 ,*河南大学生命科学学院/棉花生物学国家重点实验室/植物逆境生物学重点实验室, 河南开封475004
,*河南大学生命科学学院/棉花生物学国家重点实验室/植物逆境生物学重点实验室, 河南开封475004Functional Analysis of Hypocotyl Phototropism Modulated by RPT2-Interacting Protein RIP1 in Arabidopsis thaliana L.
ZHAO Xiang, ZHU Zi-Yi, WANG Xiao-Nan, MU Shi-Chao, ZHANG Xiao ,*Key Laboratory of Plant Stress Biology / State Key Laboratory of Cotton Biology / College of Life Sciences, Henan University, Kaifeng 475004, Henan, China
,*Key Laboratory of Plant Stress Biology / State Key Laboratory of Cotton Biology / College of Life Sciences, Henan University, Kaifeng 475004, Henan, China通讯作者:
第一联系人:
收稿日期:2018-03-21接受日期:2018-08-20网络出版日期:2018-09-26
| 基金资助: |
Received:2018-03-21Accepted:2018-08-20Online:2018-09-26
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (2574KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
赵翔, 朱自亿, 王潇楠, 慕世超, 张骁. 拟南芥RPT2与RIP1互作调节下胚轴向光弯曲的功能鉴定[J]. 作物学报, 2018, 44(12): 1802-1808. doi:10.3724/SP.J.1006.2018.01802
ZHAO Xiang, ZHU Zi-Yi, WANG Xiao-Nan, MU Shi-Chao, ZHANG Xiao.
光作为一个调控植物生长发育的重要环境因子, 主要体现在作为能源参与植物光合作用和作为信号被植物所感受以调控植物的生长周期和优化光捕获等[1,2]。蓝光对植物来说很重要, 因为它可以通过调节植物的光形态建成、植物的运动以及生物钟等诸多生理反应, 来增加植物的光捕获或降低光伤害, 优化植物在极弱或极强光逆境下生长[3,4,5,6,7,8,9]。拟南芥蓝光受体向光素(PHOT1和PHOT2)的C末端含有Ser/Thr蛋白激酶区域[3], N端含有与FMN (flavin mononucleotide)结合对光照、氧气及电压差敏感的2个LOV (light, oxygen, voltage)区, 可调节其C末端激酶活性[4]。蓝光激发引起一个可逆的光循环反应, FMN和LOV区内保守的半胱氨酸之间形成共价结合, 诱导蛋白构象变化, 激活C端激酶区, 引起受体自磷酸化[5], 从而引起相应生理反应, 如植物向光性[6,7]、气孔运动[8]、叶绿体运动[6]、叶片伸展[2,9]等。
蓝光受体PHOT1和PHOT2在介导强蓝光诱导的下胚轴向光弯曲方面表现为功能冗余, 而phot1单突变体向光弯曲增强, phot2单突变体表型与野生型类似[10], 表明PHOT1有介导和抑制下胚轴向光弯曲的双重作用。目前, 关于PHOT1和PHOT2介导的下胚轴向光弯曲信号传递方面的研究已取得一定进展[11,12,13,14,15,16], 然而PHOT1介导的强光抑制反应机制并不清楚[10]。RPT2 (ROOT PHOTOTROPISM2)是一个与NPH3 (NONPHOTOTROPIC HYPOCOTYL3)具有高度同源性的蛋白, 与NPH3属于同一植物蛋白家族, 该家族含有32个成员, N端有一个BTB/POZ区(broad complex, tramtrack, Bric-à-brac/poxvirus and zinc finger), 而C端有一个卷曲螺旋域。已有研究证实NPH3可与PHOT1、PHOT2以及RPT2互作[17,2], 调节植物向光性和叶片伸展与定位等[9,18-19]。
NPH3基因突变拟南芥缺失任何强度蓝光反应[18,20-21], 而RPT2基因突变, 拟南芥仅缺失强蓝光诱导的下胚轴向光弯曲反应, 表现为rpt2单突变体存在光强依赖的下胚轴向光弯曲反应现象, 即随着蓝光强度增加下胚轴弯曲度减小, 高强光不弯曲[11,16,18]。RPT2的表达依赖于光敏色素和隐花色素调控[11,16,22], 但是RPT2调节强光诱导的下胚轴弯曲主要通过与NPH3以及PHOT1形成的复合物来发挥功能的[11,16,18,20]。其中, PHOT1感受蓝光调节NPH3从膜上的解离, RPT2介导NPH3的回膜过程[16,23]。有趣的是, 强蓝光处理时, rpt2-2单突变表型同phot1 phot2和phot2 rpt2-2双突变体一样, 缺失下胚轴向光弯曲, 然而phot1 rpt2-2双突变体恢复向光弯曲[18], 暗示在RPT2上游存在受PHOT1抑制的旁路调节途径。即在rpt2-2背景下突变PHOT1基因(PHOT1调节的强蓝光抑制被解除), phot1 rpt2-2双突变体恢复向光弯曲。
为此, 我们以RPT2蛋白作为诱饵基因, 通过酵母文库筛选, 寻找RPT2相互作用的蛋白, 以期获得介导PHOT1蓝光抑制反应的下游信号分子。目前, 已成功筛选到与RPT2相互作用的蛋白, 对该蛋白的研究将为揭示蓝光受体PHOT1介导和抑制植物向光性的信号转导交叉调控模式提供重要理论依据。
1 材料与方法
1.1 材料与试剂
拟南芥(Arabidopsis thaliana L.) Columbia-0生态型用作野生型对照, 拟南芥突变体种子phot1 (phot1-5)、phot2 (phot2-1), phot1phot2 (phot1-5 phot2-1)和rpt2-2由Ken-ichiro Shimazaki (日本九州大学)惠赠。大肠杆菌(Escherichia coli)菌株DH5α、酵母(Saccharomyces cerevisiae)菌株Y2H、酵母双杂交载体pGADT-7和pGBKT-7, 阳性和阴性对照载体pGADT-7、pGBKT-Lam和pGBKT-53均为本实验室保存; X-α-Gal和Aureobasidin A (AbA)及酵母库购自Clontech公司; 酵母质粒提取试剂盒和各种氨基试剂购自索莱宝公司; KOD-Plus DNA聚合酶试剂盒购自Promega公司; 限制性内切酶Pst I、EcoR I、BamH I、Kpn I和Xba I等购自TaKaRa公司。1.2 酵母库筛选
参照He等[24]的方法进行酵母库筛选。提取拟南芥Col-0的两周幼苗的总RNA反转录为cDNA作为模板, 用RPT2引物RPT2-pGBKT7-LP: 5'-TGCCATG GAGATGGCAACAGAAGGAAAAAAC-3'和RPT2- pGBKT7-RP: 5'-GGCTGCAGTTAAGAGATTGAGA ATCTTCGTCTC-3', 扩增并回收PCR产物, 用Nco I和Pst I酶切后, 与载体pGBKT7-DNA-BD连接, 构建pGBKT7-RPT2载体。分别用pGBKT7-RPT2和空载体pGBKT7转化酵母感受态细胞, 取酵母混悬液涂布于SD/-Leu或SD/-Trp固体平板上, 30℃恒温培养箱中倒置培养3~4 d观察酵母菌的生长, 判断诱饵蛋白对酵母菌株的毒性。以pGBKT7-53/pGADT7-T为阳性对照、pGBKT7-Lam/pGADT7-T为阴性对照, 将pGBKT7-RPT2与pGADT7空载共同转化到酵母菌株Y2H中, 并将菌液涂布在DDO和QDO缺陷型培养基上, 30℃恒温箱中培养3~4 d, 观察两种培养基上菌落生长判断是否有自激活。1.3 酵母双杂交验证
参照Wang等[25]的方法对筛选到的蛋白与RPT2进行酵母双杂交验证。1.4 下胚轴向光弯曲度测量
参照Zhao等[10]的方法测量下胚轴向光弯曲度, 将黄化3 d生长约5~8 mm的拟南芥幼苗用镊子小心移至0.8% MS培养基上, 使下胚轴与根部都紧贴培养基表面, 垂直放于23℃暗室, 水平单侧蓝光照射12 h。用数码相机照相, 在电脑上用电子软件E-尺测量弯曲度数。试验重复3~5次, 统计其平均值。用t检验进行差异显著性分析。2 结果与分析
2.1 RPT2上游存在受PHOT1抑制的旁路调节下胚轴向光弯曲的途径
phot1单突变体表型同phot1 rpt2-2双突变体和phot1 phot2双突变体一样, 缺失弱蓝光诱导的下胚轴向光弯曲(图1-B, D)[11], 暗示PHOT1介导弱蓝光诱导的下胚轴弯曲。同时发现基因RPT2突变拟南芥极弱蓝光诱导的下胚轴弯曲反应正常(图1-B, D)。有趣的是, rpt2-2单突变体完全缺失强蓝光诱导的下胚轴弯曲反应, 在rpt2-2背景下突变PHOT1构建phot1 rpt2-2双突变体则恢复rpt2-2向光弯曲, 然而rpt2-2背景下突变PHOT2基因并不能恢复向光弯曲(图1-A, C) [18], 暗示在RPT2上游存在受PHOT1抑制的旁路调节途径, 且不受PHOT2调节。为此, 以RPT2为诱饵蛋白, 通过酵母库构建筛选与RPT2互作蛋白, 鉴定互作蛋白功能, 有望解析PHOT1调节强光抑制机制。图1
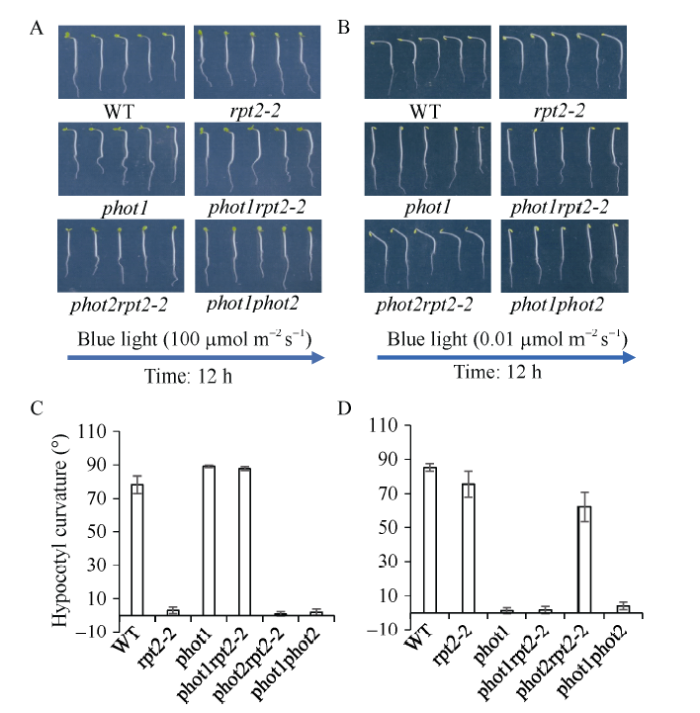 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1单侧强弱蓝光差异诱导拟南芥野生型和突变体下胚轴向光弯曲
A和B: 拟南芥野生型和突变体rpt2-2、phot1、phot1 rpt2-2、phot2 rpt2-2和phot1 phot2响应单侧蓝光(A: 100 μmol m-2 s-1; B: 0.01 μmol m-2 s-1)的下胚轴向光弯曲表型。C和D: 蓝光(C: 100 μmol m-2 s-1; D: 0.01 μmol m-2 s-1)诱导下胚轴向光弯曲度测量统计结果。图中每个数据分别来自3次独立重复试验, 大约15~20颗苗的平均值±标准误。
Fig. 1Hypocotyl phototropic curvature in wild type (WT) and mutant seedlings in response to unilateral blue light at the indicated fluence rates
A and B: phototropic phenotype of WT, rpt2-2, phot1, phot1 rpt2-2, phot2 rpt2-2, and phot1 phot2 to 100 μmol m-2 s-1 (A) or 0.01 μmol m-2 s-1 (B) unilateral blue light for 12 h. C and D: bar graph of the phototropic curvature of seedlings from A (C) or B (D). Each column represents an average of three experiments (15-20 measurements each) ±SD.
2.2 RPT2诱饵蛋白表达载体的构建及诱饵蛋白载体的毒性和自激活活性检测
将pGBKT7-RPT2连接产物转化大肠杆菌后, 采用载体两端的引物进行菌落PCR分析, 得到大小为1000~2000 bp的PCR产物(图2-A), 由于RPT2的cDNA全长为1782 bp, 表明得到的克隆为潜在的阳性克隆。将该潜在阳性克隆进行摇菌培养, 提取质粒后进行双酶切检测。表明, 用Nco I和Pst I双酶切得到的片段约为1800 bp, 符合预期的片段大小(图2-A)。重组酵母双杂交载体pGBKT7-RPT2转化Y2H酵母菌株生长状况与阳性对照载体pGBKT7转化后的酵母菌生长状况基本一致, 形成的菌落直径约为1.5~2.0 mm, 表明融合蛋白对Y2H酵母菌没有毒性作用(图2-B)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2拟南芥RPT2互作蛋白酵母库筛选体系建立
A: 重组酵母双杂交载体的构建; B: 表达蛋白的毒性检测; C: 酵母库筛选诱饵蛋白自激活验证; D: 酵母库筛选结果显色分析。
Fig. 2Screening of proteins interacted with RPT2 by yeast two hybrid
A: construction of recombinant yeast two hybrid vector; B: analysis of toxicity of recombinant yeast two-hybrid vector; C: validation of autonomously activate of bait gene; D: the color analysis of vectors of Yeast library screening.
诱饵蛋白自激活检验表明, 阳性对照在DDO和QDO缺陷型培养基上均有单菌落长出, 而阴性对照与pGBKT7-RPT2/pGADT7具有相同的现象, 只有DDO培养基上有单菌落长出, QDO培养基中没有(图2-C)。上述结果说明, 在两种质粒已经确定共转到酵母菌株Y2H的情况下, 诱饵蛋白RPT2没有自激活现象, 可用于酵母库筛选。
2.3 酵母库筛选
按照1.2的实验方法对诱饵蛋白进行了酵母库筛选。将筛选到的酵母菌落在QDO/X-α-Gal/AbA培养基上进行显色分析(图 2-D), 挑取与阳性对照显色相似的单菌落(图中箭头所示)摇菌, 用酵母质粒提取试剂盒提取酵母质粒, 用通用引物(T7和3'AD)进行PCR鉴定, 确定提取的质粒中除了诱饵基因是否还含有捕获基因。测序结果显示, 以RPT2为诱饵蛋白进行酵母库筛选, 得到的潜在互作蛋白, 通过拟南芥网站进行了初步功能分析(表 1)。Table 1
表1
表1RPT2互作蛋白筛选目标蛋白列表
Table 1
| 目的基因 Bait gene | 筛选基因 Prey gene | 功能注释 Annotation |
|---|---|---|
| RIP4/PHOT1 | 蓝光受体PHOT1, 可调节强弱蓝光诱导的下胚轴向光反应, 调节弱光诱导的叶绿体聚光运动、向光性。 Blue light photoreceptor. Mediates blue light-induced phototropism, chloroplast accumulation, stomatal opening, and leaf flattening. | |
| RIP2/KIX8 | 编码KIX8蛋白, 与PPD2相互作用, 调节植物拟分生组织分裂, 调控植物叶片大小发育。 Encodes KIX8. Interacts with PPD2. Regulates meristem division and leaf size. | |
| RIP1 | 功能未知。Unknown protein. | |
| RPT2 | RIP3 | 功能未知。Unknown protein. |
| RIP6 | 核蛋白, 功能未知。Hypothetical nuclear protein. | |
| RIP5/JAC1 | JAC1蛋白, 定位在细胞质及叶绿体中, 参与叶绿体聚光运动但不参与叶绿体避光运动, 同时响应蓝光诱导。 Located in the chloroplast and cytoplasm. Involved in chloroplast accumulation, chloroplast avoidance movement, and cellular response to blue light. |
新窗口打开|下载CSV
2.4 酵母互作验证
为了验证PHOT1、JAC1、RIP1和RIP6蛋白与RPT2相互作用, 构建了相应的酵母载体, 并与RPT2进行酵母双杂交验证。通过多次实验重复, 发现PHOT1与RPT2结合后酵母菌在QDO/AbA/X-α-Gal缺陷型培养基上可显蓝色(图3-A), 验证了PHOT1与RPT2具有相互作用, 这与之前文献报道的一致[16,18]。同样的方法验证RPT2与JAC1、RIP1和RIP6蛋白的相互作用(图3-B, C, D)。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3RPT2与PHOT1、JAC1、RIP1和RIP6蛋白相互作用
A: RPT2与PHOT1相互作用; B: RPT2与RIP1相互作用; C: RPT2与JAC1相互作用; D: RPT2与RIP6相互作用。
Fig. 3RPT2 interacted with PHOT1, JAC1, RIP1, and RIP6 proteins
A: RPT2 interacted with PHOT1; B: RPT2 interacted with RIP1; C: RPT2 interacted with JAC1; D: RPT2 interacted with RIP6.
2.5 酵母库筛选基因表型验证
根据酵母库筛选结果, 定购了部分基因相关的突变体。经纯合体鉴定后, 本研究对突变体在强蓝光(100 μmol m-2 s-1)及弱蓝光(0.01 μmol m-2 s-1)下的下胚轴向光弯曲表型进行分析。由图4可知, 弱蓝光(0.01 μmol m-2 s-1)单侧处理, 明显诱导野生型WT、rpt2-2突变体以及筛选到的基因RIP1对应的突变体rip1-1和rip1-2下胚轴向光弯曲(图4-B, C)。强蓝光(100 μmol m-2 s-1)单侧处理, 与野生型WT相比, rpt2-2单突变体表现缺失向光弯曲(图4-A, C), 与rpt2-2单突变体表型不同, 突变体rip1-1和rip1-2表现下胚轴向光弯曲类似于野生型, 暗示并没有明显单突变表型(图4-A, C)。为证明突变体rip1-1和rip1-2对应蛋白参与调节蓝光受体PHOT1调节的强蓝光抑制表型, 把突变体rip1-1和rip1-2分别与突变体rpt2-2杂交, 获得rpt2-2 rip1-1和rpt2-2 rip1-2双突变体。检测强蓝光下向光弯曲表型, 发现在突变体rpt2-2背景下突变RIP1基因, 部分地恢复了rpt2-2向光弯曲(图5-A, B), 暗示RIP1蛋白可能参与PHOT1介导强蓝光抑制弯曲调控。图4
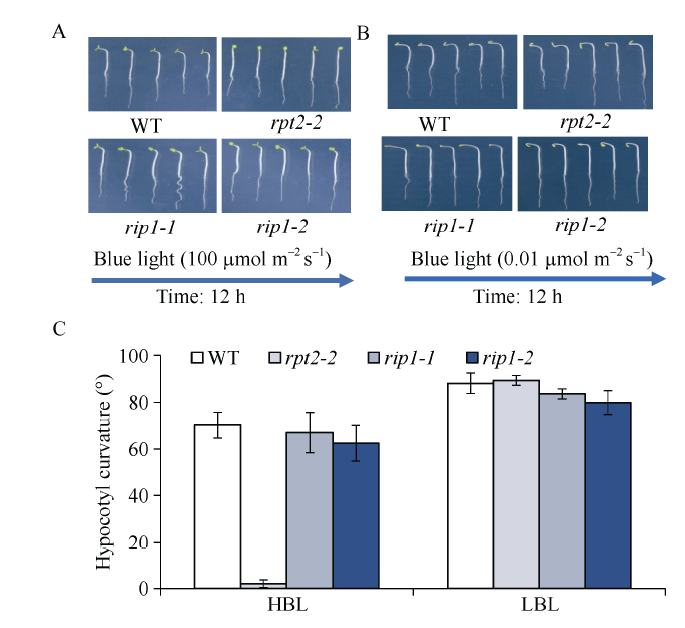 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4拟南芥rip1突变体下胚轴弯曲反应
A和B: 拟南芥野生型和突变体 rpt2-2、rip1-1和rip1-2响应单侧蓝光(A: 100 μmol m-2 s-1; B: 0.01 μmol m-2 s-1)的下胚轴向光弯曲表型; C: 弯曲度的测量统计结果。图中每个数据分别来自3次独立重复试验, 大约15~20颗苗的平均值±标准误。
Fig. 4Hypocotyl phototropism induced by blue light in rip1 mutant of Arabidopsis
A and B: phototropic phenotype of WT, rpt2-2, rip1-1, and rip1-2 in response to blue light (100 μmol m-2 s-1 for A and 0.01 μmol m-2 s-1 for B); C: measurement of phototropic curvature for A and B. The values are the average of three independent experiments (15-20 measurements each) ±SD.
图5
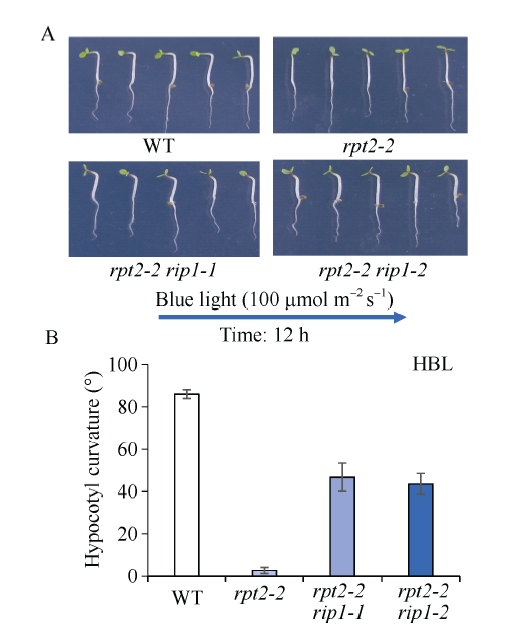 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5拟南芥双突变体rpt2-2 rip1恢复下胚轴向光弯曲反应
A: 拟南芥野生型和突变体 rpt2-2、 rpt2-2 rip1-1和rpt2-2 rip1-2响应单侧蓝光(100 μmol m-2 s-1)的下胚轴向光弯曲表型; B: 弯曲度的测量统计结果。图中每个数据分别来自3次独立重复试验, 大约15~20颗苗的平均值±标准误。
Fig. 5Arabidopsis double mutant rpt2-2 rip1 recovered hypocotyl phototropisim induced by high blue light
A: phototropic phenotype of WT, rpt2-2, rpt2-2 rip1-1, and rpt2-2 rip1-2 in response to 100 μmol m-2 s-1 blue light; B: measurement of phototropic curvature for A. The values are the average of three independent experiments (15-20 measurements each) ±SD.
3 讨论
目前, 研究向光素PHOT1和PHOT2在调控植物运动反应, 如植物向光性、气孔运动、叶绿体运动和叶片的伸展及定位等方面已取得一定的进展[26,27,28]。在植物生长发育过程中, PHOT1和PHOT2既可通过不同的信号通路调节蓝光诱导的不同生理反应, 又可以共同的信号分子调节强蓝光诱导下胚轴向光弯曲实现功能互补[10,26,29]。早期报道称RPT2基因突变导致拟南芥缺失较强蓝光(光照强度大于0.1 μmol m-2 s-1)诱导的下胚轴弯曲反应, 极弱蓝光(0.01 μmol m-2 s-1以下光强)诱导的下胚轴弯曲反应正常[11,18], 暗示RPT2调节较强蓝光诱导的反应。我们知道PHOT1可以介导较宽范围蓝光反应, 而PHOT2仅介导强蓝光(光照强度大于1 μmol m-2 s-1)诱导的反应[10,24,27], 表现出PHOT1和PHOT2调节蓝光诱导的下胚轴弯曲既存在功能冗余又存在差异调节机制。Inada等[18]发现RPT2基因突变导致拟南芥缺失较强蓝光反应, 然而在突变体rpt2-2背景下突变PHOT1基因, 拟南芥恢复强蓝光诱导的下胚轴弯曲反应, 然而rpt2-2背景下突变PHOT2基因并不能恢复向光弯曲(图1-A, C), 说明在RPT2上游存在受PHOT1抑制的旁路调节途径, 该途径并不受PHOT2调节。我们前期研究证实PHOT1单基因突变拟南芥向光弯曲增强, 而PHOT2基因突变部分抑制强蓝光诱导的下胚轴弯曲反应[10], 表明PHOT1确实有介导和抑制下胚轴向光弯曲的双重作用, 然而PHOT1抑制强蓝光的机制并不清楚。基于对在突变体rpt2-2背景下突变PHOT1基因, 拟南芥恢复强蓝光诱导的下胚轴弯曲反应, 我们推测PHOT1和RPT2信号通路应该有调节关系。为此, 本研究以RPT2蛋白作为诱饵基因, 筛选酵母文库, 寻找RPT2互作蛋白, 以期获得介导PHOT1蓝光抑制反应的下游信号分子。目前, 已成功筛选到与RPT2相互作用的蛋白PHOT1、JAC1、RIP1和RIP6。酵母双杂交试验也证实, RPT2可与上述4种蛋白发生相互作用(图3)。检测RPT2互作蛋白对应突变体的下胚轴向光弯曲反应显示, 强蓝光(100 μmol m-2 s-1)单侧处理, 单突变体rip1-1和rip1-2表现下胚轴弯曲正常, 类似于野生型(图4-A, B), 而rpt2-2 rip1-1和rpt2-2 rip1-2这两种突变体表型类似于phot1 rpt2-2双突变体, 恢复拟南芥下胚轴向光弯曲反应(图5-A, B), 暗示突变体rip1-1和rip1-2对应蛋白可能位于蓝光受体PHOT1下游介导强蓝光诱导的下胚轴弯曲抑制反应。此外, 已经证明与RPT2互作的蛋白JAC1参与蓝光诱导的叶绿体聚光运动[27]。基因AT3G24150编码KIX8蛋白, KIX8蛋白属于KIX (KID interaction, KID作用区)蛋白家族, 在拟南芥中该蛋白家族共有11个成员, 在其N端有一个KIX域[30]。KIX8可以与PPD2发生相互作用, 调节植物拟分生组织分裂, 调控植物叶片大小发育[31]。下一步鉴定获得与RPT2互作蛋白的可能生物学功能, 明确其与PHOT1和RPT2调控关系, 将为解析PHOT1介导光抑制的作用机制提供重要的基础。
4 结论
拟南芥rpt2-2突变体缺失强蓝光诱导的下胚轴向光弯曲, 而phot1 rpt2-2双突变体恢复下胚轴向光弯曲, 表明RPT2上游存在受PHOT1抑制的旁路调节途径。筛选到包括JAC1和PHOT1在内的6个与RPT2互作蛋白RIPs, 酵母互作验证显示其中有4个蛋白可以与RPT2相互作用。rip1-1和rip1-2单突变体下胚轴向光弯曲正常, 而rpt2-2 rip1-1和rpt2-2 rip1-2双突变体表型类似于phot1 rpt2-2双突变体, 恢复拟南芥下胚轴向光弯曲反应。初步推测RIP1可能调节PHOT1介导强蓝光抑制反应。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1105/tpc.104.030049URLPMID:15749755 [本文引用: 1]

Phototropins (phot1 and phot2) are plant-specific blue light receptors for phototropism, chloroplast movement, leaf expansion, and stomatal opening. All these responses are thought to optimize photosynthesis by helping to capture light energy efficiently, reduce photodamage, and acquire CO2. However, experimental evidence for the promotion of plant growth through phototropins is lacking. Here, we report dramatic phototropin-dependent effects on plant growth. When plants of Arabidopsis thaliana wild type, the phot1 and phot2 mutants, and the phot1 phot2 double mutant were grown under red light, no significant growth differences were observed. However, if a very low intensity of blue light (0.1 mol m-2s-1) was superimposed on red light, large increases in fresh weight up to threefold were found in those plants that carried functional PHOT1 genes. When the intensity of blue light was increased to 1 mol m-2s-1, the growth enhancement was also found in the phot1 single mutant, but not in the double mutant, indicating that phot2 mediated similar responses as phot1 with a lower sensitivity. The effects occurred under low photosynthetically active radiation in particular. The well-known physiological phototropin-mediated responses, including chloroplast movement, stomatal opening, and leaf expansion, in the different lines tested indicated an involvement of these responses in the blue light-induced growth enhancement. We conclude that phototropins promote plant growth by controlling and integrating a variety of responses that optimize photosynthetic performance under low photosynthetically active radiation in the natural environment.
DOI:10.1104/pp.109.150441URL [本文引用: 3]

In Arabidopsis (Arabidopsis thaliana), the blue light photoreceptor phototropins (phot1 and phot2) fine-tune the photosynthetic status of the plant by controlling several important adaptive processes in response to environmental light variations. These processes include stem and petiole phototropism (leaf positioning), leaf flattening, stomatal opening, and chloroplast movements. The PHYTOCHROME KINASE SUBSTRATE (PKS) protein family comprises four members in Arabidopsis (PKS1 KS4). PKS1 is a novel phot1 signaling element during phototropism, as it interacts with phot1 and the important signaling element NONPHOTOTROPIC HYPOCOTYL3 (NPH3) and is required for normal phot1-mediated phototropism. In this study, we have analyzed more globally the role of three PKS members (PKS1, PKS2, and PKS4). Systematic analysis of mutants reveals that PKS2 (and to a lesser extent PKS1) act in the same subset of phototropin-controlled responses as NPH3, namely leaf flattening and positioning. PKS1, PKS2, and NPH3 coimmunoprecipitate with both phot1-green fluorescent protein and phot2-green fluorescent protein in leaf extracts. Genetic experiments position PKS2 within phot1 and phot2 pathways controlling leaf positioning and leaf flattening, respectively. NPH3 can act in both phot1 and phot2 pathways, and synergistic interactions observed between pks2 and nph3 mutants suggest complementary roles of PKS2 and NPH3 during phototropin signaling. Finally, several observations further suggest that PKS2 may regulate leaf flattening and positioning by controlling auxin homeostasis. Together with previous findings, our results indicate that the PKS proteins represent an important family of phototropin signaling proteins.
DOI:10.1126/science.278.5346.2120URLPMID:9405347 [本文引用: 2]

The NPH1 (nonphototropic hypocotyl 1) gene encodes an essential component acting very early in the signal-transduction chain for phototropism. Arabidopsis NPH1 contains a serine-threonine kinase domain and LOV1 and LOV2 repeats that share similarity (36 to 56 percent) with Halobacterium salinarium Bat, Azotobacter vinelandii NIFL, Neurospora crassa White Collar-1, Escherichia coli Aer, and the Eag family of potassium-channel proteins from Drosophila and mammals. Sequence similarity with a known (NIFL) and a suspected (Aer) flavoprotein suggests that NPH1 LOV1 and LOV2 may be flavin-binding domains that regulate kinase activity in response to blue light-induced redox changes.
DOI:10.1016/j.pbi.2008.09.002URLPMID:18930433 [本文引用: 2]

Higher plants use several classes of blue light receptors to modulate a wide variety of physiological responses. Among them, both the phototropins and members of the Zeitlupe (ZTL) family use light oxygen voltage (LOV) photosensory domains. In Arabidopsis, these families comprise phot1, phot2 and ZTL, LOV Kelch Protein 2 (LKP2), and Flavin-binding Kelch F-box1 (FKF1). It has now been convincingly shown that blue-light-induced autophosphorylation of the phot1 kinase domain is an essential step in signal transduction. Recent experiments also shed light on the partially distinct photosensory specificities of phot1 and phot2. Phototropin signaling branches rapidly following photoreceptor activation to mediate distinct responses such as chloroplast movements or phototropism. Light activation of the LOV domain in ZTL family members modulates their capacity to interact with GIGANTEA (GI) and their ubiquitin E3 ligase activity. A complex between GI and FKF1 is required to trigger the degradation of a repressor of CO ( CONSTANS) expression and thus modulates flowering time. In contrast, light-regulated complex formation between ZTL and GI appears to limit the capacity of ZTL to degrade its targets, which are part of the circadian oscillator.
DOI:10.1146/annurev.arplant.58.032806.103951URL [本文引用: 2]
URL [本文引用: 3]
[本文引用: 2]
DOI:10.1038/414656aURLPMID:11740564 [本文引用: 2]

The stomatal pores of higher plants allow for gaseous exchange into and out of leaves. Situated in the epidermis, they are surrounded by a pair of guard cells which control their opening in response to many environmental stimuli, including blue light. Opening of the pores is mediated by Kaccumulation in guard cells through a Kchannel and driven by an inside-negative electrical potential. Blue light causes phosphorylation and activation of the plasma membrane H-ATPase that creates this potential. Thus far, no blue light receptor mediating stomatal opening has been identified, although the carotenoid, zeaxanthin, has been proposed. Arabidopsis mutants deficient in specific blue-light-mediated responses have identified four blue light receptors, cryptochrome 1 (cry1), cryptochrome 2 (cry2), phot1 and phot2. Here we show that in a double mutant of phot1 and phot2 stomata do not respond to blue light although single mutants are phenotypically normal. These results demonstrate that phot1 and phot2 act redundantly as blue light receptors mediating stomatal opening.
DOI:10.1073/pnas.1336802100URLPMID:166272 [本文引用: 3]

Phototropins (phot1 and phot2) are blue light (BL) receptors that mediate phototropism, chloroplast movements, and stomatal opening in Arabidopsis thaliana. Physiological studies have suggested that Ca2+in the cytoplasm plays a pivotal role in these BL-induced responses. A phot1-mediated increase in cytosolic Ca2+was reported in deetiolated seedlings of A. thaliana; however, the contribution of phot2 remains unknown. We examined a BL-induced transient increase in cytosolic free Ca2+in leaves of transgenic A. thaliana of WT plants, phot1 and phot2 mutants, and phot1 phot2 double mutants expressing the Ca2+-sensitive luminescent protein aequorin. phot1 and phot2 had different photosensitivities: phot1 increased cytosolic Ca2+at lower fluence rates$(0.1\!-\!50\>\mu mol\!\cdot\!m^{-2}\!\cdot\!s^{-1})$and phot2 increased it at higher fluence rates$(1\!-\!250\>\mu mol\!\cdot\!m^{-2}\!\cdot\!s^{-1})$. By using Ca2+channel blockers, Ca2+chelating agents, and inhibitors of phospholipase C, we further demonstrated that both phot1 and phot2 could induce Ca2+influx from the apoplast through the Ca2+channel in the plasma membrane, whereas phot2 alone induced phospholipase C-mediated phosphoinositide signaling, which might result in Ca2+release from internal Ca2+stores. These results suggest that phot1 and phot2 mediate the BL-induced increase in cytosolic free Ca2+differently.
DOI:10.1104/pp.113.216556URLPMID:23674105 [本文引用: 6]

Phototropins (phot1 and phot2), the blue light receptors in plants, regulate hypocotyl phototropism in a fluence-dependent manner. Especially under high fluence rates of blue light (HBL), the redundant function mediated by both phot1 and phot2 drastically restricts the understanding of the roles of phot2. Here, systematic analysis of phototropin-related mutants and overexpression transgenic lines revealed that HBL specifically induced a transient increase in cytosolic Ca2+ concentration ([Ca2+](cyt)) in Arabidopsis (Arabidopsis thaliana) hypocotyls and that the increase in [Ca2+](cyt) was primarily attributed to phot2. Pharmacological and genetic experiments illustrated that HBL-induced Ca2+ increases were modulated differently by phot1 and phot2. Phot2 mediated the HBL-induced increase in [Ca2+](cyt) mainly by an inner store-dependent Ca2+-release pathway, not by activating plasma membrane Ca2+ channels. Further analysis showed that the increase in [Ca2+](cyt) was possibly responsible for HBL-induced hypocotyl phototropism. An inhibitor of auxin efflux carrier exhibited significant inhibitions of both phototropism and increases in [Ca2+](cyt), which indicates that polar auxin transport is possibly involved in HBL-induced responses. Moreover, PHYTOCHROME KINASE SUBSTRATE1 (PKS1), the phototropin-related signaling element identified, interacted physically with phototropins, auxin efflux carrier PIN-FORMED1 and calcium-binding protein CALMODULIN4, in vitro and in vivo, respectively, and HBL-induced phototropism was impaired in pks multiple mutants, indicating the role of the PKS family in HBL-induced phototropism. Together, these results provide new insights into the functions of phototropins and highlight a potential integration point through which Ca2+ signaling-related HBL modulates hypocotyl phototropic responses.
[本文引用: 6]
DOI:10.1016/j.pbi.2005.07.014URL [本文引用: 1]
DOI:10.1105/tpc.105.039669URL [本文引用: 1]
DOI:10.1093/mp/ssm013URLPMID:20031920 [本文引用: 1]

Phototropism represents a differential growth response by which plant organs can respond adaptively to changes in the direction of incident light to optimize leaf/stem positioning for photosynthetic light capture and root growth orientation for water/nutrient acquisition. Studies over the past few years have identified a number of components in the signaling pathway(s) leading to development of phototropic curvatures in hypocotyls. These include the phototropin photoreceptors (phot1 and phot2) that perceive directional blue-light (BL) cues and then stimulate signaling, leading to relocalization of the plant hormone auxin, as well as the auxin response factor NPH4/ARF7 that responds to changes in local auxin concentrations to directly mediate expression of genes likely encoding proteins necessary for development of phototropic curvatures. While null mutations in NPH4/ARF7 condition an aphototropic response to unidirectional BL, seedlings carrying the same mutations recover BL-dependent phototropic responsiveness if co-irradiated with red light (RL) or pre-treated with either ethylene. In the present study, we identify second-site enhancer mutations in the nph4 background that abrogate these recovery responses. One of these mutations—map1 (modifier of arf7 phenotypes 1)—was found to represent a missense allele of AUX1—a gene encoding a high-affinity auxin influx carrier previously associated with a number of root responses. Pharmocological studies and analyses of additional aux1 mutants confirmed that AUX1 functions as a modulator of hypocotyl phototropism. Moreover, we have found that the strength of dependence of hypocotyl phototropism on AUX1-mediated auxin influx is directly related to the auxin responsiveness of the seedling in question.
DOI:10.1104/pp.113.230573URL [本文引用: 1]
DOI:10.1105/tpc.15.00178URLPMID:25873385 [本文引用: 6]

Living organisms adapt to changing light environments via mechanisms that enhance photosensitivity under darkness and attenuate photosensitivity under bright light conditions. In hypocotyl phototropism, phototropin1 (phot1) blue light photoreceptors mediate both the pulse light-induced, first positive phototropism and the continuous light-induced, second positive phototropism, suggesting the existence of a mechanism that alters their photosensitivity. Here, we show that light induction of ROOT PHOTOTROPISM2 (RPT2) underlies photosensory adaptation in hypocotyl phototropism of Arabidopsisthaliana. rpt2 loss-of-function mutants exhibited increased photosensitivity to very low fluence blue light but were insensitive to low fluence blue light. Expression of RPT2 prior to phototropic stimulation in etiolated seedlings reduced photosensitivity during first positive phototropism and accelerated second positive phototropism. Our microscopy and biochemical analyses indicated that blue light irradiation causes dephosphorylation of NONPHOTOTROPIC HYPOCOTYL3 (NPH3) proteins and mediates their release from the plasma membrane. These phenomena correlate closely with the desensitization of phot1 signaling during the transition period from first positive phototropism to second positive phototropism. RPT2 modulated the phosphorylation of NPH3 and promoted reconstruction of the phot1-NPH3 complex on the plasma membrane. We conclude that photosensitivity is increased in the absence of RPT2 and that this results in the desensitization of phot1. Light-mediated induction of RPT2 then reduces the photosensitivity of phot1, which is required for second positive phototropism under bright light conditions.
DOI:10.1073/pnas.0603799103URLPMID:16777956 [本文引用: 1]

Phototropism, or plant growth in response to unidirectional light, is an adaptive response of crucial importance. Lateral differences in low fluence rates of blue light are detected by phototropin 1 (phot1) in Arabidopsis. Only NONPHOTOTROPIC HYPOCOTYL 3 (NPH3) and root phototropism 2, both belonging to the same family of proteins, have been previously identified as phototropininteracting signal transducers involved in phototropism. PHYTOCHROME KINASE SUBSTRATE (PKS) 1 and PKS2 are two phytochrome signaling components belonging to a small gene family in Arabidopsis (PKS1-PKS4). The strong enhancement of PKS1 expression by blue light and its light induction in the elongation zone of the hypocotyl prompted us to study the function of this gene family during phototropism. Photobiological experiments show that the PKS proteins are critical for hypocotyl phototropism. Furthermore, PKS1 interacts with photl and NPH3 in vivo at the plasma membrane and in vitro, indicating that the PKS proteins may function directly with photl and NPH3 to mediate phototropism. The phytochromes are known to influence phototropism but the mechanism involved is still unclear. We show that PKS1 induction by a pulse of blue light is phytochrome A-dependent, suggesting that the PKS proteins may provide a molecular link between these two photoreceptor families.
DOI:10.1105/tpc.019901URLPMID:15031408 [本文引用: 9]

Phototropin 1 (phot1) and phot2, which are blue light receptor kinases, function in blue light-induced hypocotyl phototropism, chloroplast relocation, and stomatal opening in Arabidopsis (Arabidopsis thaliana). Previous studies have shown that the proteins RPT2 (for ROOT PHOTOTROPISM2) and NPH3 (for NONPHOTOTROPIC HYPOCOTYL3) transduce signals downstream of phototropins to induce the phototropic response. However, the involvement of RPT2 and NPH3 in stomatal opening and in chloroplast relocation mediated by phot1 and phot2 was unknown. Genetic analysis of the rpt2 mutant and of a series of double mutants indicates that RPT2 is involved in the phot1-induced phototropic response and stomatal opening but not in chloroplast relocation or phot2-induced movements. Biochemical analyses indicate that RPT2 is purified in the crude microsomal fraction, as well as phot1 and NPH3, and that RPT2 makes a complex with phot1 in vivo. On the other hand, NPH3 is not necessary for stomatal opening or chloroplast relocation. Thus, these results suggest that phot1 and phot2 choose different signal transducers to induce three responses: phototropic response of hypocotyl, stomatal opening, and chloroplast relocation.
DOI:10.1093/mp/ssm001URLPMID:20031912 [本文引用: 1]

Appropriate leaf positioning is essential for optimizing photosynthesis and plant growth. However, it has not been elucidated how green leaves reach and maintain their position for capturing light. We show here the regulation of leaf positioning under blue light stimuli. When 1-week-old Arabidopsis seedlings grown under white light were transferred to red light (25 μmol m612 s611) for 5 d, new petioles that appeared were almost horizontal and their leaves were curled and slanted downward. However, when a weak blue light from above (0.1 μmol m612 s611) was superimposed on red light, the new petioles grew obliquely upward and the leaves were flat and horizontal. The leaf positioning required both phototropin1 (phot1) and nonphototropic hypocotyl 3 (NPH3), and resulted in enhanced plant growth. In an nph3 mutant, neither optimal leaf positioning nor leaf flattening by blue light was found, and blue light-induced growth enhancement was drastically reduced. When blue light was increased from 0.1 to 5 μmol m612 s611normal leaf positioning and leaf flattening were induced in both phot1 and nph3 mutants, suggesting that phot2 signaling became functional and that the signaling was independent of phot1 and NPH3 in these responses. When plants were irradiated with blue light (0.1 μmol m612 s611) from the side and red light from above, the new leaves became oriented toward the source of blue light. When we transferred these plants to both blue light and red light from above, the leaf surface changed its orientation to the new blue light source within a few hours, whereas the petioles initially were unchanged but then gradually rotated, suggesting the plasticity of leaf positioning in response to blue light. We showed the tissue expression of NPH3 and its plasma membrane localization via the coiled-coil domain and the C-terminal region. We conclude that NPH3-mediated phototropin signaling optimizes the efficiency of light perception by inducing both optimal leaf positioning and leaf flattening, and enhances plant growth.
.
DOI:10.1126/science.286.5441.961URLPMID:10542152 [本文引用: 2]

Phototropism of Arabidopsis thaliana seedlings in response to a blue light source is initiated by nonphototropic hypocotyl 1 (NPH1), a light-activated serinethreonine protein kinase. Mutations in three loci [NPH2, root phototropism 2 (RPT2), and NPH3] disrupt early signaling occurring downstream of the NPH1 photoreceptor. The NPH3 gene, now cloned, encodes a NPH1-interacting protein. NPH3 is a member of a large protein family, apparently specific to higher plants, and may function as an adapter or scaffold protein to bring together the enzymatic components of a NPH1-activated phosphorelay.
DOI:10.3724/SP.J.1006.2015.00585URL [本文引用: 1]

phot1pignaling . The gene turned out to be the allelic of lost the phototropism to unilateral high
DOI:10.3724/SP.J.1006.2015.00585URL [本文引用: 1]

phot1pignaling . The gene turned out to be the allelic of lost the phototropism to unilateral high
DOI:10.1111/j.1365-313X.2010.04180.xURLPMID:20202166 [本文引用: 1]

Unilateral blue-light irradiation activates phototropin (phot) photoreceptors, resulting in asymmetric distribution of the phytohormone auxin and induction of a phototropic response in higher plants. Other photoreceptors, including phytochrome (phy) and cryptochrome (cry), have been proposed as modulators of phototropic responses. We show here that either phy or cry is required for hypocotyl phototropism in Arabidopsis thaliana under high fluence rates of blue light, and that constitutive expression of ROOT PHOTOTROPISM 2 (RPT2) and treatment with the phytohormone gibberellin (GA) biosynthesis inhibitor paclobutrazol partially and independently complement the non-phototropic hypocotyl phenotype of the phyA cry1 cry2 mutant under high fluence rates of blue light. Our results indicate that induction of RPT2 and reduction in the GA are crucial for hypocotyl phototropic regulation by phy and cry. We also show that GA suppresses hypocotyl bending via destabilization of DELLA transcriptional regulators under darkness, but does not suppress the phototropic response in the presence of either phyA or cryptochromes, suggesting that these photoreceptors control not only the GA content but also the GA sensing and/or signaling that affects hypocotyl phototropism. The metabolic and signaling regulation of not only auxin but also GA by photoreceptors therefore appears to determine the hypocotyl growth pattern, including phototropic and gravitropic responses and inhibition of hypocotyl elongation, for adaptation to various light environments.
DOI:10.1111/jipb.12639URLPMID:29393576 [本文引用: 1]

Abstract Two redundant blue-light receptors, known as phototropins (phot1 and phot2), influence a variety of physiological responses, including phototropism, chloroplast positioning, and stomatal opening in Arabidopsis thaliana. Whereas phot1 functions in both low- and high-intensity blue light (HBL), phot2 functions primarily in HBL. Here, we aimed to elucidate phot2-specific functions by screening for HBL-insensitive mutants among mutagenized Arabidopsis phot1 mutants. One of the resulting phot2 signaling associated (p2sa) double mutants, phot1 p2sa2, exhibited phototropic defects that could be restored by constitutively expressing NON-PHOTOTROPIC HYPOCOTYL 3 (NPH3), indicating that P2SA2 was allelic to NPH3. It was observed that NPH3-GFP signal mainly localized to and clustered on the plasma membrane in darkness. This NPH3 clustering on the plasma membrane was not affected by mutations in genes encoding proteins that interact with NPH3, including PHOT1, PHOT2 and ROOT PHOTOTROPISM 2 (RPT2). However, the HBL irradiation-mediated release of NPH3 proteins into the cytoplasm was inhibited in phot1 mutants and enhanced in phot2 and rpt2-2 mutants. Furthermore, HBL-induced hypocotyl phototropism was enhanced in phot1 mutants and inhibited in the phot2 and rpt2-2 mutants. Our findings indicate that phot1 regulates the dissociation of NPH3 from the plasma membrane, whereas phot2 mediates the stabilization and relocation of NPH3 to the plasma membrane to acclimate to HBL.
DOI:10.3864/j.issn.0578-1752.2014.24.005URL [本文引用: 2]

利用酵母双杂交系统筛选与苹果褪绿叶斑病毒(Apple chlorotic leaf spot virus,ACLSV)外壳蛋白(coat protein,CP)互作的寄主因子,了解其在病毒与寄主互作过程中可能发挥的作用.首先构建ACLSV CP基因的无白激活性、无毒性的酵母双杂交诱饵载体pGBK77-CP;然后通过顺序转化的方法白前期已构建好的苏俄苹果(Malus sylvestriscv.R12740-7A) cDNA文库中筛选互作因子,在GenBank中对互作寄主因子进行BLAST分析,根据基因注释推断其在病毒与寄主互作过程中可能发挥的作用.本实验成 功构建了酵母双杂交诱饵载体pGBKT7 -CP;筛选到了69个互作寄主基因,包括转录因子活性、碳固定活性、细胞内铁离子平衡、防御反应、水解酶活性、氧化酶活性、氧化还原酶活性、光系统Ⅱ组 件、绑定蛋白等10类蛋白,其中,8个寄主基因可能在病毒与寄主互作过程中起重要作用.分析筛选到的重要寄主蛋白功能,推测ACLSV CP与BZIP类、MYB类转录因子、病程相关蛋白(PR-5、PR-8、PR-10)互作,可能调控了寄主对病毒的抗性作用;与光系统Ⅱ (PSⅡ)的稳定性/装配因子蛋白、铁结合蛋白互作,影响植物光系统的稳定性及叶绿素的形成,从而降低光合作用.这将为揭示ACLSV导致寄主产生褪绿叶 斑及树体衰退的原因提供理论依据.
DOI:10.3864/j.issn.0578-1752.2014.24.005URL [本文引用: 2]

利用酵母双杂交系统筛选与苹果褪绿叶斑病毒(Apple chlorotic leaf spot virus,ACLSV)外壳蛋白(coat protein,CP)互作的寄主因子,了解其在病毒与寄主互作过程中可能发挥的作用.首先构建ACLSV CP基因的无白激活性、无毒性的酵母双杂交诱饵载体pGBK77-CP;然后通过顺序转化的方法白前期已构建好的苏俄苹果(Malus sylvestriscv.R12740-7A) cDNA文库中筛选互作因子,在GenBank中对互作寄主因子进行BLAST分析,根据基因注释推断其在病毒与寄主互作过程中可能发挥的作用.本实验成 功构建了酵母双杂交诱饵载体pGBKT7 -CP;筛选到了69个互作寄主基因,包括转录因子活性、碳固定活性、细胞内铁离子平衡、防御反应、水解酶活性、氧化酶活性、氧化还原酶活性、光系统Ⅱ组 件、绑定蛋白等10类蛋白,其中,8个寄主基因可能在病毒与寄主互作过程中起重要作用.分析筛选到的重要寄主蛋白功能,推测ACLSV CP与BZIP类、MYB类转录因子、病程相关蛋白(PR-5、PR-8、PR-10)互作,可能调控了寄主对病毒的抗性作用;与光系统Ⅱ (PSⅡ)的稳定性/装配因子蛋白、铁结合蛋白互作,影响植物光系统的稳定性及叶绿素的形成,从而降低光合作用.这将为揭示ACLSV导致寄主产生褪绿叶 斑及树体衰退的原因提供理论依据.
URL [本文引用: 1]

根据NCBI数据库公布的R2R3-MYB类转录因子Sm PAP1基因序列(Gen Bank登录号GU218694.2),构建丹参Sm PAP1的酵母双杂交诱饵表达载体p GBKT7-Sm PAP1,检测p GBKT7-Sm PAP1的自激活活性及其对酵母AH109细胞的毒性。以Sm PAP1为诱饵筛选构建到p GADK7中的丹参c DNA文库,挑取阳性克隆进行测序,并在NCBI数据库中进行BLAST比对分析。利用Uniprot在线网站,对筛选到的60个基因进行了功能注释。对其中4个候选互作蛋白,包括病程相关类蛋白AP2/ERF,氧化还原酶类蛋白Sm C4H,逆境胁迫相关类蛋白Sm WRKY及b HLH转录因子类蛋白Sm MYC2进行回转验证实验。通过酵母双杂交实验,确定Sm PAP1与AP2/ERF类、Sm C4H、Sm WRKY及Sm MYC2相互作用。这些数据为深入探究Sm PAP1调控丹参中酚酸类化合物合成的分子机理提供了参考。
URL [本文引用: 1]

根据NCBI数据库公布的R2R3-MYB类转录因子Sm PAP1基因序列(Gen Bank登录号GU218694.2),构建丹参Sm PAP1的酵母双杂交诱饵表达载体p GBKT7-Sm PAP1,检测p GBKT7-Sm PAP1的自激活活性及其对酵母AH109细胞的毒性。以Sm PAP1为诱饵筛选构建到p GADK7中的丹参c DNA文库,挑取阳性克隆进行测序,并在NCBI数据库中进行BLAST比对分析。利用Uniprot在线网站,对筛选到的60个基因进行了功能注释。对其中4个候选互作蛋白,包括病程相关类蛋白AP2/ERF,氧化还原酶类蛋白Sm C4H,逆境胁迫相关类蛋白Sm WRKY及b HLH转录因子类蛋白Sm MYC2进行回转验证实验。通过酵母双杂交实验,确定Sm PAP1与AP2/ERF类、Sm C4H、Sm WRKY及Sm MYC2相互作用。这些数据为深入探究Sm PAP1调控丹参中酚酸类化合物合成的分子机理提供了参考。
DOI:10.1109/9.341804URLPMID:11424903 [本文引用: 2]

The past decade has seen dramatic advances in our knowledge of plant photoreceptors and in our understanding of the signal transduction pathways that they activate (Briggs and Olney, 2001). A major part of these advances has been the identification and characterization of photoreceptors that respond to signals from the blue region of the electromagnetic spectrum. We now know that there are at least two classes of blue light photoreceptors: the cryptochromes and the phototropins. The purpose of this letter is to establish a uniform terminology for the phototropins. Knowledge of their occurrence across the plant kingdom, their structure, and their unique photochemistry is growing at a rapid rate, and it is timely to provide a consistent and simpie nomenclature based on their known properties, both structural and functional. In 1995, Liscum and Briggs identified a genetic locus, designated NPH1 (nonphototropic hypocotyl 1), which encodes a plasma membrane-associated protein known to be essential for most phototropic responses in Arabidopsis. The phototropism mutant JK224, previously described by Khurana and Poff (1989), turned out to be mutated at the NPH1 locus. When plasma membrane preparations from dark-grown seedlings are irradiated before adding ATP, a 120-kD plasma membrane protein becomes heavily phosphorylated. This prorein is present at most in trace amounts in the mutant JK224 (Reymond et al., 1992), providing genetic evidence for its role in phototropism. Cloning and sequencing of the NPH1 gene showed that the encoded protein contains all 11 classic motifs expected of a serine/threonine protein kinase (Hanks and Hunter, 1995), indicating that the protein itself was the kinase involved and that the light-activated reaction was an autophosphorylation event (Huala et al., 1997). When the protein was expressed in insect cells via Baculovirus transfection, it still retained light-activated phosphorylation, leading Christie et al. (1998) to conclude that it was it
DOI:10.1073/pnas.101137598URLPMID:11371609 [本文引用: 3]

UV-A/blue light acts to regulate a number of physiological processes in higher plants. These include light-driven chloroplast movement and phototropism. The NPH1 gene of Arabidopsis encodes an autophosphorylating protein kinase that functions as a photoreceptor for phototropism in response to low-intensity blue light. However, nph1 mutants have been reported to exhibit normal phototropic curvature under high-intensity blue light, indicating the presence of an additional phototropic receptor. A likely candidate is the nph1 homologue, npl1, which has recently been shown to mediate the avoidance response of chloroplasts to high-intensity blue light in Arabidopsis. Here we demonstrate that npl1, like nph1, noncovalently binds the chromophore flavin mononucleotide (FMN) within two specialized PAS domains, termed LOV domains. Furthermore, when expressed in insect cells, npl1, like nph1, undergoes light-dependent autophosphorylation, indicating that npl1 also functions as a light receptor kinase. Consistent with this conclusion, we show that a nph1 npl1 double mutant exhibits an impaired phototropic response under both low- and high-intensity blue light. Hence, npl1 functions as a second phototropic receptor under high fluence rate conditions and is, in part, functionally redundant to nph1. We also demonstrate that both chloroplast accumulation in response to low-intensity light and chloroplast avoidance movement in response to high-intensity light are lacking in the nph1 npl1 double mutant. Our findings therefore indicate that nph1 and npl1 show partially overlapping functions in two different responses, phototropism and chloroplast relocation, in a fluence rate-dependent manner.
DOI:10.1016/S1360-1385(02)02245-8URLPMID:11992825 [本文引用: 1]

Novel family of blue-light receptors, exclusive to plants-photoreceptors that mediate phototropism, chloroplast movement, stomatal opening and probably other plant blue-light responses.
DOI:10.3389/fpls.2016.00561URL [本文引用: 1]

During the course of green plant evolution, numerous light responses have arisen that optimize their growth under fluctuating light conditions. The blue light receptor phototropin mediates several photomovement responses at the tissue, cellular and organelle levels. Chloroplast photorelocation movement is one such photomovement response, and is found not only in most green plants, but also in some red algae and photosynthetic stramenopiles. In general, chloroplasts move toward weak light to maximally capture photosynthetically active radiation (the chloroplast accumulation response), and they move away from strong light to avoid photodamage (the avoidance response). In land plants, chloroplast movement is dependent on specialized actin filaments, chloroplast-actin filaments (cp-actin filaments). Through molecular genetic analysis usingArabidopsis thaliana, many molecular factors that regulate chloroplast photorelocation were identified. In this Perspective, we discuss the evolutionary history of the molecular mechanism for chloroplast photorelocation movement in green plants in view of cp-actin filaments.
DOI:10.1007/s00438-013-0753-9URLPMID:23756993 [本文引用: 1]

The KIX domain, which mediates protein-protein interactions, was first discovered as a motif in the large multidomain transcriptional activator histone acetyltransferase p300/CBP. Later, the domain was also found in Mediator subunit MED15, where it interacts with many transcription factors. In both proteins, the KIX domain is a target of activation domains of diverse transcription activators. It was found to be an essential component of several specific gene-activation pathways in fungi and metazoans. Not much is known about KIX domain proteins in plants. This study aims to characterize all the KIX domain proteins encoded by the genomes of Arabidopsis and rice. All identified KIX domain proteins are presented, together with their chromosomal locations, phylogenetic analysis, expression and SNP analyses. KIX domains were found not only in p300/CBP- and MED15-like plant proteins, but also in F-box proteins in rice and DNA helicase in Arabidopsis, suggesting roles of KIX domains in ubiquitin-mediated proteasomal degradation and genome stability. Expression analysis revealed overlapping expression of OsKIX_3, OsKIX_5 and OsKIX_7 in different stages of rice seeds development. Moreover, an association analysis of 136 in silico mined SNP loci in 23 different rice genotypes with grain-length information identified three non-synonymous SNP loci in these three rice genes showing strong association with long- and short-grain differentiation. Interestingly, these SNPs were located within KIX domain encoding sequences. Overall, this study lays a foundation for functional analysis of KIX domain proteins in plants.
[本文引用: 1]
