 ,1,*, 郭秀林
,1,*, 郭秀林 ,1,*
,1,*Characterization and Regulatory Roles in Thermotolerance of Wheat Heat Shock Transcription Factor Gene TaHsfA2e
ZHANG Yu-Jie1,2,**, ZHANG Yuan-Yuan1,3,**, ZHANG Hua-Ning1, QIN Ning1,2, LI Guo-Liang ,1,*, GUO Xiu-Lin
,1,*, GUO Xiu-Lin ,1,*
,1,*通讯作者:
第一联系人:
收稿日期:2018-04-19接受日期:2018-07-20网络出版日期:2018-08-02
| 基金资助: |
Received:2018-04-19Accepted:2018-07-20Online:2018-08-02
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (3012KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张玉杰, 张园园, 张华宁, 秦宁, 李国良, 郭秀林. 小麦热激转录因子基因TaHsfA2e特性及耐热性功能初探[J]. 作物学报, 2018, 44(12): 1818-1828. doi:10.3724/SP.J.1006.2018.01818
ZHANG Yu-Jie, ZHANG Yuan-Yuan, ZHANG Hua-Ning, QIN Ning, LI Guo-Liang, GUO Xiu-Lin.
小麦生育期内经常遭受高温和干旱双重胁迫, 导致产量和品质下降。然而, 作物对生长逆境有一定的适应能力。适当的高温锻炼能诱导植株体内大量热激蛋白和保护酶等相关基因的表达而获得耐热性, 从而更好地适应高温环境[1]。热激转录因子(Hsf)在这一过程中起重要的调控作用, 因Hsf能够与热激蛋白(Hsp)或其他相关蛋白启动子区域的热激元件(Hse)结合而直接激活下游基因的表达, 启动热激反应, 因此成为生物体在热胁迫和其他逆境胁迫下基因转录激活信号转导途径中重要的调节因子, 在传递逆境信号尤其是热胁迫信号以及提高植物抗逆性方面发挥重要的调控作用[2,3,4]。Hsf在植物中普遍存在, 自20世纪80年代后期酵母Hsf基因被克隆以来, 已相继获得多个物种的Hsf基因[5,6,7,8,9,10,11,12]。植物Hsf基因属于多基因家族, 依据其结构可分为3个家族A、B和C, 不同家族又分为多个亚族。Hsf家族基因数目因物种不同差异较大, 酵母和果蝇中各有1个, 脊椎动物中有4个[13], 而植物中数量较多, 如拟南芥中21个[14], 番茄中16个[15], 玉米中30个[16]。
前人对A2亚族基因的研究主要集中在模式植物番茄和拟南芥, 对HsfA2功能的了解非常有限, 近年来对其他作物HsfA2的报道逐渐增加[17,18,19]。番茄HsfA2稳定性强, 严格受热诱导上调表达, 在热胁迫后期及恢复阶段大量积累, HsfA2细胞质定位信号强, 热激条件下转位入核必须依赖与HsfA1结合形成异源寡聚体[20,21]。HsfA1为组成型表达, 在植株抵御热胁迫过程中发挥主要调控作用, HsfA1提高耐热性是通过诱导激活HsfA2和HsfFB1的合成, 进而诱导热激蛋白的表达而实现的[22]。在拟南芥中, Hsf1、Hsf3、HsfA2和HsfA3都与植物耐热性相关, AtHsfA2受热诱导表达最强, 过表达AtHsfA2不仅提高植株基础和获得耐热性, 同时可增强根系愈伤组织的耐盐和耐渗透胁迫能力, AtHsfA2突变导致植株基础和获得耐热性以及抗氧化能力明显降低[23]。拟南芥在长期热胁迫、重复循环热胁迫和热胁迫恢复期都会积累大量的HsfA2, HsfA2表达依赖于HsfA1, AtHsfA2在热激反应的后期, 即植物获得耐热性过程中起重要调控作用[24,25]。通过对AtHsfA1和AtHsfA2功能的深入鉴定, 发现AtHsfA1对诱导AtHsfA2等热激基因表达起主要作用, 而一旦AtHsfA2被激活, 就成为主要的调控因子, 激活下游Hsp的表达[26]。事实上, 在AtHsfA1缺失条件下, AtHsfA2也可从细胞质进入细胞核, 行使转录激活功能, 调控一系列Hsp或伴侣基因的表达[27]。AtHsfA1和AtHsfA2的功能既存在相同点又具特异性, AtHsfA1的功能主要集中在胁迫初期的转录起始方面, 而AtHsfA2主要激活氧稳态、碳水化合物和脂类代谢相关基因的表达, 从而维持热胁迫后期细胞的稳定性[28]。在不同热激范围及氧化胁迫中, AtHsfA2可替代AtHsfA1行使功能, 对部分恢复AtHsfA1突变体表型的研究表明, AtHsfA2可能比AtHsfA1参与更广谱的耐热性调控过程[29]。玉米有4个A2亚族成员, ZmHsf04在多个组织器官中组成型表达, 受多种逆境胁迫上调, 在酵母细胞中可被半乳糖诱导, 其表达显著提高转基因酵母的耐热性[30]。
小麦Hsf的研究起步晚, 对其家族基因特性和功能了解甚少。Qin等[31]在研究小麦不同耐热品种热胁迫下的基因表达谱时, 鉴定出6560个热胁迫响应基因, 包括7个Hsf基因, 其中一个在40℃热胁迫后上调表达128倍, 暗示该类基因在小麦耐热性中的调控作用。小麦TaHsfA4a的表达受镉胁迫上调, 参与小麦耐镉反应[32]; 转TaHsfB2d酵母细胞的耐热性显著增强, 过表达B2亚族TaHsf3可增强拟南芥植株耐热性和抗冻性[33]; 转TaHsfA2d拟南芥植株表现出较强的耐中等高温、耐盐和抗旱性, 中等高温条件下转基因植株能积累相对较高的生物量和产量[34]。Xue等[5]通过电子表达谱从小麦中识别出56个Hsf成员, A2、B2和A6亚族基因受热激明显上调表达, TaHsfA6f作为转录激活因子直接调控小麦TaHsps、TaGAAP和TaRof1类基因, 并增强植株耐热性。正常生长条件下, 4个小麦A2亚族Hsf在胚乳中高表达, 同时伴随着Hsp基因的表达, 暗示A2亚族基因在小麦耐热过程中对下游热激基因的调控作用[5]。小麦3个TaHsfC2a同源基因在灌浆期高表达, 被热胁迫和ABA显著上调, 其过表达可上调一系列旱、热和ABA诱导基因的表达[35]。本实验室近期研究发现, 小麦Hsf家族至少有81个成员, A2亚族至少有18个, RNA-seq分析表明, 多数A2亚族成员的表达受高温和H2O2上调(未发表)。本研究在对玉米A2亚族基因特性及耐热性调控功能研究[36,37,38]的基础上, 克隆了小麦A2亚族成员, 并比较不同作物相同亚族成员之间特性与功能异同, 为加速大田作物Hsf家族基因研究提供理论依据和技术支撑。
1 材料与方法
1.1 材料及处理
供试小麦品种为沧6005, 半冬性, 晚熟, 耐热性较强, 由河北省沧州市农业科学院提供。健康饱满的种子经0.1% HgCl2表面消毒及自来水反复冲洗后, 浸泡吸胀12 h, 用Hoagland营养液于25℃培养箱中培养。待幼苗长至二叶一心时, 参照李慧聪等[37]描述的方法进行热胁迫处理, 方法略有改进。将生长一致的幼苗分成3组, 第1组于37℃培养箱中进行热胁迫处理(营养液于37℃下提前预热), 第2组在培养液中加入水杨酸(终浓度0.8 mmol L-1), 第3组在培养液中加入H2O2 (终浓度10 mmol L-1), 各组处理时间均为30、60、90、120和240 min。剪取所有处理的第二展开叶, 液氮速冻后用于基因定量分析。25℃正常生长的幼苗作对照。
2016年10月中旬在河北省农林科学院大河试验站进行田间试验, 常规种植, 于不同生育期取样, 液氮中速冻后用于不同组织器官相关基因的表达量分析。
1.2 TaHsfA2e的cDNA序列扩增与测序
采用RNArose Reagent Systems试剂盒(上海华舜生物工程公司)提取总RNA, 经DNase I (TaKaRa)处理除去残留的基因组DNA, 然后取1 μg RNA, 用SuperScript IV First-Strand Synthesis System反转录试剂盒(Invitrogen)合成cDNA第1链。用DNAman设计特异引物(F: 5′-CACTGTGCTGAG CAATTCTTTCTTTG-3′; R: 5′-GGCCTGAGTATGA ACTAATTAAGA-3′)。PCR体系25 μL, 含10× Pyrobest buffer 2.5 μL、dNTP mixture (2.5 mmol L-1) 2 μL、1st strand cDNA 2 μL、引物(20 μmol L-1)各0.25 μL、Pyrobest DNA polymerase (TaKaRa) 0.25 μL和ddH2O 17.75 μL。反应程序为98℃ 10 s, 55℃ 15 s, 72℃ 2 min, 30个循环。将扩增产物连接T载体后(pEasy-Blunt Simple Cloning Kit, TransGen Biotech), 送上海生工生物工程技术服务有限公司测序, 测3个重复克隆。1.3 小麦qRT-PCR分析
依据Xue等[5]对小麦Hsf基因的分析结果, 设计TaHsfA2e的特异引物(F: 5′-TACTCTGATGATCT TAATTAG-3′; R: 5′-GCACACTACACCAAAGGCCT C-3′)。PCR体系20 μL, 含SYBR Premix Ex Taq II 10 μL、10 μmol L-1引物各0.8 μL、1st strand cDNA 1 μL和ddH2O 7.4 μL。在7500 Real-time PCR System (Applied Biosystems, USA)上进行扩增, 反应程序为预变性95℃ 10 min; 变性95℃ 5 s; 退火/延伸60℃ 1 min, 45个循环。采用2-ΔΔCt法计算基因相对表达水平, 内参基因为TaRP15 (F: 5°-GCACACGTGCTT TGCAGATAAG-3°; R: 5°-GCCCTCAAGCTCAAC CATAACT-3°)。每组试验3个生物学样本, 每个生物学样本3次技术重复。数据为3个生物学重复的平均值±标准误, 组织表达试验以幼根表达量为1, 其余试验以0 min的表达量为1。1.4 TaHsfA2e亚细胞定位分析
带有绿色荧光蛋白(GFP)基因的植物表达载体pJIT163-hGFP通过35S启动子驱动目的基因和GFP基因的融合表达[39], 显示目的蛋白的亚细胞定位。利用设计的特异引物(F: 5′-TGGAGAGGACAG CCCAAGCTTATGGACCGGGTGCTGCTG-3′; R: 5′- GCCCTTGCTCACCATGGATCCCTACGCGTCGAAACAT-3′)扩增TaHsfA2e编码序列, 产物经限制性内切酶Hind III和BamH I消化后, 连接到表达载体pJIT163-hGFP上。参照李慧聪等[37]描述的方法进行金粉包埋和基因枪转化。经瞬时表达处理的洋葱表皮细胞于22℃暗培养16 h, 然后放入浓度为10 μg mL-1的4’,6-二脒基-2-苯基吲哚(4’,6-diam idino-2-ph enylindole, DAPI)染色液中染色3~5 min, 用生理盐水冲洗干净, 于激光共聚焦显微镜(Zeiss META510)下观察荧光。1.5 TaHsfA2e转化酵母及耐热性鉴定
1.5.1 酵母表达载体的构建 酵母表达载体pYES2 (Invitrogen, 美国)用于酿酒酵母中目的蛋白的表达, 其特点在于GAL1启动子能够在酿酒酵母中被半乳糖高水平诱导从而驱动目的蛋白表达, 同时能够被葡萄糖抑制表达, 可利用URA3基因筛选带有ura3基因型的酵母宿主菌株转化子。结合ClonExpress II重组反应系统(诺唯赞生物科技有限公司)设计扩增TaHsfA2e特异引物(F: 5′-GGGA ATATTAAGCTTGGTACCATGGACCGGGTGCTGCTG-3′; R: 5′-TGATGGATATCTGCAGAATTCCTACG CGTCGAAACAT-3′), 利用高保真PCR聚合酶扩增, 获得TaHsfB2d的PCR产物。利用限制性内切酶Kpn I和EcoR I (NEB)对载体pYES2进行酶切, 电泳后回收得到线性化载体。将PCR产物与线性化载体按 1:2的摩尔比混合, 利用ClonExpress II快速克隆技术进行重组反应。于冰水浴中配制反应体系, 含4 μL 5× ClonExpress II buffer, 50~200 ng线性化载体, 20~200 ng插入片段扩增产物, 2 μL Exnase II, 加无菌水至总体积2 μL。混匀各组分后, 于37℃反应30 min, 立即置冰浴中冷却5 min, 利用热激法将反应产物直接转化大肠杆菌TOP10感受态细胞, 37℃倒置培养过夜。用无菌的牙签将单个菌落挑至100 μL新鲜的LB培养基中, 混匀, 取2 μL作为模板进行PCR扩增, 根据电泳条带大小选择正确的克隆进行序列测定。1.5.2 转TaHsfA2e酵母耐热性鉴定 参照Gietz等[40]的方法, 将测序正确的重组载体转化酵母INVSc1感受态细胞, 然后将细胞均匀涂抹在SC-Glu-Ura-筛选平板上, 30℃培养2~3 d。采用菌落PCR方法鉴定阳性克隆, 分别以重组载体pYES2-TaHsfA2e和空载体pYES2转化酵母细胞INVSc1, 筛选阳性酵母克隆。参照赵立娜等[41]描述的方法进行耐热性鉴定。
1.6 TaHsfA2e转化拟南芥及功能验证
1.6.1 转TaHsfA2e拟南芥植株获得 利用1.3中的特异引物进行RT-PCR扩增, 将扩增产物克隆到T载体上, 并进行序列验证。质粒经Xba I/Sac I消化和纯化后, 构建到双价载体pCAMBIA1300上, 载体侵染农杆菌GV3101后, 通过真空蘸花法转化拟南芥(Arabidopsis thaliana L., 生态型为Columbia), 利用含有25 μg mL-1潮霉素的MS培养基筛选转化植株, 直至纯合, 收获种子。选取过表达纯合株系用于耐热性鉴定。1.6.2 转基因拟南芥耐热性鉴定 将消毒后的拟南芥播种于含25 mL培养基(0.8%琼脂培养基)的培养皿中, 再在培养箱中培养(昼/夜温度为22℃/18℃,光/暗周期16 h/8 h)。取5 d龄幼苗, 分别进行基础耐热性(45℃ 40 min, 再于22℃恢复8 d)和获得耐热性(37℃ 1 h, 22℃下恢复2 d, 46℃ 50 min, 最后22℃恢复8 d)鉴定, 每个株系至少40株幼苗, 3次重复。
经基础耐热性和获得耐热性处理的幼苗, 观察处理后的生长情况并照相; 收集地上部莲座, 测定叶绿素含量, 用DDS-II型电导率仪(上海雷磁仪器厂)测定相对电导率(REC)[38]。
1.6.3 拟南芥热激蛋白基因表达分析 热相关基因及其引物信息见附表1。内参基因为AtActin 8 (F: 5′-GCCAGATCTTCATCGTCGTG-3′; R: 5′-TCTCCA GCGAATCCAGCCTT-3′)。预备实验结果表明, 热处理后22°C下恢复培养0~8 h连续取样测定, 发现处理后0 h和2 h热激蛋白基因出现表达峰值。因此, 本研究选择8 d龄幼苗经基础性和获得热处理后恢复分别0 h和2 h取样。拟南芥转基因株系为12_2, 以野生型(WT)为对照, 对照的相当表达量设为1。
Supplementary table 1
附表 1
附表 1拟南芥耐热性相关基因及其定量分析引物对
Supplementary table 1
| 基因 Gene | 正向序列 Forward sequence (5°-3°) | 反向序列 Reverse sequence (5°-3°) |
|---|---|---|
| AtHsp18.2 | GCAGATTAGCGGAGAGAGGA | CCTTCACTTCTTCCATCTTTGC |
| AtHsp21 | AAGTCCGCTACACCGTTCTC | CCAACAATCCGAAAGGAGAG |
| AtHsfa32 | GCGAAGTTGGTTGAGTGGTT | GGAGGAACTGAGAACAGATTGG |
| AtERDJ3A | CTCCTGTTTGTATCATTGGTGC | TGTGTCCTGAGAACCTGTGG |
| AtHsp25.3 | GACGTCTCTCCTTTCGGATTGT | CTCCACTTCCTCCTCTGTTTCTTC |
| AtHsp70T | TGATTGAGGTGAGGATGCC | CCACTTCAACGACAAACCC |
| AtHsp90 | CCCTCTCTTCTTCATAAATCAACA | CCATCGCAACGAACTTTG |
| AtHsp101 | TGTCTTCAACACTCTGCTCCA | CACTTCCATTGTTACTTTCCCAG |
新窗口打开|下载CSV
2 结果与分析
2.1 小麦热激转录因子TaHsfA2e的cDNA序列分析与亚细胞定位
从经37℃热胁迫处理1.5 h的小麦二叶一心幼苗叶片中克隆获得A2亚族热激转录因子TaHsfA2e (GenBank序列号为MG700614)的完整编码序列, 序列长1026 bp, 编码341个氨基酸残基。序列相似性比对发现, 该蛋白序列与小麦HsfA6f (AIY25733)、小麦HsfA2d (AHB61248)和节节麦HsfA2d-like (XP_020178141)的相似性较高, 分别为96%、94%和94% (图1)。图1
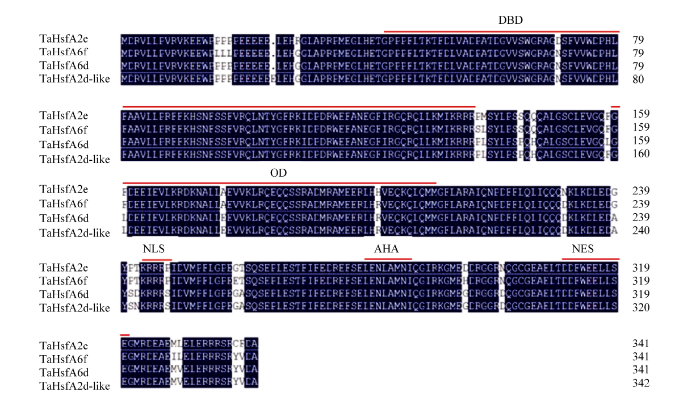 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1小麦TaHsfA2e 的氨基酸序列及其结构域
DBD: Hsf 家族保守DNA 结合域; OD: 寡聚域; NLS: 核定位信号序列; AHA: 激活结构域; NES: 核输出信号序列。
Fig. 1Amino acid sequences of TaHsfA2e from wheat and structure domains
DBD: conserved DNA binding domain of Hsf family; OD: oligomerization; NLS: nuclear localization signal; AHA: aromatic, large hydrophobic and acidic amino residues; NES: nuclear export signal.
通过构建瞬时表达载体并转化洋葱内表皮, 借助激光共聚焦显微镜观察发现, 正常条件下TaHsfA2e定位在细胞核(图2)。
图2
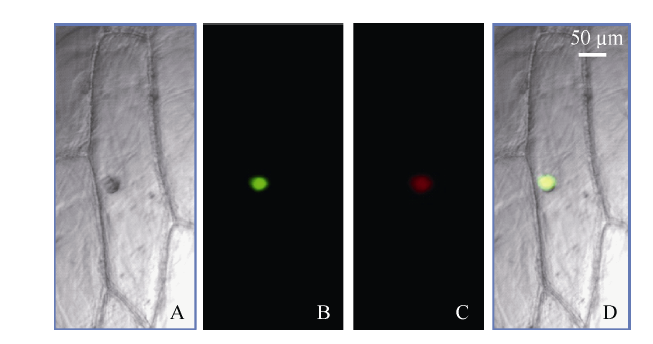 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2小麦TaHsfA2e 蛋白质在洋葱表皮细胞中亚细胞定位
A: 明场; B: GFP绿色荧光; C:细胞核DAPI 红色荧光; D: 叠加图像。
Fig. 2Subcellular localization of TaHsfA2e in onion epidermal cells under normal growth conditions
A: bright field; B: green fluorescence of GFP; C: red fluorescence of DAPI; D: merged image.
2.2 TaHsfA2e在小麦不同组织器官及逆境胁迫下的表达分析
qRT-PCR分析结果表明, TaHsfA2e除了在小麦成熟种子中表达量较高(约为幼根中的75倍)外, 在其他组织和器官中表达量均非常低(附图1)。附图1
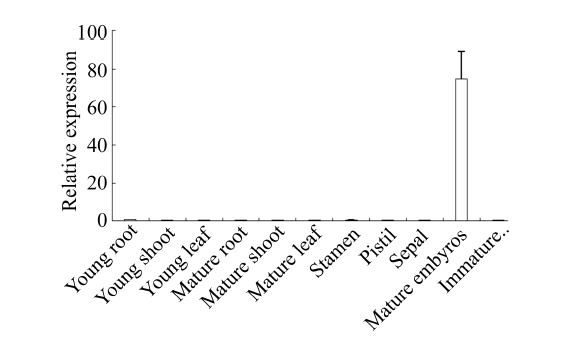 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT附图1正常生长条件下幼苗期和开花期不同组织器官中TaHsfA2e 的相对表达水平
每个样本设3次重复, 数据为3个生物学重复平均值±标准误, 幼根中基因的表达量为对照, 设为1。
Supplementary fig. 1Expression levels of TaHsfA2e in different tissues and organs at seedling and anthesis stages under normal growth conditions
There are three replicates for each sample and the data are mean ± standard error. The relative expression level of TaHsfA2e by qRT-PCR in young roots was set to 1 as controls.
37℃热处理显著上调叶片TaHsfA2e的表达, 处理60 min时达峰值, 最大值达初始值的300倍(图3-A); 0.8 mmol L-1水杨酸(图3-B)和10 mmol L-1 H2O2 (图3-C)单独处理均下调TaHsfA2e的表达。
图3
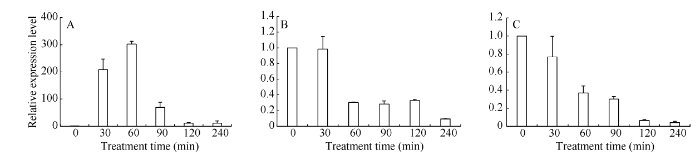 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图337℃热胁迫(A), 0.8 mmol L-1 SA (B)和10 mmol L-1 H2O2 (C)分别处理对小麦叶片TaHsfA2e表达量的影响
数据为3个生物学重复平均值±标准差。0 h作为对照, 相对表达量为“1”。
Fig. 3Changes of TaHsfA2e relative expression levels in leaves of wheat seedlings subject to heat stress at 37 °C (A), 0.8 mmol L-1 SA (B), and 10 mmol L-1 H2O2 (C)
Each bar value represents ±SD of triplicate experiments. The relative expression level of TaHsfA2e by qRT-PCR of 0 h was set to 1 as controls.
2.3 小麦热激转录因子TaHsfA2e在酵母中的耐热性鉴定
通过构建酵母表达载体并将TaHsfA2e在酵母中进行遗传转化, 对阳性菌株进行耐热性鉴定发现, 正常生长条件下, 转TaHsfA2e酵母与转空载体对照酵母菌斑的长势没有明显差异(图4-A)。50℃水浴热处理后, 转TaHsfA2e酵母与对照长势均降低, 但转基因酵母的生长势明显好于对照, 菌液浓度稀释为0.05时, 转空载体酵母菌斑明显减少(图4-B)。从酵细胞生长曲线也可看出, 正常培养条件下转pYES2和pYES2-TaHsfA2e酵母细胞生长较快, 生长速度没有明显差异(图4-C); 50℃热激处理后细胞生长速度均降低, 但处理18 h后转pYES2-TaHsfA2e酵母细胞生长速率明显高于转空载体对照细胞(图4-D), 表明TaHsfA2e能在酵母细胞中被诱导表达, 且能显著提高酵母细胞的耐热性。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图450℃热激处理后转基因TaHsfA2e和空载体酵母细胞耐热性观察
A: 30°C下正常生长, B: 50°C热胁迫 45 min, 于30°C恢复生长 3 d; C: 30°C下正常生长测定酵母细胞OD600值; D: 50°C热胁迫 45 min后于30°C恢复生长测定酵母细胞 OD600值。数据为3个生物学重复平均值±标准差。*表示转基因酵母与对照(转空载体)差异显著(P<0.05)
Fig. 4Thermotolerances of yeast harboring pYES2-TaHsfA2e and pYES2 after heat shock at 50°C
A: culture at 30 °C; B: HS at 50 °C for 45 min, then culture at 30 °C for 3 days; C: OD600 of transformed yeast cells at 30 °C; D: OD600 of transformed yeast cells at 50 °C for 45 min and then culture at 30 °C. The error bar represents ±SD of triplicate experiments. * means significant difference between transgenic yeast cells and the controls (harboring pYES2) (P<0.05)
2.4 小麦热激转录因子TaHsfA2e在拟南芥中的耐热性鉴定
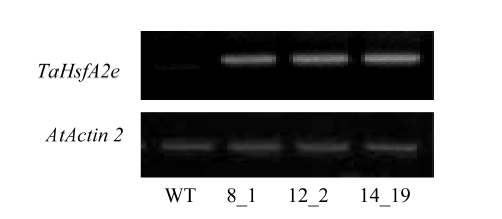
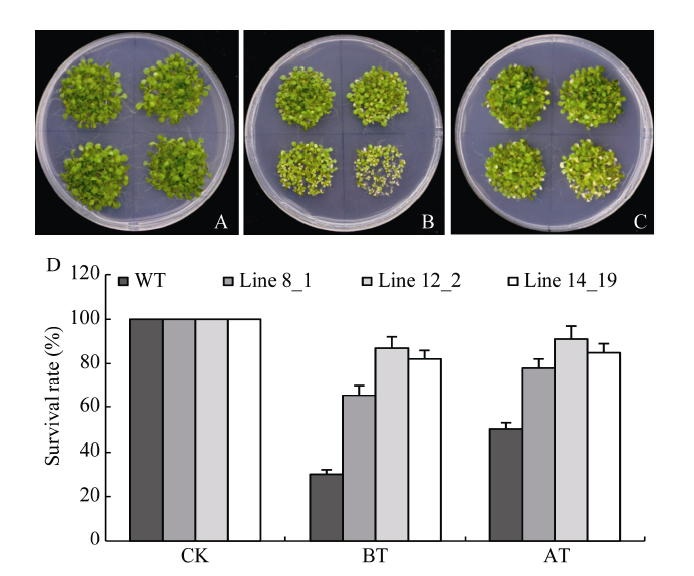
RT-PCR (附图2)和半定量阳性检测证实3个拟南芥株系(8_1、12_2和14_19)为转TaHsfA2e纯合系, 进一步进行耐热性鉴定。正常条件下, 转基因株系与野生型(WT)的生长势没有明显差异(图5-A)。5 d龄幼苗经基础性热处理后, WT地上部明显萎蔫, 而3个转基因株系则保持较好的持绿性, 尤其是株系12_2 (图5-B)。经获得耐热性处理后, 转基因株系表现出类似耐热表型, 均强于野生型(图5-C)。不同热处理后幼苗存活率与上述表型观察结果一致(图5-D)。附图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT附图2RT-PCR检测拟南芥不同转基因株系TaHsfA2e的相对表达
Supplementary fig. 2TaHsfA2e relative expression in different Arabidopsis TaHsfA2e transgenic lines by semi-RT-PCR
The wild type (WT) was used as negative control.
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图53个转TaHsfA2e拟南芥株系及野生型的基础耐热性和获得耐热性鉴定
转基因系(8_1, 12_2, 14_19)和野生型种子播种于平皿培养基上, 经3 d黑暗孵育、光照5 d后分别进行热激处理。A: 正常生长的植株; B: 基础耐热性处理的植株(45℃ 40 min, 再于22℃恢复8 d); C: 获得耐热性处理植株(37℃ 1 h, 22℃下恢复2 d, 46℃ 50 min, 最后22℃恢复8 d); D: 转基因株系和野生型在正常生长(CK)、基础耐热性处理(BT)和获得耐热性处理(AT)条件下的存活率, 实验重复3次, 每重复至少15株苗。
Fig. 5Assays of basal and acquired thermotolerance in three TaHsfA2e-transgenic Arabidopsis and its wild type (WT)
The seeds of TaHsfA2e transgenic Arabidopsis lines (8_1, 12_2, and 14_19) and wild type were planted in plate, incubated for 3 d, and grew for 5 d. Then all seedlings were subjected to heat stress. A: plants under normal growth conditions; B: plants subjected to HS treatment of BT (45°C 40 min, recovered growth for 8 d at 22°C); C: plants subjected to HS treatment of AT (37°C 1 h, recovered growth for 2 d at 22°C, and 46°C 50 min, then recovered growth for 8 d at 22°C); D: survival rates of transgenic and wild type plants at normal conditions and subjected to BT and AT treatments. The represented values are the means of at least 15 individual plants of each line, and the experiment was repeated three times.
正常条件下, 野生型和3个转基因系的叶绿素含量为0.82~0.89 μg mg-1 FW, 差异不大。两种热处理均使幼苗叶绿素含量降低, 但3个TaHsfA2e转基因系降低幅度小于野生型, 且获得耐热性处理植株叶绿素含量总体略高于基础耐热性植株(图6-A)。
图6
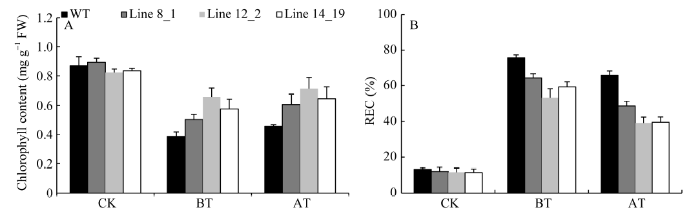 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6不同热处理后转TaHsfA2e拟南芥及野生型植株叶绿素的含量(A)及相对电导率(B)
CK: 正常生长; BT: 基础耐热性处理; AT: 获得耐热性处理。ERC: 相对电导率。数值为至少15株幼苗平均值, 重复3次。
Fig. 6Chlorophyll content (A) and REC (B) of transgenic Arabidopsis lines overexpressing TaHsfA2e and wild type subject to different heat shock regimes
CK: normal condition; BT: heat shock regime for basal thermotolerance; AT: heat shock regime for acquired thermotolerance. REC: electrical relative conductivity. The represented values are the means of at least 15 individual plants of each line, and the experiment was repeated three times.
正常条件下, 野生型和3个转基因系的相对电导值差异不明显(11%~13%); 经基础耐热性和获得耐热性处理后, 幼苗电导率均显著升高, 3个转基因系的升高幅度明显低于野生型(图6-B)。
2.5 小麦热激转录因子TaHsfA2e对拟南芥热激蛋白基因表达的影响
基础热处理后恢复0 h和获得热处理后恢复2 h分别取样, 分别检测8个与植物耐热性关系密切热激蛋白基因的定量表达, 以不同处理的野生型为对照。基础性热处理后, TaHsfA2e的转入能不同程度上调拟南芥植株热相关蛋白基因的表达, 尤其是AtHsp70T的表达量高达对照的40多倍。获得性热处理后, TaHsfA2e的转入也能上调AtHsp25.3、AtHsp32、AtERDJ3A和AtHsp70T的表达, 但上调幅度明显小于基础热处理(图7)。
图7
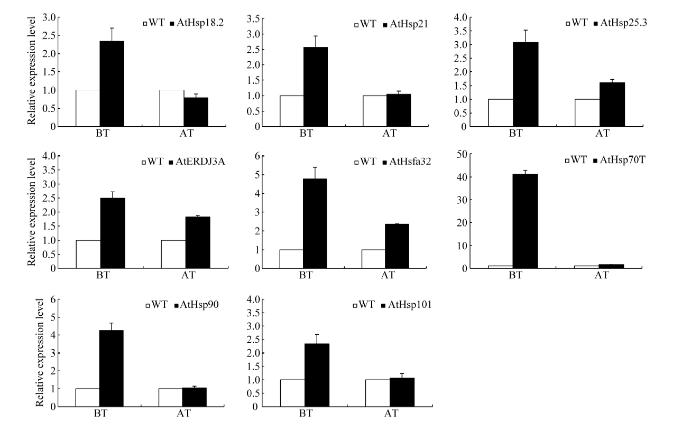 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7基础耐热性(BT)和获得耐热性(AT)处理后转TaHsfA2e拟南芥植株中8个热相关蛋白基因的表达
所有BT值为处理后0 h数值、所有AT值为处理后2 h数值, 均以相同热处理的野生型为对照。数值为至少15株幼苗平均值, 重复3次。
Fig. 7Relative expression levels of eight Hsps in transgenic Arabidopsis line overexpressing TaHsfA2e subject to heat shock regimes for basal (BT) and acquired thermotolerance (AT)
All values represent that at 0 h after heat stress in BT and at 2 h after heat stress in AT. Wild type subjected to same heat stress was used as controls. The represented values are the means of at least fifteen individual plants of each line, and the experiment was repeated three times.
3 讨论
热激转录因子Hsf已被证实在植物耐热性响应过程中发挥重要的调控作用, 其研究结果主要来自模式作物, 而且集中在A1和A2亚族成员[6,8,20,22,26]。Hsf不仅介导耐热性, 还参与多种逆境胁迫响应过程, 且亚家族基因功能差别较大, 因此对不同作物热激转录因子基因的生物学功能了解还远远不够。随着多种作物基因组测序完成, 越来越多物种的热激转录因子家族基因被推测并进行了遗传分析, 对A1和A2以外其他亚族成员也开始了功能研究[35,41]。大田作物热激转录因子基因的研究起步较晚, 2011年Lin等[16]推测玉米中该类基因至少有25个, 后来数量增加为30个[4]。2014年, Xue等[5]推测小麦中至少有56个Hsf, 其中A2亚族有9个, 从数量上远超于其他物种。本实验室近年研究结果暗示A2亚族成员更多, 目前我们已经同源克隆获得18个A2亚族成员, 基因之间生物学特性存在差异, RNA-seq初步分析发现多数基因被热胁迫上调表达, 但响应模式不同(尚未发表), 进一步说明大田作物Hsf家族基因特性与功能具有多样性和复杂性。本研究在克隆获得小麦B族基因TaHsfB2d [41]的基础上, 进一步克隆获得A2亚族TaHsfA2e基因, 该基因编码序列全长1026 bp, 编码341个氨基酸残基, 蛋白质序列含有DBD、NLS、NES及AHA完整的功能结构域, 充分体现A2亚族成员结构特性。TaHsfB2d只有DBD和NLS, 结构上明显不同于TaHsfA2e, 但正常条件下二者均被定位在细胞核。尚未发现关于该基因生物学特性及功能的研究报道。
植物Hsf分为A、B、C三族, 不同家族基因特性与功能差异较大, 相同家族不同亚族基因也表现不同。对模式植物的研究表明, A1亚族一般呈组成型表达, 表达量不高, 在拟南芥花粉中表达较高; 而A2亚族属于热胁迫诱导因子, 受胁迫植株恢复阶段和根系中表达较高[8]。本实验室前期研究发现TaHsfB2d在小麦多个组织器官中组成型表达, 其中在成熟植株根系中表达量较高, 37℃热胁迫、外源SA和H2O2处理均不同程度上调 TaHsfB2d的表达[41]。本研究结果显示, TaHsfA2e在小麦多个组织器官中表达量均很低, 但在成熟种子中高表达, 叶片中TaHsfA2e的表达受37℃热胁迫显著上调, 符合A2亚族基因特性, 但其表达受SA和H2O2下调。可见, 不同亚族基因表达特性存在明显差异, 基因生物学特性不同预示其功能上的差异性。对玉米A2亚族基因的定量表达研究发现[30], ZmHsf04在幼嫩花粉中高表达, 根系和叶片中基因的表达均可被42℃热胁迫上调, 且根系中表达量高于叶片中, 基因表达同时受ABA和H2O2上调, 充分说明同一亚族基因在不同作物中表达特性也不相同, 何况TaHsfA2e与玉米A2亚族基因的同源性很低。
酵母中只有1个Hsf, 因此酵母转化系统能较快验证Hsf功能[40]。本研究通过将TaHsfA2e转化酵母细胞pYES2并进行表型观察和生长曲线分析发现, 正常生长条件下, 转TaHsfA2e酵母和转空载体对照细胞的长势没有明显差异; 经50℃热激处理45 min 后, 转基因酵母细胞和空载体对照细胞的生长速度均受到抑制, 但前者受抑制程度明显小于后者, 表明TaHsfA2e能在酵母细胞中被诱导表达, 且能显著提高酵母细胞的耐热性, 初步显示出TaHsfA2e的耐热性调控功能。热胁迫后, 转基因酵母菌斑与对照没有明显差异, 说明导入TaHsfA2e不影响酵母细胞的生长发育, 而引入玉米ZmHsf04则显著影响酵母细胞的生长发育进程[30]。
将TaHsfA2e进一步转化拟南芥野生型, 并进行基础和获得耐热性鉴定, 发现TaHsfA2e不仅能提高37℃热胁迫下转基因株系的基础耐热性, 同时能提高获得耐热性, 不同株系热胁迫后地上部叶绿素含量和相对电导率为表型提供了生理证据, 进一步证实了酵母中研究基因功能的正确性。拟南芥热激相关蛋白基因表达的定量检测发现, 基础性热处理下, TaHsfA2e显著上调拟南芥植株热激蛋白基因的表达, 上调幅度明显大于获得性热处理。另外, 通过不同热处理后恢复阶段连续取样检测发现, TaHsfA2e大幅上调热激蛋白表达的时间是基础热处理后恢复0 h和获得热处理后恢复2 h, 之后热激蛋白表达量逐渐降低(数据未列出)。总体来看, 基础性热处理下TaHsfA2e上调下游基因表达比较快, 这可能与基础性热处理强度大且快速, 而获得性热处理持续时间较长有关。TaHsfA2e的基础耐热性强还是获得耐热性更强, 仅仅依靠上述结果还无法界定, 需要深入探讨。本研究转TaHsfA2e基因拟南芥中未发现植株矮化现象, 而拟南芥中过表达AtHsfA2植株矮化明显, 但基础耐热性却显著增强[42]。拟南芥只有1个A2亚族Hsf, 而小麦中有多个, 其功能分化可能更为精细, 本研究只初步证明TaHsfA2e的耐热性功能, 其抗逆性调控功能及其分子机制正在通过转基因小麦进一步研究。
4 结论
小麦A2亚族热激转录因子基因TaHsfA2e的cDNA序列全长1026 bp, 编码341个氨基酸残基。蛋白质序列含有DNA结合结构域、核定位信号序列、核输出信号序列和结合结构域。TaHsfA2e与小麦TaHsfA6f相似性最高, 达96%。正常条件下, 在小麦成熟种子中TaHsfA2e表达量较高, 基因表达受37℃热胁迫显著上调, 受外源水杨酸和H2O2下调。正常条件下TaHsfA2e蛋白定位于细胞核。TaHsfA2e的引入能不同程度提高转基因株系的耐热性, 同时上调热胁迫条件下热激蛋白基因的表达。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.tibs.2011.11.007URLPMID:22236506 [本文引用: 1]

In plants, the heat stress response (HSR) is highly conserved and involves multiple pathways, regulatory networks and cellular compartments. At least four putative sensors have recently been proposed to trigger the HSR. They include a plasma membrane channel that initiates an inward calcium flux, a histone sensor in the nucleus, and two unfolded protein sensors in the endoplasmic reticulum and the cytosol. Each of these putative sensors is thought to activate a similar set of HSR genes leading to enhanced thermotolerance, but the relationship between the different pathways and their hierarchical order is unclear. In this review, we explore the possible involvement of different thermosensors in the plant response to warming and heat stress.
DOI:10.1016/j.pbi.2007.04.011URLPMID:17482504 [本文引用: 1]

Plants have evolved a variety of responses to elevated temperatures that minimize damage and ensure protection of cellular homeostasis. New information about the structure and function of heat stress proteins and molecular chaperones has become available. At the same time, transcriptome analysis of Arabidopsis has revealed the involvement of factors other than classical heat stress responsive genes in thermotolerance. Recent reports suggest that both plant hormones and reactive oxygen species also contribute to heat stress signaling. Additionally, an increasing number of mutants that have altered thermotolerance have extended our understanding of the complexity of the heat stress response in plants.
DOI:10.1379/1466-1268(1996)0012.3.CO;2URLPMID:9222607 [本文引用: 1]

Based on the partial or complete sequences of 14 plant heat stress transcription factors (Hsfs) from tomato, soybean, Arabidopsis and maize we propose a general nomenclature with two basic classes, i.e. classes A and B each containing two or more types of Hsfs (HsfA1, HsfA2 etc.). Despite some plant-specific peculiarities, essential functional domains and modules of these proteins are conserved among plants, yeast, Drosophila and vertebrates. A revised terminology of these parts follows recommendations agreed upon among the authors and representatives from other laboratories working in this field (see legend to Fig. 1). Similar to the situation with the small heat shock proteins (sHsps), the complexity of the hsf gene family in plants appears to be higher than in other eukaryotic organisms.
DOI:10.3389/fpls.2016.00114URLPMID:26904076 [本文引用: 2]

Abiotic stresses such as high temperature, salinity and drought adversely affect the survival, growth and reproduction of plants. Plants respond to such unfavorable changes through developmental, physiological and biochemical ways, and these responses require expression of stress-responsive genes, which are regulated by a network of transcription factors (TFs), including heat stress transcription factors (HSFs). HSFs play a crucial role in plants response to several abiotic stresses by regulating the expression of stress-responsive genes, such as heat shock proteins (Hsps). In this review, we describe the conserved structure of plant HSFs, the identification of HSF gene families from various plant species, their expression profiling under abiotic stress conditions, regulation at different levels and function in abiotic stresses. Despite plant HSFs share highly conserved structure, their remarkable diversification across plants reflects their numerous functions as well as their integration into the complex stress signaling and response networks, which can be employed in crop improvement strategies via biotechnological intervention.
DOI:10.1093/jxb/ert399URLPMID:24323502 [本文引用: 5]

Abstract Heat shock factors (Hsfs) play a central regulatory role in acquired thermotolerance. To understand the role of the major molecular players in wheat adaptation to heat stress, the Hsf family was investigated in Triticum aestivum. Bioinformatic and phylogenetic analyses identified 56 TaHsf members, which are classified into A, B, and C classes. Many TaHsfs were constitutively expressed. Subclass A6 members were predominantly expressed in the endosperm under non-stress conditions. Upon heat stress, the transcript levels of A2 and A6 members became the dominant Hsfs, suggesting an important regulatory role during heat stress. Many TaHsfA members as well as B1, C1, and C2 members were also up-regulated during drought and salt stresses. The heat-induced expression profiles of many heat shock protein (Hsp) genes were paralleled by those of A2 and A6 members. Transactivation analysis revealed that in addition to TaHsfA members (A2b and A4e), overexpression of TaHsfC2a activated expression of TaHsp promoter-driven reporter genes under non-stress conditions, while TaHsfB1b and TaHsfC1b did not. Functional heat shock elements (HSEs) interacting with TaHsfA2b were identified in four TaHsp promoters. Promoter mutagenesis analysis demonstrated that an atypical HSE (GAACATTTTGGAA) in the TaHsp17 promoter is functional for heat-inducible expression and transactivation by Hsf proteins. The transactivation of Hsp promoter-driven reporter genes by TaHsfC2a also relied on the presence of HSE. An activation motif in the C-terminal domain of TaHsfC2a was identified by amino residue substitution analysis. These data demonstrate the role of HsfA and HsfC2 in regulation of Hsp genes in wheat.
DOI:10.1111/j.1365-313X.2006.02889.xURLPMID:17059409 [本文引用: 2]

We isolated 76 high-light and heat-shock (HL + HS) stress-inducible genes, including a putative heat-shock transcription factor ( HsfA2 ), by suppression-subtractive hybridization from Arabidopsis. The transcript level of HsfA2 was significantly increased under the several stress conditions or by the H 2 O 2 treatment. Furthermore, the induction of HsfA2 expression was highest among those of other class A HSFs in response to HL + HS stress conditions. The promoter assay revealed that HsfA2 is induced mainly in rosette leaves under HL + HS stress conditions. In the HsfA2 -overexpressing Arabidopsis ( Pro 35 S :HsfA2 ) plants, 46 genes, including a large number of heat-shock proteins, ascorbate peroxidase 2 and galactinol synthase 1 and 2, were highly expressed compared with those in the wild-type plants. The transcript levels of the HsfA2 target genes are highly correlated with those of HsfA2 in the Pro 35 S :HsfA2 plants. The transcript levels of the HsfA2 target genes, as well as HsfA2 transcripts, were induced by treating with exogenous H 2 O 2 . In the knockout HsfA2 Arabidopsis plants, the induction of 26 HsfA2 target genes was strongly reduced for up to 2 h under HL + HS stress conditions. Furthermore, the Pro 35 S :HsfA2 plants showed increased tolerance to combined environmental stresses. Our present results indicate that HsfA2 is a key regulator in the induction of the defence system under several types of environmental stress.
DOI:10.1128/MCB.21.5.1759-1768.2001URL [本文引用: 1]
DOI:10.1111/j.1365-3040.2011.02278.xURLPMID:21241330 [本文引用: 3]

In Arabidopsis, there are four homologs of class A1 heat shock factor (HSFA1) genes, which likely encode the master regulators of heat shock response (HSR). However, previous studies with double knockout (KO) mutants were unable to confirm this point probably due to functional redundancy. Here, we generated a quadruple KO (QK) and four triple KO mutants to dissect their functions. Our data show that members of the HSFA1 group not only play a pivotal role in HSR but also are involved in growth and development. Alterations in morphology and retardation in growth were observed in the quadruple but not in triple KO mutants. The basal and acquired thermotolerance capacity was dramatically decreased in the QK mutant but varied in triple KO mutants at different developmental stages. The transcriptomics profiles suggested that more than 65% of the heat stress (HS)-up-regulated genes were HSFA1 dependent. HSFA1s were also involved in the expression of several HS genes induced by H2O2, salt and mannitol, which is consistent with the increased sensitive phenotype of the QK mutant to the stress factors. In conclusion, the Arabidopsis HSFA1s function as the master regulators of HSR and participate as important components in other abiotic stress responses as well.
DOI:10.1007/s11103-014-0202-0URLPMID:24874772 [本文引用: 1]

Heat stress transcription factors (HSFs) are central regulators of the heat stress response. Plant HSFs of subgroup B lack a conserved sequence motif present in the transcriptional activation domain of class A-HSFs. Arabidopsis members were found to be involved in non-heat shock functions. In the present analysis we investigated the expression, regulation and function of HSFB2a. HSFB2a expression was counteracted by a natural long non-coding antisense RNA, asHSFB2a . In leaves, the antisense RNA gene is only expressed after heat stress and dependent on the activity of HSFA1a/HSFA1b. HSFB2a and asHSFB2a RNAs were also present in the absence of heat stress in the female gametophyte. Transgenic overexpression of HSFB2a resulted in a complete knock down of the asHSFB2a expression. Conversely, asHSFB2a overexpression leads to the absence of HSFB2a RNA. The knockdown of HSFB2a by asHSFB2a correlated with an improved, knockdown of asHSFB2a by HSFB2a overexpression with an impaired biomass production early in vegetative development. In both cases the development of female gametophytes was impaired. A T-DNA knock-out line did not segregate homozygous mutant plants, only heterozygots hsfB2a - tt1 /+ were viable. Approximately 5002% of the female gametophytes were arrested in early development, before mitosis 3, resulting in 4502% of sterile ovules. Our analysis indicates that the “Yin–Yang” regulation of gene expression at the HSFB2a locus influences vegetative and gametophytic development in Arabidopsis.
[本文引用: 1]
DOI:10.1093/mp/ssn095URLPMID:19529832 [本文引用: 1]

In order to assess the functional roles of heat stress-induced class B-heat shock factors in Arabidopsis, we investigated T-DNA knockout mutants of AtHsfB1 and AtHsfB2b. Micorarray analysis of double knockout hsfB1/hsfB2b plants revealed as strong an up-regulation of the basal mRNA-levels of the defensin genes Pdf1.2a/b in mutant plants. The Pdf expression was further enhanced by jasmonic acid treatment or infection with the necrotrophic fungus Alternaria brassicicola. The single mutant hsfB2b and the double mutant hsfB1/B2b were significantly improved in disease resistance after A. brassicicola infection. There was no indication for a direct interaction of Hsf with the promoter of Pdf1.2, which is devoid of perfect HSE consensus Hsf-binding sequences. However, changes in the formation of late HsfA2-dependent HSE binding were detected in hsfB1/B2b plants. This suggests that HsfB1/B2b may interact with class A-Hsf in regulating the shut-off of the heat shock response. The identification of Pdf genes as targets of Hsf-dependent negative regulation is the first evidence for an interconnection of Hsf in the regulation of biotic and abiotic responses.
DOI:10.1111/j.1365-3040.2012.02533.xURLPMID:22571635 [本文引用: 1]

Plants have as many as 20 heat shock factors (Hsfs) grouped into three classes, A, B and C, based on sequence similarity and modular structures. Through screening for cell death-inducing factor(s) in Nicotiana benthamiana, we identified Arabidopsis HsfB2b and thus subjected all other members of Arabidopsis Hsf class B (HsfB1, HsfB2a, HsfB2b, HsfB3 and HsfB4) to the same cell death assay. When expressed in N. enthamiana leaves, only HsfB1 and HsfB2b elicited mild cell death. Simultaneously we found that HsfB1 has a post-transcriptional control mechanism, in which a sequence-conserved upstream open-reading frame (sc-uORF) is involved. The known repressor function of the respective HsfBs was confirmed and the difference in cell death-inducing activity of HsfBs was explained by the fact that HsfB1 and HsfB2b are transcriptional repressors but the others are not. Indeed, the cell death symptom by HsfB1 and HsfB2b required not only their repression activity but also their nuclear localization activity. HsfB1 expression was drastically and transiently induced by heat shock (HS) and the intactness of sc-uORF was required for its HS response. Based on the results, the physiological significance of cell death-inducing activity of HsfB1 and HsfB2b and the sc-uORF in the HsfB1 transcript during HS response is discussed.
DOI:10.3389/fpls.2016.00114URLPMID:26904076 [本文引用: 1]

Abiotic stresses such as high temperature, salinity and drought adversely affect the survival, growth and reproduction of plants. Plants respond to such unfavorable changes through developmental, physiological and biochemical ways, and these responses require expression of stress-responsive genes, which are regulated by a network of transcription factors (TFs), including heat stress transcription factors (HSFs). HSFs play a crucial role in plants response to several abiotic stresses by regulating the expression of stress-responsive genes, such as heat shock proteins (Hsps). In this review, we describe the conserved structure of plant HSFs, the identification of HSF gene families from various plant species, their expression profiling under abiotic stress conditions, regulation at different levels and function in abiotic stresses. Despite plant HSFs share highly conserved structure, their remarkable diversification across plants reflects their numerous functions as well as their integration into the complex stress signaling and response networks, which can be employed in crop improvement strategies via biotechnological intervention.
DOI:10.1093/pcp/pcp048URLPMID:19324928 [本文引用: 1]

Abstract We showed previously that the ERF-associated amphiphilic repression (EAR) motif is a plant-specific repression domain that contains the conserved amino acid sequence LXLXL. In this report, we describe the identification of a novel repression domain, L/VR/KLFGVXM/V/L, which is different from known EAR motifs, in B3 DNA-binding domain transcription factors in Arabidopsis. Database analysis revealed that 29 Arabidopsis transcription factors, which included members of the RAV, ARF, Hsf and MYB families, contain the R/KLFGV conserved motif found in the novel repression domain. We demonstrated that factors that contain the R/KLFGV motif, namely, RAV1, RAV2, HsfB1 and HsfB2b, exhibited the repressive activity.
DOI:10.3389/fpls.2016.00490URLPMID:4836240 [本文引用: 1]

Heat shock transcription factors (Hsfs) play vital roles in the regulation of tolerance to various stresses in living organisms. To dissect the mechanisms of the Hsfs in potato adaptation to abiotic stresses, genome and transcriptome analyses ofHsfgene family were investigated inSolanum tuberosumL. Twenty-seven StHsf members were identified by bioinformatics and phylogenetic analyses and were classified into A, B, and C groups according to their structural and phylogenetic features. StHsfs in the same class shared similar gene structures and conserved motifs. The chromosomal location analysis showed that 27Hsfswere located in 10 of 12 chromosomes (except chromosome 1 and chromosome 5) and that 18 of these genes formed 9 paralogous pairs. Expression profiles ofStHsfsin 12 different organs and tissues uncovered distinct spatial expression patterns of these genes and their potential roles in the process of growth and development. Promoter and quantitative real-time polymerase chain reaction (qRT-PCR) detections ofStHsfswere conducted and demonstrated that these genes were all responsive to various stresses.StHsf004, StHsf007, StHsf009, StHsf014, andStHsf019were constitutively expressed under non-stress conditions, and some specificHsfsbecame the predominantHsfsin response to different abiotic stresses, indicating their important and diverse regulatory roles in adverse conditions. A co-expression network betweenStHsfsandStHsf-co-expressed genes was generated based on the publicly-available potato transcriptomic databases and identified key candidateStHsfsfor further functional studies.
DOI:10.1186/1471-2164-12-76URLPMID:3039612 [本文引用: 2]

pAbstract/p pBackground/p pHeat shock response in eukaryotes is transcriptionally regulated by conserved heat shock transcription factors (Hsfs). Hsf genes are represented by a large multigene family in plants and investigation of the Hsf gene family will serve to elucidate the mechanisms by which plants respond to stress. In recent years, reports of genome-wide structural and evolutionary analysis of the entire Hsf gene family have been generated in two model plant systems, itArabidopsis /itand rice. Maize, an important cereal crop, has represented a model plant for genetics and evolutionary research. Although some Hsf genes have been characterized in maize, analysis of the entire Hsf gene family were not completed following Maize (B73) Genome Sequencing Project./p pResults/p pA genome-wide analysis was carried out in the present study to identify all Hsfs maize genes. Due to the availability of complete maize genome sequences, 25 nonredundant Hsf genes, named itZmHsfs /itwere identified. Chromosomal location, protein domain and motif organization of ZmHsfs were analyzed in maize genome. The phylogenetic relationships, gene duplications and expression profiles of itZmHsf /itgenes were also presented in this study. Twenty-five ZmHsfs were classified into three major classes (class A, B, and C) according to their structural characteristics and phylogenetic comparisons, and class A was further subdivided into 10 subclasses. Moreover, phylogenetic analysis indicated that the orthologs from the three species (maize, itArabidopsis /itand rice) were distributed in all three classes, it also revealed diverse Hsf gene family expression patterns in classes and subclasses. Chromosomal/segmental duplications played a key role in Hsf gene family expansion in maize by investigation of gene duplication events. Furthermore, the transcripts of 25 itZmHsf /itgenes were detected in the leaves by heat shock using quantitative real-time PCR. The result demonstrated that itZmHsf /itgenes exhibit different expression levels in heat stress treatment./p pConclusions/p pOverall, data obtained from our investigation contributes to a better understanding of the complexity of the maize Hsf gene family and provides the first step towards directing future experimentation designed to perform systematic analysis of the functions of the Hsf gene family./p
DOI:10.1007/s11105-015-0892-8URL [本文引用: 1]

<span >Heat shock transcription factor (HSF) plays an essential role on the increased tolerance against heat stress by<span >regulating the expression of heat-responsive genes. In this <span >study, a HSF gene, <span >CarHSFB2<span >, was isolated and characterized<span >in chickpea. CarHSFB2 was a nuclear protein with a predicted <span >polypeptide of 267 amino acids and encoded by a single/low<span >copy genes. Phylogenetic analysis showed that CarHSFB2 <span >belonged to the class B HSFs. It had little or no any transcription activation activity due to lack of aromatic, hydrophobic, <span >and acidic amino acid (AHA) motifs. <span >CarHSFB2 <span >showed different expression patterns among different developmental processes (leaf senescence, developing seed, and embryo of germinating seed). It was induced by the stress of heat, salt, <span >wound and drought, and the treatment of H<span >2<span >O<span >2<span >, IAA, and <span >GA3, respectively, while inhibited by 6-BA. However, the <span >other stress and chemical treatments (cold, ABA, MeJA, Et, <span >and SA) had no obvious effect on its expression. Overexpression of <span >CarHSFB2 <span >in <span >Arabidopsis <span >seedlings showed the increased tolerance to drought and heat stress. Additionally, <span >stress-responsive genes, <span >RD22<span >, <span >RD26<span >, and <span >RD29A<span >, showed <span >significantly higher expression levels in transgenic <span >Arabidopsis <span >seedlings than in the wild type (WT) under<span >drought stress, whereas <span >HsfA2<span >, <span >HsfB2a<span >, and <span >HsfA7a <span >in transgenic <span >Arabidopsis <span >seedlings were markedly accumulated in<span >transcript level than in the WT under heat stress. All these<span >results indicate that CarHSFB2, a class B HSF, positively<span >functions in different developmental processes and various <span >stress responses, especially in positive response to heat and<span >drought stresses, in chickpea.
DOI:10.1016/j.plaphy.2009.05.003URLPMID:19539489 [本文引用: 1]

Binding of heat shock factors (HSFs) with heat shock element sequence is critical for the transcriptional induction of heat shock genes. Rice genome sequence shows 26 OsHsf genes out of which 25 possess various important domains noted in HSFs i.e. DNA binding domain (DBD), oligomerization domain (OD), nuclear localization signal (NLS), nuclear export signal (NES) and AHA type activation domain. OsHsf entry LOC_Os06g226100 has the oligomerization domain but lacks the above other domains. Also, there are no ESTs or full-length cDNA noted for this entry in database. Expression profiling showed that 22 OsHsf genes are induced by high temperature. Induction of 10 and 14 OsHsf genes was also noted against low temperature stress and oxidative stress, respectively. All OsHsf genes induced by oxidative stress were also induced by high temperature. On the other hand, induction of 6 and 1 OsHsf genes was noted to be exclusive to high and low temperature stresses, respectively. Seven OsHsf genes showed induced expression in response to all the three stresses examined. While in silico promoter analysis showed that OsHsf genes contain upstream regulatory elements corresponding to different abiotic stresses, there was lack of correlation noted between the in silico profiling of the elements and their corresponding transcript expression patterns. Apart from stress inducibility, EST database suggests that various OsHsf genes are developmentally regulated in diverse tissue types.
DOI:10.1073/pnas.1418483111URL [本文引用: 1]

The circadian clock perceives environmental signals to reset to local time, but the underlying molecular mechanisms are not well understood. Here we present data revealing that a member of the heat shock factor (Hsf) family is involved in the input pathway to the plant circadian clock. Using the yeast one-hybrid approach, we isolated several Hsfs, including Heat Shock Factor B2b (HsfB2b), a transcriptional repressor that binds the promoter of Pseudo Response Regulator 7 (PRR7) at a conserved binding site. The constitutive expression of HsfB2b leads to severely reduced levels of the PRR7 transcript and late flowering and elongated hypocotyls. HsfB2b function is important during heat and salt stress because HsfB2b overexpression sustains circadian rhythms, and the hsfB2b mutant has a short circadian period under these conditions. HsfB2b is also involved in the regulation of hypocotyl growth under warm, short days. Our findings highlight the role of the circadian clock as an integrator of ambient abiotic stress signals important for the growth and fitness of plants.
DOI:10.3354/ame032011URLPMID:121470 [本文引用: 2]

In heat-stressed (HS) tomato (Lycopersicon peruvianum) cell cultures, the constitutively expressed HS transcription factor HsfA1 is complemented by two HS-inducible forms, HsfA2 and HsfB1. Because of its stability, HsfA2 accumulates to fairly high levels in the course of a prolonged HS and recovery regimen. Using immunofluorescence and cell fractionation experiments, we identified three states of HsfA2: (i) a soluble, cytoplasmic form in preinduced cultures maintained at 25 degrees C, (ii) a salt-resistant, nuclear form found in HS cells, and (iii) a stored form of HsfA2 in cytoplasmic HS granules. The efficient nuclear transport of HsfA2 evidently requires interaction with HsfA1. When expressed in tobacco protoplasts by use of a transient-expression system, HsfA2 is mainly retained in the cytoplasm unless it is coexpressed with HsfA1. The essential parts for the interaction and nuclear cotransport of the two Hsfs are the homologous oligomerization domain (HR-A/B region of the A-type Hsfs) and functional nuclear localization signal motifs of both partners. Direct physical interaction of the two Hsfs with formation of relatively stabile hetero-oligomers was shown by a two-hybrid test in Saccharomyces cerevisiae as well as by coimmunoprecipitation using tomato and tobacco whole-cell lysates.
[本文引用: 1]
DOI:10.1101/gad.228802URLPMID:12080093 [本文引用: 2]

Abstract We generated transgenic tomato plants with altered expression of heat stress transcription factor HsfA1. Plants with 10-fold overexpression of HsfA1 (OE plants) were characterized by a single HsfA1 transgene cassette, whereas plants harboring a tandem inverted repeat of the cassette showed cosuppression (CS plants) by posttranscriptional silencing of the HsfA1 gene connected with formation of small interfering RNAs. Under normal growth conditions, major developmental parameters were similar for wild-type (WT), OE, and CS plants. However, CS plants and fruits were extremely sensitive to elevated temperatures, because heat stress-induced synthesis of chaperones and Hsfs was strongly reduced or lacking. Despite the complexity of the plant Hsf family with at least 17 members in tomato, HsfA1 has a unique function as master regulator for induced thermotolerance. Using transient reporter assays with mesophyll protoplasts from WT tomato, we demonstrated that plasmid-encoded HsfA1 and HsfA2 were well expressed. However, in CS protoplasts the cosuppression phenomenon was faithfully reproduced. Only transformation with HsfA2 expression plasmid led to normal expression of the transcription factor and reporter gene activation, whereas even high amounts of HsfA1 expression plasmids were silenced. Thermotolerance in CS protoplasts was restored by plasmid-borne HsfA2, resulting in expression of chaperones, thermoprotection of firefly luciferase, and assembly of heat stress granules.
DOI:10.1093/jxb/erm184URL [本文引用: 1]

Heat shock transcription factors (Hsfs) are the central regulators of the heat shock (HS) stress response in all eukaryotic organisms. HsfA2 is one of the Arabidopsis class A Hsfs, and the induction of HsfA2 expression in response to HS stress is highest among all 21 Arabidopsis Hsfs. In this study, it is reported that basal and acquired thermotolerance was significantly enhanced in high-level HsfA2-overexpressed transgenic lines (El2Omega::HsfA2) in comparison with wild-type plants. By contrast, the dominant negative mutants of HsfA2 (El2Omega::HsfA2DeltaC264) plants displayed reduced thermotolerance. These results indicate that the HsfA2 gene plays a role in the HS stress response. Microarray analysis of the El2Omega::HsfA2 plants identified putative target genes, which included HS stress-inducible genes and other stress-responsive genes. Salt and osmotic stress induced HsfA2 gene expression. In fact, the El2Omega::HsfA2 plants showed enhanced tolerance to these stresses, suggesting that HsfA2 was involved in multiple stress tolerance. El2Omega::HsfA2 plants showed accelerated callus growth from root explants compared with the wild type, unlike the El2Omega::HsfA2DeltaC264 plants whose growth was delayed. These observations suggest that HsfA2 plays, in addition to its role in stress tolerance, an important role in cell proliferation.
[本文引用: 1]
DOI:10.1111/j.1365-313X.2006.02889.xURLPMID:17059409 [本文引用: 1]

We isolated 76 high-light and heat-shock (HL + HS) stress-inducible genes, including a putative heat-shock transcription factor ( HsfA2 ), by suppression-subtractive hybridization from Arabidopsis. The transcript level of HsfA2 was significantly increased under the several stress conditions or by the H 2 O 2 treatment. Furthermore, the induction of HsfA2 expression was highest among those of other class A HSFs in response to HL + HS stress conditions. The promoter assay revealed that HsfA2 is induced mainly in rosette leaves under HL + HS stress conditions. In the HsfA2 -overexpressing Arabidopsis ( Pro 35 S :HsfA2 ) plants, 46 genes, including a large number of heat-shock proteins, ascorbate peroxidase 2 and galactinol synthase 1 and 2, were highly expressed compared with those in the wild-type plants. The transcript levels of the HsfA2 target genes are highly correlated with those of HsfA2 in the Pro 35 S :HsfA2 plants. The transcript levels of the HsfA2 target genes, as well as HsfA2 transcripts, were induced by treating with exogenous H 2 O 2 . In the knockout HsfA2 Arabidopsis plants, the induction of 26 HsfA2 target genes was strongly reduced for up to 2 h under HL + HS stress conditions. Furthermore, the Pro 35 S :HsfA2 plants showed increased tolerance to combined environmental stresses. Our present results indicate that HsfA2 is a key regulator in the induction of the defence system under several types of environmental stress.
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3969/j.issn.1674-7968.2017.09.004URL [本文引用: 3]

植物热激转录因子(heat shock transcription factor,Hsf)能够与热激蛋白基因启动子区域的热激元件结合而直接启动热激反应,因此成为热胁迫下基因转录激活信号转导通路中重要的调控因子。前人推测玉米(Zeamaxs)中至少有30个Hsf家族成员,其中A族18个,A2亚族4个。本实验室在前期对A1亚族ZmHsf06结构、特性及其抗旱耐热性功能研究的基础上,采用同源克隆技术,从玉米幼叶中克隆获得A2亚族基因ZmHsf04(GenBank登录号:GRMZM2G010871P01)的完整编码序列,序列全长1074bp,编码357个氨基酸残基。蛋白质结构包含Hsf家族DNA结合结构域,含有核定位信号(KRKELEDTISKKRRR)、核输出信号(LAQQLGYL)和激活结构域(LKMFESGVLN)等完整的功能结构域。蛋白序列与高粱(Sorghumbicolor)SORBIDRAFT的同源性最高,达。荧光定量分析_01g02149090%表明,正常生长条件下ZmHsf04在玉米多个组织器官中表达,幼嫩花粉中表达较高,约为幼嫩根系对照的16倍;ZmHsf04的表达水平受42℃热胁迫和外源脱落酸(abscisicacid,ABA)显著上调,最高表达量达正常对照的340倍,叶片峰值出现的时间分别为30min和24h;ZmHsf04也受水杨酸(SA)和H2O上调表达,上调幅度低于热处理,最高表达量不超过12倍,显著低于热激或ABA处理,峰值出现相对滞后;用SA和H2O2分别预处理再热激,ZmHsf04的表达水平无明显差异。通过在洋葱(Allium cepa)表皮细胞中瞬时表达并观察绿色荧光蛋白(green fluorescence protein,GFP)荧光发现,ZmHsf04定位于细胞核。通过将ZmHsf04转化酵母(Saccharomyces cerevisiae)并进行50℃水浴热胁迫处理发现,ZmHsf04在酵母中可被半乳糖诱导蛋白表达,热胁迫同时降低正常和转ZmHsf04酵母的生长势,但与转空载体对照相比,转ZmHsf04基因酵母表现更强的耐热性。研究结果表明,ZmHsf04在植株花粉发育和热胁17
DOI:10.3969/j.issn.1674-7968.2017.09.004URL [本文引用: 3]

植物热激转录因子(heat shock transcription factor,Hsf)能够与热激蛋白基因启动子区域的热激元件结合而直接启动热激反应,因此成为热胁迫下基因转录激活信号转导通路中重要的调控因子。前人推测玉米(Zeamaxs)中至少有30个Hsf家族成员,其中A族18个,A2亚族4个。本实验室在前期对A1亚族ZmHsf06结构、特性及其抗旱耐热性功能研究的基础上,采用同源克隆技术,从玉米幼叶中克隆获得A2亚族基因ZmHsf04(GenBank登录号:GRMZM2G010871P01)的完整编码序列,序列全长1074bp,编码357个氨基酸残基。蛋白质结构包含Hsf家族DNA结合结构域,含有核定位信号(KRKELEDTISKKRRR)、核输出信号(LAQQLGYL)和激活结构域(LKMFESGVLN)等完整的功能结构域。蛋白序列与高粱(Sorghumbicolor)SORBIDRAFT的同源性最高,达。荧光定量分析_01g02149090%表明,正常生长条件下ZmHsf04在玉米多个组织器官中表达,幼嫩花粉中表达较高,约为幼嫩根系对照的16倍;ZmHsf04的表达水平受42℃热胁迫和外源脱落酸(abscisicacid,ABA)显著上调,最高表达量达正常对照的340倍,叶片峰值出现的时间分别为30min和24h;ZmHsf04也受水杨酸(SA)和H2O上调表达,上调幅度低于热处理,最高表达量不超过12倍,显著低于热激或ABA处理,峰值出现相对滞后;用SA和H2O2分别预处理再热激,ZmHsf04的表达水平无明显差异。通过在洋葱(Allium cepa)表皮细胞中瞬时表达并观察绿色荧光蛋白(green fluorescence protein,GFP)荧光发现,ZmHsf04定位于细胞核。通过将ZmHsf04转化酵母(Saccharomyces cerevisiae)并进行50℃水浴热胁迫处理发现,ZmHsf04在酵母中可被半乳糖诱导蛋白表达,热胁迫同时降低正常和转ZmHsf04酵母的生长势,但与转空载体对照相比,转ZmHsf04基因酵母表现更强的耐热性。研究结果表明,ZmHsf04在植株花粉发育和热胁17
[本文引用: 1]
DOI:10.1105/tpc.109.066902URLPMID:20028842 [本文引用: 1]

Cadmium (Cd) is a widespread soil pollutant; thus, the underlying molecular controls of plant Cd tolerance are of substantial interest. A screen for wheat [Triticum aestivum) genes that confer Cd tolerance to a Cd hypersensitive yeast strain identified Heat shock transcription factor A4a (HsfA4a). Ta HsfA4a is most similar to the class A4 Hsfs from monocots. The most closely related rice [Oryza sativa) homolog, Os HsfA4a, conferred Cd tolerance in yeast, as did Ta HsfA4a, but the second most closely related rice homolog, Os HsfA4d, did not. Cd tolerance was enhanced in rice plants expressing Ta HsfA4a and decreased in rice plants with knocked-down expression of Os HsfA4a. An analysis of the functional domain using chimeric proteins constructed from Ta HsfA4a and Os HsfA4d revealed that the DNA binding domain (DBD) of HsfA4a is critical for Cd tolerance, and within the DBD, Ala-31 and Leu-42 are important for Cd tolerance. Moreover, Ta HsfA4a-mediated Cd resistance in yeast requires metallothionein (MT). In the roots of wheat and rice, Cd stress caused increases in HsfA4a expression, together the MT genes. Our findings thus suggest that HsfA4a of wheat and rice confers Cd tolerance by upregulating MT gene expression in planta.
DOI:10.1007/s11105-012-0546-zURL [本文引用: 1]

Heat shock factors (HSFs) in plants regulate heat stress response by mediating expression of a set of heat shock protein (HSP) genes. In the present study, we isolated a novel heat shock gene, TaHSF3 , encoding a protein of 315 amino acids in wheat. Phylogenetic analysis showed that TaHSF3 belonged to HSF class B2. Subcellular localization analysis indicated that TaHSF3 localized in nuclei. TaHSF3 was highly expressed in wheat spikes and showed intermediate expression levels in roots, stems, and leaves under normal conditions. It was highly upregulated in wheat seedlings by heat and cold and to a lesser extent by drought and NaCl and ABA treatments. Overexpression of TaHSF3 in Arabidopsis enhanced tolerance to extreme temperatures. Frequency of survival of three TaHSF3 transgenic Arabidopsis lines was 75–9102% after heat treatment and 85–9502% after freezing treatment compared to 25 and 1002%, respectively, in wild-type plants (WT). Leaf chlorophyll contents of the transformants were higher (0.52–0.6702mg/g) than WT (0.3502mg/g) after heat treatment, and the relative electrical conductivities of the transformants after freezing treatment were lower (from 17.56 to 18.602%) than those of WT (37.502%). The TaHSF3 gene from wheat therefore confers tolerance to extreme temperatures in transgenic Arabidopsis by activating HSPs, such as HSP70 .
DOI:10.1371/journal.pone.0079577URLPMID:24265778 [本文引用: 1]

Reduction in crop yield and quality due to various abiotic stresses is a worldwide phenomenon. In the present investigation, a heat shock factor (HSF) gene expressing preferentially in developing seed tissues of wheat grown under high temperatures was cloned. This newly identified heat shock factor possesses the characteristic domains of class A type plant HSFs and shows high similarity to rice OsHsfA2d, hence named as TaHsfA2d. The transcription factor activity of TaHsfA2d was confirmed through transactivation assay in yeast. Transgenic Arabidopsis plants overexpressing TaHsfA2d not only possess higher tolerance towards high temperature but also showed considerable tolerance to salinity and drought stresses, they also showed higher yield and biomass accumulation under constant heat stress conditions. Analysis of putative target genes of AtHSFA2 through quantitative RT-PCR showed higher and constitutive expression of several abiotic stress responsive genes in transgenic Arabidopsis plants over-expressing TaHsfA2d. Under stress conditions, TaHsfA2d can also functionally complement the T-DNA insertion mutants of AtHsfA2, although partially. These observations suggest that TaHsfA2d may be useful in molecular breeding of crop plants, especially wheat, to improve yield under abiotic stress conditions.
DOI:10.1111/pce.v41.1URL [本文引用: 2]
DOI:10.3724/SP.J.1006.2014.00622URL [本文引用: 1]

Based on our previous findings of cloning, expression characteristics and subcellular-location of gene wasO. Up-regulating gene expression by heat shock of 42 02C was dependent on existence of HO, while that PEG-6000 treatment was not. Up-regulating gene expression with ABA was partially dependent on HO. The relative expression of gene too, and chelating Ca with methyleneglycol-bis- (2-aminoethylether)-N,N,N07,N07-tetraacetic acid (EGTA) and blocking Ca intracellular transport with verapamil (Vp) did not decrease gene expression up-regulated by heat shock, PEG and ABA treatments. Those results illustrated that O signal transduction pathway. While treated with HO, the treatment, suggesting that they are the downstream binding proteins of ZmHSF-Likeresponding to Ca. Those results further indicated that ZmHSF-Like makes response to different stresses through binding different HSPs.
DOI:10.3724/SP.J.1006.2014.00622URL [本文引用: 1]

Based on our previous findings of cloning, expression characteristics and subcellular-location of gene wasO. Up-regulating gene expression by heat shock of 42 02C was dependent on existence of HO, while that PEG-6000 treatment was not. Up-regulating gene expression with ABA was partially dependent on HO. The relative expression of gene too, and chelating Ca with methyleneglycol-bis- (2-aminoethylether)-N,N,N07,N07-tetraacetic acid (EGTA) and blocking Ca intracellular transport with verapamil (Vp) did not decrease gene expression up-regulated by heat shock, PEG and ABA treatments. Those results illustrated that O signal transduction pathway. While treated with HO, the treatment, suggesting that they are the downstream binding proteins of ZmHSF-Likeresponding to Ca. Those results further indicated that ZmHSF-Like makes response to different stresses through binding different HSPs.
DOI:10.3969/j.issn.1674-7968.2015.01.005URL [本文引用: 3]

Heat shock transcription factors (Hsfs) widely exist in plants and play a key role under extreme environmental conditions, especially under heat shock stress. Most researches focused on model plants such as Arabidopsis and tomato (Lycopersicon esculentum). There were few reports about maize Hsfs so far. In this study, a Hsf gene, named ZmHsf06 (GenBank accession: GRMZM2G115456_T01), was cloned from maize (Zea mays) young leaves treated by heat shock at 42 for 1 h using homologous cloning methods. The patterns of ZmHsf06 expression level in different organs and its subcellular location were analyzed. Sequence analysis showed that the coding sequences (CDS) of ZmHsf06 was 1 584 bp encoding a protein of 527 amino acids. ZmHsf06 contained not only the most conserved and typical DNA-binding domain (DBD) of Hsf family, but also other functional domains such as a nuclear localization signal (NLS) KKRR peptide, a nuclear export signal (NES) IGDLTEQM peptide and an aromatic, large hydrophobic and acidic amino residues (AHA) DSFWEQFL peptide. Real-time quantitative PCR (qRT-PCR) analysis showed that under normal growth conditions, ZmHsf06 expressed in roots, stems and leaves of maize seedlings with the highest expression level in roots, and also in functional leaves, ears, immature embryo and pollen at anther period with the highest expression level in pollen and the lowest in leaves. The expression level of ZmHsf06 was up-regulated by 42 heat shock, abscisic acid(ABA) and salt stress, respectively. In details, under heat shock of 42 , the highest expression level of ZmHsf06 in roots was 16 folds of that in leaves, and it appeared later than in leaves. While treated with ABA, the highest expression level in leaves was 2 folds of that in roots, and it appeared later than in roots. Under salt stress, the expression levels of ZmHsf06 inleaves and roots were increased significantly and shared the similar patterns. Based on transient expression assay using onion (Allium cepa L.) epidermis, it was found that the ZmHsf06 was located in nucleus specifically both under normal condition and heat shock at 37 within 1 h. It suggested that ZmHsf06 probably participates in signal transduction process of pollen development and responses to abiotic stresses at transcription level, and functions in nucleus. The results of this work provide basic data for isolating and characterizing more Hsfs in maize, as well as exploring their biological functions in abiotic stresses.
DOI:10.3969/j.issn.1674-7968.2015.01.005URL [本文引用: 3]

Heat shock transcription factors (Hsfs) widely exist in plants and play a key role under extreme environmental conditions, especially under heat shock stress. Most researches focused on model plants such as Arabidopsis and tomato (Lycopersicon esculentum). There were few reports about maize Hsfs so far. In this study, a Hsf gene, named ZmHsf06 (GenBank accession: GRMZM2G115456_T01), was cloned from maize (Zea mays) young leaves treated by heat shock at 42 for 1 h using homologous cloning methods. The patterns of ZmHsf06 expression level in different organs and its subcellular location were analyzed. Sequence analysis showed that the coding sequences (CDS) of ZmHsf06 was 1 584 bp encoding a protein of 527 amino acids. ZmHsf06 contained not only the most conserved and typical DNA-binding domain (DBD) of Hsf family, but also other functional domains such as a nuclear localization signal (NLS) KKRR peptide, a nuclear export signal (NES) IGDLTEQM peptide and an aromatic, large hydrophobic and acidic amino residues (AHA) DSFWEQFL peptide. Real-time quantitative PCR (qRT-PCR) analysis showed that under normal growth conditions, ZmHsf06 expressed in roots, stems and leaves of maize seedlings with the highest expression level in roots, and also in functional leaves, ears, immature embryo and pollen at anther period with the highest expression level in pollen and the lowest in leaves. The expression level of ZmHsf06 was up-regulated by 42 heat shock, abscisic acid(ABA) and salt stress, respectively. In details, under heat shock of 42 , the highest expression level of ZmHsf06 in roots was 16 folds of that in leaves, and it appeared later than in leaves. While treated with ABA, the highest expression level in leaves was 2 folds of that in roots, and it appeared later than in roots. Under salt stress, the expression levels of ZmHsf06 inleaves and roots were increased significantly and shared the similar patterns. Based on transient expression assay using onion (Allium cepa L.) epidermis, it was found that the ZmHsf06 was located in nucleus specifically both under normal condition and heat shock at 37 within 1 h. It suggested that ZmHsf06 probably participates in signal transduction process of pollen development and responses to abiotic stresses at transcription level, and functions in nucleus. The results of this work provide basic data for isolating and characterizing more Hsfs in maize, as well as exploring their biological functions in abiotic stresses.
DOI:10.1071/FP15080URL [本文引用: 2]

plant sciences , function , microbial , plant , biology , plant biology , physiology , functional , ecophysiology , metabolomics , genomics , molecular biology , biochemistry , biophysics , developmental biology , cell biology , genetics , plant environment interactions , plant microbe interactions , CSIRO , CSIRO PUBLISHING , publications , science , educational , scientific , journal , journals , Australia , Australian , international
DOI:10.1111/pce.12263URLPMID:243720251 [本文引用: 1]

Copper is an essential micronutrient for plant growth and development, and copper transporter plays a pivotal role for keeping copper homeostasis. However, little is known about copper transporters in wheat. Here, we report a novel copper transporter gene family, TaCT1, in common wheat. Three TaCT1 homoeologous genes were isolated and assigned to group 5 chromosomes. Each of the TaCT1 genes (TaCT1-5A, 5B or 5D) possesses 12 transmembrane domains. TaCT1 genes exhibited higher transcript levels in leaf than in root, culm and spikelet. Excess copper down-regulated the transcript levels of TaCT1 and copper deficiency-induced TaCT1 expression. Subcellular experiments localized the TaCT1 to the Golgi apparatus. Yeast expression experiments and virus-induced gene silencing analysis indicated that the TaCT1 functioned in copper transport. Site-directed mutagenesis demonstrated that three amino acid residues, Met35, Met38 and Cys365, are required for TaCT1 function. Phylogenetic and functional analyses suggested that homologous genes shared high similarity with TaCT1 may exist exclusively in monocot plants. Our work reveals a novel wheat gene family encoding major facilitator superfamily (MFS)-type copper transporters, and provides evidence for their functional involvement in promoting copper uptake and keeping copper homeostasis in common wheat.
DOI:10.1093/nar/20.6.1425URLPMID:312198 [本文引用: 2]

Nucleic Acids Res. 1992 Mar 25;20(6):1425. Research Support, Non-U.S. Gov't
[本文引用: 4]
[本文引用: 4]
DOI:10.1093/jxb/erm184URL [本文引用: 1]

Heat shock transcription factors (Hsfs) are the central regulators of the heat shock (HS) stress response in all eukaryotic organisms. HsfA2 is one of the Arabidopsis class A Hsfs, and the induction of HsfA2 expression in response to HS stress is highest among all 21 Arabidopsis Hsfs. In this study, it is reported that basal and acquired thermotolerance was significantly enhanced in high-level HsfA2-overexpressed transgenic lines (El2Omega::HsfA2) in comparison with wild-type plants. By contrast, the dominant negative mutants of HsfA2 (El2Omega::HsfA2DeltaC264) plants displayed reduced thermotolerance. These results indicate that the HsfA2 gene plays a role in the HS stress response. Microarray analysis of the El2Omega::HsfA2 plants identified putative target genes, which included HS stress-inducible genes and other stress-responsive genes. Salt and osmotic stress induced HsfA2 gene expression. In fact, the El2Omega::HsfA2 plants showed enhanced tolerance to these stresses, suggesting that HsfA2 was involved in multiple stress tolerance. El2Omega::HsfA2 plants showed accelerated callus growth from root explants compared with the wild type, unlike the El2Omega::HsfA2DeltaC264 plants whose growth was delayed. These observations suggest that HsfA2 plays, in addition to its role in stress tolerance, an important role in cell proliferation.
