 ,*, 彭昆鹏*, 张孟珂*, 贾妍*, 王净净*信阳师范学院生命科学学院 / 大别山农业生物资源保护与利用研究院, 河南信阳 464000
,*, 彭昆鹏*, 张孟珂*, 贾妍*, 王净净*信阳师范学院生命科学学院 / 大别山农业生物资源保护与利用研究院, 河南信阳 464000Cloning and Salt Resistance Function Identification of GmHDL57 Gene from Glycine max
KE Dan-Xia ,*, PENG Kun-Peng*, ZHANG Meng-Ke*, JIA Yan*, WANG Jing-Jing*College of Life Sciences / Institute for Conservation and Utilization of Agro-bioresources in Dabie Mountains, Xinyang Normal University, Xinyang 464000, Henan, China
,*, PENG Kun-Peng*, ZHANG Meng-Ke*, JIA Yan*, WANG Jing-Jing*College of Life Sciences / Institute for Conservation and Utilization of Agro-bioresources in Dabie Mountains, Xinyang Normal University, Xinyang 464000, Henan, China通讯作者:
收稿日期:2017-11-3接受日期:2018-06-12网络出版日期:2018-06-30
| 基金资助: |
Received:2017-11-3Accepted:2018-06-12Online:2018-06-30
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (4817KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
柯丹霞, 彭昆鹏, 张孟珂, 贾妍, 王净净. 大豆GmHDL57基因的克隆及抗盐功能鉴定[J]. 作物学报, 2018, 44(9): 1347-1356. doi:10.3724/SP.J.1006.2018.01347
KE Dan-Xia, PENG Kun-Peng, ZHANG Meng-Ke, JIA Yan, WANG Jing-Jing.
大豆是世界上重要的油料、粮食和经济作物, 高盐、干旱、低温等非生物胁迫严重影响了大豆的生长和产量。植物转录因子是一类重要的调控蛋白, 通过与靶基因互作以及不同转录因子之间形成复合物, 从而激活或抑制下游特定的信号转导通路。研究表明, 转录因子在作物抵抗非生物胁迫信号途径中发挥重要作用[1]。因此, 利用生物技术手段超量表达某一特定抗逆转录因子, 是培育大豆耐逆新品种, 提高大豆产量和品质的一条行之有效的途径。
同源异型域亮氨酸拉链蛋白HD-Zip (homeodomain leucine-zipper)是植物特有的一类转录因子, 包含一个由60个或61个氨基酸组成的同源异型结构域HD和一个亮氨酸拉链结构域LZ[2]。参与非生物胁迫应答的主要是HD-Zip I类蛋白[3]。拟南芥中有17个HD-Zip I类蛋白[4], 其中, ATHB5-7和ATHB12受干旱和外源ABA诱导表达, 盐胁迫和渗透胁迫能够诱导ATHB7的表达[5,6]。烟草HD-Zip I类基因NaHD20受失水胁迫诱导, 正调控ABA在叶片中的积累[7]。蒺藜苜蓿MtHB1受NaCl胁迫诱导, 在根中表达量呈上调趋势[8]。紫花苜蓿MsHB2受NaCl和ABA诱导, 负调控植株对盐胁迫的应答反应[9]。野生大豆HD-Zip I类基因Gshdz4受碱胁迫诱导在叶片和根中高表达, 超量表达Gshdz4提高了转基因拟南芥对碳酸盐的耐受性, 这一新发现为揭示野生大豆耐盐碱胁迫机制提供了线索[10]。
目前, 在大豆中鉴定到35个HD-Zip I类转录因子[11,12], 但是有关该类转录因子参与抗逆生理方面的研究报道甚少。早期研究发现1个大豆HD-Zip I类转录因子GmHZ-1正调控大豆花叶病毒(SMV)的感染[13]。我们在前期工作中克隆得到1个大豆HD-Zip I类盐胁迫应答基因GmHAT5, 超量表达GmHAT5显著增强了豆科模式植物百脉根的抗盐能力[14,15]。目前, HD-Zip I类转录因子在拟南芥中的抗逆分子机理研究日趋成熟, 而在大豆中的研究相对滞后。本研究克隆得到大豆的另一个HD-Zip I类基因GmHDL57, 从基因编码蛋白序列结构、系统进化树、基因的时空表达特性等方面进行了生物信息学分析。通过外源脱落酸、NaCl、PEG、冷等模拟非生物胁迫, 探究GmHDL57基因表达对非生物胁迫的响应特征。构建了Ubiquitin启动子驱动GmHDL57基因的植物表达载体, 获得稳定转化的模式豆科植物百脉根, 鉴定了该基因的抗盐功能, 揭示了GmHDL57基因在转基因百脉根抗盐胁迫中的调控机制, 为进一步利用GmHDL57基因创制抗盐大豆新种质奠定了基础。
1 材料与方法
1.1 材料及处理
栽培大豆Williams 82种子经消毒后, 于1/2 Hoagland培养基中萌发生长, 光照培养箱参数设置为温度25℃, 相对湿度60%, 18 h光照/6 h黑暗。待幼苗生长至V1期(第一复叶期)时进行非生物胁迫处理。其中, 3组幼苗分别使用含100 μmol L-1 ABA、含100 mmol L-1 NaCl、含30% PEG-6000的1/2 Hoagland培养液处理。另外1组幼苗移至1/2 Hoagland培养液中, 于4℃培养箱冷处理。每组试验处理18棵苗, 分别于胁迫前0 h、胁迫后1、6、12、24和48 h这6个时间点取样, 每次随机挑选3棵幼苗, 剪取根部组织, 液氮速冻后于-80℃冰箱储存备用。试验重复3次。对另外一批生长至V1期的大豆幼苗, 用含100 mmol L-1 NaCl的1/2 Hoagland培养液处理, 方法同上。设置3个重复, 每个重复18棵苗, 分别于盐胁迫前0 h、胁迫后12、24、48、72和96 h取样, 每个时间点随机挑选3棵幼苗, 分别收集幼苗根、茎、叶组织, 液氮速冻后于-80℃冰箱储存备用。
1.2 GmHDL57基因的克隆与重组质粒的构建
参照TaKaRa公司RNA抽提试剂盒操作说明, 提取各胁迫样品的总RNA。使用天根公司的反转录试剂盒制备cDNA第1链。根据NCBI网站公布的GmHDL57基因序列(GenBank: XM_006574409.2), 设计引物F-GmHDL57 (5°-ATGGCGAGTGGCAAGC TTTATGC-3°)和R-GmHDL57 (5°-TCAATAGGGCCA GAAACAG-3°), 以cDNA第1链为模板扩增目的片段, 大小为1038 bp。目的片段回收纯化后连接T载体测序验证。将测序正确的目的基因插入植物表达载体p1301U中, 上下游引物分别为F-GmHDL57- OX (5°-CGggatccATGGCGAGTGGCAAG-3°)和R- GmHDL57-OX (5°-GGggtaccTCAATAGGGCCAGAA AC-3°), 小写字母区域为酶切位点序列BamH I和Kpn I, 构建过表达载体p1301U- GmHDL57。1.3 GmHDL57基因的生物信息学分析
利用DNAMAN软件确定GmHDL57基因的开放读码框以及编码蛋白的分子量大小; 利用SignaIP-4.1软件预测蛋白的信号肽及切割位点; 利用PSORT 软件分析蛋白的亚细胞定位情况; 利用在线软件ProtScale 预测蛋白的亲水、疏水氨基酸组成情况; 分别运用DictyOGlyc 1.1 Server、NetPhos 2.0 Server在线软件预测GmHDL57基因翻译后磷酸化修饰情况; 使用DNAMAN软件进行同源蛋白多序列比对分析以及系统进化树的构建; 利用SWISS MODEL Workspace 软件在线预测GmHDL57蛋白的三级结构模型。1.4 GmHDL57基因的时空及胁迫表达检测
从转录组数据中[12]获取GmHDL57基因在幼叶、花、1 cm豆荚、开花后10、14 d荚壳、开花后10、14、21、25、28、35、42 d种子、根和根瘤共14个组织中的表达数据, 利用Excel表绘图分析GmHDL57基因的时空表达特征。根据GmHDL57基因序列, 利用Primer 5.0软件设计1对实时荧光定量PCR引物, F-GmHDL57-rt (5°-GAAAACCTTTTGATGACC-3°)和R-GmHDL57- rt (5°-TCAATAGGGCCAGAAACAG-3°), 以各个胁迫样品的cDNA 第1链为模板, 按照TaKaRa公司PrimeScript RT reagent Kit操作说明进行。以大豆肌动蛋白11 (ACT11)为内参基因, 实时荧光定量PCR上游引物为F-ACT11 (5°-ATTTTGACTGAGCGTG GTTATTCC-3°); 下游引物为R-ACT11 (5°-GCTGGT CCTGGCTGTCTCC-3°)。根据相对定量法(ΔΔCt)公式计算结果, 利用Microsoft Excel绘图分析GmHDL57基因在非生物胁迫下的表达特征。
1.5 百脉根稳定转化及盐胁迫处理
采用根癌农杆菌介导的子叶节转化法将GmHDL57基因导入百脉根中, 详细方法参见文献[16]。以转基因和野生型百脉根植株叶片DNA为模板, PCR扩增Gus基因, 引物为F-Gus (5°-ATGT TACGTCCTGTAGAAAC-3°)和R-Gus (5°-TCATT GTTTGCCTCCCTG-3°), PCR产物大小为1812 bp。提取部分PCR阳性植株叶片RNA, 进行GmHDL57基因表达分析(引物同1.4 F-GmHDL57-rt和R-GmHDL57-rt), PCR产物大小为138 bp。以百脉根GPDH为内参基因, 引物为F-GPDH (5°-GCCTCATT CAACATCATTCC-3°)和R-GPDH (5°-CTATGAACA ACAAAAGGTTGC-3°), PCR产物大小为112 bp。收集RT-PCR检测为阳性的植株种子即T0代转基因百脉根种子, 将其与野生型种子表面灭菌, 移入MS培养基上待种子萌发。1周后将幼苗移入含有基质的盆钵中, 3周后选取长势一致的幼苗, 将转基因和野生型植株各分成3组, 每组24 株, 分别用终浓度为0、100和200 mmol L-1 NaCl 的1/8 Hoagland 营养液处理, 设置3个重复, 14 d后照相并测定相关生理生化指标。
1.6 转基因百脉根生理指标的测定
丙二醛含量测定、叶片质膜透性(相对电导率)测定、叶绿素含量测定、根系活力检测以及阳离子Na+、K+和Ca2+含量测定的具体操作方法见参考文献[14]。2 结果与分析
2.1 GmHDL57基因的序列分析
根据基因的已知序列设计引物, 以Williams 82大豆cDNA为模板, 克隆得到与NCBI数据库中公布的基因序列完全一致的GmHDL57基因。该基因包含1个1038 bp的开放读码框, 编码345个氨基酸。GmHDL57蛋白的理论分子量为39.08 kD。利用SignaIP-4.1软件预测GmHDL57蛋白不存在信号肽及切割位点, 不属于分泌蛋白。经在线软件ProtScale 预测GmHDL57蛋白除了C端303~314位和中间215~241位氨基酸为疏水区, 其余大部分区域均为亲水区。运用DictyOGlyc 1.1 Server和NetPhos 2.0 Server 在线软件预测GmHDL57基因翻译后磷酸化修饰情况表明, GmHDL57蛋白有可能在24个位点发生磷酸化修饰, 并且磷酸化位点集中分布在疏水区域。利用PSORT软件预测GmHDL57蛋白的亚细胞定位情况, 发现其定位于细胞核。采用SWISS MODEL Workspace 软件在线预测蛋白的三级结构模型显示, GmHDL57蛋白主要由3个α螺旋、不规则卷曲与延伸链组成(图1)。3个α螺旋是由GmHDL57蛋白的HD区域折叠而成的三维空间结构, 这里是靶基因5°上游区顺式作用元件的专一识别位点, 起着与DNA结合的功能, 因此α螺旋是该蛋白行使生物学功能的基础。LZ区的亮氨酸残基侧链伸出, 与α螺旋紧密相连, 二者共同作用形成一个稳定的拉链状的疏水作用区域。综上, GmHDL57是一个典型的HD-Zip类转录因子。图1
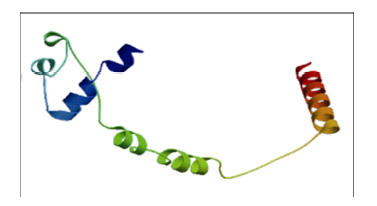 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1GmHDL57蛋白质的三级空间结构预测
Fig. 1Predicted spatial structure of GmHDL57
2.2 GmHDL57的同源蛋白比对及进化树分析
将大豆GmHDL57蛋白与另外10种豆科植物同源蛋白的保守结构域进行序列比对发现, GmHDL57蛋白与野生大豆、木豆、赤豆等物种的同源蛋白氨基酸序列相似性较高, 并且该类蛋白在不同物种中都有2个高度保守的区域, 分别为同源异型框结构域HD和亮氨酸拉链结构域LZ (图2)。图2
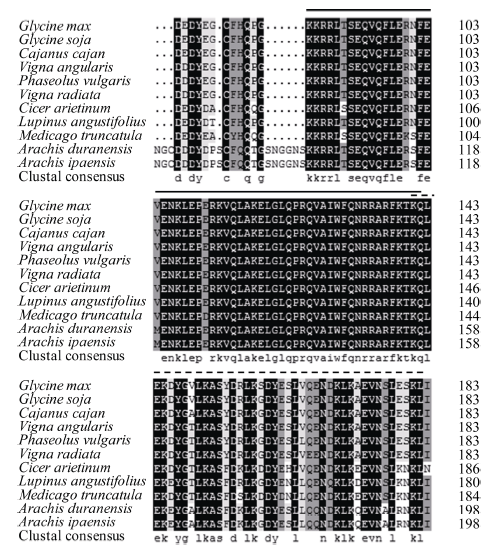 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2大豆GmHDL57与其他植物同源蛋白的保守序列比对分析
Glycine max: 栽培大豆; Glycine soja: 野生大豆; Cajanus cajan: 木豆; Vigna angularis: 赤豆; Phaseolus vulgaris: 菜豆; Vigna radiata: 绿豆; Cicer arietinum: 鹰嘴豆; Lupinus angustifolius: 狭叶羽扇豆; Medicago truncatula: 蒺藜苜蓿; Arachis duranensis: 蔓花生; Arachis ipaensis: 落花生。黑线部分为同源异型框结构域序列, 虚线部分为同源异型框结合类亮氨酸拉链结构域序列。
Fig. 2Conserved amino acid sequence alignment analysis of GmHDL57 and its homologous proteins in some other plants
The sequence marked with solid line demonstrates the homeobox domain, and the sequence marked with dotted line demonstrates the homeobox associated leucine zipper domain.
选取与GmHDL57基因编码的氨基酸序列相似性较高的几种植物的HD-Zip蛋白进行进化树分析。结果表明, 大豆GmHDL57蛋白与野生大豆亲缘关系最近, 其次是木豆、赤豆、菜豆和绿豆, 与鹰嘴豆、狭叶羽扇豆和蒺藜苜蓿的亲缘关系较远, 与蔓花生和落花生的亲缘关系最远(图3)。
图3
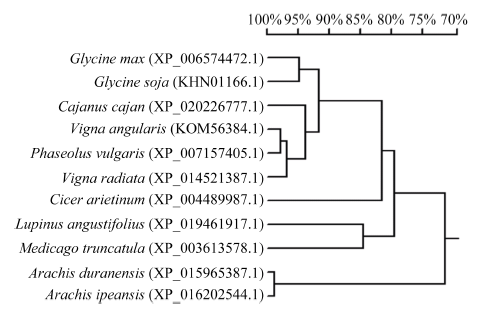 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3大豆GmHDL57与同系物的系统进化分析
Glycine max: 栽培大豆; Glycine soja: 野生大豆; Cajanus cajan: 木豆; Vigna angularis: 赤豆; Phaseolus vulgaris: 菜豆; Vigna radiata: 绿豆; Cicer arietinum: 鹰嘴豆; Lupinus angustifolius: 狭叶羽扇豆; Medicago truncatula: 蒺藜苜蓿; Arachis duranensis: 蔓花生; Arachis ipaensis: 落花生。标尺代表遗传相似性, 表明不同物种间同系物进化关系的远近。
Fig. 3Phylogenetic tree of GmHDL57 and its homologs
The scale represents genetic similarity, indicating the proximity relationships among species.
2.3 GmHDL57基因的时空表达分析
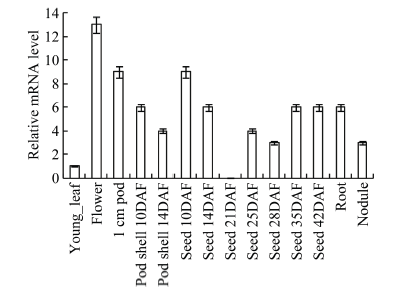
根据转录组数据[12]获取GmHDL57基因的时空表达情况, 从图4可以看出, 在幼叶、花、1 cm豆荚、根、根瘤以及开花后荚壳和种子中都能检测到GmHDL57基因的表达。在生殖器官花、豆荚和10 d种子中该基因表达量较高, 在营养器官幼叶中基因表达量较低。其中, GmHDL57基因在开花后10 d荚壳中的表达量高于14 d荚壳, 在开花后10 d种子中的表达量高于14 d、35 d和42 d种子, 在开花后25 d和28 d种子中表达量较低, 而在开花后21 d种子中未检测到该基因的表达。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4大豆GmHDL57基因在不同器官中的表达分析
横坐标依次为: 幼叶、花、1 cm豆荚、开花后10 d、14 d荚壳、开花后10 d、14 d、21 d、25d、28 d、35 d、42 d种子、根和根瘤; DAF代表开花后的天数。
Fig. 4Relative expression levels of GmHDL57 in different tissues
Abscissa represented young leaf, flower, 1 cm pod, pod shell at 10 d, pod shell at 14 d, seed at 10 d, seed at 14 d, seed at 21 d, seed at 25 d, seed at 28 d, seed at 3 d, seed at 42 d, root and nodule. DAF means days after flower.
2.4 GmHDL57基因在不同胁迫下的表达特征分析
NaCl和PEG胁迫处理1 h时, GmHDL57基因的表达量迅速上升, 且随处理时间的延长表达量持续升高, 至48 h 时达到最大值, 分别为胁迫前的8.5倍和7.2倍。而在ABA胁迫下, GmHDL57基因的表达量在1 h和6 h两个时间点增加缓慢, 至12 h时明显上升, 后又缓缓升高, 至48 h达到胁迫前的3.9倍。4℃冷胁迫处理时, GmHDL57基因的表达量持续下降, 至12 h达最小值, 为胁迫前的0.5倍, 之后趋于稳定, 表现出对冷胁迫的适应性(图5)。图5
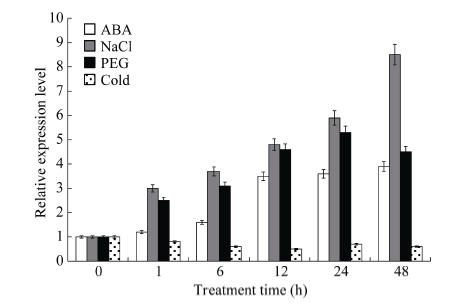 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5大豆GmHDL57基因在非生物胁迫下的表达分析
Fig. 5Expression of GmHDL57 treated with various abiotic stresses
由图5可知, 大豆GmHDL57基因在响应高盐胁迫时基因表达量变化最为明显, 因此我们进一步对盐胁迫下幼苗期大豆根、茎、叶不同组织中GmHDL57基因的表达特征进行了分析。鉴于盐胁迫下该基因的表达量在48 h内一直持续上升, 并没有出现拐点, 因此, 我们延长胁迫处理取样时间至96 h。由图6可知, 盐胁迫前后GmHDL57基因在大豆根中的表达量明显高于茎和叶, 在盐胁迫处理48 h时, GmHDL57基因在根、茎、叶中的表达水平均达到峰值, 在72 h和96 h时表达量缓慢下降, 但仍高于盐胁迫处理前的表达水平。由此推断, 盐胁迫条件下GmHDL57基因具有组织表达特异性, 可能主要在根中参与盐胁迫应答反应。
图6
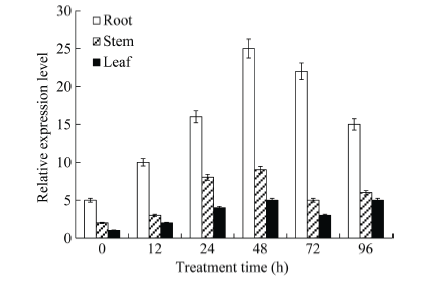 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6盐胁迫下GmHDL57基因在大豆不同组织中的表达特征分析
Fig. 6Expression of GmHDL57 gene in different tissues of soybean under salt stress
2.5 转基因百脉根的获得及抗盐表型分析
通过根癌农杆菌介导的百脉根子叶节转化法, 将构建成功的植物表达载体p1301U-GmHDL57导入百脉根中, 共获得46株卡那霉素抗性植株。经PCR鉴定, 获得28株PCR阳性植株(图7-A)。从中随机挑选5株进行RT-PCR检测, 均能检测到GmHDL57基因的表达(图7-B)。收集RT-PCR检测为阳性的植株种子, 不同株系的转基因种子与野生型种子经消毒在MS培养基上生长1周, 盆钵中生长3周后, 用0、100、200 mmol L-1 NaCl进行盐胁迫处理。14 d后, 100 mmol L-1 NaCl处理的野生型百脉根叶片开始发黄枯萎, 而转基因的2个株系生长状态优于对照; 随着盐浓度的升高, 200 mmol L-1 NaCl处理的野生型百脉根生长受到明显抑制, 大部分植株茎叶枯黄, 生长矮小, 甚至死亡, 而转基因株系生长状态较好, 只有小部分植株叶片开始发黄干枯(图7-C)。图7
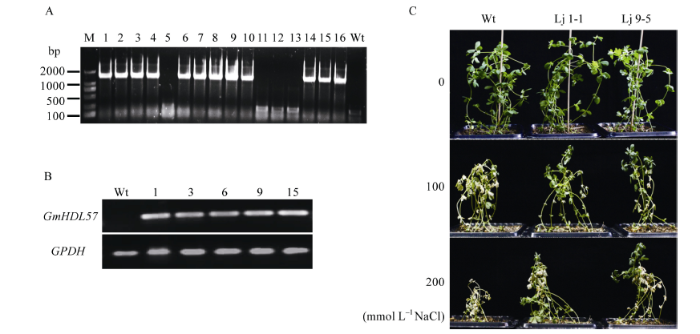 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7转基因百脉根阳性植株检测及抗盐表型鉴定
A: PCR检测植株中GUS基因的表达; B: RT-PCR检测植株中GmHDL57基因的表达; C: 不同盐浓度处理14 d后百脉根的生长状态。Lj 9-5, Lj 1-1: 转基因株系。M: DL2000 DNA marker。
Fig. 7Positive plants detection and salt stress phenotype analysis of transgenic Lotus japonicus
A: detection of GUS gene expression in plants by PCR; B: detection of GmHDL57 gene expression in plants by RT-PCR; C: growth state of Lotus japonicus in different salt concentration treatment for 14 d. Lj 9-5, Lj 1-1: transgenic lines. M: DL2000 DNA marker.
进一步对转基因和野生型百脉根的株高和根长测量统计发现, 在不含NaCl的基质中, 转基因株系和野生型百脉根株高和根长并无明显差异, 随着NaCl浓度的增加, 野生型株高和根长受到明显抑制, 而转基因株系株高和根长受抑制程度较轻(图8)。以上结果表明, GmHDL57基因的过表达能够减缓盐胁迫对植株的毒害作用, 植株的耐盐能力增强。
图8
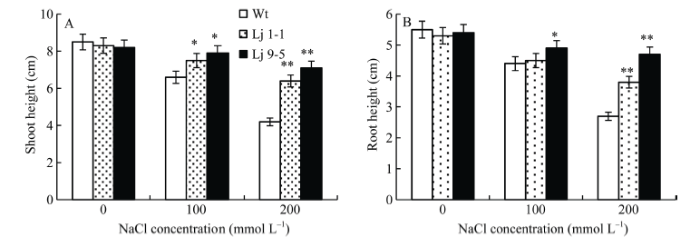 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8盐胁迫对转基因百脉根株高(A)和根长(B)的影响
Lj 9-5, Lj 1-1: 转基因株系。*代表差异显著(P<0.05), **代表差异极显著(P<0.01)。
Fig. 8Changes of shoot height (A) and root length (B) of transgenic Lotus japonicus under salt stress
Lj 9-5, Lj 1-1: transgenic lines. * means significant difference (P<0.05) and ** means extremely significant difference (P<0.01).
2.6 盐胁迫下转基因百脉根相关生理指标检测
从图9可以看出, 在正常条件下, 转基因和野生株系间丙二醛(MDA)含量、叶绿素含量、相对质膜透性以及根系活力的差异不显著。随着盐浓度的增加, 植株叶片MDA含量和相对质膜透性呈升高趋势, 但转基因株系升高幅度显著低于野生型。表明盐胁迫条件下, 转基因百脉根细胞膜的氧化损伤程度较轻, 能够维持较好的细胞膜生理活性。与之相反, 植株叶片叶绿素含量和根系活力随着盐浓度的增加而下降, 但转基因株系下降的幅度显著低于野生型, 表明转基因株系在盐胁迫条件下具备较强的光合能力以及较高的根系活力。图9
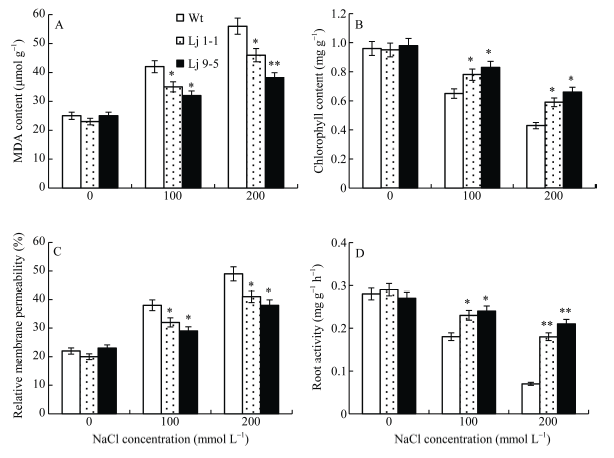 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9盐胁迫下转基因百脉根的生理指标
A: 丙二醛含量; B: 相对质膜透性; C: 叶绿素含量; D: 根系活力。Lj 9-5, Lj 1-1: 转基因株系。*代表差异显著(P<0.05), **代表差异极显著(P<0.01)。
Fig. 9Physiological characteristics of transgenic Lotus japonicus under salt stress
A: MDA content; B: relative membrane permeability; C: chlorophyll content; D: root activity. Lj 9-5, Lj 1-1: transgenic lines. * means significant difference (P<0.05), ** means extremely significant difference (P<0.01).
2.7 盐胁迫下转基因百脉根中阳离子的含量分析
在无盐胁迫下, 转基因和野生型百脉根叶片和根系中Na+、K+和Ca2+含量无明显差异。随着盐浓度的升高, Na+和Ca2+含量均呈现升高趋势, 而K+含量呈下降趋势。与野生型对照相比, 转基因百脉根中Na+含量的增加幅度较小, Ca2+的增加幅度较大, 而K+的下降幅度较小(图10)。以上结果表明, 在高盐胁迫下, GmHDL57基因的过表达能够调节转基因百脉根体内的阳离子含量, 从而增强转基因百脉根对盐的耐受性。图10
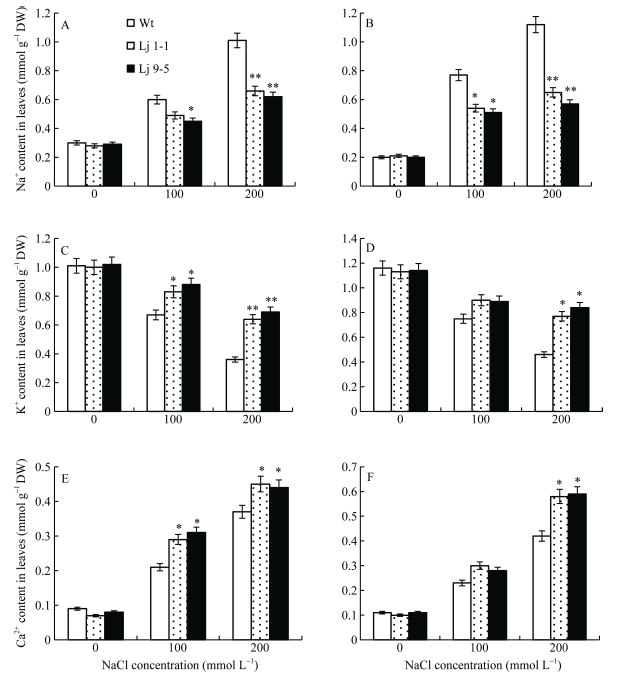 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10盐胁迫下转基因百脉根的阳离子含量
A: 叶片中Na+含量; B: 根中Na+含量; C: 叶片中K+含量; D: 根中K+含量; E: 叶片中Ca2+含量; F: 根中Ca2+含量。Lj 9-5, Lj 1-1: 转基因株系。*代表差异显著(P<0.05), **代表差异极显著(P<0.01)。
Fig. 10Cation content in transgenic Lotus japonicus under salt stress
A: Na+ content in leaves; B: Na + content in roots; C: K + content in leaves; D: K + content in roots; E: Ca2+ content in leaves; F: Ca2+ content in roots. Lj 9-5, Lj 1-1: transgenic lines. * means significant difference (P<0.05) and ** means extremely significant difference (P<0.01).
3 讨论
近年来, 关于植物HD-Zip类转录因子参与非生物胁迫应答反应已成为研究热点。目前的研究主要集中在拟南芥、水稻[17,18]、玉米[19]、杨树[20]、黄瓜[21,22]等植物, 对于大豆中该类蛋白的功能研究报道较少, 尚处于起步阶段。本研究克隆得到1个HD-Zip I类基因GmHDL57, 其编码蛋白具有HD-Zip类家族蛋白典型的保守结构域。GmHDL57基因在大豆植株的各个不同生长阶段及不同器官中均有表达, 生殖生长较营养生长阶段表达量高。拟南芥ATHB2和ATHB7在营养生长和生殖生长阶段均有表达, ATHB3主要在根和茎的皮层中表达, ATHB5和ATHB6主要在根、叶和花中表达, 而ATHB12在根、茎、叶、花、子叶等各部位均有表达[23]。HD-Zip类家族基因表达规律的复杂性决定了该类转录因子功能的多样性。研究表明, 用ABA、NaCl或者低温处理拟南芥幼苗, HD-Zip类基因ATHB6、ATHB7、ATHB12、ATHB 40和ATHB53的表达量上调至对照的2~25倍, 而ATHB52基因的表达量减少为对照的一半。ATHB3、ATHB5和ATHB23受ABA或者NaCl影响, 其表达量下调为对照的0.5倍。ATHB1和ATHB16 的表达不受外源ABA的影响, 经盐胁迫和低温处理后其表达量减少[24]。本研究中大豆GmHDL57基因在外源ABA、NaCl和PEG胁迫处理下, 表达量呈上升趋势, 48 h时达到对照的3.9~8.5倍。而在4℃冷胁迫处理时, GmHDL57基因的表达量整体呈下降趋势, 12 h时降至最低, 为对照的0.5倍。说明大豆GmHDL57基因的表达在盐和干旱胁迫下与ABA胁迫有着相似的应答模式, 而在冷胁迫下与ABA胁迫有着相反的应答模式。进一步对盐胁迫下大豆根、茎、叶不同组织中GmHDL57基因的表达特征分析发现, 盐胁迫条件下GmHDL57基因在根中表达量较高, 具有组织表达特异性, 可能主要在根中发挥功能。
本研究构建了GmHDL57基因的植物表达载体, 通过稳定转化获得了转基因百脉根植株, 并希望通过盐胁迫下转基因及野生型对照株系各项生理指标的测定揭示GmHDL57基因的抗盐功能及调控机制。通常高盐条件下, 植株的丙二醛含量和相对质膜透性会迅速增加, 导致质膜结构的破坏, 影响功能的发挥。本研究中转基因百脉根的丙二醛含量和相对质膜透性明显低于野生型对照, 说明盐胁迫下, 转GmHDL57基因百脉根的膜脂过氧化程度较低, 细胞膜受损程度较小。此外, 在高盐胁迫下, 转基因株系的叶绿素含量和根系活力显著高于野生型对照, 说明转GmHDL57基因百脉根在盐胁迫下具备较强的光合能力以及较高的根系活力。通过对以上生理指标的监测发现, GmHDL57基因的过表达减缓了盐胁迫对百脉根生长的抑制, 从而使转基因百脉根保持较好的生长状态。高盐胁迫影响植株对Na+、K+和Ca2+等阳离子的吸收。细胞质内Na+含量过高会对植物造成不利影响[25]。在植物细胞质内K+以相对较高的浓度存在, 参与调节离子平衡以及稳定细胞内环境[26,27]。Ca2+也是植物生长所必需的, 能够促进细胞对K+的吸收、调节水分平衡、充当第二信使等[28]。因此, 维持细胞质内较低的Na+水平以及较高的K+、Ca2+水平, 对植物抵抗盐胁迫具有重要意义[29]。本试验中, 在同一高盐浓度条件下, 转基因植株中Na+含量明显低于对照, 而K+和Ca2+含量显著高于对照, 说明在高盐胁迫下转基因植株维持较低的Na+水平, 较高的K+和Ca2+水平, 减轻了过量Na+对植株的毒害作用, 从而维持植株的正常生理功能。从盐胁迫诱导、组织表达特征和阳离子含量分析推断, 盐胁迫条件下GmHDL57基因主要在根中发挥功能, 控制Na+向地上部分运输和积累, 调节植物体内离子平衡, 进而降低盐胁迫对植株带来的伤害。
我们推断GmHDL57基因的超量表达能够提高转基因百脉根的耐盐性。目前, 大豆遗传转化效率低仍然极大制约了大豆抗逆分子改良的发展[30,31,32,33,34], 因此优化大豆子叶节遗传转化体系, 获得转GmHDL57基因大豆植株, 进一步探究该基因在大豆体内的功能及其调控机制, 是我们下一步工作的重点。
4 结论
克隆得到一个大豆HD-Zip I类基因GmHDL57, 该基因在大豆植株的各个不同时期及不同器官中均有表达。GmHDL57基因的表达在盐和干旱胁迫下与ABA胁迫应答模式相似, 而在冷胁迫下与ABA胁迫应答模式相反。盐胁迫条件下GmHDL57基因在根中表达量较高, 具有组织表达特异性。在高盐胁迫下, 转基因百脉根的株高、根长、叶绿素含量、根系活力以及阳离子K+、Ca2+含量均显著高于野生型, 而丙二醛含量、相对质膜透性以及Na+的含量明显低于野生型。说明GmHDL57基因可能以不同机制参与了大豆对非生物胁迫的应答过程, 过量表达GmHDL57基因能够显著提高百脉根的抗盐能力。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URL [本文引用: 1]
DOI:10.1093/molbev/msp201URLPMID:2775110 [本文引用: 1]

The full complement of homeobox transcription factor sequences, including genes and pseudogenes, was determined from the analysis of 10 complete genomes from flowering plants, moss,Selaginella, unicellular green algae, and red algae. Our exhaustive genome-wide searches resulted in the discovery in each class of a greater number of homeobox genes than previously reported. All homeobox genes can be unambiguously classified by sequence evolutionary analysis into 14 distinct classes also characterized by conserved intron xon structure and by unique codomain architectures. We identified many new genes belonging to previously defined classes (HD-ZIP I to IV, BEL, KNOX, PLINC, WOX). Other newly identified genes allowed us to characterize PHD, DDT, NDX, and LD genes as members of four new evolutionary classes and to define two additional classes, which we named SAWADEE and PINTOX. Our comprehensive analysis allowed us to identify several newly characterized conserved motifs, including novel zinc finger motifs in SAWADEE and DDT. Members of the BEL and KNOX classes were found in Chlorobionta (green plants) and in Rhodophyta. We found representatives of the DDT, WOX, and PINTOX classes only in green plants, including unicellular green algae, moss, and vascular plants. All 14 homeobox gene classes were represented in flowering plants,Selaginella, and moss, suggesting that they had already differentiated in the last common ancestor of moss and vascular plants.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
DOI:10.1007/s00425-005-1496-6URL [本文引用: 1]

By using cDNA-AFLP, we analyzed a recombinant inbred line population of soybean that was derived from a soybean mosaic virus (SMV) resistant cultivar Kefeng No.1 and a susceptible cultivar Nannong1138-2. One hundred and eight fragments showing polymorphism between SMV resistant and susceptible pools were identified. One fragment w27 was 96 bp in length and showed homology to homeobox ggth with a coding region of 738 bp, encoding a protein of 245 amino acids. The genomic sequence analysis defined an intron of 521 bp in the coding region. GmHZ1 was characterized by the presence of a homeodomain (HD) with a closely linked leucine zipper motif (Zip). Southern blot analysis indicated that there was a single copy of GmHZ1 in the soybean genome. When inoculated with SMV strain N3, resistant and susceptible varieties showed reduced and increased expression of the GmHZ1, respectively. The fusion protein of GmHZ1 with GFP was targeted only in nucleus. Yeast two hybrid studies revealed that the GmHZ1 had transcriptional activation activity and can form homodimer. GmHZ1 can bind two 9-bp pseudopalindromic elements (CAAT(A/T)ATTG and CAAT(C/G)ATTG) with different affinity. Using GUS as a reporter gene, GmHZ1 was proved to be a transcriptional activator and enhanced GUS expression by binding with the two elements in plant cells. These results indicate that the GmHZ1 may have a transcriptional activator function in plant response to SMV infection.
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
DOI:10.1007/s11103-012-9967-1URLPMID:23109182 [本文引用: 1]

Oshox22 belongs to the homeodomain-leucine zipper (HD-Zip) family I of transcription factors, most of which have unknown functions. Here we show that the expression of Oshox22 is strongly induced by salt stress, abscisic acid (ABA), and polyethylene glycol treatment (PEG), and weakly by cold stress. Trans-activation assays in yeast and transient expression analyses in rice protoplasts demonstrated that Oshox22 is able to bind the CAAT(G/C)ATTG element and acts as a transcriptional activator that requires both the HD and Zip domains. Rice plants homozygous for a T-DNA insertion in the promoter region of Oshox22 showed reduced Oshox22 expression and ABA content, decreased sensitivity to ABA, and enhanced tolerance to drought and salt stresses at the seedling stage. In contrast, transgenic rice over-expressing Oshox22 showed increased sensitivity to ABA, increased ABA content, and decreased drought and salt tolerances. Based on these results, we conclude that Oshox22 affects ABA biosynthesis and regulates drought and salt responses through ABA-mediated signal transduction pathways.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.4161/psb.4.2.7692URLPMID:2637487 [本文引用: 1]

Homeodomain-leucine zipper (HD-Zip) proteins are transcription factors unique to plants and are encoded by more than 25 genes in Arabidopsis thaliana. Based on sequence analyses these proteins have been classified into four distinct groups: HD-Zip 0205-0406V. HD-Zip proteins are characterized by the presence of two functional domains; a homeodomain (HD) responsible for DNA binding and a leucine zipper domain (Zip) located immediately C-terminal to the homeodomain and involved in protein-protein interaction. Despite sequence similarities HD-ZIP proteins participate in a variety of processes during plant growth and development. HD-Zip 0205 proteins are generally involved in responses related to abiotic stress, abscisic acid (ABA), blue light, de-etiolation, and embryogenesis. HD-Zip 02050205 proteins participate in light response, shade avoidance, and auxin signalling. Members of the third group (HD-ZipIII) control embryogenesis, leaf polarity, lateral organ initiation, and meristem function. HD-Zip 0205V proteins play significant roles during anthocyanin accumulation, differentiation of epidermal cells, trichome formation, and root development.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1073/pnas.052564899URLPMID:122654 [本文引用: 1]

Environmental stimuli such as UV, pathogen attack, and gravity can induce rapid changes in hydrogen peroxide (H2O2) levels, leading to a variety of physiological responses in plants. Catalase, which is involved in the degradation of H2O2into water and oxygen, is the major H2O2-scavenging enzyme in all aerobic organisms. A close interaction exists between intracellular H2O2and cytosolic calcium in response to biotic and abiotic stresses. Studies indicate that an increase in cytosolic calcium boosts the generation of H2O2. Here we report that calmodulin (CAM), a ubiquitous calcium-binding protein, binds to and activates some plant catalases in the presence of calcium, but calcium/CaM does not have any effect on bacterial, fungal, bovine, or human catalase. These results document that calcium/CaM can down-regulate H2O2levels in plants by stimulating the catalytic activity of plant catalase. Furthermore, these results provide evidence indicating that calcium has dual functions in regulating H2O2homeostasis, which in turn influences redox signaling in response to environmental signals in plants.
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00425-002-0922-2URLPMID:12624759 [本文引用: 1]

The efficiency of soybean [Glycine max (L.) Merrill] transformation was significantly increased from an average of 0.7% to 16.4% by combining strategies to enhance Agrobacterium tumefaciens-mediated T-DNA delivery into cotyledonary-node cells with the development of a rapid, efficient selection protocol based on hygromycin B. Wounded cotyledonary-node explants were inoculated with A. tumefaciens carrying either a standard-binary or super-binary plasmid and co-cultivated in the presence of mixtures of the thiol compounds, L-cysteine, dithiothreitol, and sodium thiosulfate. Transformed shoots began elongating only 8 weeks after co-cultivation. Southern analysis confirmed integration of the T-DNA into genomic DNA and revealed no correlation between the complexity of the integration pattern and thiol treatment applied at co-cultivation. All T0 plants were fertile and the majority of the lines transmitted the 尾-glucuronidase (GUS) phenotype in 3:1 or 15:1 ratios to their progenies.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
