 ,1,*, 韩天富
,1,*, 韩天富 ,2,*
,2,*Characterization of Growth Period Structure and Identification of E Genes of MGIII Soybean Varieties from Different Geographic Regions
JIANG Hong1,2,**, SUN Shi2,**, SONG Wen-Wen2,**, WU Cun-Xiang2, WU Ting-Ting2, HU Shui-Xiu ,1,*, HAN Tian-Fu
,1,*, HAN Tian-Fu ,2,*
,2,*通讯作者:
第一联系人:
收稿日期:2018-02-14接受日期:2018-06-12网络出版日期:2018-07-02
| 基金资助: |
Received:2018-02-14Accepted:2018-06-12Online:2018-07-02
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (351KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
江红, 孙石, 宋雯雯, 吴存祥, 武婷婷, 胡水秀, 韩天富. 不同地理来源MGIII组大豆品种生育期结构分析及E基因型鉴定[J]. 作物学报, 2018, 44(10): 1448-1458. doi:10.3724/SP.J.1006.2018.01448
JIANG Hong, SUN Shi, SONG Wen-Wen, WU Cun-Xiang, WU Ting-Ting, HU Shui-Xiu, HAN Tian-Fu.
生育期性状包括全生育期、营养生长期(V期)、生殖生长期(R期)[1]和生育期结构(R/V)[2,3], 是大豆最重要的生态性状。我国大豆生产区域广大, 地理环境和耕作制度复杂, 各地大豆品种在生育期性状上形成了丰富的多样性[4,5]。宋雯雯等[6]在全国36个试验点对共计840个大豆品种进行生育期组精细划分, 证明我国大豆品种生育期组范围为MG000-IX。Jia等[7]在中国东北高纬度冷凉地区及俄罗斯远东大豆品种中发现早于MG000组的材料。宋雯雯等[6]进一步证明, MGIII是覆盖大豆品种数量最多的生育期组。部分****发现, 同一生育期组品种在生育期结构上表现出明显差异[2-3,8]。大豆的生育期性状由包括E1~E10和J在内的多个主效基因[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]及微效多基因控制[26]。除E6和E9外, 显性E基因都对开花和成熟起到不同程度的延迟作用[13,16], 且在不同的生育期阶段存在加性效应[12], 其表达效应还受光周期、温度等环境因素[27,28]及播期的影响[19]。随着E1[20]、E2[21]、E3[22]、E4[23]、E9[16]、J[24,25]等相继被克隆, 对不同大豆品种的E基因型鉴定更加容易, 已发现丰富的E位点等位变异[29,30,31,32,33]。其中E1的等位变异有e1-as、e1-fs、e1-nl、e1-re等, 这些变异均会引起大豆光周期敏感性不同程度的弱化[20,30,34]; E2的等位变异包括可造成功能缺失的e2-ns和功能尚不清楚的E2-in及E2-dl[30,31]; E3的等位变异有E3-Mi、E3-Ha、引起功能缺失的e3-tr, 以及引起光周期敏感度变化的e3-ns和e3-fs[22,32]; E4的等位变异有e4-SORE-1及在高纬度大豆品种中发现的e4-oto、e4-tsu、e4-kam和e4-kes[23,33]。近年研究发现, E1~E4等位变异组合对大豆品种的生育期组归属有决定性影响[35], E基因的构成、基因之间及基因与环境因子(如光周期、温度)的互作也与生育期结构有关[3,36-37]。Jiang等[35]在北美生育期组标准品种中发现同一种E1~E4基因型对应于1个以上生育期组的现象; Jia等[7]在中国东北高纬度冷凉地区及俄罗斯远东地区MG0000-0组早熟大豆品种中发现1个生育期组对应多种E1~E4基因型的情况。然而, 对于中晚熟大豆品种E基因型多态性尚未进行深入的研究。本试验收集到我国覆盖纬度范围最大、播季类型最多、最能体现我国生态类型和种植制度多样性的MGIII大豆代表性品种[5-6,38-39], 在北京地区种植, 对其生育期性状特点和E基因型进行分析, 并比较不同来源MGIII组品种的农艺性状表现, 以期深入了解不同生态类型品种的生育期特点及与基因型和环境的关系, 为大豆生育期性状分子设计育种提供理论依据。
1 材料与方法
1.1 供试材料
60个供试材料分别来自美国和我国不同大豆生态区(表1), 这些品种经过多年多点生育期组鉴定试验, 已明确生育期组归属为MGIII[6]。Table 1
表1
表1用于鉴定E1、E2、E3和E4基因型的引物
Table 1
| 基因座 Locus | 等位基因 Allele | 引物序列 Primer sequence (5°-3°) | 参考文献 Reference |
|---|---|---|---|
| E1 | e1-nl/e1-as/e1-fs | F: CACTCAAATTAAGCCCTTTCA; R: TCCGATCTCATCACCTTTCC | [20], [31] |
| E2 | e2 | F: ACAGCCTCCCGTGCTG; R: TCCCATGAGTTGCGGAATGG | |
| E3 | e3-ns | F: GTCTTTTGTCCTTGTCATTTGTGT; R: CAAAGCATCATCCAATACCCTCC | |
| e3-fs | F: GGGATAGTTCTGATGCTGTTCAA; R: CCTTGTATCGATAGCATATGTGCT | [31] | |
| E3-Mi | F: TGGGTCTTCAGTTCAGTTGG; R: TGCTTCCTTTCACTTTCTGATG | [31] | |
| E3-Ha | F: TGGAGGGTATTGGATGATGC; R: CGGTCAAGAGCCAACATGAG | ||
| e3-tr | F: TGGAGGGTATTGGATGATGC; R: GTCCTATACAATTCTTTACGACG | ||
| E4 | e4-oto/e4-tsu/e4-kam/e4-kes | F: AGACGTAGTGCTAGGGCTAT; R: GCATCTCGCATCACCAGATCA | [23] |
| e4-SORE--1 | F: GCATCTCGCATCACCAGATCA; R: GCTCATCCCTTCGAATTCAG | [32] |
新窗口打开|下载CSV
1.2 种植方法
田间试验于2014年和2015年进行, 春播均在中国农业科学院作物科学研究所院部试验农场进行, 播期分别为2014年4月30日和2015年5月4日; 夏播在该所昌平试验基地进行, 播期分别为2014年5月20日和2015年6月17日。春播时每品种1行, 行长2 m, 重复3次, 每行40粒, 定苗20株。夏播时每品种3行, 行长6 m, 重复3次, 每行120粒, 定苗60株。2年试验结果趋势一致, 但2014年大部分品种鼓粒至成熟期病虫害较重, 致使农艺性状数据不够完整, 故在结果分析中以2015年数据为主。1.3 记载项目及标准
根据Fehr和Carviness[1]提出的大豆生育时期分期标准分株挂牌调查大豆的出苗期(VE)、初花期(R1)、生理成熟期(R7)和完熟期(R8)。昌平夏播收获时, 选取30份供试材料(9个北方品种, 15个黄淮海品种, 6个南方品种), 于每行随机取10株, 按照《大豆种质资源描述规范和数据标准》, 对株高、底荚高度、主茎节数、分枝数、总荚数、秕荚数及百粒重等产量相关性状进行考种。1.4 DNA提取
用CTAB法提取大豆幼叶中的DNA, 1%的琼脂糖凝胶电泳检测DNA质量, 并用Naco 2000超微量分光光度计测定DNA的浓度后稀释成工作液25 ng μL-1。1.5 E1、E2、E3和E4基因型的鉴定
根据Xu等[31]报道的方法, 用E1~E4基因特异性引物扩增待鉴定品种的DNA, 再经琼脂糖凝胶电泳检测和测序分析, 对E1、E2、E3和E4进行基因型鉴定, 引物见表1。1.6 数据统计分析
利用IBM SPSS数据处理系统(IBM SPSS Statistics 19.0)对2015年供试品种的生育期性状及农艺性状分区域进行多重比较分析, 对生育期性状和农艺性状进行Pearson相关性分析。在分析E基因显性位点的平均效应时, 选取背景基因型相同且仅1个基因在显、隐性位点上有差别的基因型计算二者表型差值, 再以该基因在不同背景基因型中表型差值的平均数作为该基因位点的效应值[27]。2 结果与分析
2.1 不同地理来源MGIII组大豆品种生育期性状的比较
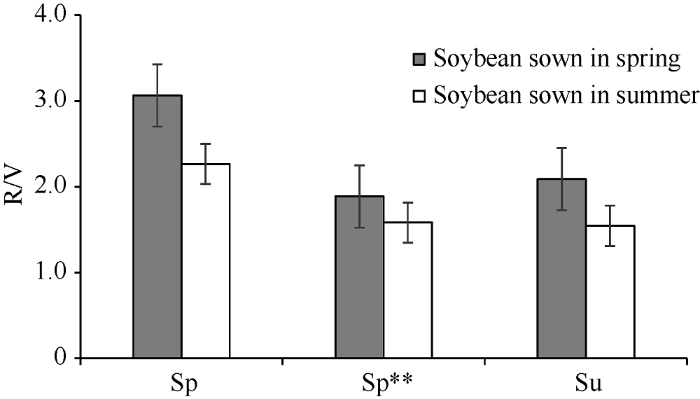
从表2可以看出, 不同地理来源的MGIII组大豆品种成熟期相近, 但V期、R期及R/V值存在明显差异且受播季影响。在春播条件下, 中国北方和北美春大豆MGIII组品种V期相近, 中国黄淮海和中国南方品种V期相近且均比中国北方及北美品种长, 而在R期上则情况相反; 中国北方和北美春大豆品种R/V值相近, 均值皆为3.1; 中国黄淮海和中国南方品种R/V值相近, 分别为2.1和1.9, 小于中国北方地区。在夏播条件下, 中国北方品种V期较短, R期较长, R/V值为2.3; 而中国黄淮海和中国南方品种的R/V值分别为1.6和1.4。夏播供试品种总体上比春播时V期延长, R期缩短, 全生育期缩短, R/V值变小, 属于“前延后促”[40]。Table 2
表2
表2不同地理来源MGIII大豆品种在北京春播和夏播条件下的生育期性状表现
Table 2
| 地理来源 Region | 品种 Variety | 播季类型 Sowing type | 春播 Spring-sowing | 夏播Summer-sowing | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VE-R7 | VE-R1 | R1-R7 | R/V | VE-R7 | VE-R1 | R1-R7 | R/V | ||||||||||||||||||||||
| 中国北方 North China | 吉38 Ji 38 | Sp | 98.8±3.9 | 23.9±2.0 | 75.0±2.6 | 3.2±0.2 | 95.0±3.4 | 25.9±2.6 | 69.4±4.4 | 2.7±0.4 | |||||||||||||||||||
| 吉82 Ji 82 | Sp | 99.5±9.2 | 25.3±2.1 | 75.2±7.4 | 3.1±0.1 | 104.1±0.4 | 30.2±2.3 | 75.1±1.7 | 2.6±0.2 | ||||||||||||||||||||
| 吉91 Ji 91 | Sp | 101.8±3.0 | 21.5±1.7 | 80.2±4.7 | 3.8±0.5 | 90.8±1.2 | 24.6±1.2 | 66.2±2.3 | 2.7±0.2 | ||||||||||||||||||||
| 吉农11 Jinong 11 | Sp | 97.1±1.5 | 20.9±0.2 | 76.3±1.8 | 3.7±0.1 | 87.9±3.1 | 26.3±2.2 | 61.6±4.9 | 2.4±0.4 | ||||||||||||||||||||
| 九农24 Jiunong 24 | Sp | 94.8±2.9 | 20.8±1.5 | 74.0±1.6 | 3.6±0.2 | 91.0±1.7 | 24.6±1.1 | 66.8±1.8 | 2.8±0.1 | ||||||||||||||||||||
| 长农8号 Changnong 8 | Sp | 106.6±7.2 | 21.9±1.6 | 84.8±7.5 | 3.9±0.5 | 91.7±4.3 | 26.4±0.8 | 65.3±3.5 | 2.5±0.1 | ||||||||||||||||||||
| 蒙豆24 Mengdou 24 | Sp | 95.7±3.9 | 20.6±2.0 | 75.1±2.1 | 3.7±0.3 | 95.0±3.6 | 25.5±1.1 | 69.4±4.7 | 2.7±0.3 | ||||||||||||||||||||
| 抚97-16 Fu 97-16 | Sp | 99.0±3.6 | 22.7±0.6 | 76.3±4.1 | 3.4±0.3 | 89.3±2.5 | 25.2±1.8 | 64.1±2.3 | 2.6±0.2 | ||||||||||||||||||||
| 通农12 Tongnong 12 | Sp | 100.4±3.1 | 28.3±2.9 | 72.1±2.4 | 2.6±0.3 | 89.7±2.8 | 35.3±0.7 | 54.3±2.7 | 1.5±0.1 | ||||||||||||||||||||
| 铁丰31 Tiefeng 31 | Sp | 102.8±5.6 | 22.9±2.8 | 79.9±3.0 | 3.5±0.4 | 104.4±2.8 | 25.2±0.4 | 79.3±2.5 | 3.1±0.1 | ||||||||||||||||||||
| 铁丰33 Tiefeng 33 | Sp | 94.8±9.0 | 21.5±1.6 | 73.3±7.5 | 3.4±0.1 | 92.5±0.8 | 25.6±0.5 | 66.9±1.2 | 2.6±0.1 | ||||||||||||||||||||
| 中黄30 Zhonghuang 30 | Sp | 102.0±6.1 | 24.2±1.2 | 77.8±5.8 | 3.2±0.3 | 92.4±2.3 | 30.0±0.3 | 62.4±2.1 | 2.1±0.1 | ||||||||||||||||||||
| 中黄35 Zhonghuang 35 | Sp | 112.5±9.2 | 54.5±1.3 | 58.1±10.5 | 1.1±0.2 | 92.3±1.5 | 51.3±0.7 | 40.9±1.0 | 0.8±0.0 | ||||||||||||||||||||
| 辽豆15 Liaodou 15 | Sp | 111.6±1.1 | 41.9±5.9 | 69.7±6.6 | 1.7±0.4 | 98.4±2.3 | 39.1±0.7 | 59.3±1.5 | 1.5±0.0 | ||||||||||||||||||||
| 辽豆31 Liaodou 31 | Sp | 112.0±5.5 | 39.9±3.0 | 72.1±3.1 | 1.8±0.1 | 101.7±2.2 | 39.4±0.7 | 62.3±2.6 | 1.6±0.1 | ||||||||||||||||||||
| 晋豆19 Jindou 19 | Sp | 102.6±2.7 | 22.4±0.7 | 80.2±3.2 | 3.6±0.3 | 91.3±2.0 | 29.7±0.8 | 61.6±1.5 | 2.1±0.1 | ||||||||||||||||||||
| 平均值 Mean | 102.0±5.9 b | 27.1±9.7 b | 75.0±5.9 ab | 3.1±0.8 a | 94.2±5.2 b | 30.3±7.5 b | 64.1±8.6 a | 2.3±0.6 a | |||||||||||||||||||||
| 中国黄淮海 Yellow-Huai- Hai River Valley of China | 诱变30 Youbian 30 | Su | 114.7±4.4 | 47.7±4.6 | 67.0±2.1 | 1.4±0.1 | 108.4±1.9 | 52.3±0.7 | 56.1±2.5 | 1.1±0.1 | |||||||||||||||||||
| 中黄37 Zhonghuang 37 | Su | 114.6±0.9 | 40.7±0.5 | 74.0±1.0 | 1.8±0.1 | 105.1±0.6 | 40.2±0.6 | 64.9±1.0 | 1.6±0.0 | ||||||||||||||||||||
| 中黄39 Zhonghuang 39 | Su | 111.1±3.9 | 43.9±1.4 | 67.3±3.4 | 1.5±0.1 | 104.3±0.6 | 43.3±0.6 | 61.1±0.3 | 1.4±0.0 | ||||||||||||||||||||
| 中黄13 Zhonghuang 13 | Su | 108.0±1.7 | 41.3±1.5 | 66.8±3.1 | 1.6±0.1 | 99.7±0.1 | 42.5±0.4 | 57.2±0.6 | 1.3±0.0 | ||||||||||||||||||||
| 沧豆6号 Cangdou 6 | Su | 110.6±2.0 | 30.7±2.2 | 79.8±2.4 | 2.6±0.2 | 91.1±2.6 | 37.3±0.7 | 54.0±2.4 | 1.5±0.1 | ||||||||||||||||||||
| 冀豆12 Jidou 12 | Su | 109.5±6.2 | 29.7±2.7 | 79.9±3.6 | 2.7±0.1 | 98.3±2.8 | 37.9±0.3 | 60.4±2.9 | 1.6±0.1 | ||||||||||||||||||||
| 邯豆5号 Handou 5 | Su | 106.6±9.6 | 24.2±4.1 | 82.4±9.2 | 3.5±0.8 | 96.7±3.4 | 29.2±1.3 | 67.5±4.3 | 2.3±0.2 | ||||||||||||||||||||
| 丰收黄 Fengshouhuang | Su | 110.0±4.5 | 28.7±1.4 | 81.3±2.3 | 2.8±0.2 | 99.5±2.7 | 35.1±1.0 | 64.2±3.3 | 1.8±0.1 | ||||||||||||||||||||
| 鲁豆4号 Ludou 10 | Su | 99.1±2.4 | 34.3±1.8 | 64.8±3.9 | 1.9±0.2 | 89.6±1.1 | 34.8±1.4 | 54.8±1.6 | 1.6±0.1 | ||||||||||||||||||||
| 临豆10号 Lindou 10 | Su | 108.7±7.0 | 34.3±5.6 | 74.4±1.6 | 2.2±0.3 | 104.0±1.3 | 41.3±0.7 | 62.7±1.1 | 1.5±0.0 | ||||||||||||||||||||
| 齐黄34 Qihuang 34 | Su | 108.4±2.3 | 38.5±3.6 | 70.0±2.1 | 1.8±0.2 | 104.3±0.8 | 42.0±1.0 | 62.3±0.3 | 1.5±0.0 | ||||||||||||||||||||
| 齐黄35 Qihuang 35 | Su | 106.3±3.3 | 38.1±3.1 | 68.1±0.3 | 1.8±0.1 | 103.0±0.7 | 38.8±1.1 | 64.2±1.7 | 1.7±0.1 | ||||||||||||||||||||
| 山宁11 Shanning 11 | Su | 107.5±7.2 | 41.0±1.2 | 66.5±7.7 | 1.6±0.2 | 101.3±1.1 | 39.4±0.4 | 61.9±0.9 | 1.6±0.0 | ||||||||||||||||||||
| 山宁14 Shanning 14 | Su | 105.5±3.1 | 32.6±6.4 | 72.9±8.2 | 2.3±0.8 | 100.1±2.8 | 36.4±2.4 | 63.8±4.6 | 1.8±0.2 | ||||||||||||||||||||
| 山宁12 Shanning 12 | Su | 110.5±7.8 | 37.9±3.5 | 72.6±4.6 | 1.9±0.1 | 97.1±2.0 | 40.3±0.7 | 57.1±2.0 | 1.4±0.0 | ||||||||||||||||||||
| 冀豆17 Jidou 17 | Su | 104.7±0.8 | 30.3±0.8 | 74.4±0.4 | 2.5±0.1 | 95.5±3.0 | 33.3±0.7 | 62.4±2.7 | 1.9±0.1 | ||||||||||||||||||||
| 菏豆19 Hedou 19 | Su | 107.1±9.7 | 33.2±4.0 | 73.9±5.7 | 2.2±0.1 | 102.7±0.8 | 38.6±1.0 | 64.0±1.8 | 1.7±0.1 | ||||||||||||||||||||
| 菏豆12 Hedou 12 | Su | 112.6±0.3 | 43.9±3.4 | 68.8±3.2 | 1.6±0.2 | 106.5±2.1 | 45.3±0.7 | 61.4±2.0 | 1.4±0.0 | ||||||||||||||||||||
| 菏豆13 Hedou 13 | Su | 108.0±3.6 | 39.1±1.7 | 68.9±1.9 | 1.8±0.0 | 96.1±1.9 | 43.3±0.7 | 53.0±1.6 | 1.2±0.0 | ||||||||||||||||||||
| 徐豆18 Xudou 18 | Su | 113.5±2.8 | 33.9±3.9 | 79.6±2.1 | 2.4±0.3 | 106.0±1.0 | 41.3±0.7 | 64.7±0.5 | 1.6±0.0 | ||||||||||||||||||||
| 淮豆9号 Huaidou 9 | Su | 114.1±2.4 | 45.1±2.9 | 69.0±6.5 | 1.5±0.3 | 106.4±1.8 | 42.9±0.1 | 63.4±1.9 | 1.5±0.0 | ||||||||||||||||||||
| 徐豆14 Xudou 14 | Su | 115.3±3.4 | 34.5±4.3 | 80.8±3.9 | 2.4±0.4 | 103.2±1.0 | 39.1±1.0 | 63.6±0.1 | 1.6±0.0 | ||||||||||||||||||||
| 徐豆15 Xudou 15 | Su | 112.7±4.1 | 33.7±3.2 | 79.0±3.3 | 2.4±0.2 | 85.3±1.1 | 27.2±2.8 | 57.8±2.7 | 2.1±0.3 | ||||||||||||||||||||
| 徐豆9号 Xudou 9 | Su | 108.4±4.0 | 32.0±3.9 | 76.4±4.1 | 2.4±0.4 | 103.6±2.1 | 40.3±0.7 | 63.3±2.1 | 1.6±0.1 | ||||||||||||||||||||
| 皖宿2156 Wansu 2156 | Su | 111.4±4.9 | 42.4±1.0 | 69±4.6 | 1.6±0.1 | 99.9±3.4 | 42.4±0.9 | 57.5±3.8 | 1.4±0.1 | ||||||||||||||||||||
| 皖宿5717 Wansu 5717 | Su | 106.9±1.0 | 32.1±0.3 | 74.8±0.7 | 2.3±0.1 | 102.3±2.4 | 36.7±1.1 | 65.6±3.4 | 1.8±0.1 | ||||||||||||||||||||
| 平均值 Mean | 109.5±3.7 a | 36.3±5.8 a | 73.2±5.4 bc | 2.1±0.5 b | 100.4±5.5 a | 39.3±5.0 a | 61.1±3.9 a | 1.6±0.3 b | |||||||||||||||||||||
| 地理来源 Region | 品种 Variety | 播季类型 Sowing type | 春播 Spring-sowing | 夏播Summer-sowing | |||||||||||||||||||||||||
| VE-R7 | VE-R1 | R1-R7 | R/V | VE-R7 | VE-R1 | R1-R7 | R/V | ||||||||||||||||||||||
| 中国南方 South China | 浙H0634 Zhe H0634 | Sp** | 96.4±2.4 | 21.7±0.3 | 74.7±2.2 | 3.4±0.1 | 89.9±1.7 | 26.3±0.7 | 63.6±1.7 | 2.4±0.1 | |||||||||||||||||||
| 中豆39 Zhongdou 39 | Sp** | 115.1±0.1 | 43.0±2.1 | 70.7±0.1 | 1.6±0.1 | 100.5±1.8 | 43.0±1.8 | 57.5±0.5 | 1.3±0.1 | ||||||||||||||||||||
| 川豆15 Chuandou 15 | Sp** | 113.9±6.0 | 45.2±3.3 | 68.7±9.1 | 1.5±0.3 | 108.0±2.8 | 46.6±1.1 | 62.0±2.8 | 1.3±0.1 | ||||||||||||||||||||
| 贡豆22 Gongdou 22 | Sp** | 105.1±3.5 | 35.8±1.8 | 69.3±4.3 | 1.9±0.2 | 91.4±0.3 | 40.8±1.7 | 50.5±2.1 | 1.2±0.1 | ||||||||||||||||||||
| 贡豆21 Gongdou 21 | Sp** | 112.7±4.2 | 43.1±3.0 | 69.6±6.3 | 1.6±0.2 | 100.3±0.8 | 42.3±0.7 | 58.3±0.8 | 1.4±0.0 | ||||||||||||||||||||
| 南豆21 Nandou 21 | Sp** | 98.9±5.7 | 39.4±2.2 | 59.5±6.9 | 1.5±0.2 | 100.5±1.0 | 43.3±0.7 | 57.5±1.0 | 1.3±0.0 | ||||||||||||||||||||
| 福豆310 Fudou 310 | Sp** | 113.5±3.6 | 44.7±1.6 | 68.7±2.7 | 1.5±0.1 | 92.7±3.1 | 42.7±0.8 | 50.0±3.9 | 1.2±0.1 | ||||||||||||||||||||
| 黔豆3号 Qiandou 3 | Sp** | 109.6±2.5 | 37.5±1.4 | 72.2±3.0 | 1.9±0.1 | 90.7±0.5 | 41.2±0.4 | 49.4±0.7 | 1.2±0.0 | ||||||||||||||||||||
| 黔豆5号 Qiandou 5 | Sp** | 101.0±3.6 | 36.5±1.3 | 64.5±2.6 | 1.8±0.1 | 87.2±1.9 | 39.6±1.2 | 47.6±1.5 | 1.2±0.1 | ||||||||||||||||||||
| 黔豆1号 Qiandou 1 | Sp** | 111.5±0.7 | 36.7±2.9 | 74.1±4.4 | 2.0±0.3 | 91.5±9.9 | 39.2±0.4 | 52.3±10.1 | 1.3±0.3 | ||||||||||||||||||||
| 平均值 Mean | 107.8±6.9 a | 38.4±6.9 a | 69.2±4.5 c | 1.9±0.6 b | 95.3±6.6 b | 40.5±5.4 a | 54.9±5.6 b | 1.4±0.4 b | |||||||||||||||||||||
| 北美 North America | Athow | Sp | 101.3±2.7 | 26.2±0.3 | 75.3±3.5 | 2.9±0.1 | — | — | — | — | |||||||||||||||||||
| Dilworth | Sp | 104.6±2.0 | 25.5±0.7 | 80.2±0.3 | 3.2±0.1 | — | — | — | — | ||||||||||||||||||||
| Iroquois | Sp | 98.2±4.2 | 22.7±0.4 | 77.3±3.5 | 3.4±0.1 | — | — | — | — | ||||||||||||||||||||
| Kottman | Sp | 102.9±2.5 | 23.4±0.3 | 81.0±0.1 | 3.5±0.1 | — | — | — | — | ||||||||||||||||||||
| Macon | Sp | 106.3±1.1 | 25.1±0.1 | 81.6±1.1 | 3.3±0.1 | — | — | — | — | ||||||||||||||||||||
| Will | Sp | 101.1±2.7 | 26.7±0.7 | 75.7±3.0 | 2.8±0.2 | — | — | — | — | ||||||||||||||||||||
| Williams 82 | Sp | 105.2±5.2 | 27.4±0.6 | 80.8±0.2 | 3.0±0.1 | — | — | — | — | ||||||||||||||||||||
| Zane | Sp | 104.5±2.3 | 26.1±1.8 | 79.5±0.1 | 3.1±0.2 | — | — | — | — | ||||||||||||||||||||
| 平均值 Mean | 103.0±2.8 b | 25.4±1.6 b | 78.9±2.5 a | 3.1±0.2 a | — | — | — | — | |||||||||||||||||||||
新窗口打开|下载CSV
2.2 不同播季类型MGIII组大豆品种生育期性状的差异
从表3可以看出, 不同播季类型MGIII组的大豆品种成熟期相近, 但V期、R期及R/V值差异较大, 且受播期影响。在春播条件下, 北方春大豆(Sp) V期较短, R期较长, R/V平均值在各类品种中最大; 黄淮海夏大豆(Su)和南方春大豆(Sp**) V期相近且较北方春大豆(Sp)长, R期则相近且较北方春大豆(Sp)短, R/V值分别为2.1和1.9, 均比北方春大豆(Sp)小。在夏播条件下, 北方春大豆(Sp)V期最短, R期最长, R/V值最大; 黄淮海夏大豆(Su)和南方春大豆(Sp**)的V期相近且较北方春大豆(Sp)长, R期则较北方春大豆(Sp)短, R/V值分别为1.6和1.4, 均比北方春大豆(Sp)小。与春播相比, 夏播时存在“前延后促”现象[40], 在各播期类型品种中, 北方春播类型(Sp)“前延”幅度最大、“后促”幅度最小。Table 3
表3
表3不同播季类型MGIII大豆在北京春播和夏播条件下的生育期结构性状表现
Table 3
| 播季类型 Sowing type | 春播 Spring-sowing | 夏播 Summer-sowing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VE-R7 | VE-R1 | R1-R7 | R/V | VE-R7 | VE-R1 | R1-R7 | R/V | ||
| 北方春大豆(Sp) | 102.3±5.0 b | 26.5±8.0 b | 76.3±5.3 a | 3.1±0.7 a | 94.2±5.2 b | 30.3±7.5 b | 64.1±8.6 a | 2.3±0.6 a | |
| 黄淮海夏大豆(Su) | 109.5±3.7 a | 36.3±5.8 a | 73.2±5.4 a | 2.1±0.5 b | 100.4±5.5 a | 39.3±5.0 a | 61.1±3.9 ab | 1.6±0.3 b | |
| 南方春大豆(Sp**) | 107.8±6.9 a | 38.4±6.9 a | 69.2±4.5b | 1.9±0.6 b | 95.3±6.6 b | 40.5±5.4 a | 54.9±5.6 b | 1.4±0.4 b | |
| 平均值 Mean | 106.2±5.9 | 32.7±8.6 | 73.7±5.7 | 2.5±0.8 | 97.5±6.3 | 36.7±7.3 | 60.8±6.7 | 1.8±0.5 | |
新窗口打开|下载CSV
2.3 不同地理来源MGIII组大豆品种的E基因型构成
从表4可看出, 不同来源的中国MGIII组大豆品种的生育期主效基因(E1~E4)组成存在一定差异, 共有9种组合, 其中E3-Mi和E3-Ha编码同种氨基酸, 表现的基因功能一致, 故用E3表示这2种等位变异, 最终将E基因型分为6种组合(9-3 = 6)。其中, E1e2E3E4和e1-asE2E3E4占的比例最大, 分别为78.8%和9.6%, 在我国分布区域广, 覆盖播季类型多; E1E2E3E4、e1-ase2E3E4、E1e2e3-trE4、e1-ase2e3-trE4所占的比例较低, 只分别在1~2个品种中被检测出。而在美国春大豆品种中只鉴定出e1-asE2E3E4, 与中国东北部分品种一致, 表现出该地区大豆品种生育期基因型的单一性。Table 4
表4
表4MGIII大豆品种的E基因型分类和区域分布情况
Table 4
| 基因型 Genotype | 品种所属地区 Region | |||
|---|---|---|---|---|
| 美国 USA | 中国北方 North China | 中国黄淮海 Yellow-Huai-Hai River Valley of China | 中国南方 South China | |
| E1E2E3E4 | 菏豆12 Hedou 12 | 黔豆3号 Qiandou 3 | ||
| e1-asE2E3E4 | Athow, Zane, Dilworth, Iroquois, Williams 82, Kottman, Macon, Will | 蒙豆24, 铁丰31, 铁丰33, 中黄30 Mengdou 24, Tiefeng 31, Tiefeng 33, Zhonghuang 30 | 冀豆17 Jidou 17 | |
| e1-ase2E3E4 | 邯豆5号 Handou 5 | |||
| E1e2E3E4 | 吉38, 吉82, 吉91, 吉农11, 九农24, 长农8号, 抚97-16, 通农12, 中黄35, 辽豆15, 辽豆31, 晋豆19 Ji 38, Ji 82, Ji 91, Jinong 11, Jiunong 24, Changnong 8, Fu 97-16, Tongnong 12, Zhonghuang 35, Liaodou 15, Liaodou 31, Jindou 19 | 诱变30, 中黄37, 中黄39, 中黄13, 沧豆6号, 冀豆12, 鲁豆4号, 临豆10号, 齐黄34, 齐黄35, 山宁11, 山宁14, 山宁12, 菏豆19 Hedou 19, 菏豆13, 徐豆18, 淮豆9号, 徐豆14, 徐豆15, 徐豆9号, 皖宿2156, 皖宿5717 Youbian 30, Zhonghuang 37, Zhonghuang 39, Zhonghuang 13, Cangdou 6, Jidou 12, Ludou 4, Lindou 10, Qihuang 34, Qihuang 35, Shanning 11, Shanning 14, Shanning 12, Hedou 19, Hedou 13, Xudou 18, Huaidou 9, Xudou 14, Xudou 15, Xudou 9, Wansu 2156, Wansu 5717 | 中豆39, 川豆15, 贡豆21, 南豆21, 福豆310, 黔豆1号, 黔豆5号 Zhongdou 39, Chuandou 15, Gongdou 21, Nandou 21, Fudou 310, Qiandou 1, Qiandou 5 | |
| E1e2e3-trE4 | 丰收黄 Fengshouhuang | 贡豆22 Gongdou 22 | ||
| e1-ase2e3-trE4 | 浙H0634 Zhe H0634 | |||
新窗口打开|下载CSV
图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1春播和夏播时不同播季类型大豆品种在北京的生育期 性状
SpV: 春播时营养生长期; SpR: 春播时生殖生长期; SuV: 夏播时营养生长期; SuR: 夏播时生殖生长期; Sp: 北方春播类型; Sp** 南方春播类型; Su: 黄淮海夏播类型。
Fig. 1Maturity-related traits of different ecotypes of MGIII soybean under spring-sowing and summer-sowing conditions in Beijing
SpV: vegetative growth period of spring-sowing soybean; SpR: reproductive growth period of spring-sowing soybean; SuV: vegetative growth period of summer-sowing soybean; SuR: reproductive growth period of summer-sowing soybean; Sp: North spring-sowing soybean; Sp**: South spring-sowing soybean; Su: Yellow-Huai-Hai River Valley summer-sowing soybean.
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2春播和夏播时不同生态类型大豆品种的R/V值
Sp: 北方春播类型; Sp** 南方春播类型; Su: 黄淮海夏播类型。
Fig. 2R/V values of different ecotypes of MGIII soybean under spring-sowing and summer-sowing conditions in Beijing
Sp: North spring-sowing soybean; Sp**: South spring-sowing soybean; Su: Yellow-Huai-Hai River Valley summer-sowing soybean.
2.4 MGIII组大豆品种生育期结构与E基因型的关系
通过比较分析不同生育期主效基因型在春播和夏播条件下的全生育期、V期和R期发现, 大豆各生育期主效基因对大豆发育的不同阶段效应大小不一, 且存在明显的加性效应。总体而言, 含显性位点越多的材料, 其V期越长、R期越短、R/V值越小, 不同基因的增效作用大小不尽相同。含e1-as的大豆品种R/V值较大(3.3~3.5), 明显高于E1完全显性的大豆品种(R/V值在1.8~2.4之间)。此外, 基因与环境因子之间也存在明显的互作, 同一E基因型大豆品种在夏播时的V期比在春播时长, R期则比春播时短, R/V值减小。不同基因型大豆品种受播季影响的程度也存在差异, 如E1E2E3E4在夏播条件下V期比春播延长了2 d, R期缩短了15 d, R/V值减小0.5, 而携带e1-ase2e3-trE4的大豆品种在夏播时V期比春播延长4 d, R期缩短11 d, R/V值减小1.0 (表5)。Table 5
表 5
表 5不同E基因型的MGIII大豆品种在北京春播和夏播条件下的生育期性状表现
Table 5
| 基因型 Genotype | 春播 Spring-sowing | 夏播 Summer-sowing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VE-R7 | VE-R1 | R1-R7 | R/V | VE-R7 | VE-R1 | R1-R7 | R/V | ||
| e1-ase2e3-trE4 | 96.4±3.8 c | 21.7±1.2 c | 74.7±2.9 b | 3.4±0.3 a | 89.9±4.0 a | 26.3±7.0 c | 63.6±2.1 ab | 2.4±0.2 a | |
| e1-ase2E3E4 | 106.6±6.9 ab | 24.2±3.5 bc | 82.4±3.6 a | 3.5±0.4 a | 96.7±3.7 a | 29.2±2.4 bc | 67.5±1.3 a | 2.3±0.2 a | |
| e1-asE2E3E4 | 100.0±4.4 bc | 23.9±3.8 bc | 76.1±2.7 ab | 3.3±0.5 a | 96.0±4.9 a | 27.9±3.6 c | 68.1±6.9 a | 2.5±0.5 a | |
| E1e2e3-trE4 | 107.5±3.4 a | 32.2±5.0 ab | 75.3±8.5 b | 2.4±0.6 b | 95.5±5.7 a | 38.0±4.0 ab | 57.3±9.7 b | 1.5±0.4 b | |
| E1e2E3E4 | 107.7±5.7 a | 35.5±8.2 a | 72.2±5.8 b | 2.2±0.7 b | 98.0±6.4 a | 38.0±6.9 ab | 60.1±6.1 ab | 1.7±0.5 b | |
| E1E2E3E4 | 111.1±2.1 a | 40.7±4.5 a | 70.5±2.4 b | 1.8±0.2 b | 98.6±11.2 a | 43.3±2.9 a | 55.4±8.5 b | 1.3±0.1 b | |
新窗口打开|下载CSV
2.5 E基因等位变异对大豆生育期性状的影响及环境效应
从表6可以看出, 不同E基因的表达效应在不同生育期阶段存在差异, 且受背景基因型影响, 如E1在不同背景基因型中的显性基因相对于隐性基因的平均效应值在V期是12.8, 在R期则是-5.0, 对R/V值的效应是-1.3; 当背景基因型为E2E3E4时, E1对V期的效应值是13.8, 对R期是-5.6, 对R/V值的效应是-1.5; 当背景基因型为e2e3-trE4时, E1对V期的效应值是10.5, 对R期是0.7, 对R/V值的效应值是 -1.0。同时还可以看出, E基因的表达效应与播期有关。在春播条件下, E1、E2能够延长V期、缩短R期、降低R/V值, 对生育期性状贡献值较大; E3能够延迟开花和成熟, 降低R/V值, 对生育期性状的贡献相对较小。在夏播条件下, E1、E2、E3在延长V期和降低R/V值上的平均效应比春播时小, E1在缩短R期上的平均效应比春播时大, E2在夏播时的效应比春播时小, E3在延长R期上的平均效应比春播时大。Table 6
表6
表6不同播期下E基因对MGIII大豆品种生育期结构相关性状的效应
Table 6
| 基因 Gene | 背景基因型 Background genotype | 春播 Spring-sowing | 夏播 Summer-sowing | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VE-R7(d) | VE-R1 (d) | R1-R7 (d) | R/V | VE-R7 (d) | VE-R1 (d) | R1-R7 (d) | R/V | |||
| E1 | E2E3E4 | 11.3 | 13.8 | -5.6 | -1.5 | 2.6 | 15.3 | -12.7 | -1.2 | |
| e2E3E4 | 1.7 | 15.0 | -10.1 | -1.3 | 1.3 | 8.7 | -7.4 | -0.6 | ||
| e2e3-trE4 | 11.5 | 10.5 | 0.7 | -1.0 | 5.6 | 11.7 | -6.3 | -0.9 | ||
| 平均值 Mean | 8.2 | 12.8 | -5.0 | -1.3 | 3.1 | 11.9 | -8.8 | -0.9 | ||
| E2 | e1-asE3E4 | -5.8 | -0.3 | -6.3 | -0.2 | -0.7 | -1.2 | 0.6 | 0.2 | |
| E1E3E4 | 3.8 | 5.3 | -1.9 | -0.5 | 0.6 | 5.4 | -4.7 | -0.4 | ||
| 平均值 Mean | -1.0 | 2.5 | -4.1 | -0.4 | 0.0 | 2.1 | -2.1 | -0.1 | ||
| E3 | E1e2E4 | 0.2 | 3.1 | -3.0 | -0.2 | 2.5 | -0.1 | 2.8 | 0.1 | |
| e1-ase2E4 | 10.0 | 2.4 | 7.8 | 0.1 | 6.8 | 2.9 | 3.9 | -0.1 | ||
| 平均值 Mean | 5.1 | 2.8 | 2.4 | -0.1 | 4.6 | 1.4 | 3.4 | 0.0 | ||
新窗口打开|下载CSV
2.6 不同地理来源MGIII大豆品种生育期结构与农艺性状的关系
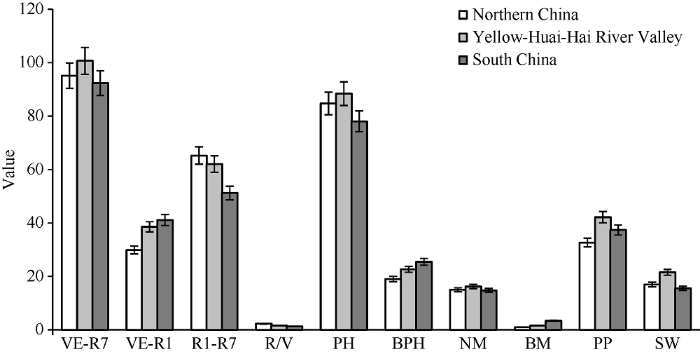
本研究选用的材料均属MGIII组, 生育期相近, 便于分析生育期结构与农艺性状的关系。从图3可以看出, 地理来源和生育期结构不同的MGIII组品种在农艺性状上存在明显差异。北方春大豆品种营养生长期较短, 生殖生长期较长, R/V值较大, 分枝数、总荚数较少, 百粒重偏小, 底荚较低; 南方品种营养生长期较长, 生殖生长期较短, 株高偏矮, 分枝多, 底荚较高, 百粒重较小; 黄淮海品种R/V值介于北方和黄淮海品种之间, 植株较高, 主茎节数、总荚数较多, 百粒重较大。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3夏播条件下不同来源MGIII大豆品种农艺性状的比较
PH: 株高; BPH: 底荚高度; NM: 主茎节数; BM: 分枝数; PP: 单株荚数; SW: 百粒重。
Fig. 3Comparison of agronomic traits among MGIII varieties from different regions under summer-sowing condition
PH: plant height; BPH: bottom pods height; NM: node number on the main stem; BM: branch number; PP: pod number per plant; SW: 100-seed weight.
相关分析表明, MGIII组大豆品种的农艺性状与生育期结构之间存在一定的相关性。在夏播条件下, 北方春大豆品种的底荚高度与R/V值极呈显著负相关(P<0.01), 单株荚数与R/V值呈极显著正相关(P<0.01); 黄淮海大豆品种的底荚高度、分枝数、单株荚数、百粒重与R/V间无显著相关性; 南方大豆品种分枝数与V期呈显著负相关(P<0.05)。从全国平均水平总体来看, 大豆品种底荚高度、分枝数与R/V值呈极显著负相关(P<0.01)(表7)。
Table 7
表7
表7不同地理来源MGIII组大豆品种生育期结构与其他农艺性状间的相关系数
Table 7
| 来源地区 Region | 性状 Trait | 株高 PH (cm) | 底荚高度 BPH (cm) | 主茎节数 NM | 分枝数 BM | 单株荚数 PP | 秕荚数 BP | 百粒重 SW (g) |
|---|---|---|---|---|---|---|---|---|
| 北方 Northern China | VE-R1 | -0.244 | 0.937** | 0.185 | -0.370 | -0.847** | -0.819** | 0.451 |
| R1-R7 | -0.303 | -0.674* | -0.412 | 0.527 | 0.688* | 0.681* | -0.783* | |
| R/V | 0.006 | -0.902** | -0.333 | 0.494 | 0.870** | 0.870** | -0.647 | |
| 黄淮海 Yellow-Huai- Hai River Valley | VE-R1 | -0.210 | 0.242 | 0.204 | -0.014 | 0.077 | -0.013 | 0.416 |
| R1-R7 | 0.175 | -0.148 | -0.088 | -0.059 | -0.232 | 0.313 | 0.306 | |
| R/V | 0.250 | -0.220 | -0.152 | -0.036 | -0.189 | 0.100 | -0.148 | |
| 南方 South China | VE-R1 | 0.577 | -0.166 | 0.687 | -0.836* | -0.008 | 0.264 | 0.672 |
| R1-R7 | 0.377 | 0.113 | 0.457 | -0.783 | -0.173 | -0.198 | 0.587 | |
| R/V | 0.069 | 0.251 | 0.083 | -0.350 | -0.207 | -0.381 | 0.214 | |
| 全国平均 Average in China | VE-R1 | -0.118 | 0.738** | 0.268 | 0.351 | 0.110 | 0.337 | -0.118 |
| R1-R7 | 0.306 | -0.597** | 0.078 | -0.597** | -0.013 | 0.228 | 0.306 | |
| R/V | 0.164 | -0.765** | -0.196 | -0.439* | -0.079 | -0.194 | 0.164 |
新窗口打开|下载CSV
3 讨论
3.1 不同来源MGIII组大豆品种生育期结构相关性状的多样性
MGIII组大豆品种分布广泛, 播季类型众多, 它们通过生育期结构的变化, 适应所在地区的自然环境和栽培制度。中国北方春大豆生长在长日、冷凉条件下, 相对较低的温度弱化了长日照对开花的抑制作用[41], 使这些品种在夏至后很快开花, 以保证大豆有足够长的结荚、鼓粒时间, 并在霜前正常成熟。这类品种开花较早, 生殖生长期较长, R/V较高。中国南方春大豆MGIII品种多分布在长江流域, 在春播条件下, 南方春大豆前期生长通常处于温度较低、日照较短的条件下, 低温弱化了短日对大豆发育的促进作用, 导致营养生长期相对较长, 有利于形成较大的营养体, 积累较多的干物质, 其较短的生殖生长期有利于大豆快速鼓粒、成熟, 缓解高温高湿等不利气象条件对种子发育的影响。这类品种开花较晚, R/V较小。美国大豆的祖先亲本主要从我国东北地区引进, 即北美MGIII组大豆品种的选育是以中国东北大豆品种为基础的[42,43], 其生育期性状与中国东北品种相似。以上皆表明, 不同地理来源的大豆品种, 尽管生育期组相同, 但在生育期结构上存在丰富的遗传变异。R/V值的合理调整, 可使大豆的生殖生长阶段处于较好的气象条件下, 规避干旱等逆境的影响。通过选用生育期结构合适的品种或调整播期等措施实现“花雨相遇”是大豆充分利用自然降水的重要措施。在相近生育期组品种分布地区进行穿梭育种则可望选育出广适应品种。中国MGIII组品种分布在北方、黄淮海和南方地区, 可在该组品种中率先开展跨区穿梭育种。3.2 MGIII组大豆品种生育期基因型的复杂性
本研究发现, 中国MGIII大豆品种存在6种E基因型, 而在北美标准品种中只鉴定出一种E基因型, 且与中国北方春大豆部分品种一致。吴存祥[44]利用与大豆成熟期有关的SSR标记, 分析中国国家区域试验对照品种与北美大豆熟期组标准品种的基因型, 发现中国大豆品种在生育期性状上存在更为丰富的遗传变异。Jiang等[35]研究报道, 北美标准品种生育期组与E基因型拟合程度较好, 能较真实地反映基因型的特征, 而中国品种基因型与表型的拟合程度相对较差。正如我们所知, 北美地区大豆生育期组分布区域和纬度是基本平行的[5], 而我国MGIII大豆品种覆盖纬度范围大, 表明生育期性状的遗传变异受地理纬度影响。Liu等[39]研究发现, MGIII大豆品种根据“开花期占全生育期的比重”可被划分出2个亚组, 亚组的划分与品种的复种制度有关, 春播类型前期短后期长, 夏秋播类型前期长后期短。我国各地种植制度多样, 大豆生育期基因型丰富。而北美大豆生产大多采用一年一熟制, 大豆品种类型单一, 生育期结构及基因型相对简单。3.3 生育期主效基因在大豆生育期结构调整和适应性改良中的应用
Li等[38]研究证明E基因组合是决定大豆生育期组归属的主要遗传因素。本研究进一步证明, 不同E基因对大豆的开花期和成熟期均有影响, 且基因间存在加性效应, 这与前人研究结果一致[12,27]。E1可延长大豆的营养生长期, 缩短生殖生长期, 降低R/V值。E1的突变型e1-as可缩短营养生长期, 延长生殖生长期, 提高R/V值, 可作为大豆生育期结构调整的重要基因资源。E2可延迟开花和成熟期, 降低R/V值, 对大豆生育期性状的贡献值仅次于E1。E3延迟开花和成熟期, 但对生育期结构的影响较小。E4延迟开花和成熟期, 在开花后受光周期影响较大。Saindon等[45]研究发现E3的效应大于E4, 且对E4有上位作用。此外, E1~E4同一基因型的大豆品种在生育期性状上表现存在一定差异, 其中, E1e2E3E4类型的MGIII组品种数目最多, 品种间开花期变幅较大, 表明除E1、E2、E3和E4外, 还有其他重要基因参与对大豆生育期性状和光周期反应的调控[27]。在不断挖掘大豆生育期相关基因的基础上, 可针对目标地区的环境条件和耕作制度开展品种生育期结构设计育种, 并根据品种基因型预测其生育期组归属和在特定条件下的开花期、成熟期。当前, 在以北美标准品种为对照、根据生育期相对长度对大豆品种进行生育期组划分时, 可以结合生育期基因型, 进一步校验大豆品种熟期组归属, 为大豆品种跨区适应性评价和相互引种提供依据。对MGIII大豆品种生育期性状与产量性状的相关性分析发现, R/V值与农艺性状的关系在不同地区和播期类型品种中有一定区别, 且以底荚高度、分枝数与R/V值负相关程度最大, 而合适的底荚高度和分枝数对于提高品种的抗逆性和满足人们利用需求有重要意义, 以上研究结果可以为大豆生育期相关基因的有效利用和生育期结构的定向和定量改良提供依据, 为进一步研究R/V与大豆抗逆性的关系以及选育广适应抗逆品种提供帮助。
4 结论
MGIII组大豆品种在生育期结构和生育期基因型方面存在丰富的遗传多样性, 这些变异是复杂多样的地理环境、耕作制度及产量选择共同作用的结果。中国北方和北美春大豆品种开花较早, R/V较高; 黄淮海品种和南方品种开花期相近且较晚, R/V较低。在中国大豆品种中鉴定出6种E基因型, 而供试的美国大豆品种只有一种E基因型。不同E基因在不同背景基因型、生育期阶段和播期下的效应值不尽相同。含显性E位点越多的材料, 其营养生长期越长、生殖生长期越短、R/V值越小, 含e1-as位点的大豆品种R/V值比较大。R/V值与产量性状存在显著的相关性, 从总体上看, 大豆品种的R/V值与底荚高度、分枝数呈显著负相关。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
URL [本文引用: 2]
URL [本文引用: 2]
[本文引用: 3]
[本文引用: 3]
URL [本文引用: 1]

正 本研究于1957年以生育期不同的六个品种,进行了八个不同组合的杂交(表3)。于F_1世代记载了成熟期等性状。于 F_2世代大豆开始成熟时,按不同成熟期组(每五日为一组)分别收获,各组并分别混合脱粒。次年自每成熟期组按组的大小比例随机抽取一定量的 F_2种籽分别种下。于 F_3世代成熟时,除将各组合的各成熟期组材料,仍按不同成熟期分批收获外,对于“东农47-1 G×丰地黄”组合,还记载了开花期株高等性状,以作为
URL [本文引用: 1]

正 本研究于1957年以生育期不同的六个品种,进行了八个不同组合的杂交(表3)。于F_1世代记载了成熟期等性状。于 F_2世代大豆开始成熟时,按不同成熟期组(每五日为一组)分别收获,各组并分别混合脱粒。次年自每成熟期组按组的大小比例随机抽取一定量的 F_2种籽分别种下。于 F_3世代成熟时,除将各组合的各成熟期组材料,仍按不同成熟期分批收获外,对于“东农47-1 G×丰地黄”组合,还记载了开花期株高等性状,以作为
[本文引用: 3]
[本文引用: 3]
URL [本文引用: 4]

大豆属于光温敏感型作物,单一品种适应区域狭窄,跨区引种常导致成熟期过早或过晚,引起产量降低或生产风险。为明确不同地区大豆品种的适宜种植区域,本研究采用国际通用的大豆生育期组划分体系,借助全国大豆产业技术体系试验技术平台,于2011至2015年在全国36个试验点开展大豆生育期组精细划分工作。并根据鉴定结果分析了各生育期组大豆品种的产量和品质分布规律。具体结果如下:(1)根据北美大豆生育期组代表品种在全国不同试验点的表现,明确了不同生态区域自然光照条件下可鉴定大豆生育期组的范围,筛选出黑河、扎兰屯、绥化、长春、北京、徐州、南充、武汉8个试验点作为我国不同大豆产区的代表性鉴定点。2014-2015年,用新引入的北美大豆生育期组标准品种(生育期组精确至0.1)在这8个试验点分别进行春播试验。根据不同试验点大豆生育日数(VE-R7)与生育期组之间的相关关系,分别建立线性回归模型,对原有的生育期尚未精确至0.1的代表品种进行生育期组精细划分,并沿用此法在北方春大豆组19个试验点、黄淮海夏大豆区9个试验点及南方多作大豆区8个试验点对全国共840个大豆主要栽培品种进行了生育期组精细划分。(2)在全国784个地点大豆生产田调查取样,结合品种生育期组信息,利用GIS中Kriging插值的方法对生育期组的分布区域进行可视化分析,完成了基于生育期组的大豆生态区划。在各生育期组中,MGIII和MGIV品种在我国分布区域最广。与美国不同生育期组大豆品种区域分布对比发现,在42°N以北地区,两国大豆生育期组分布情况一致,而在42°N以南地区,因种植制度不同,差异较大。(3)在我国大豆主要产区的17个试验点选取各地适宜生育期组品种及比其早1组和2组的品种进行分期播种试验,明确了各地晚播不同时间段条件下能够安全成熟的品种生育期组范围,并在此基础上形成各地救灾补种品种生育期组搭配方案。研究结果可为各地救灾补种工作及建立救灾备荒种子储备库提供参考。(4)通过分析各生育期组大豆品种的产量及相关的农艺性状可知,各生育期组大豆品种单产水平存在较大差异。其中,MGII大豆品种的单产居第一位,其次为MG0和MGI品种,MGV-IX品种的单产水平最低。MGII和MGV-IX品种的产量变异系数较高,组内产量差异大。比较不同生育期组大豆品种的农艺性状发现,MG000-II的早熟品种株高明显高于MGIII以后的品种。MGV-IX品种的单株荚数最多,MG0品种最少;MGIII和MGIV品种的平均百粒重最高。多元逐步回归结果表明,影响MG0和MGV-IX品种产量的主要因素为种植密度,影响MGI品种产量的主要因素为百粒重,而对MGII-IV品种来说,影响产量的主要因素为单株粒数。(5)对2010-2013年从全国大豆主产区收集到的763份大豆样品进行化学品质检测,分析不同生育期组大豆品种主要品质性状的分布情况。结果表明,我国大豆粗蛋白含量以MGV-IX品种最高,脂肪含量以MGII品种最高。分析大豆蛋白质和脂肪含量与气象因子的关系表明,大豆粗蛋白、水溶性蛋白及蛋脂总和与≥15oC积温、日平均温度呈显著正相关关系,与累计日照时数和昼夜温差呈负相关关系;粗脂肪则呈现出相反的关系。进一步研究表明,大豆粗脂肪含量与日平均温度呈一元二次回归关系,当日平均温度低于19.7oC时,大豆粗脂肪含量与日平均温度呈正相关。通径分析结果表明,昼夜温差是影响大豆粗蛋白、粗脂肪含量及蛋脂总和的最直接的气象因子。根据不同产区的分析结果,明确了各区域大豆品质的主要影响因素,并提出了各区改善大豆品质的农艺措施。
URL [本文引用: 4]

大豆属于光温敏感型作物,单一品种适应区域狭窄,跨区引种常导致成熟期过早或过晚,引起产量降低或生产风险。为明确不同地区大豆品种的适宜种植区域,本研究采用国际通用的大豆生育期组划分体系,借助全国大豆产业技术体系试验技术平台,于2011至2015年在全国36个试验点开展大豆生育期组精细划分工作。并根据鉴定结果分析了各生育期组大豆品种的产量和品质分布规律。具体结果如下:(1)根据北美大豆生育期组代表品种在全国不同试验点的表现,明确了不同生态区域自然光照条件下可鉴定大豆生育期组的范围,筛选出黑河、扎兰屯、绥化、长春、北京、徐州、南充、武汉8个试验点作为我国不同大豆产区的代表性鉴定点。2014-2015年,用新引入的北美大豆生育期组标准品种(生育期组精确至0.1)在这8个试验点分别进行春播试验。根据不同试验点大豆生育日数(VE-R7)与生育期组之间的相关关系,分别建立线性回归模型,对原有的生育期尚未精确至0.1的代表品种进行生育期组精细划分,并沿用此法在北方春大豆组19个试验点、黄淮海夏大豆区9个试验点及南方多作大豆区8个试验点对全国共840个大豆主要栽培品种进行了生育期组精细划分。(2)在全国784个地点大豆生产田调查取样,结合品种生育期组信息,利用GIS中Kriging插值的方法对生育期组的分布区域进行可视化分析,完成了基于生育期组的大豆生态区划。在各生育期组中,MGIII和MGIV品种在我国分布区域最广。与美国不同生育期组大豆品种区域分布对比发现,在42°N以北地区,两国大豆生育期组分布情况一致,而在42°N以南地区,因种植制度不同,差异较大。(3)在我国大豆主要产区的17个试验点选取各地适宜生育期组品种及比其早1组和2组的品种进行分期播种试验,明确了各地晚播不同时间段条件下能够安全成熟的品种生育期组范围,并在此基础上形成各地救灾补种品种生育期组搭配方案。研究结果可为各地救灾补种工作及建立救灾备荒种子储备库提供参考。(4)通过分析各生育期组大豆品种的产量及相关的农艺性状可知,各生育期组大豆品种单产水平存在较大差异。其中,MGII大豆品种的单产居第一位,其次为MG0和MGI品种,MGV-IX品种的单产水平最低。MGII和MGV-IX品种的产量变异系数较高,组内产量差异大。比较不同生育期组大豆品种的农艺性状发现,MG000-II的早熟品种株高明显高于MGIII以后的品种。MGV-IX品种的单株荚数最多,MG0品种最少;MGIII和MGIV品种的平均百粒重最高。多元逐步回归结果表明,影响MG0和MGV-IX品种产量的主要因素为种植密度,影响MGI品种产量的主要因素为百粒重,而对MGII-IV品种来说,影响产量的主要因素为单株粒数。(5)对2010-2013年从全国大豆主产区收集到的763份大豆样品进行化学品质检测,分析不同生育期组大豆品种主要品质性状的分布情况。结果表明,我国大豆粗蛋白含量以MGV-IX品种最高,脂肪含量以MGII品种最高。分析大豆蛋白质和脂肪含量与气象因子的关系表明,大豆粗蛋白、水溶性蛋白及蛋脂总和与≥15oC积温、日平均温度呈显著正相关关系,与累计日照时数和昼夜温差呈负相关关系;粗脂肪则呈现出相反的关系。进一步研究表明,大豆粗脂肪含量与日平均温度呈一元二次回归关系,当日平均温度低于19.7oC时,大豆粗脂肪含量与日平均温度呈正相关。通径分析结果表明,昼夜温差是影响大豆粗蛋白、粗脂肪含量及蛋脂总和的最直接的气象因子。根据不同产区的分析结果,明确了各区域大豆品质的主要影响因素,并提出了各区改善大豆品质的农艺措施。
DOI:10.1371/journal.pone.0094139URL [本文引用: 2]
[本文引用: 1]
DOI:10.2135/cropsci1971.0011183X001100020022xURL [本文引用: 1]

Two independent gene pairs affecting time of flowering and maturity in soybeans (Glycine max (L.) Merr.) were identified and studied in a common genetic background developed by backcrossing to the commercial variety ‘Clark.’ E1, a gene for lateness linked to pubescence color (T t), was transferred from strain T175, and e2, a gene for earliness, from T245. The late allele at each locus was partially dominant in most combinations. Flowering and maturity dates for the homozygotes based on 2 years data at Urbana, Ill., are summarized below as number of days earlier (–) or later (+) than Clark.
DOI:10.1080/00222937100770511URL [本文引用: 1]

The inheritance of flowering time was studied in the short-day soybean, Glycine max (L.) Merr., under long-day conditions in the greenhouse using natural day length extended to 20 hours with cool-white fluorescent light. A single, major gene with two alleles was found to control the flowering response. The dominant allele which gave a fluorescent-sensitive response of delayed flowering also resulted in later field maturity whereas the recessive allele which gave an insensitive response resulted in earlier maturity. The maturity symbols E3 and e3 are proposed for these alleles. Isolines have been developed.
URL [本文引用: 1]

In the soybean (Glycine max (L.) Merr.), the gene combination Fg1 Fg3 is responsible for the glycosylation in the biosynthesis of kaempferol triglucoside (K9) in leaves. The presence of K9 is associated with reduction in chlorophyll content, specific leaf mass, photosynthetic rate and stomatal frequency. Blocking the action of Fg1 Fg3 with the magenta flower gene wm prevents formation of K9... [Show full abstract]
DOI:10.2135/cropsci1987.0011183X002700060008xURL [本文引用: 3]

Maturity genes in soybean [Glycine max (L.) Merr.] have been discovered through accidental cotransfer during backcrossing of other traits. This study evaluated a test for identification of maturity genes in soybean strains that does not require backcrossing such genes into a common genetic background. All eight maturity isolines with homozygous combinations of the genes E1 el, E2 e2, and E3 e3 have been developed by backcrossing with the cultivar ‘Clark’. These isolines were used in testcrosses with Clark, ‘Harosoy’, or ‘Mukden’. Maturity dates (R8) for single field-grown plants of parental and populations were taken in 1982. In 1983, flowering dates (RI) were also recorded, and pod-picked bulk F3 populations were included for the Harosoy testcrosses and Clark isolines. Genotypes were proposed after examining the changes in ranges and variances between testcrosses, and by comparison with the Harosoy and Clark testcross progenies in which the segregating loci were known. Harosoy and Clark were confirmed to have the alleles el e2 E3 and el E2 E3, respectively. The genes El and E2 could be detected singly or together using either flowering or maturity data. The gene E3 could not be reliably detected unless a photoperiod sensitivity follow-up test was utilized. Mukden had the genotype e1e2e3. This test will allow the maturity genotype of unrelated soybean strains to be determined, and make discovery of other genes possible without backcrossing into a common genetic background.
[本文引用: 2]
DOI:10.2135/cropsci2001.413698xURL [本文引用: 1]

An association between early maturity and tawny pubescence has been observed in short-season soybean [Glycine max (L.) Merr.]. The objectives of this study were to determine if a single locus controls early maturity; and if there is a new locus linked to E1 and T or, alternatively, a third allele at the E1 locus. A cross was made between `Harosoy' isolines OT89-5 (e3e3 e4e4) and OT94-47. PI 196529 was the donor of early maturity in the backcrossing program which developed OT94-47. A total of 229 [F.sub.2] plants and the parents were grown under 20-h photoperiods produced by incandescent lamps. OT94-47 flowered in 43 [+ or -] 1.4 d, OT89-5 flowered later in 57 [+ or -] 4.2 d, and the [F.sub.2] population fit a 3 late: 1 early flowering ratio. Early-flowering [F.sub.2] plants produced [F.sub.3] families that flowered similarly to OT94-47. Later-flowering [F.sub. 2] plants either segregated for flowering date or flowered similarly to OT89-5. To test for ailelism with E1 and linkage with T, a cross was made between OT93-26 (E1E1 e3e3 e4e4 TT) and OT94-47. [F.sub.2] plants were classified as parental types or intermediate and equivalent to OT89-5 in maturity. Maturity and pubescence color were recorded in 376 [F.sub.3] progeny rows. The data did not fit a single locus model or a two loci dominant epistasis model (12-3:1). The E7 allele was partially, but not completely, dominant over the e7 allele. Therefore, a new locus E7 is proposed. Using the [F.sub.3] segregation data, linkage between T and E1 was estimated to be 1.3 [+ or -] 0.6 centimorgan (cM). Linkage between E1 and E7 was estimated to be 6.2 cM and linkage between E7 and T was estimated to be 3.9 cM. E7 is a new flowering, maturity, and photoperiod sensitivity locus tightly linked to both E1 and T. E7E7 results in later flowering and maturity, and sensitivity to long photoperiods produced by incandescent lamps when compared to e7e7.
DOI:10.2135/cropsci2009.04.0174URL [本文引用: 1]

The genetic model for maturity in [(L.) Merr.] is a series of near-isogenic lines, but they do not span the natural variation for early maturity. The objectives of this study were to determine if a single gene in OT98-17 controls early maturity and if this is a new locus. A cross was made between ‘Maple Presto’ and OT98-17, an early-maturing Maple Presto–derived backcross line. A total of 201 F3 progeny rows from this population and Maple Presto were grown at Ottawa, ON, in 1999. In 2000, F4 progeny rows were grown and 150 late-maturing and 51 early-maturing families were observed to fit a 3:1 ratio (n = 201, X2 = 0.01, P = 0.90). The early-maturing allele was transferred to a ‘Harosoy’ background, and isolines were grown from 2002 to 2006 at Ottawa, ON. The isolines were 9 and 6 d earlier maturing in Maple Presto and Harosoy backgrounds, respectively. To determine the independence of this locus, simple sequence repeat molecular markers were used to identify three candidate regions. The gene E8 specifically mapped to linkage group C1 between Sat_404 and Satt136. No other maturity gene has been mapped to this region. The two other candidate regions were both related to maturity quantitative trait loci on molecular linkage group L and may be inadvertently selected along with early maturity. The gene symbol E8e8 has been assigned by the Genetics Committee. E8E8 results in later maturity and e8e8 results in early maturity. The earliest Harosoy maturity isoline is now rated as maturity group 000.
DOI:10.2135/cropsci2014.03.0228URL [本文引用: 3]

Abstract Adaptability of soybean to a wide range of latitudes is attributed to the natural variation in the major genes and quantitative trait loci (QTLs) which control flowering time and maturity. Identification of novel genes and understanding their molecular basis are very critical to improve soybean productivity. We identified a new locus conditioning days to flowering and maturity that was detected in a hybrid progeny between cultivated and wild soybeans. A backcross was made between the recurrent parent Tokei 780 and two early-flowering RILs (from the cross Tokei 780 x Hidaka 4, a wild accession), all of which possessed an identical genotype at the major four maturity loci, E1 to E4. The segregation patterns observed in the F2 and F3 progeny derived from the two crosses revealed that early-flowering was controlled by a single dominant gene E9. The new gene was further fine-mapped to a 245-kb interval between markers M5 and M7 on Gm16. A tagging marker ID1 was significantly associated with the variation in days to flowering and maturity in the F2 population. The new early-flowering gene and its tagging marker are very useful for molecular breeding towards early maturity and stable productivity of soybean under high latitude environments. The results presented here has been reviewed by Soybean Genetic Committee and the gene symbol E9e9 has been assigned. E9E9 results in early maturity and e9e9 results in late maturity.
[本文引用: 1]
DOI:10.2135/cropsci1995.0011183X003500040012xURL [本文引用: 1]

Abstract The long-juvenile (LJ) trait in soybean [Glycine mar (L.) Merr.] has been defined as delayed flowering under short-day conditions. The genetic control of this trait remains ambiguous in the literature, and this study was undertaken to determine the genetic control of the LJ trait. Three field experiments were conducted between 1984 and 1998. In the first experiment, segregation patterns of the LJ trait were examined in six F-2 populations from crosses between conventional juvenile (CJ) lines and PI 159925 (source of the LJ trait). Results indicated that the LJ trait is controlled by a single recessive gene influenced by the genetic background in which it occurs. In the second experiment, the confounding effect of other genes segregating for flowering was eliminated by studying F-2 populations from crosses between members of four pairs of near isogenic Lines primarily differing in the presence of the LJ trait. A total of 1954 F-2 plants were observed from eight crosses. Within all F-2 populations, the segregation patterns for the LJ trait had a good fit to a 3:1 ratio of CJ/LJ expected for a single recessive gene. The dominance relationship of the alleles was examined in the third experiment using F-5 and F-6 families developed from seven different crosses. Results indicated that the J allele, which conditions the CJ phenotype, is nearly completely dominant. Our results indicated that the LJ trait is controlled by a single recessive gene and the symbol J/j has been assigned for the alleles conditioning the flowering response (J-, CJ and jj, LJ).
[本文引用: 2]
[本文引用: 2]
[本文引用: 3]
[本文引用: 2]
DOI:10.1534/genetics.108.098772URLPMID:19474204 [本文引用: 3]

Abstract Photosensitivity plays an essential role in the response of plants to their changing environments throughout their life cycle. In soybean [Glycine max (L.) Merrill], several associations between photosensitivity and maturity loci are known, but only limited information at the molecular level is available. The FT3 locus is one of the quantitative trait loci (QTL) for flowering time that corresponds to the maturity locus E3. To identify the gene responsible for this QTL, a map-based cloning strategy was undertaken. One phytochrome A gene (GmPhyA3) was considered a strong candidate for the FT3 locus. Allelism tests and gene sequence comparisons showed that alleles of Misuzudaizu (FT3/FT3; JP28856) and Harosoy (E3/E3; PI548573) were identical. The GmPhyA3 alleles of Moshidou Gong 503 (ft3/ft3; JP27603) and L62-667 (e3/e3; PI547716) showed weak or complete loss of function, respectively. High red/far-red (R/FR) long-day conditions enhanced the effects of the E3/FT3 alleles in various genetic backgrounds. Moreover, a mutant line harboring the nonfunctional GmPhyA3 flowered earlier than the original Bay (E3/E3; PI553043) under similar conditions. These results suggest that the variation in phytochrome A may contribute to the complex systems of soybean flowering response and geographic adaptation.
[本文引用: 3]
[本文引用: 2]
DOI:10.1038/ng.3819URLPMID:28319089 [本文引用: 2]

Soybean is a major legume crop originating in temperate regions, and photoperiod responsiveness is a key factor in its latitudinal adaptation. Varieties from temperate regions introduced to lower latitudes mature early and have extremely low grain yields. Introduction of the long-juvenile (LJ) trait extends the vegetative phase and improves yield under short-day conditions, thereby enabling expansion of cultivation in tropical regions. Here we report the cloning and characterization of J, the major classical locus conferring the LJ trait, and identify J as the ortholog of Arabidopsis thaliana EARLY FLOWERING 3 (ELF3). J depends genetically on the legume-specific flowering repressor E1, and J protein physically associates with the E1 promoter to downregulate its transcription, relieving repression of two important FLOWERING LOCUS T (FT) genes and promoting flowering under short days. Our findings identify an important new component in flowering-time control in soybean and provide new insight into soybean adaptation to tropical regions.
DOI:10.1007/s00122-011-1594-8URL [本文引用: 1]
URL [本文引用: 4]

以大豆生育期近等基因系为材 料,比较12h短日照(SD)及16h长日照(LD)条件下E1/e1、E2/e2、E3/e3、E4/e4、E5/e5、E7/e7等6对生育期相关主 基因的效应。结果表明,在大多数生育期基因型中,显性位点延迟大豆的开花期和成熟期,隐性位点提早开花期和成熟期。同一基因在不同遗传背景下的效应值不 同,显性位点可增强其他基因的效应,说明各基因间存在互作。生育期基因的效应受光周期影响很大,长日照可增强大豆生育期相关基因的效应,短日照则相反。此 外,光周期对基因效应的影响因发育阶段不同而变化,其中,E1基因在大豆营养生长阶段、E4基因在生殖生长阶段受光周期影响较大,而E3基因在营养生长和 生殖发育阶段均受光周期的严格调控。不同光照条件下生育期基因效应的分析结果,可为不同生态区大豆品种生育期性状的定量设计提供依据。
URL [本文引用: 4]

以大豆生育期近等基因系为材 料,比较12h短日照(SD)及16h长日照(LD)条件下E1/e1、E2/e2、E3/e3、E4/e4、E5/e5、E7/e7等6对生育期相关主 基因的效应。结果表明,在大多数生育期基因型中,显性位点延迟大豆的开花期和成熟期,隐性位点提早开花期和成熟期。同一基因在不同遗传背景下的效应值不 同,显性位点可增强其他基因的效应,说明各基因间存在互作。生育期基因的效应受光周期影响很大,长日照可增强大豆生育期相关基因的效应,短日照则相反。此 外,光周期对基因效应的影响因发育阶段不同而变化,其中,E1基因在大豆营养生长阶段、E4基因在生殖生长阶段受光周期影响较大,而E3基因在营养生长和 生殖发育阶段均受光周期的严格调控。不同光照条件下生育期基因效应的分析结果,可为不同生态区大豆品种生育期性状的定量设计提供依据。
DOI:10.1186/s12864-017-3778-3URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/aob/mct269URLPMID:24284817 [本文引用: 3]

Abstract BACKGROUND AND AIMS: The timing of flowering has a direct impact on successful seed production in plants. Flowering of soybean (Glycine max) is controlled by several E loci, and previous studies identified the genes responsible for the flowering loci E1, E2, E3 and E4. However, natural variation in these genes has not been fully elucidated. The aims of this study were the identification of new alleles, establishment of allele diagnoses, examination of allelic combinations for adaptability, and analysis of the integrated effect of these loci on flowering. METHODS: The sequences of these genes and their flanking regions were determined for 39 accessions by primer walking. Systematic discrimination among alleles was performed using DNA markers. Genotypes at the E1-E4 loci were determined for 63 accessions covering several ecological types using DNA markers and sequencing, and flowering times of these accessions at three sowing times were recorded. KEY RESULTS: A new allele with an insertion of a long interspersed nuclear element (LINE) at the promoter of the E1 locus (e1-re) was identified. Insertion and deletion of 36 bases in the eighth intron (E2-in and E2-dl) were observed at the E2 locus. Systematic discrimination among the alleles at the E1-E3 loci was achieved using PCR-based markers. Allelic combinations at the E1-E4 loci were found to be associated with ecological types, and about 62-66 % of variation of flowering time could be attributed to these loci. CONCLUSIONS: The study advances understanding of the combined roles of the E1-E4 loci in flowering and geographic adaptation, and suggests the existence of unidentified genes for flowering in soybean.
DOI:10.1186/1471-2229-13-91URL [本文引用: 3]
In: Krezhovaed D eds.
DOI:10.5772/21085URL [本文引用: 2]

Positional Cloning of the Responsible Genes for Maturity Loci E1, E2 and E3 in Soybean - Author Details | InTechOpen, Published on: 2011-11-07. Authors: Kyuya Harada, Satoshi Watanabe, Xia Zhengjun, et
[本文引用: 2]
[本文引用: 1]
[本文引用: 3]
DOI:10.2135/cropsci1996.0011183X003600050042xURL [本文引用: 1]
DOI:10.1073/pnas.1000088107URLPMID:20421496 [本文引用: 1]

Abstract Determinacy is an agronomically important trait associated with the domestication in soybean (Glycine max). Most soybean cultivars are classifiable into indeterminate and determinate growth habit, whereas Glycine soja, the wild progenitor of soybean, is indeterminate. Indeterminate (Dt1/Dt1) and determinate (dt1/dt1) genotypes, when mated, produce progeny that segregate in a monogenic pattern. Here, we show evidence that Dt1 is a homolog (designated as GmTfl1) of Arabidopsis terminal flower 1 (TFL1), a regulatory gene encoding a signaling protein of shoot meristems. The transition from indeterminate to determinate phenotypes in soybean is associated with independent human selections of four distinct single-nucleotide substitutions in the GmTfl1 gene, each of which led to a single amino acid change. Genetic diversity of a minicore collection of Chinese soybean landraces assessed by simple sequence repeat (SSR) markers and allelic variation at the GmTfl1 locus suggest that human selection for determinacy took place at early stages of landrace radiation. The GmTfl1 allele introduced into a determinate-type (tfl1/tfl1) Arabidopsis mutants fully restored the wild-type (TFL1/TFL1) phenotype, but the Gmtfl1 allele in tfl1/tfl1 mutants did not result in apparent phenotypic change. These observations indicate that GmTfl1 complements the functions of TFL1 in Arabidopsis. However, the GmTfl1 homeolog, despite its more recent divergence from GmTfl1 than from Arabidopsis TFL1, appears to be sub- or neo-functionalized, as revealed by the differential expression of the two genes at multiple plant developmental stages and by allelic analysis at both loci.
[本文引用: 2]
DOI:10.1270/jsbbs.16167URLPMID:5515309 [本文引用: 2]

The maturity date of soybean (Glycine max(L.) Merr.) is sensitive to photoperiod, which varies with latitude and growing seasons. The maturity group (MG) system, composed of 13 MGs, is a major approach in characterizing varieties’ ecological properties and adaptable areas. A total of 512 world soybean varieties, including 48 MG checks, were tested at a major site (Nanjing, 32.04°N) with portions tested in supplementary sites (Heihe, 50.22°N; Mudanjiang, 44.60°N; Jining, 35.38°N and Nanning, 22.84°N) in China to explore the world-wide MG distribution. The maturity date of the world soybean varied greatly (75–201 d) in Nanjing. Along with soybeans disseminated to new areas, the MGs further expanded during the last 70 years from MG I–VII to the early MG 0–000 in the north continents and to the late MG VIII–X in the south continents with the growth period structure differentiated into two subgroups in each MG 0–VIII except V. The cluster analysis among MGs and subgroups using genome-wide markers validated the MG sequential emergence order and the subgroup differentiation in eight MGs. For future evaluation, in addition to one major site (Nanjing), one supplementary southern site (Nanning) and one supplementary northern site (Heihe) are sufficient.
Magsci [本文引用: 2]

组织了濒海与内陆及海拔高低等四组大豆引种对比试验,选同纬度的点两两对此。试验证实了由内陆向海边,由低处向高处引种,出苗到始花发育延迟,始花到成熟发育加速,即前延后促,反向引种则前促后延的推论。试验结果对大豆引种有一定指导意义。
Magsci [本文引用: 2]

组织了濒海与内陆及海拔高低等四组大豆引种对比试验,选同纬度的点两两对此。试验证实了由内陆向海边,由低处向高处引种,出苗到始花发育延迟,始花到成熟发育加速,即前延后促,反向引种则前促后延的推论。试验结果对大豆引种有一定指导意义。
DOI:10.3724/SP.J.1006.2009.01525URL [本文引用: 1]

Soybean [ comprehensive response sensitivity (PTCRS) between ecotypes were observed. The above three indices of spring sowing soybean varieties from the Northeast were all lower than those of summer sowing varieties from Yellow-Huai-Hai River Valleys. However, the differences between TRS values under the two photoperiod treatments and that between PRS values under the two temperature conditions in spring sowing soybean varieties from the Northeast were both larger than those in summer sowing varieties from the Valleys, and it indicated that there was higher photoperiod temperature interaction in the spring sowing varieties. The relationship between photothermal responses of soybean varieties and their ecological adaptability was discussed, and it proposed that, in breeding program, emphasis should be paid not only on the identification of responses of soybean varieties to the individual photoperiod or temperature factor but also on the photothermal interaction.
DOI:10.3724/SP.J.1006.2009.01525URL [本文引用: 1]

Soybean [ comprehensive response sensitivity (PTCRS) between ecotypes were observed. The above three indices of spring sowing soybean varieties from the Northeast were all lower than those of summer sowing varieties from Yellow-Huai-Hai River Valleys. However, the differences between TRS values under the two photoperiod treatments and that between PRS values under the two temperature conditions in spring sowing soybean varieties from the Northeast were both larger than those in summer sowing varieties from the Valleys, and it indicated that there was higher photoperiod temperature interaction in the spring sowing varieties. The relationship between photothermal responses of soybean varieties and their ecological adaptability was discussed, and it proposed that, in breeding program, emphasis should be paid not only on the identification of responses of soybean varieties to the individual photoperiod or temperature factor but also on the photothermal interaction.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
.
[本文引用: 1]
DOI:10.7666/d.Y1499762URL [本文引用: 1]

大豆品种的生态适应性是指大豆在不同生态条件下,协调自身的生长发育与环境之间关系的能力。由于我国大豆种植制度复杂等原因,造成不同生态类型大豆品种生态适应性差异较大。本试验采用分期播种,对中国不同生态类型大豆品种的生态适应性尤其是生育期性状进行研究,以期进一步探讨不同生态类型大豆品种的光温反应模式,建立中国国家大豆品种区域试验品种生态适应性和试点设置合理性的评价体系,解析生育期及生育期结构性状对大豆产量及构成因子的影响,为大豆广适应性育种及不同生态类型品种的合理利用提供理论和技术支撑。 ⑴不同生态类型大豆品种...
DOI:10.7666/d.Y1499762URL [本文引用: 1]

大豆品种的生态适应性是指大豆在不同生态条件下,协调自身的生长发育与环境之间关系的能力。由于我国大豆种植制度复杂等原因,造成不同生态类型大豆品种生态适应性差异较大。本试验采用分期播种,对中国不同生态类型大豆品种的生态适应性尤其是生育期性状进行研究,以期进一步探讨不同生态类型大豆品种的光温反应模式,建立中国国家大豆品种区域试验品种生态适应性和试点设置合理性的评价体系,解析生育期及生育期结构性状对大豆产量及构成因子的影响,为大豆广适应性育种及不同生态类型品种的合理利用提供理论和技术支撑。 ⑴不同生态类型大豆品种...
DOI:10.2135/cropsci1989.0011183X002900060021xURL [本文引用: 1]

Seeds of an open pollinated crop of potato (Solanum tuberosum L.) TPS-2, cv. Kufri Jyoti, were graded into four sizes. There were significant differences between grades for 100-seed weight, the concentrations of total proteins and ethanol soluble proteins, and total lipids and phospholipids. Large seeds contained higher levels (% dry weight) of total proteins, ethanol soluble proteins and alkali soluble proteins than small seeds and they germinated faster and had the highest percentage of germination. Small seeds had the lowest levels of total lipids, phospholipids and water soluble proteins, the longest water saturation time and the lowest germination.
