants strongly support an apoplastic model for phloem loading, in which the sucrose transporter located at the phloem plasma membrane represents the primary route for sugar uptake into the long distance distribution network.
URLPMID:12529519 [本文引用: 1]
DOI:10.1073/pnas.250473797URLPMID:11087840 [本文引用: 1]

A major question in plant physiology is how the large amount of sucrose made in leaves is transported to the rest of the plant. Although physiological, biochemical, and anatomical investigations have been performed in this field, to date there have been very few genetic studies. Using a reverse genetic screen, we have identified mutant Arabidopsis plants containing transferred DNA insertions in the gene encoding a phloem-specific sucrose transporter, SUC2. SUC2 is thought to function in loading sugar from the apoplast into the conducting sieve tubes. In the homozygous state, these mutations resulted in stunted growth, retarded development, and sterility. The source leaves of mutant plants contained a great excess of starch, and radiolabeled sugar failed to be transported efficiently to roots and inflorescences. These data provide genetic proof that apoplastic phloem loading is critical for growth, development, and reproduction in Arabidopsis and that SUC2 is at least partially responsible for this step.
DOI:10.1104/pp.118.1.59URLPMID:9733526 [本文引用: 1]

In many species translocation of sucrose from the mesophyll to the phloem is carrier mediated. A sucrose/H+-symporter cDNA, NtSUT1, was isolated from tobacco (Nicotiana tabacum) and shown to be highly expressed in mature leaves and at low levels in other tissues, including floral organs. To study the in vivo function of NtSUT1, tobacco plants were transformed with a SUT1 antisense construct under control of the cauliflower mosaic virus 35S promoter. Upon maturation, leaves of transformants expressing reduced amounts of SUT1 mRNA curled downward, and strongly affected plants developed chloroses and necroses that led to death. The leaves exhibited impaired ability to export recently fixed 14CO2 and were unable to export transient starch during extended periods of darkness. As a consequence, soluble carbohydrates accumulated and photosynthesis was reduced. Autoradiographs of leaves show a heterogenous pattern of CO2 fixation even after a 24-h chase. The 14C pattern does not change with time, suggesting that movement of photosynthate between mesophyll cells may also be impaired. The affected lines show a reduction in the development of the root system and delayed or impaired flowering. Taken together, the effects observed in a seed plant (tobacco) demonstrate the importance of SUT1 for sucrose loading into the phloem via an apoplastic route and possibly for intermesophyll transport as well.
[本文引用: 1]
DOI:10.1104/pp.122.4.1447URLPMID:10759540 [本文引用: 1]

In celery (Apium graveolens L.), long-distance transport of reduced carbon occurs both in the form of sucrose (Suc) and mannitol. The presence of mannitol has been related to the resistance of celery to salt stress. To investigate the transport events occurring during salt stress, we have cloned the H(+)/Suc transporter of celery AgSUT1 (A. graveolens Suc uptake transport 1) from a mature leaf cDNA library. The function of the encoded protein was confirmed by expression in yeast. AgSUT1 is a H(+)/Suc transporter with a high affinity for Suc (K(m) of 139 microM). Another closely related cDNA (AgSUT2) was also identified. AgSUT1 is mainly expressed in mature leaves and phloem of petioles, but also in sink organs such as roots. When celery plants were subjected to salt stress conditions (30 d watering with 300 mM NaCl) favoring mannitol accumulation (J.D. Everard, R. Gucci, S.C. Kann, J.A. Flore, W.H. Loescher [1994] Plant Physiol 106: 281-292), AgSUT1 expression was decreased in all organs, but markedly in roots. The results are discussed in relation to the physiology of celery.
